User login
Program for poor can boost hospital profits

Credit: Petr Kratochvil
A federal program designed to help the poor may actually be used to help US hospitals increase their profits, according to research published in Health Affairs.
Researchers examined enrollment in the 340B program, which provides deep discounts on outpatient drug purchases.
They found that hospitals and clinics that joined the program since 2004 currently serve more affluent and well-insured communities than those that qualified for the program in previous years.
This supports the idea that the program is changing from one that serves patients in need to one that enriches hospitals and their affiliated clinics, according to the researchers.
“This study provides the first nationally representative empirical evidence suggesting that the program’s original intent is being eroded by the actions of certain hospitals,” said study author Rena M. Conti, PhD, of the University of Chicago in Illinois.
This study follows work by Dr Conti and Peter B. Bach, MD, of Memorial Sloan-Kettering Cancer Center in New York, that was published in JAMA last year.
The JAMA study explained how 340B-qualified hospital-affiliated clinics can boost profits thanks to discounts on expensive anticancer drugs. The facilities receive the discounts under the expectation that the savings will be passed on to poor patients.
“Hospitals qualify for the program based on the poverty of their inpatient census only,” Dr Conti said. “The affiliated clinics are the only 340B institutions not required to pass the discounts off to patients or their insurers, nor do they have to report to the government exactly how these profits are used to serve the poor. Insurers’ and their patients’ payments for outpatient drug treatment don’t reflect the discounts the hospital receives.”
The 340B program, which began in 1992, was designed to help selected hospitals and their outpatient clinics serve low-income and uninsured patients by providing discounts of 30% to 50% on outpatient drugs.
About a decade ago, enrollment in 340B began to increase rapidly. Now, more than a third of the 4375 US non-federal hospitals are 340B-qualified. Recent Congressional and news reports suggest that, for selected hospitals, profits off the 340B program can be significant.
For their new study, Drs Conti and Bach examined the populations served by hospitals and clinics qualifying for 340B before and after the decade-long growth spurt. They matched data for all hospitals and clinics registered with the 340B program to socioeconomic data from the US Census Bureau.
The results showed that communities served by hospital-affiliated clinics joining the program in 2004 or later tended to have higher household incomes, much less unemployment, and higher rates of health insurance.
The researchers said their findings are consistent with recent complaints that, rather than serving vulnerable communities, the 340B program is being used to increase profits for hospitals and their affiliated clinics. ![]()

Credit: Petr Kratochvil
A federal program designed to help the poor may actually be used to help US hospitals increase their profits, according to research published in Health Affairs.
Researchers examined enrollment in the 340B program, which provides deep discounts on outpatient drug purchases.
They found that hospitals and clinics that joined the program since 2004 currently serve more affluent and well-insured communities than those that qualified for the program in previous years.
This supports the idea that the program is changing from one that serves patients in need to one that enriches hospitals and their affiliated clinics, according to the researchers.
“This study provides the first nationally representative empirical evidence suggesting that the program’s original intent is being eroded by the actions of certain hospitals,” said study author Rena M. Conti, PhD, of the University of Chicago in Illinois.
This study follows work by Dr Conti and Peter B. Bach, MD, of Memorial Sloan-Kettering Cancer Center in New York, that was published in JAMA last year.
The JAMA study explained how 340B-qualified hospital-affiliated clinics can boost profits thanks to discounts on expensive anticancer drugs. The facilities receive the discounts under the expectation that the savings will be passed on to poor patients.
“Hospitals qualify for the program based on the poverty of their inpatient census only,” Dr Conti said. “The affiliated clinics are the only 340B institutions not required to pass the discounts off to patients or their insurers, nor do they have to report to the government exactly how these profits are used to serve the poor. Insurers’ and their patients’ payments for outpatient drug treatment don’t reflect the discounts the hospital receives.”
The 340B program, which began in 1992, was designed to help selected hospitals and their outpatient clinics serve low-income and uninsured patients by providing discounts of 30% to 50% on outpatient drugs.
About a decade ago, enrollment in 340B began to increase rapidly. Now, more than a third of the 4375 US non-federal hospitals are 340B-qualified. Recent Congressional and news reports suggest that, for selected hospitals, profits off the 340B program can be significant.
For their new study, Drs Conti and Bach examined the populations served by hospitals and clinics qualifying for 340B before and after the decade-long growth spurt. They matched data for all hospitals and clinics registered with the 340B program to socioeconomic data from the US Census Bureau.
The results showed that communities served by hospital-affiliated clinics joining the program in 2004 or later tended to have higher household incomes, much less unemployment, and higher rates of health insurance.
The researchers said their findings are consistent with recent complaints that, rather than serving vulnerable communities, the 340B program is being used to increase profits for hospitals and their affiliated clinics. ![]()

Credit: Petr Kratochvil
A federal program designed to help the poor may actually be used to help US hospitals increase their profits, according to research published in Health Affairs.
Researchers examined enrollment in the 340B program, which provides deep discounts on outpatient drug purchases.
They found that hospitals and clinics that joined the program since 2004 currently serve more affluent and well-insured communities than those that qualified for the program in previous years.
This supports the idea that the program is changing from one that serves patients in need to one that enriches hospitals and their affiliated clinics, according to the researchers.
“This study provides the first nationally representative empirical evidence suggesting that the program’s original intent is being eroded by the actions of certain hospitals,” said study author Rena M. Conti, PhD, of the University of Chicago in Illinois.
This study follows work by Dr Conti and Peter B. Bach, MD, of Memorial Sloan-Kettering Cancer Center in New York, that was published in JAMA last year.
The JAMA study explained how 340B-qualified hospital-affiliated clinics can boost profits thanks to discounts on expensive anticancer drugs. The facilities receive the discounts under the expectation that the savings will be passed on to poor patients.
“Hospitals qualify for the program based on the poverty of their inpatient census only,” Dr Conti said. “The affiliated clinics are the only 340B institutions not required to pass the discounts off to patients or their insurers, nor do they have to report to the government exactly how these profits are used to serve the poor. Insurers’ and their patients’ payments for outpatient drug treatment don’t reflect the discounts the hospital receives.”
The 340B program, which began in 1992, was designed to help selected hospitals and their outpatient clinics serve low-income and uninsured patients by providing discounts of 30% to 50% on outpatient drugs.
About a decade ago, enrollment in 340B began to increase rapidly. Now, more than a third of the 4375 US non-federal hospitals are 340B-qualified. Recent Congressional and news reports suggest that, for selected hospitals, profits off the 340B program can be significant.
For their new study, Drs Conti and Bach examined the populations served by hospitals and clinics qualifying for 340B before and after the decade-long growth spurt. They matched data for all hospitals and clinics registered with the 340B program to socioeconomic data from the US Census Bureau.
The results showed that communities served by hospital-affiliated clinics joining the program in 2004 or later tended to have higher household incomes, much less unemployment, and higher rates of health insurance.
The researchers said their findings are consistent with recent complaints that, rather than serving vulnerable communities, the 340B program is being used to increase profits for hospitals and their affiliated clinics. ![]()
Mental health challenges fairly common in cancer patients

chemotherapy
Credit: Rhoda Baer
In a large German study, investigators found that nearly a third of cancer patients experienced some form of clinically relevant mental health
challenge.
Of the more than 2100 cancer patients interviewed, 32% had experienced a clinically meaningful level of mental or emotional distress in the previous 4 weeks.
This prevalence is higher than that observed in the general population, and the difference is primarily due to a higher rate of anxiety and adjustment disorders.
The incidence of mental health issues varied by cancer type. The highest was among patients with breast cancer (42%) and head and neck cancer (41%), followed by malignant melanoma (39%).
The lowest prevalence was seen among patients with prostate cancer (22%), stomach cancers (21%), and pancreatic cancer (20%).
These results appear in the Journal of Clinical Oncology.
“These findings reinforce that, as doctors, we need to be very aware of signs and symptoms of mental and emotional distress,” said lead study author Anja Mehnert, PhD, of the University of Leipzig in Germany.
“We must encourage patients to seek evaluation, support, and treatment if necessary, as there are long-term risks often associated with more severe, untreated mental health disorders. This research also sheds light on which patients we should watch more closely.”
Dr Mehnert and her colleagues conducted this study in 2141 cancer patients who were 18 to 75 years of age. The team conducted face-to-face interviews in hospitals, outpatient cancer care centers, and rehabilitation centers in Germany.
Interview answers were immediately entered into a computer based-diagnostic program. The test assessed various psychological symptoms over the previous 4-week period. Patients’ diagnoses were classified according to the Diagnostic and Statistical Manual of Mental Disorders, the standard classification used by mental health professionals.
The patients had a range of cancer types, with the most common being breast cancer (44%), prostate cancer (15%), and colorectal cancer (14%). The average time since cancer diagnosis was 13.5 months, and 51% of the participants were women.
The researchers found that 32% of patients experienced at least one clinically meaningful mental health issue (defined in the study as a mental health disorder). This is a higher prevalence than in the general population, in which 18% to 20% of people are estimated to have a clinically meaningful mental disorder.
In the 4-week period prior to the interview, 11.5% of patients experienced an anxiety disorder. Eleven percent met the criteria for an adjustment disorder, a predominantly mixed anxiety-depressive syndrome that persisted for at least 4 weeks in response to a significant life change. And 6.5% of patients had signs of a mood disorder such as major depression.
The 11.5% rate of anxiety disorders—such as phobia, panic, or generalized anxiety disorder—was slightly higher than in the general population (9%), while the prevalence of other mental health diagnoses was similar to rates in the general population.
It is likely that the prevalence of adjustment disorders (11%), which is rarely assessed in general population surveys, significantly contributed to the overall higher prevalence rate of mental disorders in this population of patients with cancer.
Dr Mehnert said it was surprising that patients with a more treatable malignancy, such as breast cancer, experienced more distress than people with cancers that are more challenging to treat, such as stomach and pancreatic cancers. So more research is needed to interpret these findings.
The investigators believe the study’s results may be useful for planning future support programs for cancer patients, and they can provide additional information to guide programs for people with specific cancer types.
The team also believes the findings can likely be generalized to patients in the US because the prevalence of mental health diagnoses is similar between the 2 countries. ![]()

chemotherapy
Credit: Rhoda Baer
In a large German study, investigators found that nearly a third of cancer patients experienced some form of clinically relevant mental health
challenge.
Of the more than 2100 cancer patients interviewed, 32% had experienced a clinically meaningful level of mental or emotional distress in the previous 4 weeks.
This prevalence is higher than that observed in the general population, and the difference is primarily due to a higher rate of anxiety and adjustment disorders.
The incidence of mental health issues varied by cancer type. The highest was among patients with breast cancer (42%) and head and neck cancer (41%), followed by malignant melanoma (39%).
The lowest prevalence was seen among patients with prostate cancer (22%), stomach cancers (21%), and pancreatic cancer (20%).
These results appear in the Journal of Clinical Oncology.
“These findings reinforce that, as doctors, we need to be very aware of signs and symptoms of mental and emotional distress,” said lead study author Anja Mehnert, PhD, of the University of Leipzig in Germany.
“We must encourage patients to seek evaluation, support, and treatment if necessary, as there are long-term risks often associated with more severe, untreated mental health disorders. This research also sheds light on which patients we should watch more closely.”
Dr Mehnert and her colleagues conducted this study in 2141 cancer patients who were 18 to 75 years of age. The team conducted face-to-face interviews in hospitals, outpatient cancer care centers, and rehabilitation centers in Germany.
Interview answers were immediately entered into a computer based-diagnostic program. The test assessed various psychological symptoms over the previous 4-week period. Patients’ diagnoses were classified according to the Diagnostic and Statistical Manual of Mental Disorders, the standard classification used by mental health professionals.
The patients had a range of cancer types, with the most common being breast cancer (44%), prostate cancer (15%), and colorectal cancer (14%). The average time since cancer diagnosis was 13.5 months, and 51% of the participants were women.
The researchers found that 32% of patients experienced at least one clinically meaningful mental health issue (defined in the study as a mental health disorder). This is a higher prevalence than in the general population, in which 18% to 20% of people are estimated to have a clinically meaningful mental disorder.
In the 4-week period prior to the interview, 11.5% of patients experienced an anxiety disorder. Eleven percent met the criteria for an adjustment disorder, a predominantly mixed anxiety-depressive syndrome that persisted for at least 4 weeks in response to a significant life change. And 6.5% of patients had signs of a mood disorder such as major depression.
The 11.5% rate of anxiety disorders—such as phobia, panic, or generalized anxiety disorder—was slightly higher than in the general population (9%), while the prevalence of other mental health diagnoses was similar to rates in the general population.
It is likely that the prevalence of adjustment disorders (11%), which is rarely assessed in general population surveys, significantly contributed to the overall higher prevalence rate of mental disorders in this population of patients with cancer.
Dr Mehnert said it was surprising that patients with a more treatable malignancy, such as breast cancer, experienced more distress than people with cancers that are more challenging to treat, such as stomach and pancreatic cancers. So more research is needed to interpret these findings.
The investigators believe the study’s results may be useful for planning future support programs for cancer patients, and they can provide additional information to guide programs for people with specific cancer types.
The team also believes the findings can likely be generalized to patients in the US because the prevalence of mental health diagnoses is similar between the 2 countries. ![]()

chemotherapy
Credit: Rhoda Baer
In a large German study, investigators found that nearly a third of cancer patients experienced some form of clinically relevant mental health
challenge.
Of the more than 2100 cancer patients interviewed, 32% had experienced a clinically meaningful level of mental or emotional distress in the previous 4 weeks.
This prevalence is higher than that observed in the general population, and the difference is primarily due to a higher rate of anxiety and adjustment disorders.
The incidence of mental health issues varied by cancer type. The highest was among patients with breast cancer (42%) and head and neck cancer (41%), followed by malignant melanoma (39%).
The lowest prevalence was seen among patients with prostate cancer (22%), stomach cancers (21%), and pancreatic cancer (20%).
These results appear in the Journal of Clinical Oncology.
“These findings reinforce that, as doctors, we need to be very aware of signs and symptoms of mental and emotional distress,” said lead study author Anja Mehnert, PhD, of the University of Leipzig in Germany.
“We must encourage patients to seek evaluation, support, and treatment if necessary, as there are long-term risks often associated with more severe, untreated mental health disorders. This research also sheds light on which patients we should watch more closely.”
Dr Mehnert and her colleagues conducted this study in 2141 cancer patients who were 18 to 75 years of age. The team conducted face-to-face interviews in hospitals, outpatient cancer care centers, and rehabilitation centers in Germany.
Interview answers were immediately entered into a computer based-diagnostic program. The test assessed various psychological symptoms over the previous 4-week period. Patients’ diagnoses were classified according to the Diagnostic and Statistical Manual of Mental Disorders, the standard classification used by mental health professionals.
The patients had a range of cancer types, with the most common being breast cancer (44%), prostate cancer (15%), and colorectal cancer (14%). The average time since cancer diagnosis was 13.5 months, and 51% of the participants were women.
The researchers found that 32% of patients experienced at least one clinically meaningful mental health issue (defined in the study as a mental health disorder). This is a higher prevalence than in the general population, in which 18% to 20% of people are estimated to have a clinically meaningful mental disorder.
In the 4-week period prior to the interview, 11.5% of patients experienced an anxiety disorder. Eleven percent met the criteria for an adjustment disorder, a predominantly mixed anxiety-depressive syndrome that persisted for at least 4 weeks in response to a significant life change. And 6.5% of patients had signs of a mood disorder such as major depression.
The 11.5% rate of anxiety disorders—such as phobia, panic, or generalized anxiety disorder—was slightly higher than in the general population (9%), while the prevalence of other mental health diagnoses was similar to rates in the general population.
It is likely that the prevalence of adjustment disorders (11%), which is rarely assessed in general population surveys, significantly contributed to the overall higher prevalence rate of mental disorders in this population of patients with cancer.
Dr Mehnert said it was surprising that patients with a more treatable malignancy, such as breast cancer, experienced more distress than people with cancers that are more challenging to treat, such as stomach and pancreatic cancers. So more research is needed to interpret these findings.
The investigators believe the study’s results may be useful for planning future support programs for cancer patients, and they can provide additional information to guide programs for people with specific cancer types.
The team also believes the findings can likely be generalized to patients in the US because the prevalence of mental health diagnoses is similar between the 2 countries. ![]()
FDA allows access to pathogen inactivation system

The US Food and Drug Administration (FDA) has authorized the use of a pathogen inactivation system in regions of the US and its territories affected by outbreaks of Chikungunya and dengue virus.
The INTERCEPT Blood System for platelets is used for the preparation and storage of whole blood-derived and apheresis platelets.
The system can inactivate a range of viruses, bacteria, and parasites to reduce the risk of transmission via platelet transfusion. It can also prevent transfusion-associated graft-vs-host disease and reduce the risk of other adverse effects due to transfusion of contaminating donor leukocytes.
The INTERCEPT Blood System for platelets has not been granted full FDA approval. The agency has approved use of the system via an investigational device exemption (IDE).
This allows for early access to a device not yet approved in the US when no satisfactory alternative is available to treat patients with serious or life-threatening conditions.
The INTERCEPT Blood System for platelets will initially be available to sites in Puerto Rico that agree to participate in a clinical study. Depending on the scope of participation there, the system may be made available to sites in other areas where cases of Chikungunya and dengue have been reported, such as Florida and Texas.
“We are pleased to provide US blood centers and hospitals early access to INTERCEPT for the treatment of platelet components in light of the escalating threat of Chikungunya and dengue transfusion-transmitted infections,” said Carol Moore, of Cerus Corporation, the company developing the INTERCEPT system.
“With this expeditious approval of our IDE, we hope to initiate our first study site before year-end.”
About the system
The INTERCEPT Blood System for platelets is based on the premise that platelets don’t require functional DNA or RNA, but pathogens and contaminating leukocytes do. The system deploys proprietary molecules that, when activated, bind to and block the replication of DNA and RNA in the blood.
The system uses amotosalen HCl (a photoactive compound) and long-wavelength ultraviolet illumination to photochemically treat platelet components, rendering susceptible pathogens incapable of replicating and causing disease.
Published studies have demonstrated INTERCEPT inactivation of >6.4 log of Chikungunya and >5.3 log of dengue infectious titers, both in excess of observed titers in asymptomatic donors.
The INTERCEPT platelet system has been approved in Europe since 2002 and is currently used at more than 100 blood centers in 20 countries. The system is under regulatory review in the US, Canada, Brazil, and China.
About dengue and Chikungunya
Dengue virus is endemic to the Caribbean region. Local transmission of Chikungunya virus was detected in the Caribbean for the first time in February 2014. Both viruses are spread by species of mosquitoes common in tropical climates and regions within the continental US.
As of September 30, 2014, the Centers for Disease Control and Prevention has reported 11 confirmed locally transmitted cases of Chikungunya in
Florida, 421 cases in Puerto Rico, and 45 cases in the US Virgin Islands. Local transmission of dengue has also been reported in Texas and Florida.
Chikungunya virus causes high fevers, joint pain and swelling, headaches, and a rash. Symptoms have been reported to persist for up to 2 years in chronic cases. Rarely, Chikungunya can be fatal.
Symptoms of dengue include high fever, headaches, joint and muscle pain, vomiting, and a rash. In some cases, dengue infection is life-threatening due to dengue hemorrhagic fever, which causes bleeding from the nose, gums, or under the skin. ![]()

The US Food and Drug Administration (FDA) has authorized the use of a pathogen inactivation system in regions of the US and its territories affected by outbreaks of Chikungunya and dengue virus.
The INTERCEPT Blood System for platelets is used for the preparation and storage of whole blood-derived and apheresis platelets.
The system can inactivate a range of viruses, bacteria, and parasites to reduce the risk of transmission via platelet transfusion. It can also prevent transfusion-associated graft-vs-host disease and reduce the risk of other adverse effects due to transfusion of contaminating donor leukocytes.
The INTERCEPT Blood System for platelets has not been granted full FDA approval. The agency has approved use of the system via an investigational device exemption (IDE).
This allows for early access to a device not yet approved in the US when no satisfactory alternative is available to treat patients with serious or life-threatening conditions.
The INTERCEPT Blood System for platelets will initially be available to sites in Puerto Rico that agree to participate in a clinical study. Depending on the scope of participation there, the system may be made available to sites in other areas where cases of Chikungunya and dengue have been reported, such as Florida and Texas.
“We are pleased to provide US blood centers and hospitals early access to INTERCEPT for the treatment of platelet components in light of the escalating threat of Chikungunya and dengue transfusion-transmitted infections,” said Carol Moore, of Cerus Corporation, the company developing the INTERCEPT system.
“With this expeditious approval of our IDE, we hope to initiate our first study site before year-end.”
About the system
The INTERCEPT Blood System for platelets is based on the premise that platelets don’t require functional DNA or RNA, but pathogens and contaminating leukocytes do. The system deploys proprietary molecules that, when activated, bind to and block the replication of DNA and RNA in the blood.
The system uses amotosalen HCl (a photoactive compound) and long-wavelength ultraviolet illumination to photochemically treat platelet components, rendering susceptible pathogens incapable of replicating and causing disease.
Published studies have demonstrated INTERCEPT inactivation of >6.4 log of Chikungunya and >5.3 log of dengue infectious titers, both in excess of observed titers in asymptomatic donors.
The INTERCEPT platelet system has been approved in Europe since 2002 and is currently used at more than 100 blood centers in 20 countries. The system is under regulatory review in the US, Canada, Brazil, and China.
About dengue and Chikungunya
Dengue virus is endemic to the Caribbean region. Local transmission of Chikungunya virus was detected in the Caribbean for the first time in February 2014. Both viruses are spread by species of mosquitoes common in tropical climates and regions within the continental US.
As of September 30, 2014, the Centers for Disease Control and Prevention has reported 11 confirmed locally transmitted cases of Chikungunya in
Florida, 421 cases in Puerto Rico, and 45 cases in the US Virgin Islands. Local transmission of dengue has also been reported in Texas and Florida.
Chikungunya virus causes high fevers, joint pain and swelling, headaches, and a rash. Symptoms have been reported to persist for up to 2 years in chronic cases. Rarely, Chikungunya can be fatal.
Symptoms of dengue include high fever, headaches, joint and muscle pain, vomiting, and a rash. In some cases, dengue infection is life-threatening due to dengue hemorrhagic fever, which causes bleeding from the nose, gums, or under the skin. ![]()

The US Food and Drug Administration (FDA) has authorized the use of a pathogen inactivation system in regions of the US and its territories affected by outbreaks of Chikungunya and dengue virus.
The INTERCEPT Blood System for platelets is used for the preparation and storage of whole blood-derived and apheresis platelets.
The system can inactivate a range of viruses, bacteria, and parasites to reduce the risk of transmission via platelet transfusion. It can also prevent transfusion-associated graft-vs-host disease and reduce the risk of other adverse effects due to transfusion of contaminating donor leukocytes.
The INTERCEPT Blood System for platelets has not been granted full FDA approval. The agency has approved use of the system via an investigational device exemption (IDE).
This allows for early access to a device not yet approved in the US when no satisfactory alternative is available to treat patients with serious or life-threatening conditions.
The INTERCEPT Blood System for platelets will initially be available to sites in Puerto Rico that agree to participate in a clinical study. Depending on the scope of participation there, the system may be made available to sites in other areas where cases of Chikungunya and dengue have been reported, such as Florida and Texas.
“We are pleased to provide US blood centers and hospitals early access to INTERCEPT for the treatment of platelet components in light of the escalating threat of Chikungunya and dengue transfusion-transmitted infections,” said Carol Moore, of Cerus Corporation, the company developing the INTERCEPT system.
“With this expeditious approval of our IDE, we hope to initiate our first study site before year-end.”
About the system
The INTERCEPT Blood System for platelets is based on the premise that platelets don’t require functional DNA or RNA, but pathogens and contaminating leukocytes do. The system deploys proprietary molecules that, when activated, bind to and block the replication of DNA and RNA in the blood.
The system uses amotosalen HCl (a photoactive compound) and long-wavelength ultraviolet illumination to photochemically treat platelet components, rendering susceptible pathogens incapable of replicating and causing disease.
Published studies have demonstrated INTERCEPT inactivation of >6.4 log of Chikungunya and >5.3 log of dengue infectious titers, both in excess of observed titers in asymptomatic donors.
The INTERCEPT platelet system has been approved in Europe since 2002 and is currently used at more than 100 blood centers in 20 countries. The system is under regulatory review in the US, Canada, Brazil, and China.
About dengue and Chikungunya
Dengue virus is endemic to the Caribbean region. Local transmission of Chikungunya virus was detected in the Caribbean for the first time in February 2014. Both viruses are spread by species of mosquitoes common in tropical climates and regions within the continental US.
As of September 30, 2014, the Centers for Disease Control and Prevention has reported 11 confirmed locally transmitted cases of Chikungunya in
Florida, 421 cases in Puerto Rico, and 45 cases in the US Virgin Islands. Local transmission of dengue has also been reported in Texas and Florida.
Chikungunya virus causes high fevers, joint pain and swelling, headaches, and a rash. Symptoms have been reported to persist for up to 2 years in chronic cases. Rarely, Chikungunya can be fatal.
Symptoms of dengue include high fever, headaches, joint and muscle pain, vomiting, and a rash. In some cases, dengue infection is life-threatening due to dengue hemorrhagic fever, which causes bleeding from the nose, gums, or under the skin. ![]()
Granulomatous Changes Associated With Pigmented Purpuric Dermatosis
Pigmented purpuric dermatoses (PPDs) are a group of common chronic disorders characterized by speckled, cayenne pepper–like petechiae and orange-brown discoloration of the skin resulting from capillaritis.1 Pigmented purpuric dermatoses typically occur in the absence of underlying vascular insufficiency or other hematologic dysfunction. The 5 well-known clinicopathologic variants of PPD include Schamberg disease; purpura annularis telangiectodes of Majocchi; pigmented purpuric lichenoid dermatitis of Gougerot and Blum; eczematoidlike purpura of Doucas and Kapetanakis; and lichen aureus.2 All PPDs share common characteristic clinical and histologic features. Clinically, patients generally present with symmetric petechiae and/or pigmented macules. All 5 PPD variants share similar histologic findings, including a vasculocentric lymphocytic infiltrate in the papillary dermis, swelling of the endothelial cells, erythrocyte extravasation, and often hemosiderin-laden macrophages.1 Despite these clinical and histopathologic similarities, each variant contains additional distinctive features, such as telangiectasia (purpura annularis telangiectodes of Majocchi), a lichenoid infiltrate (pigmented purpuric lichenoid dermatitis of Gougerot and Blum), eczematous changes (eczematoidlike purpura of Doucas and Kapetanakis), and marked hemosiderin deposition (lichen aureus).
Granulomatous pigmented purpuric dermatosis (GPPD) is a rare variant of PPD.3-7 Clinically, these lesions appear similar to other PPDs; however, in addition to the characteristic changes associated with conventional PPD, histologic examination of GPPD reveals a granulomatous inflammatory reaction pattern. Although the pathogenesis of GPPD is not well understood, its association with hyperlipidemia may suggest a granulomatous response to capillaritis mediated by lipid deposition in the microvasculature.6,7
We present 3 cases of GPPD and provide a review of the literature. In all of our patients, biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin by standard methodologies, and all stains were performed on sections by standard methodologies. Based on a PubMed search of articles indexed for MEDLINE using the terms granulomatous pigmented purpuric dermatosis, sarcoidosis, pigmented purpuric dermatosis, granulomas, and pigmented purpuric dermatosis, we review 5 additional reports describing 10 total patients.3-7
Case Reports
Patient 1
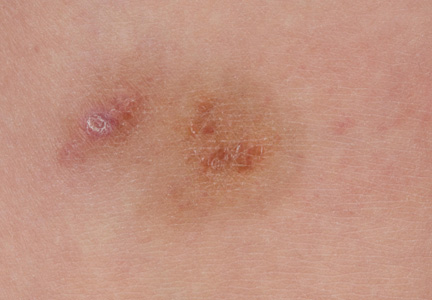
A 9-year-old white boy presented with a 3-cm asymptomatic light brown patch with a nonblanching violaceous center on the right posterior thigh that was studded with pinpoint yellow papules (Figure, A). The lesion appeared 3 to 4 years prior to presentation but had become progressively darker and centrally indurated over the last 2 years. The patient and his mother denied any history of trauma to the area. His medical history was unremarkable, and his current medications included fish oil and multivitamin tablets.
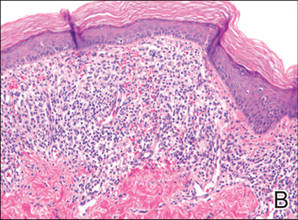
Histologic examination of a punch biopsy specimen taken from the center of the lesion revealed a lichenoid lymphohistiocytic infiltrate with marked red blood cell (RBC) extravasation and associated hemosiderin-laden macrophages. The lymphocytes comprising this infiltrate lacked cytologic atypia and exhibited minimal epidermotropism (Figure, B). Additionally, there was a superficial and deep perivascular mononuclear inflammatory infiltrate intermixed with numerous small granulomas comprised ofepithelioid histiocytes in the mid and deep dermis (Figure, C). Periodic acid–Schiff, acid-fast bacilli (AFB), and Fite stains were negative for organisms. Polarization was negative for refractile foreign material. Due to the patient’s age, no treatment was performed, and the lesion remains unchanged 1 year after biopsy.
Patient 2
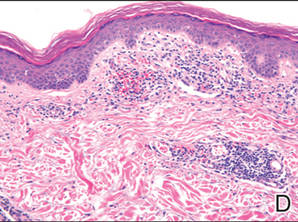
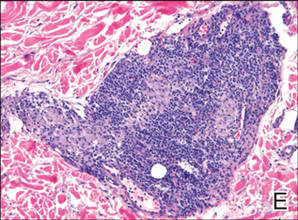
A 49-year-old white woman presented with a 2-cm yellow-brown patch with a faint, nonblanchable, violaceous center on the right lateral thigh of 4 months’ duration. The patch initially appeared as a small asymptomatic purple papule. The patient denied any history of trauma to the area. A purified protein derivative (tuberculin) skin test was negative at the time of examination. The patient’s medical history was remarkable for renal calculi. Her current medications included progesterone; estradiol; lansoprazole; prenatal vitamins; vitamins C and E; zinc; and calcium. The patient had no family history of sarcoidosis. Complete blood cell count, urinalysis, liver function tests, and angiotensin-converting enzyme levels were unremarkable. Pulmonary function tests were normal, and there was no evidence of sarcoidosis on chest radiography. Initial biopsy of the lesion revealed a perivascular and interstitial lymphohistiocytic infiltrate with abundant extravasated RBCs in the papillary dermis (Figure, D). Similar to patient 1, the infiltrate lacked cytologic atypia and did not involve the overlying epidermis. There was perivascular granulomatous inflammation in the mid dermis (Figure, E). Periodic acid–Schiff, Warthin-Starry, and AFB stains were negative for organisms. Polarization was negative for refractile foreign material.
The lesion was treated with clobetasol propionate ointment 0.05% twice daily for 6 weeks with transient improvement, but the lesion recurred upon treatment cessation. Subsequent treatment with intralesional triamcinolone resulted in slight improvement of the lesion. A therapeutic trial of targeted pulsed dye laser treatment was ineffective. The lesion gradually increased in size over the next year with no therapy, and a repeat biopsy revealed a lichenoid lymphohistiocytic infiltrate with abundant extravasated RBCs consistent with persistent PPD. A granulomatous infiltrate was not evident in the superficial shave biopsy specimen.
Patient 3
A 75-year-old white woman presented with scattered, speckled, cayenne pepper–like, red-brown macules on the legs. Two years prior to presentation, a few scattered symmetrical macules appeared on the dorsal aspects of the feet, which gradually increased in number to form larger confluent patches that spread to the lower legs. The patient denied itching or burning but reported that the patches became painful when scratched and were aggravated by sun exposure. Her medical history was remarkable for asthma, chronic renal insufficiency, coronary artery disease, Barrett esophagus, obstructive sleep apnea, hypothyroidism, renal calculi, type 2 diabetes mellitus, and hyperlipidemia. Her current medications included carvedilol, valsartan, levothyroxine, aspirin, clopidogrel, furosemide, nitrofurantoin, temazepam, insulin, ezetimibe-simvastatin, and lansoprazole. Computed tomography of the chest revealed no signs of sarcoidosis. Pulmonary function tests revealed moderate obstructive lung disease. An ophthalmology examination showed no evidence of sarcoidosis. Laboratory results revealed an elevated glucose, blood urea nitrogen, creatinine, and triglyceride levels, as well as low hematocrit and vitamin D levels. Urinalysis, thyroid-stimulating hormone (thyrotropin) test, liver function tests, angiotensin-converting enzyme test, hepatitis B surface antigen, and IFN-g release assay were normal.
Histologic examination of a punch biopsy specimen revealed an inflammatory infiltrate confined to the papillary dermis. This infiltrate was comprised of an admixture of lymphocytes and histiocytes in a perivascular distribution with associated RBC extravasation and intimately associated granulomas (Figure, F). Additional inflammation in the deeper aspects of the dermis was not identified. Periodic acid–Schiff, AFB, and Fite stains were negative for organisms. Polarization was negative for refractile foreign material.

| 
| 
| ||
| 
| 
| ||
| A 3-cm asymptomatic light brown patch with a nonblanching violaceous center on the right posterior thigh that was studded with pinpoint yellow papules (A). Lichenoid lymphohistiocytic infiltrate in the papillary dermis with marked red blood cell extravasation (B)(H&E, original magnification ×20). Superficial and deep perivascular mononuclear inflammatory infiltrate intermixed with numerous small granulomas comprised of epithelioid histiocytes in the mid and deep dermis (C)(H&E, original magnification ×20). Perivascular and interstitial lymphohistiocytic infiltrate with abundant extravasated red blood cells in the papillary dermis (D)(H&E, original magnification ×10). Perivascular lymphohistiocytic inflammation with epithelioid granulomas in the mid dermis (E)(H&E, original magnification ×20). Lymphohistiocytic inflammation in the papillary dermis comprised of an admixture of lymphocytes and histiocytes in a perivascular distribution with associated red blood cell extravasation and intimately associated granulomas (F)(H&E, original magnification ×20). | ||||
The patient was treated with topical steroids and minocycline 50 mg twice daily without improvement. The lesions improved after the patient underwent treatment with oral corticosteroids for pulmonary disease.
Comment
Pigmented purpuric dermatoses comprise a spectrum of clinical and pathologic conditions.1,2 Granulomatous PPD is a much less common variant, characterized by a granulomatous infiltrate admixed with PPD. We report 3 additional cases and review the literature on this rare and interesting variant of PPD.
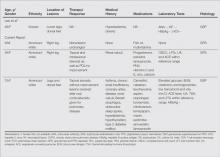
We noted several unifying clinicopathologic features among our patients and those previously reported in the literature (Table).3-7 Including our cases, our review yielded 13 GPPD patients ranging in age from 9 to 75 years, with a mean age of 49.1 years. Two of our patients—patients 1 and 3—were the youngest and oldest patients, respectively, among the cases we reviewed. The majority of the cases we reviewed included patients of East Asian descent (6 Taiwanese; 2 Japanese; 1 Korean) as well as 4 white patients. No distinctive gender predilection was apparent, as our review included 8 females and 5 males.
Our review revealed that GPPD lesions typically involve the lower extremities and usually are asymptomatic, with the exception of occasional pruritus. Additional lesions have been reported on the dorsal aspect of the hands, and 1 case noted exclusive involvement of the wrist.6 Lesions of GPPD can range in their clinical appearance. Three of 13 patients presented with purpuric papules and 2 had brown pigmentation with hemorrhagic papules3,4,6; the remaining 8 patients had erythematous or brown macules, papules, or plaques.5-7 The most commonly associated disease condition was hyperlipidemia, which was reported in 7 of 13 cases.5-7 Additional reported comorbidities included meningioma, renal calculi, obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, hepatitis C virus, ulcerative colitis, thrombocytopenia, and hyperuricemia. Reported serologic abnormalities included a rare positive antinuclear antibody, rheumatoid factor, and cryoglobulins.3,6 Therapeutic efficacy in the management of GPPD has not been well described; however, for the rare cases in which therapies were described, they were largely unsuccessful, with 1 patient exhibiting spontaneous improvement.3,4
Granulomatous PPD also appears to exhibit a range of histologic findings. All cases of GPPD shared fundamental components, such as a brisk perivascular infiltrate accompanied by RBC extravasation with variable hemosiderin-laden macrophages and a granulomatous infiltrate. All of the reports we reviewed described an intimate association between these components, with the granulomas being essentially superimposed on typical PPD. As for other types of PPD, obvious vasculitis characterized by a vasculocentric inflammatory infiltrate and evidence of vascular injury, such as fibrinoid necrosis of the vessel wall, has not been described in GPPD.3-7 Finally, histologic changes suggestive of a relationship with cutaneous T-cell lymphoma, cytologic atypia, and epidermotropism have been described for some forms of PPD but have not been described for GPPD.3-8
Our case reports expand the histologic spectrum of GPPD. Although patient 3 exhibited a relatively intimate association of granulomas and PPD, 2 of our cases (patients 1 and 2) demonstrated a granulomatous infiltrate in the mid to deep dermis, which is separate from the more superficially situated lichenoid lymphohistiocytic infiltrate, RBC extravasation, and hemosiderin-laden macrophages noted in the papillary dermis. Considered along with the absence of an obvious clinicopathologic explanation for the granulomatous infiltrates (eg, polarizable material, infectious organisms, systemic disease), these 2 cases (patients 1 and 2) suggest a composite form of PPD that combines the lichenoid pattern of PPD of Gougerot and Blum with a deep granulomatous component of GPPD. The importance of this distinction lies in the potential for physicians to overlook this potentially informative histologic pattern if only a superficial biopsy is performed. The clinical relevance is unclear; however, in our experience, it has been challenging to treat this relatively small subset of patients who have exhibited a limited response to treatment with topical steroids, intralesional steroids, pulsed dye laser, and vitamin supplementation.
The cause of the granulomatous infiltrate in GPPD is poorly understood. Seven of 13 cases included in our review occurred in patients with a history of hyperlipidemia.5-7 Some have postulated that the constellation of findings of GPPD in hyperlipidemic patients reflects an underlying vascular injury process induced by lipid deposition in the endothelial cells with subsequent RBC extravasation and a secondary granulomatous response to the lipid deposits.6,7 However, given the occurrence of GPPD in patients without hyperlipidemia, other mechanisms also must be considered in the pathogenesis of GPPD, including a reaction to medications, systemic diseases, and infectious etiologies (eg, hepatitis B virus).4,6 As additional cases are described in the literature, other unifying clinical etiologies for this histopathologic reaction pattern may emerge.
Conclusion
Granulomatous PPD may comprise an underrecognized variant of PPD in cases when only a superficial biopsy is evaluated. Clinicians and pathologists should be aware of this variant, and in refractory cases of PPD, deeper sampling may be warranted to identify granulomas.
1. Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
2. Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. London, England: Mosby; 2008:321-330.
3. Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
4. Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
5. Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
6. Lin WL, Kou TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia [published online ahead of print May 29, 2007]. Clin Exp Dermatol. 2007;32:513-515.
7. Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
8. Toro JR, Sander CA, LeBoit PE. Persistent pigmented dermatoses and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
Pigmented purpuric dermatoses (PPDs) are a group of common chronic disorders characterized by speckled, cayenne pepper–like petechiae and orange-brown discoloration of the skin resulting from capillaritis.1 Pigmented purpuric dermatoses typically occur in the absence of underlying vascular insufficiency or other hematologic dysfunction. The 5 well-known clinicopathologic variants of PPD include Schamberg disease; purpura annularis telangiectodes of Majocchi; pigmented purpuric lichenoid dermatitis of Gougerot and Blum; eczematoidlike purpura of Doucas and Kapetanakis; and lichen aureus.2 All PPDs share common characteristic clinical and histologic features. Clinically, patients generally present with symmetric petechiae and/or pigmented macules. All 5 PPD variants share similar histologic findings, including a vasculocentric lymphocytic infiltrate in the papillary dermis, swelling of the endothelial cells, erythrocyte extravasation, and often hemosiderin-laden macrophages.1 Despite these clinical and histopathologic similarities, each variant contains additional distinctive features, such as telangiectasia (purpura annularis telangiectodes of Majocchi), a lichenoid infiltrate (pigmented purpuric lichenoid dermatitis of Gougerot and Blum), eczematous changes (eczematoidlike purpura of Doucas and Kapetanakis), and marked hemosiderin deposition (lichen aureus).
Granulomatous pigmented purpuric dermatosis (GPPD) is a rare variant of PPD.3-7 Clinically, these lesions appear similar to other PPDs; however, in addition to the characteristic changes associated with conventional PPD, histologic examination of GPPD reveals a granulomatous inflammatory reaction pattern. Although the pathogenesis of GPPD is not well understood, its association with hyperlipidemia may suggest a granulomatous response to capillaritis mediated by lipid deposition in the microvasculature.6,7
We present 3 cases of GPPD and provide a review of the literature. In all of our patients, biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin by standard methodologies, and all stains were performed on sections by standard methodologies. Based on a PubMed search of articles indexed for MEDLINE using the terms granulomatous pigmented purpuric dermatosis, sarcoidosis, pigmented purpuric dermatosis, granulomas, and pigmented purpuric dermatosis, we review 5 additional reports describing 10 total patients.3-7
Case Reports
Patient 1
A 9-year-old white boy presented with a 3-cm asymptomatic light brown patch with a nonblanching violaceous center on the right posterior thigh that was studded with pinpoint yellow papules (Figure, A). The lesion appeared 3 to 4 years prior to presentation but had become progressively darker and centrally indurated over the last 2 years. The patient and his mother denied any history of trauma to the area. His medical history was unremarkable, and his current medications included fish oil and multivitamin tablets.
Histologic examination of a punch biopsy specimen taken from the center of the lesion revealed a lichenoid lymphohistiocytic infiltrate with marked red blood cell (RBC) extravasation and associated hemosiderin-laden macrophages. The lymphocytes comprising this infiltrate lacked cytologic atypia and exhibited minimal epidermotropism (Figure, B). Additionally, there was a superficial and deep perivascular mononuclear inflammatory infiltrate intermixed with numerous small granulomas comprised ofepithelioid histiocytes in the mid and deep dermis (Figure, C). Periodic acid–Schiff, acid-fast bacilli (AFB), and Fite stains were negative for organisms. Polarization was negative for refractile foreign material. Due to the patient’s age, no treatment was performed, and the lesion remains unchanged 1 year after biopsy.
Patient 2
A 49-year-old white woman presented with a 2-cm yellow-brown patch with a faint, nonblanchable, violaceous center on the right lateral thigh of 4 months’ duration. The patch initially appeared as a small asymptomatic purple papule. The patient denied any history of trauma to the area. A purified protein derivative (tuberculin) skin test was negative at the time of examination. The patient’s medical history was remarkable for renal calculi. Her current medications included progesterone; estradiol; lansoprazole; prenatal vitamins; vitamins C and E; zinc; and calcium. The patient had no family history of sarcoidosis. Complete blood cell count, urinalysis, liver function tests, and angiotensin-converting enzyme levels were unremarkable. Pulmonary function tests were normal, and there was no evidence of sarcoidosis on chest radiography. Initial biopsy of the lesion revealed a perivascular and interstitial lymphohistiocytic infiltrate with abundant extravasated RBCs in the papillary dermis (Figure, D). Similar to patient 1, the infiltrate lacked cytologic atypia and did not involve the overlying epidermis. There was perivascular granulomatous inflammation in the mid dermis (Figure, E). Periodic acid–Schiff, Warthin-Starry, and AFB stains were negative for organisms. Polarization was negative for refractile foreign material.
The lesion was treated with clobetasol propionate ointment 0.05% twice daily for 6 weeks with transient improvement, but the lesion recurred upon treatment cessation. Subsequent treatment with intralesional triamcinolone resulted in slight improvement of the lesion. A therapeutic trial of targeted pulsed dye laser treatment was ineffective. The lesion gradually increased in size over the next year with no therapy, and a repeat biopsy revealed a lichenoid lymphohistiocytic infiltrate with abundant extravasated RBCs consistent with persistent PPD. A granulomatous infiltrate was not evident in the superficial shave biopsy specimen.
Patient 3
A 75-year-old white woman presented with scattered, speckled, cayenne pepper–like, red-brown macules on the legs. Two years prior to presentation, a few scattered symmetrical macules appeared on the dorsal aspects of the feet, which gradually increased in number to form larger confluent patches that spread to the lower legs. The patient denied itching or burning but reported that the patches became painful when scratched and were aggravated by sun exposure. Her medical history was remarkable for asthma, chronic renal insufficiency, coronary artery disease, Barrett esophagus, obstructive sleep apnea, hypothyroidism, renal calculi, type 2 diabetes mellitus, and hyperlipidemia. Her current medications included carvedilol, valsartan, levothyroxine, aspirin, clopidogrel, furosemide, nitrofurantoin, temazepam, insulin, ezetimibe-simvastatin, and lansoprazole. Computed tomography of the chest revealed no signs of sarcoidosis. Pulmonary function tests revealed moderate obstructive lung disease. An ophthalmology examination showed no evidence of sarcoidosis. Laboratory results revealed an elevated glucose, blood urea nitrogen, creatinine, and triglyceride levels, as well as low hematocrit and vitamin D levels. Urinalysis, thyroid-stimulating hormone (thyrotropin) test, liver function tests, angiotensin-converting enzyme test, hepatitis B surface antigen, and IFN-g release assay were normal.
Histologic examination of a punch biopsy specimen revealed an inflammatory infiltrate confined to the papillary dermis. This infiltrate was comprised of an admixture of lymphocytes and histiocytes in a perivascular distribution with associated RBC extravasation and intimately associated granulomas (Figure, F). Additional inflammation in the deeper aspects of the dermis was not identified. Periodic acid–Schiff, AFB, and Fite stains were negative for organisms. Polarization was negative for refractile foreign material.

| 
| 
| ||

| 
| 
| ||
| A 3-cm asymptomatic light brown patch with a nonblanching violaceous center on the right posterior thigh that was studded with pinpoint yellow papules (A). Lichenoid lymphohistiocytic infiltrate in the papillary dermis with marked red blood cell extravasation (B)(H&E, original magnification ×20). Superficial and deep perivascular mononuclear inflammatory infiltrate intermixed with numerous small granulomas comprised of epithelioid histiocytes in the mid and deep dermis (C)(H&E, original magnification ×20). Perivascular and interstitial lymphohistiocytic infiltrate with abundant extravasated red blood cells in the papillary dermis (D)(H&E, original magnification ×10). Perivascular lymphohistiocytic inflammation with epithelioid granulomas in the mid dermis (E)(H&E, original magnification ×20). Lymphohistiocytic inflammation in the papillary dermis comprised of an admixture of lymphocytes and histiocytes in a perivascular distribution with associated red blood cell extravasation and intimately associated granulomas (F)(H&E, original magnification ×20). | ||||
The patient was treated with topical steroids and minocycline 50 mg twice daily without improvement. The lesions improved after the patient underwent treatment with oral corticosteroids for pulmonary disease.
Comment
Pigmented purpuric dermatoses comprise a spectrum of clinical and pathologic conditions.1,2 Granulomatous PPD is a much less common variant, characterized by a granulomatous infiltrate admixed with PPD. We report 3 additional cases and review the literature on this rare and interesting variant of PPD.
We noted several unifying clinicopathologic features among our patients and those previously reported in the literature (Table).3-7 Including our cases, our review yielded 13 GPPD patients ranging in age from 9 to 75 years, with a mean age of 49.1 years. Two of our patients—patients 1 and 3—were the youngest and oldest patients, respectively, among the cases we reviewed. The majority of the cases we reviewed included patients of East Asian descent (6 Taiwanese; 2 Japanese; 1 Korean) as well as 4 white patients. No distinctive gender predilection was apparent, as our review included 8 females and 5 males.
Our review revealed that GPPD lesions typically involve the lower extremities and usually are asymptomatic, with the exception of occasional pruritus. Additional lesions have been reported on the dorsal aspect of the hands, and 1 case noted exclusive involvement of the wrist.6 Lesions of GPPD can range in their clinical appearance. Three of 13 patients presented with purpuric papules and 2 had brown pigmentation with hemorrhagic papules3,4,6; the remaining 8 patients had erythematous or brown macules, papules, or plaques.5-7 The most commonly associated disease condition was hyperlipidemia, which was reported in 7 of 13 cases.5-7 Additional reported comorbidities included meningioma, renal calculi, obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, hepatitis C virus, ulcerative colitis, thrombocytopenia, and hyperuricemia. Reported serologic abnormalities included a rare positive antinuclear antibody, rheumatoid factor, and cryoglobulins.3,6 Therapeutic efficacy in the management of GPPD has not been well described; however, for the rare cases in which therapies were described, they were largely unsuccessful, with 1 patient exhibiting spontaneous improvement.3,4
Granulomatous PPD also appears to exhibit a range of histologic findings. All cases of GPPD shared fundamental components, such as a brisk perivascular infiltrate accompanied by RBC extravasation with variable hemosiderin-laden macrophages and a granulomatous infiltrate. All of the reports we reviewed described an intimate association between these components, with the granulomas being essentially superimposed on typical PPD. As for other types of PPD, obvious vasculitis characterized by a vasculocentric inflammatory infiltrate and evidence of vascular injury, such as fibrinoid necrosis of the vessel wall, has not been described in GPPD.3-7 Finally, histologic changes suggestive of a relationship with cutaneous T-cell lymphoma, cytologic atypia, and epidermotropism have been described for some forms of PPD but have not been described for GPPD.3-8
Our case reports expand the histologic spectrum of GPPD. Although patient 3 exhibited a relatively intimate association of granulomas and PPD, 2 of our cases (patients 1 and 2) demonstrated a granulomatous infiltrate in the mid to deep dermis, which is separate from the more superficially situated lichenoid lymphohistiocytic infiltrate, RBC extravasation, and hemosiderin-laden macrophages noted in the papillary dermis. Considered along with the absence of an obvious clinicopathologic explanation for the granulomatous infiltrates (eg, polarizable material, infectious organisms, systemic disease), these 2 cases (patients 1 and 2) suggest a composite form of PPD that combines the lichenoid pattern of PPD of Gougerot and Blum with a deep granulomatous component of GPPD. The importance of this distinction lies in the potential for physicians to overlook this potentially informative histologic pattern if only a superficial biopsy is performed. The clinical relevance is unclear; however, in our experience, it has been challenging to treat this relatively small subset of patients who have exhibited a limited response to treatment with topical steroids, intralesional steroids, pulsed dye laser, and vitamin supplementation.
The cause of the granulomatous infiltrate in GPPD is poorly understood. Seven of 13 cases included in our review occurred in patients with a history of hyperlipidemia.5-7 Some have postulated that the constellation of findings of GPPD in hyperlipidemic patients reflects an underlying vascular injury process induced by lipid deposition in the endothelial cells with subsequent RBC extravasation and a secondary granulomatous response to the lipid deposits.6,7 However, given the occurrence of GPPD in patients without hyperlipidemia, other mechanisms also must be considered in the pathogenesis of GPPD, including a reaction to medications, systemic diseases, and infectious etiologies (eg, hepatitis B virus).4,6 As additional cases are described in the literature, other unifying clinical etiologies for this histopathologic reaction pattern may emerge.
Conclusion
Granulomatous PPD may comprise an underrecognized variant of PPD in cases when only a superficial biopsy is evaluated. Clinicians and pathologists should be aware of this variant, and in refractory cases of PPD, deeper sampling may be warranted to identify granulomas.
Pigmented purpuric dermatoses (PPDs) are a group of common chronic disorders characterized by speckled, cayenne pepper–like petechiae and orange-brown discoloration of the skin resulting from capillaritis.1 Pigmented purpuric dermatoses typically occur in the absence of underlying vascular insufficiency or other hematologic dysfunction. The 5 well-known clinicopathologic variants of PPD include Schamberg disease; purpura annularis telangiectodes of Majocchi; pigmented purpuric lichenoid dermatitis of Gougerot and Blum; eczematoidlike purpura of Doucas and Kapetanakis; and lichen aureus.2 All PPDs share common characteristic clinical and histologic features. Clinically, patients generally present with symmetric petechiae and/or pigmented macules. All 5 PPD variants share similar histologic findings, including a vasculocentric lymphocytic infiltrate in the papillary dermis, swelling of the endothelial cells, erythrocyte extravasation, and often hemosiderin-laden macrophages.1 Despite these clinical and histopathologic similarities, each variant contains additional distinctive features, such as telangiectasia (purpura annularis telangiectodes of Majocchi), a lichenoid infiltrate (pigmented purpuric lichenoid dermatitis of Gougerot and Blum), eczematous changes (eczematoidlike purpura of Doucas and Kapetanakis), and marked hemosiderin deposition (lichen aureus).
Granulomatous pigmented purpuric dermatosis (GPPD) is a rare variant of PPD.3-7 Clinically, these lesions appear similar to other PPDs; however, in addition to the characteristic changes associated with conventional PPD, histologic examination of GPPD reveals a granulomatous inflammatory reaction pattern. Although the pathogenesis of GPPD is not well understood, its association with hyperlipidemia may suggest a granulomatous response to capillaritis mediated by lipid deposition in the microvasculature.6,7
We present 3 cases of GPPD and provide a review of the literature. In all of our patients, biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin by standard methodologies, and all stains were performed on sections by standard methodologies. Based on a PubMed search of articles indexed for MEDLINE using the terms granulomatous pigmented purpuric dermatosis, sarcoidosis, pigmented purpuric dermatosis, granulomas, and pigmented purpuric dermatosis, we review 5 additional reports describing 10 total patients.3-7
Case Reports
Patient 1
A 9-year-old white boy presented with a 3-cm asymptomatic light brown patch with a nonblanching violaceous center on the right posterior thigh that was studded with pinpoint yellow papules (Figure, A). The lesion appeared 3 to 4 years prior to presentation but had become progressively darker and centrally indurated over the last 2 years. The patient and his mother denied any history of trauma to the area. His medical history was unremarkable, and his current medications included fish oil and multivitamin tablets.
Histologic examination of a punch biopsy specimen taken from the center of the lesion revealed a lichenoid lymphohistiocytic infiltrate with marked red blood cell (RBC) extravasation and associated hemosiderin-laden macrophages. The lymphocytes comprising this infiltrate lacked cytologic atypia and exhibited minimal epidermotropism (Figure, B). Additionally, there was a superficial and deep perivascular mononuclear inflammatory infiltrate intermixed with numerous small granulomas comprised ofepithelioid histiocytes in the mid and deep dermis (Figure, C). Periodic acid–Schiff, acid-fast bacilli (AFB), and Fite stains were negative for organisms. Polarization was negative for refractile foreign material. Due to the patient’s age, no treatment was performed, and the lesion remains unchanged 1 year after biopsy.
Patient 2
A 49-year-old white woman presented with a 2-cm yellow-brown patch with a faint, nonblanchable, violaceous center on the right lateral thigh of 4 months’ duration. The patch initially appeared as a small asymptomatic purple papule. The patient denied any history of trauma to the area. A purified protein derivative (tuberculin) skin test was negative at the time of examination. The patient’s medical history was remarkable for renal calculi. Her current medications included progesterone; estradiol; lansoprazole; prenatal vitamins; vitamins C and E; zinc; and calcium. The patient had no family history of sarcoidosis. Complete blood cell count, urinalysis, liver function tests, and angiotensin-converting enzyme levels were unremarkable. Pulmonary function tests were normal, and there was no evidence of sarcoidosis on chest radiography. Initial biopsy of the lesion revealed a perivascular and interstitial lymphohistiocytic infiltrate with abundant extravasated RBCs in the papillary dermis (Figure, D). Similar to patient 1, the infiltrate lacked cytologic atypia and did not involve the overlying epidermis. There was perivascular granulomatous inflammation in the mid dermis (Figure, E). Periodic acid–Schiff, Warthin-Starry, and AFB stains were negative for organisms. Polarization was negative for refractile foreign material.
The lesion was treated with clobetasol propionate ointment 0.05% twice daily for 6 weeks with transient improvement, but the lesion recurred upon treatment cessation. Subsequent treatment with intralesional triamcinolone resulted in slight improvement of the lesion. A therapeutic trial of targeted pulsed dye laser treatment was ineffective. The lesion gradually increased in size over the next year with no therapy, and a repeat biopsy revealed a lichenoid lymphohistiocytic infiltrate with abundant extravasated RBCs consistent with persistent PPD. A granulomatous infiltrate was not evident in the superficial shave biopsy specimen.
Patient 3
A 75-year-old white woman presented with scattered, speckled, cayenne pepper–like, red-brown macules on the legs. Two years prior to presentation, a few scattered symmetrical macules appeared on the dorsal aspects of the feet, which gradually increased in number to form larger confluent patches that spread to the lower legs. The patient denied itching or burning but reported that the patches became painful when scratched and were aggravated by sun exposure. Her medical history was remarkable for asthma, chronic renal insufficiency, coronary artery disease, Barrett esophagus, obstructive sleep apnea, hypothyroidism, renal calculi, type 2 diabetes mellitus, and hyperlipidemia. Her current medications included carvedilol, valsartan, levothyroxine, aspirin, clopidogrel, furosemide, nitrofurantoin, temazepam, insulin, ezetimibe-simvastatin, and lansoprazole. Computed tomography of the chest revealed no signs of sarcoidosis. Pulmonary function tests revealed moderate obstructive lung disease. An ophthalmology examination showed no evidence of sarcoidosis. Laboratory results revealed an elevated glucose, blood urea nitrogen, creatinine, and triglyceride levels, as well as low hematocrit and vitamin D levels. Urinalysis, thyroid-stimulating hormone (thyrotropin) test, liver function tests, angiotensin-converting enzyme test, hepatitis B surface antigen, and IFN-g release assay were normal.
Histologic examination of a punch biopsy specimen revealed an inflammatory infiltrate confined to the papillary dermis. This infiltrate was comprised of an admixture of lymphocytes and histiocytes in a perivascular distribution with associated RBC extravasation and intimately associated granulomas (Figure, F). Additional inflammation in the deeper aspects of the dermis was not identified. Periodic acid–Schiff, AFB, and Fite stains were negative for organisms. Polarization was negative for refractile foreign material.

| 
| 
| ||

| 
| 
| ||
| A 3-cm asymptomatic light brown patch with a nonblanching violaceous center on the right posterior thigh that was studded with pinpoint yellow papules (A). Lichenoid lymphohistiocytic infiltrate in the papillary dermis with marked red blood cell extravasation (B)(H&E, original magnification ×20). Superficial and deep perivascular mononuclear inflammatory infiltrate intermixed with numerous small granulomas comprised of epithelioid histiocytes in the mid and deep dermis (C)(H&E, original magnification ×20). Perivascular and interstitial lymphohistiocytic infiltrate with abundant extravasated red blood cells in the papillary dermis (D)(H&E, original magnification ×10). Perivascular lymphohistiocytic inflammation with epithelioid granulomas in the mid dermis (E)(H&E, original magnification ×20). Lymphohistiocytic inflammation in the papillary dermis comprised of an admixture of lymphocytes and histiocytes in a perivascular distribution with associated red blood cell extravasation and intimately associated granulomas (F)(H&E, original magnification ×20). | ||||
The patient was treated with topical steroids and minocycline 50 mg twice daily without improvement. The lesions improved after the patient underwent treatment with oral corticosteroids for pulmonary disease.
Comment
Pigmented purpuric dermatoses comprise a spectrum of clinical and pathologic conditions.1,2 Granulomatous PPD is a much less common variant, characterized by a granulomatous infiltrate admixed with PPD. We report 3 additional cases and review the literature on this rare and interesting variant of PPD.
We noted several unifying clinicopathologic features among our patients and those previously reported in the literature (Table).3-7 Including our cases, our review yielded 13 GPPD patients ranging in age from 9 to 75 years, with a mean age of 49.1 years. Two of our patients—patients 1 and 3—were the youngest and oldest patients, respectively, among the cases we reviewed. The majority of the cases we reviewed included patients of East Asian descent (6 Taiwanese; 2 Japanese; 1 Korean) as well as 4 white patients. No distinctive gender predilection was apparent, as our review included 8 females and 5 males.
Our review revealed that GPPD lesions typically involve the lower extremities and usually are asymptomatic, with the exception of occasional pruritus. Additional lesions have been reported on the dorsal aspect of the hands, and 1 case noted exclusive involvement of the wrist.6 Lesions of GPPD can range in their clinical appearance. Three of 13 patients presented with purpuric papules and 2 had brown pigmentation with hemorrhagic papules3,4,6; the remaining 8 patients had erythematous or brown macules, papules, or plaques.5-7 The most commonly associated disease condition was hyperlipidemia, which was reported in 7 of 13 cases.5-7 Additional reported comorbidities included meningioma, renal calculi, obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, hepatitis C virus, ulcerative colitis, thrombocytopenia, and hyperuricemia. Reported serologic abnormalities included a rare positive antinuclear antibody, rheumatoid factor, and cryoglobulins.3,6 Therapeutic efficacy in the management of GPPD has not been well described; however, for the rare cases in which therapies were described, they were largely unsuccessful, with 1 patient exhibiting spontaneous improvement.3,4
Granulomatous PPD also appears to exhibit a range of histologic findings. All cases of GPPD shared fundamental components, such as a brisk perivascular infiltrate accompanied by RBC extravasation with variable hemosiderin-laden macrophages and a granulomatous infiltrate. All of the reports we reviewed described an intimate association between these components, with the granulomas being essentially superimposed on typical PPD. As for other types of PPD, obvious vasculitis characterized by a vasculocentric inflammatory infiltrate and evidence of vascular injury, such as fibrinoid necrosis of the vessel wall, has not been described in GPPD.3-7 Finally, histologic changes suggestive of a relationship with cutaneous T-cell lymphoma, cytologic atypia, and epidermotropism have been described for some forms of PPD but have not been described for GPPD.3-8
Our case reports expand the histologic spectrum of GPPD. Although patient 3 exhibited a relatively intimate association of granulomas and PPD, 2 of our cases (patients 1 and 2) demonstrated a granulomatous infiltrate in the mid to deep dermis, which is separate from the more superficially situated lichenoid lymphohistiocytic infiltrate, RBC extravasation, and hemosiderin-laden macrophages noted in the papillary dermis. Considered along with the absence of an obvious clinicopathologic explanation for the granulomatous infiltrates (eg, polarizable material, infectious organisms, systemic disease), these 2 cases (patients 1 and 2) suggest a composite form of PPD that combines the lichenoid pattern of PPD of Gougerot and Blum with a deep granulomatous component of GPPD. The importance of this distinction lies in the potential for physicians to overlook this potentially informative histologic pattern if only a superficial biopsy is performed. The clinical relevance is unclear; however, in our experience, it has been challenging to treat this relatively small subset of patients who have exhibited a limited response to treatment with topical steroids, intralesional steroids, pulsed dye laser, and vitamin supplementation.
The cause of the granulomatous infiltrate in GPPD is poorly understood. Seven of 13 cases included in our review occurred in patients with a history of hyperlipidemia.5-7 Some have postulated that the constellation of findings of GPPD in hyperlipidemic patients reflects an underlying vascular injury process induced by lipid deposition in the endothelial cells with subsequent RBC extravasation and a secondary granulomatous response to the lipid deposits.6,7 However, given the occurrence of GPPD in patients without hyperlipidemia, other mechanisms also must be considered in the pathogenesis of GPPD, including a reaction to medications, systemic diseases, and infectious etiologies (eg, hepatitis B virus).4,6 As additional cases are described in the literature, other unifying clinical etiologies for this histopathologic reaction pattern may emerge.
Conclusion
Granulomatous PPD may comprise an underrecognized variant of PPD in cases when only a superficial biopsy is evaluated. Clinicians and pathologists should be aware of this variant, and in refractory cases of PPD, deeper sampling may be warranted to identify granulomas.
1. Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
2. Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. London, England: Mosby; 2008:321-330.
3. Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
4. Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
5. Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
6. Lin WL, Kou TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia [published online ahead of print May 29, 2007]. Clin Exp Dermatol. 2007;32:513-515.
7. Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
8. Toro JR, Sander CA, LeBoit PE. Persistent pigmented dermatoses and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
1. Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
2. Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. London, England: Mosby; 2008:321-330.
3. Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
4. Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
5. Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
6. Lin WL, Kou TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia [published online ahead of print May 29, 2007]. Clin Exp Dermatol. 2007;32:513-515.
7. Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
8. Toro JR, Sander CA, LeBoit PE. Persistent pigmented dermatoses and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
Practice Points
- Consider a punch biopsy when sampling suspected inflammatory dermatoses, such as pigmented purpuric dermatosis, to allow deeper sampling.
- Provide all clinical details to the dermatopathologist to assist with clinicopathologic correlation and diagnostic accuracy.
Should LCZ696 receive a level I indication?
LAS VEGAS – Additional findings from the landmark PARADIGM-HF trial presented at the annual meeting of the Heart Failure Society of America provided what many observers deemed a persuasive case for the novel angiotensin receptor neprilysin inhibitor known for now as LCZ696 as deserving of a level I indication in the next update of the major heart failure management guidelines.
At a special session added late to the meeting program in the wake of the spectacularly positive top-line results of PARADIGM-HF presented just a few weeks earlier at the European Society of Cardiology meeting in Barcelona, an international panel of heart failure heavyweights tackled questions about the study’s implications, including whether the results need replication in a second randomized controlled trial before LCZ696 can win regulatory approval. And once approved, should guidelines committees give it a level I, must-use indication? How applicable are the PARADIGM-HF results to the broader population of heart failure patients, and in particular black patients and older individuals with class III/IV heart failure? And what about patients with heart failure with preserved ejection fraction (HFpEF) ?
In other words, does PARADIGM-HF, with more than 8,400 randomized subjects, represent a true paradigm shift in heart failure management?
For coprincipal investigator Dr. Milton Packer, the answer is a resounding yes.
“For the past 25 years, the magnitude of the effect of ACE inhibitors on cardiovascular mortality – about an 18% reduction – has created an ethical mandate for their use in all patients with chronic heart failure who could tolerate treatment with these drugs. The finding that LCZ696 has a 20% greater effect on cardiovascular mortality than ACE inhibitors strongly supports the conclusion that LCZ696 should replace the current use of ACE inhibitors and angiotensin receptor blockers in the management of chronic heart failure,” said Dr. Packer, professor and chair of the department of clinical sciences at University of Texas Southwestern Medical Center, Dallas.
His coprincipal investigator, Dr. John J.V. McMurray, cited the statistical strength of the PARADIGM-HF results for the primary composite outcome of cardiovascular death or heart failure hospitalization, which had an extraordinary P value of .0000004, in making the case that the trial findings are sufficient to win regulatory approval without a confirmatory study.
He noted that the regulatory standard in the United States and Europe is that a positive clinical trial having a P value of less than .05 requires replication in a second study that also yields outcomes with a P value of less than .05.
“If, however, you have a large single trial, you can win approval by meeting a standard of P less than .00125. The strength of the result of PARADIGM-HF, with a P of .0000004, is equivalent to between four and five single trials replicated at P less than .05. And for the endpoint of cardiovascular mortality, where the PARADIGM-HF result was significant at a P of .00008, that’s equivalent to between two and three trials replicated at P less than .05. So in my view PARADIGM-HF easily meets the criteria for a level IA indication,” said Dr. McMurray, professor of cardiology at the University of Glasgow.
He presented for the first time a new analysis with a major wow factor. This was an imputed placebo analysis providing the answer to a question many cardiologists have asked him since the presentation of the top-line PARADIGM-HF results in Barcelona: namely, how would LCZ696 have stacked up in a placebo-controlled trial?
Such a study wouldn’t be ethical now, of course, but it’s possible to make inferences by comparing LCZ696’s superiority to enalapril at 10 mg b.i.d. in PARADIGM-HF to enalapril’s performance at the same dose relative to placebo in the earlier 2,569-patient SOLVD-Treatment trial, which featured the same composite primary endpoint (N. Engl. J. Med. 1991;325:293-302).
In SOLVD-Treatment, enalapril resulted in a 28% relative risk reduction in the composite endpoint, compared with placebo. Through indirect comparison, LCZ696 would have an imputed 43% relative risk reduction, compared with placebo. For the endpoint of cardiovascular mortality, enalapril showed a 17% risk reduction relative to placebo; when the PARADIGM-HF results are factored in, this translates to an inferred 34% relative risk reduction for LCZ696 versus placebo.
Similarly, in the CHARM-Alternative trial (Lancet 2003;362:772-6), which featured 2,028 patients on more contemporary guideline–recommended background therapy than in SOLVD-Treatment, patients on the angiotensin receptor blocker candesartan showed a 23% relative risk reduction in the composite endpoint, compared with placebo, along with a 15% reduction in cardiovascular mortality. In the imputed placebo analysis, this translated to relative risk reductions of 49% and 34%, respectively, for LCZ696 versus placebo.
“We see a doubling in the reduction in cardiovascular mortality with this new therapy over and above that obtained with an ACE inhibitor or ARB [angiotensin receptor blocker],” Dr. McMurray emphasized.
Panelist Dr. Lynne W. Stevenson wasn’t convinced.
“I don’t believe it is time to replace ACE inhibitors and ARBs. I don’t think LCZ696 is ready for a level I [treatment should be performed] indication; that is a higher bar. ... I think we could see a level IIa [treatment is reasonable to perform] indication based on the strong results that we’ve seen,” said Dr. Stevenson, director of the heart failure and cardiomyopathy program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
She estimated that fewer than 10% of U.S. heart failure patients fit the description of PARADIGM-HF participants, with mild to moderate heart failure with reduced ejection fraction. Importantly, the run-in process employed in the study ensured that only patients with a demonstrated ability to tolerate enalapril in therapeutic doses were enrolled. And even in that filtered population, there was a substantial dropout rate in the LCZ696 arm due to hypotension during follow-up.
“I certainly don’t think we have any information about patients newly diagnosed with heart failure. I don’t think if you put new heart failure patients on LCZ696, they’d necessarily be able to stand up, and if they could stand up I’m not sure we could get them on the appropriate dose of beta blockers,” Dr. Stevenson added.
Noting that only 5% of PARADIGM-HF participants were black, she said that “clearly this is something we will need to watch as we get more experience with this drug, but there was no signal of concern.”
Dr. Marvin A. Konstam, professor of medicine at Tufts University, Boston, shared one of Dr. Stevenson’s concerns: “How do we know what will happen with ACE inhibitor virgins in the real world where you don’t get a run-in period?”
Panelist Dr. John G.F. Cleland said, “I don’t want to second-guess the guideline committees, but surely this must be a IA [data derived from multiple clinical trials or meta-analyses] indication. What intrigues me is what will the indication for ACE inhibitors look like in future guidelines? Is it also going to be IA in the same group of patients? That’s something the guidelines committees are going to have to sort out.”
“A lot of these questions and people’s concerns will either be increased or reduced once we start to get the medicine into clinical practice. What I find quite distressing is that we might be sitting here at this time next year and still not be in a position to prescribe this agent because it may still be going through the regulatory process,” said Dr. Cleland, professor of cardiology at the University of Hull (England).
LAS VEGAS – Additional findings from the landmark PARADIGM-HF trial presented at the annual meeting of the Heart Failure Society of America provided what many observers deemed a persuasive case for the novel angiotensin receptor neprilysin inhibitor known for now as LCZ696 as deserving of a level I indication in the next update of the major heart failure management guidelines.
At a special session added late to the meeting program in the wake of the spectacularly positive top-line results of PARADIGM-HF presented just a few weeks earlier at the European Society of Cardiology meeting in Barcelona, an international panel of heart failure heavyweights tackled questions about the study’s implications, including whether the results need replication in a second randomized controlled trial before LCZ696 can win regulatory approval. And once approved, should guidelines committees give it a level I, must-use indication? How applicable are the PARADIGM-HF results to the broader population of heart failure patients, and in particular black patients and older individuals with class III/IV heart failure? And what about patients with heart failure with preserved ejection fraction (HFpEF) ?
In other words, does PARADIGM-HF, with more than 8,400 randomized subjects, represent a true paradigm shift in heart failure management?
For coprincipal investigator Dr. Milton Packer, the answer is a resounding yes.
“For the past 25 years, the magnitude of the effect of ACE inhibitors on cardiovascular mortality – about an 18% reduction – has created an ethical mandate for their use in all patients with chronic heart failure who could tolerate treatment with these drugs. The finding that LCZ696 has a 20% greater effect on cardiovascular mortality than ACE inhibitors strongly supports the conclusion that LCZ696 should replace the current use of ACE inhibitors and angiotensin receptor blockers in the management of chronic heart failure,” said Dr. Packer, professor and chair of the department of clinical sciences at University of Texas Southwestern Medical Center, Dallas.
His coprincipal investigator, Dr. John J.V. McMurray, cited the statistical strength of the PARADIGM-HF results for the primary composite outcome of cardiovascular death or heart failure hospitalization, which had an extraordinary P value of .0000004, in making the case that the trial findings are sufficient to win regulatory approval without a confirmatory study.
He noted that the regulatory standard in the United States and Europe is that a positive clinical trial having a P value of less than .05 requires replication in a second study that also yields outcomes with a P value of less than .05.
“If, however, you have a large single trial, you can win approval by meeting a standard of P less than .00125. The strength of the result of PARADIGM-HF, with a P of .0000004, is equivalent to between four and five single trials replicated at P less than .05. And for the endpoint of cardiovascular mortality, where the PARADIGM-HF result was significant at a P of .00008, that’s equivalent to between two and three trials replicated at P less than .05. So in my view PARADIGM-HF easily meets the criteria for a level IA indication,” said Dr. McMurray, professor of cardiology at the University of Glasgow.
He presented for the first time a new analysis with a major wow factor. This was an imputed placebo analysis providing the answer to a question many cardiologists have asked him since the presentation of the top-line PARADIGM-HF results in Barcelona: namely, how would LCZ696 have stacked up in a placebo-controlled trial?
Such a study wouldn’t be ethical now, of course, but it’s possible to make inferences by comparing LCZ696’s superiority to enalapril at 10 mg b.i.d. in PARADIGM-HF to enalapril’s performance at the same dose relative to placebo in the earlier 2,569-patient SOLVD-Treatment trial, which featured the same composite primary endpoint (N. Engl. J. Med. 1991;325:293-302).
In SOLVD-Treatment, enalapril resulted in a 28% relative risk reduction in the composite endpoint, compared with placebo. Through indirect comparison, LCZ696 would have an imputed 43% relative risk reduction, compared with placebo. For the endpoint of cardiovascular mortality, enalapril showed a 17% risk reduction relative to placebo; when the PARADIGM-HF results are factored in, this translates to an inferred 34% relative risk reduction for LCZ696 versus placebo.
Similarly, in the CHARM-Alternative trial (Lancet 2003;362:772-6), which featured 2,028 patients on more contemporary guideline–recommended background therapy than in SOLVD-Treatment, patients on the angiotensin receptor blocker candesartan showed a 23% relative risk reduction in the composite endpoint, compared with placebo, along with a 15% reduction in cardiovascular mortality. In the imputed placebo analysis, this translated to relative risk reductions of 49% and 34%, respectively, for LCZ696 versus placebo.
“We see a doubling in the reduction in cardiovascular mortality with this new therapy over and above that obtained with an ACE inhibitor or ARB [angiotensin receptor blocker],” Dr. McMurray emphasized.
Panelist Dr. Lynne W. Stevenson wasn’t convinced.
“I don’t believe it is time to replace ACE inhibitors and ARBs. I don’t think LCZ696 is ready for a level I [treatment should be performed] indication; that is a higher bar. ... I think we could see a level IIa [treatment is reasonable to perform] indication based on the strong results that we’ve seen,” said Dr. Stevenson, director of the heart failure and cardiomyopathy program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
She estimated that fewer than 10% of U.S. heart failure patients fit the description of PARADIGM-HF participants, with mild to moderate heart failure with reduced ejection fraction. Importantly, the run-in process employed in the study ensured that only patients with a demonstrated ability to tolerate enalapril in therapeutic doses were enrolled. And even in that filtered population, there was a substantial dropout rate in the LCZ696 arm due to hypotension during follow-up.
“I certainly don’t think we have any information about patients newly diagnosed with heart failure. I don’t think if you put new heart failure patients on LCZ696, they’d necessarily be able to stand up, and if they could stand up I’m not sure we could get them on the appropriate dose of beta blockers,” Dr. Stevenson added.
Noting that only 5% of PARADIGM-HF participants were black, she said that “clearly this is something we will need to watch as we get more experience with this drug, but there was no signal of concern.”
Dr. Marvin A. Konstam, professor of medicine at Tufts University, Boston, shared one of Dr. Stevenson’s concerns: “How do we know what will happen with ACE inhibitor virgins in the real world where you don’t get a run-in period?”
Panelist Dr. John G.F. Cleland said, “I don’t want to second-guess the guideline committees, but surely this must be a IA [data derived from multiple clinical trials or meta-analyses] indication. What intrigues me is what will the indication for ACE inhibitors look like in future guidelines? Is it also going to be IA in the same group of patients? That’s something the guidelines committees are going to have to sort out.”
“A lot of these questions and people’s concerns will either be increased or reduced once we start to get the medicine into clinical practice. What I find quite distressing is that we might be sitting here at this time next year and still not be in a position to prescribe this agent because it may still be going through the regulatory process,” said Dr. Cleland, professor of cardiology at the University of Hull (England).
LAS VEGAS – Additional findings from the landmark PARADIGM-HF trial presented at the annual meeting of the Heart Failure Society of America provided what many observers deemed a persuasive case for the novel angiotensin receptor neprilysin inhibitor known for now as LCZ696 as deserving of a level I indication in the next update of the major heart failure management guidelines.
At a special session added late to the meeting program in the wake of the spectacularly positive top-line results of PARADIGM-HF presented just a few weeks earlier at the European Society of Cardiology meeting in Barcelona, an international panel of heart failure heavyweights tackled questions about the study’s implications, including whether the results need replication in a second randomized controlled trial before LCZ696 can win regulatory approval. And once approved, should guidelines committees give it a level I, must-use indication? How applicable are the PARADIGM-HF results to the broader population of heart failure patients, and in particular black patients and older individuals with class III/IV heart failure? And what about patients with heart failure with preserved ejection fraction (HFpEF) ?
In other words, does PARADIGM-HF, with more than 8,400 randomized subjects, represent a true paradigm shift in heart failure management?
For coprincipal investigator Dr. Milton Packer, the answer is a resounding yes.
“For the past 25 years, the magnitude of the effect of ACE inhibitors on cardiovascular mortality – about an 18% reduction – has created an ethical mandate for their use in all patients with chronic heart failure who could tolerate treatment with these drugs. The finding that LCZ696 has a 20% greater effect on cardiovascular mortality than ACE inhibitors strongly supports the conclusion that LCZ696 should replace the current use of ACE inhibitors and angiotensin receptor blockers in the management of chronic heart failure,” said Dr. Packer, professor and chair of the department of clinical sciences at University of Texas Southwestern Medical Center, Dallas.
His coprincipal investigator, Dr. John J.V. McMurray, cited the statistical strength of the PARADIGM-HF results for the primary composite outcome of cardiovascular death or heart failure hospitalization, which had an extraordinary P value of .0000004, in making the case that the trial findings are sufficient to win regulatory approval without a confirmatory study.
He noted that the regulatory standard in the United States and Europe is that a positive clinical trial having a P value of less than .05 requires replication in a second study that also yields outcomes with a P value of less than .05.
“If, however, you have a large single trial, you can win approval by meeting a standard of P less than .00125. The strength of the result of PARADIGM-HF, with a P of .0000004, is equivalent to between four and five single trials replicated at P less than .05. And for the endpoint of cardiovascular mortality, where the PARADIGM-HF result was significant at a P of .00008, that’s equivalent to between two and three trials replicated at P less than .05. So in my view PARADIGM-HF easily meets the criteria for a level IA indication,” said Dr. McMurray, professor of cardiology at the University of Glasgow.
He presented for the first time a new analysis with a major wow factor. This was an imputed placebo analysis providing the answer to a question many cardiologists have asked him since the presentation of the top-line PARADIGM-HF results in Barcelona: namely, how would LCZ696 have stacked up in a placebo-controlled trial?
Such a study wouldn’t be ethical now, of course, but it’s possible to make inferences by comparing LCZ696’s superiority to enalapril at 10 mg b.i.d. in PARADIGM-HF to enalapril’s performance at the same dose relative to placebo in the earlier 2,569-patient SOLVD-Treatment trial, which featured the same composite primary endpoint (N. Engl. J. Med. 1991;325:293-302).
In SOLVD-Treatment, enalapril resulted in a 28% relative risk reduction in the composite endpoint, compared with placebo. Through indirect comparison, LCZ696 would have an imputed 43% relative risk reduction, compared with placebo. For the endpoint of cardiovascular mortality, enalapril showed a 17% risk reduction relative to placebo; when the PARADIGM-HF results are factored in, this translates to an inferred 34% relative risk reduction for LCZ696 versus placebo.
Similarly, in the CHARM-Alternative trial (Lancet 2003;362:772-6), which featured 2,028 patients on more contemporary guideline–recommended background therapy than in SOLVD-Treatment, patients on the angiotensin receptor blocker candesartan showed a 23% relative risk reduction in the composite endpoint, compared with placebo, along with a 15% reduction in cardiovascular mortality. In the imputed placebo analysis, this translated to relative risk reductions of 49% and 34%, respectively, for LCZ696 versus placebo.
“We see a doubling in the reduction in cardiovascular mortality with this new therapy over and above that obtained with an ACE inhibitor or ARB [angiotensin receptor blocker],” Dr. McMurray emphasized.
Panelist Dr. Lynne W. Stevenson wasn’t convinced.
“I don’t believe it is time to replace ACE inhibitors and ARBs. I don’t think LCZ696 is ready for a level I [treatment should be performed] indication; that is a higher bar. ... I think we could see a level IIa [treatment is reasonable to perform] indication based on the strong results that we’ve seen,” said Dr. Stevenson, director of the heart failure and cardiomyopathy program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
She estimated that fewer than 10% of U.S. heart failure patients fit the description of PARADIGM-HF participants, with mild to moderate heart failure with reduced ejection fraction. Importantly, the run-in process employed in the study ensured that only patients with a demonstrated ability to tolerate enalapril in therapeutic doses were enrolled. And even in that filtered population, there was a substantial dropout rate in the LCZ696 arm due to hypotension during follow-up.
“I certainly don’t think we have any information about patients newly diagnosed with heart failure. I don’t think if you put new heart failure patients on LCZ696, they’d necessarily be able to stand up, and if they could stand up I’m not sure we could get them on the appropriate dose of beta blockers,” Dr. Stevenson added.
Noting that only 5% of PARADIGM-HF participants were black, she said that “clearly this is something we will need to watch as we get more experience with this drug, but there was no signal of concern.”
Dr. Marvin A. Konstam, professor of medicine at Tufts University, Boston, shared one of Dr. Stevenson’s concerns: “How do we know what will happen with ACE inhibitor virgins in the real world where you don’t get a run-in period?”
Panelist Dr. John G.F. Cleland said, “I don’t want to second-guess the guideline committees, but surely this must be a IA [data derived from multiple clinical trials or meta-analyses] indication. What intrigues me is what will the indication for ACE inhibitors look like in future guidelines? Is it also going to be IA in the same group of patients? That’s something the guidelines committees are going to have to sort out.”
“A lot of these questions and people’s concerns will either be increased or reduced once we start to get the medicine into clinical practice. What I find quite distressing is that we might be sitting here at this time next year and still not be in a position to prescribe this agent because it may still be going through the regulatory process,” said Dr. Cleland, professor of cardiology at the University of Hull (England).
EXPERT OPINION FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Digital Dermatology: Online service recovery
We can learn a lot from a car rentals. Like medicine, they are a service industry. And all service industries have the same problem: Service is delivered in real time, and the quality of that service depends on variables that may or may not be in the company’s control.
Even so, one bad experience can result in termination of a life-long customer, or in our case, patient. Worse, the patient can now go online and write a scathing review, criticizing everything from your bedside manner to the artwork in your waiting room.
What can you do when a visit goes wrong? Employ service-recovery techniques. Service recovery is the act of trying to resuscitate an encounter once things have gone badly. It happens to physicians and to restaurants and to car rentals.
While on vacation with my wife in Salt Lake City, we rented a car from a company (let’s call them “Discount Cars”). We don’t usually book with them; however, we got double airline points for choosing them, so we bit.
At the airport rental terminal, we waited for 15 minutes before being helped. When we reached the counter, we were told that our reserved car was not ready yet. (I immediately thought of the Seinfeld episode when Jerry says: “So you can take a reservation, but you can’t keep a reservation?!”) We were advised that our car was being washed and would be ready in 15 minutes “tops.” Thirty minutes later, my miffed wife pushed through the line to the counter. “It’s still being washed,” she was told. So she asked for another car and was offered a full-size pickup truck. My wife, who drives a teeny Honda Fit at home, said no thanks. Another 30 minutes passed and my incensed wife returned to the counter. “It’s been over an hour! This is unacceptable!” A different representative replied it was our fault for declining the pickup truck. There would be more cars soon, so they promised.
We were too far to walk to any airport bars, and the situation was rapidly deteriorating. I decided to take action. I fired up Twitter and let her rip:
“Closing in on 1 hr for a car promised in 15 min. Which we reserved ahead. This isn’t the first time, @DiscountCars #operations #fail.”
Within minutes, they replied by Twitter:
Them: @Dermdoc We are so sorry for the wait! What location are you at?
My wife, along with five other equally incensed wives, continued to wait for a response (and a car) from the live representatives at the counter.
Nearly 1 hour and 20 minutes later, we got a car. It was much larger than we wanted, but we were done waiting. After signing the papers, we got inside – it reeked of smoke. Oh, this is no bueno, I thought. We requested a different car. Twenty more minutes passed before our smoke-free vehicle arrived. The gas tank was 7/8’s full. And the carpets were littered with twigs and leaves.
Now I’m thinking, this is so bad, I should write an article about it. From the front seat of our faulty but moving vehicle, I fired again: “Dear @DiscountCars we waited 1+ hrs. Not the car we wanted. Then tank not full. Yet, not a single apology from anyone. Really?”
Them: @Dermdoc, we are sorry.
Them: @Dermdoc Please e-mail us the details and your RA# to [email protected] so we can look into this for you!
Me: @Discount Thank you! Will do.
I sent a list of grievances to the e-mail as they requested. Within an hour they offered us a $50 credit on a future rental.
What’s remarkable about this story is that not a single live person was able to assuage us, but their digital team managed to apologize and save us as customers. There might have been legitimate reasons for their service failure, but it didn’t matter. What mattered was that they responded to me personally, apologized, and made amends. This is an important lesson for us physicians. Patients will expect that your digital channels are legitimate ways to express their level of satisfaction with your practice. The stakes are higher for us in health care in particular because of the risks of violating patients’ privacy. However, as you can see from the rental car example, it can effectively be done without revealing any information about the customer or the experience. The goal is to recover the service publicly and take all of the information offline and manage it in a secure, private fashion.
The formula is simple: Believe the customer. Listen. Apologize for not satisfying the customer. Even if you’ve done nothing wrong, you have in some way failed to satisfy the customer’s needs. Ask for more information in a secure, private manner, never on a public platform. Do what you can reasonably do to remedy the problem and remediate the situation.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
We can learn a lot from a car rentals. Like medicine, they are a service industry. And all service industries have the same problem: Service is delivered in real time, and the quality of that service depends on variables that may or may not be in the company’s control.
Even so, one bad experience can result in termination of a life-long customer, or in our case, patient. Worse, the patient can now go online and write a scathing review, criticizing everything from your bedside manner to the artwork in your waiting room.
What can you do when a visit goes wrong? Employ service-recovery techniques. Service recovery is the act of trying to resuscitate an encounter once things have gone badly. It happens to physicians and to restaurants and to car rentals.
While on vacation with my wife in Salt Lake City, we rented a car from a company (let’s call them “Discount Cars”). We don’t usually book with them; however, we got double airline points for choosing them, so we bit.
At the airport rental terminal, we waited for 15 minutes before being helped. When we reached the counter, we were told that our reserved car was not ready yet. (I immediately thought of the Seinfeld episode when Jerry says: “So you can take a reservation, but you can’t keep a reservation?!”) We were advised that our car was being washed and would be ready in 15 minutes “tops.” Thirty minutes later, my miffed wife pushed through the line to the counter. “It’s still being washed,” she was told. So she asked for another car and was offered a full-size pickup truck. My wife, who drives a teeny Honda Fit at home, said no thanks. Another 30 minutes passed and my incensed wife returned to the counter. “It’s been over an hour! This is unacceptable!” A different representative replied it was our fault for declining the pickup truck. There would be more cars soon, so they promised.
We were too far to walk to any airport bars, and the situation was rapidly deteriorating. I decided to take action. I fired up Twitter and let her rip:
“Closing in on 1 hr for a car promised in 15 min. Which we reserved ahead. This isn’t the first time, @DiscountCars #operations #fail.”
Within minutes, they replied by Twitter:
Them: @Dermdoc We are so sorry for the wait! What location are you at?
My wife, along with five other equally incensed wives, continued to wait for a response (and a car) from the live representatives at the counter.
Nearly 1 hour and 20 minutes later, we got a car. It was much larger than we wanted, but we were done waiting. After signing the papers, we got inside – it reeked of smoke. Oh, this is no bueno, I thought. We requested a different car. Twenty more minutes passed before our smoke-free vehicle arrived. The gas tank was 7/8’s full. And the carpets were littered with twigs and leaves.
Now I’m thinking, this is so bad, I should write an article about it. From the front seat of our faulty but moving vehicle, I fired again: “Dear @DiscountCars we waited 1+ hrs. Not the car we wanted. Then tank not full. Yet, not a single apology from anyone. Really?”
Them: @Dermdoc, we are sorry.
Them: @Dermdoc Please e-mail us the details and your RA# to [email protected] so we can look into this for you!
Me: @Discount Thank you! Will do.
I sent a list of grievances to the e-mail as they requested. Within an hour they offered us a $50 credit on a future rental.
What’s remarkable about this story is that not a single live person was able to assuage us, but their digital team managed to apologize and save us as customers. There might have been legitimate reasons for their service failure, but it didn’t matter. What mattered was that they responded to me personally, apologized, and made amends. This is an important lesson for us physicians. Patients will expect that your digital channels are legitimate ways to express their level of satisfaction with your practice. The stakes are higher for us in health care in particular because of the risks of violating patients’ privacy. However, as you can see from the rental car example, it can effectively be done without revealing any information about the customer or the experience. The goal is to recover the service publicly and take all of the information offline and manage it in a secure, private fashion.
The formula is simple: Believe the customer. Listen. Apologize for not satisfying the customer. Even if you’ve done nothing wrong, you have in some way failed to satisfy the customer’s needs. Ask for more information in a secure, private manner, never on a public platform. Do what you can reasonably do to remedy the problem and remediate the situation.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
We can learn a lot from a car rentals. Like medicine, they are a service industry. And all service industries have the same problem: Service is delivered in real time, and the quality of that service depends on variables that may or may not be in the company’s control.
Even so, one bad experience can result in termination of a life-long customer, or in our case, patient. Worse, the patient can now go online and write a scathing review, criticizing everything from your bedside manner to the artwork in your waiting room.
What can you do when a visit goes wrong? Employ service-recovery techniques. Service recovery is the act of trying to resuscitate an encounter once things have gone badly. It happens to physicians and to restaurants and to car rentals.
While on vacation with my wife in Salt Lake City, we rented a car from a company (let’s call them “Discount Cars”). We don’t usually book with them; however, we got double airline points for choosing them, so we bit.
At the airport rental terminal, we waited for 15 minutes before being helped. When we reached the counter, we were told that our reserved car was not ready yet. (I immediately thought of the Seinfeld episode when Jerry says: “So you can take a reservation, but you can’t keep a reservation?!”) We were advised that our car was being washed and would be ready in 15 minutes “tops.” Thirty minutes later, my miffed wife pushed through the line to the counter. “It’s still being washed,” she was told. So she asked for another car and was offered a full-size pickup truck. My wife, who drives a teeny Honda Fit at home, said no thanks. Another 30 minutes passed and my incensed wife returned to the counter. “It’s been over an hour! This is unacceptable!” A different representative replied it was our fault for declining the pickup truck. There would be more cars soon, so they promised.
We were too far to walk to any airport bars, and the situation was rapidly deteriorating. I decided to take action. I fired up Twitter and let her rip:
“Closing in on 1 hr for a car promised in 15 min. Which we reserved ahead. This isn’t the first time, @DiscountCars #operations #fail.”
Within minutes, they replied by Twitter:
Them: @Dermdoc We are so sorry for the wait! What location are you at?
My wife, along with five other equally incensed wives, continued to wait for a response (and a car) from the live representatives at the counter.
Nearly 1 hour and 20 minutes later, we got a car. It was much larger than we wanted, but we were done waiting. After signing the papers, we got inside – it reeked of smoke. Oh, this is no bueno, I thought. We requested a different car. Twenty more minutes passed before our smoke-free vehicle arrived. The gas tank was 7/8’s full. And the carpets were littered with twigs and leaves.
Now I’m thinking, this is so bad, I should write an article about it. From the front seat of our faulty but moving vehicle, I fired again: “Dear @DiscountCars we waited 1+ hrs. Not the car we wanted. Then tank not full. Yet, not a single apology from anyone. Really?”
Them: @Dermdoc, we are sorry.
Them: @Dermdoc Please e-mail us the details and your RA# to [email protected] so we can look into this for you!
Me: @Discount Thank you! Will do.
I sent a list of grievances to the e-mail as they requested. Within an hour they offered us a $50 credit on a future rental.
What’s remarkable about this story is that not a single live person was able to assuage us, but their digital team managed to apologize and save us as customers. There might have been legitimate reasons for their service failure, but it didn’t matter. What mattered was that they responded to me personally, apologized, and made amends. This is an important lesson for us physicians. Patients will expect that your digital channels are legitimate ways to express their level of satisfaction with your practice. The stakes are higher for us in health care in particular because of the risks of violating patients’ privacy. However, as you can see from the rental car example, it can effectively be done without revealing any information about the customer or the experience. The goal is to recover the service publicly and take all of the information offline and manage it in a secure, private fashion.
The formula is simple: Believe the customer. Listen. Apologize for not satisfying the customer. Even if you’ve done nothing wrong, you have in some way failed to satisfy the customer’s needs. Ask for more information in a secure, private manner, never on a public platform. Do what you can reasonably do to remedy the problem and remediate the situation.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Hypercalcemia From Diuretics and Vitamin D
African Americans have higher rates of high blood pressure and lower levels of 25-hydroxyvitamin D, compared with those of whites, and may be prescribed both thiazide diuretics and vitamin D supplements concurrently. But with thiazide diuretics, the kidneys excrete less calcium, and with vitamin D, the intestines absorb more calcium. Is there a risk of hypercalcemia for these patients?
To find out, researchers from Brigham and Women’s Hospital, Harvard Medical School and School of Public Health, Massachusetts General Hospital, and Dana-Farber Cancer Institute, all in Boston, Massachusetts; Michigan State University in East Lansing; Washington University School of Medicine in St. Louis, Missouri; Duke University in Durham, North Carolina; and Medical University of South Carolina in Charleston assigned 328 healthy African American volunteers to receive 1,000 IU, 2,000 IU, or 4,000 IU of vitamin D or placebo once a day for 3 months during the winters from 2007 to 2010. Of the participants, 84 were taking hydrochlorothiazide (HCTZ) and had serum calcium levels assessed. A comparison group of 44 participants who were not taking HCTZ had serum calcium measurements at 3 months but not at baseline. Participants were assessed for adverse events in person at the beginning of each month and by telephone during the second week of each month.
Five of the participants taking HCTZ had a serum calcium level above the upper limit of normal. The 4 participants who had hypercalcemia during month 1 were asked to stop taking the study medication and were withdrawn from the study.
Only 5.9% of the participants taking concurrent HCTZ and vitamin D developed hypercalcemia. At 1 month, 3 of the HCTZ participants in the 1,000 IU group and 1 in the 2,000 IU group had hypercalcemia. After 1 month of the vitamin D supplementation, 4 participants taking HCTZ had modestly elevated serum calcium levels, ranging from 10.7 mg/dL to 11.0 mg/dL. At 3 months, only 1 HCTZ participant had elevated calcium. The late appearance of 1 case of hypercalcemia may mean hypercalcemia can occur later in therapy, or it may have been a random event, the researchers say.
This is the first analysis to directly assess the effect of concurrent use of vitamin D and HCTZ in otherwise healthy adults with hypertension. Although the optimal plasma levels of vitamin D have yet to be established, their study is critical, the researchers say, because lower doses of vitamin D may not be enough to correct the vitamin D deficiency common in African Americans.
Source
Chandler PD, Scott JB, Drake BF, et al. Am J Med. 2014;127(8):772-778.
doi: 10.1016/j.amjmed.2014.02.044.
African Americans have higher rates of high blood pressure and lower levels of 25-hydroxyvitamin D, compared with those of whites, and may be prescribed both thiazide diuretics and vitamin D supplements concurrently. But with thiazide diuretics, the kidneys excrete less calcium, and with vitamin D, the intestines absorb more calcium. Is there a risk of hypercalcemia for these patients?
To find out, researchers from Brigham and Women’s Hospital, Harvard Medical School and School of Public Health, Massachusetts General Hospital, and Dana-Farber Cancer Institute, all in Boston, Massachusetts; Michigan State University in East Lansing; Washington University School of Medicine in St. Louis, Missouri; Duke University in Durham, North Carolina; and Medical University of South Carolina in Charleston assigned 328 healthy African American volunteers to receive 1,000 IU, 2,000 IU, or 4,000 IU of vitamin D or placebo once a day for 3 months during the winters from 2007 to 2010. Of the participants, 84 were taking hydrochlorothiazide (HCTZ) and had serum calcium levels assessed. A comparison group of 44 participants who were not taking HCTZ had serum calcium measurements at 3 months but not at baseline. Participants were assessed for adverse events in person at the beginning of each month and by telephone during the second week of each month.
Five of the participants taking HCTZ had a serum calcium level above the upper limit of normal. The 4 participants who had hypercalcemia during month 1 were asked to stop taking the study medication and were withdrawn from the study.
Only 5.9% of the participants taking concurrent HCTZ and vitamin D developed hypercalcemia. At 1 month, 3 of the HCTZ participants in the 1,000 IU group and 1 in the 2,000 IU group had hypercalcemia. After 1 month of the vitamin D supplementation, 4 participants taking HCTZ had modestly elevated serum calcium levels, ranging from 10.7 mg/dL to 11.0 mg/dL. At 3 months, only 1 HCTZ participant had elevated calcium. The late appearance of 1 case of hypercalcemia may mean hypercalcemia can occur later in therapy, or it may have been a random event, the researchers say.
This is the first analysis to directly assess the effect of concurrent use of vitamin D and HCTZ in otherwise healthy adults with hypertension. Although the optimal plasma levels of vitamin D have yet to be established, their study is critical, the researchers say, because lower doses of vitamin D may not be enough to correct the vitamin D deficiency common in African Americans.
Source
Chandler PD, Scott JB, Drake BF, et al. Am J Med. 2014;127(8):772-778.
doi: 10.1016/j.amjmed.2014.02.044.
African Americans have higher rates of high blood pressure and lower levels of 25-hydroxyvitamin D, compared with those of whites, and may be prescribed both thiazide diuretics and vitamin D supplements concurrently. But with thiazide diuretics, the kidneys excrete less calcium, and with vitamin D, the intestines absorb more calcium. Is there a risk of hypercalcemia for these patients?
To find out, researchers from Brigham and Women’s Hospital, Harvard Medical School and School of Public Health, Massachusetts General Hospital, and Dana-Farber Cancer Institute, all in Boston, Massachusetts; Michigan State University in East Lansing; Washington University School of Medicine in St. Louis, Missouri; Duke University in Durham, North Carolina; and Medical University of South Carolina in Charleston assigned 328 healthy African American volunteers to receive 1,000 IU, 2,000 IU, or 4,000 IU of vitamin D or placebo once a day for 3 months during the winters from 2007 to 2010. Of the participants, 84 were taking hydrochlorothiazide (HCTZ) and had serum calcium levels assessed. A comparison group of 44 participants who were not taking HCTZ had serum calcium measurements at 3 months but not at baseline. Participants were assessed for adverse events in person at the beginning of each month and by telephone during the second week of each month.
Five of the participants taking HCTZ had a serum calcium level above the upper limit of normal. The 4 participants who had hypercalcemia during month 1 were asked to stop taking the study medication and were withdrawn from the study.
Only 5.9% of the participants taking concurrent HCTZ and vitamin D developed hypercalcemia. At 1 month, 3 of the HCTZ participants in the 1,000 IU group and 1 in the 2,000 IU group had hypercalcemia. After 1 month of the vitamin D supplementation, 4 participants taking HCTZ had modestly elevated serum calcium levels, ranging from 10.7 mg/dL to 11.0 mg/dL. At 3 months, only 1 HCTZ participant had elevated calcium. The late appearance of 1 case of hypercalcemia may mean hypercalcemia can occur later in therapy, or it may have been a random event, the researchers say.
This is the first analysis to directly assess the effect of concurrent use of vitamin D and HCTZ in otherwise healthy adults with hypertension. Although the optimal plasma levels of vitamin D have yet to be established, their study is critical, the researchers say, because lower doses of vitamin D may not be enough to correct the vitamin D deficiency common in African Americans.
Source
Chandler PD, Scott JB, Drake BF, et al. Am J Med. 2014;127(8):772-778.
doi: 10.1016/j.amjmed.2014.02.044.
A single cell can cause MPNs

Credit: Aaron Logan
Scientists have found that a single cell containing the JAK2-V617F mutation can cause myeloproliferative neoplasms (MPNs) in mice.
The team isolated hematopoietic stem cells (HSCs) from malignant MPNs and transplanted a single cell with mutated JAK2 into groups of healthy mice.
A subset of the mice developed MPNs, which also bore the JAK2 mutation.
The researchers described this work in The Journal of Experimental Medicine.
Pontus Lundberg, PhD, of University Hospital Basel in Switzerland, and his colleagues performed several sets of experiments in which they transplanted JAK2-V617F-expressing cells in dilutions of wild-type bone marrow cells.
In the 1:125 dilution experiment, 24% of mice (4/17) developed polycythemia vera (PV). In the 1:250 dilution experiment, 44% of mice developed either PV or essential thrombocythemia (ET).
The researchers were interested to find that erythrocytosis and thrombocytosis were mutually exclusive in individual mice.
Additional investigation revealed that MPN development did not depend on the acquisition of additional somatic mutations.
To confirm their findings, the researchers then transplanted a single JAK2-V617F-expressing HSC into a total of 113 mice in 4 independent experiments.
Only 1 mouse developed an ET phenotype. Two other mice had transient high chimerism in erythrocytes and/or platelets, but they did not develop ET or PV.
Furthermore, transplanting bone marrow from the mouse with the ET phenotype into secondary recipients did not result in an MPN phenotype.
The researchers also analyzed JAK2-V617F-expressing HSCs in further detail, and they found these HSCs promoted cell division and increased DNA damage.
Higher JAK2-V617F expression was associated with a short-term HSC signature and increased myeloid bias. Lower JAK2-V617F expression was associated with the capacity to stably engraft in secondary recipients.
Dr Lundberg and his colleagues said this research shows that JAK2-V617F has complex effects on HSC biology and that MPNs can develop from a single JAK2-V617F-expressing cell. ![]()

Credit: Aaron Logan
Scientists have found that a single cell containing the JAK2-V617F mutation can cause myeloproliferative neoplasms (MPNs) in mice.
The team isolated hematopoietic stem cells (HSCs) from malignant MPNs and transplanted a single cell with mutated JAK2 into groups of healthy mice.
A subset of the mice developed MPNs, which also bore the JAK2 mutation.
The researchers described this work in The Journal of Experimental Medicine.
Pontus Lundberg, PhD, of University Hospital Basel in Switzerland, and his colleagues performed several sets of experiments in which they transplanted JAK2-V617F-expressing cells in dilutions of wild-type bone marrow cells.
In the 1:125 dilution experiment, 24% of mice (4/17) developed polycythemia vera (PV). In the 1:250 dilution experiment, 44% of mice developed either PV or essential thrombocythemia (ET).
The researchers were interested to find that erythrocytosis and thrombocytosis were mutually exclusive in individual mice.
Additional investigation revealed that MPN development did not depend on the acquisition of additional somatic mutations.
To confirm their findings, the researchers then transplanted a single JAK2-V617F-expressing HSC into a total of 113 mice in 4 independent experiments.
Only 1 mouse developed an ET phenotype. Two other mice had transient high chimerism in erythrocytes and/or platelets, but they did not develop ET or PV.
Furthermore, transplanting bone marrow from the mouse with the ET phenotype into secondary recipients did not result in an MPN phenotype.
The researchers also analyzed JAK2-V617F-expressing HSCs in further detail, and they found these HSCs promoted cell division and increased DNA damage.
Higher JAK2-V617F expression was associated with a short-term HSC signature and increased myeloid bias. Lower JAK2-V617F expression was associated with the capacity to stably engraft in secondary recipients.
Dr Lundberg and his colleagues said this research shows that JAK2-V617F has complex effects on HSC biology and that MPNs can develop from a single JAK2-V617F-expressing cell. ![]()

Credit: Aaron Logan
Scientists have found that a single cell containing the JAK2-V617F mutation can cause myeloproliferative neoplasms (MPNs) in mice.
The team isolated hematopoietic stem cells (HSCs) from malignant MPNs and transplanted a single cell with mutated JAK2 into groups of healthy mice.
A subset of the mice developed MPNs, which also bore the JAK2 mutation.
The researchers described this work in The Journal of Experimental Medicine.
Pontus Lundberg, PhD, of University Hospital Basel in Switzerland, and his colleagues performed several sets of experiments in which they transplanted JAK2-V617F-expressing cells in dilutions of wild-type bone marrow cells.
In the 1:125 dilution experiment, 24% of mice (4/17) developed polycythemia vera (PV). In the 1:250 dilution experiment, 44% of mice developed either PV or essential thrombocythemia (ET).
The researchers were interested to find that erythrocytosis and thrombocytosis were mutually exclusive in individual mice.
Additional investigation revealed that MPN development did not depend on the acquisition of additional somatic mutations.
To confirm their findings, the researchers then transplanted a single JAK2-V617F-expressing HSC into a total of 113 mice in 4 independent experiments.
Only 1 mouse developed an ET phenotype. Two other mice had transient high chimerism in erythrocytes and/or platelets, but they did not develop ET or PV.
Furthermore, transplanting bone marrow from the mouse with the ET phenotype into secondary recipients did not result in an MPN phenotype.
The researchers also analyzed JAK2-V617F-expressing HSCs in further detail, and they found these HSCs promoted cell division and increased DNA damage.
Higher JAK2-V617F expression was associated with a short-term HSC signature and increased myeloid bias. Lower JAK2-V617F expression was associated with the capacity to stably engraft in secondary recipients.
Dr Lundberg and his colleagues said this research shows that JAK2-V617F has complex effects on HSC biology and that MPNs can develop from a single JAK2-V617F-expressing cell. ![]()
Survey shows lack of adherence to safety guidelines

Credit: Bill Branson
Healthcare professionals do not consistently follow the recommended safe handling practices for antineoplastic drugs, according to a survey published in the Journal of Occupational and Environmental Hygiene.
Researchers surveyed more than 2000 healthcare workers and found that a majority do not always use the recommended personal protective equipment when they are administering antineoplastic drugs.
Furthermore, some respondents reported spills or leaks of a drug during administration, and a small percentage said they had experienced skin
contact with an antineoplastic drug.
“Chemotherapy drugs save lives of cancer patients but also can result in adverse health outcomes in workers who are exposed to these drugs, including cancer, reproductive problems, and organ damage when recommended safe handling guidelines are not followed,” said John Howard, MD, director of the National Institute for Occupational Safety and Health (NIOSH).
NIOSH researchers conducted this study, which included 2069 healthcare personnel who completed the 2011 Health and Safety Practices Survey of Healthcare Workers.
Results showed that, despite the longstanding availability of authoritative safe handling guidelines (ASHP, NIOSH, ONS, OSHA), recommended exposure controls were not always used.
For example, 80% of respondents said they do not always wear 2 pairs of chemotherapy gloves, and 15% said they don’t always wear a single pair.
Forty-two percent of respondents said they don’t always wear a nonabsorbent gown with a closed front and tight-fitting cuffs, and 12% had taken home potentially contaminated clothing.
Twelve percent of respondents reported a spill or leak of an antineoplastic drug during administration, and 4% reported skin contact with an antineoplastic drug.
Six percent of respondents said they primed intravenous tubing with an antineoplastic drug instead of a non-drug containing liquid, and 12% said the pharmacy department followed this practice.
Taking these and other findings into account, the researchers concluded that better risk communication is needed to ensure that employers and employees are fully aware of the hazards and the availability of precautionary measures to minimize exposure to antineoplastic drugs. ![]()

Credit: Bill Branson
Healthcare professionals do not consistently follow the recommended safe handling practices for antineoplastic drugs, according to a survey published in the Journal of Occupational and Environmental Hygiene.
Researchers surveyed more than 2000 healthcare workers and found that a majority do not always use the recommended personal protective equipment when they are administering antineoplastic drugs.
Furthermore, some respondents reported spills or leaks of a drug during administration, and a small percentage said they had experienced skin
contact with an antineoplastic drug.
“Chemotherapy drugs save lives of cancer patients but also can result in adverse health outcomes in workers who are exposed to these drugs, including cancer, reproductive problems, and organ damage when recommended safe handling guidelines are not followed,” said John Howard, MD, director of the National Institute for Occupational Safety and Health (NIOSH).
NIOSH researchers conducted this study, which included 2069 healthcare personnel who completed the 2011 Health and Safety Practices Survey of Healthcare Workers.
Results showed that, despite the longstanding availability of authoritative safe handling guidelines (ASHP, NIOSH, ONS, OSHA), recommended exposure controls were not always used.
For example, 80% of respondents said they do not always wear 2 pairs of chemotherapy gloves, and 15% said they don’t always wear a single pair.
Forty-two percent of respondents said they don’t always wear a nonabsorbent gown with a closed front and tight-fitting cuffs, and 12% had taken home potentially contaminated clothing.
Twelve percent of respondents reported a spill or leak of an antineoplastic drug during administration, and 4% reported skin contact with an antineoplastic drug.
Six percent of respondents said they primed intravenous tubing with an antineoplastic drug instead of a non-drug containing liquid, and 12% said the pharmacy department followed this practice.
Taking these and other findings into account, the researchers concluded that better risk communication is needed to ensure that employers and employees are fully aware of the hazards and the availability of precautionary measures to minimize exposure to antineoplastic drugs. ![]()

Credit: Bill Branson
Healthcare professionals do not consistently follow the recommended safe handling practices for antineoplastic drugs, according to a survey published in the Journal of Occupational and Environmental Hygiene.
Researchers surveyed more than 2000 healthcare workers and found that a majority do not always use the recommended personal protective equipment when they are administering antineoplastic drugs.
Furthermore, some respondents reported spills or leaks of a drug during administration, and a small percentage said they had experienced skin
contact with an antineoplastic drug.
“Chemotherapy drugs save lives of cancer patients but also can result in adverse health outcomes in workers who are exposed to these drugs, including cancer, reproductive problems, and organ damage when recommended safe handling guidelines are not followed,” said John Howard, MD, director of the National Institute for Occupational Safety and Health (NIOSH).
NIOSH researchers conducted this study, which included 2069 healthcare personnel who completed the 2011 Health and Safety Practices Survey of Healthcare Workers.
Results showed that, despite the longstanding availability of authoritative safe handling guidelines (ASHP, NIOSH, ONS, OSHA), recommended exposure controls were not always used.
For example, 80% of respondents said they do not always wear 2 pairs of chemotherapy gloves, and 15% said they don’t always wear a single pair.
Forty-two percent of respondents said they don’t always wear a nonabsorbent gown with a closed front and tight-fitting cuffs, and 12% had taken home potentially contaminated clothing.
Twelve percent of respondents reported a spill or leak of an antineoplastic drug during administration, and 4% reported skin contact with an antineoplastic drug.
Six percent of respondents said they primed intravenous tubing with an antineoplastic drug instead of a non-drug containing liquid, and 12% said the pharmacy department followed this practice.
Taking these and other findings into account, the researchers concluded that better risk communication is needed to ensure that employers and employees are fully aware of the hazards and the availability of precautionary measures to minimize exposure to antineoplastic drugs. ![]()
Animal studies help explain chemo brain

californica
releasing inkafter being disturbed
Results of preclinical research appear to explain how the anticancer agent doxorubicin can cause chemo brain.
Neuroscientists conducted experiments in cells from rats and Aplysia californica, a marine mollusk that has many of the same memory mechanisms as humans.
This revealed memory mechanisms that are inhibited by doxorubicin, as well as a method of unblocking these mechanisms—administering a drug known as SB203580.
“Our research has implications in the care of people given to cognitive deficits following drug treatment for cancer,” said John H. Byrne, PhD, of the University of Texas Health Medical School.
He added that understanding how drugs like doxorubicin impact the brain is an important first step in alleviating chemo brain, which is characterized by forgetfulness, trouble concentrating, and difficulty multitasking.
Dr Byrne and his colleagues explained this first step in The Journal of Neuroscience.
The researchers knew that, in non-neuronal cells, doxorubicin inhibits the expression of MAPK phosphatases, thereby inhibiting the dephosphorylation of ERK and p38 MAPK, 2 MAPK isoforms that are important for long-term memory.
To evaluate doxorubicin’s effects on levels of phosphorylated ERK and p38 MAPK, the team used cultures of cortical neurons from rats and sensory neurons from Aplysia californica.
Experiments showed that doxorubicin elevated levels of phosphorylated ERK and phosphorylated p38 MAPK in sensory neurons and cortical neurons. In addition, the drug increased phosphorylation of the downstream transcriptional repressor CREB2 in sensory neurons.
The researchers also assessed doxorubicin’s effects on long-term enhanced excitability, long-term synaptic facilitation, and long-term
synaptic depression.
They found that doxorubicin enhanced long-term synaptic depression induced by the neuropeptide Phe-Met-Arg-Phe-NH2. And the drug inhibited long-term synaptic facilitation induced by serotonin.
However, the researchers were able to restore long-term synaptic facilitation with SB203580, an inhibitor of p38 MAPK.
Unfortunately, SB203580 would not be appropriate for human use, Dr Byrne noted, adding that his team would like to identify other drugs that might have the same effect as SB203580.
The researchers also hope to determine if doxorubicin works the same way in humans as it did in these experiments. ![]()

californica
releasing inkafter being disturbed
Results of preclinical research appear to explain how the anticancer agent doxorubicin can cause chemo brain.
Neuroscientists conducted experiments in cells from rats and Aplysia californica, a marine mollusk that has many of the same memory mechanisms as humans.
This revealed memory mechanisms that are inhibited by doxorubicin, as well as a method of unblocking these mechanisms—administering a drug known as SB203580.
“Our research has implications in the care of people given to cognitive deficits following drug treatment for cancer,” said John H. Byrne, PhD, of the University of Texas Health Medical School.
He added that understanding how drugs like doxorubicin impact the brain is an important first step in alleviating chemo brain, which is characterized by forgetfulness, trouble concentrating, and difficulty multitasking.
Dr Byrne and his colleagues explained this first step in The Journal of Neuroscience.
The researchers knew that, in non-neuronal cells, doxorubicin inhibits the expression of MAPK phosphatases, thereby inhibiting the dephosphorylation of ERK and p38 MAPK, 2 MAPK isoforms that are important for long-term memory.
To evaluate doxorubicin’s effects on levels of phosphorylated ERK and p38 MAPK, the team used cultures of cortical neurons from rats and sensory neurons from Aplysia californica.
Experiments showed that doxorubicin elevated levels of phosphorylated ERK and phosphorylated p38 MAPK in sensory neurons and cortical neurons. In addition, the drug increased phosphorylation of the downstream transcriptional repressor CREB2 in sensory neurons.
The researchers also assessed doxorubicin’s effects on long-term enhanced excitability, long-term synaptic facilitation, and long-term
synaptic depression.
They found that doxorubicin enhanced long-term synaptic depression induced by the neuropeptide Phe-Met-Arg-Phe-NH2. And the drug inhibited long-term synaptic facilitation induced by serotonin.
However, the researchers were able to restore long-term synaptic facilitation with SB203580, an inhibitor of p38 MAPK.
Unfortunately, SB203580 would not be appropriate for human use, Dr Byrne noted, adding that his team would like to identify other drugs that might have the same effect as SB203580.
The researchers also hope to determine if doxorubicin works the same way in humans as it did in these experiments. ![]()

californica
releasing inkafter being disturbed
Results of preclinical research appear to explain how the anticancer agent doxorubicin can cause chemo brain.
Neuroscientists conducted experiments in cells from rats and Aplysia californica, a marine mollusk that has many of the same memory mechanisms as humans.
This revealed memory mechanisms that are inhibited by doxorubicin, as well as a method of unblocking these mechanisms—administering a drug known as SB203580.
“Our research has implications in the care of people given to cognitive deficits following drug treatment for cancer,” said John H. Byrne, PhD, of the University of Texas Health Medical School.
He added that understanding how drugs like doxorubicin impact the brain is an important first step in alleviating chemo brain, which is characterized by forgetfulness, trouble concentrating, and difficulty multitasking.
Dr Byrne and his colleagues explained this first step in The Journal of Neuroscience.
The researchers knew that, in non-neuronal cells, doxorubicin inhibits the expression of MAPK phosphatases, thereby inhibiting the dephosphorylation of ERK and p38 MAPK, 2 MAPK isoforms that are important for long-term memory.
To evaluate doxorubicin’s effects on levels of phosphorylated ERK and p38 MAPK, the team used cultures of cortical neurons from rats and sensory neurons from Aplysia californica.
Experiments showed that doxorubicin elevated levels of phosphorylated ERK and phosphorylated p38 MAPK in sensory neurons and cortical neurons. In addition, the drug increased phosphorylation of the downstream transcriptional repressor CREB2 in sensory neurons.
The researchers also assessed doxorubicin’s effects on long-term enhanced excitability, long-term synaptic facilitation, and long-term
synaptic depression.
They found that doxorubicin enhanced long-term synaptic depression induced by the neuropeptide Phe-Met-Arg-Phe-NH2. And the drug inhibited long-term synaptic facilitation induced by serotonin.
However, the researchers were able to restore long-term synaptic facilitation with SB203580, an inhibitor of p38 MAPK.
Unfortunately, SB203580 would not be appropriate for human use, Dr Byrne noted, adding that his team would like to identify other drugs that might have the same effect as SB203580.
The researchers also hope to determine if doxorubicin works the same way in humans as it did in these experiments. ![]()