User login
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
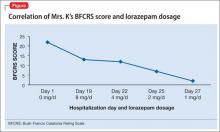
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
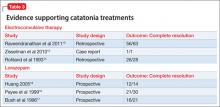
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Mute and unresponsive
Mrs. K, a 42-year-old Haitian who is 31 weeks pregnant, presents with a 4-week history of progressive mutism and psychomotor retardation. At inpatient admission, she is awake and alert but does not speak and resists the treatment team’s attempts to engage her. Mrs. K’s eyes are open, but she has a vacant stare and avoids eye contact. Her affect is flat and nonreactive and she appears internally preoccupied. Mrs. K exhibits motoric immobility, displays a rigid posture, and resists attempts to get her to move. Features of catatonic excitement, echo phenomena, posturing, stereotypies, and mannerisms are absent during the initial evaluation.
Mrs. K’s husband reports that they had been on vacation for 6 days before he brought her for psychiatric evaluation. He denies any recent evidence of psychosis or mood disturbance, stating that his wife was excited when she learned of the pregnancy, and attended all prenatal appointments. He reports that when this episode began, Mrs. K stopped talking to her 3-year-old daughter, did not respond to her name, and did not pay attention to those around her.
According to her husband, a similar episode occurred 2 years earlier, during which Mrs. K was selectively mute for approximately 1 month. She did not eat for 5 days and neglected the care of her daughter. There were 2 additional brief periods of prominent psychomotor retardation for which she was hospitalized in Haiti. According to the patient’s aunt, Mrs. K complained that her husband had cast a “voodoo spell” on her because he wanted sole custody of their daughter. Her husband recounted an episode, when they lived in Haiti, during which his wife became paranoid, left the house, and wandered the streets for 2 days.
The medical history is significant for a cervical polyp that was removed 2 years ago. Mrs. K has no history of substance abuse. She was born and raised in Haiti where she studied medicine. Her family reports that Mrs. K’s husband is “controlling,” which causes her distress.
a) major depressive disorder, severe with psychosis, with catatonia
b) schizophrenia, with catatonia
c) conversion disorder, with catatonia
d) bipolar I disorder, with psychosis and catatonia
The authors' observations
Catatonia is a neuropsychiatric syndrome that can occur in schizophrenia, mood disorders, mental retardation, neurologic disease, metabolic conditions, and drug intoxication.1 Catatonia can present in several ways, from catatonic stupor to extreme purposeless agitation; more than 60 catatonic signs and symptoms have been described.1 According to DSM-52 catatonia is characterized by 3 or more of the following symptoms:
• stupor
• catalepsy
• waxy flexibility
• mutism
• negativism
• posturing
• mannerism
• stereotypy
• agitation, not influenced by external stimuli
• grimacing
• echolalia
• echopraxia.
Mrs. K exhibited stupor, mutism, posturing, and grimacing (Table 1).2 We thought that her catatonic features were secondary to schizophrenia because she had paranoid delusions and displayed disorganized behavior while in Haiti. There was no evidence of past or current mood disorder, metabolic condition, neurologic illness, or substance abuse.
Catatonia and pregnancy
There is little available information to guide clinicians who are treating a pregnant woman who has a catatonic syndrome. Espinola-Nadurille and co-workers described a 22-year-old pregnant (21 weeks) woman from rural Mexico who was hospitalized with agitation, disorganized speech, restlessness, and hallucinations after several weeks of alternating agitation and withdrawal with mutism and refusal to eat or drink.3 This patient developed malignant catatonia with creatine phosphokinase elevation and leukocytosis and eventually responded to treatment with lorazepam and electroconvulsive therapy (ECT). She was given a diagnosis of schizophreniform disorder. Treating her catatonic symptoms did not result in any adverse effects on the pregnancy or the fetus.
Exacerbation of schizophrenia during pregnancy can lead to neglect of pregnancy and prenatal care,4 imminent harm to the fetus because of malnutrition and dehydration, and risk of preterm delivery and low weight at birth. Prolonged catatonia can cause medical complications such as decubitus ulcers, incontinence, recurrent urinary tract infections, aspiration pneumonia, increased risk of deep venous thrombosis, malnutrition, and ocular complications because of prolonged staring and reduced blinking (Table 25-10). For these reasons, it is important to treat this condition early and aggressively to improve pregnancy outcome and infant well-being.
of Mrs. K’s symptoms?
a) Positive and Negative Symptom Scale
b) The Northoff Catatonia Rating Scale
c) The Bush-Francis Catatonia Rating Scale (BFCRS)
d) The Rogers Catatonia Scale
EVALUATION: Flat affect
The mental status examination on admission describes a tall, black, Haitian woman with unkempt hair and fair hygiene. Mrs. K has a prominent abdomen, consistent with a 31-week pregnancy. She exhibits a blank stare without direct eye contact; she is mute, and exhibits flat affect. We cannot evaluate her thought processes and content because Mrs. K is mute, although she does appear internally preoccupied.
Physical examination on admission is unremarkable; vital signs are stable and within normal limits. Laboratory work-up reveals a urinary tract infection, which is treated with ceftriaxone. Mrs. K also has macrocytic anemia (hemoglobin, 11.7 g/dL; hematocrit, 34.7%; mean corpuscular volume, 99.2 μm3). Albumin is low at 2.6 g/dL. Urine drug toxicology screen is negative. Fingerstick glucose reading is 139 mg/dL. Mrs. K is given a presumptive diagnosis of schizophrenia with catatonia.
Mrs. K’s BFCRS11 score is 22 at admission. She is mute, holds postures for longer than a minute, and is resistant to repositioning. She also has extreme hypoactivity and does not interact with others. She has a fixed, blank stare, and exhibits mild grimacing.
The authors' observations
BFCRS defines each catatonic sign, rates its severity, and provides a standardized schema for clinical examination.11 The BFCRS is preferred for routine use because of its validity, reliability, and ease of administration.12 The treatment team rated Mrs. K at admission, during the course of treatment, and at discharge, showing a substantial improvement at the end of the hospitalization (Figure).
a) ECT
b) lorazepam
c) haloperidol
d) olanzapine
The authors' observations
Benzodiazepines (particularly lorazepam) and ECT are considered the treatment of choice for catatonic symptoms.11 More than 72% of patients with catatonic symptoms remit after a trial of a benzodiazepine.1 ECT is considered when patients do not respond to a benzodiazepine after 48 to 72 hours.3 Several cases of complete resolution of catatonic symptoms have been linked to ECT (Table 3).13-18
A recent retrospective review revealed that patients who do not respond to lorazepam are more likely to come from a rural setting, have a longer duration of illness, exhibit mutism, and exhibit third-person auditory hallucinations and made phenomena (the feeling that some aspect of the individual is under the external control of others).19 Case reports of treatment of catatonic patients with ECT vs lorazepam are listed in Table 3.13-18
In pregnancy, ECT can be considered early in the course of illness. A review of the literature on ECT during pregnancy reported at least partial remission in 78% of studies reporting efficacy data.20 Among these 339 patients, there were 25 fetal or neonatal complications—only 11 of these were related to ECT—and 20 maternal complications, of which 18 were related to ECT. The authors of this review concluded that 1) ECT is an effective treatment for severe mental illness during pregnancy and 2) the risks to fetus and mother are low.
A 2007 study identified 1,979 infants whose mothers reported use of benzodiazepines or hypnotic benzodiazepine-receptor agonists during early pregnancy.21 An additional 401 infants born to mothers who were prescribed these medications during late pregnancy also were included in this study. Women who took these medications were at an increased risk for preterm birth and low birth weight. The rate of congenital malformations in this study was moderately increased among infants exposed in early pregnancy (adjusted odds ratio = 1.24 [95% confidence interval, 1.00 to 1.55]).
Because catatonic symptoms can appear during the course of schizophrenia, several antipsychotics have been used to treat this condition. The efficacy and safety of antipsychotics for treating catatonia remains largely unknown, however.1
OUTCOME: Recovery, baby girl
We begin oral haloperidol, 10 mg/d, for Mrs. K, which we then increase to 20 mg/d. Because she shows little response to haloperidol, we suggest a trial of ECT, but her husband refuses to consent. She is started on IM lorazepam, 6 mg/d.
Mrs. K gradually improves and increases her intake of food and liquids. After 10 days of lorazepam treatment, her BFCRS score decreases to 13. Mrs. K begins to speak and her gaze is less fixed. Negativistic behaviors are nearly absent.
Because we are concerned about Mrs. K’s pregnancy, lab tests are repeated. A complete metabolic panel shows an elevated glucose level (122 mg/dL); urinalysis reveals glycosuria (glucose, 1,000 mg/dL), proteinuria (protein, 10 mg/dL), and ketonuria, (ketones, 20 mg/dL). She is transferred to the obstetrics service for evaluation of gestational diabetes.
Psychotropics are continued while Mrs. K is on the obstetrics service; she returns to the inpatient psychiatric unit on an insulin regimen. IM lorazepam is increased to 8 mg/d, and haloperidol is decreased from 20 mg/d, to 10 mg/d, to prevent worsening of catatonia, which can occur when catatonic patients receive a psychotropic.11 Three days later, Mrs. K’s BFCRS score is 12 and she shows only mild rigidity. Mrs. K briefly interacts with staff, particularly when she wants something.
Lorazepam is decreased to 1 mg/d in anticipation of cesarean delivery. Mrs. K becomes more adherent with her medications; often, she takes the oral dose of haloperidol, rather than the IM formulation. On mental status examination she exhibits poor eye contact, rather than a fixed gaze, and her BFCRS score decreases to 7 by day 25.
By the end of lorazepam treatment, Mrs. K has fully recovered from her catatonic state. She interacts with staff, engages with the treatment team, and is excited to go home. At discharge, she is given a diagnosis of schizophrenia with catatonia, and is taking haloperidol, 5 mg, twice a day. She gives birth to a healthy girl.
The authors' observations
Mrs. K was treated initially with haloperidol for several reasons. Haloperidol is relatively safe during pregnancy (FDA pregnancy category C) as shown by a recent multicenter, prospective, cohort study in which babies exposed in utero to haloperidol showed a congenital malformation (limb defect) rate within the expected baseline risk for the general population.22 Lorazepam is FDA category D for use in pregnancy and can cause preterm delivery,23 floppy infant syndrome, and withdrawal syndromes.24 We did not use a second-generation antipsychotic (SGA) because it could have made Mrs. K’s hyperglycemia worse. SGAs can induce gestational diabetes and increase the incidence of large-for-gestational-age newborns, compared with first-generation antipsychotics.24 Last, Mrs. K’s family rejected ECT.
Because of Mrs. K’s poor response to haloperidol, the treatment team decided to start IM lorazepam, which eventually was increased to 8 mg/d. The haloperidol dose was reduced by half to avoid worsening of catatonia and reduce the risk of neuroleptic malignant syndrome.1,25 When clinical response was achieved, lorazepam was tapered and Mrs. K was discharged with only haloperidol.
In the absence of well-designed prospective follow-up studies, information on the potential impact of prenatal exposure to antipsychotics and benzodiazepines on a child’s cognitive development is limited.26 This case adds to the scant literature on the treatment of catatonia during pregnancy and illustrates how the BFCRS can be utilized during serial patient evaluations to monitor clinical improvement.
Bottom Line
Psychosis and catatonia during pregnancy are associated with complications to mother and child. The Bush-Francis Catatonia Rating Scale can be used to identify and track catatonic symptoms. Lorazepam and electroconvulsive therapy have been used safely and with good outcomes in mentally ill pregnant women when used appropriately.
Related Resources
- Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54(7):437-445.
- Seethalakshmi R, Dhavale S, Suggu K, et al. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry. 2008;20(1):5-8.
- Salam S, Kilzieh N. Lorazepam treatment of psychogenic catatonia: an update. J Clin Psychiatry, 1988;49(suppl):16-21.
Drug Brand Names
Ceftriaxone • Rocephin Olanzapine • Zyprexa
Haloperidol • Haldol Short-acting Insulin • Novolin, Humulin
Lorazepam • Ativan
Disclosures
Dr. Runyan receives grant support from Lippincott, Williams, & Wilkins. Drs. Durant, Prudent, and Sotelo report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.
1. Weder N, Muralee S, Penland H, et al. Catatonia: a review. Ann Clin Psychiatry. 2008;20(2):97-107.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
3. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL. Pregnancy and malignant catatonia. Gen Hosp Psychiatry. 2007;29(1):69-71.
4. Solari H, Dickson KE, Miller L. Understanding and treating women with schizophrenia during pregnancy and postpartum. Can J Clin Pharmacol. 2009;16(1):e23-e32.
5. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(12):
153-155.
6. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387-391.
7. Lachner C, Sandson NB. Medical complications of catatonia: a case of catatonia-induced deep venous thrombosis. Psychosomatics. 2003;44(6):512-4.
8. Morioka H, Nagatomo I, Yamada K, et al. Deep venous thrombosis of the leg due to psychiatric stupor. Psychiatry Clin Neurosci, 1997;51(5):323-326.
9. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):
486-487.
10. Srivastava A, Gupta A, Murthy P, et al. Catatonia and multiple pressure ulcers: a rare complication in psychiatric setting. Indian J Psychiatry. 2009;51(3):206-208.
11. Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12(3):180-185.
12. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1):1-9.
13. Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127-132.
14. Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord. 1993;29(4):255-261.
15. Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425-430.
16. Payee H, Chandrasekaran R, Raju GV. Catatonic syndrome: treatment response to lorazepam. Indian J Psychiatry. 1999; 41(1):49-53.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Huang TL. Lorazepam and diazepam rapidly relieve catatonic signs in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(1):52-55.
19. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312-316.
20. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71:
235-242.
21. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:
1203-1210.
22. Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:
518-544.
23. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201:579e1-8.
24. Howland RH. Prescribing psychotropic medications during pregnancy and lactation: principles and guidelines. J Psychosoc Nurs Ment Health Serv. 2009;47(5):19-23.
25. White DA, Robins AH. Catatonia: harbinger of the neuroleptic malignant syndrome. Br J Psychiatry. 1991; 158:419-421.
26. Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010; 27(7):675-686.