User login
Survey sheds light on clinical neurophysiology fellowship training
HOUSTON – Fellowship training in clinical neurophysiology delivered high rates of satisfaction, but recommended areas for improvements include more focus on training in sleep, brain mapping, and evoked potentials, results from a survey of current trainees found.
“There has been no systematic evaluation of neurophysiology fellowships,” Zulfi Haneef, MD, said in an interview in advance of the annual meeting of the American Epilepsy Society. “In fact, there are some studies on neurology residency, but not on any of the neurology fellowships. This study opens a window into what the situation is after residency: what makes the residents decide on a fellowship, and how they feel they have done with the choice of fellowship.”
Overall, 87% of respondents expressed satisfaction with their current program and rated it as 4 or a 5 on a 1-5 Likert scale. “It seems that choosing a program based on the location may not have been the best thing to do,” he said. “Satisfaction scores (on a 1-5 scale for the training) were lower among those who chose a program based on location.” Less time spent in the epilepsy monitoring unit and EEG monitoring was also associated with higher satisfaction scores. “Rather than a lack of interest in epilepsy monitoring and EEG, this may reflect overemphasis on these at the expense of other areas, as these were also the areas that appeared to be most stressed during training,” Dr. Haneef said. The researchers observed no differences between male and female respondents in their answers to the various survey questions.
Based on the survey results, better clinical neurophysiology training in some areas were deemed necessary by the responding fellows, including sleep, brain mapping, and evoked potentials. Dr. Haneef acknowledged certain limitations of the study, including the fact that it was voluntary. “As such there could be some self-selection of fellows who have stronger viewpoints that pushed them to respond to a survey,” he said. He reported having no financial disclosures related to the study.
HOUSTON – Fellowship training in clinical neurophysiology delivered high rates of satisfaction, but recommended areas for improvements include more focus on training in sleep, brain mapping, and evoked potentials, results from a survey of current trainees found.
“There has been no systematic evaluation of neurophysiology fellowships,” Zulfi Haneef, MD, said in an interview in advance of the annual meeting of the American Epilepsy Society. “In fact, there are some studies on neurology residency, but not on any of the neurology fellowships. This study opens a window into what the situation is after residency: what makes the residents decide on a fellowship, and how they feel they have done with the choice of fellowship.”
Overall, 87% of respondents expressed satisfaction with their current program and rated it as 4 or a 5 on a 1-5 Likert scale. “It seems that choosing a program based on the location may not have been the best thing to do,” he said. “Satisfaction scores (on a 1-5 scale for the training) were lower among those who chose a program based on location.” Less time spent in the epilepsy monitoring unit and EEG monitoring was also associated with higher satisfaction scores. “Rather than a lack of interest in epilepsy monitoring and EEG, this may reflect overemphasis on these at the expense of other areas, as these were also the areas that appeared to be most stressed during training,” Dr. Haneef said. The researchers observed no differences between male and female respondents in their answers to the various survey questions.
Based on the survey results, better clinical neurophysiology training in some areas were deemed necessary by the responding fellows, including sleep, brain mapping, and evoked potentials. Dr. Haneef acknowledged certain limitations of the study, including the fact that it was voluntary. “As such there could be some self-selection of fellows who have stronger viewpoints that pushed them to respond to a survey,” he said. He reported having no financial disclosures related to the study.
HOUSTON – Fellowship training in clinical neurophysiology delivered high rates of satisfaction, but recommended areas for improvements include more focus on training in sleep, brain mapping, and evoked potentials, results from a survey of current trainees found.
“There has been no systematic evaluation of neurophysiology fellowships,” Zulfi Haneef, MD, said in an interview in advance of the annual meeting of the American Epilepsy Society. “In fact, there are some studies on neurology residency, but not on any of the neurology fellowships. This study opens a window into what the situation is after residency: what makes the residents decide on a fellowship, and how they feel they have done with the choice of fellowship.”
Overall, 87% of respondents expressed satisfaction with their current program and rated it as 4 or a 5 on a 1-5 Likert scale. “It seems that choosing a program based on the location may not have been the best thing to do,” he said. “Satisfaction scores (on a 1-5 scale for the training) were lower among those who chose a program based on location.” Less time spent in the epilepsy monitoring unit and EEG monitoring was also associated with higher satisfaction scores. “Rather than a lack of interest in epilepsy monitoring and EEG, this may reflect overemphasis on these at the expense of other areas, as these were also the areas that appeared to be most stressed during training,” Dr. Haneef said. The researchers observed no differences between male and female respondents in their answers to the various survey questions.
Based on the survey results, better clinical neurophysiology training in some areas were deemed necessary by the responding fellows, including sleep, brain mapping, and evoked potentials. Dr. Haneef acknowledged certain limitations of the study, including the fact that it was voluntary. “As such there could be some self-selection of fellows who have stronger viewpoints that pushed them to respond to a survey,” he said. He reported having no financial disclosures related to the study.
AT AES 2016
Key clinical point:
Major finding: Overall, 87% of clinical neurophysiology fellows expressed satisfaction with their current program and rated it as 4 or a 5 on a 1-5 Likert scale.
Data source: An Internet-based survey of 49 clinical neurophysiology fellows in the United States.
Disclosures: Dr. Haneef reported having no financial disclosures related to the study.
VIDEO: Artificial blood cells clear first phase of animal testing
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Halving the TKI dose safe, cost effective in CML patients with stable remissions
SAN DIEGO – For some chronic myeloid leukemia patients with solid, stable remissions, halving their dose of a tyrosine kinase inhibitor – or even stopping therapy altogether, at least temporarily – appears to be safe and to offer both health and financial benefits, European investigators said at the annual meeting of the American Society of Hematology.
In the British De-escalation and Stopping Treatment of Imatinib, Nilotinib, or Sprycel [dasatinib], or Destiny Study, a total of 12 molecular relapses occurred between the second and twelfth month of dose reduction among 174 patients with either an MR3 or MR4 molecular response, and all 12 patients had restoration of molecular remissions after resumption of full dose TKIs, reported co-investigator Mhairi Copland, MD, PhD, from the University of Glasgow, Scotland.
“What we wanted to explore in the Destiny study is cutting the dose of tyrosine kinase inhibitor therapy in CML by half, followed by stopping therapy not just in patients with undetectable disease but also with stable low levels of disease,” Dr. Copland said during a briefing at the meeting.
“We hypothesized that more patients would be able to reduce therapy safely, and a proportion of these would be able to go on to stop therapy; also, that the patients on half-dose therapy would have reduced amount of side effects compared to those on full-dose therapy,” she added.
Several recent studies, including the EURO-SKI trial, have shown that it is safe to stop TKI therapy in those patients who are optimally responding and have undetectable levels of the BCR-ABL transcript.
Rendezvous with Destiny
In Destiny, the investigators enrolled patients with “good, but not perfect” molecular responses: MR3 or better, defined as a minimum of 3 consecutive tests each with greater than 10,000 ABL control transcripts following a minimum of 3 years on a TKI at standard prescribed doses. The median overall duration of TKI therapy was 7 years.
Participants on imatinib had their daily doses reduced to 200 mg, those on nilotinib had their doses cut back to 200 mg twice daily, and those on dasatinib had their quotidian doses halved to 50 mg.
After 12 months of half-dose therapy, molecular recurrence, defined as a loss of MR3 on two consecutive samples, was detected in 9 of 49 patients (18.4%) with MR3 but not MR4 remissions, compared with 3 of 125 patients (2.4%) with MR4 or better remissions (P less than .001).
The median time to relapse was 4.4 months among MR3/not 4 patients vs. 8.7 months for MR4 or better patients.
The probability of molecular recurrence on dose reduction was unrelated to either age, sex, performance status, type of TKI, or the duration of TKI therapy (median 7 years overall).
No patients experienced either progression to advanced phase disease or loss of cytogenetic response. During the course of follow-up, one patient died, and there were 15 serious adverse events, but these were determined to be unrelated to either CML or TKI treatment.
All 12 patients who experienced molecular recurrence regained MR3 within 4 months of resuming TKI therapy at the full dose.
As noted before, patient-reported side effects such as lethargy, diarrhea, rash, nausea, periorbital edema, and hair thinning decreased during the first 3 months of de-escalation, but not thereafter. Dr. Copland said that patients had generally good quality-of-life scores at study entry, suggesting that they were likely not especially bothered by TKI side effects in the first place.
The investigators calculated that for the 174 patients, halving treatment would save an estimated £1,943,364 ($2,474,679) from an expected TKI budget of £4,156,969 ($5,293,484), a savings of 46.7%. Estimated savings were similar for patients with MR4 or better alone (47.7%) and for those with a major molecular response (44.2%).
EURO-SKI Update
Also at ASH 2016, Francois-Xavier Mahon, MD, PhD, from the University of Bordeaux, France, reported additional follow-up data from the EURO-SKI trial, results of which were first reported at the 2016 annual meeting of the European Hematology Association in Copenhagen.
The investigators found that 50% of 755 assessable patients with CML were free of molecular recurrence at 24 months, as were 47% at 36 months.
As reported previously, patients who had been on a TKI for more than 5.8 years before attempting to stop had a lower rate of relapse (34.5%) than patients who had been on therapy for less than 5.8 years (57.4%). Each additional year of TKI therapy was associated with an approximately 16% better chance of successful TKI cessation.
“With inclusion and relapse criteria less strict than in many previous trials, and with decentralized but standardized PCR monitoring, stopping of TKI therapy in a large cohort of CML patients appears feasible and safe,” Dr. Mahon said at the briefing.
The British Destiny Study was supported by Newcastle University. Dr. Copland reported honoraria, advisory board memberships, and/or research funding from Amgen, Pfizer, Shire, BMS, and Ariad.
EURO-SKI was sponsored by the European LeukemiaNet. Dr. Mahon has previously disclosed being on the scientific advisory board and receiving honoraria from Novartis Oncology and BMS, and serving as consultant to those companies and to Pfizer.
SAN DIEGO – For some chronic myeloid leukemia patients with solid, stable remissions, halving their dose of a tyrosine kinase inhibitor – or even stopping therapy altogether, at least temporarily – appears to be safe and to offer both health and financial benefits, European investigators said at the annual meeting of the American Society of Hematology.
In the British De-escalation and Stopping Treatment of Imatinib, Nilotinib, or Sprycel [dasatinib], or Destiny Study, a total of 12 molecular relapses occurred between the second and twelfth month of dose reduction among 174 patients with either an MR3 or MR4 molecular response, and all 12 patients had restoration of molecular remissions after resumption of full dose TKIs, reported co-investigator Mhairi Copland, MD, PhD, from the University of Glasgow, Scotland.
“What we wanted to explore in the Destiny study is cutting the dose of tyrosine kinase inhibitor therapy in CML by half, followed by stopping therapy not just in patients with undetectable disease but also with stable low levels of disease,” Dr. Copland said during a briefing at the meeting.
“We hypothesized that more patients would be able to reduce therapy safely, and a proportion of these would be able to go on to stop therapy; also, that the patients on half-dose therapy would have reduced amount of side effects compared to those on full-dose therapy,” she added.
Several recent studies, including the EURO-SKI trial, have shown that it is safe to stop TKI therapy in those patients who are optimally responding and have undetectable levels of the BCR-ABL transcript.
Rendezvous with Destiny
In Destiny, the investigators enrolled patients with “good, but not perfect” molecular responses: MR3 or better, defined as a minimum of 3 consecutive tests each with greater than 10,000 ABL control transcripts following a minimum of 3 years on a TKI at standard prescribed doses. The median overall duration of TKI therapy was 7 years.
Participants on imatinib had their daily doses reduced to 200 mg, those on nilotinib had their doses cut back to 200 mg twice daily, and those on dasatinib had their quotidian doses halved to 50 mg.
After 12 months of half-dose therapy, molecular recurrence, defined as a loss of MR3 on two consecutive samples, was detected in 9 of 49 patients (18.4%) with MR3 but not MR4 remissions, compared with 3 of 125 patients (2.4%) with MR4 or better remissions (P less than .001).
The median time to relapse was 4.4 months among MR3/not 4 patients vs. 8.7 months for MR4 or better patients.
The probability of molecular recurrence on dose reduction was unrelated to either age, sex, performance status, type of TKI, or the duration of TKI therapy (median 7 years overall).
No patients experienced either progression to advanced phase disease or loss of cytogenetic response. During the course of follow-up, one patient died, and there were 15 serious adverse events, but these were determined to be unrelated to either CML or TKI treatment.
All 12 patients who experienced molecular recurrence regained MR3 within 4 months of resuming TKI therapy at the full dose.
As noted before, patient-reported side effects such as lethargy, diarrhea, rash, nausea, periorbital edema, and hair thinning decreased during the first 3 months of de-escalation, but not thereafter. Dr. Copland said that patients had generally good quality-of-life scores at study entry, suggesting that they were likely not especially bothered by TKI side effects in the first place.
The investigators calculated that for the 174 patients, halving treatment would save an estimated £1,943,364 ($2,474,679) from an expected TKI budget of £4,156,969 ($5,293,484), a savings of 46.7%. Estimated savings were similar for patients with MR4 or better alone (47.7%) and for those with a major molecular response (44.2%).
EURO-SKI Update
Also at ASH 2016, Francois-Xavier Mahon, MD, PhD, from the University of Bordeaux, France, reported additional follow-up data from the EURO-SKI trial, results of which were first reported at the 2016 annual meeting of the European Hematology Association in Copenhagen.
The investigators found that 50% of 755 assessable patients with CML were free of molecular recurrence at 24 months, as were 47% at 36 months.
As reported previously, patients who had been on a TKI for more than 5.8 years before attempting to stop had a lower rate of relapse (34.5%) than patients who had been on therapy for less than 5.8 years (57.4%). Each additional year of TKI therapy was associated with an approximately 16% better chance of successful TKI cessation.
“With inclusion and relapse criteria less strict than in many previous trials, and with decentralized but standardized PCR monitoring, stopping of TKI therapy in a large cohort of CML patients appears feasible and safe,” Dr. Mahon said at the briefing.
The British Destiny Study was supported by Newcastle University. Dr. Copland reported honoraria, advisory board memberships, and/or research funding from Amgen, Pfizer, Shire, BMS, and Ariad.
EURO-SKI was sponsored by the European LeukemiaNet. Dr. Mahon has previously disclosed being on the scientific advisory board and receiving honoraria from Novartis Oncology and BMS, and serving as consultant to those companies and to Pfizer.
SAN DIEGO – For some chronic myeloid leukemia patients with solid, stable remissions, halving their dose of a tyrosine kinase inhibitor – or even stopping therapy altogether, at least temporarily – appears to be safe and to offer both health and financial benefits, European investigators said at the annual meeting of the American Society of Hematology.
In the British De-escalation and Stopping Treatment of Imatinib, Nilotinib, or Sprycel [dasatinib], or Destiny Study, a total of 12 molecular relapses occurred between the second and twelfth month of dose reduction among 174 patients with either an MR3 or MR4 molecular response, and all 12 patients had restoration of molecular remissions after resumption of full dose TKIs, reported co-investigator Mhairi Copland, MD, PhD, from the University of Glasgow, Scotland.
“What we wanted to explore in the Destiny study is cutting the dose of tyrosine kinase inhibitor therapy in CML by half, followed by stopping therapy not just in patients with undetectable disease but also with stable low levels of disease,” Dr. Copland said during a briefing at the meeting.
“We hypothesized that more patients would be able to reduce therapy safely, and a proportion of these would be able to go on to stop therapy; also, that the patients on half-dose therapy would have reduced amount of side effects compared to those on full-dose therapy,” she added.
Several recent studies, including the EURO-SKI trial, have shown that it is safe to stop TKI therapy in those patients who are optimally responding and have undetectable levels of the BCR-ABL transcript.
Rendezvous with Destiny
In Destiny, the investigators enrolled patients with “good, but not perfect” molecular responses: MR3 or better, defined as a minimum of 3 consecutive tests each with greater than 10,000 ABL control transcripts following a minimum of 3 years on a TKI at standard prescribed doses. The median overall duration of TKI therapy was 7 years.
Participants on imatinib had their daily doses reduced to 200 mg, those on nilotinib had their doses cut back to 200 mg twice daily, and those on dasatinib had their quotidian doses halved to 50 mg.
After 12 months of half-dose therapy, molecular recurrence, defined as a loss of MR3 on two consecutive samples, was detected in 9 of 49 patients (18.4%) with MR3 but not MR4 remissions, compared with 3 of 125 patients (2.4%) with MR4 or better remissions (P less than .001).
The median time to relapse was 4.4 months among MR3/not 4 patients vs. 8.7 months for MR4 or better patients.
The probability of molecular recurrence on dose reduction was unrelated to either age, sex, performance status, type of TKI, or the duration of TKI therapy (median 7 years overall).
No patients experienced either progression to advanced phase disease or loss of cytogenetic response. During the course of follow-up, one patient died, and there were 15 serious adverse events, but these were determined to be unrelated to either CML or TKI treatment.
All 12 patients who experienced molecular recurrence regained MR3 within 4 months of resuming TKI therapy at the full dose.
As noted before, patient-reported side effects such as lethargy, diarrhea, rash, nausea, periorbital edema, and hair thinning decreased during the first 3 months of de-escalation, but not thereafter. Dr. Copland said that patients had generally good quality-of-life scores at study entry, suggesting that they were likely not especially bothered by TKI side effects in the first place.
The investigators calculated that for the 174 patients, halving treatment would save an estimated £1,943,364 ($2,474,679) from an expected TKI budget of £4,156,969 ($5,293,484), a savings of 46.7%. Estimated savings were similar for patients with MR4 or better alone (47.7%) and for those with a major molecular response (44.2%).
EURO-SKI Update
Also at ASH 2016, Francois-Xavier Mahon, MD, PhD, from the University of Bordeaux, France, reported additional follow-up data from the EURO-SKI trial, results of which were first reported at the 2016 annual meeting of the European Hematology Association in Copenhagen.
The investigators found that 50% of 755 assessable patients with CML were free of molecular recurrence at 24 months, as were 47% at 36 months.
As reported previously, patients who had been on a TKI for more than 5.8 years before attempting to stop had a lower rate of relapse (34.5%) than patients who had been on therapy for less than 5.8 years (57.4%). Each additional year of TKI therapy was associated with an approximately 16% better chance of successful TKI cessation.
“With inclusion and relapse criteria less strict than in many previous trials, and with decentralized but standardized PCR monitoring, stopping of TKI therapy in a large cohort of CML patients appears feasible and safe,” Dr. Mahon said at the briefing.
The British Destiny Study was supported by Newcastle University. Dr. Copland reported honoraria, advisory board memberships, and/or research funding from Amgen, Pfizer, Shire, BMS, and Ariad.
EURO-SKI was sponsored by the European LeukemiaNet. Dr. Mahon has previously disclosed being on the scientific advisory board and receiving honoraria from Novartis Oncology and BMS, and serving as consultant to those companies and to Pfizer.
FROM ASH 2016
Key clinical point: Halving TKI doses in patients with chronic myeloid leukemia in stable remission is safe and cost effective.
Major finding: After halving TKI doses, there were 12 molecular relapses among 174 patients with an MR3 or better molecular response.
Data source: Prospective dose-reduction study in 174 patients with CML in MR3 remission or better.
Disclosures: The British Destiny Study was supported by Newcastle University. Dr. Copland reported honoraria, advisory board memberships, and/or research funding from Amgen, Pfizer, Shire, BMS, and Ariad. EURO-SKI was sponsored by the European LeukemiaNet. Dr. Mahon has previously disclosed being on the scientific advisory board and receiving honoraria from Novartis Oncology and BMS, and serving as consultant to those companies and to Pfizer.
Anti-CD22 CAR T-cells shift ALL into complete remission
SAN DIEGO – When one CAR stops one working, try another: chimeric antigen receptor (CAR) T-cell therapy for children and young adults with acute lymphoblastic leukemia is driving forward with a novel anti-CD22 target that in an early dose-finding trial has induced complete remissions in some patients with relapsed or refractory disease, including patients previously treated with anti-CD19 CAR-T therapy.
In the first-in-humans trial, CAR T-cell therapy directed against CD22 was shown to be safe and was associated with minimal residual disease (MRD)-negative complete remissions in eight of 10 children and young adults with relapsed/refractory B-precursor acute lymphoblastic leukemia treated at the highest dose level.
“This is the first successful salvage CAR therapy for CD19-negative B-[lineage] ALL,” said co-principal investigator Terry J. Fry, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
Preliminary experience with anti-CD22 immunotherapy suggests that it is comparable in potency to anti-CD19 CAR, and investigators are exploring the possibility that the two chimeric antigen targets could be combined for greater efficacy, he said during a briefing at the annual meeting of the American Society of Hematology.
Tough target
As reported previously from the 2013 ASH annual meeting, anti-CD19 CAR T cells induced complete responses in 10 of 16 children and young adults with relapsed/refractory ALL, and in a second study, CD19-targeted T cells induced complete molecular responses in 12 of 16 adults with B-lineage ALL refractory to chemotherapy.
In current phase 2 trials, anti-CD19 CAR-T therapy is associated with complete remission rates of 80% to 90% of those treated.
However, “we’re learning now that one of the limitations of this approach is the loss of CD19 expression occurring in a substantial number of patients, although it has not been systematically analyzed,” Dr. Fry said.
CD22, an antigen restricted to B-lineage cells, is a promising alternative to CD19 as a target, but finding just the right anti-CD22 CAR was tricky, Dr. Fry said in an interview. The investigators found that many candidate antigens bound well to T cells but had no efficacy, and it took several years of trying before they identified the current version of the antigen
In the phase I trial, the investigators enrolled 16 children and young adults (ages 7 to 22 years) with relapsed/refractory CD22-positive hematologic malignancies. All patients had previously undergone at least one allogeneic stem cell transplant, 11 had previously received anti-CD19 CAR-T cell therapy, and 9 were CD19-negative or had reduced CD19 expression on ALL cells.
The patients underwent peripheral blood mononuclear cells (PBMCs) collected through autologous leukapheresis. The cells were then enriched and expanded, and transduced with a lentiviral vector containing an anti-CD22 CAR for 7 to 10 days, allowing the cells to identify and bind to CD22 expressed on ALL blasts.
The patients then underwent lymphodepletion with fludarabine, and cyclophosphamide, and received infusions of the transduced T-cells at one of three dose levels, starting at 3 x 105 transduced T-cells per recipient weight in kilograms (DL-1), 1 x 106/kg (DL-2), and 3 x 106/kg (DL-3).
The complete remission rate at DL-2 and -3 combined was 80%, with the cytokine-release syndrome (CRS) at a maximum of grade 2.
As noted before, three of the remissions were comparatively durable, with one lasting more than a year.
There were no dose-limiting toxicities at DL-2, and grade 4 hypoxia at DL-3 was seen in one patient.There was one death from sepsis and multi-organ failure in one patient in an expansion cohort. There have been no cases of severe neurotoxicity thus far.
In five patients who experienced relapse, one treated at DL-1 had a loss of CAR cells, and four had changes in CD22 expression, primarily a decrease in site density that may cause the CD22 expression to fall below the threshold for CAR activity, Dr. Fry said.
“At least in our eyes, this may not be best used as a salvage therapy, but we’re beginning to think about how this should be included with CD19 in the upfront CAR treatment,” he said.
The study was funded by the National Institutes of Health with support from Lentigen and Juno Therapeutics. Dr. Fry reported no relevant disclosures.
SAN DIEGO – When one CAR stops one working, try another: chimeric antigen receptor (CAR) T-cell therapy for children and young adults with acute lymphoblastic leukemia is driving forward with a novel anti-CD22 target that in an early dose-finding trial has induced complete remissions in some patients with relapsed or refractory disease, including patients previously treated with anti-CD19 CAR-T therapy.
In the first-in-humans trial, CAR T-cell therapy directed against CD22 was shown to be safe and was associated with minimal residual disease (MRD)-negative complete remissions in eight of 10 children and young adults with relapsed/refractory B-precursor acute lymphoblastic leukemia treated at the highest dose level.
“This is the first successful salvage CAR therapy for CD19-negative B-[lineage] ALL,” said co-principal investigator Terry J. Fry, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
Preliminary experience with anti-CD22 immunotherapy suggests that it is comparable in potency to anti-CD19 CAR, and investigators are exploring the possibility that the two chimeric antigen targets could be combined for greater efficacy, he said during a briefing at the annual meeting of the American Society of Hematology.
Tough target
As reported previously from the 2013 ASH annual meeting, anti-CD19 CAR T cells induced complete responses in 10 of 16 children and young adults with relapsed/refractory ALL, and in a second study, CD19-targeted T cells induced complete molecular responses in 12 of 16 adults with B-lineage ALL refractory to chemotherapy.
In current phase 2 trials, anti-CD19 CAR-T therapy is associated with complete remission rates of 80% to 90% of those treated.
However, “we’re learning now that one of the limitations of this approach is the loss of CD19 expression occurring in a substantial number of patients, although it has not been systematically analyzed,” Dr. Fry said.
CD22, an antigen restricted to B-lineage cells, is a promising alternative to CD19 as a target, but finding just the right anti-CD22 CAR was tricky, Dr. Fry said in an interview. The investigators found that many candidate antigens bound well to T cells but had no efficacy, and it took several years of trying before they identified the current version of the antigen
In the phase I trial, the investigators enrolled 16 children and young adults (ages 7 to 22 years) with relapsed/refractory CD22-positive hematologic malignancies. All patients had previously undergone at least one allogeneic stem cell transplant, 11 had previously received anti-CD19 CAR-T cell therapy, and 9 were CD19-negative or had reduced CD19 expression on ALL cells.
The patients underwent peripheral blood mononuclear cells (PBMCs) collected through autologous leukapheresis. The cells were then enriched and expanded, and transduced with a lentiviral vector containing an anti-CD22 CAR for 7 to 10 days, allowing the cells to identify and bind to CD22 expressed on ALL blasts.
The patients then underwent lymphodepletion with fludarabine, and cyclophosphamide, and received infusions of the transduced T-cells at one of three dose levels, starting at 3 x 105 transduced T-cells per recipient weight in kilograms (DL-1), 1 x 106/kg (DL-2), and 3 x 106/kg (DL-3).
The complete remission rate at DL-2 and -3 combined was 80%, with the cytokine-release syndrome (CRS) at a maximum of grade 2.
As noted before, three of the remissions were comparatively durable, with one lasting more than a year.
There were no dose-limiting toxicities at DL-2, and grade 4 hypoxia at DL-3 was seen in one patient.There was one death from sepsis and multi-organ failure in one patient in an expansion cohort. There have been no cases of severe neurotoxicity thus far.
In five patients who experienced relapse, one treated at DL-1 had a loss of CAR cells, and four had changes in CD22 expression, primarily a decrease in site density that may cause the CD22 expression to fall below the threshold for CAR activity, Dr. Fry said.
“At least in our eyes, this may not be best used as a salvage therapy, but we’re beginning to think about how this should be included with CD19 in the upfront CAR treatment,” he said.
The study was funded by the National Institutes of Health with support from Lentigen and Juno Therapeutics. Dr. Fry reported no relevant disclosures.
SAN DIEGO – When one CAR stops one working, try another: chimeric antigen receptor (CAR) T-cell therapy for children and young adults with acute lymphoblastic leukemia is driving forward with a novel anti-CD22 target that in an early dose-finding trial has induced complete remissions in some patients with relapsed or refractory disease, including patients previously treated with anti-CD19 CAR-T therapy.
In the first-in-humans trial, CAR T-cell therapy directed against CD22 was shown to be safe and was associated with minimal residual disease (MRD)-negative complete remissions in eight of 10 children and young adults with relapsed/refractory B-precursor acute lymphoblastic leukemia treated at the highest dose level.
“This is the first successful salvage CAR therapy for CD19-negative B-[lineage] ALL,” said co-principal investigator Terry J. Fry, MD, from the Center for Cancer Research at the National Cancer Institute in Bethesda, Md.
Preliminary experience with anti-CD22 immunotherapy suggests that it is comparable in potency to anti-CD19 CAR, and investigators are exploring the possibility that the two chimeric antigen targets could be combined for greater efficacy, he said during a briefing at the annual meeting of the American Society of Hematology.
Tough target
As reported previously from the 2013 ASH annual meeting, anti-CD19 CAR T cells induced complete responses in 10 of 16 children and young adults with relapsed/refractory ALL, and in a second study, CD19-targeted T cells induced complete molecular responses in 12 of 16 adults with B-lineage ALL refractory to chemotherapy.
In current phase 2 trials, anti-CD19 CAR-T therapy is associated with complete remission rates of 80% to 90% of those treated.
However, “we’re learning now that one of the limitations of this approach is the loss of CD19 expression occurring in a substantial number of patients, although it has not been systematically analyzed,” Dr. Fry said.
CD22, an antigen restricted to B-lineage cells, is a promising alternative to CD19 as a target, but finding just the right anti-CD22 CAR was tricky, Dr. Fry said in an interview. The investigators found that many candidate antigens bound well to T cells but had no efficacy, and it took several years of trying before they identified the current version of the antigen
In the phase I trial, the investigators enrolled 16 children and young adults (ages 7 to 22 years) with relapsed/refractory CD22-positive hematologic malignancies. All patients had previously undergone at least one allogeneic stem cell transplant, 11 had previously received anti-CD19 CAR-T cell therapy, and 9 were CD19-negative or had reduced CD19 expression on ALL cells.
The patients underwent peripheral blood mononuclear cells (PBMCs) collected through autologous leukapheresis. The cells were then enriched and expanded, and transduced with a lentiviral vector containing an anti-CD22 CAR for 7 to 10 days, allowing the cells to identify and bind to CD22 expressed on ALL blasts.
The patients then underwent lymphodepletion with fludarabine, and cyclophosphamide, and received infusions of the transduced T-cells at one of three dose levels, starting at 3 x 105 transduced T-cells per recipient weight in kilograms (DL-1), 1 x 106/kg (DL-2), and 3 x 106/kg (DL-3).
The complete remission rate at DL-2 and -3 combined was 80%, with the cytokine-release syndrome (CRS) at a maximum of grade 2.
As noted before, three of the remissions were comparatively durable, with one lasting more than a year.
There were no dose-limiting toxicities at DL-2, and grade 4 hypoxia at DL-3 was seen in one patient.There was one death from sepsis and multi-organ failure in one patient in an expansion cohort. There have been no cases of severe neurotoxicity thus far.
In five patients who experienced relapse, one treated at DL-1 had a loss of CAR cells, and four had changes in CD22 expression, primarily a decrease in site density that may cause the CD22 expression to fall below the threshold for CAR activity, Dr. Fry said.
“At least in our eyes, this may not be best used as a salvage therapy, but we’re beginning to think about how this should be included with CD19 in the upfront CAR treatment,” he said.
The study was funded by the National Institutes of Health with support from Lentigen and Juno Therapeutics. Dr. Fry reported no relevant disclosures.
FROM ASH 2016
Key clinical point: CAR T-cell therapy with an anti-CD22 antigen induced complete, MRD-negative remissions in children/young adults with acute lymphoblastic leukemia.
Major finding: The complete remission rate among patients treated at the two highest dose levels was 80%.
Data source: Phase 1 dose-finding trial in 16 children/young adults with relapsed/refractory ALL or diffuse large B-cell lymphoma.
Disclosures The study was funded by the National Institutes of Health with support from Lentigen and Juno Therapeutics. Dr. Fry reported no relevant disclosures views
Malaria elimination in sub-Saharan Africa is possible, study suggests

Kariba area of southern Zambia
where malaria elimination
programs are underway.
Photo from Milen Nikolov
Malaria elimination in historically high transmission areas like southern Africa is possible with tools that are already available, according to new research.
The study suggests that high levels of vector control are key, and mass drug campaigns cannot make much of an impact without proper vector control.
Milen Nikolov, of the Institute for Disease Modeling in Bellevue, Washington, and colleagues reported these findings in PLOS Computational Biology.
The researchers said the Lake Kariba region of Southern Province, Zambia, is part of a multi-country malaria elimination effort. However, elimination in this area is challenging because villages with high and low malaria burden are interconnected through human travel.
With this in mind, the researchers combined a mathematical model of malaria transmission with field data from Zambia to test a variety of strategies for eliminating malaria in the Lake Kariba region.
The team used detailed spatial surveillance data from field studies—including household locations, climate, clinical malaria incidence, prevalence of malaria infections, and bednet usage rates—to construct a model of interconnected villages, then tested a variety of intervention scenarios to see which ones could lead to elimination.
The results indicate that elimination requires high, yet realistic, levels of vector control. And mass drug campaigns deployed to kill parasites in the human population can boost the chances of achieving elimination as long as vector control is well-implemented.
The researchers said this work suggests that elimination programs in sub-Saharan Africa should focus on how to achieve and maintain excellent coverage of vector control measures rather than spending resources on mass drug campaigns that are predicted to have little effect without well-implemented vector control already in place.
Human movement within the region should be targeted to achieve elimination, as should the importation of infections from outside the region. This is because both impact the likelihood of achieving elimination and understanding regional movement patterns can help guide strategies on targeting specific groups of at-risk people.
While no sub-Saharan African country has yet eliminated malaria, the researchers predict that regional malaria elimination is within reach with current tools, provided the efficacy and operational efficiency attained in southern Zambia can be extended and targeted to other key areas. ![]()

Kariba area of southern Zambia
where malaria elimination
programs are underway.
Photo from Milen Nikolov
Malaria elimination in historically high transmission areas like southern Africa is possible with tools that are already available, according to new research.
The study suggests that high levels of vector control are key, and mass drug campaigns cannot make much of an impact without proper vector control.
Milen Nikolov, of the Institute for Disease Modeling in Bellevue, Washington, and colleagues reported these findings in PLOS Computational Biology.
The researchers said the Lake Kariba region of Southern Province, Zambia, is part of a multi-country malaria elimination effort. However, elimination in this area is challenging because villages with high and low malaria burden are interconnected through human travel.
With this in mind, the researchers combined a mathematical model of malaria transmission with field data from Zambia to test a variety of strategies for eliminating malaria in the Lake Kariba region.
The team used detailed spatial surveillance data from field studies—including household locations, climate, clinical malaria incidence, prevalence of malaria infections, and bednet usage rates—to construct a model of interconnected villages, then tested a variety of intervention scenarios to see which ones could lead to elimination.
The results indicate that elimination requires high, yet realistic, levels of vector control. And mass drug campaigns deployed to kill parasites in the human population can boost the chances of achieving elimination as long as vector control is well-implemented.
The researchers said this work suggests that elimination programs in sub-Saharan Africa should focus on how to achieve and maintain excellent coverage of vector control measures rather than spending resources on mass drug campaigns that are predicted to have little effect without well-implemented vector control already in place.
Human movement within the region should be targeted to achieve elimination, as should the importation of infections from outside the region. This is because both impact the likelihood of achieving elimination and understanding regional movement patterns can help guide strategies on targeting specific groups of at-risk people.
While no sub-Saharan African country has yet eliminated malaria, the researchers predict that regional malaria elimination is within reach with current tools, provided the efficacy and operational efficiency attained in southern Zambia can be extended and targeted to other key areas. ![]()

Kariba area of southern Zambia
where malaria elimination
programs are underway.
Photo from Milen Nikolov
Malaria elimination in historically high transmission areas like southern Africa is possible with tools that are already available, according to new research.
The study suggests that high levels of vector control are key, and mass drug campaigns cannot make much of an impact without proper vector control.
Milen Nikolov, of the Institute for Disease Modeling in Bellevue, Washington, and colleagues reported these findings in PLOS Computational Biology.
The researchers said the Lake Kariba region of Southern Province, Zambia, is part of a multi-country malaria elimination effort. However, elimination in this area is challenging because villages with high and low malaria burden are interconnected through human travel.
With this in mind, the researchers combined a mathematical model of malaria transmission with field data from Zambia to test a variety of strategies for eliminating malaria in the Lake Kariba region.
The team used detailed spatial surveillance data from field studies—including household locations, climate, clinical malaria incidence, prevalence of malaria infections, and bednet usage rates—to construct a model of interconnected villages, then tested a variety of intervention scenarios to see which ones could lead to elimination.
The results indicate that elimination requires high, yet realistic, levels of vector control. And mass drug campaigns deployed to kill parasites in the human population can boost the chances of achieving elimination as long as vector control is well-implemented.
The researchers said this work suggests that elimination programs in sub-Saharan Africa should focus on how to achieve and maintain excellent coverage of vector control measures rather than spending resources on mass drug campaigns that are predicted to have little effect without well-implemented vector control already in place.
Human movement within the region should be targeted to achieve elimination, as should the importation of infections from outside the region. This is because both impact the likelihood of achieving elimination and understanding regional movement patterns can help guide strategies on targeting specific groups of at-risk people.
While no sub-Saharan African country has yet eliminated malaria, the researchers predict that regional malaria elimination is within reach with current tools, provided the efficacy and operational efficiency attained in southern Zambia can be extended and targeted to other key areas. ![]()
VIDEO: Anti-CD22 CAR for R/R ALL impresses in early trial
SAN DIEGO – In a first-in-humans trial, chimeric antigen receptor (CAR) T-cell therapy directed against CD22 was shown to be safe and was associated with minimal residual disease (MRD)–negative complete remissions in 8 of 10 children and young adults with relapsed/refractory B-precursor acute lymphoblastic leukemia treated at the highest dose levels. One patient remains in remission more than 1 year of treatment, one had a 6-month remission, and one had a remission lasting 3 months.
In a video interview, co-principal investigator Terry J. Fry, MD, of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md., discusses the rationale behind using an alternative antigen target in salvage therapy for ALL, and the potential for combining antigen targets to treat patients with relapsed/refractory ALL.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – In a first-in-humans trial, chimeric antigen receptor (CAR) T-cell therapy directed against CD22 was shown to be safe and was associated with minimal residual disease (MRD)–negative complete remissions in 8 of 10 children and young adults with relapsed/refractory B-precursor acute lymphoblastic leukemia treated at the highest dose levels. One patient remains in remission more than 1 year of treatment, one had a 6-month remission, and one had a remission lasting 3 months.
In a video interview, co-principal investigator Terry J. Fry, MD, of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md., discusses the rationale behind using an alternative antigen target in salvage therapy for ALL, and the potential for combining antigen targets to treat patients with relapsed/refractory ALL.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – In a first-in-humans trial, chimeric antigen receptor (CAR) T-cell therapy directed against CD22 was shown to be safe and was associated with minimal residual disease (MRD)–negative complete remissions in 8 of 10 children and young adults with relapsed/refractory B-precursor acute lymphoblastic leukemia treated at the highest dose levels. One patient remains in remission more than 1 year of treatment, one had a 6-month remission, and one had a remission lasting 3 months.
In a video interview, co-principal investigator Terry J. Fry, MD, of the Center for Cancer Research at the National Cancer Institute in Bethesda, Md., discusses the rationale behind using an alternative antigen target in salvage therapy for ALL, and the potential for combining antigen targets to treat patients with relapsed/refractory ALL.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
ASH: Hemophilia B gene therapy posts strong update
SAN DIEGO – Patients with hemophilia B who received a single 1-hour infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the 12% steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, said during a press briefing at the annual meeting of the American Society of Hematology. One patient infused himself once with factor IX after developing a suspected ankle bleed 2 days after treatment, Dr. High and her associates reported in the accompanying abstract.
This therapy works at a lower dose than previous factor IX gene transfer products and therefore has not caused the hepatotoxicity that halted their development, according to Dr. High, president and chief scientific officer of Spark Therapeutics, which makes SPK-9001. Two of nine patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels, but the immune response was halted by tapering doses of corticosteroids, and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor, she said.
Because the virus capsid breaks down over time, a transient immune response to it “is not really a safety issue, but is an efficacy issue,” Dr. High emphasized. “If it is not caught in time, and patients are not given steroids promptly, they can lose the donated gene. Therefore, quick recognition is key.” Patients who develop an immune response to the viral capsid show sharp declines in factor IX activity levels, rises in baseline AST and ALT, and mononuclear cell reactivity, she explained during an interview.
The current standard of care for hemophilia B involves the cost and treatment burden of intravenous factor IX injections given one to three times weekly. Previous work evaluated factor IX gene transfer mediated by adeno-associated virus, but long-term factor IX activity levels did not reach the trough levels typically achieved with long-acting factor IX prophylaxis. Simply escalating the vector dose did not work because the viral capsid triggered immune-mediated hepatotoxicity, Dr. High noted.
To develop a more efficient product that works at lower doses, she and her associates created a recombinant vector containing a bioengineered adeno-associated virus capsid and a DNA sequence with a promoter designed to drive hepatic expression of a highly active variant of factor IX. To test the product, researchers in Mississippi, Pennsylvania, and California enrolled men aged 18-52 years with a confirmed diagnosis of hemophilia B (no more than 2 IU/dL or 2% endogenous factor IX) who had received at least 50 days of exposure to factor IX products and averaged at least four bleeding events per year requiring factor IX treatment or prophylaxis. Patients had no measurable inhibitory antibodies but otherwise represented the “general hemophilia B population,” Dr. High said. Five of nine patients had multiple target joints, liver disease associated with hepatitis C virus infection, or both. Each patient received a 1-hour infusion of 5 x 1011 vector genomes per body weight and was followed for 7-52 weeks.
Among seven patients who, by Nov. 30, 2016, had surpassed the 12 weeks needed to reach steady state factor IX expression levels, median steady-state level was 30% (range, 13%-38%), Dr. High reported. “Now we can give one quarter the dose [of adeno-associated virus vector] that was given before, and its driving factor IX expression levels five to eight times higher,” she concluded. Results for the first seven treated patients prompted Food and Drug Administration to give the product orphan drug designation in July 2016. Plans for phase III trials are underway, and researchers also are planning to investigate this approach to gene therapy in hemophilia A, Dr. High said.
Spark Therapeutics Inc. and Pfizer sponsored the study. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
SAN DIEGO – Patients with hemophilia B who received a single 1-hour infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the 12% steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, said during a press briefing at the annual meeting of the American Society of Hematology. One patient infused himself once with factor IX after developing a suspected ankle bleed 2 days after treatment, Dr. High and her associates reported in the accompanying abstract.
This therapy works at a lower dose than previous factor IX gene transfer products and therefore has not caused the hepatotoxicity that halted their development, according to Dr. High, president and chief scientific officer of Spark Therapeutics, which makes SPK-9001. Two of nine patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels, but the immune response was halted by tapering doses of corticosteroids, and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor, she said.
Because the virus capsid breaks down over time, a transient immune response to it “is not really a safety issue, but is an efficacy issue,” Dr. High emphasized. “If it is not caught in time, and patients are not given steroids promptly, they can lose the donated gene. Therefore, quick recognition is key.” Patients who develop an immune response to the viral capsid show sharp declines in factor IX activity levels, rises in baseline AST and ALT, and mononuclear cell reactivity, she explained during an interview.
The current standard of care for hemophilia B involves the cost and treatment burden of intravenous factor IX injections given one to three times weekly. Previous work evaluated factor IX gene transfer mediated by adeno-associated virus, but long-term factor IX activity levels did not reach the trough levels typically achieved with long-acting factor IX prophylaxis. Simply escalating the vector dose did not work because the viral capsid triggered immune-mediated hepatotoxicity, Dr. High noted.
To develop a more efficient product that works at lower doses, she and her associates created a recombinant vector containing a bioengineered adeno-associated virus capsid and a DNA sequence with a promoter designed to drive hepatic expression of a highly active variant of factor IX. To test the product, researchers in Mississippi, Pennsylvania, and California enrolled men aged 18-52 years with a confirmed diagnosis of hemophilia B (no more than 2 IU/dL or 2% endogenous factor IX) who had received at least 50 days of exposure to factor IX products and averaged at least four bleeding events per year requiring factor IX treatment or prophylaxis. Patients had no measurable inhibitory antibodies but otherwise represented the “general hemophilia B population,” Dr. High said. Five of nine patients had multiple target joints, liver disease associated with hepatitis C virus infection, or both. Each patient received a 1-hour infusion of 5 x 1011 vector genomes per body weight and was followed for 7-52 weeks.
Among seven patients who, by Nov. 30, 2016, had surpassed the 12 weeks needed to reach steady state factor IX expression levels, median steady-state level was 30% (range, 13%-38%), Dr. High reported. “Now we can give one quarter the dose [of adeno-associated virus vector] that was given before, and its driving factor IX expression levels five to eight times higher,” she concluded. Results for the first seven treated patients prompted Food and Drug Administration to give the product orphan drug designation in July 2016. Plans for phase III trials are underway, and researchers also are planning to investigate this approach to gene therapy in hemophilia A, Dr. High said.
Spark Therapeutics Inc. and Pfizer sponsored the study. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
SAN DIEGO – Patients with hemophilia B who received a single 1-hour infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the 12% steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, said during a press briefing at the annual meeting of the American Society of Hematology. One patient infused himself once with factor IX after developing a suspected ankle bleed 2 days after treatment, Dr. High and her associates reported in the accompanying abstract.
This therapy works at a lower dose than previous factor IX gene transfer products and therefore has not caused the hepatotoxicity that halted their development, according to Dr. High, president and chief scientific officer of Spark Therapeutics, which makes SPK-9001. Two of nine patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels, but the immune response was halted by tapering doses of corticosteroids, and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor, she said.
Because the virus capsid breaks down over time, a transient immune response to it “is not really a safety issue, but is an efficacy issue,” Dr. High emphasized. “If it is not caught in time, and patients are not given steroids promptly, they can lose the donated gene. Therefore, quick recognition is key.” Patients who develop an immune response to the viral capsid show sharp declines in factor IX activity levels, rises in baseline AST and ALT, and mononuclear cell reactivity, she explained during an interview.
The current standard of care for hemophilia B involves the cost and treatment burden of intravenous factor IX injections given one to three times weekly. Previous work evaluated factor IX gene transfer mediated by adeno-associated virus, but long-term factor IX activity levels did not reach the trough levels typically achieved with long-acting factor IX prophylaxis. Simply escalating the vector dose did not work because the viral capsid triggered immune-mediated hepatotoxicity, Dr. High noted.
To develop a more efficient product that works at lower doses, she and her associates created a recombinant vector containing a bioengineered adeno-associated virus capsid and a DNA sequence with a promoter designed to drive hepatic expression of a highly active variant of factor IX. To test the product, researchers in Mississippi, Pennsylvania, and California enrolled men aged 18-52 years with a confirmed diagnosis of hemophilia B (no more than 2 IU/dL or 2% endogenous factor IX) who had received at least 50 days of exposure to factor IX products and averaged at least four bleeding events per year requiring factor IX treatment or prophylaxis. Patients had no measurable inhibitory antibodies but otherwise represented the “general hemophilia B population,” Dr. High said. Five of nine patients had multiple target joints, liver disease associated with hepatitis C virus infection, or both. Each patient received a 1-hour infusion of 5 x 1011 vector genomes per body weight and was followed for 7-52 weeks.
Among seven patients who, by Nov. 30, 2016, had surpassed the 12 weeks needed to reach steady state factor IX expression levels, median steady-state level was 30% (range, 13%-38%), Dr. High reported. “Now we can give one quarter the dose [of adeno-associated virus vector] that was given before, and its driving factor IX expression levels five to eight times higher,” she concluded. Results for the first seven treated patients prompted Food and Drug Administration to give the product orphan drug designation in July 2016. Plans for phase III trials are underway, and researchers also are planning to investigate this approach to gene therapy in hemophilia A, Dr. High said.
Spark Therapeutics Inc. and Pfizer sponsored the study. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
AT ASH 2016
Key clinical point: Gene therapy with SPK-9001 continues to post strong results in patients with moderate to severe hemophilia B.
Major finding: As of Nov. 30, median steady-state factor IX levels were 30% (range, 13% to 38%). Two of nine patients developed an immune response to the adeno-associated virus capsid that appears to have been halted with tapering doses of corticosteroids.
Data source: An ongoing phase I/II trial of SPK-9001, dosed at 5 x 1011 vector genomes (vg)/kg body weight.
Disclosures: Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. Dr. George had no relevant financial disclosures.
VIDEO: Hemophilia B gene therapy maintains factor IX levels averaging 28%
SAN DIEGO – Patients with hemophilia B who received a single infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, reported at the American Society of Hematology. There have been no confirmed bleeds, all patients remain off prophylactic factor IX, none have developed factor IX inhibitory antibodies, and Enzyme-Linked ImmunoSpot testing has uncovered no evidence of emergent reactivity to the gene product. Two patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels. Tapering doses of corticosteroids halted the immune response and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor.
Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. She discussed the trial in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Patients with hemophilia B who received a single infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, reported at the American Society of Hematology. There have been no confirmed bleeds, all patients remain off prophylactic factor IX, none have developed factor IX inhibitory antibodies, and Enzyme-Linked ImmunoSpot testing has uncovered no evidence of emergent reactivity to the gene product. Two patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels. Tapering doses of corticosteroids halted the immune response and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor.
Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. She discussed the trial in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Patients with hemophilia B who received a single infusion of the gene transfer therapy SPK-9001 achieved steady-state factor IX activity levels averaging 28% and persisting over 1,650 cumulative days of observation, according to updated results from a phase I/II trial.
All nine patients treated to date have exceeded the steady-state factor IX activity level typically needed to prevent breakthrough bleeds, Katherine A. High, MD, reported at the American Society of Hematology. There have been no confirmed bleeds, all patients remain off prophylactic factor IX, none have developed factor IX inhibitory antibodies, and Enzyme-Linked ImmunoSpot testing has uncovered no evidence of emergent reactivity to the gene product. Two patients developed an immune response to the viral capsid in the product, with a corresponding drop in factor IX activity levels. Tapering doses of corticosteroids halted the immune response and patients maintained sufficient levels of factor IX activity to prevent breakthrough bleeds or the need for replacement factor.
Spark Therapeutics Inc. and Pfizer sponsored the work. Dr. High is president and chief scientific officer of Spark. She discussed the trial in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
ASH: Novel microcapsules show promise in hemophilia A with inhibitory antibodies
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in a model of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” Ms. Hansen said at a press briefing.
To create the microcapsules, the investigators deposited alternatingly charged layers of polyelectrolytes, poly-L-lysine, and poly-L-glutamic acid onto a calcium carbonate core covered with factor VIII and dextran. They added fibrinogen to the final polyelectrolyte layer and then chelated out the innermost core, leaving the dextran layer as a shield between factor VIII and the outside of the microcapsule. Initial in vitro experiments showed that the microcapsules adhered to platelets and were incorporated into fibrin networks when platelets were activated, Ms. Hansen reported. Because the microcapsules only ruptured upon platelet contraction, factor VIII was only delivered to actively forming clots as intended, she added.
As a next step, the researchers perfused recalcified whole blood and platelet-poor plasma into a collagen and tissue factor patch designed to mimic vascular injury, and then measured fibrin fluorescence on the patch. Microcapsules lacking dextran, fibrinogen, or loaded factor VIII did not work – a treated sample and a phosphate-buffered saline (PBS) control yielded statistically similar fibrin production. However, complete microcapsules loaded with 0.01 U/mL factor VIII produced four times more fibrin than systemic infusion of 0.05 U/mL factor VIII.
“These were really promising results but we want to take a step back and see if a clot would form in the presence of inhibitory antibodies,” Ms. Hansen said. Accordingly, they added factor VIII inhibitory antibody 2-76 into blood samples from healthy donors. The microcapsules triggered 2.7 times more fibrin production in this setting than systemic treatment did (P less than .05). “This increased efficacy is likely due to the microcapsule shielding effect on factor VIII, preventing exposure to inhibitory antibodies,” Ms. Hansen and her associates concluded in their abstract.
The investigators are now studying the extent to which the microcapsules induce thrombin production, and how agents such as blebbistatin, ROCK, and myosin affect platelet contraction force and the efficiency of the microcapsule.
Ms. Hansen had no disclosures.
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in a model of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” Ms. Hansen said at a press briefing.
To create the microcapsules, the investigators deposited alternatingly charged layers of polyelectrolytes, poly-L-lysine, and poly-L-glutamic acid onto a calcium carbonate core covered with factor VIII and dextran. They added fibrinogen to the final polyelectrolyte layer and then chelated out the innermost core, leaving the dextran layer as a shield between factor VIII and the outside of the microcapsule. Initial in vitro experiments showed that the microcapsules adhered to platelets and were incorporated into fibrin networks when platelets were activated, Ms. Hansen reported. Because the microcapsules only ruptured upon platelet contraction, factor VIII was only delivered to actively forming clots as intended, she added.
As a next step, the researchers perfused recalcified whole blood and platelet-poor plasma into a collagen and tissue factor patch designed to mimic vascular injury, and then measured fibrin fluorescence on the patch. Microcapsules lacking dextran, fibrinogen, or loaded factor VIII did not work – a treated sample and a phosphate-buffered saline (PBS) control yielded statistically similar fibrin production. However, complete microcapsules loaded with 0.01 U/mL factor VIII produced four times more fibrin than systemic infusion of 0.05 U/mL factor VIII.
“These were really promising results but we want to take a step back and see if a clot would form in the presence of inhibitory antibodies,” Ms. Hansen said. Accordingly, they added factor VIII inhibitory antibody 2-76 into blood samples from healthy donors. The microcapsules triggered 2.7 times more fibrin production in this setting than systemic treatment did (P less than .05). “This increased efficacy is likely due to the microcapsule shielding effect on factor VIII, preventing exposure to inhibitory antibodies,” Ms. Hansen and her associates concluded in their abstract.
The investigators are now studying the extent to which the microcapsules induce thrombin production, and how agents such as blebbistatin, ROCK, and myosin affect platelet contraction force and the efficiency of the microcapsule.
Ms. Hansen had no disclosures.
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in a model of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” Ms. Hansen said at a press briefing.
To create the microcapsules, the investigators deposited alternatingly charged layers of polyelectrolytes, poly-L-lysine, and poly-L-glutamic acid onto a calcium carbonate core covered with factor VIII and dextran. They added fibrinogen to the final polyelectrolyte layer and then chelated out the innermost core, leaving the dextran layer as a shield between factor VIII and the outside of the microcapsule. Initial in vitro experiments showed that the microcapsules adhered to platelets and were incorporated into fibrin networks when platelets were activated, Ms. Hansen reported. Because the microcapsules only ruptured upon platelet contraction, factor VIII was only delivered to actively forming clots as intended, she added.
As a next step, the researchers perfused recalcified whole blood and platelet-poor plasma into a collagen and tissue factor patch designed to mimic vascular injury, and then measured fibrin fluorescence on the patch. Microcapsules lacking dextran, fibrinogen, or loaded factor VIII did not work – a treated sample and a phosphate-buffered saline (PBS) control yielded statistically similar fibrin production. However, complete microcapsules loaded with 0.01 U/mL factor VIII produced four times more fibrin than systemic infusion of 0.05 U/mL factor VIII.
“These were really promising results but we want to take a step back and see if a clot would form in the presence of inhibitory antibodies,” Ms. Hansen said. Accordingly, they added factor VIII inhibitory antibody 2-76 into blood samples from healthy donors. The microcapsules triggered 2.7 times more fibrin production in this setting than systemic treatment did (P less than .05). “This increased efficacy is likely due to the microcapsule shielding effect on factor VIII, preventing exposure to inhibitory antibodies,” Ms. Hansen and her associates concluded in their abstract.
The investigators are now studying the extent to which the microcapsules induce thrombin production, and how agents such as blebbistatin, ROCK, and myosin affect platelet contraction force and the efficiency of the microcapsule.
Ms. Hansen had no disclosures.
AT ASH 2016
Key clinical point: Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in an in vitro model of hemophilia A with inhibitory antibodies.
Major finding: In an in vitro model of this disease state, the microcapsules triggered 2.7 times more fibrin production than systemic treatment with factor VIII (P less than .05).
Data source: A multicenter laboratory study.
Disclosures: Ms. Hansen had no relevant financial disclosures.
VIDEO: Novel microcapsules show promise in hemophilia A with inhibitory antibodies
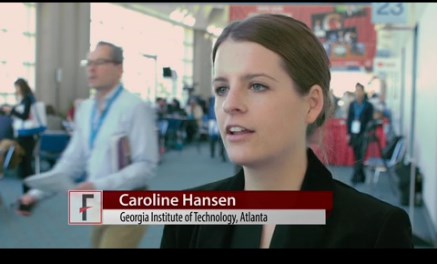
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in models of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” said Ms. Hansen of Georgia Institute of Technology, Atlanta.
The microcapsules are designed to mechanically shield factor VIII from the immune system. When they reached a modeled site of vascular injury, they contracted and released factor VIII. Initial work showed that this approach triggered significantly more fibrin production in a developing clot than did systemic infusions of factor VIII.
Ms. Hansen had no disclosures. She discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in models of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” said Ms. Hansen of Georgia Institute of Technology, Atlanta.
The microcapsules are designed to mechanically shield factor VIII from the immune system. When they reached a modeled site of vascular injury, they contracted and released factor VIII. Initial work showed that this approach triggered significantly more fibrin production in a developing clot than did systemic infusions of factor VIII.
Ms. Hansen had no disclosures. She discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in models of hemophilia A with inhibitory antibodies, Caroline E. Hansen reported at the annual meeting of the American Society of Hematology.
“This is a completely new paradigm that uses platelet biomechanics to target and deliver a drug,” said Ms. Hansen of Georgia Institute of Technology, Atlanta.
The microcapsules are designed to mechanically shield factor VIII from the immune system. When they reached a modeled site of vascular injury, they contracted and released factor VIII. Initial work showed that this approach triggered significantly more fibrin production in a developing clot than did systemic infusions of factor VIII.
Ms. Hansen had no disclosures. She discussed the findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Novel microcapsules loaded with factor VIII outperformed systemic factor VIII infusions in an in vitro model of hemophilia A with inhibitory antibodies.
Major finding: In an in vitro model, the microcapsules triggered 2.7 times more fibrin production than systemic treatment with factor VIII (P less than .05).
Data source: A multicenter laboratory study.
Disclosures: Ms. Hansen had no relevant financial disclosures.