User login
A New Technique for Obtaining Bone Graft in Cases of Distal Femur Nonunion: Passing a Reamer/Irrigator/Aspirator Retrograde Through the Nonunion Site
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
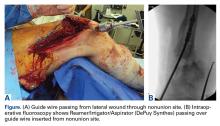
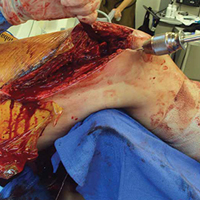
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
VIDEO: Obinutuzumab bests rituximab for PFS in follicular lymphoma
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Obinutuzumab bests rituximab in FL study

ASH Annual Meeting
SAN DIEGO—Interim results of the phase 3 GALLIUM trial suggest an obinutuzumab-based treatment regimen provides a progression-free survival (PFS) benefit over a rituximab-based regimen for patients with previously untreated follicular lymphoma (FL).
According to investigators, patients who received obinutuzumab plus chemotherapy followed by obinutuzumab maintenance had a “clinically meaningful” improvement in PFS, when compared to patients who received rituximab plus chemotherapy followed by rituximab maintenance.
However, there was no significant difference between the treatment arms with regard to overall survival. And the incidence of non-fatal adverse events (AEs) was higher among the patients who received obinutuzumab.
Nevertheless, the data suggest that obinutuzumab-based therapy significantly improves outcomes and should be considered as a first-line treatment for FL, according to Robert Marcus, MBBS, of King’s College Hospital in London, UK.
Dr Marcus presented data from GALLIUM during the plenary session at the 2016 ASH Annual Meeting (abstract 6). GALLIUM is sponsored by Hoffmann-La Roche.
Patients and treatment
The study has enrolled 1401 patients with previously untreated, indolent non-Hodgkin lymphoma, including 1202 with FL.
Half of the FL patients (n=601) were randomized to obinutuzumab plus chemotherapy followed by obinutuzumab alone for up to 2 years, and half were randomized to rituximab plus chemotherapy followed by rituximab alone for up to 2 years.
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisolone), and bendamustine. The regimens were selected by each participating study site prior to beginning enrollment.
Baseline characteristics were similar between the treatment arms. The median age was about 60 (overall range, 23-88), roughly 40% of patients had high-risk disease, and the median time from diagnosis to randomization was about 1.5 months.
A total of 341 patients in the rituximab arm and 361 patients in the obinutuzumab arm completed maintenance therapy.
The median follow-up was 34.5 months. Maintenance is ongoing in 114 patients—54 on rituximab and 60 on obinutuzumab.
Efficacy
At the end of induction, the overall response rate was 86.9% in the rituximab arm and 88.5% in the obinutuzumab arm. The complete response rates were 23.8% and 19.5%, respectively. And the rates of stable disease were 1.3% and 0.5%, respectively.
The study’s primary endpoint is investigator-assessed PFS. The 3-year PFS rate is 73.3% in the rituximab arm and 80% in the obinutuzumab arm (hazard ratio [HR]=0.66, P=0.0012).
According to an independent review committee, the 3-year PFS is 77.9% in the rituximab arm and 81.9% in the obinutuzumab arm (HR=0.71, P=0.0138).
The 3-year overall survival is 92.1% in the rituximab arm and 94% in the obinutuzumab arm (HR=0.75, P=0.21).
Safety
The overall incidence of AEs was 98.3% in the rituximab arm and 99.5% in the obinutuzumab arm. The incidence of serious AEs was 39.9% and 46.1%, respectively.
The incidence of AEs leading to treatment discontinuation was 14.2% and 16.3%, respectively. And the incidence of second neoplasms was 2.7% and 4.7%, respectively.
Grade 5 AEs occurred in 3.4% of patients in the rituximab arm and 4.0% of patients in the obinutuzumab arm. The investigators found that fatal AEs were more common in patients taking bendamustine, regardless of the treatment arm.
Grade 3 or higher AEs occurring in at least 5% of patients in either arm (rituximab and obinutuzumab, respectively) included neutropenia (67.8% and 74.6%), leukopenia (37.9% and 43.9%), febrile neutropenia (4.9% and 6.9%), infections and infestations (3.7% and 6.7%), and thrombocytopenia (2.7% and 6.1%). ![]()

ASH Annual Meeting
SAN DIEGO—Interim results of the phase 3 GALLIUM trial suggest an obinutuzumab-based treatment regimen provides a progression-free survival (PFS) benefit over a rituximab-based regimen for patients with previously untreated follicular lymphoma (FL).
According to investigators, patients who received obinutuzumab plus chemotherapy followed by obinutuzumab maintenance had a “clinically meaningful” improvement in PFS, when compared to patients who received rituximab plus chemotherapy followed by rituximab maintenance.
However, there was no significant difference between the treatment arms with regard to overall survival. And the incidence of non-fatal adverse events (AEs) was higher among the patients who received obinutuzumab.
Nevertheless, the data suggest that obinutuzumab-based therapy significantly improves outcomes and should be considered as a first-line treatment for FL, according to Robert Marcus, MBBS, of King’s College Hospital in London, UK.
Dr Marcus presented data from GALLIUM during the plenary session at the 2016 ASH Annual Meeting (abstract 6). GALLIUM is sponsored by Hoffmann-La Roche.
Patients and treatment
The study has enrolled 1401 patients with previously untreated, indolent non-Hodgkin lymphoma, including 1202 with FL.
Half of the FL patients (n=601) were randomized to obinutuzumab plus chemotherapy followed by obinutuzumab alone for up to 2 years, and half were randomized to rituximab plus chemotherapy followed by rituximab alone for up to 2 years.
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisolone), and bendamustine. The regimens were selected by each participating study site prior to beginning enrollment.
Baseline characteristics were similar between the treatment arms. The median age was about 60 (overall range, 23-88), roughly 40% of patients had high-risk disease, and the median time from diagnosis to randomization was about 1.5 months.
A total of 341 patients in the rituximab arm and 361 patients in the obinutuzumab arm completed maintenance therapy.
The median follow-up was 34.5 months. Maintenance is ongoing in 114 patients—54 on rituximab and 60 on obinutuzumab.
Efficacy
At the end of induction, the overall response rate was 86.9% in the rituximab arm and 88.5% in the obinutuzumab arm. The complete response rates were 23.8% and 19.5%, respectively. And the rates of stable disease were 1.3% and 0.5%, respectively.
The study’s primary endpoint is investigator-assessed PFS. The 3-year PFS rate is 73.3% in the rituximab arm and 80% in the obinutuzumab arm (hazard ratio [HR]=0.66, P=0.0012).
According to an independent review committee, the 3-year PFS is 77.9% in the rituximab arm and 81.9% in the obinutuzumab arm (HR=0.71, P=0.0138).
The 3-year overall survival is 92.1% in the rituximab arm and 94% in the obinutuzumab arm (HR=0.75, P=0.21).
Safety
The overall incidence of AEs was 98.3% in the rituximab arm and 99.5% in the obinutuzumab arm. The incidence of serious AEs was 39.9% and 46.1%, respectively.
The incidence of AEs leading to treatment discontinuation was 14.2% and 16.3%, respectively. And the incidence of second neoplasms was 2.7% and 4.7%, respectively.
Grade 5 AEs occurred in 3.4% of patients in the rituximab arm and 4.0% of patients in the obinutuzumab arm. The investigators found that fatal AEs were more common in patients taking bendamustine, regardless of the treatment arm.
Grade 3 or higher AEs occurring in at least 5% of patients in either arm (rituximab and obinutuzumab, respectively) included neutropenia (67.8% and 74.6%), leukopenia (37.9% and 43.9%), febrile neutropenia (4.9% and 6.9%), infections and infestations (3.7% and 6.7%), and thrombocytopenia (2.7% and 6.1%). ![]()

ASH Annual Meeting
SAN DIEGO—Interim results of the phase 3 GALLIUM trial suggest an obinutuzumab-based treatment regimen provides a progression-free survival (PFS) benefit over a rituximab-based regimen for patients with previously untreated follicular lymphoma (FL).
According to investigators, patients who received obinutuzumab plus chemotherapy followed by obinutuzumab maintenance had a “clinically meaningful” improvement in PFS, when compared to patients who received rituximab plus chemotherapy followed by rituximab maintenance.
However, there was no significant difference between the treatment arms with regard to overall survival. And the incidence of non-fatal adverse events (AEs) was higher among the patients who received obinutuzumab.
Nevertheless, the data suggest that obinutuzumab-based therapy significantly improves outcomes and should be considered as a first-line treatment for FL, according to Robert Marcus, MBBS, of King’s College Hospital in London, UK.
Dr Marcus presented data from GALLIUM during the plenary session at the 2016 ASH Annual Meeting (abstract 6). GALLIUM is sponsored by Hoffmann-La Roche.
Patients and treatment
The study has enrolled 1401 patients with previously untreated, indolent non-Hodgkin lymphoma, including 1202 with FL.
Half of the FL patients (n=601) were randomized to obinutuzumab plus chemotherapy followed by obinutuzumab alone for up to 2 years, and half were randomized to rituximab plus chemotherapy followed by rituximab alone for up to 2 years.
The different chemotherapies used were CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), CVP (cyclophosphamide, vincristine, and prednisolone), and bendamustine. The regimens were selected by each participating study site prior to beginning enrollment.
Baseline characteristics were similar between the treatment arms. The median age was about 60 (overall range, 23-88), roughly 40% of patients had high-risk disease, and the median time from diagnosis to randomization was about 1.5 months.
A total of 341 patients in the rituximab arm and 361 patients in the obinutuzumab arm completed maintenance therapy.
The median follow-up was 34.5 months. Maintenance is ongoing in 114 patients—54 on rituximab and 60 on obinutuzumab.
Efficacy
At the end of induction, the overall response rate was 86.9% in the rituximab arm and 88.5% in the obinutuzumab arm. The complete response rates were 23.8% and 19.5%, respectively. And the rates of stable disease were 1.3% and 0.5%, respectively.
The study’s primary endpoint is investigator-assessed PFS. The 3-year PFS rate is 73.3% in the rituximab arm and 80% in the obinutuzumab arm (hazard ratio [HR]=0.66, P=0.0012).
According to an independent review committee, the 3-year PFS is 77.9% in the rituximab arm and 81.9% in the obinutuzumab arm (HR=0.71, P=0.0138).
The 3-year overall survival is 92.1% in the rituximab arm and 94% in the obinutuzumab arm (HR=0.75, P=0.21).
Safety
The overall incidence of AEs was 98.3% in the rituximab arm and 99.5% in the obinutuzumab arm. The incidence of serious AEs was 39.9% and 46.1%, respectively.
The incidence of AEs leading to treatment discontinuation was 14.2% and 16.3%, respectively. And the incidence of second neoplasms was 2.7% and 4.7%, respectively.
Grade 5 AEs occurred in 3.4% of patients in the rituximab arm and 4.0% of patients in the obinutuzumab arm. The investigators found that fatal AEs were more common in patients taking bendamustine, regardless of the treatment arm.
Grade 3 or higher AEs occurring in at least 5% of patients in either arm (rituximab and obinutuzumab, respectively) included neutropenia (67.8% and 74.6%), leukopenia (37.9% and 43.9%), febrile neutropenia (4.9% and 6.9%), infections and infestations (3.7% and 6.7%), and thrombocytopenia (2.7% and 6.1%). ![]()
Fanconi anemia linked to cancer gene
Researchers say they have discovered an important molecular link between Fanconi anemia (FA) and PTEN, a gene associated with uterine, prostate, and brain cancer.
They say this discovery enhances our understanding of the molecular basis of Fanconi anemia and could lead to improved treatment outcomes for both Fanconi anemia and cancer patients.
The researchers detailed their discovery in Scientific Reports.
They explained that Fanconi anemia proteins function primarily in DNA interstrand crosslink (ICL) repair, and they wanted to determine the role of the PTEN phosphatase in this process.
“The PTEN gene codes for a phosphatase—an enzyme that removes phosphate groups from proteins,” said study author Niall Howlett, PhD, of the University of Rhode Island in Kingston, Rhode Island.
“Many Fanconi anemia proteins have phosphate groups attached to them when they become activated. However, how these phosphate groups are removed is poorly understood.”
With this in mind, the researchers performed an experiment to determine if Fanconi anemia and PTEN are biochemically linked.
The team knew that cells from Fanconi anemia patients are sensitive to ICL-inducing agents, so they set out to determine if PTEN-deficient cells are sensitive to these agents as well.
“By testing if cells with mutations in the PTEN gene were also sensitive to [ICL-inducing] agents, we discovered that Fanconi anemia patient cells and PTEN-deficient cells were practically indistinguishable in terms of sensitivity to these drugs,” Dr Howlett said.
“This strongly suggested that the Fanconi anemia proteins and PTEN might work together to repair the DNA damage caused by [ICL-inducing] agents.”
Using epistasis analysis, Dr Howlett and his colleagues found that Fanconi anemia proteins and PTEN do indeed function together in ICL repair.
“Before this work, Fanconi anemia and PTEN weren’t even on the same radar,” Dr Howlett said. “This is really important to understanding how this disease arises and what its molecular underpinnings are. The more we can find out about its molecular basis, the more likely we are to come up with strategies to treat the disease.”
Dr Howlett and his colleagues believe their research is equally important to cancer patients. Since this study showed that cells missing PTEN are highly sensitive to ICL-inducing agents, the team believes it should be possible to predict whether a particular cancer patient will respond to this class of drugs by conducting a simple DNA test.
“We can now predict that if a patient has cancer associated with mutations in PTEN, then it is likely that the cancer will be sensitive to [ICL-inducing] agents,” Dr Howlett said. “This could lead to improved outcomes for patients with certain types of PTEN mutations.” ![]()

Researchers say they have discovered an important molecular link between Fanconi anemia (FA) and PTEN, a gene associated with uterine, prostate, and brain cancer.
They say this discovery enhances our understanding of the molecular basis of Fanconi anemia and could lead to improved treatment outcomes for both Fanconi anemia and cancer patients.
The researchers detailed their discovery in Scientific Reports.
They explained that Fanconi anemia proteins function primarily in DNA interstrand crosslink (ICL) repair, and they wanted to determine the role of the PTEN phosphatase in this process.
“The PTEN gene codes for a phosphatase—an enzyme that removes phosphate groups from proteins,” said study author Niall Howlett, PhD, of the University of Rhode Island in Kingston, Rhode Island.
“Many Fanconi anemia proteins have phosphate groups attached to them when they become activated. However, how these phosphate groups are removed is poorly understood.”
With this in mind, the researchers performed an experiment to determine if Fanconi anemia and PTEN are biochemically linked.
The team knew that cells from Fanconi anemia patients are sensitive to ICL-inducing agents, so they set out to determine if PTEN-deficient cells are sensitive to these agents as well.
“By testing if cells with mutations in the PTEN gene were also sensitive to [ICL-inducing] agents, we discovered that Fanconi anemia patient cells and PTEN-deficient cells were practically indistinguishable in terms of sensitivity to these drugs,” Dr Howlett said.
“This strongly suggested that the Fanconi anemia proteins and PTEN might work together to repair the DNA damage caused by [ICL-inducing] agents.”
Using epistasis analysis, Dr Howlett and his colleagues found that Fanconi anemia proteins and PTEN do indeed function together in ICL repair.
“Before this work, Fanconi anemia and PTEN weren’t even on the same radar,” Dr Howlett said. “This is really important to understanding how this disease arises and what its molecular underpinnings are. The more we can find out about its molecular basis, the more likely we are to come up with strategies to treat the disease.”
Dr Howlett and his colleagues believe their research is equally important to cancer patients. Since this study showed that cells missing PTEN are highly sensitive to ICL-inducing agents, the team believes it should be possible to predict whether a particular cancer patient will respond to this class of drugs by conducting a simple DNA test.
“We can now predict that if a patient has cancer associated with mutations in PTEN, then it is likely that the cancer will be sensitive to [ICL-inducing] agents,” Dr Howlett said. “This could lead to improved outcomes for patients with certain types of PTEN mutations.” ![]()

Researchers say they have discovered an important molecular link between Fanconi anemia (FA) and PTEN, a gene associated with uterine, prostate, and brain cancer.
They say this discovery enhances our understanding of the molecular basis of Fanconi anemia and could lead to improved treatment outcomes for both Fanconi anemia and cancer patients.
The researchers detailed their discovery in Scientific Reports.
They explained that Fanconi anemia proteins function primarily in DNA interstrand crosslink (ICL) repair, and they wanted to determine the role of the PTEN phosphatase in this process.
“The PTEN gene codes for a phosphatase—an enzyme that removes phosphate groups from proteins,” said study author Niall Howlett, PhD, of the University of Rhode Island in Kingston, Rhode Island.
“Many Fanconi anemia proteins have phosphate groups attached to them when they become activated. However, how these phosphate groups are removed is poorly understood.”
With this in mind, the researchers performed an experiment to determine if Fanconi anemia and PTEN are biochemically linked.
The team knew that cells from Fanconi anemia patients are sensitive to ICL-inducing agents, so they set out to determine if PTEN-deficient cells are sensitive to these agents as well.
“By testing if cells with mutations in the PTEN gene were also sensitive to [ICL-inducing] agents, we discovered that Fanconi anemia patient cells and PTEN-deficient cells were practically indistinguishable in terms of sensitivity to these drugs,” Dr Howlett said.
“This strongly suggested that the Fanconi anemia proteins and PTEN might work together to repair the DNA damage caused by [ICL-inducing] agents.”
Using epistasis analysis, Dr Howlett and his colleagues found that Fanconi anemia proteins and PTEN do indeed function together in ICL repair.
“Before this work, Fanconi anemia and PTEN weren’t even on the same radar,” Dr Howlett said. “This is really important to understanding how this disease arises and what its molecular underpinnings are. The more we can find out about its molecular basis, the more likely we are to come up with strategies to treat the disease.”
Dr Howlett and his colleagues believe their research is equally important to cancer patients. Since this study showed that cells missing PTEN are highly sensitive to ICL-inducing agents, the team believes it should be possible to predict whether a particular cancer patient will respond to this class of drugs by conducting a simple DNA test.
“We can now predict that if a patient has cancer associated with mutations in PTEN, then it is likely that the cancer will be sensitive to [ICL-inducing] agents,” Dr Howlett said. “This could lead to improved outcomes for patients with certain types of PTEN mutations.” ![]()
VA Highlights Cancer Treatment Innovation, Best Practices at Launch Pad Event
The connections forged by the Cancer Moonshot will outlive the Obama administration, Greg Simon, executive director of the Cancer Moonshot Task Force, told a group of VA, nonprofit, and health care industry experts at the Launch Pad: Pathways to Cancer Innovation summit last month in Washington, DC. The event, cosponsored by the VA and the Prostate Cancer Foundation (PCF), was a forum for discussing possible new approaches to oncology care and touting progress that has already occurred at the VA.
Related: Innovation and Cancer Moonshot Highlight AVAHO Conference
At the event, the VA and PCF also signed an agreement for a $50 million Precision Oncology Program that will expand prostate cancer clinical research among veterans and develop new treatment options and cures for prostate cancer patients.
Speakers at the summit included VA Secretary Robert McDonald VA Undersecretary of Health David J. Shulkin, MD; and Deputy Under Secretary for Health for Policy and Services Jennifer S. Lee, MD. According to Dr. Shulkin, the Million Veteran Program (MVP) has already surpassed 520,000 enrollees and has contracted with the U.S. Department of Energy to use its supercomputers to speed analysis and computation. In 2016, the VA managed 181,000 prostate cancer cases, and 26,000 deaths are projected. According to Shulkin, the VA also has developed the Center for Compassionate Innovation to enhance the health of veterans and their well-being by offering emerging therapies that are safe and ethical, particularly after traditional treatments have been unsuccessful.
Related: Building Better Models for Innovation in Health Care
At the meeting, a number of health care providers also discussed ongoing oncology programs that the VA hopes to expand. Drew Moghanaki, MD, MPH, director of clinical radiation oncology research at Hunter Holmes McGuire VAMC in Richmond, Virginia, discussed efforts to make radiation oncology more precise for patients with lung cancer. Bruce Montgomery, MD, of the VA Puget Sound in Seattle, Washington, presented on the germline DNA testing of veterans with advanced prostate cancer, and Durham VAMC’s Neil Spector, MD, provided an update on using the Precision Oncology Program for more targeted therapies. Jennifer MacDonald, MD, VA’s director of clinical innovations and education, discussed the pilot and growth of virtual tumor boards to speed diagnosis and treatment to rural veterans with suspected cancers. The virtual tumor board was a recent example of a program developed through the Diffusion of Best Practices initiative spearheaded by Shereef Elnahal, MD, across the VA.
“Fighting and treating cancer among our veterans is a team effort, which is why this Launch Pad event and this partnership are so important,” Secretary McDonald told the group. “To effectively serve our veterans and to keep VA on the cutting edge of medical research, we need government, corporate, and nonprofit organizations working together. We are truly grateful to the Prostate Cancer Foundation for this important show of support. Our work together will save veterans’ lives.”
The connections forged by the Cancer Moonshot will outlive the Obama administration, Greg Simon, executive director of the Cancer Moonshot Task Force, told a group of VA, nonprofit, and health care industry experts at the Launch Pad: Pathways to Cancer Innovation summit last month in Washington, DC. The event, cosponsored by the VA and the Prostate Cancer Foundation (PCF), was a forum for discussing possible new approaches to oncology care and touting progress that has already occurred at the VA.
Related: Innovation and Cancer Moonshot Highlight AVAHO Conference
At the event, the VA and PCF also signed an agreement for a $50 million Precision Oncology Program that will expand prostate cancer clinical research among veterans and develop new treatment options and cures for prostate cancer patients.
Speakers at the summit included VA Secretary Robert McDonald VA Undersecretary of Health David J. Shulkin, MD; and Deputy Under Secretary for Health for Policy and Services Jennifer S. Lee, MD. According to Dr. Shulkin, the Million Veteran Program (MVP) has already surpassed 520,000 enrollees and has contracted with the U.S. Department of Energy to use its supercomputers to speed analysis and computation. In 2016, the VA managed 181,000 prostate cancer cases, and 26,000 deaths are projected. According to Shulkin, the VA also has developed the Center for Compassionate Innovation to enhance the health of veterans and their well-being by offering emerging therapies that are safe and ethical, particularly after traditional treatments have been unsuccessful.
Related: Building Better Models for Innovation in Health Care
At the meeting, a number of health care providers also discussed ongoing oncology programs that the VA hopes to expand. Drew Moghanaki, MD, MPH, director of clinical radiation oncology research at Hunter Holmes McGuire VAMC in Richmond, Virginia, discussed efforts to make radiation oncology more precise for patients with lung cancer. Bruce Montgomery, MD, of the VA Puget Sound in Seattle, Washington, presented on the germline DNA testing of veterans with advanced prostate cancer, and Durham VAMC’s Neil Spector, MD, provided an update on using the Precision Oncology Program for more targeted therapies. Jennifer MacDonald, MD, VA’s director of clinical innovations and education, discussed the pilot and growth of virtual tumor boards to speed diagnosis and treatment to rural veterans with suspected cancers. The virtual tumor board was a recent example of a program developed through the Diffusion of Best Practices initiative spearheaded by Shereef Elnahal, MD, across the VA.
“Fighting and treating cancer among our veterans is a team effort, which is why this Launch Pad event and this partnership are so important,” Secretary McDonald told the group. “To effectively serve our veterans and to keep VA on the cutting edge of medical research, we need government, corporate, and nonprofit organizations working together. We are truly grateful to the Prostate Cancer Foundation for this important show of support. Our work together will save veterans’ lives.”
The connections forged by the Cancer Moonshot will outlive the Obama administration, Greg Simon, executive director of the Cancer Moonshot Task Force, told a group of VA, nonprofit, and health care industry experts at the Launch Pad: Pathways to Cancer Innovation summit last month in Washington, DC. The event, cosponsored by the VA and the Prostate Cancer Foundation (PCF), was a forum for discussing possible new approaches to oncology care and touting progress that has already occurred at the VA.
Related: Innovation and Cancer Moonshot Highlight AVAHO Conference
At the event, the VA and PCF also signed an agreement for a $50 million Precision Oncology Program that will expand prostate cancer clinical research among veterans and develop new treatment options and cures for prostate cancer patients.
Speakers at the summit included VA Secretary Robert McDonald VA Undersecretary of Health David J. Shulkin, MD; and Deputy Under Secretary for Health for Policy and Services Jennifer S. Lee, MD. According to Dr. Shulkin, the Million Veteran Program (MVP) has already surpassed 520,000 enrollees and has contracted with the U.S. Department of Energy to use its supercomputers to speed analysis and computation. In 2016, the VA managed 181,000 prostate cancer cases, and 26,000 deaths are projected. According to Shulkin, the VA also has developed the Center for Compassionate Innovation to enhance the health of veterans and their well-being by offering emerging therapies that are safe and ethical, particularly after traditional treatments have been unsuccessful.
Related: Building Better Models for Innovation in Health Care
At the meeting, a number of health care providers also discussed ongoing oncology programs that the VA hopes to expand. Drew Moghanaki, MD, MPH, director of clinical radiation oncology research at Hunter Holmes McGuire VAMC in Richmond, Virginia, discussed efforts to make radiation oncology more precise for patients with lung cancer. Bruce Montgomery, MD, of the VA Puget Sound in Seattle, Washington, presented on the germline DNA testing of veterans with advanced prostate cancer, and Durham VAMC’s Neil Spector, MD, provided an update on using the Precision Oncology Program for more targeted therapies. Jennifer MacDonald, MD, VA’s director of clinical innovations and education, discussed the pilot and growth of virtual tumor boards to speed diagnosis and treatment to rural veterans with suspected cancers. The virtual tumor board was a recent example of a program developed through the Diffusion of Best Practices initiative spearheaded by Shereef Elnahal, MD, across the VA.
“Fighting and treating cancer among our veterans is a team effort, which is why this Launch Pad event and this partnership are so important,” Secretary McDonald told the group. “To effectively serve our veterans and to keep VA on the cutting edge of medical research, we need government, corporate, and nonprofit organizations working together. We are truly grateful to the Prostate Cancer Foundation for this important show of support. Our work together will save veterans’ lives.”
Shulkin: VA "Not a Political Issue”
Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD at the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016. As the clock winds down on the current administration, the interview covered a wide range of topic. The below video that discusses VA progress over the past 18 months since Shulkin was confirmed and the prospects for change in the new administration. Future videos will cover the Veterans Choice Program, employee morale and recruitment challenges, improving rural care, transparency, and the unique nature of VA’s mission and care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD at the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016. As the clock winds down on the current administration, the interview covered a wide range of topic. The below video that discusses VA progress over the past 18 months since Shulkin was confirmed and the prospects for change in the new administration. Future videos will cover the Veterans Choice Program, employee morale and recruitment challenges, improving rural care, transparency, and the unique nature of VA’s mission and care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD at the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016. As the clock winds down on the current administration, the interview covered a wide range of topic. The below video that discusses VA progress over the past 18 months since Shulkin was confirmed and the prospects for change in the new administration. Future videos will cover the Veterans Choice Program, employee morale and recruitment challenges, improving rural care, transparency, and the unique nature of VA’s mission and care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Combo shows early promise in newly diagnosed AML

© Todd Buchanan 2016
SAN DIEGO—A targeted therapy combined with standard chemotherapy can produce rapid, deep remissions in patients with newly diagnosed acute myeloid leukemia (AML), according to research presented at the 2016 ASH Annual Meeting.
In this phase 1b study, investigators tested vadastuximab talirine, an antibody drug conjugate targeting CD33, in combination with 7+3 chemotherapy—a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days.
The combination produced a high rate of response, which included minimal residual disease (MRD)-negative complete remissions (CRs).
The treatment also resulted in “acceptable” on-target myelosuppression and non-hematologic adverse events (AEs) similar to what would be expected with 7+3 alone, according to study investigator Harry Erba, MD, PhD, of the University of Alabama at Birmingham.
Dr Erba presented these results in abstract 211.* The research was sponsored by Seattle Genetics, Inc.
The study included 42 newly diagnosed AML patients with a median age of 45.5. Half the patients had intermediate-risk karyotypes, 36% had adverse karyotypes, and 17% had secondary AML.
Patients received escalating doses of vadastuximab talirine (10+10 mcg/kg [n=4] and 20+10 mcg/kg [n=38]) in combination with 7+3 induction (cytarabine at 100 mg/m2 and daunorubicin at 60 mg/m2) on days 1 and 4 of a 28-day treatment cycle. Responses were assessed on days 15 and 28.
A second induction regimen and post-remission therapies were prescribed according to investigator choice and did not include vadastuximab talirine.
Results
The maximum tolerated dose of vadastuximab talirine was 20+10 mcg/kg.
Hematologic treatment-related AEs included febrile neutropenia (43%, grade 1-3), thrombocytopenia (38%, grade 3-4), anemia (24%, grade 3), and neutropenia (17%, grade 3-4).
Non-hematologic treatment-related AEs included nausea (17%), fatigue (14%), diarrhea (7%), and decreased appetite (7%). All of these AEs were grade 1-2.
None of the patients experienced infusion-related reactions, veno-occlusive disease, or significant liver damage.
A total of 76% of patients responded to treatment, with 60% percent achieving a CR and 17% achieving a CR with incomplete blood count recovery (CRi).
The 76% response rate is close to what would be expected for a well-chosen population fit for a clinical trial, Dr Erba said.
There was a hint of additional benefit as well, he added.
“The first hint was that 30 out of the 32 patients [who achieved a CR/CRi] required only 1 round of chemotherapy to achieve that remission,” Dr Erba said. “This also suggested that deeper remissions may be possible.”
MRD assessments using a sensitive flow cytometric assay revealed that 25 of the 32 patients (78%) who achieved a CR/CRi were MRD-negative.
Dr Erba said a randomized, phase 2 trial of vadastuximab talirine plus 7+3 versus 7+3 alone is planned for the first quarter of 2017. ![]()
*Information presented at the meeting differs from the abstract.

© Todd Buchanan 2016
SAN DIEGO—A targeted therapy combined with standard chemotherapy can produce rapid, deep remissions in patients with newly diagnosed acute myeloid leukemia (AML), according to research presented at the 2016 ASH Annual Meeting.
In this phase 1b study, investigators tested vadastuximab talirine, an antibody drug conjugate targeting CD33, in combination with 7+3 chemotherapy—a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days.
The combination produced a high rate of response, which included minimal residual disease (MRD)-negative complete remissions (CRs).
The treatment also resulted in “acceptable” on-target myelosuppression and non-hematologic adverse events (AEs) similar to what would be expected with 7+3 alone, according to study investigator Harry Erba, MD, PhD, of the University of Alabama at Birmingham.
Dr Erba presented these results in abstract 211.* The research was sponsored by Seattle Genetics, Inc.
The study included 42 newly diagnosed AML patients with a median age of 45.5. Half the patients had intermediate-risk karyotypes, 36% had adverse karyotypes, and 17% had secondary AML.
Patients received escalating doses of vadastuximab talirine (10+10 mcg/kg [n=4] and 20+10 mcg/kg [n=38]) in combination with 7+3 induction (cytarabine at 100 mg/m2 and daunorubicin at 60 mg/m2) on days 1 and 4 of a 28-day treatment cycle. Responses were assessed on days 15 and 28.
A second induction regimen and post-remission therapies were prescribed according to investigator choice and did not include vadastuximab talirine.
Results
The maximum tolerated dose of vadastuximab talirine was 20+10 mcg/kg.
Hematologic treatment-related AEs included febrile neutropenia (43%, grade 1-3), thrombocytopenia (38%, grade 3-4), anemia (24%, grade 3), and neutropenia (17%, grade 3-4).
Non-hematologic treatment-related AEs included nausea (17%), fatigue (14%), diarrhea (7%), and decreased appetite (7%). All of these AEs were grade 1-2.
None of the patients experienced infusion-related reactions, veno-occlusive disease, or significant liver damage.
A total of 76% of patients responded to treatment, with 60% percent achieving a CR and 17% achieving a CR with incomplete blood count recovery (CRi).
The 76% response rate is close to what would be expected for a well-chosen population fit for a clinical trial, Dr Erba said.
There was a hint of additional benefit as well, he added.
“The first hint was that 30 out of the 32 patients [who achieved a CR/CRi] required only 1 round of chemotherapy to achieve that remission,” Dr Erba said. “This also suggested that deeper remissions may be possible.”
MRD assessments using a sensitive flow cytometric assay revealed that 25 of the 32 patients (78%) who achieved a CR/CRi were MRD-negative.
Dr Erba said a randomized, phase 2 trial of vadastuximab talirine plus 7+3 versus 7+3 alone is planned for the first quarter of 2017. ![]()
*Information presented at the meeting differs from the abstract.

© Todd Buchanan 2016
SAN DIEGO—A targeted therapy combined with standard chemotherapy can produce rapid, deep remissions in patients with newly diagnosed acute myeloid leukemia (AML), according to research presented at the 2016 ASH Annual Meeting.
In this phase 1b study, investigators tested vadastuximab talirine, an antibody drug conjugate targeting CD33, in combination with 7+3 chemotherapy—a continuous infusion of cytarabine for 7 days plus daunorubicin for 3 days.
The combination produced a high rate of response, which included minimal residual disease (MRD)-negative complete remissions (CRs).
The treatment also resulted in “acceptable” on-target myelosuppression and non-hematologic adverse events (AEs) similar to what would be expected with 7+3 alone, according to study investigator Harry Erba, MD, PhD, of the University of Alabama at Birmingham.
Dr Erba presented these results in abstract 211.* The research was sponsored by Seattle Genetics, Inc.
The study included 42 newly diagnosed AML patients with a median age of 45.5. Half the patients had intermediate-risk karyotypes, 36% had adverse karyotypes, and 17% had secondary AML.
Patients received escalating doses of vadastuximab talirine (10+10 mcg/kg [n=4] and 20+10 mcg/kg [n=38]) in combination with 7+3 induction (cytarabine at 100 mg/m2 and daunorubicin at 60 mg/m2) on days 1 and 4 of a 28-day treatment cycle. Responses were assessed on days 15 and 28.
A second induction regimen and post-remission therapies were prescribed according to investigator choice and did not include vadastuximab talirine.
Results
The maximum tolerated dose of vadastuximab talirine was 20+10 mcg/kg.
Hematologic treatment-related AEs included febrile neutropenia (43%, grade 1-3), thrombocytopenia (38%, grade 3-4), anemia (24%, grade 3), and neutropenia (17%, grade 3-4).
Non-hematologic treatment-related AEs included nausea (17%), fatigue (14%), diarrhea (7%), and decreased appetite (7%). All of these AEs were grade 1-2.
None of the patients experienced infusion-related reactions, veno-occlusive disease, or significant liver damage.
A total of 76% of patients responded to treatment, with 60% percent achieving a CR and 17% achieving a CR with incomplete blood count recovery (CRi).
The 76% response rate is close to what would be expected for a well-chosen population fit for a clinical trial, Dr Erba said.
There was a hint of additional benefit as well, he added.
“The first hint was that 30 out of the 32 patients [who achieved a CR/CRi] required only 1 round of chemotherapy to achieve that remission,” Dr Erba said. “This also suggested that deeper remissions may be possible.”
MRD assessments using a sensitive flow cytometric assay revealed that 25 of the 32 patients (78%) who achieved a CR/CRi were MRD-negative.
Dr Erba said a randomized, phase 2 trial of vadastuximab talirine plus 7+3 versus 7+3 alone is planned for the first quarter of 2017. ![]()
*Information presented at the meeting differs from the abstract.
Negotiating The Professional Contract
For the freshly minted NP or PA, finding the right place to practice and negotiating a reasonable professional contract can be a challenge. The keys to successful negotiation are similar to those for attaining proficiency in your clinical practice—providing insight into your personality, an evaluation of your personal and professional goals, and a commitment of time for preparation. For most NPs and PAs, employment opportunities do not just happen. Preparation, persistence, and personal contacts are basic requirements for finding the right position.
Of great interest to NPs and PAs—especially those with looming loan payments—is the compensation package. There are many important questions and topics to discuss regarding compensation (see Table 1). However, salaries are often determined by the “going rate” for particular services in your geographic region, in addition to your specialty, experience, and credentials. Your professional association (AAPA for PAs, AANP for NPs) has robust data on salaries in your particular specialty, practice setting, and geographic region; the average salary for both professions is currently about $97,000.1,2
Familiarize yourself with the statutes and regulations that govern the scope of practice in your state—this is especially important if there are specific supervision or collaboration rules. Be prepared to present applicable statutes, rules, and regulations to the physician and/or office manager. Know whether any reimbursement restrictions exist. Be sure to review IRS guidelines for employee status versus independent contractor status.
The diversity of NP and PA practices means one size does not fit all, so it is best to identify the practice that complements your own personality. So, before you open negotiations, it is important that you research the practice. (For suggestions on what to inquire about, see Table 2). It is also a good idea to check the Docinfo website (http://docinfo.org/#/search/query), sponsored by the Federation of State Medical Boards, to research disciplinary records of the physician(s). Additional information can be acquired at each state regulatory board site.
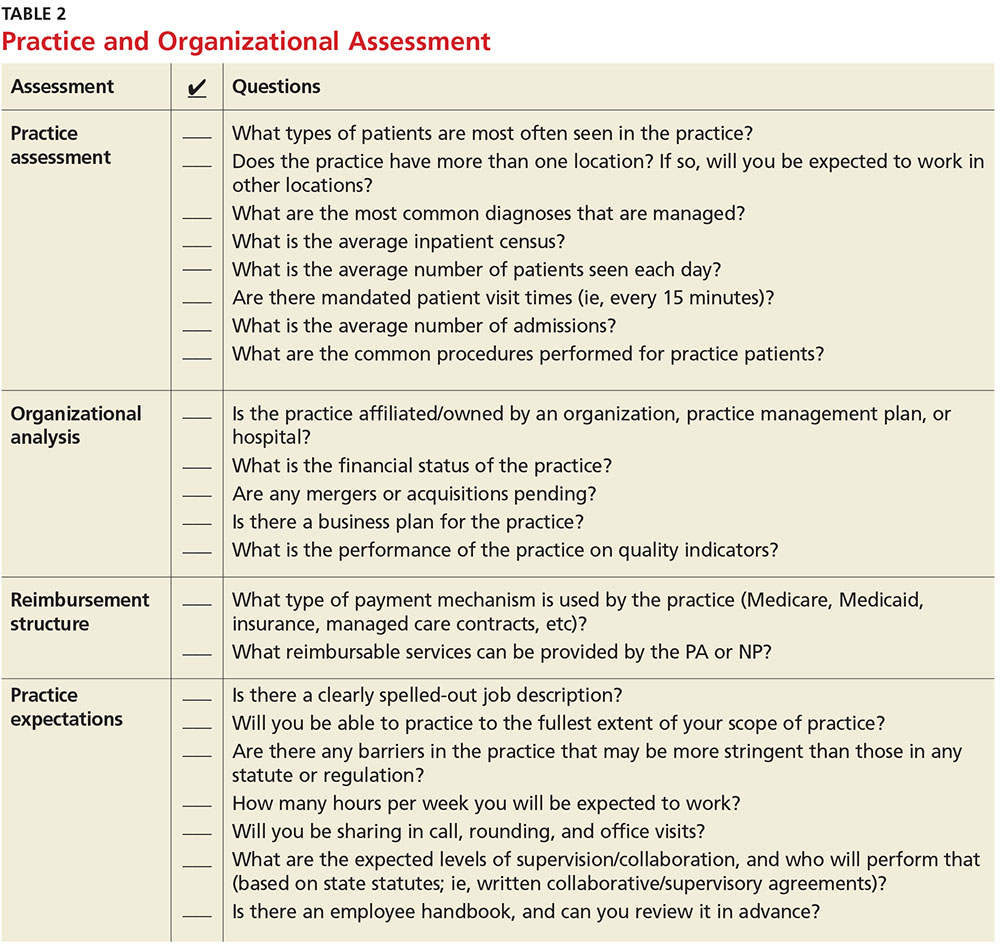
NEGOTIATION
When you’ve decided which employment offer to pursue, it’s time to think about how you want to negotiate your contract. Many people feel that negotiation is equivalent to confrontation, inevitably leading to an awkward disagreement with the practice. This is rarely the case; negotiation is simply a professional conversation, best had one-on-one with the key decision maker, rather than a group.
Never assume that your compensation package is predetermined. Whether you are starting a new job or looking to enhance your current situation, you can make a difference by asking for what you need.3 Knowing the local market and data is essential. Research the average salary in the region (for experienced versus new NP or PA). Be sure to think beyond salary and evaluate which benefits you’d like to have as part of your compensation package (see Table 3, as well as our survey results).
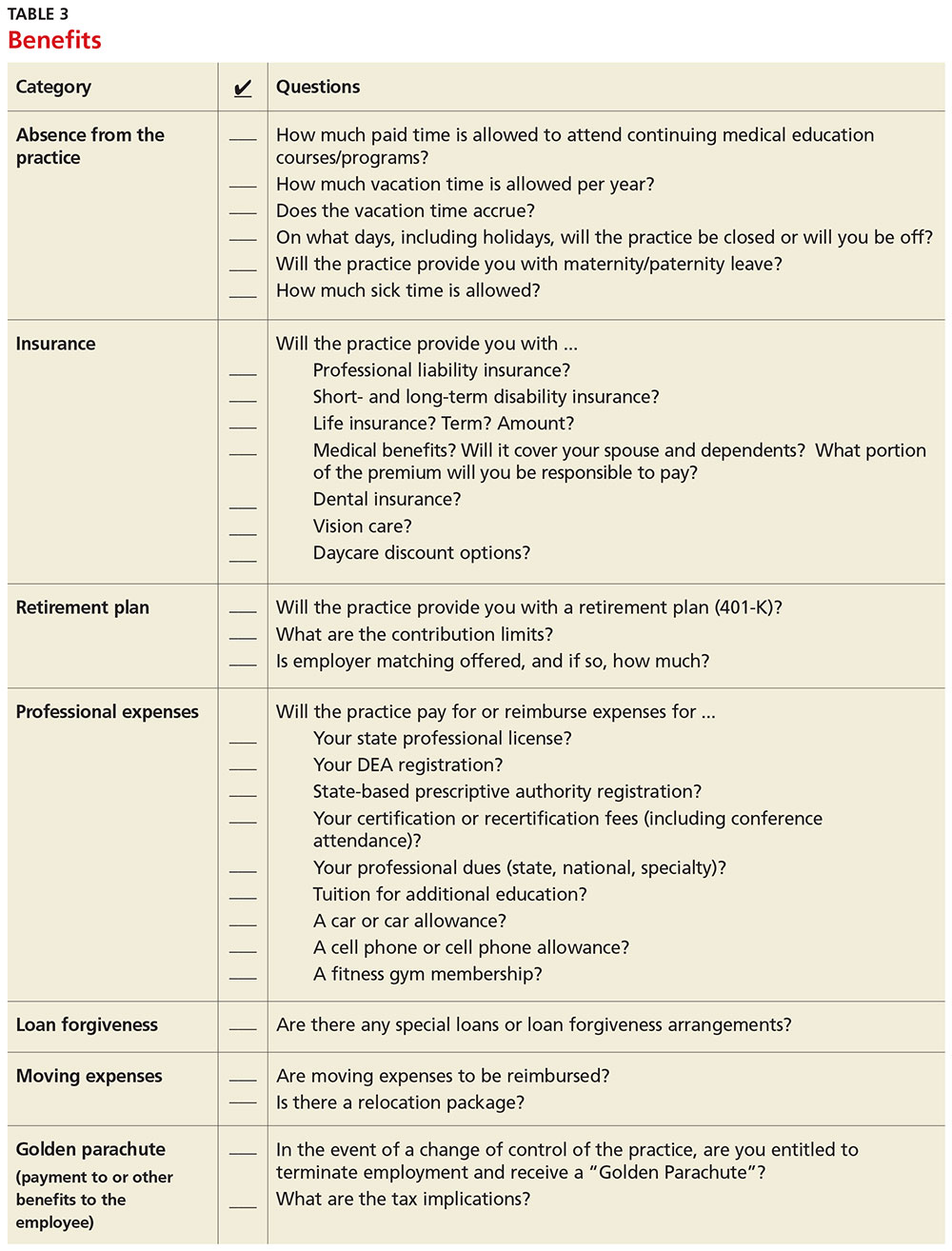
Carolyn Buppert, a specialist in legal and reimbursement issues for NPs and PAs, suggests three “P’s” of negotiation: Prepare, probe, and propose.4
Prepare. Learn how to calculate the projected revenue you would bring into a practice. You can determine the profit you generate by asking the practice administrator for data, noting Current Procedural Terminology codes and dates and becoming familiar with the fee schedule. According to Buppert, your salary and benefits should amount to about one-third of your total billings, and the benefits should equate to about 25% of your base salary.4 It is worthwhile to discuss nonmonetary contributions to the practice, such as improvements in patient satisfaction or reduction in no-shows.
Probe. Ask about the practice’s financial health during your interview and determine employer expectations for profits.
Propose. Once your prospective employer proves their value to you and after you have done due diligence researching the practice, do not hesitate to ask for what you deserve. When doing this, it is important to maintain neutral body language, keep an unemotional tone of voice, and convey an understanding of the employer’s point of view.4
Most successful negotiations occur over a couple of meetings. Careful listening is essential to avoid misunderstandings and false assumptions. By listening intently, you can identify the issues most important to the employer (eg, pay may be negotiable, but moonlighting not; or control over shift schedules is a hot button, but pay is not). Evaluating and weighing those against your own requirements may avoid an unnecessary impasse and result in a better outcome for you.
One question lives at the heart of negotiations: Should agreements be in writing? Written agreements carry more weight and prevent misunderstandings, a benefit to both parties involved. Formal, written negotiation of a contract forces the parties to discuss issues and provides a record for future reference.5
CONTRACTS
Because courses in negotiations, contract law, and business principles are rarely taught in educational programs, you should consider consulting an attorney who is familiar with contract and business issues. Avoid attorneys who have to research the laws regarding NP and PA practice—there are many who are well versed in these contracts. It is also usually more acceptable to conduct initial negotiations yourself, rather than through an attorney.
First, ask the attorney to review the contract and advise you of any troublesome provision or obvious omissions (see Table 4; a sample contract is also available on our website).6-8 Many attorneys with experience reviewing and negotiating such contracts will be willing to do an initial read and consultation for a fixed, predetermined fee. Following that consultation, it is best to discuss your concerns and questions directly with your potential employer. If you can come to a general agreement on revisions, either your lawyer or the employer’s can make the necessary changes.
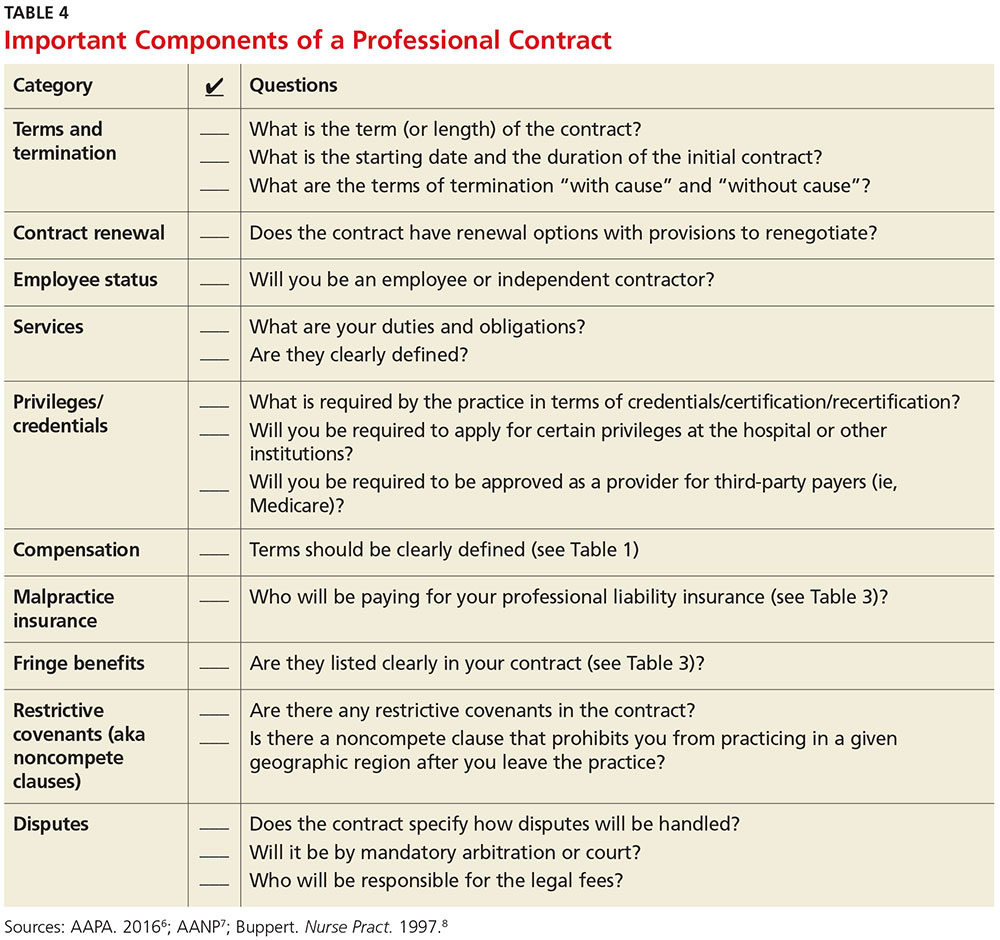
When considering an employment agreement, there are specific issues and potential hurdles to look out for. Following is a brief discussion of some of them.
Liability insurance
Find out which type of liability insurance is offered (occurrence-based or claims-made), as well as the limits of liability. Occurrence-based insurance is usually recommended. However, if the policy is claims-made, it’s important to know if the tail coverage will be paid by the employer, or if there is a rider on the physician’s policy. Determine if your contract will allow for the cost of the tail to be deducted from final amounts that may be owed to you upon termination. Be prepared to acquire the tail, if needed, to ensure coverage.
Restrictive covenants (noncompete clauses)
Unfortunately, not all employment relationships work out in the long term. In recognition of this, many professional contracts contain an agreement known as a restrictive covenant, which impedes the NP’s or PA’s ability to practice in the community following a relationship cessation. Depending on where the practice is located, such restrictions can be devastating and may even require the NP or PA to move in order to pursue a career. The law of restrictive covenants varies greatly from state to state. Your attorney can help you evaluate the enforceability and effect of a restrictive covenant, advise you on what is or isn’t reasonable in the community, and give you suggestions on how to negotiate a more reasonable one.
Moonlighting
Whether or not you can moonlight—and where—is often negotiable. An employer certainly has the right to require that moonlighting not interfere with your regular duties or schedule; endorse competitors of your employer or potentially divert patients; or expose the employer to malpractice liability. If you can assure your employer that these three situations will not transpire, then moonlighting (with advance scheduling notice and permission) should be acceptable.
Training/continuing education
It is typical for the employer to reimburse some or all of the cost of continuing education, up to a maximum annual amount. It is also reasonable for the employer (if paying for the CME) to pre-approve the curriculum, as well as the timing to avoid scheduling problems.
Salary and bonus
Compensation systems can be entirely objective (ie, production based), subjective (entirely up to the discretion of the employer based on internal criteria), or a combination of both. It is important to get a clear understanding of the system so that you know what is expected of you and what the rewards are for meeting performance goals.
Income guarantee/advanced expenses/repayment obligation
It is not uncommon for a local hospital to help a medical practice by guaranteeing the salary for a certain period of time. In this situation, the guarantee can be structured in a number of different ways—but it typically involves an obligation to repay a predetermined amount if you leave the practice area before the expiration of the term. Often, the practice will seek to pass that liability on to you. The same may apply for advanced moving expenses, CME, etc. A practice may require you to be employed for a specified amount of time to “earn” those advanced expenses, or to pay them back if an early termination occurs. In any case, it is important to know what the expectations are, and what circumstances could lead to an early termination and/or repayment obligation.
Terms and termination
Pay particular attention to the terms of your agreement. In one paragraph, it may say that the contract is for one year with annual renewals, but later on it explains that it can be terminated by either party upon 30 days notice. In that case, it is not a one-year contract; it is a 30-day contract. If there is a possibility of early termination and a restrictive covenant, or even a repayment obligation, you could really be at risk. Pay attention to whether early termination is “for cause” or “without cause.” If it is only for cause, inquire what constitutes a cause and whether there are any opportunities to rectify a perceived problem.
CONCLUSION
Constant changes in the health care marketplace will continue to generate opportunities for NPs and PAs. This is especially true for clinicians who demonstrate competence, enthusiasm, and commitment to quality patient care. The same clinical skills you learned in school and practice will help you find a clinical position and negotiate a good professional contract. Attention to detail, evidence-based research, and excellent communication skills will enable you to land a position beneficial to both parties.
1. American Academy of Physician Assistants. 2016 AAPA Salary Report. www.aapa.org/research/salary-report. Accessed November 4, 2016.
2. American Academy of Nurse Practitioners. Annual income for full-time nurse practitioners up 10% since 2011. www.aanp.org/press-room/press-releases/166-press-room/2015-press-releases/1803-annual- income-for-full-time-nurse-practitioners-up-10-since-2011. Accessed November 4, 2016.
3. Bourne H. A Great Deal! Compensation Negotiation for Nurse Practitioners & Physician Assistants. 3rd ed. Arcata, CA: Open Spaces; 1998.
4. Buppert C. Nurse Practitioner’s Business Practice and Legal Guide. 5th ed. Burlington, MA: Jones & Bartlett Learning; 2015.
5. Henley MB. Finding your ideal job and negotiating your contract: where to get the information and numbers you need to know. J Orthop Trauma. 2012;26(1):S9-S13.
6. AAPA. Contacts and contracts: a guide to your PA career. www.aapa.org/WorkArea/DownloadAsset.aspx?id=2147486883. Accessed November 4, 2016.
7. AANP. Employment negotiations. www.aanp.org/practice/reimbursement/68-articles/579-employment-negotiations. Accessed November 4, 2016.
8. Buppert C. Employment agreements: clauses that can change an NP’s life. Nurse Pract. 1997;22(8):108-109.
For the freshly minted NP or PA, finding the right place to practice and negotiating a reasonable professional contract can be a challenge. The keys to successful negotiation are similar to those for attaining proficiency in your clinical practice—providing insight into your personality, an evaluation of your personal and professional goals, and a commitment of time for preparation. For most NPs and PAs, employment opportunities do not just happen. Preparation, persistence, and personal contacts are basic requirements for finding the right position.
Of great interest to NPs and PAs—especially those with looming loan payments—is the compensation package. There are many important questions and topics to discuss regarding compensation (see Table 1). However, salaries are often determined by the “going rate” for particular services in your geographic region, in addition to your specialty, experience, and credentials. Your professional association (AAPA for PAs, AANP for NPs) has robust data on salaries in your particular specialty, practice setting, and geographic region; the average salary for both professions is currently about $97,000.1,2

Familiarize yourself with the statutes and regulations that govern the scope of practice in your state—this is especially important if there are specific supervision or collaboration rules. Be prepared to present applicable statutes, rules, and regulations to the physician and/or office manager. Know whether any reimbursement restrictions exist. Be sure to review IRS guidelines for employee status versus independent contractor status.
The diversity of NP and PA practices means one size does not fit all, so it is best to identify the practice that complements your own personality. So, before you open negotiations, it is important that you research the practice. (For suggestions on what to inquire about, see Table 2). It is also a good idea to check the Docinfo website (http://docinfo.org/#/search/query), sponsored by the Federation of State Medical Boards, to research disciplinary records of the physician(s). Additional information can be acquired at each state regulatory board site.

NEGOTIATION
When you’ve decided which employment offer to pursue, it’s time to think about how you want to negotiate your contract. Many people feel that negotiation is equivalent to confrontation, inevitably leading to an awkward disagreement with the practice. This is rarely the case; negotiation is simply a professional conversation, best had one-on-one with the key decision maker, rather than a group.
Never assume that your compensation package is predetermined. Whether you are starting a new job or looking to enhance your current situation, you can make a difference by asking for what you need.3 Knowing the local market and data is essential. Research the average salary in the region (for experienced versus new NP or PA). Be sure to think beyond salary and evaluate which benefits you’d like to have as part of your compensation package (see Table 3, as well as our survey results).

Carolyn Buppert, a specialist in legal and reimbursement issues for NPs and PAs, suggests three “P’s” of negotiation: Prepare, probe, and propose.4
Prepare. Learn how to calculate the projected revenue you would bring into a practice. You can determine the profit you generate by asking the practice administrator for data, noting Current Procedural Terminology codes and dates and becoming familiar with the fee schedule. According to Buppert, your salary and benefits should amount to about one-third of your total billings, and the benefits should equate to about 25% of your base salary.4 It is worthwhile to discuss nonmonetary contributions to the practice, such as improvements in patient satisfaction or reduction in no-shows.
Probe. Ask about the practice’s financial health during your interview and determine employer expectations for profits.
Propose. Once your prospective employer proves their value to you and after you have done due diligence researching the practice, do not hesitate to ask for what you deserve. When doing this, it is important to maintain neutral body language, keep an unemotional tone of voice, and convey an understanding of the employer’s point of view.4
Most successful negotiations occur over a couple of meetings. Careful listening is essential to avoid misunderstandings and false assumptions. By listening intently, you can identify the issues most important to the employer (eg, pay may be negotiable, but moonlighting not; or control over shift schedules is a hot button, but pay is not). Evaluating and weighing those against your own requirements may avoid an unnecessary impasse and result in a better outcome for you.
One question lives at the heart of negotiations: Should agreements be in writing? Written agreements carry more weight and prevent misunderstandings, a benefit to both parties involved. Formal, written negotiation of a contract forces the parties to discuss issues and provides a record for future reference.5
CONTRACTS
Because courses in negotiations, contract law, and business principles are rarely taught in educational programs, you should consider consulting an attorney who is familiar with contract and business issues. Avoid attorneys who have to research the laws regarding NP and PA practice—there are many who are well versed in these contracts. It is also usually more acceptable to conduct initial negotiations yourself, rather than through an attorney.
First, ask the attorney to review the contract and advise you of any troublesome provision or obvious omissions (see Table 4; a sample contract is also available on our website).6-8 Many attorneys with experience reviewing and negotiating such contracts will be willing to do an initial read and consultation for a fixed, predetermined fee. Following that consultation, it is best to discuss your concerns and questions directly with your potential employer. If you can come to a general agreement on revisions, either your lawyer or the employer’s can make the necessary changes.

When considering an employment agreement, there are specific issues and potential hurdles to look out for. Following is a brief discussion of some of them.
Liability insurance
Find out which type of liability insurance is offered (occurrence-based or claims-made), as well as the limits of liability. Occurrence-based insurance is usually recommended. However, if the policy is claims-made, it’s important to know if the tail coverage will be paid by the employer, or if there is a rider on the physician’s policy. Determine if your contract will allow for the cost of the tail to be deducted from final amounts that may be owed to you upon termination. Be prepared to acquire the tail, if needed, to ensure coverage.
Restrictive covenants (noncompete clauses)
Unfortunately, not all employment relationships work out in the long term. In recognition of this, many professional contracts contain an agreement known as a restrictive covenant, which impedes the NP’s or PA’s ability to practice in the community following a relationship cessation. Depending on where the practice is located, such restrictions can be devastating and may even require the NP or PA to move in order to pursue a career. The law of restrictive covenants varies greatly from state to state. Your attorney can help you evaluate the enforceability and effect of a restrictive covenant, advise you on what is or isn’t reasonable in the community, and give you suggestions on how to negotiate a more reasonable one.
Moonlighting
Whether or not you can moonlight—and where—is often negotiable. An employer certainly has the right to require that moonlighting not interfere with your regular duties or schedule; endorse competitors of your employer or potentially divert patients; or expose the employer to malpractice liability. If you can assure your employer that these three situations will not transpire, then moonlighting (with advance scheduling notice and permission) should be acceptable.
Training/continuing education
It is typical for the employer to reimburse some or all of the cost of continuing education, up to a maximum annual amount. It is also reasonable for the employer (if paying for the CME) to pre-approve the curriculum, as well as the timing to avoid scheduling problems.
Salary and bonus
Compensation systems can be entirely objective (ie, production based), subjective (entirely up to the discretion of the employer based on internal criteria), or a combination of both. It is important to get a clear understanding of the system so that you know what is expected of you and what the rewards are for meeting performance goals.
Income guarantee/advanced expenses/repayment obligation
It is not uncommon for a local hospital to help a medical practice by guaranteeing the salary for a certain period of time. In this situation, the guarantee can be structured in a number of different ways—but it typically involves an obligation to repay a predetermined amount if you leave the practice area before the expiration of the term. Often, the practice will seek to pass that liability on to you. The same may apply for advanced moving expenses, CME, etc. A practice may require you to be employed for a specified amount of time to “earn” those advanced expenses, or to pay them back if an early termination occurs. In any case, it is important to know what the expectations are, and what circumstances could lead to an early termination and/or repayment obligation.
Terms and termination
Pay particular attention to the terms of your agreement. In one paragraph, it may say that the contract is for one year with annual renewals, but later on it explains that it can be terminated by either party upon 30 days notice. In that case, it is not a one-year contract; it is a 30-day contract. If there is a possibility of early termination and a restrictive covenant, or even a repayment obligation, you could really be at risk. Pay attention to whether early termination is “for cause” or “without cause.” If it is only for cause, inquire what constitutes a cause and whether there are any opportunities to rectify a perceived problem.
CONCLUSION
Constant changes in the health care marketplace will continue to generate opportunities for NPs and PAs. This is especially true for clinicians who demonstrate competence, enthusiasm, and commitment to quality patient care. The same clinical skills you learned in school and practice will help you find a clinical position and negotiate a good professional contract. Attention to detail, evidence-based research, and excellent communication skills will enable you to land a position beneficial to both parties.
For the freshly minted NP or PA, finding the right place to practice and negotiating a reasonable professional contract can be a challenge. The keys to successful negotiation are similar to those for attaining proficiency in your clinical practice—providing insight into your personality, an evaluation of your personal and professional goals, and a commitment of time for preparation. For most NPs and PAs, employment opportunities do not just happen. Preparation, persistence, and personal contacts are basic requirements for finding the right position.
Of great interest to NPs and PAs—especially those with looming loan payments—is the compensation package. There are many important questions and topics to discuss regarding compensation (see Table 1). However, salaries are often determined by the “going rate” for particular services in your geographic region, in addition to your specialty, experience, and credentials. Your professional association (AAPA for PAs, AANP for NPs) has robust data on salaries in your particular specialty, practice setting, and geographic region; the average salary for both professions is currently about $97,000.1,2

Familiarize yourself with the statutes and regulations that govern the scope of practice in your state—this is especially important if there are specific supervision or collaboration rules. Be prepared to present applicable statutes, rules, and regulations to the physician and/or office manager. Know whether any reimbursement restrictions exist. Be sure to review IRS guidelines for employee status versus independent contractor status.
The diversity of NP and PA practices means one size does not fit all, so it is best to identify the practice that complements your own personality. So, before you open negotiations, it is important that you research the practice. (For suggestions on what to inquire about, see Table 2). It is also a good idea to check the Docinfo website (http://docinfo.org/#/search/query), sponsored by the Federation of State Medical Boards, to research disciplinary records of the physician(s). Additional information can be acquired at each state regulatory board site.

NEGOTIATION
When you’ve decided which employment offer to pursue, it’s time to think about how you want to negotiate your contract. Many people feel that negotiation is equivalent to confrontation, inevitably leading to an awkward disagreement with the practice. This is rarely the case; negotiation is simply a professional conversation, best had one-on-one with the key decision maker, rather than a group.
Never assume that your compensation package is predetermined. Whether you are starting a new job or looking to enhance your current situation, you can make a difference by asking for what you need.3 Knowing the local market and data is essential. Research the average salary in the region (for experienced versus new NP or PA). Be sure to think beyond salary and evaluate which benefits you’d like to have as part of your compensation package (see Table 3, as well as our survey results).

Carolyn Buppert, a specialist in legal and reimbursement issues for NPs and PAs, suggests three “P’s” of negotiation: Prepare, probe, and propose.4
Prepare. Learn how to calculate the projected revenue you would bring into a practice. You can determine the profit you generate by asking the practice administrator for data, noting Current Procedural Terminology codes and dates and becoming familiar with the fee schedule. According to Buppert, your salary and benefits should amount to about one-third of your total billings, and the benefits should equate to about 25% of your base salary.4 It is worthwhile to discuss nonmonetary contributions to the practice, such as improvements in patient satisfaction or reduction in no-shows.
Probe. Ask about the practice’s financial health during your interview and determine employer expectations for profits.
Propose. Once your prospective employer proves their value to you and after you have done due diligence researching the practice, do not hesitate to ask for what you deserve. When doing this, it is important to maintain neutral body language, keep an unemotional tone of voice, and convey an understanding of the employer’s point of view.4
Most successful negotiations occur over a couple of meetings. Careful listening is essential to avoid misunderstandings and false assumptions. By listening intently, you can identify the issues most important to the employer (eg, pay may be negotiable, but moonlighting not; or control over shift schedules is a hot button, but pay is not). Evaluating and weighing those against your own requirements may avoid an unnecessary impasse and result in a better outcome for you.
One question lives at the heart of negotiations: Should agreements be in writing? Written agreements carry more weight and prevent misunderstandings, a benefit to both parties involved. Formal, written negotiation of a contract forces the parties to discuss issues and provides a record for future reference.5
CONTRACTS
Because courses in negotiations, contract law, and business principles are rarely taught in educational programs, you should consider consulting an attorney who is familiar with contract and business issues. Avoid attorneys who have to research the laws regarding NP and PA practice—there are many who are well versed in these contracts. It is also usually more acceptable to conduct initial negotiations yourself, rather than through an attorney.
First, ask the attorney to review the contract and advise you of any troublesome provision or obvious omissions (see Table 4; a sample contract is also available on our website).6-8 Many attorneys with experience reviewing and negotiating such contracts will be willing to do an initial read and consultation for a fixed, predetermined fee. Following that consultation, it is best to discuss your concerns and questions directly with your potential employer. If you can come to a general agreement on revisions, either your lawyer or the employer’s can make the necessary changes.

When considering an employment agreement, there are specific issues and potential hurdles to look out for. Following is a brief discussion of some of them.
Liability insurance
Find out which type of liability insurance is offered (occurrence-based or claims-made), as well as the limits of liability. Occurrence-based insurance is usually recommended. However, if the policy is claims-made, it’s important to know if the tail coverage will be paid by the employer, or if there is a rider on the physician’s policy. Determine if your contract will allow for the cost of the tail to be deducted from final amounts that may be owed to you upon termination. Be prepared to acquire the tail, if needed, to ensure coverage.
Restrictive covenants (noncompete clauses)
Unfortunately, not all employment relationships work out in the long term. In recognition of this, many professional contracts contain an agreement known as a restrictive covenant, which impedes the NP’s or PA’s ability to practice in the community following a relationship cessation. Depending on where the practice is located, such restrictions can be devastating and may even require the NP or PA to move in order to pursue a career. The law of restrictive covenants varies greatly from state to state. Your attorney can help you evaluate the enforceability and effect of a restrictive covenant, advise you on what is or isn’t reasonable in the community, and give you suggestions on how to negotiate a more reasonable one.
Moonlighting
Whether or not you can moonlight—and where—is often negotiable. An employer certainly has the right to require that moonlighting not interfere with your regular duties or schedule; endorse competitors of your employer or potentially divert patients; or expose the employer to malpractice liability. If you can assure your employer that these three situations will not transpire, then moonlighting (with advance scheduling notice and permission) should be acceptable.
Training/continuing education
It is typical for the employer to reimburse some or all of the cost of continuing education, up to a maximum annual amount. It is also reasonable for the employer (if paying for the CME) to pre-approve the curriculum, as well as the timing to avoid scheduling problems.
Salary and bonus
Compensation systems can be entirely objective (ie, production based), subjective (entirely up to the discretion of the employer based on internal criteria), or a combination of both. It is important to get a clear understanding of the system so that you know what is expected of you and what the rewards are for meeting performance goals.
Income guarantee/advanced expenses/repayment obligation
It is not uncommon for a local hospital to help a medical practice by guaranteeing the salary for a certain period of time. In this situation, the guarantee can be structured in a number of different ways—but it typically involves an obligation to repay a predetermined amount if you leave the practice area before the expiration of the term. Often, the practice will seek to pass that liability on to you. The same may apply for advanced moving expenses, CME, etc. A practice may require you to be employed for a specified amount of time to “earn” those advanced expenses, or to pay them back if an early termination occurs. In any case, it is important to know what the expectations are, and what circumstances could lead to an early termination and/or repayment obligation.
Terms and termination
Pay particular attention to the terms of your agreement. In one paragraph, it may say that the contract is for one year with annual renewals, but later on it explains that it can be terminated by either party upon 30 days notice. In that case, it is not a one-year contract; it is a 30-day contract. If there is a possibility of early termination and a restrictive covenant, or even a repayment obligation, you could really be at risk. Pay attention to whether early termination is “for cause” or “without cause.” If it is only for cause, inquire what constitutes a cause and whether there are any opportunities to rectify a perceived problem.
CONCLUSION
Constant changes in the health care marketplace will continue to generate opportunities for NPs and PAs. This is especially true for clinicians who demonstrate competence, enthusiasm, and commitment to quality patient care. The same clinical skills you learned in school and practice will help you find a clinical position and negotiate a good professional contract. Attention to detail, evidence-based research, and excellent communication skills will enable you to land a position beneficial to both parties.
1. American Academy of Physician Assistants. 2016 AAPA Salary Report. www.aapa.org/research/salary-report. Accessed November 4, 2016.
2. American Academy of Nurse Practitioners. Annual income for full-time nurse practitioners up 10% since 2011. www.aanp.org/press-room/press-releases/166-press-room/2015-press-releases/1803-annual- income-for-full-time-nurse-practitioners-up-10-since-2011. Accessed November 4, 2016.
3. Bourne H. A Great Deal! Compensation Negotiation for Nurse Practitioners & Physician Assistants. 3rd ed. Arcata, CA: Open Spaces; 1998.
4. Buppert C. Nurse Practitioner’s Business Practice and Legal Guide. 5th ed. Burlington, MA: Jones & Bartlett Learning; 2015.
5. Henley MB. Finding your ideal job and negotiating your contract: where to get the information and numbers you need to know. J Orthop Trauma. 2012;26(1):S9-S13.
6. AAPA. Contacts and contracts: a guide to your PA career. www.aapa.org/WorkArea/DownloadAsset.aspx?id=2147486883. Accessed November 4, 2016.
7. AANP. Employment negotiations. www.aanp.org/practice/reimbursement/68-articles/579-employment-negotiations. Accessed November 4, 2016.
8. Buppert C. Employment agreements: clauses that can change an NP’s life. Nurse Pract. 1997;22(8):108-109.
1. American Academy of Physician Assistants. 2016 AAPA Salary Report. www.aapa.org/research/salary-report. Accessed November 4, 2016.
2. American Academy of Nurse Practitioners. Annual income for full-time nurse practitioners up 10% since 2011. www.aanp.org/press-room/press-releases/166-press-room/2015-press-releases/1803-annual- income-for-full-time-nurse-practitioners-up-10-since-2011. Accessed November 4, 2016.
3. Bourne H. A Great Deal! Compensation Negotiation for Nurse Practitioners & Physician Assistants. 3rd ed. Arcata, CA: Open Spaces; 1998.
4. Buppert C. Nurse Practitioner’s Business Practice and Legal Guide. 5th ed. Burlington, MA: Jones & Bartlett Learning; 2015.
5. Henley MB. Finding your ideal job and negotiating your contract: where to get the information and numbers you need to know. J Orthop Trauma. 2012;26(1):S9-S13.
6. AAPA. Contacts and contracts: a guide to your PA career. www.aapa.org/WorkArea/DownloadAsset.aspx?id=2147486883. Accessed November 4, 2016.
7. AANP. Employment negotiations. www.aanp.org/practice/reimbursement/68-articles/579-employment-negotiations. Accessed November 4, 2016.
8. Buppert C. Employment agreements: clauses that can change an NP’s life. Nurse Pract. 1997;22(8):108-109.
Antibody face-off in follicular lymphoma gives PFS, but not OS, edge to obinutuzumab
SAN DIEGO – Obinutuzumab, a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, offered a progression-free survival (PFS) edge over rituximab when combined with standard chemotherapy in patients with previously untreated advanced follicular lymphoma.
But other clinicians and investigators who
attended the presentation of the GALLIUM data at a plenary session during the American Society of Hematology annual meeting indicated that despite the data, they weren’t ready to make a switch to the newer, costlier antibody.
“I feel that it is not convincing for practice-changing,” said Kanti R. Rai, MD, professor of medicine and molecular medicine at Hofstra University, Hempstead, N.Y.
“Unless we have evidence of a survival advantage in indolent disease, progression-free survivorship is not an adequate reason to jump to another antibody,” he said in an interview.
In GALLIUM, the primary endpoint of investigator-assessed 3-year PFS at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio of 0.68 favoring obinutuzumab (P = .0012).
Respective 3-year overall survival rates were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
Indolent lymphoma trial
The GALLIUM trial is a phase III study comparing obinutuzumab with rituximab when paired with one of three standard chemotherapy regimens for indolent non-Hodgkin lymphomas, including follicular lymphoma and splenic, nodal, or extranodal marginal zone lymphoma. Dr. Marcus presented data on patients with follicular lymphoma only.
The antibodies were delivered in combination with either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; 33.1% of patients), CVP (cyclophosphamide, vincristine, prednisone; 9.8%) or bendamustine alone (B; 57.1%) as the chemotherapy backbone. The choice of regimen was at the discretion of the treating center.
A total of 1,202 patients with follicular lymphoma were enrolled and randomized to treatment and were included in an intention-to-treat analysis.
The treatment arms were well balanced with regard to distribution of patients characteristics, with approximately 21% in each arm having Follicular Lymphoma International Prognostic Index low-risk disease; 37% having intermediate-risk disease; and 34% having high-risk disease.
Roughly half of patients in each arm had bone marrow involvement, and two-thirds had extranodal involvement.
Obinutuzumab was dosed 1,000 mg IV on days 1, 8, and 15 of cycle one, and either on day 1 of cycles two through eight every 3 weeks, or every 4 weeks during cycles two through six.
Overall response rates at the end of induction were 86.9% with rituximab and 88.5% with obinutuzumab, with complete responses of 23.8% and 19.5%, respectively.
As noted before, investigator-assessed PFS favored obinutuzumab, as did PFS assessed by independent reviewer, at 81.9% vs. 77.9% for rituximab (HR, 0.71; P = .0138).
The newer antibody also had a slight edge in time to new treatment, with 87.1% of patients on obinutuzumab not starting on new therapy, compared with 81.2% of patients on rituximab.
More bendamustine deaths
Nearly all patients in each arm had an adverse event, with grade 3 or greater events occurring in 74.6% of patients on obinutuzumab vs. 67.8% on rituximab. Rates of neutropenia, leukopenia, febrile neutropenia, infusion reactions, and thrombocytopenia were all slightly higher with obinutuzumab. Grade 3 or greater infections occurred in 20% with obinutuzumab, compared with 15.6% with rituximab.
“What we did note, however, was a high level of mortality in patients receiving either obinutuzumab-based therapy or rituximab-based therapy, which were no different between the two arms and were somewhat higher than one might expect from patients receiving induction treatment in follicular lymphoma. Hence, we did a more detailed analysis of safety by treatment regimen,” Dr. Marcus said.
There were more deaths among patients treated with bendamustine (5.6% for patients in the B-obinutuzumab cohort, and 4.4% of patients in the B-rituximab cohort) vs. 1.6% and 2.0%, respectively, for patients on CHOP, and 1.6 and 1.8% for patients on CVP.
Dose effect?
John P. Leonard, MD, from Cornell University, New York , who introduced Dr. Marcus, commented that PFS may not be the ideal endpoint for patients with follicular lymphoma.
He pointed out that in trials comparing rituximab with obinutuzumab for other diseases, results have been mixed, with obinutuzumab showing superiority in chronic lymphocytic leukemia, but in data presented elsewhere at ASH 2016, obinutuzumab was not superior to rituximab for treatment of diffuse large B-cell lymphoma.
“One question is whether obinutuzumab, which is generally administered at a higher mg dose to patients, is in fact a better antibody or if it is in fact a dose effect,” he said.
In response to a similar question following his presentation, Dr. Marcus replied that, despite sharing a target, the two antibodies are different, with different mechanisms of action. He also noted that there is no evidence to suggest that rituximab potency would be greater in follicular lymphoma if it were given at higher doses.
The GALLIUM trial is sponsored by Hoffmann-La Roche, Dr, Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
SAN DIEGO – Obinutuzumab, a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, offered a progression-free survival (PFS) edge over rituximab when combined with standard chemotherapy in patients with previously untreated advanced follicular lymphoma.
But other clinicians and investigators who
attended the presentation of the GALLIUM data at a plenary session during the American Society of Hematology annual meeting indicated that despite the data, they weren’t ready to make a switch to the newer, costlier antibody.
“I feel that it is not convincing for practice-changing,” said Kanti R. Rai, MD, professor of medicine and molecular medicine at Hofstra University, Hempstead, N.Y.
“Unless we have evidence of a survival advantage in indolent disease, progression-free survivorship is not an adequate reason to jump to another antibody,” he said in an interview.
In GALLIUM, the primary endpoint of investigator-assessed 3-year PFS at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio of 0.68 favoring obinutuzumab (P = .0012).
Respective 3-year overall survival rates were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
Indolent lymphoma trial
The GALLIUM trial is a phase III study comparing obinutuzumab with rituximab when paired with one of three standard chemotherapy regimens for indolent non-Hodgkin lymphomas, including follicular lymphoma and splenic, nodal, or extranodal marginal zone lymphoma. Dr. Marcus presented data on patients with follicular lymphoma only.
The antibodies were delivered in combination with either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; 33.1% of patients), CVP (cyclophosphamide, vincristine, prednisone; 9.8%) or bendamustine alone (B; 57.1%) as the chemotherapy backbone. The choice of regimen was at the discretion of the treating center.
A total of 1,202 patients with follicular lymphoma were enrolled and randomized to treatment and were included in an intention-to-treat analysis.
The treatment arms were well balanced with regard to distribution of patients characteristics, with approximately 21% in each arm having Follicular Lymphoma International Prognostic Index low-risk disease; 37% having intermediate-risk disease; and 34% having high-risk disease.
Roughly half of patients in each arm had bone marrow involvement, and two-thirds had extranodal involvement.
Obinutuzumab was dosed 1,000 mg IV on days 1, 8, and 15 of cycle one, and either on day 1 of cycles two through eight every 3 weeks, or every 4 weeks during cycles two through six.
Overall response rates at the end of induction were 86.9% with rituximab and 88.5% with obinutuzumab, with complete responses of 23.8% and 19.5%, respectively.
As noted before, investigator-assessed PFS favored obinutuzumab, as did PFS assessed by independent reviewer, at 81.9% vs. 77.9% for rituximab (HR, 0.71; P = .0138).
The newer antibody also had a slight edge in time to new treatment, with 87.1% of patients on obinutuzumab not starting on new therapy, compared with 81.2% of patients on rituximab.
More bendamustine deaths
Nearly all patients in each arm had an adverse event, with grade 3 or greater events occurring in 74.6% of patients on obinutuzumab vs. 67.8% on rituximab. Rates of neutropenia, leukopenia, febrile neutropenia, infusion reactions, and thrombocytopenia were all slightly higher with obinutuzumab. Grade 3 or greater infections occurred in 20% with obinutuzumab, compared with 15.6% with rituximab.
“What we did note, however, was a high level of mortality in patients receiving either obinutuzumab-based therapy or rituximab-based therapy, which were no different between the two arms and were somewhat higher than one might expect from patients receiving induction treatment in follicular lymphoma. Hence, we did a more detailed analysis of safety by treatment regimen,” Dr. Marcus said.
There were more deaths among patients treated with bendamustine (5.6% for patients in the B-obinutuzumab cohort, and 4.4% of patients in the B-rituximab cohort) vs. 1.6% and 2.0%, respectively, for patients on CHOP, and 1.6 and 1.8% for patients on CVP.
Dose effect?
John P. Leonard, MD, from Cornell University, New York , who introduced Dr. Marcus, commented that PFS may not be the ideal endpoint for patients with follicular lymphoma.
He pointed out that in trials comparing rituximab with obinutuzumab for other diseases, results have been mixed, with obinutuzumab showing superiority in chronic lymphocytic leukemia, but in data presented elsewhere at ASH 2016, obinutuzumab was not superior to rituximab for treatment of diffuse large B-cell lymphoma.
“One question is whether obinutuzumab, which is generally administered at a higher mg dose to patients, is in fact a better antibody or if it is in fact a dose effect,” he said.
In response to a similar question following his presentation, Dr. Marcus replied that, despite sharing a target, the two antibodies are different, with different mechanisms of action. He also noted that there is no evidence to suggest that rituximab potency would be greater in follicular lymphoma if it were given at higher doses.
The GALLIUM trial is sponsored by Hoffmann-La Roche, Dr, Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
SAN DIEGO – Obinutuzumab, a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, offered a progression-free survival (PFS) edge over rituximab when combined with standard chemotherapy in patients with previously untreated advanced follicular lymphoma.
But other clinicians and investigators who
attended the presentation of the GALLIUM data at a plenary session during the American Society of Hematology annual meeting indicated that despite the data, they weren’t ready to make a switch to the newer, costlier antibody.
“I feel that it is not convincing for practice-changing,” said Kanti R. Rai, MD, professor of medicine and molecular medicine at Hofstra University, Hempstead, N.Y.
“Unless we have evidence of a survival advantage in indolent disease, progression-free survivorship is not an adequate reason to jump to another antibody,” he said in an interview.
In GALLIUM, the primary endpoint of investigator-assessed 3-year PFS at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio of 0.68 favoring obinutuzumab (P = .0012).
Respective 3-year overall survival rates were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
Indolent lymphoma trial
The GALLIUM trial is a phase III study comparing obinutuzumab with rituximab when paired with one of three standard chemotherapy regimens for indolent non-Hodgkin lymphomas, including follicular lymphoma and splenic, nodal, or extranodal marginal zone lymphoma. Dr. Marcus presented data on patients with follicular lymphoma only.
The antibodies were delivered in combination with either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; 33.1% of patients), CVP (cyclophosphamide, vincristine, prednisone; 9.8%) or bendamustine alone (B; 57.1%) as the chemotherapy backbone. The choice of regimen was at the discretion of the treating center.
A total of 1,202 patients with follicular lymphoma were enrolled and randomized to treatment and were included in an intention-to-treat analysis.
The treatment arms were well balanced with regard to distribution of patients characteristics, with approximately 21% in each arm having Follicular Lymphoma International Prognostic Index low-risk disease; 37% having intermediate-risk disease; and 34% having high-risk disease.
Roughly half of patients in each arm had bone marrow involvement, and two-thirds had extranodal involvement.
Obinutuzumab was dosed 1,000 mg IV on days 1, 8, and 15 of cycle one, and either on day 1 of cycles two through eight every 3 weeks, or every 4 weeks during cycles two through six.
Overall response rates at the end of induction were 86.9% with rituximab and 88.5% with obinutuzumab, with complete responses of 23.8% and 19.5%, respectively.
As noted before, investigator-assessed PFS favored obinutuzumab, as did PFS assessed by independent reviewer, at 81.9% vs. 77.9% for rituximab (HR, 0.71; P = .0138).
The newer antibody also had a slight edge in time to new treatment, with 87.1% of patients on obinutuzumab not starting on new therapy, compared with 81.2% of patients on rituximab.
More bendamustine deaths
Nearly all patients in each arm had an adverse event, with grade 3 or greater events occurring in 74.6% of patients on obinutuzumab vs. 67.8% on rituximab. Rates of neutropenia, leukopenia, febrile neutropenia, infusion reactions, and thrombocytopenia were all slightly higher with obinutuzumab. Grade 3 or greater infections occurred in 20% with obinutuzumab, compared with 15.6% with rituximab.
“What we did note, however, was a high level of mortality in patients receiving either obinutuzumab-based therapy or rituximab-based therapy, which were no different between the two arms and were somewhat higher than one might expect from patients receiving induction treatment in follicular lymphoma. Hence, we did a more detailed analysis of safety by treatment regimen,” Dr. Marcus said.
There were more deaths among patients treated with bendamustine (5.6% for patients in the B-obinutuzumab cohort, and 4.4% of patients in the B-rituximab cohort) vs. 1.6% and 2.0%, respectively, for patients on CHOP, and 1.6 and 1.8% for patients on CVP.
Dose effect?
John P. Leonard, MD, from Cornell University, New York , who introduced Dr. Marcus, commented that PFS may not be the ideal endpoint for patients with follicular lymphoma.
He pointed out that in trials comparing rituximab with obinutuzumab for other diseases, results have been mixed, with obinutuzumab showing superiority in chronic lymphocytic leukemia, but in data presented elsewhere at ASH 2016, obinutuzumab was not superior to rituximab for treatment of diffuse large B-cell lymphoma.
“One question is whether obinutuzumab, which is generally administered at a higher mg dose to patients, is in fact a better antibody or if it is in fact a dose effect,” he said.
In response to a similar question following his presentation, Dr. Marcus replied that, despite sharing a target, the two antibodies are different, with different mechanisms of action. He also noted that there is no evidence to suggest that rituximab potency would be greater in follicular lymphoma if it were given at higher doses.
The GALLIUM trial is sponsored by Hoffmann-La Roche, Dr, Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
AT ASH 2016
Key clinical point: Obinutuzumab plus chemotherapy was associated with better 3-year progression-free survival in patients with untreated follicular lymphoma.
Major finding: Obinutuzumab/chemo was associated with a hazard ratio for investigator-assessed PFS of 0.68 (P = .0012)
Data source: Randomized phase III trial in 1202 patients with previously untreated follicular lymphoma.
Disclosures: The GALLIUM trial was sponsored by Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
Laxative Use with Patient-Controlled Analgesia in the Hospital and Associated Outcomes
From the Division of General Internal Medicine (Dr. Lenz), Division of Biomedical Statistics and Informatics (Mr. Schroeder), and the Division of Hospital Internal Medicine (Ms. Lawson and Dr. Yu), Mayo Clinic, Rochester, MN.
Abstract
- Objective: To describe prophylactic laxative effectiveness and prescribing patterns in patients initiated on intravenous (IV) opioid analgesia.
- Design: Retrospective cohort study.
- Setting and participants: All patients who were on IV narcotics with a patient-controlled pump while admitted to a general medicine service at the Mayo Clinic in Rochester in 2011 and 2012 were identified. Patients were excluded if constipation or diarrhea were diagnosed prior to IV opioid analgesia initiation.
- Measurements: Prophylactic laxatives were defined as laxatives prescribed within 24 hours of IV opioid analgesia initiation to be given even in the absence of constipation. Constipation was recorded when diagnosed during the hospitalization. Severe constipation was defined as constipation resulting in an abdominal CT or X-ray; abdominal distension, pain, or bloating; or if an enema was performed during the hospitalization.
- Results: Of 283 patients, 101 (36%) received prophylactic laxatives and 182 (64%) did not. Constipation occurred in 61 (34%) not on prophylactic laxatives and in 49 (49%) on prophylactic laxatives (P = 0.015). Severe constipation occurred in 23 (13%) not on prophylactic laxatives and in 33 (33%) on prophylactic laxatives (P < 0.001).
- Conclusion: A large percentage of patients are not receiving prophylactic laxatives when receiving IV opioid analgesia in the hospital. Current laxative strategies are not effectively preventing constipation in patients when prescribed.
Key words: constipation; opioids; hospital medicine; patient-controlled analgesia; laxatives.
Opioid-induced constipation (OIC) is defined as a change, when initiating opioid therapy, from baseline bowel habits and defecation patterns that is characterized by any of the following: reduced bowel frequency; development or worsening of straining; a sense of incomplete evacuation; or a patient’s perception of distress related to bowel habits [1]. It is an important side effect to consider when initiating narcotic analgesia. It has been estimated that approximately 3% to 4% of the population is on chronic narcotic pain relievers in the outpatient setting [2,3], and 37% to 81% of these patients will experience constipation [3–9]. Because of the high incidence of constipation, the prophylactic prescription of laxatives with initiation of opioid pain relievers is frequently recommended [10–15]. Furthermore, it has been shown that among patients admitted to the hospital with cancer, there is a lower incidence of constipation amongst those who have received prophylactic laxatives [16]. However, there is no evidence in the literature that prophylactic laxatives improve outcomes in patients on opioid analgesia in the general medicine inpatient setting. Furthermore, studies have illustrated that recommendations for prophylactic laxative use are not reliably followed [3,9].
While the incidence of OIC is well described in the outpatient setting [17,18], there are few studies looking at the incidence of OIC in the hospital setting. It has been shown, however, that occurrence during even a brief hospitalization is possible [4,6]. Acute constipation while hospitalized can theoretically lead to longer hospitalizations, increased pain, and decreased quality of life [6,7,19]. Recent research has focused heavily on the use of novel agents such as peripherally acting mu-opioid receptor antagonists in the treatment of OIC [20–23]. However, the expense of these agents makes them less than ideal in the prophylactic setting. This study will assess the effectiveness and prescribing patterns of prophylactic laxatives in the inpatient general medicine setting over a 2-year period at our institution in patients initiated on patient-controlled analgesia with hydromorphone, morphine, or fentanyl.
Methods
This study was approved by the institutional review board at the Mayo Clinic Rochester. All patients who were initiated on intravenous analgesia with an electronic patient-controlled opioid pump (PCA) while admitted to a general medicine service in 2011 and 2012 were identified. Patients who received PCA therapy were identified through a pharmacy database. Only patients older than 18 years of age were included in the study. PCA therapy was selected for our analysis because PCA therapy is not regularly administered on an outpatient basis. All of these patients, therefore, had a change in their narcotic regimen on admission to the hospital. Patients were excluded from the study if they were on a PCA for less than 24 hours; had a PCA initiated on a service other than a general medicine service; were on a scheduled laxative regimen prior to admission; or carried a diagnosis of bowel obstruction, chronic diarrhea, constipation, or intestinal discontinuity (eg, those with previous diversions or ostomies).
A retrospective review of each patient’s chart was conducted with the assistance of a team of nurse abstractors. Basic demographic data were recorded for each patient. Date of hospital admission and discharge; scheduled laxatives ordered and administered (any dose of sennosides, polyethylene glycol, docusate, bisacodyl, lactulose, or magnesium citrate); abdominal X-rays and abdominal CT scans performed for constipation; and any administration of enemas were recorded. Fiber supplements were not considered laxatives. If a patient was documented to have constipation during their hospitalization this was recorded. Patients were classified as having severe constipation if an abdominal CT or x-ray was performed for the indication of constipation; if abdominal distension, pain, or bloating were documented due to constipation; or if an enema was performed during the hospitalization.
For analysis purposes, patients who started receiving scheduled laxatives (as opposed to laxatives “as needed”)on or before the day of PCA initiation were classified as receiving prophylactic laxatives. Baseline patient characteristics and outcomes were compared using the chi-square test for nominal variables and the rank sum test for continuous variables. In all cases, 2-tailed tests were performed with P values ≤ 0.05 considered statistically significant. A nominal logistic regression model was utilized to assess for independent association of risk factors with the outcome of constipation.
Results
Discussion
Patients initiated on opioid therapy were not prescribed prophylactic laxatives in 64% of our cohort in the inpatient setting. When prescribed, current laxative strategies did not effectively prevent constipation with 49% experiencing OIC. Our data serves as a strong reminder of the magnitude of the problem of OIC in the inpatient setting.
The strength of our paper lies in its role as a magnitude assessment. This retrospective review reveals for that among a diverse group of patients hospitalized within a large academic institution, OIC remains prevalent. Furthermore, the high incidence of severe constipation indicates the potential for increased health care costs and patient discomfort secondary to OIC emphasizing the importance of prevention of OIC. Recent guidelines have made a push toward prophylactic laxative utilization earlier. Specifically, the European Palliative Research Collaborative offers a “strong recommendation to routinely prescribe laxatives for the management or prophylaxis of opioid-induced constipation” [10]. Additionally, the American Society of Interventional Pain Physicians suggests that “a physician should consider the initiation of a bowel regimen even before the development of constipation and definitely after the development of constipation” [11]. Our manuscript serves as a reminder that OIC remains a very prevalent problem and that prophylactic laxatives are still being underutilized.
This is a retrospective study and thus has inherent limitations. Specifically, we are limited to those cases of constipation that were documented in the medical record. The presentation of constipation is varied between patients. This variation in presentation of OIC is inherent to the disease process as is demonstrated in the broad definition for OIC [1]. The cases of constipation that we are reporting clearly were bothersome enough to warrant documentation in the medical record, and while there may have been cases that escaped documentation, we can be confident that the cases of OIC we are reporting are true cases of OIC. The numbers we report can therefore be taken to represent a minimum number of cases of constipation occurring in our study population.
It has been suggested that OIC prevalence varies with type of opioid and duration of opioid therapy [24]. We did not compare dose, type, or duration of opioid therapy in this study. This could certainly account for the seemingly higher rate of constipation within the group treated with prophylactic laxatives as compared with those not treated with prophylactic laxatives. Physicians likely have a higher propensity to prescribe prophylactic laxatives to patients receiving high doses of opioids who are in turn at higher risk for OIC. We cannot say whether differences in efficacy exist between prophylactic laxative regimens or which opioids (dose and duration) cause the most constipation based upon our data. Future studies incorporating dose, duration, and opioid type along with the variables we collected in this study could potentially construct successful logistic regression models with predictive power to identify those at highest risk of OIC.
Our rate of OIC is consistent with previously published figures [3–9]. However, we demonstrate for the first time that prophylactic laxatives are prescribed infrequently and unsuccessfully in the inpatient setting. This is consistent with prescribing rates in the outpatient setting [9,25]. Furthermore, we observed a higher rate of constipation in those treated with prophylactic laxatives compared to those that did not receive prophylactic laxatives. Pottegard et al similarly demonstrated an increased rate of constipation in those utilizing laxative therapy [25]. This is likely secondary to providers recommending prophylactic laxatives to those patients most likely to develop constipation. Despite being able to recognize high-risk patients, providers are unable to prevent OIC as little is known regarding optimal laxative strategies. Previous studies comparing treatment regimens for the relief of constipation in the palliative care population have been largely inconclusive [26]. There have been no studies to date comparing different prophylactic laxatives in the inpatient setting.
Future directions for research in this area would ideally take the form of randomized controlled trials investigating efficacy of different prophylactic laxatives in the inpatient setting. These trials would ideally include well-defined patient groups receiving specific narcotics for specific reasons. These studies would be best if powered to assess the effect of narcotic dosage and duration of therapy as well. Alternatively, larger retrospective chart reviews could be performed including narcotic dosage, type, and duration of therapy with a planned logistic regression model attempting to account for likely independent variables.
Conclusion
Our study demonstrates for the first time that prophylactic laxatives are not being prescribed frequently to patients on opioid analgesia in the inpatient general medicine setting. Additionally, while providers seem to be identifying patients at higher risk of constipation, they are still unable to prevent constipation in a high percentage of patients. Further research into this area would be beneficial to prevent this uncomfortable, costly, and preventable complication of opioid analgesia.
Corresponding author: Roger Yu, MD, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, [email protected].
Funding/support: This research was supported by the Mayo Clinic Return to Work program nurses for data abstraction.
Financial disclosures: None.
1. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016.
2. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75.
3. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 2009;104:1199–204.
4. Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer 2008;16:453–9.
5. Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 1998;12:375–82.
6. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42.
7. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32.
8. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005:7:R1046–51.
9. Bouvy ML, Buurma H, Egberts TC. Laxative prescribing in relation to opioid use and the influence of pharmacy-based intervention. J Clin Pharm Ther 2002;27:107–10.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012:13:e58–68.
11. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician 2012;15(3 Suppl):S67–116.
12. Cameron JC. Constipation related to narcotic therapy. A protocol for nurses and patients. Cancer Nurs 1992;15:372–7.
13. Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996;335:1124–32.
14. Swegle JM, Logemann D. Management of common opioid-induced adverse effects. Am Fam Physician 2006;74:1347–54.
15. Donnelly S, Davis MP, Walsh D, Naughton M. Morphine in cancer pain management: a practical guide. Support Care Cancer 2002;10:13–35.
16. Ishihara M, Ikesue H Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–81.
17. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–80.
18. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–30, e96.
19. Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847–65.
20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42.
21. Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011(1):CD003448.
22. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013;108:1566–74.
23. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185–93.
24. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95.
25. Pottegard A, Knudsen TB, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014;36:291–4.
26. Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015(5):CD003448.
From the Division of General Internal Medicine (Dr. Lenz), Division of Biomedical Statistics and Informatics (Mr. Schroeder), and the Division of Hospital Internal Medicine (Ms. Lawson and Dr. Yu), Mayo Clinic, Rochester, MN.
Abstract
- Objective: To describe prophylactic laxative effectiveness and prescribing patterns in patients initiated on intravenous (IV) opioid analgesia.
- Design: Retrospective cohort study.
- Setting and participants: All patients who were on IV narcotics with a patient-controlled pump while admitted to a general medicine service at the Mayo Clinic in Rochester in 2011 and 2012 were identified. Patients were excluded if constipation or diarrhea were diagnosed prior to IV opioid analgesia initiation.
- Measurements: Prophylactic laxatives were defined as laxatives prescribed within 24 hours of IV opioid analgesia initiation to be given even in the absence of constipation. Constipation was recorded when diagnosed during the hospitalization. Severe constipation was defined as constipation resulting in an abdominal CT or X-ray; abdominal distension, pain, or bloating; or if an enema was performed during the hospitalization.
- Results: Of 283 patients, 101 (36%) received prophylactic laxatives and 182 (64%) did not. Constipation occurred in 61 (34%) not on prophylactic laxatives and in 49 (49%) on prophylactic laxatives (P = 0.015). Severe constipation occurred in 23 (13%) not on prophylactic laxatives and in 33 (33%) on prophylactic laxatives (P < 0.001).
- Conclusion: A large percentage of patients are not receiving prophylactic laxatives when receiving IV opioid analgesia in the hospital. Current laxative strategies are not effectively preventing constipation in patients when prescribed.
Key words: constipation; opioids; hospital medicine; patient-controlled analgesia; laxatives.
Opioid-induced constipation (OIC) is defined as a change, when initiating opioid therapy, from baseline bowel habits and defecation patterns that is characterized by any of the following: reduced bowel frequency; development or worsening of straining; a sense of incomplete evacuation; or a patient’s perception of distress related to bowel habits [1]. It is an important side effect to consider when initiating narcotic analgesia. It has been estimated that approximately 3% to 4% of the population is on chronic narcotic pain relievers in the outpatient setting [2,3], and 37% to 81% of these patients will experience constipation [3–9]. Because of the high incidence of constipation, the prophylactic prescription of laxatives with initiation of opioid pain relievers is frequently recommended [10–15]. Furthermore, it has been shown that among patients admitted to the hospital with cancer, there is a lower incidence of constipation amongst those who have received prophylactic laxatives [16]. However, there is no evidence in the literature that prophylactic laxatives improve outcomes in patients on opioid analgesia in the general medicine inpatient setting. Furthermore, studies have illustrated that recommendations for prophylactic laxative use are not reliably followed [3,9].
While the incidence of OIC is well described in the outpatient setting [17,18], there are few studies looking at the incidence of OIC in the hospital setting. It has been shown, however, that occurrence during even a brief hospitalization is possible [4,6]. Acute constipation while hospitalized can theoretically lead to longer hospitalizations, increased pain, and decreased quality of life [6,7,19]. Recent research has focused heavily on the use of novel agents such as peripherally acting mu-opioid receptor antagonists in the treatment of OIC [20–23]. However, the expense of these agents makes them less than ideal in the prophylactic setting. This study will assess the effectiveness and prescribing patterns of prophylactic laxatives in the inpatient general medicine setting over a 2-year period at our institution in patients initiated on patient-controlled analgesia with hydromorphone, morphine, or fentanyl.
Methods
This study was approved by the institutional review board at the Mayo Clinic Rochester. All patients who were initiated on intravenous analgesia with an electronic patient-controlled opioid pump (PCA) while admitted to a general medicine service in 2011 and 2012 were identified. Patients who received PCA therapy were identified through a pharmacy database. Only patients older than 18 years of age were included in the study. PCA therapy was selected for our analysis because PCA therapy is not regularly administered on an outpatient basis. All of these patients, therefore, had a change in their narcotic regimen on admission to the hospital. Patients were excluded from the study if they were on a PCA for less than 24 hours; had a PCA initiated on a service other than a general medicine service; were on a scheduled laxative regimen prior to admission; or carried a diagnosis of bowel obstruction, chronic diarrhea, constipation, or intestinal discontinuity (eg, those with previous diversions or ostomies).
A retrospective review of each patient’s chart was conducted with the assistance of a team of nurse abstractors. Basic demographic data were recorded for each patient. Date of hospital admission and discharge; scheduled laxatives ordered and administered (any dose of sennosides, polyethylene glycol, docusate, bisacodyl, lactulose, or magnesium citrate); abdominal X-rays and abdominal CT scans performed for constipation; and any administration of enemas were recorded. Fiber supplements were not considered laxatives. If a patient was documented to have constipation during their hospitalization this was recorded. Patients were classified as having severe constipation if an abdominal CT or x-ray was performed for the indication of constipation; if abdominal distension, pain, or bloating were documented due to constipation; or if an enema was performed during the hospitalization.
For analysis purposes, patients who started receiving scheduled laxatives (as opposed to laxatives “as needed”)on or before the day of PCA initiation were classified as receiving prophylactic laxatives. Baseline patient characteristics and outcomes were compared using the chi-square test for nominal variables and the rank sum test for continuous variables. In all cases, 2-tailed tests were performed with P values ≤ 0.05 considered statistically significant. A nominal logistic regression model was utilized to assess for independent association of risk factors with the outcome of constipation.
Results
Discussion
Patients initiated on opioid therapy were not prescribed prophylactic laxatives in 64% of our cohort in the inpatient setting. When prescribed, current laxative strategies did not effectively prevent constipation with 49% experiencing OIC. Our data serves as a strong reminder of the magnitude of the problem of OIC in the inpatient setting.
The strength of our paper lies in its role as a magnitude assessment. This retrospective review reveals for that among a diverse group of patients hospitalized within a large academic institution, OIC remains prevalent. Furthermore, the high incidence of severe constipation indicates the potential for increased health care costs and patient discomfort secondary to OIC emphasizing the importance of prevention of OIC. Recent guidelines have made a push toward prophylactic laxative utilization earlier. Specifically, the European Palliative Research Collaborative offers a “strong recommendation to routinely prescribe laxatives for the management or prophylaxis of opioid-induced constipation” [10]. Additionally, the American Society of Interventional Pain Physicians suggests that “a physician should consider the initiation of a bowel regimen even before the development of constipation and definitely after the development of constipation” [11]. Our manuscript serves as a reminder that OIC remains a very prevalent problem and that prophylactic laxatives are still being underutilized.
This is a retrospective study and thus has inherent limitations. Specifically, we are limited to those cases of constipation that were documented in the medical record. The presentation of constipation is varied between patients. This variation in presentation of OIC is inherent to the disease process as is demonstrated in the broad definition for OIC [1]. The cases of constipation that we are reporting clearly were bothersome enough to warrant documentation in the medical record, and while there may have been cases that escaped documentation, we can be confident that the cases of OIC we are reporting are true cases of OIC. The numbers we report can therefore be taken to represent a minimum number of cases of constipation occurring in our study population.
It has been suggested that OIC prevalence varies with type of opioid and duration of opioid therapy [24]. We did not compare dose, type, or duration of opioid therapy in this study. This could certainly account for the seemingly higher rate of constipation within the group treated with prophylactic laxatives as compared with those not treated with prophylactic laxatives. Physicians likely have a higher propensity to prescribe prophylactic laxatives to patients receiving high doses of opioids who are in turn at higher risk for OIC. We cannot say whether differences in efficacy exist between prophylactic laxative regimens or which opioids (dose and duration) cause the most constipation based upon our data. Future studies incorporating dose, duration, and opioid type along with the variables we collected in this study could potentially construct successful logistic regression models with predictive power to identify those at highest risk of OIC.
Our rate of OIC is consistent with previously published figures [3–9]. However, we demonstrate for the first time that prophylactic laxatives are prescribed infrequently and unsuccessfully in the inpatient setting. This is consistent with prescribing rates in the outpatient setting [9,25]. Furthermore, we observed a higher rate of constipation in those treated with prophylactic laxatives compared to those that did not receive prophylactic laxatives. Pottegard et al similarly demonstrated an increased rate of constipation in those utilizing laxative therapy [25]. This is likely secondary to providers recommending prophylactic laxatives to those patients most likely to develop constipation. Despite being able to recognize high-risk patients, providers are unable to prevent OIC as little is known regarding optimal laxative strategies. Previous studies comparing treatment regimens for the relief of constipation in the palliative care population have been largely inconclusive [26]. There have been no studies to date comparing different prophylactic laxatives in the inpatient setting.
Future directions for research in this area would ideally take the form of randomized controlled trials investigating efficacy of different prophylactic laxatives in the inpatient setting. These trials would ideally include well-defined patient groups receiving specific narcotics for specific reasons. These studies would be best if powered to assess the effect of narcotic dosage and duration of therapy as well. Alternatively, larger retrospective chart reviews could be performed including narcotic dosage, type, and duration of therapy with a planned logistic regression model attempting to account for likely independent variables.
Conclusion
Our study demonstrates for the first time that prophylactic laxatives are not being prescribed frequently to patients on opioid analgesia in the inpatient general medicine setting. Additionally, while providers seem to be identifying patients at higher risk of constipation, they are still unable to prevent constipation in a high percentage of patients. Further research into this area would be beneficial to prevent this uncomfortable, costly, and preventable complication of opioid analgesia.
Corresponding author: Roger Yu, MD, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, [email protected].
Funding/support: This research was supported by the Mayo Clinic Return to Work program nurses for data abstraction.
Financial disclosures: None.
From the Division of General Internal Medicine (Dr. Lenz), Division of Biomedical Statistics and Informatics (Mr. Schroeder), and the Division of Hospital Internal Medicine (Ms. Lawson and Dr. Yu), Mayo Clinic, Rochester, MN.
Abstract
- Objective: To describe prophylactic laxative effectiveness and prescribing patterns in patients initiated on intravenous (IV) opioid analgesia.
- Design: Retrospective cohort study.
- Setting and participants: All patients who were on IV narcotics with a patient-controlled pump while admitted to a general medicine service at the Mayo Clinic in Rochester in 2011 and 2012 were identified. Patients were excluded if constipation or diarrhea were diagnosed prior to IV opioid analgesia initiation.
- Measurements: Prophylactic laxatives were defined as laxatives prescribed within 24 hours of IV opioid analgesia initiation to be given even in the absence of constipation. Constipation was recorded when diagnosed during the hospitalization. Severe constipation was defined as constipation resulting in an abdominal CT or X-ray; abdominal distension, pain, or bloating; or if an enema was performed during the hospitalization.
- Results: Of 283 patients, 101 (36%) received prophylactic laxatives and 182 (64%) did not. Constipation occurred in 61 (34%) not on prophylactic laxatives and in 49 (49%) on prophylactic laxatives (P = 0.015). Severe constipation occurred in 23 (13%) not on prophylactic laxatives and in 33 (33%) on prophylactic laxatives (P < 0.001).
- Conclusion: A large percentage of patients are not receiving prophylactic laxatives when receiving IV opioid analgesia in the hospital. Current laxative strategies are not effectively preventing constipation in patients when prescribed.
Key words: constipation; opioids; hospital medicine; patient-controlled analgesia; laxatives.
Opioid-induced constipation (OIC) is defined as a change, when initiating opioid therapy, from baseline bowel habits and defecation patterns that is characterized by any of the following: reduced bowel frequency; development or worsening of straining; a sense of incomplete evacuation; or a patient’s perception of distress related to bowel habits [1]. It is an important side effect to consider when initiating narcotic analgesia. It has been estimated that approximately 3% to 4% of the population is on chronic narcotic pain relievers in the outpatient setting [2,3], and 37% to 81% of these patients will experience constipation [3–9]. Because of the high incidence of constipation, the prophylactic prescription of laxatives with initiation of opioid pain relievers is frequently recommended [10–15]. Furthermore, it has been shown that among patients admitted to the hospital with cancer, there is a lower incidence of constipation amongst those who have received prophylactic laxatives [16]. However, there is no evidence in the literature that prophylactic laxatives improve outcomes in patients on opioid analgesia in the general medicine inpatient setting. Furthermore, studies have illustrated that recommendations for prophylactic laxative use are not reliably followed [3,9].
While the incidence of OIC is well described in the outpatient setting [17,18], there are few studies looking at the incidence of OIC in the hospital setting. It has been shown, however, that occurrence during even a brief hospitalization is possible [4,6]. Acute constipation while hospitalized can theoretically lead to longer hospitalizations, increased pain, and decreased quality of life [6,7,19]. Recent research has focused heavily on the use of novel agents such as peripherally acting mu-opioid receptor antagonists in the treatment of OIC [20–23]. However, the expense of these agents makes them less than ideal in the prophylactic setting. This study will assess the effectiveness and prescribing patterns of prophylactic laxatives in the inpatient general medicine setting over a 2-year period at our institution in patients initiated on patient-controlled analgesia with hydromorphone, morphine, or fentanyl.
Methods
This study was approved by the institutional review board at the Mayo Clinic Rochester. All patients who were initiated on intravenous analgesia with an electronic patient-controlled opioid pump (PCA) while admitted to a general medicine service in 2011 and 2012 were identified. Patients who received PCA therapy were identified through a pharmacy database. Only patients older than 18 years of age were included in the study. PCA therapy was selected for our analysis because PCA therapy is not regularly administered on an outpatient basis. All of these patients, therefore, had a change in their narcotic regimen on admission to the hospital. Patients were excluded from the study if they were on a PCA for less than 24 hours; had a PCA initiated on a service other than a general medicine service; were on a scheduled laxative regimen prior to admission; or carried a diagnosis of bowel obstruction, chronic diarrhea, constipation, or intestinal discontinuity (eg, those with previous diversions or ostomies).
A retrospective review of each patient’s chart was conducted with the assistance of a team of nurse abstractors. Basic demographic data were recorded for each patient. Date of hospital admission and discharge; scheduled laxatives ordered and administered (any dose of sennosides, polyethylene glycol, docusate, bisacodyl, lactulose, or magnesium citrate); abdominal X-rays and abdominal CT scans performed for constipation; and any administration of enemas were recorded. Fiber supplements were not considered laxatives. If a patient was documented to have constipation during their hospitalization this was recorded. Patients were classified as having severe constipation if an abdominal CT or x-ray was performed for the indication of constipation; if abdominal distension, pain, or bloating were documented due to constipation; or if an enema was performed during the hospitalization.
For analysis purposes, patients who started receiving scheduled laxatives (as opposed to laxatives “as needed”)on or before the day of PCA initiation were classified as receiving prophylactic laxatives. Baseline patient characteristics and outcomes were compared using the chi-square test for nominal variables and the rank sum test for continuous variables. In all cases, 2-tailed tests were performed with P values ≤ 0.05 considered statistically significant. A nominal logistic regression model was utilized to assess for independent association of risk factors with the outcome of constipation.
Results
Discussion
Patients initiated on opioid therapy were not prescribed prophylactic laxatives in 64% of our cohort in the inpatient setting. When prescribed, current laxative strategies did not effectively prevent constipation with 49% experiencing OIC. Our data serves as a strong reminder of the magnitude of the problem of OIC in the inpatient setting.
The strength of our paper lies in its role as a magnitude assessment. This retrospective review reveals for that among a diverse group of patients hospitalized within a large academic institution, OIC remains prevalent. Furthermore, the high incidence of severe constipation indicates the potential for increased health care costs and patient discomfort secondary to OIC emphasizing the importance of prevention of OIC. Recent guidelines have made a push toward prophylactic laxative utilization earlier. Specifically, the European Palliative Research Collaborative offers a “strong recommendation to routinely prescribe laxatives for the management or prophylaxis of opioid-induced constipation” [10]. Additionally, the American Society of Interventional Pain Physicians suggests that “a physician should consider the initiation of a bowel regimen even before the development of constipation and definitely after the development of constipation” [11]. Our manuscript serves as a reminder that OIC remains a very prevalent problem and that prophylactic laxatives are still being underutilized.
This is a retrospective study and thus has inherent limitations. Specifically, we are limited to those cases of constipation that were documented in the medical record. The presentation of constipation is varied between patients. This variation in presentation of OIC is inherent to the disease process as is demonstrated in the broad definition for OIC [1]. The cases of constipation that we are reporting clearly were bothersome enough to warrant documentation in the medical record, and while there may have been cases that escaped documentation, we can be confident that the cases of OIC we are reporting are true cases of OIC. The numbers we report can therefore be taken to represent a minimum number of cases of constipation occurring in our study population.
It has been suggested that OIC prevalence varies with type of opioid and duration of opioid therapy [24]. We did not compare dose, type, or duration of opioid therapy in this study. This could certainly account for the seemingly higher rate of constipation within the group treated with prophylactic laxatives as compared with those not treated with prophylactic laxatives. Physicians likely have a higher propensity to prescribe prophylactic laxatives to patients receiving high doses of opioids who are in turn at higher risk for OIC. We cannot say whether differences in efficacy exist between prophylactic laxative regimens or which opioids (dose and duration) cause the most constipation based upon our data. Future studies incorporating dose, duration, and opioid type along with the variables we collected in this study could potentially construct successful logistic regression models with predictive power to identify those at highest risk of OIC.
Our rate of OIC is consistent with previously published figures [3–9]. However, we demonstrate for the first time that prophylactic laxatives are prescribed infrequently and unsuccessfully in the inpatient setting. This is consistent with prescribing rates in the outpatient setting [9,25]. Furthermore, we observed a higher rate of constipation in those treated with prophylactic laxatives compared to those that did not receive prophylactic laxatives. Pottegard et al similarly demonstrated an increased rate of constipation in those utilizing laxative therapy [25]. This is likely secondary to providers recommending prophylactic laxatives to those patients most likely to develop constipation. Despite being able to recognize high-risk patients, providers are unable to prevent OIC as little is known regarding optimal laxative strategies. Previous studies comparing treatment regimens for the relief of constipation in the palliative care population have been largely inconclusive [26]. There have been no studies to date comparing different prophylactic laxatives in the inpatient setting.
Future directions for research in this area would ideally take the form of randomized controlled trials investigating efficacy of different prophylactic laxatives in the inpatient setting. These trials would ideally include well-defined patient groups receiving specific narcotics for specific reasons. These studies would be best if powered to assess the effect of narcotic dosage and duration of therapy as well. Alternatively, larger retrospective chart reviews could be performed including narcotic dosage, type, and duration of therapy with a planned logistic regression model attempting to account for likely independent variables.
Conclusion
Our study demonstrates for the first time that prophylactic laxatives are not being prescribed frequently to patients on opioid analgesia in the inpatient general medicine setting. Additionally, while providers seem to be identifying patients at higher risk of constipation, they are still unable to prevent constipation in a high percentage of patients. Further research into this area would be beneficial to prevent this uncomfortable, costly, and preventable complication of opioid analgesia.
Corresponding author: Roger Yu, MD, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, [email protected].
Funding/support: This research was supported by the Mayo Clinic Return to Work program nurses for data abstraction.
Financial disclosures: None.
1. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016.
2. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75.
3. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 2009;104:1199–204.
4. Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer 2008;16:453–9.
5. Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 1998;12:375–82.
6. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42.
7. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32.
8. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005:7:R1046–51.
9. Bouvy ML, Buurma H, Egberts TC. Laxative prescribing in relation to opioid use and the influence of pharmacy-based intervention. J Clin Pharm Ther 2002;27:107–10.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012:13:e58–68.
11. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician 2012;15(3 Suppl):S67–116.
12. Cameron JC. Constipation related to narcotic therapy. A protocol for nurses and patients. Cancer Nurs 1992;15:372–7.
13. Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996;335:1124–32.
14. Swegle JM, Logemann D. Management of common opioid-induced adverse effects. Am Fam Physician 2006;74:1347–54.
15. Donnelly S, Davis MP, Walsh D, Naughton M. Morphine in cancer pain management: a practical guide. Support Care Cancer 2002;10:13–35.
16. Ishihara M, Ikesue H Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–81.
17. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–80.
18. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–30, e96.
19. Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847–65.
20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42.
21. Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011(1):CD003448.
22. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013;108:1566–74.
23. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185–93.
24. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95.
25. Pottegard A, Knudsen TB, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014;36:291–4.
26. Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015(5):CD003448.
1. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016.
2. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75.
3. Choung RS, Locke GR 3rd, Zinsmeister AR, et al. Opioid bowel dysfunction and narcotic bowel syndrome: a population-based study. Am J Gastroenterol 2009;104:1199–204.
4. Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer 2008;16:453–9.
5. Sykes NP. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat Med 1998;12:375–82.
6. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42.
7. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32.
8. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005:7:R1046–51.
9. Bouvy ML, Buurma H, Egberts TC. Laxative prescribing in relation to opioid use and the influence of pharmacy-based intervention. J Clin Pharm Ther 2002;27:107–10.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012:13:e58–68.
11. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician 2012;15(3 Suppl):S67–116.
12. Cameron JC. Constipation related to narcotic therapy. A protocol for nurses and patients. Cancer Nurs 1992;15:372–7.
13. Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996;335:1124–32.
14. Swegle JM, Logemann D. Management of common opioid-induced adverse effects. Am Fam Physician 2006;74:1347–54.
15. Donnelly S, Davis MP, Walsh D, Naughton M. Morphine in cancer pain management: a practical guide. Support Care Cancer 2002;10:13–35.
16. Ishihara M, Ikesue H Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain 2012;28:373–81.
17. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–80.
18. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–30, e96.
19. Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847–65.
20. Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42.
21. Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011(1):CD003448.
22. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013;108:1566–74.
23. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185–93.
24. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95.
25. Pottegard A, Knudsen TB, van Heesch K, et al. Information on risk of constipation for Danish users of opioids, and their laxative use. Int J Clin Pharm 2014;36:291–4.
26. Candy B, Jones L, Larkin PJ, et al. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev 2015(5):CD003448.