User login
Elevated cardiovascular risks linked to hidradenitis suppurativa
The inflammatory skin disease hidradenitis suppurativa is associated with significantly increased risks of adverse cardiovascular outcomes such as ischemic stroke, myocardial infarction, and cardiovascular mortality, according to results of a population-based study.
A population-based cohort study of 5,964 patients with hidradenitis suppurativa showed that, after adjusting for confounders such as age, sex, smoking, and other comorbidities, hidradenitis suppurativa was associated with a 57% greater risk of myocardial infarction, 33% greater risk of ischemic stroke, 53% increase in major adverse cardiovascular events, and 35% increase in all-cause mortality over a mean 7.1 years of follow-up.
The study, published online Feb. 17 in JAMA Dermatology, also showed a significant increase in cardiovascular-associated death, which was the only adverse outcome that remained significantly elevated (incidence rate ratio, 1.58) in patients with hidradenitis suppurativa when compared with a control group of individuals with severe psoriasis (JAMA Dermatol. 2016 Feb 17. doi: 10.1001/jamadermatol.2015.6264).
“Studies have suggested that, in hidradenitis suppurativa, atrophy of the sebaceous glands, follicular hyperkeratinization, and subsequent hair follicle destruction are associated with deep-seated inflammation, increased susceptibility to secondary infections, and chronic perpetuation of the inflammatory response,” wrote Dr. Alexander Egeberg of the University of Copenhagen and coauthors.
The researchers suggested that there was a “conspicuous absence” of research reports on the risk of cardiovascular disease in hidradenitis suppurativa, especially in light of accumulating evidence of the association between cardiovascular disease and other chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
No conflicts of interest were declared.
The inflammatory skin disease hidradenitis suppurativa is associated with significantly increased risks of adverse cardiovascular outcomes such as ischemic stroke, myocardial infarction, and cardiovascular mortality, according to results of a population-based study.
A population-based cohort study of 5,964 patients with hidradenitis suppurativa showed that, after adjusting for confounders such as age, sex, smoking, and other comorbidities, hidradenitis suppurativa was associated with a 57% greater risk of myocardial infarction, 33% greater risk of ischemic stroke, 53% increase in major adverse cardiovascular events, and 35% increase in all-cause mortality over a mean 7.1 years of follow-up.
The study, published online Feb. 17 in JAMA Dermatology, also showed a significant increase in cardiovascular-associated death, which was the only adverse outcome that remained significantly elevated (incidence rate ratio, 1.58) in patients with hidradenitis suppurativa when compared with a control group of individuals with severe psoriasis (JAMA Dermatol. 2016 Feb 17. doi: 10.1001/jamadermatol.2015.6264).
“Studies have suggested that, in hidradenitis suppurativa, atrophy of the sebaceous glands, follicular hyperkeratinization, and subsequent hair follicle destruction are associated with deep-seated inflammation, increased susceptibility to secondary infections, and chronic perpetuation of the inflammatory response,” wrote Dr. Alexander Egeberg of the University of Copenhagen and coauthors.
The researchers suggested that there was a “conspicuous absence” of research reports on the risk of cardiovascular disease in hidradenitis suppurativa, especially in light of accumulating evidence of the association between cardiovascular disease and other chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
No conflicts of interest were declared.
The inflammatory skin disease hidradenitis suppurativa is associated with significantly increased risks of adverse cardiovascular outcomes such as ischemic stroke, myocardial infarction, and cardiovascular mortality, according to results of a population-based study.
A population-based cohort study of 5,964 patients with hidradenitis suppurativa showed that, after adjusting for confounders such as age, sex, smoking, and other comorbidities, hidradenitis suppurativa was associated with a 57% greater risk of myocardial infarction, 33% greater risk of ischemic stroke, 53% increase in major adverse cardiovascular events, and 35% increase in all-cause mortality over a mean 7.1 years of follow-up.
The study, published online Feb. 17 in JAMA Dermatology, also showed a significant increase in cardiovascular-associated death, which was the only adverse outcome that remained significantly elevated (incidence rate ratio, 1.58) in patients with hidradenitis suppurativa when compared with a control group of individuals with severe psoriasis (JAMA Dermatol. 2016 Feb 17. doi: 10.1001/jamadermatol.2015.6264).
“Studies have suggested that, in hidradenitis suppurativa, atrophy of the sebaceous glands, follicular hyperkeratinization, and subsequent hair follicle destruction are associated with deep-seated inflammation, increased susceptibility to secondary infections, and chronic perpetuation of the inflammatory response,” wrote Dr. Alexander Egeberg of the University of Copenhagen and coauthors.
The researchers suggested that there was a “conspicuous absence” of research reports on the risk of cardiovascular disease in hidradenitis suppurativa, especially in light of accumulating evidence of the association between cardiovascular disease and other chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
No conflicts of interest were declared.
FROM JAMA DERMATOLOGY
Key clinical point: Hidradenitis suppurativa is associated with a significantly increased risk of adverse cardiovascular events and all-cause mortality.
Major finding: Individuals with hidradenitis suppurativa had a 57% greater risk of myocardial infarction and 33% greater risk of ischemic stroke, compared with the general population.
Data source: A population-based cohort study in 5,964 patients with hidradenitis suppurativa.
Disclosures: No conflicts of interest were declared.
Meniscal Root Tears: Identification and Repair
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
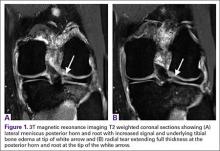
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
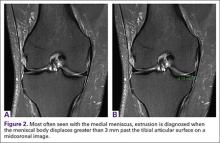
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
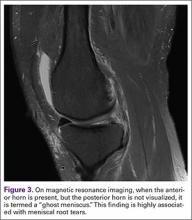
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
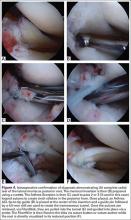
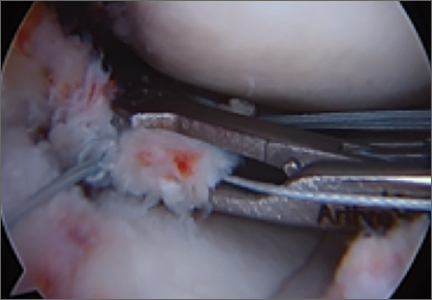
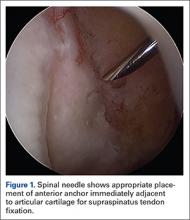
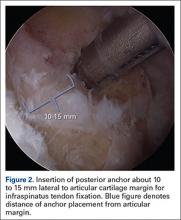
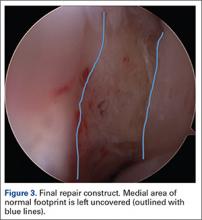
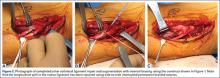
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
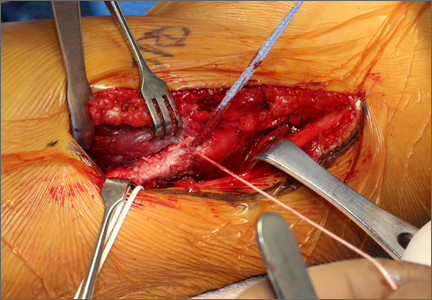
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
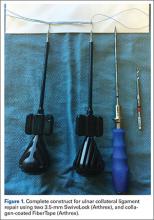
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
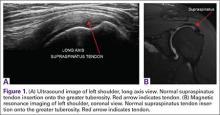
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
Intact and well functioning menisci are essential for optimal knee function. Articular cartilage damage and rapid joint degeneration have been observed in knees after meniscectomy.1-5 Meniscal root tears and avulsions are now increasingly recognized as a functional equivalent to total meniscectomy, and will follow a similar course if left untreated.6-8
The menisci provide shock absorption and stability through their unique anatomy and physiology. Their essential role in dissipation of the axial load encountered during daily activities is accomplished via generation of circumferential hoop stress.4,5,9 Tears of the horn or body may diminish this ability depending on the size and location, but a tear or an avulsion that renders the root incompetent will leave the meniscus unable to generate hoop stress.10 Likewise, as the menisci have been shown to be important secondary stabilizers for both translation and rotation, this function is lost or significantly diminished in the setting of a root tear.6,11,12
Despite their clinical and biomechanical implications, meniscal root tears can be difficult to identify, particularly when they are not actively sought. The goal of this article is to highlight the current diagnostic workup and treatment in patients with suspected meniscal root pathology. We will also aim to emphasize important anatomic and biomechanical considerations when attempting a meniscal root repair.
Anatomy
The menisci are 2 fibrocartilage wedge-shaped structures that surround the medial and lateral tibial plateau’s weight-bearing surfaces. They are attached at many points along their periphery via coronary ligaments that comprise a continuous junction of the meniscus to the capsule to the tibial plateau. Each meniscus has an anterior and a posterior horn that are securely anchored to the tibial intercondylar region via strong ligaments known as the roots.
The anterior medial root attaches just anterior and medial to the medial tibial spine. The anterior lateral root attaches just anterior to the lateral tibial spine. The medial and lateral anterior horns of the menisci are also connected via the anterior intermeniscal ligament (AIML).13-15 Recent cadaveric biomechanical studies have questioned the importance of the AIML, demonstrating no significant change in contact pressure or area before and after sectioning.16 Another important consideration with respect to the anterior root insertion of the lateral meniscus is its intimate relationship with the tibial insertion of the anterior cruciate ligament (ACL). The anterior lateral root and the ACL share over 60% of their tibial footprints.13,17
When the menisci are competent, they absorb between 40% to 70% of the contact force generated between the femur and tibia.1 By providing strong anchor points, the meniscal roots allow the horns and bodies of the menisci to maintain a stable position that maximizes congruency with the femoral condyles.
Pathology
The conversion of axial load to circumferential hoop stresses occur as the resilient, yet pliable, menisci are squeezed between the femoral condyle and tibial plateau. However, this function is dependent on secure attachment sites at the roots. In the setting of root tear, there is no restraint to the peripheral distortion of the menisci, and meniscal extrusion can occur.18
Clinical evidence and biomechanical evidence strongly show the consequences of meniscectomy. Multiple studies have shown similar findings and have proven that a meniscal root tear or avulsion is the biomechanical equivalent to total meniscectomy.3 With meniscectomy, not only do peak pressures within compartments increase significantly, it has been demonstrated that other compartments within the knee with intact menisci do not have increases in compartment pressures, lending more evidence to the menisci functioning as separate units.16 It has also been found that anterior/posterior translation is increased with medial meniscal root tears. When lateral meniscus root tears were studied with associated ACL tear, the pivot shift motion was found to be exaggerated.6
However, the finding of utmost importance in these biomechanical studies is that peak pressures and excessive tibiofemoral motion are restored to normal levels after meniscal root repair. Therefore, repair of meniscal root tears restores native knee biomechanics and will potentially prevent arthritic sequelae from developing.3,4,7,19
Epidemiology
Tears of the posterior root of either menisci are more common than their anterior counterparts, and have been more extensively studied. However, there are situations that can lead to anterior root tears, specifically during ACL reconstruction and during medullary nailing of the tibia.20,21 Barring iatrogenic injury, the anterior horn is less at risk for injury than the posterior horn given the biomechanical environment of the knee.3
Medial meniscus posterior root tears are more common than lateral tears. However, these are often more chronic in nature and not associated with an acute event. Risk factors for medial meniscus root tear include increased body mass index, varus mechanical axis, female gender, and low activity level.22
Lateral meniscus root tears more commonly occur during trauma with sprains and/or tears of knee ligaments.23 Along with increased recognition of meniscal root injuries associated with knee ligamentous injury comes the recognition that certain ligamentous reconstructions—namely the ACL—are more prone to failure and have higher stresses when a root tear is left untreated.17,24
Diagnosis
The gold standard for diagnosis of a meniscal root lesion is under direct visualization during arthroscopy.18 The meniscal roots must be probed and stressed to assess their integrity regardless of the initial indication for knee arthroscopy. In most cases, however, the diagnosis of meniscal root tears should occur prior to proceeding to the operating room.
Magnetic resonance imaging (MRI) has been used to aid in diagnosis of meniscal root tears since the early 1990s.25 Now, with the widespread use of MRI, understanding and diagnosis of meniscal root pathology has increased. All sequences should be reviewed, but T2 weighted coronal sections should provide the best visualization of the posterior roots (Figures 1A, 1B). Sagittal sections may also be helpful in this diagnosis. Increased signal within the root or horn may represent partial or full thickness tears, or may show a more degenerative process with fraying.14,15,26,27
MRI does have limitations, however. When compared to arthroscopy, the sensitivity of 3T MRI to identify posterior root tears is 77%, and specificity is 73%. Medial root tears are more readily identified on MRI than lateral tears.28 This further highlights the need for high suspicion during arthroscopy with the requisite equipment on standby should it be needed.
A concerning finding that may be observed on MRI includes meniscal extrusion (Figures 2A, 2B). Most often seen with the medial meniscus, extrusion is diagnosed when the meniscal body displaces greater than 3 mm past the tibial articular surface on a midcoronal image.26,27 Over 50% of patients with medial meniscal extrusion on MRI will have medial meniscal root tears.26,27 Conversely, meniscal extrusion is less common in lateral menisci for multiple reasons. The lateral compartment of the knee does not have as high contact pressure as the medial compartment, so the lateral meniscus is not as likely to be extruded from the joint. Additionally, the posterior lateral root has the added benefit of further stability from meniscofemoral ligaments.11 They provide a restraint to meniscal extrusion, with a reported rate of 14% lateral meniscus extrusion when they are intact. If the meniscofemoral ligaments are not present or torn in the setting of posterior root tear, the lateral meniscus extrusion rate quadruples and approaches that of medial meniscal extrusion.15
Another finding indicative of meniscal root tear is the “ghost meniscus” (Figure 3). The posterior horn and anterior horn should both be visible in sagittal cuts on MRI. When the anterior horn is present, but the posterior horn is not visualized, it is termed a “ghost meniscus.” This MRI finding is highly associated with meniscal root tears, and will often be found along with meniscal extrusion on coronal sequencing.27,28
Treatment
Historically, large meniscal tears, extruded menisci, or root avulsions have been treated with conservative observation if asymptomatic, or with meniscectomy when symptomatic. With a meniscal root tear, both forms of treatment will not provide lasting benefit and rapid joint degeneration ensues. Evidence now supports repair over meniscectomy when treating root tears.7,8,19,29
Patients who have meniscal root tears that are likely sequelae of an arthritic process are not candidates for meniscal root repair. These patients will often have known arthritis with an intact meniscus and then progress to meniscal pathology, most often medially. Because arthritis is the cause of these meniscal tears, a repair will not reverse this process; such repairs will likely fail, and the patient will re-tear the meniscus. For this subset of patients, physical therapy and activity modification are appropriate treatment.
Repair is indicated for patients with acute tears, with or without associated soft tissue injury to the knee, and those with chronic or acute on chronic tears with minimal arthritis within the knee. The authors’ preferred method of repair is via suture fixation through transosseous tunnel (Figures 4A-4F).
Once a root tear has been identified during arthroscopy, it should be probed and/or grasped and pulled to confirm its integrity. A shaver is then used to debride any fraying of the meniscus and to debride the anatomic footprint of the root. Curettes and rasps are used to prepare the meniscal bed at the center of its insertion and the undersurface of the meniscal root. Once the attachment site of the root insertion has been prepared, an ACL tip-to-tip drill guide is placed over the prepared bed. For repair of a medial meniscus posterior root, a 2.4-mm drill tip guide pin is inserted through the guide via an incision made at the anteromedial tibia. For repair of the lateral meniscus posterior root, the pin is inserted through an incision at the anterolateral aspect of the tibia.
Once the guide pin has been inserted and is visualized at the center of the root footprint, it is held in place by a hemostat or grasper placed intra-articularly. Next, the guide pin is overreamed with a 4.5-mm cannulated drill bit. The transosseous tunnel is then further prepared using a shaver to remove excess soft tissue surrounding the tunnel entrance at the tibial plateau. Further rasping around the edges of the tunnel is performed to make final preparations.
Attention is then turned back to the meniscal root. Using a FastPass Scorpion (Arthrex), 2 or 3 size 0 fiber wire sutures are passed through the root, and a cinch stitch is then secured leaving four to six stands (2 from each Scorpion pass) in the root. A FiberStick is then introduced into the tibial bone tunnel and each strand of the 0 fiberwire is retrieved. Once the FiberWire attached to the meniscal root is in the tunnel, the meniscus should be directly visualized as the appropriate tension is toggled to reduce the meniscal root into its footprint. In order to securely fasten the meniscal root, an Arthrex SwiveLock 4.75-mm suture anchor is used. The meniscus is again probed to assess the integrity of the repair. Of note, an alternative method of fixation is accomplished by tying the fiberwire over an Arthrex suture button at the anterior tibia.
Postoperatively, weight bearing restriction is warranted, along with range of motion restrictions. During the first 2 weeks, patients will be counseled to be touch down weight bearing with the use of crutches or a walker. During this period, range of motion will be restricted by hinged knee brace to 30° of flexion and full extension. The next 2-week period will advance to progressive partial weight bearing, again with crutches or a walker. Range of motion will also be expanded to 60° of flexion. After a month, the patient will then be allowed to be full weight bearing as tolerated and be weaned from assistive ambulation devices. Range of motion will then be 90° of flexion. It is paramount that full extension be achieved and maintained in the early postoperative period. Quadriceps strengthening should also proceed with unlimited straight leg raises throughout this period as well.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
1. Kidron A, Thein R. Radial tears associated with cleavage tears of the medial meniscus in athletes. Arthroscopy. 2002;18(3):254-256.
2. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B(4):664-670.
3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg. 2008;90(9):1922-1931.
4. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124-129.
5. Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18(3):189-192.
6. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905-911.
7. Vyas D, Harner CD. Meniscus root repair. Sports Med Arthrosc Rev. 2012;20(2):86-94.
8. Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032.
9. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990;(252):19-31.
10. Weaver JB. Ossification of the internal semilunar cartilage. J Bone Joint Surg. 1935;17(1):195-198.
11. Ahn JH, Lee YS, Chang JY, Chang MJ, Eun SS, Kim SM. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80.
12. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate–deficient knee: a review of the literature. Am J Orthop. 1997;26(1):18-23.
13. LaPrade CM, Ellman MB, Rasmussen MT, et al. Anatomy of the anterior root attachments of the medial and lateral menisci: a quantitative analysis. Am J Sports Med. 2014;42(10):2386-2392.
14. Brody JM, Hulstyn MJ, Fleming BC, Tung GA. The meniscal roots: Gross anatomic correlation with 3-T MRI findings. AJR Am J Roentgenol. 2007;188(5):W446-W450.
15. Brody JM, Lin HM, Hulstyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810.
16. Poh S-Y, Yew K-SA, Wong P-LK, et al. Role of the anterior intermeniscal ligament in tibiofemoral contact mechanics during axial joint loading. Knee. 2012;19(2):135-139.
17. Naranje S, Mittal R, Nag H, Sharma R. Arthroscopic and magnetic resonance imaging evaluation of meniscus lesions in the chronic anterior ligament–deficient knee. Arthroscopy. 2008;24(9):1045-1051.
18. Magee T. MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging. 2008;28(2):466-470.
19. Kim SB, Ha JK, Lee SW, et al. Medial meniscus root tear refixation: comparison of clinical, radiologic, and arthroscopic findings with medial meniscectomy. Arthroscopy. 2011;27(3):346-354.
20. LaPrade CM, Smith SD, Rasmussen MT, et al. Consequences of tibial tunnel reaming on the meniscal roots during cruciate ligament reconstruction in a cadaveric model, part 1: the anterior cruciate ligament. Am J Sports Med. 2015;43(1):200-206.
21. Ellman MB, James EW, Laprade CM, Laprade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191.
22. Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40(7):1606-1610.
23. Binfield PM, Maffulli N, King JB. Patterns of meniscal tears associated with anterior cruciate ligament lesions in athletes. Injury. 1993;24(8):557-561.
24. Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30(6):845-850.
25. Pagnani MJ, Cooper DE, Warren RF. Extrusion of the medial meniscus. Arthroscopy. 1991;7(3):297-300.
26. Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33(10):569-574.
27. Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol. 2004;183(1):17-23.
28. LaPrade RF, Ho CP, James E, Crespo B, LaPrade CM, Matheny LM. Diagnostic accuracy of 3.0 T magnetic resonance imaging for the detection of meniscus posterior root pathology. Knee Surg Sports Traumatol Arthroscopy. 2015;23(1):152-157.
29. Chung KS, Ha JK, Yeom CH, et al. Comparison of clinical and radiologic results between partial meniscectomy and refixation of medial mensicus posterior root tears: a minimum 5-year follow-up. Arthroscopy. 2015;31(10):1941-1950.
A Guide to Ultrasound of the Shoulder, Part 1: Coding and Reimbursement
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
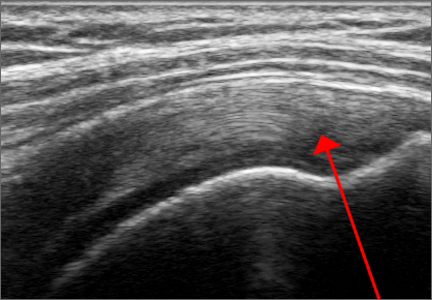
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
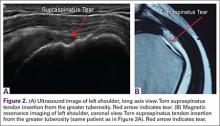
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
A novel approach to overcoming cervical stenosis and false passages

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by ![]()
STS: Valved conduit shows right ventricular outflow durability
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.

|
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.

|
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.

|
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: A prosthetic conduit with a porcine valve showed excellent durability for congenital heart defect repairs at intermediate-term follow-up.
Major finding: After an average 7-year follow-up, the replacement valve and conduit had a 92% rate of freedom from valve dysfunction.
Data source: Single-center series of 100 patients.
Disclosures: Dr. Schubmehl and Dr. Alfieris had no disclosures.
Survival is Heightened with the Use of Bisphophonate
NEW YORK (Reuters Health) - Bisphosphonate use is associated with better survival in patients admitted to the intensive care unit (ICU), according to Australian researchers.
As Dr. Paul Lee told Reuters Health by email, "Bone loss in critical illness may have wider effects on the body beyond bone itself, and bisphosphonates, by reducing bone loss, may attenuate these potentially adverse effects on the body."
Increased bone resorption is known to predict mortality in the community setting, Dr. Lee of the Gavan Institute of Medical Research in Sydney and colleagues note in the Journal of Clinical Endocrinology and Metabolism, online January 18. The team theorized that mortality would be lower among patients treated with bisphosphonates prior to their acute illness.
To investigate, they examined data on more than 7,800 patients admitted to the ICU between 2003 and 2014; 245 had received bisphosphonates before admission.
The bisphosphonate users were older and had more co-morbidities, yet their in-hospital mortality rate was significantly lower than that of non-users(mortality rate ratio, 0.41; p<0.01). The difference remained significant after adjusting for factors including age, sex, and principal diagnosis.
Bisphosphonate-associated survival benefit was independent of vitamin D use, but bisphosphonate and vitamin D co-use was associated with a further reduction in mortality (MRR, 0.38).
A substudy involving CT scans of 37 patients with preadmission bisphosphonate use and 74 matched patients without such use found that baseline bone density was significantly lower among bisphosphonate users. However, all users survived admission whereas six of the non-users died.
The researchers speculate that the apparent benefits of bisphosphonate "may be partly related to modulation of systemic inflammation through antibone resorption."
However, Dr. Lee made it clear that "causality is not proven in the study, and prospective intervention trials are required to evaluate effects of bisphosphonates in critical illness."
NEW YORK (Reuters Health) - Bisphosphonate use is associated with better survival in patients admitted to the intensive care unit (ICU), according to Australian researchers.
As Dr. Paul Lee told Reuters Health by email, "Bone loss in critical illness may have wider effects on the body beyond bone itself, and bisphosphonates, by reducing bone loss, may attenuate these potentially adverse effects on the body."
Increased bone resorption is known to predict mortality in the community setting, Dr. Lee of the Gavan Institute of Medical Research in Sydney and colleagues note in the Journal of Clinical Endocrinology and Metabolism, online January 18. The team theorized that mortality would be lower among patients treated with bisphosphonates prior to their acute illness.
To investigate, they examined data on more than 7,800 patients admitted to the ICU between 2003 and 2014; 245 had received bisphosphonates before admission.
The bisphosphonate users were older and had more co-morbidities, yet their in-hospital mortality rate was significantly lower than that of non-users(mortality rate ratio, 0.41; p<0.01). The difference remained significant after adjusting for factors including age, sex, and principal diagnosis.
Bisphosphonate-associated survival benefit was independent of vitamin D use, but bisphosphonate and vitamin D co-use was associated with a further reduction in mortality (MRR, 0.38).
A substudy involving CT scans of 37 patients with preadmission bisphosphonate use and 74 matched patients without such use found that baseline bone density was significantly lower among bisphosphonate users. However, all users survived admission whereas six of the non-users died.
The researchers speculate that the apparent benefits of bisphosphonate "may be partly related to modulation of systemic inflammation through antibone resorption."
However, Dr. Lee made it clear that "causality is not proven in the study, and prospective intervention trials are required to evaluate effects of bisphosphonates in critical illness."
NEW YORK (Reuters Health) - Bisphosphonate use is associated with better survival in patients admitted to the intensive care unit (ICU), according to Australian researchers.
As Dr. Paul Lee told Reuters Health by email, "Bone loss in critical illness may have wider effects on the body beyond bone itself, and bisphosphonates, by reducing bone loss, may attenuate these potentially adverse effects on the body."
Increased bone resorption is known to predict mortality in the community setting, Dr. Lee of the Gavan Institute of Medical Research in Sydney and colleagues note in the Journal of Clinical Endocrinology and Metabolism, online January 18. The team theorized that mortality would be lower among patients treated with bisphosphonates prior to their acute illness.
To investigate, they examined data on more than 7,800 patients admitted to the ICU between 2003 and 2014; 245 had received bisphosphonates before admission.
The bisphosphonate users were older and had more co-morbidities, yet their in-hospital mortality rate was significantly lower than that of non-users(mortality rate ratio, 0.41; p<0.01). The difference remained significant after adjusting for factors including age, sex, and principal diagnosis.
Bisphosphonate-associated survival benefit was independent of vitamin D use, but bisphosphonate and vitamin D co-use was associated with a further reduction in mortality (MRR, 0.38).
A substudy involving CT scans of 37 patients with preadmission bisphosphonate use and 74 matched patients without such use found that baseline bone density was significantly lower among bisphosphonate users. However, all users survived admission whereas six of the non-users died.
The researchers speculate that the apparent benefits of bisphosphonate "may be partly related to modulation of systemic inflammation through antibone resorption."
However, Dr. Lee made it clear that "causality is not proven in the study, and prospective intervention trials are required to evaluate effects of bisphosphonates in critical illness."
VIDEO: New topical acne therapies will target sebum
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
AT SDEF HAWAII DERMATOLOGY SEMINAR
Dr. Hospitalist: Routine Provider Evaluations Are a Necessary, Valuable Tool
Dear Dr. Hospitalist:
We have several physicians in our large academic group whom I hate to follow when picking up teams. There have only been a few situations when I thought there was a clear knowledge deficit, but the most irritating problem is that they don’t discharge patients. I’ve only been in the group for several years, so I don’t want to come across as a complainer. However, I am concerned about poor patient care and the work left to me to discharge patients. How can I help these physicians improve without damaging my relationship with them?
Dr. Frustrated
Dr. Hospitalist responds:
You bring up a problem that I’m certain many of us in hospital medicine have experienced at some point in our career. Since the “practice” of medicine can often be done with much variability, there are many gray areas that occur during the care of patients. However, we all know it is the transitioning of patients into and out of the hospital that is the most labor-intensive period of their care. If at all possible, the discharge process is best performed by the person with the most longitudinal knowledge of the patient’s hospital course.
Your leadership team has the responsibility to assess the quality and quantity of work of all team members. The periodic assessment of a clinician’s skill and aptitude, as well as the safety of care delivered to patients, can be done in several ways. Typically, the initial assessment is done by focused professional practice evaluations (FPPEs) and later by ongoing professional practice evaluations (OPPEs). The Joint Commission created these tools in 2007 to help determine if the quality of care by clinicians fell below an acceptable level.
FPPEs, as defined by the commission, are “the time limited evaluation of practitioner competence in performing a specific privilege.” They are usually done three to six months after the initial credentialing period, when a new or additional privilege is requested after the initial appointment, or when a condition or issue affecting the delivery of safe and high-quality care is identified.
OPPEs, as the name suggests, are typically done on an ongoing basis (usually annually). These practitioner-specific reports are best utilized as screening tools, and when unusual or aberrant tendencies are observed, a more detailed analysis typically is required.
Although these formal evaluations are carried out by chart review and analysis of data collected by the hospital, they should always be supported by discreet and candid conversations with other frontline team members. It is during these sessions that individuals should take the opportunity to express their opinions regarding the care delivered by their colleagues. From my experience, because of the shared care of patients in hospital medicine, if there is a problem with an individual’s professionalism or clinical abilities, it is usually well-known by others in the group.
If for some reason group leaders are not performing these mandated evaluations (and thus risking regulatory sanctions) or don’t have a formal mechanism in place, I would encourage them to establish one. In the interim, I would discreetly address the individuals and share your concerns. Many times, the problems you mention can be resolved with awareness, mentoring, and/or proctoring, but like any needed corrective actions, they must first be acknowledged.
Good luck! TH
Dear Dr. Hospitalist:
We have several physicians in our large academic group whom I hate to follow when picking up teams. There have only been a few situations when I thought there was a clear knowledge deficit, but the most irritating problem is that they don’t discharge patients. I’ve only been in the group for several years, so I don’t want to come across as a complainer. However, I am concerned about poor patient care and the work left to me to discharge patients. How can I help these physicians improve without damaging my relationship with them?
Dr. Frustrated
Dr. Hospitalist responds:
You bring up a problem that I’m certain many of us in hospital medicine have experienced at some point in our career. Since the “practice” of medicine can often be done with much variability, there are many gray areas that occur during the care of patients. However, we all know it is the transitioning of patients into and out of the hospital that is the most labor-intensive period of their care. If at all possible, the discharge process is best performed by the person with the most longitudinal knowledge of the patient’s hospital course.
Your leadership team has the responsibility to assess the quality and quantity of work of all team members. The periodic assessment of a clinician’s skill and aptitude, as well as the safety of care delivered to patients, can be done in several ways. Typically, the initial assessment is done by focused professional practice evaluations (FPPEs) and later by ongoing professional practice evaluations (OPPEs). The Joint Commission created these tools in 2007 to help determine if the quality of care by clinicians fell below an acceptable level.
FPPEs, as defined by the commission, are “the time limited evaluation of practitioner competence in performing a specific privilege.” They are usually done three to six months after the initial credentialing period, when a new or additional privilege is requested after the initial appointment, or when a condition or issue affecting the delivery of safe and high-quality care is identified.
OPPEs, as the name suggests, are typically done on an ongoing basis (usually annually). These practitioner-specific reports are best utilized as screening tools, and when unusual or aberrant tendencies are observed, a more detailed analysis typically is required.
Although these formal evaluations are carried out by chart review and analysis of data collected by the hospital, they should always be supported by discreet and candid conversations with other frontline team members. It is during these sessions that individuals should take the opportunity to express their opinions regarding the care delivered by their colleagues. From my experience, because of the shared care of patients in hospital medicine, if there is a problem with an individual’s professionalism or clinical abilities, it is usually well-known by others in the group.
If for some reason group leaders are not performing these mandated evaluations (and thus risking regulatory sanctions) or don’t have a formal mechanism in place, I would encourage them to establish one. In the interim, I would discreetly address the individuals and share your concerns. Many times, the problems you mention can be resolved with awareness, mentoring, and/or proctoring, but like any needed corrective actions, they must first be acknowledged.
Good luck! TH
Dear Dr. Hospitalist:
We have several physicians in our large academic group whom I hate to follow when picking up teams. There have only been a few situations when I thought there was a clear knowledge deficit, but the most irritating problem is that they don’t discharge patients. I’ve only been in the group for several years, so I don’t want to come across as a complainer. However, I am concerned about poor patient care and the work left to me to discharge patients. How can I help these physicians improve without damaging my relationship with them?
Dr. Frustrated
Dr. Hospitalist responds:
You bring up a problem that I’m certain many of us in hospital medicine have experienced at some point in our career. Since the “practice” of medicine can often be done with much variability, there are many gray areas that occur during the care of patients. However, we all know it is the transitioning of patients into and out of the hospital that is the most labor-intensive period of their care. If at all possible, the discharge process is best performed by the person with the most longitudinal knowledge of the patient’s hospital course.
Your leadership team has the responsibility to assess the quality and quantity of work of all team members. The periodic assessment of a clinician’s skill and aptitude, as well as the safety of care delivered to patients, can be done in several ways. Typically, the initial assessment is done by focused professional practice evaluations (FPPEs) and later by ongoing professional practice evaluations (OPPEs). The Joint Commission created these tools in 2007 to help determine if the quality of care by clinicians fell below an acceptable level.
FPPEs, as defined by the commission, are “the time limited evaluation of practitioner competence in performing a specific privilege.” They are usually done three to six months after the initial credentialing period, when a new or additional privilege is requested after the initial appointment, or when a condition or issue affecting the delivery of safe and high-quality care is identified.
OPPEs, as the name suggests, are typically done on an ongoing basis (usually annually). These practitioner-specific reports are best utilized as screening tools, and when unusual or aberrant tendencies are observed, a more detailed analysis typically is required.
Although these formal evaluations are carried out by chart review and analysis of data collected by the hospital, they should always be supported by discreet and candid conversations with other frontline team members. It is during these sessions that individuals should take the opportunity to express their opinions regarding the care delivered by their colleagues. From my experience, because of the shared care of patients in hospital medicine, if there is a problem with an individual’s professionalism or clinical abilities, it is usually well-known by others in the group.
If for some reason group leaders are not performing these mandated evaluations (and thus risking regulatory sanctions) or don’t have a formal mechanism in place, I would encourage them to establish one. In the interim, I would discreetly address the individuals and share your concerns. Many times, the problems you mention can be resolved with awareness, mentoring, and/or proctoring, but like any needed corrective actions, they must first be acknowledged.
Good luck! TH