User login
Switching to upadacitinib from dupilumab improves atopic dermatitis
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous upadacitinib treatment was safe and provided sustained responses through 40 weeks and switch to upadacitinib treatment improved outcomes irrespective of prior dupilumab response.
Major finding: At open-label extension week 16 vs Heads Up week 24, the mean Eczema Area and Severity Index scores were similar with continuous upadacitinib treatment (2.7 vs 2.6) and improved with a switch to upadacitinib from dupilumab (1.09 vs 3.29). Most patients without minimal threshold or adequate response with dupilumab achieved it with upadacitinib. No new safety signals were reported.
Study details: This 16-week interim analysis of a 52-week open-label extension study of the Heads Up trial included adults with moderate-to-severe AD who were assigned to continue receiving upadacitinib (n = 239) or switch to upadacitinib after 24 weeks of dupilumab (n = 245).
Disclosures: This study was supported by AbbVie Inc. Some authors reported ties with various organizations, including AbbVie. Eight authors declared being employees of or holding stock or stock options in AbbVie.
Source: Blauvelt A et al. Efficacy and safety of switching from dupilumab to upadacitinib versus continuous upadacitinib in moderate-to-severe atopic dermatitis: Results from an open-label extension of the phase 3, randomized, controlled trial (Heads Up). J Am Acad Dermatol. 2023 (May 22). doi: 10.1016/j.jaad.2023.05.033
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous upadacitinib treatment was safe and provided sustained responses through 40 weeks and switch to upadacitinib treatment improved outcomes irrespective of prior dupilumab response.
Major finding: At open-label extension week 16 vs Heads Up week 24, the mean Eczema Area and Severity Index scores were similar with continuous upadacitinib treatment (2.7 vs 2.6) and improved with a switch to upadacitinib from dupilumab (1.09 vs 3.29). Most patients without minimal threshold or adequate response with dupilumab achieved it with upadacitinib. No new safety signals were reported.
Study details: This 16-week interim analysis of a 52-week open-label extension study of the Heads Up trial included adults with moderate-to-severe AD who were assigned to continue receiving upadacitinib (n = 239) or switch to upadacitinib after 24 weeks of dupilumab (n = 245).
Disclosures: This study was supported by AbbVie Inc. Some authors reported ties with various organizations, including AbbVie. Eight authors declared being employees of or holding stock or stock options in AbbVie.
Source: Blauvelt A et al. Efficacy and safety of switching from dupilumab to upadacitinib versus continuous upadacitinib in moderate-to-severe atopic dermatitis: Results from an open-label extension of the phase 3, randomized, controlled trial (Heads Up). J Am Acad Dermatol. 2023 (May 22). doi: 10.1016/j.jaad.2023.05.033
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous upadacitinib treatment was safe and provided sustained responses through 40 weeks and switch to upadacitinib treatment improved outcomes irrespective of prior dupilumab response.
Major finding: At open-label extension week 16 vs Heads Up week 24, the mean Eczema Area and Severity Index scores were similar with continuous upadacitinib treatment (2.7 vs 2.6) and improved with a switch to upadacitinib from dupilumab (1.09 vs 3.29). Most patients without minimal threshold or adequate response with dupilumab achieved it with upadacitinib. No new safety signals were reported.
Study details: This 16-week interim analysis of a 52-week open-label extension study of the Heads Up trial included adults with moderate-to-severe AD who were assigned to continue receiving upadacitinib (n = 239) or switch to upadacitinib after 24 weeks of dupilumab (n = 245).
Disclosures: This study was supported by AbbVie Inc. Some authors reported ties with various organizations, including AbbVie. Eight authors declared being employees of or holding stock or stock options in AbbVie.
Source: Blauvelt A et al. Efficacy and safety of switching from dupilumab to upadacitinib versus continuous upadacitinib in moderate-to-severe atopic dermatitis: Results from an open-label extension of the phase 3, randomized, controlled trial (Heads Up). J Am Acad Dermatol. 2023 (May 22). doi: 10.1016/j.jaad.2023.05.033
Crisaborole once daily an effective long-term maintenance therapy for atopic dermatitis
Key clinical point: Maintenance therapy with once-daily crisaborole is safe and effective in adult and pediatric patients with mild-to-moderate atopic dermatitis (AD) who have previously responded to twice-daily crisaborole treatment.
Major finding: The crisaborole vs vehicle group had a significantly longer median flare-free maintenance time (111 vs 30 days; P = .0034), higher mean number of flare-free days (234.0 vs 199.4 days; P = .0346), and lower mean number of flares (0.95 vs 1.36; P = .0042). No new safety signals were reported.
Study details: This phase 3 study (CrisADe CONTROL) included 270 patients age ≥3 months with mild-to-moderate AD who received twice-daily crisaborole for a maximum of 8 weeks; the responders were randomly assigned to receive once-daily crisaborole 2% ointment (n = 135) or vehicle (n = 135) for 52 weeks.
Disclosures: This study was funded by Pfizer Inc. Some authors declared serving as investigators, speakers, or consultants for or receiving research grants from various sources, including Pfizer. Six authors declared being employees of and shareholders in Pfizer.
Source: Eichenfield LF et al. Once‑daily crisaborole ointment, 2%, as a long‑term maintenance treatment in patients aged ≥ 3 months with mild‑to‑moderate atopic dermatitis: A 52-week clinical study. Am J Clin Dermatol. 2023 (May 15). doi: 10.1007/s40257-023-00780-w
Key clinical point: Maintenance therapy with once-daily crisaborole is safe and effective in adult and pediatric patients with mild-to-moderate atopic dermatitis (AD) who have previously responded to twice-daily crisaborole treatment.
Major finding: The crisaborole vs vehicle group had a significantly longer median flare-free maintenance time (111 vs 30 days; P = .0034), higher mean number of flare-free days (234.0 vs 199.4 days; P = .0346), and lower mean number of flares (0.95 vs 1.36; P = .0042). No new safety signals were reported.
Study details: This phase 3 study (CrisADe CONTROL) included 270 patients age ≥3 months with mild-to-moderate AD who received twice-daily crisaborole for a maximum of 8 weeks; the responders were randomly assigned to receive once-daily crisaborole 2% ointment (n = 135) or vehicle (n = 135) for 52 weeks.
Disclosures: This study was funded by Pfizer Inc. Some authors declared serving as investigators, speakers, or consultants for or receiving research grants from various sources, including Pfizer. Six authors declared being employees of and shareholders in Pfizer.
Source: Eichenfield LF et al. Once‑daily crisaborole ointment, 2%, as a long‑term maintenance treatment in patients aged ≥ 3 months with mild‑to‑moderate atopic dermatitis: A 52-week clinical study. Am J Clin Dermatol. 2023 (May 15). doi: 10.1007/s40257-023-00780-w
Key clinical point: Maintenance therapy with once-daily crisaborole is safe and effective in adult and pediatric patients with mild-to-moderate atopic dermatitis (AD) who have previously responded to twice-daily crisaborole treatment.
Major finding: The crisaborole vs vehicle group had a significantly longer median flare-free maintenance time (111 vs 30 days; P = .0034), higher mean number of flare-free days (234.0 vs 199.4 days; P = .0346), and lower mean number of flares (0.95 vs 1.36; P = .0042). No new safety signals were reported.
Study details: This phase 3 study (CrisADe CONTROL) included 270 patients age ≥3 months with mild-to-moderate AD who received twice-daily crisaborole for a maximum of 8 weeks; the responders were randomly assigned to receive once-daily crisaborole 2% ointment (n = 135) or vehicle (n = 135) for 52 weeks.
Disclosures: This study was funded by Pfizer Inc. Some authors declared serving as investigators, speakers, or consultants for or receiving research grants from various sources, including Pfizer. Six authors declared being employees of and shareholders in Pfizer.
Source: Eichenfield LF et al. Once‑daily crisaborole ointment, 2%, as a long‑term maintenance treatment in patients aged ≥ 3 months with mild‑to‑moderate atopic dermatitis: A 52-week clinical study. Am J Clin Dermatol. 2023 (May 15). doi: 10.1007/s40257-023-00780-w
Atopic dermatitis positively linked with the risk for incident venous thromboembolism
Key clinical point: Adults with atopic dermatitis (AD) have a 1.28-fold increased risk for incident venous thromboembolism (VTE) compared with those without AD.
Major finding: Patients with AD vs control individuals without AD had an increased risk for incident VTE (hazard ratio [HR] 1.28; 95% CI 1.17-1.40), with the risk being elevated for both deep vein thrombosis (HR 1.26; 95% CI 1.14-1.40) and pulmonary embolism (HR 1.30; 95% CI 1.08-1.57).
Study details: The data come from a retrospective cohort study that included 142,429 patients age ≥ 20 years with AD and 142,429 matched control individuals without AD.
Disclosures: This study was funded by Hualien Tzu Chi Hospital, Taiwan. The authors declared no conflicts of interest.
Source: Chen TL et al. Risk of venous thromboembolism among adults with atopic dermatitis. JAMA Dermatol. 2023 (May 31). doi: 10.1001/jamadermatol.2023.1300.
Key clinical point: Adults with atopic dermatitis (AD) have a 1.28-fold increased risk for incident venous thromboembolism (VTE) compared with those without AD.
Major finding: Patients with AD vs control individuals without AD had an increased risk for incident VTE (hazard ratio [HR] 1.28; 95% CI 1.17-1.40), with the risk being elevated for both deep vein thrombosis (HR 1.26; 95% CI 1.14-1.40) and pulmonary embolism (HR 1.30; 95% CI 1.08-1.57).
Study details: The data come from a retrospective cohort study that included 142,429 patients age ≥ 20 years with AD and 142,429 matched control individuals without AD.
Disclosures: This study was funded by Hualien Tzu Chi Hospital, Taiwan. The authors declared no conflicts of interest.
Source: Chen TL et al. Risk of venous thromboembolism among adults with atopic dermatitis. JAMA Dermatol. 2023 (May 31). doi: 10.1001/jamadermatol.2023.1300.
Key clinical point: Adults with atopic dermatitis (AD) have a 1.28-fold increased risk for incident venous thromboembolism (VTE) compared with those without AD.
Major finding: Patients with AD vs control individuals without AD had an increased risk for incident VTE (hazard ratio [HR] 1.28; 95% CI 1.17-1.40), with the risk being elevated for both deep vein thrombosis (HR 1.26; 95% CI 1.14-1.40) and pulmonary embolism (HR 1.30; 95% CI 1.08-1.57).
Study details: The data come from a retrospective cohort study that included 142,429 patients age ≥ 20 years with AD and 142,429 matched control individuals without AD.
Disclosures: This study was funded by Hualien Tzu Chi Hospital, Taiwan. The authors declared no conflicts of interest.
Source: Chen TL et al. Risk of venous thromboembolism among adults with atopic dermatitis. JAMA Dermatol. 2023 (May 31). doi: 10.1001/jamadermatol.2023.1300.
A live topical biotherapeutic spray improves pruritus in atopic dermatitis
Key clinical point: A topical biotherapeutic spray containing live ammonia-oxidizing bacteria (B244) was safe and meaningfully improved pruritus at both high and low dose levels in patients with mild-to-moderate atopic dermatitis (AD) and moderate-to-severe pruritus.
Major finding: At week 4, treatment with low dose (optical density [OD] at 600 nm 5.0) and high dose (OD at 600 nm 20.0) spray vs vehicle showed a significant treatment effect (P = .015 and P = .014, respectively), with a 34% mean reduction in Worst Itch Numeric Rating Scale scores from baseline in both treatment groups. No serious adverse events were reported.
Study details: This multicenter phase 2b randomized controlled trial included 547 adult patients with mild-to-moderate AD and moderate-to-severe pruritus who were randomly assigned to receive low dose B244, high dose B244, or vehicle for 4 weeks.
Disclosures: This study was funded by AOBiome Therapeutics. Some authors reported ties with various organizations, including AOBiome. Six authors declared being current or former employees of or holding stock or stock options in AOBiome.
Source: Silverberg JI et al. Efficacy and safety of topically applied therapeutic ammonia oxidising bacteria in adults with mild-to-moderate atopic dermatitis and moderate-to-severe pruritus: A randomised, double-blind, placebo-controlled, dose-ranging, phase 2b trial. EClinicalMedicine. 2023 (May 16). doi:10.1016/j.eclinm.2023.102002
Key clinical point: A topical biotherapeutic spray containing live ammonia-oxidizing bacteria (B244) was safe and meaningfully improved pruritus at both high and low dose levels in patients with mild-to-moderate atopic dermatitis (AD) and moderate-to-severe pruritus.
Major finding: At week 4, treatment with low dose (optical density [OD] at 600 nm 5.0) and high dose (OD at 600 nm 20.0) spray vs vehicle showed a significant treatment effect (P = .015 and P = .014, respectively), with a 34% mean reduction in Worst Itch Numeric Rating Scale scores from baseline in both treatment groups. No serious adverse events were reported.
Study details: This multicenter phase 2b randomized controlled trial included 547 adult patients with mild-to-moderate AD and moderate-to-severe pruritus who were randomly assigned to receive low dose B244, high dose B244, or vehicle for 4 weeks.
Disclosures: This study was funded by AOBiome Therapeutics. Some authors reported ties with various organizations, including AOBiome. Six authors declared being current or former employees of or holding stock or stock options in AOBiome.
Source: Silverberg JI et al. Efficacy and safety of topically applied therapeutic ammonia oxidising bacteria in adults with mild-to-moderate atopic dermatitis and moderate-to-severe pruritus: A randomised, double-blind, placebo-controlled, dose-ranging, phase 2b trial. EClinicalMedicine. 2023 (May 16). doi:10.1016/j.eclinm.2023.102002
Key clinical point: A topical biotherapeutic spray containing live ammonia-oxidizing bacteria (B244) was safe and meaningfully improved pruritus at both high and low dose levels in patients with mild-to-moderate atopic dermatitis (AD) and moderate-to-severe pruritus.
Major finding: At week 4, treatment with low dose (optical density [OD] at 600 nm 5.0) and high dose (OD at 600 nm 20.0) spray vs vehicle showed a significant treatment effect (P = .015 and P = .014, respectively), with a 34% mean reduction in Worst Itch Numeric Rating Scale scores from baseline in both treatment groups. No serious adverse events were reported.
Study details: This multicenter phase 2b randomized controlled trial included 547 adult patients with mild-to-moderate AD and moderate-to-severe pruritus who were randomly assigned to receive low dose B244, high dose B244, or vehicle for 4 weeks.
Disclosures: This study was funded by AOBiome Therapeutics. Some authors reported ties with various organizations, including AOBiome. Six authors declared being current or former employees of or holding stock or stock options in AOBiome.
Source: Silverberg JI et al. Efficacy and safety of topically applied therapeutic ammonia oxidising bacteria in adults with mild-to-moderate atopic dermatitis and moderate-to-severe pruritus: A randomised, double-blind, placebo-controlled, dose-ranging, phase 2b trial. EClinicalMedicine. 2023 (May 16). doi:10.1016/j.eclinm.2023.102002
Cancer drug shortages spur worry, rationing, and tough choices
CHICAGO – Oncologist Denise Yardley, MD, isn’t used to expressing uncertainty when she tells patients about what’s in store for them in terms of drug treatment. But things are dramatically different now amid a severe national shortage of carboplatin and cisplatin, two common and crucial cancer drugs.
“There’s a regimen I’m thinking about,” Dr. Yardley told a new patient recently, “but we’ll have to wait until you finish your staging evaluation to see whether I can deliver this. Another regimen that’s a little more toxic is my second choice.” And, she added, the alternative chemotherapy treatment – anthracycline instead of carboplatin – requires a longer treatment period.
This ambiguity is hardly ideal, said Dr. Yardley, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville. “It’s another factor in being overwhelmed in a first-time visit and wanting to know the details about what your treatment is going to look like. You’re not walking out knowing exactly what you’re going to take or the exact timing so you can start mapping out your calendar and work schedule.”
This kind of scenario is becoming all too familiar this spring, according to oncologists who gathered at the annual meeting of the American Society of Clinical Oncology (ASCO). In interviews, these physicians said the limited supply of multiple cancer drugs – including the chemotherapies carboplatin and cisplatin – is having an unprecedented negative effect since their use is so widespread in cancer care.
“Every patient could get impacted. That’s why we need to address this sooner rather than later,” said oncologist Aditya Baria, MBBS, MPH, director of the Breast Cancer Research Program at Massachusetts General Hospital, Boston.
Shortages of cancer drugs are not unusual. Three-quarters of oncology pharmacists at 68 organizations surveyed from 2019 to 2020 said shortages prompted treatment delays, reduced doses, or alternative regimens. But the current shortages are having a much wider impact.
The National Comprehensive Cancer Network recently reported that 93% of 27 member institutions surveyed in late May are short on carboplatin, and 70% have reported a shortage of cisplatin. Plus, 20% of 19 centers said they weren’t able to continue carboplatin regimens for all patients.
The drugs are mainstays of multiple types of treatment for a long list of cancer types including lung, breast, gynecologic, and many others.
Several scenarios are possible when the drugs are in short supply, said Dr. Yardley, who noted that the shortage is more severe than any she’s seen in her medical career of more than 3 decades. Patients may need to be switched to regimens with more side effects, even when they’re in the middle of a treatment, she said. Or patients might have to go longer between treatments.
In some cases, Dr. Yardley said, the shortage is forcing patients to go without an important component of a larger combination therapy regimen. “The Keynote 522 neoadjuvant regimen for triple-negative breast cancer has carboplatin given with Taxol [paclitaxel] and Keytruda [pembrolizumab]. We are just deleting the carboplatin.”
She added that carboplatin is part of the following so-called TCHP regimen for HER2+ early-stage breast cancer: Taxotere (docetaxel), carboplatin, Herceptin (trastuzumab), and Perjeta (pertuzumab).
“You can delete [carboplatin] or consider substituting cyclophosphamide for carboplatin,” she said. But she cautioned the Keynote 522 and TCHP regimens haven’t been tested without carboplatin in curative-intent trials.
At Duke University in Durham, N.C., doses of carboplatin for many patients are being lowered by a third to the level that’s commonly used for older and frail patients, said oncologist Arif Kamal, MD, MBA, MHS, who works at the academic center and is the chief patient officer at the American Cancer Society.
“We don’t know if [the lower doses will negatively affect cancer patients’ outcomes]. What’s amazing is how many patients [are understanding about having to take smaller amounts of the chemotherapy],” he said.
Medical organizations are offering guidance. The Society of Gynecologic Oncology, for example, in late April recommended that oncologists increase intervals between chemotherapy treatments when appropriate, round down vial sizes to ensure “efficient use,” and eliminate or minimize use of cisplatin and carboplatin in certain platinum-resistant cancers.
In early June, ASCO published guidance regarding alternatives to cisplatin, carboplatin, and 5-fluorouracil, which is also in short supply, in gastrointestinal cancer. As the guidance notes, some alternatives are more untested or more toxic than ideal treatments.
In addition, ASCO has a webpage devoted to news and resources about shortages of cancer drugs. It offers drug availability updates, general guidance, and breast cancer guidance. ASCO also offers ethical guidance about handling drug shortages.
Patients in clinical trials and those who hope to join them are especially vulnerable to the drug shortage, oncologists interviewed for this story said. Cisplatin and carboplatin are the backbones of many clinical trials, Dr. Yardley said. “When you can’t supply a drug in one of the [trial] arms, that puts the whole trial on pause.”
Even clinics that have managed to find adequate supplies of the drugs are planning for when they run out.
“Our institution and other institutions are trying to come up with a rationing protocol, deciding which patients are going to get access, and which ones have reasonable alternatives,” radiation oncologist Corey Speers MD, PhD, of University Hospitals Seidman Cancer Center and Case Western Reserve University, Cleveland, said in an interview. “In some settings, there really isn’t an effective alternative. Or the alternatives are tens of thousands of dollars more expensive.”
Oncologists also noted that cisplatin and carboplatin aren’t the only cancer drugs in short supply.
“Methotrexate is critically low, and 5FU [fluorouracil] is critically low,” Dr. Yardley said, referring to drugs that each treat several types of cancer. According to the May NCNN survey, 67% of respondents reported low supplies of methotrexate, and 26% said they were low on 5FU.
“Viscous lidocaine is a component of many supportive care mouth rinses for the stomatitis caused by our drugs but is not available at all,” Dr. Yardley said.
She added that there are also low supplies of fludarabine, which is used to treat chronic lymphocytic lymphom; clofarabine, which is used to treat acute lymphoblastic leukemia; and rasburicase, which is used to treat high levels of uric acid in patients on chemotherapy.
Dr. Speers said his institution is facing a shortage of capecitabine, which is used to treat several types of cancer.
“Numerous trials have demonstrated the improved, safety, efficacy, and convenience of oral capecitabine. With the shortage we’re having to use infusional 5FU, which not only is less convenient but also ends up being more costly and requires infusion room space or continuous infusion pumps. This impacts our ability to treat cancer patients,” he said. “Our capacity is becoming more limited to accommodate these added patients, and we have to use infusional formulations of a drug that previously was readily available via an oral formulation. Patients and caregivers now have to come to the cancer center for appointments and infusions that previously weren’t needed as they could take an oral pill.”
Dr. Speers added that his institution is rationing methotrexate. “We are now prioritizing patients being treated with curative intent and adjusting protocols to use the lowest allowable doses to conserve supply,” he said.
The roots of the platinum chemotherapy drug shortage link back to the India-based Intas Pharmaceuticals company, a major manufacturer of cisplatin and carboplatin. According to Kellyann Zuzulo, spokeperson for Accord Healthcare, an Instas U.S. subsidiary, a facility inspection in December 2022 prompted a decision to temporarily stop making the drugs. The inspection identified multiple problems.
“Intas and Accord are working with the FDA on a plan to return to manufacturing,” Ms. Zuzulo said in an interview. “This will allow for continued production of products that will be prioritized based on medical necessity. A date has not yet been confirmed in which the facility will return to manufacturing for cisplatin, carboplatin or any other products.”
Ms. Zuzulo said the company is not a health care provider and cannot offer advice to patients about alternatives.
Other companies that make cisplatin and carboplatin have also reported shortages. In interviews, representatives for Fresenius Kabi and Pfizer said the companies have limited supplies because of increased demand – not because of manufacturing problems.
On June 12, the American Society of Health-System Pharmacists (ASHP) reported that carboplatin remains in short supply, with all five companies that sell the drug listed as having limited or back-ordered supplies. Cisplatin is also in short supply, the organization reported in a June 9 update, although some is available.
In a June 12 update on methotrexate, ASHP said manufacturing delays at Accord have caused a shortage, and other companies are running low due to increased demand.
As for the future, Congress and the Biden administration, according to a report by Bloomberg, are trying to figure out what to do regarding shortages of cheap generic drugs such as cisplatin and carboplatin. The FDA is exploring a partnership with a Chinese drugmaker to make cisplatin, NBC News reported.
However, fixes will be challenging, according to former FDA commissioner and Pfizer board member, Scott Gottlieb, MD.
“This generic business, particularly for these complex drugs, these complex formulations, is not a healthy business right now. Yet it’s a vital business from a public standpoint,” he told CBS News.
In an interview, Dr. Kamal said that there is even talk about boosting the prices of cheap generic drugs “to ensure that there’s enough incentive for multiple manufacturers to be involved.”
Dr. Kamal said he is crossing his fingers that cutting chemotherapy doses at his clinic doesn’t result in worse outcomes for his patients.
“Right now, I think dropping someone by 25% or 30% is okay. And for some patients, particularly in a curative setting, we try to keep them at as much as 100% as possible. But there’s just a lot of unknowns,” he said.
CHICAGO – Oncologist Denise Yardley, MD, isn’t used to expressing uncertainty when she tells patients about what’s in store for them in terms of drug treatment. But things are dramatically different now amid a severe national shortage of carboplatin and cisplatin, two common and crucial cancer drugs.
“There’s a regimen I’m thinking about,” Dr. Yardley told a new patient recently, “but we’ll have to wait until you finish your staging evaluation to see whether I can deliver this. Another regimen that’s a little more toxic is my second choice.” And, she added, the alternative chemotherapy treatment – anthracycline instead of carboplatin – requires a longer treatment period.
This ambiguity is hardly ideal, said Dr. Yardley, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville. “It’s another factor in being overwhelmed in a first-time visit and wanting to know the details about what your treatment is going to look like. You’re not walking out knowing exactly what you’re going to take or the exact timing so you can start mapping out your calendar and work schedule.”
This kind of scenario is becoming all too familiar this spring, according to oncologists who gathered at the annual meeting of the American Society of Clinical Oncology (ASCO). In interviews, these physicians said the limited supply of multiple cancer drugs – including the chemotherapies carboplatin and cisplatin – is having an unprecedented negative effect since their use is so widespread in cancer care.
“Every patient could get impacted. That’s why we need to address this sooner rather than later,” said oncologist Aditya Baria, MBBS, MPH, director of the Breast Cancer Research Program at Massachusetts General Hospital, Boston.
Shortages of cancer drugs are not unusual. Three-quarters of oncology pharmacists at 68 organizations surveyed from 2019 to 2020 said shortages prompted treatment delays, reduced doses, or alternative regimens. But the current shortages are having a much wider impact.
The National Comprehensive Cancer Network recently reported that 93% of 27 member institutions surveyed in late May are short on carboplatin, and 70% have reported a shortage of cisplatin. Plus, 20% of 19 centers said they weren’t able to continue carboplatin regimens for all patients.
The drugs are mainstays of multiple types of treatment for a long list of cancer types including lung, breast, gynecologic, and many others.
Several scenarios are possible when the drugs are in short supply, said Dr. Yardley, who noted that the shortage is more severe than any she’s seen in her medical career of more than 3 decades. Patients may need to be switched to regimens with more side effects, even when they’re in the middle of a treatment, she said. Or patients might have to go longer between treatments.
In some cases, Dr. Yardley said, the shortage is forcing patients to go without an important component of a larger combination therapy regimen. “The Keynote 522 neoadjuvant regimen for triple-negative breast cancer has carboplatin given with Taxol [paclitaxel] and Keytruda [pembrolizumab]. We are just deleting the carboplatin.”
She added that carboplatin is part of the following so-called TCHP regimen for HER2+ early-stage breast cancer: Taxotere (docetaxel), carboplatin, Herceptin (trastuzumab), and Perjeta (pertuzumab).
“You can delete [carboplatin] or consider substituting cyclophosphamide for carboplatin,” she said. But she cautioned the Keynote 522 and TCHP regimens haven’t been tested without carboplatin in curative-intent trials.
At Duke University in Durham, N.C., doses of carboplatin for many patients are being lowered by a third to the level that’s commonly used for older and frail patients, said oncologist Arif Kamal, MD, MBA, MHS, who works at the academic center and is the chief patient officer at the American Cancer Society.
“We don’t know if [the lower doses will negatively affect cancer patients’ outcomes]. What’s amazing is how many patients [are understanding about having to take smaller amounts of the chemotherapy],” he said.
Medical organizations are offering guidance. The Society of Gynecologic Oncology, for example, in late April recommended that oncologists increase intervals between chemotherapy treatments when appropriate, round down vial sizes to ensure “efficient use,” and eliminate or minimize use of cisplatin and carboplatin in certain platinum-resistant cancers.
In early June, ASCO published guidance regarding alternatives to cisplatin, carboplatin, and 5-fluorouracil, which is also in short supply, in gastrointestinal cancer. As the guidance notes, some alternatives are more untested or more toxic than ideal treatments.
In addition, ASCO has a webpage devoted to news and resources about shortages of cancer drugs. It offers drug availability updates, general guidance, and breast cancer guidance. ASCO also offers ethical guidance about handling drug shortages.
Patients in clinical trials and those who hope to join them are especially vulnerable to the drug shortage, oncologists interviewed for this story said. Cisplatin and carboplatin are the backbones of many clinical trials, Dr. Yardley said. “When you can’t supply a drug in one of the [trial] arms, that puts the whole trial on pause.”
Even clinics that have managed to find adequate supplies of the drugs are planning for when they run out.
“Our institution and other institutions are trying to come up with a rationing protocol, deciding which patients are going to get access, and which ones have reasonable alternatives,” radiation oncologist Corey Speers MD, PhD, of University Hospitals Seidman Cancer Center and Case Western Reserve University, Cleveland, said in an interview. “In some settings, there really isn’t an effective alternative. Or the alternatives are tens of thousands of dollars more expensive.”
Oncologists also noted that cisplatin and carboplatin aren’t the only cancer drugs in short supply.
“Methotrexate is critically low, and 5FU [fluorouracil] is critically low,” Dr. Yardley said, referring to drugs that each treat several types of cancer. According to the May NCNN survey, 67% of respondents reported low supplies of methotrexate, and 26% said they were low on 5FU.
“Viscous lidocaine is a component of many supportive care mouth rinses for the stomatitis caused by our drugs but is not available at all,” Dr. Yardley said.
She added that there are also low supplies of fludarabine, which is used to treat chronic lymphocytic lymphom; clofarabine, which is used to treat acute lymphoblastic leukemia; and rasburicase, which is used to treat high levels of uric acid in patients on chemotherapy.
Dr. Speers said his institution is facing a shortage of capecitabine, which is used to treat several types of cancer.
“Numerous trials have demonstrated the improved, safety, efficacy, and convenience of oral capecitabine. With the shortage we’re having to use infusional 5FU, which not only is less convenient but also ends up being more costly and requires infusion room space or continuous infusion pumps. This impacts our ability to treat cancer patients,” he said. “Our capacity is becoming more limited to accommodate these added patients, and we have to use infusional formulations of a drug that previously was readily available via an oral formulation. Patients and caregivers now have to come to the cancer center for appointments and infusions that previously weren’t needed as they could take an oral pill.”
Dr. Speers added that his institution is rationing methotrexate. “We are now prioritizing patients being treated with curative intent and adjusting protocols to use the lowest allowable doses to conserve supply,” he said.
The roots of the platinum chemotherapy drug shortage link back to the India-based Intas Pharmaceuticals company, a major manufacturer of cisplatin and carboplatin. According to Kellyann Zuzulo, spokeperson for Accord Healthcare, an Instas U.S. subsidiary, a facility inspection in December 2022 prompted a decision to temporarily stop making the drugs. The inspection identified multiple problems.
“Intas and Accord are working with the FDA on a plan to return to manufacturing,” Ms. Zuzulo said in an interview. “This will allow for continued production of products that will be prioritized based on medical necessity. A date has not yet been confirmed in which the facility will return to manufacturing for cisplatin, carboplatin or any other products.”
Ms. Zuzulo said the company is not a health care provider and cannot offer advice to patients about alternatives.
Other companies that make cisplatin and carboplatin have also reported shortages. In interviews, representatives for Fresenius Kabi and Pfizer said the companies have limited supplies because of increased demand – not because of manufacturing problems.
On June 12, the American Society of Health-System Pharmacists (ASHP) reported that carboplatin remains in short supply, with all five companies that sell the drug listed as having limited or back-ordered supplies. Cisplatin is also in short supply, the organization reported in a June 9 update, although some is available.
In a June 12 update on methotrexate, ASHP said manufacturing delays at Accord have caused a shortage, and other companies are running low due to increased demand.
As for the future, Congress and the Biden administration, according to a report by Bloomberg, are trying to figure out what to do regarding shortages of cheap generic drugs such as cisplatin and carboplatin. The FDA is exploring a partnership with a Chinese drugmaker to make cisplatin, NBC News reported.
However, fixes will be challenging, according to former FDA commissioner and Pfizer board member, Scott Gottlieb, MD.
“This generic business, particularly for these complex drugs, these complex formulations, is not a healthy business right now. Yet it’s a vital business from a public standpoint,” he told CBS News.
In an interview, Dr. Kamal said that there is even talk about boosting the prices of cheap generic drugs “to ensure that there’s enough incentive for multiple manufacturers to be involved.”
Dr. Kamal said he is crossing his fingers that cutting chemotherapy doses at his clinic doesn’t result in worse outcomes for his patients.
“Right now, I think dropping someone by 25% or 30% is okay. And for some patients, particularly in a curative setting, we try to keep them at as much as 100% as possible. But there’s just a lot of unknowns,” he said.
CHICAGO – Oncologist Denise Yardley, MD, isn’t used to expressing uncertainty when she tells patients about what’s in store for them in terms of drug treatment. But things are dramatically different now amid a severe national shortage of carboplatin and cisplatin, two common and crucial cancer drugs.
“There’s a regimen I’m thinking about,” Dr. Yardley told a new patient recently, “but we’ll have to wait until you finish your staging evaluation to see whether I can deliver this. Another regimen that’s a little more toxic is my second choice.” And, she added, the alternative chemotherapy treatment – anthracycline instead of carboplatin – requires a longer treatment period.
This ambiguity is hardly ideal, said Dr. Yardley, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville. “It’s another factor in being overwhelmed in a first-time visit and wanting to know the details about what your treatment is going to look like. You’re not walking out knowing exactly what you’re going to take or the exact timing so you can start mapping out your calendar and work schedule.”
This kind of scenario is becoming all too familiar this spring, according to oncologists who gathered at the annual meeting of the American Society of Clinical Oncology (ASCO). In interviews, these physicians said the limited supply of multiple cancer drugs – including the chemotherapies carboplatin and cisplatin – is having an unprecedented negative effect since their use is so widespread in cancer care.
“Every patient could get impacted. That’s why we need to address this sooner rather than later,” said oncologist Aditya Baria, MBBS, MPH, director of the Breast Cancer Research Program at Massachusetts General Hospital, Boston.
Shortages of cancer drugs are not unusual. Three-quarters of oncology pharmacists at 68 organizations surveyed from 2019 to 2020 said shortages prompted treatment delays, reduced doses, or alternative regimens. But the current shortages are having a much wider impact.
The National Comprehensive Cancer Network recently reported that 93% of 27 member institutions surveyed in late May are short on carboplatin, and 70% have reported a shortage of cisplatin. Plus, 20% of 19 centers said they weren’t able to continue carboplatin regimens for all patients.
The drugs are mainstays of multiple types of treatment for a long list of cancer types including lung, breast, gynecologic, and many others.
Several scenarios are possible when the drugs are in short supply, said Dr. Yardley, who noted that the shortage is more severe than any she’s seen in her medical career of more than 3 decades. Patients may need to be switched to regimens with more side effects, even when they’re in the middle of a treatment, she said. Or patients might have to go longer between treatments.
In some cases, Dr. Yardley said, the shortage is forcing patients to go without an important component of a larger combination therapy regimen. “The Keynote 522 neoadjuvant regimen for triple-negative breast cancer has carboplatin given with Taxol [paclitaxel] and Keytruda [pembrolizumab]. We are just deleting the carboplatin.”
She added that carboplatin is part of the following so-called TCHP regimen for HER2+ early-stage breast cancer: Taxotere (docetaxel), carboplatin, Herceptin (trastuzumab), and Perjeta (pertuzumab).
“You can delete [carboplatin] or consider substituting cyclophosphamide for carboplatin,” she said. But she cautioned the Keynote 522 and TCHP regimens haven’t been tested without carboplatin in curative-intent trials.
At Duke University in Durham, N.C., doses of carboplatin for many patients are being lowered by a third to the level that’s commonly used for older and frail patients, said oncologist Arif Kamal, MD, MBA, MHS, who works at the academic center and is the chief patient officer at the American Cancer Society.
“We don’t know if [the lower doses will negatively affect cancer patients’ outcomes]. What’s amazing is how many patients [are understanding about having to take smaller amounts of the chemotherapy],” he said.
Medical organizations are offering guidance. The Society of Gynecologic Oncology, for example, in late April recommended that oncologists increase intervals between chemotherapy treatments when appropriate, round down vial sizes to ensure “efficient use,” and eliminate or minimize use of cisplatin and carboplatin in certain platinum-resistant cancers.
In early June, ASCO published guidance regarding alternatives to cisplatin, carboplatin, and 5-fluorouracil, which is also in short supply, in gastrointestinal cancer. As the guidance notes, some alternatives are more untested or more toxic than ideal treatments.
In addition, ASCO has a webpage devoted to news and resources about shortages of cancer drugs. It offers drug availability updates, general guidance, and breast cancer guidance. ASCO also offers ethical guidance about handling drug shortages.
Patients in clinical trials and those who hope to join them are especially vulnerable to the drug shortage, oncologists interviewed for this story said. Cisplatin and carboplatin are the backbones of many clinical trials, Dr. Yardley said. “When you can’t supply a drug in one of the [trial] arms, that puts the whole trial on pause.”
Even clinics that have managed to find adequate supplies of the drugs are planning for when they run out.
“Our institution and other institutions are trying to come up with a rationing protocol, deciding which patients are going to get access, and which ones have reasonable alternatives,” radiation oncologist Corey Speers MD, PhD, of University Hospitals Seidman Cancer Center and Case Western Reserve University, Cleveland, said in an interview. “In some settings, there really isn’t an effective alternative. Or the alternatives are tens of thousands of dollars more expensive.”
Oncologists also noted that cisplatin and carboplatin aren’t the only cancer drugs in short supply.
“Methotrexate is critically low, and 5FU [fluorouracil] is critically low,” Dr. Yardley said, referring to drugs that each treat several types of cancer. According to the May NCNN survey, 67% of respondents reported low supplies of methotrexate, and 26% said they were low on 5FU.
“Viscous lidocaine is a component of many supportive care mouth rinses for the stomatitis caused by our drugs but is not available at all,” Dr. Yardley said.
She added that there are also low supplies of fludarabine, which is used to treat chronic lymphocytic lymphom; clofarabine, which is used to treat acute lymphoblastic leukemia; and rasburicase, which is used to treat high levels of uric acid in patients on chemotherapy.
Dr. Speers said his institution is facing a shortage of capecitabine, which is used to treat several types of cancer.
“Numerous trials have demonstrated the improved, safety, efficacy, and convenience of oral capecitabine. With the shortage we’re having to use infusional 5FU, which not only is less convenient but also ends up being more costly and requires infusion room space or continuous infusion pumps. This impacts our ability to treat cancer patients,” he said. “Our capacity is becoming more limited to accommodate these added patients, and we have to use infusional formulations of a drug that previously was readily available via an oral formulation. Patients and caregivers now have to come to the cancer center for appointments and infusions that previously weren’t needed as they could take an oral pill.”
Dr. Speers added that his institution is rationing methotrexate. “We are now prioritizing patients being treated with curative intent and adjusting protocols to use the lowest allowable doses to conserve supply,” he said.
The roots of the platinum chemotherapy drug shortage link back to the India-based Intas Pharmaceuticals company, a major manufacturer of cisplatin and carboplatin. According to Kellyann Zuzulo, spokeperson for Accord Healthcare, an Instas U.S. subsidiary, a facility inspection in December 2022 prompted a decision to temporarily stop making the drugs. The inspection identified multiple problems.
“Intas and Accord are working with the FDA on a plan to return to manufacturing,” Ms. Zuzulo said in an interview. “This will allow for continued production of products that will be prioritized based on medical necessity. A date has not yet been confirmed in which the facility will return to manufacturing for cisplatin, carboplatin or any other products.”
Ms. Zuzulo said the company is not a health care provider and cannot offer advice to patients about alternatives.
Other companies that make cisplatin and carboplatin have also reported shortages. In interviews, representatives for Fresenius Kabi and Pfizer said the companies have limited supplies because of increased demand – not because of manufacturing problems.
On June 12, the American Society of Health-System Pharmacists (ASHP) reported that carboplatin remains in short supply, with all five companies that sell the drug listed as having limited or back-ordered supplies. Cisplatin is also in short supply, the organization reported in a June 9 update, although some is available.
In a June 12 update on methotrexate, ASHP said manufacturing delays at Accord have caused a shortage, and other companies are running low due to increased demand.
As for the future, Congress and the Biden administration, according to a report by Bloomberg, are trying to figure out what to do regarding shortages of cheap generic drugs such as cisplatin and carboplatin. The FDA is exploring a partnership with a Chinese drugmaker to make cisplatin, NBC News reported.
However, fixes will be challenging, according to former FDA commissioner and Pfizer board member, Scott Gottlieb, MD.
“This generic business, particularly for these complex drugs, these complex formulations, is not a healthy business right now. Yet it’s a vital business from a public standpoint,” he told CBS News.
In an interview, Dr. Kamal said that there is even talk about boosting the prices of cheap generic drugs “to ensure that there’s enough incentive for multiple manufacturers to be involved.”
Dr. Kamal said he is crossing his fingers that cutting chemotherapy doses at his clinic doesn’t result in worse outcomes for his patients.
“Right now, I think dropping someone by 25% or 30% is okay. And for some patients, particularly in a curative setting, we try to keep them at as much as 100% as possible. But there’s just a lot of unknowns,” he said.
AT ASCO 2023
Annual physical exam
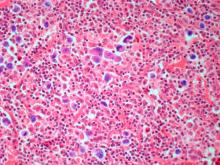
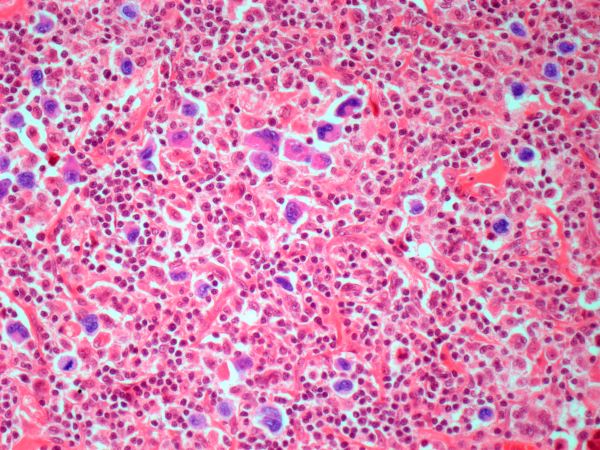
The history and findings in this case are suggestive of leukemic non-nodal mantle cell lymphoma (MCL).
MCL is a rare mature B-cell neoplasm characterized by t(11;14) (q13;q32) and cyclin D1 overexpression in more than 95% of cases. It accounts for approximately 5%-7% of all lymphomas, with an annual incidence of one case per 200,000 people. In North America and Europe, the incidence of MCL is like that of noncutaneous peripheral T-cell lymphomas. MCL occurs more frequently in men than in women (3:1), and the median age at diagnosis ranges from ages 60-70 years.
In recent years, MCL has been categorized into two major subgroups that have distinct clinical presentation and molecular features: nodal MCL and leukemic non-nodal MCL. Nodal MCL is a common variant with an aggressive disease course. Unmutated IGHV gene rearrangement, SOX11 overexpression, a higher degree of genomic instability (eg, ATM, CDKN2A, chromatin modifier mutations), and other oncogenic mutations and epigenetic modifications are seen in patients with this variant.
Leukemic non-nodal MCL is seen in 10%-20% of patients with MCL. Patients frequently present with lymphocytosis and splenomegaly. In most cases, it is associated with an indolent disease course and superior outcome. This subtype is largely IGHV mutated and mostly SOX11-negative, with positive expression of CD200, peripheral blood, bone marrow, and splenic involvement, low tumor burden, and a low Ki-67 index.
Recognition of the leukemic non-nodal MCL immunophenotype enables it to be differentiated from other CD5-positive B-cell cancers, particularly classical MCL and chronic lymphocytic leukemia (CLL). The overexpression of cyclin D1, the presence of the t(11;14) translocation, and the absence of chromosomal markers typically present in CLL differentiate leukemic non-nodal MCL from CLL. Moreover, CLL has high expression of CD23 and is negative for SOX11 and CD200.
Pathologic features of MCL include small- to medium-size lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Observed growth patterns include diffuse, nodular (more vague and less discrete than that found in follicular lymphomas), mantle-zone lymphoma with expansion of mantle zones, and in situ mantle-cell neoplasia [typical cells with the characteristic t(11;14) translocation, scattered in the mantle zone of otherwise normal-appearing lymph nodes]. Cytologic subtypes include classic MCL, the blastoid subtype (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic subtype (cells of variable sizes, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli).
MCL is a challenging disease to treat. Despite treatment advances, it is largely incurable, with a median overall survival of 1.8-9.4 years, depending on whether it is aggressive or indolent MCL. The aggressiveness of the disease, the patient's performance status, age, and mantle cell international prognostic index score should all be considered when selecting treatment because there is no standard curative treatment.
According to the 2023 guidelines from the National Comprehensive Cancer Network (NCCN), for patients with indolent disease (eg, IGHV mutated and mostly SOX11-negative with leukemic and non-nodal presentation), observation is reasonable when patients are asymptomatic and have no indications for treatment. For patients with symptomatic disease or other indications for treatment, induction therapy with aggressive regimens is recommended when patients do not have a TP53 mutation. The optimum approach for patients with TP53 mutation is not yet known; induction therapy followed by high-dose therapy with autologous stem cell transplant or less aggressive regimens could be an option for these patients.
Treatment options for relapsed/refractory MCL include radiotherapy; traditional chemotherapy regimens, with or without rituximab; and newer targeted therapies. These include Bruton tyrosine kinase inhibitors (ibrutinib, zanubrutinib, acalabrutinib, pirtobrutinib), lenalidomide, bortezomib, the mammalian target of rapamycin inhibitors temsirolimus and everolimus, the phosphatidylinositol 3–kinase inhibitors idelalisib and umbralisib, and the B-cell lymphoma 2 inhibitor venetoclax. These agents are frequently administered in combination with rituximab or another anti-CD20 antibody.
For comprehensive guidance on the treatment of MCL, consult the complete NCCN guidelines.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of leukemic non-nodal mantle cell lymphoma (MCL).
MCL is a rare mature B-cell neoplasm characterized by t(11;14) (q13;q32) and cyclin D1 overexpression in more than 95% of cases. It accounts for approximately 5%-7% of all lymphomas, with an annual incidence of one case per 200,000 people. In North America and Europe, the incidence of MCL is like that of noncutaneous peripheral T-cell lymphomas. MCL occurs more frequently in men than in women (3:1), and the median age at diagnosis ranges from ages 60-70 years.
In recent years, MCL has been categorized into two major subgroups that have distinct clinical presentation and molecular features: nodal MCL and leukemic non-nodal MCL. Nodal MCL is a common variant with an aggressive disease course. Unmutated IGHV gene rearrangement, SOX11 overexpression, a higher degree of genomic instability (eg, ATM, CDKN2A, chromatin modifier mutations), and other oncogenic mutations and epigenetic modifications are seen in patients with this variant.
Leukemic non-nodal MCL is seen in 10%-20% of patients with MCL. Patients frequently present with lymphocytosis and splenomegaly. In most cases, it is associated with an indolent disease course and superior outcome. This subtype is largely IGHV mutated and mostly SOX11-negative, with positive expression of CD200, peripheral blood, bone marrow, and splenic involvement, low tumor burden, and a low Ki-67 index.
Recognition of the leukemic non-nodal MCL immunophenotype enables it to be differentiated from other CD5-positive B-cell cancers, particularly classical MCL and chronic lymphocytic leukemia (CLL). The overexpression of cyclin D1, the presence of the t(11;14) translocation, and the absence of chromosomal markers typically present in CLL differentiate leukemic non-nodal MCL from CLL. Moreover, CLL has high expression of CD23 and is negative for SOX11 and CD200.
Pathologic features of MCL include small- to medium-size lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Observed growth patterns include diffuse, nodular (more vague and less discrete than that found in follicular lymphomas), mantle-zone lymphoma with expansion of mantle zones, and in situ mantle-cell neoplasia [typical cells with the characteristic t(11;14) translocation, scattered in the mantle zone of otherwise normal-appearing lymph nodes]. Cytologic subtypes include classic MCL, the blastoid subtype (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic subtype (cells of variable sizes, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli).
MCL is a challenging disease to treat. Despite treatment advances, it is largely incurable, with a median overall survival of 1.8-9.4 years, depending on whether it is aggressive or indolent MCL. The aggressiveness of the disease, the patient's performance status, age, and mantle cell international prognostic index score should all be considered when selecting treatment because there is no standard curative treatment.
According to the 2023 guidelines from the National Comprehensive Cancer Network (NCCN), for patients with indolent disease (eg, IGHV mutated and mostly SOX11-negative with leukemic and non-nodal presentation), observation is reasonable when patients are asymptomatic and have no indications for treatment. For patients with symptomatic disease or other indications for treatment, induction therapy with aggressive regimens is recommended when patients do not have a TP53 mutation. The optimum approach for patients with TP53 mutation is not yet known; induction therapy followed by high-dose therapy with autologous stem cell transplant or less aggressive regimens could be an option for these patients.
Treatment options for relapsed/refractory MCL include radiotherapy; traditional chemotherapy regimens, with or without rituximab; and newer targeted therapies. These include Bruton tyrosine kinase inhibitors (ibrutinib, zanubrutinib, acalabrutinib, pirtobrutinib), lenalidomide, bortezomib, the mammalian target of rapamycin inhibitors temsirolimus and everolimus, the phosphatidylinositol 3–kinase inhibitors idelalisib and umbralisib, and the B-cell lymphoma 2 inhibitor venetoclax. These agents are frequently administered in combination with rituximab or another anti-CD20 antibody.
For comprehensive guidance on the treatment of MCL, consult the complete NCCN guidelines.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of leukemic non-nodal mantle cell lymphoma (MCL).
MCL is a rare mature B-cell neoplasm characterized by t(11;14) (q13;q32) and cyclin D1 overexpression in more than 95% of cases. It accounts for approximately 5%-7% of all lymphomas, with an annual incidence of one case per 200,000 people. In North America and Europe, the incidence of MCL is like that of noncutaneous peripheral T-cell lymphomas. MCL occurs more frequently in men than in women (3:1), and the median age at diagnosis ranges from ages 60-70 years.
In recent years, MCL has been categorized into two major subgroups that have distinct clinical presentation and molecular features: nodal MCL and leukemic non-nodal MCL. Nodal MCL is a common variant with an aggressive disease course. Unmutated IGHV gene rearrangement, SOX11 overexpression, a higher degree of genomic instability (eg, ATM, CDKN2A, chromatin modifier mutations), and other oncogenic mutations and epigenetic modifications are seen in patients with this variant.
Leukemic non-nodal MCL is seen in 10%-20% of patients with MCL. Patients frequently present with lymphocytosis and splenomegaly. In most cases, it is associated with an indolent disease course and superior outcome. This subtype is largely IGHV mutated and mostly SOX11-negative, with positive expression of CD200, peripheral blood, bone marrow, and splenic involvement, low tumor burden, and a low Ki-67 index.
Recognition of the leukemic non-nodal MCL immunophenotype enables it to be differentiated from other CD5-positive B-cell cancers, particularly classical MCL and chronic lymphocytic leukemia (CLL). The overexpression of cyclin D1, the presence of the t(11;14) translocation, and the absence of chromosomal markers typically present in CLL differentiate leukemic non-nodal MCL from CLL. Moreover, CLL has high expression of CD23 and is negative for SOX11 and CD200.
Pathologic features of MCL include small- to medium-size lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Observed growth patterns include diffuse, nodular (more vague and less discrete than that found in follicular lymphomas), mantle-zone lymphoma with expansion of mantle zones, and in situ mantle-cell neoplasia [typical cells with the characteristic t(11;14) translocation, scattered in the mantle zone of otherwise normal-appearing lymph nodes]. Cytologic subtypes include classic MCL, the blastoid subtype (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic subtype (cells of variable sizes, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli).
MCL is a challenging disease to treat. Despite treatment advances, it is largely incurable, with a median overall survival of 1.8-9.4 years, depending on whether it is aggressive or indolent MCL. The aggressiveness of the disease, the patient's performance status, age, and mantle cell international prognostic index score should all be considered when selecting treatment because there is no standard curative treatment.
According to the 2023 guidelines from the National Comprehensive Cancer Network (NCCN), for patients with indolent disease (eg, IGHV mutated and mostly SOX11-negative with leukemic and non-nodal presentation), observation is reasonable when patients are asymptomatic and have no indications for treatment. For patients with symptomatic disease or other indications for treatment, induction therapy with aggressive regimens is recommended when patients do not have a TP53 mutation. The optimum approach for patients with TP53 mutation is not yet known; induction therapy followed by high-dose therapy with autologous stem cell transplant or less aggressive regimens could be an option for these patients.
Treatment options for relapsed/refractory MCL include radiotherapy; traditional chemotherapy regimens, with or without rituximab; and newer targeted therapies. These include Bruton tyrosine kinase inhibitors (ibrutinib, zanubrutinib, acalabrutinib, pirtobrutinib), lenalidomide, bortezomib, the mammalian target of rapamycin inhibitors temsirolimus and everolimus, the phosphatidylinositol 3–kinase inhibitors idelalisib and umbralisib, and the B-cell lymphoma 2 inhibitor venetoclax. These agents are frequently administered in combination with rituximab or another anti-CD20 antibody.
For comprehensive guidance on the treatment of MCL, consult the complete NCCN guidelines.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 67-year-old White man presents for his annual physical examination. The patient is a physical therapist and reports regular exercise and adherence to a healthy diet. His previous medical history is unremarkable. There is a family history of non-Hodgkin lymphoma (paternal uncle). The patient has no complaints or concerns about his health.
Physical examination reveals non-tender abdominal distention and splenomegaly. Pertinent laboratory findings include hemoglobin = 10/g/dL; red blood cell = 3.28 M/mcL; mean corpuscular volume = 54.2 fL, hematocrit = 34%; and absolute lymphocyte count = 4820/µL.
Flow cytometry showed high positivity for CD5, no expression of SOX11, low expression of CD23 and CD200, and overexpression of cyclin D1. A bone marrow biopsy is performed and show an abnormal B-lymphoid infiltrate. Fluorescence in situ hybridization analysis revealed t(11;14)(q13;q32) and mutated IGHV. A blood smear showed abnormal mononuclear cells and atypical lymphocytes.
Upadacitinib shows promise in treatment-resistant UC and Crohn’s
according to results from a real-world study at a Chicago treatment center.
The results suggest that upadacitinib may be an appropriate salvage treatment for patients who have failed other advanced therapies, including tofacitinib.
For their research, published in Clinical Gastroenterology and Hepatology, Scott Friedberg, MD, and colleagues at the University of Chicago’s Inflammatory Bowel Disease Center, looked at results from 44 patients diagnosed with ulcerative colitis and 40 with Crohn’s disease, all with active luminal or perianal disease. All patients in the study had previous exposure to tumor necrosis factor inhibitors, and nearly 90% had exposure to two or more advanced therapies, including tofacitinib (n = 17), before being switched to upadacitinib.
Upadacitinib (Rinvoq, AbbVie) is the second small-molecule Janus kinase (JAK) inhibitor approved for ulcerative colitis by the Food and Drug Administration in March 2022 after tofacitinib (Xeljanz, Pfizer) in 2018. Upadacitinib received an additional indication in May 2023 as a treatment for Crohn’s disease. It selectively inhibits JAK1, while tofacitinib inhibits JAK1 and JAK3.
Among the ulcerative colitis patients in Dr. Friedberg and colleagues’ study (mean age, 39 years; 48% female), 85% had a clinical response and 82% achieved clinical remission by week 8. Of nine patients previously treated with tofacitinib, seven (78%) achieved remission at 8 weeks.
Some 76% of the Crohn’s disease patients in the study (mean age, 37 years; 53% female) saw clinical response by 8 weeks, and 71% achieved remission by that time. More than 60% of all participants who had increased fecal calprotectin and C-reactive protein levels at baseline saw normalization of these biomarkers by week 8.
Some patients saw an especially fast response, with 36% of the ulcerative colitis patients and 56% of the Crohn’s patients experiencing clinical remission by week 2.
Acne was the most common reported adverse event, occurring in 23% of patients. Only one serious adverse event, an anemia requiring hospitalization, occurred during the study.
No wash-out period occurred before starting patients on upadacitinib. There were no adverse events seen associated with this strategy, Dr. Friedberg and colleagues noted, a finding with important implications for real-world practice.
“When patients with active IBD are sick, starting a new therapy as soon as it is available is not only reasonable, it is required,” the investigators wrote. Additionally, the findings support the use of upadacitinib in ulcerative colitis patients with previous exposure to tofacitinib, as “selectivity of JAK targets may have different effectiveness profiles.”
Upadacitinib’s rapid onset “has multiple advantages,” the investigators wrote, “not only by being an option for severely active disease but also by allowing for a rapid taper or complete avoidance of corticosteroids.”
The authors noted their study’s small sample size as a key limitation. Several of Dr. Friedberg’s coauthors disclosed financial relationships with drug manufacturers, including AbbVie.
Understanding the efficacy, onset of action and safety of newly approved inflammatory bowel disease (IBD) therapies is difficult in the absence of real-world data as clinical trial populations are much more restrictive and typically do not reflect the patient populations seen in most IBD clinics. This single-center study by Friedberg and colleagues reports on their experience with upadacitinib use in patients with ulcerative colitis (UC) and Crohn’s disease (CD). One key finding of this study is the rapid onset of action with high rates of clinical response and remission within 2 weeks of initiation (60% and 36%) for UC and (50% and 56%) for CD. Further, these high rates of clinical response and remission were noted despite exposure to multiple prior therapies (including prior tofacitinib use), which has been a limitation with other IBD therapies.
With the concerns for safety of tofacitinib use, another Janus kinase inhibitor, raised by the ORAL surveillance study, many patients and practitioners are concerned about the safety of upadacitinib use. This study highlighted the low rate of adverse events including no incidences of herpes zoster infection, venous thromboembolism or major adverse cardiovascular events. Acne was noted to be the most common adverse event, occurring in 22% of the study population.
Further research is needed to assess the long term clinical and endoscopic response rates as well as long-term safety assessments, however these results will facilitate conversations with patients who could potentially benefit from treatment with this new therapy.
Jill K. J. Gaidos, MD, FACG, AGAF, is associate professor of medicine, vice chief of clinical research, section of digestive diseases, Yale University, and director of clinical research, Yale Inflammatory Bowel Disease Program, New Haven, Conn.
Understanding the efficacy, onset of action and safety of newly approved inflammatory bowel disease (IBD) therapies is difficult in the absence of real-world data as clinical trial populations are much more restrictive and typically do not reflect the patient populations seen in most IBD clinics. This single-center study by Friedberg and colleagues reports on their experience with upadacitinib use in patients with ulcerative colitis (UC) and Crohn’s disease (CD). One key finding of this study is the rapid onset of action with high rates of clinical response and remission within 2 weeks of initiation (60% and 36%) for UC and (50% and 56%) for CD. Further, these high rates of clinical response and remission were noted despite exposure to multiple prior therapies (including prior tofacitinib use), which has been a limitation with other IBD therapies.
With the concerns for safety of tofacitinib use, another Janus kinase inhibitor, raised by the ORAL surveillance study, many patients and practitioners are concerned about the safety of upadacitinib use. This study highlighted the low rate of adverse events including no incidences of herpes zoster infection, venous thromboembolism or major adverse cardiovascular events. Acne was noted to be the most common adverse event, occurring in 22% of the study population.
Further research is needed to assess the long term clinical and endoscopic response rates as well as long-term safety assessments, however these results will facilitate conversations with patients who could potentially benefit from treatment with this new therapy.
Jill K. J. Gaidos, MD, FACG, AGAF, is associate professor of medicine, vice chief of clinical research, section of digestive diseases, Yale University, and director of clinical research, Yale Inflammatory Bowel Disease Program, New Haven, Conn.
Understanding the efficacy, onset of action and safety of newly approved inflammatory bowel disease (IBD) therapies is difficult in the absence of real-world data as clinical trial populations are much more restrictive and typically do not reflect the patient populations seen in most IBD clinics. This single-center study by Friedberg and colleagues reports on their experience with upadacitinib use in patients with ulcerative colitis (UC) and Crohn’s disease (CD). One key finding of this study is the rapid onset of action with high rates of clinical response and remission within 2 weeks of initiation (60% and 36%) for UC and (50% and 56%) for CD. Further, these high rates of clinical response and remission were noted despite exposure to multiple prior therapies (including prior tofacitinib use), which has been a limitation with other IBD therapies.
With the concerns for safety of tofacitinib use, another Janus kinase inhibitor, raised by the ORAL surveillance study, many patients and practitioners are concerned about the safety of upadacitinib use. This study highlighted the low rate of adverse events including no incidences of herpes zoster infection, venous thromboembolism or major adverse cardiovascular events. Acne was noted to be the most common adverse event, occurring in 22% of the study population.
Further research is needed to assess the long term clinical and endoscopic response rates as well as long-term safety assessments, however these results will facilitate conversations with patients who could potentially benefit from treatment with this new therapy.
Jill K. J. Gaidos, MD, FACG, AGAF, is associate professor of medicine, vice chief of clinical research, section of digestive diseases, Yale University, and director of clinical research, Yale Inflammatory Bowel Disease Program, New Haven, Conn.
according to results from a real-world study at a Chicago treatment center.
The results suggest that upadacitinib may be an appropriate salvage treatment for patients who have failed other advanced therapies, including tofacitinib.
For their research, published in Clinical Gastroenterology and Hepatology, Scott Friedberg, MD, and colleagues at the University of Chicago’s Inflammatory Bowel Disease Center, looked at results from 44 patients diagnosed with ulcerative colitis and 40 with Crohn’s disease, all with active luminal or perianal disease. All patients in the study had previous exposure to tumor necrosis factor inhibitors, and nearly 90% had exposure to two or more advanced therapies, including tofacitinib (n = 17), before being switched to upadacitinib.
Upadacitinib (Rinvoq, AbbVie) is the second small-molecule Janus kinase (JAK) inhibitor approved for ulcerative colitis by the Food and Drug Administration in March 2022 after tofacitinib (Xeljanz, Pfizer) in 2018. Upadacitinib received an additional indication in May 2023 as a treatment for Crohn’s disease. It selectively inhibits JAK1, while tofacitinib inhibits JAK1 and JAK3.
Among the ulcerative colitis patients in Dr. Friedberg and colleagues’ study (mean age, 39 years; 48% female), 85% had a clinical response and 82% achieved clinical remission by week 8. Of nine patients previously treated with tofacitinib, seven (78%) achieved remission at 8 weeks.
Some 76% of the Crohn’s disease patients in the study (mean age, 37 years; 53% female) saw clinical response by 8 weeks, and 71% achieved remission by that time. More than 60% of all participants who had increased fecal calprotectin and C-reactive protein levels at baseline saw normalization of these biomarkers by week 8.
Some patients saw an especially fast response, with 36% of the ulcerative colitis patients and 56% of the Crohn’s patients experiencing clinical remission by week 2.
Acne was the most common reported adverse event, occurring in 23% of patients. Only one serious adverse event, an anemia requiring hospitalization, occurred during the study.
No wash-out period occurred before starting patients on upadacitinib. There were no adverse events seen associated with this strategy, Dr. Friedberg and colleagues noted, a finding with important implications for real-world practice.
“When patients with active IBD are sick, starting a new therapy as soon as it is available is not only reasonable, it is required,” the investigators wrote. Additionally, the findings support the use of upadacitinib in ulcerative colitis patients with previous exposure to tofacitinib, as “selectivity of JAK targets may have different effectiveness profiles.”
Upadacitinib’s rapid onset “has multiple advantages,” the investigators wrote, “not only by being an option for severely active disease but also by allowing for a rapid taper or complete avoidance of corticosteroids.”
The authors noted their study’s small sample size as a key limitation. Several of Dr. Friedberg’s coauthors disclosed financial relationships with drug manufacturers, including AbbVie.
according to results from a real-world study at a Chicago treatment center.
The results suggest that upadacitinib may be an appropriate salvage treatment for patients who have failed other advanced therapies, including tofacitinib.
For their research, published in Clinical Gastroenterology and Hepatology, Scott Friedberg, MD, and colleagues at the University of Chicago’s Inflammatory Bowel Disease Center, looked at results from 44 patients diagnosed with ulcerative colitis and 40 with Crohn’s disease, all with active luminal or perianal disease. All patients in the study had previous exposure to tumor necrosis factor inhibitors, and nearly 90% had exposure to two or more advanced therapies, including tofacitinib (n = 17), before being switched to upadacitinib.
Upadacitinib (Rinvoq, AbbVie) is the second small-molecule Janus kinase (JAK) inhibitor approved for ulcerative colitis by the Food and Drug Administration in March 2022 after tofacitinib (Xeljanz, Pfizer) in 2018. Upadacitinib received an additional indication in May 2023 as a treatment for Crohn’s disease. It selectively inhibits JAK1, while tofacitinib inhibits JAK1 and JAK3.
Among the ulcerative colitis patients in Dr. Friedberg and colleagues’ study (mean age, 39 years; 48% female), 85% had a clinical response and 82% achieved clinical remission by week 8. Of nine patients previously treated with tofacitinib, seven (78%) achieved remission at 8 weeks.
Some 76% of the Crohn’s disease patients in the study (mean age, 37 years; 53% female) saw clinical response by 8 weeks, and 71% achieved remission by that time. More than 60% of all participants who had increased fecal calprotectin and C-reactive protein levels at baseline saw normalization of these biomarkers by week 8.
Some patients saw an especially fast response, with 36% of the ulcerative colitis patients and 56% of the Crohn’s patients experiencing clinical remission by week 2.
Acne was the most common reported adverse event, occurring in 23% of patients. Only one serious adverse event, an anemia requiring hospitalization, occurred during the study.
No wash-out period occurred before starting patients on upadacitinib. There were no adverse events seen associated with this strategy, Dr. Friedberg and colleagues noted, a finding with important implications for real-world practice.
“When patients with active IBD are sick, starting a new therapy as soon as it is available is not only reasonable, it is required,” the investigators wrote. Additionally, the findings support the use of upadacitinib in ulcerative colitis patients with previous exposure to tofacitinib, as “selectivity of JAK targets may have different effectiveness profiles.”
Upadacitinib’s rapid onset “has multiple advantages,” the investigators wrote, “not only by being an option for severely active disease but also by allowing for a rapid taper or complete avoidance of corticosteroids.”
The authors noted their study’s small sample size as a key limitation. Several of Dr. Friedberg’s coauthors disclosed financial relationships with drug manufacturers, including AbbVie.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
News & Perspectives from Ob.Gyn. News
NEWS FROM THE FDA/CDC
New USPSTF draft suggests mammography start at 40, not 50
The US Preventive Services Task Force (USPSTF) on May 9 released a draft recommendation statement and evidence review that provides critical updates to its breast cancer screening recommendations.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
FDA approves OTC naloxone, but will cost be a barrier?
The US Food and Drug Administration has approved over-the-counter sales of the overdose reversal agent Narcan (naloxone, Emergent BioSolutions). Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on US shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022—to $373.7 million—blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
CONFERENCE COVERAGE
Should you prescribe bioidentical hormones for menopause?
The off-label prescribing of compounded, bioidentical hormone therapy—in pills, creams, or pellets—for symptoms of perimenopause or menopause can put physicians at legal risk because the products lack scientific backing, according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
Once-daily nifedipine sufficient for hypertension in pregnancy
A single 60-mg daily dose of nifedipine appeared similarly effective as taking a 30-mg dose twice daily for treating hypertensive disorders in pregnancy, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose—the most common adjustment—and 20.7% needed both an increase in nifedipine and an additional medication. ●
NEWS FROM THE FDA/CDC
New USPSTF draft suggests mammography start at 40, not 50
The US Preventive Services Task Force (USPSTF) on May 9 released a draft recommendation statement and evidence review that provides critical updates to its breast cancer screening recommendations.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
FDA approves OTC naloxone, but will cost be a barrier?
The US Food and Drug Administration has approved over-the-counter sales of the overdose reversal agent Narcan (naloxone, Emergent BioSolutions). Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on US shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022—to $373.7 million—blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
CONFERENCE COVERAGE
Should you prescribe bioidentical hormones for menopause?
The off-label prescribing of compounded, bioidentical hormone therapy—in pills, creams, or pellets—for symptoms of perimenopause or menopause can put physicians at legal risk because the products lack scientific backing, according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
Once-daily nifedipine sufficient for hypertension in pregnancy
A single 60-mg daily dose of nifedipine appeared similarly effective as taking a 30-mg dose twice daily for treating hypertensive disorders in pregnancy, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose—the most common adjustment—and 20.7% needed both an increase in nifedipine and an additional medication. ●
NEWS FROM THE FDA/CDC
New USPSTF draft suggests mammography start at 40, not 50
The US Preventive Services Task Force (USPSTF) on May 9 released a draft recommendation statement and evidence review that provides critical updates to its breast cancer screening recommendations.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
FDA approves OTC naloxone, but will cost be a barrier?
The US Food and Drug Administration has approved over-the-counter sales of the overdose reversal agent Narcan (naloxone, Emergent BioSolutions). Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on US shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022—to $373.7 million—blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
CONFERENCE COVERAGE
Should you prescribe bioidentical hormones for menopause?
The off-label prescribing of compounded, bioidentical hormone therapy—in pills, creams, or pellets—for symptoms of perimenopause or menopause can put physicians at legal risk because the products lack scientific backing, according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
Once-daily nifedipine sufficient for hypertension in pregnancy
A single 60-mg daily dose of nifedipine appeared similarly effective as taking a 30-mg dose twice daily for treating hypertensive disorders in pregnancy, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose—the most common adjustment—and 20.7% needed both an increase in nifedipine and an additional medication. ●
10 ways in which ObGyn care can be more environmentally sustainable
Climate change has been called the biggest health threat of the 21st century.1 The health care sector is a huge contributor to global carbon emissions, accounting for almost double the emissions of global aviation. While other industries and countries are implementing mitigation measures to decrease their emissions, health care is currently on track to double its carbon emissions by 2050, even though it should be carbon neutral by that time to comply with the Paris Climate Agreement.2 There have been some national efforts to curb health care emissions, including the creation of the Office of Climate Change and Health Equity in 2021 and the passage of the Inflation Reduction Act in 2022.3 These are top-down, administrative approaches, and to be successful we will also need clinicians to understand and address this problem.
The negative impacts of heat, air pollution, and exposure to toxic substances on human health have been well documented in multiple regions across multiple specialties.4-7 The United States makes up 27% of the global health care carbon footprint—more emissions than the entire United Kingdom as a country—despite having only 4% of the world’s population.2 Culture and incentives for an overabundance of single-use supplies, not evidence for patient safety, have led to this uniquely American problem. It is evident that our health care industry is an excellent place to implement mitigation measures for carbon emissions that contribute to climate change and can improve health outcomes.
In this article, we recommend 10 practices that can decrease our carbon footprint in ObGyn. We focus on the classic motto of “Reduce, Reuse, Recycle,” while adding “Remove” and “Reimagine” to classify the ways in which we can reduce emissions while not compromising our care to patients.
Reduce
1. Minimize opened materials and single-use devices in the OR and labor and delivery
Health care is a unique setting where a culture of infection prevention and efficiency has led low-cost, single-use supplies to dominate over reusable items. While single-use items can have inexpensive purchasing costs compared to reusable items, the environmental costs required for the production and disposal of the former are often much greater. In operating rooms (ORs) and labor and delivery (LD) units, single-use items are omnipresent. Over the past decade, researchers and clinicians have started to take a closer look at these items and their carbon footprint. One group evaluated hysterectomy through a waste audit and found that the vast majority of waste from all of the cases was Spunbond Meltblown Spunbond, or SMS; plastic materialthat comprises gowns; blue wraps; and drapes; followed by hard plastic material that comprises trays and packaging.8 Moreover, production and manufacturing processes contributed to 95% of the environmental impacts of these items.8
In an effort to be time efficient, OR staff will open sterile surgical packs and individual peel-pack items prior to surgery to minimize having to find items during surgery. However, this creates an inordinate amount of waste. One group of neurosurgeons who evaluated their opened but unused supplies found that 85% of their unused items were individually opened items, leading to a waste of $2.9 million per year.9 Minor procedures like dilation and curettage, cystoscopy, and hysteroscopy do not need such a large sterile field, as these procedures are also safe to perform in the office. Hand surgeons have been quick to lead in this space, particularly with minor procedures such as carpal tunnel release. One division was able to eliminate 2.8 tons of waste and save $13,000 in a 2-year period by reducing the sterile field.10 ObGyns can work with OR and LD staff to create custom packs that minimize unused or underutilized items, helping to reduce both the carbon footprint and health care spending.
Bottom line: ObGyns can help foster a culture of having supplies available but not opened until needed during a case.
Continue to: 2. Decrease regulated medical waste...
2. Decrease regulated medical waste
Health care is unique from other fields in that there are multiple waste streams to consider. Infectious waste and items saturated in blood or capable of causing infection must be placed into regulated medical waste (RMW), or more commonly, red biohazard bags. RMW is autoclaved or incinerated prior to disposal in a landfill. This process is more financially and environmentally costly than general municipal waste (GMW). This process also requires more transport—1 study revealed that GMW traveled 20 km to a landfill for disposal, compared with the 50 km that RMW traveled for sterilized-prior-to-landfill disposal.11
Unfortunately, the vast majority of items placed in RMW are incorrectly triaged and should instead be disposed in GMW.12,13 One study performed in an emergency department revealed that 85% of waste was incorrectly placed in the RMW.12
Bottom line: ObGyns can avoid placing items in RMW that may not qualify and advocate for institution policy changes to remove RMW from places such as waiting rooms, at the patient bedside, or next to scrub sinks.
3. Reduce energy use
ORs and LD units use a lot of energy, and numerous studies have demonstrated that the heating, ventilation, and air conditioning (HVAC) system plays a large role in emissions.8,11 This can easily be fixed by “HVAC setbacks” and powering down rooms when not in use. One institution powered down ORs when not in use and reduced 234 metric tons of CO2 emissions and saved $33,000 per year.14 Transitioning to light-emitting diode (LED) lights reduced energy usage at 1 institution by almost 50%.15 Finally, computers in clinical offices, examination rooms, and administrative offices can be powered down at the end of the day. One study found that in 1 radiology department, 29 computers left on overnight and on weekends emitted 17.7 tons of CO2 emissions in 1 year.16
Bottom line: We as ObGyns can advocate for how energy can be saved outside of surgical cases, including powering down ORs and LD units, transitioning to LED lighting, and powering down workstations.
Reuse
4. Choose reusable equipment
In ObGyn practice, the most commonly used tool is the speculum. Given its omnipresence, the speculum is a great place to start to decrease our carbon footprint. Two studies have evaluated the environmental impact of reusable versus single-use disposable specula, and both demonstrated that the stainless-steel versions have less global warming potential than the acrylic varieties.17,18 Donahue and colleagues17 demonstrated that it only took 2 to 3 pelvic examinations for the cost of stainless-steel specula to break even, even when sterilized in a half-filled autoclave tray. Rodriquez, et al18 revealed that, compared with an acrylic model, the stainless-steel specula had fewer negative impacts in terms of global warming, acidification, respiratory effects, smog, and fossil fuel depletion.18
Bottom line: Strongly consider using stainless-steel specula to reduce costs and carbon emissions.
In addition to specula, ObGyns can choose reusable equipment in the OR. For example, surgeons can use stainless-steel trocars instead of disposable trocars.19 In vaginal cases, Breisky-Navratil retractors can be used instead of disposable self-retaining retractors. Plastic basins that often are included in sterile supply packs can be replaced with stainless-steel basins, which could have profound positive effects on the carbon footprint of gynecologic surgery.8 One study of ObGyns demonstrated that 95% of physicians supported waste-reduction efforts, and 66% supported utilizing reusable surgical tools instead of disposable tools.20
Bottom line: As surgeons, ObGyns have influence over what they want to use in the OR, and they can petition for reusable options over disposable options.
5. Launder the sterile blue towels
Sterile blue towels, which are made of cotton, have the largest environmental footprint compared with other disposable materials, such as plastics, and contribute greatly to toxicity in human health.8,11 Although these towels cannot be laundered and sterilized again for use in a sterile surgical field, they can be laundered and repurposed, including by environmental services to clean hospital rooms. Blue towels should be able to be laundered no matter how saturated in body fluids they are.
Bottom line: ObGyns should strive to always launder the blue towels and educate trainees and other staff in the OR to do the same.
Recycle
6. Recycle and reprocess materials and devices
While recycling is immensely important, it requires a large amount of energy to break down a material to its raw components for manufacturing. It likely reduces our carbon footprint from OR procedures by only 5%.8 However, recycling is still a good way to divert appropriate materials from landfill, saving costs and emissions at the end of a material’s life. One example is sterile blue wrap, which is a petroleum product with a recycling number of 6 and a filtration rating of N99. Blue wrap can be recycled into plastic pellets, or it can be recreated into other hospital supplies, such as gowns.
Bottom line: ObGyns can petition their hospitals to work with suppliers and waste-processing companies who have recycling programs built into their supply chains.
By contrast, reprocessing can have a much larger impact on carbon emissions. Complex items, such as advanced energy devices that can be reprocessed, result in a greater reduction in carbon emissions due to the reuse of their complex materials and manufacturing when compared with such devices that cannot be reprocessed. Recycling and reprocessing programs are already in place for several devices (TABLE). Authors of a systematic review showed that there is no evidence to support the use of single-use supplies and instruments over reprocessed items when considering instrument function, ease of use, patient safety, transmission of infection, or long-term patient outcomes.21
Bottom line: ObGyns can choose to use reprocessed items in ORs instead of single-use devices and educate staff on the safety of these items.
Continue to: Remove...
Remove
7. Remove desflurane and other volatile gases from formularies
Volatile anesthetic gases, such as desflurane, isoflurane, and nitrous oxide, are themselves potent greenhouse gases, comprising a large portion of the carbon emissions that come from the OR.22 Desflurane was developed to have a rapid onset for induction and quick recovery; however, studies have shown no clinical benefit over other gases.23 Furthermore, the costs and greenhouse gas potential are substantial. Desflurane costs 2 to 3 times more and has more than 20 times the global warming potential of the other volatile gases (FIGURE).8 Using 1 hour of desflurane is equivalent to driving 378 miles in a gas-powered vehicle, while the use of isoflurane and sevoflurane create equivalents of only 15 and 8 miles, respectively.23
Nitrous oxide is another powerful greenhouse gas that is a direct ozone depletor and can stay in the atmosphere for 114 years.22 Nitrous oxide has limited clinical use in hospitals, but it is often stored in central hospital piping. Most of the impact of nitrous oxide comes through leaks in a poor system design rather than patient delivery. One estimate reveals that more than 13 million liters of nitrous oxide are lost annually from leaks in European hospitals.22 The American Society of Anesthesiologists recommends decommissioning central piping of nitrous oxide in favor of cylinders at the point of care.24
Literature on enhanced recovery after surgery in gynecology promotes the use of propofol over volatile gases for our patients because of the high rate of postoperative nausea and vomiting seen with gases.25 Volatile gases should be a last-choice anesthetic for our patients.
Bottom line: It is critical that ObGyns work with colleagues in anesthesia to develop climate- and patient-friendly protocols for procedures.
8. Remove endocrine-disrupting chemicals from clinical supplies
Endocrine-disrupting chemicals (EDCs) are a type of chemical that alter the hormonal systems of humans, which can result in adverse health effects. Multiple studies and reviews have tied EDCs to reproductive abnormalities, such as the effects of bisphenol A (BPA) on estradiol levels, antral follicle counts, oocyte quality, and implantation rates; phthalates on fibroid burden; triclosan on embryo quality; parabens on live birth rates; and perfluoroalkylsubstances (PFAS or “forever substances”) on hypertensive disorders of pregnancy.5,26,27
What might be most shocking is that these EDCs are incorporated into medical supplies and pharmaceuticals. For example, BPA is known to line dialysis and ointment tubes, parabens are used for their antimicrobial properties in ultrasound gel and hep-locks, and phthalates are found in up to 40% of medical-use plastics and controlled-release medications. Authors of an observational study found that 74% of patients admitted to an LD unit were exposed to EDCs. In a neonatal intensive care unit (NICU), most of the supplies contained an EDC, and urinary BPA levels were elevated in neonates admitted to a NICU, raising concerns about long-term health risks.5
Bottom line: Physicians and health care institutions have an obligation to petition industry partners and suppliers to remove EDCs from their supply chains.
Reimagine
9. Educate
The field of health care sustainability remains in its infancy, but from 2007 to 2019, publications on climate change and health in academia increased by a factor of 8.29 Additionally, through waste audits, quality-improvement projects, and life cycle analyses (analytical tools to evaluate product or process emissions from materials extraction to disposal), we have gained insight into the scope of the problem, with evidence showing that our practices are largely derived from culture. It is time to provide formal education on health care sustainability to medical trainees, staff, and clinicians alike, who desire to see this topic reflected in their formal curricula.30 Start talking about it!
Bottom line: Commentaries, webinars, formal didactics sessions, in-services, and hospital workgroups to introduce this topic are a good way to teach others about the carbon footprint of our care and solutions to minimize it.
10. Engage in advocacy
Physicians have an ethical duty to advocate for change at the local, regional, and national levels if we want to see a better future for our patients, their children, and even ourselves. We should reimagine this work as an important public health initiative.31 Surveys of physicians, including ObGyns, reveal a concern about the sustainability of health care and a commitment to addressing this issue.20 ObGyns are on the frontlines of delivering care every day, so we are poised to implement changes that can impact our patients, especially when we can lead and petition hospital or local committees.20,28,32 There is much to be done, but every voice counts and can make impactful changes at every level. ●
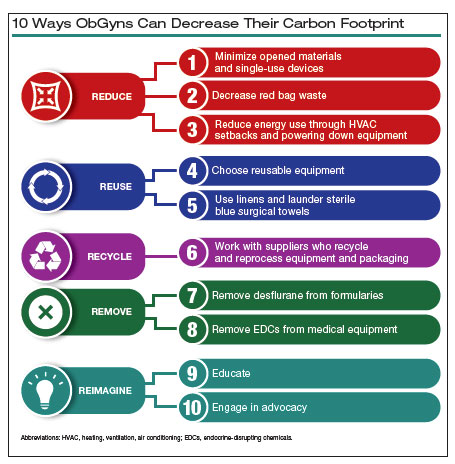
- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733.
- Health care climate footprint report. Health Care Without Harm website. https://www.noharm.org/ClimateFootprintReport. Accessed May 12, 2023.
- Balbus JM, McCannon CJ, Mataka A, et al. After COP26—putting health and equity at the center of the climate movement. N Engl J Med. 2022;386:1295-1297.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3:e208243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822-e3827.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:30263035.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786.
- Zygourakis CC, Yoon S, Valencia V, et al. Operating room waste: disposable supply utilization in neurosurgical procedures. J Neurosurg. 2017;126:620-625.
- van Demark RE, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg. 2018;43:179-181.
- Campion N, Thiel CL, DeBlois J, et al. Life cycle assessment perspectives on delivering an infant in the US. Sci Total Environ. 2012;425:191198.
- Hsu S, Thiel CL, Mello MJ, Slutzman JE. Dumpster diving in the emergency department. West J Emerg Med. 2020;21:1211-1217.
- Mcgain F, Story D, Hendel S. An audit of intensive care unit recyclable waste. Anaesthesia. 2009;64:1299-1302.
- Wormer BA, Augenstein VA, Carpenter CL, et al. The green operating room: simple changes to reduce cost and our carbon footprint. Am Surg. 2013;79:666-671.
- Kagoma Y, Stall N, Rubinstein E, et al. People, planet and profits: the case for greening operating rooms. Can Med Assoc J. 2012;184:19051911.
- McCarthy CJ, Gerstenmaier JF, O’ Neill AC, et al. “EcoRadiology”— pulling the plug on wasted energy in the radiology department. Acad Radiol. 2014;21:1563-1566.
- Donahue LM, Hilton S, Bell SG, et al. A comparative carbon footprint analysis of disposable and reusable vaginal specula. Am J Obstet Gynecol. 2020;223:225.e1-225.e7.
- Rodriguez Morris MI, Hicks A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ Res Commun. 2022;4:025002.
- MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381-e388.
- Thiel C, Duncan P, Woods N. Attitude of US obstetricians and gynaecologists to global warming and medical waste. J Health Serv Res Policy. 2017;22:162-167.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98.
- Meyer MJ. Desflurane should des-appear: global and financial rationale. Anesth Analg. 2020;131:1317-1322.
- Rollins MD, Arendt KW, Carvalho B, et al. ASA Committee on Obstetric Anesthesia Working Group. Nitrous oxide. American Society of Anesthesiologists website. Accessed May 12, 2023. https://www .asahq.org/about-asa/governance-and-committees/asa-committees /committee-on-obstetric-anesthesia/nitrous-oxide.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121.
- Bommartio PA, Ferguson KK, Meeker JD, et al. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ Health Perspect. 2021;129:107004.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129-170.
- Ryan EC, Dubrow R, Sherman JD. Medical, nursing, and physician assistant student knowledge and attitudes toward climate change, pollution, and resource conservation in health care. BMC Med Educ. 2020;20:200.
- Giudice LC, Llamas-Clark EF, DeNicola Net al; FIGO Committee on Climate Change and Toxic Environmental Exposures. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155:345-356.
- Yates EF, Bowder AN, Roa L, et al. Empowering surgeons, anesthesiologists, and obstetricians to incorporate environmental sustainability in the operating room. Ann Surg. 2021;273:1108-1114.
Climate change has been called the biggest health threat of the 21st century.1 The health care sector is a huge contributor to global carbon emissions, accounting for almost double the emissions of global aviation. While other industries and countries are implementing mitigation measures to decrease their emissions, health care is currently on track to double its carbon emissions by 2050, even though it should be carbon neutral by that time to comply with the Paris Climate Agreement.2 There have been some national efforts to curb health care emissions, including the creation of the Office of Climate Change and Health Equity in 2021 and the passage of the Inflation Reduction Act in 2022.3 These are top-down, administrative approaches, and to be successful we will also need clinicians to understand and address this problem.
The negative impacts of heat, air pollution, and exposure to toxic substances on human health have been well documented in multiple regions across multiple specialties.4-7 The United States makes up 27% of the global health care carbon footprint—more emissions than the entire United Kingdom as a country—despite having only 4% of the world’s population.2 Culture and incentives for an overabundance of single-use supplies, not evidence for patient safety, have led to this uniquely American problem. It is evident that our health care industry is an excellent place to implement mitigation measures for carbon emissions that contribute to climate change and can improve health outcomes.
In this article, we recommend 10 practices that can decrease our carbon footprint in ObGyn. We focus on the classic motto of “Reduce, Reuse, Recycle,” while adding “Remove” and “Reimagine” to classify the ways in which we can reduce emissions while not compromising our care to patients.
Reduce
1. Minimize opened materials and single-use devices in the OR and labor and delivery
Health care is a unique setting where a culture of infection prevention and efficiency has led low-cost, single-use supplies to dominate over reusable items. While single-use items can have inexpensive purchasing costs compared to reusable items, the environmental costs required for the production and disposal of the former are often much greater. In operating rooms (ORs) and labor and delivery (LD) units, single-use items are omnipresent. Over the past decade, researchers and clinicians have started to take a closer look at these items and their carbon footprint. One group evaluated hysterectomy through a waste audit and found that the vast majority of waste from all of the cases was Spunbond Meltblown Spunbond, or SMS; plastic materialthat comprises gowns; blue wraps; and drapes; followed by hard plastic material that comprises trays and packaging.8 Moreover, production and manufacturing processes contributed to 95% of the environmental impacts of these items.8
In an effort to be time efficient, OR staff will open sterile surgical packs and individual peel-pack items prior to surgery to minimize having to find items during surgery. However, this creates an inordinate amount of waste. One group of neurosurgeons who evaluated their opened but unused supplies found that 85% of their unused items were individually opened items, leading to a waste of $2.9 million per year.9 Minor procedures like dilation and curettage, cystoscopy, and hysteroscopy do not need such a large sterile field, as these procedures are also safe to perform in the office. Hand surgeons have been quick to lead in this space, particularly with minor procedures such as carpal tunnel release. One division was able to eliminate 2.8 tons of waste and save $13,000 in a 2-year period by reducing the sterile field.10 ObGyns can work with OR and LD staff to create custom packs that minimize unused or underutilized items, helping to reduce both the carbon footprint and health care spending.
Bottom line: ObGyns can help foster a culture of having supplies available but not opened until needed during a case.
Continue to: 2. Decrease regulated medical waste...
2. Decrease regulated medical waste
Health care is unique from other fields in that there are multiple waste streams to consider. Infectious waste and items saturated in blood or capable of causing infection must be placed into regulated medical waste (RMW), or more commonly, red biohazard bags. RMW is autoclaved or incinerated prior to disposal in a landfill. This process is more financially and environmentally costly than general municipal waste (GMW). This process also requires more transport—1 study revealed that GMW traveled 20 km to a landfill for disposal, compared with the 50 km that RMW traveled for sterilized-prior-to-landfill disposal.11
Unfortunately, the vast majority of items placed in RMW are incorrectly triaged and should instead be disposed in GMW.12,13 One study performed in an emergency department revealed that 85% of waste was incorrectly placed in the RMW.12
Bottom line: ObGyns can avoid placing items in RMW that may not qualify and advocate for institution policy changes to remove RMW from places such as waiting rooms, at the patient bedside, or next to scrub sinks.
3. Reduce energy use
ORs and LD units use a lot of energy, and numerous studies have demonstrated that the heating, ventilation, and air conditioning (HVAC) system plays a large role in emissions.8,11 This can easily be fixed by “HVAC setbacks” and powering down rooms when not in use. One institution powered down ORs when not in use and reduced 234 metric tons of CO2 emissions and saved $33,000 per year.14 Transitioning to light-emitting diode (LED) lights reduced energy usage at 1 institution by almost 50%.15 Finally, computers in clinical offices, examination rooms, and administrative offices can be powered down at the end of the day. One study found that in 1 radiology department, 29 computers left on overnight and on weekends emitted 17.7 tons of CO2 emissions in 1 year.16
Bottom line: We as ObGyns can advocate for how energy can be saved outside of surgical cases, including powering down ORs and LD units, transitioning to LED lighting, and powering down workstations.
Reuse
4. Choose reusable equipment
In ObGyn practice, the most commonly used tool is the speculum. Given its omnipresence, the speculum is a great place to start to decrease our carbon footprint. Two studies have evaluated the environmental impact of reusable versus single-use disposable specula, and both demonstrated that the stainless-steel versions have less global warming potential than the acrylic varieties.17,18 Donahue and colleagues17 demonstrated that it only took 2 to 3 pelvic examinations for the cost of stainless-steel specula to break even, even when sterilized in a half-filled autoclave tray. Rodriquez, et al18 revealed that, compared with an acrylic model, the stainless-steel specula had fewer negative impacts in terms of global warming, acidification, respiratory effects, smog, and fossil fuel depletion.18
Bottom line: Strongly consider using stainless-steel specula to reduce costs and carbon emissions.
In addition to specula, ObGyns can choose reusable equipment in the OR. For example, surgeons can use stainless-steel trocars instead of disposable trocars.19 In vaginal cases, Breisky-Navratil retractors can be used instead of disposable self-retaining retractors. Plastic basins that often are included in sterile supply packs can be replaced with stainless-steel basins, which could have profound positive effects on the carbon footprint of gynecologic surgery.8 One study of ObGyns demonstrated that 95% of physicians supported waste-reduction efforts, and 66% supported utilizing reusable surgical tools instead of disposable tools.20
Bottom line: As surgeons, ObGyns have influence over what they want to use in the OR, and they can petition for reusable options over disposable options.
5. Launder the sterile blue towels
Sterile blue towels, which are made of cotton, have the largest environmental footprint compared with other disposable materials, such as plastics, and contribute greatly to toxicity in human health.8,11 Although these towels cannot be laundered and sterilized again for use in a sterile surgical field, they can be laundered and repurposed, including by environmental services to clean hospital rooms. Blue towels should be able to be laundered no matter how saturated in body fluids they are.
Bottom line: ObGyns should strive to always launder the blue towels and educate trainees and other staff in the OR to do the same.
Recycle
6. Recycle and reprocess materials and devices
While recycling is immensely important, it requires a large amount of energy to break down a material to its raw components for manufacturing. It likely reduces our carbon footprint from OR procedures by only 5%.8 However, recycling is still a good way to divert appropriate materials from landfill, saving costs and emissions at the end of a material’s life. One example is sterile blue wrap, which is a petroleum product with a recycling number of 6 and a filtration rating of N99. Blue wrap can be recycled into plastic pellets, or it can be recreated into other hospital supplies, such as gowns.
Bottom line: ObGyns can petition their hospitals to work with suppliers and waste-processing companies who have recycling programs built into their supply chains.
By contrast, reprocessing can have a much larger impact on carbon emissions. Complex items, such as advanced energy devices that can be reprocessed, result in a greater reduction in carbon emissions due to the reuse of their complex materials and manufacturing when compared with such devices that cannot be reprocessed. Recycling and reprocessing programs are already in place for several devices (TABLE). Authors of a systematic review showed that there is no evidence to support the use of single-use supplies and instruments over reprocessed items when considering instrument function, ease of use, patient safety, transmission of infection, or long-term patient outcomes.21

Bottom line: ObGyns can choose to use reprocessed items in ORs instead of single-use devices and educate staff on the safety of these items.
Continue to: Remove...
Remove
7. Remove desflurane and other volatile gases from formularies
Volatile anesthetic gases, such as desflurane, isoflurane, and nitrous oxide, are themselves potent greenhouse gases, comprising a large portion of the carbon emissions that come from the OR.22 Desflurane was developed to have a rapid onset for induction and quick recovery; however, studies have shown no clinical benefit over other gases.23 Furthermore, the costs and greenhouse gas potential are substantial. Desflurane costs 2 to 3 times more and has more than 20 times the global warming potential of the other volatile gases (FIGURE).8 Using 1 hour of desflurane is equivalent to driving 378 miles in a gas-powered vehicle, while the use of isoflurane and sevoflurane create equivalents of only 15 and 8 miles, respectively.23

Nitrous oxide is another powerful greenhouse gas that is a direct ozone depletor and can stay in the atmosphere for 114 years.22 Nitrous oxide has limited clinical use in hospitals, but it is often stored in central hospital piping. Most of the impact of nitrous oxide comes through leaks in a poor system design rather than patient delivery. One estimate reveals that more than 13 million liters of nitrous oxide are lost annually from leaks in European hospitals.22 The American Society of Anesthesiologists recommends decommissioning central piping of nitrous oxide in favor of cylinders at the point of care.24
Literature on enhanced recovery after surgery in gynecology promotes the use of propofol over volatile gases for our patients because of the high rate of postoperative nausea and vomiting seen with gases.25 Volatile gases should be a last-choice anesthetic for our patients.
Bottom line: It is critical that ObGyns work with colleagues in anesthesia to develop climate- and patient-friendly protocols for procedures.
8. Remove endocrine-disrupting chemicals from clinical supplies
Endocrine-disrupting chemicals (EDCs) are a type of chemical that alter the hormonal systems of humans, which can result in adverse health effects. Multiple studies and reviews have tied EDCs to reproductive abnormalities, such as the effects of bisphenol A (BPA) on estradiol levels, antral follicle counts, oocyte quality, and implantation rates; phthalates on fibroid burden; triclosan on embryo quality; parabens on live birth rates; and perfluoroalkylsubstances (PFAS or “forever substances”) on hypertensive disorders of pregnancy.5,26,27
What might be most shocking is that these EDCs are incorporated into medical supplies and pharmaceuticals. For example, BPA is known to line dialysis and ointment tubes, parabens are used for their antimicrobial properties in ultrasound gel and hep-locks, and phthalates are found in up to 40% of medical-use plastics and controlled-release medications. Authors of an observational study found that 74% of patients admitted to an LD unit were exposed to EDCs. In a neonatal intensive care unit (NICU), most of the supplies contained an EDC, and urinary BPA levels were elevated in neonates admitted to a NICU, raising concerns about long-term health risks.5
Bottom line: Physicians and health care institutions have an obligation to petition industry partners and suppliers to remove EDCs from their supply chains.
Reimagine
9. Educate
The field of health care sustainability remains in its infancy, but from 2007 to 2019, publications on climate change and health in academia increased by a factor of 8.29 Additionally, through waste audits, quality-improvement projects, and life cycle analyses (analytical tools to evaluate product or process emissions from materials extraction to disposal), we have gained insight into the scope of the problem, with evidence showing that our practices are largely derived from culture. It is time to provide formal education on health care sustainability to medical trainees, staff, and clinicians alike, who desire to see this topic reflected in their formal curricula.30 Start talking about it!
Bottom line: Commentaries, webinars, formal didactics sessions, in-services, and hospital workgroups to introduce this topic are a good way to teach others about the carbon footprint of our care and solutions to minimize it.
10. Engage in advocacy
Physicians have an ethical duty to advocate for change at the local, regional, and national levels if we want to see a better future for our patients, their children, and even ourselves. We should reimagine this work as an important public health initiative.31 Surveys of physicians, including ObGyns, reveal a concern about the sustainability of health care and a commitment to addressing this issue.20 ObGyns are on the frontlines of delivering care every day, so we are poised to implement changes that can impact our patients, especially when we can lead and petition hospital or local committees.20,28,32 There is much to be done, but every voice counts and can make impactful changes at every level. ●

Climate change has been called the biggest health threat of the 21st century.1 The health care sector is a huge contributor to global carbon emissions, accounting for almost double the emissions of global aviation. While other industries and countries are implementing mitigation measures to decrease their emissions, health care is currently on track to double its carbon emissions by 2050, even though it should be carbon neutral by that time to comply with the Paris Climate Agreement.2 There have been some national efforts to curb health care emissions, including the creation of the Office of Climate Change and Health Equity in 2021 and the passage of the Inflation Reduction Act in 2022.3 These are top-down, administrative approaches, and to be successful we will also need clinicians to understand and address this problem.
The negative impacts of heat, air pollution, and exposure to toxic substances on human health have been well documented in multiple regions across multiple specialties.4-7 The United States makes up 27% of the global health care carbon footprint—more emissions than the entire United Kingdom as a country—despite having only 4% of the world’s population.2 Culture and incentives for an overabundance of single-use supplies, not evidence for patient safety, have led to this uniquely American problem. It is evident that our health care industry is an excellent place to implement mitigation measures for carbon emissions that contribute to climate change and can improve health outcomes.
In this article, we recommend 10 practices that can decrease our carbon footprint in ObGyn. We focus on the classic motto of “Reduce, Reuse, Recycle,” while adding “Remove” and “Reimagine” to classify the ways in which we can reduce emissions while not compromising our care to patients.
Reduce
1. Minimize opened materials and single-use devices in the OR and labor and delivery
Health care is a unique setting where a culture of infection prevention and efficiency has led low-cost, single-use supplies to dominate over reusable items. While single-use items can have inexpensive purchasing costs compared to reusable items, the environmental costs required for the production and disposal of the former are often much greater. In operating rooms (ORs) and labor and delivery (LD) units, single-use items are omnipresent. Over the past decade, researchers and clinicians have started to take a closer look at these items and their carbon footprint. One group evaluated hysterectomy through a waste audit and found that the vast majority of waste from all of the cases was Spunbond Meltblown Spunbond, or SMS; plastic materialthat comprises gowns; blue wraps; and drapes; followed by hard plastic material that comprises trays and packaging.8 Moreover, production and manufacturing processes contributed to 95% of the environmental impacts of these items.8
In an effort to be time efficient, OR staff will open sterile surgical packs and individual peel-pack items prior to surgery to minimize having to find items during surgery. However, this creates an inordinate amount of waste. One group of neurosurgeons who evaluated their opened but unused supplies found that 85% of their unused items were individually opened items, leading to a waste of $2.9 million per year.9 Minor procedures like dilation and curettage, cystoscopy, and hysteroscopy do not need such a large sterile field, as these procedures are also safe to perform in the office. Hand surgeons have been quick to lead in this space, particularly with minor procedures such as carpal tunnel release. One division was able to eliminate 2.8 tons of waste and save $13,000 in a 2-year period by reducing the sterile field.10 ObGyns can work with OR and LD staff to create custom packs that minimize unused or underutilized items, helping to reduce both the carbon footprint and health care spending.
Bottom line: ObGyns can help foster a culture of having supplies available but not opened until needed during a case.
Continue to: 2. Decrease regulated medical waste...
2. Decrease regulated medical waste
Health care is unique from other fields in that there are multiple waste streams to consider. Infectious waste and items saturated in blood or capable of causing infection must be placed into regulated medical waste (RMW), or more commonly, red biohazard bags. RMW is autoclaved or incinerated prior to disposal in a landfill. This process is more financially and environmentally costly than general municipal waste (GMW). This process also requires more transport—1 study revealed that GMW traveled 20 km to a landfill for disposal, compared with the 50 km that RMW traveled for sterilized-prior-to-landfill disposal.11
Unfortunately, the vast majority of items placed in RMW are incorrectly triaged and should instead be disposed in GMW.12,13 One study performed in an emergency department revealed that 85% of waste was incorrectly placed in the RMW.12
Bottom line: ObGyns can avoid placing items in RMW that may not qualify and advocate for institution policy changes to remove RMW from places such as waiting rooms, at the patient bedside, or next to scrub sinks.
3. Reduce energy use
ORs and LD units use a lot of energy, and numerous studies have demonstrated that the heating, ventilation, and air conditioning (HVAC) system plays a large role in emissions.8,11 This can easily be fixed by “HVAC setbacks” and powering down rooms when not in use. One institution powered down ORs when not in use and reduced 234 metric tons of CO2 emissions and saved $33,000 per year.14 Transitioning to light-emitting diode (LED) lights reduced energy usage at 1 institution by almost 50%.15 Finally, computers in clinical offices, examination rooms, and administrative offices can be powered down at the end of the day. One study found that in 1 radiology department, 29 computers left on overnight and on weekends emitted 17.7 tons of CO2 emissions in 1 year.16
Bottom line: We as ObGyns can advocate for how energy can be saved outside of surgical cases, including powering down ORs and LD units, transitioning to LED lighting, and powering down workstations.
Reuse
4. Choose reusable equipment
In ObGyn practice, the most commonly used tool is the speculum. Given its omnipresence, the speculum is a great place to start to decrease our carbon footprint. Two studies have evaluated the environmental impact of reusable versus single-use disposable specula, and both demonstrated that the stainless-steel versions have less global warming potential than the acrylic varieties.17,18 Donahue and colleagues17 demonstrated that it only took 2 to 3 pelvic examinations for the cost of stainless-steel specula to break even, even when sterilized in a half-filled autoclave tray. Rodriquez, et al18 revealed that, compared with an acrylic model, the stainless-steel specula had fewer negative impacts in terms of global warming, acidification, respiratory effects, smog, and fossil fuel depletion.18
Bottom line: Strongly consider using stainless-steel specula to reduce costs and carbon emissions.
In addition to specula, ObGyns can choose reusable equipment in the OR. For example, surgeons can use stainless-steel trocars instead of disposable trocars.19 In vaginal cases, Breisky-Navratil retractors can be used instead of disposable self-retaining retractors. Plastic basins that often are included in sterile supply packs can be replaced with stainless-steel basins, which could have profound positive effects on the carbon footprint of gynecologic surgery.8 One study of ObGyns demonstrated that 95% of physicians supported waste-reduction efforts, and 66% supported utilizing reusable surgical tools instead of disposable tools.20
Bottom line: As surgeons, ObGyns have influence over what they want to use in the OR, and they can petition for reusable options over disposable options.
5. Launder the sterile blue towels
Sterile blue towels, which are made of cotton, have the largest environmental footprint compared with other disposable materials, such as plastics, and contribute greatly to toxicity in human health.8,11 Although these towels cannot be laundered and sterilized again for use in a sterile surgical field, they can be laundered and repurposed, including by environmental services to clean hospital rooms. Blue towels should be able to be laundered no matter how saturated in body fluids they are.
Bottom line: ObGyns should strive to always launder the blue towels and educate trainees and other staff in the OR to do the same.
Recycle
6. Recycle and reprocess materials and devices
While recycling is immensely important, it requires a large amount of energy to break down a material to its raw components for manufacturing. It likely reduces our carbon footprint from OR procedures by only 5%.8 However, recycling is still a good way to divert appropriate materials from landfill, saving costs and emissions at the end of a material’s life. One example is sterile blue wrap, which is a petroleum product with a recycling number of 6 and a filtration rating of N99. Blue wrap can be recycled into plastic pellets, or it can be recreated into other hospital supplies, such as gowns.
Bottom line: ObGyns can petition their hospitals to work with suppliers and waste-processing companies who have recycling programs built into their supply chains.
By contrast, reprocessing can have a much larger impact on carbon emissions. Complex items, such as advanced energy devices that can be reprocessed, result in a greater reduction in carbon emissions due to the reuse of their complex materials and manufacturing when compared with such devices that cannot be reprocessed. Recycling and reprocessing programs are already in place for several devices (TABLE). Authors of a systematic review showed that there is no evidence to support the use of single-use supplies and instruments over reprocessed items when considering instrument function, ease of use, patient safety, transmission of infection, or long-term patient outcomes.21

Bottom line: ObGyns can choose to use reprocessed items in ORs instead of single-use devices and educate staff on the safety of these items.
Continue to: Remove...
Remove
7. Remove desflurane and other volatile gases from formularies
Volatile anesthetic gases, such as desflurane, isoflurane, and nitrous oxide, are themselves potent greenhouse gases, comprising a large portion of the carbon emissions that come from the OR.22 Desflurane was developed to have a rapid onset for induction and quick recovery; however, studies have shown no clinical benefit over other gases.23 Furthermore, the costs and greenhouse gas potential are substantial. Desflurane costs 2 to 3 times more and has more than 20 times the global warming potential of the other volatile gases (FIGURE).8 Using 1 hour of desflurane is equivalent to driving 378 miles in a gas-powered vehicle, while the use of isoflurane and sevoflurane create equivalents of only 15 and 8 miles, respectively.23

Nitrous oxide is another powerful greenhouse gas that is a direct ozone depletor and can stay in the atmosphere for 114 years.22 Nitrous oxide has limited clinical use in hospitals, but it is often stored in central hospital piping. Most of the impact of nitrous oxide comes through leaks in a poor system design rather than patient delivery. One estimate reveals that more than 13 million liters of nitrous oxide are lost annually from leaks in European hospitals.22 The American Society of Anesthesiologists recommends decommissioning central piping of nitrous oxide in favor of cylinders at the point of care.24
Literature on enhanced recovery after surgery in gynecology promotes the use of propofol over volatile gases for our patients because of the high rate of postoperative nausea and vomiting seen with gases.25 Volatile gases should be a last-choice anesthetic for our patients.
Bottom line: It is critical that ObGyns work with colleagues in anesthesia to develop climate- and patient-friendly protocols for procedures.
8. Remove endocrine-disrupting chemicals from clinical supplies
Endocrine-disrupting chemicals (EDCs) are a type of chemical that alter the hormonal systems of humans, which can result in adverse health effects. Multiple studies and reviews have tied EDCs to reproductive abnormalities, such as the effects of bisphenol A (BPA) on estradiol levels, antral follicle counts, oocyte quality, and implantation rates; phthalates on fibroid burden; triclosan on embryo quality; parabens on live birth rates; and perfluoroalkylsubstances (PFAS or “forever substances”) on hypertensive disorders of pregnancy.5,26,27
What might be most shocking is that these EDCs are incorporated into medical supplies and pharmaceuticals. For example, BPA is known to line dialysis and ointment tubes, parabens are used for their antimicrobial properties in ultrasound gel and hep-locks, and phthalates are found in up to 40% of medical-use plastics and controlled-release medications. Authors of an observational study found that 74% of patients admitted to an LD unit were exposed to EDCs. In a neonatal intensive care unit (NICU), most of the supplies contained an EDC, and urinary BPA levels were elevated in neonates admitted to a NICU, raising concerns about long-term health risks.5
Bottom line: Physicians and health care institutions have an obligation to petition industry partners and suppliers to remove EDCs from their supply chains.
Reimagine
9. Educate
The field of health care sustainability remains in its infancy, but from 2007 to 2019, publications on climate change and health in academia increased by a factor of 8.29 Additionally, through waste audits, quality-improvement projects, and life cycle analyses (analytical tools to evaluate product or process emissions from materials extraction to disposal), we have gained insight into the scope of the problem, with evidence showing that our practices are largely derived from culture. It is time to provide formal education on health care sustainability to medical trainees, staff, and clinicians alike, who desire to see this topic reflected in their formal curricula.30 Start talking about it!
Bottom line: Commentaries, webinars, formal didactics sessions, in-services, and hospital workgroups to introduce this topic are a good way to teach others about the carbon footprint of our care and solutions to minimize it.
10. Engage in advocacy
Physicians have an ethical duty to advocate for change at the local, regional, and national levels if we want to see a better future for our patients, their children, and even ourselves. We should reimagine this work as an important public health initiative.31 Surveys of physicians, including ObGyns, reveal a concern about the sustainability of health care and a commitment to addressing this issue.20 ObGyns are on the frontlines of delivering care every day, so we are poised to implement changes that can impact our patients, especially when we can lead and petition hospital or local committees.20,28,32 There is much to be done, but every voice counts and can make impactful changes at every level. ●

- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733.
- Health care climate footprint report. Health Care Without Harm website. https://www.noharm.org/ClimateFootprintReport. Accessed May 12, 2023.
- Balbus JM, McCannon CJ, Mataka A, et al. After COP26—putting health and equity at the center of the climate movement. N Engl J Med. 2022;386:1295-1297.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3:e208243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822-e3827.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:30263035.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786.
- Zygourakis CC, Yoon S, Valencia V, et al. Operating room waste: disposable supply utilization in neurosurgical procedures. J Neurosurg. 2017;126:620-625.
- van Demark RE, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg. 2018;43:179-181.
- Campion N, Thiel CL, DeBlois J, et al. Life cycle assessment perspectives on delivering an infant in the US. Sci Total Environ. 2012;425:191198.
- Hsu S, Thiel CL, Mello MJ, Slutzman JE. Dumpster diving in the emergency department. West J Emerg Med. 2020;21:1211-1217.
- Mcgain F, Story D, Hendel S. An audit of intensive care unit recyclable waste. Anaesthesia. 2009;64:1299-1302.
- Wormer BA, Augenstein VA, Carpenter CL, et al. The green operating room: simple changes to reduce cost and our carbon footprint. Am Surg. 2013;79:666-671.
- Kagoma Y, Stall N, Rubinstein E, et al. People, planet and profits: the case for greening operating rooms. Can Med Assoc J. 2012;184:19051911.
- McCarthy CJ, Gerstenmaier JF, O’ Neill AC, et al. “EcoRadiology”— pulling the plug on wasted energy in the radiology department. Acad Radiol. 2014;21:1563-1566.
- Donahue LM, Hilton S, Bell SG, et al. A comparative carbon footprint analysis of disposable and reusable vaginal specula. Am J Obstet Gynecol. 2020;223:225.e1-225.e7.
- Rodriguez Morris MI, Hicks A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ Res Commun. 2022;4:025002.
- MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381-e388.
- Thiel C, Duncan P, Woods N. Attitude of US obstetricians and gynaecologists to global warming and medical waste. J Health Serv Res Policy. 2017;22:162-167.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98.
- Meyer MJ. Desflurane should des-appear: global and financial rationale. Anesth Analg. 2020;131:1317-1322.
- Rollins MD, Arendt KW, Carvalho B, et al. ASA Committee on Obstetric Anesthesia Working Group. Nitrous oxide. American Society of Anesthesiologists website. Accessed May 12, 2023. https://www .asahq.org/about-asa/governance-and-committees/asa-committees /committee-on-obstetric-anesthesia/nitrous-oxide.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121.
- Bommartio PA, Ferguson KK, Meeker JD, et al. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ Health Perspect. 2021;129:107004.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129-170.
- Ryan EC, Dubrow R, Sherman JD. Medical, nursing, and physician assistant student knowledge and attitudes toward climate change, pollution, and resource conservation in health care. BMC Med Educ. 2020;20:200.
- Giudice LC, Llamas-Clark EF, DeNicola Net al; FIGO Committee on Climate Change and Toxic Environmental Exposures. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155:345-356.
- Yates EF, Bowder AN, Roa L, et al. Empowering surgeons, anesthesiologists, and obstetricians to incorporate environmental sustainability in the operating room. Ann Surg. 2021;273:1108-1114.
- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733.
- Health care climate footprint report. Health Care Without Harm website. https://www.noharm.org/ClimateFootprintReport. Accessed May 12, 2023.
- Balbus JM, McCannon CJ, Mataka A, et al. After COP26—putting health and equity at the center of the climate movement. N Engl J Med. 2022;386:1295-1297.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3:e208243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822-e3827.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:30263035.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786.
- Zygourakis CC, Yoon S, Valencia V, et al. Operating room waste: disposable supply utilization in neurosurgical procedures. J Neurosurg. 2017;126:620-625.
- van Demark RE, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg. 2018;43:179-181.
- Campion N, Thiel CL, DeBlois J, et al. Life cycle assessment perspectives on delivering an infant in the US. Sci Total Environ. 2012;425:191198.
- Hsu S, Thiel CL, Mello MJ, Slutzman JE. Dumpster diving in the emergency department. West J Emerg Med. 2020;21:1211-1217.
- Mcgain F, Story D, Hendel S. An audit of intensive care unit recyclable waste. Anaesthesia. 2009;64:1299-1302.
- Wormer BA, Augenstein VA, Carpenter CL, et al. The green operating room: simple changes to reduce cost and our carbon footprint. Am Surg. 2013;79:666-671.
- Kagoma Y, Stall N, Rubinstein E, et al. People, planet and profits: the case for greening operating rooms. Can Med Assoc J. 2012;184:19051911.
- McCarthy CJ, Gerstenmaier JF, O’ Neill AC, et al. “EcoRadiology”— pulling the plug on wasted energy in the radiology department. Acad Radiol. 2014;21:1563-1566.
- Donahue LM, Hilton S, Bell SG, et al. A comparative carbon footprint analysis of disposable and reusable vaginal specula. Am J Obstet Gynecol. 2020;223:225.e1-225.e7.
- Rodriguez Morris MI, Hicks A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ Res Commun. 2022;4:025002.
- MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381-e388.
- Thiel C, Duncan P, Woods N. Attitude of US obstetricians and gynaecologists to global warming and medical waste. J Health Serv Res Policy. 2017;22:162-167.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98.
- Meyer MJ. Desflurane should des-appear: global and financial rationale. Anesth Analg. 2020;131:1317-1322.
- Rollins MD, Arendt KW, Carvalho B, et al. ASA Committee on Obstetric Anesthesia Working Group. Nitrous oxide. American Society of Anesthesiologists website. Accessed May 12, 2023. https://www .asahq.org/about-asa/governance-and-committees/asa-committees /committee-on-obstetric-anesthesia/nitrous-oxide.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121.
- Bommartio PA, Ferguson KK, Meeker JD, et al. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ Health Perspect. 2021;129:107004.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129-170.
- Ryan EC, Dubrow R, Sherman JD. Medical, nursing, and physician assistant student knowledge and attitudes toward climate change, pollution, and resource conservation in health care. BMC Med Educ. 2020;20:200.
- Giudice LC, Llamas-Clark EF, DeNicola Net al; FIGO Committee on Climate Change and Toxic Environmental Exposures. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155:345-356.
- Yates EF, Bowder AN, Roa L, et al. Empowering surgeons, anesthesiologists, and obstetricians to incorporate environmental sustainability in the operating room. Ann Surg. 2021;273:1108-1114.
Did ob.gyn. residencies take a hit from abortion bans?
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.