User login
Common Epilepsies Share Genetic Overlap With Rare Types
Several genes previously implicated only in rare, severe forms of pediatric epilepsy may also contribute to common forms of the disorder, according to a report published online ahead of print January 13 in Lancet Neurology. “Our findings raise hopes that the emerging paradigm for the treatment of rare epilepsies, where therapies are targeted to the precise genetic cause of disease, may also extend to a proportion of common epilepsy syndromes,” said study leader David B. Goldstein, PhD, Director of the Institute for Genomic Medicine and Professor in the Departments of Genetics and Development and Neurology at Columbia University Medical Center in New York City.
In recent years, researchers have identified dozens of genes that, alone or in combination with other factors, cause rare pediatric epilepsies. These discoveries have led to the use of targeted therapies for some seizure disorders, such as the ketogenic diet for patients with Dravet syndrome or GLUT-1 deficiency syndrome. Other therapies such as quinidine, a medication to treat heart arrhythmias, and memantine, an Alzheimer’s disease treatment, have been tried in children with certain gene mutations. These attempts have not proved universally effective for all patients with these mutations, but suggest the potential to repurpose existing medicines to treat rare genetic forms of epilepsy.
“Unlike very rare types of epilepsies, previous studies had shed little light on the genetic underpinnings of common epilepsies, which suggested that this precision medicine paradigm may have a very narrow application,” said Dr. Goldstein.
To learn more about the genetics of epilepsy, Dr. Goldstein and his colleagues conducted a study to identify the genetic contributions to more common forms of epilepsy. Analyses were conducted at Columbia University Medical Center’s Institute for Genomic Medicine, in collaboration with NewYork-Presbyterian, as part of Epi4K, an international consortium of epilepsy clinicians and researchers. Most of the patients were recruited through the Epilepsy Phenome/Genome Project.
The researchers separately compared the sequence data from 640 individuals with familial genetic generalized epilepsy and 525 individuals with familial non-acquired focal epilepsy to the same group of 3,877 controls. The researchers found significantly higher rates of ultra-rare deleterious variation in genes established as causative for dominant epilepsy disorders (familial genetic generalized epilepsy, odd ratio 2.3; familial non-acquired focal epilepsy, odds ratio 3.6). Comparison of an additional cohort of 662 individuals with sporadic non-acquired focal epilepsy to controls did not identify study-wide significant signals.
Five Genes Implicated
For the individuals with familial non-acquired focal epilepsy, the researchers found that five known epilepsy genes—DEPDC5, LG11, PCDH19, SCN1A, and GRIN2A—ranked as the top five genes enriched for ultra-rare deleterious variation. “After accounting for the control carrier rate, we estimate that these five genes contribute to the risk of epilepsy in approximately 8% of individuals with familial non-acquired focal epilepsy,” said Erin Heinzen Cox, PhD, Assistant Professor in the Department of Pathology and Cell Biology and Deputy Director of the Institute for Genomic Medicine at Columbia University Medical Center.
Treatment Targeted to Epilepsy Subtype
The findings have important implications for clinical practice and for research. “At present, all common epilepsies are treated the same way, with the same group of medications,” said Dr. Goldstein. “But as we identify more of these epilepsy genes that span a much wider range of types of epilepsy than previously thought, we can begin to try targeted therapies across these patient populations. As this genetically driven treatment paradigm becomes more established, our field, which is accustomed to undertaking large clinical trials in broad patient populations, will need to take a new approach to clinical research, focusing on patients based on their genetic subtype.”
“This is a very exciting breakthrough in the treatment of epilepsy, in which current treatment is based on whether a child has focal seizures … or generalized seizures,” said James J. Riviello, MD, the Sergievsky Family Professor of Neurology and Pediatrics and Chief of Child Neurology at NewYork-Presbyterian Morgan Stanley Children’s Hospital in New York City. “Genetic testing for epilepsy may allow us to identify the specific anticonvulsant medication that potentially works best for an individual patient. We have already identified children in whom knowing the underlying genetic basis of the epilepsy has guided our treatment choices.”
Additional studies, which will analyze 10,000 to 12,000 samples, are planned for the coming year. “With a larger analysis, we expect to find additional genetic variations that contribute to common epilepsies,” said Dr. Goldstein.
Suggested Reading
Epi4K consortium, Epilepsy Phenome/Genome Project. Ultra-rare genetic variation in common epilepsies: a case-control sequencing study. Lancet Neurol. 2017;16(2):135-143.
Several genes previously implicated only in rare, severe forms of pediatric epilepsy may also contribute to common forms of the disorder, according to a report published online ahead of print January 13 in Lancet Neurology. “Our findings raise hopes that the emerging paradigm for the treatment of rare epilepsies, where therapies are targeted to the precise genetic cause of disease, may also extend to a proportion of common epilepsy syndromes,” said study leader David B. Goldstein, PhD, Director of the Institute for Genomic Medicine and Professor in the Departments of Genetics and Development and Neurology at Columbia University Medical Center in New York City.
In recent years, researchers have identified dozens of genes that, alone or in combination with other factors, cause rare pediatric epilepsies. These discoveries have led to the use of targeted therapies for some seizure disorders, such as the ketogenic diet for patients with Dravet syndrome or GLUT-1 deficiency syndrome. Other therapies such as quinidine, a medication to treat heart arrhythmias, and memantine, an Alzheimer’s disease treatment, have been tried in children with certain gene mutations. These attempts have not proved universally effective for all patients with these mutations, but suggest the potential to repurpose existing medicines to treat rare genetic forms of epilepsy.
“Unlike very rare types of epilepsies, previous studies had shed little light on the genetic underpinnings of common epilepsies, which suggested that this precision medicine paradigm may have a very narrow application,” said Dr. Goldstein.
To learn more about the genetics of epilepsy, Dr. Goldstein and his colleagues conducted a study to identify the genetic contributions to more common forms of epilepsy. Analyses were conducted at Columbia University Medical Center’s Institute for Genomic Medicine, in collaboration with NewYork-Presbyterian, as part of Epi4K, an international consortium of epilepsy clinicians and researchers. Most of the patients were recruited through the Epilepsy Phenome/Genome Project.
The researchers separately compared the sequence data from 640 individuals with familial genetic generalized epilepsy and 525 individuals with familial non-acquired focal epilepsy to the same group of 3,877 controls. The researchers found significantly higher rates of ultra-rare deleterious variation in genes established as causative for dominant epilepsy disorders (familial genetic generalized epilepsy, odd ratio 2.3; familial non-acquired focal epilepsy, odds ratio 3.6). Comparison of an additional cohort of 662 individuals with sporadic non-acquired focal epilepsy to controls did not identify study-wide significant signals.
Five Genes Implicated
For the individuals with familial non-acquired focal epilepsy, the researchers found that five known epilepsy genes—DEPDC5, LG11, PCDH19, SCN1A, and GRIN2A—ranked as the top five genes enriched for ultra-rare deleterious variation. “After accounting for the control carrier rate, we estimate that these five genes contribute to the risk of epilepsy in approximately 8% of individuals with familial non-acquired focal epilepsy,” said Erin Heinzen Cox, PhD, Assistant Professor in the Department of Pathology and Cell Biology and Deputy Director of the Institute for Genomic Medicine at Columbia University Medical Center.
Treatment Targeted to Epilepsy Subtype
The findings have important implications for clinical practice and for research. “At present, all common epilepsies are treated the same way, with the same group of medications,” said Dr. Goldstein. “But as we identify more of these epilepsy genes that span a much wider range of types of epilepsy than previously thought, we can begin to try targeted therapies across these patient populations. As this genetically driven treatment paradigm becomes more established, our field, which is accustomed to undertaking large clinical trials in broad patient populations, will need to take a new approach to clinical research, focusing on patients based on their genetic subtype.”
“This is a very exciting breakthrough in the treatment of epilepsy, in which current treatment is based on whether a child has focal seizures … or generalized seizures,” said James J. Riviello, MD, the Sergievsky Family Professor of Neurology and Pediatrics and Chief of Child Neurology at NewYork-Presbyterian Morgan Stanley Children’s Hospital in New York City. “Genetic testing for epilepsy may allow us to identify the specific anticonvulsant medication that potentially works best for an individual patient. We have already identified children in whom knowing the underlying genetic basis of the epilepsy has guided our treatment choices.”
Additional studies, which will analyze 10,000 to 12,000 samples, are planned for the coming year. “With a larger analysis, we expect to find additional genetic variations that contribute to common epilepsies,” said Dr. Goldstein.
Suggested Reading
Epi4K consortium, Epilepsy Phenome/Genome Project. Ultra-rare genetic variation in common epilepsies: a case-control sequencing study. Lancet Neurol. 2017;16(2):135-143.
Several genes previously implicated only in rare, severe forms of pediatric epilepsy may also contribute to common forms of the disorder, according to a report published online ahead of print January 13 in Lancet Neurology. “Our findings raise hopes that the emerging paradigm for the treatment of rare epilepsies, where therapies are targeted to the precise genetic cause of disease, may also extend to a proportion of common epilepsy syndromes,” said study leader David B. Goldstein, PhD, Director of the Institute for Genomic Medicine and Professor in the Departments of Genetics and Development and Neurology at Columbia University Medical Center in New York City.
In recent years, researchers have identified dozens of genes that, alone or in combination with other factors, cause rare pediatric epilepsies. These discoveries have led to the use of targeted therapies for some seizure disorders, such as the ketogenic diet for patients with Dravet syndrome or GLUT-1 deficiency syndrome. Other therapies such as quinidine, a medication to treat heart arrhythmias, and memantine, an Alzheimer’s disease treatment, have been tried in children with certain gene mutations. These attempts have not proved universally effective for all patients with these mutations, but suggest the potential to repurpose existing medicines to treat rare genetic forms of epilepsy.
“Unlike very rare types of epilepsies, previous studies had shed little light on the genetic underpinnings of common epilepsies, which suggested that this precision medicine paradigm may have a very narrow application,” said Dr. Goldstein.
To learn more about the genetics of epilepsy, Dr. Goldstein and his colleagues conducted a study to identify the genetic contributions to more common forms of epilepsy. Analyses were conducted at Columbia University Medical Center’s Institute for Genomic Medicine, in collaboration with NewYork-Presbyterian, as part of Epi4K, an international consortium of epilepsy clinicians and researchers. Most of the patients were recruited through the Epilepsy Phenome/Genome Project.
The researchers separately compared the sequence data from 640 individuals with familial genetic generalized epilepsy and 525 individuals with familial non-acquired focal epilepsy to the same group of 3,877 controls. The researchers found significantly higher rates of ultra-rare deleterious variation in genes established as causative for dominant epilepsy disorders (familial genetic generalized epilepsy, odd ratio 2.3; familial non-acquired focal epilepsy, odds ratio 3.6). Comparison of an additional cohort of 662 individuals with sporadic non-acquired focal epilepsy to controls did not identify study-wide significant signals.
Five Genes Implicated
For the individuals with familial non-acquired focal epilepsy, the researchers found that five known epilepsy genes—DEPDC5, LG11, PCDH19, SCN1A, and GRIN2A—ranked as the top five genes enriched for ultra-rare deleterious variation. “After accounting for the control carrier rate, we estimate that these five genes contribute to the risk of epilepsy in approximately 8% of individuals with familial non-acquired focal epilepsy,” said Erin Heinzen Cox, PhD, Assistant Professor in the Department of Pathology and Cell Biology and Deputy Director of the Institute for Genomic Medicine at Columbia University Medical Center.
Treatment Targeted to Epilepsy Subtype
The findings have important implications for clinical practice and for research. “At present, all common epilepsies are treated the same way, with the same group of medications,” said Dr. Goldstein. “But as we identify more of these epilepsy genes that span a much wider range of types of epilepsy than previously thought, we can begin to try targeted therapies across these patient populations. As this genetically driven treatment paradigm becomes more established, our field, which is accustomed to undertaking large clinical trials in broad patient populations, will need to take a new approach to clinical research, focusing on patients based on their genetic subtype.”
“This is a very exciting breakthrough in the treatment of epilepsy, in which current treatment is based on whether a child has focal seizures … or generalized seizures,” said James J. Riviello, MD, the Sergievsky Family Professor of Neurology and Pediatrics and Chief of Child Neurology at NewYork-Presbyterian Morgan Stanley Children’s Hospital in New York City. “Genetic testing for epilepsy may allow us to identify the specific anticonvulsant medication that potentially works best for an individual patient. We have already identified children in whom knowing the underlying genetic basis of the epilepsy has guided our treatment choices.”
Additional studies, which will analyze 10,000 to 12,000 samples, are planned for the coming year. “With a larger analysis, we expect to find additional genetic variations that contribute to common epilepsies,” said Dr. Goldstein.
Suggested Reading
Epi4K consortium, Epilepsy Phenome/Genome Project. Ultra-rare genetic variation in common epilepsies: a case-control sequencing study. Lancet Neurol. 2017;16(2):135-143.
Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
In November 2016, Bayer, the manufacturer of the permanent birth control tubal implant system (Essure), revised the Essure product labeling in accordance with a US Food and Drug Administration (FDA) guidance document.1 The FDA developed its labeling guidance based on its examination of an increasing number of reported adverse events associated with the system’s use (such as persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions) and its evaluation of a trade complaint regarding allegations initially made in a Citizen Petition.
Changes to the new FDA-approved labeling for Essure include:
- the addition of a boxed warning listing adverse events that have been reported either in clinical studies or through postmarket surveillance (see Box)
- updated Instructions for Use document for clinicians and Patient Information Booklet, which contain additional information on safety (contraindications, warnings, and precautions), clinical data, and instructions2,3
- a Patient-Doctor Discussion Checklist (included within the Patient Information Booklet), designed to support appropriate patient counseling, facilitate the patient’s understanding of birth control options, and explain the benefits and risks associated with the device and what to expect during and after the implantation procedure.3
How will these labeling changes impact clinicians and patients? OBG
Reference
1. Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link%20Essure%20IFU.pdf. Accessed January 5, 2017.
OBG Management: What does the new product labeling mean for clinicians who offer tubal implants as an option for permanent sterilization?
Linda D. Bradley, MD: The FDA-approved revised labeling for the Essure system means that physicians should have a very detailed, in-depth conversation with their patients who are contemplating hysteroscopic tubal insert placement for permanent sterilization. This counseling really should not differ from what doctors were doing before the label was revised. However, physicians can now use the new Patient-Doctor Discussion Checklist as a guide in reviewing the benefits of the device, its known risks and potential risks, outcomes of the insertion procedure, and the possible need for future surgical intervention if device placement–related issues arise.
For clinicians, this counseling adds just a few more minutes to the visit. The Patient-Doctor Discussion Checklist will become an inherent part of the informed consent process, aiding in the review of the device’s benefits, potential risks, and more importantly its permanence.
In the past, there was some concern that perhaps patients did not receive enough guidance for informed consent, so one of the first things listed on the checklist is confirmation—in the form of a printed line where the patient can sign her initials—that she understands that Essure is a permanent form of birth control. The checklist covers additional important issues, including that the doctor has indeed shared with the patient other options for birth control or sterilization, such as laparoscopic sterilization, vasectomy for her male partner, an intrauterine device (IUD), and birth control pills. This is an opportunity to reinforce the fact that tubal implants are a permanent form of birth control, and if the patient is uncertain about ending her fertility, the clinician can inform her about reversible options. The checklist also includes for discussion the pregnancy risk with use of the device, what the patient can expect during the implant insertion procedure and for the days afterwards (such as cramping, mild to moderate pain, nausea and vomiting), and the need for a confirmation test 3 months after device placement.
Other discussion points covered include long-term risks and benefits of the device, the potential for complications, and the possibility (due to pelvic pain) that the hysteroscopically placed devices may need to be removed with a surgical procedure requiring general anesthesia.
Incorporating the checklist into our clinical practice shows that we have listened to patients and complied with recommendations made by the FDA review panel, and we can use this document to have a more complete discussion with our patients.
OBG Management: Do you agree with some clinicians who say that physicians who place the device also should have the skills required to remove it if necessary?
Dr. Bradley: Essure placement—which is a hysteroscopic procedure—is done very differently than a laparoscopic procedure. In the past, among women who needed to have the Essure system removed, most procedures would be done laparoscopically. Since we work collaboratively in teams, someone within the team or division would have the clinical expertise to remove the devices. An ObGyn who does laparoscopy with salpingectomy and/or cornual resection would best be able to remove the devices.
The clinician who does hysteroscopy is not always the same one who does laparoscopy. Someone within the division who is interested in removing the device will develop an expertise and algorithm that suits the practice, so that person in the practice becomes the expert. This is no different from many other things that physicians do. In our clinical practice, for example, we have a pelvic pain specialist, a sexual counselor, someone interested in menopause and management, and someone interested in alternatives to hysterectomies. Those who practice their craft and their art become proficient at it. So if you do not perform a particular procedure such as a tubal implant removal, know the expert to whom you can make a referral.
OBG Management: How do you now advise your colleagues to counsel patients on permanent sterilization?
Dr. Bradley: Hysteroscopic tubal implant sterilization, a minimally invasive procedure, is an excellent and viable option for women who meet the inclusion criteria and who do not have the exclusion criteria for placement. It is overall safe and extremely effective. If a patient has issues after undergoing implant placement—just like with any other surgery or procedure—for example, if she is not feeling better or is not doing as well as anticipated, we must not forget the patient. It is important for our patients to be listened to and to be heard. Postprocedure issues are generally transient and related to pain and discomfort or abnormal bleeding. If they are persistent, then further evaluation is needed.
Tell the patient to contact you if she has questions or issues, and have a tiered approach for working up any problems that she may present with. In addition, reiterate that the patient must use another form of birth control for 3 months until she undergoes the confirmation test and until the results verify that the implants can be relied on for contraception. I am still placing the device. Before I perform the procedure, I speak with my patients—as I did before the checklist was developed—about all of the informed consent issues, the risk−benefit profile, and ruling out contraindications to use. I think this is good medical and surgical practice. The new labeling means we need to have a critical conversation with our patients, and we should be doing that for all procedures.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization: guidance for industry and Food and Drug Administration staff. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM488020.pdf. Published October 31, 2016. Accessed January 5, 2017.
- Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link_Essure_IFU.pdf. Accessed January 5, 2017.
- Essure patient information booklet. http://labeling.bayerhealthcare.com/html/products/pi/essure_pib_en.pdf. Accessed January 5, 2017.
In November 2016, Bayer, the manufacturer of the permanent birth control tubal implant system (Essure), revised the Essure product labeling in accordance with a US Food and Drug Administration (FDA) guidance document.1 The FDA developed its labeling guidance based on its examination of an increasing number of reported adverse events associated with the system’s use (such as persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions) and its evaluation of a trade complaint regarding allegations initially made in a Citizen Petition.
Changes to the new FDA-approved labeling for Essure include:
- the addition of a boxed warning listing adverse events that have been reported either in clinical studies or through postmarket surveillance (see Box)
- updated Instructions for Use document for clinicians and Patient Information Booklet, which contain additional information on safety (contraindications, warnings, and precautions), clinical data, and instructions2,3
- a Patient-Doctor Discussion Checklist (included within the Patient Information Booklet), designed to support appropriate patient counseling, facilitate the patient’s understanding of birth control options, and explain the benefits and risks associated with the device and what to expect during and after the implantation procedure.3
How will these labeling changes impact clinicians and patients? OBG
Reference
1. Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link%20Essure%20IFU.pdf. Accessed January 5, 2017.
OBG Management: What does the new product labeling mean for clinicians who offer tubal implants as an option for permanent sterilization?
Linda D. Bradley, MD: The FDA-approved revised labeling for the Essure system means that physicians should have a very detailed, in-depth conversation with their patients who are contemplating hysteroscopic tubal insert placement for permanent sterilization. This counseling really should not differ from what doctors were doing before the label was revised. However, physicians can now use the new Patient-Doctor Discussion Checklist as a guide in reviewing the benefits of the device, its known risks and potential risks, outcomes of the insertion procedure, and the possible need for future surgical intervention if device placement–related issues arise.
For clinicians, this counseling adds just a few more minutes to the visit. The Patient-Doctor Discussion Checklist will become an inherent part of the informed consent process, aiding in the review of the device’s benefits, potential risks, and more importantly its permanence.
In the past, there was some concern that perhaps patients did not receive enough guidance for informed consent, so one of the first things listed on the checklist is confirmation—in the form of a printed line where the patient can sign her initials—that she understands that Essure is a permanent form of birth control. The checklist covers additional important issues, including that the doctor has indeed shared with the patient other options for birth control or sterilization, such as laparoscopic sterilization, vasectomy for her male partner, an intrauterine device (IUD), and birth control pills. This is an opportunity to reinforce the fact that tubal implants are a permanent form of birth control, and if the patient is uncertain about ending her fertility, the clinician can inform her about reversible options. The checklist also includes for discussion the pregnancy risk with use of the device, what the patient can expect during the implant insertion procedure and for the days afterwards (such as cramping, mild to moderate pain, nausea and vomiting), and the need for a confirmation test 3 months after device placement.
Other discussion points covered include long-term risks and benefits of the device, the potential for complications, and the possibility (due to pelvic pain) that the hysteroscopically placed devices may need to be removed with a surgical procedure requiring general anesthesia.
Incorporating the checklist into our clinical practice shows that we have listened to patients and complied with recommendations made by the FDA review panel, and we can use this document to have a more complete discussion with our patients.
OBG Management: Do you agree with some clinicians who say that physicians who place the device also should have the skills required to remove it if necessary?
Dr. Bradley: Essure placement—which is a hysteroscopic procedure—is done very differently than a laparoscopic procedure. In the past, among women who needed to have the Essure system removed, most procedures would be done laparoscopically. Since we work collaboratively in teams, someone within the team or division would have the clinical expertise to remove the devices. An ObGyn who does laparoscopy with salpingectomy and/or cornual resection would best be able to remove the devices.
The clinician who does hysteroscopy is not always the same one who does laparoscopy. Someone within the division who is interested in removing the device will develop an expertise and algorithm that suits the practice, so that person in the practice becomes the expert. This is no different from many other things that physicians do. In our clinical practice, for example, we have a pelvic pain specialist, a sexual counselor, someone interested in menopause and management, and someone interested in alternatives to hysterectomies. Those who practice their craft and their art become proficient at it. So if you do not perform a particular procedure such as a tubal implant removal, know the expert to whom you can make a referral.
OBG Management: How do you now advise your colleagues to counsel patients on permanent sterilization?
Dr. Bradley: Hysteroscopic tubal implant sterilization, a minimally invasive procedure, is an excellent and viable option for women who meet the inclusion criteria and who do not have the exclusion criteria for placement. It is overall safe and extremely effective. If a patient has issues after undergoing implant placement—just like with any other surgery or procedure—for example, if she is not feeling better or is not doing as well as anticipated, we must not forget the patient. It is important for our patients to be listened to and to be heard. Postprocedure issues are generally transient and related to pain and discomfort or abnormal bleeding. If they are persistent, then further evaluation is needed.
Tell the patient to contact you if she has questions or issues, and have a tiered approach for working up any problems that she may present with. In addition, reiterate that the patient must use another form of birth control for 3 months until she undergoes the confirmation test and until the results verify that the implants can be relied on for contraception. I am still placing the device. Before I perform the procedure, I speak with my patients—as I did before the checklist was developed—about all of the informed consent issues, the risk−benefit profile, and ruling out contraindications to use. I think this is good medical and surgical practice. The new labeling means we need to have a critical conversation with our patients, and we should be doing that for all procedures.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In November 2016, Bayer, the manufacturer of the permanent birth control tubal implant system (Essure), revised the Essure product labeling in accordance with a US Food and Drug Administration (FDA) guidance document.1 The FDA developed its labeling guidance based on its examination of an increasing number of reported adverse events associated with the system’s use (such as persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions) and its evaluation of a trade complaint regarding allegations initially made in a Citizen Petition.
Changes to the new FDA-approved labeling for Essure include:
- the addition of a boxed warning listing adverse events that have been reported either in clinical studies or through postmarket surveillance (see Box)
- updated Instructions for Use document for clinicians and Patient Information Booklet, which contain additional information on safety (contraindications, warnings, and precautions), clinical data, and instructions2,3
- a Patient-Doctor Discussion Checklist (included within the Patient Information Booklet), designed to support appropriate patient counseling, facilitate the patient’s understanding of birth control options, and explain the benefits and risks associated with the device and what to expect during and after the implantation procedure.3
How will these labeling changes impact clinicians and patients? OBG
Reference
1. Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link%20Essure%20IFU.pdf. Accessed January 5, 2017.
OBG Management: What does the new product labeling mean for clinicians who offer tubal implants as an option for permanent sterilization?
Linda D. Bradley, MD: The FDA-approved revised labeling for the Essure system means that physicians should have a very detailed, in-depth conversation with their patients who are contemplating hysteroscopic tubal insert placement for permanent sterilization. This counseling really should not differ from what doctors were doing before the label was revised. However, physicians can now use the new Patient-Doctor Discussion Checklist as a guide in reviewing the benefits of the device, its known risks and potential risks, outcomes of the insertion procedure, and the possible need for future surgical intervention if device placement–related issues arise.
For clinicians, this counseling adds just a few more minutes to the visit. The Patient-Doctor Discussion Checklist will become an inherent part of the informed consent process, aiding in the review of the device’s benefits, potential risks, and more importantly its permanence.
In the past, there was some concern that perhaps patients did not receive enough guidance for informed consent, so one of the first things listed on the checklist is confirmation—in the form of a printed line where the patient can sign her initials—that she understands that Essure is a permanent form of birth control. The checklist covers additional important issues, including that the doctor has indeed shared with the patient other options for birth control or sterilization, such as laparoscopic sterilization, vasectomy for her male partner, an intrauterine device (IUD), and birth control pills. This is an opportunity to reinforce the fact that tubal implants are a permanent form of birth control, and if the patient is uncertain about ending her fertility, the clinician can inform her about reversible options. The checklist also includes for discussion the pregnancy risk with use of the device, what the patient can expect during the implant insertion procedure and for the days afterwards (such as cramping, mild to moderate pain, nausea and vomiting), and the need for a confirmation test 3 months after device placement.
Other discussion points covered include long-term risks and benefits of the device, the potential for complications, and the possibility (due to pelvic pain) that the hysteroscopically placed devices may need to be removed with a surgical procedure requiring general anesthesia.
Incorporating the checklist into our clinical practice shows that we have listened to patients and complied with recommendations made by the FDA review panel, and we can use this document to have a more complete discussion with our patients.
OBG Management: Do you agree with some clinicians who say that physicians who place the device also should have the skills required to remove it if necessary?
Dr. Bradley: Essure placement—which is a hysteroscopic procedure—is done very differently than a laparoscopic procedure. In the past, among women who needed to have the Essure system removed, most procedures would be done laparoscopically. Since we work collaboratively in teams, someone within the team or division would have the clinical expertise to remove the devices. An ObGyn who does laparoscopy with salpingectomy and/or cornual resection would best be able to remove the devices.
The clinician who does hysteroscopy is not always the same one who does laparoscopy. Someone within the division who is interested in removing the device will develop an expertise and algorithm that suits the practice, so that person in the practice becomes the expert. This is no different from many other things that physicians do. In our clinical practice, for example, we have a pelvic pain specialist, a sexual counselor, someone interested in menopause and management, and someone interested in alternatives to hysterectomies. Those who practice their craft and their art become proficient at it. So if you do not perform a particular procedure such as a tubal implant removal, know the expert to whom you can make a referral.
OBG Management: How do you now advise your colleagues to counsel patients on permanent sterilization?
Dr. Bradley: Hysteroscopic tubal implant sterilization, a minimally invasive procedure, is an excellent and viable option for women who meet the inclusion criteria and who do not have the exclusion criteria for placement. It is overall safe and extremely effective. If a patient has issues after undergoing implant placement—just like with any other surgery or procedure—for example, if she is not feeling better or is not doing as well as anticipated, we must not forget the patient. It is important for our patients to be listened to and to be heard. Postprocedure issues are generally transient and related to pain and discomfort or abnormal bleeding. If they are persistent, then further evaluation is needed.
Tell the patient to contact you if she has questions or issues, and have a tiered approach for working up any problems that she may present with. In addition, reiterate that the patient must use another form of birth control for 3 months until she undergoes the confirmation test and until the results verify that the implants can be relied on for contraception. I am still placing the device. Before I perform the procedure, I speak with my patients—as I did before the checklist was developed—about all of the informed consent issues, the risk−benefit profile, and ruling out contraindications to use. I think this is good medical and surgical practice. The new labeling means we need to have a critical conversation with our patients, and we should be doing that for all procedures.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization: guidance for industry and Food and Drug Administration staff. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM488020.pdf. Published October 31, 2016. Accessed January 5, 2017.
- Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link_Essure_IFU.pdf. Accessed January 5, 2017.
- Essure patient information booklet. http://labeling.bayerhealthcare.com/html/products/pi/essure_pib_en.pdf. Accessed January 5, 2017.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization: guidance for industry and Food and Drug Administration staff. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM488020.pdf. Published October 31, 2016. Accessed January 5, 2017.
- Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link_Essure_IFU.pdf. Accessed January 5, 2017.
- Essure patient information booklet. http://labeling.bayerhealthcare.com/html/products/pi/essure_pib_en.pdf. Accessed January 5, 2017.
2017 Update on fertility
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
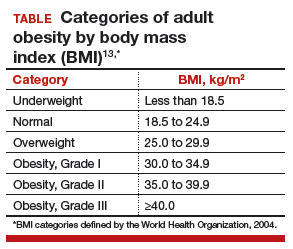
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.
A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
As-needed anticoagulation for intermittent Afib raises concerns
ORLANDO – A pilot study that suggested as-needed anticoagulation could be effective in preventing stroke in at least some patients after successful ablation of atrial fibrillation (AF) was received with caution at the annual International AF Symposium.
The positive findings, originally reported at the 2016 annual meeting of the Heart Rhythm Society (HRS), were updated at AF Symposium 2017 by Francis Marchlinski, MD, director of cardiac electrophysiology at the University of Pennsylvania. When delivering the data, he provided several caveats before other AF experts added their own.
The study was conducted in response to the substantial number of patients who request discontinuing anticoagulation therapy after a successful ablation for atrial fibrillation, according to Dr. Marchlinski. Current guidelines recommend anticoagulation in AF patients following ablation if they have risk factors for stroke even if their AF is controlled. However, according to Dr. Marchlinski, who cited five observational studies, the risk of stroke in patients with a negative electrocardiogram after ablation appears to be “in the neighborhood of 0.1%.”
“There are no randomized prospective trials that have assessed the safety of stopping anticoagulants, but the fact is that this is a pretty low event rate if the observational studies are accurate, and even if they are off by severalfold, it is likely that we would be unable to show the benefit of continuing anticoagulants in these patients,” Dr. Marchlinski observed.
A strategy of as-needed anticoagulants has been made practical by the introduction of novel oral anticoagulants (NOACs), which have a rapid onset of action relative to warfarin and would, therefore, be expected to provide rapid protection against AF-related stroke risk if initiated upon AF onset, according to Dr. Marchlinski. To test this approach, 105 “highly motivated” AF patients were selected for the pilot study.
In addition to 3 weeks of ECG monitoring to confirm the absence of AF, patients participating in the trial were required to demonstrate skill in pulse assessment, which they agreed to perform on a twice-daily basis. Use of a smartphone app that can detect AF was encouraged but not required. All patients were required to fill a prescription for a NOAC and told to initiate therapy for any AF episode of more than 1 hour.
Of the 105 patients, four were noncompliant with AF monitoring and removed from the study. Another two patients voluntarily requested to return to daily NOAC treatment. The remaining 99 were followed for 30 months. Of these, 18 had multiple episodes of AF and were transitioned back to daily NOAC therapy, 15 used NOAC on an as-needed basis at least once but remained off daily therapy, and the remaining 66 did not have an episode of AF that triggered a course of NOAC therapy.
In 263 patient years of follow-up, there was a single cerebrovascular accident (CVA). This occurred in an 81-year-old patient with a history of hypertrophic cardiomyopathy and an atherosclerotic aortic arch on imaging. The patient presented with neurologic symptoms but had a negative ECG. The CVA symptoms resolved with treatment.
In presenting these data, Dr. Marchlinski said, “PRN use of NOACs may be safe and effective to maintain a low risk of stroke when patients are adherent to diligent pulse monitoring.” However, he reiterated that the study group consisted of “a select group of motivated patients,” and he emphasized the patients must be followed closely.
In a discussion that followed this presentation, several experts expressed the usual caution about drawing conclusions from a single uncontrolled study, but Elaine M. Hylek, MD, professor of medicine, Boston University, added additional reservations to the “pill in a pocket” strategy. In particular, she noted an imperfect correlation between onset of AF and stroke risk. “I think this makes us [reluctant] to stop oral anticoagulation,” she said.
According to Daniel Singer, MD, chief of epidemiology, Harvard School of Public Health, Boston, the available data suggest that “once the AF is gone, the risk of stroke recedes,” but he indicated that all the variables of risk may not be fully understood. He said more “hard data” are needed to endorse a wider application of on-demand anticoagulation in patients like those entered into this study.
The fact that patients without AF following ablation remain at substantial risk of AF recurrences, including asymptomatic episodes, is a liability of as-needed anticoagulation, conceded Dr. Marchlinski. However, these initial results provide promise for the substantial proportion of patients without AF after ablation that wish to avoid anticoagulants and are willing to consider risks and benefits.
Dr. Marchlinski reports financial relationships with Abbott, Biosense Webster, Biotronik, Boston Scientific, St. Jude Medical, and Medtronic.
ORLANDO – A pilot study that suggested as-needed anticoagulation could be effective in preventing stroke in at least some patients after successful ablation of atrial fibrillation (AF) was received with caution at the annual International AF Symposium.
The positive findings, originally reported at the 2016 annual meeting of the Heart Rhythm Society (HRS), were updated at AF Symposium 2017 by Francis Marchlinski, MD, director of cardiac electrophysiology at the University of Pennsylvania. When delivering the data, he provided several caveats before other AF experts added their own.
The study was conducted in response to the substantial number of patients who request discontinuing anticoagulation therapy after a successful ablation for atrial fibrillation, according to Dr. Marchlinski. Current guidelines recommend anticoagulation in AF patients following ablation if they have risk factors for stroke even if their AF is controlled. However, according to Dr. Marchlinski, who cited five observational studies, the risk of stroke in patients with a negative electrocardiogram after ablation appears to be “in the neighborhood of 0.1%.”
“There are no randomized prospective trials that have assessed the safety of stopping anticoagulants, but the fact is that this is a pretty low event rate if the observational studies are accurate, and even if they are off by severalfold, it is likely that we would be unable to show the benefit of continuing anticoagulants in these patients,” Dr. Marchlinski observed.
A strategy of as-needed anticoagulants has been made practical by the introduction of novel oral anticoagulants (NOACs), which have a rapid onset of action relative to warfarin and would, therefore, be expected to provide rapid protection against AF-related stroke risk if initiated upon AF onset, according to Dr. Marchlinski. To test this approach, 105 “highly motivated” AF patients were selected for the pilot study.
In addition to 3 weeks of ECG monitoring to confirm the absence of AF, patients participating in the trial were required to demonstrate skill in pulse assessment, which they agreed to perform on a twice-daily basis. Use of a smartphone app that can detect AF was encouraged but not required. All patients were required to fill a prescription for a NOAC and told to initiate therapy for any AF episode of more than 1 hour.
Of the 105 patients, four were noncompliant with AF monitoring and removed from the study. Another two patients voluntarily requested to return to daily NOAC treatment. The remaining 99 were followed for 30 months. Of these, 18 had multiple episodes of AF and were transitioned back to daily NOAC therapy, 15 used NOAC on an as-needed basis at least once but remained off daily therapy, and the remaining 66 did not have an episode of AF that triggered a course of NOAC therapy.
In 263 patient years of follow-up, there was a single cerebrovascular accident (CVA). This occurred in an 81-year-old patient with a history of hypertrophic cardiomyopathy and an atherosclerotic aortic arch on imaging. The patient presented with neurologic symptoms but had a negative ECG. The CVA symptoms resolved with treatment.
In presenting these data, Dr. Marchlinski said, “PRN use of NOACs may be safe and effective to maintain a low risk of stroke when patients are adherent to diligent pulse monitoring.” However, he reiterated that the study group consisted of “a select group of motivated patients,” and he emphasized the patients must be followed closely.
In a discussion that followed this presentation, several experts expressed the usual caution about drawing conclusions from a single uncontrolled study, but Elaine M. Hylek, MD, professor of medicine, Boston University, added additional reservations to the “pill in a pocket” strategy. In particular, she noted an imperfect correlation between onset of AF and stroke risk. “I think this makes us [reluctant] to stop oral anticoagulation,” she said.
According to Daniel Singer, MD, chief of epidemiology, Harvard School of Public Health, Boston, the available data suggest that “once the AF is gone, the risk of stroke recedes,” but he indicated that all the variables of risk may not be fully understood. He said more “hard data” are needed to endorse a wider application of on-demand anticoagulation in patients like those entered into this study.
The fact that patients without AF following ablation remain at substantial risk of AF recurrences, including asymptomatic episodes, is a liability of as-needed anticoagulation, conceded Dr. Marchlinski. However, these initial results provide promise for the substantial proportion of patients without AF after ablation that wish to avoid anticoagulants and are willing to consider risks and benefits.
Dr. Marchlinski reports financial relationships with Abbott, Biosense Webster, Biotronik, Boston Scientific, St. Jude Medical, and Medtronic.
ORLANDO – A pilot study that suggested as-needed anticoagulation could be effective in preventing stroke in at least some patients after successful ablation of atrial fibrillation (AF) was received with caution at the annual International AF Symposium.
The positive findings, originally reported at the 2016 annual meeting of the Heart Rhythm Society (HRS), were updated at AF Symposium 2017 by Francis Marchlinski, MD, director of cardiac electrophysiology at the University of Pennsylvania. When delivering the data, he provided several caveats before other AF experts added their own.
The study was conducted in response to the substantial number of patients who request discontinuing anticoagulation therapy after a successful ablation for atrial fibrillation, according to Dr. Marchlinski. Current guidelines recommend anticoagulation in AF patients following ablation if they have risk factors for stroke even if their AF is controlled. However, according to Dr. Marchlinski, who cited five observational studies, the risk of stroke in patients with a negative electrocardiogram after ablation appears to be “in the neighborhood of 0.1%.”
“There are no randomized prospective trials that have assessed the safety of stopping anticoagulants, but the fact is that this is a pretty low event rate if the observational studies are accurate, and even if they are off by severalfold, it is likely that we would be unable to show the benefit of continuing anticoagulants in these patients,” Dr. Marchlinski observed.
A strategy of as-needed anticoagulants has been made practical by the introduction of novel oral anticoagulants (NOACs), which have a rapid onset of action relative to warfarin and would, therefore, be expected to provide rapid protection against AF-related stroke risk if initiated upon AF onset, according to Dr. Marchlinski. To test this approach, 105 “highly motivated” AF patients were selected for the pilot study.
In addition to 3 weeks of ECG monitoring to confirm the absence of AF, patients participating in the trial were required to demonstrate skill in pulse assessment, which they agreed to perform on a twice-daily basis. Use of a smartphone app that can detect AF was encouraged but not required. All patients were required to fill a prescription for a NOAC and told to initiate therapy for any AF episode of more than 1 hour.
Of the 105 patients, four were noncompliant with AF monitoring and removed from the study. Another two patients voluntarily requested to return to daily NOAC treatment. The remaining 99 were followed for 30 months. Of these, 18 had multiple episodes of AF and were transitioned back to daily NOAC therapy, 15 used NOAC on an as-needed basis at least once but remained off daily therapy, and the remaining 66 did not have an episode of AF that triggered a course of NOAC therapy.
In 263 patient years of follow-up, there was a single cerebrovascular accident (CVA). This occurred in an 81-year-old patient with a history of hypertrophic cardiomyopathy and an atherosclerotic aortic arch on imaging. The patient presented with neurologic symptoms but had a negative ECG. The CVA symptoms resolved with treatment.
In presenting these data, Dr. Marchlinski said, “PRN use of NOACs may be safe and effective to maintain a low risk of stroke when patients are adherent to diligent pulse monitoring.” However, he reiterated that the study group consisted of “a select group of motivated patients,” and he emphasized the patients must be followed closely.
In a discussion that followed this presentation, several experts expressed the usual caution about drawing conclusions from a single uncontrolled study, but Elaine M. Hylek, MD, professor of medicine, Boston University, added additional reservations to the “pill in a pocket” strategy. In particular, she noted an imperfect correlation between onset of AF and stroke risk. “I think this makes us [reluctant] to stop oral anticoagulation,” she said.
According to Daniel Singer, MD, chief of epidemiology, Harvard School of Public Health, Boston, the available data suggest that “once the AF is gone, the risk of stroke recedes,” but he indicated that all the variables of risk may not be fully understood. He said more “hard data” are needed to endorse a wider application of on-demand anticoagulation in patients like those entered into this study.
The fact that patients without AF following ablation remain at substantial risk of AF recurrences, including asymptomatic episodes, is a liability of as-needed anticoagulation, conceded Dr. Marchlinski. However, these initial results provide promise for the substantial proportion of patients without AF after ablation that wish to avoid anticoagulants and are willing to consider risks and benefits.
Dr. Marchlinski reports financial relationships with Abbott, Biosense Webster, Biotronik, Boston Scientific, St. Jude Medical, and Medtronic.
Key clinical point: Despite a positive pilot study, the efficacy of intermittent anticoagulation for stroke prevention after ablation for atrial fibrillation will be difficult to validate in a definitive fashion.
Major finding: Sixty-six percent of atrial fibrillation patients entirely avoided anticoagulation over 30 months of follow-up, but there are at least theoretical concerns.
Data source: A prospective, nonrandomized study.
Disclosures: Dr. Marchlinski reported financial relationships with Abbott, Biosense Webster, Biotronik, Boston Scientific, St. Jude Medical, and Medtronic.
Promoting mental well-being in LGBTQ youth
For many the beginning of a new year is a time to set goals and resolutions for the upcoming year. Often these resolutions are related to health, for example, quit smoking, work out more, lose weight. It is sometimes easy to overlook mental health and well-being as an integral part of overall wellness. This month’s column will focus on how as pediatric providers we can help promote the mental well-being of our patients in practice.
Mental health problems are a significant cause of morbidity and mortality in youth. In 2014, suicide was the second leading cause of death for all youth 10-14 years and 15-24 years.1 While most lesbian, gay, bisexual, transgender, and questioning (LGBTQ) persons live healthy, happy lives, LGBTQ youth are at disproportionate risk for mental illness, probably related to lack of support and to stigma related to their sexual minority and gender minority identities. Studies suggest that LGBTQ youth have suicidality rates two to five times higher than their heterosexual cisgender peers.2,3,4
• Principle 1. A comprehensive diagnostic evaluation should include an age-appropriate assessment of psychosexual development for all youths.
While pediatric providers are unlikely to perform a comprehensive mental health diagnostic evaluation, psychosocial development should regularly be assessed at well visits. It may not be readily apparent which youth are struggling with development of their sexual and gender identity. Nonassuming questions regarding development in theses domains should ideally be integrated into the psychosocial assessment. For example, begin a sexual history by asking, “Are you romantically attracted to males, females, both, or neither?”
• Principle 2. The need for confidentiality in the clinical alliance is a special consideration in the assessment of sexual and gender minority youth.
Confidentiality is important when talking to any youth about their sexual and gender identity. LGBTQ youth in particular may have concerns of family or provider rejection, and they may look for cues that they can safely discuss their sexuality or gender identity without fear of being judged or shamed. Clinicians should be aware of confidentiality practices for minors when discussing these issues. Potential risks of premature disclosure to family and support systems, such as rejection or alienation, also should be considered.
• Principle 3. Family dynamics pertinent to sexual orientation, gender nonconformity, and gender identity should be explored in the context of the cultural values of the youth, family, and community.
Families can have a variety of responses to their child’s sexual minority or gender minority identity, ranging from acceptance to rejection, with some youth being forced to leave home. Many families need to alter their ideas and expectations for a child after their child comes out, and this can lead to feelings of loss and grief accompanied by feelings of anxiety, anger, shame, and guilt.5 Over time, however, the majority of families become affirming and supportive and are not distressed.7 Recognizing that family support reduces negative health outcomes for youth, providers should aim to support and preserve positive family relationships when possible. This may involve education and support for families as well as youth. It is important to be aware that sexual and gender minority youth who are also members of ethnic minorities may face additional challenges.
• Principle 4. Clinicians should inquire about circumstances commonly encountered by youth with sexual and gender minority status that confer increased psychiatric risk.
Providers should recognize that LGBTQ youth are at disproportionate risk of bullying, suicide, substance use, high-risk sexual behaviors, running away, and becoming homeless. Providers should assess for these risks and address them as appropriate.
• Principle 5. Clinicians should aim to foster healthy psychosexual development in sexual and gender minority youth, and protect these individuals’ full capacity for integrated identity formation and functioning.
Providers should support healthy youth development and self-discovery, recognizing that there is a spectrum of sexual and gender identities, with the goal of helping youth achieve their full developmental potential.
• Principle 6. Clinicians should be aware that there is no evidence that sexual orientation can be altered through therapy, and attempts to do so may be harmful.
Therapies targeted at altering sexual orientation or gender identity, often referred to as reparative therapies, can encourage family rejection and decrease self-esteem and connectedness, all of which have been identified as risk factors for suicidality. Providers should educate parents about the potential harm of these types of therapies and ensure that mental health providers to whom patients are being referred are not practicing these potentially harmful therapies.
• Principle 7. Clinicians should be aware of current evidence on the natural course of gender discordance and associated psychopathology in children and adolescents in choosing the treatment goals and modality.
Variation in gender role behavior (for example, dress preference, toy preference, types of play) is typical in early childhood and should be distinguished from gender dysphoria, in which a child expresses distress related to a gender identity that is different from or does not fully align with the child’s sex assigned at birth. Assessing gender development in childhood and the best approach to treatment is best done by professionals with experience and training in gender development, and providers should be familiar with resources in their area. For some, gender identity concerns may not be recognized until adolescence when the onset of puberty and secondary sex characteristics result in increased dysphoria. Best practice guidelines exist for treatment of youth with gender discordance, and there is limited but growing evidence to support best practices. Providers should ensure that the providers and specialists to whom families are referred practice according to current best practices.
• Principle 8. Clinicians should be prepared to consult and act as a liaison with schools, community agencies, and other health care providers, advocating for the unique needs of sexual and gender minority youth and their families.
Pediatric providers can work with mental health professionals to be advocates for their gender and sexual minority patients and raise awareness of issues affecting these special populations such as bullying and suicidality.
• Principle 9. Mental health professionals should be aware of community and professional resources relevant to sexual and gender minority youth.
As medical providers, we have a limited amount of time to see and assess patients, and often are able to best serve our patients and families by connecting them to specialists in the medical community and resources available in the school and community. It is important to know what resources exist in the community to be able to appropriately refer and connect patients.
Resources for providers
• American Academy of Child and Adolescent Psychiatry Practice Parameter on lesbian, gay, bisexual, and transgender youth .
• National LGBT Health Education Center: Training materials and modules with continuing education credits.
Resources for families
• Gay, Lesbian, and Straight Education Network.• Parents, Friends, Families of Lesbians and Gays (PFLAG).
References
1. “10 Leading Causes of Death by Age Group, United States – 2014,” National Center for Injury Prevention and Control, Centers for Disease Control and Prevention.
2. Lesbian, Gay, Bisexual, and Transgender Health: LGBT Youth, Centers for Disease Control and Prevention, Nov. 12, 2014.
3. Am J Public Health. 2001 Aug;91(8):1276-81.
4. Am J Prev Med. 2012 Mar;42(3):221-8.
5. J. Am Acad Child Adolesc Psychiatry. 2012;51(9):957–74.
6. Pediatr Clin North Am. 2016 Dec;63(6):971-83.
7. “Mom, Dad. I’m Gay: How Families Negotiate Coming Out” (Washington, DC: American Psychological Association, 2001).
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
For many the beginning of a new year is a time to set goals and resolutions for the upcoming year. Often these resolutions are related to health, for example, quit smoking, work out more, lose weight. It is sometimes easy to overlook mental health and well-being as an integral part of overall wellness. This month’s column will focus on how as pediatric providers we can help promote the mental well-being of our patients in practice.
Mental health problems are a significant cause of morbidity and mortality in youth. In 2014, suicide was the second leading cause of death for all youth 10-14 years and 15-24 years.1 While most lesbian, gay, bisexual, transgender, and questioning (LGBTQ) persons live healthy, happy lives, LGBTQ youth are at disproportionate risk for mental illness, probably related to lack of support and to stigma related to their sexual minority and gender minority identities. Studies suggest that LGBTQ youth have suicidality rates two to five times higher than their heterosexual cisgender peers.2,3,4
• Principle 1. A comprehensive diagnostic evaluation should include an age-appropriate assessment of psychosexual development for all youths.
While pediatric providers are unlikely to perform a comprehensive mental health diagnostic evaluation, psychosocial development should regularly be assessed at well visits. It may not be readily apparent which youth are struggling with development of their sexual and gender identity. Nonassuming questions regarding development in theses domains should ideally be integrated into the psychosocial assessment. For example, begin a sexual history by asking, “Are you romantically attracted to males, females, both, or neither?”
• Principle 2. The need for confidentiality in the clinical alliance is a special consideration in the assessment of sexual and gender minority youth.
Confidentiality is important when talking to any youth about their sexual and gender identity. LGBTQ youth in particular may have concerns of family or provider rejection, and they may look for cues that they can safely discuss their sexuality or gender identity without fear of being judged or shamed. Clinicians should be aware of confidentiality practices for minors when discussing these issues. Potential risks of premature disclosure to family and support systems, such as rejection or alienation, also should be considered.
• Principle 3. Family dynamics pertinent to sexual orientation, gender nonconformity, and gender identity should be explored in the context of the cultural values of the youth, family, and community.
Families can have a variety of responses to their child’s sexual minority or gender minority identity, ranging from acceptance to rejection, with some youth being forced to leave home. Many families need to alter their ideas and expectations for a child after their child comes out, and this can lead to feelings of loss and grief accompanied by feelings of anxiety, anger, shame, and guilt.5 Over time, however, the majority of families become affirming and supportive and are not distressed.7 Recognizing that family support reduces negative health outcomes for youth, providers should aim to support and preserve positive family relationships when possible. This may involve education and support for families as well as youth. It is important to be aware that sexual and gender minority youth who are also members of ethnic minorities may face additional challenges.
• Principle 4. Clinicians should inquire about circumstances commonly encountered by youth with sexual and gender minority status that confer increased psychiatric risk.
Providers should recognize that LGBTQ youth are at disproportionate risk of bullying, suicide, substance use, high-risk sexual behaviors, running away, and becoming homeless. Providers should assess for these risks and address them as appropriate.
• Principle 5. Clinicians should aim to foster healthy psychosexual development in sexual and gender minority youth, and protect these individuals’ full capacity for integrated identity formation and functioning.
Providers should support healthy youth development and self-discovery, recognizing that there is a spectrum of sexual and gender identities, with the goal of helping youth achieve their full developmental potential.
• Principle 6. Clinicians should be aware that there is no evidence that sexual orientation can be altered through therapy, and attempts to do so may be harmful.
Therapies targeted at altering sexual orientation or gender identity, often referred to as reparative therapies, can encourage family rejection and decrease self-esteem and connectedness, all of which have been identified as risk factors for suicidality. Providers should educate parents about the potential harm of these types of therapies and ensure that mental health providers to whom patients are being referred are not practicing these potentially harmful therapies.
• Principle 7. Clinicians should be aware of current evidence on the natural course of gender discordance and associated psychopathology in children and adolescents in choosing the treatment goals and modality.
Variation in gender role behavior (for example, dress preference, toy preference, types of play) is typical in early childhood and should be distinguished from gender dysphoria, in which a child expresses distress related to a gender identity that is different from or does not fully align with the child’s sex assigned at birth. Assessing gender development in childhood and the best approach to treatment is best done by professionals with experience and training in gender development, and providers should be familiar with resources in their area. For some, gender identity concerns may not be recognized until adolescence when the onset of puberty and secondary sex characteristics result in increased dysphoria. Best practice guidelines exist for treatment of youth with gender discordance, and there is limited but growing evidence to support best practices. Providers should ensure that the providers and specialists to whom families are referred practice according to current best practices.
• Principle 8. Clinicians should be prepared to consult and act as a liaison with schools, community agencies, and other health care providers, advocating for the unique needs of sexual and gender minority youth and their families.
Pediatric providers can work with mental health professionals to be advocates for their gender and sexual minority patients and raise awareness of issues affecting these special populations such as bullying and suicidality.
• Principle 9. Mental health professionals should be aware of community and professional resources relevant to sexual and gender minority youth.
As medical providers, we have a limited amount of time to see and assess patients, and often are able to best serve our patients and families by connecting them to specialists in the medical community and resources available in the school and community. It is important to know what resources exist in the community to be able to appropriately refer and connect patients.
Resources for providers
• American Academy of Child and Adolescent Psychiatry Practice Parameter on lesbian, gay, bisexual, and transgender youth .
• National LGBT Health Education Center: Training materials and modules with continuing education credits.
Resources for families
• Gay, Lesbian, and Straight Education Network.• Parents, Friends, Families of Lesbians and Gays (PFLAG).
References
1. “10 Leading Causes of Death by Age Group, United States – 2014,” National Center for Injury Prevention and Control, Centers for Disease Control and Prevention.
2. Lesbian, Gay, Bisexual, and Transgender Health: LGBT Youth, Centers for Disease Control and Prevention, Nov. 12, 2014.
3. Am J Public Health. 2001 Aug;91(8):1276-81.
4. Am J Prev Med. 2012 Mar;42(3):221-8.
5. J. Am Acad Child Adolesc Psychiatry. 2012;51(9):957–74.
6. Pediatr Clin North Am. 2016 Dec;63(6):971-83.
7. “Mom, Dad. I’m Gay: How Families Negotiate Coming Out” (Washington, DC: American Psychological Association, 2001).
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
For many the beginning of a new year is a time to set goals and resolutions for the upcoming year. Often these resolutions are related to health, for example, quit smoking, work out more, lose weight. It is sometimes easy to overlook mental health and well-being as an integral part of overall wellness. This month’s column will focus on how as pediatric providers we can help promote the mental well-being of our patients in practice.
Mental health problems are a significant cause of morbidity and mortality in youth. In 2014, suicide was the second leading cause of death for all youth 10-14 years and 15-24 years.1 While most lesbian, gay, bisexual, transgender, and questioning (LGBTQ) persons live healthy, happy lives, LGBTQ youth are at disproportionate risk for mental illness, probably related to lack of support and to stigma related to their sexual minority and gender minority identities. Studies suggest that LGBTQ youth have suicidality rates two to five times higher than their heterosexual cisgender peers.2,3,4
• Principle 1. A comprehensive diagnostic evaluation should include an age-appropriate assessment of psychosexual development for all youths.
While pediatric providers are unlikely to perform a comprehensive mental health diagnostic evaluation, psychosocial development should regularly be assessed at well visits. It may not be readily apparent which youth are struggling with development of their sexual and gender identity. Nonassuming questions regarding development in theses domains should ideally be integrated into the psychosocial assessment. For example, begin a sexual history by asking, “Are you romantically attracted to males, females, both, or neither?”
• Principle 2. The need for confidentiality in the clinical alliance is a special consideration in the assessment of sexual and gender minority youth.
Confidentiality is important when talking to any youth about their sexual and gender identity. LGBTQ youth in particular may have concerns of family or provider rejection, and they may look for cues that they can safely discuss their sexuality or gender identity without fear of being judged or shamed. Clinicians should be aware of confidentiality practices for minors when discussing these issues. Potential risks of premature disclosure to family and support systems, such as rejection or alienation, also should be considered.
• Principle 3. Family dynamics pertinent to sexual orientation, gender nonconformity, and gender identity should be explored in the context of the cultural values of the youth, family, and community.
Families can have a variety of responses to their child’s sexual minority or gender minority identity, ranging from acceptance to rejection, with some youth being forced to leave home. Many families need to alter their ideas and expectations for a child after their child comes out, and this can lead to feelings of loss and grief accompanied by feelings of anxiety, anger, shame, and guilt.5 Over time, however, the majority of families become affirming and supportive and are not distressed.7 Recognizing that family support reduces negative health outcomes for youth, providers should aim to support and preserve positive family relationships when possible. This may involve education and support for families as well as youth. It is important to be aware that sexual and gender minority youth who are also members of ethnic minorities may face additional challenges.
• Principle 4. Clinicians should inquire about circumstances commonly encountered by youth with sexual and gender minority status that confer increased psychiatric risk.
Providers should recognize that LGBTQ youth are at disproportionate risk of bullying, suicide, substance use, high-risk sexual behaviors, running away, and becoming homeless. Providers should assess for these risks and address them as appropriate.
• Principle 5. Clinicians should aim to foster healthy psychosexual development in sexual and gender minority youth, and protect these individuals’ full capacity for integrated identity formation and functioning.
Providers should support healthy youth development and self-discovery, recognizing that there is a spectrum of sexual and gender identities, with the goal of helping youth achieve their full developmental potential.
• Principle 6. Clinicians should be aware that there is no evidence that sexual orientation can be altered through therapy, and attempts to do so may be harmful.
Therapies targeted at altering sexual orientation or gender identity, often referred to as reparative therapies, can encourage family rejection and decrease self-esteem and connectedness, all of which have been identified as risk factors for suicidality. Providers should educate parents about the potential harm of these types of therapies and ensure that mental health providers to whom patients are being referred are not practicing these potentially harmful therapies.
• Principle 7. Clinicians should be aware of current evidence on the natural course of gender discordance and associated psychopathology in children and adolescents in choosing the treatment goals and modality.
Variation in gender role behavior (for example, dress preference, toy preference, types of play) is typical in early childhood and should be distinguished from gender dysphoria, in which a child expresses distress related to a gender identity that is different from or does not fully align with the child’s sex assigned at birth. Assessing gender development in childhood and the best approach to treatment is best done by professionals with experience and training in gender development, and providers should be familiar with resources in their area. For some, gender identity concerns may not be recognized until adolescence when the onset of puberty and secondary sex characteristics result in increased dysphoria. Best practice guidelines exist for treatment of youth with gender discordance, and there is limited but growing evidence to support best practices. Providers should ensure that the providers and specialists to whom families are referred practice according to current best practices.
• Principle 8. Clinicians should be prepared to consult and act as a liaison with schools, community agencies, and other health care providers, advocating for the unique needs of sexual and gender minority youth and their families.
Pediatric providers can work with mental health professionals to be advocates for their gender and sexual minority patients and raise awareness of issues affecting these special populations such as bullying and suicidality.
• Principle 9. Mental health professionals should be aware of community and professional resources relevant to sexual and gender minority youth.
As medical providers, we have a limited amount of time to see and assess patients, and often are able to best serve our patients and families by connecting them to specialists in the medical community and resources available in the school and community. It is important to know what resources exist in the community to be able to appropriately refer and connect patients.
Resources for providers
• American Academy of Child and Adolescent Psychiatry Practice Parameter on lesbian, gay, bisexual, and transgender youth .
• National LGBT Health Education Center: Training materials and modules with continuing education credits.
Resources for families
• Gay, Lesbian, and Straight Education Network.• Parents, Friends, Families of Lesbians and Gays (PFLAG).
References
1. “10 Leading Causes of Death by Age Group, United States – 2014,” National Center for Injury Prevention and Control, Centers for Disease Control and Prevention.
2. Lesbian, Gay, Bisexual, and Transgender Health: LGBT Youth, Centers for Disease Control and Prevention, Nov. 12, 2014.
3. Am J Public Health. 2001 Aug;91(8):1276-81.
4. Am J Prev Med. 2012 Mar;42(3):221-8.
5. J. Am Acad Child Adolesc Psychiatry. 2012;51(9):957–74.
6. Pediatr Clin North Am. 2016 Dec;63(6):971-83.
7. “Mom, Dad. I’m Gay: How Families Negotiate Coming Out” (Washington, DC: American Psychological Association, 2001).
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
What lies ahead for women's health? Challenges, and opportunities, as ACOG and US leadership transition
The United States undoubtedly will undergo tremendous change under its new President and Congress. These branches of government are already indicating their determination to rewrite a vast array of health care laws. If carried out, proposals regarding the Affordable Care Act, Medicare, and Medicaid will dramatically alter the landscape of women’s health care and significantly affect ObGyns and their patients.
These shifts are coming as ACOG’s top leadership undergoes its annual transition. In May 2017, we will thank American College of Obstetricians and Gynecologists (ACOG) President Thomas Gellhaus, MD, for his tremendous service and welcome Haywood Brown, MD, as our new President.
With so much uncertainty ahead, I recently asked these 2 leaders for their reflections, predictions, hardest challenges, and plans to help our specialty surmount any new obstacles to move forward with positive initiatives.
What have we faced down in the past year; what faces us now?
Thomas Gellhaus, MD: When I took this position almost 1 year ago, our specialty and our ability to care for patients were challenged on 3 fronts: MACRA, workforce, and politics.
Through the efforts of a united American Medical Association, we saw MACRA, the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act of 2015, replace the sustainable growth rate formula, which would have drastically reduced provider payments under Medicare’s Physician Fee Schedule. With this change, a major challenge was crossed off our list and a major new one added. Now, we must ensure proper enactment of MACRA’s new payment system and be continually vigilant with respect to its implementation.
Second, we must ensure there are enough ObGyns to care for patients nationally, across all areas of the country. We must find new ways to recruit ObGyns and maintain and expand our workforce.
Third, we must stand firm against political interference. The future of our patients, our practices, and our specialty depend on it. Only 2 people are allowed in the exam room—the patient and her ObGyn—and we must close its door to politicians who want to make decisions about the kind of care our patients need and how we should provide it. We must defeat the many state efforts to decree or limit care. Patient’s access to care, including reproductive care, must not be subject to politics.
These challenges can be opportunities. For example, the more we make MACRA work for practicing ObGyns now, the brighter our future will be, as private payers likely will be following suit.

We stand with patients on reproductive rights and access to care
Haywood Brown, MD: We face several challenges. First, when we become ObGyns, we join our patients in taking on the everyday challenges of women’s health care, reproduction, and reproductive rights. These will continue to be major issues for our specialty. In addition, in our continued efforts to address health care disparities, we must take the lead at ACOG as well as enlist other women’s health care providers as partners. Too often, access to high-quality perinatal, cancer, and other women’s health care is determined or influenced by a patient’s race, residence, or income. This must change.
A future for team-based care
Another challenge is to define clinical practice expectations for the newer generations of Fellows. We must impart to Junior Fellows that team-based care will:
- help them see obstetrics and gynecology as an immensely satisfying calling and career rather than as just a job
- contribute to the expansion of care to women in all communities
- allow for the maintenance of a good work–life balance for many years. (See “Steps to address ObGyn work–life balance” on page 30.)
It’s a new Washington. We are poised for new challenges.
Dr. Gellhaus: With the new US President, Congress, governors, and state legislators in office, a new challenge is to maintain the women’s health protections in the Affordable Care Act—particularly insurance coverage for maternity care and preventive and contraceptive services. We must guard against a resurgence in the number of the uninsured and underinsured, including patients whose health insurance is cut off once they become seriously ill or reach their annual or lifetime coverage limit.
We also must be ready to fight the anticipated onslaught of state and federal attempts to limit our patients’ access to reproductive health care. Many of us remember the days of illegal, unsafe abortions and their devastating effects on women’s health. No lawmaker should doubt that having access to contraceptives helps reduce the rates of unwanted pregnancies and abortions, improves maternal and infant outcomes, and decreases health care costs. It’s a win-win!
Groundwork has been laid, and opportunities will surface
Dr. Gellhaus: The best opportunities will emerge from our long-standing history of bipartisan political cooperation in this area, as we work with lawmakers from both parties on issues that benefit ObGyns and their patients. Over the years, ACOG has well positioned itself and developed important long-term relationships with Republican and Democratic lawmakers to focus on women’s health as a central issue. This position will give ACOG a voice in the new federal Administration, in Congress, and in state houses.
We also will have opportunities to educate elected officials, debunk many of the false or mistaken ideas surrounding contraceptive methods, and reinforce the need for politicians to stop interfering with the sacred physician–patient relationship and stay out of our exam rooms.
Our commitment to our patients is strong
Dr. Brown: It is more important than ever for ACOG to affirm its commitment to women’s reproductive choices and their access to contraceptives—especially with respect to programs funded by Medicaid and Title X. In the United States, Medicaid covers 48% of all births, as well as preventive and screening services provided by Planned Parenthood clinics. Over the next years, these programs will be challenged and could fall into jeopardy. We must remind our Fellows that both Title X contraception coverage and abortion choice have been the law of the land for almost 50 years. We cannot allow politicians to return us to an era that was far worse for our patients.
I asked Haywood Brown, MD, the incoming ACOG President as of May 2017, how he views ACOG's role in addressing a main reason for ObGyn burnout--the increasing burden of compliance with required electronic documentation.
Haywood Brown, MD: We must develop new models of care that foster the ability of ObGyns to embrace practicing the depth and breadth of ObGyn long term, and maintain their enthusiasm about providing care to women throughout the life span. Compliance and electronic documentation requirements can enhance patient care by allowing us to better communicate with our practice partners and our patients, improve quality, safety, and patient satisfaction. It can also quickly wear us down.
Burnout can be reduced through shared practice models, development of niches within shared practices, and being creative with better incorporating advanced practice providers into team-based care. I sincerely believe that if we embrace new models of clinical practice, the satisfaction for what we are trained to do will improve and burnout will be less a threat to our specialty over time.
All-In for Advocacy tops presidential initiatives list
Dr. Gellhaus: With political legislative interference increasing across all of medicine, we need a unified, powerful, cohesive voice. Advocacy is at the top of my presidential initiatives list. The voice of our national organization can have a huge impact on maintaining and improving women’s health care in the United States. That is why All-In for Advocacy is vital. This initiative has significantly increased members’ involvement in ACOG. We will harness this power to pass vital legislation to help the women we serve and our specialty.
Addressing health disparities drives career and presidential focus
Dr. Brown: My presidential initiatives are rooted in my career decision to focus on health disparities, particularly race-based health disparities.
Maternal morbidity and mortality and infant mortality are complex issues shrouded in the social determinants of health. Access to care, fragmentation of care, and quality of care are other factors relevant in disparity. There is evidence of an implicit bias in health care delivery.
My telehealth initiative will focus on implementing Levels of Maternal Care (of which there are 4), the National Partnership for Maternal Safety (NPMS), and the Alliance for Innovation on Maternal Health (AIM) as well as redefining healthy pregnancies and postpartum periods as the gateway to women’s long-term health. The February 2015 Levels of Maternal Care Obstetric Care Consensus Statement, jointly issued by ACOG and the Society for Maternal–Fetal Medicine (SMFM),1 proposes a classification system for birth centers, from basic to specialized regional perinatal health care centers. ACOG is working with hospitals throughout the country to designate the Levels of Maternal Care and thereby ensure each patient receives the appropriate level of care.
The AIM program, led by ACOG and funded by the Health Resources and Services Administration (HRSA), reduces obstetric complications by encouraging hospitals to adopt defined evidence-based patient safety measures. The goal is to prevent 100,000 severe labor and delivery complications and 1,000 maternal deaths over 4 years.
- ACOG's Congressional Leadership Conference, The President's Conference. This 3-day conference, held in Washington, DC, connects you with lawmakers on the important issues facing ObGyns. To find out more, access www.acog.org/clc.
- McCain and Gellhaus Fellowships. Spend 2 to 4 weeks as a member of ACOG's Government Affairs team in Washington, DC. To apply, go to www.acog.org/ateam.
- Ob-Gyn PAC. ACOG's political action committee helps elect state and federal candidates who support our specialty. For more information, visit www.obgynpac.org.
- ACOG News. Don't miss these updates on federal and state legislative developments. To sign up, access www.acog.org/advonews.
- Advocacy webpage. All of this information and more can be found at www.acog.org/advocacy!
ACOG is committed to a “big tent” approach
Dr. Gellhaus: Like US citizens, ACOG represents members with many points of view. ACOG can best represent our members’ diversity in the future by remaining the moderate voice, and by opposing federal and state proposals that are inconsistent with facts and science. We need to bring to our membership’s attention the federal and state successes we have had, show how they have helped our patients and our specialty, and make it clear that our successes are due to our bipartisan work over the years, which we have achieved regardless of which political party has been in office.
For ACOG to continue to be a leader in women’s health care and our specialty, we must remain vigilant against political interference in the patient–physician relationship and be ready to counter with science, facts, and evidence.
Compassion and passion lie at the heart of member similarities
Dr. Brown: First and foremost, we ObGyns care about our patients and, regardless of personal politics, most of us understand the reproductive health challenges facing the women of this country as well as our history with respect to those challenges. All of us must be willing to provide counsel to patients when needed, and that counseling must be nonjudgmental. We also must be willing to protect confidentially and to refer patients whose decisions are at odds with our personal views.
ACOG recognizes that we are a melting pot of specialists and subspecialists and that we are all guided by our personal beliefs and values. Choosing to become an exclusive women’s health care physician requires our passion and compassion.
Let us use our collective voice!
Dr. Gellhaus: Our members must realize the power of our collective voice and that we must use it to deliver a unified, cohesive message. We cannot sit on the sidelines and expect others to speak for us. If we are not part of the solution, then we cede our future to others and have no right to complain about the result. Our members need to commit to advocating outside their exam rooms. A good first step is to see how ACOG makes advocacy easy. When thousands of ACOG members contact elected officials about important issues, officials listen.
Dr. Brown: Yes! We all need to get involved in ACOG and in our communities. Together, we will accomplish many important things.
[polldaddy:9703185]
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Menard MK, Kilpatrick S, Saade G, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Levels of maternal care. Am J Obstet Gynecol. 2015;212(3)259–271.
The United States undoubtedly will undergo tremendous change under its new President and Congress. These branches of government are already indicating their determination to rewrite a vast array of health care laws. If carried out, proposals regarding the Affordable Care Act, Medicare, and Medicaid will dramatically alter the landscape of women’s health care and significantly affect ObGyns and their patients.
These shifts are coming as ACOG’s top leadership undergoes its annual transition. In May 2017, we will thank American College of Obstetricians and Gynecologists (ACOG) President Thomas Gellhaus, MD, for his tremendous service and welcome Haywood Brown, MD, as our new President.
With so much uncertainty ahead, I recently asked these 2 leaders for their reflections, predictions, hardest challenges, and plans to help our specialty surmount any new obstacles to move forward with positive initiatives.
What have we faced down in the past year; what faces us now?
Thomas Gellhaus, MD: When I took this position almost 1 year ago, our specialty and our ability to care for patients were challenged on 3 fronts: MACRA, workforce, and politics.
Through the efforts of a united American Medical Association, we saw MACRA, the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act of 2015, replace the sustainable growth rate formula, which would have drastically reduced provider payments under Medicare’s Physician Fee Schedule. With this change, a major challenge was crossed off our list and a major new one added. Now, we must ensure proper enactment of MACRA’s new payment system and be continually vigilant with respect to its implementation.
Second, we must ensure there are enough ObGyns to care for patients nationally, across all areas of the country. We must find new ways to recruit ObGyns and maintain and expand our workforce.
Third, we must stand firm against political interference. The future of our patients, our practices, and our specialty depend on it. Only 2 people are allowed in the exam room—the patient and her ObGyn—and we must close its door to politicians who want to make decisions about the kind of care our patients need and how we should provide it. We must defeat the many state efforts to decree or limit care. Patient’s access to care, including reproductive care, must not be subject to politics.
These challenges can be opportunities. For example, the more we make MACRA work for practicing ObGyns now, the brighter our future will be, as private payers likely will be following suit.

We stand with patients on reproductive rights and access to care
Haywood Brown, MD: We face several challenges. First, when we become ObGyns, we join our patients in taking on the everyday challenges of women’s health care, reproduction, and reproductive rights. These will continue to be major issues for our specialty. In addition, in our continued efforts to address health care disparities, we must take the lead at ACOG as well as enlist other women’s health care providers as partners. Too often, access to high-quality perinatal, cancer, and other women’s health care is determined or influenced by a patient’s race, residence, or income. This must change.
A future for team-based care
Another challenge is to define clinical practice expectations for the newer generations of Fellows. We must impart to Junior Fellows that team-based care will:
- help them see obstetrics and gynecology as an immensely satisfying calling and career rather than as just a job
- contribute to the expansion of care to women in all communities
- allow for the maintenance of a good work–life balance for many years. (See “Steps to address ObGyn work–life balance” on page 30.)
It’s a new Washington. We are poised for new challenges.
Dr. Gellhaus: With the new US President, Congress, governors, and state legislators in office, a new challenge is to maintain the women’s health protections in the Affordable Care Act—particularly insurance coverage for maternity care and preventive and contraceptive services. We must guard against a resurgence in the number of the uninsured and underinsured, including patients whose health insurance is cut off once they become seriously ill or reach their annual or lifetime coverage limit.
We also must be ready to fight the anticipated onslaught of state and federal attempts to limit our patients’ access to reproductive health care. Many of us remember the days of illegal, unsafe abortions and their devastating effects on women’s health. No lawmaker should doubt that having access to contraceptives helps reduce the rates of unwanted pregnancies and abortions, improves maternal and infant outcomes, and decreases health care costs. It’s a win-win!
Groundwork has been laid, and opportunities will surface
Dr. Gellhaus: The best opportunities will emerge from our long-standing history of bipartisan political cooperation in this area, as we work with lawmakers from both parties on issues that benefit ObGyns and their patients. Over the years, ACOG has well positioned itself and developed important long-term relationships with Republican and Democratic lawmakers to focus on women’s health as a central issue. This position will give ACOG a voice in the new federal Administration, in Congress, and in state houses.
We also will have opportunities to educate elected officials, debunk many of the false or mistaken ideas surrounding contraceptive methods, and reinforce the need for politicians to stop interfering with the sacred physician–patient relationship and stay out of our exam rooms.
Our commitment to our patients is strong
Dr. Brown: It is more important than ever for ACOG to affirm its commitment to women’s reproductive choices and their access to contraceptives—especially with respect to programs funded by Medicaid and Title X. In the United States, Medicaid covers 48% of all births, as well as preventive and screening services provided by Planned Parenthood clinics. Over the next years, these programs will be challenged and could fall into jeopardy. We must remind our Fellows that both Title X contraception coverage and abortion choice have been the law of the land for almost 50 years. We cannot allow politicians to return us to an era that was far worse for our patients.
I asked Haywood Brown, MD, the incoming ACOG President as of May 2017, how he views ACOG's role in addressing a main reason for ObGyn burnout--the increasing burden of compliance with required electronic documentation.
Haywood Brown, MD: We must develop new models of care that foster the ability of ObGyns to embrace practicing the depth and breadth of ObGyn long term, and maintain their enthusiasm about providing care to women throughout the life span. Compliance and electronic documentation requirements can enhance patient care by allowing us to better communicate with our practice partners and our patients, improve quality, safety, and patient satisfaction. It can also quickly wear us down.
Burnout can be reduced through shared practice models, development of niches within shared practices, and being creative with better incorporating advanced practice providers into team-based care. I sincerely believe that if we embrace new models of clinical practice, the satisfaction for what we are trained to do will improve and burnout will be less a threat to our specialty over time.
All-In for Advocacy tops presidential initiatives list
Dr. Gellhaus: With political legislative interference increasing across all of medicine, we need a unified, powerful, cohesive voice. Advocacy is at the top of my presidential initiatives list. The voice of our national organization can have a huge impact on maintaining and improving women’s health care in the United States. That is why All-In for Advocacy is vital. This initiative has significantly increased members’ involvement in ACOG. We will harness this power to pass vital legislation to help the women we serve and our specialty.
Addressing health disparities drives career and presidential focus
Dr. Brown: My presidential initiatives are rooted in my career decision to focus on health disparities, particularly race-based health disparities.
Maternal morbidity and mortality and infant mortality are complex issues shrouded in the social determinants of health. Access to care, fragmentation of care, and quality of care are other factors relevant in disparity. There is evidence of an implicit bias in health care delivery.
My telehealth initiative will focus on implementing Levels of Maternal Care (of which there are 4), the National Partnership for Maternal Safety (NPMS), and the Alliance for Innovation on Maternal Health (AIM) as well as redefining healthy pregnancies and postpartum periods as the gateway to women’s long-term health. The February 2015 Levels of Maternal Care Obstetric Care Consensus Statement, jointly issued by ACOG and the Society for Maternal–Fetal Medicine (SMFM),1 proposes a classification system for birth centers, from basic to specialized regional perinatal health care centers. ACOG is working with hospitals throughout the country to designate the Levels of Maternal Care and thereby ensure each patient receives the appropriate level of care.
The AIM program, led by ACOG and funded by the Health Resources and Services Administration (HRSA), reduces obstetric complications by encouraging hospitals to adopt defined evidence-based patient safety measures. The goal is to prevent 100,000 severe labor and delivery complications and 1,000 maternal deaths over 4 years.
- ACOG's Congressional Leadership Conference, The President's Conference. This 3-day conference, held in Washington, DC, connects you with lawmakers on the important issues facing ObGyns. To find out more, access www.acog.org/clc.
- McCain and Gellhaus Fellowships. Spend 2 to 4 weeks as a member of ACOG's Government Affairs team in Washington, DC. To apply, go to www.acog.org/ateam.
- Ob-Gyn PAC. ACOG's political action committee helps elect state and federal candidates who support our specialty. For more information, visit www.obgynpac.org.
- ACOG News. Don't miss these updates on federal and state legislative developments. To sign up, access www.acog.org/advonews.
- Advocacy webpage. All of this information and more can be found at www.acog.org/advocacy!
ACOG is committed to a “big tent” approach
Dr. Gellhaus: Like US citizens, ACOG represents members with many points of view. ACOG can best represent our members’ diversity in the future by remaining the moderate voice, and by opposing federal and state proposals that are inconsistent with facts and science. We need to bring to our membership’s attention the federal and state successes we have had, show how they have helped our patients and our specialty, and make it clear that our successes are due to our bipartisan work over the years, which we have achieved regardless of which political party has been in office.
For ACOG to continue to be a leader in women’s health care and our specialty, we must remain vigilant against political interference in the patient–physician relationship and be ready to counter with science, facts, and evidence.
Compassion and passion lie at the heart of member similarities
Dr. Brown: First and foremost, we ObGyns care about our patients and, regardless of personal politics, most of us understand the reproductive health challenges facing the women of this country as well as our history with respect to those challenges. All of us must be willing to provide counsel to patients when needed, and that counseling must be nonjudgmental. We also must be willing to protect confidentially and to refer patients whose decisions are at odds with our personal views.
ACOG recognizes that we are a melting pot of specialists and subspecialists and that we are all guided by our personal beliefs and values. Choosing to become an exclusive women’s health care physician requires our passion and compassion.
Let us use our collective voice!
Dr. Gellhaus: Our members must realize the power of our collective voice and that we must use it to deliver a unified, cohesive message. We cannot sit on the sidelines and expect others to speak for us. If we are not part of the solution, then we cede our future to others and have no right to complain about the result. Our members need to commit to advocating outside their exam rooms. A good first step is to see how ACOG makes advocacy easy. When thousands of ACOG members contact elected officials about important issues, officials listen.
Dr. Brown: Yes! We all need to get involved in ACOG and in our communities. Together, we will accomplish many important things.
[polldaddy:9703185]
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
The United States undoubtedly will undergo tremendous change under its new President and Congress. These branches of government are already indicating their determination to rewrite a vast array of health care laws. If carried out, proposals regarding the Affordable Care Act, Medicare, and Medicaid will dramatically alter the landscape of women’s health care and significantly affect ObGyns and their patients.
These shifts are coming as ACOG’s top leadership undergoes its annual transition. In May 2017, we will thank American College of Obstetricians and Gynecologists (ACOG) President Thomas Gellhaus, MD, for his tremendous service and welcome Haywood Brown, MD, as our new President.
With so much uncertainty ahead, I recently asked these 2 leaders for their reflections, predictions, hardest challenges, and plans to help our specialty surmount any new obstacles to move forward with positive initiatives.
What have we faced down in the past year; what faces us now?
Thomas Gellhaus, MD: When I took this position almost 1 year ago, our specialty and our ability to care for patients were challenged on 3 fronts: MACRA, workforce, and politics.
Through the efforts of a united American Medical Association, we saw MACRA, the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act of 2015, replace the sustainable growth rate formula, which would have drastically reduced provider payments under Medicare’s Physician Fee Schedule. With this change, a major challenge was crossed off our list and a major new one added. Now, we must ensure proper enactment of MACRA’s new payment system and be continually vigilant with respect to its implementation.
Second, we must ensure there are enough ObGyns to care for patients nationally, across all areas of the country. We must find new ways to recruit ObGyns and maintain and expand our workforce.
Third, we must stand firm against political interference. The future of our patients, our practices, and our specialty depend on it. Only 2 people are allowed in the exam room—the patient and her ObGyn—and we must close its door to politicians who want to make decisions about the kind of care our patients need and how we should provide it. We must defeat the many state efforts to decree or limit care. Patient’s access to care, including reproductive care, must not be subject to politics.
These challenges can be opportunities. For example, the more we make MACRA work for practicing ObGyns now, the brighter our future will be, as private payers likely will be following suit.

We stand with patients on reproductive rights and access to care
Haywood Brown, MD: We face several challenges. First, when we become ObGyns, we join our patients in taking on the everyday challenges of women’s health care, reproduction, and reproductive rights. These will continue to be major issues for our specialty. In addition, in our continued efforts to address health care disparities, we must take the lead at ACOG as well as enlist other women’s health care providers as partners. Too often, access to high-quality perinatal, cancer, and other women’s health care is determined or influenced by a patient’s race, residence, or income. This must change.
A future for team-based care
Another challenge is to define clinical practice expectations for the newer generations of Fellows. We must impart to Junior Fellows that team-based care will:
- help them see obstetrics and gynecology as an immensely satisfying calling and career rather than as just a job
- contribute to the expansion of care to women in all communities
- allow for the maintenance of a good work–life balance for many years. (See “Steps to address ObGyn work–life balance” on page 30.)
It’s a new Washington. We are poised for new challenges.
Dr. Gellhaus: With the new US President, Congress, governors, and state legislators in office, a new challenge is to maintain the women’s health protections in the Affordable Care Act—particularly insurance coverage for maternity care and preventive and contraceptive services. We must guard against a resurgence in the number of the uninsured and underinsured, including patients whose health insurance is cut off once they become seriously ill or reach their annual or lifetime coverage limit.
We also must be ready to fight the anticipated onslaught of state and federal attempts to limit our patients’ access to reproductive health care. Many of us remember the days of illegal, unsafe abortions and their devastating effects on women’s health. No lawmaker should doubt that having access to contraceptives helps reduce the rates of unwanted pregnancies and abortions, improves maternal and infant outcomes, and decreases health care costs. It’s a win-win!
Groundwork has been laid, and opportunities will surface
Dr. Gellhaus: The best opportunities will emerge from our long-standing history of bipartisan political cooperation in this area, as we work with lawmakers from both parties on issues that benefit ObGyns and their patients. Over the years, ACOG has well positioned itself and developed important long-term relationships with Republican and Democratic lawmakers to focus on women’s health as a central issue. This position will give ACOG a voice in the new federal Administration, in Congress, and in state houses.
We also will have opportunities to educate elected officials, debunk many of the false or mistaken ideas surrounding contraceptive methods, and reinforce the need for politicians to stop interfering with the sacred physician–patient relationship and stay out of our exam rooms.
Our commitment to our patients is strong
Dr. Brown: It is more important than ever for ACOG to affirm its commitment to women’s reproductive choices and their access to contraceptives—especially with respect to programs funded by Medicaid and Title X. In the United States, Medicaid covers 48% of all births, as well as preventive and screening services provided by Planned Parenthood clinics. Over the next years, these programs will be challenged and could fall into jeopardy. We must remind our Fellows that both Title X contraception coverage and abortion choice have been the law of the land for almost 50 years. We cannot allow politicians to return us to an era that was far worse for our patients.
I asked Haywood Brown, MD, the incoming ACOG President as of May 2017, how he views ACOG's role in addressing a main reason for ObGyn burnout--the increasing burden of compliance with required electronic documentation.
Haywood Brown, MD: We must develop new models of care that foster the ability of ObGyns to embrace practicing the depth and breadth of ObGyn long term, and maintain their enthusiasm about providing care to women throughout the life span. Compliance and electronic documentation requirements can enhance patient care by allowing us to better communicate with our practice partners and our patients, improve quality, safety, and patient satisfaction. It can also quickly wear us down.
Burnout can be reduced through shared practice models, development of niches within shared practices, and being creative with better incorporating advanced practice providers into team-based care. I sincerely believe that if we embrace new models of clinical practice, the satisfaction for what we are trained to do will improve and burnout will be less a threat to our specialty over time.
All-In for Advocacy tops presidential initiatives list
Dr. Gellhaus: With political legislative interference increasing across all of medicine, we need a unified, powerful, cohesive voice. Advocacy is at the top of my presidential initiatives list. The voice of our national organization can have a huge impact on maintaining and improving women’s health care in the United States. That is why All-In for Advocacy is vital. This initiative has significantly increased members’ involvement in ACOG. We will harness this power to pass vital legislation to help the women we serve and our specialty.
Addressing health disparities drives career and presidential focus
Dr. Brown: My presidential initiatives are rooted in my career decision to focus on health disparities, particularly race-based health disparities.
Maternal morbidity and mortality and infant mortality are complex issues shrouded in the social determinants of health. Access to care, fragmentation of care, and quality of care are other factors relevant in disparity. There is evidence of an implicit bias in health care delivery.
My telehealth initiative will focus on implementing Levels of Maternal Care (of which there are 4), the National Partnership for Maternal Safety (NPMS), and the Alliance for Innovation on Maternal Health (AIM) as well as redefining healthy pregnancies and postpartum periods as the gateway to women’s long-term health. The February 2015 Levels of Maternal Care Obstetric Care Consensus Statement, jointly issued by ACOG and the Society for Maternal–Fetal Medicine (SMFM),1 proposes a classification system for birth centers, from basic to specialized regional perinatal health care centers. ACOG is working with hospitals throughout the country to designate the Levels of Maternal Care and thereby ensure each patient receives the appropriate level of care.
The AIM program, led by ACOG and funded by the Health Resources and Services Administration (HRSA), reduces obstetric complications by encouraging hospitals to adopt defined evidence-based patient safety measures. The goal is to prevent 100,000 severe labor and delivery complications and 1,000 maternal deaths over 4 years.
- ACOG's Congressional Leadership Conference, The President's Conference. This 3-day conference, held in Washington, DC, connects you with lawmakers on the important issues facing ObGyns. To find out more, access www.acog.org/clc.
- McCain and Gellhaus Fellowships. Spend 2 to 4 weeks as a member of ACOG's Government Affairs team in Washington, DC. To apply, go to www.acog.org/ateam.
- Ob-Gyn PAC. ACOG's political action committee helps elect state and federal candidates who support our specialty. For more information, visit www.obgynpac.org.
- ACOG News. Don't miss these updates on federal and state legislative developments. To sign up, access www.acog.org/advonews.
- Advocacy webpage. All of this information and more can be found at www.acog.org/advocacy!
ACOG is committed to a “big tent” approach
Dr. Gellhaus: Like US citizens, ACOG represents members with many points of view. ACOG can best represent our members’ diversity in the future by remaining the moderate voice, and by opposing federal and state proposals that are inconsistent with facts and science. We need to bring to our membership’s attention the federal and state successes we have had, show how they have helped our patients and our specialty, and make it clear that our successes are due to our bipartisan work over the years, which we have achieved regardless of which political party has been in office.
For ACOG to continue to be a leader in women’s health care and our specialty, we must remain vigilant against political interference in the patient–physician relationship and be ready to counter with science, facts, and evidence.
Compassion and passion lie at the heart of member similarities
Dr. Brown: First and foremost, we ObGyns care about our patients and, regardless of personal politics, most of us understand the reproductive health challenges facing the women of this country as well as our history with respect to those challenges. All of us must be willing to provide counsel to patients when needed, and that counseling must be nonjudgmental. We also must be willing to protect confidentially and to refer patients whose decisions are at odds with our personal views.
ACOG recognizes that we are a melting pot of specialists and subspecialists and that we are all guided by our personal beliefs and values. Choosing to become an exclusive women’s health care physician requires our passion and compassion.
Let us use our collective voice!
Dr. Gellhaus: Our members must realize the power of our collective voice and that we must use it to deliver a unified, cohesive message. We cannot sit on the sidelines and expect others to speak for us. If we are not part of the solution, then we cede our future to others and have no right to complain about the result. Our members need to commit to advocating outside their exam rooms. A good first step is to see how ACOG makes advocacy easy. When thousands of ACOG members contact elected officials about important issues, officials listen.
Dr. Brown: Yes! We all need to get involved in ACOG and in our communities. Together, we will accomplish many important things.
[polldaddy:9703185]
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Menard MK, Kilpatrick S, Saade G, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Levels of maternal care. Am J Obstet Gynecol. 2015;212(3)259–271.
- Menard MK, Kilpatrick S, Saade G, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Levels of maternal care. Am J Obstet Gynecol. 2015;212(3)259–271.
Refractory RA patients respond well to peficitinib in phase II study
A 12-week course of daily oral peficitinib improved the severity of refractory rheumatoid arthritis in a manufacturer-sponsored phase II trial of the investigational drug, which inhibits all of the intracellular signaling molecules in the Janus kinase family.
The randomized, double-blind dose-finding study was performed at 41 sites in six countries and involved 289 adults with long-standing moderate to severe rheumatoid arthritis (RA) who had not responded to or who were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). The participants were permitted to take nonsteroidal anti-inflammatory drugs, hydroxychloroquine, chloroquine, sulfasalazine, and/or oral corticosteroids (10 mg or less of prednisone or equivalent) as needed throughout the trial, but not methotrexate, gold, penicillamine, leflunomide, azathioprine, minocycline, and cyclophosphamide. The patients also were allowed to have previously taken a biologic and were not required to have had an inadequate response or intolerance to a previous biologic, said Mark C. Genovese, MD, of the division of immunology and rheumatology at Stanford University, Palo Alto, Calif., and his associates.
Patients receiving the two highest doses of peficitinib also showed a significant improvement in 28-joint Disease Activity Score, compared with those receiving placebo.
On safety outcomes, the investigators reported that peficitinib “demonstrated satisfactory tolerability” with an overall incidence of adverse events similar to that of the placebo group, and there were no new safety signals that differed from the other two previous phase II trials of peficitinib for RA. There were dose-dependent decreases in absolute neutrophil count, platelet count, and white blood cell count, as well as dose-dependent increases in creatine phosphokinase, high-density lipoprotein, and creatinine levels. Three cases of grade 3 or higher hypertriglyceridemia were considered to be drug related, and 10 patients discontinued peficitinib because of dyspepsia, nausea, vomiting, abdominal hernia, hidradenitis, skin lesions, MI, or limb abscess.
Peficitinib inhibits JAK1, JAK2, JAK3, and Tyk2 enzyme activities, with a moderate selectivity for JAK3. Two other phase II efficacy and safety studies of peficitinib have been conducted previously. One of these involved Japanese patients who were not required to fail one or more csDMARDs and received the same doses as in the current study. The 12-week study resulted in statistically significant improvements in ACR20 responses in patients who took 50-mg, 100-mg, and 150-mg doses of peficitinib monotherapy. The second 12-week phase II trial of peficitinib plus methotrexate in patients who had an inadequate response to methotrexate found that the combination was statistically significantly better than placebo plus methotrexate in ACR20 response rate only among those who took 50 mg.
Larger and longer-term phase III trials of the 100-mg and 150-mg doses of peficitinib are now underway, Dr. Genovese and his associates added. One trial is comparing peficitinib alone or in combination with DMARDs against placebo or an open-label active comparator, etanercept, for 52 weeks in patients who had an inadequate response to DMARDs. The other is a 52-week trial comparing peficitinib in combination with methotrexate versus placebo and methotrexate in patients who had an inadequate response to methotrexate.
The phase II trial was sponsored by Astellas Pharma. Dr. Genovese reported ties to Astellas, AbbVie, Johnson & Johnson, Pfizer, Eli Lilly, Vertex, Galapagos NV, and Gilead Sciences; his associates reported ties to numerous industry sources.
A 12-week course of daily oral peficitinib improved the severity of refractory rheumatoid arthritis in a manufacturer-sponsored phase II trial of the investigational drug, which inhibits all of the intracellular signaling molecules in the Janus kinase family.
The randomized, double-blind dose-finding study was performed at 41 sites in six countries and involved 289 adults with long-standing moderate to severe rheumatoid arthritis (RA) who had not responded to or who were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). The participants were permitted to take nonsteroidal anti-inflammatory drugs, hydroxychloroquine, chloroquine, sulfasalazine, and/or oral corticosteroids (10 mg or less of prednisone or equivalent) as needed throughout the trial, but not methotrexate, gold, penicillamine, leflunomide, azathioprine, minocycline, and cyclophosphamide. The patients also were allowed to have previously taken a biologic and were not required to have had an inadequate response or intolerance to a previous biologic, said Mark C. Genovese, MD, of the division of immunology and rheumatology at Stanford University, Palo Alto, Calif., and his associates.
Patients receiving the two highest doses of peficitinib also showed a significant improvement in 28-joint Disease Activity Score, compared with those receiving placebo.
On safety outcomes, the investigators reported that peficitinib “demonstrated satisfactory tolerability” with an overall incidence of adverse events similar to that of the placebo group, and there were no new safety signals that differed from the other two previous phase II trials of peficitinib for RA. There were dose-dependent decreases in absolute neutrophil count, platelet count, and white blood cell count, as well as dose-dependent increases in creatine phosphokinase, high-density lipoprotein, and creatinine levels. Three cases of grade 3 or higher hypertriglyceridemia were considered to be drug related, and 10 patients discontinued peficitinib because of dyspepsia, nausea, vomiting, abdominal hernia, hidradenitis, skin lesions, MI, or limb abscess.
Peficitinib inhibits JAK1, JAK2, JAK3, and Tyk2 enzyme activities, with a moderate selectivity for JAK3. Two other phase II efficacy and safety studies of peficitinib have been conducted previously. One of these involved Japanese patients who were not required to fail one or more csDMARDs and received the same doses as in the current study. The 12-week study resulted in statistically significant improvements in ACR20 responses in patients who took 50-mg, 100-mg, and 150-mg doses of peficitinib monotherapy. The second 12-week phase II trial of peficitinib plus methotrexate in patients who had an inadequate response to methotrexate found that the combination was statistically significantly better than placebo plus methotrexate in ACR20 response rate only among those who took 50 mg.
Larger and longer-term phase III trials of the 100-mg and 150-mg doses of peficitinib are now underway, Dr. Genovese and his associates added. One trial is comparing peficitinib alone or in combination with DMARDs against placebo or an open-label active comparator, etanercept, for 52 weeks in patients who had an inadequate response to DMARDs. The other is a 52-week trial comparing peficitinib in combination with methotrexate versus placebo and methotrexate in patients who had an inadequate response to methotrexate.
The phase II trial was sponsored by Astellas Pharma. Dr. Genovese reported ties to Astellas, AbbVie, Johnson & Johnson, Pfizer, Eli Lilly, Vertex, Galapagos NV, and Gilead Sciences; his associates reported ties to numerous industry sources.
A 12-week course of daily oral peficitinib improved the severity of refractory rheumatoid arthritis in a manufacturer-sponsored phase II trial of the investigational drug, which inhibits all of the intracellular signaling molecules in the Janus kinase family.
The randomized, double-blind dose-finding study was performed at 41 sites in six countries and involved 289 adults with long-standing moderate to severe rheumatoid arthritis (RA) who had not responded to or who were intolerant of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). The participants were permitted to take nonsteroidal anti-inflammatory drugs, hydroxychloroquine, chloroquine, sulfasalazine, and/or oral corticosteroids (10 mg or less of prednisone or equivalent) as needed throughout the trial, but not methotrexate, gold, penicillamine, leflunomide, azathioprine, minocycline, and cyclophosphamide. The patients also were allowed to have previously taken a biologic and were not required to have had an inadequate response or intolerance to a previous biologic, said Mark C. Genovese, MD, of the division of immunology and rheumatology at Stanford University, Palo Alto, Calif., and his associates.
Patients receiving the two highest doses of peficitinib also showed a significant improvement in 28-joint Disease Activity Score, compared with those receiving placebo.
On safety outcomes, the investigators reported that peficitinib “demonstrated satisfactory tolerability” with an overall incidence of adverse events similar to that of the placebo group, and there were no new safety signals that differed from the other two previous phase II trials of peficitinib for RA. There were dose-dependent decreases in absolute neutrophil count, platelet count, and white blood cell count, as well as dose-dependent increases in creatine phosphokinase, high-density lipoprotein, and creatinine levels. Three cases of grade 3 or higher hypertriglyceridemia were considered to be drug related, and 10 patients discontinued peficitinib because of dyspepsia, nausea, vomiting, abdominal hernia, hidradenitis, skin lesions, MI, or limb abscess.
Peficitinib inhibits JAK1, JAK2, JAK3, and Tyk2 enzyme activities, with a moderate selectivity for JAK3. Two other phase II efficacy and safety studies of peficitinib have been conducted previously. One of these involved Japanese patients who were not required to fail one or more csDMARDs and received the same doses as in the current study. The 12-week study resulted in statistically significant improvements in ACR20 responses in patients who took 50-mg, 100-mg, and 150-mg doses of peficitinib monotherapy. The second 12-week phase II trial of peficitinib plus methotrexate in patients who had an inadequate response to methotrexate found that the combination was statistically significantly better than placebo plus methotrexate in ACR20 response rate only among those who took 50 mg.
Larger and longer-term phase III trials of the 100-mg and 150-mg doses of peficitinib are now underway, Dr. Genovese and his associates added. One trial is comparing peficitinib alone or in combination with DMARDs against placebo or an open-label active comparator, etanercept, for 52 weeks in patients who had an inadequate response to DMARDs. The other is a 52-week trial comparing peficitinib in combination with methotrexate versus placebo and methotrexate in patients who had an inadequate response to methotrexate.
The phase II trial was sponsored by Astellas Pharma. Dr. Genovese reported ties to Astellas, AbbVie, Johnson & Johnson, Pfizer, Eli Lilly, Vertex, Galapagos NV, and Gilead Sciences; his associates reported ties to numerous industry sources.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: The primary efficacy endpoint – the percentage of patients achieving an ACR20 level of improvement at week 12 – was significantly higher in patients taking the 100-mg dose (48.3%) and the 150-mg dose (56.3%) than in those taking placebo (29.4%).
Data source: An international, manufacturer-sponsored, randomized, double-blind, placebo-controlled phase II trial involving 289 adults with RA.
Disclosures: The phase II trial was sponsored by Astellas Pharma. Dr. Genovese reported ties to Astellas, AbbVie, Johnson & Johnson, Pfizer, Eli Lilly, Vertex, Galapagos NV, and Gilead Sciences; his associates reported ties to numerous industry sources.
Bursectomy provides no benefit over omentectomy for gastric cancers
SAN FRANCISCO – Bursectomy was not found to be superior to omentectomy for improving survival in patients with subserosal/serosal gastric cancer in a Japanese randomized phase III study.
The procedure – the dissection of the peritoneal lining covering the pancreas and anterior plane of the transverse mesocolon to prevent peritoneal metastasis – was common worldwide and considered standard in Japan from the 1950s until the mid-1990s, but was replaced by omentectomy following publication of reports questioning its value, according to Masanori Terashima, MD, of Shizuoka Cancer Center, Nagaizumi, Japan.
However, interest in bursectomy was rekindled when a 2012 noninferiority phase II study suggested that bursectomy may improve survival; the authors concluded that it should not be abandoned as a futile procedure until more definitive data could be obtained (Gastric Cancer. 2012;15[1]:42-8).
“So based on this result, [Japanese Clinical Oncology Group] conducted a large-scale randomized phase III trial [JCOG1001] to evaluate the efficacy of bursectomy. The objective of this study was to confirm the superiority of bursectomy for clinical T3 or clinical T4a gastric cancer patients in terms of overall survival,” Dr. Terashima said at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and Society of Surgical Oncology.
At a planned interim analysis when 522 patients had been enrolled, the Data and Safety Monitoring Committee approved continued enrollment. However, at a second interim analysis when 54% of expected events had been observed, the committee recommended early release of the survival results because “there was little possibility of demonstration of the superiority of bursectomy,” he said.
Overall 3-year survival was 83.3% among 601 patients in the bursectomy arm, compared with 86% in 600 patients in the nonbursectomy arm (hazard ratio, 1.07). The predictive probability of superiority of bursectomy was 12.7%.
Study subjects were adults aged 20-80 years (median of 65-66 years) with histologically proven adenocarcinoma of the stomach and clinical T3 or T4 disease. They enrolled from 57 institutions and were intraoperatively randomized to the bursectomy or nonbursectomy arm after confirmation of tumor stage. All received adjuvant chemotherapy with S-1 for 1 year for pathologic stage II/III disease.
Patients with bulky nodal metastases, Borrmann type 4 and 8 cm or larger Borrmann type 3 tumors were excluded, as their poor prognosis made them candidates for other clinical trials, Dr. Terashima said.
Patients’ background and operative procedures were well balanced between the arms, he noted.
For those in the bursectomy group, operation time was longer (median, 254 vs. 222 minutes), and blood loss was larger (330 vs. 230 mL), although the incidence of blood transfusion was not significantly different between the groups (4.5% vs. 4.8%).
The incidence of grade 3 or higher complications was slightly higher in the bursectomy arm (13.3% vs. 11.6%). This was due largely to the incidence of pancreatic fistulas, which was nearly double in the bursectomy arm (4.8% vs. 2.5% ).
Mortality was “quite low” in both groups (0.2 vs. 0.8%).
“There was no significant difference in overall survival between the arms. Bursectomy seemed to be a bit inferior to the nonbursectomy arm. Relapse-free survival also demonstrated no significant difference between the arms. Again, bursectomy seemed to be a bit worse than nonbursectomy,” he said.
The most common site of recurrence for all patient was the peritoneum, with 63 and 56 patients in the bursectomy and nonbursectomy arms, respectively, experiencing peritoneal recurrence.
“We could not detect any subgroup who may have a benefit from bursectomy,” Dr. Terashima said.
“Bursectomy is not recommended as a standard treatment for clinical T3 or clinical T4 gastric cancer, while complete omentectomy remains a part of standard procedure,” he concluded.
Dr. Terashima reported receiving honoraria, and/or research funding from Chugai Pharma, Eisai; Lilly, Otsuka, Taiho Pharmaceutical, Takeda, and Yakult Honsha.
SAN FRANCISCO – Bursectomy was not found to be superior to omentectomy for improving survival in patients with subserosal/serosal gastric cancer in a Japanese randomized phase III study.
The procedure – the dissection of the peritoneal lining covering the pancreas and anterior plane of the transverse mesocolon to prevent peritoneal metastasis – was common worldwide and considered standard in Japan from the 1950s until the mid-1990s, but was replaced by omentectomy following publication of reports questioning its value, according to Masanori Terashima, MD, of Shizuoka Cancer Center, Nagaizumi, Japan.
However, interest in bursectomy was rekindled when a 2012 noninferiority phase II study suggested that bursectomy may improve survival; the authors concluded that it should not be abandoned as a futile procedure until more definitive data could be obtained (Gastric Cancer. 2012;15[1]:42-8).
“So based on this result, [Japanese Clinical Oncology Group] conducted a large-scale randomized phase III trial [JCOG1001] to evaluate the efficacy of bursectomy. The objective of this study was to confirm the superiority of bursectomy for clinical T3 or clinical T4a gastric cancer patients in terms of overall survival,” Dr. Terashima said at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and Society of Surgical Oncology.
At a planned interim analysis when 522 patients had been enrolled, the Data and Safety Monitoring Committee approved continued enrollment. However, at a second interim analysis when 54% of expected events had been observed, the committee recommended early release of the survival results because “there was little possibility of demonstration of the superiority of bursectomy,” he said.
Overall 3-year survival was 83.3% among 601 patients in the bursectomy arm, compared with 86% in 600 patients in the nonbursectomy arm (hazard ratio, 1.07). The predictive probability of superiority of bursectomy was 12.7%.
Study subjects were adults aged 20-80 years (median of 65-66 years) with histologically proven adenocarcinoma of the stomach and clinical T3 or T4 disease. They enrolled from 57 institutions and were intraoperatively randomized to the bursectomy or nonbursectomy arm after confirmation of tumor stage. All received adjuvant chemotherapy with S-1 for 1 year for pathologic stage II/III disease.
Patients with bulky nodal metastases, Borrmann type 4 and 8 cm or larger Borrmann type 3 tumors were excluded, as their poor prognosis made them candidates for other clinical trials, Dr. Terashima said.
Patients’ background and operative procedures were well balanced between the arms, he noted.
For those in the bursectomy group, operation time was longer (median, 254 vs. 222 minutes), and blood loss was larger (330 vs. 230 mL), although the incidence of blood transfusion was not significantly different between the groups (4.5% vs. 4.8%).
The incidence of grade 3 or higher complications was slightly higher in the bursectomy arm (13.3% vs. 11.6%). This was due largely to the incidence of pancreatic fistulas, which was nearly double in the bursectomy arm (4.8% vs. 2.5% ).
Mortality was “quite low” in both groups (0.2 vs. 0.8%).
“There was no significant difference in overall survival between the arms. Bursectomy seemed to be a bit inferior to the nonbursectomy arm. Relapse-free survival also demonstrated no significant difference between the arms. Again, bursectomy seemed to be a bit worse than nonbursectomy,” he said.
The most common site of recurrence for all patient was the peritoneum, with 63 and 56 patients in the bursectomy and nonbursectomy arms, respectively, experiencing peritoneal recurrence.
“We could not detect any subgroup who may have a benefit from bursectomy,” Dr. Terashima said.
“Bursectomy is not recommended as a standard treatment for clinical T3 or clinical T4 gastric cancer, while complete omentectomy remains a part of standard procedure,” he concluded.
Dr. Terashima reported receiving honoraria, and/or research funding from Chugai Pharma, Eisai; Lilly, Otsuka, Taiho Pharmaceutical, Takeda, and Yakult Honsha.
SAN FRANCISCO – Bursectomy was not found to be superior to omentectomy for improving survival in patients with subserosal/serosal gastric cancer in a Japanese randomized phase III study.
The procedure – the dissection of the peritoneal lining covering the pancreas and anterior plane of the transverse mesocolon to prevent peritoneal metastasis – was common worldwide and considered standard in Japan from the 1950s until the mid-1990s, but was replaced by omentectomy following publication of reports questioning its value, according to Masanori Terashima, MD, of Shizuoka Cancer Center, Nagaizumi, Japan.
However, interest in bursectomy was rekindled when a 2012 noninferiority phase II study suggested that bursectomy may improve survival; the authors concluded that it should not be abandoned as a futile procedure until more definitive data could be obtained (Gastric Cancer. 2012;15[1]:42-8).
“So based on this result, [Japanese Clinical Oncology Group] conducted a large-scale randomized phase III trial [JCOG1001] to evaluate the efficacy of bursectomy. The objective of this study was to confirm the superiority of bursectomy for clinical T3 or clinical T4a gastric cancer patients in terms of overall survival,” Dr. Terashima said at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and Society of Surgical Oncology.
At a planned interim analysis when 522 patients had been enrolled, the Data and Safety Monitoring Committee approved continued enrollment. However, at a second interim analysis when 54% of expected events had been observed, the committee recommended early release of the survival results because “there was little possibility of demonstration of the superiority of bursectomy,” he said.
Overall 3-year survival was 83.3% among 601 patients in the bursectomy arm, compared with 86% in 600 patients in the nonbursectomy arm (hazard ratio, 1.07). The predictive probability of superiority of bursectomy was 12.7%.
Study subjects were adults aged 20-80 years (median of 65-66 years) with histologically proven adenocarcinoma of the stomach and clinical T3 or T4 disease. They enrolled from 57 institutions and were intraoperatively randomized to the bursectomy or nonbursectomy arm after confirmation of tumor stage. All received adjuvant chemotherapy with S-1 for 1 year for pathologic stage II/III disease.
Patients with bulky nodal metastases, Borrmann type 4 and 8 cm or larger Borrmann type 3 tumors were excluded, as their poor prognosis made them candidates for other clinical trials, Dr. Terashima said.
Patients’ background and operative procedures were well balanced between the arms, he noted.
For those in the bursectomy group, operation time was longer (median, 254 vs. 222 minutes), and blood loss was larger (330 vs. 230 mL), although the incidence of blood transfusion was not significantly different between the groups (4.5% vs. 4.8%).
The incidence of grade 3 or higher complications was slightly higher in the bursectomy arm (13.3% vs. 11.6%). This was due largely to the incidence of pancreatic fistulas, which was nearly double in the bursectomy arm (4.8% vs. 2.5% ).
Mortality was “quite low” in both groups (0.2 vs. 0.8%).
“There was no significant difference in overall survival between the arms. Bursectomy seemed to be a bit inferior to the nonbursectomy arm. Relapse-free survival also demonstrated no significant difference between the arms. Again, bursectomy seemed to be a bit worse than nonbursectomy,” he said.
The most common site of recurrence for all patient was the peritoneum, with 63 and 56 patients in the bursectomy and nonbursectomy arms, respectively, experiencing peritoneal recurrence.
“We could not detect any subgroup who may have a benefit from bursectomy,” Dr. Terashima said.
“Bursectomy is not recommended as a standard treatment for clinical T3 or clinical T4 gastric cancer, while complete omentectomy remains a part of standard procedure,” he concluded.
Dr. Terashima reported receiving honoraria, and/or research funding from Chugai Pharma, Eisai; Lilly, Otsuka, Taiho Pharmaceutical, Takeda, and Yakult Honsha.
AT THE 2017 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point:
Major finding: Overall 3-year survival was 83.3% and 86% in the bursectomy and nonbursectomy arms, respectively (hazard ratio, 1.07).
Data source: The randomized phase III JCOG1001 trial with more than 1,200 subjects.
Disclosures: Dr. Terashima reported receiving honoraria, and/or research funding from Chugai Pharma, Eisai; Lilly, Otsuka, Taiho Pharmaceutical, Takeda, and Yakult Honsha.
Contact dermatitis gets personal
Parabens have been eliminated from many personal care products because of health concerns, but from a contact dermatitis standpoint, they have “very low rates of irritancy and allergenicity” and are considered safe and well tolerated, according to Jonathan I. Silverberg, MD, of Northwestern University, Chicago.
“I almost never see a positive reaction to parabens,” Dr. Silverberg said in a presentation on contact dermatitis at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
However, the confirmation of estrogenic activity associated with parabens has led to their replacement in many products – especially personal care products – with other
“MCI/MI is now a common cause of contact dermatitis and can cause severe reactions,” said Dr. Silverberg, director of the Northwestern Medicine Multidisciplinary Eczema Center in Chicago.
An alternative to MCI/MI – methyldibromoglutaronitrile/phenoxyethanol (Euxyl K 400) – also appears in personal care products, such as soaps and shampoos, as well as industrial products such as paints, glues, wood preservatives, and metal-working fluids, Dr. Silverberg noted. This preservative is relatively uncommon in the United States, and in Europe it has been banned from leave-on products since 2005 and from rinse-off products since 2007, he said.
Another paraben alternative, iodopropynyl butylcarbamate, is a relatively uncommon preservative, but it frequently occurs as a positive patch test reaction, Dr. Silverberg said.
Lanolin, a natural ingredient used in topical skin emollients and cosmetics, also has been associated with skin reactions, he added. In addition to personal care products, the increasing range of personal technology products can be sources of contact dermatitis, Dr. Silverberg pointed out. Consider nickel exposure not only from jewelry, but from items such as iPads, iPhones, laptops, and Xbox controllers, when evaluating contact dermatitis in adults and children, he said.
Dr. Silverberg disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, Galderma, GlaxoSmithKline, Lilly, Puricore, Medimmune-AstraZeneca, Pfizer, Proctor & Gamble, Puricore, Hoffmann-La Roche, and Regeneron-Sanofi.
SDEF and this news organization are owned by the same parent company.
Parabens have been eliminated from many personal care products because of health concerns, but from a contact dermatitis standpoint, they have “very low rates of irritancy and allergenicity” and are considered safe and well tolerated, according to Jonathan I. Silverberg, MD, of Northwestern University, Chicago.
“I almost never see a positive reaction to parabens,” Dr. Silverberg said in a presentation on contact dermatitis at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
However, the confirmation of estrogenic activity associated with parabens has led to their replacement in many products – especially personal care products – with other
“MCI/MI is now a common cause of contact dermatitis and can cause severe reactions,” said Dr. Silverberg, director of the Northwestern Medicine Multidisciplinary Eczema Center in Chicago.
An alternative to MCI/MI – methyldibromoglutaronitrile/phenoxyethanol (Euxyl K 400) – also appears in personal care products, such as soaps and shampoos, as well as industrial products such as paints, glues, wood preservatives, and metal-working fluids, Dr. Silverberg noted. This preservative is relatively uncommon in the United States, and in Europe it has been banned from leave-on products since 2005 and from rinse-off products since 2007, he said.
Another paraben alternative, iodopropynyl butylcarbamate, is a relatively uncommon preservative, but it frequently occurs as a positive patch test reaction, Dr. Silverberg said.
Lanolin, a natural ingredient used in topical skin emollients and cosmetics, also has been associated with skin reactions, he added. In addition to personal care products, the increasing range of personal technology products can be sources of contact dermatitis, Dr. Silverberg pointed out. Consider nickel exposure not only from jewelry, but from items such as iPads, iPhones, laptops, and Xbox controllers, when evaluating contact dermatitis in adults and children, he said.
Dr. Silverberg disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, Galderma, GlaxoSmithKline, Lilly, Puricore, Medimmune-AstraZeneca, Pfizer, Proctor & Gamble, Puricore, Hoffmann-La Roche, and Regeneron-Sanofi.
SDEF and this news organization are owned by the same parent company.
Parabens have been eliminated from many personal care products because of health concerns, but from a contact dermatitis standpoint, they have “very low rates of irritancy and allergenicity” and are considered safe and well tolerated, according to Jonathan I. Silverberg, MD, of Northwestern University, Chicago.
“I almost never see a positive reaction to parabens,” Dr. Silverberg said in a presentation on contact dermatitis at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
However, the confirmation of estrogenic activity associated with parabens has led to their replacement in many products – especially personal care products – with other
“MCI/MI is now a common cause of contact dermatitis and can cause severe reactions,” said Dr. Silverberg, director of the Northwestern Medicine Multidisciplinary Eczema Center in Chicago.
An alternative to MCI/MI – methyldibromoglutaronitrile/phenoxyethanol (Euxyl K 400) – also appears in personal care products, such as soaps and shampoos, as well as industrial products such as paints, glues, wood preservatives, and metal-working fluids, Dr. Silverberg noted. This preservative is relatively uncommon in the United States, and in Europe it has been banned from leave-on products since 2005 and from rinse-off products since 2007, he said.
Another paraben alternative, iodopropynyl butylcarbamate, is a relatively uncommon preservative, but it frequently occurs as a positive patch test reaction, Dr. Silverberg said.
Lanolin, a natural ingredient used in topical skin emollients and cosmetics, also has been associated with skin reactions, he added. In addition to personal care products, the increasing range of personal technology products can be sources of contact dermatitis, Dr. Silverberg pointed out. Consider nickel exposure not only from jewelry, but from items such as iPads, iPhones, laptops, and Xbox controllers, when evaluating contact dermatitis in adults and children, he said.
Dr. Silverberg disclosed relationships with companies including AbbVie, Anacor, Celgene, Chugai, Galderma, GlaxoSmithKline, Lilly, Puricore, Medimmune-AstraZeneca, Pfizer, Proctor & Gamble, Puricore, Hoffmann-La Roche, and Regeneron-Sanofi.
SDEF and this news organization are owned by the same parent company.
FROM SDEF HAWAII DERMATOLOGY SEMINAR
Improving sunscreen use entails patient counseling
WAILEA, HAWAII – When sunscreens are tested for their SPF, testers apply 2 mg/cm2, but most people use only 20%-50% of that amount, which significantly reduces their protection, according to Dr. Julie C. Harper, director of the Dermatology & Skin Care Center of Birmingham, Ala.
The correct amount is 1 teaspoon of sunscreen on the face/head/neck, 1 teaspoon on each arm, 2 teaspoons on the torso, and 2 teaspoons on each leg, Dr. Harper said in a presentation at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Reapplication every 90 minutes to 2 hours is key to effective protection, Dr. Harper said.
However, “most people use less than one bottle of sunscreen per year,” she noted.
Prompting patients to improve their sunscreen use involves disproving some myths, Dr. Harper pointed out. When patients cite concerns about low vitamin D as a reason to avoid sunscreens, she recommended that they be counseled that there are three sources of vitamin D: foods such as fatty fish, vitamin D fortified foods, cheese, and egg yolks; vitamin D supplements; and skin synthesis through UVB exposure; and that only one of these – UVB exposure – is a known carcinogen.
Also, some patients express concern that sunscreen itself may be a carcinogen. Oxybenzone, a common sunscreen ingredient, has demonstrated some estrogenic effects in vitro and in vivo studies. However, the rat studies often cited in support of that finding involved the use of very high doses – approximately the equivalent of 277 years of daily sunscreen application with 6% oxybenzone, a much higher concentration than is found in commercial sunscreens, she said.
For patients interested in nontopical sun protection, polypodium leucotomos extract (PLE) is an option, Dr. Harper said. PLE, an antioxidant extract from a tropical fern, can be part of a skin cancer prevention strategy that also includes good sunscreen and protective clothing. PLE works by counteracting UV-induced immunosuppression, activating the tumor suppressor p53 gene, and inhibiting cyclooxygenase-2, all of which can help protect the skin from burning.
In addition, oral nicotinamide has been shown to help repair DNA damage in human keratinocytes, and in a clinical trial, has been associated with fewer actinic keratoses and squamous cell carcinoma, compared with placebo, she said.
However, more research in these options is needed, and patients should be encouraged to follow consistent sun protection practices, Dr. Harper emphasized.
Dr. Harper disclosed relationships with companies including Allergan, Bayer, Galderma, LaRoche-Posay, Promius, Valeant, and BioPharmX.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – When sunscreens are tested for their SPF, testers apply 2 mg/cm2, but most people use only 20%-50% of that amount, which significantly reduces their protection, according to Dr. Julie C. Harper, director of the Dermatology & Skin Care Center of Birmingham, Ala.
The correct amount is 1 teaspoon of sunscreen on the face/head/neck, 1 teaspoon on each arm, 2 teaspoons on the torso, and 2 teaspoons on each leg, Dr. Harper said in a presentation at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Reapplication every 90 minutes to 2 hours is key to effective protection, Dr. Harper said.
However, “most people use less than one bottle of sunscreen per year,” she noted.
Prompting patients to improve their sunscreen use involves disproving some myths, Dr. Harper pointed out. When patients cite concerns about low vitamin D as a reason to avoid sunscreens, she recommended that they be counseled that there are three sources of vitamin D: foods such as fatty fish, vitamin D fortified foods, cheese, and egg yolks; vitamin D supplements; and skin synthesis through UVB exposure; and that only one of these – UVB exposure – is a known carcinogen.
Also, some patients express concern that sunscreen itself may be a carcinogen. Oxybenzone, a common sunscreen ingredient, has demonstrated some estrogenic effects in vitro and in vivo studies. However, the rat studies often cited in support of that finding involved the use of very high doses – approximately the equivalent of 277 years of daily sunscreen application with 6% oxybenzone, a much higher concentration than is found in commercial sunscreens, she said.
For patients interested in nontopical sun protection, polypodium leucotomos extract (PLE) is an option, Dr. Harper said. PLE, an antioxidant extract from a tropical fern, can be part of a skin cancer prevention strategy that also includes good sunscreen and protective clothing. PLE works by counteracting UV-induced immunosuppression, activating the tumor suppressor p53 gene, and inhibiting cyclooxygenase-2, all of which can help protect the skin from burning.
In addition, oral nicotinamide has been shown to help repair DNA damage in human keratinocytes, and in a clinical trial, has been associated with fewer actinic keratoses and squamous cell carcinoma, compared with placebo, she said.
However, more research in these options is needed, and patients should be encouraged to follow consistent sun protection practices, Dr. Harper emphasized.
Dr. Harper disclosed relationships with companies including Allergan, Bayer, Galderma, LaRoche-Posay, Promius, Valeant, and BioPharmX.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – When sunscreens are tested for their SPF, testers apply 2 mg/cm2, but most people use only 20%-50% of that amount, which significantly reduces their protection, according to Dr. Julie C. Harper, director of the Dermatology & Skin Care Center of Birmingham, Ala.
The correct amount is 1 teaspoon of sunscreen on the face/head/neck, 1 teaspoon on each arm, 2 teaspoons on the torso, and 2 teaspoons on each leg, Dr. Harper said in a presentation at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Reapplication every 90 minutes to 2 hours is key to effective protection, Dr. Harper said.
However, “most people use less than one bottle of sunscreen per year,” she noted.
Prompting patients to improve their sunscreen use involves disproving some myths, Dr. Harper pointed out. When patients cite concerns about low vitamin D as a reason to avoid sunscreens, she recommended that they be counseled that there are three sources of vitamin D: foods such as fatty fish, vitamin D fortified foods, cheese, and egg yolks; vitamin D supplements; and skin synthesis through UVB exposure; and that only one of these – UVB exposure – is a known carcinogen.
Also, some patients express concern that sunscreen itself may be a carcinogen. Oxybenzone, a common sunscreen ingredient, has demonstrated some estrogenic effects in vitro and in vivo studies. However, the rat studies often cited in support of that finding involved the use of very high doses – approximately the equivalent of 277 years of daily sunscreen application with 6% oxybenzone, a much higher concentration than is found in commercial sunscreens, she said.
For patients interested in nontopical sun protection, polypodium leucotomos extract (PLE) is an option, Dr. Harper said. PLE, an antioxidant extract from a tropical fern, can be part of a skin cancer prevention strategy that also includes good sunscreen and protective clothing. PLE works by counteracting UV-induced immunosuppression, activating the tumor suppressor p53 gene, and inhibiting cyclooxygenase-2, all of which can help protect the skin from burning.
In addition, oral nicotinamide has been shown to help repair DNA damage in human keratinocytes, and in a clinical trial, has been associated with fewer actinic keratoses and squamous cell carcinoma, compared with placebo, she said.
However, more research in these options is needed, and patients should be encouraged to follow consistent sun protection practices, Dr. Harper emphasized.
Dr. Harper disclosed relationships with companies including Allergan, Bayer, Galderma, LaRoche-Posay, Promius, Valeant, and BioPharmX.
SDEF and this news organization are owned by the same parent company.