User login
A medication change, then involuntary lip smacking and tongue rolling
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
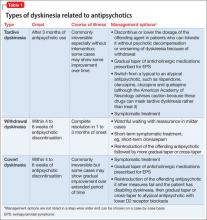
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
A depressed adolescent who won’t eat and reacts slowly
CASE A fainting spell
Ms. A, age 13, is admitted to a pediatric unit after fainting and losing consciousness for 5 minutes in the shower, during which time she was non-responsive. She reports feeling nauseated and having blurry vision before dropping to the floor.
Ms. A reports intentional self-restriction of calories, self-induced vomiting, and other purging behaviors, such as laxative abuse and excessive exercising.
During the mental status examination, Ms. A is lying in bed wearing hospital clothes, legs flexed at the knee, hands on her side, and a fixed gaze at the ceiling with poor eye contact. She is of slender stature and tall, seems slightly older than her stated age, and is poorly groomed.
Throughout the interview, Ms. A has significant psychomotor retardation, reports her mood as tired, and has a blunted affect. She speaks at a low volume and has poverty of speech; she takes deep sighs before answering questions. Her thought process is linear and she cooperates with the interview. She has poor recall, including delayed 3-minute recall and poor sustained attention. Her abstraction capacity is fair and her intellect is average and comparable with her age group. Ms. A is preoccupied that eating will cause weight gain. She denies hallucinations but reports passive death wishes with self-harm by scratching.
What is the differential diagnosis to explain Ms. A’s presentation?
a) syncope
b) seizures
c) dehydration
d) hypotension
HISTORY Preoccupied with weight
Ms. A reports vomiting twice a day, while showering and at night when no one is around, every day for 2 months. She stopped eating and taking in fluids 3 days before admission to the medical unit. Also, she reports restricting her diet to 700 to 1,000 calories a day, skipping lunch at school, and eating minimally at night. Ms. A uses raspberry ketones and green coffee beans, which are advertised to aid weight loss, and laxative pills from her mother’s medicine cabinet once or twice a week when her throat is sore from vomiting. She reports exercising excessively, which includes running, crunches, and lifting weights. She has lost approximately 30 lb in the last 2 months.
Ms. A says she fears gaining weight and feels increased guilt after eating a meal. She said that looking at food induced “anxiety attack” symptoms of increased heart rate, sweaty palms, feeling of choking, nervousness, and shakiness. She adds that she does not want to be “bigger” than her classmates. Her understanding of the consequences of not eating is, “It will get worse, I will shut down and die. I do not fear death, I only fear getting bigger than others.”
She reports that her fixation on avoiding food started when she realized that she was the tallest girl in her class and the only girl in her class running on the track team, after which she quit athletics. She reports that depression symptoms pre-dated her eating disorder symptoms; onset of significant depression likely was precipitated by her grandfather’s death a year earlier, and then exacerbated by the recent death of a family pet.
Ms. A’s depressive symptoms are described as anhedonia (avoiding being outside and not enjoying drawing anymore), decreased energy, tearfulness, sadness, decreased concentration, and passive suicidal thoughts. Her mother is supportive and motivates her daughter to “get better.” Ms. A denies any symptoms of psychosis, other anxiety symptoms, other mood disorder symptoms, substance abuse, or homicidality.
Ms. A’s mother says she felt that, recently, her daughter has been having some difficulty with confused thoughts and significantly delayed responses. However, the mother reports that her daughter always had somewhat delayed responses from what she felt is typical. Her mother adds that Ms. A’s suicidal thoughts have worsened since her daughter started restricting her diet.
Which diagnosis likely accounts for Ms. A’s presentation?
a) major depressive disorder (MDD)
b) eating disorder, not otherwise specified (NOS)
c) anorexia nervosa, purging type
d) catatonia, unspecified
e) anxiety disorder NOS
f) cognitive disorder
g) psychosis NOS
The authors’ observations
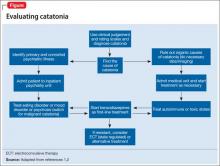
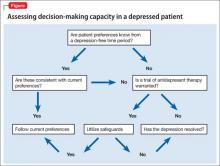
There are many reported causes of catatonia in children and adolescents, including those that are psychiatric, medical, or neurological, as well as drugs (Table 1).1,2 Affective disorders have been associated with catatonia in adults, but has not been widely reported in children and adolescents.1,3 Organic and neurologic causes, such as neurological tumors and cerebral hemorrhage, should be ruled out first because, although rare, they can be fatal (Table 2).2 If the cause of catatonia is not recognized quickly (Figure,1,2) effective treatment could be delayed.4
Catatonia involves psychomotor abnormalities, which are listed in Table 3.1,4
Presentation in adults and adolescents is similar.
An eating disorder could be comorbid with another psychiatric disorder, such as MDD, dysthymia, or panic disorder.5 Ms. A’s report of depression before she began restricting food favored a primary diagnosis of MDD. Her depressive symptoms of low appetite or low self-worth could have led to her preoccupation with body image.
There has been evidence that negative self-image and eating disorders are associated, but data are limited and the connection remains unclear.6 Ms. A’s self-esteem was very low. Her fixation on restricting food could have been perpetuated by her self-criticism and by being excluded from her peer group in school. Her weight loss could have brought anxiety symptoms to the forefront because of physiologic changes that accompany extreme weight loss.
The treatment team was concerned about her delayed responses, which could be explained by the catatonic features that reflected the severity of her depression. She had no obvious symptoms of psychosis, but her intrusive thoughts and obsessions with avoiding food did not completely rule out psychosis.
Childhood-onset schizophrenia, although rare, has been associated with catatonia; following up with a catatonia rating scale, such as the Catatonia Rating Scale or the Bush- Francis Catatonia Rating Scale (BFCRS), would be useful for tracking symptom progress. In Ms. A’s case, her mood disorder was primary, but did not rule out psychosis-like prodromal symptoms.7
Ms. A is diagnosed with MDD, single episode, severe, with catatonic features, and without psychosis, and eating disorder, NOS.
EVALUATION Mostly normal
Ms. A does not have a history of mental illness and was not seeing a psychiatrist or therapist, nor did she have any prior psychiatric admissions. She denies suicide attempts, but reports self-injurious behavior involving scratching her skin, which started during the current mood episode. She has never taken any psychotropic medications. Ms. A lives at home with her biological mother and father and 17-year-old brother. She attends middle school with average grades and has no history of disciplinary actions. She has no history of bullying or teasing, although she did report some previous difficulty with relational aggression toward her peers in the 5th grade. Her mother has a history of anorexia nervosa that began when she was a teenager, but these symptoms are stable and under control. There is additionally a family history of bipolar disorder.
Ms. A has a family history of coronary artery disease and diabetes in the mother and maternal relatives. Her grandfather died from liver cancer. She was allergic to sulfa drugs and was taking a multivitamin and minocycline for acne.
Physical examination reveals some superficial scratches but otherwise was within normal limits. Initial lab results reveal a normal complete blood count and differential. Thyroid-stimulating hormone is 1.29 mIU/L and free T4 is 0.96 mg/dL, both within normal limits. Urinalysis is within normal limits and urine pregnancy test is negative. A comprehensive metabolic panel shows mild elevation in aspartate aminotransferase (AST) at 60 U/L and alanine aminotransferase (ALT) at 92 U/L, respectively. Phosphorus level is within normal limits. Prealbumin level is slightly low at 15.1 mg/dL.
Which treatment plan would you recommend for Ms. A?
a) discharge with outpatient psychiatric treatment
b) recommend medical stabilization with follow-up from the psychosomatic team and then outpatient psychiatric follow-up
c) admit her to the psychiatric acute inpatient hospital with psychiatric outpatient discharge follow-up plan
d) discharge her home with follow-up with her primary care physician
e) recommend follow-up from the psychosomatic team while on medical floor with acute inpatient admission and psychiatric outpatient follow-up at discharge
The authors’ observations
Scarcity of data and reporting of cases of adolescent catatonia limits guidance for diagnosis and treatment.8 There are several rating scales with variability in definition, but that overall provide a guiding tool for detecting catatonia. The Brief Cognitive Rating Scale is considered the most versatile because it is more valid, reliable, and requires less time to complete than other rating scales.9
Ms. A’s symptoms were a combination of depressive symptoms with severity defined by catatonic features, eating disorder with worsening course, anxiety symptoms, and genetic loading of eating disorder in her mother. The challenge of this case was making an accurate diagnosis and treating Ms. A, which required continuous observation following an eating disorder protocol, resolution of her catatonia, resuming a normal diet, and decreasing her suicidality. Retrospectively, her scores on the BFCRS were high on screening items 1 to 14, which measure presence or absence and severity of symptoms.
The best option was to admit Ms. A to an inpatient psychiatric facility after she is cleared medically with outpatient services to follow up.
How would you treat Ms. A’s symptoms?
a) aggressively treat catatonia
b) address her eating disorder
c) work to resolve her depression
The authors’ observations
The challenge was to choose the psychotropic medication that would target her depression, obsessive, rigid thoughts, and catatonia. Administering an antidepressant with an antipsychotic would have relieved her depressive and obsessive symptoms but would not have improved her catatonia. The psychosomatic medicine team recommended starting a selective serotonin reuptake inhibitor and a benzodiazepine to target both the depression and the catatonic symptoms. Ms. A received sertraline, 12.5 mg/d, which was increased to 25 mg/d on the third day. IV lorazepam, 1 mg, 3 times a day, was recommended but the pediatric team prescribed an oral formulation. The hospital’s eating disorder protocol was instituted on the day of admission.
Treatment options for catatonia
Benzodiazepines are the first line of treatment for catatonia and other neuroleptics, specifically antipsychotics, have been considered dangerous.10 Benzodiazepine-resistant catatonia, which is sometimes seen in patients with autism, might respond to electroconvulsive therapy (ECT),11 although in some states it cannot be administered to children age <18.12 Benzodiazepines have shown dramatic improvement within hours, as has ECT.8,13 Additionally, if patients do not respond to a benzodiazepine or ECT, consider other options such as zolpidem, olanzapine,14 or sensory integration system (in adolescents with autism).15
Ms. A did not need ECT or an alternative treatment because she responded well to 3 doses of oral lorazepam. Her amotivation, negativism, and rigidity with prolonged posturing improved. Her psychomotor retardation improved overall, although she reported some dizziness and had some postural hypotension, which was attributed to her eating issues and dehydration.
OUTCOME Feeling motivated
Ms. A is transferred to psychiatric inpatient unit. She tolerates sertraline, which is titrated to 50 mg/d. She is placed on the hospital’s standard eating disorder protocol. She continues to eat well with adequate intake of solids and liquid and exhibits only some anxiety associated with meals. During the course of hospitalization, she attends group therapy and her catatonic symptoms completely resolve. She says she thinks that her thoughts are improving and that she is not longer feeling confused. She reports being motivated to continue to improve her eating disorder symptoms.
The treatment team holds a family session during which family dynamic issues that are stressful to Ms. A are discussed, such as some conflict with her parents as well as some negative interactions between Ms. A and her father. Repeat comprehensive metabolic panel on admission to the inpatient psychiatric hospital reveals persistent elevation of AST at 92 U/L and ALT at 143 U/L. Ms. A is discharged home with follow-up with a psychiatrist and a therapist. The treatment team also recommends that she follow up in a program that specializes in eating disorders.
4-month follow-up. Ms. A returns to inpatient psychiatric hospital after overdose of sertraline and aripiprazole, which were started by an outpatient psychiatrist. She reports severe depressive symptoms because of school stressors. She denies any problems eating and did not show any symptoms of catatonia. In her chart, there is a mention of “cloudy thoughts” and quietness. At this admission, her ALT is 17 U/L and AST is 19 U/L. Sertraline is increased to 150 mg/d and aripiprazole is reduced to 2 mg/d and then later increased to 5 mg/d, after which she is discharged home with an outpatient psychiatric follow-up.
1-year follow-up. Ms. A has been following up with an outpatient psychiatrist and is receiving sertraline, 150 mg/d, aripiprazole, 2.5 mg/d, and extended-release methylphenidate, 36 mg/d, along with L-methylfolate, multivitamins, and omega-3 fish oil as adjuvants for her depressive symptoms. Ms. A does not show symptoms of an eating disorder or catatonia, and her depression and psychomotor activity have improved, with better overall functionality, after adding the stimulant and adjunctives to the antidepressant.
The authors’ observations
The importance of including catatonia NOS with its various specifiers, such as medical, metabolic, toxic, affective, etc., has been discussed.16,17 In Ms. A’s case, instead of treating the specific symptoms—affective or eating disorder or obsessive quality of thought content, mimicking psychotic-like symptoms—addressing the catatonia initially had a better outcome. More studies related to chronic and acute catatonia in adolescents are needed because of the risk of increased morbidity and premature death.18 Early recognition of catatonia is needed19 because it often is underdiagnosed.20
Eating disorders often become worse over the first 5 years, and close monitoring and assessment is needed for adolescents.21 Also, prodromal psychotic symptoms require follow-up because techniques for early detection and intervention for children and adolescents are still in their infancy.22
Bottom Line
Catatonia in adolescents should be addressed early, when it is treatable and the outcome is favorable. It is important to recognize catatonia in an emergency department or inpatient medical unit setting in a hospital because it is often underdiagnosed or misdiagnosed. The presentation of catatonia is similar in adolescents and adults. Benzodiazepines are first-line treatment for catatonia; consider electroconvulsive therapy if patients do not respond to drug therapy.
Related Resources
• Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517. doi: 10.1155/2014/309517.
• Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
Drug Brand Names
Aripiprazole • Abilify Minocycline • Minocin
L-methylfolate • Deplin Olanzapine • Zyprexa
Lorazepam • Ativan Sertraline • Zoloft
Methylphenidate • Ritalin, Concerta Zolpidem • Ambien, Intermezzo
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dhossche D, Wilson C, Wachtel LE. Catatonia in childhood and adolescence: implications for the DSM-5. Primary Psychiatry. http://primarypsychiatry.com/catatonia-in-childhood-and-adolescence-implications-for-the-dsm-5. Published May 21, 2013. Accessed July 2, 2015.
2. Lahutte B, Cornic F, Bonnot O, et al. Multidisciplinary approach of organic catatonia in children and adolescents may improve treatment decision making. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1393-1398.
3. Brake JA, Abidi S. A case of adolescent catatonia. J Can Acad Child Adolesc Psychiatry. 2010;19(2):138-140.
4. Consoli A, Raffin M, Laurent C, et al. Medical and developmental risk factors of catatonia in children and adolescents: a prospective case-control study. Schizophr Res. 2012;137(1-3):151-158.
5. Zaider TI, Johnson JG, Cockell SJ. Psychiatric comorbidity associated with eating disorder symptomatology among adolescents in the community. Int J Eat Disord. 2000;28(1):58-67.
6. Forsén Mantilla E, Bergsten K, Birgegård A. Self-image and eating disorder symptoms in normal and clinical adolescents. Eat Behav. 2014;15(1):125-131.
7. Bonnot O, Tanguy ML, Consoli A, et al. Does catatonia influence the phenomenology of childhood onset schizophrenia beyond motor symptoms? Psychiatry Res. 2008;158(3):356-362.
8. Singh LK, Praharaj SK. Immediate response to lorazepam in a patient with 17 years of chronic catatonia. J Neuropsychiatry Clin Neurosci. 2013;25(3):E47-E48.
9. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1-3):1-9.
10. Cottencin O, Warembourg F, de Chouly de Lenclave MB, et al. Catatonia and consultation-liaison psychiatry study of 12 cases. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1170-1176.
11. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
12. Wachtel LE, Dhossche DM, Kellner CH. When is electroconvulsive therapy appropriate for children and adolescents? Med Hypotheses. 2011;76(3):395-399.
13. Takaoka K, Takata T. Catatonia in childhood and adolescence. Psychiatry Clin Neurosci. 2003;57(2):129-137.
14. Ceylan MF, Kul M, Kultur SE, et al. Major depression with catatonic features in a child remitted with olanzapine. J Child Adolesc Psychopharmacol. 2010;20(3):225-227.
15. Consoli A, Gheorghiev C, Jutard C, et al. Lorazepam, fluoxetine and packing therapy in an adolescent with pervasive developmental disorder and catatonia. J Physiol Paris. 2010;104(6):309-314.
16. Dhossche D, Cohen D, Ghaziuddin N, et al. The study of pediatric catatonia supports a home of its own for catatonia in DSM-5. Med Hypotheses. 2010;75(6):558-560.
17. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
18. Cornic F, Consoli A, Tanguy ML, et al. Association of adolescent catatonia with increased mortality and morbidity: evidence from a prospective follow-up study. Schizophr Res. 2009;113(2-3):233-240.
19. Quigley J, Lommel KM, Coffey B. Catatonia in an adolescent with Asperger’s disorder. J Child Adolesc Psychopharmacol. 2009;19(1):93-96.
20. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
21. Ackard DM, Fulkerson JA, Neumark-Sztainer D. Stability of eating disorder diagnostic classifications in adolescents: five-year longitudinal findings from a population-based study. Eat Disord. 2011;19(4):308-322.
22. Schimmelmann BG, Schultze-Lutter F. Early detection and intervention of psychosis in children and adolescents: urgent need for studies. Eur Child Adolesc Psychiatry. 2012;21(5):239-241.
CASE A fainting spell
Ms. A, age 13, is admitted to a pediatric unit after fainting and losing consciousness for 5 minutes in the shower, during which time she was non-responsive. She reports feeling nauseated and having blurry vision before dropping to the floor.
Ms. A reports intentional self-restriction of calories, self-induced vomiting, and other purging behaviors, such as laxative abuse and excessive exercising.
During the mental status examination, Ms. A is lying in bed wearing hospital clothes, legs flexed at the knee, hands on her side, and a fixed gaze at the ceiling with poor eye contact. She is of slender stature and tall, seems slightly older than her stated age, and is poorly groomed.
Throughout the interview, Ms. A has significant psychomotor retardation, reports her mood as tired, and has a blunted affect. She speaks at a low volume and has poverty of speech; she takes deep sighs before answering questions. Her thought process is linear and she cooperates with the interview. She has poor recall, including delayed 3-minute recall and poor sustained attention. Her abstraction capacity is fair and her intellect is average and comparable with her age group. Ms. A is preoccupied that eating will cause weight gain. She denies hallucinations but reports passive death wishes with self-harm by scratching.
What is the differential diagnosis to explain Ms. A’s presentation?
a) syncope
b) seizures
c) dehydration
d) hypotension
HISTORY Preoccupied with weight
Ms. A reports vomiting twice a day, while showering and at night when no one is around, every day for 2 months. She stopped eating and taking in fluids 3 days before admission to the medical unit. Also, she reports restricting her diet to 700 to 1,000 calories a day, skipping lunch at school, and eating minimally at night. Ms. A uses raspberry ketones and green coffee beans, which are advertised to aid weight loss, and laxative pills from her mother’s medicine cabinet once or twice a week when her throat is sore from vomiting. She reports exercising excessively, which includes running, crunches, and lifting weights. She has lost approximately 30 lb in the last 2 months.
Ms. A says she fears gaining weight and feels increased guilt after eating a meal. She said that looking at food induced “anxiety attack” symptoms of increased heart rate, sweaty palms, feeling of choking, nervousness, and shakiness. She adds that she does not want to be “bigger” than her classmates. Her understanding of the consequences of not eating is, “It will get worse, I will shut down and die. I do not fear death, I only fear getting bigger than others.”
She reports that her fixation on avoiding food started when she realized that she was the tallest girl in her class and the only girl in her class running on the track team, after which she quit athletics. She reports that depression symptoms pre-dated her eating disorder symptoms; onset of significant depression likely was precipitated by her grandfather’s death a year earlier, and then exacerbated by the recent death of a family pet.
Ms. A’s depressive symptoms are described as anhedonia (avoiding being outside and not enjoying drawing anymore), decreased energy, tearfulness, sadness, decreased concentration, and passive suicidal thoughts. Her mother is supportive and motivates her daughter to “get better.” Ms. A denies any symptoms of psychosis, other anxiety symptoms, other mood disorder symptoms, substance abuse, or homicidality.
Ms. A’s mother says she felt that, recently, her daughter has been having some difficulty with confused thoughts and significantly delayed responses. However, the mother reports that her daughter always had somewhat delayed responses from what she felt is typical. Her mother adds that Ms. A’s suicidal thoughts have worsened since her daughter started restricting her diet.
Which diagnosis likely accounts for Ms. A’s presentation?
a) major depressive disorder (MDD)
b) eating disorder, not otherwise specified (NOS)
c) anorexia nervosa, purging type
d) catatonia, unspecified
e) anxiety disorder NOS
f) cognitive disorder
g) psychosis NOS
The authors’ observations
There are many reported causes of catatonia in children and adolescents, including those that are psychiatric, medical, or neurological, as well as drugs (Table 1).1,2 Affective disorders have been associated with catatonia in adults, but has not been widely reported in children and adolescents.1,3 Organic and neurologic causes, such as neurological tumors and cerebral hemorrhage, should be ruled out first because, although rare, they can be fatal (Table 2).2 If the cause of catatonia is not recognized quickly (Figure,1,2) effective treatment could be delayed.4
Catatonia involves psychomotor abnormalities, which are listed in Table 3.1,4
Presentation in adults and adolescents is similar.
An eating disorder could be comorbid with another psychiatric disorder, such as MDD, dysthymia, or panic disorder.5 Ms. A’s report of depression before she began restricting food favored a primary diagnosis of MDD. Her depressive symptoms of low appetite or low self-worth could have led to her preoccupation with body image.
There has been evidence that negative self-image and eating disorders are associated, but data are limited and the connection remains unclear.6 Ms. A’s self-esteem was very low. Her fixation on restricting food could have been perpetuated by her self-criticism and by being excluded from her peer group in school. Her weight loss could have brought anxiety symptoms to the forefront because of physiologic changes that accompany extreme weight loss.
The treatment team was concerned about her delayed responses, which could be explained by the catatonic features that reflected the severity of her depression. She had no obvious symptoms of psychosis, but her intrusive thoughts and obsessions with avoiding food did not completely rule out psychosis.
Childhood-onset schizophrenia, although rare, has been associated with catatonia; following up with a catatonia rating scale, such as the Catatonia Rating Scale or the Bush- Francis Catatonia Rating Scale (BFCRS), would be useful for tracking symptom progress. In Ms. A’s case, her mood disorder was primary, but did not rule out psychosis-like prodromal symptoms.7
Ms. A is diagnosed with MDD, single episode, severe, with catatonic features, and without psychosis, and eating disorder, NOS.
EVALUATION Mostly normal
Ms. A does not have a history of mental illness and was not seeing a psychiatrist or therapist, nor did she have any prior psychiatric admissions. She denies suicide attempts, but reports self-injurious behavior involving scratching her skin, which started during the current mood episode. She has never taken any psychotropic medications. Ms. A lives at home with her biological mother and father and 17-year-old brother. She attends middle school with average grades and has no history of disciplinary actions. She has no history of bullying or teasing, although she did report some previous difficulty with relational aggression toward her peers in the 5th grade. Her mother has a history of anorexia nervosa that began when she was a teenager, but these symptoms are stable and under control. There is additionally a family history of bipolar disorder.
Ms. A has a family history of coronary artery disease and diabetes in the mother and maternal relatives. Her grandfather died from liver cancer. She was allergic to sulfa drugs and was taking a multivitamin and minocycline for acne.
Physical examination reveals some superficial scratches but otherwise was within normal limits. Initial lab results reveal a normal complete blood count and differential. Thyroid-stimulating hormone is 1.29 mIU/L and free T4 is 0.96 mg/dL, both within normal limits. Urinalysis is within normal limits and urine pregnancy test is negative. A comprehensive metabolic panel shows mild elevation in aspartate aminotransferase (AST) at 60 U/L and alanine aminotransferase (ALT) at 92 U/L, respectively. Phosphorus level is within normal limits. Prealbumin level is slightly low at 15.1 mg/dL.
Which treatment plan would you recommend for Ms. A?
a) discharge with outpatient psychiatric treatment
b) recommend medical stabilization with follow-up from the psychosomatic team and then outpatient psychiatric follow-up
c) admit her to the psychiatric acute inpatient hospital with psychiatric outpatient discharge follow-up plan
d) discharge her home with follow-up with her primary care physician
e) recommend follow-up from the psychosomatic team while on medical floor with acute inpatient admission and psychiatric outpatient follow-up at discharge
The authors’ observations
Scarcity of data and reporting of cases of adolescent catatonia limits guidance for diagnosis and treatment.8 There are several rating scales with variability in definition, but that overall provide a guiding tool for detecting catatonia. The Brief Cognitive Rating Scale is considered the most versatile because it is more valid, reliable, and requires less time to complete than other rating scales.9
Ms. A’s symptoms were a combination of depressive symptoms with severity defined by catatonic features, eating disorder with worsening course, anxiety symptoms, and genetic loading of eating disorder in her mother. The challenge of this case was making an accurate diagnosis and treating Ms. A, which required continuous observation following an eating disorder protocol, resolution of her catatonia, resuming a normal diet, and decreasing her suicidality. Retrospectively, her scores on the BFCRS were high on screening items 1 to 14, which measure presence or absence and severity of symptoms.
The best option was to admit Ms. A to an inpatient psychiatric facility after she is cleared medically with outpatient services to follow up.
How would you treat Ms. A’s symptoms?
a) aggressively treat catatonia
b) address her eating disorder
c) work to resolve her depression
The authors’ observations
The challenge was to choose the psychotropic medication that would target her depression, obsessive, rigid thoughts, and catatonia. Administering an antidepressant with an antipsychotic would have relieved her depressive and obsessive symptoms but would not have improved her catatonia. The psychosomatic medicine team recommended starting a selective serotonin reuptake inhibitor and a benzodiazepine to target both the depression and the catatonic symptoms. Ms. A received sertraline, 12.5 mg/d, which was increased to 25 mg/d on the third day. IV lorazepam, 1 mg, 3 times a day, was recommended but the pediatric team prescribed an oral formulation. The hospital’s eating disorder protocol was instituted on the day of admission.
Treatment options for catatonia
Benzodiazepines are the first line of treatment for catatonia and other neuroleptics, specifically antipsychotics, have been considered dangerous.10 Benzodiazepine-resistant catatonia, which is sometimes seen in patients with autism, might respond to electroconvulsive therapy (ECT),11 although in some states it cannot be administered to children age <18.12 Benzodiazepines have shown dramatic improvement within hours, as has ECT.8,13 Additionally, if patients do not respond to a benzodiazepine or ECT, consider other options such as zolpidem, olanzapine,14 or sensory integration system (in adolescents with autism).15
Ms. A did not need ECT or an alternative treatment because she responded well to 3 doses of oral lorazepam. Her amotivation, negativism, and rigidity with prolonged posturing improved. Her psychomotor retardation improved overall, although she reported some dizziness and had some postural hypotension, which was attributed to her eating issues and dehydration.
OUTCOME Feeling motivated
Ms. A is transferred to psychiatric inpatient unit. She tolerates sertraline, which is titrated to 50 mg/d. She is placed on the hospital’s standard eating disorder protocol. She continues to eat well with adequate intake of solids and liquid and exhibits only some anxiety associated with meals. During the course of hospitalization, she attends group therapy and her catatonic symptoms completely resolve. She says she thinks that her thoughts are improving and that she is not longer feeling confused. She reports being motivated to continue to improve her eating disorder symptoms.
The treatment team holds a family session during which family dynamic issues that are stressful to Ms. A are discussed, such as some conflict with her parents as well as some negative interactions between Ms. A and her father. Repeat comprehensive metabolic panel on admission to the inpatient psychiatric hospital reveals persistent elevation of AST at 92 U/L and ALT at 143 U/L. Ms. A is discharged home with follow-up with a psychiatrist and a therapist. The treatment team also recommends that she follow up in a program that specializes in eating disorders.
4-month follow-up. Ms. A returns to inpatient psychiatric hospital after overdose of sertraline and aripiprazole, which were started by an outpatient psychiatrist. She reports severe depressive symptoms because of school stressors. She denies any problems eating and did not show any symptoms of catatonia. In her chart, there is a mention of “cloudy thoughts” and quietness. At this admission, her ALT is 17 U/L and AST is 19 U/L. Sertraline is increased to 150 mg/d and aripiprazole is reduced to 2 mg/d and then later increased to 5 mg/d, after which she is discharged home with an outpatient psychiatric follow-up.
1-year follow-up. Ms. A has been following up with an outpatient psychiatrist and is receiving sertraline, 150 mg/d, aripiprazole, 2.5 mg/d, and extended-release methylphenidate, 36 mg/d, along with L-methylfolate, multivitamins, and omega-3 fish oil as adjuvants for her depressive symptoms. Ms. A does not show symptoms of an eating disorder or catatonia, and her depression and psychomotor activity have improved, with better overall functionality, after adding the stimulant and adjunctives to the antidepressant.
The authors’ observations
The importance of including catatonia NOS with its various specifiers, such as medical, metabolic, toxic, affective, etc., has been discussed.16,17 In Ms. A’s case, instead of treating the specific symptoms—affective or eating disorder or obsessive quality of thought content, mimicking psychotic-like symptoms—addressing the catatonia initially had a better outcome. More studies related to chronic and acute catatonia in adolescents are needed because of the risk of increased morbidity and premature death.18 Early recognition of catatonia is needed19 because it often is underdiagnosed.20
Eating disorders often become worse over the first 5 years, and close monitoring and assessment is needed for adolescents.21 Also, prodromal psychotic symptoms require follow-up because techniques for early detection and intervention for children and adolescents are still in their infancy.22
Bottom Line
Catatonia in adolescents should be addressed early, when it is treatable and the outcome is favorable. It is important to recognize catatonia in an emergency department or inpatient medical unit setting in a hospital because it is often underdiagnosed or misdiagnosed. The presentation of catatonia is similar in adolescents and adults. Benzodiazepines are first-line treatment for catatonia; consider electroconvulsive therapy if patients do not respond to drug therapy.
Related Resources
• Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517. doi: 10.1155/2014/309517.
• Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
Drug Brand Names
Aripiprazole • Abilify Minocycline • Minocin
L-methylfolate • Deplin Olanzapine • Zyprexa
Lorazepam • Ativan Sertraline • Zoloft
Methylphenidate • Ritalin, Concerta Zolpidem • Ambien, Intermezzo
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE A fainting spell
Ms. A, age 13, is admitted to a pediatric unit after fainting and losing consciousness for 5 minutes in the shower, during which time she was non-responsive. She reports feeling nauseated and having blurry vision before dropping to the floor.
Ms. A reports intentional self-restriction of calories, self-induced vomiting, and other purging behaviors, such as laxative abuse and excessive exercising.
During the mental status examination, Ms. A is lying in bed wearing hospital clothes, legs flexed at the knee, hands on her side, and a fixed gaze at the ceiling with poor eye contact. She is of slender stature and tall, seems slightly older than her stated age, and is poorly groomed.
Throughout the interview, Ms. A has significant psychomotor retardation, reports her mood as tired, and has a blunted affect. She speaks at a low volume and has poverty of speech; she takes deep sighs before answering questions. Her thought process is linear and she cooperates with the interview. She has poor recall, including delayed 3-minute recall and poor sustained attention. Her abstraction capacity is fair and her intellect is average and comparable with her age group. Ms. A is preoccupied that eating will cause weight gain. She denies hallucinations but reports passive death wishes with self-harm by scratching.
What is the differential diagnosis to explain Ms. A’s presentation?
a) syncope
b) seizures
c) dehydration
d) hypotension
HISTORY Preoccupied with weight
Ms. A reports vomiting twice a day, while showering and at night when no one is around, every day for 2 months. She stopped eating and taking in fluids 3 days before admission to the medical unit. Also, she reports restricting her diet to 700 to 1,000 calories a day, skipping lunch at school, and eating minimally at night. Ms. A uses raspberry ketones and green coffee beans, which are advertised to aid weight loss, and laxative pills from her mother’s medicine cabinet once or twice a week when her throat is sore from vomiting. She reports exercising excessively, which includes running, crunches, and lifting weights. She has lost approximately 30 lb in the last 2 months.
Ms. A says she fears gaining weight and feels increased guilt after eating a meal. She said that looking at food induced “anxiety attack” symptoms of increased heart rate, sweaty palms, feeling of choking, nervousness, and shakiness. She adds that she does not want to be “bigger” than her classmates. Her understanding of the consequences of not eating is, “It will get worse, I will shut down and die. I do not fear death, I only fear getting bigger than others.”
She reports that her fixation on avoiding food started when she realized that she was the tallest girl in her class and the only girl in her class running on the track team, after which she quit athletics. She reports that depression symptoms pre-dated her eating disorder symptoms; onset of significant depression likely was precipitated by her grandfather’s death a year earlier, and then exacerbated by the recent death of a family pet.
Ms. A’s depressive symptoms are described as anhedonia (avoiding being outside and not enjoying drawing anymore), decreased energy, tearfulness, sadness, decreased concentration, and passive suicidal thoughts. Her mother is supportive and motivates her daughter to “get better.” Ms. A denies any symptoms of psychosis, other anxiety symptoms, other mood disorder symptoms, substance abuse, or homicidality.
Ms. A’s mother says she felt that, recently, her daughter has been having some difficulty with confused thoughts and significantly delayed responses. However, the mother reports that her daughter always had somewhat delayed responses from what she felt is typical. Her mother adds that Ms. A’s suicidal thoughts have worsened since her daughter started restricting her diet.
Which diagnosis likely accounts for Ms. A’s presentation?
a) major depressive disorder (MDD)
b) eating disorder, not otherwise specified (NOS)
c) anorexia nervosa, purging type
d) catatonia, unspecified
e) anxiety disorder NOS
f) cognitive disorder
g) psychosis NOS
The authors’ observations
There are many reported causes of catatonia in children and adolescents, including those that are psychiatric, medical, or neurological, as well as drugs (Table 1).1,2 Affective disorders have been associated with catatonia in adults, but has not been widely reported in children and adolescents.1,3 Organic and neurologic causes, such as neurological tumors and cerebral hemorrhage, should be ruled out first because, although rare, they can be fatal (Table 2).2 If the cause of catatonia is not recognized quickly (Figure,1,2) effective treatment could be delayed.4
Catatonia involves psychomotor abnormalities, which are listed in Table 3.1,4
Presentation in adults and adolescents is similar.
An eating disorder could be comorbid with another psychiatric disorder, such as MDD, dysthymia, or panic disorder.5 Ms. A’s report of depression before she began restricting food favored a primary diagnosis of MDD. Her depressive symptoms of low appetite or low self-worth could have led to her preoccupation with body image.
There has been evidence that negative self-image and eating disorders are associated, but data are limited and the connection remains unclear.6 Ms. A’s self-esteem was very low. Her fixation on restricting food could have been perpetuated by her self-criticism and by being excluded from her peer group in school. Her weight loss could have brought anxiety symptoms to the forefront because of physiologic changes that accompany extreme weight loss.
The treatment team was concerned about her delayed responses, which could be explained by the catatonic features that reflected the severity of her depression. She had no obvious symptoms of psychosis, but her intrusive thoughts and obsessions with avoiding food did not completely rule out psychosis.
Childhood-onset schizophrenia, although rare, has been associated with catatonia; following up with a catatonia rating scale, such as the Catatonia Rating Scale or the Bush- Francis Catatonia Rating Scale (BFCRS), would be useful for tracking symptom progress. In Ms. A’s case, her mood disorder was primary, but did not rule out psychosis-like prodromal symptoms.7
Ms. A is diagnosed with MDD, single episode, severe, with catatonic features, and without psychosis, and eating disorder, NOS.
EVALUATION Mostly normal
Ms. A does not have a history of mental illness and was not seeing a psychiatrist or therapist, nor did she have any prior psychiatric admissions. She denies suicide attempts, but reports self-injurious behavior involving scratching her skin, which started during the current mood episode. She has never taken any psychotropic medications. Ms. A lives at home with her biological mother and father and 17-year-old brother. She attends middle school with average grades and has no history of disciplinary actions. She has no history of bullying or teasing, although she did report some previous difficulty with relational aggression toward her peers in the 5th grade. Her mother has a history of anorexia nervosa that began when she was a teenager, but these symptoms are stable and under control. There is additionally a family history of bipolar disorder.
Ms. A has a family history of coronary artery disease and diabetes in the mother and maternal relatives. Her grandfather died from liver cancer. She was allergic to sulfa drugs and was taking a multivitamin and minocycline for acne.
Physical examination reveals some superficial scratches but otherwise was within normal limits. Initial lab results reveal a normal complete blood count and differential. Thyroid-stimulating hormone is 1.29 mIU/L and free T4 is 0.96 mg/dL, both within normal limits. Urinalysis is within normal limits and urine pregnancy test is negative. A comprehensive metabolic panel shows mild elevation in aspartate aminotransferase (AST) at 60 U/L and alanine aminotransferase (ALT) at 92 U/L, respectively. Phosphorus level is within normal limits. Prealbumin level is slightly low at 15.1 mg/dL.
Which treatment plan would you recommend for Ms. A?
a) discharge with outpatient psychiatric treatment
b) recommend medical stabilization with follow-up from the psychosomatic team and then outpatient psychiatric follow-up
c) admit her to the psychiatric acute inpatient hospital with psychiatric outpatient discharge follow-up plan
d) discharge her home with follow-up with her primary care physician
e) recommend follow-up from the psychosomatic team while on medical floor with acute inpatient admission and psychiatric outpatient follow-up at discharge
The authors’ observations
Scarcity of data and reporting of cases of adolescent catatonia limits guidance for diagnosis and treatment.8 There are several rating scales with variability in definition, but that overall provide a guiding tool for detecting catatonia. The Brief Cognitive Rating Scale is considered the most versatile because it is more valid, reliable, and requires less time to complete than other rating scales.9
Ms. A’s symptoms were a combination of depressive symptoms with severity defined by catatonic features, eating disorder with worsening course, anxiety symptoms, and genetic loading of eating disorder in her mother. The challenge of this case was making an accurate diagnosis and treating Ms. A, which required continuous observation following an eating disorder protocol, resolution of her catatonia, resuming a normal diet, and decreasing her suicidality. Retrospectively, her scores on the BFCRS were high on screening items 1 to 14, which measure presence or absence and severity of symptoms.
The best option was to admit Ms. A to an inpatient psychiatric facility after she is cleared medically with outpatient services to follow up.
How would you treat Ms. A’s symptoms?
a) aggressively treat catatonia
b) address her eating disorder
c) work to resolve her depression
The authors’ observations
The challenge was to choose the psychotropic medication that would target her depression, obsessive, rigid thoughts, and catatonia. Administering an antidepressant with an antipsychotic would have relieved her depressive and obsessive symptoms but would not have improved her catatonia. The psychosomatic medicine team recommended starting a selective serotonin reuptake inhibitor and a benzodiazepine to target both the depression and the catatonic symptoms. Ms. A received sertraline, 12.5 mg/d, which was increased to 25 mg/d on the third day. IV lorazepam, 1 mg, 3 times a day, was recommended but the pediatric team prescribed an oral formulation. The hospital’s eating disorder protocol was instituted on the day of admission.
Treatment options for catatonia
Benzodiazepines are the first line of treatment for catatonia and other neuroleptics, specifically antipsychotics, have been considered dangerous.10 Benzodiazepine-resistant catatonia, which is sometimes seen in patients with autism, might respond to electroconvulsive therapy (ECT),11 although in some states it cannot be administered to children age <18.12 Benzodiazepines have shown dramatic improvement within hours, as has ECT.8,13 Additionally, if patients do not respond to a benzodiazepine or ECT, consider other options such as zolpidem, olanzapine,14 or sensory integration system (in adolescents with autism).15
Ms. A did not need ECT or an alternative treatment because she responded well to 3 doses of oral lorazepam. Her amotivation, negativism, and rigidity with prolonged posturing improved. Her psychomotor retardation improved overall, although she reported some dizziness and had some postural hypotension, which was attributed to her eating issues and dehydration.
OUTCOME Feeling motivated
Ms. A is transferred to psychiatric inpatient unit. She tolerates sertraline, which is titrated to 50 mg/d. She is placed on the hospital’s standard eating disorder protocol. She continues to eat well with adequate intake of solids and liquid and exhibits only some anxiety associated with meals. During the course of hospitalization, she attends group therapy and her catatonic symptoms completely resolve. She says she thinks that her thoughts are improving and that she is not longer feeling confused. She reports being motivated to continue to improve her eating disorder symptoms.
The treatment team holds a family session during which family dynamic issues that are stressful to Ms. A are discussed, such as some conflict with her parents as well as some negative interactions between Ms. A and her father. Repeat comprehensive metabolic panel on admission to the inpatient psychiatric hospital reveals persistent elevation of AST at 92 U/L and ALT at 143 U/L. Ms. A is discharged home with follow-up with a psychiatrist and a therapist. The treatment team also recommends that she follow up in a program that specializes in eating disorders.
4-month follow-up. Ms. A returns to inpatient psychiatric hospital after overdose of sertraline and aripiprazole, which were started by an outpatient psychiatrist. She reports severe depressive symptoms because of school stressors. She denies any problems eating and did not show any symptoms of catatonia. In her chart, there is a mention of “cloudy thoughts” and quietness. At this admission, her ALT is 17 U/L and AST is 19 U/L. Sertraline is increased to 150 mg/d and aripiprazole is reduced to 2 mg/d and then later increased to 5 mg/d, after which she is discharged home with an outpatient psychiatric follow-up.
1-year follow-up. Ms. A has been following up with an outpatient psychiatrist and is receiving sertraline, 150 mg/d, aripiprazole, 2.5 mg/d, and extended-release methylphenidate, 36 mg/d, along with L-methylfolate, multivitamins, and omega-3 fish oil as adjuvants for her depressive symptoms. Ms. A does not show symptoms of an eating disorder or catatonia, and her depression and psychomotor activity have improved, with better overall functionality, after adding the stimulant and adjunctives to the antidepressant.
The authors’ observations
The importance of including catatonia NOS with its various specifiers, such as medical, metabolic, toxic, affective, etc., has been discussed.16,17 In Ms. A’s case, instead of treating the specific symptoms—affective or eating disorder or obsessive quality of thought content, mimicking psychotic-like symptoms—addressing the catatonia initially had a better outcome. More studies related to chronic and acute catatonia in adolescents are needed because of the risk of increased morbidity and premature death.18 Early recognition of catatonia is needed19 because it often is underdiagnosed.20
Eating disorders often become worse over the first 5 years, and close monitoring and assessment is needed for adolescents.21 Also, prodromal psychotic symptoms require follow-up because techniques for early detection and intervention for children and adolescents are still in their infancy.22
Bottom Line
Catatonia in adolescents should be addressed early, when it is treatable and the outcome is favorable. It is important to recognize catatonia in an emergency department or inpatient medical unit setting in a hospital because it is often underdiagnosed or misdiagnosed. The presentation of catatonia is similar in adolescents and adults. Benzodiazepines are first-line treatment for catatonia; consider electroconvulsive therapy if patients do not respond to drug therapy.
Related Resources
• Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517. doi: 10.1155/2014/309517.
• Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
Drug Brand Names
Aripiprazole • Abilify Minocycline • Minocin
L-methylfolate • Deplin Olanzapine • Zyprexa
Lorazepam • Ativan Sertraline • Zoloft
Methylphenidate • Ritalin, Concerta Zolpidem • Ambien, Intermezzo
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dhossche D, Wilson C, Wachtel LE. Catatonia in childhood and adolescence: implications for the DSM-5. Primary Psychiatry. http://primarypsychiatry.com/catatonia-in-childhood-and-adolescence-implications-for-the-dsm-5. Published May 21, 2013. Accessed July 2, 2015.
2. Lahutte B, Cornic F, Bonnot O, et al. Multidisciplinary approach of organic catatonia in children and adolescents may improve treatment decision making. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1393-1398.
3. Brake JA, Abidi S. A case of adolescent catatonia. J Can Acad Child Adolesc Psychiatry. 2010;19(2):138-140.
4. Consoli A, Raffin M, Laurent C, et al. Medical and developmental risk factors of catatonia in children and adolescents: a prospective case-control study. Schizophr Res. 2012;137(1-3):151-158.
5. Zaider TI, Johnson JG, Cockell SJ. Psychiatric comorbidity associated with eating disorder symptomatology among adolescents in the community. Int J Eat Disord. 2000;28(1):58-67.
6. Forsén Mantilla E, Bergsten K, Birgegård A. Self-image and eating disorder symptoms in normal and clinical adolescents. Eat Behav. 2014;15(1):125-131.
7. Bonnot O, Tanguy ML, Consoli A, et al. Does catatonia influence the phenomenology of childhood onset schizophrenia beyond motor symptoms? Psychiatry Res. 2008;158(3):356-362.
8. Singh LK, Praharaj SK. Immediate response to lorazepam in a patient with 17 years of chronic catatonia. J Neuropsychiatry Clin Neurosci. 2013;25(3):E47-E48.
9. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1-3):1-9.
10. Cottencin O, Warembourg F, de Chouly de Lenclave MB, et al. Catatonia and consultation-liaison psychiatry study of 12 cases. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1170-1176.
11. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
12. Wachtel LE, Dhossche DM, Kellner CH. When is electroconvulsive therapy appropriate for children and adolescents? Med Hypotheses. 2011;76(3):395-399.
13. Takaoka K, Takata T. Catatonia in childhood and adolescence. Psychiatry Clin Neurosci. 2003;57(2):129-137.
14. Ceylan MF, Kul M, Kultur SE, et al. Major depression with catatonic features in a child remitted with olanzapine. J Child Adolesc Psychopharmacol. 2010;20(3):225-227.
15. Consoli A, Gheorghiev C, Jutard C, et al. Lorazepam, fluoxetine and packing therapy in an adolescent with pervasive developmental disorder and catatonia. J Physiol Paris. 2010;104(6):309-314.
16. Dhossche D, Cohen D, Ghaziuddin N, et al. The study of pediatric catatonia supports a home of its own for catatonia in DSM-5. Med Hypotheses. 2010;75(6):558-560.
17. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
18. Cornic F, Consoli A, Tanguy ML, et al. Association of adolescent catatonia with increased mortality and morbidity: evidence from a prospective follow-up study. Schizophr Res. 2009;113(2-3):233-240.
19. Quigley J, Lommel KM, Coffey B. Catatonia in an adolescent with Asperger’s disorder. J Child Adolesc Psychopharmacol. 2009;19(1):93-96.
20. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
21. Ackard DM, Fulkerson JA, Neumark-Sztainer D. Stability of eating disorder diagnostic classifications in adolescents: five-year longitudinal findings from a population-based study. Eat Disord. 2011;19(4):308-322.
22. Schimmelmann BG, Schultze-Lutter F. Early detection and intervention of psychosis in children and adolescents: urgent need for studies. Eur Child Adolesc Psychiatry. 2012;21(5):239-241.
1. Dhossche D, Wilson C, Wachtel LE. Catatonia in childhood and adolescence: implications for the DSM-5. Primary Psychiatry. http://primarypsychiatry.com/catatonia-in-childhood-and-adolescence-implications-for-the-dsm-5. Published May 21, 2013. Accessed July 2, 2015.
2. Lahutte B, Cornic F, Bonnot O, et al. Multidisciplinary approach of organic catatonia in children and adolescents may improve treatment decision making. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1393-1398.
3. Brake JA, Abidi S. A case of adolescent catatonia. J Can Acad Child Adolesc Psychiatry. 2010;19(2):138-140.
4. Consoli A, Raffin M, Laurent C, et al. Medical and developmental risk factors of catatonia in children and adolescents: a prospective case-control study. Schizophr Res. 2012;137(1-3):151-158.
5. Zaider TI, Johnson JG, Cockell SJ. Psychiatric comorbidity associated with eating disorder symptomatology among adolescents in the community. Int J Eat Disord. 2000;28(1):58-67.
6. Forsén Mantilla E, Bergsten K, Birgegård A. Self-image and eating disorder symptoms in normal and clinical adolescents. Eat Behav. 2014;15(1):125-131.
7. Bonnot O, Tanguy ML, Consoli A, et al. Does catatonia influence the phenomenology of childhood onset schizophrenia beyond motor symptoms? Psychiatry Res. 2008;158(3):356-362.
8. Singh LK, Praharaj SK. Immediate response to lorazepam in a patient with 17 years of chronic catatonia. J Neuropsychiatry Clin Neurosci. 2013;25(3):E47-E48.
9. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1-3):1-9.
10. Cottencin O, Warembourg F, de Chouly de Lenclave MB, et al. Catatonia and consultation-liaison psychiatry study of 12 cases. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1170-1176.
11. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
12. Wachtel LE, Dhossche DM, Kellner CH. When is electroconvulsive therapy appropriate for children and adolescents? Med Hypotheses. 2011;76(3):395-399.
13. Takaoka K, Takata T. Catatonia in childhood and adolescence. Psychiatry Clin Neurosci. 2003;57(2):129-137.
14. Ceylan MF, Kul M, Kultur SE, et al. Major depression with catatonic features in a child remitted with olanzapine. J Child Adolesc Psychopharmacol. 2010;20(3):225-227.
15. Consoli A, Gheorghiev C, Jutard C, et al. Lorazepam, fluoxetine and packing therapy in an adolescent with pervasive developmental disorder and catatonia. J Physiol Paris. 2010;104(6):309-314.
16. Dhossche D, Cohen D, Ghaziuddin N, et al. The study of pediatric catatonia supports a home of its own for catatonia in DSM-5. Med Hypotheses. 2010;75(6):558-560.
17. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
18. Cornic F, Consoli A, Tanguy ML, et al. Association of adolescent catatonia with increased mortality and morbidity: evidence from a prospective follow-up study. Schizophr Res. 2009;113(2-3):233-240.
19. Quigley J, Lommel KM, Coffey B. Catatonia in an adolescent with Asperger’s disorder. J Child Adolesc Psychopharmacol. 2009;19(1):93-96.
20. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
21. Ackard DM, Fulkerson JA, Neumark-Sztainer D. Stability of eating disorder diagnostic classifications in adolescents: five-year longitudinal findings from a population-based study. Eat Disord. 2011;19(4):308-322.
22. Schimmelmann BG, Schultze-Lutter F. Early detection and intervention of psychosis in children and adolescents: urgent need for studies. Eur Child Adolesc Psychiatry. 2012;21(5):239-241.
Depressed and confused, and dizzy while walking the dog
CASE Light-headed
Mr. M, age 73, is a retired project manager who feels light-headed while walking his dog, causing him to go to the emergency department. His history is significant for hypertension, coronary artery disease (CAD), 3-vessel coronary artery bypass graft surgery (CABG), hyperlipidemia, erectile dysfunction, open-angle glaucoma, hemiretinal vein occlusion, symptoms suggesting rapid eye-movement behavior disorder (RBD), and major depressive disorder (MDD).
The psychiatry consultation-liaison service is asked to help manage Mr. M’s psychiatric medications in the context of orthostatic hypotension and cognitive deficits.
What could be causing Mr. M’s symptoms?
a) drug adverse effect
b) progressive cardiovascular disease
c) MDD
d) all of the above
HISTORY Depression, heart disease
15 years ago. Mr. M experienced his first major depressive episode. His primary care physician (PCP) commented on a history of falling asleep while driving and 1 episode of sleepwalking. His depression was treated to remission with fluoxetine and methylphenidate (dosages were not recorded), the latter also addressed his falling asleep while driving.
5 years ago. Mr. M had another depressive episode characterized by anxiety, difficulty sleeping, and irritability. He also described chest pain; a cardiac work-up revealed extensive CAD, which led to 3-vessel CABG later that year. He also reported dizziness upon standing, which was treated with compression stockings and an increase in sodium intake.
Mr. M continued to express feelings of depression. His cardiologist started him on paroxetine, 10 mg/d, which he took for 2 months and decided to stop because he felt better. He declined psychiatric referral.
4 years ago. Mr. M’s PCP referred him to a psychiatrist for depressed mood, anhedonia, decreased appetite, decreased energy, and difficulty concentrating. Immediate and delayed recall were found to be intact. The psychiatrist diagnosed MDD and Mr. M started escitalopram, 5 mg/d, titrated to 15 mg/d, and trazodone, 50 mg/d.
After starting treatment, Mr. M reported decreased libido. Sustained-release bupropion, 150 mg/d, was added to boost the effects of escitalopram and counteract sexual side effects.
At follow-up, Mr. M reported that his depressive symptoms and libido had improved, but that he had been experiencing unsteady gait when getting out of his car, which he had been noticing “for a while”—before he began trazodone. Mr. M was referred to his PCP, who attributed his symptoms to orthostasis. No treatment was indicated at the time because Mr. M’s lightheadedness had resolved.
3 years ago. Mr. M reported a syncopal attack and continued “dizziness.” His PCP prescribed fludrocortisone, 0.1 mg/d, later to be dosed 0.2 mg/d, and symptoms improved.
Although Mr. M had a history of orthostatic hypotension, he was later noted to have supine hypertension. Mr. M’s PCP was concerned that fludrocortisone could be causing the supine hypertension but that decreasing the dosage would cause his orthostatic hypotension to return.
The PCP also was concerned that the psychiatric medications (escitalopram, trazodone, and bupropion) could be causing orthostasis. There was discussion among Mr. M, his PCP, and his psychiatrist of stopping the psychotropics to see if the symptoms would remit; however, because of concerns about Mr. M’s depression, the medications were continued. Mr. M monitored his blood pressure at home and was referred to a neurologist for work-up of potential autonomic dysfunction.
Shortly afterward, Mr. M reported intermittent difficulty keeping track of his thoughts and finishing sentences. His psychiatrist ordered an MRI, which showed chronic small vessel ischemic changes, and started him on donepezil, 5 mg/d.
Neuropsychological testing revealed decreased processing speed and poor recognition memory; otherwise, results showed above-average intellectual ability and average or above-average performance in measures of language, attention, visuospatial/constructional functions, and executive functions—a pattern typically attributable to psychogenic factors, such as depression.
Mr. M reported to his neurologist that he forgets directions while driving but can focus better if he makes a conscious effort. Physical exam was significant hypotension; flat affect; deficits in concentration and short-term recall; mild impairment of Luria motor sequence (composed of a go/no-go and a reciprocal motor task); and vertical and horizontal saccades.1
Mr. M consulted with an ophthalmologist for anterior iridocyclitis and ocular hypertension, which was controlled with travoprost. He continued to experience trouble with his vision and was given a diagnosis of right inferior hemiretinal vein occlusion, macular edema, and suspected glaucoma. Subsequent notes recorded a history of Posner-Schlossman syndrome (a disease characterized by recurrent attacks of increased intraocular pressure in 1 eye with concomitant anterior chamber inflammation). His vision deteriorated until he was diagnosed with ocular hypertension, open-angle glaucoma, and dermatochalasis.
The authors’ observations
Involvement of multiple specialties in a patient’s care brings to question one’s philosophy on medical diagnosis. Interdisciplinary communication would seem to promote the principle of diagnostic parsimony, or Occam’s razor, which suggests a unifying diagnosis to explain all of the patient’s symptoms. Lack of communication might favor Hickam’s dictum, which states that “patients can have as many diseases as they damn well please.”
HISTORY Low energy, forgetfulness
2 years ago. Mr. M noticed low energy and motivation. He continued to work full-time but thought that it was taking him longer to get work done. He was tapered off escitalopram and started on desvenlafaxine, 50 mg/d; donepezil was increased to 10 mg/d.
The syncopal episodes resolved but blood pressure measured at home averaged 150/70 mm Hg. Mr. M was advised to decrease fludrocortisone from 0.2 mg/d to 0.1 mg/d. He tolerated the change and blood pressure measured at home dropped on average to 120 to 130/70 mm Hg.
1 year ago. Mr. M reported that his memory loss had become worse. He perceived having more stress because of forgetfulness and visual difficulties, which had led him to stop driving at night.
At a follow-up appointment with his psychiatrist, Mr. M reported that, first, he had not tapered escitalopram as discussed and, second, he forgot to increase the dosage of desvenlafaxine. A home blood pressure log revealed consistent hypotension; the psychiatrist was concerned that hypotension could be the cause of concentration difficulties and malaise. The psychiatrist advised Mr. M to follow-up with his PCP and neurologist.
Current admission. Shortly after the visit to the psychiatrist, Mr. M presented to the emergency department for increased syncopal events. Work-up was negative for a cardiac cause. A cosyntropin stimulation test was negative, showing that adrenal insufficiency did not cause his orthostatic hypotension. Chart review showed he had been having blood pressure problems for many years, independent of antidepressants. Physical exam revealed lower extremity ataxia and a bilateral extensor plantar reflex.
What diagnosis explains Mr. M’s symptoms?
a) Parkinson’s disease
b) multiple system atrophy (MSA)
c) depression due to a general medical condition
d) dementia
The authors’ observations
MSA, previously referred to as Shy-Drager syndrome, is a rare, rapidly progressive neurodegenerative disorder with an estimated prevalence of 3.7 cases for every 100,000 people worldwide.2 MSA primarily affects middle-aged patients; because it has no cure, most patients die in 7 to 10 years.3
MSA has 2 clinical variants4,5:
• parkinsonian type (MSA-P), characterized by striatonigral degeneration and increased spasticity
• cerebellar type (MSA-C), characterized by more autonomic dysfunction.
MSA has a range of symptoms, making it a challenging diagnosis (Table).6 Although psychiatric symptoms are not part of the diagnostic criteria, they can aid in its diagnosis. In Mr. M’s case, depression, anxiety, orthostatic hypotension, and ataxia support a diagnosis of MSA.
Gilman et al6 delineated 3 diagnostic categories for MSA: definite MSA, probable MSA, and possible MSA. Clinical criteria shared by the 3 diagnostic categories are sporadic and progressive onset after age 30.
Definite MSA requires “neuropathological findings of widespread and abundant CNS alpha-synuclein-positive glial cytoplasmic inclusions,” along with “neurodegenerative changes in striatonigral or olivopontocerebellar structures” at autopsy.6
Probable MSA. Without autopsy findings required for definite MSA, the next most specific diagnostic category is probable MSA. Probable MSA also specifies that the patient show either autonomic failure involving urinary incontinence—this includes erectile dysfunction in men—or, if autonomic failure is absent, orthostatic hypotension within 3 minutes of standing by at least 30 mm Hg systolic pressure or 15 mm Hg diastolic pressure.
Possible MSA has less stringent criteria for orthostatic hypotension. The category includes patients who have only 1 symptom that suggests autonomic failure. Criteria for possible MSA include parkinsonism or a cerebellar syndrome in addition to symptoms of MSA listed in the Table, whereas probable MSA has specific criteria of either a poorly levodopa-responsive parkinsonism (MSA-P) or a cerebellar syndrome (MSA-C). In addition to having parkinsonism or a cerebellar syndrome, and 1 sign of autonomic failure or orthostatic hypotension, patients also must have ≥1 additional feature to be assigned a diagnosis of possible MSA, including:
• rapidly progressive parkinsonism
• poor response to levodopa
• postural instability within 3 years of motor onset
• gait ataxia, cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction
• dysphagia within 5 years of motor onset
• atrophy on MRI of putamen, middle cerebellar peduncle, pons, or cerebellum
• hypometabolism on fluorodeoxyglucose- PET in putamen, brainstem, or cerebellum.6
Diagnosing MSA can be challenging because its features are similar to those of many other disorders. Nonetheless, Gilman et al6 lists specific criteria for probable MSA, including autonomic dysfunction, orthostatic hypotension, and either parkinsonism or cerebellar syndrome symptoms. Although a definite MSA diagnosis only can be made by postmortem brain specimen analysis, Osaki et al7 found that a probable MSA diagnosis has a positive predictive value of 92% with a sensitivity of 22% for definite MSA.
Mr. M’s symptoms were consistent with a diagnosis of probable MSA, cerebellar type (Figure).
Psychiatric manifestations of MSA
There are a few case reports of depression identified early in patients who were later given a diagnosis of MSA.8
Depression. In a study by Benrud-Larson et al9 (N = 99), 49% of patients who had MSA reported moderate or severe depression, as indicated by a score of ≥17 on the Beck Depression Inventory (BDI); 80% reported at least mild depression (BDI ≥10, mean 17.0, standard deviation, 8.7).
In a similar study, by Balas et al,10 depression was reported as a common symptom and was statistically significant in MSA-P patients compared with controls (P = .013).
Anxiety, another symptom that was reported by Mr. M, is another psychiatric manifestation described by Balas et al10 and Chang et al.11 Balas et al10 noted that MSA-C and MSA-P patients had significantly more state anxiety (P = .009 and P = .022, respectively) compared with controls, although Chang et al11 noted higher anxiety scores in MSA-C patients compared with controls and MSA-P patients (P < .01).
Balas et al10 hypothesized that anxiety and depression contribute to cognitive decline; their study showed that MSA-C patients had difficulty learning new verbal information (P < .022) and controlling attention (P < .023). Mr. M exhibited some of these cognitive difficulties in his reports of losing track of conversations, forgetting the topic of a conversation when speaking, trouble focusing, and difficulty concentrating when driving.
Mr. M had depression and anxiety well before onset of autonomic dysfunction (orthostatic hypotension and erectile dysfunction), which eventually led to an MSA diagnosis. Psychiatrists should understand additional manifestations of MSA so that they can use psychiatric symptoms to identify these conditions in their patients. One of the most well-known and early manifestations of MSA is autonomic dysfunction; among men, another early sign is erectile dysfunction.6 Our patient also exhibited other less well-known symptoms linked to MSA and autonomic dysregulation, including RBD and ocular symptoms (iridocyclitis, glaucoma, decreased visual acuity).
Rapid eye-movement behavior disorder. Psychiatrists should consider screening for RBD during assessment of sleep problems. Identifying RBD is important because early studies have shown a strong association between RBD and development of a neurodegenerative disorder. Mr. M’s clinicians did not consider RBD, although his symptoms of sleepwalking and falling asleep while driving suggest a possible diagnosis. Also, considering this diagnosis would aid in diagnosing a synucleinopathy disorder because a higher incidence of RBD was noted in patients who developed synucleinopathy disorders (eg, Parkinson’s disease [PD] and dementia with Lewy bodies [DLB]) compared with patients who developed non-synucleinopathies (eg, frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, mild cognitive impairment, primary progressive aphasia, and posterior cortical atrophy) or tauopathies (eg, Alzheimer’s disease).12
Zanigni et al13 reported similar findings in a later study that classified patients with RBD as having idiopathic RBD (IRBD) or RBD secondary to an underlying neurodegenerative disorder, particularly an α-synucleinopathy: PD, MSA, and DLB. Most IRBD patients developed 1 of the above mentioned neurodegenerative disorders as long as 10 years after a diagnosis of RBD.
In a study by Iranzo et al,14 patients with MSA were noted to have more severe RBD compared with PD patients. Severity is illustrated by greater periodic leg movements during sleep (P = .001), less total sleep time (P = .023), longer sleep onset latency (P = .023), and a higher percentage of REM sleep without atonia (RSWA, P = .001). McCarter et al15 also noted a higher incidence of RSWA in patients with MSA.
Patients with MSA might therefore be more likely to exhibit difficulty initiating and maintaining sleep and as having RSWA years before the MSA diagnosis.
Several psychotropics (eg, first-generation antipsychotics, tricyclic antidepressants, lithium, benzodiazepines, carbamazepine, topiramate, and selective serotonin reuptake inhibitors) can cause adverse ocular effects, such as closed-angle glaucoma in predisposed persons and retinopathy.16 Therefore, it is important for psychiatrists to ask about ocular symptoms because they might be an early sign of autonomic dysfunction.
Posner and Schlossman17 theorized a causal relationship between autonomic dysfunction and ocular diseases after studying a group of patients who had intermittent unilateral attacks of iridocyclitis and glaucoma (now known as Posner-Schlossman syndrome). They hypothesized that a central cause in the hypothalamus, combined with underlying autonomic dysregulation, could cause the intermittent attacks.
Gherghel et al18 noted a significant difference in ocular blood flow and blood pressure in patients with primary open-angle glaucoma (POAG) compared with controls. Patients with POAG did not show an increase in blood pressure or ocular blood flow when challenged by cold water, which should have increased their sympathetic activity. Gherghel et al18 concluded that this indicated possible systemic autonomic dysfunction in patients with POAG. In a study by Fischer et al,19 MSA patients also were noted to have significant loss of nasal retinal nerve fiber layer thickness vs controls (P < .05), leading to decreased peripheral vision sensitivity.
Bottom Line
Although psychiatric symptoms are not part of the diagnostic criteria for multiple system atrophy (MSA), they may serve as a clue to consider when they occur with other MSA symptoms. Evaluate the importance of psychiatric symptoms in terms of the whole picture of the patient. Although the diagnosis might not alter the patient’s course, it can allow family members to understand the patient’s condition and prepare for complications that will arise.
Related Resources
• The MSA Coalition. www.multiplesystematrophy.org.
• National Institute of Neurological Disorders and Stroke. Multiple system atrophy fact sheet. www.ninds.nih.gov/disorders/msa/detailmsa.htm.
• Wenning GK, Fanciulli A, eds. Multiple system atrophy. Vienna, Austria: Springer-Verlag Wien; 2014.
Drug Brand Names
Bupropion • Wellbutrin Lithium • Eskalith, Lithobid
Carbamazepine • Tegretol Methylphenidate • Ritalin
Desvenlafaxine • Pristiq Paroxetine • Paxil
Donepezil • Aricept Travoprost • Travatan
Escitalopram • Lexapro Trazodone • Desyrel, Oleptro
Fludrocortisone • Florinef Topiramate • Topamax
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Hynan LS, Rossetti H, et al. Luria’s three-step test: what is it and what does it tell us? Int Psychogeriatr. 2011;23(10):1602-1606.
2. Orphanet Report Series. Prevalence of rare diseases: bibliographic data. http://www.orpha.net/orphacom/ cahiers/docs/GB/Prevalence_of_rare_diseases_by_ alphabetical_list.pdf. Published May 2014. Accessed May 27, 2015.
3. National Institute of Neurological Disorders and Stroke. Multiple system atrophy with orthostatic hypotension information page. http://www.ninds.nih.gov/disorders/ msa_orthostatic_hypotension/msa_orthostatic_ hypotension.htm?css=print. Updated December 5, 2013. Accessed May 27, 2015.
4. Flaherty AW, Rost NS. The Massachusetts Hospital handbook of neurology. 2nd ed. Lippincott Williams & Wilkins: Boston, MA; 2007:79.
5. Hemingway J, Franco K, Chmelik E. Shy-Drager syndrome: multisystem atrophy with comorbid depression. Psychosomatics. 2005;46(1):73-76.
6. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670-676.
7. Osaki Y, Wenning GK, Daniel SE, et al. Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology. 2002;59(10):1486-1491.
8. Goto K, Ueki A, Shimode H, et al. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci. 2000;54(4):507-511.
9. Benrud-Larson LM, Sandroni P, Schrag A, et al. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord. 2005;20(8):951-957.
10. Balas M, Balash Y, Giladi N, et al. Cognition in multiple system atrophy: neuropsychological profile and interaction with mood. J Neural Transm. 2010;117(3):369-375.
11. Chang CC, Chang YY, Chang WN, et al. Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur J Neurol. 2009;16(10):1144-1150.
12. Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61(1):40-45.
13. Zanigni S, Calandra-Buonaura G, Grimaldi D, et al. REM behaviour disorder and neurodegenerative diseases. Sleep Med. 2011;12(suppl 2):S54-S58.
14. Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65(2):247-252.
15. McCarter SJ, St. Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12(2):182-192.
16. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501-526.
17. Posner A, Schlossman A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthal. 1948;39(4):517-535.
18. Gherghel D, Hosking SL, Cunliffe IA. Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci. 2004;45(10):3546-3554.
19. Fischer MD, Synofzik M, Kernstock C, et al. Decreased retinal sensitivity and loss of retinal nerve fibers in multiple system atrophy. Graefes Arch Clin Exp Opthalmol. 2013;251(1):235-241.
CASE Light-headed
Mr. M, age 73, is a retired project manager who feels light-headed while walking his dog, causing him to go to the emergency department. His history is significant for hypertension, coronary artery disease (CAD), 3-vessel coronary artery bypass graft surgery (CABG), hyperlipidemia, erectile dysfunction, open-angle glaucoma, hemiretinal vein occlusion, symptoms suggesting rapid eye-movement behavior disorder (RBD), and major depressive disorder (MDD).
The psychiatry consultation-liaison service is asked to help manage Mr. M’s psychiatric medications in the context of orthostatic hypotension and cognitive deficits.
What could be causing Mr. M’s symptoms?
a) drug adverse effect
b) progressive cardiovascular disease
c) MDD
d) all of the above
HISTORY Depression, heart disease
15 years ago. Mr. M experienced his first major depressive episode. His primary care physician (PCP) commented on a history of falling asleep while driving and 1 episode of sleepwalking. His depression was treated to remission with fluoxetine and methylphenidate (dosages were not recorded), the latter also addressed his falling asleep while driving.
5 years ago. Mr. M had another depressive episode characterized by anxiety, difficulty sleeping, and irritability. He also described chest pain; a cardiac work-up revealed extensive CAD, which led to 3-vessel CABG later that year. He also reported dizziness upon standing, which was treated with compression stockings and an increase in sodium intake.
Mr. M continued to express feelings of depression. His cardiologist started him on paroxetine, 10 mg/d, which he took for 2 months and decided to stop because he felt better. He declined psychiatric referral.
4 years ago. Mr. M’s PCP referred him to a psychiatrist for depressed mood, anhedonia, decreased appetite, decreased energy, and difficulty concentrating. Immediate and delayed recall were found to be intact. The psychiatrist diagnosed MDD and Mr. M started escitalopram, 5 mg/d, titrated to 15 mg/d, and trazodone, 50 mg/d.
After starting treatment, Mr. M reported decreased libido. Sustained-release bupropion, 150 mg/d, was added to boost the effects of escitalopram and counteract sexual side effects.
At follow-up, Mr. M reported that his depressive symptoms and libido had improved, but that he had been experiencing unsteady gait when getting out of his car, which he had been noticing “for a while”—before he began trazodone. Mr. M was referred to his PCP, who attributed his symptoms to orthostasis. No treatment was indicated at the time because Mr. M’s lightheadedness had resolved.
3 years ago. Mr. M reported a syncopal attack and continued “dizziness.” His PCP prescribed fludrocortisone, 0.1 mg/d, later to be dosed 0.2 mg/d, and symptoms improved.
Although Mr. M had a history of orthostatic hypotension, he was later noted to have supine hypertension. Mr. M’s PCP was concerned that fludrocortisone could be causing the supine hypertension but that decreasing the dosage would cause his orthostatic hypotension to return.
The PCP also was concerned that the psychiatric medications (escitalopram, trazodone, and bupropion) could be causing orthostasis. There was discussion among Mr. M, his PCP, and his psychiatrist of stopping the psychotropics to see if the symptoms would remit; however, because of concerns about Mr. M’s depression, the medications were continued. Mr. M monitored his blood pressure at home and was referred to a neurologist for work-up of potential autonomic dysfunction.
Shortly afterward, Mr. M reported intermittent difficulty keeping track of his thoughts and finishing sentences. His psychiatrist ordered an MRI, which showed chronic small vessel ischemic changes, and started him on donepezil, 5 mg/d.
Neuropsychological testing revealed decreased processing speed and poor recognition memory; otherwise, results showed above-average intellectual ability and average or above-average performance in measures of language, attention, visuospatial/constructional functions, and executive functions—a pattern typically attributable to psychogenic factors, such as depression.
Mr. M reported to his neurologist that he forgets directions while driving but can focus better if he makes a conscious effort. Physical exam was significant hypotension; flat affect; deficits in concentration and short-term recall; mild impairment of Luria motor sequence (composed of a go/no-go and a reciprocal motor task); and vertical and horizontal saccades.1
Mr. M consulted with an ophthalmologist for anterior iridocyclitis and ocular hypertension, which was controlled with travoprost. He continued to experience trouble with his vision and was given a diagnosis of right inferior hemiretinal vein occlusion, macular edema, and suspected glaucoma. Subsequent notes recorded a history of Posner-Schlossman syndrome (a disease characterized by recurrent attacks of increased intraocular pressure in 1 eye with concomitant anterior chamber inflammation). His vision deteriorated until he was diagnosed with ocular hypertension, open-angle glaucoma, and dermatochalasis.
The authors’ observations
Involvement of multiple specialties in a patient’s care brings to question one’s philosophy on medical diagnosis. Interdisciplinary communication would seem to promote the principle of diagnostic parsimony, or Occam’s razor, which suggests a unifying diagnosis to explain all of the patient’s symptoms. Lack of communication might favor Hickam’s dictum, which states that “patients can have as many diseases as they damn well please.”
HISTORY Low energy, forgetfulness
2 years ago. Mr. M noticed low energy and motivation. He continued to work full-time but thought that it was taking him longer to get work done. He was tapered off escitalopram and started on desvenlafaxine, 50 mg/d; donepezil was increased to 10 mg/d.
The syncopal episodes resolved but blood pressure measured at home averaged 150/70 mm Hg. Mr. M was advised to decrease fludrocortisone from 0.2 mg/d to 0.1 mg/d. He tolerated the change and blood pressure measured at home dropped on average to 120 to 130/70 mm Hg.
1 year ago. Mr. M reported that his memory loss had become worse. He perceived having more stress because of forgetfulness and visual difficulties, which had led him to stop driving at night.
At a follow-up appointment with his psychiatrist, Mr. M reported that, first, he had not tapered escitalopram as discussed and, second, he forgot to increase the dosage of desvenlafaxine. A home blood pressure log revealed consistent hypotension; the psychiatrist was concerned that hypotension could be the cause of concentration difficulties and malaise. The psychiatrist advised Mr. M to follow-up with his PCP and neurologist.
Current admission. Shortly after the visit to the psychiatrist, Mr. M presented to the emergency department for increased syncopal events. Work-up was negative for a cardiac cause. A cosyntropin stimulation test was negative, showing that adrenal insufficiency did not cause his orthostatic hypotension. Chart review showed he had been having blood pressure problems for many years, independent of antidepressants. Physical exam revealed lower extremity ataxia and a bilateral extensor plantar reflex.
What diagnosis explains Mr. M’s symptoms?
a) Parkinson’s disease
b) multiple system atrophy (MSA)
c) depression due to a general medical condition
d) dementia
The authors’ observations
MSA, previously referred to as Shy-Drager syndrome, is a rare, rapidly progressive neurodegenerative disorder with an estimated prevalence of 3.7 cases for every 100,000 people worldwide.2 MSA primarily affects middle-aged patients; because it has no cure, most patients die in 7 to 10 years.3
MSA has 2 clinical variants4,5:
• parkinsonian type (MSA-P), characterized by striatonigral degeneration and increased spasticity
• cerebellar type (MSA-C), characterized by more autonomic dysfunction.
MSA has a range of symptoms, making it a challenging diagnosis (Table).6 Although psychiatric symptoms are not part of the diagnostic criteria, they can aid in its diagnosis. In Mr. M’s case, depression, anxiety, orthostatic hypotension, and ataxia support a diagnosis of MSA.
Gilman et al6 delineated 3 diagnostic categories for MSA: definite MSA, probable MSA, and possible MSA. Clinical criteria shared by the 3 diagnostic categories are sporadic and progressive onset after age 30.
Definite MSA requires “neuropathological findings of widespread and abundant CNS alpha-synuclein-positive glial cytoplasmic inclusions,” along with “neurodegenerative changes in striatonigral or olivopontocerebellar structures” at autopsy.6
Probable MSA. Without autopsy findings required for definite MSA, the next most specific diagnostic category is probable MSA. Probable MSA also specifies that the patient show either autonomic failure involving urinary incontinence—this includes erectile dysfunction in men—or, if autonomic failure is absent, orthostatic hypotension within 3 minutes of standing by at least 30 mm Hg systolic pressure or 15 mm Hg diastolic pressure.
Possible MSA has less stringent criteria for orthostatic hypotension. The category includes patients who have only 1 symptom that suggests autonomic failure. Criteria for possible MSA include parkinsonism or a cerebellar syndrome in addition to symptoms of MSA listed in the Table, whereas probable MSA has specific criteria of either a poorly levodopa-responsive parkinsonism (MSA-P) or a cerebellar syndrome (MSA-C). In addition to having parkinsonism or a cerebellar syndrome, and 1 sign of autonomic failure or orthostatic hypotension, patients also must have ≥1 additional feature to be assigned a diagnosis of possible MSA, including:
• rapidly progressive parkinsonism
• poor response to levodopa
• postural instability within 3 years of motor onset
• gait ataxia, cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction
• dysphagia within 5 years of motor onset
• atrophy on MRI of putamen, middle cerebellar peduncle, pons, or cerebellum
• hypometabolism on fluorodeoxyglucose- PET in putamen, brainstem, or cerebellum.6
Diagnosing MSA can be challenging because its features are similar to those of many other disorders. Nonetheless, Gilman et al6 lists specific criteria for probable MSA, including autonomic dysfunction, orthostatic hypotension, and either parkinsonism or cerebellar syndrome symptoms. Although a definite MSA diagnosis only can be made by postmortem brain specimen analysis, Osaki et al7 found that a probable MSA diagnosis has a positive predictive value of 92% with a sensitivity of 22% for definite MSA.
Mr. M’s symptoms were consistent with a diagnosis of probable MSA, cerebellar type (Figure).
Psychiatric manifestations of MSA
There are a few case reports of depression identified early in patients who were later given a diagnosis of MSA.8
Depression. In a study by Benrud-Larson et al9 (N = 99), 49% of patients who had MSA reported moderate or severe depression, as indicated by a score of ≥17 on the Beck Depression Inventory (BDI); 80% reported at least mild depression (BDI ≥10, mean 17.0, standard deviation, 8.7).
In a similar study, by Balas et al,10 depression was reported as a common symptom and was statistically significant in MSA-P patients compared with controls (P = .013).
Anxiety, another symptom that was reported by Mr. M, is another psychiatric manifestation described by Balas et al10 and Chang et al.11 Balas et al10 noted that MSA-C and MSA-P patients had significantly more state anxiety (P = .009 and P = .022, respectively) compared with controls, although Chang et al11 noted higher anxiety scores in MSA-C patients compared with controls and MSA-P patients (P < .01).
Balas et al10 hypothesized that anxiety and depression contribute to cognitive decline; their study showed that MSA-C patients had difficulty learning new verbal information (P < .022) and controlling attention (P < .023). Mr. M exhibited some of these cognitive difficulties in his reports of losing track of conversations, forgetting the topic of a conversation when speaking, trouble focusing, and difficulty concentrating when driving.
Mr. M had depression and anxiety well before onset of autonomic dysfunction (orthostatic hypotension and erectile dysfunction), which eventually led to an MSA diagnosis. Psychiatrists should understand additional manifestations of MSA so that they can use psychiatric symptoms to identify these conditions in their patients. One of the most well-known and early manifestations of MSA is autonomic dysfunction; among men, another early sign is erectile dysfunction.6 Our patient also exhibited other less well-known symptoms linked to MSA and autonomic dysregulation, including RBD and ocular symptoms (iridocyclitis, glaucoma, decreased visual acuity).
Rapid eye-movement behavior disorder. Psychiatrists should consider screening for RBD during assessment of sleep problems. Identifying RBD is important because early studies have shown a strong association between RBD and development of a neurodegenerative disorder. Mr. M’s clinicians did not consider RBD, although his symptoms of sleepwalking and falling asleep while driving suggest a possible diagnosis. Also, considering this diagnosis would aid in diagnosing a synucleinopathy disorder because a higher incidence of RBD was noted in patients who developed synucleinopathy disorders (eg, Parkinson’s disease [PD] and dementia with Lewy bodies [DLB]) compared with patients who developed non-synucleinopathies (eg, frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, mild cognitive impairment, primary progressive aphasia, and posterior cortical atrophy) or tauopathies (eg, Alzheimer’s disease).12
Zanigni et al13 reported similar findings in a later study that classified patients with RBD as having idiopathic RBD (IRBD) or RBD secondary to an underlying neurodegenerative disorder, particularly an α-synucleinopathy: PD, MSA, and DLB. Most IRBD patients developed 1 of the above mentioned neurodegenerative disorders as long as 10 years after a diagnosis of RBD.
In a study by Iranzo et al,14 patients with MSA were noted to have more severe RBD compared with PD patients. Severity is illustrated by greater periodic leg movements during sleep (P = .001), less total sleep time (P = .023), longer sleep onset latency (P = .023), and a higher percentage of REM sleep without atonia (RSWA, P = .001). McCarter et al15 also noted a higher incidence of RSWA in patients with MSA.
Patients with MSA might therefore be more likely to exhibit difficulty initiating and maintaining sleep and as having RSWA years before the MSA diagnosis.
Several psychotropics (eg, first-generation antipsychotics, tricyclic antidepressants, lithium, benzodiazepines, carbamazepine, topiramate, and selective serotonin reuptake inhibitors) can cause adverse ocular effects, such as closed-angle glaucoma in predisposed persons and retinopathy.16 Therefore, it is important for psychiatrists to ask about ocular symptoms because they might be an early sign of autonomic dysfunction.
Posner and Schlossman17 theorized a causal relationship between autonomic dysfunction and ocular diseases after studying a group of patients who had intermittent unilateral attacks of iridocyclitis and glaucoma (now known as Posner-Schlossman syndrome). They hypothesized that a central cause in the hypothalamus, combined with underlying autonomic dysregulation, could cause the intermittent attacks.
Gherghel et al18 noted a significant difference in ocular blood flow and blood pressure in patients with primary open-angle glaucoma (POAG) compared with controls. Patients with POAG did not show an increase in blood pressure or ocular blood flow when challenged by cold water, which should have increased their sympathetic activity. Gherghel et al18 concluded that this indicated possible systemic autonomic dysfunction in patients with POAG. In a study by Fischer et al,19 MSA patients also were noted to have significant loss of nasal retinal nerve fiber layer thickness vs controls (P < .05), leading to decreased peripheral vision sensitivity.
Bottom Line
Although psychiatric symptoms are not part of the diagnostic criteria for multiple system atrophy (MSA), they may serve as a clue to consider when they occur with other MSA symptoms. Evaluate the importance of psychiatric symptoms in terms of the whole picture of the patient. Although the diagnosis might not alter the patient’s course, it can allow family members to understand the patient’s condition and prepare for complications that will arise.
Related Resources
• The MSA Coalition. www.multiplesystematrophy.org.
• National Institute of Neurological Disorders and Stroke. Multiple system atrophy fact sheet. www.ninds.nih.gov/disorders/msa/detailmsa.htm.
• Wenning GK, Fanciulli A, eds. Multiple system atrophy. Vienna, Austria: Springer-Verlag Wien; 2014.
Drug Brand Names
Bupropion • Wellbutrin Lithium • Eskalith, Lithobid
Carbamazepine • Tegretol Methylphenidate • Ritalin
Desvenlafaxine • Pristiq Paroxetine • Paxil
Donepezil • Aricept Travoprost • Travatan
Escitalopram • Lexapro Trazodone • Desyrel, Oleptro
Fludrocortisone • Florinef Topiramate • Topamax
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Light-headed
Mr. M, age 73, is a retired project manager who feels light-headed while walking his dog, causing him to go to the emergency department. His history is significant for hypertension, coronary artery disease (CAD), 3-vessel coronary artery bypass graft surgery (CABG), hyperlipidemia, erectile dysfunction, open-angle glaucoma, hemiretinal vein occlusion, symptoms suggesting rapid eye-movement behavior disorder (RBD), and major depressive disorder (MDD).
The psychiatry consultation-liaison service is asked to help manage Mr. M’s psychiatric medications in the context of orthostatic hypotension and cognitive deficits.
What could be causing Mr. M’s symptoms?
a) drug adverse effect
b) progressive cardiovascular disease
c) MDD
d) all of the above
HISTORY Depression, heart disease
15 years ago. Mr. M experienced his first major depressive episode. His primary care physician (PCP) commented on a history of falling asleep while driving and 1 episode of sleepwalking. His depression was treated to remission with fluoxetine and methylphenidate (dosages were not recorded), the latter also addressed his falling asleep while driving.
5 years ago. Mr. M had another depressive episode characterized by anxiety, difficulty sleeping, and irritability. He also described chest pain; a cardiac work-up revealed extensive CAD, which led to 3-vessel CABG later that year. He also reported dizziness upon standing, which was treated with compression stockings and an increase in sodium intake.
Mr. M continued to express feelings of depression. His cardiologist started him on paroxetine, 10 mg/d, which he took for 2 months and decided to stop because he felt better. He declined psychiatric referral.
4 years ago. Mr. M’s PCP referred him to a psychiatrist for depressed mood, anhedonia, decreased appetite, decreased energy, and difficulty concentrating. Immediate and delayed recall were found to be intact. The psychiatrist diagnosed MDD and Mr. M started escitalopram, 5 mg/d, titrated to 15 mg/d, and trazodone, 50 mg/d.
After starting treatment, Mr. M reported decreased libido. Sustained-release bupropion, 150 mg/d, was added to boost the effects of escitalopram and counteract sexual side effects.
At follow-up, Mr. M reported that his depressive symptoms and libido had improved, but that he had been experiencing unsteady gait when getting out of his car, which he had been noticing “for a while”—before he began trazodone. Mr. M was referred to his PCP, who attributed his symptoms to orthostasis. No treatment was indicated at the time because Mr. M’s lightheadedness had resolved.
3 years ago. Mr. M reported a syncopal attack and continued “dizziness.” His PCP prescribed fludrocortisone, 0.1 mg/d, later to be dosed 0.2 mg/d, and symptoms improved.
Although Mr. M had a history of orthostatic hypotension, he was later noted to have supine hypertension. Mr. M’s PCP was concerned that fludrocortisone could be causing the supine hypertension but that decreasing the dosage would cause his orthostatic hypotension to return.
The PCP also was concerned that the psychiatric medications (escitalopram, trazodone, and bupropion) could be causing orthostasis. There was discussion among Mr. M, his PCP, and his psychiatrist of stopping the psychotropics to see if the symptoms would remit; however, because of concerns about Mr. M’s depression, the medications were continued. Mr. M monitored his blood pressure at home and was referred to a neurologist for work-up of potential autonomic dysfunction.
Shortly afterward, Mr. M reported intermittent difficulty keeping track of his thoughts and finishing sentences. His psychiatrist ordered an MRI, which showed chronic small vessel ischemic changes, and started him on donepezil, 5 mg/d.
Neuropsychological testing revealed decreased processing speed and poor recognition memory; otherwise, results showed above-average intellectual ability and average or above-average performance in measures of language, attention, visuospatial/constructional functions, and executive functions—a pattern typically attributable to psychogenic factors, such as depression.
Mr. M reported to his neurologist that he forgets directions while driving but can focus better if he makes a conscious effort. Physical exam was significant hypotension; flat affect; deficits in concentration and short-term recall; mild impairment of Luria motor sequence (composed of a go/no-go and a reciprocal motor task); and vertical and horizontal saccades.1
Mr. M consulted with an ophthalmologist for anterior iridocyclitis and ocular hypertension, which was controlled with travoprost. He continued to experience trouble with his vision and was given a diagnosis of right inferior hemiretinal vein occlusion, macular edema, and suspected glaucoma. Subsequent notes recorded a history of Posner-Schlossman syndrome (a disease characterized by recurrent attacks of increased intraocular pressure in 1 eye with concomitant anterior chamber inflammation). His vision deteriorated until he was diagnosed with ocular hypertension, open-angle glaucoma, and dermatochalasis.
The authors’ observations
Involvement of multiple specialties in a patient’s care brings to question one’s philosophy on medical diagnosis. Interdisciplinary communication would seem to promote the principle of diagnostic parsimony, or Occam’s razor, which suggests a unifying diagnosis to explain all of the patient’s symptoms. Lack of communication might favor Hickam’s dictum, which states that “patients can have as many diseases as they damn well please.”
HISTORY Low energy, forgetfulness
2 years ago. Mr. M noticed low energy and motivation. He continued to work full-time but thought that it was taking him longer to get work done. He was tapered off escitalopram and started on desvenlafaxine, 50 mg/d; donepezil was increased to 10 mg/d.
The syncopal episodes resolved but blood pressure measured at home averaged 150/70 mm Hg. Mr. M was advised to decrease fludrocortisone from 0.2 mg/d to 0.1 mg/d. He tolerated the change and blood pressure measured at home dropped on average to 120 to 130/70 mm Hg.
1 year ago. Mr. M reported that his memory loss had become worse. He perceived having more stress because of forgetfulness and visual difficulties, which had led him to stop driving at night.
At a follow-up appointment with his psychiatrist, Mr. M reported that, first, he had not tapered escitalopram as discussed and, second, he forgot to increase the dosage of desvenlafaxine. A home blood pressure log revealed consistent hypotension; the psychiatrist was concerned that hypotension could be the cause of concentration difficulties and malaise. The psychiatrist advised Mr. M to follow-up with his PCP and neurologist.
Current admission. Shortly after the visit to the psychiatrist, Mr. M presented to the emergency department for increased syncopal events. Work-up was negative for a cardiac cause. A cosyntropin stimulation test was negative, showing that adrenal insufficiency did not cause his orthostatic hypotension. Chart review showed he had been having blood pressure problems for many years, independent of antidepressants. Physical exam revealed lower extremity ataxia and a bilateral extensor plantar reflex.
What diagnosis explains Mr. M’s symptoms?
a) Parkinson’s disease
b) multiple system atrophy (MSA)
c) depression due to a general medical condition
d) dementia
The authors’ observations
MSA, previously referred to as Shy-Drager syndrome, is a rare, rapidly progressive neurodegenerative disorder with an estimated prevalence of 3.7 cases for every 100,000 people worldwide.2 MSA primarily affects middle-aged patients; because it has no cure, most patients die in 7 to 10 years.3
MSA has 2 clinical variants4,5:
• parkinsonian type (MSA-P), characterized by striatonigral degeneration and increased spasticity
• cerebellar type (MSA-C), characterized by more autonomic dysfunction.
MSA has a range of symptoms, making it a challenging diagnosis (Table).6 Although psychiatric symptoms are not part of the diagnostic criteria, they can aid in its diagnosis. In Mr. M’s case, depression, anxiety, orthostatic hypotension, and ataxia support a diagnosis of MSA.
Gilman et al6 delineated 3 diagnostic categories for MSA: definite MSA, probable MSA, and possible MSA. Clinical criteria shared by the 3 diagnostic categories are sporadic and progressive onset after age 30.
Definite MSA requires “neuropathological findings of widespread and abundant CNS alpha-synuclein-positive glial cytoplasmic inclusions,” along with “neurodegenerative changes in striatonigral or olivopontocerebellar structures” at autopsy.6
Probable MSA. Without autopsy findings required for definite MSA, the next most specific diagnostic category is probable MSA. Probable MSA also specifies that the patient show either autonomic failure involving urinary incontinence—this includes erectile dysfunction in men—or, if autonomic failure is absent, orthostatic hypotension within 3 minutes of standing by at least 30 mm Hg systolic pressure or 15 mm Hg diastolic pressure.
Possible MSA has less stringent criteria for orthostatic hypotension. The category includes patients who have only 1 symptom that suggests autonomic failure. Criteria for possible MSA include parkinsonism or a cerebellar syndrome in addition to symptoms of MSA listed in the Table, whereas probable MSA has specific criteria of either a poorly levodopa-responsive parkinsonism (MSA-P) or a cerebellar syndrome (MSA-C). In addition to having parkinsonism or a cerebellar syndrome, and 1 sign of autonomic failure or orthostatic hypotension, patients also must have ≥1 additional feature to be assigned a diagnosis of possible MSA, including:
• rapidly progressive parkinsonism
• poor response to levodopa
• postural instability within 3 years of motor onset
• gait ataxia, cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction
• dysphagia within 5 years of motor onset
• atrophy on MRI of putamen, middle cerebellar peduncle, pons, or cerebellum
• hypometabolism on fluorodeoxyglucose- PET in putamen, brainstem, or cerebellum.6
Diagnosing MSA can be challenging because its features are similar to those of many other disorders. Nonetheless, Gilman et al6 lists specific criteria for probable MSA, including autonomic dysfunction, orthostatic hypotension, and either parkinsonism or cerebellar syndrome symptoms. Although a definite MSA diagnosis only can be made by postmortem brain specimen analysis, Osaki et al7 found that a probable MSA diagnosis has a positive predictive value of 92% with a sensitivity of 22% for definite MSA.
Mr. M’s symptoms were consistent with a diagnosis of probable MSA, cerebellar type (Figure).
Psychiatric manifestations of MSA
There are a few case reports of depression identified early in patients who were later given a diagnosis of MSA.8
Depression. In a study by Benrud-Larson et al9 (N = 99), 49% of patients who had MSA reported moderate or severe depression, as indicated by a score of ≥17 on the Beck Depression Inventory (BDI); 80% reported at least mild depression (BDI ≥10, mean 17.0, standard deviation, 8.7).
In a similar study, by Balas et al,10 depression was reported as a common symptom and was statistically significant in MSA-P patients compared with controls (P = .013).
Anxiety, another symptom that was reported by Mr. M, is another psychiatric manifestation described by Balas et al10 and Chang et al.11 Balas et al10 noted that MSA-C and MSA-P patients had significantly more state anxiety (P = .009 and P = .022, respectively) compared with controls, although Chang et al11 noted higher anxiety scores in MSA-C patients compared with controls and MSA-P patients (P < .01).
Balas et al10 hypothesized that anxiety and depression contribute to cognitive decline; their study showed that MSA-C patients had difficulty learning new verbal information (P < .022) and controlling attention (P < .023). Mr. M exhibited some of these cognitive difficulties in his reports of losing track of conversations, forgetting the topic of a conversation when speaking, trouble focusing, and difficulty concentrating when driving.
Mr. M had depression and anxiety well before onset of autonomic dysfunction (orthostatic hypotension and erectile dysfunction), which eventually led to an MSA diagnosis. Psychiatrists should understand additional manifestations of MSA so that they can use psychiatric symptoms to identify these conditions in their patients. One of the most well-known and early manifestations of MSA is autonomic dysfunction; among men, another early sign is erectile dysfunction.6 Our patient also exhibited other less well-known symptoms linked to MSA and autonomic dysregulation, including RBD and ocular symptoms (iridocyclitis, glaucoma, decreased visual acuity).
Rapid eye-movement behavior disorder. Psychiatrists should consider screening for RBD during assessment of sleep problems. Identifying RBD is important because early studies have shown a strong association between RBD and development of a neurodegenerative disorder. Mr. M’s clinicians did not consider RBD, although his symptoms of sleepwalking and falling asleep while driving suggest a possible diagnosis. Also, considering this diagnosis would aid in diagnosing a synucleinopathy disorder because a higher incidence of RBD was noted in patients who developed synucleinopathy disorders (eg, Parkinson’s disease [PD] and dementia with Lewy bodies [DLB]) compared with patients who developed non-synucleinopathies (eg, frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, mild cognitive impairment, primary progressive aphasia, and posterior cortical atrophy) or tauopathies (eg, Alzheimer’s disease).12
Zanigni et al13 reported similar findings in a later study that classified patients with RBD as having idiopathic RBD (IRBD) or RBD secondary to an underlying neurodegenerative disorder, particularly an α-synucleinopathy: PD, MSA, and DLB. Most IRBD patients developed 1 of the above mentioned neurodegenerative disorders as long as 10 years after a diagnosis of RBD.
In a study by Iranzo et al,14 patients with MSA were noted to have more severe RBD compared with PD patients. Severity is illustrated by greater periodic leg movements during sleep (P = .001), less total sleep time (P = .023), longer sleep onset latency (P = .023), and a higher percentage of REM sleep without atonia (RSWA, P = .001). McCarter et al15 also noted a higher incidence of RSWA in patients with MSA.
Patients with MSA might therefore be more likely to exhibit difficulty initiating and maintaining sleep and as having RSWA years before the MSA diagnosis.
Several psychotropics (eg, first-generation antipsychotics, tricyclic antidepressants, lithium, benzodiazepines, carbamazepine, topiramate, and selective serotonin reuptake inhibitors) can cause adverse ocular effects, such as closed-angle glaucoma in predisposed persons and retinopathy.16 Therefore, it is important for psychiatrists to ask about ocular symptoms because they might be an early sign of autonomic dysfunction.
Posner and Schlossman17 theorized a causal relationship between autonomic dysfunction and ocular diseases after studying a group of patients who had intermittent unilateral attacks of iridocyclitis and glaucoma (now known as Posner-Schlossman syndrome). They hypothesized that a central cause in the hypothalamus, combined with underlying autonomic dysregulation, could cause the intermittent attacks.
Gherghel et al18 noted a significant difference in ocular blood flow and blood pressure in patients with primary open-angle glaucoma (POAG) compared with controls. Patients with POAG did not show an increase in blood pressure or ocular blood flow when challenged by cold water, which should have increased their sympathetic activity. Gherghel et al18 concluded that this indicated possible systemic autonomic dysfunction in patients with POAG. In a study by Fischer et al,19 MSA patients also were noted to have significant loss of nasal retinal nerve fiber layer thickness vs controls (P < .05), leading to decreased peripheral vision sensitivity.
Bottom Line
Although psychiatric symptoms are not part of the diagnostic criteria for multiple system atrophy (MSA), they may serve as a clue to consider when they occur with other MSA symptoms. Evaluate the importance of psychiatric symptoms in terms of the whole picture of the patient. Although the diagnosis might not alter the patient’s course, it can allow family members to understand the patient’s condition and prepare for complications that will arise.
Related Resources
• The MSA Coalition. www.multiplesystematrophy.org.
• National Institute of Neurological Disorders and Stroke. Multiple system atrophy fact sheet. www.ninds.nih.gov/disorders/msa/detailmsa.htm.
• Wenning GK, Fanciulli A, eds. Multiple system atrophy. Vienna, Austria: Springer-Verlag Wien; 2014.
Drug Brand Names
Bupropion • Wellbutrin Lithium • Eskalith, Lithobid
Carbamazepine • Tegretol Methylphenidate • Ritalin
Desvenlafaxine • Pristiq Paroxetine • Paxil
Donepezil • Aricept Travoprost • Travatan
Escitalopram • Lexapro Trazodone • Desyrel, Oleptro
Fludrocortisone • Florinef Topiramate • Topamax
Fluoxetine • Prozac
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Hynan LS, Rossetti H, et al. Luria’s three-step test: what is it and what does it tell us? Int Psychogeriatr. 2011;23(10):1602-1606.
2. Orphanet Report Series. Prevalence of rare diseases: bibliographic data. http://www.orpha.net/orphacom/ cahiers/docs/GB/Prevalence_of_rare_diseases_by_ alphabetical_list.pdf. Published May 2014. Accessed May 27, 2015.
3. National Institute of Neurological Disorders and Stroke. Multiple system atrophy with orthostatic hypotension information page. http://www.ninds.nih.gov/disorders/ msa_orthostatic_hypotension/msa_orthostatic_ hypotension.htm?css=print. Updated December 5, 2013. Accessed May 27, 2015.
4. Flaherty AW, Rost NS. The Massachusetts Hospital handbook of neurology. 2nd ed. Lippincott Williams & Wilkins: Boston, MA; 2007:79.
5. Hemingway J, Franco K, Chmelik E. Shy-Drager syndrome: multisystem atrophy with comorbid depression. Psychosomatics. 2005;46(1):73-76.
6. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670-676.
7. Osaki Y, Wenning GK, Daniel SE, et al. Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology. 2002;59(10):1486-1491.
8. Goto K, Ueki A, Shimode H, et al. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci. 2000;54(4):507-511.
9. Benrud-Larson LM, Sandroni P, Schrag A, et al. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord. 2005;20(8):951-957.
10. Balas M, Balash Y, Giladi N, et al. Cognition in multiple system atrophy: neuropsychological profile and interaction with mood. J Neural Transm. 2010;117(3):369-375.
11. Chang CC, Chang YY, Chang WN, et al. Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur J Neurol. 2009;16(10):1144-1150.
12. Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61(1):40-45.
13. Zanigni S, Calandra-Buonaura G, Grimaldi D, et al. REM behaviour disorder and neurodegenerative diseases. Sleep Med. 2011;12(suppl 2):S54-S58.
14. Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65(2):247-252.
15. McCarter SJ, St. Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12(2):182-192.
16. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501-526.
17. Posner A, Schlossman A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthal. 1948;39(4):517-535.
18. Gherghel D, Hosking SL, Cunliffe IA. Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci. 2004;45(10):3546-3554.
19. Fischer MD, Synofzik M, Kernstock C, et al. Decreased retinal sensitivity and loss of retinal nerve fibers in multiple system atrophy. Graefes Arch Clin Exp Opthalmol. 2013;251(1):235-241.
1. Weiner MF, Hynan LS, Rossetti H, et al. Luria’s three-step test: what is it and what does it tell us? Int Psychogeriatr. 2011;23(10):1602-1606.
2. Orphanet Report Series. Prevalence of rare diseases: bibliographic data. http://www.orpha.net/orphacom/ cahiers/docs/GB/Prevalence_of_rare_diseases_by_ alphabetical_list.pdf. Published May 2014. Accessed May 27, 2015.
3. National Institute of Neurological Disorders and Stroke. Multiple system atrophy with orthostatic hypotension information page. http://www.ninds.nih.gov/disorders/ msa_orthostatic_hypotension/msa_orthostatic_ hypotension.htm?css=print. Updated December 5, 2013. Accessed May 27, 2015.
4. Flaherty AW, Rost NS. The Massachusetts Hospital handbook of neurology. 2nd ed. Lippincott Williams & Wilkins: Boston, MA; 2007:79.
5. Hemingway J, Franco K, Chmelik E. Shy-Drager syndrome: multisystem atrophy with comorbid depression. Psychosomatics. 2005;46(1):73-76.
6. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670-676.
7. Osaki Y, Wenning GK, Daniel SE, et al. Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology. 2002;59(10):1486-1491.
8. Goto K, Ueki A, Shimode H, et al. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci. 2000;54(4):507-511.
9. Benrud-Larson LM, Sandroni P, Schrag A, et al. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord. 2005;20(8):951-957.
10. Balas M, Balash Y, Giladi N, et al. Cognition in multiple system atrophy: neuropsychological profile and interaction with mood. J Neural Transm. 2010;117(3):369-375.
11. Chang CC, Chang YY, Chang WN, et al. Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur J Neurol. 2009;16(10):1144-1150.
12. Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61(1):40-45.
13. Zanigni S, Calandra-Buonaura G, Grimaldi D, et al. REM behaviour disorder and neurodegenerative diseases. Sleep Med. 2011;12(suppl 2):S54-S58.
14. Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65(2):247-252.
15. McCarter SJ, St. Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12(2):182-192.
16. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501-526.
17. Posner A, Schlossman A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthal. 1948;39(4):517-535.
18. Gherghel D, Hosking SL, Cunliffe IA. Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci. 2004;45(10):3546-3554.
19. Fischer MD, Synofzik M, Kernstock C, et al. Decreased retinal sensitivity and loss of retinal nerve fibers in multiple system atrophy. Graefes Arch Clin Exp Opthalmol. 2013;251(1):235-241.
A physician who feels hopeless and worthless and complains of pain
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
Sober today, but lethargic and confused
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
Depressed and sick with ‘nothing to live for’
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE ‘I’ve had enough’
The psychiatry consultation team is asked to evaluate Mr. M, age 76, for a passive death wish and depression 2 months after he was admitted to the hospital after a traumatic fall.
Mr. M has several chronic medical conditions, including hypertension, type 2 diabetes mellitus, and coronary artery disease. Within 2 weeks of his admission, he developed Proteus mirabilis pneumonia and persistent respiratory failure requiring tracheostomy. Records indicate that Mr. M has told family and his treatment team, “I’m tired, just let me go.” He then developed antibiotic-induced Clostridium difficile colitis and acute renal failure requiring temporary renal replacement therapy (RRT).
Mr. M’s clinical status improves, allowing his transfer to a transitional unit, where he continues to state, “I have had enough. I’m done.” He asks for the tracheostomy tube to be removed and RRT discontinued. He is treated again for persistent C. difficile colitis and, within 2 weeks, develops hypotension, hypoxia, emesis, and abdominal distension, requiring transfer to the ICU for management of ileus.
He is stabilized with vasopressors and artificial nutritional support by nasogastric tube. Renal function improves, RRT is discontinued, and he is transferred to the general medical floor.
After a few days on the general medical floor, Mr. M develops a urinary tract infection and develops antibiotic-induced acute renal failure requiring re-initiation of RRT. A percutaneous endoscopic gastrostomy (PEG) tube is placed for nutrition when he shows little improvement with swallowing exercises. Two days after placing the PEG tube, he develops respiratory failure secondary to a left-sided pneumothorax and is transferred to the ICU for the third time, where he undergoes repeated bronchoscopies and requires pressure support ventilation.
One week later, Mr. M is weaned off the ventilator and transferred to the general medical floor with aggressive respiratory therapy, tube feeding, and RRT. Mr. M’s chart indicates that he expresses an ongoing desire to withdraw RRT, the tracheostomy, and feeding tube.
Which of the following would you consider when assessing Mr. M’s decision-making capacity (DMC)?
a) his ability to understand information relevant to treatment decision-making
b) his ability to appreciate the significance of his diagnoses and treatment options and consequences in the context of his own life circumstances
c) his ability to communicate a preference
d) his ability to reason through the relevant information to weigh the potential costs and benefits of treatment options
e) all of the above
HISTORY Guilt and regret
Mr. M reports a 30-year history of depression that has responded poorly to a variety of medications, outpatient psychotherapy, and electroconvulsive therapy. Before admission, he says, he was adherent to citalopram, 20 mg/d, and buspirone, 30 mg/d. Citalopram is continued throughout his hospitalization, although buspirone was discontinued for unknown reasons during admission.
Mr. M is undergoing hemodialysis during his initial encounter with the psychiatry team. He struggles to communicate clearly because of the tracheostomy but is alert, oriented to person and location, answers questions appropriately, maintains good eye contact, and does not demonstrate any psychomotor abnormalities. He describes his disposition as “tired,” and is on the verge of tears during the interview.
Mr. M denies physical discomfort and states, “I have just had enough. I do not want all of this done.” He clarifies that he is not suicidal and denies a history of suicidal or self-injurious behaviors.
Mr. M describes having low mood, anhedonia, and insomnia to varying degrees throughout his adult life. He also reports feeling guilt and regret about earlier experiences, but does not elaborate. He denies symptoms of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, mania, or hypomania. He reports an episode of visual hallucinations during an earlier hospitalization, likely a symptom of delirium, but denies any recent visual disturbances.
Mr. M’s thought process is linear and logical, with intact abstract reasoning and no evidence of delusions. Attention and concentration are intact for most of the interview but diminish as he becomes fatigued. Mr. M can describe past treatments in detail and recounts the events leading to this hospitalization.
The authors’ observations
Literature on assessment of DMC recently has centered on the 4-ability model, proposed by Grisso and Appelbaum.1 With this approach, impairment to any of the 4 processes of understanding, appreciation, ability to express a choice, and ability to use reasoning to weigh treatment options could interfere with capacity to make decisions. Few studies have clarified the mechanism and degree to which depression may impair these 4 elements, making capacity assessments in a depressed patient challenging.
Preliminary evidence suggests that depression severity, not the presence of depression, determines the degree to which DMC is impaired, if at all. In several studies, depressed patients did not demonstrate more impaired DMC compared with non-depressed patients based on standardized assessments.2-4 In depressed patients who lack DMC, case reports5-7 and cross-sectional studies8 indicate that appreciation—one’s ability to comprehend the personal relevance of illness and potential consequences of treatments in the context of one’s life—is most often impaired. Other studies suggest that the ability to reason through decision-specific information and weigh the risks and benefits of treatment options is commonly impaired in depressed patients.9,10
Even when a depressed patient demonstrates the 4 elements of DMC, providers might be concerned that the patient’s preferences are skewed by the negative emotions associated with depression.11-13 In such a case, the patient’s expressed wishes might not be consistent with views and priorities that were expressed during an earlier, euthymic period.
Rather than focusing on whether cognitive elements of DMC are impaired, some experts advocate for assessing how depression might lead to “unbalanced” decision-making that is impaired by a patient’s tendency to undervalue positive outcomes and overvalue negative ones.14 Some depressed patients will decide to forego additional medical interventions because they do not see the potential benefits of treatment, view events through a negative lens, and lack hope for the future; however, studies indicate this is not typically the case.15-17
In a study of >2,500 patients age >65 with chronic medical conditions, Garrett et al15 found that those who were depressed communicated a desire for more treatment compared with non-depressed patients. Another study of patients’ wishes for life-sustaining treatment among those who had mild or moderate depression found that most patients did not express a greater desire for life-sustaining medical interventions after their depressive episode remitted. An increased desire for life-sustaining medical interventions occurred only among the most severely depressed patients.16 Similarly, Lee and Ganzini17 found that treatment preferences among patients with mild or moderate depression and serious physical illness were unchanged after the mood disorder was treated.
These findings demonstrate that a clinician charged with assessing DMC must evaluate the severity of a patient’s depression and carefully consider how mood is influencing his (her) perspective and cognitive abilities. It is important to observe how the depressed patient perceives feelings of sadness or hopelessness in the context of decision-making, and how he (she) integrates these feelings when assigning relative value to potential outcomes and alternative treatment options. Because the intensity of depression could vary over time, assessment of the depressed patient’s decision-making abilities must be viewed as a dynamic process.
Clinical application
Recent studies indicate that, although the in-hospital mortality rate for critically ill patients who develop acute renal failure is high, it is variable, ranging from 28% to 90%.18 In one study, patients who required more interventions over the course of a hospital stay (eg, mechanical ventilation, vasopressors) had an in-hospital mortality rate closer to 60% after initiating RRT.19 In a similar trial,20,21 mean survival for critically ill patients with acute renal failure was 32 days from initiation of dialysis; only 27% of these patients were alive 6 months later.21
Given his complicated hospital course, the medical team estimates that Mr. M has a reasonable chance of surviving to discharge, although his longer-term prognosis is poor.
EVALUATION Conflicting preferences
Mr. M expresses reasonable understanding of the medical indications for temporary RRT, respiratory therapy, and enteral tube feedings, and the consequences of withdrawing these interventions. He understands that the primary team recommended ongoing but temporary use of life-sustaining interventions, anticipating that he would recover from his acute medical conditions. Mr. M clearly articulates that he wants to terminate RRT knowing that this would cause a buildup of urea and other toxins, to resume eating by mouth despite the risk of aspiration, and to be allowed to die “naturally.”
Mr. M declines to speak with a clergy member, explaining that he preferred direct contact with God and had reconciled himself to the “consequences” of his actions. He reports having “nothing left to live for” and “nothing left to do.” He says that he is “tired of being a burden” to his wife and son, regrets the way he treated them in the past, and believes they would be better off without him.
Although Mr. M’s abilities to understand, reason, and express a preference are intact, the psychiatry team is concerned that depression could be influencing his perspective, thereby compromising his appreciation for the personal relevance of his request to withdraw life-sustaining treatments. The psychiatrist shares this concern with Mr. M, who voices an understanding that undertreated depression could lead him to make irreversible decisions about his medical treatment that he might not make if he were not depressed; nevertheless, he continues to state that he is “ready” to die. With his permission, the team seeks additional information from Mr. M’s family.
Mr. M’s wife recalls a conversation with her husband 5 years ago in which he said that, were he to become seriously ill, “he would want everything done.” However, she also reports that Mr. M has been expressing a passive death wish “for years,” as he was struggling with chronic medical conditions that led to recurrent hospital admissions.
“He has always been a negative person,” she adds, and confirms that he has been depressed for most of their marriage.
The conflict between Mr. M’s earlier expressed preference for full care and his current wish to withdraw life-sustaining therapies and experience a “natural death” raises significant concern that depression could explain this change in perspective. When asked about this discrepancy, Mr. M admits that he “wanted everything done” in the past, when he was younger and healthier, but his preferences changed as his chronic medical problems progressed.
OUTCOME Better mood, discharge
We encourage Mr. M to continue discussing his treatment preferences with his family, while meeting with the palliative care team to address medical conditions that could be exacerbating depression and to clarify his goals of care. The medical team and Mr. M report feeling relieved when a palliative care consult is suggested, although his wife and son ask that it be delayed until Mr. M is more medically stable. The treatment team acknowledges the competing risks of proceeding too hastily with Mr. M’s request to withdraw life-sustaining treatments because of depression, and of delaying his decision, which could prolong suffering and violate his right to refuse medical treatment.
Mr. M agrees to increase citalopram to 40 mg/d to target depressive symptoms. We monitor Mr. M for treatment response and side effects, to provide ongoing support, to facilitate communication with the medical team, and to evaluate the influence of depression on treatment preferences and decision-making.
As Mr. M is stabilized over the next 3 weeks, he begins to reply, “I’m alive,” when asked about passive death wish. His renal function improves and RRT is discontinued. Mr. M reports a slight improvement in his mood and is discharged to a skilled nursing facility, with plans for closing his tracheostomy.
The authors’ observations
Capacity assessments can be challenging in depressed patients, often because of the uncertain role of features such as hopelessness, anhedonia, and passive death wish in the decision-making process. Depressed patients do not automatically lack DMC, and existing studies suggest that decisions regarding life-saving interventions typically are stable across time. The 4-ability model for capacity assessment is a useful starting point, but additional considerations are warranted in depressed patients with chronic illness (Figure). There is no evidence to date to guide these assessments in chronically depressed or dysthymic patients; therefore additional safeguards may be needed (Table).
In Mr. M’s case, the team’s decision to optimize depression treatment while continuing unwanted life-sustaining therapies led to improved mood and a positive health outcome. In some cases, patients do not respond quickly, if at all, to depression treatment. Also, what constitutes a reasonable attempt to treat depression, or an appropriate delay in decision-making related to life-sustaining therapies, is debatable.
When positive outcomes are not achieved or ethical dilemmas arise, health care providers could experience high moral distress.21 In Mr. M’s case, the consultation team felt moral distress because of the delayed involvement of palliative care, especially because this decision was driven by the family rather than the patient.
Related Resources
• Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
• American Academy of Hospice and Palliative Medicine. www. aahpm.org.
Drug Brand Names
Buspirone • Buspar Citalopram • Celexa
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
1. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen BJ, McGarvey El, Pinkerton RC, et al. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32(2):134-143.
3. Lapid MI, Rummans TA, Poole KL, et al. Decisional capacity of severely depressed patients requiring electroconvulsive therapy. J ECT. 2003;19(2):67-72.
4. Appelbaum PS, Grisso T, Frank E, et al. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384.
5. Leeman CP. Depression and the right to die. Gen Hosp Psychiatry. 1999;21(2):112-115.
6. Young EW, Corby JC, Johnson R. Does depression invalidate competence? Consultants’ ethical, psychiatric, and legal considerations. Camb Q Healthc Ethics. 1993;2(4):505-515.
7. Halpern J. When concretized emotion-belief complexes derail decision-making capacity. Bioethics. 2012;26(2):108-116.
8. Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19(2):149-174.
9. Bean G, Nishisato S, Rector NA, et al. The assessment of competence to make a treatment decision: an empirical approach. Can J Psychiatry. 1996;41(2):85-92.
10. Vollmann J, Bauer A, Danker-Hopfe H, et al. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463-1471.
11. Sullivan MD, Youngner SJ. Depression, competence, and the right to refuse lifesaving medical-treatment. Am J Psychiatry. 1994;151(7):971-978.
12. Meynen G. Depression, possibilities, and competence: a phenomenological perspective. Theor Med Bioeth. 2011;32(3):181-193.
13. Elliott C. Caring about risks. Are severely depressed patients competent to consent to research? Arch Gen Psychiatry. 1997;54(2):113-116.
14. Bursztajn HJ, Harding HP Jr, Gutheil TG, et al. Beyond cognition: the role of disordered affective states in impairing competence to consent to treatment. Bull Am Acad Psychiatry Law. 1991;19(4):383-388.
15. Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361-368.
16. Ganzini L, Lee MA, Heintz RT, et al. The effect of depression treatment on elderly patients’ p for life-sustaining medical therapy. Am J Psychiatry. 1994;151(11):1631-1636.
17. Lee M, Ganzini L. The effect of recovery from depression on p for life-sustaining therapy in older patients. J Gerontol. 1994;49(1):M15-M21.
18. Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2003;30(9):2051-2058.
19. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
20. The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and p for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
21. Kälvemark S, Höglund AT, Hansson MG, et al. Living the conflicts-ethical dilemmas and moral distress in the health care system. Soc Sci Med. 2004;58(6):1075-1084.
A teen with seizures, amnesia, and troubled family dynamics
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Seizures, amnesia
Ms. A, age 13, who has a history of seizures, presents to the emergency department (ED) with sudden onset of memory loss. Her family reports that she had been spending a normal evening at home with family and friends. After going to the bathroom, Ms. A became acutely confused and extremely upset, had slurred speech, and did not recognize anyone in the room except her mother.
Initial neurologic examination in the ED reports that Ms. A does not remember recent or remote past events. Her family denies any recent stressors.
Vital signs are within normal range. She has mild muscle soreness and gait instability, which is attributed to a presumed postictal phase. Her medication regimen includes: levetiracetam, 500 mg, 3 times a day; valproic acid, 1,000 mg/d; and oxcarbazepine, 2,400 mg/d, for seizure management.
Complete blood count and comprehensive metabolic panel are within normal limits. Pregnancy test is negative. Urine toxicology report is negative. Serum valproic acid level is 71 μg/mL; oxcarbazepine level, <2 μg/mL; ammonia level, 71 μg/dL (reference range, 15 to 45 μg/dL). Other than the aforementioned deficits, she is neurologically intact. The team thinks that her symptoms are part of a postictal phase of an unwitnessed seizure.
Ms. A is admitted to the inpatient medical unit for further work up. Along with the memory loss and seizures, she reports visual hallucinations.
What could be causing Ms. A’s amnesia?
a) a seizure disorder
b) malingering
c) posttraumatic stress disorder
d) traumatic brain injury
HISTORY Repeat ED visits
Ms. A’s mother reports that 3 years ago her daughter was treated for tics with quetiapine and aripiprazole, prescribed by a primary care physician. She received a short course of counseling 6 years ago after her sister was sexually abused by her grandfather. Approximately 6 months ago, Ms. A engaged in self-injurious behavior by cutting herself, and she briefly received counseling. There is no history of suicide attempts, psychiatric hospitalization, or a psychiatric diagnosis.
Medical and surgical history include viral meningitis at age 6 months. Medical records show a visit to the ED for abdominal pain after a classmate punched her in the abdomen, which resolved with supportive care. She was given a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections 6 years ago.
Ms. A developed multiple recurrent methicillin-resistant Staphylococcus aureus abscesses a year ago, which lasted for 4 months; it was noted that she was self-inoculating by scratching eczema. She had a possible syncopal episode 5 months ago, but the medical work-up was normal. The pediatric neurology service diagnosed and treated seizures 4 months ago.
Levetiracetam was prescribed after a possible syncopal episode followed by a tonic-clonic seizure. Because she was still having seizure-like episodes with a single antiepileptic drug (AED), oxcarbazepine, then valproic acid were added. Whether her seizures were generalized or partial was inconclusive. The seizures were followed by a postictal phase lasting 3 minutes to 1 hour. Her last generalized tonic-clonic seizure was 1 month before admission.
Ms. A had 3 MRI studies of the brain over the past 3 years, which showed consistent and unchanged multifocal punctate white matter lesions. The findings represented gliosis from an old perivascular inflammation, trauma, or ischemic damage. There is no history of traumatic brain injury.
Her perinatal history is unremarkable, with normal vaginal delivery at 36 weeks (pre-term birth). All developmental milestones were on target.
Ms. A lives at home with her mother, 6-year-old brother, and stepfather. Her parents are divorced, but her biological father has been involved in her upbringing. She is in seventh grade, but is home schooled after she withdrew from school because of multiple seizure episodes. Ms. A denied bullying at school although she had been punched by a peer. It was unclear if it was a single incident or bullying continued and she was hesitant to disclose it.
The authors’ observations
We focus on the amnesia because it has an acute onset and it seems this is the first time Ms. A presented with this symptom. There is no need to wait for neurology consultation, even though organic causes of amnesia need to be ruled out. Our plan is to develop rapport with Ms. A, and then administer a mental status examination focusing on memory assessment. We understand that, because Ms. A’s chief concern is amnesia, she might not be able to provide many details. We start the initial interview with the family in the patient’s room to understand family dynamics, and then interview Ms. A alone.
EVALUATION Memory problems
On initial psychiatric interview, Ms. A can recognize some of her family members. She is seen in clean attire, with short hair, lying in the bed with good eye contact and a calm demeanor. She seems to be difficult to engage because of her reserved nature.
Ms. A displays some psychomotor retardation. She reports her mood as tired, and her affect is flat and mood incongruent. She is alert and oriented to person only; not to place, time, or situation. She can do a simple spelling task, perform 5-minute recall of 3 words, complete serial 3 subtractions, repeat phrases, read aloud, focus on a coin task, and name simple objects. She does not compare similar objects or answer simple historical or factual questions.
Ms. A replies “I don’t know” to most historical questions, such as her birthday, favorite color, and family members; she does not answer when asked how many legs a dog has, who is the current or past president, what month the Fourth of July is in, or when Christmas is. She can complete some memory tasks on the Mini-Mental State Examination, but does not attempt many others. Ms. A says she is upset about her memory deficit, but her affect was flat. Her mood and her affect were incongruent. She describes a vision of a “girl with black holes [for eyes]” in the corner of her hospital room telling her not to believe anyone and that the interviewers are lying to her. Also, she reports that “the girl” tells her to hurt herself and others, but she is not going to act on the commands because she knows it is not the right thing to do. When we ask Ms. A about a history of substance abuse, she says she has never heard of drugs or alcohol.
Overall, she displays multiple apparent deficits in declarative memory, both episodic and semantic. Regarding non-declarative or procedural memory, she can dress herself, use the bathroom independently, order meals off the menu, and feed herself, among other routine tasks, without difficulty.
According to Ms. A’s mother, Ms. A has shown a decline in overall functioning and personality changes during the past 5 months. She started to cut herself superficially on her forearms 6 months ago and also tried to change her appearance with a new hairstyle when school started. She displayed noticeably intense and disturbing writings, artwork, and conversations with others over 3 to 4 months.
She started experiencing seizures, with 3 to 4 seizures a day; however, she could attend sleepovers seizure-free. She had prolonged periods of seizures lasting up to an hour, much longer than would be expected clinically. She also had requested to go to the cemetery for unclear reasons (because the spirit wanted her to visit), and was observed mumbling under her breath.
Six years ago, Ms. A’s 6-year-old sister tried to suffocate her infant brother. Child protective services was involved and the sister was hospitalized in a psychiatric facility, where she was given a diagnosis of bipolar disorder; she was then transferred to foster care, and later placed in residential treatment. Her mother relinquished her parental rights and gave custody of Ms. A’s sister to the state.
Ms. A’s mother has a history of depression, but her younger brother is healthy. There is no history of autism, attention problems, tics, substance abuse, brain tumor, or intellectual disabilities in the family.
Which diagnosis does Ms. A’s presentation and history suggest?
a) dissociative amnesia
b) factitious disorder imposed on self
c) conversion disorder (neurological symptom disorder)
d) psychosis not otherwise specified
e) malingering
The authors’ observations
The history of unwitnessed seizures, sudden onset of visual hallucinations, and transient amnesia points to a possible postictal cause. Selective amnesia brings up the question of whether psychological components are driving the symptoms.
Her psychotic symptoms appear to be mediated by anxiety and possibly related to the trauma of losing her only sister when her mother relinquished custody to the state; the circumstances might have aroused feelings of insecurity or fear of abandonment and raised questions about her mother’s love toward her. Her sister’s abuse by a family member might have created reticence to trust others. These background experiences could be intensely conflicting at this age when the second separation individuation process commences, especially in an emotionally immature adolescent.
OUTCOME Medication change
The neurology team recommends discontinuing levetiracetam because the visual hallucinations, mood disturbance, and personality change could be adverse effects of the drug. Because of generalized uncontrolled body movements with staring episodes and unresponsiveness, an EEG is ordered to rule out ongoing seizures.
Ms. A recognizes the psychosomatic medicine team members when they interview her again. The team employs consistent reassurance and a non-confrontational approach. She spends 3 days in the medical unit during which she reports that the frequency of visual and auditory hallucinations decreases and her memory symptoms resolve. Her 24-hour EEG is negative for seizure activity, and the 24-hour video EEG does not show any signs of epileptogenic foci. Ms. A’s family declines inpatient psychiatric hospitalization.
Because of gradual improvement in Ms. A’s symptoms and no imminent safety concerns, she is discharged home with valproic acid, 1,000 mg/d, and oxcarbazepine, 1,200 mg/d, and follow-up appointments with her primary care physician, a neurologist, and a psychiatrist.
The authors’ observations
Dissociative amnesia
Generalized dissociative amnesia is difficult to differentiate from factitious disorder or malingering. According to DSM-5, there is loss of episodic memory in dissociative amnesia, in which the person is unable to recall the stressful event after trauma (Table 1).1 Although there have been case reports of dissociative amnesia with loss of semantic and procedural memory, episodic memory is the last to return.2 In Ms. A’s case, there was no immediate basis to explain amnesia onset, although she had experienced the trauma of losing her sister. She had episodic and mostly semantic memory loss.
Although organic causes can precipitate amnesia,3 Ms. A’s EEG and MRI results did not reflect that. Patients with a dissociative disorder often report some physical, sexual, or emotional abuse.4 Although Ms. A did not report any abuse, it cannot be completely ruled out because of her sister’s history of abuse.
Suicidality or self-injurious behavior is common among adults with dissociative amnesia, although it is not well studied in children.4,5 Generally, the constellation of primary dissociative symptoms that patients develop are forgetfulness, fragmentation, and emotional numbing. Ms. A presented with some of these features; did she, in fact, have dissociative amnesia?
Factitious amnesia
Factious amnesia (Table 2)6 is a symptom of factious disorder in which amnesia appears with the motivation to assume a sick role.3 Ms. A’s amnesia garnered significant attention from her mother and other family members; this may have been related to insecurity in her family relationships because her sister was given up to the state. She also could be afraid of entering adolescence and leaving her sister behind. Did she want more time to bond with her mother? Did she experience emotional benefit from being cared for by medical professionals?7 Her affect during interviews was blunted and her attitude was nonchalant, and her multiple visits to the hospital since childhood for abdominal pain, abscesses (it isn’t clear whether the abscesses were related to self-injury and scratching), tics, seizures, and, recently, amnesia and hallucinations indicated some desire to occupy a sick role. Furthermore, the severity of her symptoms seemed to be increasing over time, from somatic to neurologic (seizure-like episodes) to significant and less frequent psychiatric symptoms (amnesia and hallucinations). One could speculate that her symptoms were escalating because she was not receiving the attention she needed.
Malingered amnesia
Although malingering is not a psychiatric diagnosis, it can be a focus of clinical attention. It is challenging to identify malingered cognitive impairments.8 Children often have difficulty malingering symptoms because they have limited understanding of the illness they are trying to simulate.9 Many malingerers do not want to participate in their medical work up and might exhibit a hostile attitude toward examiners (Table 26). Clinicians could rely on family to provide information regarding history and inconsistencies in clinical deficits.9 The clinical interview, mental status examination, and collateral information are crucial for identifying malingering.
Most of Ms. A’s seizure-like episodes happened in specific contexts, such as in school, but not at friends’ houses, raising the question of whether she is aware of her episodes. Ms. A’s grades are consistently good; because she is being home schooled, there is no secondary gain from not going to school. There is no other reason to speculate that she was malingering.
The inconsistency of Ms. A’s symptoms and her compliance with assessment and treatment did not reflect malingering. Interestingly, Ms. A’s amnesia was retrograde in nature. There have been more studies on malingered anterograde amnesia8 than on retrograde amnesia, making her presentation even more unusual.
Amnesia presenting as conversion disorder
Amnesia as a symptom of conversion disorder is referred as psychogenic amnesia; the memory loss mostly is isolated retrograde amnesia.10 Ms. A likely had unconsciously produced symptoms of non-epileptic seizures, followed by auditory and visual hallucinations not related to her seizures, and then later developed selective transient amnesia. Conversion disorder seemed to be the diagnosis most consistent with her indifference (“la belle indifference”) and the significant attention she gained from the acute memory loss (Table 3).1 It seemed that she developed multiple symptoms in progression leading toward a conversion disorder diagnosis. The question arises whether Ms. A’s presentation is a gradually increasing cry for help or reflects depressive or anxiety symptoms, which often are comorbid with conversion disorder.
FOLLOW-UP Suicide attempt
Ms. A has frequent visits to the ED with symptoms of syncope and seizures and undergoes medical work-up and multiple EEGs. A prolonged 5-day video EEG is performed to assess seizure episodes after AEDs were withdrawn, but no seizure activity is elicited. She also has an ED visit for recurrent tic emergence.
The last visit in the ED is for a suicide attempt with overdose of an unknown quantity of unspecified pills. Ms. A talks to a social worker, who reports that Ms. A needed answers to such questions as why her grandfather abused her sister? Could she have stopped them and made a difference for the family?
The authors’ observations
Conversion disorder arises from unconscious psychological conflicts, needs, or responses to trauma. Ms. A’s consistent conflict about her sister and grandfather’s relationship was evident from occasions when she tried to confide in hospital staff. During an ED visit, she reported her sister’s abuse to a staff member. Another time, while recovering from sedation, she spontaneously spoke about her sister’s abuse. When asked again, she said she did not remember saying it.
Freud said that patients develop conversion disorder to avoid unacceptable conflicting thoughts and feelings.10 It appeared that Ms. A was struggling with these questions because she brought them up again when she visited the ED after the suicide attempt.
Dissociative symptoms arise from unstable parenting and disciplining styles with variable family dynamics. Patients show extreme detachment and emotional unresponsiveness akin to attachment disorder.11 Ms. A had inconsistent parenting because both her stepfather and biological father were involved with her care. Her mother had relinquished her parental rights to her sister, which indicated some attachment issues.
Ms. A’s idea that her mother was indifferent stemmed from her uncaring approach toward her sister and not able to understand her emotionally. Her amnesia could be thought of as “I don’t know you because I don’t remember that I am related to you.” The traumas of infancy (referred to as hidden traumas) that were a result of parent-child mismatch of needs and availability at times of distress might not be obvious to the examiner.11
Although Ms. A’s infancy was reported to be unremarkable, there always is a question, especially in a consultation-liaison setting, of whether conversion disorder might be masking an attachment problem. Perhaps with long-term psychotherapy, an attachment issue would be revealed.
Excluding an organic cause or a neurologic disorder is important when diagnosing conversion disorder10; Ms. A’s negative neurologic tests favored a diagnosis of amnesia due to conversion disorder. It appears that, although Ms. A presented with “transient amnesia,” she had underlying psychiatric symptoms, likely depression or anxiety. We were concerned about possible psychiatric comorbidity and recommended inpatient hospitalization to clarify the diagnosis and provide intensive therapy, but her family declined. She may have received outpatient services, but that was not documented.
Bottom Line
Psychogenic amnesia can be a form of conversion disorder or a symptom of
malingering; can occur in dissociative disorder; and can be factitious in nature.
Regardless of the cause, the condition requires continuous close follow up. Although organic causes of amnesia should be ruled out, mental health care can help address comorbid psychiatric symptoms and might change the course of the illness.
Related Resources
• Byatt N, Toor R. Young, pregnant, ataxic—and jilted. Current Psychiatry. 2015;14(1):44-49.
• Leipsic J. A teen who is wasting away. Current Psychiatry. 2013;12(6):40-45.
Drug Brand Names
Aripiprazole • Abilify Quetiapine • Seroquel
Levetiracetam • Keppra Valproic acid • Depakote
Oxcarbazepine • Trileptal
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. van der Hart O, Nijenhuis E. Generalized dissociative amnesia: episodic, semantic and procedural memories lost and found. Aust N Z J Psychiatry. 2001;35(5):589-560.
3. Ehrlich S, Pfeiffer E, Salbach H, et al. Factitious disorder in children and adolescents: a retrospective study. Psychosomatics. 2008;45(5):392-398.
4. Sar V, Akyüz G, Kundakçi T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
5. Kisiel CL, Lyons JS. Dissociation as a mediator of psychopathology among sexually abused children and adolescents. Am J Psychiatry. 2001;158(7):1034-1039.
6. Worley CB, Feldman MD, Hamilton JC. The case of factitious disorder versus malingering. http://www.psychiatrictimes. com/munchausen-syndrome/case-factitious-disorder-versus-malingering. Published October 30, 2009. Accessed January 27, 2015.
7. Hagglund LA. Challenges in the treatment of factitious disorder: a case study. Arch Psychiatr Nurs. 2009;23(1):58-64.
8. Jenkins KG, Kapur N, Kopelman MD. Retrograde amnesia and malingering. Curr Opin Neurol. 2009;22(6):601-605.
9. Walker JS. Malingering in children: fibs and faking. Child Adolesc Psychiatr Clin N Am. 2011;20(3):547-556.
10. Levenson JL. Psychiatric issues in neurology, part 4: amnestic syndromes and conversion disorder. Primary Psychiatry. http://primarypsychiatry.com/psychiatric-issues-in-neurology-part-4-amnestic-syndromes-and-conversion-disorder. Published March 1, 2008. Accessed February 3, 2015.
11. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
Delusional and aggressive, while playing the lottery
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
Young, pregnant, ataxic—and jilted
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
He’s been making new ‘friends’
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.