User login
Readers weigh in on vaginal cleansing prior to cesarean delivery
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
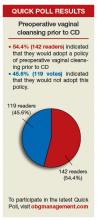
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
“SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?”
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
In his January 2016 Editorial, Editor in Chief Robert L. Barbieri, MD, presented evidence supporting the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery (CD) to prevent postoperative endometritis. He then asked readers if they would consider adopting such a practice. More than 250 readers weighed in through the Quick Poll at obgmanagement.com, and many readers sent in letters with follow-up questions and comments on controlling bacterial contamination, vaginal seeding, etc. Here are some of the letters, along with Dr. Barbieri’s response and the Quick Poll results.
A contradiction in definitions?
There seems to be a contradiction in definitions. The second sentence of the article defines endometritis as the presence of fever plus low abdominal tenderness. However, the studies presented state that vaginal cleansing pre-CD decreased endometritis but did not decrease postpartum fever. Is this not a discrepancy?
Nancy Kerr, MD, MPH
Albuquerque, New Mexico
A question about povidone-iodine
Have any studies been done on newborn iodine levels after vaginal cleansing with povidone-iodine prior to CD?
G. Millard Simmons Jr, MD
Hilton Head, Bluffton, South Carolina
Additional tips for controlling bacterial contamination
Dr. Barbieri’s editorial on vaginal cleansing prior to CD is eye opening. I have a few additional suggestions to control bacterial contamination.
First, I examine my patients in labor as few times as necessary, and I ask the nurses (RNs) not to place their fingers in the patient’s vagina while she is pushing. I remove the Foley catheter when I feel progress (descent of fetal head) is being achieved. In addition, physicians as well as RNs should consider changing their scrubs between deliveries, as I believe that bacterial contamination is splattered all over the place, especially into the birth canal. These methods have worked for me in my over-20 years of practice.
I also firmly remind the RN circulator to perform a generous vaginal cleanse with povidone-iodine, in addition to the usual intravenous prophylaxis, before hysterectomy.
Luis Leyva Jr, MD
Miami, Florida
Mixed feelings
My first reaction to this Editorial was: Is this a solution in search of a problem? That is to say, how much of a clinical problem is endometritis after CD? Are we really treating the proposed problem, and does treatment affect long-term outcomes?
Upon reflection, I have concluded that vaginal cleansing pre-CD does intuitively make sense. What sways me in this direction is that the practice is simple, easy, and inexpensive. Since we typically have the patient positioned for Foley catheter insertion, performing vaginal cleansing as we put in the Foley would be easy. If vaginal cleansing were to be done, I definitely would be in favor of doing such practice liberally—for all CDs to make vaginal cleansing part of the “routine.”
Keep in mind that we are still chasing a problem of little clinical significance.
The biggest accomplishment has been to get everyone to give antibiotics preoperatively rather than after cutting the umbilical cord. We knew that this was best practice as early as the late 1980s/early 1990s, and I have been fighting this battle ever since. Believe it or not, there are still a few holdouts.
George H. Davis, DO
Johnson City, Tennessee
Would vaginal cleansing benefit all women in labor?
Vaginal cleansing before CD reminds me of my residency days when all women having hysterectomies were admitted early and given povidone-iodine (Betadine) douches the evening before surgery (unless an iodine allergy was present).
While reading your Editorial, I had several thoughts and questions. 1) Since vaginal cleansing seems to benefit CD patients, might it not benefit all laboring patients? 2) Is the timing of vaginal cleansing critical? 3) Should we do vaginal cleansing on all laboring patients if timing is not critical?
I plan to bring up the topic of vaginal cleansing for CD with my colleagues at our next department meeting, since it seems like such a simple, logical, inexpensive, and beneficial thing to do.
Douglas G. Tolley, MD
Yuba City, California
An early study on using povidone-iodine gel before CD
When I was a chief resident at Kings County Hospital in 1973, we had a very high rate of post-CD endometritis. I conducted a small study on the use of povidone-iodine gel in the last month of pregnancy. Before commencing, we confirmed that the gel did not interfere with diagnosing ruptured membranes.
Obstetric service patients were randomly divided into “A” and “B” groups. The A patients were asked to use povidone-iodine gel at night for the last 2 weeks before their estimated due date. When admitted in labor, they were asked to confirm its use. When a resident diagnosed post-CD endometritis, we kept track of which group the patient was in and whether or not that patient had used povidone-iodine. Approximately 100 infected patients were evaluated from each group.
As it turned out, there were about 3 times the number of infections among the patients who did not use povidone-iodine than among those who said they used it. It did not seem to matter how many times povidone-iodine was used. The “As” who did not use povidone-iodine had results similar to the “Bs.”
It was many years ago, and the study design was crude. However, it does seem to support the suggestion for vaginal cleansing.
Steve Ross, MD
Port Jefferson, New York
Two different ideas about the vaginal biome
This Editorial is timely in that Dr. Dominguez-Bello and colleagues recently published an article in Nature Medicine titled, “Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer.”1 Dr. Dominguez-Bello is one of the founders of the idea of “vaginal seeding,” or using the natural biome of the vagina on a newborn immediately after CD by swabbing the baby with the bacteria from the vagina.
I find it interesting that there are two very different ideas about the biome at this time. Vaginal seeding is a new trend that a few patients have asked about during prenatal care. The jury is still out on seeding, but a larger study is currently underway at New York University. Of course, infection is one of the risks of seeding. I appreciate hearing both sides of the issue.
Deborah Herchelroath, DO
Harrisburg, Pennsylvania
Reference
- Dominguez-Bello MG, De Jesus-Labor KM, Shen N, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer [published online ahead of print February 1, 2016]. Nat Med. doi:10.1038/nm.4039.
Dr. Barbieri responds
I would like to thank our readers for taking the time from their busy schedules to write about their clinical experiences and current practices for reducing infectious complications following CD.
Dr. Kerr raises the important issue of the apparent contradictory finding of the beneficial impact of vaginal cleansing on endometritis without a beneficial effect on the overall rate of fever. In the trial reported by Starr,1 fever was defined as a temperature above 38˚C at any time after CD and endometritis was defined as a temperature above 38.4˚C PLUS uterine tenderness occurring more than 24 hours after CD. Given these 2 definitions one can understand the differential effect of vaginal cleansing on fever versus endometritis.
Dr. Simmons raises the intriguing question of the impact of an iodine-containing surgical preparation on newborn thyroid function. There are few studies addressing this issue. One study reports a transient increase in thyroid-stimulating hormone (TSH) levels in a small percentage of newborns whose mothers received an iodine preparation.2 Another study reports no effect of an iodine surgical preparation on newborn thyroid function indices.3
I agree with the guidance of Drs. Leyva and Davis that we can help prevent postcesarean endometritis by minimizing the number of cervical examinations, changing scrubs between deliveries, and by ensuring that an intravenous anti‑ biotic is given before skin incision.
Dr. Tolley wonders if all women should receive vaginal cleansing, regardless of delivery route. It is possible that such an approach would be effective and it deserves study. Given the lower rate of endometritis following vaginal delivery compared with CD, many more women having a vaginal delivery would need to be treated to prevent one case of endometritis. Dr. Ross mentions his experience with the benefit of outpatient vaginal cleansing in the 2 weeks prior to delivery. Many general surgeons are recommending that their patients shower with chlorhexidine the day before surgery in order to reduce the rate of postoperative infection. Short-term and long-term outpatient vaginal cleansing prior to delivery deserves additional study.
Dr. Herchelroath raises the possibility that vaginal cleansing will decrease the ability of the newborn to develop a normal microbiome because it may not be exposed to sufficient vaginal bacteria. This possibility certainly deserves additional study.
The questions and guidance of our readers were incredibly helpful and stimulating. Thank you for sharing your perspective.
References
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Nili F, Hantoushzadeh S, Alimohamadi A, et al. Iodine-containing disinfectants in preparation for cesarean section: impact on thyroid profile in cord blood. Postgrad Med J. 2015;91(1082):681–684.
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area. Thyroid. 2007;17(11):1097–1102.
“CELL-FREE DNA SCREENING FOR WOMEN AT LOW RISK FOR FETAL ANEUPLOIDY” MARY E. NORTON, MD (JANUARY 2016)
The price of cfDNA screening is dropping
I found Dr. Norton’s article on cell-free DNA (cfDNA) screening for women at low risk for fetal abnormalities to be enlightening and educational. The section addressing cost-effectiveness, however, was somewhat obsolete. The referenced study by Cuckle and colleagues,1 which estimated the cost of cfDNA per case of Down syndrome in low-risk patients at $3.6 million, was published in 2013. With 4 major companies in the market, the cost/benefit ratio has been changing rapidly. At least one company has dropped the cost of the cfDNA test nearly 80% from 2015 to 2016, making the above reference irrelevant. Recently, Ariosa dropped the price of their Harmony cfDNA test to just $119 in our area, regardless of a patient’s insurance or poverty level. This is significantly less than the cost of performing an early screen and is being welcomed by my patients even after substantial counseling on the test’s limitations in the low-risk population. Natera, another laboratory with a similar test, offers a low-cost option. However, patients must provide proof that their income is below a specified level.
Guidelines from the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) likely will have a hard time keeping up with the cost-effectiveness of noninvasive prenatal testing, as the price continues to be dynamic.
Samuel Wolf, DO
Panama City, Florida
Reference
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
“DOES THE DISCONTINUATION OF MENOPAUSAL HORMONE THERAPY AFFECT A WOMAN’S CARDIOVASCULAR RISK?”
ANDREW M. KAUNITZ, MD; JOANN E. MANSON, MD, DRPH; AND CYNTHIA A. STUENKEL, MD(EXAMINING THE EVIDENCE; DECEMBER 2015)
Disagrees with conclusion
In their expert commentary, Drs. Kaunitz, Manson, and Stuenkel state:
They support this claim by citing Heiss 2008.1 In fact, however, the Women’s Health Initiative (WHI) data show opposite to their statement: In the WHI, all-cause mortality was increased among the women who were assigned to estrogen-progestin therapy (EPT) relative to those who were assigned to placebo within the 3 years of EPT cessation (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.95–1.39). More importantly, mortality was significantly increased among women who were originally assigned to EPT relative to those who were assigned to placebo and were at least 80% adherent with intervention (HR, 1.53; 95% CI, 1.04–2.24). Thus, the statement by Drs. Kaunitz, Manson, and Stuenkel is incorrect.
In addition to the WHI studies, data are available from at least 2 other randomized controlled trials addressing the issue of HT withdrawal. In the Heart and Estrogen/progestin Replacement Study (HERS) II,2 the unblinded 2.7-year follow-up to the HERS trial, women originally assigned to EPT had a 3.3-fold higher rate of ventricular arrhythmia requiring resuscitation than women assigned to placebo (HR, 3.30; 95% CI, 1.08–10.10). During the first 6 months of posttrial follow-up of the Women’s Estrogen for Stroke Trial (WEST),3 there were 3 fatal strokes and 18 nonfatal strokes among the women originally randomized to estradiol therapy; there were 9 strokes (1 fatal and 8 nonfatal) among the women originally assigned to placebo (HR, 2.3; 95% CI, 1.1–5.0; P = .03).
In our study we detected that women who stopped HT, compared with women who continued HT, had a 2.3-fold (95% CI, 2.12–2.50) greater risk of cardiac death within the first post-HT year and a 1.3-fold (95% CI, 1.21–1.31) greater risk of cardiac death more than 1 year after stopping HT.4 In addition, women who stopped HT, compared with women who continuedHT, had a 2.5-fold (95% CI, 2.28–2.77) greater risk of dying from stroke within the first post-HT year and a 1.3-fold (95% CI, 1.19–1.31) greater risk of dying from stroke more than 1 year after stopping HT. We believe that these data substantially further our understanding of the posttrial data from WHI, as well as HERS and WEST. Thus, cumulative data support that HT withdrawal potentially has detrimental implications for women. In total, the data are highly informative when counseling women regarding use or discontinuation of HT.
Tomi Mikkola, MD
Helsinki, Finland
References
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
- Grady D, Herrington D, Bittner V, et al; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002;288(9):1064]. JAMA. 2002;288(1):49–57.
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249.
- Mikkola TS, Tuomikoski P, Lyytinen H, et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594.
Drs. Kaunitz, Manson, and Stuenkel respond
We thank Dr. Mikkola for his response to our commentary, but we do not agree with his interpretation of the WHI reports or our conclusions. As we originally stated, the WHI trial of estrogen-only therapy (ET) and EPT provides an opportunity to observe outcomes in the largest randomized controlled trial of HT in healthy postmenopausal women. Our commentary was based on the most recent, 13-year follow-up of the WHI trials,1 and we are confident in the accuracy of our presentation of the results.
As the debate apparently focuses on the safety of stopping HT, we wish to reiterate, for those who may not be familiar with the data, that, in the ET trial, all-cause mortality declined (although not significantly) after stopping ET, as summarized here:
HR (95% CI) | |
Intervention phase | 1.03 (0.88–1.21) |
Postintervention phase (after stopping study medication) | 0.96 (0.84–1.10) |
Cumulative 13 years of follow-up | 0.99 (0.90–1.10) |
Similarly, in the EPT trial, as the following findings indicate, stopping HT did not increase all-cause mortality:
HR (95% CI) | |
Intervention phase | 0.97 (0.81–1.16) |
Postintervention phase (afterstopping study medication) | 1.01 (0.91–1.11) |
Cumulative 13 years of follow-up | 0.99 (0.91–1.08) |
Again, these findings from the largest randomized trial of HT in healthy postmenopausal women are adequate for us to conclude that stopping HT does not elevate risk of mortality. Among all women participating in the WHI HT trials, HRs for coronary heart disease, pulmonary embolism, stroke, and cardiovascular disease mortality likewise were lower (better) after stopping treatment than during the intervention phase. The results for these outcomes in younger women followed similar patterns but, due to smaller numbers of events, could not be tested formally for differences in time trends.
Moreover, the data Dr. Mikkola cites from analyses conducted 3 years postcessation2 reflected a borderline increased risk of cancer mortality that emerged in the EPT trial after stopping treatment. This clearly was related to the prolonged effects of EPT on breast cancer and other cancers, given the known latency period for cancer, and was not observed in the ET trial postcessation. The risk elevation in the EPT trial became attenuated with longer follow-up and, as of 13 years, the HRs for cancer mortality were 1.07 (0.93–1.23) in the EPT trial and 0.95 (0.81–1.13) in the ET trial.
It is interesting that Dr. Mikkola now inculcates his interpretation of his findings3 with those from secondary prevention trials such as the Heart and Estrogen/progestin Replacement Study and the Women’s Estrogen for Stroke Trial, neither of which was included as corroborative evidence in the discussion section of his originally published manuscript, and neither of which is considered applicable to healthy postmenopausal women taking HT for treatment of menopausal symptoms. Based on these findings, we do not recommend that clinicians counsel women that stopping HT increases their risk of cardiovascular or overall mortality. Thank you for the opportunity to clarify the evidence and our position.
References
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Heiss G, Wallace R, Anderson GL, et al; WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045.
Keep cancer prominent in differential diagnoses of pediatric rheumatic complaints
MAUI, HAWAII – The first thing nonpediatric rheumatologists need to understand about a child who presents with rheumatic complaints is the importance of ruling out malignancy, Dr. Anne M. Stevens stressed at the 2016 Rheumatology Winter Clinical Symposium.
“This is something I think we in pediatric rheumatology worry about a lot more than adult rheumatologists: malignancy and how to distinguish it from rheumatic diseases,” said Dr. Stevens, a pediatric rheumatologist at Seattle Children’s Hospital and the University of Washington.
And with there being only about 250 pediatric rheumatologists in the entire United States, and a handful of states having none at all, it’s important that physicians in other specialties be familiar with key differences between pediatric and adult rheumatic diseases, she added.
A diverse group of malignancies in children and teens can present with swollen joints or other rheumatic features. One of the biggest red flags suggestive of an underlying malignancy is disproportionate pain, especially nonarticular bone pain or tenderness or back pain as a major presenting feature.
The source of this bone or back pain may be a reactive arthritis in response to local bony changes caused by an osteosarcoma or neuroblastoma, or malignant effusions as a result of leukemia or lymphoma, Dr. Stevens explained.
Other atypical features that get her thinking about the possibility of underlying malignancy rather than juvenile idiopathic arthritis include weight loss, night sweats, fatigue, fever, and night pain. Overall, young patients with an undetected cancer just seem sicker than those with rheumatic disease, she continued.
In a classic retrospective study of 29 children and teens who initially presented to pediatric rheumatologists at the University of British Columbia and were ultimately found to have malignancy, the most common provisional rheumatologic diagnosis was juvenile rheumatoid arthritis in 12 of the 29. Five patients were thought by referring physicians to have a connective tissue disease, and three each were believed to have discitis or spondyloarthropathy. Other provisional diagnoses included systemic lupus erythematosus in two patients; Kawasaki disease in two; and Lyme disease, mixed connective tissue disease, and dermatomyositis in one each.
The final diagnoses included leukemia in 13 patients, neuroblastoma in 6, lymphoma in 3, Ewing sarcoma in 3, and single cases of ependymoma, thalamic glioma, epithelioma, and sarcoma (J Pediatr. 1999 Jan;134[1]:53-7).
Working backwards, the investigators developed a set of clinical clues helpful in detecting malignancy. Nonarticular bone pain was a prominent presenting complaint in 20 of the 29, bone tenderness in 8, and back pain in 9.
“Bone tenderness is not seen in juvenile idiopathic arthritis at all, and children under about age 10 just don’t get low back pain. That really alerts us to malignancy concern,” Dr. Stevens said.
Night sweats were present in four patients, severe constitutional symptoms in nine.
Two patients had true juvenile idiopathic arthritis, so that finding doesn’t rule out malignancy.
Surprisingly, the CBC was normal in three-quarters of patients. Antinuclear antibody testing is not helpful, as it can be strongly positive in the setting of pediatric malignancy, but lactate dehydrogenase and uric acid tests are important in making the differential diagnosis.
If there are any surprising findings raising concerns about possible malignancy, a bone marrow biopsy is essential.
“We have a lot of fights with our hematologists when we’re trying to get a bone marrow biopsy and they say, ‘No, the CBC is normal so you don’t need a bone marrow biopsy.’ But you have to get that bone marrow biopsy. A strategy that works is for us to say, ‘Could you please include a note in the chart that it’s okay for us to give steroids because you’re sure it’s not a lymphoma?’ Then we usually get it scheduled for the next day,” Dr. Stevens said.
She reported having no relevant financial disclosures.
MAUI, HAWAII – The first thing nonpediatric rheumatologists need to understand about a child who presents with rheumatic complaints is the importance of ruling out malignancy, Dr. Anne M. Stevens stressed at the 2016 Rheumatology Winter Clinical Symposium.
“This is something I think we in pediatric rheumatology worry about a lot more than adult rheumatologists: malignancy and how to distinguish it from rheumatic diseases,” said Dr. Stevens, a pediatric rheumatologist at Seattle Children’s Hospital and the University of Washington.
And with there being only about 250 pediatric rheumatologists in the entire United States, and a handful of states having none at all, it’s important that physicians in other specialties be familiar with key differences between pediatric and adult rheumatic diseases, she added.
A diverse group of malignancies in children and teens can present with swollen joints or other rheumatic features. One of the biggest red flags suggestive of an underlying malignancy is disproportionate pain, especially nonarticular bone pain or tenderness or back pain as a major presenting feature.
The source of this bone or back pain may be a reactive arthritis in response to local bony changes caused by an osteosarcoma or neuroblastoma, or malignant effusions as a result of leukemia or lymphoma, Dr. Stevens explained.
Other atypical features that get her thinking about the possibility of underlying malignancy rather than juvenile idiopathic arthritis include weight loss, night sweats, fatigue, fever, and night pain. Overall, young patients with an undetected cancer just seem sicker than those with rheumatic disease, she continued.
In a classic retrospective study of 29 children and teens who initially presented to pediatric rheumatologists at the University of British Columbia and were ultimately found to have malignancy, the most common provisional rheumatologic diagnosis was juvenile rheumatoid arthritis in 12 of the 29. Five patients were thought by referring physicians to have a connective tissue disease, and three each were believed to have discitis or spondyloarthropathy. Other provisional diagnoses included systemic lupus erythematosus in two patients; Kawasaki disease in two; and Lyme disease, mixed connective tissue disease, and dermatomyositis in one each.
The final diagnoses included leukemia in 13 patients, neuroblastoma in 6, lymphoma in 3, Ewing sarcoma in 3, and single cases of ependymoma, thalamic glioma, epithelioma, and sarcoma (J Pediatr. 1999 Jan;134[1]:53-7).
Working backwards, the investigators developed a set of clinical clues helpful in detecting malignancy. Nonarticular bone pain was a prominent presenting complaint in 20 of the 29, bone tenderness in 8, and back pain in 9.
“Bone tenderness is not seen in juvenile idiopathic arthritis at all, and children under about age 10 just don’t get low back pain. That really alerts us to malignancy concern,” Dr. Stevens said.
Night sweats were present in four patients, severe constitutional symptoms in nine.
Two patients had true juvenile idiopathic arthritis, so that finding doesn’t rule out malignancy.
Surprisingly, the CBC was normal in three-quarters of patients. Antinuclear antibody testing is not helpful, as it can be strongly positive in the setting of pediatric malignancy, but lactate dehydrogenase and uric acid tests are important in making the differential diagnosis.
If there are any surprising findings raising concerns about possible malignancy, a bone marrow biopsy is essential.
“We have a lot of fights with our hematologists when we’re trying to get a bone marrow biopsy and they say, ‘No, the CBC is normal so you don’t need a bone marrow biopsy.’ But you have to get that bone marrow biopsy. A strategy that works is for us to say, ‘Could you please include a note in the chart that it’s okay for us to give steroids because you’re sure it’s not a lymphoma?’ Then we usually get it scheduled for the next day,” Dr. Stevens said.
She reported having no relevant financial disclosures.
MAUI, HAWAII – The first thing nonpediatric rheumatologists need to understand about a child who presents with rheumatic complaints is the importance of ruling out malignancy, Dr. Anne M. Stevens stressed at the 2016 Rheumatology Winter Clinical Symposium.
“This is something I think we in pediatric rheumatology worry about a lot more than adult rheumatologists: malignancy and how to distinguish it from rheumatic diseases,” said Dr. Stevens, a pediatric rheumatologist at Seattle Children’s Hospital and the University of Washington.
And with there being only about 250 pediatric rheumatologists in the entire United States, and a handful of states having none at all, it’s important that physicians in other specialties be familiar with key differences between pediatric and adult rheumatic diseases, she added.
A diverse group of malignancies in children and teens can present with swollen joints or other rheumatic features. One of the biggest red flags suggestive of an underlying malignancy is disproportionate pain, especially nonarticular bone pain or tenderness or back pain as a major presenting feature.
The source of this bone or back pain may be a reactive arthritis in response to local bony changes caused by an osteosarcoma or neuroblastoma, or malignant effusions as a result of leukemia or lymphoma, Dr. Stevens explained.
Other atypical features that get her thinking about the possibility of underlying malignancy rather than juvenile idiopathic arthritis include weight loss, night sweats, fatigue, fever, and night pain. Overall, young patients with an undetected cancer just seem sicker than those with rheumatic disease, she continued.
In a classic retrospective study of 29 children and teens who initially presented to pediatric rheumatologists at the University of British Columbia and were ultimately found to have malignancy, the most common provisional rheumatologic diagnosis was juvenile rheumatoid arthritis in 12 of the 29. Five patients were thought by referring physicians to have a connective tissue disease, and three each were believed to have discitis or spondyloarthropathy. Other provisional diagnoses included systemic lupus erythematosus in two patients; Kawasaki disease in two; and Lyme disease, mixed connective tissue disease, and dermatomyositis in one each.
The final diagnoses included leukemia in 13 patients, neuroblastoma in 6, lymphoma in 3, Ewing sarcoma in 3, and single cases of ependymoma, thalamic glioma, epithelioma, and sarcoma (J Pediatr. 1999 Jan;134[1]:53-7).
Working backwards, the investigators developed a set of clinical clues helpful in detecting malignancy. Nonarticular bone pain was a prominent presenting complaint in 20 of the 29, bone tenderness in 8, and back pain in 9.
“Bone tenderness is not seen in juvenile idiopathic arthritis at all, and children under about age 10 just don’t get low back pain. That really alerts us to malignancy concern,” Dr. Stevens said.
Night sweats were present in four patients, severe constitutional symptoms in nine.
Two patients had true juvenile idiopathic arthritis, so that finding doesn’t rule out malignancy.
Surprisingly, the CBC was normal in three-quarters of patients. Antinuclear antibody testing is not helpful, as it can be strongly positive in the setting of pediatric malignancy, but lactate dehydrogenase and uric acid tests are important in making the differential diagnosis.
If there are any surprising findings raising concerns about possible malignancy, a bone marrow biopsy is essential.
“We have a lot of fights with our hematologists when we’re trying to get a bone marrow biopsy and they say, ‘No, the CBC is normal so you don’t need a bone marrow biopsy.’ But you have to get that bone marrow biopsy. A strategy that works is for us to say, ‘Could you please include a note in the chart that it’s okay for us to give steroids because you’re sure it’s not a lymphoma?’ Then we usually get it scheduled for the next day,” Dr. Stevens said.
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM RWCS 2016
Romosozumab, coming ACR guidelines mark recent high points in osteoporosis
MAUI, HAWAII – The investigational bone-building agent romosozumab provided the therapeutic highlight in the field of osteoporosis during the past year, Dr. Martin J. Bergman said at the 2016 Rheumatology Winter Clinical Symposium.
Romosozumab is a monoclonal antibody directed against sclerostin, a glycoprotein that prevents mesenchymal cells from becoming osteoblasts. By inhibiting sclerostin, romosozumab promotes osteoblast production. The result is increased bone mineral density and bone formation coupled with decreased bone resorption, providing physicians with a promising new avenue for rapidly building strong bone, explained Dr. Bergman of Drexel University in Philadelphia and chief of the section of rheumatology at Taylor Hospital in Ridley Park, Pa.
Romosozumab caught his eye in a 12-month randomized trial presented last fall at the annual meeting of the American College of Rheumatology. The 430 postmenopausal participants were assigned to blinded romosozumab at 210 mg delivered by subcutaneous injection once per month, blinded placebo, or open-label teriparatide (Forteo). The primary endpoint in this secondary analysis was change in bone strength as measured using the Food and Drug Administration–approved method of finite element analysis based upon quantitative CT imaging.
Romosozumab boosted bone strength at the spine by 27.3% at 12 months, compared with a 3.9% reduction from baseline with placebo and an 18.5% increase with teriparatide. At the hip, romosozumab delivered a 3.6% increase in bone strength versus no significant change from baseline in the other two study arms. Thus, romosozumab increased bone strength both in the cortical and trabecular compartments even more than did teriparatide, the most potent drug currently available for building bone mass.
“The numbers are very impressive,” Dr. Bergman observed. “Trabecular bone, cortical bone, whole bone – across the board, we haven’t seen similar numbers before. I think this is going to be a very exciting new approach to the treatment of osteoporosis. We need to keep an eye on this.”
Romosozumab, which is being codeveloped by Amgen and UCB, is now in phase III testing.
The other big news in osteoporosis is that later this year the ACR will undertake a revision of its 2010 guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis (Arthritis Care Res [Hoboken]. 2010 Nov;62[11]:1515-26).
Among the actions that need to be taken are the incorporation of denosumab (Prolia) and ibandronate (Boniva) into the treatment recommendations, as well as clarification of the recommendation for supplemental calcium in light of recent evidence of an association between high serum calcium and increased cardiovascular risk. Most of the lifestyle modification recommendations in the current guidelines are supported by a weak level of evidence C, meaning “expert opinion,” and the hope is that the evidence has become stronger since 2010, he said.
Dr. Bergman reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – The investigational bone-building agent romosozumab provided the therapeutic highlight in the field of osteoporosis during the past year, Dr. Martin J. Bergman said at the 2016 Rheumatology Winter Clinical Symposium.
Romosozumab is a monoclonal antibody directed against sclerostin, a glycoprotein that prevents mesenchymal cells from becoming osteoblasts. By inhibiting sclerostin, romosozumab promotes osteoblast production. The result is increased bone mineral density and bone formation coupled with decreased bone resorption, providing physicians with a promising new avenue for rapidly building strong bone, explained Dr. Bergman of Drexel University in Philadelphia and chief of the section of rheumatology at Taylor Hospital in Ridley Park, Pa.
Romosozumab caught his eye in a 12-month randomized trial presented last fall at the annual meeting of the American College of Rheumatology. The 430 postmenopausal participants were assigned to blinded romosozumab at 210 mg delivered by subcutaneous injection once per month, blinded placebo, or open-label teriparatide (Forteo). The primary endpoint in this secondary analysis was change in bone strength as measured using the Food and Drug Administration–approved method of finite element analysis based upon quantitative CT imaging.
Romosozumab boosted bone strength at the spine by 27.3% at 12 months, compared with a 3.9% reduction from baseline with placebo and an 18.5% increase with teriparatide. At the hip, romosozumab delivered a 3.6% increase in bone strength versus no significant change from baseline in the other two study arms. Thus, romosozumab increased bone strength both in the cortical and trabecular compartments even more than did teriparatide, the most potent drug currently available for building bone mass.
“The numbers are very impressive,” Dr. Bergman observed. “Trabecular bone, cortical bone, whole bone – across the board, we haven’t seen similar numbers before. I think this is going to be a very exciting new approach to the treatment of osteoporosis. We need to keep an eye on this.”
Romosozumab, which is being codeveloped by Amgen and UCB, is now in phase III testing.
The other big news in osteoporosis is that later this year the ACR will undertake a revision of its 2010 guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis (Arthritis Care Res [Hoboken]. 2010 Nov;62[11]:1515-26).
Among the actions that need to be taken are the incorporation of denosumab (Prolia) and ibandronate (Boniva) into the treatment recommendations, as well as clarification of the recommendation for supplemental calcium in light of recent evidence of an association between high serum calcium and increased cardiovascular risk. Most of the lifestyle modification recommendations in the current guidelines are supported by a weak level of evidence C, meaning “expert opinion,” and the hope is that the evidence has become stronger since 2010, he said.
Dr. Bergman reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – The investigational bone-building agent romosozumab provided the therapeutic highlight in the field of osteoporosis during the past year, Dr. Martin J. Bergman said at the 2016 Rheumatology Winter Clinical Symposium.
Romosozumab is a monoclonal antibody directed against sclerostin, a glycoprotein that prevents mesenchymal cells from becoming osteoblasts. By inhibiting sclerostin, romosozumab promotes osteoblast production. The result is increased bone mineral density and bone formation coupled with decreased bone resorption, providing physicians with a promising new avenue for rapidly building strong bone, explained Dr. Bergman of Drexel University in Philadelphia and chief of the section of rheumatology at Taylor Hospital in Ridley Park, Pa.
Romosozumab caught his eye in a 12-month randomized trial presented last fall at the annual meeting of the American College of Rheumatology. The 430 postmenopausal participants were assigned to blinded romosozumab at 210 mg delivered by subcutaneous injection once per month, blinded placebo, or open-label teriparatide (Forteo). The primary endpoint in this secondary analysis was change in bone strength as measured using the Food and Drug Administration–approved method of finite element analysis based upon quantitative CT imaging.
Romosozumab boosted bone strength at the spine by 27.3% at 12 months, compared with a 3.9% reduction from baseline with placebo and an 18.5% increase with teriparatide. At the hip, romosozumab delivered a 3.6% increase in bone strength versus no significant change from baseline in the other two study arms. Thus, romosozumab increased bone strength both in the cortical and trabecular compartments even more than did teriparatide, the most potent drug currently available for building bone mass.
“The numbers are very impressive,” Dr. Bergman observed. “Trabecular bone, cortical bone, whole bone – across the board, we haven’t seen similar numbers before. I think this is going to be a very exciting new approach to the treatment of osteoporosis. We need to keep an eye on this.”
Romosozumab, which is being codeveloped by Amgen and UCB, is now in phase III testing.
The other big news in osteoporosis is that later this year the ACR will undertake a revision of its 2010 guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis (Arthritis Care Res [Hoboken]. 2010 Nov;62[11]:1515-26).
Among the actions that need to be taken are the incorporation of denosumab (Prolia) and ibandronate (Boniva) into the treatment recommendations, as well as clarification of the recommendation for supplemental calcium in light of recent evidence of an association between high serum calcium and increased cardiovascular risk. Most of the lifestyle modification recommendations in the current guidelines are supported by a weak level of evidence C, meaning “expert opinion,” and the hope is that the evidence has become stronger since 2010, he said.
Dr. Bergman reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM RWCS 2016
Resilience
It has been clear for a long time that a child who grows up in an environment dominated by adversity is more likely to enter adulthood scarred psychologically, and as a result is less likely to succeed. This well-described association has in the last few years become a hot button topic. A 2012 American Academy of Pediatrics policy statement alerted pediatricians to their potential role in identifying and managing what is now referred to as “toxic stress” (“Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health”).
Although a childhood in which challenges outnumber advantages is often followed by an adult life characterized by failure and dysfunction, there are a few individuals who not only survive a disadvantaged childhood unscathed but somehow manage to thrive in its wake. For example, Joe Rantz, the central figure in Daniel James Brown’s nonfiction best seller “The Boys in the Boat” (New York: Viking Press, 2013) was abandoned several times by his family but emerged to power the University of Washington crew team to victory in the 1936 Olympics. Intrigued by these outliers, a developmental psychologist and clinician from the University of Minnesota named Norman Garmezy began looking for features that may have allowed these exceptional people to succeed and even excel despite incredibly difficult circumstances (“How People Learn to Become Resilient,” Maria Konnikova, The New Yorker, Feb. 11, 2016). His search for the characteristics that might have protected these individuals as children from the acute and chronic environmental threats of their disadvantaged childhoods has spawned a breed of developmental psychologists who devote their research to a quality now referred to as “resilience.”
In 1989, Emmy E. Werner, Ph.D., published a study of 698 children on the island of Kauai in Hawaii and identified several elements that might predict resilience (“Children of the Garden Island,” Sci Am. 1989;260[4]:106-11). Not surprisingly, one factor was the good luck of having formed a strong bond with a supportive person such as a caregiver or mentor. However, Dr. Werner also discovered that resilient individuals possessed a set of psychological characteristics that included a positive social orientation prompting them to “meet the world on their own terms.” They were likely to be autonomous and independent and had the attitude that “they, and not their circumstances, affected their achievements.”
These findings lead to the obvious question of whether those attributes that can protect against adversity can be taught. George Bonanno, a clinical psychologist at Columbia University’s Teachers College, found that an individual’s perception of the situation is the key element in resilience. In the New Yorker article on resilience, he was quoted in an interview as saying, “Events are not traumatic until we experience them as traumatic.” In his studies he has found that individuals can be taught how to reframe an event in positive terms that was initially perceived as negative. Unfortunately, the reverse can occur, and as Dr. Bonanno also said in the interview, “We can create or exaggerate stressors very easily in our own minds.” Every event is potentially traumatic if we perceive it that way.
Could it be that in some situations our behavior as adults, parents, and professionals creates an environment that transforms an event into one that is more easily perceived by a child as traumatizing? While it is important to be on the lookout for children who have been emotionally traumatized by an unfortunate event such as a school shooting, we must be careful to keep our responses measured and positive. Children should be reminded that it is they who control their own behavior and achievements, not the circumstances in which they find themselves.
Parents should be reminded that hovering and overinvolvement in their children’s lives is preventing the development of independence and a sense of autonomy, two important characteristics of resilience. The trend in education that emphasizes group solutions may be helping some children learn to cooperate with others and function as a team. But, we must also remember to offer each individual child abundant opportunities to learn so that he or she can also rely on himself or herself to solve problems.
Few of us will ever have the capacity for resiliency demonstrated by Louis Zamperini in the nonfiction best seller Unbroken, but we can and should be doing a better job helping children learn that even in the most adverse conditions, they have some control – if not over the circumstance, then at least over their perception of it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
It has been clear for a long time that a child who grows up in an environment dominated by adversity is more likely to enter adulthood scarred psychologically, and as a result is less likely to succeed. This well-described association has in the last few years become a hot button topic. A 2012 American Academy of Pediatrics policy statement alerted pediatricians to their potential role in identifying and managing what is now referred to as “toxic stress” (“Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health”).
Although a childhood in which challenges outnumber advantages is often followed by an adult life characterized by failure and dysfunction, there are a few individuals who not only survive a disadvantaged childhood unscathed but somehow manage to thrive in its wake. For example, Joe Rantz, the central figure in Daniel James Brown’s nonfiction best seller “The Boys in the Boat” (New York: Viking Press, 2013) was abandoned several times by his family but emerged to power the University of Washington crew team to victory in the 1936 Olympics. Intrigued by these outliers, a developmental psychologist and clinician from the University of Minnesota named Norman Garmezy began looking for features that may have allowed these exceptional people to succeed and even excel despite incredibly difficult circumstances (“How People Learn to Become Resilient,” Maria Konnikova, The New Yorker, Feb. 11, 2016). His search for the characteristics that might have protected these individuals as children from the acute and chronic environmental threats of their disadvantaged childhoods has spawned a breed of developmental psychologists who devote their research to a quality now referred to as “resilience.”
In 1989, Emmy E. Werner, Ph.D., published a study of 698 children on the island of Kauai in Hawaii and identified several elements that might predict resilience (“Children of the Garden Island,” Sci Am. 1989;260[4]:106-11). Not surprisingly, one factor was the good luck of having formed a strong bond with a supportive person such as a caregiver or mentor. However, Dr. Werner also discovered that resilient individuals possessed a set of psychological characteristics that included a positive social orientation prompting them to “meet the world on their own terms.” They were likely to be autonomous and independent and had the attitude that “they, and not their circumstances, affected their achievements.”
These findings lead to the obvious question of whether those attributes that can protect against adversity can be taught. George Bonanno, a clinical psychologist at Columbia University’s Teachers College, found that an individual’s perception of the situation is the key element in resilience. In the New Yorker article on resilience, he was quoted in an interview as saying, “Events are not traumatic until we experience them as traumatic.” In his studies he has found that individuals can be taught how to reframe an event in positive terms that was initially perceived as negative. Unfortunately, the reverse can occur, and as Dr. Bonanno also said in the interview, “We can create or exaggerate stressors very easily in our own minds.” Every event is potentially traumatic if we perceive it that way.
Could it be that in some situations our behavior as adults, parents, and professionals creates an environment that transforms an event into one that is more easily perceived by a child as traumatizing? While it is important to be on the lookout for children who have been emotionally traumatized by an unfortunate event such as a school shooting, we must be careful to keep our responses measured and positive. Children should be reminded that it is they who control their own behavior and achievements, not the circumstances in which they find themselves.
Parents should be reminded that hovering and overinvolvement in their children’s lives is preventing the development of independence and a sense of autonomy, two important characteristics of resilience. The trend in education that emphasizes group solutions may be helping some children learn to cooperate with others and function as a team. But, we must also remember to offer each individual child abundant opportunities to learn so that he or she can also rely on himself or herself to solve problems.
Few of us will ever have the capacity for resiliency demonstrated by Louis Zamperini in the nonfiction best seller Unbroken, but we can and should be doing a better job helping children learn that even in the most adverse conditions, they have some control – if not over the circumstance, then at least over their perception of it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
It has been clear for a long time that a child who grows up in an environment dominated by adversity is more likely to enter adulthood scarred psychologically, and as a result is less likely to succeed. This well-described association has in the last few years become a hot button topic. A 2012 American Academy of Pediatrics policy statement alerted pediatricians to their potential role in identifying and managing what is now referred to as “toxic stress” (“Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health”).
Although a childhood in which challenges outnumber advantages is often followed by an adult life characterized by failure and dysfunction, there are a few individuals who not only survive a disadvantaged childhood unscathed but somehow manage to thrive in its wake. For example, Joe Rantz, the central figure in Daniel James Brown’s nonfiction best seller “The Boys in the Boat” (New York: Viking Press, 2013) was abandoned several times by his family but emerged to power the University of Washington crew team to victory in the 1936 Olympics. Intrigued by these outliers, a developmental psychologist and clinician from the University of Minnesota named Norman Garmezy began looking for features that may have allowed these exceptional people to succeed and even excel despite incredibly difficult circumstances (“How People Learn to Become Resilient,” Maria Konnikova, The New Yorker, Feb. 11, 2016). His search for the characteristics that might have protected these individuals as children from the acute and chronic environmental threats of their disadvantaged childhoods has spawned a breed of developmental psychologists who devote their research to a quality now referred to as “resilience.”
In 1989, Emmy E. Werner, Ph.D., published a study of 698 children on the island of Kauai in Hawaii and identified several elements that might predict resilience (“Children of the Garden Island,” Sci Am. 1989;260[4]:106-11). Not surprisingly, one factor was the good luck of having formed a strong bond with a supportive person such as a caregiver or mentor. However, Dr. Werner also discovered that resilient individuals possessed a set of psychological characteristics that included a positive social orientation prompting them to “meet the world on their own terms.” They were likely to be autonomous and independent and had the attitude that “they, and not their circumstances, affected their achievements.”
These findings lead to the obvious question of whether those attributes that can protect against adversity can be taught. George Bonanno, a clinical psychologist at Columbia University’s Teachers College, found that an individual’s perception of the situation is the key element in resilience. In the New Yorker article on resilience, he was quoted in an interview as saying, “Events are not traumatic until we experience them as traumatic.” In his studies he has found that individuals can be taught how to reframe an event in positive terms that was initially perceived as negative. Unfortunately, the reverse can occur, and as Dr. Bonanno also said in the interview, “We can create or exaggerate stressors very easily in our own minds.” Every event is potentially traumatic if we perceive it that way.
Could it be that in some situations our behavior as adults, parents, and professionals creates an environment that transforms an event into one that is more easily perceived by a child as traumatizing? While it is important to be on the lookout for children who have been emotionally traumatized by an unfortunate event such as a school shooting, we must be careful to keep our responses measured and positive. Children should be reminded that it is they who control their own behavior and achievements, not the circumstances in which they find themselves.
Parents should be reminded that hovering and overinvolvement in their children’s lives is preventing the development of independence and a sense of autonomy, two important characteristics of resilience. The trend in education that emphasizes group solutions may be helping some children learn to cooperate with others and function as a team. But, we must also remember to offer each individual child abundant opportunities to learn so that he or she can also rely on himself or herself to solve problems.
Few of us will ever have the capacity for resiliency demonstrated by Louis Zamperini in the nonfiction best seller Unbroken, but we can and should be doing a better job helping children learn that even in the most adverse conditions, they have some control – if not over the circumstance, then at least over their perception of it.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
Combining inhibitors to treat AML

Preclinical research has revealed a treatment approach that could prove effective against acute myeloid leukemia (AML).
Researchers tested the IAP inhibitor birinapant in combination with p38 inhibitors and observed antileukemic activity in mouse models of AML and samples from patients with the disease.
Combination treatment proved more effective than either agent alone, and the combination was less toxic than single-agent chemotherapy.
Najoua Lalaoui, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and her colleagues conducted this research and relayed the results in an article published in Cancer Cell.
The researchers generated several mouse models of AML—MLL-ENL ± NRasG12D, MLL-AF9 ± NrasG12D, AML1-ETO9a + NrasG12D, CBFβ-MYH11 + NrasG12D, NUP98-HoxA9, and HoxA9/Meis1.
In these models, the team tested birinapant with 1 of 2 p38 inhibitors—LY2228820 or SCIO-469—or with the MK2 inhibitor PF-3644022. They said each combination “dramatically” increased cell death, when compared to birinapant alone, in most models. The exceptions were AML1-ETO9a + NrasG12D and CBFβ-MYH11 + NrasG12D.
Next, the researchers tested LY2228820 plus birinapant in samples from 8 AML patients. The samples had FLT3-ITD mutations (patients 1, 2, 4, 6, and 7), a FLT3 D835 missense mutation (patient 4), nucleophosmin exon-12 mutations (patients 2 and 4), an MLL translocation (patient 3), inv(3) (patient 1), and inv(16) (patient 8).
All 8 samples were sensitive to birinapant alone. And although LY2228820 alone did not induce cell death in any of the samples, the drug had a synergistic effect with birinapant in 4 of the samples (patients 2, 3, 4, and 7).
The researchers also found that peripheral blood mononuclear cells from healthy donors proved more resistant to combination LY2228820 (at 10 µM) and birinapant (at 500 nM) than to cytarabine (10 µM), daunorubicin (at 0.4 µM), or idarubicin (at 0.4 µM).
In addition, 4 weeks of treatment with birinapant and LY2228820 was well-tolerated in mice without tumors.
Finally, the researchers tested birinapant and LY2228820, either alone or in combination, in mouse models of MLL-ENL, MLL-AF9, and NRasG12D mutant/MLL-AF9/Luc AML.
Combination treatment prolonged survival in all 3 models, when compared with mice that received single agents or no treatment. However, unlike in the MLL-ENL and MLL-AF9 models, the combination was unable to cure NRasG12D mutant/MLL-AF9/Luc mice of their leukemia.
“Our findings have made us hopeful that a combination of birinapant and a p38 inhibitor may be more effective in treating AML than current therapies and also have less toxicity for patients,” Dr Lalaoui said.
“We tested forms of AML that are highly resistant to chemotherapy and found that birinapant and p38 inhibitors could even kill these cancer cells, which is great news.”
Birinapant is being developed by TetraLogic Pharmaceuticals Corporation, and some of the researchers involved in this work reported relationships with the company. ![]()

Preclinical research has revealed a treatment approach that could prove effective against acute myeloid leukemia (AML).
Researchers tested the IAP inhibitor birinapant in combination with p38 inhibitors and observed antileukemic activity in mouse models of AML and samples from patients with the disease.
Combination treatment proved more effective than either agent alone, and the combination was less toxic than single-agent chemotherapy.
Najoua Lalaoui, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and her colleagues conducted this research and relayed the results in an article published in Cancer Cell.
The researchers generated several mouse models of AML—MLL-ENL ± NRasG12D, MLL-AF9 ± NrasG12D, AML1-ETO9a + NrasG12D, CBFβ-MYH11 + NrasG12D, NUP98-HoxA9, and HoxA9/Meis1.
In these models, the team tested birinapant with 1 of 2 p38 inhibitors—LY2228820 or SCIO-469—or with the MK2 inhibitor PF-3644022. They said each combination “dramatically” increased cell death, when compared to birinapant alone, in most models. The exceptions were AML1-ETO9a + NrasG12D and CBFβ-MYH11 + NrasG12D.
Next, the researchers tested LY2228820 plus birinapant in samples from 8 AML patients. The samples had FLT3-ITD mutations (patients 1, 2, 4, 6, and 7), a FLT3 D835 missense mutation (patient 4), nucleophosmin exon-12 mutations (patients 2 and 4), an MLL translocation (patient 3), inv(3) (patient 1), and inv(16) (patient 8).
All 8 samples were sensitive to birinapant alone. And although LY2228820 alone did not induce cell death in any of the samples, the drug had a synergistic effect with birinapant in 4 of the samples (patients 2, 3, 4, and 7).
The researchers also found that peripheral blood mononuclear cells from healthy donors proved more resistant to combination LY2228820 (at 10 µM) and birinapant (at 500 nM) than to cytarabine (10 µM), daunorubicin (at 0.4 µM), or idarubicin (at 0.4 µM).
In addition, 4 weeks of treatment with birinapant and LY2228820 was well-tolerated in mice without tumors.
Finally, the researchers tested birinapant and LY2228820, either alone or in combination, in mouse models of MLL-ENL, MLL-AF9, and NRasG12D mutant/MLL-AF9/Luc AML.
Combination treatment prolonged survival in all 3 models, when compared with mice that received single agents or no treatment. However, unlike in the MLL-ENL and MLL-AF9 models, the combination was unable to cure NRasG12D mutant/MLL-AF9/Luc mice of their leukemia.
“Our findings have made us hopeful that a combination of birinapant and a p38 inhibitor may be more effective in treating AML than current therapies and also have less toxicity for patients,” Dr Lalaoui said.
“We tested forms of AML that are highly resistant to chemotherapy and found that birinapant and p38 inhibitors could even kill these cancer cells, which is great news.”
Birinapant is being developed by TetraLogic Pharmaceuticals Corporation, and some of the researchers involved in this work reported relationships with the company. ![]()

Preclinical research has revealed a treatment approach that could prove effective against acute myeloid leukemia (AML).
Researchers tested the IAP inhibitor birinapant in combination with p38 inhibitors and observed antileukemic activity in mouse models of AML and samples from patients with the disease.
Combination treatment proved more effective than either agent alone, and the combination was less toxic than single-agent chemotherapy.
Najoua Lalaoui, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and her colleagues conducted this research and relayed the results in an article published in Cancer Cell.
The researchers generated several mouse models of AML—MLL-ENL ± NRasG12D, MLL-AF9 ± NrasG12D, AML1-ETO9a + NrasG12D, CBFβ-MYH11 + NrasG12D, NUP98-HoxA9, and HoxA9/Meis1.
In these models, the team tested birinapant with 1 of 2 p38 inhibitors—LY2228820 or SCIO-469—or with the MK2 inhibitor PF-3644022. They said each combination “dramatically” increased cell death, when compared to birinapant alone, in most models. The exceptions were AML1-ETO9a + NrasG12D and CBFβ-MYH11 + NrasG12D.
Next, the researchers tested LY2228820 plus birinapant in samples from 8 AML patients. The samples had FLT3-ITD mutations (patients 1, 2, 4, 6, and 7), a FLT3 D835 missense mutation (patient 4), nucleophosmin exon-12 mutations (patients 2 and 4), an MLL translocation (patient 3), inv(3) (patient 1), and inv(16) (patient 8).
All 8 samples were sensitive to birinapant alone. And although LY2228820 alone did not induce cell death in any of the samples, the drug had a synergistic effect with birinapant in 4 of the samples (patients 2, 3, 4, and 7).
The researchers also found that peripheral blood mononuclear cells from healthy donors proved more resistant to combination LY2228820 (at 10 µM) and birinapant (at 500 nM) than to cytarabine (10 µM), daunorubicin (at 0.4 µM), or idarubicin (at 0.4 µM).
In addition, 4 weeks of treatment with birinapant and LY2228820 was well-tolerated in mice without tumors.
Finally, the researchers tested birinapant and LY2228820, either alone or in combination, in mouse models of MLL-ENL, MLL-AF9, and NRasG12D mutant/MLL-AF9/Luc AML.
Combination treatment prolonged survival in all 3 models, when compared with mice that received single agents or no treatment. However, unlike in the MLL-ENL and MLL-AF9 models, the combination was unable to cure NRasG12D mutant/MLL-AF9/Luc mice of their leukemia.
“Our findings have made us hopeful that a combination of birinapant and a p38 inhibitor may be more effective in treating AML than current therapies and also have less toxicity for patients,” Dr Lalaoui said.
“We tested forms of AML that are highly resistant to chemotherapy and found that birinapant and p38 inhibitors could even kill these cancer cells, which is great news.”
Birinapant is being developed by TetraLogic Pharmaceuticals Corporation, and some of the researchers involved in this work reported relationships with the company. ![]()
Drug granted orphan designation for hemolytic anemia

The European Commission (EC) has granted orphan drug designation for TNT009 to treat autoimmune hemolytic anemia, including cold agglutinin disease.
TNT009 is a monoclonal antibody that selectively inhibits the classical complement pathway by targeting C1s, a serine protease within the C1-complex in the complement pathway.
The drug thereby prevents downstream disease processes involving phagocytosis, inflammation, and cell lysis.
TNT009 is being developed by True North Therapeutics.
The drug is currently in development for the treatment of autoimmune hemolytic anemia, which is characterized by the premature destruction of healthy red blood cells by autoantibodies.
In cold agglutinin disease, this destruction of red blood cells results in anemia, fatigue, and potentially fatal thrombosis.
TNT009 is also being evaluated in patients with bullous pemphigoid and end-stage renal disease.
Top-line results from a phase 1b trial of TNT009 are expected in mid-2016.
About orphan designation
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market. ![]()

The European Commission (EC) has granted orphan drug designation for TNT009 to treat autoimmune hemolytic anemia, including cold agglutinin disease.
TNT009 is a monoclonal antibody that selectively inhibits the classical complement pathway by targeting C1s, a serine protease within the C1-complex in the complement pathway.
The drug thereby prevents downstream disease processes involving phagocytosis, inflammation, and cell lysis.
TNT009 is being developed by True North Therapeutics.
The drug is currently in development for the treatment of autoimmune hemolytic anemia, which is characterized by the premature destruction of healthy red blood cells by autoantibodies.
In cold agglutinin disease, this destruction of red blood cells results in anemia, fatigue, and potentially fatal thrombosis.
TNT009 is also being evaluated in patients with bullous pemphigoid and end-stage renal disease.
Top-line results from a phase 1b trial of TNT009 are expected in mid-2016.
About orphan designation
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market. ![]()

The European Commission (EC) has granted orphan drug designation for TNT009 to treat autoimmune hemolytic anemia, including cold agglutinin disease.
TNT009 is a monoclonal antibody that selectively inhibits the classical complement pathway by targeting C1s, a serine protease within the C1-complex in the complement pathway.
The drug thereby prevents downstream disease processes involving phagocytosis, inflammation, and cell lysis.
TNT009 is being developed by True North Therapeutics.
The drug is currently in development for the treatment of autoimmune hemolytic anemia, which is characterized by the premature destruction of healthy red blood cells by autoantibodies.
In cold agglutinin disease, this destruction of red blood cells results in anemia, fatigue, and potentially fatal thrombosis.
TNT009 is also being evaluated in patients with bullous pemphigoid and end-stage renal disease.
Top-line results from a phase 1b trial of TNT009 are expected in mid-2016.
About orphan designation
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market. ![]()
SHM Offering Webinars on Reducing Readmissions, Optimizing Glycemic Control
This April, the Society of Hospital Medicine (SHM) will offer two free live webinars on how two of its signature mentored implementation programs are changing the way hospitals manage two key issues: readmissions and glycemic control.
Project BOOST is an evidence-based approach to reduce preventable admissions, decrease average length of stay, and improve patient satisfaction. It includes one year of individualized mentoring from a physician leader with expertise in clinical quality, on-site mentoring and training from leaders in the field, access to an online tool kit with clinical resources, and more. Find out how to get involved with Project BOOST and take the first steps toward reducing readmissions with our complimentary webinar in April.
Learn more at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation Program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide. Added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls and live webinars, and access to an online web-based glycemic control collaborative to share best practices.
Join more than 100 hospitals working with SHM to improve glycemic control at an upcoming free live webinar. More information is available at www.hospitalmedicine.org/gc.
A comprehensive suite of mentored implementation programs offered through SHM’s Center for Hospital Innovation and Improvement is designed to provide institutions with coaching by national physician experts to map current processes, identify root causes of deficiencies, and tailor interventions to the unique needs of the institution for sustainable results.
For more information, visit www.hospitalmedicine.org and click on Quality & Innovation.
This April, the Society of Hospital Medicine (SHM) will offer two free live webinars on how two of its signature mentored implementation programs are changing the way hospitals manage two key issues: readmissions and glycemic control.
Project BOOST is an evidence-based approach to reduce preventable admissions, decrease average length of stay, and improve patient satisfaction. It includes one year of individualized mentoring from a physician leader with expertise in clinical quality, on-site mentoring and training from leaders in the field, access to an online tool kit with clinical resources, and more. Find out how to get involved with Project BOOST and take the first steps toward reducing readmissions with our complimentary webinar in April.
Learn more at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation Program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide. Added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls and live webinars, and access to an online web-based glycemic control collaborative to share best practices.
Join more than 100 hospitals working with SHM to improve glycemic control at an upcoming free live webinar. More information is available at www.hospitalmedicine.org/gc.
A comprehensive suite of mentored implementation programs offered through SHM’s Center for Hospital Innovation and Improvement is designed to provide institutions with coaching by national physician experts to map current processes, identify root causes of deficiencies, and tailor interventions to the unique needs of the institution for sustainable results.
For more information, visit www.hospitalmedicine.org and click on Quality & Innovation.
This April, the Society of Hospital Medicine (SHM) will offer two free live webinars on how two of its signature mentored implementation programs are changing the way hospitals manage two key issues: readmissions and glycemic control.
Project BOOST is an evidence-based approach to reduce preventable admissions, decrease average length of stay, and improve patient satisfaction. It includes one year of individualized mentoring from a physician leader with expertise in clinical quality, on-site mentoring and training from leaders in the field, access to an online tool kit with clinical resources, and more. Find out how to get involved with Project BOOST and take the first steps toward reducing readmissions with our complimentary webinar in April.
Learn more at www.hospitalmedicine.org/BOOST.
Another signature program, SHM’s Glycemic Control Mentored Implementation Program, has supported the development and implementation of glycemic control in more than 100 hospitals nationwide. Added benefits include data collection and analysis tools, monthly coaching calls with mentors, SHM-facilitated calls and live webinars, and access to an online web-based glycemic control collaborative to share best practices.
Join more than 100 hospitals working with SHM to improve glycemic control at an upcoming free live webinar. More information is available at www.hospitalmedicine.org/gc.
A comprehensive suite of mentored implementation programs offered through SHM’s Center for Hospital Innovation and Improvement is designed to provide institutions with coaching by national physician experts to map current processes, identify root causes of deficiencies, and tailor interventions to the unique needs of the institution for sustainable results.
For more information, visit www.hospitalmedicine.org and click on Quality & Innovation.
Study Shows Non-diabetics can Benefit from Taking the Diabetes Drug Pioglitaztione
NEW YORK (Reuters Health) - The diabetes drug pioglitazone, given to non-diabetics with a recent history of stroke or transient ischemic attack (TIA), prevented subsequent strokes and reduced their odds of developing type 2 diabetes, a long-term multicenter study has concluded.
But the drug also increased the risk of fracture, weight gain, and edema.
After nearly five years of follow-up, the rate of stroke or heart attack was 11.8% with placebo and 9.0% with the drug (p=0.007). The target dose was 45 mg daily.
"That 25% relative reduction is a huge effect for a stroke trial," coauthor Dr. Wayne Clark, director of the Oregon Stroke Center at Oregon Health and Science University, told Reuters Health by phone. "That's on the same realm as aspirin and a big effect for stroke.
"We're always expecting negative results these days," because so many stroke drugs have failed in previous tests, he said. "This was a positive surprise."
Dr. Clark said he was particularly taken aback by the rate that diabetes developed in pioglitazone recipients. It manifested in 3.8% of drug recipients versus 7.7% of placebo
recipients (p<0.001).
"I didn't expect that at all," he said. "That has much wider implications and might take confirmatory studies."
The 3,876 volunteers studied at 179 sites worldwide were not diabetic but they had developed insulin resistance at the time of enrollment.
Drug therapy did not reduce mortality.The results of the study, known as IRIS, were presented February 17 at the American Heart Association and the American
Stroke Association's International Stroke Conference in Los Angeles, and online in the New England Journal of Medicine.
"The findings suggest that the administration of pioglitazone in 100 patients similar to those in our trial for about five years could prevent three patients from having a
stroke or myocardial infarction," the researchers wrote in the Journal. "However, during the same period, the treatment would be expected to result in bone fractures requiring surgery or hospitalization in two patients.
"It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy," they concluded.
Serious fractures occurred in 5.1% of drug recipients versus 3.2% among placebo patients (p=0.003). A weight gain of more than 4.5 kg was seen in 52.2% of pioglitazone recipientscompared with 33.7% for placebo, and rates of edema were 35.6% with the drug versus 24.9% with placebo (both p<0.001).
The drug has been plagued by suspicions that it might increase the risk of heart failure and bladder cancer. In this study, 74 pioglitazone recipients developed heart failure versus 71 in the placebo group (p=0.80). A dozen drug recipients were diagnosed with bladder cancer compared with eight cases in the placebo group (p=0.37).
Dr. Clark said, "All of the stuff we're doing for risk-factor reduction -- blood pressure reduction, stop smoking and giving aspirin -- they're all on the same level of relative improvement, and all of those are widely used. Aspirin has a list of side effects that will fill up three pages."
At the start of the study, all of the volunteers were insulin resistant, at least 40 years old, and had experienced an ischemic stroke or TIA in the previous six months. Diabetics were excluded as were patients with heart failure, active liver disease, and an increased risk of bladder cancer.
By the end of the study, 60% of the pioglitazone patients were still taking their medicine compared with 67% of placebo recipients. The most common reason for discontinuing was edema or weight gain.
The National Institute of Neurological Disorders and Stroke funded this study. Eleven coauthors reported disclosures.
NEW YORK (Reuters Health) - The diabetes drug pioglitazone, given to non-diabetics with a recent history of stroke or transient ischemic attack (TIA), prevented subsequent strokes and reduced their odds of developing type 2 diabetes, a long-term multicenter study has concluded.
But the drug also increased the risk of fracture, weight gain, and edema.
After nearly five years of follow-up, the rate of stroke or heart attack was 11.8% with placebo and 9.0% with the drug (p=0.007). The target dose was 45 mg daily.
"That 25% relative reduction is a huge effect for a stroke trial," coauthor Dr. Wayne Clark, director of the Oregon Stroke Center at Oregon Health and Science University, told Reuters Health by phone. "That's on the same realm as aspirin and a big effect for stroke.
"We're always expecting negative results these days," because so many stroke drugs have failed in previous tests, he said. "This was a positive surprise."
Dr. Clark said he was particularly taken aback by the rate that diabetes developed in pioglitazone recipients. It manifested in 3.8% of drug recipients versus 7.7% of placebo
recipients (p<0.001).
"I didn't expect that at all," he said. "That has much wider implications and might take confirmatory studies."
The 3,876 volunteers studied at 179 sites worldwide were not diabetic but they had developed insulin resistance at the time of enrollment.
Drug therapy did not reduce mortality.The results of the study, known as IRIS, were presented February 17 at the American Heart Association and the American
Stroke Association's International Stroke Conference in Los Angeles, and online in the New England Journal of Medicine.
"The findings suggest that the administration of pioglitazone in 100 patients similar to those in our trial for about five years could prevent three patients from having a
stroke or myocardial infarction," the researchers wrote in the Journal. "However, during the same period, the treatment would be expected to result in bone fractures requiring surgery or hospitalization in two patients.
"It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy," they concluded.
Serious fractures occurred in 5.1% of drug recipients versus 3.2% among placebo patients (p=0.003). A weight gain of more than 4.5 kg was seen in 52.2% of pioglitazone recipientscompared with 33.7% for placebo, and rates of edema were 35.6% with the drug versus 24.9% with placebo (both p<0.001).
The drug has been plagued by suspicions that it might increase the risk of heart failure and bladder cancer. In this study, 74 pioglitazone recipients developed heart failure versus 71 in the placebo group (p=0.80). A dozen drug recipients were diagnosed with bladder cancer compared with eight cases in the placebo group (p=0.37).
Dr. Clark said, "All of the stuff we're doing for risk-factor reduction -- blood pressure reduction, stop smoking and giving aspirin -- they're all on the same level of relative improvement, and all of those are widely used. Aspirin has a list of side effects that will fill up three pages."
At the start of the study, all of the volunteers were insulin resistant, at least 40 years old, and had experienced an ischemic stroke or TIA in the previous six months. Diabetics were excluded as were patients with heart failure, active liver disease, and an increased risk of bladder cancer.
By the end of the study, 60% of the pioglitazone patients were still taking their medicine compared with 67% of placebo recipients. The most common reason for discontinuing was edema or weight gain.
The National Institute of Neurological Disorders and Stroke funded this study. Eleven coauthors reported disclosures.
NEW YORK (Reuters Health) - The diabetes drug pioglitazone, given to non-diabetics with a recent history of stroke or transient ischemic attack (TIA), prevented subsequent strokes and reduced their odds of developing type 2 diabetes, a long-term multicenter study has concluded.
But the drug also increased the risk of fracture, weight gain, and edema.
After nearly five years of follow-up, the rate of stroke or heart attack was 11.8% with placebo and 9.0% with the drug (p=0.007). The target dose was 45 mg daily.
"That 25% relative reduction is a huge effect for a stroke trial," coauthor Dr. Wayne Clark, director of the Oregon Stroke Center at Oregon Health and Science University, told Reuters Health by phone. "That's on the same realm as aspirin and a big effect for stroke.
"We're always expecting negative results these days," because so many stroke drugs have failed in previous tests, he said. "This was a positive surprise."
Dr. Clark said he was particularly taken aback by the rate that diabetes developed in pioglitazone recipients. It manifested in 3.8% of drug recipients versus 7.7% of placebo
recipients (p<0.001).
"I didn't expect that at all," he said. "That has much wider implications and might take confirmatory studies."
The 3,876 volunteers studied at 179 sites worldwide were not diabetic but they had developed insulin resistance at the time of enrollment.
Drug therapy did not reduce mortality.The results of the study, known as IRIS, were presented February 17 at the American Heart Association and the American
Stroke Association's International Stroke Conference in Los Angeles, and online in the New England Journal of Medicine.
"The findings suggest that the administration of pioglitazone in 100 patients similar to those in our trial for about five years could prevent three patients from having a
stroke or myocardial infarction," the researchers wrote in the Journal. "However, during the same period, the treatment would be expected to result in bone fractures requiring surgery or hospitalization in two patients.
"It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy," they concluded.
Serious fractures occurred in 5.1% of drug recipients versus 3.2% among placebo patients (p=0.003). A weight gain of more than 4.5 kg was seen in 52.2% of pioglitazone recipientscompared with 33.7% for placebo, and rates of edema were 35.6% with the drug versus 24.9% with placebo (both p<0.001).
The drug has been plagued by suspicions that it might increase the risk of heart failure and bladder cancer. In this study, 74 pioglitazone recipients developed heart failure versus 71 in the placebo group (p=0.80). A dozen drug recipients were diagnosed with bladder cancer compared with eight cases in the placebo group (p=0.37).
Dr. Clark said, "All of the stuff we're doing for risk-factor reduction -- blood pressure reduction, stop smoking and giving aspirin -- they're all on the same level of relative improvement, and all of those are widely used. Aspirin has a list of side effects that will fill up three pages."
At the start of the study, all of the volunteers were insulin resistant, at least 40 years old, and had experienced an ischemic stroke or TIA in the previous six months. Diabetics were excluded as were patients with heart failure, active liver disease, and an increased risk of bladder cancer.
By the end of the study, 60% of the pioglitazone patients were still taking their medicine compared with 67% of placebo recipients. The most common reason for discontinuing was edema or weight gain.
The National Institute of Neurological Disorders and Stroke funded this study. Eleven coauthors reported disclosures.
EC grants venetoclax orphan designation for AML

The European Commission has granted orphan drug designation for the oral BCL-2 inhibitor venetoclax to treat acute myeloid leukemia (AML).
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the
European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche.
Phase 2 study
Results from a phase 2 study of venetoclax in AML were presented at ASH 2014. At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3/4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events. ![]()

The European Commission has granted orphan drug designation for the oral BCL-2 inhibitor venetoclax to treat acute myeloid leukemia (AML).
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the
European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche.
Phase 2 study
Results from a phase 2 study of venetoclax in AML were presented at ASH 2014. At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3/4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events. ![]()

The European Commission has granted orphan drug designation for the oral BCL-2 inhibitor venetoclax to treat acute myeloid leukemia (AML).
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the
European Union. The product must provide significant benefit to those affected by the condition.
Orphan drug designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is on the market.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche.
Phase 2 study
Results from a phase 2 study of venetoclax in AML were presented at ASH 2014. At that time, the trial had enrolled 32 patients, 30 of whom had relapsed or refractory disease. Patients had a median age of 71 (range, 19 to 84), and half were male.
The overall response rate was 15.5%, with 1 patient achieving a complete response (CR) and 4 achieving a CR with incomplete count recovery (CRi).
The researchers noted that 3 of the patients who had a CR/CRi had IDH mutations. Two of these patients also achieved minimal residual disease negativity.
The median bone marrow blast count in evaluable patients decreased 36% after treatment, and 6 patients (19%) had at least a 50% reduction in bone marrow blasts.
Common adverse events following treatment (occurring in at least 25% of patients) included nausea, diarrhea, fatigue, neutropenia, and vomiting.
Grade 3/4 adverse events (occurring in 3 or more patients) included febrile neutropenia, anemia, and pneumonia. No patient died as a result of treatment-related adverse events. ![]()
CHMP recommends fusion protein for hemophilia B

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that albutrepenonacog alfa (Idelvion) receive marketing authorization to treat patients with hemophilia B.
Albutrepenonacog alfa is a recombinant fusion protein linking coagulation factor IX with albumin.
The CHMP’s recommendation will be reviewed by the European Commission, which usually follows the CHMP’s advice.
In 2010, the European Commission granted albutrepenonacog alfa orphan designation as a treatment for hemophilia B.
Albutrepenonacog alfa is being developed by CSL Behring. The product is approved for use in Canada. Regulatory agencies in the US, Australia, Switzerland, and Japan are reviewing applications for the drug.
Phase 3 trial
The CHMP’s recommendation to approve albutrepenonacog alfa is based on the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were recently published in Blood. This study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10- or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that albutrepenonacog alfa (Idelvion) receive marketing authorization to treat patients with hemophilia B.
Albutrepenonacog alfa is a recombinant fusion protein linking coagulation factor IX with albumin.
The CHMP’s recommendation will be reviewed by the European Commission, which usually follows the CHMP’s advice.
In 2010, the European Commission granted albutrepenonacog alfa orphan designation as a treatment for hemophilia B.
Albutrepenonacog alfa is being developed by CSL Behring. The product is approved for use in Canada. Regulatory agencies in the US, Australia, Switzerland, and Japan are reviewing applications for the drug.
Phase 3 trial
The CHMP’s recommendation to approve albutrepenonacog alfa is based on the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were recently published in Blood. This study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10- or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that albutrepenonacog alfa (Idelvion) receive marketing authorization to treat patients with hemophilia B.
Albutrepenonacog alfa is a recombinant fusion protein linking coagulation factor IX with albumin.
The CHMP’s recommendation will be reviewed by the European Commission, which usually follows the CHMP’s advice.
In 2010, the European Commission granted albutrepenonacog alfa orphan designation as a treatment for hemophilia B.
Albutrepenonacog alfa is being developed by CSL Behring. The product is approved for use in Canada. Regulatory agencies in the US, Australia, Switzerland, and Japan are reviewing applications for the drug.
Phase 3 trial
The CHMP’s recommendation to approve albutrepenonacog alfa is based on the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of albutrepenonacog alfa in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were recently published in Blood. This study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with albutrepenonacog alfa once every 7 days for 26 weeks, followed by a 7-, 10- or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with albutrepenonacog alfa for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of albutrepenonacog alfa.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to albutrepenonacog alfa. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()