User login
Tips for avoiding nerve injuries in gynecologic surgery
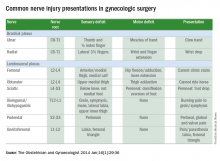
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Upper- and lower-extremity injuries can occur during gynecologic surgery. The incidence of lower-extremity injury is 1.1%-1.9% and upper-extremity injuries can occur in 0.16% of cases.1-5 Fortunately, most of the injuries are transient, sensory injuries that resolve spontaneously. However, a small percentage of injuries result in long-term sequelae.
The pathophysiology of the nerve injuries can be mechanistically separated into three categories: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia results from nerve demyelination at the site of injury because of compression and typically resolves within weeks to months as the nerve is remyelinated. Axonotmesis results from severe compression with axon damage. This may take up to a year to resolve as axonal regeneration proceeds at the rate of 1 mm per day. This can be separated into second and third degree and refers to the severity of damage and the resultant persistent deficit. Neurotmesis results from complete transection and is associated with a poor prognosis without reparative surgery.
Brachial plexus
Stretch injury is the most common reason for a brachial plexus injury. This can occur if the arm board is extended to greater than 90 degrees from the patient’s torso or if the patient’s arm falls off of the arm board. Careful positioning and securing the patient’s arm on the arm board before draping can avoid this injury. A brachial plexus injury can also occur if shoulder braces are placed too laterally during minimally invasive surgery. Radial nerve injuries can occur if there is too much pressure on the humerus during positioning. Ulnar injuries arise from pressure placed on the medial aspect of the elbow.
Tip #1: When tucking a patient’s arm for minimally invasive surgery, appropriate padding should be placed around the elbow and wrist, and the arm should be in the “thumbs-up” position.
Tip #2: Shoulder blocks should be placed over the acromioclavicular (AC) joint.
Lumbosacral plexus
The femoral nerve is the nerve most commonly injured during gynecologic surgery and this usually occurs because of compression of the nerve from the lateral blades of self-retaining retractors. One study showed an 8% incidence of injury from self-retaining retractors, compared with less than 1% when the retractors were not used.6 The femoral nerve can also be stretched when patients are placed in the lithotomy position and the hip is hyperflexed.
As with brachial injury prevention, patients should be positioned prior to draping and care must be taken to not hyperflex or externally rotate the hip during minimally invasive surgical procedures. With the introduction of robot-assisted surgery, care must be taken when docking the robot and surgeons must resist excessive movement of the stirrups.
Tip #3: During laparotomy, surgeons should use the shortest blades that allow for adequate visualization and check the blades during the procedure to ensure that excessive pressure is not placed on the psoas muscle. Consider intermittently releasing the pressure on the lateral blades during other portions of the procedure.
Tip #4: Make sure the stirrups are at the same height and that the leg is in line with the patient’s contralateral shoulder.
Obturator nerve injuries can occur during retroperitoneal dissection for pelvic lymphadenectomy (obturator nodes) and can be either a transection or a cautery injury. It can also be injured during urogynecologic procedures including paravaginal defect repairs and during the placement of transobturator tapes.
The sciatic nerve and its branch, the common peroneal, are generally injured because of excessive stretch or pressure. Both nerves can be injured from hyperflexion of the thigh and the common peroneal can suffer a pressure injury as it courses around the lateral head of the fibula. Therefore, care during lithotomy positioning with both candy cane and Allen stirrups is critical during vaginal surgery.
Tip #5: Ensure that the lateral fibula is not touching the stirrup or that padding is placed between the fibular head and the stirrup.
The ilioinguinal and iliohypogastric nerves are typically injured via suture entrapment from low transverse skin incisions, though laparoscopic injury has also been reported. The incidence after a Pfannenstiel incision is about 3.7%.7
Tip #6: Avoid extending the low transverse incision beyond the lateral margin of the rectus muscle, and do not extend the fascial closure suture more than 1.5 cm from the lateral edge of the fascial incision to avoid catching the nerve with the suture.
The pudendal nerve is most commonly injured during vaginal procedures such as sacrospinous fixation. Pain is typically worse when seated.
The genitofemoral nerve is typically injured during retroperitoneal lymph node dissection, particularly the external iliac nodes. The nerve is small and runs lateral to the external iliac artery. It can suffer cautery and transection injuries. Usually, the paresthesias over the mons pubis, labia majora, and medial inner thigh are temporary.
Tip #7: Care should be taken to identify and spare the nerve during retroperitoneal dissection or external iliac node removal.
Nerve injuries during gynecologic surgery are common and are a significant cause of potential morbidity. While occasionally unavoidable and inherent to the surgical procedure, many times the injury could be prevented with proper attention and care to patient positioning and retractor use. Gynecologists should be aware of the risks and have a through understanding of the anatomy. However, should an injury occur, the patient can be reassured that most are self-limited and full recovery is generally expected. In a prospective study, the median time to resolution of symptoms was 31.5 days (range, 1 day to 6 months).5
References
1. The Obstetrician & Gynaecologist 2014;16:29-36.
2. Gynecol Oncol. 1988 Nov;31(3):462-6.
3. Fertil Steril. 1993 Oct;60(4):729-32.
4. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664-72.
5. Am J Obstet Gynecol. 2009 Nov;201(5):531.e1-7.
6. Eur J Obstet Gynecol Reprod Biol. 1985 Dec;20(6):385-92.
7. Obstet Gynecol. 2008 Apr;111(4):839-46.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no financial disclosures relevant to this column. Email her at [email protected].
Examining the safety of lipid-lowering drugs in pregnancy
Lipid-lowering medications are some of the most commonly prescribed drugs in the United States. But while much is known about their general safety, the data are limited when it comes to pregnancy and breastfeeding.
Antilipemic agents are a pharmacologic class that contains 18 drugs. The class is divided into eight subclasses: bile acid sequestrants; fibric acid derivatives, HMG-CoA inhibitors; immunoglobulins; monoclonal antibodies; oligonucleotide inhibitors; vitamins; as well as two miscellaneous drugs, ezetimibe (Zetia) and lomitapide (Juxtapid). Another antilipemic – dextrothyroxine – has been removed from the market by the manufacturer.
Bile acid sequestrants
Bile acid sequestrants include cholestyramine (Prevalite, Questran), colesevelam (Welchol), and colestipol (Colestid). These drugs have the potential to cause fetal toxicity. This assessment is based on their mechanism of action. These agents are not absorbed systemically, or absorption is very poor and they bind bile acids into a nonabsorbable complex. This action can reduce intestinal absorption of fat-soluble vitamins A, D, E, and K.
Reports of fetal harm have not been located for the other two agents in this class, but there is only one case report involving five women for colesevelam and no reports for colestipol. Nevertheless, both of these drugs have the potential to cause fetal hemorrhage if they are taken for prolonged periods in pregnancy.
Fibric acid derivatives
The fibric acid derivatives subclass includes fenofibrate (Tricor, Lofibra) and gemfibrozil (Lopid).
Six reports, involving 13 pregnancies, have described the use of gemfibrozil during all phases of pregnancy. No teratogenic effects were observed in these cases. In one woman, similar concentrations of gemfibrozil and its active metabolite were found in the umbilical vein and artery at levels within the normal reference for adults.
Statins
There are seven HMG-CoA inhibitors, known as statins: atorvastatin (Lipitor), fluvastatin (Lescol), lovastatin (Mevacor), pitavastatin (Livalo), pravastatin (Pravachol), rosuvastatin (Crestor), and simvastatin (Zocor).
The interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia. Moreover, cholesterol and products synthesized by cholesterol are important during fetal development as shown by the rise in maternal cholesterol levels during pregnancy. Although the potential for embryo-fetal harm has not been clearly documented, and that potential may eventually be confirmed as low, the use of these agents in the first trimester are best classified as contraindicated.
One consideration in estimating the embryo-fetal risk of statins is their classification as either lipophilic or hydrophilic. Three of the seven statins are hydrophilic (fluvastatin, pravastatin, and rosuvastatin); the remaining four agents are lipophilic. In a 2004 review of 70 reports, all adverse birth outcomes were reported following exposure to lipophilic statins (atorvastatin, lovastatin, or simvastatin) and none with the hydrophilic pravastatin. The authors stated that the findings were due to the fact that lipophilic agents equilibrate between maternal and embryonic compartments, whereas pravastatin is minimally present in the embryo.3 If this is indeed the case, and a statin must be used during pregnancy, fluvastatin, pravastatin, or rosuvastatin appears to be best.
Pravastatin also has been used for the prevention and treatment of preeclampsia.5,6 Although the teratogenic potential of these agents has not been fully determined, the risk for birth defects, if any, appears to be low even when exposure occurs during organogenesis.7,8,9 Nevertheless, avoiding these products during the first trimester appears to be best.
Immunoglobulins
The only immunoglobulin in the antilipemic class is evolocumab (Repatha), which has no human pregnancy data. It is an immunoglobulin G2 that is indicated as an adjunct to diet and maximally tolerated statin therapy. It is also indicated as an adjunct to diet and other low-density lipoprotein–lowering therapies in patients with homozygous familial hypercholesterolemia who require additional lowering. No adverse embryo-fetal effects were observed in monkeys. Because statins are contraindicated in the first trimester, the drug, if combined with a statin, can also be classified as contraindicated. However, if the drug is used alone, the embryo-fetal risk appears to be low based on the animal data.
Monoclonal antibodies
The protein alirocumab (Praluent) is a human monoclonal antibody. It is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease. There are no human pregnancy data. The animal data in rats and monkeys suggest low embryo-fetal risk. However, suppression of the humoral immune response to keyhole hemocyanin antigen was observed in infant monkeys at 4-6 months of age. The significance of this in human infants is apparently unknown. Because statins are contraindicated in the first trimester, the drug should not be used with these agents during that period.
Oligonucleotide inhibitors
No reports describing the use of mipomersen (Kynamro), an oligonucleotide inhibitor of apolipoprotein B-100 synthesis, in human pregnancy have been located. The drug is indicated as an adjunct to lipid-lowering medications and diet to reduce low-density lipoprotein cholesterol, apolipoprotein B, total cholesterol, and non–high-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. It has a very long (1-2 months) elimination half-life. The drug caused fetal toxicity in rats, but not in mice or rabbits.
Vitamins
Niacin is a water-soluble B complex vitamin that is converted in vivo to niacinamide. Niacin has no known embryo-fetal risk.
Miscellaneous agents
The two agents in the miscellaneous category are ezetimibe and lomitapide. Ezetimibe is indicated, either alone or in combination with a statin, as adjunctive therapy to diet for the reduction of cholesterol and triglycerides. Statins are contraindicated in the first trimester, but ezetimibe alone could be used during that period if treatment of the mother was mandated. The drug caused no problems in rabbits, but in rats, a dose 10 times the human exposure increased the incidence of skeletal abnormalities. In one report, a woman with homozygous familial hypercholesterolemia was treated with direct adsorption of lipoprotein apheresis, ezetimibe, and rosuvastatin. When pregnancy was discovered (gestational age not specified), the two drugs were stopped but biweekly apheresis was continued. At 37 weeks’ gestation, the patient gave birth to a healthy 2,400-g male infant.10
There are no human pregnancy data with lomitapide. It is indicated as an adjunct to a low-fat diet and other lipid-lowering treatments, including low-density lipoprotein apheresis where available, to reduce LDL cholesterol, total cholesterol, apolipoprotein B, and non–high-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. At doses less than 10 times the human dose, the drug caused congenital malformations and embryo-fetal death in rats, rabbits, and ferrets. The manufacturer classifies the drug as contraindicated in pregnancy because of the animal data.
Breastfeeding
Only niacin, pravastatin, and rosuvastatin have data regarding human milk concentrations. Niacin and its active form – niacinamide – are excreted into breast milk.
The average peak milk level in 11 lactating women given pravastatin 20 mg twice daily for 2.5 days was 3.9 mcg/L, whereas the level for the active metabolite was 2.1 mcg/L. Based on these data, a fully breastfed infant would receive daily about 1.4% of the mother’s weight-adjusted dose.11
A 31-year-old woman was treated with rosuvastatin for familial hypercholesterolemia while breastfeeding her infant. The drug was stopped during breastfeeding but was restarted at 33 days post partum. Breast milk concentrations of the drug were 1.2 times serum levels (about 22 ng/mL vs. 18 ng/mL). Unfortunately, no information was provided on the status of the nursing infant.12
Three of the above agents have high molecular weights - alirocumab, evolocumab, and mipomersen - and are probably not excreted into mature breast milk. Moreover, colesevelam is not absorbed, and very small amounts of colestipol are absorbed by mothers. Several antilipemic agents have characteristics (for example, low molecular weight or long elimination half-life) that suggest they will be excreted into breast milk: ezetimibe, fenofibric acid (active metabolite of fenofibrate), gemfibrozil, lomitapide, and all the statins.
Taken in sum, all of the antilipemics, with the exception of niacin, have the potential to cause a deficiency of fat-soluble vitamins (A, D, E, K) in mother’s milk and in the nursing infant. Deficiency is a concern for all of these vitamins, but especially for vitamin K, because it could cause bruising, petechiae, hematomas, and bleeding in the nursing infant. In addition, antilipemics could cause low levels in milk of cholesterol and lipids, which are required by a nursing infant. Consequently, they should not be used by mothers who are breastfeeding an infant.
References
1. Br J Obstet Gynaecol. 1995 Feb;102(2):169-70.
2. J Matern Fetal Neonatal Med. 2015 May;28(8):954-8.
3. Am J Med Genet A. 2004 Dec 15;131(3):287-98.
4. J Clin Invest. 2016 Aug 1;126(8):2933-40.
5. Hypertension. 2015 Sep;66(3):687-97.
6. Am J Obstet Gynecol. 2016 Jun;214(6):720.e1-720.e17.
7. Birth Defects Res A Clin Mol Teratol. 2005 Nov;73(11):888-96.
8. Reprod Toxicol. 2008 Oct;26(2):175-7.
9. Ann Pharmacother. 2012 Oct;46(10):1419-24.
10. Open Cardiovasc Med J. 2015 Dec 29;9:114-7.
11. J Clin Pharmacol. 1988;28:942.
12. Am J Med. 2013 Sep;126(9):e7-e8.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Lipid-lowering medications are some of the most commonly prescribed drugs in the United States. But while much is known about their general safety, the data are limited when it comes to pregnancy and breastfeeding.
Antilipemic agents are a pharmacologic class that contains 18 drugs. The class is divided into eight subclasses: bile acid sequestrants; fibric acid derivatives, HMG-CoA inhibitors; immunoglobulins; monoclonal antibodies; oligonucleotide inhibitors; vitamins; as well as two miscellaneous drugs, ezetimibe (Zetia) and lomitapide (Juxtapid). Another antilipemic – dextrothyroxine – has been removed from the market by the manufacturer.
Bile acid sequestrants
Bile acid sequestrants include cholestyramine (Prevalite, Questran), colesevelam (Welchol), and colestipol (Colestid). These drugs have the potential to cause fetal toxicity. This assessment is based on their mechanism of action. These agents are not absorbed systemically, or absorption is very poor and they bind bile acids into a nonabsorbable complex. This action can reduce intestinal absorption of fat-soluble vitamins A, D, E, and K.
Reports of fetal harm have not been located for the other two agents in this class, but there is only one case report involving five women for colesevelam and no reports for colestipol. Nevertheless, both of these drugs have the potential to cause fetal hemorrhage if they are taken for prolonged periods in pregnancy.
Fibric acid derivatives
The fibric acid derivatives subclass includes fenofibrate (Tricor, Lofibra) and gemfibrozil (Lopid).
Six reports, involving 13 pregnancies, have described the use of gemfibrozil during all phases of pregnancy. No teratogenic effects were observed in these cases. In one woman, similar concentrations of gemfibrozil and its active metabolite were found in the umbilical vein and artery at levels within the normal reference for adults.
Statins
There are seven HMG-CoA inhibitors, known as statins: atorvastatin (Lipitor), fluvastatin (Lescol), lovastatin (Mevacor), pitavastatin (Livalo), pravastatin (Pravachol), rosuvastatin (Crestor), and simvastatin (Zocor).
The interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia. Moreover, cholesterol and products synthesized by cholesterol are important during fetal development as shown by the rise in maternal cholesterol levels during pregnancy. Although the potential for embryo-fetal harm has not been clearly documented, and that potential may eventually be confirmed as low, the use of these agents in the first trimester are best classified as contraindicated.
One consideration in estimating the embryo-fetal risk of statins is their classification as either lipophilic or hydrophilic. Three of the seven statins are hydrophilic (fluvastatin, pravastatin, and rosuvastatin); the remaining four agents are lipophilic. In a 2004 review of 70 reports, all adverse birth outcomes were reported following exposure to lipophilic statins (atorvastatin, lovastatin, or simvastatin) and none with the hydrophilic pravastatin. The authors stated that the findings were due to the fact that lipophilic agents equilibrate between maternal and embryonic compartments, whereas pravastatin is minimally present in the embryo.3 If this is indeed the case, and a statin must be used during pregnancy, fluvastatin, pravastatin, or rosuvastatin appears to be best.
Pravastatin also has been used for the prevention and treatment of preeclampsia.5,6 Although the teratogenic potential of these agents has not been fully determined, the risk for birth defects, if any, appears to be low even when exposure occurs during organogenesis.7,8,9 Nevertheless, avoiding these products during the first trimester appears to be best.
Immunoglobulins
The only immunoglobulin in the antilipemic class is evolocumab (Repatha), which has no human pregnancy data. It is an immunoglobulin G2 that is indicated as an adjunct to diet and maximally tolerated statin therapy. It is also indicated as an adjunct to diet and other low-density lipoprotein–lowering therapies in patients with homozygous familial hypercholesterolemia who require additional lowering. No adverse embryo-fetal effects were observed in monkeys. Because statins are contraindicated in the first trimester, the drug, if combined with a statin, can also be classified as contraindicated. However, if the drug is used alone, the embryo-fetal risk appears to be low based on the animal data.
Monoclonal antibodies
The protein alirocumab (Praluent) is a human monoclonal antibody. It is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease. There are no human pregnancy data. The animal data in rats and monkeys suggest low embryo-fetal risk. However, suppression of the humoral immune response to keyhole hemocyanin antigen was observed in infant monkeys at 4-6 months of age. The significance of this in human infants is apparently unknown. Because statins are contraindicated in the first trimester, the drug should not be used with these agents during that period.
Oligonucleotide inhibitors
No reports describing the use of mipomersen (Kynamro), an oligonucleotide inhibitor of apolipoprotein B-100 synthesis, in human pregnancy have been located. The drug is indicated as an adjunct to lipid-lowering medications and diet to reduce low-density lipoprotein cholesterol, apolipoprotein B, total cholesterol, and non–high-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. It has a very long (1-2 months) elimination half-life. The drug caused fetal toxicity in rats, but not in mice or rabbits.
Vitamins
Niacin is a water-soluble B complex vitamin that is converted in vivo to niacinamide. Niacin has no known embryo-fetal risk.
Miscellaneous agents
The two agents in the miscellaneous category are ezetimibe and lomitapide. Ezetimibe is indicated, either alone or in combination with a statin, as adjunctive therapy to diet for the reduction of cholesterol and triglycerides. Statins are contraindicated in the first trimester, but ezetimibe alone could be used during that period if treatment of the mother was mandated. The drug caused no problems in rabbits, but in rats, a dose 10 times the human exposure increased the incidence of skeletal abnormalities. In one report, a woman with homozygous familial hypercholesterolemia was treated with direct adsorption of lipoprotein apheresis, ezetimibe, and rosuvastatin. When pregnancy was discovered (gestational age not specified), the two drugs were stopped but biweekly apheresis was continued. At 37 weeks’ gestation, the patient gave birth to a healthy 2,400-g male infant.10
There are no human pregnancy data with lomitapide. It is indicated as an adjunct to a low-fat diet and other lipid-lowering treatments, including low-density lipoprotein apheresis where available, to reduce LDL cholesterol, total cholesterol, apolipoprotein B, and non–high-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. At doses less than 10 times the human dose, the drug caused congenital malformations and embryo-fetal death in rats, rabbits, and ferrets. The manufacturer classifies the drug as contraindicated in pregnancy because of the animal data.
Breastfeeding
Only niacin, pravastatin, and rosuvastatin have data regarding human milk concentrations. Niacin and its active form – niacinamide – are excreted into breast milk.
The average peak milk level in 11 lactating women given pravastatin 20 mg twice daily for 2.5 days was 3.9 mcg/L, whereas the level for the active metabolite was 2.1 mcg/L. Based on these data, a fully breastfed infant would receive daily about 1.4% of the mother’s weight-adjusted dose.11
A 31-year-old woman was treated with rosuvastatin for familial hypercholesterolemia while breastfeeding her infant. The drug was stopped during breastfeeding but was restarted at 33 days post partum. Breast milk concentrations of the drug were 1.2 times serum levels (about 22 ng/mL vs. 18 ng/mL). Unfortunately, no information was provided on the status of the nursing infant.12
Three of the above agents have high molecular weights - alirocumab, evolocumab, and mipomersen - and are probably not excreted into mature breast milk. Moreover, colesevelam is not absorbed, and very small amounts of colestipol are absorbed by mothers. Several antilipemic agents have characteristics (for example, low molecular weight or long elimination half-life) that suggest they will be excreted into breast milk: ezetimibe, fenofibric acid (active metabolite of fenofibrate), gemfibrozil, lomitapide, and all the statins.
Taken in sum, all of the antilipemics, with the exception of niacin, have the potential to cause a deficiency of fat-soluble vitamins (A, D, E, K) in mother’s milk and in the nursing infant. Deficiency is a concern for all of these vitamins, but especially for vitamin K, because it could cause bruising, petechiae, hematomas, and bleeding in the nursing infant. In addition, antilipemics could cause low levels in milk of cholesterol and lipids, which are required by a nursing infant. Consequently, they should not be used by mothers who are breastfeeding an infant.
References
1. Br J Obstet Gynaecol. 1995 Feb;102(2):169-70.
2. J Matern Fetal Neonatal Med. 2015 May;28(8):954-8.
3. Am J Med Genet A. 2004 Dec 15;131(3):287-98.
4. J Clin Invest. 2016 Aug 1;126(8):2933-40.
5. Hypertension. 2015 Sep;66(3):687-97.
6. Am J Obstet Gynecol. 2016 Jun;214(6):720.e1-720.e17.
7. Birth Defects Res A Clin Mol Teratol. 2005 Nov;73(11):888-96.
8. Reprod Toxicol. 2008 Oct;26(2):175-7.
9. Ann Pharmacother. 2012 Oct;46(10):1419-24.
10. Open Cardiovasc Med J. 2015 Dec 29;9:114-7.
11. J Clin Pharmacol. 1988;28:942.
12. Am J Med. 2013 Sep;126(9):e7-e8.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Lipid-lowering medications are some of the most commonly prescribed drugs in the United States. But while much is known about their general safety, the data are limited when it comes to pregnancy and breastfeeding.
Antilipemic agents are a pharmacologic class that contains 18 drugs. The class is divided into eight subclasses: bile acid sequestrants; fibric acid derivatives, HMG-CoA inhibitors; immunoglobulins; monoclonal antibodies; oligonucleotide inhibitors; vitamins; as well as two miscellaneous drugs, ezetimibe (Zetia) and lomitapide (Juxtapid). Another antilipemic – dextrothyroxine – has been removed from the market by the manufacturer.
Bile acid sequestrants
Bile acid sequestrants include cholestyramine (Prevalite, Questran), colesevelam (Welchol), and colestipol (Colestid). These drugs have the potential to cause fetal toxicity. This assessment is based on their mechanism of action. These agents are not absorbed systemically, or absorption is very poor and they bind bile acids into a nonabsorbable complex. This action can reduce intestinal absorption of fat-soluble vitamins A, D, E, and K.
Reports of fetal harm have not been located for the other two agents in this class, but there is only one case report involving five women for colesevelam and no reports for colestipol. Nevertheless, both of these drugs have the potential to cause fetal hemorrhage if they are taken for prolonged periods in pregnancy.
Fibric acid derivatives
The fibric acid derivatives subclass includes fenofibrate (Tricor, Lofibra) and gemfibrozil (Lopid).
Six reports, involving 13 pregnancies, have described the use of gemfibrozil during all phases of pregnancy. No teratogenic effects were observed in these cases. In one woman, similar concentrations of gemfibrozil and its active metabolite were found in the umbilical vein and artery at levels within the normal reference for adults.
Statins
There are seven HMG-CoA inhibitors, known as statins: atorvastatin (Lipitor), fluvastatin (Lescol), lovastatin (Mevacor), pitavastatin (Livalo), pravastatin (Pravachol), rosuvastatin (Crestor), and simvastatin (Zocor).
The interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia. Moreover, cholesterol and products synthesized by cholesterol are important during fetal development as shown by the rise in maternal cholesterol levels during pregnancy. Although the potential for embryo-fetal harm has not been clearly documented, and that potential may eventually be confirmed as low, the use of these agents in the first trimester are best classified as contraindicated.
One consideration in estimating the embryo-fetal risk of statins is their classification as either lipophilic or hydrophilic. Three of the seven statins are hydrophilic (fluvastatin, pravastatin, and rosuvastatin); the remaining four agents are lipophilic. In a 2004 review of 70 reports, all adverse birth outcomes were reported following exposure to lipophilic statins (atorvastatin, lovastatin, or simvastatin) and none with the hydrophilic pravastatin. The authors stated that the findings were due to the fact that lipophilic agents equilibrate between maternal and embryonic compartments, whereas pravastatin is minimally present in the embryo.3 If this is indeed the case, and a statin must be used during pregnancy, fluvastatin, pravastatin, or rosuvastatin appears to be best.
Pravastatin also has been used for the prevention and treatment of preeclampsia.5,6 Although the teratogenic potential of these agents has not been fully determined, the risk for birth defects, if any, appears to be low even when exposure occurs during organogenesis.7,8,9 Nevertheless, avoiding these products during the first trimester appears to be best.
Immunoglobulins
The only immunoglobulin in the antilipemic class is evolocumab (Repatha), which has no human pregnancy data. It is an immunoglobulin G2 that is indicated as an adjunct to diet and maximally tolerated statin therapy. It is also indicated as an adjunct to diet and other low-density lipoprotein–lowering therapies in patients with homozygous familial hypercholesterolemia who require additional lowering. No adverse embryo-fetal effects were observed in monkeys. Because statins are contraindicated in the first trimester, the drug, if combined with a statin, can also be classified as contraindicated. However, if the drug is used alone, the embryo-fetal risk appears to be low based on the animal data.
Monoclonal antibodies
The protein alirocumab (Praluent) is a human monoclonal antibody. It is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease. There are no human pregnancy data. The animal data in rats and monkeys suggest low embryo-fetal risk. However, suppression of the humoral immune response to keyhole hemocyanin antigen was observed in infant monkeys at 4-6 months of age. The significance of this in human infants is apparently unknown. Because statins are contraindicated in the first trimester, the drug should not be used with these agents during that period.
Oligonucleotide inhibitors
No reports describing the use of mipomersen (Kynamro), an oligonucleotide inhibitor of apolipoprotein B-100 synthesis, in human pregnancy have been located. The drug is indicated as an adjunct to lipid-lowering medications and diet to reduce low-density lipoprotein cholesterol, apolipoprotein B, total cholesterol, and non–high-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. It has a very long (1-2 months) elimination half-life. The drug caused fetal toxicity in rats, but not in mice or rabbits.
Vitamins
Niacin is a water-soluble B complex vitamin that is converted in vivo to niacinamide. Niacin has no known embryo-fetal risk.
Miscellaneous agents
The two agents in the miscellaneous category are ezetimibe and lomitapide. Ezetimibe is indicated, either alone or in combination with a statin, as adjunctive therapy to diet for the reduction of cholesterol and triglycerides. Statins are contraindicated in the first trimester, but ezetimibe alone could be used during that period if treatment of the mother was mandated. The drug caused no problems in rabbits, but in rats, a dose 10 times the human exposure increased the incidence of skeletal abnormalities. In one report, a woman with homozygous familial hypercholesterolemia was treated with direct adsorption of lipoprotein apheresis, ezetimibe, and rosuvastatin. When pregnancy was discovered (gestational age not specified), the two drugs were stopped but biweekly apheresis was continued. At 37 weeks’ gestation, the patient gave birth to a healthy 2,400-g male infant.10
There are no human pregnancy data with lomitapide. It is indicated as an adjunct to a low-fat diet and other lipid-lowering treatments, including low-density lipoprotein apheresis where available, to reduce LDL cholesterol, total cholesterol, apolipoprotein B, and non–high-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. At doses less than 10 times the human dose, the drug caused congenital malformations and embryo-fetal death in rats, rabbits, and ferrets. The manufacturer classifies the drug as contraindicated in pregnancy because of the animal data.
Breastfeeding
Only niacin, pravastatin, and rosuvastatin have data regarding human milk concentrations. Niacin and its active form – niacinamide – are excreted into breast milk.
The average peak milk level in 11 lactating women given pravastatin 20 mg twice daily for 2.5 days was 3.9 mcg/L, whereas the level for the active metabolite was 2.1 mcg/L. Based on these data, a fully breastfed infant would receive daily about 1.4% of the mother’s weight-adjusted dose.11
A 31-year-old woman was treated with rosuvastatin for familial hypercholesterolemia while breastfeeding her infant. The drug was stopped during breastfeeding but was restarted at 33 days post partum. Breast milk concentrations of the drug were 1.2 times serum levels (about 22 ng/mL vs. 18 ng/mL). Unfortunately, no information was provided on the status of the nursing infant.12
Three of the above agents have high molecular weights - alirocumab, evolocumab, and mipomersen - and are probably not excreted into mature breast milk. Moreover, colesevelam is not absorbed, and very small amounts of colestipol are absorbed by mothers. Several antilipemic agents have characteristics (for example, low molecular weight or long elimination half-life) that suggest they will be excreted into breast milk: ezetimibe, fenofibric acid (active metabolite of fenofibrate), gemfibrozil, lomitapide, and all the statins.
Taken in sum, all of the antilipemics, with the exception of niacin, have the potential to cause a deficiency of fat-soluble vitamins (A, D, E, K) in mother’s milk and in the nursing infant. Deficiency is a concern for all of these vitamins, but especially for vitamin K, because it could cause bruising, petechiae, hematomas, and bleeding in the nursing infant. In addition, antilipemics could cause low levels in milk of cholesterol and lipids, which are required by a nursing infant. Consequently, they should not be used by mothers who are breastfeeding an infant.
References
1. Br J Obstet Gynaecol. 1995 Feb;102(2):169-70.
2. J Matern Fetal Neonatal Med. 2015 May;28(8):954-8.
3. Am J Med Genet A. 2004 Dec 15;131(3):287-98.
4. J Clin Invest. 2016 Aug 1;126(8):2933-40.
5. Hypertension. 2015 Sep;66(3):687-97.
6. Am J Obstet Gynecol. 2016 Jun;214(6):720.e1-720.e17.
7. Birth Defects Res A Clin Mol Teratol. 2005 Nov;73(11):888-96.
8. Reprod Toxicol. 2008 Oct;26(2):175-7.
9. Ann Pharmacother. 2012 Oct;46(10):1419-24.
10. Open Cardiovasc Med J. 2015 Dec 29;9:114-7.
11. J Clin Pharmacol. 1988;28:942.
12. Am J Med. 2013 Sep;126(9):e7-e8.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Alarming gaps in gestational diabetes care
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
50 years of ob.gyn.: Has practice changed for the better?
As a practicing obstetrician-gynecologist for nearly 40 years, Leonard Brabson, MD, has watched his specialty transform in ways both large and small.
For starters, his regular work attire in 1977 – a shirt and tie – is now scrubs. The paper charts that once filled his office shelves have been replaced with electronic records. And the cumbersome machines that once took blurry, still pictures of a fetus have advanced by leaps and bounds and become a staple of prenatal care.
“Back then, we were expected to be generalists. If [a patient] had a cancer, you took care of it. If [she] had a fertility problem or problem with endocrinology, you took care of it. Of course, now if I have a female cancer, we refer them to the gyn-oncologist. But when you go back 40 years and beyond, we did mostly everything.”
The ob.gyns. of today are practicing in a vastly different environment than their predecessors, and while many of the differences have improved patient care and enhanced efficiency, physicians also note that some changes have harmed the doctor-patient relationship and created career dissatisfaction.
“Certainly, the advances of modern medicine have enabled the current physician to provide the patient a level of care unparalleled in history,” said Charles E. Miller, MD, a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
More volume, less time
Most long-time physicians agree that higher patient volumes and increasing administrative burdens have diminished the time they are able to spend with patients.
Rising clinical documentation and coding are the top administrative tasks taking away from one-on-one patient care, said Kristen Zeligs, MD, chair of the American Congress of Obstetricians and Gynecologists’ Junior Fellow Congress Advisory Council and a gynecologic oncology fellow at Walter Reed National Military Medical Center in Bethesda, Md.
But there are other factors straining the doctor-patient relationship. Decades ago, first-time mothers often stayed with one doctor for a lifetime, Dr. Brabson recalled, having all of her babies delivered by a single ob.gyn. The same can’t be said for today, where insurance changes, job relocations, and a lack of connections often lead patients to switch physicians frequently.
“Now a lot of patients [move on] from one year to the next,” Dr. Brabson said. “There’s not that same loyalty.”
Doctors, too, traditionally stuck with patients over the long haul, he added. In the past, if an ob.gyn treated a patient during pregnancy, that same physician was present during the delivery, even if it meant leaving a vacation early or coming in on a night off.
“Now the younger docs, especially those that have families with small children, when it’s not their night on call and it’s 5 o’clock, they’re checking out,” Dr. Brabson said. “One of my partners right now, she started inducing someone yesterday. Well today’s her day off, so I’m going to do the delivery. That’s been a big change.”
Highs and lows of liability premiums
Another pressure on the specialty over the last couple of decades has been the high cost of liability insurance.
“2003 was really the peak of the liability crisis,” Dr. Montgomery said. “Hospitals were closing. Doctors were giving up practice. It’s a little better now than it was.”
While ob.gyns. still pay higher premiums than many other specialties, the legal climate has improved in recent years, said Paul Greve Jr., executive vice president and senior consultant for the Willis Healthcare Practice, and author of the 2016 Medical Liability Monitor, an annual report that surveys medical liability premiums.
“The best way to characterize the overall environment for medical professional liability is stable,” he said. “In the area of obstetrics, for the first time ever in recent years, we are seeing lower frequency and lower severity [of lawsuits].”
The lower number of filings are largely due to patient safety initiatives among ob.gyn. programs and tort reforms – many of which have been upheld by courts in the last decade, Mr. Greve said.
Of course, the rate of premiums greatly differs depending on location, with ob.gyns. in Eastern New York paying a high of $214,999 and ob.gyns. in Minnesota paying a low of $16,449 in 2016, according to the Medical Liability Monitor.
“The environment is specific to the region,” Mr. Greve said. “New York City is still very problematic. Chicago is still very problematic. There are some pockets around the country where there’s no damage caps, and it’s really tough to defend claims.”
Technology ups and downs
Another fairly new pressure on ob.gyns. is the integration of the electronic health record and the federal reporting requirements that go along with it.
“Most practicing ob.gyns. are really fed up with the computerization of medicine and the tasking and the charting,” Dr. Montgomery said. “For every hour you spend seeing patients, you spend 1 or 2 hours doing computer chart work and paperwork. Most doctors don’t go into medicine so they can type; they go into medicine to take care of patients.”
The Internet age also poses challenges when it comes to patients conducting their own “research,” said Megan Evans, MD, an ob.gyn. at Tufts Medical Center, Boston.
Protecting the security of patients’ medical records in the digital age is another worry, she said.
But for Dr. Evans, who completed her residency training in 2015, having dozens of digital tools at her disposal as she treats patients is definitely an upside to today’s practice environment.
“I can review a practice bulletin, look up the latest treatment regimens, and contact my colleagues with a quick question – all on my iPhone,” she said. “I also believe there is so much potential for electronic medical records and how they communicate with each other.”
Advancements in ultrasound, fetal monitoring, and other medical technologies have also allowed ob.gyns. to intervene earlier and save lives.
Dr. Brabson recalled the helplessness he and other physicians felt in the 1970s when it came to delivering extremely premature babies.
“We didn’t really feel like you could save a baby under 2 pounds,” he said. “When I was a medical student, if you had a baby under 2 pounds, very commonly what they would do is lay the baby up on the table and watch and see how vigorous it was going to be, and if it really did breathe and carry on for awhile, then you might take it to the nursery. The equipment that we have to save babies with today, compared to 40 years ago, that’s a dramatic change.”
A changing focus for the future
If current trends continue, Dr. Brabson’s early experience of being a generalist ob.gyn. won’t be the norm. Instead, more ob.gyns. will choose to subspecialize. Whether this change is positive or negative for the specialty depends on who you ask.
“You could argue the pros and cons for both sides,” Dr. Zeligs said. “For me, it takes away from what drew me to the specialty – the breadth that ob.gyn. offers, both as a primary care specialty and as a surgical subspecialty.”
However, choosing one focus may offer some doctors a way to capture that elusive professional and personal balance, she added.
Despite the changing landscape of clinical duties and business operations, some parts of ob.gyn. practice have remained intact, according to Dr. Brabson. “The most rewarding and enjoyable part of the job is developing a relationship of mutual trust and respect,” he said. “As a result of developing such a relationship, both the patient and the doctor come away with positive feelings. This has not changed.”
[email protected]
On Twitter @legal_med
As a practicing obstetrician-gynecologist for nearly 40 years, Leonard Brabson, MD, has watched his specialty transform in ways both large and small.
For starters, his regular work attire in 1977 – a shirt and tie – is now scrubs. The paper charts that once filled his office shelves have been replaced with electronic records. And the cumbersome machines that once took blurry, still pictures of a fetus have advanced by leaps and bounds and become a staple of prenatal care.
“Back then, we were expected to be generalists. If [a patient] had a cancer, you took care of it. If [she] had a fertility problem or problem with endocrinology, you took care of it. Of course, now if I have a female cancer, we refer them to the gyn-oncologist. But when you go back 40 years and beyond, we did mostly everything.”
The ob.gyns. of today are practicing in a vastly different environment than their predecessors, and while many of the differences have improved patient care and enhanced efficiency, physicians also note that some changes have harmed the doctor-patient relationship and created career dissatisfaction.
“Certainly, the advances of modern medicine have enabled the current physician to provide the patient a level of care unparalleled in history,” said Charles E. Miller, MD, a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
More volume, less time
Most long-time physicians agree that higher patient volumes and increasing administrative burdens have diminished the time they are able to spend with patients.
Rising clinical documentation and coding are the top administrative tasks taking away from one-on-one patient care, said Kristen Zeligs, MD, chair of the American Congress of Obstetricians and Gynecologists’ Junior Fellow Congress Advisory Council and a gynecologic oncology fellow at Walter Reed National Military Medical Center in Bethesda, Md.
But there are other factors straining the doctor-patient relationship. Decades ago, first-time mothers often stayed with one doctor for a lifetime, Dr. Brabson recalled, having all of her babies delivered by a single ob.gyn. The same can’t be said for today, where insurance changes, job relocations, and a lack of connections often lead patients to switch physicians frequently.
“Now a lot of patients [move on] from one year to the next,” Dr. Brabson said. “There’s not that same loyalty.”
Doctors, too, traditionally stuck with patients over the long haul, he added. In the past, if an ob.gyn treated a patient during pregnancy, that same physician was present during the delivery, even if it meant leaving a vacation early or coming in on a night off.
“Now the younger docs, especially those that have families with small children, when it’s not their night on call and it’s 5 o’clock, they’re checking out,” Dr. Brabson said. “One of my partners right now, she started inducing someone yesterday. Well today’s her day off, so I’m going to do the delivery. That’s been a big change.”
Highs and lows of liability premiums
Another pressure on the specialty over the last couple of decades has been the high cost of liability insurance.
“2003 was really the peak of the liability crisis,” Dr. Montgomery said. “Hospitals were closing. Doctors were giving up practice. It’s a little better now than it was.”
While ob.gyns. still pay higher premiums than many other specialties, the legal climate has improved in recent years, said Paul Greve Jr., executive vice president and senior consultant for the Willis Healthcare Practice, and author of the 2016 Medical Liability Monitor, an annual report that surveys medical liability premiums.
“The best way to characterize the overall environment for medical professional liability is stable,” he said. “In the area of obstetrics, for the first time ever in recent years, we are seeing lower frequency and lower severity [of lawsuits].”
The lower number of filings are largely due to patient safety initiatives among ob.gyn. programs and tort reforms – many of which have been upheld by courts in the last decade, Mr. Greve said.
Of course, the rate of premiums greatly differs depending on location, with ob.gyns. in Eastern New York paying a high of $214,999 and ob.gyns. in Minnesota paying a low of $16,449 in 2016, according to the Medical Liability Monitor.
“The environment is specific to the region,” Mr. Greve said. “New York City is still very problematic. Chicago is still very problematic. There are some pockets around the country where there’s no damage caps, and it’s really tough to defend claims.”
Technology ups and downs
Another fairly new pressure on ob.gyns. is the integration of the electronic health record and the federal reporting requirements that go along with it.
“Most practicing ob.gyns. are really fed up with the computerization of medicine and the tasking and the charting,” Dr. Montgomery said. “For every hour you spend seeing patients, you spend 1 or 2 hours doing computer chart work and paperwork. Most doctors don’t go into medicine so they can type; they go into medicine to take care of patients.”
The Internet age also poses challenges when it comes to patients conducting their own “research,” said Megan Evans, MD, an ob.gyn. at Tufts Medical Center, Boston.
Protecting the security of patients’ medical records in the digital age is another worry, she said.
But for Dr. Evans, who completed her residency training in 2015, having dozens of digital tools at her disposal as she treats patients is definitely an upside to today’s practice environment.
“I can review a practice bulletin, look up the latest treatment regimens, and contact my colleagues with a quick question – all on my iPhone,” she said. “I also believe there is so much potential for electronic medical records and how they communicate with each other.”
Advancements in ultrasound, fetal monitoring, and other medical technologies have also allowed ob.gyns. to intervene earlier and save lives.
Dr. Brabson recalled the helplessness he and other physicians felt in the 1970s when it came to delivering extremely premature babies.
“We didn’t really feel like you could save a baby under 2 pounds,” he said. “When I was a medical student, if you had a baby under 2 pounds, very commonly what they would do is lay the baby up on the table and watch and see how vigorous it was going to be, and if it really did breathe and carry on for awhile, then you might take it to the nursery. The equipment that we have to save babies with today, compared to 40 years ago, that’s a dramatic change.”
A changing focus for the future
If current trends continue, Dr. Brabson’s early experience of being a generalist ob.gyn. won’t be the norm. Instead, more ob.gyns. will choose to subspecialize. Whether this change is positive or negative for the specialty depends on who you ask.
“You could argue the pros and cons for both sides,” Dr. Zeligs said. “For me, it takes away from what drew me to the specialty – the breadth that ob.gyn. offers, both as a primary care specialty and as a surgical subspecialty.”
However, choosing one focus may offer some doctors a way to capture that elusive professional and personal balance, she added.
Despite the changing landscape of clinical duties and business operations, some parts of ob.gyn. practice have remained intact, according to Dr. Brabson. “The most rewarding and enjoyable part of the job is developing a relationship of mutual trust and respect,” he said. “As a result of developing such a relationship, both the patient and the doctor come away with positive feelings. This has not changed.”
[email protected]
On Twitter @legal_med
As a practicing obstetrician-gynecologist for nearly 40 years, Leonard Brabson, MD, has watched his specialty transform in ways both large and small.
For starters, his regular work attire in 1977 – a shirt and tie – is now scrubs. The paper charts that once filled his office shelves have been replaced with electronic records. And the cumbersome machines that once took blurry, still pictures of a fetus have advanced by leaps and bounds and become a staple of prenatal care.
“Back then, we were expected to be generalists. If [a patient] had a cancer, you took care of it. If [she] had a fertility problem or problem with endocrinology, you took care of it. Of course, now if I have a female cancer, we refer them to the gyn-oncologist. But when you go back 40 years and beyond, we did mostly everything.”
The ob.gyns. of today are practicing in a vastly different environment than their predecessors, and while many of the differences have improved patient care and enhanced efficiency, physicians also note that some changes have harmed the doctor-patient relationship and created career dissatisfaction.
“Certainly, the advances of modern medicine have enabled the current physician to provide the patient a level of care unparalleled in history,” said Charles E. Miller, MD, a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
More volume, less time
Most long-time physicians agree that higher patient volumes and increasing administrative burdens have diminished the time they are able to spend with patients.
Rising clinical documentation and coding are the top administrative tasks taking away from one-on-one patient care, said Kristen Zeligs, MD, chair of the American Congress of Obstetricians and Gynecologists’ Junior Fellow Congress Advisory Council and a gynecologic oncology fellow at Walter Reed National Military Medical Center in Bethesda, Md.
But there are other factors straining the doctor-patient relationship. Decades ago, first-time mothers often stayed with one doctor for a lifetime, Dr. Brabson recalled, having all of her babies delivered by a single ob.gyn. The same can’t be said for today, where insurance changes, job relocations, and a lack of connections often lead patients to switch physicians frequently.
“Now a lot of patients [move on] from one year to the next,” Dr. Brabson said. “There’s not that same loyalty.”
Doctors, too, traditionally stuck with patients over the long haul, he added. In the past, if an ob.gyn treated a patient during pregnancy, that same physician was present during the delivery, even if it meant leaving a vacation early or coming in on a night off.
“Now the younger docs, especially those that have families with small children, when it’s not their night on call and it’s 5 o’clock, they’re checking out,” Dr. Brabson said. “One of my partners right now, she started inducing someone yesterday. Well today’s her day off, so I’m going to do the delivery. That’s been a big change.”
Highs and lows of liability premiums
Another pressure on the specialty over the last couple of decades has been the high cost of liability insurance.
“2003 was really the peak of the liability crisis,” Dr. Montgomery said. “Hospitals were closing. Doctors were giving up practice. It’s a little better now than it was.”
While ob.gyns. still pay higher premiums than many other specialties, the legal climate has improved in recent years, said Paul Greve Jr., executive vice president and senior consultant for the Willis Healthcare Practice, and author of the 2016 Medical Liability Monitor, an annual report that surveys medical liability premiums.
“The best way to characterize the overall environment for medical professional liability is stable,” he said. “In the area of obstetrics, for the first time ever in recent years, we are seeing lower frequency and lower severity [of lawsuits].”
The lower number of filings are largely due to patient safety initiatives among ob.gyn. programs and tort reforms – many of which have been upheld by courts in the last decade, Mr. Greve said.
Of course, the rate of premiums greatly differs depending on location, with ob.gyns. in Eastern New York paying a high of $214,999 and ob.gyns. in Minnesota paying a low of $16,449 in 2016, according to the Medical Liability Monitor.
“The environment is specific to the region,” Mr. Greve said. “New York City is still very problematic. Chicago is still very problematic. There are some pockets around the country where there’s no damage caps, and it’s really tough to defend claims.”
Technology ups and downs
Another fairly new pressure on ob.gyns. is the integration of the electronic health record and the federal reporting requirements that go along with it.
“Most practicing ob.gyns. are really fed up with the computerization of medicine and the tasking and the charting,” Dr. Montgomery said. “For every hour you spend seeing patients, you spend 1 or 2 hours doing computer chart work and paperwork. Most doctors don’t go into medicine so they can type; they go into medicine to take care of patients.”
The Internet age also poses challenges when it comes to patients conducting their own “research,” said Megan Evans, MD, an ob.gyn. at Tufts Medical Center, Boston.
Protecting the security of patients’ medical records in the digital age is another worry, she said.
But for Dr. Evans, who completed her residency training in 2015, having dozens of digital tools at her disposal as she treats patients is definitely an upside to today’s practice environment.
“I can review a practice bulletin, look up the latest treatment regimens, and contact my colleagues with a quick question – all on my iPhone,” she said. “I also believe there is so much potential for electronic medical records and how they communicate with each other.”
Advancements in ultrasound, fetal monitoring, and other medical technologies have also allowed ob.gyns. to intervene earlier and save lives.
Dr. Brabson recalled the helplessness he and other physicians felt in the 1970s when it came to delivering extremely premature babies.
“We didn’t really feel like you could save a baby under 2 pounds,” he said. “When I was a medical student, if you had a baby under 2 pounds, very commonly what they would do is lay the baby up on the table and watch and see how vigorous it was going to be, and if it really did breathe and carry on for awhile, then you might take it to the nursery. The equipment that we have to save babies with today, compared to 40 years ago, that’s a dramatic change.”
A changing focus for the future
If current trends continue, Dr. Brabson’s early experience of being a generalist ob.gyn. won’t be the norm. Instead, more ob.gyns. will choose to subspecialize. Whether this change is positive or negative for the specialty depends on who you ask.
“You could argue the pros and cons for both sides,” Dr. Zeligs said. “For me, it takes away from what drew me to the specialty – the breadth that ob.gyn. offers, both as a primary care specialty and as a surgical subspecialty.”
However, choosing one focus may offer some doctors a way to capture that elusive professional and personal balance, she added.
Despite the changing landscape of clinical duties and business operations, some parts of ob.gyn. practice have remained intact, according to Dr. Brabson. “The most rewarding and enjoyable part of the job is developing a relationship of mutual trust and respect,” he said. “As a result of developing such a relationship, both the patient and the doctor come away with positive feelings. This has not changed.”
[email protected]
On Twitter @legal_med
Vitamin D supplementation recommended in all children, teens
SAN FRANCISCO – Vitamin D deficiency is common among children and adolescents, particularly those with chronic disease, Catherine Gordon, MD, said at the annual meeting of the American Academy of Pediatrics.
Yet the precise definition of vitamin D deficiency and the healthy threshold for vitamin D levels lack universally agreed-upon standards. Generally speaking, levels of at least 30 ng/mL (75 nmol/L) appear safe and reasonable for children with chronic disease, and additional research is confirming whether this range is appropriate for other pediatric groups as well. Although too much vitamin D can lead to hypercalcemia, vitamin D intoxication is very rare, said Dr. Gordon, director of the division of adolescents and transition medicine at the University of Cincinnati.
Severe vitamin D deficiency can lead to rickets, when bones have insufficient calcium and phosphorus levels, resulting in bone softening and weakening before growth plates close. If not treated with vitamin D and calcium supplementation, rickets becomes osteomalacia after the growth plates close.
Vitamin D deficiency rates vary by population
It’s difficult to pin down rates of vitamin D deficiency. One 2004 study of just over 300 children found nearly a quarter of them (24%) were deficient based on a threshold of levels below 15 ng/mL, and another 42% had insufficient levels, defined as 20 ng/mL or lower, but all were asymptomatic. Another 2008 study using different cut-offs found that 12% of healthy 8- to 24-month-olds were deficient, defined as levels below 20 ng/mL. Forty percent of the children had suboptimal levels below 30 ng/mL. Overall, a third of the children showed demineralization on their x-rays. While the season of the year and race/ethnicity did not emerge as predictors of vitamin D insufficiency, breastfeeding without supplementation and lack of milk consumption did.
Because the vitamin D content in human breast milk is low, breastfed infants typically develop low vitamin D levels unless they receive supplementation or plenty of exposure to sunlight. A maternal dose of 6,400 IU of vitamin D is needed for breastfed infants to reach normal vitamin D levels, Dr. Gordon said. Babies born to mothers with vitamin D deficiency have the highest risk of becoming deficient themselves, although formula-fed babies usually receive plenty through the vitamin D fortification in infant formula.
Among adolescents, obesity remains a common risk factor, and those with obesity require higher doses to correct deficiency or insufficiency. A study in the Journal of Pediatrics this year found that adult-sized teens need at least 5,000 IU of vitamin D3 a day for 8 weeks to correct deficiency. Similarly, a small 2012 study of 61 children and adolescents with inflammatory bowel disease found that supplementation of 2,000 IU of vitamin D3 daily or 50,000 IU of D2 weekly, for 6 weeks, more effectively corrected vitamin D deficiency than 2,000 IU daily of vitamin D2 without any changes to parathyroid hormone suppression.
How much to supplement
Much debate and uncertainty surround how much (if at all) healthy infants, children, and adolescents should be supplemented with vitamin D. The American Academy of Pediatrics recommends daily supplementation of 400 IU of vitamin D from birth through adolescence for all children and teens, although that’s far below the safe upper limit of vitamin D intake, Dr. Gordon said.
The health and sciences division (formerly the Institute of Medicine) of the National Academies of Sciences, Engineering, and Medicine, by contrast, recommends a daily intake of 400 IU of vitamin D for the first year of life and then 600 IU for age 1 through old age. The safe upper limits set by the health and sciences division include 1,000 IU for infants up to 6 months old, 1,500 IU for infants aged 6 months to 1 year, 2,500 IU for toddlers up to 3 years, 3,000 IU for children aged 4-8 years, and 4,000 IU for those 9 years and older.
Yet the Endocrine Society recommends a greater amount of supplementation for children at risk for vitamin D deficiency or low bone density mass: from 400 to 1,000 IU for children 1 year and younger, and 600-1,000 IU for all older children, adolescents, and adults. The Endocrine Society also cites a higher safe upper limit of 2,000 IU for infants up to 12 months and 4,000 IU for those aged 1 year and up.
Part of the discordance in these recommendations lies in what populations they are aimed at, Dr. Gordon explained. While the health and sciences division recommendations were written for healthy children and adolescents, the Endocrine Society is specifically addressing those in risk groups, such as transplant recipients, those with chronic conditions that can cause malabsorption, and those taking anticonvulsants or receiving other treatments that can threaten bone health. Among older children and adolescents, anorexia nervosa is also a risk factor for inadequate vitamin D levels.
Dr. Gordon recommended 600 IU of vitamin D daily for all healthy children and teens while noting that those in risk groups may require 1,000-2,000 IU to prevent vitamin D deficiency.
Additional concerns with inadequate vitamin D
Aside from bone mineral density and levels of 25(OH)D (25-hydroxy vitamin D) and parathyroid hormone, vitamin D insufficiency may be suspected based on several other biomarkers, including fractures or falls, intestinal calcium absorption, dental health, insulin sensitivity, beta-cell or immune functioning, respiratory disease such as wheezing or tuberculosis, and possibly hypertension.
Researchers have developed new interest in exploring whether factors during childhood and adolescence – critical years for bone acquisition – such as vitamin D levels might influence the risk for osteoporosis later in life, Dr. Gordon said.
Both males and females reach their peak bone mass and skeletal strength in their early to mid-20s and maintain these through about their mid-40s. While individuals have no control over intrinsic factors that help determine their bone mass, such as sex, family history, and ethnicity, other extrinsic factors are also bone mass determinants, including diet, body mass, a particular individual’s hormonal mix, illnesses and their treatments, physical activity level, and lifestyle choices.
Therefore, health providers should encourage patients to regularly exercise, maintain a healthy weight, eat healthfully, and take daily supplements, Dr. Gordon said. She only recommended testing 25(OH)D levels in those at risk for deficiency and/or low bone mass.
Dr. Gordon reported no relevant financial disclosures.
SAN FRANCISCO – Vitamin D deficiency is common among children and adolescents, particularly those with chronic disease, Catherine Gordon, MD, said at the annual meeting of the American Academy of Pediatrics.
Yet the precise definition of vitamin D deficiency and the healthy threshold for vitamin D levels lack universally agreed-upon standards. Generally speaking, levels of at least 30 ng/mL (75 nmol/L) appear safe and reasonable for children with chronic disease, and additional research is confirming whether this range is appropriate for other pediatric groups as well. Although too much vitamin D can lead to hypercalcemia, vitamin D intoxication is very rare, said Dr. Gordon, director of the division of adolescents and transition medicine at the University of Cincinnati.
Severe vitamin D deficiency can lead to rickets, when bones have insufficient calcium and phosphorus levels, resulting in bone softening and weakening before growth plates close. If not treated with vitamin D and calcium supplementation, rickets becomes osteomalacia after the growth plates close.
Vitamin D deficiency rates vary by population
It’s difficult to pin down rates of vitamin D deficiency. One 2004 study of just over 300 children found nearly a quarter of them (24%) were deficient based on a threshold of levels below 15 ng/mL, and another 42% had insufficient levels, defined as 20 ng/mL or lower, but all were asymptomatic. Another 2008 study using different cut-offs found that 12% of healthy 8- to 24-month-olds were deficient, defined as levels below 20 ng/mL. Forty percent of the children had suboptimal levels below 30 ng/mL. Overall, a third of the children showed demineralization on their x-rays. While the season of the year and race/ethnicity did not emerge as predictors of vitamin D insufficiency, breastfeeding without supplementation and lack of milk consumption did.
Because the vitamin D content in human breast milk is low, breastfed infants typically develop low vitamin D levels unless they receive supplementation or plenty of exposure to sunlight. A maternal dose of 6,400 IU of vitamin D is needed for breastfed infants to reach normal vitamin D levels, Dr. Gordon said. Babies born to mothers with vitamin D deficiency have the highest risk of becoming deficient themselves, although formula-fed babies usually receive plenty through the vitamin D fortification in infant formula.
Among adolescents, obesity remains a common risk factor, and those with obesity require higher doses to correct deficiency or insufficiency. A study in the Journal of Pediatrics this year found that adult-sized teens need at least 5,000 IU of vitamin D3 a day for 8 weeks to correct deficiency. Similarly, a small 2012 study of 61 children and adolescents with inflammatory bowel disease found that supplementation of 2,000 IU of vitamin D3 daily or 50,000 IU of D2 weekly, for 6 weeks, more effectively corrected vitamin D deficiency than 2,000 IU daily of vitamin D2 without any changes to parathyroid hormone suppression.
How much to supplement
Much debate and uncertainty surround how much (if at all) healthy infants, children, and adolescents should be supplemented with vitamin D. The American Academy of Pediatrics recommends daily supplementation of 400 IU of vitamin D from birth through adolescence for all children and teens, although that’s far below the safe upper limit of vitamin D intake, Dr. Gordon said.
The health and sciences division (formerly the Institute of Medicine) of the National Academies of Sciences, Engineering, and Medicine, by contrast, recommends a daily intake of 400 IU of vitamin D for the first year of life and then 600 IU for age 1 through old age. The safe upper limits set by the health and sciences division include 1,000 IU for infants up to 6 months old, 1,500 IU for infants aged 6 months to 1 year, 2,500 IU for toddlers up to 3 years, 3,000 IU for children aged 4-8 years, and 4,000 IU for those 9 years and older.
Yet the Endocrine Society recommends a greater amount of supplementation for children at risk for vitamin D deficiency or low bone density mass: from 400 to 1,000 IU for children 1 year and younger, and 600-1,000 IU for all older children, adolescents, and adults. The Endocrine Society also cites a higher safe upper limit of 2,000 IU for infants up to 12 months and 4,000 IU for those aged 1 year and up.
Part of the discordance in these recommendations lies in what populations they are aimed at, Dr. Gordon explained. While the health and sciences division recommendations were written for healthy children and adolescents, the Endocrine Society is specifically addressing those in risk groups, such as transplant recipients, those with chronic conditions that can cause malabsorption, and those taking anticonvulsants or receiving other treatments that can threaten bone health. Among older children and adolescents, anorexia nervosa is also a risk factor for inadequate vitamin D levels.
Dr. Gordon recommended 600 IU of vitamin D daily for all healthy children and teens while noting that those in risk groups may require 1,000-2,000 IU to prevent vitamin D deficiency.
Additional concerns with inadequate vitamin D
Aside from bone mineral density and levels of 25(OH)D (25-hydroxy vitamin D) and parathyroid hormone, vitamin D insufficiency may be suspected based on several other biomarkers, including fractures or falls, intestinal calcium absorption, dental health, insulin sensitivity, beta-cell or immune functioning, respiratory disease such as wheezing or tuberculosis, and possibly hypertension.
Researchers have developed new interest in exploring whether factors during childhood and adolescence – critical years for bone acquisition – such as vitamin D levels might influence the risk for osteoporosis later in life, Dr. Gordon said.
Both males and females reach their peak bone mass and skeletal strength in their early to mid-20s and maintain these through about their mid-40s. While individuals have no control over intrinsic factors that help determine their bone mass, such as sex, family history, and ethnicity, other extrinsic factors are also bone mass determinants, including diet, body mass, a particular individual’s hormonal mix, illnesses and their treatments, physical activity level, and lifestyle choices.
Therefore, health providers should encourage patients to regularly exercise, maintain a healthy weight, eat healthfully, and take daily supplements, Dr. Gordon said. She only recommended testing 25(OH)D levels in those at risk for deficiency and/or low bone mass.
Dr. Gordon reported no relevant financial disclosures.
SAN FRANCISCO – Vitamin D deficiency is common among children and adolescents, particularly those with chronic disease, Catherine Gordon, MD, said at the annual meeting of the American Academy of Pediatrics.
Yet the precise definition of vitamin D deficiency and the healthy threshold for vitamin D levels lack universally agreed-upon standards. Generally speaking, levels of at least 30 ng/mL (75 nmol/L) appear safe and reasonable for children with chronic disease, and additional research is confirming whether this range is appropriate for other pediatric groups as well. Although too much vitamin D can lead to hypercalcemia, vitamin D intoxication is very rare, said Dr. Gordon, director of the division of adolescents and transition medicine at the University of Cincinnati.
Severe vitamin D deficiency can lead to rickets, when bones have insufficient calcium and phosphorus levels, resulting in bone softening and weakening before growth plates close. If not treated with vitamin D and calcium supplementation, rickets becomes osteomalacia after the growth plates close.
Vitamin D deficiency rates vary by population
It’s difficult to pin down rates of vitamin D deficiency. One 2004 study of just over 300 children found nearly a quarter of them (24%) were deficient based on a threshold of levels below 15 ng/mL, and another 42% had insufficient levels, defined as 20 ng/mL or lower, but all were asymptomatic. Another 2008 study using different cut-offs found that 12% of healthy 8- to 24-month-olds were deficient, defined as levels below 20 ng/mL. Forty percent of the children had suboptimal levels below 30 ng/mL. Overall, a third of the children showed demineralization on their x-rays. While the season of the year and race/ethnicity did not emerge as predictors of vitamin D insufficiency, breastfeeding without supplementation and lack of milk consumption did.
Because the vitamin D content in human breast milk is low, breastfed infants typically develop low vitamin D levels unless they receive supplementation or plenty of exposure to sunlight. A maternal dose of 6,400 IU of vitamin D is needed for breastfed infants to reach normal vitamin D levels, Dr. Gordon said. Babies born to mothers with vitamin D deficiency have the highest risk of becoming deficient themselves, although formula-fed babies usually receive plenty through the vitamin D fortification in infant formula.
Among adolescents, obesity remains a common risk factor, and those with obesity require higher doses to correct deficiency or insufficiency. A study in the Journal of Pediatrics this year found that adult-sized teens need at least 5,000 IU of vitamin D3 a day for 8 weeks to correct deficiency. Similarly, a small 2012 study of 61 children and adolescents with inflammatory bowel disease found that supplementation of 2,000 IU of vitamin D3 daily or 50,000 IU of D2 weekly, for 6 weeks, more effectively corrected vitamin D deficiency than 2,000 IU daily of vitamin D2 without any changes to parathyroid hormone suppression.
How much to supplement
Much debate and uncertainty surround how much (if at all) healthy infants, children, and adolescents should be supplemented with vitamin D. The American Academy of Pediatrics recommends daily supplementation of 400 IU of vitamin D from birth through adolescence for all children and teens, although that’s far below the safe upper limit of vitamin D intake, Dr. Gordon said.
The health and sciences division (formerly the Institute of Medicine) of the National Academies of Sciences, Engineering, and Medicine, by contrast, recommends a daily intake of 400 IU of vitamin D for the first year of life and then 600 IU for age 1 through old age. The safe upper limits set by the health and sciences division include 1,000 IU for infants up to 6 months old, 1,500 IU for infants aged 6 months to 1 year, 2,500 IU for toddlers up to 3 years, 3,000 IU for children aged 4-8 years, and 4,000 IU for those 9 years and older.
Yet the Endocrine Society recommends a greater amount of supplementation for children at risk for vitamin D deficiency or low bone density mass: from 400 to 1,000 IU for children 1 year and younger, and 600-1,000 IU for all older children, adolescents, and adults. The Endocrine Society also cites a higher safe upper limit of 2,000 IU for infants up to 12 months and 4,000 IU for those aged 1 year and up.
Part of the discordance in these recommendations lies in what populations they are aimed at, Dr. Gordon explained. While the health and sciences division recommendations were written for healthy children and adolescents, the Endocrine Society is specifically addressing those in risk groups, such as transplant recipients, those with chronic conditions that can cause malabsorption, and those taking anticonvulsants or receiving other treatments that can threaten bone health. Among older children and adolescents, anorexia nervosa is also a risk factor for inadequate vitamin D levels.
Dr. Gordon recommended 600 IU of vitamin D daily for all healthy children and teens while noting that those in risk groups may require 1,000-2,000 IU to prevent vitamin D deficiency.
Additional concerns with inadequate vitamin D
Aside from bone mineral density and levels of 25(OH)D (25-hydroxy vitamin D) and parathyroid hormone, vitamin D insufficiency may be suspected based on several other biomarkers, including fractures or falls, intestinal calcium absorption, dental health, insulin sensitivity, beta-cell or immune functioning, respiratory disease such as wheezing or tuberculosis, and possibly hypertension.
Researchers have developed new interest in exploring whether factors during childhood and adolescence – critical years for bone acquisition – such as vitamin D levels might influence the risk for osteoporosis later in life, Dr. Gordon said.
Both males and females reach their peak bone mass and skeletal strength in their early to mid-20s and maintain these through about their mid-40s. While individuals have no control over intrinsic factors that help determine their bone mass, such as sex, family history, and ethnicity, other extrinsic factors are also bone mass determinants, including diet, body mass, a particular individual’s hormonal mix, illnesses and their treatments, physical activity level, and lifestyle choices.
Therefore, health providers should encourage patients to regularly exercise, maintain a healthy weight, eat healthfully, and take daily supplements, Dr. Gordon said. She only recommended testing 25(OH)D levels in those at risk for deficiency and/or low bone mass.
Dr. Gordon reported no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 16
Microsensor perfectly distinguished coagulopathy patients from controls
SAN DIEGO – Using less than a drop of blood, a portable microsensor provided a comprehensive coagulation profile in less than 15 minutes and perfectly distinguished various coagulopathies from normal blood samples – handily beating the results with both activated partial thromboplastin time (aPTT) and prothrombin time (PT).
Dubbed ClotChip, the disposable device detects coagulation factors and platelet activity using dielectric spectroscopy, Evi X. Stavrou, MD, said at the annual meeting of the American Society of Hematology. The development points the way for comprehensive, rapid, point-of-care assessment of critically ill or severely injured patients and those who need ongoing monitoring to evaluate response to anticoagulant therapy, she added.
Existing point-of-care coagulation assays have several shortcomings, Dr. Stavrou, of Case Western Reserve University, Cleveland, said during a press briefing at the conference. They are relatively insensitive, fail to measure platelet activity, or are only approved for specific subgroups of patients, such as those on warfarin, she specified.
To develop an alternative, Dr. Stavrou and her associates added a parallel-plate capacitive sensing structure to an inexpensive, disposable microfluidic biochip designed to test 9 microliters (less than one drop) of blood. They built the microsensor from biocompatible and chemically inert materials to minimize the chances of artificial contact activation.
To test the device, the researchers used calcium dichloride to induce coagulation in whole blood samples from 11 controls with normal aPTT and PT values. Time curves of output from the microsensor showed that coagulation consistently peaked within 4.5 to 6 minutes.
Next, the investigators tested blood from 12 patients with coagulopathies, including hemophilia A, hemophilia B, acquired von Willebrand factor defect, and congenital hypodysfibrinogenemia. These samples all yielded abnormal curves, with prolonged times to peak that ranged between 7 and 15 minutes – significantly exceeding those of healthy controls (P = .0002).
By plotting rates of true positives against rates of true negatives, the researchers obtained areas under the receiver operating curves of 100% for ClotChip, 78% for aPTT, and 57% for PT. In other words, ClotChip correctly identified all cases and controls in this small patient cohort, which neither aPTT or PT did.
Finally, the researchers used the microsensor to measure coagulation activity in normal blood samples that they treated with prostaglandin E2 to inhibit platelet aggregation. Normalized permittivity (an electrical measure) was significantly lower than in untreated control samples (P = .03), but time to peak values were the same in both groups. This finding confirms that the chip can identify abnormal platelet function, Dr. Stavrou said. “ClotChip is sensitive to the complete hemostasis process, exhibits better sensitivity and specificity than conventional coagulation assays, and discriminates between coagulation and platelet defects,” she concluded.
The investigators are recruiting volunteers for an expanded round of testing for the device, and are working to optimize construction to further enhance its sensitivity.
Dr. Stavrou and her coinvestigators had no relevant financial disclosures.
SAN DIEGO – Using less than a drop of blood, a portable microsensor provided a comprehensive coagulation profile in less than 15 minutes and perfectly distinguished various coagulopathies from normal blood samples – handily beating the results with both activated partial thromboplastin time (aPTT) and prothrombin time (PT).
Dubbed ClotChip, the disposable device detects coagulation factors and platelet activity using dielectric spectroscopy, Evi X. Stavrou, MD, said at the annual meeting of the American Society of Hematology. The development points the way for comprehensive, rapid, point-of-care assessment of critically ill or severely injured patients and those who need ongoing monitoring to evaluate response to anticoagulant therapy, she added.
Existing point-of-care coagulation assays have several shortcomings, Dr. Stavrou, of Case Western Reserve University, Cleveland, said during a press briefing at the conference. They are relatively insensitive, fail to measure platelet activity, or are only approved for specific subgroups of patients, such as those on warfarin, she specified.
To develop an alternative, Dr. Stavrou and her associates added a parallel-plate capacitive sensing structure to an inexpensive, disposable microfluidic biochip designed to test 9 microliters (less than one drop) of blood. They built the microsensor from biocompatible and chemically inert materials to minimize the chances of artificial contact activation.
To test the device, the researchers used calcium dichloride to induce coagulation in whole blood samples from 11 controls with normal aPTT and PT values. Time curves of output from the microsensor showed that coagulation consistently peaked within 4.5 to 6 minutes.
Next, the investigators tested blood from 12 patients with coagulopathies, including hemophilia A, hemophilia B, acquired von Willebrand factor defect, and congenital hypodysfibrinogenemia. These samples all yielded abnormal curves, with prolonged times to peak that ranged between 7 and 15 minutes – significantly exceeding those of healthy controls (P = .0002).
By plotting rates of true positives against rates of true negatives, the researchers obtained areas under the receiver operating curves of 100% for ClotChip, 78% for aPTT, and 57% for PT. In other words, ClotChip correctly identified all cases and controls in this small patient cohort, which neither aPTT or PT did.
Finally, the researchers used the microsensor to measure coagulation activity in normal blood samples that they treated with prostaglandin E2 to inhibit platelet aggregation. Normalized permittivity (an electrical measure) was significantly lower than in untreated control samples (P = .03), but time to peak values were the same in both groups. This finding confirms that the chip can identify abnormal platelet function, Dr. Stavrou said. “ClotChip is sensitive to the complete hemostasis process, exhibits better sensitivity and specificity than conventional coagulation assays, and discriminates between coagulation and platelet defects,” she concluded.
The investigators are recruiting volunteers for an expanded round of testing for the device, and are working to optimize construction to further enhance its sensitivity.
Dr. Stavrou and her coinvestigators had no relevant financial disclosures.
SAN DIEGO – Using less than a drop of blood, a portable microsensor provided a comprehensive coagulation profile in less than 15 minutes and perfectly distinguished various coagulopathies from normal blood samples – handily beating the results with both activated partial thromboplastin time (aPTT) and prothrombin time (PT).
Dubbed ClotChip, the disposable device detects coagulation factors and platelet activity using dielectric spectroscopy, Evi X. Stavrou, MD, said at the annual meeting of the American Society of Hematology. The development points the way for comprehensive, rapid, point-of-care assessment of critically ill or severely injured patients and those who need ongoing monitoring to evaluate response to anticoagulant therapy, she added.
Existing point-of-care coagulation assays have several shortcomings, Dr. Stavrou, of Case Western Reserve University, Cleveland, said during a press briefing at the conference. They are relatively insensitive, fail to measure platelet activity, or are only approved for specific subgroups of patients, such as those on warfarin, she specified.
To develop an alternative, Dr. Stavrou and her associates added a parallel-plate capacitive sensing structure to an inexpensive, disposable microfluidic biochip designed to test 9 microliters (less than one drop) of blood. They built the microsensor from biocompatible and chemically inert materials to minimize the chances of artificial contact activation.
To test the device, the researchers used calcium dichloride to induce coagulation in whole blood samples from 11 controls with normal aPTT and PT values. Time curves of output from the microsensor showed that coagulation consistently peaked within 4.5 to 6 minutes.
Next, the investigators tested blood from 12 patients with coagulopathies, including hemophilia A, hemophilia B, acquired von Willebrand factor defect, and congenital hypodysfibrinogenemia. These samples all yielded abnormal curves, with prolonged times to peak that ranged between 7 and 15 minutes – significantly exceeding those of healthy controls (P = .0002).
By plotting rates of true positives against rates of true negatives, the researchers obtained areas under the receiver operating curves of 100% for ClotChip, 78% for aPTT, and 57% for PT. In other words, ClotChip correctly identified all cases and controls in this small patient cohort, which neither aPTT or PT did.
Finally, the researchers used the microsensor to measure coagulation activity in normal blood samples that they treated with prostaglandin E2 to inhibit platelet aggregation. Normalized permittivity (an electrical measure) was significantly lower than in untreated control samples (P = .03), but time to peak values were the same in both groups. This finding confirms that the chip can identify abnormal platelet function, Dr. Stavrou said. “ClotChip is sensitive to the complete hemostasis process, exhibits better sensitivity and specificity than conventional coagulation assays, and discriminates between coagulation and platelet defects,” she concluded.
The investigators are recruiting volunteers for an expanded round of testing for the device, and are working to optimize construction to further enhance its sensitivity.
Dr. Stavrou and her coinvestigators had no relevant financial disclosures.
AT ASH 2016
Key clinical point: A prototype point-of-care microsensor perfectly distinguished patients with various coagulopathies from healthy controls.
Major finding: The area under the receiver operating characteristic curve was 100%, compared with 78% for aPTT and 59% for PT.
Data source: Oral and poster sessions at ASH 2016.
Disclosures: None of the investigators had relevant financial disclosures.
RSV is top cause of severe respiratory disease in preterm infants
Respiratory syncytial virus is the number one virus causing severe lower respiratory disease in preterm infants, while those of younger age and those exposed to young children are at greatest risk, Eric A. F. Simões, MD, of the University of Colorado at Denver, Aurora, and his coauthors reported in the Nov. 29 edition of PLOS ONE.
“These data demonstrate that higher risk for 32 to 35 wGA [weeks gestational age] infants can be easily identified by age or birth month and significant exposure to other young children,” they wrote. “These infants would benefit from targeted efforts to prevent severe RSV disease.”
The overall rates of lower respiratory infections per 100 infant-seasons – a season being 5 months of observation from November 1 to March 31 in 2009-2010 or 2010-2011 – were 13.7 for respiratory syncytial virus (RSV), 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, 1.3 for human metapneumovirus, and 0.3 for parainfluenza virus type 2 (PLoS One. 2016 Nov 29. doi: 10.1371/journal.pone.0166226).
Infants who had been exposed to young children, either through attending day care or living with non–multiple birth preschool-age siblings, had a twofold higher risk of RSV and human metapneumovirus, and a 3.3-fold greater risk of adenovirus.
The youngest infants showed the highest rate of hospitalizations with RSV: the incidence ranged from 8.2 per 100 infant season in those aged less than 1 month to 2.3 per 100 infant seasons in those aged 10 months of age. Similarly, the incidence of admission to ICU was significantly higher among younger infants.
Infants born in May, before the RSV season, had a much lower incidence of hospitalization, compared with those born in the height of RSV season in February. ICU admission rates also were higher among those born in February, compared with those born in May.
The highest overall rates of hospitalization with RSV – 19 per 100 infant-seasons – were among those born in February, and also those who were exposed to other young children.
“The current results are unique in that they provide continuous age-based risk models for outpatient and inpatient disease for infants with and without young child exposure,” wrote Dr. Simões and his coauthors.
They argued that their findings refute earlier suggestions that the rate of RSV infection in preterm infants is similar to the rate in term infants, and suggested that the limitations of their study may have even underestimated the incidence in preterm babies.
The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
Respiratory syncytial virus is the number one virus causing severe lower respiratory disease in preterm infants, while those of younger age and those exposed to young children are at greatest risk, Eric A. F. Simões, MD, of the University of Colorado at Denver, Aurora, and his coauthors reported in the Nov. 29 edition of PLOS ONE.
“These data demonstrate that higher risk for 32 to 35 wGA [weeks gestational age] infants can be easily identified by age or birth month and significant exposure to other young children,” they wrote. “These infants would benefit from targeted efforts to prevent severe RSV disease.”
The overall rates of lower respiratory infections per 100 infant-seasons – a season being 5 months of observation from November 1 to March 31 in 2009-2010 or 2010-2011 – were 13.7 for respiratory syncytial virus (RSV), 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, 1.3 for human metapneumovirus, and 0.3 for parainfluenza virus type 2 (PLoS One. 2016 Nov 29. doi: 10.1371/journal.pone.0166226).
Infants who had been exposed to young children, either through attending day care or living with non–multiple birth preschool-age siblings, had a twofold higher risk of RSV and human metapneumovirus, and a 3.3-fold greater risk of adenovirus.
The youngest infants showed the highest rate of hospitalizations with RSV: the incidence ranged from 8.2 per 100 infant season in those aged less than 1 month to 2.3 per 100 infant seasons in those aged 10 months of age. Similarly, the incidence of admission to ICU was significantly higher among younger infants.
Infants born in May, before the RSV season, had a much lower incidence of hospitalization, compared with those born in the height of RSV season in February. ICU admission rates also were higher among those born in February, compared with those born in May.
The highest overall rates of hospitalization with RSV – 19 per 100 infant-seasons – were among those born in February, and also those who were exposed to other young children.
“The current results are unique in that they provide continuous age-based risk models for outpatient and inpatient disease for infants with and without young child exposure,” wrote Dr. Simões and his coauthors.
They argued that their findings refute earlier suggestions that the rate of RSV infection in preterm infants is similar to the rate in term infants, and suggested that the limitations of their study may have even underestimated the incidence in preterm babies.
The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
Respiratory syncytial virus is the number one virus causing severe lower respiratory disease in preterm infants, while those of younger age and those exposed to young children are at greatest risk, Eric A. F. Simões, MD, of the University of Colorado at Denver, Aurora, and his coauthors reported in the Nov. 29 edition of PLOS ONE.
“These data demonstrate that higher risk for 32 to 35 wGA [weeks gestational age] infants can be easily identified by age or birth month and significant exposure to other young children,” they wrote. “These infants would benefit from targeted efforts to prevent severe RSV disease.”
The overall rates of lower respiratory infections per 100 infant-seasons – a season being 5 months of observation from November 1 to March 31 in 2009-2010 or 2010-2011 – were 13.7 for respiratory syncytial virus (RSV), 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, 1.3 for human metapneumovirus, and 0.3 for parainfluenza virus type 2 (PLoS One. 2016 Nov 29. doi: 10.1371/journal.pone.0166226).
Infants who had been exposed to young children, either through attending day care or living with non–multiple birth preschool-age siblings, had a twofold higher risk of RSV and human metapneumovirus, and a 3.3-fold greater risk of adenovirus.
The youngest infants showed the highest rate of hospitalizations with RSV: the incidence ranged from 8.2 per 100 infant season in those aged less than 1 month to 2.3 per 100 infant seasons in those aged 10 months of age. Similarly, the incidence of admission to ICU was significantly higher among younger infants.
Infants born in May, before the RSV season, had a much lower incidence of hospitalization, compared with those born in the height of RSV season in February. ICU admission rates also were higher among those born in February, compared with those born in May.
The highest overall rates of hospitalization with RSV – 19 per 100 infant-seasons – were among those born in February, and also those who were exposed to other young children.
“The current results are unique in that they provide continuous age-based risk models for outpatient and inpatient disease for infants with and without young child exposure,” wrote Dr. Simões and his coauthors.
They argued that their findings refute earlier suggestions that the rate of RSV infection in preterm infants is similar to the rate in term infants, and suggested that the limitations of their study may have even underestimated the incidence in preterm babies.
The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
FROM PLOS ONE
Key clinical point: Respiratory syncytial virus is the leading viral cause of severe lower respiratory disease in preterm infants, with those of younger age and those exposed to young children at greatest risk.
Major finding: The rates of lower respiratory infections per 100 infant-seasons were 13.7 for RSV, 2.9 for adenovirus, 1.7 for parainfluenza virus type 2, and 1.3 for human metapneumovirus.
Data source: The prospective RSV Respiratory Events Among Preterm Infants Outcomes and Risk Tracking (REPORT) study, in 1,642 preterm infants with medically attended acute respiratory illness.
Disclosures: The study was supported by AstraZeneca, parent company of MedImmune. Two authors declared grant support and research funding from AstraZeneca, one author was a former employee of AstraZeneca, and one author was a former employee of MedImmune and now contractor to AstraZeneca. One author was a current employee of AstraZeneca and holds stock options. Two authors also declared funding and consultancies with AbbVie.
Presume parents want HPV vaccines for tweens
Clinics in which providers presented human papillomavirus (HPV) vaccination as an assumed part of tween health care had a 5% increase in HPV vaccination coverage, compared with clinics that did not receive “announcement” training, based on data from a parallel-group, randomized trial of 30 pediatric and family medicine clinics in North Carolina.
Many providers hesitate to recommend HPV vaccination for 11- to 12-year-olds for a number of reasons, including lack of time and anticipation of a lengthy conversation about sex, wrote Noel T. Brewer, PhD, of the University of North Carolina, Chapel Hill, and his colleagues.
At 6 months after the training period, 17,173 children aged 11-12 years and 37,796 children aged 13-17 years were seen at the clinics. Overall, clinics that underwent announcement training increased HPV vaccine initiation for 11- and 12-year-olds by 5.4% over the control clinics. Clinics that received conversation training showed no significant increase in vaccine initiation, compared with controls. Intervention groups did not differ from the controls in terms of other ages (adolescents aged 13-17 years) or other immunization coverage, including HPV series completion, Tdap, and meningococcal vaccines.
The findings were limited by several factors, including the collection of data from a single Southeastern state that may not be generalizable to other areas, the researchers noted. The results, however, “support training providers to use announcements as an approach to address low HPV vaccination uptake in primary care clinics,” especially at the recommended ages for routine vaccination, Dr. Brewer and his associates said.
Read the full study here (Pediatrics 2016;139:e20161764. doi: 10.1542/peds.2016-1764).
Clinics in which providers presented human papillomavirus (HPV) vaccination as an assumed part of tween health care had a 5% increase in HPV vaccination coverage, compared with clinics that did not receive “announcement” training, based on data from a parallel-group, randomized trial of 30 pediatric and family medicine clinics in North Carolina.
Many providers hesitate to recommend HPV vaccination for 11- to 12-year-olds for a number of reasons, including lack of time and anticipation of a lengthy conversation about sex, wrote Noel T. Brewer, PhD, of the University of North Carolina, Chapel Hill, and his colleagues.
At 6 months after the training period, 17,173 children aged 11-12 years and 37,796 children aged 13-17 years were seen at the clinics. Overall, clinics that underwent announcement training increased HPV vaccine initiation for 11- and 12-year-olds by 5.4% over the control clinics. Clinics that received conversation training showed no significant increase in vaccine initiation, compared with controls. Intervention groups did not differ from the controls in terms of other ages (adolescents aged 13-17 years) or other immunization coverage, including HPV series completion, Tdap, and meningococcal vaccines.
The findings were limited by several factors, including the collection of data from a single Southeastern state that may not be generalizable to other areas, the researchers noted. The results, however, “support training providers to use announcements as an approach to address low HPV vaccination uptake in primary care clinics,” especially at the recommended ages for routine vaccination, Dr. Brewer and his associates said.
Read the full study here (Pediatrics 2016;139:e20161764. doi: 10.1542/peds.2016-1764).
Clinics in which providers presented human papillomavirus (HPV) vaccination as an assumed part of tween health care had a 5% increase in HPV vaccination coverage, compared with clinics that did not receive “announcement” training, based on data from a parallel-group, randomized trial of 30 pediatric and family medicine clinics in North Carolina.
Many providers hesitate to recommend HPV vaccination for 11- to 12-year-olds for a number of reasons, including lack of time and anticipation of a lengthy conversation about sex, wrote Noel T. Brewer, PhD, of the University of North Carolina, Chapel Hill, and his colleagues.
At 6 months after the training period, 17,173 children aged 11-12 years and 37,796 children aged 13-17 years were seen at the clinics. Overall, clinics that underwent announcement training increased HPV vaccine initiation for 11- and 12-year-olds by 5.4% over the control clinics. Clinics that received conversation training showed no significant increase in vaccine initiation, compared with controls. Intervention groups did not differ from the controls in terms of other ages (adolescents aged 13-17 years) or other immunization coverage, including HPV series completion, Tdap, and meningococcal vaccines.
The findings were limited by several factors, including the collection of data from a single Southeastern state that may not be generalizable to other areas, the researchers noted. The results, however, “support training providers to use announcements as an approach to address low HPV vaccination uptake in primary care clinics,” especially at the recommended ages for routine vaccination, Dr. Brewer and his associates said.
Read the full study here (Pediatrics 2016;139:e20161764. doi: 10.1542/peds.2016-1764).
FROM PEDIATRICS
Beyond Salary—Are You Happy With Your Work?
Would you say you're satisfied with your job? If you had to do it all over again, what—if anything—would you change? We asked, you answered! Here are the results of our first annual Job Satisfaction Survey, including a comprehensive list of the nonmonetary benefits you deem most important.
Would you say you're satisfied with your job? If you had to do it all over again, what—if anything—would you change? We asked, you answered! Here are the results of our first annual Job Satisfaction Survey, including a comprehensive list of the nonmonetary benefits you deem most important.
Would you say you're satisfied with your job? If you had to do it all over again, what—if anything—would you change? We asked, you answered! Here are the results of our first annual Job Satisfaction Survey, including a comprehensive list of the nonmonetary benefits you deem most important.
Critical Care Commentary
According to IBM, over 2 quintillion bytes of data are generated every day (that’s a 2 with 18 zeros!), with over 90% of the data in the world today generated in the past 2 years alone.
In our private lives, much of this information is generated through online shopping, web surfing, and popular websites such as Facebook and Twitter. Companies are making incredible efforts to collect these data and to use it to improve how they relate to customers and, ultimately, to make more money. For example, companies like Google, Amazon, Facebook, and Netflix collect enormous amounts of data and then use algorithms to provide real-time suggestions for what their customers might want to rent, buy, or click on. These algorithms, which companies use for anything from predicting customer behavior to facial recognition, were developed in the field of machine learning, a branch of computer science that focuses on how to learn from data.
Big data and critical care
Although the “big data” revolution has proliferated across the private sector, medicine has been slow to utilize the data we painstakingly collect in hospitals every day in order to improve patient care.
Clinicians typically rely on their intuition and the few clinical trials that their patients would have been included in to make decisions, and evidence-based clinical decision support tools are often not available or not used. The tools and scores we have at our disposal are often oversimplified so that they can be calculated by hand and usually rely on the clinician to manually gather information from the electronic health record (EHR) to calculate the score. However, this is starting to change. From partnerships between IBM Watson and hospitals, to groups developing and implementing clinical decision support tools in the EHR, it is clear that hospitals are becoming increasingly interested in learning from and using the enormous amount of data that are just sitting in the hospital records.
Although there are many areas in medicine that stand to benefit from harnessing the data available in the EHR to improve patient care, critical care should be one of the specialties that benefits the most. With the variety and frequency of monitoring that critically ill patients receive, there are large swaths of data available to collect, analyze, and harness to improve patient care. The current glut of information results in data overload and alarm fatigue for today’s clinicians, but intelligent use of these data holds promise for making care safer and more efficient and effective.
Groups have already begun using these data to develop tools to identify patients with ARDS (Herasevich V, et al. Intensive Care Med. 2009;35[6]:1018-23), patients at risk of adverse drug reactions (Harinstein LM, et al. J Crit Care. 2012;27[3]:242-9), and those with sepsis (Tafelski S, et al. J Int Med Res. 2010;38:1605-16).
Furthermore, groups have begun “crowdsourcing” critical care problems by making large datasets publicly available, such as the Multi-parameter Intelligent Monitoring in Intensive Care (MIMIC) database, which now holds clinical data from over 40,000 ICU stays from Beth Israel Deaconess Medical Center. Continued efforts to utilize data from patients in the ICU have the potential to revolutionize the care in hospitals today.
An important area of critical care that has seen a rapid rise in the use of EHR data to create decision support tools is in the early detection of critical illness. Given that many in-hospital cardiac arrests occur outside the ICU and delays in transferring critically ill patients to the ICU increase morbidity and mortality (Churpek MM, et al. J Hosp Med. 2016;11[11]:757-62), detecting critical illness early is incredibly important.
For millennia, clinicians have relied on their intuition and experience to determine which patients have a poor prognosis or need increased levels of care. In the 1990s, rapid response teams (RRTs) were developed, with the goal of identifying and treating critical illness earlier. Along with them came early warning scores, which are objective tools that typically use vital sign abnormalities to detect patients at high risk of clinical deterioration. RRTs and the early warning scores used to activate them have proliferated around the world, including in the United States, and scores like the Modified Early Warning Score (MEWS) are available for automatic calculation in the EHR.
However, taking a tool such as the MEWS that can easily be calculated by hand and making our expensive EHRs calculate it is a lot like buying a Ferrari just to drive it around the parking lot. There is no reason to limit our decision support tools to simple algorithms with only a few variables, especially when patients’ lives are at stake.
Several groups around the country have, therefore, begun to utilize other variables in the EHR, such as laboratory values, to create integrated decision support tools for the early identification of critical illness. For example, Kollef and colleagues developed a statistical model to identify critical illness and implemented it on the wards to activate their RRT, which resulted in decreased lengths of stay in the intervention group (Kollef MH, et al. J Hosp Med. 2014;9[7]:424-9).
Escobar et al. developed a model to predict ICU transfer or non-DNR deaths in the Kaiser system and found it to be more accurate than the MEWS in a validation cohort (Escobar GJ, et al. J Hosp Med. 2012;7[5]:388-95). A clinical trial of their system is ongoing.
Finally, our group developed a model called eCART in a multicenter study of over 250,000 patients and has since implemented it in our hospital. An early “black-box” study found that eCART detected more patients who went on to experience a cardiac arrest or ICU transfer than our usual care RRT and it did so 24 hours earlier (Kang MA, et al. Crit Care Med. 2016;44[8]:1468-73). These scores and many more will likely become commonplace in hospitals to provide an objective and accurate way to identify critically ill patients earlier, which may result in decreased preventable morbidity and mortality.
Future directions
There are several important future directions at the intersection of big data and critical care.
First, efforts to collect, store, and share the highly granular data in the ICU are paramount for successful and generalizable research collaborations. Although there are often institutional barriers to data sharing to surmount, efforts such as the MIMIC database provide a roadmap for how ICU data can be shared and problems “crowdsourced” in order to allow researchers access to these data for high quality research.
Second, efforts to fuse randomized controlled trials with big data, such as randomized, embedded, multifactorial, adaptive platform (REMAP) trials, have the potential to greatly enhance the way trials are done in the future. REMAP trials would be embedded in the EHR, provide the ability to study multiple therapies at once, and adapt the randomization scheme to ensure that patients are not harmed by interventions that are clearly detrimental while the study is ongoing (Angus DC. JAMA. 2015;314[8]:767-8).
Finally, it is important that we move beyond the classic statistical methods that are commonly used to develop decision support tools and increase our use of more modern machine learning techniques that companies in the private sector use every day. For example, our group found that classic regression methods were the least accurate of all the methods we studied for detecting clinical deterioration on the wards (Churpek MM, et al. Crit Care Med. 2016;44[2]:368-74). In the future, methods such as the random forest and neural network should become commonplace in the critical care literature.
The big data revolution is here, both in our private lives and in the hospital. The future will bring continued efforts to use data to identify critical illness earlier, improve the care of patients in the ICU, and implement smarter and more efficient clinical trials. This should rapidly increase the generation and utilization of new knowledge and will have a profound impact on the way we care for critically ill patients.
Dr. Churpek is assistant professor, section of pulmonary and critical care medicine, department of medicine at University of Chicago.
Editor’s comment
Why should busy ICU clinicians bother with big data? Isn’t this simply a “flash in the pan” phenomenon that has sprung up in the aftermath of the electronic medical records (EMRs) mandated by the Affordable Care Act? Are concerns valid that clinical data–based algorithms will lead to an endless stream of alerts akin to the ubiquitous pop-up ads for mortgage refinancing, herbal Viagra, and online gambling that has resulted from commercial data mining?
In this Critical Care Commentary, Dr. Matthew Churpek convincingly outlines the potential inherent in the big data generated by our collective ICUs. These benefits are manifesting themselves not just in the data populated within the EMR – but also in the novel ways we can now design and execute studies. And for those who aren’t yet convinced, recall that payers already use the treasure trove of information within our EMRs against us in the forms of self-serving quality metrics, punitive reimbursement, and unvalidated hospital comparison sites.
Lee E. Morrow, MD, FCCP, is the editor of the Critical Care Commentary section of CHEST Physician.
According to IBM, over 2 quintillion bytes of data are generated every day (that’s a 2 with 18 zeros!), with over 90% of the data in the world today generated in the past 2 years alone.
In our private lives, much of this information is generated through online shopping, web surfing, and popular websites such as Facebook and Twitter. Companies are making incredible efforts to collect these data and to use it to improve how they relate to customers and, ultimately, to make more money. For example, companies like Google, Amazon, Facebook, and Netflix collect enormous amounts of data and then use algorithms to provide real-time suggestions for what their customers might want to rent, buy, or click on. These algorithms, which companies use for anything from predicting customer behavior to facial recognition, were developed in the field of machine learning, a branch of computer science that focuses on how to learn from data.
Big data and critical care
Although the “big data” revolution has proliferated across the private sector, medicine has been slow to utilize the data we painstakingly collect in hospitals every day in order to improve patient care.
Clinicians typically rely on their intuition and the few clinical trials that their patients would have been included in to make decisions, and evidence-based clinical decision support tools are often not available or not used. The tools and scores we have at our disposal are often oversimplified so that they can be calculated by hand and usually rely on the clinician to manually gather information from the electronic health record (EHR) to calculate the score. However, this is starting to change. From partnerships between IBM Watson and hospitals, to groups developing and implementing clinical decision support tools in the EHR, it is clear that hospitals are becoming increasingly interested in learning from and using the enormous amount of data that are just sitting in the hospital records.
Although there are many areas in medicine that stand to benefit from harnessing the data available in the EHR to improve patient care, critical care should be one of the specialties that benefits the most. With the variety and frequency of monitoring that critically ill patients receive, there are large swaths of data available to collect, analyze, and harness to improve patient care. The current glut of information results in data overload and alarm fatigue for today’s clinicians, but intelligent use of these data holds promise for making care safer and more efficient and effective.
Groups have already begun using these data to develop tools to identify patients with ARDS (Herasevich V, et al. Intensive Care Med. 2009;35[6]:1018-23), patients at risk of adverse drug reactions (Harinstein LM, et al. J Crit Care. 2012;27[3]:242-9), and those with sepsis (Tafelski S, et al. J Int Med Res. 2010;38:1605-16).
Furthermore, groups have begun “crowdsourcing” critical care problems by making large datasets publicly available, such as the Multi-parameter Intelligent Monitoring in Intensive Care (MIMIC) database, which now holds clinical data from over 40,000 ICU stays from Beth Israel Deaconess Medical Center. Continued efforts to utilize data from patients in the ICU have the potential to revolutionize the care in hospitals today.
An important area of critical care that has seen a rapid rise in the use of EHR data to create decision support tools is in the early detection of critical illness. Given that many in-hospital cardiac arrests occur outside the ICU and delays in transferring critically ill patients to the ICU increase morbidity and mortality (Churpek MM, et al. J Hosp Med. 2016;11[11]:757-62), detecting critical illness early is incredibly important.
For millennia, clinicians have relied on their intuition and experience to determine which patients have a poor prognosis or need increased levels of care. In the 1990s, rapid response teams (RRTs) were developed, with the goal of identifying and treating critical illness earlier. Along with them came early warning scores, which are objective tools that typically use vital sign abnormalities to detect patients at high risk of clinical deterioration. RRTs and the early warning scores used to activate them have proliferated around the world, including in the United States, and scores like the Modified Early Warning Score (MEWS) are available for automatic calculation in the EHR.
However, taking a tool such as the MEWS that can easily be calculated by hand and making our expensive EHRs calculate it is a lot like buying a Ferrari just to drive it around the parking lot. There is no reason to limit our decision support tools to simple algorithms with only a few variables, especially when patients’ lives are at stake.
Several groups around the country have, therefore, begun to utilize other variables in the EHR, such as laboratory values, to create integrated decision support tools for the early identification of critical illness. For example, Kollef and colleagues developed a statistical model to identify critical illness and implemented it on the wards to activate their RRT, which resulted in decreased lengths of stay in the intervention group (Kollef MH, et al. J Hosp Med. 2014;9[7]:424-9).
Escobar et al. developed a model to predict ICU transfer or non-DNR deaths in the Kaiser system and found it to be more accurate than the MEWS in a validation cohort (Escobar GJ, et al. J Hosp Med. 2012;7[5]:388-95). A clinical trial of their system is ongoing.
Finally, our group developed a model called eCART in a multicenter study of over 250,000 patients and has since implemented it in our hospital. An early “black-box” study found that eCART detected more patients who went on to experience a cardiac arrest or ICU transfer than our usual care RRT and it did so 24 hours earlier (Kang MA, et al. Crit Care Med. 2016;44[8]:1468-73). These scores and many more will likely become commonplace in hospitals to provide an objective and accurate way to identify critically ill patients earlier, which may result in decreased preventable morbidity and mortality.
Future directions
There are several important future directions at the intersection of big data and critical care.
First, efforts to collect, store, and share the highly granular data in the ICU are paramount for successful and generalizable research collaborations. Although there are often institutional barriers to data sharing to surmount, efforts such as the MIMIC database provide a roadmap for how ICU data can be shared and problems “crowdsourced” in order to allow researchers access to these data for high quality research.
Second, efforts to fuse randomized controlled trials with big data, such as randomized, embedded, multifactorial, adaptive platform (REMAP) trials, have the potential to greatly enhance the way trials are done in the future. REMAP trials would be embedded in the EHR, provide the ability to study multiple therapies at once, and adapt the randomization scheme to ensure that patients are not harmed by interventions that are clearly detrimental while the study is ongoing (Angus DC. JAMA. 2015;314[8]:767-8).
Finally, it is important that we move beyond the classic statistical methods that are commonly used to develop decision support tools and increase our use of more modern machine learning techniques that companies in the private sector use every day. For example, our group found that classic regression methods were the least accurate of all the methods we studied for detecting clinical deterioration on the wards (Churpek MM, et al. Crit Care Med. 2016;44[2]:368-74). In the future, methods such as the random forest and neural network should become commonplace in the critical care literature.
The big data revolution is here, both in our private lives and in the hospital. The future will bring continued efforts to use data to identify critical illness earlier, improve the care of patients in the ICU, and implement smarter and more efficient clinical trials. This should rapidly increase the generation and utilization of new knowledge and will have a profound impact on the way we care for critically ill patients.
Dr. Churpek is assistant professor, section of pulmonary and critical care medicine, department of medicine at University of Chicago.
Editor’s comment
Why should busy ICU clinicians bother with big data? Isn’t this simply a “flash in the pan” phenomenon that has sprung up in the aftermath of the electronic medical records (EMRs) mandated by the Affordable Care Act? Are concerns valid that clinical data–based algorithms will lead to an endless stream of alerts akin to the ubiquitous pop-up ads for mortgage refinancing, herbal Viagra, and online gambling that has resulted from commercial data mining?
In this Critical Care Commentary, Dr. Matthew Churpek convincingly outlines the potential inherent in the big data generated by our collective ICUs. These benefits are manifesting themselves not just in the data populated within the EMR – but also in the novel ways we can now design and execute studies. And for those who aren’t yet convinced, recall that payers already use the treasure trove of information within our EMRs against us in the forms of self-serving quality metrics, punitive reimbursement, and unvalidated hospital comparison sites.
Lee E. Morrow, MD, FCCP, is the editor of the Critical Care Commentary section of CHEST Physician.
According to IBM, over 2 quintillion bytes of data are generated every day (that’s a 2 with 18 zeros!), with over 90% of the data in the world today generated in the past 2 years alone.
In our private lives, much of this information is generated through online shopping, web surfing, and popular websites such as Facebook and Twitter. Companies are making incredible efforts to collect these data and to use it to improve how they relate to customers and, ultimately, to make more money. For example, companies like Google, Amazon, Facebook, and Netflix collect enormous amounts of data and then use algorithms to provide real-time suggestions for what their customers might want to rent, buy, or click on. These algorithms, which companies use for anything from predicting customer behavior to facial recognition, were developed in the field of machine learning, a branch of computer science that focuses on how to learn from data.
Big data and critical care
Although the “big data” revolution has proliferated across the private sector, medicine has been slow to utilize the data we painstakingly collect in hospitals every day in order to improve patient care.
Clinicians typically rely on their intuition and the few clinical trials that their patients would have been included in to make decisions, and evidence-based clinical decision support tools are often not available or not used. The tools and scores we have at our disposal are often oversimplified so that they can be calculated by hand and usually rely on the clinician to manually gather information from the electronic health record (EHR) to calculate the score. However, this is starting to change. From partnerships between IBM Watson and hospitals, to groups developing and implementing clinical decision support tools in the EHR, it is clear that hospitals are becoming increasingly interested in learning from and using the enormous amount of data that are just sitting in the hospital records.
Although there are many areas in medicine that stand to benefit from harnessing the data available in the EHR to improve patient care, critical care should be one of the specialties that benefits the most. With the variety and frequency of monitoring that critically ill patients receive, there are large swaths of data available to collect, analyze, and harness to improve patient care. The current glut of information results in data overload and alarm fatigue for today’s clinicians, but intelligent use of these data holds promise for making care safer and more efficient and effective.
Groups have already begun using these data to develop tools to identify patients with ARDS (Herasevich V, et al. Intensive Care Med. 2009;35[6]:1018-23), patients at risk of adverse drug reactions (Harinstein LM, et al. J Crit Care. 2012;27[3]:242-9), and those with sepsis (Tafelski S, et al. J Int Med Res. 2010;38:1605-16).
Furthermore, groups have begun “crowdsourcing” critical care problems by making large datasets publicly available, such as the Multi-parameter Intelligent Monitoring in Intensive Care (MIMIC) database, which now holds clinical data from over 40,000 ICU stays from Beth Israel Deaconess Medical Center. Continued efforts to utilize data from patients in the ICU have the potential to revolutionize the care in hospitals today.
An important area of critical care that has seen a rapid rise in the use of EHR data to create decision support tools is in the early detection of critical illness. Given that many in-hospital cardiac arrests occur outside the ICU and delays in transferring critically ill patients to the ICU increase morbidity and mortality (Churpek MM, et al. J Hosp Med. 2016;11[11]:757-62), detecting critical illness early is incredibly important.
For millennia, clinicians have relied on their intuition and experience to determine which patients have a poor prognosis or need increased levels of care. In the 1990s, rapid response teams (RRTs) were developed, with the goal of identifying and treating critical illness earlier. Along with them came early warning scores, which are objective tools that typically use vital sign abnormalities to detect patients at high risk of clinical deterioration. RRTs and the early warning scores used to activate them have proliferated around the world, including in the United States, and scores like the Modified Early Warning Score (MEWS) are available for automatic calculation in the EHR.
However, taking a tool such as the MEWS that can easily be calculated by hand and making our expensive EHRs calculate it is a lot like buying a Ferrari just to drive it around the parking lot. There is no reason to limit our decision support tools to simple algorithms with only a few variables, especially when patients’ lives are at stake.
Several groups around the country have, therefore, begun to utilize other variables in the EHR, such as laboratory values, to create integrated decision support tools for the early identification of critical illness. For example, Kollef and colleagues developed a statistical model to identify critical illness and implemented it on the wards to activate their RRT, which resulted in decreased lengths of stay in the intervention group (Kollef MH, et al. J Hosp Med. 2014;9[7]:424-9).
Escobar et al. developed a model to predict ICU transfer or non-DNR deaths in the Kaiser system and found it to be more accurate than the MEWS in a validation cohort (Escobar GJ, et al. J Hosp Med. 2012;7[5]:388-95). A clinical trial of their system is ongoing.
Finally, our group developed a model called eCART in a multicenter study of over 250,000 patients and has since implemented it in our hospital. An early “black-box” study found that eCART detected more patients who went on to experience a cardiac arrest or ICU transfer than our usual care RRT and it did so 24 hours earlier (Kang MA, et al. Crit Care Med. 2016;44[8]:1468-73). These scores and many more will likely become commonplace in hospitals to provide an objective and accurate way to identify critically ill patients earlier, which may result in decreased preventable morbidity and mortality.
Future directions
There are several important future directions at the intersection of big data and critical care.
First, efforts to collect, store, and share the highly granular data in the ICU are paramount for successful and generalizable research collaborations. Although there are often institutional barriers to data sharing to surmount, efforts such as the MIMIC database provide a roadmap for how ICU data can be shared and problems “crowdsourced” in order to allow researchers access to these data for high quality research.
Second, efforts to fuse randomized controlled trials with big data, such as randomized, embedded, multifactorial, adaptive platform (REMAP) trials, have the potential to greatly enhance the way trials are done in the future. REMAP trials would be embedded in the EHR, provide the ability to study multiple therapies at once, and adapt the randomization scheme to ensure that patients are not harmed by interventions that are clearly detrimental while the study is ongoing (Angus DC. JAMA. 2015;314[8]:767-8).
Finally, it is important that we move beyond the classic statistical methods that are commonly used to develop decision support tools and increase our use of more modern machine learning techniques that companies in the private sector use every day. For example, our group found that classic regression methods were the least accurate of all the methods we studied for detecting clinical deterioration on the wards (Churpek MM, et al. Crit Care Med. 2016;44[2]:368-74). In the future, methods such as the random forest and neural network should become commonplace in the critical care literature.
The big data revolution is here, both in our private lives and in the hospital. The future will bring continued efforts to use data to identify critical illness earlier, improve the care of patients in the ICU, and implement smarter and more efficient clinical trials. This should rapidly increase the generation and utilization of new knowledge and will have a profound impact on the way we care for critically ill patients.
Dr. Churpek is assistant professor, section of pulmonary and critical care medicine, department of medicine at University of Chicago.
Editor’s comment
Why should busy ICU clinicians bother with big data? Isn’t this simply a “flash in the pan” phenomenon that has sprung up in the aftermath of the electronic medical records (EMRs) mandated by the Affordable Care Act? Are concerns valid that clinical data–based algorithms will lead to an endless stream of alerts akin to the ubiquitous pop-up ads for mortgage refinancing, herbal Viagra, and online gambling that has resulted from commercial data mining?
In this Critical Care Commentary, Dr. Matthew Churpek convincingly outlines the potential inherent in the big data generated by our collective ICUs. These benefits are manifesting themselves not just in the data populated within the EMR – but also in the novel ways we can now design and execute studies. And for those who aren’t yet convinced, recall that payers already use the treasure trove of information within our EMRs against us in the forms of self-serving quality metrics, punitive reimbursement, and unvalidated hospital comparison sites.
Lee E. Morrow, MD, FCCP, is the editor of the Critical Care Commentary section of CHEST Physician.