User login
Acting strange after trying to ‘get numb’
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
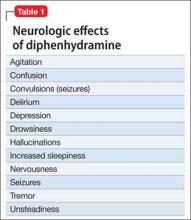
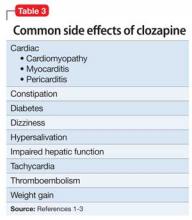
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Numb and confused
Mr. L, age 17, is admitted to the hospital after ingesting 24 diphenhydramine 25-mg tablets in 3 hours as a possible suicide attempt. His parents witnessed him behaving strangely and brought him to the hospital. They state that their son was visibly agitated and acting inappropriately. He was seen talking to birds, trees, and the walls of the house.
Mr. L says he is upset because he broke up with his girlfriend a week earlier after she asked if they could “take a break.” He says that he took the diphenhydramine because he wanted to “get numb” to deal with the emotional stress caused by the break-up.
After the break-up, Mr. L experienced middle-to-late insomnia and was unable to get more than 3 or 4 hours of sleep a night. He reports significant fatigue, depressed mood, anhedonia, impaired concentration, and psychomotor retardation. He denies homicidal ideation or auditory and visual hallucinations.
As an aside, Mr. L reports that, for the past year, he had difficulties with gender identity, sometimes thinking that he might be better off if he had been born a girl and that he felt uncomfortable in a male body.
Which treatment option would you choose for Mr. L’s substance abuse?
a) refer him to a 12-step program
b) begin supportive measures
c) administer activated charcoal
d) prescribe a benzodiazepine to control agitation
The authors’ observations
As youths gain increasing access to medical and pharmaceutical knowledge through the Internet and other sources, it appears that adolescent drug abuse has, in part, shifted toward more easily attainable over-the-counter (OTC) medications. Diphenhydramine, a first-generation antihistamine, can be abused for its effects on the CNS, such as disturbed coordination, irritability, paresthesia, blurred vision, and depression. Effects of diphenhydramine are increased by the presence of alcohol, monoamine oxidase inhibitors, diazepam, hypnotics, sedatives, tranquilizers, and other CNS depressants. In 2011, diphenhydramine abuse was involved in 19,012 emergency room visits, of which 9,301 were for drug-related suicide attempts.1
Diphenhydramine is an inverse agonist of the histamine H1 receptor.2 It is a member of the ethanolamine subclass of antihistaminergic agents.3 By reversing the effects of histamine on capillaries, diphenhydramine can reduce the intensity of allergic symptoms. Diphenhydramine also crosses the blood–brain barrier and antagonizes H1 receptors centrally.
Used as a common sleep aid and allergy medication, the drug works primarily as an H1 receptor partial agonist, but also is a strong competitive antagonist at muscarinic acetylcholine receptors.4 It is abused for its sedative effects and its capacity to cause delirium and hallucinations.5 Diphenhydramine can have a stimulatory effect in children and young adults, instead of the sedating properties seen in adults.6 Such misuse is concerning because diphenhydramine overdose can lead to delirium, confusion, and hallucinations, tachycardia, seizures, mydriasis, xerostomia, urinary retention, ileus, anhidrosis, and hyperthermia. In severe cases it has been associated with cardiac arrhythmias, rhabdomyolysis, status epilepticus, and death.4,6 Neurologic symptoms of diphenhydramine overdose are listed in Table 1.
HISTORY Polysubstance abuse
Mr. L has a 2-year history of major depressive disorder and a history of Cannabis abuse with physiological dependence; Robitussin (base active ingredient, guaifenesin) and hydrocodone abuse with physiological dependence; 3,4-methylenedioxymethamphetamine (MDMA) abuse; and diphenhydramine abuse. He also has a history of gender dysphoria, although he reports that these feelings have become less severe over the past year.
Mr. L attends bi-weekly appointments with an outpatient psychiatrist and reportedly adheres to his medication regimen: fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime. He denies previous suicidal ideation, suicide attempts, homicidal ideation, or homicidal attempts. He reports no history of physical, sexual, or emotional abuse. He gets good grades in school and has no outstanding academic problems.
Mr. L began using Cannabis at age 14; his last use was 3 weeks before admission. He is guarded about his use of Robitussin, hydrocodone, and MDMA. However, Mr. L reports that he has researched diphenhydramine on the internet and believes that he can safely take up to 1,200 mg without overdosing. He reports normally taking 450 mg of diphenhydramine daily. Mr. L reports difficulty urinating after using diphenhydramine but no other physical complaints.
Mr. L lives with his father and stepmother and has a history of one psychiatric hospitalization at a different facility 2 months ago, followed by outpatient therapy. He obtained his Graduate Equivalency Diploma (GED) and plans to attend college.
At age 5, Mr. L emigrated from Turkey to the United States with his parents. His mother returned to Turkey when he was age 6 and has had no contact with her son since. Whenever Mr. L visits Turkey with his father, the patient refuses to see her, as per collaterals. He gets along well with his stepmother, who is his maternal aunt. Mr. L has been bullied at school and reportedly has few friends.
On mental status examination, Mr. L has an appropriate appearance and appears to be his stated age. He shows good eye contact and is cooperative. Muscle tone and gait are within normal limits. He has no abnormal movements. Speech, thought processes, and associations are normal. He denies auditory hallucinations, visual hallucinations, suicidal ideation (although he presented with a probable suicide attempt), or homicidal ideation. No delusions are elicited.
Mr. L shows poor judgment about his drug use and situation. He demonstrates limited insight, because he says his only goal is to get out of the hospital. He is alert, awake, and oriented to person, place, and time. He shows no memory or knowledge impairment. He appears euthymic with an inappropriate and constricted affect. On neurologic exam, he had mild tremors in his hands. The authors’ observationsTreatment for diphenhydramine overdose should begin quickly to prevent life-threatening effects and reduce the risk for mortality. The toxin can be removed from the patient’s GI tract with activated charcoal or gastric lavage if the patient presents within 1 hour of ingesting the substance. Administering IV fluids will prevent dehydration. Cardiac functioning is monitored and benzodiazepines could be administered to manage seizures.
Key elements of a toxicologic physical examination include:
• eyes: pupillary size, symmetry, and response to light (vertical or horizontal nystagmus)
• oropharynx: moist or dry mucous membranes, presence or absence of the gag reflex, distinctive odors
• abdomen: presence or absence and quality of bowel sounds
• skin: warm and dry, warm and sweaty, or cool
• neurologic: level of consciousness and mental status, presence of tremors, seizures, or other movement disorders, presence or absence and quality of deep tendon reflexes.7
If a child or adolescent patient cannot communicate how much of a drug he (she) has ingested, questions to ask parents or other informants include:
• Was the medication purchased recently, and if so was the bottle or box full before the patient took the pills?
• If the medication was not new, how many pills were in the bottle before the patient got to it?
• If the medication was prescribed, how many pills were originally prescribed, when was the medication prescribed, and how many pills were already taken prior to the patient getting to the bottle?
• How many pills were left in the bottle?
• How many pills were seen around the area where the patient was found?
• How many pills were found in the patient’s mouth?7
Recommendations
It is well known that OTC medication abuse is a growing medical problem (Table 2). Antihistamines, including diphenhydramine, are readily available to minors and adults. Because of the powerful sedating effects of antihistamines, many adolescent health practitioners give them to patients who have insomnia as a sleep aid.8 As in our case, antihistamines are used recreationally for their hallucinogenic effects, at dosages of 300 to 700 mg.9 Severe symptoms of toxicity, such as delirium and psychosis, seizures, and coma, occur at dosages ≥1,000 mg.9
With growing abuse of these medications, we aim to encourage detailed history taking about abuse of OTC drugs, especially diphenhydramine in adolescent patients.
Outcome Improvement, discharge
Mr. L is given a dual diagnosis of diphenhydramine-induced psychotic disorder with
hallucinations and diphenhydramine-induced depressive disorder, both with onset during intoxication. He also is given a provisional diagnosis of psychotic disorder not otherwise specified and major depressive disorder. Last, he is given a diagnosis of Cannabis dependence with physiological dependence, MDMA abuse, hydrocodone abuse, and Robitussin abuse.
Mr. L is maintained on fluoxetine, 40 mg/d, and risperidone, 1 mg at bedtime and 0.5 mg in the morning. He receives milieu, individual, group, recreational, and medical therapy while in the hospital. Symptoms abate and he is discharged with a plan to follow up with outpatient providers.
Bottom Line
Abuse of over-the-counter (OTC) drugs, such as diphenhydramine, among youths is a growing problem. Remember to question adolescents who appear intoxicated or to have overdosed not only about abuse of alcohol and illicit substances but also of common—and easily and legally accessible—OTC drugs.
Related Resources
• Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184-188.
• Thomas A, Nallur DG, Jones N, et al. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23(1):101-105.
Drug Brand Names
Diazepam • Valium Hydrocodone • Vicodin
Diphenhydramine • Benadryl Risperidone • Risperdal
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
1. U.S. Department of Health and Human Services. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. http://www.samhsa. gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Published May 2013. Accessed on September 29, 2014.
2. Yamashiro K, Kiryu J, Tsujikawa A, et al. Suppressive effects of histamine H1 receptor antagonist diphenhydramine on the leukocyte infiltration during endotoxin-induced uveitis. Exp Eye Res. 2001;73(1):69-80.
3. Skidgel RA, Kaplan AP, Erdos EG. Histamine, bradykinin, and their antagonists. In: Brunton L, Chabner B, Knollman B, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw Hill; 2011: 911-935.
4. Vearrier D, Curtis JA. Case files of the medical toxicology fellowship at Drexel University. Rhabdomyolysis and compartment syndrome following acute diphenhydramine overdose. J Med Toxicol. 2011;7(3):213-219.
5. Ho M, Tsai K, Liu C. Diphenhydramine overdose related delirium: a case report. Journal of Emergency and Critical Care Medicine. 2006;17(2):77-79.
6. Krenzelok EP, Anderson GM, Mirick M. Massive diphenhydramine overdose resulting in death. Ann Emerg Med. 1982;11(4):212-213.
7. Inaba AS. Toxicologic teasers: Testing your knowledge of clinical toxicology. Hawaii Med J. 1998;57(4):471-473.
8. Kaplan SL. Busner J. The use of prn and stat medication in three child psychiatric inpatient settings. Psychopharmacol Bull. 1997;33(1):161-164.
9. Radovanovic D, Meier PJ, Guirguis M, et al. Dose-dependent toxicity of diphenhydramine overdose. Hum Exp Toxicol. 2000;19(9):489-495.
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
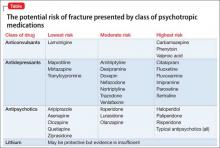
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
A young man with psychosis whose heart is racing
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Opioid use remits, depression remains
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
Case Forgetful and depressed
Mr. B, age 55, has been a patient at our clinic for 8 years, where he has been under our care for treatment-resistant depression and opioid addiction [read about earlier events in his case in “A life of drugs and ‘downtime’” Current Psychiatry, August 2007, p. 98-103].1 He reports feeling intermittently depressed since his teens and has had 3 near-fatal suicide attempts.
Three years ago, Mr. B reported severe depressive symptoms and short-term memory loss, which undermined his job performance and contributed to interpersonal conflict with his wife. The episode has been continuously severe for 10 months. He was taking sertraline, 150 mg/d, and duloxetine, 60 mg/d, for major depressive disorder (MDD) and sublingual buprenorphine/naloxone, 20 mg/d, for opioid dependence, which was in sustained full remission.2 Mr. B scored 24/30 in the Mini- Mental State Examination, indicating mild cognitive deficit. Negative results of a complete routine laboratory workup rule out an organic cause for his deteriorating cognition.
How would you diagnose Mr. B’s condition at this point?
a) treatment-resistant MDD
b) cognitive disorder not otherwise specified
c) opioid use disorder
d) a and c
The authors' observations
Relapse is a core feature of substance use disorders (SUDs) that contributes significantly to the longstanding functional impairment in patients with a mood disorder. With the relapse rate following substance use treatment estimated at more than 60%,3 SUDs often are described as chronic relapsing conditions. In chronic stress, corticotropin-releasing factor (CRF) is over-sensitized; we believe that acute stress can cause an unhealthy response to an over-expressed CRF system.
To prevent relapse in patients with an over-expressed CRF system, it is crucial to manage stress. One treatment option to consider in preventing relapse is mindfulness-based interventions (MBI). Mindfulness has been described as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.” In the event of a relapse, awareness and acceptance fostered by mindfulness may aid in recognizing and minimizing unhealthy responses, such as negative thinking that can increase the risk of relapse.
History Remission, then relapse
Mr. B was admitted to inpatient psychiatric unit after a near-fatal suicide attempt 8 years ago and given a diagnosis of MDD recurrent, severe without psychotic features. Trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
Before he presented to our clinic 8 years ago, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist added methylphenidate, 40 mg/d, to augment the antidepressants, but this did not alleviate Mr. B’s depression.
At age 40, he entered a methadone program, began working steadily, and got married. Five years later, he stopped methadone (it is unclear from the chart if his psychiatrist initiated this change). Mr. B’s depression persisted while using opioids and became worse after stopping methadone.
We considered electroconvulsive therapy (ECT) at the time, but switching the antidepressant or starting ECT would address only the persistent depression; buprenorphine/naloxone would target opioid craving. We started a trial of buprenorphine/ naloxone, a partial μ opioid agonist and ĸ opioid antagonist; ĸ receptor antagonism serves as an antidepressant. He responded well to augmentation of his current regimen (mirtazapine, 30 mg at bedtime, and venlafaxine, 225 mg/d) with buprenorphine/naloxone, 16 mg/d.4,5 he reported no anergia and said he felt more motivated and productive.
Mr. B took buprenorphine/naloxone, 32 mg/d, for 4 years until, because of concern for daytime sedation, his outpatient psychiatrist reduced the dose to 20 mg/d. With the lower dosage of buprenorphine/naloxone initiated 4 years ago, Mr. B reported irritability, anhedonia, insomnia, increased self-criticism, and decreased self-care.
How would you treat Mr. B’s depression at this point?
a) switch to a daytime antidepressant
b) adjust the dosage of buprenorphine/ naloxone
c) try ECT
d) try mindfulness-based cognitive therapy
The authors’ observations
Mindfulness meditation (MM) is a meditation practice that cultivates awareness. While learning MM, the practitioner intentionally focuses on awareness—a way of purposely paying attention to the present moment, non-judgmentally, to nurture calmness and self-acceptance. Being conscious of what the practitioner is doing while he is doing it is the core of mindfulness practice.6
Mindfulness-based interventions. We recommended the following forms of MBI to treat Mr. B:
• Mindfulness-based cognitive therapy (MBCT). MBCT is designed to help people who suffer repeated bouts of depression and chronic unhappiness. It combines the ideas of cognitive-behavioral therapy (CBT) with MM practices and attitudes based on cultivating mindfulness.7
• Mindfulness-based stress reduction (MBSR). MBSR brings together MM and physical/breathing exercises to relax body and mind.6
Chronic stress and drug addiction
The literature demonstrates a significant association between acute and chronic stress and motivation to abuse substances. Stress mobilizes the CRF system to stimulate the hypothalamic-pituitary-adrenal (HPA) axis, and extra-hypothalamic actions of CRF can kindle the neuronal circuits responsible for stress-induced anxiety, dysphoria, and drug abuse behaviors.8
A study to evaluate effects of mindfulness on young adult romantic partners’ HPA responses to conflict stress showed that MM has sex-specific effects on neuroendocrine response to interpersonal stress.9 Research has shown that MM practice can decrease stress, increase well-being, and affect brain structure and function.10 Meta-analysis of studies of animal models and humans described how specific interventions intended to encourage pro-social behavior and well-being might produce plasticity-related changes in the brain.11 This work concluded that, by taking responsibility for the mind and the brain by participating in regular mental exercise, plastic changes in the brain promoted could produce lasting beneficial consequences for social and emotional behavior.11
What could be perpetuating Mr. B’s depression?
a) psychosocial stressors
b) over-expression of CRF gene due to psychosocial stressors
c) a and b
Treatment Mindfulness practice
Mr. B was started on CBT to manage anxiety symptoms and cognitive distortions. After 2 months, he reports no improvements in anxiety, depression, or cognitive distortions.
We consider MBI for Mr. B, which was developed by Segal et al7 to help prevent relapse of depression and gain the benefits of MM. There is evidence that MBI can prevent relapse of SUDs.12 Mr. B’s MBI practice is based on MBCT, as outlined by Segal et al.7 He attends biweekly, 45-minute therapy sessions at our outpatient clinic. During these sessions, MM is practiced for 10 minutes under a psychiatrist’s supervision. The MBCT manual calls for 45 minutes of MM practice but, during the 10-minute session, we instruct Mr. B to independently practice MM at home. Mr. B is assessed for relapses, and drug cravings; a urine toxicology screen is performed every 6 months.
We score Mr. B’s day-to-day level of mindfulness experience, depression, and anxiety symptoms before starting MBI and after 8 weeks of practicing MBI (Figure 1). Mindfulness is scored with the Mindful Attention Awareness Scale (MAAS), a valid, reliable scale.13 The MAAS comprises 15 items designed to reflect mindfulness in everyday experiences, including awareness and attention to thoughts, emotions, actions, and physical states. Items are rated on a 6-point Likert-type scale of 1 (“almost never”) to 6 (“almost always”). A typical item on MAAS is “I find myself doing things without paying attention.”
Depression and anxiety symptoms are measured using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Scale-7 (GAD-7) Item Scale. Mr. B scores a 23 on PHQ-9, indicating severe depression (he reports that he finds it ‘‘extremely difficult” to function) (Figure 2).
There is evidence to support the use of PHQ-9 for measurement-based care in the psychiatric population.14 PHQ-9 does not capture anxiety, which is a strong predicator of suicidal behavior; therefore, we use GAD-7 to measure the severity of Mr. B’s subjective anxiety.15 He scores a 14 on GAD-7 and reports that it is “very difficult” for him to function.
Mr. B is retested after 8 weeks. During those 8 weeks, he was instructed by audio guidance in body scan technique. He practices MBI techniques for 45 minutes every morning between 5 AM and 6 AM.6
After 3 months of MBI, Mr. B is promoted at work and reports that he is handling more responsibilities. He is stressed at his new job and, subsequently, experiences a relapse of anxiety symptoms and insomnia. Partly, this is because Mr. B is not able to consistently practice MBI and misses a few outpatient appointments. In the meantime, he has difficulties with sleep and concentration and anxiety symptoms.
The treating psychiatrist reassures Mr. B and provides support to restart MBI. He manages to attend outpatient clinic appointments consistently and shows interest in practicing MBI daily. Later, he reports practicing MBI consistently along with his routine treatment at our clinic. The timeline of Mr. B’s history and treatment are summarized in Figure 3.
The authors’ observations
Mr. B’s CRF may have been down-regulated by MBI. This, in turn, decreased his depressive and anxiety symptoms, thereby helping to prevent relapse of depression and substance abuse. He benefited from MBI practices in several areas of his life, which can be described with the acronym FACES.10
Flexible. Mr. B became more cognitively flexible. He started to realize that “thoughts are not facts.”7 This change was reflected in his relationship with his wife. His wife came to one of our sessions because she noticed significant change in his attitude toward her. Their marriage of 15 years was riddled with conflict and his wife was excited to see the improvement he achieved within the short time of practicing MBI.
Adaptive. He became more adaptive to changes at the work place and reported that he is enjoying his work. This is a change from his feeling that his job was a burden, as he observed in our earlier sessions.
Coherent. He became more cognitively rational. He reported improvement in his memory and concentration. Five months after initiation of MBI and MM training, he was promoted and could cope with the stress at work.
Energized. Initially, he had said that he never wanted to be part of his extended family. During a session toward the end of the treatment, he mentioned that he made an effort to contact his extended family and reported that he found it more meaningful now to be reconnected with them.
Stable. He became more emotionally stable. He did not have the urge to use drugs and he did not relapse.
As we hypothesized, for Mr. B, practicing MBI was associated with abstinence from substance use, increased mindfulness, acceptance of mental health problems, and remission of psychiatric symptoms.
Bottom Line
Mindfulness-based interventions provide patients with tools to target symptoms such as poor affect regulation, poor impulse control, and rumination. Evidence supports that using MBI in addition to the usual treatment can prevent relapse of a substance use disorder.
Related Resources
• Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiatry. 2012;57(2):63-69.• Lau MA, Grabovac AD. Mindfulness-based interventions: Effective for depression and anxiety. Current Psychiatry. 2009;8(12):39-55.
Drug Brand Names
Aripiprazole • Abilify Mirtazapine • Remeron
Buprenorphine/naloxone • Quetiapine • Seroquel
Suboxone
Bupropion • Wellbutrin Sertraline • Zoloft
Duloxetine • Cymbalta Trazodone • Desyrel
Methadone • Dolophine Venlafaxine • Effexor
Methylphenidate • Ritalin, Concerta
Acknowledgement
The manuscript preparation of Maju Mathew Koola, MD, DPM was supported by the NIMH T32 grant MH067533-07 (PI: William T. Carpenter, MD) and the American Psychiatric Association/Kempf Fund Award for Research Development in Psychobiological Psychiatry (PI: Koola). The treating Psychiatrist PGY-5 (2011-2012) Addiction Psychiatry fellow (Dr. Varghese) was supervised by Dr. Eiger. Drs. Koola and Varghese contributed equally with the manuscript preparation and are joint first authors. Dr. Varghese received a second prize for a poster presentation of this case report at the 34th Indo American Psychiatric Association meeting in San Francisco, CA, May 19, 2013. Christina Mathew, MD, also contributed with manuscript preparation.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufactures of competing products.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
1. Tan EM, Eiger RI, Roth JD. A life of drugs and ‘downtime.’ Current Psychiatry. 2007;6(8):98-103.
2. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC, American Psychiatric Association; 2000.
3. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
4. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects—a possible opioid involvement in severe depression? J Mol Neurosci. 2002; 18(1-2):143-149.
5. Sikka P, Kaushik S, Kumar G, et al. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412-416.
6. Kabat-Zinn J. Full catastrophe living. 15th ed. New York, NY: Bantam Books; 1990.
7. Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach for preventing relapse. New York, NY: Guilford Press; 2002.
8. Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3-14.
9. Laurent H, Laurent S, Hertz R, et al. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology. 2013;38(12):2905-2913.
10. Siegel DJ. The mindful brain: reflection and attunement in the cultivation of well-being. New York, NY: W.W. Norton & Company; 2007.
11. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689-695.
12. Bowen S, Chawla N, Collins SE, et al. Mindfulness-based prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295-305.
13. Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2001). Psychol Assess. 2011;23(4):1034-1040; discussion 1041-1046.
14. Koola MM, Fawcett JA, Kelly DL. Case report on the management of depression in schizoaffective disorder, bipolar type focusing on lithium levels and measurement-based care. J Nerv Ment Dis. 2011;199(12):989-990.
15. Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123.
Confused and nearly naked after going on spending sprees
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Nearly naked
Mr. A, age 68, is found sitting in his car, wearing only a jacket, underpants, and boots. He speaks of spreading a message about Osama bin Laden and “taking a census.” Police officers bring him to a hospital emergency department for evaluation.
The examining clinician determines that Mr. A is a danger to himself and others because of mental illness, leading to admission to our state psychiatric hospital.
Mr. A’s wife describes recent spending sprees with large purchases. She had obtained a restraining order against her husband because of his threatening remarks and behaviors. Within days of the order issuance, he got a home equity loan and purchased a $300,000 house.
The medical history is notable for type 2 diabetes mellitus. Although he is not taking medications, his blood sugar is well controlled. Other than an initial resting heart rate of 116 beats per minute, vital signs are stable and within normal limits. Physical examination is unremarkable. Screening laboratory studies are notable for mildly elevated hepatic function, which approaches normal range several days after admission.
Mr. A reports a remote history of alcohol abuse but says he had not been drinking recently, and does not detail his pattern of use. Urine toxicology screen is negative for all substances of abuse.
Mental status examination reveals disheveled appearance, motor agitation, pressured speech, labile affect, loosening of associations, grandiose delusions, and auditory hallucinations. Mr. A’s thought processes are grossly disorganized, such that we could not gather a meaningful history. He believes God is speaking directly to him about plans to build a carousel at Disney World. He makes strange gestures with his hands throughout the interview, as if attempting to trace the shapes of letters and numbers. He frequently speaks of seeing an array of colors. Cognitive examination reveals a score of 5 of 30 on the Montreal Cognitive Assessment (Figure 1), indicating a severe impairment in neurocognitive functioning. He demonstrates limited insight and markedly impaired judgment, and denies having a mental illness.
What should be the next step in managing Mr. A?
a) obtain records from other facilities and collateral history
b) start an antipsychotic
c) order a brain MRI
d) start an alcohol withdrawal protocol
The authors’ observations
Mr. A showed elements of mania, psychosis, and delirium. We considered a broad differential diagnosis (Table). Mr. A initially could not provide reliable or accurate information. The least invasive next step was to obtain additional history from his wife and other medical records to refine the differential diagnosis.
HISTORY Bizarre behavior
Mr. A allows staff to speak with his wife and obtain records from a psychiatric hospitalization 3 years earlier. Mrs. A reports significant and rapid changes in her husband’s behavior and personality over 3 months, but does not describe a recent alcohol relapse. Mr. A sleeps very little, remaining awake and active throughout the night. He frequently rearranges the furniture in their home for no clear reason. Once, he knocked on the door of a young female neighbor asking if she found him attractive.
Mr. A has a significant criminal history. Approximately 30 years ago, he was charged with attempted murder of his ex-wife and he had faced charges of attempted kidnapping and assaulting a police officer. However, he has no recent legal issues.
Mr. A has a history of episodes that are similar to this presentation. Seven years ago, he impulsively purchased a $650,000 house after his fourth wife died. He then had a $90,000 heart-shaped pool installed. He also drove a tractor through his stepdaughter’s car for no apparent reason. Also, 3 years ago, he displayed symptoms similar to his current presentation, including insomnia, irritability, and grandiosity. He engaged in strange behaviors, such as dressing up and imitating homeless people at his church.
During the hospitalization 3 years ago, clinicians gave Mr. A a diagnosis of bipolar disorder, current episode manic, and delirium of an unclear cause. A medical workup, including brain MRI, did not uncover a basis for his delirium. Antipsychotics (risperidone and perphenazine) and mood stabilizers (lithium and valproic acid), stabilized his condition; after 7 weeks, Mr. A was discharged, but he did not pursue outpatient psychiatric care.
What is the most likely DSM-5 diagnosis?
a) major neurocognitive disorder (dementia)
b) alcohol use disorder (eg, Wernicke- Korsakoff syndrome)
c) delirium secondary to mania
d) psychotic disorder
The authors’ observations
DSM-51 suggests a stepwise approach to diagnosis, with consideration of:
• signs and symptoms
• substance use
• general medical condition
• developmental conflict or stage
• whether a mental disorder is present.
Mr. A’s age and severe cognitive impairment raise the possibility of dementia. Rapid onset, history of similar episodes, and apparent inter-episode recovery make dementia unlikely. The history of alcohol abuse and mildly elevated hepatic function tests suggest a substance use disorder such as Wernicke-Korsakoff syndrome or a withdrawal syndrome. However, there is no evidence of excessive alcohol use over the past several months, toxicology studies were negative, and vital signs were stable. General medical causes for Mr. A’s presentation, such as hypoglycemia, head trauma, intracranial infection, and metabolic disturbance were considered, but physical examination and laboratory studies did not suggest any condition that would explain his condition.
Mr. A’s previous psychiatric hospitalization is critical in clarifying the more likely diagnosis. A similar presentation yielded the diagnosis of bipolar disorder, manic phase. Our working diagnosis, therefore, was bipolar disorder with features of delirious mania.
Delirious mania
Delirious mania was first described by Luther Bell in 1849 and is characterized by an acute and simultaneous onset of mania— severe insomnia, poor judgment, grandiosity, excitement, emotional lability, bizarre hallucinations, and delusions—and delirium—altered consciousness, disorientation, and confusion.2,3 Although there are no diagnostic criteria, some authors suggest that delirious mania is characterized by inappropriate toileting, denudation, profound lack of sleep, and episodic memory impairment that can last hours or days.4 Catatonia frequently is seen with delirious mania.5 Initial case descriptions described a high mortality rate, approaching 75% of patients.6 There is little published literature and no classification of delirious mania in DSM-5.1 Estimates are that delirium is concomitant in 20% to 33% of patients with mania.7,8
Several theories try to clarify the underlying etiology of delirious mania. Jacobowski et al9 summarized the etiology and proposed that it is:
• 1 of 3 types of mania, including: acute and delusional manias, as initially proposed by Kraeplin
• a severe form of catatonia
• a condition akin to, but distinct from, delirium with similar underlying medical causes
• a primary psychiatric disorder underlying the cause of delirium.
EVALUATION Brain changes
For several days, Mr. A continues to engage in strange behavior. He tries to take patients’ belongings, is denudative, crawls on floors, licks walls, is unable to feed himself, and exhibits odd motor movements with purposeless motor activity.
We consult our internal medicine team to identify treatable, medical causes. Results of serum B12, thyroid-stimulating hormone, and rapid plasma reagin studies are within normal limits. Urinalysis is negative. A brain MRI reveals numerous white-matter T2-weighted and FLAIR hyperintensities, indicating small-vessel ischemic changes that are consistent with the findings of an MRI 3 years ago. A sleep-deprived EEG with temporal leads obtained on Day 4 of hospitalization demonstrates a diffusely slow and marginally to poorly organized background, believed to indicate global cerebral dysfunction that is most consistent with nonfocal global encephalopathy. There is no seizure activity. We do not perform a lumbar puncture because of Mr. A’s absence of focal neurologic deficits, lack of fever, and normal white blood cell count.
What is the most appropriate treatment?
a) electroconvulsive therapy (ECT)
b) high-dose benzodiazepine
c) mood stabilizer
d) antipsychotic
The authors’ observations
We strongly suspect that Mr. A has delirious mania. Symptoms and signs of mania include labile mood, excessive spending, grandiosity, insomnia, and psychosis together with delirium (marked disorientation, confusion). We ascribed Mr. A’s odd motor behaviors to catatonia, a hallmark of delirious mania. The literature has little description of EEG findings in suspected cases of delirious mania; however, abnormal EEG tracings have been reported.10 We also speculated that Mr. A’s EEG reflected effects produced by his prescribed antipsychotic regimen.
Treatment
There is no clear consensus on treating delirious mania. Because catatonia is a key feature of delirious mania—whether etiologically or as a prominent sign of the condition—ECT and benzodiazepines are proposed as primary treatments. In a study of 16 patients with delirious mania, Karmacharya et al4 found ECT to be effective, with patients showing improvement after 1 to 4 treatments. Lee et al10 reported similar findings. Although a high-dose benzodiazepine is not as effective as ECT, a 1-time oral dose of 3 to 4 mg of lorazepam has been used to treat delirious mania.
The efficacy of antipsychotic and mood-stabilizing pharmacotherapy is not clear. Bond3 described 3 cases in which patients were treated effectively with a typical antipsychotic (haloperidol or chlorpromazine) and lithium. Jung and Lee11demonstrated the efficacy of atypical antipsychotics, with a marked improvement in symptoms within 1 week. However, other studies do not support these findings. Karmacharya et al4 found that typical antipsychotics 1) make the clinical picture worse by increasing extrapyramidal symptoms and 2) produce inconsistent effects. Mood stabilizers sometimes proved beneficial.
Karmacharya et al4 further argued that the delay in improvement seen with any antipsychotics and mood stabilizers suggest they should not be considered a first-line treatment. These discordant findings are the result of a small number of studies and a lack of understanding of the exact nature of delirious mania.
TREATMENT Quick Response
Mr. A’s symptoms rapidly resolve with a combination of quetiapine, 800 mg/d, haloperidol, 10 mg/d, and lithium, 1,200 mg/d. His mood returns to euthymia and his psychotic symptoms abate. He is able to attend to all activities of daily living. Mental status clears and he is fully oriented and able to hold a logical conversation. He scores 28 out of 30 on a subsequent Montreal Cognitive Assessment, administered 11 days after the initial assessment (Figure 2), indicating normal neurocognitive function. He returns to his baseline level of functioning and is discharged in psychiatrically stable condition. Mr. A has no recollection of the bizarre behaviors he displayed earlier in his hospitalization.
The authors’ observations
We started Mr. A on antipsychotics because of his initial level of agitation. In reviewing pharmacotherapy options for Mr. A’s mania and delirium, we contemplated several options. Quetiapine and lithium were chosen after a review of outside hospital records demonstrated a combination of a mood stabilizer and an antipsychotic was effective in treating a previous similar episode, which led to remission of Mr. A’s symptoms. We chose quetiapine because of it highly sedating properties, suspecting that it would help treat his insomnia. We thought that the risk that lithium would make delirium worse was mitigated by Mr. A’s previous therapeutic response to it. Haloperidol was added for treating delirium, given its more potent D2 antagonism. Mr. A responded quickly to these interventions.
We did not consider ECT at the beginning of Mr. A’s admission, and we avoided sedative-hypnotic agents because we were concerned that a benzodiazepine might make his delirium worse. In light of available data suggesting that ECT and benzodiazepines are preferred treatments for delirious mania, it is noteworthy that Mr. A responded so robustly and rapidly to an antipsychotic and a mood stabilizer.
Bottom Line
Consider delirious mania in any patient who has a history of bipolar disorder presenting with co-occuring symptoms of mania and delirium. Collateral information is vital to establishing a diagnosis. With suspected delirium, rule out concomitant reversible medical problems. Electroconvulsive therapy, high-dose benzodiazepines, antipsychotics, and mood stabilizers have shown efficacy.
Related Resources
• Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
• Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
Drug Brand Names
Chlorpromazine • Thorazine Perphenazine • Trilafon
Haloperidol • Haldol Quetiapine • Seroquel
Lithium • Eskalith Risperidone • Risperdal
Lorazepam • Ativan Valproic acid • Depakene
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Aggressive and delusional about his alien origins, but refusing treatment
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
Psychosis resolves, but menses stop
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
Deaf and self-signing
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
The confused binge drinker
CASE Paranoid and confused
Mr. P, age 46, presents to the emergency department (ED) with a chief complaint of feeling “very weird.” Although he has seen a number of psychiatrists in the past, he does not recall being given a specific diagnosis. He describes his feelings as “1 minute I am fine and the next minute I am confused.” He endorses feeling paranoid for the past 6 to 12 months and reports a history of passive suicidal ideations. On the day he presents to the ED, however, he has a specific plan to shoot himself. He does not report audiovisual hallucinations, but has noticed that he talks to himself often.
Mr. P reports feeling worthless at times. He has a history of manic symptoms, including decreased need for sleep and hypersexuality. He describes verbal and sexual abuse by his foster parents. Mr. P reports using Cannabis and opioids occasionally and to drinking every “now and then” but not every day. He denies using benzodiazepines. When he is evaluated, he is not taking any medication and has no significant medical problems. Mr. P reports a history of several hospitalizations, but he could not describe the reasons or timing of past admissions.
Mr. P has a 10th-grade education. He lives with his fiancée, who reports that he has been behaving oddly for some time. She noticed that he has memory problems and describes violent behavior, such as shaking his fist at her, breaking the television, and attempting to cut his throat once when he was “intoxicated.” She says she does not feel safe around him because of his labile mood and history of
aggression. She confirms that Mr. P does not drink daily but binge-drinks at times.
Initial mental status examination of evaluation reveals hyperverbal, rapid speech. Mr. P is circumstantial and tangential in his thought process. He has poor judgment and insight and exhibits suicidal ideations with a plan. Toxicology screening reveals a blood alcohol level of 50 mg/dL and is positive for Cannabis and opiates.
Which condition most likely accounts for Mr. P’s presentation?
a) bipolar disorder, currently manic
b) substance-induced mood disorder
c) cognitive disorder
d) delirium
TREATMENT Rapid improvement
From the ED, Mr. P was admitted to an inpatient psychiatric unit, where he was found initially to be disoriented to time, place, and person. His thought process remained disorganized and irrational, with significant memory difficulties. He is noted to have an unsteady gait. Nursing staff observes that Mr. P has significant difficulties with activities of daily living and requires assistance. He talks in circles
and uses nonsensical words.
His serum vitamin B12 level, folate level, rapid plasma reagin, magnesium level, and thiamine level are within normal limits; CT scan of the brain is unremarkable. Neuropsychological testing reveals significant and diffuse cognitive deficits suggestive of frontal lobe dysfunction. He is deemed to not have decision-making capacity; because he has no family, his fiancée is appointed as his temporary health care proxy.
Thiamine and lorazepam are prescribed as needed because of Mr. P’s history of alcohol abuse. However, it’s determined that he does not need lorazepam because his vital signs are stable and there is no evidence of alcohol withdrawal symptoms.
During the course of his 10-day hospitalization, Mr. P’s cognitive difficulties resolved. He regains orientation to time, place, and person. He gains skill in all his activities of daily living, to the point of independence, and is discharged with minimal supervision. Vitamin B supplementation is prescribed, with close follow up in an outpatient day program. MRI/SPECT scan is considered to rule out frontotemporal dementia as recommended by the results of his neurocognitive testing profile.
Which condition likely account for Mr. P’s presentation during inpatient hospitalization?
a) Wernicke’s encephalopathy
b) Korsakoff’s syndrome
c) malingering
d) frontotemporal dementia
e) a neurodegenerative disease
The author's observations
Mr. P’s fluctuating mental status, gait instability, and confabulation create high suspicion for Wernicke’s encephalopathy; his dramatic improvement with IV thiamine supports that diagnosis. Mr. P attends the outpatient day program once after his discharge, and is then lost to follow-up.
During inpatient stay, Mr. P eventually admits to binge drinking several times a week, and drinking early in the morning, which would continue throughout the day. His significant cognitive deficits revealed by neuropsychological testing suggests consideration of a differential diagnosis of multifactorial cognitive dysfunction because of:
• long-term substance use
• Korsakoff’s syndrome
• frontotemporal dementia
• a neurodegenerative disease
• malingering (Table 1).
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening neurologic disorder caused by thiamine deficiency. The disease is rare, catastrophic in onset, and clinically complex1; as in Mr. P’s case, diagnosis often is delayed. In autopsy studies, the reported prevalence of Wernicke’s encephalopathy is 0.4% to 2.8%.1 Wernicke’s encephalopathy was suspected before death in 33% of alcohol-dependent patientsand 6% of nonalcoholics.1 Other causes of Wernicke’s encephalopathy include cancer, gastrointestinal surgery, hyperemesis gravidarum, a starvation or malnutrition state, GI tract disease, AIDS, parenteral nutrition, repetitive vomiting, and infection.1
Diagnosis. Making the correct diagnosis is challenging because the clinical presentation can be variable. No lab or imaging studies confirm the diagnosis. The triad of signs considered to support the diagnosis include ocular signs such as nystagmus, cerebellar signs, and confusion. These signs occur in only 8% to 10% of patients in whom the diagnosis likely.1,2
Attempts to increase the likelihood of making an accurate lifetime diagnosis of
Wernicke’s encephalopathy include expanding the focus to 8 clinical domains:
• dietary deficiency
• eye signs
• cerebellar signs
• seizures
• frontal lobe dysfunction
• amnesia
• mild memory impairment
• altered mental status.1
The sensitivity of making a correct diagnosis increases to 85% if at least 2 of 4 features—namely dietary deficiency, eye signs, cerebellar signs, memory impairment, and altered mental status—are present. These criteria can be applied to alcoholic and nonalcoholic patients.1Table 23 lists common and uncommon symptoms of Wernicke’s encephalopathy.
Although CT scan of the brain is not a reliable test for the disorder, MRI can be powerful tool that could support a diagnosis of acute Wernicke’s encephalopathy.1 We did not consider MRI in Mr. P’s case because the consulting neurologist thought this was unnecessary because of the quick improvement in his cognitive status with IV thiamine—although MRI might have helped to detect the disease earlier. In some studies, brain MRI revealed lesions in two-thirds of Wernicke’s encephalopathy patients.1 Typically, lesions are symmetrical and seen in the thalamus, mammillary body, and periaqueductal areas.1,4 Atypical lesions commonly are seen in the cerebellum, dentate nuclei, caudate nucleus, and cerebral cortex.1
Treatment. Evidence supports use of IV thiamine, 200 mg 3 times a day, when the disease is suspected or established.1,2 Thiamine has been associated with sporadic anaphylactic reactions, and should be administered when resuscitation facilities are available. Do not delay treatment because resuscitation measures are unavailable because you risk causing irreversible brain damage.1
In Mr. P’s case, prompt recognition of the need for thiamine likely led to a better outcome. Thiamine supplementation can prevent Wernicke’s encephalopathy in some patients. Prophylactic parenteral administration of thiamine before administration of glucose in the ED is recommended, as well as vitamin B supplementation with thiamine included upon discharge.1,2 Studies support several treatment regimens for patients with Wernicke’s encephalopathy and those at risk of it.1,3,5
Neither the optimal dosage of thiamine nor the appropriate duration of treatment have been determined by randomized, double-blind, controlled studies; empirical clinical practice and recommendations by Royal College of Physicians, London, suggest that a more prolonged course of thiamine—administered as long as improvement continues—might be beneficial.6
Left untreated, Wernicke’s encephalopathy can lead to irreversible brain damage.2
Mortality has been reported as 17% to 20%; 82% of patients develop Korsakoff’s syndrome, a chronic condition characterized by short-term memory loss. One-quarter of patients who develop Korsakoff’s syndrome require long-term residential care because of permanent brain damage.2
Making a diagnosis of Wernicke’s encephalopathy is a challenge because no specific symptom or diagnostic test can be relied upon to confirm the diagnosis. Also, patients might deny that they have an alcohol problem or give an inaccurate history of their alcohol use,2 as Mr. P did. The disorder is substantially underdiagnosed; as a consequence, patients are at risk of brain damage.2
Bottom Line
Not all patients who present with aggressive behavior, mania, and psychiatric
symptoms have a primary psychiatric diagnosis. It is important to consider
nutritional deficiencies caused by chronic alcohol abuse in patients presenting
with acute onset of confusion or altered mental status. Wernicke’s encephalopathy
might be the result of alcohol abuse and can be treated with IV thiamine.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Galvin R, Bråthen G, Ivashynka A, et al; EFNS. Guidelines for diagnosis, therapy and prevention of Wernicke’s encephalopathy. Eur J Neurol. 2010;17(12):
1408-1418.
2. Robinson K. Wernicke’s encephalopathy. Emerg Nurse. 2003;11(5):30-33.
3. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
4. Celik Y, Kaya M. Brain SPECT findings in Wernicke’s encephalopathy. Neurol Sci. 2004;25(1):23-26.
5. Thomson AD, Guerrini I, Marshall JE. Wernicke’s encephalopathy: role of thiamine. Practical Gastroenterology. 2009;33(6):21-30.
6. Thomson AD, Cook CCH, Guerrini I, et al. Wernicke’s encephalopathy: ‘plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008;43:180-186.
CASE Paranoid and confused
Mr. P, age 46, presents to the emergency department (ED) with a chief complaint of feeling “very weird.” Although he has seen a number of psychiatrists in the past, he does not recall being given a specific diagnosis. He describes his feelings as “1 minute I am fine and the next minute I am confused.” He endorses feeling paranoid for the past 6 to 12 months and reports a history of passive suicidal ideations. On the day he presents to the ED, however, he has a specific plan to shoot himself. He does not report audiovisual hallucinations, but has noticed that he talks to himself often.
Mr. P reports feeling worthless at times. He has a history of manic symptoms, including decreased need for sleep and hypersexuality. He describes verbal and sexual abuse by his foster parents. Mr. P reports using Cannabis and opioids occasionally and to drinking every “now and then” but not every day. He denies using benzodiazepines. When he is evaluated, he is not taking any medication and has no significant medical problems. Mr. P reports a history of several hospitalizations, but he could not describe the reasons or timing of past admissions.
Mr. P has a 10th-grade education. He lives with his fiancée, who reports that he has been behaving oddly for some time. She noticed that he has memory problems and describes violent behavior, such as shaking his fist at her, breaking the television, and attempting to cut his throat once when he was “intoxicated.” She says she does not feel safe around him because of his labile mood and history of
aggression. She confirms that Mr. P does not drink daily but binge-drinks at times.
Initial mental status examination of evaluation reveals hyperverbal, rapid speech. Mr. P is circumstantial and tangential in his thought process. He has poor judgment and insight and exhibits suicidal ideations with a plan. Toxicology screening reveals a blood alcohol level of 50 mg/dL and is positive for Cannabis and opiates.
Which condition most likely accounts for Mr. P’s presentation?
a) bipolar disorder, currently manic
b) substance-induced mood disorder
c) cognitive disorder
d) delirium
TREATMENT Rapid improvement
From the ED, Mr. P was admitted to an inpatient psychiatric unit, where he was found initially to be disoriented to time, place, and person. His thought process remained disorganized and irrational, with significant memory difficulties. He is noted to have an unsteady gait. Nursing staff observes that Mr. P has significant difficulties with activities of daily living and requires assistance. He talks in circles
and uses nonsensical words.
His serum vitamin B12 level, folate level, rapid plasma reagin, magnesium level, and thiamine level are within normal limits; CT scan of the brain is unremarkable. Neuropsychological testing reveals significant and diffuse cognitive deficits suggestive of frontal lobe dysfunction. He is deemed to not have decision-making capacity; because he has no family, his fiancée is appointed as his temporary health care proxy.
Thiamine and lorazepam are prescribed as needed because of Mr. P’s history of alcohol abuse. However, it’s determined that he does not need lorazepam because his vital signs are stable and there is no evidence of alcohol withdrawal symptoms.
During the course of his 10-day hospitalization, Mr. P’s cognitive difficulties resolved. He regains orientation to time, place, and person. He gains skill in all his activities of daily living, to the point of independence, and is discharged with minimal supervision. Vitamin B supplementation is prescribed, with close follow up in an outpatient day program. MRI/SPECT scan is considered to rule out frontotemporal dementia as recommended by the results of his neurocognitive testing profile.
Which condition likely account for Mr. P’s presentation during inpatient hospitalization?
a) Wernicke’s encephalopathy
b) Korsakoff’s syndrome
c) malingering
d) frontotemporal dementia
e) a neurodegenerative disease
The author's observations
Mr. P’s fluctuating mental status, gait instability, and confabulation create high suspicion for Wernicke’s encephalopathy; his dramatic improvement with IV thiamine supports that diagnosis. Mr. P attends the outpatient day program once after his discharge, and is then lost to follow-up.
During inpatient stay, Mr. P eventually admits to binge drinking several times a week, and drinking early in the morning, which would continue throughout the day. His significant cognitive deficits revealed by neuropsychological testing suggests consideration of a differential diagnosis of multifactorial cognitive dysfunction because of:
• long-term substance use
• Korsakoff’s syndrome
• frontotemporal dementia
• a neurodegenerative disease
• malingering (Table 1).
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening neurologic disorder caused by thiamine deficiency. The disease is rare, catastrophic in onset, and clinically complex1; as in Mr. P’s case, diagnosis often is delayed. In autopsy studies, the reported prevalence of Wernicke’s encephalopathy is 0.4% to 2.8%.1 Wernicke’s encephalopathy was suspected before death in 33% of alcohol-dependent patientsand 6% of nonalcoholics.1 Other causes of Wernicke’s encephalopathy include cancer, gastrointestinal surgery, hyperemesis gravidarum, a starvation or malnutrition state, GI tract disease, AIDS, parenteral nutrition, repetitive vomiting, and infection.1
Diagnosis. Making the correct diagnosis is challenging because the clinical presentation can be variable. No lab or imaging studies confirm the diagnosis. The triad of signs considered to support the diagnosis include ocular signs such as nystagmus, cerebellar signs, and confusion. These signs occur in only 8% to 10% of patients in whom the diagnosis likely.1,2
Attempts to increase the likelihood of making an accurate lifetime diagnosis of
Wernicke’s encephalopathy include expanding the focus to 8 clinical domains:
• dietary deficiency
• eye signs
• cerebellar signs
• seizures
• frontal lobe dysfunction
• amnesia
• mild memory impairment
• altered mental status.1
The sensitivity of making a correct diagnosis increases to 85% if at least 2 of 4 features—namely dietary deficiency, eye signs, cerebellar signs, memory impairment, and altered mental status—are present. These criteria can be applied to alcoholic and nonalcoholic patients.1Table 23 lists common and uncommon symptoms of Wernicke’s encephalopathy.
Although CT scan of the brain is not a reliable test for the disorder, MRI can be powerful tool that could support a diagnosis of acute Wernicke’s encephalopathy.1 We did not consider MRI in Mr. P’s case because the consulting neurologist thought this was unnecessary because of the quick improvement in his cognitive status with IV thiamine—although MRI might have helped to detect the disease earlier. In some studies, brain MRI revealed lesions in two-thirds of Wernicke’s encephalopathy patients.1 Typically, lesions are symmetrical and seen in the thalamus, mammillary body, and periaqueductal areas.1,4 Atypical lesions commonly are seen in the cerebellum, dentate nuclei, caudate nucleus, and cerebral cortex.1
Treatment. Evidence supports use of IV thiamine, 200 mg 3 times a day, when the disease is suspected or established.1,2 Thiamine has been associated with sporadic anaphylactic reactions, and should be administered when resuscitation facilities are available. Do not delay treatment because resuscitation measures are unavailable because you risk causing irreversible brain damage.1
In Mr. P’s case, prompt recognition of the need for thiamine likely led to a better outcome. Thiamine supplementation can prevent Wernicke’s encephalopathy in some patients. Prophylactic parenteral administration of thiamine before administration of glucose in the ED is recommended, as well as vitamin B supplementation with thiamine included upon discharge.1,2 Studies support several treatment regimens for patients with Wernicke’s encephalopathy and those at risk of it.1,3,5
Neither the optimal dosage of thiamine nor the appropriate duration of treatment have been determined by randomized, double-blind, controlled studies; empirical clinical practice and recommendations by Royal College of Physicians, London, suggest that a more prolonged course of thiamine—administered as long as improvement continues—might be beneficial.6
Left untreated, Wernicke’s encephalopathy can lead to irreversible brain damage.2
Mortality has been reported as 17% to 20%; 82% of patients develop Korsakoff’s syndrome, a chronic condition characterized by short-term memory loss. One-quarter of patients who develop Korsakoff’s syndrome require long-term residential care because of permanent brain damage.2
Making a diagnosis of Wernicke’s encephalopathy is a challenge because no specific symptom or diagnostic test can be relied upon to confirm the diagnosis. Also, patients might deny that they have an alcohol problem or give an inaccurate history of their alcohol use,2 as Mr. P did. The disorder is substantially underdiagnosed; as a consequence, patients are at risk of brain damage.2
Bottom Line
Not all patients who present with aggressive behavior, mania, and psychiatric
symptoms have a primary psychiatric diagnosis. It is important to consider
nutritional deficiencies caused by chronic alcohol abuse in patients presenting
with acute onset of confusion or altered mental status. Wernicke’s encephalopathy
might be the result of alcohol abuse and can be treated with IV thiamine.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Paranoid and confused
Mr. P, age 46, presents to the emergency department (ED) with a chief complaint of feeling “very weird.” Although he has seen a number of psychiatrists in the past, he does not recall being given a specific diagnosis. He describes his feelings as “1 minute I am fine and the next minute I am confused.” He endorses feeling paranoid for the past 6 to 12 months and reports a history of passive suicidal ideations. On the day he presents to the ED, however, he has a specific plan to shoot himself. He does not report audiovisual hallucinations, but has noticed that he talks to himself often.
Mr. P reports feeling worthless at times. He has a history of manic symptoms, including decreased need for sleep and hypersexuality. He describes verbal and sexual abuse by his foster parents. Mr. P reports using Cannabis and opioids occasionally and to drinking every “now and then” but not every day. He denies using benzodiazepines. When he is evaluated, he is not taking any medication and has no significant medical problems. Mr. P reports a history of several hospitalizations, but he could not describe the reasons or timing of past admissions.
Mr. P has a 10th-grade education. He lives with his fiancée, who reports that he has been behaving oddly for some time. She noticed that he has memory problems and describes violent behavior, such as shaking his fist at her, breaking the television, and attempting to cut his throat once when he was “intoxicated.” She says she does not feel safe around him because of his labile mood and history of
aggression. She confirms that Mr. P does not drink daily but binge-drinks at times.
Initial mental status examination of evaluation reveals hyperverbal, rapid speech. Mr. P is circumstantial and tangential in his thought process. He has poor judgment and insight and exhibits suicidal ideations with a plan. Toxicology screening reveals a blood alcohol level of 50 mg/dL and is positive for Cannabis and opiates.
Which condition most likely accounts for Mr. P’s presentation?
a) bipolar disorder, currently manic
b) substance-induced mood disorder
c) cognitive disorder
d) delirium
TREATMENT Rapid improvement
From the ED, Mr. P was admitted to an inpatient psychiatric unit, where he was found initially to be disoriented to time, place, and person. His thought process remained disorganized and irrational, with significant memory difficulties. He is noted to have an unsteady gait. Nursing staff observes that Mr. P has significant difficulties with activities of daily living and requires assistance. He talks in circles
and uses nonsensical words.
His serum vitamin B12 level, folate level, rapid plasma reagin, magnesium level, and thiamine level are within normal limits; CT scan of the brain is unremarkable. Neuropsychological testing reveals significant and diffuse cognitive deficits suggestive of frontal lobe dysfunction. He is deemed to not have decision-making capacity; because he has no family, his fiancée is appointed as his temporary health care proxy.
Thiamine and lorazepam are prescribed as needed because of Mr. P’s history of alcohol abuse. However, it’s determined that he does not need lorazepam because his vital signs are stable and there is no evidence of alcohol withdrawal symptoms.
During the course of his 10-day hospitalization, Mr. P’s cognitive difficulties resolved. He regains orientation to time, place, and person. He gains skill in all his activities of daily living, to the point of independence, and is discharged with minimal supervision. Vitamin B supplementation is prescribed, with close follow up in an outpatient day program. MRI/SPECT scan is considered to rule out frontotemporal dementia as recommended by the results of his neurocognitive testing profile.
Which condition likely account for Mr. P’s presentation during inpatient hospitalization?
a) Wernicke’s encephalopathy
b) Korsakoff’s syndrome
c) malingering
d) frontotemporal dementia
e) a neurodegenerative disease
The author's observations
Mr. P’s fluctuating mental status, gait instability, and confabulation create high suspicion for Wernicke’s encephalopathy; his dramatic improvement with IV thiamine supports that diagnosis. Mr. P attends the outpatient day program once after his discharge, and is then lost to follow-up.
During inpatient stay, Mr. P eventually admits to binge drinking several times a week, and drinking early in the morning, which would continue throughout the day. His significant cognitive deficits revealed by neuropsychological testing suggests consideration of a differential diagnosis of multifactorial cognitive dysfunction because of:
• long-term substance use
• Korsakoff’s syndrome
• frontotemporal dementia
• a neurodegenerative disease
• malingering (Table 1).
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening neurologic disorder caused by thiamine deficiency. The disease is rare, catastrophic in onset, and clinically complex1; as in Mr. P’s case, diagnosis often is delayed. In autopsy studies, the reported prevalence of Wernicke’s encephalopathy is 0.4% to 2.8%.1 Wernicke’s encephalopathy was suspected before death in 33% of alcohol-dependent patientsand 6% of nonalcoholics.1 Other causes of Wernicke’s encephalopathy include cancer, gastrointestinal surgery, hyperemesis gravidarum, a starvation or malnutrition state, GI tract disease, AIDS, parenteral nutrition, repetitive vomiting, and infection.1
Diagnosis. Making the correct diagnosis is challenging because the clinical presentation can be variable. No lab or imaging studies confirm the diagnosis. The triad of signs considered to support the diagnosis include ocular signs such as nystagmus, cerebellar signs, and confusion. These signs occur in only 8% to 10% of patients in whom the diagnosis likely.1,2
Attempts to increase the likelihood of making an accurate lifetime diagnosis of
Wernicke’s encephalopathy include expanding the focus to 8 clinical domains:
• dietary deficiency
• eye signs
• cerebellar signs
• seizures
• frontal lobe dysfunction
• amnesia
• mild memory impairment
• altered mental status.1
The sensitivity of making a correct diagnosis increases to 85% if at least 2 of 4 features—namely dietary deficiency, eye signs, cerebellar signs, memory impairment, and altered mental status—are present. These criteria can be applied to alcoholic and nonalcoholic patients.1Table 23 lists common and uncommon symptoms of Wernicke’s encephalopathy.
Although CT scan of the brain is not a reliable test for the disorder, MRI can be powerful tool that could support a diagnosis of acute Wernicke’s encephalopathy.1 We did not consider MRI in Mr. P’s case because the consulting neurologist thought this was unnecessary because of the quick improvement in his cognitive status with IV thiamine—although MRI might have helped to detect the disease earlier. In some studies, brain MRI revealed lesions in two-thirds of Wernicke’s encephalopathy patients.1 Typically, lesions are symmetrical and seen in the thalamus, mammillary body, and periaqueductal areas.1,4 Atypical lesions commonly are seen in the cerebellum, dentate nuclei, caudate nucleus, and cerebral cortex.1
Treatment. Evidence supports use of IV thiamine, 200 mg 3 times a day, when the disease is suspected or established.1,2 Thiamine has been associated with sporadic anaphylactic reactions, and should be administered when resuscitation facilities are available. Do not delay treatment because resuscitation measures are unavailable because you risk causing irreversible brain damage.1
In Mr. P’s case, prompt recognition of the need for thiamine likely led to a better outcome. Thiamine supplementation can prevent Wernicke’s encephalopathy in some patients. Prophylactic parenteral administration of thiamine before administration of glucose in the ED is recommended, as well as vitamin B supplementation with thiamine included upon discharge.1,2 Studies support several treatment regimens for patients with Wernicke’s encephalopathy and those at risk of it.1,3,5
Neither the optimal dosage of thiamine nor the appropriate duration of treatment have been determined by randomized, double-blind, controlled studies; empirical clinical practice and recommendations by Royal College of Physicians, London, suggest that a more prolonged course of thiamine—administered as long as improvement continues—might be beneficial.6
Left untreated, Wernicke’s encephalopathy can lead to irreversible brain damage.2
Mortality has been reported as 17% to 20%; 82% of patients develop Korsakoff’s syndrome, a chronic condition characterized by short-term memory loss. One-quarter of patients who develop Korsakoff’s syndrome require long-term residential care because of permanent brain damage.2
Making a diagnosis of Wernicke’s encephalopathy is a challenge because no specific symptom or diagnostic test can be relied upon to confirm the diagnosis. Also, patients might deny that they have an alcohol problem or give an inaccurate history of their alcohol use,2 as Mr. P did. The disorder is substantially underdiagnosed; as a consequence, patients are at risk of brain damage.2
Bottom Line
Not all patients who present with aggressive behavior, mania, and psychiatric
symptoms have a primary psychiatric diagnosis. It is important to consider
nutritional deficiencies caused by chronic alcohol abuse in patients presenting
with acute onset of confusion or altered mental status. Wernicke’s encephalopathy
might be the result of alcohol abuse and can be treated with IV thiamine.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Galvin R, Bråthen G, Ivashynka A, et al; EFNS. Guidelines for diagnosis, therapy and prevention of Wernicke’s encephalopathy. Eur J Neurol. 2010;17(12):
1408-1418.
2. Robinson K. Wernicke’s encephalopathy. Emerg Nurse. 2003;11(5):30-33.
3. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
4. Celik Y, Kaya M. Brain SPECT findings in Wernicke’s encephalopathy. Neurol Sci. 2004;25(1):23-26.
5. Thomson AD, Guerrini I, Marshall JE. Wernicke’s encephalopathy: role of thiamine. Practical Gastroenterology. 2009;33(6):21-30.
6. Thomson AD, Cook CCH, Guerrini I, et al. Wernicke’s encephalopathy: ‘plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008;43:180-186.
1. Galvin R, Bråthen G, Ivashynka A, et al; EFNS. Guidelines for diagnosis, therapy and prevention of Wernicke’s encephalopathy. Eur J Neurol. 2010;17(12):
1408-1418.
2. Robinson K. Wernicke’s encephalopathy. Emerg Nurse. 2003;11(5):30-33.
3. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
4. Celik Y, Kaya M. Brain SPECT findings in Wernicke’s encephalopathy. Neurol Sci. 2004;25(1):23-26.
5. Thomson AD, Guerrini I, Marshall JE. Wernicke’s encephalopathy: role of thiamine. Practical Gastroenterology. 2009;33(6):21-30.
6. Thomson AD, Cook CCH, Guerrini I, et al. Wernicke’s encephalopathy: ‘plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008;43:180-186.
Confused, cold, and lethargic
CASE Confused and cold
Ms. K, age 48, is brought to the emergency department (ED) by her husband because she has become increasingly lethargic over the past 2 weeks and cannot attend to activities of daily living. She is incontinent of stool and poorly responsive.
Ms. K’s husband reports that lethargy culminated in his wife sleeping 30 continuous hours. She has a history of a ruptured cerebral arteriovenous malformation (AVM) complicated by a secondary infarct 7 years ago, with residual symptoms of frontal lobe syndrome. Until 2 weeks ago, however, she was in her usual state of health.
Symptoms have included depression, mood lability, impulsivity, disinhibition, poor focus, and apathy. An outpatient psychiatrist has managed these symptoms with antidepressants and atypical antipsychotics.
When Ms. K arrives in the ED, she is taking citalopram, 30 mg/d, and paliperidone,
6 mg/d. Her psychiatrist started paliperidone 2 months ago, increasing the dosage to 6 mg/d 6 weeks before presentation because of worsening mood lability, disinhibition, and paranoia regarding her caregivers. Her husband denies any other medication changes or exposure to environmental toxins.
In the ED, Ms. K is confused and oriented only to person. Vital signs are: pulse 46 bpm; blood pressure, 66/51 mm Hg; respirations, 12/min; and temperature, 29.9ºC (85.8ºF) via bladder probe.
a) major depressive disorder, severe, with catatonic features
b) exposure to cold
c) hypothyroidism
d) drug-induced hypothermia
e) stroke
f) sepsis
g) delirium
The authors’ observations
Hypothermia is core body temperature <35ºC (95ºF).1 It often is caused by exposure to low ambient temperature (Table 1),1 but Ms. K’s husband denied that she had been exposed to cold. Because of Ms. K’s neurologic history, stroke was high on the differential diagnosis, but physical examination did not reveal evidence of focal dysfunction and was significant only for altered mental status.
Ms. K had no posturing, rigidity, negativism, or excessive motor activity that would suggest catatonia. Before she became lethargic, her husband had not noted any deterioration of mood, although she did exhibit other behavioral changes that prompted her outpatient psychiatrist to increase the dosage of paliperidone. Although Ms. K began experiencing persecutory delusions—she believed that her caregivers were trying to harm her—she and her family denied perceptual disturbances. On examination, she did not appear responsive to auditory or visual hallucinations.
Frontal lobe syndrome is defined as a set of changes in the cognitive, behavioral, or emotional domains, often leading to disturbed affect, alteration of attention, aphasia, perseveration, disinhibition, and personality changes.2 These symptoms are not specific to lesions in the frontal lobes but can arise from lesions anywhere in the frontal-striatal-thalamic circuit.3 Causes include traumatic brain injury, neurodegenerative disorders, cerebrovascular disease, tumors, and aging.2 Recommended treatment incorporates psychosocial interventions with drug treatment to target specific symptoms. Medications reported to be effective include typical and atypical antipsychotics to target aggression and agitation; benzodiazepines to reduce arousal; antidepressants for mood symptoms, dopamine agonists (eg, bromocriptine) to decrease apathy, and mood stabilizers to target mood lability.4
Before her AVM rupture, review of Ms. K’s psychiatric history revealed no psychiatric symptoms or impaired functioning. When hospitalized for the AVM repair, she was started on sertraline. She began seeing a psychiatrist 2 years later because of increased agitation and behavioral disturbances, and aripiprazole was added. Persistent agitation prompted a trial of divalproex sodium, which was discontinued because of slurred speech and increased distractibility. Aripiprazole was tapered and replaced with paliperidone because of poor response. Citalopram was initiated 1 year before she presented to the ED.
a) brain MRI
b) infectious evaluation (lumbar puncture with analysis of cerebrospinal fluid, complete blood count, blood cultures, chest radiographs)
c) endocrine panel
d) urine toxicology screen
EVALUATION Hypothermia
Laboratory tests reveal multiple abnormalities, including thrombocytopenia (platelet level, 53 ×103/μL), altered coagulation (partial thromboplastin time, 55.6 s), elevated levels of hepatic transaminases (aspartate aminotransferase, 168 U/L; alanine aminotransferase, 357 U/L), and increased alkaline phosphatase (206 U/L). Other mild metabolic disturbances include: sodium, 149 mEq/L; CO2, 33 mEq/L; and blood urea nitrogen, 24 mg/dL.
These laboratory values are consistent with complications of hypothermia.1
ECG reveals sinus bradycardia (40 bpm) and Osborn waves (additional deflection at the end of the QRS complex), which are seen often in hypothermia.1 Head CT and brain MRI show chronic changes after Ms. K’s right temporoparietal AVM rupture, but no acute abnormality. Urinalysis, blood cultures, and chest radiographs are negative for infection. Urine toxicology screen is negative. Results of thyroid function tests and pituitary hormones studies are significant only for hyperprolactinemia of 155.7 ng/mL, a known adverse effect of antipsychotics.5
Ms. K is admitted and rewarmed passively and with warm IV fluids; by day 10 of hospitalization, temperature is stable (>35.1ºC [95.2ºF]). Thrombocytopenia, transaminitis, and altered mental status resolve.
Ms. K’s oral medications, including citalopram and paliperidone, have been held since admission because of her altered mental status. The psychiatry service is consulted to evaluate whether her presentation could be related to her change of medication.
A literature search reveals no report of paliperidone-induced hypothermia, but we consider it a possible explanation for Ms. K’s presentation. Lamotrigine (titrated to 50 mg/d), a benzodiazepine (oral lorazepam as needed), and discontinuing antipsychotics are recommended. After she returns to her baseline functioning, Ms. K is discharged to a skilled nursing facility.
Ms. K presents to the ED 2 days after discharge with altered mental status. Vital signs are: blood pressure, 90/55 mm Hg; pulse, 59 bpm; respiratory rate, 14/min; and temperature, 34.4ºC (93.9ºF) via bladder probe (Figure). Laboratory tests were significant for hepatic transaminitis (aspartate aminotransferase, 75 U/L; alanine aminotransferase, 122 U/L) and elevated alkaline phosphatase (226 U/L). A review of records from the nursing facility revealed that Ms. K was receiving paliperidone because of an error in the discharge summary, which recommended restarting all prior medications.
The authors’ observations
The Naranjo Causality Scale,6 which categorizes the probability that an adverse event is related to a drug (based on several variables, including timing of the drug administration with the onset of event, drug dosage and levels, response relationships to a drug, including re-challenge when possible, and previous patient experience with the medication), often is used to evaluate whether an adverse clinical event has been caused by a drug (Table 2). We applied the Scale to Ms. K’s case, which revealed a score of 7—indicating a probable adverse drug reaction. The sequence of events in Ms. K’s case that led to a paliperidone challenge-dechallenge-rechallenge, and the resulting hypothermia, are, we concluded, evidence of an adverse drug reaction.
Using the World Health Organization database for adverse drug reactions, van Marum et al7 found 480 reports hypothermia with antipsychotics as of 2007 (compared with 524 reports of hyperthermia in the same period); 55% involved atypical antipsychotics, mainly risperidone. There are no case reports of paliperidone-induced hypothermia; however, several reports of hypothermia have been attributed to risperidone, and paliperidone is the primary active metabolite of risperidone.5
To identify risk factors for hypothermia with antipsychotic use, van Marum et al7 performed a literature search for case reports of antipsychotic-induced hypothermia, which revealed no association with age or sex. The most common diagnosis in cases of antipsychotic-induced hypothermia was schizophrenia (51%). In 73% of the cases, hypothermia followed the start or dosage increase of the antipsychotic. These observations have been noted in case reports and case series of hypothermia associated with antipsychotic use.8-12
Mechanism of action
One proposed mechanism for antipsychotic-induced hypothermia includes preferential 5-HT2A receptor antagonism over D2 receptor antagonism.7,12 It has been believed that, under normal conditions, the action of dopamine to reduce body temperature and the action of serotonin to elevate it are in balance.9
Another possible mechanism is peripheral á2-adrenergic blockade, which might increase the hypothermic effect by inhibiting peripheral responses to cooling, such as vasoconstriction and shivering.7,8 Boschi et al13 found that antipsychotics cause hypothermia in rats when the drug is administered intraperitoneally but not when given intrathecally. Perhaps for these reasons, in the early 1950s, before its psychotropic properties were known, chlorpromazine was used during surgery to induce artificial hibernation and suppress the body’s response to cooling.7 The therapeutic activity of paliperidone is mediated though a D2, 5-HT2A, and á2-receptor antagonism5; these mechanisms could, therefore, be contributing to Ms. K’s hypothermia.
Patients with preexisting brain damage— such as Ms. K—might be at increased risk of antipsychotic-induced hypothermia.7,8 This includes focal damage to central thermoregulatory centers, such as the pre-optic anterior hypothalamic region,14 and more diffuse damage seen in patients with cognitive impairment or a seizure disorder.8
Studies of people with schizophrenia show a decrease in core temperature after administration of an antipsychotic,15 raising the possibility of an impairment of baseline thermoregulatory control. Such thermal dysregulation in patients with schizophrenia might be explained by changes in neurotensin levels.7
The neuropeptide neurotensin has been implicated in the regulation of prolactin release and interacts to a significant degree with the dopaminergic system.16 When administered to animals, neurotensin suppresses heat production and increases heat loss.17 The neurotensin level in CSF was found to be lower in non-medicated patients with schizophrenia than in healthy controls, with an inverse correlation between the severity of symptoms and the neurotensin level.18
Additionally, persons with schizophrenia might be at increased risk of developing hypothermia when exposed to a low environmental temperature.7,8 Kudoh et al19 investigated temperature regulation during anesthesia in patients with chronic (≥7 years) schizophrenia receiving antipsychotics, and compared findings against what was seen in controls. The team reported that patients with schizophrenia had significantly lower intraoperative temperatures.
A published analysis of cases and studies of antipsychotic-induced hypothermia describes the combination of drug variables, patient variables, and environmental variables that contribute to thermal dysregulation (Table 3).7-12,15 The recommendation for practitioners is that, when considering an antipsychotic for a patient at high risk of thermal dysregulation, your choice of an agent should take that risk into account, especially when that drug is one that has comparatively stronger serotonergic and peripheral á-adrenergic effects. You should monitor patients closely for hypothermia after starting and when increasing the dosage of the drug. In patients with schizophrenia who might have a problem with baseline thermoregulation, advise them to take measures to counteract their increased susceptibility to low ambient temperatures.
OUTCOME Readmission
Ms. K was readmitted, rewarmed, and discharged to a skilled nursing facility 4 days later, after baseline function returned to normal and temperature stabilized. Paliperidone is now listed in her electronic medical record as “drug intolerance.”
This case also highlights the importance of adequate medication reconciliation at
admission and discharge, especially when using an electronic medical record system, because what might otherwise be considered a minor mistake can have devastating consequences.
Bottom Line
Thermal dysregulation—hyperthermia and hypothermia—can occur secondary to an antipsychotic. Determining whether a patient is at increased risk of either of these adverse effects is important when deciding to use antipsychotics. Recognizing agents that can cause hypothermia is essential, because management requires prompt discontinuation of the offending drug.
Related Resource
- Espay AJ, et al. Frontal lobe syndromes. http://emedicine.medscape.com/article/1135866-overview. Updated September 17, 2012. Accessed November 3, 2012.
Drug Brand Names
Aripiprazole • Abilify Lamotrigine • Lamictal
Bromocriptine • Parlodel Lorazepam • Ativan
Chlorpromazine • Thorazine Paliperidone • Invega
Citalopram • Celexa Risperidone • Risperdal
Clozapine • Clozaril Sertraline • Zoloft
Divalproex sodium • Depakote Thioridazine • Mellaril
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Aslam AF, Aslam AK, Vasavada BC, et al. Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med. 2006;119(4):297-301.
2. Hanna-Pladdy B. Dysexecutive syndromes in neurologic disease. J Neurol Phys Ther. 2007;31(3):119-127.
3. Salloway SP. Diagnosis and treatment of patients with “frontal lobe” syndromes. J Neuropsychiatry Clin Neurosci. 1994;6(4):388-398.
4. Campbell JJ, Duffy JD, Salloway SP. Treatment strategies for patients with dysexecutive syndromes. In: Salloway SP, Malloy PF, Duffy JD, eds. The frontal lobes and neuropsychiatric illness. Washington, DC: American Psychiatric Press; 2001:153-163.
5. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 3rd ed. New York, NY: Cambridge University Press; 2000:336.
6. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
7. van Marum RJ, Wegewijs MA, Loonen AJM, et al. Hypothermia following antipsychotic drug use. Eur J Clin Pharmacol. 2007;63(6):627-631.
8. Kreuzer P, Landgrebe M, Wittmann M, et al. Hypothermia associated with antipsychotic drug use: a clinical case series and review of current literature. J Clin Pharmacol. 2012;52(7)1090-1097.
9. Hung CF, Huang TY, Lin PY. Hypothermia and rhabdomyolysis following olanzapine injection in an adolescent with schizophreniform disorder. Gen Hosp Psychiatry. 2009;31(4):376-378.
10. Razaq M, Samma M. A case of risperidone-induced hypothermia. Am J Ther. 2004;11(3):229-230.
11. Schwaninger M, Weisbrod M, Schwab S, et al. Hypothermia induced by atypical neuroleptics. Clin Neuropharmacol. 1998;21(6):344-346.
12. Bookstaver PB, Miller AD. Possible long-acting risperidone-induced hypothermia precipitating phenytoin toxicity in an elderly patient. J Clin Pharm Ther. 2011; 36(3):426-429.
13. Boschi G, Launay N, Rips R. Neuroleptic-induced hypothermia in mice: lack of evidence for a central mechanism. Br J Pharmacol. 1987;90(4):745-751.
14. Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37(suppl 7):S203-S210.
15. Shiloh R, Weizman A, Epstein Y, et al. Abnormal thermoregulation in drug-free male schizophrenia patients. Eur Neuropsychopharmacol. 2001;11(4):285-288.
16. McCann SM, Vijayan E. Control of anterior pituitary hormone secretion by neurotensin. Ann N Y Acad Sci. 1992; 668:287-297.
17. Chandra A, Chou HC, Chang C, et al. Effecst of intraventricular administration of neurotensin and somatostatin on thermoregulation in the rat. Neuropharmacology. 1981;20(7):715-718.
18. Sharma RP, Janicak PG, Bissette G, et al. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1997; 154(7):1019-1021.
19. Kudoh A, Takase H, Takazawa T. Chronic treatment with antipsychotics enhances intraoperative core hypothermia. Anesth Analg. 2004;98(1):111-115.
CASE Confused and cold
Ms. K, age 48, is brought to the emergency department (ED) by her husband because she has become increasingly lethargic over the past 2 weeks and cannot attend to activities of daily living. She is incontinent of stool and poorly responsive.
Ms. K’s husband reports that lethargy culminated in his wife sleeping 30 continuous hours. She has a history of a ruptured cerebral arteriovenous malformation (AVM) complicated by a secondary infarct 7 years ago, with residual symptoms of frontal lobe syndrome. Until 2 weeks ago, however, she was in her usual state of health.
Symptoms have included depression, mood lability, impulsivity, disinhibition, poor focus, and apathy. An outpatient psychiatrist has managed these symptoms with antidepressants and atypical antipsychotics.
When Ms. K arrives in the ED, she is taking citalopram, 30 mg/d, and paliperidone,
6 mg/d. Her psychiatrist started paliperidone 2 months ago, increasing the dosage to 6 mg/d 6 weeks before presentation because of worsening mood lability, disinhibition, and paranoia regarding her caregivers. Her husband denies any other medication changes or exposure to environmental toxins.
In the ED, Ms. K is confused and oriented only to person. Vital signs are: pulse 46 bpm; blood pressure, 66/51 mm Hg; respirations, 12/min; and temperature, 29.9ºC (85.8ºF) via bladder probe.
a) major depressive disorder, severe, with catatonic features
b) exposure to cold
c) hypothyroidism
d) drug-induced hypothermia
e) stroke
f) sepsis
g) delirium
The authors’ observations
Hypothermia is core body temperature <35ºC (95ºF).1 It often is caused by exposure to low ambient temperature (Table 1),1 but Ms. K’s husband denied that she had been exposed to cold. Because of Ms. K’s neurologic history, stroke was high on the differential diagnosis, but physical examination did not reveal evidence of focal dysfunction and was significant only for altered mental status.
Ms. K had no posturing, rigidity, negativism, or excessive motor activity that would suggest catatonia. Before she became lethargic, her husband had not noted any deterioration of mood, although she did exhibit other behavioral changes that prompted her outpatient psychiatrist to increase the dosage of paliperidone. Although Ms. K began experiencing persecutory delusions—she believed that her caregivers were trying to harm her—she and her family denied perceptual disturbances. On examination, she did not appear responsive to auditory or visual hallucinations.
Frontal lobe syndrome is defined as a set of changes in the cognitive, behavioral, or emotional domains, often leading to disturbed affect, alteration of attention, aphasia, perseveration, disinhibition, and personality changes.2 These symptoms are not specific to lesions in the frontal lobes but can arise from lesions anywhere in the frontal-striatal-thalamic circuit.3 Causes include traumatic brain injury, neurodegenerative disorders, cerebrovascular disease, tumors, and aging.2 Recommended treatment incorporates psychosocial interventions with drug treatment to target specific symptoms. Medications reported to be effective include typical and atypical antipsychotics to target aggression and agitation; benzodiazepines to reduce arousal; antidepressants for mood symptoms, dopamine agonists (eg, bromocriptine) to decrease apathy, and mood stabilizers to target mood lability.4
Before her AVM rupture, review of Ms. K’s psychiatric history revealed no psychiatric symptoms or impaired functioning. When hospitalized for the AVM repair, she was started on sertraline. She began seeing a psychiatrist 2 years later because of increased agitation and behavioral disturbances, and aripiprazole was added. Persistent agitation prompted a trial of divalproex sodium, which was discontinued because of slurred speech and increased distractibility. Aripiprazole was tapered and replaced with paliperidone because of poor response. Citalopram was initiated 1 year before she presented to the ED.
a) brain MRI
b) infectious evaluation (lumbar puncture with analysis of cerebrospinal fluid, complete blood count, blood cultures, chest radiographs)
c) endocrine panel
d) urine toxicology screen
EVALUATION Hypothermia
Laboratory tests reveal multiple abnormalities, including thrombocytopenia (platelet level, 53 ×103/μL), altered coagulation (partial thromboplastin time, 55.6 s), elevated levels of hepatic transaminases (aspartate aminotransferase, 168 U/L; alanine aminotransferase, 357 U/L), and increased alkaline phosphatase (206 U/L). Other mild metabolic disturbances include: sodium, 149 mEq/L; CO2, 33 mEq/L; and blood urea nitrogen, 24 mg/dL.
These laboratory values are consistent with complications of hypothermia.1
ECG reveals sinus bradycardia (40 bpm) and Osborn waves (additional deflection at the end of the QRS complex), which are seen often in hypothermia.1 Head CT and brain MRI show chronic changes after Ms. K’s right temporoparietal AVM rupture, but no acute abnormality. Urinalysis, blood cultures, and chest radiographs are negative for infection. Urine toxicology screen is negative. Results of thyroid function tests and pituitary hormones studies are significant only for hyperprolactinemia of 155.7 ng/mL, a known adverse effect of antipsychotics.5
Ms. K is admitted and rewarmed passively and with warm IV fluids; by day 10 of hospitalization, temperature is stable (>35.1ºC [95.2ºF]). Thrombocytopenia, transaminitis, and altered mental status resolve.
Ms. K’s oral medications, including citalopram and paliperidone, have been held since admission because of her altered mental status. The psychiatry service is consulted to evaluate whether her presentation could be related to her change of medication.
A literature search reveals no report of paliperidone-induced hypothermia, but we consider it a possible explanation for Ms. K’s presentation. Lamotrigine (titrated to 50 mg/d), a benzodiazepine (oral lorazepam as needed), and discontinuing antipsychotics are recommended. After she returns to her baseline functioning, Ms. K is discharged to a skilled nursing facility.
Ms. K presents to the ED 2 days after discharge with altered mental status. Vital signs are: blood pressure, 90/55 mm Hg; pulse, 59 bpm; respiratory rate, 14/min; and temperature, 34.4ºC (93.9ºF) via bladder probe (Figure). Laboratory tests were significant for hepatic transaminitis (aspartate aminotransferase, 75 U/L; alanine aminotransferase, 122 U/L) and elevated alkaline phosphatase (226 U/L). A review of records from the nursing facility revealed that Ms. K was receiving paliperidone because of an error in the discharge summary, which recommended restarting all prior medications.
The authors’ observations
The Naranjo Causality Scale,6 which categorizes the probability that an adverse event is related to a drug (based on several variables, including timing of the drug administration with the onset of event, drug dosage and levels, response relationships to a drug, including re-challenge when possible, and previous patient experience with the medication), often is used to evaluate whether an adverse clinical event has been caused by a drug (Table 2). We applied the Scale to Ms. K’s case, which revealed a score of 7—indicating a probable adverse drug reaction. The sequence of events in Ms. K’s case that led to a paliperidone challenge-dechallenge-rechallenge, and the resulting hypothermia, are, we concluded, evidence of an adverse drug reaction.
Using the World Health Organization database for adverse drug reactions, van Marum et al7 found 480 reports hypothermia with antipsychotics as of 2007 (compared with 524 reports of hyperthermia in the same period); 55% involved atypical antipsychotics, mainly risperidone. There are no case reports of paliperidone-induced hypothermia; however, several reports of hypothermia have been attributed to risperidone, and paliperidone is the primary active metabolite of risperidone.5
To identify risk factors for hypothermia with antipsychotic use, van Marum et al7 performed a literature search for case reports of antipsychotic-induced hypothermia, which revealed no association with age or sex. The most common diagnosis in cases of antipsychotic-induced hypothermia was schizophrenia (51%). In 73% of the cases, hypothermia followed the start or dosage increase of the antipsychotic. These observations have been noted in case reports and case series of hypothermia associated with antipsychotic use.8-12
Mechanism of action
One proposed mechanism for antipsychotic-induced hypothermia includes preferential 5-HT2A receptor antagonism over D2 receptor antagonism.7,12 It has been believed that, under normal conditions, the action of dopamine to reduce body temperature and the action of serotonin to elevate it are in balance.9
Another possible mechanism is peripheral á2-adrenergic blockade, which might increase the hypothermic effect by inhibiting peripheral responses to cooling, such as vasoconstriction and shivering.7,8 Boschi et al13 found that antipsychotics cause hypothermia in rats when the drug is administered intraperitoneally but not when given intrathecally. Perhaps for these reasons, in the early 1950s, before its psychotropic properties were known, chlorpromazine was used during surgery to induce artificial hibernation and suppress the body’s response to cooling.7 The therapeutic activity of paliperidone is mediated though a D2, 5-HT2A, and á2-receptor antagonism5; these mechanisms could, therefore, be contributing to Ms. K’s hypothermia.
Patients with preexisting brain damage— such as Ms. K—might be at increased risk of antipsychotic-induced hypothermia.7,8 This includes focal damage to central thermoregulatory centers, such as the pre-optic anterior hypothalamic region,14 and more diffuse damage seen in patients with cognitive impairment or a seizure disorder.8
Studies of people with schizophrenia show a decrease in core temperature after administration of an antipsychotic,15 raising the possibility of an impairment of baseline thermoregulatory control. Such thermal dysregulation in patients with schizophrenia might be explained by changes in neurotensin levels.7
The neuropeptide neurotensin has been implicated in the regulation of prolactin release and interacts to a significant degree with the dopaminergic system.16 When administered to animals, neurotensin suppresses heat production and increases heat loss.17 The neurotensin level in CSF was found to be lower in non-medicated patients with schizophrenia than in healthy controls, with an inverse correlation between the severity of symptoms and the neurotensin level.18
Additionally, persons with schizophrenia might be at increased risk of developing hypothermia when exposed to a low environmental temperature.7,8 Kudoh et al19 investigated temperature regulation during anesthesia in patients with chronic (≥7 years) schizophrenia receiving antipsychotics, and compared findings against what was seen in controls. The team reported that patients with schizophrenia had significantly lower intraoperative temperatures.
A published analysis of cases and studies of antipsychotic-induced hypothermia describes the combination of drug variables, patient variables, and environmental variables that contribute to thermal dysregulation (Table 3).7-12,15 The recommendation for practitioners is that, when considering an antipsychotic for a patient at high risk of thermal dysregulation, your choice of an agent should take that risk into account, especially when that drug is one that has comparatively stronger serotonergic and peripheral á-adrenergic effects. You should monitor patients closely for hypothermia after starting and when increasing the dosage of the drug. In patients with schizophrenia who might have a problem with baseline thermoregulation, advise them to take measures to counteract their increased susceptibility to low ambient temperatures.
OUTCOME Readmission
Ms. K was readmitted, rewarmed, and discharged to a skilled nursing facility 4 days later, after baseline function returned to normal and temperature stabilized. Paliperidone is now listed in her electronic medical record as “drug intolerance.”
This case also highlights the importance of adequate medication reconciliation at
admission and discharge, especially when using an electronic medical record system, because what might otherwise be considered a minor mistake can have devastating consequences.
Bottom Line
Thermal dysregulation—hyperthermia and hypothermia—can occur secondary to an antipsychotic. Determining whether a patient is at increased risk of either of these adverse effects is important when deciding to use antipsychotics. Recognizing agents that can cause hypothermia is essential, because management requires prompt discontinuation of the offending drug.
Related Resource
- Espay AJ, et al. Frontal lobe syndromes. http://emedicine.medscape.com/article/1135866-overview. Updated September 17, 2012. Accessed November 3, 2012.
Drug Brand Names
Aripiprazole • Abilify Lamotrigine • Lamictal
Bromocriptine • Parlodel Lorazepam • Ativan
Chlorpromazine • Thorazine Paliperidone • Invega
Citalopram • Celexa Risperidone • Risperdal
Clozapine • Clozaril Sertraline • Zoloft
Divalproex sodium • Depakote Thioridazine • Mellaril
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Confused and cold
Ms. K, age 48, is brought to the emergency department (ED) by her husband because she has become increasingly lethargic over the past 2 weeks and cannot attend to activities of daily living. She is incontinent of stool and poorly responsive.
Ms. K’s husband reports that lethargy culminated in his wife sleeping 30 continuous hours. She has a history of a ruptured cerebral arteriovenous malformation (AVM) complicated by a secondary infarct 7 years ago, with residual symptoms of frontal lobe syndrome. Until 2 weeks ago, however, she was in her usual state of health.
Symptoms have included depression, mood lability, impulsivity, disinhibition, poor focus, and apathy. An outpatient psychiatrist has managed these symptoms with antidepressants and atypical antipsychotics.
When Ms. K arrives in the ED, she is taking citalopram, 30 mg/d, and paliperidone,
6 mg/d. Her psychiatrist started paliperidone 2 months ago, increasing the dosage to 6 mg/d 6 weeks before presentation because of worsening mood lability, disinhibition, and paranoia regarding her caregivers. Her husband denies any other medication changes or exposure to environmental toxins.
In the ED, Ms. K is confused and oriented only to person. Vital signs are: pulse 46 bpm; blood pressure, 66/51 mm Hg; respirations, 12/min; and temperature, 29.9ºC (85.8ºF) via bladder probe.
a) major depressive disorder, severe, with catatonic features
b) exposure to cold
c) hypothyroidism
d) drug-induced hypothermia
e) stroke
f) sepsis
g) delirium
The authors’ observations
Hypothermia is core body temperature <35ºC (95ºF).1 It often is caused by exposure to low ambient temperature (Table 1),1 but Ms. K’s husband denied that she had been exposed to cold. Because of Ms. K’s neurologic history, stroke was high on the differential diagnosis, but physical examination did not reveal evidence of focal dysfunction and was significant only for altered mental status.
Ms. K had no posturing, rigidity, negativism, or excessive motor activity that would suggest catatonia. Before she became lethargic, her husband had not noted any deterioration of mood, although she did exhibit other behavioral changes that prompted her outpatient psychiatrist to increase the dosage of paliperidone. Although Ms. K began experiencing persecutory delusions—she believed that her caregivers were trying to harm her—she and her family denied perceptual disturbances. On examination, she did not appear responsive to auditory or visual hallucinations.
Frontal lobe syndrome is defined as a set of changes in the cognitive, behavioral, or emotional domains, often leading to disturbed affect, alteration of attention, aphasia, perseveration, disinhibition, and personality changes.2 These symptoms are not specific to lesions in the frontal lobes but can arise from lesions anywhere in the frontal-striatal-thalamic circuit.3 Causes include traumatic brain injury, neurodegenerative disorders, cerebrovascular disease, tumors, and aging.2 Recommended treatment incorporates psychosocial interventions with drug treatment to target specific symptoms. Medications reported to be effective include typical and atypical antipsychotics to target aggression and agitation; benzodiazepines to reduce arousal; antidepressants for mood symptoms, dopamine agonists (eg, bromocriptine) to decrease apathy, and mood stabilizers to target mood lability.4
Before her AVM rupture, review of Ms. K’s psychiatric history revealed no psychiatric symptoms or impaired functioning. When hospitalized for the AVM repair, she was started on sertraline. She began seeing a psychiatrist 2 years later because of increased agitation and behavioral disturbances, and aripiprazole was added. Persistent agitation prompted a trial of divalproex sodium, which was discontinued because of slurred speech and increased distractibility. Aripiprazole was tapered and replaced with paliperidone because of poor response. Citalopram was initiated 1 year before she presented to the ED.
a) brain MRI
b) infectious evaluation (lumbar puncture with analysis of cerebrospinal fluid, complete blood count, blood cultures, chest radiographs)
c) endocrine panel
d) urine toxicology screen
EVALUATION Hypothermia
Laboratory tests reveal multiple abnormalities, including thrombocytopenia (platelet level, 53 ×103/μL), altered coagulation (partial thromboplastin time, 55.6 s), elevated levels of hepatic transaminases (aspartate aminotransferase, 168 U/L; alanine aminotransferase, 357 U/L), and increased alkaline phosphatase (206 U/L). Other mild metabolic disturbances include: sodium, 149 mEq/L; CO2, 33 mEq/L; and blood urea nitrogen, 24 mg/dL.
These laboratory values are consistent with complications of hypothermia.1
ECG reveals sinus bradycardia (40 bpm) and Osborn waves (additional deflection at the end of the QRS complex), which are seen often in hypothermia.1 Head CT and brain MRI show chronic changes after Ms. K’s right temporoparietal AVM rupture, but no acute abnormality. Urinalysis, blood cultures, and chest radiographs are negative for infection. Urine toxicology screen is negative. Results of thyroid function tests and pituitary hormones studies are significant only for hyperprolactinemia of 155.7 ng/mL, a known adverse effect of antipsychotics.5
Ms. K is admitted and rewarmed passively and with warm IV fluids; by day 10 of hospitalization, temperature is stable (>35.1ºC [95.2ºF]). Thrombocytopenia, transaminitis, and altered mental status resolve.
Ms. K’s oral medications, including citalopram and paliperidone, have been held since admission because of her altered mental status. The psychiatry service is consulted to evaluate whether her presentation could be related to her change of medication.
A literature search reveals no report of paliperidone-induced hypothermia, but we consider it a possible explanation for Ms. K’s presentation. Lamotrigine (titrated to 50 mg/d), a benzodiazepine (oral lorazepam as needed), and discontinuing antipsychotics are recommended. After she returns to her baseline functioning, Ms. K is discharged to a skilled nursing facility.
Ms. K presents to the ED 2 days after discharge with altered mental status. Vital signs are: blood pressure, 90/55 mm Hg; pulse, 59 bpm; respiratory rate, 14/min; and temperature, 34.4ºC (93.9ºF) via bladder probe (Figure). Laboratory tests were significant for hepatic transaminitis (aspartate aminotransferase, 75 U/L; alanine aminotransferase, 122 U/L) and elevated alkaline phosphatase (226 U/L). A review of records from the nursing facility revealed that Ms. K was receiving paliperidone because of an error in the discharge summary, which recommended restarting all prior medications.
The authors’ observations
The Naranjo Causality Scale,6 which categorizes the probability that an adverse event is related to a drug (based on several variables, including timing of the drug administration with the onset of event, drug dosage and levels, response relationships to a drug, including re-challenge when possible, and previous patient experience with the medication), often is used to evaluate whether an adverse clinical event has been caused by a drug (Table 2). We applied the Scale to Ms. K’s case, which revealed a score of 7—indicating a probable adverse drug reaction. The sequence of events in Ms. K’s case that led to a paliperidone challenge-dechallenge-rechallenge, and the resulting hypothermia, are, we concluded, evidence of an adverse drug reaction.
Using the World Health Organization database for adverse drug reactions, van Marum et al7 found 480 reports hypothermia with antipsychotics as of 2007 (compared with 524 reports of hyperthermia in the same period); 55% involved atypical antipsychotics, mainly risperidone. There are no case reports of paliperidone-induced hypothermia; however, several reports of hypothermia have been attributed to risperidone, and paliperidone is the primary active metabolite of risperidone.5
To identify risk factors for hypothermia with antipsychotic use, van Marum et al7 performed a literature search for case reports of antipsychotic-induced hypothermia, which revealed no association with age or sex. The most common diagnosis in cases of antipsychotic-induced hypothermia was schizophrenia (51%). In 73% of the cases, hypothermia followed the start or dosage increase of the antipsychotic. These observations have been noted in case reports and case series of hypothermia associated with antipsychotic use.8-12
Mechanism of action
One proposed mechanism for antipsychotic-induced hypothermia includes preferential 5-HT2A receptor antagonism over D2 receptor antagonism.7,12 It has been believed that, under normal conditions, the action of dopamine to reduce body temperature and the action of serotonin to elevate it are in balance.9
Another possible mechanism is peripheral á2-adrenergic blockade, which might increase the hypothermic effect by inhibiting peripheral responses to cooling, such as vasoconstriction and shivering.7,8 Boschi et al13 found that antipsychotics cause hypothermia in rats when the drug is administered intraperitoneally but not when given intrathecally. Perhaps for these reasons, in the early 1950s, before its psychotropic properties were known, chlorpromazine was used during surgery to induce artificial hibernation and suppress the body’s response to cooling.7 The therapeutic activity of paliperidone is mediated though a D2, 5-HT2A, and á2-receptor antagonism5; these mechanisms could, therefore, be contributing to Ms. K’s hypothermia.
Patients with preexisting brain damage— such as Ms. K—might be at increased risk of antipsychotic-induced hypothermia.7,8 This includes focal damage to central thermoregulatory centers, such as the pre-optic anterior hypothalamic region,14 and more diffuse damage seen in patients with cognitive impairment or a seizure disorder.8
Studies of people with schizophrenia show a decrease in core temperature after administration of an antipsychotic,15 raising the possibility of an impairment of baseline thermoregulatory control. Such thermal dysregulation in patients with schizophrenia might be explained by changes in neurotensin levels.7
The neuropeptide neurotensin has been implicated in the regulation of prolactin release and interacts to a significant degree with the dopaminergic system.16 When administered to animals, neurotensin suppresses heat production and increases heat loss.17 The neurotensin level in CSF was found to be lower in non-medicated patients with schizophrenia than in healthy controls, with an inverse correlation between the severity of symptoms and the neurotensin level.18
Additionally, persons with schizophrenia might be at increased risk of developing hypothermia when exposed to a low environmental temperature.7,8 Kudoh et al19 investigated temperature regulation during anesthesia in patients with chronic (≥7 years) schizophrenia receiving antipsychotics, and compared findings against what was seen in controls. The team reported that patients with schizophrenia had significantly lower intraoperative temperatures.
A published analysis of cases and studies of antipsychotic-induced hypothermia describes the combination of drug variables, patient variables, and environmental variables that contribute to thermal dysregulation (Table 3).7-12,15 The recommendation for practitioners is that, when considering an antipsychotic for a patient at high risk of thermal dysregulation, your choice of an agent should take that risk into account, especially when that drug is one that has comparatively stronger serotonergic and peripheral á-adrenergic effects. You should monitor patients closely for hypothermia after starting and when increasing the dosage of the drug. In patients with schizophrenia who might have a problem with baseline thermoregulation, advise them to take measures to counteract their increased susceptibility to low ambient temperatures.
OUTCOME Readmission
Ms. K was readmitted, rewarmed, and discharged to a skilled nursing facility 4 days later, after baseline function returned to normal and temperature stabilized. Paliperidone is now listed in her electronic medical record as “drug intolerance.”
This case also highlights the importance of adequate medication reconciliation at
admission and discharge, especially when using an electronic medical record system, because what might otherwise be considered a minor mistake can have devastating consequences.
Bottom Line
Thermal dysregulation—hyperthermia and hypothermia—can occur secondary to an antipsychotic. Determining whether a patient is at increased risk of either of these adverse effects is important when deciding to use antipsychotics. Recognizing agents that can cause hypothermia is essential, because management requires prompt discontinuation of the offending drug.
Related Resource
- Espay AJ, et al. Frontal lobe syndromes. http://emedicine.medscape.com/article/1135866-overview. Updated September 17, 2012. Accessed November 3, 2012.
Drug Brand Names
Aripiprazole • Abilify Lamotrigine • Lamictal
Bromocriptine • Parlodel Lorazepam • Ativan
Chlorpromazine • Thorazine Paliperidone • Invega
Citalopram • Celexa Risperidone • Risperdal
Clozapine • Clozaril Sertraline • Zoloft
Divalproex sodium • Depakote Thioridazine • Mellaril
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Aslam AF, Aslam AK, Vasavada BC, et al. Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med. 2006;119(4):297-301.
2. Hanna-Pladdy B. Dysexecutive syndromes in neurologic disease. J Neurol Phys Ther. 2007;31(3):119-127.
3. Salloway SP. Diagnosis and treatment of patients with “frontal lobe” syndromes. J Neuropsychiatry Clin Neurosci. 1994;6(4):388-398.
4. Campbell JJ, Duffy JD, Salloway SP. Treatment strategies for patients with dysexecutive syndromes. In: Salloway SP, Malloy PF, Duffy JD, eds. The frontal lobes and neuropsychiatric illness. Washington, DC: American Psychiatric Press; 2001:153-163.
5. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 3rd ed. New York, NY: Cambridge University Press; 2000:336.
6. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
7. van Marum RJ, Wegewijs MA, Loonen AJM, et al. Hypothermia following antipsychotic drug use. Eur J Clin Pharmacol. 2007;63(6):627-631.
8. Kreuzer P, Landgrebe M, Wittmann M, et al. Hypothermia associated with antipsychotic drug use: a clinical case series and review of current literature. J Clin Pharmacol. 2012;52(7)1090-1097.
9. Hung CF, Huang TY, Lin PY. Hypothermia and rhabdomyolysis following olanzapine injection in an adolescent with schizophreniform disorder. Gen Hosp Psychiatry. 2009;31(4):376-378.
10. Razaq M, Samma M. A case of risperidone-induced hypothermia. Am J Ther. 2004;11(3):229-230.
11. Schwaninger M, Weisbrod M, Schwab S, et al. Hypothermia induced by atypical neuroleptics. Clin Neuropharmacol. 1998;21(6):344-346.
12. Bookstaver PB, Miller AD. Possible long-acting risperidone-induced hypothermia precipitating phenytoin toxicity in an elderly patient. J Clin Pharm Ther. 2011; 36(3):426-429.
13. Boschi G, Launay N, Rips R. Neuroleptic-induced hypothermia in mice: lack of evidence for a central mechanism. Br J Pharmacol. 1987;90(4):745-751.
14. Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37(suppl 7):S203-S210.
15. Shiloh R, Weizman A, Epstein Y, et al. Abnormal thermoregulation in drug-free male schizophrenia patients. Eur Neuropsychopharmacol. 2001;11(4):285-288.
16. McCann SM, Vijayan E. Control of anterior pituitary hormone secretion by neurotensin. Ann N Y Acad Sci. 1992; 668:287-297.
17. Chandra A, Chou HC, Chang C, et al. Effecst of intraventricular administration of neurotensin and somatostatin on thermoregulation in the rat. Neuropharmacology. 1981;20(7):715-718.
18. Sharma RP, Janicak PG, Bissette G, et al. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1997; 154(7):1019-1021.
19. Kudoh A, Takase H, Takazawa T. Chronic treatment with antipsychotics enhances intraoperative core hypothermia. Anesth Analg. 2004;98(1):111-115.
1. Aslam AF, Aslam AK, Vasavada BC, et al. Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med. 2006;119(4):297-301.
2. Hanna-Pladdy B. Dysexecutive syndromes in neurologic disease. J Neurol Phys Ther. 2007;31(3):119-127.
3. Salloway SP. Diagnosis and treatment of patients with “frontal lobe” syndromes. J Neuropsychiatry Clin Neurosci. 1994;6(4):388-398.
4. Campbell JJ, Duffy JD, Salloway SP. Treatment strategies for patients with dysexecutive syndromes. In: Salloway SP, Malloy PF, Duffy JD, eds. The frontal lobes and neuropsychiatric illness. Washington, DC: American Psychiatric Press; 2001:153-163.
5. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 3rd ed. New York, NY: Cambridge University Press; 2000:336.
6. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
7. van Marum RJ, Wegewijs MA, Loonen AJM, et al. Hypothermia following antipsychotic drug use. Eur J Clin Pharmacol. 2007;63(6):627-631.
8. Kreuzer P, Landgrebe M, Wittmann M, et al. Hypothermia associated with antipsychotic drug use: a clinical case series and review of current literature. J Clin Pharmacol. 2012;52(7)1090-1097.
9. Hung CF, Huang TY, Lin PY. Hypothermia and rhabdomyolysis following olanzapine injection in an adolescent with schizophreniform disorder. Gen Hosp Psychiatry. 2009;31(4):376-378.
10. Razaq M, Samma M. A case of risperidone-induced hypothermia. Am J Ther. 2004;11(3):229-230.
11. Schwaninger M, Weisbrod M, Schwab S, et al. Hypothermia induced by atypical neuroleptics. Clin Neuropharmacol. 1998;21(6):344-346.
12. Bookstaver PB, Miller AD. Possible long-acting risperidone-induced hypothermia precipitating phenytoin toxicity in an elderly patient. J Clin Pharm Ther. 2011; 36(3):426-429.
13. Boschi G, Launay N, Rips R. Neuroleptic-induced hypothermia in mice: lack of evidence for a central mechanism. Br J Pharmacol. 1987;90(4):745-751.
14. Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37(suppl 7):S203-S210.
15. Shiloh R, Weizman A, Epstein Y, et al. Abnormal thermoregulation in drug-free male schizophrenia patients. Eur Neuropsychopharmacol. 2001;11(4):285-288.
16. McCann SM, Vijayan E. Control of anterior pituitary hormone secretion by neurotensin. Ann N Y Acad Sci. 1992; 668:287-297.
17. Chandra A, Chou HC, Chang C, et al. Effecst of intraventricular administration of neurotensin and somatostatin on thermoregulation in the rat. Neuropharmacology. 1981;20(7):715-718.
18. Sharma RP, Janicak PG, Bissette G, et al. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1997; 154(7):1019-1021.
19. Kudoh A, Takase H, Takazawa T. Chronic treatment with antipsychotics enhances intraoperative core hypothermia. Anesth Analg. 2004;98(1):111-115.