User login
Lung Ultrasound for Diagnosing Pneumonia in Children: A Meta-Analysis
Clinical question: Can bedside ultrasound reliably diagnose pneumonia in children?
Background: Pneumonia is the most common cause of death in children worldwide, one of the most common causes of inpatient admission for children in the U.S., and carries estimated costs of about $1 billion per year. Its diagnosis can challenge clinicians and require radiographic and laboratory studies. Chest radiographs require specialized equipment, expertise to interpret, and ionizing radiation exposure. Radiographic findings can also lag behind the clinical picture. Ultrasound, a rapid, noninvasive, portable, and inexpensive imaging modality that can be taught quickly, is widely used to diagnose such pediatric conditions as appendicitis and abscess and may be useful in diagnosing pediatric pneumonia.
Study design: Meta-analysis.
Setting: Eight studies reviewed and synthesized (three ED, two regular inpatient, one PICU, two NICU).
Synopsis: Lung ultrasound was compared to chest radiograph, lab findings, and clinical findings for detection of pneumonia. In pooled analysis, lung ultrasound was 96% sensitive and 93% specific for the diagnosis of pneumonia with area under the receiver operating characteristic (ROC) curve of 0.98 (95% CI 0.96-1). Subgroup analysis showed that having physicians who have had experience with more than 100 ultrasounds or radiologists performing the ultrasounds increased the sensitivity to 97% and the specificity to 99%. Novice users could detect pneumonia with less reliability.
Bottom line: Lung ultrasound is a promising and potentially reliable modality for diagnosis of pneumonia. This may prove especially useful in resource-poor settings or in clinics without access to chest radiographs.
Citation: Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta analysis. Pediatrics. 2015;135(4):714-722.

Clinical question: Can bedside ultrasound reliably diagnose pneumonia in children?
Background: Pneumonia is the most common cause of death in children worldwide, one of the most common causes of inpatient admission for children in the U.S., and carries estimated costs of about $1 billion per year. Its diagnosis can challenge clinicians and require radiographic and laboratory studies. Chest radiographs require specialized equipment, expertise to interpret, and ionizing radiation exposure. Radiographic findings can also lag behind the clinical picture. Ultrasound, a rapid, noninvasive, portable, and inexpensive imaging modality that can be taught quickly, is widely used to diagnose such pediatric conditions as appendicitis and abscess and may be useful in diagnosing pediatric pneumonia.
Study design: Meta-analysis.
Setting: Eight studies reviewed and synthesized (three ED, two regular inpatient, one PICU, two NICU).
Synopsis: Lung ultrasound was compared to chest radiograph, lab findings, and clinical findings for detection of pneumonia. In pooled analysis, lung ultrasound was 96% sensitive and 93% specific for the diagnosis of pneumonia with area under the receiver operating characteristic (ROC) curve of 0.98 (95% CI 0.96-1). Subgroup analysis showed that having physicians who have had experience with more than 100 ultrasounds or radiologists performing the ultrasounds increased the sensitivity to 97% and the specificity to 99%. Novice users could detect pneumonia with less reliability.
Bottom line: Lung ultrasound is a promising and potentially reliable modality for diagnosis of pneumonia. This may prove especially useful in resource-poor settings or in clinics without access to chest radiographs.
Citation: Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta analysis. Pediatrics. 2015;135(4):714-722.

Clinical question: Can bedside ultrasound reliably diagnose pneumonia in children?
Background: Pneumonia is the most common cause of death in children worldwide, one of the most common causes of inpatient admission for children in the U.S., and carries estimated costs of about $1 billion per year. Its diagnosis can challenge clinicians and require radiographic and laboratory studies. Chest radiographs require specialized equipment, expertise to interpret, and ionizing radiation exposure. Radiographic findings can also lag behind the clinical picture. Ultrasound, a rapid, noninvasive, portable, and inexpensive imaging modality that can be taught quickly, is widely used to diagnose such pediatric conditions as appendicitis and abscess and may be useful in diagnosing pediatric pneumonia.
Study design: Meta-analysis.
Setting: Eight studies reviewed and synthesized (three ED, two regular inpatient, one PICU, two NICU).
Synopsis: Lung ultrasound was compared to chest radiograph, lab findings, and clinical findings for detection of pneumonia. In pooled analysis, lung ultrasound was 96% sensitive and 93% specific for the diagnosis of pneumonia with area under the receiver operating characteristic (ROC) curve of 0.98 (95% CI 0.96-1). Subgroup analysis showed that having physicians who have had experience with more than 100 ultrasounds or radiologists performing the ultrasounds increased the sensitivity to 97% and the specificity to 99%. Novice users could detect pneumonia with less reliability.
Bottom line: Lung ultrasound is a promising and potentially reliable modality for diagnosis of pneumonia. This may prove especially useful in resource-poor settings or in clinics without access to chest radiographs.
Citation: Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta analysis. Pediatrics. 2015;135(4):714-722.

How Hospitalists Can Support the Hospital Medicine Movement
There are all kinds of ways to show your support for the hospital medicine movement, including the following opportunities:
- Visit and share the new Future of Hospital Medicine website (www.futureofhospitalmedicine.org) with medical students, residents, and colleagues.
- Plan to attend a Future of Hospital Medicine event in your city:
- Chicago: Rush University, Sept. 24
- Los Angeles: UCLA, Oct. 22
- Apply for FHM or SFHM status today! Now’s the time to begin the application process for Fellow in Hospital Medicine or Senior Fellow. Visit www.hospitalmedicine.org/fellows for details.
- Recruit others to join the movement. Active members can receive dues credits and special recognition for recruiting others. For details, visit www.hospitalmedicine.org/membership.
- Get the gear! Visit www.hospitalmedicine.org for the latest in hospitalist apparel, including new hoodies, coffee tumblers, water bottles, and tablet cases.
There are all kinds of ways to show your support for the hospital medicine movement, including the following opportunities:
- Visit and share the new Future of Hospital Medicine website (www.futureofhospitalmedicine.org) with medical students, residents, and colleagues.
- Plan to attend a Future of Hospital Medicine event in your city:
- Chicago: Rush University, Sept. 24
- Los Angeles: UCLA, Oct. 22
- Apply for FHM or SFHM status today! Now’s the time to begin the application process for Fellow in Hospital Medicine or Senior Fellow. Visit www.hospitalmedicine.org/fellows for details.
- Recruit others to join the movement. Active members can receive dues credits and special recognition for recruiting others. For details, visit www.hospitalmedicine.org/membership.
- Get the gear! Visit www.hospitalmedicine.org for the latest in hospitalist apparel, including new hoodies, coffee tumblers, water bottles, and tablet cases.
There are all kinds of ways to show your support for the hospital medicine movement, including the following opportunities:
- Visit and share the new Future of Hospital Medicine website (www.futureofhospitalmedicine.org) with medical students, residents, and colleagues.
- Plan to attend a Future of Hospital Medicine event in your city:
- Chicago: Rush University, Sept. 24
- Los Angeles: UCLA, Oct. 22
- Apply for FHM or SFHM status today! Now’s the time to begin the application process for Fellow in Hospital Medicine or Senior Fellow. Visit www.hospitalmedicine.org/fellows for details.
- Recruit others to join the movement. Active members can receive dues credits and special recognition for recruiting others. For details, visit www.hospitalmedicine.org/membership.
- Get the gear! Visit www.hospitalmedicine.org for the latest in hospitalist apparel, including new hoodies, coffee tumblers, water bottles, and tablet cases.
Healthcare's Main Contributors to Wasteful Spending
Three studies, same conclusion. Three separate studies largely agreed that unnecessary care or overtreatment represents the top contributor to wasteful healthcare in the U.S.
1 Main contributors to $600 to $800 billion in annual healthcare waste
40% Unnecessary care (“Unwarranted treatment, such as the over-use of antibiotics and the use of diagnostic lab tests to protect against malpractice exposure”)
19% Fraud
17% Administrative inefficiency
12% Healthcare provider errors
6% Preventable conditions
6% Lack of care coordination
Source: Thomson Reuters, 2010
2 Main contributors to $765 billion in annual healthcare waste
27% Unnecessary services
25% Excessive administrative costs
17% Inefficiently delivered services
14% Prices that are too high
10% Fraud
7% Missed prevention opportunities
Source: Institute of Medicine working group, 2011
3 Main contributors to $558 billion (low estimate) - $1263 billion (high estimate) in healthcare waste for 2011
28% (low) or 18% (high) Overtreatment (“Waste that comes from subjecting patients to care that, according to ound science and the patients’ own preferences, cannot possibly help them—care rooted in outmoded habits, supply-driven behaviors, and ignoring science”)
19% (low) or 31% (high) Administrative complexity
18% (low) or 12% (high) Failures of care delivery
15% (low) or 14% (high) Pricing failures
15% (low) or 22% (high) Fraud and abuse
4% (low) or 4% (high) Failures of care coordination
Source: RAND Corporation, 2012
A fourth study digs into some of the key drivers of overutilization:
1 Physician training and culture
2 Cultural preference for technological solutions
3 Direct-to-consumer marketing
4 Physician-directed pharmaceutical marketing
5 Fee-for-service payment structure
“The reality is that we are all human beings in the end. If I get paid more to do more, even if I don’t think I’m going to do more, I’m going to do more, because getting paid is very influential.”
—Brandon Combs, MD
6 Medical malpractice laws and defensive medicine
“People’s perspective of how likely they are to get sued drives behaviors, whether or not they actually are likely to get sued, and this has been shown many times.”
—Christopher Moriates, MD
7 Lack of cost transparency “It’s not about knowing the exact dollars and cents—that actually doesn’t matter. But it is about having some idea of magnitude, like an MRI is twice as expensive as a CT. When is it worth twice as much? When is it high value?”
—Christopher Moriates, MD
Three studies, same conclusion. Three separate studies largely agreed that unnecessary care or overtreatment represents the top contributor to wasteful healthcare in the U.S.
1 Main contributors to $600 to $800 billion in annual healthcare waste
40% Unnecessary care (“Unwarranted treatment, such as the over-use of antibiotics and the use of diagnostic lab tests to protect against malpractice exposure”)
19% Fraud
17% Administrative inefficiency
12% Healthcare provider errors
6% Preventable conditions
6% Lack of care coordination
Source: Thomson Reuters, 2010
2 Main contributors to $765 billion in annual healthcare waste
27% Unnecessary services
25% Excessive administrative costs
17% Inefficiently delivered services
14% Prices that are too high
10% Fraud
7% Missed prevention opportunities
Source: Institute of Medicine working group, 2011
3 Main contributors to $558 billion (low estimate) - $1263 billion (high estimate) in healthcare waste for 2011
28% (low) or 18% (high) Overtreatment (“Waste that comes from subjecting patients to care that, according to ound science and the patients’ own preferences, cannot possibly help them—care rooted in outmoded habits, supply-driven behaviors, and ignoring science”)
19% (low) or 31% (high) Administrative complexity
18% (low) or 12% (high) Failures of care delivery
15% (low) or 14% (high) Pricing failures
15% (low) or 22% (high) Fraud and abuse
4% (low) or 4% (high) Failures of care coordination
Source: RAND Corporation, 2012
A fourth study digs into some of the key drivers of overutilization:
1 Physician training and culture
2 Cultural preference for technological solutions
3 Direct-to-consumer marketing
4 Physician-directed pharmaceutical marketing
5 Fee-for-service payment structure
“The reality is that we are all human beings in the end. If I get paid more to do more, even if I don’t think I’m going to do more, I’m going to do more, because getting paid is very influential.”
—Brandon Combs, MD
6 Medical malpractice laws and defensive medicine
“People’s perspective of how likely they are to get sued drives behaviors, whether or not they actually are likely to get sued, and this has been shown many times.”
—Christopher Moriates, MD
7 Lack of cost transparency “It’s not about knowing the exact dollars and cents—that actually doesn’t matter. But it is about having some idea of magnitude, like an MRI is twice as expensive as a CT. When is it worth twice as much? When is it high value?”
—Christopher Moriates, MD
Three studies, same conclusion. Three separate studies largely agreed that unnecessary care or overtreatment represents the top contributor to wasteful healthcare in the U.S.
1 Main contributors to $600 to $800 billion in annual healthcare waste
40% Unnecessary care (“Unwarranted treatment, such as the over-use of antibiotics and the use of diagnostic lab tests to protect against malpractice exposure”)
19% Fraud
17% Administrative inefficiency
12% Healthcare provider errors
6% Preventable conditions
6% Lack of care coordination
Source: Thomson Reuters, 2010
2 Main contributors to $765 billion in annual healthcare waste
27% Unnecessary services
25% Excessive administrative costs
17% Inefficiently delivered services
14% Prices that are too high
10% Fraud
7% Missed prevention opportunities
Source: Institute of Medicine working group, 2011
3 Main contributors to $558 billion (low estimate) - $1263 billion (high estimate) in healthcare waste for 2011
28% (low) or 18% (high) Overtreatment (“Waste that comes from subjecting patients to care that, according to ound science and the patients’ own preferences, cannot possibly help them—care rooted in outmoded habits, supply-driven behaviors, and ignoring science”)
19% (low) or 31% (high) Administrative complexity
18% (low) or 12% (high) Failures of care delivery
15% (low) or 14% (high) Pricing failures
15% (low) or 22% (high) Fraud and abuse
4% (low) or 4% (high) Failures of care coordination
Source: RAND Corporation, 2012
A fourth study digs into some of the key drivers of overutilization:
1 Physician training and culture
2 Cultural preference for technological solutions
3 Direct-to-consumer marketing
4 Physician-directed pharmaceutical marketing
5 Fee-for-service payment structure
“The reality is that we are all human beings in the end. If I get paid more to do more, even if I don’t think I’m going to do more, I’m going to do more, because getting paid is very influential.”
—Brandon Combs, MD
6 Medical malpractice laws and defensive medicine
“People’s perspective of how likely they are to get sued drives behaviors, whether or not they actually are likely to get sued, and this has been shown many times.”
—Christopher Moriates, MD
7 Lack of cost transparency “It’s not about knowing the exact dollars and cents—that actually doesn’t matter. But it is about having some idea of magnitude, like an MRI is twice as expensive as a CT. When is it worth twice as much? When is it high value?”
—Christopher Moriates, MD
Treatment of Patients with Atrial Fibrillation, Low CHA2DS2-VASc Scores
Clinical question: Is anticoagulation beneficial for patients with atrial fibrillation (Afib) and low CHA2DS2-VASc score (0 for men, 1 for women) or for those with one additional stroke risk factor?
Background: Guidelines nearly universally recommend anticoagulation for patients with a CHA2DS2-VASc of >2, but differ on recommendation for patients with a CHA2DS2-VASc of 1.
Study design: Cohort study.
Setting: Multiple national registries in Denmark.
Synopsis: Based on analysis, patients with very low stroke risk using the CHA2DS2-VASc score (0 for men, 1 for women) had particularly low stroke risk and did not appear to benefit from additional therapy with aspirin or warfarin, both at one year and at full follow-up (mean 5.9 years).
The addition of one stroke risk factor increased stroke risk without treatment significantly (three-fold increase). Hazard ratios favored treatment with warfarin in these patients, most notably with a reduction in all-cause mortality (though this was more significant at one year than at full follow-up).
Bottom line: Although guidelines differ on treatment strategy for patients with Afib and one stroke risk factor (i.e., CHA2DS2-VASc score of 1 for men, 2 for women), this study supports treatment with warfarin.
Citation: Lip GY, Skjöth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394.
Clinical question: Is anticoagulation beneficial for patients with atrial fibrillation (Afib) and low CHA2DS2-VASc score (0 for men, 1 for women) or for those with one additional stroke risk factor?
Background: Guidelines nearly universally recommend anticoagulation for patients with a CHA2DS2-VASc of >2, but differ on recommendation for patients with a CHA2DS2-VASc of 1.
Study design: Cohort study.
Setting: Multiple national registries in Denmark.
Synopsis: Based on analysis, patients with very low stroke risk using the CHA2DS2-VASc score (0 for men, 1 for women) had particularly low stroke risk and did not appear to benefit from additional therapy with aspirin or warfarin, both at one year and at full follow-up (mean 5.9 years).
The addition of one stroke risk factor increased stroke risk without treatment significantly (three-fold increase). Hazard ratios favored treatment with warfarin in these patients, most notably with a reduction in all-cause mortality (though this was more significant at one year than at full follow-up).
Bottom line: Although guidelines differ on treatment strategy for patients with Afib and one stroke risk factor (i.e., CHA2DS2-VASc score of 1 for men, 2 for women), this study supports treatment with warfarin.
Citation: Lip GY, Skjöth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394.
Clinical question: Is anticoagulation beneficial for patients with atrial fibrillation (Afib) and low CHA2DS2-VASc score (0 for men, 1 for women) or for those with one additional stroke risk factor?
Background: Guidelines nearly universally recommend anticoagulation for patients with a CHA2DS2-VASc of >2, but differ on recommendation for patients with a CHA2DS2-VASc of 1.
Study design: Cohort study.
Setting: Multiple national registries in Denmark.
Synopsis: Based on analysis, patients with very low stroke risk using the CHA2DS2-VASc score (0 for men, 1 for women) had particularly low stroke risk and did not appear to benefit from additional therapy with aspirin or warfarin, both at one year and at full follow-up (mean 5.9 years).
The addition of one stroke risk factor increased stroke risk without treatment significantly (three-fold increase). Hazard ratios favored treatment with warfarin in these patients, most notably with a reduction in all-cause mortality (though this was more significant at one year than at full follow-up).
Bottom line: Although guidelines differ on treatment strategy for patients with Afib and one stroke risk factor (i.e., CHA2DS2-VASc score of 1 for men, 2 for women), this study supports treatment with warfarin.
Citation: Lip GY, Skjöth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394.
Research Review: Ticagrelor for Post-Myocardial Infarction
Clinical question: Is ticagrelor for secondary prevention indicated for more than one year after myocardial infarction (MI)?
Background: The efficacy and safety of ticagrelor combined with low-dose aspirin more than one year after MI for secondary prevention has not previously been established.
Study design: Randomized, double-blinded, placebo-controlled, clinical trial.
Setting: Multi-center across 31 countries.
Synopsis: Investigators randomized 21,162 patients one to three years after first MI to a 90 mg, twice daily dose; a 60 mg, twice daily dose; or placebo. Patients also received low-dose aspirin (75 mg to 100 mg). Interestingly, a number of patients had been off dual antiplatelet therapy prior to the start of the trial, because most patients were enrolled closer to two years after primary MI. The manufacturer of ticagrelor sponsored the trial.
The study authors’ analysis showed that treating 10,000 patients with the 90 mg dose would prevent 40 cardiac events (cardiovascular death, MI, or stroke), while the 60 mg dose would prevent 42 events; however, the 90 mg dose would cause 41 major bleeding events and the 60 mg dose 31 major bleeding events. Fatal bleeding was less than 1% in all groups, though patients with increased bleeding risk were excluded.
In addition, patients on either dose of ticagrelor had a significantly higher rate of dyspnea, which resulted in increases in drug discontinuation.
Bottom line: Use of ticagrelor with aspirin for secondary prevention greater than one year after myocardial infarction reduced rates of cardiovascular death, MI, and stroke but increased the risk of major bleeding.
Citation: Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
Clinical question: Is ticagrelor for secondary prevention indicated for more than one year after myocardial infarction (MI)?
Background: The efficacy and safety of ticagrelor combined with low-dose aspirin more than one year after MI for secondary prevention has not previously been established.
Study design: Randomized, double-blinded, placebo-controlled, clinical trial.
Setting: Multi-center across 31 countries.
Synopsis: Investigators randomized 21,162 patients one to three years after first MI to a 90 mg, twice daily dose; a 60 mg, twice daily dose; or placebo. Patients also received low-dose aspirin (75 mg to 100 mg). Interestingly, a number of patients had been off dual antiplatelet therapy prior to the start of the trial, because most patients were enrolled closer to two years after primary MI. The manufacturer of ticagrelor sponsored the trial.
The study authors’ analysis showed that treating 10,000 patients with the 90 mg dose would prevent 40 cardiac events (cardiovascular death, MI, or stroke), while the 60 mg dose would prevent 42 events; however, the 90 mg dose would cause 41 major bleeding events and the 60 mg dose 31 major bleeding events. Fatal bleeding was less than 1% in all groups, though patients with increased bleeding risk were excluded.
In addition, patients on either dose of ticagrelor had a significantly higher rate of dyspnea, which resulted in increases in drug discontinuation.
Bottom line: Use of ticagrelor with aspirin for secondary prevention greater than one year after myocardial infarction reduced rates of cardiovascular death, MI, and stroke but increased the risk of major bleeding.
Citation: Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
Clinical question: Is ticagrelor for secondary prevention indicated for more than one year after myocardial infarction (MI)?
Background: The efficacy and safety of ticagrelor combined with low-dose aspirin more than one year after MI for secondary prevention has not previously been established.
Study design: Randomized, double-blinded, placebo-controlled, clinical trial.
Setting: Multi-center across 31 countries.
Synopsis: Investigators randomized 21,162 patients one to three years after first MI to a 90 mg, twice daily dose; a 60 mg, twice daily dose; or placebo. Patients also received low-dose aspirin (75 mg to 100 mg). Interestingly, a number of patients had been off dual antiplatelet therapy prior to the start of the trial, because most patients were enrolled closer to two years after primary MI. The manufacturer of ticagrelor sponsored the trial.
The study authors’ analysis showed that treating 10,000 patients with the 90 mg dose would prevent 40 cardiac events (cardiovascular death, MI, or stroke), while the 60 mg dose would prevent 42 events; however, the 90 mg dose would cause 41 major bleeding events and the 60 mg dose 31 major bleeding events. Fatal bleeding was less than 1% in all groups, though patients with increased bleeding risk were excluded.
In addition, patients on either dose of ticagrelor had a significantly higher rate of dyspnea, which resulted in increases in drug discontinuation.
Bottom line: Use of ticagrelor with aspirin for secondary prevention greater than one year after myocardial infarction reduced rates of cardiovascular death, MI, and stroke but increased the risk of major bleeding.
Citation: Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
Bundled Payment and Hospital Medicine, Pt. 2
Editor’s note: Second in a two-part series examining bundled payments and hospital medicine. In full disclosure, Dr. Whitcomb works for a company that is an Awardee Convener in the CMS Bundled Payments for Care Improvement (BPCI) Initiative.
In part one of this series, we discussed the basics of the BPCI program. Now we will delve into specific roles and opportunities for hospitalists in bundled payment programs in general, and the BPCI program in particular.
The bundled payment model can be hard to explain. One example that might make it clearer is that of LASIK vision correction surgery, where a single bundled payment covers the fees of the ophthalmologist, the operating facility, and any other services (like optometry) and medications (like eye drops). Another example is the diagnosis-related group (DRG) payment for hospital care, in which all facility costs are bundled together into a single payment.
A simplistic way to differentiate bundled payment from accountable care organization (ACOs) is that the former is typically initiated by an acute medical or surgical event and concludes after a recovery period—often 30, 60, or 90 days. Conversely, the latter generally covers the care of individuals within a population over time, often focusing on the management of chronic conditions.
The Opportunity
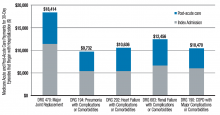
Two major opportunities for hospitalists to improve value (quality/cost) present themselves through the BPCI initiative. One is in post-acute facility utilization, and the other is in reducing readmissions. Figure 1 shows that for 30-day episodes starting with a hospitalization for five common conditions, payments for post-acute care are surprisingly close in amount to those for the preceding hospitalization.1
Much of the cost of post-acute care comes from skilled nursing facilities (SNFs) and, to a lesser degree, inpatient rehabilitation facilities. A broad range of research studies has demonstrated that inpatient care managed by hospitalists—compared with the traditional model—is associated with a decrease in inpatient costs; however, recent research indicates that the hospital cost savings generated by hospitalists are offset by more spending in the 30 days post discharge, specifically on more SNF care and increased readmissions.2 As another indicator that post-acute care needs a closer look, a 2013 Institute of Medicine report concluded that spending on post-acute care was responsible for the majority of Medicare’s overall regional variation in spending.1,3
Of course, success in a bundled payment model will also be derived from reducing costs in the hospital setting, such as those stemming from unnecessary or duplicative testing and imaging, injudicious use of consultants, and practices identified in programs such as Choosing Wisely.
How Your Practice Can Drive Bundled Payment Success
The aforementioned observations point to the need to improve the value of post-acute care by optimizing post-acute spending—driven mostly by SNF costs—and minimizing avoidable readmissions. I offer the following inpatient interventions to achieve these goals:
- Speak with patients early and often regarding expectations for recovery post discharge. When possible, set a goal of home discharge with the needed support.
- Write orders for early ambulation. Develop an early ambulation program with nursing and physical therapy.
- Address goals of care during the patient/family meeting. For appropriate patients with life-limiting illness, involve the palliative care service or equivalent and discuss the role of future aggressive interventions, including hospitalization, so that the course set is consistent with the patients’ goals and wishes.
- Lead the in-hospital team, instead of defaulting to others, like case management, in making an informed decision about ideal post-discharge location by factoring in caregiver availability, independence, and SNF needs. Marshal resources to enable a home recovery (i.e., home health evaluation), whether or not there is an intervening SNF stay. If patients go to a SNF, set expectations for length of stay in the facility.
- Adhere to best practices for care transitions, such as those in Project BOOST, including thorough medication reconciliation.
Beyond the Four Walls
As you aim for a high-value (high quality and affordable) discharge, your hospital medicine practice may consider new approaches to filling longstanding gaps in post-acute care. Forward-looking hospitalist groups have implemented the following approaches:
- Establish a post-discharge clinic where patients are seen after discharge, in the interim before they have an opportunity for primary care follow-up;
- Send teams to work in SNFs;
- Call patients after discharge to ensure they are following their plan of care;
- Leverage newer current procedural terminology (CPT) codes, like the Transitional Care Management or Chronic Care Management codes, to support your transitional care services;
- Provide home visits for high-risk patients; and
- Access waivers for G-codes for home visits and/or telemedicine outside of rural areas. These waivers exist under the BPCI initiative.
Shift from ‘Traditional’ Hospitalist to ‘Value’ Hospitalist
If some of the changes in practice needed to succeed in a bundled payment world seem daunting to you, it may be helpful to realize that with the challenge comes an opportunity. This opportunity for hospitalists parallels that of the early days of the specialty, when reducing length of stay created substantial support from hospital leaders and was a factor leading to the rapid growth in the number of hospitalists. In January, the U.S. Secretary of Health and Human Services set a goal to tie 50% of Medicare payments to ‘alternative payment models’ like bundled payments by 2018. In April, as part of the sustainable growth rate fix, Medicare announced it would create substantial new bonuses for physicians who have at least 25% of their revenue in such models.1
As healthcare policy aligns behind ‘alternative payment models,’ bundled payment programs are likely to be a potent driver of an evolving hospitalist specialty. Next-generation hospitalists will be asked to take a leadership role in addressing ‘value’ with responsibility for improving care coordination and affordability over an episode of illness.
Now may be the time to take to heart the words of computer scientist Alan Kay: “The best way to predict the future is to invent it.”

References
- Mechanic R. Post-acute care–the next frontier for controlling Medicare spending. N Engl J Med. 2014;370(8):692-694.
- Kuo YF, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152-159.
- Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368(16):1465-1468.
Editor’s note: Second in a two-part series examining bundled payments and hospital medicine. In full disclosure, Dr. Whitcomb works for a company that is an Awardee Convener in the CMS Bundled Payments for Care Improvement (BPCI) Initiative.
In part one of this series, we discussed the basics of the BPCI program. Now we will delve into specific roles and opportunities for hospitalists in bundled payment programs in general, and the BPCI program in particular.
The bundled payment model can be hard to explain. One example that might make it clearer is that of LASIK vision correction surgery, where a single bundled payment covers the fees of the ophthalmologist, the operating facility, and any other services (like optometry) and medications (like eye drops). Another example is the diagnosis-related group (DRG) payment for hospital care, in which all facility costs are bundled together into a single payment.
A simplistic way to differentiate bundled payment from accountable care organization (ACOs) is that the former is typically initiated by an acute medical or surgical event and concludes after a recovery period—often 30, 60, or 90 days. Conversely, the latter generally covers the care of individuals within a population over time, often focusing on the management of chronic conditions.
The Opportunity
Two major opportunities for hospitalists to improve value (quality/cost) present themselves through the BPCI initiative. One is in post-acute facility utilization, and the other is in reducing readmissions. Figure 1 shows that for 30-day episodes starting with a hospitalization for five common conditions, payments for post-acute care are surprisingly close in amount to those for the preceding hospitalization.1
Much of the cost of post-acute care comes from skilled nursing facilities (SNFs) and, to a lesser degree, inpatient rehabilitation facilities. A broad range of research studies has demonstrated that inpatient care managed by hospitalists—compared with the traditional model—is associated with a decrease in inpatient costs; however, recent research indicates that the hospital cost savings generated by hospitalists are offset by more spending in the 30 days post discharge, specifically on more SNF care and increased readmissions.2 As another indicator that post-acute care needs a closer look, a 2013 Institute of Medicine report concluded that spending on post-acute care was responsible for the majority of Medicare’s overall regional variation in spending.1,3
Of course, success in a bundled payment model will also be derived from reducing costs in the hospital setting, such as those stemming from unnecessary or duplicative testing and imaging, injudicious use of consultants, and practices identified in programs such as Choosing Wisely.
How Your Practice Can Drive Bundled Payment Success
The aforementioned observations point to the need to improve the value of post-acute care by optimizing post-acute spending—driven mostly by SNF costs—and minimizing avoidable readmissions. I offer the following inpatient interventions to achieve these goals:
- Speak with patients early and often regarding expectations for recovery post discharge. When possible, set a goal of home discharge with the needed support.
- Write orders for early ambulation. Develop an early ambulation program with nursing and physical therapy.
- Address goals of care during the patient/family meeting. For appropriate patients with life-limiting illness, involve the palliative care service or equivalent and discuss the role of future aggressive interventions, including hospitalization, so that the course set is consistent with the patients’ goals and wishes.
- Lead the in-hospital team, instead of defaulting to others, like case management, in making an informed decision about ideal post-discharge location by factoring in caregiver availability, independence, and SNF needs. Marshal resources to enable a home recovery (i.e., home health evaluation), whether or not there is an intervening SNF stay. If patients go to a SNF, set expectations for length of stay in the facility.
- Adhere to best practices for care transitions, such as those in Project BOOST, including thorough medication reconciliation.
Beyond the Four Walls
As you aim for a high-value (high quality and affordable) discharge, your hospital medicine practice may consider new approaches to filling longstanding gaps in post-acute care. Forward-looking hospitalist groups have implemented the following approaches:
- Establish a post-discharge clinic where patients are seen after discharge, in the interim before they have an opportunity for primary care follow-up;
- Send teams to work in SNFs;
- Call patients after discharge to ensure they are following their plan of care;
- Leverage newer current procedural terminology (CPT) codes, like the Transitional Care Management or Chronic Care Management codes, to support your transitional care services;
- Provide home visits for high-risk patients; and
- Access waivers for G-codes for home visits and/or telemedicine outside of rural areas. These waivers exist under the BPCI initiative.
Shift from ‘Traditional’ Hospitalist to ‘Value’ Hospitalist
If some of the changes in practice needed to succeed in a bundled payment world seem daunting to you, it may be helpful to realize that with the challenge comes an opportunity. This opportunity for hospitalists parallels that of the early days of the specialty, when reducing length of stay created substantial support from hospital leaders and was a factor leading to the rapid growth in the number of hospitalists. In January, the U.S. Secretary of Health and Human Services set a goal to tie 50% of Medicare payments to ‘alternative payment models’ like bundled payments by 2018. In April, as part of the sustainable growth rate fix, Medicare announced it would create substantial new bonuses for physicians who have at least 25% of their revenue in such models.1
As healthcare policy aligns behind ‘alternative payment models,’ bundled payment programs are likely to be a potent driver of an evolving hospitalist specialty. Next-generation hospitalists will be asked to take a leadership role in addressing ‘value’ with responsibility for improving care coordination and affordability over an episode of illness.
Now may be the time to take to heart the words of computer scientist Alan Kay: “The best way to predict the future is to invent it.”

References
- Mechanic R. Post-acute care–the next frontier for controlling Medicare spending. N Engl J Med. 2014;370(8):692-694.
- Kuo YF, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152-159.
- Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368(16):1465-1468.
Editor’s note: Second in a two-part series examining bundled payments and hospital medicine. In full disclosure, Dr. Whitcomb works for a company that is an Awardee Convener in the CMS Bundled Payments for Care Improvement (BPCI) Initiative.
In part one of this series, we discussed the basics of the BPCI program. Now we will delve into specific roles and opportunities for hospitalists in bundled payment programs in general, and the BPCI program in particular.
The bundled payment model can be hard to explain. One example that might make it clearer is that of LASIK vision correction surgery, where a single bundled payment covers the fees of the ophthalmologist, the operating facility, and any other services (like optometry) and medications (like eye drops). Another example is the diagnosis-related group (DRG) payment for hospital care, in which all facility costs are bundled together into a single payment.
A simplistic way to differentiate bundled payment from accountable care organization (ACOs) is that the former is typically initiated by an acute medical or surgical event and concludes after a recovery period—often 30, 60, or 90 days. Conversely, the latter generally covers the care of individuals within a population over time, often focusing on the management of chronic conditions.
The Opportunity
Two major opportunities for hospitalists to improve value (quality/cost) present themselves through the BPCI initiative. One is in post-acute facility utilization, and the other is in reducing readmissions. Figure 1 shows that for 30-day episodes starting with a hospitalization for five common conditions, payments for post-acute care are surprisingly close in amount to those for the preceding hospitalization.1
Much of the cost of post-acute care comes from skilled nursing facilities (SNFs) and, to a lesser degree, inpatient rehabilitation facilities. A broad range of research studies has demonstrated that inpatient care managed by hospitalists—compared with the traditional model—is associated with a decrease in inpatient costs; however, recent research indicates that the hospital cost savings generated by hospitalists are offset by more spending in the 30 days post discharge, specifically on more SNF care and increased readmissions.2 As another indicator that post-acute care needs a closer look, a 2013 Institute of Medicine report concluded that spending on post-acute care was responsible for the majority of Medicare’s overall regional variation in spending.1,3
Of course, success in a bundled payment model will also be derived from reducing costs in the hospital setting, such as those stemming from unnecessary or duplicative testing and imaging, injudicious use of consultants, and practices identified in programs such as Choosing Wisely.
How Your Practice Can Drive Bundled Payment Success
The aforementioned observations point to the need to improve the value of post-acute care by optimizing post-acute spending—driven mostly by SNF costs—and minimizing avoidable readmissions. I offer the following inpatient interventions to achieve these goals:
- Speak with patients early and often regarding expectations for recovery post discharge. When possible, set a goal of home discharge with the needed support.
- Write orders for early ambulation. Develop an early ambulation program with nursing and physical therapy.
- Address goals of care during the patient/family meeting. For appropriate patients with life-limiting illness, involve the palliative care service or equivalent and discuss the role of future aggressive interventions, including hospitalization, so that the course set is consistent with the patients’ goals and wishes.
- Lead the in-hospital team, instead of defaulting to others, like case management, in making an informed decision about ideal post-discharge location by factoring in caregiver availability, independence, and SNF needs. Marshal resources to enable a home recovery (i.e., home health evaluation), whether or not there is an intervening SNF stay. If patients go to a SNF, set expectations for length of stay in the facility.
- Adhere to best practices for care transitions, such as those in Project BOOST, including thorough medication reconciliation.
Beyond the Four Walls
As you aim for a high-value (high quality and affordable) discharge, your hospital medicine practice may consider new approaches to filling longstanding gaps in post-acute care. Forward-looking hospitalist groups have implemented the following approaches:
- Establish a post-discharge clinic where patients are seen after discharge, in the interim before they have an opportunity for primary care follow-up;
- Send teams to work in SNFs;
- Call patients after discharge to ensure they are following their plan of care;
- Leverage newer current procedural terminology (CPT) codes, like the Transitional Care Management or Chronic Care Management codes, to support your transitional care services;
- Provide home visits for high-risk patients; and
- Access waivers for G-codes for home visits and/or telemedicine outside of rural areas. These waivers exist under the BPCI initiative.
Shift from ‘Traditional’ Hospitalist to ‘Value’ Hospitalist
If some of the changes in practice needed to succeed in a bundled payment world seem daunting to you, it may be helpful to realize that with the challenge comes an opportunity. This opportunity for hospitalists parallels that of the early days of the specialty, when reducing length of stay created substantial support from hospital leaders and was a factor leading to the rapid growth in the number of hospitalists. In January, the U.S. Secretary of Health and Human Services set a goal to tie 50% of Medicare payments to ‘alternative payment models’ like bundled payments by 2018. In April, as part of the sustainable growth rate fix, Medicare announced it would create substantial new bonuses for physicians who have at least 25% of their revenue in such models.1
As healthcare policy aligns behind ‘alternative payment models,’ bundled payment programs are likely to be a potent driver of an evolving hospitalist specialty. Next-generation hospitalists will be asked to take a leadership role in addressing ‘value’ with responsibility for improving care coordination and affordability over an episode of illness.
Now may be the time to take to heart the words of computer scientist Alan Kay: “The best way to predict the future is to invent it.”

References
- Mechanic R. Post-acute care–the next frontier for controlling Medicare spending. N Engl J Med. 2014;370(8):692-694.
- Kuo YF, Goodwin JS. Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152-159.
- Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368(16):1465-1468.
Changing the Paradigm: New Thoughts on Pathophysiology and Drugable Targets in Acne
Who is tired of the same old stuff when it comes to acne? Innovation in therapy has been stagnant with a flurry of “me too” reformulated fixed combinations. The only true advance has been in drug delivery, with new vehicles allowing for the solubilization of established drugs such as dapsone or the combination of incompatible actives such as benzoyl peroxide and a retinoid. Before we can welcome new drugs with open arms, we must first expand the construct of acne pathophysiology to identify more appropriate targets for said new drugs. In a recent article published online in the Journal of the American Academy of Dermatology in June, Metiko et al highlight this sentiment. Generations of dermatologists were taught the 3- to 4-step process (depending on the teacher) through which an acne lesion forms: (1) follicular epidermal hyperproliferation, (2) Propionibacterium acnes colonization, and (3) inflammation. However, the molecular underpinnings of this theory have been challenged for more than a decade, with research highlighting the presence of preclinical inflammation, most recently found to be mediated by IL-1ß through a specific inflammasome pathway, NLRP3 (NOD-like receptor family, pyrin domain containing 3). Maybe we are missing a bridge between this stellar basic science and the clinical dermatologist who contends that the pesky microcomedone is the acne instigator. This short but sweet letter once again calls this antiquated prose into question in a highly visible clinical dermatology journal.
In thinking of new pathways and targets, Gupta et al published an article online in Archives of Dermatological Research on May 19 on the role of peroxisome proliferator-activated receptors (PPARs) and PPAR agonists in the treatment of multiple dermatologic diseases. For our purposes, I will highlight the section on acne and will start at the end: More research is needed. Peroxisome proliferator-activated receptors are nuclear hormone receptors that regulate gene expression, cell growth and differentiation, apoptosis, inflammatory responses, and tumorigenesis after binding with specific ligands. With respect to acne specifically, PPARs influence 2 of the pathophysiologic factors—sebum production and inflammation—due to their effect on lipid deposition in the sebocytes and inhibition of proinflammatory gene expression and downregulation of inflammatory cytokines. It appears that activation or inhibition of specific PPAR subtypes can either increase or decrease sebum production or be pro- or anti-inflammatory. The tough part is which receptors to activate and which to inhibit. This review related to an interesting clinical study that evaluated oral zileuton 600 mg administered 4 times daily for 3 months for acne. Zileuton inhibits leukotriene B4 production, which, as it turns out, is a natural ligand for PPARα. The idea here is that this blockade would be anti-inflammatory and indirectly inhibit the sebum production via PPARα suppression. The pilot study was reported as successful, with a decrease in the papulopustular acne severity index in a time-dependent manner in subjects evaluated.
What’s the issue?
So, what’s the point of this long-winded, double-paper review? We need to expand our acne horizons. We need new bench-to-bedside approaches. Which is your favorite target?
Who is tired of the same old stuff when it comes to acne? Innovation in therapy has been stagnant with a flurry of “me too” reformulated fixed combinations. The only true advance has been in drug delivery, with new vehicles allowing for the solubilization of established drugs such as dapsone or the combination of incompatible actives such as benzoyl peroxide and a retinoid. Before we can welcome new drugs with open arms, we must first expand the construct of acne pathophysiology to identify more appropriate targets for said new drugs. In a recent article published online in the Journal of the American Academy of Dermatology in June, Metiko et al highlight this sentiment. Generations of dermatologists were taught the 3- to 4-step process (depending on the teacher) through which an acne lesion forms: (1) follicular epidermal hyperproliferation, (2) Propionibacterium acnes colonization, and (3) inflammation. However, the molecular underpinnings of this theory have been challenged for more than a decade, with research highlighting the presence of preclinical inflammation, most recently found to be mediated by IL-1ß through a specific inflammasome pathway, NLRP3 (NOD-like receptor family, pyrin domain containing 3). Maybe we are missing a bridge between this stellar basic science and the clinical dermatologist who contends that the pesky microcomedone is the acne instigator. This short but sweet letter once again calls this antiquated prose into question in a highly visible clinical dermatology journal.
In thinking of new pathways and targets, Gupta et al published an article online in Archives of Dermatological Research on May 19 on the role of peroxisome proliferator-activated receptors (PPARs) and PPAR agonists in the treatment of multiple dermatologic diseases. For our purposes, I will highlight the section on acne and will start at the end: More research is needed. Peroxisome proliferator-activated receptors are nuclear hormone receptors that regulate gene expression, cell growth and differentiation, apoptosis, inflammatory responses, and tumorigenesis after binding with specific ligands. With respect to acne specifically, PPARs influence 2 of the pathophysiologic factors—sebum production and inflammation—due to their effect on lipid deposition in the sebocytes and inhibition of proinflammatory gene expression and downregulation of inflammatory cytokines. It appears that activation or inhibition of specific PPAR subtypes can either increase or decrease sebum production or be pro- or anti-inflammatory. The tough part is which receptors to activate and which to inhibit. This review related to an interesting clinical study that evaluated oral zileuton 600 mg administered 4 times daily for 3 months for acne. Zileuton inhibits leukotriene B4 production, which, as it turns out, is a natural ligand for PPARα. The idea here is that this blockade would be anti-inflammatory and indirectly inhibit the sebum production via PPARα suppression. The pilot study was reported as successful, with a decrease in the papulopustular acne severity index in a time-dependent manner in subjects evaluated.
What’s the issue?
So, what’s the point of this long-winded, double-paper review? We need to expand our acne horizons. We need new bench-to-bedside approaches. Which is your favorite target?
Who is tired of the same old stuff when it comes to acne? Innovation in therapy has been stagnant with a flurry of “me too” reformulated fixed combinations. The only true advance has been in drug delivery, with new vehicles allowing for the solubilization of established drugs such as dapsone or the combination of incompatible actives such as benzoyl peroxide and a retinoid. Before we can welcome new drugs with open arms, we must first expand the construct of acne pathophysiology to identify more appropriate targets for said new drugs. In a recent article published online in the Journal of the American Academy of Dermatology in June, Metiko et al highlight this sentiment. Generations of dermatologists were taught the 3- to 4-step process (depending on the teacher) through which an acne lesion forms: (1) follicular epidermal hyperproliferation, (2) Propionibacterium acnes colonization, and (3) inflammation. However, the molecular underpinnings of this theory have been challenged for more than a decade, with research highlighting the presence of preclinical inflammation, most recently found to be mediated by IL-1ß through a specific inflammasome pathway, NLRP3 (NOD-like receptor family, pyrin domain containing 3). Maybe we are missing a bridge between this stellar basic science and the clinical dermatologist who contends that the pesky microcomedone is the acne instigator. This short but sweet letter once again calls this antiquated prose into question in a highly visible clinical dermatology journal.
In thinking of new pathways and targets, Gupta et al published an article online in Archives of Dermatological Research on May 19 on the role of peroxisome proliferator-activated receptors (PPARs) and PPAR agonists in the treatment of multiple dermatologic diseases. For our purposes, I will highlight the section on acne and will start at the end: More research is needed. Peroxisome proliferator-activated receptors are nuclear hormone receptors that regulate gene expression, cell growth and differentiation, apoptosis, inflammatory responses, and tumorigenesis after binding with specific ligands. With respect to acne specifically, PPARs influence 2 of the pathophysiologic factors—sebum production and inflammation—due to their effect on lipid deposition in the sebocytes and inhibition of proinflammatory gene expression and downregulation of inflammatory cytokines. It appears that activation or inhibition of specific PPAR subtypes can either increase or decrease sebum production or be pro- or anti-inflammatory. The tough part is which receptors to activate and which to inhibit. This review related to an interesting clinical study that evaluated oral zileuton 600 mg administered 4 times daily for 3 months for acne. Zileuton inhibits leukotriene B4 production, which, as it turns out, is a natural ligand for PPARα. The idea here is that this blockade would be anti-inflammatory and indirectly inhibit the sebum production via PPARα suppression. The pilot study was reported as successful, with a decrease in the papulopustular acne severity index in a time-dependent manner in subjects evaluated.
What’s the issue?
So, what’s the point of this long-winded, double-paper review? We need to expand our acne horizons. We need new bench-to-bedside approaches. Which is your favorite target?
Prenatal test can detect lymphoma in mothers

Photo by Graham Colm
GLASGOW—A non-invasive prenatal test (NIPT) used to identify chromosomal fetal disorders can detect maternal cancers before symptoms appear, a new study has shown.
Testing revealed chromosomal abnormalities in 3 women that bore a resemblance to abnormalities observed in cancers. And additional testing confirmed the women had cancer—Hodgkin lymphoma (HL), follicular lymphoma (FL), and ovarian carcinoma.
This research was presented at the European Human Genetics Conference 2015 and published simultaneously in JAMA Oncology.
Nathalie Brison, PhD, of University Hospitals Leuven in Belgium, and her colleagues conducted this research with the goal of improving the NIPT test. They wanted to overcome some of the technical problems that can cause the test to produce false-negative or false-positive results.
“Even though it is very reliable, we believed that we could make it even better, and, in doing so, we could also find other chromosomal abnormalities apart from the traditional trisomy syndromes—Down’s [trisomy 21], Edward’s [trisomy 18], and Patau [trisomy 13],” Dr Brison said.
“Using the new, adapted test in over 6000 pregnancies, and looking at other chromosomes, we identified 3 different genomic abnormalities in 3 women that could not be linked to either the maternal or fetal genomic profile. We realized that the abnormalities bore a resemblance to those found in cancer and referred the women to the oncology unit.”
Further examination, including whole-body MRI scanning and pathological and genetic investigations, revealed the presence of 3 different early stage cancers in the women: ovarian carcinoma, FL, and HL.
The researchers said that, without NIPT, these cancers likely would not have been detected until the women developed symptoms.
“Considering the bad prognosis of some cancers when detected later, and given that we know that it is both possible and safe to treat the disease during pregnancy, this is an important added advantage of NIPT,” said study author Joris Vermeesch, PhD, also of University Hospitals Leuven.
“During pregnancy, cancer-related symptoms may well be masked. Fatigue, nausea, abdominal pain, and vaginal blood loss are easily interpretable as a normal part of being pregnant. NIPT offers an opportunity for the accurate screening of high-risk women for cancer, allowing us to overcome the challenge of early diagnosis in pregnant women.”
Two of the 3 women diagnosed with cancer were treated. The woman with ovarian cancer was treated after delivery.
The woman with HL was treated during pregnancy and ultimately gave birth to a healthy girl. The woman with FL has indolent disease and may not require treatment for years, according to the researchers.
Follow-up investigations in the treated women showed that NIPT had the additional advantage of allowing for treatment monitoring. The researchers were able to see that chromosomal profiles became normal during and after chemotherapy.
Because NIPT involves looking at chromosomes other than 13, 18, and 21, the women taking part in this study were informed about the possibility of incidental findings.
“However, our study feeds into the ethical debate about whether or not to report incidental findings to patients and also has implications for the current political discussions concerning reimbursement and funding of NIPT by national healthcare systems,” Dr Vermeesch said.
The results also suggest that NIPT might enable the detection of pre-symptomatic cancers in the general population.
“We now know that it is possible to offer the accurate detection of chromosomally imbalanced cancers to the general population via minimally invasive screening methods,” Dr Brison said. “The normalization of the NIPT profile in these patients following treatment indicates that we can also measure response to treatment as early as after the first administration of chemotherapy.”
“Of course, larger-scale studies will be required to validate these results further, but we are confident that we have made an important step towards the possibility of wide-scale, effective, non-invasive cancer screening capable of detecting disease at an early stage.” ![]()

Photo by Graham Colm
GLASGOW—A non-invasive prenatal test (NIPT) used to identify chromosomal fetal disorders can detect maternal cancers before symptoms appear, a new study has shown.
Testing revealed chromosomal abnormalities in 3 women that bore a resemblance to abnormalities observed in cancers. And additional testing confirmed the women had cancer—Hodgkin lymphoma (HL), follicular lymphoma (FL), and ovarian carcinoma.
This research was presented at the European Human Genetics Conference 2015 and published simultaneously in JAMA Oncology.
Nathalie Brison, PhD, of University Hospitals Leuven in Belgium, and her colleagues conducted this research with the goal of improving the NIPT test. They wanted to overcome some of the technical problems that can cause the test to produce false-negative or false-positive results.
“Even though it is very reliable, we believed that we could make it even better, and, in doing so, we could also find other chromosomal abnormalities apart from the traditional trisomy syndromes—Down’s [trisomy 21], Edward’s [trisomy 18], and Patau [trisomy 13],” Dr Brison said.
“Using the new, adapted test in over 6000 pregnancies, and looking at other chromosomes, we identified 3 different genomic abnormalities in 3 women that could not be linked to either the maternal or fetal genomic profile. We realized that the abnormalities bore a resemblance to those found in cancer and referred the women to the oncology unit.”
Further examination, including whole-body MRI scanning and pathological and genetic investigations, revealed the presence of 3 different early stage cancers in the women: ovarian carcinoma, FL, and HL.
The researchers said that, without NIPT, these cancers likely would not have been detected until the women developed symptoms.
“Considering the bad prognosis of some cancers when detected later, and given that we know that it is both possible and safe to treat the disease during pregnancy, this is an important added advantage of NIPT,” said study author Joris Vermeesch, PhD, also of University Hospitals Leuven.
“During pregnancy, cancer-related symptoms may well be masked. Fatigue, nausea, abdominal pain, and vaginal blood loss are easily interpretable as a normal part of being pregnant. NIPT offers an opportunity for the accurate screening of high-risk women for cancer, allowing us to overcome the challenge of early diagnosis in pregnant women.”
Two of the 3 women diagnosed with cancer were treated. The woman with ovarian cancer was treated after delivery.
The woman with HL was treated during pregnancy and ultimately gave birth to a healthy girl. The woman with FL has indolent disease and may not require treatment for years, according to the researchers.
Follow-up investigations in the treated women showed that NIPT had the additional advantage of allowing for treatment monitoring. The researchers were able to see that chromosomal profiles became normal during and after chemotherapy.
Because NIPT involves looking at chromosomes other than 13, 18, and 21, the women taking part in this study were informed about the possibility of incidental findings.
“However, our study feeds into the ethical debate about whether or not to report incidental findings to patients and also has implications for the current political discussions concerning reimbursement and funding of NIPT by national healthcare systems,” Dr Vermeesch said.
The results also suggest that NIPT might enable the detection of pre-symptomatic cancers in the general population.
“We now know that it is possible to offer the accurate detection of chromosomally imbalanced cancers to the general population via minimally invasive screening methods,” Dr Brison said. “The normalization of the NIPT profile in these patients following treatment indicates that we can also measure response to treatment as early as after the first administration of chemotherapy.”
“Of course, larger-scale studies will be required to validate these results further, but we are confident that we have made an important step towards the possibility of wide-scale, effective, non-invasive cancer screening capable of detecting disease at an early stage.” ![]()

Photo by Graham Colm
GLASGOW—A non-invasive prenatal test (NIPT) used to identify chromosomal fetal disorders can detect maternal cancers before symptoms appear, a new study has shown.
Testing revealed chromosomal abnormalities in 3 women that bore a resemblance to abnormalities observed in cancers. And additional testing confirmed the women had cancer—Hodgkin lymphoma (HL), follicular lymphoma (FL), and ovarian carcinoma.
This research was presented at the European Human Genetics Conference 2015 and published simultaneously in JAMA Oncology.
Nathalie Brison, PhD, of University Hospitals Leuven in Belgium, and her colleagues conducted this research with the goal of improving the NIPT test. They wanted to overcome some of the technical problems that can cause the test to produce false-negative or false-positive results.
“Even though it is very reliable, we believed that we could make it even better, and, in doing so, we could also find other chromosomal abnormalities apart from the traditional trisomy syndromes—Down’s [trisomy 21], Edward’s [trisomy 18], and Patau [trisomy 13],” Dr Brison said.
“Using the new, adapted test in over 6000 pregnancies, and looking at other chromosomes, we identified 3 different genomic abnormalities in 3 women that could not be linked to either the maternal or fetal genomic profile. We realized that the abnormalities bore a resemblance to those found in cancer and referred the women to the oncology unit.”
Further examination, including whole-body MRI scanning and pathological and genetic investigations, revealed the presence of 3 different early stage cancers in the women: ovarian carcinoma, FL, and HL.
The researchers said that, without NIPT, these cancers likely would not have been detected until the women developed symptoms.
“Considering the bad prognosis of some cancers when detected later, and given that we know that it is both possible and safe to treat the disease during pregnancy, this is an important added advantage of NIPT,” said study author Joris Vermeesch, PhD, also of University Hospitals Leuven.
“During pregnancy, cancer-related symptoms may well be masked. Fatigue, nausea, abdominal pain, and vaginal blood loss are easily interpretable as a normal part of being pregnant. NIPT offers an opportunity for the accurate screening of high-risk women for cancer, allowing us to overcome the challenge of early diagnosis in pregnant women.”
Two of the 3 women diagnosed with cancer were treated. The woman with ovarian cancer was treated after delivery.
The woman with HL was treated during pregnancy and ultimately gave birth to a healthy girl. The woman with FL has indolent disease and may not require treatment for years, according to the researchers.
Follow-up investigations in the treated women showed that NIPT had the additional advantage of allowing for treatment monitoring. The researchers were able to see that chromosomal profiles became normal during and after chemotherapy.
Because NIPT involves looking at chromosomes other than 13, 18, and 21, the women taking part in this study were informed about the possibility of incidental findings.
“However, our study feeds into the ethical debate about whether or not to report incidental findings to patients and also has implications for the current political discussions concerning reimbursement and funding of NIPT by national healthcare systems,” Dr Vermeesch said.
The results also suggest that NIPT might enable the detection of pre-symptomatic cancers in the general population.
“We now know that it is possible to offer the accurate detection of chromosomally imbalanced cancers to the general population via minimally invasive screening methods,” Dr Brison said. “The normalization of the NIPT profile in these patients following treatment indicates that we can also measure response to treatment as early as after the first administration of chemotherapy.”
“Of course, larger-scale studies will be required to validate these results further, but we are confident that we have made an important step towards the possibility of wide-scale, effective, non-invasive cancer screening capable of detecting disease at an early stage.” ![]()
Host cell type may impact malaria treatment

Image by Peter H. Seeberger
A study published in PLOS Pathogens suggests the different metabolic states of reticulocytes and erythrocytes provide different growth conditions for the malaria parasites Plasmodium vivax and Plasmodium falciparum.
As P vivax grows exclusively in reticulocytes, and P falciparum grows primarily in erythrocytes, the research suggests drugs that work against one species might fail to be effective against the other.
Andrew Waters, PhD, of the University of Glasgow in the UK, and his colleagues set out to determine whether the 2 classes of host red blood cells offer different resources for parasite survival and whether these resources could influence antimalarial drug efficacy.
To do that, the team analyzed the metabolites present in reticulocytes and erythrocytes. They found that reticulocytes contain elevated levels of many metabolites that could potentially be scavenged by the invading and growing malaria parasite.
And there was a marked overlap in metabolic pathways observed in the reticulocyte and those predicted in the parasite. The researchers thought these common pathways might be uniquely dispensable to Plasmodium during its growth in reticulocytes but essential—and therefore a good drug target—for growth in erythrocytes.
To test this hypothesis, the team disrupted some of the overlapping pathways in P berghei, a species that causes malaria in mice and, similar to P vivax, has a strong preference for growth in reticulocytes.
They found the mutant P berghei strains could grow in mouse reticulocytes (utilizing the host’s metabolic products).
The researchers also compared the sensitivity of P berghei and P falciparum to a drug known to target one of the overlapping pathways, the pyrimidine biosynthesis inhibitor 5-fluoroorotate (5FOA).
They found that P berghei was considerably less sensitive to 5FOA than P falciparum. The IC50 value of 5FOA in vitro was almost 90-fold higher in P berghei than in P falciparum.
This was presumably because P berghei was able to scavenge the metabolites from their reticulocyte host environment, but no such external sources were available in the erythrocyte host cells invaded by P falciparum.
The researchers said their results indicate that reticulocytes provide a highly enriched host cell environment for Plasmodium parasites, and the availability of the reticulocyte metabolome might reduce or block the efficacy of antimalarial drugs that target parasite metabolism. ![]()

Image by Peter H. Seeberger
A study published in PLOS Pathogens suggests the different metabolic states of reticulocytes and erythrocytes provide different growth conditions for the malaria parasites Plasmodium vivax and Plasmodium falciparum.
As P vivax grows exclusively in reticulocytes, and P falciparum grows primarily in erythrocytes, the research suggests drugs that work against one species might fail to be effective against the other.
Andrew Waters, PhD, of the University of Glasgow in the UK, and his colleagues set out to determine whether the 2 classes of host red blood cells offer different resources for parasite survival and whether these resources could influence antimalarial drug efficacy.
To do that, the team analyzed the metabolites present in reticulocytes and erythrocytes. They found that reticulocytes contain elevated levels of many metabolites that could potentially be scavenged by the invading and growing malaria parasite.
And there was a marked overlap in metabolic pathways observed in the reticulocyte and those predicted in the parasite. The researchers thought these common pathways might be uniquely dispensable to Plasmodium during its growth in reticulocytes but essential—and therefore a good drug target—for growth in erythrocytes.
To test this hypothesis, the team disrupted some of the overlapping pathways in P berghei, a species that causes malaria in mice and, similar to P vivax, has a strong preference for growth in reticulocytes.
They found the mutant P berghei strains could grow in mouse reticulocytes (utilizing the host’s metabolic products).
The researchers also compared the sensitivity of P berghei and P falciparum to a drug known to target one of the overlapping pathways, the pyrimidine biosynthesis inhibitor 5-fluoroorotate (5FOA).
They found that P berghei was considerably less sensitive to 5FOA than P falciparum. The IC50 value of 5FOA in vitro was almost 90-fold higher in P berghei than in P falciparum.
This was presumably because P berghei was able to scavenge the metabolites from their reticulocyte host environment, but no such external sources were available in the erythrocyte host cells invaded by P falciparum.
The researchers said their results indicate that reticulocytes provide a highly enriched host cell environment for Plasmodium parasites, and the availability of the reticulocyte metabolome might reduce or block the efficacy of antimalarial drugs that target parasite metabolism. ![]()

Image by Peter H. Seeberger
A study published in PLOS Pathogens suggests the different metabolic states of reticulocytes and erythrocytes provide different growth conditions for the malaria parasites Plasmodium vivax and Plasmodium falciparum.
As P vivax grows exclusively in reticulocytes, and P falciparum grows primarily in erythrocytes, the research suggests drugs that work against one species might fail to be effective against the other.
Andrew Waters, PhD, of the University of Glasgow in the UK, and his colleagues set out to determine whether the 2 classes of host red blood cells offer different resources for parasite survival and whether these resources could influence antimalarial drug efficacy.
To do that, the team analyzed the metabolites present in reticulocytes and erythrocytes. They found that reticulocytes contain elevated levels of many metabolites that could potentially be scavenged by the invading and growing malaria parasite.
And there was a marked overlap in metabolic pathways observed in the reticulocyte and those predicted in the parasite. The researchers thought these common pathways might be uniquely dispensable to Plasmodium during its growth in reticulocytes but essential—and therefore a good drug target—for growth in erythrocytes.
To test this hypothesis, the team disrupted some of the overlapping pathways in P berghei, a species that causes malaria in mice and, similar to P vivax, has a strong preference for growth in reticulocytes.
They found the mutant P berghei strains could grow in mouse reticulocytes (utilizing the host’s metabolic products).
The researchers also compared the sensitivity of P berghei and P falciparum to a drug known to target one of the overlapping pathways, the pyrimidine biosynthesis inhibitor 5-fluoroorotate (5FOA).
They found that P berghei was considerably less sensitive to 5FOA than P falciparum. The IC50 value of 5FOA in vitro was almost 90-fold higher in P berghei than in P falciparum.
This was presumably because P berghei was able to scavenge the metabolites from their reticulocyte host environment, but no such external sources were available in the erythrocyte host cells invaded by P falciparum.
The researchers said their results indicate that reticulocytes provide a highly enriched host cell environment for Plasmodium parasites, and the availability of the reticulocyte metabolome might reduce or block the efficacy of antimalarial drugs that target parasite metabolism. ![]()
CMSC: Many menopausal and MS symptoms overlap
INDIANAPOLIS – About 50% of women with multiple sclerosis are postmenopausal, but objective data about the impact of menopause on the course of multiple sclerosis are lacking.
“We don’t know anything about the impact of menopause on the MS course; there is wide variability in patient-reported outcomes, but nothing written about comorbidities or symptom management,” Dr. Riley Bove, a neurologist at Brigham and Women’s Hospital, Boston, said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We’ve been working on this. A lot of it is unknown.”
She described menopause is “an opportunity for providers to tackle symptoms and improve well-being and discuss meaningful quality of life and priorities with patients. It can be a good time to talk about these things.”
In a study that Dr. Bove conducted with the online patient-powered research platform PatientsLikeMe, female MS patients were asked to describe the impact of menopause on their disease. Among the themes that emerged were an occasional perimenopausal onset of MS (“menopause and MS symptoms were pretty much simultaneous,” one 58-year-old respondent said); the effect of hot flashes on MS symptoms (“I confused the two, especially hot flashes,” said a 53-year-old woman); and worsening of MS-related disability after menopause, particularly surgical (“Before I stopped taking birth control pills I was working and able to walk and house clean , etc.,” a 53-year-old respondent said. “I had the surgery, and I started to progress toward being completely wheelchair bound.”)
When it comes to solid recommendations for how to manage MS symptoms that overlap with menopause, “we’re in an evidence-free zone,” Dr. Bove said. “Symptoms in MS are kind of like dominoes: You have difficulty sleeping and then your day is off, fatigue is up and your mood is off, and everything can spiral. In the clinic, perimenopausal women often say things like, ‘I feel like I’m falling apart’ or ‘something has to give’ or ‘I need a change.’ ”
Common overlapping symptoms include vasomotor manifestations such as hot flashes, cold flashes, vascular instability, and rapid heartbeat. “The leading mechanistic explanation for the vasomotor symptoms is that abrupt hormone deprivation will result in the loss of negative feedback over hypothalamic NA [noradrenaline] synthesis,” Dr. Bove explained. “The proximity of the hypothalamic thermoregulatory center to luteinizing hormone–releasing hormone-producing areas may also be involved.”
Sleep disturbances and insomnia in menopausal MS patients can impact fatigue and mood. Also, hot flashes can directly exacerbate the MS symptoms, fluctuating over the day or the week.
According to recommendations from the North American Menopause Society, estradiol is the most effective therapy for treating vasomotor symptoms. Other options include selective serotonin reuptake inhibitors (SSRIs) and selective noradrenergic reuptake inhibitors (SNRIs). “Probably the best studied SNRI is venlafaxine,” Dr. Bove said. “It seems to have modest effects on sleep quality and insomnia perimenopausally, as well as on hot flashes.”
Other alternatives include clonidine and gabapentin.
“If a patient wants to use [hormone therapy] for menopausal symptoms, it has to be an individualized approach,” Dr. Bove said. “You have to communicate with the primary care physician to understand what else is going on in this woman’s medical history. The current recommendations are to treat the symptoms with as low a dose as possible for as short a duration as possible. Our MS patients are also at risk for neurodegeneration, for brain volume loss, for cognitive decline, and for worsening function over time.”
With respect to cognition, observational studies have demonstrated that during a certain window of opportunity – defined as within 5 years of the last menstrual period – hormone therapy (HT) may have protective effects against Alzheimer’s disease and against cognitive decline in general. “Beyond this window of time, perhaps due to estrogen receptor down regulation, HT can be harmful, with an increased risk of stroke and dementia reported later on,” she said.
Against this, the Women’s Health Initiative Memory Study (WHIMS) looked at unopposed estrogen, and estrogen and progestin combination, compared with placebo. It found that in women who initiated HT at the age of 65 or older faced an increased risk of dementia from any cause, and of cognitive decline. “This study really put the kibosh on HT as a form of neuroprotection,” Dr. Bove said. “But perhaps it’s unfair to the many patients who are at risk for cognitive decline and neurodegeneration, because the WHIMS did not find an increased risk of adverse cognitive events in women in whom HT was started perimenopausally. Longitudinal, placebo-controlled trials of the effects of HT within the window of opportunity are required to either prove or refute the observational studies that already exist that suggest HT may be neuroprotective. This is an important point to discuss with patients.”
The impact of perimenopausal sleep disturbance on MS symptoms also is unknown. A practical approach to managing sleep disturbance in perimenopausal MS patients is to identify and assess the triggers. “If the bladder symptoms are the major trigger versus mood disturbances such as depression and anxiety, the intervention will be different,” she said. “Consider counseling and/or consultation with a sleep specialist.” Some patients may benefit from pharmacologic treatment to “get them over the hump and get them sleeping better for a little while, versus longer term management if they have a life history of insomnia,” she said. “If the problem is sleep management, you’ll need a drug with a longer half-life. Consider other comorbidities such as anxiety and restless leg syndrome.” Classes of medications to consider include benzodiazepines, nonbenzodiazepines, tricyclic antidepressants, SSRIs and SNRIs.
Mood symptoms commonly overlap in menopausal patients with MS, especially those related to depression and anxiety. “They may be underdiagnosed and undertreated,” Dr. Bove said. “It’s been shown that depression influences the perceived severity of other MS symptoms. Depression is a strong predictor of cognitive and sexual dysfunction, so our perimenopausal MS women have a vulnerability to more severe mood symptoms.” Managing mood symptoms “needs to be multifaceted” and may include psychotherapy to optimize coping abilities, antidepressants, support groups, fatigue and sleep optimization, and social work “to see how employment or financial stressors may be playing a role in a person’s mood.”
In addition, menopausal women may report changes in attention, executive function, multitasking, word finding difficulties, and memory problems, especially in the first year after the final menstrual period. “It’s known that about half of MS patients experience some degree of cognitive impairment,” she said. “Neurocognitive testing may help to identify particular areas of dysfunction that would be amenable to some kind of cognitive rehabilitation.”
Bladder symptoms also can impact postmenopausal patients, especially increasing bladder irritability and incontinence (stress and urge). In MS, “the baseline bladder dysfunction may be magnified,” Dr. Bove said. “If you’re trying to tease out whether the postmenopausal bladder symptoms are from menopause or MS, the MS relapses tend to have more urgency, frequency, and urge incontinence, and the presentation will be more acute. Urodynamic testing can be used to tease this out. The big lifestyle piece that urologists like to hone in on is that people in America drink too much fluid. A practical guideline is that after 3 p.m. just drink for thirst; don’t worry that everything will fall apart if you don’t get your eight glasses of water per day in. If you’re not thirsty, you probably don’t need it.”
While postmenopausal women face an increased risk for osteoporosis, that risk is magnified for MS patients because of the cumulative effect of steroid use – particularly for those who were diagnosed in the pre–disease-modifying-therapy era – being sedentary, and being deconditioned. “Other MS issues such as balance, vision problems, strength or cognitive impairments may all impact gait and compound the risk of falls,” Dr. Bove said. “Osteoporosis prevention and screening should be encouraged in these patients.”
Dr. Bove concluded her presentation by noting that in general, women with disabilities are less likely to be up to date on Pap tests, mammograms, and other important preventive screening tests. “The magnitude of disparities is greater for women with complex limitations,” she said. “Women with MS may have a lower cancer risk, but a larger tumor size at diagnosis.” For example, “is this because the patient is uncomfortable getting on an exam table to get a Pap smear, or is the physician not thinking about other aspects of the person’s life because the focus is on the MS?”
Dr. Bove disclosed that she has received funding from the National Multiple Sclerosis Society, the National Institutes of Health, and from the Harvard Clinical Investigator Training Program.
On Twitter @dougbrunk
INDIANAPOLIS – About 50% of women with multiple sclerosis are postmenopausal, but objective data about the impact of menopause on the course of multiple sclerosis are lacking.
“We don’t know anything about the impact of menopause on the MS course; there is wide variability in patient-reported outcomes, but nothing written about comorbidities or symptom management,” Dr. Riley Bove, a neurologist at Brigham and Women’s Hospital, Boston, said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We’ve been working on this. A lot of it is unknown.”
She described menopause is “an opportunity for providers to tackle symptoms and improve well-being and discuss meaningful quality of life and priorities with patients. It can be a good time to talk about these things.”
In a study that Dr. Bove conducted with the online patient-powered research platform PatientsLikeMe, female MS patients were asked to describe the impact of menopause on their disease. Among the themes that emerged were an occasional perimenopausal onset of MS (“menopause and MS symptoms were pretty much simultaneous,” one 58-year-old respondent said); the effect of hot flashes on MS symptoms (“I confused the two, especially hot flashes,” said a 53-year-old woman); and worsening of MS-related disability after menopause, particularly surgical (“Before I stopped taking birth control pills I was working and able to walk and house clean , etc.,” a 53-year-old respondent said. “I had the surgery, and I started to progress toward being completely wheelchair bound.”)
When it comes to solid recommendations for how to manage MS symptoms that overlap with menopause, “we’re in an evidence-free zone,” Dr. Bove said. “Symptoms in MS are kind of like dominoes: You have difficulty sleeping and then your day is off, fatigue is up and your mood is off, and everything can spiral. In the clinic, perimenopausal women often say things like, ‘I feel like I’m falling apart’ or ‘something has to give’ or ‘I need a change.’ ”
Common overlapping symptoms include vasomotor manifestations such as hot flashes, cold flashes, vascular instability, and rapid heartbeat. “The leading mechanistic explanation for the vasomotor symptoms is that abrupt hormone deprivation will result in the loss of negative feedback over hypothalamic NA [noradrenaline] synthesis,” Dr. Bove explained. “The proximity of the hypothalamic thermoregulatory center to luteinizing hormone–releasing hormone-producing areas may also be involved.”
Sleep disturbances and insomnia in menopausal MS patients can impact fatigue and mood. Also, hot flashes can directly exacerbate the MS symptoms, fluctuating over the day or the week.
According to recommendations from the North American Menopause Society, estradiol is the most effective therapy for treating vasomotor symptoms. Other options include selective serotonin reuptake inhibitors (SSRIs) and selective noradrenergic reuptake inhibitors (SNRIs). “Probably the best studied SNRI is venlafaxine,” Dr. Bove said. “It seems to have modest effects on sleep quality and insomnia perimenopausally, as well as on hot flashes.”
Other alternatives include clonidine and gabapentin.
“If a patient wants to use [hormone therapy] for menopausal symptoms, it has to be an individualized approach,” Dr. Bove said. “You have to communicate with the primary care physician to understand what else is going on in this woman’s medical history. The current recommendations are to treat the symptoms with as low a dose as possible for as short a duration as possible. Our MS patients are also at risk for neurodegeneration, for brain volume loss, for cognitive decline, and for worsening function over time.”
With respect to cognition, observational studies have demonstrated that during a certain window of opportunity – defined as within 5 years of the last menstrual period – hormone therapy (HT) may have protective effects against Alzheimer’s disease and against cognitive decline in general. “Beyond this window of time, perhaps due to estrogen receptor down regulation, HT can be harmful, with an increased risk of stroke and dementia reported later on,” she said.
Against this, the Women’s Health Initiative Memory Study (WHIMS) looked at unopposed estrogen, and estrogen and progestin combination, compared with placebo. It found that in women who initiated HT at the age of 65 or older faced an increased risk of dementia from any cause, and of cognitive decline. “This study really put the kibosh on HT as a form of neuroprotection,” Dr. Bove said. “But perhaps it’s unfair to the many patients who are at risk for cognitive decline and neurodegeneration, because the WHIMS did not find an increased risk of adverse cognitive events in women in whom HT was started perimenopausally. Longitudinal, placebo-controlled trials of the effects of HT within the window of opportunity are required to either prove or refute the observational studies that already exist that suggest HT may be neuroprotective. This is an important point to discuss with patients.”
The impact of perimenopausal sleep disturbance on MS symptoms also is unknown. A practical approach to managing sleep disturbance in perimenopausal MS patients is to identify and assess the triggers. “If the bladder symptoms are the major trigger versus mood disturbances such as depression and anxiety, the intervention will be different,” she said. “Consider counseling and/or consultation with a sleep specialist.” Some patients may benefit from pharmacologic treatment to “get them over the hump and get them sleeping better for a little while, versus longer term management if they have a life history of insomnia,” she said. “If the problem is sleep management, you’ll need a drug with a longer half-life. Consider other comorbidities such as anxiety and restless leg syndrome.” Classes of medications to consider include benzodiazepines, nonbenzodiazepines, tricyclic antidepressants, SSRIs and SNRIs.
Mood symptoms commonly overlap in menopausal patients with MS, especially those related to depression and anxiety. “They may be underdiagnosed and undertreated,” Dr. Bove said. “It’s been shown that depression influences the perceived severity of other MS symptoms. Depression is a strong predictor of cognitive and sexual dysfunction, so our perimenopausal MS women have a vulnerability to more severe mood symptoms.” Managing mood symptoms “needs to be multifaceted” and may include psychotherapy to optimize coping abilities, antidepressants, support groups, fatigue and sleep optimization, and social work “to see how employment or financial stressors may be playing a role in a person’s mood.”
In addition, menopausal women may report changes in attention, executive function, multitasking, word finding difficulties, and memory problems, especially in the first year after the final menstrual period. “It’s known that about half of MS patients experience some degree of cognitive impairment,” she said. “Neurocognitive testing may help to identify particular areas of dysfunction that would be amenable to some kind of cognitive rehabilitation.”
Bladder symptoms also can impact postmenopausal patients, especially increasing bladder irritability and incontinence (stress and urge). In MS, “the baseline bladder dysfunction may be magnified,” Dr. Bove said. “If you’re trying to tease out whether the postmenopausal bladder symptoms are from menopause or MS, the MS relapses tend to have more urgency, frequency, and urge incontinence, and the presentation will be more acute. Urodynamic testing can be used to tease this out. The big lifestyle piece that urologists like to hone in on is that people in America drink too much fluid. A practical guideline is that after 3 p.m. just drink for thirst; don’t worry that everything will fall apart if you don’t get your eight glasses of water per day in. If you’re not thirsty, you probably don’t need it.”
While postmenopausal women face an increased risk for osteoporosis, that risk is magnified for MS patients because of the cumulative effect of steroid use – particularly for those who were diagnosed in the pre–disease-modifying-therapy era – being sedentary, and being deconditioned. “Other MS issues such as balance, vision problems, strength or cognitive impairments may all impact gait and compound the risk of falls,” Dr. Bove said. “Osteoporosis prevention and screening should be encouraged in these patients.”
Dr. Bove concluded her presentation by noting that in general, women with disabilities are less likely to be up to date on Pap tests, mammograms, and other important preventive screening tests. “The magnitude of disparities is greater for women with complex limitations,” she said. “Women with MS may have a lower cancer risk, but a larger tumor size at diagnosis.” For example, “is this because the patient is uncomfortable getting on an exam table to get a Pap smear, or is the physician not thinking about other aspects of the person’s life because the focus is on the MS?”
Dr. Bove disclosed that she has received funding from the National Multiple Sclerosis Society, the National Institutes of Health, and from the Harvard Clinical Investigator Training Program.
On Twitter @dougbrunk
INDIANAPOLIS – About 50% of women with multiple sclerosis are postmenopausal, but objective data about the impact of menopause on the course of multiple sclerosis are lacking.
“We don’t know anything about the impact of menopause on the MS course; there is wide variability in patient-reported outcomes, but nothing written about comorbidities or symptom management,” Dr. Riley Bove, a neurologist at Brigham and Women’s Hospital, Boston, said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We’ve been working on this. A lot of it is unknown.”
She described menopause is “an opportunity for providers to tackle symptoms and improve well-being and discuss meaningful quality of life and priorities with patients. It can be a good time to talk about these things.”
In a study that Dr. Bove conducted with the online patient-powered research platform PatientsLikeMe, female MS patients were asked to describe the impact of menopause on their disease. Among the themes that emerged were an occasional perimenopausal onset of MS (“menopause and MS symptoms were pretty much simultaneous,” one 58-year-old respondent said); the effect of hot flashes on MS symptoms (“I confused the two, especially hot flashes,” said a 53-year-old woman); and worsening of MS-related disability after menopause, particularly surgical (“Before I stopped taking birth control pills I was working and able to walk and house clean , etc.,” a 53-year-old respondent said. “I had the surgery, and I started to progress toward being completely wheelchair bound.”)
When it comes to solid recommendations for how to manage MS symptoms that overlap with menopause, “we’re in an evidence-free zone,” Dr. Bove said. “Symptoms in MS are kind of like dominoes: You have difficulty sleeping and then your day is off, fatigue is up and your mood is off, and everything can spiral. In the clinic, perimenopausal women often say things like, ‘I feel like I’m falling apart’ or ‘something has to give’ or ‘I need a change.’ ”
Common overlapping symptoms include vasomotor manifestations such as hot flashes, cold flashes, vascular instability, and rapid heartbeat. “The leading mechanistic explanation for the vasomotor symptoms is that abrupt hormone deprivation will result in the loss of negative feedback over hypothalamic NA [noradrenaline] synthesis,” Dr. Bove explained. “The proximity of the hypothalamic thermoregulatory center to luteinizing hormone–releasing hormone-producing areas may also be involved.”
Sleep disturbances and insomnia in menopausal MS patients can impact fatigue and mood. Also, hot flashes can directly exacerbate the MS symptoms, fluctuating over the day or the week.
According to recommendations from the North American Menopause Society, estradiol is the most effective therapy for treating vasomotor symptoms. Other options include selective serotonin reuptake inhibitors (SSRIs) and selective noradrenergic reuptake inhibitors (SNRIs). “Probably the best studied SNRI is venlafaxine,” Dr. Bove said. “It seems to have modest effects on sleep quality and insomnia perimenopausally, as well as on hot flashes.”
Other alternatives include clonidine and gabapentin.
“If a patient wants to use [hormone therapy] for menopausal symptoms, it has to be an individualized approach,” Dr. Bove said. “You have to communicate with the primary care physician to understand what else is going on in this woman’s medical history. The current recommendations are to treat the symptoms with as low a dose as possible for as short a duration as possible. Our MS patients are also at risk for neurodegeneration, for brain volume loss, for cognitive decline, and for worsening function over time.”
With respect to cognition, observational studies have demonstrated that during a certain window of opportunity – defined as within 5 years of the last menstrual period – hormone therapy (HT) may have protective effects against Alzheimer’s disease and against cognitive decline in general. “Beyond this window of time, perhaps due to estrogen receptor down regulation, HT can be harmful, with an increased risk of stroke and dementia reported later on,” she said.
Against this, the Women’s Health Initiative Memory Study (WHIMS) looked at unopposed estrogen, and estrogen and progestin combination, compared with placebo. It found that in women who initiated HT at the age of 65 or older faced an increased risk of dementia from any cause, and of cognitive decline. “This study really put the kibosh on HT as a form of neuroprotection,” Dr. Bove said. “But perhaps it’s unfair to the many patients who are at risk for cognitive decline and neurodegeneration, because the WHIMS did not find an increased risk of adverse cognitive events in women in whom HT was started perimenopausally. Longitudinal, placebo-controlled trials of the effects of HT within the window of opportunity are required to either prove or refute the observational studies that already exist that suggest HT may be neuroprotective. This is an important point to discuss with patients.”
The impact of perimenopausal sleep disturbance on MS symptoms also is unknown. A practical approach to managing sleep disturbance in perimenopausal MS patients is to identify and assess the triggers. “If the bladder symptoms are the major trigger versus mood disturbances such as depression and anxiety, the intervention will be different,” she said. “Consider counseling and/or consultation with a sleep specialist.” Some patients may benefit from pharmacologic treatment to “get them over the hump and get them sleeping better for a little while, versus longer term management if they have a life history of insomnia,” she said. “If the problem is sleep management, you’ll need a drug with a longer half-life. Consider other comorbidities such as anxiety and restless leg syndrome.” Classes of medications to consider include benzodiazepines, nonbenzodiazepines, tricyclic antidepressants, SSRIs and SNRIs.
Mood symptoms commonly overlap in menopausal patients with MS, especially those related to depression and anxiety. “They may be underdiagnosed and undertreated,” Dr. Bove said. “It’s been shown that depression influences the perceived severity of other MS symptoms. Depression is a strong predictor of cognitive and sexual dysfunction, so our perimenopausal MS women have a vulnerability to more severe mood symptoms.” Managing mood symptoms “needs to be multifaceted” and may include psychotherapy to optimize coping abilities, antidepressants, support groups, fatigue and sleep optimization, and social work “to see how employment or financial stressors may be playing a role in a person’s mood.”
In addition, menopausal women may report changes in attention, executive function, multitasking, word finding difficulties, and memory problems, especially in the first year after the final menstrual period. “It’s known that about half of MS patients experience some degree of cognitive impairment,” she said. “Neurocognitive testing may help to identify particular areas of dysfunction that would be amenable to some kind of cognitive rehabilitation.”
Bladder symptoms also can impact postmenopausal patients, especially increasing bladder irritability and incontinence (stress and urge). In MS, “the baseline bladder dysfunction may be magnified,” Dr. Bove said. “If you’re trying to tease out whether the postmenopausal bladder symptoms are from menopause or MS, the MS relapses tend to have more urgency, frequency, and urge incontinence, and the presentation will be more acute. Urodynamic testing can be used to tease this out. The big lifestyle piece that urologists like to hone in on is that people in America drink too much fluid. A practical guideline is that after 3 p.m. just drink for thirst; don’t worry that everything will fall apart if you don’t get your eight glasses of water per day in. If you’re not thirsty, you probably don’t need it.”
While postmenopausal women face an increased risk for osteoporosis, that risk is magnified for MS patients because of the cumulative effect of steroid use – particularly for those who were diagnosed in the pre–disease-modifying-therapy era – being sedentary, and being deconditioned. “Other MS issues such as balance, vision problems, strength or cognitive impairments may all impact gait and compound the risk of falls,” Dr. Bove said. “Osteoporosis prevention and screening should be encouraged in these patients.”
Dr. Bove concluded her presentation by noting that in general, women with disabilities are less likely to be up to date on Pap tests, mammograms, and other important preventive screening tests. “The magnitude of disparities is greater for women with complex limitations,” she said. “Women with MS may have a lower cancer risk, but a larger tumor size at diagnosis.” For example, “is this because the patient is uncomfortable getting on an exam table to get a Pap smear, or is the physician not thinking about other aspects of the person’s life because the focus is on the MS?”
Dr. Bove disclosed that she has received funding from the National Multiple Sclerosis Society, the National Institutes of Health, and from the Harvard Clinical Investigator Training Program.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE CMSC ANNUAL MEETING