User login
VIDEO: ACTRIMS Forum focuses on progressive MS
NEW ORLEANS – A focus of the 2016 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum is the pathogenic mechanisms involved in progressive forms of MS, including the genetic and environmental underpinnings and the immunopathologic processes involved, according to ACTRIMS president, Dr. Suhayl Dhib-Jalbut.
In particular, the role of B cells in the pathogenesis of progressive disease will be addressed as recent studies targeting B lymphocytes are providing important new information about the importance of B cells in the pathogenesis of progressive MS.
In this video interview, Dr. Dhib-Jalbut, professor and chair of the department of neurology at the Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., notes that it is hypothesized that in progressive MS, there is a depletion of energy in the central nervous system. Studies of medications that can restore mitochondrial function and energy pools and perhaps have an impact on disease progression will be presented during the forum, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A focus of the 2016 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum is the pathogenic mechanisms involved in progressive forms of MS, including the genetic and environmental underpinnings and the immunopathologic processes involved, according to ACTRIMS president, Dr. Suhayl Dhib-Jalbut.
In particular, the role of B cells in the pathogenesis of progressive disease will be addressed as recent studies targeting B lymphocytes are providing important new information about the importance of B cells in the pathogenesis of progressive MS.
In this video interview, Dr. Dhib-Jalbut, professor and chair of the department of neurology at the Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., notes that it is hypothesized that in progressive MS, there is a depletion of energy in the central nervous system. Studies of medications that can restore mitochondrial function and energy pools and perhaps have an impact on disease progression will be presented during the forum, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A focus of the 2016 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum is the pathogenic mechanisms involved in progressive forms of MS, including the genetic and environmental underpinnings and the immunopathologic processes involved, according to ACTRIMS president, Dr. Suhayl Dhib-Jalbut.
In particular, the role of B cells in the pathogenesis of progressive disease will be addressed as recent studies targeting B lymphocytes are providing important new information about the importance of B cells in the pathogenesis of progressive MS.
In this video interview, Dr. Dhib-Jalbut, professor and chair of the department of neurology at the Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., notes that it is hypothesized that in progressive MS, there is a depletion of energy in the central nervous system. Studies of medications that can restore mitochondrial function and energy pools and perhaps have an impact on disease progression will be presented during the forum, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE ACTRIMS 2016 FORUM
VIDEO: Post-stroke pioglitazone modestly protective against secondary vascular events
LOS ANGELES – Nondiabetic, insulin-resistant patients who started pioglitazone within 6 months of an ischemic stroke or transient ischemic attack had almost a 3% absolute risk reduction in secondary strokes and myocardial infarctions after 5 years, in a randomized, clinical trial published online Feb. 17 in the New England Journal of Medicine.
Stroke or MI – the study’s primary combined outcome – occurred in 175 of 1,939 (9.0%) pioglitazone (Actos) patients, but 228 of 1,937 (11.8%) placebo patients (hazard ratio, 0.76; P = 0.007). There was no significant difference in all-cause mortality (HR, 0.93; P = 0.52).
Seventy-three pioglitazone patients (3.8%) developed diabetes, compared with 149 (7.7%) in the placebo group (HR, 0.48; P less than 0.001). That wasn’t a surprise; pioglitazone has been shown to protect insulin resistant patients against diabetes, the study investigators noted (N Engl J Med. 2016 Feb 17; doi: 10.1056/NEJMoa1506930).
Known side effects showed up as well. Pioglitazone was associated with a greater frequency of weight gain exceeding 4.5 kg (52.2% versus 33.7%, P less than 0.001), edema (35.6% versus 24.9%, P less than 0.001), and bone fracture requiring surgery or hospitalization (5.1% versus 3.2%, P = 0.003).
Heart failure – another known risk with the drug – did not show up in the trial; people with heart failure histories or other risk factors were excluded.
The median baseline modified Rankin Scale in both groups was 1, and the median NIH Stroke Scale score was 0. The pioglitazone target dose was 45 mg daily.
Insulin resistance is a risk factor for heart attack and stroke, which may help explain the thiazolidinedione’s apparent protective effects. It was defined in the trial as a score greater than 3.0 on the homeostasis model assessment of insulin resistance (HOMA-IR) index.
So, should pioglitazone be a part of routine post-stroke care?
In a video interview at the International Stroke Conference, lead investigator Dr. Walter N. Kernan, a professor of general medicine at Yale University, New Haven, Conn., shared his thoughts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LOS ANGELES – Nondiabetic, insulin-resistant patients who started pioglitazone within 6 months of an ischemic stroke or transient ischemic attack had almost a 3% absolute risk reduction in secondary strokes and myocardial infarctions after 5 years, in a randomized, clinical trial published online Feb. 17 in the New England Journal of Medicine.
Stroke or MI – the study’s primary combined outcome – occurred in 175 of 1,939 (9.0%) pioglitazone (Actos) patients, but 228 of 1,937 (11.8%) placebo patients (hazard ratio, 0.76; P = 0.007). There was no significant difference in all-cause mortality (HR, 0.93; P = 0.52).
Seventy-three pioglitazone patients (3.8%) developed diabetes, compared with 149 (7.7%) in the placebo group (HR, 0.48; P less than 0.001). That wasn’t a surprise; pioglitazone has been shown to protect insulin resistant patients against diabetes, the study investigators noted (N Engl J Med. 2016 Feb 17; doi: 10.1056/NEJMoa1506930).
Known side effects showed up as well. Pioglitazone was associated with a greater frequency of weight gain exceeding 4.5 kg (52.2% versus 33.7%, P less than 0.001), edema (35.6% versus 24.9%, P less than 0.001), and bone fracture requiring surgery or hospitalization (5.1% versus 3.2%, P = 0.003).
Heart failure – another known risk with the drug – did not show up in the trial; people with heart failure histories or other risk factors were excluded.
The median baseline modified Rankin Scale in both groups was 1, and the median NIH Stroke Scale score was 0. The pioglitazone target dose was 45 mg daily.
Insulin resistance is a risk factor for heart attack and stroke, which may help explain the thiazolidinedione’s apparent protective effects. It was defined in the trial as a score greater than 3.0 on the homeostasis model assessment of insulin resistance (HOMA-IR) index.
So, should pioglitazone be a part of routine post-stroke care?
In a video interview at the International Stroke Conference, lead investigator Dr. Walter N. Kernan, a professor of general medicine at Yale University, New Haven, Conn., shared his thoughts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LOS ANGELES – Nondiabetic, insulin-resistant patients who started pioglitazone within 6 months of an ischemic stroke or transient ischemic attack had almost a 3% absolute risk reduction in secondary strokes and myocardial infarctions after 5 years, in a randomized, clinical trial published online Feb. 17 in the New England Journal of Medicine.
Stroke or MI – the study’s primary combined outcome – occurred in 175 of 1,939 (9.0%) pioglitazone (Actos) patients, but 228 of 1,937 (11.8%) placebo patients (hazard ratio, 0.76; P = 0.007). There was no significant difference in all-cause mortality (HR, 0.93; P = 0.52).
Seventy-three pioglitazone patients (3.8%) developed diabetes, compared with 149 (7.7%) in the placebo group (HR, 0.48; P less than 0.001). That wasn’t a surprise; pioglitazone has been shown to protect insulin resistant patients against diabetes, the study investigators noted (N Engl J Med. 2016 Feb 17; doi: 10.1056/NEJMoa1506930).
Known side effects showed up as well. Pioglitazone was associated with a greater frequency of weight gain exceeding 4.5 kg (52.2% versus 33.7%, P less than 0.001), edema (35.6% versus 24.9%, P less than 0.001), and bone fracture requiring surgery or hospitalization (5.1% versus 3.2%, P = 0.003).
Heart failure – another known risk with the drug – did not show up in the trial; people with heart failure histories or other risk factors were excluded.
The median baseline modified Rankin Scale in both groups was 1, and the median NIH Stroke Scale score was 0. The pioglitazone target dose was 45 mg daily.
Insulin resistance is a risk factor for heart attack and stroke, which may help explain the thiazolidinedione’s apparent protective effects. It was defined in the trial as a score greater than 3.0 on the homeostasis model assessment of insulin resistance (HOMA-IR) index.
So, should pioglitazone be a part of routine post-stroke care?
In a video interview at the International Stroke Conference, lead investigator Dr. Walter N. Kernan, a professor of general medicine at Yale University, New Haven, Conn., shared his thoughts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE INTERNATIONAL STROKE CONFERENCE
Pediatric BMI increases linked to rises in blood pressure, hypertension risk
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
FROM PEDIATRICS
Key clinical point: The risk of blood pressure increases and even hypertension rises with increasing BMI among youth aged 3-17 years.
Major finding: Incident hypertension risk doubled for children and adolescents with obesity (HR, 2.02 and HR, 2.20, respectively) and quadrupled for those with severe obesity (HR, 4.42 and HR, 4.46, respectively).
Data source: The findings are based on a retrospective cohort study of 100,606 individuals, aged 3-17 years, from one of three U.S. health systems who were tracked over a median 3.1 years.
Disclosures: The study was funded by the National Heart, Lung, and Blood Institute. Dr. Lo has received previous research funding from Sanofi.
Targeting EZH2 to treat ETP-ALL

The gene EZH2 is a driver of, and potential therapeutic target for, early T-cell precursor acute lymphoblastic leukemia (ETP-ALL), according to a new study.
A previous study, published in Nature in 2012, suggested that nearly half of ETP-ALLs have inactivating alterations in EZH2.
Loss of EZH2 function can inactivate Polycomb repressive complex 2 (PRC2), but it was not clear how PRC2 loss-of-function mutations would aid leukemia growth.
The new study, published in Cell Reports, provides some insight.
Tobias Neff, MD, of the University of Colorado Denver in Aurora, and his colleagues developed a mouse model of NRASQ61K-driven leukemia that recapitulates phenotypic and transcriptional features of ETP-ALL.
Experiments with this model revealed that inactivation of EZH2 helps accelerate leukemia development and enhances a stem-cell-related transcriptional program.
“We have 2 major features of [ETP-ALL]—stem-like cells and increased growth—and, now, we show an actor implicated in both—namely, EZH2/PRC2,” Dr Neff said.
“How exactly the stem-cell-like gene expression profile contributes to the aggressiveness of ETP-ALL is unknown, but we’ve known that these stem-like cells are associated with poor prognosis in acute leukemia.”
The researchers also found that EZH2 inactivation resulted in increased activation of STAT3 by tyrosine 705 phosphorylation. This led them to wonder whether the JAK/STAT pathway might be important in their ETP-ALL model.
The team tested the JAK1/2 inhibitor ruxolitinib in NRASQ61K cells with EZH2 deletion and observed inhibition of cell growth.
“Ruxolitinib is unlikely to treat the disease by itself, but this model will help us test possible drug combinations that could eventually benefit ETP-ALL patients,” Dr Neff said.
He and his colleagues also plan to test the activity of different drugs against other cell types with inactivated EZH2.
“In addition to our specific finding in this disease, we are excited to now have a model that allows us to explore consequences of EZH2 inactivation that may enrich our understanding of a number of other conditions with a similar set of genetic changes,” Dr Neff said.
He and his colleagues noted that EZH2 is known to be inactivated in myelodysplastic syndromes, myeloproliferative neoplasms, and other hematologic malignancies. ![]()

The gene EZH2 is a driver of, and potential therapeutic target for, early T-cell precursor acute lymphoblastic leukemia (ETP-ALL), according to a new study.
A previous study, published in Nature in 2012, suggested that nearly half of ETP-ALLs have inactivating alterations in EZH2.
Loss of EZH2 function can inactivate Polycomb repressive complex 2 (PRC2), but it was not clear how PRC2 loss-of-function mutations would aid leukemia growth.
The new study, published in Cell Reports, provides some insight.
Tobias Neff, MD, of the University of Colorado Denver in Aurora, and his colleagues developed a mouse model of NRASQ61K-driven leukemia that recapitulates phenotypic and transcriptional features of ETP-ALL.
Experiments with this model revealed that inactivation of EZH2 helps accelerate leukemia development and enhances a stem-cell-related transcriptional program.
“We have 2 major features of [ETP-ALL]—stem-like cells and increased growth—and, now, we show an actor implicated in both—namely, EZH2/PRC2,” Dr Neff said.
“How exactly the stem-cell-like gene expression profile contributes to the aggressiveness of ETP-ALL is unknown, but we’ve known that these stem-like cells are associated with poor prognosis in acute leukemia.”
The researchers also found that EZH2 inactivation resulted in increased activation of STAT3 by tyrosine 705 phosphorylation. This led them to wonder whether the JAK/STAT pathway might be important in their ETP-ALL model.
The team tested the JAK1/2 inhibitor ruxolitinib in NRASQ61K cells with EZH2 deletion and observed inhibition of cell growth.
“Ruxolitinib is unlikely to treat the disease by itself, but this model will help us test possible drug combinations that could eventually benefit ETP-ALL patients,” Dr Neff said.
He and his colleagues also plan to test the activity of different drugs against other cell types with inactivated EZH2.
“In addition to our specific finding in this disease, we are excited to now have a model that allows us to explore consequences of EZH2 inactivation that may enrich our understanding of a number of other conditions with a similar set of genetic changes,” Dr Neff said.
He and his colleagues noted that EZH2 is known to be inactivated in myelodysplastic syndromes, myeloproliferative neoplasms, and other hematologic malignancies. ![]()

The gene EZH2 is a driver of, and potential therapeutic target for, early T-cell precursor acute lymphoblastic leukemia (ETP-ALL), according to a new study.
A previous study, published in Nature in 2012, suggested that nearly half of ETP-ALLs have inactivating alterations in EZH2.
Loss of EZH2 function can inactivate Polycomb repressive complex 2 (PRC2), but it was not clear how PRC2 loss-of-function mutations would aid leukemia growth.
The new study, published in Cell Reports, provides some insight.
Tobias Neff, MD, of the University of Colorado Denver in Aurora, and his colleagues developed a mouse model of NRASQ61K-driven leukemia that recapitulates phenotypic and transcriptional features of ETP-ALL.
Experiments with this model revealed that inactivation of EZH2 helps accelerate leukemia development and enhances a stem-cell-related transcriptional program.
“We have 2 major features of [ETP-ALL]—stem-like cells and increased growth—and, now, we show an actor implicated in both—namely, EZH2/PRC2,” Dr Neff said.
“How exactly the stem-cell-like gene expression profile contributes to the aggressiveness of ETP-ALL is unknown, but we’ve known that these stem-like cells are associated with poor prognosis in acute leukemia.”
The researchers also found that EZH2 inactivation resulted in increased activation of STAT3 by tyrosine 705 phosphorylation. This led them to wonder whether the JAK/STAT pathway might be important in their ETP-ALL model.
The team tested the JAK1/2 inhibitor ruxolitinib in NRASQ61K cells with EZH2 deletion and observed inhibition of cell growth.
“Ruxolitinib is unlikely to treat the disease by itself, but this model will help us test possible drug combinations that could eventually benefit ETP-ALL patients,” Dr Neff said.
He and his colleagues also plan to test the activity of different drugs against other cell types with inactivated EZH2.
“In addition to our specific finding in this disease, we are excited to now have a model that allows us to explore consequences of EZH2 inactivation that may enrich our understanding of a number of other conditions with a similar set of genetic changes,” Dr Neff said.
He and his colleagues noted that EZH2 is known to be inactivated in myelodysplastic syndromes, myeloproliferative neoplasms, and other hematologic malignancies. ![]()
Does Life, Liberty, and the Pursuit of Happiness Apply to Hospital Medicine?
Every American knows this well-known phrase from the Declaration of Independence, which describes the three “unalienable rights” ordained on humans by their Creator and which governments are bound to dutifully protect. But I wonder if the last unalienable right has implications for career happiness in the healthcare industry, particularly for hospitalists. With the phrase now being 240 years old, it has understandably permeated every inch of American society and affected every crevice of the American psyche. Despite having this decreed inalienable right of the pursuit of happiness, there is evidence of widespread dissatisfaction and unhappiness within our profession.
Speaking of happiness, I was listening to a 60 Minutes podcast entitled “Heroin in the Heartland.” It described a widespread affliction of heroin among mainstream middle- and upper-class suburban youths.1 During the piece, they interviewed several addicted youngsters and their parents. I was struck by the story of a young woman named Hannah; she described how and why she became addicted to heroin in her upper-middle-class high school in Columbus, Ohio. She described how heroin made her feel. On a scale of 1–10 in happiness, she said it made her feel like a “26.” She and many of her friends became addicted to the feeling of happiness that was infused into them, a feeling that could not be replicated without the use of the drug. She and her friends started their road to addiction in a quest for their unalienable right of the pursuit of happiness.
Contrast that story with the “unhappiness factor” that plagues U.S. physicians. A 2014 survey found that 54% of physicians reported at least one symptom of burnout.2 That figure was up from 46% in a 2011 survey. From 2011 to 2014, satisfaction with work-life balance dropped to 41% from 49%. Within that same time frame, burnout and dissatisfaction showed very little change in other U.S. working adults, widening the gap in dissatisfaction between physicians and non-physicians. Even after adjusting for age, sex, relationship status, and hours worked, physicians still were almost twice as likely to experience burnout than other working U.S. adults, and they only had an odds ratio of satisfaction of 0.68 (95% CI, 0.62–0.75) compared with non-physicians. In another recent (and sobering) meta-analysis, researchers found that about a third of all resident physicians report depression or depressive symptoms during their training (ranging from 21% to 43%, depending on the instrument used).3
Could it be that physicians in the U.S., in their quest for the pursuit of happiness, are looking for happiness in all the wrong ways? I read an article recently on DailyGood entitled “Does Trying to Be Happy Make Us Unhappy?”4 It describes several studies that purport that the more value people place on trying to become happy, the less happy they actually become. It turns out that in order for us to figure out if we are happy, we are forced to evaluate our current level of happiness and set that against some benchmark (usually from our own past) to analyze where we are. The mere act of doing this moves us from an experiential mode to an evaluation mode, which puts us out of touch with those things in life that can bring us joy and contentment.
Social scientists have found that when we are immersed in the present, we don’t report being happy in that moment, but we do report happiness later when reflecting on those moments. Ruminating about whether we are unhappy, depressed, burned out, or unsatisfied makes us inwardly focused and makes us lose the ability to become immersed in the present.
Scientists also have found that we tend to overestimate how external influences, such as getting a promotion or moving into a new job, will inflate our happiness and that we all adapt to new experiences and quickly return to our baseline happiness (as if the change never occurred). They’ve also found that when we pursue happiness as an individual state, we become inwardly focused and less likely to actually achieve happiness. People who are more outwardly focused on how others feel (and not how they themselves feel) are much more likely to achieve a state of sustained happiness.
Finally, researchers have found that happiness is more likely achieved by pursuing frequent positive emotions rather than intense positive emotions. Many of us search for single intense emotional experiences (the winning of a gold medal) in the pursuit of happiness, but researchers found that the frequency of positive emotions are much more important than the intensity of positive emotions.
So maybe, as physicians in pursuit of happiness, we are going about this pursuit all wrong, with resultant depression, dissatisfaction, and burnout. We can’t change the Declaration of Independence or the American psyche, but we can change how we perceive that pursuit.
Happiness is not a goal to be achieved but a state of mind to be savored. Immersing ourselves in our daily life, we should be outwardly focused on our colleagues and our patients. If we take this approach, there is no other profession better suited to actually achieving sustained happiness. TH
References
1. Preview: heroin in the heartland. CBS News website. Available at: www.cbsnews.com/videos/preview-heroin-in-the-heartland. Accessed Feb. 1, 2016.
2. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600-1613. doi:10.1016/j.maocop.2015.08.023.
3. Mata DA, Ramos MA, Bansal N. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383. doi:10.1001/jama.2015.15845.
4. Grant A. Does trying to be happy make us unhappy? DailyGood website. Available at: http://www.dailygood.org/story/1187/does-trying-to-be-happy-make-us-unhappy-adam-grant/. Accessed Feb. 1, 2016.

Every American knows this well-known phrase from the Declaration of Independence, which describes the three “unalienable rights” ordained on humans by their Creator and which governments are bound to dutifully protect. But I wonder if the last unalienable right has implications for career happiness in the healthcare industry, particularly for hospitalists. With the phrase now being 240 years old, it has understandably permeated every inch of American society and affected every crevice of the American psyche. Despite having this decreed inalienable right of the pursuit of happiness, there is evidence of widespread dissatisfaction and unhappiness within our profession.
Speaking of happiness, I was listening to a 60 Minutes podcast entitled “Heroin in the Heartland.” It described a widespread affliction of heroin among mainstream middle- and upper-class suburban youths.1 During the piece, they interviewed several addicted youngsters and their parents. I was struck by the story of a young woman named Hannah; she described how and why she became addicted to heroin in her upper-middle-class high school in Columbus, Ohio. She described how heroin made her feel. On a scale of 1–10 in happiness, she said it made her feel like a “26.” She and many of her friends became addicted to the feeling of happiness that was infused into them, a feeling that could not be replicated without the use of the drug. She and her friends started their road to addiction in a quest for their unalienable right of the pursuit of happiness.
Contrast that story with the “unhappiness factor” that plagues U.S. physicians. A 2014 survey found that 54% of physicians reported at least one symptom of burnout.2 That figure was up from 46% in a 2011 survey. From 2011 to 2014, satisfaction with work-life balance dropped to 41% from 49%. Within that same time frame, burnout and dissatisfaction showed very little change in other U.S. working adults, widening the gap in dissatisfaction between physicians and non-physicians. Even after adjusting for age, sex, relationship status, and hours worked, physicians still were almost twice as likely to experience burnout than other working U.S. adults, and they only had an odds ratio of satisfaction of 0.68 (95% CI, 0.62–0.75) compared with non-physicians. In another recent (and sobering) meta-analysis, researchers found that about a third of all resident physicians report depression or depressive symptoms during their training (ranging from 21% to 43%, depending on the instrument used).3
Could it be that physicians in the U.S., in their quest for the pursuit of happiness, are looking for happiness in all the wrong ways? I read an article recently on DailyGood entitled “Does Trying to Be Happy Make Us Unhappy?”4 It describes several studies that purport that the more value people place on trying to become happy, the less happy they actually become. It turns out that in order for us to figure out if we are happy, we are forced to evaluate our current level of happiness and set that against some benchmark (usually from our own past) to analyze where we are. The mere act of doing this moves us from an experiential mode to an evaluation mode, which puts us out of touch with those things in life that can bring us joy and contentment.
Social scientists have found that when we are immersed in the present, we don’t report being happy in that moment, but we do report happiness later when reflecting on those moments. Ruminating about whether we are unhappy, depressed, burned out, or unsatisfied makes us inwardly focused and makes us lose the ability to become immersed in the present.
Scientists also have found that we tend to overestimate how external influences, such as getting a promotion or moving into a new job, will inflate our happiness and that we all adapt to new experiences and quickly return to our baseline happiness (as if the change never occurred). They’ve also found that when we pursue happiness as an individual state, we become inwardly focused and less likely to actually achieve happiness. People who are more outwardly focused on how others feel (and not how they themselves feel) are much more likely to achieve a state of sustained happiness.
Finally, researchers have found that happiness is more likely achieved by pursuing frequent positive emotions rather than intense positive emotions. Many of us search for single intense emotional experiences (the winning of a gold medal) in the pursuit of happiness, but researchers found that the frequency of positive emotions are much more important than the intensity of positive emotions.
So maybe, as physicians in pursuit of happiness, we are going about this pursuit all wrong, with resultant depression, dissatisfaction, and burnout. We can’t change the Declaration of Independence or the American psyche, but we can change how we perceive that pursuit.
Happiness is not a goal to be achieved but a state of mind to be savored. Immersing ourselves in our daily life, we should be outwardly focused on our colleagues and our patients. If we take this approach, there is no other profession better suited to actually achieving sustained happiness. TH
References
1. Preview: heroin in the heartland. CBS News website. Available at: www.cbsnews.com/videos/preview-heroin-in-the-heartland. Accessed Feb. 1, 2016.
2. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600-1613. doi:10.1016/j.maocop.2015.08.023.
3. Mata DA, Ramos MA, Bansal N. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383. doi:10.1001/jama.2015.15845.
4. Grant A. Does trying to be happy make us unhappy? DailyGood website. Available at: http://www.dailygood.org/story/1187/does-trying-to-be-happy-make-us-unhappy-adam-grant/. Accessed Feb. 1, 2016.

Every American knows this well-known phrase from the Declaration of Independence, which describes the three “unalienable rights” ordained on humans by their Creator and which governments are bound to dutifully protect. But I wonder if the last unalienable right has implications for career happiness in the healthcare industry, particularly for hospitalists. With the phrase now being 240 years old, it has understandably permeated every inch of American society and affected every crevice of the American psyche. Despite having this decreed inalienable right of the pursuit of happiness, there is evidence of widespread dissatisfaction and unhappiness within our profession.
Speaking of happiness, I was listening to a 60 Minutes podcast entitled “Heroin in the Heartland.” It described a widespread affliction of heroin among mainstream middle- and upper-class suburban youths.1 During the piece, they interviewed several addicted youngsters and their parents. I was struck by the story of a young woman named Hannah; she described how and why she became addicted to heroin in her upper-middle-class high school in Columbus, Ohio. She described how heroin made her feel. On a scale of 1–10 in happiness, she said it made her feel like a “26.” She and many of her friends became addicted to the feeling of happiness that was infused into them, a feeling that could not be replicated without the use of the drug. She and her friends started their road to addiction in a quest for their unalienable right of the pursuit of happiness.
Contrast that story with the “unhappiness factor” that plagues U.S. physicians. A 2014 survey found that 54% of physicians reported at least one symptom of burnout.2 That figure was up from 46% in a 2011 survey. From 2011 to 2014, satisfaction with work-life balance dropped to 41% from 49%. Within that same time frame, burnout and dissatisfaction showed very little change in other U.S. working adults, widening the gap in dissatisfaction between physicians and non-physicians. Even after adjusting for age, sex, relationship status, and hours worked, physicians still were almost twice as likely to experience burnout than other working U.S. adults, and they only had an odds ratio of satisfaction of 0.68 (95% CI, 0.62–0.75) compared with non-physicians. In another recent (and sobering) meta-analysis, researchers found that about a third of all resident physicians report depression or depressive symptoms during their training (ranging from 21% to 43%, depending on the instrument used).3
Could it be that physicians in the U.S., in their quest for the pursuit of happiness, are looking for happiness in all the wrong ways? I read an article recently on DailyGood entitled “Does Trying to Be Happy Make Us Unhappy?”4 It describes several studies that purport that the more value people place on trying to become happy, the less happy they actually become. It turns out that in order for us to figure out if we are happy, we are forced to evaluate our current level of happiness and set that against some benchmark (usually from our own past) to analyze where we are. The mere act of doing this moves us from an experiential mode to an evaluation mode, which puts us out of touch with those things in life that can bring us joy and contentment.
Social scientists have found that when we are immersed in the present, we don’t report being happy in that moment, but we do report happiness later when reflecting on those moments. Ruminating about whether we are unhappy, depressed, burned out, or unsatisfied makes us inwardly focused and makes us lose the ability to become immersed in the present.
Scientists also have found that we tend to overestimate how external influences, such as getting a promotion or moving into a new job, will inflate our happiness and that we all adapt to new experiences and quickly return to our baseline happiness (as if the change never occurred). They’ve also found that when we pursue happiness as an individual state, we become inwardly focused and less likely to actually achieve happiness. People who are more outwardly focused on how others feel (and not how they themselves feel) are much more likely to achieve a state of sustained happiness.
Finally, researchers have found that happiness is more likely achieved by pursuing frequent positive emotions rather than intense positive emotions. Many of us search for single intense emotional experiences (the winning of a gold medal) in the pursuit of happiness, but researchers found that the frequency of positive emotions are much more important than the intensity of positive emotions.
So maybe, as physicians in pursuit of happiness, we are going about this pursuit all wrong, with resultant depression, dissatisfaction, and burnout. We can’t change the Declaration of Independence or the American psyche, but we can change how we perceive that pursuit.
Happiness is not a goal to be achieved but a state of mind to be savored. Immersing ourselves in our daily life, we should be outwardly focused on our colleagues and our patients. If we take this approach, there is no other profession better suited to actually achieving sustained happiness. TH
References
1. Preview: heroin in the heartland. CBS News website. Available at: www.cbsnews.com/videos/preview-heroin-in-the-heartland. Accessed Feb. 1, 2016.
2. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600-1613. doi:10.1016/j.maocop.2015.08.023.
3. Mata DA, Ramos MA, Bansal N. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383. doi:10.1001/jama.2015.15845.
4. Grant A. Does trying to be happy make us unhappy? DailyGood website. Available at: http://www.dailygood.org/story/1187/does-trying-to-be-happy-make-us-unhappy-adam-grant/. Accessed Feb. 1, 2016.

Dabigatran and Warfarin are Both Used for Stroke-prevention in Patients with AF but their Side effects Differ
NEW YORK (Reuters Health) - Dabigatran and warfarin offer similar stroke-prevention efficacy in patients with atrial fibrillation (AF), but their side effect profiles differ, according to a systematic review and meta-analysis of real-world clinical practice.
"There could be many reasons for the differences in our findings, such as differences in the quality of evidence of observational studies and randomized controlled trials (RCTs) or differences in the included study populations between the observational studies in our review and the RE-LY trial," Dr. Robert J. Romanelli from Palo Alto Medical Foundation Research Institute, California, told Reuters Health by email.
The RE-LY trial is the only RCT to have evaluated dabigatran in stroke prevention, and RCTs are prone to selection biases less likely to be present in well designed observational studies, Dr. Romanelli and colleagues note in Circulation:Cardiovascular and Quality Outcomes, online January 26.
The team used data from seven retrospective cohort studies to compare the effectiveness and safety of dabigatran and warfarin among more than 348,750 patients with nonvalvular AF.
During an overall mean follow-up of 794 days, dabigatran 150mg or 110 mg was similar to warfarin in ischemic stroke prevention.
Both the higher and lower dabigatran doses had significantly lower hazards of intracranial bleeding compared with warfarin (pooled hazard ratio, 0.44 and 0.49, respectively). But the hazard of gastrointestinal bleeding was significantly greater for dabigatran 150 mg (but not for 110 mg) than for warfarin (pHR, 1.23). The 110 mg dose of dabigatran was only available during the trial; it's now sold in 150 mg or 75 mg capsules.
The increased risk of gastrointestinal bleeding with the higher dose of dabigatran was significant only in older populations (75 years or older).
"Data presented in this review reflect relative risk, which is not always clinically meaningful," the researchers caution. "It is important to bear in mind that event rates for the outcome of interest are low under standard treatment."
"I don't think the findings from this one reviewshould change clinical practice," Dr. Romanelli said. "If anything, this study revealed areas for future research.
NEW YORK (Reuters Health) - Dabigatran and warfarin offer similar stroke-prevention efficacy in patients with atrial fibrillation (AF), but their side effect profiles differ, according to a systematic review and meta-analysis of real-world clinical practice.
"There could be many reasons for the differences in our findings, such as differences in the quality of evidence of observational studies and randomized controlled trials (RCTs) or differences in the included study populations between the observational studies in our review and the RE-LY trial," Dr. Robert J. Romanelli from Palo Alto Medical Foundation Research Institute, California, told Reuters Health by email.
The RE-LY trial is the only RCT to have evaluated dabigatran in stroke prevention, and RCTs are prone to selection biases less likely to be present in well designed observational studies, Dr. Romanelli and colleagues note in Circulation:Cardiovascular and Quality Outcomes, online January 26.
The team used data from seven retrospective cohort studies to compare the effectiveness and safety of dabigatran and warfarin among more than 348,750 patients with nonvalvular AF.
During an overall mean follow-up of 794 days, dabigatran 150mg or 110 mg was similar to warfarin in ischemic stroke prevention.
Both the higher and lower dabigatran doses had significantly lower hazards of intracranial bleeding compared with warfarin (pooled hazard ratio, 0.44 and 0.49, respectively). But the hazard of gastrointestinal bleeding was significantly greater for dabigatran 150 mg (but not for 110 mg) than for warfarin (pHR, 1.23). The 110 mg dose of dabigatran was only available during the trial; it's now sold in 150 mg or 75 mg capsules.
The increased risk of gastrointestinal bleeding with the higher dose of dabigatran was significant only in older populations (75 years or older).
"Data presented in this review reflect relative risk, which is not always clinically meaningful," the researchers caution. "It is important to bear in mind that event rates for the outcome of interest are low under standard treatment."
"I don't think the findings from this one reviewshould change clinical practice," Dr. Romanelli said. "If anything, this study revealed areas for future research.
NEW YORK (Reuters Health) - Dabigatran and warfarin offer similar stroke-prevention efficacy in patients with atrial fibrillation (AF), but their side effect profiles differ, according to a systematic review and meta-analysis of real-world clinical practice.
"There could be many reasons for the differences in our findings, such as differences in the quality of evidence of observational studies and randomized controlled trials (RCTs) or differences in the included study populations between the observational studies in our review and the RE-LY trial," Dr. Robert J. Romanelli from Palo Alto Medical Foundation Research Institute, California, told Reuters Health by email.
The RE-LY trial is the only RCT to have evaluated dabigatran in stroke prevention, and RCTs are prone to selection biases less likely to be present in well designed observational studies, Dr. Romanelli and colleagues note in Circulation:Cardiovascular and Quality Outcomes, online January 26.
The team used data from seven retrospective cohort studies to compare the effectiveness and safety of dabigatran and warfarin among more than 348,750 patients with nonvalvular AF.
During an overall mean follow-up of 794 days, dabigatran 150mg or 110 mg was similar to warfarin in ischemic stroke prevention.
Both the higher and lower dabigatran doses had significantly lower hazards of intracranial bleeding compared with warfarin (pooled hazard ratio, 0.44 and 0.49, respectively). But the hazard of gastrointestinal bleeding was significantly greater for dabigatran 150 mg (but not for 110 mg) than for warfarin (pHR, 1.23). The 110 mg dose of dabigatran was only available during the trial; it's now sold in 150 mg or 75 mg capsules.
The increased risk of gastrointestinal bleeding with the higher dose of dabigatran was significant only in older populations (75 years or older).
"Data presented in this review reflect relative risk, which is not always clinically meaningful," the researchers caution. "It is important to bear in mind that event rates for the outcome of interest are low under standard treatment."
"I don't think the findings from this one reviewshould change clinical practice," Dr. Romanelli said. "If anything, this study revealed areas for future research.
Health Canada approves ruxolitinib for PV

Image courtesy of AFIP
Health Canada has approved the JAK1/2 inhibitor ruxolitinib (Jakavi) for the control of hematocrit in adult patients with polycythemia vera (PV) that is resistant to or intolerant of a cytoreductive agent.
Ruxolitinib is the first targeted treatment approved to treat PV in Canada.
The approval is based on results of the phase 3 RESPONSE trial, which showed that ruxolitinib could provide hematocrit control without phlebotomy in patients with PV.
For RESPONSE, researchers compared ruxolitinib to best available therapy (BAT) for PV. The trial was sponsored by Incyte Corporation and Novartis Pharmaceuticals, the companies developing ruxolitinib.
The study’s primary endpoint was the proportion of patients who achieved hematocrit control and were not eligible for phlebotomy from weeks 8 through 32 (with no more than 1 instance of phlebotomy eligibility between randomization and week 8) and who saw a 35% or greater reduction in spleen volume from baseline, as assessed by imaging at week 32.
The primary endpoint was met by significantly more patients in the ruxolitinib arm than the BAT arm— 20.9% and 0.9%, respectively (P<0.0001).
Sixty percent of patients in the ruxolitinib arm achieved hematocrit control, as did 19.6% of patients in the BAT arm. The percentage of patients who had at least a 35% reduction in spleen volume was 38.2% in the ruxolitinib arm and 0.9% in the BAT arm.
The proportion of patients achieving a complete hematologic remission at week 32 was 23.6% in the ruxolitinib arm and 8.9% in the BAT arm (P=0.0028). The proportion of patients achieving a durable primary response at week 48 was 19.1% in the ruxolitinib arm and 0.9% in the BAT arm (P<0.0001).
At 80 weeks, the most common adverse events in the ruxolitinib arm were headache (22%), diarrhea (20%), pruritus (20%), and fatigue (17%). Grade 3 or 4 anemia and thrombocytopenia occurred in 2% and 6% of patients, respectively. Five percent of patients discontinued ruxolitinib due to adverse events. ![]()

Image courtesy of AFIP
Health Canada has approved the JAK1/2 inhibitor ruxolitinib (Jakavi) for the control of hematocrit in adult patients with polycythemia vera (PV) that is resistant to or intolerant of a cytoreductive agent.
Ruxolitinib is the first targeted treatment approved to treat PV in Canada.
The approval is based on results of the phase 3 RESPONSE trial, which showed that ruxolitinib could provide hematocrit control without phlebotomy in patients with PV.
For RESPONSE, researchers compared ruxolitinib to best available therapy (BAT) for PV. The trial was sponsored by Incyte Corporation and Novartis Pharmaceuticals, the companies developing ruxolitinib.
The study’s primary endpoint was the proportion of patients who achieved hematocrit control and were not eligible for phlebotomy from weeks 8 through 32 (with no more than 1 instance of phlebotomy eligibility between randomization and week 8) and who saw a 35% or greater reduction in spleen volume from baseline, as assessed by imaging at week 32.
The primary endpoint was met by significantly more patients in the ruxolitinib arm than the BAT arm— 20.9% and 0.9%, respectively (P<0.0001).
Sixty percent of patients in the ruxolitinib arm achieved hematocrit control, as did 19.6% of patients in the BAT arm. The percentage of patients who had at least a 35% reduction in spleen volume was 38.2% in the ruxolitinib arm and 0.9% in the BAT arm.
The proportion of patients achieving a complete hematologic remission at week 32 was 23.6% in the ruxolitinib arm and 8.9% in the BAT arm (P=0.0028). The proportion of patients achieving a durable primary response at week 48 was 19.1% in the ruxolitinib arm and 0.9% in the BAT arm (P<0.0001).
At 80 weeks, the most common adverse events in the ruxolitinib arm were headache (22%), diarrhea (20%), pruritus (20%), and fatigue (17%). Grade 3 or 4 anemia and thrombocytopenia occurred in 2% and 6% of patients, respectively. Five percent of patients discontinued ruxolitinib due to adverse events. ![]()

Image courtesy of AFIP
Health Canada has approved the JAK1/2 inhibitor ruxolitinib (Jakavi) for the control of hematocrit in adult patients with polycythemia vera (PV) that is resistant to or intolerant of a cytoreductive agent.
Ruxolitinib is the first targeted treatment approved to treat PV in Canada.
The approval is based on results of the phase 3 RESPONSE trial, which showed that ruxolitinib could provide hematocrit control without phlebotomy in patients with PV.
For RESPONSE, researchers compared ruxolitinib to best available therapy (BAT) for PV. The trial was sponsored by Incyte Corporation and Novartis Pharmaceuticals, the companies developing ruxolitinib.
The study’s primary endpoint was the proportion of patients who achieved hematocrit control and were not eligible for phlebotomy from weeks 8 through 32 (with no more than 1 instance of phlebotomy eligibility between randomization and week 8) and who saw a 35% or greater reduction in spleen volume from baseline, as assessed by imaging at week 32.
The primary endpoint was met by significantly more patients in the ruxolitinib arm than the BAT arm— 20.9% and 0.9%, respectively (P<0.0001).
Sixty percent of patients in the ruxolitinib arm achieved hematocrit control, as did 19.6% of patients in the BAT arm. The percentage of patients who had at least a 35% reduction in spleen volume was 38.2% in the ruxolitinib arm and 0.9% in the BAT arm.
The proportion of patients achieving a complete hematologic remission at week 32 was 23.6% in the ruxolitinib arm and 8.9% in the BAT arm (P=0.0028). The proportion of patients achieving a durable primary response at week 48 was 19.1% in the ruxolitinib arm and 0.9% in the BAT arm (P<0.0001).
At 80 weeks, the most common adverse events in the ruxolitinib arm were headache (22%), diarrhea (20%), pruritus (20%), and fatigue (17%). Grade 3 or 4 anemia and thrombocytopenia occurred in 2% and 6% of patients, respectively. Five percent of patients discontinued ruxolitinib due to adverse events. ![]()
Blood collection set gets FDA clearance, CE mark

Photo by Graham Colm
A new blood collection set has received 510(k) clearance from the US Food and Drug Administration as well as the CE mark, which means it can be marketed within the European Economic Area.
The BD Vacutainer® UltraTouch™ Push Button Blood Collection Set is engineered to minimize patient discomfort during blood collection.
The set uses proprietary needle technology—Pentapoint™ Comfort and RightGauge™ Ultra-Thin Wall technology.
According to the manufacturer, BD, this technology can reduce penetration forces without compromising tube fill times or sample quality.
Research has shown that PentaPoint™ Comfort 5-bevel needle technology helps reduce the chance of a painful injection by creating a flatter, thinner surface to help penetrate the skin with significantly greater ease.1
When combined with RightGauge™ technology, which increases the needle’s inner diameter and enables clinicians to select a smaller gauge needle without sacrificing sample quality and blood flow, the BD Vacutainer® UltraTouch™ Push Button Blood Collection Set has been shown to reduce penetration forces by up to 32% when compared to another blood collection set.2
“The ability to use smaller gauge needles should also help clinicians access veins more successfully,” said Ana K. Stankovic, MD, PhD, worldwide vice president of Medical Affairs for BD Life Sciences – Preanalytical Systems and Global Health.
“This could prove especially valuable in patient populations—such as oncology, geriatric, and pediatric—that often have difficult or fragile veins.”
Dr Stankovic also noted that clinicians may be reluctant to use smaller gauge needles for fear of increasing hemolysis as the blood passes slowly through the narrow cannula.
“With BD Vacutainer® UltraTouch™ Push Button Blood Collection Sets, clinicians can select the gauge that is most appropriate for their patients, without compromising sample quality, testing accuracy, and their own efficiency,” she said. ![]()
1. Hirsch LJ, et al. Journal of Diabetes Science and Technology. 2012, 6(2):328-35.
2. 2015 BD bench testing versus BD Vacutainer® Push Button Blood Collection Sets.

Photo by Graham Colm
A new blood collection set has received 510(k) clearance from the US Food and Drug Administration as well as the CE mark, which means it can be marketed within the European Economic Area.
The BD Vacutainer® UltraTouch™ Push Button Blood Collection Set is engineered to minimize patient discomfort during blood collection.
The set uses proprietary needle technology—Pentapoint™ Comfort and RightGauge™ Ultra-Thin Wall technology.
According to the manufacturer, BD, this technology can reduce penetration forces without compromising tube fill times or sample quality.
Research has shown that PentaPoint™ Comfort 5-bevel needle technology helps reduce the chance of a painful injection by creating a flatter, thinner surface to help penetrate the skin with significantly greater ease.1
When combined with RightGauge™ technology, which increases the needle’s inner diameter and enables clinicians to select a smaller gauge needle without sacrificing sample quality and blood flow, the BD Vacutainer® UltraTouch™ Push Button Blood Collection Set has been shown to reduce penetration forces by up to 32% when compared to another blood collection set.2
“The ability to use smaller gauge needles should also help clinicians access veins more successfully,” said Ana K. Stankovic, MD, PhD, worldwide vice president of Medical Affairs for BD Life Sciences – Preanalytical Systems and Global Health.
“This could prove especially valuable in patient populations—such as oncology, geriatric, and pediatric—that often have difficult or fragile veins.”
Dr Stankovic also noted that clinicians may be reluctant to use smaller gauge needles for fear of increasing hemolysis as the blood passes slowly through the narrow cannula.
“With BD Vacutainer® UltraTouch™ Push Button Blood Collection Sets, clinicians can select the gauge that is most appropriate for their patients, without compromising sample quality, testing accuracy, and their own efficiency,” she said. ![]()
1. Hirsch LJ, et al. Journal of Diabetes Science and Technology. 2012, 6(2):328-35.
2. 2015 BD bench testing versus BD Vacutainer® Push Button Blood Collection Sets.

Photo by Graham Colm
A new blood collection set has received 510(k) clearance from the US Food and Drug Administration as well as the CE mark, which means it can be marketed within the European Economic Area.
The BD Vacutainer® UltraTouch™ Push Button Blood Collection Set is engineered to minimize patient discomfort during blood collection.
The set uses proprietary needle technology—Pentapoint™ Comfort and RightGauge™ Ultra-Thin Wall technology.
According to the manufacturer, BD, this technology can reduce penetration forces without compromising tube fill times or sample quality.
Research has shown that PentaPoint™ Comfort 5-bevel needle technology helps reduce the chance of a painful injection by creating a flatter, thinner surface to help penetrate the skin with significantly greater ease.1
When combined with RightGauge™ technology, which increases the needle’s inner diameter and enables clinicians to select a smaller gauge needle without sacrificing sample quality and blood flow, the BD Vacutainer® UltraTouch™ Push Button Blood Collection Set has been shown to reduce penetration forces by up to 32% when compared to another blood collection set.2
“The ability to use smaller gauge needles should also help clinicians access veins more successfully,” said Ana K. Stankovic, MD, PhD, worldwide vice president of Medical Affairs for BD Life Sciences – Preanalytical Systems and Global Health.
“This could prove especially valuable in patient populations—such as oncology, geriatric, and pediatric—that often have difficult or fragile veins.”
Dr Stankovic also noted that clinicians may be reluctant to use smaller gauge needles for fear of increasing hemolysis as the blood passes slowly through the narrow cannula.
“With BD Vacutainer® UltraTouch™ Push Button Blood Collection Sets, clinicians can select the gauge that is most appropriate for their patients, without compromising sample quality, testing accuracy, and their own efficiency,” she said. ![]()
1. Hirsch LJ, et al. Journal of Diabetes Science and Technology. 2012, 6(2):328-35.
2. 2015 BD bench testing versus BD Vacutainer® Push Button Blood Collection Sets.
Risk of reproductive problems in male cancer survivors

A study of Norwegian men has revealed several factors that may help predict reproductive problems among males diagnosed with cancer before age 25.
Cancer type, age at diagnosis, and time period of diagnosis were all associated with the likelihood of paternity.
And although cancer survivors were less likely to reproduce and more likely to use assisted reproductive technology, their first offspring were no less healthy than the offspring of control subjects.
This research was published in the British Journal of Cancer.
The study began with all Norwegian men born between 1965 and 1985 (n=626,495). The researchers excluded men who emigrated or died before reaching fertile age, which left 2687 men who were diagnosed with cancer before age 25 and 607,668 cancer-free controls.
The most common cancers were testicular cancer (27%), CNS tumors (18%), lymphoma (15%), and leukemia (13%). Thirty percent of the cancer cases were diagnosed in childhood (0–14 years of age), 26% in adolescence (15–19 years), and 43% in young adulthood (20–24 years).
Nine percent (n=247) of cancer cases were diagnosed from 1965 through 1979, 50% (n=1346) from 1980 through 1994, and 41% (n=1094) from 1995 through 2007.
The cancer survivors were less likely to have children than controls, with a hazard ratio (HR) of 0.72.
The reduction in paternity was significant for survivors of leukemia (HR=0.78), lymphoma (HR=0.78), testicular cancer (HR=0.77), CNS tumors (HR=0.45), bone tumors (HR=0.69), sympathetic nervous system tumors (HR=0.50), and retinoblastoma (HR=0.52).
The reduction in paternity was also more pronounced for cancer patients diagnosed before 1995. The HR was 0.61 for those diagnosed from 1965 through 1979 and 0.66 for those diagnosed from 1980 through 1994.
Patients who were diagnosed before age 15 were less likely to reproduce as well, with an HR of 0.59.
“These finds are important for male cancer survivors, seeing as we can identify groups at risk of having reproduction problems,” said study author Maria Winther Gunnes, a PhD candidate at the University of Bergen in Norway.
Another finding was that male cancer survivors were more likely than controls to have pregnancies resulting from assisted reproductive technology. The relative risk was 3.32.
When assessed by cancer type, the relative risk was 2.29 for leukemia, 3.79 for lymphoma, 2.41 for CNS tumors, 5.71 for sympathetic nervous system tumors, 2.20 for renal tumors, 4.77 for bone tumors, 1.32 for soft tissue sarcomas, 3.70 for testicular cancer, 4.36 for thyroid carcinoma, and 0.45 for malignant melanoma.
There was no increased risk among the first offspring of cancer survivors for perinatal death, congenital malformations, being small for gestational age, low birth weight, or preterm birth.
“It is important to be able to assure young male cancer survivors that their illness and treatment will not have a negative impact on their own children,” Gunnes said. ![]()

A study of Norwegian men has revealed several factors that may help predict reproductive problems among males diagnosed with cancer before age 25.
Cancer type, age at diagnosis, and time period of diagnosis were all associated with the likelihood of paternity.
And although cancer survivors were less likely to reproduce and more likely to use assisted reproductive technology, their first offspring were no less healthy than the offspring of control subjects.
This research was published in the British Journal of Cancer.
The study began with all Norwegian men born between 1965 and 1985 (n=626,495). The researchers excluded men who emigrated or died before reaching fertile age, which left 2687 men who were diagnosed with cancer before age 25 and 607,668 cancer-free controls.
The most common cancers were testicular cancer (27%), CNS tumors (18%), lymphoma (15%), and leukemia (13%). Thirty percent of the cancer cases were diagnosed in childhood (0–14 years of age), 26% in adolescence (15–19 years), and 43% in young adulthood (20–24 years).
Nine percent (n=247) of cancer cases were diagnosed from 1965 through 1979, 50% (n=1346) from 1980 through 1994, and 41% (n=1094) from 1995 through 2007.
The cancer survivors were less likely to have children than controls, with a hazard ratio (HR) of 0.72.
The reduction in paternity was significant for survivors of leukemia (HR=0.78), lymphoma (HR=0.78), testicular cancer (HR=0.77), CNS tumors (HR=0.45), bone tumors (HR=0.69), sympathetic nervous system tumors (HR=0.50), and retinoblastoma (HR=0.52).
The reduction in paternity was also more pronounced for cancer patients diagnosed before 1995. The HR was 0.61 for those diagnosed from 1965 through 1979 and 0.66 for those diagnosed from 1980 through 1994.
Patients who were diagnosed before age 15 were less likely to reproduce as well, with an HR of 0.59.
“These finds are important for male cancer survivors, seeing as we can identify groups at risk of having reproduction problems,” said study author Maria Winther Gunnes, a PhD candidate at the University of Bergen in Norway.
Another finding was that male cancer survivors were more likely than controls to have pregnancies resulting from assisted reproductive technology. The relative risk was 3.32.
When assessed by cancer type, the relative risk was 2.29 for leukemia, 3.79 for lymphoma, 2.41 for CNS tumors, 5.71 for sympathetic nervous system tumors, 2.20 for renal tumors, 4.77 for bone tumors, 1.32 for soft tissue sarcomas, 3.70 for testicular cancer, 4.36 for thyroid carcinoma, and 0.45 for malignant melanoma.
There was no increased risk among the first offspring of cancer survivors for perinatal death, congenital malformations, being small for gestational age, low birth weight, or preterm birth.
“It is important to be able to assure young male cancer survivors that their illness and treatment will not have a negative impact on their own children,” Gunnes said. ![]()

A study of Norwegian men has revealed several factors that may help predict reproductive problems among males diagnosed with cancer before age 25.
Cancer type, age at diagnosis, and time period of diagnosis were all associated with the likelihood of paternity.
And although cancer survivors were less likely to reproduce and more likely to use assisted reproductive technology, their first offspring were no less healthy than the offspring of control subjects.
This research was published in the British Journal of Cancer.
The study began with all Norwegian men born between 1965 and 1985 (n=626,495). The researchers excluded men who emigrated or died before reaching fertile age, which left 2687 men who were diagnosed with cancer before age 25 and 607,668 cancer-free controls.
The most common cancers were testicular cancer (27%), CNS tumors (18%), lymphoma (15%), and leukemia (13%). Thirty percent of the cancer cases were diagnosed in childhood (0–14 years of age), 26% in adolescence (15–19 years), and 43% in young adulthood (20–24 years).
Nine percent (n=247) of cancer cases were diagnosed from 1965 through 1979, 50% (n=1346) from 1980 through 1994, and 41% (n=1094) from 1995 through 2007.
The cancer survivors were less likely to have children than controls, with a hazard ratio (HR) of 0.72.
The reduction in paternity was significant for survivors of leukemia (HR=0.78), lymphoma (HR=0.78), testicular cancer (HR=0.77), CNS tumors (HR=0.45), bone tumors (HR=0.69), sympathetic nervous system tumors (HR=0.50), and retinoblastoma (HR=0.52).
The reduction in paternity was also more pronounced for cancer patients diagnosed before 1995. The HR was 0.61 for those diagnosed from 1965 through 1979 and 0.66 for those diagnosed from 1980 through 1994.
Patients who were diagnosed before age 15 were less likely to reproduce as well, with an HR of 0.59.
“These finds are important for male cancer survivors, seeing as we can identify groups at risk of having reproduction problems,” said study author Maria Winther Gunnes, a PhD candidate at the University of Bergen in Norway.
Another finding was that male cancer survivors were more likely than controls to have pregnancies resulting from assisted reproductive technology. The relative risk was 3.32.
When assessed by cancer type, the relative risk was 2.29 for leukemia, 3.79 for lymphoma, 2.41 for CNS tumors, 5.71 for sympathetic nervous system tumors, 2.20 for renal tumors, 4.77 for bone tumors, 1.32 for soft tissue sarcomas, 3.70 for testicular cancer, 4.36 for thyroid carcinoma, and 0.45 for malignant melanoma.
There was no increased risk among the first offspring of cancer survivors for perinatal death, congenital malformations, being small for gestational age, low birth weight, or preterm birth.
“It is important to be able to assure young male cancer survivors that their illness and treatment will not have a negative impact on their own children,” Gunnes said. ![]()
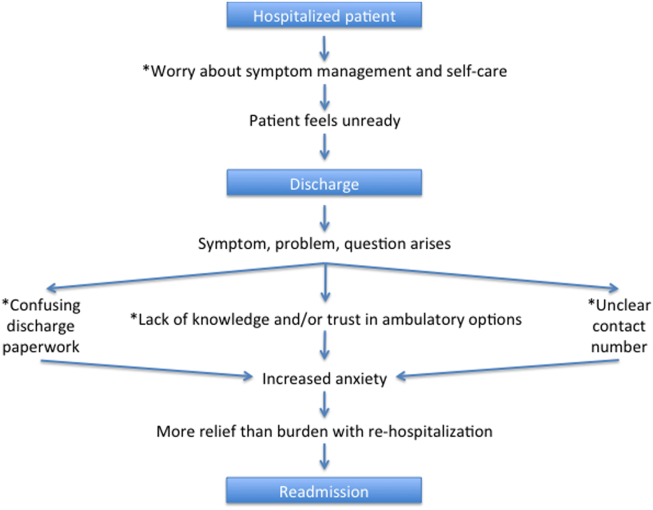
A Patient's Perspective on Readmissions
Years into the national discourse on reducing readmissions, hospitals and providers are still struggling with how to sustainably reduce 30‐day readmissions.[1] All‐cause hospital readmission rates for Medicare benificiaries averaged 19% from 2007 through 2011 and showed only a modest improvement to 18.4% in 2012.[2] A review of 43 studies in 2011 concluded that no single intervention was reliably associated with reducing readmission rates.[3] However, although no institution has found a magic bullet for reducing readmissions, progress has been made. A 2014 meta‐analysis of randomized trials aimed at preventing 30‐day readmissions found that overall readmission interventions are effective, and that the most successful interventions are more complex in nature and focus on empowering patients to engage in self‐care after discharge.[4] Readmission reduction efforts for patients with specific diagnoses have also made gains. Among patients with heart failure, for instance, higher rates of early outpatient follow‐up and care‐transition interventions for high‐risk patients have been shown to reduce 30‐day readmissions.[5, 6]
An emerging, yet still underexplored, area in readmissions is the importance of evaluating patient perspectives. The patient has intimate knowledge of the circumstances surrounding their readmission and can be a valuable resource. This is particularly true given evidence that patient perspectives do not always align with those of providers.[7, 8] Coleman's Care Transitions Intervention was one of the earliest care‐transition models demonstrating value in engaging patients to become actively involved in their care.[9] Since then, others have begun to analyze transitions of care from the patient perspective, identifying patient‐reported needs in anticipation of discharge and after they are home.[10, 11, 12, 13, 14] However, still only a few studies have endeavored to gain a thorough understanding of the readmitted patient perspective.[7, 15, 16] These studies have already identified important issues such as lack of patient readiness for discharge and the need for additional advanced care planning and caregiver resources. A few smaller studies have interviewed readmitted patients with specific diagnoses and have also shed light on disease‐specific issues.[17, 18, 19, 20] Outside the field of readmissions, improving patient‐centered communication has been shown to reduce expenditures on diagnostic tests,[21, 22] increase adherence to treatment,[23] and improve health outcomes.[24, 25] It is time for us to incorporate the patient voice into all areas of care.
In 2014, our group published the results of a study aimed at understanding the patient perspective surrounding readmissions. In this study, 27% of patients believed their readmission could have been prevented. This opinion was associated with not feeling ready for discharge, not having a follow‐up appointment scheduled, and poor satisfaction with the discharging team.[7] A key observation in these initial interviews was that patients often expressed sentiments of relief rather than frustration when they returned to the hospital. With the results of this previous study in mind, we designed a more comprehensive evaluation to investigate why patients felt unprepared for discharge, explore reasons for and attitudes surrounding readmissions, and identify patient‐centered interventions that could prevent future readmissions.
METHODS
Study Design and Recruitment
We designed the study as an in‐person survey of readmitted patients. Over a 7‐month period (February 11, 2014September 8, 2014), we identified all patients readmitted within 30 days to general medicine and cardiology services through daily queries from the electronic health record. The study took place in a 540‐bed tertiary academic medical center, as well as a 266‐bed affiliated community hospital. We reviewed the discharge summary from the index admission and the history and physical documentation from the readmission for exclusion criteria. Patients were excluded if they were: (1) readmitted to the intensive care unit, (2) had a planned readmission, (3) received an organ transplant in the preceding 3 months, (4) did not speak English, or (5) had a physical or mental incapacity preventing interview and no family member or caregiver was available to interview.
Patient Interviews
Five trained study volunteers approached all eligible patients for an interview starting the day after the patient was readmitted. Prior to the start of the interview, we obtained verbal consent from all patients. Interviews typically lasted 10 to 30 minutes in the patient's hospital room. Caregivers and/or family members were allowed to respond to interview questions if the patient granted them permission or if the patient was unable to participate. The interviewers were not part of the patient's medical team and the patients could refuse the interview at any time. According to the University of California Los Angeles (UCLA) Institutional Review Board, this work met criteria for quality‐improvement activities and was deemed to be exempt.
The survey was comprised of 24 questions addressing causes, preventability, and attitudes toward readmissions, readiness for discharge, quality of the discharge process, outpatient resources, and follow‐up care (see Supporting Information in the online version of this article). These areas of focus were chosen based on a pilot study of 98 patient interviews in which these topics emerged as worthy of further investigation.[7] With regard to patient readiness for discharge, we investigated correlations between patient readiness and symptom resolution, pain control, discharge location, level of support at home, and concerns about independent self‐care after discharge.
Data Analysis
We administered the surveys, collected and managed the data using REDCap (Research Electronic Data Capture) hosted at UCLA.[26] We collected demographic data, including race, ethnicity, and insurance status retrospectively though automated chart abstraction.
We summarized descriptive characteristics by mean and standard deviation (SD) for continuous variables (except for length of stay, which was summarized by median and range) and by proportions for categorical variables. To compare demographic variables between interviewed participants and those not interviewed (not available, not approached, refused, or excluded) we used Pearson 2 tests and Fisher exact tests for categorical variables and Student t tests for the only continuous variable, age. In evaluating patient readiness for discharge, we divided patients into groups of ready and not ready as determined by interview responses, then performed Pearson 2 tests and Fisher exact tests where appropriate.
For comparing the extent of burden and relief patients endorsed upon being readmitted, we subtracted the burden score (110) from the relief score (110) for each patient, resulting in a net relief score. We then performed a 1‐sample t test to determine whether the net relief was significantly different from 0. A P value of<0.05 was considered to be statistically significant. All statistical analyses were performed using R version 3.0.2 (
RESULTS
Patient Characteristics
Eight hundred nineteen patients were readmitted to general medicine and cardiology services over the 7‐month study period at both institutions. Two hundred thirty‐five patients (29%) were excluded based on the predetermined exclusion criteria, and 105 patients (13%) were not approached for interview due to time constraints. Of the 479 eligible patients approached for interview, 164 patients (34%) could not be interviewed because they were unavailable, and 85 patients (18%) refused. We interviewed 230 patients (48%). We conducted 115 interviews at our academic medical center and 115 at our community affiliate. The only significant demographic difference between interviewed and not‐interviewed patients was race (P=0.004).
Interviewed patients had a mean (SD) age of 63 (SD 20) years, and 45% were male. Sixty‐three percent of interviewees were white, 21% black, 8% Asian, and 8% other. The index admission median length of stay was 4 days, and the average time between admission and readmission was 13 days (Table 1). Seventy‐nine percent of the interviews were performed directly with the patient, and 21% were conducted predominantly with the patient's caregiver.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean (SD) | 62.9 (20.2) |
| Female, n (%) | 127 (55.2) |
| Insurance status, n (%) | |
| Commercial | 36 (16.3) |
| Medi‐Cal/Medicaid | 31 (14.0) |
| Medicare | 123 (55.7) |
| Other | 5 (2.3) |
| UCLA managed care | 26 (11.8) |
| Missing | 9 |
| Race, n (%) | |
| Asian | 18 (7.9) |
| Black or African American | 48 (21.1) |
| Other/refused | 19 (8.3) |
| White or Caucasian | 143 (62.7) |
| Missing | 2 |
| Index length of stay, d, median (maximum, minimum) | 4 (1, 49) |
| Time between discharge and readmission, d, mean (SD) | 13 (9) |
| Discharge location following index admission, n (%) | |
| Home | 202 (88.2) |
| Skilled nursing facility | 3 (1.3) |
| Acute rehab facility | 17 (7.4) |
| Assisted living facility | 2 (0.9) |
| Other | 5 (2.2) |
| Missing | 1 |
Patient Readiness
Twenty‐eight percent of patients reported feeling unready for discharge from their index admission. Patients who felt that their readmission was preventable were significantly more likely to report feeling unready at the time of discharge compared to those who did not classify their readmission as preventable (53% vs 17%, P<0.01). Among patients who did not feel ready for discharge, over two‐thirds felt their symptoms were not adequately resolved. Conversely, among patients who did feel ready for discharge, only 8% felt their symptoms were not resolved (P<0.01). Patients who felt they were not ready for discharge were also significantly more likely to endorse poor pain control (43% vs 7%, P<0.01). The location of discharge (ie, home, rehab facility, or skilled nursing facility) and having someone to help take care of them at home did not significantly correlate with patient readiness. Over 80% of patients in both groups reported having someone to help at home, but patients who felt unready for discharge were significantly more likely to have concerns about taking care of themselves at home (54% vs 25%, P<0.001) (Table 2).
| All Participants, n=230 | Ready, n=164 | Not Ready, n=65 | P Value | |
|---|---|---|---|---|
| Symptoms were resolved enough to leave the hospital, n=227 | 170 (74.9%) | 149 (92.0%) | 21 (32.3%) | <0.01 |
| Felt pain was under control when left the hospital, n=229 | 190 (83.0%) | 153 (93.3%) | 37 (56.9%) | <0.01 |
| Discharged to home following index admission, n=229 | 202 (88.2%) | 146 (89.6%) | 56 (86.2%) | 0.62 |
| If discharged home, had someone at home able to help, n=202 | 178 (88.1%) | 132 (90.4%) | 46 (82.1%) | 0.17 |
| If discharged home, had concerns about being able to take of themselves at home or not being strong enough to go home, n=202 | 67 (33.2%) | 37 (25.3%) | 30 (53.6%) | <0.01 |
| Thought something could have been done to prevent them from coming back to the hospital, n=228 | 75 (32.9%) | 35 (21.6%) | 39 (60.0%) | <0.01 |
Discharge Instructions
Twenty‐nine percent of patients did not recall a physician talking to them about their discharge, and 35% did not remember receiving and reviewing the discharge paperwork. Of those who read the discharge paperwork, 23% noted difficulty identifying contact phone numbers, and 22% could not locate warning symptoms indicating when to seek medical attention. Patients were able to identify medications and follow‐up appointments on the discharge paperwork a majority of the time (92% and 85%, respectively).
Ambulatory Resources and Utilization
Patients were asked about their access to outpatient resources as well as their reason(s) for returning to the hospital. Eighty‐five percent of patients reported having a primary care doctor that they would feel comfortable calling if their symptoms worsened at home. Of the patients who indicated that they were given a contact number by their discharging team, only 56% contacted a doctor before returning to the emergency room. One‐third of patients reported knowing where to obtain urgent or same‐day care besides the emergency room. Among those who did report knowledge of same‐day care centers, 89% still chose not to utilize them.
Attitudes About Readmission
To investigate the patient experience with readmissions, patients were asked to rate the extent of the burden they felt upon returning to the hospital on a scale of 1 to 10, where 1 was no burden and 10 was extreme burden. Patients were also asked to evaluate the extent of relief they felt upon readmission using the same scale. On average, patients rated their sense of relief 1.8 points higher than their sense of burden upon readmission to the hospital (7.7 [SD 2.8] vs 5.9 [SD 3.4], P<0.001). The relief of readmission was rated as equal to or greater than the burden of readmission in 79% of cases. Lastly, patients' mean (SD) overall satisfaction with their medical care was 8.5 (SD 2.0) on a scale of 1 to 10, where 1 was the least satisfied and 10 was the most satisfied a patient could imagine.
DISCUSSION
This study performs a comprehensive evaluation of the patient perspective on 30‐day readmissions. Our previous work indicated that patients associate preventable readmissions with lack of preparedness at the time of discharge.[7] This study further evaluates the basis of this association. We found that nearly 1 in 3 readmitted patients did not feel ready to leave the hospital at the time of initial discharge. Feelings of inadequate symptom resolution and poor pain control appear to be major contributors to this sentiment. Furthermore, although 88% of patients endorse having a caretaker at home, patients with concerns about taking care of themselves are more likely to feel unready at discharge. Presumably, when healthcare providers discharge patients, they believe that the patient is ready to be discharged. However, our findings suggest that often patients do not agree, highlighting a gap between the beliefs of patients and those of healthcare providers. Creating patient‐centered education on symptom management and engaging patients in developing skills for independent self‐care may minimize this gap and allow patients to feel more prepared at discharge. Future research investigating provider opinions and the steps providers take when there is a disagreement over discharge readiness would also be useful.
One way to enhance education at the time of discharge is through improvements in printed discharge instructions. Jha et al. previously showed that chart documentation of providing discharge instructions does not correlate with patients reporting receiving discharge instructions.[27] Our study echoes this finding, with only 65% of the patients remembering receiving and reviewing the discharge paperwork. Horwitz et al. have also previously demonstrated poor comprehension of discharge planning and postdischarge care among patients discharged from an academic medical center.[28] Ensuring that all patients understand and retain their discharge instructions is an essential step in improving the patient experience and potentially decreasing readmissions. Our surveys have illuminated potential shortcomings in our own center's discharge instructions. Interventions aimed at clarifying critical pieces of information on the discharge paperwork, such as warning symptoms, contact phone numbers and follow‐up appointments, could be especially helpful.
After discharge, our findings suggest that only about half of patients will call a physician before returning to the hospital. Furthermore, there is limited knowledge and poor utilization of same‐day treatment centers besides the emergency room. In previous studies, Long et al. found that frequently readmitted patients self‐triage to the emergency room because they believe primary care clinics cannot treat acute illness.[11] Another study concluded that low‐income patients prefer hospital care to ambulatory care because of a greater sense of trust in inpatient care.[29]
Our patients' attitudes about readmission may also be different from those of providers. For patients, coming back to the hospital is not a significant burden, and satisfaction with their medical care remains high despite readmission. Additional research is needed to further explore the complex emotions patients have when coming back to the hospital and why patients may not be as upset with returning to the hospital as providers may expect. Ultimately, if patients continue to feel more comfortable being hospitalized, there are few incentives for patients to stay out of the hospital, and readmission rates will remain elevated.
Based on our survey results we have hypothesized a potential framework for studying readmissions from a patient‐centered approach (Figure 1). This figure is not meant to imply causality, but rather to highlight a potential journey from discharge to readmission for a patient who does not feel ready to go home. This schema principally applies to patients who are worried about symptom management and/or self‐care before discharge and may not apply to everyone. Each asterisk in this framework represents an area where an intervention could be designed to improve the patient experience and possibly reduce readmissions. Such interventions should be centered around increasing patient education about symptom management and self‐care at the time of discharge, improving printed discharge instructions, increasing patient awareness of outpatient resources, enhancing communication after discharge, and changing patients' attitudes about readmissions.
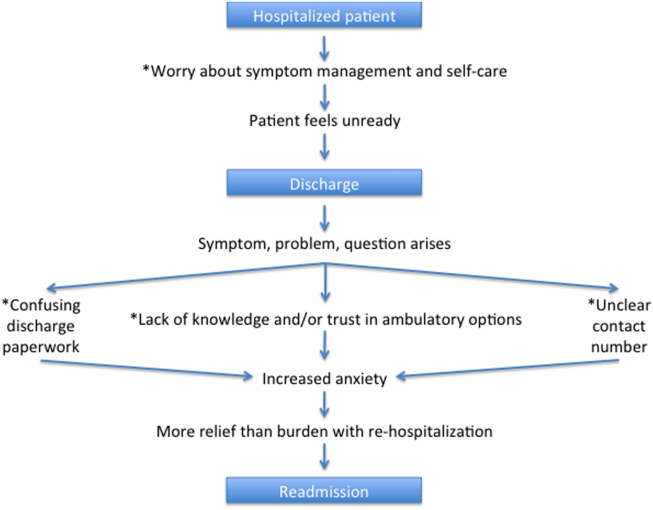
This study's limitations include that it is a single‐institution study focusing on patients admitted to a large academic medical center and its partner community hospital. Only English‐speaking patients were included, and thus our results may not be generalizable to other populations. All patients were interviewed at the time of readmission, potentially introducing recall bias regarding their prior discharge. For example, patients might be more likely to state they were not ready for discharge once they have been readmitted to the hospital. Lastly, because there are only a few prior studies interviewing readmitted patients, our survey instrument was not previously validated. Nevertheless, we believe this study offers a unique view on 30‐day readmissions from the patient perspective, with a focus on identifying areas for quality‐improvement interventions.
In conclusion, this study has enabled us to understand readmissions from a patient‐centered perspective. This perspective helps to challenge provider assumptions and gives much‐needed insight into the patient experience. For example, prior to surveying patients, one might assume that if a patient has a caregiver at home, they are unlikely to have concerns about taking care of themselves. We now know this is not the case. Similarly, we have discovered sections of our discharge paperwork that are confusing. Additionally, this study has revealed that patient attitudes regarding readmission can vary significantly from provider attitudes. By exploring the patient perspective and creating a new transition framework, we have identified specific target areas for interventions that would be meaningful to patients. As the nation continues to strive to identify sustainable solutions to reduce readmissions, the way to redesign care must always start and end with the patient.
Acknowledgements
The authors acknowledge Puneet Rana, James Haggerty‐Skeans, Jae Kim, Rhea Mathew, and Anna Do (UCLA volunteers) for helping to perform the patient interviews. We acknowledge Sandy Berry, MA (Senior Behavioral Scientist at RAND Corporation) for her help in reviewing our patient interview script. Additionally Anna Dermenchyan, RN, BSN (Senior Clinical Quality Specialist in the Department of Medicine at UCLA) provided significant administrative support.
Disclosures: This project was supported by a Patient Experience Grant from the Beryl Institute awarded to Jessica Howard‐Anderson, Sarah Lonowski, Ashley Busuttil, and Nasim Afsar‐manesh. Dr. Howard‐Anderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All coauthors have seen and agree with the contents of the article. The article is not under review by any other publication. An earlier version of this work was written as a research report (not peer reviewed) for the Beryl Institute (available at:
- , . What will it take to move the needle on hospital readmissions? Am J Med Qual. 2013;29(4):357–359.
- , , , , , . Medicare readmission rates showed meaningful decline in 2012. Medicare Medicaid Res Rev. 2013;3(2):E1–E12.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , , et al. Allocating scarce resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , , , . Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Comparing perspectives of patients, caregivers, and clinicians on heart failure management [published online October 23, 2015]. J Card Fail. doi: 10.1016/j.cardfail.2015.10.011.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , . Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization? J Hosp Med. 2007;2(5):297–304.
- , , . Reasons for readmission in an underserved high‐risk population: a qualitative analysis of a series of inpatient interviews. BMJ Open. 2013;3(9):e003212.
- , , , , , . Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62(8):1556–1561.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , . Reasons for readmission in heart failure: perspectives of patients, caregivers, cardiologists, and heart failure nurses. Heart Lung. 2009;38(5):427–434.
- , , , et al. Patient‐identified factors related to heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2013;6(2):171–177.
- , , , , . Early readmission among patients with diabetes: a qualitative assessment of contributing factors. J Diabetes Complications. 2014;28(6):869–873.
- , , , , . “Because I was sick”: seriously ill veterans' perspectives on reason for 30‐day readmissions. J Am Geriatr Soc. 2015;63(3):537–542.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Patient‐centered communication and diagnostic testing. Ann Fam Med. 2005;3(5):415–421.
- , . Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826–834.
- , , , et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90.
- , , , , . Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–457.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , , . Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637–2645.
- , , , et al. Quality of discharge practices and patient understanding at an academic medical center. JAMA Intern Med. 2013;173(18):1715–1722
- , , , , , . Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
Years into the national discourse on reducing readmissions, hospitals and providers are still struggling with how to sustainably reduce 30‐day readmissions.[1] All‐cause hospital readmission rates for Medicare benificiaries averaged 19% from 2007 through 2011 and showed only a modest improvement to 18.4% in 2012.[2] A review of 43 studies in 2011 concluded that no single intervention was reliably associated with reducing readmission rates.[3] However, although no institution has found a magic bullet for reducing readmissions, progress has been made. A 2014 meta‐analysis of randomized trials aimed at preventing 30‐day readmissions found that overall readmission interventions are effective, and that the most successful interventions are more complex in nature and focus on empowering patients to engage in self‐care after discharge.[4] Readmission reduction efforts for patients with specific diagnoses have also made gains. Among patients with heart failure, for instance, higher rates of early outpatient follow‐up and care‐transition interventions for high‐risk patients have been shown to reduce 30‐day readmissions.[5, 6]
An emerging, yet still underexplored, area in readmissions is the importance of evaluating patient perspectives. The patient has intimate knowledge of the circumstances surrounding their readmission and can be a valuable resource. This is particularly true given evidence that patient perspectives do not always align with those of providers.[7, 8] Coleman's Care Transitions Intervention was one of the earliest care‐transition models demonstrating value in engaging patients to become actively involved in their care.[9] Since then, others have begun to analyze transitions of care from the patient perspective, identifying patient‐reported needs in anticipation of discharge and after they are home.[10, 11, 12, 13, 14] However, still only a few studies have endeavored to gain a thorough understanding of the readmitted patient perspective.[7, 15, 16] These studies have already identified important issues such as lack of patient readiness for discharge and the need for additional advanced care planning and caregiver resources. A few smaller studies have interviewed readmitted patients with specific diagnoses and have also shed light on disease‐specific issues.[17, 18, 19, 20] Outside the field of readmissions, improving patient‐centered communication has been shown to reduce expenditures on diagnostic tests,[21, 22] increase adherence to treatment,[23] and improve health outcomes.[24, 25] It is time for us to incorporate the patient voice into all areas of care.
In 2014, our group published the results of a study aimed at understanding the patient perspective surrounding readmissions. In this study, 27% of patients believed their readmission could have been prevented. This opinion was associated with not feeling ready for discharge, not having a follow‐up appointment scheduled, and poor satisfaction with the discharging team.[7] A key observation in these initial interviews was that patients often expressed sentiments of relief rather than frustration when they returned to the hospital. With the results of this previous study in mind, we designed a more comprehensive evaluation to investigate why patients felt unprepared for discharge, explore reasons for and attitudes surrounding readmissions, and identify patient‐centered interventions that could prevent future readmissions.
METHODS
Study Design and Recruitment
We designed the study as an in‐person survey of readmitted patients. Over a 7‐month period (February 11, 2014September 8, 2014), we identified all patients readmitted within 30 days to general medicine and cardiology services through daily queries from the electronic health record. The study took place in a 540‐bed tertiary academic medical center, as well as a 266‐bed affiliated community hospital. We reviewed the discharge summary from the index admission and the history and physical documentation from the readmission for exclusion criteria. Patients were excluded if they were: (1) readmitted to the intensive care unit, (2) had a planned readmission, (3) received an organ transplant in the preceding 3 months, (4) did not speak English, or (5) had a physical or mental incapacity preventing interview and no family member or caregiver was available to interview.
Patient Interviews
Five trained study volunteers approached all eligible patients for an interview starting the day after the patient was readmitted. Prior to the start of the interview, we obtained verbal consent from all patients. Interviews typically lasted 10 to 30 minutes in the patient's hospital room. Caregivers and/or family members were allowed to respond to interview questions if the patient granted them permission or if the patient was unable to participate. The interviewers were not part of the patient's medical team and the patients could refuse the interview at any time. According to the University of California Los Angeles (UCLA) Institutional Review Board, this work met criteria for quality‐improvement activities and was deemed to be exempt.
The survey was comprised of 24 questions addressing causes, preventability, and attitudes toward readmissions, readiness for discharge, quality of the discharge process, outpatient resources, and follow‐up care (see Supporting Information in the online version of this article). These areas of focus were chosen based on a pilot study of 98 patient interviews in which these topics emerged as worthy of further investigation.[7] With regard to patient readiness for discharge, we investigated correlations between patient readiness and symptom resolution, pain control, discharge location, level of support at home, and concerns about independent self‐care after discharge.
Data Analysis
We administered the surveys, collected and managed the data using REDCap (Research Electronic Data Capture) hosted at UCLA.[26] We collected demographic data, including race, ethnicity, and insurance status retrospectively though automated chart abstraction.
We summarized descriptive characteristics by mean and standard deviation (SD) for continuous variables (except for length of stay, which was summarized by median and range) and by proportions for categorical variables. To compare demographic variables between interviewed participants and those not interviewed (not available, not approached, refused, or excluded) we used Pearson 2 tests and Fisher exact tests for categorical variables and Student t tests for the only continuous variable, age. In evaluating patient readiness for discharge, we divided patients into groups of ready and not ready as determined by interview responses, then performed Pearson 2 tests and Fisher exact tests where appropriate.
For comparing the extent of burden and relief patients endorsed upon being readmitted, we subtracted the burden score (110) from the relief score (110) for each patient, resulting in a net relief score. We then performed a 1‐sample t test to determine whether the net relief was significantly different from 0. A P value of<0.05 was considered to be statistically significant. All statistical analyses were performed using R version 3.0.2 (
RESULTS
Patient Characteristics
Eight hundred nineteen patients were readmitted to general medicine and cardiology services over the 7‐month study period at both institutions. Two hundred thirty‐five patients (29%) were excluded based on the predetermined exclusion criteria, and 105 patients (13%) were not approached for interview due to time constraints. Of the 479 eligible patients approached for interview, 164 patients (34%) could not be interviewed because they were unavailable, and 85 patients (18%) refused. We interviewed 230 patients (48%). We conducted 115 interviews at our academic medical center and 115 at our community affiliate. The only significant demographic difference between interviewed and not‐interviewed patients was race (P=0.004).
Interviewed patients had a mean (SD) age of 63 (SD 20) years, and 45% were male. Sixty‐three percent of interviewees were white, 21% black, 8% Asian, and 8% other. The index admission median length of stay was 4 days, and the average time between admission and readmission was 13 days (Table 1). Seventy‐nine percent of the interviews were performed directly with the patient, and 21% were conducted predominantly with the patient's caregiver.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean (SD) | 62.9 (20.2) |
| Female, n (%) | 127 (55.2) |
| Insurance status, n (%) | |
| Commercial | 36 (16.3) |
| Medi‐Cal/Medicaid | 31 (14.0) |
| Medicare | 123 (55.7) |
| Other | 5 (2.3) |
| UCLA managed care | 26 (11.8) |
| Missing | 9 |
| Race, n (%) | |
| Asian | 18 (7.9) |
| Black or African American | 48 (21.1) |
| Other/refused | 19 (8.3) |
| White or Caucasian | 143 (62.7) |
| Missing | 2 |
| Index length of stay, d, median (maximum, minimum) | 4 (1, 49) |
| Time between discharge and readmission, d, mean (SD) | 13 (9) |
| Discharge location following index admission, n (%) | |
| Home | 202 (88.2) |
| Skilled nursing facility | 3 (1.3) |
| Acute rehab facility | 17 (7.4) |
| Assisted living facility | 2 (0.9) |
| Other | 5 (2.2) |
| Missing | 1 |
Patient Readiness
Twenty‐eight percent of patients reported feeling unready for discharge from their index admission. Patients who felt that their readmission was preventable were significantly more likely to report feeling unready at the time of discharge compared to those who did not classify their readmission as preventable (53% vs 17%, P<0.01). Among patients who did not feel ready for discharge, over two‐thirds felt their symptoms were not adequately resolved. Conversely, among patients who did feel ready for discharge, only 8% felt their symptoms were not resolved (P<0.01). Patients who felt they were not ready for discharge were also significantly more likely to endorse poor pain control (43% vs 7%, P<0.01). The location of discharge (ie, home, rehab facility, or skilled nursing facility) and having someone to help take care of them at home did not significantly correlate with patient readiness. Over 80% of patients in both groups reported having someone to help at home, but patients who felt unready for discharge were significantly more likely to have concerns about taking care of themselves at home (54% vs 25%, P<0.001) (Table 2).
| All Participants, n=230 | Ready, n=164 | Not Ready, n=65 | P Value | |
|---|---|---|---|---|
| Symptoms were resolved enough to leave the hospital, n=227 | 170 (74.9%) | 149 (92.0%) | 21 (32.3%) | <0.01 |
| Felt pain was under control when left the hospital, n=229 | 190 (83.0%) | 153 (93.3%) | 37 (56.9%) | <0.01 |
| Discharged to home following index admission, n=229 | 202 (88.2%) | 146 (89.6%) | 56 (86.2%) | 0.62 |
| If discharged home, had someone at home able to help, n=202 | 178 (88.1%) | 132 (90.4%) | 46 (82.1%) | 0.17 |
| If discharged home, had concerns about being able to take of themselves at home or not being strong enough to go home, n=202 | 67 (33.2%) | 37 (25.3%) | 30 (53.6%) | <0.01 |
| Thought something could have been done to prevent them from coming back to the hospital, n=228 | 75 (32.9%) | 35 (21.6%) | 39 (60.0%) | <0.01 |
Discharge Instructions
Twenty‐nine percent of patients did not recall a physician talking to them about their discharge, and 35% did not remember receiving and reviewing the discharge paperwork. Of those who read the discharge paperwork, 23% noted difficulty identifying contact phone numbers, and 22% could not locate warning symptoms indicating when to seek medical attention. Patients were able to identify medications and follow‐up appointments on the discharge paperwork a majority of the time (92% and 85%, respectively).
Ambulatory Resources and Utilization
Patients were asked about their access to outpatient resources as well as their reason(s) for returning to the hospital. Eighty‐five percent of patients reported having a primary care doctor that they would feel comfortable calling if their symptoms worsened at home. Of the patients who indicated that they were given a contact number by their discharging team, only 56% contacted a doctor before returning to the emergency room. One‐third of patients reported knowing where to obtain urgent or same‐day care besides the emergency room. Among those who did report knowledge of same‐day care centers, 89% still chose not to utilize them.
Attitudes About Readmission
To investigate the patient experience with readmissions, patients were asked to rate the extent of the burden they felt upon returning to the hospital on a scale of 1 to 10, where 1 was no burden and 10 was extreme burden. Patients were also asked to evaluate the extent of relief they felt upon readmission using the same scale. On average, patients rated their sense of relief 1.8 points higher than their sense of burden upon readmission to the hospital (7.7 [SD 2.8] vs 5.9 [SD 3.4], P<0.001). The relief of readmission was rated as equal to or greater than the burden of readmission in 79% of cases. Lastly, patients' mean (SD) overall satisfaction with their medical care was 8.5 (SD 2.0) on a scale of 1 to 10, where 1 was the least satisfied and 10 was the most satisfied a patient could imagine.
DISCUSSION
This study performs a comprehensive evaluation of the patient perspective on 30‐day readmissions. Our previous work indicated that patients associate preventable readmissions with lack of preparedness at the time of discharge.[7] This study further evaluates the basis of this association. We found that nearly 1 in 3 readmitted patients did not feel ready to leave the hospital at the time of initial discharge. Feelings of inadequate symptom resolution and poor pain control appear to be major contributors to this sentiment. Furthermore, although 88% of patients endorse having a caretaker at home, patients with concerns about taking care of themselves are more likely to feel unready at discharge. Presumably, when healthcare providers discharge patients, they believe that the patient is ready to be discharged. However, our findings suggest that often patients do not agree, highlighting a gap between the beliefs of patients and those of healthcare providers. Creating patient‐centered education on symptom management and engaging patients in developing skills for independent self‐care may minimize this gap and allow patients to feel more prepared at discharge. Future research investigating provider opinions and the steps providers take when there is a disagreement over discharge readiness would also be useful.
One way to enhance education at the time of discharge is through improvements in printed discharge instructions. Jha et al. previously showed that chart documentation of providing discharge instructions does not correlate with patients reporting receiving discharge instructions.[27] Our study echoes this finding, with only 65% of the patients remembering receiving and reviewing the discharge paperwork. Horwitz et al. have also previously demonstrated poor comprehension of discharge planning and postdischarge care among patients discharged from an academic medical center.[28] Ensuring that all patients understand and retain their discharge instructions is an essential step in improving the patient experience and potentially decreasing readmissions. Our surveys have illuminated potential shortcomings in our own center's discharge instructions. Interventions aimed at clarifying critical pieces of information on the discharge paperwork, such as warning symptoms, contact phone numbers and follow‐up appointments, could be especially helpful.
After discharge, our findings suggest that only about half of patients will call a physician before returning to the hospital. Furthermore, there is limited knowledge and poor utilization of same‐day treatment centers besides the emergency room. In previous studies, Long et al. found that frequently readmitted patients self‐triage to the emergency room because they believe primary care clinics cannot treat acute illness.[11] Another study concluded that low‐income patients prefer hospital care to ambulatory care because of a greater sense of trust in inpatient care.[29]
Our patients' attitudes about readmission may also be different from those of providers. For patients, coming back to the hospital is not a significant burden, and satisfaction with their medical care remains high despite readmission. Additional research is needed to further explore the complex emotions patients have when coming back to the hospital and why patients may not be as upset with returning to the hospital as providers may expect. Ultimately, if patients continue to feel more comfortable being hospitalized, there are few incentives for patients to stay out of the hospital, and readmission rates will remain elevated.
Based on our survey results we have hypothesized a potential framework for studying readmissions from a patient‐centered approach (Figure 1). This figure is not meant to imply causality, but rather to highlight a potential journey from discharge to readmission for a patient who does not feel ready to go home. This schema principally applies to patients who are worried about symptom management and/or self‐care before discharge and may not apply to everyone. Each asterisk in this framework represents an area where an intervention could be designed to improve the patient experience and possibly reduce readmissions. Such interventions should be centered around increasing patient education about symptom management and self‐care at the time of discharge, improving printed discharge instructions, increasing patient awareness of outpatient resources, enhancing communication after discharge, and changing patients' attitudes about readmissions.

This study's limitations include that it is a single‐institution study focusing on patients admitted to a large academic medical center and its partner community hospital. Only English‐speaking patients were included, and thus our results may not be generalizable to other populations. All patients were interviewed at the time of readmission, potentially introducing recall bias regarding their prior discharge. For example, patients might be more likely to state they were not ready for discharge once they have been readmitted to the hospital. Lastly, because there are only a few prior studies interviewing readmitted patients, our survey instrument was not previously validated. Nevertheless, we believe this study offers a unique view on 30‐day readmissions from the patient perspective, with a focus on identifying areas for quality‐improvement interventions.
In conclusion, this study has enabled us to understand readmissions from a patient‐centered perspective. This perspective helps to challenge provider assumptions and gives much‐needed insight into the patient experience. For example, prior to surveying patients, one might assume that if a patient has a caregiver at home, they are unlikely to have concerns about taking care of themselves. We now know this is not the case. Similarly, we have discovered sections of our discharge paperwork that are confusing. Additionally, this study has revealed that patient attitudes regarding readmission can vary significantly from provider attitudes. By exploring the patient perspective and creating a new transition framework, we have identified specific target areas for interventions that would be meaningful to patients. As the nation continues to strive to identify sustainable solutions to reduce readmissions, the way to redesign care must always start and end with the patient.
Acknowledgements
The authors acknowledge Puneet Rana, James Haggerty‐Skeans, Jae Kim, Rhea Mathew, and Anna Do (UCLA volunteers) for helping to perform the patient interviews. We acknowledge Sandy Berry, MA (Senior Behavioral Scientist at RAND Corporation) for her help in reviewing our patient interview script. Additionally Anna Dermenchyan, RN, BSN (Senior Clinical Quality Specialist in the Department of Medicine at UCLA) provided significant administrative support.
Disclosures: This project was supported by a Patient Experience Grant from the Beryl Institute awarded to Jessica Howard‐Anderson, Sarah Lonowski, Ashley Busuttil, and Nasim Afsar‐manesh. Dr. Howard‐Anderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All coauthors have seen and agree with the contents of the article. The article is not under review by any other publication. An earlier version of this work was written as a research report (not peer reviewed) for the Beryl Institute (available at:
Years into the national discourse on reducing readmissions, hospitals and providers are still struggling with how to sustainably reduce 30‐day readmissions.[1] All‐cause hospital readmission rates for Medicare benificiaries averaged 19% from 2007 through 2011 and showed only a modest improvement to 18.4% in 2012.[2] A review of 43 studies in 2011 concluded that no single intervention was reliably associated with reducing readmission rates.[3] However, although no institution has found a magic bullet for reducing readmissions, progress has been made. A 2014 meta‐analysis of randomized trials aimed at preventing 30‐day readmissions found that overall readmission interventions are effective, and that the most successful interventions are more complex in nature and focus on empowering patients to engage in self‐care after discharge.[4] Readmission reduction efforts for patients with specific diagnoses have also made gains. Among patients with heart failure, for instance, higher rates of early outpatient follow‐up and care‐transition interventions for high‐risk patients have been shown to reduce 30‐day readmissions.[5, 6]
An emerging, yet still underexplored, area in readmissions is the importance of evaluating patient perspectives. The patient has intimate knowledge of the circumstances surrounding their readmission and can be a valuable resource. This is particularly true given evidence that patient perspectives do not always align with those of providers.[7, 8] Coleman's Care Transitions Intervention was one of the earliest care‐transition models demonstrating value in engaging patients to become actively involved in their care.[9] Since then, others have begun to analyze transitions of care from the patient perspective, identifying patient‐reported needs in anticipation of discharge and after they are home.[10, 11, 12, 13, 14] However, still only a few studies have endeavored to gain a thorough understanding of the readmitted patient perspective.[7, 15, 16] These studies have already identified important issues such as lack of patient readiness for discharge and the need for additional advanced care planning and caregiver resources. A few smaller studies have interviewed readmitted patients with specific diagnoses and have also shed light on disease‐specific issues.[17, 18, 19, 20] Outside the field of readmissions, improving patient‐centered communication has been shown to reduce expenditures on diagnostic tests,[21, 22] increase adherence to treatment,[23] and improve health outcomes.[24, 25] It is time for us to incorporate the patient voice into all areas of care.
In 2014, our group published the results of a study aimed at understanding the patient perspective surrounding readmissions. In this study, 27% of patients believed their readmission could have been prevented. This opinion was associated with not feeling ready for discharge, not having a follow‐up appointment scheduled, and poor satisfaction with the discharging team.[7] A key observation in these initial interviews was that patients often expressed sentiments of relief rather than frustration when they returned to the hospital. With the results of this previous study in mind, we designed a more comprehensive evaluation to investigate why patients felt unprepared for discharge, explore reasons for and attitudes surrounding readmissions, and identify patient‐centered interventions that could prevent future readmissions.
METHODS
Study Design and Recruitment
We designed the study as an in‐person survey of readmitted patients. Over a 7‐month period (February 11, 2014September 8, 2014), we identified all patients readmitted within 30 days to general medicine and cardiology services through daily queries from the electronic health record. The study took place in a 540‐bed tertiary academic medical center, as well as a 266‐bed affiliated community hospital. We reviewed the discharge summary from the index admission and the history and physical documentation from the readmission for exclusion criteria. Patients were excluded if they were: (1) readmitted to the intensive care unit, (2) had a planned readmission, (3) received an organ transplant in the preceding 3 months, (4) did not speak English, or (5) had a physical or mental incapacity preventing interview and no family member or caregiver was available to interview.
Patient Interviews
Five trained study volunteers approached all eligible patients for an interview starting the day after the patient was readmitted. Prior to the start of the interview, we obtained verbal consent from all patients. Interviews typically lasted 10 to 30 minutes in the patient's hospital room. Caregivers and/or family members were allowed to respond to interview questions if the patient granted them permission or if the patient was unable to participate. The interviewers were not part of the patient's medical team and the patients could refuse the interview at any time. According to the University of California Los Angeles (UCLA) Institutional Review Board, this work met criteria for quality‐improvement activities and was deemed to be exempt.
The survey was comprised of 24 questions addressing causes, preventability, and attitudes toward readmissions, readiness for discharge, quality of the discharge process, outpatient resources, and follow‐up care (see Supporting Information in the online version of this article). These areas of focus were chosen based on a pilot study of 98 patient interviews in which these topics emerged as worthy of further investigation.[7] With regard to patient readiness for discharge, we investigated correlations between patient readiness and symptom resolution, pain control, discharge location, level of support at home, and concerns about independent self‐care after discharge.
Data Analysis
We administered the surveys, collected and managed the data using REDCap (Research Electronic Data Capture) hosted at UCLA.[26] We collected demographic data, including race, ethnicity, and insurance status retrospectively though automated chart abstraction.
We summarized descriptive characteristics by mean and standard deviation (SD) for continuous variables (except for length of stay, which was summarized by median and range) and by proportions for categorical variables. To compare demographic variables between interviewed participants and those not interviewed (not available, not approached, refused, or excluded) we used Pearson 2 tests and Fisher exact tests for categorical variables and Student t tests for the only continuous variable, age. In evaluating patient readiness for discharge, we divided patients into groups of ready and not ready as determined by interview responses, then performed Pearson 2 tests and Fisher exact tests where appropriate.
For comparing the extent of burden and relief patients endorsed upon being readmitted, we subtracted the burden score (110) from the relief score (110) for each patient, resulting in a net relief score. We then performed a 1‐sample t test to determine whether the net relief was significantly different from 0. A P value of<0.05 was considered to be statistically significant. All statistical analyses were performed using R version 3.0.2 (
RESULTS
Patient Characteristics
Eight hundred nineteen patients were readmitted to general medicine and cardiology services over the 7‐month study period at both institutions. Two hundred thirty‐five patients (29%) were excluded based on the predetermined exclusion criteria, and 105 patients (13%) were not approached for interview due to time constraints. Of the 479 eligible patients approached for interview, 164 patients (34%) could not be interviewed because they were unavailable, and 85 patients (18%) refused. We interviewed 230 patients (48%). We conducted 115 interviews at our academic medical center and 115 at our community affiliate. The only significant demographic difference between interviewed and not‐interviewed patients was race (P=0.004).
Interviewed patients had a mean (SD) age of 63 (SD 20) years, and 45% were male. Sixty‐three percent of interviewees were white, 21% black, 8% Asian, and 8% other. The index admission median length of stay was 4 days, and the average time between admission and readmission was 13 days (Table 1). Seventy‐nine percent of the interviews were performed directly with the patient, and 21% were conducted predominantly with the patient's caregiver.
| Characteristic | Value |
|---|---|
| |
| Age, y, mean (SD) | 62.9 (20.2) |
| Female, n (%) | 127 (55.2) |
| Insurance status, n (%) | |
| Commercial | 36 (16.3) |
| Medi‐Cal/Medicaid | 31 (14.0) |
| Medicare | 123 (55.7) |
| Other | 5 (2.3) |
| UCLA managed care | 26 (11.8) |
| Missing | 9 |
| Race, n (%) | |
| Asian | 18 (7.9) |
| Black or African American | 48 (21.1) |
| Other/refused | 19 (8.3) |
| White or Caucasian | 143 (62.7) |
| Missing | 2 |
| Index length of stay, d, median (maximum, minimum) | 4 (1, 49) |
| Time between discharge and readmission, d, mean (SD) | 13 (9) |
| Discharge location following index admission, n (%) | |
| Home | 202 (88.2) |
| Skilled nursing facility | 3 (1.3) |
| Acute rehab facility | 17 (7.4) |
| Assisted living facility | 2 (0.9) |
| Other | 5 (2.2) |
| Missing | 1 |
Patient Readiness
Twenty‐eight percent of patients reported feeling unready for discharge from their index admission. Patients who felt that their readmission was preventable were significantly more likely to report feeling unready at the time of discharge compared to those who did not classify their readmission as preventable (53% vs 17%, P<0.01). Among patients who did not feel ready for discharge, over two‐thirds felt their symptoms were not adequately resolved. Conversely, among patients who did feel ready for discharge, only 8% felt their symptoms were not resolved (P<0.01). Patients who felt they were not ready for discharge were also significantly more likely to endorse poor pain control (43% vs 7%, P<0.01). The location of discharge (ie, home, rehab facility, or skilled nursing facility) and having someone to help take care of them at home did not significantly correlate with patient readiness. Over 80% of patients in both groups reported having someone to help at home, but patients who felt unready for discharge were significantly more likely to have concerns about taking care of themselves at home (54% vs 25%, P<0.001) (Table 2).
| All Participants, n=230 | Ready, n=164 | Not Ready, n=65 | P Value | |
|---|---|---|---|---|
| Symptoms were resolved enough to leave the hospital, n=227 | 170 (74.9%) | 149 (92.0%) | 21 (32.3%) | <0.01 |
| Felt pain was under control when left the hospital, n=229 | 190 (83.0%) | 153 (93.3%) | 37 (56.9%) | <0.01 |
| Discharged to home following index admission, n=229 | 202 (88.2%) | 146 (89.6%) | 56 (86.2%) | 0.62 |
| If discharged home, had someone at home able to help, n=202 | 178 (88.1%) | 132 (90.4%) | 46 (82.1%) | 0.17 |
| If discharged home, had concerns about being able to take of themselves at home or not being strong enough to go home, n=202 | 67 (33.2%) | 37 (25.3%) | 30 (53.6%) | <0.01 |
| Thought something could have been done to prevent them from coming back to the hospital, n=228 | 75 (32.9%) | 35 (21.6%) | 39 (60.0%) | <0.01 |
Discharge Instructions
Twenty‐nine percent of patients did not recall a physician talking to them about their discharge, and 35% did not remember receiving and reviewing the discharge paperwork. Of those who read the discharge paperwork, 23% noted difficulty identifying contact phone numbers, and 22% could not locate warning symptoms indicating when to seek medical attention. Patients were able to identify medications and follow‐up appointments on the discharge paperwork a majority of the time (92% and 85%, respectively).
Ambulatory Resources and Utilization
Patients were asked about their access to outpatient resources as well as their reason(s) for returning to the hospital. Eighty‐five percent of patients reported having a primary care doctor that they would feel comfortable calling if their symptoms worsened at home. Of the patients who indicated that they were given a contact number by their discharging team, only 56% contacted a doctor before returning to the emergency room. One‐third of patients reported knowing where to obtain urgent or same‐day care besides the emergency room. Among those who did report knowledge of same‐day care centers, 89% still chose not to utilize them.
Attitudes About Readmission
To investigate the patient experience with readmissions, patients were asked to rate the extent of the burden they felt upon returning to the hospital on a scale of 1 to 10, where 1 was no burden and 10 was extreme burden. Patients were also asked to evaluate the extent of relief they felt upon readmission using the same scale. On average, patients rated their sense of relief 1.8 points higher than their sense of burden upon readmission to the hospital (7.7 [SD 2.8] vs 5.9 [SD 3.4], P<0.001). The relief of readmission was rated as equal to or greater than the burden of readmission in 79% of cases. Lastly, patients' mean (SD) overall satisfaction with their medical care was 8.5 (SD 2.0) on a scale of 1 to 10, where 1 was the least satisfied and 10 was the most satisfied a patient could imagine.
DISCUSSION
This study performs a comprehensive evaluation of the patient perspective on 30‐day readmissions. Our previous work indicated that patients associate preventable readmissions with lack of preparedness at the time of discharge.[7] This study further evaluates the basis of this association. We found that nearly 1 in 3 readmitted patients did not feel ready to leave the hospital at the time of initial discharge. Feelings of inadequate symptom resolution and poor pain control appear to be major contributors to this sentiment. Furthermore, although 88% of patients endorse having a caretaker at home, patients with concerns about taking care of themselves are more likely to feel unready at discharge. Presumably, when healthcare providers discharge patients, they believe that the patient is ready to be discharged. However, our findings suggest that often patients do not agree, highlighting a gap between the beliefs of patients and those of healthcare providers. Creating patient‐centered education on symptom management and engaging patients in developing skills for independent self‐care may minimize this gap and allow patients to feel more prepared at discharge. Future research investigating provider opinions and the steps providers take when there is a disagreement over discharge readiness would also be useful.
One way to enhance education at the time of discharge is through improvements in printed discharge instructions. Jha et al. previously showed that chart documentation of providing discharge instructions does not correlate with patients reporting receiving discharge instructions.[27] Our study echoes this finding, with only 65% of the patients remembering receiving and reviewing the discharge paperwork. Horwitz et al. have also previously demonstrated poor comprehension of discharge planning and postdischarge care among patients discharged from an academic medical center.[28] Ensuring that all patients understand and retain their discharge instructions is an essential step in improving the patient experience and potentially decreasing readmissions. Our surveys have illuminated potential shortcomings in our own center's discharge instructions. Interventions aimed at clarifying critical pieces of information on the discharge paperwork, such as warning symptoms, contact phone numbers and follow‐up appointments, could be especially helpful.
After discharge, our findings suggest that only about half of patients will call a physician before returning to the hospital. Furthermore, there is limited knowledge and poor utilization of same‐day treatment centers besides the emergency room. In previous studies, Long et al. found that frequently readmitted patients self‐triage to the emergency room because they believe primary care clinics cannot treat acute illness.[11] Another study concluded that low‐income patients prefer hospital care to ambulatory care because of a greater sense of trust in inpatient care.[29]
Our patients' attitudes about readmission may also be different from those of providers. For patients, coming back to the hospital is not a significant burden, and satisfaction with their medical care remains high despite readmission. Additional research is needed to further explore the complex emotions patients have when coming back to the hospital and why patients may not be as upset with returning to the hospital as providers may expect. Ultimately, if patients continue to feel more comfortable being hospitalized, there are few incentives for patients to stay out of the hospital, and readmission rates will remain elevated.
Based on our survey results we have hypothesized a potential framework for studying readmissions from a patient‐centered approach (Figure 1). This figure is not meant to imply causality, but rather to highlight a potential journey from discharge to readmission for a patient who does not feel ready to go home. This schema principally applies to patients who are worried about symptom management and/or self‐care before discharge and may not apply to everyone. Each asterisk in this framework represents an area where an intervention could be designed to improve the patient experience and possibly reduce readmissions. Such interventions should be centered around increasing patient education about symptom management and self‐care at the time of discharge, improving printed discharge instructions, increasing patient awareness of outpatient resources, enhancing communication after discharge, and changing patients' attitudes about readmissions.

This study's limitations include that it is a single‐institution study focusing on patients admitted to a large academic medical center and its partner community hospital. Only English‐speaking patients were included, and thus our results may not be generalizable to other populations. All patients were interviewed at the time of readmission, potentially introducing recall bias regarding their prior discharge. For example, patients might be more likely to state they were not ready for discharge once they have been readmitted to the hospital. Lastly, because there are only a few prior studies interviewing readmitted patients, our survey instrument was not previously validated. Nevertheless, we believe this study offers a unique view on 30‐day readmissions from the patient perspective, with a focus on identifying areas for quality‐improvement interventions.
In conclusion, this study has enabled us to understand readmissions from a patient‐centered perspective. This perspective helps to challenge provider assumptions and gives much‐needed insight into the patient experience. For example, prior to surveying patients, one might assume that if a patient has a caregiver at home, they are unlikely to have concerns about taking care of themselves. We now know this is not the case. Similarly, we have discovered sections of our discharge paperwork that are confusing. Additionally, this study has revealed that patient attitudes regarding readmission can vary significantly from provider attitudes. By exploring the patient perspective and creating a new transition framework, we have identified specific target areas for interventions that would be meaningful to patients. As the nation continues to strive to identify sustainable solutions to reduce readmissions, the way to redesign care must always start and end with the patient.
Acknowledgements
The authors acknowledge Puneet Rana, James Haggerty‐Skeans, Jae Kim, Rhea Mathew, and Anna Do (UCLA volunteers) for helping to perform the patient interviews. We acknowledge Sandy Berry, MA (Senior Behavioral Scientist at RAND Corporation) for her help in reviewing our patient interview script. Additionally Anna Dermenchyan, RN, BSN (Senior Clinical Quality Specialist in the Department of Medicine at UCLA) provided significant administrative support.
Disclosures: This project was supported by a Patient Experience Grant from the Beryl Institute awarded to Jessica Howard‐Anderson, Sarah Lonowski, Ashley Busuttil, and Nasim Afsar‐manesh. Dr. Howard‐Anderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All coauthors have seen and agree with the contents of the article. The article is not under review by any other publication. An earlier version of this work was written as a research report (not peer reviewed) for the Beryl Institute (available at:
- , . What will it take to move the needle on hospital readmissions? Am J Med Qual. 2013;29(4):357–359.
- , , , , , . Medicare readmission rates showed meaningful decline in 2012. Medicare Medicaid Res Rev. 2013;3(2):E1–E12.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , , et al. Allocating scarce resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , , , . Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Comparing perspectives of patients, caregivers, and clinicians on heart failure management [published online October 23, 2015]. J Card Fail. doi: 10.1016/j.cardfail.2015.10.011.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , . Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization? J Hosp Med. 2007;2(5):297–304.
- , , . Reasons for readmission in an underserved high‐risk population: a qualitative analysis of a series of inpatient interviews. BMJ Open. 2013;3(9):e003212.
- , , , , , . Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62(8):1556–1561.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , . Reasons for readmission in heart failure: perspectives of patients, caregivers, cardiologists, and heart failure nurses. Heart Lung. 2009;38(5):427–434.
- , , , et al. Patient‐identified factors related to heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2013;6(2):171–177.
- , , , , . Early readmission among patients with diabetes: a qualitative assessment of contributing factors. J Diabetes Complications. 2014;28(6):869–873.
- , , , , . “Because I was sick”: seriously ill veterans' perspectives on reason for 30‐day readmissions. J Am Geriatr Soc. 2015;63(3):537–542.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Patient‐centered communication and diagnostic testing. Ann Fam Med. 2005;3(5):415–421.
- , . Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826–834.
- , , , et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90.
- , , , , . Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–457.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , , . Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637–2645.
- , , , et al. Quality of discharge practices and patient understanding at an academic medical center. JAMA Intern Med. 2013;173(18):1715–1722
- , , , , , . Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
- , . What will it take to move the needle on hospital readmissions? Am J Med Qual. 2013;29(4):357–359.
- , , , , , . Medicare readmission rates showed meaningful decline in 2012. Medicare Medicaid Res Rev. 2013;3(2):E1–E12.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , , et al. Allocating scarce resources in real‐time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005.
- , , , , , . Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Comparing perspectives of patients, caregivers, and clinicians on heart failure management [published online October 23, 2015]. J Card Fail. doi: 10.1016/j.cardfail.2015.10.011.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , . Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization? J Hosp Med. 2007;2(5):297–304.
- , , . Reasons for readmission in an underserved high‐risk population: a qualitative analysis of a series of inpatient interviews. BMJ Open. 2013;3(9):e003212.
- , , , , , . Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62(8):1556–1561.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605.
- , , . Reasons for readmission in heart failure: perspectives of patients, caregivers, cardiologists, and heart failure nurses. Heart Lung. 2009;38(5):427–434.
- , , , et al. Patient‐identified factors related to heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2013;6(2):171–177.
- , , , , . Early readmission among patients with diabetes: a qualitative assessment of contributing factors. J Diabetes Complications. 2014;28(6):869–873.
- , , , , . “Because I was sick”: seriously ill veterans' perspectives on reason for 30‐day readmissions. J Am Geriatr Soc. 2015;63(3):537–542.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Patient‐centered communication and diagnostic testing. Ann Fam Med. 2005;3(5):415–421.
- , . Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826–834.
- , , , et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90.
- , , , , . Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–457.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , , . Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637–2645.
- , , , et al. Quality of discharge practices and patient understanding at an academic medical center. JAMA Intern Med. 2013;173(18):1715–1722
- , , , , , . Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
© 2016 Society of Hospital Medicine