User login
Feeling disconnected? Focus on what you can do
This is the exciting time of year when we graduate new classes of medical students and residents. Med school graduation brings mixed emotions; the new doctors and I both know residency will bring growth and challenges. Residency graduation is a wistful passage as well. It is so rewarding to welcome the newly board-certified family physicians to family medicine, but we miss them even as we orient a new class.
Every year, a few months (or even a few years) after graduation, I hear from a former resident, sometimes several. They ask to talk and, although it can be hard for them to explain exactly the ennui and disillusionment they’re feeling, their concerns boil down to: Is this all there is?
They are not burnt out, exactly, but they were hoping for more from their careers in family medicine.1 They find their hopes and expectations are not fulfilled by seeing patients in the office 8 hours per day, 4.5 days per week. Even those who report rewarding relationships with patients express less overall enthusiasm for jobs they were excited to start just months or years earlier.
Some of the difficulties I hear the graduates report are expected growing pains. It is a transition to go from supervised practice with attending backup to a setting where you are on your own, typically with a 4-fold increase in volume compared with residency. But the monotony is real for family physicians in full-time outpatient practice.
Research suggests an expanded scope of practice—including hospital medicine, obstetrics, and procedures—is associated with physician well-being.2,3 A broad scope of practice can bring stress, but it also brings meaning, and that meaning is protective to our well-being. However, a robust scope of practice is not always supported by medical groups or hospital systems, who prefer a more compartmentalized, widgetized physician.4 It would be easier for their algorithms if family physicians picked a lane and stayed in it. Alas, the broader our scope of practice, the healthier our population, the more equitable our care,5,6 and the happier our physicians.
The disconnect and hopelessness experienced by family physicians is more concerning. Many of my graduates report feeling disconnected from their patients, because they begin to feel disillusioned by the demands and requests that practice and patients place on them. The paperwork, “permission slips,” and requests for tests and studies not only feel overwhelming and exhausting but also create distance between physicians and patients.7 We want to help our patients, so we do the forms and order the tests. As the quantity of forms, slips, and requests adds up, we begin to feel resentful at what the forms take away: time with our patients, perhaps, or time with our families. We get angry at the forms and the “asks,” and then begin to get angry at the patients simply for having needs. Administrative burden is a hassle, but it is also insidiously destructive.8
Family physicians confront hopelessness when, day after day, we diagnose problems that no physician is likely to fix in a single office visit: chronic stress, family dysfunction, violence, unemployment, poverty, racism, loneliness, and the hopelessness of the patients themselves. This is not to say that we ignore these concerns or their impact on health. It is because we see and feel them, and deeply understand their consequences for our patients, that we grow frustrated with the lack of solutions.9,10
Thankfully, we have strong teams working at the policy level to improve the primary care and public health infrastructure so that we can maintain some hope that it will be better in the future. Sometimes when I counsel a former resident, they decide to join those teams so that they can work on the solutions. Others decide to expand their scope of practice. Others seek out virtual scribes to streamline charting and regain time. Some build better boundaries with their EHR inboxes.
The key is figuring out what we can do and making peace with our limits. When disillusionment hits, what we can do includes seeking connection and social contact and remembering that we are not trapped in our situation, even if we are practicing in a less-than-functional health care system. There are many ways to “be” a family physician—if what you’re doing isn’t working for you, look for opportunities (big or small) that make it better. We can all reach out to coaches, therapists, colleagues, and friends for support to remain steadfast in our purpose as family physicians. This support and the power of change means that from residency to the latter parts of our careers, we will continue to bring the tremendous good of family medicine to the communities we serve.
1. Coutinho AJ, Cochrane A, Stelter K, et al. Comparison of intended scope of practice for family medicine residents with reported scope of practice among practicing family physicians. JAMA. 2015;314:2364-2372. doi: 10.1001/jama.2015.13734
2. Weidner AKH, Phillips RL, Fang B, et al. Burnout and scope of practice in new family physicians. Ann Fam Med. 2018;16:200-205. doi: 10.1370/afm.2221
3. Zomahoun HT, Samson I, Sawadogo J, et al. Effects of the scope of practice on family physicians: a systematic review. BMC Family Practice. 2021;22. doi: 10.1186/s12875-020-01328-1
4. Killeen D, Jetty A, Peterson LE, et al. The association of practice type and the scope of care of family physicians. J Am Board Fam Med. 2023;36:79-87. doi: 10.3122/jabfm.2022.220172R1
5. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502. doi: 10.1111/j.1468-0009.2005.00409.x
6. Ferrer RL. Pursuing equity: contact with primary care and specialist clinicians by demographics, insurance, and health status. Ann Fam Med. 2007;5:492-502. doi: 10.1370/afm.746
7. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92:237-243. doi: 10.1097/ACM.0000000000001461
8. McMahon LF, Rize K, Irby-Johnson N, et al. Designed to fail? The future of primary care. J Gen Intern Med. 2021;36:515-517. doi: 10.1007/s11606-020-06077-6
9. Welles CC, Tong A, Brereton E, et al. Sources of clinician burnout in providing care for underserved patients in a safety-net healthcare system. J Gen Intern Med. 2023;38:1468-1475. doi: 10.1007/s11606-022-07896-5
10. Kung A, Cheung T, Knox M, et al. Capacity to address social needs affects primary care clinician burnout. Ann Fam Med. 2019;17:487-494. doi: 10.1370/afm.2470
This is the exciting time of year when we graduate new classes of medical students and residents. Med school graduation brings mixed emotions; the new doctors and I both know residency will bring growth and challenges. Residency graduation is a wistful passage as well. It is so rewarding to welcome the newly board-certified family physicians to family medicine, but we miss them even as we orient a new class.
Every year, a few months (or even a few years) after graduation, I hear from a former resident, sometimes several. They ask to talk and, although it can be hard for them to explain exactly the ennui and disillusionment they’re feeling, their concerns boil down to: Is this all there is?
They are not burnt out, exactly, but they were hoping for more from their careers in family medicine.1 They find their hopes and expectations are not fulfilled by seeing patients in the office 8 hours per day, 4.5 days per week. Even those who report rewarding relationships with patients express less overall enthusiasm for jobs they were excited to start just months or years earlier.
Some of the difficulties I hear the graduates report are expected growing pains. It is a transition to go from supervised practice with attending backup to a setting where you are on your own, typically with a 4-fold increase in volume compared with residency. But the monotony is real for family physicians in full-time outpatient practice.
Research suggests an expanded scope of practice—including hospital medicine, obstetrics, and procedures—is associated with physician well-being.2,3 A broad scope of practice can bring stress, but it also brings meaning, and that meaning is protective to our well-being. However, a robust scope of practice is not always supported by medical groups or hospital systems, who prefer a more compartmentalized, widgetized physician.4 It would be easier for their algorithms if family physicians picked a lane and stayed in it. Alas, the broader our scope of practice, the healthier our population, the more equitable our care,5,6 and the happier our physicians.
The disconnect and hopelessness experienced by family physicians is more concerning. Many of my graduates report feeling disconnected from their patients, because they begin to feel disillusioned by the demands and requests that practice and patients place on them. The paperwork, “permission slips,” and requests for tests and studies not only feel overwhelming and exhausting but also create distance between physicians and patients.7 We want to help our patients, so we do the forms and order the tests. As the quantity of forms, slips, and requests adds up, we begin to feel resentful at what the forms take away: time with our patients, perhaps, or time with our families. We get angry at the forms and the “asks,” and then begin to get angry at the patients simply for having needs. Administrative burden is a hassle, but it is also insidiously destructive.8
Family physicians confront hopelessness when, day after day, we diagnose problems that no physician is likely to fix in a single office visit: chronic stress, family dysfunction, violence, unemployment, poverty, racism, loneliness, and the hopelessness of the patients themselves. This is not to say that we ignore these concerns or their impact on health. It is because we see and feel them, and deeply understand their consequences for our patients, that we grow frustrated with the lack of solutions.9,10
Thankfully, we have strong teams working at the policy level to improve the primary care and public health infrastructure so that we can maintain some hope that it will be better in the future. Sometimes when I counsel a former resident, they decide to join those teams so that they can work on the solutions. Others decide to expand their scope of practice. Others seek out virtual scribes to streamline charting and regain time. Some build better boundaries with their EHR inboxes.
The key is figuring out what we can do and making peace with our limits. When disillusionment hits, what we can do includes seeking connection and social contact and remembering that we are not trapped in our situation, even if we are practicing in a less-than-functional health care system. There are many ways to “be” a family physician—if what you’re doing isn’t working for you, look for opportunities (big or small) that make it better. We can all reach out to coaches, therapists, colleagues, and friends for support to remain steadfast in our purpose as family physicians. This support and the power of change means that from residency to the latter parts of our careers, we will continue to bring the tremendous good of family medicine to the communities we serve.
This is the exciting time of year when we graduate new classes of medical students and residents. Med school graduation brings mixed emotions; the new doctors and I both know residency will bring growth and challenges. Residency graduation is a wistful passage as well. It is so rewarding to welcome the newly board-certified family physicians to family medicine, but we miss them even as we orient a new class.
Every year, a few months (or even a few years) after graduation, I hear from a former resident, sometimes several. They ask to talk and, although it can be hard for them to explain exactly the ennui and disillusionment they’re feeling, their concerns boil down to: Is this all there is?
They are not burnt out, exactly, but they were hoping for more from their careers in family medicine.1 They find their hopes and expectations are not fulfilled by seeing patients in the office 8 hours per day, 4.5 days per week. Even those who report rewarding relationships with patients express less overall enthusiasm for jobs they were excited to start just months or years earlier.
Some of the difficulties I hear the graduates report are expected growing pains. It is a transition to go from supervised practice with attending backup to a setting where you are on your own, typically with a 4-fold increase in volume compared with residency. But the monotony is real for family physicians in full-time outpatient practice.
Research suggests an expanded scope of practice—including hospital medicine, obstetrics, and procedures—is associated with physician well-being.2,3 A broad scope of practice can bring stress, but it also brings meaning, and that meaning is protective to our well-being. However, a robust scope of practice is not always supported by medical groups or hospital systems, who prefer a more compartmentalized, widgetized physician.4 It would be easier for their algorithms if family physicians picked a lane and stayed in it. Alas, the broader our scope of practice, the healthier our population, the more equitable our care,5,6 and the happier our physicians.
The disconnect and hopelessness experienced by family physicians is more concerning. Many of my graduates report feeling disconnected from their patients, because they begin to feel disillusioned by the demands and requests that practice and patients place on them. The paperwork, “permission slips,” and requests for tests and studies not only feel overwhelming and exhausting but also create distance between physicians and patients.7 We want to help our patients, so we do the forms and order the tests. As the quantity of forms, slips, and requests adds up, we begin to feel resentful at what the forms take away: time with our patients, perhaps, or time with our families. We get angry at the forms and the “asks,” and then begin to get angry at the patients simply for having needs. Administrative burden is a hassle, but it is also insidiously destructive.8
Family physicians confront hopelessness when, day after day, we diagnose problems that no physician is likely to fix in a single office visit: chronic stress, family dysfunction, violence, unemployment, poverty, racism, loneliness, and the hopelessness of the patients themselves. This is not to say that we ignore these concerns or their impact on health. It is because we see and feel them, and deeply understand their consequences for our patients, that we grow frustrated with the lack of solutions.9,10
Thankfully, we have strong teams working at the policy level to improve the primary care and public health infrastructure so that we can maintain some hope that it will be better in the future. Sometimes when I counsel a former resident, they decide to join those teams so that they can work on the solutions. Others decide to expand their scope of practice. Others seek out virtual scribes to streamline charting and regain time. Some build better boundaries with their EHR inboxes.
The key is figuring out what we can do and making peace with our limits. When disillusionment hits, what we can do includes seeking connection and social contact and remembering that we are not trapped in our situation, even if we are practicing in a less-than-functional health care system. There are many ways to “be” a family physician—if what you’re doing isn’t working for you, look for opportunities (big or small) that make it better. We can all reach out to coaches, therapists, colleagues, and friends for support to remain steadfast in our purpose as family physicians. This support and the power of change means that from residency to the latter parts of our careers, we will continue to bring the tremendous good of family medicine to the communities we serve.
1. Coutinho AJ, Cochrane A, Stelter K, et al. Comparison of intended scope of practice for family medicine residents with reported scope of practice among practicing family physicians. JAMA. 2015;314:2364-2372. doi: 10.1001/jama.2015.13734
2. Weidner AKH, Phillips RL, Fang B, et al. Burnout and scope of practice in new family physicians. Ann Fam Med. 2018;16:200-205. doi: 10.1370/afm.2221
3. Zomahoun HT, Samson I, Sawadogo J, et al. Effects of the scope of practice on family physicians: a systematic review. BMC Family Practice. 2021;22. doi: 10.1186/s12875-020-01328-1
4. Killeen D, Jetty A, Peterson LE, et al. The association of practice type and the scope of care of family physicians. J Am Board Fam Med. 2023;36:79-87. doi: 10.3122/jabfm.2022.220172R1
5. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502. doi: 10.1111/j.1468-0009.2005.00409.x
6. Ferrer RL. Pursuing equity: contact with primary care and specialist clinicians by demographics, insurance, and health status. Ann Fam Med. 2007;5:492-502. doi: 10.1370/afm.746
7. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92:237-243. doi: 10.1097/ACM.0000000000001461
8. McMahon LF, Rize K, Irby-Johnson N, et al. Designed to fail? The future of primary care. J Gen Intern Med. 2021;36:515-517. doi: 10.1007/s11606-020-06077-6
9. Welles CC, Tong A, Brereton E, et al. Sources of clinician burnout in providing care for underserved patients in a safety-net healthcare system. J Gen Intern Med. 2023;38:1468-1475. doi: 10.1007/s11606-022-07896-5
10. Kung A, Cheung T, Knox M, et al. Capacity to address social needs affects primary care clinician burnout. Ann Fam Med. 2019;17:487-494. doi: 10.1370/afm.2470
1. Coutinho AJ, Cochrane A, Stelter K, et al. Comparison of intended scope of practice for family medicine residents with reported scope of practice among practicing family physicians. JAMA. 2015;314:2364-2372. doi: 10.1001/jama.2015.13734
2. Weidner AKH, Phillips RL, Fang B, et al. Burnout and scope of practice in new family physicians. Ann Fam Med. 2018;16:200-205. doi: 10.1370/afm.2221
3. Zomahoun HT, Samson I, Sawadogo J, et al. Effects of the scope of practice on family physicians: a systematic review. BMC Family Practice. 2021;22. doi: 10.1186/s12875-020-01328-1
4. Killeen D, Jetty A, Peterson LE, et al. The association of practice type and the scope of care of family physicians. J Am Board Fam Med. 2023;36:79-87. doi: 10.3122/jabfm.2022.220172R1
5. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502. doi: 10.1111/j.1468-0009.2005.00409.x
6. Ferrer RL. Pursuing equity: contact with primary care and specialist clinicians by demographics, insurance, and health status. Ann Fam Med. 2007;5:492-502. doi: 10.1370/afm.746
7. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92:237-243. doi: 10.1097/ACM.0000000000001461
8. McMahon LF, Rize K, Irby-Johnson N, et al. Designed to fail? The future of primary care. J Gen Intern Med. 2021;36:515-517. doi: 10.1007/s11606-020-06077-6
9. Welles CC, Tong A, Brereton E, et al. Sources of clinician burnout in providing care for underserved patients in a safety-net healthcare system. J Gen Intern Med. 2023;38:1468-1475. doi: 10.1007/s11606-022-07896-5
10. Kung A, Cheung T, Knox M, et al. Capacity to address social needs affects primary care clinician burnout. Ann Fam Med. 2019;17:487-494. doi: 10.1370/afm.2470
Pedunculated gluteal mass
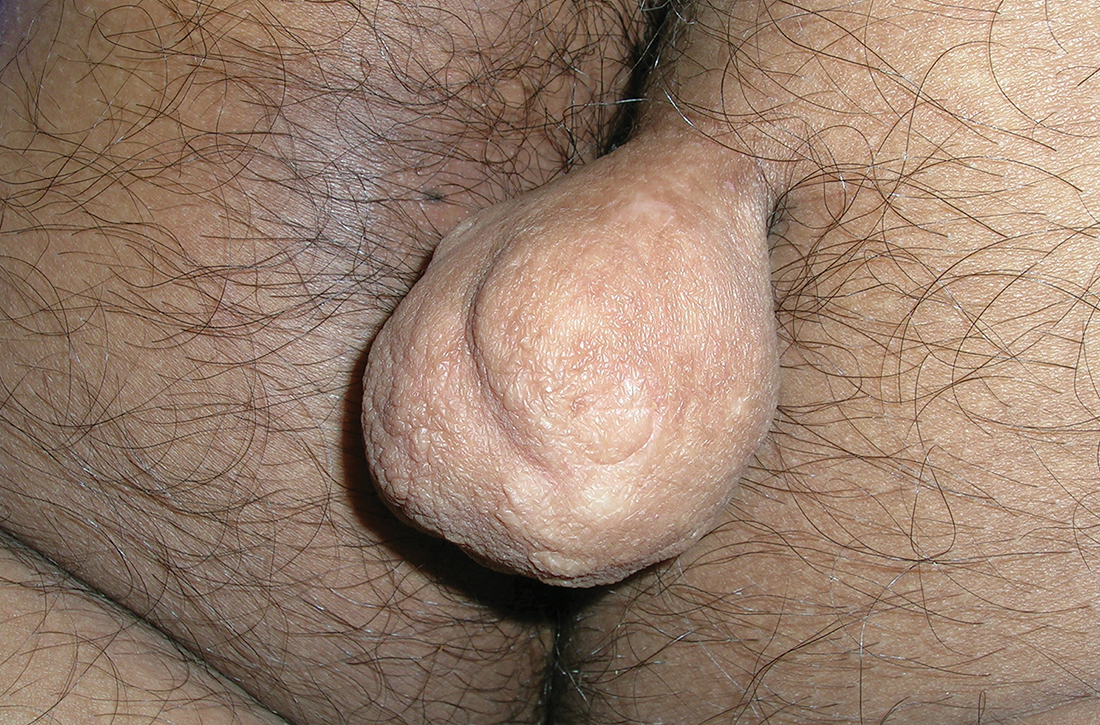
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.
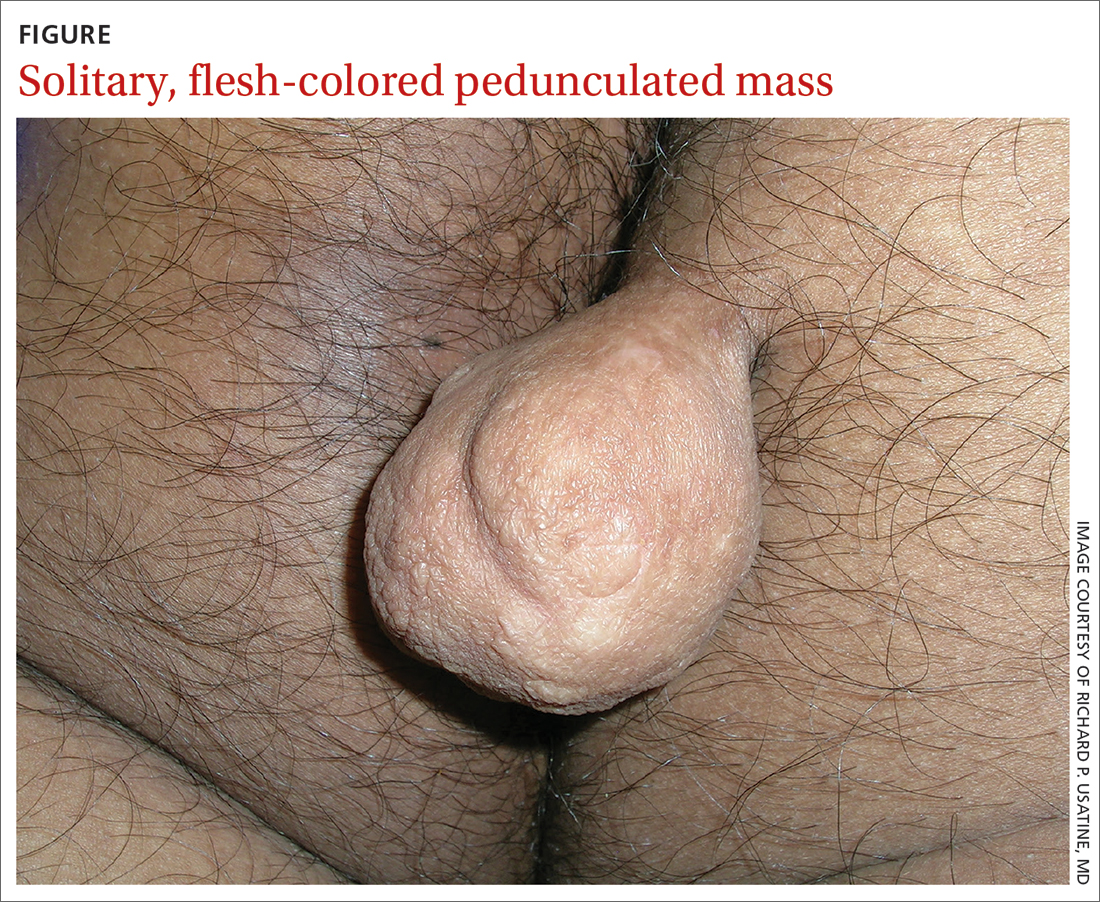
On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.

On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.

On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
64-year-old woman • hot flashes, facial flushing, excessive sweating, and palpitations • daily headaches • history of hypertension • Dx?
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.
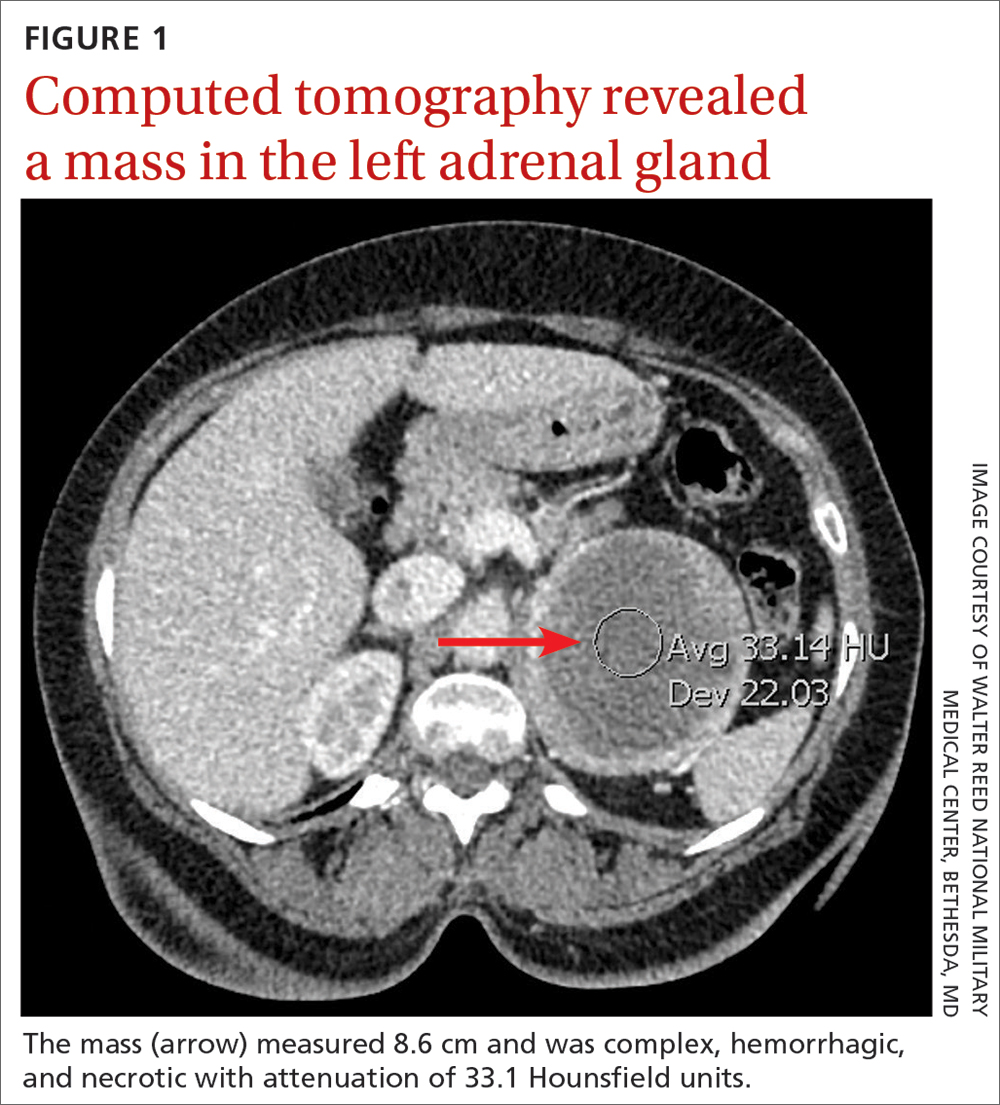
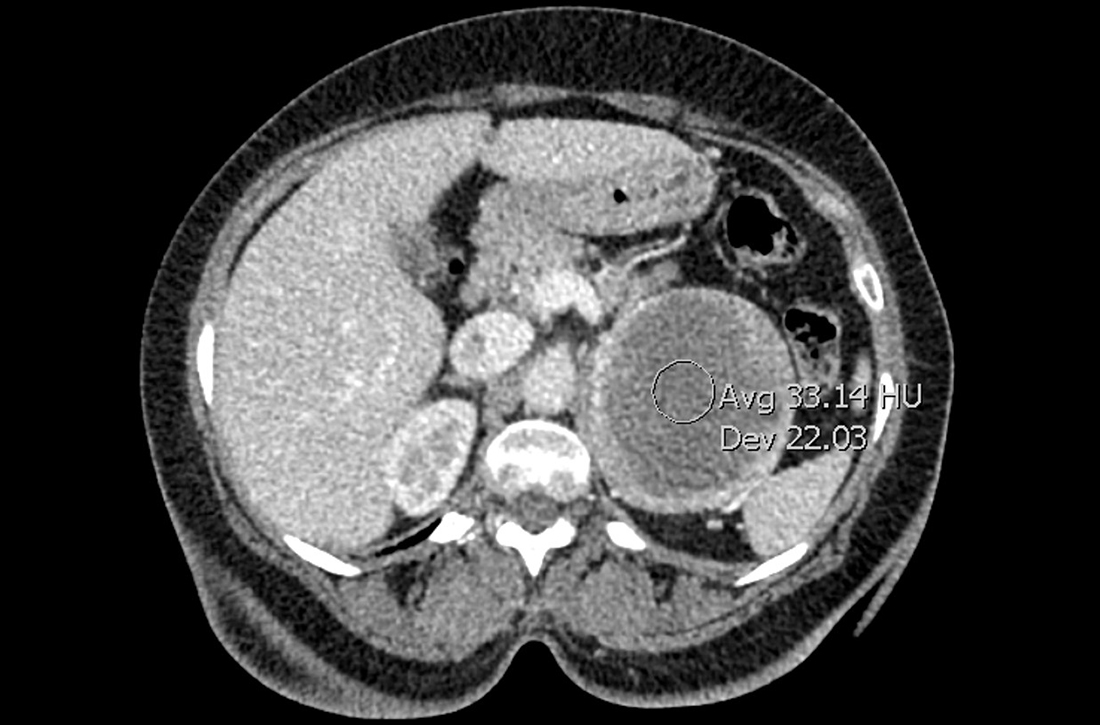
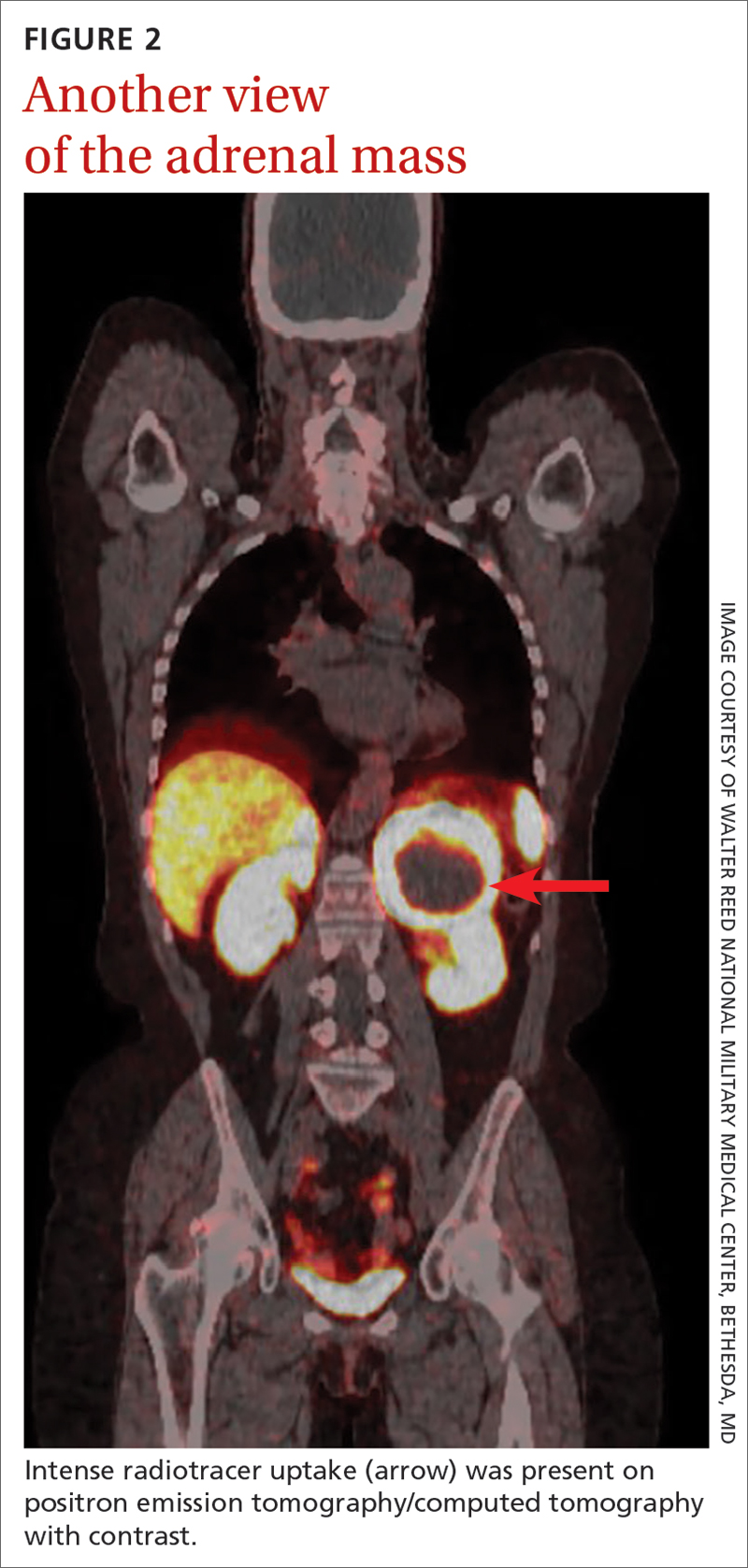
Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).
THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
► Hot flashes, facial flushing, excessive sweating, and palpitations
► Daily headaches
► History of hypertension
Acute Achilles tendon rupture: Skip the surgery?
ILLUSTRATIVE CASE
An otherwise healthy 45-year-old man sustained an acute right-side Achilles tendon rupture while playing tennis. He has not taken quinolones recently, has no history of previous Achilles tendon rupture, and prior to this injury had no difficulty walking. He presents initially to his primary care physician and wants advice: Does he need surgery?
Acute Achilles tendon rupture manifests as acute-onset pain and impaired plantar flexion.2 Older, active, male patients are at increased risk. There is disagreement among treating physicians regarding best practices for managing this common and debilitating injury. Prior clinical trials comparing operative to nonoperative management, as well as those comparing different surgical techniques, were limited by small sample sizes.3-5
A 2019 systematic review and meta-analysis that relied heavily on observational data suggested that nonoperative management carries greater risk for rerupture but lower risk for complications than surgical treatment, without differences in patient-reported functional outcomes.5 This 2022 RCT adds certainty to comparisons of surgical and nonoperative treatment.
STUDY SUMMARY
Equivalent outcomes but higher rates of rerupture for nonoperative patients
Norwegian investigators conducted a prospective, single-blind RCT at 4 treating facilities among patients ages 18 to 60 years with unilateral acute Achilles tendon rupture. A total of 554 patients were randomized in a 1:1:1 ratio to 1 of 3 groups: nonoperative treatment, open-repair surgery, or minimally invasive surgery. Ultimately, 526 patients who completed the intervention and at least 1 follow-up survey were included in the final analysis, which exceeded the number needed according to the pre-study 80% power calculation. Seventy-four percent of the patients were male, and the average age at time of injury was 40 years. Nearly all patients were classified as healthy or having only mild or well-controlled chronic illnesses.
Before randomization, patients completed the 10-item Achilles tendon Total Rupture Score (ATRS) questionnaire to gauge their pre-injury baseline function. ATRS is scored 0 to 100, with lower scores indicating more limitation in function; a clinically important difference is 8 to 10 points. There were no statistically significant differences in pre-injury baseline ATRS (92.7, 93.9, and 94.2 for the nonoperative, open-repair, and minimally invasive groups, respectively) or other patient characteristics among the 3 groups.
For all participants, application of a below-the-knee equinus cast with plantar flexion was performed within 72 hours after the injury. Patients in the surgical arms had surgery within 8 days, followed by application of a new cast. For all study groups, the cast was maintained for a total of 2 weeks, followed by 6 weeks of weight-bearing in an ankle-foot orthosis with heel wedges that were gradually reduced in number. All patients were treated with identical serial immobilization and physical therapy programs for 36 weeks.
The primary study outcome was change from baseline ATRS at 12 months after injury. Secondary outcomes included ATRS at 3 and 6 months and domain-specific quality-of-life scores (from the 36-Item Short Form Health Survey; SF-36) at 6 and 12 months. Patients also underwent physical testing of their Achilles tendon function at 6 and 12 months, during which they wore knee-high socks in order to blind the evaluators. Reruptures were recorded as secondary outcomes as well.
Continue to: There were no significant...
There were no significant differences between groups in the primary outcome. The mean changes in ATRS were −2.6 points (95% CI, −6.5 to 2.0) for nonoperative treatment compared with minimally invasive surgery, and 1.0 point (95% CI, −5.2 to 3.1) for nonoperative treatment compared with open repair.
All groups had similar secondary self-reported ATRS at 3 and 6 months and SF-36 scores at 6 and 12 months. Blinded physical test results also were similar between groups at 6 and 12 months.
Tendon rerupture within 12 months was more common in the nonoperative arm than in the 2 surgical arms (6.2% vs 0.6% in both operative groups; 5.6% difference; 95% CI for difference, 1.9-10.2 for open repair and 1.8-10.2 for minimally invasive surgery). Risk for nerve injury was higher in both the minimally invasive surgery group (5.2%) and the open-repair surgery group (2.8%) compared with the nonoperative group (0.6%; no P value given for comparison).
WHAT’S NEW
Largest RCT to date showed effectiveness of nonoperative Tx
This study is the largest well-powered and rigorously conducted RCT to show that nonoperative management of acute Achilles tendon rupture offers equivalent patient-reported outcomes at 12 months after injury. Nonoperative management was associated with a lower risk for nerve injury but higher risk for tendon rerupture.
These findings support previous studies on the topic. As previously mentioned, a 2019 systematic review and meta-analysis of 10 RCTs (N = 944) and 19 observational studies (N = 14,918) examined operative compared with nonoperative treatment of acute Achilles tendon rupture and found a lower rerupture rate in the operative group but a higher complication rate.5 An underpowered 2010 RCT (N = 97) of operative vs nonoperative treatment of acute Achilles tendon rupture found no statistical difference in ATRS.3 Another underpowered RCT conducted in 2013 (N = 100) compared surgical treatment, accelerated rehabilitation, and nonsurgical treatment in acute Achilles tendon rupture and found no statistical difference in ATRS.4
CAVEATS
Study results may not apply to some patient groups
These findings may not apply to patients older than 60 years, who were excluded from this RCT, or patients with debilitation or significant chronic disease. Patients with prior Achilles rupture also were excluded.
The study population in Norway, which is more physically active than nearby countries, may not be generalizable worldwide.6 Patients wishing to minimize the risk for rerupture may still prefer to have surgery after acute Achilles tendon rupture.
CHALLENGES TO IMPLEMENTATION
Potentially limited options for patients
Most patients with acute Achilles tendon rupture are evaluated by orthopedic surgeons, who may or may not offer nonoperative management. Availability of practitioners to provide serial casting, appropriate heel wedges, and rehabilitation may vary regionally. All patients in this study were evaluated within 72 hours of injury; these findings may not be applicable for patients at a longer time since injury.
1. Myhrvold SB, Brouwer EF, Andresen TKM, et al. Nonoperative or surgical treatment of acute Achilles’ tendon rupture. N Engl J Med. 2022;386:1409-1420. doi: 10.1056/NEJMoa2108447
2. Huttunen TT, Kannus P, Rolf C, et al. Acute achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42:2419-2423. doi: 10.1177/0363546514540599
3. Nilsson-Helander K, Silbernagel KG, Thomeé R, et al. Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38:2186-2193. doi: 10.1177/0363546510376052
4. Olsson N, Silbernagel KG, Eriksson BI, et al. Stable surgical repair with accelerated rehabilitation versus nonsurgical treatment for acute Achilles tendon ruptures: a randomized controlled study. Am J Sports Med. 2013;41:2867-2876. doi: 10.1177/0363546513503282
5. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120
6. Urbaniak-Brekke AM, Pluta B, Krzykała M, et al. Physical activity of Polish and Norwegian local communities in the context of self-government authorities’ projects. Int J Environ Res Public Health. 2019;16:1710. doi: 10.3390/ijerph16101710
ILLUSTRATIVE CASE
An otherwise healthy 45-year-old man sustained an acute right-side Achilles tendon rupture while playing tennis. He has not taken quinolones recently, has no history of previous Achilles tendon rupture, and prior to this injury had no difficulty walking. He presents initially to his primary care physician and wants advice: Does he need surgery?
Acute Achilles tendon rupture manifests as acute-onset pain and impaired plantar flexion.2 Older, active, male patients are at increased risk. There is disagreement among treating physicians regarding best practices for managing this common and debilitating injury. Prior clinical trials comparing operative to nonoperative management, as well as those comparing different surgical techniques, were limited by small sample sizes.3-5
A 2019 systematic review and meta-analysis that relied heavily on observational data suggested that nonoperative management carries greater risk for rerupture but lower risk for complications than surgical treatment, without differences in patient-reported functional outcomes.5 This 2022 RCT adds certainty to comparisons of surgical and nonoperative treatment.
STUDY SUMMARY
Equivalent outcomes but higher rates of rerupture for nonoperative patients
Norwegian investigators conducted a prospective, single-blind RCT at 4 treating facilities among patients ages 18 to 60 years with unilateral acute Achilles tendon rupture. A total of 554 patients were randomized in a 1:1:1 ratio to 1 of 3 groups: nonoperative treatment, open-repair surgery, or minimally invasive surgery. Ultimately, 526 patients who completed the intervention and at least 1 follow-up survey were included in the final analysis, which exceeded the number needed according to the pre-study 80% power calculation. Seventy-four percent of the patients were male, and the average age at time of injury was 40 years. Nearly all patients were classified as healthy or having only mild or well-controlled chronic illnesses.
Before randomization, patients completed the 10-item Achilles tendon Total Rupture Score (ATRS) questionnaire to gauge their pre-injury baseline function. ATRS is scored 0 to 100, with lower scores indicating more limitation in function; a clinically important difference is 8 to 10 points. There were no statistically significant differences in pre-injury baseline ATRS (92.7, 93.9, and 94.2 for the nonoperative, open-repair, and minimally invasive groups, respectively) or other patient characteristics among the 3 groups.
For all participants, application of a below-the-knee equinus cast with plantar flexion was performed within 72 hours after the injury. Patients in the surgical arms had surgery within 8 days, followed by application of a new cast. For all study groups, the cast was maintained for a total of 2 weeks, followed by 6 weeks of weight-bearing in an ankle-foot orthosis with heel wedges that were gradually reduced in number. All patients were treated with identical serial immobilization and physical therapy programs for 36 weeks.
The primary study outcome was change from baseline ATRS at 12 months after injury. Secondary outcomes included ATRS at 3 and 6 months and domain-specific quality-of-life scores (from the 36-Item Short Form Health Survey; SF-36) at 6 and 12 months. Patients also underwent physical testing of their Achilles tendon function at 6 and 12 months, during which they wore knee-high socks in order to blind the evaluators. Reruptures were recorded as secondary outcomes as well.
Continue to: There were no significant...
There were no significant differences between groups in the primary outcome. The mean changes in ATRS were −2.6 points (95% CI, −6.5 to 2.0) for nonoperative treatment compared with minimally invasive surgery, and 1.0 point (95% CI, −5.2 to 3.1) for nonoperative treatment compared with open repair.
All groups had similar secondary self-reported ATRS at 3 and 6 months and SF-36 scores at 6 and 12 months. Blinded physical test results also were similar between groups at 6 and 12 months.
Tendon rerupture within 12 months was more common in the nonoperative arm than in the 2 surgical arms (6.2% vs 0.6% in both operative groups; 5.6% difference; 95% CI for difference, 1.9-10.2 for open repair and 1.8-10.2 for minimally invasive surgery). Risk for nerve injury was higher in both the minimally invasive surgery group (5.2%) and the open-repair surgery group (2.8%) compared with the nonoperative group (0.6%; no P value given for comparison).
WHAT’S NEW
Largest RCT to date showed effectiveness of nonoperative Tx
This study is the largest well-powered and rigorously conducted RCT to show that nonoperative management of acute Achilles tendon rupture offers equivalent patient-reported outcomes at 12 months after injury. Nonoperative management was associated with a lower risk for nerve injury but higher risk for tendon rerupture.
These findings support previous studies on the topic. As previously mentioned, a 2019 systematic review and meta-analysis of 10 RCTs (N = 944) and 19 observational studies (N = 14,918) examined operative compared with nonoperative treatment of acute Achilles tendon rupture and found a lower rerupture rate in the operative group but a higher complication rate.5 An underpowered 2010 RCT (N = 97) of operative vs nonoperative treatment of acute Achilles tendon rupture found no statistical difference in ATRS.3 Another underpowered RCT conducted in 2013 (N = 100) compared surgical treatment, accelerated rehabilitation, and nonsurgical treatment in acute Achilles tendon rupture and found no statistical difference in ATRS.4
CAVEATS
Study results may not apply to some patient groups
These findings may not apply to patients older than 60 years, who were excluded from this RCT, or patients with debilitation or significant chronic disease. Patients with prior Achilles rupture also were excluded.
The study population in Norway, which is more physically active than nearby countries, may not be generalizable worldwide.6 Patients wishing to minimize the risk for rerupture may still prefer to have surgery after acute Achilles tendon rupture.
CHALLENGES TO IMPLEMENTATION
Potentially limited options for patients
Most patients with acute Achilles tendon rupture are evaluated by orthopedic surgeons, who may or may not offer nonoperative management. Availability of practitioners to provide serial casting, appropriate heel wedges, and rehabilitation may vary regionally. All patients in this study were evaluated within 72 hours of injury; these findings may not be applicable for patients at a longer time since injury.
ILLUSTRATIVE CASE
An otherwise healthy 45-year-old man sustained an acute right-side Achilles tendon rupture while playing tennis. He has not taken quinolones recently, has no history of previous Achilles tendon rupture, and prior to this injury had no difficulty walking. He presents initially to his primary care physician and wants advice: Does he need surgery?
Acute Achilles tendon rupture manifests as acute-onset pain and impaired plantar flexion.2 Older, active, male patients are at increased risk. There is disagreement among treating physicians regarding best practices for managing this common and debilitating injury. Prior clinical trials comparing operative to nonoperative management, as well as those comparing different surgical techniques, were limited by small sample sizes.3-5
A 2019 systematic review and meta-analysis that relied heavily on observational data suggested that nonoperative management carries greater risk for rerupture but lower risk for complications than surgical treatment, without differences in patient-reported functional outcomes.5 This 2022 RCT adds certainty to comparisons of surgical and nonoperative treatment.
STUDY SUMMARY
Equivalent outcomes but higher rates of rerupture for nonoperative patients
Norwegian investigators conducted a prospective, single-blind RCT at 4 treating facilities among patients ages 18 to 60 years with unilateral acute Achilles tendon rupture. A total of 554 patients were randomized in a 1:1:1 ratio to 1 of 3 groups: nonoperative treatment, open-repair surgery, or minimally invasive surgery. Ultimately, 526 patients who completed the intervention and at least 1 follow-up survey were included in the final analysis, which exceeded the number needed according to the pre-study 80% power calculation. Seventy-four percent of the patients were male, and the average age at time of injury was 40 years. Nearly all patients were classified as healthy or having only mild or well-controlled chronic illnesses.
Before randomization, patients completed the 10-item Achilles tendon Total Rupture Score (ATRS) questionnaire to gauge their pre-injury baseline function. ATRS is scored 0 to 100, with lower scores indicating more limitation in function; a clinically important difference is 8 to 10 points. There were no statistically significant differences in pre-injury baseline ATRS (92.7, 93.9, and 94.2 for the nonoperative, open-repair, and minimally invasive groups, respectively) or other patient characteristics among the 3 groups.
For all participants, application of a below-the-knee equinus cast with plantar flexion was performed within 72 hours after the injury. Patients in the surgical arms had surgery within 8 days, followed by application of a new cast. For all study groups, the cast was maintained for a total of 2 weeks, followed by 6 weeks of weight-bearing in an ankle-foot orthosis with heel wedges that were gradually reduced in number. All patients were treated with identical serial immobilization and physical therapy programs for 36 weeks.
The primary study outcome was change from baseline ATRS at 12 months after injury. Secondary outcomes included ATRS at 3 and 6 months and domain-specific quality-of-life scores (from the 36-Item Short Form Health Survey; SF-36) at 6 and 12 months. Patients also underwent physical testing of their Achilles tendon function at 6 and 12 months, during which they wore knee-high socks in order to blind the evaluators. Reruptures were recorded as secondary outcomes as well.
Continue to: There were no significant...
There were no significant differences between groups in the primary outcome. The mean changes in ATRS were −2.6 points (95% CI, −6.5 to 2.0) for nonoperative treatment compared with minimally invasive surgery, and 1.0 point (95% CI, −5.2 to 3.1) for nonoperative treatment compared with open repair.
All groups had similar secondary self-reported ATRS at 3 and 6 months and SF-36 scores at 6 and 12 months. Blinded physical test results also were similar between groups at 6 and 12 months.
Tendon rerupture within 12 months was more common in the nonoperative arm than in the 2 surgical arms (6.2% vs 0.6% in both operative groups; 5.6% difference; 95% CI for difference, 1.9-10.2 for open repair and 1.8-10.2 for minimally invasive surgery). Risk for nerve injury was higher in both the minimally invasive surgery group (5.2%) and the open-repair surgery group (2.8%) compared with the nonoperative group (0.6%; no P value given for comparison).
WHAT’S NEW
Largest RCT to date showed effectiveness of nonoperative Tx
This study is the largest well-powered and rigorously conducted RCT to show that nonoperative management of acute Achilles tendon rupture offers equivalent patient-reported outcomes at 12 months after injury. Nonoperative management was associated with a lower risk for nerve injury but higher risk for tendon rerupture.
These findings support previous studies on the topic. As previously mentioned, a 2019 systematic review and meta-analysis of 10 RCTs (N = 944) and 19 observational studies (N = 14,918) examined operative compared with nonoperative treatment of acute Achilles tendon rupture and found a lower rerupture rate in the operative group but a higher complication rate.5 An underpowered 2010 RCT (N = 97) of operative vs nonoperative treatment of acute Achilles tendon rupture found no statistical difference in ATRS.3 Another underpowered RCT conducted in 2013 (N = 100) compared surgical treatment, accelerated rehabilitation, and nonsurgical treatment in acute Achilles tendon rupture and found no statistical difference in ATRS.4
CAVEATS
Study results may not apply to some patient groups
These findings may not apply to patients older than 60 years, who were excluded from this RCT, or patients with debilitation or significant chronic disease. Patients with prior Achilles rupture also were excluded.
The study population in Norway, which is more physically active than nearby countries, may not be generalizable worldwide.6 Patients wishing to minimize the risk for rerupture may still prefer to have surgery after acute Achilles tendon rupture.
CHALLENGES TO IMPLEMENTATION
Potentially limited options for patients
Most patients with acute Achilles tendon rupture are evaluated by orthopedic surgeons, who may or may not offer nonoperative management. Availability of practitioners to provide serial casting, appropriate heel wedges, and rehabilitation may vary regionally. All patients in this study were evaluated within 72 hours of injury; these findings may not be applicable for patients at a longer time since injury.
1. Myhrvold SB, Brouwer EF, Andresen TKM, et al. Nonoperative or surgical treatment of acute Achilles’ tendon rupture. N Engl J Med. 2022;386:1409-1420. doi: 10.1056/NEJMoa2108447
2. Huttunen TT, Kannus P, Rolf C, et al. Acute achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42:2419-2423. doi: 10.1177/0363546514540599
3. Nilsson-Helander K, Silbernagel KG, Thomeé R, et al. Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38:2186-2193. doi: 10.1177/0363546510376052
4. Olsson N, Silbernagel KG, Eriksson BI, et al. Stable surgical repair with accelerated rehabilitation versus nonsurgical treatment for acute Achilles tendon ruptures: a randomized controlled study. Am J Sports Med. 2013;41:2867-2876. doi: 10.1177/0363546513503282
5. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120
6. Urbaniak-Brekke AM, Pluta B, Krzykała M, et al. Physical activity of Polish and Norwegian local communities in the context of self-government authorities’ projects. Int J Environ Res Public Health. 2019;16:1710. doi: 10.3390/ijerph16101710
1. Myhrvold SB, Brouwer EF, Andresen TKM, et al. Nonoperative or surgical treatment of acute Achilles’ tendon rupture. N Engl J Med. 2022;386:1409-1420. doi: 10.1056/NEJMoa2108447
2. Huttunen TT, Kannus P, Rolf C, et al. Acute achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42:2419-2423. doi: 10.1177/0363546514540599
3. Nilsson-Helander K, Silbernagel KG, Thomeé R, et al. Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38:2186-2193. doi: 10.1177/0363546510376052
4. Olsson N, Silbernagel KG, Eriksson BI, et al. Stable surgical repair with accelerated rehabilitation versus nonsurgical treatment for acute Achilles tendon ruptures: a randomized controlled study. Am J Sports Med. 2013;41:2867-2876. doi: 10.1177/0363546513503282
5. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120
6. Urbaniak-Brekke AM, Pluta B, Krzykała M, et al. Physical activity of Polish and Norwegian local communities in the context of self-government authorities’ projects. Int J Environ Res Public Health. 2019;16:1710. doi: 10.3390/ijerph16101710
PRACTICE CHANGER
For healthy patients ages 18 to 60 years with acute Achilles tendon rupture, consider nonoperative immobilization, which offered a benefit in function comparable to open-repair or minimally invasive surgery in this randomized controlled trial (RCT).
STRENGTH OF RECOMMENDATION
B: Based on a single RCT.1
Myhrvold SB, Brouwer EF, Andresen TKM, et al. Nonoperative or surgical treatment of acute Achilles’ tendon rupture. N Engl J Med. 2022;386:1409-1420. doi: 10.1056/NEJMoa2108447
Caring for the caregiver in dementia
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-
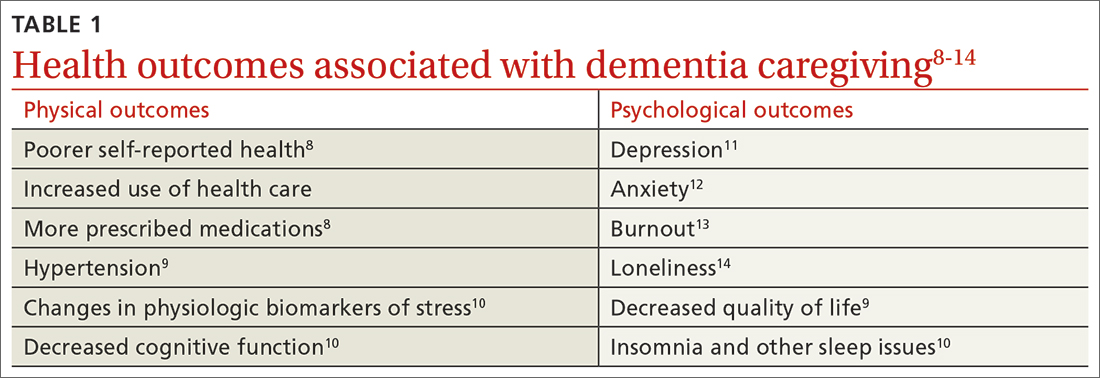
Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17
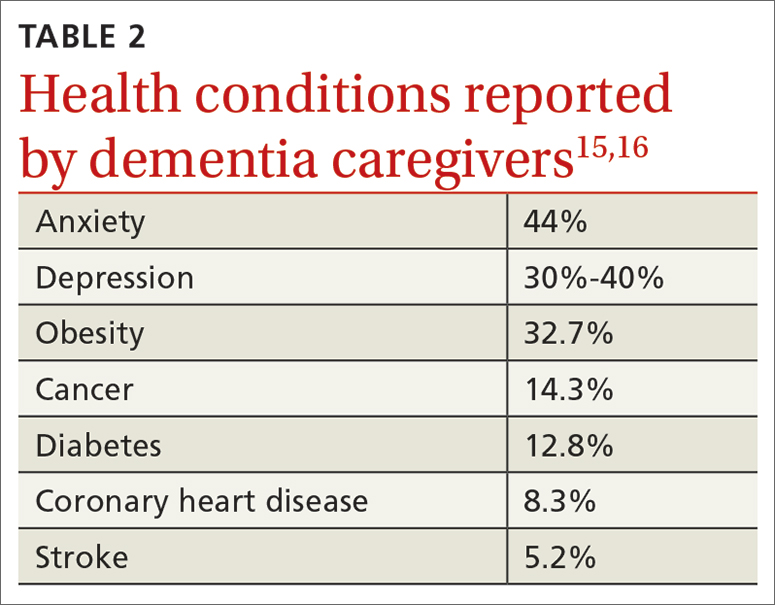
Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; [email protected]
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-

Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17

Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; [email protected]
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-

Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17

Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; [email protected]
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
How telehealth can work best for our patients
Social distancing measures instituted during the COVID-19 pandemic challenged the usual way of operating in primary care. To continue delivering medical services, physicians had to transition quickly to forms of remote interaction with patients. Use of technology appeared to be the answer. And it gave clinicians the ability to do what many had long hoped for: offer patients the option of telehealth.
The terms telemedicine and telehealth have similar definitions and are commonly used interchangeably. We think most practices probably would have adopted telehealth earlier were it not for reimbursement barriers. In this article, we adopt the World Health Organization’s definition of telemedicine as: “The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.”1
To provide family medicine clinicians with evidence-based recommendations about telehealth, we conducted a critical review of the literature published through April 30, 2021. The scope of this review includes studies found using the PubMed and Google Scholar databases. In addition, we used the keywords “telehealth,” “telemedicine,” “family medicine,” and “primary care.” We divided this review into 6 sections, including focus areas on implementation in primary care, remote diagnostic accuracy, conditions lending themselves to telehealth, physician and patient perceptions, disparities in telehealth, and finally, the conclusions.
Telehealth implementation in primary care
Telehealth in various forms had been around for years before the pandemic, mainly in the form of commercial telehealth businesses. Telehealth was being used in rural and remote areas where it could be difficult to see a primary care provider—let alone a specialist. The family medicine department of the University of Colorado was an early adopter of telehealth and had navigated this transition since 2017, with clinical champions guiding the process. By 2019, 54% of their clinicians were conducting telehealth encounters.2
However, telehealth implementation elsewhere was not accepted so readily. Before the pandemic, a cross-sectional study of more than 1.1 million patients in Northern California showed that 86% preferred in-person care over video.3 Even as the pandemic began and social distancing measures were implemented, a quality improvement project at a family medicine residency clinic in Florida documented that clinicians still preferred telephone interviews despite the capacity for video visits.4 And many primary care systems were simply unprepared to adopt telehealth technologies.
With time, however, family physicians began to improvise using popular videoconferencing technologies (eg, Zoom) that were readily available and familiar to patients, and medical centers began to repurpose their existing videoconferencing systems.5 The Ohio State University Wexner Medical Center launched a virtual health initiative just before the pandemic struck, at which time fewer than 5% of patient visits were conducted through telehealth. Weeks later, nearly 93% of patient visits were offered through telehealth.6
Reimbursement. Another significant impediment to early telehealth uptake was the late reaction by the Centers for Medicare and Medicaid Services (CMS) in changing the payment system. Hectic expansion of telehealth in response to the crisis pointed to the lack of policies that supported primary care with payments based on outcomes rather than fee-for-service models.7 By the end of April 2020, CMS finally announced that video visits would be reimbursed at the same rate as in-person visits. However, telephone-only visits are still very limited in coverage, and appropriate codes should be verified with payers.
Continue to: Remote diagnosis comes with a caveat
Remote diagnosis comes with a caveat
Some primary care practices have found that images of skin lesions submitted by patients (usually by cell phone) suffice for accurate diagnosis in lieu of office visits.8 With chronic conditions, home-based remote monitoring of vital signs may assist in diagnosing and managing acute issues. More efficient triage of patients is increasingly possible with the receipt of still images or video files of concerning lesions (eg, burns, rash, chronic wounds) sent from smartphones alone9,10 or with devices attached to smartphones (eg, parent-managed otoscopes).11,12
Family physicians historically have relied on in-person visits for holistic assessment and diagnosis. Telehealth video visits have the potential to assist with this goal, but there are risks. For example, one patient cut her foot while swimming and the wound became infected.
Specific conditions usually suitable for telehealth evaluation
The pandemic helped us understand that some situations and conditions are better suited than others to coverage by telehealth. The National Ambulatory Medical Care Survey examined 850 million patient–physician encounters and found that 66% of all ambulatory primary care visits required in-office care,15 suggesting that about one-third of patient encounters could be treated via telehealth.
As an example, our southeastern Wisconsin urban clinic has about 20,000 office visits per year. We launched telehealth in March 2020 in direct response to the pandemic. Telehealth usage peaked at the beginning of the pandemic (FIGURE), fell gradually, hit a lower peak in November and December as COVID case counts increased, and then decreased again as our community changed from a “quarantine/lockdown” mentality to “opening up/back to new normal.
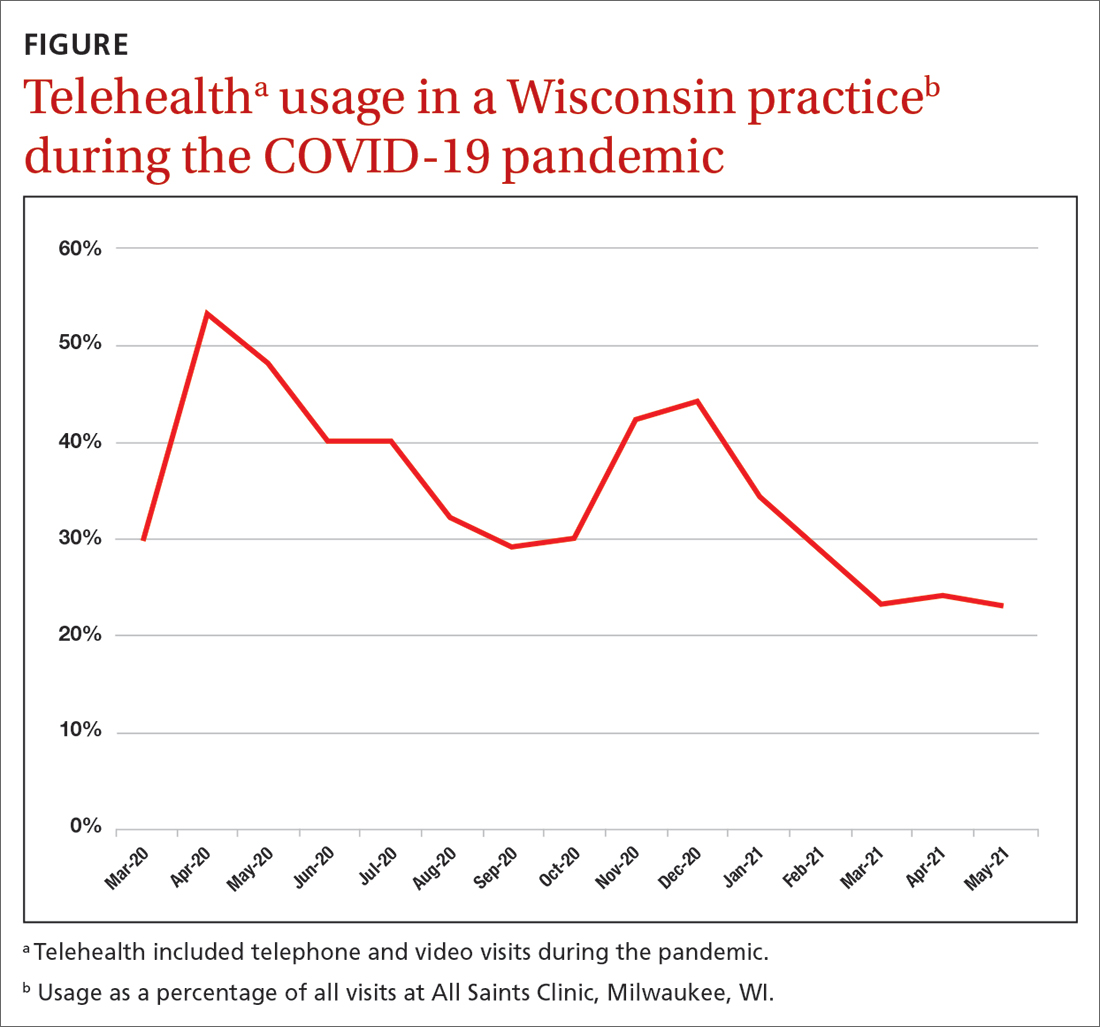
Some conditions can be managed favorably with the telehealth format:
Infectious diseases may be treatable remotely.16,17 Following an initial telehealth visit, the physician can evaluate and recommend further care.
Stable, chronic conditions. Telehealth can be used for stable, chronic conditions such as diabetes, chronic obstructive pulmonary disease, and heart failure when lab or imaging studies are not needed.18
Mental health. Telehealth can be useful in counseling and providing mental health and social support.18 Safeguards can be put in place to protect patient privacy in this setting.19
Behavioral change. Telehealth can be effective in providing support for patients actively trying to quit smoking or lose weight, and for caregivers. A physician who “checks in” can be a positive motivator and can promote a patient’s continued success.20
Continue to: Telehealth is less beneficial...
Telehealth is less beneficial when a physical exam is needed to assess pain, tenderness, strength, or other sensations. Office visits also are required for lab assays and imaging, as in periodic checks of A1C levels in patients with diabetes. As technology advances, home-based laboratory kits and sensors likely will change this picture. New patients may be better served through an initial office visit to develop the patient–physician relationship.
Visual assessment of conditions may be limited by telehealth depending on the quality of the devices used. For example, rashes may be difficult to assess given the clarity of the picture on the device and the ability to see only in 2D. There is still a need for more controlled trials to clarify which conditions can be evaluated and managed by telehealth and which ones need in-person care.21
Physician and patient perceptions of telehealth encounters
Research into family physicians’ perceptions of telehealth is scant. However, 3 studies published in 2021 reveal some advantages and challenges for telehealth adoption.
- A qualitative study found that physicians valued the increased access to care for some patients, changes to reimbursement practices not covered before, and the opportunity to see patients’ home environments.22 Disadvantages included an inability to examine the patient, problems with diagnostic accuracy, hindrances to developing personal connections, and the potential for burnout with on-demand care.22 The researchers suggested that telehealth might better serve to augment in-person care.
- A second study found that clinicians are satisfied with the use of telehealth in general. However, it also noted that the lack of physical examination could hinder accurate diagnosis and treatment.23
- A third study surveyed 109 family physicians, reinforcing the importance of physical exams and highlighting the lack of body language as another barrier.24
In addition, all 3 studies noted that video visits are typically briefer than in-person visits. Previous research predominantly done in specialty and mental health care showed that the benefits of telehealth for physicians include an increase in efficiency, reduced commute time, and improved work-life balance.25
Patient perspectives. Many patients have reported that they prefer telehealth because of lower costs, decreased travel time, and faster health care access.26,27 However, patients also have expressed concerns that the telehealth environment may reduce physician attention, can limit personal interaction (and impart a sense of being rushed), and lacks the physical examination that may be key to an adequate diagnosis.28
Continue to: A survey of 223 patients showed...
A survey of 223 patients showed that sicker patients choose in-person care because they want more in-depth visits with more attention to detail than healthier patients do.29 In a Veterans Affairs health care system qualitative study, patients voiced concerns about communicating with physicians via telehealth, including the potential for errors, less attention paid to their needs, audio difficulties, and challenges to establishing a physician–patient relationship.30 Some patients thought telehealth inhibited their personal expression or that the clinician was not attentive enough. These patient reports underscore the importance of patient–clinician relationships developed in person.31 The perceived level of complexity involved in a visit appears to be an essential factor in a patient opting for telehealth—or not.
In light of these known physician and patient perspectives, it seems wise to develop a hybrid model approach in which visits alternate between telehealth and office.
Patient disparities that may limit the use of telehealth
Race and ethnicity is a major factor in telehealth use. Patients who are Black or Hispanic use telehealth services less often than patients who are White.32,33 A study that looked at patients with chronic conditions—hypertension and diabetes—that disproportionately affect Black and Hispanic patients found that patients in these populations with either of these conditions had a lower prevalence of Internet use when compared with White patients.34 However, subpopulations can vary in their usage. For example, a study in East Harlem, New York, found that Hispanic pregnant women used telehealth frequently for prenatal care and perceived the care as satisfactory.35
Age is also a significant variable in the adoption of telehealth, with pre-COVID-19 studies finding lower use of technology among older adults. However, a study performed at the University of Missouri during the first months of the pandemic found an increase in telehealth use in seniors,32 although the increase was in telephone use and not full video sessions.
Many patients in need of health care services may have older devices and/or low-speed or no Internet access; they also may lack the technical know-how to conduct a telehealth visit.4,36 For example, regardless of race or ethnicity, patients on government insurance (Medicaid and Medicare) have been shown to complete more telephone than video visits,37 underscoring the importance of telehealth practice flexibility and the need for increased technology support to decrease the digital divide. Even with adequate technological support and patient training, telehealth may be more complicated if patients have such comorbidities as hearing, visual, or cognitive impairment.31 Patients from a lower socioeconomic status may feel uncomfortable with providers seeing their home environment on video.38
Overall, incorporating telehealth for the care of older and/or vulnerable patients will present a unique set of challenges that organizations must address. Efforts must be made to understand the available technologies and patients’ comfort in using them. A hybrid model offering telehealth and in-office encounters may be the best solution.
Hernan Barenboim, PhD, KPC Health Group, 301 North San Jacinto Street, Hemet, CA 92543; [email protected]
1. WHO. A health telematics policy: in support of WHO’s Health-for-All strategy for global health development. 1997. Accessed February 8, 2023. https://apps.who.int/iris/bitstream/handle/10665/63857/WHO_DGO_98.1.pdf?sequence=1&isAllowed=y
2. Knierim K, Palmer C, Kramer ES, et al. Lessons learned during COVID-19 that can move telehealth in primary care forward. J Am Board Fam Med. Supplement 2021;34(suppl):S196-S202. doi: 10.3122/jabfm.2021.S1.200419
3. Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3:e205873. doi: 10.1001/jamanetworkopen.2020.5873
4. Silver SL, Lewis MN, Ledford CJ. A stepwise transition to telemedicine in response to COVID-19. J Am Board Fam Med. 2021;34(suppl):S152-S161. doi: 10.3122/jabfm.2021.S1.200358
5. Hron JD, Parsons CR, Williams LA, et al. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;3:452-459. doi: 10.1055/s-0040-1713635
6. Olayiwola JN, Magaña C, Harmon A, et al. Telehealth as a bright spot of the COVID-19 pandemic: recommendations from the virtual frontlines (“Frontweb”). JMIR Public Health Surveill. 2020;6:e19045. doi: 10.2196/19045
7. Gausvik C, Jabbarpour Y. COVID-19 timeline: Centers for Medicare and Medicaid Services (CMS) changes and primary care support were not enough to prevent practice losses. J Am Board Fam Med. 2021;34(suppl):S7-S9. doi: 10.3122/jabfm.2021.S1.200305
8. Marin-Gomez FX, Vidal-Alaball J, Poch PR, et al. Diagnosis of skin lesions using photographs taken with a mobile phone: an online survey of primary care physicians. J Prim Care Community Health. 2020;11:2150132720937831. doi: 10.1177/2150132720937831
9. Garber RN, Garcia E, Goodwin CW, et al. (2020). Pictures do influence the decision to transfer: outcomes of a telemedicine program serving an eight-state rural population. J Burn Care Res. 2020;41:690-694. doi: 10.1093/jbcr/iraa017
10. Felix F, Greenblatt M, Shin L. Saving limbs in the time of COVID. 2020. Accessed February 8, 2023. https://podiatrym.com/pdf/2020/7/FelixGreenblattShin820web.pdf
11. Erkkola-Anttinen N, Irjala H, Laine MK, et al. Smartphone otoscopy performed by parents. Telemed J E Health. 2019;25:477-484. doi: 10.1089/tmj.2018.0062
12. Verzantvoort NC, Teunis T, Verheij TJ, et al. Self-triage for acute primary care via a smartphone application: practical, safe and efficient? PLoS One. 2018;13:e0199284. doi: 10.1371/journal.pone.0199284
13. Hickner J. When patients don’t get the care they should. J Fam Pract. 2020;69:427.
14. Pappan N, Benkhadra R, Papincak D, et al. Values and limits of telemedicine: a case report. SN Compr Clin Med. 2021;3:317-319. doi: 10.1007/s42399-020-00725-y
15. Jabbarpour Y, Jetty A, Westfall M, et al. Not telehealth: which primary care visits need in-person care? J Am Board Fam Med. 2021;34(suppl):S162-S169. doi: 10.3122/jabfm.2021.S1.200247
16. Parmar P, Mackie D, Varghese S, et al. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084-1094. doi: 10.1093/cid/ciu1143
17. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin Infect Dis. 2019;68:1437-1443. doi: 10.1093/cid/ciy907
18. ARHQ. Telehealth: mapping the evidence for patient outcomes from systematic reviews. 2016. Accessed March 27, 2023. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/telehealth_technical-brief.pdf
19. Lustgarten SD, Garrison YL, Sinnard MT, et al. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol. 2020;36:25-31. doi: 10.1016/j.copsyc.2020.03.012
20. Baird A, Xia Y, Cheng Y. Consumer perceptions of telehealth for mental health or substance abuse: a Twitter-based topic modeling analysis. JAMIA Open. 2022;5:ooac028. doi: 10.1093/jamiaopen/ooac028
21. Flumignan CD, da Rocha AP, Pinto AC, et al. What do Cochrane systematic reviews say about telemedicine for healthcare? Sao Paulo Med J. 2019;137:184-192. doi: 10.1590/1516-3180.0177240419
22. Gomez T, Anaya YB, Shih KJ, et al. A qualitative study of primary care physicians’ experiences with telemedicine during COVID-19. J Am Board Fam Med. 2021;34(suppl):S61-S70. doi: 10.3122/jabfm.2021.S1.200517
23. Malliaras P, Merolli M, Williams CM, et al. ‘It’s not hands-on therapy, so it’s very limited’: telehealth use and views among allied health clinicians during the coronavirus pandemic. Musculoskelet Sci Pract. 2021;52:102340. doi: 10.1016/j.msksp.2021.102340
24. Gold KJ, Laurie AR, Kinney DR, et al. Video visits: family physician experiences with uptake during the COVID-19 pandemic. Fam Med. 53:207-210. doi: 10.22454/FamMed.2021.613099
25. Björndell C, Premberg A. Physicians’ experiences of video consultation with patients at a public virtual primary care clinic: a qualitative interview study. Scand J Prim Health Care. 2021;39:67-76. doi: 10.1080/02813432.2021.1882082
26. Powell RE, Henstenburg JM, Cooper G, et al. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15:225-229. doi: 10.1370/afm.2095
27. Imlach F, McKinlay E, Middleton L, et al. Telehealth consultations in general practice during a pandemic lockdown: survey and interviews on patient experiences and preferences. BMC Fam Pract. 2020;21:1-14. doi: 10.1186/s12875-020-01336-1
28. Gordon HS, Solanki P, Bokhour BG, et al. “I’m not feeling like I’m part of the conversation” patients’ perspectives on communicating in clinical video telehealth visits. J Gen Intern Med. 2020;35:1751-1758. doi: 10.1007/s11606-020-05673-w
29. Volcy J, Smith W, Mills K, et al. Assessment of patient and provider satisfaction with the change to telehealth from in-person visits at an academic safety net institution during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(suppl):S71-S76. doi: 10.3122/jabfm.2021.S1.200393
30. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17:582-587. doi: 10.1016/j.nurpra.2021.02.020
31. Edgoose JY. Exploring the face-to-face: revisiting patient-doctor relationships in a time of expanding telemedicine. J Am Board Fam Med. 2021;34(suppl):S252-S254. doi: 10.3122/jabfm.2021.S1.200398
32. Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare. 2023;29:3-9. doi: 10.1177/1357633X20963893
33. Lame M, Leyden D, Platt SL. Geocode maps spotlight disparities in telehealth utilization during the COVID-19 pandemic in New York City. Telemed J E Health. 2021;27:251-253. doi: 10.1089/tmj.2020.0297
34. Jain V, Al Rifai M, Lee MT, et al. Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care. 2021;44:e15-e17. doi: 10.2337/dc20-2016
35. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in East Harlem. Am J Perinatol. 2021;38:88-92. doi: 10.1055/s-0040-1718695
36. Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93-99. doi: 10.1007/s10620-021-06842-5.
37. ASPE. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Issue brief: February 21, 2022. Accessed March 27, 2023. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf
38. Ukoha EP, Davis K, Yinger M, et al. Ensuring equitable implementation of telemedicine in perinatal care. Obstet Gynecol. 2021;137:487-492. doi: 10.1097/AOG.0000000000004276
Social distancing measures instituted during the COVID-19 pandemic challenged the usual way of operating in primary care. To continue delivering medical services, physicians had to transition quickly to forms of remote interaction with patients. Use of technology appeared to be the answer. And it gave clinicians the ability to do what many had long hoped for: offer patients the option of telehealth.
The terms telemedicine and telehealth have similar definitions and are commonly used interchangeably. We think most practices probably would have adopted telehealth earlier were it not for reimbursement barriers. In this article, we adopt the World Health Organization’s definition of telemedicine as: “The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.”1
To provide family medicine clinicians with evidence-based recommendations about telehealth, we conducted a critical review of the literature published through April 30, 2021. The scope of this review includes studies found using the PubMed and Google Scholar databases. In addition, we used the keywords “telehealth,” “telemedicine,” “family medicine,” and “primary care.” We divided this review into 6 sections, including focus areas on implementation in primary care, remote diagnostic accuracy, conditions lending themselves to telehealth, physician and patient perceptions, disparities in telehealth, and finally, the conclusions.
Telehealth implementation in primary care
Telehealth in various forms had been around for years before the pandemic, mainly in the form of commercial telehealth businesses. Telehealth was being used in rural and remote areas where it could be difficult to see a primary care provider—let alone a specialist. The family medicine department of the University of Colorado was an early adopter of telehealth and had navigated this transition since 2017, with clinical champions guiding the process. By 2019, 54% of their clinicians were conducting telehealth encounters.2
However, telehealth implementation elsewhere was not accepted so readily. Before the pandemic, a cross-sectional study of more than 1.1 million patients in Northern California showed that 86% preferred in-person care over video.3 Even as the pandemic began and social distancing measures were implemented, a quality improvement project at a family medicine residency clinic in Florida documented that clinicians still preferred telephone interviews despite the capacity for video visits.4 And many primary care systems were simply unprepared to adopt telehealth technologies.
With time, however, family physicians began to improvise using popular videoconferencing technologies (eg, Zoom) that were readily available and familiar to patients, and medical centers began to repurpose their existing videoconferencing systems.5 The Ohio State University Wexner Medical Center launched a virtual health initiative just before the pandemic struck, at which time fewer than 5% of patient visits were conducted through telehealth. Weeks later, nearly 93% of patient visits were offered through telehealth.6
Reimbursement. Another significant impediment to early telehealth uptake was the late reaction by the Centers for Medicare and Medicaid Services (CMS) in changing the payment system. Hectic expansion of telehealth in response to the crisis pointed to the lack of policies that supported primary care with payments based on outcomes rather than fee-for-service models.7 By the end of April 2020, CMS finally announced that video visits would be reimbursed at the same rate as in-person visits. However, telephone-only visits are still very limited in coverage, and appropriate codes should be verified with payers.
Continue to: Remote diagnosis comes with a caveat
Remote diagnosis comes with a caveat
Some primary care practices have found that images of skin lesions submitted by patients (usually by cell phone) suffice for accurate diagnosis in lieu of office visits.8 With chronic conditions, home-based remote monitoring of vital signs may assist in diagnosing and managing acute issues. More efficient triage of patients is increasingly possible with the receipt of still images or video files of concerning lesions (eg, burns, rash, chronic wounds) sent from smartphones alone9,10 or with devices attached to smartphones (eg, parent-managed otoscopes).11,12
Family physicians historically have relied on in-person visits for holistic assessment and diagnosis. Telehealth video visits have the potential to assist with this goal, but there are risks. For example, one patient cut her foot while swimming and the wound became infected.
Specific conditions usually suitable for telehealth evaluation
The pandemic helped us understand that some situations and conditions are better suited than others to coverage by telehealth. The National Ambulatory Medical Care Survey examined 850 million patient–physician encounters and found that 66% of all ambulatory primary care visits required in-office care,15 suggesting that about one-third of patient encounters could be treated via telehealth.
As an example, our southeastern Wisconsin urban clinic has about 20,000 office visits per year. We launched telehealth in March 2020 in direct response to the pandemic. Telehealth usage peaked at the beginning of the pandemic (FIGURE), fell gradually, hit a lower peak in November and December as COVID case counts increased, and then decreased again as our community changed from a “quarantine/lockdown” mentality to “opening up/back to new normal.

Some conditions can be managed favorably with the telehealth format:
Infectious diseases may be treatable remotely.16,17 Following an initial telehealth visit, the physician can evaluate and recommend further care.
Stable, chronic conditions. Telehealth can be used for stable, chronic conditions such as diabetes, chronic obstructive pulmonary disease, and heart failure when lab or imaging studies are not needed.18
Mental health. Telehealth can be useful in counseling and providing mental health and social support.18 Safeguards can be put in place to protect patient privacy in this setting.19
Behavioral change. Telehealth can be effective in providing support for patients actively trying to quit smoking or lose weight, and for caregivers. A physician who “checks in” can be a positive motivator and can promote a patient’s continued success.20
Continue to: Telehealth is less beneficial...
Telehealth is less beneficial when a physical exam is needed to assess pain, tenderness, strength, or other sensations. Office visits also are required for lab assays and imaging, as in periodic checks of A1C levels in patients with diabetes. As technology advances, home-based laboratory kits and sensors likely will change this picture. New patients may be better served through an initial office visit to develop the patient–physician relationship.
Visual assessment of conditions may be limited by telehealth depending on the quality of the devices used. For example, rashes may be difficult to assess given the clarity of the picture on the device and the ability to see only in 2D. There is still a need for more controlled trials to clarify which conditions can be evaluated and managed by telehealth and which ones need in-person care.21
Physician and patient perceptions of telehealth encounters
Research into family physicians’ perceptions of telehealth is scant. However, 3 studies published in 2021 reveal some advantages and challenges for telehealth adoption.
- A qualitative study found that physicians valued the increased access to care for some patients, changes to reimbursement practices not covered before, and the opportunity to see patients’ home environments.22 Disadvantages included an inability to examine the patient, problems with diagnostic accuracy, hindrances to developing personal connections, and the potential for burnout with on-demand care.22 The researchers suggested that telehealth might better serve to augment in-person care.
- A second study found that clinicians are satisfied with the use of telehealth in general. However, it also noted that the lack of physical examination could hinder accurate diagnosis and treatment.23
- A third study surveyed 109 family physicians, reinforcing the importance of physical exams and highlighting the lack of body language as another barrier.24
In addition, all 3 studies noted that video visits are typically briefer than in-person visits. Previous research predominantly done in specialty and mental health care showed that the benefits of telehealth for physicians include an increase in efficiency, reduced commute time, and improved work-life balance.25
Patient perspectives. Many patients have reported that they prefer telehealth because of lower costs, decreased travel time, and faster health care access.26,27 However, patients also have expressed concerns that the telehealth environment may reduce physician attention, can limit personal interaction (and impart a sense of being rushed), and lacks the physical examination that may be key to an adequate diagnosis.28
Continue to: A survey of 223 patients showed...
A survey of 223 patients showed that sicker patients choose in-person care because they want more in-depth visits with more attention to detail than healthier patients do.29 In a Veterans Affairs health care system qualitative study, patients voiced concerns about communicating with physicians via telehealth, including the potential for errors, less attention paid to their needs, audio difficulties, and challenges to establishing a physician–patient relationship.30 Some patients thought telehealth inhibited their personal expression or that the clinician was not attentive enough. These patient reports underscore the importance of patient–clinician relationships developed in person.31 The perceived level of complexity involved in a visit appears to be an essential factor in a patient opting for telehealth—or not.
In light of these known physician and patient perspectives, it seems wise to develop a hybrid model approach in which visits alternate between telehealth and office.
Patient disparities that may limit the use of telehealth
Race and ethnicity is a major factor in telehealth use. Patients who are Black or Hispanic use telehealth services less often than patients who are White.32,33 A study that looked at patients with chronic conditions—hypertension and diabetes—that disproportionately affect Black and Hispanic patients found that patients in these populations with either of these conditions had a lower prevalence of Internet use when compared with White patients.34 However, subpopulations can vary in their usage. For example, a study in East Harlem, New York, found that Hispanic pregnant women used telehealth frequently for prenatal care and perceived the care as satisfactory.35
Age is also a significant variable in the adoption of telehealth, with pre-COVID-19 studies finding lower use of technology among older adults. However, a study performed at the University of Missouri during the first months of the pandemic found an increase in telehealth use in seniors,32 although the increase was in telephone use and not full video sessions.
Many patients in need of health care services may have older devices and/or low-speed or no Internet access; they also may lack the technical know-how to conduct a telehealth visit.4,36 For example, regardless of race or ethnicity, patients on government insurance (Medicaid and Medicare) have been shown to complete more telephone than video visits,37 underscoring the importance of telehealth practice flexibility and the need for increased technology support to decrease the digital divide. Even with adequate technological support and patient training, telehealth may be more complicated if patients have such comorbidities as hearing, visual, or cognitive impairment.31 Patients from a lower socioeconomic status may feel uncomfortable with providers seeing their home environment on video.38
Overall, incorporating telehealth for the care of older and/or vulnerable patients will present a unique set of challenges that organizations must address. Efforts must be made to understand the available technologies and patients’ comfort in using them. A hybrid model offering telehealth and in-office encounters may be the best solution.
Hernan Barenboim, PhD, KPC Health Group, 301 North San Jacinto Street, Hemet, CA 92543; [email protected]
Social distancing measures instituted during the COVID-19 pandemic challenged the usual way of operating in primary care. To continue delivering medical services, physicians had to transition quickly to forms of remote interaction with patients. Use of technology appeared to be the answer. And it gave clinicians the ability to do what many had long hoped for: offer patients the option of telehealth.
The terms telemedicine and telehealth have similar definitions and are commonly used interchangeably. We think most practices probably would have adopted telehealth earlier were it not for reimbursement barriers. In this article, we adopt the World Health Organization’s definition of telemedicine as: “The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.”1
To provide family medicine clinicians with evidence-based recommendations about telehealth, we conducted a critical review of the literature published through April 30, 2021. The scope of this review includes studies found using the PubMed and Google Scholar databases. In addition, we used the keywords “telehealth,” “telemedicine,” “family medicine,” and “primary care.” We divided this review into 6 sections, including focus areas on implementation in primary care, remote diagnostic accuracy, conditions lending themselves to telehealth, physician and patient perceptions, disparities in telehealth, and finally, the conclusions.
Telehealth implementation in primary care
Telehealth in various forms had been around for years before the pandemic, mainly in the form of commercial telehealth businesses. Telehealth was being used in rural and remote areas where it could be difficult to see a primary care provider—let alone a specialist. The family medicine department of the University of Colorado was an early adopter of telehealth and had navigated this transition since 2017, with clinical champions guiding the process. By 2019, 54% of their clinicians were conducting telehealth encounters.2
However, telehealth implementation elsewhere was not accepted so readily. Before the pandemic, a cross-sectional study of more than 1.1 million patients in Northern California showed that 86% preferred in-person care over video.3 Even as the pandemic began and social distancing measures were implemented, a quality improvement project at a family medicine residency clinic in Florida documented that clinicians still preferred telephone interviews despite the capacity for video visits.4 And many primary care systems were simply unprepared to adopt telehealth technologies.
With time, however, family physicians began to improvise using popular videoconferencing technologies (eg, Zoom) that were readily available and familiar to patients, and medical centers began to repurpose their existing videoconferencing systems.5 The Ohio State University Wexner Medical Center launched a virtual health initiative just before the pandemic struck, at which time fewer than 5% of patient visits were conducted through telehealth. Weeks later, nearly 93% of patient visits were offered through telehealth.6
Reimbursement. Another significant impediment to early telehealth uptake was the late reaction by the Centers for Medicare and Medicaid Services (CMS) in changing the payment system. Hectic expansion of telehealth in response to the crisis pointed to the lack of policies that supported primary care with payments based on outcomes rather than fee-for-service models.7 By the end of April 2020, CMS finally announced that video visits would be reimbursed at the same rate as in-person visits. However, telephone-only visits are still very limited in coverage, and appropriate codes should be verified with payers.
Continue to: Remote diagnosis comes with a caveat
Remote diagnosis comes with a caveat
Some primary care practices have found that images of skin lesions submitted by patients (usually by cell phone) suffice for accurate diagnosis in lieu of office visits.8 With chronic conditions, home-based remote monitoring of vital signs may assist in diagnosing and managing acute issues. More efficient triage of patients is increasingly possible with the receipt of still images or video files of concerning lesions (eg, burns, rash, chronic wounds) sent from smartphones alone9,10 or with devices attached to smartphones (eg, parent-managed otoscopes).11,12
Family physicians historically have relied on in-person visits for holistic assessment and diagnosis. Telehealth video visits have the potential to assist with this goal, but there are risks. For example, one patient cut her foot while swimming and the wound became infected.
Specific conditions usually suitable for telehealth evaluation
The pandemic helped us understand that some situations and conditions are better suited than others to coverage by telehealth. The National Ambulatory Medical Care Survey examined 850 million patient–physician encounters and found that 66% of all ambulatory primary care visits required in-office care,15 suggesting that about one-third of patient encounters could be treated via telehealth.
As an example, our southeastern Wisconsin urban clinic has about 20,000 office visits per year. We launched telehealth in March 2020 in direct response to the pandemic. Telehealth usage peaked at the beginning of the pandemic (FIGURE), fell gradually, hit a lower peak in November and December as COVID case counts increased, and then decreased again as our community changed from a “quarantine/lockdown” mentality to “opening up/back to new normal.

Some conditions can be managed favorably with the telehealth format:
Infectious diseases may be treatable remotely.16,17 Following an initial telehealth visit, the physician can evaluate and recommend further care.
Stable, chronic conditions. Telehealth can be used for stable, chronic conditions such as diabetes, chronic obstructive pulmonary disease, and heart failure when lab or imaging studies are not needed.18
Mental health. Telehealth can be useful in counseling and providing mental health and social support.18 Safeguards can be put in place to protect patient privacy in this setting.19
Behavioral change. Telehealth can be effective in providing support for patients actively trying to quit smoking or lose weight, and for caregivers. A physician who “checks in” can be a positive motivator and can promote a patient’s continued success.20
Continue to: Telehealth is less beneficial...
Telehealth is less beneficial when a physical exam is needed to assess pain, tenderness, strength, or other sensations. Office visits also are required for lab assays and imaging, as in periodic checks of A1C levels in patients with diabetes. As technology advances, home-based laboratory kits and sensors likely will change this picture. New patients may be better served through an initial office visit to develop the patient–physician relationship.
Visual assessment of conditions may be limited by telehealth depending on the quality of the devices used. For example, rashes may be difficult to assess given the clarity of the picture on the device and the ability to see only in 2D. There is still a need for more controlled trials to clarify which conditions can be evaluated and managed by telehealth and which ones need in-person care.21
Physician and patient perceptions of telehealth encounters
Research into family physicians’ perceptions of telehealth is scant. However, 3 studies published in 2021 reveal some advantages and challenges for telehealth adoption.
- A qualitative study found that physicians valued the increased access to care for some patients, changes to reimbursement practices not covered before, and the opportunity to see patients’ home environments.22 Disadvantages included an inability to examine the patient, problems with diagnostic accuracy, hindrances to developing personal connections, and the potential for burnout with on-demand care.22 The researchers suggested that telehealth might better serve to augment in-person care.
- A second study found that clinicians are satisfied with the use of telehealth in general. However, it also noted that the lack of physical examination could hinder accurate diagnosis and treatment.23
- A third study surveyed 109 family physicians, reinforcing the importance of physical exams and highlighting the lack of body language as another barrier.24
In addition, all 3 studies noted that video visits are typically briefer than in-person visits. Previous research predominantly done in specialty and mental health care showed that the benefits of telehealth for physicians include an increase in efficiency, reduced commute time, and improved work-life balance.25
Patient perspectives. Many patients have reported that they prefer telehealth because of lower costs, decreased travel time, and faster health care access.26,27 However, patients also have expressed concerns that the telehealth environment may reduce physician attention, can limit personal interaction (and impart a sense of being rushed), and lacks the physical examination that may be key to an adequate diagnosis.28
Continue to: A survey of 223 patients showed...
A survey of 223 patients showed that sicker patients choose in-person care because they want more in-depth visits with more attention to detail than healthier patients do.29 In a Veterans Affairs health care system qualitative study, patients voiced concerns about communicating with physicians via telehealth, including the potential for errors, less attention paid to their needs, audio difficulties, and challenges to establishing a physician–patient relationship.30 Some patients thought telehealth inhibited their personal expression or that the clinician was not attentive enough. These patient reports underscore the importance of patient–clinician relationships developed in person.31 The perceived level of complexity involved in a visit appears to be an essential factor in a patient opting for telehealth—or not.
In light of these known physician and patient perspectives, it seems wise to develop a hybrid model approach in which visits alternate between telehealth and office.
Patient disparities that may limit the use of telehealth
Race and ethnicity is a major factor in telehealth use. Patients who are Black or Hispanic use telehealth services less often than patients who are White.32,33 A study that looked at patients with chronic conditions—hypertension and diabetes—that disproportionately affect Black and Hispanic patients found that patients in these populations with either of these conditions had a lower prevalence of Internet use when compared with White patients.34 However, subpopulations can vary in their usage. For example, a study in East Harlem, New York, found that Hispanic pregnant women used telehealth frequently for prenatal care and perceived the care as satisfactory.35
Age is also a significant variable in the adoption of telehealth, with pre-COVID-19 studies finding lower use of technology among older adults. However, a study performed at the University of Missouri during the first months of the pandemic found an increase in telehealth use in seniors,32 although the increase was in telephone use and not full video sessions.
Many patients in need of health care services may have older devices and/or low-speed or no Internet access; they also may lack the technical know-how to conduct a telehealth visit.4,36 For example, regardless of race or ethnicity, patients on government insurance (Medicaid and Medicare) have been shown to complete more telephone than video visits,37 underscoring the importance of telehealth practice flexibility and the need for increased technology support to decrease the digital divide. Even with adequate technological support and patient training, telehealth may be more complicated if patients have such comorbidities as hearing, visual, or cognitive impairment.31 Patients from a lower socioeconomic status may feel uncomfortable with providers seeing their home environment on video.38
Overall, incorporating telehealth for the care of older and/or vulnerable patients will present a unique set of challenges that organizations must address. Efforts must be made to understand the available technologies and patients’ comfort in using them. A hybrid model offering telehealth and in-office encounters may be the best solution.
Hernan Barenboim, PhD, KPC Health Group, 301 North San Jacinto Street, Hemet, CA 92543; [email protected]
1. WHO. A health telematics policy: in support of WHO’s Health-for-All strategy for global health development. 1997. Accessed February 8, 2023. https://apps.who.int/iris/bitstream/handle/10665/63857/WHO_DGO_98.1.pdf?sequence=1&isAllowed=y
2. Knierim K, Palmer C, Kramer ES, et al. Lessons learned during COVID-19 that can move telehealth in primary care forward. J Am Board Fam Med. Supplement 2021;34(suppl):S196-S202. doi: 10.3122/jabfm.2021.S1.200419
3. Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3:e205873. doi: 10.1001/jamanetworkopen.2020.5873
4. Silver SL, Lewis MN, Ledford CJ. A stepwise transition to telemedicine in response to COVID-19. J Am Board Fam Med. 2021;34(suppl):S152-S161. doi: 10.3122/jabfm.2021.S1.200358
5. Hron JD, Parsons CR, Williams LA, et al. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;3:452-459. doi: 10.1055/s-0040-1713635
6. Olayiwola JN, Magaña C, Harmon A, et al. Telehealth as a bright spot of the COVID-19 pandemic: recommendations from the virtual frontlines (“Frontweb”). JMIR Public Health Surveill. 2020;6:e19045. doi: 10.2196/19045
7. Gausvik C, Jabbarpour Y. COVID-19 timeline: Centers for Medicare and Medicaid Services (CMS) changes and primary care support were not enough to prevent practice losses. J Am Board Fam Med. 2021;34(suppl):S7-S9. doi: 10.3122/jabfm.2021.S1.200305
8. Marin-Gomez FX, Vidal-Alaball J, Poch PR, et al. Diagnosis of skin lesions using photographs taken with a mobile phone: an online survey of primary care physicians. J Prim Care Community Health. 2020;11:2150132720937831. doi: 10.1177/2150132720937831
9. Garber RN, Garcia E, Goodwin CW, et al. (2020). Pictures do influence the decision to transfer: outcomes of a telemedicine program serving an eight-state rural population. J Burn Care Res. 2020;41:690-694. doi: 10.1093/jbcr/iraa017
10. Felix F, Greenblatt M, Shin L. Saving limbs in the time of COVID. 2020. Accessed February 8, 2023. https://podiatrym.com/pdf/2020/7/FelixGreenblattShin820web.pdf
11. Erkkola-Anttinen N, Irjala H, Laine MK, et al. Smartphone otoscopy performed by parents. Telemed J E Health. 2019;25:477-484. doi: 10.1089/tmj.2018.0062
12. Verzantvoort NC, Teunis T, Verheij TJ, et al. Self-triage for acute primary care via a smartphone application: practical, safe and efficient? PLoS One. 2018;13:e0199284. doi: 10.1371/journal.pone.0199284
13. Hickner J. When patients don’t get the care they should. J Fam Pract. 2020;69:427.
14. Pappan N, Benkhadra R, Papincak D, et al. Values and limits of telemedicine: a case report. SN Compr Clin Med. 2021;3:317-319. doi: 10.1007/s42399-020-00725-y
15. Jabbarpour Y, Jetty A, Westfall M, et al. Not telehealth: which primary care visits need in-person care? J Am Board Fam Med. 2021;34(suppl):S162-S169. doi: 10.3122/jabfm.2021.S1.200247
16. Parmar P, Mackie D, Varghese S, et al. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084-1094. doi: 10.1093/cid/ciu1143
17. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin Infect Dis. 2019;68:1437-1443. doi: 10.1093/cid/ciy907
18. ARHQ. Telehealth: mapping the evidence for patient outcomes from systematic reviews. 2016. Accessed March 27, 2023. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/telehealth_technical-brief.pdf
19. Lustgarten SD, Garrison YL, Sinnard MT, et al. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol. 2020;36:25-31. doi: 10.1016/j.copsyc.2020.03.012
20. Baird A, Xia Y, Cheng Y. Consumer perceptions of telehealth for mental health or substance abuse: a Twitter-based topic modeling analysis. JAMIA Open. 2022;5:ooac028. doi: 10.1093/jamiaopen/ooac028
21. Flumignan CD, da Rocha AP, Pinto AC, et al. What do Cochrane systematic reviews say about telemedicine for healthcare? Sao Paulo Med J. 2019;137:184-192. doi: 10.1590/1516-3180.0177240419
22. Gomez T, Anaya YB, Shih KJ, et al. A qualitative study of primary care physicians’ experiences with telemedicine during COVID-19. J Am Board Fam Med. 2021;34(suppl):S61-S70. doi: 10.3122/jabfm.2021.S1.200517
23. Malliaras P, Merolli M, Williams CM, et al. ‘It’s not hands-on therapy, so it’s very limited’: telehealth use and views among allied health clinicians during the coronavirus pandemic. Musculoskelet Sci Pract. 2021;52:102340. doi: 10.1016/j.msksp.2021.102340
24. Gold KJ, Laurie AR, Kinney DR, et al. Video visits: family physician experiences with uptake during the COVID-19 pandemic. Fam Med. 53:207-210. doi: 10.22454/FamMed.2021.613099
25. Björndell C, Premberg A. Physicians’ experiences of video consultation with patients at a public virtual primary care clinic: a qualitative interview study. Scand J Prim Health Care. 2021;39:67-76. doi: 10.1080/02813432.2021.1882082
26. Powell RE, Henstenburg JM, Cooper G, et al. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15:225-229. doi: 10.1370/afm.2095
27. Imlach F, McKinlay E, Middleton L, et al. Telehealth consultations in general practice during a pandemic lockdown: survey and interviews on patient experiences and preferences. BMC Fam Pract. 2020;21:1-14. doi: 10.1186/s12875-020-01336-1
28. Gordon HS, Solanki P, Bokhour BG, et al. “I’m not feeling like I’m part of the conversation” patients’ perspectives on communicating in clinical video telehealth visits. J Gen Intern Med. 2020;35:1751-1758. doi: 10.1007/s11606-020-05673-w
29. Volcy J, Smith W, Mills K, et al. Assessment of patient and provider satisfaction with the change to telehealth from in-person visits at an academic safety net institution during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(suppl):S71-S76. doi: 10.3122/jabfm.2021.S1.200393
30. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17:582-587. doi: 10.1016/j.nurpra.2021.02.020
31. Edgoose JY. Exploring the face-to-face: revisiting patient-doctor relationships in a time of expanding telemedicine. J Am Board Fam Med. 2021;34(suppl):S252-S254. doi: 10.3122/jabfm.2021.S1.200398
32. Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare. 2023;29:3-9. doi: 10.1177/1357633X20963893
33. Lame M, Leyden D, Platt SL. Geocode maps spotlight disparities in telehealth utilization during the COVID-19 pandemic in New York City. Telemed J E Health. 2021;27:251-253. doi: 10.1089/tmj.2020.0297
34. Jain V, Al Rifai M, Lee MT, et al. Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care. 2021;44:e15-e17. doi: 10.2337/dc20-2016
35. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in East Harlem. Am J Perinatol. 2021;38:88-92. doi: 10.1055/s-0040-1718695
36. Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93-99. doi: 10.1007/s10620-021-06842-5.
37. ASPE. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Issue brief: February 21, 2022. Accessed March 27, 2023. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf
38. Ukoha EP, Davis K, Yinger M, et al. Ensuring equitable implementation of telemedicine in perinatal care. Obstet Gynecol. 2021;137:487-492. doi: 10.1097/AOG.0000000000004276
1. WHO. A health telematics policy: in support of WHO’s Health-for-All strategy for global health development. 1997. Accessed February 8, 2023. https://apps.who.int/iris/bitstream/handle/10665/63857/WHO_DGO_98.1.pdf?sequence=1&isAllowed=y
2. Knierim K, Palmer C, Kramer ES, et al. Lessons learned during COVID-19 that can move telehealth in primary care forward. J Am Board Fam Med. Supplement 2021;34(suppl):S196-S202. doi: 10.3122/jabfm.2021.S1.200419
3. Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3:e205873. doi: 10.1001/jamanetworkopen.2020.5873
4. Silver SL, Lewis MN, Ledford CJ. A stepwise transition to telemedicine in response to COVID-19. J Am Board Fam Med. 2021;34(suppl):S152-S161. doi: 10.3122/jabfm.2021.S1.200358
5. Hron JD, Parsons CR, Williams LA, et al. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;3:452-459. doi: 10.1055/s-0040-1713635
6. Olayiwola JN, Magaña C, Harmon A, et al. Telehealth as a bright spot of the COVID-19 pandemic: recommendations from the virtual frontlines (“Frontweb”). JMIR Public Health Surveill. 2020;6:e19045. doi: 10.2196/19045
7. Gausvik C, Jabbarpour Y. COVID-19 timeline: Centers for Medicare and Medicaid Services (CMS) changes and primary care support were not enough to prevent practice losses. J Am Board Fam Med. 2021;34(suppl):S7-S9. doi: 10.3122/jabfm.2021.S1.200305
8. Marin-Gomez FX, Vidal-Alaball J, Poch PR, et al. Diagnosis of skin lesions using photographs taken with a mobile phone: an online survey of primary care physicians. J Prim Care Community Health. 2020;11:2150132720937831. doi: 10.1177/2150132720937831
9. Garber RN, Garcia E, Goodwin CW, et al. (2020). Pictures do influence the decision to transfer: outcomes of a telemedicine program serving an eight-state rural population. J Burn Care Res. 2020;41:690-694. doi: 10.1093/jbcr/iraa017
10. Felix F, Greenblatt M, Shin L. Saving limbs in the time of COVID. 2020. Accessed February 8, 2023. https://podiatrym.com/pdf/2020/7/FelixGreenblattShin820web.pdf
11. Erkkola-Anttinen N, Irjala H, Laine MK, et al. Smartphone otoscopy performed by parents. Telemed J E Health. 2019;25:477-484. doi: 10.1089/tmj.2018.0062
12. Verzantvoort NC, Teunis T, Verheij TJ, et al. Self-triage for acute primary care via a smartphone application: practical, safe and efficient? PLoS One. 2018;13:e0199284. doi: 10.1371/journal.pone.0199284
13. Hickner J. When patients don’t get the care they should. J Fam Pract. 2020;69:427.
14. Pappan N, Benkhadra R, Papincak D, et al. Values and limits of telemedicine: a case report. SN Compr Clin Med. 2021;3:317-319. doi: 10.1007/s42399-020-00725-y
15. Jabbarpour Y, Jetty A, Westfall M, et al. Not telehealth: which primary care visits need in-person care? J Am Board Fam Med. 2021;34(suppl):S162-S169. doi: 10.3122/jabfm.2021.S1.200247
16. Parmar P, Mackie D, Varghese S, et al. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084-1094. doi: 10.1093/cid/ciu1143
17. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin Infect Dis. 2019;68:1437-1443. doi: 10.1093/cid/ciy907
18. ARHQ. Telehealth: mapping the evidence for patient outcomes from systematic reviews. 2016. Accessed March 27, 2023. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/telehealth_technical-brief.pdf
19. Lustgarten SD, Garrison YL, Sinnard MT, et al. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol. 2020;36:25-31. doi: 10.1016/j.copsyc.2020.03.012
20. Baird A, Xia Y, Cheng Y. Consumer perceptions of telehealth for mental health or substance abuse: a Twitter-based topic modeling analysis. JAMIA Open. 2022;5:ooac028. doi: 10.1093/jamiaopen/ooac028
21. Flumignan CD, da Rocha AP, Pinto AC, et al. What do Cochrane systematic reviews say about telemedicine for healthcare? Sao Paulo Med J. 2019;137:184-192. doi: 10.1590/1516-3180.0177240419
22. Gomez T, Anaya YB, Shih KJ, et al. A qualitative study of primary care physicians’ experiences with telemedicine during COVID-19. J Am Board Fam Med. 2021;34(suppl):S61-S70. doi: 10.3122/jabfm.2021.S1.200517
23. Malliaras P, Merolli M, Williams CM, et al. ‘It’s not hands-on therapy, so it’s very limited’: telehealth use and views among allied health clinicians during the coronavirus pandemic. Musculoskelet Sci Pract. 2021;52:102340. doi: 10.1016/j.msksp.2021.102340
24. Gold KJ, Laurie AR, Kinney DR, et al. Video visits: family physician experiences with uptake during the COVID-19 pandemic. Fam Med. 53:207-210. doi: 10.22454/FamMed.2021.613099
25. Björndell C, Premberg A. Physicians’ experiences of video consultation with patients at a public virtual primary care clinic: a qualitative interview study. Scand J Prim Health Care. 2021;39:67-76. doi: 10.1080/02813432.2021.1882082
26. Powell RE, Henstenburg JM, Cooper G, et al. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15:225-229. doi: 10.1370/afm.2095
27. Imlach F, McKinlay E, Middleton L, et al. Telehealth consultations in general practice during a pandemic lockdown: survey and interviews on patient experiences and preferences. BMC Fam Pract. 2020;21:1-14. doi: 10.1186/s12875-020-01336-1
28. Gordon HS, Solanki P, Bokhour BG, et al. “I’m not feeling like I’m part of the conversation” patients’ perspectives on communicating in clinical video telehealth visits. J Gen Intern Med. 2020;35:1751-1758. doi: 10.1007/s11606-020-05673-w
29. Volcy J, Smith W, Mills K, et al. Assessment of patient and provider satisfaction with the change to telehealth from in-person visits at an academic safety net institution during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(suppl):S71-S76. doi: 10.3122/jabfm.2021.S1.200393
30. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17:582-587. doi: 10.1016/j.nurpra.2021.02.020
31. Edgoose JY. Exploring the face-to-face: revisiting patient-doctor relationships in a time of expanding telemedicine. J Am Board Fam Med. 2021;34(suppl):S252-S254. doi: 10.3122/jabfm.2021.S1.200398
32. Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare. 2023;29:3-9. doi: 10.1177/1357633X20963893
33. Lame M, Leyden D, Platt SL. Geocode maps spotlight disparities in telehealth utilization during the COVID-19 pandemic in New York City. Telemed J E Health. 2021;27:251-253. doi: 10.1089/tmj.2020.0297
34. Jain V, Al Rifai M, Lee MT, et al. Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care. 2021;44:e15-e17. doi: 10.2337/dc20-2016
35. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in East Harlem. Am J Perinatol. 2021;38:88-92. doi: 10.1055/s-0040-1718695
36. Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93-99. doi: 10.1007/s10620-021-06842-5.
37. ASPE. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Issue brief: February 21, 2022. Accessed March 27, 2023. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf
38. Ukoha EP, Davis K, Yinger M, et al. Ensuring equitable implementation of telemedicine in perinatal care. Obstet Gynecol. 2021;137:487-492. doi: 10.1097/AOG.0000000000004276
PRACTICE RECOMMENDATIONS
› Consider using telehealth encounters for diagnosing and treating infectious diseases and for monitoring stable chronic conditions. C
› Consider telehealth “check-ins” to encourage patients working on behavioral change, such as smoking cessation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patient with newly diagnosed type 2 diabetes? Remember these steps
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8
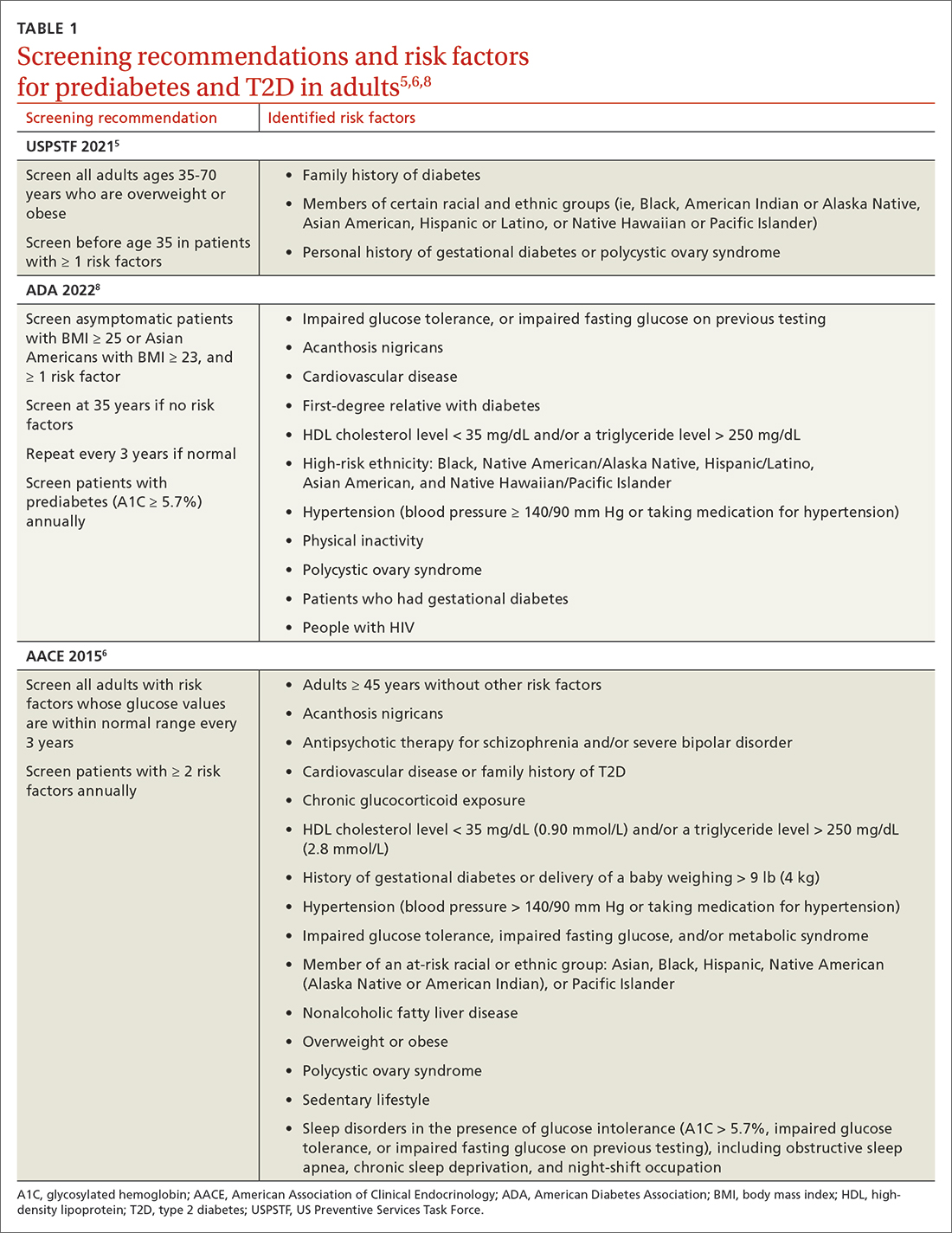
Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.
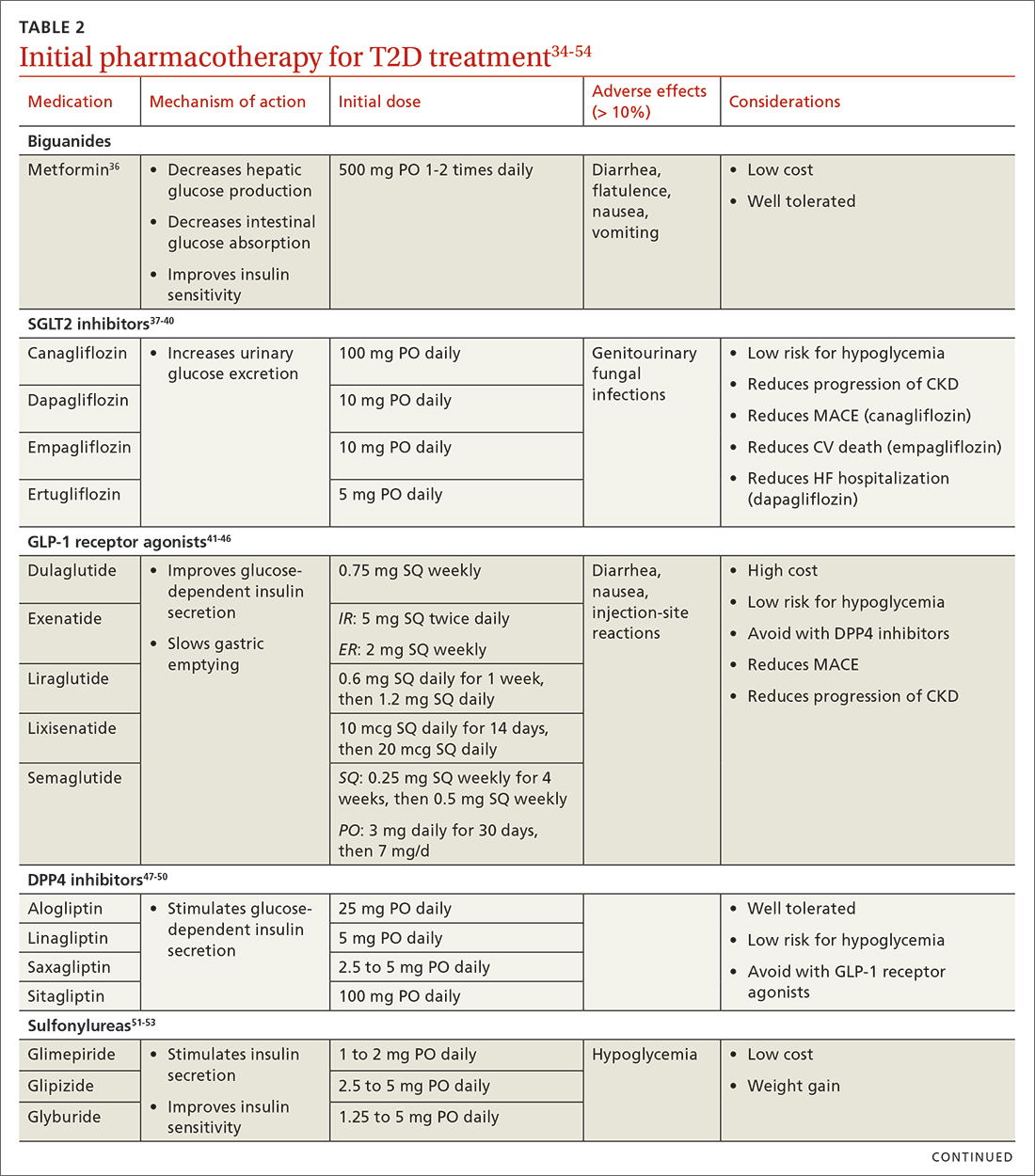
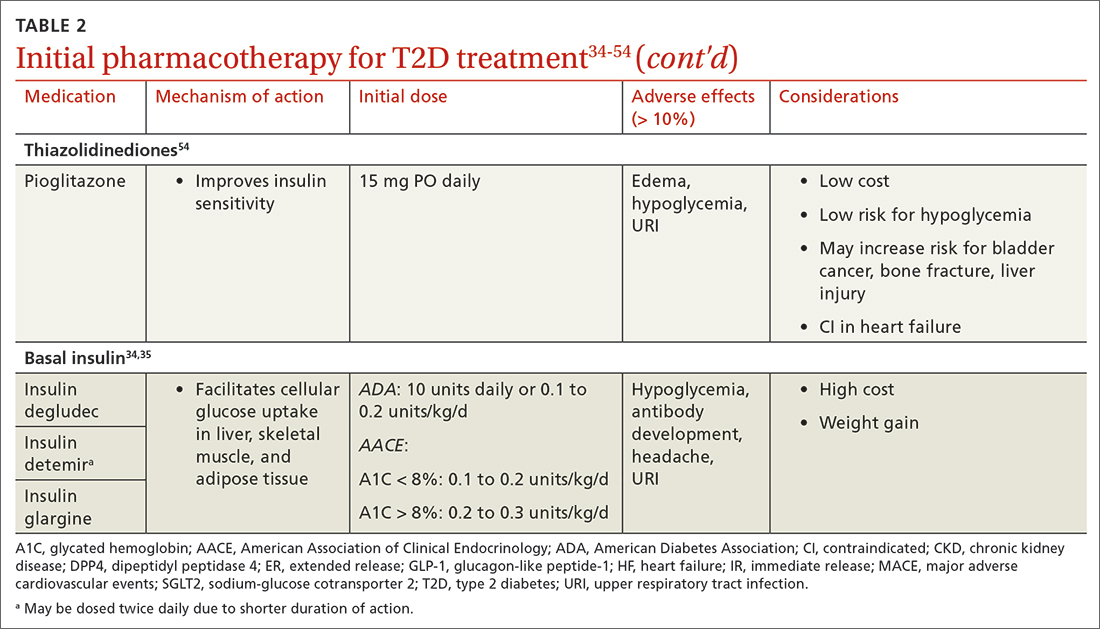
Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.
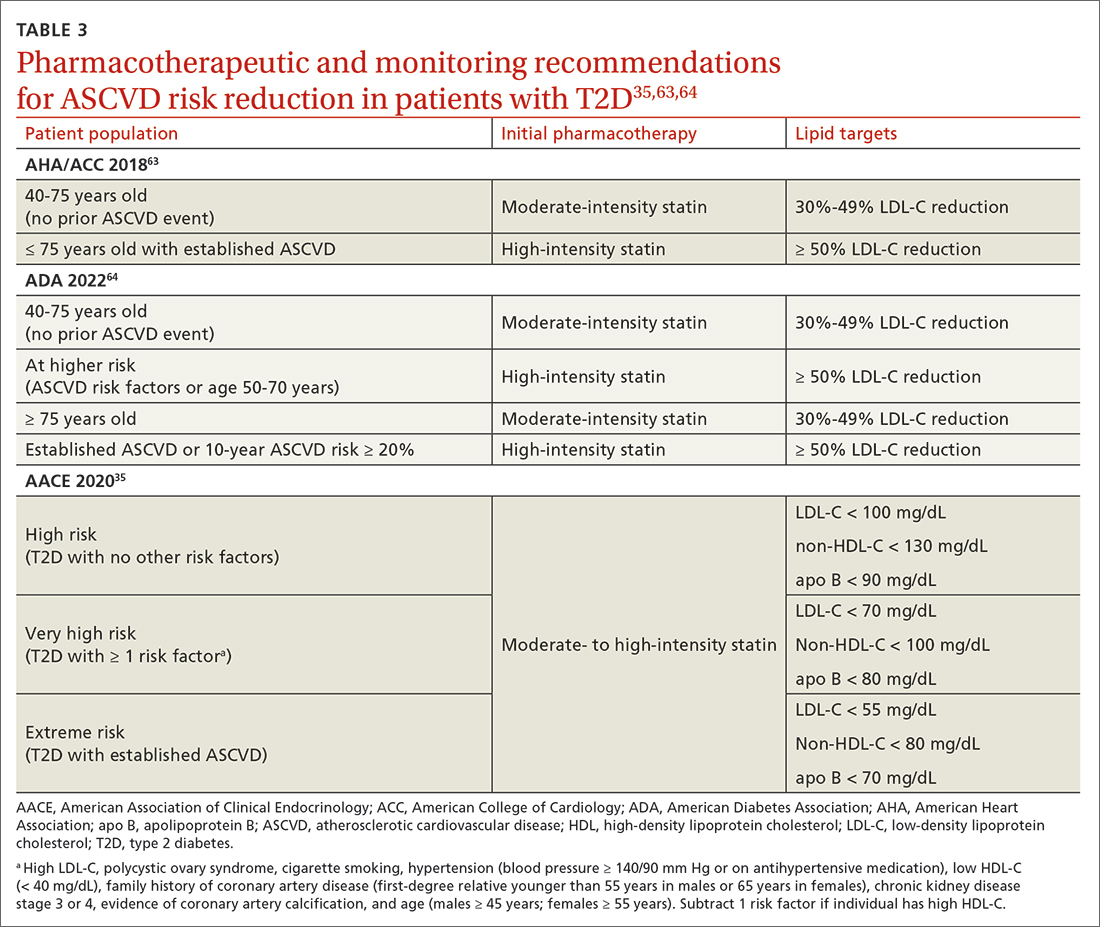
It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
PRACTICE RECOMMENDATIONS
› Individualize lifestyle modifications, considering personal and cultural experiences, health literacy, access to healthy foods, willingness and ability to make behavior changes, and barriers to change. C
› Initiate medication therapy at diagnosis, considering medication efficacy and cost, hypoglycemia risk, weight effects, benefits in cardiovascular and kidney disease, and patient-specific comorbidities. C
› Start basal insulin as first-line therapy in patients with severe baseline hyperglycemia, symptoms of hyperglycemia, or evidence of catabolism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Which patients might benefit from platelet-rich plasma?
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediators that enhance the natural healing process.2
When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93). The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03). However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001). The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma. Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).
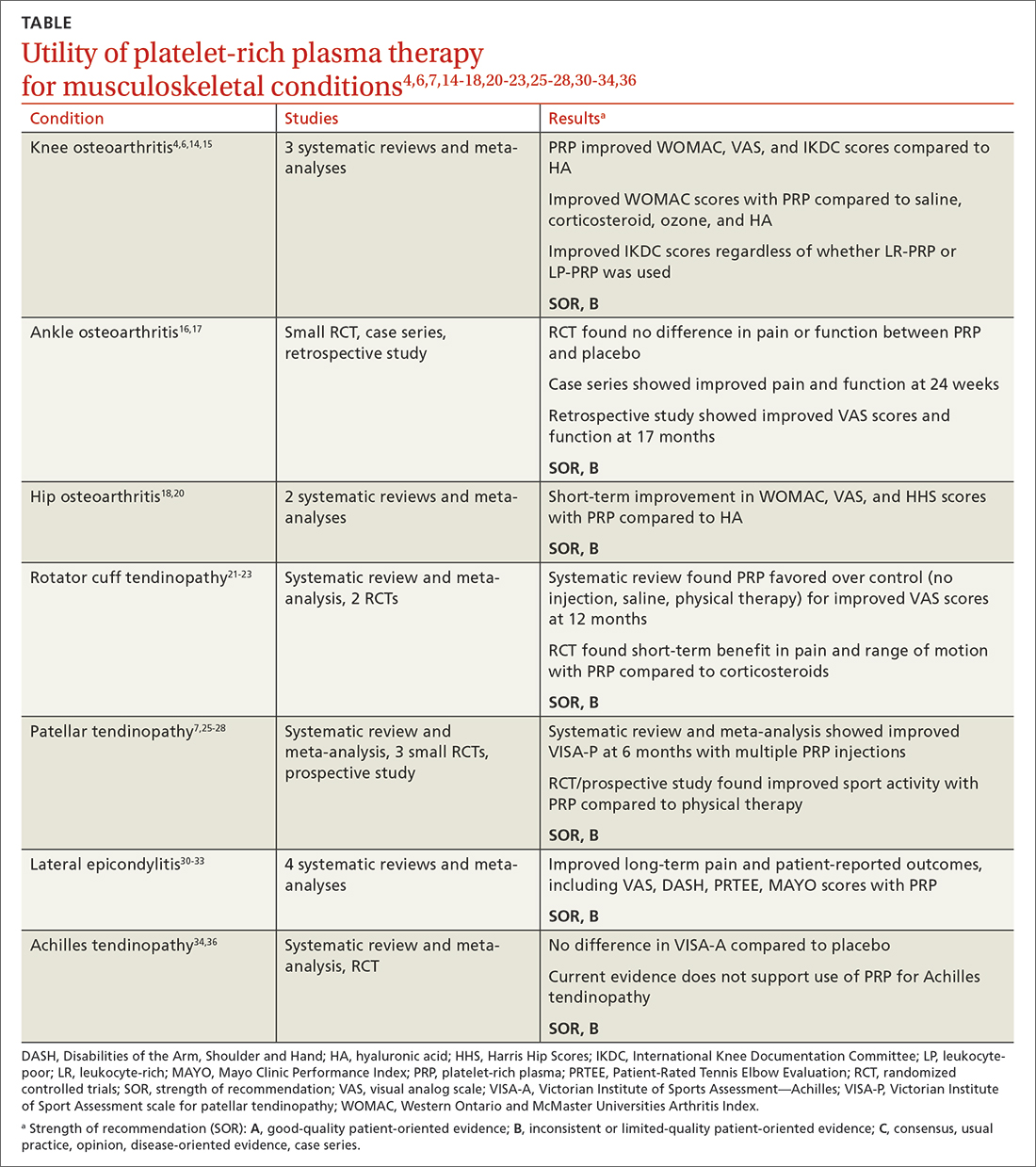
Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; [email protected]
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediators that enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93). The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03). However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001). The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma. Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; [email protected]
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediators that enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93). The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03). However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001). The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma. Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; [email protected]
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
PRACTICE RECOMMENDATIONS
› Consider plateletrich plasma (PRP) for conservative management of knee osteoarthritis and lateral epicondylitis. B
› Consider giving multiple injections of PRP for longterm pain relief and expedited return to sport in patellar tendinopathy. B
› Do not use PRP for Achilles tendinopathy due to a lack of clinical evidence. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12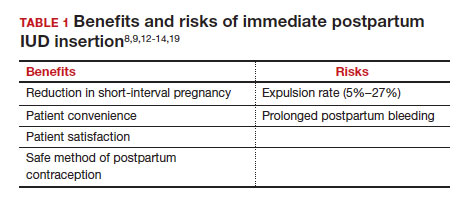
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
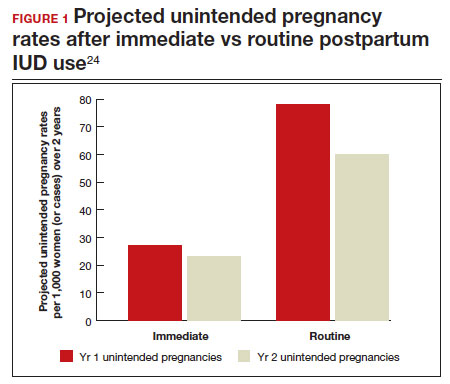
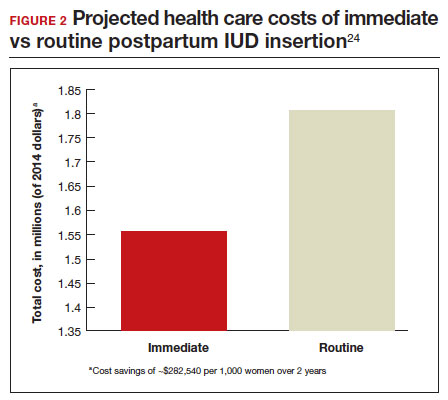
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
Breast cancer experts and other HCPs disagree on treatment strategies for early BC
The discrepancy suggests that many providers aren’t aware of the findings of recent landmark trials that formed the basis of the panel’s opinions, said study coauthor Denise A. Yardley, MD, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville, in an interview. The findings, based on responses to a treatment decision tool, were presented in a poster at the annual meeting of the American Society of Clinical Oncology.
Study methods and results
For the new study, researchers analyzed how 547 providers – and the panel – responded to 10 case scenarios in high-risk HER2– early breast cancer between June 2022 and January 2023.
Among the providers surveyed, 72% identified as physicians, including oncologists, hematologists/oncologists, surgery oncologists, radiation oncologists, and pathologists. One percent said they were nurse practitioners or physician assistants, 7% said they were pharmacists, 1% were nurses, and the specific roles of the remaining 19% were unknown, but included medical students, according to Dr. Yardley, who is a breast cancer oncologist.
The study authors developed the free decision tool – available via the medical education company Clinical Care Options – to help oncologists navigate new treatment options for high-risk HER2– early breast cancer. The Food and Drug Administration has recently approved drugs such as abemaciclib, olaparib, and pembrolizumab for the condition.
Health care providers enter details into the tool about their patients along with their intended treatment plans. The tool then shows them recommendations for treatment from a panel of five oncologists with expertise in oncology. The members of the panel based their perspectives on the findings of the KEYNOTE-522 (pembrolizumab), OlympiA (olaparib), and monarchE (abemaciclib) trials.
The oncologists with expertise in breast cancer, who provided recommendations in March 2022, generally agreed about the best treatments, Dr. Yardley said.
The other health care providers surveyed didn’t agree with the breast cancer experts about the best treatment 58.8% of the time.
For example, one scenario describes a HR+, HER2– patient with no deleterious BRCA mutation – or unknown status – who fits the monarchE high-risk criteria. All the breast cancer experts on the panel recommended abemaciclib and endocrine therapy. But 203 providers supported a variety of strategies: endocrine therapy alone (9%), chemotherapy followed by endocrine therapy (49%), and olaparib and endocrine therapy (2%). Only 37% opted for abemaciclib and endocrine therapy, and 4% were uncertain.
Another scenario describes a patient with triple-negative breast cancer with no residual disease after neoadjuvant chemotherapy. All the experts agreed on a strategy of no adjuvant therapy plus observation. Forty percent of 25 providers agreed with this approach, but 24% were uncertain, 12% chose pembrolizumab, and 24% chose capecitabine.
In many cases, providers chose more intensive treatment options than the experts did, Dr. Yardley said.
Overtreatment in cancer is often a reflex for oncologists, she said, although “we’re learning to deescalate these treatment algorithms where there is really no benefit [to extra treatment].”
“It’s a challenge for some of these oncologists who are busy and dealing with multiple solid tumor types to keep up with the nuances of a rapidly changing field,” Dr. Yardley noted.
Many community oncologists aren’t specialists in one type of cancer and must try to keep up with treatment recommendations regarding multiple types, she continued.
Decision tool’s value explained
According to the study, 32% of providers changed their treatment choices in clinical practice after they learned about the expert perspectives via the decision tool; 46% said the expert opinions confirmed that their choices were best practice.
The value of the tool is its ability to help providers make better decisions about patient care, Dr. Yardley said. “There seems to be a need for this kind of support.”
In an interview, University of Pittsburgh Medical Center oncologist Adam M. Brufsky, MD, PhD – who wasn’t involved with the study – said he was surprised by the amount of disagreement between the expert and provider perspectives on treatment. However, he noted that community oncologists – unlike the breast cancer experts – often don’t see just one type of cancer.
“You just have to know so much now as an oncologist,” Dr. Brufsky said. He recommended that colleagues take advantage of decision support tools, such as cancer treatment pathways.
The study was funded by AstraZeneca, Lilly, and Merck Sharp & Dohme. Dr. Yardley has no disclosures, and disclosure information from other authors was not available. Dr. Brufsky discloses consulting support from AstraZeneca, Lilly, and Merck and grants from AstraZeneca.
The discrepancy suggests that many providers aren’t aware of the findings of recent landmark trials that formed the basis of the panel’s opinions, said study coauthor Denise A. Yardley, MD, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville, in an interview. The findings, based on responses to a treatment decision tool, were presented in a poster at the annual meeting of the American Society of Clinical Oncology.
Study methods and results
For the new study, researchers analyzed how 547 providers – and the panel – responded to 10 case scenarios in high-risk HER2– early breast cancer between June 2022 and January 2023.
Among the providers surveyed, 72% identified as physicians, including oncologists, hematologists/oncologists, surgery oncologists, radiation oncologists, and pathologists. One percent said they were nurse practitioners or physician assistants, 7% said they were pharmacists, 1% were nurses, and the specific roles of the remaining 19% were unknown, but included medical students, according to Dr. Yardley, who is a breast cancer oncologist.
The study authors developed the free decision tool – available via the medical education company Clinical Care Options – to help oncologists navigate new treatment options for high-risk HER2– early breast cancer. The Food and Drug Administration has recently approved drugs such as abemaciclib, olaparib, and pembrolizumab for the condition.
Health care providers enter details into the tool about their patients along with their intended treatment plans. The tool then shows them recommendations for treatment from a panel of five oncologists with expertise in oncology. The members of the panel based their perspectives on the findings of the KEYNOTE-522 (pembrolizumab), OlympiA (olaparib), and monarchE (abemaciclib) trials.
The oncologists with expertise in breast cancer, who provided recommendations in March 2022, generally agreed about the best treatments, Dr. Yardley said.
The other health care providers surveyed didn’t agree with the breast cancer experts about the best treatment 58.8% of the time.
For example, one scenario describes a HR+, HER2– patient with no deleterious BRCA mutation – or unknown status – who fits the monarchE high-risk criteria. All the breast cancer experts on the panel recommended abemaciclib and endocrine therapy. But 203 providers supported a variety of strategies: endocrine therapy alone (9%), chemotherapy followed by endocrine therapy (49%), and olaparib and endocrine therapy (2%). Only 37% opted for abemaciclib and endocrine therapy, and 4% were uncertain.
Another scenario describes a patient with triple-negative breast cancer with no residual disease after neoadjuvant chemotherapy. All the experts agreed on a strategy of no adjuvant therapy plus observation. Forty percent of 25 providers agreed with this approach, but 24% were uncertain, 12% chose pembrolizumab, and 24% chose capecitabine.
In many cases, providers chose more intensive treatment options than the experts did, Dr. Yardley said.
Overtreatment in cancer is often a reflex for oncologists, she said, although “we’re learning to deescalate these treatment algorithms where there is really no benefit [to extra treatment].”
“It’s a challenge for some of these oncologists who are busy and dealing with multiple solid tumor types to keep up with the nuances of a rapidly changing field,” Dr. Yardley noted.
Many community oncologists aren’t specialists in one type of cancer and must try to keep up with treatment recommendations regarding multiple types, she continued.
Decision tool’s value explained
According to the study, 32% of providers changed their treatment choices in clinical practice after they learned about the expert perspectives via the decision tool; 46% said the expert opinions confirmed that their choices were best practice.
The value of the tool is its ability to help providers make better decisions about patient care, Dr. Yardley said. “There seems to be a need for this kind of support.”
In an interview, University of Pittsburgh Medical Center oncologist Adam M. Brufsky, MD, PhD – who wasn’t involved with the study – said he was surprised by the amount of disagreement between the expert and provider perspectives on treatment. However, he noted that community oncologists – unlike the breast cancer experts – often don’t see just one type of cancer.
“You just have to know so much now as an oncologist,” Dr. Brufsky said. He recommended that colleagues take advantage of decision support tools, such as cancer treatment pathways.
The study was funded by AstraZeneca, Lilly, and Merck Sharp & Dohme. Dr. Yardley has no disclosures, and disclosure information from other authors was not available. Dr. Brufsky discloses consulting support from AstraZeneca, Lilly, and Merck and grants from AstraZeneca.
The discrepancy suggests that many providers aren’t aware of the findings of recent landmark trials that formed the basis of the panel’s opinions, said study coauthor Denise A. Yardley, MD, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville, in an interview. The findings, based on responses to a treatment decision tool, were presented in a poster at the annual meeting of the American Society of Clinical Oncology.
Study methods and results
For the new study, researchers analyzed how 547 providers – and the panel – responded to 10 case scenarios in high-risk HER2– early breast cancer between June 2022 and January 2023.
Among the providers surveyed, 72% identified as physicians, including oncologists, hematologists/oncologists, surgery oncologists, radiation oncologists, and pathologists. One percent said they were nurse practitioners or physician assistants, 7% said they were pharmacists, 1% were nurses, and the specific roles of the remaining 19% were unknown, but included medical students, according to Dr. Yardley, who is a breast cancer oncologist.
The study authors developed the free decision tool – available via the medical education company Clinical Care Options – to help oncologists navigate new treatment options for high-risk HER2– early breast cancer. The Food and Drug Administration has recently approved drugs such as abemaciclib, olaparib, and pembrolizumab for the condition.
Health care providers enter details into the tool about their patients along with their intended treatment plans. The tool then shows them recommendations for treatment from a panel of five oncologists with expertise in oncology. The members of the panel based their perspectives on the findings of the KEYNOTE-522 (pembrolizumab), OlympiA (olaparib), and monarchE (abemaciclib) trials.
The oncologists with expertise in breast cancer, who provided recommendations in March 2022, generally agreed about the best treatments, Dr. Yardley said.
The other health care providers surveyed didn’t agree with the breast cancer experts about the best treatment 58.8% of the time.
For example, one scenario describes a HR+, HER2– patient with no deleterious BRCA mutation – or unknown status – who fits the monarchE high-risk criteria. All the breast cancer experts on the panel recommended abemaciclib and endocrine therapy. But 203 providers supported a variety of strategies: endocrine therapy alone (9%), chemotherapy followed by endocrine therapy (49%), and olaparib and endocrine therapy (2%). Only 37% opted for abemaciclib and endocrine therapy, and 4% were uncertain.
Another scenario describes a patient with triple-negative breast cancer with no residual disease after neoadjuvant chemotherapy. All the experts agreed on a strategy of no adjuvant therapy plus observation. Forty percent of 25 providers agreed with this approach, but 24% were uncertain, 12% chose pembrolizumab, and 24% chose capecitabine.
In many cases, providers chose more intensive treatment options than the experts did, Dr. Yardley said.
Overtreatment in cancer is often a reflex for oncologists, she said, although “we’re learning to deescalate these treatment algorithms where there is really no benefit [to extra treatment].”
“It’s a challenge for some of these oncologists who are busy and dealing with multiple solid tumor types to keep up with the nuances of a rapidly changing field,” Dr. Yardley noted.
Many community oncologists aren’t specialists in one type of cancer and must try to keep up with treatment recommendations regarding multiple types, she continued.
Decision tool’s value explained
According to the study, 32% of providers changed their treatment choices in clinical practice after they learned about the expert perspectives via the decision tool; 46% said the expert opinions confirmed that their choices were best practice.
The value of the tool is its ability to help providers make better decisions about patient care, Dr. Yardley said. “There seems to be a need for this kind of support.”
In an interview, University of Pittsburgh Medical Center oncologist Adam M. Brufsky, MD, PhD – who wasn’t involved with the study – said he was surprised by the amount of disagreement between the expert and provider perspectives on treatment. However, he noted that community oncologists – unlike the breast cancer experts – often don’t see just one type of cancer.
“You just have to know so much now as an oncologist,” Dr. Brufsky said. He recommended that colleagues take advantage of decision support tools, such as cancer treatment pathways.
The study was funded by AstraZeneca, Lilly, and Merck Sharp & Dohme. Dr. Yardley has no disclosures, and disclosure information from other authors was not available. Dr. Brufsky discloses consulting support from AstraZeneca, Lilly, and Merck and grants from AstraZeneca.
AT ASCO 2023