User login
Musculoskeletal Hand Pain Group Visits: An Adaptive Health Care Model
From Cooper Medical School of Rowan University (Dr. Patel, Dr. Fuller) and Cooper University Hospital (Dr. Kaufman), Camden, NJ.
Abstract
- Objective: To describe an adaptive musculoskeletal hand clinic that offers accessible and economically viable musculoskeletal care for an underserved, urban population.
- Methods: Descriptive report.
- Results: An enhanced access group visit model was developed offering both nonsurgical and surgical care pathways for patients with musculoskeletal disorders of the hand. Both patient education and care were provided in the group environment. Staffing included an orthopedic surgeon, nurse practitioner, medical student, orthopedic technician, and medical assistant. Over a 12-month period, group visit efficiency improved to accommodate an equivalent number of patients as compared to the traditional model. Access (time to appointment) was improved in the group visit. The model allows for the addition of non-physician clinical staff to improve access and limit costs in a manner not feasible with traditional office visits.
- Conclusion: A group visit model may offer a sustainable process to increase patient access to musculoskeletal subspecialty care and accommodate care of greater numbers of patients while maintaining quality. The group model offers flexible staffing, enhanced access, and educational benefit.
Key words: Group medical visit, team-based care, hand pain, access, underserved populations.
Group visits are a relatively new health care delivery model [1–3]. The term is applied to a wide variety of visits designed for groups of patients, rather than individual patient-provider appointments. The group visit format has been used for various disease- or condition-specific populations. Group visits can increase access to care and have been associated with improved clinical outcomes [4].
The Urban Health Institute, a dedicated business unit at Cooper University Health System in Camden, NJ, was established to focus on care of the underserved. The business unit is working to reduce cost of care delivery, increase access, and improve quality through the use of workflow redesign, task shifting, dashboards, and other methods. With a large startup grant from the Nicholson Foundation, the Urban Health Institute launched the Cooper Advanced Care Center to provide the local under-served population with access to a collaborative practice of 23 medical and surgical specialties under one roof. The center incorporates traditional one-on-one provider visits with innovative models of care, including group visits. Multiple partners were required in the group visit design and implementation starting with buy-in from Cooper Health System’s leadership for existing space to be redesigned for the group visit activities.
The Cooper Advanced Care Center, which had high no-show rates of 30% to 40%, and low reimbursement for a primarily Medicaid and self-pay population, initially operated at a financial loss. Meanwhile, most physicians had extended lag time until their next available appointment. In a traditional patient–physician office visit, additional physician time is required to increase access to care. This costly solution is often not financially viable. Group visits were considered as a means of using an interdisciplinary team to increase access while controlling or decreasing the per-visit cost.
Musculoskeletal medicine was identified as an area of need in part due to limited access to care. Patients were waiting more than 2 months to see a musculoskeletal specialist and were being scheduled beyond capacity in our existing traditional weekly hand and knee/sports medicine clinics. Within musculoskeletal medicine, 4 areas of musculoskeletal medicine were considered for group visits: back pain, knee pain, hand pain, and foot and ankle disorders. The decision was made to focus the subspecialty musculoskeletal clinic on disorders of the hand and wrist to provide nonsurgical and surgical care options for atraumatic and traumatic disorders of the hand in a teaching environment at an urban hospital. The purpose of this article is to discuss the design and implementation of a hand pain group visit model to increase access to care without increasing cost.
Setting and Patients
Camden City, New Jersey, is a medically underserved, resource-poor community. The population is 48% African American and 47% Hispanic and nearly 40% of individuals live below the poverty level [5]. The group visit was intentionally set up as a means to provide access to the un- or underinsured. Patients attending the group visits were 33% African American, 33% Hispanic, and 30% Caucasian. Most patients had Medicaid insurance (67%) with the remaining patients covered by commercial insurance (15%), dual Medicare/Medicaid (11%), Medicare (5%), or self pay (2%).
Group Visit Staffing and Structure
In a traditional office visit, used nearly ubiquitously in outpatient medical offices, patients arrive at individual appointment times for a prescribed time encounter with the physician, are registered and roomed by support staff, and are then seen by a clinician for diagnosis and treatment. While assistants and trainees participate in the patient’s care with attending physician supervision, the majority of direct care falls to the physician. Access is coupled to physician availability; increasing access to care requires crowding the schedule with additional patients. We used this model as the benchmark for volume and scheduling against which to compare the group visit.
The group visit staffing was the same as for the traditional visit: hand surgeon, nurse practitioner, orthopedic technician, medical student, and medical assistant. However, each clinical session consists of four 1-hour, consecutive group visits scheduled once a month on a Monday morning. Up to 10 people could be scheduled for each 1-hour group visit. We continued to offer our traditional office visit clinic on the other 3 Mondays in the month.
The hand surgeon begins the group visit with a 10-minute educational session and group discussion held in a meeting room. He reviews common disorders of the hand, including carpal tunnel syndrome, trigger fingers, hand arthritis, cysts, sprains and fractures, how they are treated, and risks and benefits of treatments. Patients sign a confidentiality agreement at check-in. Time is allowed for questions and experiential sharing is encouraged. Expectations are set at the start of the visit to honor each patient’s input to provide a safe environment for asking questions and expressing concerns about their shared health condition to enhance the learning experience [6]. A medical assistant enters the chief complaint using an electronic standardized questionnaire into the EMR along with basic vital signs for each patient either prior to, during, or after the group presentation.
After the group educational session with the surgeon, patients transition to a large, open clinical room with 6 separate workstations, each consisting of a small table with 4 chairs and a laptop computer. Small procedures can be performed on the table (suture removal, dressing changes, injections) and the table is appropriately sized to accommodate a care provider, the patient, and their support person(s). Tables are spaced comfortably such that conversations do not carry much from one to the other. The clinical space has white noise speakers for sound dampening while patients receive individual history, vital signs, physical examination, and review of relevant studies. Patients may see the clinicians in a private exam room if they wish or require.
In a traditional office setting, workflow through the major tasks (check-in, data gathering, diagnosis, treatment) is often linear, as dictated by the configuration of individual patient rooms and the patient’s expectations of a traditional doctor’s visit. In the group visit, major tasks are performed simultaneously by the advance practice providers (nurse practitioners, physician assistants) in conjunction with supervision of the attending physician. The workstations (tables, chairs, laptops) in the open clinical room allows for greater efficiency; providers can easily transition to other tasks from one workstation to another during time that may have been spent waiting for other team members in the more linear, traditional clinic. For example, while waiting for the attending physician’s approval of a diagnosis and treatment plan for one patient, a nurse practitioner may be able to begin assessing and gathering data with a second patient until the physician becomes available.
Scheduling and Access
A primary aim of the group visit pilot was to develop a model of care delivery that allowed scheduling beyond capacity for the traditional office hand clinic. At the inception of the group visit, all patients were offered a visit in either the traditional office or group visit model by our scheduling secretaries based upon availability, with emphasis on scheduling a defined underserved population into the group visit.
In traditional 1:1 appointments, the number of people who can access care is dependent on physician availability. The team-based model uncouples the number of scheduled patients from the physician availability, allowing increased efficiency in the model and/or additional staff to increase the number of patients the group visit can accommodate. Thus, patients were essentially guaranteed an appointment in the next clinic because there was no cap on the number of patients that could be scheduled into the group visit. If the number of patients exceeded the limit of 10 per hour, a non-physician clinician was added to accommodate the patient demand. As our group visit matures, the ability to increase the staffing model enables patients to get care without adding more physician time.
Task Shifting
A central pillar of making the group visit a sustainable model for more accessible care is shifting tasks to non-physician health care workers. Adding specialist time enhances access but drives up the cost of care. Non-physician clinical staff members in subspecialty orthopedic offices with experience diagnosing and treating common conditions are capable of providing the standard of care for those conditions with variable physician oversight [7].
In the group visit, there is a deliberate assignment of patients to clinical staff by the physician based on anticipated level of care required. Given the attending physician’s experience with the most common disorders related to hand pain, it is possible to anticipate the approximate complexity of care required for each patient based on the nature and duration of the presenting complaint. With some degree of clinical supervision by the physician, members of the team operate maximally independently to assist patients. The nurse practitioner can operate largely autonomously in the group visit. The overall goal is to encourage all team members to function at the top of their licenses and abilities. Task shifting in this manner increases the productivity of all members of the team and minimizes redundancy. Despite more autonomy of mid-level providers and support staff in the group visit, there is still direct supervision of care by the attending physician.
The current body of literature in task shifting to non-physician health care workers has mostly concerned low-income countries with marked physician and provider shortages [8]. However, given the increased patient volume already seen with the Affordable Care Act and further expected increases, the health care system is likely to see provider shortages, especially in primary care [9–11]. This will necessitate the adoption of strategies to increase access, maintain quality care, and decrease systemic cost. Task shifting provides one such strategy.
Patient Satisfaction
One concern with shifting clinical duties to non-physician clinical staff is patient satisfaction. An abbreviated interaction with physicians can make patients dissatisfied with medical care independent of eventual clinical outcome [12]. However, it has also been demonstrated in an outpatient hand surgery clinic that quality of time spent with the physician may have a greater impact on satisfaction than quantity of time [13].
Our group visit is structured to allow high physician-patient visibility and interaction. The introductory remarks by the physician engage patients with the physician early in the visit and establish a group and individual rapport. The physician introduces the clinical team and the idea of patients being seen by other clinical staff up front, which establishes comfort for later patient–staff interaction. This is also an important time for patient education, which has been shown as a significant determinant of patient satisfaction in the outpatient setting [14]. The patient education at the beginning of the visit answers questions by one person that another person may not have considered, and generates patient questions to be addressed individually with a clinician. One common example is when a person considering carpal tunnel surgery hears from a person who has recently completed the procedure and can talk about their operative experience.
In the group room, the physician and staff can move between patients quickly and efficiently without waiting for turnover of rooms and resources. The structure of the visit allows staff to dedicate more time to patient care by bypassing the extra time required when patients are roomed individually. The group/communal structure also allows patients to see the staff at work, as compared to time spent waiting alone in an exam room in the traditional office. This enables patients to appreciate the efforts of the clinical staff and avoids giving the impression that the physician is inattentive or cursory in patient interaction.
Medical Education
An important consideration at any academic medical center is education. However, education often introduces redundancies and inefficiency into the medical care visit. The work a trainee does is either extensively overseen or the clinical questions are repeated by a supervising provider. However, it is possible to increase efficiency and utility of trainees in the group visit setting while maintaining educational value.
Given the relatively narrow scope and the nature of conditions encountered in the hand clinic, medical decision making for many patients is limited to a “straightforward” or “low complexity” level. These designations assume a limited number of diagnoses, management options, and amount and complexity of diagnostic workup. Most importantly, risk of complications or morbidity/mortality at these levels is minimal to low. For these conditions, a supervising physician can allow a trainee more independence to practice employing simple treatment and management guidelines and progress to working independently when addressing simpler conditions as the trainee’s experience grows. As independence grows, trainees can build confidence in medical management as well as focus on other core educational competencies once they are comfortable with the evaluation and management of a limited scope of conditions. Conditions such as trigger fingers, hand arthritis, ganglion cysts, and carpal tunnel are those the trainee is likely to encounter in a primary care practice. While there may be a decrease in direct physician teaching, the trainee gains clinical autonomy and experience in educational core competencies such as patient communication, patient education, systems-based practice, procedural skill, cultural competency, and interdisciplinary teamwork [15,16].
Lessons Learned
The success of the group visit required buy-in from hospital and physician leadership, the clinical team, and multiple partners in the hospital system. The hospital administration supported group visits as an integral component of the Urban Health Institute. Buy-in from key hospital leadership ensured resources and dedicated space for the group visit program. Grant support allowed additional programmatic support to acquire the necessary assistance from information services, EMR support, legal, and marketing. Physician buy-in was the most significant piece to the success of an individual group visit. Accepting the movement away from physician autonomy to team-based care is challenging for many providers. Physician willingness to start a high-demand group visit, recognition of the start-up inefficiencies, and working with the administrative and clinical team on program improvement strategies has succeeded in launching a sustaining group visit model.
Conclusion
There is a need for an adaptive and economically viable model of patient care to meet increasing demand, as well as provide care for indigent populations in a way that is more economically sustainable than providing care through the emergency department. The development and implementation of an urban hand group visit at our institution has demonstrated that such a model, based on group visit models more commonly seen in primary care, can be effectively implemented in a subspecialty care setting. This model is capable of increasing patient access to care and effectively handling increased patient volume with room for cost-effective growth in the future, all while maintaining quality of care. We anticipate further subspecialty clinics within hand pain to emerge, such as a group visit dedicated specifically to carpal tunnel syndrome or hand arthritis. This will allow each group to be more focused and will streamline education and mutual support among the patients.
Corresponding author: Steven Kaufman, MD, 3 Cooper Plaza, Suite 211, Camden, NJ 08103, [email protected].
Funding/support: The Nicholson Foundation.
Financial disclosures: None reported.
1. Gardiner P, Dresner D, Barnett KG, et al. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med 2014;3:20–6.
2. Remick RA, Remick AK. Do patients really prefer individual outpatient follow-up visits, compared with group medical visits?. Can J Psychiatry 2014;59:50–3.
3. Thompson C, Meeuwisse I, Dahlke R, Drummond N. Group medical visits in primary care for patients with diabetes and low socioeconomic status: users’ perspectives and lessons for practitioners. Can J Diabetes 2014;38:198–204.
4. Eisenstat S, Lipps SA, Carlson K, Ulman K. Putting group visits into practice: a practical overview to preparation, implementation, and maintenance of group visits at Massachusetts General Hospital. Women’s Health Associates, The John D. Stoeckle Center for Primary Care Innovation, Massachusetts General Hospital; January 2012.
5. U.S. Census Bureau. State and city Quickfacts: Camden (city), New Jersey. Accessed 29 Sep 2015 at http://quickfacts.census.gov/qfd/states/34/3410000.html.
6. Slocum YS. A survey of expectations about group therapy among clinical and nonclinical populations. Int J Group Psychother 1987;37:39–54.
7. Newhouse RP, Stanik-hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. Nurs Econ 2011;29:230–50.
8. Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS ONE 2014;9:e103754.
9. Hofer AN, Abraham JM, Moscovice I. Expansion of coverage under the Patient Protection and Affordable Care Act and primary care utilization. Milbank Q 2011;89:69–89.
10. Kushnir T, Greenberg D, Madjar N, et al. Is burnout associated with referral rates among primary care physicians in community clinics?. Fam Pract 2014;31:44–50.
11. Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am 2012;94:2177–84.
12. Lin CT, Albertson GA, Schilling LM, et al. Is patients’ perception of time spent with the physician a determinant of ambulatory patient satisfaction?. Arch Intern Med 2001;161:1437–42.
13. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated with patient satisfaction. Clin Orthop Relat Res 2014.
14. Murdock A, Griffin B. How is patient education linked to patient satisfaction?. Nursing 2013;43:43–5.
15. Accreditation Council for Graduate Medical Education. Common program requirements. Approved 2014. Available at www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_07012016.pdf.
16. Liaison Committee on Medical Education. Functions and structure of a medical school: standards for accreditation of medical education programs leading to the M.D. degree. [updated June 2013]. Available at www.lcme.org/publications/functions.pdf.
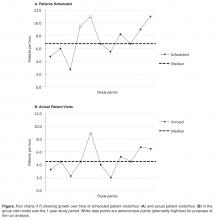
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51.
From Cooper Medical School of Rowan University (Dr. Patel, Dr. Fuller) and Cooper University Hospital (Dr. Kaufman), Camden, NJ.
Abstract
- Objective: To describe an adaptive musculoskeletal hand clinic that offers accessible and economically viable musculoskeletal care for an underserved, urban population.
- Methods: Descriptive report.
- Results: An enhanced access group visit model was developed offering both nonsurgical and surgical care pathways for patients with musculoskeletal disorders of the hand. Both patient education and care were provided in the group environment. Staffing included an orthopedic surgeon, nurse practitioner, medical student, orthopedic technician, and medical assistant. Over a 12-month period, group visit efficiency improved to accommodate an equivalent number of patients as compared to the traditional model. Access (time to appointment) was improved in the group visit. The model allows for the addition of non-physician clinical staff to improve access and limit costs in a manner not feasible with traditional office visits.
- Conclusion: A group visit model may offer a sustainable process to increase patient access to musculoskeletal subspecialty care and accommodate care of greater numbers of patients while maintaining quality. The group model offers flexible staffing, enhanced access, and educational benefit.
Key words: Group medical visit, team-based care, hand pain, access, underserved populations.
Group visits are a relatively new health care delivery model [1–3]. The term is applied to a wide variety of visits designed for groups of patients, rather than individual patient-provider appointments. The group visit format has been used for various disease- or condition-specific populations. Group visits can increase access to care and have been associated with improved clinical outcomes [4].
The Urban Health Institute, a dedicated business unit at Cooper University Health System in Camden, NJ, was established to focus on care of the underserved. The business unit is working to reduce cost of care delivery, increase access, and improve quality through the use of workflow redesign, task shifting, dashboards, and other methods. With a large startup grant from the Nicholson Foundation, the Urban Health Institute launched the Cooper Advanced Care Center to provide the local under-served population with access to a collaborative practice of 23 medical and surgical specialties under one roof. The center incorporates traditional one-on-one provider visits with innovative models of care, including group visits. Multiple partners were required in the group visit design and implementation starting with buy-in from Cooper Health System’s leadership for existing space to be redesigned for the group visit activities.
The Cooper Advanced Care Center, which had high no-show rates of 30% to 40%, and low reimbursement for a primarily Medicaid and self-pay population, initially operated at a financial loss. Meanwhile, most physicians had extended lag time until their next available appointment. In a traditional patient–physician office visit, additional physician time is required to increase access to care. This costly solution is often not financially viable. Group visits were considered as a means of using an interdisciplinary team to increase access while controlling or decreasing the per-visit cost.
Musculoskeletal medicine was identified as an area of need in part due to limited access to care. Patients were waiting more than 2 months to see a musculoskeletal specialist and were being scheduled beyond capacity in our existing traditional weekly hand and knee/sports medicine clinics. Within musculoskeletal medicine, 4 areas of musculoskeletal medicine were considered for group visits: back pain, knee pain, hand pain, and foot and ankle disorders. The decision was made to focus the subspecialty musculoskeletal clinic on disorders of the hand and wrist to provide nonsurgical and surgical care options for atraumatic and traumatic disorders of the hand in a teaching environment at an urban hospital. The purpose of this article is to discuss the design and implementation of a hand pain group visit model to increase access to care without increasing cost.
Setting and Patients
Camden City, New Jersey, is a medically underserved, resource-poor community. The population is 48% African American and 47% Hispanic and nearly 40% of individuals live below the poverty level [5]. The group visit was intentionally set up as a means to provide access to the un- or underinsured. Patients attending the group visits were 33% African American, 33% Hispanic, and 30% Caucasian. Most patients had Medicaid insurance (67%) with the remaining patients covered by commercial insurance (15%), dual Medicare/Medicaid (11%), Medicare (5%), or self pay (2%).
Group Visit Staffing and Structure
In a traditional office visit, used nearly ubiquitously in outpatient medical offices, patients arrive at individual appointment times for a prescribed time encounter with the physician, are registered and roomed by support staff, and are then seen by a clinician for diagnosis and treatment. While assistants and trainees participate in the patient’s care with attending physician supervision, the majority of direct care falls to the physician. Access is coupled to physician availability; increasing access to care requires crowding the schedule with additional patients. We used this model as the benchmark for volume and scheduling against which to compare the group visit.
The group visit staffing was the same as for the traditional visit: hand surgeon, nurse practitioner, orthopedic technician, medical student, and medical assistant. However, each clinical session consists of four 1-hour, consecutive group visits scheduled once a month on a Monday morning. Up to 10 people could be scheduled for each 1-hour group visit. We continued to offer our traditional office visit clinic on the other 3 Mondays in the month.
The hand surgeon begins the group visit with a 10-minute educational session and group discussion held in a meeting room. He reviews common disorders of the hand, including carpal tunnel syndrome, trigger fingers, hand arthritis, cysts, sprains and fractures, how they are treated, and risks and benefits of treatments. Patients sign a confidentiality agreement at check-in. Time is allowed for questions and experiential sharing is encouraged. Expectations are set at the start of the visit to honor each patient’s input to provide a safe environment for asking questions and expressing concerns about their shared health condition to enhance the learning experience [6]. A medical assistant enters the chief complaint using an electronic standardized questionnaire into the EMR along with basic vital signs for each patient either prior to, during, or after the group presentation.
After the group educational session with the surgeon, patients transition to a large, open clinical room with 6 separate workstations, each consisting of a small table with 4 chairs and a laptop computer. Small procedures can be performed on the table (suture removal, dressing changes, injections) and the table is appropriately sized to accommodate a care provider, the patient, and their support person(s). Tables are spaced comfortably such that conversations do not carry much from one to the other. The clinical space has white noise speakers for sound dampening while patients receive individual history, vital signs, physical examination, and review of relevant studies. Patients may see the clinicians in a private exam room if they wish or require.
In a traditional office setting, workflow through the major tasks (check-in, data gathering, diagnosis, treatment) is often linear, as dictated by the configuration of individual patient rooms and the patient’s expectations of a traditional doctor’s visit. In the group visit, major tasks are performed simultaneously by the advance practice providers (nurse practitioners, physician assistants) in conjunction with supervision of the attending physician. The workstations (tables, chairs, laptops) in the open clinical room allows for greater efficiency; providers can easily transition to other tasks from one workstation to another during time that may have been spent waiting for other team members in the more linear, traditional clinic. For example, while waiting for the attending physician’s approval of a diagnosis and treatment plan for one patient, a nurse practitioner may be able to begin assessing and gathering data with a second patient until the physician becomes available.
Scheduling and Access
A primary aim of the group visit pilot was to develop a model of care delivery that allowed scheduling beyond capacity for the traditional office hand clinic. At the inception of the group visit, all patients were offered a visit in either the traditional office or group visit model by our scheduling secretaries based upon availability, with emphasis on scheduling a defined underserved population into the group visit.
In traditional 1:1 appointments, the number of people who can access care is dependent on physician availability. The team-based model uncouples the number of scheduled patients from the physician availability, allowing increased efficiency in the model and/or additional staff to increase the number of patients the group visit can accommodate. Thus, patients were essentially guaranteed an appointment in the next clinic because there was no cap on the number of patients that could be scheduled into the group visit. If the number of patients exceeded the limit of 10 per hour, a non-physician clinician was added to accommodate the patient demand. As our group visit matures, the ability to increase the staffing model enables patients to get care without adding more physician time.
Task Shifting
A central pillar of making the group visit a sustainable model for more accessible care is shifting tasks to non-physician health care workers. Adding specialist time enhances access but drives up the cost of care. Non-physician clinical staff members in subspecialty orthopedic offices with experience diagnosing and treating common conditions are capable of providing the standard of care for those conditions with variable physician oversight [7].
In the group visit, there is a deliberate assignment of patients to clinical staff by the physician based on anticipated level of care required. Given the attending physician’s experience with the most common disorders related to hand pain, it is possible to anticipate the approximate complexity of care required for each patient based on the nature and duration of the presenting complaint. With some degree of clinical supervision by the physician, members of the team operate maximally independently to assist patients. The nurse practitioner can operate largely autonomously in the group visit. The overall goal is to encourage all team members to function at the top of their licenses and abilities. Task shifting in this manner increases the productivity of all members of the team and minimizes redundancy. Despite more autonomy of mid-level providers and support staff in the group visit, there is still direct supervision of care by the attending physician.
The current body of literature in task shifting to non-physician health care workers has mostly concerned low-income countries with marked physician and provider shortages [8]. However, given the increased patient volume already seen with the Affordable Care Act and further expected increases, the health care system is likely to see provider shortages, especially in primary care [9–11]. This will necessitate the adoption of strategies to increase access, maintain quality care, and decrease systemic cost. Task shifting provides one such strategy.
Patient Satisfaction
One concern with shifting clinical duties to non-physician clinical staff is patient satisfaction. An abbreviated interaction with physicians can make patients dissatisfied with medical care independent of eventual clinical outcome [12]. However, it has also been demonstrated in an outpatient hand surgery clinic that quality of time spent with the physician may have a greater impact on satisfaction than quantity of time [13].
Our group visit is structured to allow high physician-patient visibility and interaction. The introductory remarks by the physician engage patients with the physician early in the visit and establish a group and individual rapport. The physician introduces the clinical team and the idea of patients being seen by other clinical staff up front, which establishes comfort for later patient–staff interaction. This is also an important time for patient education, which has been shown as a significant determinant of patient satisfaction in the outpatient setting [14]. The patient education at the beginning of the visit answers questions by one person that another person may not have considered, and generates patient questions to be addressed individually with a clinician. One common example is when a person considering carpal tunnel surgery hears from a person who has recently completed the procedure and can talk about their operative experience.
In the group room, the physician and staff can move between patients quickly and efficiently without waiting for turnover of rooms and resources. The structure of the visit allows staff to dedicate more time to patient care by bypassing the extra time required when patients are roomed individually. The group/communal structure also allows patients to see the staff at work, as compared to time spent waiting alone in an exam room in the traditional office. This enables patients to appreciate the efforts of the clinical staff and avoids giving the impression that the physician is inattentive or cursory in patient interaction.
Medical Education
An important consideration at any academic medical center is education. However, education often introduces redundancies and inefficiency into the medical care visit. The work a trainee does is either extensively overseen or the clinical questions are repeated by a supervising provider. However, it is possible to increase efficiency and utility of trainees in the group visit setting while maintaining educational value.
Given the relatively narrow scope and the nature of conditions encountered in the hand clinic, medical decision making for many patients is limited to a “straightforward” or “low complexity” level. These designations assume a limited number of diagnoses, management options, and amount and complexity of diagnostic workup. Most importantly, risk of complications or morbidity/mortality at these levels is minimal to low. For these conditions, a supervising physician can allow a trainee more independence to practice employing simple treatment and management guidelines and progress to working independently when addressing simpler conditions as the trainee’s experience grows. As independence grows, trainees can build confidence in medical management as well as focus on other core educational competencies once they are comfortable with the evaluation and management of a limited scope of conditions. Conditions such as trigger fingers, hand arthritis, ganglion cysts, and carpal tunnel are those the trainee is likely to encounter in a primary care practice. While there may be a decrease in direct physician teaching, the trainee gains clinical autonomy and experience in educational core competencies such as patient communication, patient education, systems-based practice, procedural skill, cultural competency, and interdisciplinary teamwork [15,16].
Lessons Learned
The success of the group visit required buy-in from hospital and physician leadership, the clinical team, and multiple partners in the hospital system. The hospital administration supported group visits as an integral component of the Urban Health Institute. Buy-in from key hospital leadership ensured resources and dedicated space for the group visit program. Grant support allowed additional programmatic support to acquire the necessary assistance from information services, EMR support, legal, and marketing. Physician buy-in was the most significant piece to the success of an individual group visit. Accepting the movement away from physician autonomy to team-based care is challenging for many providers. Physician willingness to start a high-demand group visit, recognition of the start-up inefficiencies, and working with the administrative and clinical team on program improvement strategies has succeeded in launching a sustaining group visit model.
Conclusion
There is a need for an adaptive and economically viable model of patient care to meet increasing demand, as well as provide care for indigent populations in a way that is more economically sustainable than providing care through the emergency department. The development and implementation of an urban hand group visit at our institution has demonstrated that such a model, based on group visit models more commonly seen in primary care, can be effectively implemented in a subspecialty care setting. This model is capable of increasing patient access to care and effectively handling increased patient volume with room for cost-effective growth in the future, all while maintaining quality of care. We anticipate further subspecialty clinics within hand pain to emerge, such as a group visit dedicated specifically to carpal tunnel syndrome or hand arthritis. This will allow each group to be more focused and will streamline education and mutual support among the patients.
Corresponding author: Steven Kaufman, MD, 3 Cooper Plaza, Suite 211, Camden, NJ 08103, [email protected].
Funding/support: The Nicholson Foundation.
Financial disclosures: None reported.
From Cooper Medical School of Rowan University (Dr. Patel, Dr. Fuller) and Cooper University Hospital (Dr. Kaufman), Camden, NJ.
Abstract
- Objective: To describe an adaptive musculoskeletal hand clinic that offers accessible and economically viable musculoskeletal care for an underserved, urban population.
- Methods: Descriptive report.
- Results: An enhanced access group visit model was developed offering both nonsurgical and surgical care pathways for patients with musculoskeletal disorders of the hand. Both patient education and care were provided in the group environment. Staffing included an orthopedic surgeon, nurse practitioner, medical student, orthopedic technician, and medical assistant. Over a 12-month period, group visit efficiency improved to accommodate an equivalent number of patients as compared to the traditional model. Access (time to appointment) was improved in the group visit. The model allows for the addition of non-physician clinical staff to improve access and limit costs in a manner not feasible with traditional office visits.
- Conclusion: A group visit model may offer a sustainable process to increase patient access to musculoskeletal subspecialty care and accommodate care of greater numbers of patients while maintaining quality. The group model offers flexible staffing, enhanced access, and educational benefit.
Key words: Group medical visit, team-based care, hand pain, access, underserved populations.
Group visits are a relatively new health care delivery model [1–3]. The term is applied to a wide variety of visits designed for groups of patients, rather than individual patient-provider appointments. The group visit format has been used for various disease- or condition-specific populations. Group visits can increase access to care and have been associated with improved clinical outcomes [4].
The Urban Health Institute, a dedicated business unit at Cooper University Health System in Camden, NJ, was established to focus on care of the underserved. The business unit is working to reduce cost of care delivery, increase access, and improve quality through the use of workflow redesign, task shifting, dashboards, and other methods. With a large startup grant from the Nicholson Foundation, the Urban Health Institute launched the Cooper Advanced Care Center to provide the local under-served population with access to a collaborative practice of 23 medical and surgical specialties under one roof. The center incorporates traditional one-on-one provider visits with innovative models of care, including group visits. Multiple partners were required in the group visit design and implementation starting with buy-in from Cooper Health System’s leadership for existing space to be redesigned for the group visit activities.
The Cooper Advanced Care Center, which had high no-show rates of 30% to 40%, and low reimbursement for a primarily Medicaid and self-pay population, initially operated at a financial loss. Meanwhile, most physicians had extended lag time until their next available appointment. In a traditional patient–physician office visit, additional physician time is required to increase access to care. This costly solution is often not financially viable. Group visits were considered as a means of using an interdisciplinary team to increase access while controlling or decreasing the per-visit cost.
Musculoskeletal medicine was identified as an area of need in part due to limited access to care. Patients were waiting more than 2 months to see a musculoskeletal specialist and were being scheduled beyond capacity in our existing traditional weekly hand and knee/sports medicine clinics. Within musculoskeletal medicine, 4 areas of musculoskeletal medicine were considered for group visits: back pain, knee pain, hand pain, and foot and ankle disorders. The decision was made to focus the subspecialty musculoskeletal clinic on disorders of the hand and wrist to provide nonsurgical and surgical care options for atraumatic and traumatic disorders of the hand in a teaching environment at an urban hospital. The purpose of this article is to discuss the design and implementation of a hand pain group visit model to increase access to care without increasing cost.
Setting and Patients
Camden City, New Jersey, is a medically underserved, resource-poor community. The population is 48% African American and 47% Hispanic and nearly 40% of individuals live below the poverty level [5]. The group visit was intentionally set up as a means to provide access to the un- or underinsured. Patients attending the group visits were 33% African American, 33% Hispanic, and 30% Caucasian. Most patients had Medicaid insurance (67%) with the remaining patients covered by commercial insurance (15%), dual Medicare/Medicaid (11%), Medicare (5%), or self pay (2%).
Group Visit Staffing and Structure
In a traditional office visit, used nearly ubiquitously in outpatient medical offices, patients arrive at individual appointment times for a prescribed time encounter with the physician, are registered and roomed by support staff, and are then seen by a clinician for diagnosis and treatment. While assistants and trainees participate in the patient’s care with attending physician supervision, the majority of direct care falls to the physician. Access is coupled to physician availability; increasing access to care requires crowding the schedule with additional patients. We used this model as the benchmark for volume and scheduling against which to compare the group visit.
The group visit staffing was the same as for the traditional visit: hand surgeon, nurse practitioner, orthopedic technician, medical student, and medical assistant. However, each clinical session consists of four 1-hour, consecutive group visits scheduled once a month on a Monday morning. Up to 10 people could be scheduled for each 1-hour group visit. We continued to offer our traditional office visit clinic on the other 3 Mondays in the month.
The hand surgeon begins the group visit with a 10-minute educational session and group discussion held in a meeting room. He reviews common disorders of the hand, including carpal tunnel syndrome, trigger fingers, hand arthritis, cysts, sprains and fractures, how they are treated, and risks and benefits of treatments. Patients sign a confidentiality agreement at check-in. Time is allowed for questions and experiential sharing is encouraged. Expectations are set at the start of the visit to honor each patient’s input to provide a safe environment for asking questions and expressing concerns about their shared health condition to enhance the learning experience [6]. A medical assistant enters the chief complaint using an electronic standardized questionnaire into the EMR along with basic vital signs for each patient either prior to, during, or after the group presentation.
After the group educational session with the surgeon, patients transition to a large, open clinical room with 6 separate workstations, each consisting of a small table with 4 chairs and a laptop computer. Small procedures can be performed on the table (suture removal, dressing changes, injections) and the table is appropriately sized to accommodate a care provider, the patient, and their support person(s). Tables are spaced comfortably such that conversations do not carry much from one to the other. The clinical space has white noise speakers for sound dampening while patients receive individual history, vital signs, physical examination, and review of relevant studies. Patients may see the clinicians in a private exam room if they wish or require.
In a traditional office setting, workflow through the major tasks (check-in, data gathering, diagnosis, treatment) is often linear, as dictated by the configuration of individual patient rooms and the patient’s expectations of a traditional doctor’s visit. In the group visit, major tasks are performed simultaneously by the advance practice providers (nurse practitioners, physician assistants) in conjunction with supervision of the attending physician. The workstations (tables, chairs, laptops) in the open clinical room allows for greater efficiency; providers can easily transition to other tasks from one workstation to another during time that may have been spent waiting for other team members in the more linear, traditional clinic. For example, while waiting for the attending physician’s approval of a diagnosis and treatment plan for one patient, a nurse practitioner may be able to begin assessing and gathering data with a second patient until the physician becomes available.
Scheduling and Access
A primary aim of the group visit pilot was to develop a model of care delivery that allowed scheduling beyond capacity for the traditional office hand clinic. At the inception of the group visit, all patients were offered a visit in either the traditional office or group visit model by our scheduling secretaries based upon availability, with emphasis on scheduling a defined underserved population into the group visit.
In traditional 1:1 appointments, the number of people who can access care is dependent on physician availability. The team-based model uncouples the number of scheduled patients from the physician availability, allowing increased efficiency in the model and/or additional staff to increase the number of patients the group visit can accommodate. Thus, patients were essentially guaranteed an appointment in the next clinic because there was no cap on the number of patients that could be scheduled into the group visit. If the number of patients exceeded the limit of 10 per hour, a non-physician clinician was added to accommodate the patient demand. As our group visit matures, the ability to increase the staffing model enables patients to get care without adding more physician time.
Task Shifting
A central pillar of making the group visit a sustainable model for more accessible care is shifting tasks to non-physician health care workers. Adding specialist time enhances access but drives up the cost of care. Non-physician clinical staff members in subspecialty orthopedic offices with experience diagnosing and treating common conditions are capable of providing the standard of care for those conditions with variable physician oversight [7].
In the group visit, there is a deliberate assignment of patients to clinical staff by the physician based on anticipated level of care required. Given the attending physician’s experience with the most common disorders related to hand pain, it is possible to anticipate the approximate complexity of care required for each patient based on the nature and duration of the presenting complaint. With some degree of clinical supervision by the physician, members of the team operate maximally independently to assist patients. The nurse practitioner can operate largely autonomously in the group visit. The overall goal is to encourage all team members to function at the top of their licenses and abilities. Task shifting in this manner increases the productivity of all members of the team and minimizes redundancy. Despite more autonomy of mid-level providers and support staff in the group visit, there is still direct supervision of care by the attending physician.
The current body of literature in task shifting to non-physician health care workers has mostly concerned low-income countries with marked physician and provider shortages [8]. However, given the increased patient volume already seen with the Affordable Care Act and further expected increases, the health care system is likely to see provider shortages, especially in primary care [9–11]. This will necessitate the adoption of strategies to increase access, maintain quality care, and decrease systemic cost. Task shifting provides one such strategy.
Patient Satisfaction
One concern with shifting clinical duties to non-physician clinical staff is patient satisfaction. An abbreviated interaction with physicians can make patients dissatisfied with medical care independent of eventual clinical outcome [12]. However, it has also been demonstrated in an outpatient hand surgery clinic that quality of time spent with the physician may have a greater impact on satisfaction than quantity of time [13].
Our group visit is structured to allow high physician-patient visibility and interaction. The introductory remarks by the physician engage patients with the physician early in the visit and establish a group and individual rapport. The physician introduces the clinical team and the idea of patients being seen by other clinical staff up front, which establishes comfort for later patient–staff interaction. This is also an important time for patient education, which has been shown as a significant determinant of patient satisfaction in the outpatient setting [14]. The patient education at the beginning of the visit answers questions by one person that another person may not have considered, and generates patient questions to be addressed individually with a clinician. One common example is when a person considering carpal tunnel surgery hears from a person who has recently completed the procedure and can talk about their operative experience.
In the group room, the physician and staff can move between patients quickly and efficiently without waiting for turnover of rooms and resources. The structure of the visit allows staff to dedicate more time to patient care by bypassing the extra time required when patients are roomed individually. The group/communal structure also allows patients to see the staff at work, as compared to time spent waiting alone in an exam room in the traditional office. This enables patients to appreciate the efforts of the clinical staff and avoids giving the impression that the physician is inattentive or cursory in patient interaction.
Medical Education
An important consideration at any academic medical center is education. However, education often introduces redundancies and inefficiency into the medical care visit. The work a trainee does is either extensively overseen or the clinical questions are repeated by a supervising provider. However, it is possible to increase efficiency and utility of trainees in the group visit setting while maintaining educational value.
Given the relatively narrow scope and the nature of conditions encountered in the hand clinic, medical decision making for many patients is limited to a “straightforward” or “low complexity” level. These designations assume a limited number of diagnoses, management options, and amount and complexity of diagnostic workup. Most importantly, risk of complications or morbidity/mortality at these levels is minimal to low. For these conditions, a supervising physician can allow a trainee more independence to practice employing simple treatment and management guidelines and progress to working independently when addressing simpler conditions as the trainee’s experience grows. As independence grows, trainees can build confidence in medical management as well as focus on other core educational competencies once they are comfortable with the evaluation and management of a limited scope of conditions. Conditions such as trigger fingers, hand arthritis, ganglion cysts, and carpal tunnel are those the trainee is likely to encounter in a primary care practice. While there may be a decrease in direct physician teaching, the trainee gains clinical autonomy and experience in educational core competencies such as patient communication, patient education, systems-based practice, procedural skill, cultural competency, and interdisciplinary teamwork [15,16].
Lessons Learned
The success of the group visit required buy-in from hospital and physician leadership, the clinical team, and multiple partners in the hospital system. The hospital administration supported group visits as an integral component of the Urban Health Institute. Buy-in from key hospital leadership ensured resources and dedicated space for the group visit program. Grant support allowed additional programmatic support to acquire the necessary assistance from information services, EMR support, legal, and marketing. Physician buy-in was the most significant piece to the success of an individual group visit. Accepting the movement away from physician autonomy to team-based care is challenging for many providers. Physician willingness to start a high-demand group visit, recognition of the start-up inefficiencies, and working with the administrative and clinical team on program improvement strategies has succeeded in launching a sustaining group visit model.
Conclusion
There is a need for an adaptive and economically viable model of patient care to meet increasing demand, as well as provide care for indigent populations in a way that is more economically sustainable than providing care through the emergency department. The development and implementation of an urban hand group visit at our institution has demonstrated that such a model, based on group visit models more commonly seen in primary care, can be effectively implemented in a subspecialty care setting. This model is capable of increasing patient access to care and effectively handling increased patient volume with room for cost-effective growth in the future, all while maintaining quality of care. We anticipate further subspecialty clinics within hand pain to emerge, such as a group visit dedicated specifically to carpal tunnel syndrome or hand arthritis. This will allow each group to be more focused and will streamline education and mutual support among the patients.
Corresponding author: Steven Kaufman, MD, 3 Cooper Plaza, Suite 211, Camden, NJ 08103, [email protected].
Funding/support: The Nicholson Foundation.
Financial disclosures: None reported.
1. Gardiner P, Dresner D, Barnett KG, et al. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med 2014;3:20–6.
2. Remick RA, Remick AK. Do patients really prefer individual outpatient follow-up visits, compared with group medical visits?. Can J Psychiatry 2014;59:50–3.
3. Thompson C, Meeuwisse I, Dahlke R, Drummond N. Group medical visits in primary care for patients with diabetes and low socioeconomic status: users’ perspectives and lessons for practitioners. Can J Diabetes 2014;38:198–204.
4. Eisenstat S, Lipps SA, Carlson K, Ulman K. Putting group visits into practice: a practical overview to preparation, implementation, and maintenance of group visits at Massachusetts General Hospital. Women’s Health Associates, The John D. Stoeckle Center for Primary Care Innovation, Massachusetts General Hospital; January 2012.
5. U.S. Census Bureau. State and city Quickfacts: Camden (city), New Jersey. Accessed 29 Sep 2015 at http://quickfacts.census.gov/qfd/states/34/3410000.html.
6. Slocum YS. A survey of expectations about group therapy among clinical and nonclinical populations. Int J Group Psychother 1987;37:39–54.
7. Newhouse RP, Stanik-hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. Nurs Econ 2011;29:230–50.
8. Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS ONE 2014;9:e103754.
9. Hofer AN, Abraham JM, Moscovice I. Expansion of coverage under the Patient Protection and Affordable Care Act and primary care utilization. Milbank Q 2011;89:69–89.
10. Kushnir T, Greenberg D, Madjar N, et al. Is burnout associated with referral rates among primary care physicians in community clinics?. Fam Pract 2014;31:44–50.
11. Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am 2012;94:2177–84.
12. Lin CT, Albertson GA, Schilling LM, et al. Is patients’ perception of time spent with the physician a determinant of ambulatory patient satisfaction?. Arch Intern Med 2001;161:1437–42.
13. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated with patient satisfaction. Clin Orthop Relat Res 2014.
14. Murdock A, Griffin B. How is patient education linked to patient satisfaction?. Nursing 2013;43:43–5.
15. Accreditation Council for Graduate Medical Education. Common program requirements. Approved 2014. Available at www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_07012016.pdf.
16. Liaison Committee on Medical Education. Functions and structure of a medical school: standards for accreditation of medical education programs leading to the M.D. degree. [updated June 2013]. Available at www.lcme.org/publications/functions.pdf.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51.
1. Gardiner P, Dresner D, Barnett KG, et al. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med 2014;3:20–6.
2. Remick RA, Remick AK. Do patients really prefer individual outpatient follow-up visits, compared with group medical visits?. Can J Psychiatry 2014;59:50–3.
3. Thompson C, Meeuwisse I, Dahlke R, Drummond N. Group medical visits in primary care for patients with diabetes and low socioeconomic status: users’ perspectives and lessons for practitioners. Can J Diabetes 2014;38:198–204.
4. Eisenstat S, Lipps SA, Carlson K, Ulman K. Putting group visits into practice: a practical overview to preparation, implementation, and maintenance of group visits at Massachusetts General Hospital. Women’s Health Associates, The John D. Stoeckle Center for Primary Care Innovation, Massachusetts General Hospital; January 2012.
5. U.S. Census Bureau. State and city Quickfacts: Camden (city), New Jersey. Accessed 29 Sep 2015 at http://quickfacts.census.gov/qfd/states/34/3410000.html.
6. Slocum YS. A survey of expectations about group therapy among clinical and nonclinical populations. Int J Group Psychother 1987;37:39–54.
7. Newhouse RP, Stanik-hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. Nurs Econ 2011;29:230–50.
8. Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS ONE 2014;9:e103754.
9. Hofer AN, Abraham JM, Moscovice I. Expansion of coverage under the Patient Protection and Affordable Care Act and primary care utilization. Milbank Q 2011;89:69–89.
10. Kushnir T, Greenberg D, Madjar N, et al. Is burnout associated with referral rates among primary care physicians in community clinics?. Fam Pract 2014;31:44–50.
11. Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am 2012;94:2177–84.
12. Lin CT, Albertson GA, Schilling LM, et al. Is patients’ perception of time spent with the physician a determinant of ambulatory patient satisfaction?. Arch Intern Med 2001;161:1437–42.
13. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated with patient satisfaction. Clin Orthop Relat Res 2014.
14. Murdock A, Griffin B. How is patient education linked to patient satisfaction?. Nursing 2013;43:43–5.
15. Accreditation Council for Graduate Medical Education. Common program requirements. Approved 2014. Available at www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_07012016.pdf.
16. Liaison Committee on Medical Education. Functions and structure of a medical school: standards for accreditation of medical education programs leading to the M.D. degree. [updated June 2013]. Available at www.lcme.org/publications/functions.pdf.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51.
Hand-foot syndrome less with S-1 than capecitabine in mCRC
AMSTERDAM – For patients with metastatic colorectal cancer, S-1 was comparable in efficacy to capecitabine (Xeloda), and was associated with a lower incidence of all grades of hand-foot syndrome, reported investigators.
Among 161 patients with untreated metastatic colorectal cancer, the investigator-assessed incidence of all grades of hand-foot syndrome was 73% for patients randomly assigned to capecitabine, compared with 45% for patients randomized to S-1 (P = .0005).
Patient-assessed symptoms also were lower with S-1 than with capecitabine, reported Robert Jan Kwakman, MD, of the Academic Medical Center in Amsterdam.
“We conclude that treatment with S-1 is a useful alternative to capecitabine in the treatment of metastatic colorectal cancer,” he said at an annual congress sponsored by the European Cancer Organisation.
S-1 is an oral fluoropyrimidine consisting of tegafur, a prodrug of 5-fluorauracil (5-FU), combined with two 5-FU biochemical modulators. It is associated with a lower incidence of hand-foot syndrome than capecitabine, and has shown efficacy comparable to that of other fluoropyrimidines in Asian patients with gastrointestinal cancers, Dr. Kwakman noted.
Hand-foot syndrome can vary in severity from grade 1, marked by minimal skin changes or dermatitis without pain, to grade 3, characterized by severe skin changes with pain and significant limits to self care during activities of daily living.
In the randomized phase III SALTO trial (S1 Versus Capecitabine in the First Line Treatment of Metastatic Colorectal Cancer Patients), investigators in the Dutch Colorectal Cancer Group enrolled patients with untreated metastatic colorectal cancer with World Health Organization performance status 0-2 who were scheduled for treatment with fluoropyrimidine monotherapy. The patients were assigned to receive either capecitabine 1,250 mg/m2 for patients younger than 70, or 1,000 mg/m2 for those 70 and older, twice a day for days 1-14 of a 3-week cycle, or to S-1 30 mg/m2 twice daily on the same schedule.
In each arm, investigators could, at their discretion, also prescribe bevacizumab 7.5 mg/kg on day 1. In each arm, 59% of patients were scheduled to receive bevacizumab.
Patients were stratified by bevacizumab status, lactate dehydrogenase levels (normal vs. abnormal), performance status (0-1 vs. 2) and institution.
Patients were asked to keep diaries and record whether during the past 3 weeks they had experienced symptoms in their hands and/or feet such as tingling/numbness, pain, redness, swelling, and desquamation, and if so, whether the symptoms interfered with daily activities.
After a median follow-up of 16.1 months, investigators assessed hand-foot syndrome rates by grade were as follows:
• Grade 1: 21% for the capecitabine arm vs. 28% for the S-1 arm (not significant).
• Grade 2: 30% vs. 14% (P = .02).
• Grade 3: 21% vs. 4% (P = .003).
The incidence of patient-assessed hand-foot syndrome of all grades was 84% in the capecitabine arm and 58% in the S-1 arm (P = .004). Patient-reported grade 3 hand-foot syndrome occurred in 18% vs. 5%, respectively (P = .05).
The only other toxicity occurring more frequently among patients on S-1 was anorexia, which occurred in 29% of patients on capecitabine compared with 41% with S-1. Grade 3 or greater anorexia occurred in 3% vs. 13%, respectively (P = .03).
Significantly more dose reductions were required for patients on capecitabine (69% vs. 41%, P = .0008). The median relative dose intensity was higher for patients on S-1 (89% vs. 95%, P = .035).
Among all patients, median progression-free survival was 8.18 months with capecitabine vs. 8.39 months with S-1, a difference that was not significant. There was a trend toward better progression-free survival for patients in each arm who received bevacizumab (8.74 vs. 6.37 months without bevacizumab), but this difference was also not significant.
Overall survival rates at 12 months were 67% with capecitabine and 62% with S-1 (not significantly different), and respective rates at 18 months were 50% vs. 39%. The hazard ratio for mortality with S-1 was 1.28 (not significant).
The study was sponsored by the Dutch Colorectal Cancer Group, with research funds supplied by Nordic Pharma BV. Dr. Kwakman disclosed receiving an honorarium from the company.
AMSTERDAM – For patients with metastatic colorectal cancer, S-1 was comparable in efficacy to capecitabine (Xeloda), and was associated with a lower incidence of all grades of hand-foot syndrome, reported investigators.
Among 161 patients with untreated metastatic colorectal cancer, the investigator-assessed incidence of all grades of hand-foot syndrome was 73% for patients randomly assigned to capecitabine, compared with 45% for patients randomized to S-1 (P = .0005).
Patient-assessed symptoms also were lower with S-1 than with capecitabine, reported Robert Jan Kwakman, MD, of the Academic Medical Center in Amsterdam.
“We conclude that treatment with S-1 is a useful alternative to capecitabine in the treatment of metastatic colorectal cancer,” he said at an annual congress sponsored by the European Cancer Organisation.
S-1 is an oral fluoropyrimidine consisting of tegafur, a prodrug of 5-fluorauracil (5-FU), combined with two 5-FU biochemical modulators. It is associated with a lower incidence of hand-foot syndrome than capecitabine, and has shown efficacy comparable to that of other fluoropyrimidines in Asian patients with gastrointestinal cancers, Dr. Kwakman noted.
Hand-foot syndrome can vary in severity from grade 1, marked by minimal skin changes or dermatitis without pain, to grade 3, characterized by severe skin changes with pain and significant limits to self care during activities of daily living.
In the randomized phase III SALTO trial (S1 Versus Capecitabine in the First Line Treatment of Metastatic Colorectal Cancer Patients), investigators in the Dutch Colorectal Cancer Group enrolled patients with untreated metastatic colorectal cancer with World Health Organization performance status 0-2 who were scheduled for treatment with fluoropyrimidine monotherapy. The patients were assigned to receive either capecitabine 1,250 mg/m2 for patients younger than 70, or 1,000 mg/m2 for those 70 and older, twice a day for days 1-14 of a 3-week cycle, or to S-1 30 mg/m2 twice daily on the same schedule.
In each arm, investigators could, at their discretion, also prescribe bevacizumab 7.5 mg/kg on day 1. In each arm, 59% of patients were scheduled to receive bevacizumab.
Patients were stratified by bevacizumab status, lactate dehydrogenase levels (normal vs. abnormal), performance status (0-1 vs. 2) and institution.
Patients were asked to keep diaries and record whether during the past 3 weeks they had experienced symptoms in their hands and/or feet such as tingling/numbness, pain, redness, swelling, and desquamation, and if so, whether the symptoms interfered with daily activities.
After a median follow-up of 16.1 months, investigators assessed hand-foot syndrome rates by grade were as follows:
• Grade 1: 21% for the capecitabine arm vs. 28% for the S-1 arm (not significant).
• Grade 2: 30% vs. 14% (P = .02).
• Grade 3: 21% vs. 4% (P = .003).
The incidence of patient-assessed hand-foot syndrome of all grades was 84% in the capecitabine arm and 58% in the S-1 arm (P = .004). Patient-reported grade 3 hand-foot syndrome occurred in 18% vs. 5%, respectively (P = .05).
The only other toxicity occurring more frequently among patients on S-1 was anorexia, which occurred in 29% of patients on capecitabine compared with 41% with S-1. Grade 3 or greater anorexia occurred in 3% vs. 13%, respectively (P = .03).
Significantly more dose reductions were required for patients on capecitabine (69% vs. 41%, P = .0008). The median relative dose intensity was higher for patients on S-1 (89% vs. 95%, P = .035).
Among all patients, median progression-free survival was 8.18 months with capecitabine vs. 8.39 months with S-1, a difference that was not significant. There was a trend toward better progression-free survival for patients in each arm who received bevacizumab (8.74 vs. 6.37 months without bevacizumab), but this difference was also not significant.
Overall survival rates at 12 months were 67% with capecitabine and 62% with S-1 (not significantly different), and respective rates at 18 months were 50% vs. 39%. The hazard ratio for mortality with S-1 was 1.28 (not significant).
The study was sponsored by the Dutch Colorectal Cancer Group, with research funds supplied by Nordic Pharma BV. Dr. Kwakman disclosed receiving an honorarium from the company.
AMSTERDAM – For patients with metastatic colorectal cancer, S-1 was comparable in efficacy to capecitabine (Xeloda), and was associated with a lower incidence of all grades of hand-foot syndrome, reported investigators.
Among 161 patients with untreated metastatic colorectal cancer, the investigator-assessed incidence of all grades of hand-foot syndrome was 73% for patients randomly assigned to capecitabine, compared with 45% for patients randomized to S-1 (P = .0005).
Patient-assessed symptoms also were lower with S-1 than with capecitabine, reported Robert Jan Kwakman, MD, of the Academic Medical Center in Amsterdam.
“We conclude that treatment with S-1 is a useful alternative to capecitabine in the treatment of metastatic colorectal cancer,” he said at an annual congress sponsored by the European Cancer Organisation.
S-1 is an oral fluoropyrimidine consisting of tegafur, a prodrug of 5-fluorauracil (5-FU), combined with two 5-FU biochemical modulators. It is associated with a lower incidence of hand-foot syndrome than capecitabine, and has shown efficacy comparable to that of other fluoropyrimidines in Asian patients with gastrointestinal cancers, Dr. Kwakman noted.
Hand-foot syndrome can vary in severity from grade 1, marked by minimal skin changes or dermatitis without pain, to grade 3, characterized by severe skin changes with pain and significant limits to self care during activities of daily living.
In the randomized phase III SALTO trial (S1 Versus Capecitabine in the First Line Treatment of Metastatic Colorectal Cancer Patients), investigators in the Dutch Colorectal Cancer Group enrolled patients with untreated metastatic colorectal cancer with World Health Organization performance status 0-2 who were scheduled for treatment with fluoropyrimidine monotherapy. The patients were assigned to receive either capecitabine 1,250 mg/m2 for patients younger than 70, or 1,000 mg/m2 for those 70 and older, twice a day for days 1-14 of a 3-week cycle, or to S-1 30 mg/m2 twice daily on the same schedule.
In each arm, investigators could, at their discretion, also prescribe bevacizumab 7.5 mg/kg on day 1. In each arm, 59% of patients were scheduled to receive bevacizumab.
Patients were stratified by bevacizumab status, lactate dehydrogenase levels (normal vs. abnormal), performance status (0-1 vs. 2) and institution.
Patients were asked to keep diaries and record whether during the past 3 weeks they had experienced symptoms in their hands and/or feet such as tingling/numbness, pain, redness, swelling, and desquamation, and if so, whether the symptoms interfered with daily activities.
After a median follow-up of 16.1 months, investigators assessed hand-foot syndrome rates by grade were as follows:
• Grade 1: 21% for the capecitabine arm vs. 28% for the S-1 arm (not significant).
• Grade 2: 30% vs. 14% (P = .02).
• Grade 3: 21% vs. 4% (P = .003).
The incidence of patient-assessed hand-foot syndrome of all grades was 84% in the capecitabine arm and 58% in the S-1 arm (P = .004). Patient-reported grade 3 hand-foot syndrome occurred in 18% vs. 5%, respectively (P = .05).
The only other toxicity occurring more frequently among patients on S-1 was anorexia, which occurred in 29% of patients on capecitabine compared with 41% with S-1. Grade 3 or greater anorexia occurred in 3% vs. 13%, respectively (P = .03).
Significantly more dose reductions were required for patients on capecitabine (69% vs. 41%, P = .0008). The median relative dose intensity was higher for patients on S-1 (89% vs. 95%, P = .035).
Among all patients, median progression-free survival was 8.18 months with capecitabine vs. 8.39 months with S-1, a difference that was not significant. There was a trend toward better progression-free survival for patients in each arm who received bevacizumab (8.74 vs. 6.37 months without bevacizumab), but this difference was also not significant.
Overall survival rates at 12 months were 67% with capecitabine and 62% with S-1 (not significantly different), and respective rates at 18 months were 50% vs. 39%. The hazard ratio for mortality with S-1 was 1.28 (not significant).
The study was sponsored by the Dutch Colorectal Cancer Group, with research funds supplied by Nordic Pharma BV. Dr. Kwakman disclosed receiving an honorarium from the company.
AT ECCO2017
Key clinical point: S-1 was associated with a lower incidence of hand-foot syndrome than was capecitabine in patients with metastatic colorectal cancer.
Major finding: Hand-foot syndrome of any grade occurred in 73% of patients on capecitabine vs. 45% on S-1 (P = .0005).
Data source: Randomized phase III trial of 161 patients with previously untreated metastatic colorectal cancer.
Disclosures: The study was sponsored by the Dutch Colorectal Cancer Group, with research funds supplied by Nordic Pharma BV. Dr. Kwakman disclosed receiving an honorarium from the company.
Postpartum Recovery Trends in Women with Hypertensive Disorders of Pregnancy
From the Department of Obstetrics and Gynecology, Kasturba Medical College, Manipal, Karnataka, India.
Abstracts
- Objective: To examine the association of the patient’s obstetric profile and time to normalization of blood pressure in the postnatal period among women with hypertensive disorders in pregnancy.
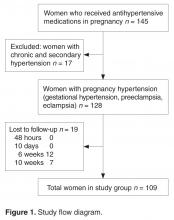
- Methods: We conducted a prospective cohort study at a tertiary level hospital between November 2014 and May 2015. Women with pregnancy hypertension who required antihypertensive treatment were recruited after delivery. The normalization trends in blood pressure were tested for associations with patient demographic data and details of pregnancy hypertension.
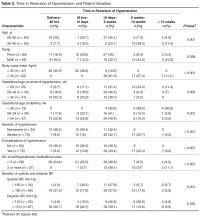
- Results: Among 109 women included in the study, earlier gestational age at onset of hypertension and earlier gestational age at delivery was correlated with slower resolution of hypertension. Time to resolution also was correlated with age, BMI, severity of hypertension, associated complications, and the number of antihypertensive medications received. There was no correlation with highest recorded systolic or diastolic blood pressures. Only 15% of women with gestational hypertension had persistent hypertension beyond 6 weeks. In the groups with nonsevere preeclampsia, severe preeclampsia, and eclampsia, blood pressure remained high after 6 weeks in 26%, 14%, and 50% of women, respectively.
- Conclusion: Women with advanced age, higher body mass index, early gestational age at the onset of hypertension, severe hypertension and who had complications of hypertension require prolonged monitoring and treatment when indicated for hypertension in postnatal period.
Key words: intensive care unit; communication; family meeting; critical illness; decision making; end of life care.
Hypertension is the most common medical problem encountered during pregnancy, complicating up to 10% of pregnancies worldwide [1]. The disorders of hypertension in pregnancy are generally classified as chronic hypertension, preeclampsia–eclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension. The hypertensive disorders of pregnancy are a leading cause of mortality and morbidity in the perinatal period.
Women with hypertensive disorders in pregnancy show varying trends of blood pressure normalization, with the recovery period ranging from a few hours to several months after delivery. In one study, nearly one-fourth of women with preeclampsia/eclampsia had persistent high blood pressure after puerperium [2]. Identifying the obstetric risk factors for persistent hypertension will help in focusing care and research in this group of patients.
We undertook a prospective study to assess possible correlations of obstetric profile with time to normalization of blood pressure in the postnatal period among women with hypertensive disorders in pregnancy.
Methods
Setting
This prospective cohort study was conducted in the department of obstetrics and gynecology at Kasturba Hospital, Manipal, between November 2014 and May 2015. Permission for the study was obtained from the Institution Ethical Committee (IEC264/2015).
Patients
Women who had hypertension in pregnancy and required antihypertensive treatment were approached on the first postnatal day and invited to participate in the study. Women with chronic hypertension (women with known pre-pregnancy hypertension and with hypertension diagnosed before 20 weeks gestation) or secondary hypertension were excluded. After granting informed consent, enrolled women were followed until the time they no longer required antihypertensive medication (“reversion of hypertension”) or until 10 weeks postpartum, whichever came first.
During the hospital stay in the postnatal period, women had their blood pressure monitored and antihypertensives were adjusted as needed. After discharge from the hospital, blood pressure was monitored by the family physician who also made decisions regarding antihypertensive management. All women had a follow-up visit in the hospital in the 6th postnatal week as per the postnatal clinic protocol.
Definitions
Hypertension was defined as BP ≥ 140/90 mm Hg. The hypertension disorders of pregnancy were defined as follows:
- Gestational hypertension: hypertension after 20 weeks gestation on two occasions 4 hours apart without meeting criteria for preeclampsia.
- Preeclampsia: hypertension after 20 weeks gestation on two occasions 4 hours apart with proteinuria (≥ 300 mg/24 hour) or, in the absence of proteinuria, new onset of any of the following: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms [1]. Severe preeclampsia was defined as preeclampsia with any of the following: systolic blood pressure > 160 mm Hg diastolic BP > 110 mm Hg or more on 2 occasions 4 hours apart, thrombocytopenia (platelet count < 100,00/mL), renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms. Preeclampsia without any of these features was considered nonsevere preeclampsia.
- Eclampsia: Women with hypertension with epigastric pain, headache, vomiting, and blurring of vision were diagnosed with imminent eclampsia and those with hypertension-related convulsions were diagnosed with eclampsia.
- Complications of preeclampsia included eclampsia, placental abruption, pulmonary edema, thrombocytopenia, HELLP syndrome, disseminated intravascular coagulation, multiorgan failure, severe intrauterine growth restriction, and fetal demise.
Main Outcome Measure
Time to reversion of hypertension was the main outcome measure. We defined the reversion date as the day that hypertension medications were stopped. This information was obtained via in-person questioning on the 2nd postpartum day and at the 6-week postnatal visit and via telephonic survey on the 10th postnatal day and at 10 weeks postdelivery. Women who missed the 6-week postnatal visit were also followed up by telephone.
Data Collection
Demographic details (age, parity, BMI) as well as information regarding gestational age at onset of hypertension, severity, highest systolic and diastolic blood pressure recordings, treatment received, complications related to hypertension, pregnancy termination and delivery was obtained from the medical charts and/or via telephonic follow-up.
Analysis
We used Pearson’s chi-square test to assess the association between recovery trends in blood pressure and the patient’s demographic profile and details of pregnancy hypertension. Statistical analysis was done using SPSS16.
Results
In our study, earlier the gestational age at onset of hypertension and earlier gestation at delivery was associated with slower recovery from hypertension (Table 2). Time taken for recovery also was associated with age, BMI, severity of hypertension, associated complications, and the number of antihypertensive medications received (Table 2). Among women who received more than 3 antiphypertensives in pregnancy, nearly 50% continued to have hypertension beyond 6 weeks (Table 2).
On testing for strength of correlation, it was found that body mass index and time to blood pressure normalization had a strong positive correlation (r = 0.8). The remaining parameters (ie, gestational age at onset, gestational age at delivery, severity and complications of hypertension and number of antihypertensive medications) and time to recovery were weakly correlated (r = 0.3 to 0.5 [+/–]).
Women with gestational hypertension and mild preeclampsia had faster normalization of blood pressure compared to those with severe preeclampsia and eclampsia (Figure 2). Only 15% of women with gestational hypertension had persistent hypertension beyond 6 weeks, whereas in the groups with nonsevere preeclampsia, severe preeclampsia, and eclampsia, blood pressure
Eighteen women had additional medical problems: gestational diabetes (n = 5), anemia (n = 3), hypothyroidism (n = 4), rheumatic heart disease (n = 2), antiphospholipid antibody syndrome (n = 1) chronic kidney disease (n = 1), post atrial septal defect closure (n = 1), and tricuspid valve prolapse (TVP) with regurgitation and pulmonary arterial hypertension (n = 1). With the exception of the woman with chronic kidney disease, all reverted to normal blood pressure by 6 weeks; the woman with TVP reverted after corrective cardiac surgery in puerperium.
Discussion
In the present study we assessed possible correlations of obstetric profile with time to postpartum recovery of blood pressure in women with pregnancy hypertension. Women with advanced age, higher body mass index, early gestational age at the onset of hypertension, early gestational age at delivery, severe hypertension, and those with complications of hypertension took longer time in the postnatal period for normalization of blood pressure.
The strength of this study was its prospective design and high rate of follow-up. Those who missed a visit were followed up over telephone. However, 19 women were not available even by phone. A limitation of this study is that the information regarding when the antihypertensive was stopped was obtained by patient recall, raising the possibility of recall bias. However, as the range of recovery times was wide, an error of few days may not be significant.
In the study we noted that women with preeclampsia took a longer time to recovery compared with women with gestational hypertension. Earlier and more severe disease was associated with delay to recovery or persistence of hypertension beyond 10 weeks postpartum.
Similar to our observation, other authors have observed a consistent association of time to reversion of hypertension and early-onset hypertension in pregnancy [3–5]. Ferrazzani explained the longer time to normalization of blood pressure in preeclampsia compared to gestational hypertension as the recovery time of the endothelial damage in preeclampsia [4].
Berks et al [6] found a correlation of maximum diastolic blood pressure, maximum proteinuria in pregnancy, and diagnosis-to-delivery interval with time taken for resolution of hypertension; however, they did not find that time to resolution was correlated with gestational age at onset of preeclampsia. They opined that their observations reflected endothelial recovery after preeclampsia. They also suggested further research in the area of temporizing management of preeclampsia to determine if a conservative approach increases remote cardiovascular risk [6]. We did not study the diagnosis-to-delivery interval, but those with early delivery in our group had late postpartum recovery, indicating that they had severe/complicated preeclampsia that demanded early termination.
In conclusion, women with advanced age, higher body mass index, early gestational age at the onset of hypertension, severe and with complications of hypertension require prolonged monitoring and treatment when indicated for hypertension in the postnatal period. Women with a history of pregnancy hypertension have increased risk of stroke, cardiac ischemia, venous thrombosis within 10 to 20 years after pregnancy and higher risk of hypertension and type 2 diabetes mellitus [7–9]. Extended postnatal follow-up and regular monitoring is recommended to address the needs of these high-risk women.
Corresponding author: Dr. Shyamala Guruvare, 1-167 (C4), Lahari, Eshakripa Road, Parkala, Udupi District, Karnataka, India 576107, [email protected].
Financial disclosures: None reported.
1. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31.
2. Ndayambagye EB, Nakalembe M, Kaye DK. Factors associated with persistent hypertension after puerperium among women with preeclampsia/ eclampsia in Mulago Hospital, Uganda. BMC Pregnancy Childbirth 2010;10:12.
3. Mikami Y,Takagi K, Itaya Y, et al. Post-partum recovery course in patients with gestational hypertension and preeclampsia. J Obstet Gynaecol Res 2014;40:919–25.
4. Ferrazzini S, Carolis SD, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: Relationship with renal impairment and week of delivery. Am J Obstet Gynecol 1994;171:506–12.
5. Kaze FF, Njukeng FA , Kengne A, et al. Post-partum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in Sub-Saharan Africa: a 6-months cohort study.BMC Pregnancy Childbirth 2014;14:134
6. Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 2009;114:1307–14.
7. Gongora MC, Wenger NK. Cardiovascular complications of pregnancy. Int J Mol Sci 2015;16:23905–28.
8. Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep 2013;114–21.
9. Zandstra M, Stekkinger E, van der Vlugt MJ, et al. Cardiac diastolic dysfunction and metabolic syndrome in young women after placental syndrome. Obstet Gynecol 2010;115:101–8.
From the Department of Obstetrics and Gynecology, Kasturba Medical College, Manipal, Karnataka, India.
Abstracts
- Objective: To examine the association of the patient’s obstetric profile and time to normalization of blood pressure in the postnatal period among women with hypertensive disorders in pregnancy.
- Methods: We conducted a prospective cohort study at a tertiary level hospital between November 2014 and May 2015. Women with pregnancy hypertension who required antihypertensive treatment were recruited after delivery. The normalization trends in blood pressure were tested for associations with patient demographic data and details of pregnancy hypertension.
- Results: Among 109 women included in the study, earlier gestational age at onset of hypertension and earlier gestational age at delivery was correlated with slower resolution of hypertension. Time to resolution also was correlated with age, BMI, severity of hypertension, associated complications, and the number of antihypertensive medications received. There was no correlation with highest recorded systolic or diastolic blood pressures. Only 15% of women with gestational hypertension had persistent hypertension beyond 6 weeks. In the groups with nonsevere preeclampsia, severe preeclampsia, and eclampsia, blood pressure remained high after 6 weeks in 26%, 14%, and 50% of women, respectively.
- Conclusion: Women with advanced age, higher body mass index, early gestational age at the onset of hypertension, severe hypertension and who had complications of hypertension require prolonged monitoring and treatment when indicated for hypertension in postnatal period.
Key words: intensive care unit; communication; family meeting; critical illness; decision making; end of life care.
Hypertension is the most common medical problem encountered during pregnancy, complicating up to 10% of pregnancies worldwide [1]. The disorders of hypertension in pregnancy are generally classified as chronic hypertension, preeclampsia–eclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension. The hypertensive disorders of pregnancy are a leading cause of mortality and morbidity in the perinatal period.
Women with hypertensive disorders in pregnancy show varying trends of blood pressure normalization, with the recovery period ranging from a few hours to several months after delivery. In one study, nearly one-fourth of women with preeclampsia/eclampsia had persistent high blood pressure after puerperium [2]. Identifying the obstetric risk factors for persistent hypertension will help in focusing care and research in this group of patients.
We undertook a prospective study to assess possible correlations of obstetric profile with time to normalization of blood pressure in the postnatal period among women with hypertensive disorders in pregnancy.
Methods
Setting
This prospective cohort study was conducted in the department of obstetrics and gynecology at Kasturba Hospital, Manipal, between November 2014 and May 2015. Permission for the study was obtained from the Institution Ethical Committee (IEC264/2015).
Patients
Women who had hypertension in pregnancy and required antihypertensive treatment were approached on the first postnatal day and invited to participate in the study. Women with chronic hypertension (women with known pre-pregnancy hypertension and with hypertension diagnosed before 20 weeks gestation) or secondary hypertension were excluded. After granting informed consent, enrolled women were followed until the time they no longer required antihypertensive medication (“reversion of hypertension”) or until 10 weeks postpartum, whichever came first.
During the hospital stay in the postnatal period, women had their blood pressure monitored and antihypertensives were adjusted as needed. After discharge from the hospital, blood pressure was monitored by the family physician who also made decisions regarding antihypertensive management. All women had a follow-up visit in the hospital in the 6th postnatal week as per the postnatal clinic protocol.
Definitions
Hypertension was defined as BP ≥ 140/90 mm Hg. The hypertension disorders of pregnancy were defined as follows:
- Gestational hypertension: hypertension after 20 weeks gestation on two occasions 4 hours apart without meeting criteria for preeclampsia.
- Preeclampsia: hypertension after 20 weeks gestation on two occasions 4 hours apart with proteinuria (≥ 300 mg/24 hour) or, in the absence of proteinuria, new onset of any of the following: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms [1]. Severe preeclampsia was defined as preeclampsia with any of the following: systolic blood pressure > 160 mm Hg diastolic BP > 110 mm Hg or more on 2 occasions 4 hours apart, thrombocytopenia (platelet count < 100,00/mL), renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms. Preeclampsia without any of these features was considered nonsevere preeclampsia.
- Eclampsia: Women with hypertension with epigastric pain, headache, vomiting, and blurring of vision were diagnosed with imminent eclampsia and those with hypertension-related convulsions were diagnosed with eclampsia.
- Complications of preeclampsia included eclampsia, placental abruption, pulmonary edema, thrombocytopenia, HELLP syndrome, disseminated intravascular coagulation, multiorgan failure, severe intrauterine growth restriction, and fetal demise.
Main Outcome Measure
Time to reversion of hypertension was the main outcome measure. We defined the reversion date as the day that hypertension medications were stopped. This information was obtained via in-person questioning on the 2nd postpartum day and at the 6-week postnatal visit and via telephonic survey on the 10th postnatal day and at 10 weeks postdelivery. Women who missed the 6-week postnatal visit were also followed up by telephone.
Data Collection
Demographic details (age, parity, BMI) as well as information regarding gestational age at onset of hypertension, severity, highest systolic and diastolic blood pressure recordings, treatment received, complications related to hypertension, pregnancy termination and delivery was obtained from the medical charts and/or via telephonic follow-up.
Analysis
We used Pearson’s chi-square test to assess the association between recovery trends in blood pressure and the patient’s demographic profile and details of pregnancy hypertension. Statistical analysis was done using SPSS16.
Results
In our study, earlier the gestational age at onset of hypertension and earlier gestation at delivery was associated with slower recovery from hypertension (Table 2). Time taken for recovery also was associated with age, BMI, severity of hypertension, associated complications, and the number of antihypertensive medications received (Table 2). Among women who received more than 3 antiphypertensives in pregnancy, nearly 50% continued to have hypertension beyond 6 weeks (Table 2).
On testing for strength of correlation, it was found that body mass index and time to blood pressure normalization had a strong positive correlation (r = 0.8). The remaining parameters (ie, gestational age at onset, gestational age at delivery, severity and complications of hypertension and number of antihypertensive medications) and time to recovery were weakly correlated (r = 0.3 to 0.5 [+/–]).
Women with gestational hypertension and mild preeclampsia had faster normalization of blood pressure compared to those with severe preeclampsia and eclampsia (Figure 2). Only 15% of women with gestational hypertension had persistent hypertension beyond 6 weeks, whereas in the groups with nonsevere preeclampsia, severe preeclampsia, and eclampsia, blood pressure
Eighteen women had additional medical problems: gestational diabetes (n = 5), anemia (n = 3), hypothyroidism (n = 4), rheumatic heart disease (n = 2), antiphospholipid antibody syndrome (n = 1) chronic kidney disease (n = 1), post atrial septal defect closure (n = 1), and tricuspid valve prolapse (TVP) with regurgitation and pulmonary arterial hypertension (n = 1). With the exception of the woman with chronic kidney disease, all reverted to normal blood pressure by 6 weeks; the woman with TVP reverted after corrective cardiac surgery in puerperium.
Discussion
In the present study we assessed possible correlations of obstetric profile with time to postpartum recovery of blood pressure in women with pregnancy hypertension. Women with advanced age, higher body mass index, early gestational age at the onset of hypertension, early gestational age at delivery, severe hypertension, and those with complications of hypertension took longer time in the postnatal period for normalization of blood pressure.
The strength of this study was its prospective design and high rate of follow-up. Those who missed a visit were followed up over telephone. However, 19 women were not available even by phone. A limitation of this study is that the information regarding when the antihypertensive was stopped was obtained by patient recall, raising the possibility of recall bias. However, as the range of recovery times was wide, an error of few days may not be significant.
In the study we noted that women with preeclampsia took a longer time to recovery compared with women with gestational hypertension. Earlier and more severe disease was associated with delay to recovery or persistence of hypertension beyond 10 weeks postpartum.
Similar to our observation, other authors have observed a consistent association of time to reversion of hypertension and early-onset hypertension in pregnancy [3–5]. Ferrazzani explained the longer time to normalization of blood pressure in preeclampsia compared to gestational hypertension as the recovery time of the endothelial damage in preeclampsia [4].
Berks et al [6] found a correlation of maximum diastolic blood pressure, maximum proteinuria in pregnancy, and diagnosis-to-delivery interval with time taken for resolution of hypertension; however, they did not find that time to resolution was correlated with gestational age at onset of preeclampsia. They opined that their observations reflected endothelial recovery after preeclampsia. They also suggested further research in the area of temporizing management of preeclampsia to determine if a conservative approach increases remote cardiovascular risk [6]. We did not study the diagnosis-to-delivery interval, but those with early delivery in our group had late postpartum recovery, indicating that they had severe/complicated preeclampsia that demanded early termination.
In conclusion, women with advanced age, higher body mass index, early gestational age at the onset of hypertension, severe and with complications of hypertension require prolonged monitoring and treatment when indicated for hypertension in the postnatal period. Women with a history of pregnancy hypertension have increased risk of stroke, cardiac ischemia, venous thrombosis within 10 to 20 years after pregnancy and higher risk of hypertension and type 2 diabetes mellitus [7–9]. Extended postnatal follow-up and regular monitoring is recommended to address the needs of these high-risk women.
Corresponding author: Dr. Shyamala Guruvare, 1-167 (C4), Lahari, Eshakripa Road, Parkala, Udupi District, Karnataka, India 576107, [email protected].
Financial disclosures: None reported.
From the Department of Obstetrics and Gynecology, Kasturba Medical College, Manipal, Karnataka, India.
Abstracts
- Objective: To examine the association of the patient’s obstetric profile and time to normalization of blood pressure in the postnatal period among women with hypertensive disorders in pregnancy.
- Methods: We conducted a prospective cohort study at a tertiary level hospital between November 2014 and May 2015. Women with pregnancy hypertension who required antihypertensive treatment were recruited after delivery. The normalization trends in blood pressure were tested for associations with patient demographic data and details of pregnancy hypertension.
- Results: Among 109 women included in the study, earlier gestational age at onset of hypertension and earlier gestational age at delivery was correlated with slower resolution of hypertension. Time to resolution also was correlated with age, BMI, severity of hypertension, associated complications, and the number of antihypertensive medications received. There was no correlation with highest recorded systolic or diastolic blood pressures. Only 15% of women with gestational hypertension had persistent hypertension beyond 6 weeks. In the groups with nonsevere preeclampsia, severe preeclampsia, and eclampsia, blood pressure remained high after 6 weeks in 26%, 14%, and 50% of women, respectively.
- Conclusion: Women with advanced age, higher body mass index, early gestational age at the onset of hypertension, severe hypertension and who had complications of hypertension require prolonged monitoring and treatment when indicated for hypertension in postnatal period.
Key words: intensive care unit; communication; family meeting; critical illness; decision making; end of life care.
Hypertension is the most common medical problem encountered during pregnancy, complicating up to 10% of pregnancies worldwide [1]. The disorders of hypertension in pregnancy are generally classified as chronic hypertension, preeclampsia–eclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension. The hypertensive disorders of pregnancy are a leading cause of mortality and morbidity in the perinatal period.
Women with hypertensive disorders in pregnancy show varying trends of blood pressure normalization, with the recovery period ranging from a few hours to several months after delivery. In one study, nearly one-fourth of women with preeclampsia/eclampsia had persistent high blood pressure after puerperium [2]. Identifying the obstetric risk factors for persistent hypertension will help in focusing care and research in this group of patients.
We undertook a prospective study to assess possible correlations of obstetric profile with time to normalization of blood pressure in the postnatal period among women with hypertensive disorders in pregnancy.
Methods
Setting
This prospective cohort study was conducted in the department of obstetrics and gynecology at Kasturba Hospital, Manipal, between November 2014 and May 2015. Permission for the study was obtained from the Institution Ethical Committee (IEC264/2015).
Patients
Women who had hypertension in pregnancy and required antihypertensive treatment were approached on the first postnatal day and invited to participate in the study. Women with chronic hypertension (women with known pre-pregnancy hypertension and with hypertension diagnosed before 20 weeks gestation) or secondary hypertension were excluded. After granting informed consent, enrolled women were followed until the time they no longer required antihypertensive medication (“reversion of hypertension”) or until 10 weeks postpartum, whichever came first.
During the hospital stay in the postnatal period, women had their blood pressure monitored and antihypertensives were adjusted as needed. After discharge from the hospital, blood pressure was monitored by the family physician who also made decisions regarding antihypertensive management. All women had a follow-up visit in the hospital in the 6th postnatal week as per the postnatal clinic protocol.
Definitions
Hypertension was defined as BP ≥ 140/90 mm Hg. The hypertension disorders of pregnancy were defined as follows:
- Gestational hypertension: hypertension after 20 weeks gestation on two occasions 4 hours apart without meeting criteria for preeclampsia.
- Preeclampsia: hypertension after 20 weeks gestation on two occasions 4 hours apart with proteinuria (≥ 300 mg/24 hour) or, in the absence of proteinuria, new onset of any of the following: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms [1]. Severe preeclampsia was defined as preeclampsia with any of the following: systolic blood pressure > 160 mm Hg diastolic BP > 110 mm Hg or more on 2 occasions 4 hours apart, thrombocytopenia (platelet count < 100,00/mL), renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms. Preeclampsia without any of these features was considered nonsevere preeclampsia.
- Eclampsia: Women with hypertension with epigastric pain, headache, vomiting, and blurring of vision were diagnosed with imminent eclampsia and those with hypertension-related convulsions were diagnosed with eclampsia.
- Complications of preeclampsia included eclampsia, placental abruption, pulmonary edema, thrombocytopenia, HELLP syndrome, disseminated intravascular coagulation, multiorgan failure, severe intrauterine growth restriction, and fetal demise.
Main Outcome Measure
Time to reversion of hypertension was the main outcome measure. We defined the reversion date as the day that hypertension medications were stopped. This information was obtained via in-person questioning on the 2nd postpartum day and at the 6-week postnatal visit and via telephonic survey on the 10th postnatal day and at 10 weeks postdelivery. Women who missed the 6-week postnatal visit were also followed up by telephone.
Data Collection
Demographic details (age, parity, BMI) as well as information regarding gestational age at onset of hypertension, severity, highest systolic and diastolic blood pressure recordings, treatment received, complications related to hypertension, pregnancy termination and delivery was obtained from the medical charts and/or via telephonic follow-up.
Analysis
We used Pearson’s chi-square test to assess the association between recovery trends in blood pressure and the patient’s demographic profile and details of pregnancy hypertension. Statistical analysis was done using SPSS16.
Results
In our study, earlier the gestational age at onset of hypertension and earlier gestation at delivery was associated with slower recovery from hypertension (Table 2). Time taken for recovery also was associated with age, BMI, severity of hypertension, associated complications, and the number of antihypertensive medications received (Table 2). Among women who received more than 3 antiphypertensives in pregnancy, nearly 50% continued to have hypertension beyond 6 weeks (Table 2).
On testing for strength of correlation, it was found that body mass index and time to blood pressure normalization had a strong positive correlation (r = 0.8). The remaining parameters (ie, gestational age at onset, gestational age at delivery, severity and complications of hypertension and number of antihypertensive medications) and time to recovery were weakly correlated (r = 0.3 to 0.5 [+/–]).
Women with gestational hypertension and mild preeclampsia had faster normalization of blood pressure compared to those with severe preeclampsia and eclampsia (Figure 2). Only 15% of women with gestational hypertension had persistent hypertension beyond 6 weeks, whereas in the groups with nonsevere preeclampsia, severe preeclampsia, and eclampsia, blood pressure
Eighteen women had additional medical problems: gestational diabetes (n = 5), anemia (n = 3), hypothyroidism (n = 4), rheumatic heart disease (n = 2), antiphospholipid antibody syndrome (n = 1) chronic kidney disease (n = 1), post atrial septal defect closure (n = 1), and tricuspid valve prolapse (TVP) with regurgitation and pulmonary arterial hypertension (n = 1). With the exception of the woman with chronic kidney disease, all reverted to normal blood pressure by 6 weeks; the woman with TVP reverted after corrective cardiac surgery in puerperium.
Discussion
In the present study we assessed possible correlations of obstetric profile with time to postpartum recovery of blood pressure in women with pregnancy hypertension. Women with advanced age, higher body mass index, early gestational age at the onset of hypertension, early gestational age at delivery, severe hypertension, and those with complications of hypertension took longer time in the postnatal period for normalization of blood pressure.
The strength of this study was its prospective design and high rate of follow-up. Those who missed a visit were followed up over telephone. However, 19 women were not available even by phone. A limitation of this study is that the information regarding when the antihypertensive was stopped was obtained by patient recall, raising the possibility of recall bias. However, as the range of recovery times was wide, an error of few days may not be significant.
In the study we noted that women with preeclampsia took a longer time to recovery compared with women with gestational hypertension. Earlier and more severe disease was associated with delay to recovery or persistence of hypertension beyond 10 weeks postpartum.
Similar to our observation, other authors have observed a consistent association of time to reversion of hypertension and early-onset hypertension in pregnancy [3–5]. Ferrazzani explained the longer time to normalization of blood pressure in preeclampsia compared to gestational hypertension as the recovery time of the endothelial damage in preeclampsia [4].
Berks et al [6] found a correlation of maximum diastolic blood pressure, maximum proteinuria in pregnancy, and diagnosis-to-delivery interval with time taken for resolution of hypertension; however, they did not find that time to resolution was correlated with gestational age at onset of preeclampsia. They opined that their observations reflected endothelial recovery after preeclampsia. They also suggested further research in the area of temporizing management of preeclampsia to determine if a conservative approach increases remote cardiovascular risk [6]. We did not study the diagnosis-to-delivery interval, but those with early delivery in our group had late postpartum recovery, indicating that they had severe/complicated preeclampsia that demanded early termination.
In conclusion, women with advanced age, higher body mass index, early gestational age at the onset of hypertension, severe and with complications of hypertension require prolonged monitoring and treatment when indicated for hypertension in the postnatal period. Women with a history of pregnancy hypertension have increased risk of stroke, cardiac ischemia, venous thrombosis within 10 to 20 years after pregnancy and higher risk of hypertension and type 2 diabetes mellitus [7–9]. Extended postnatal follow-up and regular monitoring is recommended to address the needs of these high-risk women.
Corresponding author: Dr. Shyamala Guruvare, 1-167 (C4), Lahari, Eshakripa Road, Parkala, Udupi District, Karnataka, India 576107, [email protected].
Financial disclosures: None reported.
1. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31.
2. Ndayambagye EB, Nakalembe M, Kaye DK. Factors associated with persistent hypertension after puerperium among women with preeclampsia/ eclampsia in Mulago Hospital, Uganda. BMC Pregnancy Childbirth 2010;10:12.
3. Mikami Y,Takagi K, Itaya Y, et al. Post-partum recovery course in patients with gestational hypertension and preeclampsia. J Obstet Gynaecol Res 2014;40:919–25.
4. Ferrazzini S, Carolis SD, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: Relationship with renal impairment and week of delivery. Am J Obstet Gynecol 1994;171:506–12.
5. Kaze FF, Njukeng FA , Kengne A, et al. Post-partum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in Sub-Saharan Africa: a 6-months cohort study.BMC Pregnancy Childbirth 2014;14:134
6. Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 2009;114:1307–14.
7. Gongora MC, Wenger NK. Cardiovascular complications of pregnancy. Int J Mol Sci 2015;16:23905–28.
8. Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep 2013;114–21.
9. Zandstra M, Stekkinger E, van der Vlugt MJ, et al. Cardiac diastolic dysfunction and metabolic syndrome in young women after placental syndrome. Obstet Gynecol 2010;115:101–8.
1. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31.
2. Ndayambagye EB, Nakalembe M, Kaye DK. Factors associated with persistent hypertension after puerperium among women with preeclampsia/ eclampsia in Mulago Hospital, Uganda. BMC Pregnancy Childbirth 2010;10:12.
3. Mikami Y,Takagi K, Itaya Y, et al. Post-partum recovery course in patients with gestational hypertension and preeclampsia. J Obstet Gynaecol Res 2014;40:919–25.
4. Ferrazzini S, Carolis SD, Pomini F, et al. The duration of hypertension in the puerperium of preeclamptic women: Relationship with renal impairment and week of delivery. Am J Obstet Gynecol 1994;171:506–12.
5. Kaze FF, Njukeng FA , Kengne A, et al. Post-partum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in Sub-Saharan Africa: a 6-months cohort study.BMC Pregnancy Childbirth 2014;14:134
6. Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 2009;114:1307–14.
7. Gongora MC, Wenger NK. Cardiovascular complications of pregnancy. Int J Mol Sci 2015;16:23905–28.
8. Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep 2013;114–21.
9. Zandstra M, Stekkinger E, van der Vlugt MJ, et al. Cardiac diastolic dysfunction and metabolic syndrome in young women after placental syndrome. Obstet Gynecol 2010;115:101–8.
Are There Racial/Ethnic Differences in Weight-Related Care Encounters Reported by Patients?
Study Overview
Objective. To compare patients’ health care experiences related to their weight across racial and ethnic groups.
Design. Cross-sectional survey-based study.
Setting and participants. Between March and July 2015, 5400 individuals were randomly sampled from the Patient Outcomes to Advance Learning (PORTAL) obesity cohort, which includes over 5 million adults. The PORTAL network is a clinical data research network funded by the Patient Centered Outcomes Research Institute to promote collaboration across several large health systems with electronic medical records (EMRs), including all the Kaiser Permanente regions, Group Health Cooperative, Health Partners, and Denver Health. The selected 5400 cohort members were equally distributed across 3 geographically diverse Kaiser Permanente regions (Southwest, Northern and Southern California, Hawaii, Colorado, and Northwest) and Denver sites. Selected individuals were non-pregnant English or Spanish speakers with a body mass index (BMI) ≥ 25 kg/m2 (per their EMR) who were members of a participating health plan and had at least 1 outpatient visit in the last 12 months. Patients with BMI ≥ 40 kg/m2 were oversampled. Individuals were mailed a written 10-minute survey (offered in English or Spanish based on a patient’s written language preference noted in their EMR), consisting of 36 multiple-choice and fill-in-the-blank items. Telephone contact for verbal administration was attempted if a mailed response was not received within 4 weeks.
Main measures and analysis. The primary independent variable was a respondent’s racial/ethnic group, categorized as (1) non-Hispanic white (White), 2) non-Hispanic black (Black), 3) Hispanic, 4) Asian, or 5) Native Hawaiian/Other Pacific Islanders/American Indian/Native Alaskan (NA/PI).
Dependent variables focused on patients’ perceptions of the health care experience (based on services received at their usual place of care from their primary care providers) related to being overweight or obese using items based on the Rudd Center’s Patient Survey of Weight-Sensitive Healthcare Practices. Respondents described (1) whether and how often they avoid coming to their provider because they do not want to be weighed or have a discussion about their weight; (2) how often does their provider ask their permission before discussion their weight; (3) how often has their provider been supportive of their weight concerns and efforts to be healthy; (4) whether they think that their provider understands the physical and emotional challenges faced by individuals who are overweight or obese; (5) how often has their provider brought up their weight during a clinic visit; (6) whether their provider has ever given or discussed resources on healthy eating and weight loss; and (7) what types of weight loss resources were discussed with their provider and which types did they want more information about (ie, dietary changes, physical activity, classes, medications, meal replacements, and bariatric surgery). Covariate variables derived from EMR data included sex, age category, diabetes, hypertension, Charlson Index score (overall measures of morbidity), Medicaid enrollment, language preferences, site, and BMI. Survey-derived covariate variables included emotional well-being, perceived weight status, and educational attainment.
Descriptive statistics were generated and compared across racial/ethnic groups using Kruskal-Wallis and chi-square testing, as appropriate. To evaluate the association between a patient’s race/ethnicity and their perceived weight management experience, multinomial logistic regression adjusted for covariates was used to estimate odds ratios (OR).
Main results. From the original sample (n = 5400), 1569 individuals (29%) did not respond, 925 (17%) refused, and 114 (2%) were ineligible, leaving an eligible sample pool of 5286 individuals. The overall response rate was 53% (2197 written; 614 phone, n = 2811). Those with missing data were excluded (6 with missing race/ethnicity; 80 missing other covariates), leaving a final group of 2725 respondents for analysis. Mean age was 52.7 years (SD 15), almost 62% of participants were female, 51.7% identified as White, 21.1% identified as Black, 14.6% identified as Hispanic, 5.8% identified as Asian, and 6.7% identified as NA/PI. About a quarter (24.4%) had diabetes, less than half (43.5%) had hypertension, and most (86.2%) perceived themselves to be overweight. There were significant differences in measured baseline covariates by racial/ethnic groups including mean BMI, diabetes, and being a Medicaid beneficiary.
In response to the 7 key areas assessed regarding patients’ perceptions of the health care experience related to being overweight or obese:
- Black respondents were less likely than Whites to report that they frequently avoided care from their provider because they did not want to be weighed or discuss their weight (OR 0.49 [95% confidence interval, 0.26–0.90]), with a trend toward all groups being less likely to report frequent avoidance compared to Whites.
- While just over half of respondents (59.3%) indicated that their providers never asked for their permission before discussing their weight, Asians and NA/PI were more likely to report that their providers either frequently (Asians: OR 2.7 [1.3–5.6]; NA/PI: OR 2.3 [1.1–5.0]) or sometimes (Asians: OR 2.3 [1.2–4.3]; NA/PI: OR 2.1 [1.1–4.1]) asked their permission before discussing their weight compared to Whites.
- Over half (61.9%) indicated that their providers were sometimes or frequently supportive of their weight concerns, with no significant differences among racial/ethnic groups.
- Just over half (52.0%) indicated they felt their providers understood the physical and emotional challenges faced by people who are overweight/obese, with Blacks more likely to feel this way (OR 1.8 [1.2–2.8]) compared to Whites.
- Black patients were more likely than Whites (OR 2.0 [1.4–2.8]) to report that their providers discussed their weight with them at a clinic visit.
- While over half (59.7%) indicated that their providers had given or discussed resources with them on healthy eating and weight loss, Black and Asian respondents were more likely than Whites to recall these discussions (Black: OR 1.6 [1.2–2.1]; Asians: OR 1.8 [1.1–2.9]).
- Most weight loss resources or recommendations received were related to lifestyle changes, with very few resources given related to weight loss medications, meal replacement products, or bariatric surgery—few differences across racial/ethnic groups were identified. However, respondents from racial/ethnic minority groups were more likely than Whites to say that they wanted more information about lifestyle changes, classes, and meal replacements. Other than Blacks, all other racial/ethnic groups were also more likely than Whites to indicate that they wanted more information about bariatric surgery.
Conclusions. Most patients across racial/ethnic groups are having positive experiences with weight-related care. However, race/ethnicity correlates with patients’ perception of weight-related care and discussions in clinic encounters.
Commentary
The obesity epidemic in the United States is well-established [1], and recent data from 2014 show that over 37% of adults in the US are obese (defined as having a body mass index greater than 30 kg/m2) [2]. However, while obesity prevalence rates have increased over the past several decades across all genders, ethnicities, income levels, and education levels, important racial/ethnic disparities exist [2,3]. Primary care physicians (PCPs) are ideally situated to promote weight loss via effective obesity counseling since multiple clinic visits over time have the potential to enable rapport building and behavioral change management [4]. In fact, the US Preventive Services Task Force (USPTF) recommends that all patients be screened for obesity and offered intensive lifestyle counseling, as modest weight loss can have significant health benefits [5]. However, some studies have found racial/ethnic differences and disparities in weight-related diagnoses, counseling, and treatment by providers, but also patient perceptions of care and preferred interventions [6–10]. Other studies have described racial/ethnic differences in weight-related concerns and behaviors, body satisfaction, and body image [11–13]. Thus, research is needed to examine these differences.
This cross-sectional study contributes to the limited literature examining the potential for heterogeneity of care according to patient characteristics like race and ethnicity. Key strengths of the design include a large and both geographically and racially/ethnically diverse sample of patients (increased generalizability), the use of mailed brief surveys (reduces non-response rate and reporting bias) and telephone follow-up for verbal administration (reduces non-response rate, though it increases interviewer bias), oversampling of respondents with BMI ≥ 40 kg/m2, and the controlling of key covariates including sex, age, Medicaid enrollment, site, and BMI.
However, there are several important limitations, many of which are acknowledged by the authors. While respondents were overall representative of the targeted sample population, the final respondent population was comprised of mostly older females who received managed care, which may have contributed to selection bias and impacted generalizability of findings. Further, Whites were overrepresented, Hispanics were underrepresented, and the small combined sample of NA/PI may have masked important distinctions between these subpopulations. Importantly, this study only provided the survey in English and Spanish and did not include other language translations (eg, Chinese, Japanese, Tagalog), which likely contributed to underrepresented perspectives of immigrants and ESL patients who may struggle with receiving/discussing weight management counseling and resources. The use of a surveys collected subjective and self-reported data on patient encounters as opposed to objective observations. Lastly, the study did not adjust for individual provider factors or assess the potential impact of provider-level differences on care, such as provider-patient concordance on race, ethnicity, language, and/or weight. The incorporation of qualitative interviewers or focus groups with a subsample of each racial/ethnic may have also provided relevant context to understand differences in weight-related care experiences.
Applications for Clinical Practice
As the authors suggest, this study highlights several opportunities to continue improving weight-related care and weight management counseling. PCPs should engage all overweight/obese patients in weight management discussions, and in particular, high-risk minority patients who may desire these conversations and more weight loss advice and resources. However, these discussions require sensitivity and can benefit from the simple practice of asking permission of the patient to talk about their weight in order to reduce care avoidance and improve perceptions of care. Providers should also be mindful of patient priorities and assess patient preferences for all the different weight loss strategies, including lifestyle changes, meal replacements, medications, and surgery.
—Katrina F. Mateo, MPH
1. Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717–32.
2. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284.
3. Wong RJ, Chou C, Ahmed A. Long term trends and racial/ethnic disparities in the prevalence of obesity. J Community Health 2014;39:1150–60.
4. Schlair S, Moore S, Mcmacken M, Jay M. How to deliver high quality obesity counseling using the 5As framework. J Clin Outcomes Manag 2012;19:221–9.
5. Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373–8.
6. Davis NJ, Wildman RP, Forbes BF, Schechter CB. Trends and disparities in provider diagnosis of overweight analysis of NHANES 1999–2004. Obesity 2009;17:2110–3.
7. Wee CC, Huskey KW, Bolcic-Jankovic D, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
8. Johnson RL, Saha S, Arbelaez JJ, et al. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med 2004;19:101–10.
9. Chugh M, Friedman AM, Clemow LP, Ferrante JM. Women weigh in: obese african american and white women’s perspectives on physicians’ roles in weight management. J Am Board Fam Med 2013;26:421–8.
10. Blixen CE, Singh A, Xu M, et al. What women want: understanding obesity and preferences for primary care weight reduction interventions among African-American and Caucasian women. J Natl Med Assoc 2006;98:1160–70.
11. Arcan C, Larson N, Bauer K, et al. Dietary and weight-related behaviors and body mass index among Hispanic, Hmong, Somali, and White adolescents. J Acad Nutr Diet 2014;114:375–83.
12. Kronenfeld LW, Reba-Harrelson L, Von Holle A, et al. Ethnic and racial differences in body size perception and satisfaction. Body Image 2010;7:131–6.
13. Gluck ME, Geliebter A. Racial/ethnic differences in body image and eating behaviors. Eat Behav 2002;3:143–51.
Study Overview
Objective. To compare patients’ health care experiences related to their weight across racial and ethnic groups.
Design. Cross-sectional survey-based study.
Setting and participants. Between March and July 2015, 5400 individuals were randomly sampled from the Patient Outcomes to Advance Learning (PORTAL) obesity cohort, which includes over 5 million adults. The PORTAL network is a clinical data research network funded by the Patient Centered Outcomes Research Institute to promote collaboration across several large health systems with electronic medical records (EMRs), including all the Kaiser Permanente regions, Group Health Cooperative, Health Partners, and Denver Health. The selected 5400 cohort members were equally distributed across 3 geographically diverse Kaiser Permanente regions (Southwest, Northern and Southern California, Hawaii, Colorado, and Northwest) and Denver sites. Selected individuals were non-pregnant English or Spanish speakers with a body mass index (BMI) ≥ 25 kg/m2 (per their EMR) who were members of a participating health plan and had at least 1 outpatient visit in the last 12 months. Patients with BMI ≥ 40 kg/m2 were oversampled. Individuals were mailed a written 10-minute survey (offered in English or Spanish based on a patient’s written language preference noted in their EMR), consisting of 36 multiple-choice and fill-in-the-blank items. Telephone contact for verbal administration was attempted if a mailed response was not received within 4 weeks.
Main measures and analysis. The primary independent variable was a respondent’s racial/ethnic group, categorized as (1) non-Hispanic white (White), 2) non-Hispanic black (Black), 3) Hispanic, 4) Asian, or 5) Native Hawaiian/Other Pacific Islanders/American Indian/Native Alaskan (NA/PI).
Dependent variables focused on patients’ perceptions of the health care experience (based on services received at their usual place of care from their primary care providers) related to being overweight or obese using items based on the Rudd Center’s Patient Survey of Weight-Sensitive Healthcare Practices. Respondents described (1) whether and how often they avoid coming to their provider because they do not want to be weighed or have a discussion about their weight; (2) how often does their provider ask their permission before discussion their weight; (3) how often has their provider been supportive of their weight concerns and efforts to be healthy; (4) whether they think that their provider understands the physical and emotional challenges faced by individuals who are overweight or obese; (5) how often has their provider brought up their weight during a clinic visit; (6) whether their provider has ever given or discussed resources on healthy eating and weight loss; and (7) what types of weight loss resources were discussed with their provider and which types did they want more information about (ie, dietary changes, physical activity, classes, medications, meal replacements, and bariatric surgery). Covariate variables derived from EMR data included sex, age category, diabetes, hypertension, Charlson Index score (overall measures of morbidity), Medicaid enrollment, language preferences, site, and BMI. Survey-derived covariate variables included emotional well-being, perceived weight status, and educational attainment.
Descriptive statistics were generated and compared across racial/ethnic groups using Kruskal-Wallis and chi-square testing, as appropriate. To evaluate the association between a patient’s race/ethnicity and their perceived weight management experience, multinomial logistic regression adjusted for covariates was used to estimate odds ratios (OR).
Main results. From the original sample (n = 5400), 1569 individuals (29%) did not respond, 925 (17%) refused, and 114 (2%) were ineligible, leaving an eligible sample pool of 5286 individuals. The overall response rate was 53% (2197 written; 614 phone, n = 2811). Those with missing data were excluded (6 with missing race/ethnicity; 80 missing other covariates), leaving a final group of 2725 respondents for analysis. Mean age was 52.7 years (SD 15), almost 62% of participants were female, 51.7% identified as White, 21.1% identified as Black, 14.6% identified as Hispanic, 5.8% identified as Asian, and 6.7% identified as NA/PI. About a quarter (24.4%) had diabetes, less than half (43.5%) had hypertension, and most (86.2%) perceived themselves to be overweight. There were significant differences in measured baseline covariates by racial/ethnic groups including mean BMI, diabetes, and being a Medicaid beneficiary.
In response to the 7 key areas assessed regarding patients’ perceptions of the health care experience related to being overweight or obese:
- Black respondents were less likely than Whites to report that they frequently avoided care from their provider because they did not want to be weighed or discuss their weight (OR 0.49 [95% confidence interval, 0.26–0.90]), with a trend toward all groups being less likely to report frequent avoidance compared to Whites.
- While just over half of respondents (59.3%) indicated that their providers never asked for their permission before discussing their weight, Asians and NA/PI were more likely to report that their providers either frequently (Asians: OR 2.7 [1.3–5.6]; NA/PI: OR 2.3 [1.1–5.0]) or sometimes (Asians: OR 2.3 [1.2–4.3]; NA/PI: OR 2.1 [1.1–4.1]) asked their permission before discussing their weight compared to Whites.
- Over half (61.9%) indicated that their providers were sometimes or frequently supportive of their weight concerns, with no significant differences among racial/ethnic groups.
- Just over half (52.0%) indicated they felt their providers understood the physical and emotional challenges faced by people who are overweight/obese, with Blacks more likely to feel this way (OR 1.8 [1.2–2.8]) compared to Whites.
- Black patients were more likely than Whites (OR 2.0 [1.4–2.8]) to report that their providers discussed their weight with them at a clinic visit.
- While over half (59.7%) indicated that their providers had given or discussed resources with them on healthy eating and weight loss, Black and Asian respondents were more likely than Whites to recall these discussions (Black: OR 1.6 [1.2–2.1]; Asians: OR 1.8 [1.1–2.9]).
- Most weight loss resources or recommendations received were related to lifestyle changes, with very few resources given related to weight loss medications, meal replacement products, or bariatric surgery—few differences across racial/ethnic groups were identified. However, respondents from racial/ethnic minority groups were more likely than Whites to say that they wanted more information about lifestyle changes, classes, and meal replacements. Other than Blacks, all other racial/ethnic groups were also more likely than Whites to indicate that they wanted more information about bariatric surgery.
Conclusions. Most patients across racial/ethnic groups are having positive experiences with weight-related care. However, race/ethnicity correlates with patients’ perception of weight-related care and discussions in clinic encounters.
Commentary
The obesity epidemic in the United States is well-established [1], and recent data from 2014 show that over 37% of adults in the US are obese (defined as having a body mass index greater than 30 kg/m2) [2]. However, while obesity prevalence rates have increased over the past several decades across all genders, ethnicities, income levels, and education levels, important racial/ethnic disparities exist [2,3]. Primary care physicians (PCPs) are ideally situated to promote weight loss via effective obesity counseling since multiple clinic visits over time have the potential to enable rapport building and behavioral change management [4]. In fact, the US Preventive Services Task Force (USPTF) recommends that all patients be screened for obesity and offered intensive lifestyle counseling, as modest weight loss can have significant health benefits [5]. However, some studies have found racial/ethnic differences and disparities in weight-related diagnoses, counseling, and treatment by providers, but also patient perceptions of care and preferred interventions [6–10]. Other studies have described racial/ethnic differences in weight-related concerns and behaviors, body satisfaction, and body image [11–13]. Thus, research is needed to examine these differences.
This cross-sectional study contributes to the limited literature examining the potential for heterogeneity of care according to patient characteristics like race and ethnicity. Key strengths of the design include a large and both geographically and racially/ethnically diverse sample of patients (increased generalizability), the use of mailed brief surveys (reduces non-response rate and reporting bias) and telephone follow-up for verbal administration (reduces non-response rate, though it increases interviewer bias), oversampling of respondents with BMI ≥ 40 kg/m2, and the controlling of key covariates including sex, age, Medicaid enrollment, site, and BMI.
However, there are several important limitations, many of which are acknowledged by the authors. While respondents were overall representative of the targeted sample population, the final respondent population was comprised of mostly older females who received managed care, which may have contributed to selection bias and impacted generalizability of findings. Further, Whites were overrepresented, Hispanics were underrepresented, and the small combined sample of NA/PI may have masked important distinctions between these subpopulations. Importantly, this study only provided the survey in English and Spanish and did not include other language translations (eg, Chinese, Japanese, Tagalog), which likely contributed to underrepresented perspectives of immigrants and ESL patients who may struggle with receiving/discussing weight management counseling and resources. The use of a surveys collected subjective and self-reported data on patient encounters as opposed to objective observations. Lastly, the study did not adjust for individual provider factors or assess the potential impact of provider-level differences on care, such as provider-patient concordance on race, ethnicity, language, and/or weight. The incorporation of qualitative interviewers or focus groups with a subsample of each racial/ethnic may have also provided relevant context to understand differences in weight-related care experiences.
Applications for Clinical Practice
As the authors suggest, this study highlights several opportunities to continue improving weight-related care and weight management counseling. PCPs should engage all overweight/obese patients in weight management discussions, and in particular, high-risk minority patients who may desire these conversations and more weight loss advice and resources. However, these discussions require sensitivity and can benefit from the simple practice of asking permission of the patient to talk about their weight in order to reduce care avoidance and improve perceptions of care. Providers should also be mindful of patient priorities and assess patient preferences for all the different weight loss strategies, including lifestyle changes, meal replacements, medications, and surgery.
—Katrina F. Mateo, MPH
Study Overview
Objective. To compare patients’ health care experiences related to their weight across racial and ethnic groups.
Design. Cross-sectional survey-based study.
Setting and participants. Between March and July 2015, 5400 individuals were randomly sampled from the Patient Outcomes to Advance Learning (PORTAL) obesity cohort, which includes over 5 million adults. The PORTAL network is a clinical data research network funded by the Patient Centered Outcomes Research Institute to promote collaboration across several large health systems with electronic medical records (EMRs), including all the Kaiser Permanente regions, Group Health Cooperative, Health Partners, and Denver Health. The selected 5400 cohort members were equally distributed across 3 geographically diverse Kaiser Permanente regions (Southwest, Northern and Southern California, Hawaii, Colorado, and Northwest) and Denver sites. Selected individuals were non-pregnant English or Spanish speakers with a body mass index (BMI) ≥ 25 kg/m2 (per their EMR) who were members of a participating health plan and had at least 1 outpatient visit in the last 12 months. Patients with BMI ≥ 40 kg/m2 were oversampled. Individuals were mailed a written 10-minute survey (offered in English or Spanish based on a patient’s written language preference noted in their EMR), consisting of 36 multiple-choice and fill-in-the-blank items. Telephone contact for verbal administration was attempted if a mailed response was not received within 4 weeks.
Main measures and analysis. The primary independent variable was a respondent’s racial/ethnic group, categorized as (1) non-Hispanic white (White), 2) non-Hispanic black (Black), 3) Hispanic, 4) Asian, or 5) Native Hawaiian/Other Pacific Islanders/American Indian/Native Alaskan (NA/PI).
Dependent variables focused on patients’ perceptions of the health care experience (based on services received at their usual place of care from their primary care providers) related to being overweight or obese using items based on the Rudd Center’s Patient Survey of Weight-Sensitive Healthcare Practices. Respondents described (1) whether and how often they avoid coming to their provider because they do not want to be weighed or have a discussion about their weight; (2) how often does their provider ask their permission before discussion their weight; (3) how often has their provider been supportive of their weight concerns and efforts to be healthy; (4) whether they think that their provider understands the physical and emotional challenges faced by individuals who are overweight or obese; (5) how often has their provider brought up their weight during a clinic visit; (6) whether their provider has ever given or discussed resources on healthy eating and weight loss; and (7) what types of weight loss resources were discussed with their provider and which types did they want more information about (ie, dietary changes, physical activity, classes, medications, meal replacements, and bariatric surgery). Covariate variables derived from EMR data included sex, age category, diabetes, hypertension, Charlson Index score (overall measures of morbidity), Medicaid enrollment, language preferences, site, and BMI. Survey-derived covariate variables included emotional well-being, perceived weight status, and educational attainment.
Descriptive statistics were generated and compared across racial/ethnic groups using Kruskal-Wallis and chi-square testing, as appropriate. To evaluate the association between a patient’s race/ethnicity and their perceived weight management experience, multinomial logistic regression adjusted for covariates was used to estimate odds ratios (OR).
Main results. From the original sample (n = 5400), 1569 individuals (29%) did not respond, 925 (17%) refused, and 114 (2%) were ineligible, leaving an eligible sample pool of 5286 individuals. The overall response rate was 53% (2197 written; 614 phone, n = 2811). Those with missing data were excluded (6 with missing race/ethnicity; 80 missing other covariates), leaving a final group of 2725 respondents for analysis. Mean age was 52.7 years (SD 15), almost 62% of participants were female, 51.7% identified as White, 21.1% identified as Black, 14.6% identified as Hispanic, 5.8% identified as Asian, and 6.7% identified as NA/PI. About a quarter (24.4%) had diabetes, less than half (43.5%) had hypertension, and most (86.2%) perceived themselves to be overweight. There were significant differences in measured baseline covariates by racial/ethnic groups including mean BMI, diabetes, and being a Medicaid beneficiary.
In response to the 7 key areas assessed regarding patients’ perceptions of the health care experience related to being overweight or obese:
- Black respondents were less likely than Whites to report that they frequently avoided care from their provider because they did not want to be weighed or discuss their weight (OR 0.49 [95% confidence interval, 0.26–0.90]), with a trend toward all groups being less likely to report frequent avoidance compared to Whites.
- While just over half of respondents (59.3%) indicated that their providers never asked for their permission before discussing their weight, Asians and NA/PI were more likely to report that their providers either frequently (Asians: OR 2.7 [1.3–5.6]; NA/PI: OR 2.3 [1.1–5.0]) or sometimes (Asians: OR 2.3 [1.2–4.3]; NA/PI: OR 2.1 [1.1–4.1]) asked their permission before discussing their weight compared to Whites.
- Over half (61.9%) indicated that their providers were sometimes or frequently supportive of their weight concerns, with no significant differences among racial/ethnic groups.
- Just over half (52.0%) indicated they felt their providers understood the physical and emotional challenges faced by people who are overweight/obese, with Blacks more likely to feel this way (OR 1.8 [1.2–2.8]) compared to Whites.
- Black patients were more likely than Whites (OR 2.0 [1.4–2.8]) to report that their providers discussed their weight with them at a clinic visit.
- While over half (59.7%) indicated that their providers had given or discussed resources with them on healthy eating and weight loss, Black and Asian respondents were more likely than Whites to recall these discussions (Black: OR 1.6 [1.2–2.1]; Asians: OR 1.8 [1.1–2.9]).
- Most weight loss resources or recommendations received were related to lifestyle changes, with very few resources given related to weight loss medications, meal replacement products, or bariatric surgery—few differences across racial/ethnic groups were identified. However, respondents from racial/ethnic minority groups were more likely than Whites to say that they wanted more information about lifestyle changes, classes, and meal replacements. Other than Blacks, all other racial/ethnic groups were also more likely than Whites to indicate that they wanted more information about bariatric surgery.
Conclusions. Most patients across racial/ethnic groups are having positive experiences with weight-related care. However, race/ethnicity correlates with patients’ perception of weight-related care and discussions in clinic encounters.
Commentary
The obesity epidemic in the United States is well-established [1], and recent data from 2014 show that over 37% of adults in the US are obese (defined as having a body mass index greater than 30 kg/m2) [2]. However, while obesity prevalence rates have increased over the past several decades across all genders, ethnicities, income levels, and education levels, important racial/ethnic disparities exist [2,3]. Primary care physicians (PCPs) are ideally situated to promote weight loss via effective obesity counseling since multiple clinic visits over time have the potential to enable rapport building and behavioral change management [4]. In fact, the US Preventive Services Task Force (USPTF) recommends that all patients be screened for obesity and offered intensive lifestyle counseling, as modest weight loss can have significant health benefits [5]. However, some studies have found racial/ethnic differences and disparities in weight-related diagnoses, counseling, and treatment by providers, but also patient perceptions of care and preferred interventions [6–10]. Other studies have described racial/ethnic differences in weight-related concerns and behaviors, body satisfaction, and body image [11–13]. Thus, research is needed to examine these differences.
This cross-sectional study contributes to the limited literature examining the potential for heterogeneity of care according to patient characteristics like race and ethnicity. Key strengths of the design include a large and both geographically and racially/ethnically diverse sample of patients (increased generalizability), the use of mailed brief surveys (reduces non-response rate and reporting bias) and telephone follow-up for verbal administration (reduces non-response rate, though it increases interviewer bias), oversampling of respondents with BMI ≥ 40 kg/m2, and the controlling of key covariates including sex, age, Medicaid enrollment, site, and BMI.
However, there are several important limitations, many of which are acknowledged by the authors. While respondents were overall representative of the targeted sample population, the final respondent population was comprised of mostly older females who received managed care, which may have contributed to selection bias and impacted generalizability of findings. Further, Whites were overrepresented, Hispanics were underrepresented, and the small combined sample of NA/PI may have masked important distinctions between these subpopulations. Importantly, this study only provided the survey in English and Spanish and did not include other language translations (eg, Chinese, Japanese, Tagalog), which likely contributed to underrepresented perspectives of immigrants and ESL patients who may struggle with receiving/discussing weight management counseling and resources. The use of a surveys collected subjective and self-reported data on patient encounters as opposed to objective observations. Lastly, the study did not adjust for individual provider factors or assess the potential impact of provider-level differences on care, such as provider-patient concordance on race, ethnicity, language, and/or weight. The incorporation of qualitative interviewers or focus groups with a subsample of each racial/ethnic may have also provided relevant context to understand differences in weight-related care experiences.
Applications for Clinical Practice
As the authors suggest, this study highlights several opportunities to continue improving weight-related care and weight management counseling. PCPs should engage all overweight/obese patients in weight management discussions, and in particular, high-risk minority patients who may desire these conversations and more weight loss advice and resources. However, these discussions require sensitivity and can benefit from the simple practice of asking permission of the patient to talk about their weight in order to reduce care avoidance and improve perceptions of care. Providers should also be mindful of patient priorities and assess patient preferences for all the different weight loss strategies, including lifestyle changes, meal replacements, medications, and surgery.
—Katrina F. Mateo, MPH
1. Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717–32.
2. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284.
3. Wong RJ, Chou C, Ahmed A. Long term trends and racial/ethnic disparities in the prevalence of obesity. J Community Health 2014;39:1150–60.
4. Schlair S, Moore S, Mcmacken M, Jay M. How to deliver high quality obesity counseling using the 5As framework. J Clin Outcomes Manag 2012;19:221–9.
5. Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373–8.
6. Davis NJ, Wildman RP, Forbes BF, Schechter CB. Trends and disparities in provider diagnosis of overweight analysis of NHANES 1999–2004. Obesity 2009;17:2110–3.
7. Wee CC, Huskey KW, Bolcic-Jankovic D, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
8. Johnson RL, Saha S, Arbelaez JJ, et al. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med 2004;19:101–10.
9. Chugh M, Friedman AM, Clemow LP, Ferrante JM. Women weigh in: obese african american and white women’s perspectives on physicians’ roles in weight management. J Am Board Fam Med 2013;26:421–8.
10. Blixen CE, Singh A, Xu M, et al. What women want: understanding obesity and preferences for primary care weight reduction interventions among African-American and Caucasian women. J Natl Med Assoc 2006;98:1160–70.
11. Arcan C, Larson N, Bauer K, et al. Dietary and weight-related behaviors and body mass index among Hispanic, Hmong, Somali, and White adolescents. J Acad Nutr Diet 2014;114:375–83.
12. Kronenfeld LW, Reba-Harrelson L, Von Holle A, et al. Ethnic and racial differences in body size perception and satisfaction. Body Image 2010;7:131–6.
13. Gluck ME, Geliebter A. Racial/ethnic differences in body image and eating behaviors. Eat Behav 2002;3:143–51.
1. Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717–32.
2. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284.
3. Wong RJ, Chou C, Ahmed A. Long term trends and racial/ethnic disparities in the prevalence of obesity. J Community Health 2014;39:1150–60.
4. Schlair S, Moore S, Mcmacken M, Jay M. How to deliver high quality obesity counseling using the 5As framework. J Clin Outcomes Manag 2012;19:221–9.
5. Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373–8.
6. Davis NJ, Wildman RP, Forbes BF, Schechter CB. Trends and disparities in provider diagnosis of overweight analysis of NHANES 1999–2004. Obesity 2009;17:2110–3.
7. Wee CC, Huskey KW, Bolcic-Jankovic D, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
8. Johnson RL, Saha S, Arbelaez JJ, et al. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med 2004;19:101–10.
9. Chugh M, Friedman AM, Clemow LP, Ferrante JM. Women weigh in: obese african american and white women’s perspectives on physicians’ roles in weight management. J Am Board Fam Med 2013;26:421–8.
10. Blixen CE, Singh A, Xu M, et al. What women want: understanding obesity and preferences for primary care weight reduction interventions among African-American and Caucasian women. J Natl Med Assoc 2006;98:1160–70.
11. Arcan C, Larson N, Bauer K, et al. Dietary and weight-related behaviors and body mass index among Hispanic, Hmong, Somali, and White adolescents. J Acad Nutr Diet 2014;114:375–83.
12. Kronenfeld LW, Reba-Harrelson L, Von Holle A, et al. Ethnic and racial differences in body size perception and satisfaction. Body Image 2010;7:131–6.
13. Gluck ME, Geliebter A. Racial/ethnic differences in body image and eating behaviors. Eat Behav 2002;3:143–51.
It isn’t over until it’s over
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Intermittent breaks from sunitinib feasible in metastatic RCC
Intermittent breaks from sunitinib therapy are feasible and don’t appear to compromise the agent’s clinical efficacy against metastatic renal cell carcinoma, according to investigators.
One of the greatest challenges in treating this cancer is balancing treatment-related toxicity against efficacy “in a setting ... in which patients are largely incurable and thus subjected to chronic therapy,” said Moshe C. Ornstein, MD, and his associates at the Cleveland Clinic Taussig Cancer Institute.
They performed a single-center phase II study that they described as the first prospective trial to assess treatment interruptions whenever tumor burden was reduced by 10% or more, followed by resumption of treatment if tumors then progressed 10% or more. This approach “may result in reduced toxicities, improved quality of life, cost savings, and potentially improved clinical outcomes.”
The study involved 37 adults (median age, 63 years) who were given 50 mg sunitinib once daily for the first 28 days of a 42-day cycle, with dose adjustments allowed to minimize toxicities. Twenty patients (54%) who showed a 10% or greater reduction in tumor burden after four cycles suspended treatment until scans showed a 10% or greater progression, at which point treatment was resumed. These 20 patients were able to take up to 11 treatment breaks (median, 3 breaks per patient), with each break having a median duration of more than 8 weeks (range, 4.7-192.1 weeks).
Seven patients were able to have extended drug holidays lasting 3-43 months, and three have never had to resume sunitinib therapy after their first break. “The overall sum of all the breaks was 1,296.6 weeks, which corresponds to 216 6-week cycles with a median of 9 saved cycles per patient,” Dr. Ornstein and his associates said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.1184).
Given that the average wholesale price of sunitinib is $17,811.83 per 28-day cycle, “the 9 cycles saved per patient translates into a median cost saving of $160,302 per patient and $3.85 million overall, not including the cost of toxicity management that may have been incurred without breaks,” they noted.
The median progression-free survival was 22.4 months for the entire study cohort and 37.6 months for the 20 patients who had intermittent therapy.
Patients who showed tumor progression during a treatment break were transitioned back to standard dosing without showing any adverse clinical effects. This suggests that “with close clinical and radiographic monitoring, patients can safely take a therapy break for extended periods of time,” the investigators said.
Intermittent breaks from sunitinib therapy are feasible and don’t appear to compromise the agent’s clinical efficacy against metastatic renal cell carcinoma, according to investigators.
One of the greatest challenges in treating this cancer is balancing treatment-related toxicity against efficacy “in a setting ... in which patients are largely incurable and thus subjected to chronic therapy,” said Moshe C. Ornstein, MD, and his associates at the Cleveland Clinic Taussig Cancer Institute.
They performed a single-center phase II study that they described as the first prospective trial to assess treatment interruptions whenever tumor burden was reduced by 10% or more, followed by resumption of treatment if tumors then progressed 10% or more. This approach “may result in reduced toxicities, improved quality of life, cost savings, and potentially improved clinical outcomes.”
The study involved 37 adults (median age, 63 years) who were given 50 mg sunitinib once daily for the first 28 days of a 42-day cycle, with dose adjustments allowed to minimize toxicities. Twenty patients (54%) who showed a 10% or greater reduction in tumor burden after four cycles suspended treatment until scans showed a 10% or greater progression, at which point treatment was resumed. These 20 patients were able to take up to 11 treatment breaks (median, 3 breaks per patient), with each break having a median duration of more than 8 weeks (range, 4.7-192.1 weeks).
Seven patients were able to have extended drug holidays lasting 3-43 months, and three have never had to resume sunitinib therapy after their first break. “The overall sum of all the breaks was 1,296.6 weeks, which corresponds to 216 6-week cycles with a median of 9 saved cycles per patient,” Dr. Ornstein and his associates said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.1184).
Given that the average wholesale price of sunitinib is $17,811.83 per 28-day cycle, “the 9 cycles saved per patient translates into a median cost saving of $160,302 per patient and $3.85 million overall, not including the cost of toxicity management that may have been incurred without breaks,” they noted.
The median progression-free survival was 22.4 months for the entire study cohort and 37.6 months for the 20 patients who had intermittent therapy.
Patients who showed tumor progression during a treatment break were transitioned back to standard dosing without showing any adverse clinical effects. This suggests that “with close clinical and radiographic monitoring, patients can safely take a therapy break for extended periods of time,” the investigators said.
Intermittent breaks from sunitinib therapy are feasible and don’t appear to compromise the agent’s clinical efficacy against metastatic renal cell carcinoma, according to investigators.
One of the greatest challenges in treating this cancer is balancing treatment-related toxicity against efficacy “in a setting ... in which patients are largely incurable and thus subjected to chronic therapy,” said Moshe C. Ornstein, MD, and his associates at the Cleveland Clinic Taussig Cancer Institute.
They performed a single-center phase II study that they described as the first prospective trial to assess treatment interruptions whenever tumor burden was reduced by 10% or more, followed by resumption of treatment if tumors then progressed 10% or more. This approach “may result in reduced toxicities, improved quality of life, cost savings, and potentially improved clinical outcomes.”
The study involved 37 adults (median age, 63 years) who were given 50 mg sunitinib once daily for the first 28 days of a 42-day cycle, with dose adjustments allowed to minimize toxicities. Twenty patients (54%) who showed a 10% or greater reduction in tumor burden after four cycles suspended treatment until scans showed a 10% or greater progression, at which point treatment was resumed. These 20 patients were able to take up to 11 treatment breaks (median, 3 breaks per patient), with each break having a median duration of more than 8 weeks (range, 4.7-192.1 weeks).
Seven patients were able to have extended drug holidays lasting 3-43 months, and three have never had to resume sunitinib therapy after their first break. “The overall sum of all the breaks was 1,296.6 weeks, which corresponds to 216 6-week cycles with a median of 9 saved cycles per patient,” Dr. Ornstein and his associates said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.1184).
Given that the average wholesale price of sunitinib is $17,811.83 per 28-day cycle, “the 9 cycles saved per patient translates into a median cost saving of $160,302 per patient and $3.85 million overall, not including the cost of toxicity management that may have been incurred without breaks,” they noted.
The median progression-free survival was 22.4 months for the entire study cohort and 37.6 months for the 20 patients who had intermittent therapy.
Patients who showed tumor progression during a treatment break were transitioned back to standard dosing without showing any adverse clinical effects. This suggests that “with close clinical and radiographic monitoring, patients can safely take a therapy break for extended periods of time,” the investigators said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Intermittent breaks from sunitinib therapy are feasible and don’t appear to compromise the agent’s clinical efficacy against metastatic renal cell carcinoma.
Major finding: Twenty patients were able to take up to 11 treatment breaks (median, 3 drug holidays per patient), with each break having a median duration of more than 8 weeks (range, 5-192 weeks).
Data source: A 2-year single-center prospective phase II trial involving 37 adults with metastatic renal cell carcinoma.
Disclosures: The Cleveland Clinic Taussig Cancer Institute supported the trial. Dr. Ornstein reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
What Are the Key Elements of an Initial Pediatric Concussion Evaluation?
VANCOUVER—Initial pediatric concussion evaluations provide an opportunity to recognize premorbid conditions that may be exacerbated by the injury; address problems with prior management, such as excessive rest or NSAID overuse; and identify treatment approaches, according to a seminar delivered at the 45th Annual Meeting of the Child Neurology Society.
Neurologists also may decide whether imaging is warranted and note risk factors for prolonged recovery, said Sharief Taraman, MD, Director of the Children’s Concussion Program at Children’s Hospital of Orange County in Orange, California, and Assistant Clinical Professor of Pediatrics at the University of California, Irvine.
Imaging Likely Not Needed
In most cases, a CT scan is not necessary, Dr. Taraman said. He encouraged neurologists to work with their emergency department colleagues to ensure that patients only undergo CT scans when appropriate. His department uses Pediatric Emergency Care Applied Research Network (PECARN) criteria to determine when a CT scan is warranted. Many patients do not have signs of altered mental status in the emergency department, and “there is really no good reason to scan many of these kids,” he said. Patients also typically do not undergo MRI unless “a significant neurologic finding … suggests that there might have been a stronger mechanism of action.”
History taking is a vital component of initial management, and identifying premorbid conditions is a key factor, Dr. Taraman said. “What we have seen is that concussion symptoms act as a magnifying glass. If I have migraine and I get a concussion, my migraine will likely become exacerbated,” he said. Patients may also present for evaluation of concussion but have an alternate diagnosis that better explains their symptoms. For example, Dr. Taraman described a patient who had persistent symptoms following a concussion. “Listening to the story, it was clearly sleep apnea,” he said. The child underwent polysomnography and received continuous positive airway pressure treatment because he had 40 apneas in an hour.
During the evaluation, neurologists can recognize poor initial management of the injury, such as excessive bed rest or removal from activities. NSAID overuse also is a big problem. Emergency departments may tell patients to take ibuprofen every eight hours for five weeks, which can lead to rebound headaches, Dr. Taraman said.
Facilitate Recovery
Recognition of certain symptoms can inform the patient’s prognosis and suggest ways to speed recovery. For example, neurologists should look for vestibular dysfunction or balance problems and decide whether to address these symptoms. Neurologists also should check for and address cervical strain and ocular dysfunction. Treating severe convergence insufficiency or excess may help patients recover faster.
Anxiety and mood disorders suggest a prolonged recovery. Some patients develop adjustment disorder after concussion. “Interestingly, we see that patients who have more severe traumatic brain injury … are unaware of their deficits,” whereas high-functioning patients who feel slightly off perceive their deficits, which “causes a lot of discomfort for them,” he said.
Symptoms from concussions that involve assaults and litigation tend to take longer to resolve. Some patients’ symptoms persist until litigation ends, although typically not due to malingering but rather due to increased psychological stress.
Poor headache control, sleep disturbances, prior concussions, and a history of prolonged concussion recovery are other risk factors for prolonged recovery.
The Sport Concussion Assessment Tool 3 (SCAT3) is a free, standardized way of assessing symptoms. Developed as a sideline assessment tool, the SCAT3 also works well as a symptoms form, Dr. Taraman said. The tool includes a quick cognitive assessment and balance exam, and online video tutorials explain how to perform the assessment. After assessing a patient’s symptoms, including cognition, concentration, balance, and convergence insufficiency, “then you can decide, how … to triage the patient and start managing them.”
—Jake Remaly
Suggested Reading
Bressan S, Romanato S, Mion T, et al. Implementation of adapted PECARN decision rule for children with minor head injury in the pediatric emergency department. Acad Emerg Med. 2012;19(7):801-807.
Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-1170.
Yengo-Kahn AM, Hale AT, Zalneraitis BH, et al. The Sport Concussion Assessment Tool: a systematic review. Neurosurg Focus. 2016;40(4):E6.
VANCOUVER—Initial pediatric concussion evaluations provide an opportunity to recognize premorbid conditions that may be exacerbated by the injury; address problems with prior management, such as excessive rest or NSAID overuse; and identify treatment approaches, according to a seminar delivered at the 45th Annual Meeting of the Child Neurology Society.
Neurologists also may decide whether imaging is warranted and note risk factors for prolonged recovery, said Sharief Taraman, MD, Director of the Children’s Concussion Program at Children’s Hospital of Orange County in Orange, California, and Assistant Clinical Professor of Pediatrics at the University of California, Irvine.
Imaging Likely Not Needed
In most cases, a CT scan is not necessary, Dr. Taraman said. He encouraged neurologists to work with their emergency department colleagues to ensure that patients only undergo CT scans when appropriate. His department uses Pediatric Emergency Care Applied Research Network (PECARN) criteria to determine when a CT scan is warranted. Many patients do not have signs of altered mental status in the emergency department, and “there is really no good reason to scan many of these kids,” he said. Patients also typically do not undergo MRI unless “a significant neurologic finding … suggests that there might have been a stronger mechanism of action.”
History taking is a vital component of initial management, and identifying premorbid conditions is a key factor, Dr. Taraman said. “What we have seen is that concussion symptoms act as a magnifying glass. If I have migraine and I get a concussion, my migraine will likely become exacerbated,” he said. Patients may also present for evaluation of concussion but have an alternate diagnosis that better explains their symptoms. For example, Dr. Taraman described a patient who had persistent symptoms following a concussion. “Listening to the story, it was clearly sleep apnea,” he said. The child underwent polysomnography and received continuous positive airway pressure treatment because he had 40 apneas in an hour.
During the evaluation, neurologists can recognize poor initial management of the injury, such as excessive bed rest or removal from activities. NSAID overuse also is a big problem. Emergency departments may tell patients to take ibuprofen every eight hours for five weeks, which can lead to rebound headaches, Dr. Taraman said.
Facilitate Recovery
Recognition of certain symptoms can inform the patient’s prognosis and suggest ways to speed recovery. For example, neurologists should look for vestibular dysfunction or balance problems and decide whether to address these symptoms. Neurologists also should check for and address cervical strain and ocular dysfunction. Treating severe convergence insufficiency or excess may help patients recover faster.
Anxiety and mood disorders suggest a prolonged recovery. Some patients develop adjustment disorder after concussion. “Interestingly, we see that patients who have more severe traumatic brain injury … are unaware of their deficits,” whereas high-functioning patients who feel slightly off perceive their deficits, which “causes a lot of discomfort for them,” he said.
Symptoms from concussions that involve assaults and litigation tend to take longer to resolve. Some patients’ symptoms persist until litigation ends, although typically not due to malingering but rather due to increased psychological stress.
Poor headache control, sleep disturbances, prior concussions, and a history of prolonged concussion recovery are other risk factors for prolonged recovery.
The Sport Concussion Assessment Tool 3 (SCAT3) is a free, standardized way of assessing symptoms. Developed as a sideline assessment tool, the SCAT3 also works well as a symptoms form, Dr. Taraman said. The tool includes a quick cognitive assessment and balance exam, and online video tutorials explain how to perform the assessment. After assessing a patient’s symptoms, including cognition, concentration, balance, and convergence insufficiency, “then you can decide, how … to triage the patient and start managing them.”
—Jake Remaly
Suggested Reading
Bressan S, Romanato S, Mion T, et al. Implementation of adapted PECARN decision rule for children with minor head injury in the pediatric emergency department. Acad Emerg Med. 2012;19(7):801-807.
Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-1170.
Yengo-Kahn AM, Hale AT, Zalneraitis BH, et al. The Sport Concussion Assessment Tool: a systematic review. Neurosurg Focus. 2016;40(4):E6.
VANCOUVER—Initial pediatric concussion evaluations provide an opportunity to recognize premorbid conditions that may be exacerbated by the injury; address problems with prior management, such as excessive rest or NSAID overuse; and identify treatment approaches, according to a seminar delivered at the 45th Annual Meeting of the Child Neurology Society.
Neurologists also may decide whether imaging is warranted and note risk factors for prolonged recovery, said Sharief Taraman, MD, Director of the Children’s Concussion Program at Children’s Hospital of Orange County in Orange, California, and Assistant Clinical Professor of Pediatrics at the University of California, Irvine.
Imaging Likely Not Needed
In most cases, a CT scan is not necessary, Dr. Taraman said. He encouraged neurologists to work with their emergency department colleagues to ensure that patients only undergo CT scans when appropriate. His department uses Pediatric Emergency Care Applied Research Network (PECARN) criteria to determine when a CT scan is warranted. Many patients do not have signs of altered mental status in the emergency department, and “there is really no good reason to scan many of these kids,” he said. Patients also typically do not undergo MRI unless “a significant neurologic finding … suggests that there might have been a stronger mechanism of action.”
History taking is a vital component of initial management, and identifying premorbid conditions is a key factor, Dr. Taraman said. “What we have seen is that concussion symptoms act as a magnifying glass. If I have migraine and I get a concussion, my migraine will likely become exacerbated,” he said. Patients may also present for evaluation of concussion but have an alternate diagnosis that better explains their symptoms. For example, Dr. Taraman described a patient who had persistent symptoms following a concussion. “Listening to the story, it was clearly sleep apnea,” he said. The child underwent polysomnography and received continuous positive airway pressure treatment because he had 40 apneas in an hour.
During the evaluation, neurologists can recognize poor initial management of the injury, such as excessive bed rest or removal from activities. NSAID overuse also is a big problem. Emergency departments may tell patients to take ibuprofen every eight hours for five weeks, which can lead to rebound headaches, Dr. Taraman said.
Facilitate Recovery
Recognition of certain symptoms can inform the patient’s prognosis and suggest ways to speed recovery. For example, neurologists should look for vestibular dysfunction or balance problems and decide whether to address these symptoms. Neurologists also should check for and address cervical strain and ocular dysfunction. Treating severe convergence insufficiency or excess may help patients recover faster.
Anxiety and mood disorders suggest a prolonged recovery. Some patients develop adjustment disorder after concussion. “Interestingly, we see that patients who have more severe traumatic brain injury … are unaware of their deficits,” whereas high-functioning patients who feel slightly off perceive their deficits, which “causes a lot of discomfort for them,” he said.
Symptoms from concussions that involve assaults and litigation tend to take longer to resolve. Some patients’ symptoms persist until litigation ends, although typically not due to malingering but rather due to increased psychological stress.
Poor headache control, sleep disturbances, prior concussions, and a history of prolonged concussion recovery are other risk factors for prolonged recovery.
The Sport Concussion Assessment Tool 3 (SCAT3) is a free, standardized way of assessing symptoms. Developed as a sideline assessment tool, the SCAT3 also works well as a symptoms form, Dr. Taraman said. The tool includes a quick cognitive assessment and balance exam, and online video tutorials explain how to perform the assessment. After assessing a patient’s symptoms, including cognition, concentration, balance, and convergence insufficiency, “then you can decide, how … to triage the patient and start managing them.”
—Jake Remaly
Suggested Reading
Bressan S, Romanato S, Mion T, et al. Implementation of adapted PECARN decision rule for children with minor head injury in the pediatric emergency department. Acad Emerg Med. 2012;19(7):801-807.
Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-1170.
Yengo-Kahn AM, Hale AT, Zalneraitis BH, et al. The Sport Concussion Assessment Tool: a systematic review. Neurosurg Focus. 2016;40(4):E6.
Charging for medical records: For whom and at what cost?
Do you charge for medical records?
You probably do, and so do I, at times.
Generally, I’m willing to give a patient one copy of their records or transfer them to another doctor for continuation of care, at no charge. People move away. They change insurance or doctors. They have urgent hospital admissions. To me, charging to forward records in these cases is like withholding care.
That’s not to say I don’t lose money on them. It takes a few minutes (or more) of staff time to print them up and fax them. If they need to be mailed, postage costs money. And then there’s paper, printer ink, and so on. I’m sure it adds up to something over the course of the year, although I have no idea how much.
How much you can charge is a more complex issue, with each state setting its own rules. A recent article published in JAMA Internal Medicine noted that a patient in Georgia could pay up to $111.68 for a 100-page record. Hitting someone up for that amount, who’s already having health problems and may be relocating or trying to find a new doctor, seems like making an already difficult situation worse.
But we’re in the digital age now. So how much does it cost to send records? Most files (.doc, .pdf, .jpg, and so on) are interchangeable between Mac and Windows.
Things get iffy here. I mean, it’s easy to send a .pdf file by email, but that’s not particularly secure. And I hate having to sign up and create passwords for the many allegedly safer file-sharing services out there.
Burning records on a CD or DVD certainly saves postage, though takes about the same amount of computer time as printing them up. Not only that, but this seems to be a format that’s on its way out. The last three computers I’ve bought didn’t even have optical drives. CD/DVD’s are starting to resemble VHS tapes in the late 1990s.
Flash drives are the present and immediate future of transferred records. Small, lightweight, and capable of holding a lot. But they still need to be mailed, and are more expensive than paper. They also have security risks that concern me. When a patient hands me one and asks me to plug it in, I never do. There could be a virus or spyware that can compromise the security and privacy of my office, and cost a fortune to reverse the damage.
And so, at the end of that chain of thought, paper still appears to be king. It’s not going to carry ransomware into my office. It can be mailed or faxed, and is easily adaptable to any system (like mine) with a scanner. The paper world may hypothetically no longer exist, but for many things in medicine it still does, and is critical.
Some ultimate solutions, such as a universal database of health care data on all patients or a complete interchangeability between systems, sound great. No one would need to transfer records between doctors and all would have access to their own charts. But at this point in time, while technologically achievable, the privacy concerns and high-stakes security risks make such a thing impossible.
It’s easy to hope that the age of electronic medical records will lead to, as the article states, “easy, inexpensive” reproduction of medical records. But things never seem to be that simple, for some of the reasons I’ve mentioned above.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Do you charge for medical records?
You probably do, and so do I, at times.
Generally, I’m willing to give a patient one copy of their records or transfer them to another doctor for continuation of care, at no charge. People move away. They change insurance or doctors. They have urgent hospital admissions. To me, charging to forward records in these cases is like withholding care.
That’s not to say I don’t lose money on them. It takes a few minutes (or more) of staff time to print them up and fax them. If they need to be mailed, postage costs money. And then there’s paper, printer ink, and so on. I’m sure it adds up to something over the course of the year, although I have no idea how much.
How much you can charge is a more complex issue, with each state setting its own rules. A recent article published in JAMA Internal Medicine noted that a patient in Georgia could pay up to $111.68 for a 100-page record. Hitting someone up for that amount, who’s already having health problems and may be relocating or trying to find a new doctor, seems like making an already difficult situation worse.
But we’re in the digital age now. So how much does it cost to send records? Most files (.doc, .pdf, .jpg, and so on) are interchangeable between Mac and Windows.
Things get iffy here. I mean, it’s easy to send a .pdf file by email, but that’s not particularly secure. And I hate having to sign up and create passwords for the many allegedly safer file-sharing services out there.
Burning records on a CD or DVD certainly saves postage, though takes about the same amount of computer time as printing them up. Not only that, but this seems to be a format that’s on its way out. The last three computers I’ve bought didn’t even have optical drives. CD/DVD’s are starting to resemble VHS tapes in the late 1990s.
Flash drives are the present and immediate future of transferred records. Small, lightweight, and capable of holding a lot. But they still need to be mailed, and are more expensive than paper. They also have security risks that concern me. When a patient hands me one and asks me to plug it in, I never do. There could be a virus or spyware that can compromise the security and privacy of my office, and cost a fortune to reverse the damage.
And so, at the end of that chain of thought, paper still appears to be king. It’s not going to carry ransomware into my office. It can be mailed or faxed, and is easily adaptable to any system (like mine) with a scanner. The paper world may hypothetically no longer exist, but for many things in medicine it still does, and is critical.
Some ultimate solutions, such as a universal database of health care data on all patients or a complete interchangeability between systems, sound great. No one would need to transfer records between doctors and all would have access to their own charts. But at this point in time, while technologically achievable, the privacy concerns and high-stakes security risks make such a thing impossible.
It’s easy to hope that the age of electronic medical records will lead to, as the article states, “easy, inexpensive” reproduction of medical records. But things never seem to be that simple, for some of the reasons I’ve mentioned above.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Do you charge for medical records?
You probably do, and so do I, at times.
Generally, I’m willing to give a patient one copy of their records or transfer them to another doctor for continuation of care, at no charge. People move away. They change insurance or doctors. They have urgent hospital admissions. To me, charging to forward records in these cases is like withholding care.
That’s not to say I don’t lose money on them. It takes a few minutes (or more) of staff time to print them up and fax them. If they need to be mailed, postage costs money. And then there’s paper, printer ink, and so on. I’m sure it adds up to something over the course of the year, although I have no idea how much.
How much you can charge is a more complex issue, with each state setting its own rules. A recent article published in JAMA Internal Medicine noted that a patient in Georgia could pay up to $111.68 for a 100-page record. Hitting someone up for that amount, who’s already having health problems and may be relocating or trying to find a new doctor, seems like making an already difficult situation worse.
But we’re in the digital age now. So how much does it cost to send records? Most files (.doc, .pdf, .jpg, and so on) are interchangeable between Mac and Windows.
Things get iffy here. I mean, it’s easy to send a .pdf file by email, but that’s not particularly secure. And I hate having to sign up and create passwords for the many allegedly safer file-sharing services out there.
Burning records on a CD or DVD certainly saves postage, though takes about the same amount of computer time as printing them up. Not only that, but this seems to be a format that’s on its way out. The last three computers I’ve bought didn’t even have optical drives. CD/DVD’s are starting to resemble VHS tapes in the late 1990s.
Flash drives are the present and immediate future of transferred records. Small, lightweight, and capable of holding a lot. But they still need to be mailed, and are more expensive than paper. They also have security risks that concern me. When a patient hands me one and asks me to plug it in, I never do. There could be a virus or spyware that can compromise the security and privacy of my office, and cost a fortune to reverse the damage.
And so, at the end of that chain of thought, paper still appears to be king. It’s not going to carry ransomware into my office. It can be mailed or faxed, and is easily adaptable to any system (like mine) with a scanner. The paper world may hypothetically no longer exist, but for many things in medicine it still does, and is critical.
Some ultimate solutions, such as a universal database of health care data on all patients or a complete interchangeability between systems, sound great. No one would need to transfer records between doctors and all would have access to their own charts. But at this point in time, while technologically achievable, the privacy concerns and high-stakes security risks make such a thing impossible.
It’s easy to hope that the age of electronic medical records will lead to, as the article states, “easy, inexpensive” reproduction of medical records. But things never seem to be that simple, for some of the reasons I’ve mentioned above.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Substantial long-term increase seen in multiple myeloma survival
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).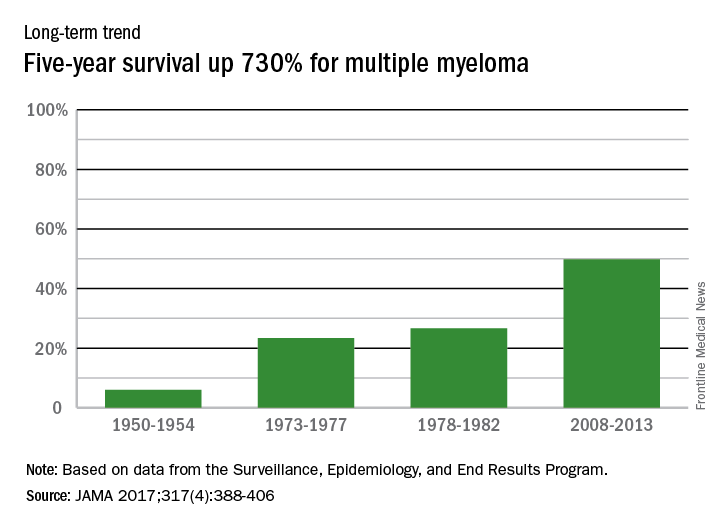
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The 5-year survival rate for multiple myeloma increased eightfold over an approximately 60-year span starting in the early 1950s, said Ali H. Mokdad, PhD, and his associates.
Patients with multiple myeloma had a 5-year relative survival rate of 6% in 1950-1954, compared with 49.8% in 2008-2013, according to data from the Surveillance, Epidemiology, and End Results Program (JAMA 2017;317[4]:388-406).
In 2014, there were about 13,000 deaths resulting from multiple myeloma, with 219,000 years of life lost, which ranked 17th among the 29 selected cancers, noted Dr. Mokdad and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA
AML leads percent gains in 5-year survival among leukemias
Over the 60-year span from the early 1950s to 2013, the 5-year survival rate for all leukemias increased by 500%, according to data from the Surveillance, Epidemiology, and End Results Program.
For 2008-2013, the 5-year relative survival rate for all leukemias was 60.1%, compared with 10% during 1950-1954, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle (JAMA 2017;317[4]:388-406).
Over the 60-year span from the early 1950s to 2013, the 5-year survival rate for all leukemias increased by 500%, according to data from the Surveillance, Epidemiology, and End Results Program.
For 2008-2013, the 5-year relative survival rate for all leukemias was 60.1%, compared with 10% during 1950-1954, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle (JAMA 2017;317[4]:388-406).
Over the 60-year span from the early 1950s to 2013, the 5-year survival rate for all leukemias increased by 500%, according to data from the Surveillance, Epidemiology, and End Results Program.
For 2008-2013, the 5-year relative survival rate for all leukemias was 60.1%, compared with 10% during 1950-1954, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle (JAMA 2017;317[4]:388-406).