User login
A girl refuses to eat solid food because she is afraid of choking
CASE Refusing solid food
Ms. B, age 11, is admitted to a pediatric medical inpatient unit for unintentional weight loss of 14 lb (15% total body weight) over the past month. She reports having 2 traumatic episodes last month: choking on a piece of cheese and having a swab specimen taken for a rapid strep test, which required several people to restrain her (the test was positive). Since then, she has refused to ingest solids, despite hunger and a desire to eat.
Ms. B reports diffuse abdominal pain merely “at the sight of food” and a fear of swallowing solids. She denies difficulty or pain upon swallowing, nausea, vomiting, or any change in bowel habits.
Her mother reports that, on the rare occasion that Ms. B has attempted to eat solid food, she spent as long as an hour cutting it into small pieces before bringing it to her mouth—after which she put the food down without eating. Her mother also witnessed Ms. B holding food in her mouth for “a very long time,” then spitting it out.
Ms. B says she is distressed about the weight loss and recognizes that her fear of solid food is excessive.
What would your diagnosis of Ms. B’s problem be?
a) anorexia nervosa
b) avoidant/restrictive food intake disorder (ARFID)
c) specific phobia (swallowing solids or choking)
d) generalized anxiety disorder (GAD)
The authors’ observations
DSM-5 describes a new eating disorder called ARFID, which replaces the DSM-IV-TR diagnosis of feeding disorder of infancy or early childhood</keyword>. DSM-5 diagnostic criteria define ARFID as:
An eating or feeding disturbance (eg, avoidance based on the sensory characteristics of food…) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with at least one of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning.1
DSM-5 also specifies that the disorder cannot be caused by lack of available food or traditional cultural practices; cannot coexist with anorexia or bulimia nervosa; and is not attributable to a concurrent medical or psychiatric disorder.
Because it is a newly defined diagnosis, the epidemiology of ARFID is unclear. Patients with ARFID have a wide variety of eating symptoms that do not meet diagnostic criteria for anorexia or bulimia nervosa. One study found that, among a cohort of mostly adolescent patients who presented for evaluation of an eating disorder, 14% met diagnostic criteria for ARFID.2 Another retrospective case-control study found a similar prevalence among patients age 8 to 18 (13.8% of 712 patients).3 Because of the variety of maladaptive feeding behaviors seen in ARFID, there is little evidence that pharmacotherapy is effective.4
HISTORY Premature birth
Ms. B’s medical history states that she is twin A of a premature birth at 26 weeks (birth weight, 1,060 g), with a 90-day neonatal intensive care unit hospitalization, during which she required supplemental oxygen and nasogastric tube feeding. She has mild cerebral palsy, and had motor delay of walking at 2.5 years old. Currently, she has no motor difficulties.
Ms. B does not have a psychiatric history and does not take medications. Her mother has a history of major depressive disorder that is well controlled with an unspecified selective serotonin reuptake inhibitor. Ms. B’s maternal uncle has poorly controlled schizophrenia.
During Ms. B’s 6-day hospitalization, her mental status exams are unremarkable. She is shy but cooperative and open. Her mood ranges from “sad” and “nervous” on admission to “fine” with mood-congruent affect. She denies suicidal or homicidal thoughts and hallucinations, and demonstrates good insight and judgment. All laboratory values are within normal limits except for mild hypophosphatemia (3.7 mg/dL) and mild hyperalbuminemia (4.9 g/dL) on admission, which may have been related to her nutritional status.
DIAGNOSIS Not solely psychiatric
The psychiatric differential diagnosis includes:
- ARFID
- specific phobia of swallowing solids or choking (pseudodysphagia)
- GAD
- unspecified feeding disorder.
Ms. B meets diagnostic criteria for ARFID, particularly that of profound acute weight loss due to restrictive eating behaviors. Her presentation also is similar to that of a specific phobia, namely profound anxiety upon even the thought of solid food (phobic stimulus) and recognition that her fear is excessive. However, she fails to meet diagnostic criteria for phobia in children, which require duration of at least 6 months. GAD also is less likely because she has had symptoms for 1 month (also requires 6-month duration) and her anxiety is limited to feeding behaviors.
The treatment team starts exposure therapy, encouraging Ms. B to begin taking small bites of textured foods, such as oatmeal.
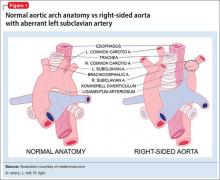
On hospital Day 5, barium esophagogram reveals extrinsic compression of the esophagus. To identify the precise cause of compression, chest magnetic resonance angiogram reveals that Ms. B has a right-sided aortic arch with an aberrant left subclavian artery at T4 that, with the ligamentum arteriosum and left pulmonary artery, form a vascular ring impinging around the esophagus.
The authors’ observations
Dysphagia lusoria, coined in 1789 from the root for “natural abnormality,”5 is caused by an aberrant right subclavian artery, which persists because of abnormal involution of the right fourth aortic arch during embryogenesis6 (Figure 1). The condition is diagnosed incidentally in most affected adults, who are asymptomatic throughout life and do not require operative management.6
Symptoms of dysphagia lusoria can include dysphagia and recurrent aspiration if significant esophageal impingement is present.7 It is thought that patients tend to show more symptoms as they age because of sclerosis, aneurysm, or atherosclerosis of the impinging vessel.7 Cough and dyspnea caused by impingement of the trachea also have been reported, and might be more common in children because of tracheal flexibility.5
This case illustrates the complex interrelationship of physical and psychiatric conditions. After the treatment team discovered a physical cause of Ms. B’s symptoms, the initial psychiatric diagnosis became problematic, but remained critically important for long-term treatment of her comorbid eating phobia.
TREATMENT Therapy, surgery
The treatment team is faced with the question of whether dysphagia lusoria fully accounts for Ms. B’s status. The anatomic anomaly might explain the initial choking incident if the food particle was lodged at the site of impingement, but does not account for development of a severe acute eating disorder and subsequent malnutrition.
Because of the presence of dysphagia lusoria, the team concludes that Ms. B does not meet diagnostic criteria for ARFID. She is given a diagnosis of unspecified eating disorder.
Ms. B is discharged and referred to a pediatric cardiothoracic surgeon for operative consult of symptomatic dysphagia lusoria. By discharge, she is successfully eating yogurt with fruit chunks, which she had rejected earlier. She also expresses optimism about eating cake at her birthday party, scheduled for 2 weeks after discharge.
The treatment team strongly recommends outpatient psychiatric follow-up to manage Ms. B’s unspecified eating disorder with cognitive-behavioral therapy and exposure and response prevention, which helped to mildly decrease her anxiety during the hospital stay.
Ms. B’s surgeon deems the case severe enough to warrant surgery. Surgery for dysphagia lusoria can be indicated to prevent progression of symptoms into adulthood8; options include division of the ligamentum arteriosum to loosen the vascular ring and re-implantation of the aberrant subclavian artery.9,10 (On the other hand, dietary modification might be therapeutic in patients whose symptoms are mild.5)
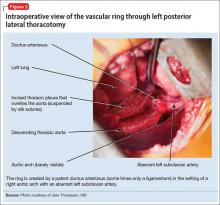
The surgeon elects to divide the ligamentum arteriosum to relieve the pressure of the vascular ring on the esophagus. Intraoperatively, he discovers that Ms. B has a mildly patent ductus arteriosus (PDA) (Figure 2), which is more common in premature infants than in full-term births. The PDA is clipped on both sides and divided. This immediately causes the vascular ring created by the aberrant left subclavian artery, right-sided aorta, and PDA to spring open, releasing pressure on the esophagus.
The aberrant left subclavian artery emerges from an abnormal bulging of aorta, known as Kommerell’s diverticulum. After the vascular ring is released, the diverticulum is not observed to impinge on the esophagus; however, as a preventive measure, the surgeon sutures it to the anterior spinous ligament. This will prevent the diverticulum from enlarging and impinging on the esophagus as Ms. B grows to adulthood.
The surgery is completed without complications. Ms. B tolerates the procedure well.
Ten days after surgery, Ms. B is recovering well. She and her mother report satisfaction with the procedure to release the vascular ring. She discontinues her pain medication after 2 days and slowly begins reintroducing solid foods. Her fear of dysphagia and choking rapidly diminish.
Bottom Line
The diagnosis and management of eating disorders, including avoidant/restrictive food intake disorder and choking phobia, can be challenging. Furthermore, if an anatomical or organic anomaly is found, it is important to question how a patient’s eating disorder can be managed best through interdisciplinary collaboration between medical and behavioral specialties.
Related Resources
• Bryant-Waugh R. Feeding and eating disorders in children. Curr Opin Psychiatry. 2013;26(6):537-542.
• Norris ML, Robinson A, Obeid N, et al. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47(5):495-499.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health. 2013;53(2):303-305.
3. Fisher MM, Rosen DS, Ornstein RM, et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49-52.
4. Kelly NR, Shank LM, Bakalar JL, et al. Pediatric feeding and eating disorders: current state of diagnosis and treatment. Curr Psychiatry Rep. 2014;16(5):446.
5. Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7(2):106-109.
7. Calleja F, Eguaras M, Montero J, et al. Aberrant right subclavian artery associated with common carotid trunk. A rare cause of vascular ring. Eur J Cardiothorac Surg. 1990;4(10):568-570.
8. Jalaie H, Grommes J, Sailer A, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48(5):521-526.
9. Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532-534.
10. Morrris CD, Kanter KR, Miller JI Jr. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71(2):710-712.
CASE Refusing solid food
Ms. B, age 11, is admitted to a pediatric medical inpatient unit for unintentional weight loss of 14 lb (15% total body weight) over the past month. She reports having 2 traumatic episodes last month: choking on a piece of cheese and having a swab specimen taken for a rapid strep test, which required several people to restrain her (the test was positive). Since then, she has refused to ingest solids, despite hunger and a desire to eat.
Ms. B reports diffuse abdominal pain merely “at the sight of food” and a fear of swallowing solids. She denies difficulty or pain upon swallowing, nausea, vomiting, or any change in bowel habits.
Her mother reports that, on the rare occasion that Ms. B has attempted to eat solid food, she spent as long as an hour cutting it into small pieces before bringing it to her mouth—after which she put the food down without eating. Her mother also witnessed Ms. B holding food in her mouth for “a very long time,” then spitting it out.
Ms. B says she is distressed about the weight loss and recognizes that her fear of solid food is excessive.
What would your diagnosis of Ms. B’s problem be?
a) anorexia nervosa
b) avoidant/restrictive food intake disorder (ARFID)
c) specific phobia (swallowing solids or choking)
d) generalized anxiety disorder (GAD)
The authors’ observations
DSM-5 describes a new eating disorder called ARFID, which replaces the DSM-IV-TR diagnosis of feeding disorder of infancy or early childhood</keyword>. DSM-5 diagnostic criteria define ARFID as:
An eating or feeding disturbance (eg, avoidance based on the sensory characteristics of food…) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with at least one of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning.1
DSM-5 also specifies that the disorder cannot be caused by lack of available food or traditional cultural practices; cannot coexist with anorexia or bulimia nervosa; and is not attributable to a concurrent medical or psychiatric disorder.
Because it is a newly defined diagnosis, the epidemiology of ARFID is unclear. Patients with ARFID have a wide variety of eating symptoms that do not meet diagnostic criteria for anorexia or bulimia nervosa. One study found that, among a cohort of mostly adolescent patients who presented for evaluation of an eating disorder, 14% met diagnostic criteria for ARFID.2 Another retrospective case-control study found a similar prevalence among patients age 8 to 18 (13.8% of 712 patients).3 Because of the variety of maladaptive feeding behaviors seen in ARFID, there is little evidence that pharmacotherapy is effective.4
HISTORY Premature birth
Ms. B’s medical history states that she is twin A of a premature birth at 26 weeks (birth weight, 1,060 g), with a 90-day neonatal intensive care unit hospitalization, during which she required supplemental oxygen and nasogastric tube feeding. She has mild cerebral palsy, and had motor delay of walking at 2.5 years old. Currently, she has no motor difficulties.
Ms. B does not have a psychiatric history and does not take medications. Her mother has a history of major depressive disorder that is well controlled with an unspecified selective serotonin reuptake inhibitor. Ms. B’s maternal uncle has poorly controlled schizophrenia.
During Ms. B’s 6-day hospitalization, her mental status exams are unremarkable. She is shy but cooperative and open. Her mood ranges from “sad” and “nervous” on admission to “fine” with mood-congruent affect. She denies suicidal or homicidal thoughts and hallucinations, and demonstrates good insight and judgment. All laboratory values are within normal limits except for mild hypophosphatemia (3.7 mg/dL) and mild hyperalbuminemia (4.9 g/dL) on admission, which may have been related to her nutritional status.
DIAGNOSIS Not solely psychiatric
The psychiatric differential diagnosis includes:
- ARFID
- specific phobia of swallowing solids or choking (pseudodysphagia)
- GAD
- unspecified feeding disorder.
Ms. B meets diagnostic criteria for ARFID, particularly that of profound acute weight loss due to restrictive eating behaviors. Her presentation also is similar to that of a specific phobia, namely profound anxiety upon even the thought of solid food (phobic stimulus) and recognition that her fear is excessive. However, she fails to meet diagnostic criteria for phobia in children, which require duration of at least 6 months. GAD also is less likely because she has had symptoms for 1 month (also requires 6-month duration) and her anxiety is limited to feeding behaviors.
The treatment team starts exposure therapy, encouraging Ms. B to begin taking small bites of textured foods, such as oatmeal.
On hospital Day 5, barium esophagogram reveals extrinsic compression of the esophagus. To identify the precise cause of compression, chest magnetic resonance angiogram reveals that Ms. B has a right-sided aortic arch with an aberrant left subclavian artery at T4 that, with the ligamentum arteriosum and left pulmonary artery, form a vascular ring impinging around the esophagus.
The authors’ observations
Dysphagia lusoria, coined in 1789 from the root for “natural abnormality,”5 is caused by an aberrant right subclavian artery, which persists because of abnormal involution of the right fourth aortic arch during embryogenesis6 (Figure 1). The condition is diagnosed incidentally in most affected adults, who are asymptomatic throughout life and do not require operative management.6
Symptoms of dysphagia lusoria can include dysphagia and recurrent aspiration if significant esophageal impingement is present.7 It is thought that patients tend to show more symptoms as they age because of sclerosis, aneurysm, or atherosclerosis of the impinging vessel.7 Cough and dyspnea caused by impingement of the trachea also have been reported, and might be more common in children because of tracheal flexibility.5
This case illustrates the complex interrelationship of physical and psychiatric conditions. After the treatment team discovered a physical cause of Ms. B’s symptoms, the initial psychiatric diagnosis became problematic, but remained critically important for long-term treatment of her comorbid eating phobia.
TREATMENT Therapy, surgery
The treatment team is faced with the question of whether dysphagia lusoria fully accounts for Ms. B’s status. The anatomic anomaly might explain the initial choking incident if the food particle was lodged at the site of impingement, but does not account for development of a severe acute eating disorder and subsequent malnutrition.
Because of the presence of dysphagia lusoria, the team concludes that Ms. B does not meet diagnostic criteria for ARFID. She is given a diagnosis of unspecified eating disorder.
Ms. B is discharged and referred to a pediatric cardiothoracic surgeon for operative consult of symptomatic dysphagia lusoria. By discharge, she is successfully eating yogurt with fruit chunks, which she had rejected earlier. She also expresses optimism about eating cake at her birthday party, scheduled for 2 weeks after discharge.
The treatment team strongly recommends outpatient psychiatric follow-up to manage Ms. B’s unspecified eating disorder with cognitive-behavioral therapy and exposure and response prevention, which helped to mildly decrease her anxiety during the hospital stay.
Ms. B’s surgeon deems the case severe enough to warrant surgery. Surgery for dysphagia lusoria can be indicated to prevent progression of symptoms into adulthood8; options include division of the ligamentum arteriosum to loosen the vascular ring and re-implantation of the aberrant subclavian artery.9,10 (On the other hand, dietary modification might be therapeutic in patients whose symptoms are mild.5)
The surgeon elects to divide the ligamentum arteriosum to relieve the pressure of the vascular ring on the esophagus. Intraoperatively, he discovers that Ms. B has a mildly patent ductus arteriosus (PDA) (Figure 2), which is more common in premature infants than in full-term births. The PDA is clipped on both sides and divided. This immediately causes the vascular ring created by the aberrant left subclavian artery, right-sided aorta, and PDA to spring open, releasing pressure on the esophagus.
The aberrant left subclavian artery emerges from an abnormal bulging of aorta, known as Kommerell’s diverticulum. After the vascular ring is released, the diverticulum is not observed to impinge on the esophagus; however, as a preventive measure, the surgeon sutures it to the anterior spinous ligament. This will prevent the diverticulum from enlarging and impinging on the esophagus as Ms. B grows to adulthood.
The surgery is completed without complications. Ms. B tolerates the procedure well.
Ten days after surgery, Ms. B is recovering well. She and her mother report satisfaction with the procedure to release the vascular ring. She discontinues her pain medication after 2 days and slowly begins reintroducing solid foods. Her fear of dysphagia and choking rapidly diminish.
Bottom Line
The diagnosis and management of eating disorders, including avoidant/restrictive food intake disorder and choking phobia, can be challenging. Furthermore, if an anatomical or organic anomaly is found, it is important to question how a patient’s eating disorder can be managed best through interdisciplinary collaboration between medical and behavioral specialties.
Related Resources
• Bryant-Waugh R. Feeding and eating disorders in children. Curr Opin Psychiatry. 2013;26(6):537-542.
• Norris ML, Robinson A, Obeid N, et al. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47(5):495-499.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Refusing solid food
Ms. B, age 11, is admitted to a pediatric medical inpatient unit for unintentional weight loss of 14 lb (15% total body weight) over the past month. She reports having 2 traumatic episodes last month: choking on a piece of cheese and having a swab specimen taken for a rapid strep test, which required several people to restrain her (the test was positive). Since then, she has refused to ingest solids, despite hunger and a desire to eat.
Ms. B reports diffuse abdominal pain merely “at the sight of food” and a fear of swallowing solids. She denies difficulty or pain upon swallowing, nausea, vomiting, or any change in bowel habits.
Her mother reports that, on the rare occasion that Ms. B has attempted to eat solid food, she spent as long as an hour cutting it into small pieces before bringing it to her mouth—after which she put the food down without eating. Her mother also witnessed Ms. B holding food in her mouth for “a very long time,” then spitting it out.
Ms. B says she is distressed about the weight loss and recognizes that her fear of solid food is excessive.
What would your diagnosis of Ms. B’s problem be?
a) anorexia nervosa
b) avoidant/restrictive food intake disorder (ARFID)
c) specific phobia (swallowing solids or choking)
d) generalized anxiety disorder (GAD)
The authors’ observations
DSM-5 describes a new eating disorder called ARFID, which replaces the DSM-IV-TR diagnosis of feeding disorder of infancy or early childhood</keyword>. DSM-5 diagnostic criteria define ARFID as:
An eating or feeding disturbance (eg, avoidance based on the sensory characteristics of food…) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with at least one of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning.1
DSM-5 also specifies that the disorder cannot be caused by lack of available food or traditional cultural practices; cannot coexist with anorexia or bulimia nervosa; and is not attributable to a concurrent medical or psychiatric disorder.
Because it is a newly defined diagnosis, the epidemiology of ARFID is unclear. Patients with ARFID have a wide variety of eating symptoms that do not meet diagnostic criteria for anorexia or bulimia nervosa. One study found that, among a cohort of mostly adolescent patients who presented for evaluation of an eating disorder, 14% met diagnostic criteria for ARFID.2 Another retrospective case-control study found a similar prevalence among patients age 8 to 18 (13.8% of 712 patients).3 Because of the variety of maladaptive feeding behaviors seen in ARFID, there is little evidence that pharmacotherapy is effective.4
HISTORY Premature birth
Ms. B’s medical history states that she is twin A of a premature birth at 26 weeks (birth weight, 1,060 g), with a 90-day neonatal intensive care unit hospitalization, during which she required supplemental oxygen and nasogastric tube feeding. She has mild cerebral palsy, and had motor delay of walking at 2.5 years old. Currently, she has no motor difficulties.
Ms. B does not have a psychiatric history and does not take medications. Her mother has a history of major depressive disorder that is well controlled with an unspecified selective serotonin reuptake inhibitor. Ms. B’s maternal uncle has poorly controlled schizophrenia.
During Ms. B’s 6-day hospitalization, her mental status exams are unremarkable. She is shy but cooperative and open. Her mood ranges from “sad” and “nervous” on admission to “fine” with mood-congruent affect. She denies suicidal or homicidal thoughts and hallucinations, and demonstrates good insight and judgment. All laboratory values are within normal limits except for mild hypophosphatemia (3.7 mg/dL) and mild hyperalbuminemia (4.9 g/dL) on admission, which may have been related to her nutritional status.
DIAGNOSIS Not solely psychiatric
The psychiatric differential diagnosis includes:
- ARFID
- specific phobia of swallowing solids or choking (pseudodysphagia)
- GAD
- unspecified feeding disorder.
Ms. B meets diagnostic criteria for ARFID, particularly that of profound acute weight loss due to restrictive eating behaviors. Her presentation also is similar to that of a specific phobia, namely profound anxiety upon even the thought of solid food (phobic stimulus) and recognition that her fear is excessive. However, she fails to meet diagnostic criteria for phobia in children, which require duration of at least 6 months. GAD also is less likely because she has had symptoms for 1 month (also requires 6-month duration) and her anxiety is limited to feeding behaviors.
The treatment team starts exposure therapy, encouraging Ms. B to begin taking small bites of textured foods, such as oatmeal.
On hospital Day 5, barium esophagogram reveals extrinsic compression of the esophagus. To identify the precise cause of compression, chest magnetic resonance angiogram reveals that Ms. B has a right-sided aortic arch with an aberrant left subclavian artery at T4 that, with the ligamentum arteriosum and left pulmonary artery, form a vascular ring impinging around the esophagus.
The authors’ observations
Dysphagia lusoria, coined in 1789 from the root for “natural abnormality,”5 is caused by an aberrant right subclavian artery, which persists because of abnormal involution of the right fourth aortic arch during embryogenesis6 (Figure 1). The condition is diagnosed incidentally in most affected adults, who are asymptomatic throughout life and do not require operative management.6
Symptoms of dysphagia lusoria can include dysphagia and recurrent aspiration if significant esophageal impingement is present.7 It is thought that patients tend to show more symptoms as they age because of sclerosis, aneurysm, or atherosclerosis of the impinging vessel.7 Cough and dyspnea caused by impingement of the trachea also have been reported, and might be more common in children because of tracheal flexibility.5
This case illustrates the complex interrelationship of physical and psychiatric conditions. After the treatment team discovered a physical cause of Ms. B’s symptoms, the initial psychiatric diagnosis became problematic, but remained critically important for long-term treatment of her comorbid eating phobia.
TREATMENT Therapy, surgery
The treatment team is faced with the question of whether dysphagia lusoria fully accounts for Ms. B’s status. The anatomic anomaly might explain the initial choking incident if the food particle was lodged at the site of impingement, but does not account for development of a severe acute eating disorder and subsequent malnutrition.
Because of the presence of dysphagia lusoria, the team concludes that Ms. B does not meet diagnostic criteria for ARFID. She is given a diagnosis of unspecified eating disorder.
Ms. B is discharged and referred to a pediatric cardiothoracic surgeon for operative consult of symptomatic dysphagia lusoria. By discharge, she is successfully eating yogurt with fruit chunks, which she had rejected earlier. She also expresses optimism about eating cake at her birthday party, scheduled for 2 weeks after discharge.
The treatment team strongly recommends outpatient psychiatric follow-up to manage Ms. B’s unspecified eating disorder with cognitive-behavioral therapy and exposure and response prevention, which helped to mildly decrease her anxiety during the hospital stay.
Ms. B’s surgeon deems the case severe enough to warrant surgery. Surgery for dysphagia lusoria can be indicated to prevent progression of symptoms into adulthood8; options include division of the ligamentum arteriosum to loosen the vascular ring and re-implantation of the aberrant subclavian artery.9,10 (On the other hand, dietary modification might be therapeutic in patients whose symptoms are mild.5)
The surgeon elects to divide the ligamentum arteriosum to relieve the pressure of the vascular ring on the esophagus. Intraoperatively, he discovers that Ms. B has a mildly patent ductus arteriosus (PDA) (Figure 2), which is more common in premature infants than in full-term births. The PDA is clipped on both sides and divided. This immediately causes the vascular ring created by the aberrant left subclavian artery, right-sided aorta, and PDA to spring open, releasing pressure on the esophagus.
The aberrant left subclavian artery emerges from an abnormal bulging of aorta, known as Kommerell’s diverticulum. After the vascular ring is released, the diverticulum is not observed to impinge on the esophagus; however, as a preventive measure, the surgeon sutures it to the anterior spinous ligament. This will prevent the diverticulum from enlarging and impinging on the esophagus as Ms. B grows to adulthood.
The surgery is completed without complications. Ms. B tolerates the procedure well.
Ten days after surgery, Ms. B is recovering well. She and her mother report satisfaction with the procedure to release the vascular ring. She discontinues her pain medication after 2 days and slowly begins reintroducing solid foods. Her fear of dysphagia and choking rapidly diminish.
Bottom Line
The diagnosis and management of eating disorders, including avoidant/restrictive food intake disorder and choking phobia, can be challenging. Furthermore, if an anatomical or organic anomaly is found, it is important to question how a patient’s eating disorder can be managed best through interdisciplinary collaboration between medical and behavioral specialties.
Related Resources
• Bryant-Waugh R. Feeding and eating disorders in children. Curr Opin Psychiatry. 2013;26(6):537-542.
• Norris ML, Robinson A, Obeid N, et al. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47(5):495-499.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health. 2013;53(2):303-305.
3. Fisher MM, Rosen DS, Ornstein RM, et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49-52.
4. Kelly NR, Shank LM, Bakalar JL, et al. Pediatric feeding and eating disorders: current state of diagnosis and treatment. Curr Psychiatry Rep. 2014;16(5):446.
5. Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7(2):106-109.
7. Calleja F, Eguaras M, Montero J, et al. Aberrant right subclavian artery associated with common carotid trunk. A rare cause of vascular ring. Eur J Cardiothorac Surg. 1990;4(10):568-570.
8. Jalaie H, Grommes J, Sailer A, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48(5):521-526.
9. Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532-534.
10. Morrris CD, Kanter KR, Miller JI Jr. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71(2):710-712.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health. 2013;53(2):303-305.
3. Fisher MM, Rosen DS, Ornstein RM, et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49-52.
4. Kelly NR, Shank LM, Bakalar JL, et al. Pediatric feeding and eating disorders: current state of diagnosis and treatment. Curr Psychiatry Rep. 2014;16(5):446.
5. Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7(2):106-109.
7. Calleja F, Eguaras M, Montero J, et al. Aberrant right subclavian artery associated with common carotid trunk. A rare cause of vascular ring. Eur J Cardiothorac Surg. 1990;4(10):568-570.
8. Jalaie H, Grommes J, Sailer A, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48(5):521-526.
9. Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532-534.
10. Morrris CD, Kanter KR, Miller JI Jr. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71(2):710-712.
A teen who is wasting away
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.