User login
Weighing the Options for Obesity Meds
In June 2013, the American Medical Association classified obesity as a disease. Since then, several medical societies have published guidelines to help clinicians improve care of affected patients. One avenue is, of course, pharmacologic treatment.
Until recently, there was only one FDA-approved medication for chronic weight loss on the market: orlistat, which was approved in 1999. (Phentermine and diethylpropion are only indicated for short-term use). After a long hiatus, the FDA approved two additional agents (phentermine/topiramate and lorcaserin)in 2012 and another two (liraglutide and bupropion/naltrexone) in 2014.
While clinicians appreciate having options for managing their patients’ conditions, in this case, many are overwhelmed by the choices. Most health care providers have not received formal training in obesity management. This column will attempt to fill the information gap in terms of what agents are available and what factors should be assessed before prescribing any of them.
Proviso: Experts claim obesity is a chronic disease, similar to hypertension, and should be managed as such. Although not discussed here, the most important aspect of weight loss and maintenance is lifestyle intervention (diet, exercise, and behavioral modification). It should be emphasized that no medication works by itself; all should be used as an adjunct tool to reinforce adherence to lifestyle changes.1 Furthermore, patients may be disappointed to learn that without these changes, the weight may return when they cease medication use.
CASE Deb, age 61, presents to your office for routine follow-up. She has a history of type 2 diabetes, dyslipidemia, hypertension, atrial fibrillation, depression, and chronic back pain due to a herniated disc. Her medications include insulin glargine, glyburide, pioglitazone, atorvastatin, metoprolol, paroxetine, and acetaminophen/hydrocodone.
Her vital signs include a blood pressure of 143/91 mm Hg and a pulse of 93 beats/min. She has a BMI of 37 and a waist circumference of 35 in.
Deb, concerned about her weight, would like to discuss weight-loss options. She has tried three different commercial programs; each time, she was able to lose 30 to 50 lb in three to six months but regained the weight once she stopped the program. She reports excessive appetite as the main reason for her rebound weight gain. Her exercise is limited due to her back pain.
She recently tried OTC orlistat but could not tolerate it due to flatulence and fecal urgency. She reports an incident in which she couldn’t reach the bathroom in time.
Continue for Discussion >>
DISCUSSION
The Endocrine Society’s recommended approaches to obesity management include diet, exercise, and behavioral modification for patients with a BMI ≥ 25. The addition of pharmacotherapy can be considered for those with a BMI ≥ 30 or with a BMI ≥ 27 and one or more weight-related comorbidities (eg, diabetes, dyslipidemia, hypertension). This matches the FDA-approved product labeling for chronic weight-loss medications. Bariatric surgery should be considered for patients with a BMI ≥ 40 or with a BMI ≥ 35 and at least one weight-related comorbidity.
Orlistat
Orlistat is available OTC in a 60-mg thrice-daily form. A higher dosage (120 mg tid) is available via prescription. Orlistat decreases fat absorption in the gastrointestinal (GI) tract by inhibiting GI lipase. Average weight loss with orlistat is 3% at first and second year, and, when compared with placebo, 2.4% greater at four years.2
Orlistat should be prescribed with a multivitamin due to decreased absorption of fat-soluble vitamins. It is contraindicated in patients with malabsorption syndrome and gallbladder disease (> 2% incidence3). It can increase cyclosporine exposure, and rare cases of liver failure have been reported. The most common adverse effect is related to steatorrhea. Of the available options, orlistat is the only medication that has no effect on neurohormonal regulation in appetite control and metabolic rate, which may be a limiting factor.
CASE POINT Due to Deb’s intolerance of and embarrassment with GI adverse effects, she requests an alternative medication.
Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist that reduces appetite by affecting anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Of note, lorcaserin “selects” the 2C receptor instead of 2A and 2B; 2B receptors are found in both aortic and mitral valves, which may explain the association between fenfluramine/phentermine (commonly known as “fen/phen” and withdrawn from the market in 1997) and possible cardiac valvulopathy. (Fenfluramine is an amphetamine derivative that nonselectively stimulates serotonin release and inhibits reuptake.)
Lorcaserin comes in a 10-mg twice-daily dosage. In studies, patients taking lorcaserin had an average weight loss of 3.3% more than those taking placebo at one year; weight loss was maintained through the second year for those who continued on medication. However, those who stopped the medication at one year had regained their weight by the two-year mark.4
It is recommended that the medication be discontinued if patients don’t achieve a loss of more than 5% of body weight by 12 weeks.
In a study that enrolled diabetic patients, lorcaserin also demonstrated a 0.9% reduction in A1C, which is similar to or even better than some oral antidiabetic medications.4 However, since the manufacturer was not planning for an antidiabetic indication, A1C was only a secondary endpoint. The reduction is most likely due to decreased caloric intake and weight loss.
The most common adverse effects of lorcaserin include headache, dizziness, and fatigue. The discontinuation rate due to intolerance was 8.6%, compared to 6.7% with placebo.5
Although this was not observed in clinical studies, co-administration of lorcaserin (a serotonin receptor agonist) with other serotonergic or antidopaminergic agents can theoretically cause serotonin syndrome or neuroleptic malignant syndrome–like reactions. Caution is therefore advisable when prescribing these agents. The package insert carries a warning for cardiac valvulopathy due to fen/phen’s history and a lack of long-term cardiovascular safety data.
CASE POINT Deb is taking paroxetine (an SSRI) for her depression. Since you are concerned about serotonin syndrome, you decide to keep exploring options. Checking the package insert for phentermine/topiramate, you learn that it does not have a potential adverse reaction related to co-administration with SSRIs.
Phentermine/Topiramate
Phentermine, a sympathomimetic medication, was approved for short-term (12-week) use for weight loss in the 1960s. Topiramate, an antiseizure and migraine prophylactic medication, enhances appetite suppression—although the exact mechanism of action is unknown.1
Four once-daily doses are available: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, and 15/92 mg. Dosing starts with 3.75/23 mg for two weeks, then increases to 7.5/46 mg. If a loss of 5% or more of body weight is achieved, the patient can continue the dosage; if not, it can be increased to 11.25/69 mg for two weeks and then to 15/92 mg. The average weight loss for mid and maximum dose was 6.6% and 8.6% greater than placebo at one year.5
Commonly reported adverse effects include paraesthesia, dysgeusia (distortion of sense of taste), dizziness, insomnia, constipation, and dry mouth. Due to phentermine’s sympathomimetic action, mild increases in heart rate and blood pressure were reported. The Endocrine Society recommends against the use of phentermine in patients with uncontrolled hypertension and a history of heart disease.1
Weight loss is generally not recommended during pregnancy, and all weight loss medications are classified as category X for pregnancy. Strict caution is advised with this particular agent, as topiramate has known teratogenicity and therefore comes with a Risk Evaluation Mitigation Strategy. Patients must be advised to use appropriate contraception while taking topiramate, and a pregnancy test should be performed before medication commencement and monthly thereafter.
Abrupt cessation of topiramate can cause seizure. When taking the 15/92-mg dosage, the patient should reduce to one tablet every other day for at least one week before discontinuation.
CASE POINT Deb’s blood pressure is still not at goal. This, along with her history of atrial fibrillation and high pulse, prompts you to consider another option.
Bupropion/Naltrexone
Bupropion, a widely used antidepressant, inhibits the uptake of norepinephrine and dopamine and thereby blocks the reward pathway that various foods can induce. Naltrexone, an opioid antagonist, blocks the opioid pathway and can be helpful in enhancing weight loss.
This combination comes in an 8/90-mg tablet. The suggested titration regimen is to start with one tablet per day and increase by one tablet every week, up to the maximum dosage of two tablets twice a day. Average weight loss was 3.1% greater than placebo at one year with the maximum dosage. An A1C reduction of 0.6% was seen in diabetic patients.6 It is recommended to stop the medication and seek an alternative treatment option if > 5% loss of body weight is not achieved by 12 weeks.
GI adverse effects (eg, nausea and vomiting) are common; these can be reduced with a slower titration regimen or by prescribing a maximum of one tablet twice daily (instead of two). Every antidepressant carries suicidal risk, and caution is advised with their use. Bupropion can also lower the seizure threshold, and it is contraindicated for patients with seizure disorder. It is also contraindicated in patients who are undergoing abrupt cessation of alcohol, benzodiazepines, or barbiturates. It can increase pulse and blood pressure during early titration; regular blood pressure monitoring is warranted.
CASE POINT Due to Deb’s opioid usage and uncontrolled hypertension, you discuss a final option that was recently approved for weight loss.
Liraglutide
This glucagon-like peptide-1 (GLP1) receptor agonist affects the brain to suppress/control appetite, slows down gastric emptying, and induces early satiety. A 3-mg dosage was approved in December 2014, but 0.6-, 1.2-, and 1.8-mg dosages have been available since 2010 for patients with type 2 diabetes.
Average weight loss was 4.5% greater than placebo at one year.7 If < 4% weight loss is achieved by 16 weeks, consider using an alternative agent.
The most common adverse effect is GI upset, which could be related to the mechanism of action (slower gastric emptying). Although self-reported GI upset was high (39%), the actual discontinuation rate was low (2.9% for nausea, 1.7% for vomiting, and 1.4% for diarrhea).3
This adverse effect could, in certain contexts, be considered “wanted,” since it discourages overeating or eating too quickly. My clinical pearl is to tell patients taking liraglutide that they are “trapped” and have to eat smaller portions and eat more slowly or they will be more prone to GI effects. With this strategy, we can encourage portion control and responsibility for behavior. (Please note that this is my experience with the diabetic dosage of liraglutide; I do not have any clinical experience with the obesity dosage, which was not clinically available at the time of writing.)
Both branded versions of liraglutide carry a black-box warning for thyroid C-cell tumors, which were observed in rodents but unproven in humans. The medication is contraindicated in patients with medullary thyroid cancer or with multiple endocrine neoplasia 2 syndrome. Increased rates of acute pancreatitis, cholecystitis, and cholelithiasis were seen in studies, and caution is advised.
Continue for A Word About Meds That Cause Weight Gain >>
A WORD ABOUT MEDS THAT CAUSE WEIGHT GAIN
The Endocrine Society has published a list of medications that can influence weight gain, along with suggestions for alternative agents that are either weight neutral or promote weight loss.
Note that our case patient, Deb, is taking insulin, a sulfonylurea (glyburide), and thiazolidinedione (pioglitazone) for diabetes—all of which can promote weight gain. Guidelines suggest choosing metformin, DPP4 inhibitors, GLP1 agonists, amylin analog, and SGLT2 inhibitors instead when weight gain is a major concern.1
Guidelines also suggest using ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers instead of β-blockers as firstline antihypertensive therapy for diabetic patients.1 Adequate blood pressure and lipid control are imperative in diabetes management.
CASE POINT Deb would need better hypertension control before she considers weight-loss medication. Since she is also taking paroxetine, which among SSRIs is associated with greatest weight gain, a changed to fluoxetine or sertraline should be considered.2
Next page: Conclusion >>
CONCLUSION
There are now five medications approved by the FDA for chronic weight loss, with more to come. Agents with different mechanisms of action give us options to help obese patients and hopefully reduce and prevent obesity-related complications. It is important for clinicians to be competent in managing obesity, especially since we live in an era in which the disease is considered pandemic.
REFERENCES
1. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
2. Xenical [package insert]. South San Francisco, CA: Genentech USA, Inc; 2012.
3. Fujioka K. Safety and tolerability of medications approved for chronic weight management. Obesity (Silver Spring). 2015;22 (suppl 1):S7-S11.
4. Belviq [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2015.
5. Qsymia [package insert]. Mountain View, CA: Vivus, Inc; 2013.
6. Contrave [package insert]. Deerfield, IL: Takeda USA, Inc; 2014.
7. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk; 2014.
In June 2013, the American Medical Association classified obesity as a disease. Since then, several medical societies have published guidelines to help clinicians improve care of affected patients. One avenue is, of course, pharmacologic treatment.
Until recently, there was only one FDA-approved medication for chronic weight loss on the market: orlistat, which was approved in 1999. (Phentermine and diethylpropion are only indicated for short-term use). After a long hiatus, the FDA approved two additional agents (phentermine/topiramate and lorcaserin)in 2012 and another two (liraglutide and bupropion/naltrexone) in 2014.
While clinicians appreciate having options for managing their patients’ conditions, in this case, many are overwhelmed by the choices. Most health care providers have not received formal training in obesity management. This column will attempt to fill the information gap in terms of what agents are available and what factors should be assessed before prescribing any of them.
Proviso: Experts claim obesity is a chronic disease, similar to hypertension, and should be managed as such. Although not discussed here, the most important aspect of weight loss and maintenance is lifestyle intervention (diet, exercise, and behavioral modification). It should be emphasized that no medication works by itself; all should be used as an adjunct tool to reinforce adherence to lifestyle changes.1 Furthermore, patients may be disappointed to learn that without these changes, the weight may return when they cease medication use.
CASE Deb, age 61, presents to your office for routine follow-up. She has a history of type 2 diabetes, dyslipidemia, hypertension, atrial fibrillation, depression, and chronic back pain due to a herniated disc. Her medications include insulin glargine, glyburide, pioglitazone, atorvastatin, metoprolol, paroxetine, and acetaminophen/hydrocodone.
Her vital signs include a blood pressure of 143/91 mm Hg and a pulse of 93 beats/min. She has a BMI of 37 and a waist circumference of 35 in.
Deb, concerned about her weight, would like to discuss weight-loss options. She has tried three different commercial programs; each time, she was able to lose 30 to 50 lb in three to six months but regained the weight once she stopped the program. She reports excessive appetite as the main reason for her rebound weight gain. Her exercise is limited due to her back pain.
She recently tried OTC orlistat but could not tolerate it due to flatulence and fecal urgency. She reports an incident in which she couldn’t reach the bathroom in time.
Continue for Discussion >>
DISCUSSION
The Endocrine Society’s recommended approaches to obesity management include diet, exercise, and behavioral modification for patients with a BMI ≥ 25. The addition of pharmacotherapy can be considered for those with a BMI ≥ 30 or with a BMI ≥ 27 and one or more weight-related comorbidities (eg, diabetes, dyslipidemia, hypertension). This matches the FDA-approved product labeling for chronic weight-loss medications. Bariatric surgery should be considered for patients with a BMI ≥ 40 or with a BMI ≥ 35 and at least one weight-related comorbidity.
Orlistat
Orlistat is available OTC in a 60-mg thrice-daily form. A higher dosage (120 mg tid) is available via prescription. Orlistat decreases fat absorption in the gastrointestinal (GI) tract by inhibiting GI lipase. Average weight loss with orlistat is 3% at first and second year, and, when compared with placebo, 2.4% greater at four years.2
Orlistat should be prescribed with a multivitamin due to decreased absorption of fat-soluble vitamins. It is contraindicated in patients with malabsorption syndrome and gallbladder disease (> 2% incidence3). It can increase cyclosporine exposure, and rare cases of liver failure have been reported. The most common adverse effect is related to steatorrhea. Of the available options, orlistat is the only medication that has no effect on neurohormonal regulation in appetite control and metabolic rate, which may be a limiting factor.
CASE POINT Due to Deb’s intolerance of and embarrassment with GI adverse effects, she requests an alternative medication.
Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist that reduces appetite by affecting anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Of note, lorcaserin “selects” the 2C receptor instead of 2A and 2B; 2B receptors are found in both aortic and mitral valves, which may explain the association between fenfluramine/phentermine (commonly known as “fen/phen” and withdrawn from the market in 1997) and possible cardiac valvulopathy. (Fenfluramine is an amphetamine derivative that nonselectively stimulates serotonin release and inhibits reuptake.)
Lorcaserin comes in a 10-mg twice-daily dosage. In studies, patients taking lorcaserin had an average weight loss of 3.3% more than those taking placebo at one year; weight loss was maintained through the second year for those who continued on medication. However, those who stopped the medication at one year had regained their weight by the two-year mark.4
It is recommended that the medication be discontinued if patients don’t achieve a loss of more than 5% of body weight by 12 weeks.
In a study that enrolled diabetic patients, lorcaserin also demonstrated a 0.9% reduction in A1C, which is similar to or even better than some oral antidiabetic medications.4 However, since the manufacturer was not planning for an antidiabetic indication, A1C was only a secondary endpoint. The reduction is most likely due to decreased caloric intake and weight loss.
The most common adverse effects of lorcaserin include headache, dizziness, and fatigue. The discontinuation rate due to intolerance was 8.6%, compared to 6.7% with placebo.5
Although this was not observed in clinical studies, co-administration of lorcaserin (a serotonin receptor agonist) with other serotonergic or antidopaminergic agents can theoretically cause serotonin syndrome or neuroleptic malignant syndrome–like reactions. Caution is therefore advisable when prescribing these agents. The package insert carries a warning for cardiac valvulopathy due to fen/phen’s history and a lack of long-term cardiovascular safety data.
CASE POINT Deb is taking paroxetine (an SSRI) for her depression. Since you are concerned about serotonin syndrome, you decide to keep exploring options. Checking the package insert for phentermine/topiramate, you learn that it does not have a potential adverse reaction related to co-administration with SSRIs.
Phentermine/Topiramate
Phentermine, a sympathomimetic medication, was approved for short-term (12-week) use for weight loss in the 1960s. Topiramate, an antiseizure and migraine prophylactic medication, enhances appetite suppression—although the exact mechanism of action is unknown.1
Four once-daily doses are available: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, and 15/92 mg. Dosing starts with 3.75/23 mg for two weeks, then increases to 7.5/46 mg. If a loss of 5% or more of body weight is achieved, the patient can continue the dosage; if not, it can be increased to 11.25/69 mg for two weeks and then to 15/92 mg. The average weight loss for mid and maximum dose was 6.6% and 8.6% greater than placebo at one year.5
Commonly reported adverse effects include paraesthesia, dysgeusia (distortion of sense of taste), dizziness, insomnia, constipation, and dry mouth. Due to phentermine’s sympathomimetic action, mild increases in heart rate and blood pressure were reported. The Endocrine Society recommends against the use of phentermine in patients with uncontrolled hypertension and a history of heart disease.1
Weight loss is generally not recommended during pregnancy, and all weight loss medications are classified as category X for pregnancy. Strict caution is advised with this particular agent, as topiramate has known teratogenicity and therefore comes with a Risk Evaluation Mitigation Strategy. Patients must be advised to use appropriate contraception while taking topiramate, and a pregnancy test should be performed before medication commencement and monthly thereafter.
Abrupt cessation of topiramate can cause seizure. When taking the 15/92-mg dosage, the patient should reduce to one tablet every other day for at least one week before discontinuation.
CASE POINT Deb’s blood pressure is still not at goal. This, along with her history of atrial fibrillation and high pulse, prompts you to consider another option.
Bupropion/Naltrexone
Bupropion, a widely used antidepressant, inhibits the uptake of norepinephrine and dopamine and thereby blocks the reward pathway that various foods can induce. Naltrexone, an opioid antagonist, blocks the opioid pathway and can be helpful in enhancing weight loss.
This combination comes in an 8/90-mg tablet. The suggested titration regimen is to start with one tablet per day and increase by one tablet every week, up to the maximum dosage of two tablets twice a day. Average weight loss was 3.1% greater than placebo at one year with the maximum dosage. An A1C reduction of 0.6% was seen in diabetic patients.6 It is recommended to stop the medication and seek an alternative treatment option if > 5% loss of body weight is not achieved by 12 weeks.
GI adverse effects (eg, nausea and vomiting) are common; these can be reduced with a slower titration regimen or by prescribing a maximum of one tablet twice daily (instead of two). Every antidepressant carries suicidal risk, and caution is advised with their use. Bupropion can also lower the seizure threshold, and it is contraindicated for patients with seizure disorder. It is also contraindicated in patients who are undergoing abrupt cessation of alcohol, benzodiazepines, or barbiturates. It can increase pulse and blood pressure during early titration; regular blood pressure monitoring is warranted.
CASE POINT Due to Deb’s opioid usage and uncontrolled hypertension, you discuss a final option that was recently approved for weight loss.
Liraglutide
This glucagon-like peptide-1 (GLP1) receptor agonist affects the brain to suppress/control appetite, slows down gastric emptying, and induces early satiety. A 3-mg dosage was approved in December 2014, but 0.6-, 1.2-, and 1.8-mg dosages have been available since 2010 for patients with type 2 diabetes.
Average weight loss was 4.5% greater than placebo at one year.7 If < 4% weight loss is achieved by 16 weeks, consider using an alternative agent.
The most common adverse effect is GI upset, which could be related to the mechanism of action (slower gastric emptying). Although self-reported GI upset was high (39%), the actual discontinuation rate was low (2.9% for nausea, 1.7% for vomiting, and 1.4% for diarrhea).3
This adverse effect could, in certain contexts, be considered “wanted,” since it discourages overeating or eating too quickly. My clinical pearl is to tell patients taking liraglutide that they are “trapped” and have to eat smaller portions and eat more slowly or they will be more prone to GI effects. With this strategy, we can encourage portion control and responsibility for behavior. (Please note that this is my experience with the diabetic dosage of liraglutide; I do not have any clinical experience with the obesity dosage, which was not clinically available at the time of writing.)
Both branded versions of liraglutide carry a black-box warning for thyroid C-cell tumors, which were observed in rodents but unproven in humans. The medication is contraindicated in patients with medullary thyroid cancer or with multiple endocrine neoplasia 2 syndrome. Increased rates of acute pancreatitis, cholecystitis, and cholelithiasis were seen in studies, and caution is advised.
Continue for A Word About Meds That Cause Weight Gain >>
A WORD ABOUT MEDS THAT CAUSE WEIGHT GAIN
The Endocrine Society has published a list of medications that can influence weight gain, along with suggestions for alternative agents that are either weight neutral or promote weight loss.
Note that our case patient, Deb, is taking insulin, a sulfonylurea (glyburide), and thiazolidinedione (pioglitazone) for diabetes—all of which can promote weight gain. Guidelines suggest choosing metformin, DPP4 inhibitors, GLP1 agonists, amylin analog, and SGLT2 inhibitors instead when weight gain is a major concern.1
Guidelines also suggest using ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers instead of β-blockers as firstline antihypertensive therapy for diabetic patients.1 Adequate blood pressure and lipid control are imperative in diabetes management.
CASE POINT Deb would need better hypertension control before she considers weight-loss medication. Since she is also taking paroxetine, which among SSRIs is associated with greatest weight gain, a changed to fluoxetine or sertraline should be considered.2
Next page: Conclusion >>
CONCLUSION
There are now five medications approved by the FDA for chronic weight loss, with more to come. Agents with different mechanisms of action give us options to help obese patients and hopefully reduce and prevent obesity-related complications. It is important for clinicians to be competent in managing obesity, especially since we live in an era in which the disease is considered pandemic.
REFERENCES
1. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
2. Xenical [package insert]. South San Francisco, CA: Genentech USA, Inc; 2012.
3. Fujioka K. Safety and tolerability of medications approved for chronic weight management. Obesity (Silver Spring). 2015;22 (suppl 1):S7-S11.
4. Belviq [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2015.
5. Qsymia [package insert]. Mountain View, CA: Vivus, Inc; 2013.
6. Contrave [package insert]. Deerfield, IL: Takeda USA, Inc; 2014.
7. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk; 2014.
In June 2013, the American Medical Association classified obesity as a disease. Since then, several medical societies have published guidelines to help clinicians improve care of affected patients. One avenue is, of course, pharmacologic treatment.
Until recently, there was only one FDA-approved medication for chronic weight loss on the market: orlistat, which was approved in 1999. (Phentermine and diethylpropion are only indicated for short-term use). After a long hiatus, the FDA approved two additional agents (phentermine/topiramate and lorcaserin)in 2012 and another two (liraglutide and bupropion/naltrexone) in 2014.
While clinicians appreciate having options for managing their patients’ conditions, in this case, many are overwhelmed by the choices. Most health care providers have not received formal training in obesity management. This column will attempt to fill the information gap in terms of what agents are available and what factors should be assessed before prescribing any of them.
Proviso: Experts claim obesity is a chronic disease, similar to hypertension, and should be managed as such. Although not discussed here, the most important aspect of weight loss and maintenance is lifestyle intervention (diet, exercise, and behavioral modification). It should be emphasized that no medication works by itself; all should be used as an adjunct tool to reinforce adherence to lifestyle changes.1 Furthermore, patients may be disappointed to learn that without these changes, the weight may return when they cease medication use.
CASE Deb, age 61, presents to your office for routine follow-up. She has a history of type 2 diabetes, dyslipidemia, hypertension, atrial fibrillation, depression, and chronic back pain due to a herniated disc. Her medications include insulin glargine, glyburide, pioglitazone, atorvastatin, metoprolol, paroxetine, and acetaminophen/hydrocodone.
Her vital signs include a blood pressure of 143/91 mm Hg and a pulse of 93 beats/min. She has a BMI of 37 and a waist circumference of 35 in.
Deb, concerned about her weight, would like to discuss weight-loss options. She has tried three different commercial programs; each time, she was able to lose 30 to 50 lb in three to six months but regained the weight once she stopped the program. She reports excessive appetite as the main reason for her rebound weight gain. Her exercise is limited due to her back pain.
She recently tried OTC orlistat but could not tolerate it due to flatulence and fecal urgency. She reports an incident in which she couldn’t reach the bathroom in time.
Continue for Discussion >>
DISCUSSION
The Endocrine Society’s recommended approaches to obesity management include diet, exercise, and behavioral modification for patients with a BMI ≥ 25. The addition of pharmacotherapy can be considered for those with a BMI ≥ 30 or with a BMI ≥ 27 and one or more weight-related comorbidities (eg, diabetes, dyslipidemia, hypertension). This matches the FDA-approved product labeling for chronic weight-loss medications. Bariatric surgery should be considered for patients with a BMI ≥ 40 or with a BMI ≥ 35 and at least one weight-related comorbidity.
Orlistat
Orlistat is available OTC in a 60-mg thrice-daily form. A higher dosage (120 mg tid) is available via prescription. Orlistat decreases fat absorption in the gastrointestinal (GI) tract by inhibiting GI lipase. Average weight loss with orlistat is 3% at first and second year, and, when compared with placebo, 2.4% greater at four years.2
Orlistat should be prescribed with a multivitamin due to decreased absorption of fat-soluble vitamins. It is contraindicated in patients with malabsorption syndrome and gallbladder disease (> 2% incidence3). It can increase cyclosporine exposure, and rare cases of liver failure have been reported. The most common adverse effect is related to steatorrhea. Of the available options, orlistat is the only medication that has no effect on neurohormonal regulation in appetite control and metabolic rate, which may be a limiting factor.
CASE POINT Due to Deb’s intolerance of and embarrassment with GI adverse effects, she requests an alternative medication.
Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist that reduces appetite by affecting anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. Of note, lorcaserin “selects” the 2C receptor instead of 2A and 2B; 2B receptors are found in both aortic and mitral valves, which may explain the association between fenfluramine/phentermine (commonly known as “fen/phen” and withdrawn from the market in 1997) and possible cardiac valvulopathy. (Fenfluramine is an amphetamine derivative that nonselectively stimulates serotonin release and inhibits reuptake.)
Lorcaserin comes in a 10-mg twice-daily dosage. In studies, patients taking lorcaserin had an average weight loss of 3.3% more than those taking placebo at one year; weight loss was maintained through the second year for those who continued on medication. However, those who stopped the medication at one year had regained their weight by the two-year mark.4
It is recommended that the medication be discontinued if patients don’t achieve a loss of more than 5% of body weight by 12 weeks.
In a study that enrolled diabetic patients, lorcaserin also demonstrated a 0.9% reduction in A1C, which is similar to or even better than some oral antidiabetic medications.4 However, since the manufacturer was not planning for an antidiabetic indication, A1C was only a secondary endpoint. The reduction is most likely due to decreased caloric intake and weight loss.
The most common adverse effects of lorcaserin include headache, dizziness, and fatigue. The discontinuation rate due to intolerance was 8.6%, compared to 6.7% with placebo.5
Although this was not observed in clinical studies, co-administration of lorcaserin (a serotonin receptor agonist) with other serotonergic or antidopaminergic agents can theoretically cause serotonin syndrome or neuroleptic malignant syndrome–like reactions. Caution is therefore advisable when prescribing these agents. The package insert carries a warning for cardiac valvulopathy due to fen/phen’s history and a lack of long-term cardiovascular safety data.
CASE POINT Deb is taking paroxetine (an SSRI) for her depression. Since you are concerned about serotonin syndrome, you decide to keep exploring options. Checking the package insert for phentermine/topiramate, you learn that it does not have a potential adverse reaction related to co-administration with SSRIs.
Phentermine/Topiramate
Phentermine, a sympathomimetic medication, was approved for short-term (12-week) use for weight loss in the 1960s. Topiramate, an antiseizure and migraine prophylactic medication, enhances appetite suppression—although the exact mechanism of action is unknown.1
Four once-daily doses are available: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, and 15/92 mg. Dosing starts with 3.75/23 mg for two weeks, then increases to 7.5/46 mg. If a loss of 5% or more of body weight is achieved, the patient can continue the dosage; if not, it can be increased to 11.25/69 mg for two weeks and then to 15/92 mg. The average weight loss for mid and maximum dose was 6.6% and 8.6% greater than placebo at one year.5
Commonly reported adverse effects include paraesthesia, dysgeusia (distortion of sense of taste), dizziness, insomnia, constipation, and dry mouth. Due to phentermine’s sympathomimetic action, mild increases in heart rate and blood pressure were reported. The Endocrine Society recommends against the use of phentermine in patients with uncontrolled hypertension and a history of heart disease.1
Weight loss is generally not recommended during pregnancy, and all weight loss medications are classified as category X for pregnancy. Strict caution is advised with this particular agent, as topiramate has known teratogenicity and therefore comes with a Risk Evaluation Mitigation Strategy. Patients must be advised to use appropriate contraception while taking topiramate, and a pregnancy test should be performed before medication commencement and monthly thereafter.
Abrupt cessation of topiramate can cause seizure. When taking the 15/92-mg dosage, the patient should reduce to one tablet every other day for at least one week before discontinuation.
CASE POINT Deb’s blood pressure is still not at goal. This, along with her history of atrial fibrillation and high pulse, prompts you to consider another option.
Bupropion/Naltrexone
Bupropion, a widely used antidepressant, inhibits the uptake of norepinephrine and dopamine and thereby blocks the reward pathway that various foods can induce. Naltrexone, an opioid antagonist, blocks the opioid pathway and can be helpful in enhancing weight loss.
This combination comes in an 8/90-mg tablet. The suggested titration regimen is to start with one tablet per day and increase by one tablet every week, up to the maximum dosage of two tablets twice a day. Average weight loss was 3.1% greater than placebo at one year with the maximum dosage. An A1C reduction of 0.6% was seen in diabetic patients.6 It is recommended to stop the medication and seek an alternative treatment option if > 5% loss of body weight is not achieved by 12 weeks.
GI adverse effects (eg, nausea and vomiting) are common; these can be reduced with a slower titration regimen or by prescribing a maximum of one tablet twice daily (instead of two). Every antidepressant carries suicidal risk, and caution is advised with their use. Bupropion can also lower the seizure threshold, and it is contraindicated for patients with seizure disorder. It is also contraindicated in patients who are undergoing abrupt cessation of alcohol, benzodiazepines, or barbiturates. It can increase pulse and blood pressure during early titration; regular blood pressure monitoring is warranted.
CASE POINT Due to Deb’s opioid usage and uncontrolled hypertension, you discuss a final option that was recently approved for weight loss.
Liraglutide
This glucagon-like peptide-1 (GLP1) receptor agonist affects the brain to suppress/control appetite, slows down gastric emptying, and induces early satiety. A 3-mg dosage was approved in December 2014, but 0.6-, 1.2-, and 1.8-mg dosages have been available since 2010 for patients with type 2 diabetes.
Average weight loss was 4.5% greater than placebo at one year.7 If < 4% weight loss is achieved by 16 weeks, consider using an alternative agent.
The most common adverse effect is GI upset, which could be related to the mechanism of action (slower gastric emptying). Although self-reported GI upset was high (39%), the actual discontinuation rate was low (2.9% for nausea, 1.7% for vomiting, and 1.4% for diarrhea).3
This adverse effect could, in certain contexts, be considered “wanted,” since it discourages overeating or eating too quickly. My clinical pearl is to tell patients taking liraglutide that they are “trapped” and have to eat smaller portions and eat more slowly or they will be more prone to GI effects. With this strategy, we can encourage portion control and responsibility for behavior. (Please note that this is my experience with the diabetic dosage of liraglutide; I do not have any clinical experience with the obesity dosage, which was not clinically available at the time of writing.)
Both branded versions of liraglutide carry a black-box warning for thyroid C-cell tumors, which were observed in rodents but unproven in humans. The medication is contraindicated in patients with medullary thyroid cancer or with multiple endocrine neoplasia 2 syndrome. Increased rates of acute pancreatitis, cholecystitis, and cholelithiasis were seen in studies, and caution is advised.
Continue for A Word About Meds That Cause Weight Gain >>
A WORD ABOUT MEDS THAT CAUSE WEIGHT GAIN
The Endocrine Society has published a list of medications that can influence weight gain, along with suggestions for alternative agents that are either weight neutral or promote weight loss.
Note that our case patient, Deb, is taking insulin, a sulfonylurea (glyburide), and thiazolidinedione (pioglitazone) for diabetes—all of which can promote weight gain. Guidelines suggest choosing metformin, DPP4 inhibitors, GLP1 agonists, amylin analog, and SGLT2 inhibitors instead when weight gain is a major concern.1
Guidelines also suggest using ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers instead of β-blockers as firstline antihypertensive therapy for diabetic patients.1 Adequate blood pressure and lipid control are imperative in diabetes management.
CASE POINT Deb would need better hypertension control before she considers weight-loss medication. Since she is also taking paroxetine, which among SSRIs is associated with greatest weight gain, a changed to fluoxetine or sertraline should be considered.2
Next page: Conclusion >>
CONCLUSION
There are now five medications approved by the FDA for chronic weight loss, with more to come. Agents with different mechanisms of action give us options to help obese patients and hopefully reduce and prevent obesity-related complications. It is important for clinicians to be competent in managing obesity, especially since we live in an era in which the disease is considered pandemic.
REFERENCES
1. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
2. Xenical [package insert]. South San Francisco, CA: Genentech USA, Inc; 2012.
3. Fujioka K. Safety and tolerability of medications approved for chronic weight management. Obesity (Silver Spring). 2015;22 (suppl 1):S7-S11.
4. Belviq [package insert]. Woodcliff Lake, NJ: Eisai Inc; 2015.
5. Qsymia [package insert]. Mountain View, CA: Vivus, Inc; 2013.
6. Contrave [package insert]. Deerfield, IL: Takeda USA, Inc; 2014.
7. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk; 2014.
Woman Complains of Knee Pain Following Fight
ANSWER
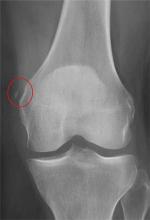
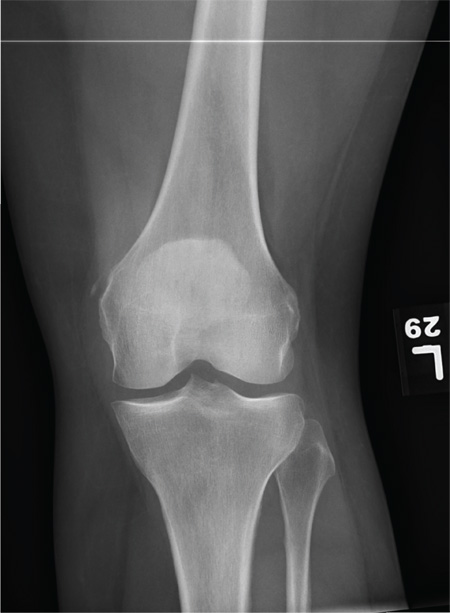
The radiograph shows a small calcification along the medial aspect of the medial collateral ligament. This finding is known as a Pellegrini-Stieda lesion. While it certainly could represent a small avulsion fracture, the lack of joint fluid and soft-tissue swelling makes this diagnosis less likely. The patient was treated symptomatically with anti-inflammatory medications.
ANSWER
The radiograph shows a small calcification along the medial aspect of the medial collateral ligament. This finding is known as a Pellegrini-Stieda lesion. While it certainly could represent a small avulsion fracture, the lack of joint fluid and soft-tissue swelling makes this diagnosis less likely. The patient was treated symptomatically with anti-inflammatory medications.
ANSWER
The radiograph shows a small calcification along the medial aspect of the medial collateral ligament. This finding is known as a Pellegrini-Stieda lesion. While it certainly could represent a small avulsion fracture, the lack of joint fluid and soft-tissue swelling makes this diagnosis less likely. The patient was treated symptomatically with anti-inflammatory medications.
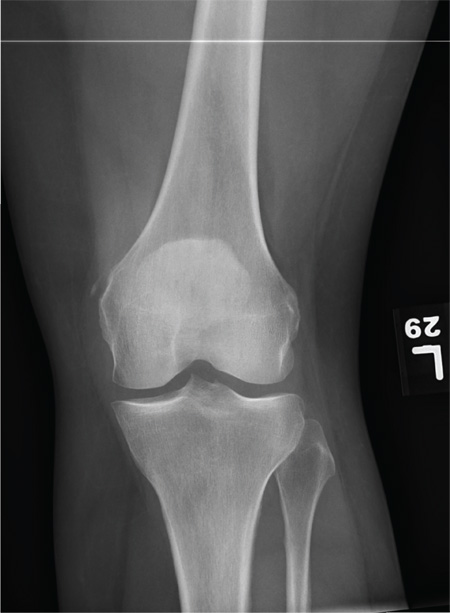
A 35-year-old woman presents for evaluation of left knee pain secondary to an assault. She says she was involved in a fight and was struck multiple times throughout her whole body. She states she is “sore all over,” but her knee bothers her the most, as it is difficult and painful to bear weight. The patient’s medical history is unremarkable. Physical exam shows a young female who is uncomfortable but in no obvious distress. Her vital signs are normal. You note bruises throughout her body. Inspection of her left knee shows no obvious deformity or swelling. There is some mild bruising and pain present to palpation. She has limited flexion and extension secondary to pain. However, the joint itself appears stable. Radiographs of the knee are obtained. What is your impression?
Neighbor Finds Man on Knees, Vomiting
ANSWER
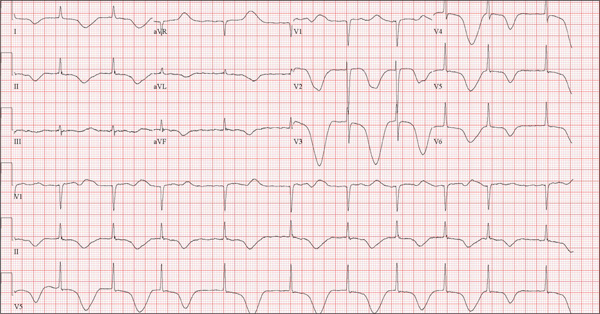
The correct interpretation of this ECG includes atrial fibrillation and ST- and T-wave changes consistent with a central nervous system hemorrhage, as well as a markedly prolonged QT interval.
The most common ECG alterations seen in cases of acute subarachnoid hemorrhage include repolarization abnormalities due to imbalance of autonomic cardiovascular control. While ST depression is more common in patients with poor outcomes, it is not predictive.
ANSWER
The correct interpretation of this ECG includes atrial fibrillation and ST- and T-wave changes consistent with a central nervous system hemorrhage, as well as a markedly prolonged QT interval.
The most common ECG alterations seen in cases of acute subarachnoid hemorrhage include repolarization abnormalities due to imbalance of autonomic cardiovascular control. While ST depression is more common in patients with poor outcomes, it is not predictive.
ANSWER
The correct interpretation of this ECG includes atrial fibrillation and ST- and T-wave changes consistent with a central nervous system hemorrhage, as well as a markedly prolonged QT interval.
The most common ECG alterations seen in cases of acute subarachnoid hemorrhage include repolarization abnormalities due to imbalance of autonomic cardiovascular control. While ST depression is more common in patients with poor outcomes, it is not predictive.
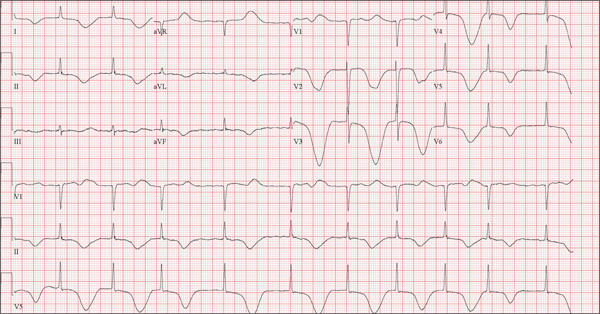
A 68-year-old man was found on his knees, vomiting, in his backyard by a neighbor early this morning. When asked if he was OK, the patient responded that he suddenly felt nauseated and had a headache. The neighbor helped him to his feet and, noticing that his left leg and arm were weak, called 911. During that time, the neighbor observed slurred speech. As they waited for the ambulance to arrive, the patient was able to say that this was the worst headache he’d ever had. When the paramedics arrived, they recorded a blood pressure of 198/120 mm Hg, consistent in both arms. Medical history is remarkable for hypertension, paroxysmal atrial fibrillation, adult-onset diabetes, and nephrolithiasis. Surgical history is remarkable for appendectomy, cholecystectomy, and lithotripsy. The patient is a retired civil engineer who lives at home alone. His wife died three years ago of complications from uterine cancer. He has one adult son who is in excellent health but lives on the opposite coast. The patient has never smoked and drinks approximately one six-pack of beer per month. Current medications include hydrochlorothiazide, metformin, and rivaroxaban. He is allergic to sulfa-containing medications. A complete review of systems is not obtained, given the patient’s slurred speech and progressive aphasia. He is able to nod yes or no to specific questions, and no significant symptoms or findings are identified. At the hospital, the patient is agitated and in apparent distress, with severe headache and aphasia. Vital signs include a blood pressure of 180/110 mm Hg; pulse, 60 beats/min; respiratory rate, 14 breaths/min; and temperature, 98.4°F. The O2 saturation is 96.4% on room air. Pertinent physical findings include aphasia, oculomotor nerve palsy, nuchal rigidity, and findings of a left-sided (right hemispheric) progressive hemiparesis. The remainder of the physical examination is noncontributory. Samples are drawn for laboratory testing, and an ECG is obtained prior to the patient’s transfer to radiology for CT of the head. The ECG findings include a ventricular rate of 62 beats/min; QRS duration, 88 ms; QT/QTc interval, 718/728 ms; P axis, not measured; R axis, 35°; and T axis, 209°. What is your interpretation of this ECG?
Healthcare Industry Agents of Change Promote Responsible Spending
1 Caring Wisely Program
http://healthvalue.ucsf.edu/caring-wisely
- Started in 2012 within the division of hospital medicine at the University of California, San Francisco (UCSF), the program sponsored or collaborated on six high-value care projects within its first year. “We don’t shy away from the fact that part of what we do is address cost, but it is about making sure that we’ve got the right mindset and right frame, which is that we’re going to improve quality while decreasing costs and keeping it really patient centered,” says Christopher Moriates, MD, program director and an assistant clinical professor.
- Beyond its successful Nebs No More After 24 project, Caring Wisely helped hospital pharmacists and the UCSF Medication Outcomes Center develop and implement an evidence-based initiative to cut inappropriate stress ulcer prophylaxis in intensive care unit patients. After its first month, the program had cut unnecessary use of the medication from 19% to 6.6%.
2 Choosing Wisely Program
- Launched in 2012 as an initiative of the ABIM Foundation and based on a pilot project by the National Physicians Alliance, Choosing Wisely was designed to encourage more proactive conversations between providers and patients. The goal is to help patients choose care that is both evidence-based and necessary, while minimizing harm and avoiding duplication of tests or procedures.
- Since its debut, the program has gathered nearly 60 specialty society lists of “Five Things Physicians and Patients Should Question,” including two lists compiled by SHM for adult and pediatric hospital medicine. As a complement, Consumer Reports and many of the specialty societies have collaborated on 75 patient-friendly reports that dispense advice about whether a test, treatment, or procedure is really needed.
3 Costs of Care
- Founded in 2009 by Neel Shah, MD, an assistant professor at Harvard Medical School, the nonprofit got its start by collecting stories from patients and physicians about unnecessary or inflated healthcare costs. “It had a manifesto about what the role of physicians ought to be and thinking about healthcare costs, and that message actually really resonated with a lot of people,” Dr. Shah says. “That basic message that we decide what goes on the bill, patients have to pay for it, and yet we don’t know what it’s costing them—that just seemed crazy and we heard from a lot of people, both from patients with whom that message resonated and physicians who were like, ‘Yeah.’”
- In 2010, the organizers hosted their first essay contest and ended up receiving more than 300 entries; several were subsequently included as case reports in a report on healthcare waste by the Institute of Medicine. The nonprofit, supported by the ABIM Foundation and other institutions, has since led to an educational venture called the Teaching Value Project, a textbook titled Understanding Value-Based Care (McGraw-Hill), and a “Costs of Care” iPhone app—all designed to help clinicians make high-value clinical decisions and increase price transparency.
4 The Do No Harm Project
http://www.ucdenver.edu/academics/colleges/medicalschool/departments/medicine/
GIM/education/DoNoHarmProject/Pages/Welcome.aspx
- Launched in 2012 at the University of Colorado by Brandon Combs, MD, and Tanner Caverly, MD, MPH, the project is aimed at medical trainees. Starting with the internal medicine program, the physicians asked medical residents to reflect on a patient who had suffered an adverse consequence from medical overuse. “This was reasonable care that was nevertheless unneeded or unwanted by a fully informed patient,” Dr. Combs says. “So this isn’t errors or malpractice; this is the stuff that flies under the radar, the stuff that people might miss.”
- The project uses clinical vignettes written by medical trainees (including those found in the “Teachable Moments” section of JAMA Internal Medicine) to improve the recognition of potential harm from overuse and to spur a culture change. In 2013, the Teaching Value and Choosing Wisely Competition, jointly sponsored by Costs of Care and the ABIM Foundation, recognized the project as one of its Innovations award winners; so far, five internal medicine and emergency medicine programs around the country have adopted the model.
5 I-CARE
- The Interactive Cost-Awareness Resident Exercise (I-CARE) was launched in 2011 by Yale hospitalist Robert Fogerty, MD, MPH, and colleagues. The friendly competition among medical students, interns, residents, and attending physicians uses a traditional morning report structure and charge data. At these conferences, the providers compete to come up with the correct diagnosis using the fewest resources possible. In 2013, the Teaching Value and Choosing Wisely competition, jointly sponsored by Costs of Care and the ABIM Foundation, recognized I-CARE as one of its Innovations award winners.
- “Physicians tend not to have a lot of business training,” Dr. Fogerty says. “They don’t have a lot of financial training. They don’t have a lot of economics background, and when you tell them that healthcare expense is 18% of GDP [gross domestic product], they don’t really know what that means. When you tell them that that would be in the top 10 of world economies, now they’re starting to get a picture of it. And when you tell them that that CAT scan you just ordered is going to cost your patient $1,200, that’s an eye-opening number that they can understand. So I think the purpose behind I-CARE was to take this seemingly insurmountable problem and to begin to digest it into small enough bits of information that allowed this problem to be accessible to the trainees.”
6 Providers for Responsible Ordering (PRO)
www.providersforresponsibleordering.org
- The organization launched in 2009 with a mission to “promote high-value care and create a culture that minimizes unnecessary or potentially-harmful diagnostic tests and interventions.” By the end of 2014, five chapters had been established and more than 150 providers had signed the PRO pledge that asks signatories, in part, “to provide my patients with all of the care that they need and none that they do not, thereby protecting them from unnecessary diagnostic tests and treatments.”
- “Our model is simple and yet powerful. It’s a grass-roots effort that any interested provider can join, and it builds on a peer-to-peer approach of establishment of chapters that solve local problems and reporting of those solutions back to the national group,” says Anthony Accurso, MD, PRO faculty director at Johns Hopkins Bayview Medical Center in Baltimore.
1 Caring Wisely Program
http://healthvalue.ucsf.edu/caring-wisely
- Started in 2012 within the division of hospital medicine at the University of California, San Francisco (UCSF), the program sponsored or collaborated on six high-value care projects within its first year. “We don’t shy away from the fact that part of what we do is address cost, but it is about making sure that we’ve got the right mindset and right frame, which is that we’re going to improve quality while decreasing costs and keeping it really patient centered,” says Christopher Moriates, MD, program director and an assistant clinical professor.
- Beyond its successful Nebs No More After 24 project, Caring Wisely helped hospital pharmacists and the UCSF Medication Outcomes Center develop and implement an evidence-based initiative to cut inappropriate stress ulcer prophylaxis in intensive care unit patients. After its first month, the program had cut unnecessary use of the medication from 19% to 6.6%.
2 Choosing Wisely Program
- Launched in 2012 as an initiative of the ABIM Foundation and based on a pilot project by the National Physicians Alliance, Choosing Wisely was designed to encourage more proactive conversations between providers and patients. The goal is to help patients choose care that is both evidence-based and necessary, while minimizing harm and avoiding duplication of tests or procedures.
- Since its debut, the program has gathered nearly 60 specialty society lists of “Five Things Physicians and Patients Should Question,” including two lists compiled by SHM for adult and pediatric hospital medicine. As a complement, Consumer Reports and many of the specialty societies have collaborated on 75 patient-friendly reports that dispense advice about whether a test, treatment, or procedure is really needed.
3 Costs of Care
- Founded in 2009 by Neel Shah, MD, an assistant professor at Harvard Medical School, the nonprofit got its start by collecting stories from patients and physicians about unnecessary or inflated healthcare costs. “It had a manifesto about what the role of physicians ought to be and thinking about healthcare costs, and that message actually really resonated with a lot of people,” Dr. Shah says. “That basic message that we decide what goes on the bill, patients have to pay for it, and yet we don’t know what it’s costing them—that just seemed crazy and we heard from a lot of people, both from patients with whom that message resonated and physicians who were like, ‘Yeah.’”
- In 2010, the organizers hosted their first essay contest and ended up receiving more than 300 entries; several were subsequently included as case reports in a report on healthcare waste by the Institute of Medicine. The nonprofit, supported by the ABIM Foundation and other institutions, has since led to an educational venture called the Teaching Value Project, a textbook titled Understanding Value-Based Care (McGraw-Hill), and a “Costs of Care” iPhone app—all designed to help clinicians make high-value clinical decisions and increase price transparency.
4 The Do No Harm Project
http://www.ucdenver.edu/academics/colleges/medicalschool/departments/medicine/
GIM/education/DoNoHarmProject/Pages/Welcome.aspx
- Launched in 2012 at the University of Colorado by Brandon Combs, MD, and Tanner Caverly, MD, MPH, the project is aimed at medical trainees. Starting with the internal medicine program, the physicians asked medical residents to reflect on a patient who had suffered an adverse consequence from medical overuse. “This was reasonable care that was nevertheless unneeded or unwanted by a fully informed patient,” Dr. Combs says. “So this isn’t errors or malpractice; this is the stuff that flies under the radar, the stuff that people might miss.”
- The project uses clinical vignettes written by medical trainees (including those found in the “Teachable Moments” section of JAMA Internal Medicine) to improve the recognition of potential harm from overuse and to spur a culture change. In 2013, the Teaching Value and Choosing Wisely Competition, jointly sponsored by Costs of Care and the ABIM Foundation, recognized the project as one of its Innovations award winners; so far, five internal medicine and emergency medicine programs around the country have adopted the model.
5 I-CARE
- The Interactive Cost-Awareness Resident Exercise (I-CARE) was launched in 2011 by Yale hospitalist Robert Fogerty, MD, MPH, and colleagues. The friendly competition among medical students, interns, residents, and attending physicians uses a traditional morning report structure and charge data. At these conferences, the providers compete to come up with the correct diagnosis using the fewest resources possible. In 2013, the Teaching Value and Choosing Wisely competition, jointly sponsored by Costs of Care and the ABIM Foundation, recognized I-CARE as one of its Innovations award winners.
- “Physicians tend not to have a lot of business training,” Dr. Fogerty says. “They don’t have a lot of financial training. They don’t have a lot of economics background, and when you tell them that healthcare expense is 18% of GDP [gross domestic product], they don’t really know what that means. When you tell them that that would be in the top 10 of world economies, now they’re starting to get a picture of it. And when you tell them that that CAT scan you just ordered is going to cost your patient $1,200, that’s an eye-opening number that they can understand. So I think the purpose behind I-CARE was to take this seemingly insurmountable problem and to begin to digest it into small enough bits of information that allowed this problem to be accessible to the trainees.”
6 Providers for Responsible Ordering (PRO)
www.providersforresponsibleordering.org
- The organization launched in 2009 with a mission to “promote high-value care and create a culture that minimizes unnecessary or potentially-harmful diagnostic tests and interventions.” By the end of 2014, five chapters had been established and more than 150 providers had signed the PRO pledge that asks signatories, in part, “to provide my patients with all of the care that they need and none that they do not, thereby protecting them from unnecessary diagnostic tests and treatments.”
- “Our model is simple and yet powerful. It’s a grass-roots effort that any interested provider can join, and it builds on a peer-to-peer approach of establishment of chapters that solve local problems and reporting of those solutions back to the national group,” says Anthony Accurso, MD, PRO faculty director at Johns Hopkins Bayview Medical Center in Baltimore.
1 Caring Wisely Program
http://healthvalue.ucsf.edu/caring-wisely
- Started in 2012 within the division of hospital medicine at the University of California, San Francisco (UCSF), the program sponsored or collaborated on six high-value care projects within its first year. “We don’t shy away from the fact that part of what we do is address cost, but it is about making sure that we’ve got the right mindset and right frame, which is that we’re going to improve quality while decreasing costs and keeping it really patient centered,” says Christopher Moriates, MD, program director and an assistant clinical professor.
- Beyond its successful Nebs No More After 24 project, Caring Wisely helped hospital pharmacists and the UCSF Medication Outcomes Center develop and implement an evidence-based initiative to cut inappropriate stress ulcer prophylaxis in intensive care unit patients. After its first month, the program had cut unnecessary use of the medication from 19% to 6.6%.
2 Choosing Wisely Program
- Launched in 2012 as an initiative of the ABIM Foundation and based on a pilot project by the National Physicians Alliance, Choosing Wisely was designed to encourage more proactive conversations between providers and patients. The goal is to help patients choose care that is both evidence-based and necessary, while minimizing harm and avoiding duplication of tests or procedures.
- Since its debut, the program has gathered nearly 60 specialty society lists of “Five Things Physicians and Patients Should Question,” including two lists compiled by SHM for adult and pediatric hospital medicine. As a complement, Consumer Reports and many of the specialty societies have collaborated on 75 patient-friendly reports that dispense advice about whether a test, treatment, or procedure is really needed.
3 Costs of Care
- Founded in 2009 by Neel Shah, MD, an assistant professor at Harvard Medical School, the nonprofit got its start by collecting stories from patients and physicians about unnecessary or inflated healthcare costs. “It had a manifesto about what the role of physicians ought to be and thinking about healthcare costs, and that message actually really resonated with a lot of people,” Dr. Shah says. “That basic message that we decide what goes on the bill, patients have to pay for it, and yet we don’t know what it’s costing them—that just seemed crazy and we heard from a lot of people, both from patients with whom that message resonated and physicians who were like, ‘Yeah.’”
- In 2010, the organizers hosted their first essay contest and ended up receiving more than 300 entries; several were subsequently included as case reports in a report on healthcare waste by the Institute of Medicine. The nonprofit, supported by the ABIM Foundation and other institutions, has since led to an educational venture called the Teaching Value Project, a textbook titled Understanding Value-Based Care (McGraw-Hill), and a “Costs of Care” iPhone app—all designed to help clinicians make high-value clinical decisions and increase price transparency.
4 The Do No Harm Project
http://www.ucdenver.edu/academics/colleges/medicalschool/departments/medicine/
GIM/education/DoNoHarmProject/Pages/Welcome.aspx
- Launched in 2012 at the University of Colorado by Brandon Combs, MD, and Tanner Caverly, MD, MPH, the project is aimed at medical trainees. Starting with the internal medicine program, the physicians asked medical residents to reflect on a patient who had suffered an adverse consequence from medical overuse. “This was reasonable care that was nevertheless unneeded or unwanted by a fully informed patient,” Dr. Combs says. “So this isn’t errors or malpractice; this is the stuff that flies under the radar, the stuff that people might miss.”
- The project uses clinical vignettes written by medical trainees (including those found in the “Teachable Moments” section of JAMA Internal Medicine) to improve the recognition of potential harm from overuse and to spur a culture change. In 2013, the Teaching Value and Choosing Wisely Competition, jointly sponsored by Costs of Care and the ABIM Foundation, recognized the project as one of its Innovations award winners; so far, five internal medicine and emergency medicine programs around the country have adopted the model.
5 I-CARE
- The Interactive Cost-Awareness Resident Exercise (I-CARE) was launched in 2011 by Yale hospitalist Robert Fogerty, MD, MPH, and colleagues. The friendly competition among medical students, interns, residents, and attending physicians uses a traditional morning report structure and charge data. At these conferences, the providers compete to come up with the correct diagnosis using the fewest resources possible. In 2013, the Teaching Value and Choosing Wisely competition, jointly sponsored by Costs of Care and the ABIM Foundation, recognized I-CARE as one of its Innovations award winners.
- “Physicians tend not to have a lot of business training,” Dr. Fogerty says. “They don’t have a lot of financial training. They don’t have a lot of economics background, and when you tell them that healthcare expense is 18% of GDP [gross domestic product], they don’t really know what that means. When you tell them that that would be in the top 10 of world economies, now they’re starting to get a picture of it. And when you tell them that that CAT scan you just ordered is going to cost your patient $1,200, that’s an eye-opening number that they can understand. So I think the purpose behind I-CARE was to take this seemingly insurmountable problem and to begin to digest it into small enough bits of information that allowed this problem to be accessible to the trainees.”
6 Providers for Responsible Ordering (PRO)
www.providersforresponsibleordering.org
- The organization launched in 2009 with a mission to “promote high-value care and create a culture that minimizes unnecessary or potentially-harmful diagnostic tests and interventions.” By the end of 2014, five chapters had been established and more than 150 providers had signed the PRO pledge that asks signatories, in part, “to provide my patients with all of the care that they need and none that they do not, thereby protecting them from unnecessary diagnostic tests and treatments.”
- “Our model is simple and yet powerful. It’s a grass-roots effort that any interested provider can join, and it builds on a peer-to-peer approach of establishment of chapters that solve local problems and reporting of those solutions back to the national group,” says Anthony Accurso, MD, PRO faculty director at Johns Hopkins Bayview Medical Center in Baltimore.
Practical Approaches in the Management of Bipolar Depression: Overcoming Challenges and Avoiding Pitfalls
In the past 2 decades, the burden of care for psychiatric complaints in primary care—including bipolar depression—has increased considerably. The prevalence of bipolar disorder (BPD) in primary care has been recently estimated to range up to 4.3%, and in studies with broader definitions of the disorder or in populations with higher-than-usual psychiatric disorders, the prevalence has been reported to be up to 11.4%. Even though BPD is seen commonly in primary care, there are still profound disparities in the delivery of care, including underdiagnosis, misdiagnosis, and inappropriate treatments. There is abundant evidence that BPD can be successfully managed in the primary care setting when adequate physician education, collaborative care teams, and patient education are employed. Efficacious and well-tolerated pharmacologic treatments for BPD are available, and evidence-based pharmacotherapy can be optimally managed by the primary care provider. In this supplement, experts in BPD discuss the recognition and management of bipolar depression and associated comorbidities in the primary care setting.
In the past 2 decades, the burden of care for psychiatric complaints in primary care—including bipolar depression—has increased considerably. The prevalence of bipolar disorder (BPD) in primary care has been recently estimated to range up to 4.3%, and in studies with broader definitions of the disorder or in populations with higher-than-usual psychiatric disorders, the prevalence has been reported to be up to 11.4%. Even though BPD is seen commonly in primary care, there are still profound disparities in the delivery of care, including underdiagnosis, misdiagnosis, and inappropriate treatments. There is abundant evidence that BPD can be successfully managed in the primary care setting when adequate physician education, collaborative care teams, and patient education are employed. Efficacious and well-tolerated pharmacologic treatments for BPD are available, and evidence-based pharmacotherapy can be optimally managed by the primary care provider. In this supplement, experts in BPD discuss the recognition and management of bipolar depression and associated comorbidities in the primary care setting.
In the past 2 decades, the burden of care for psychiatric complaints in primary care—including bipolar depression—has increased considerably. The prevalence of bipolar disorder (BPD) in primary care has been recently estimated to range up to 4.3%, and in studies with broader definitions of the disorder or in populations with higher-than-usual psychiatric disorders, the prevalence has been reported to be up to 11.4%. Even though BPD is seen commonly in primary care, there are still profound disparities in the delivery of care, including underdiagnosis, misdiagnosis, and inappropriate treatments. There is abundant evidence that BPD can be successfully managed in the primary care setting when adequate physician education, collaborative care teams, and patient education are employed. Efficacious and well-tolerated pharmacologic treatments for BPD are available, and evidence-based pharmacotherapy can be optimally managed by the primary care provider. In this supplement, experts in BPD discuss the recognition and management of bipolar depression and associated comorbidities in the primary care setting.
Standard Text Messaging for Smartphones Not HIPAA Compliant
Doctors were the first to begin using pagers and, along with drug dealers, appear to be the last to give them up. But we really need to get rid of them.
Sadly, for the foreseeable future, we will need a pager replacement, but, in the longer term, I’m hopeful that we can:
- Reduce the frequency of electronic interruptions—all forms of interruptions—and the adverse effects that reliably accompany them, and
- Ensure that each interruption has value—that is, reduce or eliminate the many low value and non-urgent messages we all get (e.g. the ones informing you of a lab result you’ve already seen).
Death to the Pager
I can’t imagine anyone who will be more pleased than I will if pagers go the way of now rare hospital-wide PA announcements. Some hospitals have eliminated these announcements entirely, and even critical messages like “code blue” announcements are sent directly to each responder via a pager or other personal device.
Around the time the first iPhone was born, hospital signs banning cell phones began coming down. It seems the fear that they would disrupt hospital electronics, such as telemetry and other monitoring devices, has proven largely unfounded (though, along with things like computer keyboards and stethoscopes, pagers and cell phones can serve as dangerous repositories of bacteria).
Now nearly everyone, from staff to patients, keeps a cell phone with them while in the hospital. I think that is the most important step toward getting rid of pagers. Many doctors already are using the standard text messaging apps that come with the phone to communicate with one another efficiently.
“Regular” Texting Won’t Cut It
Unfortunately, the standard text messaging that comes with every smartphone is not HIPAA compliant. Though I certainly don’t know how anyone would do it, it is apparently too easy for another person to intercept the message. So, if you’re texting information related to your clinical work, you need to make sure it doesn’t include anything that could be considered protected health information. It isn’t enough just to leave the patient’s name off the message. If you’re in the habit of regularly texting doctors, nurses, and other healthcare personnel about patient care, you are at high risk of violating HIPAA, even if you try hard to avoid it.
Another big drawback is that there isn’t a good way to turn off work-related texting when you’re off duty, while leaving your texting app open for communication with your friends and family. Hospital staff will sometimes fail to check whether you’re on duty before texting, and that will lead to your personal time being interrupted by work reminders.
I think these shortcomings mean that none of us should rely on the standard text messaging apps that come with our phones.
But in order for a different app or service to be of any value, we will need to ensure that most providers associated with our hospital are on the same messaging system. That is a tall order, but fortunately there are a lot of companies trying to produce an attractive product that makes it as easy as possible to attract a critical mass of users at your institution.
HIPAA-Compliant Texting Vendors
Many healthcare tech companies provide secure messaging, usually at no additional cost, as an add-on to their main products, such as charge capture software (e.g. IngeniousMed), or physician social networking (e.g. Doximity). Something like 30 companies now offer a dedicated HIPAA-compliant texting option, including IM Your Doc, Voalte, Telmediq, PerfectServe, Vocera, and TigerText. There are so many that it is awfully tough to understand all of their strengths and shortcomings in detail, but I’m having fun trying to do just that. And I anticipate there will be significant consolidation in vendors within the next two to three years.
The dedicated HIPAA-compliant texting services range in price from free for basic features to a monthly fee per user that varies depending on the features you choose to enable. Some offer integration with the hospital’s EHR, which can let a message sender who only knows the patient’s name to see which doctor, nurse, or other caregiver is currently responsible for the patient. Some offer integration with a call schedule and answering service, or even replace an answering service.
No pager replacement will be viable if there are sites in the hospital or elsewhere where it is out of contact; a solution that works on both cellular networks and Wi-Fi is essential. Some vendors offer the ability for messages not delivered to or acknowledged by the recipient to escalate to other forms of delivery after a specified period of time.
I would love to see a feature that I don’t think any vendor offers yet. It would be great if all messages the sender hasn’t marked “stat” or “urgent” first went to a queue in the EHR rather than immediately interrupting the recipient. That way a doctor or other caregiver could see messages while already working in the EHR, rather than glancing at each new message as it arrives, something that all too often needlessly interrupts another important task such as talking with a patient.
And, since most work in EHRs is done in front of a larger device with a full keyboard, it would be easier to type a quick reply message than it would be to rely on a smartphone keyboard for return messaging. Protocols could be established such that messages waiting in the EHR without a reply or dismissal after a specified time would then be sent to the recipient’s personal device.
A Texting Ecosystem
In nearly every case, the hospital will select the text messaging vendor, though hospitalists and nurses, who will typically be among the highest-volume users, should participate in the decision. But the real value of the system hinges on ensuring its wide adoption by most, or nearly all, hospital caregivers and affiliated ambulatory providers.
I would enjoy hearing from those who are already using a HIPAA-secure texting and pager replacement service now, as well as those still researching their options. This has the potential to meaningfully change the way hospitalists and others do their work.

Doctors were the first to begin using pagers and, along with drug dealers, appear to be the last to give them up. But we really need to get rid of them.
Sadly, for the foreseeable future, we will need a pager replacement, but, in the longer term, I’m hopeful that we can:
- Reduce the frequency of electronic interruptions—all forms of interruptions—and the adverse effects that reliably accompany them, and
- Ensure that each interruption has value—that is, reduce or eliminate the many low value and non-urgent messages we all get (e.g. the ones informing you of a lab result you’ve already seen).
Death to the Pager
I can’t imagine anyone who will be more pleased than I will if pagers go the way of now rare hospital-wide PA announcements. Some hospitals have eliminated these announcements entirely, and even critical messages like “code blue” announcements are sent directly to each responder via a pager or other personal device.
Around the time the first iPhone was born, hospital signs banning cell phones began coming down. It seems the fear that they would disrupt hospital electronics, such as telemetry and other monitoring devices, has proven largely unfounded (though, along with things like computer keyboards and stethoscopes, pagers and cell phones can serve as dangerous repositories of bacteria).
Now nearly everyone, from staff to patients, keeps a cell phone with them while in the hospital. I think that is the most important step toward getting rid of pagers. Many doctors already are using the standard text messaging apps that come with the phone to communicate with one another efficiently.
“Regular” Texting Won’t Cut It
Unfortunately, the standard text messaging that comes with every smartphone is not HIPAA compliant. Though I certainly don’t know how anyone would do it, it is apparently too easy for another person to intercept the message. So, if you’re texting information related to your clinical work, you need to make sure it doesn’t include anything that could be considered protected health information. It isn’t enough just to leave the patient’s name off the message. If you’re in the habit of regularly texting doctors, nurses, and other healthcare personnel about patient care, you are at high risk of violating HIPAA, even if you try hard to avoid it.
Another big drawback is that there isn’t a good way to turn off work-related texting when you’re off duty, while leaving your texting app open for communication with your friends and family. Hospital staff will sometimes fail to check whether you’re on duty before texting, and that will lead to your personal time being interrupted by work reminders.
I think these shortcomings mean that none of us should rely on the standard text messaging apps that come with our phones.
But in order for a different app or service to be of any value, we will need to ensure that most providers associated with our hospital are on the same messaging system. That is a tall order, but fortunately there are a lot of companies trying to produce an attractive product that makes it as easy as possible to attract a critical mass of users at your institution.
HIPAA-Compliant Texting Vendors
Many healthcare tech companies provide secure messaging, usually at no additional cost, as an add-on to their main products, such as charge capture software (e.g. IngeniousMed), or physician social networking (e.g. Doximity). Something like 30 companies now offer a dedicated HIPAA-compliant texting option, including IM Your Doc, Voalte, Telmediq, PerfectServe, Vocera, and TigerText. There are so many that it is awfully tough to understand all of their strengths and shortcomings in detail, but I’m having fun trying to do just that. And I anticipate there will be significant consolidation in vendors within the next two to three years.
The dedicated HIPAA-compliant texting services range in price from free for basic features to a monthly fee per user that varies depending on the features you choose to enable. Some offer integration with the hospital’s EHR, which can let a message sender who only knows the patient’s name to see which doctor, nurse, or other caregiver is currently responsible for the patient. Some offer integration with a call schedule and answering service, or even replace an answering service.
No pager replacement will be viable if there are sites in the hospital or elsewhere where it is out of contact; a solution that works on both cellular networks and Wi-Fi is essential. Some vendors offer the ability for messages not delivered to or acknowledged by the recipient to escalate to other forms of delivery after a specified period of time.
I would love to see a feature that I don’t think any vendor offers yet. It would be great if all messages the sender hasn’t marked “stat” or “urgent” first went to a queue in the EHR rather than immediately interrupting the recipient. That way a doctor or other caregiver could see messages while already working in the EHR, rather than glancing at each new message as it arrives, something that all too often needlessly interrupts another important task such as talking with a patient.
And, since most work in EHRs is done in front of a larger device with a full keyboard, it would be easier to type a quick reply message than it would be to rely on a smartphone keyboard for return messaging. Protocols could be established such that messages waiting in the EHR without a reply or dismissal after a specified time would then be sent to the recipient’s personal device.
A Texting Ecosystem
In nearly every case, the hospital will select the text messaging vendor, though hospitalists and nurses, who will typically be among the highest-volume users, should participate in the decision. But the real value of the system hinges on ensuring its wide adoption by most, or nearly all, hospital caregivers and affiliated ambulatory providers.
I would enjoy hearing from those who are already using a HIPAA-secure texting and pager replacement service now, as well as those still researching their options. This has the potential to meaningfully change the way hospitalists and others do their work.

Doctors were the first to begin using pagers and, along with drug dealers, appear to be the last to give them up. But we really need to get rid of them.
Sadly, for the foreseeable future, we will need a pager replacement, but, in the longer term, I’m hopeful that we can:
- Reduce the frequency of electronic interruptions—all forms of interruptions—and the adverse effects that reliably accompany them, and
- Ensure that each interruption has value—that is, reduce or eliminate the many low value and non-urgent messages we all get (e.g. the ones informing you of a lab result you’ve already seen).
Death to the Pager
I can’t imagine anyone who will be more pleased than I will if pagers go the way of now rare hospital-wide PA announcements. Some hospitals have eliminated these announcements entirely, and even critical messages like “code blue” announcements are sent directly to each responder via a pager or other personal device.
Around the time the first iPhone was born, hospital signs banning cell phones began coming down. It seems the fear that they would disrupt hospital electronics, such as telemetry and other monitoring devices, has proven largely unfounded (though, along with things like computer keyboards and stethoscopes, pagers and cell phones can serve as dangerous repositories of bacteria).
Now nearly everyone, from staff to patients, keeps a cell phone with them while in the hospital. I think that is the most important step toward getting rid of pagers. Many doctors already are using the standard text messaging apps that come with the phone to communicate with one another efficiently.
“Regular” Texting Won’t Cut It
Unfortunately, the standard text messaging that comes with every smartphone is not HIPAA compliant. Though I certainly don’t know how anyone would do it, it is apparently too easy for another person to intercept the message. So, if you’re texting information related to your clinical work, you need to make sure it doesn’t include anything that could be considered protected health information. It isn’t enough just to leave the patient’s name off the message. If you’re in the habit of regularly texting doctors, nurses, and other healthcare personnel about patient care, you are at high risk of violating HIPAA, even if you try hard to avoid it.
Another big drawback is that there isn’t a good way to turn off work-related texting when you’re off duty, while leaving your texting app open for communication with your friends and family. Hospital staff will sometimes fail to check whether you’re on duty before texting, and that will lead to your personal time being interrupted by work reminders.
I think these shortcomings mean that none of us should rely on the standard text messaging apps that come with our phones.
But in order for a different app or service to be of any value, we will need to ensure that most providers associated with our hospital are on the same messaging system. That is a tall order, but fortunately there are a lot of companies trying to produce an attractive product that makes it as easy as possible to attract a critical mass of users at your institution.
HIPAA-Compliant Texting Vendors
Many healthcare tech companies provide secure messaging, usually at no additional cost, as an add-on to their main products, such as charge capture software (e.g. IngeniousMed), or physician social networking (e.g. Doximity). Something like 30 companies now offer a dedicated HIPAA-compliant texting option, including IM Your Doc, Voalte, Telmediq, PerfectServe, Vocera, and TigerText. There are so many that it is awfully tough to understand all of their strengths and shortcomings in detail, but I’m having fun trying to do just that. And I anticipate there will be significant consolidation in vendors within the next two to three years.
The dedicated HIPAA-compliant texting services range in price from free for basic features to a monthly fee per user that varies depending on the features you choose to enable. Some offer integration with the hospital’s EHR, which can let a message sender who only knows the patient’s name to see which doctor, nurse, or other caregiver is currently responsible for the patient. Some offer integration with a call schedule and answering service, or even replace an answering service.
No pager replacement will be viable if there are sites in the hospital or elsewhere where it is out of contact; a solution that works on both cellular networks and Wi-Fi is essential. Some vendors offer the ability for messages not delivered to or acknowledged by the recipient to escalate to other forms of delivery after a specified period of time.
I would love to see a feature that I don’t think any vendor offers yet. It would be great if all messages the sender hasn’t marked “stat” or “urgent” first went to a queue in the EHR rather than immediately interrupting the recipient. That way a doctor or other caregiver could see messages while already working in the EHR, rather than glancing at each new message as it arrives, something that all too often needlessly interrupts another important task such as talking with a patient.
And, since most work in EHRs is done in front of a larger device with a full keyboard, it would be easier to type a quick reply message than it would be to rely on a smartphone keyboard for return messaging. Protocols could be established such that messages waiting in the EHR without a reply or dismissal after a specified time would then be sent to the recipient’s personal device.
A Texting Ecosystem
In nearly every case, the hospital will select the text messaging vendor, though hospitalists and nurses, who will typically be among the highest-volume users, should participate in the decision. But the real value of the system hinges on ensuring its wide adoption by most, or nearly all, hospital caregivers and affiliated ambulatory providers.
I would enjoy hearing from those who are already using a HIPAA-secure texting and pager replacement service now, as well as those still researching their options. This has the potential to meaningfully change the way hospitalists and others do their work.

Consider ACO Participation As Medicare Weighs Changes to Shared Savings Program
In December 2014, nearly three years since its launch, the Centers for Medicare and Medicaid Services (CMS) issued the first proposed rule changes to the Shared Savings Program. The changes, if approved, would take effect in the 2016 performance year and would focus on a host of alterations impacting participating accountable care organizations (ACOs), including reduced administrative burden, improved function and transparency, and enhanced incentives to participate in risk-based models.
Experts say the changes could address some of the biggest flaws in the program but also may not go far enough to incentivize more healthcare providers to participate—or protect them from the risk of financial loss. The rules are under review following a public comment period.
“Many features about the original rules weaken the incentives to participate in ACOs,” says Michael McWilliams, MD, PhD, associate professor of healthcare policy and medicine at Harvard Medical School and a practicing primary care physician at Brigham and Women’s Hospital in Boston.
The ACO model encourages providers to realize savings under fee-for-service Medicare through better-coordinated care and improvements in metrics related to utilization and quality. Any savings relative to a benchmark year are shared between the ACO and CMS.
This year, 424 ACOs are participating in the program nationwide, and while the number of Pioneer ACOs has fallen in recent years (Pioneer ACOs wager higher savings for participants at the risk of greater financial loss), a new independent report commissioned by CMS shows the program saved more than $300 annually per beneficiary in its first two years, achieving $384 million in savings.1 In a statement, CMS concluded this meets the criteria for expanding the Pioneer program; however, Dr. McWilliams says policy changes may still be needed to encourage participation in ACOs with downside risk.
In January, Dr. McWilliams and colleagues published a study in Health Affairs that demonstrated that existing benchmark rules may actually encourage higher Medicare spending as ACOs try to “fatten up” so they have more improvements to make and, therefore, more chance of success at realizing savings.2
Currently, providers’ performance is stacked against their performance and cost benchmarks established in the year prior to forming an ACO. As improvements are made, it becomes increasingly challenging for ACOs to do better. Dr. McWilliams says ACOs should instead be compared to other ACOs and providers.
It’s a “melting ice cube problem,” says Gregory Burke, MPA, director of innovation strategies for New York-based United Health Fund (UHF), a research and philanthropic organization focused on advancing healthcare.
—Gregory Burke, MPA, director of innovation Strategies, United Health Fund
“You are punishing the good, lean providers that are efficient,” he adds, “and rewarding people who are less efficient, in terms of cost of care and utilization of services.”
Burke and a colleague at UHF, health policy analyst Suzanne Brundage, recently completed qualitative and quantitative reports on ACOs in the state of New York, which currently make up 20% of Medicare fee-for-service beneficiaries.3,4
Through their analysis, which included structured interviews with 17 Pioneer ACO leaders, Burke and Brundage found ACO rules could change in the following ways to make the program sustainable and more attractive to providers:
- Patients should be attributed to PCPs within the ACO;
- Risk adjustment should be made for ACO providers serving a sicker population of patients; and
- Benchmark rules should be altered.
Additionally, Dr. McWilliams says the shared savings rate realized by ACOs should be higher than 50%, which is especially true for hospitals within an ACO, since the goals of the program are to reduce hospital visits, extensive specialist services, and testing services.
“For a hospital system, the bulk of the money comes from inpatient care,” Burke says. “We’re saying: ‘You stomp on your own air hose today, and a year from now I’ll give you 50% oxygen—and you have to share with your buddy.’”
The 2014 State of Hospital Medicine report indicates that 36% of adult hospitalist medicine groups are in hospitals either already involved in or considering involvement in an ACO; however, respondents in that report also reflect no clear role for hospitalists in the ACO model.
This is a point disputed by Val Akopov, MD, vice president and chief of hospital medicine at WellStar Health System, a not-for-profit organization in northwestern Atlanta and a participating ACO. Dr. Akopov highlights ways in which hospitals and hospitalists could take advantage of the model.
“Five measures fall into the domain of care coordination that are directly, unequivocally related to what hospitalists do, and these metrics are part of, in my opinion, what any hospital medicine program should have as a value proposition,” Dr. Akopov says.
At WellStar, for example, hospitalists have become part of the ACO structure by serving as medical directors and attending physicians at skilled nursing facilities (SNFs). They are “solely responsible to attend to patients in SNFs, and we have seen a dramatic improvement in readmission rates and quality metrics in nursing homes, such as incidence of falls, use of antipsychotics, and 30-day unplanned readmissions to acute care hospitals,” Dr. Akopov explains.
Additionally, WellStar hospitalists work with each inpatient to ensure they have primary care follow-up scheduled before discharge, Dr. Akopov says, noting that the model is a good opportunity to explore changes to the way hospitals and providers deliver care.
“There are roughly 38,000 Medicare patients in [our] ACO; it’s much easier to work out the kinks with innovations on a limited patient population and then extrapolate findings on 1.5 million annually, rather than trying to bite too much,” he says.
Despite the challenges, experts are optimistic the ACO model can—and will—work. In their reporting, Burke and Brundage found healthcare leaders participating in ACOs remain optimistic.
“It’s a post-Copernican universe, where the world no longer revolves around the hospital, so balancing the equation is a little different,” Burke says. “But they’re staying in the game, because that’s where the puck is going to be.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Affordable Care Act payment model saves more than $384 million in two years, meets criteria for first-ever expansion. Centers for Medicare & Medicaid Services website. Published May 4, 2015. Accessed May 11, 2015.
- Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff. 2015;34(1):143-149.
- Burke, G, Brundage S. Accountable care in New York state: emerging themes and issues. United Hospital Fund. Accessed May 9, 2015.
- Burke, G and Brundage S. New York’s Medicare ACOs: participants and performance. United Hospital Fund. Accessed May 9, 2015.
In December 2014, nearly three years since its launch, the Centers for Medicare and Medicaid Services (CMS) issued the first proposed rule changes to the Shared Savings Program. The changes, if approved, would take effect in the 2016 performance year and would focus on a host of alterations impacting participating accountable care organizations (ACOs), including reduced administrative burden, improved function and transparency, and enhanced incentives to participate in risk-based models.
Experts say the changes could address some of the biggest flaws in the program but also may not go far enough to incentivize more healthcare providers to participate—or protect them from the risk of financial loss. The rules are under review following a public comment period.
“Many features about the original rules weaken the incentives to participate in ACOs,” says Michael McWilliams, MD, PhD, associate professor of healthcare policy and medicine at Harvard Medical School and a practicing primary care physician at Brigham and Women’s Hospital in Boston.
The ACO model encourages providers to realize savings under fee-for-service Medicare through better-coordinated care and improvements in metrics related to utilization and quality. Any savings relative to a benchmark year are shared between the ACO and CMS.
This year, 424 ACOs are participating in the program nationwide, and while the number of Pioneer ACOs has fallen in recent years (Pioneer ACOs wager higher savings for participants at the risk of greater financial loss), a new independent report commissioned by CMS shows the program saved more than $300 annually per beneficiary in its first two years, achieving $384 million in savings.1 In a statement, CMS concluded this meets the criteria for expanding the Pioneer program; however, Dr. McWilliams says policy changes may still be needed to encourage participation in ACOs with downside risk.
In January, Dr. McWilliams and colleagues published a study in Health Affairs that demonstrated that existing benchmark rules may actually encourage higher Medicare spending as ACOs try to “fatten up” so they have more improvements to make and, therefore, more chance of success at realizing savings.2
Currently, providers’ performance is stacked against their performance and cost benchmarks established in the year prior to forming an ACO. As improvements are made, it becomes increasingly challenging for ACOs to do better. Dr. McWilliams says ACOs should instead be compared to other ACOs and providers.
It’s a “melting ice cube problem,” says Gregory Burke, MPA, director of innovation strategies for New York-based United Health Fund (UHF), a research and philanthropic organization focused on advancing healthcare.
—Gregory Burke, MPA, director of innovation Strategies, United Health Fund
“You are punishing the good, lean providers that are efficient,” he adds, “and rewarding people who are less efficient, in terms of cost of care and utilization of services.”
Burke and a colleague at UHF, health policy analyst Suzanne Brundage, recently completed qualitative and quantitative reports on ACOs in the state of New York, which currently make up 20% of Medicare fee-for-service beneficiaries.3,4
Through their analysis, which included structured interviews with 17 Pioneer ACO leaders, Burke and Brundage found ACO rules could change in the following ways to make the program sustainable and more attractive to providers:
- Patients should be attributed to PCPs within the ACO;
- Risk adjustment should be made for ACO providers serving a sicker population of patients; and
- Benchmark rules should be altered.
Additionally, Dr. McWilliams says the shared savings rate realized by ACOs should be higher than 50%, which is especially true for hospitals within an ACO, since the goals of the program are to reduce hospital visits, extensive specialist services, and testing services.
“For a hospital system, the bulk of the money comes from inpatient care,” Burke says. “We’re saying: ‘You stomp on your own air hose today, and a year from now I’ll give you 50% oxygen—and you have to share with your buddy.’”
The 2014 State of Hospital Medicine report indicates that 36% of adult hospitalist medicine groups are in hospitals either already involved in or considering involvement in an ACO; however, respondents in that report also reflect no clear role for hospitalists in the ACO model.
This is a point disputed by Val Akopov, MD, vice president and chief of hospital medicine at WellStar Health System, a not-for-profit organization in northwestern Atlanta and a participating ACO. Dr. Akopov highlights ways in which hospitals and hospitalists could take advantage of the model.
“Five measures fall into the domain of care coordination that are directly, unequivocally related to what hospitalists do, and these metrics are part of, in my opinion, what any hospital medicine program should have as a value proposition,” Dr. Akopov says.
At WellStar, for example, hospitalists have become part of the ACO structure by serving as medical directors and attending physicians at skilled nursing facilities (SNFs). They are “solely responsible to attend to patients in SNFs, and we have seen a dramatic improvement in readmission rates and quality metrics in nursing homes, such as incidence of falls, use of antipsychotics, and 30-day unplanned readmissions to acute care hospitals,” Dr. Akopov explains.
Additionally, WellStar hospitalists work with each inpatient to ensure they have primary care follow-up scheduled before discharge, Dr. Akopov says, noting that the model is a good opportunity to explore changes to the way hospitals and providers deliver care.
“There are roughly 38,000 Medicare patients in [our] ACO; it’s much easier to work out the kinks with innovations on a limited patient population and then extrapolate findings on 1.5 million annually, rather than trying to bite too much,” he says.
Despite the challenges, experts are optimistic the ACO model can—and will—work. In their reporting, Burke and Brundage found healthcare leaders participating in ACOs remain optimistic.
“It’s a post-Copernican universe, where the world no longer revolves around the hospital, so balancing the equation is a little different,” Burke says. “But they’re staying in the game, because that’s where the puck is going to be.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Affordable Care Act payment model saves more than $384 million in two years, meets criteria for first-ever expansion. Centers for Medicare & Medicaid Services website. Published May 4, 2015. Accessed May 11, 2015.
- Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff. 2015;34(1):143-149.
- Burke, G, Brundage S. Accountable care in New York state: emerging themes and issues. United Hospital Fund. Accessed May 9, 2015.
- Burke, G and Brundage S. New York’s Medicare ACOs: participants and performance. United Hospital Fund. Accessed May 9, 2015.
In December 2014, nearly three years since its launch, the Centers for Medicare and Medicaid Services (CMS) issued the first proposed rule changes to the Shared Savings Program. The changes, if approved, would take effect in the 2016 performance year and would focus on a host of alterations impacting participating accountable care organizations (ACOs), including reduced administrative burden, improved function and transparency, and enhanced incentives to participate in risk-based models.
Experts say the changes could address some of the biggest flaws in the program but also may not go far enough to incentivize more healthcare providers to participate—or protect them from the risk of financial loss. The rules are under review following a public comment period.
“Many features about the original rules weaken the incentives to participate in ACOs,” says Michael McWilliams, MD, PhD, associate professor of healthcare policy and medicine at Harvard Medical School and a practicing primary care physician at Brigham and Women’s Hospital in Boston.
The ACO model encourages providers to realize savings under fee-for-service Medicare through better-coordinated care and improvements in metrics related to utilization and quality. Any savings relative to a benchmark year are shared between the ACO and CMS.
This year, 424 ACOs are participating in the program nationwide, and while the number of Pioneer ACOs has fallen in recent years (Pioneer ACOs wager higher savings for participants at the risk of greater financial loss), a new independent report commissioned by CMS shows the program saved more than $300 annually per beneficiary in its first two years, achieving $384 million in savings.1 In a statement, CMS concluded this meets the criteria for expanding the Pioneer program; however, Dr. McWilliams says policy changes may still be needed to encourage participation in ACOs with downside risk.
In January, Dr. McWilliams and colleagues published a study in Health Affairs that demonstrated that existing benchmark rules may actually encourage higher Medicare spending as ACOs try to “fatten up” so they have more improvements to make and, therefore, more chance of success at realizing savings.2
Currently, providers’ performance is stacked against their performance and cost benchmarks established in the year prior to forming an ACO. As improvements are made, it becomes increasingly challenging for ACOs to do better. Dr. McWilliams says ACOs should instead be compared to other ACOs and providers.
It’s a “melting ice cube problem,” says Gregory Burke, MPA, director of innovation strategies for New York-based United Health Fund (UHF), a research and philanthropic organization focused on advancing healthcare.
—Gregory Burke, MPA, director of innovation Strategies, United Health Fund
“You are punishing the good, lean providers that are efficient,” he adds, “and rewarding people who are less efficient, in terms of cost of care and utilization of services.”
Burke and a colleague at UHF, health policy analyst Suzanne Brundage, recently completed qualitative and quantitative reports on ACOs in the state of New York, which currently make up 20% of Medicare fee-for-service beneficiaries.3,4
Through their analysis, which included structured interviews with 17 Pioneer ACO leaders, Burke and Brundage found ACO rules could change in the following ways to make the program sustainable and more attractive to providers:
- Patients should be attributed to PCPs within the ACO;
- Risk adjustment should be made for ACO providers serving a sicker population of patients; and
- Benchmark rules should be altered.
Additionally, Dr. McWilliams says the shared savings rate realized by ACOs should be higher than 50%, which is especially true for hospitals within an ACO, since the goals of the program are to reduce hospital visits, extensive specialist services, and testing services.
“For a hospital system, the bulk of the money comes from inpatient care,” Burke says. “We’re saying: ‘You stomp on your own air hose today, and a year from now I’ll give you 50% oxygen—and you have to share with your buddy.’”
The 2014 State of Hospital Medicine report indicates that 36% of adult hospitalist medicine groups are in hospitals either already involved in or considering involvement in an ACO; however, respondents in that report also reflect no clear role for hospitalists in the ACO model.
This is a point disputed by Val Akopov, MD, vice president and chief of hospital medicine at WellStar Health System, a not-for-profit organization in northwestern Atlanta and a participating ACO. Dr. Akopov highlights ways in which hospitals and hospitalists could take advantage of the model.
“Five measures fall into the domain of care coordination that are directly, unequivocally related to what hospitalists do, and these metrics are part of, in my opinion, what any hospital medicine program should have as a value proposition,” Dr. Akopov says.
At WellStar, for example, hospitalists have become part of the ACO structure by serving as medical directors and attending physicians at skilled nursing facilities (SNFs). They are “solely responsible to attend to patients in SNFs, and we have seen a dramatic improvement in readmission rates and quality metrics in nursing homes, such as incidence of falls, use of antipsychotics, and 30-day unplanned readmissions to acute care hospitals,” Dr. Akopov explains.
Additionally, WellStar hospitalists work with each inpatient to ensure they have primary care follow-up scheduled before discharge, Dr. Akopov says, noting that the model is a good opportunity to explore changes to the way hospitals and providers deliver care.
“There are roughly 38,000 Medicare patients in [our] ACO; it’s much easier to work out the kinks with innovations on a limited patient population and then extrapolate findings on 1.5 million annually, rather than trying to bite too much,” he says.
Despite the challenges, experts are optimistic the ACO model can—and will—work. In their reporting, Burke and Brundage found healthcare leaders participating in ACOs remain optimistic.
“It’s a post-Copernican universe, where the world no longer revolves around the hospital, so balancing the equation is a little different,” Burke says. “But they’re staying in the game, because that’s where the puck is going to be.”
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Affordable Care Act payment model saves more than $384 million in two years, meets criteria for first-ever expansion. Centers for Medicare & Medicaid Services website. Published May 4, 2015. Accessed May 11, 2015.
- Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff. 2015;34(1):143-149.
- Burke, G, Brundage S. Accountable care in New York state: emerging themes and issues. United Hospital Fund. Accessed May 9, 2015.
- Burke, G and Brundage S. New York’s Medicare ACOs: participants and performance. United Hospital Fund. Accessed May 9, 2015.
Hospitals’ Uncompensated Costs Estimated at $27.3 Billion in 2014
The estimated total amount of uncompensated costs incurred by hospitals in 2014 was $27.3 billion, which is $7.4 billion, or 21 percent, less than uncompensated hospital care would have been in 2014 at 2013 levels, before Accountable Care Act Medicaid coverage provisions took effect. Federal data reported by CNBC on March 23 indicate most of the reduction came in the 28 states and the District of Columbia that expanded their Medicare programs under the act to cover nearly all poor people in their states, while those that did not could have seen their revenues decline by an additional $1.4 billion.
The estimated total amount of uncompensated costs incurred by hospitals in 2014 was $27.3 billion, which is $7.4 billion, or 21 percent, less than uncompensated hospital care would have been in 2014 at 2013 levels, before Accountable Care Act Medicaid coverage provisions took effect. Federal data reported by CNBC on March 23 indicate most of the reduction came in the 28 states and the District of Columbia that expanded their Medicare programs under the act to cover nearly all poor people in their states, while those that did not could have seen their revenues decline by an additional $1.4 billion.
The estimated total amount of uncompensated costs incurred by hospitals in 2014 was $27.3 billion, which is $7.4 billion, or 21 percent, less than uncompensated hospital care would have been in 2014 at 2013 levels, before Accountable Care Act Medicaid coverage provisions took effect. Federal data reported by CNBC on March 23 indicate most of the reduction came in the 28 states and the District of Columbia that expanded their Medicare programs under the act to cover nearly all poor people in their states, while those that did not could have seen their revenues decline by an additional $1.4 billion.
Quality Data Dashboards Provide Performance Feedback to Physicians
A best-of-research plenary presentation at HM15 in National Harbor, Md., described a project to link physicians’ schedules to the electronic health record (EHR) in order to provide real-time, individualized performance feedback on key quality improvement and value metrics.
The abstract’s lead author, Victoria Valencia, MPH, a research data and project manager at the University of California San Francisco (UCSF), explains that quality improvement priorities have driven feedback of quality metrics at the department level.
“Where I came in was to try to get the same quality metrics down to the level of the team,” she says. “We take data from our EPIC EHR, clean it up by removing outliers, merge it with our online scheduling program, and provide a robust visual presentation of individualized, real-time performance feedback to the clinical team.”
–Dr. Valencia
One example is counting the total number of phlebotomy “sticks” per day, per patient. Reporting this data helped to reduce the number of “sticks per day” by 20%, to 1.6 from 2.0. A similar approach is used for care transitions and the percentage of discharges with high-quality, after-visit summaries.
“The feedback is timely and actionable and allows the teams to address areas needing improvement,” Valencia says.
How have the doctors responded to this feedback?
“Our division is used to receiving quality feedback as part of an ongoing process that includes working meetings where the metrics are reviewed,” she says, adding that there hasn’t been pushback from the teams over these reports.
A best-of-research plenary presentation at HM15 in National Harbor, Md., described a project to link physicians’ schedules to the electronic health record (EHR) in order to provide real-time, individualized performance feedback on key quality improvement and value metrics.
The abstract’s lead author, Victoria Valencia, MPH, a research data and project manager at the University of California San Francisco (UCSF), explains that quality improvement priorities have driven feedback of quality metrics at the department level.
“Where I came in was to try to get the same quality metrics down to the level of the team,” she says. “We take data from our EPIC EHR, clean it up by removing outliers, merge it with our online scheduling program, and provide a robust visual presentation of individualized, real-time performance feedback to the clinical team.”
–Dr. Valencia
One example is counting the total number of phlebotomy “sticks” per day, per patient. Reporting this data helped to reduce the number of “sticks per day” by 20%, to 1.6 from 2.0. A similar approach is used for care transitions and the percentage of discharges with high-quality, after-visit summaries.
“The feedback is timely and actionable and allows the teams to address areas needing improvement,” Valencia says.
How have the doctors responded to this feedback?
“Our division is used to receiving quality feedback as part of an ongoing process that includes working meetings where the metrics are reviewed,” she says, adding that there hasn’t been pushback from the teams over these reports.
A best-of-research plenary presentation at HM15 in National Harbor, Md., described a project to link physicians’ schedules to the electronic health record (EHR) in order to provide real-time, individualized performance feedback on key quality improvement and value metrics.
The abstract’s lead author, Victoria Valencia, MPH, a research data and project manager at the University of California San Francisco (UCSF), explains that quality improvement priorities have driven feedback of quality metrics at the department level.
“Where I came in was to try to get the same quality metrics down to the level of the team,” she says. “We take data from our EPIC EHR, clean it up by removing outliers, merge it with our online scheduling program, and provide a robust visual presentation of individualized, real-time performance feedback to the clinical team.”
–Dr. Valencia
One example is counting the total number of phlebotomy “sticks” per day, per patient. Reporting this data helped to reduce the number of “sticks per day” by 20%, to 1.6 from 2.0. A similar approach is used for care transitions and the percentage of discharges with high-quality, after-visit summaries.
“The feedback is timely and actionable and allows the teams to address areas needing improvement,” Valencia says.
How have the doctors responded to this feedback?
“Our division is used to receiving quality feedback as part of an ongoing process that includes working meetings where the metrics are reviewed,” she says, adding that there hasn’t been pushback from the teams over these reports.
Why Physicians Override Best Practice Alerts
Research published earlier this year in the Journal of Hospital Medicine finds that rationales offered by physicians for overriding interruptive, computerized best practice alerts (BPAs) regarding whether or not to give blood transfusions vary widely, including specialty service protocolized behaviors, anticipation of surgical or procedural interventions, and imminent hospital transfers.
The electronic health record at Stanford University Medical Center in Palo Alto, Calif., has an automated alert function to check reported hemoglobin level and trigger a pop-up reminder when a doctor orders a transfusion for a patient with a hemoglobin level of 9 or above—outside of the recognized guidelines—prompting the doctor to either abort the transfusion or provide a reason for the override, explains co-author Lisa Shieh, MD, PhD, FHM, medical director of quality in the department of medicine at Stanford.
“Our study was trying to understand why providers still transfuse, even when we provide just-in-time education on transfusion recommendations,” she says. “We can’t say that all of these orders are inappropriate. But, for many reasons, blood has harms and is costly.
“We want to convey an overall understanding about why this issue is important.”
Although a substantial number of transfusions continue outside of the recommended guidelines, Stanford has reduced its numbers significantly.
“I’m a big believer in clinical decision support … if it’s designed well and doesn’t add to alert fatigue,” Dr. Shieh says. “I think this BPA was effective in education and making people stop and think why they were ordering transfusions. Our next step will be to look at the outlier practices and maybe have a conversation with them, doctor to doctor.”
Stanford is looking at sepsis treatment as a next target.
Research published earlier this year in the Journal of Hospital Medicine finds that rationales offered by physicians for overriding interruptive, computerized best practice alerts (BPAs) regarding whether or not to give blood transfusions vary widely, including specialty service protocolized behaviors, anticipation of surgical or procedural interventions, and imminent hospital transfers.
The electronic health record at Stanford University Medical Center in Palo Alto, Calif., has an automated alert function to check reported hemoglobin level and trigger a pop-up reminder when a doctor orders a transfusion for a patient with a hemoglobin level of 9 or above—outside of the recognized guidelines—prompting the doctor to either abort the transfusion or provide a reason for the override, explains co-author Lisa Shieh, MD, PhD, FHM, medical director of quality in the department of medicine at Stanford.
“Our study was trying to understand why providers still transfuse, even when we provide just-in-time education on transfusion recommendations,” she says. “We can’t say that all of these orders are inappropriate. But, for many reasons, blood has harms and is costly.
“We want to convey an overall understanding about why this issue is important.”
Although a substantial number of transfusions continue outside of the recommended guidelines, Stanford has reduced its numbers significantly.
“I’m a big believer in clinical decision support … if it’s designed well and doesn’t add to alert fatigue,” Dr. Shieh says. “I think this BPA was effective in education and making people stop and think why they were ordering transfusions. Our next step will be to look at the outlier practices and maybe have a conversation with them, doctor to doctor.”
Stanford is looking at sepsis treatment as a next target.
Research published earlier this year in the Journal of Hospital Medicine finds that rationales offered by physicians for overriding interruptive, computerized best practice alerts (BPAs) regarding whether or not to give blood transfusions vary widely, including specialty service protocolized behaviors, anticipation of surgical or procedural interventions, and imminent hospital transfers.
The electronic health record at Stanford University Medical Center in Palo Alto, Calif., has an automated alert function to check reported hemoglobin level and trigger a pop-up reminder when a doctor orders a transfusion for a patient with a hemoglobin level of 9 or above—outside of the recognized guidelines—prompting the doctor to either abort the transfusion or provide a reason for the override, explains co-author Lisa Shieh, MD, PhD, FHM, medical director of quality in the department of medicine at Stanford.
“Our study was trying to understand why providers still transfuse, even when we provide just-in-time education on transfusion recommendations,” she says. “We can’t say that all of these orders are inappropriate. But, for many reasons, blood has harms and is costly.
“We want to convey an overall understanding about why this issue is important.”
Although a substantial number of transfusions continue outside of the recommended guidelines, Stanford has reduced its numbers significantly.
“I’m a big believer in clinical decision support … if it’s designed well and doesn’t add to alert fatigue,” Dr. Shieh says. “I think this BPA was effective in education and making people stop and think why they were ordering transfusions. Our next step will be to look at the outlier practices and maybe have a conversation with them, doctor to doctor.”
Stanford is looking at sepsis treatment as a next target.