User login
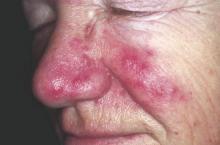
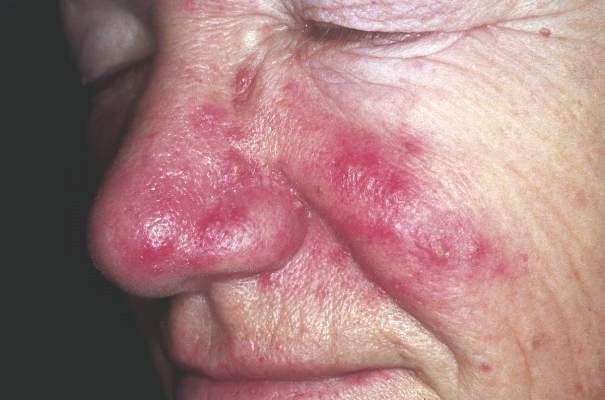
Danish study finds increased glioma risk in a rosacea population
An increased focus on neurologic symptoms in patients with rosacea may be warranted, according to Danish researchers, who found a significantly increased risk of glioma associated with rosacea, in a nationwide study of Danish citizens.
The observational study followed 5,484,910 Danish adults from January 1997 through December 2011; 68,372 were diagnosed with rosacea, and the remaining 5,416,538 were the reference group. The incidence rate of glioma per 10,000 person-years (adjusted for age, sex, and socioeconomic status) was 3.34 in the reference population, but was 4.99 among those with rosacea, reported Dr. Alexander Egeberg of the department of dermatoallergology, Herlev and Gentofte University Hospital, University of Copenhagen, Hellerup, and his coauthors (JAMA Dermatol. 2016 Jan 27. doi: 10.1001/jamadermatol.2015.5549).
The adjusted incidence rate ratio (IRR) of glioma in patients with rosacea was 1.36 (P less than .001). When the researchers limited the analysis to patients who had been diagnosed by a hospital dermatologist, the adjusted IRR was 1.82. The results remained significant after sensitivity analyses and after adjustment for potential confounders.
Among the patients with rosacea, men had an increased risk of glioma, compared with women (an incidence rate per 10,000 person-years of 6.45 vs. 4.30), although “gliomas and rosacea were generally more common among women,” the authors reported.
The association might be partially mediated by mechanisms dependent on matrix metalloproteinases (MMPs), the authors said, referring to studies indicating that MMPs, in particular MMP-9, “play a pivotal role in rosacea and regulation of the invasiveness of malignant glioma cells.” While speculative, “mechanisms dependent on MMPs may contribute to the link between rosacea and the risk for glioma,” the investigators added.
An increased focus on neurologic symptoms such as headaches, memory loss, visual symptoms, cognitive decline, and personality changes in patients with rosacea “and timely referral to relevant specialists may be warranted,” they concluded.
Limitations of the study included the observational design, which cannot establish causation, the authors noted. Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
An increased focus on neurologic symptoms in patients with rosacea may be warranted, according to Danish researchers, who found a significantly increased risk of glioma associated with rosacea, in a nationwide study of Danish citizens.
The observational study followed 5,484,910 Danish adults from January 1997 through December 2011; 68,372 were diagnosed with rosacea, and the remaining 5,416,538 were the reference group. The incidence rate of glioma per 10,000 person-years (adjusted for age, sex, and socioeconomic status) was 3.34 in the reference population, but was 4.99 among those with rosacea, reported Dr. Alexander Egeberg of the department of dermatoallergology, Herlev and Gentofte University Hospital, University of Copenhagen, Hellerup, and his coauthors (JAMA Dermatol. 2016 Jan 27. doi: 10.1001/jamadermatol.2015.5549).
The adjusted incidence rate ratio (IRR) of glioma in patients with rosacea was 1.36 (P less than .001). When the researchers limited the analysis to patients who had been diagnosed by a hospital dermatologist, the adjusted IRR was 1.82. The results remained significant after sensitivity analyses and after adjustment for potential confounders.
Among the patients with rosacea, men had an increased risk of glioma, compared with women (an incidence rate per 10,000 person-years of 6.45 vs. 4.30), although “gliomas and rosacea were generally more common among women,” the authors reported.
The association might be partially mediated by mechanisms dependent on matrix metalloproteinases (MMPs), the authors said, referring to studies indicating that MMPs, in particular MMP-9, “play a pivotal role in rosacea and regulation of the invasiveness of malignant glioma cells.” While speculative, “mechanisms dependent on MMPs may contribute to the link between rosacea and the risk for glioma,” the investigators added.
An increased focus on neurologic symptoms such as headaches, memory loss, visual symptoms, cognitive decline, and personality changes in patients with rosacea “and timely referral to relevant specialists may be warranted,” they concluded.
Limitations of the study included the observational design, which cannot establish causation, the authors noted. Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
An increased focus on neurologic symptoms in patients with rosacea may be warranted, according to Danish researchers, who found a significantly increased risk of glioma associated with rosacea, in a nationwide study of Danish citizens.
The observational study followed 5,484,910 Danish adults from January 1997 through December 2011; 68,372 were diagnosed with rosacea, and the remaining 5,416,538 were the reference group. The incidence rate of glioma per 10,000 person-years (adjusted for age, sex, and socioeconomic status) was 3.34 in the reference population, but was 4.99 among those with rosacea, reported Dr. Alexander Egeberg of the department of dermatoallergology, Herlev and Gentofte University Hospital, University of Copenhagen, Hellerup, and his coauthors (JAMA Dermatol. 2016 Jan 27. doi: 10.1001/jamadermatol.2015.5549).
The adjusted incidence rate ratio (IRR) of glioma in patients with rosacea was 1.36 (P less than .001). When the researchers limited the analysis to patients who had been diagnosed by a hospital dermatologist, the adjusted IRR was 1.82. The results remained significant after sensitivity analyses and after adjustment for potential confounders.
Among the patients with rosacea, men had an increased risk of glioma, compared with women (an incidence rate per 10,000 person-years of 6.45 vs. 4.30), although “gliomas and rosacea were generally more common among women,” the authors reported.
The association might be partially mediated by mechanisms dependent on matrix metalloproteinases (MMPs), the authors said, referring to studies indicating that MMPs, in particular MMP-9, “play a pivotal role in rosacea and regulation of the invasiveness of malignant glioma cells.” While speculative, “mechanisms dependent on MMPs may contribute to the link between rosacea and the risk for glioma,” the investigators added.
An increased focus on neurologic symptoms such as headaches, memory loss, visual symptoms, cognitive decline, and personality changes in patients with rosacea “and timely referral to relevant specialists may be warranted,” they concluded.
Limitations of the study included the observational design, which cannot establish causation, the authors noted. Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
FROM JAMA DERMATOLOGY
Key clinical point: Clinicians should be mindful of neurologic symptoms in patients with rosacea because of a significant association between glioma and rosacea.
Major finding: The incidence rate ratio of glioma per 10,000 person-years was 3.34 in the reference population and 4.99 in patients with rosacea.
Data source: A nationwide cohort study that followed 5,484 910 Danish adults from 1997 through 2011.
Disclosures: Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
Guidelines in works to tackle ruptured AAA transfers
CHICAGO – Adoption of an organized, systematic approach to ruptured abdominal aortic aneurysm has been inconsistent.
In a recent survey of vascular physicians in the western United States, 60% who accept ruptured abdominal aortic aneurysm (rAAA) transfers do not have a formal protocol for treatment and 70% do not use a transfer protocol or clinical guidelines (J Vasc Surg. 2015 Aug;62:326-30).
Guidelines for the management and transfer of patients with rAAA have been developed in the United Kingdom, but no such guidelines currently exist in the United States.
To address this disparity, the Western Vascular Society used the survey results, existing European guidelines, and a literature review to develop a set of 15 best practice steps for rAAA transfer. The “guidelines” were endorsed by the society members in September 2015 and are to be published early in 2016, Dr. Matthew Mell of Stanford (Calif.) University Medical Center said at a symposium on vascular surgery sponsored by Northwestern University. The guidelines identify four key components to a successful transfer: an organized inter-facility system of care including rapid triage and transport, defined clinical criteria for transfer, standard resuscitation protocols for the transport, and appropriate resources at the receiving hospital.
During transport, aim for a systolic blood pressure of 70 mm Hg to 90 mm Hg, establish peripheral intravenous access, and avoid aggressive fluid resuscitation, the guidelines advise. Blood products may delay the transfer.
Receiving hospitals should provide a simple and reliable method of referral and have formal protocols in place for the treatment of transferred patients.
Centers that receive patients should have endovascular aortic repair capabilities for ruptured aneurysms, including the ability to perform EVAR under local anesthesia, as well as appropriate facilities and expertise, Dr. Mell said. This advice is based mainly on outcomes observed in the IMPROVE trial (Br J Surg. 2014;101;216-24).
Successful programs tend to repair more than 20-25 ruptures per year, have on-site EVAR inventory, and, for the most part, have vascular surgeons able to perform dual open and endovascular repair. Hospital resources in these successful programs have a single phone number for transfer requests, electronic image transfer, immediately available blood products, hospital policy to accept all requests regardless of bed capacity, a contingency plan to create bed capacity after repair, and real-time management between the transfer center, bed control, and clinicians.
“This is really important because a lot of tertiary centers struggle with bed capacity if bottlenecked and a significant number [about one-third] of transfer requests are declined because of lack of capacity or dedicated room,” Dr. Mell said.
In a more recent study, nearly 20% of 4,439 patients who presented with rAAA in New York, California, and Florida were transferred for definitive care. Transfer rates rose yearly during the study period from 14% in 2005 to 22% in 2010 (J Vasc Surg. 2014;60:553-7).
“Transfer is increasingly utilized as a means for definitive care,” Dr. Mell said.
However, one in six of those transferred died without receiving treatment.
In adjusted analyses, inter-facility transfer was associated with significantly lower mortality when only patients receiving treatment were analyzed (adjusted odds ratio, 0.81; P = .02), but was actually associated with higher mortality when patients who died without treatment were also included (aOR, 1.30; P = .01).
“Outcomes after transfer can be improved by better patient selection and more efficient systems of care,” Dr. Mell concluded. “Guidelines may help; it’s too soon to know, but successful transfer programs require forethought, resources, and alignment of all stakeholders.”
Dr. Mell reported having no conflicts of interest.
CHICAGO – Adoption of an organized, systematic approach to ruptured abdominal aortic aneurysm has been inconsistent.
In a recent survey of vascular physicians in the western United States, 60% who accept ruptured abdominal aortic aneurysm (rAAA) transfers do not have a formal protocol for treatment and 70% do not use a transfer protocol or clinical guidelines (J Vasc Surg. 2015 Aug;62:326-30).
Guidelines for the management and transfer of patients with rAAA have been developed in the United Kingdom, but no such guidelines currently exist in the United States.
To address this disparity, the Western Vascular Society used the survey results, existing European guidelines, and a literature review to develop a set of 15 best practice steps for rAAA transfer. The “guidelines” were endorsed by the society members in September 2015 and are to be published early in 2016, Dr. Matthew Mell of Stanford (Calif.) University Medical Center said at a symposium on vascular surgery sponsored by Northwestern University. The guidelines identify four key components to a successful transfer: an organized inter-facility system of care including rapid triage and transport, defined clinical criteria for transfer, standard resuscitation protocols for the transport, and appropriate resources at the receiving hospital.
During transport, aim for a systolic blood pressure of 70 mm Hg to 90 mm Hg, establish peripheral intravenous access, and avoid aggressive fluid resuscitation, the guidelines advise. Blood products may delay the transfer.
Receiving hospitals should provide a simple and reliable method of referral and have formal protocols in place for the treatment of transferred patients.
Centers that receive patients should have endovascular aortic repair capabilities for ruptured aneurysms, including the ability to perform EVAR under local anesthesia, as well as appropriate facilities and expertise, Dr. Mell said. This advice is based mainly on outcomes observed in the IMPROVE trial (Br J Surg. 2014;101;216-24).
Successful programs tend to repair more than 20-25 ruptures per year, have on-site EVAR inventory, and, for the most part, have vascular surgeons able to perform dual open and endovascular repair. Hospital resources in these successful programs have a single phone number for transfer requests, electronic image transfer, immediately available blood products, hospital policy to accept all requests regardless of bed capacity, a contingency plan to create bed capacity after repair, and real-time management between the transfer center, bed control, and clinicians.
“This is really important because a lot of tertiary centers struggle with bed capacity if bottlenecked and a significant number [about one-third] of transfer requests are declined because of lack of capacity or dedicated room,” Dr. Mell said.
In a more recent study, nearly 20% of 4,439 patients who presented with rAAA in New York, California, and Florida were transferred for definitive care. Transfer rates rose yearly during the study period from 14% in 2005 to 22% in 2010 (J Vasc Surg. 2014;60:553-7).
“Transfer is increasingly utilized as a means for definitive care,” Dr. Mell said.
However, one in six of those transferred died without receiving treatment.
In adjusted analyses, inter-facility transfer was associated with significantly lower mortality when only patients receiving treatment were analyzed (adjusted odds ratio, 0.81; P = .02), but was actually associated with higher mortality when patients who died without treatment were also included (aOR, 1.30; P = .01).
“Outcomes after transfer can be improved by better patient selection and more efficient systems of care,” Dr. Mell concluded. “Guidelines may help; it’s too soon to know, but successful transfer programs require forethought, resources, and alignment of all stakeholders.”
Dr. Mell reported having no conflicts of interest.
CHICAGO – Adoption of an organized, systematic approach to ruptured abdominal aortic aneurysm has been inconsistent.
In a recent survey of vascular physicians in the western United States, 60% who accept ruptured abdominal aortic aneurysm (rAAA) transfers do not have a formal protocol for treatment and 70% do not use a transfer protocol or clinical guidelines (J Vasc Surg. 2015 Aug;62:326-30).
Guidelines for the management and transfer of patients with rAAA have been developed in the United Kingdom, but no such guidelines currently exist in the United States.
To address this disparity, the Western Vascular Society used the survey results, existing European guidelines, and a literature review to develop a set of 15 best practice steps for rAAA transfer. The “guidelines” were endorsed by the society members in September 2015 and are to be published early in 2016, Dr. Matthew Mell of Stanford (Calif.) University Medical Center said at a symposium on vascular surgery sponsored by Northwestern University. The guidelines identify four key components to a successful transfer: an organized inter-facility system of care including rapid triage and transport, defined clinical criteria for transfer, standard resuscitation protocols for the transport, and appropriate resources at the receiving hospital.
During transport, aim for a systolic blood pressure of 70 mm Hg to 90 mm Hg, establish peripheral intravenous access, and avoid aggressive fluid resuscitation, the guidelines advise. Blood products may delay the transfer.
Receiving hospitals should provide a simple and reliable method of referral and have formal protocols in place for the treatment of transferred patients.
Centers that receive patients should have endovascular aortic repair capabilities for ruptured aneurysms, including the ability to perform EVAR under local anesthesia, as well as appropriate facilities and expertise, Dr. Mell said. This advice is based mainly on outcomes observed in the IMPROVE trial (Br J Surg. 2014;101;216-24).
Successful programs tend to repair more than 20-25 ruptures per year, have on-site EVAR inventory, and, for the most part, have vascular surgeons able to perform dual open and endovascular repair. Hospital resources in these successful programs have a single phone number for transfer requests, electronic image transfer, immediately available blood products, hospital policy to accept all requests regardless of bed capacity, a contingency plan to create bed capacity after repair, and real-time management between the transfer center, bed control, and clinicians.
“This is really important because a lot of tertiary centers struggle with bed capacity if bottlenecked and a significant number [about one-third] of transfer requests are declined because of lack of capacity or dedicated room,” Dr. Mell said.
In a more recent study, nearly 20% of 4,439 patients who presented with rAAA in New York, California, and Florida were transferred for definitive care. Transfer rates rose yearly during the study period from 14% in 2005 to 22% in 2010 (J Vasc Surg. 2014;60:553-7).
“Transfer is increasingly utilized as a means for definitive care,” Dr. Mell said.
However, one in six of those transferred died without receiving treatment.
In adjusted analyses, inter-facility transfer was associated with significantly lower mortality when only patients receiving treatment were analyzed (adjusted odds ratio, 0.81; P = .02), but was actually associated with higher mortality when patients who died without treatment were also included (aOR, 1.30; P = .01).
“Outcomes after transfer can be improved by better patient selection and more efficient systems of care,” Dr. Mell concluded. “Guidelines may help; it’s too soon to know, but successful transfer programs require forethought, resources, and alignment of all stakeholders.”
Dr. Mell reported having no conflicts of interest.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Resources limit availability of bone marrow transplants
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
FROM JAMA
Key clinical point: In regions with limited resources, peripheral blood stem cells were used more frequently than bone marrow in hematopoietic stem cell transplantation for bone marrow failure.
Major finding: For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Data source: Data on 3,282 allogeneic HSCTs for bone marrow failure performed in 2009 and 2010 were collected by retrospective surveys by the Worldwide Network for Blood and Marrow Transplantation, and by direct contact with transplant centers in countries without registries.
Disclosures: Funding was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Stent-retriever Therapy Improves the Rate of Functional Independence for Acute Ischemic Patients
NEW YORK (Reuters Health) - Stent-retriever therapy for the treatment of acute ischemic stroke improves the rate of functional independence at 90 days, according to a systematic
review and meta-analysis.
Stent retrievers are deployed in an occluded vessel, temporarily expanded into the body of a thrombus, and then retracted along with the thrombus.
Dr. Mark J. Eisenberg, from Jewish General Hospital/McGill University, Montreal, Quebec, Canada, and colleagues compared stent retrievers with intravenous recombinant tissue plasminogen activator (rtPA) versus rtPA alone for the treatment of acute ischemic stroke in their systematic review and meta-analysis of five randomized controlled trials (RCTs) with a total of 1,287 patients.
In all five trials, patients randomized to stent-retriever therapy had significantly better functional independence (a modified Rankin Scale (mRS) score of 0-2) at 90 days than did patients randomized to rtPA alone.
Stent-retriever therapy also doubled the likelihood of a one-unit improvement in mRS score at 90 days, according to the January 25 JAMA Neurology online report.
In pooled analyses, there were no significant differences between treatment groups in all-cause mortality, intracranial hemorrhage, or parenchymal hematoma rates at 90 days.
The number needed to treat to achieve an mRS score of 0 to 2 at 90 days was six.
"Given the totality of the evidence regarding the benefits and risks of stent retrievers, our results suggest that the use of these devices in patients with acute ischemic stroke is warranted," the researchers conclude.
Dr. Raphael A. Carandang, from the University of Massachusetts Medical School, Worcester, who wrote an editorial related to this report, told Reuters Health by email, "The data from these five RCTs (as the meta-analysis confirms) provides level 1 class A evidence that in the properly selected patients, stent retriever treatment is superior to the current standard of care with intravenous rtPA and would endorse that it should be considered in all acute ischemic stroke patients that are eligible for it. As with any therapy, proper patient selection is needed, but I do think it changes the landscape of acute stroke treatment going forward. I think that systems of care should be organized in stroke centers around this new therapy."
"The current technology for acute stroke care has reached the point where effective interventional therapies are clearly and unequivocally beneficial in the properly selected patients, but the key takeaway is still that the patients need to be selected properly, and the biggest factor continues to be time to recanalization, which means that all practitioners and systems of care need to focus on getting patients to treatment sooner than ever before," Dr. Carandang concluded.
Dr. Woong Yoon, from Chonnam National University Hospital, Gwangju, Korea, recently found no improvement in outcomes with stent-retriever therapy for patients with acute anterior circulation stroke (http://bit.ly/1OT7M5I). He told Reuters Health by email, "Not all patients with acute ischemic stroke can benefit from this new treatment. Patients with acute stroke due to occlusions of intracranial large vessels such as internal carotid artery, middle cerebral artery, or basilar artery and who presented within six-eight hours of stroke onset can benefit from thrombectomy with stent retrievers."
"We should realize that we are facing the moment of change in the paradigm for acute stroke treatment," Dr. Yoon concluded."Further refinement in the patient selection for stent retrieverthrombectomy is needed in the near future."
Dr. Mayank Goyal, from the University of Calgary, Alberta, Canada, coauthored two of the studies included in the current review. He told Reuters Health by email," There are several additional data coming out on this issue in the near future, which will in fact be more powerful than what is mentioned in this study."
Dr. Goyal said, "However, the key issues going into the future are: how should those patients who were not included in the current trials be treated; how should we as a collective evaluate new devices/technologies; and how do societies/countries who cannot afford stent retrievers implement endovascular stroke treatment."
Dr. Eisenberg was unavailable for comment.
The authors reported no funding. Three coauthors reported disclosures.
NEW YORK (Reuters Health) - Stent-retriever therapy for the treatment of acute ischemic stroke improves the rate of functional independence at 90 days, according to a systematic
review and meta-analysis.
Stent retrievers are deployed in an occluded vessel, temporarily expanded into the body of a thrombus, and then retracted along with the thrombus.
Dr. Mark J. Eisenberg, from Jewish General Hospital/McGill University, Montreal, Quebec, Canada, and colleagues compared stent retrievers with intravenous recombinant tissue plasminogen activator (rtPA) versus rtPA alone for the treatment of acute ischemic stroke in their systematic review and meta-analysis of five randomized controlled trials (RCTs) with a total of 1,287 patients.
In all five trials, patients randomized to stent-retriever therapy had significantly better functional independence (a modified Rankin Scale (mRS) score of 0-2) at 90 days than did patients randomized to rtPA alone.
Stent-retriever therapy also doubled the likelihood of a one-unit improvement in mRS score at 90 days, according to the January 25 JAMA Neurology online report.
In pooled analyses, there were no significant differences between treatment groups in all-cause mortality, intracranial hemorrhage, or parenchymal hematoma rates at 90 days.
The number needed to treat to achieve an mRS score of 0 to 2 at 90 days was six.
"Given the totality of the evidence regarding the benefits and risks of stent retrievers, our results suggest that the use of these devices in patients with acute ischemic stroke is warranted," the researchers conclude.
Dr. Raphael A. Carandang, from the University of Massachusetts Medical School, Worcester, who wrote an editorial related to this report, told Reuters Health by email, "The data from these five RCTs (as the meta-analysis confirms) provides level 1 class A evidence that in the properly selected patients, stent retriever treatment is superior to the current standard of care with intravenous rtPA and would endorse that it should be considered in all acute ischemic stroke patients that are eligible for it. As with any therapy, proper patient selection is needed, but I do think it changes the landscape of acute stroke treatment going forward. I think that systems of care should be organized in stroke centers around this new therapy."
"The current technology for acute stroke care has reached the point where effective interventional therapies are clearly and unequivocally beneficial in the properly selected patients, but the key takeaway is still that the patients need to be selected properly, and the biggest factor continues to be time to recanalization, which means that all practitioners and systems of care need to focus on getting patients to treatment sooner than ever before," Dr. Carandang concluded.
Dr. Woong Yoon, from Chonnam National University Hospital, Gwangju, Korea, recently found no improvement in outcomes with stent-retriever therapy for patients with acute anterior circulation stroke (http://bit.ly/1OT7M5I). He told Reuters Health by email, "Not all patients with acute ischemic stroke can benefit from this new treatment. Patients with acute stroke due to occlusions of intracranial large vessels such as internal carotid artery, middle cerebral artery, or basilar artery and who presented within six-eight hours of stroke onset can benefit from thrombectomy with stent retrievers."
"We should realize that we are facing the moment of change in the paradigm for acute stroke treatment," Dr. Yoon concluded."Further refinement in the patient selection for stent retrieverthrombectomy is needed in the near future."
Dr. Mayank Goyal, from the University of Calgary, Alberta, Canada, coauthored two of the studies included in the current review. He told Reuters Health by email," There are several additional data coming out on this issue in the near future, which will in fact be more powerful than what is mentioned in this study."
Dr. Goyal said, "However, the key issues going into the future are: how should those patients who were not included in the current trials be treated; how should we as a collective evaluate new devices/technologies; and how do societies/countries who cannot afford stent retrievers implement endovascular stroke treatment."
Dr. Eisenberg was unavailable for comment.
The authors reported no funding. Three coauthors reported disclosures.
NEW YORK (Reuters Health) - Stent-retriever therapy for the treatment of acute ischemic stroke improves the rate of functional independence at 90 days, according to a systematic
review and meta-analysis.
Stent retrievers are deployed in an occluded vessel, temporarily expanded into the body of a thrombus, and then retracted along with the thrombus.
Dr. Mark J. Eisenberg, from Jewish General Hospital/McGill University, Montreal, Quebec, Canada, and colleagues compared stent retrievers with intravenous recombinant tissue plasminogen activator (rtPA) versus rtPA alone for the treatment of acute ischemic stroke in their systematic review and meta-analysis of five randomized controlled trials (RCTs) with a total of 1,287 patients.
In all five trials, patients randomized to stent-retriever therapy had significantly better functional independence (a modified Rankin Scale (mRS) score of 0-2) at 90 days than did patients randomized to rtPA alone.
Stent-retriever therapy also doubled the likelihood of a one-unit improvement in mRS score at 90 days, according to the January 25 JAMA Neurology online report.
In pooled analyses, there were no significant differences between treatment groups in all-cause mortality, intracranial hemorrhage, or parenchymal hematoma rates at 90 days.
The number needed to treat to achieve an mRS score of 0 to 2 at 90 days was six.
"Given the totality of the evidence regarding the benefits and risks of stent retrievers, our results suggest that the use of these devices in patients with acute ischemic stroke is warranted," the researchers conclude.
Dr. Raphael A. Carandang, from the University of Massachusetts Medical School, Worcester, who wrote an editorial related to this report, told Reuters Health by email, "The data from these five RCTs (as the meta-analysis confirms) provides level 1 class A evidence that in the properly selected patients, stent retriever treatment is superior to the current standard of care with intravenous rtPA and would endorse that it should be considered in all acute ischemic stroke patients that are eligible for it. As with any therapy, proper patient selection is needed, but I do think it changes the landscape of acute stroke treatment going forward. I think that systems of care should be organized in stroke centers around this new therapy."
"The current technology for acute stroke care has reached the point where effective interventional therapies are clearly and unequivocally beneficial in the properly selected patients, but the key takeaway is still that the patients need to be selected properly, and the biggest factor continues to be time to recanalization, which means that all practitioners and systems of care need to focus on getting patients to treatment sooner than ever before," Dr. Carandang concluded.
Dr. Woong Yoon, from Chonnam National University Hospital, Gwangju, Korea, recently found no improvement in outcomes with stent-retriever therapy for patients with acute anterior circulation stroke (http://bit.ly/1OT7M5I). He told Reuters Health by email, "Not all patients with acute ischemic stroke can benefit from this new treatment. Patients with acute stroke due to occlusions of intracranial large vessels such as internal carotid artery, middle cerebral artery, or basilar artery and who presented within six-eight hours of stroke onset can benefit from thrombectomy with stent retrievers."
"We should realize that we are facing the moment of change in the paradigm for acute stroke treatment," Dr. Yoon concluded."Further refinement in the patient selection for stent retrieverthrombectomy is needed in the near future."
Dr. Mayank Goyal, from the University of Calgary, Alberta, Canada, coauthored two of the studies included in the current review. He told Reuters Health by email," There are several additional data coming out on this issue in the near future, which will in fact be more powerful than what is mentioned in this study."
Dr. Goyal said, "However, the key issues going into the future are: how should those patients who were not included in the current trials be treated; how should we as a collective evaluate new devices/technologies; and how do societies/countries who cannot afford stent retrievers implement endovascular stroke treatment."
Dr. Eisenberg was unavailable for comment.
The authors reported no funding. Three coauthors reported disclosures.
Dual inhibitor could treat ATLL

Photo by Larry Young
SAN FRANCISCO—Preclinical research suggests a compound that inhibits both EZH1 and EZH2 could be effective against adult T-cell leukemia/lymphoma (ATLL).
The compound, known as OR-S1, has demonstrated activity against ATLL in vitro and in vivo.
Researchers said OR-S1 reversed epigenetic disruption in ATLL cells, selectively eliminated both ATLL cells and cells infected with human T-cell leukemia virus type I (HTLV-1), and inhibited tumor growth in mouse models of ATLL.
Based on these results, the researchers are planning a phase 1 study of the compound.
Makoto Yamagishi, PhD, of The University of Tokyo in Japan, described the preclinical research with OR-S1 and discussed the rationale for developing the compound at the 8th Annual T-cell Lymphoma Forum. The work was carried out in collaboration with Daiichi Sankyo Co., Ltd.
“We do not precisely understand the molecular mechanism of ATLL development, including genetic and epigenetic abnormalities,” Dr Yamagishi noted.
To gain some insight, he and his colleagues performed microRNA profiling, gene expression profiling, and histone methylation/epigenetic factor profiling on cells from ATLL patients and CD4+ T cells from healthy donors.
The team found that PRC2 factors were significantly upregulated in ATLL. EZH2 was the most upregulated histone methyltransferase, but ATLL cells did not have active mutations in the EZH2 gene. Dr Yamagishi said this suggests EZH2 upregulation is critical for the ATLL-specific epigenome.
“At long last, we determined the epigenetic pattern of ATLL,” he said. “ATLL cells showed specific and significant reprogramming of the epigenome, especially H3K27me3 gain. We found abnormal H3K27me3 change in half of genes, and gain was dominant.”
“But, interestingly, the methylated genes are specific in ATLL and do not overlap with other EZH2-dependent cell types, such as embryonic stem cells and diffuse large B-cell lymphoma cells. So ATLL has a very unique epigenome.”
Further investigation revealed that both EZH1 and EZH2 contribute to ATLL-specific epigenetic deregulation. More than 80% of H3K27me3 accumulated genes are occupied by EZH1 and/or EZH2.
So the researchers decided to examine the effects of knocking down EZH1 and EZH2 in ATLL cells.
Compared with knockdown of either gene alone, double knockdown synergistically influenced target gene expression. It led to complete dysfunction of the Polycomb family and had a significant impact on ATLL cell survival.
The researchers also found that EZH1 depletion enhanced ATLL cells’ sensitivity to the EZH2 inhibitor GSK126.
So the team decided to develop a dual EZH1/EZH2 inhibitor. They created OR-S1, which showed “strong activity” against EZH1 and EZH2 but none of the other histone methyltransferases tested.
In in vitro experiments, OR-S1 completely removed H3K27me3 and significantly reduced cell growth in the ATLL-derived cell line TL-Om1.
The drug also reduced cell viability in primary ATLL cells. All 15 samples tested proved sensitive to OR-S1. In addition, OR-S1 treatment selectively removed HTLV-1-infected cells from samples taken from 16 asymptomatic carriers.
Finally, OR-S1 proved active in mice. The drug prevented engraftment of ATLL cells in immunocompromised mice. All 6 OR-S1-treated mice were alive and tumor-free at 49 days, whereas 5 of 6 control mice had died (P=0.0041).
In mice treated after ATLL cell engraftment, OR-S1 reduced tumor growth without causing notable weight loss.
“Synthetic lethality by targeting EZH1 and EZH2 is promising [for ATLL],” Dr Yamagishi said. “Toxicity tests suggest the EZH1/2 dual inhibitor may be sufficient for clinical use, so we are now planning a phase 1 study.” ![]()

Photo by Larry Young
SAN FRANCISCO—Preclinical research suggests a compound that inhibits both EZH1 and EZH2 could be effective against adult T-cell leukemia/lymphoma (ATLL).
The compound, known as OR-S1, has demonstrated activity against ATLL in vitro and in vivo.
Researchers said OR-S1 reversed epigenetic disruption in ATLL cells, selectively eliminated both ATLL cells and cells infected with human T-cell leukemia virus type I (HTLV-1), and inhibited tumor growth in mouse models of ATLL.
Based on these results, the researchers are planning a phase 1 study of the compound.
Makoto Yamagishi, PhD, of The University of Tokyo in Japan, described the preclinical research with OR-S1 and discussed the rationale for developing the compound at the 8th Annual T-cell Lymphoma Forum. The work was carried out in collaboration with Daiichi Sankyo Co., Ltd.
“We do not precisely understand the molecular mechanism of ATLL development, including genetic and epigenetic abnormalities,” Dr Yamagishi noted.
To gain some insight, he and his colleagues performed microRNA profiling, gene expression profiling, and histone methylation/epigenetic factor profiling on cells from ATLL patients and CD4+ T cells from healthy donors.
The team found that PRC2 factors were significantly upregulated in ATLL. EZH2 was the most upregulated histone methyltransferase, but ATLL cells did not have active mutations in the EZH2 gene. Dr Yamagishi said this suggests EZH2 upregulation is critical for the ATLL-specific epigenome.
“At long last, we determined the epigenetic pattern of ATLL,” he said. “ATLL cells showed specific and significant reprogramming of the epigenome, especially H3K27me3 gain. We found abnormal H3K27me3 change in half of genes, and gain was dominant.”
“But, interestingly, the methylated genes are specific in ATLL and do not overlap with other EZH2-dependent cell types, such as embryonic stem cells and diffuse large B-cell lymphoma cells. So ATLL has a very unique epigenome.”
Further investigation revealed that both EZH1 and EZH2 contribute to ATLL-specific epigenetic deregulation. More than 80% of H3K27me3 accumulated genes are occupied by EZH1 and/or EZH2.
So the researchers decided to examine the effects of knocking down EZH1 and EZH2 in ATLL cells.
Compared with knockdown of either gene alone, double knockdown synergistically influenced target gene expression. It led to complete dysfunction of the Polycomb family and had a significant impact on ATLL cell survival.
The researchers also found that EZH1 depletion enhanced ATLL cells’ sensitivity to the EZH2 inhibitor GSK126.
So the team decided to develop a dual EZH1/EZH2 inhibitor. They created OR-S1, which showed “strong activity” against EZH1 and EZH2 but none of the other histone methyltransferases tested.
In in vitro experiments, OR-S1 completely removed H3K27me3 and significantly reduced cell growth in the ATLL-derived cell line TL-Om1.
The drug also reduced cell viability in primary ATLL cells. All 15 samples tested proved sensitive to OR-S1. In addition, OR-S1 treatment selectively removed HTLV-1-infected cells from samples taken from 16 asymptomatic carriers.
Finally, OR-S1 proved active in mice. The drug prevented engraftment of ATLL cells in immunocompromised mice. All 6 OR-S1-treated mice were alive and tumor-free at 49 days, whereas 5 of 6 control mice had died (P=0.0041).
In mice treated after ATLL cell engraftment, OR-S1 reduced tumor growth without causing notable weight loss.
“Synthetic lethality by targeting EZH1 and EZH2 is promising [for ATLL],” Dr Yamagishi said. “Toxicity tests suggest the EZH1/2 dual inhibitor may be sufficient for clinical use, so we are now planning a phase 1 study.” ![]()

Photo by Larry Young
SAN FRANCISCO—Preclinical research suggests a compound that inhibits both EZH1 and EZH2 could be effective against adult T-cell leukemia/lymphoma (ATLL).
The compound, known as OR-S1, has demonstrated activity against ATLL in vitro and in vivo.
Researchers said OR-S1 reversed epigenetic disruption in ATLL cells, selectively eliminated both ATLL cells and cells infected with human T-cell leukemia virus type I (HTLV-1), and inhibited tumor growth in mouse models of ATLL.
Based on these results, the researchers are planning a phase 1 study of the compound.
Makoto Yamagishi, PhD, of The University of Tokyo in Japan, described the preclinical research with OR-S1 and discussed the rationale for developing the compound at the 8th Annual T-cell Lymphoma Forum. The work was carried out in collaboration with Daiichi Sankyo Co., Ltd.
“We do not precisely understand the molecular mechanism of ATLL development, including genetic and epigenetic abnormalities,” Dr Yamagishi noted.
To gain some insight, he and his colleagues performed microRNA profiling, gene expression profiling, and histone methylation/epigenetic factor profiling on cells from ATLL patients and CD4+ T cells from healthy donors.
The team found that PRC2 factors were significantly upregulated in ATLL. EZH2 was the most upregulated histone methyltransferase, but ATLL cells did not have active mutations in the EZH2 gene. Dr Yamagishi said this suggests EZH2 upregulation is critical for the ATLL-specific epigenome.
“At long last, we determined the epigenetic pattern of ATLL,” he said. “ATLL cells showed specific and significant reprogramming of the epigenome, especially H3K27me3 gain. We found abnormal H3K27me3 change in half of genes, and gain was dominant.”
“But, interestingly, the methylated genes are specific in ATLL and do not overlap with other EZH2-dependent cell types, such as embryonic stem cells and diffuse large B-cell lymphoma cells. So ATLL has a very unique epigenome.”
Further investigation revealed that both EZH1 and EZH2 contribute to ATLL-specific epigenetic deregulation. More than 80% of H3K27me3 accumulated genes are occupied by EZH1 and/or EZH2.
So the researchers decided to examine the effects of knocking down EZH1 and EZH2 in ATLL cells.
Compared with knockdown of either gene alone, double knockdown synergistically influenced target gene expression. It led to complete dysfunction of the Polycomb family and had a significant impact on ATLL cell survival.
The researchers also found that EZH1 depletion enhanced ATLL cells’ sensitivity to the EZH2 inhibitor GSK126.
So the team decided to develop a dual EZH1/EZH2 inhibitor. They created OR-S1, which showed “strong activity” against EZH1 and EZH2 but none of the other histone methyltransferases tested.
In in vitro experiments, OR-S1 completely removed H3K27me3 and significantly reduced cell growth in the ATLL-derived cell line TL-Om1.
The drug also reduced cell viability in primary ATLL cells. All 15 samples tested proved sensitive to OR-S1. In addition, OR-S1 treatment selectively removed HTLV-1-infected cells from samples taken from 16 asymptomatic carriers.
Finally, OR-S1 proved active in mice. The drug prevented engraftment of ATLL cells in immunocompromised mice. All 6 OR-S1-treated mice were alive and tumor-free at 49 days, whereas 5 of 6 control mice had died (P=0.0041).
In mice treated after ATLL cell engraftment, OR-S1 reduced tumor growth without causing notable weight loss.
“Synthetic lethality by targeting EZH1 and EZH2 is promising [for ATLL],” Dr Yamagishi said. “Toxicity tests suggest the EZH1/2 dual inhibitor may be sufficient for clinical use, so we are now planning a phase 1 study.” ![]()
New insight into Ph-like ALL could lead to new treatment

Photo courtesy of St. Jude
Children’s Research Hospital
Research published in Cancer Cell appears to explain how the abnormal breakage and rearrangement of chromosomes in white blood cells triggers Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL).
Genomic analysis revealed 4 chromosomal rearrangements that all resulted in a truncated version of the erythropoietin receptor (EPOR) gene and drove white blood cells to proliferate out of control.
“To our knowledge, this is a previously unknown mechanism for leukemia,” said study author Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our search of cancer genomic data has shown that there are many other examples of chromosomal rearrangements that alter genes’ structure, but this type—where a truncating rearrangement leads to activation—is new.”
Although Dr Mullighan and his colleagues had previously identified an abnormal chromosome rearrangement in Ph-like ALL, little was known about the biological effects of that rearrangement. So they set out to pinpoint those effects by studying human leukemic cells and mouse cells engineered to mimic Ph-like ALL.
The investigators discovered the 4 rearrangements of EPOR, all of which resulted in truncation of the cytoplasmic tail of EPOR at residues similar to those mutated in primary familial congenital polycythemia. The proximal tyrosine essential for receptor activation was preserved, but distal regulatory residues were lost.
The team said these rearrangements resulted in deregulated EPOR expression, hypersensitivity to erythropoietin stimulation, and heightened JAK-STAT activation.
The investigators noted that the rearrangements were present in all of the leukemic cells from patients, which suggests these changes were fundamental to Ph-like ALL development. The team also showed that introducing truncated EPOR in mouse B-cell progenitors gave rise to ALL in mice.
Further investigation revealed that EPOR rearrangements arise early in the development of Ph-like ALL and persist as the disease progresses.
“That finding was important because it suggests that treatments for this leukemia targeting this receptor won’t just impact a subset of the leukemia cells, allowing others to keep proliferating,” said study author Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital.
The investigators then found that human leukemic cells with EPOR rearrangements were sensitive to JAK-STAT inhibition via treatment with ruxolitinib.
The team also cited the case of an adult patient treated at MD Anderson Cancer Research Center in Houston, Texas, whose genetic analysis revealed EPOR-rearranged ALL. That patient had not responded significantly to other chemotherapy drugs. But, when given ruxolitinib, the patient showed a major drop in leukemia cells.
In experiments with leukemic cells, the investigators found that ruxolitinib worked synergistically with 3 chemotherapeutic agents—dexamethasone, vincristine, and daunorubicin.
“We think these findings provide a useful road map for planning more accurate testing of combination chemotherapies,” Dr Mullighan said.
“These findings expand the number of ALL patients who should be amenable to precision medicine therapies that add targeted inhibitors to chemotherapy for ALL patents with specific genetic changes in the leukemia cells,” added study author Stephen Hunger, MD, of Children’s Hospital of Philadelphia in Pennsylvania.
Dr Hunger said the Children’s Oncology Group has developed a clinical trial testing this strategy with ruxolitinib, which will begin treating patients in mid-2016. Based on the results of the Cancer Cell research, the trial will include children with ALL and EPOR rearrangements. ![]()

Photo courtesy of St. Jude
Children’s Research Hospital
Research published in Cancer Cell appears to explain how the abnormal breakage and rearrangement of chromosomes in white blood cells triggers Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL).
Genomic analysis revealed 4 chromosomal rearrangements that all resulted in a truncated version of the erythropoietin receptor (EPOR) gene and drove white blood cells to proliferate out of control.
“To our knowledge, this is a previously unknown mechanism for leukemia,” said study author Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our search of cancer genomic data has shown that there are many other examples of chromosomal rearrangements that alter genes’ structure, but this type—where a truncating rearrangement leads to activation—is new.”
Although Dr Mullighan and his colleagues had previously identified an abnormal chromosome rearrangement in Ph-like ALL, little was known about the biological effects of that rearrangement. So they set out to pinpoint those effects by studying human leukemic cells and mouse cells engineered to mimic Ph-like ALL.
The investigators discovered the 4 rearrangements of EPOR, all of which resulted in truncation of the cytoplasmic tail of EPOR at residues similar to those mutated in primary familial congenital polycythemia. The proximal tyrosine essential for receptor activation was preserved, but distal regulatory residues were lost.
The team said these rearrangements resulted in deregulated EPOR expression, hypersensitivity to erythropoietin stimulation, and heightened JAK-STAT activation.
The investigators noted that the rearrangements were present in all of the leukemic cells from patients, which suggests these changes were fundamental to Ph-like ALL development. The team also showed that introducing truncated EPOR in mouse B-cell progenitors gave rise to ALL in mice.
Further investigation revealed that EPOR rearrangements arise early in the development of Ph-like ALL and persist as the disease progresses.
“That finding was important because it suggests that treatments for this leukemia targeting this receptor won’t just impact a subset of the leukemia cells, allowing others to keep proliferating,” said study author Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital.
The investigators then found that human leukemic cells with EPOR rearrangements were sensitive to JAK-STAT inhibition via treatment with ruxolitinib.
The team also cited the case of an adult patient treated at MD Anderson Cancer Research Center in Houston, Texas, whose genetic analysis revealed EPOR-rearranged ALL. That patient had not responded significantly to other chemotherapy drugs. But, when given ruxolitinib, the patient showed a major drop in leukemia cells.
In experiments with leukemic cells, the investigators found that ruxolitinib worked synergistically with 3 chemotherapeutic agents—dexamethasone, vincristine, and daunorubicin.
“We think these findings provide a useful road map for planning more accurate testing of combination chemotherapies,” Dr Mullighan said.
“These findings expand the number of ALL patients who should be amenable to precision medicine therapies that add targeted inhibitors to chemotherapy for ALL patents with specific genetic changes in the leukemia cells,” added study author Stephen Hunger, MD, of Children’s Hospital of Philadelphia in Pennsylvania.
Dr Hunger said the Children’s Oncology Group has developed a clinical trial testing this strategy with ruxolitinib, which will begin treating patients in mid-2016. Based on the results of the Cancer Cell research, the trial will include children with ALL and EPOR rearrangements. ![]()

Photo courtesy of St. Jude
Children’s Research Hospital
Research published in Cancer Cell appears to explain how the abnormal breakage and rearrangement of chromosomes in white blood cells triggers Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL).
Genomic analysis revealed 4 chromosomal rearrangements that all resulted in a truncated version of the erythropoietin receptor (EPOR) gene and drove white blood cells to proliferate out of control.
“To our knowledge, this is a previously unknown mechanism for leukemia,” said study author Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Our search of cancer genomic data has shown that there are many other examples of chromosomal rearrangements that alter genes’ structure, but this type—where a truncating rearrangement leads to activation—is new.”
Although Dr Mullighan and his colleagues had previously identified an abnormal chromosome rearrangement in Ph-like ALL, little was known about the biological effects of that rearrangement. So they set out to pinpoint those effects by studying human leukemic cells and mouse cells engineered to mimic Ph-like ALL.
The investigators discovered the 4 rearrangements of EPOR, all of which resulted in truncation of the cytoplasmic tail of EPOR at residues similar to those mutated in primary familial congenital polycythemia. The proximal tyrosine essential for receptor activation was preserved, but distal regulatory residues were lost.
The team said these rearrangements resulted in deregulated EPOR expression, hypersensitivity to erythropoietin stimulation, and heightened JAK-STAT activation.
The investigators noted that the rearrangements were present in all of the leukemic cells from patients, which suggests these changes were fundamental to Ph-like ALL development. The team also showed that introducing truncated EPOR in mouse B-cell progenitors gave rise to ALL in mice.
Further investigation revealed that EPOR rearrangements arise early in the development of Ph-like ALL and persist as the disease progresses.
“That finding was important because it suggests that treatments for this leukemia targeting this receptor won’t just impact a subset of the leukemia cells, allowing others to keep proliferating,” said study author Ilaria Iacobucci, PhD, of St. Jude Children’s Research Hospital.
The investigators then found that human leukemic cells with EPOR rearrangements were sensitive to JAK-STAT inhibition via treatment with ruxolitinib.
The team also cited the case of an adult patient treated at MD Anderson Cancer Research Center in Houston, Texas, whose genetic analysis revealed EPOR-rearranged ALL. That patient had not responded significantly to other chemotherapy drugs. But, when given ruxolitinib, the patient showed a major drop in leukemia cells.
In experiments with leukemic cells, the investigators found that ruxolitinib worked synergistically with 3 chemotherapeutic agents—dexamethasone, vincristine, and daunorubicin.
“We think these findings provide a useful road map for planning more accurate testing of combination chemotherapies,” Dr Mullighan said.
“These findings expand the number of ALL patients who should be amenable to precision medicine therapies that add targeted inhibitors to chemotherapy for ALL patents with specific genetic changes in the leukemia cells,” added study author Stephen Hunger, MD, of Children’s Hospital of Philadelphia in Pennsylvania.
Dr Hunger said the Children’s Oncology Group has developed a clinical trial testing this strategy with ruxolitinib, which will begin treating patients in mid-2016. Based on the results of the Cancer Cell research, the trial will include children with ALL and EPOR rearrangements. ![]()
Study reveals delays in emergency blood transfusions

Photo courtesy of UAB Hospital
A new study suggests that as few as 2% of patients with life-threatening bleeding after serious injury receive optimal blood transfusion therapy in England and Wales.
Researchers estimate that nearly 5000 trauma patients sustain a major hemorrhage in England and Wales each year, and one-third of those patients die.
The current study, published in the British Journal of Surgery, highlights how delays in transfusions may contribute to this death rate.
“The rapid and consistent delivery of blood, plasma, platelets, and other clotting products to trauma patients is essential to maintain clotting during hemorrhage and has been shown to halve mortality,” said study author Karim Brohi, MBBS, of Queen Mary University of London in the UK.
“However, we found that only 2% of patients with massive hemorrhage received the optimal type of blood transfusion for their resuscitation. There is a clear opportunity for clinicians to improve the delivery of blood and clotting products during resuscitation for major hemorrhage.”
Dr Brohi and his colleagues analyzed 442 patients treated at 22 hospitals in England and Wales. The patients had experienced major hemorrhage as a result of injuries and received at least 4 units of packed red blood cells (PRBCs) in the first 24 hours of admission.
The patients’ median age was 38 (range, 24-54), and 74% were men. Thirty-three percent of patients (n=146) had massive hemorrhage.
Mortality from bleeding tended to occur early in these patients. Twenty-seven percent of patients (n=117) died in hospital—18% (n=79) within the first 24 hours. The 30-day mortality rate was about 27% (n=119), and 33% of evaluable patients had died at 1 year (127/383).
All 442 patients received PRBCs. The median number of PRBC units transfused within 24 hours was 7 (range, 5-11), and the median number of PRBC units given in 30 days was 9 (range, 6-15).
The average time to transfusion of PRBCs was longer than expected, at 41 minutes (range, 1-122).
Similarly, the researchers found the administration of blood components such as plasma and platelets to be significantly delayed, occurring, on average, 2 to 3 hours after admission.
Three-quarters of patients (n=330) received fresh-frozen plasma (FFP). The median number of FFP units given within 24 hours was 4 (range, 0-7), and the time to first FFP transfusion was 87 minutes (range, 42.5-229).
About 45% of patients (n=197) received platelets. The median dose was 0 (range, 0-1), and the time to first platelet transfusion was 146 minutes (range, 72.5-364).
About 28% of patients (n=122) received cryoprecipitate. The median dose was 0 (range, 0-1), and the time to first cryoprecipitate infusion was 179.5 minutes (range, 84.5-333.5).
“The rapid delivery of the right mix of blood components in an emergency environment is extremely challenging,” Dr Brohi said.
“Some transfusion components have to be thawed and, at present, aren’t always available for the patient quickly enough. More research is also needed into techniques and devices to control bleeding earlier, even at the scene of injury.”
The researchers noted that this study had its limitations, such as incomplete data for some patients. ![]()

Photo courtesy of UAB Hospital
A new study suggests that as few as 2% of patients with life-threatening bleeding after serious injury receive optimal blood transfusion therapy in England and Wales.
Researchers estimate that nearly 5000 trauma patients sustain a major hemorrhage in England and Wales each year, and one-third of those patients die.
The current study, published in the British Journal of Surgery, highlights how delays in transfusions may contribute to this death rate.
“The rapid and consistent delivery of blood, plasma, platelets, and other clotting products to trauma patients is essential to maintain clotting during hemorrhage and has been shown to halve mortality,” said study author Karim Brohi, MBBS, of Queen Mary University of London in the UK.
“However, we found that only 2% of patients with massive hemorrhage received the optimal type of blood transfusion for their resuscitation. There is a clear opportunity for clinicians to improve the delivery of blood and clotting products during resuscitation for major hemorrhage.”
Dr Brohi and his colleagues analyzed 442 patients treated at 22 hospitals in England and Wales. The patients had experienced major hemorrhage as a result of injuries and received at least 4 units of packed red blood cells (PRBCs) in the first 24 hours of admission.
The patients’ median age was 38 (range, 24-54), and 74% were men. Thirty-three percent of patients (n=146) had massive hemorrhage.
Mortality from bleeding tended to occur early in these patients. Twenty-seven percent of patients (n=117) died in hospital—18% (n=79) within the first 24 hours. The 30-day mortality rate was about 27% (n=119), and 33% of evaluable patients had died at 1 year (127/383).
All 442 patients received PRBCs. The median number of PRBC units transfused within 24 hours was 7 (range, 5-11), and the median number of PRBC units given in 30 days was 9 (range, 6-15).
The average time to transfusion of PRBCs was longer than expected, at 41 minutes (range, 1-122).
Similarly, the researchers found the administration of blood components such as plasma and platelets to be significantly delayed, occurring, on average, 2 to 3 hours after admission.
Three-quarters of patients (n=330) received fresh-frozen plasma (FFP). The median number of FFP units given within 24 hours was 4 (range, 0-7), and the time to first FFP transfusion was 87 minutes (range, 42.5-229).
About 45% of patients (n=197) received platelets. The median dose was 0 (range, 0-1), and the time to first platelet transfusion was 146 minutes (range, 72.5-364).
About 28% of patients (n=122) received cryoprecipitate. The median dose was 0 (range, 0-1), and the time to first cryoprecipitate infusion was 179.5 minutes (range, 84.5-333.5).
“The rapid delivery of the right mix of blood components in an emergency environment is extremely challenging,” Dr Brohi said.
“Some transfusion components have to be thawed and, at present, aren’t always available for the patient quickly enough. More research is also needed into techniques and devices to control bleeding earlier, even at the scene of injury.”
The researchers noted that this study had its limitations, such as incomplete data for some patients. ![]()

Photo courtesy of UAB Hospital
A new study suggests that as few as 2% of patients with life-threatening bleeding after serious injury receive optimal blood transfusion therapy in England and Wales.
Researchers estimate that nearly 5000 trauma patients sustain a major hemorrhage in England and Wales each year, and one-third of those patients die.
The current study, published in the British Journal of Surgery, highlights how delays in transfusions may contribute to this death rate.
“The rapid and consistent delivery of blood, plasma, platelets, and other clotting products to trauma patients is essential to maintain clotting during hemorrhage and has been shown to halve mortality,” said study author Karim Brohi, MBBS, of Queen Mary University of London in the UK.
“However, we found that only 2% of patients with massive hemorrhage received the optimal type of blood transfusion for their resuscitation. There is a clear opportunity for clinicians to improve the delivery of blood and clotting products during resuscitation for major hemorrhage.”
Dr Brohi and his colleagues analyzed 442 patients treated at 22 hospitals in England and Wales. The patients had experienced major hemorrhage as a result of injuries and received at least 4 units of packed red blood cells (PRBCs) in the first 24 hours of admission.
The patients’ median age was 38 (range, 24-54), and 74% were men. Thirty-three percent of patients (n=146) had massive hemorrhage.
Mortality from bleeding tended to occur early in these patients. Twenty-seven percent of patients (n=117) died in hospital—18% (n=79) within the first 24 hours. The 30-day mortality rate was about 27% (n=119), and 33% of evaluable patients had died at 1 year (127/383).
All 442 patients received PRBCs. The median number of PRBC units transfused within 24 hours was 7 (range, 5-11), and the median number of PRBC units given in 30 days was 9 (range, 6-15).
The average time to transfusion of PRBCs was longer than expected, at 41 minutes (range, 1-122).
Similarly, the researchers found the administration of blood components such as plasma and platelets to be significantly delayed, occurring, on average, 2 to 3 hours after admission.
Three-quarters of patients (n=330) received fresh-frozen plasma (FFP). The median number of FFP units given within 24 hours was 4 (range, 0-7), and the time to first FFP transfusion was 87 minutes (range, 42.5-229).
About 45% of patients (n=197) received platelets. The median dose was 0 (range, 0-1), and the time to first platelet transfusion was 146 minutes (range, 72.5-364).
About 28% of patients (n=122) received cryoprecipitate. The median dose was 0 (range, 0-1), and the time to first cryoprecipitate infusion was 179.5 minutes (range, 84.5-333.5).
“The rapid delivery of the right mix of blood components in an emergency environment is extremely challenging,” Dr Brohi said.
“Some transfusion components have to be thawed and, at present, aren’t always available for the patient quickly enough. More research is also needed into techniques and devices to control bleeding earlier, even at the scene of injury.”
The researchers noted that this study had its limitations, such as incomplete data for some patients. ![]()
MF drug trials placed on partial clinical hold

The US Food and Drug Administration (FDA) has placed a partial clinical hold on trials conducted under the investigational new drug
(IND) application for pacritinib.
Pacritinib is a JAK2/FLT3 inhibitor being developed by CTI BioPharma for the treatment of myelofibrosis (MF).
The partial clinical hold impacts part of the clinical work currently being conducted under the pacritinib IND and will also affect planned clinical trials.
The FDA said the reasons for the partial clinical hold are excess mortality and other adverse events in pacritinib-treated patients (compared to the control arm) in the PERSIST-1 trial.
The excess mortality was most evident during the non-randomized crossover period following the initial 24 weeks of randomized treatment, during which patients in the control arm could switch to pacritinib treatment.
Under the partial clinical hold, investigators may not enroll new patients or start pacritinib as initial or crossover treatment, and patients not deriving benefit after 30 weeks of pacritinib treatment must stop using pacritinib.
In addition, the FDA has recommended that CTI BioPharma make certain modifications to protocols, provide certain notifications, revise relevant statements in the related investigator’s brochure and informed consent documents, and take certain other actions.
CTI BioPharma said it intends to implement the FDA’s recommendations, and all clinical investigators worldwide have been notified of the partial clinical hold.
Just before the FDA notified CTI BioPharma of the partial clinical hold, the company completed enrollment in the phase 3 PERSIST-2 trial.
In PERSIST-2, researchers are comparing the efficacy and safety of pacritinib and best available therapy in patients with thrombocytopenia and primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
Under the partial clinical hold, patients on this trial who are currently receiving pacritinib may continue to do so unless they are not deriving benefit after 30 weeks of pacritinib treatment, and crossover of patients from the control arm to the pacritinib arm will not be allowed. ![]()

The US Food and Drug Administration (FDA) has placed a partial clinical hold on trials conducted under the investigational new drug
(IND) application for pacritinib.
Pacritinib is a JAK2/FLT3 inhibitor being developed by CTI BioPharma for the treatment of myelofibrosis (MF).
The partial clinical hold impacts part of the clinical work currently being conducted under the pacritinib IND and will also affect planned clinical trials.
The FDA said the reasons for the partial clinical hold are excess mortality and other adverse events in pacritinib-treated patients (compared to the control arm) in the PERSIST-1 trial.
The excess mortality was most evident during the non-randomized crossover period following the initial 24 weeks of randomized treatment, during which patients in the control arm could switch to pacritinib treatment.
Under the partial clinical hold, investigators may not enroll new patients or start pacritinib as initial or crossover treatment, and patients not deriving benefit after 30 weeks of pacritinib treatment must stop using pacritinib.
In addition, the FDA has recommended that CTI BioPharma make certain modifications to protocols, provide certain notifications, revise relevant statements in the related investigator’s brochure and informed consent documents, and take certain other actions.
CTI BioPharma said it intends to implement the FDA’s recommendations, and all clinical investigators worldwide have been notified of the partial clinical hold.
Just before the FDA notified CTI BioPharma of the partial clinical hold, the company completed enrollment in the phase 3 PERSIST-2 trial.
In PERSIST-2, researchers are comparing the efficacy and safety of pacritinib and best available therapy in patients with thrombocytopenia and primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
Under the partial clinical hold, patients on this trial who are currently receiving pacritinib may continue to do so unless they are not deriving benefit after 30 weeks of pacritinib treatment, and crossover of patients from the control arm to the pacritinib arm will not be allowed. ![]()

The US Food and Drug Administration (FDA) has placed a partial clinical hold on trials conducted under the investigational new drug
(IND) application for pacritinib.
Pacritinib is a JAK2/FLT3 inhibitor being developed by CTI BioPharma for the treatment of myelofibrosis (MF).
The partial clinical hold impacts part of the clinical work currently being conducted under the pacritinib IND and will also affect planned clinical trials.
The FDA said the reasons for the partial clinical hold are excess mortality and other adverse events in pacritinib-treated patients (compared to the control arm) in the PERSIST-1 trial.
The excess mortality was most evident during the non-randomized crossover period following the initial 24 weeks of randomized treatment, during which patients in the control arm could switch to pacritinib treatment.
Under the partial clinical hold, investigators may not enroll new patients or start pacritinib as initial or crossover treatment, and patients not deriving benefit after 30 weeks of pacritinib treatment must stop using pacritinib.
In addition, the FDA has recommended that CTI BioPharma make certain modifications to protocols, provide certain notifications, revise relevant statements in the related investigator’s brochure and informed consent documents, and take certain other actions.
CTI BioPharma said it intends to implement the FDA’s recommendations, and all clinical investigators worldwide have been notified of the partial clinical hold.
Just before the FDA notified CTI BioPharma of the partial clinical hold, the company completed enrollment in the phase 3 PERSIST-2 trial.
In PERSIST-2, researchers are comparing the efficacy and safety of pacritinib and best available therapy in patients with thrombocytopenia and primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
Under the partial clinical hold, patients on this trial who are currently receiving pacritinib may continue to do so unless they are not deriving benefit after 30 weeks of pacritinib treatment, and crossover of patients from the control arm to the pacritinib arm will not be allowed. ![]()
Average person with atopic dermatitis has no increased risk of actinic keratosis or nonmelanoma skin cancer
People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer, according to a recent population-based, cross-sectional study.
“This is the first study to examine the association between atopic dermatitis and actinic keratosis [AK]. Our findings suggest that within a population-based sample, atopic dermatitis patients do not have more AKs than the rest of the population. Patients with atopic dermatitis were not found to have more AKs or keratotic cancers [basal or squamous cell cancers]. Moreover, individuals with atopic dermatitis seem to be less likely to develop multiple AKs,” said Dr. Enes Hajdarbegovic and his associates of the Erasmus Medical Centre, Rotterdam, the Netherlands.
The study is part of an ongoing, prospective, Dutch population-based cohort study that follows people in a district of Rotterdam since 1990. There are now 14,926 participants in the database. The current study included 4,375 participants who had undergone full body skin examinations; 56% of patients were female, and the mean age was 68 years (Br J Dermatol. 2016 Jan 29. doi: 10.1111/bjd.14423).
Twenty-four percent had 1 or more AKs; 57% had 1-3 of these lesions; 23% had 4-9, and 20% had more than 10. The mean age of participants with AK was significantly higher, compared with those without AK (73 years vs. 66 years; P less than .01).
Of the 4,375 participants screened, 6.3% met the diagnostic criteria for atopic dermatitis. A lower proportion of those with atopic dermatitis had AK: 16% vs. 24%, respectively (P = .002). In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more AKs than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted odds ratio, 0.71) and squamous cell cancer (adjusted OR, 1.54).
The authors explained that it is already known that patients with severe atopic dermatitis exposed to ultraviolet light and immunosuppressants are at increased risk of keratinocyte malignancies. This study shows that a community-dwelling person with moderate atopic dermatitis does not develop more AKs or keratinocyte cancers.
The investigators said they had no relevant financial disclosures.
People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer, according to a recent population-based, cross-sectional study.
“This is the first study to examine the association between atopic dermatitis and actinic keratosis [AK]. Our findings suggest that within a population-based sample, atopic dermatitis patients do not have more AKs than the rest of the population. Patients with atopic dermatitis were not found to have more AKs or keratotic cancers [basal or squamous cell cancers]. Moreover, individuals with atopic dermatitis seem to be less likely to develop multiple AKs,” said Dr. Enes Hajdarbegovic and his associates of the Erasmus Medical Centre, Rotterdam, the Netherlands.
The study is part of an ongoing, prospective, Dutch population-based cohort study that follows people in a district of Rotterdam since 1990. There are now 14,926 participants in the database. The current study included 4,375 participants who had undergone full body skin examinations; 56% of patients were female, and the mean age was 68 years (Br J Dermatol. 2016 Jan 29. doi: 10.1111/bjd.14423).
Twenty-four percent had 1 or more AKs; 57% had 1-3 of these lesions; 23% had 4-9, and 20% had more than 10. The mean age of participants with AK was significantly higher, compared with those without AK (73 years vs. 66 years; P less than .01).
Of the 4,375 participants screened, 6.3% met the diagnostic criteria for atopic dermatitis. A lower proportion of those with atopic dermatitis had AK: 16% vs. 24%, respectively (P = .002). In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more AKs than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted odds ratio, 0.71) and squamous cell cancer (adjusted OR, 1.54).
The authors explained that it is already known that patients with severe atopic dermatitis exposed to ultraviolet light and immunosuppressants are at increased risk of keratinocyte malignancies. This study shows that a community-dwelling person with moderate atopic dermatitis does not develop more AKs or keratinocyte cancers.
The investigators said they had no relevant financial disclosures.
People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer, according to a recent population-based, cross-sectional study.
“This is the first study to examine the association between atopic dermatitis and actinic keratosis [AK]. Our findings suggest that within a population-based sample, atopic dermatitis patients do not have more AKs than the rest of the population. Patients with atopic dermatitis were not found to have more AKs or keratotic cancers [basal or squamous cell cancers]. Moreover, individuals with atopic dermatitis seem to be less likely to develop multiple AKs,” said Dr. Enes Hajdarbegovic and his associates of the Erasmus Medical Centre, Rotterdam, the Netherlands.
The study is part of an ongoing, prospective, Dutch population-based cohort study that follows people in a district of Rotterdam since 1990. There are now 14,926 participants in the database. The current study included 4,375 participants who had undergone full body skin examinations; 56% of patients were female, and the mean age was 68 years (Br J Dermatol. 2016 Jan 29. doi: 10.1111/bjd.14423).
Twenty-four percent had 1 or more AKs; 57% had 1-3 of these lesions; 23% had 4-9, and 20% had more than 10. The mean age of participants with AK was significantly higher, compared with those without AK (73 years vs. 66 years; P less than .01).
Of the 4,375 participants screened, 6.3% met the diagnostic criteria for atopic dermatitis. A lower proportion of those with atopic dermatitis had AK: 16% vs. 24%, respectively (P = .002). In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more AKs than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted odds ratio, 0.71) and squamous cell cancer (adjusted OR, 1.54).
The authors explained that it is already known that patients with severe atopic dermatitis exposed to ultraviolet light and immunosuppressants are at increased risk of keratinocyte malignancies. This study shows that a community-dwelling person with moderate atopic dermatitis does not develop more AKs or keratinocyte cancers.
The investigators said they had no relevant financial disclosures.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer.
Major finding: In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more actinic keratoses than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted OR, 0.71) and squamous cell cancer (adjusted OR, 1.54).
Data source: A prospective, Dutch population-based cohort study of 4,375 participants who had undergone full body skin examinations.
Disclosures: The investigators said they had no relevant financial disclosures.
USPSTF: Screen all adults for depression
All adults, including pregnant and postpartum women, should be screened for depression, according to new recommendations of the U.S. Preventive Services Task Force.
The recommendation also calls for screening to be coupled with “adequate systems” to ensure diagnosis, treatment, and follow-up (JAMA. 2016 Jan 26;315[4]:380-7).
The depression screening recommendation, authored by Dr. Albert L. Siu and the other members of the USPSTF, is a level B recommendation, meaning that it has either high certainty of moderate net benefit, or moderate certainty of moderate to substantial net benefit.
The new guidance in screening for depression helps address a disorder that is “the leading cause of disability among adults in high-income countries,” said Dr. Siu and his coauthors. Lost productivity attributable to depression cost $23 billion in the United States in 2011, and $22.8 billion was spent on treatments for depression in 2009, the last year for which figures are available.
Dr. Siu, chair of geriatrics and palliative medicine at Icahn School of Medicine at Mount Sinai, New York, and his coauthors cited “convincing evidence that screening improves the accurate identification of adult patients with depression in primary care settings, including pregnant and postpartum women.”
In addition, the task force found convincing evidence that for older adults as well as the general adult population, treatment of “depression identified through screening in primary care settings with antidepressants, psychotherapy, or both decreases clinical morbidity.”
For pregnant and postpartum women with depression, Dr. Siu and his coauthors found “adequate” evidence that cognitive behavioral therapy (CBT) improves outcomes.
The recommendation does not identify optimal timing and intervals for depression screening, citing a need for more research in this area. However, “a pragmatic approach might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” explained Dr. Siu and his coauthors.
The new depression screening recommendation from USPSTF updates the 2009 recommendation, which recommended universal screening if “staff-assisted depression care supports” were in place, and targeted screening based on clinical judgment and patient preference if such support were unavailable.
The rationale for the current recommendation of universal screening for those 18 years and older is the “recognition that such support is now much more widely available and accepted as part of mental health care,” the task force members said.
Any potential harms of screening, said Dr. Siu and his coauthors, were minimal to nonexistent.
Overall, the USPSTF assigned a small to moderate risk to the use of medication in depression. However, the use of “second-generation” antidepressants – mostly SSRIs – was associated with some harms, including increased risk of suicidal behavior in young adults and of gastrointestinal bleeding in older adults, as well as potential fetal harms in pregnant women taking antidepressants.
Using CBT to treat depression in pregnant and postpartum women was also associated with minimal to no harm.
The USPSTF screening recommendation is aligned with the American Academy of Family Physicians’ recommendation to screen the general adult population for depression, and with the American Academy of Pediatrics’ recommendation that pediatricians screen mothers for depression at their babies’ 1-, 2-, and 4-month office visits.
Released in draft form in July 2015, the depression screening recommendation was available for public comment for a period of 4 weeks. In response to public input, the final recommendation’s implementation section clarifies and characterizes an “adequate system” of screening, and gives more resources for evidence-based depression screening and treatment.
The Agency for Healthcare Research and Quality supports the operations of the USPSTF, but the task force’s recommendations are independent of the federal government. Dr. Siu and the other task force members reported no conflicts of interest.
On Twitter @karioakes
Arthritis affects one in five adults and is one of the most frequent reasons for ambulatory visits to the primary care physician. Arthritis affects patients both physically and psychologically and often leads to depressed mood with subsequent worse health outcomes, including increased mortality. Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease, myocardial infarction, and suicide. Patients with arthritis and associated depression have increased health service utilization and are less likely to be adherent with their medications. In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the U.K. National Institute of Clinical Excellence. The U.S. Preventive Service Task Force and the Canadian Task Force for Preventive Health Care recommend depression screening in all adults. However, before screening for depression in specific patient groups can be recommended, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening. Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent with rates ranging from 18%-42%. Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.
Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 minutes to a visit. Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments. Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes. Thus, provision of or referral to treatment is a necessary follow-up to screening.
It has been shown that there is no difference in depression screening rates in patients with arthritis, compared with the general population, despite patients with arthritis being considered “high risk.” Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase the recognition of depression in high-risk groups and the use of appropriate interventions, such as mental health referrals and/or treatment with antidepressants.
Dr. Mary Margaretten is an associate professor of medicine in the division of rheumatology at the University of California, San Francisco. She has no relevant disclosures.
Arthritis affects one in five adults and is one of the most frequent reasons for ambulatory visits to the primary care physician. Arthritis affects patients both physically and psychologically and often leads to depressed mood with subsequent worse health outcomes, including increased mortality. Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease, myocardial infarction, and suicide. Patients with arthritis and associated depression have increased health service utilization and are less likely to be adherent with their medications. In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the U.K. National Institute of Clinical Excellence. The U.S. Preventive Service Task Force and the Canadian Task Force for Preventive Health Care recommend depression screening in all adults. However, before screening for depression in specific patient groups can be recommended, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening. Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent with rates ranging from 18%-42%. Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.
Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 minutes to a visit. Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments. Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes. Thus, provision of or referral to treatment is a necessary follow-up to screening.
It has been shown that there is no difference in depression screening rates in patients with arthritis, compared with the general population, despite patients with arthritis being considered “high risk.” Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase the recognition of depression in high-risk groups and the use of appropriate interventions, such as mental health referrals and/or treatment with antidepressants.
Dr. Mary Margaretten is an associate professor of medicine in the division of rheumatology at the University of California, San Francisco. She has no relevant disclosures.
Arthritis affects one in five adults and is one of the most frequent reasons for ambulatory visits to the primary care physician. Arthritis affects patients both physically and psychologically and often leads to depressed mood with subsequent worse health outcomes, including increased mortality. Specifically, depression in patients with arthritis is an independent risk factor for cardiovascular disease, myocardial infarction, and suicide. Patients with arthritis and associated depression have increased health service utilization and are less likely to be adherent with their medications. In addition to these negative health consequences, depression may contribute to unemployment, loss of work productivity, and increased healthcare costs in persons with arthritis.
Depression screening guidelines for adults with chronic musculoskeletal diseases such as arthritis have been endorsed by the U.K. National Institute of Clinical Excellence. The U.S. Preventive Service Task Force and the Canadian Task Force for Preventive Health Care recommend depression screening in all adults. However, before screening for depression in specific patient groups can be recommended, well-established criteria should be met. Generally, screening is reasonable if the condition, depression in this case, is important and prevalent, can be effectively treated, and cannot be readily detected without screening. Comorbid depression in patients with arthritis meets these criteria. It is highly prevalent with rates ranging from 18%-42%. Depression with inflammatory arthritis, such as rheumatoid arthritis (RA), occurs more frequently than with osteoarthritis but even though it is more prevalent, depression with RA is often unrecognized and/or untreated.
Performing depression screening should not unduly burden physicians because, on average, depression screening adds less than 3 minutes to a visit. Asking two simple questions about mood and anhedonia (“Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?”) is as effective as using more formal instruments. Implicit in the use of depression screening is the assumption that screening will increase recognition of depression and that recognized patients would benefit from treatment. It has been shown that patients who screen positive but were not in treatment had high rates of depression and overall poor mental health outcomes. Thus, provision of or referral to treatment is a necessary follow-up to screening.
It has been shown that there is no difference in depression screening rates in patients with arthritis, compared with the general population, despite patients with arthritis being considered “high risk.” Given the endorsement of national guidelines for depression screening, quality improvement initiatives should target physicians and non-physicians to increase the recognition of depression in high-risk groups and the use of appropriate interventions, such as mental health referrals and/or treatment with antidepressants.
Dr. Mary Margaretten is an associate professor of medicine in the division of rheumatology at the University of California, San Francisco. She has no relevant disclosures.
All adults, including pregnant and postpartum women, should be screened for depression, according to new recommendations of the U.S. Preventive Services Task Force.
The recommendation also calls for screening to be coupled with “adequate systems” to ensure diagnosis, treatment, and follow-up (JAMA. 2016 Jan 26;315[4]:380-7).
The depression screening recommendation, authored by Dr. Albert L. Siu and the other members of the USPSTF, is a level B recommendation, meaning that it has either high certainty of moderate net benefit, or moderate certainty of moderate to substantial net benefit.
The new guidance in screening for depression helps address a disorder that is “the leading cause of disability among adults in high-income countries,” said Dr. Siu and his coauthors. Lost productivity attributable to depression cost $23 billion in the United States in 2011, and $22.8 billion was spent on treatments for depression in 2009, the last year for which figures are available.
Dr. Siu, chair of geriatrics and palliative medicine at Icahn School of Medicine at Mount Sinai, New York, and his coauthors cited “convincing evidence that screening improves the accurate identification of adult patients with depression in primary care settings, including pregnant and postpartum women.”
In addition, the task force found convincing evidence that for older adults as well as the general adult population, treatment of “depression identified through screening in primary care settings with antidepressants, psychotherapy, or both decreases clinical morbidity.”
For pregnant and postpartum women with depression, Dr. Siu and his coauthors found “adequate” evidence that cognitive behavioral therapy (CBT) improves outcomes.
The recommendation does not identify optimal timing and intervals for depression screening, citing a need for more research in this area. However, “a pragmatic approach might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” explained Dr. Siu and his coauthors.
The new depression screening recommendation from USPSTF updates the 2009 recommendation, which recommended universal screening if “staff-assisted depression care supports” were in place, and targeted screening based on clinical judgment and patient preference if such support were unavailable.
The rationale for the current recommendation of universal screening for those 18 years and older is the “recognition that such support is now much more widely available and accepted as part of mental health care,” the task force members said.
Any potential harms of screening, said Dr. Siu and his coauthors, were minimal to nonexistent.
Overall, the USPSTF assigned a small to moderate risk to the use of medication in depression. However, the use of “second-generation” antidepressants – mostly SSRIs – was associated with some harms, including increased risk of suicidal behavior in young adults and of gastrointestinal bleeding in older adults, as well as potential fetal harms in pregnant women taking antidepressants.
Using CBT to treat depression in pregnant and postpartum women was also associated with minimal to no harm.
The USPSTF screening recommendation is aligned with the American Academy of Family Physicians’ recommendation to screen the general adult population for depression, and with the American Academy of Pediatrics’ recommendation that pediatricians screen mothers for depression at their babies’ 1-, 2-, and 4-month office visits.
Released in draft form in July 2015, the depression screening recommendation was available for public comment for a period of 4 weeks. In response to public input, the final recommendation’s implementation section clarifies and characterizes an “adequate system” of screening, and gives more resources for evidence-based depression screening and treatment.
The Agency for Healthcare Research and Quality supports the operations of the USPSTF, but the task force’s recommendations are independent of the federal government. Dr. Siu and the other task force members reported no conflicts of interest.
On Twitter @karioakes
All adults, including pregnant and postpartum women, should be screened for depression, according to new recommendations of the U.S. Preventive Services Task Force.
The recommendation also calls for screening to be coupled with “adequate systems” to ensure diagnosis, treatment, and follow-up (JAMA. 2016 Jan 26;315[4]:380-7).
The depression screening recommendation, authored by Dr. Albert L. Siu and the other members of the USPSTF, is a level B recommendation, meaning that it has either high certainty of moderate net benefit, or moderate certainty of moderate to substantial net benefit.
The new guidance in screening for depression helps address a disorder that is “the leading cause of disability among adults in high-income countries,” said Dr. Siu and his coauthors. Lost productivity attributable to depression cost $23 billion in the United States in 2011, and $22.8 billion was spent on treatments for depression in 2009, the last year for which figures are available.
Dr. Siu, chair of geriatrics and palliative medicine at Icahn School of Medicine at Mount Sinai, New York, and his coauthors cited “convincing evidence that screening improves the accurate identification of adult patients with depression in primary care settings, including pregnant and postpartum women.”
In addition, the task force found convincing evidence that for older adults as well as the general adult population, treatment of “depression identified through screening in primary care settings with antidepressants, psychotherapy, or both decreases clinical morbidity.”
For pregnant and postpartum women with depression, Dr. Siu and his coauthors found “adequate” evidence that cognitive behavioral therapy (CBT) improves outcomes.
The recommendation does not identify optimal timing and intervals for depression screening, citing a need for more research in this area. However, “a pragmatic approach might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” explained Dr. Siu and his coauthors.
The new depression screening recommendation from USPSTF updates the 2009 recommendation, which recommended universal screening if “staff-assisted depression care supports” were in place, and targeted screening based on clinical judgment and patient preference if such support were unavailable.
The rationale for the current recommendation of universal screening for those 18 years and older is the “recognition that such support is now much more widely available and accepted as part of mental health care,” the task force members said.
Any potential harms of screening, said Dr. Siu and his coauthors, were minimal to nonexistent.
Overall, the USPSTF assigned a small to moderate risk to the use of medication in depression. However, the use of “second-generation” antidepressants – mostly SSRIs – was associated with some harms, including increased risk of suicidal behavior in young adults and of gastrointestinal bleeding in older adults, as well as potential fetal harms in pregnant women taking antidepressants.
Using CBT to treat depression in pregnant and postpartum women was also associated with minimal to no harm.
The USPSTF screening recommendation is aligned with the American Academy of Family Physicians’ recommendation to screen the general adult population for depression, and with the American Academy of Pediatrics’ recommendation that pediatricians screen mothers for depression at their babies’ 1-, 2-, and 4-month office visits.
Released in draft form in July 2015, the depression screening recommendation was available for public comment for a period of 4 weeks. In response to public input, the final recommendation’s implementation section clarifies and characterizes an “adequate system” of screening, and gives more resources for evidence-based depression screening and treatment.
The Agency for Healthcare Research and Quality supports the operations of the USPSTF, but the task force’s recommendations are independent of the federal government. Dr. Siu and the other task force members reported no conflicts of interest.
On Twitter @karioakes
FROM JAMA