User login
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
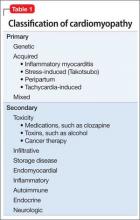
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
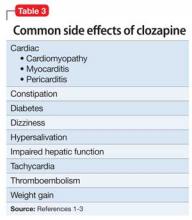
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.