User login
Failure to properly manage a patient’s hypertension
Failure to properly manage a patient’s hypertension
A 44-YEAR-OLD MAN WHO WEIGHED >450 POUNDS went to his internist for treatment of hypertension. At a work-related physical the previous day, his blood pressure had been 160/110 mm Hg. After examination, the internist wrote a 30-day prescription for amlodipine, 5 mg/d, with 3 refills. The patient saw the physician 2 weeks later but not again until 3 months later. At that visit, the internist prescribed amlodipine, 5 mg/d, for 90 days with 2 refills. The patient missed his next appointment, which was set for 4 months later, but when his medication was about to run out, he was able to get a prescription for 10 months’ worth of amlodipine by phone. The patient died 2 months before the prescription ran out.
PLAINTIFF’S CLAIM The physician failed to properly manage and monitor the patient’s hypertension. The dosage of amlodipine was insufficient.
THE DEFENSE The patient was noncompliant and failed to show for follow-up appointments. The dosage of amlodipine was sufficient. The cause of death was unknown because no autopsy was performed.
VERDICT $136,000 New Jersey verdict.
COMMENT If we accept a patient into our practice, we need to have reasonable policies for patients to show up for follow-up, and to consider having them find another physician if they do not.
Did the patient’s age discourage proper evaluation?
THREE MONTHS AFTER NOTICING BLOOD IN HER STOOL, a 19-year-old woman went to see her physician. Without ordering a flexible sigmoidoscopy or colonoscopy, the physician diagnosed a healing anal fissure. Approximately 4 years later, the patient developed bloody diarrhea and went to a gastroenterologist, who found a 2.6 cm lesion in her rectum during a flexible sigmoidoscopy. Biopsy confirmed a low-grade adenocarcinoma. Imaging studies revealed that the cancer had spread to her lungs and liver, and she was diagnosed with Stage IV rectal cancer. After 2 years of extensive treatment that included surgical resection, conventional and experimental chemotherapy, and radiation therapy, the patient died.
PLAINTIFF’S CLAIM If the physician had ordered endoscopy exams when the patient first presented for treatment, testing could have identified a polyp or early-stage cancer.
THE DEFENSE No information about the defense is available.
VERDICT $2.5 million Maryland verdict.
COMMENT Colon cancer in a 19-year-old is extraordinarily rare. I doubt that the patient didn’t experience any more rectal bleeding until 4 years after she first sought treatment. A lesson in this tragic case is to be sure to document when you tell patients to “come back to see me right away if this happens again.”
23-year-old dies when myocarditis is mistaken for bronchitis
A 23-YEAR-OLD MAN PRESENTED TO THE EMERGENCY DEPARTMENT (ED) with chest tightness, cough, and fever. After a chest x-ray, the ED physician diagnosed bronchitis and sent the patient home with prescriptions for hydrocodone/acetaminophen and antibiotics. He was found dead in his bed less than 24 hours later. An autopsy determined the cause of death was myocarditis.
PLAINTIFF’S CLAIM The physician didn’t perform an electrocardiogram (EKG), which is a routine evaluation for a patient with chest pain. The EKG would have detected myocarditis.
THE DEFENSE The patient was evaluated properly. An EKG was not necessary.
VERDICT $2.9 million Massachusetts verdict.
COMMENT I think the jury got this one wrong. I don’t think an EKG is necessary for every case of acute bronchitis. However, I do wonder if the chest x-ray showed a large heart shadow.
Failure to properly manage a patient’s hypertension
A 44-YEAR-OLD MAN WHO WEIGHED >450 POUNDS went to his internist for treatment of hypertension. At a work-related physical the previous day, his blood pressure had been 160/110 mm Hg. After examination, the internist wrote a 30-day prescription for amlodipine, 5 mg/d, with 3 refills. The patient saw the physician 2 weeks later but not again until 3 months later. At that visit, the internist prescribed amlodipine, 5 mg/d, for 90 days with 2 refills. The patient missed his next appointment, which was set for 4 months later, but when his medication was about to run out, he was able to get a prescription for 10 months’ worth of amlodipine by phone. The patient died 2 months before the prescription ran out.
PLAINTIFF’S CLAIM The physician failed to properly manage and monitor the patient’s hypertension. The dosage of amlodipine was insufficient.
THE DEFENSE The patient was noncompliant and failed to show for follow-up appointments. The dosage of amlodipine was sufficient. The cause of death was unknown because no autopsy was performed.
VERDICT $136,000 New Jersey verdict.
COMMENT If we accept a patient into our practice, we need to have reasonable policies for patients to show up for follow-up, and to consider having them find another physician if they do not.
Did the patient’s age discourage proper evaluation?
THREE MONTHS AFTER NOTICING BLOOD IN HER STOOL, a 19-year-old woman went to see her physician. Without ordering a flexible sigmoidoscopy or colonoscopy, the physician diagnosed a healing anal fissure. Approximately 4 years later, the patient developed bloody diarrhea and went to a gastroenterologist, who found a 2.6 cm lesion in her rectum during a flexible sigmoidoscopy. Biopsy confirmed a low-grade adenocarcinoma. Imaging studies revealed that the cancer had spread to her lungs and liver, and she was diagnosed with Stage IV rectal cancer. After 2 years of extensive treatment that included surgical resection, conventional and experimental chemotherapy, and radiation therapy, the patient died.
PLAINTIFF’S CLAIM If the physician had ordered endoscopy exams when the patient first presented for treatment, testing could have identified a polyp or early-stage cancer.
THE DEFENSE No information about the defense is available.
VERDICT $2.5 million Maryland verdict.
COMMENT Colon cancer in a 19-year-old is extraordinarily rare. I doubt that the patient didn’t experience any more rectal bleeding until 4 years after she first sought treatment. A lesson in this tragic case is to be sure to document when you tell patients to “come back to see me right away if this happens again.”
23-year-old dies when myocarditis is mistaken for bronchitis
A 23-YEAR-OLD MAN PRESENTED TO THE EMERGENCY DEPARTMENT (ED) with chest tightness, cough, and fever. After a chest x-ray, the ED physician diagnosed bronchitis and sent the patient home with prescriptions for hydrocodone/acetaminophen and antibiotics. He was found dead in his bed less than 24 hours later. An autopsy determined the cause of death was myocarditis.
PLAINTIFF’S CLAIM The physician didn’t perform an electrocardiogram (EKG), which is a routine evaluation for a patient with chest pain. The EKG would have detected myocarditis.
THE DEFENSE The patient was evaluated properly. An EKG was not necessary.
VERDICT $2.9 million Massachusetts verdict.
COMMENT I think the jury got this one wrong. I don’t think an EKG is necessary for every case of acute bronchitis. However, I do wonder if the chest x-ray showed a large heart shadow.
Failure to properly manage a patient’s hypertension
A 44-YEAR-OLD MAN WHO WEIGHED >450 POUNDS went to his internist for treatment of hypertension. At a work-related physical the previous day, his blood pressure had been 160/110 mm Hg. After examination, the internist wrote a 30-day prescription for amlodipine, 5 mg/d, with 3 refills. The patient saw the physician 2 weeks later but not again until 3 months later. At that visit, the internist prescribed amlodipine, 5 mg/d, for 90 days with 2 refills. The patient missed his next appointment, which was set for 4 months later, but when his medication was about to run out, he was able to get a prescription for 10 months’ worth of amlodipine by phone. The patient died 2 months before the prescription ran out.
PLAINTIFF’S CLAIM The physician failed to properly manage and monitor the patient’s hypertension. The dosage of amlodipine was insufficient.
THE DEFENSE The patient was noncompliant and failed to show for follow-up appointments. The dosage of amlodipine was sufficient. The cause of death was unknown because no autopsy was performed.
VERDICT $136,000 New Jersey verdict.
COMMENT If we accept a patient into our practice, we need to have reasonable policies for patients to show up for follow-up, and to consider having them find another physician if they do not.
Did the patient’s age discourage proper evaluation?
THREE MONTHS AFTER NOTICING BLOOD IN HER STOOL, a 19-year-old woman went to see her physician. Without ordering a flexible sigmoidoscopy or colonoscopy, the physician diagnosed a healing anal fissure. Approximately 4 years later, the patient developed bloody diarrhea and went to a gastroenterologist, who found a 2.6 cm lesion in her rectum during a flexible sigmoidoscopy. Biopsy confirmed a low-grade adenocarcinoma. Imaging studies revealed that the cancer had spread to her lungs and liver, and she was diagnosed with Stage IV rectal cancer. After 2 years of extensive treatment that included surgical resection, conventional and experimental chemotherapy, and radiation therapy, the patient died.
PLAINTIFF’S CLAIM If the physician had ordered endoscopy exams when the patient first presented for treatment, testing could have identified a polyp or early-stage cancer.
THE DEFENSE No information about the defense is available.
VERDICT $2.5 million Maryland verdict.
COMMENT Colon cancer in a 19-year-old is extraordinarily rare. I doubt that the patient didn’t experience any more rectal bleeding until 4 years after she first sought treatment. A lesson in this tragic case is to be sure to document when you tell patients to “come back to see me right away if this happens again.”
23-year-old dies when myocarditis is mistaken for bronchitis
A 23-YEAR-OLD MAN PRESENTED TO THE EMERGENCY DEPARTMENT (ED) with chest tightness, cough, and fever. After a chest x-ray, the ED physician diagnosed bronchitis and sent the patient home with prescriptions for hydrocodone/acetaminophen and antibiotics. He was found dead in his bed less than 24 hours later. An autopsy determined the cause of death was myocarditis.
PLAINTIFF’S CLAIM The physician didn’t perform an electrocardiogram (EKG), which is a routine evaluation for a patient with chest pain. The EKG would have detected myocarditis.
THE DEFENSE The patient was evaluated properly. An EKG was not necessary.
VERDICT $2.9 million Massachusetts verdict.
COMMENT I think the jury got this one wrong. I don’t think an EKG is necessary for every case of acute bronchitis. However, I do wonder if the chest x-ray showed a large heart shadow.
Probiotics for colic? A PURL update
In “Colicky baby? Here’s a surprising remedy” (J Fam Pract. 2011;60:34-36), we summarized a 2010 double-blind randomized controlled trial (RCT) that found the probiotic Lactobacillus reuteri DSM 17938 reduced daily crying time in colicky, exclusively breastfed infants.1
A recently published RCT of the same probiotic by Sung et al2 adds to the body of evidence and suggests that the jury may still be out as to the value of probiotics for colicky babies.
The newer study (which also measured colic using modified Wessel’s criteria) included babies who were formula-fed as well as those who were breastfed. When researchers looked at all babies as a single group, those who received probiotics fussed significantly more than those who received placebo at nearly all of the postintervention time points. However, when they delved deeper, the researchers noted that an increase in fussing occurred only among infants on formula. On the other hand, the time that breastfed infants spent crying or fussing did not vary significantly between those who received probiotics and those who received placebo.
Both the 2010 and 2014 studies used valid RCT methods with low risk for bias, so we’re not clear why the results (especially for breastfed infants) differed. The 2010 study was done in Italy and required breastfeeding moms to avoid cow’s milk, while the 2014 Sung et al2 study was conducted in Australia and did not have this requirement, so environmental factors may have played a role. The reporting method in the Sung et al2 study—a well-validated, detailed diary of infant behaviors—may have led to less parent recall error than the diary used in the 2010 study. All in all, we can only conclude that it is unclear whether probiotics work to reduce crying in colicky infants.
A safe bet may be to avoid recommending probiotics for colicky formula-fed infants, since no study of this population has shown probiotics are effective, and in the Sung et al2 study, they appeared to worsen symptoms. For breastfed babies, there is no evidence of harm, and mixed evidence on whether probiotics help.
1. Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533.
2. Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107.
In “Colicky baby? Here’s a surprising remedy” (J Fam Pract. 2011;60:34-36), we summarized a 2010 double-blind randomized controlled trial (RCT) that found the probiotic Lactobacillus reuteri DSM 17938 reduced daily crying time in colicky, exclusively breastfed infants.1
A recently published RCT of the same probiotic by Sung et al2 adds to the body of evidence and suggests that the jury may still be out as to the value of probiotics for colicky babies.
The newer study (which also measured colic using modified Wessel’s criteria) included babies who were formula-fed as well as those who were breastfed. When researchers looked at all babies as a single group, those who received probiotics fussed significantly more than those who received placebo at nearly all of the postintervention time points. However, when they delved deeper, the researchers noted that an increase in fussing occurred only among infants on formula. On the other hand, the time that breastfed infants spent crying or fussing did not vary significantly between those who received probiotics and those who received placebo.
Both the 2010 and 2014 studies used valid RCT methods with low risk for bias, so we’re not clear why the results (especially for breastfed infants) differed. The 2010 study was done in Italy and required breastfeeding moms to avoid cow’s milk, while the 2014 Sung et al2 study was conducted in Australia and did not have this requirement, so environmental factors may have played a role. The reporting method in the Sung et al2 study—a well-validated, detailed diary of infant behaviors—may have led to less parent recall error than the diary used in the 2010 study. All in all, we can only conclude that it is unclear whether probiotics work to reduce crying in colicky infants.
A safe bet may be to avoid recommending probiotics for colicky formula-fed infants, since no study of this population has shown probiotics are effective, and in the Sung et al2 study, they appeared to worsen symptoms. For breastfed babies, there is no evidence of harm, and mixed evidence on whether probiotics help.
In “Colicky baby? Here’s a surprising remedy” (J Fam Pract. 2011;60:34-36), we summarized a 2010 double-blind randomized controlled trial (RCT) that found the probiotic Lactobacillus reuteri DSM 17938 reduced daily crying time in colicky, exclusively breastfed infants.1
A recently published RCT of the same probiotic by Sung et al2 adds to the body of evidence and suggests that the jury may still be out as to the value of probiotics for colicky babies.
The newer study (which also measured colic using modified Wessel’s criteria) included babies who were formula-fed as well as those who were breastfed. When researchers looked at all babies as a single group, those who received probiotics fussed significantly more than those who received placebo at nearly all of the postintervention time points. However, when they delved deeper, the researchers noted that an increase in fussing occurred only among infants on formula. On the other hand, the time that breastfed infants spent crying or fussing did not vary significantly between those who received probiotics and those who received placebo.
Both the 2010 and 2014 studies used valid RCT methods with low risk for bias, so we’re not clear why the results (especially for breastfed infants) differed. The 2010 study was done in Italy and required breastfeeding moms to avoid cow’s milk, while the 2014 Sung et al2 study was conducted in Australia and did not have this requirement, so environmental factors may have played a role. The reporting method in the Sung et al2 study—a well-validated, detailed diary of infant behaviors—may have led to less parent recall error than the diary used in the 2010 study. All in all, we can only conclude that it is unclear whether probiotics work to reduce crying in colicky infants.
A safe bet may be to avoid recommending probiotics for colicky formula-fed infants, since no study of this population has shown probiotics are effective, and in the Sung et al2 study, they appeared to worsen symptoms. For breastfed babies, there is no evidence of harm, and mixed evidence on whether probiotics help.
1. Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533.
2. Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107.
1. Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533.
2. Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Dutch study clarifies risk of attempted vaginal birth of breech fetus
Should a baby that presents breech at term be delivered vaginally or by cesarean delivery? In October 2000, results of the Term Breech Trial (TBT), the largest randomized controlled trial to investigate the effect of delivery mode for term breech deliveries on neonatal and maternal outcomes, essentially answered this question, showing that planned cesarean delivery was safer than planned vaginal delivery (with combined perinatal morbidity and mortality scores of 5% vs 16%, respectively).1 These study results affected national guidelines for choosing delivery mode in the Netherlands as well as other countries around the world.
What has been the impact on mode of delivery and neonatal outcome in the Netherlands since the TBT’s publication? Furthermore, are there antepartum parameters that can distinguish which women are at high risk versus low risk for adverse neonatal outcomes when presenting breech at delivery? Vlemmix and colleagues sought to answer these questions, using retrospective data from the Netherlands Perinatal Registry (PRN) from 1999 through 2007.1
Perinatal death decreased over time—but only for women electing cesarean
During the study period, approximately 4% of all births were breech. The researchers studied 58,320 women with term breech delivery, using the PRN. They noted an increase in the elective cesarean delivery (ECD) rate for these women, from 24% before October 2000 to 60% after December 2000, and as a consequence, found that overall perinatal mortality decreased from 1.3% to 0.7% (odds ratio [OR] 0.51; 95% confidence interval [CI] 0.28–0.93).1
However, among the women who underwent planned vaginal delivery, the overall perinatal mortality remained stable (1.7% vs 1.6%; OR, 0.96; 95% CI, 0.52–1.76). “Despite the lower percentage of women opting for or offered a vaginal delivery, and despite a higher emergency cesarean rate during vaginal breech birth, neonatal outcome within the planned vaginal birth group did not improve,” state the authors.1
Putting the results in absolute numbers
The investigators say that the 40% of Dutch women with breech presentation at term who still attempt vaginal birth do so without improved neonatal outcome; these deliveries generate a 10-fold higher fetal mortality rate compared with ECD. Further, no subgroup of women, when evaluating parity, onset of labor, type of breech presentation, and birthweight, could be identified with a low risk of poor neonatal outcome during planned vaginal delivery compared with ECD.1
Since the TBT, 1,692 more combined elective and emergency cesarean deliveries were performed annually, leading to five less neonatal deaths per year (number needed to treat, 338). If all women who still undergo planned vaginal birth receive ECD, 6,490 more ECDs would be performed, with 10 less neonatal deaths, 116 less neonates with low Apgar score, and 20 less neonates with birth trauma per year, according to the researchers.1
They suggest that clinicians use the results of this study when counseling women with a term breech presentation on mode of delivery. “To properly inform patients, a combination of risk presentation (absolute risks, relative risks and figures) is necessary to enable individual informed decision making.”1
Share your thoughts on this news! Send your Letter to the Editor to [email protected]. Please include your name, and the city and state in which you practice.
Reference
- Vlemmix F, Bergenhenegouwen L, Schaaf JM, et al. Term breech deliveries in the Netherlands: did the increased cesarean rate affect neonatal outcome? A population-based cohort study. Acta Obstet Gynecol Scand. 2014;93:888–896.
Should a baby that presents breech at term be delivered vaginally or by cesarean delivery? In October 2000, results of the Term Breech Trial (TBT), the largest randomized controlled trial to investigate the effect of delivery mode for term breech deliveries on neonatal and maternal outcomes, essentially answered this question, showing that planned cesarean delivery was safer than planned vaginal delivery (with combined perinatal morbidity and mortality scores of 5% vs 16%, respectively).1 These study results affected national guidelines for choosing delivery mode in the Netherlands as well as other countries around the world.
What has been the impact on mode of delivery and neonatal outcome in the Netherlands since the TBT’s publication? Furthermore, are there antepartum parameters that can distinguish which women are at high risk versus low risk for adverse neonatal outcomes when presenting breech at delivery? Vlemmix and colleagues sought to answer these questions, using retrospective data from the Netherlands Perinatal Registry (PRN) from 1999 through 2007.1
Perinatal death decreased over time—but only for women electing cesarean
During the study period, approximately 4% of all births were breech. The researchers studied 58,320 women with term breech delivery, using the PRN. They noted an increase in the elective cesarean delivery (ECD) rate for these women, from 24% before October 2000 to 60% after December 2000, and as a consequence, found that overall perinatal mortality decreased from 1.3% to 0.7% (odds ratio [OR] 0.51; 95% confidence interval [CI] 0.28–0.93).1
However, among the women who underwent planned vaginal delivery, the overall perinatal mortality remained stable (1.7% vs 1.6%; OR, 0.96; 95% CI, 0.52–1.76). “Despite the lower percentage of women opting for or offered a vaginal delivery, and despite a higher emergency cesarean rate during vaginal breech birth, neonatal outcome within the planned vaginal birth group did not improve,” state the authors.1
Putting the results in absolute numbers
The investigators say that the 40% of Dutch women with breech presentation at term who still attempt vaginal birth do so without improved neonatal outcome; these deliveries generate a 10-fold higher fetal mortality rate compared with ECD. Further, no subgroup of women, when evaluating parity, onset of labor, type of breech presentation, and birthweight, could be identified with a low risk of poor neonatal outcome during planned vaginal delivery compared with ECD.1
Since the TBT, 1,692 more combined elective and emergency cesarean deliveries were performed annually, leading to five less neonatal deaths per year (number needed to treat, 338). If all women who still undergo planned vaginal birth receive ECD, 6,490 more ECDs would be performed, with 10 less neonatal deaths, 116 less neonates with low Apgar score, and 20 less neonates with birth trauma per year, according to the researchers.1
They suggest that clinicians use the results of this study when counseling women with a term breech presentation on mode of delivery. “To properly inform patients, a combination of risk presentation (absolute risks, relative risks and figures) is necessary to enable individual informed decision making.”1
Share your thoughts on this news! Send your Letter to the Editor to [email protected]. Please include your name, and the city and state in which you practice.
Should a baby that presents breech at term be delivered vaginally or by cesarean delivery? In October 2000, results of the Term Breech Trial (TBT), the largest randomized controlled trial to investigate the effect of delivery mode for term breech deliveries on neonatal and maternal outcomes, essentially answered this question, showing that planned cesarean delivery was safer than planned vaginal delivery (with combined perinatal morbidity and mortality scores of 5% vs 16%, respectively).1 These study results affected national guidelines for choosing delivery mode in the Netherlands as well as other countries around the world.
What has been the impact on mode of delivery and neonatal outcome in the Netherlands since the TBT’s publication? Furthermore, are there antepartum parameters that can distinguish which women are at high risk versus low risk for adverse neonatal outcomes when presenting breech at delivery? Vlemmix and colleagues sought to answer these questions, using retrospective data from the Netherlands Perinatal Registry (PRN) from 1999 through 2007.1
Perinatal death decreased over time—but only for women electing cesarean
During the study period, approximately 4% of all births were breech. The researchers studied 58,320 women with term breech delivery, using the PRN. They noted an increase in the elective cesarean delivery (ECD) rate for these women, from 24% before October 2000 to 60% after December 2000, and as a consequence, found that overall perinatal mortality decreased from 1.3% to 0.7% (odds ratio [OR] 0.51; 95% confidence interval [CI] 0.28–0.93).1
However, among the women who underwent planned vaginal delivery, the overall perinatal mortality remained stable (1.7% vs 1.6%; OR, 0.96; 95% CI, 0.52–1.76). “Despite the lower percentage of women opting for or offered a vaginal delivery, and despite a higher emergency cesarean rate during vaginal breech birth, neonatal outcome within the planned vaginal birth group did not improve,” state the authors.1
Putting the results in absolute numbers
The investigators say that the 40% of Dutch women with breech presentation at term who still attempt vaginal birth do so without improved neonatal outcome; these deliveries generate a 10-fold higher fetal mortality rate compared with ECD. Further, no subgroup of women, when evaluating parity, onset of labor, type of breech presentation, and birthweight, could be identified with a low risk of poor neonatal outcome during planned vaginal delivery compared with ECD.1
Since the TBT, 1,692 more combined elective and emergency cesarean deliveries were performed annually, leading to five less neonatal deaths per year (number needed to treat, 338). If all women who still undergo planned vaginal birth receive ECD, 6,490 more ECDs would be performed, with 10 less neonatal deaths, 116 less neonates with low Apgar score, and 20 less neonates with birth trauma per year, according to the researchers.1
They suggest that clinicians use the results of this study when counseling women with a term breech presentation on mode of delivery. “To properly inform patients, a combination of risk presentation (absolute risks, relative risks and figures) is necessary to enable individual informed decision making.”1
Share your thoughts on this news! Send your Letter to the Editor to [email protected]. Please include your name, and the city and state in which you practice.
Reference
- Vlemmix F, Bergenhenegouwen L, Schaaf JM, et al. Term breech deliveries in the Netherlands: did the increased cesarean rate affect neonatal outcome? A population-based cohort study. Acta Obstet Gynecol Scand. 2014;93:888–896.
Reference
- Vlemmix F, Bergenhenegouwen L, Schaaf JM, et al. Term breech deliveries in the Netherlands: did the increased cesarean rate affect neonatal outcome? A population-based cohort study. Acta Obstet Gynecol Scand. 2014;93:888–896.
Total abdominal hysterectomy the Mayo Clinic way
The abdominal approach to hysterectomy remains the most common route to hysterectomy in the United States. Its greatest advantage: It allows the uterus to be removed intact.1–3
The recent US Food and Drug Administration (FDA) warning against the use of power morcellation in women with known or suspected uterine malignancy has left many gynecologic surgeons wondering what might be the optimal approach to the removal of a large uterus.4
Although most hysterectomies are performed for benign conditions—namely, uterine fibroids—malignancy should be considered in the differential diagnosis. When hysterectomy is performed laparoscopically, a large uterus must be morcellated intraperitoneally. Since the FDA safety communication was issued, some hospitals have imposed a moratorium on the use of power morcellators for removal of uterine tissue until more definitive evidence is put forth regarding safety and best practices. This chain of events allows us an opportunity to review the basics of abdominal hysterectomy.
For the sake of this discussion, I will assume that the hysterectomy is being performed for a benign indication as I highlight the Mayo Clinic approach to total abdominal hysterectomy (TAH).5
Preoperative considerations
The patient should be medically able to undergo operative intervention. If she has preexisting medical conditions, preoperative clearance should be obtained from her primary care provider, and her medical conditions should be optimized prior to surgical intervention.
Baseline laboratory studies include a complete blood count, electrolyte panel, glucose assessment, and an electrocardiogram (EKG). Bowel prep typically is not required. Provisions should be made to prevent deep venous thrombosis (DVT), usually by utilizing sequential compression devices, based on the individual patient’s risk factors.6,7
A prophylactic antibiotic to prevent surgical site infection (often a first-generation cephalosporin) should be given as a single intravenous (IV) dose prior to the incision.8 If bacterial vaginosis is present, treatment prior to surgery can reduce the frequency of vaginal cuff infection.9
Again, for the sake of this discussion, I will assume that malignancy has been ruled out.
Positioning and preparation
After induction of anesthesia, position the patient either in a dorsal supine (traditional) or lithotomy (yellow-fin stirrups) position and reexamine her to confirm the findings of the pelvic exam. If the patient is positioned in the supine position, use ankle straps to prevent her from moving as the Trendelenburg position advances during the procedure.
Prep the abdominal skin with a bactericidal agent (most often a povidone-iodine solution). Also prep the vagina with a povidone-iodine solution because the vaginal cuff will be opened during the TAH. Place a transurethral catheter to drain urine throughout the case. Use of a three-way catheter allows the bladder to be easily backfilled during the procedure for identification of its borders or assessment of its integrity.
Last, incorporate a surgical pause prior to the incision to confirm that you have the right patient, know the procedure and incision planned, and are aware of any allergies. Also confirm that antibiotics have been given.
Operative technique
Intraoperative principles
A planned approach avoids wasteful time and motion, and an adequate incision allows for sufficient exposure, which is critical but often underappreciated by the novice surgeon. We prefer a midline incision because it allows the most flexibility to adapt to intraoperative findings, but a Pfannenstiel incision also is an option.
Fixed retraction is paramount to “set up” exposure for the remainder of the case. We prefer a Balfour fixed retractor but, with smaller uteri, a self-retaining Alexis retractor (Applied Medical, Rancho Santa Margarita, California) affords decent exposure and may cause less postoperative abdominal wall discomfort; it also avoids the possibility of retractor-related neuropathy.
Moistened abdominal packing allows the bowel to be packed into the upper abdomen for the remainder of the case, which facilitates consistent exposure of the operative field. Adequate lighting is essential, as is one or more knowledgeable assistants.
Use sharp dissection throughout the procedure. Clean, sharp dissection averts injury to adjacent structures, such as the ureter, bladder, and rectum, and promotes recognition of any injuries, permitting immediate repair.
The application of proper traction and counter-traction on tissues allows accurate definition of the correct tissue planes and facilitates identification of important anatomic structures. Vital structures should be identified and, if necessary, mobilized before any clamps are placed or pedicles transected. Adhesions should be sharply lysed to facilitate exposure.
Freeing the bladder anteriorly and the rectum posteriorly prevents their inadvertent inclusion in closure of the vagina and minimizes the risk of fistula formation. The bladder and rectum should be sharply mobilized at least 1 cm beyond the site of planned vaginal transection.
Last, excellent support of the vaginal wall can be provided by securing the uterosacral-cardinal ligaments to the corners of the vaginal vault.
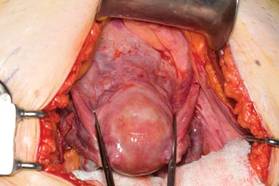
|
| FIGURE 1: Place straight Kocher clamps to facilitate traction during the operation. |
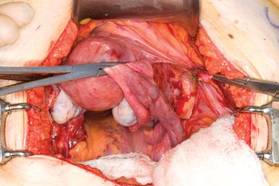
|
| FIGURE 2: Clamp and divide the right round ligament, opening the broad ligament. |
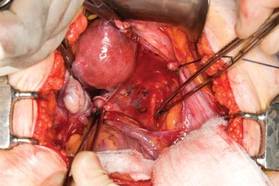
|
| FIGURE 3: Identify the right ureter along the medial leaf of the broad ligament. |
Identifying the ureter
Once good exposure and adequate Trendelenburg position are achieved, place Kocher clamps across the cornual portion of the uterus (incorporating the round ligament, tube, and utero-ovarian pedicle) (FIGURE 1). This facilitates continuous traction and prevents back bleeding throughout the case.
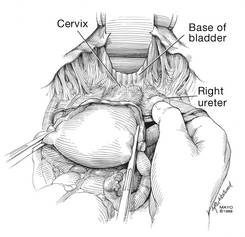
With traction applied to the left, identify the right round ligament, clamp it with a Kocher clamp, and transect it. Incise the peritoneum parallel to the uterus and gonadal vessels (FIGURE 2). This opens the broad ligament and allows identification of the critical underlying structures (ureter, external and internal iliac vessels). Following the medial leaf of the broad ligament downward, identify the ureter by both visualization and palpation (FIGURE 3).
Although I do not discuss salpingo-oophorectomy in this article, be aware that the ureter is at risk when clamping the gonadal vessels near the pelvic brim.
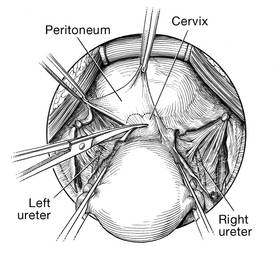
Once the ureter is identified, create a window in the broad ligament above the ureter. In a medial to lateral fashion, place your index finger through that peritoneal window, making certain the ureter is below and out of the way. Place a Kocher clamp across the tube and utero-ovarian pedicle, and transect and suture-ligate the pedicle (preserving the tube and ovary). Repeat this procedure on the patient’s left side, using traction and counter-traction to facilitate exposure (FIGURE 4).
Mobilizing the bladder
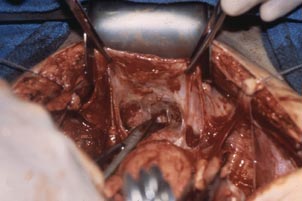
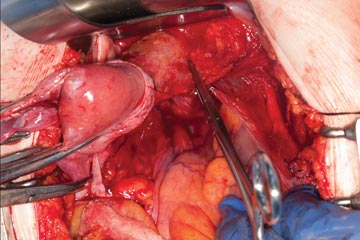
With the assistant providing upward traction on the uterus, use Russian forceps to elevate the peritoneum overlying the bladder. Undermine and incise the peritoneum from the patient’s left to the right (FIGURE 5). Begin sharp dissection of the loose areolar tissue. By gently spreading the tissue using the tips of the scissors, and snipping the tissue in the midline, you allow the dissection to proceed down the lower uterine segment (FIGURE 6).
Any bleeding usually means you are too close to the bladder or have ventured too far laterally. If the patient has had a previous cesarean delivery, this area may be densely scarred. Often, it is easiest to dissect laterally around the scar on each side, where there is less dense scarring, and mobilize the tissue until the denser central scarring can be dissected. Note that the bladder attachment curves upward on each side and lateral to the cervix, over the lateral vagina and the uterine vessels.
It is absolutely critical to dissect and expose 1 or 2 cm of the entire anterior vaginal wall below the level of the cervix to be certain that the bladder has been fully mobilized and to prevent later incorporation into the vaginal cuff closure. The exact location of the cervix is best detected by placing a finger behind the uterus and using the thumb to compress the area of the anterior portion of the cervix under the bladder.
Fibroids can cause distortion of the anatomic planes we utilize. Be aware of the distortion and adjust your dissection accordingly. The structures of the urinary tract are most often affected; sharp dissection is necessary to mobilize the ureter and bladder in these cases. (See the case discussions)
| 
|
FIGURE 5: Upward traction on the peritoneum overlying the bladder facilitates development of the bladder flap off of the lower uterus. | FIGURE 6: Dissect the bladder off the lower uterus |
Large intramural or pedunculated myomas can be difficult surgical challenges. Broad-ligament myomas, however, are unique. Significant anatomic distortion can occur. Always consider the possibility of some degree of ureteral obstruction and be on the lookout for unrecognized bladder injury.
Case 1
This very large myoma essentially filled the pelvis but seems to arise from the left side of the uterus, distorting the anatomy. Note the attenuation of the round ligaments and the normal appearance of the tubes and ovaries (the left tube has a distal paratubal cyst.) Note also the bladder, particularly how sharp dissection will be required to mobilize it off the underlying mass.
To manage removal, at case outset, we placed bilateral external ureteral stents and used a lucite vaginal dilator to aid in respective ureter and vaginal apex identification. The bladder was attenuated over this large mass and was rather easily dissected, given the defined mass around it. The ureters were well lateral and inferior and readily identified with stent palpation. The cervix was certainly elongated and, after the uterine vessels were removed, the hysterectomy was completed without incident.
Surgical pearl: To extract very large masses during total abdominal hysterectomy, sometimes you have more “room” if the fixed retractor is removed. You can then use a series of handheld retractors (Deavor, Harrington, etc) on the side you are operating until the mass has been mobilized enough to place a fixed retractor.
CASE 2
| 
|
| 
|
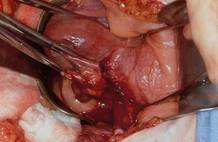
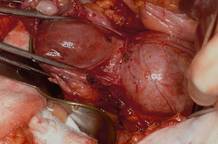
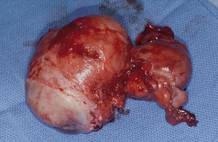
This large cervical myoma is creating urinary urgency, frequency, and moderate obstruction of the right ureter. Sharp dissection is critical to mobilize the bladder well free of the myoma. We placed bilateral ureteral stents to start the case to aid in identification of the ureter.
The first illustration at right (top left) shows the operative appearance before the bladder flap was taken down. The second photo (top right) reveals the extent of this large myoma after the bladder has been sharply dissected free of the mass. The third photo (bottom left) displays the specimen sent to pathology (be sure to minimize the amount of vaginal tissue taken with the specimen). Note the distortion of the endocervical canal and cervix. The last photo (bottom right) reveals the sectioned specimen.
Surgical pearl: Use a three-way catheter and backfill the bladder for identification during the procedure and at the conclusion of the case to rule out bladder injury. A few drops of methylene blue added to the solution makes recognition easier.
Ligation of the uterine arteries
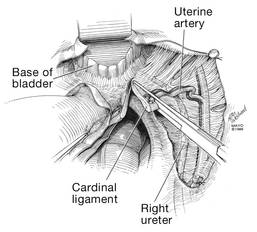
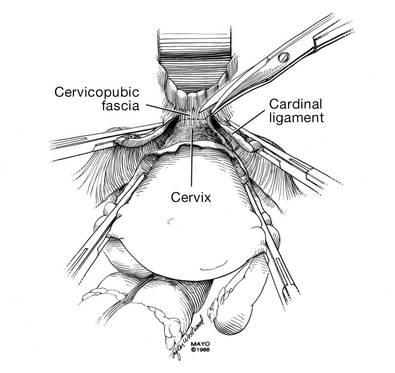
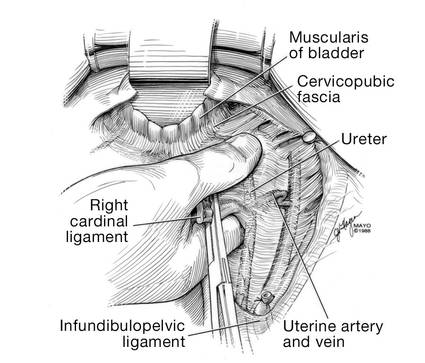
Apply cephalad traction to the uterus and place a Harrington retractor anteriorly to retract the bladder away from the cervix on the upper portion of the vagina. With the uterus pulled first to the left, palpate the right ureter between the thumb and index finger at the level of the uterine artery (FIGURE 7). Once you have determined the course of the ureter, place a Kocher clamp well down on the right side of the lower cervix at about a 45° angle, sliding off the side of the cervix (FIGURE 8). The clamp should now include the superior portion of the cardinal ligament with the uterine vessels and paracolpium immediately above the lateral vaginal fornix.
Transect the cardinal pedicle. Repeat the procedure on the left side after adjusting the Harrington retractor slightly to the left and identifying the course of the ureter where the Kocher clamp will be placed. Thus, a single Kocher clamp is placed on each side to control the blood supply.
It is paramount that you know the location of the ureter prior to placement and transection of the uterine vessels to prevent inadvertent injury or obstruction of the ureters.
Divide the uterine vessel–cardinal ligament complex close to the cervix (medial to the Kocher clamp), slightly undercutting the tip (FIGURE 9). This creates a fascial window, the anterior edge of which is the pubocervical fascia. This is subsequently developed and managed as a separate layer during the vaginal vault closure.
Repeat this procedure on the left side.
| 
| 
|
| FIGURE 7: After mobilizing the bladder, palpate the right ureter prior to placement of a Kocher clamp on the cardinal vascular pedicle. | FIGURE 8: Place a single Kocher clamp on the right uterine vessels. See the right ureter well lateral of the clamp. | FIGURE 9: The right uterine vessels have been clamped and transected. The clamp is slightly undercut to allow access to the vaginal fornix. |
Preparing and opening the vaginal cuff
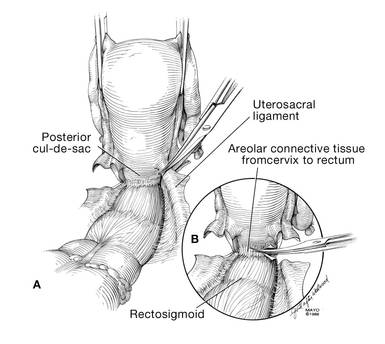
Develop the anterior pubocervical fascia by cutting with a scissors horizontally across the anterior vaginal wall at the level of the cervix (FIGURE 10). This layer of tissue will be utilized to close the vaginal cuff in a secondary layer later on. This technique will serve to close the pubocervical fascial ring at the vaginal vault and, with support of the uterosacral ligaments, provide support to the vaginal apex. At this point, the vagina has not yet been entered. The remaining tissue beneath the mobilized layer and the anterior cervix is the anterior vaginal wall.
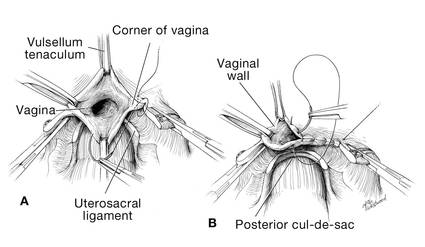
Pull the uterus up and anterior toward the pubic symphysis to make the uterosacral ligaments prominent. Then transect the uterosacral ligaments close to the uterus (FIGURE 11). You may encounter minor bleeding, but there is no need to ligate the stumps at this point. Cut the tissue between the ligaments horizontally, similarly to the anterior dissection. With gentle finger dissection, as necessary, this should free the rectosigmoid colon from the posterior vaginal wall.
If this is a new technique for you, it may serve you well to place a stitch in each uterosacral ligament, below the spot where you will transect it, prior to cutting. When you have the uterus on tension, the ligaments are most recognizable, and these sutures can then be incorporated into the vaginal angles.
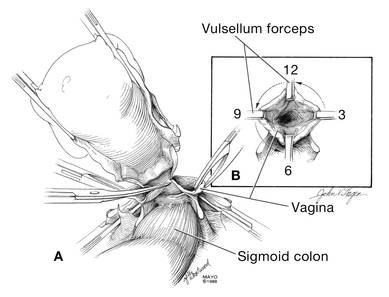
At this point there should be a circular area just beneath the cervix that is the vaginal wall at the apex of the vagina. Retract the uterus anteriorly and to the left, and enter the vagina posteriorly and laterally, just above the stump of the right uterosacral ligament (FIGURE 12). The uterus now can be removed by circumcising the vagina as close to the cervix as possible to avoid vaginal shortening. As the uterus is being removed, place four vulsellum tenacula successively at the 3, 12, 9, and 6 o’clock positions of the vaginal cuff as it is developed (FIGURE 13). Then swab povidone-iodine on the vaginal cuff and canal.
| 
|
| FIGURE 10: After mobilizing the bladder and securing the vascular pedicles, develop the anterior endopelvic fascia. It will be used as a second layer to close the vaginal cuff. | FIGURE 11: A. Transect the uterosacral ligaments. B. Mobilize the rectum posteriorly. |
| 
|
| FIGURE 12: Enter the right vaginal fornix and grasp it with a vulsellum tenaculum. | FIGURE 13: A. After entering the right vaginal fornix, extend the incision in a counter-clockwise fashion, preserving vaginal length. B. Placement of the tenaculum as the incision proceeds. |
Cuff closure
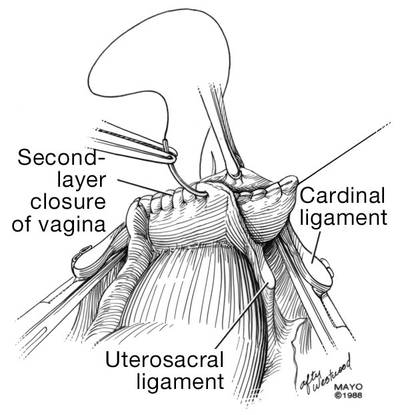
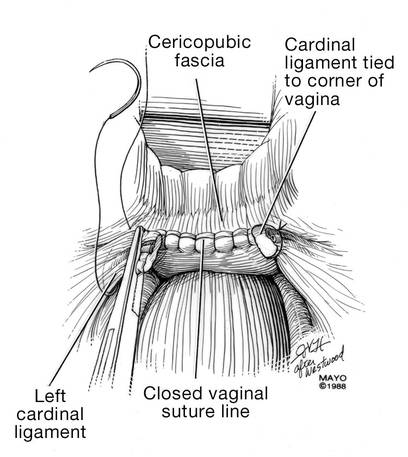
Place a lap salt sponge over the Kocher clamps to prevent suture entanglement. Use a 36-inch continuous 0-polyglactin suture to close the vaginal vault and achieve hemostasis. Begin suturing with a right vaginal angle stitch, placed so that the small vessels are ligated and the lateral supporting tissues from the base of the cardinal ligament are attached to the right vaginal angle. This closes the “window” that was created with slight undercutting of the cardinal ligament earlier. Continue suturing toward the left, with each bite placed submucosally so that the epithelial edges are approximated and inverted into the vagina. The suture does not enter the vagina (FIGURE 14). As you reach the left vaginal angle, obtain a healthy purchase of the left uterosacral ligament and then pass the needle laterally to the vaginal fornix angle, through the lateral supporting tissues at the base of the cardinal ligament, as was done on the right vaginal angle. Then lock the suture and return across the vaginal vault. This will plicate together the anterior and posterior pubocervical fascia layers developed earlier, creating a second layer, before closing the fascial ring at the vaginal apex. Incorporate the right uterosacral ligament into the right vaginal angle and tie the suture (FIGURES 15 AND 16).
Close the cuff and verify hemostasis. Use the lap salt sponge covering the Kocher clamps on the cardinal ligament pedicles to wipe the surgical field clean. Then use light cautery along the cuff and the base and back of the bladder. If there is some bleeding at the very corners of the vagina, it can usually be managed during suturing of the cardinal ligament pedicles into the corners of the vault.
Elevate the Kocher clamp containing the cardinal ligament and uterine vessels toward the midline. Then palpate the ureter between your index finger and thumb as it courses through the cardinal ligament toward the bladder. This step provides a second check on the location of the ureter (the first was when the clamp was originally placed) before the cardinal ligament is tied to the corner of the vault (FIGURE 17). The needle should enter the peritoneum, right uterosacral ligament, and full thickness of the right angle of the vagina just lateral to the suture used to close the vaginal apex. Bring the pedicle over the corner of the vagina and tie it close to the lateral aspect of the Kocher clamp, leaving an adequate stump. Then free-tie the stump of the cardinal ligament to add a double ligation of this vascular pedicle. Repeat this procedure on the opposite side (FIGURE 18). Then verify hemostasis throughout the operative field.
Take the patient out of Trendelenburg position and place her flat, and copiously irrigate the pelvis. Once needle and sponge counts are completed, close the abdomen in a layered fashion. Place a wound dressing and a Foley catheter, leaving the latter in place overnight.
| 
|
| FIGURE 14: A. Begin in the right vaginal corner, closing the area that was “undercut” (see FIGURE 10). B. Close the first layer in in a subcuticular manner. | FIGURE 15: The second layer of cuff closure utilizes the anterior and posterior endopelvic fascia and imbricates the first layer. |
| |
FIGURE 16: Two-layer closure of the vaginal cuff. | |
| 
|
| FIGURE 17: Prior to suture ligation of the right uterine vessels, palpate the ureter again to identify its location. | FIGURE 18: Suture-ligate the uterine pedicles into the corners of the vaginal cuff. |
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095.
2. Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol. 2008;111(3):753–767.
3. Unger JB, Paul R, Caldito G. Hysterectomy for the massive leiomyomatous uterus. Obstet Gynecol. 2002;100(6):1271–1275.
4. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed September 15, 2014.
5. Webb MJ. Mayo Clinic Manual of Pelvic Surgery. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:55–72.
6. Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84. Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 pt 1):429–440.
7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
8. Committee on Practice Bulletins, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108(1):225–234.
9. Larsson PG, Carlsson B. Does pre- and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis? Infect Dis Obstet Gynecol. 2002;10(3):133–140.
The abdominal approach to hysterectomy remains the most common route to hysterectomy in the United States. Its greatest advantage: It allows the uterus to be removed intact.1–3
The recent US Food and Drug Administration (FDA) warning against the use of power morcellation in women with known or suspected uterine malignancy has left many gynecologic surgeons wondering what might be the optimal approach to the removal of a large uterus.4
Although most hysterectomies are performed for benign conditions—namely, uterine fibroids—malignancy should be considered in the differential diagnosis. When hysterectomy is performed laparoscopically, a large uterus must be morcellated intraperitoneally. Since the FDA safety communication was issued, some hospitals have imposed a moratorium on the use of power morcellators for removal of uterine tissue until more definitive evidence is put forth regarding safety and best practices. This chain of events allows us an opportunity to review the basics of abdominal hysterectomy.
For the sake of this discussion, I will assume that the hysterectomy is being performed for a benign indication as I highlight the Mayo Clinic approach to total abdominal hysterectomy (TAH).5
Preoperative considerations
The patient should be medically able to undergo operative intervention. If she has preexisting medical conditions, preoperative clearance should be obtained from her primary care provider, and her medical conditions should be optimized prior to surgical intervention.
Baseline laboratory studies include a complete blood count, electrolyte panel, glucose assessment, and an electrocardiogram (EKG). Bowel prep typically is not required. Provisions should be made to prevent deep venous thrombosis (DVT), usually by utilizing sequential compression devices, based on the individual patient’s risk factors.6,7
A prophylactic antibiotic to prevent surgical site infection (often a first-generation cephalosporin) should be given as a single intravenous (IV) dose prior to the incision.8 If bacterial vaginosis is present, treatment prior to surgery can reduce the frequency of vaginal cuff infection.9
Again, for the sake of this discussion, I will assume that malignancy has been ruled out.
Positioning and preparation
After induction of anesthesia, position the patient either in a dorsal supine (traditional) or lithotomy (yellow-fin stirrups) position and reexamine her to confirm the findings of the pelvic exam. If the patient is positioned in the supine position, use ankle straps to prevent her from moving as the Trendelenburg position advances during the procedure.
Prep the abdominal skin with a bactericidal agent (most often a povidone-iodine solution). Also prep the vagina with a povidone-iodine solution because the vaginal cuff will be opened during the TAH. Place a transurethral catheter to drain urine throughout the case. Use of a three-way catheter allows the bladder to be easily backfilled during the procedure for identification of its borders or assessment of its integrity.
Last, incorporate a surgical pause prior to the incision to confirm that you have the right patient, know the procedure and incision planned, and are aware of any allergies. Also confirm that antibiotics have been given.
Operative technique
Intraoperative principles
A planned approach avoids wasteful time and motion, and an adequate incision allows for sufficient exposure, which is critical but often underappreciated by the novice surgeon. We prefer a midline incision because it allows the most flexibility to adapt to intraoperative findings, but a Pfannenstiel incision also is an option.
Fixed retraction is paramount to “set up” exposure for the remainder of the case. We prefer a Balfour fixed retractor but, with smaller uteri, a self-retaining Alexis retractor (Applied Medical, Rancho Santa Margarita, California) affords decent exposure and may cause less postoperative abdominal wall discomfort; it also avoids the possibility of retractor-related neuropathy.
Moistened abdominal packing allows the bowel to be packed into the upper abdomen for the remainder of the case, which facilitates consistent exposure of the operative field. Adequate lighting is essential, as is one or more knowledgeable assistants.
Use sharp dissection throughout the procedure. Clean, sharp dissection averts injury to adjacent structures, such as the ureter, bladder, and rectum, and promotes recognition of any injuries, permitting immediate repair.
The application of proper traction and counter-traction on tissues allows accurate definition of the correct tissue planes and facilitates identification of important anatomic structures. Vital structures should be identified and, if necessary, mobilized before any clamps are placed or pedicles transected. Adhesions should be sharply lysed to facilitate exposure.
Freeing the bladder anteriorly and the rectum posteriorly prevents their inadvertent inclusion in closure of the vagina and minimizes the risk of fistula formation. The bladder and rectum should be sharply mobilized at least 1 cm beyond the site of planned vaginal transection.
Last, excellent support of the vaginal wall can be provided by securing the uterosacral-cardinal ligaments to the corners of the vaginal vault.

|
| FIGURE 1: Place straight Kocher clamps to facilitate traction during the operation. |

|
| FIGURE 2: Clamp and divide the right round ligament, opening the broad ligament. |

|
| FIGURE 3: Identify the right ureter along the medial leaf of the broad ligament. |
Identifying the ureter
Once good exposure and adequate Trendelenburg position are achieved, place Kocher clamps across the cornual portion of the uterus (incorporating the round ligament, tube, and utero-ovarian pedicle) (FIGURE 1). This facilitates continuous traction and prevents back bleeding throughout the case.
With traction applied to the left, identify the right round ligament, clamp it with a Kocher clamp, and transect it. Incise the peritoneum parallel to the uterus and gonadal vessels (FIGURE 2). This opens the broad ligament and allows identification of the critical underlying structures (ureter, external and internal iliac vessels). Following the medial leaf of the broad ligament downward, identify the ureter by both visualization and palpation (FIGURE 3).
Although I do not discuss salpingo-oophorectomy in this article, be aware that the ureter is at risk when clamping the gonadal vessels near the pelvic brim.
Once the ureter is identified, create a window in the broad ligament above the ureter. In a medial to lateral fashion, place your index finger through that peritoneal window, making certain the ureter is below and out of the way. Place a Kocher clamp across the tube and utero-ovarian pedicle, and transect and suture-ligate the pedicle (preserving the tube and ovary). Repeat this procedure on the patient’s left side, using traction and counter-traction to facilitate exposure (FIGURE 4).
Mobilizing the bladder
With the assistant providing upward traction on the uterus, use Russian forceps to elevate the peritoneum overlying the bladder. Undermine and incise the peritoneum from the patient’s left to the right (FIGURE 5). Begin sharp dissection of the loose areolar tissue. By gently spreading the tissue using the tips of the scissors, and snipping the tissue in the midline, you allow the dissection to proceed down the lower uterine segment (FIGURE 6).
Any bleeding usually means you are too close to the bladder or have ventured too far laterally. If the patient has had a previous cesarean delivery, this area may be densely scarred. Often, it is easiest to dissect laterally around the scar on each side, where there is less dense scarring, and mobilize the tissue until the denser central scarring can be dissected. Note that the bladder attachment curves upward on each side and lateral to the cervix, over the lateral vagina and the uterine vessels.
It is absolutely critical to dissect and expose 1 or 2 cm of the entire anterior vaginal wall below the level of the cervix to be certain that the bladder has been fully mobilized and to prevent later incorporation into the vaginal cuff closure. The exact location of the cervix is best detected by placing a finger behind the uterus and using the thumb to compress the area of the anterior portion of the cervix under the bladder.
Fibroids can cause distortion of the anatomic planes we utilize. Be aware of the distortion and adjust your dissection accordingly. The structures of the urinary tract are most often affected; sharp dissection is necessary to mobilize the ureter and bladder in these cases. (See the case discussions)

| 
|
FIGURE 5: Upward traction on the peritoneum overlying the bladder facilitates development of the bladder flap off of the lower uterus. | FIGURE 6: Dissect the bladder off the lower uterus |
Large intramural or pedunculated myomas can be difficult surgical challenges. Broad-ligament myomas, however, are unique. Significant anatomic distortion can occur. Always consider the possibility of some degree of ureteral obstruction and be on the lookout for unrecognized bladder injury.
Case 1
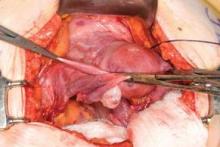
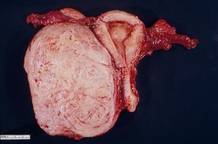
This very large myoma essentially filled the pelvis but seems to arise from the left side of the uterus, distorting the anatomy. Note the attenuation of the round ligaments and the normal appearance of the tubes and ovaries (the left tube has a distal paratubal cyst.) Note also the bladder, particularly how sharp dissection will be required to mobilize it off the underlying mass.
To manage removal, at case outset, we placed bilateral external ureteral stents and used a lucite vaginal dilator to aid in respective ureter and vaginal apex identification. The bladder was attenuated over this large mass and was rather easily dissected, given the defined mass around it. The ureters were well lateral and inferior and readily identified with stent palpation. The cervix was certainly elongated and, after the uterine vessels were removed, the hysterectomy was completed without incident.
Surgical pearl: To extract very large masses during total abdominal hysterectomy, sometimes you have more “room” if the fixed retractor is removed. You can then use a series of handheld retractors (Deavor, Harrington, etc) on the side you are operating until the mass has been mobilized enough to place a fixed retractor.
CASE 2

| 
|

| 
|
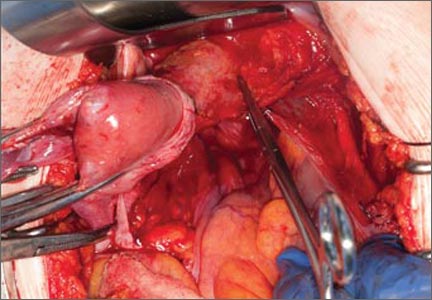
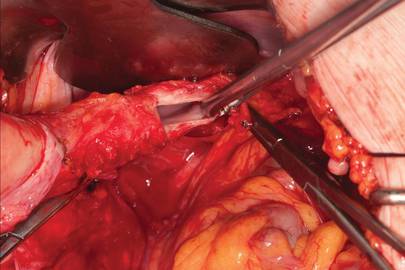
This large cervical myoma is creating urinary urgency, frequency, and moderate obstruction of the right ureter. Sharp dissection is critical to mobilize the bladder well free of the myoma. We placed bilateral ureteral stents to start the case to aid in identification of the ureter.
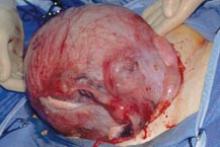
The first illustration at right (top left) shows the operative appearance before the bladder flap was taken down. The second photo (top right) reveals the extent of this large myoma after the bladder has been sharply dissected free of the mass. The third photo (bottom left) displays the specimen sent to pathology (be sure to minimize the amount of vaginal tissue taken with the specimen). Note the distortion of the endocervical canal and cervix. The last photo (bottom right) reveals the sectioned specimen.
Surgical pearl: Use a three-way catheter and backfill the bladder for identification during the procedure and at the conclusion of the case to rule out bladder injury. A few drops of methylene blue added to the solution makes recognition easier.
Ligation of the uterine arteries
Apply cephalad traction to the uterus and place a Harrington retractor anteriorly to retract the bladder away from the cervix on the upper portion of the vagina. With the uterus pulled first to the left, palpate the right ureter between the thumb and index finger at the level of the uterine artery (FIGURE 7). Once you have determined the course of the ureter, place a Kocher clamp well down on the right side of the lower cervix at about a 45° angle, sliding off the side of the cervix (FIGURE 8). The clamp should now include the superior portion of the cardinal ligament with the uterine vessels and paracolpium immediately above the lateral vaginal fornix.
Transect the cardinal pedicle. Repeat the procedure on the left side after adjusting the Harrington retractor slightly to the left and identifying the course of the ureter where the Kocher clamp will be placed. Thus, a single Kocher clamp is placed on each side to control the blood supply.
It is paramount that you know the location of the ureter prior to placement and transection of the uterine vessels to prevent inadvertent injury or obstruction of the ureters.
Divide the uterine vessel–cardinal ligament complex close to the cervix (medial to the Kocher clamp), slightly undercutting the tip (FIGURE 9). This creates a fascial window, the anterior edge of which is the pubocervical fascia. This is subsequently developed and managed as a separate layer during the vaginal vault closure.
Repeat this procedure on the left side.

| 
| 
|
| FIGURE 7: After mobilizing the bladder, palpate the right ureter prior to placement of a Kocher clamp on the cardinal vascular pedicle. | FIGURE 8: Place a single Kocher clamp on the right uterine vessels. See the right ureter well lateral of the clamp. | FIGURE 9: The right uterine vessels have been clamped and transected. The clamp is slightly undercut to allow access to the vaginal fornix. |
Preparing and opening the vaginal cuff
Develop the anterior pubocervical fascia by cutting with a scissors horizontally across the anterior vaginal wall at the level of the cervix (FIGURE 10). This layer of tissue will be utilized to close the vaginal cuff in a secondary layer later on. This technique will serve to close the pubocervical fascial ring at the vaginal vault and, with support of the uterosacral ligaments, provide support to the vaginal apex. At this point, the vagina has not yet been entered. The remaining tissue beneath the mobilized layer and the anterior cervix is the anterior vaginal wall.
Pull the uterus up and anterior toward the pubic symphysis to make the uterosacral ligaments prominent. Then transect the uterosacral ligaments close to the uterus (FIGURE 11). You may encounter minor bleeding, but there is no need to ligate the stumps at this point. Cut the tissue between the ligaments horizontally, similarly to the anterior dissection. With gentle finger dissection, as necessary, this should free the rectosigmoid colon from the posterior vaginal wall.
If this is a new technique for you, it may serve you well to place a stitch in each uterosacral ligament, below the spot where you will transect it, prior to cutting. When you have the uterus on tension, the ligaments are most recognizable, and these sutures can then be incorporated into the vaginal angles.
At this point there should be a circular area just beneath the cervix that is the vaginal wall at the apex of the vagina. Retract the uterus anteriorly and to the left, and enter the vagina posteriorly and laterally, just above the stump of the right uterosacral ligament (FIGURE 12). The uterus now can be removed by circumcising the vagina as close to the cervix as possible to avoid vaginal shortening. As the uterus is being removed, place four vulsellum tenacula successively at the 3, 12, 9, and 6 o’clock positions of the vaginal cuff as it is developed (FIGURE 13). Then swab povidone-iodine on the vaginal cuff and canal.

| 
|
| FIGURE 10: After mobilizing the bladder and securing the vascular pedicles, develop the anterior endopelvic fascia. It will be used as a second layer to close the vaginal cuff. | FIGURE 11: A. Transect the uterosacral ligaments. B. Mobilize the rectum posteriorly. |

| 
|
| FIGURE 12: Enter the right vaginal fornix and grasp it with a vulsellum tenaculum. | FIGURE 13: A. After entering the right vaginal fornix, extend the incision in a counter-clockwise fashion, preserving vaginal length. B. Placement of the tenaculum as the incision proceeds. |
Cuff closure
Place a lap salt sponge over the Kocher clamps to prevent suture entanglement. Use a 36-inch continuous 0-polyglactin suture to close the vaginal vault and achieve hemostasis. Begin suturing with a right vaginal angle stitch, placed so that the small vessels are ligated and the lateral supporting tissues from the base of the cardinal ligament are attached to the right vaginal angle. This closes the “window” that was created with slight undercutting of the cardinal ligament earlier. Continue suturing toward the left, with each bite placed submucosally so that the epithelial edges are approximated and inverted into the vagina. The suture does not enter the vagina (FIGURE 14). As you reach the left vaginal angle, obtain a healthy purchase of the left uterosacral ligament and then pass the needle laterally to the vaginal fornix angle, through the lateral supporting tissues at the base of the cardinal ligament, as was done on the right vaginal angle. Then lock the suture and return across the vaginal vault. This will plicate together the anterior and posterior pubocervical fascia layers developed earlier, creating a second layer, before closing the fascial ring at the vaginal apex. Incorporate the right uterosacral ligament into the right vaginal angle and tie the suture (FIGURES 15 AND 16).
Close the cuff and verify hemostasis. Use the lap salt sponge covering the Kocher clamps on the cardinal ligament pedicles to wipe the surgical field clean. Then use light cautery along the cuff and the base and back of the bladder. If there is some bleeding at the very corners of the vagina, it can usually be managed during suturing of the cardinal ligament pedicles into the corners of the vault.
Elevate the Kocher clamp containing the cardinal ligament and uterine vessels toward the midline. Then palpate the ureter between your index finger and thumb as it courses through the cardinal ligament toward the bladder. This step provides a second check on the location of the ureter (the first was when the clamp was originally placed) before the cardinal ligament is tied to the corner of the vault (FIGURE 17). The needle should enter the peritoneum, right uterosacral ligament, and full thickness of the right angle of the vagina just lateral to the suture used to close the vaginal apex. Bring the pedicle over the corner of the vagina and tie it close to the lateral aspect of the Kocher clamp, leaving an adequate stump. Then free-tie the stump of the cardinal ligament to add a double ligation of this vascular pedicle. Repeat this procedure on the opposite side (FIGURE 18). Then verify hemostasis throughout the operative field.
Take the patient out of Trendelenburg position and place her flat, and copiously irrigate the pelvis. Once needle and sponge counts are completed, close the abdomen in a layered fashion. Place a wound dressing and a Foley catheter, leaving the latter in place overnight.

| 
|
| FIGURE 14: A. Begin in the right vaginal corner, closing the area that was “undercut” (see FIGURE 10). B. Close the first layer in in a subcuticular manner. | FIGURE 15: The second layer of cuff closure utilizes the anterior and posterior endopelvic fascia and imbricates the first layer. |

| |
FIGURE 16: Two-layer closure of the vaginal cuff. | |

| 
|
| FIGURE 17: Prior to suture ligation of the right uterine vessels, palpate the ureter again to identify its location. | FIGURE 18: Suture-ligate the uterine pedicles into the corners of the vaginal cuff. |
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The abdominal approach to hysterectomy remains the most common route to hysterectomy in the United States. Its greatest advantage: It allows the uterus to be removed intact.1–3
The recent US Food and Drug Administration (FDA) warning against the use of power morcellation in women with known or suspected uterine malignancy has left many gynecologic surgeons wondering what might be the optimal approach to the removal of a large uterus.4
Although most hysterectomies are performed for benign conditions—namely, uterine fibroids—malignancy should be considered in the differential diagnosis. When hysterectomy is performed laparoscopically, a large uterus must be morcellated intraperitoneally. Since the FDA safety communication was issued, some hospitals have imposed a moratorium on the use of power morcellators for removal of uterine tissue until more definitive evidence is put forth regarding safety and best practices. This chain of events allows us an opportunity to review the basics of abdominal hysterectomy.
For the sake of this discussion, I will assume that the hysterectomy is being performed for a benign indication as I highlight the Mayo Clinic approach to total abdominal hysterectomy (TAH).5
Preoperative considerations
The patient should be medically able to undergo operative intervention. If she has preexisting medical conditions, preoperative clearance should be obtained from her primary care provider, and her medical conditions should be optimized prior to surgical intervention.
Baseline laboratory studies include a complete blood count, electrolyte panel, glucose assessment, and an electrocardiogram (EKG). Bowel prep typically is not required. Provisions should be made to prevent deep venous thrombosis (DVT), usually by utilizing sequential compression devices, based on the individual patient’s risk factors.6,7
A prophylactic antibiotic to prevent surgical site infection (often a first-generation cephalosporin) should be given as a single intravenous (IV) dose prior to the incision.8 If bacterial vaginosis is present, treatment prior to surgery can reduce the frequency of vaginal cuff infection.9
Again, for the sake of this discussion, I will assume that malignancy has been ruled out.
Positioning and preparation
After induction of anesthesia, position the patient either in a dorsal supine (traditional) or lithotomy (yellow-fin stirrups) position and reexamine her to confirm the findings of the pelvic exam. If the patient is positioned in the supine position, use ankle straps to prevent her from moving as the Trendelenburg position advances during the procedure.
Prep the abdominal skin with a bactericidal agent (most often a povidone-iodine solution). Also prep the vagina with a povidone-iodine solution because the vaginal cuff will be opened during the TAH. Place a transurethral catheter to drain urine throughout the case. Use of a three-way catheter allows the bladder to be easily backfilled during the procedure for identification of its borders or assessment of its integrity.
Last, incorporate a surgical pause prior to the incision to confirm that you have the right patient, know the procedure and incision planned, and are aware of any allergies. Also confirm that antibiotics have been given.
Operative technique
Intraoperative principles
A planned approach avoids wasteful time and motion, and an adequate incision allows for sufficient exposure, which is critical but often underappreciated by the novice surgeon. We prefer a midline incision because it allows the most flexibility to adapt to intraoperative findings, but a Pfannenstiel incision also is an option.
Fixed retraction is paramount to “set up” exposure for the remainder of the case. We prefer a Balfour fixed retractor but, with smaller uteri, a self-retaining Alexis retractor (Applied Medical, Rancho Santa Margarita, California) affords decent exposure and may cause less postoperative abdominal wall discomfort; it also avoids the possibility of retractor-related neuropathy.
Moistened abdominal packing allows the bowel to be packed into the upper abdomen for the remainder of the case, which facilitates consistent exposure of the operative field. Adequate lighting is essential, as is one or more knowledgeable assistants.
Use sharp dissection throughout the procedure. Clean, sharp dissection averts injury to adjacent structures, such as the ureter, bladder, and rectum, and promotes recognition of any injuries, permitting immediate repair.
The application of proper traction and counter-traction on tissues allows accurate definition of the correct tissue planes and facilitates identification of important anatomic structures. Vital structures should be identified and, if necessary, mobilized before any clamps are placed or pedicles transected. Adhesions should be sharply lysed to facilitate exposure.
Freeing the bladder anteriorly and the rectum posteriorly prevents their inadvertent inclusion in closure of the vagina and minimizes the risk of fistula formation. The bladder and rectum should be sharply mobilized at least 1 cm beyond the site of planned vaginal transection.
Last, excellent support of the vaginal wall can be provided by securing the uterosacral-cardinal ligaments to the corners of the vaginal vault.

|
| FIGURE 1: Place straight Kocher clamps to facilitate traction during the operation. |

|
| FIGURE 2: Clamp and divide the right round ligament, opening the broad ligament. |

|
| FIGURE 3: Identify the right ureter along the medial leaf of the broad ligament. |
Identifying the ureter
Once good exposure and adequate Trendelenburg position are achieved, place Kocher clamps across the cornual portion of the uterus (incorporating the round ligament, tube, and utero-ovarian pedicle) (FIGURE 1). This facilitates continuous traction and prevents back bleeding throughout the case.
With traction applied to the left, identify the right round ligament, clamp it with a Kocher clamp, and transect it. Incise the peritoneum parallel to the uterus and gonadal vessels (FIGURE 2). This opens the broad ligament and allows identification of the critical underlying structures (ureter, external and internal iliac vessels). Following the medial leaf of the broad ligament downward, identify the ureter by both visualization and palpation (FIGURE 3).
Although I do not discuss salpingo-oophorectomy in this article, be aware that the ureter is at risk when clamping the gonadal vessels near the pelvic brim.
Once the ureter is identified, create a window in the broad ligament above the ureter. In a medial to lateral fashion, place your index finger through that peritoneal window, making certain the ureter is below and out of the way. Place a Kocher clamp across the tube and utero-ovarian pedicle, and transect and suture-ligate the pedicle (preserving the tube and ovary). Repeat this procedure on the patient’s left side, using traction and counter-traction to facilitate exposure (FIGURE 4).
Mobilizing the bladder
With the assistant providing upward traction on the uterus, use Russian forceps to elevate the peritoneum overlying the bladder. Undermine and incise the peritoneum from the patient’s left to the right (FIGURE 5). Begin sharp dissection of the loose areolar tissue. By gently spreading the tissue using the tips of the scissors, and snipping the tissue in the midline, you allow the dissection to proceed down the lower uterine segment (FIGURE 6).
Any bleeding usually means you are too close to the bladder or have ventured too far laterally. If the patient has had a previous cesarean delivery, this area may be densely scarred. Often, it is easiest to dissect laterally around the scar on each side, where there is less dense scarring, and mobilize the tissue until the denser central scarring can be dissected. Note that the bladder attachment curves upward on each side and lateral to the cervix, over the lateral vagina and the uterine vessels.
It is absolutely critical to dissect and expose 1 or 2 cm of the entire anterior vaginal wall below the level of the cervix to be certain that the bladder has been fully mobilized and to prevent later incorporation into the vaginal cuff closure. The exact location of the cervix is best detected by placing a finger behind the uterus and using the thumb to compress the area of the anterior portion of the cervix under the bladder.
Fibroids can cause distortion of the anatomic planes we utilize. Be aware of the distortion and adjust your dissection accordingly. The structures of the urinary tract are most often affected; sharp dissection is necessary to mobilize the ureter and bladder in these cases. (See the case discussions)

| 
|
FIGURE 5: Upward traction on the peritoneum overlying the bladder facilitates development of the bladder flap off of the lower uterus. | FIGURE 6: Dissect the bladder off the lower uterus |
Large intramural or pedunculated myomas can be difficult surgical challenges. Broad-ligament myomas, however, are unique. Significant anatomic distortion can occur. Always consider the possibility of some degree of ureteral obstruction and be on the lookout for unrecognized bladder injury.
Case 1
This very large myoma essentially filled the pelvis but seems to arise from the left side of the uterus, distorting the anatomy. Note the attenuation of the round ligaments and the normal appearance of the tubes and ovaries (the left tube has a distal paratubal cyst.) Note also the bladder, particularly how sharp dissection will be required to mobilize it off the underlying mass.
To manage removal, at case outset, we placed bilateral external ureteral stents and used a lucite vaginal dilator to aid in respective ureter and vaginal apex identification. The bladder was attenuated over this large mass and was rather easily dissected, given the defined mass around it. The ureters were well lateral and inferior and readily identified with stent palpation. The cervix was certainly elongated and, after the uterine vessels were removed, the hysterectomy was completed without incident.
Surgical pearl: To extract very large masses during total abdominal hysterectomy, sometimes you have more “room” if the fixed retractor is removed. You can then use a series of handheld retractors (Deavor, Harrington, etc) on the side you are operating until the mass has been mobilized enough to place a fixed retractor.
CASE 2

| 
|

| 
|
This large cervical myoma is creating urinary urgency, frequency, and moderate obstruction of the right ureter. Sharp dissection is critical to mobilize the bladder well free of the myoma. We placed bilateral ureteral stents to start the case to aid in identification of the ureter.
The first illustration at right (top left) shows the operative appearance before the bladder flap was taken down. The second photo (top right) reveals the extent of this large myoma after the bladder has been sharply dissected free of the mass. The third photo (bottom left) displays the specimen sent to pathology (be sure to minimize the amount of vaginal tissue taken with the specimen). Note the distortion of the endocervical canal and cervix. The last photo (bottom right) reveals the sectioned specimen.
Surgical pearl: Use a three-way catheter and backfill the bladder for identification during the procedure and at the conclusion of the case to rule out bladder injury. A few drops of methylene blue added to the solution makes recognition easier.
Ligation of the uterine arteries
Apply cephalad traction to the uterus and place a Harrington retractor anteriorly to retract the bladder away from the cervix on the upper portion of the vagina. With the uterus pulled first to the left, palpate the right ureter between the thumb and index finger at the level of the uterine artery (FIGURE 7). Once you have determined the course of the ureter, place a Kocher clamp well down on the right side of the lower cervix at about a 45° angle, sliding off the side of the cervix (FIGURE 8). The clamp should now include the superior portion of the cardinal ligament with the uterine vessels and paracolpium immediately above the lateral vaginal fornix.
Transect the cardinal pedicle. Repeat the procedure on the left side after adjusting the Harrington retractor slightly to the left and identifying the course of the ureter where the Kocher clamp will be placed. Thus, a single Kocher clamp is placed on each side to control the blood supply.
It is paramount that you know the location of the ureter prior to placement and transection of the uterine vessels to prevent inadvertent injury or obstruction of the ureters.
Divide the uterine vessel–cardinal ligament complex close to the cervix (medial to the Kocher clamp), slightly undercutting the tip (FIGURE 9). This creates a fascial window, the anterior edge of which is the pubocervical fascia. This is subsequently developed and managed as a separate layer during the vaginal vault closure.
Repeat this procedure on the left side.

| 
| 
|
| FIGURE 7: After mobilizing the bladder, palpate the right ureter prior to placement of a Kocher clamp on the cardinal vascular pedicle. | FIGURE 8: Place a single Kocher clamp on the right uterine vessels. See the right ureter well lateral of the clamp. | FIGURE 9: The right uterine vessels have been clamped and transected. The clamp is slightly undercut to allow access to the vaginal fornix. |
Preparing and opening the vaginal cuff
Develop the anterior pubocervical fascia by cutting with a scissors horizontally across the anterior vaginal wall at the level of the cervix (FIGURE 10). This layer of tissue will be utilized to close the vaginal cuff in a secondary layer later on. This technique will serve to close the pubocervical fascial ring at the vaginal vault and, with support of the uterosacral ligaments, provide support to the vaginal apex. At this point, the vagina has not yet been entered. The remaining tissue beneath the mobilized layer and the anterior cervix is the anterior vaginal wall.
Pull the uterus up and anterior toward the pubic symphysis to make the uterosacral ligaments prominent. Then transect the uterosacral ligaments close to the uterus (FIGURE 11). You may encounter minor bleeding, but there is no need to ligate the stumps at this point. Cut the tissue between the ligaments horizontally, similarly to the anterior dissection. With gentle finger dissection, as necessary, this should free the rectosigmoid colon from the posterior vaginal wall.
If this is a new technique for you, it may serve you well to place a stitch in each uterosacral ligament, below the spot where you will transect it, prior to cutting. When you have the uterus on tension, the ligaments are most recognizable, and these sutures can then be incorporated into the vaginal angles.
At this point there should be a circular area just beneath the cervix that is the vaginal wall at the apex of the vagina. Retract the uterus anteriorly and to the left, and enter the vagina posteriorly and laterally, just above the stump of the right uterosacral ligament (FIGURE 12). The uterus now can be removed by circumcising the vagina as close to the cervix as possible to avoid vaginal shortening. As the uterus is being removed, place four vulsellum tenacula successively at the 3, 12, 9, and 6 o’clock positions of the vaginal cuff as it is developed (FIGURE 13). Then swab povidone-iodine on the vaginal cuff and canal.

| 
|
| FIGURE 10: After mobilizing the bladder and securing the vascular pedicles, develop the anterior endopelvic fascia. It will be used as a second layer to close the vaginal cuff. | FIGURE 11: A. Transect the uterosacral ligaments. B. Mobilize the rectum posteriorly. |

| 
|
| FIGURE 12: Enter the right vaginal fornix and grasp it with a vulsellum tenaculum. | FIGURE 13: A. After entering the right vaginal fornix, extend the incision in a counter-clockwise fashion, preserving vaginal length. B. Placement of the tenaculum as the incision proceeds. |
Cuff closure
Place a lap salt sponge over the Kocher clamps to prevent suture entanglement. Use a 36-inch continuous 0-polyglactin suture to close the vaginal vault and achieve hemostasis. Begin suturing with a right vaginal angle stitch, placed so that the small vessels are ligated and the lateral supporting tissues from the base of the cardinal ligament are attached to the right vaginal angle. This closes the “window” that was created with slight undercutting of the cardinal ligament earlier. Continue suturing toward the left, with each bite placed submucosally so that the epithelial edges are approximated and inverted into the vagina. The suture does not enter the vagina (FIGURE 14). As you reach the left vaginal angle, obtain a healthy purchase of the left uterosacral ligament and then pass the needle laterally to the vaginal fornix angle, through the lateral supporting tissues at the base of the cardinal ligament, as was done on the right vaginal angle. Then lock the suture and return across the vaginal vault. This will plicate together the anterior and posterior pubocervical fascia layers developed earlier, creating a second layer, before closing the fascial ring at the vaginal apex. Incorporate the right uterosacral ligament into the right vaginal angle and tie the suture (FIGURES 15 AND 16).
Close the cuff and verify hemostasis. Use the lap salt sponge covering the Kocher clamps on the cardinal ligament pedicles to wipe the surgical field clean. Then use light cautery along the cuff and the base and back of the bladder. If there is some bleeding at the very corners of the vagina, it can usually be managed during suturing of the cardinal ligament pedicles into the corners of the vault.
Elevate the Kocher clamp containing the cardinal ligament and uterine vessels toward the midline. Then palpate the ureter between your index finger and thumb as it courses through the cardinal ligament toward the bladder. This step provides a second check on the location of the ureter (the first was when the clamp was originally placed) before the cardinal ligament is tied to the corner of the vault (FIGURE 17). The needle should enter the peritoneum, right uterosacral ligament, and full thickness of the right angle of the vagina just lateral to the suture used to close the vaginal apex. Bring the pedicle over the corner of the vagina and tie it close to the lateral aspect of the Kocher clamp, leaving an adequate stump. Then free-tie the stump of the cardinal ligament to add a double ligation of this vascular pedicle. Repeat this procedure on the opposite side (FIGURE 18). Then verify hemostasis throughout the operative field.
Take the patient out of Trendelenburg position and place her flat, and copiously irrigate the pelvis. Once needle and sponge counts are completed, close the abdomen in a layered fashion. Place a wound dressing and a Foley catheter, leaving the latter in place overnight.

| 
|
| FIGURE 14: A. Begin in the right vaginal corner, closing the area that was “undercut” (see FIGURE 10). B. Close the first layer in in a subcuticular manner. | FIGURE 15: The second layer of cuff closure utilizes the anterior and posterior endopelvic fascia and imbricates the first layer. |

| |
FIGURE 16: Two-layer closure of the vaginal cuff. | |

| 
|
| FIGURE 17: Prior to suture ligation of the right uterine vessels, palpate the ureter again to identify its location. | FIGURE 18: Suture-ligate the uterine pedicles into the corners of the vaginal cuff. |
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095.
2. Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol. 2008;111(3):753–767.
3. Unger JB, Paul R, Caldito G. Hysterectomy for the massive leiomyomatous uterus. Obstet Gynecol. 2002;100(6):1271–1275.
4. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed September 15, 2014.
5. Webb MJ. Mayo Clinic Manual of Pelvic Surgery. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:55–72.
6. Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84. Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 pt 1):429–440.
7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
8. Committee on Practice Bulletins, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108(1):225–234.
9. Larsson PG, Carlsson B. Does pre- and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis? Infect Dis Obstet Gynecol. 2002;10(3):133–140.
1. Wu JM, Wechter ME, Geller EJ, et al. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095.
2. Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol. 2008;111(3):753–767.
3. Unger JB, Paul R, Caldito G. Hysterectomy for the massive leiomyomatous uterus. Obstet Gynecol. 2002;100(6):1271–1275.
4. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed September 15, 2014.
5. Webb MJ. Mayo Clinic Manual of Pelvic Surgery. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:55–72.
6. Committee on Practice Bulletins–Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 84. Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 pt 1):429–440.
7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
8. Committee on Practice Bulletins, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 74. Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2006;108(1):225–234.
9. Larsson PG, Carlsson B. Does pre- and postoperative metronidazole treatment lower vaginal cuff infection rate after abdominal hysterectomy among women with bacterial vaginosis? Infect Dis Obstet Gynecol. 2002;10(3):133–140.
An antiemetic for irritable bowel syndrome?
Consider prescribing ondansetron up to 24 mg/d for patients who have irritable bowel syndrome with diarrhea (IBS-D).1
Strength of recommendation
B: Based on a well-done double-blind, placebo-controlled randomized controlled trial (RCT).
Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625.
Illustrative case
A 23-year-old woman who was diagnosed with irritable bowel syndrome (IBS) comes to your clinic with complaints of increased frequency of defecation with watery stools and generalized, cramping abdominal pain. She also notes increased passage of mucus and a sensation of incomplete evacuation. She says the only thing that relieves her pain is defecation. She has tried loperamide, acetaminophen, and ibuprofen without relief. She does not have Crohn’s disease or ulcerative colitis. What else can you offer her that is safe and effective?
IBS is a chronic, episodic functional gastrointestinal disorder characterized by abdominal pain or discomfort and altered bowel habits (constipation [IBS-C], diarrhea [IBS-D], or alternating periods of both—mixed [IBS-M]).2 It is diagnosed based on Rome III criteria—recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months associated with ≥2 of the following: improvement with defecation, onset associated with a change in frequency of stool, and onset associated with a change in form (appearance) of stool.3 IBS often is unrecognized or untreated, and as few as 25% of patients with IBS seek care.4
IBS-D affects approximately 5% of the general population in North America.5,6 IBS-D is associated with a considerably decreased quality of life and is a common cause of work absenteeism.7,8 Because many conditions can cause diarrhea, patients typically undergo numerous tests before receiving an accurate diagnosis, which creates a financial burden.9
For many patients, current IBS treatments, which include fiber supplements, laxatives, antidiarrheal medications, antispasmodics, and antidepressants such as tricyclics and selective serotonin reuptake inhibitors, are unsatisfactory.10 Alosetron, a 5-hydroxytryptamine 3 (5HT3) receptor antagonist, has been used to treat IBS-D,11 but this medication was voluntarily withdrawn from the US market in 2000 due to concerns of ischemic colitis and severe constipation.12 It was reintroduced in 2002, but can be prescribed only by physicians who enroll in a prescribing program provided by the manufacturer, and the drug has restrictions on its use.
Ondansetron—a different 5HT3 receptor antagonist used to treat nausea and vomiting caused by chemotherapy—may be another option for treating IBS-D. Garsed et al1 recently conducted a RCT to evaluate the efficacy of ondansetron for patients with IBS-D.
STUDY SUMMARY: Ondansetron improves stool consistency, severity of IBS symptoms
In a 5-week, double-blind crossover RCT, Garsed et al1 compared ondansetron vs placebo for symptom relief in 120 patients who met Rome III criteria for IBS-D. All patients were ages 18 to 75 and had no evidence of inflammatory bowel disease. Exclusion criteria were pregnancy or breastfeeding, unwillingness to stop antidiarrheal medication, prior abdominal surgery other than appendectomy or cholecystectomy, or being in another trial. Patients were started on ondansetron 4 mg/d with dose titration up to 24 mg/d based on response; no dose adjustments were allowed during the last 2 weeks of the study. There was a 2- to 3-week washout between treatment periods.
The primary endpoint was average stool consistency in the last 2 weeks of treatment, as measured by the Bristol Stool Form (BSF) scale.13 The BSF is a visual scale that depicts stool as hard (Type 1) to watery (Type 7); types 3 and 4 describe normal stools. The study also looked at urgency and frequency of defecation, bowel transit time, and pain scores.
Treatment with ondansetron resulted in a small but statistically significant improvement in stool consistency. The mean difference in BSF score between ondansetron and placebo was -0.9 (95% confidence interval [CI], -1.1 to -0.6; P<.001), indicating slightly more formed stool with use of ondansetron. The IBS Severity Scoring System score (maximum score 500 points, with mild, moderate, and severe cases indicated by scores of 75-175, 175-300, and >300, respectively) was reduced by more points with ondansetron than placebo (83 ± 9.8 vs 37 ± 9.7; P=.001). Although this mean difference of 46 points fell just short of the 50-point threshold that is considered clinically significant, many patients exceeded this threshold.
Compared to those who received placebo, patients who took ondansetron also had less frequent defecation (P=.002) and lower urgency scores (P<.001). Gut transit time was lengthened in the ondansetron group by 10 hours more than in the placebo group (95% CI, 6-14 hours; P<.001). Pain scores did not change significantly for patients taking ondansetron, although they experienced significantly fewer days of urgency and bloating. Symptoms typically improved in as little as 7 days but returned after stopping ondansetron, typically within 2 weeks. Sixty-five percent of patients reported adequate relief with ondansetron, compared to 14% with placebo.
Patients whose diarrhea was more severe at baseline didn’t respond as well to ondansetron as did those whose diarrhea was less severe. The only frequent adverse effect was constipation, which occurred in 9% of patients receiving ondansetron and 2% of those on placebo.
WHAT’S NEW: Another option for IBS patients with diarrhea
A prior, smaller study of ondansetron that used a lower dosage (12 mg/d) suggested benefit in IBS-D.14 In that study, ondansetron decreased diarrhea and functional dyspepsia. The study by Garsed et al1 is the first large RCT to show significantly improved stool consistency, less frequent defecation, and less urgency and bloating from using ondansetron to treat IBS-D.
CAVEATS: Ondansetron doesn’t appear to reduce pain
In Garsed et al,1 patients who received ondansetron did not experience relief from pain, which is one of the main complaints of IBS. However, this study did find slight improvement in formed stools, symptom relief that approached—but did not quite reach—clinical significance, fewer days with urgency and bloating, and less frequent defecation. This study did not evaluate the long-term effects of ondansetron use. However, ondansetron has been used for other indications for more than 25 years and has been reported to have a low risk of adverse effects.15
CHALLENGES TO IMPLEMENTATION: Remember ondansetron is not for IBS patients with constipation
Proper use of this drug among patients with IBS is key. The primary benefits of ondansetron are limited to IBS patients who suffer from diarrhea, and not constipation. Ondansetron should not be prescribed to IBS patients who experience constipation, or those with mixed symptoms.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625.
2. Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60:77-81.
3. Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237-241.
4. Luscombe FA. Health-related quality of life and associated psychosocial factors in irritable bowel syndrome: a review. Qual Life Res. 2000;9:161-176.
5. Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816-2824.
6. Thompson WG, Heaton KW, Smyth GT, et al. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78-82.
7. Tillisch K, Labus JS, Naliboff BD, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005;100:896-904.
8. Schuster MM. Diagnostic evaluation of the irritable bowel syndrome. Gastroenterol Clin North Am. 1991;20:269-278.
9. Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511.
10. Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:750-758.
11. Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545-555.
12. Chang L, Chey WD, Harris L, et al. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069-1079.
13. Heaton KW, O’Donnell LJ. An office guide to whole-gut transit time. Patients’ recollection of their stool form. J Clin Gastroenterol. 1994;19:28-30.
14. Maxton DG, Morris J, Whorwell PJ. Selective 5‐hydroxytryptamine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther. 1996;10:595-599.
15. Gill SK, Einarson A. The safety of drugs for the treatment of nausea and vomiting of pregnancy. Expert Opin Drug Saf. 2007;6:685-694.
Consider prescribing ondansetron up to 24 mg/d for patients who have irritable bowel syndrome with diarrhea (IBS-D).1
Strength of recommendation
B: Based on a well-done double-blind, placebo-controlled randomized controlled trial (RCT).
Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625.
Illustrative case
A 23-year-old woman who was diagnosed with irritable bowel syndrome (IBS) comes to your clinic with complaints of increased frequency of defecation with watery stools and generalized, cramping abdominal pain. She also notes increased passage of mucus and a sensation of incomplete evacuation. She says the only thing that relieves her pain is defecation. She has tried loperamide, acetaminophen, and ibuprofen without relief. She does not have Crohn’s disease or ulcerative colitis. What else can you offer her that is safe and effective?
IBS is a chronic, episodic functional gastrointestinal disorder characterized by abdominal pain or discomfort and altered bowel habits (constipation [IBS-C], diarrhea [IBS-D], or alternating periods of both—mixed [IBS-M]).2 It is diagnosed based on Rome III criteria—recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months associated with ≥2 of the following: improvement with defecation, onset associated with a change in frequency of stool, and onset associated with a change in form (appearance) of stool.3 IBS often is unrecognized or untreated, and as few as 25% of patients with IBS seek care.4
IBS-D affects approximately 5% of the general population in North America.5,6 IBS-D is associated with a considerably decreased quality of life and is a common cause of work absenteeism.7,8 Because many conditions can cause diarrhea, patients typically undergo numerous tests before receiving an accurate diagnosis, which creates a financial burden.9
For many patients, current IBS treatments, which include fiber supplements, laxatives, antidiarrheal medications, antispasmodics, and antidepressants such as tricyclics and selective serotonin reuptake inhibitors, are unsatisfactory.10 Alosetron, a 5-hydroxytryptamine 3 (5HT3) receptor antagonist, has been used to treat IBS-D,11 but this medication was voluntarily withdrawn from the US market in 2000 due to concerns of ischemic colitis and severe constipation.12 It was reintroduced in 2002, but can be prescribed only by physicians who enroll in a prescribing program provided by the manufacturer, and the drug has restrictions on its use.
Ondansetron—a different 5HT3 receptor antagonist used to treat nausea and vomiting caused by chemotherapy—may be another option for treating IBS-D. Garsed et al1 recently conducted a RCT to evaluate the efficacy of ondansetron for patients with IBS-D.
STUDY SUMMARY: Ondansetron improves stool consistency, severity of IBS symptoms
In a 5-week, double-blind crossover RCT, Garsed et al1 compared ondansetron vs placebo for symptom relief in 120 patients who met Rome III criteria for IBS-D. All patients were ages 18 to 75 and had no evidence of inflammatory bowel disease. Exclusion criteria were pregnancy or breastfeeding, unwillingness to stop antidiarrheal medication, prior abdominal surgery other than appendectomy or cholecystectomy, or being in another trial. Patients were started on ondansetron 4 mg/d with dose titration up to 24 mg/d based on response; no dose adjustments were allowed during the last 2 weeks of the study. There was a 2- to 3-week washout between treatment periods.
The primary endpoint was average stool consistency in the last 2 weeks of treatment, as measured by the Bristol Stool Form (BSF) scale.13 The BSF is a visual scale that depicts stool as hard (Type 1) to watery (Type 7); types 3 and 4 describe normal stools. The study also looked at urgency and frequency of defecation, bowel transit time, and pain scores.
Treatment with ondansetron resulted in a small but statistically significant improvement in stool consistency. The mean difference in BSF score between ondansetron and placebo was -0.9 (95% confidence interval [CI], -1.1 to -0.6; P<.001), indicating slightly more formed stool with use of ondansetron. The IBS Severity Scoring System score (maximum score 500 points, with mild, moderate, and severe cases indicated by scores of 75-175, 175-300, and >300, respectively) was reduced by more points with ondansetron than placebo (83 ± 9.8 vs 37 ± 9.7; P=.001). Although this mean difference of 46 points fell just short of the 50-point threshold that is considered clinically significant, many patients exceeded this threshold.
Compared to those who received placebo, patients who took ondansetron also had less frequent defecation (P=.002) and lower urgency scores (P<.001). Gut transit time was lengthened in the ondansetron group by 10 hours more than in the placebo group (95% CI, 6-14 hours; P<.001). Pain scores did not change significantly for patients taking ondansetron, although they experienced significantly fewer days of urgency and bloating. Symptoms typically improved in as little as 7 days but returned after stopping ondansetron, typically within 2 weeks. Sixty-five percent of patients reported adequate relief with ondansetron, compared to 14% with placebo.
Patients whose diarrhea was more severe at baseline didn’t respond as well to ondansetron as did those whose diarrhea was less severe. The only frequent adverse effect was constipation, which occurred in 9% of patients receiving ondansetron and 2% of those on placebo.
WHAT’S NEW: Another option for IBS patients with diarrhea
A prior, smaller study of ondansetron that used a lower dosage (12 mg/d) suggested benefit in IBS-D.14 In that study, ondansetron decreased diarrhea and functional dyspepsia. The study by Garsed et al1 is the first large RCT to show significantly improved stool consistency, less frequent defecation, and less urgency and bloating from using ondansetron to treat IBS-D.
CAVEATS: Ondansetron doesn’t appear to reduce pain
In Garsed et al,1 patients who received ondansetron did not experience relief from pain, which is one of the main complaints of IBS. However, this study did find slight improvement in formed stools, symptom relief that approached—but did not quite reach—clinical significance, fewer days with urgency and bloating, and less frequent defecation. This study did not evaluate the long-term effects of ondansetron use. However, ondansetron has been used for other indications for more than 25 years and has been reported to have a low risk of adverse effects.15
CHALLENGES TO IMPLEMENTATION: Remember ondansetron is not for IBS patients with constipation
Proper use of this drug among patients with IBS is key. The primary benefits of ondansetron are limited to IBS patients who suffer from diarrhea, and not constipation. Ondansetron should not be prescribed to IBS patients who experience constipation, or those with mixed symptoms.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
Consider prescribing ondansetron up to 24 mg/d for patients who have irritable bowel syndrome with diarrhea (IBS-D).1
Strength of recommendation
B: Based on a well-done double-blind, placebo-controlled randomized controlled trial (RCT).
Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625.
Illustrative case
A 23-year-old woman who was diagnosed with irritable bowel syndrome (IBS) comes to your clinic with complaints of increased frequency of defecation with watery stools and generalized, cramping abdominal pain. She also notes increased passage of mucus and a sensation of incomplete evacuation. She says the only thing that relieves her pain is defecation. She has tried loperamide, acetaminophen, and ibuprofen without relief. She does not have Crohn’s disease or ulcerative colitis. What else can you offer her that is safe and effective?
IBS is a chronic, episodic functional gastrointestinal disorder characterized by abdominal pain or discomfort and altered bowel habits (constipation [IBS-C], diarrhea [IBS-D], or alternating periods of both—mixed [IBS-M]).2 It is diagnosed based on Rome III criteria—recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months associated with ≥2 of the following: improvement with defecation, onset associated with a change in frequency of stool, and onset associated with a change in form (appearance) of stool.3 IBS often is unrecognized or untreated, and as few as 25% of patients with IBS seek care.4
IBS-D affects approximately 5% of the general population in North America.5,6 IBS-D is associated with a considerably decreased quality of life and is a common cause of work absenteeism.7,8 Because many conditions can cause diarrhea, patients typically undergo numerous tests before receiving an accurate diagnosis, which creates a financial burden.9
For many patients, current IBS treatments, which include fiber supplements, laxatives, antidiarrheal medications, antispasmodics, and antidepressants such as tricyclics and selective serotonin reuptake inhibitors, are unsatisfactory.10 Alosetron, a 5-hydroxytryptamine 3 (5HT3) receptor antagonist, has been used to treat IBS-D,11 but this medication was voluntarily withdrawn from the US market in 2000 due to concerns of ischemic colitis and severe constipation.12 It was reintroduced in 2002, but can be prescribed only by physicians who enroll in a prescribing program provided by the manufacturer, and the drug has restrictions on its use.
Ondansetron—a different 5HT3 receptor antagonist used to treat nausea and vomiting caused by chemotherapy—may be another option for treating IBS-D. Garsed et al1 recently conducted a RCT to evaluate the efficacy of ondansetron for patients with IBS-D.
STUDY SUMMARY: Ondansetron improves stool consistency, severity of IBS symptoms
In a 5-week, double-blind crossover RCT, Garsed et al1 compared ondansetron vs placebo for symptom relief in 120 patients who met Rome III criteria for IBS-D. All patients were ages 18 to 75 and had no evidence of inflammatory bowel disease. Exclusion criteria were pregnancy or breastfeeding, unwillingness to stop antidiarrheal medication, prior abdominal surgery other than appendectomy or cholecystectomy, or being in another trial. Patients were started on ondansetron 4 mg/d with dose titration up to 24 mg/d based on response; no dose adjustments were allowed during the last 2 weeks of the study. There was a 2- to 3-week washout between treatment periods.
The primary endpoint was average stool consistency in the last 2 weeks of treatment, as measured by the Bristol Stool Form (BSF) scale.13 The BSF is a visual scale that depicts stool as hard (Type 1) to watery (Type 7); types 3 and 4 describe normal stools. The study also looked at urgency and frequency of defecation, bowel transit time, and pain scores.
Treatment with ondansetron resulted in a small but statistically significant improvement in stool consistency. The mean difference in BSF score between ondansetron and placebo was -0.9 (95% confidence interval [CI], -1.1 to -0.6; P<.001), indicating slightly more formed stool with use of ondansetron. The IBS Severity Scoring System score (maximum score 500 points, with mild, moderate, and severe cases indicated by scores of 75-175, 175-300, and >300, respectively) was reduced by more points with ondansetron than placebo (83 ± 9.8 vs 37 ± 9.7; P=.001). Although this mean difference of 46 points fell just short of the 50-point threshold that is considered clinically significant, many patients exceeded this threshold.
Compared to those who received placebo, patients who took ondansetron also had less frequent defecation (P=.002) and lower urgency scores (P<.001). Gut transit time was lengthened in the ondansetron group by 10 hours more than in the placebo group (95% CI, 6-14 hours; P<.001). Pain scores did not change significantly for patients taking ondansetron, although they experienced significantly fewer days of urgency and bloating. Symptoms typically improved in as little as 7 days but returned after stopping ondansetron, typically within 2 weeks. Sixty-five percent of patients reported adequate relief with ondansetron, compared to 14% with placebo.
Patients whose diarrhea was more severe at baseline didn’t respond as well to ondansetron as did those whose diarrhea was less severe. The only frequent adverse effect was constipation, which occurred in 9% of patients receiving ondansetron and 2% of those on placebo.
WHAT’S NEW: Another option for IBS patients with diarrhea
A prior, smaller study of ondansetron that used a lower dosage (12 mg/d) suggested benefit in IBS-D.14 In that study, ondansetron decreased diarrhea and functional dyspepsia. The study by Garsed et al1 is the first large RCT to show significantly improved stool consistency, less frequent defecation, and less urgency and bloating from using ondansetron to treat IBS-D.
CAVEATS: Ondansetron doesn’t appear to reduce pain
In Garsed et al,1 patients who received ondansetron did not experience relief from pain, which is one of the main complaints of IBS. However, this study did find slight improvement in formed stools, symptom relief that approached—but did not quite reach—clinical significance, fewer days with urgency and bloating, and less frequent defecation. This study did not evaluate the long-term effects of ondansetron use. However, ondansetron has been used for other indications for more than 25 years and has been reported to have a low risk of adverse effects.15
CHALLENGES TO IMPLEMENTATION: Remember ondansetron is not for IBS patients with constipation
Proper use of this drug among patients with IBS is key. The primary benefits of ondansetron are limited to IBS patients who suffer from diarrhea, and not constipation. Ondansetron should not be prescribed to IBS patients who experience constipation, or those with mixed symptoms.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625.
2. Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60:77-81.
3. Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237-241.
4. Luscombe FA. Health-related quality of life and associated psychosocial factors in irritable bowel syndrome: a review. Qual Life Res. 2000;9:161-176.
5. Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816-2824.
6. Thompson WG, Heaton KW, Smyth GT, et al. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78-82.
7. Tillisch K, Labus JS, Naliboff BD, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005;100:896-904.
8. Schuster MM. Diagnostic evaluation of the irritable bowel syndrome. Gastroenterol Clin North Am. 1991;20:269-278.
9. Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511.
10. Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:750-758.
11. Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545-555.
12. Chang L, Chey WD, Harris L, et al. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069-1079.
13. Heaton KW, O’Donnell LJ. An office guide to whole-gut transit time. Patients’ recollection of their stool form. J Clin Gastroenterol. 1994;19:28-30.
14. Maxton DG, Morris J, Whorwell PJ. Selective 5‐hydroxytryptamine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther. 1996;10:595-599.
15. Gill SK, Einarson A. The safety of drugs for the treatment of nausea and vomiting of pregnancy. Expert Opin Drug Saf. 2007;6:685-694.
1. Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625.
2. Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60:77-81.
3. Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237-241.
4. Luscombe FA. Health-related quality of life and associated psychosocial factors in irritable bowel syndrome: a review. Qual Life Res. 2000;9:161-176.
5. Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816-2824.
6. Thompson WG, Heaton KW, Smyth GT, et al. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78-82.
7. Tillisch K, Labus JS, Naliboff BD, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005;100:896-904.
8. Schuster MM. Diagnostic evaluation of the irritable bowel syndrome. Gastroenterol Clin North Am. 1991;20:269-278.
9. Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511.
10. Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:750-758.
11. Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545-555.
12. Chang L, Chey WD, Harris L, et al. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069-1079.
13. Heaton KW, O’Donnell LJ. An office guide to whole-gut transit time. Patients’ recollection of their stool form. J Clin Gastroenterol. 1994;19:28-30.
14. Maxton DG, Morris J, Whorwell PJ. Selective 5‐hydroxytryptamine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther. 1996;10:595-599.
15. Gill SK, Einarson A. The safety of drugs for the treatment of nausea and vomiting of pregnancy. Expert Opin Drug Saf. 2007;6:685-694.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
ACOG issues guidelines for managing listeriosis during pregnancy
The first medical management guidelines for treating the bacteria listeria during pregnancy were recently released by the American College of Obstetricians and Gynecologists (ACOG).1
The new guidelines were developed in response to recent reports of recalls of listeria-contaminated food and concern because data show the incidence of listeriosis among pregnant women is approximately 13 times higher than in the general population.1 Maternal listeriosis can cause significant fetal and perinatal complications, including miscarriage, preterm labor, stillbirth, as well as neonatal listeriosis and neonatal death.2
“It is essential for ObGyns not only to be aware of the best ways to manage the care of a pregnant patient who has been exposed to listeria bacteria, but, just as important, to counsel pregnant women regarding how to avoid potential exposure,” said Jeffrey L. Ecker, MD, chair of the ACOG Committee on Obstetric Practice.2
Symptoms of listeriosis are similar to a flu-like infection and can include fever, muscle pain, backache, headache, and gastrointestinal symptoms, including diarrhea.2
The Committee Opinion offers management recommendations for three scenarios1:
- Asymptomatic women who have been exposed to listeria: No testing is necessary unless symptoms develop within 2 months of exposure. Listeriosis-related fetal surveillance is unnecessary.
- No fever, with mild symptoms consistent with listeriosis: A pregnant woman who has been exposed to a listeria-containing food and is displaying mild symptoms, but does not have a fever, does not require culture testing (although it may be done). If her blood is tested, the laboratory should be informed about the listeriosis threat so that the bacteria is not confused with a contaminant.
- Fever, with or without symptoms consistent with listeriosis: A pregnant woman who has been exposed and has a fever exceeding 100.6°F should undergo blood culture testing. Because results will not be available for several days, the Committee Opinion recommends simultaneous listeriosis treatment for the patient and surveillance of the fetus.
Preventive measures to avoid listeria exposure include not eating the following foods1:
- hot dogs, lunch meats, cold cuts served cold or heated to less than 165°F
- refrigerated pate and meat spreads
- refrigerated smoked seafood
- raw (unpasteurized) milk
- unpasteurized soft cheese (feta, queso blanco, Brie, blue-veined cheeses)
- unwashed raw produce (when eating raw fruits and vegetables, skin should be washed thoroughly in running tap water, even if it will be peeled or cut).
Share your thoughts on this news! Send your Letter to the Editor to [email protected]. Please include your name, and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion. Management of pregnant women with presumptive exposure to Listeria monocytogenes [published online ahead of print August 5, 2014]. http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Management-of-Pregnant-Women-With-Presumptive-Exposure-to-Listeria-monocytogenes. Accessed September 29, 2014.
- American College of Obstetricians and Gynecologists. Ob-Gyns address management of listeria during pregnancy [press release]. http://www.acog.org/About-ACOG/News-Room/News-Releases/2014/Ob-Gyns-Address-Management-of-Listeria-During-Pregnancy. Published August 6, 2014. Accessed September 29, 2014.
The first medical management guidelines for treating the bacteria listeria during pregnancy were recently released by the American College of Obstetricians and Gynecologists (ACOG).1
The new guidelines were developed in response to recent reports of recalls of listeria-contaminated food and concern because data show the incidence of listeriosis among pregnant women is approximately 13 times higher than in the general population.1 Maternal listeriosis can cause significant fetal and perinatal complications, including miscarriage, preterm labor, stillbirth, as well as neonatal listeriosis and neonatal death.2
“It is essential for ObGyns not only to be aware of the best ways to manage the care of a pregnant patient who has been exposed to listeria bacteria, but, just as important, to counsel pregnant women regarding how to avoid potential exposure,” said Jeffrey L. Ecker, MD, chair of the ACOG Committee on Obstetric Practice.2
Symptoms of listeriosis are similar to a flu-like infection and can include fever, muscle pain, backache, headache, and gastrointestinal symptoms, including diarrhea.2
The Committee Opinion offers management recommendations for three scenarios1:
- Asymptomatic women who have been exposed to listeria: No testing is necessary unless symptoms develop within 2 months of exposure. Listeriosis-related fetal surveillance is unnecessary.
- No fever, with mild symptoms consistent with listeriosis: A pregnant woman who has been exposed to a listeria-containing food and is displaying mild symptoms, but does not have a fever, does not require culture testing (although it may be done). If her blood is tested, the laboratory should be informed about the listeriosis threat so that the bacteria is not confused with a contaminant.
- Fever, with or without symptoms consistent with listeriosis: A pregnant woman who has been exposed and has a fever exceeding 100.6°F should undergo blood culture testing. Because results will not be available for several days, the Committee Opinion recommends simultaneous listeriosis treatment for the patient and surveillance of the fetus.
Preventive measures to avoid listeria exposure include not eating the following foods1:
- hot dogs, lunch meats, cold cuts served cold or heated to less than 165°F
- refrigerated pate and meat spreads
- refrigerated smoked seafood
- raw (unpasteurized) milk
- unpasteurized soft cheese (feta, queso blanco, Brie, blue-veined cheeses)
- unwashed raw produce (when eating raw fruits and vegetables, skin should be washed thoroughly in running tap water, even if it will be peeled or cut).
Share your thoughts on this news! Send your Letter to the Editor to [email protected]. Please include your name, and the city and state in which you practice.
The first medical management guidelines for treating the bacteria listeria during pregnancy were recently released by the American College of Obstetricians and Gynecologists (ACOG).1
The new guidelines were developed in response to recent reports of recalls of listeria-contaminated food and concern because data show the incidence of listeriosis among pregnant women is approximately 13 times higher than in the general population.1 Maternal listeriosis can cause significant fetal and perinatal complications, including miscarriage, preterm labor, stillbirth, as well as neonatal listeriosis and neonatal death.2
“It is essential for ObGyns not only to be aware of the best ways to manage the care of a pregnant patient who has been exposed to listeria bacteria, but, just as important, to counsel pregnant women regarding how to avoid potential exposure,” said Jeffrey L. Ecker, MD, chair of the ACOG Committee on Obstetric Practice.2
Symptoms of listeriosis are similar to a flu-like infection and can include fever, muscle pain, backache, headache, and gastrointestinal symptoms, including diarrhea.2
The Committee Opinion offers management recommendations for three scenarios1:
- Asymptomatic women who have been exposed to listeria: No testing is necessary unless symptoms develop within 2 months of exposure. Listeriosis-related fetal surveillance is unnecessary.
- No fever, with mild symptoms consistent with listeriosis: A pregnant woman who has been exposed to a listeria-containing food and is displaying mild symptoms, but does not have a fever, does not require culture testing (although it may be done). If her blood is tested, the laboratory should be informed about the listeriosis threat so that the bacteria is not confused with a contaminant.
- Fever, with or without symptoms consistent with listeriosis: A pregnant woman who has been exposed and has a fever exceeding 100.6°F should undergo blood culture testing. Because results will not be available for several days, the Committee Opinion recommends simultaneous listeriosis treatment for the patient and surveillance of the fetus.
Preventive measures to avoid listeria exposure include not eating the following foods1:
- hot dogs, lunch meats, cold cuts served cold or heated to less than 165°F
- refrigerated pate and meat spreads
- refrigerated smoked seafood
- raw (unpasteurized) milk
- unpasteurized soft cheese (feta, queso blanco, Brie, blue-veined cheeses)
- unwashed raw produce (when eating raw fruits and vegetables, skin should be washed thoroughly in running tap water, even if it will be peeled or cut).
Share your thoughts on this news! Send your Letter to the Editor to [email protected]. Please include your name, and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion. Management of pregnant women with presumptive exposure to Listeria monocytogenes [published online ahead of print August 5, 2014]. http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Management-of-Pregnant-Women-With-Presumptive-Exposure-to-Listeria-monocytogenes. Accessed September 29, 2014.
- American College of Obstetricians and Gynecologists. Ob-Gyns address management of listeria during pregnancy [press release]. http://www.acog.org/About-ACOG/News-Room/News-Releases/2014/Ob-Gyns-Address-Management-of-Listeria-During-Pregnancy. Published August 6, 2014. Accessed September 29, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion. Management of pregnant women with presumptive exposure to Listeria monocytogenes [published online ahead of print August 5, 2014]. http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Management-of-Pregnant-Women-With-Presumptive-Exposure-to-Listeria-monocytogenes. Accessed September 29, 2014.
- American College of Obstetricians and Gynecologists. Ob-Gyns address management of listeria during pregnancy [press release]. http://www.acog.org/About-ACOG/News-Room/News-Releases/2014/Ob-Gyns-Address-Management-of-Listeria-During-Pregnancy. Published August 6, 2014. Accessed September 29, 2014.
E-cigarettes: What to tell your patients
EHRs: Something’s gotta give
Clicking on a checklist of symptoms seldom provides sufficient information about the patient’s illness. “Hypertension” and “type 2 diabetes” are not assessments; they are diagnoses that do not tell the person reading the electronic health record (EHR) how the patient is doing. A diagnosis of “abdominal pain” without a prioritized differential is inadequate, especially in court.
Why is visit documentation too often inadequate? I am convinced it is rarely due to clinician incompetence, laziness, or lack of knowledge, but nearly always due to a combination of inadequate EHR formats and billing documentation requirements that encourage quantity rather than quality. Documentation is no longer driven by the essential need to record the care provided.
I’m sure a lot of you are nodding your heads in agreement. You all know what those EHR notes look like—cluttered with cut-and-pasted information drawn from prior encounters that document no end of details regarding medical, family, and social histories, facts that are often completely irrelevant to the reason the patient is in the office today. And unless one meticulously updates those other elements of the patient’s record, this information pulled into the note may be inaccurate.
I am not the only one complaining. The American Medical Association just published Improving Care: Priorities to Improve Electronic Health Record Usability,1 which outlines 8 priorities for EHR improvement. The first is to “Enhance physicians’ ability to provide high-quality patient care.” I could not agree more. I think today’s EHRs are like an old-fashioned crank phone and what we really need is an iPhone.
Something has got to change.
So tell me: Have any of you figured out how to use your EHR to enhance the quality of your documentation?
Reference
1. American Medical Association. Improving Care: Priorities to Improve Electronic Health Record Usability. American Medical Association Web site. Available at: https://download.ama-assn.org/resources/doc/ps2/x-pub/ehr-priorities.pdf. Accessed September 18, 2014.
Clicking on a checklist of symptoms seldom provides sufficient information about the patient’s illness. “Hypertension” and “type 2 diabetes” are not assessments; they are diagnoses that do not tell the person reading the electronic health record (EHR) how the patient is doing. A diagnosis of “abdominal pain” without a prioritized differential is inadequate, especially in court.
Why is visit documentation too often inadequate? I am convinced it is rarely due to clinician incompetence, laziness, or lack of knowledge, but nearly always due to a combination of inadequate EHR formats and billing documentation requirements that encourage quantity rather than quality. Documentation is no longer driven by the essential need to record the care provided.
I’m sure a lot of you are nodding your heads in agreement. You all know what those EHR notes look like—cluttered with cut-and-pasted information drawn from prior encounters that document no end of details regarding medical, family, and social histories, facts that are often completely irrelevant to the reason the patient is in the office today. And unless one meticulously updates those other elements of the patient’s record, this information pulled into the note may be inaccurate.
I am not the only one complaining. The American Medical Association just published Improving Care: Priorities to Improve Electronic Health Record Usability,1 which outlines 8 priorities for EHR improvement. The first is to “Enhance physicians’ ability to provide high-quality patient care.” I could not agree more. I think today’s EHRs are like an old-fashioned crank phone and what we really need is an iPhone.
Something has got to change.
So tell me: Have any of you figured out how to use your EHR to enhance the quality of your documentation?
Clicking on a checklist of symptoms seldom provides sufficient information about the patient’s illness. “Hypertension” and “type 2 diabetes” are not assessments; they are diagnoses that do not tell the person reading the electronic health record (EHR) how the patient is doing. A diagnosis of “abdominal pain” without a prioritized differential is inadequate, especially in court.
Why is visit documentation too often inadequate? I am convinced it is rarely due to clinician incompetence, laziness, or lack of knowledge, but nearly always due to a combination of inadequate EHR formats and billing documentation requirements that encourage quantity rather than quality. Documentation is no longer driven by the essential need to record the care provided.
I’m sure a lot of you are nodding your heads in agreement. You all know what those EHR notes look like—cluttered with cut-and-pasted information drawn from prior encounters that document no end of details regarding medical, family, and social histories, facts that are often completely irrelevant to the reason the patient is in the office today. And unless one meticulously updates those other elements of the patient’s record, this information pulled into the note may be inaccurate.
I am not the only one complaining. The American Medical Association just published Improving Care: Priorities to Improve Electronic Health Record Usability,1 which outlines 8 priorities for EHR improvement. The first is to “Enhance physicians’ ability to provide high-quality patient care.” I could not agree more. I think today’s EHRs are like an old-fashioned crank phone and what we really need is an iPhone.
Something has got to change.
So tell me: Have any of you figured out how to use your EHR to enhance the quality of your documentation?
Reference
1. American Medical Association. Improving Care: Priorities to Improve Electronic Health Record Usability. American Medical Association Web site. Available at: https://download.ama-assn.org/resources/doc/ps2/x-pub/ehr-priorities.pdf. Accessed September 18, 2014.
Reference
1. American Medical Association. Improving Care: Priorities to Improve Electronic Health Record Usability. American Medical Association Web site. Available at: https://download.ama-assn.org/resources/doc/ps2/x-pub/ehr-priorities.pdf. Accessed September 18, 2014.
Uterine rupture, child stillborn: $3.8M net award
Uterine rupture, child stillborn: $3.8M net award
At 35 weeks' gestation, a woman went to the emergency department (ED) with abdominal pain, fast heartbeat, and irregular contractions. Her history included three cesarean deliveries, including one with a vertical incision. She was admitted, and a cesarean delivery was planned for the next day. After 8 hours, during which the patient’s condition worsened, an emergency cesarean delivery was undertaken. A full rupture of the uterus was found; the baby’s body had extruded into the mother’s abdomen. The child was stillborn.
PARENTS’ CLAIM The stillbirth could have been avoided if the nurses had communicated the mother’s worsening condition to the physicians.
DEFENDANTS’ DEFENSE After the hospital and physicians settled prior to trial, the case continued against the nurse in charge of the mother’s care and the nurse-staffing group. Negligence was denied; all protocols were followed.
VERDICT A $2.9 million Illinois verdict was returned. With a $900,000 settlement from the hospital and physicians, the net award was $3.8 million.
_______________
Where did rare strep A infection come from?
A 36-year-old woman reported heavy vaginal bleeding to her ObGyn. She underwent endometrial ablation in her physician’s office.
The next day, the woman called the office to report abdominal pain. She was told to stop the medication she was taking, and if the pain continued to the next day, to go to an ED. The next day, the patient went to the ED and was found to be in septic shock. During emergency laparotomy, 50 mL of purulent fluid were drained and an emergency hysterectomy was performed. Three days later, the patient died from pulmonary arrest caused by toxic shock syndrome. An autopsy revealed that the patient’s sepsis was caused by group A streptococci (GAS) infection.
ESTATE’S CLAIM The patient was not a proper candidate for endometrial ablation because of her history of chronic cervical infection. The ObGyn perforated the cervix during the procedure and tried to conceal it. At autopsy, bone wax was found in the rectal lumen that had been used to cover up damage to the cervix. The ObGyn introduced GAS bacteria into the patient’s system. The ObGyn’s staff failed to ask the proper questions when she called the day after the procedure. She should have been told to go directly to the ED.
DEFENDANTS’ DEFENSE The ObGyn did not perforate the cervix or uterus during the procedure. GAS infection is so rare that it would have been difficult to foresee or diagnose. Potentially, the patient had a chronic
cervical infection before ablation.
VERDICT A Texas defense verdict was returned.
_______________
DURING INSERTION, IUD PERFORATES UTERINE WALL; LATER FOUND BELOW LIVER
On July 21, a 46-year-old woman went to an ObGyn for placement of an intrauterine device (IUD). Shortly after the ObGyn inserted the levonorgestrel-releasing intrauterine system (Mirena, Bayer HealthCare), the patient reported severe pelvic and abdominal pain. On July 26, the patient underwent surgical removal of the IUD.
She was discharged on July 29 but continued to report pain. She was readmitted to the hospital the next day and treated for pain. She was bed ridden for 3 weeks after IUD-removal surgery, and had a 3-month recovery before feeling pain free.
PATIENT’S CLAIM The ObGyn was negligent in perforating the patient’s uterine wall during IUD insertion, causing the device to ultimately migrate under the patient’s liver.
DEFENDANTS’ DEFENSE Uterine perforation is a known complication of IUD insertion. The IUD escaped from the patient’s uterus at a later time and not during the insertion procedure.
VERDICT A Florida verdict of $208,839 was returned; the amount was reduced to $161,058 because the medical expenses were written off by the health-care providers.
_______________
Was travel appropriate for this pregnant woman?
A woman with a history of two premature deliveries and one miscarriage became pregnant again. She received prenatal care at an Army hospital. She traveled to Spain, where the baby was born at 31 weeks’ gestation. The baby required treatment in a neonatal intensive care unit (NICU) for 17 days. The child has cerebral palsy, with tetraplegia of all four extremities. She cannot walk without assistance and suffers severe cognitive and vision impairment.
PARENTS’ CLAIM The ObGyn at the Army hospital should not have approved the mother’s request for travel; he did so, despite knowing that the mother was at high risk for premature birth. The military medical hospital to which she was assigned in Spain could not manage a high-risk pregnancy, didn’t have a NICU, and didn’t have specialists to treat premature infants.
DEFENDANTS’ DEFENSE The ObGyn argued that he did not have access to the medical records showing the mother’s history. The patient countered that the ObGyn did indeed have the patient’s records, as he had discussed them with her.
VERDICT A $10,409,700 California verdict was returned against the ObGyn and the government facility.
_______________
Triple-negative BrCa not diagnosed until metastasized: $5.2M
After finding lumps in both breasts, a woman in her 30s saw a nurse practitioner (NP) at an Army hospital. A radiologist reported no mass in the right breast and multiple benign-appearing anechoic lesions in the left breast after bilateral mammography and ultrasonography (US) in July 2008. The Chief of Mammography Services recommended referral to a breast surgeon, but the patient never received the letter. It was placed in her mammography file, not in the treatment file.
In November 2008, the patient returned to the clinic. Bilateral diagnostic mammography and US were ordered, but for unknown reasons, cancelled. US of the left breast was interpreted as benign in January 2009.
After imaging in March 2010, followed by a needle biopsy of the right breast, a radiologist reported finding intermediate-grade infiltrating ductal carcinoma.
The patient sought care outside the military medical system at a large university hospital. In April 2010, stage 3 triple-negative invasive ductal carcinoma (IDC) was identified. The patient underwent chemotherapy, a double mastectomy, removal of 21 lymph nodes, and breast reconstruction. She was given a 60% chance of recurrence in 5–7 years.
PATIENT’S CLAIM It was negligent to not inform her of imaging results. Biopsy should have been performed in 2008, when the IDC was likely at stage 1; treatment would have been far less aggressive. Electronic medical records showed that the 2008 mammography and US results had been “signed off” by an NP at the clinic.
DEFENDANTS’ DEFENSE While unable to concede liability, the government agency did not contest the point.
VERDICT A $5.2 million Tennessee federal court bench verdict was returned, citing failures in communication, poor and improper record keeping and retention, failure to follow-up, and an unexplained cancellation of a medical order.
_______________
Woman dies from cervical cancer: $2.3M
In 2001, a 41-year-old woman had abnormal Pap smear results but her gynecologist did not order more testing. The patient was told to return in 3 months, but she did not return until 2007—reporting abnormal bleeding, vaginal discharge, and pain. Her Pap results were normal, however, and the gynecologist did not order further testing. In 2009, the patient was found to have advanced cervical cancer. She died 2 years later.
ESTATE’S CLAIM Further testing should have been ordered in 2001, which would have likely revealed dysplasia, which can lead to cancer. The laboratory incorrectly interpreted the 2007 Pap test; if the results had been properly reported, additional testing could have been ordered.
DEFENDANTS’ DEFENSE The laboratory and patient’s estate settled for a confidential amount before trial. The gynecologist denied negligence.
VERDICT A New Jersey jury found the gynecologist 40% at fault for his actions in 2007. The jury found the laboratory 50% at fault, and the patient 10% at fault. A gross verdict of $2.33 million was returned.
_______________
Bowel injury after cesarean delivery; mother dies of sepsis
At 40 4/7 weeks' gestation, a 37-year-old woman gave birth to a healthy child by cesarean delivery. The next day, the patient had an elevated white blood cell (WBC) count with a left shift, her abdomen was tympanic but soft, and she was passing flatus and belching. The ObGyn ordered a Fleet enema; only flatus was released. A covering ObGyn ordered an abdominal radiograph, which the radiologist reported as showing postoperative ileus and mild constipation. The patient was given a second Fleet enema the next day, resulting in watery stool. She vomited 300 mL of dark green fluid.
After a rectal tube was placed 2 days later, one hard brown stool and several brown, pasty, loose, and liquid stools were returned. She vomited several times that day, and was found to have hypoactive bowel sounds with continued tympanic quality in the upper quadrants. Laboratory testing revealed continued elevated WBC count with left shift. The next day, she had hypoactive bowel sounds with brown liquid stools. Later that morning, she was able to tolerate clear liquids. The ObGyn decided to discharge her home with instructions to continue on a clear liquid diet for 2 more days before advancing her diet.
The day after discharge, she was found unresponsive at home. She was taken to the hospital, but resuscitation attempts failed. She died. An autopsy revealed that the cause of death was sepsis.
ESTATE’S CLAIM The ObGyn was negligent in failing to diagnose and treat a postoperative intra-abdominal infection caused by bowel perforation. A surgical consult should have been obtained. The woman was prematurely discharged. The radiologist failed to report the presence of free air on the abdominal x-ray.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million Maryland settlement was reached.
_______________
Right ureter injury detected and repaired
During laparoscopic-assisted vaginal hysterectomy, the ObGyn detected and repaired an injury to the right ureter. The patient’s recovery was delayed by the injury.
PATIENT’S CLAIM The ObGyn was negligent in using a Kleppinger bipolar cauterizing instrument to cauterize the vaginal cuff. Thermal overspray from the instrument or the instrument itself damaged the ureter. The ObGyn was also negligent in not performing diagnostic cystoscopy to confirm patency of the ureter after the repair was made.
PHYSICIAN’S DEFENSE Ureter injury is a known risk of the procedure. All procedures were performed according to protocol.
VERDICT A Florida defense verdict was returned.
_______________
Failure to detect inflammatory BrCa; woman dies
A 42-year-old woman underwent mammography in February 2002 after reporting pain, discoloration, inflammation, and swelling in her left breast. The radiologist who interpreted the mammography suggested a biopsy for a differential diagnosis of mastitis or inflammatory carcinoma. The biopsy results were negative.
The patient’s symptoms persisted, and she underwent US in late May 2002. Another radiologist interpreted the US, noting that the patient could not tolerate compression, which led to less than optimal evaluation. The radiologist suggested that mastitis was the likely cause of the patient’s symptoms.
The patient then consulted a surgeon, who ordered mammography and magnetic resonance imaging (MRI) followed by biopsy, which indicated cancer. The patient underwent a mastectomy but metastasis had already occurred. She died at age 50 prior to the trial.
ESTATE’S CLAIM If the cancer had been diagnosed earlier, the outcome would have been better. Both radiologists misinterpreted the mammographies.
DEFENDANTS’ DEFENSE The mammographies had been properly interpreted. Any missed diagnosis would not have impacted the outcome due to the type of cancer. The scans had been released to the patient, but were subsequently lost; an adverse interference instruction was given to the jury.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine rupture, child stillborn: $3.8M net award
At 35 weeks' gestation, a woman went to the emergency department (ED) with abdominal pain, fast heartbeat, and irregular contractions. Her history included three cesarean deliveries, including one with a vertical incision. She was admitted, and a cesarean delivery was planned for the next day. After 8 hours, during which the patient’s condition worsened, an emergency cesarean delivery was undertaken. A full rupture of the uterus was found; the baby’s body had extruded into the mother’s abdomen. The child was stillborn.
PARENTS’ CLAIM The stillbirth could have been avoided if the nurses had communicated the mother’s worsening condition to the physicians.
DEFENDANTS’ DEFENSE After the hospital and physicians settled prior to trial, the case continued against the nurse in charge of the mother’s care and the nurse-staffing group. Negligence was denied; all protocols were followed.
VERDICT A $2.9 million Illinois verdict was returned. With a $900,000 settlement from the hospital and physicians, the net award was $3.8 million.
_______________
Where did rare strep A infection come from?
A 36-year-old woman reported heavy vaginal bleeding to her ObGyn. She underwent endometrial ablation in her physician’s office.
The next day, the woman called the office to report abdominal pain. She was told to stop the medication she was taking, and if the pain continued to the next day, to go to an ED. The next day, the patient went to the ED and was found to be in septic shock. During emergency laparotomy, 50 mL of purulent fluid were drained and an emergency hysterectomy was performed. Three days later, the patient died from pulmonary arrest caused by toxic shock syndrome. An autopsy revealed that the patient’s sepsis was caused by group A streptococci (GAS) infection.
ESTATE’S CLAIM The patient was not a proper candidate for endometrial ablation because of her history of chronic cervical infection. The ObGyn perforated the cervix during the procedure and tried to conceal it. At autopsy, bone wax was found in the rectal lumen that had been used to cover up damage to the cervix. The ObGyn introduced GAS bacteria into the patient’s system. The ObGyn’s staff failed to ask the proper questions when she called the day after the procedure. She should have been told to go directly to the ED.
DEFENDANTS’ DEFENSE The ObGyn did not perforate the cervix or uterus during the procedure. GAS infection is so rare that it would have been difficult to foresee or diagnose. Potentially, the patient had a chronic
cervical infection before ablation.
VERDICT A Texas defense verdict was returned.
_______________
DURING INSERTION, IUD PERFORATES UTERINE WALL; LATER FOUND BELOW LIVER
On July 21, a 46-year-old woman went to an ObGyn for placement of an intrauterine device (IUD). Shortly after the ObGyn inserted the levonorgestrel-releasing intrauterine system (Mirena, Bayer HealthCare), the patient reported severe pelvic and abdominal pain. On July 26, the patient underwent surgical removal of the IUD.
She was discharged on July 29 but continued to report pain. She was readmitted to the hospital the next day and treated for pain. She was bed ridden for 3 weeks after IUD-removal surgery, and had a 3-month recovery before feeling pain free.
PATIENT’S CLAIM The ObGyn was negligent in perforating the patient’s uterine wall during IUD insertion, causing the device to ultimately migrate under the patient’s liver.
DEFENDANTS’ DEFENSE Uterine perforation is a known complication of IUD insertion. The IUD escaped from the patient’s uterus at a later time and not during the insertion procedure.
VERDICT A Florida verdict of $208,839 was returned; the amount was reduced to $161,058 because the medical expenses were written off by the health-care providers.
_______________
Was travel appropriate for this pregnant woman?
A woman with a history of two premature deliveries and one miscarriage became pregnant again. She received prenatal care at an Army hospital. She traveled to Spain, where the baby was born at 31 weeks’ gestation. The baby required treatment in a neonatal intensive care unit (NICU) for 17 days. The child has cerebral palsy, with tetraplegia of all four extremities. She cannot walk without assistance and suffers severe cognitive and vision impairment.
PARENTS’ CLAIM The ObGyn at the Army hospital should not have approved the mother’s request for travel; he did so, despite knowing that the mother was at high risk for premature birth. The military medical hospital to which she was assigned in Spain could not manage a high-risk pregnancy, didn’t have a NICU, and didn’t have specialists to treat premature infants.
DEFENDANTS’ DEFENSE The ObGyn argued that he did not have access to the medical records showing the mother’s history. The patient countered that the ObGyn did indeed have the patient’s records, as he had discussed them with her.
VERDICT A $10,409,700 California verdict was returned against the ObGyn and the government facility.
_______________
Triple-negative BrCa not diagnosed until metastasized: $5.2M
After finding lumps in both breasts, a woman in her 30s saw a nurse practitioner (NP) at an Army hospital. A radiologist reported no mass in the right breast and multiple benign-appearing anechoic lesions in the left breast after bilateral mammography and ultrasonography (US) in July 2008. The Chief of Mammography Services recommended referral to a breast surgeon, but the patient never received the letter. It was placed in her mammography file, not in the treatment file.
In November 2008, the patient returned to the clinic. Bilateral diagnostic mammography and US were ordered, but for unknown reasons, cancelled. US of the left breast was interpreted as benign in January 2009.
After imaging in March 2010, followed by a needle biopsy of the right breast, a radiologist reported finding intermediate-grade infiltrating ductal carcinoma.
The patient sought care outside the military medical system at a large university hospital. In April 2010, stage 3 triple-negative invasive ductal carcinoma (IDC) was identified. The patient underwent chemotherapy, a double mastectomy, removal of 21 lymph nodes, and breast reconstruction. She was given a 60% chance of recurrence in 5–7 years.
PATIENT’S CLAIM It was negligent to not inform her of imaging results. Biopsy should have been performed in 2008, when the IDC was likely at stage 1; treatment would have been far less aggressive. Electronic medical records showed that the 2008 mammography and US results had been “signed off” by an NP at the clinic.
DEFENDANTS’ DEFENSE While unable to concede liability, the government agency did not contest the point.
VERDICT A $5.2 million Tennessee federal court bench verdict was returned, citing failures in communication, poor and improper record keeping and retention, failure to follow-up, and an unexplained cancellation of a medical order.
_______________
Woman dies from cervical cancer: $2.3M
In 2001, a 41-year-old woman had abnormal Pap smear results but her gynecologist did not order more testing. The patient was told to return in 3 months, but she did not return until 2007—reporting abnormal bleeding, vaginal discharge, and pain. Her Pap results were normal, however, and the gynecologist did not order further testing. In 2009, the patient was found to have advanced cervical cancer. She died 2 years later.
ESTATE’S CLAIM Further testing should have been ordered in 2001, which would have likely revealed dysplasia, which can lead to cancer. The laboratory incorrectly interpreted the 2007 Pap test; if the results had been properly reported, additional testing could have been ordered.
DEFENDANTS’ DEFENSE The laboratory and patient’s estate settled for a confidential amount before trial. The gynecologist denied negligence.
VERDICT A New Jersey jury found the gynecologist 40% at fault for his actions in 2007. The jury found the laboratory 50% at fault, and the patient 10% at fault. A gross verdict of $2.33 million was returned.
_______________
Bowel injury after cesarean delivery; mother dies of sepsis
At 40 4/7 weeks' gestation, a 37-year-old woman gave birth to a healthy child by cesarean delivery. The next day, the patient had an elevated white blood cell (WBC) count with a left shift, her abdomen was tympanic but soft, and she was passing flatus and belching. The ObGyn ordered a Fleet enema; only flatus was released. A covering ObGyn ordered an abdominal radiograph, which the radiologist reported as showing postoperative ileus and mild constipation. The patient was given a second Fleet enema the next day, resulting in watery stool. She vomited 300 mL of dark green fluid.
After a rectal tube was placed 2 days later, one hard brown stool and several brown, pasty, loose, and liquid stools were returned. She vomited several times that day, and was found to have hypoactive bowel sounds with continued tympanic quality in the upper quadrants. Laboratory testing revealed continued elevated WBC count with left shift. The next day, she had hypoactive bowel sounds with brown liquid stools. Later that morning, she was able to tolerate clear liquids. The ObGyn decided to discharge her home with instructions to continue on a clear liquid diet for 2 more days before advancing her diet.
The day after discharge, she was found unresponsive at home. She was taken to the hospital, but resuscitation attempts failed. She died. An autopsy revealed that the cause of death was sepsis.
ESTATE’S CLAIM The ObGyn was negligent in failing to diagnose and treat a postoperative intra-abdominal infection caused by bowel perforation. A surgical consult should have been obtained. The woman was prematurely discharged. The radiologist failed to report the presence of free air on the abdominal x-ray.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million Maryland settlement was reached.
_______________
Right ureter injury detected and repaired
During laparoscopic-assisted vaginal hysterectomy, the ObGyn detected and repaired an injury to the right ureter. The patient’s recovery was delayed by the injury.
PATIENT’S CLAIM The ObGyn was negligent in using a Kleppinger bipolar cauterizing instrument to cauterize the vaginal cuff. Thermal overspray from the instrument or the instrument itself damaged the ureter. The ObGyn was also negligent in not performing diagnostic cystoscopy to confirm patency of the ureter after the repair was made.
PHYSICIAN’S DEFENSE Ureter injury is a known risk of the procedure. All procedures were performed according to protocol.
VERDICT A Florida defense verdict was returned.
_______________
Failure to detect inflammatory BrCa; woman dies
A 42-year-old woman underwent mammography in February 2002 after reporting pain, discoloration, inflammation, and swelling in her left breast. The radiologist who interpreted the mammography suggested a biopsy for a differential diagnosis of mastitis or inflammatory carcinoma. The biopsy results were negative.
The patient’s symptoms persisted, and she underwent US in late May 2002. Another radiologist interpreted the US, noting that the patient could not tolerate compression, which led to less than optimal evaluation. The radiologist suggested that mastitis was the likely cause of the patient’s symptoms.
The patient then consulted a surgeon, who ordered mammography and magnetic resonance imaging (MRI) followed by biopsy, which indicated cancer. The patient underwent a mastectomy but metastasis had already occurred. She died at age 50 prior to the trial.
ESTATE’S CLAIM If the cancer had been diagnosed earlier, the outcome would have been better. Both radiologists misinterpreted the mammographies.
DEFENDANTS’ DEFENSE The mammographies had been properly interpreted. Any missed diagnosis would not have impacted the outcome due to the type of cancer. The scans had been released to the patient, but were subsequently lost; an adverse interference instruction was given to the jury.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine rupture, child stillborn: $3.8M net award
At 35 weeks' gestation, a woman went to the emergency department (ED) with abdominal pain, fast heartbeat, and irregular contractions. Her history included three cesarean deliveries, including one with a vertical incision. She was admitted, and a cesarean delivery was planned for the next day. After 8 hours, during which the patient’s condition worsened, an emergency cesarean delivery was undertaken. A full rupture of the uterus was found; the baby’s body had extruded into the mother’s abdomen. The child was stillborn.
PARENTS’ CLAIM The stillbirth could have been avoided if the nurses had communicated the mother’s worsening condition to the physicians.
DEFENDANTS’ DEFENSE After the hospital and physicians settled prior to trial, the case continued against the nurse in charge of the mother’s care and the nurse-staffing group. Negligence was denied; all protocols were followed.
VERDICT A $2.9 million Illinois verdict was returned. With a $900,000 settlement from the hospital and physicians, the net award was $3.8 million.
_______________
Where did rare strep A infection come from?
A 36-year-old woman reported heavy vaginal bleeding to her ObGyn. She underwent endometrial ablation in her physician’s office.
The next day, the woman called the office to report abdominal pain. She was told to stop the medication she was taking, and if the pain continued to the next day, to go to an ED. The next day, the patient went to the ED and was found to be in septic shock. During emergency laparotomy, 50 mL of purulent fluid were drained and an emergency hysterectomy was performed. Three days later, the patient died from pulmonary arrest caused by toxic shock syndrome. An autopsy revealed that the patient’s sepsis was caused by group A streptococci (GAS) infection.
ESTATE’S CLAIM The patient was not a proper candidate for endometrial ablation because of her history of chronic cervical infection. The ObGyn perforated the cervix during the procedure and tried to conceal it. At autopsy, bone wax was found in the rectal lumen that had been used to cover up damage to the cervix. The ObGyn introduced GAS bacteria into the patient’s system. The ObGyn’s staff failed to ask the proper questions when she called the day after the procedure. She should have been told to go directly to the ED.
DEFENDANTS’ DEFENSE The ObGyn did not perforate the cervix or uterus during the procedure. GAS infection is so rare that it would have been difficult to foresee or diagnose. Potentially, the patient had a chronic
cervical infection before ablation.
VERDICT A Texas defense verdict was returned.
_______________
DURING INSERTION, IUD PERFORATES UTERINE WALL; LATER FOUND BELOW LIVER
On July 21, a 46-year-old woman went to an ObGyn for placement of an intrauterine device (IUD). Shortly after the ObGyn inserted the levonorgestrel-releasing intrauterine system (Mirena, Bayer HealthCare), the patient reported severe pelvic and abdominal pain. On July 26, the patient underwent surgical removal of the IUD.
She was discharged on July 29 but continued to report pain. She was readmitted to the hospital the next day and treated for pain. She was bed ridden for 3 weeks after IUD-removal surgery, and had a 3-month recovery before feeling pain free.
PATIENT’S CLAIM The ObGyn was negligent in perforating the patient’s uterine wall during IUD insertion, causing the device to ultimately migrate under the patient’s liver.
DEFENDANTS’ DEFENSE Uterine perforation is a known complication of IUD insertion. The IUD escaped from the patient’s uterus at a later time and not during the insertion procedure.
VERDICT A Florida verdict of $208,839 was returned; the amount was reduced to $161,058 because the medical expenses were written off by the health-care providers.
_______________
Was travel appropriate for this pregnant woman?
A woman with a history of two premature deliveries and one miscarriage became pregnant again. She received prenatal care at an Army hospital. She traveled to Spain, where the baby was born at 31 weeks’ gestation. The baby required treatment in a neonatal intensive care unit (NICU) for 17 days. The child has cerebral palsy, with tetraplegia of all four extremities. She cannot walk without assistance and suffers severe cognitive and vision impairment.
PARENTS’ CLAIM The ObGyn at the Army hospital should not have approved the mother’s request for travel; he did so, despite knowing that the mother was at high risk for premature birth. The military medical hospital to which she was assigned in Spain could not manage a high-risk pregnancy, didn’t have a NICU, and didn’t have specialists to treat premature infants.
DEFENDANTS’ DEFENSE The ObGyn argued that he did not have access to the medical records showing the mother’s history. The patient countered that the ObGyn did indeed have the patient’s records, as he had discussed them with her.
VERDICT A $10,409,700 California verdict was returned against the ObGyn and the government facility.
_______________
Triple-negative BrCa not diagnosed until metastasized: $5.2M
After finding lumps in both breasts, a woman in her 30s saw a nurse practitioner (NP) at an Army hospital. A radiologist reported no mass in the right breast and multiple benign-appearing anechoic lesions in the left breast after bilateral mammography and ultrasonography (US) in July 2008. The Chief of Mammography Services recommended referral to a breast surgeon, but the patient never received the letter. It was placed in her mammography file, not in the treatment file.
In November 2008, the patient returned to the clinic. Bilateral diagnostic mammography and US were ordered, but for unknown reasons, cancelled. US of the left breast was interpreted as benign in January 2009.
After imaging in March 2010, followed by a needle biopsy of the right breast, a radiologist reported finding intermediate-grade infiltrating ductal carcinoma.
The patient sought care outside the military medical system at a large university hospital. In April 2010, stage 3 triple-negative invasive ductal carcinoma (IDC) was identified. The patient underwent chemotherapy, a double mastectomy, removal of 21 lymph nodes, and breast reconstruction. She was given a 60% chance of recurrence in 5–7 years.
PATIENT’S CLAIM It was negligent to not inform her of imaging results. Biopsy should have been performed in 2008, when the IDC was likely at stage 1; treatment would have been far less aggressive. Electronic medical records showed that the 2008 mammography and US results had been “signed off” by an NP at the clinic.
DEFENDANTS’ DEFENSE While unable to concede liability, the government agency did not contest the point.
VERDICT A $5.2 million Tennessee federal court bench verdict was returned, citing failures in communication, poor and improper record keeping and retention, failure to follow-up, and an unexplained cancellation of a medical order.
_______________
Woman dies from cervical cancer: $2.3M
In 2001, a 41-year-old woman had abnormal Pap smear results but her gynecologist did not order more testing. The patient was told to return in 3 months, but she did not return until 2007—reporting abnormal bleeding, vaginal discharge, and pain. Her Pap results were normal, however, and the gynecologist did not order further testing. In 2009, the patient was found to have advanced cervical cancer. She died 2 years later.
ESTATE’S CLAIM Further testing should have been ordered in 2001, which would have likely revealed dysplasia, which can lead to cancer. The laboratory incorrectly interpreted the 2007 Pap test; if the results had been properly reported, additional testing could have been ordered.
DEFENDANTS’ DEFENSE The laboratory and patient’s estate settled for a confidential amount before trial. The gynecologist denied negligence.
VERDICT A New Jersey jury found the gynecologist 40% at fault for his actions in 2007. The jury found the laboratory 50% at fault, and the patient 10% at fault. A gross verdict of $2.33 million was returned.
_______________
Bowel injury after cesarean delivery; mother dies of sepsis
At 40 4/7 weeks' gestation, a 37-year-old woman gave birth to a healthy child by cesarean delivery. The next day, the patient had an elevated white blood cell (WBC) count with a left shift, her abdomen was tympanic but soft, and she was passing flatus and belching. The ObGyn ordered a Fleet enema; only flatus was released. A covering ObGyn ordered an abdominal radiograph, which the radiologist reported as showing postoperative ileus and mild constipation. The patient was given a second Fleet enema the next day, resulting in watery stool. She vomited 300 mL of dark green fluid.
After a rectal tube was placed 2 days later, one hard brown stool and several brown, pasty, loose, and liquid stools were returned. She vomited several times that day, and was found to have hypoactive bowel sounds with continued tympanic quality in the upper quadrants. Laboratory testing revealed continued elevated WBC count with left shift. The next day, she had hypoactive bowel sounds with brown liquid stools. Later that morning, she was able to tolerate clear liquids. The ObGyn decided to discharge her home with instructions to continue on a clear liquid diet for 2 more days before advancing her diet.
The day after discharge, she was found unresponsive at home. She was taken to the hospital, but resuscitation attempts failed. She died. An autopsy revealed that the cause of death was sepsis.
ESTATE’S CLAIM The ObGyn was negligent in failing to diagnose and treat a postoperative intra-abdominal infection caused by bowel perforation. A surgical consult should have been obtained. The woman was prematurely discharged. The radiologist failed to report the presence of free air on the abdominal x-ray.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $1 million Maryland settlement was reached.
_______________
Right ureter injury detected and repaired
During laparoscopic-assisted vaginal hysterectomy, the ObGyn detected and repaired an injury to the right ureter. The patient’s recovery was delayed by the injury.
PATIENT’S CLAIM The ObGyn was negligent in using a Kleppinger bipolar cauterizing instrument to cauterize the vaginal cuff. Thermal overspray from the instrument or the instrument itself damaged the ureter. The ObGyn was also negligent in not performing diagnostic cystoscopy to confirm patency of the ureter after the repair was made.
PHYSICIAN’S DEFENSE Ureter injury is a known risk of the procedure. All procedures were performed according to protocol.
VERDICT A Florida defense verdict was returned.
_______________
Failure to detect inflammatory BrCa; woman dies
A 42-year-old woman underwent mammography in February 2002 after reporting pain, discoloration, inflammation, and swelling in her left breast. The radiologist who interpreted the mammography suggested a biopsy for a differential diagnosis of mastitis or inflammatory carcinoma. The biopsy results were negative.
The patient’s symptoms persisted, and she underwent US in late May 2002. Another radiologist interpreted the US, noting that the patient could not tolerate compression, which led to less than optimal evaluation. The radiologist suggested that mastitis was the likely cause of the patient’s symptoms.
The patient then consulted a surgeon, who ordered mammography and magnetic resonance imaging (MRI) followed by biopsy, which indicated cancer. The patient underwent a mastectomy but metastasis had already occurred. She died at age 50 prior to the trial.
ESTATE’S CLAIM If the cancer had been diagnosed earlier, the outcome would have been better. Both radiologists misinterpreted the mammographies.
DEFENDANTS’ DEFENSE The mammographies had been properly interpreted. Any missed diagnosis would not have impacted the outcome due to the type of cancer. The scans had been released to the patient, but were subsequently lost; an adverse interference instruction was given to the jury.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
More inclusions:
- Where did rare strep A infection come from?
- During insertion, IUD perforates uterine wall; Later found below liver
- Was travel appropriate for this pregnant woman?
- Triple-negative BrCa not diagnosed until metastasized: $5.2M
- Woman dies from cervical cancer: $2.3M
- Bowel injury after cesarean delivery; mother dies of sepsis
- Right ureter injury detected and repaired
- Failure to detect inflammatory BrCa; woman dies
What treatments relieve arthritis and fatigue associated with systemic lupus erythematosus?
EVIDENCE-BASED ANSWER:
Hydroxychloroquine and chloroquine improve the arthritis associated with mild systemic lupus erythematosus (SLE)—producing a 50% reduction in arthritis flares and articular involvement—and have few adverse effects (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Methotrexate reduces arthralgias by as much as 79%, but produces adverse effects in up to 70% of patients (SOR: B, systematic review of RCTs with limited patient-oriented evidence).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used for SLE joint pain (SOR: C, expert opinion).
Omega-3 fatty acids may reduce arthritis symptoms by about 35% (SOR: B, RCTs with inconsistent evidence).
Abatacept and dehydroepiandrosterone don’t produce clinically meaningful improvements in fatigue associated with SLE, and abatacept causes significant adverse effects (SOR: B, posthoc analysis of a single RCT).
Aerobic exercise may help fatigue (SOR: B, systematic review with inconsistent evidence).
EVIDENCE SUMMARY
A systematic review of pharmacotherapy for joint pain in patients with SLE found 4 poor-quality RCTs that evaluated hydroxychloroquine, chloroquine, and methotrexate.1 Of the 2 studies that examined the effect of hydroxychloroquine, one (47 patients) showed a statistically significant 50% reduction in SLE flares (including arthritis, pleuritis, and cutaneous symptoms) over 24 weeks in patients treated with hydroxychloroquine compared with placebo (TABLE1-8). The second study (71 subjects) found a nonquantified decrease in self-reported pain when hydroxychloroquine was compared with placebo, although some of the patients were also taking prednisone (10 mg/d).
An RCT that evaluated the effect of chloroquine showed a statistically significant reduction in unspecified “articular involvement” compared with placebo.
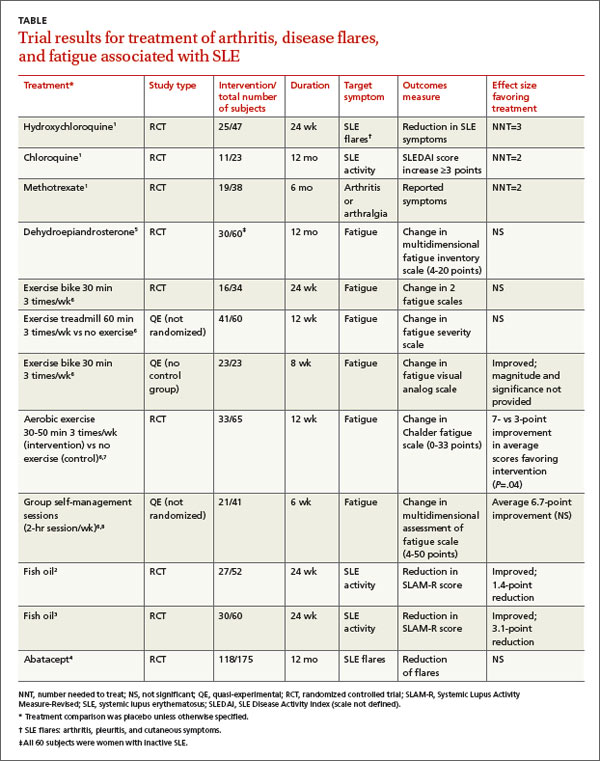
The fourth RCT, assessing methotrexate, found a statistically significant reduction by as much as 79% in patients with residual arthritis or arthralgia at 6 months compared with placebo, although 70% of patients taking methotrexate developed significant adverse effects, including infections, gastrointestinal symptoms, and elevated transaminases compared with 14% on placebo (number needed to harm [NNH]=2).
The authors of the review noted that consensus opinion holds that oral corticosteroids and NSAIDs reduce SLE-associated joint pain, but they found no studies that objectively evaluated either of these interventions.1
Fish oil also helps arthritis
Two RCTs on the effects of 3 g/d of omega-3 polyunsaturated fatty acids (fish oil) for 24 weeks in SLE patients with mild disease found a reduction in Systemic Lupus Activity Measure-Revised (SLAM-R) scores.2,3 SLAM-R is a validated measure of SLE disease activity, rated on a scale from 0 to 81, including 23 clinical and 7 laboratory manifestations of disease.
In the first study (52 subjects), disease activity decreased from an average SLAM-R score of 6.1 at baseline to 4.7 (P<.05). The second study (60 subjects) found a similar reduction in mean SLAM-R scores from 9.4 to 6.3 (P<.001) and joint pain scores from 1.27 to 0.83 (P=.047).
Drug treatments don’t significantly relieve fatigue
An industry-sponsored RCT that compared abatacept with placebo found improvements in fatigue that weren’t clinically meaningful in posthoc analysis (-9.45 points difference on a self-reported 0-to-100 visual analog scale; 95% confidence interval, -17.65 to -1.25, with a 10-point reduction considered to be clinically meaningful). Abatacept also had a high rate of serious adverse events, including facial edema, polyneuropathy, and serious infections (24/121 with abatacept vs 4/59 placebo; NNH=8).4
Another RCT found no effect of dehydroepiandrosterone on fatigue in women with inactive SLE.5
Nondrug treatments for fatigue produce mixed results
Studies of nondrug treatment of SLE-associated fatigue show inconsistent results. A systematic review of nonpharmacologic interventions for fatigue in several chronic diseases found 2 RCTs and 4 quasi-experimental studies that included 324 patients with SLE.6 Of 4 studies that evaluated the effect of exercise, 2 showed improvement and 2 didn’t. Neither group self-management nor relaxation therapy and telephone counseling significantly relieved fatigue.6-8 A small RCT (24 patients) found no benefit for acupuncture over sham needling in treating pain and fatigue in SLE.9
RECOMMENDATIONS
The American College of Rheumatology guideline for referral and management of SLE states that “NSAIDs are sometimes helpful for control of fever, arthritis, and mild serositis. Antimalarial agents (eg, hydroxychloroquine) are useful for skin and joint manifestations of SLE, for preventing flares, and for other constitutional symptoms of the disease. They may also reduce fatigue.”10
The European League Against Rheumatism recommends antimalarials or glucocorticoids to treat patients with SLE without major organ manifestations. They also say clinicians may try NSAIDs for limited periods of time in patients at low risk for the drugs’ complications.11
1. Madhok R, Wu O. Systemic lupus erythematosus. Clin Evid. 2009;7:1123.
2. Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with omega-3 fish oils and/ or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551-1556.
3. Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841-848.
4. Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077-3087.
5. Hartkamp A, Geenen R, Godaert GL, et al. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomized controlled trial. Ann Rheum Dis. 2010;69:1144-1147.
6. Neill J, Belan I, Reid K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosis: a systematic review. J Adv Nurs. 2006;56:617-635.
7. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42:1050-1054.
8. Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs. 2003;42:479-486.
9. Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17:1108-1116.
10. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
11. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
EVIDENCE-BASED ANSWER:
Hydroxychloroquine and chloroquine improve the arthritis associated with mild systemic lupus erythematosus (SLE)—producing a 50% reduction in arthritis flares and articular involvement—and have few adverse effects (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Methotrexate reduces arthralgias by as much as 79%, but produces adverse effects in up to 70% of patients (SOR: B, systematic review of RCTs with limited patient-oriented evidence).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used for SLE joint pain (SOR: C, expert opinion).
Omega-3 fatty acids may reduce arthritis symptoms by about 35% (SOR: B, RCTs with inconsistent evidence).
Abatacept and dehydroepiandrosterone don’t produce clinically meaningful improvements in fatigue associated with SLE, and abatacept causes significant adverse effects (SOR: B, posthoc analysis of a single RCT).
Aerobic exercise may help fatigue (SOR: B, systematic review with inconsistent evidence).
EVIDENCE SUMMARY
A systematic review of pharmacotherapy for joint pain in patients with SLE found 4 poor-quality RCTs that evaluated hydroxychloroquine, chloroquine, and methotrexate.1 Of the 2 studies that examined the effect of hydroxychloroquine, one (47 patients) showed a statistically significant 50% reduction in SLE flares (including arthritis, pleuritis, and cutaneous symptoms) over 24 weeks in patients treated with hydroxychloroquine compared with placebo (TABLE1-8). The second study (71 subjects) found a nonquantified decrease in self-reported pain when hydroxychloroquine was compared with placebo, although some of the patients were also taking prednisone (10 mg/d).
An RCT that evaluated the effect of chloroquine showed a statistically significant reduction in unspecified “articular involvement” compared with placebo.

The fourth RCT, assessing methotrexate, found a statistically significant reduction by as much as 79% in patients with residual arthritis or arthralgia at 6 months compared with placebo, although 70% of patients taking methotrexate developed significant adverse effects, including infections, gastrointestinal symptoms, and elevated transaminases compared with 14% on placebo (number needed to harm [NNH]=2).
The authors of the review noted that consensus opinion holds that oral corticosteroids and NSAIDs reduce SLE-associated joint pain, but they found no studies that objectively evaluated either of these interventions.1
Fish oil also helps arthritis
Two RCTs on the effects of 3 g/d of omega-3 polyunsaturated fatty acids (fish oil) for 24 weeks in SLE patients with mild disease found a reduction in Systemic Lupus Activity Measure-Revised (SLAM-R) scores.2,3 SLAM-R is a validated measure of SLE disease activity, rated on a scale from 0 to 81, including 23 clinical and 7 laboratory manifestations of disease.
In the first study (52 subjects), disease activity decreased from an average SLAM-R score of 6.1 at baseline to 4.7 (P<.05). The second study (60 subjects) found a similar reduction in mean SLAM-R scores from 9.4 to 6.3 (P<.001) and joint pain scores from 1.27 to 0.83 (P=.047).
Drug treatments don’t significantly relieve fatigue
An industry-sponsored RCT that compared abatacept with placebo found improvements in fatigue that weren’t clinically meaningful in posthoc analysis (-9.45 points difference on a self-reported 0-to-100 visual analog scale; 95% confidence interval, -17.65 to -1.25, with a 10-point reduction considered to be clinically meaningful). Abatacept also had a high rate of serious adverse events, including facial edema, polyneuropathy, and serious infections (24/121 with abatacept vs 4/59 placebo; NNH=8).4
Another RCT found no effect of dehydroepiandrosterone on fatigue in women with inactive SLE.5
Nondrug treatments for fatigue produce mixed results
Studies of nondrug treatment of SLE-associated fatigue show inconsistent results. A systematic review of nonpharmacologic interventions for fatigue in several chronic diseases found 2 RCTs and 4 quasi-experimental studies that included 324 patients with SLE.6 Of 4 studies that evaluated the effect of exercise, 2 showed improvement and 2 didn’t. Neither group self-management nor relaxation therapy and telephone counseling significantly relieved fatigue.6-8 A small RCT (24 patients) found no benefit for acupuncture over sham needling in treating pain and fatigue in SLE.9
RECOMMENDATIONS
The American College of Rheumatology guideline for referral and management of SLE states that “NSAIDs are sometimes helpful for control of fever, arthritis, and mild serositis. Antimalarial agents (eg, hydroxychloroquine) are useful for skin and joint manifestations of SLE, for preventing flares, and for other constitutional symptoms of the disease. They may also reduce fatigue.”10
The European League Against Rheumatism recommends antimalarials or glucocorticoids to treat patients with SLE without major organ manifestations. They also say clinicians may try NSAIDs for limited periods of time in patients at low risk for the drugs’ complications.11
EVIDENCE-BASED ANSWER:
Hydroxychloroquine and chloroquine improve the arthritis associated with mild systemic lupus erythematosus (SLE)—producing a 50% reduction in arthritis flares and articular involvement—and have few adverse effects (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Methotrexate reduces arthralgias by as much as 79%, but produces adverse effects in up to 70% of patients (SOR: B, systematic review of RCTs with limited patient-oriented evidence).
Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are often used for SLE joint pain (SOR: C, expert opinion).
Omega-3 fatty acids may reduce arthritis symptoms by about 35% (SOR: B, RCTs with inconsistent evidence).
Abatacept and dehydroepiandrosterone don’t produce clinically meaningful improvements in fatigue associated with SLE, and abatacept causes significant adverse effects (SOR: B, posthoc analysis of a single RCT).
Aerobic exercise may help fatigue (SOR: B, systematic review with inconsistent evidence).
EVIDENCE SUMMARY
A systematic review of pharmacotherapy for joint pain in patients with SLE found 4 poor-quality RCTs that evaluated hydroxychloroquine, chloroquine, and methotrexate.1 Of the 2 studies that examined the effect of hydroxychloroquine, one (47 patients) showed a statistically significant 50% reduction in SLE flares (including arthritis, pleuritis, and cutaneous symptoms) over 24 weeks in patients treated with hydroxychloroquine compared with placebo (TABLE1-8). The second study (71 subjects) found a nonquantified decrease in self-reported pain when hydroxychloroquine was compared with placebo, although some of the patients were also taking prednisone (10 mg/d).
An RCT that evaluated the effect of chloroquine showed a statistically significant reduction in unspecified “articular involvement” compared with placebo.

The fourth RCT, assessing methotrexate, found a statistically significant reduction by as much as 79% in patients with residual arthritis or arthralgia at 6 months compared with placebo, although 70% of patients taking methotrexate developed significant adverse effects, including infections, gastrointestinal symptoms, and elevated transaminases compared with 14% on placebo (number needed to harm [NNH]=2).
The authors of the review noted that consensus opinion holds that oral corticosteroids and NSAIDs reduce SLE-associated joint pain, but they found no studies that objectively evaluated either of these interventions.1
Fish oil also helps arthritis
Two RCTs on the effects of 3 g/d of omega-3 polyunsaturated fatty acids (fish oil) for 24 weeks in SLE patients with mild disease found a reduction in Systemic Lupus Activity Measure-Revised (SLAM-R) scores.2,3 SLAM-R is a validated measure of SLE disease activity, rated on a scale from 0 to 81, including 23 clinical and 7 laboratory manifestations of disease.
In the first study (52 subjects), disease activity decreased from an average SLAM-R score of 6.1 at baseline to 4.7 (P<.05). The second study (60 subjects) found a similar reduction in mean SLAM-R scores from 9.4 to 6.3 (P<.001) and joint pain scores from 1.27 to 0.83 (P=.047).
Drug treatments don’t significantly relieve fatigue
An industry-sponsored RCT that compared abatacept with placebo found improvements in fatigue that weren’t clinically meaningful in posthoc analysis (-9.45 points difference on a self-reported 0-to-100 visual analog scale; 95% confidence interval, -17.65 to -1.25, with a 10-point reduction considered to be clinically meaningful). Abatacept also had a high rate of serious adverse events, including facial edema, polyneuropathy, and serious infections (24/121 with abatacept vs 4/59 placebo; NNH=8).4
Another RCT found no effect of dehydroepiandrosterone on fatigue in women with inactive SLE.5
Nondrug treatments for fatigue produce mixed results
Studies of nondrug treatment of SLE-associated fatigue show inconsistent results. A systematic review of nonpharmacologic interventions for fatigue in several chronic diseases found 2 RCTs and 4 quasi-experimental studies that included 324 patients with SLE.6 Of 4 studies that evaluated the effect of exercise, 2 showed improvement and 2 didn’t. Neither group self-management nor relaxation therapy and telephone counseling significantly relieved fatigue.6-8 A small RCT (24 patients) found no benefit for acupuncture over sham needling in treating pain and fatigue in SLE.9
RECOMMENDATIONS
The American College of Rheumatology guideline for referral and management of SLE states that “NSAIDs are sometimes helpful for control of fever, arthritis, and mild serositis. Antimalarial agents (eg, hydroxychloroquine) are useful for skin and joint manifestations of SLE, for preventing flares, and for other constitutional symptoms of the disease. They may also reduce fatigue.”10
The European League Against Rheumatism recommends antimalarials or glucocorticoids to treat patients with SLE without major organ manifestations. They also say clinicians may try NSAIDs for limited periods of time in patients at low risk for the drugs’ complications.11
1. Madhok R, Wu O. Systemic lupus erythematosus. Clin Evid. 2009;7:1123.
2. Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with omega-3 fish oils and/ or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551-1556.
3. Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841-848.
4. Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077-3087.
5. Hartkamp A, Geenen R, Godaert GL, et al. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomized controlled trial. Ann Rheum Dis. 2010;69:1144-1147.
6. Neill J, Belan I, Reid K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosis: a systematic review. J Adv Nurs. 2006;56:617-635.
7. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42:1050-1054.
8. Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs. 2003;42:479-486.
9. Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17:1108-1116.
10. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
11. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
1. Madhok R, Wu O. Systemic lupus erythematosus. Clin Evid. 2009;7:1123.
2. Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with omega-3 fish oils and/ or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551-1556.
3. Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67:841-848.
4. Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077-3087.
5. Hartkamp A, Geenen R, Godaert GL, et al. Effects of dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomized controlled trial. Ann Rheum Dis. 2010;69:1144-1147.
6. Neill J, Belan I, Reid K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosis: a systematic review. J Adv Nurs. 2006;56:617-635.
7. Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42:1050-1054.
8. Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs. 2003;42:479-486.
9. Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17:1108-1116.
10. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785-1796.
11. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
Evidence-based answers from the Family Physicians Inquiries Network