User login
Blood test screens for many viruses simultaneously
Photo by Максим Кукушкин
Scientists have reported that a new test can screen patients for current and past infection with more than 1000 viral strains, using a single drop of blood and for a cost of about $25 per blood sample.
The investigators used the test, known as VirScan, to screen more than 500 people from around the world and found that, on average, participants had been exposed to about 10 viral species over their lifetimes.
“Using this method, we can take a tiny drop of blood and determine what viruses a person has been infected with over the course of many years,” said Stephen Elledge, PhD, of Harvard Medical School in Boston, Massachusetts.
“What makes this so unique is the scale. Right now, a physician needs to guess what virus might be at play and individually test for it. With VirScan, we can look for virtually all viruses, even rare ones, with a single test.”
Dr Elledge and his colleagues described their work with VirScan in Science.
To develop VirScan, the group synthesized more than 93,000 short pieces of DNA encoding different segments of viral proteins. They introduced those pieces of DNA into bacteriophages.
Each bacteriophage manufactured a peptide and displayed it on the bacteriophage surface. As a group, the bacteriophages displayed all of the protein sequences found in the more than 1000 known strains of human viruses.
To perform the VirScan analysis, all of the peptide-displaying bacteriophages are allowed to mingle with a blood sample. Antiviral antibodies in the blood find and bind to their target epitopes within the displayed peptides.
The scientists then retrieve the antibodies and wash away everything except the few bacteriophages that cling to them. By sequencing the DNA of those bacteriophages, the team can identify which viral protein pieces are bound to antibodies in the blood sample.
That reveals which viruses a person’s immune system has previously encountered, either through infection or vaccination.
Dr Elledge estimated that it would take about 2 to 3 days to process 100 samples with VirScan, assuming sequencing is working optimally. He said he is optimistic the speed of the assay will increase with further development.
Putting VirScan to the test
To test VirScan, Dr Elledge and his colleagues used it to analyze blood samples from patients known to be infected with particular viruses, including HIV and hepatitis C.
“It turns out that it works really well,” Dr Elledge said. “We were in the sensitivity range of 95% to 100% for those, and the specificity was good. We didn’t falsely identify people who were negative. That gave us confidence that we could detect other viruses, and when we did see them, we would know they were real.”
So the investigators used VirScan to analyze the antibodies in 569 people from 4 countries (Peru, South Africa, Thailand, and the US), examining about 100 million potential antibody/epitope interactions.
The team found that, on average, each person had antibodies to 10 different species of viruses. As expected, antibodies against certain viruses were common among adults but not in children, suggesting that children had not yet been exposed to those viruses.
Individuals residing in South Africa, Peru, and Thailand tended to have antibodies against more viruses than people in the US. And people infected with HIV had antibodies against many more viruses than people without HIV.
Dr Elledge and his colleagues were surprised to find that antibody responses against specific viruses were similar between individuals, with different people’s antibodies recognizing identical amino acids in the viral peptides.
“In this paper alone, we identified more antibody/peptide interactions to viral proteins than had been identified in the previous history of all viral exploration,” Dr Elledge said.
The reproducibility of those interactions allowed the scientists to refine their analysis and improve the sensitivity of VirScan, Dr Elledge said, adding that the method will continue to improve as his team analyzes more samples.
The investigators also noted that their work is not limited to antiviral antibodies. They are now using it to look for antibodies that attack the body’s tissue in autoimmune diseases associated with cancers. A similar approach could be used to screen for antibodies against other types of pathogens as well. ![]()

Photo by Максим Кукушкин
Scientists have reported that a new test can screen patients for current and past infection with more than 1000 viral strains, using a single drop of blood and for a cost of about $25 per blood sample.
The investigators used the test, known as VirScan, to screen more than 500 people from around the world and found that, on average, participants had been exposed to about 10 viral species over their lifetimes.
“Using this method, we can take a tiny drop of blood and determine what viruses a person has been infected with over the course of many years,” said Stephen Elledge, PhD, of Harvard Medical School in Boston, Massachusetts.
“What makes this so unique is the scale. Right now, a physician needs to guess what virus might be at play and individually test for it. With VirScan, we can look for virtually all viruses, even rare ones, with a single test.”
Dr Elledge and his colleagues described their work with VirScan in Science.
To develop VirScan, the group synthesized more than 93,000 short pieces of DNA encoding different segments of viral proteins. They introduced those pieces of DNA into bacteriophages.
Each bacteriophage manufactured a peptide and displayed it on the bacteriophage surface. As a group, the bacteriophages displayed all of the protein sequences found in the more than 1000 known strains of human viruses.
To perform the VirScan analysis, all of the peptide-displaying bacteriophages are allowed to mingle with a blood sample. Antiviral antibodies in the blood find and bind to their target epitopes within the displayed peptides.
The scientists then retrieve the antibodies and wash away everything except the few bacteriophages that cling to them. By sequencing the DNA of those bacteriophages, the team can identify which viral protein pieces are bound to antibodies in the blood sample.
That reveals which viruses a person’s immune system has previously encountered, either through infection or vaccination.
Dr Elledge estimated that it would take about 2 to 3 days to process 100 samples with VirScan, assuming sequencing is working optimally. He said he is optimistic the speed of the assay will increase with further development.
Putting VirScan to the test
To test VirScan, Dr Elledge and his colleagues used it to analyze blood samples from patients known to be infected with particular viruses, including HIV and hepatitis C.
“It turns out that it works really well,” Dr Elledge said. “We were in the sensitivity range of 95% to 100% for those, and the specificity was good. We didn’t falsely identify people who were negative. That gave us confidence that we could detect other viruses, and when we did see them, we would know they were real.”
So the investigators used VirScan to analyze the antibodies in 569 people from 4 countries (Peru, South Africa, Thailand, and the US), examining about 100 million potential antibody/epitope interactions.
The team found that, on average, each person had antibodies to 10 different species of viruses. As expected, antibodies against certain viruses were common among adults but not in children, suggesting that children had not yet been exposed to those viruses.
Individuals residing in South Africa, Peru, and Thailand tended to have antibodies against more viruses than people in the US. And people infected with HIV had antibodies against many more viruses than people without HIV.
Dr Elledge and his colleagues were surprised to find that antibody responses against specific viruses were similar between individuals, with different people’s antibodies recognizing identical amino acids in the viral peptides.
“In this paper alone, we identified more antibody/peptide interactions to viral proteins than had been identified in the previous history of all viral exploration,” Dr Elledge said.
The reproducibility of those interactions allowed the scientists to refine their analysis and improve the sensitivity of VirScan, Dr Elledge said, adding that the method will continue to improve as his team analyzes more samples.
The investigators also noted that their work is not limited to antiviral antibodies. They are now using it to look for antibodies that attack the body’s tissue in autoimmune diseases associated with cancers. A similar approach could be used to screen for antibodies against other types of pathogens as well. ![]()

Photo by Максим Кукушкин
Scientists have reported that a new test can screen patients for current and past infection with more than 1000 viral strains, using a single drop of blood and for a cost of about $25 per blood sample.
The investigators used the test, known as VirScan, to screen more than 500 people from around the world and found that, on average, participants had been exposed to about 10 viral species over their lifetimes.
“Using this method, we can take a tiny drop of blood and determine what viruses a person has been infected with over the course of many years,” said Stephen Elledge, PhD, of Harvard Medical School in Boston, Massachusetts.
“What makes this so unique is the scale. Right now, a physician needs to guess what virus might be at play and individually test for it. With VirScan, we can look for virtually all viruses, even rare ones, with a single test.”
Dr Elledge and his colleagues described their work with VirScan in Science.
To develop VirScan, the group synthesized more than 93,000 short pieces of DNA encoding different segments of viral proteins. They introduced those pieces of DNA into bacteriophages.
Each bacteriophage manufactured a peptide and displayed it on the bacteriophage surface. As a group, the bacteriophages displayed all of the protein sequences found in the more than 1000 known strains of human viruses.
To perform the VirScan analysis, all of the peptide-displaying bacteriophages are allowed to mingle with a blood sample. Antiviral antibodies in the blood find and bind to their target epitopes within the displayed peptides.
The scientists then retrieve the antibodies and wash away everything except the few bacteriophages that cling to them. By sequencing the DNA of those bacteriophages, the team can identify which viral protein pieces are bound to antibodies in the blood sample.
That reveals which viruses a person’s immune system has previously encountered, either through infection or vaccination.
Dr Elledge estimated that it would take about 2 to 3 days to process 100 samples with VirScan, assuming sequencing is working optimally. He said he is optimistic the speed of the assay will increase with further development.
Putting VirScan to the test
To test VirScan, Dr Elledge and his colleagues used it to analyze blood samples from patients known to be infected with particular viruses, including HIV and hepatitis C.
“It turns out that it works really well,” Dr Elledge said. “We were in the sensitivity range of 95% to 100% for those, and the specificity was good. We didn’t falsely identify people who were negative. That gave us confidence that we could detect other viruses, and when we did see them, we would know they were real.”
So the investigators used VirScan to analyze the antibodies in 569 people from 4 countries (Peru, South Africa, Thailand, and the US), examining about 100 million potential antibody/epitope interactions.
The team found that, on average, each person had antibodies to 10 different species of viruses. As expected, antibodies against certain viruses were common among adults but not in children, suggesting that children had not yet been exposed to those viruses.
Individuals residing in South Africa, Peru, and Thailand tended to have antibodies against more viruses than people in the US. And people infected with HIV had antibodies against many more viruses than people without HIV.
Dr Elledge and his colleagues were surprised to find that antibody responses against specific viruses were similar between individuals, with different people’s antibodies recognizing identical amino acids in the viral peptides.
“In this paper alone, we identified more antibody/peptide interactions to viral proteins than had been identified in the previous history of all viral exploration,” Dr Elledge said.
The reproducibility of those interactions allowed the scientists to refine their analysis and improve the sensitivity of VirScan, Dr Elledge said, adding that the method will continue to improve as his team analyzes more samples.
The investigators also noted that their work is not limited to antiviral antibodies. They are now using it to look for antibodies that attack the body’s tissue in autoimmune diseases associated with cancers. A similar approach could be used to screen for antibodies against other types of pathogens as well. ![]()
Medicolegal issues: The consequences of AAA repair
Dr. Risley together with associate editor Dr. O. William Brown describe a case involving failure to document, a common complaint in malpractice litigation.
A 63-year-old white male, actively working, presented with a 5.3-cm infra-renal abdominal aortic aneurysm (AAA). His anatomy was deemed appropriate for endovascular AAA repair and was cleared by his cardiologist for that procedure. The patient underwent a thorough informed consent regarding endovascular and open options as well as the option for continued AAA surveillance. Of note, the patient never had his family with him at any time during the decision-making process, nor with him in the preoperative holding area.
He underwent an aorto- bi- iliac unibody endovascular graft placement. During delivery of the main body, the right common iliac artery ruptured. Initial attempts at endovascular control and subsequent right retroperitoneal exposure and repair of the iliac rupture were unsuccessful, ultimately requiring laparotomy, explant of the device, and aorto- bi-femoral bypass graft. The first time the surgeon met the large family was at the completion of the procedure to explain the complications that were encountered. The patient had a very “rocky” ICU course with complications of ARDS (acute respiratory distress syndrome), acute renal insufficiency, anemia, encephalopathy, and thrombocytopenia. He was initially started on Lovenox on POD # 2 in addition to SCDs (sequential compression devices) for DVT prophylaxis.
Lovenox was stopped because of thrombocytopenia, and mechanical SCD prophylaxis was maintained. As his platelet count recovered and his HIT (heparin-induced thrombocytopenia) screen came back negative, discussion was undertaken to resume his chemical thromboprophylaxis.
It was elected to leave him on only the SCDs because of potential bleeding complications. He continued to improve, but developed a swollen left lower extremity as a result of a DVT. He was placed on heparin drip and converted to Coumadin. Despite an INR of 2.7 on the day he was to be discharged to the rehabilitation center, and shortly after a walk with the therapists and his family, he suffered a fatal pulmonary embolism.
Ultimately, the hospital, the intensivist and the surgeon were sued for failure to adequately provide prophylaxis and treat the DVT. The case was settled. The criticisms were that the physicians failed to provide both mechanical and chemical thromboprophylaxis, particularly after the HIT panel was negative. No criticisms of the intra-operative complications were levied.
The plaintiff’s expert in this case was a physician who was board certified in internal medicine, anesthesiology, and critical care. He had no training in general surgery or vascular surgery. Accordingly, although he may have made suggestions regarding post operative surgical care he had no experience in being the responsible physician for making a postoperative surgical decision. In addition, assertions regarding the incidence of DVT in specific patient populations were exaggerated. Yet the case still settled.
This case emphasizes several important issues. First, communication is paramount in potentially avoiding medical malpractice litigation. The surgeon never met anyone from the patient’s large and involved family until he had to explain the reason his 2-hour planned procedure took 8 hours. The patient had clearly minimized to his family any of the risks discussed with him preoperatively. Prior to proceeding with any surgical intervention it is important that, if possible, a family member be informed of the risks and benefits of the planned procedure and that this conversation be documented in the chart.
Documentation remains very important not only during the informed consent process prior to surgery, but also in decision making regarding postoperative care. Although the physicians discussed the options of resuming the patient’s chemothromboprophylaxis, nothing was documented in the chart. If the discussion was documented, they may have been able to refute the allegation of failure to prevent the DVT.
Finally, in certain instances even when the facts support the judgments that were made regarding the care given, it may be the best “business decision” to settle a case. This is perhaps the most difficult concept for practicing surgeons to grasp. Whether physicians like it or not, medicine is a business and decisions made regarding defending or settling a law suit should not be based on emotion. We don’t do that in the operating room, and we should not do it in the courtroom.
Dr. Risley is the medical director of the Jacksonville Vein Center, Fla.
The opinions expressed by the author neither imply nor establish a standard of care. Cases have been modified and may be fictional in order to maintain HIPPA and confidentiality regulations.
Dr. Risley together with associate editor Dr. O. William Brown describe a case involving failure to document, a common complaint in malpractice litigation.
A 63-year-old white male, actively working, presented with a 5.3-cm infra-renal abdominal aortic aneurysm (AAA). His anatomy was deemed appropriate for endovascular AAA repair and was cleared by his cardiologist for that procedure. The patient underwent a thorough informed consent regarding endovascular and open options as well as the option for continued AAA surveillance. Of note, the patient never had his family with him at any time during the decision-making process, nor with him in the preoperative holding area.
He underwent an aorto- bi- iliac unibody endovascular graft placement. During delivery of the main body, the right common iliac artery ruptured. Initial attempts at endovascular control and subsequent right retroperitoneal exposure and repair of the iliac rupture were unsuccessful, ultimately requiring laparotomy, explant of the device, and aorto- bi-femoral bypass graft. The first time the surgeon met the large family was at the completion of the procedure to explain the complications that were encountered. The patient had a very “rocky” ICU course with complications of ARDS (acute respiratory distress syndrome), acute renal insufficiency, anemia, encephalopathy, and thrombocytopenia. He was initially started on Lovenox on POD # 2 in addition to SCDs (sequential compression devices) for DVT prophylaxis.
Lovenox was stopped because of thrombocytopenia, and mechanical SCD prophylaxis was maintained. As his platelet count recovered and his HIT (heparin-induced thrombocytopenia) screen came back negative, discussion was undertaken to resume his chemical thromboprophylaxis.
It was elected to leave him on only the SCDs because of potential bleeding complications. He continued to improve, but developed a swollen left lower extremity as a result of a DVT. He was placed on heparin drip and converted to Coumadin. Despite an INR of 2.7 on the day he was to be discharged to the rehabilitation center, and shortly after a walk with the therapists and his family, he suffered a fatal pulmonary embolism.
Ultimately, the hospital, the intensivist and the surgeon were sued for failure to adequately provide prophylaxis and treat the DVT. The case was settled. The criticisms were that the physicians failed to provide both mechanical and chemical thromboprophylaxis, particularly after the HIT panel was negative. No criticisms of the intra-operative complications were levied.
The plaintiff’s expert in this case was a physician who was board certified in internal medicine, anesthesiology, and critical care. He had no training in general surgery or vascular surgery. Accordingly, although he may have made suggestions regarding post operative surgical care he had no experience in being the responsible physician for making a postoperative surgical decision. In addition, assertions regarding the incidence of DVT in specific patient populations were exaggerated. Yet the case still settled.
This case emphasizes several important issues. First, communication is paramount in potentially avoiding medical malpractice litigation. The surgeon never met anyone from the patient’s large and involved family until he had to explain the reason his 2-hour planned procedure took 8 hours. The patient had clearly minimized to his family any of the risks discussed with him preoperatively. Prior to proceeding with any surgical intervention it is important that, if possible, a family member be informed of the risks and benefits of the planned procedure and that this conversation be documented in the chart.
Documentation remains very important not only during the informed consent process prior to surgery, but also in decision making regarding postoperative care. Although the physicians discussed the options of resuming the patient’s chemothromboprophylaxis, nothing was documented in the chart. If the discussion was documented, they may have been able to refute the allegation of failure to prevent the DVT.
Finally, in certain instances even when the facts support the judgments that were made regarding the care given, it may be the best “business decision” to settle a case. This is perhaps the most difficult concept for practicing surgeons to grasp. Whether physicians like it or not, medicine is a business and decisions made regarding defending or settling a law suit should not be based on emotion. We don’t do that in the operating room, and we should not do it in the courtroom.
Dr. Risley is the medical director of the Jacksonville Vein Center, Fla.
The opinions expressed by the author neither imply nor establish a standard of care. Cases have been modified and may be fictional in order to maintain HIPPA and confidentiality regulations.
Dr. Risley together with associate editor Dr. O. William Brown describe a case involving failure to document, a common complaint in malpractice litigation.
A 63-year-old white male, actively working, presented with a 5.3-cm infra-renal abdominal aortic aneurysm (AAA). His anatomy was deemed appropriate for endovascular AAA repair and was cleared by his cardiologist for that procedure. The patient underwent a thorough informed consent regarding endovascular and open options as well as the option for continued AAA surveillance. Of note, the patient never had his family with him at any time during the decision-making process, nor with him in the preoperative holding area.
He underwent an aorto- bi- iliac unibody endovascular graft placement. During delivery of the main body, the right common iliac artery ruptured. Initial attempts at endovascular control and subsequent right retroperitoneal exposure and repair of the iliac rupture were unsuccessful, ultimately requiring laparotomy, explant of the device, and aorto- bi-femoral bypass graft. The first time the surgeon met the large family was at the completion of the procedure to explain the complications that were encountered. The patient had a very “rocky” ICU course with complications of ARDS (acute respiratory distress syndrome), acute renal insufficiency, anemia, encephalopathy, and thrombocytopenia. He was initially started on Lovenox on POD # 2 in addition to SCDs (sequential compression devices) for DVT prophylaxis.
Lovenox was stopped because of thrombocytopenia, and mechanical SCD prophylaxis was maintained. As his platelet count recovered and his HIT (heparin-induced thrombocytopenia) screen came back negative, discussion was undertaken to resume his chemical thromboprophylaxis.
It was elected to leave him on only the SCDs because of potential bleeding complications. He continued to improve, but developed a swollen left lower extremity as a result of a DVT. He was placed on heparin drip and converted to Coumadin. Despite an INR of 2.7 on the day he was to be discharged to the rehabilitation center, and shortly after a walk with the therapists and his family, he suffered a fatal pulmonary embolism.
Ultimately, the hospital, the intensivist and the surgeon were sued for failure to adequately provide prophylaxis and treat the DVT. The case was settled. The criticisms were that the physicians failed to provide both mechanical and chemical thromboprophylaxis, particularly after the HIT panel was negative. No criticisms of the intra-operative complications were levied.
The plaintiff’s expert in this case was a physician who was board certified in internal medicine, anesthesiology, and critical care. He had no training in general surgery or vascular surgery. Accordingly, although he may have made suggestions regarding post operative surgical care he had no experience in being the responsible physician for making a postoperative surgical decision. In addition, assertions regarding the incidence of DVT in specific patient populations were exaggerated. Yet the case still settled.
This case emphasizes several important issues. First, communication is paramount in potentially avoiding medical malpractice litigation. The surgeon never met anyone from the patient’s large and involved family until he had to explain the reason his 2-hour planned procedure took 8 hours. The patient had clearly minimized to his family any of the risks discussed with him preoperatively. Prior to proceeding with any surgical intervention it is important that, if possible, a family member be informed of the risks and benefits of the planned procedure and that this conversation be documented in the chart.
Documentation remains very important not only during the informed consent process prior to surgery, but also in decision making regarding postoperative care. Although the physicians discussed the options of resuming the patient’s chemothromboprophylaxis, nothing was documented in the chart. If the discussion was documented, they may have been able to refute the allegation of failure to prevent the DVT.
Finally, in certain instances even when the facts support the judgments that were made regarding the care given, it may be the best “business decision” to settle a case. This is perhaps the most difficult concept for practicing surgeons to grasp. Whether physicians like it or not, medicine is a business and decisions made regarding defending or settling a law suit should not be based on emotion. We don’t do that in the operating room, and we should not do it in the courtroom.
Dr. Risley is the medical director of the Jacksonville Vein Center, Fla.
The opinions expressed by the author neither imply nor establish a standard of care. Cases have been modified and may be fictional in order to maintain HIPPA and confidentiality regulations.
Patients in nursing homes show poor vascular outcomes
A substantial number of nursing home residents undergo lower-extremity revascularization each year, but very few of them gain any function and approximately half die within the year, according to an online report in JAMA Internal Medicine.
In a population-based analysis of Medicare claims and a database that tracks virtually all U.S. nursing homes, 82% of residents who underwent lower-extremity revascularization during a 3-year period had either died or were unable to walk a year afterward.
Most showed a clinically significant decline in function within 3 months of having the procedure, said Dr. Lawrence Oresanya of the department of surgery, University of California, San Francisco, and his associates. “Our findings can inform conversations between physicians, patients, and families about the risks and expected outcomes of surgery and whether the surgery is likely to be worthwhile. Our findings also highlight the importance of carefully considering a prognosis independent of vascular disease and assessing the goals of care. Ambulatory function … may be impossible to attain,” they wrote.
Lower-extremity revascularization is usually performed to maintain elderly patients’ functional independence by preserving their limbs. But a closer examination of these procedures is warranted in the nursing home population “because nursing home residents, in general, have high levels of functional dependence unrelated to peripheral arterial disease, and higher rates of mortality after most invasive procedures,” according to the investigators.
Dr. Oresanya and his colleagues identified 10,784 nursing home residents across the country who underwent lower-extremity revascularization.
The procedure was elective in 67% of cases and emergent or urgent in 33%. An endovascular approach was used in 56%, and an open approach in the remainder, with the endovacular approach being more associated with clinical success than open surgery.
The mean patient age was 82 years, and serious comorbidities were very common: 60% had cognitive impairment, 57% had heart failure, and 29% had renal failure. Three-fourths of the patients were nonambulatory at the time of surgery.
The investigators assumed that most patients in this setting had critical limb ischemia rather than claudication. They did not have information about the severity of the lower-extremity ischemia, or about the prevalence or duration of nonhealing wounds or gangrene.
One year after lower-extremity revascularization, mortality was 51% among ambulatory patients and 53% among nonambulatory patients.
Only 13% of the entire cohort were able to walk, and only 18% had maintained or improved their presurgical functional status.
“Revascularization rarely allowed a nonambulatory resident to become ambulatory,” Dr. Oresanya and his associates wrote (JAMA Intern. Med. 2015 April 6 [doi:10.1001/jamainternmed.2015.0486]).
The researchers were unable to determine whether these poor outcomes resulted from the surgery itself or were due to these patients’ “insufficient physiologic reserve.”
They also cautioned that they confined their study strictly to functional outcomes of lower-extremity revascularization, namely ambulation and mortality. Some patients may have derived other benefits from the procedure, such as relief of pain, healing of wounds, and avoidance of major amputation.
The authors reported having no relevant financial disclosures.
The recent study by Oresanya et al. is very controversial, especially in light of the recent NY Times article suggesting too many inappropriate procedures are occurring for PAD. However, the study must be looked at in context. This is a database study, which, as such, has inherent limitations. The authors admit they had no data on pain, wound healing, or avoidance of major amputations. Fully one-third of the cases in this study were urgent/emergent, again suggesting the critical nature of impaired perfusion in these patients. The only outcomes assessed were mortality and functional status. Seventy-five percent of the patients were already nonambulatory, again suggesting that the indications for intervention were likely critical limb ischemia. If this is correct, as the authors have also assumed, then the issue is not one of mortality or improving functional status, but rather one of limb salvage, and pain control – issues of quality of life. Further, the finding of endovascular cases being more successful suggests that the endovascular first approach is clearly appropriate in this debilitated population.
It is, unfortunately, not possible from this type of database study to ascertain whether the patients treated with open bypass were candidates for endovascular interventions, or had already failed an endovascular approach. It is also not possible to determine whether “success” was achieved, with pain relief, avoidance of major limb amputation with the inherent increased mortality risk, and wound healing.
However, what we can glean from this study is information to better counsel patients and families regarding functional status and life expectancy, as some families and patients have unrealistic expectations and push surgeons to intervene, despite initial attempts at counseling. Having additional data may allow surgeons to correctly convince some patients, whose pain is controlled and tissue loss is not immediately life or limb threatening, that because of limited life expectancy, medical management alone may be appropriate. This study, like most database studies, should lead to further evaluation in a situation in which clinical outcomes may be better assessed, such as a study of the Vascular Quality Initiative database, which does collect this type of information.
Dr. Linda Harris is professor of surgery and chief, division vascular surgery, University at Buffalo, State University of New York, and an associate medical editor for Vascular Specialist.
The recent study by Oresanya et al. is very controversial, especially in light of the recent NY Times article suggesting too many inappropriate procedures are occurring for PAD. However, the study must be looked at in context. This is a database study, which, as such, has inherent limitations. The authors admit they had no data on pain, wound healing, or avoidance of major amputations. Fully one-third of the cases in this study were urgent/emergent, again suggesting the critical nature of impaired perfusion in these patients. The only outcomes assessed were mortality and functional status. Seventy-five percent of the patients were already nonambulatory, again suggesting that the indications for intervention were likely critical limb ischemia. If this is correct, as the authors have also assumed, then the issue is not one of mortality or improving functional status, but rather one of limb salvage, and pain control – issues of quality of life. Further, the finding of endovascular cases being more successful suggests that the endovascular first approach is clearly appropriate in this debilitated population.
It is, unfortunately, not possible from this type of database study to ascertain whether the patients treated with open bypass were candidates for endovascular interventions, or had already failed an endovascular approach. It is also not possible to determine whether “success” was achieved, with pain relief, avoidance of major limb amputation with the inherent increased mortality risk, and wound healing.
However, what we can glean from this study is information to better counsel patients and families regarding functional status and life expectancy, as some families and patients have unrealistic expectations and push surgeons to intervene, despite initial attempts at counseling. Having additional data may allow surgeons to correctly convince some patients, whose pain is controlled and tissue loss is not immediately life or limb threatening, that because of limited life expectancy, medical management alone may be appropriate. This study, like most database studies, should lead to further evaluation in a situation in which clinical outcomes may be better assessed, such as a study of the Vascular Quality Initiative database, which does collect this type of information.
Dr. Linda Harris is professor of surgery and chief, division vascular surgery, University at Buffalo, State University of New York, and an associate medical editor for Vascular Specialist.
The recent study by Oresanya et al. is very controversial, especially in light of the recent NY Times article suggesting too many inappropriate procedures are occurring for PAD. However, the study must be looked at in context. This is a database study, which, as such, has inherent limitations. The authors admit they had no data on pain, wound healing, or avoidance of major amputations. Fully one-third of the cases in this study were urgent/emergent, again suggesting the critical nature of impaired perfusion in these patients. The only outcomes assessed were mortality and functional status. Seventy-five percent of the patients were already nonambulatory, again suggesting that the indications for intervention were likely critical limb ischemia. If this is correct, as the authors have also assumed, then the issue is not one of mortality or improving functional status, but rather one of limb salvage, and pain control – issues of quality of life. Further, the finding of endovascular cases being more successful suggests that the endovascular first approach is clearly appropriate in this debilitated population.
It is, unfortunately, not possible from this type of database study to ascertain whether the patients treated with open bypass were candidates for endovascular interventions, or had already failed an endovascular approach. It is also not possible to determine whether “success” was achieved, with pain relief, avoidance of major limb amputation with the inherent increased mortality risk, and wound healing.
However, what we can glean from this study is information to better counsel patients and families regarding functional status and life expectancy, as some families and patients have unrealistic expectations and push surgeons to intervene, despite initial attempts at counseling. Having additional data may allow surgeons to correctly convince some patients, whose pain is controlled and tissue loss is not immediately life or limb threatening, that because of limited life expectancy, medical management alone may be appropriate. This study, like most database studies, should lead to further evaluation in a situation in which clinical outcomes may be better assessed, such as a study of the Vascular Quality Initiative database, which does collect this type of information.
Dr. Linda Harris is professor of surgery and chief, division vascular surgery, University at Buffalo, State University of New York, and an associate medical editor for Vascular Specialist.
A substantial number of nursing home residents undergo lower-extremity revascularization each year, but very few of them gain any function and approximately half die within the year, according to an online report in JAMA Internal Medicine.
In a population-based analysis of Medicare claims and a database that tracks virtually all U.S. nursing homes, 82% of residents who underwent lower-extremity revascularization during a 3-year period had either died or were unable to walk a year afterward.
Most showed a clinically significant decline in function within 3 months of having the procedure, said Dr. Lawrence Oresanya of the department of surgery, University of California, San Francisco, and his associates. “Our findings can inform conversations between physicians, patients, and families about the risks and expected outcomes of surgery and whether the surgery is likely to be worthwhile. Our findings also highlight the importance of carefully considering a prognosis independent of vascular disease and assessing the goals of care. Ambulatory function … may be impossible to attain,” they wrote.
Lower-extremity revascularization is usually performed to maintain elderly patients’ functional independence by preserving their limbs. But a closer examination of these procedures is warranted in the nursing home population “because nursing home residents, in general, have high levels of functional dependence unrelated to peripheral arterial disease, and higher rates of mortality after most invasive procedures,” according to the investigators.
Dr. Oresanya and his colleagues identified 10,784 nursing home residents across the country who underwent lower-extremity revascularization.
The procedure was elective in 67% of cases and emergent or urgent in 33%. An endovascular approach was used in 56%, and an open approach in the remainder, with the endovacular approach being more associated with clinical success than open surgery.
The mean patient age was 82 years, and serious comorbidities were very common: 60% had cognitive impairment, 57% had heart failure, and 29% had renal failure. Three-fourths of the patients were nonambulatory at the time of surgery.
The investigators assumed that most patients in this setting had critical limb ischemia rather than claudication. They did not have information about the severity of the lower-extremity ischemia, or about the prevalence or duration of nonhealing wounds or gangrene.
One year after lower-extremity revascularization, mortality was 51% among ambulatory patients and 53% among nonambulatory patients.
Only 13% of the entire cohort were able to walk, and only 18% had maintained or improved their presurgical functional status.
“Revascularization rarely allowed a nonambulatory resident to become ambulatory,” Dr. Oresanya and his associates wrote (JAMA Intern. Med. 2015 April 6 [doi:10.1001/jamainternmed.2015.0486]).
The researchers were unable to determine whether these poor outcomes resulted from the surgery itself or were due to these patients’ “insufficient physiologic reserve.”
They also cautioned that they confined their study strictly to functional outcomes of lower-extremity revascularization, namely ambulation and mortality. Some patients may have derived other benefits from the procedure, such as relief of pain, healing of wounds, and avoidance of major amputation.
The authors reported having no relevant financial disclosures.
A substantial number of nursing home residents undergo lower-extremity revascularization each year, but very few of them gain any function and approximately half die within the year, according to an online report in JAMA Internal Medicine.
In a population-based analysis of Medicare claims and a database that tracks virtually all U.S. nursing homes, 82% of residents who underwent lower-extremity revascularization during a 3-year period had either died or were unable to walk a year afterward.
Most showed a clinically significant decline in function within 3 months of having the procedure, said Dr. Lawrence Oresanya of the department of surgery, University of California, San Francisco, and his associates. “Our findings can inform conversations between physicians, patients, and families about the risks and expected outcomes of surgery and whether the surgery is likely to be worthwhile. Our findings also highlight the importance of carefully considering a prognosis independent of vascular disease and assessing the goals of care. Ambulatory function … may be impossible to attain,” they wrote.
Lower-extremity revascularization is usually performed to maintain elderly patients’ functional independence by preserving their limbs. But a closer examination of these procedures is warranted in the nursing home population “because nursing home residents, in general, have high levels of functional dependence unrelated to peripheral arterial disease, and higher rates of mortality after most invasive procedures,” according to the investigators.
Dr. Oresanya and his colleagues identified 10,784 nursing home residents across the country who underwent lower-extremity revascularization.
The procedure was elective in 67% of cases and emergent or urgent in 33%. An endovascular approach was used in 56%, and an open approach in the remainder, with the endovacular approach being more associated with clinical success than open surgery.
The mean patient age was 82 years, and serious comorbidities were very common: 60% had cognitive impairment, 57% had heart failure, and 29% had renal failure. Three-fourths of the patients were nonambulatory at the time of surgery.
The investigators assumed that most patients in this setting had critical limb ischemia rather than claudication. They did not have information about the severity of the lower-extremity ischemia, or about the prevalence or duration of nonhealing wounds or gangrene.
One year after lower-extremity revascularization, mortality was 51% among ambulatory patients and 53% among nonambulatory patients.
Only 13% of the entire cohort were able to walk, and only 18% had maintained or improved their presurgical functional status.
“Revascularization rarely allowed a nonambulatory resident to become ambulatory,” Dr. Oresanya and his associates wrote (JAMA Intern. Med. 2015 April 6 [doi:10.1001/jamainternmed.2015.0486]).
The researchers were unable to determine whether these poor outcomes resulted from the surgery itself or were due to these patients’ “insufficient physiologic reserve.”
They also cautioned that they confined their study strictly to functional outcomes of lower-extremity revascularization, namely ambulation and mortality. Some patients may have derived other benefits from the procedure, such as relief of pain, healing of wounds, and avoidance of major amputation.
The authors reported having no relevant financial disclosures.
With Rashes, Consider the Source (Or Lack Thereof)
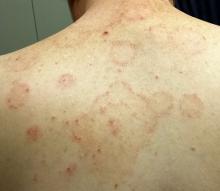
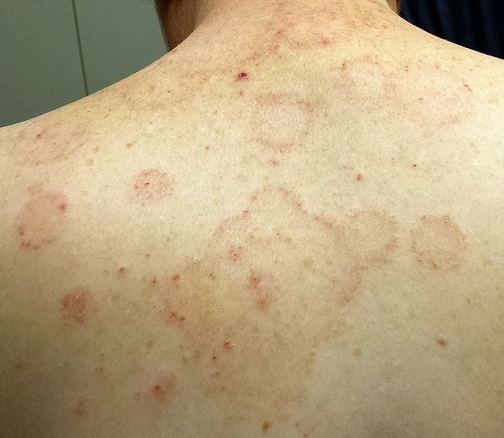
A 44-year-old man presents with what his primary care provider called “ringworm.” The condition has not responded to topical and oral antifungal medications (clotrimazole and oral terbinafine). Fortunately, the lesions are not particularly symptomatic.
The patient, who works in an oil field, claims to be in otherwise good health. He has no pets at home, and no one else in his household has been similarly affected. He is taking no medications at this time.
EXAMINATION
Multiple arcuate and annular scaly lesions are seen, most densely distributed across the patient’s upper back and posterior shoulders. They thin out inferiorly and are missing entirely below the waist.
Many of the lesions are larger than 10 cm (though on average half that size). They have well-defined, scaly margins and mostly clear centers. There is minimal erythema.
A KOH prep of the lesions does not show fungal elements. A 4-mm punch biopsy is performed, the results of which will be discussed in the next section.
What is the diagnosis?
DISCUSSION
This case nicely illustrates a common dilemma in dermatology: “ringworm” that is neither fungal nor any other kind of infection. Presented with this case, the vast majority of primary care providers would do what this man’s clinician did: treat it as fungal. This attempt would fail, for one basic reason: There is an extensive differential for round, scaly lesions, of which dermatophytosis (superficial fungal infection) is but one item.
In this case, there was no source from which our patient could have acquired a fungal infection (animal, child, soil) and no particular susceptibility by virtue of immune suppression. To these facts we add the negative KOH, forcing us to consider other items in the differential, including psoriasis, T-cell lymphoma, erythema annulare centrifugum, chronic cutaneous lupus, and nummular eczema, just to name a few.
One way to settle the issue is to obtain a skin biopsy, which in this case showed results most consistent with eczema and effectively ruled out items such as lupus or cutaneous T-cell lymphoma. This provides extremely useful treatment guidance, as well as reassurance to the patient. Stains for fungal organisms were requested and obtained as part of specimen processing—and, of course, results were negative.
In most derm clinics, skin biopsies are routinely done—but what really sets the specialty apart is the fact that a full differential is considered, not just the first (and often only) diagnostic idea that comes to mind. More often than not, a reasonable diagnosis can be found without biopsy, using corroborative history and physical data.
This patient responded nicely to the application of a topical steroid lotion (fluocinonide 0.05%) and the use of emollients. In the process of history-taking, it was discovered that the patient had a habit of taking long, hot showers while standing with his back to the nozzle. Since this certainly can contribute to dry skin, he was advised to reduce the temperature to a more moderate level and limit his time spent in the shower.
TAKE-HOME LEARNING POINTS
• Just because a rash presents with round, scaly lesions doesn’t mean it’s fungal.
• The rather extensive differential for such rashes includes lupus, psoriasis, eczema, and a type of skin cancer called cutaneous T-cell lymphoma.
• Biopsy, performed under local anesthetic and with a 4-mm punch (closed with two sutures), can be extremely useful in sorting through the differential.
• A KOH prep is a helpful first step (one that would confirm or rule out fungal origin).
• In the absence of a source or indications of susceptibility, fungal infection is an unlikely diagnosis in these cases.
A 44-year-old man presents with what his primary care provider called “ringworm.” The condition has not responded to topical and oral antifungal medications (clotrimazole and oral terbinafine). Fortunately, the lesions are not particularly symptomatic.
The patient, who works in an oil field, claims to be in otherwise good health. He has no pets at home, and no one else in his household has been similarly affected. He is taking no medications at this time.
EXAMINATION
Multiple arcuate and annular scaly lesions are seen, most densely distributed across the patient’s upper back and posterior shoulders. They thin out inferiorly and are missing entirely below the waist.
Many of the lesions are larger than 10 cm (though on average half that size). They have well-defined, scaly margins and mostly clear centers. There is minimal erythema.
A KOH prep of the lesions does not show fungal elements. A 4-mm punch biopsy is performed, the results of which will be discussed in the next section.
What is the diagnosis?
DISCUSSION
This case nicely illustrates a common dilemma in dermatology: “ringworm” that is neither fungal nor any other kind of infection. Presented with this case, the vast majority of primary care providers would do what this man’s clinician did: treat it as fungal. This attempt would fail, for one basic reason: There is an extensive differential for round, scaly lesions, of which dermatophytosis (superficial fungal infection) is but one item.
In this case, there was no source from which our patient could have acquired a fungal infection (animal, child, soil) and no particular susceptibility by virtue of immune suppression. To these facts we add the negative KOH, forcing us to consider other items in the differential, including psoriasis, T-cell lymphoma, erythema annulare centrifugum, chronic cutaneous lupus, and nummular eczema, just to name a few.
One way to settle the issue is to obtain a skin biopsy, which in this case showed results most consistent with eczema and effectively ruled out items such as lupus or cutaneous T-cell lymphoma. This provides extremely useful treatment guidance, as well as reassurance to the patient. Stains for fungal organisms were requested and obtained as part of specimen processing—and, of course, results were negative.
In most derm clinics, skin biopsies are routinely done—but what really sets the specialty apart is the fact that a full differential is considered, not just the first (and often only) diagnostic idea that comes to mind. More often than not, a reasonable diagnosis can be found without biopsy, using corroborative history and physical data.
This patient responded nicely to the application of a topical steroid lotion (fluocinonide 0.05%) and the use of emollients. In the process of history-taking, it was discovered that the patient had a habit of taking long, hot showers while standing with his back to the nozzle. Since this certainly can contribute to dry skin, he was advised to reduce the temperature to a more moderate level and limit his time spent in the shower.
TAKE-HOME LEARNING POINTS
• Just because a rash presents with round, scaly lesions doesn’t mean it’s fungal.
• The rather extensive differential for such rashes includes lupus, psoriasis, eczema, and a type of skin cancer called cutaneous T-cell lymphoma.
• Biopsy, performed under local anesthetic and with a 4-mm punch (closed with two sutures), can be extremely useful in sorting through the differential.
• A KOH prep is a helpful first step (one that would confirm or rule out fungal origin).
• In the absence of a source or indications of susceptibility, fungal infection is an unlikely diagnosis in these cases.
A 44-year-old man presents with what his primary care provider called “ringworm.” The condition has not responded to topical and oral antifungal medications (clotrimazole and oral terbinafine). Fortunately, the lesions are not particularly symptomatic.
The patient, who works in an oil field, claims to be in otherwise good health. He has no pets at home, and no one else in his household has been similarly affected. He is taking no medications at this time.
EXAMINATION
Multiple arcuate and annular scaly lesions are seen, most densely distributed across the patient’s upper back and posterior shoulders. They thin out inferiorly and are missing entirely below the waist.
Many of the lesions are larger than 10 cm (though on average half that size). They have well-defined, scaly margins and mostly clear centers. There is minimal erythema.
A KOH prep of the lesions does not show fungal elements. A 4-mm punch biopsy is performed, the results of which will be discussed in the next section.
What is the diagnosis?
DISCUSSION
This case nicely illustrates a common dilemma in dermatology: “ringworm” that is neither fungal nor any other kind of infection. Presented with this case, the vast majority of primary care providers would do what this man’s clinician did: treat it as fungal. This attempt would fail, for one basic reason: There is an extensive differential for round, scaly lesions, of which dermatophytosis (superficial fungal infection) is but one item.
In this case, there was no source from which our patient could have acquired a fungal infection (animal, child, soil) and no particular susceptibility by virtue of immune suppression. To these facts we add the negative KOH, forcing us to consider other items in the differential, including psoriasis, T-cell lymphoma, erythema annulare centrifugum, chronic cutaneous lupus, and nummular eczema, just to name a few.
One way to settle the issue is to obtain a skin biopsy, which in this case showed results most consistent with eczema and effectively ruled out items such as lupus or cutaneous T-cell lymphoma. This provides extremely useful treatment guidance, as well as reassurance to the patient. Stains for fungal organisms were requested and obtained as part of specimen processing—and, of course, results were negative.
In most derm clinics, skin biopsies are routinely done—but what really sets the specialty apart is the fact that a full differential is considered, not just the first (and often only) diagnostic idea that comes to mind. More often than not, a reasonable diagnosis can be found without biopsy, using corroborative history and physical data.
This patient responded nicely to the application of a topical steroid lotion (fluocinonide 0.05%) and the use of emollients. In the process of history-taking, it was discovered that the patient had a habit of taking long, hot showers while standing with his back to the nozzle. Since this certainly can contribute to dry skin, he was advised to reduce the temperature to a more moderate level and limit his time spent in the shower.
TAKE-HOME LEARNING POINTS
• Just because a rash presents with round, scaly lesions doesn’t mean it’s fungal.
• The rather extensive differential for such rashes includes lupus, psoriasis, eczema, and a type of skin cancer called cutaneous T-cell lymphoma.
• Biopsy, performed under local anesthetic and with a 4-mm punch (closed with two sutures), can be extremely useful in sorting through the differential.
• A KOH prep is a helpful first step (one that would confirm or rule out fungal origin).
• In the absence of a source or indications of susceptibility, fungal infection is an unlikely diagnosis in these cases.
LSC phenotypes correlate with prognosis in AML

© ASCO/Rodney White
CHICAGO—Researchers say they have identified 3 leukemia stem cell (LSC) phenotypes that are correlated with cytogenetic/molecular abnormalities and prognosis in acute myeloid leukemia (AML).
The investigators believe this knowledge could aid risk stratification of AML patients, particularly those without identifiable cytogenetic or molecular risk factors.
The findings may also pave the way for scientists to identify novel therapeutic targets on LSCs and monitor LSCs in response to therapy.
Jonathan Michael Gerber, MD, of Levine Cancer Institute in Charlotte, North Carolina, presented the findings at the 2015 ASCO Annual Meeting (abstract 7000*).
Via previous research, Dr Gerber and his colleagues identified 3 different LSC phenotypes in AML:
- LSCs that are CD34-negative
- LSCs that are CD34-positive, CD38-negative, and have intermediate levels of aldehyde dehydrogenase (ALDHint)
- LSCs that are CD34-positive, CD38-negative, and have high levels of ALDH (ALDHhigh).
With the current study, the researchers wanted to determine if these phenotypes correlate with cytogenetic/molecular features and treatment outcomes.
So they analyzed diagnostic samples from 98 patients with newly diagnosed AML who had normal or unfavorable cytogenetics. The patients were enrolled on a phase 2 trial comparing FLAM and 7+3 (Zeidner et al, haematologica 2015).
Dr Gerber and his colleagues identified 22 patients with CD34- LSCs, 43 with ALDHint LSCs, and 33 with ALDHhigh LSCs.
Risk factors
“We found that leukemia stem cell phenotype indeed correlated quite strongly with cytogenetic and molecular risk factors,” Dr Gerber said.
NPM1 mutations were more common in patients with CD34- LSCs (64%) than those with ALDHint LSCs (14%) or ALDHhigh LSCs (6%, P<0.001). NPM1 mutations were the sole abnormality in 50% of patients with CD34- LSCs.
Poor-risk cytogenetics and/or FLT3-ITD mutations were more common in patients with ALDHhigh LSCs (85%) than those with ALDHint LCSs (35%) or CD34- LSCs (18%, P<0.001).
Nine percent of patients in the CD34- LSC group fell into the European Leukemia Network poor-risk category, compared to 73% of patients in the ALDHhigh LSC group.
Only 2 patients had 11q23, and both had CD34- LSCs. Fifty-five percent of patients with ALDHhigh LSCs had prior myelodysplastic syndromes or myeloproliferative neoplasms.
Prognosis
“We found that leukemia stem cell phenotype correlated strongly with outcomes as well,” Dr Gerber said. “It turned out that CD34- patients fared more favorably overall.”
Patients with CD34- LSCs had the highest complete response rate (86%), followed by those with ALDHint LSCs (67%) and ALDHhigh LSCs (45%, P<0.01).
Patients in the CD34- group also had a higher rate of event-free survival at 2 years (46%) than patients in the ALDHint group (26%) or the ALDHhigh group (0%). The median event-free survival was 13 months, 11.3 months, and 2.2 months, respectively (P<0.01).
The rate of overall survival at 2 years was best for the CD34- group (76%), followed by the ALDHint group (38%) and the ALDHhigh group (34%). The median overall survival was not reached, 18.7 months, and 9.4 months, respectively (P=0.02).
Dr Gerber also noted that ALDHhigh patients fared much better if they underwent hematopoietic stem cell transplant.
“There is 0% leukemia-free survival at the 2-year mark for the ALDHhigh patients who were not transplanted,” he said. “Those that were transplanted fared about the same as everyone else in the series. So it was very striking that there were no chemotherapy survivors in that group.”
In closing, Dr Gerber said this research suggests the 3 LSC phenotypes are mutually exclusive and correlate with cytogenetic and molecular risk factors as well as outcomes in patients with AML.
“This [discovery] may allow for rapid risk stratification in this explosive disease, facilitate enrollment onto induction protocols . . . , and allow us to divert those ALDHhigh, very high-risk patients earlier to novel therapies and/or transplant, given that they’re not really helped much by conventional chemotherapy.” ![]()
*Information in the abstract differs from that presented at the meeting.

© ASCO/Rodney White
CHICAGO—Researchers say they have identified 3 leukemia stem cell (LSC) phenotypes that are correlated with cytogenetic/molecular abnormalities and prognosis in acute myeloid leukemia (AML).
The investigators believe this knowledge could aid risk stratification of AML patients, particularly those without identifiable cytogenetic or molecular risk factors.
The findings may also pave the way for scientists to identify novel therapeutic targets on LSCs and monitor LSCs in response to therapy.
Jonathan Michael Gerber, MD, of Levine Cancer Institute in Charlotte, North Carolina, presented the findings at the 2015 ASCO Annual Meeting (abstract 7000*).
Via previous research, Dr Gerber and his colleagues identified 3 different LSC phenotypes in AML:
- LSCs that are CD34-negative
- LSCs that are CD34-positive, CD38-negative, and have intermediate levels of aldehyde dehydrogenase (ALDHint)
- LSCs that are CD34-positive, CD38-negative, and have high levels of ALDH (ALDHhigh).
With the current study, the researchers wanted to determine if these phenotypes correlate with cytogenetic/molecular features and treatment outcomes.
So they analyzed diagnostic samples from 98 patients with newly diagnosed AML who had normal or unfavorable cytogenetics. The patients were enrolled on a phase 2 trial comparing FLAM and 7+3 (Zeidner et al, haematologica 2015).
Dr Gerber and his colleagues identified 22 patients with CD34- LSCs, 43 with ALDHint LSCs, and 33 with ALDHhigh LSCs.
Risk factors
“We found that leukemia stem cell phenotype indeed correlated quite strongly with cytogenetic and molecular risk factors,” Dr Gerber said.
NPM1 mutations were more common in patients with CD34- LSCs (64%) than those with ALDHint LSCs (14%) or ALDHhigh LSCs (6%, P<0.001). NPM1 mutations were the sole abnormality in 50% of patients with CD34- LSCs.
Poor-risk cytogenetics and/or FLT3-ITD mutations were more common in patients with ALDHhigh LSCs (85%) than those with ALDHint LCSs (35%) or CD34- LSCs (18%, P<0.001).
Nine percent of patients in the CD34- LSC group fell into the European Leukemia Network poor-risk category, compared to 73% of patients in the ALDHhigh LSC group.
Only 2 patients had 11q23, and both had CD34- LSCs. Fifty-five percent of patients with ALDHhigh LSCs had prior myelodysplastic syndromes or myeloproliferative neoplasms.
Prognosis
“We found that leukemia stem cell phenotype correlated strongly with outcomes as well,” Dr Gerber said. “It turned out that CD34- patients fared more favorably overall.”
Patients with CD34- LSCs had the highest complete response rate (86%), followed by those with ALDHint LSCs (67%) and ALDHhigh LSCs (45%, P<0.01).
Patients in the CD34- group also had a higher rate of event-free survival at 2 years (46%) than patients in the ALDHint group (26%) or the ALDHhigh group (0%). The median event-free survival was 13 months, 11.3 months, and 2.2 months, respectively (P<0.01).
The rate of overall survival at 2 years was best for the CD34- group (76%), followed by the ALDHint group (38%) and the ALDHhigh group (34%). The median overall survival was not reached, 18.7 months, and 9.4 months, respectively (P=0.02).
Dr Gerber also noted that ALDHhigh patients fared much better if they underwent hematopoietic stem cell transplant.
“There is 0% leukemia-free survival at the 2-year mark for the ALDHhigh patients who were not transplanted,” he said. “Those that were transplanted fared about the same as everyone else in the series. So it was very striking that there were no chemotherapy survivors in that group.”
In closing, Dr Gerber said this research suggests the 3 LSC phenotypes are mutually exclusive and correlate with cytogenetic and molecular risk factors as well as outcomes in patients with AML.
“This [discovery] may allow for rapid risk stratification in this explosive disease, facilitate enrollment onto induction protocols . . . , and allow us to divert those ALDHhigh, very high-risk patients earlier to novel therapies and/or transplant, given that they’re not really helped much by conventional chemotherapy.” ![]()
*Information in the abstract differs from that presented at the meeting.

© ASCO/Rodney White
CHICAGO—Researchers say they have identified 3 leukemia stem cell (LSC) phenotypes that are correlated with cytogenetic/molecular abnormalities and prognosis in acute myeloid leukemia (AML).
The investigators believe this knowledge could aid risk stratification of AML patients, particularly those without identifiable cytogenetic or molecular risk factors.
The findings may also pave the way for scientists to identify novel therapeutic targets on LSCs and monitor LSCs in response to therapy.
Jonathan Michael Gerber, MD, of Levine Cancer Institute in Charlotte, North Carolina, presented the findings at the 2015 ASCO Annual Meeting (abstract 7000*).
Via previous research, Dr Gerber and his colleagues identified 3 different LSC phenotypes in AML:
- LSCs that are CD34-negative
- LSCs that are CD34-positive, CD38-negative, and have intermediate levels of aldehyde dehydrogenase (ALDHint)
- LSCs that are CD34-positive, CD38-negative, and have high levels of ALDH (ALDHhigh).
With the current study, the researchers wanted to determine if these phenotypes correlate with cytogenetic/molecular features and treatment outcomes.
So they analyzed diagnostic samples from 98 patients with newly diagnosed AML who had normal or unfavorable cytogenetics. The patients were enrolled on a phase 2 trial comparing FLAM and 7+3 (Zeidner et al, haematologica 2015).
Dr Gerber and his colleagues identified 22 patients with CD34- LSCs, 43 with ALDHint LSCs, and 33 with ALDHhigh LSCs.
Risk factors
“We found that leukemia stem cell phenotype indeed correlated quite strongly with cytogenetic and molecular risk factors,” Dr Gerber said.
NPM1 mutations were more common in patients with CD34- LSCs (64%) than those with ALDHint LSCs (14%) or ALDHhigh LSCs (6%, P<0.001). NPM1 mutations were the sole abnormality in 50% of patients with CD34- LSCs.
Poor-risk cytogenetics and/or FLT3-ITD mutations were more common in patients with ALDHhigh LSCs (85%) than those with ALDHint LCSs (35%) or CD34- LSCs (18%, P<0.001).
Nine percent of patients in the CD34- LSC group fell into the European Leukemia Network poor-risk category, compared to 73% of patients in the ALDHhigh LSC group.
Only 2 patients had 11q23, and both had CD34- LSCs. Fifty-five percent of patients with ALDHhigh LSCs had prior myelodysplastic syndromes or myeloproliferative neoplasms.
Prognosis
“We found that leukemia stem cell phenotype correlated strongly with outcomes as well,” Dr Gerber said. “It turned out that CD34- patients fared more favorably overall.”
Patients with CD34- LSCs had the highest complete response rate (86%), followed by those with ALDHint LSCs (67%) and ALDHhigh LSCs (45%, P<0.01).
Patients in the CD34- group also had a higher rate of event-free survival at 2 years (46%) than patients in the ALDHint group (26%) or the ALDHhigh group (0%). The median event-free survival was 13 months, 11.3 months, and 2.2 months, respectively (P<0.01).
The rate of overall survival at 2 years was best for the CD34- group (76%), followed by the ALDHint group (38%) and the ALDHhigh group (34%). The median overall survival was not reached, 18.7 months, and 9.4 months, respectively (P=0.02).
Dr Gerber also noted that ALDHhigh patients fared much better if they underwent hematopoietic stem cell transplant.
“There is 0% leukemia-free survival at the 2-year mark for the ALDHhigh patients who were not transplanted,” he said. “Those that were transplanted fared about the same as everyone else in the series. So it was very striking that there were no chemotherapy survivors in that group.”
In closing, Dr Gerber said this research suggests the 3 LSC phenotypes are mutually exclusive and correlate with cytogenetic and molecular risk factors as well as outcomes in patients with AML.
“This [discovery] may allow for rapid risk stratification in this explosive disease, facilitate enrollment onto induction protocols . . . , and allow us to divert those ALDHhigh, very high-risk patients earlier to novel therapies and/or transplant, given that they’re not really helped much by conventional chemotherapy.” ![]()
*Information in the abstract differs from that presented at the meeting.
Biochemist Irwin Rose dies at 88

Photo courtesy of UCI
Biochemist and Nobel laureate Irwin “Ernie” Rose, PhD, has passed away at the age of 88.
Dr Rose and colleagues from Israel won the Nobel Prize in Chemistry in 2004 for their discovery of ubiquitin-mediated protein degradation.
This research has wide-ranging implications for medicine and led to the development of anticancer drugs such as bortezomib, which is approved in the US to treat multiple myeloma and mantle cell lymphoma.
According to his friends and colleagues, Dr Rose was humble, generous, and endlessly curious.
“Ernie was not interested in personal fame and was oblivious to the politics of science,” said Ann Skalka, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“His total satisfaction came from solving intricate biochemical puzzles. Although Ernie was an intellectual leader on the project that ultimately won him the Nobel, he took no personal credit. He was rather surprised at being recognized, but all of us at Fox Chase knew that the Nobel Committee had gotten it right.”
Dr Rose was born in Brooklyn, New York, on July 16, 1926. His scientific ambitions began to take shape after he moved to Spokane, Washington, at 13. While in high school, he spent summers working at a local hospital. And this inspired him to pursue a career that involved “solving medical problems.”
Dr Rose attended Washington State College for his undergraduate work and went on to earn a doctoral degree at the University of Chicago, after a brief stint in the Navy. He spent the better part of his career as a research scientist at the Fox Chase Cancer Center.
There, during the late 1970s and early 1980s, Dr Rose helped reveal how ubiquitin molecules facilitate the breakdown of old and damaged proteins. The discovery of this process fostered a new understanding of the molecular activity present in cancers and other diseases.
For the work, Dr Rose shared the 2004 Nobel Prize in Chemistry with Avram Hershko, MD, PhD, and Aaron Ciechanover, MD, PhD, of the Israel Institute of Technology.
“Ernie had a genius for asking the right questions,” said Jonathan Chernoff, MD, PhD, of Fox Chase Cancer Center.
“In the mid-1950s, when many scientists were interested in how proteins are synthesized, Ernie became fascinated with the opposite issue—how are proteins degraded? With the collaboration of his Israeli colleagues, he cracked that problem with the discovery of the ubiquitin conjugating system.”
After retiring to Laguna Woods, California, in 1997, Dr Rose accepted a special research position with the University of California Irvine (UCI).
There, he studied the mechanisms of fumarase, an enzyme involved in the citric acid cycle, the cellular pathway by which higher organisms convert food into energy. And he quickly became a beloved colleague and mentor to students and faculty.
“[B]oth prior to and after winning the Nobel Prize, he would help any student or young postdoctoral researcher who was having a hard time with an experiment,” said Ralph Bradshaw, PhD, a former professor at UCI.
“It was a lot of fun working with him,” said James Nowick, PhD, of UCI. “He worked with his own hands, not relying on others, with old instrumentation, and was able to do literally superb science.”
“He was the quintessential scientist—perseverant, soft-spoken, and interested in science for science’s sake,” Dr Chernoff said. “We will miss him very much.”
Dr Rose died in his sleep on June 2 in Deerfield, Massachusetts. He is survived by his wife, Zelda; their sons, Howard, Frederic, and Robert; and 5 grandchildren. Dr Rose’s daughter, Sarah, died in 2005. ![]()

Photo courtesy of UCI
Biochemist and Nobel laureate Irwin “Ernie” Rose, PhD, has passed away at the age of 88.
Dr Rose and colleagues from Israel won the Nobel Prize in Chemistry in 2004 for their discovery of ubiquitin-mediated protein degradation.
This research has wide-ranging implications for medicine and led to the development of anticancer drugs such as bortezomib, which is approved in the US to treat multiple myeloma and mantle cell lymphoma.
According to his friends and colleagues, Dr Rose was humble, generous, and endlessly curious.
“Ernie was not interested in personal fame and was oblivious to the politics of science,” said Ann Skalka, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“His total satisfaction came from solving intricate biochemical puzzles. Although Ernie was an intellectual leader on the project that ultimately won him the Nobel, he took no personal credit. He was rather surprised at being recognized, but all of us at Fox Chase knew that the Nobel Committee had gotten it right.”
Dr Rose was born in Brooklyn, New York, on July 16, 1926. His scientific ambitions began to take shape after he moved to Spokane, Washington, at 13. While in high school, he spent summers working at a local hospital. And this inspired him to pursue a career that involved “solving medical problems.”
Dr Rose attended Washington State College for his undergraduate work and went on to earn a doctoral degree at the University of Chicago, after a brief stint in the Navy. He spent the better part of his career as a research scientist at the Fox Chase Cancer Center.
There, during the late 1970s and early 1980s, Dr Rose helped reveal how ubiquitin molecules facilitate the breakdown of old and damaged proteins. The discovery of this process fostered a new understanding of the molecular activity present in cancers and other diseases.
For the work, Dr Rose shared the 2004 Nobel Prize in Chemistry with Avram Hershko, MD, PhD, and Aaron Ciechanover, MD, PhD, of the Israel Institute of Technology.
“Ernie had a genius for asking the right questions,” said Jonathan Chernoff, MD, PhD, of Fox Chase Cancer Center.
“In the mid-1950s, when many scientists were interested in how proteins are synthesized, Ernie became fascinated with the opposite issue—how are proteins degraded? With the collaboration of his Israeli colleagues, he cracked that problem with the discovery of the ubiquitin conjugating system.”
After retiring to Laguna Woods, California, in 1997, Dr Rose accepted a special research position with the University of California Irvine (UCI).
There, he studied the mechanisms of fumarase, an enzyme involved in the citric acid cycle, the cellular pathway by which higher organisms convert food into energy. And he quickly became a beloved colleague and mentor to students and faculty.
“[B]oth prior to and after winning the Nobel Prize, he would help any student or young postdoctoral researcher who was having a hard time with an experiment,” said Ralph Bradshaw, PhD, a former professor at UCI.
“It was a lot of fun working with him,” said James Nowick, PhD, of UCI. “He worked with his own hands, not relying on others, with old instrumentation, and was able to do literally superb science.”
“He was the quintessential scientist—perseverant, soft-spoken, and interested in science for science’s sake,” Dr Chernoff said. “We will miss him very much.”
Dr Rose died in his sleep on June 2 in Deerfield, Massachusetts. He is survived by his wife, Zelda; their sons, Howard, Frederic, and Robert; and 5 grandchildren. Dr Rose’s daughter, Sarah, died in 2005. ![]()

Photo courtesy of UCI
Biochemist and Nobel laureate Irwin “Ernie” Rose, PhD, has passed away at the age of 88.
Dr Rose and colleagues from Israel won the Nobel Prize in Chemistry in 2004 for their discovery of ubiquitin-mediated protein degradation.
This research has wide-ranging implications for medicine and led to the development of anticancer drugs such as bortezomib, which is approved in the US to treat multiple myeloma and mantle cell lymphoma.
According to his friends and colleagues, Dr Rose was humble, generous, and endlessly curious.
“Ernie was not interested in personal fame and was oblivious to the politics of science,” said Ann Skalka, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“His total satisfaction came from solving intricate biochemical puzzles. Although Ernie was an intellectual leader on the project that ultimately won him the Nobel, he took no personal credit. He was rather surprised at being recognized, but all of us at Fox Chase knew that the Nobel Committee had gotten it right.”
Dr Rose was born in Brooklyn, New York, on July 16, 1926. His scientific ambitions began to take shape after he moved to Spokane, Washington, at 13. While in high school, he spent summers working at a local hospital. And this inspired him to pursue a career that involved “solving medical problems.”
Dr Rose attended Washington State College for his undergraduate work and went on to earn a doctoral degree at the University of Chicago, after a brief stint in the Navy. He spent the better part of his career as a research scientist at the Fox Chase Cancer Center.
There, during the late 1970s and early 1980s, Dr Rose helped reveal how ubiquitin molecules facilitate the breakdown of old and damaged proteins. The discovery of this process fostered a new understanding of the molecular activity present in cancers and other diseases.
For the work, Dr Rose shared the 2004 Nobel Prize in Chemistry with Avram Hershko, MD, PhD, and Aaron Ciechanover, MD, PhD, of the Israel Institute of Technology.
“Ernie had a genius for asking the right questions,” said Jonathan Chernoff, MD, PhD, of Fox Chase Cancer Center.
“In the mid-1950s, when many scientists were interested in how proteins are synthesized, Ernie became fascinated with the opposite issue—how are proteins degraded? With the collaboration of his Israeli colleagues, he cracked that problem with the discovery of the ubiquitin conjugating system.”
After retiring to Laguna Woods, California, in 1997, Dr Rose accepted a special research position with the University of California Irvine (UCI).
There, he studied the mechanisms of fumarase, an enzyme involved in the citric acid cycle, the cellular pathway by which higher organisms convert food into energy. And he quickly became a beloved colleague and mentor to students and faculty.
“[B]oth prior to and after winning the Nobel Prize, he would help any student or young postdoctoral researcher who was having a hard time with an experiment,” said Ralph Bradshaw, PhD, a former professor at UCI.
“It was a lot of fun working with him,” said James Nowick, PhD, of UCI. “He worked with his own hands, not relying on others, with old instrumentation, and was able to do literally superb science.”
“He was the quintessential scientist—perseverant, soft-spoken, and interested in science for science’s sake,” Dr Chernoff said. “We will miss him very much.”
Dr Rose died in his sleep on June 2 in Deerfield, Massachusetts. He is survived by his wife, Zelda; their sons, Howard, Frederic, and Robert; and 5 grandchildren. Dr Rose’s daughter, Sarah, died in 2005. ![]()
Nutrition source could improve platinum-based nanodrugs

A parenteral nutrition source can reduce the toxicity and increase the bioavailability of platinum-based anticancer nanodrugs, according to preclinical research published in Scientific Reports.
Many of the side effects of platinum-based drugs occur when they settle in healthy tissue.
To deliver these drugs in a more targeted way, researchers have created nanoscale delivery systems engineered to make the drugs accumulate at tumor sites.
However, tests of these nanodrugs show that between 1% and 10% of the drugs are delivered to the tumor site, with most of the remainder being diverted to the liver and spleen.
“The body’s immune system, especially the liver and spleen, has been one of the biggest stumbling blocks in developing nanoscale chemotherapy drug delivery systems,” said Chien Ho, PhD, of Carnegie Mellon University in Pittsburg, Pennsylvania.
“When the drugs collect in those organs, they become less available to treat the cancer and can also cause toxicity.”
But Dr Ho and his colleagues have found evidence to suggest that Intralipid, a fat emulsion used as a parenteral nutrition source, can help prevent that.
While developing cellular nanotags to help detect organ rejection, Dr Ho noticed that Intralipid reduced the amount of nanoparticles that were being cleared by the liver and spleen by about 50%. As a result, the nanoparticles remained in the bloodstream for longer periods of time.
So he and his colleagues decided to see if Intralipid had the same effect on platinum-based anticancer nanodrugs.
In the newly published study, the researchers administered a single, clinical dose of Intralipid to Sprague Dawley rats. One hour later, they administered a dose of a platinum-based chemotherapy drug that had been incorporated into a nanoparticle to both Intralipid-treated rats and controls.
Twenty-four hours after the drug was administered, rats pretreated with Intralipid had experienced reduced accumulation of the platinum-based drug compared to controls.
Drug accumulation decreased by 20.4% in the liver, 42.5% in the spleen, and 31.2% in the kidney. Consequently, in these organs, the toxic side effects of the nanodrug were significantly decreased compared to controls.
Furthermore, Intralipid pretreatment allowed more of the drug to remain available and active in the body for longer periods of time.
After 5 hours, the drug’s bioavailability increased by 18.7% in Intralipid-treated mice compared to controls. After 24 hours, bioavailability was 9.4% higher in Intralipid-treated mice than in controls.
The researchers believe this increased bioavailability will allow more of the drug to reach the tumor site and could perhaps allow clinicians to reduce the dosage needed to treat a patient. The team is now investigating the possibility of bringing this research to a clinical trial. ![]()

A parenteral nutrition source can reduce the toxicity and increase the bioavailability of platinum-based anticancer nanodrugs, according to preclinical research published in Scientific Reports.
Many of the side effects of platinum-based drugs occur when they settle in healthy tissue.
To deliver these drugs in a more targeted way, researchers have created nanoscale delivery systems engineered to make the drugs accumulate at tumor sites.
However, tests of these nanodrugs show that between 1% and 10% of the drugs are delivered to the tumor site, with most of the remainder being diverted to the liver and spleen.
“The body’s immune system, especially the liver and spleen, has been one of the biggest stumbling blocks in developing nanoscale chemotherapy drug delivery systems,” said Chien Ho, PhD, of Carnegie Mellon University in Pittsburg, Pennsylvania.
“When the drugs collect in those organs, they become less available to treat the cancer and can also cause toxicity.”
But Dr Ho and his colleagues have found evidence to suggest that Intralipid, a fat emulsion used as a parenteral nutrition source, can help prevent that.
While developing cellular nanotags to help detect organ rejection, Dr Ho noticed that Intralipid reduced the amount of nanoparticles that were being cleared by the liver and spleen by about 50%. As a result, the nanoparticles remained in the bloodstream for longer periods of time.
So he and his colleagues decided to see if Intralipid had the same effect on platinum-based anticancer nanodrugs.
In the newly published study, the researchers administered a single, clinical dose of Intralipid to Sprague Dawley rats. One hour later, they administered a dose of a platinum-based chemotherapy drug that had been incorporated into a nanoparticle to both Intralipid-treated rats and controls.
Twenty-four hours after the drug was administered, rats pretreated with Intralipid had experienced reduced accumulation of the platinum-based drug compared to controls.
Drug accumulation decreased by 20.4% in the liver, 42.5% in the spleen, and 31.2% in the kidney. Consequently, in these organs, the toxic side effects of the nanodrug were significantly decreased compared to controls.
Furthermore, Intralipid pretreatment allowed more of the drug to remain available and active in the body for longer periods of time.
After 5 hours, the drug’s bioavailability increased by 18.7% in Intralipid-treated mice compared to controls. After 24 hours, bioavailability was 9.4% higher in Intralipid-treated mice than in controls.
The researchers believe this increased bioavailability will allow more of the drug to reach the tumor site and could perhaps allow clinicians to reduce the dosage needed to treat a patient. The team is now investigating the possibility of bringing this research to a clinical trial. ![]()

A parenteral nutrition source can reduce the toxicity and increase the bioavailability of platinum-based anticancer nanodrugs, according to preclinical research published in Scientific Reports.
Many of the side effects of platinum-based drugs occur when they settle in healthy tissue.
To deliver these drugs in a more targeted way, researchers have created nanoscale delivery systems engineered to make the drugs accumulate at tumor sites.
However, tests of these nanodrugs show that between 1% and 10% of the drugs are delivered to the tumor site, with most of the remainder being diverted to the liver and spleen.
“The body’s immune system, especially the liver and spleen, has been one of the biggest stumbling blocks in developing nanoscale chemotherapy drug delivery systems,” said Chien Ho, PhD, of Carnegie Mellon University in Pittsburg, Pennsylvania.
“When the drugs collect in those organs, they become less available to treat the cancer and can also cause toxicity.”
But Dr Ho and his colleagues have found evidence to suggest that Intralipid, a fat emulsion used as a parenteral nutrition source, can help prevent that.
While developing cellular nanotags to help detect organ rejection, Dr Ho noticed that Intralipid reduced the amount of nanoparticles that were being cleared by the liver and spleen by about 50%. As a result, the nanoparticles remained in the bloodstream for longer periods of time.
So he and his colleagues decided to see if Intralipid had the same effect on platinum-based anticancer nanodrugs.
In the newly published study, the researchers administered a single, clinical dose of Intralipid to Sprague Dawley rats. One hour later, they administered a dose of a platinum-based chemotherapy drug that had been incorporated into a nanoparticle to both Intralipid-treated rats and controls.
Twenty-four hours after the drug was administered, rats pretreated with Intralipid had experienced reduced accumulation of the platinum-based drug compared to controls.
Drug accumulation decreased by 20.4% in the liver, 42.5% in the spleen, and 31.2% in the kidney. Consequently, in these organs, the toxic side effects of the nanodrug were significantly decreased compared to controls.
Furthermore, Intralipid pretreatment allowed more of the drug to remain available and active in the body for longer periods of time.
After 5 hours, the drug’s bioavailability increased by 18.7% in Intralipid-treated mice compared to controls. After 24 hours, bioavailability was 9.4% higher in Intralipid-treated mice than in controls.
The researchers believe this increased bioavailability will allow more of the drug to reach the tumor site and could perhaps allow clinicians to reduce the dosage needed to treat a patient. The team is now investigating the possibility of bringing this research to a clinical trial. ![]()
Letter to the Editor
I read Beck et al.'s article titled Redesigning an Inpatient Pediatric Service Using Lean to Improve Throughput Efficiency with great interest.[1] Redesigning the rounding process using Lean not only created a standard workflow including seeing dischargeable patients first, involving interdisciplinary huddle, completing the discharge checklist at bedside, but also added a second attending physician, thereby decreasing the workload. Stein et al. demonstrated that restructured floor‐based patient care including unit‐based teams, interdisciplinary bedside rounds, unit‐level performance reporting, and unit‐level nurse and physician coleadership all improved workflow with an average of 12.9 patients per physician.[2] Another study showed that increased workload was associated with prolonged length of stay with a recommended number of patients per day per physician at 15.[3] I want to point out the number of patients per physician in these studies. Today's hospitalists in community hospitals are expected to see >18 patients per day, with the additional pressure of decreasing costs, readmission rates, length of stays, and time for discharge while increasing productivity and patient satisfaction. Michtalik et al.'s survey showed that 40% of hospitalists reported exceeding their own safe numbers. Regardless of any assistance, physicians reported that they could safely see 15 patients per shift if their effort was 100% clinical.[4] Therefore, despite the outstanding results of the above studies, I am hesitant as to whether similar interventions would be as successful in community hospitals with higher patient loads. We need further studies to determine the optimum number of patients per hospitalist for nonteaching community hospitals. Another concern is how to adopt the successful examples of academic centers in nonteaching community hospitals in the absence of interns. Expecting hospitalists to replace the intern role is worrisome for job satisfaction, especially in the presence of high burnout rates.
Eliminating waste and redesigning the rounding process initiatives will definitely be the norm over the next years. We need to define center‐specific right patient/hospitalist ratios with proper roles and responsibilities for hospitalists. What works in the presence of residents may not work for nonteaching community hospitals. Caution should be taken while restructuring hospital medicine.
- , . Redesigning an inpatient pediatric service using Lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220–227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- , , , . Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med. 2013;173(5):375–377.
I read Beck et al.'s article titled Redesigning an Inpatient Pediatric Service Using Lean to Improve Throughput Efficiency with great interest.[1] Redesigning the rounding process using Lean not only created a standard workflow including seeing dischargeable patients first, involving interdisciplinary huddle, completing the discharge checklist at bedside, but also added a second attending physician, thereby decreasing the workload. Stein et al. demonstrated that restructured floor‐based patient care including unit‐based teams, interdisciplinary bedside rounds, unit‐level performance reporting, and unit‐level nurse and physician coleadership all improved workflow with an average of 12.9 patients per physician.[2] Another study showed that increased workload was associated with prolonged length of stay with a recommended number of patients per day per physician at 15.[3] I want to point out the number of patients per physician in these studies. Today's hospitalists in community hospitals are expected to see >18 patients per day, with the additional pressure of decreasing costs, readmission rates, length of stays, and time for discharge while increasing productivity and patient satisfaction. Michtalik et al.'s survey showed that 40% of hospitalists reported exceeding their own safe numbers. Regardless of any assistance, physicians reported that they could safely see 15 patients per shift if their effort was 100% clinical.[4] Therefore, despite the outstanding results of the above studies, I am hesitant as to whether similar interventions would be as successful in community hospitals with higher patient loads. We need further studies to determine the optimum number of patients per hospitalist for nonteaching community hospitals. Another concern is how to adopt the successful examples of academic centers in nonteaching community hospitals in the absence of interns. Expecting hospitalists to replace the intern role is worrisome for job satisfaction, especially in the presence of high burnout rates.
Eliminating waste and redesigning the rounding process initiatives will definitely be the norm over the next years. We need to define center‐specific right patient/hospitalist ratios with proper roles and responsibilities for hospitalists. What works in the presence of residents may not work for nonteaching community hospitals. Caution should be taken while restructuring hospital medicine.
I read Beck et al.'s article titled Redesigning an Inpatient Pediatric Service Using Lean to Improve Throughput Efficiency with great interest.[1] Redesigning the rounding process using Lean not only created a standard workflow including seeing dischargeable patients first, involving interdisciplinary huddle, completing the discharge checklist at bedside, but also added a second attending physician, thereby decreasing the workload. Stein et al. demonstrated that restructured floor‐based patient care including unit‐based teams, interdisciplinary bedside rounds, unit‐level performance reporting, and unit‐level nurse and physician coleadership all improved workflow with an average of 12.9 patients per physician.[2] Another study showed that increased workload was associated with prolonged length of stay with a recommended number of patients per day per physician at 15.[3] I want to point out the number of patients per physician in these studies. Today's hospitalists in community hospitals are expected to see >18 patients per day, with the additional pressure of decreasing costs, readmission rates, length of stays, and time for discharge while increasing productivity and patient satisfaction. Michtalik et al.'s survey showed that 40% of hospitalists reported exceeding their own safe numbers. Regardless of any assistance, physicians reported that they could safely see 15 patients per shift if their effort was 100% clinical.[4] Therefore, despite the outstanding results of the above studies, I am hesitant as to whether similar interventions would be as successful in community hospitals with higher patient loads. We need further studies to determine the optimum number of patients per hospitalist for nonteaching community hospitals. Another concern is how to adopt the successful examples of academic centers in nonteaching community hospitals in the absence of interns. Expecting hospitalists to replace the intern role is worrisome for job satisfaction, especially in the presence of high burnout rates.
Eliminating waste and redesigning the rounding process initiatives will definitely be the norm over the next years. We need to define center‐specific right patient/hospitalist ratios with proper roles and responsibilities for hospitalists. What works in the presence of residents may not work for nonteaching community hospitals. Caution should be taken while restructuring hospital medicine.
- , . Redesigning an inpatient pediatric service using Lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220–227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- , , , . Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med. 2013;173(5):375–377.
- , . Redesigning an inpatient pediatric service using Lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220–227.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- , , , . Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med. 2013;173(5):375–377.
Tight glycemic control: Somewhat fewer CV events, same mortality
Tight glycemic control modestly reduced the rate of major cardiovascular events but didn’t improve mortality in an extended follow-up of a clinical trial involving 1,791 veterans with type 2 diabetes, which was published online June 3 in the New England Journal of Medicine.
At the conclusion of the treatment phase of the Veteran Affairs Diabetes Trial in 2008, the primary outcome – the rate of a first major CV event – was nonsignificantly lower with intensive glycemic control than with standard glycemic control. Researchers now report the findings after an additional 7.5 years of follow-up of 92% of the participants in that multicenter unblended randomized controlled trial.
During the treatment phase of the study, median glycated hemoglobin level differed by 1.5 percentage points between patients who received intensive therapy (6.9%) and patients who received standard therapy (8.4%). During follow-up, this difference declined to only 0.2-0.3 percentage points. “Even with the support of a dedicated research team, only approximately half the participants [achieved] a glycated hemoglobin level of less than 7%,” said Dr. Rodney A. Hayward of the VA Center for Clinical Management Research, VA Ann Arbor (Mich.) Healthcare System, and his associates.
During extended follow-up, there were 253 major CV events in the group randomly assigned to intensive therapy and 288 in the group assigned to standard therapy. Tight glycemic control using a multidrug regimen was associated with a significant, though modest, 17% relative reduction in a the primary composite outcome of heart attack, stroke, new or worsening congestive heart failure, death from CV causes, or amputation due to ischemic gangrene. This represents 8.6 CV events prevented per 1,000 person-years.
However, there was no evidence of any reduction in either cardiovascular or all-cause mortality. In addition, treatment effects were no different between patients at high and those at low cardiovascular risk, the investigators said (N. Engl. J. Med. 2015 June 3 [doi:10.1056/NEJMoa1414266]).
“In the absence of a reduction in total mortality, a small to moderate reduction in the rate of CV events needs to be weighed against potential harm due to overly aggressive care and the burden, long-term safety profile, and side effects of treatment, including weight gain and hypoglycemia,” they added.
Tight glycemic control modestly reduced the rate of major cardiovascular events but didn’t improve mortality in an extended follow-up of a clinical trial involving 1,791 veterans with type 2 diabetes, which was published online June 3 in the New England Journal of Medicine.
At the conclusion of the treatment phase of the Veteran Affairs Diabetes Trial in 2008, the primary outcome – the rate of a first major CV event – was nonsignificantly lower with intensive glycemic control than with standard glycemic control. Researchers now report the findings after an additional 7.5 years of follow-up of 92% of the participants in that multicenter unblended randomized controlled trial.
During the treatment phase of the study, median glycated hemoglobin level differed by 1.5 percentage points between patients who received intensive therapy (6.9%) and patients who received standard therapy (8.4%). During follow-up, this difference declined to only 0.2-0.3 percentage points. “Even with the support of a dedicated research team, only approximately half the participants [achieved] a glycated hemoglobin level of less than 7%,” said Dr. Rodney A. Hayward of the VA Center for Clinical Management Research, VA Ann Arbor (Mich.) Healthcare System, and his associates.
During extended follow-up, there were 253 major CV events in the group randomly assigned to intensive therapy and 288 in the group assigned to standard therapy. Tight glycemic control using a multidrug regimen was associated with a significant, though modest, 17% relative reduction in a the primary composite outcome of heart attack, stroke, new or worsening congestive heart failure, death from CV causes, or amputation due to ischemic gangrene. This represents 8.6 CV events prevented per 1,000 person-years.
However, there was no evidence of any reduction in either cardiovascular or all-cause mortality. In addition, treatment effects were no different between patients at high and those at low cardiovascular risk, the investigators said (N. Engl. J. Med. 2015 June 3 [doi:10.1056/NEJMoa1414266]).
“In the absence of a reduction in total mortality, a small to moderate reduction in the rate of CV events needs to be weighed against potential harm due to overly aggressive care and the burden, long-term safety profile, and side effects of treatment, including weight gain and hypoglycemia,” they added.
Tight glycemic control modestly reduced the rate of major cardiovascular events but didn’t improve mortality in an extended follow-up of a clinical trial involving 1,791 veterans with type 2 diabetes, which was published online June 3 in the New England Journal of Medicine.
At the conclusion of the treatment phase of the Veteran Affairs Diabetes Trial in 2008, the primary outcome – the rate of a first major CV event – was nonsignificantly lower with intensive glycemic control than with standard glycemic control. Researchers now report the findings after an additional 7.5 years of follow-up of 92% of the participants in that multicenter unblended randomized controlled trial.
During the treatment phase of the study, median glycated hemoglobin level differed by 1.5 percentage points between patients who received intensive therapy (6.9%) and patients who received standard therapy (8.4%). During follow-up, this difference declined to only 0.2-0.3 percentage points. “Even with the support of a dedicated research team, only approximately half the participants [achieved] a glycated hemoglobin level of less than 7%,” said Dr. Rodney A. Hayward of the VA Center for Clinical Management Research, VA Ann Arbor (Mich.) Healthcare System, and his associates.
During extended follow-up, there were 253 major CV events in the group randomly assigned to intensive therapy and 288 in the group assigned to standard therapy. Tight glycemic control using a multidrug regimen was associated with a significant, though modest, 17% relative reduction in a the primary composite outcome of heart attack, stroke, new or worsening congestive heart failure, death from CV causes, or amputation due to ischemic gangrene. This represents 8.6 CV events prevented per 1,000 person-years.
However, there was no evidence of any reduction in either cardiovascular or all-cause mortality. In addition, treatment effects were no different between patients at high and those at low cardiovascular risk, the investigators said (N. Engl. J. Med. 2015 June 3 [doi:10.1056/NEJMoa1414266]).
“In the absence of a reduction in total mortality, a small to moderate reduction in the rate of CV events needs to be weighed against potential harm due to overly aggressive care and the burden, long-term safety profile, and side effects of treatment, including weight gain and hypoglycemia,” they added.
Key clinical point: Tight glycemic control cut the rate of major cardiovascular events by 17% but didn’t improve mortality in patients with type 2 diabetes.
Major finding: Compared with standard glycemic control, tight glycemic control prevented 8.6 CV events per 1,000 person-years.
Data source: Extended follow-up of an unblinded, multicenter, randomized, controlled trial involving 1,791 veterans with type 2 diabetes.
Disclosures: This study was supported by the VA Cooperative Studies Program, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institutes of Health. Dr. Hayward reported having no relevant financial disclosures; two of his associates reported ties to Amgen, AstraZeneca, Merck, and Novo Nordisk.
No Advantage to Routine Thrombectomy Prior to Percutaneous Coronary Intervention for STEMI
Clinical question: Does the use of routine thrombectomy for patients presenting with ST-segment elevation myocardial infarction improve outcomes?
Bottom line: For patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI), the routine use of manual thrombectomy improves some electrocardiographic and angiographic outcomes, but ultimately does not result in improved cardiovascular morbidity or mortality. Moreover, thrombectomy may increase the risk of stroke. (LOE = 1b)
Reference: Jolly SS, Cairns JA, Yusuf S, et al, for the TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372(15):1389–1398.
Study design: Randomized controlled trial (nonblinded)
Funding source: Industry + govt
Allocation: Concealed
Setting: Inpatient (any location) with outpatient follow-up
Synopsis
Manual thrombectomy with aspiration of thrombus prior to PCI is thought to prevent distal embolization and improve microvascular perfusion. Whether this results in clinical benefit is unclear. In this study, the investigators randomized patients presenting with STEMI to undergo either routine thrombus aspiration followed by PCI or PCI alone. Those who had a previous history of coronary-artery bypass grafting or those who had received fibrinolytics were excluded. The 2 groups were balanced at baseline, with almost 80% of patients in each group noted to have a high thrombus burden. A modified intention-to-treat analysis was used that included only those patients who actually underwent PCI for the index STEMI.
Although electrocardiographic and angiographic outcomes improved with thrombectomy (eg, increased ST-segment resolution, decreased distal embolization), no clinical benefit was found. Specifically, for the primary outcome of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or New York Heart Association class IV heart failure within 180 days of randomization, there were no significant differences detected between the 2 groups. The components of the composite outcome taken individually were also similar in each group. These results persisted across prespecified analyses of the as-treated population, per-protocol population, and the subgroup with high thrombus burden. Additionally, patients in the thrombectomy group were more likely to have a stroke within 30 days and 180 days, although the number of events was relatively small (for 30 days: 0.7% vs 0.3%, P = .02; for 180 days: 1% vs 0.5%, P = .002).
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Does the use of routine thrombectomy for patients presenting with ST-segment elevation myocardial infarction improve outcomes?
Bottom line: For patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI), the routine use of manual thrombectomy improves some electrocardiographic and angiographic outcomes, but ultimately does not result in improved cardiovascular morbidity or mortality. Moreover, thrombectomy may increase the risk of stroke. (LOE = 1b)
Reference: Jolly SS, Cairns JA, Yusuf S, et al, for the TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372(15):1389–1398.
Study design: Randomized controlled trial (nonblinded)
Funding source: Industry + govt
Allocation: Concealed
Setting: Inpatient (any location) with outpatient follow-up
Synopsis
Manual thrombectomy with aspiration of thrombus prior to PCI is thought to prevent distal embolization and improve microvascular perfusion. Whether this results in clinical benefit is unclear. In this study, the investigators randomized patients presenting with STEMI to undergo either routine thrombus aspiration followed by PCI or PCI alone. Those who had a previous history of coronary-artery bypass grafting or those who had received fibrinolytics were excluded. The 2 groups were balanced at baseline, with almost 80% of patients in each group noted to have a high thrombus burden. A modified intention-to-treat analysis was used that included only those patients who actually underwent PCI for the index STEMI.
Although electrocardiographic and angiographic outcomes improved with thrombectomy (eg, increased ST-segment resolution, decreased distal embolization), no clinical benefit was found. Specifically, for the primary outcome of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or New York Heart Association class IV heart failure within 180 days of randomization, there were no significant differences detected between the 2 groups. The components of the composite outcome taken individually were also similar in each group. These results persisted across prespecified analyses of the as-treated population, per-protocol population, and the subgroup with high thrombus burden. Additionally, patients in the thrombectomy group were more likely to have a stroke within 30 days and 180 days, although the number of events was relatively small (for 30 days: 0.7% vs 0.3%, P = .02; for 180 days: 1% vs 0.5%, P = .002).
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Does the use of routine thrombectomy for patients presenting with ST-segment elevation myocardial infarction improve outcomes?
Bottom line: For patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI), the routine use of manual thrombectomy improves some electrocardiographic and angiographic outcomes, but ultimately does not result in improved cardiovascular morbidity or mortality. Moreover, thrombectomy may increase the risk of stroke. (LOE = 1b)
Reference: Jolly SS, Cairns JA, Yusuf S, et al, for the TOTAL Investigators. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372(15):1389–1398.
Study design: Randomized controlled trial (nonblinded)
Funding source: Industry + govt
Allocation: Concealed
Setting: Inpatient (any location) with outpatient follow-up
Synopsis
Manual thrombectomy with aspiration of thrombus prior to PCI is thought to prevent distal embolization and improve microvascular perfusion. Whether this results in clinical benefit is unclear. In this study, the investigators randomized patients presenting with STEMI to undergo either routine thrombus aspiration followed by PCI or PCI alone. Those who had a previous history of coronary-artery bypass grafting or those who had received fibrinolytics were excluded. The 2 groups were balanced at baseline, with almost 80% of patients in each group noted to have a high thrombus burden. A modified intention-to-treat analysis was used that included only those patients who actually underwent PCI for the index STEMI.
Although electrocardiographic and angiographic outcomes improved with thrombectomy (eg, increased ST-segment resolution, decreased distal embolization), no clinical benefit was found. Specifically, for the primary outcome of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or New York Heart Association class IV heart failure within 180 days of randomization, there were no significant differences detected between the 2 groups. The components of the composite outcome taken individually were also similar in each group. These results persisted across prespecified analyses of the as-treated population, per-protocol population, and the subgroup with high thrombus burden. Additionally, patients in the thrombectomy group were more likely to have a stroke within 30 days and 180 days, although the number of events was relatively small (for 30 days: 0.7% vs 0.3%, P = .02; for 180 days: 1% vs 0.5%, P = .002).
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.