User login
Ticagrelor may reduce brain lesions after carotid stenting
MUNICH – secondary endpoint results of the PRECISE-MRI trial suggest.
More than 200 patients with carotid artery stenosis underwent MRI and were randomized to ticagrelor or clopidogrel before undergoing CAS. They then had two follow-up MRIs to assess the presence of emergent ischemic lesions.
Although the trial, which was stopped early, failed to show a difference between the two treatments in the primary endpoint – occurrence of at least one ischemic lesion – it did show that ticagrelor was associated with significant reductions in secondary endpoints including the total number and total volume of new lesions.
There were also significantly fewer cases of a composite of adverse clinical events with ticagrelor versus clopidogrel, but no difference in rates of hemorrhagic bleeds.
The research was presented at the annual European Stroke Organisation Conference .
Highlighting the failure of the trial to meet its primary endpoint, study presenter Leo Bonati, MD, head of the Stroke Center, Rena Rheinfelden, University Hospital Basel (Switzerland), pointed out that the proportion of patients with one or more ischemic brain lesions was “much higher than expected.”
Based on the secondary outcomes, the study nevertheless indicates that, “compared with clopidogrel, ticagrelor reduces the total burden of ischemic brain lesions occurring during CAS,” he said.
Ticagrelor is therefore a “safe alternative to clopidogrel as an add-on to aspirin to cover carotid artery stent procedures.”
Dr. Bonati cautioned, however, that the findings are preliminary.
‘Promising’ results
Session cochair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the stroke unit, department of neurology, Oslo University Hospital, called the results “interesting” and “promising.”
She said in an interview that they “also provide us with an additional option” in the management of patients undergoing CAS.
Dr. Sandset suggested that “it may have been a little bit hard to prove the primary endpoint” chosen for the trial, but believes that the secondary endpoint results “are very interesting.”
“Of course, we would need more data and further trials to provide some reassurance that we can use ticagrelor in this fashion,” she said.
Major complication
Dr. Bonati began by noting that the major procedural complication of CAS is embolic stroke, but this may be prevented with optimized antiplatelet therapy.
Previous studies have shown that ticagrelor is superior to clopidogrel as an add-on to aspirin in reducing rates of major adverse cardiovascular events in acute coronary syndrome patients undergoing percutaneous coronary intervention.
Adding the drug to aspirin is also superior to aspirin alone in preventing recurrent stroke in patients with minor stroke or transient ischemic attack, Dr. Bonati said.
To examine whether ticagrelor is superior to clopidogrel as an add-on to aspirin in preventing ischemic brain lesions during CAS, the team conducted a randomized, open, active-controlled trial.
They recruited patients with ≥ 50% symptomatic or asymptomatic carotid stenosis undergoing CAS in line with local guidelines and performed a baseline MRI scan and clinical examination.
The patients were then randomized to ticagrelor or clopidogrel plus aspirin 1-3 days before undergoing CAS. A second MRI and clinical examination, as well as an ultrasound scan, was performed at 1 to 3 days post-CAS, with a third set of examinations performed at 28-32 days after the procedure.
The study included 14 sites in Belgium, Germany, Italy, the Netherlands, Switzerland, and the United Kingdom. Enrollment was stopped after 209 of the originally planned 370 patients, “due to slow recruitment and a lack of further funding,” Dr. Bonati said.
Of those, 207 patients were included in the intention-to-treat safety analysis, and 172 in the per-protocol efficacy analysis.
The mean age of the patients was 69.0-69.5 years in the two treatment groups, and 67%-71% were male. Dr. Bonati noted that 52%-55% of the patients had symptomatic stenosis, and that in 83%-88% the stenosis was severe.
The majority (79%-82%) of patients had hypertension, alongside hypercholesterolemia, at 76% in both treatment groups.
Dr. Bonati showed that there was no significant difference in the primary efficacy outcome of the presence of at least new ischemic brain lesion on the second or third MRI, at 74.7% for patients given ticagrelor versus 79.8% with clopidogrel, or a relative risk of 0.94 (95% confidence interval, 0.79-1.10; P = .43).
However, there was a significant reduction in the number of new ischemic lesions, at a median of 2 (interquartile range, 0.5-5.5) with ticagrelor versus 3 with clopidogrel (IQR, 1-8), or an exponential beta value of 0.63 (95% CI, 0.42-0.95; P = .027).
Ticagrelor was also associated with a significant reduction in the total volume of lesions, at a median of 66 mcL (IQR, 2.5-2.19) versus 91 mcL (IQR, 25-394) for clopidogrel, or an exponential beta value of 0.30 (95% CI, 0.10-0.92; P = .030).
Patients assigned to ticagrelor also had a significantly lower rate of the primary clinical safety outcome, a composite of stroke, myocardial infarction, major bleeding, or cardiovascular death, at 2.9% versus 7.8% (relative risk, 0.36; 95% CI, 0.08-1.20). This was driven by a reduction in rates of post-CAS stroke.
Dr. Bonati noted that there was no significant difference in the presence of at least one hemorrhagic lesion after CAS, at 42.7% with ticagrelor and 47.6% in the clopidogrel group (RR, 0.90; 95% CI, 0.63-1.26).
There was also a similar rate of microbleeds between the two treatment groups, at 36.6% in patients given ticagrelor and 47.6% in those assigned to clopidogrel.
The study was investigator initiated and funded by an unrestricted research grant from AstraZeneca. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH – secondary endpoint results of the PRECISE-MRI trial suggest.
More than 200 patients with carotid artery stenosis underwent MRI and were randomized to ticagrelor or clopidogrel before undergoing CAS. They then had two follow-up MRIs to assess the presence of emergent ischemic lesions.
Although the trial, which was stopped early, failed to show a difference between the two treatments in the primary endpoint – occurrence of at least one ischemic lesion – it did show that ticagrelor was associated with significant reductions in secondary endpoints including the total number and total volume of new lesions.
There were also significantly fewer cases of a composite of adverse clinical events with ticagrelor versus clopidogrel, but no difference in rates of hemorrhagic bleeds.
The research was presented at the annual European Stroke Organisation Conference .
Highlighting the failure of the trial to meet its primary endpoint, study presenter Leo Bonati, MD, head of the Stroke Center, Rena Rheinfelden, University Hospital Basel (Switzerland), pointed out that the proportion of patients with one or more ischemic brain lesions was “much higher than expected.”
Based on the secondary outcomes, the study nevertheless indicates that, “compared with clopidogrel, ticagrelor reduces the total burden of ischemic brain lesions occurring during CAS,” he said.
Ticagrelor is therefore a “safe alternative to clopidogrel as an add-on to aspirin to cover carotid artery stent procedures.”
Dr. Bonati cautioned, however, that the findings are preliminary.
‘Promising’ results
Session cochair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the stroke unit, department of neurology, Oslo University Hospital, called the results “interesting” and “promising.”
She said in an interview that they “also provide us with an additional option” in the management of patients undergoing CAS.
Dr. Sandset suggested that “it may have been a little bit hard to prove the primary endpoint” chosen for the trial, but believes that the secondary endpoint results “are very interesting.”
“Of course, we would need more data and further trials to provide some reassurance that we can use ticagrelor in this fashion,” she said.
Major complication
Dr. Bonati began by noting that the major procedural complication of CAS is embolic stroke, but this may be prevented with optimized antiplatelet therapy.
Previous studies have shown that ticagrelor is superior to clopidogrel as an add-on to aspirin in reducing rates of major adverse cardiovascular events in acute coronary syndrome patients undergoing percutaneous coronary intervention.
Adding the drug to aspirin is also superior to aspirin alone in preventing recurrent stroke in patients with minor stroke or transient ischemic attack, Dr. Bonati said.
To examine whether ticagrelor is superior to clopidogrel as an add-on to aspirin in preventing ischemic brain lesions during CAS, the team conducted a randomized, open, active-controlled trial.
They recruited patients with ≥ 50% symptomatic or asymptomatic carotid stenosis undergoing CAS in line with local guidelines and performed a baseline MRI scan and clinical examination.
The patients were then randomized to ticagrelor or clopidogrel plus aspirin 1-3 days before undergoing CAS. A second MRI and clinical examination, as well as an ultrasound scan, was performed at 1 to 3 days post-CAS, with a third set of examinations performed at 28-32 days after the procedure.
The study included 14 sites in Belgium, Germany, Italy, the Netherlands, Switzerland, and the United Kingdom. Enrollment was stopped after 209 of the originally planned 370 patients, “due to slow recruitment and a lack of further funding,” Dr. Bonati said.
Of those, 207 patients were included in the intention-to-treat safety analysis, and 172 in the per-protocol efficacy analysis.
The mean age of the patients was 69.0-69.5 years in the two treatment groups, and 67%-71% were male. Dr. Bonati noted that 52%-55% of the patients had symptomatic stenosis, and that in 83%-88% the stenosis was severe.
The majority (79%-82%) of patients had hypertension, alongside hypercholesterolemia, at 76% in both treatment groups.
Dr. Bonati showed that there was no significant difference in the primary efficacy outcome of the presence of at least new ischemic brain lesion on the second or third MRI, at 74.7% for patients given ticagrelor versus 79.8% with clopidogrel, or a relative risk of 0.94 (95% confidence interval, 0.79-1.10; P = .43).
However, there was a significant reduction in the number of new ischemic lesions, at a median of 2 (interquartile range, 0.5-5.5) with ticagrelor versus 3 with clopidogrel (IQR, 1-8), or an exponential beta value of 0.63 (95% CI, 0.42-0.95; P = .027).
Ticagrelor was also associated with a significant reduction in the total volume of lesions, at a median of 66 mcL (IQR, 2.5-2.19) versus 91 mcL (IQR, 25-394) for clopidogrel, or an exponential beta value of 0.30 (95% CI, 0.10-0.92; P = .030).
Patients assigned to ticagrelor also had a significantly lower rate of the primary clinical safety outcome, a composite of stroke, myocardial infarction, major bleeding, or cardiovascular death, at 2.9% versus 7.8% (relative risk, 0.36; 95% CI, 0.08-1.20). This was driven by a reduction in rates of post-CAS stroke.
Dr. Bonati noted that there was no significant difference in the presence of at least one hemorrhagic lesion after CAS, at 42.7% with ticagrelor and 47.6% in the clopidogrel group (RR, 0.90; 95% CI, 0.63-1.26).
There was also a similar rate of microbleeds between the two treatment groups, at 36.6% in patients given ticagrelor and 47.6% in those assigned to clopidogrel.
The study was investigator initiated and funded by an unrestricted research grant from AstraZeneca. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH – secondary endpoint results of the PRECISE-MRI trial suggest.
More than 200 patients with carotid artery stenosis underwent MRI and were randomized to ticagrelor or clopidogrel before undergoing CAS. They then had two follow-up MRIs to assess the presence of emergent ischemic lesions.
Although the trial, which was stopped early, failed to show a difference between the two treatments in the primary endpoint – occurrence of at least one ischemic lesion – it did show that ticagrelor was associated with significant reductions in secondary endpoints including the total number and total volume of new lesions.
There were also significantly fewer cases of a composite of adverse clinical events with ticagrelor versus clopidogrel, but no difference in rates of hemorrhagic bleeds.
The research was presented at the annual European Stroke Organisation Conference .
Highlighting the failure of the trial to meet its primary endpoint, study presenter Leo Bonati, MD, head of the Stroke Center, Rena Rheinfelden, University Hospital Basel (Switzerland), pointed out that the proportion of patients with one or more ischemic brain lesions was “much higher than expected.”
Based on the secondary outcomes, the study nevertheless indicates that, “compared with clopidogrel, ticagrelor reduces the total burden of ischemic brain lesions occurring during CAS,” he said.
Ticagrelor is therefore a “safe alternative to clopidogrel as an add-on to aspirin to cover carotid artery stent procedures.”
Dr. Bonati cautioned, however, that the findings are preliminary.
‘Promising’ results
Session cochair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the stroke unit, department of neurology, Oslo University Hospital, called the results “interesting” and “promising.”
She said in an interview that they “also provide us with an additional option” in the management of patients undergoing CAS.
Dr. Sandset suggested that “it may have been a little bit hard to prove the primary endpoint” chosen for the trial, but believes that the secondary endpoint results “are very interesting.”
“Of course, we would need more data and further trials to provide some reassurance that we can use ticagrelor in this fashion,” she said.
Major complication
Dr. Bonati began by noting that the major procedural complication of CAS is embolic stroke, but this may be prevented with optimized antiplatelet therapy.
Previous studies have shown that ticagrelor is superior to clopidogrel as an add-on to aspirin in reducing rates of major adverse cardiovascular events in acute coronary syndrome patients undergoing percutaneous coronary intervention.
Adding the drug to aspirin is also superior to aspirin alone in preventing recurrent stroke in patients with minor stroke or transient ischemic attack, Dr. Bonati said.
To examine whether ticagrelor is superior to clopidogrel as an add-on to aspirin in preventing ischemic brain lesions during CAS, the team conducted a randomized, open, active-controlled trial.
They recruited patients with ≥ 50% symptomatic or asymptomatic carotid stenosis undergoing CAS in line with local guidelines and performed a baseline MRI scan and clinical examination.
The patients were then randomized to ticagrelor or clopidogrel plus aspirin 1-3 days before undergoing CAS. A second MRI and clinical examination, as well as an ultrasound scan, was performed at 1 to 3 days post-CAS, with a third set of examinations performed at 28-32 days after the procedure.
The study included 14 sites in Belgium, Germany, Italy, the Netherlands, Switzerland, and the United Kingdom. Enrollment was stopped after 209 of the originally planned 370 patients, “due to slow recruitment and a lack of further funding,” Dr. Bonati said.
Of those, 207 patients were included in the intention-to-treat safety analysis, and 172 in the per-protocol efficacy analysis.
The mean age of the patients was 69.0-69.5 years in the two treatment groups, and 67%-71% were male. Dr. Bonati noted that 52%-55% of the patients had symptomatic stenosis, and that in 83%-88% the stenosis was severe.
The majority (79%-82%) of patients had hypertension, alongside hypercholesterolemia, at 76% in both treatment groups.
Dr. Bonati showed that there was no significant difference in the primary efficacy outcome of the presence of at least new ischemic brain lesion on the second or third MRI, at 74.7% for patients given ticagrelor versus 79.8% with clopidogrel, or a relative risk of 0.94 (95% confidence interval, 0.79-1.10; P = .43).
However, there was a significant reduction in the number of new ischemic lesions, at a median of 2 (interquartile range, 0.5-5.5) with ticagrelor versus 3 with clopidogrel (IQR, 1-8), or an exponential beta value of 0.63 (95% CI, 0.42-0.95; P = .027).
Ticagrelor was also associated with a significant reduction in the total volume of lesions, at a median of 66 mcL (IQR, 2.5-2.19) versus 91 mcL (IQR, 25-394) for clopidogrel, or an exponential beta value of 0.30 (95% CI, 0.10-0.92; P = .030).
Patients assigned to ticagrelor also had a significantly lower rate of the primary clinical safety outcome, a composite of stroke, myocardial infarction, major bleeding, or cardiovascular death, at 2.9% versus 7.8% (relative risk, 0.36; 95% CI, 0.08-1.20). This was driven by a reduction in rates of post-CAS stroke.
Dr. Bonati noted that there was no significant difference in the presence of at least one hemorrhagic lesion after CAS, at 42.7% with ticagrelor and 47.6% in the clopidogrel group (RR, 0.90; 95% CI, 0.63-1.26).
There was also a similar rate of microbleeds between the two treatment groups, at 36.6% in patients given ticagrelor and 47.6% in those assigned to clopidogrel.
The study was investigator initiated and funded by an unrestricted research grant from AstraZeneca. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Alcohol may curb stress signaling in brain to protect heart
The study shows that light to moderate drinking was associated with lower major adverse cardiovascular events (MACE), and this was partly mediated by decreased stress signaling in the brain.
In addition, the benefit of light to moderate drinking with respect to MACE was most pronounced among people with a history of anxiety, a condition known to be associated with higher stress signaling in the brain.
However, the apparent CVD benefits of light to moderate drinking were counterbalanced by an increased risk of cancer.
“There is no safe level of alcohol consumption,” senior author and cardiologist Ahmed Tawakol, MD, codirector of the Cardiovascular Imaging Research Center at Massachusetts General Hospital, Boston, said in an interview.
“We see cancer risk even at the level that we see some protection from heart disease. And higher amounts of alcohol clearly increase heart disease risk,” Dr. Tawakol said.
The study was published online in the Journal of the American College of Cardiology.
Clear mechanistic link
Chronic stress is associated with MACE via stress-related neural network activity (SNA). Light to moderate alcohol consumption has been linked to lower MACE risk, but the mechanisms behind this connection remain unclear.
“We know that when the neural centers of stress are activated, they trigger downstream changes that result in heart disease. And we’ve long appreciated that alcohol in the short term reduces stress, so we hypothesized that maybe alcohol impacts those stress systems chronically and that might explain its cardiovascular effects,” Dr. Tawakol explained.
The study included roughly 53,000 adults (mean age, 60 years; 60% women) from the Mass General Brigham Biobank. The researchers first evaluated the relationship between light to moderate alcohol consumption and MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic factors.
During mean follow-up of 3.4 years, 1,914 individuals experienced MACE. Light to moderate alcohol consumption (compared to none/minimal) was associated with lower MACE risk (hazard ratio [HR], 0.786; 95% confidence interval [CI], 0.717-0.862; P < .0001) after adjustment for cardiovascular risk factors.
The researchers then studied a subset of 713 individuals who had undergone previous PET/CT brain imaging (primarily for cancer surveillance) to determine the effect of light to moderate alcohol consumption on resting SNA.
They found that light to moderate alcohol consumption correlated with decreased SNA (standardized beta, –0.192; 95% CI, –0.338 to 0.046; P = .01). Lower SNA partially mediated the beneficial effect of light to moderate alcohol intake on MACE risk (odds ratio [OR], –0.040; 95% CI, –0.097 to –0.003; P < .05).
Light to moderate alcohol consumption was associated with larger decreases in MACE risk among individuals with a history of anxiety (HR, 0.60; 95% CI, 0.50-0.72, vs. HR, 1.78; 95% CI, 0.73-0.80; P = .003).
The coauthors of an editorial say the discovery of a “new possible mechanism of action” for why light to moderate alcohol consumption might protect the heart “deserves closer attention in future investigations.”
However, Giovanni de Gaetano, MD, PhD, department of epidemiology and prevention, IRCCS NEUROMED, Pozzilli, Italy, one of the authors, emphasized that individuals who consume alcohol should not “exceed the recommended daily dose limits suggested in many countries and that no abstainer should start to drink, even in moderation, solely for the purpose of improving his/her health outcomes.”
Dr. Tawakol and colleagues said that, given alcohol’s adverse health effects, such as heightened cancer risk, new interventions that have positive effects on the neurobiology of stress but without the harmful effects of alcohol are needed.
To that end, they are studying the effect of exercise, stress-reduction interventions such as meditation, and pharmacologic therapies on stress-associated neural networks, and how they might induce CV benefits.
Dr. Tawakol said in an interview that one “additional important message is that anxiety and other related conditions like depression have really substantial health consequences, including increased MACE. Safer interventions that reduce anxiety may yet prove to reduce the risk of heart disease very nicely.”
The study was supported by the National Institutes of Health. Dr. Tawakol and Dr. de Gaetano have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The study shows that light to moderate drinking was associated with lower major adverse cardiovascular events (MACE), and this was partly mediated by decreased stress signaling in the brain.
In addition, the benefit of light to moderate drinking with respect to MACE was most pronounced among people with a history of anxiety, a condition known to be associated with higher stress signaling in the brain.
However, the apparent CVD benefits of light to moderate drinking were counterbalanced by an increased risk of cancer.
“There is no safe level of alcohol consumption,” senior author and cardiologist Ahmed Tawakol, MD, codirector of the Cardiovascular Imaging Research Center at Massachusetts General Hospital, Boston, said in an interview.
“We see cancer risk even at the level that we see some protection from heart disease. And higher amounts of alcohol clearly increase heart disease risk,” Dr. Tawakol said.
The study was published online in the Journal of the American College of Cardiology.
Clear mechanistic link
Chronic stress is associated with MACE via stress-related neural network activity (SNA). Light to moderate alcohol consumption has been linked to lower MACE risk, but the mechanisms behind this connection remain unclear.
“We know that when the neural centers of stress are activated, they trigger downstream changes that result in heart disease. And we’ve long appreciated that alcohol in the short term reduces stress, so we hypothesized that maybe alcohol impacts those stress systems chronically and that might explain its cardiovascular effects,” Dr. Tawakol explained.
The study included roughly 53,000 adults (mean age, 60 years; 60% women) from the Mass General Brigham Biobank. The researchers first evaluated the relationship between light to moderate alcohol consumption and MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic factors.
During mean follow-up of 3.4 years, 1,914 individuals experienced MACE. Light to moderate alcohol consumption (compared to none/minimal) was associated with lower MACE risk (hazard ratio [HR], 0.786; 95% confidence interval [CI], 0.717-0.862; P < .0001) after adjustment for cardiovascular risk factors.
The researchers then studied a subset of 713 individuals who had undergone previous PET/CT brain imaging (primarily for cancer surveillance) to determine the effect of light to moderate alcohol consumption on resting SNA.
They found that light to moderate alcohol consumption correlated with decreased SNA (standardized beta, –0.192; 95% CI, –0.338 to 0.046; P = .01). Lower SNA partially mediated the beneficial effect of light to moderate alcohol intake on MACE risk (odds ratio [OR], –0.040; 95% CI, –0.097 to –0.003; P < .05).
Light to moderate alcohol consumption was associated with larger decreases in MACE risk among individuals with a history of anxiety (HR, 0.60; 95% CI, 0.50-0.72, vs. HR, 1.78; 95% CI, 0.73-0.80; P = .003).
The coauthors of an editorial say the discovery of a “new possible mechanism of action” for why light to moderate alcohol consumption might protect the heart “deserves closer attention in future investigations.”
However, Giovanni de Gaetano, MD, PhD, department of epidemiology and prevention, IRCCS NEUROMED, Pozzilli, Italy, one of the authors, emphasized that individuals who consume alcohol should not “exceed the recommended daily dose limits suggested in many countries and that no abstainer should start to drink, even in moderation, solely for the purpose of improving his/her health outcomes.”
Dr. Tawakol and colleagues said that, given alcohol’s adverse health effects, such as heightened cancer risk, new interventions that have positive effects on the neurobiology of stress but without the harmful effects of alcohol are needed.
To that end, they are studying the effect of exercise, stress-reduction interventions such as meditation, and pharmacologic therapies on stress-associated neural networks, and how they might induce CV benefits.
Dr. Tawakol said in an interview that one “additional important message is that anxiety and other related conditions like depression have really substantial health consequences, including increased MACE. Safer interventions that reduce anxiety may yet prove to reduce the risk of heart disease very nicely.”
The study was supported by the National Institutes of Health. Dr. Tawakol and Dr. de Gaetano have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The study shows that light to moderate drinking was associated with lower major adverse cardiovascular events (MACE), and this was partly mediated by decreased stress signaling in the brain.
In addition, the benefit of light to moderate drinking with respect to MACE was most pronounced among people with a history of anxiety, a condition known to be associated with higher stress signaling in the brain.
However, the apparent CVD benefits of light to moderate drinking were counterbalanced by an increased risk of cancer.
“There is no safe level of alcohol consumption,” senior author and cardiologist Ahmed Tawakol, MD, codirector of the Cardiovascular Imaging Research Center at Massachusetts General Hospital, Boston, said in an interview.
“We see cancer risk even at the level that we see some protection from heart disease. And higher amounts of alcohol clearly increase heart disease risk,” Dr. Tawakol said.
The study was published online in the Journal of the American College of Cardiology.
Clear mechanistic link
Chronic stress is associated with MACE via stress-related neural network activity (SNA). Light to moderate alcohol consumption has been linked to lower MACE risk, but the mechanisms behind this connection remain unclear.
“We know that when the neural centers of stress are activated, they trigger downstream changes that result in heart disease. And we’ve long appreciated that alcohol in the short term reduces stress, so we hypothesized that maybe alcohol impacts those stress systems chronically and that might explain its cardiovascular effects,” Dr. Tawakol explained.
The study included roughly 53,000 adults (mean age, 60 years; 60% women) from the Mass General Brigham Biobank. The researchers first evaluated the relationship between light to moderate alcohol consumption and MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic factors.
During mean follow-up of 3.4 years, 1,914 individuals experienced MACE. Light to moderate alcohol consumption (compared to none/minimal) was associated with lower MACE risk (hazard ratio [HR], 0.786; 95% confidence interval [CI], 0.717-0.862; P < .0001) after adjustment for cardiovascular risk factors.
The researchers then studied a subset of 713 individuals who had undergone previous PET/CT brain imaging (primarily for cancer surveillance) to determine the effect of light to moderate alcohol consumption on resting SNA.
They found that light to moderate alcohol consumption correlated with decreased SNA (standardized beta, –0.192; 95% CI, –0.338 to 0.046; P = .01). Lower SNA partially mediated the beneficial effect of light to moderate alcohol intake on MACE risk (odds ratio [OR], –0.040; 95% CI, –0.097 to –0.003; P < .05).
Light to moderate alcohol consumption was associated with larger decreases in MACE risk among individuals with a history of anxiety (HR, 0.60; 95% CI, 0.50-0.72, vs. HR, 1.78; 95% CI, 0.73-0.80; P = .003).
The coauthors of an editorial say the discovery of a “new possible mechanism of action” for why light to moderate alcohol consumption might protect the heart “deserves closer attention in future investigations.”
However, Giovanni de Gaetano, MD, PhD, department of epidemiology and prevention, IRCCS NEUROMED, Pozzilli, Italy, one of the authors, emphasized that individuals who consume alcohol should not “exceed the recommended daily dose limits suggested in many countries and that no abstainer should start to drink, even in moderation, solely for the purpose of improving his/her health outcomes.”
Dr. Tawakol and colleagues said that, given alcohol’s adverse health effects, such as heightened cancer risk, new interventions that have positive effects on the neurobiology of stress but without the harmful effects of alcohol are needed.
To that end, they are studying the effect of exercise, stress-reduction interventions such as meditation, and pharmacologic therapies on stress-associated neural networks, and how they might induce CV benefits.
Dr. Tawakol said in an interview that one “additional important message is that anxiety and other related conditions like depression have really substantial health consequences, including increased MACE. Safer interventions that reduce anxiety may yet prove to reduce the risk of heart disease very nicely.”
The study was supported by the National Institutes of Health. Dr. Tawakol and Dr. de Gaetano have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
The cardiopulmonary effects of mask wearing
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
There was a time when I would have had to explain to you what an N95 mask is, how it is designed to filter out 95% of fine particles, defined as stuff in the air less than 2.5 microns in size.
But of course, you know that now. The N95 had its moment – a moment that seemed to be passing as the concentration of airborne coronavirus particles decreased.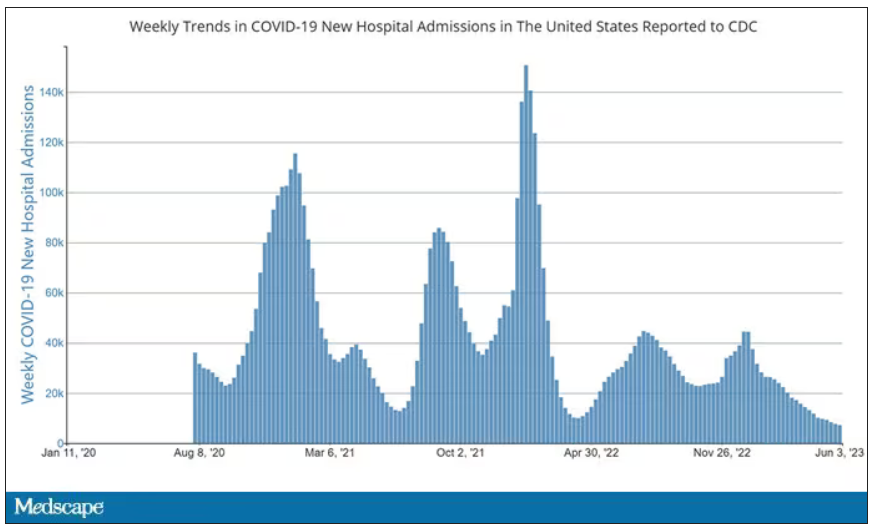
But, as the poet said, all that is less than 2.5 microns in size is not coronavirus. Wildfire smoke is also chock full of fine particulate matter. And so, N95s are having something of a comeback.
That’s why an article that took a deep look at what happens to our cardiovascular system when we wear N95 masks caught my eye.
Mask wearing has been the subject of intense debate around the country. While the vast majority of evidence, as well as the personal experience of thousands of doctors, suggests that wearing a mask has no significant physiologic effects, it’s not hard to find those who suggest that mask wearing depletes oxygen levels, or leads to infection, or has other bizarre effects.
In a world of conflicting opinions, a controlled study is a wonderful thing, and that’s what appeared in JAMA Network Open.
This isn’t a huge study, but it’s big enough to make some important conclusions. Thirty individuals, all young and healthy, half female, were enrolled. Each participant spent 3 days in a metabolic chamber; this is essentially a giant, airtight room where all the inputs (oxygen levels and so on) and outputs (carbon dioxide levels and so on) can be precisely measured.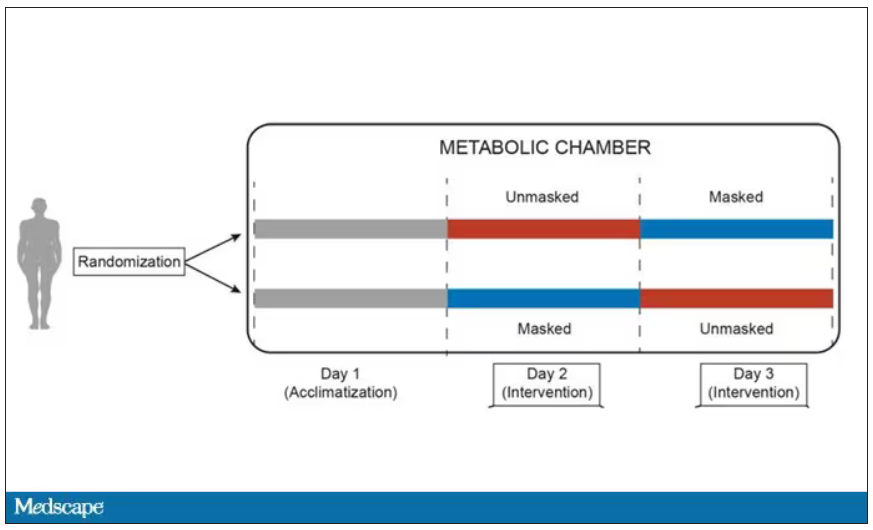
After a day of getting used to the environment, the participants spent a day either wearing an N95 mask or not for 16 waking hours. On the next day, they switched. Every other variable was controlled, from the calories in their diet to the temperature of the room itself.
They engaged in light exercise twice during the day – riding a stationary bike – and a host of physiologic parameters were measured. The question being, would the wearing of the mask for 16 hours straight change anything?
And the answer is yes, some things changed, but not by much.
Here’s a graph of the heart rate over time. You can see some separation, with higher heart rates during the mask-wearing day, particularly around 11 a.m. – when light exercise was scheduled.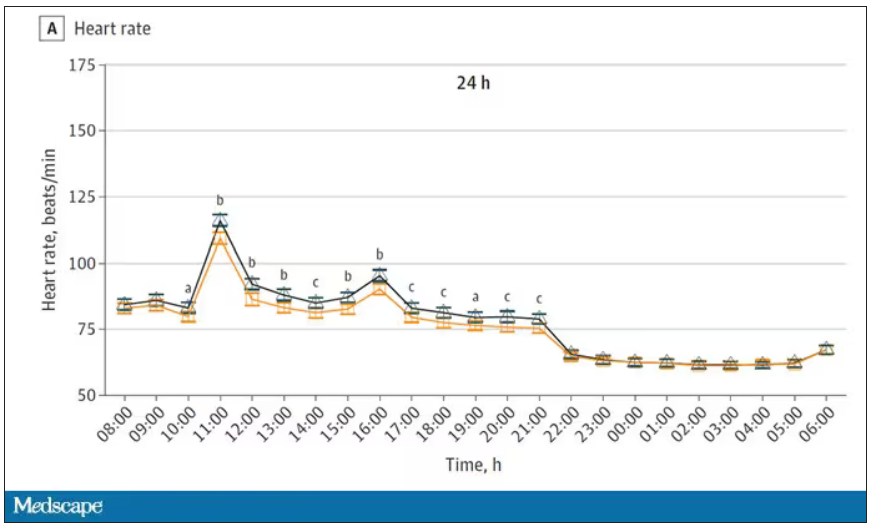
Zooming in on the exercise period makes the difference more clear. The heart rate was about eight beats/min higher while masked and engaging in exercise. Systolic blood pressure was about 6 mm Hg higher. Oxygen saturation was lower by 0.7%.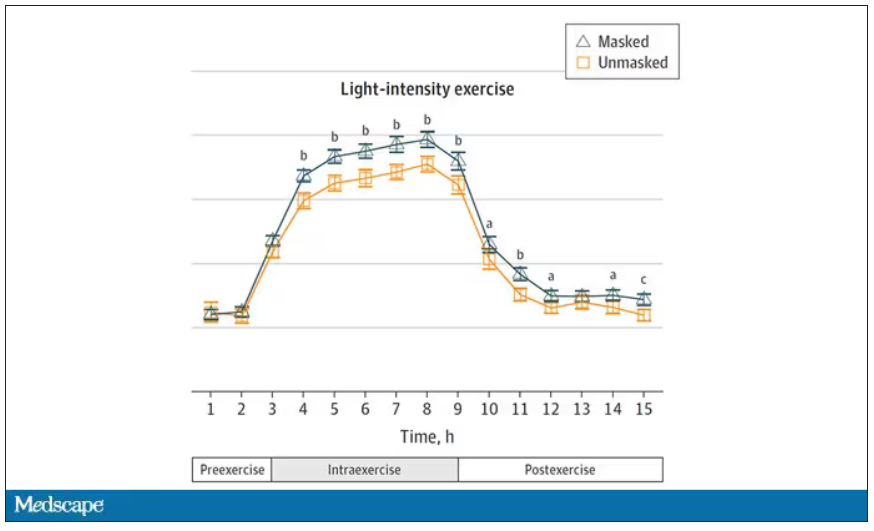
So yes, exercising while wearing an N95 mask might be different from exercising without an N95 mask. But nothing here looks dangerous to me. The 0.7% decrease in oxygen saturation is smaller than the typical measurement error of a pulse oximeter. The authors write that venous pH decreased during the masked day, which is of more interest to me as a nephrologist, but they don’t show that data even in the supplement. I suspect it didn’t decrease much.
They also showed that respiratory rate during exercise decreased in the masked condition. That doesn’t really make sense when you think about it in the context of the other findings, which are all suggestive of increased metabolic rate and sympathetic drive. Does that call the whole procedure into question? No, but it’s worth noting.
These were young, healthy people. You could certainly argue that those with more vulnerable cardiopulmonary status might have had different effects from mask wearing, but without a specific study in those people, it’s just conjecture. Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise.
But remember that, in reality, we are wearing masks for a reason. One could imagine a study where this metabolic chamber was filled with wildfire smoke at a concentration similar to what we saw in New York. In that situation, we might find that wearing an N95 is quite helpful. The thing is, studying masks in isolation is useful because you can control so many variables. But masks aren’t used in isolation. In fact, that’s sort of their defining characteristic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
There was a time when I would have had to explain to you what an N95 mask is, how it is designed to filter out 95% of fine particles, defined as stuff in the air less than 2.5 microns in size.
But of course, you know that now. The N95 had its moment – a moment that seemed to be passing as the concentration of airborne coronavirus particles decreased.
But, as the poet said, all that is less than 2.5 microns in size is not coronavirus. Wildfire smoke is also chock full of fine particulate matter. And so, N95s are having something of a comeback.
That’s why an article that took a deep look at what happens to our cardiovascular system when we wear N95 masks caught my eye.
Mask wearing has been the subject of intense debate around the country. While the vast majority of evidence, as well as the personal experience of thousands of doctors, suggests that wearing a mask has no significant physiologic effects, it’s not hard to find those who suggest that mask wearing depletes oxygen levels, or leads to infection, or has other bizarre effects.
In a world of conflicting opinions, a controlled study is a wonderful thing, and that’s what appeared in JAMA Network Open.
This isn’t a huge study, but it’s big enough to make some important conclusions. Thirty individuals, all young and healthy, half female, were enrolled. Each participant spent 3 days in a metabolic chamber; this is essentially a giant, airtight room where all the inputs (oxygen levels and so on) and outputs (carbon dioxide levels and so on) can be precisely measured.
After a day of getting used to the environment, the participants spent a day either wearing an N95 mask or not for 16 waking hours. On the next day, they switched. Every other variable was controlled, from the calories in their diet to the temperature of the room itself.
They engaged in light exercise twice during the day – riding a stationary bike – and a host of physiologic parameters were measured. The question being, would the wearing of the mask for 16 hours straight change anything?
And the answer is yes, some things changed, but not by much.
Here’s a graph of the heart rate over time. You can see some separation, with higher heart rates during the mask-wearing day, particularly around 11 a.m. – when light exercise was scheduled.
Zooming in on the exercise period makes the difference more clear. The heart rate was about eight beats/min higher while masked and engaging in exercise. Systolic blood pressure was about 6 mm Hg higher. Oxygen saturation was lower by 0.7%.
So yes, exercising while wearing an N95 mask might be different from exercising without an N95 mask. But nothing here looks dangerous to me. The 0.7% decrease in oxygen saturation is smaller than the typical measurement error of a pulse oximeter. The authors write that venous pH decreased during the masked day, which is of more interest to me as a nephrologist, but they don’t show that data even in the supplement. I suspect it didn’t decrease much.
They also showed that respiratory rate during exercise decreased in the masked condition. That doesn’t really make sense when you think about it in the context of the other findings, which are all suggestive of increased metabolic rate and sympathetic drive. Does that call the whole procedure into question? No, but it’s worth noting.
These were young, healthy people. You could certainly argue that those with more vulnerable cardiopulmonary status might have had different effects from mask wearing, but without a specific study in those people, it’s just conjecture. Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise.
But remember that, in reality, we are wearing masks for a reason. One could imagine a study where this metabolic chamber was filled with wildfire smoke at a concentration similar to what we saw in New York. In that situation, we might find that wearing an N95 is quite helpful. The thing is, studying masks in isolation is useful because you can control so many variables. But masks aren’t used in isolation. In fact, that’s sort of their defining characteristic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
There was a time when I would have had to explain to you what an N95 mask is, how it is designed to filter out 95% of fine particles, defined as stuff in the air less than 2.5 microns in size.
But of course, you know that now. The N95 had its moment – a moment that seemed to be passing as the concentration of airborne coronavirus particles decreased.
But, as the poet said, all that is less than 2.5 microns in size is not coronavirus. Wildfire smoke is also chock full of fine particulate matter. And so, N95s are having something of a comeback.
That’s why an article that took a deep look at what happens to our cardiovascular system when we wear N95 masks caught my eye.
Mask wearing has been the subject of intense debate around the country. While the vast majority of evidence, as well as the personal experience of thousands of doctors, suggests that wearing a mask has no significant physiologic effects, it’s not hard to find those who suggest that mask wearing depletes oxygen levels, or leads to infection, or has other bizarre effects.
In a world of conflicting opinions, a controlled study is a wonderful thing, and that’s what appeared in JAMA Network Open.
This isn’t a huge study, but it’s big enough to make some important conclusions. Thirty individuals, all young and healthy, half female, were enrolled. Each participant spent 3 days in a metabolic chamber; this is essentially a giant, airtight room where all the inputs (oxygen levels and so on) and outputs (carbon dioxide levels and so on) can be precisely measured.
After a day of getting used to the environment, the participants spent a day either wearing an N95 mask or not for 16 waking hours. On the next day, they switched. Every other variable was controlled, from the calories in their diet to the temperature of the room itself.
They engaged in light exercise twice during the day – riding a stationary bike – and a host of physiologic parameters were measured. The question being, would the wearing of the mask for 16 hours straight change anything?
And the answer is yes, some things changed, but not by much.
Here’s a graph of the heart rate over time. You can see some separation, with higher heart rates during the mask-wearing day, particularly around 11 a.m. – when light exercise was scheduled.
Zooming in on the exercise period makes the difference more clear. The heart rate was about eight beats/min higher while masked and engaging in exercise. Systolic blood pressure was about 6 mm Hg higher. Oxygen saturation was lower by 0.7%.
So yes, exercising while wearing an N95 mask might be different from exercising without an N95 mask. But nothing here looks dangerous to me. The 0.7% decrease in oxygen saturation is smaller than the typical measurement error of a pulse oximeter. The authors write that venous pH decreased during the masked day, which is of more interest to me as a nephrologist, but they don’t show that data even in the supplement. I suspect it didn’t decrease much.
They also showed that respiratory rate during exercise decreased in the masked condition. That doesn’t really make sense when you think about it in the context of the other findings, which are all suggestive of increased metabolic rate and sympathetic drive. Does that call the whole procedure into question? No, but it’s worth noting.
These were young, healthy people. You could certainly argue that those with more vulnerable cardiopulmonary status might have had different effects from mask wearing, but without a specific study in those people, it’s just conjecture. Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise.
But remember that, in reality, we are wearing masks for a reason. One could imagine a study where this metabolic chamber was filled with wildfire smoke at a concentration similar to what we saw in New York. In that situation, we might find that wearing an N95 is quite helpful. The thing is, studying masks in isolation is useful because you can control so many variables. But masks aren’t used in isolation. In fact, that’s sort of their defining characteristic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Insurers poised to crack down on off-label Ozempic prescriptions
The warning letters, first reported by The Washington Post, include threats such as the possibility of reporting “suspected inappropriate or fraudulent activity ... to the state licensure board, federal and/or state law enforcement.”
It’s the latest chapter in the story of the popular, highly effective, and very expensive drug intended for diabetes that results in quick weight loss. Off-label prescribing means a medicine has been prescribed for a reason other than the uses approved by the Food and Drug Administration. The practice is common and legal (the U.S. Agency for Healthcare Research and Quality says one in five prescriptions in the U.S. are off label).
But insurance companies are pushing back because many do not cover weight loss medications, while they do cover diabetes treatments. The insurance company letters suggest that prescribers are failing to document in a person’s medical record that the person actually has diabetes.
Ozempic, which is FDA approved for treatment of diabetes, is similar to the drug Wegovy, which is approved to be used for weight loss. Ozempic typically costs more than $900 per month. Both Wegovy and Ozempic contain semaglutide, which mimics a hormone that helps the brain regulate appetite and food intake. Clinical studies show that after taking semaglutide for more than 5 years, people lose on average 17% of their body weight. But once they stop taking it, most people regain much of the weight.
Demand for both Ozempic and Wegovy has been surging, leading to shortages and tactics to acquire the drugs outside of the United States, as well as warnings from public health officials about the dangers of knockoff versions of the drugs. The Centers for Disease Control and Prevention says 42% of people in the United States are obese.
“Obesity is a complex disease involving an excessive amount of body fat,” the Mayo Clinic explained. “Obesity isn’t just a cosmetic concern. It’s a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure, and certain cancers.”
A version of this article first appeared on WebMD.com.
The warning letters, first reported by The Washington Post, include threats such as the possibility of reporting “suspected inappropriate or fraudulent activity ... to the state licensure board, federal and/or state law enforcement.”
It’s the latest chapter in the story of the popular, highly effective, and very expensive drug intended for diabetes that results in quick weight loss. Off-label prescribing means a medicine has been prescribed for a reason other than the uses approved by the Food and Drug Administration. The practice is common and legal (the U.S. Agency for Healthcare Research and Quality says one in five prescriptions in the U.S. are off label).
But insurance companies are pushing back because many do not cover weight loss medications, while they do cover diabetes treatments. The insurance company letters suggest that prescribers are failing to document in a person’s medical record that the person actually has diabetes.
Ozempic, which is FDA approved for treatment of diabetes, is similar to the drug Wegovy, which is approved to be used for weight loss. Ozempic typically costs more than $900 per month. Both Wegovy and Ozempic contain semaglutide, which mimics a hormone that helps the brain regulate appetite and food intake. Clinical studies show that after taking semaglutide for more than 5 years, people lose on average 17% of their body weight. But once they stop taking it, most people regain much of the weight.
Demand for both Ozempic and Wegovy has been surging, leading to shortages and tactics to acquire the drugs outside of the United States, as well as warnings from public health officials about the dangers of knockoff versions of the drugs. The Centers for Disease Control and Prevention says 42% of people in the United States are obese.
“Obesity is a complex disease involving an excessive amount of body fat,” the Mayo Clinic explained. “Obesity isn’t just a cosmetic concern. It’s a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure, and certain cancers.”
A version of this article first appeared on WebMD.com.
The warning letters, first reported by The Washington Post, include threats such as the possibility of reporting “suspected inappropriate or fraudulent activity ... to the state licensure board, federal and/or state law enforcement.”
It’s the latest chapter in the story of the popular, highly effective, and very expensive drug intended for diabetes that results in quick weight loss. Off-label prescribing means a medicine has been prescribed for a reason other than the uses approved by the Food and Drug Administration. The practice is common and legal (the U.S. Agency for Healthcare Research and Quality says one in five prescriptions in the U.S. are off label).
But insurance companies are pushing back because many do not cover weight loss medications, while they do cover diabetes treatments. The insurance company letters suggest that prescribers are failing to document in a person’s medical record that the person actually has diabetes.
Ozempic, which is FDA approved for treatment of diabetes, is similar to the drug Wegovy, which is approved to be used for weight loss. Ozempic typically costs more than $900 per month. Both Wegovy and Ozempic contain semaglutide, which mimics a hormone that helps the brain regulate appetite and food intake. Clinical studies show that after taking semaglutide for more than 5 years, people lose on average 17% of their body weight. But once they stop taking it, most people regain much of the weight.
Demand for both Ozempic and Wegovy has been surging, leading to shortages and tactics to acquire the drugs outside of the United States, as well as warnings from public health officials about the dangers of knockoff versions of the drugs. The Centers for Disease Control and Prevention says 42% of people in the United States are obese.
“Obesity is a complex disease involving an excessive amount of body fat,” the Mayo Clinic explained. “Obesity isn’t just a cosmetic concern. It’s a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure, and certain cancers.”
A version of this article first appeared on WebMD.com.
Popular weight loss drugs can carry some unpleasant side effects
Johnna Mendenall had never been “the skinny friend,” she said, but the demands of motherhood – along with a sedentary desk job – made weight management even more difficult. Worried that family type 2 diabetes would catch up with her, she decided to start Wegovy shots for weight loss.
She was nervous about potential side effects. It took 5 days of staring at the Wegovy pen before she worked up the nerve for her first .25-milligram shot. And sure enough, the side effects came on strong.
“The nausea kicked in,” she said. “When I increased my dose to 1 milligram, I spent the entire night from 10 p.m. to 5 a.m. vomiting. I almost quit that day.”
While gastrointestinal (GI) symptoms seem to be the most common, a laundry list of others has been discussed in the news, on TikTok, and across online forums. Those include “Ozempic face,” or the gaunt look some get after taking the medication, along with hair loss, anxiety, depression, and debilitating fatigue.
Ms. Mendenall’s primary side effects have been vomiting, fatigue, and severe constipation, but she has also seen some positive changes: The “food noise,” or the urge to eat when she isn’t hungry, is gone. Since her first dose 12 weeks ago, she has gone from 236 pounds to 215.
Warning label
Wegovy’s active ingredient, semaglutide, mimics the role of a natural hormone called glucagonlike peptide–1 (GLP-1), which helps you feel well fed. Semaglutide is used at a lower dose under the brand name Ozempic, which is approved for type 2 diabetes and used off-label for weight loss.
Both Ozempic and Wegovy come with a warning label for potential side effects, the most common ones being nausea, diarrhea, stomach pain, and vomiting.
With the surging popularity of semaglutide, more people are getting prescriptions through telemedicine companies, forgoing more in-depth consultations, leading to more side effects, said Caroline Apovian, MD, professor of medicine at Harvard Medical School and codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, Boston.
Specialists say starting with low doses and gradually increasing over time helps avoid side effects, but insurance companies often require a faster timeline to continue covering the medication, Dr. Apovian said.
“Insurance companies are practicing medicine for us by demanding the patient go up in dosage [too quickly],” she explained.
Ms. Mendenall’s insurance has paid for her Wegovy shots, but without that coverage, she said it would cost her $1,200 per month.
There are similar medications on the market, such as liraglutide, sold under the name Saxenda. But it is a daily, rather than a weekly, shot and also comes with side effects and has been shown to be less effective. In one clinical trial, the people being studied saw their average body weight over 68 weeks drop by 15.8% with semaglutide, and by 6.4% with liraglutide.
Tirzepatide, branded Mounjaro – a type 2 diabetes drug made by Eli Lilly that may soon gain Food and Drug Administration approval for weight loss – could have fewer side effects. In clinical trials, 44% of those taking semaglutide had nausea and 31% reported diarrhea, compared with 33% and 23% of those taking tirzepatide, although no trial has directly compared the two agents.
Loss of bowel control
For now, Wegovy and Saxenda are the only GLP-1 agonist shots authorized for weight loss, and their maker, Danish drug company Novo Nordisk, is facing its second shortage of Wegovy amid growing demand.
Personal stories online about semaglutide range from overwhelmingly positive – just what some need to win a lifelong battle with obesity – to harsh scenarios with potentially long-term health consequences, and everything in between.
One private community on Reddit is dedicated to a particularly unpleasant side effect: loss of bowel control while sleeping. Others have reported uncontrollable vomiting.
Kimberly Carew of Clearwater, Fla., started on .5 milligrams of Ozempic last year after her rheumatologist and endocrinologist suggested it to treat her type 2 diabetes. She was told it came with the bonus of weight loss, which she was hoping would help with her joint and back pain.
But after she increased the dose to 1 milligram, her GI symptoms, which started out mild, became unbearable. She couldn’t keep food down, and when she vomited, the food would often come up whole, she said.
“One night I ate ramen before bed. And the next morning, it came out just as it went down,” said Ms. Carew, 42, a registered mental health counseling intern. “I was getting severe heartburn and could not take a couple bites of food without getting nauseous.”
She also had “sulfur burps,” a side effect discussed by some Ozempic users, causing her to taste rotten egg sometimes.
She was diagnosed with gastroparesis. Some types of gastroparesis can be resolved by discontinuing GLP-1 medications, as referenced in two case reports in the Journal of Investigative Medicine.
Gut hormone
GI symptoms are most common with semaglutide because the hormone it imitates, GLP-1, is secreted by cells in the stomach, small intestines, and pancreas, said Anne Peters, MD, director of the USC Clinical Diabetes Programs.
“This is the deal: The side effects are real because it’s a gut hormone. It’s increasing the level of something your body already has,” she said.
But, like Dr. Apovian, Dr. Peters said those side effects can likely be avoided if shots are started at the lowest doses and gradually adjusted up.
While the average starting dose is .25 milligrams, Dr. Peters said she often starts her patients on about an eighth of that – just “a whiff of a dose.”
“It’ll take them months to get up to the starting dose, but what’s the rush?”
Dr. Peters said she also avoids giving diabetes patients the maximum dose, which is 2 milligrams per week for Ozempic (and 2.4 milligrams for Wegovy for weight loss).
When asked about the drugs’ side effects, Novo Nordisk responded that “GLP-1 receptor agonists are a well-established class of medicines, which have demonstrated long-term safety in clinical trials. The most common adverse reactions, as with all GLP-1 [agonists], are gastrointestinal related.”
Is it the drug or the weight loss?
Still, non-gastrointestinal side effects such as hair loss, mood changes, and sunken facial features are reported among semaglutide users across the Internet. While these cases are often anecdotal, they can be very heartfelt.
Celina Horvath Myers, also known as CelinaSpookyBoo, a Canadian YouTuber who took Ozempic for type 2 diabetes, said she began having intense panic attacks and depression after starting the medication.
“Who I have been these last couple weeks, has probably been the scariest time of my life,” she said on her YouTube channel.
While severe depression and anxiety are not established side effects of the medication, some people get anhedonia, said W. Scott Butsch, MD, MSc, director of obesity medicine in the Bariatric and Metabolic Institute at Cleveland Clinic. But that could be a natural consequence of lower appetite, he said, given that food gives most people pleasure in the moment.
Many other reported changes come from the weight loss itself, not the medication, said Dr. Butsch.
“These are drugs that change the body’s weight regulatory system,” he said. “When someone loses weight, you get the shrinking of the fat cells, as well as the atrophy of the muscles. This rapid weight loss may give the appearance of one’s face changing.”
For some people, like Ms. Mendenall, the side effects are worth it. For others, like Ms. Carew, they’re intolerable.
Ms. Carew said she stopped the medication after about 7 months, and gradually worked up to eating solid foods again.
“It’s the American way, we’ve all got to be thin and beautiful,” she said. “But I feel like it’s very unsafe because we just don’t know how seriously our bodies will react to these things in the long term. People see it as a quick fix, but it comes with risks.”
A version of this article first appeared on WebMD.com.
Johnna Mendenall had never been “the skinny friend,” she said, but the demands of motherhood – along with a sedentary desk job – made weight management even more difficult. Worried that family type 2 diabetes would catch up with her, she decided to start Wegovy shots for weight loss.
She was nervous about potential side effects. It took 5 days of staring at the Wegovy pen before she worked up the nerve for her first .25-milligram shot. And sure enough, the side effects came on strong.
“The nausea kicked in,” she said. “When I increased my dose to 1 milligram, I spent the entire night from 10 p.m. to 5 a.m. vomiting. I almost quit that day.”
While gastrointestinal (GI) symptoms seem to be the most common, a laundry list of others has been discussed in the news, on TikTok, and across online forums. Those include “Ozempic face,” or the gaunt look some get after taking the medication, along with hair loss, anxiety, depression, and debilitating fatigue.
Ms. Mendenall’s primary side effects have been vomiting, fatigue, and severe constipation, but she has also seen some positive changes: The “food noise,” or the urge to eat when she isn’t hungry, is gone. Since her first dose 12 weeks ago, she has gone from 236 pounds to 215.
Warning label
Wegovy’s active ingredient, semaglutide, mimics the role of a natural hormone called glucagonlike peptide–1 (GLP-1), which helps you feel well fed. Semaglutide is used at a lower dose under the brand name Ozempic, which is approved for type 2 diabetes and used off-label for weight loss.
Both Ozempic and Wegovy come with a warning label for potential side effects, the most common ones being nausea, diarrhea, stomach pain, and vomiting.
With the surging popularity of semaglutide, more people are getting prescriptions through telemedicine companies, forgoing more in-depth consultations, leading to more side effects, said Caroline Apovian, MD, professor of medicine at Harvard Medical School and codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, Boston.
Specialists say starting with low doses and gradually increasing over time helps avoid side effects, but insurance companies often require a faster timeline to continue covering the medication, Dr. Apovian said.
“Insurance companies are practicing medicine for us by demanding the patient go up in dosage [too quickly],” she explained.
Ms. Mendenall’s insurance has paid for her Wegovy shots, but without that coverage, she said it would cost her $1,200 per month.
There are similar medications on the market, such as liraglutide, sold under the name Saxenda. But it is a daily, rather than a weekly, shot and also comes with side effects and has been shown to be less effective. In one clinical trial, the people being studied saw their average body weight over 68 weeks drop by 15.8% with semaglutide, and by 6.4% with liraglutide.
Tirzepatide, branded Mounjaro – a type 2 diabetes drug made by Eli Lilly that may soon gain Food and Drug Administration approval for weight loss – could have fewer side effects. In clinical trials, 44% of those taking semaglutide had nausea and 31% reported diarrhea, compared with 33% and 23% of those taking tirzepatide, although no trial has directly compared the two agents.
Loss of bowel control
For now, Wegovy and Saxenda are the only GLP-1 agonist shots authorized for weight loss, and their maker, Danish drug company Novo Nordisk, is facing its second shortage of Wegovy amid growing demand.
Personal stories online about semaglutide range from overwhelmingly positive – just what some need to win a lifelong battle with obesity – to harsh scenarios with potentially long-term health consequences, and everything in between.
One private community on Reddit is dedicated to a particularly unpleasant side effect: loss of bowel control while sleeping. Others have reported uncontrollable vomiting.
Kimberly Carew of Clearwater, Fla., started on .5 milligrams of Ozempic last year after her rheumatologist and endocrinologist suggested it to treat her type 2 diabetes. She was told it came with the bonus of weight loss, which she was hoping would help with her joint and back pain.
But after she increased the dose to 1 milligram, her GI symptoms, which started out mild, became unbearable. She couldn’t keep food down, and when she vomited, the food would often come up whole, she said.
“One night I ate ramen before bed. And the next morning, it came out just as it went down,” said Ms. Carew, 42, a registered mental health counseling intern. “I was getting severe heartburn and could not take a couple bites of food without getting nauseous.”
She also had “sulfur burps,” a side effect discussed by some Ozempic users, causing her to taste rotten egg sometimes.
She was diagnosed with gastroparesis. Some types of gastroparesis can be resolved by discontinuing GLP-1 medications, as referenced in two case reports in the Journal of Investigative Medicine.
Gut hormone
GI symptoms are most common with semaglutide because the hormone it imitates, GLP-1, is secreted by cells in the stomach, small intestines, and pancreas, said Anne Peters, MD, director of the USC Clinical Diabetes Programs.
“This is the deal: The side effects are real because it’s a gut hormone. It’s increasing the level of something your body already has,” she said.
But, like Dr. Apovian, Dr. Peters said those side effects can likely be avoided if shots are started at the lowest doses and gradually adjusted up.
While the average starting dose is .25 milligrams, Dr. Peters said she often starts her patients on about an eighth of that – just “a whiff of a dose.”
“It’ll take them months to get up to the starting dose, but what’s the rush?”
Dr. Peters said she also avoids giving diabetes patients the maximum dose, which is 2 milligrams per week for Ozempic (and 2.4 milligrams for Wegovy for weight loss).
When asked about the drugs’ side effects, Novo Nordisk responded that “GLP-1 receptor agonists are a well-established class of medicines, which have demonstrated long-term safety in clinical trials. The most common adverse reactions, as with all GLP-1 [agonists], are gastrointestinal related.”
Is it the drug or the weight loss?
Still, non-gastrointestinal side effects such as hair loss, mood changes, and sunken facial features are reported among semaglutide users across the Internet. While these cases are often anecdotal, they can be very heartfelt.
Celina Horvath Myers, also known as CelinaSpookyBoo, a Canadian YouTuber who took Ozempic for type 2 diabetes, said she began having intense panic attacks and depression after starting the medication.
“Who I have been these last couple weeks, has probably been the scariest time of my life,” she said on her YouTube channel.
While severe depression and anxiety are not established side effects of the medication, some people get anhedonia, said W. Scott Butsch, MD, MSc, director of obesity medicine in the Bariatric and Metabolic Institute at Cleveland Clinic. But that could be a natural consequence of lower appetite, he said, given that food gives most people pleasure in the moment.
Many other reported changes come from the weight loss itself, not the medication, said Dr. Butsch.
“These are drugs that change the body’s weight regulatory system,” he said. “When someone loses weight, you get the shrinking of the fat cells, as well as the atrophy of the muscles. This rapid weight loss may give the appearance of one’s face changing.”
For some people, like Ms. Mendenall, the side effects are worth it. For others, like Ms. Carew, they’re intolerable.
Ms. Carew said she stopped the medication after about 7 months, and gradually worked up to eating solid foods again.
“It’s the American way, we’ve all got to be thin and beautiful,” she said. “But I feel like it’s very unsafe because we just don’t know how seriously our bodies will react to these things in the long term. People see it as a quick fix, but it comes with risks.”
A version of this article first appeared on WebMD.com.
Johnna Mendenall had never been “the skinny friend,” she said, but the demands of motherhood – along with a sedentary desk job – made weight management even more difficult. Worried that family type 2 diabetes would catch up with her, she decided to start Wegovy shots for weight loss.
She was nervous about potential side effects. It took 5 days of staring at the Wegovy pen before she worked up the nerve for her first .25-milligram shot. And sure enough, the side effects came on strong.
“The nausea kicked in,” she said. “When I increased my dose to 1 milligram, I spent the entire night from 10 p.m. to 5 a.m. vomiting. I almost quit that day.”
While gastrointestinal (GI) symptoms seem to be the most common, a laundry list of others has been discussed in the news, on TikTok, and across online forums. Those include “Ozempic face,” or the gaunt look some get after taking the medication, along with hair loss, anxiety, depression, and debilitating fatigue.
Ms. Mendenall’s primary side effects have been vomiting, fatigue, and severe constipation, but she has also seen some positive changes: The “food noise,” or the urge to eat when she isn’t hungry, is gone. Since her first dose 12 weeks ago, she has gone from 236 pounds to 215.
Warning label
Wegovy’s active ingredient, semaglutide, mimics the role of a natural hormone called glucagonlike peptide–1 (GLP-1), which helps you feel well fed. Semaglutide is used at a lower dose under the brand name Ozempic, which is approved for type 2 diabetes and used off-label for weight loss.
Both Ozempic and Wegovy come with a warning label for potential side effects, the most common ones being nausea, diarrhea, stomach pain, and vomiting.
With the surging popularity of semaglutide, more people are getting prescriptions through telemedicine companies, forgoing more in-depth consultations, leading to more side effects, said Caroline Apovian, MD, professor of medicine at Harvard Medical School and codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, Boston.
Specialists say starting with low doses and gradually increasing over time helps avoid side effects, but insurance companies often require a faster timeline to continue covering the medication, Dr. Apovian said.
“Insurance companies are practicing medicine for us by demanding the patient go up in dosage [too quickly],” she explained.
Ms. Mendenall’s insurance has paid for her Wegovy shots, but without that coverage, she said it would cost her $1,200 per month.
There are similar medications on the market, such as liraglutide, sold under the name Saxenda. But it is a daily, rather than a weekly, shot and also comes with side effects and has been shown to be less effective. In one clinical trial, the people being studied saw their average body weight over 68 weeks drop by 15.8% with semaglutide, and by 6.4% with liraglutide.
Tirzepatide, branded Mounjaro – a type 2 diabetes drug made by Eli Lilly that may soon gain Food and Drug Administration approval for weight loss – could have fewer side effects. In clinical trials, 44% of those taking semaglutide had nausea and 31% reported diarrhea, compared with 33% and 23% of those taking tirzepatide, although no trial has directly compared the two agents.
Loss of bowel control
For now, Wegovy and Saxenda are the only GLP-1 agonist shots authorized for weight loss, and their maker, Danish drug company Novo Nordisk, is facing its second shortage of Wegovy amid growing demand.
Personal stories online about semaglutide range from overwhelmingly positive – just what some need to win a lifelong battle with obesity – to harsh scenarios with potentially long-term health consequences, and everything in between.
One private community on Reddit is dedicated to a particularly unpleasant side effect: loss of bowel control while sleeping. Others have reported uncontrollable vomiting.
Kimberly Carew of Clearwater, Fla., started on .5 milligrams of Ozempic last year after her rheumatologist and endocrinologist suggested it to treat her type 2 diabetes. She was told it came with the bonus of weight loss, which she was hoping would help with her joint and back pain.
But after she increased the dose to 1 milligram, her GI symptoms, which started out mild, became unbearable. She couldn’t keep food down, and when she vomited, the food would often come up whole, she said.
“One night I ate ramen before bed. And the next morning, it came out just as it went down,” said Ms. Carew, 42, a registered mental health counseling intern. “I was getting severe heartburn and could not take a couple bites of food without getting nauseous.”
She also had “sulfur burps,” a side effect discussed by some Ozempic users, causing her to taste rotten egg sometimes.
She was diagnosed with gastroparesis. Some types of gastroparesis can be resolved by discontinuing GLP-1 medications, as referenced in two case reports in the Journal of Investigative Medicine.
Gut hormone
GI symptoms are most common with semaglutide because the hormone it imitates, GLP-1, is secreted by cells in the stomach, small intestines, and pancreas, said Anne Peters, MD, director of the USC Clinical Diabetes Programs.
“This is the deal: The side effects are real because it’s a gut hormone. It’s increasing the level of something your body already has,” she said.
But, like Dr. Apovian, Dr. Peters said those side effects can likely be avoided if shots are started at the lowest doses and gradually adjusted up.
While the average starting dose is .25 milligrams, Dr. Peters said she often starts her patients on about an eighth of that – just “a whiff of a dose.”
“It’ll take them months to get up to the starting dose, but what’s the rush?”
Dr. Peters said she also avoids giving diabetes patients the maximum dose, which is 2 milligrams per week for Ozempic (and 2.4 milligrams for Wegovy for weight loss).
When asked about the drugs’ side effects, Novo Nordisk responded that “GLP-1 receptor agonists are a well-established class of medicines, which have demonstrated long-term safety in clinical trials. The most common adverse reactions, as with all GLP-1 [agonists], are gastrointestinal related.”
Is it the drug or the weight loss?
Still, non-gastrointestinal side effects such as hair loss, mood changes, and sunken facial features are reported among semaglutide users across the Internet. While these cases are often anecdotal, they can be very heartfelt.
Celina Horvath Myers, also known as CelinaSpookyBoo, a Canadian YouTuber who took Ozempic for type 2 diabetes, said she began having intense panic attacks and depression after starting the medication.
“Who I have been these last couple weeks, has probably been the scariest time of my life,” she said on her YouTube channel.
While severe depression and anxiety are not established side effects of the medication, some people get anhedonia, said W. Scott Butsch, MD, MSc, director of obesity medicine in the Bariatric and Metabolic Institute at Cleveland Clinic. But that could be a natural consequence of lower appetite, he said, given that food gives most people pleasure in the moment.
Many other reported changes come from the weight loss itself, not the medication, said Dr. Butsch.
“These are drugs that change the body’s weight regulatory system,” he said. “When someone loses weight, you get the shrinking of the fat cells, as well as the atrophy of the muscles. This rapid weight loss may give the appearance of one’s face changing.”
For some people, like Ms. Mendenall, the side effects are worth it. For others, like Ms. Carew, they’re intolerable.
Ms. Carew said she stopped the medication after about 7 months, and gradually worked up to eating solid foods again.
“It’s the American way, we’ve all got to be thin and beautiful,” she said. “But I feel like it’s very unsafe because we just don’t know how seriously our bodies will react to these things in the long term. People see it as a quick fix, but it comes with risks.”
A version of this article first appeared on WebMD.com.
PTSD: Children, adolescents, and all of us may be at risk
Not everyone will suffer an episode of posttraumatic stress disorder, even though everyday American life is characterized by a lot of uncertainty these days, particularly considering the proliferation of gun violence.
Also, everyone who does experience a traumatic event will not suffer an episode of PTSD – just as not everyone develops a heart attack or cancer, nor will everyone get every illness.
The data suggest that of those exposed to trauma, up to 25% of people will develop PTSD, according to Massachusetts General/McLean Hospital, Belmont, psychiatrist Kerry J. Ressler, MD, PhD, chief of the division of depression and anxiety disorders.
As I wrote in December 2022, our “kids” are not all right and psychiatry can help. I would say that many adolescents, and adults as well, may not be all right as we are terrorized not only by mass school shootings, but shootings happening almost anywhere and everywhere in our country: in supermarkets, hospitals, and shopping malls, at graduation parties, and on the streets.
According to a report published in Clinical Psychiatry News, a poll conducted by the American Psychiatric Association showed that most American adults [70%] reported that they were anxious or extremely anxious about keeping themselves or their families safe. APA President Rebecca W. Brendel, MD, JD, pointed out that there is “a lot of worry out there about economic uncertainty, about violence and how we are going to come out of this time period.”
Meanwhile, PTSD is still defined in the DSM-5 as exposure to actual or threatened death, serious injury, or sexual violence experienced directly, witnessing the traumatic event as it occurs to others, learning that a traumatic event occurred to a close family member or friend, or experiencing of traumatic events plus extreme exposure to aversive details of the event.
Examples of traumatic events can be numerous. They include natural disasters, man-made disasters, various types of assaults, war trauma, and severe illness with ICU experiences. I would add encounters with racism and bigotry – including homophobia when one fears for their very life or physical injury. This list includes only a few triggers that may invoke this disorder.
Interestingly, the DSM-5 excludes aversive exposure through electronic media, television, movies, or pictures. Including these aspects of trauma exposure would indeed increase PTSD diagnoses, and I believe this type of exposure needs to be included, especially considering how different people process information. Some viewers of media remain “outside” the events depicted on television, movies, or electronic media while others fit directly “into” the film or TV show. Even, for example, a news program, as evidenced by those people suffering from PTSD after viewing the Sept. 11, 2001, disaster on TV.
I have interviewed numerous people who witnessed Sept. 11 tragedies on TV, some during and some after the event, and they genuinely had experienced key factors of PTSD, including nightmares and intrusive recollections of the event. It’s important to include the ways in which people process information and events in order to make a correct diagnosis, in that “one [diagnostic] size does not fit all.”
PTSD at school
In my December column, I noted the fear of death that my generation and beyond experienced with the endless threat of nuclear war, which by its very nature meant death, and if not, the saying went “the living would envy the dead” – that is, in post–nuclear war.
As I pointed out in the column, that war never came and hopefully never will, yet the intensity of those many decades of threatened terror with regular school exercises of “hide under the desk” and “don’t look at the flash” left some with intrusive fearful thoughts, nightmares, and even visualization of atomic destruction, as well as the many scenes of destruction portrayed in news casts and films of nuclear explosions.
Clearly, most U.S. school children who participate in school lockdown drills will not suffer from PTSD episodes, but some will. If that “some” approaches 20% or even 10% or less, that will amount to a lot of kids.
I decided to interview two of my grandchildren, each living in different communities and attending different school systems, but both experiencing “lockdown drills.”
Jack, who is 13 and going into eighth grade, was quite clear regarding the drills and reported that in his age group, both he and the kids in his class felt scared while in lockdown. He told me some kids looked nervous. He mentioned that they were taught in school that if the “real thing” happened, the message was “hide, run, and fight.” I was curious and asked why not run first. He was quick to answer and said if you run, you might run into danger, so it’s better to hide and wait for help to arrive. I said to myself, if not PTSD, then being scared or nervous may also lead to anxiety or even to an anxiety disorder.
Next, I interviewed almost 11-year-old Charley, who is going into sixth grade. She was very clear about not at all being fearful or nervous during these drills and was confident that her classmates felt the same way. Then she explained that the school did a great job with a security officer and had locked doors all around that only opened from the inside. She was proud of the school and not fearful or worried at all.
The diverse views of these two young people surprised me but confirm that PTSD is not at all a given based on what is occurring in society. However, it should always be considered by clinicians if a child or adolescent begins to show signs consistent with PTSD.
These two interviews were quite short, but after I finished talking with Charley, she reported spontaneously that while she and her classmates were neither worried nor scared, some of their teachers did look nervous and seemed scared.
I was quite impressed with her sharpness and nuanced observation, and as noted, adults as well may be adversely affected by the entire concept of school lockdowns, as the awareness of their purpose rests in the forefront of their minds.
The way forward
So how do we prepare kids and adolescents for potential emotional problems like PTSD arising from lockdowns, even though most children or adults will not suffer any of these PTSD issues?
First, I believe that Second, it is important that school children be aware that if they feel bad in any way emotionally, they should speak to their parents, guardians, teachers, or school nurses.
Clearly, communicating simple problems without embarrassment or shame can lead to solutions, often quickly. Larger, more complicated issues may need professional intervention. Equally important, many mental health interventions need not be long in duration but client-centered, focused, and short term.
But what needs to be emphasized is that speaking and addressing what’s going on, if your thoughts and emotions are troubling, are in themselves therapeutic. Talk therapy works – especially if you get a new perspective on the old set of problems.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Not everyone will suffer an episode of posttraumatic stress disorder, even though everyday American life is characterized by a lot of uncertainty these days, particularly considering the proliferation of gun violence.
Also, everyone who does experience a traumatic event will not suffer an episode of PTSD – just as not everyone develops a heart attack or cancer, nor will everyone get every illness.
The data suggest that of those exposed to trauma, up to 25% of people will develop PTSD, according to Massachusetts General/McLean Hospital, Belmont, psychiatrist Kerry J. Ressler, MD, PhD, chief of the division of depression and anxiety disorders.
As I wrote in December 2022, our “kids” are not all right and psychiatry can help. I would say that many adolescents, and adults as well, may not be all right as we are terrorized not only by mass school shootings, but shootings happening almost anywhere and everywhere in our country: in supermarkets, hospitals, and shopping malls, at graduation parties, and on the streets.
According to a report published in Clinical Psychiatry News, a poll conducted by the American Psychiatric Association showed that most American adults [70%] reported that they were anxious or extremely anxious about keeping themselves or their families safe. APA President Rebecca W. Brendel, MD, JD, pointed out that there is “a lot of worry out there about economic uncertainty, about violence and how we are going to come out of this time period.”
Meanwhile, PTSD is still defined in the DSM-5 as exposure to actual or threatened death, serious injury, or sexual violence experienced directly, witnessing the traumatic event as it occurs to others, learning that a traumatic event occurred to a close family member or friend, or experiencing of traumatic events plus extreme exposure to aversive details of the event.
Examples of traumatic events can be numerous. They include natural disasters, man-made disasters, various types of assaults, war trauma, and severe illness with ICU experiences. I would add encounters with racism and bigotry – including homophobia when one fears for their very life or physical injury. This list includes only a few triggers that may invoke this disorder.
Interestingly, the DSM-5 excludes aversive exposure through electronic media, television, movies, or pictures. Including these aspects of trauma exposure would indeed increase PTSD diagnoses, and I believe this type of exposure needs to be included, especially considering how different people process information. Some viewers of media remain “outside” the events depicted on television, movies, or electronic media while others fit directly “into” the film or TV show. Even, for example, a news program, as evidenced by those people suffering from PTSD after viewing the Sept. 11, 2001, disaster on TV.
I have interviewed numerous people who witnessed Sept. 11 tragedies on TV, some during and some after the event, and they genuinely had experienced key factors of PTSD, including nightmares and intrusive recollections of the event. It’s important to include the ways in which people process information and events in order to make a correct diagnosis, in that “one [diagnostic] size does not fit all.”
PTSD at school
In my December column, I noted the fear of death that my generation and beyond experienced with the endless threat of nuclear war, which by its very nature meant death, and if not, the saying went “the living would envy the dead” – that is, in post–nuclear war.
As I pointed out in the column, that war never came and hopefully never will, yet the intensity of those many decades of threatened terror with regular school exercises of “hide under the desk” and “don’t look at the flash” left some with intrusive fearful thoughts, nightmares, and even visualization of atomic destruction, as well as the many scenes of destruction portrayed in news casts and films of nuclear explosions.
Clearly, most U.S. school children who participate in school lockdown drills will not suffer from PTSD episodes, but some will. If that “some” approaches 20% or even 10% or less, that will amount to a lot of kids.
I decided to interview two of my grandchildren, each living in different communities and attending different school systems, but both experiencing “lockdown drills.”
Jack, who is 13 and going into eighth grade, was quite clear regarding the drills and reported that in his age group, both he and the kids in his class felt scared while in lockdown. He told me some kids looked nervous. He mentioned that they were taught in school that if the “real thing” happened, the message was “hide, run, and fight.” I was curious and asked why not run first. He was quick to answer and said if you run, you might run into danger, so it’s better to hide and wait for help to arrive. I said to myself, if not PTSD, then being scared or nervous may also lead to anxiety or even to an anxiety disorder.
Next, I interviewed almost 11-year-old Charley, who is going into sixth grade. She was very clear about not at all being fearful or nervous during these drills and was confident that her classmates felt the same way. Then she explained that the school did a great job with a security officer and had locked doors all around that only opened from the inside. She was proud of the school and not fearful or worried at all.
The diverse views of these two young people surprised me but confirm that PTSD is not at all a given based on what is occurring in society. However, it should always be considered by clinicians if a child or adolescent begins to show signs consistent with PTSD.
These two interviews were quite short, but after I finished talking with Charley, she reported spontaneously that while she and her classmates were neither worried nor scared, some of their teachers did look nervous and seemed scared.
I was quite impressed with her sharpness and nuanced observation, and as noted, adults as well may be adversely affected by the entire concept of school lockdowns, as the awareness of their purpose rests in the forefront of their minds.
The way forward
So how do we prepare kids and adolescents for potential emotional problems like PTSD arising from lockdowns, even though most children or adults will not suffer any of these PTSD issues?
First, I believe that Second, it is important that school children be aware that if they feel bad in any way emotionally, they should speak to their parents, guardians, teachers, or school nurses.
Clearly, communicating simple problems without embarrassment or shame can lead to solutions, often quickly. Larger, more complicated issues may need professional intervention. Equally important, many mental health interventions need not be long in duration but client-centered, focused, and short term.
But what needs to be emphasized is that speaking and addressing what’s going on, if your thoughts and emotions are troubling, are in themselves therapeutic. Talk therapy works – especially if you get a new perspective on the old set of problems.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Not everyone will suffer an episode of posttraumatic stress disorder, even though everyday American life is characterized by a lot of uncertainty these days, particularly considering the proliferation of gun violence.
Also, everyone who does experience a traumatic event will not suffer an episode of PTSD – just as not everyone develops a heart attack or cancer, nor will everyone get every illness.
The data suggest that of those exposed to trauma, up to 25% of people will develop PTSD, according to Massachusetts General/McLean Hospital, Belmont, psychiatrist Kerry J. Ressler, MD, PhD, chief of the division of depression and anxiety disorders.
As I wrote in December 2022, our “kids” are not all right and psychiatry can help. I would say that many adolescents, and adults as well, may not be all right as we are terrorized not only by mass school shootings, but shootings happening almost anywhere and everywhere in our country: in supermarkets, hospitals, and shopping malls, at graduation parties, and on the streets.
According to a report published in Clinical Psychiatry News, a poll conducted by the American Psychiatric Association showed that most American adults [70%] reported that they were anxious or extremely anxious about keeping themselves or their families safe. APA President Rebecca W. Brendel, MD, JD, pointed out that there is “a lot of worry out there about economic uncertainty, about violence and how we are going to come out of this time period.”
Meanwhile, PTSD is still defined in the DSM-5 as exposure to actual or threatened death, serious injury, or sexual violence experienced directly, witnessing the traumatic event as it occurs to others, learning that a traumatic event occurred to a close family member or friend, or experiencing of traumatic events plus extreme exposure to aversive details of the event.
Examples of traumatic events can be numerous. They include natural disasters, man-made disasters, various types of assaults, war trauma, and severe illness with ICU experiences. I would add encounters with racism and bigotry – including homophobia when one fears for their very life or physical injury. This list includes only a few triggers that may invoke this disorder.
Interestingly, the DSM-5 excludes aversive exposure through electronic media, television, movies, or pictures. Including these aspects of trauma exposure would indeed increase PTSD diagnoses, and I believe this type of exposure needs to be included, especially considering how different people process information. Some viewers of media remain “outside” the events depicted on television, movies, or electronic media while others fit directly “into” the film or TV show. Even, for example, a news program, as evidenced by those people suffering from PTSD after viewing the Sept. 11, 2001, disaster on TV.
I have interviewed numerous people who witnessed Sept. 11 tragedies on TV, some during and some after the event, and they genuinely had experienced key factors of PTSD, including nightmares and intrusive recollections of the event. It’s important to include the ways in which people process information and events in order to make a correct diagnosis, in that “one [diagnostic] size does not fit all.”
PTSD at school
In my December column, I noted the fear of death that my generation and beyond experienced with the endless threat of nuclear war, which by its very nature meant death, and if not, the saying went “the living would envy the dead” – that is, in post–nuclear war.
As I pointed out in the column, that war never came and hopefully never will, yet the intensity of those many decades of threatened terror with regular school exercises of “hide under the desk” and “don’t look at the flash” left some with intrusive fearful thoughts, nightmares, and even visualization of atomic destruction, as well as the many scenes of destruction portrayed in news casts and films of nuclear explosions.
Clearly, most U.S. school children who participate in school lockdown drills will not suffer from PTSD episodes, but some will. If that “some” approaches 20% or even 10% or less, that will amount to a lot of kids.
I decided to interview two of my grandchildren, each living in different communities and attending different school systems, but both experiencing “lockdown drills.”
Jack, who is 13 and going into eighth grade, was quite clear regarding the drills and reported that in his age group, both he and the kids in his class felt scared while in lockdown. He told me some kids looked nervous. He mentioned that they were taught in school that if the “real thing” happened, the message was “hide, run, and fight.” I was curious and asked why not run first. He was quick to answer and said if you run, you might run into danger, so it’s better to hide and wait for help to arrive. I said to myself, if not PTSD, then being scared or nervous may also lead to anxiety or even to an anxiety disorder.
Next, I interviewed almost 11-year-old Charley, who is going into sixth grade. She was very clear about not at all being fearful or nervous during these drills and was confident that her classmates felt the same way. Then she explained that the school did a great job with a security officer and had locked doors all around that only opened from the inside. She was proud of the school and not fearful or worried at all.
The diverse views of these two young people surprised me but confirm that PTSD is not at all a given based on what is occurring in society. However, it should always be considered by clinicians if a child or adolescent begins to show signs consistent with PTSD.
These two interviews were quite short, but after I finished talking with Charley, she reported spontaneously that while she and her classmates were neither worried nor scared, some of their teachers did look nervous and seemed scared.
I was quite impressed with her sharpness and nuanced observation, and as noted, adults as well may be adversely affected by the entire concept of school lockdowns, as the awareness of their purpose rests in the forefront of their minds.
The way forward
So how do we prepare kids and adolescents for potential emotional problems like PTSD arising from lockdowns, even though most children or adults will not suffer any of these PTSD issues?
First, I believe that Second, it is important that school children be aware that if they feel bad in any way emotionally, they should speak to their parents, guardians, teachers, or school nurses.
Clearly, communicating simple problems without embarrassment or shame can lead to solutions, often quickly. Larger, more complicated issues may need professional intervention. Equally important, many mental health interventions need not be long in duration but client-centered, focused, and short term.
But what needs to be emphasized is that speaking and addressing what’s going on, if your thoughts and emotions are troubling, are in themselves therapeutic. Talk therapy works – especially if you get a new perspective on the old set of problems.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Good COP, bad COP. Is this cardiorespiratory measure the best predictor of early death?
according to clinicians who champion the assessment. The COP is easier to obtain than cardiorespiratory measures that require people to exercise to their limit, advocates say; rather than running full speed, someone can walk or lightly jog on a treadmill, with a COP value obtained easily.
But other clinicians argue that maximal exercise tests have many prognostic benefits, and that physicians should do everything in their power to push patients to exercise as hard as possible. In particular, the VO2 max test captures the maximum amount of oxygen someone uses when exercising at their capacity and is the preferred method for measuring cardiovascular endurance.
The COP is a measure of the minimum number of liters of air during breathing required to move one liter of oxygen through the bloodstream. The lower the COP the better, because this means that someone is working less strenuously than someone else to transport the same amount of oxygen, denoting a more efficient interaction between their heart and lungs.
The COP for a fit person might be 15, about 20-25 for a healthy person, and 35 for someone with heart failure, according to Claudio Gil Araújo, MD, PhD, director of research and education at CLINIMEX, an exercise medicine clinic in Rio de Janeiro.
“Max VO2 is very important, that’s indisputable. But when do you use max VO2 in your daily life? Never,” Dr. Araújo said. But almost anyone can generate a COP.
Emerging uses for the COP
“I can put someone on the treadmill or bike, and after 3 or 4 minutes I have the COP. It’s like a walking pace,” Dr. Araújo said. Yet the values are obtained with roughly half the effort as VO2 max. Other clinicians argue exercising to the limits of endurance offers unique clinical insights.
“We should do everything in our power to exercise our patients to maximum. How long a patient is able to go is really important,” said Anu Lala, MD, a cardiologist who specializes in heart failure treatment at Mount Sinai Hospital in New York. A full-capacity exercise test gives useful insights into someone’s heart rate, heart rate recovery, blood pressure, and ECG response to vigorous exercise, Dr. Lala added, all of which are important clues to someone’s overall health.
In 2012 Dr. Araújo coauthored a study that first defined the COP, which is calculated by measuring expired gasses people produce while gently exercising, perhaps to the point where they begin to perspire, and then dividing their breathing capacity by their oxygen uptake every minute. The lowest value obtained during any exercise session is the COP.
Various studies show that higher COP values are associated with more severe heart lesions in patients with congenital heart disease; higher levels of mortality in seemingly healthy male adults; and with worse prognoses in patients with heart failure. These studies all appeared within the last 7 months.
The mortality study, which Dr. Araújo coauthored, compared COP in more than 3,000 U.S. men and women who completed an exercise test from 1973 to 2018 and were tracked for an average of 23 years. Although COP was introduced as an assessment in 2012, calculating the value from tests prior to that date was possible because those tests had captured the relevant breathing rate and oxygen uptake. In males aged 18-85 years, a worse COP was significantly associated with an increased risk for earlier death. This finding did not hold for females, however; Dr. Araújo noted that more research is needed to understand the discrepancy in COP’s predictive power by sex.
In the heart failure study, everyone enrolled had heart failure and completed a COP test. People with the worse COPs also had the worst symptoms of heart failure, but completing an exercise rehabilitation program improved COP values when researchers measured them again. Dr. Araújo was also part of this study, based in the Netherlands.
“I think the COP could become a novel parameter in clinical care,” for most people, said Thijs Eijsvogels, PhD, an exercise physiologist at Radboud University in Nijmegen, the Netherlands, and the senior author of the heart failure study. That said, Dr. Eijsvogels said elite athletes will always be more interested in measuring VO2 max.
Dr. Lala agreed that tests such as the COP have some value. Her own work has shown that measuring the efficiency of someone’s breathing patterns for exhaling carbon dioxide, which can also be done without making people exercise full strength, has prognostic value for patients with advanced heart failure. Even so, she said she would like to see maximal effort tests used as much as possible.
“I worry about saying we’re going to settle for a parameter that can be achieved at 50% of peak VO2 and then we don’t exercise our patients,” Dr. Lala said.
Dr. Araújo said he plans to continue to measure VO2 max but he believes COP has utility – even for elite athletes. One of his patients is a frequent Ironman competitor who competes well despite having a solid but not amazing VO2 max level. But her COP is quite low, Dr. Araújo said, which to him suggests an especially efficient interaction between her respiratory and cardiovascular systems.
“We have a new player in the game,” Dr. Araújo said.
The sources in this study report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to clinicians who champion the assessment. The COP is easier to obtain than cardiorespiratory measures that require people to exercise to their limit, advocates say; rather than running full speed, someone can walk or lightly jog on a treadmill, with a COP value obtained easily.
But other clinicians argue that maximal exercise tests have many prognostic benefits, and that physicians should do everything in their power to push patients to exercise as hard as possible. In particular, the VO2 max test captures the maximum amount of oxygen someone uses when exercising at their capacity and is the preferred method for measuring cardiovascular endurance.
The COP is a measure of the minimum number of liters of air during breathing required to move one liter of oxygen through the bloodstream. The lower the COP the better, because this means that someone is working less strenuously than someone else to transport the same amount of oxygen, denoting a more efficient interaction between their heart and lungs.
The COP for a fit person might be 15, about 20-25 for a healthy person, and 35 for someone with heart failure, according to Claudio Gil Araújo, MD, PhD, director of research and education at CLINIMEX, an exercise medicine clinic in Rio de Janeiro.
“Max VO2 is very important, that’s indisputable. But when do you use max VO2 in your daily life? Never,” Dr. Araújo said. But almost anyone can generate a COP.
Emerging uses for the COP
“I can put someone on the treadmill or bike, and after 3 or 4 minutes I have the COP. It’s like a walking pace,” Dr. Araújo said. Yet the values are obtained with roughly half the effort as VO2 max. Other clinicians argue exercising to the limits of endurance offers unique clinical insights.
“We should do everything in our power to exercise our patients to maximum. How long a patient is able to go is really important,” said Anu Lala, MD, a cardiologist who specializes in heart failure treatment at Mount Sinai Hospital in New York. A full-capacity exercise test gives useful insights into someone’s heart rate, heart rate recovery, blood pressure, and ECG response to vigorous exercise, Dr. Lala added, all of which are important clues to someone’s overall health.
In 2012 Dr. Araújo coauthored a study that first defined the COP, which is calculated by measuring expired gasses people produce while gently exercising, perhaps to the point where they begin to perspire, and then dividing their breathing capacity by their oxygen uptake every minute. The lowest value obtained during any exercise session is the COP.
Various studies show that higher COP values are associated with more severe heart lesions in patients with congenital heart disease; higher levels of mortality in seemingly healthy male adults; and with worse prognoses in patients with heart failure. These studies all appeared within the last 7 months.
The mortality study, which Dr. Araújo coauthored, compared COP in more than 3,000 U.S. men and women who completed an exercise test from 1973 to 2018 and were tracked for an average of 23 years. Although COP was introduced as an assessment in 2012, calculating the value from tests prior to that date was possible because those tests had captured the relevant breathing rate and oxygen uptake. In males aged 18-85 years, a worse COP was significantly associated with an increased risk for earlier death. This finding did not hold for females, however; Dr. Araújo noted that more research is needed to understand the discrepancy in COP’s predictive power by sex.
In the heart failure study, everyone enrolled had heart failure and completed a COP test. People with the worse COPs also had the worst symptoms of heart failure, but completing an exercise rehabilitation program improved COP values when researchers measured them again. Dr. Araújo was also part of this study, based in the Netherlands.
“I think the COP could become a novel parameter in clinical care,” for most people, said Thijs Eijsvogels, PhD, an exercise physiologist at Radboud University in Nijmegen, the Netherlands, and the senior author of the heart failure study. That said, Dr. Eijsvogels said elite athletes will always be more interested in measuring VO2 max.
Dr. Lala agreed that tests such as the COP have some value. Her own work has shown that measuring the efficiency of someone’s breathing patterns for exhaling carbon dioxide, which can also be done without making people exercise full strength, has prognostic value for patients with advanced heart failure. Even so, she said she would like to see maximal effort tests used as much as possible.
“I worry about saying we’re going to settle for a parameter that can be achieved at 50% of peak VO2 and then we don’t exercise our patients,” Dr. Lala said.
Dr. Araújo said he plans to continue to measure VO2 max but he believes COP has utility – even for elite athletes. One of his patients is a frequent Ironman competitor who competes well despite having a solid but not amazing VO2 max level. But her COP is quite low, Dr. Araújo said, which to him suggests an especially efficient interaction between her respiratory and cardiovascular systems.
“We have a new player in the game,” Dr. Araújo said.
The sources in this study report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to clinicians who champion the assessment. The COP is easier to obtain than cardiorespiratory measures that require people to exercise to their limit, advocates say; rather than running full speed, someone can walk or lightly jog on a treadmill, with a COP value obtained easily.
But other clinicians argue that maximal exercise tests have many prognostic benefits, and that physicians should do everything in their power to push patients to exercise as hard as possible. In particular, the VO2 max test captures the maximum amount of oxygen someone uses when exercising at their capacity and is the preferred method for measuring cardiovascular endurance.
The COP is a measure of the minimum number of liters of air during breathing required to move one liter of oxygen through the bloodstream. The lower the COP the better, because this means that someone is working less strenuously than someone else to transport the same amount of oxygen, denoting a more efficient interaction between their heart and lungs.
The COP for a fit person might be 15, about 20-25 for a healthy person, and 35 for someone with heart failure, according to Claudio Gil Araújo, MD, PhD, director of research and education at CLINIMEX, an exercise medicine clinic in Rio de Janeiro.
“Max VO2 is very important, that’s indisputable. But when do you use max VO2 in your daily life? Never,” Dr. Araújo said. But almost anyone can generate a COP.
Emerging uses for the COP
“I can put someone on the treadmill or bike, and after 3 or 4 minutes I have the COP. It’s like a walking pace,” Dr. Araújo said. Yet the values are obtained with roughly half the effort as VO2 max. Other clinicians argue exercising to the limits of endurance offers unique clinical insights.
“We should do everything in our power to exercise our patients to maximum. How long a patient is able to go is really important,” said Anu Lala, MD, a cardiologist who specializes in heart failure treatment at Mount Sinai Hospital in New York. A full-capacity exercise test gives useful insights into someone’s heart rate, heart rate recovery, blood pressure, and ECG response to vigorous exercise, Dr. Lala added, all of which are important clues to someone’s overall health.
In 2012 Dr. Araújo coauthored a study that first defined the COP, which is calculated by measuring expired gasses people produce while gently exercising, perhaps to the point where they begin to perspire, and then dividing their breathing capacity by their oxygen uptake every minute. The lowest value obtained during any exercise session is the COP.
Various studies show that higher COP values are associated with more severe heart lesions in patients with congenital heart disease; higher levels of mortality in seemingly healthy male adults; and with worse prognoses in patients with heart failure. These studies all appeared within the last 7 months.
The mortality study, which Dr. Araújo coauthored, compared COP in more than 3,000 U.S. men and women who completed an exercise test from 1973 to 2018 and were tracked for an average of 23 years. Although COP was introduced as an assessment in 2012, calculating the value from tests prior to that date was possible because those tests had captured the relevant breathing rate and oxygen uptake. In males aged 18-85 years, a worse COP was significantly associated with an increased risk for earlier death. This finding did not hold for females, however; Dr. Araújo noted that more research is needed to understand the discrepancy in COP’s predictive power by sex.
In the heart failure study, everyone enrolled had heart failure and completed a COP test. People with the worse COPs also had the worst symptoms of heart failure, but completing an exercise rehabilitation program improved COP values when researchers measured them again. Dr. Araújo was also part of this study, based in the Netherlands.
“I think the COP could become a novel parameter in clinical care,” for most people, said Thijs Eijsvogels, PhD, an exercise physiologist at Radboud University in Nijmegen, the Netherlands, and the senior author of the heart failure study. That said, Dr. Eijsvogels said elite athletes will always be more interested in measuring VO2 max.
Dr. Lala agreed that tests such as the COP have some value. Her own work has shown that measuring the efficiency of someone’s breathing patterns for exhaling carbon dioxide, which can also be done without making people exercise full strength, has prognostic value for patients with advanced heart failure. Even so, she said she would like to see maximal effort tests used as much as possible.
“I worry about saying we’re going to settle for a parameter that can be achieved at 50% of peak VO2 and then we don’t exercise our patients,” Dr. Lala said.
Dr. Araújo said he plans to continue to measure VO2 max but he believes COP has utility – even for elite athletes. One of his patients is a frequent Ironman competitor who competes well despite having a solid but not amazing VO2 max level. But her COP is quite low, Dr. Araújo said, which to him suggests an especially efficient interaction between her respiratory and cardiovascular systems.
“We have a new player in the game,” Dr. Araújo said.
The sources in this study report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New EULAR lupus recommendations advise using biologics, tapering steroids
MILAN – Treatment of systemic lupus erythematosus with biologics may enable steroid tapering while ensuring the achievement of remission or low disease activity in more patients with fewer flares and less organ damage, as well as leading to better responses if used early, according to the latest recommendations on the management of SLE from the European Alliance of Associations for Rheumatology (EULAR).
Dimitrios Boumpas, MD, president of the Athens Medical Society and chair of the European Task force on SLE, presented the recommendations at the annual European Congress of Rheumatology. “Although steroids save lives, it is at the expense of excessive collateral damage. They are better for short-term use as a rescue or bridging therapy but may be used in some patients at 5 mg/day of prednisone or less, rather than the previous 7.5 mg/day,” he emphasized.
The 2023 recommendations cover new treatment strategies with more ambitious goals, new data on adverse effects of chronic glucocorticoid use, and newly approved agents and combination therapies.
“Most importantly, we sourced help from experts from all over the world,” said Dr. Boumpas, describing the task force that included 35 rheumatologists, 5 nephrologists, 2 methodologists, 2 patient representatives, and 2 fellows, all brought together from across Europe, North America, Asia, and Australia.
Over 7,000 papers were reviewed, with 437 included in the systematic literature review to inform the updated recommendations.
Session moderator Robert Landewé, MD, PhD, professor of clinical immunology and rheumatology at the University of Amsterdam, said that “the underlying heterogeneity and multisystem involvement of SLE can make it difficult to demonstrate and know which drugs work in the condition. However, these latest recommendations should encourage greater confidence to taper steroids early on and perhaps consider new biologic drugs, so that more patients can achieve better results sooner to prevent flares and organ damage, improve prognosis, and enhance their quality of life.”
Dr. Boumpas provided a summary of the overarching principles that guide the recommendations. These say that SLE requires multidisciplinary individualized management; disease activity should be assessed at each visit; nonpharmacologic interventions such as sun protection, smoking cessation, and following a healthy diet are all important for improving long-term outcomes; pharmacologic interventions are to be directed by patient characteristics, type and severity of organ involvement, treatment-related harms, and patient preferences, among other factors; and early SLE diagnosis is essential to prevent flares and organ damage, improve prognosis, and enhance quality of life.
Referring to each recommendation statement in turn, Dr. Boumpas provided a detailed description of each, and highlighted any changes since the 2019 recommendations.
Hydroxychloroquine, glucocorticoids as bridging therapy, and biologics
Referring to statement 1, Dr. Boumpas reported that hydroxychloroquine should be a first-line therapy at a dose of 5 mg/kg, but this dose should be individualized based on risk of flare and retinal toxicity. “There was some discussion about monitoring blood levels, but this was to ensure adherence only,” said Dr. Boumpas.
Continuing to statement 2, he added, “here is one change. With chronic use of glucocorticoids, the maintenance dose is 5 mg/day or less or prednisone equivalent. This pertains to both new onset and relapsing disease.” Previous recommendations advised a maintenance dose of 7.5 mg/day or less.
But he pointed out that “we are discussing using glucocorticoids in lupus as a bridging therapy only, for short, limited periods of time. We should shy away from chronic use of glucocorticoids and only use them for 3 months, and to do this we need to use glucocorticoid-sparing strategies.”
This led to statement 3, which refers to glucocorticoid-sparing strategies. Dr. Boumpas explained that, in patients who are not responding to hydroxychloroquine or unable to reduce glucocorticoids further during chronic use, add immunosuppressive agents, such as methotrexate and/or biologics (for example, belimumab [Benlysta] or anifrolumab [Saphnelo]).
“To allow flexibility for patients and clinicians, it isn’t necessary to use DMARDs [disease-modifying antirheumatic drugs] first if you prefer biologics,” he continued. “We are becoming more liberal with the use of biologics because there are new data that confirm the efficacy of belimumab in extrarenal SLE, plus good data with 3-year extension with anifrolumab.”
Statement 4 says that for patients with organ- or life-threatening disease, intravenous cyclophosphamide, “our old friend,” should be considered, while in refractory cases, rituximab may be considered, Dr. Boumpas said. “It’s okay to use cyclophosphamide. It isn’t a sin.”
Statement 5 refers to skin disease, and Dr. Boumpas explained that good data suggested that biologics help, including both belimumab and anifrolumab.
Nothing has changed with statement 6 concerning neuropsychiatric lupus, said Dr. Boumpas. “Glucocorticoids, immunosuppressive, and antithrombotic therapies should be considered.”
Regarding hematologic disease (statement 7), he said, “the new kid on the block is MMF [mycophenolate mofetil]. For acute treatment, still use the same drugs, including rituximab, but for maintenance you may use rituximab, azathioprine, MMF, or cyclosporine.”
Lupus nephritis
Turning to what Dr. Boumpas described as the “reason you had all come here, and what you had been waiting for ... what’s changing with lupus nephritis?” he said.
Statement 8 describes initial therapy in active lupus nephritis. Dr. Boumpas said that low-dose, intravenous cyclophosphamide or mycophenolate should be considered, but also that belimumab or a calcineurin inhibitor (CNI) should be considered at the start. The changes were based on two successful phase 3 trials of belimumab and voclosporin, with belimumab being associated with a reduced flare rate and estimated glomerular filtration rate (eGFR).
“Changes from 2019 include that there is no distinction between classes III/IV and V, which is heretical,” he stressed. Belimumab and CNIs/voclosporin should be considered in all patents as an add-on therapy from the start. “Lupus nephritis has high morbidity, and it’s difficult to predict outcomes at the beginning, but there are clear benefits of add-on therapies. CNIs, although they can be used for all patients, might be more appropriate for membranous or nephrotic-range proteinuria.”
He went on to announce that the “million-dollar question” was whether to use belimumab or voclosporin (or other CNIs), and that this was “a question of gentle, compared with forceful, power and collateral damage.
“For me, voclosporin works very fast, but you worry about side effects, while belimumab is gentle and the response is sustained, preventing flares and organ damage,” he said, adding that “our expert panel discussions showed that nephrologists were more eager to support steroid-free regimens.”
Moving on to statement 9, Dr. Boumpas explained that after initial therapy and renal response, subsequent therapy should continue for at least 3 years. If treated with MMF alone or in combination with belimumab, then these drugs should continue. However, MMF should replace cyclophosphamide if the latter is used initially.
Regarding treat-to-target in lupus nephritis, he said that EULAR now advises to aim for a 25% drop in urine protein/creatinine ratio by 3 months, a 50% drop by 6 months, and a UPCR of less than 0.5-0.7, plus normal eGFR, by 12 months, Dr. Boumpas said.
Statement 10 advises considering high-dose intravenous cyclophosphamide in combination with pulse intravenous methylprednisolone for patients at high risk of renal failure.
Tapering drugs in sustained remission, managing antiphospholipid syndrome, giving immunizations
Statement 11 suggests to consider tapering immunosuppressive agents and glucocorticoids in patients achieving sustained remission, starting with glucocorticoids first.
There was no change to statement 12, which recommends that thrombotic antiphospholipid syndrome associated with SLE be treated with long-term vitamin K antagonists.
Statement 13 addresses immunizations and adjunct therapies. In addition to conventional immunizations, Dr. Boumpas said that renoprotection should receive attention in case of proteinuria and/or hypertension.
“With [sodium-glucose cotransporter 2] inhibitors, it’s a bit early. They’re promising, and you may consider them, although there are no data for patients with eGFR below 60 mL/min per 1.73 m2,” he remarked, completing his detailed discussion of the updated recommendations.
Dr. Boumpas reported no relevant financial relationships. Dr. Landewé served as past chair of EULAR’s Quality of Care Committee, which develops recommendations.
MILAN – Treatment of systemic lupus erythematosus with biologics may enable steroid tapering while ensuring the achievement of remission or low disease activity in more patients with fewer flares and less organ damage, as well as leading to better responses if used early, according to the latest recommendations on the management of SLE from the European Alliance of Associations for Rheumatology (EULAR).
Dimitrios Boumpas, MD, president of the Athens Medical Society and chair of the European Task force on SLE, presented the recommendations at the annual European Congress of Rheumatology. “Although steroids save lives, it is at the expense of excessive collateral damage. They are better for short-term use as a rescue or bridging therapy but may be used in some patients at 5 mg/day of prednisone or less, rather than the previous 7.5 mg/day,” he emphasized.
The 2023 recommendations cover new treatment strategies with more ambitious goals, new data on adverse effects of chronic glucocorticoid use, and newly approved agents and combination therapies.
“Most importantly, we sourced help from experts from all over the world,” said Dr. Boumpas, describing the task force that included 35 rheumatologists, 5 nephrologists, 2 methodologists, 2 patient representatives, and 2 fellows, all brought together from across Europe, North America, Asia, and Australia.
Over 7,000 papers were reviewed, with 437 included in the systematic literature review to inform the updated recommendations.
Session moderator Robert Landewé, MD, PhD, professor of clinical immunology and rheumatology at the University of Amsterdam, said that “the underlying heterogeneity and multisystem involvement of SLE can make it difficult to demonstrate and know which drugs work in the condition. However, these latest recommendations should encourage greater confidence to taper steroids early on and perhaps consider new biologic drugs, so that more patients can achieve better results sooner to prevent flares and organ damage, improve prognosis, and enhance their quality of life.”
Dr. Boumpas provided a summary of the overarching principles that guide the recommendations. These say that SLE requires multidisciplinary individualized management; disease activity should be assessed at each visit; nonpharmacologic interventions such as sun protection, smoking cessation, and following a healthy diet are all important for improving long-term outcomes; pharmacologic interventions are to be directed by patient characteristics, type and severity of organ involvement, treatment-related harms, and patient preferences, among other factors; and early SLE diagnosis is essential to prevent flares and organ damage, improve prognosis, and enhance quality of life.
Referring to each recommendation statement in turn, Dr. Boumpas provided a detailed description of each, and highlighted any changes since the 2019 recommendations.
Hydroxychloroquine, glucocorticoids as bridging therapy, and biologics
Referring to statement 1, Dr. Boumpas reported that hydroxychloroquine should be a first-line therapy at a dose of 5 mg/kg, but this dose should be individualized based on risk of flare and retinal toxicity. “There was some discussion about monitoring blood levels, but this was to ensure adherence only,” said Dr. Boumpas.
Continuing to statement 2, he added, “here is one change. With chronic use of glucocorticoids, the maintenance dose is 5 mg/day or less or prednisone equivalent. This pertains to both new onset and relapsing disease.” Previous recommendations advised a maintenance dose of 7.5 mg/day or less.
But he pointed out that “we are discussing using glucocorticoids in lupus as a bridging therapy only, for short, limited periods of time. We should shy away from chronic use of glucocorticoids and only use them for 3 months, and to do this we need to use glucocorticoid-sparing strategies.”
This led to statement 3, which refers to glucocorticoid-sparing strategies. Dr. Boumpas explained that, in patients who are not responding to hydroxychloroquine or unable to reduce glucocorticoids further during chronic use, add immunosuppressive agents, such as methotrexate and/or biologics (for example, belimumab [Benlysta] or anifrolumab [Saphnelo]).
“To allow flexibility for patients and clinicians, it isn’t necessary to use DMARDs [disease-modifying antirheumatic drugs] first if you prefer biologics,” he continued. “We are becoming more liberal with the use of biologics because there are new data that confirm the efficacy of belimumab in extrarenal SLE, plus good data with 3-year extension with anifrolumab.”
Statement 4 says that for patients with organ- or life-threatening disease, intravenous cyclophosphamide, “our old friend,” should be considered, while in refractory cases, rituximab may be considered, Dr. Boumpas said. “It’s okay to use cyclophosphamide. It isn’t a sin.”
Statement 5 refers to skin disease, and Dr. Boumpas explained that good data suggested that biologics help, including both belimumab and anifrolumab.
Nothing has changed with statement 6 concerning neuropsychiatric lupus, said Dr. Boumpas. “Glucocorticoids, immunosuppressive, and antithrombotic therapies should be considered.”
Regarding hematologic disease (statement 7), he said, “the new kid on the block is MMF [mycophenolate mofetil]. For acute treatment, still use the same drugs, including rituximab, but for maintenance you may use rituximab, azathioprine, MMF, or cyclosporine.”
Lupus nephritis
Turning to what Dr. Boumpas described as the “reason you had all come here, and what you had been waiting for ... what’s changing with lupus nephritis?” he said.
Statement 8 describes initial therapy in active lupus nephritis. Dr. Boumpas said that low-dose, intravenous cyclophosphamide or mycophenolate should be considered, but also that belimumab or a calcineurin inhibitor (CNI) should be considered at the start. The changes were based on two successful phase 3 trials of belimumab and voclosporin, with belimumab being associated with a reduced flare rate and estimated glomerular filtration rate (eGFR).
“Changes from 2019 include that there is no distinction between classes III/IV and V, which is heretical,” he stressed. Belimumab and CNIs/voclosporin should be considered in all patents as an add-on therapy from the start. “Lupus nephritis has high morbidity, and it’s difficult to predict outcomes at the beginning, but there are clear benefits of add-on therapies. CNIs, although they can be used for all patients, might be more appropriate for membranous or nephrotic-range proteinuria.”
He went on to announce that the “million-dollar question” was whether to use belimumab or voclosporin (or other CNIs), and that this was “a question of gentle, compared with forceful, power and collateral damage.
“For me, voclosporin works very fast, but you worry about side effects, while belimumab is gentle and the response is sustained, preventing flares and organ damage,” he said, adding that “our expert panel discussions showed that nephrologists were more eager to support steroid-free regimens.”
Moving on to statement 9, Dr. Boumpas explained that after initial therapy and renal response, subsequent therapy should continue for at least 3 years. If treated with MMF alone or in combination with belimumab, then these drugs should continue. However, MMF should replace cyclophosphamide if the latter is used initially.
Regarding treat-to-target in lupus nephritis, he said that EULAR now advises to aim for a 25% drop in urine protein/creatinine ratio by 3 months, a 50% drop by 6 months, and a UPCR of less than 0.5-0.7, plus normal eGFR, by 12 months, Dr. Boumpas said.
Statement 10 advises considering high-dose intravenous cyclophosphamide in combination with pulse intravenous methylprednisolone for patients at high risk of renal failure.
Tapering drugs in sustained remission, managing antiphospholipid syndrome, giving immunizations
Statement 11 suggests to consider tapering immunosuppressive agents and glucocorticoids in patients achieving sustained remission, starting with glucocorticoids first.
There was no change to statement 12, which recommends that thrombotic antiphospholipid syndrome associated with SLE be treated with long-term vitamin K antagonists.
Statement 13 addresses immunizations and adjunct therapies. In addition to conventional immunizations, Dr. Boumpas said that renoprotection should receive attention in case of proteinuria and/or hypertension.
“With [sodium-glucose cotransporter 2] inhibitors, it’s a bit early. They’re promising, and you may consider them, although there are no data for patients with eGFR below 60 mL/min per 1.73 m2,” he remarked, completing his detailed discussion of the updated recommendations.
Dr. Boumpas reported no relevant financial relationships. Dr. Landewé served as past chair of EULAR’s Quality of Care Committee, which develops recommendations.
MILAN – Treatment of systemic lupus erythematosus with biologics may enable steroid tapering while ensuring the achievement of remission or low disease activity in more patients with fewer flares and less organ damage, as well as leading to better responses if used early, according to the latest recommendations on the management of SLE from the European Alliance of Associations for Rheumatology (EULAR).
Dimitrios Boumpas, MD, president of the Athens Medical Society and chair of the European Task force on SLE, presented the recommendations at the annual European Congress of Rheumatology. “Although steroids save lives, it is at the expense of excessive collateral damage. They are better for short-term use as a rescue or bridging therapy but may be used in some patients at 5 mg/day of prednisone or less, rather than the previous 7.5 mg/day,” he emphasized.
The 2023 recommendations cover new treatment strategies with more ambitious goals, new data on adverse effects of chronic glucocorticoid use, and newly approved agents and combination therapies.
“Most importantly, we sourced help from experts from all over the world,” said Dr. Boumpas, describing the task force that included 35 rheumatologists, 5 nephrologists, 2 methodologists, 2 patient representatives, and 2 fellows, all brought together from across Europe, North America, Asia, and Australia.
Over 7,000 papers were reviewed, with 437 included in the systematic literature review to inform the updated recommendations.
Session moderator Robert Landewé, MD, PhD, professor of clinical immunology and rheumatology at the University of Amsterdam, said that “the underlying heterogeneity and multisystem involvement of SLE can make it difficult to demonstrate and know which drugs work in the condition. However, these latest recommendations should encourage greater confidence to taper steroids early on and perhaps consider new biologic drugs, so that more patients can achieve better results sooner to prevent flares and organ damage, improve prognosis, and enhance their quality of life.”
Dr. Boumpas provided a summary of the overarching principles that guide the recommendations. These say that SLE requires multidisciplinary individualized management; disease activity should be assessed at each visit; nonpharmacologic interventions such as sun protection, smoking cessation, and following a healthy diet are all important for improving long-term outcomes; pharmacologic interventions are to be directed by patient characteristics, type and severity of organ involvement, treatment-related harms, and patient preferences, among other factors; and early SLE diagnosis is essential to prevent flares and organ damage, improve prognosis, and enhance quality of life.
Referring to each recommendation statement in turn, Dr. Boumpas provided a detailed description of each, and highlighted any changes since the 2019 recommendations.
Hydroxychloroquine, glucocorticoids as bridging therapy, and biologics
Referring to statement 1, Dr. Boumpas reported that hydroxychloroquine should be a first-line therapy at a dose of 5 mg/kg, but this dose should be individualized based on risk of flare and retinal toxicity. “There was some discussion about monitoring blood levels, but this was to ensure adherence only,” said Dr. Boumpas.
Continuing to statement 2, he added, “here is one change. With chronic use of glucocorticoids, the maintenance dose is 5 mg/day or less or prednisone equivalent. This pertains to both new onset and relapsing disease.” Previous recommendations advised a maintenance dose of 7.5 mg/day or less.
But he pointed out that “we are discussing using glucocorticoids in lupus as a bridging therapy only, for short, limited periods of time. We should shy away from chronic use of glucocorticoids and only use them for 3 months, and to do this we need to use glucocorticoid-sparing strategies.”
This led to statement 3, which refers to glucocorticoid-sparing strategies. Dr. Boumpas explained that, in patients who are not responding to hydroxychloroquine or unable to reduce glucocorticoids further during chronic use, add immunosuppressive agents, such as methotrexate and/or biologics (for example, belimumab [Benlysta] or anifrolumab [Saphnelo]).
“To allow flexibility for patients and clinicians, it isn’t necessary to use DMARDs [disease-modifying antirheumatic drugs] first if you prefer biologics,” he continued. “We are becoming more liberal with the use of biologics because there are new data that confirm the efficacy of belimumab in extrarenal SLE, plus good data with 3-year extension with anifrolumab.”
Statement 4 says that for patients with organ- or life-threatening disease, intravenous cyclophosphamide, “our old friend,” should be considered, while in refractory cases, rituximab may be considered, Dr. Boumpas said. “It’s okay to use cyclophosphamide. It isn’t a sin.”
Statement 5 refers to skin disease, and Dr. Boumpas explained that good data suggested that biologics help, including both belimumab and anifrolumab.
Nothing has changed with statement 6 concerning neuropsychiatric lupus, said Dr. Boumpas. “Glucocorticoids, immunosuppressive, and antithrombotic therapies should be considered.”
Regarding hematologic disease (statement 7), he said, “the new kid on the block is MMF [mycophenolate mofetil]. For acute treatment, still use the same drugs, including rituximab, but for maintenance you may use rituximab, azathioprine, MMF, or cyclosporine.”
Lupus nephritis
Turning to what Dr. Boumpas described as the “reason you had all come here, and what you had been waiting for ... what’s changing with lupus nephritis?” he said.
Statement 8 describes initial therapy in active lupus nephritis. Dr. Boumpas said that low-dose, intravenous cyclophosphamide or mycophenolate should be considered, but also that belimumab or a calcineurin inhibitor (CNI) should be considered at the start. The changes were based on two successful phase 3 trials of belimumab and voclosporin, with belimumab being associated with a reduced flare rate and estimated glomerular filtration rate (eGFR).
“Changes from 2019 include that there is no distinction between classes III/IV and V, which is heretical,” he stressed. Belimumab and CNIs/voclosporin should be considered in all patents as an add-on therapy from the start. “Lupus nephritis has high morbidity, and it’s difficult to predict outcomes at the beginning, but there are clear benefits of add-on therapies. CNIs, although they can be used for all patients, might be more appropriate for membranous or nephrotic-range proteinuria.”
He went on to announce that the “million-dollar question” was whether to use belimumab or voclosporin (or other CNIs), and that this was “a question of gentle, compared with forceful, power and collateral damage.
“For me, voclosporin works very fast, but you worry about side effects, while belimumab is gentle and the response is sustained, preventing flares and organ damage,” he said, adding that “our expert panel discussions showed that nephrologists were more eager to support steroid-free regimens.”
Moving on to statement 9, Dr. Boumpas explained that after initial therapy and renal response, subsequent therapy should continue for at least 3 years. If treated with MMF alone or in combination with belimumab, then these drugs should continue. However, MMF should replace cyclophosphamide if the latter is used initially.
Regarding treat-to-target in lupus nephritis, he said that EULAR now advises to aim for a 25% drop in urine protein/creatinine ratio by 3 months, a 50% drop by 6 months, and a UPCR of less than 0.5-0.7, plus normal eGFR, by 12 months, Dr. Boumpas said.
Statement 10 advises considering high-dose intravenous cyclophosphamide in combination with pulse intravenous methylprednisolone for patients at high risk of renal failure.
Tapering drugs in sustained remission, managing antiphospholipid syndrome, giving immunizations
Statement 11 suggests to consider tapering immunosuppressive agents and glucocorticoids in patients achieving sustained remission, starting with glucocorticoids first.
There was no change to statement 12, which recommends that thrombotic antiphospholipid syndrome associated with SLE be treated with long-term vitamin K antagonists.
Statement 13 addresses immunizations and adjunct therapies. In addition to conventional immunizations, Dr. Boumpas said that renoprotection should receive attention in case of proteinuria and/or hypertension.
“With [sodium-glucose cotransporter 2] inhibitors, it’s a bit early. They’re promising, and you may consider them, although there are no data for patients with eGFR below 60 mL/min per 1.73 m2,” he remarked, completing his detailed discussion of the updated recommendations.
Dr. Boumpas reported no relevant financial relationships. Dr. Landewé served as past chair of EULAR’s Quality of Care Committee, which develops recommendations.
AT EULAR 2023
Low-dose oral minoxidil for hair loss soars after NYT article
.
The weekly rate of first-time low-dose oral minoxidil (LDOM) prescriptions per 10,000 outpatient encounters was “significantly higher 8 weeks after vs. 8 weeks before article publication,” at 0.9 prescriptions, compared with 0.5 per 10,000, wrote the authors of the research letter, published in JAMA Network Open. There was no similar bump for first-time finasteride or hypertension prescriptions, wrote the authors, from Harvard Medical School and Massachusetts General Hospital, Boston, and Truveta, a company that provides EHR data from U.S. health care systems.
The New York Times article noted that LDOM was relatively unknown to patients and doctors – and not approved by the Food and Drug Administration for treating hair loss – but that it was inexpensive, safe, and very effective for many individuals. “The article did not report new research findings or large-scale randomized evidence,” wrote the authors of the JAMA study.
Rodney Sinclair, MD, professor of dermatology at the University of Melbourne, who conducted the original research on LDOM and hair loss and was quoted in the Times story, told this news organization that “the sharp uplift after the New York Times article was on the back of a gradual increase.” He added that “the momentum for minoxidil prescriptions is increasing,” so much so that it has led to a global shortage of LDOM. The drug appears to still be widely available in the United States, however. It is not on the ASHP shortages list.
“There has been growing momentum for minoxidil use since I first presented our data about 6 years ago,” Dr. Sinclair said. He noted that 2022 International Society of Hair Restoration Surgery survey data found that 26% of treating physicians always or often prescribed off-label oral minoxidil, up from 10% in 2019 and 0% in 2017, while another 20% said they prescribed it sometimes.
The authors of the new study looked at prescriptions for patients at eight health care systems before and after the Times article was published in August 2022. They calculated the rate of first-time oral minoxidil prescriptions for 2.5 mg and 5 mg tablets, excluding 10 mg tablets, which are prescribed for hypertension.
Among those receiving first-time prescriptions, 2,846 received them in the 7 months before the article and 3,695 in the 5 months after publication. Men (43.6% after vs. 37.7% before publication) and White individuals (68.6% after vs. 60.8% before publication) accounted for a higher proportion of prescriptions after the article was published. There was a 2.4-fold increase in first-time prescriptions among men, and a 1.7-fold increase among females, while people with comorbidities accounted for a smaller proportion after the publication.
“Socioeconomic factors, such as access to health care and education and income levels, may be associated with individuals seeking low-dose oral minoxidil after article publication,” wrote the authors.
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said that he was not surprised to see an uptick in prescriptions after the Times article.
He and his colleagues were curious as to whether the article might have prompted newfound interest in LDOM. They experienced an uptick at George Washington, which Dr. Friedman thought could have been because he was quoted in the Times story. He and colleagues conducted a national survey of dermatologists asking if more patients had called, emailed, or come in to the office asking about LDOM after the article’s publication. “Over 85% said yes,” Dr. Friedman said in the interview. He and his coauthors also found a huge increase in Google searches for terms such as hair loss, alopecia, and minoxidil in the weeks after the article, he said.
The results are expected to published soon in the Journal of Drugs in Dermatology.
“I think a lot of people know about [LDOM] and it’s certainly has gained a lot more attention and acceptance in recent years,” said Dr. Friedman, but he added that “there’s no question” that the Times article increased interest.
That is not necessarily a bad thing, he said. “With one article, education on a common disease was disseminated worldwide in a way that no one doctor can do,” he said. The article was truthful, evidence-based, and included expert dermatologists, he noted.
“It probably got people who never thought twice about their hair thinning to actually think that there’s hope,” he said, adding that it also likely prompted them to seek care, and, more importantly, “to seek care from the person who should be taking care of this, which is the dermatologist.”
However, the article might also inspire some people to think LDOM can help when it can’t, or they might insist on a prescription when another medication is more appropriate, said Dr. Friedman.
Both he and Dr. Sinclair expect demand for LDOM to continue increasing.
“Word of mouth will drive the next wave of prescriptions,” said Dr. Sinclair. “We are continuing to do work to improve safety, to understand its mechanism of action, and identify ways to improve equity of access to treatment for men and women who are concerned about their hair loss and motivated to treat it,” he said.
Dr. Sinclair and Dr. Friedman report no relevant financial relationships.
.
The weekly rate of first-time low-dose oral minoxidil (LDOM) prescriptions per 10,000 outpatient encounters was “significantly higher 8 weeks after vs. 8 weeks before article publication,” at 0.9 prescriptions, compared with 0.5 per 10,000, wrote the authors of the research letter, published in JAMA Network Open. There was no similar bump for first-time finasteride or hypertension prescriptions, wrote the authors, from Harvard Medical School and Massachusetts General Hospital, Boston, and Truveta, a company that provides EHR data from U.S. health care systems.
The New York Times article noted that LDOM was relatively unknown to patients and doctors – and not approved by the Food and Drug Administration for treating hair loss – but that it was inexpensive, safe, and very effective for many individuals. “The article did not report new research findings or large-scale randomized evidence,” wrote the authors of the JAMA study.
Rodney Sinclair, MD, professor of dermatology at the University of Melbourne, who conducted the original research on LDOM and hair loss and was quoted in the Times story, told this news organization that “the sharp uplift after the New York Times article was on the back of a gradual increase.” He added that “the momentum for minoxidil prescriptions is increasing,” so much so that it has led to a global shortage of LDOM. The drug appears to still be widely available in the United States, however. It is not on the ASHP shortages list.
“There has been growing momentum for minoxidil use since I first presented our data about 6 years ago,” Dr. Sinclair said. He noted that 2022 International Society of Hair Restoration Surgery survey data found that 26% of treating physicians always or often prescribed off-label oral minoxidil, up from 10% in 2019 and 0% in 2017, while another 20% said they prescribed it sometimes.
The authors of the new study looked at prescriptions for patients at eight health care systems before and after the Times article was published in August 2022. They calculated the rate of first-time oral minoxidil prescriptions for 2.5 mg and 5 mg tablets, excluding 10 mg tablets, which are prescribed for hypertension.
Among those receiving first-time prescriptions, 2,846 received them in the 7 months before the article and 3,695 in the 5 months after publication. Men (43.6% after vs. 37.7% before publication) and White individuals (68.6% after vs. 60.8% before publication) accounted for a higher proportion of prescriptions after the article was published. There was a 2.4-fold increase in first-time prescriptions among men, and a 1.7-fold increase among females, while people with comorbidities accounted for a smaller proportion after the publication.
“Socioeconomic factors, such as access to health care and education and income levels, may be associated with individuals seeking low-dose oral minoxidil after article publication,” wrote the authors.
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said that he was not surprised to see an uptick in prescriptions after the Times article.
He and his colleagues were curious as to whether the article might have prompted newfound interest in LDOM. They experienced an uptick at George Washington, which Dr. Friedman thought could have been because he was quoted in the Times story. He and colleagues conducted a national survey of dermatologists asking if more patients had called, emailed, or come in to the office asking about LDOM after the article’s publication. “Over 85% said yes,” Dr. Friedman said in the interview. He and his coauthors also found a huge increase in Google searches for terms such as hair loss, alopecia, and minoxidil in the weeks after the article, he said.
The results are expected to published soon in the Journal of Drugs in Dermatology.
“I think a lot of people know about [LDOM] and it’s certainly has gained a lot more attention and acceptance in recent years,” said Dr. Friedman, but he added that “there’s no question” that the Times article increased interest.
That is not necessarily a bad thing, he said. “With one article, education on a common disease was disseminated worldwide in a way that no one doctor can do,” he said. The article was truthful, evidence-based, and included expert dermatologists, he noted.
“It probably got people who never thought twice about their hair thinning to actually think that there’s hope,” he said, adding that it also likely prompted them to seek care, and, more importantly, “to seek care from the person who should be taking care of this, which is the dermatologist.”
However, the article might also inspire some people to think LDOM can help when it can’t, or they might insist on a prescription when another medication is more appropriate, said Dr. Friedman.
Both he and Dr. Sinclair expect demand for LDOM to continue increasing.
“Word of mouth will drive the next wave of prescriptions,” said Dr. Sinclair. “We are continuing to do work to improve safety, to understand its mechanism of action, and identify ways to improve equity of access to treatment for men and women who are concerned about their hair loss and motivated to treat it,” he said.
Dr. Sinclair and Dr. Friedman report no relevant financial relationships.
.
The weekly rate of first-time low-dose oral minoxidil (LDOM) prescriptions per 10,000 outpatient encounters was “significantly higher 8 weeks after vs. 8 weeks before article publication,” at 0.9 prescriptions, compared with 0.5 per 10,000, wrote the authors of the research letter, published in JAMA Network Open. There was no similar bump for first-time finasteride or hypertension prescriptions, wrote the authors, from Harvard Medical School and Massachusetts General Hospital, Boston, and Truveta, a company that provides EHR data from U.S. health care systems.
The New York Times article noted that LDOM was relatively unknown to patients and doctors – and not approved by the Food and Drug Administration for treating hair loss – but that it was inexpensive, safe, and very effective for many individuals. “The article did not report new research findings or large-scale randomized evidence,” wrote the authors of the JAMA study.
Rodney Sinclair, MD, professor of dermatology at the University of Melbourne, who conducted the original research on LDOM and hair loss and was quoted in the Times story, told this news organization that “the sharp uplift after the New York Times article was on the back of a gradual increase.” He added that “the momentum for minoxidil prescriptions is increasing,” so much so that it has led to a global shortage of LDOM. The drug appears to still be widely available in the United States, however. It is not on the ASHP shortages list.
“There has been growing momentum for minoxidil use since I first presented our data about 6 years ago,” Dr. Sinclair said. He noted that 2022 International Society of Hair Restoration Surgery survey data found that 26% of treating physicians always or often prescribed off-label oral minoxidil, up from 10% in 2019 and 0% in 2017, while another 20% said they prescribed it sometimes.
The authors of the new study looked at prescriptions for patients at eight health care systems before and after the Times article was published in August 2022. They calculated the rate of first-time oral minoxidil prescriptions for 2.5 mg and 5 mg tablets, excluding 10 mg tablets, which are prescribed for hypertension.
Among those receiving first-time prescriptions, 2,846 received them in the 7 months before the article and 3,695 in the 5 months after publication. Men (43.6% after vs. 37.7% before publication) and White individuals (68.6% after vs. 60.8% before publication) accounted for a higher proportion of prescriptions after the article was published. There was a 2.4-fold increase in first-time prescriptions among men, and a 1.7-fold increase among females, while people with comorbidities accounted for a smaller proportion after the publication.
“Socioeconomic factors, such as access to health care and education and income levels, may be associated with individuals seeking low-dose oral minoxidil after article publication,” wrote the authors.
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said that he was not surprised to see an uptick in prescriptions after the Times article.
He and his colleagues were curious as to whether the article might have prompted newfound interest in LDOM. They experienced an uptick at George Washington, which Dr. Friedman thought could have been because he was quoted in the Times story. He and colleagues conducted a national survey of dermatologists asking if more patients had called, emailed, or come in to the office asking about LDOM after the article’s publication. “Over 85% said yes,” Dr. Friedman said in the interview. He and his coauthors also found a huge increase in Google searches for terms such as hair loss, alopecia, and minoxidil in the weeks after the article, he said.
The results are expected to published soon in the Journal of Drugs in Dermatology.
“I think a lot of people know about [LDOM] and it’s certainly has gained a lot more attention and acceptance in recent years,” said Dr. Friedman, but he added that “there’s no question” that the Times article increased interest.
That is not necessarily a bad thing, he said. “With one article, education on a common disease was disseminated worldwide in a way that no one doctor can do,” he said. The article was truthful, evidence-based, and included expert dermatologists, he noted.
“It probably got people who never thought twice about their hair thinning to actually think that there’s hope,” he said, adding that it also likely prompted them to seek care, and, more importantly, “to seek care from the person who should be taking care of this, which is the dermatologist.”
However, the article might also inspire some people to think LDOM can help when it can’t, or they might insist on a prescription when another medication is more appropriate, said Dr. Friedman.
Both he and Dr. Sinclair expect demand for LDOM to continue increasing.
“Word of mouth will drive the next wave of prescriptions,” said Dr. Sinclair. “We are continuing to do work to improve safety, to understand its mechanism of action, and identify ways to improve equity of access to treatment for men and women who are concerned about their hair loss and motivated to treat it,” he said.
Dr. Sinclair and Dr. Friedman report no relevant financial relationships.
FROM JAMA NETWORK OPEN
Patient selection key to lowering placebo response rates in lupus clinical trials
SEOUL, SOUTH KOREA – A major challenge for clinical trials in systemic lupus erythematosus (SLE) is how to get the placebo response rate down low enough that the effectiveness of a drug can actually be seen. Better patient selection may be the key.
Speaking at an international congress on SLE, Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, presented on how the heterogeneity of lupus is contributing to the ongoing failure of so many potential therapies in clinical trials.
“It’s a miracle that any drug has been successful in clinical trials,” she told the conference, comparing the few drugs approved for the treatment of lupus with the much larger numbers of approved, targeted biologics that are available for rheumatoid arthritis.
The problem is that placebo response rates in clinical trials for lupus are high – well over 40% – Dr. Merrill said, and trials aren’t showing a big difference in response rates between the treatment and placebo arms. “If the placebo response is 40%, wouldn’t an effective drug help 80%?” she said. “If it also affects only 40%, does that mean it’s a failed drug?”
Dr. Merrill suggested that better patient selection could be key to achieving lower placebo response rates, which could in turn reveal if and in whom a drug might be effective. “If we could get the placebo response rate down, at least we’d be able to see a little bit better whether the drug is effective, even if it only could work in 50% of the patients,” she said.
Data from research done by the Oklahoma Medical Research Foundation suggested that patients with SLE could be loosely categorized into seven different clusters based on patterns of gene expression in areas such as interferon expression and inflammation pathways.
For example, two of those clusters represented patients with high levels of expression for both interferons and inflammation. “Maybe those are the patients who’d want to be put in a trial for interferon inhibition,” Dr. Merrill said.
This was demonstrated in a trial of type 1 interferon inhibitor anifrolumab (Saphnelo), where patients were sorted into groups according to their level of interferon expression – either high or low – based on expression of certain interferon genes. This revealed that patients in the interferon-high group had a much higher treatment effect than patients in the interferon-low group. But the difference lay in the placebo response.
“The efficacy rate was not that different between the interferon-high and the interferon-low patients,” Dr. Merrill said. “The difference was in the placebo response rate – what they had managed to find was a great marker for sicker patients.”
This phenomenon is not limited to interferon-targeted therapies. Dr. Merrill cited another literature review which looked at subset studies within clinical trials that had delivered disappointing results. This showed consistently that patients who were considered more unwell, by virtue of higher SLE Disease Activity Index (SLEDAI) scores, for example, were more likely to show an effect of treatment.
“You begin to see bigger differences between treatment and placebo because the treatment rate might go up, but mostly because the placebo rate goes down,” she said.
Another issue that could be affecting both placebo and treatment response rates is background medication. “Subset analysis of people on less background drugs was showing lower placebo response rates and better differences between treatments and placebo,” Dr. Merrill said. For example, a recent phase 2 study of anifrolumab took the strategy of actively pursuing tapering of glucocorticoids in patients where that could be done safely. That achieved a lowering of the placebo response rate to the point where a greater difference could be seen between the placebo response and the treatment response rates.
The challenge for clinical trials is therefore to identify which patients to include. “If we could figure out which patients would be the most appropriate [to enroll to fit a particular drug’s mechanism of action], then we could really get ahead of the game,” she said.
The unique problem for lupus clinic trials is the heterogeneity of lupus as a disease, Dr. Merrill said in an interview. “We’re going to have to find combinations of treatments that fit right for each patient, and they won’t necessarily be one size fits all,” she said.
Dr. Merrill said that subset analyses at the phase 2 stage could help identify the patients who responded better to the treatment and could therefore be targeted in phase 3 trials. “Once you take that hypothesis, and if you can establish and validate it in phase 3, now you’ve got yourself a biomarker,” she said.
Richard A. Furie, MD, chief of the division of rheumatology at Northwell Health in New York, agreed that the high placebo response rate was a particular nemesis for researchers involved in lupus clinical trials.
Dr. Furie said it could be that selecting sicker patients is a solution to this, as had been suggested in the subset analysis of the anifrolumab studies – which he was involved in – that identified differences in the response rates between interferon-high and interferon-low patients.
But if that was the case, the challenge would be recruiting enough of any particular subset of patients. For example, relatively few patients in the anifrolumab trial were classified as interferon low.
If the interferon expression levels are a marker for patients who are sicker, that could serve as a way to better select patients for clinical trials, he said. But it would also make it harder to achieve recruitment targets.
“I think the major problem in SLE trials is that patients have inflated activity scores, so you can gain SLEDAI scores with a little alopecia and an oral ulcer,” he said. “You can start eliminating those parameters from counting towards entry, but then as soon as you do that, you’re going to have trouble recruiting.”
Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and anifrolumab manufacturer AstraZeneca. Dr. Furie reported financial relationships with Genentech/Roche, GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda.
SEOUL, SOUTH KOREA – A major challenge for clinical trials in systemic lupus erythematosus (SLE) is how to get the placebo response rate down low enough that the effectiveness of a drug can actually be seen. Better patient selection may be the key.
Speaking at an international congress on SLE, Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, presented on how the heterogeneity of lupus is contributing to the ongoing failure of so many potential therapies in clinical trials.
“It’s a miracle that any drug has been successful in clinical trials,” she told the conference, comparing the few drugs approved for the treatment of lupus with the much larger numbers of approved, targeted biologics that are available for rheumatoid arthritis.
The problem is that placebo response rates in clinical trials for lupus are high – well over 40% – Dr. Merrill said, and trials aren’t showing a big difference in response rates between the treatment and placebo arms. “If the placebo response is 40%, wouldn’t an effective drug help 80%?” she said. “If it also affects only 40%, does that mean it’s a failed drug?”
Dr. Merrill suggested that better patient selection could be key to achieving lower placebo response rates, which could in turn reveal if and in whom a drug might be effective. “If we could get the placebo response rate down, at least we’d be able to see a little bit better whether the drug is effective, even if it only could work in 50% of the patients,” she said.
Data from research done by the Oklahoma Medical Research Foundation suggested that patients with SLE could be loosely categorized into seven different clusters based on patterns of gene expression in areas such as interferon expression and inflammation pathways.
For example, two of those clusters represented patients with high levels of expression for both interferons and inflammation. “Maybe those are the patients who’d want to be put in a trial for interferon inhibition,” Dr. Merrill said.
This was demonstrated in a trial of type 1 interferon inhibitor anifrolumab (Saphnelo), where patients were sorted into groups according to their level of interferon expression – either high or low – based on expression of certain interferon genes. This revealed that patients in the interferon-high group had a much higher treatment effect than patients in the interferon-low group. But the difference lay in the placebo response.
“The efficacy rate was not that different between the interferon-high and the interferon-low patients,” Dr. Merrill said. “The difference was in the placebo response rate – what they had managed to find was a great marker for sicker patients.”
This phenomenon is not limited to interferon-targeted therapies. Dr. Merrill cited another literature review which looked at subset studies within clinical trials that had delivered disappointing results. This showed consistently that patients who were considered more unwell, by virtue of higher SLE Disease Activity Index (SLEDAI) scores, for example, were more likely to show an effect of treatment.
“You begin to see bigger differences between treatment and placebo because the treatment rate might go up, but mostly because the placebo rate goes down,” she said.
Another issue that could be affecting both placebo and treatment response rates is background medication. “Subset analysis of people on less background drugs was showing lower placebo response rates and better differences between treatments and placebo,” Dr. Merrill said. For example, a recent phase 2 study of anifrolumab took the strategy of actively pursuing tapering of glucocorticoids in patients where that could be done safely. That achieved a lowering of the placebo response rate to the point where a greater difference could be seen between the placebo response and the treatment response rates.
The challenge for clinical trials is therefore to identify which patients to include. “If we could figure out which patients would be the most appropriate [to enroll to fit a particular drug’s mechanism of action], then we could really get ahead of the game,” she said.
The unique problem for lupus clinic trials is the heterogeneity of lupus as a disease, Dr. Merrill said in an interview. “We’re going to have to find combinations of treatments that fit right for each patient, and they won’t necessarily be one size fits all,” she said.
Dr. Merrill said that subset analyses at the phase 2 stage could help identify the patients who responded better to the treatment and could therefore be targeted in phase 3 trials. “Once you take that hypothesis, and if you can establish and validate it in phase 3, now you’ve got yourself a biomarker,” she said.
Richard A. Furie, MD, chief of the division of rheumatology at Northwell Health in New York, agreed that the high placebo response rate was a particular nemesis for researchers involved in lupus clinical trials.
Dr. Furie said it could be that selecting sicker patients is a solution to this, as had been suggested in the subset analysis of the anifrolumab studies – which he was involved in – that identified differences in the response rates between interferon-high and interferon-low patients.
But if that was the case, the challenge would be recruiting enough of any particular subset of patients. For example, relatively few patients in the anifrolumab trial were classified as interferon low.
If the interferon expression levels are a marker for patients who are sicker, that could serve as a way to better select patients for clinical trials, he said. But it would also make it harder to achieve recruitment targets.
“I think the major problem in SLE trials is that patients have inflated activity scores, so you can gain SLEDAI scores with a little alopecia and an oral ulcer,” he said. “You can start eliminating those parameters from counting towards entry, but then as soon as you do that, you’re going to have trouble recruiting.”
Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and anifrolumab manufacturer AstraZeneca. Dr. Furie reported financial relationships with Genentech/Roche, GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda.
SEOUL, SOUTH KOREA – A major challenge for clinical trials in systemic lupus erythematosus (SLE) is how to get the placebo response rate down low enough that the effectiveness of a drug can actually be seen. Better patient selection may be the key.
Speaking at an international congress on SLE, Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, presented on how the heterogeneity of lupus is contributing to the ongoing failure of so many potential therapies in clinical trials.
“It’s a miracle that any drug has been successful in clinical trials,” she told the conference, comparing the few drugs approved for the treatment of lupus with the much larger numbers of approved, targeted biologics that are available for rheumatoid arthritis.
The problem is that placebo response rates in clinical trials for lupus are high – well over 40% – Dr. Merrill said, and trials aren’t showing a big difference in response rates between the treatment and placebo arms. “If the placebo response is 40%, wouldn’t an effective drug help 80%?” she said. “If it also affects only 40%, does that mean it’s a failed drug?”
Dr. Merrill suggested that better patient selection could be key to achieving lower placebo response rates, which could in turn reveal if and in whom a drug might be effective. “If we could get the placebo response rate down, at least we’d be able to see a little bit better whether the drug is effective, even if it only could work in 50% of the patients,” she said.
Data from research done by the Oklahoma Medical Research Foundation suggested that patients with SLE could be loosely categorized into seven different clusters based on patterns of gene expression in areas such as interferon expression and inflammation pathways.
For example, two of those clusters represented patients with high levels of expression for both interferons and inflammation. “Maybe those are the patients who’d want to be put in a trial for interferon inhibition,” Dr. Merrill said.
This was demonstrated in a trial of type 1 interferon inhibitor anifrolumab (Saphnelo), where patients were sorted into groups according to their level of interferon expression – either high or low – based on expression of certain interferon genes. This revealed that patients in the interferon-high group had a much higher treatment effect than patients in the interferon-low group. But the difference lay in the placebo response.
“The efficacy rate was not that different between the interferon-high and the interferon-low patients,” Dr. Merrill said. “The difference was in the placebo response rate – what they had managed to find was a great marker for sicker patients.”
This phenomenon is not limited to interferon-targeted therapies. Dr. Merrill cited another literature review which looked at subset studies within clinical trials that had delivered disappointing results. This showed consistently that patients who were considered more unwell, by virtue of higher SLE Disease Activity Index (SLEDAI) scores, for example, were more likely to show an effect of treatment.
“You begin to see bigger differences between treatment and placebo because the treatment rate might go up, but mostly because the placebo rate goes down,” she said.
Another issue that could be affecting both placebo and treatment response rates is background medication. “Subset analysis of people on less background drugs was showing lower placebo response rates and better differences between treatments and placebo,” Dr. Merrill said. For example, a recent phase 2 study of anifrolumab took the strategy of actively pursuing tapering of glucocorticoids in patients where that could be done safely. That achieved a lowering of the placebo response rate to the point where a greater difference could be seen between the placebo response and the treatment response rates.
The challenge for clinical trials is therefore to identify which patients to include. “If we could figure out which patients would be the most appropriate [to enroll to fit a particular drug’s mechanism of action], then we could really get ahead of the game,” she said.
The unique problem for lupus clinic trials is the heterogeneity of lupus as a disease, Dr. Merrill said in an interview. “We’re going to have to find combinations of treatments that fit right for each patient, and they won’t necessarily be one size fits all,” she said.
Dr. Merrill said that subset analyses at the phase 2 stage could help identify the patients who responded better to the treatment and could therefore be targeted in phase 3 trials. “Once you take that hypothesis, and if you can establish and validate it in phase 3, now you’ve got yourself a biomarker,” she said.
Richard A. Furie, MD, chief of the division of rheumatology at Northwell Health in New York, agreed that the high placebo response rate was a particular nemesis for researchers involved in lupus clinical trials.
Dr. Furie said it could be that selecting sicker patients is a solution to this, as had been suggested in the subset analysis of the anifrolumab studies – which he was involved in – that identified differences in the response rates between interferon-high and interferon-low patients.
But if that was the case, the challenge would be recruiting enough of any particular subset of patients. For example, relatively few patients in the anifrolumab trial were classified as interferon low.
If the interferon expression levels are a marker for patients who are sicker, that could serve as a way to better select patients for clinical trials, he said. But it would also make it harder to achieve recruitment targets.
“I think the major problem in SLE trials is that patients have inflated activity scores, so you can gain SLEDAI scores with a little alopecia and an oral ulcer,” he said. “You can start eliminating those parameters from counting towards entry, but then as soon as you do that, you’re going to have trouble recruiting.”
Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and anifrolumab manufacturer AstraZeneca. Dr. Furie reported financial relationships with Genentech/Roche, GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda.
AT LUPUS 2023