User login
Nomogram predicts conversion of first demyelinating event to definite MS
Baseline and follow-up data from a prospective, multinational cohort of more than 3,000 patients with clinically isolated events allowed researchers to develop an accurate prognostic nomogram to calculate an individual’s risk for conversion to clinically definite multiple sclerosis at 12 months.
“Identification of patient, disease, and examination factors associated with higher probability of second attack in clinical practice may enable clinicians to flag patients that could benefit from more intensive follow-up and consideration of early DMD [disease-modifying drug] treatment intervention, facilitating more favorable patient outcomes,” wrote Tim Spelman, PhD, and his associates.
Risk of relapse was decreased when clinically isolated syndrome was diagnosed later, with an adjusted hazard ratio of 0.9 for every 5 additional years of age. Other risk factors for earlier relapse include higher Expanded Disability Status Scale score at the onset of the first demyelinating event, DMD exposure prior to the first attack, multiple brain and spinal MRI criteria, and oligoclonal bands.
“These results corroborate and extend prior, albeit smaller, studies observing similar sets of predictors of clinical conversion probability,” Dr. Spelman and his coauthors wrote.
The predictive nomogram had a concordance index of 0.81 between the 12-month estimated and observed conversion probabilities.
“While our own internal validation suggested good performance, both an additional training-validation approach and an external validation through the application of the nomogram to a separate MS data set or population are required to confirm the generalizability of the nomogram,” they wrote.
Read the full study in Multiple Sclerosis Journal (2016 Nov 24. doi: 10.1177/1352458516679893).
Baseline and follow-up data from a prospective, multinational cohort of more than 3,000 patients with clinically isolated events allowed researchers to develop an accurate prognostic nomogram to calculate an individual’s risk for conversion to clinically definite multiple sclerosis at 12 months.
“Identification of patient, disease, and examination factors associated with higher probability of second attack in clinical practice may enable clinicians to flag patients that could benefit from more intensive follow-up and consideration of early DMD [disease-modifying drug] treatment intervention, facilitating more favorable patient outcomes,” wrote Tim Spelman, PhD, and his associates.
Risk of relapse was decreased when clinically isolated syndrome was diagnosed later, with an adjusted hazard ratio of 0.9 for every 5 additional years of age. Other risk factors for earlier relapse include higher Expanded Disability Status Scale score at the onset of the first demyelinating event, DMD exposure prior to the first attack, multiple brain and spinal MRI criteria, and oligoclonal bands.
“These results corroborate and extend prior, albeit smaller, studies observing similar sets of predictors of clinical conversion probability,” Dr. Spelman and his coauthors wrote.
The predictive nomogram had a concordance index of 0.81 between the 12-month estimated and observed conversion probabilities.
“While our own internal validation suggested good performance, both an additional training-validation approach and an external validation through the application of the nomogram to a separate MS data set or population are required to confirm the generalizability of the nomogram,” they wrote.
Read the full study in Multiple Sclerosis Journal (2016 Nov 24. doi: 10.1177/1352458516679893).
Baseline and follow-up data from a prospective, multinational cohort of more than 3,000 patients with clinically isolated events allowed researchers to develop an accurate prognostic nomogram to calculate an individual’s risk for conversion to clinically definite multiple sclerosis at 12 months.
“Identification of patient, disease, and examination factors associated with higher probability of second attack in clinical practice may enable clinicians to flag patients that could benefit from more intensive follow-up and consideration of early DMD [disease-modifying drug] treatment intervention, facilitating more favorable patient outcomes,” wrote Tim Spelman, PhD, and his associates.
Risk of relapse was decreased when clinically isolated syndrome was diagnosed later, with an adjusted hazard ratio of 0.9 for every 5 additional years of age. Other risk factors for earlier relapse include higher Expanded Disability Status Scale score at the onset of the first demyelinating event, DMD exposure prior to the first attack, multiple brain and spinal MRI criteria, and oligoclonal bands.
“These results corroborate and extend prior, albeit smaller, studies observing similar sets of predictors of clinical conversion probability,” Dr. Spelman and his coauthors wrote.
The predictive nomogram had a concordance index of 0.81 between the 12-month estimated and observed conversion probabilities.
“While our own internal validation suggested good performance, both an additional training-validation approach and an external validation through the application of the nomogram to a separate MS data set or population are required to confirm the generalizability of the nomogram,” they wrote.
Read the full study in Multiple Sclerosis Journal (2016 Nov 24. doi: 10.1177/1352458516679893).
FROM MULTIPLE SCLEROSIS JOURNAL
Lower analgesic use after robotic pelvic surgery
Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer, according to a report published in Gynecologic Oncology.
Researchers assessed the use of postoperative pain medication in a single-center retrospective study involving 340 consecutive robotically assisted surgeries for endometrial cancer during a 6-year period. The mean patient age was 65 years, and more than one-third of the women were aged 70 or older. Slightly more than half were obese, and 19% were morbidly obese, said Jeremie Abitbol, PhD, of the division of gynecologic oncology, Jewish General Hospital and McGill University, Montreal, and his associates.
This benefit in the use of pain medication occurred regardless of the patient’s obesity status or age, which is particularly helpful in view of the increased risk of adverse events in these two patient populations, Dr. Abitbol and his associates reported (Gynecol Oncol. 2016. doi: 10.1016/jgyno.2016.11.014).
The direct costs associated with postoperative analgesia also were commensurately lower for robotically assisted surgery ($2.52 per day) than for laparotomy ($7.89 per day).
This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer, according to a report published in Gynecologic Oncology.
Researchers assessed the use of postoperative pain medication in a single-center retrospective study involving 340 consecutive robotically assisted surgeries for endometrial cancer during a 6-year period. The mean patient age was 65 years, and more than one-third of the women were aged 70 or older. Slightly more than half were obese, and 19% were morbidly obese, said Jeremie Abitbol, PhD, of the division of gynecologic oncology, Jewish General Hospital and McGill University, Montreal, and his associates.
This benefit in the use of pain medication occurred regardless of the patient’s obesity status or age, which is particularly helpful in view of the increased risk of adverse events in these two patient populations, Dr. Abitbol and his associates reported (Gynecol Oncol. 2016. doi: 10.1016/jgyno.2016.11.014).
The direct costs associated with postoperative analgesia also were commensurately lower for robotically assisted surgery ($2.52 per day) than for laparotomy ($7.89 per day).
This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer, according to a report published in Gynecologic Oncology.
Researchers assessed the use of postoperative pain medication in a single-center retrospective study involving 340 consecutive robotically assisted surgeries for endometrial cancer during a 6-year period. The mean patient age was 65 years, and more than one-third of the women were aged 70 or older. Slightly more than half were obese, and 19% were morbidly obese, said Jeremie Abitbol, PhD, of the division of gynecologic oncology, Jewish General Hospital and McGill University, Montreal, and his associates.
This benefit in the use of pain medication occurred regardless of the patient’s obesity status or age, which is particularly helpful in view of the increased risk of adverse events in these two patient populations, Dr. Abitbol and his associates reported (Gynecol Oncol. 2016. doi: 10.1016/jgyno.2016.11.014).
The direct costs associated with postoperative analgesia also were commensurately lower for robotically assisted surgery ($2.52 per day) than for laparotomy ($7.89 per day).
This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
FROM GYNECOLOGIC ONCOLOGY
Key clinical point: Postoperative use of both opioid and nonopioid analgesics was lower after robotic surgery than after laparotomy for endometrial cancer.
Major finding: The robotic surgery cohort required significantly less opioids (12 mg vs. 71 mg), acetaminophen (2,151 mg vs. 4,810 mg), ibuprofen (377 mg vs. 1,892 mg), and naproxen (393 mg vs 1,470 mg), compared with an historical cohort of 59 women who underwent laparotomy.
Data source: A single-center retrospective cohort study involving 340 consecutive robotically assisted pelvic surgeries during a 6-year period.
Disclosures: This study was supported by grants from the Israel Cancer Research Foundation, the Gloria’s Girls Fund, the Levi Family Fund, and the Weekend to End Women’s Cancers. Dr. Abitbol reported having no relevant financial disclosures; one of his associates reported receiving a grant from Intuitive Surgical.
Few drug-resistant epilepsy patients recruitable for trials of new AEDs
HOUSTON – Fewer than 8% of adult patients with drug-resistant epilepsy would have been recruitable for recent phase II and III trials of new antiepileptic drugs (AEDs), results from a single-center study showed.
The findings underscore a need to rethink how phase II and III trials of AEDs are conducted, Bernhard J. Steinhoff, MD, said in an interview prior to the annual meeting of the American Epilepsy Society. “In spite of the marketing of numerous new antiepileptic drugs, the percentage of drug-resistant epilepsies has remained almost unchanged,” said Dr. Steinhoff of the Kork (Germany) Epilepsy Center. “I am not aware that anybody involved in the issue of clinical trials with AEDs addressed the problem [of] whether the pivotal trials really cover the patients the new AEDs are developed for: namely, patients with drug-resistant epilepsies. Every time we start with a new drug after licensing, we realize that we have no clue whether this drug will be appropriate for our difficult-to-treat patients. We wondered whether the design of the usual phase II and III randomized controlled trials covers an acceptable percentage of AED-resistant epilepsy patients.”
“The result was frightening because less than 10% [of patients] would have been covered by the trials,” Dr. Steinhoff said. “Therefore, we suggest that a bad trials strategy may be responsible for the fact that the percentage of drug-resistant patients has not been markedly reduced by more than 15 new AEDs that were introduced during the recent years.” He added that pivotal trials with new AEDs “show superiority over placebo as add-on in an extremely limited group of difficult-to-treat patients. After licensing and marketing, we start to learn whether a new AED is appropriate for our drug-resistant patients.”
Medical writing was funded by an unrestricted grant from UCB. Dr. Steinhoff reported having no financial conflicts.
HOUSTON – Fewer than 8% of adult patients with drug-resistant epilepsy would have been recruitable for recent phase II and III trials of new antiepileptic drugs (AEDs), results from a single-center study showed.
The findings underscore a need to rethink how phase II and III trials of AEDs are conducted, Bernhard J. Steinhoff, MD, said in an interview prior to the annual meeting of the American Epilepsy Society. “In spite of the marketing of numerous new antiepileptic drugs, the percentage of drug-resistant epilepsies has remained almost unchanged,” said Dr. Steinhoff of the Kork (Germany) Epilepsy Center. “I am not aware that anybody involved in the issue of clinical trials with AEDs addressed the problem [of] whether the pivotal trials really cover the patients the new AEDs are developed for: namely, patients with drug-resistant epilepsies. Every time we start with a new drug after licensing, we realize that we have no clue whether this drug will be appropriate for our difficult-to-treat patients. We wondered whether the design of the usual phase II and III randomized controlled trials covers an acceptable percentage of AED-resistant epilepsy patients.”
“The result was frightening because less than 10% [of patients] would have been covered by the trials,” Dr. Steinhoff said. “Therefore, we suggest that a bad trials strategy may be responsible for the fact that the percentage of drug-resistant patients has not been markedly reduced by more than 15 new AEDs that were introduced during the recent years.” He added that pivotal trials with new AEDs “show superiority over placebo as add-on in an extremely limited group of difficult-to-treat patients. After licensing and marketing, we start to learn whether a new AED is appropriate for our drug-resistant patients.”
Medical writing was funded by an unrestricted grant from UCB. Dr. Steinhoff reported having no financial conflicts.
HOUSTON – Fewer than 8% of adult patients with drug-resistant epilepsy would have been recruitable for recent phase II and III trials of new antiepileptic drugs (AEDs), results from a single-center study showed.
The findings underscore a need to rethink how phase II and III trials of AEDs are conducted, Bernhard J. Steinhoff, MD, said in an interview prior to the annual meeting of the American Epilepsy Society. “In spite of the marketing of numerous new antiepileptic drugs, the percentage of drug-resistant epilepsies has remained almost unchanged,” said Dr. Steinhoff of the Kork (Germany) Epilepsy Center. “I am not aware that anybody involved in the issue of clinical trials with AEDs addressed the problem [of] whether the pivotal trials really cover the patients the new AEDs are developed for: namely, patients with drug-resistant epilepsies. Every time we start with a new drug after licensing, we realize that we have no clue whether this drug will be appropriate for our difficult-to-treat patients. We wondered whether the design of the usual phase II and III randomized controlled trials covers an acceptable percentage of AED-resistant epilepsy patients.”
“The result was frightening because less than 10% [of patients] would have been covered by the trials,” Dr. Steinhoff said. “Therefore, we suggest that a bad trials strategy may be responsible for the fact that the percentage of drug-resistant patients has not been markedly reduced by more than 15 new AEDs that were introduced during the recent years.” He added that pivotal trials with new AEDs “show superiority over placebo as add-on in an extremely limited group of difficult-to-treat patients. After licensing and marketing, we start to learn whether a new AED is appropriate for our drug-resistant patients.”
Medical writing was funded by an unrestricted grant from UCB. Dr. Steinhoff reported having no financial conflicts.
AT AES 2016
Key clinical point:
Major finding: Only 7.4% of patients would have fulfilled the inclusion criteria of five phase II and III trials of new AEDs.
Data source: A review of 216 outpatients with drug-resistant epilepsies.
Disclosures: Medical writing was funded by an unrestricted grant from UCB. Dr. Steinhoff reported having no financial disclosures.
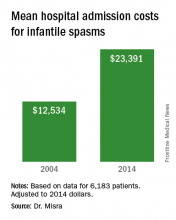
Wide variation seen in treatment of infantile spasms
HOUSTON – The types of diagnostic tests ordered and medication used for treatment of infantile spasms vary considerably, a large study of children’s hospitals showed.
“Children with infantile spasms often require extensive diagnostic work-up to determine etiology, expensive medications for treatment, and hospitalization during the initiation of certain therapies,” researchers led by Sunita N. Misra, MD, PhD, wrote in an abstract presented at the annual meeting of the American Epilepsy Society. “The common diagnostic studies and therapies have evolved over the last several decades.”
The researchers collected patient demographics, hospital length of stay, hospital admission cost, use of various diagnostic studies (such as lumbar puncture, brain MRI, and EEG), and medications used for infantile spasms (including antiepileptic drugs, corticotropin, and steroids). Cost data, calculated as a ratio of cost to charges, were collected and adjusted to 2014 dollars.
A total of 6,183 patients were included in the analysis and their average age of infantile-spasm diagnosis was 9 months. The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%). Medications were started during inpatient hospitalization in two-thirds of patients, with 33% starting on corticotropin; 29% on topiramate; and fewer than 10% of patients on an oral or intravenous steroid, zonisamide, or vigabatrin (Sabril). Use of corticotropin decreased over time, while use of oral steroids trended upwards. “We were surprised that one-third of patients did not have a medication initiated as an inpatient, given the studies showing earlier use of effective therapy has better outcomes,” Dr. Misra said in an interview in advance of the meeting.
“The cost of taking care of children with infantile spasms has increased over the study period 2004-2014,” Dr. Misra said. “Although we identified a few contributors to rising cost, there are probably other factors that need to be considered in future studies.” She acknowledged certain limitations of the analysis, including its retrospective design and the fact that it only identified cost associated with the initial admission. “Several of the diagnostic studies and medications may be initiated as an outpatient, for which we do not have the data,” she said.
Dr. Misra reported having no financial disclosures.
HOUSTON – The types of diagnostic tests ordered and medication used for treatment of infantile spasms vary considerably, a large study of children’s hospitals showed.
“Children with infantile spasms often require extensive diagnostic work-up to determine etiology, expensive medications for treatment, and hospitalization during the initiation of certain therapies,” researchers led by Sunita N. Misra, MD, PhD, wrote in an abstract presented at the annual meeting of the American Epilepsy Society. “The common diagnostic studies and therapies have evolved over the last several decades.”
The researchers collected patient demographics, hospital length of stay, hospital admission cost, use of various diagnostic studies (such as lumbar puncture, brain MRI, and EEG), and medications used for infantile spasms (including antiepileptic drugs, corticotropin, and steroids). Cost data, calculated as a ratio of cost to charges, were collected and adjusted to 2014 dollars.
A total of 6,183 patients were included in the analysis and their average age of infantile-spasm diagnosis was 9 months. The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%). Medications were started during inpatient hospitalization in two-thirds of patients, with 33% starting on corticotropin; 29% on topiramate; and fewer than 10% of patients on an oral or intravenous steroid, zonisamide, or vigabatrin (Sabril). Use of corticotropin decreased over time, while use of oral steroids trended upwards. “We were surprised that one-third of patients did not have a medication initiated as an inpatient, given the studies showing earlier use of effective therapy has better outcomes,” Dr. Misra said in an interview in advance of the meeting.
“The cost of taking care of children with infantile spasms has increased over the study period 2004-2014,” Dr. Misra said. “Although we identified a few contributors to rising cost, there are probably other factors that need to be considered in future studies.” She acknowledged certain limitations of the analysis, including its retrospective design and the fact that it only identified cost associated with the initial admission. “Several of the diagnostic studies and medications may be initiated as an outpatient, for which we do not have the data,” she said.
Dr. Misra reported having no financial disclosures.
HOUSTON – The types of diagnostic tests ordered and medication used for treatment of infantile spasms vary considerably, a large study of children’s hospitals showed.
“Children with infantile spasms often require extensive diagnostic work-up to determine etiology, expensive medications for treatment, and hospitalization during the initiation of certain therapies,” researchers led by Sunita N. Misra, MD, PhD, wrote in an abstract presented at the annual meeting of the American Epilepsy Society. “The common diagnostic studies and therapies have evolved over the last several decades.”
The researchers collected patient demographics, hospital length of stay, hospital admission cost, use of various diagnostic studies (such as lumbar puncture, brain MRI, and EEG), and medications used for infantile spasms (including antiepileptic drugs, corticotropin, and steroids). Cost data, calculated as a ratio of cost to charges, were collected and adjusted to 2014 dollars.
A total of 6,183 patients were included in the analysis and their average age of infantile-spasm diagnosis was 9 months. The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%). Medications were started during inpatient hospitalization in two-thirds of patients, with 33% starting on corticotropin; 29% on topiramate; and fewer than 10% of patients on an oral or intravenous steroid, zonisamide, or vigabatrin (Sabril). Use of corticotropin decreased over time, while use of oral steroids trended upwards. “We were surprised that one-third of patients did not have a medication initiated as an inpatient, given the studies showing earlier use of effective therapy has better outcomes,” Dr. Misra said in an interview in advance of the meeting.
“The cost of taking care of children with infantile spasms has increased over the study period 2004-2014,” Dr. Misra said. “Although we identified a few contributors to rising cost, there are probably other factors that need to be considered in future studies.” She acknowledged certain limitations of the analysis, including its retrospective design and the fact that it only identified cost associated with the initial admission. “Several of the diagnostic studies and medications may be initiated as an outpatient, for which we do not have the data,” she said.
Dr. Misra reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: The most common diagnostic test ordered was EEG (76%), followed by brain imaging (57%), organic acids (38%), and lumbar puncture (17%).
Data source: Retrospective analysis of data on 6,183 patients with infantile spasms between 2004 and 2014.
Disclosures: Dr. Misra reported having no financial disclosures.
The plan-do-study-act cycle and data display
This month’s column is the second in a series of three articles written by a group from Toronto and Houston. The series imagined that a community of gastroenterologists set out to improve the adenoma detection rates of physicians in their practice. The first article described the design and launch of the project. This month, Dr. Bollegala and her colleagues explain the plan-do-study-act (PDSA) cycle of improvement within a small practice. The PDSA cycle is a fundamental component of successful quality improvement initiatives; it allows a group to systematically analyze what works and what doesn’t. The focus of this article is squarely on small community practices (still the majority of gastrointestinal practices nationally), so its relevance is high. PDSA cycles are small, narrowly focused projects that can be accomplished by all as we strive to improve our care of the patients we serve. Next month, we will learn how to embed a quality initiative within our practices so sustained improvement can be seen.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Article 1 of our series focused on the emergence of the adenoma detection rate (ADR) as a quality indicator for colonoscopy-based colorectal cancer screening programs.1 A target ADR of 25% has been established by several national gastroenterology societies and serves as a focus area for those seeking to develop quality improvement (QI) initiatives aimed at reducing the interval incidence of colorectal cancer.2 In this series, you are a community-based urban general gastroenterologist interested in improving your current group ADR of 19% to the established target of 25% for each individual endoscopist within the group over a 12-month period.
This article focuses on a clinician-friendly description of the plan-do-study-act (PDSA) cycle, a key construct within the Model for Improvement framework for QI initiatives. It also describes the importance and key elements of QI data reporting, including the run chart. All core concepts will be framed within the series example of the development of an institutional QI initiative for ADR improvement.
Plan-Do-Study-Act cycle
Conventional scientific research in health care generally is based on large-scale projects, performed over long periods of time and producing aggregate data analyzed through summary statistics. QI-related research, as it relates to PDSA, in contrast, is characterized by smaller-scale projects performed over shorter periods of time, with iterative protocols to accommodate local context and therefore optimize intervention success. As such, the framework for their development, implementation, and continual modification requires a conceptual and methodologic shift.
The PDSA cycle is characterized by four key steps. The first step is to plan. This step involves addressing the following questions: 1) what are we trying to accomplish? (aim); 2) how will we know that a change is an improvement? (measure); and 3) what changes can we make that will lead to improvement? (change). Additional considerations include ensuring that the stated goal is attainable, relevant, and that the timeline is feasible. An important aspect of the plan stage is gaining an understanding for the current local context, key participants and their roles, and areas in which performance is excelling or is challenged. This understanding is critical to conceptually linking the identified problem with its proposed solution. Formulating an impact prediction allows subsequent learning and adaptation.
The second step is to do. This step involves execution of the identified plan over a specified period of time. It also involves rigorous qualitative and quantitative data collection, allowing the research team to assess change and document unexpected events. The identification of an implementation leader or champion to ensure protocol adherence, effective communication among team members, and coordinate accurate data collection can be critical for overall success.
The third step is to study. This step requires evaluating whether a change in the outcome measure has occurred, which intervention was successful, and whether an identified change is sustained over time. It also requires interpretation of change within the local context, specifically with respect to unintended consequences, unanticipated events, and the sustainability of any gains. To interpret study findings appropriately, feedback with involved process members, endoscopists, and/or other stakeholder groups may be necessary. This can be important for explaining the results of each cycle, identifying protocol modifications for future cycles, and optimizing the opportunity for success. Studying the data generated by a QI initiative requires clear and accurate data display and rules for interpretation.
The fourth step is to act. This final step allows team members to reflect on the results generated and decide whether the same intervention should be continued, modified, or changed, thereby incorporating lessons learned from previous PDSA cycles (Figure 1).3
Despite these challenges, the PDSA framework allows for small-scale and fast-paced initiative testing that reduces patient and institutional risk while minimizing the commitment of resources.4,7 Successful cycles improve stakeholder confidence in the probability for success with larger-scale implementation.
In our series example, step 1 of the PDSA cycle, plan, can be described as follows: Aim: increase the ADR of all group endoscopists to 25% over a 12-month period. Measure: Outcome: the proportion of endoscopists at your institution with an ADR greater than 25%; process – withdrawal time; balancing – staff satisfaction, patient satisfaction, and procedure time. Change: Successive cycles will institute the following: audible timers to ensure adequate withdrawal time, publication of an endoscopist-specific composite score, and training to improve inspection technique.8
In step 2 of the PDSA cycle, do, a physician member of the gastroenterology division incorporates QI into their job description and leads a change team charged with PDSA cycle 1. An administrative assistant calculates the endoscopist-specific ADRs for that month. Documentation of related events for this cycle such as unexpected physician absence, delays in polyp histology reporting, and so forth, is performed.
In step 3 of the PDSA cycle, study, the data generated will be represented on a run chart plotting the proportion of endoscopists with an ADR greater than 25% on the y-axis, and time (in monthly intervals) on the x-axis. This will be described in further detail in a later section.
In the final step of the PDSA cycle, act, continuation and modification of the tested changes can be represented as follows.
Displaying data
The documentation, analysis, and interpretation of data generated by multiple PDSA cycles must be displayed accurately and succinctly. The run chart has been developed as a simple technique for identifying nonrandom patterns (that is, signals), which allows QI researchers to determine the impact of each cycle of change and the stability of that change over a given time period.9 This often is contrasted with conventional statistical approaches that aggregate data and perform summary statistical comparisons at static time points. Instead, the run chart allows for an appreciation of the dynamic nature of PDSA-driven process manipulation and resulting outcome changes.
Correct interpretation of the presented data requires an understanding of common cause variation (CCV) and special cause variation (SCV). CCV occurs randomly and is present in all health care processes. It can never be eliminated completely. SCV, in contrast, is the result of external factors that are imposed on normal processes. For example, the introduction of audible timers within endoscopy rooms to ensure adequate withdrawal time may result in an increase in the ADR. The relatively stable ADR measured in both the pre-intervention and postintervention periods are subject to CCV. However, the postintervention increase in ADR is the result of SCV.10
As shown in Figure 2, the horizontal axis shows the time scale and spans the entire duration of the intervention period. The y-axis shows the outcome measure of interest. A horizontal line representing the median is shown.9 A goal line also may be depicted. Annotations to indicate the implementation of change or other important events (such as unintended consequences or unexpected events) also may be added to facilitate data interpretation.
Shift: at least six consecutive data points above or below the median line are needed (points on the median line are skipped).9 To assess a shift appropriately, at least 10 data points are required.
Trend: at least five consecutive data points all increasing in value or all decreasing in value are needed (numerically equivalent points are skipped).9
Runs: a run refers to a series of data points on one side of the median.9 If a random pattern of data points exists on the run chart, there should be an appropriate number of runs on either side of the median. Values outside of this indicate a higher probability of a nonrandom pattern.9,11
Astronomic point: this refers to a data point that subjectively is found to be obviously different from the rest and prompts consideration of the events that led to this.9
Although straightforward to construct and interpret for clinicians without statistical training, the run chart has specific limitations. It is ideal for the display of early data but cannot be used to determine its durability.9 In addition, a run chart does not reflect discrete data with no clear median.
The example run chart in Figure 2 shows that there is a shift in data points from below the median to above the median, ultimately achieving 100% group adherence to the ADR target of greater than 25%. There are only two runs for a total of 12 data points within the 12-month study period, indicating that there is a 5% or less probability that this is a random pattern.11 It appears that our interventions have resulted in incremental improvements in the ADR to exceed the target level in a nonrandom fashion. Although the cumulative effect of these interventions has been successful, it is difficult to predict the durability of this change moving forward. In addition, it would be difficult to select only a single intervention, of the many trialed, that would result in a sustained ADR of 25% or greater.
Summary and next steps
This article selectively reviews the process of change framed by the PDSA cycle. We also discuss the role of data display and interpretation using a run chart. The final article in this series will cover how to sustain change and support a culture of continuous improvement.
References
1. Corley, D.A., Jensen, C.D., Marks, A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
2. Cohen, J., Schoenfeld, P., Park, W., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
3. Module 5: Improvement Cycle. (2013). Available at: http://implementation.fpg.unc.edu/book/export/html/326. Accessed Feb. 1, 2016.
4. Taylor, M.J., McNicholas, C., Nicolay, C., et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-8.
5. Davidoff, F., Batalden, P., Stevens, D. et al. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17:i3-9.
6. Ogrinc, G., Mooney, S., Estrada, C., et al. The SQUIRE (standards for Quality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17:i13-32.
7. Nelson, E.C., Batalden, B.P., Godfrey, M.M. Quality by design: a clinical microsystems approach. Jossey-Bass, San Francisco; 2007.
8. Coe, S.G.C.J., Diehl, N.N., Wallace, M.B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219-26.
9. Perla, R.J., Provost, L.P., Murray, S.K. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51.
10. Neuhauser, D., Provost, L., Bergman, B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf. 2011;20:i36-40.
11. Swed, F.S. Eisenhart, C. Tables for testing randomness of grouping in a sequence of alternatives. Ann Math Statist. 1943;14:66-87
Dr. Bollegala is in the division of gastroenterology, department of medicine, Women’s College Hospital; Dr. Mosko is in the division of gastroenterology, department of medicine, St. Michael’s Hospital, and the Institute of Health Policy, Management, and Evaluation; Dr. Bernstein is in the division of gastroenterology, department of medicine, Sunnybrook Health Sciences Centre; Dr. Brahmania is in the Toronto Center for Liver Diseases, division of gastroenterology, department of medicine, University Health Network; Dr. Liu is in the division of gastroenterology, department of medicine, University Health Network; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management, and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, department of medicine, Mount Sinai Hospital; Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine; Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation. All are at the University of Toronto. Dr. Patel is in the division of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. The authors disclose no conflicts.
This month’s column is the second in a series of three articles written by a group from Toronto and Houston. The series imagined that a community of gastroenterologists set out to improve the adenoma detection rates of physicians in their practice. The first article described the design and launch of the project. This month, Dr. Bollegala and her colleagues explain the plan-do-study-act (PDSA) cycle of improvement within a small practice. The PDSA cycle is a fundamental component of successful quality improvement initiatives; it allows a group to systematically analyze what works and what doesn’t. The focus of this article is squarely on small community practices (still the majority of gastrointestinal practices nationally), so its relevance is high. PDSA cycles are small, narrowly focused projects that can be accomplished by all as we strive to improve our care of the patients we serve. Next month, we will learn how to embed a quality initiative within our practices so sustained improvement can be seen.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Article 1 of our series focused on the emergence of the adenoma detection rate (ADR) as a quality indicator for colonoscopy-based colorectal cancer screening programs.1 A target ADR of 25% has been established by several national gastroenterology societies and serves as a focus area for those seeking to develop quality improvement (QI) initiatives aimed at reducing the interval incidence of colorectal cancer.2 In this series, you are a community-based urban general gastroenterologist interested in improving your current group ADR of 19% to the established target of 25% for each individual endoscopist within the group over a 12-month period.
This article focuses on a clinician-friendly description of the plan-do-study-act (PDSA) cycle, a key construct within the Model for Improvement framework for QI initiatives. It also describes the importance and key elements of QI data reporting, including the run chart. All core concepts will be framed within the series example of the development of an institutional QI initiative for ADR improvement.
Plan-Do-Study-Act cycle
Conventional scientific research in health care generally is based on large-scale projects, performed over long periods of time and producing aggregate data analyzed through summary statistics. QI-related research, as it relates to PDSA, in contrast, is characterized by smaller-scale projects performed over shorter periods of time, with iterative protocols to accommodate local context and therefore optimize intervention success. As such, the framework for their development, implementation, and continual modification requires a conceptual and methodologic shift.
The PDSA cycle is characterized by four key steps. The first step is to plan. This step involves addressing the following questions: 1) what are we trying to accomplish? (aim); 2) how will we know that a change is an improvement? (measure); and 3) what changes can we make that will lead to improvement? (change). Additional considerations include ensuring that the stated goal is attainable, relevant, and that the timeline is feasible. An important aspect of the plan stage is gaining an understanding for the current local context, key participants and their roles, and areas in which performance is excelling or is challenged. This understanding is critical to conceptually linking the identified problem with its proposed solution. Formulating an impact prediction allows subsequent learning and adaptation.
The second step is to do. This step involves execution of the identified plan over a specified period of time. It also involves rigorous qualitative and quantitative data collection, allowing the research team to assess change and document unexpected events. The identification of an implementation leader or champion to ensure protocol adherence, effective communication among team members, and coordinate accurate data collection can be critical for overall success.
The third step is to study. This step requires evaluating whether a change in the outcome measure has occurred, which intervention was successful, and whether an identified change is sustained over time. It also requires interpretation of change within the local context, specifically with respect to unintended consequences, unanticipated events, and the sustainability of any gains. To interpret study findings appropriately, feedback with involved process members, endoscopists, and/or other stakeholder groups may be necessary. This can be important for explaining the results of each cycle, identifying protocol modifications for future cycles, and optimizing the opportunity for success. Studying the data generated by a QI initiative requires clear and accurate data display and rules for interpretation.
The fourth step is to act. This final step allows team members to reflect on the results generated and decide whether the same intervention should be continued, modified, or changed, thereby incorporating lessons learned from previous PDSA cycles (Figure 1).3
Despite these challenges, the PDSA framework allows for small-scale and fast-paced initiative testing that reduces patient and institutional risk while minimizing the commitment of resources.4,7 Successful cycles improve stakeholder confidence in the probability for success with larger-scale implementation.
In our series example, step 1 of the PDSA cycle, plan, can be described as follows: Aim: increase the ADR of all group endoscopists to 25% over a 12-month period. Measure: Outcome: the proportion of endoscopists at your institution with an ADR greater than 25%; process – withdrawal time; balancing – staff satisfaction, patient satisfaction, and procedure time. Change: Successive cycles will institute the following: audible timers to ensure adequate withdrawal time, publication of an endoscopist-specific composite score, and training to improve inspection technique.8
In step 2 of the PDSA cycle, do, a physician member of the gastroenterology division incorporates QI into their job description and leads a change team charged with PDSA cycle 1. An administrative assistant calculates the endoscopist-specific ADRs for that month. Documentation of related events for this cycle such as unexpected physician absence, delays in polyp histology reporting, and so forth, is performed.
In step 3 of the PDSA cycle, study, the data generated will be represented on a run chart plotting the proportion of endoscopists with an ADR greater than 25% on the y-axis, and time (in monthly intervals) on the x-axis. This will be described in further detail in a later section.
In the final step of the PDSA cycle, act, continuation and modification of the tested changes can be represented as follows.
Displaying data
The documentation, analysis, and interpretation of data generated by multiple PDSA cycles must be displayed accurately and succinctly. The run chart has been developed as a simple technique for identifying nonrandom patterns (that is, signals), which allows QI researchers to determine the impact of each cycle of change and the stability of that change over a given time period.9 This often is contrasted with conventional statistical approaches that aggregate data and perform summary statistical comparisons at static time points. Instead, the run chart allows for an appreciation of the dynamic nature of PDSA-driven process manipulation and resulting outcome changes.
Correct interpretation of the presented data requires an understanding of common cause variation (CCV) and special cause variation (SCV). CCV occurs randomly and is present in all health care processes. It can never be eliminated completely. SCV, in contrast, is the result of external factors that are imposed on normal processes. For example, the introduction of audible timers within endoscopy rooms to ensure adequate withdrawal time may result in an increase in the ADR. The relatively stable ADR measured in both the pre-intervention and postintervention periods are subject to CCV. However, the postintervention increase in ADR is the result of SCV.10
As shown in Figure 2, the horizontal axis shows the time scale and spans the entire duration of the intervention period. The y-axis shows the outcome measure of interest. A horizontal line representing the median is shown.9 A goal line also may be depicted. Annotations to indicate the implementation of change or other important events (such as unintended consequences or unexpected events) also may be added to facilitate data interpretation.
Shift: at least six consecutive data points above or below the median line are needed (points on the median line are skipped).9 To assess a shift appropriately, at least 10 data points are required.
Trend: at least five consecutive data points all increasing in value or all decreasing in value are needed (numerically equivalent points are skipped).9
Runs: a run refers to a series of data points on one side of the median.9 If a random pattern of data points exists on the run chart, there should be an appropriate number of runs on either side of the median. Values outside of this indicate a higher probability of a nonrandom pattern.9,11
Astronomic point: this refers to a data point that subjectively is found to be obviously different from the rest and prompts consideration of the events that led to this.9
Although straightforward to construct and interpret for clinicians without statistical training, the run chart has specific limitations. It is ideal for the display of early data but cannot be used to determine its durability.9 In addition, a run chart does not reflect discrete data with no clear median.
The example run chart in Figure 2 shows that there is a shift in data points from below the median to above the median, ultimately achieving 100% group adherence to the ADR target of greater than 25%. There are only two runs for a total of 12 data points within the 12-month study period, indicating that there is a 5% or less probability that this is a random pattern.11 It appears that our interventions have resulted in incremental improvements in the ADR to exceed the target level in a nonrandom fashion. Although the cumulative effect of these interventions has been successful, it is difficult to predict the durability of this change moving forward. In addition, it would be difficult to select only a single intervention, of the many trialed, that would result in a sustained ADR of 25% or greater.
Summary and next steps
This article selectively reviews the process of change framed by the PDSA cycle. We also discuss the role of data display and interpretation using a run chart. The final article in this series will cover how to sustain change and support a culture of continuous improvement.
References
1. Corley, D.A., Jensen, C.D., Marks, A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
2. Cohen, J., Schoenfeld, P., Park, W., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
3. Module 5: Improvement Cycle. (2013). Available at: http://implementation.fpg.unc.edu/book/export/html/326. Accessed Feb. 1, 2016.
4. Taylor, M.J., McNicholas, C., Nicolay, C., et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-8.
5. Davidoff, F., Batalden, P., Stevens, D. et al. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17:i3-9.
6. Ogrinc, G., Mooney, S., Estrada, C., et al. The SQUIRE (standards for Quality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17:i13-32.
7. Nelson, E.C., Batalden, B.P., Godfrey, M.M. Quality by design: a clinical microsystems approach. Jossey-Bass, San Francisco; 2007.
8. Coe, S.G.C.J., Diehl, N.N., Wallace, M.B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219-26.
9. Perla, R.J., Provost, L.P., Murray, S.K. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51.
10. Neuhauser, D., Provost, L., Bergman, B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf. 2011;20:i36-40.
11. Swed, F.S. Eisenhart, C. Tables for testing randomness of grouping in a sequence of alternatives. Ann Math Statist. 1943;14:66-87
Dr. Bollegala is in the division of gastroenterology, department of medicine, Women’s College Hospital; Dr. Mosko is in the division of gastroenterology, department of medicine, St. Michael’s Hospital, and the Institute of Health Policy, Management, and Evaluation; Dr. Bernstein is in the division of gastroenterology, department of medicine, Sunnybrook Health Sciences Centre; Dr. Brahmania is in the Toronto Center for Liver Diseases, division of gastroenterology, department of medicine, University Health Network; Dr. Liu is in the division of gastroenterology, department of medicine, University Health Network; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management, and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, department of medicine, Mount Sinai Hospital; Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine; Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation. All are at the University of Toronto. Dr. Patel is in the division of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. The authors disclose no conflicts.
This month’s column is the second in a series of three articles written by a group from Toronto and Houston. The series imagined that a community of gastroenterologists set out to improve the adenoma detection rates of physicians in their practice. The first article described the design and launch of the project. This month, Dr. Bollegala and her colleagues explain the plan-do-study-act (PDSA) cycle of improvement within a small practice. The PDSA cycle is a fundamental component of successful quality improvement initiatives; it allows a group to systematically analyze what works and what doesn’t. The focus of this article is squarely on small community practices (still the majority of gastrointestinal practices nationally), so its relevance is high. PDSA cycles are small, narrowly focused projects that can be accomplished by all as we strive to improve our care of the patients we serve. Next month, we will learn how to embed a quality initiative within our practices so sustained improvement can be seen.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Article 1 of our series focused on the emergence of the adenoma detection rate (ADR) as a quality indicator for colonoscopy-based colorectal cancer screening programs.1 A target ADR of 25% has been established by several national gastroenterology societies and serves as a focus area for those seeking to develop quality improvement (QI) initiatives aimed at reducing the interval incidence of colorectal cancer.2 In this series, you are a community-based urban general gastroenterologist interested in improving your current group ADR of 19% to the established target of 25% for each individual endoscopist within the group over a 12-month period.
This article focuses on a clinician-friendly description of the plan-do-study-act (PDSA) cycle, a key construct within the Model for Improvement framework for QI initiatives. It also describes the importance and key elements of QI data reporting, including the run chart. All core concepts will be framed within the series example of the development of an institutional QI initiative for ADR improvement.
Plan-Do-Study-Act cycle
Conventional scientific research in health care generally is based on large-scale projects, performed over long periods of time and producing aggregate data analyzed through summary statistics. QI-related research, as it relates to PDSA, in contrast, is characterized by smaller-scale projects performed over shorter periods of time, with iterative protocols to accommodate local context and therefore optimize intervention success. As such, the framework for their development, implementation, and continual modification requires a conceptual and methodologic shift.
The PDSA cycle is characterized by four key steps. The first step is to plan. This step involves addressing the following questions: 1) what are we trying to accomplish? (aim); 2) how will we know that a change is an improvement? (measure); and 3) what changes can we make that will lead to improvement? (change). Additional considerations include ensuring that the stated goal is attainable, relevant, and that the timeline is feasible. An important aspect of the plan stage is gaining an understanding for the current local context, key participants and their roles, and areas in which performance is excelling or is challenged. This understanding is critical to conceptually linking the identified problem with its proposed solution. Formulating an impact prediction allows subsequent learning and adaptation.
The second step is to do. This step involves execution of the identified plan over a specified period of time. It also involves rigorous qualitative and quantitative data collection, allowing the research team to assess change and document unexpected events. The identification of an implementation leader or champion to ensure protocol adherence, effective communication among team members, and coordinate accurate data collection can be critical for overall success.
The third step is to study. This step requires evaluating whether a change in the outcome measure has occurred, which intervention was successful, and whether an identified change is sustained over time. It also requires interpretation of change within the local context, specifically with respect to unintended consequences, unanticipated events, and the sustainability of any gains. To interpret study findings appropriately, feedback with involved process members, endoscopists, and/or other stakeholder groups may be necessary. This can be important for explaining the results of each cycle, identifying protocol modifications for future cycles, and optimizing the opportunity for success. Studying the data generated by a QI initiative requires clear and accurate data display and rules for interpretation.
The fourth step is to act. This final step allows team members to reflect on the results generated and decide whether the same intervention should be continued, modified, or changed, thereby incorporating lessons learned from previous PDSA cycles (Figure 1).3
Despite these challenges, the PDSA framework allows for small-scale and fast-paced initiative testing that reduces patient and institutional risk while minimizing the commitment of resources.4,7 Successful cycles improve stakeholder confidence in the probability for success with larger-scale implementation.
In our series example, step 1 of the PDSA cycle, plan, can be described as follows: Aim: increase the ADR of all group endoscopists to 25% over a 12-month period. Measure: Outcome: the proportion of endoscopists at your institution with an ADR greater than 25%; process – withdrawal time; balancing – staff satisfaction, patient satisfaction, and procedure time. Change: Successive cycles will institute the following: audible timers to ensure adequate withdrawal time, publication of an endoscopist-specific composite score, and training to improve inspection technique.8
In step 2 of the PDSA cycle, do, a physician member of the gastroenterology division incorporates QI into their job description and leads a change team charged with PDSA cycle 1. An administrative assistant calculates the endoscopist-specific ADRs for that month. Documentation of related events for this cycle such as unexpected physician absence, delays in polyp histology reporting, and so forth, is performed.
In step 3 of the PDSA cycle, study, the data generated will be represented on a run chart plotting the proportion of endoscopists with an ADR greater than 25% on the y-axis, and time (in monthly intervals) on the x-axis. This will be described in further detail in a later section.
In the final step of the PDSA cycle, act, continuation and modification of the tested changes can be represented as follows.
Displaying data
The documentation, analysis, and interpretation of data generated by multiple PDSA cycles must be displayed accurately and succinctly. The run chart has been developed as a simple technique for identifying nonrandom patterns (that is, signals), which allows QI researchers to determine the impact of each cycle of change and the stability of that change over a given time period.9 This often is contrasted with conventional statistical approaches that aggregate data and perform summary statistical comparisons at static time points. Instead, the run chart allows for an appreciation of the dynamic nature of PDSA-driven process manipulation and resulting outcome changes.
Correct interpretation of the presented data requires an understanding of common cause variation (CCV) and special cause variation (SCV). CCV occurs randomly and is present in all health care processes. It can never be eliminated completely. SCV, in contrast, is the result of external factors that are imposed on normal processes. For example, the introduction of audible timers within endoscopy rooms to ensure adequate withdrawal time may result in an increase in the ADR. The relatively stable ADR measured in both the pre-intervention and postintervention periods are subject to CCV. However, the postintervention increase in ADR is the result of SCV.10
As shown in Figure 2, the horizontal axis shows the time scale and spans the entire duration of the intervention period. The y-axis shows the outcome measure of interest. A horizontal line representing the median is shown.9 A goal line also may be depicted. Annotations to indicate the implementation of change or other important events (such as unintended consequences or unexpected events) also may be added to facilitate data interpretation.
Shift: at least six consecutive data points above or below the median line are needed (points on the median line are skipped).9 To assess a shift appropriately, at least 10 data points are required.
Trend: at least five consecutive data points all increasing in value or all decreasing in value are needed (numerically equivalent points are skipped).9
Runs: a run refers to a series of data points on one side of the median.9 If a random pattern of data points exists on the run chart, there should be an appropriate number of runs on either side of the median. Values outside of this indicate a higher probability of a nonrandom pattern.9,11
Astronomic point: this refers to a data point that subjectively is found to be obviously different from the rest and prompts consideration of the events that led to this.9
Although straightforward to construct and interpret for clinicians without statistical training, the run chart has specific limitations. It is ideal for the display of early data but cannot be used to determine its durability.9 In addition, a run chart does not reflect discrete data with no clear median.
The example run chart in Figure 2 shows that there is a shift in data points from below the median to above the median, ultimately achieving 100% group adherence to the ADR target of greater than 25%. There are only two runs for a total of 12 data points within the 12-month study period, indicating that there is a 5% or less probability that this is a random pattern.11 It appears that our interventions have resulted in incremental improvements in the ADR to exceed the target level in a nonrandom fashion. Although the cumulative effect of these interventions has been successful, it is difficult to predict the durability of this change moving forward. In addition, it would be difficult to select only a single intervention, of the many trialed, that would result in a sustained ADR of 25% or greater.
Summary and next steps
This article selectively reviews the process of change framed by the PDSA cycle. We also discuss the role of data display and interpretation using a run chart. The final article in this series will cover how to sustain change and support a culture of continuous improvement.
References
1. Corley, D.A., Jensen, C.D., Marks, A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
2. Cohen, J., Schoenfeld, P., Park, W., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
3. Module 5: Improvement Cycle. (2013). Available at: http://implementation.fpg.unc.edu/book/export/html/326. Accessed Feb. 1, 2016.
4. Taylor, M.J., McNicholas, C., Nicolay, C., et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-8.
5. Davidoff, F., Batalden, P., Stevens, D. et al. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17:i3-9.
6. Ogrinc, G., Mooney, S., Estrada, C., et al. The SQUIRE (standards for Quality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17:i13-32.
7. Nelson, E.C., Batalden, B.P., Godfrey, M.M. Quality by design: a clinical microsystems approach. Jossey-Bass, San Francisco; 2007.
8. Coe, S.G.C.J., Diehl, N.N., Wallace, M.B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219-26.
9. Perla, R.J., Provost, L.P., Murray, S.K. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51.
10. Neuhauser, D., Provost, L., Bergman, B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf. 2011;20:i36-40.
11. Swed, F.S. Eisenhart, C. Tables for testing randomness of grouping in a sequence of alternatives. Ann Math Statist. 1943;14:66-87
Dr. Bollegala is in the division of gastroenterology, department of medicine, Women’s College Hospital; Dr. Mosko is in the division of gastroenterology, department of medicine, St. Michael’s Hospital, and the Institute of Health Policy, Management, and Evaluation; Dr. Bernstein is in the division of gastroenterology, department of medicine, Sunnybrook Health Sciences Centre; Dr. Brahmania is in the Toronto Center for Liver Diseases, division of gastroenterology, department of medicine, University Health Network; Dr. Liu is in the division of gastroenterology, department of medicine, University Health Network; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management, and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, department of medicine, Mount Sinai Hospital; Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine; Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation. All are at the University of Toronto. Dr. Patel is in the division of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. The authors disclose no conflicts.
Herbal medicine can reduce pain, fatigue in SCD patients

Convention Center, site of
the 2016 ASH Annual Meeting
SAN DIEGO—Results of a phase 1 study suggest that SCD-101, a botanical extract based on an herbal medicine used in Nigeria to treat sickle cell disease (SCD), can reduce pain and fatigue in people with SCD.
The anti-sickling drug also improves the shape of red blood cells but doesn’t produce a change in hemoglobin, according to researchers.
Peter Gillette, MD, of SUNY Downstate in Brooklyn, New York, reported these results at the 2016 ASH Annual Meeting (abstract 121*).
A 6-month phase 2b study conducted previously in Nigeria showed that the herbal medicine Niprisan reduced pain crises and school absenteeism and raised hemoglobin levels compared to placebo.
Based on this study and positive preclinical activity, Dr Gillette and his colleagues undertook a phase 1 study to determine the safety of escalating doses of SCD-101.
Dr Gillette pointed out that Niprisan had been produced commercially in Nigeria but was later removed by the government from the commercial market because of production problems.
The researchers evaluated 23 patients with homozygous SCD or S/beta0 thalassemia.
Patients were aged 18 to 55 years with hemoglobin F of 15% or less and hemoglobin levels between 6.0 and 9.5 g/dL.
Patients could not have had hydroxyurea treatment within 6 months of enrollment, red blood cell transfusion within 3 months, or hospitalization within 4 weeks.
Patients received SCD-101 orally for 28 days administered 2 times daily (BID) or 3 times daily (TID). Doses were 550 mg BID, 1100 mg BID, 2200 mg BID, 4400 mg BID, and 2750 mg TID.
Dr Gillette explained that by distributing the highest dose 3 times over the course of a day, the researchers were able to decrease the side effects of bloating and flatulence on the highest dose.
“Interestingly, with the dose-distributed TID, we found that the hemoglobin had increased by 10%,” he said. “In other words, it appears that the effects are very short-acting, and that by going from a Q12 to a Q8 dosage, the hemoglobin suddenly looks like it might be significant, although this is not a significant change.”
Laboratory outcomes included hemoglobin and hemolysis (LDH, bilirubin, and reticulocyte measurements). Patient-reported outcomes included pain and fatigue.
The most common adverse events (AEs) were pain, flatulence, bloating, diarrhea, constipation, nausea, and headache.
Seven patients in the 2200 mg BID and 4400 mg BID cohorts had dose-related bloating, gas, flatulence or diarrhea, which subsided in a few days.
Patients in the 2750 mg TID cohort did not experience gas side effects.
The gastrointestinal symptoms were most likely dose-related from an excipient of SCD-101, Dr Gillette said.
He and his colleagues found no significant side effects after 28 days of dosing, and there were no dose reductions or interruptions due to drug-related AEs.
There were also no laboratory or electrocardiogram abnormalities.
“Almost all pain AEs stopped by day 13 in 22 of 23 patients,” Dr Gillette said.
“And unexpectedly, patients began to report that they slept better and had improved energy and cognition,” he noted.
Six patients in the 2200 and 4400 mg BID cohorts reported reduced fatigue as measured by the PROMIS fatigue questionnaire.
And 2 patients with ankle ulcers in the 2 highest dose cohorts reported improved healing.
Two weeks after treatment stopped, patients were almost back to baseline in terms of their chronic pain and fatigue levels, Dr Gillette said.
A parallel design, double-blind, placebo-controlled pilot study of the 2750 mg TID dose is ongoing.
Future studies include a crossover-design, exploratory study of the 2750 mg TID dose and a phase 2 parallel design study of the 2200 mg BID and 2750 mg TID doses.
While the researchers are uncertain about the mechanism of action of SCD-101, they hypothesize that its effects could be due to increased vascular flow, increased oxygen delivery, or a reduction in inflammation.
“This is a promising drug potentially for low-income countries or middle-income countries elsewhere in the world where gene therapy and transplant are really not that feasible,” Dr Gillette said.
Research for this study was supported in part by the National Heart, Lung, and Blood Institute and National Center for Complementary and Integrative Health of the National Institutes of Health. ![]()
*Information presented at the meeting differs from the abstract.

Convention Center, site of
the 2016 ASH Annual Meeting
SAN DIEGO—Results of a phase 1 study suggest that SCD-101, a botanical extract based on an herbal medicine used in Nigeria to treat sickle cell disease (SCD), can reduce pain and fatigue in people with SCD.
The anti-sickling drug also improves the shape of red blood cells but doesn’t produce a change in hemoglobin, according to researchers.
Peter Gillette, MD, of SUNY Downstate in Brooklyn, New York, reported these results at the 2016 ASH Annual Meeting (abstract 121*).
A 6-month phase 2b study conducted previously in Nigeria showed that the herbal medicine Niprisan reduced pain crises and school absenteeism and raised hemoglobin levels compared to placebo.
Based on this study and positive preclinical activity, Dr Gillette and his colleagues undertook a phase 1 study to determine the safety of escalating doses of SCD-101.
Dr Gillette pointed out that Niprisan had been produced commercially in Nigeria but was later removed by the government from the commercial market because of production problems.
The researchers evaluated 23 patients with homozygous SCD or S/beta0 thalassemia.
Patients were aged 18 to 55 years with hemoglobin F of 15% or less and hemoglobin levels between 6.0 and 9.5 g/dL.
Patients could not have had hydroxyurea treatment within 6 months of enrollment, red blood cell transfusion within 3 months, or hospitalization within 4 weeks.
Patients received SCD-101 orally for 28 days administered 2 times daily (BID) or 3 times daily (TID). Doses were 550 mg BID, 1100 mg BID, 2200 mg BID, 4400 mg BID, and 2750 mg TID.
Dr Gillette explained that by distributing the highest dose 3 times over the course of a day, the researchers were able to decrease the side effects of bloating and flatulence on the highest dose.
“Interestingly, with the dose-distributed TID, we found that the hemoglobin had increased by 10%,” he said. “In other words, it appears that the effects are very short-acting, and that by going from a Q12 to a Q8 dosage, the hemoglobin suddenly looks like it might be significant, although this is not a significant change.”
Laboratory outcomes included hemoglobin and hemolysis (LDH, bilirubin, and reticulocyte measurements). Patient-reported outcomes included pain and fatigue.
The most common adverse events (AEs) were pain, flatulence, bloating, diarrhea, constipation, nausea, and headache.
Seven patients in the 2200 mg BID and 4400 mg BID cohorts had dose-related bloating, gas, flatulence or diarrhea, which subsided in a few days.
Patients in the 2750 mg TID cohort did not experience gas side effects.
The gastrointestinal symptoms were most likely dose-related from an excipient of SCD-101, Dr Gillette said.
He and his colleagues found no significant side effects after 28 days of dosing, and there were no dose reductions or interruptions due to drug-related AEs.
There were also no laboratory or electrocardiogram abnormalities.
“Almost all pain AEs stopped by day 13 in 22 of 23 patients,” Dr Gillette said.
“And unexpectedly, patients began to report that they slept better and had improved energy and cognition,” he noted.
Six patients in the 2200 and 4400 mg BID cohorts reported reduced fatigue as measured by the PROMIS fatigue questionnaire.
And 2 patients with ankle ulcers in the 2 highest dose cohorts reported improved healing.
Two weeks after treatment stopped, patients were almost back to baseline in terms of their chronic pain and fatigue levels, Dr Gillette said.
A parallel design, double-blind, placebo-controlled pilot study of the 2750 mg TID dose is ongoing.
Future studies include a crossover-design, exploratory study of the 2750 mg TID dose and a phase 2 parallel design study of the 2200 mg BID and 2750 mg TID doses.
While the researchers are uncertain about the mechanism of action of SCD-101, they hypothesize that its effects could be due to increased vascular flow, increased oxygen delivery, or a reduction in inflammation.
“This is a promising drug potentially for low-income countries or middle-income countries elsewhere in the world where gene therapy and transplant are really not that feasible,” Dr Gillette said.
Research for this study was supported in part by the National Heart, Lung, and Blood Institute and National Center for Complementary and Integrative Health of the National Institutes of Health. ![]()
*Information presented at the meeting differs from the abstract.

Convention Center, site of
the 2016 ASH Annual Meeting
SAN DIEGO—Results of a phase 1 study suggest that SCD-101, a botanical extract based on an herbal medicine used in Nigeria to treat sickle cell disease (SCD), can reduce pain and fatigue in people with SCD.
The anti-sickling drug also improves the shape of red blood cells but doesn’t produce a change in hemoglobin, according to researchers.
Peter Gillette, MD, of SUNY Downstate in Brooklyn, New York, reported these results at the 2016 ASH Annual Meeting (abstract 121*).
A 6-month phase 2b study conducted previously in Nigeria showed that the herbal medicine Niprisan reduced pain crises and school absenteeism and raised hemoglobin levels compared to placebo.
Based on this study and positive preclinical activity, Dr Gillette and his colleagues undertook a phase 1 study to determine the safety of escalating doses of SCD-101.
Dr Gillette pointed out that Niprisan had been produced commercially in Nigeria but was later removed by the government from the commercial market because of production problems.
The researchers evaluated 23 patients with homozygous SCD or S/beta0 thalassemia.
Patients were aged 18 to 55 years with hemoglobin F of 15% or less and hemoglobin levels between 6.0 and 9.5 g/dL.
Patients could not have had hydroxyurea treatment within 6 months of enrollment, red blood cell transfusion within 3 months, or hospitalization within 4 weeks.
Patients received SCD-101 orally for 28 days administered 2 times daily (BID) or 3 times daily (TID). Doses were 550 mg BID, 1100 mg BID, 2200 mg BID, 4400 mg BID, and 2750 mg TID.
Dr Gillette explained that by distributing the highest dose 3 times over the course of a day, the researchers were able to decrease the side effects of bloating and flatulence on the highest dose.
“Interestingly, with the dose-distributed TID, we found that the hemoglobin had increased by 10%,” he said. “In other words, it appears that the effects are very short-acting, and that by going from a Q12 to a Q8 dosage, the hemoglobin suddenly looks like it might be significant, although this is not a significant change.”
Laboratory outcomes included hemoglobin and hemolysis (LDH, bilirubin, and reticulocyte measurements). Patient-reported outcomes included pain and fatigue.
The most common adverse events (AEs) were pain, flatulence, bloating, diarrhea, constipation, nausea, and headache.
Seven patients in the 2200 mg BID and 4400 mg BID cohorts had dose-related bloating, gas, flatulence or diarrhea, which subsided in a few days.
Patients in the 2750 mg TID cohort did not experience gas side effects.
The gastrointestinal symptoms were most likely dose-related from an excipient of SCD-101, Dr Gillette said.
He and his colleagues found no significant side effects after 28 days of dosing, and there were no dose reductions or interruptions due to drug-related AEs.
There were also no laboratory or electrocardiogram abnormalities.
“Almost all pain AEs stopped by day 13 in 22 of 23 patients,” Dr Gillette said.
“And unexpectedly, patients began to report that they slept better and had improved energy and cognition,” he noted.
Six patients in the 2200 and 4400 mg BID cohorts reported reduced fatigue as measured by the PROMIS fatigue questionnaire.
And 2 patients with ankle ulcers in the 2 highest dose cohorts reported improved healing.
Two weeks after treatment stopped, patients were almost back to baseline in terms of their chronic pain and fatigue levels, Dr Gillette said.
A parallel design, double-blind, placebo-controlled pilot study of the 2750 mg TID dose is ongoing.
Future studies include a crossover-design, exploratory study of the 2750 mg TID dose and a phase 2 parallel design study of the 2200 mg BID and 2750 mg TID doses.
While the researchers are uncertain about the mechanism of action of SCD-101, they hypothesize that its effects could be due to increased vascular flow, increased oxygen delivery, or a reduction in inflammation.
“This is a promising drug potentially for low-income countries or middle-income countries elsewhere in the world where gene therapy and transplant are really not that feasible,” Dr Gillette said.
Research for this study was supported in part by the National Heart, Lung, and Blood Institute and National Center for Complementary and Integrative Health of the National Institutes of Health. ![]()
*Information presented at the meeting differs from the abstract.
Observational hospital stays for HF linked to worse outcomes
NEW ORLEANS – The Centers for Medicare & Medicaid Services policy providing financial incentives for hospitals to readmit patients for heart failure for an observational stay rather than as an inpatient is antithetical to the patients’ best interests, according to data presented at the American Heart Association scientific sessions.
“We showed that if you get admitted under observation, the risk of you coming back is much higher than if you’re under an inpatient stay,” said Ahmad Masri, MBBS, of the University of Pittsburgh.
“Since CMS instituted this rule in 2013, there has been a surge in utilization of observational status versus inpatient status,” Dr. Masri noted.
That might make sense if the patients selected for in-hospital observation were less ill at the time than the heart failure patients admitted as inpatients, but that wasn’t the case in his large, retrospective study.
Dr. Masri reported on 21,339 patients with a total of 52,493 admissions for a primary diagnosis of heart failure during 2008-2015 in an 18-hospital health care system. After excluding admissions which involved cardiac surgery or in-hospital mortality, the total was 50,654 admissions.
Of these admissions, 5% were for in-hospital observation; 17% were inpatient admissions with discharge in less than 2 days. The two groups were similar in terms of age, comorbid conditions, and use of guideline-directed medications, although 36% of patients admitted under observation had a left ventricular ejection fraction below 40%, compared with 30% of those with an inpatient admission for less than 2 days.
The majority of patients in both groups were readmitted for heart failure within 1 year; however, the readmission rate was 23% lower in the group with an inpatient stay of less than 2 days, in an analysis adjusted for age, sex, ejection fraction, hypertension, diabetes, pneumonia, chronic obstructive pulmonary disease, liver disease, and renal failure.
Similarly, the group with an inpatient stay of less than 2 days’ duration was 24% less likely to have a cardiac readmission within 1 year than the group admitted for a penalty-free observational stay. The short inpatient stay group’s 1-year all-cause readmission rate was also 24% lower. All of these differences were statistically significant and clinically meaningful.
Yet 1-year all-cause mortality in the two groups was no different.
“This suggests that the difference between these two groups is more of an administrative distinction than a reflection of patient status at time of admission. It looks like it’s just random,” according to Dr. Masri. “There is a real need for a patient-centered, streamlined approach in evaluating and treating patients with heart failure, with a revised treatment-based algorithm and admission rules that guide physicians and shape health care policy.”
He reported having no financial conflicts of interest regarding this study.
NEW ORLEANS – The Centers for Medicare & Medicaid Services policy providing financial incentives for hospitals to readmit patients for heart failure for an observational stay rather than as an inpatient is antithetical to the patients’ best interests, according to data presented at the American Heart Association scientific sessions.
“We showed that if you get admitted under observation, the risk of you coming back is much higher than if you’re under an inpatient stay,” said Ahmad Masri, MBBS, of the University of Pittsburgh.
“Since CMS instituted this rule in 2013, there has been a surge in utilization of observational status versus inpatient status,” Dr. Masri noted.
That might make sense if the patients selected for in-hospital observation were less ill at the time than the heart failure patients admitted as inpatients, but that wasn’t the case in his large, retrospective study.
Dr. Masri reported on 21,339 patients with a total of 52,493 admissions for a primary diagnosis of heart failure during 2008-2015 in an 18-hospital health care system. After excluding admissions which involved cardiac surgery or in-hospital mortality, the total was 50,654 admissions.
Of these admissions, 5% were for in-hospital observation; 17% were inpatient admissions with discharge in less than 2 days. The two groups were similar in terms of age, comorbid conditions, and use of guideline-directed medications, although 36% of patients admitted under observation had a left ventricular ejection fraction below 40%, compared with 30% of those with an inpatient admission for less than 2 days.
The majority of patients in both groups were readmitted for heart failure within 1 year; however, the readmission rate was 23% lower in the group with an inpatient stay of less than 2 days, in an analysis adjusted for age, sex, ejection fraction, hypertension, diabetes, pneumonia, chronic obstructive pulmonary disease, liver disease, and renal failure.
Similarly, the group with an inpatient stay of less than 2 days’ duration was 24% less likely to have a cardiac readmission within 1 year than the group admitted for a penalty-free observational stay. The short inpatient stay group’s 1-year all-cause readmission rate was also 24% lower. All of these differences were statistically significant and clinically meaningful.
Yet 1-year all-cause mortality in the two groups was no different.
“This suggests that the difference between these two groups is more of an administrative distinction than a reflection of patient status at time of admission. It looks like it’s just random,” according to Dr. Masri. “There is a real need for a patient-centered, streamlined approach in evaluating and treating patients with heart failure, with a revised treatment-based algorithm and admission rules that guide physicians and shape health care policy.”
He reported having no financial conflicts of interest regarding this study.
NEW ORLEANS – The Centers for Medicare & Medicaid Services policy providing financial incentives for hospitals to readmit patients for heart failure for an observational stay rather than as an inpatient is antithetical to the patients’ best interests, according to data presented at the American Heart Association scientific sessions.
“We showed that if you get admitted under observation, the risk of you coming back is much higher than if you’re under an inpatient stay,” said Ahmad Masri, MBBS, of the University of Pittsburgh.
“Since CMS instituted this rule in 2013, there has been a surge in utilization of observational status versus inpatient status,” Dr. Masri noted.
That might make sense if the patients selected for in-hospital observation were less ill at the time than the heart failure patients admitted as inpatients, but that wasn’t the case in his large, retrospective study.
Dr. Masri reported on 21,339 patients with a total of 52,493 admissions for a primary diagnosis of heart failure during 2008-2015 in an 18-hospital health care system. After excluding admissions which involved cardiac surgery or in-hospital mortality, the total was 50,654 admissions.
Of these admissions, 5% were for in-hospital observation; 17% were inpatient admissions with discharge in less than 2 days. The two groups were similar in terms of age, comorbid conditions, and use of guideline-directed medications, although 36% of patients admitted under observation had a left ventricular ejection fraction below 40%, compared with 30% of those with an inpatient admission for less than 2 days.
The majority of patients in both groups were readmitted for heart failure within 1 year; however, the readmission rate was 23% lower in the group with an inpatient stay of less than 2 days, in an analysis adjusted for age, sex, ejection fraction, hypertension, diabetes, pneumonia, chronic obstructive pulmonary disease, liver disease, and renal failure.
Similarly, the group with an inpatient stay of less than 2 days’ duration was 24% less likely to have a cardiac readmission within 1 year than the group admitted for a penalty-free observational stay. The short inpatient stay group’s 1-year all-cause readmission rate was also 24% lower. All of these differences were statistically significant and clinically meaningful.
Yet 1-year all-cause mortality in the two groups was no different.
“This suggests that the difference between these two groups is more of an administrative distinction than a reflection of patient status at time of admission. It looks like it’s just random,” according to Dr. Masri. “There is a real need for a patient-centered, streamlined approach in evaluating and treating patients with heart failure, with a revised treatment-based algorithm and admission rules that guide physicians and shape health care policy.”
He reported having no financial conflicts of interest regarding this study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The 1-year rates of readmission for heart failure, cardiac readmission, and all-cause readmission were each 23%-24% lower in heart failure patients admitted for an inpatient stay of less than 2 days’ duration than if they were designated as being admitted under observation.
Data source: A retrospective analysis of more than 50,000 hospital admissions with a primary diagnosis of heart failure in 21,339 patients during 2008-2015.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study.
Halogenated anesthetic linked to less chronic postop mastectomy pain
The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain (CPMP), according to a paper published in the the Journal of Clinical Anesthesia.
The retrospective cross-sectional survey of 128 women who underwent mastectomy with axillary lymph node dissection set out to determine whether the anesthetic or analgesic regimen used perioperatively had any impact on the risk of long-term chronic postoperative pain.
Overall, 43.8% of the women reported chronic pain, and nearly half of these showed neuropathic characteristics with an ID Pain score greater than or equal to 2 (J Clin Anesthesia. 2016;33:20-25. doi: 10.1016/j.jclinane.2015.07.010).
Those who were given a halogenated agent for anesthesia during the operation – 64% of patients in the survey - had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain (95% CI, 0.70-0.95; P = .012).
Arnaud Steyaert, MD, and colleagues at the Catholic University of Louvain (Belgium) described this result as surprising, noting that sevoflurane use was recently found to be a risk factor for chronic pain after breast cancer surgery.
“An explanation for this discrepancy could be that the influence of sevoflurane on the development of CPMP depends on the other components of the anesthetic regimen,” the authors wrote, pointing out that the aforementioned study included the use of remifentanil in all patients, which can trigger acute opioid-induced hyperalgesia and chronic pain after surgery.
Apart from this effect, the authors said they did not see any impact from other analgesics – which included sufentanil, ketamine, clonidine, NSAIDs, and/or magnesium sulfate – on the risk of long-term chronic pain. However, patients treated with piritramide in the recovery room did have a significant 30% greater risk of chronic postoperative pain.
The study also found that patients who needed strong opioids in the postanesthesia care unit had a 30% higher risk of chronic long-term pain (95% CI, 1.11-1.53). “This was expected, as more intense acute postoperative pain is a known risk factor for developing chronic postsurgical pain, including CPMP,” the authors wrote.
Patients who had received adjuvant chemotherapy had a 32% higher incidence of chronic long-term pain, but there was no increase in risk associated with adjuvant radiotherapy. Both are known to cause neurotoxicity and therefore neuropathic pain, the authors commented.
No conflicts of interest were declared.
The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain (CPMP), according to a paper published in the the Journal of Clinical Anesthesia.
The retrospective cross-sectional survey of 128 women who underwent mastectomy with axillary lymph node dissection set out to determine whether the anesthetic or analgesic regimen used perioperatively had any impact on the risk of long-term chronic postoperative pain.
Overall, 43.8% of the women reported chronic pain, and nearly half of these showed neuropathic characteristics with an ID Pain score greater than or equal to 2 (J Clin Anesthesia. 2016;33:20-25. doi: 10.1016/j.jclinane.2015.07.010).
Those who were given a halogenated agent for anesthesia during the operation – 64% of patients in the survey - had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain (95% CI, 0.70-0.95; P = .012).
Arnaud Steyaert, MD, and colleagues at the Catholic University of Louvain (Belgium) described this result as surprising, noting that sevoflurane use was recently found to be a risk factor for chronic pain after breast cancer surgery.
“An explanation for this discrepancy could be that the influence of sevoflurane on the development of CPMP depends on the other components of the anesthetic regimen,” the authors wrote, pointing out that the aforementioned study included the use of remifentanil in all patients, which can trigger acute opioid-induced hyperalgesia and chronic pain after surgery.
Apart from this effect, the authors said they did not see any impact from other analgesics – which included sufentanil, ketamine, clonidine, NSAIDs, and/or magnesium sulfate – on the risk of long-term chronic pain. However, patients treated with piritramide in the recovery room did have a significant 30% greater risk of chronic postoperative pain.
The study also found that patients who needed strong opioids in the postanesthesia care unit had a 30% higher risk of chronic long-term pain (95% CI, 1.11-1.53). “This was expected, as more intense acute postoperative pain is a known risk factor for developing chronic postsurgical pain, including CPMP,” the authors wrote.
Patients who had received adjuvant chemotherapy had a 32% higher incidence of chronic long-term pain, but there was no increase in risk associated with adjuvant radiotherapy. Both are known to cause neurotoxicity and therefore neuropathic pain, the authors commented.
No conflicts of interest were declared.
The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain (CPMP), according to a paper published in the the Journal of Clinical Anesthesia.
The retrospective cross-sectional survey of 128 women who underwent mastectomy with axillary lymph node dissection set out to determine whether the anesthetic or analgesic regimen used perioperatively had any impact on the risk of long-term chronic postoperative pain.
Overall, 43.8% of the women reported chronic pain, and nearly half of these showed neuropathic characteristics with an ID Pain score greater than or equal to 2 (J Clin Anesthesia. 2016;33:20-25. doi: 10.1016/j.jclinane.2015.07.010).
Those who were given a halogenated agent for anesthesia during the operation – 64% of patients in the survey - had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain (95% CI, 0.70-0.95; P = .012).
Arnaud Steyaert, MD, and colleagues at the Catholic University of Louvain (Belgium) described this result as surprising, noting that sevoflurane use was recently found to be a risk factor for chronic pain after breast cancer surgery.
“An explanation for this discrepancy could be that the influence of sevoflurane on the development of CPMP depends on the other components of the anesthetic regimen,” the authors wrote, pointing out that the aforementioned study included the use of remifentanil in all patients, which can trigger acute opioid-induced hyperalgesia and chronic pain after surgery.
Apart from this effect, the authors said they did not see any impact from other analgesics – which included sufentanil, ketamine, clonidine, NSAIDs, and/or magnesium sulfate – on the risk of long-term chronic pain. However, patients treated with piritramide in the recovery room did have a significant 30% greater risk of chronic postoperative pain.
The study also found that patients who needed strong opioids in the postanesthesia care unit had a 30% higher risk of chronic long-term pain (95% CI, 1.11-1.53). “This was expected, as more intense acute postoperative pain is a known risk factor for developing chronic postsurgical pain, including CPMP,” the authors wrote.
Patients who had received adjuvant chemotherapy had a 32% higher incidence of chronic long-term pain, but there was no increase in risk associated with adjuvant radiotherapy. Both are known to cause neurotoxicity and therefore neuropathic pain, the authors commented.
No conflicts of interest were declared.
FROM THE JOURNAL OF CLINICAL ANESTHESIA
Key clinical point: The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain.
Major finding: Patients given a halogenated agent for anesthesia during a mastectomy had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain.
Data source: A retrospective cross-sectional survey.
Disclosures: No conflicts of interest were declared.
Wrist-worn system detected convulsive seizures in home setting
HOUSTON – A wrist-worn device and smartphone-based alert system worn by a patient outside of the epilepsy monitoring unit for 3 months effectively detected convulsive seizure events and minimized false alarms, results from a long-term study found.
The automated device, known as Embrace, relies on an accelerometer and electrodermal activity to detect convulsive seizures. It pairs a wrist-worn device with a smartphone-based application that provides an alert to designated caregivers when an unusual event is detected. Embrace, which is being developed by Empatica Inc., received clearance by the European Union as a medical device for convulsive seizure detection but is still under consideration by the Food and Drug Administration. Previous reports evaluating the automated detector used by Embrace have always relied on data from epilepsy monitoring units (EMUs), where it achieved sensitivity scores ranging from 92% to 100% and 0.15-2.02 false alarms per day in detecting convulsive seizures.
To find out, Dr. Picard, chief scientist at Empatica, and her associates conducted a case study of a 14-year-old patient with Dravet syndrome who was enrolled in a trial of Embrace in the outpatient setting. No data from this patient were used in training the system. The patient’s caregiver was asked to annotate the occurrence of each convulsive seizure and any activity that generated an alert. The number of false alerts was obtained by subtracting the number of correctly recognized convulsive seizures from the total alerts fired by the device. Sensitivity was the percentage of convulsive seizures that automatically triggered an alert.
“Embrace can work for patients outside the EMU, detecting events and issuing alerts through a paired smartphone to a designated caregiver. Also, for clinicians who understand machine learning, we should be clear that we used the strongest test possible: none of the patient’s data were used to train the algorithm that was used, nor was it tuned in any way special for this patient. Had we done so, the results could have possibly appeared even better. However, we chose to use this test of generalization to better get a sense of how it might work on new patients, whose data had never been seen before.”
Dr. Picard acknowledged certain limitations of the study, including the fact that it was a single case and that the patient had Dravet syndrome. She disclosed that she is a cofounder of Empatica and owns shares in the company.
HOUSTON – A wrist-worn device and smartphone-based alert system worn by a patient outside of the epilepsy monitoring unit for 3 months effectively detected convulsive seizure events and minimized false alarms, results from a long-term study found.
The automated device, known as Embrace, relies on an accelerometer and electrodermal activity to detect convulsive seizures. It pairs a wrist-worn device with a smartphone-based application that provides an alert to designated caregivers when an unusual event is detected. Embrace, which is being developed by Empatica Inc., received clearance by the European Union as a medical device for convulsive seizure detection but is still under consideration by the Food and Drug Administration. Previous reports evaluating the automated detector used by Embrace have always relied on data from epilepsy monitoring units (EMUs), where it achieved sensitivity scores ranging from 92% to 100% and 0.15-2.02 false alarms per day in detecting convulsive seizures.
To find out, Dr. Picard, chief scientist at Empatica, and her associates conducted a case study of a 14-year-old patient with Dravet syndrome who was enrolled in a trial of Embrace in the outpatient setting. No data from this patient were used in training the system. The patient’s caregiver was asked to annotate the occurrence of each convulsive seizure and any activity that generated an alert. The number of false alerts was obtained by subtracting the number of correctly recognized convulsive seizures from the total alerts fired by the device. Sensitivity was the percentage of convulsive seizures that automatically triggered an alert.
“Embrace can work for patients outside the EMU, detecting events and issuing alerts through a paired smartphone to a designated caregiver. Also, for clinicians who understand machine learning, we should be clear that we used the strongest test possible: none of the patient’s data were used to train the algorithm that was used, nor was it tuned in any way special for this patient. Had we done so, the results could have possibly appeared even better. However, we chose to use this test of generalization to better get a sense of how it might work on new patients, whose data had never been seen before.”
Dr. Picard acknowledged certain limitations of the study, including the fact that it was a single case and that the patient had Dravet syndrome. She disclosed that she is a cofounder of Empatica and owns shares in the company.
HOUSTON – A wrist-worn device and smartphone-based alert system worn by a patient outside of the epilepsy monitoring unit for 3 months effectively detected convulsive seizure events and minimized false alarms, results from a long-term study found.
The automated device, known as Embrace, relies on an accelerometer and electrodermal activity to detect convulsive seizures. It pairs a wrist-worn device with a smartphone-based application that provides an alert to designated caregivers when an unusual event is detected. Embrace, which is being developed by Empatica Inc., received clearance by the European Union as a medical device for convulsive seizure detection but is still under consideration by the Food and Drug Administration. Previous reports evaluating the automated detector used by Embrace have always relied on data from epilepsy monitoring units (EMUs), where it achieved sensitivity scores ranging from 92% to 100% and 0.15-2.02 false alarms per day in detecting convulsive seizures.
To find out, Dr. Picard, chief scientist at Empatica, and her associates conducted a case study of a 14-year-old patient with Dravet syndrome who was enrolled in a trial of Embrace in the outpatient setting. No data from this patient were used in training the system. The patient’s caregiver was asked to annotate the occurrence of each convulsive seizure and any activity that generated an alert. The number of false alerts was obtained by subtracting the number of correctly recognized convulsive seizures from the total alerts fired by the device. Sensitivity was the percentage of convulsive seizures that automatically triggered an alert.
“Embrace can work for patients outside the EMU, detecting events and issuing alerts through a paired smartphone to a designated caregiver. Also, for clinicians who understand machine learning, we should be clear that we used the strongest test possible: none of the patient’s data were used to train the algorithm that was used, nor was it tuned in any way special for this patient. Had we done so, the results could have possibly appeared even better. However, we chose to use this test of generalization to better get a sense of how it might work on new patients, whose data had never been seen before.”
Dr. Picard acknowledged certain limitations of the study, including the fact that it was a single case and that the patient had Dravet syndrome. She disclosed that she is a cofounder of Empatica and owns shares in the company.
AT AES 2016
Key clinical point:
Major finding: A device known as Embrace detected 22 of the patient’s 24 convulsive seizure events, for a sensitivity of 92% and a false-alarm rate of 0.35 false alarms per day worn.
Data source: Case study of a 14-year-old patient with Dravet syndrome who was enrolled in a trial of Embrace in the outpatient setting and used the device for 113 days.
Disclosures: Dr. Picard is a cofounder of Empatica. She also owns shares in the company.
EMTALA – statutory law
This is the first of a two-part series.
Question: Which of the following statements regarding the Emergency Medical Treatment & Labor Act (EMTALA) is correct?
A. Deals with the standard of care in emergency medicine.
B. Provides safeguards for uninsured and nonpaying patients with an emergency medical condition.
C. Mandates uniform screening and treatment stabilization prior to transfer, irrespective of the hospital’s capability.
D. Is mostly directed at hospitals and emergency department staff doctors, but excludes on-call physicians.
E. Violations can result in fines, loss of Medicare provider participation, or even imprisonment.
Answer: B. In 1985, the CBS investigative news show “60 Minutes” ran an exposé on abuses in the emergency departments of U.S. hospitals, featuring the case of Eugene Barnes, a 32-year-old man brought to the Brookside Hospital emergency department (ED) in San Pablo, Calif., with a penetrating stab wound.
In another case, William Jenness, injured in an auto accident, died after a delayed transfer to a county hospital, because the original hospital required a $1,000 deposit in advance before initiating treatment.
In response to the widespread perception that uninsured patients were being denied treatment in the nation’s emergency departments, Congress enacted the Emergency Medical Treatment & Labor Act.1
Originally referred to as the “antidumping law,” EMTALA was designed to prevent hospitals from transferring financially undesirable patients to public hospitals without providing a medical screening examination and stabilizing treatment prior to transfer.
The purpose and intent of the law is to ensure that all patients who come to the ED have access to emergency services, although the statute itself is silent on payment ability.
EMTALA is not meant to replace or override state tort law, and does not deal with quality of care issues that may arise in the emergency department. Over the 30-year period since its enactment, EMTALA has received mixed reviews, with one scholar complaining that the statute is sloppily drafted and the premise of the statute, silly at best.2
EMTALA defines an emergency medical condition as:
1. A medical condition manifesting itself by acute symptoms of sufficient severity (including severe pain, psychiatric disturbances, and/or symptoms of substance abuse) such that the absence of immediate medical attention could reasonably be expected to result in placing the health of the individual (or, with respect to a pregnant woman, the health of the woman or her unborn child) in serious jeopardy, cause serious impairment to bodily functions, or result in serious dysfunction of any bodily organ or part.
2. With respect to a pregnant woman who is having contractions, there is inadequate time to effect a safe transfer to another hospital before delivery, or that transfer may pose a threat to the health or safety of the woman or the unborn child.3
Whether an emergency medical condition exists is determined by a medical screening exam (MSE). EMTALA is about a process directed at the well-being and safety of all patients with a medical emergency who come to the ED, defined as being licensed by the state or held out to the public as a place that provides care for emergency medical conditions. Hospital-based outpatient clinics that handle less than one-third of emergency visits and physician offices are exempt.
All patients who present to the ED seeking treatment are entitled to an MSE, and EDs are required to post such notification on their premises. A triage nurse may not be qualified to conduct the MSE unless he or she possesses special competencies, and has approval from the medical staff and the hospital’s governing body.
It is important that the MSE be documented soon after the patient’s arrival to determine if the medical condition warrants immediate treatment. It is definitely not acceptable to delay performing an MSE while awaiting information on insurance coverage, and one cannot “hold” the patient and delay stabilizing treatment because of the carrier’s insistence on using only certain approved facilities.
EMTALA requires that the screening exam be “appropriate,” but the statute does not define the term except to note that it is to be “within the capability of the hospital’s emergency department.” However, it is generally recognized that triage alone is insufficient, and the screening exam should be based on the patient’s symptoms and performed by a qualified person.
The important point is that it is uniformly applied, without discrimination, to all who seek treatment in the ED. The hospital itself is expected to have in place policies addressing the broad aspects of the screening process in a nondisparate manner.
The second key issue under the EMTALA statute concerns treatment and transfer.4 If an emergency medical condition exists, treatment must be provided until the emergency is resolved or stabilized.
Under the law, a patient is considered stable for transfer (or discharge) if the treating physician determines that no material deterioration is reasonably likely to occur during or as a result of the transfer between facilities. A receiving hospital is obligated to report any individual who has been transferred in an unstable condition in violation of EMTALA.
However, in the event the hospital does not have the capability to stabilize the emergency medical condition, EMTALA mandates an appropriate transfer, under prescribed conditions, to another hospital whose specialized capabilities obligate it to cooperate. The ED physician in the sending hospital will directly request acceptance of such a transfer. If the patient is unstable, the physician must certify that the medical benefits expected from the transfer outweigh the risks, unless the patient insists on a transfer in writing after being informed of the hospital’s obligations under EMTALA and the risks of transfer.
Furthermore, the transferring hospital must: 1. provide ongoing care within its capability until transfer to minimize transfer risks, 2. provide copies of medical records, 3. confirm that the receiving facility has space and qualified personnel to treat the condition and has agreed to accept the transfer, and 4. ensure that the transfer is made with qualified personnel and appropriate medical equipment.
On-call physicians at both transferring and accepting facilities are also subject to EMTALA. The U.S. Department of Health & Human Services’ Office of Inspector General (OIG) has promulgated rules regarding on-call physicians, even touching on reimbursement.
The American College of Emergency Physicians subscribes to the view that hospitals, medical staff, and payers share an ethical responsibility for the provision of emergency care, acknowledging that EDs require a reliable on-call system that provides for the availability of medical staff members for consultation and participation in the evaluation and treatment of emergency patients.5
Penalties for EMTALA violations include fines up to $50,000 per violation, and/or nullification of Medicare provider agreements. There is a 2-year statute of limitations for civil enforcement of any violation,6 carried out by the OIG and the Centers for Medicare & Medicaid Services (CMS).
A receiving facility, having suffered financial loss as a result of another hospital’s violation of EMTALA, can bring suit to recover damages, and patients may bring private lawsuits against hospitals, though not against physicians. EMTALA, being a civil rather than a criminal statute, does not impose any prison terms.
Investigations and citations by the OIG/CMS are common, with about half of all hospitals subjected to EMTALA investigations and a quarter receiving a violation citation over a recent 10-year period.
However, a recently published study covering 2002-2015 found that, despite 40% of investigations ending up with EMTALA violations, only 3% of investigations triggered fines – and none resulted in suspension of Medicare provider participation.7
There were a total of 192 settlements, or an average of 14 per year for the 4,000 hospitals in the United States. Most were for failing to provide screening (75%) and stabilization (42%). The vast majority of violations affected hospitals, and only eight physicians were involved.
Fines against hospitals and physicians totaled $6,357,000 (averages, $33,435 and $25,625, respectively). Patient dumping attributable to insurance or financial discrimination accounted for 15.6% of settlements.
References
1. 42 USC §1395dd et seq.
2. Chest. 2015 Jun;147(6):1691-6.
3. 42 USC §1395dd(a).
4. 42 USC §1395dd(b)(c).
5. “EMTALA and On-call Responsibility for Emergency Department Patients,” American College of Emergency Physicians.
6. 42 USC §1395dd(d).
7. West J Emerg Med. 2016 May;17(3):245-51.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
This is the first of a two-part series.
Question: Which of the following statements regarding the Emergency Medical Treatment & Labor Act (EMTALA) is correct?
A. Deals with the standard of care in emergency medicine.
B. Provides safeguards for uninsured and nonpaying patients with an emergency medical condition.
C. Mandates uniform screening and treatment stabilization prior to transfer, irrespective of the hospital’s capability.
D. Is mostly directed at hospitals and emergency department staff doctors, but excludes on-call physicians.
E. Violations can result in fines, loss of Medicare provider participation, or even imprisonment.
Answer: B. In 1985, the CBS investigative news show “60 Minutes” ran an exposé on abuses in the emergency departments of U.S. hospitals, featuring the case of Eugene Barnes, a 32-year-old man brought to the Brookside Hospital emergency department (ED) in San Pablo, Calif., with a penetrating stab wound.
In another case, William Jenness, injured in an auto accident, died after a delayed transfer to a county hospital, because the original hospital required a $1,000 deposit in advance before initiating treatment.
In response to the widespread perception that uninsured patients were being denied treatment in the nation’s emergency departments, Congress enacted the Emergency Medical Treatment & Labor Act.1
Originally referred to as the “antidumping law,” EMTALA was designed to prevent hospitals from transferring financially undesirable patients to public hospitals without providing a medical screening examination and stabilizing treatment prior to transfer.
The purpose and intent of the law is to ensure that all patients who come to the ED have access to emergency services, although the statute itself is silent on payment ability.
EMTALA is not meant to replace or override state tort law, and does not deal with quality of care issues that may arise in the emergency department. Over the 30-year period since its enactment, EMTALA has received mixed reviews, with one scholar complaining that the statute is sloppily drafted and the premise of the statute, silly at best.2
EMTALA defines an emergency medical condition as:
1. A medical condition manifesting itself by acute symptoms of sufficient severity (including severe pain, psychiatric disturbances, and/or symptoms of substance abuse) such that the absence of immediate medical attention could reasonably be expected to result in placing the health of the individual (or, with respect to a pregnant woman, the health of the woman or her unborn child) in serious jeopardy, cause serious impairment to bodily functions, or result in serious dysfunction of any bodily organ or part.
2. With respect to a pregnant woman who is having contractions, there is inadequate time to effect a safe transfer to another hospital before delivery, or that transfer may pose a threat to the health or safety of the woman or the unborn child.3
Whether an emergency medical condition exists is determined by a medical screening exam (MSE). EMTALA is about a process directed at the well-being and safety of all patients with a medical emergency who come to the ED, defined as being licensed by the state or held out to the public as a place that provides care for emergency medical conditions. Hospital-based outpatient clinics that handle less than one-third of emergency visits and physician offices are exempt.
All patients who present to the ED seeking treatment are entitled to an MSE, and EDs are required to post such notification on their premises. A triage nurse may not be qualified to conduct the MSE unless he or she possesses special competencies, and has approval from the medical staff and the hospital’s governing body.
It is important that the MSE be documented soon after the patient’s arrival to determine if the medical condition warrants immediate treatment. It is definitely not acceptable to delay performing an MSE while awaiting information on insurance coverage, and one cannot “hold” the patient and delay stabilizing treatment because of the carrier’s insistence on using only certain approved facilities.
EMTALA requires that the screening exam be “appropriate,” but the statute does not define the term except to note that it is to be “within the capability of the hospital’s emergency department.” However, it is generally recognized that triage alone is insufficient, and the screening exam should be based on the patient’s symptoms and performed by a qualified person.
The important point is that it is uniformly applied, without discrimination, to all who seek treatment in the ED. The hospital itself is expected to have in place policies addressing the broad aspects of the screening process in a nondisparate manner.
The second key issue under the EMTALA statute concerns treatment and transfer.4 If an emergency medical condition exists, treatment must be provided until the emergency is resolved or stabilized.
Under the law, a patient is considered stable for transfer (or discharge) if the treating physician determines that no material deterioration is reasonably likely to occur during or as a result of the transfer between facilities. A receiving hospital is obligated to report any individual who has been transferred in an unstable condition in violation of EMTALA.
However, in the event the hospital does not have the capability to stabilize the emergency medical condition, EMTALA mandates an appropriate transfer, under prescribed conditions, to another hospital whose specialized capabilities obligate it to cooperate. The ED physician in the sending hospital will directly request acceptance of such a transfer. If the patient is unstable, the physician must certify that the medical benefits expected from the transfer outweigh the risks, unless the patient insists on a transfer in writing after being informed of the hospital’s obligations under EMTALA and the risks of transfer.
Furthermore, the transferring hospital must: 1. provide ongoing care within its capability until transfer to minimize transfer risks, 2. provide copies of medical records, 3. confirm that the receiving facility has space and qualified personnel to treat the condition and has agreed to accept the transfer, and 4. ensure that the transfer is made with qualified personnel and appropriate medical equipment.
On-call physicians at both transferring and accepting facilities are also subject to EMTALA. The U.S. Department of Health & Human Services’ Office of Inspector General (OIG) has promulgated rules regarding on-call physicians, even touching on reimbursement.
The American College of Emergency Physicians subscribes to the view that hospitals, medical staff, and payers share an ethical responsibility for the provision of emergency care, acknowledging that EDs require a reliable on-call system that provides for the availability of medical staff members for consultation and participation in the evaluation and treatment of emergency patients.5
Penalties for EMTALA violations include fines up to $50,000 per violation, and/or nullification of Medicare provider agreements. There is a 2-year statute of limitations for civil enforcement of any violation,6 carried out by the OIG and the Centers for Medicare & Medicaid Services (CMS).
A receiving facility, having suffered financial loss as a result of another hospital’s violation of EMTALA, can bring suit to recover damages, and patients may bring private lawsuits against hospitals, though not against physicians. EMTALA, being a civil rather than a criminal statute, does not impose any prison terms.
Investigations and citations by the OIG/CMS are common, with about half of all hospitals subjected to EMTALA investigations and a quarter receiving a violation citation over a recent 10-year period.
However, a recently published study covering 2002-2015 found that, despite 40% of investigations ending up with EMTALA violations, only 3% of investigations triggered fines – and none resulted in suspension of Medicare provider participation.7
There were a total of 192 settlements, or an average of 14 per year for the 4,000 hospitals in the United States. Most were for failing to provide screening (75%) and stabilization (42%). The vast majority of violations affected hospitals, and only eight physicians were involved.
Fines against hospitals and physicians totaled $6,357,000 (averages, $33,435 and $25,625, respectively). Patient dumping attributable to insurance or financial discrimination accounted for 15.6% of settlements.
References
1. 42 USC §1395dd et seq.
2. Chest. 2015 Jun;147(6):1691-6.
3. 42 USC §1395dd(a).
4. 42 USC §1395dd(b)(c).
5. “EMTALA and On-call Responsibility for Emergency Department Patients,” American College of Emergency Physicians.
6. 42 USC §1395dd(d).
7. West J Emerg Med. 2016 May;17(3):245-51.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
This is the first of a two-part series.
Question: Which of the following statements regarding the Emergency Medical Treatment & Labor Act (EMTALA) is correct?
A. Deals with the standard of care in emergency medicine.
B. Provides safeguards for uninsured and nonpaying patients with an emergency medical condition.
C. Mandates uniform screening and treatment stabilization prior to transfer, irrespective of the hospital’s capability.
D. Is mostly directed at hospitals and emergency department staff doctors, but excludes on-call physicians.
E. Violations can result in fines, loss of Medicare provider participation, or even imprisonment.
Answer: B. In 1985, the CBS investigative news show “60 Minutes” ran an exposé on abuses in the emergency departments of U.S. hospitals, featuring the case of Eugene Barnes, a 32-year-old man brought to the Brookside Hospital emergency department (ED) in San Pablo, Calif., with a penetrating stab wound.
In another case, William Jenness, injured in an auto accident, died after a delayed transfer to a county hospital, because the original hospital required a $1,000 deposit in advance before initiating treatment.
In response to the widespread perception that uninsured patients were being denied treatment in the nation’s emergency departments, Congress enacted the Emergency Medical Treatment & Labor Act.1
Originally referred to as the “antidumping law,” EMTALA was designed to prevent hospitals from transferring financially undesirable patients to public hospitals without providing a medical screening examination and stabilizing treatment prior to transfer.
The purpose and intent of the law is to ensure that all patients who come to the ED have access to emergency services, although the statute itself is silent on payment ability.
EMTALA is not meant to replace or override state tort law, and does not deal with quality of care issues that may arise in the emergency department. Over the 30-year period since its enactment, EMTALA has received mixed reviews, with one scholar complaining that the statute is sloppily drafted and the premise of the statute, silly at best.2
EMTALA defines an emergency medical condition as:
1. A medical condition manifesting itself by acute symptoms of sufficient severity (including severe pain, psychiatric disturbances, and/or symptoms of substance abuse) such that the absence of immediate medical attention could reasonably be expected to result in placing the health of the individual (or, with respect to a pregnant woman, the health of the woman or her unborn child) in serious jeopardy, cause serious impairment to bodily functions, or result in serious dysfunction of any bodily organ or part.
2. With respect to a pregnant woman who is having contractions, there is inadequate time to effect a safe transfer to another hospital before delivery, or that transfer may pose a threat to the health or safety of the woman or the unborn child.3
Whether an emergency medical condition exists is determined by a medical screening exam (MSE). EMTALA is about a process directed at the well-being and safety of all patients with a medical emergency who come to the ED, defined as being licensed by the state or held out to the public as a place that provides care for emergency medical conditions. Hospital-based outpatient clinics that handle less than one-third of emergency visits and physician offices are exempt.
All patients who present to the ED seeking treatment are entitled to an MSE, and EDs are required to post such notification on their premises. A triage nurse may not be qualified to conduct the MSE unless he or she possesses special competencies, and has approval from the medical staff and the hospital’s governing body.
It is important that the MSE be documented soon after the patient’s arrival to determine if the medical condition warrants immediate treatment. It is definitely not acceptable to delay performing an MSE while awaiting information on insurance coverage, and one cannot “hold” the patient and delay stabilizing treatment because of the carrier’s insistence on using only certain approved facilities.
EMTALA requires that the screening exam be “appropriate,” but the statute does not define the term except to note that it is to be “within the capability of the hospital’s emergency department.” However, it is generally recognized that triage alone is insufficient, and the screening exam should be based on the patient’s symptoms and performed by a qualified person.
The important point is that it is uniformly applied, without discrimination, to all who seek treatment in the ED. The hospital itself is expected to have in place policies addressing the broad aspects of the screening process in a nondisparate manner.
The second key issue under the EMTALA statute concerns treatment and transfer.4 If an emergency medical condition exists, treatment must be provided until the emergency is resolved or stabilized.
Under the law, a patient is considered stable for transfer (or discharge) if the treating physician determines that no material deterioration is reasonably likely to occur during or as a result of the transfer between facilities. A receiving hospital is obligated to report any individual who has been transferred in an unstable condition in violation of EMTALA.
However, in the event the hospital does not have the capability to stabilize the emergency medical condition, EMTALA mandates an appropriate transfer, under prescribed conditions, to another hospital whose specialized capabilities obligate it to cooperate. The ED physician in the sending hospital will directly request acceptance of such a transfer. If the patient is unstable, the physician must certify that the medical benefits expected from the transfer outweigh the risks, unless the patient insists on a transfer in writing after being informed of the hospital’s obligations under EMTALA and the risks of transfer.
Furthermore, the transferring hospital must: 1. provide ongoing care within its capability until transfer to minimize transfer risks, 2. provide copies of medical records, 3. confirm that the receiving facility has space and qualified personnel to treat the condition and has agreed to accept the transfer, and 4. ensure that the transfer is made with qualified personnel and appropriate medical equipment.
On-call physicians at both transferring and accepting facilities are also subject to EMTALA. The U.S. Department of Health & Human Services’ Office of Inspector General (OIG) has promulgated rules regarding on-call physicians, even touching on reimbursement.
The American College of Emergency Physicians subscribes to the view that hospitals, medical staff, and payers share an ethical responsibility for the provision of emergency care, acknowledging that EDs require a reliable on-call system that provides for the availability of medical staff members for consultation and participation in the evaluation and treatment of emergency patients.5
Penalties for EMTALA violations include fines up to $50,000 per violation, and/or nullification of Medicare provider agreements. There is a 2-year statute of limitations for civil enforcement of any violation,6 carried out by the OIG and the Centers for Medicare & Medicaid Services (CMS).
A receiving facility, having suffered financial loss as a result of another hospital’s violation of EMTALA, can bring suit to recover damages, and patients may bring private lawsuits against hospitals, though not against physicians. EMTALA, being a civil rather than a criminal statute, does not impose any prison terms.
Investigations and citations by the OIG/CMS are common, with about half of all hospitals subjected to EMTALA investigations and a quarter receiving a violation citation over a recent 10-year period.
However, a recently published study covering 2002-2015 found that, despite 40% of investigations ending up with EMTALA violations, only 3% of investigations triggered fines – and none resulted in suspension of Medicare provider participation.7
There were a total of 192 settlements, or an average of 14 per year for the 4,000 hospitals in the United States. Most were for failing to provide screening (75%) and stabilization (42%). The vast majority of violations affected hospitals, and only eight physicians were involved.
Fines against hospitals and physicians totaled $6,357,000 (averages, $33,435 and $25,625, respectively). Patient dumping attributable to insurance or financial discrimination accounted for 15.6% of settlements.
References
1. 42 USC §1395dd et seq.
2. Chest. 2015 Jun;147(6):1691-6.
3. 42 USC §1395dd(a).
4. 42 USC §1395dd(b)(c).
5. “EMTALA and On-call Responsibility for Emergency Department Patients,” American College of Emergency Physicians.
6. 42 USC §1395dd(d).
7. West J Emerg Med. 2016 May;17(3):245-51.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].