User login
Sober today, but lethargic and confused
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
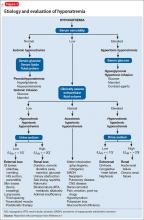
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
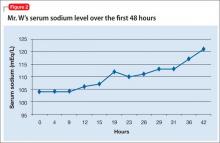
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Confused and weak
Mr. W, age 26, is brought to the emergency department (ED) by his parents for intermittent confusion, weakness, and increasing lethargy over the past 4 days. He is jaundiced with mild abdominal pain, nausea, and vomiting.
Mr. W has a history of alcohol use disorder, drinking as much as 1 L of vodka a day. Six months ago, he was hospitalized for alcoholic hepatitis and severe hyponatremia.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse 89 beats per minute; blood pressure, 117/50 mm Hg; respirations, 15 breaths per minute; and temperature, 98.5ºF. Physical examination is notable for scleral icterus, jaundice, tender hepatomegaly, and asterixis.
Mr. W is not taking any medications. He reports that his most recent drink was the day before; however, his current alcohol intake is unknown.
Laboratory tests reveal altered hepatic function, including elevated aspartate aminotransferase (251 U/L), alanine aminotransferase (56 U/L), alkaline phosphatase (179 U/L), total bilirubin (15.4 mg/dL), and ammonia (143 U/L), impaired coagulation (international normalized ratio 2.39), and decreased albumin (2.7 g/dL). Other metabolic disturbances include: sodium, 104 mEq/L; chloride, <60 mEq/L; potassium, 2.2 mEq/L; and CO2, 44.5 mEq/L.
What is your differential diagnosis for Mr. W’s altered mental status?
a) hepatic encephalopathy
b) Wernicke’s encephalopathy
c) hyponatremia
d) drug intoxication
e) head trauma
The authors’ observations
Hyponatremia is defined as a serum sodium concentration <136 mEq/L. Mr. W is considered to have severe hyponatremia because his serum sodium concentration is <125 mEq/L. Although commonly caused by an inability to suppress antidiuretic hormone, hyponatremia has several possible causes (Figure 1).1 Symptoms are nonspecific and are more visible when there is a large or rapid decrease in the serum sodium concentration. Most patients with a serum sodium concentration >125 mEq/L are asymptomatic. Mr. W, who had a serum sodium of 104 mEq/L, presented with several symptoms, including confusion, lethargy, nausea, vomiting, and weakness. Headache, muscle spasms, depressed reflexes, restlessness, and disorientation also might be observed.2
Because of Mr. W’s impaired hepatic function, elevated ammonia, and asterixis, hepatic encephalopathy could be contributing to his altered mental status. Suspect Wernicke’s encephalopathy in a patient with neurologic symptoms and a history of chronic alcohol abuse. In its classic form, Wernicke’s encephalopathy has acute onset, characterized by the triad of ataxia, global confusion, and ocular abnormalities. However, this triad is not consistently or frequently encountered.3
Which tests would you order next?
a) blood ethanol level
b) urine drug screen
c) serum osmolality
d) CT of the head
EVALUATION Sober, yet sick
To rule out intoxication as the cause of Mr. W’s altered mental status, blood ethanol level and urine drug screens are obtained and found to be negative. CT of the head is negative for acute intracranial pathology.
Mr. W is admitted to the medical intensive care unit (MICU) for severe hyponatremia and altered mental status. Serum osmolality is 220 mOsm/kg (normal range 281 to 304 mOsm/kg). To further classify his hypotonic hyponatremia, volume status is assessed, and Mr. W is determined to be euvolemic. Thyroid-stimulating hormone and cortisol are within normal limits, eliminating hypothyroidism and adrenal insufficiency as causes of his euvolemic hypotonic hyponatremia. Mr. W is treated for hyponatremia likely secondary to syndrome of inappropriate antidiuretic hormone (SIADH). SIADH is a diagnosis of exclusion that first requires ruling out hypothyroidism and glucocorticoid insufficiency (Figure 1).1
The authors’ observations
Because hypokalemia is an independent predictive factor for development of hyponatremia, it is necessary to evaluate the potassium level in all hyponatremic patients. Mr. W’s potassium level was 2.2 mEq/L on admission. Serum sodium concentration is related to total exchangeable sodium, total body water, and total exchangeable potassium. Potassium depletion causes a shift of sodium into cells with a comparable exit of potassium from cells into extracellular fluid. The reverse process occurs during potassium repletion, leading to an increase in serum sodium concentration and making hypokalemia a risk factor for developing osmotic demyelination syndrome (ODS).4
Treating hyponatremia
Hyponatremia treatment depends on its severity, presence or absence of symptoms, and whether the hyponatremia is acute (<24 hours) or chronic (>48 hours).5
Because of Mr. W’s extremely low serum sodium concentration, predisposition to hyponatremia secondary to alcoholism, and history of severe hyponatremia, it is likely he is chronically hyponatremic.
In patients with chronic hyponatremia, neurological sequelae are associated with the need for a more rapid rate of correction of serum sodium. For most patients with chronic hyponatremia, a correction rate of ≤10 to 12 mEq/L during the first 24 hours and <18 mEq/L over 48 hours is recommended to avoid ODS.6
Evidence suggests, however, that this 1-day limit might be too high for some patients. Alcoholism, hypokalemia, malnutrition, and liver disease are present in a high percentage of patients who develop
ODS after correcting hyponatremia (Table 1).6 Therefore, for patients such as Mr. W who are at high risk of ODS, experts recommend a goal of 4 to 6 mEq/L/d with a correction rate of ≤8 mEq/L in any 24-hour period (Table 2).6
TREATMENT Sodium normalizes
Mr. W receives 1 L of normal saline in the ED before admission to the MICU. Once in the MICU, despite likely chronic hyponatremia, he receives hypertonic (3%) saline, followed by normal saline. Initially, he responds when the serum sodium concentration improves. Because of his likely SIADH, Mr. W is fluid-restricted for 4 days. Serum sodium returns to normal over 7 hospital days (Figure 2). To address the profound hypokalemia, Mr. S receives 30 mEq of potassium chloride in the ED, and potassium is repeated daily throughout his stay in the MICU.
Mr. W remains lethargic, with intermittent periods of confusion throughout the hospital stay. His altered mental status is attributed to hepatic encephalopathy secondary to alcoholic hepatitis. The Maddrey discriminant function is a calculation that stratifies patients with alcoholic hepatitis for risk of mortality and the use of steroids. Because Mr. W shows a Maddrey discriminant function ≥32, he receives methylprednisolone, followed by pentoxifylline, and liver function tests trend down. He also receives lactulose throughout hospitalization.
By discharge on hospital day 9, Mr. W’s serum sodium is 138 mEq/L; serum potassium, 4.1 mEq/L. Total bilirubin and prothrombin remain elevated. Mr. W is discharged on lactulose, thiamine, folic acid, and a 1-month course of pentoxifylline, 400 mg, 3 times a day.
READMISSION Unsteady gait, nausea
Three days after discharge, Mr. W returns to the ED after experiencing a 20-second episode of total body rigidity. He has an unsteady gait and worsening nausea and vomiting.
When Mr. W arrives in the ED, he confirms he is taking his discharge medications as prescribed. His parents report that he has consumed alcohol and Cannabis since discharge and has been taking his sibling’s prescription medications, including quetiapine.
In the ED, Mr. W is awake, alert, and oriented to person, place, and time. Vital signs are: pulse, 118 beats per minute; blood pressure, 128/73 mm Hg; respirations, 16 breaths per minute; and temperature, 98.5ºF. Physical examination, again, is notable for scleral icterus, jaundice, and asterixis. No focal neurologic deficits are noted.
Consistent with Mr. W’s previous admission, laboratory values reveal altered hepatic function and impaired coagulation. The serum sodium level remains within normal limits at 136 mEq/L. However, again, metabolic disturbances include decreased chloride (97 mEq/L), potassium (2.9 mEq/L), and CO2 (18.2 mEq/L). CT on readmission is unchanged from the earlier hospitalization.
What is your differential diagnosis for Mr. W’s total body rigidity?
a) seizure
b) ODS
c) drug intoxication
d) neuroleptic malignant syndrome
EVALUATION Shaking and weakness
Once admitted to the hospital, Mr. W reports an episode of right upper-extremity “shaking,” followed by weakness. He remembers the entire event and denies tongue biting or incontinence. He is evaluated for possible seizure, given his multiple risk factors, including drug and alcohol use, ingestion of quetiapine, and history of hyponatremia. Routine EEG is negative but prolactin level is elevated.
Mr. W’s mental status continues to wax and wane, prompting a neurology consult and MRI for further evaluation. MRI of the brain without contrast reveals restricted diffusion in the pons centrally, with extension bilaterally to the midbrain and thalami—findings consistent with central pontine myelinolysis. A neurology consultation reveals quadriparesis, paraparesis, dysarthria, and diplopia on examination, all symptoms associated with central pontine myelinolysis.
The authors’ observations
ODS, including central and extrapontine myelinolysis, is a demyelinating condition that occurs because of severe osmotic stress, most commonly secondary to the overly rapid correction of hyponatremia in patients with conditions leading to nutritional or electrolyte stress.7 Mr. W is considered at high risk of developing ODS because he fulfills the 5 criteria listed in Table 1.
Several psychiatric illnesses and neuropsychiatric medications could lead to hyponatremia. Many studies8-10 have documented hyponatremia and resulting ODS in patients with alcoholism, schizophrenia, anorexia, primary psychogenic polydipsia, and MDMA (3,4-methylenedioxymethamphetamine) abuse. Hyponatremia is a side effect of several neuropsychiatric medications, including serotonin reuptake inhibitors, lithium, tricyclic antidepressants, opioids, carbamazepine, oxcarbazepine, and antipsychotic polypharmacy. Other commonly used medications associated with hyponatremia include salt-losing diuretics, nonsteroidal anti-inflammatory drugs, and acetaminophen.7
Disease severity varies from asymptomatic to coma or death. Symptoms, although some could reverse completely, typically are a combination of neuropsychiatric (ie, emotional lability, disinhibition, and other bizarre behaviors) and neurologic. Neurologic symptoms include confusion, impaired cognition, dysarthria, dysphagia, gait instability, weakness or paralysis, and generalized seizures. Severely affected patients could experience “locked-in syndrome,” in which they are awake but unable to move or communicate. Also consistent with Mr. W’s case, ODS often presents initially with delirium, seizures, or encephalopathy, followed by a lucid interval before symptoms develop.7
Diagnosis is based on the appearance of demyelinating brain lesions on CT or MRI. MRI is more sensitive than CT; however, even an MRI scan can appear normal for as long as 4 weeks after symptoms appear.7 Therefore, an initial negative radiologic study in a high-risk patient who develops neurologic symptoms does not exclude ODS. Earlier detection is possible with diffusion-weighted MRI, which is most sensitive and can detect lesions within 24 hours of developing symptoms.11 The severity of the lesion does not correlate with severity of symptoms.
Studies reveal a considerable range in prognosis of patients with clinically symptomatic ODS. A study of 44 patients with central pontine myelinolysis, of which 42 had chronic alcoholism, reported that 34% had no significant functional deficits at follow-up, 34% had minor neurologic deficits, and 31% became dependent on personal help. Outcome did not depend on the extent or severity of neurologic symptoms or the severity of concomitant systemic complications.12
Because of its poor prognosis, prevention of ODS is important. Because ODS commonly is caused by overly rapid correction of hyponatremia, it is necessary to adhere to guidelines for treating chronic hyponatremia (Table 2). If overcorrection occurs, therapeutic re-lowering of serum sodium can be considered, but has not been validated in controlled trials. Based mainly on case reports that suggest benefit from early re-lowering serum sodium in patients with ODS symptoms, experts recommend the following:
• administer desmopressin, 2 to 4 μg, every 8 hours parenterally
• replace water orally or as 5% dextrose in water intravenously (3 mL/kg/hr)
• check serum sodium hourly until serum is reduced to goal.6
Bottom Line
Hyponatremia is the most common electrolyte disorder encountered in practice. Osmotic demyelination syndrome often is preventable, with considerable morbidity and mortality. Psychiatrists should be aware of this condition because it could be an adverse effect of many psychiatric medications and there are some psychiatric illnesses in which hyponatremia is a potential risk. In hyponatremic patients with persistent nonspecific neurologic or neuropsychiatric symptoms and negative CT imaging, additional imaging, such as MRI, is warranted.
Related Resources
- Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
- Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726.
Drug Brand Names
Carbamazepine • Tegretol
Oxcarbazepine • Trileptal
Desmopressin • Stimate, DDAVP
Lithium • Eskalith, Lithobid
Pentoxifylline • Trental, Pentoxil
Methylprednisolone • Medrol
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
1. Elhassen EA, Schrier RW. Disorders of sodium and water balance. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and practice of hospital medicine. New York, NY: McGraw-Hill; 2012:2084-2093.
2. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
3. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035-1039.
4. Edelman IS, Leibman J, O’Meara MP, et al. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
5. Reynolds RM, Seckl JR. Hyponatraemia for the clinical endocrinologist. Clin Endocrinol (Oxf). 2005;63(4):366-374.
6. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1-S42.
7. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23(4):369-374.
8. Goldman MB. The assessment and treatment of water imbalance in patients with psychosis. Clin Schizophr Related Psychoses. 2010;4(2):115-123.
9. Patel AS, Matthews L, Bruce-Jones W. Central pontine myelinolysis as a complication of refeeding syndrome in a patient with anorexia nervosa. J Neuropsychiatry Clin Neurosci. 2008;20(3):371-373.
10. Bhuvaneswar CG, Baldessarini RJ, Harsh VL, et al. Adverse endocrine and metabolic effects of psychotropic drugs: selective clinical review. CNS Drugs. 2009;23(12):1003-1021.
11. Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210-213.
12. Menger H, Jörg J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J Neurol. 1999;246(8):700-705.
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder
sodium concentration, Wernicke’s
encephalopathy, osmotic demyelination syndrome, electrolyte disorder