User login
#payattention
Social media consumes the attention of the majority of teens. It is a place to express yourself, flirt, intimidate, and keep them up to date with the latest happenings in the social circles. But, teens are using social media for much more.
Instagram, a social media site comprising photographs followed by comments, is one of the most common sites used by teens. They post everything from the meal they are eating to the new love in their life and everything in between.
A hashtag is a type of label or metadata tag used on social networks and microblogging services, which makes it easier for users to find messages with a specific theme or content. Users create hashtags by placing the hash character # (the number sign) in front of a word or unspaced phrase, either in the main text of a message or at the end. Searching for that hashtag will then present each message that has been tagged with it.1 Although teens seem to prefer simple phrases, these hashtags are used to link users to what many refer to as “Secret Society.”
For example, if a teen girl was “cutting” or interested in connecting with other teens that cut, putting #cat would link her to several social communities with the related topic. Similarly, #selfharm was the initial term used to connect to this secret society. When that was shut down by the social media site, it resurfaced as #selfharmmm2.
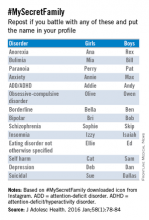
#MySecretFamily is a very popular hashtag that connects teens struggling with a variety of mental illnesses. Teens are instructed to put various names in their profile to identify which mental illness they are battling. For example, depression would be identified as “DEB” for a girl and “DAN” for a boy. The chart below lists various disorders.2
Nonsuicidal self-injury (NSSI) or deliberate destruction of one’s body in the absence of suicidal intent3 is most common in the middle school ages, and exposure to peer NSSI may increase the risk of engaging these behaviors.2,4 Although distinct from suicidal behaviors, there is a comorbidity between them. The normalization of these behaviors through social media and acceptance into the this “Secret Society” can only augment the risk of NSSI.
Parents not only need to be educated about the importance of monitoring their children’s social media but also about what to look for that may be a sign that something more serious is going on with them.
Although we hear so much of the negative impact of social media, it also can be a tool for early intervention.
References
1. Oxford English Dictionary, definition of “hashtag.”
2. J Adolesc Health. 2016 Jan;58(1):78-84.
4. Dev Psychol. 2006 May;42(3):407-17.
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
Social media consumes the attention of the majority of teens. It is a place to express yourself, flirt, intimidate, and keep them up to date with the latest happenings in the social circles. But, teens are using social media for much more.
Instagram, a social media site comprising photographs followed by comments, is one of the most common sites used by teens. They post everything from the meal they are eating to the new love in their life and everything in between.
A hashtag is a type of label or metadata tag used on social networks and microblogging services, which makes it easier for users to find messages with a specific theme or content. Users create hashtags by placing the hash character # (the number sign) in front of a word or unspaced phrase, either in the main text of a message or at the end. Searching for that hashtag will then present each message that has been tagged with it.1 Although teens seem to prefer simple phrases, these hashtags are used to link users to what many refer to as “Secret Society.”
For example, if a teen girl was “cutting” or interested in connecting with other teens that cut, putting #cat would link her to several social communities with the related topic. Similarly, #selfharm was the initial term used to connect to this secret society. When that was shut down by the social media site, it resurfaced as #selfharmmm2.
#MySecretFamily is a very popular hashtag that connects teens struggling with a variety of mental illnesses. Teens are instructed to put various names in their profile to identify which mental illness they are battling. For example, depression would be identified as “DEB” for a girl and “DAN” for a boy. The chart below lists various disorders.2
Nonsuicidal self-injury (NSSI) or deliberate destruction of one’s body in the absence of suicidal intent3 is most common in the middle school ages, and exposure to peer NSSI may increase the risk of engaging these behaviors.2,4 Although distinct from suicidal behaviors, there is a comorbidity between them. The normalization of these behaviors through social media and acceptance into the this “Secret Society” can only augment the risk of NSSI.
Parents not only need to be educated about the importance of monitoring their children’s social media but also about what to look for that may be a sign that something more serious is going on with them.
Although we hear so much of the negative impact of social media, it also can be a tool for early intervention.
References
1. Oxford English Dictionary, definition of “hashtag.”
2. J Adolesc Health. 2016 Jan;58(1):78-84.
4. Dev Psychol. 2006 May;42(3):407-17.
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
Social media consumes the attention of the majority of teens. It is a place to express yourself, flirt, intimidate, and keep them up to date with the latest happenings in the social circles. But, teens are using social media for much more.
Instagram, a social media site comprising photographs followed by comments, is one of the most common sites used by teens. They post everything from the meal they are eating to the new love in their life and everything in between.
A hashtag is a type of label or metadata tag used on social networks and microblogging services, which makes it easier for users to find messages with a specific theme or content. Users create hashtags by placing the hash character # (the number sign) in front of a word or unspaced phrase, either in the main text of a message or at the end. Searching for that hashtag will then present each message that has been tagged with it.1 Although teens seem to prefer simple phrases, these hashtags are used to link users to what many refer to as “Secret Society.”
For example, if a teen girl was “cutting” or interested in connecting with other teens that cut, putting #cat would link her to several social communities with the related topic. Similarly, #selfharm was the initial term used to connect to this secret society. When that was shut down by the social media site, it resurfaced as #selfharmmm2.
#MySecretFamily is a very popular hashtag that connects teens struggling with a variety of mental illnesses. Teens are instructed to put various names in their profile to identify which mental illness they are battling. For example, depression would be identified as “DEB” for a girl and “DAN” for a boy. The chart below lists various disorders.2
Nonsuicidal self-injury (NSSI) or deliberate destruction of one’s body in the absence of suicidal intent3 is most common in the middle school ages, and exposure to peer NSSI may increase the risk of engaging these behaviors.2,4 Although distinct from suicidal behaviors, there is a comorbidity between them. The normalization of these behaviors through social media and acceptance into the this “Secret Society” can only augment the risk of NSSI.
Parents not only need to be educated about the importance of monitoring their children’s social media but also about what to look for that may be a sign that something more serious is going on with them.
Although we hear so much of the negative impact of social media, it also can be a tool for early intervention.
References
1. Oxford English Dictionary, definition of “hashtag.”
2. J Adolesc Health. 2016 Jan;58(1):78-84.
4. Dev Psychol. 2006 May;42(3):407-17.
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
Treating influenza: A guide to antiviral safety in pregnancy
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at [email protected].
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at [email protected].
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at [email protected].
Unresponsive and mute after he smoked ‘Spice’
CASE Mute and nonresponsive
Mr. R, a 19-year-old African-American man, is brought to the emergency room (ER) because he has reduced oral intake and mutism, and is not attending to activities of daily living (ADL). His family reports gradual onset of symptoms over the past month after he began using “Spice,” a synthetic cannabinoid (Box1-8).
Mr. R has been using marijuana regularly for a few years. He has no history of psychiatric illness. The family history is positive for schizophrenia (mother).
Mr. R slowly stopped speaking and eating, and no longer responds to verbal stimulation. On examination, he responds only with unintelligible mumbling. Mr. R exhibits blunted affect and fails to maintain eye contact, looking to the side of the interviewer. He exhibits severe psychomotor retardation but without posturing or waxy flexibility. It takes him approximately 3 minutes to transfer between chairs, and he is incontinent of bladder and bowel.
Mr. R has not experienced a similar episode in the past, although he had exhibited brief paranoia while intoxicated with marijuana.
Before this episode, Mr. R had been moving between his grandmother’s and father’s homes and was attending high school classes. Recent stressful events include his brother’s incarceration and his father having re-entered his life after a long absence.
Which treatment would you initiate for Mr. R’s symptoms of catatonia?
a) dantrolene
b) a benzodiazepine
c) an antipsychotic
d) electroconvulsive therapy (ECT)
The authors’ observations
Catatonia is a common complication in a variety of psychiatric and medical contexts. It can be a feature of mood disorders, schizophrenia, metabolic disturbances, drug intoxication, neuroleptic malignant syndrome (NMS), and encephalopathy. The most common psychiatric comorbidity is bipolar disorder; as many as 25% of cases are caused by a medical or neurological condition.9 When accompanied by fever and autonomic instability, so-called malignant catatonia can lead to respiratory failure, coma, and death.
Catatonia is characterized by ≥3 of the elements outlined in Table 1.10
In DSM-5, catatonia is no longer considered a subtype of schizophrenia, but is a specifier in the following disorders: brief psychotic disorder, schizophreniform disorder, schizoaffective disorder, and substance-induced psychotic disorder. In addition, catatonia not otherwise specified is reserved for cases when the cause is not apparent; this diagnosis is intended to lead to greater recognition of catatonia and prompt initiation of treatment. DSM-5 stops short of classifying catatonia as an independent syndrome, however. Changes in clinical status can be charted with instruments such as the Bush-Francis Catatonia Rating Scale.
Workup and treatment
The initial workup of patients with catatonia is extensive. A basic metabolic panel can detect electrolyte disturbances and acute renal failure. Monitoring creatine kinase (CK) allows clinicians to assess for rhabdomyolysis. Patients should also undergo an infectious workup, including complete blood count (CBC) and chest radiography, because patients can develop pneumonia due to atelectasis or aspiration. Additional workup could include EEG, erythrocyte sedimentation rate, D-dimer, urinalysis, urine drug screen, antinuclear antibodies, magnetic resonance imaging, cerebrospinal fluid analysis, anti-N-methyl-D-aspartate receptor antibodies, and serum iron, which could predict development of NMS in patients treated with an antipsychotic.11
Treatment. In addition to supportive measures, the initial treatment of choice for catatonia is a benzodiazepine, lorazepam being the most commonly used agent; dramatic improvement in symptoms can be seen within minutes of IV administration. A high dosage of lorazepam (14 to 16 mg/d) sometimes is required for symptomatic relief. Zolpidem also has been used successfully to treat catatonia, although the supporting literature is less extensive.12
Antipsychotics generally are held during the initial stages of catatonia treatment because they can exacerbate symptoms and increase the likelihood of NMS. Glutamate antagonists, such as amantadine and memantine, also are being investigated for treating catatonia.9
ECT is effective but is reserved for when pharmacotherapy has failed or when a rapid response is required. ECT is associated with cognitive and medical complications, although current techniques have greatly mitigated the risks. Mortality is estimated to be 1 in every 10,000 patients or 1 for every 80,000 treatments, most often due to a cardiac or pulmonary cause.13 Patients receiving ECT could experience temporary anterograde amnesia and confusion as well as retrograde amnesia, particularly memories formed around the time of treatment.
Response to benzodiazepine therapy varies: Some patients experience significant improvement after 1 dose; others require a high dosage for an extended period. More than 70% of cases remit with benzodiazepines; ECT should be considered after several days or earlier if indicated.9 Some patients with catatonia require a slow benzodiazepine taper to prevent symptoms from recurring.
Patients with catatonia are at risk of dehydration and malnutrition, and might require IV fluids or parenteral nutrition. These patients also are at risk of constipation, ileus, decubitus ulcers, deep vein thrombosis, and pulmonary embolism. Encourage early ambulation and consider prescribing an antithrombotic. Some patients might require physical therapy to prevent or treat muscle contractures.
TREATMENT Benzodiazepines, ECT
Mr. R is admitted for stabilization of catatonic symptoms. A basic metabolic panel, CBC with differential, urine drug screen, urinalysis, folate level, thyroid-stimulating hormone level, vitamin B12, EEG, and a stool culture are unremarkable. Ammonia level is slightly elevated at 40 µmol/L.
Mr. R is started on IM lorazepam, 1 mg every 8 hours. Antipsychotics are held in part because of an elevated CK level (614 U/L). CK is rechecked daily and increases to 5,681 U/L by the second week. Internal medicine is consulted because Mr. R could develop NMS. However, the treatment team thinks that CK elevation is caused by immobility, because Mr. R remains afebrile, normotensive, and without leukocytosis.
After 4 days of treatment, Mr. R can follow simple commands. He nods or shakes his head when questioned. IV fluids are started because of limited oral intake. As the month progresses, Mr. R’s CK levels slowly trend downward, toward 500 U/L.
Mr. R progresses slowly with benzodiazepine therapy. He begins to ambulate, make eye contact, and look at interviewers. Lorazepam is slowly titrated to 4 mg IM every 8 hours. On hospital Day 20, his functioning reaches a plateau; Mr. R’s cognition continues to fluctuate with periods of unresponsiveness, immobility, and incontinence.
The treatment team obtains consent from the family to begin ECT. On hospital Day 24, bilateral transtemporal ECT is initiated and continued 3 times a week. Mr. R tolerates the procedure without complications. After the first treatment, he demonstrates spontaneous speech for the first time since admission. He continues to improve overall but has a variable clinical course.
By Day 30, Mr. R can state the day, month, year, and that he is in the “psych” unit. He remembers being on the unit for a long time and says that he had been attempting to talk but “it wasn’t coming out.” When further questioned about substance use, he admits to using Spice for the month before admission and marijuana regularly over several years. He denies using other illicit drugs or alcohol.
Mr. R is started on olanzapine, 2.5 mg/d, titrated to 15 mg/d. He becomes increasingly interactive, although with occasional bouts of confusion, and regains bladder and bowel control. He receives a total of 12 ECT treatments. The family is adamant that Mr. R should not receive more ECT treatments, and is not interested in maintenance therapy. Mr. R’s father and grandmother visit and believe that he is back to baseline functioning. After 51 days of inpatient treatment, Mr. R is discharged on olanzapine, 15 mg/d, and oral lorazepam, 1 mg/d.
Nine days later, Mr. R is brought to the ER because of unresponsiveness, poor oral intake, refusal of medication, bowel and bladder incontinence, and inability to perform ADL. His father reports that he administered olanzapine but, because he only recognized the brand name of lorazepam, he did not get that prescription filled. Mr. R slowly decompensates and, by the time of readmission, refuses all medications.
Over the next few months, Mr. R is readmitted several times for similar symptoms. Again, the family states they do not want further ECT; the father believes that these treatments have caused his son’s condition. Complicating the matter is that the father had been out of his son’s life for an extended period and is unaccustomed to his son’s display of psychiatric symptoms.
The authors’ observations
The use of ECT for drug-induced psychosis is not well described in the literature because substance abuse is exclusionary in many trials. The safety and efficacy of ECT has been established for adolescents with first-episode psychosis14 and with catatonia.15,16
The use of ECT in Spice-induced catatonia has been reported in 2 case studies.17,18
Case 1. A 36-year-old man with schizophrenia and Cannabis dependence was admitted for auditory hallucinations, disorganization, paranoia, and manic symptoms, which progressed to catatonia.17 His symptoms were profound, including psychomotor retardation, rigidity, posturing, waxy flexibility, and inability to perform ADL.
The patient later reported that, 3 weeks prior, he had stopped taking his psychotropic medications and started smoking “K2,” a synthetic cannabinoid, because it was cheaper and easier to obtain than Cannabis. He had never experienced disturbances in motor function or speech in the past, even during episodes of Cannabis use and medication non-adherence.
After clozapine and benzodiazepine treatment (as high as 12 mg/d of lorazepam) did not resolve his symptoms, the patient received 6 bilateral ECT treatments over 16 days, with complete resolution of catatonic symptoms. He showed marked improvement, including resumption of speech after the first treatment, although he required an additional 20 days of inpatient care. As in our case, exposure to synthetic cannabinoids was self-reported; no confirmatory tests were performed.
Case 2. A 17-year-old male with no history of psychosis exhibited catatonic symptoms after smoking an estimated 2 to 3 g/d of K2 over 2 months.18 Similar to the case of Mr. R, he plateaued after lorazepam treatment, and then received 6 ECT treatments, which resulted in complete resolution of symptoms. He was discharged with olanzapine.
As our patient, and the 2 cases cited, show, ECT seems to be an effective option for Spice-induced catatonia. Unlike those published cases, however, our patient achieved only brief resolution of symptoms after an acute course of ECT. There appears to be a subset of patients who require maintenance ECT or prolonged benzodiazepine therapy after Spice-induced catatonia.
1. Cohen J, Morrison S, Greenberg J, et al. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. 2012;129(4):e1064-e1067.
2. Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl). 2013;228(4):525-540.
3. Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the future national survey results on drug use. 2012 Overview: key findings on adolescent drug use. http://monitoringthefuture.org/pubs/monographs/mtf-overview2012.pdf. Published February 2013. Accessed February 8, 2016.
4. Hu X, Primack BA, Barnett TE, et al. College students and use of K2: an emerging drug abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16.
5. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: a case series. Am J Psychiatry. 2011;168(10):1119.
6. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Medi Biol Res. 2006;39(4):421-429.
7. Fadda P, Robinson L, Fratta W, et al. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropsychopharmacology. 2004;47(8):1170-1179.
8. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systemic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561.
9. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):499-507.
12. Thomas P, Rascle C, Mastain B, et al. Test for catatonia with zolpidem. Lancet. 1997;349(9053):702.
13. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
14. Zhang ZJ, Chen YC, Wang HN, et al. Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis—a case-control study. Schizophr Res. 2012;137(1-3):97-103.
15. Consoli A, Benmiloud M, Wachtel L, et al. Electroconvulsive therapy in adolescents with the catatonia syndrome: efficacy and ethics. J ECT. 2010;26(4):259-265.
16. Shoirah H, Hamoda HM. Electroconvulsive therapy in children and adolescents. Expert Rev Neurother. 2011;11(1):127-137.
17. Leibu E, Garakani A, McGonigle DP, et al. Electroconvulsive therapy (ECT) for catatonia in a patient with schizophrenia and synthetic cannabinoid abuse: a case report. J ECT. 2013;29(4):e61-e62. doi: 10.1097/YCT.0b013e318290fa36.
18. Smith DL, Roberts C. Synthetic marijuana use and development of catatonia in a 17-year-old male. Minn Med. 2014;97(5):38.
CASE Mute and nonresponsive
Mr. R, a 19-year-old African-American man, is brought to the emergency room (ER) because he has reduced oral intake and mutism, and is not attending to activities of daily living (ADL). His family reports gradual onset of symptoms over the past month after he began using “Spice,” a synthetic cannabinoid (Box1-8).
Mr. R has been using marijuana regularly for a few years. He has no history of psychiatric illness. The family history is positive for schizophrenia (mother).
Mr. R slowly stopped speaking and eating, and no longer responds to verbal stimulation. On examination, he responds only with unintelligible mumbling. Mr. R exhibits blunted affect and fails to maintain eye contact, looking to the side of the interviewer. He exhibits severe psychomotor retardation but without posturing or waxy flexibility. It takes him approximately 3 minutes to transfer between chairs, and he is incontinent of bladder and bowel.
Mr. R has not experienced a similar episode in the past, although he had exhibited brief paranoia while intoxicated with marijuana.
Before this episode, Mr. R had been moving between his grandmother’s and father’s homes and was attending high school classes. Recent stressful events include his brother’s incarceration and his father having re-entered his life after a long absence.
Which treatment would you initiate for Mr. R’s symptoms of catatonia?
a) dantrolene
b) a benzodiazepine
c) an antipsychotic
d) electroconvulsive therapy (ECT)
The authors’ observations
Catatonia is a common complication in a variety of psychiatric and medical contexts. It can be a feature of mood disorders, schizophrenia, metabolic disturbances, drug intoxication, neuroleptic malignant syndrome (NMS), and encephalopathy. The most common psychiatric comorbidity is bipolar disorder; as many as 25% of cases are caused by a medical or neurological condition.9 When accompanied by fever and autonomic instability, so-called malignant catatonia can lead to respiratory failure, coma, and death.
Catatonia is characterized by ≥3 of the elements outlined in Table 1.10
In DSM-5, catatonia is no longer considered a subtype of schizophrenia, but is a specifier in the following disorders: brief psychotic disorder, schizophreniform disorder, schizoaffective disorder, and substance-induced psychotic disorder. In addition, catatonia not otherwise specified is reserved for cases when the cause is not apparent; this diagnosis is intended to lead to greater recognition of catatonia and prompt initiation of treatment. DSM-5 stops short of classifying catatonia as an independent syndrome, however. Changes in clinical status can be charted with instruments such as the Bush-Francis Catatonia Rating Scale.
Workup and treatment
The initial workup of patients with catatonia is extensive. A basic metabolic panel can detect electrolyte disturbances and acute renal failure. Monitoring creatine kinase (CK) allows clinicians to assess for rhabdomyolysis. Patients should also undergo an infectious workup, including complete blood count (CBC) and chest radiography, because patients can develop pneumonia due to atelectasis or aspiration. Additional workup could include EEG, erythrocyte sedimentation rate, D-dimer, urinalysis, urine drug screen, antinuclear antibodies, magnetic resonance imaging, cerebrospinal fluid analysis, anti-N-methyl-D-aspartate receptor antibodies, and serum iron, which could predict development of NMS in patients treated with an antipsychotic.11
Treatment. In addition to supportive measures, the initial treatment of choice for catatonia is a benzodiazepine, lorazepam being the most commonly used agent; dramatic improvement in symptoms can be seen within minutes of IV administration. A high dosage of lorazepam (14 to 16 mg/d) sometimes is required for symptomatic relief. Zolpidem also has been used successfully to treat catatonia, although the supporting literature is less extensive.12
Antipsychotics generally are held during the initial stages of catatonia treatment because they can exacerbate symptoms and increase the likelihood of NMS. Glutamate antagonists, such as amantadine and memantine, also are being investigated for treating catatonia.9
ECT is effective but is reserved for when pharmacotherapy has failed or when a rapid response is required. ECT is associated with cognitive and medical complications, although current techniques have greatly mitigated the risks. Mortality is estimated to be 1 in every 10,000 patients or 1 for every 80,000 treatments, most often due to a cardiac or pulmonary cause.13 Patients receiving ECT could experience temporary anterograde amnesia and confusion as well as retrograde amnesia, particularly memories formed around the time of treatment.
Response to benzodiazepine therapy varies: Some patients experience significant improvement after 1 dose; others require a high dosage for an extended period. More than 70% of cases remit with benzodiazepines; ECT should be considered after several days or earlier if indicated.9 Some patients with catatonia require a slow benzodiazepine taper to prevent symptoms from recurring.
Patients with catatonia are at risk of dehydration and malnutrition, and might require IV fluids or parenteral nutrition. These patients also are at risk of constipation, ileus, decubitus ulcers, deep vein thrombosis, and pulmonary embolism. Encourage early ambulation and consider prescribing an antithrombotic. Some patients might require physical therapy to prevent or treat muscle contractures.
TREATMENT Benzodiazepines, ECT
Mr. R is admitted for stabilization of catatonic symptoms. A basic metabolic panel, CBC with differential, urine drug screen, urinalysis, folate level, thyroid-stimulating hormone level, vitamin B12, EEG, and a stool culture are unremarkable. Ammonia level is slightly elevated at 40 µmol/L.
Mr. R is started on IM lorazepam, 1 mg every 8 hours. Antipsychotics are held in part because of an elevated CK level (614 U/L). CK is rechecked daily and increases to 5,681 U/L by the second week. Internal medicine is consulted because Mr. R could develop NMS. However, the treatment team thinks that CK elevation is caused by immobility, because Mr. R remains afebrile, normotensive, and without leukocytosis.
After 4 days of treatment, Mr. R can follow simple commands. He nods or shakes his head when questioned. IV fluids are started because of limited oral intake. As the month progresses, Mr. R’s CK levels slowly trend downward, toward 500 U/L.
Mr. R progresses slowly with benzodiazepine therapy. He begins to ambulate, make eye contact, and look at interviewers. Lorazepam is slowly titrated to 4 mg IM every 8 hours. On hospital Day 20, his functioning reaches a plateau; Mr. R’s cognition continues to fluctuate with periods of unresponsiveness, immobility, and incontinence.
The treatment team obtains consent from the family to begin ECT. On hospital Day 24, bilateral transtemporal ECT is initiated and continued 3 times a week. Mr. R tolerates the procedure without complications. After the first treatment, he demonstrates spontaneous speech for the first time since admission. He continues to improve overall but has a variable clinical course.
By Day 30, Mr. R can state the day, month, year, and that he is in the “psych” unit. He remembers being on the unit for a long time and says that he had been attempting to talk but “it wasn’t coming out.” When further questioned about substance use, he admits to using Spice for the month before admission and marijuana regularly over several years. He denies using other illicit drugs or alcohol.
Mr. R is started on olanzapine, 2.5 mg/d, titrated to 15 mg/d. He becomes increasingly interactive, although with occasional bouts of confusion, and regains bladder and bowel control. He receives a total of 12 ECT treatments. The family is adamant that Mr. R should not receive more ECT treatments, and is not interested in maintenance therapy. Mr. R’s father and grandmother visit and believe that he is back to baseline functioning. After 51 days of inpatient treatment, Mr. R is discharged on olanzapine, 15 mg/d, and oral lorazepam, 1 mg/d.
Nine days later, Mr. R is brought to the ER because of unresponsiveness, poor oral intake, refusal of medication, bowel and bladder incontinence, and inability to perform ADL. His father reports that he administered olanzapine but, because he only recognized the brand name of lorazepam, he did not get that prescription filled. Mr. R slowly decompensates and, by the time of readmission, refuses all medications.
Over the next few months, Mr. R is readmitted several times for similar symptoms. Again, the family states they do not want further ECT; the father believes that these treatments have caused his son’s condition. Complicating the matter is that the father had been out of his son’s life for an extended period and is unaccustomed to his son’s display of psychiatric symptoms.
The authors’ observations
The use of ECT for drug-induced psychosis is not well described in the literature because substance abuse is exclusionary in many trials. The safety and efficacy of ECT has been established for adolescents with first-episode psychosis14 and with catatonia.15,16
The use of ECT in Spice-induced catatonia has been reported in 2 case studies.17,18
Case 1. A 36-year-old man with schizophrenia and Cannabis dependence was admitted for auditory hallucinations, disorganization, paranoia, and manic symptoms, which progressed to catatonia.17 His symptoms were profound, including psychomotor retardation, rigidity, posturing, waxy flexibility, and inability to perform ADL.
The patient later reported that, 3 weeks prior, he had stopped taking his psychotropic medications and started smoking “K2,” a synthetic cannabinoid, because it was cheaper and easier to obtain than Cannabis. He had never experienced disturbances in motor function or speech in the past, even during episodes of Cannabis use and medication non-adherence.
After clozapine and benzodiazepine treatment (as high as 12 mg/d of lorazepam) did not resolve his symptoms, the patient received 6 bilateral ECT treatments over 16 days, with complete resolution of catatonic symptoms. He showed marked improvement, including resumption of speech after the first treatment, although he required an additional 20 days of inpatient care. As in our case, exposure to synthetic cannabinoids was self-reported; no confirmatory tests were performed.
Case 2. A 17-year-old male with no history of psychosis exhibited catatonic symptoms after smoking an estimated 2 to 3 g/d of K2 over 2 months.18 Similar to the case of Mr. R, he plateaued after lorazepam treatment, and then received 6 ECT treatments, which resulted in complete resolution of symptoms. He was discharged with olanzapine.
As our patient, and the 2 cases cited, show, ECT seems to be an effective option for Spice-induced catatonia. Unlike those published cases, however, our patient achieved only brief resolution of symptoms after an acute course of ECT. There appears to be a subset of patients who require maintenance ECT or prolonged benzodiazepine therapy after Spice-induced catatonia.
CASE Mute and nonresponsive
Mr. R, a 19-year-old African-American man, is brought to the emergency room (ER) because he has reduced oral intake and mutism, and is not attending to activities of daily living (ADL). His family reports gradual onset of symptoms over the past month after he began using “Spice,” a synthetic cannabinoid (Box1-8).
Mr. R has been using marijuana regularly for a few years. He has no history of psychiatric illness. The family history is positive for schizophrenia (mother).
Mr. R slowly stopped speaking and eating, and no longer responds to verbal stimulation. On examination, he responds only with unintelligible mumbling. Mr. R exhibits blunted affect and fails to maintain eye contact, looking to the side of the interviewer. He exhibits severe psychomotor retardation but without posturing or waxy flexibility. It takes him approximately 3 minutes to transfer between chairs, and he is incontinent of bladder and bowel.
Mr. R has not experienced a similar episode in the past, although he had exhibited brief paranoia while intoxicated with marijuana.
Before this episode, Mr. R had been moving between his grandmother’s and father’s homes and was attending high school classes. Recent stressful events include his brother’s incarceration and his father having re-entered his life after a long absence.
Which treatment would you initiate for Mr. R’s symptoms of catatonia?
a) dantrolene
b) a benzodiazepine
c) an antipsychotic
d) electroconvulsive therapy (ECT)
The authors’ observations
Catatonia is a common complication in a variety of psychiatric and medical contexts. It can be a feature of mood disorders, schizophrenia, metabolic disturbances, drug intoxication, neuroleptic malignant syndrome (NMS), and encephalopathy. The most common psychiatric comorbidity is bipolar disorder; as many as 25% of cases are caused by a medical or neurological condition.9 When accompanied by fever and autonomic instability, so-called malignant catatonia can lead to respiratory failure, coma, and death.
Catatonia is characterized by ≥3 of the elements outlined in Table 1.10
In DSM-5, catatonia is no longer considered a subtype of schizophrenia, but is a specifier in the following disorders: brief psychotic disorder, schizophreniform disorder, schizoaffective disorder, and substance-induced psychotic disorder. In addition, catatonia not otherwise specified is reserved for cases when the cause is not apparent; this diagnosis is intended to lead to greater recognition of catatonia and prompt initiation of treatment. DSM-5 stops short of classifying catatonia as an independent syndrome, however. Changes in clinical status can be charted with instruments such as the Bush-Francis Catatonia Rating Scale.
Workup and treatment
The initial workup of patients with catatonia is extensive. A basic metabolic panel can detect electrolyte disturbances and acute renal failure. Monitoring creatine kinase (CK) allows clinicians to assess for rhabdomyolysis. Patients should also undergo an infectious workup, including complete blood count (CBC) and chest radiography, because patients can develop pneumonia due to atelectasis or aspiration. Additional workup could include EEG, erythrocyte sedimentation rate, D-dimer, urinalysis, urine drug screen, antinuclear antibodies, magnetic resonance imaging, cerebrospinal fluid analysis, anti-N-methyl-D-aspartate receptor antibodies, and serum iron, which could predict development of NMS in patients treated with an antipsychotic.11
Treatment. In addition to supportive measures, the initial treatment of choice for catatonia is a benzodiazepine, lorazepam being the most commonly used agent; dramatic improvement in symptoms can be seen within minutes of IV administration. A high dosage of lorazepam (14 to 16 mg/d) sometimes is required for symptomatic relief. Zolpidem also has been used successfully to treat catatonia, although the supporting literature is less extensive.12
Antipsychotics generally are held during the initial stages of catatonia treatment because they can exacerbate symptoms and increase the likelihood of NMS. Glutamate antagonists, such as amantadine and memantine, also are being investigated for treating catatonia.9
ECT is effective but is reserved for when pharmacotherapy has failed or when a rapid response is required. ECT is associated with cognitive and medical complications, although current techniques have greatly mitigated the risks. Mortality is estimated to be 1 in every 10,000 patients or 1 for every 80,000 treatments, most often due to a cardiac or pulmonary cause.13 Patients receiving ECT could experience temporary anterograde amnesia and confusion as well as retrograde amnesia, particularly memories formed around the time of treatment.
Response to benzodiazepine therapy varies: Some patients experience significant improvement after 1 dose; others require a high dosage for an extended period. More than 70% of cases remit with benzodiazepines; ECT should be considered after several days or earlier if indicated.9 Some patients with catatonia require a slow benzodiazepine taper to prevent symptoms from recurring.
Patients with catatonia are at risk of dehydration and malnutrition, and might require IV fluids or parenteral nutrition. These patients also are at risk of constipation, ileus, decubitus ulcers, deep vein thrombosis, and pulmonary embolism. Encourage early ambulation and consider prescribing an antithrombotic. Some patients might require physical therapy to prevent or treat muscle contractures.
TREATMENT Benzodiazepines, ECT
Mr. R is admitted for stabilization of catatonic symptoms. A basic metabolic panel, CBC with differential, urine drug screen, urinalysis, folate level, thyroid-stimulating hormone level, vitamin B12, EEG, and a stool culture are unremarkable. Ammonia level is slightly elevated at 40 µmol/L.
Mr. R is started on IM lorazepam, 1 mg every 8 hours. Antipsychotics are held in part because of an elevated CK level (614 U/L). CK is rechecked daily and increases to 5,681 U/L by the second week. Internal medicine is consulted because Mr. R could develop NMS. However, the treatment team thinks that CK elevation is caused by immobility, because Mr. R remains afebrile, normotensive, and without leukocytosis.
After 4 days of treatment, Mr. R can follow simple commands. He nods or shakes his head when questioned. IV fluids are started because of limited oral intake. As the month progresses, Mr. R’s CK levels slowly trend downward, toward 500 U/L.
Mr. R progresses slowly with benzodiazepine therapy. He begins to ambulate, make eye contact, and look at interviewers. Lorazepam is slowly titrated to 4 mg IM every 8 hours. On hospital Day 20, his functioning reaches a plateau; Mr. R’s cognition continues to fluctuate with periods of unresponsiveness, immobility, and incontinence.
The treatment team obtains consent from the family to begin ECT. On hospital Day 24, bilateral transtemporal ECT is initiated and continued 3 times a week. Mr. R tolerates the procedure without complications. After the first treatment, he demonstrates spontaneous speech for the first time since admission. He continues to improve overall but has a variable clinical course.
By Day 30, Mr. R can state the day, month, year, and that he is in the “psych” unit. He remembers being on the unit for a long time and says that he had been attempting to talk but “it wasn’t coming out.” When further questioned about substance use, he admits to using Spice for the month before admission and marijuana regularly over several years. He denies using other illicit drugs or alcohol.
Mr. R is started on olanzapine, 2.5 mg/d, titrated to 15 mg/d. He becomes increasingly interactive, although with occasional bouts of confusion, and regains bladder and bowel control. He receives a total of 12 ECT treatments. The family is adamant that Mr. R should not receive more ECT treatments, and is not interested in maintenance therapy. Mr. R’s father and grandmother visit and believe that he is back to baseline functioning. After 51 days of inpatient treatment, Mr. R is discharged on olanzapine, 15 mg/d, and oral lorazepam, 1 mg/d.
Nine days later, Mr. R is brought to the ER because of unresponsiveness, poor oral intake, refusal of medication, bowel and bladder incontinence, and inability to perform ADL. His father reports that he administered olanzapine but, because he only recognized the brand name of lorazepam, he did not get that prescription filled. Mr. R slowly decompensates and, by the time of readmission, refuses all medications.
Over the next few months, Mr. R is readmitted several times for similar symptoms. Again, the family states they do not want further ECT; the father believes that these treatments have caused his son’s condition. Complicating the matter is that the father had been out of his son’s life for an extended period and is unaccustomed to his son’s display of psychiatric symptoms.
The authors’ observations
The use of ECT for drug-induced psychosis is not well described in the literature because substance abuse is exclusionary in many trials. The safety and efficacy of ECT has been established for adolescents with first-episode psychosis14 and with catatonia.15,16
The use of ECT in Spice-induced catatonia has been reported in 2 case studies.17,18
Case 1. A 36-year-old man with schizophrenia and Cannabis dependence was admitted for auditory hallucinations, disorganization, paranoia, and manic symptoms, which progressed to catatonia.17 His symptoms were profound, including psychomotor retardation, rigidity, posturing, waxy flexibility, and inability to perform ADL.
The patient later reported that, 3 weeks prior, he had stopped taking his psychotropic medications and started smoking “K2,” a synthetic cannabinoid, because it was cheaper and easier to obtain than Cannabis. He had never experienced disturbances in motor function or speech in the past, even during episodes of Cannabis use and medication non-adherence.
After clozapine and benzodiazepine treatment (as high as 12 mg/d of lorazepam) did not resolve his symptoms, the patient received 6 bilateral ECT treatments over 16 days, with complete resolution of catatonic symptoms. He showed marked improvement, including resumption of speech after the first treatment, although he required an additional 20 days of inpatient care. As in our case, exposure to synthetic cannabinoids was self-reported; no confirmatory tests were performed.
Case 2. A 17-year-old male with no history of psychosis exhibited catatonic symptoms after smoking an estimated 2 to 3 g/d of K2 over 2 months.18 Similar to the case of Mr. R, he plateaued after lorazepam treatment, and then received 6 ECT treatments, which resulted in complete resolution of symptoms. He was discharged with olanzapine.
As our patient, and the 2 cases cited, show, ECT seems to be an effective option for Spice-induced catatonia. Unlike those published cases, however, our patient achieved only brief resolution of symptoms after an acute course of ECT. There appears to be a subset of patients who require maintenance ECT or prolonged benzodiazepine therapy after Spice-induced catatonia.
1. Cohen J, Morrison S, Greenberg J, et al. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. 2012;129(4):e1064-e1067.
2. Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl). 2013;228(4):525-540.
3. Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the future national survey results on drug use. 2012 Overview: key findings on adolescent drug use. http://monitoringthefuture.org/pubs/monographs/mtf-overview2012.pdf. Published February 2013. Accessed February 8, 2016.
4. Hu X, Primack BA, Barnett TE, et al. College students and use of K2: an emerging drug abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16.
5. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: a case series. Am J Psychiatry. 2011;168(10):1119.
6. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Medi Biol Res. 2006;39(4):421-429.
7. Fadda P, Robinson L, Fratta W, et al. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropsychopharmacology. 2004;47(8):1170-1179.
8. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systemic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561.
9. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):499-507.
12. Thomas P, Rascle C, Mastain B, et al. Test for catatonia with zolpidem. Lancet. 1997;349(9053):702.
13. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
14. Zhang ZJ, Chen YC, Wang HN, et al. Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis—a case-control study. Schizophr Res. 2012;137(1-3):97-103.
15. Consoli A, Benmiloud M, Wachtel L, et al. Electroconvulsive therapy in adolescents with the catatonia syndrome: efficacy and ethics. J ECT. 2010;26(4):259-265.
16. Shoirah H, Hamoda HM. Electroconvulsive therapy in children and adolescents. Expert Rev Neurother. 2011;11(1):127-137.
17. Leibu E, Garakani A, McGonigle DP, et al. Electroconvulsive therapy (ECT) for catatonia in a patient with schizophrenia and synthetic cannabinoid abuse: a case report. J ECT. 2013;29(4):e61-e62. doi: 10.1097/YCT.0b013e318290fa36.
18. Smith DL, Roberts C. Synthetic marijuana use and development of catatonia in a 17-year-old male. Minn Med. 2014;97(5):38.
1. Cohen J, Morrison S, Greenberg J, et al. Clinical presentation of intoxication due to synthetic cannabinoids. Pediatrics. 2012;129(4):e1064-e1067.
2. Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl). 2013;228(4):525-540.
3. Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the future national survey results on drug use. 2012 Overview: key findings on adolescent drug use. http://monitoringthefuture.org/pubs/monographs/mtf-overview2012.pdf. Published February 2013. Accessed February 8, 2016.
4. Hu X, Primack BA, Barnett TE, et al. College students and use of K2: an emerging drug abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16.
5. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: a case series. Am J Psychiatry. 2011;168(10):1119.
6. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Medi Biol Res. 2006;39(4):421-429.
7. Fadda P, Robinson L, Fratta W, et al. Differential effects of THC- or CBD-rich cannabis extracts on working memory in rats. Neuropsychopharmacology. 2004;47(8):1170-1179.
8. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systemic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561.
9. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
10. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
11. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):499-507.
12. Thomas P, Rascle C, Mastain B, et al. Test for catatonia with zolpidem. Lancet. 1997;349(9053):702.
13. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
14. Zhang ZJ, Chen YC, Wang HN, et al. Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis—a case-control study. Schizophr Res. 2012;137(1-3):97-103.
15. Consoli A, Benmiloud M, Wachtel L, et al. Electroconvulsive therapy in adolescents with the catatonia syndrome: efficacy and ethics. J ECT. 2010;26(4):259-265.
16. Shoirah H, Hamoda HM. Electroconvulsive therapy in children and adolescents. Expert Rev Neurother. 2011;11(1):127-137.
17. Leibu E, Garakani A, McGonigle DP, et al. Electroconvulsive therapy (ECT) for catatonia in a patient with schizophrenia and synthetic cannabinoid abuse: a case report. J ECT. 2013;29(4):e61-e62. doi: 10.1097/YCT.0b013e318290fa36.
18. Smith DL, Roberts C. Synthetic marijuana use and development of catatonia in a 17-year-old male. Minn Med. 2014;97(5):38.
New Study Shows PCMH Resulted in Positive Changes
NEW YORK (Reuters Health) - Implementation of a patient-centered medical home (PCMH) resulted in small changes in utilization patterns and modest quality improvements over a three-year period, according to a new report.
Dr. Lisa M. Kern of Weill Cornell Medical College in New York City and colleagues found more primary care visits, fewer specialist visits, fewer lab and radiologic tests, and fewer hospitalizations and rehospitalizations in the practices that adopted the PCMH.
Most changes occurred in the last year of the study, three years after PCMH implementation, they report in the Annals of Internal Medicine, online February 15.
The PCMH model "attempts to shift the medical paradigm from care for individual patients to care for populations, from care by physicians to care by a team of providers, from a focus on acute illness to an emphasis on chronic disease management, and from care at a single site to coordinated care across providers and settings," Dr. Kern and her team write. However, they add, studies looking at the effectiveness of the approach have had mixed results.
To date, most studies attempting to look at PCMH have had follow-up periods lasting just 1.5 to 2 years after implementation, the researchers note. "These changes take time, and studies with relatively short follow-up may have underestimated the effects of the intervention," they add.
The new study included 438 primary care physicians in 226 practices with more than 136,000 patients enrolled in five health plans. Insurers offered incentives of $2 to $10 per patient per month to practices that achieved level III PCMH recognition from the National Committee for Quality Assurance
(NCQA).
Twelve practices including 125 physicians volunteered for the PCMH initiative, and were assisted by two outside consulting groups. All of these practices achieved level III PCMH recognition. Among the remaining physicians, 87 doctors in 45 practices adopted electronic health records (EHR) without the
PCMH intervention, and 226 physicians in 169 practices continued using paper records.
For the eight quality measures the researchers looked at, two showed greater improvements over time in the PCMH group compared to one or both of the control groups: eye examination and hemoglobin A1c testing for patients with diabetes.
From 2008 to 2012, the PCMH group showed improvements over the paper group and the EHR group for six of seven utilization measures.
NCQA recognition was one aspect of the PCMH intervention in the new study, but this doesn't represent the entire intervention, Dr. Mark W. Friedberg of RAND Corporation and Brigham and Women's Hospital in Boston, who wrote an editorial accompanying the study, told Reuters Health.
"What they evaluated was a different way of paying practices, combined with some technical assistance, combined with some shared savings in the last year of the pilot," Dr. Friedberg explained. And this also requires defining what improving care means, he added, for example "better technical quality of care, better patient experience, better effectiveness of care, better professional satisfaction and lower burnout for people working in the practices. It's also hard to measure all of those, and most studies don't."
The new study is well done, according to Dr. Friedberg, but the challenge will be to understand how it fits in with the rest of the medical home literature, he said. "There's a lot of trials still out there and the results are still coming in, including some very large Medicare medical home pilots. I think we'll have a much better sense of what works in a year or two as those results come back."
Dr. Kern did not respond to an interview request by press time.
The study was funded by The Commonwealth Fund and the New York State Department of Health.
NEW YORK (Reuters Health) - Implementation of a patient-centered medical home (PCMH) resulted in small changes in utilization patterns and modest quality improvements over a three-year period, according to a new report.
Dr. Lisa M. Kern of Weill Cornell Medical College in New York City and colleagues found more primary care visits, fewer specialist visits, fewer lab and radiologic tests, and fewer hospitalizations and rehospitalizations in the practices that adopted the PCMH.
Most changes occurred in the last year of the study, three years after PCMH implementation, they report in the Annals of Internal Medicine, online February 15.
The PCMH model "attempts to shift the medical paradigm from care for individual patients to care for populations, from care by physicians to care by a team of providers, from a focus on acute illness to an emphasis on chronic disease management, and from care at a single site to coordinated care across providers and settings," Dr. Kern and her team write. However, they add, studies looking at the effectiveness of the approach have had mixed results.
To date, most studies attempting to look at PCMH have had follow-up periods lasting just 1.5 to 2 years after implementation, the researchers note. "These changes take time, and studies with relatively short follow-up may have underestimated the effects of the intervention," they add.
The new study included 438 primary care physicians in 226 practices with more than 136,000 patients enrolled in five health plans. Insurers offered incentives of $2 to $10 per patient per month to practices that achieved level III PCMH recognition from the National Committee for Quality Assurance
(NCQA).
Twelve practices including 125 physicians volunteered for the PCMH initiative, and were assisted by two outside consulting groups. All of these practices achieved level III PCMH recognition. Among the remaining physicians, 87 doctors in 45 practices adopted electronic health records (EHR) without the
PCMH intervention, and 226 physicians in 169 practices continued using paper records.
For the eight quality measures the researchers looked at, two showed greater improvements over time in the PCMH group compared to one or both of the control groups: eye examination and hemoglobin A1c testing for patients with diabetes.
From 2008 to 2012, the PCMH group showed improvements over the paper group and the EHR group for six of seven utilization measures.
NCQA recognition was one aspect of the PCMH intervention in the new study, but this doesn't represent the entire intervention, Dr. Mark W. Friedberg of RAND Corporation and Brigham and Women's Hospital in Boston, who wrote an editorial accompanying the study, told Reuters Health.
"What they evaluated was a different way of paying practices, combined with some technical assistance, combined with some shared savings in the last year of the pilot," Dr. Friedberg explained. And this also requires defining what improving care means, he added, for example "better technical quality of care, better patient experience, better effectiveness of care, better professional satisfaction and lower burnout for people working in the practices. It's also hard to measure all of those, and most studies don't."
The new study is well done, according to Dr. Friedberg, but the challenge will be to understand how it fits in with the rest of the medical home literature, he said. "There's a lot of trials still out there and the results are still coming in, including some very large Medicare medical home pilots. I think we'll have a much better sense of what works in a year or two as those results come back."
Dr. Kern did not respond to an interview request by press time.
The study was funded by The Commonwealth Fund and the New York State Department of Health.
NEW YORK (Reuters Health) - Implementation of a patient-centered medical home (PCMH) resulted in small changes in utilization patterns and modest quality improvements over a three-year period, according to a new report.
Dr. Lisa M. Kern of Weill Cornell Medical College in New York City and colleagues found more primary care visits, fewer specialist visits, fewer lab and radiologic tests, and fewer hospitalizations and rehospitalizations in the practices that adopted the PCMH.
Most changes occurred in the last year of the study, three years after PCMH implementation, they report in the Annals of Internal Medicine, online February 15.
The PCMH model "attempts to shift the medical paradigm from care for individual patients to care for populations, from care by physicians to care by a team of providers, from a focus on acute illness to an emphasis on chronic disease management, and from care at a single site to coordinated care across providers and settings," Dr. Kern and her team write. However, they add, studies looking at the effectiveness of the approach have had mixed results.
To date, most studies attempting to look at PCMH have had follow-up periods lasting just 1.5 to 2 years after implementation, the researchers note. "These changes take time, and studies with relatively short follow-up may have underestimated the effects of the intervention," they add.
The new study included 438 primary care physicians in 226 practices with more than 136,000 patients enrolled in five health plans. Insurers offered incentives of $2 to $10 per patient per month to practices that achieved level III PCMH recognition from the National Committee for Quality Assurance
(NCQA).
Twelve practices including 125 physicians volunteered for the PCMH initiative, and were assisted by two outside consulting groups. All of these practices achieved level III PCMH recognition. Among the remaining physicians, 87 doctors in 45 practices adopted electronic health records (EHR) without the
PCMH intervention, and 226 physicians in 169 practices continued using paper records.
For the eight quality measures the researchers looked at, two showed greater improvements over time in the PCMH group compared to one or both of the control groups: eye examination and hemoglobin A1c testing for patients with diabetes.
From 2008 to 2012, the PCMH group showed improvements over the paper group and the EHR group for six of seven utilization measures.
NCQA recognition was one aspect of the PCMH intervention in the new study, but this doesn't represent the entire intervention, Dr. Mark W. Friedberg of RAND Corporation and Brigham and Women's Hospital in Boston, who wrote an editorial accompanying the study, told Reuters Health.
"What they evaluated was a different way of paying practices, combined with some technical assistance, combined with some shared savings in the last year of the pilot," Dr. Friedberg explained. And this also requires defining what improving care means, he added, for example "better technical quality of care, better patient experience, better effectiveness of care, better professional satisfaction and lower burnout for people working in the practices. It's also hard to measure all of those, and most studies don't."
The new study is well done, according to Dr. Friedberg, but the challenge will be to understand how it fits in with the rest of the medical home literature, he said. "There's a lot of trials still out there and the results are still coming in, including some very large Medicare medical home pilots. I think we'll have a much better sense of what works in a year or two as those results come back."
Dr. Kern did not respond to an interview request by press time.
The study was funded by The Commonwealth Fund and the New York State Department of Health.
Chronic pain and depression: Treatment of 2 culprits in common
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
‘We need to protect the brain’ Addressing the growing problem of chronic traumatic encephalopathy
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
Imposing treatment on patients with eating disorders: What are the legal risks?
Dear Dr. Mossman,
At the general hospital where I perform consultations, the medical service asked me to fill out psychiatric “hold” documents to keep a severely malnourished young woman with anorexia nervosa from leaving the hospital. Ms. Q, whose body mass index (BMI) was 12 (yes, 12), came to the hospital to have her “electrolytes fixed.” She was willing to stay the night for electrolyte repletion, but insisted she could gain weight on her own at home.
I’m worried that she might die without prompt inpatient treatment; she needs to stay on the medical service. Should I fill out a psychiatric hold to keep her there? What legal risks could I face if Ms. Q is detained and force-fed against her will? What are the legal risks of letting her leave the hospital before she is medically stable?
Submitted by “Dr. F”
When a severely malnourished patient with an eating disorder arrives on a medical floor, treatment teams often ask psychiatric consultants to help them impose care the patient desperately needs but doesn’t want. This reaction is understandable. After all, an eating disorder is a psychiatric illness, and hospital-based psychiatrists have experience with treating involuntary patients. A psychiatric hold may seem like a sensible way to save the life of a hospitalized patient with a mental illness.
But filling out a psychiatric hold only scratches the surface of what a psychiatric consultant’s contribution should include; in Ms. Q’s case, initiating a psychiatric hold is probably the wrong thing to do.
Why would filling out a psychiatric hold be inappropriate for Ms. Q? What clinical factors and legal issues should a psychiatrist consider when helping medical colleagues provide unwanted treatment to a severely malnourished patient with an eating disorder? We’ll explore these matters as we consider the case of Ms. Q (Figure) and the following questions:
- What type of care is most appropriate for her now?
- Can she refuse medical treatment?
- What are the medicolegal risks of letting her leave the hospital?
- What are the medicolegal risks of detaining and force-feeding her against her will?
- When is a psychiatric “hold” appropriate?
What care is appropriate?
Given her state of self-starvation, Ms. Q’s treatment plan could require close monitoring of her electrolytes and cardiac status, as well as watching her for signs of “refeeding syndrome”—rapid, potentially fatal fluid shifts and metabolic derangements that malnourished patients could experience when they receive artificial refeeding.1
First, the physicians who are caring for Ms. Q should determine whether she needs more intensive medical supervision than is usually available on a psychiatric unit. If she does, but she won’t agree to stay on a medical unit for care, a psychiatric hold is the wrong step, for 2 reasons:
- Once a psychiatric hold has been executed, state statutes require the patient to be placed in a psychiatric facility—a state-approved psychiatric treatment setting, such as a psychiatric unit or free-standing psychiatric hospital—within a specified period.2,3 Most nonpsychiatric medical units would not meet state’s statutory definition for such a facility.
- A psychiatric hold only permits short-term detention. It does not provide legal authority to impose unwanted medical treatment.
Does Ms. Q have capacity?
In the United States, Ms. Q has a legal right to refuse medical care—even if she needs it urgently—provided that her refusal is made competently.4 As Appelbaum and Grisso5 explained in a now-classic 1988 article:
The legal standards for competence include the four related skills of communicating a choice, understanding relevant information, appreciating the current situation and its consequences, and manipulating information rationally.
The Table5 describes these abilities in more detail.
Only courts can make legal determinations of competence, so physicians refer to an evaluation of a patient’s competence-related abilities as a “capacity assessment.” The decision as to whether a patient has capacity ultimately rests with the primary treatment team; however, physicians in other specialties often enlist psychiatrists’ help with this matter because of their interviewing skills and knowledge of how mental illness can impair capacity.
No easy-to-use instrument for evaluating capacity is available. However, Appelbaum6 provides examples of questions that often prove useful in such assessments, and a review by Sessums et al7 on several capacity evaluation tools suggests that the Aid to Capacity Evaluation8 may be the best instrument for performing capacity assessments.
Patients with anorexia nervosa often differ substantially from healthy people in how they assign values to life and death,9 which can make it difficult to evaluate their capacity to refuse life-saving treatment. Malnutrition can alter patients’ ability to think clearly, a phenomenon that some patients with anorexia mention as a reason they are grateful (in retrospect) for the compulsory treatment they received.10 Yet, if an evaluation shows that the patient has the decision-making capacity to refuse care, then her (his) caregivers should carefully document this conclusion and the basis for it. Although caregivers might encourage her to accept the treatment they believe she needs, they should not provide treatment that conflicts with their patient’s wishes.
If evaluation shows that the patient lacks capacity, however, the findings that support this conclusion should be documented clearly. The team then should consult the hospital attorney to determine how to best proceed. The attorney might recommend that a physician on the primary treatment team initiate a “medical hold”—an order that the patient may not leave against medical advice (AMA)—and then seek an emergency guardianship to permit medical treatment, such as refeeding.
To treat or not to treat?
What are the legal risks of allowing Ms. Q to leave AMA before she reaches medical stability?
Powers and Cloak11 describe a case of a 26-year-old woman with anorexia nervosa who came to the hospital with dizziness, weakness, and a very low blood glucose level. She was discharged after 6 days without having received any feeding, only to return to the emergency department 2 days later. This time, she had a letter from her physician stating that she needed medical supervision to start refeeding, yet she was discharged from the emergency department within a few hours. She was re-admitted to the hospital the next day.
Powers and Cloak11 do not report this woman’s medical outcome. But what if she had suffered a fatal cardiac arrhythmia before her third presentation to the emergency department or suffered another injury attributable to her nutritional state: Could her physicians be found at fault?
On Cohen & Associates’ Web site, they essentially answer, “Yes.” They describe a case of “Miss McIntosh,” who had anorexia nervosa and was discharged home from a hospital despite “chronic metabolic problems and not eating properly.” She went into a “hypoglycemic encephalopathic coma” and “suffered irreversible brain damage.” A subsequent lawsuit against the hospital resulted in a 7-figure settlement,12 illustrating the potential for adverse medicolegal consequences if failure to treat a patient with anorexia nervosa could be linked to subsequent physical harm. On the other hand, could a patient with anorexia who is being force-fed take legal action against her providers? At least 3 recent British cases suggest that this is possible.13-15 A British medical student with anorexia, E, made an emergency application to the Court of Protection in London, claiming that being fed against her will was akin to reliving her past experience of sexual abuse. In E’s case, the judge ruled “that the balance tips slowly but unmistakably in the direction of life preserving treatment” and authorized feeding over her objection.6 In 2 other cases, however, British courts have ruled that force-feeding anorexic patients would be futile and disallowed the practice.14,15
Faced with possible legal action, no matter what course you take, how should you respond? Getting legal and ethical consultation is prudent if time allows. In many cases, hospital attorneys might prefer that physicians err on the side of preserving life(D. Vanderpool, MBA, JD, personal communication, February 3, 2016)—even if that means detaining a patient without clear legal authorization to do so—because attorneys would prefer to defend a doctor who acted to save someone’s life than to defend a doctor who knowingly allowed a patient to die.
When might persons with an eating disorder be civilly committed?
Suppose that Ms. Q does not need urgent nonpsychiatric medical care, or that her life-threatening physical problems now have been addressed. Her physicians strongly recommend that she undergo inpatient psychiatric treatment for her eating disorder, but she wants to leave. Would it now be appropriate to fill out paperwork to initiate a psychiatric hold?
All U.S. jurisdictions authorize “civil commitment” proceedings that can lead to involuntary psychiatric hospitalization of people who have a mental disorder and pose a risk to themselves or others because of the disorder.16
In general, to be subject to civil commitment, a person must have a substantial disorder of thought, mood, perception, orientation, or memory. In addition, that disorder must grossly impair her (his) judgment, behavior, reality testing, or ability to meet the demands of everyday life.17
People with psychosis, a severe mood disorder, or dementia often meet these criteria. However, psychiatrists do not usually consider anorexia nervosa to be a thought disorder, mood disorder, or memory disorder. Does this mean that people with anorexia nervosa cannot meet the “substantial” mental disorder criterion?
It does not. Courts interpret the words in statutes based on their “ordinary and natural meaning.”18 If Ms. Q perceived herself as fat, despite having a BMI that was far below the healthy range, most people would regard her thinking to be disordered. If, in addition, her mental disorder impaired her “judgment, behavior, and capacity to meet the ordinary demands of sustaining existence,” then her anorexia nervosa “would qualify as a mental disorder for commitment purposes.”19
To be subject to civil commitment, a person with a substantial mental disorder also must pose a risk of harm to herself or others because of the disorder. That risk can be evidenced via an action, attempt, or threat to do direct physical harm, or it might inhere in the potential for developing grave disability through neglect of one’s basic needs, such as failing to eat adequately. In Ms. Q’s case, if the evidence shows her eating-disordered behavior has placed her at imminent risk of permanent injury or death, she has satisfied the legal criteria that justify court-ordered psychiatric hospitalization.
Bottom Line
When a severely malnourished patient with anorexia nervosa does not agree to allow recommended care, an appropriate clinical response should include judgment about the urgency of the proposed treatment, what treatment setting is best suited to the patient’s condition, and whether the patient has the mental capacity to refuse potentially life-saving care.
1. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495-1498.
2. Ohio Revised Code §5122.01(F).
3. Oregon Revised Statutes §426.005(c).
4. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (N1914).
5. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
6. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
7. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
8. Community tools: Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. http://www.jcb.utoronto.ca/tools/ace_download.shtml. Updated May 8, 2008. Accessed December 21, 2015.
9. Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry. 2003;26(6):697-707.
10. Elzakkers IF, Danner UN, Hoek HW, et al. Compulsory treatment in anorexia nervosa: a review. Int J Eat Disord. 2014;47(8):845-852.
11. Powers PS, Cloak NL. Failure to feed patients with anorexia nervosa and other perils and perplexities in the medical care of eating disorder patients. Eat Disord. 2013;21(1):81-89.
12. “Failure to properly treat anorexia nervosa.” Harry S. Cohen & Associates. http://medmal1.com/article/failure-to-properly-treat-anorexia-nervosa. Accessed February 1, 2016.
13. A Local Authority v E. and Others [2012] EWHC 1639 (COP).
14. A NHS Foundation Trust v Ms. X [2014] EWCOP 35.
15. NHS Trust v L [2012] EWHC 2741 (COP).
16. Pinals DA, Mossman D. Evaluation for civil commitment. New York, NY: Oxford University Press; 2011.
17. Castellano-Hoyt DW. Enhancing police response to persons in mental health crisis: providing strategies, communication techniques, and crisis intervention preparation in overcoming institutional challenges. Springfield, IL: Charles C. Thomas Publisher, Ltd; 2003.
18. FDIC v Meyer, 510 U.S. 471 (1994).
19. Appelbaum PS, Rumpf T. Civil commitment of the anorexic patient. Gen Hosp Psychiatry. 1998;20(4):225-230.
Dear Dr. Mossman,
At the general hospital where I perform consultations, the medical service asked me to fill out psychiatric “hold” documents to keep a severely malnourished young woman with anorexia nervosa from leaving the hospital. Ms. Q, whose body mass index (BMI) was 12 (yes, 12), came to the hospital to have her “electrolytes fixed.” She was willing to stay the night for electrolyte repletion, but insisted she could gain weight on her own at home.
I’m worried that she might die without prompt inpatient treatment; she needs to stay on the medical service. Should I fill out a psychiatric hold to keep her there? What legal risks could I face if Ms. Q is detained and force-fed against her will? What are the legal risks of letting her leave the hospital before she is medically stable?
Submitted by “Dr. F”
When a severely malnourished patient with an eating disorder arrives on a medical floor, treatment teams often ask psychiatric consultants to help them impose care the patient desperately needs but doesn’t want. This reaction is understandable. After all, an eating disorder is a psychiatric illness, and hospital-based psychiatrists have experience with treating involuntary patients. A psychiatric hold may seem like a sensible way to save the life of a hospitalized patient with a mental illness.
But filling out a psychiatric hold only scratches the surface of what a psychiatric consultant’s contribution should include; in Ms. Q’s case, initiating a psychiatric hold is probably the wrong thing to do.
Why would filling out a psychiatric hold be inappropriate for Ms. Q? What clinical factors and legal issues should a psychiatrist consider when helping medical colleagues provide unwanted treatment to a severely malnourished patient with an eating disorder? We’ll explore these matters as we consider the case of Ms. Q (Figure) and the following questions:
- What type of care is most appropriate for her now?
- Can she refuse medical treatment?
- What are the medicolegal risks of letting her leave the hospital?
- What are the medicolegal risks of detaining and force-feeding her against her will?
- When is a psychiatric “hold” appropriate?
What care is appropriate?
Given her state of self-starvation, Ms. Q’s treatment plan could require close monitoring of her electrolytes and cardiac status, as well as watching her for signs of “refeeding syndrome”—rapid, potentially fatal fluid shifts and metabolic derangements that malnourished patients could experience when they receive artificial refeeding.1
First, the physicians who are caring for Ms. Q should determine whether she needs more intensive medical supervision than is usually available on a psychiatric unit. If she does, but she won’t agree to stay on a medical unit for care, a psychiatric hold is the wrong step, for 2 reasons:
- Once a psychiatric hold has been executed, state statutes require the patient to be placed in a psychiatric facility—a state-approved psychiatric treatment setting, such as a psychiatric unit or free-standing psychiatric hospital—within a specified period.2,3 Most nonpsychiatric medical units would not meet state’s statutory definition for such a facility.
- A psychiatric hold only permits short-term detention. It does not provide legal authority to impose unwanted medical treatment.
Does Ms. Q have capacity?
In the United States, Ms. Q has a legal right to refuse medical care—even if she needs it urgently—provided that her refusal is made competently.4 As Appelbaum and Grisso5 explained in a now-classic 1988 article:
The legal standards for competence include the four related skills of communicating a choice, understanding relevant information, appreciating the current situation and its consequences, and manipulating information rationally.
The Table5 describes these abilities in more detail.
Only courts can make legal determinations of competence, so physicians refer to an evaluation of a patient’s competence-related abilities as a “capacity assessment.” The decision as to whether a patient has capacity ultimately rests with the primary treatment team; however, physicians in other specialties often enlist psychiatrists’ help with this matter because of their interviewing skills and knowledge of how mental illness can impair capacity.
No easy-to-use instrument for evaluating capacity is available. However, Appelbaum6 provides examples of questions that often prove useful in such assessments, and a review by Sessums et al7 on several capacity evaluation tools suggests that the Aid to Capacity Evaluation8 may be the best instrument for performing capacity assessments.
Patients with anorexia nervosa often differ substantially from healthy people in how they assign values to life and death,9 which can make it difficult to evaluate their capacity to refuse life-saving treatment. Malnutrition can alter patients’ ability to think clearly, a phenomenon that some patients with anorexia mention as a reason they are grateful (in retrospect) for the compulsory treatment they received.10 Yet, if an evaluation shows that the patient has the decision-making capacity to refuse care, then her (his) caregivers should carefully document this conclusion and the basis for it. Although caregivers might encourage her to accept the treatment they believe she needs, they should not provide treatment that conflicts with their patient’s wishes.
If evaluation shows that the patient lacks capacity, however, the findings that support this conclusion should be documented clearly. The team then should consult the hospital attorney to determine how to best proceed. The attorney might recommend that a physician on the primary treatment team initiate a “medical hold”—an order that the patient may not leave against medical advice (AMA)—and then seek an emergency guardianship to permit medical treatment, such as refeeding.
To treat or not to treat?
What are the legal risks of allowing Ms. Q to leave AMA before she reaches medical stability?
Powers and Cloak11 describe a case of a 26-year-old woman with anorexia nervosa who came to the hospital with dizziness, weakness, and a very low blood glucose level. She was discharged after 6 days without having received any feeding, only to return to the emergency department 2 days later. This time, she had a letter from her physician stating that she needed medical supervision to start refeeding, yet she was discharged from the emergency department within a few hours. She was re-admitted to the hospital the next day.
Powers and Cloak11 do not report this woman’s medical outcome. But what if she had suffered a fatal cardiac arrhythmia before her third presentation to the emergency department or suffered another injury attributable to her nutritional state: Could her physicians be found at fault?
On Cohen & Associates’ Web site, they essentially answer, “Yes.” They describe a case of “Miss McIntosh,” who had anorexia nervosa and was discharged home from a hospital despite “chronic metabolic problems and not eating properly.” She went into a “hypoglycemic encephalopathic coma” and “suffered irreversible brain damage.” A subsequent lawsuit against the hospital resulted in a 7-figure settlement,12 illustrating the potential for adverse medicolegal consequences if failure to treat a patient with anorexia nervosa could be linked to subsequent physical harm. On the other hand, could a patient with anorexia who is being force-fed take legal action against her providers? At least 3 recent British cases suggest that this is possible.13-15 A British medical student with anorexia, E, made an emergency application to the Court of Protection in London, claiming that being fed against her will was akin to reliving her past experience of sexual abuse. In E’s case, the judge ruled “that the balance tips slowly but unmistakably in the direction of life preserving treatment” and authorized feeding over her objection.6 In 2 other cases, however, British courts have ruled that force-feeding anorexic patients would be futile and disallowed the practice.14,15
Faced with possible legal action, no matter what course you take, how should you respond? Getting legal and ethical consultation is prudent if time allows. In many cases, hospital attorneys might prefer that physicians err on the side of preserving life(D. Vanderpool, MBA, JD, personal communication, February 3, 2016)—even if that means detaining a patient without clear legal authorization to do so—because attorneys would prefer to defend a doctor who acted to save someone’s life than to defend a doctor who knowingly allowed a patient to die.
When might persons with an eating disorder be civilly committed?
Suppose that Ms. Q does not need urgent nonpsychiatric medical care, or that her life-threatening physical problems now have been addressed. Her physicians strongly recommend that she undergo inpatient psychiatric treatment for her eating disorder, but she wants to leave. Would it now be appropriate to fill out paperwork to initiate a psychiatric hold?
All U.S. jurisdictions authorize “civil commitment” proceedings that can lead to involuntary psychiatric hospitalization of people who have a mental disorder and pose a risk to themselves or others because of the disorder.16
In general, to be subject to civil commitment, a person must have a substantial disorder of thought, mood, perception, orientation, or memory. In addition, that disorder must grossly impair her (his) judgment, behavior, reality testing, or ability to meet the demands of everyday life.17
People with psychosis, a severe mood disorder, or dementia often meet these criteria. However, psychiatrists do not usually consider anorexia nervosa to be a thought disorder, mood disorder, or memory disorder. Does this mean that people with anorexia nervosa cannot meet the “substantial” mental disorder criterion?
It does not. Courts interpret the words in statutes based on their “ordinary and natural meaning.”18 If Ms. Q perceived herself as fat, despite having a BMI that was far below the healthy range, most people would regard her thinking to be disordered. If, in addition, her mental disorder impaired her “judgment, behavior, and capacity to meet the ordinary demands of sustaining existence,” then her anorexia nervosa “would qualify as a mental disorder for commitment purposes.”19
To be subject to civil commitment, a person with a substantial mental disorder also must pose a risk of harm to herself or others because of the disorder. That risk can be evidenced via an action, attempt, or threat to do direct physical harm, or it might inhere in the potential for developing grave disability through neglect of one’s basic needs, such as failing to eat adequately. In Ms. Q’s case, if the evidence shows her eating-disordered behavior has placed her at imminent risk of permanent injury or death, she has satisfied the legal criteria that justify court-ordered psychiatric hospitalization.
Bottom Line
When a severely malnourished patient with anorexia nervosa does not agree to allow recommended care, an appropriate clinical response should include judgment about the urgency of the proposed treatment, what treatment setting is best suited to the patient’s condition, and whether the patient has the mental capacity to refuse potentially life-saving care.
Dear Dr. Mossman,
At the general hospital where I perform consultations, the medical service asked me to fill out psychiatric “hold” documents to keep a severely malnourished young woman with anorexia nervosa from leaving the hospital. Ms. Q, whose body mass index (BMI) was 12 (yes, 12), came to the hospital to have her “electrolytes fixed.” She was willing to stay the night for electrolyte repletion, but insisted she could gain weight on her own at home.
I’m worried that she might die without prompt inpatient treatment; she needs to stay on the medical service. Should I fill out a psychiatric hold to keep her there? What legal risks could I face if Ms. Q is detained and force-fed against her will? What are the legal risks of letting her leave the hospital before she is medically stable?
Submitted by “Dr. F”
When a severely malnourished patient with an eating disorder arrives on a medical floor, treatment teams often ask psychiatric consultants to help them impose care the patient desperately needs but doesn’t want. This reaction is understandable. After all, an eating disorder is a psychiatric illness, and hospital-based psychiatrists have experience with treating involuntary patients. A psychiatric hold may seem like a sensible way to save the life of a hospitalized patient with a mental illness.
But filling out a psychiatric hold only scratches the surface of what a psychiatric consultant’s contribution should include; in Ms. Q’s case, initiating a psychiatric hold is probably the wrong thing to do.
Why would filling out a psychiatric hold be inappropriate for Ms. Q? What clinical factors and legal issues should a psychiatrist consider when helping medical colleagues provide unwanted treatment to a severely malnourished patient with an eating disorder? We’ll explore these matters as we consider the case of Ms. Q (Figure) and the following questions:
- What type of care is most appropriate for her now?
- Can she refuse medical treatment?
- What are the medicolegal risks of letting her leave the hospital?
- What are the medicolegal risks of detaining and force-feeding her against her will?
- When is a psychiatric “hold” appropriate?
What care is appropriate?
Given her state of self-starvation, Ms. Q’s treatment plan could require close monitoring of her electrolytes and cardiac status, as well as watching her for signs of “refeeding syndrome”—rapid, potentially fatal fluid shifts and metabolic derangements that malnourished patients could experience when they receive artificial refeeding.1
First, the physicians who are caring for Ms. Q should determine whether she needs more intensive medical supervision than is usually available on a psychiatric unit. If she does, but she won’t agree to stay on a medical unit for care, a psychiatric hold is the wrong step, for 2 reasons:
- Once a psychiatric hold has been executed, state statutes require the patient to be placed in a psychiatric facility—a state-approved psychiatric treatment setting, such as a psychiatric unit or free-standing psychiatric hospital—within a specified period.2,3 Most nonpsychiatric medical units would not meet state’s statutory definition for such a facility.
- A psychiatric hold only permits short-term detention. It does not provide legal authority to impose unwanted medical treatment.
Does Ms. Q have capacity?
In the United States, Ms. Q has a legal right to refuse medical care—even if she needs it urgently—provided that her refusal is made competently.4 As Appelbaum and Grisso5 explained in a now-classic 1988 article:
The legal standards for competence include the four related skills of communicating a choice, understanding relevant information, appreciating the current situation and its consequences, and manipulating information rationally.
The Table5 describes these abilities in more detail.
Only courts can make legal determinations of competence, so physicians refer to an evaluation of a patient’s competence-related abilities as a “capacity assessment.” The decision as to whether a patient has capacity ultimately rests with the primary treatment team; however, physicians in other specialties often enlist psychiatrists’ help with this matter because of their interviewing skills and knowledge of how mental illness can impair capacity.
No easy-to-use instrument for evaluating capacity is available. However, Appelbaum6 provides examples of questions that often prove useful in such assessments, and a review by Sessums et al7 on several capacity evaluation tools suggests that the Aid to Capacity Evaluation8 may be the best instrument for performing capacity assessments.
Patients with anorexia nervosa often differ substantially from healthy people in how they assign values to life and death,9 which can make it difficult to evaluate their capacity to refuse life-saving treatment. Malnutrition can alter patients’ ability to think clearly, a phenomenon that some patients with anorexia mention as a reason they are grateful (in retrospect) for the compulsory treatment they received.10 Yet, if an evaluation shows that the patient has the decision-making capacity to refuse care, then her (his) caregivers should carefully document this conclusion and the basis for it. Although caregivers might encourage her to accept the treatment they believe she needs, they should not provide treatment that conflicts with their patient’s wishes.
If evaluation shows that the patient lacks capacity, however, the findings that support this conclusion should be documented clearly. The team then should consult the hospital attorney to determine how to best proceed. The attorney might recommend that a physician on the primary treatment team initiate a “medical hold”—an order that the patient may not leave against medical advice (AMA)—and then seek an emergency guardianship to permit medical treatment, such as refeeding.
To treat or not to treat?
What are the legal risks of allowing Ms. Q to leave AMA before she reaches medical stability?
Powers and Cloak11 describe a case of a 26-year-old woman with anorexia nervosa who came to the hospital with dizziness, weakness, and a very low blood glucose level. She was discharged after 6 days without having received any feeding, only to return to the emergency department 2 days later. This time, she had a letter from her physician stating that she needed medical supervision to start refeeding, yet she was discharged from the emergency department within a few hours. She was re-admitted to the hospital the next day.
Powers and Cloak11 do not report this woman’s medical outcome. But what if she had suffered a fatal cardiac arrhythmia before her third presentation to the emergency department or suffered another injury attributable to her nutritional state: Could her physicians be found at fault?
On Cohen & Associates’ Web site, they essentially answer, “Yes.” They describe a case of “Miss McIntosh,” who had anorexia nervosa and was discharged home from a hospital despite “chronic metabolic problems and not eating properly.” She went into a “hypoglycemic encephalopathic coma” and “suffered irreversible brain damage.” A subsequent lawsuit against the hospital resulted in a 7-figure settlement,12 illustrating the potential for adverse medicolegal consequences if failure to treat a patient with anorexia nervosa could be linked to subsequent physical harm. On the other hand, could a patient with anorexia who is being force-fed take legal action against her providers? At least 3 recent British cases suggest that this is possible.13-15 A British medical student with anorexia, E, made an emergency application to the Court of Protection in London, claiming that being fed against her will was akin to reliving her past experience of sexual abuse. In E’s case, the judge ruled “that the balance tips slowly but unmistakably in the direction of life preserving treatment” and authorized feeding over her objection.6 In 2 other cases, however, British courts have ruled that force-feeding anorexic patients would be futile and disallowed the practice.14,15
Faced with possible legal action, no matter what course you take, how should you respond? Getting legal and ethical consultation is prudent if time allows. In many cases, hospital attorneys might prefer that physicians err on the side of preserving life(D. Vanderpool, MBA, JD, personal communication, February 3, 2016)—even if that means detaining a patient without clear legal authorization to do so—because attorneys would prefer to defend a doctor who acted to save someone’s life than to defend a doctor who knowingly allowed a patient to die.
When might persons with an eating disorder be civilly committed?
Suppose that Ms. Q does not need urgent nonpsychiatric medical care, or that her life-threatening physical problems now have been addressed. Her physicians strongly recommend that she undergo inpatient psychiatric treatment for her eating disorder, but she wants to leave. Would it now be appropriate to fill out paperwork to initiate a psychiatric hold?
All U.S. jurisdictions authorize “civil commitment” proceedings that can lead to involuntary psychiatric hospitalization of people who have a mental disorder and pose a risk to themselves or others because of the disorder.16
In general, to be subject to civil commitment, a person must have a substantial disorder of thought, mood, perception, orientation, or memory. In addition, that disorder must grossly impair her (his) judgment, behavior, reality testing, or ability to meet the demands of everyday life.17
People with psychosis, a severe mood disorder, or dementia often meet these criteria. However, psychiatrists do not usually consider anorexia nervosa to be a thought disorder, mood disorder, or memory disorder. Does this mean that people with anorexia nervosa cannot meet the “substantial” mental disorder criterion?
It does not. Courts interpret the words in statutes based on their “ordinary and natural meaning.”18 If Ms. Q perceived herself as fat, despite having a BMI that was far below the healthy range, most people would regard her thinking to be disordered. If, in addition, her mental disorder impaired her “judgment, behavior, and capacity to meet the ordinary demands of sustaining existence,” then her anorexia nervosa “would qualify as a mental disorder for commitment purposes.”19
To be subject to civil commitment, a person with a substantial mental disorder also must pose a risk of harm to herself or others because of the disorder. That risk can be evidenced via an action, attempt, or threat to do direct physical harm, or it might inhere in the potential for developing grave disability through neglect of one’s basic needs, such as failing to eat adequately. In Ms. Q’s case, if the evidence shows her eating-disordered behavior has placed her at imminent risk of permanent injury or death, she has satisfied the legal criteria that justify court-ordered psychiatric hospitalization.
Bottom Line
When a severely malnourished patient with anorexia nervosa does not agree to allow recommended care, an appropriate clinical response should include judgment about the urgency of the proposed treatment, what treatment setting is best suited to the patient’s condition, and whether the patient has the mental capacity to refuse potentially life-saving care.
1. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495-1498.
2. Ohio Revised Code §5122.01(F).
3. Oregon Revised Statutes §426.005(c).
4. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (N1914).
5. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
6. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
7. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
8. Community tools: Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. http://www.jcb.utoronto.ca/tools/ace_download.shtml. Updated May 8, 2008. Accessed December 21, 2015.
9. Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry. 2003;26(6):697-707.
10. Elzakkers IF, Danner UN, Hoek HW, et al. Compulsory treatment in anorexia nervosa: a review. Int J Eat Disord. 2014;47(8):845-852.
11. Powers PS, Cloak NL. Failure to feed patients with anorexia nervosa and other perils and perplexities in the medical care of eating disorder patients. Eat Disord. 2013;21(1):81-89.
12. “Failure to properly treat anorexia nervosa.” Harry S. Cohen & Associates. http://medmal1.com/article/failure-to-properly-treat-anorexia-nervosa. Accessed February 1, 2016.
13. A Local Authority v E. and Others [2012] EWHC 1639 (COP).
14. A NHS Foundation Trust v Ms. X [2014] EWCOP 35.
15. NHS Trust v L [2012] EWHC 2741 (COP).
16. Pinals DA, Mossman D. Evaluation for civil commitment. New York, NY: Oxford University Press; 2011.
17. Castellano-Hoyt DW. Enhancing police response to persons in mental health crisis: providing strategies, communication techniques, and crisis intervention preparation in overcoming institutional challenges. Springfield, IL: Charles C. Thomas Publisher, Ltd; 2003.
18. FDIC v Meyer, 510 U.S. 471 (1994).
19. Appelbaum PS, Rumpf T. Civil commitment of the anorexic patient. Gen Hosp Psychiatry. 1998;20(4):225-230.
1. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495-1498.
2. Ohio Revised Code §5122.01(F).
3. Oregon Revised Statutes §426.005(c).
4. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (N1914).
5. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
6. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
7. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
8. Community tools: Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. http://www.jcb.utoronto.ca/tools/ace_download.shtml. Updated May 8, 2008. Accessed December 21, 2015.
9. Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry. 2003;26(6):697-707.
10. Elzakkers IF, Danner UN, Hoek HW, et al. Compulsory treatment in anorexia nervosa: a review. Int J Eat Disord. 2014;47(8):845-852.
11. Powers PS, Cloak NL. Failure to feed patients with anorexia nervosa and other perils and perplexities in the medical care of eating disorder patients. Eat Disord. 2013;21(1):81-89.
12. “Failure to properly treat anorexia nervosa.” Harry S. Cohen & Associates. http://medmal1.com/article/failure-to-properly-treat-anorexia-nervosa. Accessed February 1, 2016.
13. A Local Authority v E. and Others [2012] EWHC 1639 (COP).
14. A NHS Foundation Trust v Ms. X [2014] EWCOP 35.
15. NHS Trust v L [2012] EWHC 2741 (COP).
16. Pinals DA, Mossman D. Evaluation for civil commitment. New York, NY: Oxford University Press; 2011.
17. Castellano-Hoyt DW. Enhancing police response to persons in mental health crisis: providing strategies, communication techniques, and crisis intervention preparation in overcoming institutional challenges. Springfield, IL: Charles C. Thomas Publisher, Ltd; 2003.
18. FDIC v Meyer, 510 U.S. 471 (1994).
19. Appelbaum PS, Rumpf T. Civil commitment of the anorexic patient. Gen Hosp Psychiatry. 1998;20(4):225-230.
VIDEO: Progressive MS trial failures provide lessons for future success
NEW ORLEANS – Findings last year from the ORATORIO trial showed for the first time that a pharmaceutical agent – ocrelizumab – was effective for slowing the rate of progression in patients with primary progressive multiple sclerosis, but there is as much to learn from the many failed trials and treatments as from this recent success, according to experts at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Insofar as we have seen failure after failure in studying progressive MS, we’ve also learned from the studies themselves how best to redesign the studies and how to target the right kind of patients to be able to see a therapeutic effect once that appropriate drug came along,” Dr. John Rinker II said in an interview at the meeting sponsored by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Rinker of the University of Alabama at Birmingham chaired a session on “the treatment pipeline” in MS, and noted in the interview that the lessons learned from failed trials could potentially be used to “re-look at some of these older drugs that had been failures in the past and, using a different trial methodology, maybe find some success in the future.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Findings last year from the ORATORIO trial showed for the first time that a pharmaceutical agent – ocrelizumab – was effective for slowing the rate of progression in patients with primary progressive multiple sclerosis, but there is as much to learn from the many failed trials and treatments as from this recent success, according to experts at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Insofar as we have seen failure after failure in studying progressive MS, we’ve also learned from the studies themselves how best to redesign the studies and how to target the right kind of patients to be able to see a therapeutic effect once that appropriate drug came along,” Dr. John Rinker II said in an interview at the meeting sponsored by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Rinker of the University of Alabama at Birmingham chaired a session on “the treatment pipeline” in MS, and noted in the interview that the lessons learned from failed trials could potentially be used to “re-look at some of these older drugs that had been failures in the past and, using a different trial methodology, maybe find some success in the future.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Findings last year from the ORATORIO trial showed for the first time that a pharmaceutical agent – ocrelizumab – was effective for slowing the rate of progression in patients with primary progressive multiple sclerosis, but there is as much to learn from the many failed trials and treatments as from this recent success, according to experts at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Insofar as we have seen failure after failure in studying progressive MS, we’ve also learned from the studies themselves how best to redesign the studies and how to target the right kind of patients to be able to see a therapeutic effect once that appropriate drug came along,” Dr. John Rinker II said in an interview at the meeting sponsored by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Rinker of the University of Alabama at Birmingham chaired a session on “the treatment pipeline” in MS, and noted in the interview that the lessons learned from failed trials could potentially be used to “re-look at some of these older drugs that had been failures in the past and, using a different trial methodology, maybe find some success in the future.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACTRIMS Forum 2016
A tool to assess behavioral problems in neurocognitive disorder and guide treatment
Non-drug treatment options, such as behavioral techniques and environment adjustment, should be considered before initiating pharmacotherapy in older patients with behavioral deregulation caused by a neurocognitive disorder. Before considering any interventions, including medical therapy, an evaluation and development of a profile of behavioral symptoms is warranted.
The purpose of such a profile is to:
- guide a patient-specific treatment plan
- measure treatment response (whether medication-related or otherwise).
Developing a profile for a patient can lead to a more tailored treatment plan. Such a plan includes identification of mitigating factors for the patient’s behavior and use of specific interventions, with a preference for non-medication interventions.
Profile assessment can guide treatment
The disruptive-behavior profile that I created (Table) can be used as an initial screening device; the score (1 through 4) in each domain indicates the intensity of intervention required. The profile also can be used to evaluate treatment response.
For example, when caring for a person with a neurocognitive disorder with agitation and disruptive behavior, this profile can be used by the caregiver as a reporting tool for the behavioral heath professional providing consultation. Based on this report, the behavioral health professional can evaluate the predisposing, precipitating, and perpetuating factors of the behavioral disturbance, and a treatment plan can be implemented. After interventions are applied, follow-up assessment with the tool can assess the response to the intervention.
The scale can aid in averting overuse of non-specific medication therapy and, if required, can guide pharmacotherapy. This assessment tool can be useful for clinicians providing care for patients with a neurocognitive disorder, not only in choosing treatment, but also to justify clinical rationale.
How does this scale compare with others?
The Neuropsychiatric Inventory (NPI), Behavioral Pathology in Alzheimer’s Disease (Behave-AD), Cohen-Mansfield Agitation Inventory, and Brief Agitation Rating Scale provide valuable information for clinical care. However:
- Use of the NPI in everyday practice is limited; time spent completing the NPI scale remains a significant impediment for the busy clinician.
- Behave-AD requires a higher level of skill for some caregivers to estimate behavioral symptoms and answer questions about severity.
- Cohen-Mansfeld and Brief Agitation Rating Scale provide a limited description of the intensity of behavioral disturbance.
Developing a treatment plan and justifying pharmacotherapy in patients with a neurocognitive disorder is a challenge for clinicians. The scale that I developed aims to (1) assist the busy clinician who must construct a targeted treatment plan and (2) avoid pharmacotherapy when it is unnecessary. If pharmacotherapy is warranted on the basis of any of the domain scores in the profile, it should be documented with a judicious rationale.
Non-drug treatment options, such as behavioral techniques and environment adjustment, should be considered before initiating pharmacotherapy in older patients with behavioral deregulation caused by a neurocognitive disorder. Before considering any interventions, including medical therapy, an evaluation and development of a profile of behavioral symptoms is warranted.
The purpose of such a profile is to:
- guide a patient-specific treatment plan
- measure treatment response (whether medication-related or otherwise).
Developing a profile for a patient can lead to a more tailored treatment plan. Such a plan includes identification of mitigating factors for the patient’s behavior and use of specific interventions, with a preference for non-medication interventions.
Profile assessment can guide treatment
The disruptive-behavior profile that I created (Table) can be used as an initial screening device; the score (1 through 4) in each domain indicates the intensity of intervention required. The profile also can be used to evaluate treatment response.
For example, when caring for a person with a neurocognitive disorder with agitation and disruptive behavior, this profile can be used by the caregiver as a reporting tool for the behavioral heath professional providing consultation. Based on this report, the behavioral health professional can evaluate the predisposing, precipitating, and perpetuating factors of the behavioral disturbance, and a treatment plan can be implemented. After interventions are applied, follow-up assessment with the tool can assess the response to the intervention.
The scale can aid in averting overuse of non-specific medication therapy and, if required, can guide pharmacotherapy. This assessment tool can be useful for clinicians providing care for patients with a neurocognitive disorder, not only in choosing treatment, but also to justify clinical rationale.
How does this scale compare with others?
The Neuropsychiatric Inventory (NPI), Behavioral Pathology in Alzheimer’s Disease (Behave-AD), Cohen-Mansfield Agitation Inventory, and Brief Agitation Rating Scale provide valuable information for clinical care. However:
- Use of the NPI in everyday practice is limited; time spent completing the NPI scale remains a significant impediment for the busy clinician.
- Behave-AD requires a higher level of skill for some caregivers to estimate behavioral symptoms and answer questions about severity.
- Cohen-Mansfeld and Brief Agitation Rating Scale provide a limited description of the intensity of behavioral disturbance.
Developing a treatment plan and justifying pharmacotherapy in patients with a neurocognitive disorder is a challenge for clinicians. The scale that I developed aims to (1) assist the busy clinician who must construct a targeted treatment plan and (2) avoid pharmacotherapy when it is unnecessary. If pharmacotherapy is warranted on the basis of any of the domain scores in the profile, it should be documented with a judicious rationale.
Non-drug treatment options, such as behavioral techniques and environment adjustment, should be considered before initiating pharmacotherapy in older patients with behavioral deregulation caused by a neurocognitive disorder. Before considering any interventions, including medical therapy, an evaluation and development of a profile of behavioral symptoms is warranted.
The purpose of such a profile is to:
- guide a patient-specific treatment plan
- measure treatment response (whether medication-related or otherwise).
Developing a profile for a patient can lead to a more tailored treatment plan. Such a plan includes identification of mitigating factors for the patient’s behavior and use of specific interventions, with a preference for non-medication interventions.
Profile assessment can guide treatment
The disruptive-behavior profile that I created (Table) can be used as an initial screening device; the score (1 through 4) in each domain indicates the intensity of intervention required. The profile also can be used to evaluate treatment response.
For example, when caring for a person with a neurocognitive disorder with agitation and disruptive behavior, this profile can be used by the caregiver as a reporting tool for the behavioral heath professional providing consultation. Based on this report, the behavioral health professional can evaluate the predisposing, precipitating, and perpetuating factors of the behavioral disturbance, and a treatment plan can be implemented. After interventions are applied, follow-up assessment with the tool can assess the response to the intervention.
The scale can aid in averting overuse of non-specific medication therapy and, if required, can guide pharmacotherapy. This assessment tool can be useful for clinicians providing care for patients with a neurocognitive disorder, not only in choosing treatment, but also to justify clinical rationale.
How does this scale compare with others?
The Neuropsychiatric Inventory (NPI), Behavioral Pathology in Alzheimer’s Disease (Behave-AD), Cohen-Mansfield Agitation Inventory, and Brief Agitation Rating Scale provide valuable information for clinical care. However:
- Use of the NPI in everyday practice is limited; time spent completing the NPI scale remains a significant impediment for the busy clinician.
- Behave-AD requires a higher level of skill for some caregivers to estimate behavioral symptoms and answer questions about severity.
- Cohen-Mansfeld and Brief Agitation Rating Scale provide a limited description of the intensity of behavioral disturbance.
Developing a treatment plan and justifying pharmacotherapy in patients with a neurocognitive disorder is a challenge for clinicians. The scale that I developed aims to (1) assist the busy clinician who must construct a targeted treatment plan and (2) avoid pharmacotherapy when it is unnecessary. If pharmacotherapy is warranted on the basis of any of the domain scores in the profile, it should be documented with a judicious rationale.