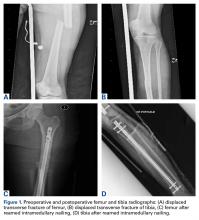
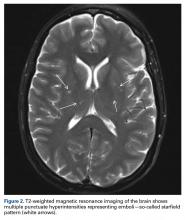
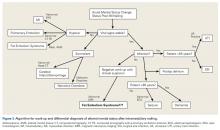
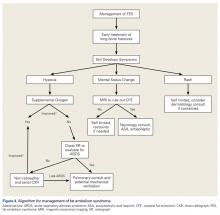
User login
Helping patients bring an end to domestic violence
As healers trained to address some of the psychosocial issues facing our patients, we need to understand various forms of domestic violence – and what we can do to stop it. One form, honor killings, remains pervasive across the globe.
In a sample of 856 ninth-grade students from 14 schools in Amman, Jordan, for example, about 40% of boys and 20% of girls believed that killing a daughter, sister, or wife who had dishonored the family was justified (Aggress Behav. 2013 Sep-Oct;39[5]:405-17). The schools were representative of students from different religions, socioeconomic statuses, and upbringings. The proportions are broadly in line with the religious affiliation of Jordanians, with 92% of the population identifying themselves as Muslims, 6% as Christians, and 2% as affiliated with other religions.1 However, researchers found that support for honor killings was more widespread among adolescents from poorer and more traditional family backgrounds.
What are honor killings?
According to Sally Elakkary, MD, and her colleagues, honor killings are “violence implicated against a female for the deviancy of her activities from the traditional cultural norms.”2 The perpetrators in these crimes are usually male relatives but may be other family members, including women. Males also can be victims of honor crimes. For example, a male can become a victim if he is the female’s lover in an extramarital relationship or if he is homosexual.
Most honor killings are reported from countries in the Maghreb region of North Africa; the Middle East (Palestine, Lebanon, Syria, Jordan, and Turkey); and Western and Central Asia (Iraq, Pakistan, Afghanistan, and India). However, honor killings also occur in countries with strong minorities from those origins. A report3 published in 2000 by the United Nations Population Fund estimated that 5,000 honor killings were carried out worldwide per year, with the largest absolute numbers reported for Pakistan and India (about 1,000 cases per year in each country).
Until the 1960s in the United States, penal codes in some states, such as Georgia, New Mexico, Texas, and Utah, justified a husband killing his wife’s lover. In those states, the law was formulated to protect the male’s honor. Honor killings are culture-based practices that are supported indirectly by that country’s legal system. In a recent New York Times op-ed4 piece, Bina Shah stated that “upending misogynistic tribal codes is the real key to finally ending the most egregious gender crime.” The Pakistan Parliament recently stiffened the punishment for honor killings. The new anti–honor killing law mandates a minimum lifetime jail sentence for perpetrators and closes a legal loophole that allowed an honor killer to walk free if the family of the victim forgave him.
However, in rural areas, a Pakistani woman accused of violating family or tribal honor can be sentenced to death by an informal village court or a gathering of tribal elders. Women have been killed by stoning, shooting, or being buried alive. Sometimes, the woman’s relatives condemn her to death without a trial. Women are killed for reasons such as wanting to marry of their own choice, divorcing abusive husbands, or speaking to a man or boy outside the family; “in one case, four young girls who were filmed dancing in a rain shower were executed by their cousin for immorality,” Ms. Shah said.
Range of behaviors
Abusive behavior can take many forms, including:
• Isolating a person from her friends and family.
• Depriving her of basic needs.
• Monitoring her time.
• Monitoring her use of online communication tools or spyware.
• Taking control over aspects of her everyday life, such as where she can go, whom she can see, what she can wear, and when she can sleep.
• Depriving her of access to support services, such as specialist support or medical services.
• Repeatedly putting her down, such as telling her that she is worthless.
• Enforcing rules and activities that humiliate, degrade, or dehumanize the victim.
• Forcing the victim to take part in criminal activity such as shoplifting, and neglecting or abusing children to encourage self-blame and prevent disclosure to authorities.
• Abusing finances, including controlling resources so that the person is allowed only a punitive allowance.
• Threatening to hurt or kill her.
• Threatening a child.
• Threatening to reveal or publish private information (for example, threatening to “out” someone).
• Assaulting the person.
• Causing criminal damage (such as destruction of household goods).
• Engaging in rape.
• Preventing a person from having access to transportation or from working.
New domestic violence law in the United Kingdom
Meanwhile, domestic violence laws in England and Wales now consider emotional and psychological abuse as legally actionable. The new legislation targets those who subject spouses, partners, and family members to psychological and emotional torment, but stop short of violence. The new law5 follows a Home Office consultation showing that 85% of participants said the existing law did not provide sufficient protection and a Citizens Advice report showing a 24% rise in people seeking advice for domestic abuse.
The new law falls under the Serious Crime Act 2015 of England and Wales. The act creates a new offense of “controlling or coercive behavior in intimate or familial relationships.” The act states: “The new offence closes a gap in the law around patterns of controlling or coercive behavior in an ongoing relationship between intimate partners or family members.”
Breaking the law carries a maximum sentence of 5 years’ imprisonment, a fine, or both. The behavior must have had a “serious effect” on the victim, meaning that it has caused the victim to fear violence will be used against them on “at least two occasions,” or it has had a “substantial adverse effect on the victim’s day to day activities.”
The alleged perpetrator must have known that his behavior would have a serious effect on the victim, or the behavior must have been such that he “ought to have known” it would have that effect.
The new law includes honor-based violence, female genital mutilation, and forced marriage. The law explicitly states that the victim may fear that the perpetrator has asked another person to commit violence against them, thus including family honor killings.
Gendered nature of domestic controlling or coercive behavior
While all legislation is gender neutral, women and girls are disproportionately affected by crimes of domestic violence and abuse. Women from black and minority ethnic backgrounds may experience barriers to reporting, such as a distrust of the police, concerns about racism, language barriers, concerns about family finding out, or fear of rejection by the wider community. A victim might be fearful about her children being taken away if she makes a report. Lesbian, gay, bisexual, and transgender individuals in relationships may be subjected to threats to reveal sexual orientation to family or others.
Interestingly, the U.K. guidelines state that victims of controlling or coercive behavior may not recognize themselves as victims. Therefore, it is important that the new offense be considered by the police and other authorities in attendance at all call-outs. Police are encouraged to ask questions about rules, decision making, norms, and fear in the relationship, rather than just what happened. The guidelines provide specific comments about handling perpetrators who are described as being “particularly adept” at manipulating professionals, agencies, and systems, and may use a range of tactics in relation to this offense, including targeting people who might be vulnerable (there may be evidence of this from previous relationships) and using the system against the victim by making false or vexatious allegations to agencies.
The Authorized Professional Practice on Investigating Domestic Abuse issued by the College of Policing states: “A manipulative perpetrator may be trying to draw the police into colluding with their coercive control of the victim. Police officers must avoid playing into the primary perpetrator’s hands and take account of all available evidence when making the decision to arrest.” Such evidence includes attempting to frustrate or interfere with the police investigation; making counterallegations against the victim; and using threats of manipulation against the victim – such as telling the victim that he will make a counterallegation against her, that the victim will not be believed by the police or other agencies, that he will inform social services, or that he will inform immigration officials where the victim does not have a right to remain.
How can psychiatrists raise awareness?
• Individual change. Abusive controlling behavior can have its origin in childhood psychological experiences, in the same way that honor killings and wife beating can have their roots in the perpetrator’s cultural experience. As a child, the adult perpetrator may have been a direct victim of violence or may have been a witness to domestic violence. Controlling abusive behavior also can occur as a choice in perpetrators with personality disorders unrelated to childhood experiences. It can occur in a person with both exposure and personality disorder. It is important to understand the origins and reasoning behind the behavior in order to understand how best to intervene.
If the behavior is based on the childhood experience of the prevailing sociocultural practice, the psychiatrist can explore values and beliefs, identifying those that are based on family and cultural factors. Beliefs that have been present during a person’s entire life can appear as the unexamined “background” of their lives. Bringing those beliefs to the fore can allow discussion. For example, does the individual hold the belief that women are possessions? What is the basis of holding such a belief? How does he account for the differences between societies?
• Family change. Individuals in the family may differ in their support of honor killings. Those who do not support honor killings may have difficulty speaking out for fear of becoming a future target. When we meet with families who have a belief in honor killings, we can discuss how patriarchal societies have encouraged families to maintain a firm hand on the behavior of their members. This practice encourages repression of women’s individuality and also may consider women to be possessions. Patriarchal societies control their populations by supporting values and beliefs in their citizens that support the patriarchal structure. In this way, they can exert social control easily by having members of the society act as enforcers. Open and clear discussion with families about the ways in which cultural practice affects individual behavior may allow families that are unsure to explore alternative new beliefs.
Families must understand that, under U.S. law, perpetrators of honor killings and domestic violence can be prosecuted.
• System change. According to the U.S. Justice Department, 9 out of 10 honor killings were victims who had become “too Westernized.” Leaders of the American Muslim community and members of the Council on American-Islamic Relations have condemned all honor killings, stating that the practice stems from sexism and tribal behavior that predates the religion. In February 2009, after the high-profile killing of Aasiya Zubair Hassan in New York, Muslim leaders began a nationwide effort entitled, “Imams Speak Out: Domestic Violence Will Not Be Tolerated in Our Communities,” asking all imams and religious leaders to discuss domestic violence in their weekly sermon or their Friday prayer services. The group, “Muslim Men Against Domestic Violence,” was founded soon after the murder, which came just a few days after she had filed for divorce. (After a 3-week trial, Mrs. Hassan’s estranged husband, Muzzammil “Mo” Hassan, was found guilty of second-degree murder and received a 25-year to life sentence).
Our laws reflect our values as a society. As citizens, we must actively work for the enforcement of domestic violence laws. Our mental health organizations can support the training of police and health agencies to identify victims and perpetrators.
References
1. The World Factbook, Central Intelligence Agency, 2009.
2 Forensic Sci Med Pathol. 2014;10(1):76-82.
3. “Lives Together, Worlds Apart: Men and Women in a Time of Change,” United Nations Population Fund report, 2000.
4. “Pakistan’s Honor-Killing Law Isn’t Enough,” The New York Times, Oct. 27, 2016.
5. “Controlling or Coercive Behaviour in an Intimate or Family Relationship,” Home Office, December 2015.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose.
As healers trained to address some of the psychosocial issues facing our patients, we need to understand various forms of domestic violence – and what we can do to stop it. One form, honor killings, remains pervasive across the globe.
In a sample of 856 ninth-grade students from 14 schools in Amman, Jordan, for example, about 40% of boys and 20% of girls believed that killing a daughter, sister, or wife who had dishonored the family was justified (Aggress Behav. 2013 Sep-Oct;39[5]:405-17). The schools were representative of students from different religions, socioeconomic statuses, and upbringings. The proportions are broadly in line with the religious affiliation of Jordanians, with 92% of the population identifying themselves as Muslims, 6% as Christians, and 2% as affiliated with other religions.1 However, researchers found that support for honor killings was more widespread among adolescents from poorer and more traditional family backgrounds.
What are honor killings?
According to Sally Elakkary, MD, and her colleagues, honor killings are “violence implicated against a female for the deviancy of her activities from the traditional cultural norms.”2 The perpetrators in these crimes are usually male relatives but may be other family members, including women. Males also can be victims of honor crimes. For example, a male can become a victim if he is the female’s lover in an extramarital relationship or if he is homosexual.
Most honor killings are reported from countries in the Maghreb region of North Africa; the Middle East (Palestine, Lebanon, Syria, Jordan, and Turkey); and Western and Central Asia (Iraq, Pakistan, Afghanistan, and India). However, honor killings also occur in countries with strong minorities from those origins. A report3 published in 2000 by the United Nations Population Fund estimated that 5,000 honor killings were carried out worldwide per year, with the largest absolute numbers reported for Pakistan and India (about 1,000 cases per year in each country).
Until the 1960s in the United States, penal codes in some states, such as Georgia, New Mexico, Texas, and Utah, justified a husband killing his wife’s lover. In those states, the law was formulated to protect the male’s honor. Honor killings are culture-based practices that are supported indirectly by that country’s legal system. In a recent New York Times op-ed4 piece, Bina Shah stated that “upending misogynistic tribal codes is the real key to finally ending the most egregious gender crime.” The Pakistan Parliament recently stiffened the punishment for honor killings. The new anti–honor killing law mandates a minimum lifetime jail sentence for perpetrators and closes a legal loophole that allowed an honor killer to walk free if the family of the victim forgave him.
However, in rural areas, a Pakistani woman accused of violating family or tribal honor can be sentenced to death by an informal village court or a gathering of tribal elders. Women have been killed by stoning, shooting, or being buried alive. Sometimes, the woman’s relatives condemn her to death without a trial. Women are killed for reasons such as wanting to marry of their own choice, divorcing abusive husbands, or speaking to a man or boy outside the family; “in one case, four young girls who were filmed dancing in a rain shower were executed by their cousin for immorality,” Ms. Shah said.
Range of behaviors
Abusive behavior can take many forms, including:
• Isolating a person from her friends and family.
• Depriving her of basic needs.
• Monitoring her time.
• Monitoring her use of online communication tools or spyware.
• Taking control over aspects of her everyday life, such as where she can go, whom she can see, what she can wear, and when she can sleep.
• Depriving her of access to support services, such as specialist support or medical services.
• Repeatedly putting her down, such as telling her that she is worthless.
• Enforcing rules and activities that humiliate, degrade, or dehumanize the victim.
• Forcing the victim to take part in criminal activity such as shoplifting, and neglecting or abusing children to encourage self-blame and prevent disclosure to authorities.
• Abusing finances, including controlling resources so that the person is allowed only a punitive allowance.
• Threatening to hurt or kill her.
• Threatening a child.
• Threatening to reveal or publish private information (for example, threatening to “out” someone).
• Assaulting the person.
• Causing criminal damage (such as destruction of household goods).
• Engaging in rape.
• Preventing a person from having access to transportation or from working.
New domestic violence law in the United Kingdom
Meanwhile, domestic violence laws in England and Wales now consider emotional and psychological abuse as legally actionable. The new legislation targets those who subject spouses, partners, and family members to psychological and emotional torment, but stop short of violence. The new law5 follows a Home Office consultation showing that 85% of participants said the existing law did not provide sufficient protection and a Citizens Advice report showing a 24% rise in people seeking advice for domestic abuse.
The new law falls under the Serious Crime Act 2015 of England and Wales. The act creates a new offense of “controlling or coercive behavior in intimate or familial relationships.” The act states: “The new offence closes a gap in the law around patterns of controlling or coercive behavior in an ongoing relationship between intimate partners or family members.”
Breaking the law carries a maximum sentence of 5 years’ imprisonment, a fine, or both. The behavior must have had a “serious effect” on the victim, meaning that it has caused the victim to fear violence will be used against them on “at least two occasions,” or it has had a “substantial adverse effect on the victim’s day to day activities.”
The alleged perpetrator must have known that his behavior would have a serious effect on the victim, or the behavior must have been such that he “ought to have known” it would have that effect.
The new law includes honor-based violence, female genital mutilation, and forced marriage. The law explicitly states that the victim may fear that the perpetrator has asked another person to commit violence against them, thus including family honor killings.
Gendered nature of domestic controlling or coercive behavior
While all legislation is gender neutral, women and girls are disproportionately affected by crimes of domestic violence and abuse. Women from black and minority ethnic backgrounds may experience barriers to reporting, such as a distrust of the police, concerns about racism, language barriers, concerns about family finding out, or fear of rejection by the wider community. A victim might be fearful about her children being taken away if she makes a report. Lesbian, gay, bisexual, and transgender individuals in relationships may be subjected to threats to reveal sexual orientation to family or others.
Interestingly, the U.K. guidelines state that victims of controlling or coercive behavior may not recognize themselves as victims. Therefore, it is important that the new offense be considered by the police and other authorities in attendance at all call-outs. Police are encouraged to ask questions about rules, decision making, norms, and fear in the relationship, rather than just what happened. The guidelines provide specific comments about handling perpetrators who are described as being “particularly adept” at manipulating professionals, agencies, and systems, and may use a range of tactics in relation to this offense, including targeting people who might be vulnerable (there may be evidence of this from previous relationships) and using the system against the victim by making false or vexatious allegations to agencies.
The Authorized Professional Practice on Investigating Domestic Abuse issued by the College of Policing states: “A manipulative perpetrator may be trying to draw the police into colluding with their coercive control of the victim. Police officers must avoid playing into the primary perpetrator’s hands and take account of all available evidence when making the decision to arrest.” Such evidence includes attempting to frustrate or interfere with the police investigation; making counterallegations against the victim; and using threats of manipulation against the victim – such as telling the victim that he will make a counterallegation against her, that the victim will not be believed by the police or other agencies, that he will inform social services, or that he will inform immigration officials where the victim does not have a right to remain.
How can psychiatrists raise awareness?
• Individual change. Abusive controlling behavior can have its origin in childhood psychological experiences, in the same way that honor killings and wife beating can have their roots in the perpetrator’s cultural experience. As a child, the adult perpetrator may have been a direct victim of violence or may have been a witness to domestic violence. Controlling abusive behavior also can occur as a choice in perpetrators with personality disorders unrelated to childhood experiences. It can occur in a person with both exposure and personality disorder. It is important to understand the origins and reasoning behind the behavior in order to understand how best to intervene.
If the behavior is based on the childhood experience of the prevailing sociocultural practice, the psychiatrist can explore values and beliefs, identifying those that are based on family and cultural factors. Beliefs that have been present during a person’s entire life can appear as the unexamined “background” of their lives. Bringing those beliefs to the fore can allow discussion. For example, does the individual hold the belief that women are possessions? What is the basis of holding such a belief? How does he account for the differences between societies?
• Family change. Individuals in the family may differ in their support of honor killings. Those who do not support honor killings may have difficulty speaking out for fear of becoming a future target. When we meet with families who have a belief in honor killings, we can discuss how patriarchal societies have encouraged families to maintain a firm hand on the behavior of their members. This practice encourages repression of women’s individuality and also may consider women to be possessions. Patriarchal societies control their populations by supporting values and beliefs in their citizens that support the patriarchal structure. In this way, they can exert social control easily by having members of the society act as enforcers. Open and clear discussion with families about the ways in which cultural practice affects individual behavior may allow families that are unsure to explore alternative new beliefs.
Families must understand that, under U.S. law, perpetrators of honor killings and domestic violence can be prosecuted.
• System change. According to the U.S. Justice Department, 9 out of 10 honor killings were victims who had become “too Westernized.” Leaders of the American Muslim community and members of the Council on American-Islamic Relations have condemned all honor killings, stating that the practice stems from sexism and tribal behavior that predates the religion. In February 2009, after the high-profile killing of Aasiya Zubair Hassan in New York, Muslim leaders began a nationwide effort entitled, “Imams Speak Out: Domestic Violence Will Not Be Tolerated in Our Communities,” asking all imams and religious leaders to discuss domestic violence in their weekly sermon or their Friday prayer services. The group, “Muslim Men Against Domestic Violence,” was founded soon after the murder, which came just a few days after she had filed for divorce. (After a 3-week trial, Mrs. Hassan’s estranged husband, Muzzammil “Mo” Hassan, was found guilty of second-degree murder and received a 25-year to life sentence).
Our laws reflect our values as a society. As citizens, we must actively work for the enforcement of domestic violence laws. Our mental health organizations can support the training of police and health agencies to identify victims and perpetrators.
References
1. The World Factbook, Central Intelligence Agency, 2009.
2 Forensic Sci Med Pathol. 2014;10(1):76-82.
3. “Lives Together, Worlds Apart: Men and Women in a Time of Change,” United Nations Population Fund report, 2000.
4. “Pakistan’s Honor-Killing Law Isn’t Enough,” The New York Times, Oct. 27, 2016.
5. “Controlling or Coercive Behaviour in an Intimate or Family Relationship,” Home Office, December 2015.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose.
As healers trained to address some of the psychosocial issues facing our patients, we need to understand various forms of domestic violence – and what we can do to stop it. One form, honor killings, remains pervasive across the globe.
In a sample of 856 ninth-grade students from 14 schools in Amman, Jordan, for example, about 40% of boys and 20% of girls believed that killing a daughter, sister, or wife who had dishonored the family was justified (Aggress Behav. 2013 Sep-Oct;39[5]:405-17). The schools were representative of students from different religions, socioeconomic statuses, and upbringings. The proportions are broadly in line with the religious affiliation of Jordanians, with 92% of the population identifying themselves as Muslims, 6% as Christians, and 2% as affiliated with other religions.1 However, researchers found that support for honor killings was more widespread among adolescents from poorer and more traditional family backgrounds.
What are honor killings?
According to Sally Elakkary, MD, and her colleagues, honor killings are “violence implicated against a female for the deviancy of her activities from the traditional cultural norms.”2 The perpetrators in these crimes are usually male relatives but may be other family members, including women. Males also can be victims of honor crimes. For example, a male can become a victim if he is the female’s lover in an extramarital relationship or if he is homosexual.
Most honor killings are reported from countries in the Maghreb region of North Africa; the Middle East (Palestine, Lebanon, Syria, Jordan, and Turkey); and Western and Central Asia (Iraq, Pakistan, Afghanistan, and India). However, honor killings also occur in countries with strong minorities from those origins. A report3 published in 2000 by the United Nations Population Fund estimated that 5,000 honor killings were carried out worldwide per year, with the largest absolute numbers reported for Pakistan and India (about 1,000 cases per year in each country).
Until the 1960s in the United States, penal codes in some states, such as Georgia, New Mexico, Texas, and Utah, justified a husband killing his wife’s lover. In those states, the law was formulated to protect the male’s honor. Honor killings are culture-based practices that are supported indirectly by that country’s legal system. In a recent New York Times op-ed4 piece, Bina Shah stated that “upending misogynistic tribal codes is the real key to finally ending the most egregious gender crime.” The Pakistan Parliament recently stiffened the punishment for honor killings. The new anti–honor killing law mandates a minimum lifetime jail sentence for perpetrators and closes a legal loophole that allowed an honor killer to walk free if the family of the victim forgave him.
However, in rural areas, a Pakistani woman accused of violating family or tribal honor can be sentenced to death by an informal village court or a gathering of tribal elders. Women have been killed by stoning, shooting, or being buried alive. Sometimes, the woman’s relatives condemn her to death without a trial. Women are killed for reasons such as wanting to marry of their own choice, divorcing abusive husbands, or speaking to a man or boy outside the family; “in one case, four young girls who were filmed dancing in a rain shower were executed by their cousin for immorality,” Ms. Shah said.
Range of behaviors
Abusive behavior can take many forms, including:
• Isolating a person from her friends and family.
• Depriving her of basic needs.
• Monitoring her time.
• Monitoring her use of online communication tools or spyware.
• Taking control over aspects of her everyday life, such as where she can go, whom she can see, what she can wear, and when she can sleep.
• Depriving her of access to support services, such as specialist support or medical services.
• Repeatedly putting her down, such as telling her that she is worthless.
• Enforcing rules and activities that humiliate, degrade, or dehumanize the victim.
• Forcing the victim to take part in criminal activity such as shoplifting, and neglecting or abusing children to encourage self-blame and prevent disclosure to authorities.
• Abusing finances, including controlling resources so that the person is allowed only a punitive allowance.
• Threatening to hurt or kill her.
• Threatening a child.
• Threatening to reveal or publish private information (for example, threatening to “out” someone).
• Assaulting the person.
• Causing criminal damage (such as destruction of household goods).
• Engaging in rape.
• Preventing a person from having access to transportation or from working.
New domestic violence law in the United Kingdom
Meanwhile, domestic violence laws in England and Wales now consider emotional and psychological abuse as legally actionable. The new legislation targets those who subject spouses, partners, and family members to psychological and emotional torment, but stop short of violence. The new law5 follows a Home Office consultation showing that 85% of participants said the existing law did not provide sufficient protection and a Citizens Advice report showing a 24% rise in people seeking advice for domestic abuse.
The new law falls under the Serious Crime Act 2015 of England and Wales. The act creates a new offense of “controlling or coercive behavior in intimate or familial relationships.” The act states: “The new offence closes a gap in the law around patterns of controlling or coercive behavior in an ongoing relationship between intimate partners or family members.”
Breaking the law carries a maximum sentence of 5 years’ imprisonment, a fine, or both. The behavior must have had a “serious effect” on the victim, meaning that it has caused the victim to fear violence will be used against them on “at least two occasions,” or it has had a “substantial adverse effect on the victim’s day to day activities.”
The alleged perpetrator must have known that his behavior would have a serious effect on the victim, or the behavior must have been such that he “ought to have known” it would have that effect.
The new law includes honor-based violence, female genital mutilation, and forced marriage. The law explicitly states that the victim may fear that the perpetrator has asked another person to commit violence against them, thus including family honor killings.
Gendered nature of domestic controlling or coercive behavior
While all legislation is gender neutral, women and girls are disproportionately affected by crimes of domestic violence and abuse. Women from black and minority ethnic backgrounds may experience barriers to reporting, such as a distrust of the police, concerns about racism, language barriers, concerns about family finding out, or fear of rejection by the wider community. A victim might be fearful about her children being taken away if she makes a report. Lesbian, gay, bisexual, and transgender individuals in relationships may be subjected to threats to reveal sexual orientation to family or others.
Interestingly, the U.K. guidelines state that victims of controlling or coercive behavior may not recognize themselves as victims. Therefore, it is important that the new offense be considered by the police and other authorities in attendance at all call-outs. Police are encouraged to ask questions about rules, decision making, norms, and fear in the relationship, rather than just what happened. The guidelines provide specific comments about handling perpetrators who are described as being “particularly adept” at manipulating professionals, agencies, and systems, and may use a range of tactics in relation to this offense, including targeting people who might be vulnerable (there may be evidence of this from previous relationships) and using the system against the victim by making false or vexatious allegations to agencies.
The Authorized Professional Practice on Investigating Domestic Abuse issued by the College of Policing states: “A manipulative perpetrator may be trying to draw the police into colluding with their coercive control of the victim. Police officers must avoid playing into the primary perpetrator’s hands and take account of all available evidence when making the decision to arrest.” Such evidence includes attempting to frustrate or interfere with the police investigation; making counterallegations against the victim; and using threats of manipulation against the victim – such as telling the victim that he will make a counterallegation against her, that the victim will not be believed by the police or other agencies, that he will inform social services, or that he will inform immigration officials where the victim does not have a right to remain.
How can psychiatrists raise awareness?
• Individual change. Abusive controlling behavior can have its origin in childhood psychological experiences, in the same way that honor killings and wife beating can have their roots in the perpetrator’s cultural experience. As a child, the adult perpetrator may have been a direct victim of violence or may have been a witness to domestic violence. Controlling abusive behavior also can occur as a choice in perpetrators with personality disorders unrelated to childhood experiences. It can occur in a person with both exposure and personality disorder. It is important to understand the origins and reasoning behind the behavior in order to understand how best to intervene.
If the behavior is based on the childhood experience of the prevailing sociocultural practice, the psychiatrist can explore values and beliefs, identifying those that are based on family and cultural factors. Beliefs that have been present during a person’s entire life can appear as the unexamined “background” of their lives. Bringing those beliefs to the fore can allow discussion. For example, does the individual hold the belief that women are possessions? What is the basis of holding such a belief? How does he account for the differences between societies?
• Family change. Individuals in the family may differ in their support of honor killings. Those who do not support honor killings may have difficulty speaking out for fear of becoming a future target. When we meet with families who have a belief in honor killings, we can discuss how patriarchal societies have encouraged families to maintain a firm hand on the behavior of their members. This practice encourages repression of women’s individuality and also may consider women to be possessions. Patriarchal societies control their populations by supporting values and beliefs in their citizens that support the patriarchal structure. In this way, they can exert social control easily by having members of the society act as enforcers. Open and clear discussion with families about the ways in which cultural practice affects individual behavior may allow families that are unsure to explore alternative new beliefs.
Families must understand that, under U.S. law, perpetrators of honor killings and domestic violence can be prosecuted.
• System change. According to the U.S. Justice Department, 9 out of 10 honor killings were victims who had become “too Westernized.” Leaders of the American Muslim community and members of the Council on American-Islamic Relations have condemned all honor killings, stating that the practice stems from sexism and tribal behavior that predates the religion. In February 2009, after the high-profile killing of Aasiya Zubair Hassan in New York, Muslim leaders began a nationwide effort entitled, “Imams Speak Out: Domestic Violence Will Not Be Tolerated in Our Communities,” asking all imams and religious leaders to discuss domestic violence in their weekly sermon or their Friday prayer services. The group, “Muslim Men Against Domestic Violence,” was founded soon after the murder, which came just a few days after she had filed for divorce. (After a 3-week trial, Mrs. Hassan’s estranged husband, Muzzammil “Mo” Hassan, was found guilty of second-degree murder and received a 25-year to life sentence).
Our laws reflect our values as a society. As citizens, we must actively work for the enforcement of domestic violence laws. Our mental health organizations can support the training of police and health agencies to identify victims and perpetrators.
References
1. The World Factbook, Central Intelligence Agency, 2009.
2 Forensic Sci Med Pathol. 2014;10(1):76-82.
3. “Lives Together, Worlds Apart: Men and Women in a Time of Change,” United Nations Population Fund report, 2000.
4. “Pakistan’s Honor-Killing Law Isn’t Enough,” The New York Times, Oct. 27, 2016.
5. “Controlling or Coercive Behaviour in an Intimate or Family Relationship,” Home Office, December 2015.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose.
Selected elderly trauma patients do well in non–ICU wards
CORONADO, CALIF. – When elderly patients are appropriately triaged, they can be selectively admitted to non–intensive care wards with acceptable outcomes, results from a single-center study showed.
“Trauma centers across the United States are caring for elderly trauma patients with greater frequency,” researchers led by Marc D. Trust, MD, wrote in an abstract presented during a poster session at the annual meeting of the Western Surgical Association.
“Previous literature showed improved outcomes in this population from aggressive care and invasive monitoring. This may have led to an increased utilization of intensive care resources for these patients,” they noted.
In an effort to assess the safety of admitting this population of patients to non–intensive care units, Dr. Trust, a surgery resident at the University of Texas at Austin, and his associates retrospectively reviewed the medical records of 3,682 trauma patients aged 65 and older who were admitted from 2006 to 2015. They compared demographic data and outcomes between patients admitted to the ICU and those admitted to the surgical ward. The primary endpoint was mortality, while secondary endpoints were transfer to higher level of care and hospital length of stay. Patients admitted only for comfort care and those with injuries thought to be terminal and irreversible were excluded from the analysis.
The mean age of the 3,682 patients was 76 years and 1,838 (50%) were admitted to the ICU, while the remaining 1,844 (50%) were admitted to the surgical ward. When the researchers compared patients admitted to the ICU with those admitted to the surgical ward, they observed significant differences in mortality (7% vs. 0.82%, respectively; P less than .001), as well as systolic blood pressure on admission (146 vs. 149 mm Hg, respectively; P = .0002), pulse (85 vs. 81 beats per minute; P less than .0001), Glasgow Coma Scale (14 vs. 15; P less than .001), Injury Severity Score (16 vs. 8; P less than .001), and hospital stay (a mean of 8 vs. 4 days; P less than .0001). In addition, fewer than 1% of patients admitted to the surgical ward required transfer to a higher level of care (P less than .0001).
Next, Dr. Trust and his associates conducted a subgroup analysis of 300 patients admitted to the ICU (28%) and 766 (72%) admitted to the surgical ward who had all-system Abbreviated Injury Scale scores of less than 3, no hypotension on admission, and a Glasgow Coma Scale of 14 or greater. Compared with those admitted to the surgical ward, those admitted to the ICU were older (77 vs. 76 years old, respectively; P = .003), more likely to be male (54% vs. 45%; P = .007), more tachycardic (HR 84 vs. 81; P = .004), more severely injured (ISS score of 5 vs. 4; P less than .0001), and more likely to have a longer hospital stay (a mean of 6 vs. 4 days; P less than .0001). Two patients admitted to the surgical ward died (0.26%; P = .0009) and none required transfer to a higher level of care.
The researchers reported having no financial disclosures.
CORONADO, CALIF. – When elderly patients are appropriately triaged, they can be selectively admitted to non–intensive care wards with acceptable outcomes, results from a single-center study showed.
“Trauma centers across the United States are caring for elderly trauma patients with greater frequency,” researchers led by Marc D. Trust, MD, wrote in an abstract presented during a poster session at the annual meeting of the Western Surgical Association.
“Previous literature showed improved outcomes in this population from aggressive care and invasive monitoring. This may have led to an increased utilization of intensive care resources for these patients,” they noted.
In an effort to assess the safety of admitting this population of patients to non–intensive care units, Dr. Trust, a surgery resident at the University of Texas at Austin, and his associates retrospectively reviewed the medical records of 3,682 trauma patients aged 65 and older who were admitted from 2006 to 2015. They compared demographic data and outcomes between patients admitted to the ICU and those admitted to the surgical ward. The primary endpoint was mortality, while secondary endpoints were transfer to higher level of care and hospital length of stay. Patients admitted only for comfort care and those with injuries thought to be terminal and irreversible were excluded from the analysis.
The mean age of the 3,682 patients was 76 years and 1,838 (50%) were admitted to the ICU, while the remaining 1,844 (50%) were admitted to the surgical ward. When the researchers compared patients admitted to the ICU with those admitted to the surgical ward, they observed significant differences in mortality (7% vs. 0.82%, respectively; P less than .001), as well as systolic blood pressure on admission (146 vs. 149 mm Hg, respectively; P = .0002), pulse (85 vs. 81 beats per minute; P less than .0001), Glasgow Coma Scale (14 vs. 15; P less than .001), Injury Severity Score (16 vs. 8; P less than .001), and hospital stay (a mean of 8 vs. 4 days; P less than .0001). In addition, fewer than 1% of patients admitted to the surgical ward required transfer to a higher level of care (P less than .0001).
Next, Dr. Trust and his associates conducted a subgroup analysis of 300 patients admitted to the ICU (28%) and 766 (72%) admitted to the surgical ward who had all-system Abbreviated Injury Scale scores of less than 3, no hypotension on admission, and a Glasgow Coma Scale of 14 or greater. Compared with those admitted to the surgical ward, those admitted to the ICU were older (77 vs. 76 years old, respectively; P = .003), more likely to be male (54% vs. 45%; P = .007), more tachycardic (HR 84 vs. 81; P = .004), more severely injured (ISS score of 5 vs. 4; P less than .0001), and more likely to have a longer hospital stay (a mean of 6 vs. 4 days; P less than .0001). Two patients admitted to the surgical ward died (0.26%; P = .0009) and none required transfer to a higher level of care.
The researchers reported having no financial disclosures.
CORONADO, CALIF. – When elderly patients are appropriately triaged, they can be selectively admitted to non–intensive care wards with acceptable outcomes, results from a single-center study showed.
“Trauma centers across the United States are caring for elderly trauma patients with greater frequency,” researchers led by Marc D. Trust, MD, wrote in an abstract presented during a poster session at the annual meeting of the Western Surgical Association.
“Previous literature showed improved outcomes in this population from aggressive care and invasive monitoring. This may have led to an increased utilization of intensive care resources for these patients,” they noted.
In an effort to assess the safety of admitting this population of patients to non–intensive care units, Dr. Trust, a surgery resident at the University of Texas at Austin, and his associates retrospectively reviewed the medical records of 3,682 trauma patients aged 65 and older who were admitted from 2006 to 2015. They compared demographic data and outcomes between patients admitted to the ICU and those admitted to the surgical ward. The primary endpoint was mortality, while secondary endpoints were transfer to higher level of care and hospital length of stay. Patients admitted only for comfort care and those with injuries thought to be terminal and irreversible were excluded from the analysis.
The mean age of the 3,682 patients was 76 years and 1,838 (50%) were admitted to the ICU, while the remaining 1,844 (50%) were admitted to the surgical ward. When the researchers compared patients admitted to the ICU with those admitted to the surgical ward, they observed significant differences in mortality (7% vs. 0.82%, respectively; P less than .001), as well as systolic blood pressure on admission (146 vs. 149 mm Hg, respectively; P = .0002), pulse (85 vs. 81 beats per minute; P less than .0001), Glasgow Coma Scale (14 vs. 15; P less than .001), Injury Severity Score (16 vs. 8; P less than .001), and hospital stay (a mean of 8 vs. 4 days; P less than .0001). In addition, fewer than 1% of patients admitted to the surgical ward required transfer to a higher level of care (P less than .0001).
Next, Dr. Trust and his associates conducted a subgroup analysis of 300 patients admitted to the ICU (28%) and 766 (72%) admitted to the surgical ward who had all-system Abbreviated Injury Scale scores of less than 3, no hypotension on admission, and a Glasgow Coma Scale of 14 or greater. Compared with those admitted to the surgical ward, those admitted to the ICU were older (77 vs. 76 years old, respectively; P = .003), more likely to be male (54% vs. 45%; P = .007), more tachycardic (HR 84 vs. 81; P = .004), more severely injured (ISS score of 5 vs. 4; P less than .0001), and more likely to have a longer hospital stay (a mean of 6 vs. 4 days; P less than .0001). Two patients admitted to the surgical ward died (0.26%; P = .0009) and none required transfer to a higher level of care.
The researchers reported having no financial disclosures.
AT WSA 2016
Key clinical point:
Major finding: Mortality rates were significantly higher among elderly trauma patients admitted to the ICU, compared with those admitted to the surgical ward (7% vs. 0.82%, respectively; P less than .001).
Data source: A retrospective review of 3,682 trauma patients aged 65 and older who were admitted from 2006 to 2015.
Disclosures: The researchers reported having no financial disclosures.
Experts share tips on minimizing the trauma of skin biopsy in children
DVDs, iPads, and toys. “Sweeties” to suck on. Buffered lidocaine, soothing talk, and a distracting “angel’s pinch.”
These are just a few of the strategies that dermatologists can use to calm children during a skin biopsy, which can be traumatic for everyone in the room. “This procedure, while minor, can be a big deal to kids,” said Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital and professor, department of pediatrics, University of Washington, Seattle. “It’s invasive. And it involves a shot and blood and discomfort, albeit relatively mild – all things that are frightening for anyone, but more so for kids.”
When a biopsy is performed in a child, “the anxiety that they bring to the situation is as much an issue as the pain,” Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and professor of dermatology and pediatrics, University of California, San Diego, said in an interview.
But there are ways to lessen the intensity of the procedures for children, their parents, and medical staff, according to the two pediatric dermatologists. Here are their tips for various age groups:
Infants
Dr. Sidbury is a big fan of papooses or wraps, as long as they are not obstructive. “Babies are used to being wrapped, and it can be an atraumatic way to restrain,” he said. “If parents are comfortable, I will have them present, talking and cooing to the baby throughout. Their voices are soothing.”
Indeed, Dr. Eichenfield says he breaks his rule about allowing parents in the room for biopsies when the children are under age 7 to 8 months. “It’s more unnerving for them to be in the room, and they’re not that calming to the baby.”
Food can be another soothing strategy. Infants may suck on “sweeties,” a glucose-rich solution known as TootSweet sucrose solution, prior to and during the procedure, Dr. Sidbury said. “EMLA cream or some form of topical anesthetic can be helpful, but the provider must remain mindful of the maximum safe amounts to apply as outlined in the package insert.”
He also advises colleagues to remember the thinness of infant skin. “Biopsying ‘down to the hub,’ as one will often do in an adult with a punch biopsy, can be too deep in some places,” he said.
Toddlers and younger children
“Two-to-six-year-olds are the toughest group,” Dr. Eichenfield noted. “They’re afraid of needles, they don’t understand why they have to have the procedure, and they don’t understand that once it’s done, it’s not going to hurt.”
Shifting away their focus is ideal, he said. “Distraction is always great. They’ll sense less pain and have less anxiety if they’re busy.” Distractions like a video on DVD can be helpful, he said, as can a “counterstimulation” technique, like a firm “angel’s pinch” that prevents them from noticing an injection. “Kids are comfortable getting pinched,” he said. “Many times I’ll block their view of the procedure, too.”
Older children
If a child is over age 6 years, Dr. Eichenfield recommends asking parents about whether the child has had any difficulty while undergoing anesthesia for dental procedures. If they don’t, “you know that they’re not coming with a history of anxiety or pain that can definitely amplify their perception and concern about the procedure.”
Dr. Sidbury also recommended distractions like iPads, movie players, video games, and music. Prizes may also help: They can be given as rewards at the end of procedures.
“Try not to show the needle,” he advised. “But this does not mean surprising kids or not letting them know a shot will be involved.”
And be aware that the numbing in older children is often the hardest part. “They will realize once it stops hurting they are OK,” he pointed out. “Hence, this part should be relatively fast. Don’t linger over the child, needle in hand, explaining things. Keep the needle and sharp, scary-looking instruments covered until needed, and then keep the needle itself covered as long as possible. Just the sight of it can be a deal breaker.”
Anesthesia tips
Regardless of the age of the child, careful use of anesthesia is recommended. “I often have the parents apply a topical anesthetic at home for a few hours before their arrival,” said Bernard Cohen, MD, professor of dermatology, Johns Hopkins University, Baltimore. “I inject deeper in the subcutaneous fat first before injecting more superficially, and I try to extend the anesthetic from the first area of injection to minimize the pain.”
For his part, Dr. Sidbury recommends using EMLA or LMX cream, in advance of 1% lidocaine with buffered epinephrine injected locally. Topical EMLA works better if used liberally – albeit within specified safe limits, he said. So instead of applying a small amount and rubbing it in, a thicker layer can be applied without rubbing it in, and when possible, the area can be occluded with a dressing or other type of covering, “while you are waiting the 30-plus minutes for it to work.” Occluding the area with something like “Press ’N’ Seal” wrap that comes off easily, instead of adhesive, is a good idea, he added, since removing an adhesive dressing can be as painful as the procedure.
Like Dr. Cohen and Dr. Eichenfield, Dr. Sidbury also supports physical distraction when the lidocaine is injected, like “having the patient cough if the movement is not problematic. Or rubbing or scratching the adjacent skin to the site of shot, or the opposite arm.”
Dr. Eichenfield, Dr. Sidbury, and Dr. Cohen reported no relevant disclosures.
DVDs, iPads, and toys. “Sweeties” to suck on. Buffered lidocaine, soothing talk, and a distracting “angel’s pinch.”
These are just a few of the strategies that dermatologists can use to calm children during a skin biopsy, which can be traumatic for everyone in the room. “This procedure, while minor, can be a big deal to kids,” said Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital and professor, department of pediatrics, University of Washington, Seattle. “It’s invasive. And it involves a shot and blood and discomfort, albeit relatively mild – all things that are frightening for anyone, but more so for kids.”
When a biopsy is performed in a child, “the anxiety that they bring to the situation is as much an issue as the pain,” Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and professor of dermatology and pediatrics, University of California, San Diego, said in an interview.
But there are ways to lessen the intensity of the procedures for children, their parents, and medical staff, according to the two pediatric dermatologists. Here are their tips for various age groups:
Infants
Dr. Sidbury is a big fan of papooses or wraps, as long as they are not obstructive. “Babies are used to being wrapped, and it can be an atraumatic way to restrain,” he said. “If parents are comfortable, I will have them present, talking and cooing to the baby throughout. Their voices are soothing.”
Indeed, Dr. Eichenfield says he breaks his rule about allowing parents in the room for biopsies when the children are under age 7 to 8 months. “It’s more unnerving for them to be in the room, and they’re not that calming to the baby.”
Food can be another soothing strategy. Infants may suck on “sweeties,” a glucose-rich solution known as TootSweet sucrose solution, prior to and during the procedure, Dr. Sidbury said. “EMLA cream or some form of topical anesthetic can be helpful, but the provider must remain mindful of the maximum safe amounts to apply as outlined in the package insert.”
He also advises colleagues to remember the thinness of infant skin. “Biopsying ‘down to the hub,’ as one will often do in an adult with a punch biopsy, can be too deep in some places,” he said.
Toddlers and younger children
“Two-to-six-year-olds are the toughest group,” Dr. Eichenfield noted. “They’re afraid of needles, they don’t understand why they have to have the procedure, and they don’t understand that once it’s done, it’s not going to hurt.”
Shifting away their focus is ideal, he said. “Distraction is always great. They’ll sense less pain and have less anxiety if they’re busy.” Distractions like a video on DVD can be helpful, he said, as can a “counterstimulation” technique, like a firm “angel’s pinch” that prevents them from noticing an injection. “Kids are comfortable getting pinched,” he said. “Many times I’ll block their view of the procedure, too.”
Older children
If a child is over age 6 years, Dr. Eichenfield recommends asking parents about whether the child has had any difficulty while undergoing anesthesia for dental procedures. If they don’t, “you know that they’re not coming with a history of anxiety or pain that can definitely amplify their perception and concern about the procedure.”
Dr. Sidbury also recommended distractions like iPads, movie players, video games, and music. Prizes may also help: They can be given as rewards at the end of procedures.
“Try not to show the needle,” he advised. “But this does not mean surprising kids or not letting them know a shot will be involved.”
And be aware that the numbing in older children is often the hardest part. “They will realize once it stops hurting they are OK,” he pointed out. “Hence, this part should be relatively fast. Don’t linger over the child, needle in hand, explaining things. Keep the needle and sharp, scary-looking instruments covered until needed, and then keep the needle itself covered as long as possible. Just the sight of it can be a deal breaker.”
Anesthesia tips
Regardless of the age of the child, careful use of anesthesia is recommended. “I often have the parents apply a topical anesthetic at home for a few hours before their arrival,” said Bernard Cohen, MD, professor of dermatology, Johns Hopkins University, Baltimore. “I inject deeper in the subcutaneous fat first before injecting more superficially, and I try to extend the anesthetic from the first area of injection to minimize the pain.”
For his part, Dr. Sidbury recommends using EMLA or LMX cream, in advance of 1% lidocaine with buffered epinephrine injected locally. Topical EMLA works better if used liberally – albeit within specified safe limits, he said. So instead of applying a small amount and rubbing it in, a thicker layer can be applied without rubbing it in, and when possible, the area can be occluded with a dressing or other type of covering, “while you are waiting the 30-plus minutes for it to work.” Occluding the area with something like “Press ’N’ Seal” wrap that comes off easily, instead of adhesive, is a good idea, he added, since removing an adhesive dressing can be as painful as the procedure.
Like Dr. Cohen and Dr. Eichenfield, Dr. Sidbury also supports physical distraction when the lidocaine is injected, like “having the patient cough if the movement is not problematic. Or rubbing or scratching the adjacent skin to the site of shot, or the opposite arm.”
Dr. Eichenfield, Dr. Sidbury, and Dr. Cohen reported no relevant disclosures.
DVDs, iPads, and toys. “Sweeties” to suck on. Buffered lidocaine, soothing talk, and a distracting “angel’s pinch.”
These are just a few of the strategies that dermatologists can use to calm children during a skin biopsy, which can be traumatic for everyone in the room. “This procedure, while minor, can be a big deal to kids,” said Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital and professor, department of pediatrics, University of Washington, Seattle. “It’s invasive. And it involves a shot and blood and discomfort, albeit relatively mild – all things that are frightening for anyone, but more so for kids.”
When a biopsy is performed in a child, “the anxiety that they bring to the situation is as much an issue as the pain,” Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and professor of dermatology and pediatrics, University of California, San Diego, said in an interview.
But there are ways to lessen the intensity of the procedures for children, their parents, and medical staff, according to the two pediatric dermatologists. Here are their tips for various age groups:
Infants
Dr. Sidbury is a big fan of papooses or wraps, as long as they are not obstructive. “Babies are used to being wrapped, and it can be an atraumatic way to restrain,” he said. “If parents are comfortable, I will have them present, talking and cooing to the baby throughout. Their voices are soothing.”
Indeed, Dr. Eichenfield says he breaks his rule about allowing parents in the room for biopsies when the children are under age 7 to 8 months. “It’s more unnerving for them to be in the room, and they’re not that calming to the baby.”
Food can be another soothing strategy. Infants may suck on “sweeties,” a glucose-rich solution known as TootSweet sucrose solution, prior to and during the procedure, Dr. Sidbury said. “EMLA cream or some form of topical anesthetic can be helpful, but the provider must remain mindful of the maximum safe amounts to apply as outlined in the package insert.”
He also advises colleagues to remember the thinness of infant skin. “Biopsying ‘down to the hub,’ as one will often do in an adult with a punch biopsy, can be too deep in some places,” he said.
Toddlers and younger children
“Two-to-six-year-olds are the toughest group,” Dr. Eichenfield noted. “They’re afraid of needles, they don’t understand why they have to have the procedure, and they don’t understand that once it’s done, it’s not going to hurt.”
Shifting away their focus is ideal, he said. “Distraction is always great. They’ll sense less pain and have less anxiety if they’re busy.” Distractions like a video on DVD can be helpful, he said, as can a “counterstimulation” technique, like a firm “angel’s pinch” that prevents them from noticing an injection. “Kids are comfortable getting pinched,” he said. “Many times I’ll block their view of the procedure, too.”
Older children
If a child is over age 6 years, Dr. Eichenfield recommends asking parents about whether the child has had any difficulty while undergoing anesthesia for dental procedures. If they don’t, “you know that they’re not coming with a history of anxiety or pain that can definitely amplify their perception and concern about the procedure.”
Dr. Sidbury also recommended distractions like iPads, movie players, video games, and music. Prizes may also help: They can be given as rewards at the end of procedures.
“Try not to show the needle,” he advised. “But this does not mean surprising kids or not letting them know a shot will be involved.”
And be aware that the numbing in older children is often the hardest part. “They will realize once it stops hurting they are OK,” he pointed out. “Hence, this part should be relatively fast. Don’t linger over the child, needle in hand, explaining things. Keep the needle and sharp, scary-looking instruments covered until needed, and then keep the needle itself covered as long as possible. Just the sight of it can be a deal breaker.”
Anesthesia tips
Regardless of the age of the child, careful use of anesthesia is recommended. “I often have the parents apply a topical anesthetic at home for a few hours before their arrival,” said Bernard Cohen, MD, professor of dermatology, Johns Hopkins University, Baltimore. “I inject deeper in the subcutaneous fat first before injecting more superficially, and I try to extend the anesthetic from the first area of injection to minimize the pain.”
For his part, Dr. Sidbury recommends using EMLA or LMX cream, in advance of 1% lidocaine with buffered epinephrine injected locally. Topical EMLA works better if used liberally – albeit within specified safe limits, he said. So instead of applying a small amount and rubbing it in, a thicker layer can be applied without rubbing it in, and when possible, the area can be occluded with a dressing or other type of covering, “while you are waiting the 30-plus minutes for it to work.” Occluding the area with something like “Press ’N’ Seal” wrap that comes off easily, instead of adhesive, is a good idea, he added, since removing an adhesive dressing can be as painful as the procedure.
Like Dr. Cohen and Dr. Eichenfield, Dr. Sidbury also supports physical distraction when the lidocaine is injected, like “having the patient cough if the movement is not problematic. Or rubbing or scratching the adjacent skin to the site of shot, or the opposite arm.”
Dr. Eichenfield, Dr. Sidbury, and Dr. Cohen reported no relevant disclosures.
Azathioprine linked to increased risk of SCC in transplant recipients
Immunosuppressive treatment with azathioprine may be associated with an increased risk of squamous cell carcinoma in organ transplant recipients, but does not appear to increase the risk of basal cell carcinoma or keratinocyte cancers overall, according to a systematic review and meta-analysis of 27 studies.
While immunosuppressive agents generally are known to contribute to an increased risk of skin carcinogenesis, azathioprine – a purine antimetabolite immunosuppressant – is thought to add to this increase through its photosensitizing effects and the accumulation of mutagenic reactive oxygen species when exposed to UVA.
To address conflicting data on whether azathioprine increases the risk of skin cancer, the authors conducted the analysis of 27 studies (23 cohort studies, 1 randomized study, and 3 case control studies) published between 1996 and 2011, which evaluated skin cancer risk associated with azathioprine in people who received an organ transplant from 1963 to 2011, at a median age of 38-54 years.
In the studies that evaluated the risk of squamous cell carcinoma, risk was elevated by 56% among patients treated with azathioprine (95% CI 1.11-2.18, P less than .001), but estimates ranged from 0.64 to 8.64. The risk was sevenfold higher in two case-control studies combined, but was not significant in eight cohort studies, reported Zainab Jiyad, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Australia, and St. George’s University of London, and coauthors. They noted that there was significant heterogeneity between the studies, probably because of differences in study design, organ transplant type, and period of transplantation (Am J Transplant. 2016 Dec;16[12]:3490-503).
“Despite the substantial heterogeneity, which would tend to dilute the observed summary risk estimate, a significant effect of azathioprine was detected,” they added. “Thus, we acknowledge that our summary estimate may be a conservative estimate of the risk associated with azathioprine.”
The subgroup analysis comparing the risk among kidney and heart transplant recipients showed the increased risk of squamous cell carcinoma was significant among kidney transplant recipients but not among heart transplant recipients.
Among the six cohort studies that evaluated the risk of basal cell carcinoma, the risk was increased in four studies, while two suggested that azathioprine was actually protective against it. When the studies were pooled, the estimated risk was 0.96.
Similarly, when researchers looked at studies of keratinocyte cancers (the combined risk of squamous cell and basal cell cancers), there was no significant association with overall risk of KC.
Older age and transplantation, fair skin type, high sun exposure, childhood sunburn, a history of skin cancer, and rejection episodes in the first year of transplantation were all risk factors for developing squamous cell carcinoma. Therefore, avoiding azathioprine in organ transplant recipients “with one or more of these risk factors may help reduce the future risk” of squamous cell cancer, they wrote.
The authors added that more high quality studies were needed and that studies should evaluate the risk of squamous cell and basal cell cancer separately, because of the apparent difference in risk.
No conflicts of interest were declared.
Immunosuppressive treatment with azathioprine may be associated with an increased risk of squamous cell carcinoma in organ transplant recipients, but does not appear to increase the risk of basal cell carcinoma or keratinocyte cancers overall, according to a systematic review and meta-analysis of 27 studies.
While immunosuppressive agents generally are known to contribute to an increased risk of skin carcinogenesis, azathioprine – a purine antimetabolite immunosuppressant – is thought to add to this increase through its photosensitizing effects and the accumulation of mutagenic reactive oxygen species when exposed to UVA.
To address conflicting data on whether azathioprine increases the risk of skin cancer, the authors conducted the analysis of 27 studies (23 cohort studies, 1 randomized study, and 3 case control studies) published between 1996 and 2011, which evaluated skin cancer risk associated with azathioprine in people who received an organ transplant from 1963 to 2011, at a median age of 38-54 years.
In the studies that evaluated the risk of squamous cell carcinoma, risk was elevated by 56% among patients treated with azathioprine (95% CI 1.11-2.18, P less than .001), but estimates ranged from 0.64 to 8.64. The risk was sevenfold higher in two case-control studies combined, but was not significant in eight cohort studies, reported Zainab Jiyad, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Australia, and St. George’s University of London, and coauthors. They noted that there was significant heterogeneity between the studies, probably because of differences in study design, organ transplant type, and period of transplantation (Am J Transplant. 2016 Dec;16[12]:3490-503).
“Despite the substantial heterogeneity, which would tend to dilute the observed summary risk estimate, a significant effect of azathioprine was detected,” they added. “Thus, we acknowledge that our summary estimate may be a conservative estimate of the risk associated with azathioprine.”
The subgroup analysis comparing the risk among kidney and heart transplant recipients showed the increased risk of squamous cell carcinoma was significant among kidney transplant recipients but not among heart transplant recipients.
Among the six cohort studies that evaluated the risk of basal cell carcinoma, the risk was increased in four studies, while two suggested that azathioprine was actually protective against it. When the studies were pooled, the estimated risk was 0.96.
Similarly, when researchers looked at studies of keratinocyte cancers (the combined risk of squamous cell and basal cell cancers), there was no significant association with overall risk of KC.
Older age and transplantation, fair skin type, high sun exposure, childhood sunburn, a history of skin cancer, and rejection episodes in the first year of transplantation were all risk factors for developing squamous cell carcinoma. Therefore, avoiding azathioprine in organ transplant recipients “with one or more of these risk factors may help reduce the future risk” of squamous cell cancer, they wrote.
The authors added that more high quality studies were needed and that studies should evaluate the risk of squamous cell and basal cell cancer separately, because of the apparent difference in risk.
No conflicts of interest were declared.
Immunosuppressive treatment with azathioprine may be associated with an increased risk of squamous cell carcinoma in organ transplant recipients, but does not appear to increase the risk of basal cell carcinoma or keratinocyte cancers overall, according to a systematic review and meta-analysis of 27 studies.
While immunosuppressive agents generally are known to contribute to an increased risk of skin carcinogenesis, azathioprine – a purine antimetabolite immunosuppressant – is thought to add to this increase through its photosensitizing effects and the accumulation of mutagenic reactive oxygen species when exposed to UVA.
To address conflicting data on whether azathioprine increases the risk of skin cancer, the authors conducted the analysis of 27 studies (23 cohort studies, 1 randomized study, and 3 case control studies) published between 1996 and 2011, which evaluated skin cancer risk associated with azathioprine in people who received an organ transplant from 1963 to 2011, at a median age of 38-54 years.
In the studies that evaluated the risk of squamous cell carcinoma, risk was elevated by 56% among patients treated with azathioprine (95% CI 1.11-2.18, P less than .001), but estimates ranged from 0.64 to 8.64. The risk was sevenfold higher in two case-control studies combined, but was not significant in eight cohort studies, reported Zainab Jiyad, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Australia, and St. George’s University of London, and coauthors. They noted that there was significant heterogeneity between the studies, probably because of differences in study design, organ transplant type, and period of transplantation (Am J Transplant. 2016 Dec;16[12]:3490-503).
“Despite the substantial heterogeneity, which would tend to dilute the observed summary risk estimate, a significant effect of azathioprine was detected,” they added. “Thus, we acknowledge that our summary estimate may be a conservative estimate of the risk associated with azathioprine.”
The subgroup analysis comparing the risk among kidney and heart transplant recipients showed the increased risk of squamous cell carcinoma was significant among kidney transplant recipients but not among heart transplant recipients.
Among the six cohort studies that evaluated the risk of basal cell carcinoma, the risk was increased in four studies, while two suggested that azathioprine was actually protective against it. When the studies were pooled, the estimated risk was 0.96.
Similarly, when researchers looked at studies of keratinocyte cancers (the combined risk of squamous cell and basal cell cancers), there was no significant association with overall risk of KC.
Older age and transplantation, fair skin type, high sun exposure, childhood sunburn, a history of skin cancer, and rejection episodes in the first year of transplantation were all risk factors for developing squamous cell carcinoma. Therefore, avoiding azathioprine in organ transplant recipients “with one or more of these risk factors may help reduce the future risk” of squamous cell cancer, they wrote.
The authors added that more high quality studies were needed and that studies should evaluate the risk of squamous cell and basal cell cancer separately, because of the apparent difference in risk.
No conflicts of interest were declared.
FROM THE AMERICAN JOURNAL OF TRANSPLANTATION
Key clinical point: Immunosuppressive treatment with azathioprine may be associated with an increased risk of squamous cell carcinoma but not basal cell carcinoma.
Major finding: Patients treated with azathioprine after an organ transplant had a 56% increased risk of squamous cell carcinoma.
Data source: A systematic review and meta-analysis of 27 studies that evaluated the risk of skin cancer in organ transplant recipients treated with azathioprine.
Disclosures: The authors had no conflicts of interest to disclose.
HM 2016: A Year in Review
From celebrating the 20th anniversary of hospital medicine’s arrival to rapid reimbursement reform to sought-after clarity for observation rules, the past 12 months have been eventful. The Hospitalist asked more than a dozen industry leaders what they thought were the biggest highlights of 2016. Here is what they said:
1. Happy Birthday, HM
August 15 marked the 20th anniversary of “The Emerging Role of ‘Hospitalists’ in the American Health Care System,”1 a New England Journal of Medicine paper authored by Lee Goldman, MD, and Robert Wachter, MD, MHM.2 It was the first time anyone defined what a hospitalist was, putting a sobriquet to an inpatient care model that had organically and independently started happening nationwide.
The paper laid the groundwork for a specialty that spread like a proverbial wildfire over the next 20 years. The model overcame early opposition from residents fearful they’d lose the ability to learn and critical-care specialists who wanted to maintain the caseloads they’d fought to build. The field has now swelled to an estimated 52,000 practitioners.
“I reflect back … and think today about what the hospitalist model brings to us,” James Merlino, MD, president and chief medical officer of Press Ganey’s strategic consulting division, told The Hospitalist. “It is an amazing transformation on how the hospitalist model really delivers.”1
2. Its Own Specialty Code
Hospitalist medicine received federal approval for its first dedicated specialty code3 in a decision hailed by many. The code, approved by the Centers for Medicare & Medicaid Services (CMS), should allow hospitalist-specific billing in 2017.
SHM leaders have long pushed for the code, particularly as healthcare transitions from fee-for-service (FFS) to quality-based payment models. Public Policy Committee Chair Ron Greeno, MD, MHM, says that a dedicated code is necessary to ensure that hospitalists are properly reimbursed for their work.
Historically, hospitalists have used codes more in line with the workload of general internal medicine, family medicine, and other specialties. But those codes don’t account for the complexity of what hospitalists deal with on a daily basis.
“We as hospitalists face unique challenges and work with patients from all demographics, often with severe illnesses, making it nearly impossible to rely on benchmarks used for these other specialties,” Dr. Greeno said.3
SHM pushed for the change and has said it is committed to educating members about how best to use it. Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians of Tacoma, Wash., said the move is great for HM but that more needs to be done to tailor billing and coding to the specialty’s actual workflow.
“A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears said. “I don’t think it’s an end-all, be-all, but it’s a place to start.”3
3. Down with SGR, Long Live MACRA
While it’s unlikely hospitalists have that printed on bumper stickers, 2016 brought the end of the oft-maligned Sustainable Growth Rate (SGR) formula. This was the first year under the Medicare Access and CHIP Reauthorization Act of 2015, better known as MACRA.4
MACRA changes the ways hospitalists and other physicians are reimbursed by Medicare by continuing the broader trend of moving from volume-based reimbursements toward a value-based payment (VBP) system. Its two tracks for physicians to change how they get paid are the Merit-Based Incentive Payment System (MIPS) and the Alternative Payments Model (APM).
MIPS probably looks familiar because it is similar to the traditional FFS model. But to make value a more important factor in the reimbursement formula, MIPS will account for both volume and quality. APM allows physicians to opt out of MIPS by participating in other approved programs where providers take on “more than nominal” financial risk, report on their quality measures, and use certified electronic health record (EHR) technology.
4. The Surgeon General Is a Hospitalist
Any specialty’s annual meeting would be lucky to get the U.S. Surgeon General as a plenary speaker, but HM16 featured one of its own in hospitalist and U.S. Surgeon General Vivek Murthy, MD, MBA.
Dr. Murthy kicked off the confab in San Diego, and some 4,000 hospitalists listened to one of their own talk about ascending the highest perch a physician can attain in the federal government.7 Dr. Murthy, previously a hospitalist at Brigham and Women’s Hospital in Boston, was confirmed as the 19th Surgeon General in December 2014.
In an address titled “Bringing Health to America,” Dr. Murthy inspired with tales of hope and encouraged hospitalists to make the pursuit of healthy appealing while improving the safety of our communities. In particular, he suggested hospitalists look to leverage their leadership to improve systems and to be a powerful force of change both inside the walls of their institution and in their communities.
“In the end, the world gets better when people choose to come together to make it better,” he said.8
5. Nurse Practitioner Joins SHM Board of Directors
At HM16, Tracy Cardin, ACNP-BC, SFHM, was the first nurse practitioner (NP) or physician assistant (PA) given voting privileges on the society’s oversight panel.
“I can’t describe to you how passionately I believe that [NPs and PAs] have a huge role moving forward,” Cardin said. “I think our representation, our visibility, has sort of been flabby and kind of under the wire for a long time. We can really impact the design of care models at the bedside in a way that’s innovative and more efficient and in a way that’s really huge. I think there’s a transformation that’s going to be coming, and we’re going to be a huge part of it.”9
With Cardin’s ascension, it has added the voice of another constituency to its board. She was previously chair of SHM’s Nurse Practitioner/Physician Assistant Committee and in 2015 received the society’s Award in Excellence in Hospital Medicine for NPs and PAs. She has been at the University of Chicago for about 10 years.
“It does send a message to the rest of our membership that SHM values those other constituencies and that this is not a physician membership organization but rather a membership organization comprised of people who are interested in improving healthcare for our hospitalized patients,” immediate past president Robert Harrington Jr., MD, SFHM said.10
6. The State of Hospital Medicine Is Strong
According to the biennial 2016 State of Hospital Medicine Report6 from SHM and partially populated by data from the Medical Group Management Association (MGMA), median compensation for adult hospitalists rose 10% to $278,746 from 2013 to 2015. The double-digit increase continues the steady climb of hospitalist pay, which is up 30% since 2010. At the same time, productivity is flattening.
And nearly all HM groups (HMGs)—96%—are still getting financial support, mostly from their host hospitals, in addition to their professional fee revenue. However, that median support, $157,535 per full-time employee (FTE), increased just 1%.
The slowing growth in that contribution is a harbinger that the level of financial support can no longer grow unabated, said Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee.
“We’re pretty close to that breaking point,” she said. “When we go around the country and do consulting work, we are hearing many more hospital leaders telling us, ‘We’re concerned about how much money this program is costing us, and we are getting to the point where we can’t afford it.’”6
7. Pay Cut for ‘Two-Midnight’ Rule for Observations Is No More
CMS announced a proposed rule in April that would have Medicare stop imposing an inpatient payment cut to hospitals under the “two-midnight” rule, according to a report in Modern Healthcare.10 The final rule went into effect in October.
The rule, which continues to be a contentious issue for hospitalists even with the pay cut being eliminated, was put in place in October 2013 to define which Medicare beneficiary hospital stays are appropriate for Medicare Part A payment by stating that if the physician expected the patient to stay for fewer than two midnights, then the services should be billed as outpatient (Medicare Part B) and not inpatient.
For two years, the only exception to the pay withhold was for those diagnoses that CMS designated as “inpatient only.” An overhaul of the rule in October 2015 stated that exceptions could be determined by the physician (or other practitioner) on a “case-by-case basis.”
But a legal challenge and withering criticism from SHM and other professional organizations prompted CMS to undo the withheld payments associated rule.11–13 In fact, hospitals also will see a one-time increase of 0.6% in fiscal 2017, making up for the 0.2% reduction to the rates of the last three years. SHM has said it will continue to advocate for further changes to the rule.
8. Medicaid Expansion Takes Hold
Thirty-one states and the District of Columbia, under the auspices of the Affordable Care Act, have expanded Medicaid. One 2014 study in states including Minnesota, Kentucky, and Arizona showed a dramatic decrease in uninsured hospital stays and a significant increase in Medicaid stays. Meanwhile, in six states that did not expand, including Florida, Georgia, and Missouri, there was no significant change in payor mix.14
“What a lot of these early studies are saying is that when you expand Medicaid, people get on Medicaid, and that’s exactly what you hope will happen when you do a major public coverage expansion,” said Sayeh Nikpay, PhD, MPH, assistant professor of health policy at Vanderbilt University School of Medicine in Nashville, Tenn., and a lead author of the 2014 Health Affairs study. “Physicians are grappling with payment issues, and it should be quite a relief that people are coming in the door with some kind of insurance rather than uninsured.”15
While expansion from 2015 to 2016 was the main story for Medicaid, many observers are now watching to see what President-elect Donald Trump will do. As a candidate, he campaigned to repeal Obamacare, and that may have significant impacts on the expansion of Medicaid.
9. Antimicrobial Stewardship Rules Upgrade
In July, The Joint Commission announced a new Medication Management (MM) standard for hospitals, critical-access hospitals, and nursing care centers that goes into effect in 2017.16,17 Its stated purpose is to improve quality and patient safety. The Joint Commission move came a year after President Barack Obama made combatting antibiotic-resistant bacteria a national issue.
“If you review the scientific literature, it will indicate that we’re in crisis mode right now because of this,” said Kelly Podgorny, DNP, MS, CPHQ, RN, project director at The Joint Commission. 16
The standard encourages the prioritization of establishing antimicrobial stewardship programs. It suggests staff members at institutions receive training and education on dispensing, administering, and monitoring antimicrobial resistance and antimicrobial stewardship practice. And, in turn, those staffers should look to educate patients and caregivers on appropriate use of antimicrobial medications.
Lastly, when possible, an antimicrobial team should take a multidisciplinary approach and include an infectious disease physician, a pharmacist, and a practitioner.
10. Febrile-Infant Care Draws a Crowd
One of the most well-attended sessions at 2016 Pediatric Hospital Medicine in Chicago was an update on the anticipated American Academy of Pediatricians (AAP) guidelines for febrile infants.18
The guidelines, for infants 7–90 days old, are aimed at providing pediatric hospitalists and others evidence-based guidelines, not rules, from the most recent literature available. The PHM16 session, presented by Kenneth Roberts, MD, highlighted the need to separate individual components of serious bacterial infections such as urinary tract infections (UTIs), bacteremia, and meningitis. Dr. Roberts noted that the incidence and clinical course can vary greatly for the different diagnoses.
The new criteria would be for full-term infants (37–43 weeks’ gestation) who are well-appearing and present with a temperature of 38°C. Exclusion criteria would include perinatal/prenatal/neonatal maternal fever, infection, or antimicrobial treatment; the presence of any evident infection; being technology-dependent; and the presence of congenital anomalies.
In particular, the new guidelines will look to risk-stratify management by age groups of 7–28 days, 29–60 days, and 61–90 days. The criteria are expected to be released in the next year.
Richard Quinn is a freelance writer in New Jersey.
References
- Quinn R. HM turns 20: a look at the evolution of hospitalist medicine. The Hospitalist website. Accessed November 14, 2016.
- Wachter M, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-517.
- Tyrrell KA. New hospitalist billing code should benefit hospitalists, patients. The Hospitalist website. Accessed November 14, 2016.
- Doctoroff L, Dutta S. MACRA provides new direction for U.S. healthcare. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. Everything you need to know about the Bundled Payments for Care Improvement Initiative. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. The state of hospital medicine is strong. The Hospitalist website. Accessed November 14, 2016.
- Scheurer D. U.S. surgeon general encourages hospitalists to remain hopeful, motivated. The Hospitalist website. Accessed November 14, 2016.
- Collins TR. HM16 speakers focus on public health, leadership, future of hospital medicine. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. SHM seats its first non-physician board member. The Hospitalist website. Accessed at November 14, 2016.
- Carris J. Centers for Medicare & Medicaid Services (CMS) eliminates two-midnight rule’s inpatient payment cuts: report. The Hospitalist website. Accessed November 14, 2016.
- Schencker L. Judge tells HHS to revisit two-midnight rule’s inpatient pay cut. Healh Affairs website. Accessed November 14, 2016.
- Tyrrell KA. Physicians critical of proposed changes to Medicare’s two-midnight rule. The Hospitalist website. Accessed November 14, 2016.
- Tyrrell KA. Ann Sheehy, MD, MS, FHM, outlines to lawmakers hospitalist concerns about two-midnight rule, Medicare policies. The Hospitalist website. Accessed November 14, 2016.
- Nikpay S, Buchmueller T, Levy HG. Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106-110.
- Tyrrell KA. Benefits of Medicaid expansion for hospitalists. The Hospitalist website. Accessed November 14, 2016.
- Bopp S. New standard announced for antimicrobial stewardship. The Hospitalist website. Accessed November 14, 2016.
- Prepublication standards – new antimicrobial stewardship standard. The Joint Commission website. Accessed November 14, 2016.
- 18. DeZure C. PHM16: the new AAP clinical practice guideline on evaluating, managing febrile infants. The Hospitalist website. Accessed November 14, 2016.
From celebrating the 20th anniversary of hospital medicine’s arrival to rapid reimbursement reform to sought-after clarity for observation rules, the past 12 months have been eventful. The Hospitalist asked more than a dozen industry leaders what they thought were the biggest highlights of 2016. Here is what they said:
1. Happy Birthday, HM
August 15 marked the 20th anniversary of “The Emerging Role of ‘Hospitalists’ in the American Health Care System,”1 a New England Journal of Medicine paper authored by Lee Goldman, MD, and Robert Wachter, MD, MHM.2 It was the first time anyone defined what a hospitalist was, putting a sobriquet to an inpatient care model that had organically and independently started happening nationwide.
The paper laid the groundwork for a specialty that spread like a proverbial wildfire over the next 20 years. The model overcame early opposition from residents fearful they’d lose the ability to learn and critical-care specialists who wanted to maintain the caseloads they’d fought to build. The field has now swelled to an estimated 52,000 practitioners.
“I reflect back … and think today about what the hospitalist model brings to us,” James Merlino, MD, president and chief medical officer of Press Ganey’s strategic consulting division, told The Hospitalist. “It is an amazing transformation on how the hospitalist model really delivers.”1
2. Its Own Specialty Code
Hospitalist medicine received federal approval for its first dedicated specialty code3 in a decision hailed by many. The code, approved by the Centers for Medicare & Medicaid Services (CMS), should allow hospitalist-specific billing in 2017.
SHM leaders have long pushed for the code, particularly as healthcare transitions from fee-for-service (FFS) to quality-based payment models. Public Policy Committee Chair Ron Greeno, MD, MHM, says that a dedicated code is necessary to ensure that hospitalists are properly reimbursed for their work.
Historically, hospitalists have used codes more in line with the workload of general internal medicine, family medicine, and other specialties. But those codes don’t account for the complexity of what hospitalists deal with on a daily basis.
“We as hospitalists face unique challenges and work with patients from all demographics, often with severe illnesses, making it nearly impossible to rely on benchmarks used for these other specialties,” Dr. Greeno said.3
SHM pushed for the change and has said it is committed to educating members about how best to use it. Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians of Tacoma, Wash., said the move is great for HM but that more needs to be done to tailor billing and coding to the specialty’s actual workflow.
“A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears said. “I don’t think it’s an end-all, be-all, but it’s a place to start.”3
3. Down with SGR, Long Live MACRA
While it’s unlikely hospitalists have that printed on bumper stickers, 2016 brought the end of the oft-maligned Sustainable Growth Rate (SGR) formula. This was the first year under the Medicare Access and CHIP Reauthorization Act of 2015, better known as MACRA.4
MACRA changes the ways hospitalists and other physicians are reimbursed by Medicare by continuing the broader trend of moving from volume-based reimbursements toward a value-based payment (VBP) system. Its two tracks for physicians to change how they get paid are the Merit-Based Incentive Payment System (MIPS) and the Alternative Payments Model (APM).
MIPS probably looks familiar because it is similar to the traditional FFS model. But to make value a more important factor in the reimbursement formula, MIPS will account for both volume and quality. APM allows physicians to opt out of MIPS by participating in other approved programs where providers take on “more than nominal” financial risk, report on their quality measures, and use certified electronic health record (EHR) technology.
4. The Surgeon General Is a Hospitalist
Any specialty’s annual meeting would be lucky to get the U.S. Surgeon General as a plenary speaker, but HM16 featured one of its own in hospitalist and U.S. Surgeon General Vivek Murthy, MD, MBA.
Dr. Murthy kicked off the confab in San Diego, and some 4,000 hospitalists listened to one of their own talk about ascending the highest perch a physician can attain in the federal government.7 Dr. Murthy, previously a hospitalist at Brigham and Women’s Hospital in Boston, was confirmed as the 19th Surgeon General in December 2014.
In an address titled “Bringing Health to America,” Dr. Murthy inspired with tales of hope and encouraged hospitalists to make the pursuit of healthy appealing while improving the safety of our communities. In particular, he suggested hospitalists look to leverage their leadership to improve systems and to be a powerful force of change both inside the walls of their institution and in their communities.
“In the end, the world gets better when people choose to come together to make it better,” he said.8
5. Nurse Practitioner Joins SHM Board of Directors
At HM16, Tracy Cardin, ACNP-BC, SFHM, was the first nurse practitioner (NP) or physician assistant (PA) given voting privileges on the society’s oversight panel.
“I can’t describe to you how passionately I believe that [NPs and PAs] have a huge role moving forward,” Cardin said. “I think our representation, our visibility, has sort of been flabby and kind of under the wire for a long time. We can really impact the design of care models at the bedside in a way that’s innovative and more efficient and in a way that’s really huge. I think there’s a transformation that’s going to be coming, and we’re going to be a huge part of it.”9
With Cardin’s ascension, it has added the voice of another constituency to its board. She was previously chair of SHM’s Nurse Practitioner/Physician Assistant Committee and in 2015 received the society’s Award in Excellence in Hospital Medicine for NPs and PAs. She has been at the University of Chicago for about 10 years.
“It does send a message to the rest of our membership that SHM values those other constituencies and that this is not a physician membership organization but rather a membership organization comprised of people who are interested in improving healthcare for our hospitalized patients,” immediate past president Robert Harrington Jr., MD, SFHM said.10
6. The State of Hospital Medicine Is Strong
According to the biennial 2016 State of Hospital Medicine Report6 from SHM and partially populated by data from the Medical Group Management Association (MGMA), median compensation for adult hospitalists rose 10% to $278,746 from 2013 to 2015. The double-digit increase continues the steady climb of hospitalist pay, which is up 30% since 2010. At the same time, productivity is flattening.
And nearly all HM groups (HMGs)—96%—are still getting financial support, mostly from their host hospitals, in addition to their professional fee revenue. However, that median support, $157,535 per full-time employee (FTE), increased just 1%.
The slowing growth in that contribution is a harbinger that the level of financial support can no longer grow unabated, said Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee.
“We’re pretty close to that breaking point,” she said. “When we go around the country and do consulting work, we are hearing many more hospital leaders telling us, ‘We’re concerned about how much money this program is costing us, and we are getting to the point where we can’t afford it.’”6
7. Pay Cut for ‘Two-Midnight’ Rule for Observations Is No More
CMS announced a proposed rule in April that would have Medicare stop imposing an inpatient payment cut to hospitals under the “two-midnight” rule, according to a report in Modern Healthcare.10 The final rule went into effect in October.
The rule, which continues to be a contentious issue for hospitalists even with the pay cut being eliminated, was put in place in October 2013 to define which Medicare beneficiary hospital stays are appropriate for Medicare Part A payment by stating that if the physician expected the patient to stay for fewer than two midnights, then the services should be billed as outpatient (Medicare Part B) and not inpatient.
For two years, the only exception to the pay withhold was for those diagnoses that CMS designated as “inpatient only.” An overhaul of the rule in October 2015 stated that exceptions could be determined by the physician (or other practitioner) on a “case-by-case basis.”
But a legal challenge and withering criticism from SHM and other professional organizations prompted CMS to undo the withheld payments associated rule.11–13 In fact, hospitals also will see a one-time increase of 0.6% in fiscal 2017, making up for the 0.2% reduction to the rates of the last three years. SHM has said it will continue to advocate for further changes to the rule.
8. Medicaid Expansion Takes Hold
Thirty-one states and the District of Columbia, under the auspices of the Affordable Care Act, have expanded Medicaid. One 2014 study in states including Minnesota, Kentucky, and Arizona showed a dramatic decrease in uninsured hospital stays and a significant increase in Medicaid stays. Meanwhile, in six states that did not expand, including Florida, Georgia, and Missouri, there was no significant change in payor mix.14
“What a lot of these early studies are saying is that when you expand Medicaid, people get on Medicaid, and that’s exactly what you hope will happen when you do a major public coverage expansion,” said Sayeh Nikpay, PhD, MPH, assistant professor of health policy at Vanderbilt University School of Medicine in Nashville, Tenn., and a lead author of the 2014 Health Affairs study. “Physicians are grappling with payment issues, and it should be quite a relief that people are coming in the door with some kind of insurance rather than uninsured.”15
While expansion from 2015 to 2016 was the main story for Medicaid, many observers are now watching to see what President-elect Donald Trump will do. As a candidate, he campaigned to repeal Obamacare, and that may have significant impacts on the expansion of Medicaid.
9. Antimicrobial Stewardship Rules Upgrade
In July, The Joint Commission announced a new Medication Management (MM) standard for hospitals, critical-access hospitals, and nursing care centers that goes into effect in 2017.16,17 Its stated purpose is to improve quality and patient safety. The Joint Commission move came a year after President Barack Obama made combatting antibiotic-resistant bacteria a national issue.
“If you review the scientific literature, it will indicate that we’re in crisis mode right now because of this,” said Kelly Podgorny, DNP, MS, CPHQ, RN, project director at The Joint Commission. 16
The standard encourages the prioritization of establishing antimicrobial stewardship programs. It suggests staff members at institutions receive training and education on dispensing, administering, and monitoring antimicrobial resistance and antimicrobial stewardship practice. And, in turn, those staffers should look to educate patients and caregivers on appropriate use of antimicrobial medications.
Lastly, when possible, an antimicrobial team should take a multidisciplinary approach and include an infectious disease physician, a pharmacist, and a practitioner.
10. Febrile-Infant Care Draws a Crowd
One of the most well-attended sessions at 2016 Pediatric Hospital Medicine in Chicago was an update on the anticipated American Academy of Pediatricians (AAP) guidelines for febrile infants.18
The guidelines, for infants 7–90 days old, are aimed at providing pediatric hospitalists and others evidence-based guidelines, not rules, from the most recent literature available. The PHM16 session, presented by Kenneth Roberts, MD, highlighted the need to separate individual components of serious bacterial infections such as urinary tract infections (UTIs), bacteremia, and meningitis. Dr. Roberts noted that the incidence and clinical course can vary greatly for the different diagnoses.
The new criteria would be for full-term infants (37–43 weeks’ gestation) who are well-appearing and present with a temperature of 38°C. Exclusion criteria would include perinatal/prenatal/neonatal maternal fever, infection, or antimicrobial treatment; the presence of any evident infection; being technology-dependent; and the presence of congenital anomalies.
In particular, the new guidelines will look to risk-stratify management by age groups of 7–28 days, 29–60 days, and 61–90 days. The criteria are expected to be released in the next year.
Richard Quinn is a freelance writer in New Jersey.
References
- Quinn R. HM turns 20: a look at the evolution of hospitalist medicine. The Hospitalist website. Accessed November 14, 2016.
- Wachter M, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-517.
- Tyrrell KA. New hospitalist billing code should benefit hospitalists, patients. The Hospitalist website. Accessed November 14, 2016.
- Doctoroff L, Dutta S. MACRA provides new direction for U.S. healthcare. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. Everything you need to know about the Bundled Payments for Care Improvement Initiative. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. The state of hospital medicine is strong. The Hospitalist website. Accessed November 14, 2016.
- Scheurer D. U.S. surgeon general encourages hospitalists to remain hopeful, motivated. The Hospitalist website. Accessed November 14, 2016.
- Collins TR. HM16 speakers focus on public health, leadership, future of hospital medicine. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. SHM seats its first non-physician board member. The Hospitalist website. Accessed at November 14, 2016.
- Carris J. Centers for Medicare & Medicaid Services (CMS) eliminates two-midnight rule’s inpatient payment cuts: report. The Hospitalist website. Accessed November 14, 2016.
- Schencker L. Judge tells HHS to revisit two-midnight rule’s inpatient pay cut. Healh Affairs website. Accessed November 14, 2016.
- Tyrrell KA. Physicians critical of proposed changes to Medicare’s two-midnight rule. The Hospitalist website. Accessed November 14, 2016.
- Tyrrell KA. Ann Sheehy, MD, MS, FHM, outlines to lawmakers hospitalist concerns about two-midnight rule, Medicare policies. The Hospitalist website. Accessed November 14, 2016.
- Nikpay S, Buchmueller T, Levy HG. Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106-110.
- Tyrrell KA. Benefits of Medicaid expansion for hospitalists. The Hospitalist website. Accessed November 14, 2016.
- Bopp S. New standard announced for antimicrobial stewardship. The Hospitalist website. Accessed November 14, 2016.
- Prepublication standards – new antimicrobial stewardship standard. The Joint Commission website. Accessed November 14, 2016.
- 18. DeZure C. PHM16: the new AAP clinical practice guideline on evaluating, managing febrile infants. The Hospitalist website. Accessed November 14, 2016.
From celebrating the 20th anniversary of hospital medicine’s arrival to rapid reimbursement reform to sought-after clarity for observation rules, the past 12 months have been eventful. The Hospitalist asked more than a dozen industry leaders what they thought were the biggest highlights of 2016. Here is what they said:
1. Happy Birthday, HM
August 15 marked the 20th anniversary of “The Emerging Role of ‘Hospitalists’ in the American Health Care System,”1 a New England Journal of Medicine paper authored by Lee Goldman, MD, and Robert Wachter, MD, MHM.2 It was the first time anyone defined what a hospitalist was, putting a sobriquet to an inpatient care model that had organically and independently started happening nationwide.
The paper laid the groundwork for a specialty that spread like a proverbial wildfire over the next 20 years. The model overcame early opposition from residents fearful they’d lose the ability to learn and critical-care specialists who wanted to maintain the caseloads they’d fought to build. The field has now swelled to an estimated 52,000 practitioners.
“I reflect back … and think today about what the hospitalist model brings to us,” James Merlino, MD, president and chief medical officer of Press Ganey’s strategic consulting division, told The Hospitalist. “It is an amazing transformation on how the hospitalist model really delivers.”1
2. Its Own Specialty Code
Hospitalist medicine received federal approval for its first dedicated specialty code3 in a decision hailed by many. The code, approved by the Centers for Medicare & Medicaid Services (CMS), should allow hospitalist-specific billing in 2017.
SHM leaders have long pushed for the code, particularly as healthcare transitions from fee-for-service (FFS) to quality-based payment models. Public Policy Committee Chair Ron Greeno, MD, MHM, says that a dedicated code is necessary to ensure that hospitalists are properly reimbursed for their work.
Historically, hospitalists have used codes more in line with the workload of general internal medicine, family medicine, and other specialties. But those codes don’t account for the complexity of what hospitalists deal with on a daily basis.
“We as hospitalists face unique challenges and work with patients from all demographics, often with severe illnesses, making it nearly impossible to rely on benchmarks used for these other specialties,” Dr. Greeno said.3
SHM pushed for the change and has said it is committed to educating members about how best to use it. Scott Sears, MD, FHM, CPE, MBA, chief clinical officer for Sound Physicians of Tacoma, Wash., said the move is great for HM but that more needs to be done to tailor billing and coding to the specialty’s actual workflow.
“A pediatric hospitalist may not want to be compared to an adult hospitalist. A critical-access hospitalist doesn’t want to be compared to a hospitalist in a tertiary academic medical center,” Dr. Sears said. “I don’t think it’s an end-all, be-all, but it’s a place to start.”3
3. Down with SGR, Long Live MACRA
While it’s unlikely hospitalists have that printed on bumper stickers, 2016 brought the end of the oft-maligned Sustainable Growth Rate (SGR) formula. This was the first year under the Medicare Access and CHIP Reauthorization Act of 2015, better known as MACRA.4
MACRA changes the ways hospitalists and other physicians are reimbursed by Medicare by continuing the broader trend of moving from volume-based reimbursements toward a value-based payment (VBP) system. Its two tracks for physicians to change how they get paid are the Merit-Based Incentive Payment System (MIPS) and the Alternative Payments Model (APM).
MIPS probably looks familiar because it is similar to the traditional FFS model. But to make value a more important factor in the reimbursement formula, MIPS will account for both volume and quality. APM allows physicians to opt out of MIPS by participating in other approved programs where providers take on “more than nominal” financial risk, report on their quality measures, and use certified electronic health record (EHR) technology.
4. The Surgeon General Is a Hospitalist
Any specialty’s annual meeting would be lucky to get the U.S. Surgeon General as a plenary speaker, but HM16 featured one of its own in hospitalist and U.S. Surgeon General Vivek Murthy, MD, MBA.
Dr. Murthy kicked off the confab in San Diego, and some 4,000 hospitalists listened to one of their own talk about ascending the highest perch a physician can attain in the federal government.7 Dr. Murthy, previously a hospitalist at Brigham and Women’s Hospital in Boston, was confirmed as the 19th Surgeon General in December 2014.
In an address titled “Bringing Health to America,” Dr. Murthy inspired with tales of hope and encouraged hospitalists to make the pursuit of healthy appealing while improving the safety of our communities. In particular, he suggested hospitalists look to leverage their leadership to improve systems and to be a powerful force of change both inside the walls of their institution and in their communities.
“In the end, the world gets better when people choose to come together to make it better,” he said.8
5. Nurse Practitioner Joins SHM Board of Directors
At HM16, Tracy Cardin, ACNP-BC, SFHM, was the first nurse practitioner (NP) or physician assistant (PA) given voting privileges on the society’s oversight panel.
“I can’t describe to you how passionately I believe that [NPs and PAs] have a huge role moving forward,” Cardin said. “I think our representation, our visibility, has sort of been flabby and kind of under the wire for a long time. We can really impact the design of care models at the bedside in a way that’s innovative and more efficient and in a way that’s really huge. I think there’s a transformation that’s going to be coming, and we’re going to be a huge part of it.”9
With Cardin’s ascension, it has added the voice of another constituency to its board. She was previously chair of SHM’s Nurse Practitioner/Physician Assistant Committee and in 2015 received the society’s Award in Excellence in Hospital Medicine for NPs and PAs. She has been at the University of Chicago for about 10 years.
“It does send a message to the rest of our membership that SHM values those other constituencies and that this is not a physician membership organization but rather a membership organization comprised of people who are interested in improving healthcare for our hospitalized patients,” immediate past president Robert Harrington Jr., MD, SFHM said.10
6. The State of Hospital Medicine Is Strong
According to the biennial 2016 State of Hospital Medicine Report6 from SHM and partially populated by data from the Medical Group Management Association (MGMA), median compensation for adult hospitalists rose 10% to $278,746 from 2013 to 2015. The double-digit increase continues the steady climb of hospitalist pay, which is up 30% since 2010. At the same time, productivity is flattening.
And nearly all HM groups (HMGs)—96%—are still getting financial support, mostly from their host hospitals, in addition to their professional fee revenue. However, that median support, $157,535 per full-time employee (FTE), increased just 1%.
The slowing growth in that contribution is a harbinger that the level of financial support can no longer grow unabated, said Leslie Flores, MHA, a partner in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee.
“We’re pretty close to that breaking point,” she said. “When we go around the country and do consulting work, we are hearing many more hospital leaders telling us, ‘We’re concerned about how much money this program is costing us, and we are getting to the point where we can’t afford it.’”6
7. Pay Cut for ‘Two-Midnight’ Rule for Observations Is No More
CMS announced a proposed rule in April that would have Medicare stop imposing an inpatient payment cut to hospitals under the “two-midnight” rule, according to a report in Modern Healthcare.10 The final rule went into effect in October.
The rule, which continues to be a contentious issue for hospitalists even with the pay cut being eliminated, was put in place in October 2013 to define which Medicare beneficiary hospital stays are appropriate for Medicare Part A payment by stating that if the physician expected the patient to stay for fewer than two midnights, then the services should be billed as outpatient (Medicare Part B) and not inpatient.
For two years, the only exception to the pay withhold was for those diagnoses that CMS designated as “inpatient only.” An overhaul of the rule in October 2015 stated that exceptions could be determined by the physician (or other practitioner) on a “case-by-case basis.”
But a legal challenge and withering criticism from SHM and other professional organizations prompted CMS to undo the withheld payments associated rule.11–13 In fact, hospitals also will see a one-time increase of 0.6% in fiscal 2017, making up for the 0.2% reduction to the rates of the last three years. SHM has said it will continue to advocate for further changes to the rule.
8. Medicaid Expansion Takes Hold
Thirty-one states and the District of Columbia, under the auspices of the Affordable Care Act, have expanded Medicaid. One 2014 study in states including Minnesota, Kentucky, and Arizona showed a dramatic decrease in uninsured hospital stays and a significant increase in Medicaid stays. Meanwhile, in six states that did not expand, including Florida, Georgia, and Missouri, there was no significant change in payor mix.14
“What a lot of these early studies are saying is that when you expand Medicaid, people get on Medicaid, and that’s exactly what you hope will happen when you do a major public coverage expansion,” said Sayeh Nikpay, PhD, MPH, assistant professor of health policy at Vanderbilt University School of Medicine in Nashville, Tenn., and a lead author of the 2014 Health Affairs study. “Physicians are grappling with payment issues, and it should be quite a relief that people are coming in the door with some kind of insurance rather than uninsured.”15
While expansion from 2015 to 2016 was the main story for Medicaid, many observers are now watching to see what President-elect Donald Trump will do. As a candidate, he campaigned to repeal Obamacare, and that may have significant impacts on the expansion of Medicaid.
9. Antimicrobial Stewardship Rules Upgrade
In July, The Joint Commission announced a new Medication Management (MM) standard for hospitals, critical-access hospitals, and nursing care centers that goes into effect in 2017.16,17 Its stated purpose is to improve quality and patient safety. The Joint Commission move came a year after President Barack Obama made combatting antibiotic-resistant bacteria a national issue.
“If you review the scientific literature, it will indicate that we’re in crisis mode right now because of this,” said Kelly Podgorny, DNP, MS, CPHQ, RN, project director at The Joint Commission. 16
The standard encourages the prioritization of establishing antimicrobial stewardship programs. It suggests staff members at institutions receive training and education on dispensing, administering, and monitoring antimicrobial resistance and antimicrobial stewardship practice. And, in turn, those staffers should look to educate patients and caregivers on appropriate use of antimicrobial medications.
Lastly, when possible, an antimicrobial team should take a multidisciplinary approach and include an infectious disease physician, a pharmacist, and a practitioner.
10. Febrile-Infant Care Draws a Crowd
One of the most well-attended sessions at 2016 Pediatric Hospital Medicine in Chicago was an update on the anticipated American Academy of Pediatricians (AAP) guidelines for febrile infants.18
The guidelines, for infants 7–90 days old, are aimed at providing pediatric hospitalists and others evidence-based guidelines, not rules, from the most recent literature available. The PHM16 session, presented by Kenneth Roberts, MD, highlighted the need to separate individual components of serious bacterial infections such as urinary tract infections (UTIs), bacteremia, and meningitis. Dr. Roberts noted that the incidence and clinical course can vary greatly for the different diagnoses.
The new criteria would be for full-term infants (37–43 weeks’ gestation) who are well-appearing and present with a temperature of 38°C. Exclusion criteria would include perinatal/prenatal/neonatal maternal fever, infection, or antimicrobial treatment; the presence of any evident infection; being technology-dependent; and the presence of congenital anomalies.
In particular, the new guidelines will look to risk-stratify management by age groups of 7–28 days, 29–60 days, and 61–90 days. The criteria are expected to be released in the next year.
Richard Quinn is a freelance writer in New Jersey.
References
- Quinn R. HM turns 20: a look at the evolution of hospitalist medicine. The Hospitalist website. Accessed November 14, 2016.
- Wachter M, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-517.
- Tyrrell KA. New hospitalist billing code should benefit hospitalists, patients. The Hospitalist website. Accessed November 14, 2016.
- Doctoroff L, Dutta S. MACRA provides new direction for U.S. healthcare. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. Everything you need to know about the Bundled Payments for Care Improvement Initiative. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. The state of hospital medicine is strong. The Hospitalist website. Accessed November 14, 2016.
- Scheurer D. U.S. surgeon general encourages hospitalists to remain hopeful, motivated. The Hospitalist website. Accessed November 14, 2016.
- Collins TR. HM16 speakers focus on public health, leadership, future of hospital medicine. The Hospitalist website. Accessed November 14, 2016.
- Quinn R. SHM seats its first non-physician board member. The Hospitalist website. Accessed at November 14, 2016.
- Carris J. Centers for Medicare & Medicaid Services (CMS) eliminates two-midnight rule’s inpatient payment cuts: report. The Hospitalist website. Accessed November 14, 2016.
- Schencker L. Judge tells HHS to revisit two-midnight rule’s inpatient pay cut. Healh Affairs website. Accessed November 14, 2016.
- Tyrrell KA. Physicians critical of proposed changes to Medicare’s two-midnight rule. The Hospitalist website. Accessed November 14, 2016.
- Tyrrell KA. Ann Sheehy, MD, MS, FHM, outlines to lawmakers hospitalist concerns about two-midnight rule, Medicare policies. The Hospitalist website. Accessed November 14, 2016.
- Nikpay S, Buchmueller T, Levy HG. Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106-110.
- Tyrrell KA. Benefits of Medicaid expansion for hospitalists. The Hospitalist website. Accessed November 14, 2016.
- Bopp S. New standard announced for antimicrobial stewardship. The Hospitalist website. Accessed November 14, 2016.
- Prepublication standards – new antimicrobial stewardship standard. The Joint Commission website. Accessed November 14, 2016.
- 18. DeZure C. PHM16: the new AAP clinical practice guideline on evaluating, managing febrile infants. The Hospitalist website. Accessed November 14, 2016.
Diabetes treatment costs doubled in Sweden since 2006
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
[email protected]
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
[email protected]
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
[email protected]
On Twitter @Alz_Gal
AT EASD 2016
Key clinical point:
Major finding: Treatment costs jumped from €608 million in 2006 to €1.27 billion in 2014.
Data source: The 8-year study used national health care data.
Disclosures: The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
Sutureless aortic valve replacement: Is ease worth the cost?
CHICAGO – Rapid deployment sutureless valves can be a good option for some patients, providing a highly functional and nearly leakproof valve with less cardiopulmonary bypass and aortic cross-clamp times than those of conventional procedures.
“Why use a sutureless valve?” asked Vinod H. Thourani, MD, speaking at Heart Valve Summit 2016. He said that for many patients, there are abundant good reasons for the choice. The rapidity of the implantation procedure is a huge plus, he said. Cardiopulmonary bypass times are reduced when sutureless valve replacement is a stand-alone procedure, added Dr. Thourani, chief of cardiovascular surgery at Emory Hospital Midtown and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
Rapid deployment is also of benefit in combined cases, or when patients have multiple comorbidities or poor left ventricular function. Sutureless valves, he said, are “optimal for multiple valve or concomitant procedures.”
Hemodynamics also are favorable, said Dr. Thourani; sutureless valves produce lower gradients than do their sutured alternatives, and work well in patients with a small aortic root.
Both sutureless valves that are currently available use bovine tissue; one, Sorin’s Perceval, uses a nitinol stent, while the Edwards’ Intuity uses stainless steel. The Perceval stent requires no sutures, while the Intuity requires just three. Also, the Perceval is collapsible, while the Intuity is not.
Removal of the pathologic valve in the sutureless procedure, he said, may contribute to the lower paravalvular leak and stroke rates than are seen in transcatheter aortic valve replacement (TAVR).
Expanded indications for sutureless valves include a calcified aortic root or a homograft; sutureless valves also can be used as an aortic valve redo, with patent grafts. Dr. Thourani said that he favors a transverse incision with a high aortotomy, about 2 cm above the sinotubular junction (STJ). In addition, off-label indications have included bicuspid aortic valve, pure aortic insufficiency, a prior mitral prosthesis or a degenerated aortic bioprosthesis, and a rescue procedure for a failed TAVR.
Dr. Thourani cited results of a trial conducted by Theodor Fischlein, MD, of Paracelsus Medical University in Nuremberg, Germany, and coauthors. These 1-year follow-up data from 628 patients participating in CAVALIER (Perceval S Valve Clinical Trial for Extended CE Mark), an international multicenter prospective trial, were presented at AATS 2016 (J Thorac Cardiovasc Surg. 2016 Jun;51[6]:1617-26.e4).
Of the 658 patients who met enrollment criteria and had a Perceval valve placement attempted, 30 wound up with a different prosthesis, most often because the correct valve size was not available. The remaining 628 patients who received the Perceval valve were included in the study. At 1 year, 549 patients remained; 50 had died, 12 had undergone valve explantation, and the remainder withdrew or were lost to follow-up.
Of the original Perceval recipients, 219 had received their valve via minimally invasive access. At 1 year, effective orifice area remained stable at the same mean 1.5 cm2 that was seen at discharge, an improvement from the mean 0.7 cm2 effective orifice area seen preoperatively. The mean pressure gradient, which was 45 mm Hg preoperatively, dropped precipitously to 10.3 mm Hg at discharge, and dropped a bit more at 1 year, to 9.2 mm Hg.
“This is a rapid and reproducible procedure: Over 20,000 implants have been performed worldwide,” said Dr. Thourani. The procedure looks good for low- to medium-risk patients, and may be the first procedure to consider for patients with a small aortic root, who have had prior coronary artery bypass surgery with patent grafts, or those with a calcified aortic root and homografts.
Questions still to be answered, he said, include whether “the cost will justify the decrease in cross-clamp times.” Also, though midrange results are good, longitudinal follow-up to track long-term valve hemodynamics is still ongoing.
Although patient demand seems to be high for a minimally invasive approach, sutureless valves still have low adoption rates, he said.
Dr. Thourani reported multiple financial relationships with medical device companies.
[email protected]
On Twitter @karioakes
CHICAGO – Rapid deployment sutureless valves can be a good option for some patients, providing a highly functional and nearly leakproof valve with less cardiopulmonary bypass and aortic cross-clamp times than those of conventional procedures.
“Why use a sutureless valve?” asked Vinod H. Thourani, MD, speaking at Heart Valve Summit 2016. He said that for many patients, there are abundant good reasons for the choice. The rapidity of the implantation procedure is a huge plus, he said. Cardiopulmonary bypass times are reduced when sutureless valve replacement is a stand-alone procedure, added Dr. Thourani, chief of cardiovascular surgery at Emory Hospital Midtown and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
Rapid deployment is also of benefit in combined cases, or when patients have multiple comorbidities or poor left ventricular function. Sutureless valves, he said, are “optimal for multiple valve or concomitant procedures.”
Hemodynamics also are favorable, said Dr. Thourani; sutureless valves produce lower gradients than do their sutured alternatives, and work well in patients with a small aortic root.
Both sutureless valves that are currently available use bovine tissue; one, Sorin’s Perceval, uses a nitinol stent, while the Edwards’ Intuity uses stainless steel. The Perceval stent requires no sutures, while the Intuity requires just three. Also, the Perceval is collapsible, while the Intuity is not.
Removal of the pathologic valve in the sutureless procedure, he said, may contribute to the lower paravalvular leak and stroke rates than are seen in transcatheter aortic valve replacement (TAVR).
Expanded indications for sutureless valves include a calcified aortic root or a homograft; sutureless valves also can be used as an aortic valve redo, with patent grafts. Dr. Thourani said that he favors a transverse incision with a high aortotomy, about 2 cm above the sinotubular junction (STJ). In addition, off-label indications have included bicuspid aortic valve, pure aortic insufficiency, a prior mitral prosthesis or a degenerated aortic bioprosthesis, and a rescue procedure for a failed TAVR.
Dr. Thourani cited results of a trial conducted by Theodor Fischlein, MD, of Paracelsus Medical University in Nuremberg, Germany, and coauthors. These 1-year follow-up data from 628 patients participating in CAVALIER (Perceval S Valve Clinical Trial for Extended CE Mark), an international multicenter prospective trial, were presented at AATS 2016 (J Thorac Cardiovasc Surg. 2016 Jun;51[6]:1617-26.e4).
Of the 658 patients who met enrollment criteria and had a Perceval valve placement attempted, 30 wound up with a different prosthesis, most often because the correct valve size was not available. The remaining 628 patients who received the Perceval valve were included in the study. At 1 year, 549 patients remained; 50 had died, 12 had undergone valve explantation, and the remainder withdrew or were lost to follow-up.
Of the original Perceval recipients, 219 had received their valve via minimally invasive access. At 1 year, effective orifice area remained stable at the same mean 1.5 cm2 that was seen at discharge, an improvement from the mean 0.7 cm2 effective orifice area seen preoperatively. The mean pressure gradient, which was 45 mm Hg preoperatively, dropped precipitously to 10.3 mm Hg at discharge, and dropped a bit more at 1 year, to 9.2 mm Hg.
“This is a rapid and reproducible procedure: Over 20,000 implants have been performed worldwide,” said Dr. Thourani. The procedure looks good for low- to medium-risk patients, and may be the first procedure to consider for patients with a small aortic root, who have had prior coronary artery bypass surgery with patent grafts, or those with a calcified aortic root and homografts.
Questions still to be answered, he said, include whether “the cost will justify the decrease in cross-clamp times.” Also, though midrange results are good, longitudinal follow-up to track long-term valve hemodynamics is still ongoing.
Although patient demand seems to be high for a minimally invasive approach, sutureless valves still have low adoption rates, he said.
Dr. Thourani reported multiple financial relationships with medical device companies.
[email protected]
On Twitter @karioakes
CHICAGO – Rapid deployment sutureless valves can be a good option for some patients, providing a highly functional and nearly leakproof valve with less cardiopulmonary bypass and aortic cross-clamp times than those of conventional procedures.
“Why use a sutureless valve?” asked Vinod H. Thourani, MD, speaking at Heart Valve Summit 2016. He said that for many patients, there are abundant good reasons for the choice. The rapidity of the implantation procedure is a huge plus, he said. Cardiopulmonary bypass times are reduced when sutureless valve replacement is a stand-alone procedure, added Dr. Thourani, chief of cardiovascular surgery at Emory Hospital Midtown and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
Rapid deployment is also of benefit in combined cases, or when patients have multiple comorbidities or poor left ventricular function. Sutureless valves, he said, are “optimal for multiple valve or concomitant procedures.”
Hemodynamics also are favorable, said Dr. Thourani; sutureless valves produce lower gradients than do their sutured alternatives, and work well in patients with a small aortic root.
Both sutureless valves that are currently available use bovine tissue; one, Sorin’s Perceval, uses a nitinol stent, while the Edwards’ Intuity uses stainless steel. The Perceval stent requires no sutures, while the Intuity requires just three. Also, the Perceval is collapsible, while the Intuity is not.
Removal of the pathologic valve in the sutureless procedure, he said, may contribute to the lower paravalvular leak and stroke rates than are seen in transcatheter aortic valve replacement (TAVR).
Expanded indications for sutureless valves include a calcified aortic root or a homograft; sutureless valves also can be used as an aortic valve redo, with patent grafts. Dr. Thourani said that he favors a transverse incision with a high aortotomy, about 2 cm above the sinotubular junction (STJ). In addition, off-label indications have included bicuspid aortic valve, pure aortic insufficiency, a prior mitral prosthesis or a degenerated aortic bioprosthesis, and a rescue procedure for a failed TAVR.
Dr. Thourani cited results of a trial conducted by Theodor Fischlein, MD, of Paracelsus Medical University in Nuremberg, Germany, and coauthors. These 1-year follow-up data from 628 patients participating in CAVALIER (Perceval S Valve Clinical Trial for Extended CE Mark), an international multicenter prospective trial, were presented at AATS 2016 (J Thorac Cardiovasc Surg. 2016 Jun;51[6]:1617-26.e4).
Of the 658 patients who met enrollment criteria and had a Perceval valve placement attempted, 30 wound up with a different prosthesis, most often because the correct valve size was not available. The remaining 628 patients who received the Perceval valve were included in the study. At 1 year, 549 patients remained; 50 had died, 12 had undergone valve explantation, and the remainder withdrew or were lost to follow-up.
Of the original Perceval recipients, 219 had received their valve via minimally invasive access. At 1 year, effective orifice area remained stable at the same mean 1.5 cm2 that was seen at discharge, an improvement from the mean 0.7 cm2 effective orifice area seen preoperatively. The mean pressure gradient, which was 45 mm Hg preoperatively, dropped precipitously to 10.3 mm Hg at discharge, and dropped a bit more at 1 year, to 9.2 mm Hg.
“This is a rapid and reproducible procedure: Over 20,000 implants have been performed worldwide,” said Dr. Thourani. The procedure looks good for low- to medium-risk patients, and may be the first procedure to consider for patients with a small aortic root, who have had prior coronary artery bypass surgery with patent grafts, or those with a calcified aortic root and homografts.
Questions still to be answered, he said, include whether “the cost will justify the decrease in cross-clamp times.” Also, though midrange results are good, longitudinal follow-up to track long-term valve hemodynamics is still ongoing.
Although patient demand seems to be high for a minimally invasive approach, sutureless valves still have low adoption rates, he said.
Dr. Thourani reported multiple financial relationships with medical device companies.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE HEART VALVE SUMMIT 2016
Nasal infantile hemangiomas develop most complications
Infantile hemangiomas of the nose develop more complications than those at all other body sites combined, according to a report published in Pediatric Dermatology.
In what they described as the largest study to date to assess nasal infantile hemangiomas, researchers assessed which traits are associated with complications and predict residual skin changes at the age of 5 years. “Nasal infantile hemangiomas pose an immediate risk of airway obstruction because infants are obligate nasal breathers, and may have long-term functional and psychosocial consequences if involution is incomplete or development of surrounding structures, such as nasal cartilage, is compromised,” said Maria S. Kryatova of the departments of pediatrics and dermatology, Johns Hopkins University, Baltimore, and her associates.
The investigators identified all patients younger than 18 years who had been treated at their academic referral center for nasal infantile hemangiomas between 2001 and 2014. They performed retrospective chart reviews, which included photographs, for 89 participants. The parents of 63 of these children were interviewed when the participants reached a median age of 5 years and provided comparison photographs taken at their entry into kindergarten.
Thirty-five children (39%) developed one or more complications at some time during follow-up, including airway compromise, compression, or functional impairment; ulceration; visual obstruction or ocular compression; and infection. In comparison, the Hemangioma Investigator Group has previously reported a 24% overall rate of complications at all body sites. Similarly, the proportion of study participants who received at least one type of treatment (propranolol, oral steroids, pulsed dye laser, surgery, topical timolol, intralesional corticosteroids, yttrium-aluminum-garnet laser, carbon dioxide laser, or fraxel laser) was markedly higher (80%) than that reported previously by the Hemangioma Investigator Group for all body sites (38%).
“Our study is the first to report a significant association between [the hemangioma’s location on the nose] and depth. Lesions on the nasal dorsum are unlikely to be deep, whereas nasal tip lesions are unlikely to be superficial. Deep vertical growth may be limited by underlying nasal bone in the dorsum but less so by the soft tissue of the nasal tip.” Alternatively, as suggested by other investigators, an embryologic explanation is also possible – “the fusion lines between neural crest–derived mesenchyme and ectoderm-derived nasal placodes may have different properties in the vicinity of the nasal dorsum and nasal tip that predispose them to the development of superficial and deep hemangiomas, respectively,” Ms. Kryatova and her associates reported (Ped Dermatol. 2016;33[6]:652-8).
Segmental- and indeterminate-type lesions were more likely than focal-type lesions to develop ulceration, compression, or functional obstruction, and mixed-depth hemangiomas were more likely than deep or superficial hemangiomas to ulcerate. Overall, the lesions had involuted by kindergarten age in 70% of the study participants but persisted in 30%, and most of the children with involution showed residual skin changes such as telangiectasia (14 children), fibrofatty tissue (11 children), and scarring (9 children).
These findings show that a multicenter study to expand on these conclusions and to determine the best treatment algorithm for nasal infantile hemangiomas is warranted, the investigators added.
Infantile hemangiomas of the nose develop more complications than those at all other body sites combined, according to a report published in Pediatric Dermatology.
In what they described as the largest study to date to assess nasal infantile hemangiomas, researchers assessed which traits are associated with complications and predict residual skin changes at the age of 5 years. “Nasal infantile hemangiomas pose an immediate risk of airway obstruction because infants are obligate nasal breathers, and may have long-term functional and psychosocial consequences if involution is incomplete or development of surrounding structures, such as nasal cartilage, is compromised,” said Maria S. Kryatova of the departments of pediatrics and dermatology, Johns Hopkins University, Baltimore, and her associates.
The investigators identified all patients younger than 18 years who had been treated at their academic referral center for nasal infantile hemangiomas between 2001 and 2014. They performed retrospective chart reviews, which included photographs, for 89 participants. The parents of 63 of these children were interviewed when the participants reached a median age of 5 years and provided comparison photographs taken at their entry into kindergarten.
Thirty-five children (39%) developed one or more complications at some time during follow-up, including airway compromise, compression, or functional impairment; ulceration; visual obstruction or ocular compression; and infection. In comparison, the Hemangioma Investigator Group has previously reported a 24% overall rate of complications at all body sites. Similarly, the proportion of study participants who received at least one type of treatment (propranolol, oral steroids, pulsed dye laser, surgery, topical timolol, intralesional corticosteroids, yttrium-aluminum-garnet laser, carbon dioxide laser, or fraxel laser) was markedly higher (80%) than that reported previously by the Hemangioma Investigator Group for all body sites (38%).
“Our study is the first to report a significant association between [the hemangioma’s location on the nose] and depth. Lesions on the nasal dorsum are unlikely to be deep, whereas nasal tip lesions are unlikely to be superficial. Deep vertical growth may be limited by underlying nasal bone in the dorsum but less so by the soft tissue of the nasal tip.” Alternatively, as suggested by other investigators, an embryologic explanation is also possible – “the fusion lines between neural crest–derived mesenchyme and ectoderm-derived nasal placodes may have different properties in the vicinity of the nasal dorsum and nasal tip that predispose them to the development of superficial and deep hemangiomas, respectively,” Ms. Kryatova and her associates reported (Ped Dermatol. 2016;33[6]:652-8).
Segmental- and indeterminate-type lesions were more likely than focal-type lesions to develop ulceration, compression, or functional obstruction, and mixed-depth hemangiomas were more likely than deep or superficial hemangiomas to ulcerate. Overall, the lesions had involuted by kindergarten age in 70% of the study participants but persisted in 30%, and most of the children with involution showed residual skin changes such as telangiectasia (14 children), fibrofatty tissue (11 children), and scarring (9 children).
These findings show that a multicenter study to expand on these conclusions and to determine the best treatment algorithm for nasal infantile hemangiomas is warranted, the investigators added.
Infantile hemangiomas of the nose develop more complications than those at all other body sites combined, according to a report published in Pediatric Dermatology.
In what they described as the largest study to date to assess nasal infantile hemangiomas, researchers assessed which traits are associated with complications and predict residual skin changes at the age of 5 years. “Nasal infantile hemangiomas pose an immediate risk of airway obstruction because infants are obligate nasal breathers, and may have long-term functional and psychosocial consequences if involution is incomplete or development of surrounding structures, such as nasal cartilage, is compromised,” said Maria S. Kryatova of the departments of pediatrics and dermatology, Johns Hopkins University, Baltimore, and her associates.
The investigators identified all patients younger than 18 years who had been treated at their academic referral center for nasal infantile hemangiomas between 2001 and 2014. They performed retrospective chart reviews, which included photographs, for 89 participants. The parents of 63 of these children were interviewed when the participants reached a median age of 5 years and provided comparison photographs taken at their entry into kindergarten.
Thirty-five children (39%) developed one or more complications at some time during follow-up, including airway compromise, compression, or functional impairment; ulceration; visual obstruction or ocular compression; and infection. In comparison, the Hemangioma Investigator Group has previously reported a 24% overall rate of complications at all body sites. Similarly, the proportion of study participants who received at least one type of treatment (propranolol, oral steroids, pulsed dye laser, surgery, topical timolol, intralesional corticosteroids, yttrium-aluminum-garnet laser, carbon dioxide laser, or fraxel laser) was markedly higher (80%) than that reported previously by the Hemangioma Investigator Group for all body sites (38%).
“Our study is the first to report a significant association between [the hemangioma’s location on the nose] and depth. Lesions on the nasal dorsum are unlikely to be deep, whereas nasal tip lesions are unlikely to be superficial. Deep vertical growth may be limited by underlying nasal bone in the dorsum but less so by the soft tissue of the nasal tip.” Alternatively, as suggested by other investigators, an embryologic explanation is also possible – “the fusion lines between neural crest–derived mesenchyme and ectoderm-derived nasal placodes may have different properties in the vicinity of the nasal dorsum and nasal tip that predispose them to the development of superficial and deep hemangiomas, respectively,” Ms. Kryatova and her associates reported (Ped Dermatol. 2016;33[6]:652-8).
Segmental- and indeterminate-type lesions were more likely than focal-type lesions to develop ulceration, compression, or functional obstruction, and mixed-depth hemangiomas were more likely than deep or superficial hemangiomas to ulcerate. Overall, the lesions had involuted by kindergarten age in 70% of the study participants but persisted in 30%, and most of the children with involution showed residual skin changes such as telangiectasia (14 children), fibrofatty tissue (11 children), and scarring (9 children).
These findings show that a multicenter study to expand on these conclusions and to determine the best treatment algorithm for nasal infantile hemangiomas is warranted, the investigators added.
Key clinical point: Infantile hemangiomas of the nose develop more complications than those at all other sites combined.
Major finding: Thirty-five children (39%) developed one or more complications at some time during follow-up, including airway compromise, compression, or functional impairment; lesion ulceration; visual obstruction or ocular compression; and infection.
Data source: A retrospective chart review involving 89 patients with nasal infantile hemangiomas who were followed up at 5 years of age.
Disclosures: No sponsor was cited for this study, and the authors didn’t report their financial disclosures.
Rosacea improved with fractional microneedling radiofrequency therapy
Fractional microneedling radiofrequency (FMR) therapy resulted in modest but clinically significant improvements in the appearance and inflammation of rosacea in a small study of patients with mild to moderate rosacea.
The treatment delivers bipolar radiofrequency energy to the dermis via an array of microneedles, without damaging the epidermis, noted Seon Yong Park, MD, and colleagues from the department of dermatology at Seoul (South Korea) National University. It has previously been associated with clinical and histological improvements in acne-associated postinflammatory erythema and is used in the treatment of cutaneous wrinkles. The authors said that, as far as they know, this is the first study to evaluate the use of FMR in patients with rosacea.
In the prospective, single-blind, randomized, split-face clinical study, 21 patients (20 females, 1 male) with mild to moderate rosacea were treated with two FMR sessions, 4 weeks apart, then assessed 4, 8, and 12 weeks after the second session. The mean age of the patients was 43 years; they had Fitzpatrick skin type III (13 patients) or IV (8 patients), and rosacea was considered mild in 12 patients and moderate in 9 patients at baseline.
Researchers saw clinical improvements in 17 (81%) of the patients on the treated side; these patients had a mean improvement in the Investigator’s Global Assessment (IGA) score of 2.47 by week 12, representing about a 20% improvement (Dermatol Surg. 2016 Dec;42[12]:1362-9).
In the group overall, mean IGA scores at weeks 4, 8, and 12 were 1.05, 1.57, and 2.00 for the treated side, compared with 0.29, 0.38, and 0.38, respectively, for the untreated side, which, the authors wrote, indicated that “there was modest but statistically significant improvements in the treated side.”
Photometric measurements of redness showed significant reductions on the treated side, compared with the untreated side and baseline, with reductions in the erythema index of 11.9%, 10.7%, and 13.6% at week 4, 8, and 12, respectively.
Histological assessment showed reduced dermal inflammation, significant reductions in average mast cell count, and an overall decrease in immunohistochemical intensity in the treated skin 8 weeks after treatment. Similarly, there were significant decreases in markers of angiogenesis, inflammation, innate immunity, and neuroimmunity on the treated side, compared with baseline.
“Fractional microneedling radiofrequency was slightly more effective in reducing erythema in patients with PPR [papulopustular rosacea] than in those with ETR [erythematotelangiectatic rosacea], suggesting that inflammatory lesions, such as papules and pustules, could be more effectively treated with this device,” the authors wrote. “This result agreed with reports showing that FMR is effective in treating inflammatory acne.”
No serious adverse effects were reported, although 19 patients (90.5%) experienced mild pain during the procedure and 17 (81%) had mild erythema that lasted for up to 5 days. Patients also reported less itching, heat, burning, or pricking on the treated side, which showed that the treatment was effective in controlling the symptoms of rosacea, Dr. Park and associates said.
The study was supported by the SNUH Research Fund and National Research Foundation of Korea. The authors, who are also in the acne and rosacea research laboratory, Seoul National University Hospital, had no conflicts to disclose.
Fractional microneedling radiofrequency (FMR) therapy resulted in modest but clinically significant improvements in the appearance and inflammation of rosacea in a small study of patients with mild to moderate rosacea.
The treatment delivers bipolar radiofrequency energy to the dermis via an array of microneedles, without damaging the epidermis, noted Seon Yong Park, MD, and colleagues from the department of dermatology at Seoul (South Korea) National University. It has previously been associated with clinical and histological improvements in acne-associated postinflammatory erythema and is used in the treatment of cutaneous wrinkles. The authors said that, as far as they know, this is the first study to evaluate the use of FMR in patients with rosacea.
In the prospective, single-blind, randomized, split-face clinical study, 21 patients (20 females, 1 male) with mild to moderate rosacea were treated with two FMR sessions, 4 weeks apart, then assessed 4, 8, and 12 weeks after the second session. The mean age of the patients was 43 years; they had Fitzpatrick skin type III (13 patients) or IV (8 patients), and rosacea was considered mild in 12 patients and moderate in 9 patients at baseline.
Researchers saw clinical improvements in 17 (81%) of the patients on the treated side; these patients had a mean improvement in the Investigator’s Global Assessment (IGA) score of 2.47 by week 12, representing about a 20% improvement (Dermatol Surg. 2016 Dec;42[12]:1362-9).
In the group overall, mean IGA scores at weeks 4, 8, and 12 were 1.05, 1.57, and 2.00 for the treated side, compared with 0.29, 0.38, and 0.38, respectively, for the untreated side, which, the authors wrote, indicated that “there was modest but statistically significant improvements in the treated side.”
Photometric measurements of redness showed significant reductions on the treated side, compared with the untreated side and baseline, with reductions in the erythema index of 11.9%, 10.7%, and 13.6% at week 4, 8, and 12, respectively.
Histological assessment showed reduced dermal inflammation, significant reductions in average mast cell count, and an overall decrease in immunohistochemical intensity in the treated skin 8 weeks after treatment. Similarly, there were significant decreases in markers of angiogenesis, inflammation, innate immunity, and neuroimmunity on the treated side, compared with baseline.
“Fractional microneedling radiofrequency was slightly more effective in reducing erythema in patients with PPR [papulopustular rosacea] than in those with ETR [erythematotelangiectatic rosacea], suggesting that inflammatory lesions, such as papules and pustules, could be more effectively treated with this device,” the authors wrote. “This result agreed with reports showing that FMR is effective in treating inflammatory acne.”
No serious adverse effects were reported, although 19 patients (90.5%) experienced mild pain during the procedure and 17 (81%) had mild erythema that lasted for up to 5 days. Patients also reported less itching, heat, burning, or pricking on the treated side, which showed that the treatment was effective in controlling the symptoms of rosacea, Dr. Park and associates said.
The study was supported by the SNUH Research Fund and National Research Foundation of Korea. The authors, who are also in the acne and rosacea research laboratory, Seoul National University Hospital, had no conflicts to disclose.
Fractional microneedling radiofrequency (FMR) therapy resulted in modest but clinically significant improvements in the appearance and inflammation of rosacea in a small study of patients with mild to moderate rosacea.
The treatment delivers bipolar radiofrequency energy to the dermis via an array of microneedles, without damaging the epidermis, noted Seon Yong Park, MD, and colleagues from the department of dermatology at Seoul (South Korea) National University. It has previously been associated with clinical and histological improvements in acne-associated postinflammatory erythema and is used in the treatment of cutaneous wrinkles. The authors said that, as far as they know, this is the first study to evaluate the use of FMR in patients with rosacea.
In the prospective, single-blind, randomized, split-face clinical study, 21 patients (20 females, 1 male) with mild to moderate rosacea were treated with two FMR sessions, 4 weeks apart, then assessed 4, 8, and 12 weeks after the second session. The mean age of the patients was 43 years; they had Fitzpatrick skin type III (13 patients) or IV (8 patients), and rosacea was considered mild in 12 patients and moderate in 9 patients at baseline.
Researchers saw clinical improvements in 17 (81%) of the patients on the treated side; these patients had a mean improvement in the Investigator’s Global Assessment (IGA) score of 2.47 by week 12, representing about a 20% improvement (Dermatol Surg. 2016 Dec;42[12]:1362-9).
In the group overall, mean IGA scores at weeks 4, 8, and 12 were 1.05, 1.57, and 2.00 for the treated side, compared with 0.29, 0.38, and 0.38, respectively, for the untreated side, which, the authors wrote, indicated that “there was modest but statistically significant improvements in the treated side.”
Photometric measurements of redness showed significant reductions on the treated side, compared with the untreated side and baseline, with reductions in the erythema index of 11.9%, 10.7%, and 13.6% at week 4, 8, and 12, respectively.
Histological assessment showed reduced dermal inflammation, significant reductions in average mast cell count, and an overall decrease in immunohistochemical intensity in the treated skin 8 weeks after treatment. Similarly, there were significant decreases in markers of angiogenesis, inflammation, innate immunity, and neuroimmunity on the treated side, compared with baseline.
“Fractional microneedling radiofrequency was slightly more effective in reducing erythema in patients with PPR [papulopustular rosacea] than in those with ETR [erythematotelangiectatic rosacea], suggesting that inflammatory lesions, such as papules and pustules, could be more effectively treated with this device,” the authors wrote. “This result agreed with reports showing that FMR is effective in treating inflammatory acne.”
No serious adverse effects were reported, although 19 patients (90.5%) experienced mild pain during the procedure and 17 (81%) had mild erythema that lasted for up to 5 days. Patients also reported less itching, heat, burning, or pricking on the treated side, which showed that the treatment was effective in controlling the symptoms of rosacea, Dr. Park and associates said.
The study was supported by the SNUH Research Fund and National Research Foundation of Korea. The authors, who are also in the acne and rosacea research laboratory, Seoul National University Hospital, had no conflicts to disclose.
FROM DERMATOLOGIC SURGERY
Key clinical point: Fractional microneedling radiofrequency therapy may achieve modest but clinically significant improvements in the appearance and inflammation of rosacea.
Major finding: Fractional microneedling radiofrequency therapy was associated with clinical improvements in 81% of treated patients.
Data source: A prospective, single-blind, randomized, split-face clinical trial in 21 patients with mild to moderate rosacea.
Disclosures: The study was supported by the SNUH Research Fund and National Research Foundation of Korea. No conflicts of interest were declared.
Fat Embolism Syndrome With Cerebral Fat Embolism Associated With Long-Bone Fracture
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.