User login
Drug exhibits activity against myeloma, solid tumors

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()
Drug shows promise for treating resistant AML, MCL

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()
NIH’s peer-review process is flawed, team says

Photo by Rhoda Baer
The peer-review process the National Institutes of Health (NIH) use to allocate government research funds to US scientists may work no better than distributing those dollars at random, according to a group of researchers.
The group said their findings, published in eLife, suggest that peer review is not necessarily funding the best science, and awarding grants by lottery might actually produce equally good, if not better, results.
“The NIH claims that they are funding the best grants by the best scientists,” said study author Arturo Casadevall, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
“While [our] data would argue that the NIH is funding a lot of very good science, they are also leaving a lot of very good science on the table. The government can’t afford to fund every good grant proposal, but the problems with the current system make it worse than awarding grants through a lottery.”
The researchers noted that the NIH rejects the majority of research grant proposals it receives. To decide which proposals to fund, the organization relies on expert panels whose members score each application. Funding decisions are made on the basis of these scores and the amount of available funds.
In recent years, the NIH has only funded those proposals ranked around the top 10%. The 2015 annual research budget for the NIH was $30.1 billion.
For their study, Dr Casadevall and his colleagues reanalyzed data on the 102,740 research project grants funded by the NIH from 1980 through 2008.
Another group of researchers previously collected the data. Their research, published in Science in 2015, suggested that peer review works, as the highest ranked research projects funded by the NIH earned the most citations.
For the current study, Dr Casadevall and his colleagues decided to look only at the top 20% of grants awarded. They found very little difference between the top-ranked projects and those projects ranked in the 20th percentile when it came to citations.
What the peer-review process can do, they determined, is discriminate between very good science and very bad science—that is, those in the top 20% versus those below the 50th percentile.
“We are not criticizing the peer reviewers,” said study author Ferric Fang, MD, of the University of Washington in Seattle.
“We are simply showing that there are limits to the ability of peer review to predict future productivity based on grant applications. This suggests that some of the resources and effort spent on ranking applications might be better spent elsewhere. While the average productivity of grants with better scores was somewhat higher, the differences were extremely small, raising questions as to whether the effort is worthwhile.”
The researchers noted that peer review isn’t cheap. The annual budget of the NIH Center for Scientific Review is $110 million. Individual NIH institutes and centers also spend money on peer review. The team said that money could be used toward more grants.
They also noted that peer review allows for substantial subjectivity. The objection of a single member of the committee can effectively kill a grant proposal, whether that objection is legitimate or not.
“When people’s opinions count a lot, we may be doing worse than choosing at random,” Dr Casadevall said. “A negative word at the table can often swing the debate. And this is how we allocate research funding in this country.”
However, Dr Casadevall and his colleagues do not recommend abandoning the peer-review process completely. They believe a way to improve the system might be to continue using peer review to identify the top proposals but then place those proposals into a lottery, with grants awarded at random. ![]()

Photo by Rhoda Baer
The peer-review process the National Institutes of Health (NIH) use to allocate government research funds to US scientists may work no better than distributing those dollars at random, according to a group of researchers.
The group said their findings, published in eLife, suggest that peer review is not necessarily funding the best science, and awarding grants by lottery might actually produce equally good, if not better, results.
“The NIH claims that they are funding the best grants by the best scientists,” said study author Arturo Casadevall, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
“While [our] data would argue that the NIH is funding a lot of very good science, they are also leaving a lot of very good science on the table. The government can’t afford to fund every good grant proposal, but the problems with the current system make it worse than awarding grants through a lottery.”
The researchers noted that the NIH rejects the majority of research grant proposals it receives. To decide which proposals to fund, the organization relies on expert panels whose members score each application. Funding decisions are made on the basis of these scores and the amount of available funds.
In recent years, the NIH has only funded those proposals ranked around the top 10%. The 2015 annual research budget for the NIH was $30.1 billion.
For their study, Dr Casadevall and his colleagues reanalyzed data on the 102,740 research project grants funded by the NIH from 1980 through 2008.
Another group of researchers previously collected the data. Their research, published in Science in 2015, suggested that peer review works, as the highest ranked research projects funded by the NIH earned the most citations.
For the current study, Dr Casadevall and his colleagues decided to look only at the top 20% of grants awarded. They found very little difference between the top-ranked projects and those projects ranked in the 20th percentile when it came to citations.
What the peer-review process can do, they determined, is discriminate between very good science and very bad science—that is, those in the top 20% versus those below the 50th percentile.
“We are not criticizing the peer reviewers,” said study author Ferric Fang, MD, of the University of Washington in Seattle.
“We are simply showing that there are limits to the ability of peer review to predict future productivity based on grant applications. This suggests that some of the resources and effort spent on ranking applications might be better spent elsewhere. While the average productivity of grants with better scores was somewhat higher, the differences were extremely small, raising questions as to whether the effort is worthwhile.”
The researchers noted that peer review isn’t cheap. The annual budget of the NIH Center for Scientific Review is $110 million. Individual NIH institutes and centers also spend money on peer review. The team said that money could be used toward more grants.
They also noted that peer review allows for substantial subjectivity. The objection of a single member of the committee can effectively kill a grant proposal, whether that objection is legitimate or not.
“When people’s opinions count a lot, we may be doing worse than choosing at random,” Dr Casadevall said. “A negative word at the table can often swing the debate. And this is how we allocate research funding in this country.”
However, Dr Casadevall and his colleagues do not recommend abandoning the peer-review process completely. They believe a way to improve the system might be to continue using peer review to identify the top proposals but then place those proposals into a lottery, with grants awarded at random. ![]()

Photo by Rhoda Baer
The peer-review process the National Institutes of Health (NIH) use to allocate government research funds to US scientists may work no better than distributing those dollars at random, according to a group of researchers.
The group said their findings, published in eLife, suggest that peer review is not necessarily funding the best science, and awarding grants by lottery might actually produce equally good, if not better, results.
“The NIH claims that they are funding the best grants by the best scientists,” said study author Arturo Casadevall, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
“While [our] data would argue that the NIH is funding a lot of very good science, they are also leaving a lot of very good science on the table. The government can’t afford to fund every good grant proposal, but the problems with the current system make it worse than awarding grants through a lottery.”
The researchers noted that the NIH rejects the majority of research grant proposals it receives. To decide which proposals to fund, the organization relies on expert panels whose members score each application. Funding decisions are made on the basis of these scores and the amount of available funds.
In recent years, the NIH has only funded those proposals ranked around the top 10%. The 2015 annual research budget for the NIH was $30.1 billion.
For their study, Dr Casadevall and his colleagues reanalyzed data on the 102,740 research project grants funded by the NIH from 1980 through 2008.
Another group of researchers previously collected the data. Their research, published in Science in 2015, suggested that peer review works, as the highest ranked research projects funded by the NIH earned the most citations.
For the current study, Dr Casadevall and his colleagues decided to look only at the top 20% of grants awarded. They found very little difference between the top-ranked projects and those projects ranked in the 20th percentile when it came to citations.
What the peer-review process can do, they determined, is discriminate between very good science and very bad science—that is, those in the top 20% versus those below the 50th percentile.
“We are not criticizing the peer reviewers,” said study author Ferric Fang, MD, of the University of Washington in Seattle.
“We are simply showing that there are limits to the ability of peer review to predict future productivity based on grant applications. This suggests that some of the resources and effort spent on ranking applications might be better spent elsewhere. While the average productivity of grants with better scores was somewhat higher, the differences were extremely small, raising questions as to whether the effort is worthwhile.”
The researchers noted that peer review isn’t cheap. The annual budget of the NIH Center for Scientific Review is $110 million. Individual NIH institutes and centers also spend money on peer review. The team said that money could be used toward more grants.
They also noted that peer review allows for substantial subjectivity. The objection of a single member of the committee can effectively kill a grant proposal, whether that objection is legitimate or not.
“When people’s opinions count a lot, we may be doing worse than choosing at random,” Dr Casadevall said. “A negative word at the table can often swing the debate. And this is how we allocate research funding in this country.”
However, Dr Casadevall and his colleagues do not recommend abandoning the peer-review process completely. They believe a way to improve the system might be to continue using peer review to identify the top proposals but then place those proposals into a lottery, with grants awarded at random. ![]()
Group identifies genes that may impact HSCT

Photo by Aaron Logan
A new screening method has revealed genes that regulate how hematopoietic stem and progenitor cells (HSPCs) grow and thrive in mice.
Researchers used this method to uncover 17 genes that are regulators of hematopoietic stem cell transplant (HSCT).
Thirteen of these genes had never before been linked to HSPC engraftment.
The researchers reported their findings in the Journal of Experimental Medicine.
“We recognized that one barrier to improving [HSCT] is a lack of understanding of how [HSPCs] successfully grow in the challenged environment of transplant, so we set out to identify the genes that control this process,” said Shannon McKinney-Freeman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr McKinney-Freeman and her colleagues transplanted more than 1300 mice with shRNA-transduced HSPCs and searched for genes that regulate HSPC repopulation.
The team identified 17 such genes—Arhgef5, Armcx1, Cadps2, Crispld1, Emcn, Foxa3, Fstl1, Glis2, Gprasp2, Gpr56, Myct1, Nbea, P2ry14, Smarca2, Sox4, Stat4, and Zfp251.
For most of these genes, knockdown yielded a loss of function. The exceptions were Armcx1 and Gprasp2, whose loss enhanced HSPC repopulation.
“Our functional screen in mice is a first step to enhancing [HSCT],” Dr McKinney-Freeman said. “If we are to improve transplant outcomes in patients, we next need to study these identified genes and the molecules they specify in much more detail.”
“The more we understand the full scope of the molecular mechanisms that regulate stable engraftment of [HSPCs], the better equipped we will be to develop and clinically test novel therapies to improve health outcomes.” ![]()

Photo by Aaron Logan
A new screening method has revealed genes that regulate how hematopoietic stem and progenitor cells (HSPCs) grow and thrive in mice.
Researchers used this method to uncover 17 genes that are regulators of hematopoietic stem cell transplant (HSCT).
Thirteen of these genes had never before been linked to HSPC engraftment.
The researchers reported their findings in the Journal of Experimental Medicine.
“We recognized that one barrier to improving [HSCT] is a lack of understanding of how [HSPCs] successfully grow in the challenged environment of transplant, so we set out to identify the genes that control this process,” said Shannon McKinney-Freeman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr McKinney-Freeman and her colleagues transplanted more than 1300 mice with shRNA-transduced HSPCs and searched for genes that regulate HSPC repopulation.
The team identified 17 such genes—Arhgef5, Armcx1, Cadps2, Crispld1, Emcn, Foxa3, Fstl1, Glis2, Gprasp2, Gpr56, Myct1, Nbea, P2ry14, Smarca2, Sox4, Stat4, and Zfp251.
For most of these genes, knockdown yielded a loss of function. The exceptions were Armcx1 and Gprasp2, whose loss enhanced HSPC repopulation.
“Our functional screen in mice is a first step to enhancing [HSCT],” Dr McKinney-Freeman said. “If we are to improve transplant outcomes in patients, we next need to study these identified genes and the molecules they specify in much more detail.”
“The more we understand the full scope of the molecular mechanisms that regulate stable engraftment of [HSPCs], the better equipped we will be to develop and clinically test novel therapies to improve health outcomes.” ![]()

Photo by Aaron Logan
A new screening method has revealed genes that regulate how hematopoietic stem and progenitor cells (HSPCs) grow and thrive in mice.
Researchers used this method to uncover 17 genes that are regulators of hematopoietic stem cell transplant (HSCT).
Thirteen of these genes had never before been linked to HSPC engraftment.
The researchers reported their findings in the Journal of Experimental Medicine.
“We recognized that one barrier to improving [HSCT] is a lack of understanding of how [HSPCs] successfully grow in the challenged environment of transplant, so we set out to identify the genes that control this process,” said Shannon McKinney-Freeman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr McKinney-Freeman and her colleagues transplanted more than 1300 mice with shRNA-transduced HSPCs and searched for genes that regulate HSPC repopulation.
The team identified 17 such genes—Arhgef5, Armcx1, Cadps2, Crispld1, Emcn, Foxa3, Fstl1, Glis2, Gprasp2, Gpr56, Myct1, Nbea, P2ry14, Smarca2, Sox4, Stat4, and Zfp251.
For most of these genes, knockdown yielded a loss of function. The exceptions were Armcx1 and Gprasp2, whose loss enhanced HSPC repopulation.
“Our functional screen in mice is a first step to enhancing [HSCT],” Dr McKinney-Freeman said. “If we are to improve transplant outcomes in patients, we next need to study these identified genes and the molecules they specify in much more detail.”
“The more we understand the full scope of the molecular mechanisms that regulate stable engraftment of [HSPCs], the better equipped we will be to develop and clinically test novel therapies to improve health outcomes.” ![]()
MI Patients who Receive Followup Care are Less Likely to be Readmitted
NEW YORK (Reuters Health) - Myocardial infarction (MI) patients who are transferred to another hospital for care are less likely to be followed up and more likely to be readmitted to the hospital, new findings show.
"This group of patients may represent a vulnerable population and we really need to come up with specific strategies to make their post-discharge transition back to their local community as seamless as possible," corresponding author Dr. Amit Vora, of Duke University in Durham, North Carolina, told Reuters Health.
Many patients admitted to their local hospital for acute MI must be transferred to another hospital for care, for example, to receive revascularization, Dr. Vora and his team note in their report, to be published online in Circulation: Cardiovascular Quality and outcomes. Logistical factors may lead to poor communication and coordination when it's time for the patient to be transferred back to their community, they add, which could be particularly problematic for older patients who may have more comorbidity and require closer follow-up after discharge.
To investigate, the researchers looked at outcomes for 39,136 acute MI patients 65 and older who were treated between 2007 and 2010 at 451 hospitals participating in Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines.
Thirty-six percent of patients were transferred to another hospital for acute MI care, traveling a median of 43 miles.Within 30 days of discharge, 69.9% of the transferred patients had received outpatient follow-up, versus 78.2% of direct-arrival patients.
The adjusted risk of readmission for any cause was 14.5% for transferred patients versus 14% for direct-admit patients, while the risk of readmission for cardiovascular causes was 9.5% for
transferred patients and 9.1% for the direct-admit patients.However, the risk adjusted 30-day mortality was 1.6% for each group.
"Post-discharge care for acute MI patients is a performance measure, and we do track how often these patients are admitted
to the hospital following their discharge," Dr. Vora said. "A big focus of quality improvement is identifying strategies to reduce rehospitalization."
The next step in the research will be to identify the specific barriers to receiving follow-up care for transferred patients, he added, and then "define clear pathways and clear plans following discharge to ensure that these patients receive the care and the follow-up that they need."
The Agency for Healthcare Research and Quality funded this research. Three coauthors reported relevant relationships.
NEW YORK (Reuters Health) - Myocardial infarction (MI) patients who are transferred to another hospital for care are less likely to be followed up and more likely to be readmitted to the hospital, new findings show.
"This group of patients may represent a vulnerable population and we really need to come up with specific strategies to make their post-discharge transition back to their local community as seamless as possible," corresponding author Dr. Amit Vora, of Duke University in Durham, North Carolina, told Reuters Health.
Many patients admitted to their local hospital for acute MI must be transferred to another hospital for care, for example, to receive revascularization, Dr. Vora and his team note in their report, to be published online in Circulation: Cardiovascular Quality and outcomes. Logistical factors may lead to poor communication and coordination when it's time for the patient to be transferred back to their community, they add, which could be particularly problematic for older patients who may have more comorbidity and require closer follow-up after discharge.
To investigate, the researchers looked at outcomes for 39,136 acute MI patients 65 and older who were treated between 2007 and 2010 at 451 hospitals participating in Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines.
Thirty-six percent of patients were transferred to another hospital for acute MI care, traveling a median of 43 miles.Within 30 days of discharge, 69.9% of the transferred patients had received outpatient follow-up, versus 78.2% of direct-arrival patients.
The adjusted risk of readmission for any cause was 14.5% for transferred patients versus 14% for direct-admit patients, while the risk of readmission for cardiovascular causes was 9.5% for
transferred patients and 9.1% for the direct-admit patients.However, the risk adjusted 30-day mortality was 1.6% for each group.
"Post-discharge care for acute MI patients is a performance measure, and we do track how often these patients are admitted
to the hospital following their discharge," Dr. Vora said. "A big focus of quality improvement is identifying strategies to reduce rehospitalization."
The next step in the research will be to identify the specific barriers to receiving follow-up care for transferred patients, he added, and then "define clear pathways and clear plans following discharge to ensure that these patients receive the care and the follow-up that they need."
The Agency for Healthcare Research and Quality funded this research. Three coauthors reported relevant relationships.
NEW YORK (Reuters Health) - Myocardial infarction (MI) patients who are transferred to another hospital for care are less likely to be followed up and more likely to be readmitted to the hospital, new findings show.
"This group of patients may represent a vulnerable population and we really need to come up with specific strategies to make their post-discharge transition back to their local community as seamless as possible," corresponding author Dr. Amit Vora, of Duke University in Durham, North Carolina, told Reuters Health.
Many patients admitted to their local hospital for acute MI must be transferred to another hospital for care, for example, to receive revascularization, Dr. Vora and his team note in their report, to be published online in Circulation: Cardiovascular Quality and outcomes. Logistical factors may lead to poor communication and coordination when it's time for the patient to be transferred back to their community, they add, which could be particularly problematic for older patients who may have more comorbidity and require closer follow-up after discharge.
To investigate, the researchers looked at outcomes for 39,136 acute MI patients 65 and older who were treated between 2007 and 2010 at 451 hospitals participating in Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines.
Thirty-six percent of patients were transferred to another hospital for acute MI care, traveling a median of 43 miles.Within 30 days of discharge, 69.9% of the transferred patients had received outpatient follow-up, versus 78.2% of direct-arrival patients.
The adjusted risk of readmission for any cause was 14.5% for transferred patients versus 14% for direct-admit patients, while the risk of readmission for cardiovascular causes was 9.5% for
transferred patients and 9.1% for the direct-admit patients.However, the risk adjusted 30-day mortality was 1.6% for each group.
"Post-discharge care for acute MI patients is a performance measure, and we do track how often these patients are admitted
to the hospital following their discharge," Dr. Vora said. "A big focus of quality improvement is identifying strategies to reduce rehospitalization."
The next step in the research will be to identify the specific barriers to receiving follow-up care for transferred patients, he added, and then "define clear pathways and clear plans following discharge to ensure that these patients receive the care and the follow-up that they need."
The Agency for Healthcare Research and Quality funded this research. Three coauthors reported relevant relationships.
The Epidemiology of Hip and Groin Injuries in Professional Baseball Players
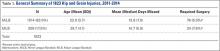
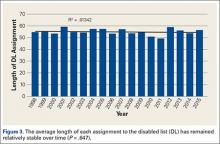
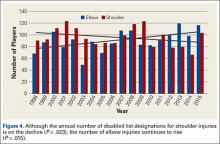
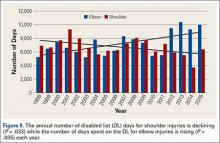
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
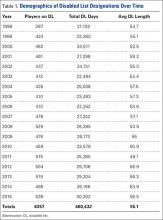
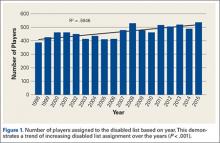
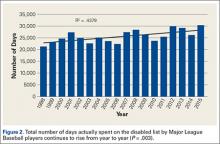
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
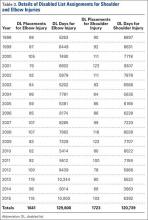
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
Injuries around the hip and groin occurring in professional baseball players can present as muscle strains, avulsions, contusions, hip subluxations or dislocations, femoroacetabular impingement (FAI) causing labral tears or chondral defects, and athletic pubalgia.1-9 Several recent articles have reported on the epidemiology of musculoskeletal injuries in Major League Baseball (MLB) players4,8,10 but with little attention to injuries to the hip and groin, likely because prior studies show only a 6.3% overall incidence for these injuries, much less than the more commonly discussed shoulder or elbow injuries.8 Despite the lower proportion of hip and groin injuries overall, these injuries lead to a relatively long period of disability for the players and often have a high rate of recurrence.4,8,9
The important contribution of hip mechanics and the surrounding muscular function in the kinetic chain during overhead athletic activities, such as a tennis serve or throwing, has recently been discussed.11,12 In sports requiring overhead activities, trunk rotation is a key component to generating force, and hip internal and external rotation is necessary for this trunk rotation to occur.12,13 Alterations in hip morphology causing constrained motion, as seen in FAI, may predispose an overhead throwing athlete to intra-articular injury such as labral tears or chondral injuries, or to a compensatory movement pattern causing an extra-articular soft tissue injury about the hip.12 Decreased hip range of motion may also lead to increased forces across the upper extremity during the throwing motion, which puts the shoulder and elbow at increased risk of injury.12
Increased awareness of hip and groin injuries, advances in diagnostic imaging, and an understanding of the relationship between the throwing motion in baseball and hip mechanics have improved our ability to appropriately identify and treat athletes with injuries of the hip and groin. Several studies on hip and groin injuries in elite athletes treated both operatively and nonoperatively have reported a high rate of return to sport.3,7,14-19 A systematic review on return to sport following hip arthroscopy for intra-articular pathology associated with FAI showed a 95% return to sport rate and a 92% rate of return to pre-injury level of play in a subgroup of professional athletes in 9 studies.20
Despite the large body of literature on upper extremity injuries, there is no study specifically focusing on the epidemiology of hip and groin injuries in MLB or Minor League Baseball (MiLB) players. The incidence of all injuries in professional baseball players has steadily increased over the last 2 decades,8 and the reported incidence of hip and groin injuries will likely increase as well. The current incidence of this injury, the positions most at risk, the mechanism of injury, and the time to return to sport are important to understand given the large number of players who participate in baseball not only at a professional level, but also at an amateur level, where this information may also be applicable. This information could improve our efforts at prevention and rehabilitation of these injuries, and can guide efforts to counsel and train players at high risk of a hip or groin injury. To address this gap in the literature, the purpose of this study was to describe the epidemiology of hip and groin injuries in MLB and MiLB players from 2011 to 2014.
Materials and Methods
Population and Sample
US MLB is comprised of the major and minor leagues. The major leagues are divided into 30 clubs, with 25 active players, for a total of 750 active players. Each club has a 40-man roster consisting of 25 active players and up to 15 additional players who are either not active or optioned to the minor leagues. The minor leagues are comprised of a network of over 200 clubs that are each affiliated with a major league club, and organized by geography and level of play. The minor leagues consist of roughly 7500 players, of whom about 6500 are actively playing at any given time. The entire population of players in the MLB who sustained a hip or groin injury over the study period was eligible for this study.
Data
The MLB’s Health and Injury Tracking System (HITS) is a centralized database that contains the de-identified medical data from the electronic medical record (EMR) system. Data on all injuries are entered into the EMR by each team’s certified athletic trainer. An injury is defined as any physical complaint sustained by a player that affects or limits participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training). The data extracted from HITS only relates to injuries that resulted in lost game time for a player and that occurred during spring training, regular season, or postseason play; off-season injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of assessing days missed because many of these players may not have been cleared to play until the beginning of the following season. For each injury, data were collected on the diagnosis, body part, activity, location, and date of injury.
Materials and Methods
Hip and groin injuries were defined as cases having a body region variable classified as “hip/groin” or a Sports Medicine Diagnostic Coding System (SMDCS) that included any “adductor” or “hernia” or “hip pointer” labels. Cases categorized as inguinal and femoral hernia (n = 26) and testicular contusions (n = 87) were excluded. Characteristics about each hip and groin injury were also extracted from HITS. These variables included level of play, player position (activity at the time of injury), field location, injury mechanism, chronicity of the injury, and days missed. Chronicity of the injury was documented as acute, overuse, or undetermined. For level of play, the injury event was categorized as the league in which the game was played when the injury occurred. Players were excluded if they had an unknown level of play or were in the amateur league. The injuries of the hip and groin were further classified as intra-articular and extra-articular. Treatment for each injury was characterized as surgical or nonsurgical, and correlated with days missed for each type of injury.
Statistical Analysis
Data for the 2011-2014 seasons were combined, and results presented for all players and separately for MiLB and MLB. Frequencies and comparative analyses for hip and groin injuries were performed across the aforementioned injury characteristics. The distribution of days missed for the variables considered was often skewed to the right, even after excluding the season-ending injuries; hence, the mean days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when hip and groin injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented. Chi-square tests were used to test the hypothesis of equal proportions between the various categories of hip and groin characteristics, with statistical significance determined at the P = .05 level.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores that are publicly available. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those hip and groin injuries that occurred during the regular season. It should be noted that the number of regular season hip and groin injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training hip and groin injuries.
Data analysis was performed in the R statistical computing Environment (R Core Team 2014). Study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
Overall Summary
A total of 1823 hip and groin injuries occurred from 2011-2014, with 83% occurring in MiLB and 17% occurring in MLB (Table 1). There were 1146 acute injuries, 252 overuse injuries, and 425 injuries of undetermined chronicity. The average age of players experiencing a hip and groin injury in MiLB was 22.9 years compared to 29.7 years in MLB. Of the 1514 hip and groin injuries in MiLB, 76 (5.0%) required surgery and of the 309 hip and groin injuries in MLB, 24 (7.8%) required surgery. Compared to league-wide injury events, hip and groin injuries ranked 6th highest in prevalence in MiLB and 8th highest in prevalence in MLB, accounting for 5.4% and 5.6%, respectively, of the 28,116 MiLB and 5507 MLB injury events that occurred between 2011-2014.
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE from 2011-2014. The overall hip and groin rate across both MLB and MiLB was 1.2 per 1000 AE, based on the 238 and 1152 regular season hip and groin injuries in MLB and MiLB, respectively. The rate of hip and groin injury was 1.5 times more likely in MiLB than in MLB (P < .0001) (rate of 1.26 per 1000 AE in MiLB and 0.86 per 1000 AE in MLB).
Characteristics of Injuries
Injury activity was based on the position being played at the time of injury, with categories of infield and outfield corresponding to fielding activities (defense), with batting and base runner categories corresponding to activities while on offense (Table 2). The occurrence of hip and groin injuries while players are fielding on defense (MiLB 33.0%, MLB 37.2%, all players 33.8%) was significantly greater compared to injuries while batting and base running on offense (MiLB 24.9%, MLB 21.7%, all players 24.3%) (all P values < .001). There was a high percentage of missing data for the event position variable, which resulted from this field not being available in HITS for 2011. Time lost due to hip and groin injuries was similar across leagues with respect to injury activity, ranging on average between 8 and 18 days.
There were statistically significant differences for MiLB and MLB separately, and combined, in the number of hip and groin injuries by field location (all P values < .0001) (Table 2). For MiLB, MLB, and across both leagues, by injury location, the majority of hip and groin injuries occurred in the infield (MiLB 34.1%, MLB 35.3%, all players 34.3%). As a single location, the pitcher’s mound accounted for a large proportion of hip and groin injuries (MiLB 19.2%, MLB 23.3%, all players 19.9%). Time lost due to hip and groin injuries was similar across leagues with respect to field location, ranging on average between about 10 and 22 days. Among all players, injuries on the pitcher’s mound resulted in the largest mean days missed after injury.
There were statistically significant differences across the mechanisms of injury for MiLB and MLB, as well as both leagues combined (all P values < .0001) (Table 2). The majority of hip and groin injuries were noncontact-related (MiLB 73.7%, MLB 75.7%, all players 74.1%) compared to those resulting from some form of contact (MiLB 11.4%, MLB 12.6%, all players 11.7%) or other mechanisms. Time lost across these mechanisms varied, ranging on average between 4 and 15 days with noncontact-related hip and groin injuries resulting in the largest time lost.
Surgery
The 1823 hip and groin injuries across both leagues were further classified using the SMDCS descriptions as intra-articular (N = 84) or extra-articular (N = 1739) (Table 3). A much larger percentage of hip and groin injuries were extra-articular (MiLB 95.6%, MLB 94.4%, all players 95.4%) compared to those classified as intra-articular (Table 3). The most common extra-articular injuries were strains or contusions of the adductor, iliopsoas, or gluteal muscles, making up 79.1% of this group of injuries. The most common intra-articular injuries were FAI and a labral tear, accounting for 80.9% of these injuries. Only a small percentage of the extra-articular cases required surgery (MiLB 3.4%, MLB 5.8%, all players 3.8%) (Table 4). This finding was in contrast to the larger percentage of intra-articular cases requiring surgery (MiLB 40.3%, MLB 41.2%, all players 40.5%). Time lost varied greatly by surgery status, as well as extra-articular or intra-articular, as would be expected even after excluding season-ending injuries. For both types of injuries, the average time lost was consistently greater for injuries that required surgery versus the ones that did not result in surgery.
Discussion
The incidence of overall injuries in MLB players is increasing.8 Injuries to the hip and groin for professional baseball players continue to be of concern both in the number of injuries and the potential for these injuries to be debilitating or to recur. The correct diagnosis of hip injuries can be challenging in these athletes due to the complex anatomy of the region. However, our understanding of the pathoanatomy of hip and groin injuries, combined with the utilization of improved magnetic resonance imaging (MRI,) has aided in making the correct diagnosis more reliable. Although upper extremity injuries have traditionally been the focus of MLB injury reporting, hip injuries have been shown to cause an average of 23 days missed per player.4 This was similar to the more commonly highlighted elbow and knee injuries in the same study (23 and 27 days, respectively). The purpose of this study was to explore the epidemiology of hip and groin injuries in MLB. The lack of existing data on this issue is important for sports injury research. Exploring these injuries increases the understanding of which players are at risk, and how we can tailor training programs for prevention or rehabilitation programs for those players who suffer these injuries.
In addition to the increased awareness of hip injuries, there has been a recent focus on the contribution of hip range of motion, leg drive, and pelvic rotation to the overall mechanics of overhead activities such as throwing, a tennis serve, or pitching.12 Pelvic rotation and leg drive have been correlated to throwing velocity,21 and therefore if hip range of motion is inhibited by pain or a structural issue such as FAI, there will likely be altered upper extremity mechanics leading to less power and possibly injury.12 Additionally, it has been shown that limited hip range of motion due to FAI is correlated with compensatory lower extremity muscular injuries such as hamstring and adductor strains as well as overload of the lumbar spine and sacroiliac joint.22
In the current study, extra-articular injuries about the hip were the most common, making up 95.4% of the total injuries. Many (79.1%) of these were strains or contusions of the adductor, iliopsoas, or gluteal muscles. This is consistent with other articles reporting hip injuries in athletes.3,9 A study on hip injuries in the National Football League found that strains and contusions comprised 92% of all hip injuries.3 Another report on European professional football found that 72% of hip injuries over a 7-season period were adductor or iliopsoas injuries.9 This prior study also reported that 15% of the hip and groin strains were re-injuries. Intra-articular injuries comprised only 4.6% of the hip injuries in our study. FAI and labral tears were the most common intra-articular diagnosis at 80.9%.
Almost all (96.2%) of the extra-articular hip injuries in this series were able to be treated nonoperatively and caused a mean of 12.4 days missed. Those which required operative treatment caused a mean of 54.6 days missed. For intra-articular injuries, 40.5% were treated surgically and these players missed a mean 122.5 days. Those treated nonsurgically missed an average of 22.2 days. Whether treated surgically or nonsurgically, the mean days missed following an intra-articular injury was approximately twice that of extra-articular injuries. Our findings regarding time or games missed are similar to other reports studying hip injuries in professional athletes.2,3,9 Intra-articular injuries such as FAI, chondral injuries, or labral tears caused between 46 and 64 days missed compared to 3 to 27 days missed for extra-articular injuries in professional soccer players.9 Feeley and colleagues3 found a mean of 5.07 to 33.6 days missed for extra-articular injuries such as strains or contusions, and 63.5 to 126.2 days missed for intra-articular injuries including arthritis, labral tears, subluxations, dislocations, and fractures. A report on National Hockey League players found that intra-articular injuries made up 10.6% of all hip and groin injuries and caused significantly more games missed than extra-articular injuries.2
In both minor and major league players, for all reported positions at the time of hip or groin injury, infield players collectively were more commonly injured than outfielders, batters, or base runners, and fielding was the most common activity being performed at the time of injury. The pitcher’s mound was the most common single location for injuries and these players had the longest time missed following injury. The correlation between hip and groin pathology and upper extremity injuries in overhead athletes has been discussed in previous studies.12,21 Interestingly, we found that the specific location on the field with the highest incidence of hip and groin injuries was the pitcher’s mound. As we follow these players over time, a future correlation between the incidence of hip and groin injuries and the incidence of shoulder and elbow injuries may be noted. A noncontact injury was the most frequent mechanism of injury. This corroborates the finding that muscle strains and contusions made up the majority of injuries in this series. Other series on hip injuries have also found that noncontact mechanisms are common.3
Although this was one of the first studies exploring the epidemiology of hip and groin injury, there are some limitations of this study. The retrospective nature of this study relied upon the reporting of injuries in the MLB database. As such, there may be underreporting of injuries into the official database by players or medical staff for a variety of reasons. Differences in technique for diagnosis and treatment among the medical staff for different teams were not controlled for. Due to the wide range of hip and groin pathology, and the often difficult diagnosis, a specific injury was not always provided. Therefore, the category of “other” hip injury was entered in to the database when symptoms were nonspecific or not all details were provided. Fortunately, this category made up a small percentage of the reported injuries, but does remain a confounding factor in describing the etiology of hip injuries in these players. Our data were taken from professional baseball players only, and so we cannot recommend extrapolation to other sports or nonprofessional baseball athletes.
Despite the inherent limitations of reporting registry data, this study serves as the initial report of the occurrence of hip and groin injuries in professional baseball players, and improves our knowledge of the positions and situations that put players at most risk for these injuries. An understanding of the overall epidemiology of these injuries serves as a platform for more focused research in this area in the future. We can now focus research on specific positions, such as pitchers, that have a high incidence of injury to determine the physiologic and environmental factors which put them at higher risk for injury in general and for more significant injuries with more days missed. This information can help to guide position-specific training programs for injury prevention as well as improve rehabilitation protocols for more efficient recovery and return to sports.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
1. Amenabar T, O’Donnell J. Return to sport in Australian football league footballers after hip arthroscopy and midterm outcome. Arthroscopy. 2013;29(7):1188-1194.
2. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
3. Feeley BT, Powell JW, Muller MS, Barnes RP, Warren RF, Kelly BT. Hip injuries and labral tears in the national football league. Am J Sports Med. 2008;36(11):2187-2195.
4. Li X, Zhou H, Williams P, et al. The epidemiology of single season musculoskeletal injuries in professional baseball. Orthop Rev (Pavia). 2013;5(1):e3.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am. 2003;85-A(7):1190-1196.
7. Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908-914.
8. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
9. Werner J, Hagglund M, Walden M, Ekstrand J. UEFA injury study: a prospective study of hip and groin injuries in professional football over seven consecutive seasons. Br J Sports Med. 2009;43(13):1036-1040.
10. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
11. Ellenbecker TS, Ellenbecker GA, Roetert EP, Silva RT, Keuter G, Sperling F. Descriptive profile of hip rotation range of motion in elite tennis players and professional baseball pitchers. Am J Sports Med. 2007;35(8):1371-1376.
12. Klingenstein GG, Martin R, Kivlan B, Kelly BT. Hip injuries in the overhead athlete. Clin Orthop Relat Res. 2012;470(6):1579-1585.
13. McCarthy J, Barsoum W, Puri L, Lee JA, Murphy S, Cooke P. The role of hip arthroscopy in the elite athlete. Clin Orthop Relat Res. 2003(406):71-74.
14. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
15. Boykin RE, Patterson D, Briggs KK, Dee A, Philippon MJ. Results of arthroscopic labral reconstruction of the hip in elite athletes. Am J Sports Med. 2013;41(10):2296-2301.
16. Malviya A, Paliobeis CP, Villar RN. Do professional athletes perform better than recreational athletes after arthroscopy for femoroacetabular impingement? Clin Orthop Relat Res. 2013;471(8):2477-2483.
17. McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915-919.
18. McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29(2):330-335.
19. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
20. Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy. 2012;28(10):1567-1576.
21. Stodden DF, Langendorfer SJ, Fleisig GS, Andrews JR. Kinematic constraints associated with the acquisition of overarm throwing part I: step and trunk actions. Res Q Exerc Sport. 2006;77(4):417-427.
22. Hammoud S, Bedi A, Voos JE, Mauro CS, Kelly BT. The recognition and evaluation of patterns of compensatory injury in patients with mechanical hip pain. Sports Health. 2014;6(2):108-118.
Injury Trends in Major League Baseball Over 18 Seasons: 1998-2015
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Levels of Immune Cells Do Not Differ Between Relapsing-Remitting and Progressive MS
NEW ORLEANS—Intrathecal levels of T and B cells are comparable in progressive multiple sclerosis (MS), compared with relapsing-remitting MS, according to research described at the ACTRIMS 2016 Forum. The major difference between the two forms of the disease, according to the researchers, is that although the immune cells are mobile in relapsing-remitting MS, they are predominantly embedded in CNS tissue in progressive MS. “This compartmentalization of the immune responses is likely the major reason for the failure of current immunomodulatory treatments in both subtypes of progressive MS,” said Mika Komori, MD, PhD, of the Neuroimmunological Unit at the National Institute of Neurological Disorders and Stroke.
Neurologists have interpreted immunomodulatory therapies’ lack of efficacy in progressive MS as evidence that neurodegeneration, rather than immunopathology, stimulates CNS tissue destruction. Dr. Komori and colleagues sought to develop a methodology that reliably quantifies immune-cell infiltration of CNS tissue by combining CSF immunophenotyping with analysis of immune-cell specific soluble markers.
The researchers collected CSF from 198 subjects, including patients with relapsing-remitting MS, secondary progressive MS, primary progressive MS, noninflammatory neurologic disorders, and other inflammatory neurologic disorders, as well as from healthy donors, and processed the samples in a blinded fashion. They optimized electroluminescent assays to quantify 19 soluble biomarkers in the CSF. Cell-specific secretion was assessed in supernatants from sorted primary immune cells. The investigators quantified absolute numbers of CSF immune cells by flow cytometry in 50-fold concentrated CSF and used them to define three ratios between the concentrations of cell-specific soluble CSF biomarkers and absolute numbers of corresponding CSF cells per milliliter of CSF (ie, sCD14:monocyte, sCD21:B-cell, and CD27:T-cell).
The sCD14:monocyte ratio did not differ among diagnostic groups, but the sCD21:B-cell and especially sCD27:T-cell ratios were significantly higher in progressive MS, compared with all other diagnostic groups. The sCD21:B-cell and sCD27:T-cell ratios differentiated patients with progressive MS from those with relapsing-remitting MS with an area under reviewer operator characteristic curve (AUC) comparable to that of current clinically used tests (AUC, 0.76–0.77). An excess of the soluble biomarkers in comparison with the number of CSF cells that produce it implies presence of the second, nonmobile pool of secreting immune cells embedded in CNS tissue. Dr. Komori and colleagues validated the interpretation that higher biomarker ratios parallel infiltration of CNS tissue by corresponding immune cells in patients with available CNS autopsy or biopsy results with 100% concordance.
NEW ORLEANS—Intrathecal levels of T and B cells are comparable in progressive multiple sclerosis (MS), compared with relapsing-remitting MS, according to research described at the ACTRIMS 2016 Forum. The major difference between the two forms of the disease, according to the researchers, is that although the immune cells are mobile in relapsing-remitting MS, they are predominantly embedded in CNS tissue in progressive MS. “This compartmentalization of the immune responses is likely the major reason for the failure of current immunomodulatory treatments in both subtypes of progressive MS,” said Mika Komori, MD, PhD, of the Neuroimmunological Unit at the National Institute of Neurological Disorders and Stroke.
Neurologists have interpreted immunomodulatory therapies’ lack of efficacy in progressive MS as evidence that neurodegeneration, rather than immunopathology, stimulates CNS tissue destruction. Dr. Komori and colleagues sought to develop a methodology that reliably quantifies immune-cell infiltration of CNS tissue by combining CSF immunophenotyping with analysis of immune-cell specific soluble markers.
The researchers collected CSF from 198 subjects, including patients with relapsing-remitting MS, secondary progressive MS, primary progressive MS, noninflammatory neurologic disorders, and other inflammatory neurologic disorders, as well as from healthy donors, and processed the samples in a blinded fashion. They optimized electroluminescent assays to quantify 19 soluble biomarkers in the CSF. Cell-specific secretion was assessed in supernatants from sorted primary immune cells. The investigators quantified absolute numbers of CSF immune cells by flow cytometry in 50-fold concentrated CSF and used them to define three ratios between the concentrations of cell-specific soluble CSF biomarkers and absolute numbers of corresponding CSF cells per milliliter of CSF (ie, sCD14:monocyte, sCD21:B-cell, and CD27:T-cell).
The sCD14:monocyte ratio did not differ among diagnostic groups, but the sCD21:B-cell and especially sCD27:T-cell ratios were significantly higher in progressive MS, compared with all other diagnostic groups. The sCD21:B-cell and sCD27:T-cell ratios differentiated patients with progressive MS from those with relapsing-remitting MS with an area under reviewer operator characteristic curve (AUC) comparable to that of current clinically used tests (AUC, 0.76–0.77). An excess of the soluble biomarkers in comparison with the number of CSF cells that produce it implies presence of the second, nonmobile pool of secreting immune cells embedded in CNS tissue. Dr. Komori and colleagues validated the interpretation that higher biomarker ratios parallel infiltration of CNS tissue by corresponding immune cells in patients with available CNS autopsy or biopsy results with 100% concordance.
NEW ORLEANS—Intrathecal levels of T and B cells are comparable in progressive multiple sclerosis (MS), compared with relapsing-remitting MS, according to research described at the ACTRIMS 2016 Forum. The major difference between the two forms of the disease, according to the researchers, is that although the immune cells are mobile in relapsing-remitting MS, they are predominantly embedded in CNS tissue in progressive MS. “This compartmentalization of the immune responses is likely the major reason for the failure of current immunomodulatory treatments in both subtypes of progressive MS,” said Mika Komori, MD, PhD, of the Neuroimmunological Unit at the National Institute of Neurological Disorders and Stroke.
Neurologists have interpreted immunomodulatory therapies’ lack of efficacy in progressive MS as evidence that neurodegeneration, rather than immunopathology, stimulates CNS tissue destruction. Dr. Komori and colleagues sought to develop a methodology that reliably quantifies immune-cell infiltration of CNS tissue by combining CSF immunophenotyping with analysis of immune-cell specific soluble markers.
The researchers collected CSF from 198 subjects, including patients with relapsing-remitting MS, secondary progressive MS, primary progressive MS, noninflammatory neurologic disorders, and other inflammatory neurologic disorders, as well as from healthy donors, and processed the samples in a blinded fashion. They optimized electroluminescent assays to quantify 19 soluble biomarkers in the CSF. Cell-specific secretion was assessed in supernatants from sorted primary immune cells. The investigators quantified absolute numbers of CSF immune cells by flow cytometry in 50-fold concentrated CSF and used them to define three ratios between the concentrations of cell-specific soluble CSF biomarkers and absolute numbers of corresponding CSF cells per milliliter of CSF (ie, sCD14:monocyte, sCD21:B-cell, and CD27:T-cell).
The sCD14:monocyte ratio did not differ among diagnostic groups, but the sCD21:B-cell and especially sCD27:T-cell ratios were significantly higher in progressive MS, compared with all other diagnostic groups. The sCD21:B-cell and sCD27:T-cell ratios differentiated patients with progressive MS from those with relapsing-remitting MS with an area under reviewer operator characteristic curve (AUC) comparable to that of current clinically used tests (AUC, 0.76–0.77). An excess of the soluble biomarkers in comparison with the number of CSF cells that produce it implies presence of the second, nonmobile pool of secreting immune cells embedded in CNS tissue. Dr. Komori and colleagues validated the interpretation that higher biomarker ratios parallel infiltration of CNS tissue by corresponding immune cells in patients with available CNS autopsy or biopsy results with 100% concordance.
Exercise May Improve Inhibitory Control in MS
NEW ORLEANS—Light-, moderate-, and vigorous-intensity treadmill walking may particularly improve inhibitory control in fully ambulatory people with multiple sclerosis (MS), according to research described at the ACTRIMS 2016 Forum. Furthermore, the increase in core body temperature associated with such exercise may not negate its potentially beneficial effects on inhibitory control.
“This [study] represents the next step in delineating the optimal exercise stimuli for improving cognition in fully ambulatory persons with MS and supports the feasibility of chronic treadmill walking exercise training for improving inhibitory control in thermosensitive persons with MS,” said Brian M. Sandroff, PhD, of the Kessler Foundation in West Orange, New Jersey.
Exercise training is a promising approach for managing cognitive impairment in persons with MS. Although preliminary evidence indicates that treadmill walking might have the greatest beneficial effects on inhibitory control, compared with other forms of exercise, in fully ambulatory persons with MS, the effects of varying intensities of treadmill walking exercise on inhibitory control are unknown. In addition, previous research has not indicated whether increases in core body temperature negate the potentially beneficial effects of treadmill walking exercise on inhibitory control in thermosensitive people with MS.
To better determine the optimal form of exercise for improving cognition in MS, Dr. Sandroff and colleagues first compared the acute effects of light-, moderate-, and vigorous-intensity treadmill walking exercise on inhibitory control in 24 participants with MS using a within-subjects, repeated-measures design. In a second study, the researchers examined the acute effects of core body temperature on inhibitory control during vigorous treadmill walking exercise in 14 thermosensitive persons with MS.
Participants in the first study completed four experimental conditions (ie, 20 minutes of light-, moderate-, and vigorous-intensity treadmill walking exercise and quiet rest) in a randomized, counterbalanced order. The investigators measured inhibitory control before and after each condition using a modified flanker task. In the second study, thermosensitive participants with MS completed two experimental conditions (ie, 20 minutes of vigorous treadmill walking exercise and 20 minutes of quiet rest) in a randomized, counterbalanced order. The researchers measured core body temperature throughout both conditions. Inhibitory control was measured before and after each condition using a modified flanker task.
In the first study, the investigators observed large, statistically significant improvements in inhibitory control for all three intensities of treadmill walking exercise, compared with quiet rest. The improvements were of similar magnitude. The second study indicated that, compared with rest, vigorous exercise was followed by improvements in inhibitory control, despite significant elevations in core body temperature (~0.6 °C) in thermosensitive persons with MS.
NEW ORLEANS—Light-, moderate-, and vigorous-intensity treadmill walking may particularly improve inhibitory control in fully ambulatory people with multiple sclerosis (MS), according to research described at the ACTRIMS 2016 Forum. Furthermore, the increase in core body temperature associated with such exercise may not negate its potentially beneficial effects on inhibitory control.
“This [study] represents the next step in delineating the optimal exercise stimuli for improving cognition in fully ambulatory persons with MS and supports the feasibility of chronic treadmill walking exercise training for improving inhibitory control in thermosensitive persons with MS,” said Brian M. Sandroff, PhD, of the Kessler Foundation in West Orange, New Jersey.
Exercise training is a promising approach for managing cognitive impairment in persons with MS. Although preliminary evidence indicates that treadmill walking might have the greatest beneficial effects on inhibitory control, compared with other forms of exercise, in fully ambulatory persons with MS, the effects of varying intensities of treadmill walking exercise on inhibitory control are unknown. In addition, previous research has not indicated whether increases in core body temperature negate the potentially beneficial effects of treadmill walking exercise on inhibitory control in thermosensitive people with MS.
To better determine the optimal form of exercise for improving cognition in MS, Dr. Sandroff and colleagues first compared the acute effects of light-, moderate-, and vigorous-intensity treadmill walking exercise on inhibitory control in 24 participants with MS using a within-subjects, repeated-measures design. In a second study, the researchers examined the acute effects of core body temperature on inhibitory control during vigorous treadmill walking exercise in 14 thermosensitive persons with MS.
Participants in the first study completed four experimental conditions (ie, 20 minutes of light-, moderate-, and vigorous-intensity treadmill walking exercise and quiet rest) in a randomized, counterbalanced order. The investigators measured inhibitory control before and after each condition using a modified flanker task. In the second study, thermosensitive participants with MS completed two experimental conditions (ie, 20 minutes of vigorous treadmill walking exercise and 20 minutes of quiet rest) in a randomized, counterbalanced order. The researchers measured core body temperature throughout both conditions. Inhibitory control was measured before and after each condition using a modified flanker task.
In the first study, the investigators observed large, statistically significant improvements in inhibitory control for all three intensities of treadmill walking exercise, compared with quiet rest. The improvements were of similar magnitude. The second study indicated that, compared with rest, vigorous exercise was followed by improvements in inhibitory control, despite significant elevations in core body temperature (~0.6 °C) in thermosensitive persons with MS.
NEW ORLEANS—Light-, moderate-, and vigorous-intensity treadmill walking may particularly improve inhibitory control in fully ambulatory people with multiple sclerosis (MS), according to research described at the ACTRIMS 2016 Forum. Furthermore, the increase in core body temperature associated with such exercise may not negate its potentially beneficial effects on inhibitory control.
“This [study] represents the next step in delineating the optimal exercise stimuli for improving cognition in fully ambulatory persons with MS and supports the feasibility of chronic treadmill walking exercise training for improving inhibitory control in thermosensitive persons with MS,” said Brian M. Sandroff, PhD, of the Kessler Foundation in West Orange, New Jersey.
Exercise training is a promising approach for managing cognitive impairment in persons with MS. Although preliminary evidence indicates that treadmill walking might have the greatest beneficial effects on inhibitory control, compared with other forms of exercise, in fully ambulatory persons with MS, the effects of varying intensities of treadmill walking exercise on inhibitory control are unknown. In addition, previous research has not indicated whether increases in core body temperature negate the potentially beneficial effects of treadmill walking exercise on inhibitory control in thermosensitive people with MS.
To better determine the optimal form of exercise for improving cognition in MS, Dr. Sandroff and colleagues first compared the acute effects of light-, moderate-, and vigorous-intensity treadmill walking exercise on inhibitory control in 24 participants with MS using a within-subjects, repeated-measures design. In a second study, the researchers examined the acute effects of core body temperature on inhibitory control during vigorous treadmill walking exercise in 14 thermosensitive persons with MS.
Participants in the first study completed four experimental conditions (ie, 20 minutes of light-, moderate-, and vigorous-intensity treadmill walking exercise and quiet rest) in a randomized, counterbalanced order. The investigators measured inhibitory control before and after each condition using a modified flanker task. In the second study, thermosensitive participants with MS completed two experimental conditions (ie, 20 minutes of vigorous treadmill walking exercise and 20 minutes of quiet rest) in a randomized, counterbalanced order. The researchers measured core body temperature throughout both conditions. Inhibitory control was measured before and after each condition using a modified flanker task.
In the first study, the investigators observed large, statistically significant improvements in inhibitory control for all three intensities of treadmill walking exercise, compared with quiet rest. The improvements were of similar magnitude. The second study indicated that, compared with rest, vigorous exercise was followed by improvements in inhibitory control, despite significant elevations in core body temperature (~0.6 °C) in thermosensitive persons with MS.
Latissimus Dorsi and Teres Major Injuries in Major League Baseball Pitchers: A Systematic Review
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
Upper extremity injuries are very common in pitchers in amateur and professional baseball. The vast majority involving labral or rotator cuff pathology.1-3 While uncommon, injuries to the latissimus dorsi (LD) (Figure) and teres major (TM) have been reported in Major League Baseball (MLB) pitchers.4 Jobe and colleagues5 demonstrated the role of the LD during the various phases of pitching. The LD is most active during the acceleration phase and remains active during the deceleration phase and follow-through.6 Anatomically, the TM lies posterior to the LD separated by bursal tissue. The tendon fibers converge and unite along their lower borders, leading to a synergistic mechanism of action.
Due to the rarity of LD and TM injuries, literature on the pathology and appropriate treatments for these injuries is limited. The goal of this review is to present the current literature on professional baseball players who have undergone either nonsurgical treatment or surgery for LD and TM strains and/or avulsion injuries. This review will ultimately assist clinicians when deciding on the optimal treatment method for professional baseball players.
Methods
We performed an extensive Medline database search with the following search algorithm: ([latissimus OR latissimus dorsi OR teres major] AND baseball). The search returned 20 citations. Inclusion criteria consisted of clinical studies that focused on professional baseball pitchers with TM and/or LD injuries that underwent either conservative nonsurgical treatment or surgical repair. There was no exclusion based on the type of injury present, such as avulsion vs strain. Any study with amateur athletes or athletes from other sports such as handball or rugby were excluded. Due to the limited amount of data available, the majority of included studies were case reports and case series.
Based on these parameters, 5 articles met criteria for inclusion. Of the 5 included studies, 3 were case reports and 2 were case series. From the eligible articles, the following information was obtained: publication year, sample size, mean age, mean follow-up duration, type of treatment (conservative vs surgical), ability to return to original level of play, time required to return to original form, and complications (Tables 1, 2).
Results
Nonoperative Management
Four of the 5 included studies implemented only conservative therapy for their patients.4,7-9 The average duration these patients were followed for during treatment and rehabilitation was 26.3 months. Malcolm and colleagues7 followed patients for 8 months, the shortest length among the 4 conservative studies in this review. Leland and colleagues8 followed patients for 17 months, and Nagda and colleagues9 had the longest length of observation of 36 months (range 12 to 82 months).Schickendantz and colleagues4 followed patients for >12 months, but the exact duration was not specified. In order to calculate the average duration of observation, each patient was assigned a duration of 12 months.
Of the 30 patients included in this review, 29 were treated conservatively. All of the included studies consisted of male patients. The mean age was 26.8 years (range 22 to 28.1 years). Of the 29 injuries treated conservatively, there were 2 LD tendon avulsions, 4 TM tendon avulsions, 1 LD and TM tendon avulsion, 7 LD intramuscular strains, 9 TM intramuscular strains, and 6 LD and TM intramuscular strains.
Treatment Protocol
The various treatment and rehabilitation programs used for the conservative patient population all followed a similar pathway. After initial injury, a rest period focused on stretching was implemented. Patients were started on steroid or anti-inflammatory medications, cryotherapy, or other therapeutic modalities. Once pain-free and full range of motion was achieved, patients began the strength and throwing components of the rehabilitation program. Reoccurrence of symptoms would halt the throwing component of the rehabilitation program until symptoms improved. Patients were progressed through a return-to-throw program and once they could throw off the mound and achieve their preinjury velocity, strength, and range of motion, they were cleared to return to competitive pitching.
In the senior author’s (MSS) practice, all throwers are managed with the same nonoperative protocol.4 Initial treatment consists of short periods of rest and symptom control via the application of cryotherapy, among other modalities. Restoration of preinjury range of motion is achieved with active-assisted stretching exercises. As range of motion begins approaching pre-injury levels, strength training is initiated with isometric strengthening of the LD and TM progressing to resistance exercises. Exercising the abdominal core, strengthening the lower body, and cardiovascular conditioning are focal points of the rehabilitation period. Once patients regain preinjury shoulder strength and range of motion without pain, they begin a throwing program that consists of 4 weeks of long toss followed by 2 weeks of throwing from the pitching mound. After completion of the throwing program, the patient is allowed to return to competitive pitching. For patients who did not suffer season-ending injury, the average time required to return to play was 99.8 days (range 72.3 to 182.6 days).
Complications and Reinjury
The patients in Leland and colleagues8 and Malcolm and colleagues7 did not suffer any complications or reinjuries. In Schickendantz and colleagues4, all but 3 of the 10 patients were able to return to full speed pitching by 3 months. The other 3 required 4, 6, and 10 months. The patient that required 10 months tore both his LD and TM and the patient that required 6 months tore his TM and was never able to regain his pre-injury throwing velocity. None of the TM tears had a recurrence, while 1 LD tear had a recurrence of injury 6 months after returning to competitive pitching. This patient was successfully treated with 6 weeks of conservative rest and rehabilitation.
In Nagda and colleagues9, 2 athletes suffered injury recurrence. One athlete with a LD strain suffered 2 subsequent LD strains, 4 months and 1 year after initial injury. The other athlete with a LD avulsion suffered a subsequent TM avulsion 13 months after initial injury. One pitcher who had an LD and TM strain suffered a superior labrum anterior and posterior (SLAP) tear and was never able to return to his prior level of play.
Surgical Treatment
Only 1 of the 5 included studies utilized surgical repair for their patient.10 The single patient suffered an avulsion injury of the distal LD tendon and its insertion on the humerus. The LD tendon was retracted approximately 5 cm from the distal humeral insertion. The TM was not involved. Eight days post-injury, the patient underwent surgical repair.11 Postoperatively, the patient started passive range of motion after 2 weeks and active range of motion after 6 weeks. He started throwing at 12 weeks and returned to play at 30 weeks after he had returned to his preinjury form in regards to muscle strength, pitch control, and velocity. The patient was able to resume pitching at a high level in MLB.
Discussion
Overhand throwing athletes, especially professional baseball players, have to constantly deal with a variety of shoulder injuries.12,13 Currently, there is minimal literature on isolated TM and LD injuries. As a result, there is still debate about the optimal treatment method for these injuries, especially in athletes who compete at the highest level. In order to treat isolated injuries of these muscles, it is important to understand their anatomic relationship, as these 2 muscles are intimately associated. The LD originates from the thoracolumbar spine and inserts on the proximal humerus between the pectoralis<hl name="2"/> major and TM tendons. The TM originates from the scapula and, similar to the LD, inserts on the proximal humerus. In an anatomic study, the TM tendon inserted into the LD tendon before its humeral insertion in the majority of cadavers.14,15
The LD is responsible for extension, adduction, and internal rotation of the humerus. The TM, while not as extensively studied, is believed to also contribute to extension, adduction, and internal rotation of the humerus.16 As Jobe and colleagues5 demonstrated, the LD is vital during the acceleration phase of pitching. While they were unable to make any conclusions about the role of the TM during the pitching cycle, it is reasonable to hypothesize that these 2 muscles work together. While it is thought that these 2 muscles work as a unit, it is significant to note that a professional pitcher can sustain an isolated injury to the TM without injury to the LD, and vice versa. This questions whether these 2 muscles work more independently than once thought. One hypothesis is that the physical size of the LD provides protection from injuries that the smaller TM cannot overcome. This is a potential area of further research.
The most common findings in patients with TM injuries include swelling, bruising, tenderness of the proximal arm, and limitations of shoulder range of motion in abduction, flexion, and external rotation. There is also weakness when resistance is applied against internal rotation and extension. Similar to the TM, common findings in patients with LD injuries include pain in the posterior shoulder, bruising, and weakness when resistance is applied against internal rotation of the shoulder. Pitchers are often able to pinpoint the occurrence of their acute pain during a specific time in the game. They commonly experience a pulling sensation and sometimes even feel a “pop” in their shoulder followed by an acute onset of pain and stiffness in the posterior aspect of the axilla. These injuries seem to be associated with the pitcher throwing a “breaking ball,” a pitch that requires greater shoulder rotation since it changes trajectory while traveling towards home plate. Despite the clear role of the LD and hypothesized role of the TM in the pitching sequence, there has been limited research on the optimal treatment of isolated injuries of these muscles in MLB pitchers. The majority of studies in this review opted for conservative treatment for both LD and TM injuries. The only study that presented a surgical option was for a LD avulsion injury.
Athletes undergoing either conservative or surgical treatment required a significant period of recovery and rehabilitation before they were able to compete at the professional level. In Leland and colleagues8, it took about 10 to 12 weeks of rehabilitation for both pitchers to return to pitching against competition. In Schickendantz and colleagues4, barring any complications or injury recurrence, it took patients 12 weeks to return to their preinjury level. In Malcolm and colleagues7, magnetic resonance imaging after 8 weeks showed marked recovery, and shortly after the pitcher was able to return to the pitching rotation. In Nagda and colleagues9, the time lost to injury ranged from 7 weeks to an entire season. Of the 9 pitchers who were lost for the season, 6 had avulsion injuries. The other 3 consisted of an LD strain, TM strain, and LD plus TM strain.9 In this study, it seems that avulsion injuries had a more significant impact on patient recovery. On average, it took 35.6 days after injury for players to begin throwing. In contrast, it took an average of 65.5 days after an avulsion injury for players to begin throwing. Ellman and colleagues10 included the only surgically repaired injury, and it was for an avulsion of the LD tendon. In the surgical case, it took slightly longer for the pitcher to return to preinjury form. It took him 12 to 16 weeks to begin light throwing and his full return to pitching took about 20 to 30 weeks. Since muscle strains and tendon avulsions are significantly different injuries in regards to the type of soft tissue damage and healing potential, they may require different treatment strategies. An avulsion injury may require more aggressive intervention, whereas a strain may only require conservative rehabilitation. Ultimately, there does not seem to be a significant benefit of one treatment option compared to the other. The majority of conservatively managed pitchers were able to return to previous form in a reasonable time frame. While each rehabilitation protocol was slightly different, multiple studies advocated for rehab programs that centered around the following goals: slowly progressing pitchers to light throwing once their pain resolved, followed by long throwing, then throwing off of the mound, and finally returning to competitive pitching. It is important to discuss with patients that rehabilitation generally takes 12 to 16 weeks before they are able to fully return to pitching against competition and that rest should immediately follow any recurrence of pain or stiffness. Once those symptoms resolve, patients may continue the rehabilitation protocol.
As with any form of treatment, there are risks involved. This holds true for both conservative and nonconservative therapy for LD and TM injuries. One risk of nonoperative treatment of an LD avulsion is the development of strength deficits in the muscle.17 While this deficit may go unnoticed in a recreational athlete, it may be more pronounced in a professional athlete, especially since the LD of a professional baseball pitcher is more active on electromyography during the acceleration phase of the pitching cycle compared to a recreational athlete.18 Another risk of conservative treatment of an LD avulsion is jeopardizing the potential for future surgery. As a result, some advocate for early surgical intervention of an acute LD avulsion.19,20 Others, however, recommend conservative management with subsequent surgical intervention if conservative measures fail. One caveat is that surgical intervention to restore the original anatomy may become difficult after a certain period of time due to the buildup of scar tissue. Surgical intervention also has associated risks, such as nerve injury, infection, vascular damage, persistent pain, and the buildup of large amounts of scar tissue. It is important to discuss these risks with patients when deciding on a treatment option.
LD and TM avulsion and tears typically present after an acute event in throwing athletes. There are a number of case reports published that demonstrate successful outcomes with both nonoperative management21 and operative repair of LD injuries in non-throwing athletes such as competitive water skiers,22,23 steer wrestlers,24 professional wrestlers,25 and recreational rock climbers.26 The 5 studies included in this review were the first ones to present LD and TM injuries in MLB pitchers. They discussed the outcomes of mainly conservative and surgical management of LD and TM avulsion and tears. Unfortunately, there remains a limited number of cases on the treatment of these injuries in highly competitive throwing athletes. Further research is required to elucidate the advantages and disadvantages of operative vs nonoperative treatment. The goal of this review is to provide clinicians with a concise summary of the current literature so that they may offer some evidence to their patients when discussing appropriate treatment plans.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.
1. Conway JE, Arthroscopic repair of partial-thickness rotator cuff tears and SLAP lesions in professional baseball players. Orthop Clin North Am. 2001;32(3):443-456.
2. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
3. Cerynik DL, Ewald TJ, Sastry A, Amin NH, Liao JG, Tom JA. Outcomes of isolated glenoid labral injuries in professional baseball pitchers. Clin J Sport Med. 2008;18(3):255-258
4. Schickendantz MS, Kaar SG, Meister K, Lund P, Beverley L. Latissimus dorsi and teres major tears in professional baseball pitchers: a case series. Am J Sports Med. 2009;37(10):2016-2020.
5. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
6. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
7. Malcolm PN, Reinus WR, London SL. Magnetic resonance imaging appearance of teres major tendon injury in a baseball pitcher. Am J Sports Med. 1999;27(1):98-100.
8. Leland JM, Ciccotti MG, Cohen SB, Zoga AC, Frederick RJ. Teres major injuries in two professional baseball pitchers. J Shoulder Elbow Surg. 2009;18(6):e1-e5.
9. Nagda SH, Cohen SB, Noonan TJ, Raasch WG, Ciccotti MG, Yocum LA. Management and outcomes of latissimus dorsi and teres major injuries in professional baseball pitchers. Am J Sports Med. 2011;39(10):2181-2186.
10. Ellman MB, Yanke A, Juhan T, et al. Open repair of an acute latissimus tendon avulsion in a Major League Baseball pitcher. J Shoulder Elbow Surg. 2013;22(7):e19-e23.
11. Ellman MB, Yanke A, Juhan T, et al. Open repair of retracted latissimus dorsi tendon avulsion. Am J Orthop. 2013;42(6):280-285.
12. Altchek DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3(3):159-165.
13. Limpisvasti O, ElAttrache NS, Jobe FW. Understanding shoulder and elbow injuries in baseball. J Am Acad Orthop Surg. 2007;15(3):139-147.
14. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
15. Morelli M, Nagamori J, Gilbart M, Miniaci A. Latissimus dorsi tendon transfer for massive irreparable cuff tears: an anatomic study. J Shoulder Elbow Surg. 2008;17(1):139-143.
16. Broome HL, Basmajian JV. The function of the teres major muscle: an electromyographic study. Anat Rec. 1971;170(3):309-310.
17. Brumback RJ, McBride MS, Ortolani NC. Functional evaluation of the shoulder after transfer of the vascularized latissimus dorsi muscle. J Bone Joint Surg Am. 1992;74(3):377-382.
18. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
19. Park JY, Lhee SH, Keum JS. Rupture of latissimus dorsi muscle in a tennis player. Orthopedics. 2008;31(10).
20. Gregory JM, Harwood DP, Sherman SL, Romeo AA. Surgical repair of a subacute latissimus dorsi tendon rupture. Tech Shoulder Elbow Surg. 2011;12(4):77-79.
21. Butterwick DJ, Mohtadi NG, Meeuwisse WH, Frizzell JB. Rupture of latissimus dorsi in an athlete. Clin J Sport Med. 2003;13(3):189-191.
22. Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion. A case report. Am J Sports Med. 2000;28(4):577-579.
23. Lim JK, Tilford ME, Hamersly SF, Sallay PI. Surgical repair of an acute latissimus dorsi tendon avulsion using suture anchors through a single incision. Am J Sports Med. 2006;34(8):1351-1355.
24. Hiemstra LA, Butterwick D, Cooke M, Walker RE. Surgical management of latissimus dorsi rupture in a steer wrestler. Clin J Sport Med. 2007;17(4):316-318.
25. Hapa O, Wijdicks CA, LaPrade RF, Braman JP. Out of the ring and into a sling: acute latissimus dorsi avulsion in a professional wrestler: a case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1146-1150.
26. Livesey J, Brownson P, Wallace WA. Traumatic latissimus dorsi tendon rupture. J Shoulder Elbow Surg. 2002;11(6):642-644.