User login
Shortness of Breath and Loss of Appetite
Answer
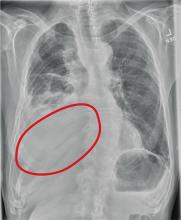
The radiograph shows several abnormalities: There is a moderate to large right pleural effusion, as well as a parenchymal density within the right lower lobe. In addition, several of the ribs have a mottled appearance.
All of these findings are highly suspicious for primary as well as metastatic carcinoma. The patient was admitted to the hospital for further workup.
Answer
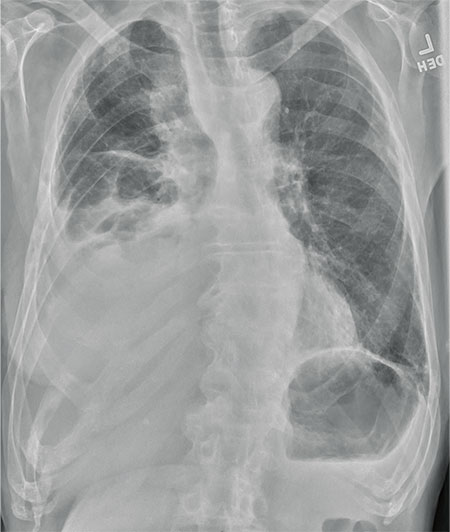
The radiograph shows several abnormalities: There is a moderate to large right pleural effusion, as well as a parenchymal density within the right lower lobe. In addition, several of the ribs have a mottled appearance.
All of these findings are highly suspicious for primary as well as metastatic carcinoma. The patient was admitted to the hospital for further workup.
Answer
The radiograph shows several abnormalities: There is a moderate to large right pleural effusion, as well as a parenchymal density within the right lower lobe. In addition, several of the ribs have a mottled appearance.
All of these findings are highly suspicious for primary as well as metastatic carcinoma. The patient was admitted to the hospital for further workup.
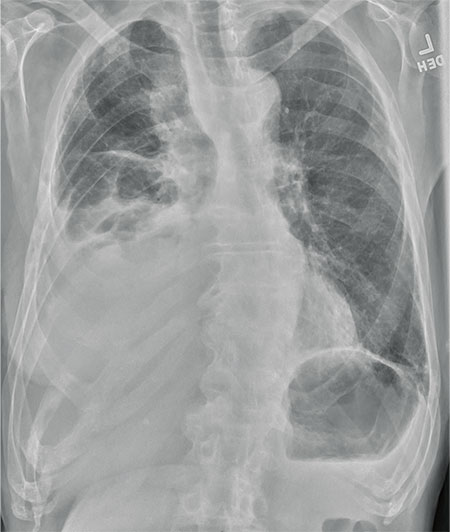
An 80-year-old man presents with a complaint of acute shortness of breath. He says he has had difficulty breathing for the past two months, but the problem has worsened in the past two days. He reports experiencing dyspnea on exertion and denies fever or chills. He says he has had no appetite lately, adding that he’s lost about 20 to 30 lb in the past couple of months. Medical history is significant for atrial fibrillation, hypothyroidism, hyperlipidemia, and remote bladder cancer. He is a former heavy smoker who quit about 30 years ago. On initial assessment, you note an elderly male in mild respiratory distress. His vital signs are stable, except for his O2 saturation, which is 90% on room air. On auscultation, you note decreased breath sounds on the right and occasional wheezing. You order some preliminary lab work, as well as a chest radiograph. What is your impression?
Cold and Fever Followed by Chest Discomfort
ANSWER
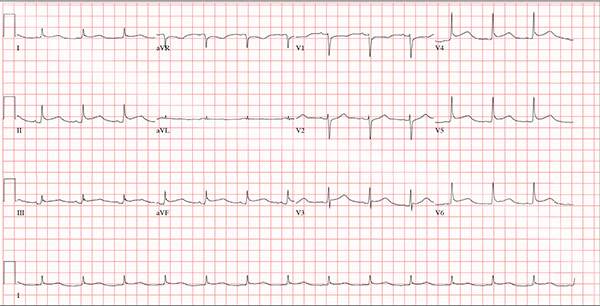
This ECG demonstrates normal sinus rhythm and diffuse ST elevations consistent with a diagnosis of pericarditis.
Although the QTc interval is long, it is due to the ST changes of pericarditis. Comparison with previous ECGs documented normal QTc intervals.
The patient’s pericarditis is most likely related to his recent viral illness. Following treatment with indomethacin, his symptoms resolved and his ECG normalized. Also, his abscess was managed by the surgical service and has since resolved.
ANSWER
This ECG demonstrates normal sinus rhythm and diffuse ST elevations consistent with a diagnosis of pericarditis.
Although the QTc interval is long, it is due to the ST changes of pericarditis. Comparison with previous ECGs documented normal QTc intervals.
The patient’s pericarditis is most likely related to his recent viral illness. Following treatment with indomethacin, his symptoms resolved and his ECG normalized. Also, his abscess was managed by the surgical service and has since resolved.
ANSWER
This ECG demonstrates normal sinus rhythm and diffuse ST elevations consistent with a diagnosis of pericarditis.
Although the QTc interval is long, it is due to the ST changes of pericarditis. Comparison with previous ECGs documented normal QTc intervals.
The patient’s pericarditis is most likely related to his recent viral illness. Following treatment with indomethacin, his symptoms resolved and his ECG normalized. Also, his abscess was managed by the surgical service and has since resolved.
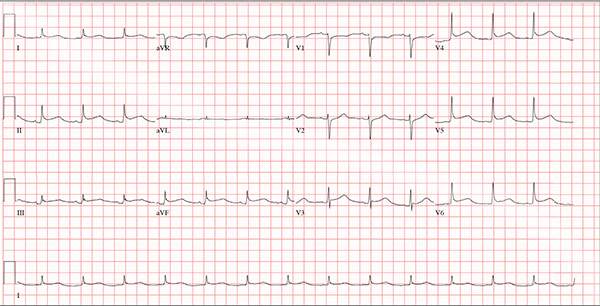
A 47-year-old man presents with a five-day history of chest discomfort that he describes as vague and achy but not painful. The discomfort does not radiate to his arm or neck and is not affected by activity. About six weeks ago, the patient says, he developed a severe viral cold that had him bedridden for several days. During his illness, his temperature reached 102°F for three or four days, and he developed a rash that subsided around the time his fever did. He had shortness of breath then, but not now. He adds, however, that if he takes a deep breath, coughs, or sneezes, he feels a shooting pain beneath his sternum. Medical history is remarkable for hypertension, type 2 diabetes, and Wolff-Parkinson-White syndrome. Surgical history includes a left inguinal hernia repair at age 6, an appendectomy for acute appendicitis at age 13, and a successful catheter ablation at age 24. The patient, a long-haul trucker, is on the road five days a week and home on weekends. He is married and has four teenage children. He does not smoke or use recreational drugs; the company he works for performs weekly drug checks and offers financial incentives to employees who do not smoke. Family history reveals that his father died at age 68 of complications of diabetes. His 64-year-old mother is alive and well and has no health issues of which he is aware. His grandparents are deceased, and he has no information on their medical history. His medication list includes metoprolol, glyburide, and metformin. He has no known drug allergies. Review of systems reveals that he has recently developed an abscess on his left buttock that he says he needs to get fixed. He wears glasses and has several teeth with dental caries. He denies any symptoms suggestive of diabetic neuropathy. The remainder of the review is normal. Physical exam reveals that he weighs 228 lb and stands 76 in tall. Vital signs include a blood pressure of 138/84 mm Hg; pulse, 80 beats/min and regular; respiratory rate, 14 breaths/min-1; temperature, 99°F; and O2 saturation, 97% on room air. Pertinent physical findings include clear lungs bilaterally and a friction rub over the entire precordium. The abdomen is soft and nontender. There is a 1-cm abscess located 2 cm left of the sacrum that is fluctuant and tender to palpation. There is no peripheral edema. All pulses are present and strong bilaterally, and there are no focal neurologic findings. Laboratory tests reveal a normal blood chemistry panel. The complete blood count is remarkable for a white blood cell count of 12,000 cells/µL. In light of the friction rub, an ECG is obtained. It shows a ventricular rate of 82 beats/min; PR interval, 130 ms; QRS duration, 90 ms; QT/QTc interval, 442/516 ms; P axis, 78°; R axis, 59°; and T axis, 73°. What is your interpretation of this ECG?
Lesion Is Tender and Bleeds Copiously
ANSWER
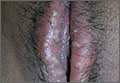
The correct answer is pyogenic granuloma (choice “d”), further discussion of which follows. Bacillary angiomatosis (choice “a”) is a lesion caused by infection with a species of Bartonella—a distinctly unusual problem. While a retained foreign body (choice “b”), such as a splinter, could trigger a similar lesion, there was no relevant history to suggest this was the case here. The most concerning differential item, melanoma (choice “c”), can present as a glistening red nodule, especially in children, but this too would be quite unusual.
DISCUSSION
Pyogenic granuloma (PG) was the name originally given to these common lesions, which are neither pyogenic (pus producing) nor truly granulomatous (demonstrating a classic histologic pattern). Rather, they are the body’s frustrated attempt to lay down new blood supply in a healing but oft-traumatized lesion (eg, acne lesion, tag, nevus, or wart).
Other names for them include sclerosing hemangioma and lobular capillary hemangioma. Their appearance can vary from the classic look seen in this case to older lesions that tend to be drier and more warty.
PGs are far more common in children than in adults and greatly favor females over males. Pregnancy appears to trigger them, especially in the mouth, but they can appear on fingers, nipples, or even the scalp. Certain drugs, such as isotretinoin and certain chemotherapy agents, predispose to their formation.
PGs removed from children (by shave technique, followed by electrodesiccation and curettage) must be sent for pathologic examination to rule out nodular melanoma. That’s what was done in this case, with the pathology report confirming the expected vascular nature of the lesion.
ANSWER
The correct answer is pyogenic granuloma (choice “d”), further discussion of which follows. Bacillary angiomatosis (choice “a”) is a lesion caused by infection with a species of Bartonella—a distinctly unusual problem. While a retained foreign body (choice “b”), such as a splinter, could trigger a similar lesion, there was no relevant history to suggest this was the case here. The most concerning differential item, melanoma (choice “c”), can present as a glistening red nodule, especially in children, but this too would be quite unusual.
DISCUSSION
Pyogenic granuloma (PG) was the name originally given to these common lesions, which are neither pyogenic (pus producing) nor truly granulomatous (demonstrating a classic histologic pattern). Rather, they are the body’s frustrated attempt to lay down new blood supply in a healing but oft-traumatized lesion (eg, acne lesion, tag, nevus, or wart).
Other names for them include sclerosing hemangioma and lobular capillary hemangioma. Their appearance can vary from the classic look seen in this case to older lesions that tend to be drier and more warty.
PGs are far more common in children than in adults and greatly favor females over males. Pregnancy appears to trigger them, especially in the mouth, but they can appear on fingers, nipples, or even the scalp. Certain drugs, such as isotretinoin and certain chemotherapy agents, predispose to their formation.
PGs removed from children (by shave technique, followed by electrodesiccation and curettage) must be sent for pathologic examination to rule out nodular melanoma. That’s what was done in this case, with the pathology report confirming the expected vascular nature of the lesion.
ANSWER
The correct answer is pyogenic granuloma (choice “d”), further discussion of which follows. Bacillary angiomatosis (choice “a”) is a lesion caused by infection with a species of Bartonella—a distinctly unusual problem. While a retained foreign body (choice “b”), such as a splinter, could trigger a similar lesion, there was no relevant history to suggest this was the case here. The most concerning differential item, melanoma (choice “c”), can present as a glistening red nodule, especially in children, but this too would be quite unusual.
DISCUSSION
Pyogenic granuloma (PG) was the name originally given to these common lesions, which are neither pyogenic (pus producing) nor truly granulomatous (demonstrating a classic histologic pattern). Rather, they are the body’s frustrated attempt to lay down new blood supply in a healing but oft-traumatized lesion (eg, acne lesion, tag, nevus, or wart).
Other names for them include sclerosing hemangioma and lobular capillary hemangioma. Their appearance can vary from the classic look seen in this case to older lesions that tend to be drier and more warty.
PGs are far more common in children than in adults and greatly favor females over males. Pregnancy appears to trigger them, especially in the mouth, but they can appear on fingers, nipples, or even the scalp. Certain drugs, such as isotretinoin and certain chemotherapy agents, predispose to their formation.
PGs removed from children (by shave technique, followed by electrodesiccation and curettage) must be sent for pathologic examination to rule out nodular melanoma. That’s what was done in this case, with the pathology report confirming the expected vascular nature of the lesion.
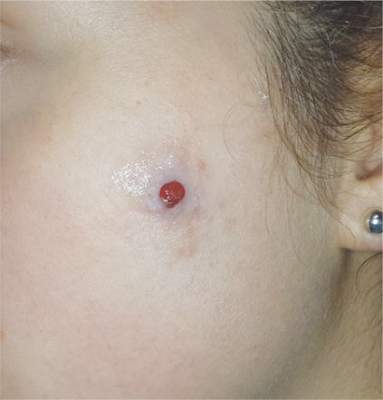
The lesion on the face of this 16-year-old girl is slightly tender to the touch and bleeds copiously with even minor trauma. It manifested several months ago and has persisted even after a course of oral antibiotics (trimethoprim/sulfa) as well as twice-daily application of mupirocin ointment. Prior to the lesion’s appearance, the girl experienced an acne flare. Her mother, who is present, says her daughter “just couldn’t leave it alone” and was often observed picking at the problem area. The patient is otherwise healthy. The lesion in question measures about 1.6 cm. It comprises a round, flesh-colored, 1-cm nodule in the center of which is a bright red, glistening 5-mm papule. There is no erythema in or around the lesion or any palpable adenopathy. The rest of the patient’s exposed skin is unremarkable.
Vulvar pain in pregnancy
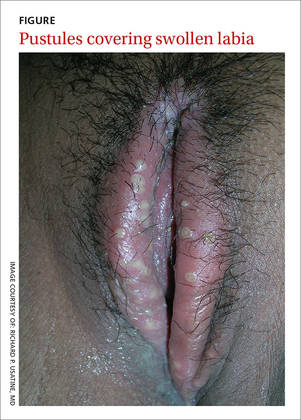
A 30-year-old pregnant woman presented to a rural Panamanian hospital with new onset genital pain, vaginal itching, and dysuria that she’d had for 48 hours. The patient was in the first trimester of her pregnancy and indicated that she’d had recent unprotected sex with a new partner who wasn’t the father of the developing fetus. The patient had never experienced symptoms like these before and denied ever having a sexually transmitted infection (STI). On physical exam, the physician noted numerous pustules covering tender, swollen labia (FIGURE). A small amount of white discharge was noted at the introitus.
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Herpes simplex virus
The physician on-call diagnosed candida vaginitis along with a bacterial skin infection, and admitted the patient to the hospital for intravenous (IV) antibiotics. Fortunately, we were there on a medical mission and were consulted on the case.
We diagnosed a primary herpes simplex virus type 2 (HSV-2) infection in this patient, based on the classic presentation of grouped pustules and vesicles on erythematous and swollen labia, and the patient’s complaint of dysuria.
Herpes cultures weren’t available in the hospital, but the clinical picture was unmistakable for HSV infection. Since multiple STIs may occur simultaneously, we ordered a serum rapid plasma reagin (RPR) test for syphilis, and tested her urine for gonorrhea and chlamydia. The tests were negative.
Differential Dx includes other STIs and a fixed drug eruption
Herpes is a common STI and most people don’t have symptoms. In 2012, an estimated 417 million people worldwide were living with genital herpes caused by HSV-2.1
The differential diagnosis for HSV infection includes primary syphilis, chancroid, folliculitis, and fixed drug eruptions.
Primary syphilis (Treponema pallidum) commonly presents with a painless, ulcerated, clean-based ulcer. While the chancre of primary syphilis can sometimes be painful, this patient did not have ulcers at the time of her presentation. Her pustules would likely ulcerate over time, but would not resemble the chancre of syphilis.
Chancroid (Haemophilus ducreyi) is a less common STI than syphilis and HSV infection. It presents with deep, sharply defined, purulent ulcers that are often associated with painful adenopathy. The ulcers can appear grey or yellowish in color.
Folliculitis presents with pustules surrounding hair follicles. Some of the pustules were surrounding hair follicles in this patient’s case, but others were independent of the hair. The patient’s marked swelling and tenderness along with dysuria also did not fit the characteristics of folliculitis.
Fixed drug eruptions can occur in the genital region, but the patient had neither bullous nor ulcerated eruptions (as one would expect with this condition). Fixed drug eruptions are usually hyperpigmented and require a history of taking medication, such as an antibiotic or a nonsteroidal anti-inflammatory drug.
Questions that help narrow the differential. Zeroing in on the cause of a patient’s genital lesions requires that you ask whether the lesions are painful, if the patient has dysuria, if there are any constitutional symptoms, and if this has happened before. Other distinguishing factors include enlarged lymph nodes and the presence of multiple (vs single) lesions.
Viral cell cultures are the preferred lab test
Common laboratory tests to make the diagnosis include viral culture, direct fluorescence antibody (DFA), polymerase chain reaction (PCR), and type-specific serologic tests.
Viral cell culture is the preferred test for suspected HSV of the skin and mucous membranes.2 PCR is the preferred test for suspected herpes meningitis or encephalitis when cerebrospinal fluid has been obtained through lumbar puncture.3 DFA and herpes culture can be ordered simultaneously. DFA can provide a quick result, and herpes culture can provide a more sensitive result (this may take 5-7 days before results are available).
No evidence that antivirals pose risk during pregnancy
Treatment with antivirals (acyclovir, famciclovir, or valacyclovir) may help to reduce the length of the outbreak. Oral antivirals are usually sufficient for uncomplicated HSV; IV antivirals may be needed in complicated cases. The current recommendation for acyclovir (the most commonly prescribed drug for HSV infection) is 400 mg 3 times daily or 200 mg 5 times daily for 7 to 10 days in a primary outbreak.3
Antiviral therapy is most effective if begun within 72 hours of symptom onset in primary herpes genitalis.4 Analgesics can help with pain control and sitz baths are helpful for women with severe dysuria.
Maternal–fetal transmission of HSV is associated with significant morbidity and mortality in children.5 The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists recommend that cesarean delivery be offered as soon as possible to women who have active HSV lesions or, in those with a history of genital herpes, symptoms of vulvar pain or burning at the time of delivery.3
There is no evidence that the use of antiviral agents in women who are pregnant and have a history of genital herpes prevents perinatal transmission of HSV to neonates.6 However, antenatal antiviral prophylaxis has been shown to reduce viral shedding, recurrences at delivery, and the need for cesarean delivery.7
Our patient was treated with oral acyclovir 400 mg 3 times a day for 10 days. One day after seeking care, she had less pain, swelling, and tenderness and was discharged. (Based on the severity of the outbreak and lack of sanitary living conditions, hospitalization was the safest and most reliable option.) The patient was counseled on the ramifications of HSV infection in pregnancy, including the fact that she might need a cesarean section. She was told that she must get prenatal care and that she needed to tell her primary care physician about her HSV infection. She was also warned about the risk of disease transmission to sexual partners and the importance of using barrier contraception to minimize the risk of future transmission.
CORRESPONDENCE
Luke Wallis, BS, 6410 Rambling Trail Drive, San Antonio, TX 78240; [email protected].
1. World Health Organization. Herpes simplex virus. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs400/en/. Accessed February 8, 2016.
2. Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
3. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
4. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
5. Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics. 2011;127:e1-e8.
6. Wenner C, Nashelsky J. Antiviral agents for pregnant women with genital herpes. Am Fam Physician. 2005;72:1807-1808.
7. Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008;CD004946.
A 30-year-old pregnant woman presented to a rural Panamanian hospital with new onset genital pain, vaginal itching, and dysuria that she’d had for 48 hours. The patient was in the first trimester of her pregnancy and indicated that she’d had recent unprotected sex with a new partner who wasn’t the father of the developing fetus. The patient had never experienced symptoms like these before and denied ever having a sexually transmitted infection (STI). On physical exam, the physician noted numerous pustules covering tender, swollen labia (FIGURE). A small amount of white discharge was noted at the introitus.

HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Herpes simplex virus
The physician on-call diagnosed candida vaginitis along with a bacterial skin infection, and admitted the patient to the hospital for intravenous (IV) antibiotics. Fortunately, we were there on a medical mission and were consulted on the case.
We diagnosed a primary herpes simplex virus type 2 (HSV-2) infection in this patient, based on the classic presentation of grouped pustules and vesicles on erythematous and swollen labia, and the patient’s complaint of dysuria.
Herpes cultures weren’t available in the hospital, but the clinical picture was unmistakable for HSV infection. Since multiple STIs may occur simultaneously, we ordered a serum rapid plasma reagin (RPR) test for syphilis, and tested her urine for gonorrhea and chlamydia. The tests were negative.
Differential Dx includes other STIs and a fixed drug eruption
Herpes is a common STI and most people don’t have symptoms. In 2012, an estimated 417 million people worldwide were living with genital herpes caused by HSV-2.1
The differential diagnosis for HSV infection includes primary syphilis, chancroid, folliculitis, and fixed drug eruptions.
Primary syphilis (Treponema pallidum) commonly presents with a painless, ulcerated, clean-based ulcer. While the chancre of primary syphilis can sometimes be painful, this patient did not have ulcers at the time of her presentation. Her pustules would likely ulcerate over time, but would not resemble the chancre of syphilis.
Chancroid (Haemophilus ducreyi) is a less common STI than syphilis and HSV infection. It presents with deep, sharply defined, purulent ulcers that are often associated with painful adenopathy. The ulcers can appear grey or yellowish in color.
Folliculitis presents with pustules surrounding hair follicles. Some of the pustules were surrounding hair follicles in this patient’s case, but others were independent of the hair. The patient’s marked swelling and tenderness along with dysuria also did not fit the characteristics of folliculitis.
Fixed drug eruptions can occur in the genital region, but the patient had neither bullous nor ulcerated eruptions (as one would expect with this condition). Fixed drug eruptions are usually hyperpigmented and require a history of taking medication, such as an antibiotic or a nonsteroidal anti-inflammatory drug.
Questions that help narrow the differential. Zeroing in on the cause of a patient’s genital lesions requires that you ask whether the lesions are painful, if the patient has dysuria, if there are any constitutional symptoms, and if this has happened before. Other distinguishing factors include enlarged lymph nodes and the presence of multiple (vs single) lesions.
Viral cell cultures are the preferred lab test
Common laboratory tests to make the diagnosis include viral culture, direct fluorescence antibody (DFA), polymerase chain reaction (PCR), and type-specific serologic tests.
Viral cell culture is the preferred test for suspected HSV of the skin and mucous membranes.2 PCR is the preferred test for suspected herpes meningitis or encephalitis when cerebrospinal fluid has been obtained through lumbar puncture.3 DFA and herpes culture can be ordered simultaneously. DFA can provide a quick result, and herpes culture can provide a more sensitive result (this may take 5-7 days before results are available).
No evidence that antivirals pose risk during pregnancy
Treatment with antivirals (acyclovir, famciclovir, or valacyclovir) may help to reduce the length of the outbreak. Oral antivirals are usually sufficient for uncomplicated HSV; IV antivirals may be needed in complicated cases. The current recommendation for acyclovir (the most commonly prescribed drug for HSV infection) is 400 mg 3 times daily or 200 mg 5 times daily for 7 to 10 days in a primary outbreak.3
Antiviral therapy is most effective if begun within 72 hours of symptom onset in primary herpes genitalis.4 Analgesics can help with pain control and sitz baths are helpful for women with severe dysuria.
Maternal–fetal transmission of HSV is associated with significant morbidity and mortality in children.5 The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists recommend that cesarean delivery be offered as soon as possible to women who have active HSV lesions or, in those with a history of genital herpes, symptoms of vulvar pain or burning at the time of delivery.3
There is no evidence that the use of antiviral agents in women who are pregnant and have a history of genital herpes prevents perinatal transmission of HSV to neonates.6 However, antenatal antiviral prophylaxis has been shown to reduce viral shedding, recurrences at delivery, and the need for cesarean delivery.7
Our patient was treated with oral acyclovir 400 mg 3 times a day for 10 days. One day after seeking care, she had less pain, swelling, and tenderness and was discharged. (Based on the severity of the outbreak and lack of sanitary living conditions, hospitalization was the safest and most reliable option.) The patient was counseled on the ramifications of HSV infection in pregnancy, including the fact that she might need a cesarean section. She was told that she must get prenatal care and that she needed to tell her primary care physician about her HSV infection. She was also warned about the risk of disease transmission to sexual partners and the importance of using barrier contraception to minimize the risk of future transmission.
CORRESPONDENCE
Luke Wallis, BS, 6410 Rambling Trail Drive, San Antonio, TX 78240; [email protected].
A 30-year-old pregnant woman presented to a rural Panamanian hospital with new onset genital pain, vaginal itching, and dysuria that she’d had for 48 hours. The patient was in the first trimester of her pregnancy and indicated that she’d had recent unprotected sex with a new partner who wasn’t the father of the developing fetus. The patient had never experienced symptoms like these before and denied ever having a sexually transmitted infection (STI). On physical exam, the physician noted numerous pustules covering tender, swollen labia (FIGURE). A small amount of white discharge was noted at the introitus.

HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Herpes simplex virus
The physician on-call diagnosed candida vaginitis along with a bacterial skin infection, and admitted the patient to the hospital for intravenous (IV) antibiotics. Fortunately, we were there on a medical mission and were consulted on the case.
We diagnosed a primary herpes simplex virus type 2 (HSV-2) infection in this patient, based on the classic presentation of grouped pustules and vesicles on erythematous and swollen labia, and the patient’s complaint of dysuria.
Herpes cultures weren’t available in the hospital, but the clinical picture was unmistakable for HSV infection. Since multiple STIs may occur simultaneously, we ordered a serum rapid plasma reagin (RPR) test for syphilis, and tested her urine for gonorrhea and chlamydia. The tests were negative.
Differential Dx includes other STIs and a fixed drug eruption
Herpes is a common STI and most people don’t have symptoms. In 2012, an estimated 417 million people worldwide were living with genital herpes caused by HSV-2.1
The differential diagnosis for HSV infection includes primary syphilis, chancroid, folliculitis, and fixed drug eruptions.
Primary syphilis (Treponema pallidum) commonly presents with a painless, ulcerated, clean-based ulcer. While the chancre of primary syphilis can sometimes be painful, this patient did not have ulcers at the time of her presentation. Her pustules would likely ulcerate over time, but would not resemble the chancre of syphilis.
Chancroid (Haemophilus ducreyi) is a less common STI than syphilis and HSV infection. It presents with deep, sharply defined, purulent ulcers that are often associated with painful adenopathy. The ulcers can appear grey or yellowish in color.
Folliculitis presents with pustules surrounding hair follicles. Some of the pustules were surrounding hair follicles in this patient’s case, but others were independent of the hair. The patient’s marked swelling and tenderness along with dysuria also did not fit the characteristics of folliculitis.
Fixed drug eruptions can occur in the genital region, but the patient had neither bullous nor ulcerated eruptions (as one would expect with this condition). Fixed drug eruptions are usually hyperpigmented and require a history of taking medication, such as an antibiotic or a nonsteroidal anti-inflammatory drug.
Questions that help narrow the differential. Zeroing in on the cause of a patient’s genital lesions requires that you ask whether the lesions are painful, if the patient has dysuria, if there are any constitutional symptoms, and if this has happened before. Other distinguishing factors include enlarged lymph nodes and the presence of multiple (vs single) lesions.
Viral cell cultures are the preferred lab test
Common laboratory tests to make the diagnosis include viral culture, direct fluorescence antibody (DFA), polymerase chain reaction (PCR), and type-specific serologic tests.
Viral cell culture is the preferred test for suspected HSV of the skin and mucous membranes.2 PCR is the preferred test for suspected herpes meningitis or encephalitis when cerebrospinal fluid has been obtained through lumbar puncture.3 DFA and herpes culture can be ordered simultaneously. DFA can provide a quick result, and herpes culture can provide a more sensitive result (this may take 5-7 days before results are available).
No evidence that antivirals pose risk during pregnancy
Treatment with antivirals (acyclovir, famciclovir, or valacyclovir) may help to reduce the length of the outbreak. Oral antivirals are usually sufficient for uncomplicated HSV; IV antivirals may be needed in complicated cases. The current recommendation for acyclovir (the most commonly prescribed drug for HSV infection) is 400 mg 3 times daily or 200 mg 5 times daily for 7 to 10 days in a primary outbreak.3
Antiviral therapy is most effective if begun within 72 hours of symptom onset in primary herpes genitalis.4 Analgesics can help with pain control and sitz baths are helpful for women with severe dysuria.
Maternal–fetal transmission of HSV is associated with significant morbidity and mortality in children.5 The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists recommend that cesarean delivery be offered as soon as possible to women who have active HSV lesions or, in those with a history of genital herpes, symptoms of vulvar pain or burning at the time of delivery.3
There is no evidence that the use of antiviral agents in women who are pregnant and have a history of genital herpes prevents perinatal transmission of HSV to neonates.6 However, antenatal antiviral prophylaxis has been shown to reduce viral shedding, recurrences at delivery, and the need for cesarean delivery.7
Our patient was treated with oral acyclovir 400 mg 3 times a day for 10 days. One day after seeking care, she had less pain, swelling, and tenderness and was discharged. (Based on the severity of the outbreak and lack of sanitary living conditions, hospitalization was the safest and most reliable option.) The patient was counseled on the ramifications of HSV infection in pregnancy, including the fact that she might need a cesarean section. She was told that she must get prenatal care and that she needed to tell her primary care physician about her HSV infection. She was also warned about the risk of disease transmission to sexual partners and the importance of using barrier contraception to minimize the risk of future transmission.
CORRESPONDENCE
Luke Wallis, BS, 6410 Rambling Trail Drive, San Antonio, TX 78240; [email protected].
1. World Health Organization. Herpes simplex virus. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs400/en/. Accessed February 8, 2016.
2. Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
3. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
4. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
5. Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics. 2011;127:e1-e8.
6. Wenner C, Nashelsky J. Antiviral agents for pregnant women with genital herpes. Am Fam Physician. 2005;72:1807-1808.
7. Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008;CD004946.
1. World Health Organization. Herpes simplex virus. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs400/en/. Accessed February 8, 2016.
2. Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
3. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1-110.
4. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
5. Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics. 2011;127:e1-e8.
6. Wenner C, Nashelsky J. Antiviral agents for pregnant women with genital herpes. Am Fam Physician. 2005;72:1807-1808.
7. Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008;CD004946.
Working with scribes—the good, the surprising
The clerical work involved in managing the electronic medical record (EMR) is clearly not at the top of the skill set for physicians, yet many office-based clinicians find themselves bogged down in this work with no easy way out.
However, practices that are adopting team-based care—where each team member works at the top of his or her skill set—are finding a solution in the form of scribing, or team documentation. This approach can ease that burden and perhaps even help to curb physician burnout in the process. But many questions still surround this approach, notably: What do we know about the quality of this documentation?
Research conducted by Misra-Hebert et al reported on in this issue provides some insight—and reason for optimism. (See “Medical scribes: How do their notes stack up?”) Their study found that scribes’ outpatient notes stack up quite well when compared to those of physicians. And having worked with this approach to documentation, I can attest to its benefits, as well.
Two approaches, one goal. There are 2 different ways that physicians can get help with documentation. One involves the use of trained scribes, who come from a variety of backgrounds and are charged with writing down, or scribing, what the physician says. The other involves training staff, usually certified medical assistants (CMAs) or licensed practical nurses (LPNs), to take on a wide variety of additional duties including refill management, care gap closure, and most of the duties concerning the EMR—including documentation.
Misra-Hebert et al studied the second approach and found important evidence that using staff in this way does not adversely affect—and may even enhance—documentation previously done entirely by physicians.
This change in the way we approach EMRs involves commitment, as I’ve seen first hand. There needs to be significant training to make this work and there needs to be more staff, since physicians require 2 of these valuable team members to function effectively. (At least that’s been our experience.)
We are in the process of implementing team-based care throughout our 32-location health care system and have found that using CMAs and LPNs to assist with documentation is a “win” for everyone.
1. A win for the patient. Patients immediately notice that their physicians are now able to focus on them during the office visit, since they no longer have to tend to the demands of the computer. In addition, since the CMAs/LPNs are with patients during the entire visit, the patients bond with them and feel the extra support from this relationship.
2. A win for the care team. Physician satisfaction has never been higher. Charts are usually closed at the end of each half-day. There is no need to take work home at night. CMAs/LPNs feel empowered and meaningfully involved in patient care. Their increase in satisfaction mirrors that of the physicians.
3. A win for the system. Not only are quality measures improving, but access improves since this team support increases efficiency. The biggest surprise of all for us was a financial one. We are able to see more patients per day and are billing at a higher level of service, since there is more time to attend to more of the patient’s needs (thanks to the additional team support).
There is much talk about putting joy back into the practice of medicine. But the benchmark of any change needs to be whether it helps our patients. I believe that team documentation does. Happier, less burned-out physicians are able to better focus on patients during their visit. As one patient recently said to me at the end of a visit, “I feel like I’ve got my doctor back.”
That’s something that patients, and doctors alike, can feel good about.
The clerical work involved in managing the electronic medical record (EMR) is clearly not at the top of the skill set for physicians, yet many office-based clinicians find themselves bogged down in this work with no easy way out.
However, practices that are adopting team-based care—where each team member works at the top of his or her skill set—are finding a solution in the form of scribing, or team documentation. This approach can ease that burden and perhaps even help to curb physician burnout in the process. But many questions still surround this approach, notably: What do we know about the quality of this documentation?
Research conducted by Misra-Hebert et al reported on in this issue provides some insight—and reason for optimism. (See “Medical scribes: How do their notes stack up?”) Their study found that scribes’ outpatient notes stack up quite well when compared to those of physicians. And having worked with this approach to documentation, I can attest to its benefits, as well.
Two approaches, one goal. There are 2 different ways that physicians can get help with documentation. One involves the use of trained scribes, who come from a variety of backgrounds and are charged with writing down, or scribing, what the physician says. The other involves training staff, usually certified medical assistants (CMAs) or licensed practical nurses (LPNs), to take on a wide variety of additional duties including refill management, care gap closure, and most of the duties concerning the EMR—including documentation.
Misra-Hebert et al studied the second approach and found important evidence that using staff in this way does not adversely affect—and may even enhance—documentation previously done entirely by physicians.
This change in the way we approach EMRs involves commitment, as I’ve seen first hand. There needs to be significant training to make this work and there needs to be more staff, since physicians require 2 of these valuable team members to function effectively. (At least that’s been our experience.)
We are in the process of implementing team-based care throughout our 32-location health care system and have found that using CMAs and LPNs to assist with documentation is a “win” for everyone.
1. A win for the patient. Patients immediately notice that their physicians are now able to focus on them during the office visit, since they no longer have to tend to the demands of the computer. In addition, since the CMAs/LPNs are with patients during the entire visit, the patients bond with them and feel the extra support from this relationship.
2. A win for the care team. Physician satisfaction has never been higher. Charts are usually closed at the end of each half-day. There is no need to take work home at night. CMAs/LPNs feel empowered and meaningfully involved in patient care. Their increase in satisfaction mirrors that of the physicians.
3. A win for the system. Not only are quality measures improving, but access improves since this team support increases efficiency. The biggest surprise of all for us was a financial one. We are able to see more patients per day and are billing at a higher level of service, since there is more time to attend to more of the patient’s needs (thanks to the additional team support).
There is much talk about putting joy back into the practice of medicine. But the benchmark of any change needs to be whether it helps our patients. I believe that team documentation does. Happier, less burned-out physicians are able to better focus on patients during their visit. As one patient recently said to me at the end of a visit, “I feel like I’ve got my doctor back.”
That’s something that patients, and doctors alike, can feel good about.
The clerical work involved in managing the electronic medical record (EMR) is clearly not at the top of the skill set for physicians, yet many office-based clinicians find themselves bogged down in this work with no easy way out.
However, practices that are adopting team-based care—where each team member works at the top of his or her skill set—are finding a solution in the form of scribing, or team documentation. This approach can ease that burden and perhaps even help to curb physician burnout in the process. But many questions still surround this approach, notably: What do we know about the quality of this documentation?
Research conducted by Misra-Hebert et al reported on in this issue provides some insight—and reason for optimism. (See “Medical scribes: How do their notes stack up?”) Their study found that scribes’ outpatient notes stack up quite well when compared to those of physicians. And having worked with this approach to documentation, I can attest to its benefits, as well.
Two approaches, one goal. There are 2 different ways that physicians can get help with documentation. One involves the use of trained scribes, who come from a variety of backgrounds and are charged with writing down, or scribing, what the physician says. The other involves training staff, usually certified medical assistants (CMAs) or licensed practical nurses (LPNs), to take on a wide variety of additional duties including refill management, care gap closure, and most of the duties concerning the EMR—including documentation.
Misra-Hebert et al studied the second approach and found important evidence that using staff in this way does not adversely affect—and may even enhance—documentation previously done entirely by physicians.
This change in the way we approach EMRs involves commitment, as I’ve seen first hand. There needs to be significant training to make this work and there needs to be more staff, since physicians require 2 of these valuable team members to function effectively. (At least that’s been our experience.)
We are in the process of implementing team-based care throughout our 32-location health care system and have found that using CMAs and LPNs to assist with documentation is a “win” for everyone.
1. A win for the patient. Patients immediately notice that their physicians are now able to focus on them during the office visit, since they no longer have to tend to the demands of the computer. In addition, since the CMAs/LPNs are with patients during the entire visit, the patients bond with them and feel the extra support from this relationship.
2. A win for the care team. Physician satisfaction has never been higher. Charts are usually closed at the end of each half-day. There is no need to take work home at night. CMAs/LPNs feel empowered and meaningfully involved in patient care. Their increase in satisfaction mirrors that of the physicians.
3. A win for the system. Not only are quality measures improving, but access improves since this team support increases efficiency. The biggest surprise of all for us was a financial one. We are able to see more patients per day and are billing at a higher level of service, since there is more time to attend to more of the patient’s needs (thanks to the additional team support).
There is much talk about putting joy back into the practice of medicine. But the benchmark of any change needs to be whether it helps our patients. I believe that team documentation does. Happier, less burned-out physicians are able to better focus on patients during their visit. As one patient recently said to me at the end of a visit, “I feel like I’ve got my doctor back.”
That’s something that patients, and doctors alike, can feel good about.
Swollen lymph nodes • patient is otherwise "healthy" • Dx?
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
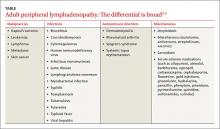
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
Taking an integrative approach to migraine headaches
› Ask all patients with migraines about their use of complementary and alternative medicine and what modalities, if any, they have found helpful. A
› Advise patients that while butterbur has been proven effective at reducing migraine frequency, its use requires caution, as products not processed properly may contain hepatotoxic compounds. A
› Caution women who are pregnant or attempting to conceive to avoid feverfew, which may cause uterine contractions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Americans who suffer from migraine headaches are far more likely than those who don’t to turn to complementary and alternative medicine (CAM). A 2007 National Health Interview Survey and a subsequent analysis of the results found that just under 50% of adults with migraine headaches used alternative therapies; among those without migraine, 34% did.1,2 What’s more, only about half of the migraine patients who reported the use of CAM modalities mentioned it to their health care providers.2
With migraine affecting some 36 million Americans,3 chances are you are caring for many of them. It is likely, too, that you are unaware of which of your headache patients are using alternative treatments, or what modalities they have tried. The only way to find out is to ask.
Women, who are 3 times more likely than men to suffer from migraine headache,4 are also the greatest users of CAM, particularly biologically based therapies and mind-body practices.5 Use is highly individualized and typically does not involve professional supervision.5
A number of alternative modalities look promising for migraine prevention. As a family physician, you are in an ideal position to guide patients in the use of safe and effective CAM therapies. To do so, however, you need to be familiar with the evidence for or against various options—many of which can be used in conjunction with pharmacotherapy.
An integrative approach to the treatment of migraine headaches makes use of the best available evidence for both conventional and alternative therapies and takes into account the whole person, including all aspects of his or her belief system and lifestyle. It also emphasizes a strong physician-patient relationship, which can have a powerful therapeutic effect.
We wrote this evidence-based update with such an approach in mind. In the text and table that follow, we present the latest findings. But first, a brief review of what constitutes migraine headache and an overview of conventional treatment.
A conventional approach to migraine
Migraine headache is a common and disabling neurologic disorder that frequently goes unrecognized and undertreated.6 It is generally characterized as recurrent headaches that are unilateral, pulsating, moderately severe, aggravated by physical activity, and associated with nausea, vomiting, photophobia, phonophobia, and sometimes a preceding aura. Conventional treatment typically includes abortive treatment for acute migraine, with medications such as the triptans and dihydroergotamine. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and the combination of acetaminophen/aspirin/caffeine are also effective. Opiates are efficacious, but not recommended.7
Prophylactic medications are generally offered to patients experiencing more than 4 migraines per month. The American Academy of Neurology cites strong evidence for the use of divalproex, valproate, topiramate, and beta-blockers, including metoprolol, propranolol, and timolol. Frovatriptan has strong evidence for prevention of menstrual-associated migraine. Common adverse effects include weight loss and parasthesias with topiramate and weight gain and somnolence with valproate, divalproex, and beta-blockers. There is also moderate evidence for the use of amitriptyline, venlafaxine, atenolol, and nadolol. Potential adverse effects must be considered to determine the optimal therapy for individual patients, and trial and error are often required.8
Addressing triggers
Conventional treatment also focuses on identifying and avoiding triggers to the extent possible. Physicians typically advise patients to keep a headache diary, recording details about diet and lifestyle, triggers, frequency and intensity of attacks, and possible patterns of headaches due to medication overuse.
Sleep disturbances and stress are common triggers, and instruction in sleep hygiene and stress reduction, as well as screening for anxiety or depression, can be beneficial. Other frequently reported factors believed to trigger or aggravate migraine attacks are skipping meals, particular foods, alcohol, weather changes, and exposure to light, sounds, and odors.
Despite the focus on migraine triggers, however, clinical studies of the role they play have shown conflicting results. A recent study involving 27 patients7 found that when attempting to provoke migraine with aura using participants’ self-reported triggers, only 3 individuals reported that the provocation actually led to a migraine.9 Additional studies suggest that exposure to headache triggers has the same effect as exposure to anxiety, with short-term exposure associated with an increased pain response and prolonged exposure leading to a decreased response.10,11 Thus, it may be beneficial to advise patients to learn to cope with controlled exposure to triggers rather than to aim for trigger avoidance.12
If noise is identified as a trigger, for instance, repeated exposure in a relaxed environment can help desensitize the patient. Triggers such as visual disturbances and odors are also good candidates for desensitization, while others, such as sleep deprivation and skipping meals, are better served by avoidance.12
CAM approaches: A look at the evidence
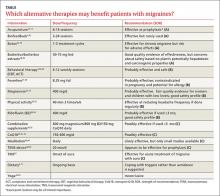
Acupuncture, butterbur, feverfew, magnesium, riboflavin, and biofeedback look promising for migraine prevention. Many of our patients are already using these and other alternative therapies. Here’s what the latest studies show (TABLE).2,9-11, 13-51
Can acupuncture help?
A 2009 Cochrane review of 22 high-quality studies with a total of 4419 participants supports the use of acupuncture for migraine prophylaxis.13 Acupuncture was found to be superior to no prophylactic treatment and acute treatment alone, and as effective as conventional preventive medications. Interestingly, though, among studies included in the Cochrane review that compared true acupuncture with sham interventions, no significant difference in results was found.
A more recent meta-analysis of 29 studies representing nearly 18,000 patients did show true acupuncture to be statistically superior to sham acupuncture, although the difference was of small clinical significance. Sham acupuncture was also shown to have a larger clinical effect than oral placebos, raising questions about the importance of exact point location.14
In a 2015 study comparing real and sham acupuncture over a 20-week period, however, the differences were more marked. Those who received real acupuncture reported significantly fewer migraine days and less severe headaches, and there were more responders in the treatment group compared with recipients of the sham procedures.15 Overall, the evidence indicates that acupuncture is at least as effective as conventional drug treatment for migraine prophylaxis, but with fewer adverse effects.13-17
Butterbur raises concerns about toxicity
Butterbur (Petasites hybridus) is one of the best-studied natural remedies for migraine. The research has primarily focused on an extract of 15% petasin and isopetasin sold under the trade name Petadolex. A study of patients using this herbal preparation for 16 weeks showed a response rate of 48% for reduction in headache frequency among those taking 75 mg twice daily and a 36% reduction in those taking 50 mg twice a day. The primary adverse effect was burping.18
Proper processing is crucial. The key concern about butterbur is that it naturally contains hepatotoxic compounds called pyrrolizidine alkaloids (PA), which may remain if the product is not properly processed.18-20 The labeling on many butterbur products states that they are “PA-free,” but because the manufacturers, rather than the US Food and Drug Administration (FDA), bear the responsibility for the safety and labeling of supplements, there is little oversight.
In fact, supplement quality is of considerable concern and subject to ongoing research. DNA bar-coding studies have confirmed that many common herbal preparations either contain ingredients not listed on the label or, conversely, fail to contain all the ingredients that are listed.52 Patients should be advised to look for evidence of quality assurance when purchasing herbal supplements, such as that offered by the US Pharmacopeia (USP) on a limited range of products. (We have not found any butterbur supplements with USP verification.)
Feverfew yields mixed results
Studies of feverfew extract for migraine have had conflicting results, probably because different extracts have been tested. A recent Cochrane review, however, cited one clinical trial (N=218) that added positive evidence to previously inconclusive findings.21
The study in question assessed a proprietary extract of feverfew (MIG-99) and found a small decrease in frequency of migraines (0.6 per month) compared with placebo. Adverse effects were gastrointestinal disturbances and mouth ulcers.22
Warn women of childbearing age that feverfew may cause uterine contractions and is contraindicated for those who are pregnant or trying to conceive.23 In addition, patients who are allergic to ragweed, chrysanthemums, or other members of the daisy family may be allergic to feverfew, as well.24
Magnesium is helpful for some
While magnesium is used for both acute relief of migraine and as prophylaxis, evidence of its efficacy is mixed. Studies have been promising in women with low magnesium levels and those who suffer from menstrual migraines, and for use in children with migraine headaches as both acute and preventive treatment.25,26
One RCT involving 160 children ages 5 to 16 years found magnesium to have a synergistic effect with acetaminophen or ibuprofen, leading to greater acute pain relief and reducing migraine frequency.27 A recent meta-analysis, however, concluded that intravenous magnesium is not likely to be effective for acute treatment.53 The main adverse effects seen with magnesium are diarrhea and soft stools.
Patients with renal disease should avoid magnesium supplementation. Food sources of magnesium include whole grains, spinach, nuts, legumes, and white potatoes.54
Riboflavin shows promise
Riboflavin (B2) plays an important role in cellular energy production and is an important antioxidant in mitochondria. Several small studies have shown promising results with high-dose (400 mg) riboflavin in migraine prevention, with evidence suggesting that it may be as effective as beta-blockers such as bisoprolol and metoprolol.28-30 Discoloration of urine, which turns bright yellow, is the primary adverse effect.
CoQ10 helps those with low levels
Like riboflavin, coenzyme Q10 (CoQ10) is involved in mitochondrial transport and plays an important role in cellular energy metabolism. Several small studies have shown efficacy in migraine prevention in doses of 150 to 300 mg/d, with response rates between 30% and 50%.31,32 Based on data in adults, the American Academy of Neurology guidelines give CoQ10 a Level C rating, indicating that it is possibly effective in preventing migraine.29
An open label study of children with migraine found that close to a third were below the reference range for CoQ10 levels. Their serum levels increased when they began taking CoQ10 supplements, resulting in a significant reduction in headache frequency and an improvement in migraine-related disability.33
Combination supplements have little efficacy
In a study published in 2015,34 a proprietary supplement containing magnesium 600 mg, riboflavin 400 mg, CoQ10 150 mg, and low-dose multivitamins, taken daily, did not show statistically significant efficacy in the reduction of migraine days. After 3 months of supplementation, however, the severity of migraine pain improved. Adverse effects included abdominal discomfort and diarrhea.
Another study compared a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg with low-dose riboflavin (25 mg) as placebo, and found that the combination did not reduce the frequency or severity of migraine any more than the placebo.35
Botox may relieve chronic migraine
Onabotulinumtoxin A (Botox) has FDA approval for the prevention of chronic migraines—ie, migraines that occur >15 days per month and at least 4 hours or more per day.55 Botox is administered by injection every 12 weeks, across 31 sites on the head and neck. The recommended dose is 155 units, with 5 units delivered into each injection site.
This protocol has been found to reduce the number of headache days by 50% in half of those being treated after one cycle, and in more than 70% of patients after 3 cycles.36 Potential adverse effects include blepharoptosis, neck muscle weakness, and the risk of botulism at sites distant from the injections.37-39
Mind-body therapies are most widely used
Of all the CAM therapies used by patients with migraine headaches, mind-body modalities are the most prevalent. Overall, 30% of headache patients use them, compared with 17% of the general population.2
Many of these modalities have been found to be effective and safe to use with the conventional migraine treatments with which patients commonly combine them.
Meditation. Both spiritual and secular forms of meditation have been studied for acute and preventive treatment of migraine and found to be effective. A recent small study suggests that spiritual meditation may be more effective,40 but secular mindfulness-based stress reduction training has also shown promise in migraine treatment.
One positive effect is that those who meditate typically use less migraine medication,41 decreasing the burden of disease. Meditation is increasingly available via a range of options, including both in-person groups and online sessions, and can easily augment conventional medical treatments.
Yoga, which typically combines physical postures, breathing techniques, and mental concentration/meditation, is increasingly widespread. While there is compelling evidence of its effect in treating chronic pain and stress-related conditions,42 studies specific to migraine are lacking. Several small studies comparing yoga to NSAIDs, educational handouts, and conventional care for headache suggest that yoga has efficacy for the treatment of migraine, but the findings are limited by methodology and sample size.42,43
Relaxation training. Various types of relaxation are described in the literature, often combining progressive muscle relaxation, diaphragmatic breathing, and relaxation-inducing imagery. Although the consensus is that these techniques are effective, differences in standards, frequency, and duration of training make it hard to draw conclusions.44
Biofeedback is similar to relaxation training, with the key difference being that it uses monitoring to train patients to alter their physiological state, thereby leading to desired changes—eg, fewer headaches and lower intensity of pain. Monitors evaluate skin temperature, electromyography, heart rate variability (blood-volume-pulse), and skin conductance, among other measures.
A robust collection of studies has shown the efficacy of skin temperature feedback, blood-volume-pulse feedback, and electromyography feedback as treatment for migraine.45 Blood-volume-pulse feedback in combination with additional home training is perhaps more effective than other modalities. Despite convincing evidence of its efficacy for migraine headaches, however, only about 1% of patients with migraine use biofeedback. That’s likely due to a lack of availability outside of urban medical centers, limited insurance coverage, and time constraints.2,45
Behavioral therapy can be of help
Cognitive behavioral therapy (CBT) focuses on adjusting maladaptive thoughts and behaviors. For migraine patients, this may include identifying and changing the patient’s response to migraine triggers such as stress, sleep deprivation, and fear of headache pain. Relaxation techniques may be incorporated into the therapy.
The effect size of CBT for prevention is comparable to prophylactic medication use, with 34% to 40% of patients achieving a clinically significant decrease in the number of attacks. Additive effects are especially promising, with more than two-thirds of patients achieving decreased frequency when CBT is combined with preventative medications.2,44,46
Acceptance and commitment therapy (ACT) is a newer variant of CBT that has recently been studied.44 Unlike CBT, in which patients are taught to control and revise their maladaptive thoughts and feelings, ACT focuses on noticing and accepting such unwanted thoughts and feelings and changing the way individuals respond to them rather than changing the thoughts themselves. Further study is needed to determine whether ACT is an effective treatment for migraine.
FDA-approved devices take aim at migraine
A transcranial magnetic stimulator (TMS) (Cerena, eNeura Inc, Sunnyvale, Calif) received FDA approval in 2013.56 The single-pulse TMS is the first device authorized for the treatment of migraine headache pain. It is geared specifically to patients suffering from migraine with aura and requires a prescription.
In a study of 201 patients, the group using the TMS device at the onset of aura had a 38% response rate, compared with a 17% response among those in the sham control group. Dizziness was reported as an adverse effect. Caution patients who express an interest in it that the device should not be used by those who are at risk for seizures or have an implanted device, such as a pacemaker or deep brain stimulator.47
Transcutaneous electrical nerve stimulation (TENS) has long been used for chronic pain, but in 2014 the FDA approved the first TENS device aimed at the prevention of migraine headaches in patients age 18 and older.57 It is also the first such device approved for use prior to the onset of pain.
The Cefaly (Cefaly US, Inc., Wilton, Conn), which requires a prescription, is worn like a headband. It is positioned on the forehead just above the eyes, using an adhesive electrode, and is worn once a day for 20 minutes. The device applies an electrical current to the skin and underlying tissues to stimulate branches of the trigeminal nerve, which can cause a tingling or massaging sensation. Several small studies have shown a decrease in migraine frequency comparable with other preventive treatments. The main adverse effect reported was sedation, but more than half of those who used it were satisfied and willing to purchase the device.48,49
Regular exercise has little downside
While physical activity can be a trigger for acute migraine, regular exercise has been shown to decrease the frequency of migraine attacks. And, although aerobic exercise is no more effective as migraine prophylaxis than conventional drug treatments, it has few adverse effects. For patients who want to stay fit and avoid taking preventive medications, exercise is a valuable adjunct to conventional treatments.50,51
CORRESPONDENCE
Laura Armstrong, MD, Memorial Hermann Family Medicine Residency Program, 14023 Southwest Freeway, Sugar Land, TX 77478; [email protected].
1. National Center for Complementary and Integrative Health. 2007 Statistics on CAM Use in the United States. National Center for Complementary and Integrative Health Web site. Available at: https://nccih.nih.gov/news/camstats/2007. Accessed January 6, 2016.
2. Wells RE, Bertisch SM, Buettner C, et al. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51:1087-1097.
3. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:S103-S122.
4. Migraine Research Foundation. Migraine in women. Migraine Research Foundation Web site. Available at: http://www.migraineresearchfoundation.org/Migraine%20in%20Women.html. Accessed January 7, 2016.
5. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015:1-16.
6. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
8. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
9. Hougaard A, Amin FM, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428-431.
10. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
11. Martin PR. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30:634-637.
12. Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483-495.
13. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218.
14. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
15. Wang Y, Xue CC, Helme R, et al. Acupuncture for frequent migraine: A randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid Based Complement Alternat Med. 2015;2015:920353.
16. Da Silva AN. Acupuncture for migraine prevention. Headache. 2015;55:470-473.
17. Meissner K, Fassler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941-1951.
18. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
19. Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6:303-310.
20. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
21. Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4:CD002286.
22. Pfaffenrath V, Diener HC, Fischer M, et al; Investigators. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523-532.
23. Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutr. 2000;3:509-514.
24. Natural Medicines. Feverfew Professional Monograph, 2016. Natural Medicines Web site. Available at: https://naturalmedicines.therapeuticresearch.com/. Accessed January 1, 2016.
25. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601-610.
26. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
27. Gallelli L, Avenoso T, Falcone D, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313-324.
28. Sándor PS, Afra J, Ambrosini A, et al. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40:30-35.
29. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
30. Boehnke C, Reuter U, Flach U, et al. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11:475-477.
31. Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64:713-715.
32. Rozen TD, Oshinsky ML, Gebeline CA, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137-141.
33. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73-80.
34. Gaul C, Diener HC, Danesch U; Migravent Study Group. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:516.
35. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.
36. Silberstein SD, Dodick DW, Aurora SK, et al. Percent of patients with chronic migraine who responded per onabotulinumtoxin A treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996-1001.
37. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
38. Aurora SK, Winner P, Freeman MC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358-1373.
39. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814.
40. Wachholtz AB, Malone CD, Pargament KI. Effect of different meditation types on migraine headache medication use. Behav Med. 2015:1-8.
41. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:13.
42. Kisan R, Sujan M, Adoor M, et al. Effect of yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126-132.
43. Büssing A, Ostermann T, Lüdtke R, et al. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13:1-9.
44. Penzien DB, Irby MB, Smitherman TA, et al. Well-established and empirically supported behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:34.
45. Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111-127.
46. Fritsche G, Kröner-Herwig B, Kropp P, et al. Psychological therapy of migraine: systematic review. Schmerz. 2013;27:263-274.
47. Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373-380.
48. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697-704.
49. Piquet M, Balestra C, Sava SL, et al. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
50. Gil-Martínez A, Kindelan-Calvo P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials. Rev Neurol. 2013;57:433-443.
51. Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
52. Newmaster SG, Grguric M, Shanmughanandhan D, et al. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
53. Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials. Eur J Emerg Med. 2014;21:2-9.
54. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4:S378-S383.
55. US Food and Drug Administration. FDA approves Botox to treat chronic migraine. US Food and Drug Administration Web site. October 15, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229782.htm. Accessed January 7, 2016.
56. US Food and Drug Administration. FDA allows marketing of first device to relieve migraine headache pain. US Food and Drug Administration Web site. December 13, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm378608.htm. Accessed January 7, 2016.
57. US Food and Drug Administration. FDA allows marketing of first medical device to prevent migraine headache. US Food and Drug Administration Web site. March 11, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm. Accessed January 7, 2016.
› Ask all patients with migraines about their use of complementary and alternative medicine and what modalities, if any, they have found helpful. A
› Advise patients that while butterbur has been proven effective at reducing migraine frequency, its use requires caution, as products not processed properly may contain hepatotoxic compounds. A
› Caution women who are pregnant or attempting to conceive to avoid feverfew, which may cause uterine contractions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Americans who suffer from migraine headaches are far more likely than those who don’t to turn to complementary and alternative medicine (CAM). A 2007 National Health Interview Survey and a subsequent analysis of the results found that just under 50% of adults with migraine headaches used alternative therapies; among those without migraine, 34% did.1,2 What’s more, only about half of the migraine patients who reported the use of CAM modalities mentioned it to their health care providers.2
With migraine affecting some 36 million Americans,3 chances are you are caring for many of them. It is likely, too, that you are unaware of which of your headache patients are using alternative treatments, or what modalities they have tried. The only way to find out is to ask.
Women, who are 3 times more likely than men to suffer from migraine headache,4 are also the greatest users of CAM, particularly biologically based therapies and mind-body practices.5 Use is highly individualized and typically does not involve professional supervision.5
A number of alternative modalities look promising for migraine prevention. As a family physician, you are in an ideal position to guide patients in the use of safe and effective CAM therapies. To do so, however, you need to be familiar with the evidence for or against various options—many of which can be used in conjunction with pharmacotherapy.
An integrative approach to the treatment of migraine headaches makes use of the best available evidence for both conventional and alternative therapies and takes into account the whole person, including all aspects of his or her belief system and lifestyle. It also emphasizes a strong physician-patient relationship, which can have a powerful therapeutic effect.
We wrote this evidence-based update with such an approach in mind. In the text and table that follow, we present the latest findings. But first, a brief review of what constitutes migraine headache and an overview of conventional treatment.
A conventional approach to migraine
Migraine headache is a common and disabling neurologic disorder that frequently goes unrecognized and undertreated.6 It is generally characterized as recurrent headaches that are unilateral, pulsating, moderately severe, aggravated by physical activity, and associated with nausea, vomiting, photophobia, phonophobia, and sometimes a preceding aura. Conventional treatment typically includes abortive treatment for acute migraine, with medications such as the triptans and dihydroergotamine. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and the combination of acetaminophen/aspirin/caffeine are also effective. Opiates are efficacious, but not recommended.7
Prophylactic medications are generally offered to patients experiencing more than 4 migraines per month. The American Academy of Neurology cites strong evidence for the use of divalproex, valproate, topiramate, and beta-blockers, including metoprolol, propranolol, and timolol. Frovatriptan has strong evidence for prevention of menstrual-associated migraine. Common adverse effects include weight loss and parasthesias with topiramate and weight gain and somnolence with valproate, divalproex, and beta-blockers. There is also moderate evidence for the use of amitriptyline, venlafaxine, atenolol, and nadolol. Potential adverse effects must be considered to determine the optimal therapy for individual patients, and trial and error are often required.8
Addressing triggers
Conventional treatment also focuses on identifying and avoiding triggers to the extent possible. Physicians typically advise patients to keep a headache diary, recording details about diet and lifestyle, triggers, frequency and intensity of attacks, and possible patterns of headaches due to medication overuse.
Sleep disturbances and stress are common triggers, and instruction in sleep hygiene and stress reduction, as well as screening for anxiety or depression, can be beneficial. Other frequently reported factors believed to trigger or aggravate migraine attacks are skipping meals, particular foods, alcohol, weather changes, and exposure to light, sounds, and odors.
Despite the focus on migraine triggers, however, clinical studies of the role they play have shown conflicting results. A recent study involving 27 patients7 found that when attempting to provoke migraine with aura using participants’ self-reported triggers, only 3 individuals reported that the provocation actually led to a migraine.9 Additional studies suggest that exposure to headache triggers has the same effect as exposure to anxiety, with short-term exposure associated with an increased pain response and prolonged exposure leading to a decreased response.10,11 Thus, it may be beneficial to advise patients to learn to cope with controlled exposure to triggers rather than to aim for trigger avoidance.12
If noise is identified as a trigger, for instance, repeated exposure in a relaxed environment can help desensitize the patient. Triggers such as visual disturbances and odors are also good candidates for desensitization, while others, such as sleep deprivation and skipping meals, are better served by avoidance.12
CAM approaches: A look at the evidence
Acupuncture, butterbur, feverfew, magnesium, riboflavin, and biofeedback look promising for migraine prevention. Many of our patients are already using these and other alternative therapies. Here’s what the latest studies show (TABLE).2,9-11, 13-51
Can acupuncture help?
A 2009 Cochrane review of 22 high-quality studies with a total of 4419 participants supports the use of acupuncture for migraine prophylaxis.13 Acupuncture was found to be superior to no prophylactic treatment and acute treatment alone, and as effective as conventional preventive medications. Interestingly, though, among studies included in the Cochrane review that compared true acupuncture with sham interventions, no significant difference in results was found.
A more recent meta-analysis of 29 studies representing nearly 18,000 patients did show true acupuncture to be statistically superior to sham acupuncture, although the difference was of small clinical significance. Sham acupuncture was also shown to have a larger clinical effect than oral placebos, raising questions about the importance of exact point location.14
In a 2015 study comparing real and sham acupuncture over a 20-week period, however, the differences were more marked. Those who received real acupuncture reported significantly fewer migraine days and less severe headaches, and there were more responders in the treatment group compared with recipients of the sham procedures.15 Overall, the evidence indicates that acupuncture is at least as effective as conventional drug treatment for migraine prophylaxis, but with fewer adverse effects.13-17
Butterbur raises concerns about toxicity
Butterbur (Petasites hybridus) is one of the best-studied natural remedies for migraine. The research has primarily focused on an extract of 15% petasin and isopetasin sold under the trade name Petadolex. A study of patients using this herbal preparation for 16 weeks showed a response rate of 48% for reduction in headache frequency among those taking 75 mg twice daily and a 36% reduction in those taking 50 mg twice a day. The primary adverse effect was burping.18
Proper processing is crucial. The key concern about butterbur is that it naturally contains hepatotoxic compounds called pyrrolizidine alkaloids (PA), which may remain if the product is not properly processed.18-20 The labeling on many butterbur products states that they are “PA-free,” but because the manufacturers, rather than the US Food and Drug Administration (FDA), bear the responsibility for the safety and labeling of supplements, there is little oversight.
In fact, supplement quality is of considerable concern and subject to ongoing research. DNA bar-coding studies have confirmed that many common herbal preparations either contain ingredients not listed on the label or, conversely, fail to contain all the ingredients that are listed.52 Patients should be advised to look for evidence of quality assurance when purchasing herbal supplements, such as that offered by the US Pharmacopeia (USP) on a limited range of products. (We have not found any butterbur supplements with USP verification.)
Feverfew yields mixed results
Studies of feverfew extract for migraine have had conflicting results, probably because different extracts have been tested. A recent Cochrane review, however, cited one clinical trial (N=218) that added positive evidence to previously inconclusive findings.21
The study in question assessed a proprietary extract of feverfew (MIG-99) and found a small decrease in frequency of migraines (0.6 per month) compared with placebo. Adverse effects were gastrointestinal disturbances and mouth ulcers.22
Warn women of childbearing age that feverfew may cause uterine contractions and is contraindicated for those who are pregnant or trying to conceive.23 In addition, patients who are allergic to ragweed, chrysanthemums, or other members of the daisy family may be allergic to feverfew, as well.24
Magnesium is helpful for some
While magnesium is used for both acute relief of migraine and as prophylaxis, evidence of its efficacy is mixed. Studies have been promising in women with low magnesium levels and those who suffer from menstrual migraines, and for use in children with migraine headaches as both acute and preventive treatment.25,26
One RCT involving 160 children ages 5 to 16 years found magnesium to have a synergistic effect with acetaminophen or ibuprofen, leading to greater acute pain relief and reducing migraine frequency.27 A recent meta-analysis, however, concluded that intravenous magnesium is not likely to be effective for acute treatment.53 The main adverse effects seen with magnesium are diarrhea and soft stools.
Patients with renal disease should avoid magnesium supplementation. Food sources of magnesium include whole grains, spinach, nuts, legumes, and white potatoes.54
Riboflavin shows promise
Riboflavin (B2) plays an important role in cellular energy production and is an important antioxidant in mitochondria. Several small studies have shown promising results with high-dose (400 mg) riboflavin in migraine prevention, with evidence suggesting that it may be as effective as beta-blockers such as bisoprolol and metoprolol.28-30 Discoloration of urine, which turns bright yellow, is the primary adverse effect.
CoQ10 helps those with low levels
Like riboflavin, coenzyme Q10 (CoQ10) is involved in mitochondrial transport and plays an important role in cellular energy metabolism. Several small studies have shown efficacy in migraine prevention in doses of 150 to 300 mg/d, with response rates between 30% and 50%.31,32 Based on data in adults, the American Academy of Neurology guidelines give CoQ10 a Level C rating, indicating that it is possibly effective in preventing migraine.29
An open label study of children with migraine found that close to a third were below the reference range for CoQ10 levels. Their serum levels increased when they began taking CoQ10 supplements, resulting in a significant reduction in headache frequency and an improvement in migraine-related disability.33
Combination supplements have little efficacy
In a study published in 2015,34 a proprietary supplement containing magnesium 600 mg, riboflavin 400 mg, CoQ10 150 mg, and low-dose multivitamins, taken daily, did not show statistically significant efficacy in the reduction of migraine days. After 3 months of supplementation, however, the severity of migraine pain improved. Adverse effects included abdominal discomfort and diarrhea.
Another study compared a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg with low-dose riboflavin (25 mg) as placebo, and found that the combination did not reduce the frequency or severity of migraine any more than the placebo.35
Botox may relieve chronic migraine
Onabotulinumtoxin A (Botox) has FDA approval for the prevention of chronic migraines—ie, migraines that occur >15 days per month and at least 4 hours or more per day.55 Botox is administered by injection every 12 weeks, across 31 sites on the head and neck. The recommended dose is 155 units, with 5 units delivered into each injection site.
This protocol has been found to reduce the number of headache days by 50% in half of those being treated after one cycle, and in more than 70% of patients after 3 cycles.36 Potential adverse effects include blepharoptosis, neck muscle weakness, and the risk of botulism at sites distant from the injections.37-39
Mind-body therapies are most widely used
Of all the CAM therapies used by patients with migraine headaches, mind-body modalities are the most prevalent. Overall, 30% of headache patients use them, compared with 17% of the general population.2
Many of these modalities have been found to be effective and safe to use with the conventional migraine treatments with which patients commonly combine them.
Meditation. Both spiritual and secular forms of meditation have been studied for acute and preventive treatment of migraine and found to be effective. A recent small study suggests that spiritual meditation may be more effective,40 but secular mindfulness-based stress reduction training has also shown promise in migraine treatment.
One positive effect is that those who meditate typically use less migraine medication,41 decreasing the burden of disease. Meditation is increasingly available via a range of options, including both in-person groups and online sessions, and can easily augment conventional medical treatments.
Yoga, which typically combines physical postures, breathing techniques, and mental concentration/meditation, is increasingly widespread. While there is compelling evidence of its effect in treating chronic pain and stress-related conditions,42 studies specific to migraine are lacking. Several small studies comparing yoga to NSAIDs, educational handouts, and conventional care for headache suggest that yoga has efficacy for the treatment of migraine, but the findings are limited by methodology and sample size.42,43
Relaxation training. Various types of relaxation are described in the literature, often combining progressive muscle relaxation, diaphragmatic breathing, and relaxation-inducing imagery. Although the consensus is that these techniques are effective, differences in standards, frequency, and duration of training make it hard to draw conclusions.44
Biofeedback is similar to relaxation training, with the key difference being that it uses monitoring to train patients to alter their physiological state, thereby leading to desired changes—eg, fewer headaches and lower intensity of pain. Monitors evaluate skin temperature, electromyography, heart rate variability (blood-volume-pulse), and skin conductance, among other measures.
A robust collection of studies has shown the efficacy of skin temperature feedback, blood-volume-pulse feedback, and electromyography feedback as treatment for migraine.45 Blood-volume-pulse feedback in combination with additional home training is perhaps more effective than other modalities. Despite convincing evidence of its efficacy for migraine headaches, however, only about 1% of patients with migraine use biofeedback. That’s likely due to a lack of availability outside of urban medical centers, limited insurance coverage, and time constraints.2,45
Behavioral therapy can be of help
Cognitive behavioral therapy (CBT) focuses on adjusting maladaptive thoughts and behaviors. For migraine patients, this may include identifying and changing the patient’s response to migraine triggers such as stress, sleep deprivation, and fear of headache pain. Relaxation techniques may be incorporated into the therapy.
The effect size of CBT for prevention is comparable to prophylactic medication use, with 34% to 40% of patients achieving a clinically significant decrease in the number of attacks. Additive effects are especially promising, with more than two-thirds of patients achieving decreased frequency when CBT is combined with preventative medications.2,44,46
Acceptance and commitment therapy (ACT) is a newer variant of CBT that has recently been studied.44 Unlike CBT, in which patients are taught to control and revise their maladaptive thoughts and feelings, ACT focuses on noticing and accepting such unwanted thoughts and feelings and changing the way individuals respond to them rather than changing the thoughts themselves. Further study is needed to determine whether ACT is an effective treatment for migraine.
FDA-approved devices take aim at migraine
A transcranial magnetic stimulator (TMS) (Cerena, eNeura Inc, Sunnyvale, Calif) received FDA approval in 2013.56 The single-pulse TMS is the first device authorized for the treatment of migraine headache pain. It is geared specifically to patients suffering from migraine with aura and requires a prescription.
In a study of 201 patients, the group using the TMS device at the onset of aura had a 38% response rate, compared with a 17% response among those in the sham control group. Dizziness was reported as an adverse effect. Caution patients who express an interest in it that the device should not be used by those who are at risk for seizures or have an implanted device, such as a pacemaker or deep brain stimulator.47
Transcutaneous electrical nerve stimulation (TENS) has long been used for chronic pain, but in 2014 the FDA approved the first TENS device aimed at the prevention of migraine headaches in patients age 18 and older.57 It is also the first such device approved for use prior to the onset of pain.
The Cefaly (Cefaly US, Inc., Wilton, Conn), which requires a prescription, is worn like a headband. It is positioned on the forehead just above the eyes, using an adhesive electrode, and is worn once a day for 20 minutes. The device applies an electrical current to the skin and underlying tissues to stimulate branches of the trigeminal nerve, which can cause a tingling or massaging sensation. Several small studies have shown a decrease in migraine frequency comparable with other preventive treatments. The main adverse effect reported was sedation, but more than half of those who used it were satisfied and willing to purchase the device.48,49
Regular exercise has little downside
While physical activity can be a trigger for acute migraine, regular exercise has been shown to decrease the frequency of migraine attacks. And, although aerobic exercise is no more effective as migraine prophylaxis than conventional drug treatments, it has few adverse effects. For patients who want to stay fit and avoid taking preventive medications, exercise is a valuable adjunct to conventional treatments.50,51
CORRESPONDENCE
Laura Armstrong, MD, Memorial Hermann Family Medicine Residency Program, 14023 Southwest Freeway, Sugar Land, TX 77478; [email protected].
› Ask all patients with migraines about their use of complementary and alternative medicine and what modalities, if any, they have found helpful. A
› Advise patients that while butterbur has been proven effective at reducing migraine frequency, its use requires caution, as products not processed properly may contain hepatotoxic compounds. A
› Caution women who are pregnant or attempting to conceive to avoid feverfew, which may cause uterine contractions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Americans who suffer from migraine headaches are far more likely than those who don’t to turn to complementary and alternative medicine (CAM). A 2007 National Health Interview Survey and a subsequent analysis of the results found that just under 50% of adults with migraine headaches used alternative therapies; among those without migraine, 34% did.1,2 What’s more, only about half of the migraine patients who reported the use of CAM modalities mentioned it to their health care providers.2
With migraine affecting some 36 million Americans,3 chances are you are caring for many of them. It is likely, too, that you are unaware of which of your headache patients are using alternative treatments, or what modalities they have tried. The only way to find out is to ask.
Women, who are 3 times more likely than men to suffer from migraine headache,4 are also the greatest users of CAM, particularly biologically based therapies and mind-body practices.5 Use is highly individualized and typically does not involve professional supervision.5
A number of alternative modalities look promising for migraine prevention. As a family physician, you are in an ideal position to guide patients in the use of safe and effective CAM therapies. To do so, however, you need to be familiar with the evidence for or against various options—many of which can be used in conjunction with pharmacotherapy.
An integrative approach to the treatment of migraine headaches makes use of the best available evidence for both conventional and alternative therapies and takes into account the whole person, including all aspects of his or her belief system and lifestyle. It also emphasizes a strong physician-patient relationship, which can have a powerful therapeutic effect.
We wrote this evidence-based update with such an approach in mind. In the text and table that follow, we present the latest findings. But first, a brief review of what constitutes migraine headache and an overview of conventional treatment.
A conventional approach to migraine
Migraine headache is a common and disabling neurologic disorder that frequently goes unrecognized and undertreated.6 It is generally characterized as recurrent headaches that are unilateral, pulsating, moderately severe, aggravated by physical activity, and associated with nausea, vomiting, photophobia, phonophobia, and sometimes a preceding aura. Conventional treatment typically includes abortive treatment for acute migraine, with medications such as the triptans and dihydroergotamine. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and the combination of acetaminophen/aspirin/caffeine are also effective. Opiates are efficacious, but not recommended.7
Prophylactic medications are generally offered to patients experiencing more than 4 migraines per month. The American Academy of Neurology cites strong evidence for the use of divalproex, valproate, topiramate, and beta-blockers, including metoprolol, propranolol, and timolol. Frovatriptan has strong evidence for prevention of menstrual-associated migraine. Common adverse effects include weight loss and parasthesias with topiramate and weight gain and somnolence with valproate, divalproex, and beta-blockers. There is also moderate evidence for the use of amitriptyline, venlafaxine, atenolol, and nadolol. Potential adverse effects must be considered to determine the optimal therapy for individual patients, and trial and error are often required.8
Addressing triggers
Conventional treatment also focuses on identifying and avoiding triggers to the extent possible. Physicians typically advise patients to keep a headache diary, recording details about diet and lifestyle, triggers, frequency and intensity of attacks, and possible patterns of headaches due to medication overuse.
Sleep disturbances and stress are common triggers, and instruction in sleep hygiene and stress reduction, as well as screening for anxiety or depression, can be beneficial. Other frequently reported factors believed to trigger or aggravate migraine attacks are skipping meals, particular foods, alcohol, weather changes, and exposure to light, sounds, and odors.
Despite the focus on migraine triggers, however, clinical studies of the role they play have shown conflicting results. A recent study involving 27 patients7 found that when attempting to provoke migraine with aura using participants’ self-reported triggers, only 3 individuals reported that the provocation actually led to a migraine.9 Additional studies suggest that exposure to headache triggers has the same effect as exposure to anxiety, with short-term exposure associated with an increased pain response and prolonged exposure leading to a decreased response.10,11 Thus, it may be beneficial to advise patients to learn to cope with controlled exposure to triggers rather than to aim for trigger avoidance.12
If noise is identified as a trigger, for instance, repeated exposure in a relaxed environment can help desensitize the patient. Triggers such as visual disturbances and odors are also good candidates for desensitization, while others, such as sleep deprivation and skipping meals, are better served by avoidance.12
CAM approaches: A look at the evidence
Acupuncture, butterbur, feverfew, magnesium, riboflavin, and biofeedback look promising for migraine prevention. Many of our patients are already using these and other alternative therapies. Here’s what the latest studies show (TABLE).2,9-11, 13-51
Can acupuncture help?
A 2009 Cochrane review of 22 high-quality studies with a total of 4419 participants supports the use of acupuncture for migraine prophylaxis.13 Acupuncture was found to be superior to no prophylactic treatment and acute treatment alone, and as effective as conventional preventive medications. Interestingly, though, among studies included in the Cochrane review that compared true acupuncture with sham interventions, no significant difference in results was found.
A more recent meta-analysis of 29 studies representing nearly 18,000 patients did show true acupuncture to be statistically superior to sham acupuncture, although the difference was of small clinical significance. Sham acupuncture was also shown to have a larger clinical effect than oral placebos, raising questions about the importance of exact point location.14
In a 2015 study comparing real and sham acupuncture over a 20-week period, however, the differences were more marked. Those who received real acupuncture reported significantly fewer migraine days and less severe headaches, and there were more responders in the treatment group compared with recipients of the sham procedures.15 Overall, the evidence indicates that acupuncture is at least as effective as conventional drug treatment for migraine prophylaxis, but with fewer adverse effects.13-17
Butterbur raises concerns about toxicity
Butterbur (Petasites hybridus) is one of the best-studied natural remedies for migraine. The research has primarily focused on an extract of 15% petasin and isopetasin sold under the trade name Petadolex. A study of patients using this herbal preparation for 16 weeks showed a response rate of 48% for reduction in headache frequency among those taking 75 mg twice daily and a 36% reduction in those taking 50 mg twice a day. The primary adverse effect was burping.18
Proper processing is crucial. The key concern about butterbur is that it naturally contains hepatotoxic compounds called pyrrolizidine alkaloids (PA), which may remain if the product is not properly processed.18-20 The labeling on many butterbur products states that they are “PA-free,” but because the manufacturers, rather than the US Food and Drug Administration (FDA), bear the responsibility for the safety and labeling of supplements, there is little oversight.
In fact, supplement quality is of considerable concern and subject to ongoing research. DNA bar-coding studies have confirmed that many common herbal preparations either contain ingredients not listed on the label or, conversely, fail to contain all the ingredients that are listed.52 Patients should be advised to look for evidence of quality assurance when purchasing herbal supplements, such as that offered by the US Pharmacopeia (USP) on a limited range of products. (We have not found any butterbur supplements with USP verification.)
Feverfew yields mixed results
Studies of feverfew extract for migraine have had conflicting results, probably because different extracts have been tested. A recent Cochrane review, however, cited one clinical trial (N=218) that added positive evidence to previously inconclusive findings.21
The study in question assessed a proprietary extract of feverfew (MIG-99) and found a small decrease in frequency of migraines (0.6 per month) compared with placebo. Adverse effects were gastrointestinal disturbances and mouth ulcers.22
Warn women of childbearing age that feverfew may cause uterine contractions and is contraindicated for those who are pregnant or trying to conceive.23 In addition, patients who are allergic to ragweed, chrysanthemums, or other members of the daisy family may be allergic to feverfew, as well.24
Magnesium is helpful for some
While magnesium is used for both acute relief of migraine and as prophylaxis, evidence of its efficacy is mixed. Studies have been promising in women with low magnesium levels and those who suffer from menstrual migraines, and for use in children with migraine headaches as both acute and preventive treatment.25,26
One RCT involving 160 children ages 5 to 16 years found magnesium to have a synergistic effect with acetaminophen or ibuprofen, leading to greater acute pain relief and reducing migraine frequency.27 A recent meta-analysis, however, concluded that intravenous magnesium is not likely to be effective for acute treatment.53 The main adverse effects seen with magnesium are diarrhea and soft stools.
Patients with renal disease should avoid magnesium supplementation. Food sources of magnesium include whole grains, spinach, nuts, legumes, and white potatoes.54
Riboflavin shows promise
Riboflavin (B2) plays an important role in cellular energy production and is an important antioxidant in mitochondria. Several small studies have shown promising results with high-dose (400 mg) riboflavin in migraine prevention, with evidence suggesting that it may be as effective as beta-blockers such as bisoprolol and metoprolol.28-30 Discoloration of urine, which turns bright yellow, is the primary adverse effect.
CoQ10 helps those with low levels
Like riboflavin, coenzyme Q10 (CoQ10) is involved in mitochondrial transport and plays an important role in cellular energy metabolism. Several small studies have shown efficacy in migraine prevention in doses of 150 to 300 mg/d, with response rates between 30% and 50%.31,32 Based on data in adults, the American Academy of Neurology guidelines give CoQ10 a Level C rating, indicating that it is possibly effective in preventing migraine.29
An open label study of children with migraine found that close to a third were below the reference range for CoQ10 levels. Their serum levels increased when they began taking CoQ10 supplements, resulting in a significant reduction in headache frequency and an improvement in migraine-related disability.33
Combination supplements have little efficacy
In a study published in 2015,34 a proprietary supplement containing magnesium 600 mg, riboflavin 400 mg, CoQ10 150 mg, and low-dose multivitamins, taken daily, did not show statistically significant efficacy in the reduction of migraine days. After 3 months of supplementation, however, the severity of migraine pain improved. Adverse effects included abdominal discomfort and diarrhea.
Another study compared a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg with low-dose riboflavin (25 mg) as placebo, and found that the combination did not reduce the frequency or severity of migraine any more than the placebo.35
Botox may relieve chronic migraine
Onabotulinumtoxin A (Botox) has FDA approval for the prevention of chronic migraines—ie, migraines that occur >15 days per month and at least 4 hours or more per day.55 Botox is administered by injection every 12 weeks, across 31 sites on the head and neck. The recommended dose is 155 units, with 5 units delivered into each injection site.
This protocol has been found to reduce the number of headache days by 50% in half of those being treated after one cycle, and in more than 70% of patients after 3 cycles.36 Potential adverse effects include blepharoptosis, neck muscle weakness, and the risk of botulism at sites distant from the injections.37-39
Mind-body therapies are most widely used
Of all the CAM therapies used by patients with migraine headaches, mind-body modalities are the most prevalent. Overall, 30% of headache patients use them, compared with 17% of the general population.2
Many of these modalities have been found to be effective and safe to use with the conventional migraine treatments with which patients commonly combine them.
Meditation. Both spiritual and secular forms of meditation have been studied for acute and preventive treatment of migraine and found to be effective. A recent small study suggests that spiritual meditation may be more effective,40 but secular mindfulness-based stress reduction training has also shown promise in migraine treatment.
One positive effect is that those who meditate typically use less migraine medication,41 decreasing the burden of disease. Meditation is increasingly available via a range of options, including both in-person groups and online sessions, and can easily augment conventional medical treatments.
Yoga, which typically combines physical postures, breathing techniques, and mental concentration/meditation, is increasingly widespread. While there is compelling evidence of its effect in treating chronic pain and stress-related conditions,42 studies specific to migraine are lacking. Several small studies comparing yoga to NSAIDs, educational handouts, and conventional care for headache suggest that yoga has efficacy for the treatment of migraine, but the findings are limited by methodology and sample size.42,43
Relaxation training. Various types of relaxation are described in the literature, often combining progressive muscle relaxation, diaphragmatic breathing, and relaxation-inducing imagery. Although the consensus is that these techniques are effective, differences in standards, frequency, and duration of training make it hard to draw conclusions.44
Biofeedback is similar to relaxation training, with the key difference being that it uses monitoring to train patients to alter their physiological state, thereby leading to desired changes—eg, fewer headaches and lower intensity of pain. Monitors evaluate skin temperature, electromyography, heart rate variability (blood-volume-pulse), and skin conductance, among other measures.
A robust collection of studies has shown the efficacy of skin temperature feedback, blood-volume-pulse feedback, and electromyography feedback as treatment for migraine.45 Blood-volume-pulse feedback in combination with additional home training is perhaps more effective than other modalities. Despite convincing evidence of its efficacy for migraine headaches, however, only about 1% of patients with migraine use biofeedback. That’s likely due to a lack of availability outside of urban medical centers, limited insurance coverage, and time constraints.2,45
Behavioral therapy can be of help
Cognitive behavioral therapy (CBT) focuses on adjusting maladaptive thoughts and behaviors. For migraine patients, this may include identifying and changing the patient’s response to migraine triggers such as stress, sleep deprivation, and fear of headache pain. Relaxation techniques may be incorporated into the therapy.
The effect size of CBT for prevention is comparable to prophylactic medication use, with 34% to 40% of patients achieving a clinically significant decrease in the number of attacks. Additive effects are especially promising, with more than two-thirds of patients achieving decreased frequency when CBT is combined with preventative medications.2,44,46
Acceptance and commitment therapy (ACT) is a newer variant of CBT that has recently been studied.44 Unlike CBT, in which patients are taught to control and revise their maladaptive thoughts and feelings, ACT focuses on noticing and accepting such unwanted thoughts and feelings and changing the way individuals respond to them rather than changing the thoughts themselves. Further study is needed to determine whether ACT is an effective treatment for migraine.
FDA-approved devices take aim at migraine
A transcranial magnetic stimulator (TMS) (Cerena, eNeura Inc, Sunnyvale, Calif) received FDA approval in 2013.56 The single-pulse TMS is the first device authorized for the treatment of migraine headache pain. It is geared specifically to patients suffering from migraine with aura and requires a prescription.
In a study of 201 patients, the group using the TMS device at the onset of aura had a 38% response rate, compared with a 17% response among those in the sham control group. Dizziness was reported as an adverse effect. Caution patients who express an interest in it that the device should not be used by those who are at risk for seizures or have an implanted device, such as a pacemaker or deep brain stimulator.47
Transcutaneous electrical nerve stimulation (TENS) has long been used for chronic pain, but in 2014 the FDA approved the first TENS device aimed at the prevention of migraine headaches in patients age 18 and older.57 It is also the first such device approved for use prior to the onset of pain.
The Cefaly (Cefaly US, Inc., Wilton, Conn), which requires a prescription, is worn like a headband. It is positioned on the forehead just above the eyes, using an adhesive electrode, and is worn once a day for 20 minutes. The device applies an electrical current to the skin and underlying tissues to stimulate branches of the trigeminal nerve, which can cause a tingling or massaging sensation. Several small studies have shown a decrease in migraine frequency comparable with other preventive treatments. The main adverse effect reported was sedation, but more than half of those who used it were satisfied and willing to purchase the device.48,49
Regular exercise has little downside
While physical activity can be a trigger for acute migraine, regular exercise has been shown to decrease the frequency of migraine attacks. And, although aerobic exercise is no more effective as migraine prophylaxis than conventional drug treatments, it has few adverse effects. For patients who want to stay fit and avoid taking preventive medications, exercise is a valuable adjunct to conventional treatments.50,51
CORRESPONDENCE
Laura Armstrong, MD, Memorial Hermann Family Medicine Residency Program, 14023 Southwest Freeway, Sugar Land, TX 77478; [email protected].
1. National Center for Complementary and Integrative Health. 2007 Statistics on CAM Use in the United States. National Center for Complementary and Integrative Health Web site. Available at: https://nccih.nih.gov/news/camstats/2007. Accessed January 6, 2016.
2. Wells RE, Bertisch SM, Buettner C, et al. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51:1087-1097.
3. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:S103-S122.
4. Migraine Research Foundation. Migraine in women. Migraine Research Foundation Web site. Available at: http://www.migraineresearchfoundation.org/Migraine%20in%20Women.html. Accessed January 7, 2016.
5. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015:1-16.
6. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
8. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
9. Hougaard A, Amin FM, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428-431.
10. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
11. Martin PR. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30:634-637.
12. Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483-495.
13. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218.
14. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
15. Wang Y, Xue CC, Helme R, et al. Acupuncture for frequent migraine: A randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid Based Complement Alternat Med. 2015;2015:920353.
16. Da Silva AN. Acupuncture for migraine prevention. Headache. 2015;55:470-473.
17. Meissner K, Fassler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941-1951.
18. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
19. Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6:303-310.
20. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
21. Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4:CD002286.
22. Pfaffenrath V, Diener HC, Fischer M, et al; Investigators. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523-532.
23. Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutr. 2000;3:509-514.
24. Natural Medicines. Feverfew Professional Monograph, 2016. Natural Medicines Web site. Available at: https://naturalmedicines.therapeuticresearch.com/. Accessed January 1, 2016.
25. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601-610.
26. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
27. Gallelli L, Avenoso T, Falcone D, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313-324.
28. Sándor PS, Afra J, Ambrosini A, et al. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40:30-35.
29. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
30. Boehnke C, Reuter U, Flach U, et al. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11:475-477.
31. Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64:713-715.
32. Rozen TD, Oshinsky ML, Gebeline CA, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137-141.
33. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73-80.
34. Gaul C, Diener HC, Danesch U; Migravent Study Group. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:516.
35. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.
36. Silberstein SD, Dodick DW, Aurora SK, et al. Percent of patients with chronic migraine who responded per onabotulinumtoxin A treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996-1001.
37. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
38. Aurora SK, Winner P, Freeman MC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358-1373.
39. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814.
40. Wachholtz AB, Malone CD, Pargament KI. Effect of different meditation types on migraine headache medication use. Behav Med. 2015:1-8.
41. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:13.
42. Kisan R, Sujan M, Adoor M, et al. Effect of yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126-132.
43. Büssing A, Ostermann T, Lüdtke R, et al. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13:1-9.
44. Penzien DB, Irby MB, Smitherman TA, et al. Well-established and empirically supported behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:34.
45. Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111-127.
46. Fritsche G, Kröner-Herwig B, Kropp P, et al. Psychological therapy of migraine: systematic review. Schmerz. 2013;27:263-274.
47. Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373-380.
48. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697-704.
49. Piquet M, Balestra C, Sava SL, et al. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
50. Gil-Martínez A, Kindelan-Calvo P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials. Rev Neurol. 2013;57:433-443.
51. Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
52. Newmaster SG, Grguric M, Shanmughanandhan D, et al. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
53. Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials. Eur J Emerg Med. 2014;21:2-9.
54. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4:S378-S383.
55. US Food and Drug Administration. FDA approves Botox to treat chronic migraine. US Food and Drug Administration Web site. October 15, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229782.htm. Accessed January 7, 2016.
56. US Food and Drug Administration. FDA allows marketing of first device to relieve migraine headache pain. US Food and Drug Administration Web site. December 13, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm378608.htm. Accessed January 7, 2016.
57. US Food and Drug Administration. FDA allows marketing of first medical device to prevent migraine headache. US Food and Drug Administration Web site. March 11, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm. Accessed January 7, 2016.
1. National Center for Complementary and Integrative Health. 2007 Statistics on CAM Use in the United States. National Center for Complementary and Integrative Health Web site. Available at: https://nccih.nih.gov/news/camstats/2007. Accessed January 6, 2016.
2. Wells RE, Bertisch SM, Buettner C, et al. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51:1087-1097.
3. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:S103-S122.
4. Migraine Research Foundation. Migraine in women. Migraine Research Foundation Web site. Available at: http://www.migraineresearchfoundation.org/Migraine%20in%20Women.html. Accessed January 7, 2016.
5. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015:1-16.
6. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
8. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
9. Hougaard A, Amin FM, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428-431.
10. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
11. Martin PR. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30:634-637.
12. Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483-495.
13. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218.
14. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
15. Wang Y, Xue CC, Helme R, et al. Acupuncture for frequent migraine: A randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid Based Complement Alternat Med. 2015;2015:920353.
16. Da Silva AN. Acupuncture for migraine prevention. Headache. 2015;55:470-473.
17. Meissner K, Fassler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941-1951.
18. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
19. Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6:303-310.
20. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
21. Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4:CD002286.
22. Pfaffenrath V, Diener HC, Fischer M, et al; Investigators. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523-532.
23. Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutr. 2000;3:509-514.
24. Natural Medicines. Feverfew Professional Monograph, 2016. Natural Medicines Web site. Available at: https://naturalmedicines.therapeuticresearch.com/. Accessed January 1, 2016.
25. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601-610.
26. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
27. Gallelli L, Avenoso T, Falcone D, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313-324.
28. Sándor PS, Afra J, Ambrosini A, et al. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40:30-35.
29. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
30. Boehnke C, Reuter U, Flach U, et al. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11:475-477.
31. Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64:713-715.
32. Rozen TD, Oshinsky ML, Gebeline CA, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137-141.
33. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73-80.
34. Gaul C, Diener HC, Danesch U; Migravent Study Group. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:516.
35. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.
36. Silberstein SD, Dodick DW, Aurora SK, et al. Percent of patients with chronic migraine who responded per onabotulinumtoxin A treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996-1001.
37. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
38. Aurora SK, Winner P, Freeman MC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358-1373.
39. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814.
40. Wachholtz AB, Malone CD, Pargament KI. Effect of different meditation types on migraine headache medication use. Behav Med. 2015:1-8.
41. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:13.
42. Kisan R, Sujan M, Adoor M, et al. Effect of yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126-132.
43. Büssing A, Ostermann T, Lüdtke R, et al. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13:1-9.
44. Penzien DB, Irby MB, Smitherman TA, et al. Well-established and empirically supported behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:34.
45. Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111-127.
46. Fritsche G, Kröner-Herwig B, Kropp P, et al. Psychological therapy of migraine: systematic review. Schmerz. 2013;27:263-274.
47. Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373-380.
48. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697-704.
49. Piquet M, Balestra C, Sava SL, et al. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
50. Gil-Martínez A, Kindelan-Calvo P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials. Rev Neurol. 2013;57:433-443.
51. Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
52. Newmaster SG, Grguric M, Shanmughanandhan D, et al. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
53. Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials. Eur J Emerg Med. 2014;21:2-9.
54. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4:S378-S383.
55. US Food and Drug Administration. FDA approves Botox to treat chronic migraine. US Food and Drug Administration Web site. October 15, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229782.htm. Accessed January 7, 2016.
56. US Food and Drug Administration. FDA allows marketing of first device to relieve migraine headache pain. US Food and Drug Administration Web site. December 13, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm378608.htm. Accessed January 7, 2016.
57. US Food and Drug Administration. FDA allows marketing of first medical device to prevent migraine headache. US Food and Drug Administration Web site. March 11, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm. Accessed January 7, 2016.
Can patients with infectious endocarditis be safely anticoagulated?
Managing anticoagulation in patients with infectious endocarditis requires an individualized approach, using a careful risk-benefit assessment on a case-by-case basis. There is a dearth of high-quality evidence; consequently, the recommendations also vary according to the clinical situation.
Newly diagnosed native valve infectious endocarditis in itself is not an indication for anticoagulation.1–3 The question of whether to anticoagulate arises in patients who have a preexisting or coexisting indication for anticoagulation such as atrial fibrillation, deep vein thrombosis, pulmonary embolism, or a mechanical prosthetic heart valve. The question becomes yet more complex in patients with cerebrovascular complications and a coexistent strong indication for anticoagulation, creating what is often a very thorny dilemma.
Based on a review of available evidence, recommendations for anticoagulation in patients with infectious endocarditis are summarized below.
AVAILABLE EVIDENCE IS SCARCE AND MIXED
Earlier observational studies suggested a significant risk of cerebral hemorrhage with anticoagulation in patients with native valve endocarditis, although none of these studies were recent (some of them took place in the 1940s), and none are methodologically compelling.4–8 Consequently, some experts have expressed skepticism regarding their findings, particularly in recent years.
In part, this skepticism arises from studies that showed a lower incidence of cerebrovascular complications and smaller vegetation size in patients with prosthetic valve infectious endocarditis, studies in which many of the patients received anticoagulation therapy.9,10 The mechanism responsible for this effect is theorized to be that the vegetation is an amalgam of destroyed cells, platelets, and fibrin, with anticoagulation preventing this aggregation from further growth and propagation.
How great is the benefit or the potential harm?
Some experts argue that the incidence of ischemic stroke with hemorrhagic transformation in patients with infectious endocarditis receiving anticoagulation is overestimated. According to this view, the beneficial effects of anticoagulation at least counterbalance the potential harmful effects.
In addition to the studies cited above, recent studies have shown that patients on anticoagulation tend to have smaller vegetations and fewer cerebrovascular complications.11–13 Snygg-Martin et al11 and Rasmussen et al12 found not only that cerebrovascular complications were less common in patients already on anticoagulation at the time infectious endocarditis was diagnosed, but also that no increase in the rate of hemorrhagic lesions was reported.
These were all nonrandomized studies, and most of the patients in them had native valve infectious endocarditis diagnosed at an early stage. Importantly, these studies found that the beneficial effects of anticoagulation were only present if the patient was receiving warfarin before infectious endocarditis was diagnosed and antibiotic therapy was initiated. No benefits from anticoagulation were demonstrated once antimicrobial therapy was begun.
Similarly, Anavekar et al14 showed that embolic events occurred significantly less often in those who were currently on continuous daily antiplatelet therapy, suggesting that receiving antiplatelet agents at baseline protects against cardioembolic events in patients who develop infective endocarditis. However, the only randomized trial examining the initiation of antiplatelet therapy in patients diagnosed with infectious endocarditis receiving antibiotic treatment showed that adding aspirin did not reduce the risk of embolic events and was associated with a trend toward increased risk of bleeding.15
A recent large cohort study suggested that infectious endocarditis patients who receive anticoagulation therapy may have a higher incidence of cerebrovascular complications (hazard ratio 1.31, 95% confidence interval 1.00–1.72, P = .048), with a particular association of anticoagulation therapy with intracranial bleeding (hazard ratio 2.71, 95% confidence interval 1.54–4.76, P = .001).16
Another provocative link supported by the same study was a higher incidence of hemorrhagic complications with anticoagulation in patients with infectious endocarditis caused by Staphylococcus aureus, an association also suggested by older data from Tornos et al,8 but not seen in a study by Rasmussen et al.12
Continuing anticoagulation is an individualized decision
The benefit or harm of anticoagulation in patients with infectious endocarditis may be determined at least in part by a complex mix of factors including the valve involved (embolic events are more common with mitral valve vegetations than with aortic valve vegetations), vegetation size (higher risk if > 1 cm), mobility of vegetations, and perhaps the virulence of the causative organism.16,17 The fact that antimicrobial therapy obviates any beneficial effect of anticoagulation speaks strongly against starting anticoagulation therapy in infectious endocarditis patients with the sole purpose of reducing stroke risk.
Without large randomized trials to better delineate the risks and benefits of continuing preexisting anticoagulation in all patients with infectious endocarditis, patients already receiving anticoagulants need a careful, individualized risk-benefit assessment. Current guidelines agree that newly diagnosed infectious endocarditis per se is not an indication for anticoagulation or aspirin therapy (Table 1).1–3
TAKE-HOME POINTS
- Starting antiplatelet and anticoagulation therapy for the sole purpose of stroke prevention is not recommended in patients with newly diagnosed infectious endocarditis.
- In most cases, anticoagulation and antiplatelet therapy should be temporarily discontinued in patients with infectious endocarditis and stroke or suspected stroke.
- Patients need careful assessment on a case-by-case basis, and the presence of risk factors predisposing patients to cerebrovascular complications (eg, large or very mobile vegetations, causative pathogens such as S aureus or Candida spp) may prompt temporary suspension of anticoagulation and antiplatelet therapy.
- If there is a clear preexisting or coexisting indication for these agents and surgery is not anticipated, consider continuing antiplatelet and anticoagulant therapy in patients with infectious endocarditis, provided they lack the risk factors described above and stroke has been excluded.
- If there is a clear preexisting or coexisting indication for these agents and surgery is being considered, consider using a short-acting anticoagulant such as intravenous or low-molecular weight heparin as a bridge to surgery.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–1486.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Rev Esp Cardiol (Engl Ed) 2016; 69:69.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e576S–e600S.
- Delahaye JP, Poncet P, Malquarti V, Beaune J, Garé JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990; 11:1074–1078.
- Loewe L. The combined use of anti-infectives and anticoagulants in the treatment of subacute bacterial endocarditis. Bull N Y Acad Med 1945; 21:59–86.
- Priest WS, Smith JM, McGee GC. The effect of anticoagulants on the penicillin therapy and the pathologic lesions of subacute bacterial endocarditis. N Engl J Med 1946; 235:699–706.
- Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine 1978; 57:329–343.
- Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999; 159:473–475.
- Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation 1978; 57:1004–1007.
- Schulz R, Werner GS, Fuchs JB, et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990’s. Eur Heart J 1996; 17:281–288.
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis 2011; 30:151–157.
- Rasmussen RV, Snygg-Martin U, Olaison L, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 2009; 114:284–291.
- Yau JW, Lee P, Wilson A, Jenkins AJ. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? Intern Med J 2011; 41:795–797.
- Anavekar NS, Tleyjeh IM, Mirzoyev Z, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis 2007; 44:1180–1186.
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42:775–780.
- Garcia-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–2284.
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112:69–75.
Managing anticoagulation in patients with infectious endocarditis requires an individualized approach, using a careful risk-benefit assessment on a case-by-case basis. There is a dearth of high-quality evidence; consequently, the recommendations also vary according to the clinical situation.
Newly diagnosed native valve infectious endocarditis in itself is not an indication for anticoagulation.1–3 The question of whether to anticoagulate arises in patients who have a preexisting or coexisting indication for anticoagulation such as atrial fibrillation, deep vein thrombosis, pulmonary embolism, or a mechanical prosthetic heart valve. The question becomes yet more complex in patients with cerebrovascular complications and a coexistent strong indication for anticoagulation, creating what is often a very thorny dilemma.
Based on a review of available evidence, recommendations for anticoagulation in patients with infectious endocarditis are summarized below.
AVAILABLE EVIDENCE IS SCARCE AND MIXED
Earlier observational studies suggested a significant risk of cerebral hemorrhage with anticoagulation in patients with native valve endocarditis, although none of these studies were recent (some of them took place in the 1940s), and none are methodologically compelling.4–8 Consequently, some experts have expressed skepticism regarding their findings, particularly in recent years.
In part, this skepticism arises from studies that showed a lower incidence of cerebrovascular complications and smaller vegetation size in patients with prosthetic valve infectious endocarditis, studies in which many of the patients received anticoagulation therapy.9,10 The mechanism responsible for this effect is theorized to be that the vegetation is an amalgam of destroyed cells, platelets, and fibrin, with anticoagulation preventing this aggregation from further growth and propagation.
How great is the benefit or the potential harm?
Some experts argue that the incidence of ischemic stroke with hemorrhagic transformation in patients with infectious endocarditis receiving anticoagulation is overestimated. According to this view, the beneficial effects of anticoagulation at least counterbalance the potential harmful effects.
In addition to the studies cited above, recent studies have shown that patients on anticoagulation tend to have smaller vegetations and fewer cerebrovascular complications.11–13 Snygg-Martin et al11 and Rasmussen et al12 found not only that cerebrovascular complications were less common in patients already on anticoagulation at the time infectious endocarditis was diagnosed, but also that no increase in the rate of hemorrhagic lesions was reported.
These were all nonrandomized studies, and most of the patients in them had native valve infectious endocarditis diagnosed at an early stage. Importantly, these studies found that the beneficial effects of anticoagulation were only present if the patient was receiving warfarin before infectious endocarditis was diagnosed and antibiotic therapy was initiated. No benefits from anticoagulation were demonstrated once antimicrobial therapy was begun.
Similarly, Anavekar et al14 showed that embolic events occurred significantly less often in those who were currently on continuous daily antiplatelet therapy, suggesting that receiving antiplatelet agents at baseline protects against cardioembolic events in patients who develop infective endocarditis. However, the only randomized trial examining the initiation of antiplatelet therapy in patients diagnosed with infectious endocarditis receiving antibiotic treatment showed that adding aspirin did not reduce the risk of embolic events and was associated with a trend toward increased risk of bleeding.15
A recent large cohort study suggested that infectious endocarditis patients who receive anticoagulation therapy may have a higher incidence of cerebrovascular complications (hazard ratio 1.31, 95% confidence interval 1.00–1.72, P = .048), with a particular association of anticoagulation therapy with intracranial bleeding (hazard ratio 2.71, 95% confidence interval 1.54–4.76, P = .001).16
Another provocative link supported by the same study was a higher incidence of hemorrhagic complications with anticoagulation in patients with infectious endocarditis caused by Staphylococcus aureus, an association also suggested by older data from Tornos et al,8 but not seen in a study by Rasmussen et al.12
Continuing anticoagulation is an individualized decision
The benefit or harm of anticoagulation in patients with infectious endocarditis may be determined at least in part by a complex mix of factors including the valve involved (embolic events are more common with mitral valve vegetations than with aortic valve vegetations), vegetation size (higher risk if > 1 cm), mobility of vegetations, and perhaps the virulence of the causative organism.16,17 The fact that antimicrobial therapy obviates any beneficial effect of anticoagulation speaks strongly against starting anticoagulation therapy in infectious endocarditis patients with the sole purpose of reducing stroke risk.
Without large randomized trials to better delineate the risks and benefits of continuing preexisting anticoagulation in all patients with infectious endocarditis, patients already receiving anticoagulants need a careful, individualized risk-benefit assessment. Current guidelines agree that newly diagnosed infectious endocarditis per se is not an indication for anticoagulation or aspirin therapy (Table 1).1–3
TAKE-HOME POINTS
- Starting antiplatelet and anticoagulation therapy for the sole purpose of stroke prevention is not recommended in patients with newly diagnosed infectious endocarditis.
- In most cases, anticoagulation and antiplatelet therapy should be temporarily discontinued in patients with infectious endocarditis and stroke or suspected stroke.
- Patients need careful assessment on a case-by-case basis, and the presence of risk factors predisposing patients to cerebrovascular complications (eg, large or very mobile vegetations, causative pathogens such as S aureus or Candida spp) may prompt temporary suspension of anticoagulation and antiplatelet therapy.
- If there is a clear preexisting or coexisting indication for these agents and surgery is not anticipated, consider continuing antiplatelet and anticoagulant therapy in patients with infectious endocarditis, provided they lack the risk factors described above and stroke has been excluded.
- If there is a clear preexisting or coexisting indication for these agents and surgery is being considered, consider using a short-acting anticoagulant such as intravenous or low-molecular weight heparin as a bridge to surgery.
Managing anticoagulation in patients with infectious endocarditis requires an individualized approach, using a careful risk-benefit assessment on a case-by-case basis. There is a dearth of high-quality evidence; consequently, the recommendations also vary according to the clinical situation.
Newly diagnosed native valve infectious endocarditis in itself is not an indication for anticoagulation.1–3 The question of whether to anticoagulate arises in patients who have a preexisting or coexisting indication for anticoagulation such as atrial fibrillation, deep vein thrombosis, pulmonary embolism, or a mechanical prosthetic heart valve. The question becomes yet more complex in patients with cerebrovascular complications and a coexistent strong indication for anticoagulation, creating what is often a very thorny dilemma.
Based on a review of available evidence, recommendations for anticoagulation in patients with infectious endocarditis are summarized below.
AVAILABLE EVIDENCE IS SCARCE AND MIXED
Earlier observational studies suggested a significant risk of cerebral hemorrhage with anticoagulation in patients with native valve endocarditis, although none of these studies were recent (some of them took place in the 1940s), and none are methodologically compelling.4–8 Consequently, some experts have expressed skepticism regarding their findings, particularly in recent years.
In part, this skepticism arises from studies that showed a lower incidence of cerebrovascular complications and smaller vegetation size in patients with prosthetic valve infectious endocarditis, studies in which many of the patients received anticoagulation therapy.9,10 The mechanism responsible for this effect is theorized to be that the vegetation is an amalgam of destroyed cells, platelets, and fibrin, with anticoagulation preventing this aggregation from further growth and propagation.
How great is the benefit or the potential harm?
Some experts argue that the incidence of ischemic stroke with hemorrhagic transformation in patients with infectious endocarditis receiving anticoagulation is overestimated. According to this view, the beneficial effects of anticoagulation at least counterbalance the potential harmful effects.
In addition to the studies cited above, recent studies have shown that patients on anticoagulation tend to have smaller vegetations and fewer cerebrovascular complications.11–13 Snygg-Martin et al11 and Rasmussen et al12 found not only that cerebrovascular complications were less common in patients already on anticoagulation at the time infectious endocarditis was diagnosed, but also that no increase in the rate of hemorrhagic lesions was reported.
These were all nonrandomized studies, and most of the patients in them had native valve infectious endocarditis diagnosed at an early stage. Importantly, these studies found that the beneficial effects of anticoagulation were only present if the patient was receiving warfarin before infectious endocarditis was diagnosed and antibiotic therapy was initiated. No benefits from anticoagulation were demonstrated once antimicrobial therapy was begun.
Similarly, Anavekar et al14 showed that embolic events occurred significantly less often in those who were currently on continuous daily antiplatelet therapy, suggesting that receiving antiplatelet agents at baseline protects against cardioembolic events in patients who develop infective endocarditis. However, the only randomized trial examining the initiation of antiplatelet therapy in patients diagnosed with infectious endocarditis receiving antibiotic treatment showed that adding aspirin did not reduce the risk of embolic events and was associated with a trend toward increased risk of bleeding.15
A recent large cohort study suggested that infectious endocarditis patients who receive anticoagulation therapy may have a higher incidence of cerebrovascular complications (hazard ratio 1.31, 95% confidence interval 1.00–1.72, P = .048), with a particular association of anticoagulation therapy with intracranial bleeding (hazard ratio 2.71, 95% confidence interval 1.54–4.76, P = .001).16
Another provocative link supported by the same study was a higher incidence of hemorrhagic complications with anticoagulation in patients with infectious endocarditis caused by Staphylococcus aureus, an association also suggested by older data from Tornos et al,8 but not seen in a study by Rasmussen et al.12
Continuing anticoagulation is an individualized decision
The benefit or harm of anticoagulation in patients with infectious endocarditis may be determined at least in part by a complex mix of factors including the valve involved (embolic events are more common with mitral valve vegetations than with aortic valve vegetations), vegetation size (higher risk if > 1 cm), mobility of vegetations, and perhaps the virulence of the causative organism.16,17 The fact that antimicrobial therapy obviates any beneficial effect of anticoagulation speaks strongly against starting anticoagulation therapy in infectious endocarditis patients with the sole purpose of reducing stroke risk.
Without large randomized trials to better delineate the risks and benefits of continuing preexisting anticoagulation in all patients with infectious endocarditis, patients already receiving anticoagulants need a careful, individualized risk-benefit assessment. Current guidelines agree that newly diagnosed infectious endocarditis per se is not an indication for anticoagulation or aspirin therapy (Table 1).1–3
TAKE-HOME POINTS
- Starting antiplatelet and anticoagulation therapy for the sole purpose of stroke prevention is not recommended in patients with newly diagnosed infectious endocarditis.
- In most cases, anticoagulation and antiplatelet therapy should be temporarily discontinued in patients with infectious endocarditis and stroke or suspected stroke.
- Patients need careful assessment on a case-by-case basis, and the presence of risk factors predisposing patients to cerebrovascular complications (eg, large or very mobile vegetations, causative pathogens such as S aureus or Candida spp) may prompt temporary suspension of anticoagulation and antiplatelet therapy.
- If there is a clear preexisting or coexisting indication for these agents and surgery is not anticipated, consider continuing antiplatelet and anticoagulant therapy in patients with infectious endocarditis, provided they lack the risk factors described above and stroke has been excluded.
- If there is a clear preexisting or coexisting indication for these agents and surgery is being considered, consider using a short-acting anticoagulant such as intravenous or low-molecular weight heparin as a bridge to surgery.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–1486.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Rev Esp Cardiol (Engl Ed) 2016; 69:69.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e576S–e600S.
- Delahaye JP, Poncet P, Malquarti V, Beaune J, Garé JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990; 11:1074–1078.
- Loewe L. The combined use of anti-infectives and anticoagulants in the treatment of subacute bacterial endocarditis. Bull N Y Acad Med 1945; 21:59–86.
- Priest WS, Smith JM, McGee GC. The effect of anticoagulants on the penicillin therapy and the pathologic lesions of subacute bacterial endocarditis. N Engl J Med 1946; 235:699–706.
- Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine 1978; 57:329–343.
- Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999; 159:473–475.
- Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation 1978; 57:1004–1007.
- Schulz R, Werner GS, Fuchs JB, et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990’s. Eur Heart J 1996; 17:281–288.
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis 2011; 30:151–157.
- Rasmussen RV, Snygg-Martin U, Olaison L, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 2009; 114:284–291.
- Yau JW, Lee P, Wilson A, Jenkins AJ. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? Intern Med J 2011; 41:795–797.
- Anavekar NS, Tleyjeh IM, Mirzoyev Z, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis 2007; 44:1180–1186.
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42:775–780.
- Garcia-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–2284.
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112:69–75.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–1486.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Rev Esp Cardiol (Engl Ed) 2016; 69:69.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e576S–e600S.
- Delahaye JP, Poncet P, Malquarti V, Beaune J, Garé JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990; 11:1074–1078.
- Loewe L. The combined use of anti-infectives and anticoagulants in the treatment of subacute bacterial endocarditis. Bull N Y Acad Med 1945; 21:59–86.
- Priest WS, Smith JM, McGee GC. The effect of anticoagulants on the penicillin therapy and the pathologic lesions of subacute bacterial endocarditis. N Engl J Med 1946; 235:699–706.
- Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine 1978; 57:329–343.
- Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999; 159:473–475.
- Wilson WR, Geraci JE, Danielson GK, et al. Anticoagulant therapy and central nervous system complications in patients with prosthetic valve endocarditis. Circulation 1978; 57:1004–1007.
- Schulz R, Werner GS, Fuchs JB, et al. Clinical outcome and echocardiographic findings of native and prosthetic valve endocarditis in the 1990’s. Eur Heart J 1996; 17:281–288.
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. Eur J Clin Microbiol Infect Dis 2011; 30:151–157.
- Rasmussen RV, Snygg-Martin U, Olaison L, et al. Major cerebral events in Staphylococcus aureus infective endocarditis: is anticoagulant therapy safe? Cardiology 2009; 114:284–291.
- Yau JW, Lee P, Wilson A, Jenkins AJ. Prosthetic valve endocarditis: what is the evidence for anticoagulant therapy? Intern Med J 2011; 41:795–797.
- Anavekar NS, Tleyjeh IM, Mirzoyev Z, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis 2007; 44:1180–1186.
- Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003; 42:775–780.
- Garcia-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127:2272–2284.
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112:69–75.
Immunization update: This year’s changes
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
Medical marijuana: A treatment worth trying?
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.