User login
RA and demyelinating disease: No consistent link to TNFi
MILAN – Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors (TNFi) does not appear to demonstrate a consistent and significant risk for demyelinating disease, according to a systematic literature review presented at the annual European Congress of Rheumatology.
The review was conducted by Isabel Castrejon, MD, of the rheumatology department at Gregorio Marañón General University Hospital, Madrid, and colleagues. “In male RA patients, a marginal and slight increase in risk was found. The low number of events provides reassurance regarding the use of these drugs. However, careful consideration is recommended for individuals at the highest risk of demyelinating diseases,” Dr. Castrejon said in an interview. “Health care providers should evaluate the potential benefits and risks of TNFi treatment on a case-by-case basis and closely monitor patients for any signs or symptoms of demyelinating events.”
The researchers performed the review because early data from biologic registries did not provide sufficient clarity, and the association between TNFi exposure and inflammatory central nervous system events remains poorly understood.
Key findings from the analyzed data
Dr. Castrejon and colleagues’ review considered 368 studies that included patients with RA, treatment with any biologic including TNFi and synthetic disease-modifying antirheumatic drugs (DMARDs), and demyelinating event.
The studies focused on assessing the risk of demyelinating events following treatment with biologics, particularly TNFi. Some studies included only patients with RA, while others examined mixed forms of arthritis. In cases involving mixed populations, patients with RA were analyzed separately. Additionally, certain studies solely considered multiple sclerosis, while others encompassed various types of demyelinating events. Dr. Castrejon said that a meta-analysis of the studies could not be performed because of their heterogeneity.
Among the 368 studies, four observational cohort studies and three nested case-control studies reported a risk of demyelinating events following treatment with biologics. Two nested case-control studies indicated an increased risk in mixed populations but did not separately analyze the subgroup of patients with RA. Two observational cohort studies revealed a marginally increased risk in men with RA who undergo TNFi treatment. In the first study, the incidence was 19.7/100,000 patient-years (95% confidence interval, 13.7-27.3) with a standardized incidence ratio of 1.38 (95% CI, 0.96-1.92), a definite case risk ratio of 0.83 (95% CI, 0.51-1.26), and an RR for male patients of 2.75 (95% CI, 1.31-5.06). The second study had an SIR of 1.11 (95% CI, 0.63-1.93), a RR for patients with RA of 0.65 (95% CI, 0.24-1.72), and male RR of 3.48 (95% CI, 1.45-8.37).
An unresolved question is whether demyelinating events are attributable to the underlying disease itself, which may not have been recognized at the time of diagnosis, or whether they are caused by DMARDs. Additionally, the articles that the reviewers analyzed did not consider patient characteristics that could interact with other factors, such as comorbidities or smoking, that might influence their susceptibility to the development of demyelinating events.
How should clinicians manage patients with RA who are at high risk of developing demyelinating diseases? “Typically, we initiate treatment with conventional synthetic disease-modifying methotrexate and then progress to other drugs,” Maya H. Buch, MBChB, PhD, professor of rheumatology at the University of Leeds (England), said in an interview. “For patients in high-risk groups, there are alternative treatment strategies, especially in comparison to TNFi, where there may be a rationale for their use.” Dr. Buch was not involved in the review.
Dr. Castrejon and colleagues reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors (TNFi) does not appear to demonstrate a consistent and significant risk for demyelinating disease, according to a systematic literature review presented at the annual European Congress of Rheumatology.
The review was conducted by Isabel Castrejon, MD, of the rheumatology department at Gregorio Marañón General University Hospital, Madrid, and colleagues. “In male RA patients, a marginal and slight increase in risk was found. The low number of events provides reassurance regarding the use of these drugs. However, careful consideration is recommended for individuals at the highest risk of demyelinating diseases,” Dr. Castrejon said in an interview. “Health care providers should evaluate the potential benefits and risks of TNFi treatment on a case-by-case basis and closely monitor patients for any signs or symptoms of demyelinating events.”
The researchers performed the review because early data from biologic registries did not provide sufficient clarity, and the association between TNFi exposure and inflammatory central nervous system events remains poorly understood.
Key findings from the analyzed data
Dr. Castrejon and colleagues’ review considered 368 studies that included patients with RA, treatment with any biologic including TNFi and synthetic disease-modifying antirheumatic drugs (DMARDs), and demyelinating event.
The studies focused on assessing the risk of demyelinating events following treatment with biologics, particularly TNFi. Some studies included only patients with RA, while others examined mixed forms of arthritis. In cases involving mixed populations, patients with RA were analyzed separately. Additionally, certain studies solely considered multiple sclerosis, while others encompassed various types of demyelinating events. Dr. Castrejon said that a meta-analysis of the studies could not be performed because of their heterogeneity.
Among the 368 studies, four observational cohort studies and three nested case-control studies reported a risk of demyelinating events following treatment with biologics. Two nested case-control studies indicated an increased risk in mixed populations but did not separately analyze the subgroup of patients with RA. Two observational cohort studies revealed a marginally increased risk in men with RA who undergo TNFi treatment. In the first study, the incidence was 19.7/100,000 patient-years (95% confidence interval, 13.7-27.3) with a standardized incidence ratio of 1.38 (95% CI, 0.96-1.92), a definite case risk ratio of 0.83 (95% CI, 0.51-1.26), and an RR for male patients of 2.75 (95% CI, 1.31-5.06). The second study had an SIR of 1.11 (95% CI, 0.63-1.93), a RR for patients with RA of 0.65 (95% CI, 0.24-1.72), and male RR of 3.48 (95% CI, 1.45-8.37).
An unresolved question is whether demyelinating events are attributable to the underlying disease itself, which may not have been recognized at the time of diagnosis, or whether they are caused by DMARDs. Additionally, the articles that the reviewers analyzed did not consider patient characteristics that could interact with other factors, such as comorbidities or smoking, that might influence their susceptibility to the development of demyelinating events.
How should clinicians manage patients with RA who are at high risk of developing demyelinating diseases? “Typically, we initiate treatment with conventional synthetic disease-modifying methotrexate and then progress to other drugs,” Maya H. Buch, MBChB, PhD, professor of rheumatology at the University of Leeds (England), said in an interview. “For patients in high-risk groups, there are alternative treatment strategies, especially in comparison to TNFi, where there may be a rationale for their use.” Dr. Buch was not involved in the review.
Dr. Castrejon and colleagues reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors (TNFi) does not appear to demonstrate a consistent and significant risk for demyelinating disease, according to a systematic literature review presented at the annual European Congress of Rheumatology.
The review was conducted by Isabel Castrejon, MD, of the rheumatology department at Gregorio Marañón General University Hospital, Madrid, and colleagues. “In male RA patients, a marginal and slight increase in risk was found. The low number of events provides reassurance regarding the use of these drugs. However, careful consideration is recommended for individuals at the highest risk of demyelinating diseases,” Dr. Castrejon said in an interview. “Health care providers should evaluate the potential benefits and risks of TNFi treatment on a case-by-case basis and closely monitor patients for any signs or symptoms of demyelinating events.”
The researchers performed the review because early data from biologic registries did not provide sufficient clarity, and the association between TNFi exposure and inflammatory central nervous system events remains poorly understood.
Key findings from the analyzed data
Dr. Castrejon and colleagues’ review considered 368 studies that included patients with RA, treatment with any biologic including TNFi and synthetic disease-modifying antirheumatic drugs (DMARDs), and demyelinating event.
The studies focused on assessing the risk of demyelinating events following treatment with biologics, particularly TNFi. Some studies included only patients with RA, while others examined mixed forms of arthritis. In cases involving mixed populations, patients with RA were analyzed separately. Additionally, certain studies solely considered multiple sclerosis, while others encompassed various types of demyelinating events. Dr. Castrejon said that a meta-analysis of the studies could not be performed because of their heterogeneity.
Among the 368 studies, four observational cohort studies and three nested case-control studies reported a risk of demyelinating events following treatment with biologics. Two nested case-control studies indicated an increased risk in mixed populations but did not separately analyze the subgroup of patients with RA. Two observational cohort studies revealed a marginally increased risk in men with RA who undergo TNFi treatment. In the first study, the incidence was 19.7/100,000 patient-years (95% confidence interval, 13.7-27.3) with a standardized incidence ratio of 1.38 (95% CI, 0.96-1.92), a definite case risk ratio of 0.83 (95% CI, 0.51-1.26), and an RR for male patients of 2.75 (95% CI, 1.31-5.06). The second study had an SIR of 1.11 (95% CI, 0.63-1.93), a RR for patients with RA of 0.65 (95% CI, 0.24-1.72), and male RR of 3.48 (95% CI, 1.45-8.37).
An unresolved question is whether demyelinating events are attributable to the underlying disease itself, which may not have been recognized at the time of diagnosis, or whether they are caused by DMARDs. Additionally, the articles that the reviewers analyzed did not consider patient characteristics that could interact with other factors, such as comorbidities or smoking, that might influence their susceptibility to the development of demyelinating events.
How should clinicians manage patients with RA who are at high risk of developing demyelinating diseases? “Typically, we initiate treatment with conventional synthetic disease-modifying methotrexate and then progress to other drugs,” Maya H. Buch, MBChB, PhD, professor of rheumatology at the University of Leeds (England), said in an interview. “For patients in high-risk groups, there are alternative treatment strategies, especially in comparison to TNFi, where there may be a rationale for their use.” Dr. Buch was not involved in the review.
Dr. Castrejon and colleagues reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
High-intensity interval training has sustainable effects in patients with inflammatory arthritis
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
Early hysterectomy linked to higher CVD, stroke risk
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Pediatric Crohn’s disease: Adalimumab plus methotrexate offers strong benefit
Children initiating treatment with adalimumab plus a low dose of methotrexate experienced a twofold reduction in treatment failure, note the authors of the largest, double-blind, randomized trial to date in pediatric Crohn’s disease. However, children initiating infliximab, another TNFi, had similar outcomes with or without methotrexate.
“We believe these results are practice changing,” said principal investigator Michael Kappelman, MD, MPH, professor of pediatrics at University of North Carolina.
All patients with pediatric Crohn’s disease starting on adalimumab, and their parents, should be informed that combining the drug with low-dose oral methotrexate improves treatment effectiveness, he said.
“Those without contraindications should be offered combination therapy, and shared decision-making should be incorporated into final treatment decisions. In contrast, most patients starting infliximab are not likely to experience added benefits from low-dose oral methotrexate,” Dr. Kappelman added.
The study was published online in Gastroenterology and was presented in May in Chicago at the annual Digestive Disease Week® (DDW).
Impactful study
“This is an important study, published in a very high-ranking journal, that will have a huge impact on how we practice,” Jacob Kurowski, MD, department of pediatric gastroenterology, hepatology and nutrition, Cleveland Clinic Children’s, who wasn’t involved in the study, said.
Treatment with a TNFi, including infliximab and adalimumab, is a mainstay of pediatric Crohn’s disease therapy. However, not all patients achieve remission, and many lose response over time.
The current trial compared the effectiveness and safety of adding a low-dose of oral methotrexate to adalimumab or infliximab vs. TNFi therapy alone in 297 children with Crohn’s disease. The mean age was 13.9 years, and about two-thirds were boys. None had a prior history of TNFi therapy.
Participants initiating infliximab or adalimumab were randomly allocated (1:1) to oral methotrexate or placebo. Of them, 110 infliximab initiators and 46 adalimumab initiators received methotrexate, while 102 infliximab initiators and 39 adalimumab initiators were given placebo. Methotrexate was administered as a weekly dose of 15 mg for children weighing 40 kg or more, 12.5 mg for children 30 kg to less than 40 kg, and 10 mg for children 20 kg to less than 30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg per day). Participants were followed for 12-36 months.
The primary outcome was a failure to achieve or maintain steroid-free remission defined by occurrence of any of the following:
- Short Pediatric Crohn’s Disease Activity Index (SPCDAI) score of less than 15 by week 26.
- Failure to complete a steroid taper by week 16.
- SPCDAI score of 15 or higher as a result of active Crohn’s disease at two or more consecutive visits beyond week 26.
- Hospitalization or surgery for Crohn’s disease beyond week 26.
- Use of corticosteroids for Crohn’s disease for 10 or more weeks cumulatively beyond week 16.
- Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity.
Overall, 88 of 297 children (30%) experienced treatment failure, including 57 of 212 (27%) on infliximab and 31 of 85 (36%) on adalimumab. Overall, 40 of 156 children (26%) on combination therapy and 48 of 141 (34%) on monotherapy experienced treatment failure.
Kaplan Meier analysis of the overall population showed a nonsignificant trend toward lower event rates with combination therapy (hazard ratio, 0.69; 95% confidence interval, 0.45-1.05; P = .08).
After stratification by TNFi, there was no difference in time to treatment failure among infliximab initiators between combination and monotherapy (HR, 0.93; 95% CI, 0.55-1.56; P = .78). In contrast, among adalimumab initiators, combination therapy was significantly associated with a longer time to treatment failure (HR, 0.40; 95% CI 0.19-0.81; P = .01).
There was a nonsignificant trend toward lower development of antidrug antibodies with combination therapy (risk ratio 0.72 with infliximab and 0.71 with adalimumab). This trend is in line with adult studies and adds substantially to the pediatric literature on this topic, the researchers note.
No differences in patient-reported outcomes were observed. There were slightly more adverse events with combination therapy, as expected, but fewer serious adverse events.
Shared decision-making
Dr. Kappelman noted that the study was not designed to answer the question of which is better – adalimumab plus methotrexate or infliximab alone.
“This is an area for future research. At this point, we believe it is an individualized decision, and appropriate counseling is needed to support shared decision-making,” he said.
The trial was not designed to evaluate the role of proactive therapeutic drug monitoring. However, proactive TDM is endorsed in the ImproveCareNow Model IBD Care guidelines and was considered standard of care at the 35 study sites.
The findings “suggest strong consideration of using combination therapy for pediatric Crohn’s disease patients initiating adalimumab, but not infliximab,” Dr. Kappelman and colleagues say.
“Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of deimplementation of combination therapy in infliximab-treated patients,” they add.
The decision about which approach to use is still very dependent on patients and their providers, Dr. Kurowski said.
“The study shows that you can safely use infliximab as monotherapy, with low risk of antibody formation, while utilizing proactive therapeutic drug monitoring and dose optimization,” he said. “The study also shows that adalimumab in combination with low-dose methotrexate can be strongly considered when needed.”
The researchers’ standardization of methotrexate doses by weight “is another significant contribution and provides a guide for clinicians,” Dr. Kurowski added.
The study was funded by grants from the Patient-Centered Outcomes Research Institute, the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kappelman has consulted for AbbVie, Janssen, Pfizer, Takeda, and Lilly; holds shares in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol-Myers Squibb, Celtrion, and Arena Pharmaceuticals. Dr. Kurowski reports no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Children initiating treatment with adalimumab plus a low dose of methotrexate experienced a twofold reduction in treatment failure, note the authors of the largest, double-blind, randomized trial to date in pediatric Crohn’s disease. However, children initiating infliximab, another TNFi, had similar outcomes with or without methotrexate.
“We believe these results are practice changing,” said principal investigator Michael Kappelman, MD, MPH, professor of pediatrics at University of North Carolina.
All patients with pediatric Crohn’s disease starting on adalimumab, and their parents, should be informed that combining the drug with low-dose oral methotrexate improves treatment effectiveness, he said.
“Those without contraindications should be offered combination therapy, and shared decision-making should be incorporated into final treatment decisions. In contrast, most patients starting infliximab are not likely to experience added benefits from low-dose oral methotrexate,” Dr. Kappelman added.
The study was published online in Gastroenterology and was presented in May in Chicago at the annual Digestive Disease Week® (DDW).
Impactful study
“This is an important study, published in a very high-ranking journal, that will have a huge impact on how we practice,” Jacob Kurowski, MD, department of pediatric gastroenterology, hepatology and nutrition, Cleveland Clinic Children’s, who wasn’t involved in the study, said.
Treatment with a TNFi, including infliximab and adalimumab, is a mainstay of pediatric Crohn’s disease therapy. However, not all patients achieve remission, and many lose response over time.
The current trial compared the effectiveness and safety of adding a low-dose of oral methotrexate to adalimumab or infliximab vs. TNFi therapy alone in 297 children with Crohn’s disease. The mean age was 13.9 years, and about two-thirds were boys. None had a prior history of TNFi therapy.
Participants initiating infliximab or adalimumab were randomly allocated (1:1) to oral methotrexate or placebo. Of them, 110 infliximab initiators and 46 adalimumab initiators received methotrexate, while 102 infliximab initiators and 39 adalimumab initiators were given placebo. Methotrexate was administered as a weekly dose of 15 mg for children weighing 40 kg or more, 12.5 mg for children 30 kg to less than 40 kg, and 10 mg for children 20 kg to less than 30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg per day). Participants were followed for 12-36 months.
The primary outcome was a failure to achieve or maintain steroid-free remission defined by occurrence of any of the following:
- Short Pediatric Crohn’s Disease Activity Index (SPCDAI) score of less than 15 by week 26.
- Failure to complete a steroid taper by week 16.
- SPCDAI score of 15 or higher as a result of active Crohn’s disease at two or more consecutive visits beyond week 26.
- Hospitalization or surgery for Crohn’s disease beyond week 26.
- Use of corticosteroids for Crohn’s disease for 10 or more weeks cumulatively beyond week 16.
- Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity.
Overall, 88 of 297 children (30%) experienced treatment failure, including 57 of 212 (27%) on infliximab and 31 of 85 (36%) on adalimumab. Overall, 40 of 156 children (26%) on combination therapy and 48 of 141 (34%) on monotherapy experienced treatment failure.
Kaplan Meier analysis of the overall population showed a nonsignificant trend toward lower event rates with combination therapy (hazard ratio, 0.69; 95% confidence interval, 0.45-1.05; P = .08).
After stratification by TNFi, there was no difference in time to treatment failure among infliximab initiators between combination and monotherapy (HR, 0.93; 95% CI, 0.55-1.56; P = .78). In contrast, among adalimumab initiators, combination therapy was significantly associated with a longer time to treatment failure (HR, 0.40; 95% CI 0.19-0.81; P = .01).
There was a nonsignificant trend toward lower development of antidrug antibodies with combination therapy (risk ratio 0.72 with infliximab and 0.71 with adalimumab). This trend is in line with adult studies and adds substantially to the pediatric literature on this topic, the researchers note.
No differences in patient-reported outcomes were observed. There were slightly more adverse events with combination therapy, as expected, but fewer serious adverse events.
Shared decision-making
Dr. Kappelman noted that the study was not designed to answer the question of which is better – adalimumab plus methotrexate or infliximab alone.
“This is an area for future research. At this point, we believe it is an individualized decision, and appropriate counseling is needed to support shared decision-making,” he said.
The trial was not designed to evaluate the role of proactive therapeutic drug monitoring. However, proactive TDM is endorsed in the ImproveCareNow Model IBD Care guidelines and was considered standard of care at the 35 study sites.
The findings “suggest strong consideration of using combination therapy for pediatric Crohn’s disease patients initiating adalimumab, but not infliximab,” Dr. Kappelman and colleagues say.
“Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of deimplementation of combination therapy in infliximab-treated patients,” they add.
The decision about which approach to use is still very dependent on patients and their providers, Dr. Kurowski said.
“The study shows that you can safely use infliximab as monotherapy, with low risk of antibody formation, while utilizing proactive therapeutic drug monitoring and dose optimization,” he said. “The study also shows that adalimumab in combination with low-dose methotrexate can be strongly considered when needed.”
The researchers’ standardization of methotrexate doses by weight “is another significant contribution and provides a guide for clinicians,” Dr. Kurowski added.
The study was funded by grants from the Patient-Centered Outcomes Research Institute, the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kappelman has consulted for AbbVie, Janssen, Pfizer, Takeda, and Lilly; holds shares in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol-Myers Squibb, Celtrion, and Arena Pharmaceuticals. Dr. Kurowski reports no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Children initiating treatment with adalimumab plus a low dose of methotrexate experienced a twofold reduction in treatment failure, note the authors of the largest, double-blind, randomized trial to date in pediatric Crohn’s disease. However, children initiating infliximab, another TNFi, had similar outcomes with or without methotrexate.
“We believe these results are practice changing,” said principal investigator Michael Kappelman, MD, MPH, professor of pediatrics at University of North Carolina.
All patients with pediatric Crohn’s disease starting on adalimumab, and their parents, should be informed that combining the drug with low-dose oral methotrexate improves treatment effectiveness, he said.
“Those without contraindications should be offered combination therapy, and shared decision-making should be incorporated into final treatment decisions. In contrast, most patients starting infliximab are not likely to experience added benefits from low-dose oral methotrexate,” Dr. Kappelman added.
The study was published online in Gastroenterology and was presented in May in Chicago at the annual Digestive Disease Week® (DDW).
Impactful study
“This is an important study, published in a very high-ranking journal, that will have a huge impact on how we practice,” Jacob Kurowski, MD, department of pediatric gastroenterology, hepatology and nutrition, Cleveland Clinic Children’s, who wasn’t involved in the study, said.
Treatment with a TNFi, including infliximab and adalimumab, is a mainstay of pediatric Crohn’s disease therapy. However, not all patients achieve remission, and many lose response over time.
The current trial compared the effectiveness and safety of adding a low-dose of oral methotrexate to adalimumab or infliximab vs. TNFi therapy alone in 297 children with Crohn’s disease. The mean age was 13.9 years, and about two-thirds were boys. None had a prior history of TNFi therapy.
Participants initiating infliximab or adalimumab were randomly allocated (1:1) to oral methotrexate or placebo. Of them, 110 infliximab initiators and 46 adalimumab initiators received methotrexate, while 102 infliximab initiators and 39 adalimumab initiators were given placebo. Methotrexate was administered as a weekly dose of 15 mg for children weighing 40 kg or more, 12.5 mg for children 30 kg to less than 40 kg, and 10 mg for children 20 kg to less than 30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg per day). Participants were followed for 12-36 months.
The primary outcome was a failure to achieve or maintain steroid-free remission defined by occurrence of any of the following:
- Short Pediatric Crohn’s Disease Activity Index (SPCDAI) score of less than 15 by week 26.
- Failure to complete a steroid taper by week 16.
- SPCDAI score of 15 or higher as a result of active Crohn’s disease at two or more consecutive visits beyond week 26.
- Hospitalization or surgery for Crohn’s disease beyond week 26.
- Use of corticosteroids for Crohn’s disease for 10 or more weeks cumulatively beyond week 16.
- Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity.
Overall, 88 of 297 children (30%) experienced treatment failure, including 57 of 212 (27%) on infliximab and 31 of 85 (36%) on adalimumab. Overall, 40 of 156 children (26%) on combination therapy and 48 of 141 (34%) on monotherapy experienced treatment failure.
Kaplan Meier analysis of the overall population showed a nonsignificant trend toward lower event rates with combination therapy (hazard ratio, 0.69; 95% confidence interval, 0.45-1.05; P = .08).
After stratification by TNFi, there was no difference in time to treatment failure among infliximab initiators between combination and monotherapy (HR, 0.93; 95% CI, 0.55-1.56; P = .78). In contrast, among adalimumab initiators, combination therapy was significantly associated with a longer time to treatment failure (HR, 0.40; 95% CI 0.19-0.81; P = .01).
There was a nonsignificant trend toward lower development of antidrug antibodies with combination therapy (risk ratio 0.72 with infliximab and 0.71 with adalimumab). This trend is in line with adult studies and adds substantially to the pediatric literature on this topic, the researchers note.
No differences in patient-reported outcomes were observed. There were slightly more adverse events with combination therapy, as expected, but fewer serious adverse events.
Shared decision-making
Dr. Kappelman noted that the study was not designed to answer the question of which is better – adalimumab plus methotrexate or infliximab alone.
“This is an area for future research. At this point, we believe it is an individualized decision, and appropriate counseling is needed to support shared decision-making,” he said.
The trial was not designed to evaluate the role of proactive therapeutic drug monitoring. However, proactive TDM is endorsed in the ImproveCareNow Model IBD Care guidelines and was considered standard of care at the 35 study sites.
The findings “suggest strong consideration of using combination therapy for pediatric Crohn’s disease patients initiating adalimumab, but not infliximab,” Dr. Kappelman and colleagues say.
“Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of deimplementation of combination therapy in infliximab-treated patients,” they add.
The decision about which approach to use is still very dependent on patients and their providers, Dr. Kurowski said.
“The study shows that you can safely use infliximab as monotherapy, with low risk of antibody formation, while utilizing proactive therapeutic drug monitoring and dose optimization,” he said. “The study also shows that adalimumab in combination with low-dose methotrexate can be strongly considered when needed.”
The researchers’ standardization of methotrexate doses by weight “is another significant contribution and provides a guide for clinicians,” Dr. Kurowski added.
The study was funded by grants from the Patient-Centered Outcomes Research Institute, the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kappelman has consulted for AbbVie, Janssen, Pfizer, Takeda, and Lilly; holds shares in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol-Myers Squibb, Celtrion, and Arena Pharmaceuticals. Dr. Kurowski reports no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
FROM DDW 2023
FDA approves first treatment for constipation in children
The recommended dosage in pediatric patients is 72 mcg orally once daily.
Functional constipation is common in children and adolescents. Symptoms include infrequent bowel movements with hard stools that can be difficult or painful to pass.
There is no known underlying organic cause and there are typically multiple contributing factors, the FDA noted in a statement announcing the approval.
The efficacy of linaclotide in children with functional constipation was demonstrated in a 12-week double-blind, placebo-controlled, randomized, multicenter clinical trial (Trial 7; NCT04026113) and supported by efficacy data from trials in adults with chronic idiopathic constipation, the FDA said.
The FDA first approved linaclotide in 2012 for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation (IBS-C) in adults.
Study details
To be eligible for the pediatric clinical trial, patients had to have experienced fewer than three spontaneous bowel movements (SBMs) per week.
They also had to experience one or more of the following at least once weekly, for at least 2 months prior to the trial screening visit:
- History of stool withholding or excessive voluntary stool retention.
- History of painful or hard bowel movements.
- History of large diameter stools that may obstruct the toilet.
- Presence of a large fecal mass in the rectum.
- At least one episode of fecal incontinence per week.
The primary efficacy endpoint was a 12-week change from baseline in SBM frequency rate. Children on linaclotide experienced greater improvement in the average number of SBMs per week than peers given placebo.
SBM frequency improved during the first week and was maintained throughout the remainder of the 12-week treatment period, the FDA said.
The most common adverse reaction is diarrhea. If severe diarrhea occurs, linaclotide should be discontinued and rehydration started.
The product’s boxed warning states that linaclotide is contraindicated in children younger than 2 years. In neonatal mice, linaclotide caused deaths due to dehydration.
Patients with known or suspected mechanical gastrointestinal obstruction should not take linaclotide.
Full prescribing information is available online.
The application for linaclotide in children received priority review.
A version of this article originally appeared on Medscape.com.
The recommended dosage in pediatric patients is 72 mcg orally once daily.
Functional constipation is common in children and adolescents. Symptoms include infrequent bowel movements with hard stools that can be difficult or painful to pass.
There is no known underlying organic cause and there are typically multiple contributing factors, the FDA noted in a statement announcing the approval.
The efficacy of linaclotide in children with functional constipation was demonstrated in a 12-week double-blind, placebo-controlled, randomized, multicenter clinical trial (Trial 7; NCT04026113) and supported by efficacy data from trials in adults with chronic idiopathic constipation, the FDA said.
The FDA first approved linaclotide in 2012 for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation (IBS-C) in adults.
Study details
To be eligible for the pediatric clinical trial, patients had to have experienced fewer than three spontaneous bowel movements (SBMs) per week.
They also had to experience one or more of the following at least once weekly, for at least 2 months prior to the trial screening visit:
- History of stool withholding or excessive voluntary stool retention.
- History of painful or hard bowel movements.
- History of large diameter stools that may obstruct the toilet.
- Presence of a large fecal mass in the rectum.
- At least one episode of fecal incontinence per week.
The primary efficacy endpoint was a 12-week change from baseline in SBM frequency rate. Children on linaclotide experienced greater improvement in the average number of SBMs per week than peers given placebo.
SBM frequency improved during the first week and was maintained throughout the remainder of the 12-week treatment period, the FDA said.
The most common adverse reaction is diarrhea. If severe diarrhea occurs, linaclotide should be discontinued and rehydration started.
The product’s boxed warning states that linaclotide is contraindicated in children younger than 2 years. In neonatal mice, linaclotide caused deaths due to dehydration.
Patients with known or suspected mechanical gastrointestinal obstruction should not take linaclotide.
Full prescribing information is available online.
The application for linaclotide in children received priority review.
A version of this article originally appeared on Medscape.com.
The recommended dosage in pediatric patients is 72 mcg orally once daily.
Functional constipation is common in children and adolescents. Symptoms include infrequent bowel movements with hard stools that can be difficult or painful to pass.
There is no known underlying organic cause and there are typically multiple contributing factors, the FDA noted in a statement announcing the approval.
The efficacy of linaclotide in children with functional constipation was demonstrated in a 12-week double-blind, placebo-controlled, randomized, multicenter clinical trial (Trial 7; NCT04026113) and supported by efficacy data from trials in adults with chronic idiopathic constipation, the FDA said.
The FDA first approved linaclotide in 2012 for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation (IBS-C) in adults.
Study details
To be eligible for the pediatric clinical trial, patients had to have experienced fewer than three spontaneous bowel movements (SBMs) per week.
They also had to experience one or more of the following at least once weekly, for at least 2 months prior to the trial screening visit:
- History of stool withholding or excessive voluntary stool retention.
- History of painful or hard bowel movements.
- History of large diameter stools that may obstruct the toilet.
- Presence of a large fecal mass in the rectum.
- At least one episode of fecal incontinence per week.
The primary efficacy endpoint was a 12-week change from baseline in SBM frequency rate. Children on linaclotide experienced greater improvement in the average number of SBMs per week than peers given placebo.
SBM frequency improved during the first week and was maintained throughout the remainder of the 12-week treatment period, the FDA said.
The most common adverse reaction is diarrhea. If severe diarrhea occurs, linaclotide should be discontinued and rehydration started.
The product’s boxed warning states that linaclotide is contraindicated in children younger than 2 years. In neonatal mice, linaclotide caused deaths due to dehydration.
Patients with known or suspected mechanical gastrointestinal obstruction should not take linaclotide.
Full prescribing information is available online.
The application for linaclotide in children received priority review.
A version of this article originally appeared on Medscape.com.
No apparent drug interaction with ozanimod and antidepressants
DENVER – , according to research presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Depression and anxiety are prevalent comorbidities occurring in up to 54% of patients with multiple sclerosis, and selective serotonin reuptake inhibitors (SSRIs)/serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments for depression and anxiety disorders,” Robert T. Naismith, MD, of Washington University in St. Louis, and his colleagues reported.
“Coadministration of ozanimod with drugs that increase serotonin could hypothetically lead to serotonin accumulation,” which can increase the likelihood of hypertension. U.S. prescribing information recommends that patients taking both ozanimod and medications that increase norepinephrine or serotonin be monitored for hypertension, an adverse reaction reported in 3.9% of patients receiving ozanimod in the phase 3 trials for relapsing MS.
Clarifying the risk
“It’s important to be aware of potential drug interactions and risks from MS disease modifying therapies,” Lauren Gluck, MD, an assistant professor and director of the division of multiple sclerosis at Montefiore Medical Center/Albert Einstein College of Medicine, New York, said in an interview. Dr. Gluck was not involved in this study but described some of the history that revealed the value of this type of research. For example, the first sphingosine-1-phosphate receptor (S1PR) modulator approved for MS, fingolimod (Gilenya), has a risk of cardiac conduction dysfunction with QTc prolongation, so people taking fingolimod with other medications that prolong QTc, such as SSRIs, need additional monitoring.
“Ozanimod is a newer, more selective S1PR modulator that initially raised concerns about interaction with serotonin-increasing drugs based on in vitro studies,” Dr. Gluck said. “This could mean that people on ozanimod and other serotonin-increasing medicine could be at risk for dangerous events like serotonin syndrome. However, in vitro studies do not always translate to how something affects the human body, so it is not clear how much risk truly exists.”
Examining open-label extension trial data
The researchers therefore evaluated the safety of taking ozanimod and SSRIs or SNRIs in a subset of patients with relapsing MS who participated in the DAYBREAK open-label extension trial. The phase 3 parent trials compared 30 mcg once weekly of intramuscular interferon beta-1a with 0.92 mg of once-daily oral ozanimod and 0.46 mg of once-daily oral ozanimod. In the DAYBREAK open-label extension, 2,256 participants underwent a dose escalation over one week until all reached 0.92 mg of ozanimod, where they remained for an average of just under 5 years of follow-up. Nearly all the participants (99.4%) were White, and two-thirds (66.5%) were female.
The researchers searched the study data for terms related to serotonin toxicity and compared the rates of adverse events related with those terms and the rates of hypertension in the 274 participants who were and the 2,032 participant who were not taking antidepressants at the same time as ozanimod.
They found that 13.9% of patients taking SSRIs or SNRIs experienced at least one treatment-emergent adverse event related to their search criteria, compared with 17.7% of patients not taking SSRIs or SNRIs. Similarly, 9.2% of trial participants not taking SSRIs or SNRIs had hypertension, compared with 4.7% of participants who were taking antidepressants. The authors further noted that “similar trends were observed when 6 weeks after the end date of concomitant SSRIs/SNRI use were included in the ‘on SSRI/SNRI’ analysis period.”
When the researchers searched specifically for three terms directly related to serotonin toxicity – “serotonin syndrome,” “neuroleptic malignant syndrome,” and “hyperthermia malignant” – they did not find any patients who had treatment-emergent adverse events related to those terms.
“SSRIs/SNRIs were freely allowed as concomitant medications in the DAYBREAK open-label extension, and among the patients from SUNBEAM or RADIANCE who were followed for up to 6 years, there have been no reported safety concerns during the concurrent administration of serotonergic antidepressants and ozanimod in patients with relapsing MS as of the data cutoff,” concluded the authors, though they also noted that the overall rate of SSRI and SNRI use was low in the extension trial.
A reassuring finding for clinicians and patients alike
“It is reassuring, if not unexpected, that there were no clinically significant rates of symptoms associated with excess serotonin in patients on ozanimod and SSRI/SNRIs,” Dr. Gluck commented. “These findings are important for both clinicians and patients – they can help [both] feel comfortable considering ozanimod if SSRI/SNRIs are already being used. There is also freedom to use SSRI/SNRIs for symptom management in patients already on ozanimod.”
The research was funded by Bristol Myers Squibb. Dr. Naismith reported consulting for Abata Therapeutics, Banner Life Sciences, BeiGene, Biogen, Bristol Myers Squibb, Celltrion, Genentech, Genzyme, GW Therapeutics, Janssen, Horizon Therapeutics, Lundbeck, NervGen, and TG Therapeutics. Six other authors reported disclosures for various pharmaceutical companies, and six other authors are employees and/or shareholders of Bristol Myers Squibb. Dr. Gluck has served on advisory boards with Genentech and EMD Serono.
DENVER – , according to research presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Depression and anxiety are prevalent comorbidities occurring in up to 54% of patients with multiple sclerosis, and selective serotonin reuptake inhibitors (SSRIs)/serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments for depression and anxiety disorders,” Robert T. Naismith, MD, of Washington University in St. Louis, and his colleagues reported.
“Coadministration of ozanimod with drugs that increase serotonin could hypothetically lead to serotonin accumulation,” which can increase the likelihood of hypertension. U.S. prescribing information recommends that patients taking both ozanimod and medications that increase norepinephrine or serotonin be monitored for hypertension, an adverse reaction reported in 3.9% of patients receiving ozanimod in the phase 3 trials for relapsing MS.
Clarifying the risk
“It’s important to be aware of potential drug interactions and risks from MS disease modifying therapies,” Lauren Gluck, MD, an assistant professor and director of the division of multiple sclerosis at Montefiore Medical Center/Albert Einstein College of Medicine, New York, said in an interview. Dr. Gluck was not involved in this study but described some of the history that revealed the value of this type of research. For example, the first sphingosine-1-phosphate receptor (S1PR) modulator approved for MS, fingolimod (Gilenya), has a risk of cardiac conduction dysfunction with QTc prolongation, so people taking fingolimod with other medications that prolong QTc, such as SSRIs, need additional monitoring.
“Ozanimod is a newer, more selective S1PR modulator that initially raised concerns about interaction with serotonin-increasing drugs based on in vitro studies,” Dr. Gluck said. “This could mean that people on ozanimod and other serotonin-increasing medicine could be at risk for dangerous events like serotonin syndrome. However, in vitro studies do not always translate to how something affects the human body, so it is not clear how much risk truly exists.”
Examining open-label extension trial data
The researchers therefore evaluated the safety of taking ozanimod and SSRIs or SNRIs in a subset of patients with relapsing MS who participated in the DAYBREAK open-label extension trial. The phase 3 parent trials compared 30 mcg once weekly of intramuscular interferon beta-1a with 0.92 mg of once-daily oral ozanimod and 0.46 mg of once-daily oral ozanimod. In the DAYBREAK open-label extension, 2,256 participants underwent a dose escalation over one week until all reached 0.92 mg of ozanimod, where they remained for an average of just under 5 years of follow-up. Nearly all the participants (99.4%) were White, and two-thirds (66.5%) were female.
The researchers searched the study data for terms related to serotonin toxicity and compared the rates of adverse events related with those terms and the rates of hypertension in the 274 participants who were and the 2,032 participant who were not taking antidepressants at the same time as ozanimod.
They found that 13.9% of patients taking SSRIs or SNRIs experienced at least one treatment-emergent adverse event related to their search criteria, compared with 17.7% of patients not taking SSRIs or SNRIs. Similarly, 9.2% of trial participants not taking SSRIs or SNRIs had hypertension, compared with 4.7% of participants who were taking antidepressants. The authors further noted that “similar trends were observed when 6 weeks after the end date of concomitant SSRIs/SNRI use were included in the ‘on SSRI/SNRI’ analysis period.”
When the researchers searched specifically for three terms directly related to serotonin toxicity – “serotonin syndrome,” “neuroleptic malignant syndrome,” and “hyperthermia malignant” – they did not find any patients who had treatment-emergent adverse events related to those terms.
“SSRIs/SNRIs were freely allowed as concomitant medications in the DAYBREAK open-label extension, and among the patients from SUNBEAM or RADIANCE who were followed for up to 6 years, there have been no reported safety concerns during the concurrent administration of serotonergic antidepressants and ozanimod in patients with relapsing MS as of the data cutoff,” concluded the authors, though they also noted that the overall rate of SSRI and SNRI use was low in the extension trial.
A reassuring finding for clinicians and patients alike
“It is reassuring, if not unexpected, that there were no clinically significant rates of symptoms associated with excess serotonin in patients on ozanimod and SSRI/SNRIs,” Dr. Gluck commented. “These findings are important for both clinicians and patients – they can help [both] feel comfortable considering ozanimod if SSRI/SNRIs are already being used. There is also freedom to use SSRI/SNRIs for symptom management in patients already on ozanimod.”
The research was funded by Bristol Myers Squibb. Dr. Naismith reported consulting for Abata Therapeutics, Banner Life Sciences, BeiGene, Biogen, Bristol Myers Squibb, Celltrion, Genentech, Genzyme, GW Therapeutics, Janssen, Horizon Therapeutics, Lundbeck, NervGen, and TG Therapeutics. Six other authors reported disclosures for various pharmaceutical companies, and six other authors are employees and/or shareholders of Bristol Myers Squibb. Dr. Gluck has served on advisory boards with Genentech and EMD Serono.
DENVER – , according to research presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Depression and anxiety are prevalent comorbidities occurring in up to 54% of patients with multiple sclerosis, and selective serotonin reuptake inhibitors (SSRIs)/serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments for depression and anxiety disorders,” Robert T. Naismith, MD, of Washington University in St. Louis, and his colleagues reported.
“Coadministration of ozanimod with drugs that increase serotonin could hypothetically lead to serotonin accumulation,” which can increase the likelihood of hypertension. U.S. prescribing information recommends that patients taking both ozanimod and medications that increase norepinephrine or serotonin be monitored for hypertension, an adverse reaction reported in 3.9% of patients receiving ozanimod in the phase 3 trials for relapsing MS.
Clarifying the risk
“It’s important to be aware of potential drug interactions and risks from MS disease modifying therapies,” Lauren Gluck, MD, an assistant professor and director of the division of multiple sclerosis at Montefiore Medical Center/Albert Einstein College of Medicine, New York, said in an interview. Dr. Gluck was not involved in this study but described some of the history that revealed the value of this type of research. For example, the first sphingosine-1-phosphate receptor (S1PR) modulator approved for MS, fingolimod (Gilenya), has a risk of cardiac conduction dysfunction with QTc prolongation, so people taking fingolimod with other medications that prolong QTc, such as SSRIs, need additional monitoring.
“Ozanimod is a newer, more selective S1PR modulator that initially raised concerns about interaction with serotonin-increasing drugs based on in vitro studies,” Dr. Gluck said. “This could mean that people on ozanimod and other serotonin-increasing medicine could be at risk for dangerous events like serotonin syndrome. However, in vitro studies do not always translate to how something affects the human body, so it is not clear how much risk truly exists.”
Examining open-label extension trial data
The researchers therefore evaluated the safety of taking ozanimod and SSRIs or SNRIs in a subset of patients with relapsing MS who participated in the DAYBREAK open-label extension trial. The phase 3 parent trials compared 30 mcg once weekly of intramuscular interferon beta-1a with 0.92 mg of once-daily oral ozanimod and 0.46 mg of once-daily oral ozanimod. In the DAYBREAK open-label extension, 2,256 participants underwent a dose escalation over one week until all reached 0.92 mg of ozanimod, where they remained for an average of just under 5 years of follow-up. Nearly all the participants (99.4%) were White, and two-thirds (66.5%) were female.
The researchers searched the study data for terms related to serotonin toxicity and compared the rates of adverse events related with those terms and the rates of hypertension in the 274 participants who were and the 2,032 participant who were not taking antidepressants at the same time as ozanimod.
They found that 13.9% of patients taking SSRIs or SNRIs experienced at least one treatment-emergent adverse event related to their search criteria, compared with 17.7% of patients not taking SSRIs or SNRIs. Similarly, 9.2% of trial participants not taking SSRIs or SNRIs had hypertension, compared with 4.7% of participants who were taking antidepressants. The authors further noted that “similar trends were observed when 6 weeks after the end date of concomitant SSRIs/SNRI use were included in the ‘on SSRI/SNRI’ analysis period.”
When the researchers searched specifically for three terms directly related to serotonin toxicity – “serotonin syndrome,” “neuroleptic malignant syndrome,” and “hyperthermia malignant” – they did not find any patients who had treatment-emergent adverse events related to those terms.
“SSRIs/SNRIs were freely allowed as concomitant medications in the DAYBREAK open-label extension, and among the patients from SUNBEAM or RADIANCE who were followed for up to 6 years, there have been no reported safety concerns during the concurrent administration of serotonergic antidepressants and ozanimod in patients with relapsing MS as of the data cutoff,” concluded the authors, though they also noted that the overall rate of SSRI and SNRI use was low in the extension trial.
A reassuring finding for clinicians and patients alike
“It is reassuring, if not unexpected, that there were no clinically significant rates of symptoms associated with excess serotonin in patients on ozanimod and SSRI/SNRIs,” Dr. Gluck commented. “These findings are important for both clinicians and patients – they can help [both] feel comfortable considering ozanimod if SSRI/SNRIs are already being used. There is also freedom to use SSRI/SNRIs for symptom management in patients already on ozanimod.”
The research was funded by Bristol Myers Squibb. Dr. Naismith reported consulting for Abata Therapeutics, Banner Life Sciences, BeiGene, Biogen, Bristol Myers Squibb, Celltrion, Genentech, Genzyme, GW Therapeutics, Janssen, Horizon Therapeutics, Lundbeck, NervGen, and TG Therapeutics. Six other authors reported disclosures for various pharmaceutical companies, and six other authors are employees and/or shareholders of Bristol Myers Squibb. Dr. Gluck has served on advisory boards with Genentech and EMD Serono.
AT CMSC 2023
The timekeeper
This little fellow greets you at my office. He’s been there for 25 years.
I don’t know where he came from originally. When I started out he was up front with the physician I subleased from and when he retired he passed him on to me (thanks, Fran!).
From the beginning he’s been the first thing I see when I arrive each morning. Because of my suprachiasmatic nucleus kicking me out of bed between 4 and 5 each morning, I’m always the first one in the office and so I update him. At this point he’s as much a part of my morning ritual as coffee and tea. I juggle the cubes to change the day (12 times a year I change the month) and once this is done I don’t think of him again until the next morning.
When I started setting him each morning I didn’t have kids. Now I have three, all grown. Patients, years, drug reps, and even a pandemic have all been marked by the clicking of his cubes when I change them each morning.
Now two-thirds of the way through my career, he’s taken on a different meaning. He’s counting down the days until I walk away and leave neurology in the hands of another generation. I don’t have a date for doing that, nor a plan to do so anytime soon, but sooner or later I’ll be changing his cubes for the last office day of my life as a neurologist.
What will happen to him then? Seems like a strange question to ask about an inanimate object, but after this much time I’ve gotten attached to the little guy. He’s come to symbolize more than just the date – he’s the passage of time. Maybe he’ll stay on a shelf at home, giving me something to do each morning of my retirement. Maybe one of my kids will want him.
Inevitably, he’ll probably end up at a charity store, awaiting a new owner. When that happens I hope he gives them something to pause, smile, and think about each day, like he did with me, as we travel around the sun together.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This little fellow greets you at my office. He’s been there for 25 years.
I don’t know where he came from originally. When I started out he was up front with the physician I subleased from and when he retired he passed him on to me (thanks, Fran!).
From the beginning he’s been the first thing I see when I arrive each morning. Because of my suprachiasmatic nucleus kicking me out of bed between 4 and 5 each morning, I’m always the first one in the office and so I update him. At this point he’s as much a part of my morning ritual as coffee and tea. I juggle the cubes to change the day (12 times a year I change the month) and once this is done I don’t think of him again until the next morning.
When I started setting him each morning I didn’t have kids. Now I have three, all grown. Patients, years, drug reps, and even a pandemic have all been marked by the clicking of his cubes when I change them each morning.
Now two-thirds of the way through my career, he’s taken on a different meaning. He’s counting down the days until I walk away and leave neurology in the hands of another generation. I don’t have a date for doing that, nor a plan to do so anytime soon, but sooner or later I’ll be changing his cubes for the last office day of my life as a neurologist.
What will happen to him then? Seems like a strange question to ask about an inanimate object, but after this much time I’ve gotten attached to the little guy. He’s come to symbolize more than just the date – he’s the passage of time. Maybe he’ll stay on a shelf at home, giving me something to do each morning of my retirement. Maybe one of my kids will want him.
Inevitably, he’ll probably end up at a charity store, awaiting a new owner. When that happens I hope he gives them something to pause, smile, and think about each day, like he did with me, as we travel around the sun together.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This little fellow greets you at my office. He’s been there for 25 years.
I don’t know where he came from originally. When I started out he was up front with the physician I subleased from and when he retired he passed him on to me (thanks, Fran!).
From the beginning he’s been the first thing I see when I arrive each morning. Because of my suprachiasmatic nucleus kicking me out of bed between 4 and 5 each morning, I’m always the first one in the office and so I update him. At this point he’s as much a part of my morning ritual as coffee and tea. I juggle the cubes to change the day (12 times a year I change the month) and once this is done I don’t think of him again until the next morning.
When I started setting him each morning I didn’t have kids. Now I have three, all grown. Patients, years, drug reps, and even a pandemic have all been marked by the clicking of his cubes when I change them each morning.
Now two-thirds of the way through my career, he’s taken on a different meaning. He’s counting down the days until I walk away and leave neurology in the hands of another generation. I don’t have a date for doing that, nor a plan to do so anytime soon, but sooner or later I’ll be changing his cubes for the last office day of my life as a neurologist.
What will happen to him then? Seems like a strange question to ask about an inanimate object, but after this much time I’ve gotten attached to the little guy. He’s come to symbolize more than just the date – he’s the passage of time. Maybe he’ll stay on a shelf at home, giving me something to do each morning of my retirement. Maybe one of my kids will want him.
Inevitably, he’ll probably end up at a charity store, awaiting a new owner. When that happens I hope he gives them something to pause, smile, and think about each day, like he did with me, as we travel around the sun together.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
55-year-old woman • unilateral nasal drainage • salty taste • nasal redness • recent COVID-19 nasal swabs • Dx?
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
► Unilateral nasal drainage
► Salty taste
► Nasal redness
► Recent COVID-19 nasal swabs
Is there benefit to adding ezetimibe to a statin for the secondary prevention of CVD?
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.
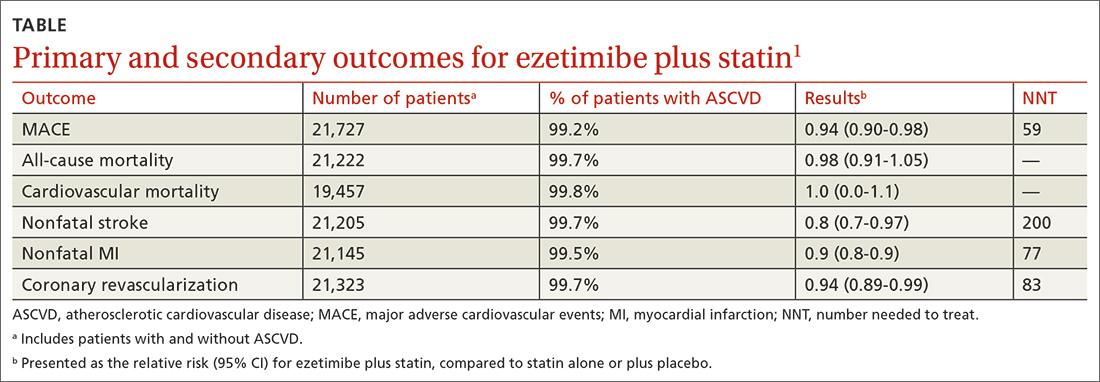
The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
EVIDENCE-BASED REVIEW:
YES. In patients with known cardio- vascular disease (CVD), ezetimibe with a statin decreases
In adults with atherosclerotic CVD (ASCVD), the combination of ezetimibe and a moderate-intensity statin (rosuvastatin 10 mg) was noninferior at decreasing cardiovascular death, major cardiovascular events, and nonfatal stroke, but was more tolerable, compared to a high-intensity statin (rosuvastatin 20 mg) alone (SOR, B; 1 RCT).