User login
Fecal calprotectin tops CRP as Crohn’s marker
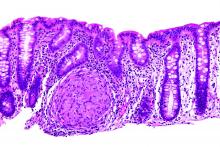
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: A fecal calprotectin cutoff of 215 mcg/g identified mucosal healing with 82.8% sensitivity, 71.4% specificity, and an AUC of 0.81.
Data source: Review of 89 Crohn’s patients
Disclosures: The investigators had no conflicts of interest.
PrEP adoption lagging behind awareness in high-risk population
NEW ORLEANS – Awareness about preexposure prophylaxis (PrEP) is steadily increasing among men who have sex with men at high risk for HIV infection, but that increased knowledge did not translate into greater willingness to take the daily pill nor did it increase engagement in high-risk behaviors.
Using questions from the National HIV Behavioral Surveillance System (NHBS), investigators surveyed men who have sex with men in urban settings in 3-year cycles. Awareness about HIV PrEP, once-daily emtricitabine/tenofovir (Truvada) increased from 21% in 2008 to 28% in 2011 to 46% in 2014.
The increase from 2011 to 2014 was statistically significant (P less than .001). The Food and Drug Administration approved the preventive regimen in 2012.
Increased knowledge did not translate to greater willingness to take the daily pill – which has held steady at about 60% of over time.
The number of men who self-reported as HIV negative and sexually active in the previous 12 months included in the survey varied from 421 in 2008, to 461 in 2011, to 451 in 2014.
For people at elevated risk for HIV infection, PrEP also represents an opportunity to take greater control over behavior, according to a recent review (Curr Opin HIV AIDS. 2016;11:3-9). “When you get people in for counseling or condoms, you give them a sense of control,” said Dr. Krotchko of Denver Health Medical Center.
Most survey respondents said they anticipated they would use condoms just as frequently as before if taking PrEP (82%, 78%, and 78%, in 2008, 2011, and 2014, respectively). Similarly, the majority of respondents anticipated having the same number of sexual partners if taking PrEP (92%, 85%, and 89%). These differences were not statistically significant.
The findings indicate availability of HIV PrEP is not increasing unhealthy behaviors, as some may fear. “Riskier behavior while on PrEP has not been borne out by the literature,” Dr. Krotchko said.
Strengths of the study include directly targeting a high-risk population and identifying those with high-risk behaviors who could benefit from use of HIV PrEP. Self-reported anticipated changes may not reflect future behavior in all cases, a potential limitation, Dr. Krotchko pointed out.
The NHBS survey is funded by the Centers for Disease Control and Prevention. IDWeek 2016 comprises the combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
NEW ORLEANS – Awareness about preexposure prophylaxis (PrEP) is steadily increasing among men who have sex with men at high risk for HIV infection, but that increased knowledge did not translate into greater willingness to take the daily pill nor did it increase engagement in high-risk behaviors.
Using questions from the National HIV Behavioral Surveillance System (NHBS), investigators surveyed men who have sex with men in urban settings in 3-year cycles. Awareness about HIV PrEP, once-daily emtricitabine/tenofovir (Truvada) increased from 21% in 2008 to 28% in 2011 to 46% in 2014.
The increase from 2011 to 2014 was statistically significant (P less than .001). The Food and Drug Administration approved the preventive regimen in 2012.
Increased knowledge did not translate to greater willingness to take the daily pill – which has held steady at about 60% of over time.
The number of men who self-reported as HIV negative and sexually active in the previous 12 months included in the survey varied from 421 in 2008, to 461 in 2011, to 451 in 2014.
For people at elevated risk for HIV infection, PrEP also represents an opportunity to take greater control over behavior, according to a recent review (Curr Opin HIV AIDS. 2016;11:3-9). “When you get people in for counseling or condoms, you give them a sense of control,” said Dr. Krotchko of Denver Health Medical Center.
Most survey respondents said they anticipated they would use condoms just as frequently as before if taking PrEP (82%, 78%, and 78%, in 2008, 2011, and 2014, respectively). Similarly, the majority of respondents anticipated having the same number of sexual partners if taking PrEP (92%, 85%, and 89%). These differences were not statistically significant.
The findings indicate availability of HIV PrEP is not increasing unhealthy behaviors, as some may fear. “Riskier behavior while on PrEP has not been borne out by the literature,” Dr. Krotchko said.
Strengths of the study include directly targeting a high-risk population and identifying those with high-risk behaviors who could benefit from use of HIV PrEP. Self-reported anticipated changes may not reflect future behavior in all cases, a potential limitation, Dr. Krotchko pointed out.
The NHBS survey is funded by the Centers for Disease Control and Prevention. IDWeek 2016 comprises the combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
NEW ORLEANS – Awareness about preexposure prophylaxis (PrEP) is steadily increasing among men who have sex with men at high risk for HIV infection, but that increased knowledge did not translate into greater willingness to take the daily pill nor did it increase engagement in high-risk behaviors.
Using questions from the National HIV Behavioral Surveillance System (NHBS), investigators surveyed men who have sex with men in urban settings in 3-year cycles. Awareness about HIV PrEP, once-daily emtricitabine/tenofovir (Truvada) increased from 21% in 2008 to 28% in 2011 to 46% in 2014.
The increase from 2011 to 2014 was statistically significant (P less than .001). The Food and Drug Administration approved the preventive regimen in 2012.
Increased knowledge did not translate to greater willingness to take the daily pill – which has held steady at about 60% of over time.
The number of men who self-reported as HIV negative and sexually active in the previous 12 months included in the survey varied from 421 in 2008, to 461 in 2011, to 451 in 2014.
For people at elevated risk for HIV infection, PrEP also represents an opportunity to take greater control over behavior, according to a recent review (Curr Opin HIV AIDS. 2016;11:3-9). “When you get people in for counseling or condoms, you give them a sense of control,” said Dr. Krotchko of Denver Health Medical Center.
Most survey respondents said they anticipated they would use condoms just as frequently as before if taking PrEP (82%, 78%, and 78%, in 2008, 2011, and 2014, respectively). Similarly, the majority of respondents anticipated having the same number of sexual partners if taking PrEP (92%, 85%, and 89%). These differences were not statistically significant.
The findings indicate availability of HIV PrEP is not increasing unhealthy behaviors, as some may fear. “Riskier behavior while on PrEP has not been borne out by the literature,” Dr. Krotchko said.
Strengths of the study include directly targeting a high-risk population and identifying those with high-risk behaviors who could benefit from use of HIV PrEP. Self-reported anticipated changes may not reflect future behavior in all cases, a potential limitation, Dr. Krotchko pointed out.
The NHBS survey is funded by the Centers for Disease Control and Prevention. IDWeek 2016 comprises the combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
AT IDWEEK 2016
Key clinical point: Adoption and use of HIV PrEP continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men.
Major finding: Despite increasing awareness, willingness to use HIV PrEP among MSM remained steady at about 60% over time in a series of national behavioral health surveys.
Data source: The National HIV Behavioral Surveillance System.
Disclosures: Dr. Krotchko had no relevant disclosures.
Thank You to Our 2016 Peer Reviewers
The editors of Emergency Medicine acknowledge the help of the journal’s editorial board members, other emergency physicians, and colleagues in other specialties who reviewed manuscripts in 2016. On behalf of our readers, who are the beneficiaries of your efforts, we thank you.
Alfred Z. Abuhamad, MD
Department of Obstetrics and Gynecology
Eastern Virginia Medical School
John E. Arbo, MD
Division of Emergency Medicine and Pulmonary Critical Care Medicine
Weill Cornell Medical College, Cornell University
David P. Calfee, MD
Division of Infectious Diseases
Weill Cornell Medical College, Cornell University
Richard M. Cantor, MD, FAAP, FACEP
Emergency Department, Pediatrics
Upstate Medical University
Wallace A. Carter, MD, FACEP
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Sunday Clark, ScD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Theodore R. Delbridge, MD
Department of Emergency
Medicine East Carolina University, Brody School of Medicine
Joseph J. Fins, MD
Division of Medical Ethics Internal Medicine
Weill Cornell Medical College, Cornell University
Ron W. Flenner, MD
Department of Internal Medicine
Eastern Virginia Medical School
E. John Gallagher, MD
Department of Emergency Medicine
Albert Einstein College of Medicine
Marianne Gausche-Hill, MD
Department of Emergency Medicine
Harbor-UCLA Medical Center
Keith D. Hentel, MD
Department of Radiology
Weill Cornell Medical College, Cornell University
Barry J. Knapp, MD
Department of Emergency Medicine
Eastern Virginia Medical School
Richard I. Lapin, MD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Anthony C. Mustalish, MD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Lewis S. Nelson, MD
Department of Emergency Medicine
Rutgers New Jersey Medical School
Debra Perina, MD
Department of Emergency Medicine
University of Virginia, Charlottesville
Constance Peterson, MA
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Shari L. Platt, MD, FAAP
Division of Pediatric Emergency Medicine
Weill Cornell Medical College, Cornell University
Rama B. Rao, MD
Division of Toxicology
Weill Cornell Medical College, Cornell University
Earl J. Reisdorff, MD
Executive Director
American Board of Emergency Medicine
Thomas M. Scalea, MD, FACS, FCCM
Program in Trauma
University of Maryland School of Medicine
Edward J. Schenk, MD
Pulmonary Critical Care Medicine
Weill Cornell Medical College, Cornell University
Christopher K. Schott, MD, MS
Department of Emergency Medicine and Critical Care Medicine
University of Pittsburgh
Adam J Singer, MD
Department of Emergency Medicine
Stony Brook University and Medical Center
Sarah A. Stahmer, MD, FACEP
Division of Emergency Medicine
Duke University Medical Center
Michael E. Stern, MD
Division of Geriatric Emergency Medicine
Weill Cornell Medical College, Cornell University
Susan Stone, MD
Emergency Medicine/Palliative Care
University of California, Los Angeles
Todd Taylor, MD
Department of Emergency Medicine
Emory University School of Medicine
The editors of Emergency Medicine acknowledge the help of the journal’s editorial board members, other emergency physicians, and colleagues in other specialties who reviewed manuscripts in 2016. On behalf of our readers, who are the beneficiaries of your efforts, we thank you.
Alfred Z. Abuhamad, MD
Department of Obstetrics and Gynecology
Eastern Virginia Medical School
John E. Arbo, MD
Division of Emergency Medicine and Pulmonary Critical Care Medicine
Weill Cornell Medical College, Cornell University
David P. Calfee, MD
Division of Infectious Diseases
Weill Cornell Medical College, Cornell University
Richard M. Cantor, MD, FAAP, FACEP
Emergency Department, Pediatrics
Upstate Medical University
Wallace A. Carter, MD, FACEP
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Sunday Clark, ScD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Theodore R. Delbridge, MD
Department of Emergency
Medicine East Carolina University, Brody School of Medicine
Joseph J. Fins, MD
Division of Medical Ethics Internal Medicine
Weill Cornell Medical College, Cornell University
Ron W. Flenner, MD
Department of Internal Medicine
Eastern Virginia Medical School
E. John Gallagher, MD
Department of Emergency Medicine
Albert Einstein College of Medicine
Marianne Gausche-Hill, MD
Department of Emergency Medicine
Harbor-UCLA Medical Center
Keith D. Hentel, MD
Department of Radiology
Weill Cornell Medical College, Cornell University
Barry J. Knapp, MD
Department of Emergency Medicine
Eastern Virginia Medical School
Richard I. Lapin, MD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Anthony C. Mustalish, MD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Lewis S. Nelson, MD
Department of Emergency Medicine
Rutgers New Jersey Medical School
Debra Perina, MD
Department of Emergency Medicine
University of Virginia, Charlottesville
Constance Peterson, MA
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Shari L. Platt, MD, FAAP
Division of Pediatric Emergency Medicine
Weill Cornell Medical College, Cornell University
Rama B. Rao, MD
Division of Toxicology
Weill Cornell Medical College, Cornell University
Earl J. Reisdorff, MD
Executive Director
American Board of Emergency Medicine
Thomas M. Scalea, MD, FACS, FCCM
Program in Trauma
University of Maryland School of Medicine
Edward J. Schenk, MD
Pulmonary Critical Care Medicine
Weill Cornell Medical College, Cornell University
Christopher K. Schott, MD, MS
Department of Emergency Medicine and Critical Care Medicine
University of Pittsburgh
Adam J Singer, MD
Department of Emergency Medicine
Stony Brook University and Medical Center
Sarah A. Stahmer, MD, FACEP
Division of Emergency Medicine
Duke University Medical Center
Michael E. Stern, MD
Division of Geriatric Emergency Medicine
Weill Cornell Medical College, Cornell University
Susan Stone, MD
Emergency Medicine/Palliative Care
University of California, Los Angeles
Todd Taylor, MD
Department of Emergency Medicine
Emory University School of Medicine
The editors of Emergency Medicine acknowledge the help of the journal’s editorial board members, other emergency physicians, and colleagues in other specialties who reviewed manuscripts in 2016. On behalf of our readers, who are the beneficiaries of your efforts, we thank you.
Alfred Z. Abuhamad, MD
Department of Obstetrics and Gynecology
Eastern Virginia Medical School
John E. Arbo, MD
Division of Emergency Medicine and Pulmonary Critical Care Medicine
Weill Cornell Medical College, Cornell University
David P. Calfee, MD
Division of Infectious Diseases
Weill Cornell Medical College, Cornell University
Richard M. Cantor, MD, FAAP, FACEP
Emergency Department, Pediatrics
Upstate Medical University
Wallace A. Carter, MD, FACEP
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Sunday Clark, ScD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Theodore R. Delbridge, MD
Department of Emergency
Medicine East Carolina University, Brody School of Medicine
Joseph J. Fins, MD
Division of Medical Ethics Internal Medicine
Weill Cornell Medical College, Cornell University
Ron W. Flenner, MD
Department of Internal Medicine
Eastern Virginia Medical School
E. John Gallagher, MD
Department of Emergency Medicine
Albert Einstein College of Medicine
Marianne Gausche-Hill, MD
Department of Emergency Medicine
Harbor-UCLA Medical Center
Keith D. Hentel, MD
Department of Radiology
Weill Cornell Medical College, Cornell University
Barry J. Knapp, MD
Department of Emergency Medicine
Eastern Virginia Medical School
Richard I. Lapin, MD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Anthony C. Mustalish, MD
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Lewis S. Nelson, MD
Department of Emergency Medicine
Rutgers New Jersey Medical School
Debra Perina, MD
Department of Emergency Medicine
University of Virginia, Charlottesville
Constance Peterson, MA
Department of Emergency Medicine
Weill Cornell Medical College, Cornell University
Shari L. Platt, MD, FAAP
Division of Pediatric Emergency Medicine
Weill Cornell Medical College, Cornell University
Rama B. Rao, MD
Division of Toxicology
Weill Cornell Medical College, Cornell University
Earl J. Reisdorff, MD
Executive Director
American Board of Emergency Medicine
Thomas M. Scalea, MD, FACS, FCCM
Program in Trauma
University of Maryland School of Medicine
Edward J. Schenk, MD
Pulmonary Critical Care Medicine
Weill Cornell Medical College, Cornell University
Christopher K. Schott, MD, MS
Department of Emergency Medicine and Critical Care Medicine
University of Pittsburgh
Adam J Singer, MD
Department of Emergency Medicine
Stony Brook University and Medical Center
Sarah A. Stahmer, MD, FACEP
Division of Emergency Medicine
Duke University Medical Center
Michael E. Stern, MD
Division of Geriatric Emergency Medicine
Weill Cornell Medical College, Cornell University
Susan Stone, MD
Emergency Medicine/Palliative Care
University of California, Los Angeles
Todd Taylor, MD
Department of Emergency Medicine
Emory University School of Medicine
Screening tool spots teens headed for substance-dependent adulthood
VIENNA – The creation of a simple risk score that accurately predicts which adolescents in the general population will develop persistent substance dependence as adults has been one of the highlights of the year in addiction medicine, Wim van den Brink, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“These predictors are not very difficult to assess. Clinicians will be interested to know that the positive predictive value of the screen is threefold greater than the persistent prevalence rate,” noted Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
The New Zealand researchers developed what they call “a universal screening tool” by working backward in an analysis of a representative group of 1,037 individuals born in Dunedin, New Zealand, in 1972-1973 and prospectively followed to age 38 years, with a 95% study retention rate. Along the way, participants were assessed for dependence on alcohol, tobacco, cannabis, or hard drugs at ages 21, 26, 32, and 38.
Persistent substance dependence in adulthood, defined as dependence at a minimum of three of the assessments, was present in 19% of subjects.
The investigators found that the presence in childhood or adolescence of any four of nine risk factors had an area under the curve of 80% for persistent substance dependence as an adult. The sensitivity was 43%, with a 93% specificity. The positive predictive value was 60%, and the negative predictive value was 87% (Psychol Med. 2016 Mar;46[4]:877-89).
The nine risk factors are low family socioeconomic status, a family history of substance dependence, childhood depression, childhood conduct disorder, early exposure to substances, adolescent frequent alcohol use, adolescent frequent cannabis use, male gender, and adolescent frequent tobacco use.
The single least potent predictor was low family socioeconomic status, with an associated 1.73-fold increased risk. The strongest predictors were adolescent frequent tobacco use, which conferred a 5.41-fold increased risk; adolescent frequent cannabis use, with a 4.25-fold risk; and childhood conduct disorder, with a 3.2-fold increased risk.
The investigators also analyzed the screening tool’s performance in predicting a modified outcome consisting of adult persistent dependence on any of the target substances except for tobacco. The predictive power of having any four of the risk factors was similar to that found in the main analysis; however, the two strongest predictors now became adolescent frequent cannabis use, with a 9.5-fold increased risk, and childhood conduct disorder, with a relative risk of 5.42.
Regarding childhood conduct disorder as a risk factor, Dr. van den Brink said, “If you are a child with conduct disorder, your chances of becoming substance dependent in coming years is more than fivefold greater than in a child without conduct disorder.”
This raises the question of whether effective treatment of childhood conduct disorder might prevent later development of persistent substance dependence in adulthood. The answer remains unknown. Although there is no approved drug therapy for conduct disorder, methylphenidate is widely prescribed, especially in young patients with comorbid attention-deficit/hyperactivity disorder.
Several years ago a meta-analysis of 15 longitudinal studies with more than 2,500 participants concluded that stimulant therapy of childhood ADHD neither increased nor reduced the risk of subsequent substance use disorders (JAMA Psychiatry. 2013 Jul;70[7]:740-9). Prescribing physicians were happy to hear they weren’t causing iatrogenic injury, but Dr. van den Brink said he was never comfortable with the investigators’ conclusion.
“There was a lot of heterogeneity in the data, so the overall conclusion might not be the best conclusion,” he said.
He said has become more convinced of that than ever as a result of a recent randomized, double-blind, placebo-controlled MRI study of cerebral blood flow in response to methylphenidate in stimulant-naive patients with childhood or adult AHDH. The investigators found that MRIs obtained 1 week after the conclusion of 16 weeks of methylphenidate therapy showed increased blood flow in the strial and thalamic areas in the pediatric ADHD patients but not in the adults with ADHD (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
This is evidence of an age-dependent sustained effect of methylphenidate therapy on dopamine striatal-thalamic circuitry in children that’s not related to the drug’s clinical effects, which were gone after a week off therapy. The question is, Does this effect represent neurotoxicity, or is it an expression of enhanced brain maturation? Dr. van den Brink said he suspects it’s the latter but cannot exclude the former possibility.
VIENNA – The creation of a simple risk score that accurately predicts which adolescents in the general population will develop persistent substance dependence as adults has been one of the highlights of the year in addiction medicine, Wim van den Brink, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“These predictors are not very difficult to assess. Clinicians will be interested to know that the positive predictive value of the screen is threefold greater than the persistent prevalence rate,” noted Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
The New Zealand researchers developed what they call “a universal screening tool” by working backward in an analysis of a representative group of 1,037 individuals born in Dunedin, New Zealand, in 1972-1973 and prospectively followed to age 38 years, with a 95% study retention rate. Along the way, participants were assessed for dependence on alcohol, tobacco, cannabis, or hard drugs at ages 21, 26, 32, and 38.
Persistent substance dependence in adulthood, defined as dependence at a minimum of three of the assessments, was present in 19% of subjects.
The investigators found that the presence in childhood or adolescence of any four of nine risk factors had an area under the curve of 80% for persistent substance dependence as an adult. The sensitivity was 43%, with a 93% specificity. The positive predictive value was 60%, and the negative predictive value was 87% (Psychol Med. 2016 Mar;46[4]:877-89).
The nine risk factors are low family socioeconomic status, a family history of substance dependence, childhood depression, childhood conduct disorder, early exposure to substances, adolescent frequent alcohol use, adolescent frequent cannabis use, male gender, and adolescent frequent tobacco use.
The single least potent predictor was low family socioeconomic status, with an associated 1.73-fold increased risk. The strongest predictors were adolescent frequent tobacco use, which conferred a 5.41-fold increased risk; adolescent frequent cannabis use, with a 4.25-fold risk; and childhood conduct disorder, with a 3.2-fold increased risk.
The investigators also analyzed the screening tool’s performance in predicting a modified outcome consisting of adult persistent dependence on any of the target substances except for tobacco. The predictive power of having any four of the risk factors was similar to that found in the main analysis; however, the two strongest predictors now became adolescent frequent cannabis use, with a 9.5-fold increased risk, and childhood conduct disorder, with a relative risk of 5.42.
Regarding childhood conduct disorder as a risk factor, Dr. van den Brink said, “If you are a child with conduct disorder, your chances of becoming substance dependent in coming years is more than fivefold greater than in a child without conduct disorder.”
This raises the question of whether effective treatment of childhood conduct disorder might prevent later development of persistent substance dependence in adulthood. The answer remains unknown. Although there is no approved drug therapy for conduct disorder, methylphenidate is widely prescribed, especially in young patients with comorbid attention-deficit/hyperactivity disorder.
Several years ago a meta-analysis of 15 longitudinal studies with more than 2,500 participants concluded that stimulant therapy of childhood ADHD neither increased nor reduced the risk of subsequent substance use disorders (JAMA Psychiatry. 2013 Jul;70[7]:740-9). Prescribing physicians were happy to hear they weren’t causing iatrogenic injury, but Dr. van den Brink said he was never comfortable with the investigators’ conclusion.
“There was a lot of heterogeneity in the data, so the overall conclusion might not be the best conclusion,” he said.
He said has become more convinced of that than ever as a result of a recent randomized, double-blind, placebo-controlled MRI study of cerebral blood flow in response to methylphenidate in stimulant-naive patients with childhood or adult AHDH. The investigators found that MRIs obtained 1 week after the conclusion of 16 weeks of methylphenidate therapy showed increased blood flow in the strial and thalamic areas in the pediatric ADHD patients but not in the adults with ADHD (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
This is evidence of an age-dependent sustained effect of methylphenidate therapy on dopamine striatal-thalamic circuitry in children that’s not related to the drug’s clinical effects, which were gone after a week off therapy. The question is, Does this effect represent neurotoxicity, or is it an expression of enhanced brain maturation? Dr. van den Brink said he suspects it’s the latter but cannot exclude the former possibility.
VIENNA – The creation of a simple risk score that accurately predicts which adolescents in the general population will develop persistent substance dependence as adults has been one of the highlights of the year in addiction medicine, Wim van den Brink, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“These predictors are not very difficult to assess. Clinicians will be interested to know that the positive predictive value of the screen is threefold greater than the persistent prevalence rate,” noted Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
The New Zealand researchers developed what they call “a universal screening tool” by working backward in an analysis of a representative group of 1,037 individuals born in Dunedin, New Zealand, in 1972-1973 and prospectively followed to age 38 years, with a 95% study retention rate. Along the way, participants were assessed for dependence on alcohol, tobacco, cannabis, or hard drugs at ages 21, 26, 32, and 38.
Persistent substance dependence in adulthood, defined as dependence at a minimum of three of the assessments, was present in 19% of subjects.
The investigators found that the presence in childhood or adolescence of any four of nine risk factors had an area under the curve of 80% for persistent substance dependence as an adult. The sensitivity was 43%, with a 93% specificity. The positive predictive value was 60%, and the negative predictive value was 87% (Psychol Med. 2016 Mar;46[4]:877-89).
The nine risk factors are low family socioeconomic status, a family history of substance dependence, childhood depression, childhood conduct disorder, early exposure to substances, adolescent frequent alcohol use, adolescent frequent cannabis use, male gender, and adolescent frequent tobacco use.
The single least potent predictor was low family socioeconomic status, with an associated 1.73-fold increased risk. The strongest predictors were adolescent frequent tobacco use, which conferred a 5.41-fold increased risk; adolescent frequent cannabis use, with a 4.25-fold risk; and childhood conduct disorder, with a 3.2-fold increased risk.
The investigators also analyzed the screening tool’s performance in predicting a modified outcome consisting of adult persistent dependence on any of the target substances except for tobacco. The predictive power of having any four of the risk factors was similar to that found in the main analysis; however, the two strongest predictors now became adolescent frequent cannabis use, with a 9.5-fold increased risk, and childhood conduct disorder, with a relative risk of 5.42.
Regarding childhood conduct disorder as a risk factor, Dr. van den Brink said, “If you are a child with conduct disorder, your chances of becoming substance dependent in coming years is more than fivefold greater than in a child without conduct disorder.”
This raises the question of whether effective treatment of childhood conduct disorder might prevent later development of persistent substance dependence in adulthood. The answer remains unknown. Although there is no approved drug therapy for conduct disorder, methylphenidate is widely prescribed, especially in young patients with comorbid attention-deficit/hyperactivity disorder.
Several years ago a meta-analysis of 15 longitudinal studies with more than 2,500 participants concluded that stimulant therapy of childhood ADHD neither increased nor reduced the risk of subsequent substance use disorders (JAMA Psychiatry. 2013 Jul;70[7]:740-9). Prescribing physicians were happy to hear they weren’t causing iatrogenic injury, but Dr. van den Brink said he was never comfortable with the investigators’ conclusion.
“There was a lot of heterogeneity in the data, so the overall conclusion might not be the best conclusion,” he said.
He said has become more convinced of that than ever as a result of a recent randomized, double-blind, placebo-controlled MRI study of cerebral blood flow in response to methylphenidate in stimulant-naive patients with childhood or adult AHDH. The investigators found that MRIs obtained 1 week after the conclusion of 16 weeks of methylphenidate therapy showed increased blood flow in the strial and thalamic areas in the pediatric ADHD patients but not in the adults with ADHD (JAMA Psychiatry. 2016 Sep 1;73[9]:955-62).
This is evidence of an age-dependent sustained effect of methylphenidate therapy on dopamine striatal-thalamic circuitry in children that’s not related to the drug’s clinical effects, which were gone after a week off therapy. The question is, Does this effect represent neurotoxicity, or is it an expression of enhanced brain maturation? Dr. van den Brink said he suspects it’s the latter but cannot exclude the former possibility.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
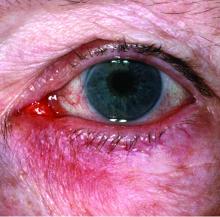
Ocular rosacea remains a stubborn foe
Few skin disorders have the power to devastate lives like ocular rosacea, a painful condition that disrupts vision and can lead to blindness, according to ophthalmologist Edward Wladis, MD.
“Patients really suffer from this diagnosis,” said Dr. Wladis, who practices in Slingerlands, N.Y.
Charles Slonim, MD, an ophthalmologist who practices in Tampa, Fla., put it this way: “We control the condition more than 50 percent of the time, but frequently patients go into periods of remission only to have a recurrence or exacerbation of their ocular rosacea.”
Dr. Wladis coauthored a 2013 report that examined treatments for ocular rosacea, which noted that estimates of the proportion of people with rosacea in the United States who develop ocular rosacea vary, ranging from 58% to 72% (US Ophthalmic Review, 2013;6[2]:86-8).
“Ocular rosacea is one of the subtypes of this disease of cutaneous inflammation,” Dr. Wladis said. “Once the skin becomes so severely inflamed, the glands that lubricate the eye become damaged, and the tear film evaporates rapidly. As a result, patients complain of the effects of a dry ocular surface, and they suffer from blurred vision, tearing, pain, and problems with glare.”
Dr. Slonim suggests that dermatologists refer rosacea patients to an ophthalmologist if they present with any eye symptom, such as dryness, burning, or itching, foreign body sensation in one or both eyes, or chronic redness of either the eyes or the eyelid margins. “They should be seen should be seen by an ophthalmologist to rule out ocular rosacea,” he said. “The ophthalmologist’s ability to look at the eye and eyelids under high magnification – a slit lamp examination – gives us an advantage in the diagnosis of ocular rosacea.”
If these patients do have ocular rosacea, their prognosis is unclear. “Unfortunately, many of our treatments haven’t been carefully vetted,” Dr. Wladis said.
He tends to begin with simpler treatments to heal the ocular surface, such as artificial tears and plugs in the tear drainage ducts to keep tears from leaving the eye quickly. Eyelid scrubs and warm compresses can also be helpful, he said, along with suggestions about lifestyle modifications to avoid the triggers that may exacerbate rosacea.
If those treatments fail, antibiotics are an option.
A 2015 Cochrane Review of studies of rosacea treatments suggested that for treating ocular rosacea, cyclosporine 0.05% ophthalmic emulsion “appeared to be more effective than artificial tears” (Cochrane Database Syst Rev. 2015 Apr 28;[3]:CD003262). And a 2015 study of 38 patients with ocular rosacea concluded that topical cyclosporine was significantly more effective in relieving symptoms and in the treatment of eyelid signs, compared with oral doxycycline (Int J Ophthalmol. 2015 Jun 18;8[3]:544-9).
Antibiotics seem to improve the eyelid’s health, “although some studies have documented that the cornea often doesn’t benefit from antibiotics, and patients’ visual acuity may not improve,” Dr. Wladis said.
Ophthalmologists also may prescribe nonsteroidal and steroidal anti-inflammatory drops, Dr. Slonim added, although “the use of topical ophthalmic steroids do carry the risk of secondary glaucoma with increased intraocular pressures.”
There are even more alternatives. “Dietary modification with omega-3 fatty acids appears to benefit the quality of the tear film,” Dr. Wladis said, referring to the results of a prospective, placebo-controlled, double-blind, randomized trial of patients with dry eye (Int J Ophthalmol. 2013 Dec 18;[6]:811-6). “Intraductal meibomian gland probing and intense pulsed light therapy have both been shown to improve ocular surface–related quality of life, although these treatments are relatively invasive and can rapidly become quite expensive for the patient.”
What’s on the horizon? Researchers have “started to unlock the mysteries of rosacea at the cellular level,” Dr. Wladis said. “Our efforts have recently focused on the cellular changes in the skin of rosacea patients. Using several methods, we assayed the activation of a wide variety of signals within the cells of the skin of these patients and found a consistent elevation of two specific signals.”
The researchers were especially pleased, he said, “that these signals appear to be activated in the outer layers of the skin, meaning that a topical preparation could be developed to selectively suppress these cell signals to turn off the disease without interfering with normal skin structure and function and without the side effects of oral or intravenous medications.”
His team is now working on a topical medication. “Ideally,” he noted, “future clinicians will be able to shift their focus from nonspecific therapies like antibiotics and steroids to really powerful, meaningful cellular therapeutics.”
Dr. Slonim reported no relevant disclosures. Dr. Wladis shares a provisional patent for the use of topical kinase inhibitors in the management of rosacea and recently co-started a biotechnology company called Praxis Biotechnology that aims to develop and test therapies for the condition. He serves as a consultant for both Bausch & Lomb and Valeant Pharmaceuticals.
Few skin disorders have the power to devastate lives like ocular rosacea, a painful condition that disrupts vision and can lead to blindness, according to ophthalmologist Edward Wladis, MD.
“Patients really suffer from this diagnosis,” said Dr. Wladis, who practices in Slingerlands, N.Y.
Charles Slonim, MD, an ophthalmologist who practices in Tampa, Fla., put it this way: “We control the condition more than 50 percent of the time, but frequently patients go into periods of remission only to have a recurrence or exacerbation of their ocular rosacea.”
Dr. Wladis coauthored a 2013 report that examined treatments for ocular rosacea, which noted that estimates of the proportion of people with rosacea in the United States who develop ocular rosacea vary, ranging from 58% to 72% (US Ophthalmic Review, 2013;6[2]:86-8).
“Ocular rosacea is one of the subtypes of this disease of cutaneous inflammation,” Dr. Wladis said. “Once the skin becomes so severely inflamed, the glands that lubricate the eye become damaged, and the tear film evaporates rapidly. As a result, patients complain of the effects of a dry ocular surface, and they suffer from blurred vision, tearing, pain, and problems with glare.”
Dr. Slonim suggests that dermatologists refer rosacea patients to an ophthalmologist if they present with any eye symptom, such as dryness, burning, or itching, foreign body sensation in one or both eyes, or chronic redness of either the eyes or the eyelid margins. “They should be seen should be seen by an ophthalmologist to rule out ocular rosacea,” he said. “The ophthalmologist’s ability to look at the eye and eyelids under high magnification – a slit lamp examination – gives us an advantage in the diagnosis of ocular rosacea.”
If these patients do have ocular rosacea, their prognosis is unclear. “Unfortunately, many of our treatments haven’t been carefully vetted,” Dr. Wladis said.
He tends to begin with simpler treatments to heal the ocular surface, such as artificial tears and plugs in the tear drainage ducts to keep tears from leaving the eye quickly. Eyelid scrubs and warm compresses can also be helpful, he said, along with suggestions about lifestyle modifications to avoid the triggers that may exacerbate rosacea.
If those treatments fail, antibiotics are an option.
A 2015 Cochrane Review of studies of rosacea treatments suggested that for treating ocular rosacea, cyclosporine 0.05% ophthalmic emulsion “appeared to be more effective than artificial tears” (Cochrane Database Syst Rev. 2015 Apr 28;[3]:CD003262). And a 2015 study of 38 patients with ocular rosacea concluded that topical cyclosporine was significantly more effective in relieving symptoms and in the treatment of eyelid signs, compared with oral doxycycline (Int J Ophthalmol. 2015 Jun 18;8[3]:544-9).
Antibiotics seem to improve the eyelid’s health, “although some studies have documented that the cornea often doesn’t benefit from antibiotics, and patients’ visual acuity may not improve,” Dr. Wladis said.
Ophthalmologists also may prescribe nonsteroidal and steroidal anti-inflammatory drops, Dr. Slonim added, although “the use of topical ophthalmic steroids do carry the risk of secondary glaucoma with increased intraocular pressures.”
There are even more alternatives. “Dietary modification with omega-3 fatty acids appears to benefit the quality of the tear film,” Dr. Wladis said, referring to the results of a prospective, placebo-controlled, double-blind, randomized trial of patients with dry eye (Int J Ophthalmol. 2013 Dec 18;[6]:811-6). “Intraductal meibomian gland probing and intense pulsed light therapy have both been shown to improve ocular surface–related quality of life, although these treatments are relatively invasive and can rapidly become quite expensive for the patient.”
What’s on the horizon? Researchers have “started to unlock the mysteries of rosacea at the cellular level,” Dr. Wladis said. “Our efforts have recently focused on the cellular changes in the skin of rosacea patients. Using several methods, we assayed the activation of a wide variety of signals within the cells of the skin of these patients and found a consistent elevation of two specific signals.”
The researchers were especially pleased, he said, “that these signals appear to be activated in the outer layers of the skin, meaning that a topical preparation could be developed to selectively suppress these cell signals to turn off the disease without interfering with normal skin structure and function and without the side effects of oral or intravenous medications.”
His team is now working on a topical medication. “Ideally,” he noted, “future clinicians will be able to shift their focus from nonspecific therapies like antibiotics and steroids to really powerful, meaningful cellular therapeutics.”
Dr. Slonim reported no relevant disclosures. Dr. Wladis shares a provisional patent for the use of topical kinase inhibitors in the management of rosacea and recently co-started a biotechnology company called Praxis Biotechnology that aims to develop and test therapies for the condition. He serves as a consultant for both Bausch & Lomb and Valeant Pharmaceuticals.
Few skin disorders have the power to devastate lives like ocular rosacea, a painful condition that disrupts vision and can lead to blindness, according to ophthalmologist Edward Wladis, MD.
“Patients really suffer from this diagnosis,” said Dr. Wladis, who practices in Slingerlands, N.Y.
Charles Slonim, MD, an ophthalmologist who practices in Tampa, Fla., put it this way: “We control the condition more than 50 percent of the time, but frequently patients go into periods of remission only to have a recurrence or exacerbation of their ocular rosacea.”
Dr. Wladis coauthored a 2013 report that examined treatments for ocular rosacea, which noted that estimates of the proportion of people with rosacea in the United States who develop ocular rosacea vary, ranging from 58% to 72% (US Ophthalmic Review, 2013;6[2]:86-8).
“Ocular rosacea is one of the subtypes of this disease of cutaneous inflammation,” Dr. Wladis said. “Once the skin becomes so severely inflamed, the glands that lubricate the eye become damaged, and the tear film evaporates rapidly. As a result, patients complain of the effects of a dry ocular surface, and they suffer from blurred vision, tearing, pain, and problems with glare.”
Dr. Slonim suggests that dermatologists refer rosacea patients to an ophthalmologist if they present with any eye symptom, such as dryness, burning, or itching, foreign body sensation in one or both eyes, or chronic redness of either the eyes or the eyelid margins. “They should be seen should be seen by an ophthalmologist to rule out ocular rosacea,” he said. “The ophthalmologist’s ability to look at the eye and eyelids under high magnification – a slit lamp examination – gives us an advantage in the diagnosis of ocular rosacea.”
If these patients do have ocular rosacea, their prognosis is unclear. “Unfortunately, many of our treatments haven’t been carefully vetted,” Dr. Wladis said.
He tends to begin with simpler treatments to heal the ocular surface, such as artificial tears and plugs in the tear drainage ducts to keep tears from leaving the eye quickly. Eyelid scrubs and warm compresses can also be helpful, he said, along with suggestions about lifestyle modifications to avoid the triggers that may exacerbate rosacea.
If those treatments fail, antibiotics are an option.
A 2015 Cochrane Review of studies of rosacea treatments suggested that for treating ocular rosacea, cyclosporine 0.05% ophthalmic emulsion “appeared to be more effective than artificial tears” (Cochrane Database Syst Rev. 2015 Apr 28;[3]:CD003262). And a 2015 study of 38 patients with ocular rosacea concluded that topical cyclosporine was significantly more effective in relieving symptoms and in the treatment of eyelid signs, compared with oral doxycycline (Int J Ophthalmol. 2015 Jun 18;8[3]:544-9).
Antibiotics seem to improve the eyelid’s health, “although some studies have documented that the cornea often doesn’t benefit from antibiotics, and patients’ visual acuity may not improve,” Dr. Wladis said.
Ophthalmologists also may prescribe nonsteroidal and steroidal anti-inflammatory drops, Dr. Slonim added, although “the use of topical ophthalmic steroids do carry the risk of secondary glaucoma with increased intraocular pressures.”
There are even more alternatives. “Dietary modification with omega-3 fatty acids appears to benefit the quality of the tear film,” Dr. Wladis said, referring to the results of a prospective, placebo-controlled, double-blind, randomized trial of patients with dry eye (Int J Ophthalmol. 2013 Dec 18;[6]:811-6). “Intraductal meibomian gland probing and intense pulsed light therapy have both been shown to improve ocular surface–related quality of life, although these treatments are relatively invasive and can rapidly become quite expensive for the patient.”
What’s on the horizon? Researchers have “started to unlock the mysteries of rosacea at the cellular level,” Dr. Wladis said. “Our efforts have recently focused on the cellular changes in the skin of rosacea patients. Using several methods, we assayed the activation of a wide variety of signals within the cells of the skin of these patients and found a consistent elevation of two specific signals.”
The researchers were especially pleased, he said, “that these signals appear to be activated in the outer layers of the skin, meaning that a topical preparation could be developed to selectively suppress these cell signals to turn off the disease without interfering with normal skin structure and function and without the side effects of oral or intravenous medications.”
His team is now working on a topical medication. “Ideally,” he noted, “future clinicians will be able to shift their focus from nonspecific therapies like antibiotics and steroids to really powerful, meaningful cellular therapeutics.”
Dr. Slonim reported no relevant disclosures. Dr. Wladis shares a provisional patent for the use of topical kinase inhibitors in the management of rosacea and recently co-started a biotechnology company called Praxis Biotechnology that aims to develop and test therapies for the condition. He serves as a consultant for both Bausch & Lomb and Valeant Pharmaceuticals.
Emergency Imaging: Facial Trauma After a Fall
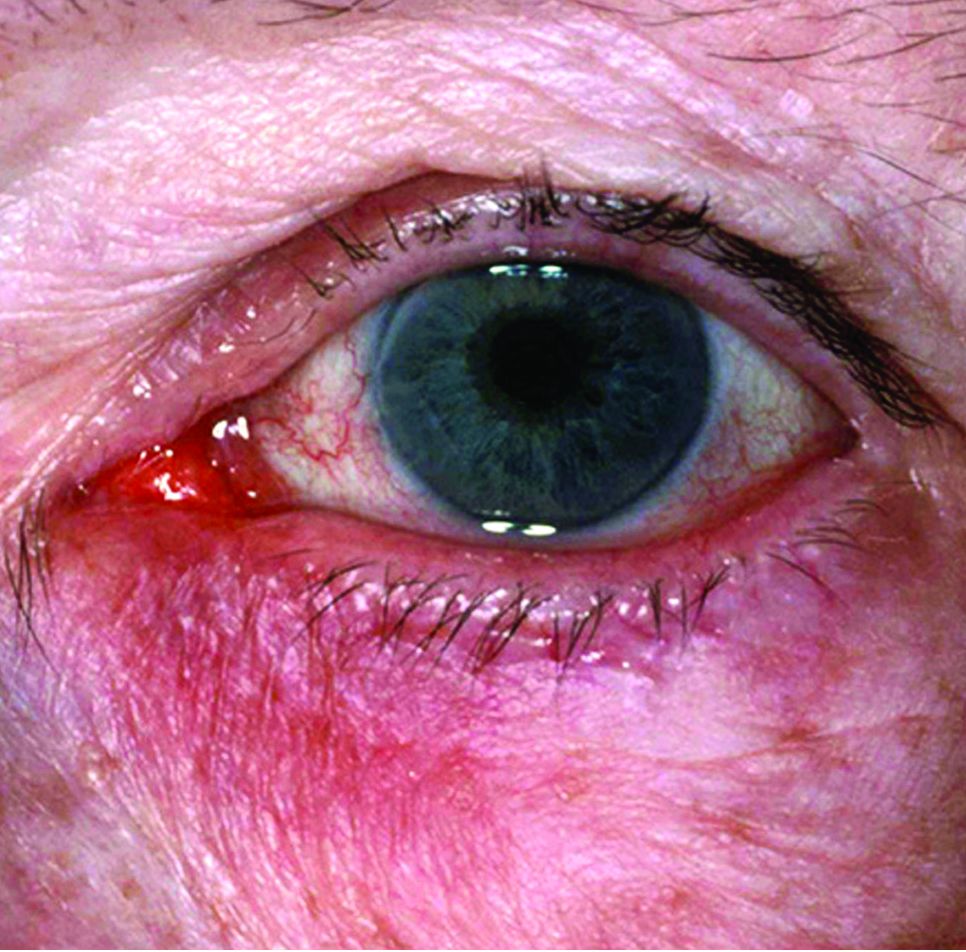
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
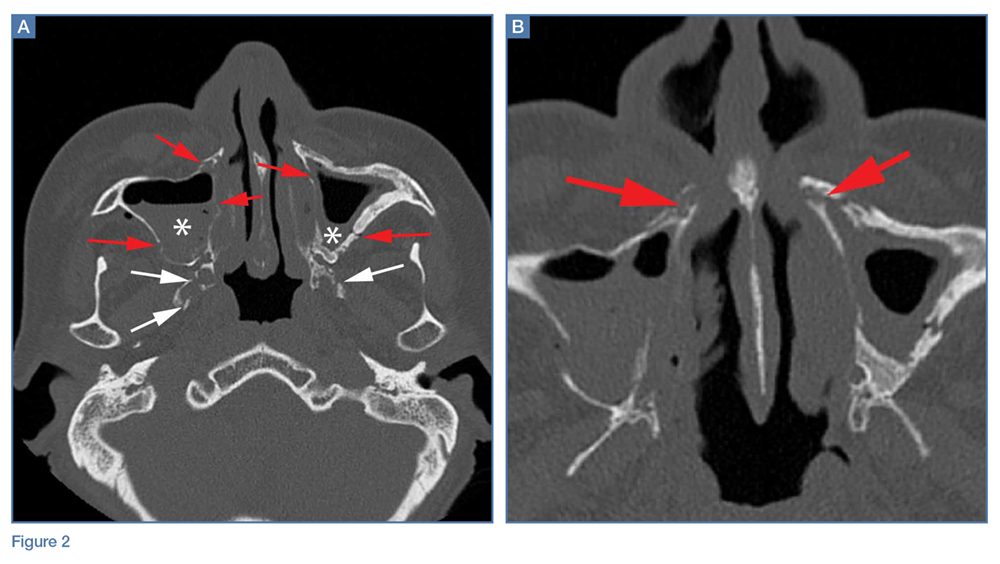
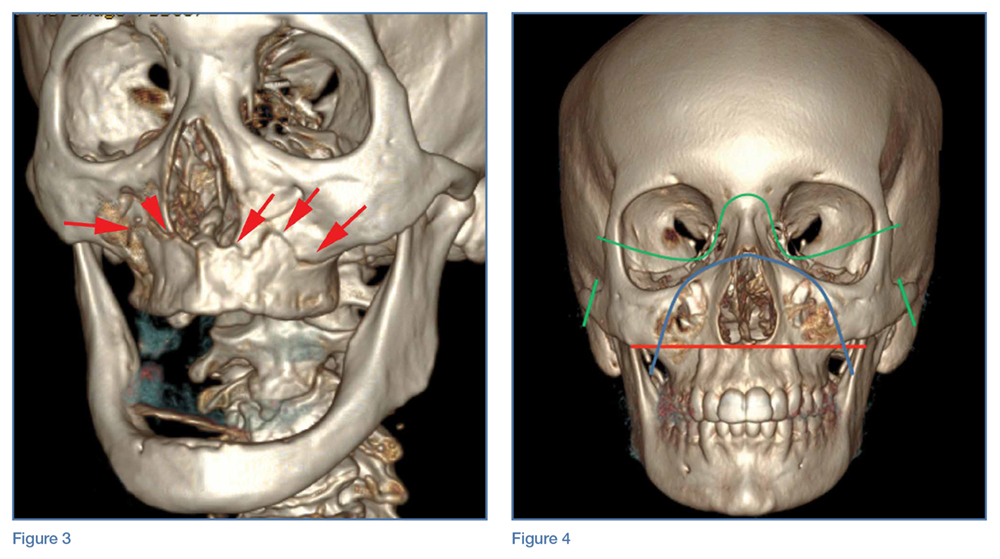
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).
Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
First EDition: Retail Clinics and Rate of ED Visits, more
Retail Clinics Have Not Decreased the Rate of Low-Acuity ED Visits
BY JEFF BAUER
The number of retail clinics—those located in pharmacies, supermarkets, and other retail settings—in the United States increased from 130 in 2006 to nearly 1,400 in 2012. However, this proliferation of retail clinics has not lead to a meaningful reduction in low-acuity ED visits, according to a recent observational study published in Annals of Emergency Medicine.1
Using information from the Healthcare Cost and Utilization Project State Emergency Department Databases, which include data on more than 2,000 EDs in 23 states from 2006 through 2013, researchers looked at the association between retail clinic penetration and the rate of treat-and-release ED visits for 11 low-acuity conditions (allergic rhinitis, bronchitis, conjunctivitis, other eye conditions, influenza, otitis externa, otitis media, pharyngitis, upper respiratory infections/sinusitis, urinary tract infections, and viral infections).
Retail clinic penetration was defined as the percentage of an ED’s catchment area (areas that accounted for up to 75% of patients who visited for low-acuity conditions) that overlapped with the 10-minute-drive radius of a retail clinic. The results were calculated as a rate ratio, which reflected the change in the rate of low-acuity ED visits associated with an ED having no retail clinic penetration to having approximately the average penetration rate within 2012. Results were controlled for the number of urgent care centers that were present in each ED catchment area, but only for hospital-associated urgent care centers, as there are no reliable data to identify all urgent care centers.
Retail clinic penetration more than doubled during the study period. Overall, increased retail clinic penetration was not associated with a change in the rate of low-acuity ED visits. Among patients with private insurance, there was a small reduction (0.3% per calendar quarter) in ED visits for low-acuity conditions, but this translated into an estimated 17 fewer ED visits by privately insured patients over 1 year for the average ED, assuming the retail clinic penetration rate increased by 40% in that year.
In an accompanying editorial,2 Jesse M. Pines, MD, suggests that visits to retail clinics may be mostly “new-use” visits, meaning many individuals who would not have otherwise received treatment seek care in a retail clinic because such clinics are available. Dr Pines proposed three reasons retail clinics may create new-use visits: they meet unmet demands for care; motivations for seeking care differ in EDs and retail clinics; and people who are more likely to use EDs for low-acuity conditions do so because they have limited access to other types of care, including retail clinics.
1. Martsolf G, Fingar KR, Coffey R, et al. Association between the opening of retail clinics and low-acuity emergency department visits. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.08.462.
2. Pines JM. Why retail clinics do not substitute for emergency department visits and what this means for value-based care. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.09.047.
Hypotension During Transport to ED Drives Mortality in Traumatic Brain Injury
MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
The severity and duration of hypotension in traumatic brain injury (TBI) patients during emergency medical service (EMS) transport to an ED has a tight and essentially linear relationship to mortality rate during subsequent weeks of recovery, according to an analysis of more than 7,500 brain-injured patients.
For each doubling of the combined severity and duration of hypotension during the prehospital period, when systolic blood pressure (BP) was <90 mm Hg, patient mortality rose by 19%, Daniel W. Spaite, MD, reported at the American Heart Association scientific sessions.
However, the results do not address whether aggressive treatment of hypotension by EMS technicians in a patient with TBI leads to reduced mortality. That question is being assessed as part of the primary endpoint of the Excellence in Prehospital Injury Care-Traumatic Brain Injury (EPIC-TBI) study, which should be completed by the end of 2017, said Dr Spaite, professor of emergency medicine at the University of Arizona in Tucson.Results from prior studies have clearly linked prehospital hypotension with worse survival in TBI patients. Until now, however, there was no appreciation of the fact that not all hypotensive episodes are equal, and that both the severity of hypotension and its duration incrementally contribute to mortality as the “dose” of hypotension a patient experiences increases. In large part, this is because prehospital hypotension has been recorded simply as a dichotomous, yes/no condition.
The innovation introduced by Dr Spaite and his associates in their analysis of the EPIC-TBI data was to drill down into each patient’s hypotensive event, made possible by the 16,711 patients enrolled in EPIC-TBI. Their calculations were limited to patients with EMS records of at least two BP measurements during prehospital transport. These data allowed Spaite et al to utilize both the extent to which systolic BP dropped below 90 mm Hg and the amount of time systolic BP was below this threshold to better define the total hypotension exposure each patient received.
This meant that a patient with a TBI and a systolic BP of 80 mm Hg for 10 minutes had twice the hypotension exposure of both a patient with a systolic BP of 85 mm Hg for 10 minutes and a patient with a systolic BP of 80 mm Hg for 5 minutes.
The analysis by Spaite et al also adjusted the relationship of total hypotensive severity and duration and subsequent mortality based on several baseline demographic and clinical variables, including age, sex, injury severity, trauma type, and head-region severity score. After adjustment, the researchers found a “strikingly linear relationship” between hypotension severity and duration and mortality, Dr Spaite said.
The EPIC-TBI enrolled TBI patients aged 10 years or older during 2007 to 2014 through participation of dozens of EMS providers throughout Arizona. For the current analysis, the researchers identified 7,521 patients from the total group who had at least two BP measurements taken during their prehospital EMS care and also met other inclusion criteria.
The best way to manage hypotension in TBI patients during the prehospital period remains unclear. Simply raising BP via intravenous (IV) fluid infusion may not necessarily help, because it could exacerbate a patient’s bleeding, Dr Spaite noted during an interview.
The primary goal of EPIC-TBI is to assess the implementation of the third edition of the TBI guidelines released in 2007 by the Brain Trauma Foundation. (The fourth edition of these guidelines came out in August 2016.) The new finding by Dr Spaite and his associates will allow the full EPIC-TBI analysis to correlate patient outcomes with the impact that acute, prehospital treatment had on the hypotension severity and duration each patient experienced, he noted.
“What’s remarkable is that the single prehospital parameter of hypotension for just a few minutes during transport can have such a strong impact on survival, given all the other factors that can influence outcomes” in TBI patients once they reach a hospital and during the period they remain hospitalized, Dr Spaite said.
1. Spaite DW. Presentation at: American Heart Association Scientific Sessions 2016. November 12-16, 2016; New Orleans, LA.
Fluid Administration in Sepsis Did Not Increase Need for Dialysis
M. Alexander Otto
FRONTLINE MEDICAL NEWS
Fluid administration of at least 1 L did not increase the incidence of acute respiratory or heart failure in severe sepsis, and actually seemed to decrease the need for dialysis in a review of 164 patients at Scott and White Memorial Hospital in Temple, Texas.
For every 1 mL of fluid administered per kilogram of body weight, the likelihood of dialysis decreased by 8.5% (odds ratio [OR], 0.915; 95% confidence interval [CI], 0.854-0.980; P = .0111), with no increase in heart or respiratory failure on univariate analysis. The 126 patients (77%) who received at least 1 L of fluid had a 68% reduction in the need for dialysis (OR, 0.32; CI, 0.117-0.890; P = .0288).
These findings come from a quality improvement project the hospital launched after researchers there realized that the benchmark Surviving Sepsis Campaign guidelines were not being met. The patients in the study had a systolic BP below 90 mm Hg or lactate level of at least 4 mmol/L. The guidelines would have called for these patients to receive 30 mL/kg of crystalloid fluids within 3 hours of presentation, but only 28 patients (17%) met that mark.
“The No. 1 reason we weren’t meeting benchmarks was fluid administration,” explained lead investigator Aruna Jahoor, MD, a pulmonary critical care and sleep medicine fellow at Texas Tech University Health Sciences Center.
Seventeen percent of patients received ≥30 mL/kg of fluid resuscitation, while 28% received ≥20 mL/kg of IV fluid resuscitation. It turned out that staff in the ED—where most of the patients were treated in the critical first 6 hours—were concerned about fluid overload and putting patients into respiratory, heart, or renal failure, Dr Jahoor said. The team found no difference in mortality rates when patients received 30 mL/kg—just over 2 L in a patient weighing 70 kg—vs 20 mL/kg or 1 L. The patients’ in-hospital mortality rate and 28-day mortality rate were 27% and 32%, respectively.
There also were no increased rates of heart failure, acute respiratory failure, or mechanical ventilation when patients received at least 1 L of fluid. “There were [also] lower rates of dialysis, which indicated that we weren’t overloading patients. Even when we looked at fluid as a continuous variable, we still didn’t see” complications, Dr Jahoor said.
The findings should be reassuring to treating physicians. “When you have pushback against 30-mL/kg administration, you can say ‘well, at least let’s give a liter.’ You don’t have to worry as much about some of the complications you are citing,’ ” she said.
For very obese patients, “it can get a little uncomfortable to be given” enough fluid to meet the 30-mL/kg goal, “but you can give at least a liter” without having to worry too much, she said. The patients in the study were treated from 2010 to 2013; normal saline was the most common resuscitation fluid. The hospital has since added the 30-mL/kg fluid resuscitation to its sepsis admission orders, and compliance has increased significantly.
A multivariate analysis is in the works to control for confounders. “We will probably [still] see you are not having increased rates of congestive heart or respiratory failure, or needing dialysis,” Dr Jahoor said. The protective effect against dialysis might drop out, “but I am hoping it doesn’t,” she said.
1. Jahoor A, Delmas T, Giri B, et al. Fluid resuscitation of at least 1 liter in septic patients decreases the need for renal replacement therapy without increasing the risk of acute congestive heart failure or acute respiratory failure. Chest. 2016;150(4_S):349A. doi:10.1016/j.chest.2016.08.362.
Survey: Antibiotic Shortages Are the New Norm
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a Web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs 73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the 2016 survey, the top 10 antimicrobials reported as being in short supply over the past 2 years were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. Trimethoprim-sulfamethoxazole and acyclovir were in short supply in 2011 and 2016.
According to respondents, the most common ways they learned about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the US Food and Drug Administration (FDA) Web site or another Web site (23%). The most common ways of learning about a shortage changed—from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr Gundlapalli said, noting ampicillin-sulbactam in 2016 and penicillin G procaine as examples.
“And then, of course, the other theme across the board...was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal—a way of life,” Dr Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive...maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
Donald Graham, MD, one of the study’s coauthors, said he believes the problem is in part the result of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it is not always in the financial best interest of a pharmaceutical company to upgrade their facilities.
1. Gundlapalli A. Presentation at: IDWeek 2016. October 26-30, 2016. New Orleans, LA.
Hospitalizations for Opioid Poisoning Tripled in Preschool Children
Richard Franki
FRONTLINE MEDICAL NEWS
From 1997 to 2012, the annual number of hospitalizations for opioid poisoning rose 178% among children aged 1 to 19 years, according to data from 13,052 discharges in the Agency for Healthcare Research and Quality’s Kids’ Inpatient Database.
In 2012, there were 2,918 hospitalizations for opioid poisoning among children aged 1 to 19 years, compared with 1,049 in 1997, reported Julie R. Gaither, PhD, MPH, RN, and her associates at Yale University in New Haven, Connecticut.
The greatest change occurred among the youngest children, as the number of those aged 1 to 4 years rose from 133 in 1997 to 421 in 2012—an increase of 217%. For those aged 15 to 19 years, the annual number of hospitalizations went from 715 to 2,171 (204%) over that time period, which included a slight drop from 2009 to 2012, according to the investigators,
The increase in hospitalizations for prescription opioid poisoning in children aged 10 to 14 years was 58% from 1997 to 2012 (rising from 171 to 272), while estimates for 5- to 9-year-old children did not meet the criteria for statistical reliability and were not included in the analysis, Dr Gaither and her associates said.
1. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016 Oct 31. Epub ahead of print. doi:10.1001/jamapediatrics.2016.2154.
Pelvic Fracture Pattern Predicts the Need for Hemorrhage Control
Doug Brunk
FRONTLINE MEDICAL NEWS
Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage-control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma (AAST). They identified three main force patterns: lateral compression, anterior posterior compression, and vertical shear.
“They were able to show that certain pelvic fractures were associated with soft-tissue injury and pelvic hemorrhage,” said Dr Costantini, of the division of trauma, surgical critical care, burns and acute care surgery at the University of California, San Diego. “Since then, several single-center studies have been conducted in an attempt to correlate fracture pattern with the risk of pelvic hemorrhage. A majority of these studies evaluated angiogram [and embolization] as the endpoint for hemorrhage control. Modern trauma care has evolved to include multiple modalities to control hemorrhage, which include pelvic external fixator placement, pelvic angiography and embolization, preperitoneal pelvic packing, and the use of the REBOA [Resuscitative Endovascular Balloon Occlusion of the Aorta] catheter as an adjunct to hemorrhage control.”
In a recently published study, Dr Costantini and his associates found wide variability in the use of pelvic hemorrhage-control methods.1 “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level 1 trauma centers over a 2-year period.2 Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over age 18 years, blunt mechanism of injury, and shock on admission defined as “...systolic blood pressure <90 mm Hg or heart rate >120 beats per minute or base deficit <-5.”1 Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr Costantini said. Next, they used univariate and multivariate logistic regression analyses to examine predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the 2-year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic BP was 93 mm Hg, their mean HR was 117 beats/min, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian vs auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage-control intervention, those who did received more transfusion of packed red blood cells (13 vs 7 units, respectively; P < .01) and fresh frozen plasma (10 U vs 5 U; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage-control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (OR, 109.43; P < .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
1. Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717-723; discussion 723-725. doi:10.1097/TA.0000000000001034.2. Costantini TW. Presentation at: 75th Annual Meeting of American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery. September 14-17, 2016. Waikoloa, Hawaii.
Retail Clinics Have Not Decreased the Rate of Low-Acuity ED Visits
BY JEFF BAUER
The number of retail clinics—those located in pharmacies, supermarkets, and other retail settings—in the United States increased from 130 in 2006 to nearly 1,400 in 2012. However, this proliferation of retail clinics has not lead to a meaningful reduction in low-acuity ED visits, according to a recent observational study published in Annals of Emergency Medicine.1
Using information from the Healthcare Cost and Utilization Project State Emergency Department Databases, which include data on more than 2,000 EDs in 23 states from 2006 through 2013, researchers looked at the association between retail clinic penetration and the rate of treat-and-release ED visits for 11 low-acuity conditions (allergic rhinitis, bronchitis, conjunctivitis, other eye conditions, influenza, otitis externa, otitis media, pharyngitis, upper respiratory infections/sinusitis, urinary tract infections, and viral infections).
Retail clinic penetration was defined as the percentage of an ED’s catchment area (areas that accounted for up to 75% of patients who visited for low-acuity conditions) that overlapped with the 10-minute-drive radius of a retail clinic. The results were calculated as a rate ratio, which reflected the change in the rate of low-acuity ED visits associated with an ED having no retail clinic penetration to having approximately the average penetration rate within 2012. Results were controlled for the number of urgent care centers that were present in each ED catchment area, but only for hospital-associated urgent care centers, as there are no reliable data to identify all urgent care centers.
Retail clinic penetration more than doubled during the study period. Overall, increased retail clinic penetration was not associated with a change in the rate of low-acuity ED visits. Among patients with private insurance, there was a small reduction (0.3% per calendar quarter) in ED visits for low-acuity conditions, but this translated into an estimated 17 fewer ED visits by privately insured patients over 1 year for the average ED, assuming the retail clinic penetration rate increased by 40% in that year.
In an accompanying editorial,2 Jesse M. Pines, MD, suggests that visits to retail clinics may be mostly “new-use” visits, meaning many individuals who would not have otherwise received treatment seek care in a retail clinic because such clinics are available. Dr Pines proposed three reasons retail clinics may create new-use visits: they meet unmet demands for care; motivations for seeking care differ in EDs and retail clinics; and people who are more likely to use EDs for low-acuity conditions do so because they have limited access to other types of care, including retail clinics.
1. Martsolf G, Fingar KR, Coffey R, et al. Association between the opening of retail clinics and low-acuity emergency department visits. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.08.462.
2. Pines JM. Why retail clinics do not substitute for emergency department visits and what this means for value-based care. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.09.047.
Hypotension During Transport to ED Drives Mortality in Traumatic Brain Injury
MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
The severity and duration of hypotension in traumatic brain injury (TBI) patients during emergency medical service (EMS) transport to an ED has a tight and essentially linear relationship to mortality rate during subsequent weeks of recovery, according to an analysis of more than 7,500 brain-injured patients.
For each doubling of the combined severity and duration of hypotension during the prehospital period, when systolic blood pressure (BP) was <90 mm Hg, patient mortality rose by 19%, Daniel W. Spaite, MD, reported at the American Heart Association scientific sessions.
However, the results do not address whether aggressive treatment of hypotension by EMS technicians in a patient with TBI leads to reduced mortality. That question is being assessed as part of the primary endpoint of the Excellence in Prehospital Injury Care-Traumatic Brain Injury (EPIC-TBI) study, which should be completed by the end of 2017, said Dr Spaite, professor of emergency medicine at the University of Arizona in Tucson.Results from prior studies have clearly linked prehospital hypotension with worse survival in TBI patients. Until now, however, there was no appreciation of the fact that not all hypotensive episodes are equal, and that both the severity of hypotension and its duration incrementally contribute to mortality as the “dose” of hypotension a patient experiences increases. In large part, this is because prehospital hypotension has been recorded simply as a dichotomous, yes/no condition.
The innovation introduced by Dr Spaite and his associates in their analysis of the EPIC-TBI data was to drill down into each patient’s hypotensive event, made possible by the 16,711 patients enrolled in EPIC-TBI. Their calculations were limited to patients with EMS records of at least two BP measurements during prehospital transport. These data allowed Spaite et al to utilize both the extent to which systolic BP dropped below 90 mm Hg and the amount of time systolic BP was below this threshold to better define the total hypotension exposure each patient received.
This meant that a patient with a TBI and a systolic BP of 80 mm Hg for 10 minutes had twice the hypotension exposure of both a patient with a systolic BP of 85 mm Hg for 10 minutes and a patient with a systolic BP of 80 mm Hg for 5 minutes.
The analysis by Spaite et al also adjusted the relationship of total hypotensive severity and duration and subsequent mortality based on several baseline demographic and clinical variables, including age, sex, injury severity, trauma type, and head-region severity score. After adjustment, the researchers found a “strikingly linear relationship” between hypotension severity and duration and mortality, Dr Spaite said.
The EPIC-TBI enrolled TBI patients aged 10 years or older during 2007 to 2014 through participation of dozens of EMS providers throughout Arizona. For the current analysis, the researchers identified 7,521 patients from the total group who had at least two BP measurements taken during their prehospital EMS care and also met other inclusion criteria.
The best way to manage hypotension in TBI patients during the prehospital period remains unclear. Simply raising BP via intravenous (IV) fluid infusion may not necessarily help, because it could exacerbate a patient’s bleeding, Dr Spaite noted during an interview.
The primary goal of EPIC-TBI is to assess the implementation of the third edition of the TBI guidelines released in 2007 by the Brain Trauma Foundation. (The fourth edition of these guidelines came out in August 2016.) The new finding by Dr Spaite and his associates will allow the full EPIC-TBI analysis to correlate patient outcomes with the impact that acute, prehospital treatment had on the hypotension severity and duration each patient experienced, he noted.
“What’s remarkable is that the single prehospital parameter of hypotension for just a few minutes during transport can have such a strong impact on survival, given all the other factors that can influence outcomes” in TBI patients once they reach a hospital and during the period they remain hospitalized, Dr Spaite said.
1. Spaite DW. Presentation at: American Heart Association Scientific Sessions 2016. November 12-16, 2016; New Orleans, LA.
Fluid Administration in Sepsis Did Not Increase Need for Dialysis
M. Alexander Otto
FRONTLINE MEDICAL NEWS
Fluid administration of at least 1 L did not increase the incidence of acute respiratory or heart failure in severe sepsis, and actually seemed to decrease the need for dialysis in a review of 164 patients at Scott and White Memorial Hospital in Temple, Texas.
For every 1 mL of fluid administered per kilogram of body weight, the likelihood of dialysis decreased by 8.5% (odds ratio [OR], 0.915; 95% confidence interval [CI], 0.854-0.980; P = .0111), with no increase in heart or respiratory failure on univariate analysis. The 126 patients (77%) who received at least 1 L of fluid had a 68% reduction in the need for dialysis (OR, 0.32; CI, 0.117-0.890; P = .0288).
These findings come from a quality improvement project the hospital launched after researchers there realized that the benchmark Surviving Sepsis Campaign guidelines were not being met. The patients in the study had a systolic BP below 90 mm Hg or lactate level of at least 4 mmol/L. The guidelines would have called for these patients to receive 30 mL/kg of crystalloid fluids within 3 hours of presentation, but only 28 patients (17%) met that mark.
“The No. 1 reason we weren’t meeting benchmarks was fluid administration,” explained lead investigator Aruna Jahoor, MD, a pulmonary critical care and sleep medicine fellow at Texas Tech University Health Sciences Center.
Seventeen percent of patients received ≥30 mL/kg of fluid resuscitation, while 28% received ≥20 mL/kg of IV fluid resuscitation. It turned out that staff in the ED—where most of the patients were treated in the critical first 6 hours—were concerned about fluid overload and putting patients into respiratory, heart, or renal failure, Dr Jahoor said. The team found no difference in mortality rates when patients received 30 mL/kg—just over 2 L in a patient weighing 70 kg—vs 20 mL/kg or 1 L. The patients’ in-hospital mortality rate and 28-day mortality rate were 27% and 32%, respectively.
There also were no increased rates of heart failure, acute respiratory failure, or mechanical ventilation when patients received at least 1 L of fluid. “There were [also] lower rates of dialysis, which indicated that we weren’t overloading patients. Even when we looked at fluid as a continuous variable, we still didn’t see” complications, Dr Jahoor said.
The findings should be reassuring to treating physicians. “When you have pushback against 30-mL/kg administration, you can say ‘well, at least let’s give a liter.’ You don’t have to worry as much about some of the complications you are citing,’ ” she said.
For very obese patients, “it can get a little uncomfortable to be given” enough fluid to meet the 30-mL/kg goal, “but you can give at least a liter” without having to worry too much, she said. The patients in the study were treated from 2010 to 2013; normal saline was the most common resuscitation fluid. The hospital has since added the 30-mL/kg fluid resuscitation to its sepsis admission orders, and compliance has increased significantly.
A multivariate analysis is in the works to control for confounders. “We will probably [still] see you are not having increased rates of congestive heart or respiratory failure, or needing dialysis,” Dr Jahoor said. The protective effect against dialysis might drop out, “but I am hoping it doesn’t,” she said.
1. Jahoor A, Delmas T, Giri B, et al. Fluid resuscitation of at least 1 liter in septic patients decreases the need for renal replacement therapy without increasing the risk of acute congestive heart failure or acute respiratory failure. Chest. 2016;150(4_S):349A. doi:10.1016/j.chest.2016.08.362.
Survey: Antibiotic Shortages Are the New Norm
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a Web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs 73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the 2016 survey, the top 10 antimicrobials reported as being in short supply over the past 2 years were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. Trimethoprim-sulfamethoxazole and acyclovir were in short supply in 2011 and 2016.
According to respondents, the most common ways they learned about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the US Food and Drug Administration (FDA) Web site or another Web site (23%). The most common ways of learning about a shortage changed—from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr Gundlapalli said, noting ampicillin-sulbactam in 2016 and penicillin G procaine as examples.
“And then, of course, the other theme across the board...was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal—a way of life,” Dr Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive...maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
Donald Graham, MD, one of the study’s coauthors, said he believes the problem is in part the result of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it is not always in the financial best interest of a pharmaceutical company to upgrade their facilities.
1. Gundlapalli A. Presentation at: IDWeek 2016. October 26-30, 2016. New Orleans, LA.
Hospitalizations for Opioid Poisoning Tripled in Preschool Children
Richard Franki
FRONTLINE MEDICAL NEWS
From 1997 to 2012, the annual number of hospitalizations for opioid poisoning rose 178% among children aged 1 to 19 years, according to data from 13,052 discharges in the Agency for Healthcare Research and Quality’s Kids’ Inpatient Database.
In 2012, there were 2,918 hospitalizations for opioid poisoning among children aged 1 to 19 years, compared with 1,049 in 1997, reported Julie R. Gaither, PhD, MPH, RN, and her associates at Yale University in New Haven, Connecticut.
The greatest change occurred among the youngest children, as the number of those aged 1 to 4 years rose from 133 in 1997 to 421 in 2012—an increase of 217%. For those aged 15 to 19 years, the annual number of hospitalizations went from 715 to 2,171 (204%) over that time period, which included a slight drop from 2009 to 2012, according to the investigators,
The increase in hospitalizations for prescription opioid poisoning in children aged 10 to 14 years was 58% from 1997 to 2012 (rising from 171 to 272), while estimates for 5- to 9-year-old children did not meet the criteria for statistical reliability and were not included in the analysis, Dr Gaither and her associates said.
1. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016 Oct 31. Epub ahead of print. doi:10.1001/jamapediatrics.2016.2154.
Pelvic Fracture Pattern Predicts the Need for Hemorrhage Control
Doug Brunk
FRONTLINE MEDICAL NEWS
Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage-control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma (AAST). They identified three main force patterns: lateral compression, anterior posterior compression, and vertical shear.
“They were able to show that certain pelvic fractures were associated with soft-tissue injury and pelvic hemorrhage,” said Dr Costantini, of the division of trauma, surgical critical care, burns and acute care surgery at the University of California, San Diego. “Since then, several single-center studies have been conducted in an attempt to correlate fracture pattern with the risk of pelvic hemorrhage. A majority of these studies evaluated angiogram [and embolization] as the endpoint for hemorrhage control. Modern trauma care has evolved to include multiple modalities to control hemorrhage, which include pelvic external fixator placement, pelvic angiography and embolization, preperitoneal pelvic packing, and the use of the REBOA [Resuscitative Endovascular Balloon Occlusion of the Aorta] catheter as an adjunct to hemorrhage control.”
In a recently published study, Dr Costantini and his associates found wide variability in the use of pelvic hemorrhage-control methods.1 “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level 1 trauma centers over a 2-year period.2 Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over age 18 years, blunt mechanism of injury, and shock on admission defined as “...systolic blood pressure <90 mm Hg or heart rate >120 beats per minute or base deficit <-5.”1 Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr Costantini said. Next, they used univariate and multivariate logistic regression analyses to examine predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the 2-year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic BP was 93 mm Hg, their mean HR was 117 beats/min, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian vs auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage-control intervention, those who did received more transfusion of packed red blood cells (13 vs 7 units, respectively; P < .01) and fresh frozen plasma (10 U vs 5 U; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage-control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (OR, 109.43; P < .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
1. Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717-723; discussion 723-725. doi:10.1097/TA.0000000000001034.2. Costantini TW. Presentation at: 75th Annual Meeting of American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery. September 14-17, 2016. Waikoloa, Hawaii.
Retail Clinics Have Not Decreased the Rate of Low-Acuity ED Visits
BY JEFF BAUER
The number of retail clinics—those located in pharmacies, supermarkets, and other retail settings—in the United States increased from 130 in 2006 to nearly 1,400 in 2012. However, this proliferation of retail clinics has not lead to a meaningful reduction in low-acuity ED visits, according to a recent observational study published in Annals of Emergency Medicine.1
Using information from the Healthcare Cost and Utilization Project State Emergency Department Databases, which include data on more than 2,000 EDs in 23 states from 2006 through 2013, researchers looked at the association between retail clinic penetration and the rate of treat-and-release ED visits for 11 low-acuity conditions (allergic rhinitis, bronchitis, conjunctivitis, other eye conditions, influenza, otitis externa, otitis media, pharyngitis, upper respiratory infections/sinusitis, urinary tract infections, and viral infections).
Retail clinic penetration was defined as the percentage of an ED’s catchment area (areas that accounted for up to 75% of patients who visited for low-acuity conditions) that overlapped with the 10-minute-drive radius of a retail clinic. The results were calculated as a rate ratio, which reflected the change in the rate of low-acuity ED visits associated with an ED having no retail clinic penetration to having approximately the average penetration rate within 2012. Results were controlled for the number of urgent care centers that were present in each ED catchment area, but only for hospital-associated urgent care centers, as there are no reliable data to identify all urgent care centers.
Retail clinic penetration more than doubled during the study period. Overall, increased retail clinic penetration was not associated with a change in the rate of low-acuity ED visits. Among patients with private insurance, there was a small reduction (0.3% per calendar quarter) in ED visits for low-acuity conditions, but this translated into an estimated 17 fewer ED visits by privately insured patients over 1 year for the average ED, assuming the retail clinic penetration rate increased by 40% in that year.
In an accompanying editorial,2 Jesse M. Pines, MD, suggests that visits to retail clinics may be mostly “new-use” visits, meaning many individuals who would not have otherwise received treatment seek care in a retail clinic because such clinics are available. Dr Pines proposed three reasons retail clinics may create new-use visits: they meet unmet demands for care; motivations for seeking care differ in EDs and retail clinics; and people who are more likely to use EDs for low-acuity conditions do so because they have limited access to other types of care, including retail clinics.
1. Martsolf G, Fingar KR, Coffey R, et al. Association between the opening of retail clinics and low-acuity emergency department visits. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.08.462.
2. Pines JM. Why retail clinics do not substitute for emergency department visits and what this means for value-based care. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.09.047.
Hypotension During Transport to ED Drives Mortality in Traumatic Brain Injury
MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
The severity and duration of hypotension in traumatic brain injury (TBI) patients during emergency medical service (EMS) transport to an ED has a tight and essentially linear relationship to mortality rate during subsequent weeks of recovery, according to an analysis of more than 7,500 brain-injured patients.
For each doubling of the combined severity and duration of hypotension during the prehospital period, when systolic blood pressure (BP) was <90 mm Hg, patient mortality rose by 19%, Daniel W. Spaite, MD, reported at the American Heart Association scientific sessions.
However, the results do not address whether aggressive treatment of hypotension by EMS technicians in a patient with TBI leads to reduced mortality. That question is being assessed as part of the primary endpoint of the Excellence in Prehospital Injury Care-Traumatic Brain Injury (EPIC-TBI) study, which should be completed by the end of 2017, said Dr Spaite, professor of emergency medicine at the University of Arizona in Tucson.Results from prior studies have clearly linked prehospital hypotension with worse survival in TBI patients. Until now, however, there was no appreciation of the fact that not all hypotensive episodes are equal, and that both the severity of hypotension and its duration incrementally contribute to mortality as the “dose” of hypotension a patient experiences increases. In large part, this is because prehospital hypotension has been recorded simply as a dichotomous, yes/no condition.
The innovation introduced by Dr Spaite and his associates in their analysis of the EPIC-TBI data was to drill down into each patient’s hypotensive event, made possible by the 16,711 patients enrolled in EPIC-TBI. Their calculations were limited to patients with EMS records of at least two BP measurements during prehospital transport. These data allowed Spaite et al to utilize both the extent to which systolic BP dropped below 90 mm Hg and the amount of time systolic BP was below this threshold to better define the total hypotension exposure each patient received.
This meant that a patient with a TBI and a systolic BP of 80 mm Hg for 10 minutes had twice the hypotension exposure of both a patient with a systolic BP of 85 mm Hg for 10 minutes and a patient with a systolic BP of 80 mm Hg for 5 minutes.
The analysis by Spaite et al also adjusted the relationship of total hypotensive severity and duration and subsequent mortality based on several baseline demographic and clinical variables, including age, sex, injury severity, trauma type, and head-region severity score. After adjustment, the researchers found a “strikingly linear relationship” between hypotension severity and duration and mortality, Dr Spaite said.
The EPIC-TBI enrolled TBI patients aged 10 years or older during 2007 to 2014 through participation of dozens of EMS providers throughout Arizona. For the current analysis, the researchers identified 7,521 patients from the total group who had at least two BP measurements taken during their prehospital EMS care and also met other inclusion criteria.
The best way to manage hypotension in TBI patients during the prehospital period remains unclear. Simply raising BP via intravenous (IV) fluid infusion may not necessarily help, because it could exacerbate a patient’s bleeding, Dr Spaite noted during an interview.
The primary goal of EPIC-TBI is to assess the implementation of the third edition of the TBI guidelines released in 2007 by the Brain Trauma Foundation. (The fourth edition of these guidelines came out in August 2016.) The new finding by Dr Spaite and his associates will allow the full EPIC-TBI analysis to correlate patient outcomes with the impact that acute, prehospital treatment had on the hypotension severity and duration each patient experienced, he noted.
“What’s remarkable is that the single prehospital parameter of hypotension for just a few minutes during transport can have such a strong impact on survival, given all the other factors that can influence outcomes” in TBI patients once they reach a hospital and during the period they remain hospitalized, Dr Spaite said.
1. Spaite DW. Presentation at: American Heart Association Scientific Sessions 2016. November 12-16, 2016; New Orleans, LA.
Fluid Administration in Sepsis Did Not Increase Need for Dialysis
M. Alexander Otto
FRONTLINE MEDICAL NEWS
Fluid administration of at least 1 L did not increase the incidence of acute respiratory or heart failure in severe sepsis, and actually seemed to decrease the need for dialysis in a review of 164 patients at Scott and White Memorial Hospital in Temple, Texas.
For every 1 mL of fluid administered per kilogram of body weight, the likelihood of dialysis decreased by 8.5% (odds ratio [OR], 0.915; 95% confidence interval [CI], 0.854-0.980; P = .0111), with no increase in heart or respiratory failure on univariate analysis. The 126 patients (77%) who received at least 1 L of fluid had a 68% reduction in the need for dialysis (OR, 0.32; CI, 0.117-0.890; P = .0288).
These findings come from a quality improvement project the hospital launched after researchers there realized that the benchmark Surviving Sepsis Campaign guidelines were not being met. The patients in the study had a systolic BP below 90 mm Hg or lactate level of at least 4 mmol/L. The guidelines would have called for these patients to receive 30 mL/kg of crystalloid fluids within 3 hours of presentation, but only 28 patients (17%) met that mark.
“The No. 1 reason we weren’t meeting benchmarks was fluid administration,” explained lead investigator Aruna Jahoor, MD, a pulmonary critical care and sleep medicine fellow at Texas Tech University Health Sciences Center.
Seventeen percent of patients received ≥30 mL/kg of fluid resuscitation, while 28% received ≥20 mL/kg of IV fluid resuscitation. It turned out that staff in the ED—where most of the patients were treated in the critical first 6 hours—were concerned about fluid overload and putting patients into respiratory, heart, or renal failure, Dr Jahoor said. The team found no difference in mortality rates when patients received 30 mL/kg—just over 2 L in a patient weighing 70 kg—vs 20 mL/kg or 1 L. The patients’ in-hospital mortality rate and 28-day mortality rate were 27% and 32%, respectively.
There also were no increased rates of heart failure, acute respiratory failure, or mechanical ventilation when patients received at least 1 L of fluid. “There were [also] lower rates of dialysis, which indicated that we weren’t overloading patients. Even when we looked at fluid as a continuous variable, we still didn’t see” complications, Dr Jahoor said.
The findings should be reassuring to treating physicians. “When you have pushback against 30-mL/kg administration, you can say ‘well, at least let’s give a liter.’ You don’t have to worry as much about some of the complications you are citing,’ ” she said.
For very obese patients, “it can get a little uncomfortable to be given” enough fluid to meet the 30-mL/kg goal, “but you can give at least a liter” without having to worry too much, she said. The patients in the study were treated from 2010 to 2013; normal saline was the most common resuscitation fluid. The hospital has since added the 30-mL/kg fluid resuscitation to its sepsis admission orders, and compliance has increased significantly.
A multivariate analysis is in the works to control for confounders. “We will probably [still] see you are not having increased rates of congestive heart or respiratory failure, or needing dialysis,” Dr Jahoor said. The protective effect against dialysis might drop out, “but I am hoping it doesn’t,” she said.
1. Jahoor A, Delmas T, Giri B, et al. Fluid resuscitation of at least 1 liter in septic patients decreases the need for renal replacement therapy without increasing the risk of acute congestive heart failure or acute respiratory failure. Chest. 2016;150(4_S):349A. doi:10.1016/j.chest.2016.08.362.
Survey: Antibiotic Shortages Are the New Norm
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a Web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs 73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the 2016 survey, the top 10 antimicrobials reported as being in short supply over the past 2 years were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. Trimethoprim-sulfamethoxazole and acyclovir were in short supply in 2011 and 2016.
According to respondents, the most common ways they learned about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the US Food and Drug Administration (FDA) Web site or another Web site (23%). The most common ways of learning about a shortage changed—from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr Gundlapalli said, noting ampicillin-sulbactam in 2016 and penicillin G procaine as examples.
“And then, of course, the other theme across the board...was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal—a way of life,” Dr Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive...maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
Donald Graham, MD, one of the study’s coauthors, said he believes the problem is in part the result of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it is not always in the financial best interest of a pharmaceutical company to upgrade their facilities.
1. Gundlapalli A. Presentation at: IDWeek 2016. October 26-30, 2016. New Orleans, LA.
Hospitalizations for Opioid Poisoning Tripled in Preschool Children
Richard Franki
FRONTLINE MEDICAL NEWS
From 1997 to 2012, the annual number of hospitalizations for opioid poisoning rose 178% among children aged 1 to 19 years, according to data from 13,052 discharges in the Agency for Healthcare Research and Quality’s Kids’ Inpatient Database.
In 2012, there were 2,918 hospitalizations for opioid poisoning among children aged 1 to 19 years, compared with 1,049 in 1997, reported Julie R. Gaither, PhD, MPH, RN, and her associates at Yale University in New Haven, Connecticut.
The greatest change occurred among the youngest children, as the number of those aged 1 to 4 years rose from 133 in 1997 to 421 in 2012—an increase of 217%. For those aged 15 to 19 years, the annual number of hospitalizations went from 715 to 2,171 (204%) over that time period, which included a slight drop from 2009 to 2012, according to the investigators,
The increase in hospitalizations for prescription opioid poisoning in children aged 10 to 14 years was 58% from 1997 to 2012 (rising from 171 to 272), while estimates for 5- to 9-year-old children did not meet the criteria for statistical reliability and were not included in the analysis, Dr Gaither and her associates said.
1. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016 Oct 31. Epub ahead of print. doi:10.1001/jamapediatrics.2016.2154.
Pelvic Fracture Pattern Predicts the Need for Hemorrhage Control
Doug Brunk
FRONTLINE MEDICAL NEWS
Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage-control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma (AAST). They identified three main force patterns: lateral compression, anterior posterior compression, and vertical shear.
“They were able to show that certain pelvic fractures were associated with soft-tissue injury and pelvic hemorrhage,” said Dr Costantini, of the division of trauma, surgical critical care, burns and acute care surgery at the University of California, San Diego. “Since then, several single-center studies have been conducted in an attempt to correlate fracture pattern with the risk of pelvic hemorrhage. A majority of these studies evaluated angiogram [and embolization] as the endpoint for hemorrhage control. Modern trauma care has evolved to include multiple modalities to control hemorrhage, which include pelvic external fixator placement, pelvic angiography and embolization, preperitoneal pelvic packing, and the use of the REBOA [Resuscitative Endovascular Balloon Occlusion of the Aorta] catheter as an adjunct to hemorrhage control.”
In a recently published study, Dr Costantini and his associates found wide variability in the use of pelvic hemorrhage-control methods.1 “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level 1 trauma centers over a 2-year period.2 Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over age 18 years, blunt mechanism of injury, and shock on admission defined as “...systolic blood pressure <90 mm Hg or heart rate >120 beats per minute or base deficit <-5.”1 Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr Costantini said. Next, they used univariate and multivariate logistic regression analyses to examine predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the 2-year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic BP was 93 mm Hg, their mean HR was 117 beats/min, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian vs auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage-control intervention, those who did received more transfusion of packed red blood cells (13 vs 7 units, respectively; P < .01) and fresh frozen plasma (10 U vs 5 U; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage-control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (OR, 109.43; P < .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
1. Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717-723; discussion 723-725. doi:10.1097/TA.0000000000001034.2. Costantini TW. Presentation at: 75th Annual Meeting of American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery. September 14-17, 2016. Waikoloa, Hawaii.
TAVR valve durability supported in large follow-up
WASHINGTON – First-generation, balloon-expandable transcatheter aortic valves had a less than 1% rate of valve failure in planned echocardiography examinations during follow-up that extended as long as 5 years after valve placement in more than 2,400 patients, a demonstration of durability that experts uniformly called “reassuring.”
This finding from patients who underwent transcatheter aortic valve replacement (TAVR) in the first U.S. pivotal trial for these devices, PARTNER 1 parts A and B, and during the subsequent continued-access program at PARTNER 1 study sites, represents the largest and longest systematic ultrasound follow-up of TAVR patients, Pamela S. Douglas, MD, said at the Transcatheter Cardiovascular Therapeutics annual meeting.
This evaluation of 2,404 TAVR patients in the PARTNER 1 trial examined by echocardiography and encompassing 6,493 patient-years of follow-up is the “largest core-lab based study of transcatheter heart valves to date. These data demonstrate excellent durability of transcatheter heart valves, suggesting that the low 5-year survival observed in this cohort is not related to adverse hemodynamics or transcatheter heart valve deterioration,” said Dr. Douglas, professor of medicine at Duke University, Durham, N.C.
Her findings showed that out of the 2,482 patients treated with TAVR (and including those without echo follow-up) either in the trial or during the continued access program and followed for a median of 2.9 years and an average of 2.6 years, 20 patients (0.8%) required a reintervention. Four of these 20 patients (0.2% of the total cohort) showed a “classic pattern” of aortic valve deterioration marked by an increased valve pressure gradient and a reduced valve area, she reported.
“Reintervention was rare, became less frequent over time, and was usually not due to structural deterioration of the transcatheter heart valve,” she said. But Dr. Douglas also cautioned that among the patients who received the first-generation, balloon expandable Sapien valve in this cohort, just 39% survived to 5 years, and a mere 282 patients (11%) actually underwent echocardiographic examination at 5 years.
“This is one of several steps we need to take to figure out the durability of transcatheter valves,” said Jeffrey J. Popma, MD, professor of medicine and an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston. He noted that data are needed from follow-up periods of 8 or 10 years, but these data will not be available until intermediate- or low-risk patients undergo TAVR in controlled circumstances and have long-term follow-up.
“Ten-year follow-up data will essentially be impossible” for the high-risk or inoperable patients treated with TAVR in the PARTNER 1 trial, which focused on the sickest patients with aortic stenosis, said Dr. Popma, lead investigator for several studies of TAVR using self-expanding aortic valves and marketed as CoreValve devices.
“We obviously need to follow patients longer. The 5-year results look terrific, and so very reassuring, but we need to keep an eye on this as we move TAVR into less sick and younger patients,” said Dr. Robert O. Bonow, professor of cardiology at Northwestern University, Chicago. “Durability is the remaining frontier in terms of moving TAVR into younger patients,” Dr. Bonow said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
These data continue to show that “transcatheter valves have looked hemodynamically superior to surgically-placed valves with respect to the VARC (Valve Academic Research Consortium)–2 criteria” for prosthetic valve function, Dr. Popma noted. “I think the benefits of surgical valves have been overstated and the benefits of transcatheter valves understated,” he said.
“Surgical valves have not been held to the same [very demanding] standard as transcatheter valves,” Dr. Douglas agreed.
The data Dr. Douglas reported contrast with longer-term follow-up reported in May 2016 for 378 patients who underwent TAVR at either of two pioneering centers in a retrospective review. Those data suggested a valve degeneration rate of about 50% after 8 years, Danny Dvir, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions. Speaking recently in an interview, Dr. Dvir acknowledged some of the challenges in trying to derive valve durability information from a relatively small number of very-high-risk patients who underwent TAVR very early during development of the procedure.
Some TAVR experts have also questioned the criteria that Dr. Dvir used to identify valve structural valve degeneration for this analysis. “The criteria he used were much more stringent that the criteria we have used to assess surgically-placed valves,” said Michael J. Reardon, MD, professor of cardiovascular surgery at Houston Methodist Hospital. “If surgically-placed aortic valves were subjected to the same criteria Dr. Dvir applied then they would perform even worse,” Dr. Reardon said in an interview.
PARTNER 1 was sponsored by Edwards Lifesciences, the company that had marketed the Sapien first-generation, balloon expandable TAVR system. Dr. Douglas has received research support from Edwards. Dr. Bonow had no disclosures. Dr. Popma has been the lead investigator for several studies of a self-expanding TAVR system sponsored by Medtronic, and he has also received research funding from several other companies, has been a consultant to Boston Scientific and Direct Flow, and owns equity in Direct Flow. Dr. Dvir has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. Dr. Reardon has been a consultant to Medtronic.
[email protected]
On Twitter @mitchelzoler
The data reported by Dr. Douglas are very important and very reassuring. It isn’t easy to evaluate long-term results in patients who underwent TAVR in the early days because that population of patients was old and at very high risk. Even when patients had successful procedures their longevity wasn’t long. Only about 10% of the starting population of 2,482 patients in Dr. Douglas’ study actually had echocardiography done after 5 years. To assess durability you need longer-term echo follow-up, but it will be very challenging to have enough patients to have statistical power to do that.
I am not nervous about long-term durability of TAVR in octogenarian patients, the most typical age for TAVR patients today and since we began using it. Durability is more of an issue for patients who are 75 or younger, and we will need data from 7- to 10-year follow-up of younger patients to have a reasonable answer. Younger patients who undergo TAVR may face more of a threat from valve deterioration simply because of their longer life expectancy. In addition, with surgical valves we know that younger age is one of the strongest predictors of valve degeneration.
Danny Dvir, MD , is an interventional cardiologist at the University of Washington in Seattle. He has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. He made these comments in an interview.
The data reported by Dr. Douglas are very important and very reassuring. It isn’t easy to evaluate long-term results in patients who underwent TAVR in the early days because that population of patients was old and at very high risk. Even when patients had successful procedures their longevity wasn’t long. Only about 10% of the starting population of 2,482 patients in Dr. Douglas’ study actually had echocardiography done after 5 years. To assess durability you need longer-term echo follow-up, but it will be very challenging to have enough patients to have statistical power to do that.
I am not nervous about long-term durability of TAVR in octogenarian patients, the most typical age for TAVR patients today and since we began using it. Durability is more of an issue for patients who are 75 or younger, and we will need data from 7- to 10-year follow-up of younger patients to have a reasonable answer. Younger patients who undergo TAVR may face more of a threat from valve deterioration simply because of their longer life expectancy. In addition, with surgical valves we know that younger age is one of the strongest predictors of valve degeneration.
Danny Dvir, MD , is an interventional cardiologist at the University of Washington in Seattle. He has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. He made these comments in an interview.
The data reported by Dr. Douglas are very important and very reassuring. It isn’t easy to evaluate long-term results in patients who underwent TAVR in the early days because that population of patients was old and at very high risk. Even when patients had successful procedures their longevity wasn’t long. Only about 10% of the starting population of 2,482 patients in Dr. Douglas’ study actually had echocardiography done after 5 years. To assess durability you need longer-term echo follow-up, but it will be very challenging to have enough patients to have statistical power to do that.
I am not nervous about long-term durability of TAVR in octogenarian patients, the most typical age for TAVR patients today and since we began using it. Durability is more of an issue for patients who are 75 or younger, and we will need data from 7- to 10-year follow-up of younger patients to have a reasonable answer. Younger patients who undergo TAVR may face more of a threat from valve deterioration simply because of their longer life expectancy. In addition, with surgical valves we know that younger age is one of the strongest predictors of valve degeneration.
Danny Dvir, MD , is an interventional cardiologist at the University of Washington in Seattle. He has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. He made these comments in an interview.
WASHINGTON – First-generation, balloon-expandable transcatheter aortic valves had a less than 1% rate of valve failure in planned echocardiography examinations during follow-up that extended as long as 5 years after valve placement in more than 2,400 patients, a demonstration of durability that experts uniformly called “reassuring.”
This finding from patients who underwent transcatheter aortic valve replacement (TAVR) in the first U.S. pivotal trial for these devices, PARTNER 1 parts A and B, and during the subsequent continued-access program at PARTNER 1 study sites, represents the largest and longest systematic ultrasound follow-up of TAVR patients, Pamela S. Douglas, MD, said at the Transcatheter Cardiovascular Therapeutics annual meeting.
This evaluation of 2,404 TAVR patients in the PARTNER 1 trial examined by echocardiography and encompassing 6,493 patient-years of follow-up is the “largest core-lab based study of transcatheter heart valves to date. These data demonstrate excellent durability of transcatheter heart valves, suggesting that the low 5-year survival observed in this cohort is not related to adverse hemodynamics or transcatheter heart valve deterioration,” said Dr. Douglas, professor of medicine at Duke University, Durham, N.C.
Her findings showed that out of the 2,482 patients treated with TAVR (and including those without echo follow-up) either in the trial or during the continued access program and followed for a median of 2.9 years and an average of 2.6 years, 20 patients (0.8%) required a reintervention. Four of these 20 patients (0.2% of the total cohort) showed a “classic pattern” of aortic valve deterioration marked by an increased valve pressure gradient and a reduced valve area, she reported.
“Reintervention was rare, became less frequent over time, and was usually not due to structural deterioration of the transcatheter heart valve,” she said. But Dr. Douglas also cautioned that among the patients who received the first-generation, balloon expandable Sapien valve in this cohort, just 39% survived to 5 years, and a mere 282 patients (11%) actually underwent echocardiographic examination at 5 years.
“This is one of several steps we need to take to figure out the durability of transcatheter valves,” said Jeffrey J. Popma, MD, professor of medicine and an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston. He noted that data are needed from follow-up periods of 8 or 10 years, but these data will not be available until intermediate- or low-risk patients undergo TAVR in controlled circumstances and have long-term follow-up.
“Ten-year follow-up data will essentially be impossible” for the high-risk or inoperable patients treated with TAVR in the PARTNER 1 trial, which focused on the sickest patients with aortic stenosis, said Dr. Popma, lead investigator for several studies of TAVR using self-expanding aortic valves and marketed as CoreValve devices.
“We obviously need to follow patients longer. The 5-year results look terrific, and so very reassuring, but we need to keep an eye on this as we move TAVR into less sick and younger patients,” said Dr. Robert O. Bonow, professor of cardiology at Northwestern University, Chicago. “Durability is the remaining frontier in terms of moving TAVR into younger patients,” Dr. Bonow said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
These data continue to show that “transcatheter valves have looked hemodynamically superior to surgically-placed valves with respect to the VARC (Valve Academic Research Consortium)–2 criteria” for prosthetic valve function, Dr. Popma noted. “I think the benefits of surgical valves have been overstated and the benefits of transcatheter valves understated,” he said.
“Surgical valves have not been held to the same [very demanding] standard as transcatheter valves,” Dr. Douglas agreed.
The data Dr. Douglas reported contrast with longer-term follow-up reported in May 2016 for 378 patients who underwent TAVR at either of two pioneering centers in a retrospective review. Those data suggested a valve degeneration rate of about 50% after 8 years, Danny Dvir, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions. Speaking recently in an interview, Dr. Dvir acknowledged some of the challenges in trying to derive valve durability information from a relatively small number of very-high-risk patients who underwent TAVR very early during development of the procedure.
Some TAVR experts have also questioned the criteria that Dr. Dvir used to identify valve structural valve degeneration for this analysis. “The criteria he used were much more stringent that the criteria we have used to assess surgically-placed valves,” said Michael J. Reardon, MD, professor of cardiovascular surgery at Houston Methodist Hospital. “If surgically-placed aortic valves were subjected to the same criteria Dr. Dvir applied then they would perform even worse,” Dr. Reardon said in an interview.
PARTNER 1 was sponsored by Edwards Lifesciences, the company that had marketed the Sapien first-generation, balloon expandable TAVR system. Dr. Douglas has received research support from Edwards. Dr. Bonow had no disclosures. Dr. Popma has been the lead investigator for several studies of a self-expanding TAVR system sponsored by Medtronic, and he has also received research funding from several other companies, has been a consultant to Boston Scientific and Direct Flow, and owns equity in Direct Flow. Dr. Dvir has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. Dr. Reardon has been a consultant to Medtronic.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – First-generation, balloon-expandable transcatheter aortic valves had a less than 1% rate of valve failure in planned echocardiography examinations during follow-up that extended as long as 5 years after valve placement in more than 2,400 patients, a demonstration of durability that experts uniformly called “reassuring.”
This finding from patients who underwent transcatheter aortic valve replacement (TAVR) in the first U.S. pivotal trial for these devices, PARTNER 1 parts A and B, and during the subsequent continued-access program at PARTNER 1 study sites, represents the largest and longest systematic ultrasound follow-up of TAVR patients, Pamela S. Douglas, MD, said at the Transcatheter Cardiovascular Therapeutics annual meeting.
This evaluation of 2,404 TAVR patients in the PARTNER 1 trial examined by echocardiography and encompassing 6,493 patient-years of follow-up is the “largest core-lab based study of transcatheter heart valves to date. These data demonstrate excellent durability of transcatheter heart valves, suggesting that the low 5-year survival observed in this cohort is not related to adverse hemodynamics or transcatheter heart valve deterioration,” said Dr. Douglas, professor of medicine at Duke University, Durham, N.C.
Her findings showed that out of the 2,482 patients treated with TAVR (and including those without echo follow-up) either in the trial or during the continued access program and followed for a median of 2.9 years and an average of 2.6 years, 20 patients (0.8%) required a reintervention. Four of these 20 patients (0.2% of the total cohort) showed a “classic pattern” of aortic valve deterioration marked by an increased valve pressure gradient and a reduced valve area, she reported.
“Reintervention was rare, became less frequent over time, and was usually not due to structural deterioration of the transcatheter heart valve,” she said. But Dr. Douglas also cautioned that among the patients who received the first-generation, balloon expandable Sapien valve in this cohort, just 39% survived to 5 years, and a mere 282 patients (11%) actually underwent echocardiographic examination at 5 years.
“This is one of several steps we need to take to figure out the durability of transcatheter valves,” said Jeffrey J. Popma, MD, professor of medicine and an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston. He noted that data are needed from follow-up periods of 8 or 10 years, but these data will not be available until intermediate- or low-risk patients undergo TAVR in controlled circumstances and have long-term follow-up.
“Ten-year follow-up data will essentially be impossible” for the high-risk or inoperable patients treated with TAVR in the PARTNER 1 trial, which focused on the sickest patients with aortic stenosis, said Dr. Popma, lead investigator for several studies of TAVR using self-expanding aortic valves and marketed as CoreValve devices.
“We obviously need to follow patients longer. The 5-year results look terrific, and so very reassuring, but we need to keep an eye on this as we move TAVR into less sick and younger patients,” said Dr. Robert O. Bonow, professor of cardiology at Northwestern University, Chicago. “Durability is the remaining frontier in terms of moving TAVR into younger patients,” Dr. Bonow said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
These data continue to show that “transcatheter valves have looked hemodynamically superior to surgically-placed valves with respect to the VARC (Valve Academic Research Consortium)–2 criteria” for prosthetic valve function, Dr. Popma noted. “I think the benefits of surgical valves have been overstated and the benefits of transcatheter valves understated,” he said.
“Surgical valves have not been held to the same [very demanding] standard as transcatheter valves,” Dr. Douglas agreed.
The data Dr. Douglas reported contrast with longer-term follow-up reported in May 2016 for 378 patients who underwent TAVR at either of two pioneering centers in a retrospective review. Those data suggested a valve degeneration rate of about 50% after 8 years, Danny Dvir, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions. Speaking recently in an interview, Dr. Dvir acknowledged some of the challenges in trying to derive valve durability information from a relatively small number of very-high-risk patients who underwent TAVR very early during development of the procedure.
Some TAVR experts have also questioned the criteria that Dr. Dvir used to identify valve structural valve degeneration for this analysis. “The criteria he used were much more stringent that the criteria we have used to assess surgically-placed valves,” said Michael J. Reardon, MD, professor of cardiovascular surgery at Houston Methodist Hospital. “If surgically-placed aortic valves were subjected to the same criteria Dr. Dvir applied then they would perform even worse,” Dr. Reardon said in an interview.
PARTNER 1 was sponsored by Edwards Lifesciences, the company that had marketed the Sapien first-generation, balloon expandable TAVR system. Dr. Douglas has received research support from Edwards. Dr. Bonow had no disclosures. Dr. Popma has been the lead investigator for several studies of a self-expanding TAVR system sponsored by Medtronic, and he has also received research funding from several other companies, has been a consultant to Boston Scientific and Direct Flow, and owns equity in Direct Flow. Dr. Dvir has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. Dr. Reardon has been a consultant to Medtronic.
[email protected]
On Twitter @mitchelzoler
AT TCT 2016
Key clinical point:
Major finding: During median follow-up of 2.9 years, 0.2% of patients had valves with classic hemodynamic signs of valve deterioration.
Data source: A total of 2,482 TAVR patients either enrolled in the PARTNER 1 trial or who underwent TAVR during a continued access program.
Disclosures: PARTNER 1 was sponsored by Edwards Lifesciences, the company that had marketed the Sapien first-generation, balloon expandable TAVR system. Dr. Douglas has received research support from Edwards. Dr. Bonow had no disclosures. Dr. Popma has been the lead investigator for several studies of a self-expanding TAVR system sponsored by Medtronic, and he has also received research funding from several other companies, has been a consultant to Boston Scientific and Direct Flow, and owns equity in Direct Flow. Dr. Dvir has been a consultant to and received research support from Edwards, Medtronic, and St. Jude. Dr. Reardon has been a consultant to Medtronic.
Malpractice Counsel: Abdominal pain in an elderly patient
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
A Holiday Visit to the ED (With Apologies to Clement Clarke Moore)
‘Twas the night before New Year, when all through the land
Every ED was busy—Can you give us a hand?
Treating chest pains, and traumas, and hot swollen knees,
While clinics were shuttered, along with UCs.
The handoffs were done with hardly a frown,
In hopes that the volume soon would slow down.
Babies were nestled all snug in a sheet,
Watching sutures applied to their hands and their feet.
And amateur athletes unpadded, uncapped,
Had brains that were rattled after balls had been snapped.
When out on the deck there arose such a clatter
We sprang from the doc box to help with the matter.
To Resusc room 1 we flew in a flash,
Tearing open the curtain before the patient could crash.
The leads on the breast of the now-fallen fellow,
Made lustrous white circles near sclerae bright yellow.
When what to our wondering ears did we hear,
But an overhead page that inspired some fear:
Notifications of a Level 1 trauma,
And several ODs, to add to the drama.
More rapid than eagles the new patients came,
All victims of poisons with rather strange names:
Poinsettia, and holly, and dried mistletoe,
Angel hair, leaded tinsel, polyacrylate snow.
And a man who was tarnished with ashes and soot,
With a cherry red color from his head to his foot.
Smoke inhalation and a toxic epoxide?
Or alcohol, cyanide, carbon monoxide?
But “Holiday Poisonings” on the pages ahead,
Soon reassured us we had nothing to dread…
When patients were discharged to families waiting,
They promised to give us all a good rating.
So to all EMTs, NPs, and PAs,
RNs, and EPs who work holidays,
And to all ED staffs who “fight the good fight,”
Have a Happy New Year, and a nice quiet night!
—Neal Flomenbaum, MD
‘Twas the night before New Year, when all through the land
Every ED was busy—Can you give us a hand?
Treating chest pains, and traumas, and hot swollen knees,
While clinics were shuttered, along with UCs.
The handoffs were done with hardly a frown,
In hopes that the volume soon would slow down.
Babies were nestled all snug in a sheet,
Watching sutures applied to their hands and their feet.
And amateur athletes unpadded, uncapped,
Had brains that were rattled after balls had been snapped.
When out on the deck there arose such a clatter
We sprang from the doc box to help with the matter.
To Resusc room 1 we flew in a flash,
Tearing open the curtain before the patient could crash.
The leads on the breast of the now-fallen fellow,
Made lustrous white circles near sclerae bright yellow.
When what to our wondering ears did we hear,
But an overhead page that inspired some fear:
Notifications of a Level 1 trauma,
And several ODs, to add to the drama.
More rapid than eagles the new patients came,
All victims of poisons with rather strange names:
Poinsettia, and holly, and dried mistletoe,
Angel hair, leaded tinsel, polyacrylate snow.
And a man who was tarnished with ashes and soot,
With a cherry red color from his head to his foot.
Smoke inhalation and a toxic epoxide?
Or alcohol, cyanide, carbon monoxide?
But “Holiday Poisonings” on the pages ahead,
Soon reassured us we had nothing to dread…
When patients were discharged to families waiting,
They promised to give us all a good rating.
So to all EMTs, NPs, and PAs,
RNs, and EPs who work holidays,
And to all ED staffs who “fight the good fight,”
Have a Happy New Year, and a nice quiet night!
—Neal Flomenbaum, MD
‘Twas the night before New Year, when all through the land
Every ED was busy—Can you give us a hand?
Treating chest pains, and traumas, and hot swollen knees,
While clinics were shuttered, along with UCs.
The handoffs were done with hardly a frown,
In hopes that the volume soon would slow down.
Babies were nestled all snug in a sheet,
Watching sutures applied to their hands and their feet.
And amateur athletes unpadded, uncapped,
Had brains that were rattled after balls had been snapped.
When out on the deck there arose such a clatter
We sprang from the doc box to help with the matter.
To Resusc room 1 we flew in a flash,
Tearing open the curtain before the patient could crash.
The leads on the breast of the now-fallen fellow,
Made lustrous white circles near sclerae bright yellow.
When what to our wondering ears did we hear,
But an overhead page that inspired some fear:
Notifications of a Level 1 trauma,
And several ODs, to add to the drama.
More rapid than eagles the new patients came,
All victims of poisons with rather strange names:
Poinsettia, and holly, and dried mistletoe,
Angel hair, leaded tinsel, polyacrylate snow.
And a man who was tarnished with ashes and soot,
With a cherry red color from his head to his foot.
Smoke inhalation and a toxic epoxide?
Or alcohol, cyanide, carbon monoxide?
But “Holiday Poisonings” on the pages ahead,
Soon reassured us we had nothing to dread…
When patients were discharged to families waiting,
They promised to give us all a good rating.
So to all EMTs, NPs, and PAs,
RNs, and EPs who work holidays,
And to all ED staffs who “fight the good fight,”
Have a Happy New Year, and a nice quiet night!
—Neal Flomenbaum, MD