User login
Combo can improve survival in certain AML patients
In a phase 3 trial, adding the quinolone derivative vosaroxin (Qinprezo) to treatment with cytarabine did not improve overall survival in patients with relapsed or refractory acute myeloid leukemia (AML).
However, the combination did confer a survival benefit when transplant patients were excluded from the analysis and in patients age 60 and older.
Results of this trial were recently announced by Sunesis Pharmaceuticals, the company developing vosaroxin.
The results are set to be presented in more detail at an upcoming scientific conference.
The trial, known as VALOR, enrolled 711 AML patients who had relapsed or become refractory to treatment for the first time. The patients were randomized to receive cytarabine plus vosaroxin or cytarabine plus placebo. They were stratified for age, geography, and disease status.
The trial did not meet its primary endpoint of demonstrating a significant improvement in overall survival. The median overall survival was 7.5 months in the vosaroxin-cytarabine arm and 6.1 months in the placebo-cytarabine arm (hazard ratio [HR]=0.865, P=0.06).
However, there was a significant benefit in complete response rate with vosaroxin over placebo—30.1% and 16.3%, respectively (P=0.0000148).
Because transplant could confound the primary analysis, the researchers planned a predefined analysis of overall survival censoring for stem cell transplant.
In this analysis, patients receiving the vosaroxin combination had a median overall survival of 6.7 months, compared to 5.3 months for placebo and cytarabine (HR=0.809, P=0.02).
Results according to age
For age, the researchers stratified patients into those age 60 years and older and those younger than 60 years at enrollment.
For the younger patients (n=260), the median overall survival was 9.1 months in the vosaroxin-cytarabine arm and 7.9 months in the placebo-cytarabine arm (HR=1.079, not significant).
The complete response rates were 26.9% and 20.8%, respectively (P=0.24). The rate of stem cell transplant was 45.8% in this age group.
For patients aged 60 years and older (n=451), the median overall survival was 7.1 months in the vosaroxin-cytarabine arm and 5.0 months in the placebo-cytarabine arm (HR=0.755, P=0.006).
The complete response rates were 31.9% and 13.8%, respectively (P=0.0000048). The rate of stem cell transplant was 20.2% for this age group.
Adverse events and mortality
In the intent-to-treat population, grade 3 or higher non-hematologic adverse events that were more common in the vosaroxin arm were gastrointestinal and infection-related toxicities. This is consistent with events observed in previous company trials.
The rate of serious adverse events was 55.5% in the vosaroxin-cytarabine arm and 35.7% in the placebo-cytarabine arm.
All-cause mortality rates were similar between the arms. Thirty-day mortality rates were 7.9% in the vosaroxin-cytarabine arm and 6.6% in the placebo-cytarabine arm. And 60-day mortality rates were 19.7% and 19.4%, respectively.
Regulatory plans
Based on the results of the trial, Sunesis plans to submit a marketing authorization application for vosaroxin to the European Medicines Agency. The company also plans to meet with the US Food and Drug Administration (FDA) to determine the appropriate regulatory path forward.
The FDA and the European Commission have already granted orphan designation to vosaroxin for the treatment of AML. The drug has been granted fast track designation by the FDA as well, for the potential treatment of relapsed or refractory AML in combination with cytarabine. ![]()
In a phase 3 trial, adding the quinolone derivative vosaroxin (Qinprezo) to treatment with cytarabine did not improve overall survival in patients with relapsed or refractory acute myeloid leukemia (AML).
However, the combination did confer a survival benefit when transplant patients were excluded from the analysis and in patients age 60 and older.
Results of this trial were recently announced by Sunesis Pharmaceuticals, the company developing vosaroxin.
The results are set to be presented in more detail at an upcoming scientific conference.
The trial, known as VALOR, enrolled 711 AML patients who had relapsed or become refractory to treatment for the first time. The patients were randomized to receive cytarabine plus vosaroxin or cytarabine plus placebo. They were stratified for age, geography, and disease status.
The trial did not meet its primary endpoint of demonstrating a significant improvement in overall survival. The median overall survival was 7.5 months in the vosaroxin-cytarabine arm and 6.1 months in the placebo-cytarabine arm (hazard ratio [HR]=0.865, P=0.06).
However, there was a significant benefit in complete response rate with vosaroxin over placebo—30.1% and 16.3%, respectively (P=0.0000148).
Because transplant could confound the primary analysis, the researchers planned a predefined analysis of overall survival censoring for stem cell transplant.
In this analysis, patients receiving the vosaroxin combination had a median overall survival of 6.7 months, compared to 5.3 months for placebo and cytarabine (HR=0.809, P=0.02).
Results according to age
For age, the researchers stratified patients into those age 60 years and older and those younger than 60 years at enrollment.
For the younger patients (n=260), the median overall survival was 9.1 months in the vosaroxin-cytarabine arm and 7.9 months in the placebo-cytarabine arm (HR=1.079, not significant).
The complete response rates were 26.9% and 20.8%, respectively (P=0.24). The rate of stem cell transplant was 45.8% in this age group.
For patients aged 60 years and older (n=451), the median overall survival was 7.1 months in the vosaroxin-cytarabine arm and 5.0 months in the placebo-cytarabine arm (HR=0.755, P=0.006).
The complete response rates were 31.9% and 13.8%, respectively (P=0.0000048). The rate of stem cell transplant was 20.2% for this age group.
Adverse events and mortality
In the intent-to-treat population, grade 3 or higher non-hematologic adverse events that were more common in the vosaroxin arm were gastrointestinal and infection-related toxicities. This is consistent with events observed in previous company trials.
The rate of serious adverse events was 55.5% in the vosaroxin-cytarabine arm and 35.7% in the placebo-cytarabine arm.
All-cause mortality rates were similar between the arms. Thirty-day mortality rates were 7.9% in the vosaroxin-cytarabine arm and 6.6% in the placebo-cytarabine arm. And 60-day mortality rates were 19.7% and 19.4%, respectively.
Regulatory plans
Based on the results of the trial, Sunesis plans to submit a marketing authorization application for vosaroxin to the European Medicines Agency. The company also plans to meet with the US Food and Drug Administration (FDA) to determine the appropriate regulatory path forward.
The FDA and the European Commission have already granted orphan designation to vosaroxin for the treatment of AML. The drug has been granted fast track designation by the FDA as well, for the potential treatment of relapsed or refractory AML in combination with cytarabine. ![]()
In a phase 3 trial, adding the quinolone derivative vosaroxin (Qinprezo) to treatment with cytarabine did not improve overall survival in patients with relapsed or refractory acute myeloid leukemia (AML).
However, the combination did confer a survival benefit when transplant patients were excluded from the analysis and in patients age 60 and older.
Results of this trial were recently announced by Sunesis Pharmaceuticals, the company developing vosaroxin.
The results are set to be presented in more detail at an upcoming scientific conference.
The trial, known as VALOR, enrolled 711 AML patients who had relapsed or become refractory to treatment for the first time. The patients were randomized to receive cytarabine plus vosaroxin or cytarabine plus placebo. They were stratified for age, geography, and disease status.
The trial did not meet its primary endpoint of demonstrating a significant improvement in overall survival. The median overall survival was 7.5 months in the vosaroxin-cytarabine arm and 6.1 months in the placebo-cytarabine arm (hazard ratio [HR]=0.865, P=0.06).
However, there was a significant benefit in complete response rate with vosaroxin over placebo—30.1% and 16.3%, respectively (P=0.0000148).
Because transplant could confound the primary analysis, the researchers planned a predefined analysis of overall survival censoring for stem cell transplant.
In this analysis, patients receiving the vosaroxin combination had a median overall survival of 6.7 months, compared to 5.3 months for placebo and cytarabine (HR=0.809, P=0.02).
Results according to age
For age, the researchers stratified patients into those age 60 years and older and those younger than 60 years at enrollment.
For the younger patients (n=260), the median overall survival was 9.1 months in the vosaroxin-cytarabine arm and 7.9 months in the placebo-cytarabine arm (HR=1.079, not significant).
The complete response rates were 26.9% and 20.8%, respectively (P=0.24). The rate of stem cell transplant was 45.8% in this age group.
For patients aged 60 years and older (n=451), the median overall survival was 7.1 months in the vosaroxin-cytarabine arm and 5.0 months in the placebo-cytarabine arm (HR=0.755, P=0.006).
The complete response rates were 31.9% and 13.8%, respectively (P=0.0000048). The rate of stem cell transplant was 20.2% for this age group.
Adverse events and mortality
In the intent-to-treat population, grade 3 or higher non-hematologic adverse events that were more common in the vosaroxin arm were gastrointestinal and infection-related toxicities. This is consistent with events observed in previous company trials.
The rate of serious adverse events was 55.5% in the vosaroxin-cytarabine arm and 35.7% in the placebo-cytarabine arm.
All-cause mortality rates were similar between the arms. Thirty-day mortality rates were 7.9% in the vosaroxin-cytarabine arm and 6.6% in the placebo-cytarabine arm. And 60-day mortality rates were 19.7% and 19.4%, respectively.
Regulatory plans
Based on the results of the trial, Sunesis plans to submit a marketing authorization application for vosaroxin to the European Medicines Agency. The company also plans to meet with the US Food and Drug Administration (FDA) to determine the appropriate regulatory path forward.
The FDA and the European Commission have already granted orphan designation to vosaroxin for the treatment of AML. The drug has been granted fast track designation by the FDA as well, for the potential treatment of relapsed or refractory AML in combination with cytarabine. ![]()
Why screen if there are no services?
Do you remember the discussion of the ethical dilemma of Huntington’s disease you probably participated in during medical school? The question was whether you would want to know that you were at risk for a chronic debilitating condition that would develop at some later age if there was nothing that could be done about it. In that discussion, you also may have heard about individuals who, after hearing about their risk status, become depressed or suicidal, depending on the story line.
Some pediatricians seem to have taken this example too far in arguing that there is no point in screening for issues of development, autism, maternal depression, or child mental health because “there are no services” available to treat them.
Despair is understandable. Physicians’ lack of knowledge about resources in the community is often a sore point among local agencies, parents, and even pediatricians themselves. In spite of United Way, state 3-1-1 programs ,and the occasionally available social worker, the resources with which we are familiar sometimes come from hard-working parents telling us about a program they found on their own. It also seems that, just when we hit upon a valuable resource, it runs out of funding, changes eligibility requirements, or loses key staff. Worse yet, we may rely on resources we know about because of our own children’s problems, activities, or friends. While the Internet is an increasingly valuable method of finding resources, there is no filter of the evidence-basis of the care provided, and the process of searching, vetting, and informing your patients is extremely time consuming, and often the patient is not eligible or has a long waiting period after all that.
There are important reasons not to succumb to throwing up one’s hands about service availability. And more important reasons to still screen even if you do not know where to refer.
Screening using validated tools is recommended by the American Academy of Pediatrics because parent concern and even clinical observation are not adequately sensitive to detect significant problems of development and mental health, even when done by experienced physicians who know families well. The process of screening sends an important message to the parents – that you care about the child’s progress and are using proven methods to ensure that it is going well and consider it part of complete medical care.
And families often already think that their child may have a problem, even when they don’t bring it up. Perhaps deep down they are afraid that somehow raising the question of autism will make it true. They may be in denial, are feeling guilty, or are under pressure from their spouse, relatives, or friends not to worry, that “he will grow out” of it, that better discipline will fix the problem, etc. They may even care so much about your positive regard that they do not want to seem overly anxious, obsessive, or be regarded as a failure for having a “defective” child. They, like you, also may be in despair about finding effective help.
But there can be serious consequences to not screening, even when you are not sure what you will do with the results. The family may push the child with delays or mental health problems beyond his abilities, and even become negative and punitive in trying to make him succeed, in the process promoting unnecessary behavior problems, discouragement, and even defiance in the child. Failure to detect also means failure to list the child on a registry for follow-up to determine progress or refer when resources become available. Some problems of development or mental health that are detected by screening may have medical causes that you can treat, even though counseling or therapy interventions are not available. Examples include hearing or vision deficits causing delays or anemia, sleep apnea, or hypothyroidism or maternal depression or attention-deficit/hyperactivity disorder (ADHD). For issues with a genetic basis, siblings may be born with same problem during the period of delay in making a diagnosis, a prime example being Fragile X. In untold cases, the family loses trust in you and in the medical system for not acknowledging a problem.
In many cases, your acknowledgment, explanation, sympathy, and advice can help enormously. Families can cope better, garner support from family or friends, deal with the child’s behavior better, and find steps to take to help their child in their own ways, even without formal services, once told that their child has a specific problem.
On a system level, it is important to realize that how services are established and maintained is far less rational than might be imagined. State programs, schools, hospitals, and insurers all have legal requirements to provide services within a certain time frame once referred. Even if the services are not there to help a your child or family right now, the referral itself adds to the data used to determine if services are adequate and to plan for additional service types or capacity. The Autism Waiver is one such example where waits are years long, but getting on the list is crucial to the future of the program.
Until you screen and give parents information – especially middle-class parents – we will never have the resources. As it was for lead paint, until we identified prevalence of elevated lead levels and the harm associated, we got no action on lead paint removal policies. Another example where complaints about access made a difference, is the relatively new Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 that requires health insurers and health plans to guarantee that financial requirements on benefits for mental health, such as copays, deductibles, and limitations on treatment benefits, are not more restrictive than those that are for medical benefits. This does not guarantee that services will be available or of high quality, but is a step toward accessibility.
You may be one of the many pediatricians who consider advocacy a basic component of your professional responsibilities. If you cannot advocate for services that you see your patients in need of, you can pass your concerns onto a group that does. Many American Academy of Pediatrics state chapters have so-called Pediatric Councils that receive ideas about system problems and put group pressure on leaders in the state to address them.
As in the historic painting of the physician leaning over the ill child whom he could not cure, after detection through screening our thoughtful evaluation, explanations, shared concern, and our patients’ advocacy have great value even when specific services are not yet available.
Dr. Howard is an assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.chadis.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
Do you remember the discussion of the ethical dilemma of Huntington’s disease you probably participated in during medical school? The question was whether you would want to know that you were at risk for a chronic debilitating condition that would develop at some later age if there was nothing that could be done about it. In that discussion, you also may have heard about individuals who, after hearing about their risk status, become depressed or suicidal, depending on the story line.
Some pediatricians seem to have taken this example too far in arguing that there is no point in screening for issues of development, autism, maternal depression, or child mental health because “there are no services” available to treat them.
Despair is understandable. Physicians’ lack of knowledge about resources in the community is often a sore point among local agencies, parents, and even pediatricians themselves. In spite of United Way, state 3-1-1 programs ,and the occasionally available social worker, the resources with which we are familiar sometimes come from hard-working parents telling us about a program they found on their own. It also seems that, just when we hit upon a valuable resource, it runs out of funding, changes eligibility requirements, or loses key staff. Worse yet, we may rely on resources we know about because of our own children’s problems, activities, or friends. While the Internet is an increasingly valuable method of finding resources, there is no filter of the evidence-basis of the care provided, and the process of searching, vetting, and informing your patients is extremely time consuming, and often the patient is not eligible or has a long waiting period after all that.
There are important reasons not to succumb to throwing up one’s hands about service availability. And more important reasons to still screen even if you do not know where to refer.
Screening using validated tools is recommended by the American Academy of Pediatrics because parent concern and even clinical observation are not adequately sensitive to detect significant problems of development and mental health, even when done by experienced physicians who know families well. The process of screening sends an important message to the parents – that you care about the child’s progress and are using proven methods to ensure that it is going well and consider it part of complete medical care.
And families often already think that their child may have a problem, even when they don’t bring it up. Perhaps deep down they are afraid that somehow raising the question of autism will make it true. They may be in denial, are feeling guilty, or are under pressure from their spouse, relatives, or friends not to worry, that “he will grow out” of it, that better discipline will fix the problem, etc. They may even care so much about your positive regard that they do not want to seem overly anxious, obsessive, or be regarded as a failure for having a “defective” child. They, like you, also may be in despair about finding effective help.
But there can be serious consequences to not screening, even when you are not sure what you will do with the results. The family may push the child with delays or mental health problems beyond his abilities, and even become negative and punitive in trying to make him succeed, in the process promoting unnecessary behavior problems, discouragement, and even defiance in the child. Failure to detect also means failure to list the child on a registry for follow-up to determine progress or refer when resources become available. Some problems of development or mental health that are detected by screening may have medical causes that you can treat, even though counseling or therapy interventions are not available. Examples include hearing or vision deficits causing delays or anemia, sleep apnea, or hypothyroidism or maternal depression or attention-deficit/hyperactivity disorder (ADHD). For issues with a genetic basis, siblings may be born with same problem during the period of delay in making a diagnosis, a prime example being Fragile X. In untold cases, the family loses trust in you and in the medical system for not acknowledging a problem.
In many cases, your acknowledgment, explanation, sympathy, and advice can help enormously. Families can cope better, garner support from family or friends, deal with the child’s behavior better, and find steps to take to help their child in their own ways, even without formal services, once told that their child has a specific problem.
On a system level, it is important to realize that how services are established and maintained is far less rational than might be imagined. State programs, schools, hospitals, and insurers all have legal requirements to provide services within a certain time frame once referred. Even if the services are not there to help a your child or family right now, the referral itself adds to the data used to determine if services are adequate and to plan for additional service types or capacity. The Autism Waiver is one such example where waits are years long, but getting on the list is crucial to the future of the program.
Until you screen and give parents information – especially middle-class parents – we will never have the resources. As it was for lead paint, until we identified prevalence of elevated lead levels and the harm associated, we got no action on lead paint removal policies. Another example where complaints about access made a difference, is the relatively new Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 that requires health insurers and health plans to guarantee that financial requirements on benefits for mental health, such as copays, deductibles, and limitations on treatment benefits, are not more restrictive than those that are for medical benefits. This does not guarantee that services will be available or of high quality, but is a step toward accessibility.
You may be one of the many pediatricians who consider advocacy a basic component of your professional responsibilities. If you cannot advocate for services that you see your patients in need of, you can pass your concerns onto a group that does. Many American Academy of Pediatrics state chapters have so-called Pediatric Councils that receive ideas about system problems and put group pressure on leaders in the state to address them.
As in the historic painting of the physician leaning over the ill child whom he could not cure, after detection through screening our thoughtful evaluation, explanations, shared concern, and our patients’ advocacy have great value even when specific services are not yet available.
Dr. Howard is an assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.chadis.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
Do you remember the discussion of the ethical dilemma of Huntington’s disease you probably participated in during medical school? The question was whether you would want to know that you were at risk for a chronic debilitating condition that would develop at some later age if there was nothing that could be done about it. In that discussion, you also may have heard about individuals who, after hearing about their risk status, become depressed or suicidal, depending on the story line.
Some pediatricians seem to have taken this example too far in arguing that there is no point in screening for issues of development, autism, maternal depression, or child mental health because “there are no services” available to treat them.
Despair is understandable. Physicians’ lack of knowledge about resources in the community is often a sore point among local agencies, parents, and even pediatricians themselves. In spite of United Way, state 3-1-1 programs ,and the occasionally available social worker, the resources with which we are familiar sometimes come from hard-working parents telling us about a program they found on their own. It also seems that, just when we hit upon a valuable resource, it runs out of funding, changes eligibility requirements, or loses key staff. Worse yet, we may rely on resources we know about because of our own children’s problems, activities, or friends. While the Internet is an increasingly valuable method of finding resources, there is no filter of the evidence-basis of the care provided, and the process of searching, vetting, and informing your patients is extremely time consuming, and often the patient is not eligible or has a long waiting period after all that.
There are important reasons not to succumb to throwing up one’s hands about service availability. And more important reasons to still screen even if you do not know where to refer.
Screening using validated tools is recommended by the American Academy of Pediatrics because parent concern and even clinical observation are not adequately sensitive to detect significant problems of development and mental health, even when done by experienced physicians who know families well. The process of screening sends an important message to the parents – that you care about the child’s progress and are using proven methods to ensure that it is going well and consider it part of complete medical care.
And families often already think that their child may have a problem, even when they don’t bring it up. Perhaps deep down they are afraid that somehow raising the question of autism will make it true. They may be in denial, are feeling guilty, or are under pressure from their spouse, relatives, or friends not to worry, that “he will grow out” of it, that better discipline will fix the problem, etc. They may even care so much about your positive regard that they do not want to seem overly anxious, obsessive, or be regarded as a failure for having a “defective” child. They, like you, also may be in despair about finding effective help.
But there can be serious consequences to not screening, even when you are not sure what you will do with the results. The family may push the child with delays or mental health problems beyond his abilities, and even become negative and punitive in trying to make him succeed, in the process promoting unnecessary behavior problems, discouragement, and even defiance in the child. Failure to detect also means failure to list the child on a registry for follow-up to determine progress or refer when resources become available. Some problems of development or mental health that are detected by screening may have medical causes that you can treat, even though counseling or therapy interventions are not available. Examples include hearing or vision deficits causing delays or anemia, sleep apnea, or hypothyroidism or maternal depression or attention-deficit/hyperactivity disorder (ADHD). For issues with a genetic basis, siblings may be born with same problem during the period of delay in making a diagnosis, a prime example being Fragile X. In untold cases, the family loses trust in you and in the medical system for not acknowledging a problem.
In many cases, your acknowledgment, explanation, sympathy, and advice can help enormously. Families can cope better, garner support from family or friends, deal with the child’s behavior better, and find steps to take to help their child in their own ways, even without formal services, once told that their child has a specific problem.
On a system level, it is important to realize that how services are established and maintained is far less rational than might be imagined. State programs, schools, hospitals, and insurers all have legal requirements to provide services within a certain time frame once referred. Even if the services are not there to help a your child or family right now, the referral itself adds to the data used to determine if services are adequate and to plan for additional service types or capacity. The Autism Waiver is one such example where waits are years long, but getting on the list is crucial to the future of the program.
Until you screen and give parents information – especially middle-class parents – we will never have the resources. As it was for lead paint, until we identified prevalence of elevated lead levels and the harm associated, we got no action on lead paint removal policies. Another example where complaints about access made a difference, is the relatively new Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008 that requires health insurers and health plans to guarantee that financial requirements on benefits for mental health, such as copays, deductibles, and limitations on treatment benefits, are not more restrictive than those that are for medical benefits. This does not guarantee that services will be available or of high quality, but is a step toward accessibility.
You may be one of the many pediatricians who consider advocacy a basic component of your professional responsibilities. If you cannot advocate for services that you see your patients in need of, you can pass your concerns onto a group that does. Many American Academy of Pediatrics state chapters have so-called Pediatric Councils that receive ideas about system problems and put group pressure on leaders in the state to address them.
As in the historic painting of the physician leaning over the ill child whom he could not cure, after detection through screening our thoughtful evaluation, explanations, shared concern, and our patients’ advocacy have great value even when specific services are not yet available.
Dr. Howard is an assistant professor of pediatrics at the Johns Hopkins University, Baltimore, and creator of CHADIS (www.chadis.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
HATS Syndrome: Hemimaxillary Enlargement, Asymmetry of the Face, Tooth Abnormalities, and Skin Findings
Case Report
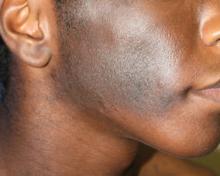
A 14-year-old adolescent boy presented to the dermatology clinic at our institution for evaluation of a hyperpigmented hairy patch on the right side of the face that had been present since birth. The patient reported the lesion originally had involved the right cheek, neck, and back but had gradually expanded to include the right side of the upper lip and oral mucosa. His medical history was remarkable for acne, which was currently being managed with topical treatments. There was no family history of similar conditions. There were no mental or developmental deformities since birth.
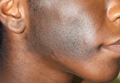
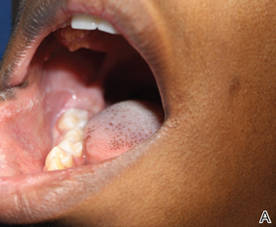
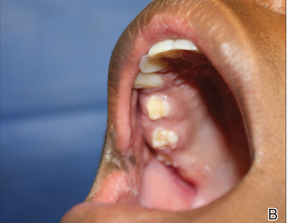
Physical examination revealed a hyperpigmented patch with hypertrichosis on the right side of the body involving the back, neck, and cheek (Figure 1), as well as hyperpigmentation involving the right side of the upper lip and oral mucosa (Figure 2A). Slight facial asymmetry also was noted. Dental examination revealed irregular spacing and decreased growth of the teeth on the right side of the mouth (Figure 2B).
| 
|
Figure 2. Some hyperpigmentation involving the oral mucosa on the right side (A) and dental abnormalities (B). | |
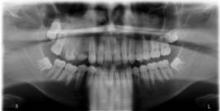
A biopsy of the hyperpigmented patch on the back revealed mild regular acanthosis, basal hypermelanosis, slight papillomatosis, and hair structures within the dermis with features that were consistent with a Becker nevus. A dental radiograph demonstrated hyperplasia of the right maxillary alveolus and basal bone area with 2 missing permanent teeth (fourth and fifth premolars)(Figure 3). Computed axial tomography revealed enlargement of the maxillary bone on the right side.
The constellation of clinical, histopathologic, and radiologic findings was consistent with a diagnosis of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS syndrome). The treatment plan involved surgical modification of the maxillary bone to correct the hyperplasia on the affected side and implanting 2 artificial premolars. Additionally, laser therapy using a Q-switched ruby laser, frequency-doubled Nd:YAG, 1550-nm erbium-doped fiber laser, or 755-nm alexandrite laser was considered to treat the hyperpigmentation associ-ated with the Becker nevus.
Comment
HATS syndrome is a rare, local developmental defect involving the first and second branchial arches. It generally is detected at birth or in early childhood and is associated with unilateral abnormalities of the bones, teeth, gums, and skin. It is more common in boys than girls (1.8:1.0 ratio), with an age range of 2 to 28 years; there is a peak in the first decade of life.1 It was first described by Miles et al2 in 1987 in a case of congenital mild facial asymmetry, unilateral enlargement of the maxillary gingiva and alveolar bone, hypoplastic teeth, and hypertrichosis in the affected area. The investigators at that time suggested the term hemimaxillofacial dysplasia (HD). In 1990, Danforth et al3 reported 8 additional cases with similar features but without known skin changes; they proposed the term segmental odontomaxillary dysplasia (SOD). In 1996, Desalvo et al4 reported a case of SOD involving a 7-year-old girl with an area of hypopigmentation of the lip on the affected side, and Packota et al5 described the radiographic features of 12 cases of SOD. In subsequent years, other cases of HD or SOD were reported in the literature.1,6-16 In 2004, Welsch and Stein17 reported 1 patient with a Becker nevus of the skin and recommended the acronym HATS. Armstrong et al18 reported 2 cases of SOD with new histopathologic findings of the teeth (eg, fibrous enlargement of the pulps, an irregular pulp-dentin interface displaying many pseudoinclusions, pulp stones). In 2008, Porwal et al19 reported a case of HD in which maxillary hypoplasia rather than hyperplasia was noted, which emphasized the variability of the maxillary dysplasia. Koenig et al20 reported a case of SOD with facial hypertrichosis, commissural lip clefting, and hyperlinear palms. Bhatia et al21 reported another case of SOD with a new finding of unilateral ectopic eyelashes.
The etiology remains unknown, but theories include an alteration that occurs in utero or in in-fancy; the possibility of a systemic or endocrine aberration; a postzygotic mutation resulting in genotypic and phenotypic mosaicism of bone and skin, similar to McCune-Albright syndrome; and viral or bacterial infection along the branches of the maxillary division of the trigeminal nerve.1,15 Bone defects include unilateral enlargement of the maxillary alveolar process and thickening of the vertically oriented trabeculae, which is detected radiographically. A reduction in size of the maxillary sinus and nasal airway was reported in about one-half of cases1 and can be detected easily by computed tomography scanning. Missing permanent premolar teeth, tooth shape abnormalities, delayed eruption of teeth, abnormal spacing of teeth, hypoplastic teeth, enlarged teeth, and gingival thickening also are common oral findings.1 The skin manifestations of HATS syndrome are not static but progress well into adolescence15 and can include facial asymmetry, hypertrichosis, Becker nevus, hairy nevus, lip hypopigmentation, discontinuity of the vermilion border, depression of the cheek, and facial erythema.17
The differential diagnosis includes hemifacial hyperplasia, monostotic fibrous dysplasia, and regional odontodysplasia.1 Little information is available concerning the treatment of patients with this condition.15 The reported treatment modalities include combined surgical and orthodontic treatment of unerupted teeth (premolar/canine), prosthodontic treatment, gingivoplasty, recontouring osteotomy for severe facial asymmetry, and reconstructive jaw surgery.1,6,11,15 Successful treatment of Becker nevi with the Q-switched ruby laser, erbium:YAG laser, and 755-nm alexandrite laser have been reported.22-24
Conclusion
There is a need for continued reporting of cases of HATS syndrome in addition to long-term follow-up to document the natural history of the condition and to establish the appropriate treatment.
1. Friedlander-Barenboim S, Kamburoglu K, Kaffe I. Clinical and radiological presentation of hemimaxillofacial dysplasia/segmental odontomaxillary dysplasia: critical analysis and report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:268-273.
2. Miles DA, Lovas JL, Cohen MM. Hemimaxillofacial dysplasia: a newly recognized disorder of facial asymmetry, hypertrichosis of the facial skin, unilateral enlargement of the maxilla, and hypoplastic teeth in two patients. Oral Surg Oral Med Oral Pathol. 1987;64:445-448.
3. Danforth RA, Melrose RJ, Abrams AM, et al. Segmental odontomaxillary dysplasia: report of eight cases and comparison with hemimaxillofacial dysplasia. Oral Surg Oral Med Oral Pathol. 1990;70:81-85.
4. Desalvo MS, Copete MA, Riesenberger RE, et al. Segmental odontomaxillary dysplasia (hemimaxillofacial dysplasia): case report. Pediatr Dent. 1996;18:154-156.
5. Packota GV, Pharoah MJ, Petrikowski CG. Radiographic features of segmental odontomaxillary dysplasia: a study of 12 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:577-584.
6. Paticoff K, Marion RW, Shprintzen RJ, et al. Hemimaxillofacial dysplasia: a report of two new cases and further delineation of the disorder. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:484-488.
7. Jones AC, Ford MJ. Simultaneous occurrence of segmental odontomaxillary dysplasia and Becker’s nevus. J Oral Maxillofac Surg. 1990;57:1251-1254.
8. Prusack N, Pringle G, Scotti V, et al. Segmental odontomaxillary dysplasia: a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:483-488.
9. Becktor KB, Reibel J, Vedel B, et al. Segmental odontomaxillary dysplasia: clinical, radiological and histological aspects of four cases. Oral Dis. 2002;8:106-110.
10. Velez I, Vedrenne D, Cralle P, et al. Segmental odontomaxillary dysplasia: report of two cases and review of the literature. Todays FDA. 2002;14:20-21.
11. Drake DL. Segmental odontomaxillary dysplasia: an unusual orthodontic challenge. Am J Orthod Dentofac Orthop. 2003;123:84-86.
12. Gavalda C. Segmental odontomaxillary dysplasia. Med Oral. 2004;9:181.
13. Ozpinar B, Gokce B, Uzel G, et al. Prosthetic rehabilitation of segmental odontomaxillary dysplasia: seven-year follow-up. Cleft Palate Craniofac J. 2009;46:103-107.
14. Kuklani RM, Nair MK. Segmental odontomaxillary dysplasia: review of the literature and case report [published online ahead of print December 14, 2010]. Int J Dent. 2010;2010:837283.
15. Minett CP, Daley TD. Hemimaxillofacial dysplasia (segmental odontomaxillary dysplasia): case study with 11 years of follow-up from primary to adult dentition. J Oral Maxillofac Surg. 2012;70:1183-1191.
16. Whitt JC, Rokos JW, Dunlap CL, et al. Segmental odontomaxillary dysplasia: report of a series of 5 cases with long-term follow-up. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2011;112:E29-E47.
17. Welsch MJ, Stein SL. A syndrome of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS). Pediatr Dermatol. 2004;21:448-451.
18. Armstrong C, Napier SS, Boyd RC, et al. Histopathology of teeth in segmental odontomaxillary dysplasia: new findings. J Oral Pathol Med. 2004;33:246-248.
19. Porwal R, Ousterhout DK, Hoffman WY, et al. Hemimaxillofacial dysplasia: a variable presentation. J Craniofac Surg. 2008;19:1554-1557.
20. Koenig LJ, Lynch DP, Yancey KB. Segmental odontomaxillary dysplasia presenting with facial hypertrichosis, commissural lip clefting, and hyperlinear palm. Pediatr Dermatol. 2008;25:491-492.
21. Bhatia SK, Drage N, Cronin AJ, et al. Case report: segmental odontomaxillary dysplasia—a rare disorder. Eur Arch Paediatr Dent. 2008;9:245-248.
22. Raulin C, Schönermark MP, Greve B, et al. Q-switched ruby laser treatment of tattoos and benign pigmented skin lesions: a critical review. Ann Plast Surg. 1998;41:555-565.
23. Trelles MA, Allones I, Moreno-Arias GA, et al. Becker’s naevus: a comparative study between erbium: YAG and Q-switched neodymium:YAG; clinical and histopathological findings. Br J Dermatol. 2005;152:308-313.
24. Choi JE, Kim JW, Seo SH, et al. Treatment of Becker’s nevi with a long-pulse alexandrite laser. Dermatol Surg. 2009;35:1105-1108.
Case Report
A 14-year-old adolescent boy presented to the dermatology clinic at our institution for evaluation of a hyperpigmented hairy patch on the right side of the face that had been present since birth. The patient reported the lesion originally had involved the right cheek, neck, and back but had gradually expanded to include the right side of the upper lip and oral mucosa. His medical history was remarkable for acne, which was currently being managed with topical treatments. There was no family history of similar conditions. There were no mental or developmental deformities since birth.
Physical examination revealed a hyperpigmented patch with hypertrichosis on the right side of the body involving the back, neck, and cheek (Figure 1), as well as hyperpigmentation involving the right side of the upper lip and oral mucosa (Figure 2A). Slight facial asymmetry also was noted. Dental examination revealed irregular spacing and decreased growth of the teeth on the right side of the mouth (Figure 2B).

| 
|
Figure 2. Some hyperpigmentation involving the oral mucosa on the right side (A) and dental abnormalities (B). | |
A biopsy of the hyperpigmented patch on the back revealed mild regular acanthosis, basal hypermelanosis, slight papillomatosis, and hair structures within the dermis with features that were consistent with a Becker nevus. A dental radiograph demonstrated hyperplasia of the right maxillary alveolus and basal bone area with 2 missing permanent teeth (fourth and fifth premolars)(Figure 3). Computed axial tomography revealed enlargement of the maxillary bone on the right side.
The constellation of clinical, histopathologic, and radiologic findings was consistent with a diagnosis of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS syndrome). The treatment plan involved surgical modification of the maxillary bone to correct the hyperplasia on the affected side and implanting 2 artificial premolars. Additionally, laser therapy using a Q-switched ruby laser, frequency-doubled Nd:YAG, 1550-nm erbium-doped fiber laser, or 755-nm alexandrite laser was considered to treat the hyperpigmentation associ-ated with the Becker nevus.
Comment
HATS syndrome is a rare, local developmental defect involving the first and second branchial arches. It generally is detected at birth or in early childhood and is associated with unilateral abnormalities of the bones, teeth, gums, and skin. It is more common in boys than girls (1.8:1.0 ratio), with an age range of 2 to 28 years; there is a peak in the first decade of life.1 It was first described by Miles et al2 in 1987 in a case of congenital mild facial asymmetry, unilateral enlargement of the maxillary gingiva and alveolar bone, hypoplastic teeth, and hypertrichosis in the affected area. The investigators at that time suggested the term hemimaxillofacial dysplasia (HD). In 1990, Danforth et al3 reported 8 additional cases with similar features but without known skin changes; they proposed the term segmental odontomaxillary dysplasia (SOD). In 1996, Desalvo et al4 reported a case of SOD involving a 7-year-old girl with an area of hypopigmentation of the lip on the affected side, and Packota et al5 described the radiographic features of 12 cases of SOD. In subsequent years, other cases of HD or SOD were reported in the literature.1,6-16 In 2004, Welsch and Stein17 reported 1 patient with a Becker nevus of the skin and recommended the acronym HATS. Armstrong et al18 reported 2 cases of SOD with new histopathologic findings of the teeth (eg, fibrous enlargement of the pulps, an irregular pulp-dentin interface displaying many pseudoinclusions, pulp stones). In 2008, Porwal et al19 reported a case of HD in which maxillary hypoplasia rather than hyperplasia was noted, which emphasized the variability of the maxillary dysplasia. Koenig et al20 reported a case of SOD with facial hypertrichosis, commissural lip clefting, and hyperlinear palms. Bhatia et al21 reported another case of SOD with a new finding of unilateral ectopic eyelashes.
The etiology remains unknown, but theories include an alteration that occurs in utero or in in-fancy; the possibility of a systemic or endocrine aberration; a postzygotic mutation resulting in genotypic and phenotypic mosaicism of bone and skin, similar to McCune-Albright syndrome; and viral or bacterial infection along the branches of the maxillary division of the trigeminal nerve.1,15 Bone defects include unilateral enlargement of the maxillary alveolar process and thickening of the vertically oriented trabeculae, which is detected radiographically. A reduction in size of the maxillary sinus and nasal airway was reported in about one-half of cases1 and can be detected easily by computed tomography scanning. Missing permanent premolar teeth, tooth shape abnormalities, delayed eruption of teeth, abnormal spacing of teeth, hypoplastic teeth, enlarged teeth, and gingival thickening also are common oral findings.1 The skin manifestations of HATS syndrome are not static but progress well into adolescence15 and can include facial asymmetry, hypertrichosis, Becker nevus, hairy nevus, lip hypopigmentation, discontinuity of the vermilion border, depression of the cheek, and facial erythema.17
The differential diagnosis includes hemifacial hyperplasia, monostotic fibrous dysplasia, and regional odontodysplasia.1 Little information is available concerning the treatment of patients with this condition.15 The reported treatment modalities include combined surgical and orthodontic treatment of unerupted teeth (premolar/canine), prosthodontic treatment, gingivoplasty, recontouring osteotomy for severe facial asymmetry, and reconstructive jaw surgery.1,6,11,15 Successful treatment of Becker nevi with the Q-switched ruby laser, erbium:YAG laser, and 755-nm alexandrite laser have been reported.22-24
Conclusion
There is a need for continued reporting of cases of HATS syndrome in addition to long-term follow-up to document the natural history of the condition and to establish the appropriate treatment.
Case Report
A 14-year-old adolescent boy presented to the dermatology clinic at our institution for evaluation of a hyperpigmented hairy patch on the right side of the face that had been present since birth. The patient reported the lesion originally had involved the right cheek, neck, and back but had gradually expanded to include the right side of the upper lip and oral mucosa. His medical history was remarkable for acne, which was currently being managed with topical treatments. There was no family history of similar conditions. There were no mental or developmental deformities since birth.
Physical examination revealed a hyperpigmented patch with hypertrichosis on the right side of the body involving the back, neck, and cheek (Figure 1), as well as hyperpigmentation involving the right side of the upper lip and oral mucosa (Figure 2A). Slight facial asymmetry also was noted. Dental examination revealed irregular spacing and decreased growth of the teeth on the right side of the mouth (Figure 2B).

| 
|
Figure 2. Some hyperpigmentation involving the oral mucosa on the right side (A) and dental abnormalities (B). | |
A biopsy of the hyperpigmented patch on the back revealed mild regular acanthosis, basal hypermelanosis, slight papillomatosis, and hair structures within the dermis with features that were consistent with a Becker nevus. A dental radiograph demonstrated hyperplasia of the right maxillary alveolus and basal bone area with 2 missing permanent teeth (fourth and fifth premolars)(Figure 3). Computed axial tomography revealed enlargement of the maxillary bone on the right side.
The constellation of clinical, histopathologic, and radiologic findings was consistent with a diagnosis of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS syndrome). The treatment plan involved surgical modification of the maxillary bone to correct the hyperplasia on the affected side and implanting 2 artificial premolars. Additionally, laser therapy using a Q-switched ruby laser, frequency-doubled Nd:YAG, 1550-nm erbium-doped fiber laser, or 755-nm alexandrite laser was considered to treat the hyperpigmentation associ-ated with the Becker nevus.
Comment
HATS syndrome is a rare, local developmental defect involving the first and second branchial arches. It generally is detected at birth or in early childhood and is associated with unilateral abnormalities of the bones, teeth, gums, and skin. It is more common in boys than girls (1.8:1.0 ratio), with an age range of 2 to 28 years; there is a peak in the first decade of life.1 It was first described by Miles et al2 in 1987 in a case of congenital mild facial asymmetry, unilateral enlargement of the maxillary gingiva and alveolar bone, hypoplastic teeth, and hypertrichosis in the affected area. The investigators at that time suggested the term hemimaxillofacial dysplasia (HD). In 1990, Danforth et al3 reported 8 additional cases with similar features but without known skin changes; they proposed the term segmental odontomaxillary dysplasia (SOD). In 1996, Desalvo et al4 reported a case of SOD involving a 7-year-old girl with an area of hypopigmentation of the lip on the affected side, and Packota et al5 described the radiographic features of 12 cases of SOD. In subsequent years, other cases of HD or SOD were reported in the literature.1,6-16 In 2004, Welsch and Stein17 reported 1 patient with a Becker nevus of the skin and recommended the acronym HATS. Armstrong et al18 reported 2 cases of SOD with new histopathologic findings of the teeth (eg, fibrous enlargement of the pulps, an irregular pulp-dentin interface displaying many pseudoinclusions, pulp stones). In 2008, Porwal et al19 reported a case of HD in which maxillary hypoplasia rather than hyperplasia was noted, which emphasized the variability of the maxillary dysplasia. Koenig et al20 reported a case of SOD with facial hypertrichosis, commissural lip clefting, and hyperlinear palms. Bhatia et al21 reported another case of SOD with a new finding of unilateral ectopic eyelashes.
The etiology remains unknown, but theories include an alteration that occurs in utero or in in-fancy; the possibility of a systemic or endocrine aberration; a postzygotic mutation resulting in genotypic and phenotypic mosaicism of bone and skin, similar to McCune-Albright syndrome; and viral or bacterial infection along the branches of the maxillary division of the trigeminal nerve.1,15 Bone defects include unilateral enlargement of the maxillary alveolar process and thickening of the vertically oriented trabeculae, which is detected radiographically. A reduction in size of the maxillary sinus and nasal airway was reported in about one-half of cases1 and can be detected easily by computed tomography scanning. Missing permanent premolar teeth, tooth shape abnormalities, delayed eruption of teeth, abnormal spacing of teeth, hypoplastic teeth, enlarged teeth, and gingival thickening also are common oral findings.1 The skin manifestations of HATS syndrome are not static but progress well into adolescence15 and can include facial asymmetry, hypertrichosis, Becker nevus, hairy nevus, lip hypopigmentation, discontinuity of the vermilion border, depression of the cheek, and facial erythema.17
The differential diagnosis includes hemifacial hyperplasia, monostotic fibrous dysplasia, and regional odontodysplasia.1 Little information is available concerning the treatment of patients with this condition.15 The reported treatment modalities include combined surgical and orthodontic treatment of unerupted teeth (premolar/canine), prosthodontic treatment, gingivoplasty, recontouring osteotomy for severe facial asymmetry, and reconstructive jaw surgery.1,6,11,15 Successful treatment of Becker nevi with the Q-switched ruby laser, erbium:YAG laser, and 755-nm alexandrite laser have been reported.22-24
Conclusion
There is a need for continued reporting of cases of HATS syndrome in addition to long-term follow-up to document the natural history of the condition and to establish the appropriate treatment.
1. Friedlander-Barenboim S, Kamburoglu K, Kaffe I. Clinical and radiological presentation of hemimaxillofacial dysplasia/segmental odontomaxillary dysplasia: critical analysis and report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:268-273.
2. Miles DA, Lovas JL, Cohen MM. Hemimaxillofacial dysplasia: a newly recognized disorder of facial asymmetry, hypertrichosis of the facial skin, unilateral enlargement of the maxilla, and hypoplastic teeth in two patients. Oral Surg Oral Med Oral Pathol. 1987;64:445-448.
3. Danforth RA, Melrose RJ, Abrams AM, et al. Segmental odontomaxillary dysplasia: report of eight cases and comparison with hemimaxillofacial dysplasia. Oral Surg Oral Med Oral Pathol. 1990;70:81-85.
4. Desalvo MS, Copete MA, Riesenberger RE, et al. Segmental odontomaxillary dysplasia (hemimaxillofacial dysplasia): case report. Pediatr Dent. 1996;18:154-156.
5. Packota GV, Pharoah MJ, Petrikowski CG. Radiographic features of segmental odontomaxillary dysplasia: a study of 12 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:577-584.
6. Paticoff K, Marion RW, Shprintzen RJ, et al. Hemimaxillofacial dysplasia: a report of two new cases and further delineation of the disorder. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:484-488.
7. Jones AC, Ford MJ. Simultaneous occurrence of segmental odontomaxillary dysplasia and Becker’s nevus. J Oral Maxillofac Surg. 1990;57:1251-1254.
8. Prusack N, Pringle G, Scotti V, et al. Segmental odontomaxillary dysplasia: a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:483-488.
9. Becktor KB, Reibel J, Vedel B, et al. Segmental odontomaxillary dysplasia: clinical, radiological and histological aspects of four cases. Oral Dis. 2002;8:106-110.
10. Velez I, Vedrenne D, Cralle P, et al. Segmental odontomaxillary dysplasia: report of two cases and review of the literature. Todays FDA. 2002;14:20-21.
11. Drake DL. Segmental odontomaxillary dysplasia: an unusual orthodontic challenge. Am J Orthod Dentofac Orthop. 2003;123:84-86.
12. Gavalda C. Segmental odontomaxillary dysplasia. Med Oral. 2004;9:181.
13. Ozpinar B, Gokce B, Uzel G, et al. Prosthetic rehabilitation of segmental odontomaxillary dysplasia: seven-year follow-up. Cleft Palate Craniofac J. 2009;46:103-107.
14. Kuklani RM, Nair MK. Segmental odontomaxillary dysplasia: review of the literature and case report [published online ahead of print December 14, 2010]. Int J Dent. 2010;2010:837283.
15. Minett CP, Daley TD. Hemimaxillofacial dysplasia (segmental odontomaxillary dysplasia): case study with 11 years of follow-up from primary to adult dentition. J Oral Maxillofac Surg. 2012;70:1183-1191.
16. Whitt JC, Rokos JW, Dunlap CL, et al. Segmental odontomaxillary dysplasia: report of a series of 5 cases with long-term follow-up. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2011;112:E29-E47.
17. Welsch MJ, Stein SL. A syndrome of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS). Pediatr Dermatol. 2004;21:448-451.
18. Armstrong C, Napier SS, Boyd RC, et al. Histopathology of teeth in segmental odontomaxillary dysplasia: new findings. J Oral Pathol Med. 2004;33:246-248.
19. Porwal R, Ousterhout DK, Hoffman WY, et al. Hemimaxillofacial dysplasia: a variable presentation. J Craniofac Surg. 2008;19:1554-1557.
20. Koenig LJ, Lynch DP, Yancey KB. Segmental odontomaxillary dysplasia presenting with facial hypertrichosis, commissural lip clefting, and hyperlinear palm. Pediatr Dermatol. 2008;25:491-492.
21. Bhatia SK, Drage N, Cronin AJ, et al. Case report: segmental odontomaxillary dysplasia—a rare disorder. Eur Arch Paediatr Dent. 2008;9:245-248.
22. Raulin C, Schönermark MP, Greve B, et al. Q-switched ruby laser treatment of tattoos and benign pigmented skin lesions: a critical review. Ann Plast Surg. 1998;41:555-565.
23. Trelles MA, Allones I, Moreno-Arias GA, et al. Becker’s naevus: a comparative study between erbium: YAG and Q-switched neodymium:YAG; clinical and histopathological findings. Br J Dermatol. 2005;152:308-313.
24. Choi JE, Kim JW, Seo SH, et al. Treatment of Becker’s nevi with a long-pulse alexandrite laser. Dermatol Surg. 2009;35:1105-1108.
1. Friedlander-Barenboim S, Kamburoglu K, Kaffe I. Clinical and radiological presentation of hemimaxillofacial dysplasia/segmental odontomaxillary dysplasia: critical analysis and report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:268-273.
2. Miles DA, Lovas JL, Cohen MM. Hemimaxillofacial dysplasia: a newly recognized disorder of facial asymmetry, hypertrichosis of the facial skin, unilateral enlargement of the maxilla, and hypoplastic teeth in two patients. Oral Surg Oral Med Oral Pathol. 1987;64:445-448.
3. Danforth RA, Melrose RJ, Abrams AM, et al. Segmental odontomaxillary dysplasia: report of eight cases and comparison with hemimaxillofacial dysplasia. Oral Surg Oral Med Oral Pathol. 1990;70:81-85.
4. Desalvo MS, Copete MA, Riesenberger RE, et al. Segmental odontomaxillary dysplasia (hemimaxillofacial dysplasia): case report. Pediatr Dent. 1996;18:154-156.
5. Packota GV, Pharoah MJ, Petrikowski CG. Radiographic features of segmental odontomaxillary dysplasia: a study of 12 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:577-584.
6. Paticoff K, Marion RW, Shprintzen RJ, et al. Hemimaxillofacial dysplasia: a report of two new cases and further delineation of the disorder. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:484-488.
7. Jones AC, Ford MJ. Simultaneous occurrence of segmental odontomaxillary dysplasia and Becker’s nevus. J Oral Maxillofac Surg. 1990;57:1251-1254.
8. Prusack N, Pringle G, Scotti V, et al. Segmental odontomaxillary dysplasia: a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:483-488.
9. Becktor KB, Reibel J, Vedel B, et al. Segmental odontomaxillary dysplasia: clinical, radiological and histological aspects of four cases. Oral Dis. 2002;8:106-110.
10. Velez I, Vedrenne D, Cralle P, et al. Segmental odontomaxillary dysplasia: report of two cases and review of the literature. Todays FDA. 2002;14:20-21.
11. Drake DL. Segmental odontomaxillary dysplasia: an unusual orthodontic challenge. Am J Orthod Dentofac Orthop. 2003;123:84-86.
12. Gavalda C. Segmental odontomaxillary dysplasia. Med Oral. 2004;9:181.
13. Ozpinar B, Gokce B, Uzel G, et al. Prosthetic rehabilitation of segmental odontomaxillary dysplasia: seven-year follow-up. Cleft Palate Craniofac J. 2009;46:103-107.
14. Kuklani RM, Nair MK. Segmental odontomaxillary dysplasia: review of the literature and case report [published online ahead of print December 14, 2010]. Int J Dent. 2010;2010:837283.
15. Minett CP, Daley TD. Hemimaxillofacial dysplasia (segmental odontomaxillary dysplasia): case study with 11 years of follow-up from primary to adult dentition. J Oral Maxillofac Surg. 2012;70:1183-1191.
16. Whitt JC, Rokos JW, Dunlap CL, et al. Segmental odontomaxillary dysplasia: report of a series of 5 cases with long-term follow-up. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2011;112:E29-E47.
17. Welsch MJ, Stein SL. A syndrome of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS). Pediatr Dermatol. 2004;21:448-451.
18. Armstrong C, Napier SS, Boyd RC, et al. Histopathology of teeth in segmental odontomaxillary dysplasia: new findings. J Oral Pathol Med. 2004;33:246-248.
19. Porwal R, Ousterhout DK, Hoffman WY, et al. Hemimaxillofacial dysplasia: a variable presentation. J Craniofac Surg. 2008;19:1554-1557.
20. Koenig LJ, Lynch DP, Yancey KB. Segmental odontomaxillary dysplasia presenting with facial hypertrichosis, commissural lip clefting, and hyperlinear palm. Pediatr Dermatol. 2008;25:491-492.
21. Bhatia SK, Drage N, Cronin AJ, et al. Case report: segmental odontomaxillary dysplasia—a rare disorder. Eur Arch Paediatr Dent. 2008;9:245-248.
22. Raulin C, Schönermark MP, Greve B, et al. Q-switched ruby laser treatment of tattoos and benign pigmented skin lesions: a critical review. Ann Plast Surg. 1998;41:555-565.
23. Trelles MA, Allones I, Moreno-Arias GA, et al. Becker’s naevus: a comparative study between erbium: YAG and Q-switched neodymium:YAG; clinical and histopathological findings. Br J Dermatol. 2005;152:308-313.
24. Choi JE, Kim JW, Seo SH, et al. Treatment of Becker’s nevi with a long-pulse alexandrite laser. Dermatol Surg. 2009;35:1105-1108.
Practice Points
- Appropriate management and treatment of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS syndrome) requires a multidisciplinary team including a dermatologist, dentist, radiologist, and orthopedic surgeon.
- Becker nevus is among the most common cutaneous manifestations of HATS syndrome and can be treated effectively with the Q-switched laser or the erbium:YAG laser.
Flurbiprofen-Induced Unilateral Eyelid Angioedema
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
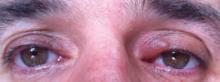
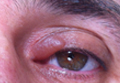
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
To the Editor:
Flurbiprofen, a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to treat fever, inflammation, and pain of arthritis.1 The exact prevalence of allergic reactions to NSAIDs in the general population is not known. Rhinoconjunctivitis, bronchospasm, urticaria, angioedema, and anaphylaxis can occur as an allergic reaction to NSAIDs. Isolated angioedema following NSAID ingestion typically involves the face, particularly the periorbital skin, lips, and mouth.2 These patients may develop urticaria and/or angioedema only after NSAID ingestion, but they do not have underlying chronic urticaria. We report a rare case of isolated unilateral eyelid angioedema with flurbiprofen.
A 39-year-old man presented with the onset of unilateral angioedema of the left upper eyelid that had developed approximately 30 minutes after taking flurbiprofen (100 mg). He reported frequent use of flurbiprofen for headaches. The patient also had a history of taking aspirin, ibuprofen, diclofenac, etodolac, and naproxen sodium as needed for migraines with no prior angioedema. He had no history of chronic urticaria or allergic disease. The patient was treated with oral pheniramine hydrogen maleate and angioedema resolved after 12 hours. Three days later, the patient used flurbiprofen again for a headache. He was readmitted to our clinic with unilateral angioedema of the left upper eyelid (Figure). The symptoms started approximately 30 minutes after taking flurbiprofen. Angioedema resolved within 1 day with oral pheniramine.
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed class of drugs in the world and are the most common cause of all adverse drug reactions.3 Urticaria, angioedema, and anaphylaxis are common adverse reactions to NSAIDs. The prevalence of urticaria and angioedema to NSAIDs has been reported to be 0.1% to 3% worldwide.4
Angioedema is an abrupt localized swelling of the skin and mucous membranes of the face, lips, mouth, throat, larynx, extremities, and genitalia. Angioedema generally develops over minutes to hours and resolves in 24 to 48 hours.5 Angioedema without urticaria is the clinical syndrome that can be caused by an adverse drug reaction. In an Italian review of 2137 reactions, NSAIDs were causative agents in 33.6% of patients with drug-induced angioedema.6 In another study, Leeyaphan et al5 reported that 50% of patients with drug-induced angioedema resulted from NSAIDs, commonly with ibuprofen and diclofenac. Although angioedema is due to inhibition of cyclooxygenase 1, overproduction of leukotrienes, and possibly IgE-mediated reactions to single drugs,7 localized unilateral eyelid angioedema with NSAIDs is rare. The exact mechanism of localized eyelid edema is not known.8 We believe that the unilateral eyelid angioedema in our patient was caused by flurbiprofen use because the reaction recurred when the drug was used again.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
1. Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339-345.
2. Asero R. Multiple sensitivity to NSAID. Allergy. 2000;55:893-894.
3. Nettis E, Colanardi MC, Ferrannini A, et al. Update on sensitivity to nonsteroidal anti-inflammatory drugs. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:233-240.
4. Kulthanan K, Jiamton S, Boochangkool K, et al. Angioedema: clinical and etiological aspects. Clin Dev Immunol. 2007;2007:26438.
5. Leeyaphan C, Kulthanan K, Jongiarearnprasert K, et al. Drug-induced angioedema without urticaria: prevalence and clinical features [published online ahead of print November 17, 2009]. J Eur Acad Dermatol Venereol. 2010;24:685-691.
6. Cutaneous reactions to analgesic-antipyretics and nonsteroidal anti-inflammatory drugs. analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology. 1993;186:164-169.
7. Stevenson OE, Finch TM. Allergic contact dermatitis from rectified camphor oil in Earex ear drops. Contact Dermatitis. 2003;49:51.
8. Tsuruta D, Oshimo T, Sowa J, et al. Unilateral eyelid angioedema with congestion of the right bulbar conjunctiva due to loxoprofen sodium. Cutis. 2011;87:41-43.
New insight into HSCs’ role in hematopoiesis
allows scientists to identify
differences in blood cell origin
Credit: Camargo Lab
By developing a tracking system for stem cells, researchers may have discovered previously unrecognized features of hematopoiesis.
Their work suggests the main drivers of steady-state hematopoiesis are not hematopoietic stem cells (HSCs) but their less pluripotent descendants, progenitor cells.
The team speculates that stable populations of long-lived progenitor cells are responsible for giving rise to specific blood cell types, while HSCs likely act as essential reserves.
The research, published in Nature, indicates that progenitor cells could be just as valuable as HSCs for blood regeneration therapies.
The work challenges what textbooks have long read: that HSCs maintain the day-to-day renewal of blood, a conclusion drawn from their importance in re-establishing blood cell populations after bone marrow transplants.
Due to a lack of tools to study how blood forms in a normal context, no one was able to track the origin of blood cells without doing a transplant.
Fernando Camargo, PhD, of Boston Children’s Hospital in Massachusetts, and his colleagues addressed this problem with a tool that generates a unique barcode in the DNA of all HSCs and their progenitor cells in a mouse.
When a tagged cell divides, all of its descendant cells possess the same barcode. This biological inventory system makes it possible to determine the number of HSCs/progenitors being used to make blood and how long they live, as well as answer fundamental questions about where individual blood cells come from.
“There’s never been such a robust experimental method that could allow people to look at lineage relationships between mature cell types in the body without doing transplantation,” said study author Jianlong Sun, PhD, also of Boston Children’s Hospital.
“One of the major directions we can now go is to revisit the entire blood cell hierarchy and see how the current knowledge holds true when we use this internal labeling system.”
“People have tried using viruses to tag blood cells in the past, but the cells needed to be taken out of the body, infected, and re-transplanted, which raised a number of issues,” Dr Camargo noted. “I wanted to figure out a way to label blood cells inside of the body, and the best idea I had was to use mobile genetic elements called transposons.”
A transposon is a piece of genetic code that can jump to a random point in DNA when exposed to the enzyme transposase. Dr Camargo’s approach works using transgenic mice that possess a single fish-derived transposon in all of their blood cells.
When one of these mice is exposed to transposase, each of its blood cells’ transposons changes location. The location in the DNA where a transposon moves acts as an individual cell’s barcode, so that if the mouse’s blood is taken a few months later, any cell with the same transposon location can be linked back to its parent cell.
Now, the researchers are planning to explore more applications for their barcode tool.
“We are also tremendously excited to use this tool to barcode and track descendants of different stem cells or progenitor cells for a range of conditions, from aging to the normal immune response,” Dr Sun said.
“We first used this technology for blood analysis. However, this system can also help address basic questions about cell populations in solid tissue. You can imagine being able to look at tumor progression or identify the precise origins of cancer cells that have broken off from a tumor and are now circulating in the blood.” ![]()

allows scientists to identify
differences in blood cell origin
Credit: Camargo Lab
By developing a tracking system for stem cells, researchers may have discovered previously unrecognized features of hematopoiesis.
Their work suggests the main drivers of steady-state hematopoiesis are not hematopoietic stem cells (HSCs) but their less pluripotent descendants, progenitor cells.
The team speculates that stable populations of long-lived progenitor cells are responsible for giving rise to specific blood cell types, while HSCs likely act as essential reserves.
The research, published in Nature, indicates that progenitor cells could be just as valuable as HSCs for blood regeneration therapies.
The work challenges what textbooks have long read: that HSCs maintain the day-to-day renewal of blood, a conclusion drawn from their importance in re-establishing blood cell populations after bone marrow transplants.
Due to a lack of tools to study how blood forms in a normal context, no one was able to track the origin of blood cells without doing a transplant.
Fernando Camargo, PhD, of Boston Children’s Hospital in Massachusetts, and his colleagues addressed this problem with a tool that generates a unique barcode in the DNA of all HSCs and their progenitor cells in a mouse.
When a tagged cell divides, all of its descendant cells possess the same barcode. This biological inventory system makes it possible to determine the number of HSCs/progenitors being used to make blood and how long they live, as well as answer fundamental questions about where individual blood cells come from.
“There’s never been such a robust experimental method that could allow people to look at lineage relationships between mature cell types in the body without doing transplantation,” said study author Jianlong Sun, PhD, also of Boston Children’s Hospital.
“One of the major directions we can now go is to revisit the entire blood cell hierarchy and see how the current knowledge holds true when we use this internal labeling system.”
“People have tried using viruses to tag blood cells in the past, but the cells needed to be taken out of the body, infected, and re-transplanted, which raised a number of issues,” Dr Camargo noted. “I wanted to figure out a way to label blood cells inside of the body, and the best idea I had was to use mobile genetic elements called transposons.”
A transposon is a piece of genetic code that can jump to a random point in DNA when exposed to the enzyme transposase. Dr Camargo’s approach works using transgenic mice that possess a single fish-derived transposon in all of their blood cells.
When one of these mice is exposed to transposase, each of its blood cells’ transposons changes location. The location in the DNA where a transposon moves acts as an individual cell’s barcode, so that if the mouse’s blood is taken a few months later, any cell with the same transposon location can be linked back to its parent cell.
Now, the researchers are planning to explore more applications for their barcode tool.
“We are also tremendously excited to use this tool to barcode and track descendants of different stem cells or progenitor cells for a range of conditions, from aging to the normal immune response,” Dr Sun said.
“We first used this technology for blood analysis. However, this system can also help address basic questions about cell populations in solid tissue. You can imagine being able to look at tumor progression or identify the precise origins of cancer cells that have broken off from a tumor and are now circulating in the blood.” ![]()

allows scientists to identify
differences in blood cell origin
Credit: Camargo Lab
By developing a tracking system for stem cells, researchers may have discovered previously unrecognized features of hematopoiesis.
Their work suggests the main drivers of steady-state hematopoiesis are not hematopoietic stem cells (HSCs) but their less pluripotent descendants, progenitor cells.
The team speculates that stable populations of long-lived progenitor cells are responsible for giving rise to specific blood cell types, while HSCs likely act as essential reserves.
The research, published in Nature, indicates that progenitor cells could be just as valuable as HSCs for blood regeneration therapies.
The work challenges what textbooks have long read: that HSCs maintain the day-to-day renewal of blood, a conclusion drawn from their importance in re-establishing blood cell populations after bone marrow transplants.
Due to a lack of tools to study how blood forms in a normal context, no one was able to track the origin of blood cells without doing a transplant.
Fernando Camargo, PhD, of Boston Children’s Hospital in Massachusetts, and his colleagues addressed this problem with a tool that generates a unique barcode in the DNA of all HSCs and their progenitor cells in a mouse.
When a tagged cell divides, all of its descendant cells possess the same barcode. This biological inventory system makes it possible to determine the number of HSCs/progenitors being used to make blood and how long they live, as well as answer fundamental questions about where individual blood cells come from.
“There’s never been such a robust experimental method that could allow people to look at lineage relationships between mature cell types in the body without doing transplantation,” said study author Jianlong Sun, PhD, also of Boston Children’s Hospital.
“One of the major directions we can now go is to revisit the entire blood cell hierarchy and see how the current knowledge holds true when we use this internal labeling system.”
“People have tried using viruses to tag blood cells in the past, but the cells needed to be taken out of the body, infected, and re-transplanted, which raised a number of issues,” Dr Camargo noted. “I wanted to figure out a way to label blood cells inside of the body, and the best idea I had was to use mobile genetic elements called transposons.”
A transposon is a piece of genetic code that can jump to a random point in DNA when exposed to the enzyme transposase. Dr Camargo’s approach works using transgenic mice that possess a single fish-derived transposon in all of their blood cells.
When one of these mice is exposed to transposase, each of its blood cells’ transposons changes location. The location in the DNA where a transposon moves acts as an individual cell’s barcode, so that if the mouse’s blood is taken a few months later, any cell with the same transposon location can be linked back to its parent cell.
Now, the researchers are planning to explore more applications for their barcode tool.
“We are also tremendously excited to use this tool to barcode and track descendants of different stem cells or progenitor cells for a range of conditions, from aging to the normal immune response,” Dr Sun said.
“We first used this technology for blood analysis. However, this system can also help address basic questions about cell populations in solid tissue. You can imagine being able to look at tumor progression or identify the precise origins of cancer cells that have broken off from a tumor and are now circulating in the blood.” ![]()
NICE rejects obinutuzumab for CLL

Credit: Linda Bartlett
In a new draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) has said it cannot recommend obinutuzumab (Gazyvaro) to treat chronic lymphocytic leukemia (CLL).
NICE CEO Sir Andrew Dillon said that although data suggest obinutuzumab is effective, there were too many “uncertainties” in the information submitted by Roche, the company developing the drug.
So NICE cannot be sure obinutuzumab would be an effective use of the National Health Service’s resources.
This is despite the fact that Roche offered to discount the drug’s list price of £26,496 per treatment course.
NICE is accepting comments on the draft guidance until 5 pm on October 23.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells. The drug induces antibody-dependent cellular cytotoxicity and caspase-independent apoptosis.
The European Commission approved obinutuzumab in July for use in combination with chlorambucil to treat patients with previously untreated CLL who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab was approved for this indication in the US in November 2013.
Obinutuzumab is marketed as Gazyvaro in the European Union and Switzerland but as Gazyva in the US and the rest of the world. ![]()

Credit: Linda Bartlett
In a new draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) has said it cannot recommend obinutuzumab (Gazyvaro) to treat chronic lymphocytic leukemia (CLL).
NICE CEO Sir Andrew Dillon said that although data suggest obinutuzumab is effective, there were too many “uncertainties” in the information submitted by Roche, the company developing the drug.
So NICE cannot be sure obinutuzumab would be an effective use of the National Health Service’s resources.
This is despite the fact that Roche offered to discount the drug’s list price of £26,496 per treatment course.
NICE is accepting comments on the draft guidance until 5 pm on October 23.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells. The drug induces antibody-dependent cellular cytotoxicity and caspase-independent apoptosis.
The European Commission approved obinutuzumab in July for use in combination with chlorambucil to treat patients with previously untreated CLL who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab was approved for this indication in the US in November 2013.
Obinutuzumab is marketed as Gazyvaro in the European Union and Switzerland but as Gazyva in the US and the rest of the world. ![]()

Credit: Linda Bartlett
In a new draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) has said it cannot recommend obinutuzumab (Gazyvaro) to treat chronic lymphocytic leukemia (CLL).
NICE CEO Sir Andrew Dillon said that although data suggest obinutuzumab is effective, there were too many “uncertainties” in the information submitted by Roche, the company developing the drug.
So NICE cannot be sure obinutuzumab would be an effective use of the National Health Service’s resources.
This is despite the fact that Roche offered to discount the drug’s list price of £26,496 per treatment course.
NICE is accepting comments on the draft guidance until 5 pm on October 23.
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells. The drug induces antibody-dependent cellular cytotoxicity and caspase-independent apoptosis.
The European Commission approved obinutuzumab in July for use in combination with chlorambucil to treat patients with previously untreated CLL who have comorbidities that make them ineligible to receive fludarabine-based therapy.
Obinutuzumab was approved for this indication in the US in November 2013.
Obinutuzumab is marketed as Gazyvaro in the European Union and Switzerland but as Gazyva in the US and the rest of the world. ![]()
Pediatric Surgical Comanagement
According to the 2012 Society of Hospital Medicine (SHM) survey, 94% of adult hospitalists and 74% of pediatric hospitalists provide inpatient care to surgical patients.[1] Many of these programs involve comanagement, which the SHM Comanagement Advisory Panel has described as a system of care featuring shared responsibility, authority, and accountability for hospitalized patients with medical and surgical needs.[2] Collaboration between medical and surgical teams for these patients has occurred commonly at some community institutions for decades, but may only be emerging at some tertiary care hospitals. The trend of comanagement appears to be increasing in popularity in adult medicine.[3] As in adult patients, comanagement for children undergoing surgical procedures, particularly those children with special healthcare needs (CSHCNs), has been proposed as a strategy for improving quality and costs. In this review, we will describe structural, quality, and financial implications of pediatric hospitalist comanagement programs, each of which include both potential benefits and drawbacks, as well as discuss a future research agenda for these programs.
ORGANIZATIONAL NEEDS AND STRUCTURE OF COMANAGEMENT PROGRAMS
Patterns of comanagement likely depend on hospital size and structure, both in adult patients[3] and in pediatrics. Children hospitalized for surgical procedures generally fall into 1 of 2 groups: those who are typically healthy and at low risk for complications, and those who are medically complex and at high risk. Healthy children often undergo high‐prevalence, low‐complexity surgical procedures such as tonsillectomy and hernia repairs;[4] these patients are commonly cared for at community hospitals by adult and pediatric surgeons. Whereas medically complex children also undergo these common procedures, they are more likely to be cared for at tertiary care centers and are also more likely to undergo higher‐complexity surgeries such as spinal fusions, hip osteotomies, and ventriculoperitoneal shunt placements.[4] Hospitalist comanagement programs at community hospitals and tertiary care centers may therefore have evolved differently in response to different needs of patients, providers, and organizations,[5] though some institutions may not fall neatly into 1 of these 2 categories.
Comanagement in Community Hospitals
A significant number of pediatric patients are hospitalized each year in community hospitals.[6] As noted above, children undergoing surgery in these settings are generally healthy, and may be cared for by surgeons with varying amounts of pediatric expertise. In this model, the surgeon may frequently be offsite when not in the operating room, necessitating some type of onsite postoperative coverage. In pediatrics, following adult models, this coverage need may be relatively straightforward: surgeons perform the procedure, followed by a medical team assuming postoperative care with surgical consultation. Because of general surgeons' varying experience with children, the American Academy of Pediatrics suggests that patients younger than 14 years or weighing less than 40 kg cared for by providers without routine pediatric experience should have a pediatric‐trained provider involved in their care,[7] though this suggestion does not mandate comanagement.
For children cared for by adult providers who have little experience with areas of pediatric‐specific care such as medication dosing and assessment of deterioration, we believe that involvement of a pediatric provider may impact care in a number of ways. A pediatric hospitalist's availability on the inpatient unit may allow him or her to better manage routine issues such as pain control and intravenous fluids without surgeon input, improving the efficiency of care. A pediatric hospitalist's general pediatric training may allow him or her to more quickly recognize when a child is medically deteriorating, or when transfer to a tertiary care center may be necessary, making care safer. However, no studies have specifically examined these variables. Future research should measure outcomes such as transfers to higher levels of care, medication errors, length of stay (LOS), and complication rates, especially in community hospital settings.
Comanagement in Tertiary Care Referral Centers
At tertiary care referral centers, surgeries in children are most often performed by pediatric surgeons. In these settings, providing routine hospitalist comanagement to all patients may be neither cost‐effective nor feasible. Adult studies have suggested that population‐targeted models can significantly improve several clinical outcomes. For example, in several studies of patients 65 years and older hospitalized with hip fractures, comanagement with a geriatric hospitalist was associated with improved clinical outcomes and shortened LOS.[8, 9, 10, 11, 12]
An analogous group of pediatric patients to the geriatric population may be CSHCNs or children who are medically complex. Several frameworks have been proposed to identify these patients.[13] Many institutions classify medically complex patients as those with complex chronic medical conditions (CCCs).[13, 14, 15] One framework to identify CSHCNs suggested including not only children with CCCs, but also those with (1) substantial service needs and/or family burden; (2) severe functional limitations; and/or (3) high rates of healthcare system utilization, often requiring the care of several subspecialty providers.[16] As the needs of these patients may be quite diverse, pediatric hospitalists may be involved in many aspects of their care, from preoperative evaluation,[17] to establishing protocols for best practices, to communicating with primary care providers, and even seeing patients in postoperative follow‐up clinics. These patients are known to be at high risk for surgical complications, readmissions,[14] medical errors,[18] lapses in communication, and high care costs. In 1 study, comanagement for children with neuromuscular scoliosis hospitalized for spinal fusion surgery has been associated with shorter LOS and less variability in LOS.[19] However, drawbacks of comanagement programs involving CSHCNs may include difficulty with consistent identification of the population who will most benefit from comanagement and higher initial costs of care.[18]
Models of Comanagement and Comanagement Agreements
A comanagement agreement should address 5 major questions: (1) Who is the primary service? (2) Who is the consulting/comanaging service? (3) Are consults as‐needed or automatic? (4) Who writes orders for the patient? (5) Which staffing model will be used for patient care?[20] Although each question above may be answered differently in different systems, the correct comanagement program is a program that aligns most closely with the patient population and care setting.[11, 20]
Several different models exist for hospitalistsurgeon comanagement programs[20, 21] (Table 1). Under the consultation model (model I), hospitalists become involved in the care of surgical patients only when requested to do so by the surgical team. Criteria for requesting this kind of consultation and the extent of responsibility afforded to the medical team are often not clearly defined, and may differ from hospital to hospital or even surgeon to surgeon.[22] Hospitalist involvement with adult patients with postoperative medical complications, which presumably employed this as‐needed model, has been associated with lower mortality and LOS[23]; whether this involvement provides similar benefits in children with postoperative complications has not been explicitly studied.
| Model | Attending Service | Consulting Service | Automatic Consultation | Who Writes Orders? | Notes |
|---|---|---|---|---|---|
| |||||
| I | Surgery | Pediatrics | No | Surgery | Similar to traditional consultation |
| II | Surgery | Pediatrics | Yes | Usually surgery | Basic comanagement, consultant may sign off |
| III | Pediatrics | Surgery | Yes | Usually pediatrics | Basic comanagement, consultant may sign off |
| IV | Combined | N/A | N/A | Each service writes own | True comanagement, no sign‐off from either service permitted |
The remaining models involve compulsory participation by both surgical and medical services. In models II and III, patients may be evaluated preoperatively; those felt to meet specific criteria for high medical complexity are either admitted to a medical service with automatic surgical consultation or admitted to a surgical service with automatic medical consultation. In both cases, writing of orders is handled in the same manner as any consultation model; depending on the service agreement, consulting services may sign off or may be required to be involved until discharge. In model IV, care is fully comanaged by medical and surgical services, with each service having ownership over orders pertaining to their discipline. Ethical concerns about such agreements outlined by the American Medical Association include whether all patients cared for under agreements II to IV will truly benefit from the cost of multispecialty care, and whether informed consent from patients themselves should be required given the cost implications.[24]
Comanagement models also vary with respect to frontline provider staffing. Models may incorporate nurse practitioners, hospitalists, physician assistants, or a combination thereof. These providers may assume a variety of roles, including preoperative patient evaluation, direct care of patients while hospitalized, and/or coordination of inpatient and outpatient postoperative care. Staffing requirements for hospitalists and/or mid‐level providers will differ significantly at different institutions based on surgical volume, patient complexity, and other local factors.
COMANAGEMENT AND QUALITY
Comanagement as a Family‐Centered Initiative
Development of a family‐centered culture of care, including care coordination, lies at the core of pediatric hospital medicine, particularly for CSHCNs.[25, 26] In the outpatient setting, family‐centered care has been associated with improved quality of care for CSHCNs.[27, 28] For families of hospitalized children, issues such as involvement in care and timely information transfer have been identified as high priorities.[29] An important tool for addressing these needs is family‐centered rounds (FCRs), which represent multidisciplinary rounds at the bedside involving families and patients as active shared decision makers in conjunction with the medical team.[30, 31] Although FCRs have not been studied in comanagement arrangements specifically, evidence suggests that this tool improves family centeredness and patient safety in nonsurgical patients,[32] and FCRs can likely have a similar impact on postoperative care.
A pediatric hospitalist comanagement program may impact quality and safety of care in a number of other ways. Hospitalists may offer improved access to clinical information for nurses and families, making care safer. One study of comanagement in adult neurosurgical patients found that access to hospitalists led to improved quality and safety of care as perceived by nurses and other members of the care team.[33] A study in pediatric patients found that nurses overwhelmingly supported having hospitalist involvement in complex children undergoing surgery; the same study found that pediatric hospitalists were particularly noted for their communication skills.[34]
Assessing Clinical Outcomes in Pediatric Hospitalist Comanagement Programs
Most studies evaluating the impact of surgical comanagement programs have focused on global metrics such as LOS, overall complication rates, and resource utilization. In adults, results of these studies have been mixed, suggesting that patient selection may be an important factor.[35] In pediatrics, 2 US studies have assessed these metrics at single centers. Simon et al. found that involvement of a pediatric hospitalist in comanagement of patients undergoing spinal fusion surgery significantly decreased LOS.[19] Rappaport et al. found that patients comanaged by hospitalists had lower utilization of laboratory tests and parenteral nutrition, though initial program costs significantly increased.[36] Studies outside the United States, including a study from Sweden,[37] have suggested that a multidisciplinary approach to children's surgical care, including the presence of pediatric specialists, reduced infection rate and other complications. These studies provide general support for the role of hospitalists in comanagement, although determining which aspects of care are most impacted may be difficult.
Comanagement programs might impact safety and quality negatively as well. Care may be fragmented, leading to provider and family dissatisfaction. Poor communication and multiple handoffs among multidisciplinary team members might interfere with the central role of the nurse in patient care.[38] Comanagement programs might lead to provider disengagement if providers feel that others will assume roles with which they may be unfamiliar or poorly trained.[35] This lack of knowledge may also affect communication with families, leading to conflicting messages among the care team and family frustration. In addition, the impact of comanagement programs on trainees such as residents, both surgical and pediatric, has received limited study.[39] Assessing pediatric comanagement programs' impact on communication, family‐centeredness, and trainees deserves further study.
FINANCIAL IMPLICATIONS OF PEDIATRIC COMANAGEMENT PROGRAMS
Children undergoing surgery require significant financial resources for their care. A study of 38 major US children's hospitals found that 3 of the top 10 conditions with the highest annual expenditures were surgical procedures.[4] The most costly procedure was spinal fusion for scoliosis, accounting for an average of $45,000 per admission and $610 million annually. Although a significant portion of these costs represented surgical devices and operating room time, these totals also included the cost of hospital services and in‐hospital complications. CSHCNs more often undergo high‐complexity procedures such as spinal fusions[36] and face greater risk for costly postoperative complications. The financial benefits that come from reductions in outcomes such as LOS and readmissions in this population are potentially large, but may depend on the payment model as described below.
Billing Models in Comanagement Programs
Several billing constructs exist in comanagement models. At many institutions, comanagement billing may resemble that for traditional consultation: the pediatric hospitalist bills for his/her services using standard initial and subsequent consultation billing coding for the child's medical conditions, and may sign off when the hospitalist feels recommendations are complete. Other models may also exist. Model IV comanagement may involve a prearranged financial agreement, in which billing modifiers are used to differentiate surgical care only (modifier 54) and postoperative medical care only (modifier 55). These modifiers, typically used for Medicare patients, indicate a split in a global surgical fee.[40]
The SHM has outlined financial considerations that should be addressed at the time of program inception and updated periodically, including identifying how each party will bill, who bills for which service, and monitoring collection rates and rejected claims.[2] Regardless of billing model, the main focus of comanagement must be quality of care, not financial considerations; situations in which the latter are emphasized at the expense of patient care may be unethical or illegal.[24] Regardless, surgical comanagement programs should seek maximal reimbursement in order to remain viable.
Value of Comanagement for a Healthcare Organization Under Fee‐for‐Service Payment
The value of any comanagement program is highly dependent on both the institution's payor mix and the healthcare organization's overarching goals. From a business perspective, a multidisciplinary approach may be perceived as resource intensive, but formal cost‐effectiveness analyses over time are limited. Theoretically, a traditional payment model involving hospitalist comanagement would be financially beneficial for a healthcare institution by allowing surgeons and surgical trainees more time to operate. However, these savings are difficult to quantify. Despite the fact that most hospitalist programs have no direct financial benefit to the institution, many hospital leaders seem willing to subsidize hospitalist programs based on measures such as patient and referring physician satisfaction with hospitalist care.[41] Postoperative complications, although unfortunate, may be a source of revenue under this model if paid by insurance companies in the usual manner, leading to a misalignment of quality and financial goals. Regardless, whether these programs are considered worthy investments to healthcare organizations will ultimately depend on evolving billing and reimbursement structures; to date, no formal survey of how comanagement programs bill and are reimbursed has been performed.
Value for an Organization in an Accountable Care Organization Model
Although a detailed discussion of accountable care organizations (ACOs) is beyond the scope of this article, stronger incentives in these structures for reducing resource utilization and complication rates may make hospitalist comanagement attractive in an ACO model.[42] ACO program evaluation is expected to be based on such data as patient surveys, documentation of care coordination, and several disease‐specific metrics.[43, 44] Several children's hospital‐based systems and mixed health systems have dedicated significant resources to establishing networks of providers that bridge inpatient and outpatient episodes of surgical care.[27, 28] One adult study has suggested lower costs associated with hospitalist comanagement for geriatric patients with hip fractures.[12] Comanagement programs may help meet quality and value goals, including enhancing care coordination between inpatient and outpatient care, and therefore may prove to be a beneficial investment for institutions. As the healthcare landscape evolves, formal study of the costs and benefits of pediatric comanagement models in ACO‐type care structures will be important.
SETTING A RESEARCH AGENDA
Pediatric hospitalist comanagement programs require vigorous study to evaluate their impact. Potential research targets include not only clinical data such as LOS, perioperative complication rates, readmission rates, and resource utilization, but also data regarding surgeon, nursing, and family satisfaction. These programs should also be evaluated in terms of how they impact trainees, both surgical and pediatric. Because of comanagement programs' complexities, we anticipate that they will impart both positive and negative effects on some of these factors. These programs will also require evaluation over time as they require significant education on the part of staff and families.[36]
In addition to affecting global metrics, pediatric hospitalists may also have a positive impact on surgical care by demonstrating leadership to improve systems of care relevant to surgical patients, including the use of guidelines. The American College of Surgeons' National Surgical Quality Improvement Program has identified 2 priorities that hospitalists may impact: surgical site infection (SSI) and pulmonary complications, which combined comprise greater than half of all 30‐day postoperative complications.[45] Regarding SSI prevention, hospitalist researchers are making valuable contributions to literature surrounding adherence to Centers for Disease Control and Prevention and Pediatric Orthopedic Society of North America guidelines.[46, 47, 48] Research is ongoing regarding how human and systems factors may impact the effectiveness of these guidelines, but also how reliably these guidelines are implemented.[49] In the area of pulmonary complications, pediatric hospitalists have followed the example of successful initiatives in adult surgery patients by developing and implementing postoperative protocols to prevent pulmonary complications such as postoperative pneumonia.[50] At 1 center, pediatric hospitalists have led efforts to implement a standardized respiratory care pathway for high‐risk orthopedic patients.[51] Evaluation of the effectiveness of such programs is currently ongoing, but early data show similar benefits to those demonstrated in adults.
CONCLUSIONS
Pediatric hospitalist comanagement programs for surgical patients have largely followed the path of adult programs. Limited data suggest that certain clinical outcomes may be improved under comanagement, but patient selection may be important. Although there is significant variety between programs, there exist several common themes, including the importance of clear delineation of roles and a central goal of improved care coordination. Ongoing research will hopefully shed more light on the impact of these programs, especially with regard to patient safety, hospitalist‐led quality‐improvement programs, and financial implications, particularly in different structures of care and reimbursement models.
Disclosure: Nothing to report.
- 2012 State of Hospital Medicine Report, Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/survey. Accessed on September 1, 2014.
- Society of Hospital Medicine Co‐Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co‐management. SHM website. Available at: http://tools.hospitalmedicine.org/Implementation/Co‐ManagementWhitePaper‐final_5‐10‐10.pdf. Accessed on September 25, 2014.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , . Pediatric hospitalist comanagement of surgical patients: challenges and opportunities. Clin Pediatr (Phila). 2008;47(2):114–121.
- , . Utilization of pediatric hospitals in New York State. Pediatrics. 2003;111(5 pt 1):1068–1071.
- ; Committee on Hospital Care. Physicians' roles in coordinating care of hospitalized children. Pediatrics. 2003;111(3):707–709.
- , , , et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219–225.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , . Geriatric co‐management of proximal femur fractures: total quality management and protocol‐driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356.
- , , , , , . Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , , . Pediatric hospital medicine and children with medical complexity: past, present, and future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):113–119.
- , , , et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140.
- , , , , . Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684–688.
- , , , , . Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , . Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189.
- . How best to design surgical comanagement services for pediatric surgical patients? Hosp Pediatr. 2013;3(3):242–243.
- , , , , , . Effects of provider characteristics on care coordination under comanagement. J Hosp Med. 2010;5(9):508–513.
- , , , . Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17(9):e333–e339.
- American Medical Association. 2008 report of the Council on Medical Service, policy sunset report for 1998 AMA socioeconomic policies. CMS Report 4, A‐08. Available at: http://www.ama‐assn.org/resources/doc/cms/a‐08cms4.pdf. Accessed on September 1, 2014.
- Committee on Hospital Care and Institute for Patient‐and Family‐Centered Care. Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129(2):394–404.
- Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460.
- , , , , , . Care coordination for CSHCN: associations with family‐provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428–S434.
- , , , et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.
- , , . Parents' priorities and satisfaction with acute pediatric care. Arch Pediatr Adolesc Med. 2005;159(2):127–131.
- . Family‐centered rounds. Pediatr Clin North Am. 2014;61(4):663–670.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- , , , , ; PIPS Group. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15(suppl 1):17–25.
- , , , et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004–2010.
- , , . Nurses' assessment of pediatric physicians: are hospitalists different? J Healthc Manag Am Coll Healthc Exec. 2008;53(1):14–24; discussion 24–25.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- , , , et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233–241.
- , , . Outcome of major spinal deformity surgery in high‐risk patients: comparison between two departments. Evid Based Spine Care J. 2010;1(3):11–18.
- , , , , , . Meeting the complex needs of the health care team: identification of nurse‐team communication practices perceived to enhance patient outcomes. Qual Health Res. 2010;20(1):15–28.
- , , , . A pediatric residency experience with surgical comanagement. Hosp Pediatr. 2013;3(2):144–148.
- U.S. Department of Health and Human Services. Centers for Medicaid and Medicare Services. MLN matters. Available at: http://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/Downloads/MM7872.pdf. Accessed on September 1,2014.
- , , ; Research Advisory Committee of the American Board of Pediatrics. Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- . Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364(16):e32.
- American Academy of Pediatrics. Accountable Care Organizations (ACOs) and Pediatricians: Evaluation and Engagement. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/Pages/Accountable‐Care‐Organizations‐and‐Pediatricians‐Evaluation‐and‐Engagement.aspx. Accessed on September 25, 2014.
- , , , , . Delivery system characteristics and their association with quality and costs of care: implications for accountable care organizations [published online ahead of print February 21, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000014.
- , , , et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121.
- , , , et al. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–278. Available at: http://www.cdc.gov/hicpac/pdf/guidelines/SSI_1999.pdf. Accessed on September 1, 2014.
- , , , et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high‐risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–478.
- , , , et al. Perioperative antibiotic use for spinal surgery procedures in US children's hospitals. Spine. 2013;38(7):609–616. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , , , , , . A human factors intervention to improve post‐operative antibiotic timing and prevent surgical site infection. Paper presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- , , , , . I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , . Early postoperative respiratory care improves outcomes, adds value for hospitalized pediatric orthopedic patients. Poster presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
According to the 2012 Society of Hospital Medicine (SHM) survey, 94% of adult hospitalists and 74% of pediatric hospitalists provide inpatient care to surgical patients.[1] Many of these programs involve comanagement, which the SHM Comanagement Advisory Panel has described as a system of care featuring shared responsibility, authority, and accountability for hospitalized patients with medical and surgical needs.[2] Collaboration between medical and surgical teams for these patients has occurred commonly at some community institutions for decades, but may only be emerging at some tertiary care hospitals. The trend of comanagement appears to be increasing in popularity in adult medicine.[3] As in adult patients, comanagement for children undergoing surgical procedures, particularly those children with special healthcare needs (CSHCNs), has been proposed as a strategy for improving quality and costs. In this review, we will describe structural, quality, and financial implications of pediatric hospitalist comanagement programs, each of which include both potential benefits and drawbacks, as well as discuss a future research agenda for these programs.
ORGANIZATIONAL NEEDS AND STRUCTURE OF COMANAGEMENT PROGRAMS
Patterns of comanagement likely depend on hospital size and structure, both in adult patients[3] and in pediatrics. Children hospitalized for surgical procedures generally fall into 1 of 2 groups: those who are typically healthy and at low risk for complications, and those who are medically complex and at high risk. Healthy children often undergo high‐prevalence, low‐complexity surgical procedures such as tonsillectomy and hernia repairs;[4] these patients are commonly cared for at community hospitals by adult and pediatric surgeons. Whereas medically complex children also undergo these common procedures, they are more likely to be cared for at tertiary care centers and are also more likely to undergo higher‐complexity surgeries such as spinal fusions, hip osteotomies, and ventriculoperitoneal shunt placements.[4] Hospitalist comanagement programs at community hospitals and tertiary care centers may therefore have evolved differently in response to different needs of patients, providers, and organizations,[5] though some institutions may not fall neatly into 1 of these 2 categories.
Comanagement in Community Hospitals
A significant number of pediatric patients are hospitalized each year in community hospitals.[6] As noted above, children undergoing surgery in these settings are generally healthy, and may be cared for by surgeons with varying amounts of pediatric expertise. In this model, the surgeon may frequently be offsite when not in the operating room, necessitating some type of onsite postoperative coverage. In pediatrics, following adult models, this coverage need may be relatively straightforward: surgeons perform the procedure, followed by a medical team assuming postoperative care with surgical consultation. Because of general surgeons' varying experience with children, the American Academy of Pediatrics suggests that patients younger than 14 years or weighing less than 40 kg cared for by providers without routine pediatric experience should have a pediatric‐trained provider involved in their care,[7] though this suggestion does not mandate comanagement.
For children cared for by adult providers who have little experience with areas of pediatric‐specific care such as medication dosing and assessment of deterioration, we believe that involvement of a pediatric provider may impact care in a number of ways. A pediatric hospitalist's availability on the inpatient unit may allow him or her to better manage routine issues such as pain control and intravenous fluids without surgeon input, improving the efficiency of care. A pediatric hospitalist's general pediatric training may allow him or her to more quickly recognize when a child is medically deteriorating, or when transfer to a tertiary care center may be necessary, making care safer. However, no studies have specifically examined these variables. Future research should measure outcomes such as transfers to higher levels of care, medication errors, length of stay (LOS), and complication rates, especially in community hospital settings.
Comanagement in Tertiary Care Referral Centers
At tertiary care referral centers, surgeries in children are most often performed by pediatric surgeons. In these settings, providing routine hospitalist comanagement to all patients may be neither cost‐effective nor feasible. Adult studies have suggested that population‐targeted models can significantly improve several clinical outcomes. For example, in several studies of patients 65 years and older hospitalized with hip fractures, comanagement with a geriatric hospitalist was associated with improved clinical outcomes and shortened LOS.[8, 9, 10, 11, 12]
An analogous group of pediatric patients to the geriatric population may be CSHCNs or children who are medically complex. Several frameworks have been proposed to identify these patients.[13] Many institutions classify medically complex patients as those with complex chronic medical conditions (CCCs).[13, 14, 15] One framework to identify CSHCNs suggested including not only children with CCCs, but also those with (1) substantial service needs and/or family burden; (2) severe functional limitations; and/or (3) high rates of healthcare system utilization, often requiring the care of several subspecialty providers.[16] As the needs of these patients may be quite diverse, pediatric hospitalists may be involved in many aspects of their care, from preoperative evaluation,[17] to establishing protocols for best practices, to communicating with primary care providers, and even seeing patients in postoperative follow‐up clinics. These patients are known to be at high risk for surgical complications, readmissions,[14] medical errors,[18] lapses in communication, and high care costs. In 1 study, comanagement for children with neuromuscular scoliosis hospitalized for spinal fusion surgery has been associated with shorter LOS and less variability in LOS.[19] However, drawbacks of comanagement programs involving CSHCNs may include difficulty with consistent identification of the population who will most benefit from comanagement and higher initial costs of care.[18]
Models of Comanagement and Comanagement Agreements
A comanagement agreement should address 5 major questions: (1) Who is the primary service? (2) Who is the consulting/comanaging service? (3) Are consults as‐needed or automatic? (4) Who writes orders for the patient? (5) Which staffing model will be used for patient care?[20] Although each question above may be answered differently in different systems, the correct comanagement program is a program that aligns most closely with the patient population and care setting.[11, 20]
Several different models exist for hospitalistsurgeon comanagement programs[20, 21] (Table 1). Under the consultation model (model I), hospitalists become involved in the care of surgical patients only when requested to do so by the surgical team. Criteria for requesting this kind of consultation and the extent of responsibility afforded to the medical team are often not clearly defined, and may differ from hospital to hospital or even surgeon to surgeon.[22] Hospitalist involvement with adult patients with postoperative medical complications, which presumably employed this as‐needed model, has been associated with lower mortality and LOS[23]; whether this involvement provides similar benefits in children with postoperative complications has not been explicitly studied.
| Model | Attending Service | Consulting Service | Automatic Consultation | Who Writes Orders? | Notes |
|---|---|---|---|---|---|
| |||||
| I | Surgery | Pediatrics | No | Surgery | Similar to traditional consultation |
| II | Surgery | Pediatrics | Yes | Usually surgery | Basic comanagement, consultant may sign off |
| III | Pediatrics | Surgery | Yes | Usually pediatrics | Basic comanagement, consultant may sign off |
| IV | Combined | N/A | N/A | Each service writes own | True comanagement, no sign‐off from either service permitted |
The remaining models involve compulsory participation by both surgical and medical services. In models II and III, patients may be evaluated preoperatively; those felt to meet specific criteria for high medical complexity are either admitted to a medical service with automatic surgical consultation or admitted to a surgical service with automatic medical consultation. In both cases, writing of orders is handled in the same manner as any consultation model; depending on the service agreement, consulting services may sign off or may be required to be involved until discharge. In model IV, care is fully comanaged by medical and surgical services, with each service having ownership over orders pertaining to their discipline. Ethical concerns about such agreements outlined by the American Medical Association include whether all patients cared for under agreements II to IV will truly benefit from the cost of multispecialty care, and whether informed consent from patients themselves should be required given the cost implications.[24]
Comanagement models also vary with respect to frontline provider staffing. Models may incorporate nurse practitioners, hospitalists, physician assistants, or a combination thereof. These providers may assume a variety of roles, including preoperative patient evaluation, direct care of patients while hospitalized, and/or coordination of inpatient and outpatient postoperative care. Staffing requirements for hospitalists and/or mid‐level providers will differ significantly at different institutions based on surgical volume, patient complexity, and other local factors.
COMANAGEMENT AND QUALITY
Comanagement as a Family‐Centered Initiative
Development of a family‐centered culture of care, including care coordination, lies at the core of pediatric hospital medicine, particularly for CSHCNs.[25, 26] In the outpatient setting, family‐centered care has been associated with improved quality of care for CSHCNs.[27, 28] For families of hospitalized children, issues such as involvement in care and timely information transfer have been identified as high priorities.[29] An important tool for addressing these needs is family‐centered rounds (FCRs), which represent multidisciplinary rounds at the bedside involving families and patients as active shared decision makers in conjunction with the medical team.[30, 31] Although FCRs have not been studied in comanagement arrangements specifically, evidence suggests that this tool improves family centeredness and patient safety in nonsurgical patients,[32] and FCRs can likely have a similar impact on postoperative care.
A pediatric hospitalist comanagement program may impact quality and safety of care in a number of other ways. Hospitalists may offer improved access to clinical information for nurses and families, making care safer. One study of comanagement in adult neurosurgical patients found that access to hospitalists led to improved quality and safety of care as perceived by nurses and other members of the care team.[33] A study in pediatric patients found that nurses overwhelmingly supported having hospitalist involvement in complex children undergoing surgery; the same study found that pediatric hospitalists were particularly noted for their communication skills.[34]
Assessing Clinical Outcomes in Pediatric Hospitalist Comanagement Programs
Most studies evaluating the impact of surgical comanagement programs have focused on global metrics such as LOS, overall complication rates, and resource utilization. In adults, results of these studies have been mixed, suggesting that patient selection may be an important factor.[35] In pediatrics, 2 US studies have assessed these metrics at single centers. Simon et al. found that involvement of a pediatric hospitalist in comanagement of patients undergoing spinal fusion surgery significantly decreased LOS.[19] Rappaport et al. found that patients comanaged by hospitalists had lower utilization of laboratory tests and parenteral nutrition, though initial program costs significantly increased.[36] Studies outside the United States, including a study from Sweden,[37] have suggested that a multidisciplinary approach to children's surgical care, including the presence of pediatric specialists, reduced infection rate and other complications. These studies provide general support for the role of hospitalists in comanagement, although determining which aspects of care are most impacted may be difficult.
Comanagement programs might impact safety and quality negatively as well. Care may be fragmented, leading to provider and family dissatisfaction. Poor communication and multiple handoffs among multidisciplinary team members might interfere with the central role of the nurse in patient care.[38] Comanagement programs might lead to provider disengagement if providers feel that others will assume roles with which they may be unfamiliar or poorly trained.[35] This lack of knowledge may also affect communication with families, leading to conflicting messages among the care team and family frustration. In addition, the impact of comanagement programs on trainees such as residents, both surgical and pediatric, has received limited study.[39] Assessing pediatric comanagement programs' impact on communication, family‐centeredness, and trainees deserves further study.
FINANCIAL IMPLICATIONS OF PEDIATRIC COMANAGEMENT PROGRAMS
Children undergoing surgery require significant financial resources for their care. A study of 38 major US children's hospitals found that 3 of the top 10 conditions with the highest annual expenditures were surgical procedures.[4] The most costly procedure was spinal fusion for scoliosis, accounting for an average of $45,000 per admission and $610 million annually. Although a significant portion of these costs represented surgical devices and operating room time, these totals also included the cost of hospital services and in‐hospital complications. CSHCNs more often undergo high‐complexity procedures such as spinal fusions[36] and face greater risk for costly postoperative complications. The financial benefits that come from reductions in outcomes such as LOS and readmissions in this population are potentially large, but may depend on the payment model as described below.
Billing Models in Comanagement Programs
Several billing constructs exist in comanagement models. At many institutions, comanagement billing may resemble that for traditional consultation: the pediatric hospitalist bills for his/her services using standard initial and subsequent consultation billing coding for the child's medical conditions, and may sign off when the hospitalist feels recommendations are complete. Other models may also exist. Model IV comanagement may involve a prearranged financial agreement, in which billing modifiers are used to differentiate surgical care only (modifier 54) and postoperative medical care only (modifier 55). These modifiers, typically used for Medicare patients, indicate a split in a global surgical fee.[40]
The SHM has outlined financial considerations that should be addressed at the time of program inception and updated periodically, including identifying how each party will bill, who bills for which service, and monitoring collection rates and rejected claims.[2] Regardless of billing model, the main focus of comanagement must be quality of care, not financial considerations; situations in which the latter are emphasized at the expense of patient care may be unethical or illegal.[24] Regardless, surgical comanagement programs should seek maximal reimbursement in order to remain viable.
Value of Comanagement for a Healthcare Organization Under Fee‐for‐Service Payment
The value of any comanagement program is highly dependent on both the institution's payor mix and the healthcare organization's overarching goals. From a business perspective, a multidisciplinary approach may be perceived as resource intensive, but formal cost‐effectiveness analyses over time are limited. Theoretically, a traditional payment model involving hospitalist comanagement would be financially beneficial for a healthcare institution by allowing surgeons and surgical trainees more time to operate. However, these savings are difficult to quantify. Despite the fact that most hospitalist programs have no direct financial benefit to the institution, many hospital leaders seem willing to subsidize hospitalist programs based on measures such as patient and referring physician satisfaction with hospitalist care.[41] Postoperative complications, although unfortunate, may be a source of revenue under this model if paid by insurance companies in the usual manner, leading to a misalignment of quality and financial goals. Regardless, whether these programs are considered worthy investments to healthcare organizations will ultimately depend on evolving billing and reimbursement structures; to date, no formal survey of how comanagement programs bill and are reimbursed has been performed.
Value for an Organization in an Accountable Care Organization Model
Although a detailed discussion of accountable care organizations (ACOs) is beyond the scope of this article, stronger incentives in these structures for reducing resource utilization and complication rates may make hospitalist comanagement attractive in an ACO model.[42] ACO program evaluation is expected to be based on such data as patient surveys, documentation of care coordination, and several disease‐specific metrics.[43, 44] Several children's hospital‐based systems and mixed health systems have dedicated significant resources to establishing networks of providers that bridge inpatient and outpatient episodes of surgical care.[27, 28] One adult study has suggested lower costs associated with hospitalist comanagement for geriatric patients with hip fractures.[12] Comanagement programs may help meet quality and value goals, including enhancing care coordination between inpatient and outpatient care, and therefore may prove to be a beneficial investment for institutions. As the healthcare landscape evolves, formal study of the costs and benefits of pediatric comanagement models in ACO‐type care structures will be important.
SETTING A RESEARCH AGENDA
Pediatric hospitalist comanagement programs require vigorous study to evaluate their impact. Potential research targets include not only clinical data such as LOS, perioperative complication rates, readmission rates, and resource utilization, but also data regarding surgeon, nursing, and family satisfaction. These programs should also be evaluated in terms of how they impact trainees, both surgical and pediatric. Because of comanagement programs' complexities, we anticipate that they will impart both positive and negative effects on some of these factors. These programs will also require evaluation over time as they require significant education on the part of staff and families.[36]
In addition to affecting global metrics, pediatric hospitalists may also have a positive impact on surgical care by demonstrating leadership to improve systems of care relevant to surgical patients, including the use of guidelines. The American College of Surgeons' National Surgical Quality Improvement Program has identified 2 priorities that hospitalists may impact: surgical site infection (SSI) and pulmonary complications, which combined comprise greater than half of all 30‐day postoperative complications.[45] Regarding SSI prevention, hospitalist researchers are making valuable contributions to literature surrounding adherence to Centers for Disease Control and Prevention and Pediatric Orthopedic Society of North America guidelines.[46, 47, 48] Research is ongoing regarding how human and systems factors may impact the effectiveness of these guidelines, but also how reliably these guidelines are implemented.[49] In the area of pulmonary complications, pediatric hospitalists have followed the example of successful initiatives in adult surgery patients by developing and implementing postoperative protocols to prevent pulmonary complications such as postoperative pneumonia.[50] At 1 center, pediatric hospitalists have led efforts to implement a standardized respiratory care pathway for high‐risk orthopedic patients.[51] Evaluation of the effectiveness of such programs is currently ongoing, but early data show similar benefits to those demonstrated in adults.
CONCLUSIONS
Pediatric hospitalist comanagement programs for surgical patients have largely followed the path of adult programs. Limited data suggest that certain clinical outcomes may be improved under comanagement, but patient selection may be important. Although there is significant variety between programs, there exist several common themes, including the importance of clear delineation of roles and a central goal of improved care coordination. Ongoing research will hopefully shed more light on the impact of these programs, especially with regard to patient safety, hospitalist‐led quality‐improvement programs, and financial implications, particularly in different structures of care and reimbursement models.
Disclosure: Nothing to report.
According to the 2012 Society of Hospital Medicine (SHM) survey, 94% of adult hospitalists and 74% of pediatric hospitalists provide inpatient care to surgical patients.[1] Many of these programs involve comanagement, which the SHM Comanagement Advisory Panel has described as a system of care featuring shared responsibility, authority, and accountability for hospitalized patients with medical and surgical needs.[2] Collaboration between medical and surgical teams for these patients has occurred commonly at some community institutions for decades, but may only be emerging at some tertiary care hospitals. The trend of comanagement appears to be increasing in popularity in adult medicine.[3] As in adult patients, comanagement for children undergoing surgical procedures, particularly those children with special healthcare needs (CSHCNs), has been proposed as a strategy for improving quality and costs. In this review, we will describe structural, quality, and financial implications of pediatric hospitalist comanagement programs, each of which include both potential benefits and drawbacks, as well as discuss a future research agenda for these programs.
ORGANIZATIONAL NEEDS AND STRUCTURE OF COMANAGEMENT PROGRAMS
Patterns of comanagement likely depend on hospital size and structure, both in adult patients[3] and in pediatrics. Children hospitalized for surgical procedures generally fall into 1 of 2 groups: those who are typically healthy and at low risk for complications, and those who are medically complex and at high risk. Healthy children often undergo high‐prevalence, low‐complexity surgical procedures such as tonsillectomy and hernia repairs;[4] these patients are commonly cared for at community hospitals by adult and pediatric surgeons. Whereas medically complex children also undergo these common procedures, they are more likely to be cared for at tertiary care centers and are also more likely to undergo higher‐complexity surgeries such as spinal fusions, hip osteotomies, and ventriculoperitoneal shunt placements.[4] Hospitalist comanagement programs at community hospitals and tertiary care centers may therefore have evolved differently in response to different needs of patients, providers, and organizations,[5] though some institutions may not fall neatly into 1 of these 2 categories.
Comanagement in Community Hospitals
A significant number of pediatric patients are hospitalized each year in community hospitals.[6] As noted above, children undergoing surgery in these settings are generally healthy, and may be cared for by surgeons with varying amounts of pediatric expertise. In this model, the surgeon may frequently be offsite when not in the operating room, necessitating some type of onsite postoperative coverage. In pediatrics, following adult models, this coverage need may be relatively straightforward: surgeons perform the procedure, followed by a medical team assuming postoperative care with surgical consultation. Because of general surgeons' varying experience with children, the American Academy of Pediatrics suggests that patients younger than 14 years or weighing less than 40 kg cared for by providers without routine pediatric experience should have a pediatric‐trained provider involved in their care,[7] though this suggestion does not mandate comanagement.
For children cared for by adult providers who have little experience with areas of pediatric‐specific care such as medication dosing and assessment of deterioration, we believe that involvement of a pediatric provider may impact care in a number of ways. A pediatric hospitalist's availability on the inpatient unit may allow him or her to better manage routine issues such as pain control and intravenous fluids without surgeon input, improving the efficiency of care. A pediatric hospitalist's general pediatric training may allow him or her to more quickly recognize when a child is medically deteriorating, or when transfer to a tertiary care center may be necessary, making care safer. However, no studies have specifically examined these variables. Future research should measure outcomes such as transfers to higher levels of care, medication errors, length of stay (LOS), and complication rates, especially in community hospital settings.
Comanagement in Tertiary Care Referral Centers
At tertiary care referral centers, surgeries in children are most often performed by pediatric surgeons. In these settings, providing routine hospitalist comanagement to all patients may be neither cost‐effective nor feasible. Adult studies have suggested that population‐targeted models can significantly improve several clinical outcomes. For example, in several studies of patients 65 years and older hospitalized with hip fractures, comanagement with a geriatric hospitalist was associated with improved clinical outcomes and shortened LOS.[8, 9, 10, 11, 12]
An analogous group of pediatric patients to the geriatric population may be CSHCNs or children who are medically complex. Several frameworks have been proposed to identify these patients.[13] Many institutions classify medically complex patients as those with complex chronic medical conditions (CCCs).[13, 14, 15] One framework to identify CSHCNs suggested including not only children with CCCs, but also those with (1) substantial service needs and/or family burden; (2) severe functional limitations; and/or (3) high rates of healthcare system utilization, often requiring the care of several subspecialty providers.[16] As the needs of these patients may be quite diverse, pediatric hospitalists may be involved in many aspects of their care, from preoperative evaluation,[17] to establishing protocols for best practices, to communicating with primary care providers, and even seeing patients in postoperative follow‐up clinics. These patients are known to be at high risk for surgical complications, readmissions,[14] medical errors,[18] lapses in communication, and high care costs. In 1 study, comanagement for children with neuromuscular scoliosis hospitalized for spinal fusion surgery has been associated with shorter LOS and less variability in LOS.[19] However, drawbacks of comanagement programs involving CSHCNs may include difficulty with consistent identification of the population who will most benefit from comanagement and higher initial costs of care.[18]
Models of Comanagement and Comanagement Agreements
A comanagement agreement should address 5 major questions: (1) Who is the primary service? (2) Who is the consulting/comanaging service? (3) Are consults as‐needed or automatic? (4) Who writes orders for the patient? (5) Which staffing model will be used for patient care?[20] Although each question above may be answered differently in different systems, the correct comanagement program is a program that aligns most closely with the patient population and care setting.[11, 20]
Several different models exist for hospitalistsurgeon comanagement programs[20, 21] (Table 1). Under the consultation model (model I), hospitalists become involved in the care of surgical patients only when requested to do so by the surgical team. Criteria for requesting this kind of consultation and the extent of responsibility afforded to the medical team are often not clearly defined, and may differ from hospital to hospital or even surgeon to surgeon.[22] Hospitalist involvement with adult patients with postoperative medical complications, which presumably employed this as‐needed model, has been associated with lower mortality and LOS[23]; whether this involvement provides similar benefits in children with postoperative complications has not been explicitly studied.
| Model | Attending Service | Consulting Service | Automatic Consultation | Who Writes Orders? | Notes |
|---|---|---|---|---|---|
| |||||
| I | Surgery | Pediatrics | No | Surgery | Similar to traditional consultation |
| II | Surgery | Pediatrics | Yes | Usually surgery | Basic comanagement, consultant may sign off |
| III | Pediatrics | Surgery | Yes | Usually pediatrics | Basic comanagement, consultant may sign off |
| IV | Combined | N/A | N/A | Each service writes own | True comanagement, no sign‐off from either service permitted |
The remaining models involve compulsory participation by both surgical and medical services. In models II and III, patients may be evaluated preoperatively; those felt to meet specific criteria for high medical complexity are either admitted to a medical service with automatic surgical consultation or admitted to a surgical service with automatic medical consultation. In both cases, writing of orders is handled in the same manner as any consultation model; depending on the service agreement, consulting services may sign off or may be required to be involved until discharge. In model IV, care is fully comanaged by medical and surgical services, with each service having ownership over orders pertaining to their discipline. Ethical concerns about such agreements outlined by the American Medical Association include whether all patients cared for under agreements II to IV will truly benefit from the cost of multispecialty care, and whether informed consent from patients themselves should be required given the cost implications.[24]
Comanagement models also vary with respect to frontline provider staffing. Models may incorporate nurse practitioners, hospitalists, physician assistants, or a combination thereof. These providers may assume a variety of roles, including preoperative patient evaluation, direct care of patients while hospitalized, and/or coordination of inpatient and outpatient postoperative care. Staffing requirements for hospitalists and/or mid‐level providers will differ significantly at different institutions based on surgical volume, patient complexity, and other local factors.
COMANAGEMENT AND QUALITY
Comanagement as a Family‐Centered Initiative
Development of a family‐centered culture of care, including care coordination, lies at the core of pediatric hospital medicine, particularly for CSHCNs.[25, 26] In the outpatient setting, family‐centered care has been associated with improved quality of care for CSHCNs.[27, 28] For families of hospitalized children, issues such as involvement in care and timely information transfer have been identified as high priorities.[29] An important tool for addressing these needs is family‐centered rounds (FCRs), which represent multidisciplinary rounds at the bedside involving families and patients as active shared decision makers in conjunction with the medical team.[30, 31] Although FCRs have not been studied in comanagement arrangements specifically, evidence suggests that this tool improves family centeredness and patient safety in nonsurgical patients,[32] and FCRs can likely have a similar impact on postoperative care.
A pediatric hospitalist comanagement program may impact quality and safety of care in a number of other ways. Hospitalists may offer improved access to clinical information for nurses and families, making care safer. One study of comanagement in adult neurosurgical patients found that access to hospitalists led to improved quality and safety of care as perceived by nurses and other members of the care team.[33] A study in pediatric patients found that nurses overwhelmingly supported having hospitalist involvement in complex children undergoing surgery; the same study found that pediatric hospitalists were particularly noted for their communication skills.[34]
Assessing Clinical Outcomes in Pediatric Hospitalist Comanagement Programs
Most studies evaluating the impact of surgical comanagement programs have focused on global metrics such as LOS, overall complication rates, and resource utilization. In adults, results of these studies have been mixed, suggesting that patient selection may be an important factor.[35] In pediatrics, 2 US studies have assessed these metrics at single centers. Simon et al. found that involvement of a pediatric hospitalist in comanagement of patients undergoing spinal fusion surgery significantly decreased LOS.[19] Rappaport et al. found that patients comanaged by hospitalists had lower utilization of laboratory tests and parenteral nutrition, though initial program costs significantly increased.[36] Studies outside the United States, including a study from Sweden,[37] have suggested that a multidisciplinary approach to children's surgical care, including the presence of pediatric specialists, reduced infection rate and other complications. These studies provide general support for the role of hospitalists in comanagement, although determining which aspects of care are most impacted may be difficult.
Comanagement programs might impact safety and quality negatively as well. Care may be fragmented, leading to provider and family dissatisfaction. Poor communication and multiple handoffs among multidisciplinary team members might interfere with the central role of the nurse in patient care.[38] Comanagement programs might lead to provider disengagement if providers feel that others will assume roles with which they may be unfamiliar or poorly trained.[35] This lack of knowledge may also affect communication with families, leading to conflicting messages among the care team and family frustration. In addition, the impact of comanagement programs on trainees such as residents, both surgical and pediatric, has received limited study.[39] Assessing pediatric comanagement programs' impact on communication, family‐centeredness, and trainees deserves further study.
FINANCIAL IMPLICATIONS OF PEDIATRIC COMANAGEMENT PROGRAMS
Children undergoing surgery require significant financial resources for their care. A study of 38 major US children's hospitals found that 3 of the top 10 conditions with the highest annual expenditures were surgical procedures.[4] The most costly procedure was spinal fusion for scoliosis, accounting for an average of $45,000 per admission and $610 million annually. Although a significant portion of these costs represented surgical devices and operating room time, these totals also included the cost of hospital services and in‐hospital complications. CSHCNs more often undergo high‐complexity procedures such as spinal fusions[36] and face greater risk for costly postoperative complications. The financial benefits that come from reductions in outcomes such as LOS and readmissions in this population are potentially large, but may depend on the payment model as described below.
Billing Models in Comanagement Programs
Several billing constructs exist in comanagement models. At many institutions, comanagement billing may resemble that for traditional consultation: the pediatric hospitalist bills for his/her services using standard initial and subsequent consultation billing coding for the child's medical conditions, and may sign off when the hospitalist feels recommendations are complete. Other models may also exist. Model IV comanagement may involve a prearranged financial agreement, in which billing modifiers are used to differentiate surgical care only (modifier 54) and postoperative medical care only (modifier 55). These modifiers, typically used for Medicare patients, indicate a split in a global surgical fee.[40]
The SHM has outlined financial considerations that should be addressed at the time of program inception and updated periodically, including identifying how each party will bill, who bills for which service, and monitoring collection rates and rejected claims.[2] Regardless of billing model, the main focus of comanagement must be quality of care, not financial considerations; situations in which the latter are emphasized at the expense of patient care may be unethical or illegal.[24] Regardless, surgical comanagement programs should seek maximal reimbursement in order to remain viable.
Value of Comanagement for a Healthcare Organization Under Fee‐for‐Service Payment
The value of any comanagement program is highly dependent on both the institution's payor mix and the healthcare organization's overarching goals. From a business perspective, a multidisciplinary approach may be perceived as resource intensive, but formal cost‐effectiveness analyses over time are limited. Theoretically, a traditional payment model involving hospitalist comanagement would be financially beneficial for a healthcare institution by allowing surgeons and surgical trainees more time to operate. However, these savings are difficult to quantify. Despite the fact that most hospitalist programs have no direct financial benefit to the institution, many hospital leaders seem willing to subsidize hospitalist programs based on measures such as patient and referring physician satisfaction with hospitalist care.[41] Postoperative complications, although unfortunate, may be a source of revenue under this model if paid by insurance companies in the usual manner, leading to a misalignment of quality and financial goals. Regardless, whether these programs are considered worthy investments to healthcare organizations will ultimately depend on evolving billing and reimbursement structures; to date, no formal survey of how comanagement programs bill and are reimbursed has been performed.
Value for an Organization in an Accountable Care Organization Model
Although a detailed discussion of accountable care organizations (ACOs) is beyond the scope of this article, stronger incentives in these structures for reducing resource utilization and complication rates may make hospitalist comanagement attractive in an ACO model.[42] ACO program evaluation is expected to be based on such data as patient surveys, documentation of care coordination, and several disease‐specific metrics.[43, 44] Several children's hospital‐based systems and mixed health systems have dedicated significant resources to establishing networks of providers that bridge inpatient and outpatient episodes of surgical care.[27, 28] One adult study has suggested lower costs associated with hospitalist comanagement for geriatric patients with hip fractures.[12] Comanagement programs may help meet quality and value goals, including enhancing care coordination between inpatient and outpatient care, and therefore may prove to be a beneficial investment for institutions. As the healthcare landscape evolves, formal study of the costs and benefits of pediatric comanagement models in ACO‐type care structures will be important.
SETTING A RESEARCH AGENDA
Pediatric hospitalist comanagement programs require vigorous study to evaluate their impact. Potential research targets include not only clinical data such as LOS, perioperative complication rates, readmission rates, and resource utilization, but also data regarding surgeon, nursing, and family satisfaction. These programs should also be evaluated in terms of how they impact trainees, both surgical and pediatric. Because of comanagement programs' complexities, we anticipate that they will impart both positive and negative effects on some of these factors. These programs will also require evaluation over time as they require significant education on the part of staff and families.[36]
In addition to affecting global metrics, pediatric hospitalists may also have a positive impact on surgical care by demonstrating leadership to improve systems of care relevant to surgical patients, including the use of guidelines. The American College of Surgeons' National Surgical Quality Improvement Program has identified 2 priorities that hospitalists may impact: surgical site infection (SSI) and pulmonary complications, which combined comprise greater than half of all 30‐day postoperative complications.[45] Regarding SSI prevention, hospitalist researchers are making valuable contributions to literature surrounding adherence to Centers for Disease Control and Prevention and Pediatric Orthopedic Society of North America guidelines.[46, 47, 48] Research is ongoing regarding how human and systems factors may impact the effectiveness of these guidelines, but also how reliably these guidelines are implemented.[49] In the area of pulmonary complications, pediatric hospitalists have followed the example of successful initiatives in adult surgery patients by developing and implementing postoperative protocols to prevent pulmonary complications such as postoperative pneumonia.[50] At 1 center, pediatric hospitalists have led efforts to implement a standardized respiratory care pathway for high‐risk orthopedic patients.[51] Evaluation of the effectiveness of such programs is currently ongoing, but early data show similar benefits to those demonstrated in adults.
CONCLUSIONS
Pediatric hospitalist comanagement programs for surgical patients have largely followed the path of adult programs. Limited data suggest that certain clinical outcomes may be improved under comanagement, but patient selection may be important. Although there is significant variety between programs, there exist several common themes, including the importance of clear delineation of roles and a central goal of improved care coordination. Ongoing research will hopefully shed more light on the impact of these programs, especially with regard to patient safety, hospitalist‐led quality‐improvement programs, and financial implications, particularly in different structures of care and reimbursement models.
Disclosure: Nothing to report.
- 2012 State of Hospital Medicine Report, Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/survey. Accessed on September 1, 2014.
- Society of Hospital Medicine Co‐Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co‐management. SHM website. Available at: http://tools.hospitalmedicine.org/Implementation/Co‐ManagementWhitePaper‐final_5‐10‐10.pdf. Accessed on September 25, 2014.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , . Pediatric hospitalist comanagement of surgical patients: challenges and opportunities. Clin Pediatr (Phila). 2008;47(2):114–121.
- , . Utilization of pediatric hospitals in New York State. Pediatrics. 2003;111(5 pt 1):1068–1071.
- ; Committee on Hospital Care. Physicians' roles in coordinating care of hospitalized children. Pediatrics. 2003;111(3):707–709.
- , , , et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219–225.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , . Geriatric co‐management of proximal femur fractures: total quality management and protocol‐driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356.
- , , , , , . Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , , . Pediatric hospital medicine and children with medical complexity: past, present, and future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):113–119.
- , , , et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140.
- , , , , . Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684–688.
- , , , , . Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , . Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189.
- . How best to design surgical comanagement services for pediatric surgical patients? Hosp Pediatr. 2013;3(3):242–243.
- , , , , , . Effects of provider characteristics on care coordination under comanagement. J Hosp Med. 2010;5(9):508–513.
- , , , . Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17(9):e333–e339.
- American Medical Association. 2008 report of the Council on Medical Service, policy sunset report for 1998 AMA socioeconomic policies. CMS Report 4, A‐08. Available at: http://www.ama‐assn.org/resources/doc/cms/a‐08cms4.pdf. Accessed on September 1, 2014.
- Committee on Hospital Care and Institute for Patient‐and Family‐Centered Care. Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129(2):394–404.
- Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460.
- , , , , , . Care coordination for CSHCN: associations with family‐provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428–S434.
- , , , et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.
- , , . Parents' priorities and satisfaction with acute pediatric care. Arch Pediatr Adolesc Med. 2005;159(2):127–131.
- . Family‐centered rounds. Pediatr Clin North Am. 2014;61(4):663–670.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- , , , , ; PIPS Group. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15(suppl 1):17–25.
- , , , et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004–2010.
- , , . Nurses' assessment of pediatric physicians: are hospitalists different? J Healthc Manag Am Coll Healthc Exec. 2008;53(1):14–24; discussion 24–25.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- , , , et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233–241.
- , , . Outcome of major spinal deformity surgery in high‐risk patients: comparison between two departments. Evid Based Spine Care J. 2010;1(3):11–18.
- , , , , , . Meeting the complex needs of the health care team: identification of nurse‐team communication practices perceived to enhance patient outcomes. Qual Health Res. 2010;20(1):15–28.
- , , , . A pediatric residency experience with surgical comanagement. Hosp Pediatr. 2013;3(2):144–148.
- U.S. Department of Health and Human Services. Centers for Medicaid and Medicare Services. MLN matters. Available at: http://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/Downloads/MM7872.pdf. Accessed on September 1,2014.
- , , ; Research Advisory Committee of the American Board of Pediatrics. Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- . Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364(16):e32.
- American Academy of Pediatrics. Accountable Care Organizations (ACOs) and Pediatricians: Evaluation and Engagement. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/Pages/Accountable‐Care‐Organizations‐and‐Pediatricians‐Evaluation‐and‐Engagement.aspx. Accessed on September 25, 2014.
- , , , , . Delivery system characteristics and their association with quality and costs of care: implications for accountable care organizations [published online ahead of print February 21, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000014.
- , , , et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121.
- , , , et al. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–278. Available at: http://www.cdc.gov/hicpac/pdf/guidelines/SSI_1999.pdf. Accessed on September 1, 2014.
- , , , et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high‐risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–478.
- , , , et al. Perioperative antibiotic use for spinal surgery procedures in US children's hospitals. Spine. 2013;38(7):609–616. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , , , , , . A human factors intervention to improve post‐operative antibiotic timing and prevent surgical site infection. Paper presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- , , , , . I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , . Early postoperative respiratory care improves outcomes, adds value for hospitalized pediatric orthopedic patients. Poster presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- 2012 State of Hospital Medicine Report, Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/survey. Accessed on September 1, 2014.
- Society of Hospital Medicine Co‐Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co‐management. SHM website. Available at: http://tools.hospitalmedicine.org/Implementation/Co‐ManagementWhitePaper‐final_5‐10‐10.pdf. Accessed on September 25, 2014.
- , , , , . Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164.
- , . Pediatric hospitalist comanagement of surgical patients: challenges and opportunities. Clin Pediatr (Phila). 2008;47(2):114–121.
- , . Utilization of pediatric hospitals in New York State. Pediatrics. 2003;111(5 pt 1):1068–1071.
- ; Committee on Hospital Care. Physicians' roles in coordinating care of hospitalized children. Pediatrics. 2003;111(3):707–709.
- , , , et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219–225.
- , , , et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796–801.
- , , , , , . Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172–178; discussion 179–180.
- , , , . Geriatric co‐management of proximal femur fractures: total quality management and protocol‐driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356.
- , , , , , . Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , , . Pediatric hospital medicine and children with medical complexity: past, present, and future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):113–119.
- , , , et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140.
- , , , , . Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684–688.
- , , , , . Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255.
- , , , , , . Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23–30.
- , . Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183–189.
- . How best to design surgical comanagement services for pediatric surgical patients? Hosp Pediatr. 2013;3(3):242–243.
- , , , , , . Effects of provider characteristics on care coordination under comanagement. J Hosp Med. 2010;5(9):508–513.
- , , , . Potential role of comanagement in “rescue” of surgical patients. Am J Manag Care. 2011;17(9):e333–e339.
- American Medical Association. 2008 report of the Council on Medical Service, policy sunset report for 1998 AMA socioeconomic policies. CMS Report 4, A‐08. Available at: http://www.ama‐assn.org/resources/doc/cms/a‐08cms4.pdf. Accessed on September 1, 2014.
- Committee on Hospital Care and Institute for Patient‐and Family‐Centered Care. Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129(2):394–404.
- Council on Children with Disabilities and Medical Home Implementation Project Advisory Committee. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460.
- , , , , , . Care coordination for CSHCN: associations with family‐provider relations and family/child outcomes. Pediatrics. 2009;124(suppl 4):S428–S434.
- , , , et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.
- , , . Parents' priorities and satisfaction with acute pediatric care. Arch Pediatr Adolesc Med. 2005;159(2):127–131.
- . Family‐centered rounds. Pediatr Clin North Am. 2014;61(4):663–670.
- , , , et al. Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126(1):37–43.
- , , , , ; PIPS Group. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. J Health Serv Res Policy. 2010;15(suppl 1):17–25.
- , , , et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004–2010.
- , , . Nurses' assessment of pediatric physicians: are hospitalists different? J Healthc Manag Am Coll Healthc Exec. 2008;53(1):14–24; discussion 24–25.
- . Just because you can, doesn't mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398–402.
- , , , et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233–241.
- , , . Outcome of major spinal deformity surgery in high‐risk patients: comparison between two departments. Evid Based Spine Care J. 2010;1(3):11–18.
- , , , , , . Meeting the complex needs of the health care team: identification of nurse‐team communication practices perceived to enhance patient outcomes. Qual Health Res. 2010;20(1):15–28.
- , , , . A pediatric residency experience with surgical comanagement. Hosp Pediatr. 2013;3(2):144–148.
- U.S. Department of Health and Human Services. Centers for Medicaid and Medicare Services. MLN matters. Available at: http://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNMattersArticles/Downloads/MM7872.pdf. Accessed on September 1,2014.
- , , ; Research Advisory Committee of the American Board of Pediatrics. Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- . Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364(16):e32.
- American Academy of Pediatrics. Accountable Care Organizations (ACOs) and Pediatricians: Evaluation and Engagement. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/Pages/Accountable‐Care‐Organizations‐and‐Pediatricians‐Evaluation‐and‐Engagement.aspx. Accessed on September 25, 2014.
- , , , , . Delivery system characteristics and their association with quality and costs of care: implications for accountable care organizations [published online ahead of print February 21, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000014.
- , , , et al. Pediatric American College of Surgeons National Surgical Quality Improvement Program: feasibility of a novel, prospective assessment of surgical outcomes. J Pediatr Surg. 2011;46(1):115–121.
- , , , et al. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20(4):247–278. Available at: http://www.cdc.gov/hicpac/pdf/guidelines/SSI_1999.pdf. Accessed on September 1, 2014.
- , , , et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high‐risk pediatric spine surgery. J Pediatr Orthop. 2013;33(5):471–478.
- , , , et al. Perioperative antibiotic use for spinal surgery procedures in US children's hospitals. Spine. 2013;38(7):609–616. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , , , , , . A human factors intervention to improve post‐operative antibiotic timing and prevent surgical site infection. Paper presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
- , , , , . I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. Available at: http://www.ihi.org/education/Conferences/Forum2013/Pages/Scientific‐Symposium.aspx. Accessed on September 26, 2014.
- , , , , , , , . Early postoperative respiratory care improves outcomes, adds value for hospitalized pediatric orthopedic patients. Poster presented at: Institute for Healthcare Improvement Scientific Symposium; 2013; Orlando, FL. Abstract.
Novel capsule could replace injections

Credit: USDA
Scientists have created a novel drug capsule coated with tiny needles that can inject drugs directly into the lining of the stomach after the capsule is swallowed.
In experiments with pigs, the capsule delivered insulin more efficiently than an injection under the skin, and there were no harmful side effects as the capsule passed through the digestive system.
The researchers anticipate the capsule would be most useful for delivering biopharmaceuticals such as antibodies to treat cancers and other disorders.
“This could be a way that the patient can circumvent the need to have an infusion or subcutaneous administration of a drug,” said Giovanni Traverso, MB BChir, PhD, of Massachusetts General Hospital in Boston.
He and his colleagues described their capsule in the Journal of Pharmaceutical Sciences.
The team had set out to design a capsule that would serve as a platform for the delivery of a wide range of therapeutics, prevent degradation of the drugs, and inject the payload directly into the lining of the gastrointestinal tract.
Their prototype acrylic capsule, 2 cm long and 1 cm in diameter, includes a reservoir for the drug and is coated with hollow, stainless steel needles about 5 mm long.
Previous studies of accidental ingestion of sharp objects in human patients have suggested that it could be safe to swallow a capsule coated with short needles. Because there are no pain receptors in the gastrointestinal tract, patients would not feel any pain from the drug injection.
To test whether this type of capsule could allow safe and effective drug delivery, the researchers tested it in pigs, with insulin as the drug payload.
It took more than a week for the capsules to move through the entire digestive tract, and the researchers found no traces of tissue damage, supporting the potential safety of this novel approach.
They also found the microneedles successfully injected insulin into the lining of the stomach, small intestine, and colon, causing the animals’ blood glucose levels to drop. This reduction in blood glucose was faster and larger than the drop seen when the same amount of glucose was given by subcutaneous injection.
“The kinetics are much better, and much faster-onset, than those seen with traditional under-the-skin administration,” Dr Traverso said. “For molecules that are particularly difficult to absorb, this would be a way of actually administering them at much higher efficiency.”
This approach could also be used to administer vaccines that normally have to be injected, the researchers said.
The team now plans to modify the capsule so that peristalsis would slowly squeeze the drug out of the capsule as it travels through the digestive tract.
They are also working on capsules with needles made of degradable polymers and sugar that would break off and become embedded in the gut lining, where they would slowly disintegrate and release the drug. This would further minimize any safety concern. ![]()

Credit: USDA
Scientists have created a novel drug capsule coated with tiny needles that can inject drugs directly into the lining of the stomach after the capsule is swallowed.
In experiments with pigs, the capsule delivered insulin more efficiently than an injection under the skin, and there were no harmful side effects as the capsule passed through the digestive system.
The researchers anticipate the capsule would be most useful for delivering biopharmaceuticals such as antibodies to treat cancers and other disorders.
“This could be a way that the patient can circumvent the need to have an infusion or subcutaneous administration of a drug,” said Giovanni Traverso, MB BChir, PhD, of Massachusetts General Hospital in Boston.
He and his colleagues described their capsule in the Journal of Pharmaceutical Sciences.
The team had set out to design a capsule that would serve as a platform for the delivery of a wide range of therapeutics, prevent degradation of the drugs, and inject the payload directly into the lining of the gastrointestinal tract.
Their prototype acrylic capsule, 2 cm long and 1 cm in diameter, includes a reservoir for the drug and is coated with hollow, stainless steel needles about 5 mm long.
Previous studies of accidental ingestion of sharp objects in human patients have suggested that it could be safe to swallow a capsule coated with short needles. Because there are no pain receptors in the gastrointestinal tract, patients would not feel any pain from the drug injection.
To test whether this type of capsule could allow safe and effective drug delivery, the researchers tested it in pigs, with insulin as the drug payload.
It took more than a week for the capsules to move through the entire digestive tract, and the researchers found no traces of tissue damage, supporting the potential safety of this novel approach.
They also found the microneedles successfully injected insulin into the lining of the stomach, small intestine, and colon, causing the animals’ blood glucose levels to drop. This reduction in blood glucose was faster and larger than the drop seen when the same amount of glucose was given by subcutaneous injection.
“The kinetics are much better, and much faster-onset, than those seen with traditional under-the-skin administration,” Dr Traverso said. “For molecules that are particularly difficult to absorb, this would be a way of actually administering them at much higher efficiency.”
This approach could also be used to administer vaccines that normally have to be injected, the researchers said.
The team now plans to modify the capsule so that peristalsis would slowly squeeze the drug out of the capsule as it travels through the digestive tract.
They are also working on capsules with needles made of degradable polymers and sugar that would break off and become embedded in the gut lining, where they would slowly disintegrate and release the drug. This would further minimize any safety concern. ![]()

Credit: USDA
Scientists have created a novel drug capsule coated with tiny needles that can inject drugs directly into the lining of the stomach after the capsule is swallowed.
In experiments with pigs, the capsule delivered insulin more efficiently than an injection under the skin, and there were no harmful side effects as the capsule passed through the digestive system.
The researchers anticipate the capsule would be most useful for delivering biopharmaceuticals such as antibodies to treat cancers and other disorders.
“This could be a way that the patient can circumvent the need to have an infusion or subcutaneous administration of a drug,” said Giovanni Traverso, MB BChir, PhD, of Massachusetts General Hospital in Boston.
He and his colleagues described their capsule in the Journal of Pharmaceutical Sciences.
The team had set out to design a capsule that would serve as a platform for the delivery of a wide range of therapeutics, prevent degradation of the drugs, and inject the payload directly into the lining of the gastrointestinal tract.
Their prototype acrylic capsule, 2 cm long and 1 cm in diameter, includes a reservoir for the drug and is coated with hollow, stainless steel needles about 5 mm long.
Previous studies of accidental ingestion of sharp objects in human patients have suggested that it could be safe to swallow a capsule coated with short needles. Because there are no pain receptors in the gastrointestinal tract, patients would not feel any pain from the drug injection.
To test whether this type of capsule could allow safe and effective drug delivery, the researchers tested it in pigs, with insulin as the drug payload.
It took more than a week for the capsules to move through the entire digestive tract, and the researchers found no traces of tissue damage, supporting the potential safety of this novel approach.
They also found the microneedles successfully injected insulin into the lining of the stomach, small intestine, and colon, causing the animals’ blood glucose levels to drop. This reduction in blood glucose was faster and larger than the drop seen when the same amount of glucose was given by subcutaneous injection.
“The kinetics are much better, and much faster-onset, than those seen with traditional under-the-skin administration,” Dr Traverso said. “For molecules that are particularly difficult to absorb, this would be a way of actually administering them at much higher efficiency.”
This approach could also be used to administer vaccines that normally have to be injected, the researchers said.
The team now plans to modify the capsule so that peristalsis would slowly squeeze the drug out of the capsule as it travels through the digestive tract.
They are also working on capsules with needles made of degradable polymers and sugar that would break off and become embedded in the gut lining, where they would slowly disintegrate and release the drug. This would further minimize any safety concern. ![]()
Biomarker predicts bone loss in premenopausal breast cancer patients
CHICAGO – A premenopausal breast cancer patient’s follicle-stimulating hormone level upon completion of chemotherapy predicts her risk of bone loss during the ensuing 12 months, Dr. Laila S. Tabatabai reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
“This may have significant implications for preserving bone health in premenopausal women with breast cancer. Appropriate use of FSH as a marker for premature ovarian failure and as a predictor of bone loss after breast cancer treatment may allow for the timely implementation of preventive measures to reduce fracture risk,” said Dr. Tabatabai of Johns Hopkins University, Baltimore.
She presented a secondary analysis from the Exercise for Bone Health: Young Breast Cancer Survivors Study, in which 206 women who were under age 55 and had completed adjuvant chemotherapy for breast cancer were randomized to a 12-month structured exercise program conducted through the YMCA or to a control group that received a monthly health newsletter.
Investigators measured baseline levels of FSH, bone turnover markers, calciotropic hormones, and high-sensitivity C-reactive protein. At 1 year follow-up, only baseline FSH level was significantly related to bone loss.
After adjustment for age, ethnicity, baseline bone mineral density, and assignment to the exercise or control arm, multivariate analysis showed that only women in the lowest tertile for baseline FSH – that is, a level of 21.1 IU/L or less – maintained their baseline bone mineral density at the lumbar spine. They averaged a 0.007% increase over 12 months. In contrast, women in the middle tertile, with a baseline FSH of 21.2-61.6 IU/L, had a mean 0.96% decrease in bone density, and those in the highest tertile, with an FSH of 61.7-124.6 IU/L, averaged a 2.2% bone loss.
“Of note, bone loss was seen with an FSH greater than 21 IU/L, a lower level than is typical of diagnostic criteria for premature ovarian failure,” Dr. Tabatabai observed.
Tamoxifen therapy, time since chemotherapy, and baseline estradiol levels were not related to bone loss or preservation. Baseline CTX (urinary C-terminal crosslinking telopeptide) was the only bone turnover marker associated with subsequent bone loss, but this relationship was marginal.
Also noteworthy was the finding that absence of menstruation did not predict bone loss, said Dr. Tabatabai. Less than 60% of women in the lowest FSH tertile reported menstruating both at baseline and at 12 months, yet they maintained bone mass.
Chemotherapy in premenopausal women often results in premature ovarian failure, bone loss, and amenorrhea. This comes about because the medications damage ovarian follicles and steroid-producing cells, with resultant reduced production of estradiol and inhibin B. This results in loss of feedback inhibition of pituitary gonadotropins along with increased FSH levels, Dr. Tabatabai explained.
She said that since hers is the first study to look at biomarkers to predict bone loss in premenopausal breast cancer patients after chemotherapy, the findings need confirmation. Further studies also should aim to pin down the optimal timing of FSH measurement in relation to breast cancer treatment.
CHICAGO – A premenopausal breast cancer patient’s follicle-stimulating hormone level upon completion of chemotherapy predicts her risk of bone loss during the ensuing 12 months, Dr. Laila S. Tabatabai reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
“This may have significant implications for preserving bone health in premenopausal women with breast cancer. Appropriate use of FSH as a marker for premature ovarian failure and as a predictor of bone loss after breast cancer treatment may allow for the timely implementation of preventive measures to reduce fracture risk,” said Dr. Tabatabai of Johns Hopkins University, Baltimore.
She presented a secondary analysis from the Exercise for Bone Health: Young Breast Cancer Survivors Study, in which 206 women who were under age 55 and had completed adjuvant chemotherapy for breast cancer were randomized to a 12-month structured exercise program conducted through the YMCA or to a control group that received a monthly health newsletter.
Investigators measured baseline levels of FSH, bone turnover markers, calciotropic hormones, and high-sensitivity C-reactive protein. At 1 year follow-up, only baseline FSH level was significantly related to bone loss.
After adjustment for age, ethnicity, baseline bone mineral density, and assignment to the exercise or control arm, multivariate analysis showed that only women in the lowest tertile for baseline FSH – that is, a level of 21.1 IU/L or less – maintained their baseline bone mineral density at the lumbar spine. They averaged a 0.007% increase over 12 months. In contrast, women in the middle tertile, with a baseline FSH of 21.2-61.6 IU/L, had a mean 0.96% decrease in bone density, and those in the highest tertile, with an FSH of 61.7-124.6 IU/L, averaged a 2.2% bone loss.
“Of note, bone loss was seen with an FSH greater than 21 IU/L, a lower level than is typical of diagnostic criteria for premature ovarian failure,” Dr. Tabatabai observed.
Tamoxifen therapy, time since chemotherapy, and baseline estradiol levels were not related to bone loss or preservation. Baseline CTX (urinary C-terminal crosslinking telopeptide) was the only bone turnover marker associated with subsequent bone loss, but this relationship was marginal.
Also noteworthy was the finding that absence of menstruation did not predict bone loss, said Dr. Tabatabai. Less than 60% of women in the lowest FSH tertile reported menstruating both at baseline and at 12 months, yet they maintained bone mass.
Chemotherapy in premenopausal women often results in premature ovarian failure, bone loss, and amenorrhea. This comes about because the medications damage ovarian follicles and steroid-producing cells, with resultant reduced production of estradiol and inhibin B. This results in loss of feedback inhibition of pituitary gonadotropins along with increased FSH levels, Dr. Tabatabai explained.
She said that since hers is the first study to look at biomarkers to predict bone loss in premenopausal breast cancer patients after chemotherapy, the findings need confirmation. Further studies also should aim to pin down the optimal timing of FSH measurement in relation to breast cancer treatment.
CHICAGO – A premenopausal breast cancer patient’s follicle-stimulating hormone level upon completion of chemotherapy predicts her risk of bone loss during the ensuing 12 months, Dr. Laila S. Tabatabai reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
“This may have significant implications for preserving bone health in premenopausal women with breast cancer. Appropriate use of FSH as a marker for premature ovarian failure and as a predictor of bone loss after breast cancer treatment may allow for the timely implementation of preventive measures to reduce fracture risk,” said Dr. Tabatabai of Johns Hopkins University, Baltimore.
She presented a secondary analysis from the Exercise for Bone Health: Young Breast Cancer Survivors Study, in which 206 women who were under age 55 and had completed adjuvant chemotherapy for breast cancer were randomized to a 12-month structured exercise program conducted through the YMCA or to a control group that received a monthly health newsletter.
Investigators measured baseline levels of FSH, bone turnover markers, calciotropic hormones, and high-sensitivity C-reactive protein. At 1 year follow-up, only baseline FSH level was significantly related to bone loss.
After adjustment for age, ethnicity, baseline bone mineral density, and assignment to the exercise or control arm, multivariate analysis showed that only women in the lowest tertile for baseline FSH – that is, a level of 21.1 IU/L or less – maintained their baseline bone mineral density at the lumbar spine. They averaged a 0.007% increase over 12 months. In contrast, women in the middle tertile, with a baseline FSH of 21.2-61.6 IU/L, had a mean 0.96% decrease in bone density, and those in the highest tertile, with an FSH of 61.7-124.6 IU/L, averaged a 2.2% bone loss.
“Of note, bone loss was seen with an FSH greater than 21 IU/L, a lower level than is typical of diagnostic criteria for premature ovarian failure,” Dr. Tabatabai observed.
Tamoxifen therapy, time since chemotherapy, and baseline estradiol levels were not related to bone loss or preservation. Baseline CTX (urinary C-terminal crosslinking telopeptide) was the only bone turnover marker associated with subsequent bone loss, but this relationship was marginal.
Also noteworthy was the finding that absence of menstruation did not predict bone loss, said Dr. Tabatabai. Less than 60% of women in the lowest FSH tertile reported menstruating both at baseline and at 12 months, yet they maintained bone mass.
Chemotherapy in premenopausal women often results in premature ovarian failure, bone loss, and amenorrhea. This comes about because the medications damage ovarian follicles and steroid-producing cells, with resultant reduced production of estradiol and inhibin B. This results in loss of feedback inhibition of pituitary gonadotropins along with increased FSH levels, Dr. Tabatabai explained.
She said that since hers is the first study to look at biomarkers to predict bone loss in premenopausal breast cancer patients after chemotherapy, the findings need confirmation. Further studies also should aim to pin down the optimal timing of FSH measurement in relation to breast cancer treatment.
Key clinical point: A premenopausal breast cancer patient’s FSH level upon completion of adjuvant chemotherapy identifies whether she ought to be placed on preventive antiosteoporosis medication to reduce fracture risk.
Major finding: Premenopausal breast cancer patients with an FSH level greater than 21.1 IU/L after completion of chemotherapy had a significant rate of bone loss during the subsequent 12 months.
Data source: A secondary analysis of a prospective, randomized, controlled trial involving 206 women who underwent adjuvant chemotherapy for premenopausal breast cancer.
Disclosures: The study was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
VIDEO: Smartphone ECG detects atrial fibrillation
SANTA CLARA, CALIF. – When Dr. Omar Dawood demonstrated the AliveCor Heart Monitor with a new app and algorithm for detecting atrial fibrillation on stage at the Health 2.0 fall conference 2014, it showed that his heart was in normal sinus rhythm – but he had a heart rate of 135 beats per minute.
Chalk it up to the excitement of speaking before an audience of physicians and technologists about this new mobile ECG tool, Dr. Dawood said. “I’m not always that anxious.”
The AliveCor device attaches to the back of iPhones or Android-based smartphones and sells for $60-$199, depending on the model of smartphone. The Food and Drug Administration approved it in 2013, and patients have used it since March 2014. The device sends an ECG reading to a cardiologist or cardiac technician, who sends a reply within 24 hours.
With the new, free app, however, patients get an immediate result from the device showing whether or not they are likely to have atrial fibrillation. The FDA cleared the algorithm for the app in August 2014, and the company launched it on the marketplace at the Health 2.0 conference.
Validation studies have shown that the AliveCor system performs comparably to a traditional 12-lead ECG, Dr. Dawood said in a video interview.
For other recent news on studies of AliveCor in clinical settings, see our Evidence-Based Apps column.
Dr. Dawood, a surgeon by training, is a clinical adviser at AliveCor and also works for a separate technology company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SANTA CLARA, CALIF. – When Dr. Omar Dawood demonstrated the AliveCor Heart Monitor with a new app and algorithm for detecting atrial fibrillation on stage at the Health 2.0 fall conference 2014, it showed that his heart was in normal sinus rhythm – but he had a heart rate of 135 beats per minute.
Chalk it up to the excitement of speaking before an audience of physicians and technologists about this new mobile ECG tool, Dr. Dawood said. “I’m not always that anxious.”
The AliveCor device attaches to the back of iPhones or Android-based smartphones and sells for $60-$199, depending on the model of smartphone. The Food and Drug Administration approved it in 2013, and patients have used it since March 2014. The device sends an ECG reading to a cardiologist or cardiac technician, who sends a reply within 24 hours.
With the new, free app, however, patients get an immediate result from the device showing whether or not they are likely to have atrial fibrillation. The FDA cleared the algorithm for the app in August 2014, and the company launched it on the marketplace at the Health 2.0 conference.
Validation studies have shown that the AliveCor system performs comparably to a traditional 12-lead ECG, Dr. Dawood said in a video interview.
For other recent news on studies of AliveCor in clinical settings, see our Evidence-Based Apps column.
Dr. Dawood, a surgeon by training, is a clinical adviser at AliveCor and also works for a separate technology company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SANTA CLARA, CALIF. – When Dr. Omar Dawood demonstrated the AliveCor Heart Monitor with a new app and algorithm for detecting atrial fibrillation on stage at the Health 2.0 fall conference 2014, it showed that his heart was in normal sinus rhythm – but he had a heart rate of 135 beats per minute.
Chalk it up to the excitement of speaking before an audience of physicians and technologists about this new mobile ECG tool, Dr. Dawood said. “I’m not always that anxious.”
The AliveCor device attaches to the back of iPhones or Android-based smartphones and sells for $60-$199, depending on the model of smartphone. The Food and Drug Administration approved it in 2013, and patients have used it since March 2014. The device sends an ECG reading to a cardiologist or cardiac technician, who sends a reply within 24 hours.
With the new, free app, however, patients get an immediate result from the device showing whether or not they are likely to have atrial fibrillation. The FDA cleared the algorithm for the app in August 2014, and the company launched it on the marketplace at the Health 2.0 conference.
Validation studies have shown that the AliveCor system performs comparably to a traditional 12-lead ECG, Dr. Dawood said in a video interview.
For other recent news on studies of AliveCor in clinical settings, see our Evidence-Based Apps column.
Dr. Dawood, a surgeon by training, is a clinical adviser at AliveCor and also works for a separate technology company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert