User login
Northwestern University: Annual Northwestern Vascular Symposium
Aortic aneurysms pose unique challenges in transplant recipients
CHICAGO – Surgeons can expect to see more abdominal organ transplant recipients presenting with aortic aneurysms, as transplant survival rates increase along with the age of organ donors and recipients.
“The consensus is that abdominal aortic aneurysms (AAAs) have a more aggressive course post-transplant and within that context, probably need to be managed more aggressively,” Dr. Michael J. Englesbe of the University of Michigan, Ann Arbor said at the annual Northwestern Vascular Symposium.
Some 270,000 Americans are living with a functioning liver or kidney graft, and their average age has risen from 47 years to 57 years over the last decade.
Though the data isn’t great, it’s hypothesized that the immunosuppression prerequisite for successful organ transplantation promotes the progression of atherosclerosis and aneurysm growth in transplant patients, he said.
New-onset diabetes, hyperlipidemia, and hypertension are all common post-transplant due to immunosuppression therapy. Aortic aneurysms are also reported to rupture at smaller sizes in transplant recipients.
Intriguingly, the opposite effect has been observed in experimental animal models, where immunosuppression with calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors has been shown to stabilize atherosclerotic lesions and inhibit aneurysm expansion.
The reason for this disparity is unclear, but immunosuppressants likely augment other cardiovascular comorbidities such as hypertension and atherosclerosis and this may trump their anti-inflammatory effects and lead to worse aneurysm disease and faster expansion in humans, Dr. Englesbe speculated in an interview.
As for when aneurysms should be fixed, kidney transplant candidates should undergo AAA repair prior to transplantation since the risk of renal complications after aneurysm repair puts the allograft at risk, Dr. Englesbe advised. Either an open or endovascular approach can be used.
In liver transplant candidates, elective AAA repair should be avoided if possible and is contraindicated if any signs of hepatic decompensation are present such as muscle wasting, ascites, platelet count less than 50 x 109/L, or encephalopathy. For well-compensated cirrhotic patients, endovascular repair is best.
One of the most important considerations for any solid-organ transplant patient undergoing aneurysm repair is perioperative management of immunosuppression, Dr. Englesbe stressed.
Transplant patients are maintained on oral calcineurin inhibitors such as cyclosporine and tacrolimus (Prograf) throughout the perioperative period to prevent organ rejection, but these drugs have nephrotoxic effects. About 10% of recipients, typically the sicker patients, will be switched to mTOR inhibitors such as everolimus (Afinitor) and sirolumus (Rapamune) as a kidney-sparing alternative.
“Part of the mechanism of these [mTOR] drugs is that they really affect fibroblast functioning, so patients that are on these medications, their wound will fall apart and they will invariably get a hernia,” Dr. Englesbe said. “You have to stop them upwards of about 6 weeks before surgical intervention, and I think this is also true for many endografts.”
He highlighted a case in which an mTOR inhibitor was started three months after liver transplant due to renal dysfunction in a patient who was fully healed, but within three weeks, “her wound fell apart, completely fell apart.” She developed several seromas underneath her incision, one of which became infected and took months to close.
“The transplant professionals – your nephrologists, your cardiologists – aren’t going to know this fact, but as a transplant surgeon it’s usually the first question we’re going to ask with respect to any post-transplant patient we’re going to operate on, so it’s something to keep in mind,” Dr. Englesbe said.
Another take-home message was the importance of maintaining kidney function in kidney recipients presenting with aortic aneurysm, as mortality in these patients is about 10-fold higher once the kidney fails, he said. A recent study reported that AAAs are significantly more common in kidney than liver transplant recipients (29.6% vs. 11.4%; P = .02), despite a similar prevalence for any aneurysm (4%) in both groups (J Vasc Surg. 2014 Mar;59;594-8).
When kidney recipients present, preoperative imaging of the aorta from the aneurysm to the kidney allograft is mandatory, he said. Endovascular repair is preferred, whenever possible.
The renal graft is typically sewn to the external iliac artery 3 cm to 10 cm from the bifurcation of the external and internal iliac arteries. Because of this, repair is challenging when aneurysmal disease involves the iliac artery, Dr. Englesbe observed. Aneurysmal dilation is less common in the external iliac, but stenting an iliac aneurysm can still compromise inflow to the transplanted kidney.
Several surgical techniques including axillofemoral bypass, aortofemoral shunt, or extracorporeal circuit have been reported to preserve renal function during open AAA repair in renal transplant recipients. These techniques are not without their own risk of complications and should be avoided in patients with low creatinine, but are appropriate in patients with marginal or impaired renal function, according to Dr. Englesbe, who reported having no relevant disclosures.
CHICAGO – Surgeons can expect to see more abdominal organ transplant recipients presenting with aortic aneurysms, as transplant survival rates increase along with the age of organ donors and recipients.
“The consensus is that abdominal aortic aneurysms (AAAs) have a more aggressive course post-transplant and within that context, probably need to be managed more aggressively,” Dr. Michael J. Englesbe of the University of Michigan, Ann Arbor said at the annual Northwestern Vascular Symposium.
Some 270,000 Americans are living with a functioning liver or kidney graft, and their average age has risen from 47 years to 57 years over the last decade.
Though the data isn’t great, it’s hypothesized that the immunosuppression prerequisite for successful organ transplantation promotes the progression of atherosclerosis and aneurysm growth in transplant patients, he said.
New-onset diabetes, hyperlipidemia, and hypertension are all common post-transplant due to immunosuppression therapy. Aortic aneurysms are also reported to rupture at smaller sizes in transplant recipients.
Intriguingly, the opposite effect has been observed in experimental animal models, where immunosuppression with calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors has been shown to stabilize atherosclerotic lesions and inhibit aneurysm expansion.
The reason for this disparity is unclear, but immunosuppressants likely augment other cardiovascular comorbidities such as hypertension and atherosclerosis and this may trump their anti-inflammatory effects and lead to worse aneurysm disease and faster expansion in humans, Dr. Englesbe speculated in an interview.
As for when aneurysms should be fixed, kidney transplant candidates should undergo AAA repair prior to transplantation since the risk of renal complications after aneurysm repair puts the allograft at risk, Dr. Englesbe advised. Either an open or endovascular approach can be used.
In liver transplant candidates, elective AAA repair should be avoided if possible and is contraindicated if any signs of hepatic decompensation are present such as muscle wasting, ascites, platelet count less than 50 x 109/L, or encephalopathy. For well-compensated cirrhotic patients, endovascular repair is best.
One of the most important considerations for any solid-organ transplant patient undergoing aneurysm repair is perioperative management of immunosuppression, Dr. Englesbe stressed.
Transplant patients are maintained on oral calcineurin inhibitors such as cyclosporine and tacrolimus (Prograf) throughout the perioperative period to prevent organ rejection, but these drugs have nephrotoxic effects. About 10% of recipients, typically the sicker patients, will be switched to mTOR inhibitors such as everolimus (Afinitor) and sirolumus (Rapamune) as a kidney-sparing alternative.
“Part of the mechanism of these [mTOR] drugs is that they really affect fibroblast functioning, so patients that are on these medications, their wound will fall apart and they will invariably get a hernia,” Dr. Englesbe said. “You have to stop them upwards of about 6 weeks before surgical intervention, and I think this is also true for many endografts.”
He highlighted a case in which an mTOR inhibitor was started three months after liver transplant due to renal dysfunction in a patient who was fully healed, but within three weeks, “her wound fell apart, completely fell apart.” She developed several seromas underneath her incision, one of which became infected and took months to close.
“The transplant professionals – your nephrologists, your cardiologists – aren’t going to know this fact, but as a transplant surgeon it’s usually the first question we’re going to ask with respect to any post-transplant patient we’re going to operate on, so it’s something to keep in mind,” Dr. Englesbe said.
Another take-home message was the importance of maintaining kidney function in kidney recipients presenting with aortic aneurysm, as mortality in these patients is about 10-fold higher once the kidney fails, he said. A recent study reported that AAAs are significantly more common in kidney than liver transplant recipients (29.6% vs. 11.4%; P = .02), despite a similar prevalence for any aneurysm (4%) in both groups (J Vasc Surg. 2014 Mar;59;594-8).
When kidney recipients present, preoperative imaging of the aorta from the aneurysm to the kidney allograft is mandatory, he said. Endovascular repair is preferred, whenever possible.
The renal graft is typically sewn to the external iliac artery 3 cm to 10 cm from the bifurcation of the external and internal iliac arteries. Because of this, repair is challenging when aneurysmal disease involves the iliac artery, Dr. Englesbe observed. Aneurysmal dilation is less common in the external iliac, but stenting an iliac aneurysm can still compromise inflow to the transplanted kidney.
Several surgical techniques including axillofemoral bypass, aortofemoral shunt, or extracorporeal circuit have been reported to preserve renal function during open AAA repair in renal transplant recipients. These techniques are not without their own risk of complications and should be avoided in patients with low creatinine, but are appropriate in patients with marginal or impaired renal function, according to Dr. Englesbe, who reported having no relevant disclosures.
CHICAGO – Surgeons can expect to see more abdominal organ transplant recipients presenting with aortic aneurysms, as transplant survival rates increase along with the age of organ donors and recipients.
“The consensus is that abdominal aortic aneurysms (AAAs) have a more aggressive course post-transplant and within that context, probably need to be managed more aggressively,” Dr. Michael J. Englesbe of the University of Michigan, Ann Arbor said at the annual Northwestern Vascular Symposium.
Some 270,000 Americans are living with a functioning liver or kidney graft, and their average age has risen from 47 years to 57 years over the last decade.
Though the data isn’t great, it’s hypothesized that the immunosuppression prerequisite for successful organ transplantation promotes the progression of atherosclerosis and aneurysm growth in transplant patients, he said.
New-onset diabetes, hyperlipidemia, and hypertension are all common post-transplant due to immunosuppression therapy. Aortic aneurysms are also reported to rupture at smaller sizes in transplant recipients.
Intriguingly, the opposite effect has been observed in experimental animal models, where immunosuppression with calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors has been shown to stabilize atherosclerotic lesions and inhibit aneurysm expansion.
The reason for this disparity is unclear, but immunosuppressants likely augment other cardiovascular comorbidities such as hypertension and atherosclerosis and this may trump their anti-inflammatory effects and lead to worse aneurysm disease and faster expansion in humans, Dr. Englesbe speculated in an interview.
As for when aneurysms should be fixed, kidney transplant candidates should undergo AAA repair prior to transplantation since the risk of renal complications after aneurysm repair puts the allograft at risk, Dr. Englesbe advised. Either an open or endovascular approach can be used.
In liver transplant candidates, elective AAA repair should be avoided if possible and is contraindicated if any signs of hepatic decompensation are present such as muscle wasting, ascites, platelet count less than 50 x 109/L, or encephalopathy. For well-compensated cirrhotic patients, endovascular repair is best.
One of the most important considerations for any solid-organ transplant patient undergoing aneurysm repair is perioperative management of immunosuppression, Dr. Englesbe stressed.
Transplant patients are maintained on oral calcineurin inhibitors such as cyclosporine and tacrolimus (Prograf) throughout the perioperative period to prevent organ rejection, but these drugs have nephrotoxic effects. About 10% of recipients, typically the sicker patients, will be switched to mTOR inhibitors such as everolimus (Afinitor) and sirolumus (Rapamune) as a kidney-sparing alternative.
“Part of the mechanism of these [mTOR] drugs is that they really affect fibroblast functioning, so patients that are on these medications, their wound will fall apart and they will invariably get a hernia,” Dr. Englesbe said. “You have to stop them upwards of about 6 weeks before surgical intervention, and I think this is also true for many endografts.”
He highlighted a case in which an mTOR inhibitor was started three months after liver transplant due to renal dysfunction in a patient who was fully healed, but within three weeks, “her wound fell apart, completely fell apart.” She developed several seromas underneath her incision, one of which became infected and took months to close.
“The transplant professionals – your nephrologists, your cardiologists – aren’t going to know this fact, but as a transplant surgeon it’s usually the first question we’re going to ask with respect to any post-transplant patient we’re going to operate on, so it’s something to keep in mind,” Dr. Englesbe said.
Another take-home message was the importance of maintaining kidney function in kidney recipients presenting with aortic aneurysm, as mortality in these patients is about 10-fold higher once the kidney fails, he said. A recent study reported that AAAs are significantly more common in kidney than liver transplant recipients (29.6% vs. 11.4%; P = .02), despite a similar prevalence for any aneurysm (4%) in both groups (J Vasc Surg. 2014 Mar;59;594-8).
When kidney recipients present, preoperative imaging of the aorta from the aneurysm to the kidney allograft is mandatory, he said. Endovascular repair is preferred, whenever possible.
The renal graft is typically sewn to the external iliac artery 3 cm to 10 cm from the bifurcation of the external and internal iliac arteries. Because of this, repair is challenging when aneurysmal disease involves the iliac artery, Dr. Englesbe observed. Aneurysmal dilation is less common in the external iliac, but stenting an iliac aneurysm can still compromise inflow to the transplanted kidney.
Several surgical techniques including axillofemoral bypass, aortofemoral shunt, or extracorporeal circuit have been reported to preserve renal function during open AAA repair in renal transplant recipients. These techniques are not without their own risk of complications and should be avoided in patients with low creatinine, but are appropriate in patients with marginal or impaired renal function, according to Dr. Englesbe, who reported having no relevant disclosures.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Guidelines in works to tackle ruptured AAA transfers
CHICAGO – Adoption of an organized, systematic approach to ruptured abdominal aortic aneurysm has been inconsistent.
In a recent survey of vascular physicians in the western United States, 60% who accept ruptured abdominal aortic aneurysm (rAAA) transfers do not have a formal protocol for treatment and 70% do not use a transfer protocol or clinical guidelines (J Vasc Surg. 2015 Aug;62:326-30).
Guidelines for the management and transfer of patients with rAAA have been developed in the United Kingdom, but no such guidelines currently exist in the United States.
To address this disparity, the Western Vascular Society used the survey results, existing European guidelines, and a literature review to develop a set of 15 best practice steps for rAAA transfer. The “guidelines” were endorsed by the society members in September 2015 and are to be published early in 2016, Dr. Matthew Mell of Stanford (Calif.) University Medical Center said at a symposium on vascular surgery sponsored by Northwestern University. The guidelines identify four key components to a successful transfer: an organized inter-facility system of care including rapid triage and transport, defined clinical criteria for transfer, standard resuscitation protocols for the transport, and appropriate resources at the receiving hospital.
During transport, aim for a systolic blood pressure of 70 mm Hg to 90 mm Hg, establish peripheral intravenous access, and avoid aggressive fluid resuscitation, the guidelines advise. Blood products may delay the transfer.
Receiving hospitals should provide a simple and reliable method of referral and have formal protocols in place for the treatment of transferred patients.
Centers that receive patients should have endovascular aortic repair capabilities for ruptured aneurysms, including the ability to perform EVAR under local anesthesia, as well as appropriate facilities and expertise, Dr. Mell said. This advice is based mainly on outcomes observed in the IMPROVE trial (Br J Surg. 2014;101;216-24).
Successful programs tend to repair more than 20-25 ruptures per year, have on-site EVAR inventory, and, for the most part, have vascular surgeons able to perform dual open and endovascular repair. Hospital resources in these successful programs have a single phone number for transfer requests, electronic image transfer, immediately available blood products, hospital policy to accept all requests regardless of bed capacity, a contingency plan to create bed capacity after repair, and real-time management between the transfer center, bed control, and clinicians.
“This is really important because a lot of tertiary centers struggle with bed capacity if bottlenecked and a significant number [about one-third] of transfer requests are declined because of lack of capacity or dedicated room,” Dr. Mell said.
In a more recent study, nearly 20% of 4,439 patients who presented with rAAA in New York, California, and Florida were transferred for definitive care. Transfer rates rose yearly during the study period from 14% in 2005 to 22% in 2010 (J Vasc Surg. 2014;60:553-7).
“Transfer is increasingly utilized as a means for definitive care,” Dr. Mell said.
However, one in six of those transferred died without receiving treatment.
In adjusted analyses, inter-facility transfer was associated with significantly lower mortality when only patients receiving treatment were analyzed (adjusted odds ratio, 0.81; P = .02), but was actually associated with higher mortality when patients who died without treatment were also included (aOR, 1.30; P = .01).
“Outcomes after transfer can be improved by better patient selection and more efficient systems of care,” Dr. Mell concluded. “Guidelines may help; it’s too soon to know, but successful transfer programs require forethought, resources, and alignment of all stakeholders.”
Dr. Mell reported having no conflicts of interest.
CHICAGO – Adoption of an organized, systematic approach to ruptured abdominal aortic aneurysm has been inconsistent.
In a recent survey of vascular physicians in the western United States, 60% who accept ruptured abdominal aortic aneurysm (rAAA) transfers do not have a formal protocol for treatment and 70% do not use a transfer protocol or clinical guidelines (J Vasc Surg. 2015 Aug;62:326-30).
Guidelines for the management and transfer of patients with rAAA have been developed in the United Kingdom, but no such guidelines currently exist in the United States.
To address this disparity, the Western Vascular Society used the survey results, existing European guidelines, and a literature review to develop a set of 15 best practice steps for rAAA transfer. The “guidelines” were endorsed by the society members in September 2015 and are to be published early in 2016, Dr. Matthew Mell of Stanford (Calif.) University Medical Center said at a symposium on vascular surgery sponsored by Northwestern University. The guidelines identify four key components to a successful transfer: an organized inter-facility system of care including rapid triage and transport, defined clinical criteria for transfer, standard resuscitation protocols for the transport, and appropriate resources at the receiving hospital.
During transport, aim for a systolic blood pressure of 70 mm Hg to 90 mm Hg, establish peripheral intravenous access, and avoid aggressive fluid resuscitation, the guidelines advise. Blood products may delay the transfer.
Receiving hospitals should provide a simple and reliable method of referral and have formal protocols in place for the treatment of transferred patients.
Centers that receive patients should have endovascular aortic repair capabilities for ruptured aneurysms, including the ability to perform EVAR under local anesthesia, as well as appropriate facilities and expertise, Dr. Mell said. This advice is based mainly on outcomes observed in the IMPROVE trial (Br J Surg. 2014;101;216-24).
Successful programs tend to repair more than 20-25 ruptures per year, have on-site EVAR inventory, and, for the most part, have vascular surgeons able to perform dual open and endovascular repair. Hospital resources in these successful programs have a single phone number for transfer requests, electronic image transfer, immediately available blood products, hospital policy to accept all requests regardless of bed capacity, a contingency plan to create bed capacity after repair, and real-time management between the transfer center, bed control, and clinicians.
“This is really important because a lot of tertiary centers struggle with bed capacity if bottlenecked and a significant number [about one-third] of transfer requests are declined because of lack of capacity or dedicated room,” Dr. Mell said.
In a more recent study, nearly 20% of 4,439 patients who presented with rAAA in New York, California, and Florida were transferred for definitive care. Transfer rates rose yearly during the study period from 14% in 2005 to 22% in 2010 (J Vasc Surg. 2014;60:553-7).
“Transfer is increasingly utilized as a means for definitive care,” Dr. Mell said.
However, one in six of those transferred died without receiving treatment.
In adjusted analyses, inter-facility transfer was associated with significantly lower mortality when only patients receiving treatment were analyzed (adjusted odds ratio, 0.81; P = .02), but was actually associated with higher mortality when patients who died without treatment were also included (aOR, 1.30; P = .01).
“Outcomes after transfer can be improved by better patient selection and more efficient systems of care,” Dr. Mell concluded. “Guidelines may help; it’s too soon to know, but successful transfer programs require forethought, resources, and alignment of all stakeholders.”
Dr. Mell reported having no conflicts of interest.
CHICAGO – Adoption of an organized, systematic approach to ruptured abdominal aortic aneurysm has been inconsistent.
In a recent survey of vascular physicians in the western United States, 60% who accept ruptured abdominal aortic aneurysm (rAAA) transfers do not have a formal protocol for treatment and 70% do not use a transfer protocol or clinical guidelines (J Vasc Surg. 2015 Aug;62:326-30).
Guidelines for the management and transfer of patients with rAAA have been developed in the United Kingdom, but no such guidelines currently exist in the United States.
To address this disparity, the Western Vascular Society used the survey results, existing European guidelines, and a literature review to develop a set of 15 best practice steps for rAAA transfer. The “guidelines” were endorsed by the society members in September 2015 and are to be published early in 2016, Dr. Matthew Mell of Stanford (Calif.) University Medical Center said at a symposium on vascular surgery sponsored by Northwestern University. The guidelines identify four key components to a successful transfer: an organized inter-facility system of care including rapid triage and transport, defined clinical criteria for transfer, standard resuscitation protocols for the transport, and appropriate resources at the receiving hospital.
During transport, aim for a systolic blood pressure of 70 mm Hg to 90 mm Hg, establish peripheral intravenous access, and avoid aggressive fluid resuscitation, the guidelines advise. Blood products may delay the transfer.
Receiving hospitals should provide a simple and reliable method of referral and have formal protocols in place for the treatment of transferred patients.
Centers that receive patients should have endovascular aortic repair capabilities for ruptured aneurysms, including the ability to perform EVAR under local anesthesia, as well as appropriate facilities and expertise, Dr. Mell said. This advice is based mainly on outcomes observed in the IMPROVE trial (Br J Surg. 2014;101;216-24).
Successful programs tend to repair more than 20-25 ruptures per year, have on-site EVAR inventory, and, for the most part, have vascular surgeons able to perform dual open and endovascular repair. Hospital resources in these successful programs have a single phone number for transfer requests, electronic image transfer, immediately available blood products, hospital policy to accept all requests regardless of bed capacity, a contingency plan to create bed capacity after repair, and real-time management between the transfer center, bed control, and clinicians.
“This is really important because a lot of tertiary centers struggle with bed capacity if bottlenecked and a significant number [about one-third] of transfer requests are declined because of lack of capacity or dedicated room,” Dr. Mell said.
In a more recent study, nearly 20% of 4,439 patients who presented with rAAA in New York, California, and Florida were transferred for definitive care. Transfer rates rose yearly during the study period from 14% in 2005 to 22% in 2010 (J Vasc Surg. 2014;60:553-7).
“Transfer is increasingly utilized as a means for definitive care,” Dr. Mell said.
However, one in six of those transferred died without receiving treatment.
In adjusted analyses, inter-facility transfer was associated with significantly lower mortality when only patients receiving treatment were analyzed (adjusted odds ratio, 0.81; P = .02), but was actually associated with higher mortality when patients who died without treatment were also included (aOR, 1.30; P = .01).
“Outcomes after transfer can be improved by better patient selection and more efficient systems of care,” Dr. Mell concluded. “Guidelines may help; it’s too soon to know, but successful transfer programs require forethought, resources, and alignment of all stakeholders.”
Dr. Mell reported having no conflicts of interest.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Lymphedema microsurgery gaining momentum
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
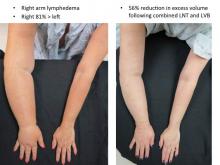
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
EXPERT ANALYSIS AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Prescribing a winning home-exercise plan for PAD patients
CHICAGO – Following a few simple steps can help patients with peripheral artery disease (PAD) get off on the right foot with a home-based exercise program.
“There is growing evidence that PAD patients can walk for exercise at home and improve their walking performance,” Dr. Mary McDermott said at a symposium on vascular surgery sponsored by Northwestern University.
Getting them to do so, however, can be challenging. Patients with peripheral artery disease have greater functional impairment and are at higher risk for cardiovascular disease than is the general population. They also frequently limit their activity to avoid leg problems, as their PAD progresses.
“It’s hard enough to get patients without peripheral artery disease to exercise. As a general internist, I’m well aware of that,” she said. “It’s even more difficult when walking is so painful and uncomfortable.”
At present, the American College of Cardiology/American Heart Association practice guidelines for the management of patients with PAD do not recommend advising patients with PAD to go home and walk.
The guidelines were published in 2005, however, and since 2011, three of four randomized clinical trials of home-based exercise programs have shown a significant gain in walking endurance in patients with PAD, Dr. McDermott of Northwestern University in Chicago, observed.
The most hands-off of these trials showed that patients with symptomatic PAD randomized to a supervised exercise program did the best at treadmill walking, but the home-exercise group who received a step monitor and in-person feedback just once per month did significantly better than did controls assigned light resistance training. Moreover, the home-exercise group had greater change in 6-minute walk distances than either of the other groups (J Am Heart Assoc. 2014 Oct;3[5]:e001107).
“I think the reason for this is that the home-based group was walking outside or perhaps in a mall and getting better at walking over ground,” Dr. McDermott. “The treadmill group was walking only on the treadmill.”
Supervised treadmill exercise may seem like an easier prescription to write, but it faces two major barriers, she said. Most medical insurers, including Medicare, do not pay for supervised exercise for people with PAD and intermittent claudication, and most PAD patients don’t participate. The burden of traveling to an exercise center three times weekly, week after week, to participate can be overwhelming.
“For all of these reasons, we really need to develop home-based exercise programs that work,” Dr. McDermott said.
Based on the successful trials, home-based programs should include monitoring, group support, and goal setting. Dr. McDermott and her colleagues added cognitive-behavioral therapy to group support in the Group Oriented Arterial Leg Study (GOALS), resulting in significant improvement in 6-minute walk distance at 6 months, physical activity over 7 days, and self-perception of walking endurance and speed among home walkers (JAMA. 2013 Jul 3;310[1]:57-65).
Before embarking on any home-exercise program, all patients with PAD should undergo a baseline cardiac stress test to rule out coronary artery ischemia, she cautioned. This also serves to identify any coronary ischemia that may develop during the new walking program.
Once this is performed, Dr. McDermott recommends clinicians:
• Advise patients to walk 5 days per week.
“This may seem like a lot, but it’s important to have them see this as part of their daily routine; just something they get in the habit of doing,” she said.
• Start with 10-15 minutes of walking per exercise session. Tailor the program to the individual patient.
• Walk to maximal leg pain or onset of ischemic pain. Stopping to rest is acceptable.
“A lot of patients, I find, have questions, “ ‘I can’t do this.’ ‘How can this be beneficial?’ So just letting them know that if they just walk and stop, walk and stop, and start with just 10 minutes of that and increase this over time, they really can see improvement,” Dr. McDermott said.
• Increase the walk time by 5 minutes each week.
Increase the duration until the patient is walking at least 30 minutes per session and preferably 45-50 minutes per session, excluding rest periods, she said.
• Advise patients to write down their walking goals.
Specify where they will walk, when they will walk, and the duration of walking to improve compliance, which can slip following hospitalizations or when patients experience acute illness.
• Have patients self-monitor, but also check in with someone for support.
“It doesn’t have to be a nurse,” Dr. McDermott said. “In our studies, we’ve used bachelor’s degree-level people, but told them what to look for. They can do this and the patient feels there is someone they’re accountable to.”
Dr. McDermott and her colleagues are testing the boundaries of support in the ongoing HONOR trial, which includes four weekly visits to an exercise center in phase I to meet the telephone coach, learn to use a Fitbit monitor, and learn the behavioral skills necessary for long-term adherence. Phase II, however, is entirely home based and includes only Fitbit self-monitoring, regular telephone calls from the coach for feedback, use of the study website, and optional group telephone calls.
The bottom line with any program is for patients to understand it must be indefinite to maintain improvement.
“Unfortunately, if they don’t stick with it, they will slide back,” she cautioned.
Dr. McDermott reported research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and Novartis.
CHICAGO – Following a few simple steps can help patients with peripheral artery disease (PAD) get off on the right foot with a home-based exercise program.
“There is growing evidence that PAD patients can walk for exercise at home and improve their walking performance,” Dr. Mary McDermott said at a symposium on vascular surgery sponsored by Northwestern University.
Getting them to do so, however, can be challenging. Patients with peripheral artery disease have greater functional impairment and are at higher risk for cardiovascular disease than is the general population. They also frequently limit their activity to avoid leg problems, as their PAD progresses.
“It’s hard enough to get patients without peripheral artery disease to exercise. As a general internist, I’m well aware of that,” she said. “It’s even more difficult when walking is so painful and uncomfortable.”
At present, the American College of Cardiology/American Heart Association practice guidelines for the management of patients with PAD do not recommend advising patients with PAD to go home and walk.
The guidelines were published in 2005, however, and since 2011, three of four randomized clinical trials of home-based exercise programs have shown a significant gain in walking endurance in patients with PAD, Dr. McDermott of Northwestern University in Chicago, observed.
The most hands-off of these trials showed that patients with symptomatic PAD randomized to a supervised exercise program did the best at treadmill walking, but the home-exercise group who received a step monitor and in-person feedback just once per month did significantly better than did controls assigned light resistance training. Moreover, the home-exercise group had greater change in 6-minute walk distances than either of the other groups (J Am Heart Assoc. 2014 Oct;3[5]:e001107).
“I think the reason for this is that the home-based group was walking outside or perhaps in a mall and getting better at walking over ground,” Dr. McDermott. “The treadmill group was walking only on the treadmill.”
Supervised treadmill exercise may seem like an easier prescription to write, but it faces two major barriers, she said. Most medical insurers, including Medicare, do not pay for supervised exercise for people with PAD and intermittent claudication, and most PAD patients don’t participate. The burden of traveling to an exercise center three times weekly, week after week, to participate can be overwhelming.
“For all of these reasons, we really need to develop home-based exercise programs that work,” Dr. McDermott said.
Based on the successful trials, home-based programs should include monitoring, group support, and goal setting. Dr. McDermott and her colleagues added cognitive-behavioral therapy to group support in the Group Oriented Arterial Leg Study (GOALS), resulting in significant improvement in 6-minute walk distance at 6 months, physical activity over 7 days, and self-perception of walking endurance and speed among home walkers (JAMA. 2013 Jul 3;310[1]:57-65).
Before embarking on any home-exercise program, all patients with PAD should undergo a baseline cardiac stress test to rule out coronary artery ischemia, she cautioned. This also serves to identify any coronary ischemia that may develop during the new walking program.
Once this is performed, Dr. McDermott recommends clinicians:
• Advise patients to walk 5 days per week.
“This may seem like a lot, but it’s important to have them see this as part of their daily routine; just something they get in the habit of doing,” she said.
• Start with 10-15 minutes of walking per exercise session. Tailor the program to the individual patient.
• Walk to maximal leg pain or onset of ischemic pain. Stopping to rest is acceptable.
“A lot of patients, I find, have questions, “ ‘I can’t do this.’ ‘How can this be beneficial?’ So just letting them know that if they just walk and stop, walk and stop, and start with just 10 minutes of that and increase this over time, they really can see improvement,” Dr. McDermott said.
• Increase the walk time by 5 minutes each week.
Increase the duration until the patient is walking at least 30 minutes per session and preferably 45-50 minutes per session, excluding rest periods, she said.
• Advise patients to write down their walking goals.
Specify where they will walk, when they will walk, and the duration of walking to improve compliance, which can slip following hospitalizations or when patients experience acute illness.
• Have patients self-monitor, but also check in with someone for support.
“It doesn’t have to be a nurse,” Dr. McDermott said. “In our studies, we’ve used bachelor’s degree-level people, but told them what to look for. They can do this and the patient feels there is someone they’re accountable to.”
Dr. McDermott and her colleagues are testing the boundaries of support in the ongoing HONOR trial, which includes four weekly visits to an exercise center in phase I to meet the telephone coach, learn to use a Fitbit monitor, and learn the behavioral skills necessary for long-term adherence. Phase II, however, is entirely home based and includes only Fitbit self-monitoring, regular telephone calls from the coach for feedback, use of the study website, and optional group telephone calls.
The bottom line with any program is for patients to understand it must be indefinite to maintain improvement.
“Unfortunately, if they don’t stick with it, they will slide back,” she cautioned.
Dr. McDermott reported research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and Novartis.
CHICAGO – Following a few simple steps can help patients with peripheral artery disease (PAD) get off on the right foot with a home-based exercise program.
“There is growing evidence that PAD patients can walk for exercise at home and improve their walking performance,” Dr. Mary McDermott said at a symposium on vascular surgery sponsored by Northwestern University.
Getting them to do so, however, can be challenging. Patients with peripheral artery disease have greater functional impairment and are at higher risk for cardiovascular disease than is the general population. They also frequently limit their activity to avoid leg problems, as their PAD progresses.
“It’s hard enough to get patients without peripheral artery disease to exercise. As a general internist, I’m well aware of that,” she said. “It’s even more difficult when walking is so painful and uncomfortable.”
At present, the American College of Cardiology/American Heart Association practice guidelines for the management of patients with PAD do not recommend advising patients with PAD to go home and walk.
The guidelines were published in 2005, however, and since 2011, three of four randomized clinical trials of home-based exercise programs have shown a significant gain in walking endurance in patients with PAD, Dr. McDermott of Northwestern University in Chicago, observed.
The most hands-off of these trials showed that patients with symptomatic PAD randomized to a supervised exercise program did the best at treadmill walking, but the home-exercise group who received a step monitor and in-person feedback just once per month did significantly better than did controls assigned light resistance training. Moreover, the home-exercise group had greater change in 6-minute walk distances than either of the other groups (J Am Heart Assoc. 2014 Oct;3[5]:e001107).
“I think the reason for this is that the home-based group was walking outside or perhaps in a mall and getting better at walking over ground,” Dr. McDermott. “The treadmill group was walking only on the treadmill.”
Supervised treadmill exercise may seem like an easier prescription to write, but it faces two major barriers, she said. Most medical insurers, including Medicare, do not pay for supervised exercise for people with PAD and intermittent claudication, and most PAD patients don’t participate. The burden of traveling to an exercise center three times weekly, week after week, to participate can be overwhelming.
“For all of these reasons, we really need to develop home-based exercise programs that work,” Dr. McDermott said.
Based on the successful trials, home-based programs should include monitoring, group support, and goal setting. Dr. McDermott and her colleagues added cognitive-behavioral therapy to group support in the Group Oriented Arterial Leg Study (GOALS), resulting in significant improvement in 6-minute walk distance at 6 months, physical activity over 7 days, and self-perception of walking endurance and speed among home walkers (JAMA. 2013 Jul 3;310[1]:57-65).
Before embarking on any home-exercise program, all patients with PAD should undergo a baseline cardiac stress test to rule out coronary artery ischemia, she cautioned. This also serves to identify any coronary ischemia that may develop during the new walking program.
Once this is performed, Dr. McDermott recommends clinicians:
• Advise patients to walk 5 days per week.
“This may seem like a lot, but it’s important to have them see this as part of their daily routine; just something they get in the habit of doing,” she said.
• Start with 10-15 minutes of walking per exercise session. Tailor the program to the individual patient.
• Walk to maximal leg pain or onset of ischemic pain. Stopping to rest is acceptable.
“A lot of patients, I find, have questions, “ ‘I can’t do this.’ ‘How can this be beneficial?’ So just letting them know that if they just walk and stop, walk and stop, and start with just 10 minutes of that and increase this over time, they really can see improvement,” Dr. McDermott said.
• Increase the walk time by 5 minutes each week.
Increase the duration until the patient is walking at least 30 minutes per session and preferably 45-50 minutes per session, excluding rest periods, she said.
• Advise patients to write down their walking goals.
Specify where they will walk, when they will walk, and the duration of walking to improve compliance, which can slip following hospitalizations or when patients experience acute illness.
• Have patients self-monitor, but also check in with someone for support.
“It doesn’t have to be a nurse,” Dr. McDermott said. “In our studies, we’ve used bachelor’s degree-level people, but told them what to look for. They can do this and the patient feels there is someone they’re accountable to.”
Dr. McDermott and her colleagues are testing the boundaries of support in the ongoing HONOR trial, which includes four weekly visits to an exercise center in phase I to meet the telephone coach, learn to use a Fitbit monitor, and learn the behavioral skills necessary for long-term adherence. Phase II, however, is entirely home based and includes only Fitbit self-monitoring, regular telephone calls from the coach for feedback, use of the study website, and optional group telephone calls.
The bottom line with any program is for patients to understand it must be indefinite to maintain improvement.
“Unfortunately, if they don’t stick with it, they will slide back,” she cautioned.
Dr. McDermott reported research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and Novartis.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM