User login
Hospitalists Should Make Commitment to Improve Healthcare Safety
Peter Pronovost, MD, PhD, FCCM, knows how to deliver a great talk. It is no wonder he is highly sought after and was asked to speak at the plenary for SHM’s annual meeting. Dr. Pronovost, also known as the “Checklist Doctor,” knows how to combine just the right amount of sadness, inspiration, and humor to make his audience feel motivated and compelled to DO something. And, in fact, he implores you—DO something.
Most of us feel excited and inspired during the annual meeting. But those feelings serve little purpose unless we translate them into actions that will make the medical industry a better place for clinicians to work and for patients to receive care. As Dr. Pronovost said, “We are the only hope that the healthcare system has of improving quality and safety.”
He was inspired years ago by the watershed event that will forever be imprinted upon Johns Hopkins Hospital in Baltimore, the preventable death of 18-month-old Josie King on Feb. 22, 2001. Years after the event, her mother, Sorrel King, a passionate patient safety advocate, wanted to know if hospitals are any safer than they were the day that Josie died. She wanted to know what patient safety experts at Hopkins had done to ensure there would not be another Josie King story.
Patients and their families consistently voice similar desires after they have suffered preventable harm. They want to know what happened, why it happened, what it means for them, and what will be done to prevent it from happening again.1 The latter question is one I am frequently asked by patients and their families at my hospital. “What are you going to do to make sure this does not happen again?”
I would venture to guess most hospitalists have been responsible for some type of preventable patient harm during their careers. We work in complex, high-volume, unpredictable, and continuously changing environments. Many of the patients and families in our care are new to us and are with us for only short periods of time. Those of us who have been responsible for preventable patient harm know that it is an unforgettable moment in time that can weigh upon your conscious. And, of course, we all want to do something to make sure it does not happen again.
That is exactly what patients and their families expect of all of us—to DO something—and they should.
But this can be an overwhelming responsibility, especially when the root causes of harm are difficult either to identify or to fix—such as a miscommunication, a diagnostic error, or an inadequate handoff.
Which gets me back to Dr. Pronovost giving a great talk. His appeal to our audience of about 3,000 hospitalists was to DO something. To make the healthcare system improve quality and safety for future patients. To not wait until we or our colleagues are involved in a preventable harm event. To do something, anything, now, that contributes to safer care, today and every day going forward.
He ended his talk with “I will….” Dr. Pronovost (and I would venture to guess patients and their families) wants each of us to fill in the blank with a statement of personal accountability for action. Unfortunately, many of us still believe that we are personally unable to make complex systems safer for patients. Many of us still believe that patients and the systems they traverse are too complex, unpredictable, unreliable, or noncompliant.
The truth is, patients and systems are indeed complex, unpredictable, unreliable, and noncompliant. The further truth is, the only way to make care safer is for each of us to start with a collective shared mental model that we can make it better—and for each of us to commit to personal accountability for action.
My “I Will”
So, while I really enjoyed Dr. Pronovost’s talk, what I enjoyed even more was reading the section in last month’s edition of The Hospitalist in which about a half dozen hospitalists interviewed after the plenary accepted the challenge of filling in the blank “I will….” A few excerpts:
- “I will let them know that everything is possible…”
- “I will improve healthcare…”
- “[I will] make sure the patient is heard…”
By a simple proclamation of personal accountability, a mere thousand hospitalists attending an annual meeting can collectively and progressively change the safety of healthcare in thousands of hospitals around the country. It starts with thinking we can do it and publicly committing to the journey. Although we are still a relatively new specialty, we have permeated almost every hospital in the country, and we have outpaced the growth of any specialty in the history of modern medicine. We are perfectly poised to be the safety change agents for every hospital system. As Margaret Meade famously said, “Never doubt that a small group of thoughtful, committed citizens can change the world; indeed, it’s the only thing that ever has….”
So don’t delay. Whether or not you had the good fortune of being inspired at the SHM annual meeting, each of us owes it to our patients to commit to improving the safety of healthcare and paving the future of hospital care. Get out your pen, craft a commitment now, follow through with it, and make hospitals safer tomorrow than they were yesterday.
I will…

Reference
- Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001-1007.
Peter Pronovost, MD, PhD, FCCM, knows how to deliver a great talk. It is no wonder he is highly sought after and was asked to speak at the plenary for SHM’s annual meeting. Dr. Pronovost, also known as the “Checklist Doctor,” knows how to combine just the right amount of sadness, inspiration, and humor to make his audience feel motivated and compelled to DO something. And, in fact, he implores you—DO something.
Most of us feel excited and inspired during the annual meeting. But those feelings serve little purpose unless we translate them into actions that will make the medical industry a better place for clinicians to work and for patients to receive care. As Dr. Pronovost said, “We are the only hope that the healthcare system has of improving quality and safety.”
He was inspired years ago by the watershed event that will forever be imprinted upon Johns Hopkins Hospital in Baltimore, the preventable death of 18-month-old Josie King on Feb. 22, 2001. Years after the event, her mother, Sorrel King, a passionate patient safety advocate, wanted to know if hospitals are any safer than they were the day that Josie died. She wanted to know what patient safety experts at Hopkins had done to ensure there would not be another Josie King story.
Patients and their families consistently voice similar desires after they have suffered preventable harm. They want to know what happened, why it happened, what it means for them, and what will be done to prevent it from happening again.1 The latter question is one I am frequently asked by patients and their families at my hospital. “What are you going to do to make sure this does not happen again?”
I would venture to guess most hospitalists have been responsible for some type of preventable patient harm during their careers. We work in complex, high-volume, unpredictable, and continuously changing environments. Many of the patients and families in our care are new to us and are with us for only short periods of time. Those of us who have been responsible for preventable patient harm know that it is an unforgettable moment in time that can weigh upon your conscious. And, of course, we all want to do something to make sure it does not happen again.
That is exactly what patients and their families expect of all of us—to DO something—and they should.
But this can be an overwhelming responsibility, especially when the root causes of harm are difficult either to identify or to fix—such as a miscommunication, a diagnostic error, or an inadequate handoff.
Which gets me back to Dr. Pronovost giving a great talk. His appeal to our audience of about 3,000 hospitalists was to DO something. To make the healthcare system improve quality and safety for future patients. To not wait until we or our colleagues are involved in a preventable harm event. To do something, anything, now, that contributes to safer care, today and every day going forward.
He ended his talk with “I will….” Dr. Pronovost (and I would venture to guess patients and their families) wants each of us to fill in the blank with a statement of personal accountability for action. Unfortunately, many of us still believe that we are personally unable to make complex systems safer for patients. Many of us still believe that patients and the systems they traverse are too complex, unpredictable, unreliable, or noncompliant.
The truth is, patients and systems are indeed complex, unpredictable, unreliable, and noncompliant. The further truth is, the only way to make care safer is for each of us to start with a collective shared mental model that we can make it better—and for each of us to commit to personal accountability for action.
My “I Will”
So, while I really enjoyed Dr. Pronovost’s talk, what I enjoyed even more was reading the section in last month’s edition of The Hospitalist in which about a half dozen hospitalists interviewed after the plenary accepted the challenge of filling in the blank “I will….” A few excerpts:
- “I will let them know that everything is possible…”
- “I will improve healthcare…”
- “[I will] make sure the patient is heard…”
By a simple proclamation of personal accountability, a mere thousand hospitalists attending an annual meeting can collectively and progressively change the safety of healthcare in thousands of hospitals around the country. It starts with thinking we can do it and publicly committing to the journey. Although we are still a relatively new specialty, we have permeated almost every hospital in the country, and we have outpaced the growth of any specialty in the history of modern medicine. We are perfectly poised to be the safety change agents for every hospital system. As Margaret Meade famously said, “Never doubt that a small group of thoughtful, committed citizens can change the world; indeed, it’s the only thing that ever has….”
So don’t delay. Whether or not you had the good fortune of being inspired at the SHM annual meeting, each of us owes it to our patients to commit to improving the safety of healthcare and paving the future of hospital care. Get out your pen, craft a commitment now, follow through with it, and make hospitals safer tomorrow than they were yesterday.
I will…

Reference
- Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001-1007.
Peter Pronovost, MD, PhD, FCCM, knows how to deliver a great talk. It is no wonder he is highly sought after and was asked to speak at the plenary for SHM’s annual meeting. Dr. Pronovost, also known as the “Checklist Doctor,” knows how to combine just the right amount of sadness, inspiration, and humor to make his audience feel motivated and compelled to DO something. And, in fact, he implores you—DO something.
Most of us feel excited and inspired during the annual meeting. But those feelings serve little purpose unless we translate them into actions that will make the medical industry a better place for clinicians to work and for patients to receive care. As Dr. Pronovost said, “We are the only hope that the healthcare system has of improving quality and safety.”
He was inspired years ago by the watershed event that will forever be imprinted upon Johns Hopkins Hospital in Baltimore, the preventable death of 18-month-old Josie King on Feb. 22, 2001. Years after the event, her mother, Sorrel King, a passionate patient safety advocate, wanted to know if hospitals are any safer than they were the day that Josie died. She wanted to know what patient safety experts at Hopkins had done to ensure there would not be another Josie King story.
Patients and their families consistently voice similar desires after they have suffered preventable harm. They want to know what happened, why it happened, what it means for them, and what will be done to prevent it from happening again.1 The latter question is one I am frequently asked by patients and their families at my hospital. “What are you going to do to make sure this does not happen again?”
I would venture to guess most hospitalists have been responsible for some type of preventable patient harm during their careers. We work in complex, high-volume, unpredictable, and continuously changing environments. Many of the patients and families in our care are new to us and are with us for only short periods of time. Those of us who have been responsible for preventable patient harm know that it is an unforgettable moment in time that can weigh upon your conscious. And, of course, we all want to do something to make sure it does not happen again.
That is exactly what patients and their families expect of all of us—to DO something—and they should.
But this can be an overwhelming responsibility, especially when the root causes of harm are difficult either to identify or to fix—such as a miscommunication, a diagnostic error, or an inadequate handoff.
Which gets me back to Dr. Pronovost giving a great talk. His appeal to our audience of about 3,000 hospitalists was to DO something. To make the healthcare system improve quality and safety for future patients. To not wait until we or our colleagues are involved in a preventable harm event. To do something, anything, now, that contributes to safer care, today and every day going forward.
He ended his talk with “I will….” Dr. Pronovost (and I would venture to guess patients and their families) wants each of us to fill in the blank with a statement of personal accountability for action. Unfortunately, many of us still believe that we are personally unable to make complex systems safer for patients. Many of us still believe that patients and the systems they traverse are too complex, unpredictable, unreliable, or noncompliant.
The truth is, patients and systems are indeed complex, unpredictable, unreliable, and noncompliant. The further truth is, the only way to make care safer is for each of us to start with a collective shared mental model that we can make it better—and for each of us to commit to personal accountability for action.
My “I Will”
So, while I really enjoyed Dr. Pronovost’s talk, what I enjoyed even more was reading the section in last month’s edition of The Hospitalist in which about a half dozen hospitalists interviewed after the plenary accepted the challenge of filling in the blank “I will….” A few excerpts:
- “I will let them know that everything is possible…”
- “I will improve healthcare…”
- “[I will] make sure the patient is heard…”
By a simple proclamation of personal accountability, a mere thousand hospitalists attending an annual meeting can collectively and progressively change the safety of healthcare in thousands of hospitals around the country. It starts with thinking we can do it and publicly committing to the journey. Although we are still a relatively new specialty, we have permeated almost every hospital in the country, and we have outpaced the growth of any specialty in the history of modern medicine. We are perfectly poised to be the safety change agents for every hospital system. As Margaret Meade famously said, “Never doubt that a small group of thoughtful, committed citizens can change the world; indeed, it’s the only thing that ever has….”
So don’t delay. Whether or not you had the good fortune of being inspired at the SHM annual meeting, each of us owes it to our patients to commit to improving the safety of healthcare and paving the future of hospital care. Get out your pen, craft a commitment now, follow through with it, and make hospitals safer tomorrow than they were yesterday.
I will…

Reference
- Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001-1007.
How Should a Patient with Pulmonary Hypertension Be Evaluated, Managed?
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
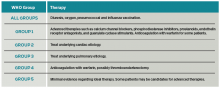
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Your postop patient is confused and agitated—next steps?
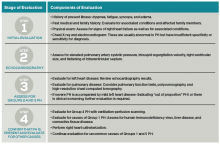
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
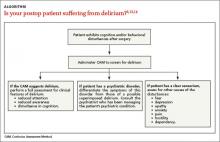
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
› Conduct a baseline cognitive assessment during your patient’s routine visits and preoperative assessments to gauge his or her risk for delirium. A
› Work with the hospital team to implement nonpharmacologic interventions, such as reorienting the patient to day and time and avoiding sensory deprivation, as an initial treatment for delirium. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Mark Q, age 80, is admitted to the hospital to undergo hemicolectomy for colon cancer. His medical history includes hypertension, benign prostatic hyperplasia, and colon cancer. He did well immediately postop, but when you make morning rounds the day after his surgery, you notice that he is confused and agitated. Mr. Q’s chart reveals that earlier that morning, he pulled out his Foley catheter and intravenous (IV) line when his nurse declined his request to walk him to the bathroom.
How would you proceed?
Up to 50% of older adults who undergo surgical procedures develop delirium—a disturbance in attention and awareness accompanied by changes in cognition.1 Older adults are at heightened risk for this postoperative complication for several reasons. For one thing, older patients have a reduced capacity for homeostatic regulation when they undergo anesthesia and surgery.2 For another, age-related changes in brain neurochemistry and drug metabolism increase the likelihood of adverse drug effects, including those that could precipitate delirirum.3
Although postop delirium is a common complication in older patients, it sometimes goes unrecognized. Missed or delayed diagnosis of delirium can result in patients exhibiting behaviors that can compromise their safety, delay recuperation, and result in longer hospital stays, a greater financial burden, and increased morbidity and mortality.4 The American Geriatric Society recently published clinical guidelines and a best practices statement for preventing and treating postop delirium in patients ages >65 years.1,5 This article describes steps family physicians can take to assess their patients’ risk of delirium before they undergo surgery, and to recognize and treat delirium in the postop period.
Defining delirium
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the criteria for delirium are:6
A. A disturbance in attention (ie, reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment).
B. The disturbance develops over a short time (usually hours to a few days), represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day.
C. An additional disturbance in cognition (eg, memory deficits, disorientation, language, visuospatial ability, perception).
D. The disturbances in attention, awareness, and cognition aren’t better explained by another preexisting or evolving neurocognitive disorder and don’t occur in the context of a severely reduced level of arousal.
E. History, physical, or laboratory findings show that the disturbance is caused by the direct physiologic consequences of a general medical condition, substance intoxication/withdrawal, exposure to a toxin, or multiple etiologies.
The 3 subtypes of delirium are based on patients’ psychomotor activity.7 In hyperactive delirium, patients exhibit heightened arousal, restlessness, agitation, hallucinations, and inappropriate behavior. Hypoactive delirium is characterized by lethargy, reduced motor activity, incoherent speech, and lack of interest. Mixed delirium consists of a combination of hyperactive and hypoactive signs and symptoms.
Gauge risk before patients undergo surgery
Family physicians can assess their patients’ risk for developing delirium by conducting baseline screening during routine office visits as well as during preoperative evaluations. Factors that increase postop delirium risk include:1
• age >65 years
• dementia
• poor vision
• decreased hearing
• severe illness
• infection.
Routine cognitive screening can be done easily and efficiently using readily available tools such as the Alzheimer Association’s Cognitive Assessment Toolkit.8 This toolkit includes 3 brief, validated screening tools to identify patients with probable cognitive impairment: the General Practitioner Assessment of Cognition, the Memory Impairment Screen, and the Mini-Cog.
If preop screening indicates that the patient is at increased risk for delirium, the family physician should work with hospital’s interdisciplinary teams to institute prevention measures, such as the Hospital Elder Life Program (HELP).9 This program offers a structured curriculum for instructing volunteers to deliver daily orientation, early mobilization, feeding assistance, therapeutic activities, and other measures to help prevent delirium.
Prompt screening after surgery is essential, too
In addition to preop delirium risk assessment, all patients who undergo surgery should receive daily delirium screening during the first postoperative week. The Confusion Assessment Method (CAM) is a quick screening tool for assessing a patient’s level of arousal and consciousness.10 Based on the results of 7 high-quality studies (N=1071), CAM has a sensitivity of 94% (95% confidence interval [CI], 91%-97%) and specificity of 89% (95% CI, 85%-94%).11,12
Feature 1 of CAM, “Acute onset and fluctuating course,” requires that you compare the patient’s current mental status to his or her pre-hospital baseline mental status; the baseline status should be obtained from a family member, caretaker, or clinician who has observed the patient over time.10 This is intended to determine if the patient has experienced an acute change in mental status (eg, attention, orientation, cognition), usually over the course of hours to days.10 Feature 2, “Inattention,” is used to determine if the patient has a reduced ability to maintain attention to external stimuli and to appropriately shift attention to new external stimuli, and if the patient is unaware or out of touch with the environment.10 Feature 3, “Disorganized thinking,” is used to assess the patient’s organization of thought as expressed by speech or writing. Disorganized thinking typically manifests as rambling and irrelevant or incoherent speech.10 Feature 4, “Altered level of consciousness,” is used to rate the patient’s alertness level.10
A positive screen for delirium requires the presence of Feature 1 (acute onset and/or fluctuation) and Feature 2, plus either Feature 3 or Feature 4.
Is delirium—or something else—at work?
If an older adult is exhibiting cognitive and/or behavioral disturbances after undergoing surgery, it’s important to discern if these manifestations are the result of delirium, a preexisting psychiatric disorder, or some other cause if the patient has a clear sensorium (ALGORITHM).6,13,14
Delirium. If a patient’s CAM screen suggests delirium, conduct a thorough assessment for the signs and symptoms of delirium to determine if the patient meets DSM-5 criteria for the diagnosis.1 In order to avoid missing hypoactive, subtle, or atypical cases of delirium, conduct a thorough medical record and medications review, and gather assessments from the nursing staff and other team members regarding the patient’s behavior.
Preexisting psychiatric disorder. It’s important to differentiate psychiatric symptoms from those of a superimposed delirium.13 Because patients with preoperative depressive symptoms may be at increased risk for postop delirium, pre-surgical psychiatric evaluations are important for identifying even subtle psychopathological symptoms.15 (The psychiatric interview is the gold standard for diagnosis.16) For patients who have an established psychiatric diagnosis, consider consulting with the psychiatrist who is managing the patient’s psychiatric care.13
Other causes. If a patient who is exhibiting postop cognitive and/or behavioral disturbances has a reasonably accurate memory and a correct orientation for time, place, and person, interviews with the patient and caregivers (along with the psychiatric interview) will likely reveal potential causes for the behavioral problems.13
Is the patient suffering from dehydration? Drug withdrawal?
Assessment for an underlying organic cause must be performed because specific treatment for the underlying diagnosis may improve delirium.17 Common causes include hypoxia, infection, dehydration, acute metabolic disturbance, endocrinopathies, cardiac or vascular disorders, and drug withdrawal.13 An appropriate diagnostic work-up might consist of serum urea, glucose, electrolytes, liver function tests, arterial blood gas analyses, urinalysis, nutritional evaluation, electrocardiogram, and a complete blood count.
Ask patients about their use of alcohol and benzodiazepines, and consider alcohol or drug withdrawal as potential etiologies.18 Patients with delirium should also be assessed for iatrogenic hospital-related factors that could be causing or contributing to the condition, such as immobilization or malnutrition.13
Medications are a common culprit: Approximately 40% of cases of delirium are related to medication use.18 Commonly used postop medications such as analgesics, sedatives, proton pump inhibitors, and others can cause delirium.19 Carefully review the patient’s medication list.13 Medication-induced delirium is influenced by the number of medications taken (generally >3),20 the use of psychoactive medications,21 and the specific agent's anticholinergic potential.22 The 2012 updated Beers Criteria (American Geriatrics Society) is a useful resource for determining if “inappropriate polypharmacy” is the cause of postop delirium.23
Inadequate pain control. In a multisite trial,24 patients who received <10 mg/d of parenteral morphine sulfate equivalents were more likely to develop delirium than patients who received more analgesia. In cognitively intact patients, severe pain significantly increased the risk of delirium. With the exception of meperidine, opioids do not precipitate delirium in patients with acute pain.24 Not treating pain or administering very low—or excessively high—doses of opioids is associated with an increased risk of delirium for both cognitively intact and impaired patients.24
Constipation can contribute to the development of delirium.25 After surgery, patients tend to be less mobile and may be receiving medications that can cause constipation, such as opioids, iron, calcium, and channel blockers. Preventing and treating constipation in postop patients can reduce delirium risk.25
Begin treatment with nonpharmacologic measures
Regardless of whether a patient suffers from hyperactive, hypoactive, or mixed delirium, nonpharmacologic interventions are firstline treatment.19 Such interventions can help patients develop a sense of control over their environment, which can help relieve agitation.13 Because environmental shifts contribute to the development of delirium, avoiding transfers and securing a single room can be helpful.19 Patients with delirium have altered perceptions, and may view normal objects and routine clinician actions as harmful and threatening. Therefore, it is helpful to avoid sensory deprivation by making sure patients have access to their eyeglasses and hearing aids, and to provide nonthreatening cognitive/environmental stimulation.1,13,19 Patients should be encouraged to resume walking as soon as possible.1,19 Other nonpharmacologic interventions are listed in the TABLE.1,13,19
Safety issues must also be addressed.17 Patients with mixed or hyperactive delirium may become agitated, which can lead them to pull tubes, drains, or lines, as occurred with Mr. Q. Patients with hypoactive delirium may be prone to wandering, or receive less attention due to their hypoactive state.17 All patients with delirium are at risk of falls.
Patients should be evaluated for these risks to determine whether assigning a "sitter" or transfer to a stepdown unit or intensive care unit is warranted.17 Restraints are not recommended because they can exacerbate delirium and lead to injuries.26
Pharmacologic treatment should be reserved for patients whose behavior compromises their safety, and implemented only when the cause of the delirium is known. The primary objectives of drug therapy are to achieve and maintain safe and rapid behavioral control so the patient can receive necessary medical care, and to enhance functional recovery.14 The choice of a specific medication is individualized and depends on each patient’s clinical condition.14
For a patient with hyperactive delirium, an antipsychotic typically is the treatment of choice because these medications are dopamine receptor antagonists, and excessive dopamine transmission has been implicated in this type of delirium.27 Haloperidol often is the preferred treatment; a low-dose oral form is recommended for older patients who exhibit severe agitation because there is less risk of QT prolongation compared to IV administration.28
Second-generation antipsychotics (eg, risperidone, olanzapine, and quetiapine) are increasingly used due to their lower risk for adverse extrapyramidal symptoms, which are common in older patients.29-31 Despite this, increasing data show that morbidity with these agents may be underestimated, and the risks of adverse effects may vary among the medications in this class.32
For hypoactive or mixed delirium, nonpharmacologic interventions should be the mainstay of treatment. When medications are used, they should be used to target the underlying etiology of delirium (eg, treating a urinary tract infection with an antibiotic).33
A few final words about medication use for delirium ... Most medications that modify symptoms of delirium can actually prolong the delirium.33 Therefore, it's important to carefully consider the balance between effectively managing symptoms and causing adverse effects. Because older adults have increased sensitivity to medications, always start with small dosages and titrate to effect.34 Benzodiazepines and other hypnotics should be avoided in older patients, except when treating alcohol or benzodiazepine withdrawal.35
CASE › Mr. Q’s postop delirium screen is positive, and assessment for underlying causes reveals that he is suffering from postoperative pain and is constipated. Due to roommate noise and insomnia, he is transferred to a private room, where quiet times are observed. He receives oxycodone 5 mg every 4 hours for his pain and senna 30 mg at bedtime and a bisacodyl rectal suppository 10 mg/d for constipation. After 3 days Mr. Q’s postop pain and delirium resolves, and he is discharged home.
CORRESPONDENCE
Jackson Ng, MD, Teresa Lang Research Center, New York Hospital Queens, 56-45 Main St., Flushing, NY 11355; [email protected]
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
1. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136-148.
2. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
3. O’Keeffe ST, Ní Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673-687.
4. Mangnall LT, Gallagher R, Stein-Parbury J. Postoperative delirium after colorectal surgery in older patients. Am J Crit Care. 2011;20:45-55.
5. American Geriatrics Society. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults: November 2014. American Geriatrics Society Web site. Available at: http://geriatricscareonline.org/ProductAbstract/americangeriatrics-society-clinical-practice-guideline-for-postoperativedelirium-in-older-adults/CL018. Accessed April 7, 2015.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
7. Potter J, George J; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med. 2006;6:303-308.
8. Alzheimer’s Association. Cognitive Assessment Toolkit: A guide to detect cognitive impairment quickly and efficiently during the Medicare Annual Wellness Visit. 1999. Alzheimer’s Association Web site. Available at: http://www.alz.org/documents_custom/The%20Cognitive%20Assessment%20Toolkit%20Copy_v1.pdf. Accessed April 6, 2015.
9. The Hospital Elder Life Program. Hospital Elder Life Program (HELP) for Prevention of Delirium. The Hospital Elder Life Program Web site. Available at: http://www.hospitalelderlifeprogram.org. Accessed April 9, 2015.
10. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
11. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823-830.
12. Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
13. Simon L, Jewell N, Brokel J. Management of acute delirium in hospitalized elderly: a process improvement project. Geriatr Nurs. 1997;18:150-154.
14. Fish DN. Treatment of delirium in the critically ill patient. Clin Pharm. 1991;10:456-466.
15. Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149-156.
16. Nordgaard J, Sass LA, Parnas J. The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci. 2013;263:353-364.
17. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interven Aging. 2008;3:351-355.
18. Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203:752-757.
19. Ghandour A, Saab R, Mehr DR. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60:726-734.
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852-857.
21. Gaudreau JD, Gagnon P, Roy MA, et al. Association between psychoactive medications and delirium in hospitalized patients: a critical review. Psychosomatics. 2005;46:302-316.
22. Tune L, Carr S, Cooper T, et al. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5:208-210.
23. Hitzeman N, Belsky K. Appropriate use of polypharmacy for older patients. Am Fam Physician. 2013;87:483-484.
24. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76-81.
25. Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part II. Constipation, delirium, and dyspnea. Am Fam Physician. 2001;64:1019-1027.
26. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1-20.
27. Mantz J, Hemmings HC, Boddaert J. Case scenario: postoperative delirium in elderly surgical patients. Anesthesiology. 2010;112:189-195.
28. Gleason OC. Delirium. Am Fam Physician. 2003;67:1027-1034.
29. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125-127.
30. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43:171-174.
31. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
32. Kohen I, Lester PE, Lam S. Antipsychotic treatments for the elderly: efficacy and safety of aripiprazole. Neuropsychiatr Dis Treat. 2010;6:47-58.
33. Farrell TW, Dosa D. The assessment and management of hypoactive delirium. Geriatrics for the Practicing Physician. 2007;90:393-395.
34. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176-1181.
35. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388-393.
Girl, 5, With Fever and Hip Pain
A 5-year-old Filipino girl was brought to a pediatric clinic for follow up of an unresolved fever and for new-onset right hip pain, which occurred intermittently for the past week and was associated with a right-sided limp. She had been experiencing nightly fevers ranging from 101°F to 105°F for the past two weeks, for which her parents had been giving ibuprofen with mixed results; she remained afebrile during daytime hours.
Using the Wong-Baker FACES pain scale, the patient rated the pain as a 4/10 in severity (“Hurts a Little More” face).1 Standing and walking aggravated the pain but did not limit activity. Although ibuprofen decreased the fever, it did not alleviate the hip pain. Other symptoms included vomiting one to two times daily, without hematemesis, and four to five episodes of diarrhea daily, without abdominal pain, hematochezia, or melena. She also experienced decreased appetite, but her parents reported no change in her dietary or fluid intake. The patient and her parents denied additional symptoms.
Further investigation revealed that the patient had been seen a week earlier by two other clinicians in the office for complaints of fever, rash, nausea, hematemesis, and diarrhea. She had been diagnosed with a herpes simplex viral (HSV) lesion of the nose, epistaxis, and viral gastroenteritis. Her treatment plan consisted of acyclovir ointment for the HSV lesion and symptomatic support for the gastroenteritis associated diarrhea. The complaint of hematemesis was attributed to postnasal drip from the epistaxis, and reassurance was provided to the patient and family. In addition, six weeks earlier, the patient had been treated for otitis media with a full course of amoxicillin.
Medical history was negative for surgeries, trauma, injuries, and chronic medical conditions. She took no medications or supplements on a regular basis. Her parents denied any known drug allergies and stated that her immunizations were up to date.
The patient lived at home with her biological parents and two brothers, all of whom were healthy, without any recent infections or illnesses. Of significance, the family had travelled to the Philippines for vacation about four months earlier. Results of a tuberculin skin test done six weeks earlier (because the patient presented with respiratory symptoms shortly after traveling to the Philippines) were negative.
Physical examination revealed a well-developed, well-nourished 40-lb girl, in no acute distress, who was active and playful with her brother while in the exam room. Vital signs were significant for a fever of 101.9°F (last dose of ibuprofen was approximately six hours earlier) but were otherwise stable. Skin exam revealed that the prior HSV lesion of the nose had resolved. HEENT, cardiovascular, and pulmonary exam findings were noncontributory. Urine dipstick was negative.
Abdominal exam revealed normoactive bowel sounds in all four quadrants, and on palpation, the abdomen was soft, nontender, and without organomegaly. Specialized abdominal exams to assess for peritonitis, including those to elicit Rovsing, rebound tenderness, obturator, and psoas signs, were all negative. Bilateral extremity exams of the hips, knees, and ankles revealed full range of motion (active and passive), with normal muscle strength throughout. The only significant finding on the physical exam was mild pain of the right anterior hip at 15° of flexion, appreciated while the patient was supine on the exam table. The patient was also observed pushing off her right lower extremity when climbing onto the exam table, and she skipped down the hall when leaving the exam.
With fever of unknown origin (FUO) and a largely negative history and physical, the working list of differential diagnoses included
• Avascular necrosis
• Bacteremia
• Juvenile idiopathic arthritis
• Osteomyelitis
• Pyelonephritis
• Reiter syndrome
• Rheumatic fever
• Rheumatoid arthritis
• Septic joint
• Urinary tract infection
To begin the diagnostic process, a number of laboratory tests and imaging procedures were ordered. Table 1 presents the results of these studies. A tuberculin skin test was not repeated. While awaiting test results, the patient was started on naproxen oral suspension (125 mg/5 mL; 4 mL bid) for fever and pain control.
Based on findings consistent with an inflammatory pattern, the history of otitis media (of possible streptococcal origin) six weeks prior to this visit, and the elevated ASO titer, the patient was started on penicillin V (250 mg bid) and instructed to return for follow up in two days.
At the follow-up visit, no improvement was noted; the patient continued to experience nightly fevers and hip pain. Rovsing, rebound tenderness, obturator, and psoas signs continued to be negative. Physical examination did, however, reveal a mild abdominal tenderness in the right lower quadrant.
Due to this new finding, an abdominal ultrasound was ordered to screen for appendicitis. Despite the parents’ appropriate concern for the child, misunderstanding about the urgent need to obtain the abdominal ultrasound led to a two-day delay in scheduling the exam. Results of ultrasonography revealed psoas abscess, and the patient was promptly admitted to the pediatric floor of the local hospital.
Continue for discussion >>
DISCUSSION
Psoas abscess is a collection of pus in the iliopsoas compartment, an extraperitoneal space containing the psoas and iliacus muscles.2 It can be life-threatening if the infection progresses to septic shock. Historically, psoas abscesses were a frequent complication of tuberculosis (TB) of the spine; but with modern TB treatment, these abscesses have become rare.2 Paradoxically, increased utilization of CT to evaluate sepsis of unknown etiology has led to a recent increase in the frequency of psoas abscess diagnosis.3
Psoas abscesses are categorized as either primary or secondary, with primary infections originating in the psoas muscle and secondary infections spreading from adjacent organs.2 In 42% to 88% of cases (depending on the study), primary psoas abscesses are caused by the hematogenous spread of Staphylococcus aureus from distant infection sites.2,4,5 The psoas muscle is particularly susceptible to this mode of infection because of its rich vascular supply.6 Children, immunosuppressed adults (ie, patients with diabetes, HIV/AIDS, or renal failure), IV drug users, and patients with a history of trauma to the muscle are most susceptible to developing a primary psoas abscess.2,5
Secondary psoas abscesses are caused by infections involving adjacent structures of the gastrointestinal, urinary, and skeletal systems. They are most frequently associated with intra-abdominal inflammatory processes, with the most common etiology being Crohn disease.5 Secondary psoas abscesses, though more diverse in their bacterial flora, tend to follow certain microbiologic patterns based on the inoculating source; Escherichia coli is the most common pathogen in secondary abscesses caused by gastrointestinal (42%) and urinary (61%) sources, and S aureus the most common (35%) from skeletal origins (ie, osteomyelitis).4,5Mycobacterium tuberculosis is the more frequently found cause in developing countries but should be considered if the patient has recently travelled outside the United States.
Review of the literature suggests that the incidence of methicillin-resistant S aureus (MRSA) as the causative agent of psoas abscesses may be increasing. However, there is a wide variance in the incidence reported, ranging from 1.1% to 12% of confirmed microbial infections.5,7,8
The classic historical presentation of psoas abscess has been described as the triad of back pain, fever, and limp5,6; however, this triad has only been described in approximately 30% of cases.5 The typical presentation consists of flank or lower limb pain (91%), fever (75%), anorexia (46%), and/or weakness (43%).4 Laboratory abnormalities include leukocytosis (67%) and elevated markers of inflammation (eg, erythrocyte sedimentation rate, seen in 73% of cases).4
Imaging via abdominal ultrasound may be helpful to screen for psoas abscess; however, its utility is limited by a low diagnostic yield of 60% or less.2,4 Direct visualization of the retroperitoneal structures, for example, can be problematic due to the presence of bowel gas.9 Abdominal CT is considered the gold standard for the definitive diagnosis of psoas abscess due to its high sensitivity (100%) and specificity (77%); it can also be used simultaneously to guide percutaneous drainage to treat the abscess if needed.7 However, some clinicians prefer abdominal MRI because of its ability to enhance soft-tissue visualization without requiring use of IV contrast.2,4
The approach to treating psoas abscess varies from a strictly antibiotic regimen to percutaneous drainage, and in rare circumstances, open surgical drainage. Antibiotic therapy without drainage or surgical intervention is a sufficient starting point for treatment of abscesses less than 3 cm in size.3
The antibiotic regimen choice depends on the suspected pathogen. In cases of suspected S aureus, empiric antistaphylococcal antibiotics should be initiated while culture results are pending.2,4 Secondary psoas abscesses thought to be derived from a urinary or gastrointestinal source should prompt use of a broader spectrum antibiotic due to the higher probability of gram-negative, anaerobic, or polymicrobial involvement.2,4
Once final culture and sensitivity results are obtained, antibiotic therapy should be modified to target the isolated pathogen(s). Treatment duration is typically six weeks but may vary, depending on serial culture results and the inoculating source.4 Review of the literature reveals that abscesses resulting from skeletal sources have traditionally been treated longer, usually with antibiotics alone, than those from urinary or gastrointestinal sources, which are often treated with the combination of antibiotics and percutaneous drainage.4
In cases of psoas abscesses larger than 3 cm, management should include both appropriate antibiotics and percutaneous drainage of the abscess.2 Percutaneous drainage is preferred to open surgical drainage because outcomes are similar, it is less invasive, and there is less risk of spreading abscess contents.2-4 In a retrospective analysis by Dietrich et al, 50% of patients treated with antibiotics and percutaneous drainage responded after one drainage, but the success rate increased to 100% after a second drainage.7 In addition, percutaneous drainage was associated with a lower mortality rate and a shorter hospital stay when compared to open surgical drainage.7
Open surgical drainage is rarely performed and usually only considered if the patient is not responding to a combination of focused antibiotic treatment and percutaneous drainage or has associated comorbidities, such as Crohn ileocolitis.2-4 In a retrospective analysis by Tabrizian et al, percutaneous drainage served as a bridge to open surgical drainage in nearly all patients with a gastrointestinal origin, such as Crohn disease, diverticulitis, appendicitis, and/or pancreatitis.6
Treatment of psoas abscesses has an overall failure rate of 15.8%, with an associated mortality rate of less than 7%.4 Overall prognosis is good, but outcomes can be negatively affected by such factors as advanced age, delay in diagnosis, bacteremia, and other comorbidities.4
Next page: Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
The patient required an 11-day hospitalization; her day-by-day course is described briefly below.
Day 1. Upon admission, abdominal MRI was ordered (see Figure 1) and empiric piperacillin/tazobactam IV was initiated. C-reactive protein (CRP) level and white blood cell (WBC) counts were elevated (see Table 2). Infectious disease, surgery, and urology consults were obtained.
Day 2. Fine-needle aspiration of the abscess was performed for cultures, and 40 mL of purulent fluid was drained. Piperacillin/tazobactam administration was continued, but the patient experienced ongoing fever and vomiting.
Day 3. Preliminary aspirate culture results revealed S aureus infection. Piperacillin/tazobactam was discontinued, and vancomycin IV was started. CRP levels and WBC counts decreased, as did fever and vomiting.
Day 4. Final aspirate culture results identified MRSA infection, sensitive to clindamycin. Vancomycin was discontinued, and clindamycin IV was started. Although the patient’s condition improved somewhat, fever and vomiting persisted.
Day 5. Both CRP levels and WBC counts increased from day 3. A surgical consult was sought.
Day 6. Repeat abdominal MRI revealed a decrease in the size of the abscess (see Figure 2, page 30. CRP levels and WBC counts remained high, with persistent fever and vomiting.
Day 7. The clinical team, in consultation with the parents, determined that placement of a peripherally inserted central catheter (PICC) line for drainage of the abscess was necessary.
Day 8. A 10-French pigtail catheter was inserted into the abscess, 20 mL of purulent fluid was drained, and a PICC line was inserted. Clindamycin IV was continued and, eight hours after the catheter was placed, fever and vomiting resolved.
Day 9. Both CRP levels and WBC counts dropped by half (WBC count was normal), while 10 mL of clear fluid drained from the catheter. The patient remained afebrile, without nausea or vomiting, on clindamycin IV.
Day 10. After 36 hours of clear drainage, the catheter was removed. CRP level further decreased. Clindamycin IV was discontinued, and the patient, now asymptomatic, was started on oral clindamycin.
Day 11. The patient was discharged on a regimen of oral clindamycin for six weeks, with weekly abdominal ultrasounds. She completed her entire course of antibiotics and fully recovered from the infection.
Next page: Conclusion >>
CONCLUSION
Since children generally compensate well during times of increased stress on the body, it is vital that persistent FUOs continue to be evaluated until a definitive source is identified, especially in this population. Early diagnosis and treatment of psoas abscess is essential for better outcomes, since delay is associated with a greater risk for sepsis.
While the likelihood of developing psoas abscess is low, it is worth keeping the diagnosis in mind for cases of unexplained lower abdominal pain, flank pain, or hip pain when more common etiologies have been excluded. This is especially important in the setting of recent travel to a developing country due to the fact that a psoas abscess can be a complication of TB of the spine.
The authors would like to thank Jeff Brand, MD, for his assistance in the preparation of this manuscript.
REFERENCES
1. Wong-Baker Faces Corporation. Wong-Baker FACES Pain Rating Scale. www.wongbakerfaces.org. Accessed May 19, 2015.
2. Mallick IH, Thoufreeq MH, Rajendren TP. Iliopsoas abscesses. Postgrad Med J. 2004;80(946):459-462.
3. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223-227.
4. Lopez VN, Ramos JM, Meseguer V, et al; The Infectious Diseases Study Group of the Spanish Society of Internal Medicine. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine. 2009;88(2):120-130.
5. Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg. 2012;10(9):466-469.
6. Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg. 2009;144(10):946-949.
7. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23(1):45-48.
8. Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19(5):416-423.
9. Woo MY. Psoas abscess. J Emerg Med. 2014;47(5):e129-e130.
A 5-year-old Filipino girl was brought to a pediatric clinic for follow up of an unresolved fever and for new-onset right hip pain, which occurred intermittently for the past week and was associated with a right-sided limp. She had been experiencing nightly fevers ranging from 101°F to 105°F for the past two weeks, for which her parents had been giving ibuprofen with mixed results; she remained afebrile during daytime hours.
Using the Wong-Baker FACES pain scale, the patient rated the pain as a 4/10 in severity (“Hurts a Little More” face).1 Standing and walking aggravated the pain but did not limit activity. Although ibuprofen decreased the fever, it did not alleviate the hip pain. Other symptoms included vomiting one to two times daily, without hematemesis, and four to five episodes of diarrhea daily, without abdominal pain, hematochezia, or melena. She also experienced decreased appetite, but her parents reported no change in her dietary or fluid intake. The patient and her parents denied additional symptoms.
Further investigation revealed that the patient had been seen a week earlier by two other clinicians in the office for complaints of fever, rash, nausea, hematemesis, and diarrhea. She had been diagnosed with a herpes simplex viral (HSV) lesion of the nose, epistaxis, and viral gastroenteritis. Her treatment plan consisted of acyclovir ointment for the HSV lesion and symptomatic support for the gastroenteritis associated diarrhea. The complaint of hematemesis was attributed to postnasal drip from the epistaxis, and reassurance was provided to the patient and family. In addition, six weeks earlier, the patient had been treated for otitis media with a full course of amoxicillin.
Medical history was negative for surgeries, trauma, injuries, and chronic medical conditions. She took no medications or supplements on a regular basis. Her parents denied any known drug allergies and stated that her immunizations were up to date.
The patient lived at home with her biological parents and two brothers, all of whom were healthy, without any recent infections or illnesses. Of significance, the family had travelled to the Philippines for vacation about four months earlier. Results of a tuberculin skin test done six weeks earlier (because the patient presented with respiratory symptoms shortly after traveling to the Philippines) were negative.
Physical examination revealed a well-developed, well-nourished 40-lb girl, in no acute distress, who was active and playful with her brother while in the exam room. Vital signs were significant for a fever of 101.9°F (last dose of ibuprofen was approximately six hours earlier) but were otherwise stable. Skin exam revealed that the prior HSV lesion of the nose had resolved. HEENT, cardiovascular, and pulmonary exam findings were noncontributory. Urine dipstick was negative.
Abdominal exam revealed normoactive bowel sounds in all four quadrants, and on palpation, the abdomen was soft, nontender, and without organomegaly. Specialized abdominal exams to assess for peritonitis, including those to elicit Rovsing, rebound tenderness, obturator, and psoas signs, were all negative. Bilateral extremity exams of the hips, knees, and ankles revealed full range of motion (active and passive), with normal muscle strength throughout. The only significant finding on the physical exam was mild pain of the right anterior hip at 15° of flexion, appreciated while the patient was supine on the exam table. The patient was also observed pushing off her right lower extremity when climbing onto the exam table, and she skipped down the hall when leaving the exam.
With fever of unknown origin (FUO) and a largely negative history and physical, the working list of differential diagnoses included
• Avascular necrosis
• Bacteremia
• Juvenile idiopathic arthritis
• Osteomyelitis
• Pyelonephritis
• Reiter syndrome
• Rheumatic fever
• Rheumatoid arthritis
• Septic joint
• Urinary tract infection
To begin the diagnostic process, a number of laboratory tests and imaging procedures were ordered. Table 1 presents the results of these studies. A tuberculin skin test was not repeated. While awaiting test results, the patient was started on naproxen oral suspension (125 mg/5 mL; 4 mL bid) for fever and pain control.
Based on findings consistent with an inflammatory pattern, the history of otitis media (of possible streptococcal origin) six weeks prior to this visit, and the elevated ASO titer, the patient was started on penicillin V (250 mg bid) and instructed to return for follow up in two days.
At the follow-up visit, no improvement was noted; the patient continued to experience nightly fevers and hip pain. Rovsing, rebound tenderness, obturator, and psoas signs continued to be negative. Physical examination did, however, reveal a mild abdominal tenderness in the right lower quadrant.
Due to this new finding, an abdominal ultrasound was ordered to screen for appendicitis. Despite the parents’ appropriate concern for the child, misunderstanding about the urgent need to obtain the abdominal ultrasound led to a two-day delay in scheduling the exam. Results of ultrasonography revealed psoas abscess, and the patient was promptly admitted to the pediatric floor of the local hospital.
Continue for discussion >>
DISCUSSION
Psoas abscess is a collection of pus in the iliopsoas compartment, an extraperitoneal space containing the psoas and iliacus muscles.2 It can be life-threatening if the infection progresses to septic shock. Historically, psoas abscesses were a frequent complication of tuberculosis (TB) of the spine; but with modern TB treatment, these abscesses have become rare.2 Paradoxically, increased utilization of CT to evaluate sepsis of unknown etiology has led to a recent increase in the frequency of psoas abscess diagnosis.3
Psoas abscesses are categorized as either primary or secondary, with primary infections originating in the psoas muscle and secondary infections spreading from adjacent organs.2 In 42% to 88% of cases (depending on the study), primary psoas abscesses are caused by the hematogenous spread of Staphylococcus aureus from distant infection sites.2,4,5 The psoas muscle is particularly susceptible to this mode of infection because of its rich vascular supply.6 Children, immunosuppressed adults (ie, patients with diabetes, HIV/AIDS, or renal failure), IV drug users, and patients with a history of trauma to the muscle are most susceptible to developing a primary psoas abscess.2,5
Secondary psoas abscesses are caused by infections involving adjacent structures of the gastrointestinal, urinary, and skeletal systems. They are most frequently associated with intra-abdominal inflammatory processes, with the most common etiology being Crohn disease.5 Secondary psoas abscesses, though more diverse in their bacterial flora, tend to follow certain microbiologic patterns based on the inoculating source; Escherichia coli is the most common pathogen in secondary abscesses caused by gastrointestinal (42%) and urinary (61%) sources, and S aureus the most common (35%) from skeletal origins (ie, osteomyelitis).4,5Mycobacterium tuberculosis is the more frequently found cause in developing countries but should be considered if the patient has recently travelled outside the United States.
Review of the literature suggests that the incidence of methicillin-resistant S aureus (MRSA) as the causative agent of psoas abscesses may be increasing. However, there is a wide variance in the incidence reported, ranging from 1.1% to 12% of confirmed microbial infections.5,7,8
The classic historical presentation of psoas abscess has been described as the triad of back pain, fever, and limp5,6; however, this triad has only been described in approximately 30% of cases.5 The typical presentation consists of flank or lower limb pain (91%), fever (75%), anorexia (46%), and/or weakness (43%).4 Laboratory abnormalities include leukocytosis (67%) and elevated markers of inflammation (eg, erythrocyte sedimentation rate, seen in 73% of cases).4
Imaging via abdominal ultrasound may be helpful to screen for psoas abscess; however, its utility is limited by a low diagnostic yield of 60% or less.2,4 Direct visualization of the retroperitoneal structures, for example, can be problematic due to the presence of bowel gas.9 Abdominal CT is considered the gold standard for the definitive diagnosis of psoas abscess due to its high sensitivity (100%) and specificity (77%); it can also be used simultaneously to guide percutaneous drainage to treat the abscess if needed.7 However, some clinicians prefer abdominal MRI because of its ability to enhance soft-tissue visualization without requiring use of IV contrast.2,4
The approach to treating psoas abscess varies from a strictly antibiotic regimen to percutaneous drainage, and in rare circumstances, open surgical drainage. Antibiotic therapy without drainage or surgical intervention is a sufficient starting point for treatment of abscesses less than 3 cm in size.3
The antibiotic regimen choice depends on the suspected pathogen. In cases of suspected S aureus, empiric antistaphylococcal antibiotics should be initiated while culture results are pending.2,4 Secondary psoas abscesses thought to be derived from a urinary or gastrointestinal source should prompt use of a broader spectrum antibiotic due to the higher probability of gram-negative, anaerobic, or polymicrobial involvement.2,4
Once final culture and sensitivity results are obtained, antibiotic therapy should be modified to target the isolated pathogen(s). Treatment duration is typically six weeks but may vary, depending on serial culture results and the inoculating source.4 Review of the literature reveals that abscesses resulting from skeletal sources have traditionally been treated longer, usually with antibiotics alone, than those from urinary or gastrointestinal sources, which are often treated with the combination of antibiotics and percutaneous drainage.4
In cases of psoas abscesses larger than 3 cm, management should include both appropriate antibiotics and percutaneous drainage of the abscess.2 Percutaneous drainage is preferred to open surgical drainage because outcomes are similar, it is less invasive, and there is less risk of spreading abscess contents.2-4 In a retrospective analysis by Dietrich et al, 50% of patients treated with antibiotics and percutaneous drainage responded after one drainage, but the success rate increased to 100% after a second drainage.7 In addition, percutaneous drainage was associated with a lower mortality rate and a shorter hospital stay when compared to open surgical drainage.7
Open surgical drainage is rarely performed and usually only considered if the patient is not responding to a combination of focused antibiotic treatment and percutaneous drainage or has associated comorbidities, such as Crohn ileocolitis.2-4 In a retrospective analysis by Tabrizian et al, percutaneous drainage served as a bridge to open surgical drainage in nearly all patients with a gastrointestinal origin, such as Crohn disease, diverticulitis, appendicitis, and/or pancreatitis.6
Treatment of psoas abscesses has an overall failure rate of 15.8%, with an associated mortality rate of less than 7%.4 Overall prognosis is good, but outcomes can be negatively affected by such factors as advanced age, delay in diagnosis, bacteremia, and other comorbidities.4
Next page: Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
The patient required an 11-day hospitalization; her day-by-day course is described briefly below.
Day 1. Upon admission, abdominal MRI was ordered (see Figure 1) and empiric piperacillin/tazobactam IV was initiated. C-reactive protein (CRP) level and white blood cell (WBC) counts were elevated (see Table 2). Infectious disease, surgery, and urology consults were obtained.
Day 2. Fine-needle aspiration of the abscess was performed for cultures, and 40 mL of purulent fluid was drained. Piperacillin/tazobactam administration was continued, but the patient experienced ongoing fever and vomiting.
Day 3. Preliminary aspirate culture results revealed S aureus infection. Piperacillin/tazobactam was discontinued, and vancomycin IV was started. CRP levels and WBC counts decreased, as did fever and vomiting.
Day 4. Final aspirate culture results identified MRSA infection, sensitive to clindamycin. Vancomycin was discontinued, and clindamycin IV was started. Although the patient’s condition improved somewhat, fever and vomiting persisted.
Day 5. Both CRP levels and WBC counts increased from day 3. A surgical consult was sought.
Day 6. Repeat abdominal MRI revealed a decrease in the size of the abscess (see Figure 2, page 30. CRP levels and WBC counts remained high, with persistent fever and vomiting.
Day 7. The clinical team, in consultation with the parents, determined that placement of a peripherally inserted central catheter (PICC) line for drainage of the abscess was necessary.
Day 8. A 10-French pigtail catheter was inserted into the abscess, 20 mL of purulent fluid was drained, and a PICC line was inserted. Clindamycin IV was continued and, eight hours after the catheter was placed, fever and vomiting resolved.
Day 9. Both CRP levels and WBC counts dropped by half (WBC count was normal), while 10 mL of clear fluid drained from the catheter. The patient remained afebrile, without nausea or vomiting, on clindamycin IV.
Day 10. After 36 hours of clear drainage, the catheter was removed. CRP level further decreased. Clindamycin IV was discontinued, and the patient, now asymptomatic, was started on oral clindamycin.
Day 11. The patient was discharged on a regimen of oral clindamycin for six weeks, with weekly abdominal ultrasounds. She completed her entire course of antibiotics and fully recovered from the infection.
Next page: Conclusion >>
CONCLUSION
Since children generally compensate well during times of increased stress on the body, it is vital that persistent FUOs continue to be evaluated until a definitive source is identified, especially in this population. Early diagnosis and treatment of psoas abscess is essential for better outcomes, since delay is associated with a greater risk for sepsis.
While the likelihood of developing psoas abscess is low, it is worth keeping the diagnosis in mind for cases of unexplained lower abdominal pain, flank pain, or hip pain when more common etiologies have been excluded. This is especially important in the setting of recent travel to a developing country due to the fact that a psoas abscess can be a complication of TB of the spine.
The authors would like to thank Jeff Brand, MD, for his assistance in the preparation of this manuscript.
REFERENCES
1. Wong-Baker Faces Corporation. Wong-Baker FACES Pain Rating Scale. www.wongbakerfaces.org. Accessed May 19, 2015.
2. Mallick IH, Thoufreeq MH, Rajendren TP. Iliopsoas abscesses. Postgrad Med J. 2004;80(946):459-462.
3. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223-227.
4. Lopez VN, Ramos JM, Meseguer V, et al; The Infectious Diseases Study Group of the Spanish Society of Internal Medicine. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine. 2009;88(2):120-130.
5. Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg. 2012;10(9):466-469.
6. Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg. 2009;144(10):946-949.
7. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23(1):45-48.
8. Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19(5):416-423.
9. Woo MY. Psoas abscess. J Emerg Med. 2014;47(5):e129-e130.
A 5-year-old Filipino girl was brought to a pediatric clinic for follow up of an unresolved fever and for new-onset right hip pain, which occurred intermittently for the past week and was associated with a right-sided limp. She had been experiencing nightly fevers ranging from 101°F to 105°F for the past two weeks, for which her parents had been giving ibuprofen with mixed results; she remained afebrile during daytime hours.
Using the Wong-Baker FACES pain scale, the patient rated the pain as a 4/10 in severity (“Hurts a Little More” face).1 Standing and walking aggravated the pain but did not limit activity. Although ibuprofen decreased the fever, it did not alleviate the hip pain. Other symptoms included vomiting one to two times daily, without hematemesis, and four to five episodes of diarrhea daily, without abdominal pain, hematochezia, or melena. She also experienced decreased appetite, but her parents reported no change in her dietary or fluid intake. The patient and her parents denied additional symptoms.
Further investigation revealed that the patient had been seen a week earlier by two other clinicians in the office for complaints of fever, rash, nausea, hematemesis, and diarrhea. She had been diagnosed with a herpes simplex viral (HSV) lesion of the nose, epistaxis, and viral gastroenteritis. Her treatment plan consisted of acyclovir ointment for the HSV lesion and symptomatic support for the gastroenteritis associated diarrhea. The complaint of hematemesis was attributed to postnasal drip from the epistaxis, and reassurance was provided to the patient and family. In addition, six weeks earlier, the patient had been treated for otitis media with a full course of amoxicillin.
Medical history was negative for surgeries, trauma, injuries, and chronic medical conditions. She took no medications or supplements on a regular basis. Her parents denied any known drug allergies and stated that her immunizations were up to date.
The patient lived at home with her biological parents and two brothers, all of whom were healthy, without any recent infections or illnesses. Of significance, the family had travelled to the Philippines for vacation about four months earlier. Results of a tuberculin skin test done six weeks earlier (because the patient presented with respiratory symptoms shortly after traveling to the Philippines) were negative.
Physical examination revealed a well-developed, well-nourished 40-lb girl, in no acute distress, who was active and playful with her brother while in the exam room. Vital signs were significant for a fever of 101.9°F (last dose of ibuprofen was approximately six hours earlier) but were otherwise stable. Skin exam revealed that the prior HSV lesion of the nose had resolved. HEENT, cardiovascular, and pulmonary exam findings were noncontributory. Urine dipstick was negative.
Abdominal exam revealed normoactive bowel sounds in all four quadrants, and on palpation, the abdomen was soft, nontender, and without organomegaly. Specialized abdominal exams to assess for peritonitis, including those to elicit Rovsing, rebound tenderness, obturator, and psoas signs, were all negative. Bilateral extremity exams of the hips, knees, and ankles revealed full range of motion (active and passive), with normal muscle strength throughout. The only significant finding on the physical exam was mild pain of the right anterior hip at 15° of flexion, appreciated while the patient was supine on the exam table. The patient was also observed pushing off her right lower extremity when climbing onto the exam table, and she skipped down the hall when leaving the exam.
With fever of unknown origin (FUO) and a largely negative history and physical, the working list of differential diagnoses included
• Avascular necrosis
• Bacteremia
• Juvenile idiopathic arthritis
• Osteomyelitis
• Pyelonephritis
• Reiter syndrome
• Rheumatic fever
• Rheumatoid arthritis
• Septic joint
• Urinary tract infection
To begin the diagnostic process, a number of laboratory tests and imaging procedures were ordered. Table 1 presents the results of these studies. A tuberculin skin test was not repeated. While awaiting test results, the patient was started on naproxen oral suspension (125 mg/5 mL; 4 mL bid) for fever and pain control.
Based on findings consistent with an inflammatory pattern, the history of otitis media (of possible streptococcal origin) six weeks prior to this visit, and the elevated ASO titer, the patient was started on penicillin V (250 mg bid) and instructed to return for follow up in two days.
At the follow-up visit, no improvement was noted; the patient continued to experience nightly fevers and hip pain. Rovsing, rebound tenderness, obturator, and psoas signs continued to be negative. Physical examination did, however, reveal a mild abdominal tenderness in the right lower quadrant.
Due to this new finding, an abdominal ultrasound was ordered to screen for appendicitis. Despite the parents’ appropriate concern for the child, misunderstanding about the urgent need to obtain the abdominal ultrasound led to a two-day delay in scheduling the exam. Results of ultrasonography revealed psoas abscess, and the patient was promptly admitted to the pediatric floor of the local hospital.
Continue for discussion >>
DISCUSSION
Psoas abscess is a collection of pus in the iliopsoas compartment, an extraperitoneal space containing the psoas and iliacus muscles.2 It can be life-threatening if the infection progresses to septic shock. Historically, psoas abscesses were a frequent complication of tuberculosis (TB) of the spine; but with modern TB treatment, these abscesses have become rare.2 Paradoxically, increased utilization of CT to evaluate sepsis of unknown etiology has led to a recent increase in the frequency of psoas abscess diagnosis.3
Psoas abscesses are categorized as either primary or secondary, with primary infections originating in the psoas muscle and secondary infections spreading from adjacent organs.2 In 42% to 88% of cases (depending on the study), primary psoas abscesses are caused by the hematogenous spread of Staphylococcus aureus from distant infection sites.2,4,5 The psoas muscle is particularly susceptible to this mode of infection because of its rich vascular supply.6 Children, immunosuppressed adults (ie, patients with diabetes, HIV/AIDS, or renal failure), IV drug users, and patients with a history of trauma to the muscle are most susceptible to developing a primary psoas abscess.2,5
Secondary psoas abscesses are caused by infections involving adjacent structures of the gastrointestinal, urinary, and skeletal systems. They are most frequently associated with intra-abdominal inflammatory processes, with the most common etiology being Crohn disease.5 Secondary psoas abscesses, though more diverse in their bacterial flora, tend to follow certain microbiologic patterns based on the inoculating source; Escherichia coli is the most common pathogen in secondary abscesses caused by gastrointestinal (42%) and urinary (61%) sources, and S aureus the most common (35%) from skeletal origins (ie, osteomyelitis).4,5Mycobacterium tuberculosis is the more frequently found cause in developing countries but should be considered if the patient has recently travelled outside the United States.
Review of the literature suggests that the incidence of methicillin-resistant S aureus (MRSA) as the causative agent of psoas abscesses may be increasing. However, there is a wide variance in the incidence reported, ranging from 1.1% to 12% of confirmed microbial infections.5,7,8
The classic historical presentation of psoas abscess has been described as the triad of back pain, fever, and limp5,6; however, this triad has only been described in approximately 30% of cases.5 The typical presentation consists of flank or lower limb pain (91%), fever (75%), anorexia (46%), and/or weakness (43%).4 Laboratory abnormalities include leukocytosis (67%) and elevated markers of inflammation (eg, erythrocyte sedimentation rate, seen in 73% of cases).4
Imaging via abdominal ultrasound may be helpful to screen for psoas abscess; however, its utility is limited by a low diagnostic yield of 60% or less.2,4 Direct visualization of the retroperitoneal structures, for example, can be problematic due to the presence of bowel gas.9 Abdominal CT is considered the gold standard for the definitive diagnosis of psoas abscess due to its high sensitivity (100%) and specificity (77%); it can also be used simultaneously to guide percutaneous drainage to treat the abscess if needed.7 However, some clinicians prefer abdominal MRI because of its ability to enhance soft-tissue visualization without requiring use of IV contrast.2,4
The approach to treating psoas abscess varies from a strictly antibiotic regimen to percutaneous drainage, and in rare circumstances, open surgical drainage. Antibiotic therapy without drainage or surgical intervention is a sufficient starting point for treatment of abscesses less than 3 cm in size.3
The antibiotic regimen choice depends on the suspected pathogen. In cases of suspected S aureus, empiric antistaphylococcal antibiotics should be initiated while culture results are pending.2,4 Secondary psoas abscesses thought to be derived from a urinary or gastrointestinal source should prompt use of a broader spectrum antibiotic due to the higher probability of gram-negative, anaerobic, or polymicrobial involvement.2,4
Once final culture and sensitivity results are obtained, antibiotic therapy should be modified to target the isolated pathogen(s). Treatment duration is typically six weeks but may vary, depending on serial culture results and the inoculating source.4 Review of the literature reveals that abscesses resulting from skeletal sources have traditionally been treated longer, usually with antibiotics alone, than those from urinary or gastrointestinal sources, which are often treated with the combination of antibiotics and percutaneous drainage.4
In cases of psoas abscesses larger than 3 cm, management should include both appropriate antibiotics and percutaneous drainage of the abscess.2 Percutaneous drainage is preferred to open surgical drainage because outcomes are similar, it is less invasive, and there is less risk of spreading abscess contents.2-4 In a retrospective analysis by Dietrich et al, 50% of patients treated with antibiotics and percutaneous drainage responded after one drainage, but the success rate increased to 100% after a second drainage.7 In addition, percutaneous drainage was associated with a lower mortality rate and a shorter hospital stay when compared to open surgical drainage.7
Open surgical drainage is rarely performed and usually only considered if the patient is not responding to a combination of focused antibiotic treatment and percutaneous drainage or has associated comorbidities, such as Crohn ileocolitis.2-4 In a retrospective analysis by Tabrizian et al, percutaneous drainage served as a bridge to open surgical drainage in nearly all patients with a gastrointestinal origin, such as Crohn disease, diverticulitis, appendicitis, and/or pancreatitis.6
Treatment of psoas abscesses has an overall failure rate of 15.8%, with an associated mortality rate of less than 7%.4 Overall prognosis is good, but outcomes can be negatively affected by such factors as advanced age, delay in diagnosis, bacteremia, and other comorbidities.4
Next page: Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
The patient required an 11-day hospitalization; her day-by-day course is described briefly below.
Day 1. Upon admission, abdominal MRI was ordered (see Figure 1) and empiric piperacillin/tazobactam IV was initiated. C-reactive protein (CRP) level and white blood cell (WBC) counts were elevated (see Table 2). Infectious disease, surgery, and urology consults were obtained.
Day 2. Fine-needle aspiration of the abscess was performed for cultures, and 40 mL of purulent fluid was drained. Piperacillin/tazobactam administration was continued, but the patient experienced ongoing fever and vomiting.
Day 3. Preliminary aspirate culture results revealed S aureus infection. Piperacillin/tazobactam was discontinued, and vancomycin IV was started. CRP levels and WBC counts decreased, as did fever and vomiting.
Day 4. Final aspirate culture results identified MRSA infection, sensitive to clindamycin. Vancomycin was discontinued, and clindamycin IV was started. Although the patient’s condition improved somewhat, fever and vomiting persisted.
Day 5. Both CRP levels and WBC counts increased from day 3. A surgical consult was sought.
Day 6. Repeat abdominal MRI revealed a decrease in the size of the abscess (see Figure 2, page 30. CRP levels and WBC counts remained high, with persistent fever and vomiting.
Day 7. The clinical team, in consultation with the parents, determined that placement of a peripherally inserted central catheter (PICC) line for drainage of the abscess was necessary.
Day 8. A 10-French pigtail catheter was inserted into the abscess, 20 mL of purulent fluid was drained, and a PICC line was inserted. Clindamycin IV was continued and, eight hours after the catheter was placed, fever and vomiting resolved.
Day 9. Both CRP levels and WBC counts dropped by half (WBC count was normal), while 10 mL of clear fluid drained from the catheter. The patient remained afebrile, without nausea or vomiting, on clindamycin IV.
Day 10. After 36 hours of clear drainage, the catheter was removed. CRP level further decreased. Clindamycin IV was discontinued, and the patient, now asymptomatic, was started on oral clindamycin.
Day 11. The patient was discharged on a regimen of oral clindamycin for six weeks, with weekly abdominal ultrasounds. She completed her entire course of antibiotics and fully recovered from the infection.
Next page: Conclusion >>
CONCLUSION
Since children generally compensate well during times of increased stress on the body, it is vital that persistent FUOs continue to be evaluated until a definitive source is identified, especially in this population. Early diagnosis and treatment of psoas abscess is essential for better outcomes, since delay is associated with a greater risk for sepsis.
While the likelihood of developing psoas abscess is low, it is worth keeping the diagnosis in mind for cases of unexplained lower abdominal pain, flank pain, or hip pain when more common etiologies have been excluded. This is especially important in the setting of recent travel to a developing country due to the fact that a psoas abscess can be a complication of TB of the spine.
The authors would like to thank Jeff Brand, MD, for his assistance in the preparation of this manuscript.
REFERENCES
1. Wong-Baker Faces Corporation. Wong-Baker FACES Pain Rating Scale. www.wongbakerfaces.org. Accessed May 19, 2015.
2. Mallick IH, Thoufreeq MH, Rajendren TP. Iliopsoas abscesses. Postgrad Med J. 2004;80(946):459-462.
3. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223-227.
4. Lopez VN, Ramos JM, Meseguer V, et al; The Infectious Diseases Study Group of the Spanish Society of Internal Medicine. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine. 2009;88(2):120-130.
5. Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg. 2012;10(9):466-469.
6. Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg. 2009;144(10):946-949.
7. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23(1):45-48.
8. Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19(5):416-423.
9. Woo MY. Psoas abscess. J Emerg Med. 2014;47(5):e129-e130.
CDC investigating accidental anthrax shipment to labs
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
The Centers for Disease Control and Prevention is investigating a shipment of anthrax mistakenly sent to labs in the United States and abroad from the Department of Defense, the agency said in a May 30 announcement.
The presence of anthrax was confirmed after a laboratory working with the DOD reported being able to grow live Bacillus anthracis bacteria, although an inactive agent was expected. The lab was working with the DOD to develop a diagnostic test to identify biological threats, the CDC reported.
The accidental shipment is not believed to pose a risk to the public, the CDC said. Samples are being sent to the CDC or Laboratory Response Network labs for testing, and CDC officials are performing onsite investigations at the laboratories involved.
Do hormonal contraceptives lead to weight gain?
It depends. Weight doesn’t appear to increase with combined oral contraception (OC) compared with nonhormonal contraception, but percent body fat may increase slightly. Depot-medroxyprogesterone acetate injection (DMPA) users experience weight gain compared with OC and nonhormonal contraception (NH) users (strength of recommendation: B, cohort studies).
DMPA users gain more weight and body fat than OC users
A 2008 prospective, nonrandomized, controlled study of 703 women compared changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio in 245 women using OC, 240 using DMPA, and 218 using NH methods of birth control.1 Over the 36-month follow-up period, 257 women were lost to follow-up, 137 discontinued participation because they wanted a different contraceptive method, and 123 didn’t complete the study for other reasons.
Compared to OC and NH users, DMPA users gained more actual weight (+5.1 kg) and body fat (+4.1 kg) and increased their percent body fat (+3.4%) and central-to-peripheral fat ratio (+0.1; P<.01 in all models). OC use wasn’t associated with weight gain compared with the NH group but did increase OC users’ percent body fat by 1.6% (P<.01) and decrease their total lean body mass by 0.36 (P<.026) (TABLE1).
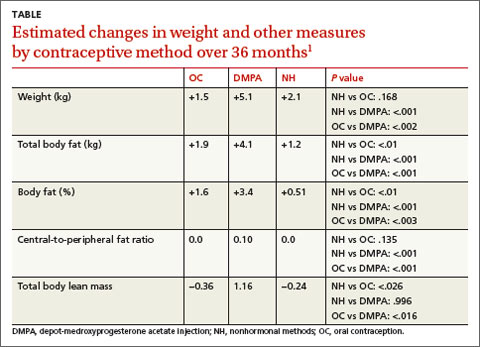
DMPA users gain more weight in specific populations
For 18 months, researchers conducting a large prospective, nonrandomized study followed American adolescents ages 12 to 18 years who used DMPA and were classified as obese (defined as a baseline body mass index [BMI] >30 kg/m2) to determine how their weight gain compared with obese combined OC users and obese controls.2
Obese DMPA users gained significantly more weight (9.4 kg) than obese combined OC users (0.2 kg; P<.001) and obese controls (3.1 kg; P<.001). Of the 450 patients, 280 (62%) identified themselves as black and 170 (38%) identified themselves as nonblack.
In another retrospective cohort study of 379 adult women from a Brazilian public family planning clinic, current or past DMPA users were matched with copper T 30A intrauterine device users for age and baseline BMI and categorized into 3 groups: G1 (BMI <25 kg/m2), G2 (25-29.9 kg/m2), or G3 (≥30 kg/m2).3
At the end of the third year of use, the mean increase in weight for the normal weight group (G1) and the overweight group (G2) was greater in DMPA users than in DMPA nonusers (4.5 kg vs 1.2 kg in G1; P<.0107; 3.4 kg vs 0.2 kg in G2; P<.0001). In the obese group (G3), the difference in weight gain between DMPA users and DMPA nonusers was minimal (1.9 kg vs 0.6 kg; P=not significant).
One limitation of these 2 studies could be that the women under investigation were from defined populations—black urban adolescents and a public family planning service.
1. Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1-8.
2. Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch PediatrAdolesc Med. 2006;160:40-45.
3. Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107-111.
It depends. Weight doesn’t appear to increase with combined oral contraception (OC) compared with nonhormonal contraception, but percent body fat may increase slightly. Depot-medroxyprogesterone acetate injection (DMPA) users experience weight gain compared with OC and nonhormonal contraception (NH) users (strength of recommendation: B, cohort studies).
DMPA users gain more weight and body fat than OC users
A 2008 prospective, nonrandomized, controlled study of 703 women compared changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio in 245 women using OC, 240 using DMPA, and 218 using NH methods of birth control.1 Over the 36-month follow-up period, 257 women were lost to follow-up, 137 discontinued participation because they wanted a different contraceptive method, and 123 didn’t complete the study for other reasons.
Compared to OC and NH users, DMPA users gained more actual weight (+5.1 kg) and body fat (+4.1 kg) and increased their percent body fat (+3.4%) and central-to-peripheral fat ratio (+0.1; P<.01 in all models). OC use wasn’t associated with weight gain compared with the NH group but did increase OC users’ percent body fat by 1.6% (P<.01) and decrease their total lean body mass by 0.36 (P<.026) (TABLE1).

DMPA users gain more weight in specific populations
For 18 months, researchers conducting a large prospective, nonrandomized study followed American adolescents ages 12 to 18 years who used DMPA and were classified as obese (defined as a baseline body mass index [BMI] >30 kg/m2) to determine how their weight gain compared with obese combined OC users and obese controls.2
Obese DMPA users gained significantly more weight (9.4 kg) than obese combined OC users (0.2 kg; P<.001) and obese controls (3.1 kg; P<.001). Of the 450 patients, 280 (62%) identified themselves as black and 170 (38%) identified themselves as nonblack.
In another retrospective cohort study of 379 adult women from a Brazilian public family planning clinic, current or past DMPA users were matched with copper T 30A intrauterine device users for age and baseline BMI and categorized into 3 groups: G1 (BMI <25 kg/m2), G2 (25-29.9 kg/m2), or G3 (≥30 kg/m2).3
At the end of the third year of use, the mean increase in weight for the normal weight group (G1) and the overweight group (G2) was greater in DMPA users than in DMPA nonusers (4.5 kg vs 1.2 kg in G1; P<.0107; 3.4 kg vs 0.2 kg in G2; P<.0001). In the obese group (G3), the difference in weight gain between DMPA users and DMPA nonusers was minimal (1.9 kg vs 0.6 kg; P=not significant).
One limitation of these 2 studies could be that the women under investigation were from defined populations—black urban adolescents and a public family planning service.
It depends. Weight doesn’t appear to increase with combined oral contraception (OC) compared with nonhormonal contraception, but percent body fat may increase slightly. Depot-medroxyprogesterone acetate injection (DMPA) users experience weight gain compared with OC and nonhormonal contraception (NH) users (strength of recommendation: B, cohort studies).
DMPA users gain more weight and body fat than OC users
A 2008 prospective, nonrandomized, controlled study of 703 women compared changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio in 245 women using OC, 240 using DMPA, and 218 using NH methods of birth control.1 Over the 36-month follow-up period, 257 women were lost to follow-up, 137 discontinued participation because they wanted a different contraceptive method, and 123 didn’t complete the study for other reasons.
Compared to OC and NH users, DMPA users gained more actual weight (+5.1 kg) and body fat (+4.1 kg) and increased their percent body fat (+3.4%) and central-to-peripheral fat ratio (+0.1; P<.01 in all models). OC use wasn’t associated with weight gain compared with the NH group but did increase OC users’ percent body fat by 1.6% (P<.01) and decrease their total lean body mass by 0.36 (P<.026) (TABLE1).

DMPA users gain more weight in specific populations
For 18 months, researchers conducting a large prospective, nonrandomized study followed American adolescents ages 12 to 18 years who used DMPA and were classified as obese (defined as a baseline body mass index [BMI] >30 kg/m2) to determine how their weight gain compared with obese combined OC users and obese controls.2
Obese DMPA users gained significantly more weight (9.4 kg) than obese combined OC users (0.2 kg; P<.001) and obese controls (3.1 kg; P<.001). Of the 450 patients, 280 (62%) identified themselves as black and 170 (38%) identified themselves as nonblack.
In another retrospective cohort study of 379 adult women from a Brazilian public family planning clinic, current or past DMPA users were matched with copper T 30A intrauterine device users for age and baseline BMI and categorized into 3 groups: G1 (BMI <25 kg/m2), G2 (25-29.9 kg/m2), or G3 (≥30 kg/m2).3
At the end of the third year of use, the mean increase in weight for the normal weight group (G1) and the overweight group (G2) was greater in DMPA users than in DMPA nonusers (4.5 kg vs 1.2 kg in G1; P<.0107; 3.4 kg vs 0.2 kg in G2; P<.0001). In the obese group (G3), the difference in weight gain between DMPA users and DMPA nonusers was minimal (1.9 kg vs 0.6 kg; P=not significant).
One limitation of these 2 studies could be that the women under investigation were from defined populations—black urban adolescents and a public family planning service.
1. Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1-8.
2. Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch PediatrAdolesc Med. 2006;160:40-45.
3. Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107-111.
1. Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. Am J Obstet Gynecol. 2009;200:329.e1-8.
2. Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch PediatrAdolesc Med. 2006;160:40-45.
3. Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107-111.
Evidence-based answers from the Family Physicians Inquiries Network
About Face
1. Papulosquamous skin disease marked by a history of waxing and waning in severity and scaling in perinasal area manifested in this patient.
Diagnosis: Seborrheic dermatitis presenting as erythema with scaling on the perinasal and perioral area. Similar to rosacea, seborrheic dermatitis is a chronic and relapsing erythematous rash with well-demarcated erythematous patches, papules, or plaques; however, unlike rosacea, the distribution varies from minimal asymptomatic scaliness of the scalp to more widespread involvement (eg, scalp, ears, upper aspect of the trunk, intertriginous areas). Also, although macular erythema and scaling involving the perinasal area may be seen in either rosacea or seborrheic dermatitis, a greasy quality to the scales and involvement of other sites such as the scalp, retroauricular skin, and eyebrows suggest a diagnosis of seborrheic dermatitis
Read more about seborrheic dermatitis at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
For the next photograph, proceed to the next page >>
2. The patient had a painful, erythematous skin rash over the bridge of her nose, spreading to the malar area, eyelids, and forehead.
Diagnosis: Erysipelas is an acute superficial cellulitis with lymphatic involvement. It is characterized by the abrupt onset of a warm, erythematous rash with a sharply demarcated, indurated, elevated margin. There are no suppurative foci; sometimes, however, there are bullae or vesicles.
In facial “butterfly” erysipelas (which this patient had), the plaques may involve the eyelids, cheeks, nose, and forehead. Upon palpation, the skin is hot and tender. As the process develops, the color becomes a dark, fiery red and vesicles appear at the advancing border and over the surface. Associated regional lymphadenopathy may be present. There is no necrosis.1
Read more about erysipelas at Painful rash on face. J Fam Pract. 2010 August;59(8):459-462.
For the next photograph, proceed to the next page >>
3. Shortly after she started taking Bikram yoga classes, this 30-year-old patient developed inflammatory papules and pustules. Her symptoms worsened after each 1-hour session. On physical exam, there were erythematous, inflammatory papules and pustules concentrated on her forehead. No comedones were present.
Diagnosis: Rosacea, an inflammatory condition of the skin that typically affects the convex portions of the central face. This chronic cutaneous disorder usually starts after age 30 in both men and women, and is more prevalent in those with fairer skin.2 In fact, an epidemiologic study showed the prevalence to be as high as 10% in the Swedish population.3 The condition, which is not life threatening, can be controlled, although not cured. Its effect on appearance may have a negative impact on a patient’s quality of life.
Read more about Rosacea at Pustular eruption on face. J Fam Pract. 2010 July;59(7):399-401.
For the next photograph, proceed to the next page >>
4. Erythematous papules, papulovesicles, and plaques appeared on sun-exposed surfaces of the skin.
Diagnosis: Polymorphous light eruption (PMLE) manifesting as erythematous papules over the cheek and dorsal aspect of the nose. Similarities between PMLE and rosacea include exacerbation by sun exposure and a higher prevalence in fair-skinned individuals.4 Also, in both conditions erythematous papules appear on the face and may be pruritic and in some instances painful; however, unlike rosacea, which is chronic, PMLE tends to be intermittent and recurrent, typically occurring in the spring and early summer months.
Read more about PMLE at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
For the next photograph, proceed to the next page >>
5. This patient presented with isolated, grouped, and multiple erythematous to violaceous papules, plaques, or nodules, in an asymmetric distribution.
Diagnosis: B-cell neoplasms with skin involvement can present as primary cutaneous lymphomas or as secondary processes, including specific infiltrates of nodal or extranodal lymphoma or leukemia.5 B-cell lymphomas involving the skin have a distinct clinical appearance, presenting as isolated, grouped, or multiple erythematous to violaceous papules, plaques, or nodules, usually in an asymmetric distribution. B-cell lymphoproliferative diseases simulating rosacea are extremely rare.5 Nevertheless, B-cell lymphoma mimicking rhinophyma has been documented in the literature.5-11
Read more about B-cell neoplasms at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
Figures 1, 4, 5 reprinted with permission from Cutis. 2014;94(1):39-45; photographs for Figures 4 and 5 courtesy of Marc Silverstein, MD. Figure 2 reprinted with permission from J Fam Pract. 2010 August;59(8):459-462; photograph courtesy of Felix B. Chang, MD. Figure 3 reprinted courtesy of J Fam Pract. 2010 July;59(7):399-401; photograph courtesy of Nikki N. Kim, MD, Heather W. Wickless, MD, MPH.
REFERENCES
1. Chang F, Lopes A. Erysipelas. In: Domino FJ, ed. The 5-Minute Clinical Consult 2008. 16th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2008:448–449.
2. Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur Acad Dermatol Venereol. 2005;19:273-285.
3. Berg M, Liden S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69:419-423.
4. Tutrone WD. Polymorphic light eruption. Dermatol Ther. 2003;16:28-39.
5. Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
6. Moulonguet I, Ghnassia M, Molina T, et al. Miliarial-type perifollicular B-cell pseudolymphoma (lymphocytoma cutis): a misleading eruption in two women. J Cutan Pathol. 2012;39:1016-1021.
7. Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
8. Rosmaninho A, Alves R, Lima M, et al. Red nose: primary cutaneous marginal zone B-cell lymphoma. Leuk Res.2010;34:682-684.
9. Ogden S, Coulson IH. B-cell lymphoma mimicking rhinophyma. Clin Exp Dermatol. 2008;33:213-214.
10. Seward JL, Malone JC, Callen JP. Rhinophymalike swelling in an 86-year-old woman. Primary cutaneous B-cell lymphoma of the nose. Arch Dermatol. 2004;140:751-756.
11. Colvin JH, Lamerson CL, Cualing H, et al. Cutaneous lymphoplasmacytoid lymphoma (immunocytoma) with Waldenström’s macroglobulinemia mimicking rosacea. J Am Acad Dermatol. 2003;49:1159-1162.
1. Papulosquamous skin disease marked by a history of waxing and waning in severity and scaling in perinasal area manifested in this patient.
Diagnosis: Seborrheic dermatitis presenting as erythema with scaling on the perinasal and perioral area. Similar to rosacea, seborrheic dermatitis is a chronic and relapsing erythematous rash with well-demarcated erythematous patches, papules, or plaques; however, unlike rosacea, the distribution varies from minimal asymptomatic scaliness of the scalp to more widespread involvement (eg, scalp, ears, upper aspect of the trunk, intertriginous areas). Also, although macular erythema and scaling involving the perinasal area may be seen in either rosacea or seborrheic dermatitis, a greasy quality to the scales and involvement of other sites such as the scalp, retroauricular skin, and eyebrows suggest a diagnosis of seborrheic dermatitis
Read more about seborrheic dermatitis at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
For the next photograph, proceed to the next page >>
2. The patient had a painful, erythematous skin rash over the bridge of her nose, spreading to the malar area, eyelids, and forehead.
Diagnosis: Erysipelas is an acute superficial cellulitis with lymphatic involvement. It is characterized by the abrupt onset of a warm, erythematous rash with a sharply demarcated, indurated, elevated margin. There are no suppurative foci; sometimes, however, there are bullae or vesicles.
In facial “butterfly” erysipelas (which this patient had), the plaques may involve the eyelids, cheeks, nose, and forehead. Upon palpation, the skin is hot and tender. As the process develops, the color becomes a dark, fiery red and vesicles appear at the advancing border and over the surface. Associated regional lymphadenopathy may be present. There is no necrosis.1
Read more about erysipelas at Painful rash on face. J Fam Pract. 2010 August;59(8):459-462.
For the next photograph, proceed to the next page >>
3. Shortly after she started taking Bikram yoga classes, this 30-year-old patient developed inflammatory papules and pustules. Her symptoms worsened after each 1-hour session. On physical exam, there were erythematous, inflammatory papules and pustules concentrated on her forehead. No comedones were present.
Diagnosis: Rosacea, an inflammatory condition of the skin that typically affects the convex portions of the central face. This chronic cutaneous disorder usually starts after age 30 in both men and women, and is more prevalent in those with fairer skin.2 In fact, an epidemiologic study showed the prevalence to be as high as 10% in the Swedish population.3 The condition, which is not life threatening, can be controlled, although not cured. Its effect on appearance may have a negative impact on a patient’s quality of life.
Read more about Rosacea at Pustular eruption on face. J Fam Pract. 2010 July;59(7):399-401.
For the next photograph, proceed to the next page >>
4. Erythematous papules, papulovesicles, and plaques appeared on sun-exposed surfaces of the skin.
Diagnosis: Polymorphous light eruption (PMLE) manifesting as erythematous papules over the cheek and dorsal aspect of the nose. Similarities between PMLE and rosacea include exacerbation by sun exposure and a higher prevalence in fair-skinned individuals.4 Also, in both conditions erythematous papules appear on the face and may be pruritic and in some instances painful; however, unlike rosacea, which is chronic, PMLE tends to be intermittent and recurrent, typically occurring in the spring and early summer months.
Read more about PMLE at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
For the next photograph, proceed to the next page >>
5. This patient presented with isolated, grouped, and multiple erythematous to violaceous papules, plaques, or nodules, in an asymmetric distribution.
Diagnosis: B-cell neoplasms with skin involvement can present as primary cutaneous lymphomas or as secondary processes, including specific infiltrates of nodal or extranodal lymphoma or leukemia.5 B-cell lymphomas involving the skin have a distinct clinical appearance, presenting as isolated, grouped, or multiple erythematous to violaceous papules, plaques, or nodules, usually in an asymmetric distribution. B-cell lymphoproliferative diseases simulating rosacea are extremely rare.5 Nevertheless, B-cell lymphoma mimicking rhinophyma has been documented in the literature.5-11
Read more about B-cell neoplasms at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
Figures 1, 4, 5 reprinted with permission from Cutis. 2014;94(1):39-45; photographs for Figures 4 and 5 courtesy of Marc Silverstein, MD. Figure 2 reprinted with permission from J Fam Pract. 2010 August;59(8):459-462; photograph courtesy of Felix B. Chang, MD. Figure 3 reprinted courtesy of J Fam Pract. 2010 July;59(7):399-401; photograph courtesy of Nikki N. Kim, MD, Heather W. Wickless, MD, MPH.
REFERENCES
1. Chang F, Lopes A. Erysipelas. In: Domino FJ, ed. The 5-Minute Clinical Consult 2008. 16th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2008:448–449.
2. Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur Acad Dermatol Venereol. 2005;19:273-285.
3. Berg M, Liden S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69:419-423.
4. Tutrone WD. Polymorphic light eruption. Dermatol Ther. 2003;16:28-39.
5. Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
6. Moulonguet I, Ghnassia M, Molina T, et al. Miliarial-type perifollicular B-cell pseudolymphoma (lymphocytoma cutis): a misleading eruption in two women. J Cutan Pathol. 2012;39:1016-1021.
7. Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
8. Rosmaninho A, Alves R, Lima M, et al. Red nose: primary cutaneous marginal zone B-cell lymphoma. Leuk Res.2010;34:682-684.
9. Ogden S, Coulson IH. B-cell lymphoma mimicking rhinophyma. Clin Exp Dermatol. 2008;33:213-214.
10. Seward JL, Malone JC, Callen JP. Rhinophymalike swelling in an 86-year-old woman. Primary cutaneous B-cell lymphoma of the nose. Arch Dermatol. 2004;140:751-756.
11. Colvin JH, Lamerson CL, Cualing H, et al. Cutaneous lymphoplasmacytoid lymphoma (immunocytoma) with Waldenström’s macroglobulinemia mimicking rosacea. J Am Acad Dermatol. 2003;49:1159-1162.
1. Papulosquamous skin disease marked by a history of waxing and waning in severity and scaling in perinasal area manifested in this patient.
Diagnosis: Seborrheic dermatitis presenting as erythema with scaling on the perinasal and perioral area. Similar to rosacea, seborrheic dermatitis is a chronic and relapsing erythematous rash with well-demarcated erythematous patches, papules, or plaques; however, unlike rosacea, the distribution varies from minimal asymptomatic scaliness of the scalp to more widespread involvement (eg, scalp, ears, upper aspect of the trunk, intertriginous areas). Also, although macular erythema and scaling involving the perinasal area may be seen in either rosacea or seborrheic dermatitis, a greasy quality to the scales and involvement of other sites such as the scalp, retroauricular skin, and eyebrows suggest a diagnosis of seborrheic dermatitis
Read more about seborrheic dermatitis at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
For the next photograph, proceed to the next page >>
2. The patient had a painful, erythematous skin rash over the bridge of her nose, spreading to the malar area, eyelids, and forehead.
Diagnosis: Erysipelas is an acute superficial cellulitis with lymphatic involvement. It is characterized by the abrupt onset of a warm, erythematous rash with a sharply demarcated, indurated, elevated margin. There are no suppurative foci; sometimes, however, there are bullae or vesicles.
In facial “butterfly” erysipelas (which this patient had), the plaques may involve the eyelids, cheeks, nose, and forehead. Upon palpation, the skin is hot and tender. As the process develops, the color becomes a dark, fiery red and vesicles appear at the advancing border and over the surface. Associated regional lymphadenopathy may be present. There is no necrosis.1
Read more about erysipelas at Painful rash on face. J Fam Pract. 2010 August;59(8):459-462.
For the next photograph, proceed to the next page >>
3. Shortly after she started taking Bikram yoga classes, this 30-year-old patient developed inflammatory papules and pustules. Her symptoms worsened after each 1-hour session. On physical exam, there were erythematous, inflammatory papules and pustules concentrated on her forehead. No comedones were present.
Diagnosis: Rosacea, an inflammatory condition of the skin that typically affects the convex portions of the central face. This chronic cutaneous disorder usually starts after age 30 in both men and women, and is more prevalent in those with fairer skin.2 In fact, an epidemiologic study showed the prevalence to be as high as 10% in the Swedish population.3 The condition, which is not life threatening, can be controlled, although not cured. Its effect on appearance may have a negative impact on a patient’s quality of life.
Read more about Rosacea at Pustular eruption on face. J Fam Pract. 2010 July;59(7):399-401.
For the next photograph, proceed to the next page >>
4. Erythematous papules, papulovesicles, and plaques appeared on sun-exposed surfaces of the skin.
Diagnosis: Polymorphous light eruption (PMLE) manifesting as erythematous papules over the cheek and dorsal aspect of the nose. Similarities between PMLE and rosacea include exacerbation by sun exposure and a higher prevalence in fair-skinned individuals.4 Also, in both conditions erythematous papules appear on the face and may be pruritic and in some instances painful; however, unlike rosacea, which is chronic, PMLE tends to be intermittent and recurrent, typically occurring in the spring and early summer months.
Read more about PMLE at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
For the next photograph, proceed to the next page >>
5. This patient presented with isolated, grouped, and multiple erythematous to violaceous papules, plaques, or nodules, in an asymmetric distribution.
Diagnosis: B-cell neoplasms with skin involvement can present as primary cutaneous lymphomas or as secondary processes, including specific infiltrates of nodal or extranodal lymphoma or leukemia.5 B-cell lymphomas involving the skin have a distinct clinical appearance, presenting as isolated, grouped, or multiple erythematous to violaceous papules, plaques, or nodules, usually in an asymmetric distribution. B-cell lymphoproliferative diseases simulating rosacea are extremely rare.5 Nevertheless, B-cell lymphoma mimicking rhinophyma has been documented in the literature.5-11
Read more about B-cell neoplasms at The great mimickers of rosacea. Cutis. 2014;94(1):39-45.
Figures 1, 4, 5 reprinted with permission from Cutis. 2014;94(1):39-45; photographs for Figures 4 and 5 courtesy of Marc Silverstein, MD. Figure 2 reprinted with permission from J Fam Pract. 2010 August;59(8):459-462; photograph courtesy of Felix B. Chang, MD. Figure 3 reprinted courtesy of J Fam Pract. 2010 July;59(7):399-401; photograph courtesy of Nikki N. Kim, MD, Heather W. Wickless, MD, MPH.
REFERENCES
1. Chang F, Lopes A. Erysipelas. In: Domino FJ, ed. The 5-Minute Clinical Consult 2008. 16th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2008:448–449.
2. Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur Acad Dermatol Venereol. 2005;19:273-285.
3. Berg M, Liden S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69:419-423.
4. Tutrone WD. Polymorphic light eruption. Dermatol Ther. 2003;16:28-39.
5. Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
6. Moulonguet I, Ghnassia M, Molina T, et al. Miliarial-type perifollicular B-cell pseudolymphoma (lymphocytoma cutis): a misleading eruption in two women. J Cutan Pathol. 2012;39:1016-1021.
7. Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
8. Rosmaninho A, Alves R, Lima M, et al. Red nose: primary cutaneous marginal zone B-cell lymphoma. Leuk Res.2010;34:682-684.
9. Ogden S, Coulson IH. B-cell lymphoma mimicking rhinophyma. Clin Exp Dermatol. 2008;33:213-214.
10. Seward JL, Malone JC, Callen JP. Rhinophymalike swelling in an 86-year-old woman. Primary cutaneous B-cell lymphoma of the nose. Arch Dermatol. 2004;140:751-756.
11. Colvin JH, Lamerson CL, Cualing H, et al. Cutaneous lymphoplasmacytoid lymphoma (immunocytoma) with Waldenström’s macroglobulinemia mimicking rosacea. J Am Acad Dermatol. 2003;49:1159-1162.
Is it safe to add long-acting β-2 agonists to inhaled corticosteroids in patients with persistent asthma?
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
Evidence-based answers from the Family Physicians Inquiries Network
New Specialty Focus - Gynecology
Enterovirus D-68 presenting with acute pancreatitis
To the Editor: We read the review on enterovirus D681 (EV-D68) with great interest, and we thought it merited comment.
During the current influenza season, we have had several adult cases of EV-D68 presenting as an influenza-like illness. EV-D68 was diagnosed by nasal swab viral film array polymerase chain reaction (PCR) testing. We agree with the authors that the clinical spectrum of enteroviral infection includes a variety of extraintestinal manifestations, eg, acute pancreatitis. As more cases of EV-D68 are described, the range of clinical manifestations will be increased.2–5
We recently saw a 27-year-old woman who presented with an influenza-like illness, but with a main complaint of right-upper-quadrant abdominal pain. She denied recent travel or contacts with sick children or adults. Her past medical history was unremarkable, and she was not taking any medications. The physical examination was unremarkable except for moderately severe tenderness in the right upper quadrant, with no rebound or guarding.
Results of laboratory testing at hospital admission included a white blood cell count of 7.3 × 109/L (49% neutrophils, 41% lymphocytes, 7% monocytes, 3% eosinophils), a normal platelet count, serum lipase 73 U/L (reference range 5.6–51.3 U/L), and serum amylase 211 U/L (37–121 U/L). Serum aminotransferase and alkaline phosphatase levels were normal. Abdominal ultrasonography was unremarkable. Nasal swab for multiplex PCR testing for respiratory viruses was positive for human rhinovirus-enterovirus. Further PCR testing was positive for EV-D68 (New York State Department of Health, Wadsworth Laboratory). Her abdominal pain was treated symptomatically; she gradually improved and was discharged.
This instance of EV-D68 in a healthy 27-year-old woman presenting with influenza-like illness and acute pain in the right upper quadrant is the first we have seen of EV-D68 presenting as acute pancreatitis. Clinicians should be aware that EV-D68, like influenza, may present with gastrointestinal manifestations.
- Foster CB, Friedman N, Carl J, Piedimonte G. Enterovirus D68: a clinically important respiratory enterovirus. Cleve Clin J Med 2015; 82:26–31.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR 2014; 63:798–799.
To the Editor: We read the review on enterovirus D681 (EV-D68) with great interest, and we thought it merited comment.
During the current influenza season, we have had several adult cases of EV-D68 presenting as an influenza-like illness. EV-D68 was diagnosed by nasal swab viral film array polymerase chain reaction (PCR) testing. We agree with the authors that the clinical spectrum of enteroviral infection includes a variety of extraintestinal manifestations, eg, acute pancreatitis. As more cases of EV-D68 are described, the range of clinical manifestations will be increased.2–5
We recently saw a 27-year-old woman who presented with an influenza-like illness, but with a main complaint of right-upper-quadrant abdominal pain. She denied recent travel or contacts with sick children or adults. Her past medical history was unremarkable, and she was not taking any medications. The physical examination was unremarkable except for moderately severe tenderness in the right upper quadrant, with no rebound or guarding.
Results of laboratory testing at hospital admission included a white blood cell count of 7.3 × 109/L (49% neutrophils, 41% lymphocytes, 7% monocytes, 3% eosinophils), a normal platelet count, serum lipase 73 U/L (reference range 5.6–51.3 U/L), and serum amylase 211 U/L (37–121 U/L). Serum aminotransferase and alkaline phosphatase levels were normal. Abdominal ultrasonography was unremarkable. Nasal swab for multiplex PCR testing for respiratory viruses was positive for human rhinovirus-enterovirus. Further PCR testing was positive for EV-D68 (New York State Department of Health, Wadsworth Laboratory). Her abdominal pain was treated symptomatically; she gradually improved and was discharged.
This instance of EV-D68 in a healthy 27-year-old woman presenting with influenza-like illness and acute pain in the right upper quadrant is the first we have seen of EV-D68 presenting as acute pancreatitis. Clinicians should be aware that EV-D68, like influenza, may present with gastrointestinal manifestations.
To the Editor: We read the review on enterovirus D681 (EV-D68) with great interest, and we thought it merited comment.
During the current influenza season, we have had several adult cases of EV-D68 presenting as an influenza-like illness. EV-D68 was diagnosed by nasal swab viral film array polymerase chain reaction (PCR) testing. We agree with the authors that the clinical spectrum of enteroviral infection includes a variety of extraintestinal manifestations, eg, acute pancreatitis. As more cases of EV-D68 are described, the range of clinical manifestations will be increased.2–5
We recently saw a 27-year-old woman who presented with an influenza-like illness, but with a main complaint of right-upper-quadrant abdominal pain. She denied recent travel or contacts with sick children or adults. Her past medical history was unremarkable, and she was not taking any medications. The physical examination was unremarkable except for moderately severe tenderness in the right upper quadrant, with no rebound or guarding.
Results of laboratory testing at hospital admission included a white blood cell count of 7.3 × 109/L (49% neutrophils, 41% lymphocytes, 7% monocytes, 3% eosinophils), a normal platelet count, serum lipase 73 U/L (reference range 5.6–51.3 U/L), and serum amylase 211 U/L (37–121 U/L). Serum aminotransferase and alkaline phosphatase levels were normal. Abdominal ultrasonography was unremarkable. Nasal swab for multiplex PCR testing for respiratory viruses was positive for human rhinovirus-enterovirus. Further PCR testing was positive for EV-D68 (New York State Department of Health, Wadsworth Laboratory). Her abdominal pain was treated symptomatically; she gradually improved and was discharged.
This instance of EV-D68 in a healthy 27-year-old woman presenting with influenza-like illness and acute pain in the right upper quadrant is the first we have seen of EV-D68 presenting as acute pancreatitis. Clinicians should be aware that EV-D68, like influenza, may present with gastrointestinal manifestations.
- Foster CB, Friedman N, Carl J, Piedimonte G. Enterovirus D68: a clinically important respiratory enterovirus. Cleve Clin J Med 2015; 82:26–31.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR 2014; 63:798–799.
- Foster CB, Friedman N, Carl J, Piedimonte G. Enterovirus D68: a clinically important respiratory enterovirus. Cleve Clin J Med 2015; 82:26–31.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR 2014; 63:798–799.