User login
Long view shows doubling of survival in non-Hodgkin lymphoma
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).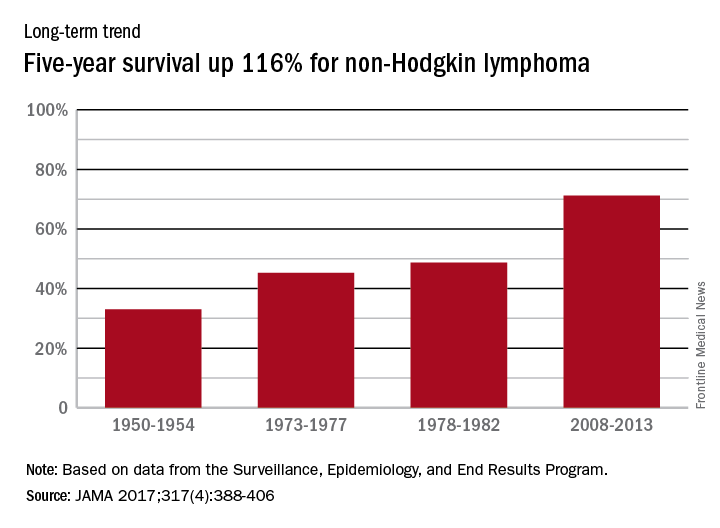
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA
How Do Patients With Epilepsy Use Herbal and Botanical Therapies?
HOUSTON—Many people with epilepsy use herbal and botanical therapies. Some patients use them to help control their seizures, while others use them to treat medication side effects or co-occurring symptoms, such as depression, anxiety, and memory problems.
“Ask your patients about the use of botanicals because they are probably using them,” said Dana Ekstein, MD, PhD, a senior neurologist at Hadassah Medical Center in Jerusalem, in an overview provided at the 70th Annual Meeting of the American Epilepsy Society. “Be ready to advise on the safety of their use.”
Efficacy and safety data for many of the treatments are limited, but educational resources are available to keep neurologists up to date. In addition, neurologists should ensure that botanicals do not interfere with conventional medication.
Widely Used
According to the 2012 American National Health Interview Survey, about a third of the population uses complementary medicine. Herbal and botanical medicines (ie, nonvitamin, nonmineral natural products) are the most commonly used complementary medicines and are used by about 18% of the population. Among people with epilepsy, studies have found that about half of adults and a third of children use complementary therapies. Patients often do not report the use of complementary medicine to their physicians, Dr. Ekstein said.
Massot-Tarrús and McLachlan examined the use of cannabis by people with epilepsy who were admitted to an epilepsy monitoring unit in Canada. More than half of them had tried marijuana. Many of them—59% of patients with epilepsy and 33% of patients with psychogenic nonepileptic seizures—used cannabis daily.Although botanicals were the mainstay of epilepsy treatment for centuries, modern data on botanicals in epilepsy are limited, Dr. Ekstein said. A 2009 Cochrane review of traditional Chinese medicine for epilepsy included only five unblinded single-center controlled studies. Although the studies reported some benefit, the probability of selection, detection, and performance bias meant that the treatment’s effect could not reliably be evaluated.
Cannabis
A 2014 Cochrane review of cannabinoids for epilepsy considered four randomized controlled trials with a total of 48 patients. Each trial included between nine and 15 patients, and investigators followed patients for between one and 12 months. The studies failed to provide efficacy evidence. In another review that considered all published studies with more than one patient, researchers analyzed eight studies with a total of 105 children and adults. Patients received placebo or cannabis compounds. Of the patients who received cannabis, 61% experienced improvement.
A retrospective trial of medical cannabis oil in Israel published in 2016 included 74 pediatric patients. Fifty-two percent of the patients had a 50% or greater reduction in seizure frequency, and one patient became seizure-free. Sixty percent of patients reported other benefits, such as improved behavior, alertness, language, communication, motor skills, and sleep.
A prospective open-label study by Devinsky et al enrolled 214 patients from 11 centers in the United States. The study included patients with drug-resistant epilepsy with onset in childhood who had at least four seizures per month. Patients received cannabidiol and were followed for three months. Of 137 patients included in the efficacy analysis, 39% had a 50% or greater reduction in seizure frequency.
Mushroom May Treat Depression
A Chinese mushroom called Xylaria nigripes has been studied for the treatment of depression in patients with epilepsy, Dr. Ekstein said. Peng et al conducted a randomized double-blind placebo-controlled trial that included 104 patients with epilepsy and depression. The mushroom significantly improved Hamilton Depression Rating Scale and quality of life scores, compared with placebo.
Although not studied in patients with epilepsy, research has shown that St. John’s wort effectively treats mild to moderate depression; kava treats generalized anxiety; and rosenroot improves attention, fatigue, mild depression, and mental performance, she said.
Safety
Many patients assume that natural products are safe, but there are important safety considerations, Dr. Ekstein said. Some botanicals have been associated with seizures, including ephedra, St. John’s wort, and ginkgo biloba. Cannabinoids, especially tetrahydrocannabinol (THC), may induce memory and executive function impairment and psychiatric symptoms. Cannabinoids also may impair plasticity in the developing brain.
Side effects in cannabis trials have been relatively common. In the retrospective Israeli trial, 46% of patients experienced adverse events (eg, seizure aggravation, somnolence, fatigue, and gastrointestinal disturbances). In the trial by Devinsky et al, 79% of patients experienced adverse events. Although most of the adverse events were mild to moderate and transient, 30% were serious adverse events (eg, status epilepticus, diarrhea, pneumonia, and weight loss).
Interactions between botanicals and antiepileptic drugs also should be taken into account, she said. Some botanicals may decrease or increase bioavailability of antiepileptic drugs. For example, cannabidiol increases concentrations of clobazam.
More clinical trials are needed to establish the efficacy and safety of botanical therapies. Cannabinoids are “on the right path” in terms of receiving further study. “There are almost no planned trials” of other botanicals, however, Dr. Ekstein said. The medical community should prioritize botanicals for further study, and neurologists should offer patients participation in clinical studies when they are available, she said.
Neurologists generally should be better educated regarding the use of botanical therapies. Online resources, such as those provided by the National Center for Complementary and Integrative Health, provide information about botanical therapies. The International League Against Epilepsy recently created the Web-based Epilepsy Naturapedia to provide information about the use of natural products in the treatment of epilepsy, Dr. Ekstein said.
—Jake Remaly
Suggested Reading
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278.
Ekstein D, Schachter SC. Natural products in epilepsy-the present situation and perspectives for the future. Pharmaceuticals (Basel). 2010;3(5):1426-1445.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
Li Q, Chen X, He L, Zhou D. Traditional Chinese medicine for epilepsy. Cochrane Database Syst Rev. 2009;(3):CD006454.
Massot-Tarrús A, McLachlan RS. Marijuana use in adults admitted to a Canadian epilepsy monitoring unit. Epilepsy Behav. 2016;63:73-78.
Peng WF, Wang X, Hong Z, et al. The anti-depression effect of Xylaria nigripes in patients with epilepsy: A multicenter randomized double-blind study. Seizure. 2015;29:26-33.
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy--from receptors to clinical response. Epilepsy Behav. 2014;41:277-282.
Tzadok M, Uliel-Siboni S, Linder I, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure. 2016;35:41-44.
HOUSTON—Many people with epilepsy use herbal and botanical therapies. Some patients use them to help control their seizures, while others use them to treat medication side effects or co-occurring symptoms, such as depression, anxiety, and memory problems.
“Ask your patients about the use of botanicals because they are probably using them,” said Dana Ekstein, MD, PhD, a senior neurologist at Hadassah Medical Center in Jerusalem, in an overview provided at the 70th Annual Meeting of the American Epilepsy Society. “Be ready to advise on the safety of their use.”
Efficacy and safety data for many of the treatments are limited, but educational resources are available to keep neurologists up to date. In addition, neurologists should ensure that botanicals do not interfere with conventional medication.
Widely Used
According to the 2012 American National Health Interview Survey, about a third of the population uses complementary medicine. Herbal and botanical medicines (ie, nonvitamin, nonmineral natural products) are the most commonly used complementary medicines and are used by about 18% of the population. Among people with epilepsy, studies have found that about half of adults and a third of children use complementary therapies. Patients often do not report the use of complementary medicine to their physicians, Dr. Ekstein said.
Massot-Tarrús and McLachlan examined the use of cannabis by people with epilepsy who were admitted to an epilepsy monitoring unit in Canada. More than half of them had tried marijuana. Many of them—59% of patients with epilepsy and 33% of patients with psychogenic nonepileptic seizures—used cannabis daily.Although botanicals were the mainstay of epilepsy treatment for centuries, modern data on botanicals in epilepsy are limited, Dr. Ekstein said. A 2009 Cochrane review of traditional Chinese medicine for epilepsy included only five unblinded single-center controlled studies. Although the studies reported some benefit, the probability of selection, detection, and performance bias meant that the treatment’s effect could not reliably be evaluated.
Cannabis
A 2014 Cochrane review of cannabinoids for epilepsy considered four randomized controlled trials with a total of 48 patients. Each trial included between nine and 15 patients, and investigators followed patients for between one and 12 months. The studies failed to provide efficacy evidence. In another review that considered all published studies with more than one patient, researchers analyzed eight studies with a total of 105 children and adults. Patients received placebo or cannabis compounds. Of the patients who received cannabis, 61% experienced improvement.
A retrospective trial of medical cannabis oil in Israel published in 2016 included 74 pediatric patients. Fifty-two percent of the patients had a 50% or greater reduction in seizure frequency, and one patient became seizure-free. Sixty percent of patients reported other benefits, such as improved behavior, alertness, language, communication, motor skills, and sleep.
A prospective open-label study by Devinsky et al enrolled 214 patients from 11 centers in the United States. The study included patients with drug-resistant epilepsy with onset in childhood who had at least four seizures per month. Patients received cannabidiol and were followed for three months. Of 137 patients included in the efficacy analysis, 39% had a 50% or greater reduction in seizure frequency.
Mushroom May Treat Depression
A Chinese mushroom called Xylaria nigripes has been studied for the treatment of depression in patients with epilepsy, Dr. Ekstein said. Peng et al conducted a randomized double-blind placebo-controlled trial that included 104 patients with epilepsy and depression. The mushroom significantly improved Hamilton Depression Rating Scale and quality of life scores, compared with placebo.
Although not studied in patients with epilepsy, research has shown that St. John’s wort effectively treats mild to moderate depression; kava treats generalized anxiety; and rosenroot improves attention, fatigue, mild depression, and mental performance, she said.
Safety
Many patients assume that natural products are safe, but there are important safety considerations, Dr. Ekstein said. Some botanicals have been associated with seizures, including ephedra, St. John’s wort, and ginkgo biloba. Cannabinoids, especially tetrahydrocannabinol (THC), may induce memory and executive function impairment and psychiatric symptoms. Cannabinoids also may impair plasticity in the developing brain.
Side effects in cannabis trials have been relatively common. In the retrospective Israeli trial, 46% of patients experienced adverse events (eg, seizure aggravation, somnolence, fatigue, and gastrointestinal disturbances). In the trial by Devinsky et al, 79% of patients experienced adverse events. Although most of the adverse events were mild to moderate and transient, 30% were serious adverse events (eg, status epilepticus, diarrhea, pneumonia, and weight loss).
Interactions between botanicals and antiepileptic drugs also should be taken into account, she said. Some botanicals may decrease or increase bioavailability of antiepileptic drugs. For example, cannabidiol increases concentrations of clobazam.
More clinical trials are needed to establish the efficacy and safety of botanical therapies. Cannabinoids are “on the right path” in terms of receiving further study. “There are almost no planned trials” of other botanicals, however, Dr. Ekstein said. The medical community should prioritize botanicals for further study, and neurologists should offer patients participation in clinical studies when they are available, she said.
Neurologists generally should be better educated regarding the use of botanical therapies. Online resources, such as those provided by the National Center for Complementary and Integrative Health, provide information about botanical therapies. The International League Against Epilepsy recently created the Web-based Epilepsy Naturapedia to provide information about the use of natural products in the treatment of epilepsy, Dr. Ekstein said.
—Jake Remaly
Suggested Reading
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278.
Ekstein D, Schachter SC. Natural products in epilepsy-the present situation and perspectives for the future. Pharmaceuticals (Basel). 2010;3(5):1426-1445.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
Li Q, Chen X, He L, Zhou D. Traditional Chinese medicine for epilepsy. Cochrane Database Syst Rev. 2009;(3):CD006454.
Massot-Tarrús A, McLachlan RS. Marijuana use in adults admitted to a Canadian epilepsy monitoring unit. Epilepsy Behav. 2016;63:73-78.
Peng WF, Wang X, Hong Z, et al. The anti-depression effect of Xylaria nigripes in patients with epilepsy: A multicenter randomized double-blind study. Seizure. 2015;29:26-33.
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy--from receptors to clinical response. Epilepsy Behav. 2014;41:277-282.
Tzadok M, Uliel-Siboni S, Linder I, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure. 2016;35:41-44.
HOUSTON—Many people with epilepsy use herbal and botanical therapies. Some patients use them to help control their seizures, while others use them to treat medication side effects or co-occurring symptoms, such as depression, anxiety, and memory problems.
“Ask your patients about the use of botanicals because they are probably using them,” said Dana Ekstein, MD, PhD, a senior neurologist at Hadassah Medical Center in Jerusalem, in an overview provided at the 70th Annual Meeting of the American Epilepsy Society. “Be ready to advise on the safety of their use.”
Efficacy and safety data for many of the treatments are limited, but educational resources are available to keep neurologists up to date. In addition, neurologists should ensure that botanicals do not interfere with conventional medication.
Widely Used
According to the 2012 American National Health Interview Survey, about a third of the population uses complementary medicine. Herbal and botanical medicines (ie, nonvitamin, nonmineral natural products) are the most commonly used complementary medicines and are used by about 18% of the population. Among people with epilepsy, studies have found that about half of adults and a third of children use complementary therapies. Patients often do not report the use of complementary medicine to their physicians, Dr. Ekstein said.
Massot-Tarrús and McLachlan examined the use of cannabis by people with epilepsy who were admitted to an epilepsy monitoring unit in Canada. More than half of them had tried marijuana. Many of them—59% of patients with epilepsy and 33% of patients with psychogenic nonepileptic seizures—used cannabis daily.Although botanicals were the mainstay of epilepsy treatment for centuries, modern data on botanicals in epilepsy are limited, Dr. Ekstein said. A 2009 Cochrane review of traditional Chinese medicine for epilepsy included only five unblinded single-center controlled studies. Although the studies reported some benefit, the probability of selection, detection, and performance bias meant that the treatment’s effect could not reliably be evaluated.
Cannabis
A 2014 Cochrane review of cannabinoids for epilepsy considered four randomized controlled trials with a total of 48 patients. Each trial included between nine and 15 patients, and investigators followed patients for between one and 12 months. The studies failed to provide efficacy evidence. In another review that considered all published studies with more than one patient, researchers analyzed eight studies with a total of 105 children and adults. Patients received placebo or cannabis compounds. Of the patients who received cannabis, 61% experienced improvement.
A retrospective trial of medical cannabis oil in Israel published in 2016 included 74 pediatric patients. Fifty-two percent of the patients had a 50% or greater reduction in seizure frequency, and one patient became seizure-free. Sixty percent of patients reported other benefits, such as improved behavior, alertness, language, communication, motor skills, and sleep.
A prospective open-label study by Devinsky et al enrolled 214 patients from 11 centers in the United States. The study included patients with drug-resistant epilepsy with onset in childhood who had at least four seizures per month. Patients received cannabidiol and were followed for three months. Of 137 patients included in the efficacy analysis, 39% had a 50% or greater reduction in seizure frequency.
Mushroom May Treat Depression
A Chinese mushroom called Xylaria nigripes has been studied for the treatment of depression in patients with epilepsy, Dr. Ekstein said. Peng et al conducted a randomized double-blind placebo-controlled trial that included 104 patients with epilepsy and depression. The mushroom significantly improved Hamilton Depression Rating Scale and quality of life scores, compared with placebo.
Although not studied in patients with epilepsy, research has shown that St. John’s wort effectively treats mild to moderate depression; kava treats generalized anxiety; and rosenroot improves attention, fatigue, mild depression, and mental performance, she said.
Safety
Many patients assume that natural products are safe, but there are important safety considerations, Dr. Ekstein said. Some botanicals have been associated with seizures, including ephedra, St. John’s wort, and ginkgo biloba. Cannabinoids, especially tetrahydrocannabinol (THC), may induce memory and executive function impairment and psychiatric symptoms. Cannabinoids also may impair plasticity in the developing brain.
Side effects in cannabis trials have been relatively common. In the retrospective Israeli trial, 46% of patients experienced adverse events (eg, seizure aggravation, somnolence, fatigue, and gastrointestinal disturbances). In the trial by Devinsky et al, 79% of patients experienced adverse events. Although most of the adverse events were mild to moderate and transient, 30% were serious adverse events (eg, status epilepticus, diarrhea, pneumonia, and weight loss).
Interactions between botanicals and antiepileptic drugs also should be taken into account, she said. Some botanicals may decrease or increase bioavailability of antiepileptic drugs. For example, cannabidiol increases concentrations of clobazam.
More clinical trials are needed to establish the efficacy and safety of botanical therapies. Cannabinoids are “on the right path” in terms of receiving further study. “There are almost no planned trials” of other botanicals, however, Dr. Ekstein said. The medical community should prioritize botanicals for further study, and neurologists should offer patients participation in clinical studies when they are available, she said.
Neurologists generally should be better educated regarding the use of botanical therapies. Online resources, such as those provided by the National Center for Complementary and Integrative Health, provide information about botanical therapies. The International League Against Epilepsy recently created the Web-based Epilepsy Naturapedia to provide information about the use of natural products in the treatment of epilepsy, Dr. Ekstein said.
—Jake Remaly
Suggested Reading
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278.
Ekstein D, Schachter SC. Natural products in epilepsy-the present situation and perspectives for the future. Pharmaceuticals (Basel). 2010;3(5):1426-1445.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
Li Q, Chen X, He L, Zhou D. Traditional Chinese medicine for epilepsy. Cochrane Database Syst Rev. 2009;(3):CD006454.
Massot-Tarrús A, McLachlan RS. Marijuana use in adults admitted to a Canadian epilepsy monitoring unit. Epilepsy Behav. 2016;63:73-78.
Peng WF, Wang X, Hong Z, et al. The anti-depression effect of Xylaria nigripes in patients with epilepsy: A multicenter randomized double-blind study. Seizure. 2015;29:26-33.
Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy--from receptors to clinical response. Epilepsy Behav. 2014;41:277-282.
Tzadok M, Uliel-Siboni S, Linder I, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure. 2016;35:41-44.
Hodgkin lymphoma survival has nearly tripled since the 1950s
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.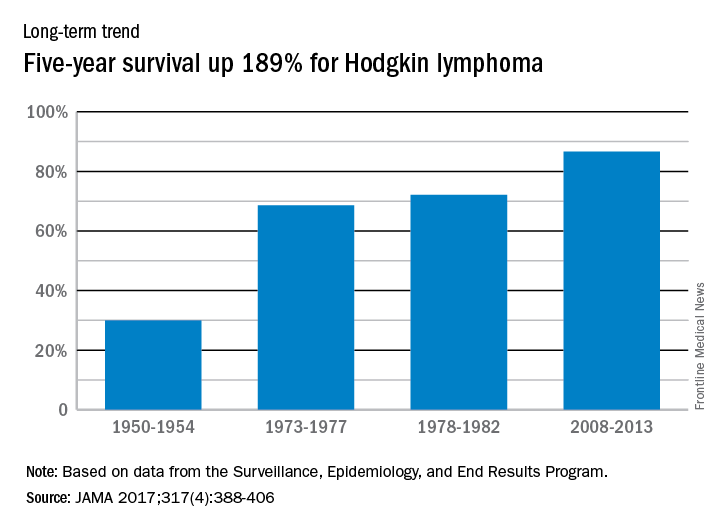
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
FROM JAMA
Sterile fecal filtrate effectively treated recurrent CDI
Sterile fecal filtrate transplantation (FFT) effectively treated five cases of symptomatic chronic-relapsing Clostridium difficile infection, investigators reported.
The procedure restored normal bowel habits and eliminated symptoms through the end of the study – that is, for at least 6 months – in all patients, Stephan J. Ott, MD, and his associates wrote (Gastroenterology. 2019. doi: 10.1053/j.gastro.2016.11.010).
Proteome analyses did not identify proteins likely to explain this efficacy, but 16S rRNA gene sequencing did demonstrate diverse bacterial DNA signatures in the filtrates, and tests of virus-like particles yielded “a complex signature of macrophages,” Dr. Ott and his associates reported. Additional tests suggested that recipients’ microbiomes continued to change weeks after FFT. “This open-label series strongly suggests that FFT should be evaluated in a controlled setting in comparison with standard fecal microbiota transplantation,” the researchers concluded.
Fecal microbiota transplantation (FMT) effectively treats recurrent Clostridium difficile infection (CDI), but even “the most rigorous and costly donor screening procedures, or defined panels of bacteria, cannot exclude the risk of transferring unknown pathogens or undetectable functional characteristics within the living microorganisms to the recipient, including bacterial or viral risk factors for metabolic diseases, cancer, atopy, or autoimmunity,” the investigators wrote.
Therefore, they performed sterile FFT in five patients who were positive on at least two of three tests: enzyme-linked immunosorbent assay for C. difficile–specific glutamate dehydrogenase; C. difficile toxin enzyme-linked immunosorbent assay; and culture of toxin-producing C. difficile. Patients chose their own stool donors, who were then screened based on published guidelines (Clin Gastroenterol Hepatol. 2011;9[12]:1044-49). Next, “slurries” were prepared from donor stool and filtered with a custom-built air pressure filtration system, yielding a “light brown, clear liquid with a subjectively less unpleasant and intensive odor” than conventional FMT stool preparations. Bacterial cultures of these filtrates yielded no growth, whereas donor stool cultures showed profuse growth of aerobic and anaerobic bacterial colonies, Dr. Ott and his associates said.
Patients became symptom-free 2-4 days after undergoing FFT. Notably, one patient had previously undergone FMT, which led to acute fever and diarrhea and recurrence of baseline symptoms after 3 months. This patient did not develop fever or diarrhea after FFT, was symptom-free after 3 days, and remained symptom-free until the study ended 2 years later, the researchers said. All other patients also remained symptom-free through the end of the study, that is, for 6 months to more than 2 years.
Analyses of 16S rRNA revealed substantial longitudinal shifts after FFT that often were present by week 1 and remained stable until week 6, the investigators said. Further tests confirmed marked shifts in bacterial phylotypes and in their relative abundance over time. Repeated virus analyses of one patient also showed that the phageome shifted over time to resemble that of the donor.
Patients were between 49 and 75 years old, three were female and two were male, and all had received more than one antibiotic before their first episode of CDI. Antibiotics for CDI had included metronidazole, vancomycin, and rifaximin. Comorbidities included pseudomembranous colitis, renal failure, HIV infection, epilepsy, and chronic heart failure, and medical histories included recurrent diverticulitis with sigmoid resection, gastric carcinoma, and colon cancer.
“It is important to keep in mind that, in contrast to conventional FMT, transferring sterile FFT filtrates cannot be expected to establish a microbiota similar to that of the donor in the receiving patient,” Dr. Ott and his associates noted. Instead, bacterial DNA in the filtrate might trigger the re-establishment of the recipient microbiome, they said. Bacterial cell wall fragments or bacteriophages also might play a role, they added.
The German Excellence Cluster and CONARIS Research Institute AG supported the work. Dr. Ott reported having lectured for Allergosan. Two coinvestigators reported employment with CONARIS. A third coinvestigator reported shareholder relationships with CONARIS, Allergosan, Danone, and Nestle and lectureship compensation from Allergosan. The other eight coinvestigators had no relevant conflicts of interest.
The remarkable efficacy of fecal microbial transplant in recurrent C. difficile infection provides a compelling example of ecologic microbiome-based therapy. Its mechanism is widely considered to be the restoration of select microbial species that suppress C. difficile colonization and virulence in healthy individuals. Identification of such suppressive microbiota is still at an early stage, with empirical studies revealing effective synthetic microbial consortia, and evidence of some modes of action, such as bile salt metabolism (Nature 2015;517:205-8; PLoS Pathog. 2012;8:e1002995).
Clouding this elegant concept is the provocative new study of Dr. Ott and his colleagues. Prompted by long-term safety concerns, they evaluated the efficacy of a donor fecal microfiltrate lacking viable intact organisms. Indeed, in five patients, long-term eradication of C. difficile was achieved with a single dose. This observation indicates that the initial action of fecal transplant may not require restoration of viable organisms into the antibiotic-damaged ecosystem.
What mechanisms might account for the therapeutic action of organism-free fecal microfiltrate? First, this material is laden with a complex, potentially distinct mix of microbial products and particulates (Cell. 2016;165[5]:1106-19) from donor origin or ex vivo processing. These biologicals may induce immune processes to promote control of C. difficile directly or via changes in other commensals of the patient's microbiome. Second, the microfiltrate retains abundant and diverse bacteria-targeting viruses of the fecal stream. Perhaps certain viruses, deficient in patients, target C. difficile and/or beneficially reshape microbial composition (Cell Mol Gastroenterol Hepatol. 2015;1[1]:28-40). So, C. difficile challenges us once more into the breach with new insights ahead for the principles and practice of ecologic microbiome therapy.
Jonathan Braun, MD, PhD, is professor and chair of pathology and laboratory medicine at the University of California, Los Angeles. He has no conflicts of interest.
The remarkable efficacy of fecal microbial transplant in recurrent C. difficile infection provides a compelling example of ecologic microbiome-based therapy. Its mechanism is widely considered to be the restoration of select microbial species that suppress C. difficile colonization and virulence in healthy individuals. Identification of such suppressive microbiota is still at an early stage, with empirical studies revealing effective synthetic microbial consortia, and evidence of some modes of action, such as bile salt metabolism (Nature 2015;517:205-8; PLoS Pathog. 2012;8:e1002995).
Clouding this elegant concept is the provocative new study of Dr. Ott and his colleagues. Prompted by long-term safety concerns, they evaluated the efficacy of a donor fecal microfiltrate lacking viable intact organisms. Indeed, in five patients, long-term eradication of C. difficile was achieved with a single dose. This observation indicates that the initial action of fecal transplant may not require restoration of viable organisms into the antibiotic-damaged ecosystem.
What mechanisms might account for the therapeutic action of organism-free fecal microfiltrate? First, this material is laden with a complex, potentially distinct mix of microbial products and particulates (Cell. 2016;165[5]:1106-19) from donor origin or ex vivo processing. These biologicals may induce immune processes to promote control of C. difficile directly or via changes in other commensals of the patient's microbiome. Second, the microfiltrate retains abundant and diverse bacteria-targeting viruses of the fecal stream. Perhaps certain viruses, deficient in patients, target C. difficile and/or beneficially reshape microbial composition (Cell Mol Gastroenterol Hepatol. 2015;1[1]:28-40). So, C. difficile challenges us once more into the breach with new insights ahead for the principles and practice of ecologic microbiome therapy.
Jonathan Braun, MD, PhD, is professor and chair of pathology and laboratory medicine at the University of California, Los Angeles. He has no conflicts of interest.
The remarkable efficacy of fecal microbial transplant in recurrent C. difficile infection provides a compelling example of ecologic microbiome-based therapy. Its mechanism is widely considered to be the restoration of select microbial species that suppress C. difficile colonization and virulence in healthy individuals. Identification of such suppressive microbiota is still at an early stage, with empirical studies revealing effective synthetic microbial consortia, and evidence of some modes of action, such as bile salt metabolism (Nature 2015;517:205-8; PLoS Pathog. 2012;8:e1002995).
Clouding this elegant concept is the provocative new study of Dr. Ott and his colleagues. Prompted by long-term safety concerns, they evaluated the efficacy of a donor fecal microfiltrate lacking viable intact organisms. Indeed, in five patients, long-term eradication of C. difficile was achieved with a single dose. This observation indicates that the initial action of fecal transplant may not require restoration of viable organisms into the antibiotic-damaged ecosystem.
What mechanisms might account for the therapeutic action of organism-free fecal microfiltrate? First, this material is laden with a complex, potentially distinct mix of microbial products and particulates (Cell. 2016;165[5]:1106-19) from donor origin or ex vivo processing. These biologicals may induce immune processes to promote control of C. difficile directly or via changes in other commensals of the patient's microbiome. Second, the microfiltrate retains abundant and diverse bacteria-targeting viruses of the fecal stream. Perhaps certain viruses, deficient in patients, target C. difficile and/or beneficially reshape microbial composition (Cell Mol Gastroenterol Hepatol. 2015;1[1]:28-40). So, C. difficile challenges us once more into the breach with new insights ahead for the principles and practice of ecologic microbiome therapy.
Jonathan Braun, MD, PhD, is professor and chair of pathology and laboratory medicine at the University of California, Los Angeles. He has no conflicts of interest.
Sterile fecal filtrate transplantation (FFT) effectively treated five cases of symptomatic chronic-relapsing Clostridium difficile infection, investigators reported.
The procedure restored normal bowel habits and eliminated symptoms through the end of the study – that is, for at least 6 months – in all patients, Stephan J. Ott, MD, and his associates wrote (Gastroenterology. 2019. doi: 10.1053/j.gastro.2016.11.010).
Proteome analyses did not identify proteins likely to explain this efficacy, but 16S rRNA gene sequencing did demonstrate diverse bacterial DNA signatures in the filtrates, and tests of virus-like particles yielded “a complex signature of macrophages,” Dr. Ott and his associates reported. Additional tests suggested that recipients’ microbiomes continued to change weeks after FFT. “This open-label series strongly suggests that FFT should be evaluated in a controlled setting in comparison with standard fecal microbiota transplantation,” the researchers concluded.
Fecal microbiota transplantation (FMT) effectively treats recurrent Clostridium difficile infection (CDI), but even “the most rigorous and costly donor screening procedures, or defined panels of bacteria, cannot exclude the risk of transferring unknown pathogens or undetectable functional characteristics within the living microorganisms to the recipient, including bacterial or viral risk factors for metabolic diseases, cancer, atopy, or autoimmunity,” the investigators wrote.
Therefore, they performed sterile FFT in five patients who were positive on at least two of three tests: enzyme-linked immunosorbent assay for C. difficile–specific glutamate dehydrogenase; C. difficile toxin enzyme-linked immunosorbent assay; and culture of toxin-producing C. difficile. Patients chose their own stool donors, who were then screened based on published guidelines (Clin Gastroenterol Hepatol. 2011;9[12]:1044-49). Next, “slurries” were prepared from donor stool and filtered with a custom-built air pressure filtration system, yielding a “light brown, clear liquid with a subjectively less unpleasant and intensive odor” than conventional FMT stool preparations. Bacterial cultures of these filtrates yielded no growth, whereas donor stool cultures showed profuse growth of aerobic and anaerobic bacterial colonies, Dr. Ott and his associates said.
Patients became symptom-free 2-4 days after undergoing FFT. Notably, one patient had previously undergone FMT, which led to acute fever and diarrhea and recurrence of baseline symptoms after 3 months. This patient did not develop fever or diarrhea after FFT, was symptom-free after 3 days, and remained symptom-free until the study ended 2 years later, the researchers said. All other patients also remained symptom-free through the end of the study, that is, for 6 months to more than 2 years.
Analyses of 16S rRNA revealed substantial longitudinal shifts after FFT that often were present by week 1 and remained stable until week 6, the investigators said. Further tests confirmed marked shifts in bacterial phylotypes and in their relative abundance over time. Repeated virus analyses of one patient also showed that the phageome shifted over time to resemble that of the donor.
Patients were between 49 and 75 years old, three were female and two were male, and all had received more than one antibiotic before their first episode of CDI. Antibiotics for CDI had included metronidazole, vancomycin, and rifaximin. Comorbidities included pseudomembranous colitis, renal failure, HIV infection, epilepsy, and chronic heart failure, and medical histories included recurrent diverticulitis with sigmoid resection, gastric carcinoma, and colon cancer.
“It is important to keep in mind that, in contrast to conventional FMT, transferring sterile FFT filtrates cannot be expected to establish a microbiota similar to that of the donor in the receiving patient,” Dr. Ott and his associates noted. Instead, bacterial DNA in the filtrate might trigger the re-establishment of the recipient microbiome, they said. Bacterial cell wall fragments or bacteriophages also might play a role, they added.
The German Excellence Cluster and CONARIS Research Institute AG supported the work. Dr. Ott reported having lectured for Allergosan. Two coinvestigators reported employment with CONARIS. A third coinvestigator reported shareholder relationships with CONARIS, Allergosan, Danone, and Nestle and lectureship compensation from Allergosan. The other eight coinvestigators had no relevant conflicts of interest.
Sterile fecal filtrate transplantation (FFT) effectively treated five cases of symptomatic chronic-relapsing Clostridium difficile infection, investigators reported.
The procedure restored normal bowel habits and eliminated symptoms through the end of the study – that is, for at least 6 months – in all patients, Stephan J. Ott, MD, and his associates wrote (Gastroenterology. 2019. doi: 10.1053/j.gastro.2016.11.010).
Proteome analyses did not identify proteins likely to explain this efficacy, but 16S rRNA gene sequencing did demonstrate diverse bacterial DNA signatures in the filtrates, and tests of virus-like particles yielded “a complex signature of macrophages,” Dr. Ott and his associates reported. Additional tests suggested that recipients’ microbiomes continued to change weeks after FFT. “This open-label series strongly suggests that FFT should be evaluated in a controlled setting in comparison with standard fecal microbiota transplantation,” the researchers concluded.
Fecal microbiota transplantation (FMT) effectively treats recurrent Clostridium difficile infection (CDI), but even “the most rigorous and costly donor screening procedures, or defined panels of bacteria, cannot exclude the risk of transferring unknown pathogens or undetectable functional characteristics within the living microorganisms to the recipient, including bacterial or viral risk factors for metabolic diseases, cancer, atopy, or autoimmunity,” the investigators wrote.
Therefore, they performed sterile FFT in five patients who were positive on at least two of three tests: enzyme-linked immunosorbent assay for C. difficile–specific glutamate dehydrogenase; C. difficile toxin enzyme-linked immunosorbent assay; and culture of toxin-producing C. difficile. Patients chose their own stool donors, who were then screened based on published guidelines (Clin Gastroenterol Hepatol. 2011;9[12]:1044-49). Next, “slurries” were prepared from donor stool and filtered with a custom-built air pressure filtration system, yielding a “light brown, clear liquid with a subjectively less unpleasant and intensive odor” than conventional FMT stool preparations. Bacterial cultures of these filtrates yielded no growth, whereas donor stool cultures showed profuse growth of aerobic and anaerobic bacterial colonies, Dr. Ott and his associates said.
Patients became symptom-free 2-4 days after undergoing FFT. Notably, one patient had previously undergone FMT, which led to acute fever and diarrhea and recurrence of baseline symptoms after 3 months. This patient did not develop fever or diarrhea after FFT, was symptom-free after 3 days, and remained symptom-free until the study ended 2 years later, the researchers said. All other patients also remained symptom-free through the end of the study, that is, for 6 months to more than 2 years.
Analyses of 16S rRNA revealed substantial longitudinal shifts after FFT that often were present by week 1 and remained stable until week 6, the investigators said. Further tests confirmed marked shifts in bacterial phylotypes and in their relative abundance over time. Repeated virus analyses of one patient also showed that the phageome shifted over time to resemble that of the donor.
Patients were between 49 and 75 years old, three were female and two were male, and all had received more than one antibiotic before their first episode of CDI. Antibiotics for CDI had included metronidazole, vancomycin, and rifaximin. Comorbidities included pseudomembranous colitis, renal failure, HIV infection, epilepsy, and chronic heart failure, and medical histories included recurrent diverticulitis with sigmoid resection, gastric carcinoma, and colon cancer.
“It is important to keep in mind that, in contrast to conventional FMT, transferring sterile FFT filtrates cannot be expected to establish a microbiota similar to that of the donor in the receiving patient,” Dr. Ott and his associates noted. Instead, bacterial DNA in the filtrate might trigger the re-establishment of the recipient microbiome, they said. Bacterial cell wall fragments or bacteriophages also might play a role, they added.
The German Excellence Cluster and CONARIS Research Institute AG supported the work. Dr. Ott reported having lectured for Allergosan. Two coinvestigators reported employment with CONARIS. A third coinvestigator reported shareholder relationships with CONARIS, Allergosan, Danone, and Nestle and lectureship compensation from Allergosan. The other eight coinvestigators had no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Sterile fecal filtrate transplantation effectively treated symptomatic chronic-relapsing Clostridium difficile infection.
Major finding: For all patients, transplantation restored normal bowel habits within 2-4 days and eliminated symptoms for at least 6 months.
Data source: A single-center cases series of five patients with recurrent symptomatic CDI.
Disclosures: The German Excellence Cluster and CONARIS Research Institute AG supported the work. Dr. Ott reported having lectured for Allergosan. Two coinvestigators reported employment with CONARIS. A third coinvestigator reported shareholder relationships with CONARIS, Allergosan, Danone, and Nestle and lectureship compensation from Allergosan. The other eight coinvestigators had no relevant conflicts of interest.
How to Ensure a Smooth Transition Into Adult Epilepsy Care
Has this ever happened to you? You are an adult neurologist who has been asked to take on the care of a pediatric neurology patient. The patient who comes to your clinic is a 20-year-old woman with a history of moderate developmental delay and intractable epilepsy. She is on numerous medications, including valproic acid,and has tried the ketogenic diet. You receive a report that she has focal epilepsy, she is having frequent seizures, and her last MRI was performed at age 2.
Prior notes talk about her summer vacations but not much about the future plans for her epilepsy. You see the patient in the clinic, and the family is not happy to be in the adult clinic. They are disappointed that you don’t spend more time with them or fill out myriad forms. You find out that they have not obtained legal guardianship for their daughter and have no plan for work placement after school. She also has various other medical comorbidities that were previously addressed by the pediatric neurologist.
Why does this happen? When patients are simply transferred instead of transitioned between providers when they get too old to be seen by pediatric specialists, the process often does not go smoothly. A true transition of care prepares the patient and the family to understand the underlying disease and everything that goes along with it to be able to successfully seek appropriate care as they move into the adult world.
There is not much evidence on the right way to do this. In 2013, the American Epilepsy Society approved a transition tool that is helpful in outlining the steps for a successful transition. In 2016, the Child Neurology Foundation put forth a consensus statement with eight principles to guide a successful transition. Transitions are an expectation of good care, and they recommend that all offices have a written policy.
Talking about transitioning should start as early as 10 to 12 years of age and should be discussed every year. Thinking about prognosis and a realistic plan for each child as they enter adult life is important. Patients and families should be able to understand how the disease affects them, what their medications are and how to independently obtain them, what comorbidities are associated with their disease, how to stay healthy, how to improve their quality of life, and how to advocate for themselves. As children become teenagers, they should have a concrete plan for ongoing education, work, women’s issues, and an understanding of decision-making capacity and whether legal guardianship or a power of attorney needs to be implemented.
When the pediatric epilepsy patient reaches young adulthood (18 years or older), the adult model of care should be implemented, even if they are still being seen in the pediatric setting. A transition packet should be created that includes a summary of the diagnosis, work-up, previous treatments, and considerations for future treatments and emergency care. Also included is a plan for who will continue to address any non–seizure-related diagnoses the pediatric neurologist may have been managing. The patient and family also have an opportunity to review and contribute to this plan. This packet enables the adult neurologist to easily understand all issues and assume care of the patient, easing this aspect of the transition.
An advance meeting of the patient and family with the adult provider should be arranged whenever possible. To address this, some centers are now creating a transition clinic staffed by both pediatric and adult neurologists and/or nurses. This ideally takes place in the adult setting and is an excellent way to ensure a smooth transition for the patient, family, and providers. Good transition is important to help prevent gaps in care, avoid reinventing the wheel, and improve satisfaction for everyone involved (patient, family, nurses, and neurologists).
The key points are that transition discussions start early, patients and families should be involved and empowered in the process, and the creation of a transition packet for the adult provider is very helpful. Care transitions are something we will be hearing a lot more about in the upcoming years. And hopefully, next time, the patient scenario seen above will go more smoothly!
Suggested Reading
Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care. Neurology. 2016;87(8):835-840.
Has this ever happened to you? You are an adult neurologist who has been asked to take on the care of a pediatric neurology patient. The patient who comes to your clinic is a 20-year-old woman with a history of moderate developmental delay and intractable epilepsy. She is on numerous medications, including valproic acid,and has tried the ketogenic diet. You receive a report that she has focal epilepsy, she is having frequent seizures, and her last MRI was performed at age 2.
Prior notes talk about her summer vacations but not much about the future plans for her epilepsy. You see the patient in the clinic, and the family is not happy to be in the adult clinic. They are disappointed that you don’t spend more time with them or fill out myriad forms. You find out that they have not obtained legal guardianship for their daughter and have no plan for work placement after school. She also has various other medical comorbidities that were previously addressed by the pediatric neurologist.
Why does this happen? When patients are simply transferred instead of transitioned between providers when they get too old to be seen by pediatric specialists, the process often does not go smoothly. A true transition of care prepares the patient and the family to understand the underlying disease and everything that goes along with it to be able to successfully seek appropriate care as they move into the adult world.
There is not much evidence on the right way to do this. In 2013, the American Epilepsy Society approved a transition tool that is helpful in outlining the steps for a successful transition. In 2016, the Child Neurology Foundation put forth a consensus statement with eight principles to guide a successful transition. Transitions are an expectation of good care, and they recommend that all offices have a written policy.
Talking about transitioning should start as early as 10 to 12 years of age and should be discussed every year. Thinking about prognosis and a realistic plan for each child as they enter adult life is important. Patients and families should be able to understand how the disease affects them, what their medications are and how to independently obtain them, what comorbidities are associated with their disease, how to stay healthy, how to improve their quality of life, and how to advocate for themselves. As children become teenagers, they should have a concrete plan for ongoing education, work, women’s issues, and an understanding of decision-making capacity and whether legal guardianship or a power of attorney needs to be implemented.
When the pediatric epilepsy patient reaches young adulthood (18 years or older), the adult model of care should be implemented, even if they are still being seen in the pediatric setting. A transition packet should be created that includes a summary of the diagnosis, work-up, previous treatments, and considerations for future treatments and emergency care. Also included is a plan for who will continue to address any non–seizure-related diagnoses the pediatric neurologist may have been managing. The patient and family also have an opportunity to review and contribute to this plan. This packet enables the adult neurologist to easily understand all issues and assume care of the patient, easing this aspect of the transition.
An advance meeting of the patient and family with the adult provider should be arranged whenever possible. To address this, some centers are now creating a transition clinic staffed by both pediatric and adult neurologists and/or nurses. This ideally takes place in the adult setting and is an excellent way to ensure a smooth transition for the patient, family, and providers. Good transition is important to help prevent gaps in care, avoid reinventing the wheel, and improve satisfaction for everyone involved (patient, family, nurses, and neurologists).
The key points are that transition discussions start early, patients and families should be involved and empowered in the process, and the creation of a transition packet for the adult provider is very helpful. Care transitions are something we will be hearing a lot more about in the upcoming years. And hopefully, next time, the patient scenario seen above will go more smoothly!
Suggested Reading
Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care. Neurology. 2016;87(8):835-840.
Has this ever happened to you? You are an adult neurologist who has been asked to take on the care of a pediatric neurology patient. The patient who comes to your clinic is a 20-year-old woman with a history of moderate developmental delay and intractable epilepsy. She is on numerous medications, including valproic acid,and has tried the ketogenic diet. You receive a report that she has focal epilepsy, she is having frequent seizures, and her last MRI was performed at age 2.
Prior notes talk about her summer vacations but not much about the future plans for her epilepsy. You see the patient in the clinic, and the family is not happy to be in the adult clinic. They are disappointed that you don’t spend more time with them or fill out myriad forms. You find out that they have not obtained legal guardianship for their daughter and have no plan for work placement after school. She also has various other medical comorbidities that were previously addressed by the pediatric neurologist.
Why does this happen? When patients are simply transferred instead of transitioned between providers when they get too old to be seen by pediatric specialists, the process often does not go smoothly. A true transition of care prepares the patient and the family to understand the underlying disease and everything that goes along with it to be able to successfully seek appropriate care as they move into the adult world.
There is not much evidence on the right way to do this. In 2013, the American Epilepsy Society approved a transition tool that is helpful in outlining the steps for a successful transition. In 2016, the Child Neurology Foundation put forth a consensus statement with eight principles to guide a successful transition. Transitions are an expectation of good care, and they recommend that all offices have a written policy.
Talking about transitioning should start as early as 10 to 12 years of age and should be discussed every year. Thinking about prognosis and a realistic plan for each child as they enter adult life is important. Patients and families should be able to understand how the disease affects them, what their medications are and how to independently obtain them, what comorbidities are associated with their disease, how to stay healthy, how to improve their quality of life, and how to advocate for themselves. As children become teenagers, they should have a concrete plan for ongoing education, work, women’s issues, and an understanding of decision-making capacity and whether legal guardianship or a power of attorney needs to be implemented.
When the pediatric epilepsy patient reaches young adulthood (18 years or older), the adult model of care should be implemented, even if they are still being seen in the pediatric setting. A transition packet should be created that includes a summary of the diagnosis, work-up, previous treatments, and considerations for future treatments and emergency care. Also included is a plan for who will continue to address any non–seizure-related diagnoses the pediatric neurologist may have been managing. The patient and family also have an opportunity to review and contribute to this plan. This packet enables the adult neurologist to easily understand all issues and assume care of the patient, easing this aspect of the transition.
An advance meeting of the patient and family with the adult provider should be arranged whenever possible. To address this, some centers are now creating a transition clinic staffed by both pediatric and adult neurologists and/or nurses. This ideally takes place in the adult setting and is an excellent way to ensure a smooth transition for the patient, family, and providers. Good transition is important to help prevent gaps in care, avoid reinventing the wheel, and improve satisfaction for everyone involved (patient, family, nurses, and neurologists).
The key points are that transition discussions start early, patients and families should be involved and empowered in the process, and the creation of a transition packet for the adult provider is very helpful. Care transitions are something we will be hearing a lot more about in the upcoming years. And hopefully, next time, the patient scenario seen above will go more smoothly!
Suggested Reading
Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care. Neurology. 2016;87(8):835-840.
Lung cancer screening a challenge to implement
A comprehensive lung cancer screening program carried out at Veterans Health Administration hospitals was taxing to implement and revealed a large number of patients with results requiring follow-up, though only 1.5% had cancers.
Investigators at eight VHA hospitals, led by Linda S. Kinsinger, MD, of the VHA’s National Center for Health Promotion and Disease Prevention in Durham, N.C., looked at records from about 93,000 primary care patients and identified 4,246 eligible for screening, based on age, medical history, and smoking history (JAMA Intern Med. 2017 Jan 30. doi: 10.1001/jamainternmed.2016.9022).
Approximately 58% of the eligible patients consented, and 2,106 underwent screening with low-dose computed tomography (LDCT). The mean age of patients was 65 years, and 96% of patients were male.
Nearly 60% of patients screened (1,257) had nodules, 1,184 patients (56.2%) required tracking, and 31 patients (1.5%) had lung cancer.
The pilot study was developed in response to a 2013 recommendation from the U.S. Preventive Services Task Force favoring annual screening with LDCT in current or former heavy smokers between 55 and 80 years old.
The recommendation sparked concerns about the practicability of implementing large-scale lung cancer screening, which Dr. Kinsinger and her colleagues’ study seemed to underscore. For example, “creating electronic tools to capture the necessary clinical data in real time … proved to be difficult, even with the VHA’s highly regarded electronic medical record,” the investigators wrote. A key measure used in the screening program – cigarette pack-years – was “not fully captured” in the system’s EMR.
The investigators also noted that if the eligibility criteria used in the pilot program were applied to the VHA nationwide, about 900,000 patients would be eligible for LDCT screening, and that fewer than 60% of patients in this study had consented. That meant that “accurately identifying these patients and discussing with them the benefits and harms of [screening] will take significant effort for primary care teams,” the researchers noted.
In addition, the required follow-up “may stress the capacity” of radiology and pulmonology services, the study authors cautioned.
Finally, “primary care will need to be involved in deciding which incidental findings need further evaluation,” they wrote. “These clinical efforts will require coordination and communication among clinical services and between patients and staff, and dedicated coordinators will need to be hired.”
The investigators noted that their findings might not be generalizable to non-VHA health care systems. The experience of the VHA, “owing to its central organizational structure, may represent a best-case scenario,” they wrote.
The Veterans Health Administration funded the study. Two of its coauthors reported commercial conflicts of interest; one of those disclosed a grant application to the Bristol-Myers Squibb Foundation related to lung cancer screening.
A comprehensive lung cancer screening program carried out at Veterans Health Administration hospitals was taxing to implement and revealed a large number of patients with results requiring follow-up, though only 1.5% had cancers.
Investigators at eight VHA hospitals, led by Linda S. Kinsinger, MD, of the VHA’s National Center for Health Promotion and Disease Prevention in Durham, N.C., looked at records from about 93,000 primary care patients and identified 4,246 eligible for screening, based on age, medical history, and smoking history (JAMA Intern Med. 2017 Jan 30. doi: 10.1001/jamainternmed.2016.9022).
Approximately 58% of the eligible patients consented, and 2,106 underwent screening with low-dose computed tomography (LDCT). The mean age of patients was 65 years, and 96% of patients were male.
Nearly 60% of patients screened (1,257) had nodules, 1,184 patients (56.2%) required tracking, and 31 patients (1.5%) had lung cancer.
The pilot study was developed in response to a 2013 recommendation from the U.S. Preventive Services Task Force favoring annual screening with LDCT in current or former heavy smokers between 55 and 80 years old.
The recommendation sparked concerns about the practicability of implementing large-scale lung cancer screening, which Dr. Kinsinger and her colleagues’ study seemed to underscore. For example, “creating electronic tools to capture the necessary clinical data in real time … proved to be difficult, even with the VHA’s highly regarded electronic medical record,” the investigators wrote. A key measure used in the screening program – cigarette pack-years – was “not fully captured” in the system’s EMR.
The investigators also noted that if the eligibility criteria used in the pilot program were applied to the VHA nationwide, about 900,000 patients would be eligible for LDCT screening, and that fewer than 60% of patients in this study had consented. That meant that “accurately identifying these patients and discussing with them the benefits and harms of [screening] will take significant effort for primary care teams,” the researchers noted.
In addition, the required follow-up “may stress the capacity” of radiology and pulmonology services, the study authors cautioned.
Finally, “primary care will need to be involved in deciding which incidental findings need further evaluation,” they wrote. “These clinical efforts will require coordination and communication among clinical services and between patients and staff, and dedicated coordinators will need to be hired.”
The investigators noted that their findings might not be generalizable to non-VHA health care systems. The experience of the VHA, “owing to its central organizational structure, may represent a best-case scenario,” they wrote.
The Veterans Health Administration funded the study. Two of its coauthors reported commercial conflicts of interest; one of those disclosed a grant application to the Bristol-Myers Squibb Foundation related to lung cancer screening.
A comprehensive lung cancer screening program carried out at Veterans Health Administration hospitals was taxing to implement and revealed a large number of patients with results requiring follow-up, though only 1.5% had cancers.
Investigators at eight VHA hospitals, led by Linda S. Kinsinger, MD, of the VHA’s National Center for Health Promotion and Disease Prevention in Durham, N.C., looked at records from about 93,000 primary care patients and identified 4,246 eligible for screening, based on age, medical history, and smoking history (JAMA Intern Med. 2017 Jan 30. doi: 10.1001/jamainternmed.2016.9022).
Approximately 58% of the eligible patients consented, and 2,106 underwent screening with low-dose computed tomography (LDCT). The mean age of patients was 65 years, and 96% of patients were male.
Nearly 60% of patients screened (1,257) had nodules, 1,184 patients (56.2%) required tracking, and 31 patients (1.5%) had lung cancer.
The pilot study was developed in response to a 2013 recommendation from the U.S. Preventive Services Task Force favoring annual screening with LDCT in current or former heavy smokers between 55 and 80 years old.
The recommendation sparked concerns about the practicability of implementing large-scale lung cancer screening, which Dr. Kinsinger and her colleagues’ study seemed to underscore. For example, “creating electronic tools to capture the necessary clinical data in real time … proved to be difficult, even with the VHA’s highly regarded electronic medical record,” the investigators wrote. A key measure used in the screening program – cigarette pack-years – was “not fully captured” in the system’s EMR.
The investigators also noted that if the eligibility criteria used in the pilot program were applied to the VHA nationwide, about 900,000 patients would be eligible for LDCT screening, and that fewer than 60% of patients in this study had consented. That meant that “accurately identifying these patients and discussing with them the benefits and harms of [screening] will take significant effort for primary care teams,” the researchers noted.
In addition, the required follow-up “may stress the capacity” of radiology and pulmonology services, the study authors cautioned.
Finally, “primary care will need to be involved in deciding which incidental findings need further evaluation,” they wrote. “These clinical efforts will require coordination and communication among clinical services and between patients and staff, and dedicated coordinators will need to be hired.”
The investigators noted that their findings might not be generalizable to non-VHA health care systems. The experience of the VHA, “owing to its central organizational structure, may represent a best-case scenario,” they wrote.
The Veterans Health Administration funded the study. Two of its coauthors reported commercial conflicts of interest; one of those disclosed a grant application to the Bristol-Myers Squibb Foundation related to lung cancer screening.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Comprehensive lung cancer screening is complex to implement in hospital primary care settings and may trigger resource-intensive follow-up.
Major finding: Of more than 2,000 patients screened, nearly 60% were positive for nodules, though only 1.5% had cancer.
Data source: A pilot study in 4,246 eligible primary care patients at eight Veterans Health Administration hospitals; 2,106 were screened using low-dose computed tomography.
Disclosures: The Veterans Health Administration funded the study. Two of its coauthors reported commercial conflicts of interest; one of those disclosed a grant application to the Bristol-Myers Squibb Foundation related to lung cancer screening.
Hypertension risk soars in offspring of early-HT parents
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.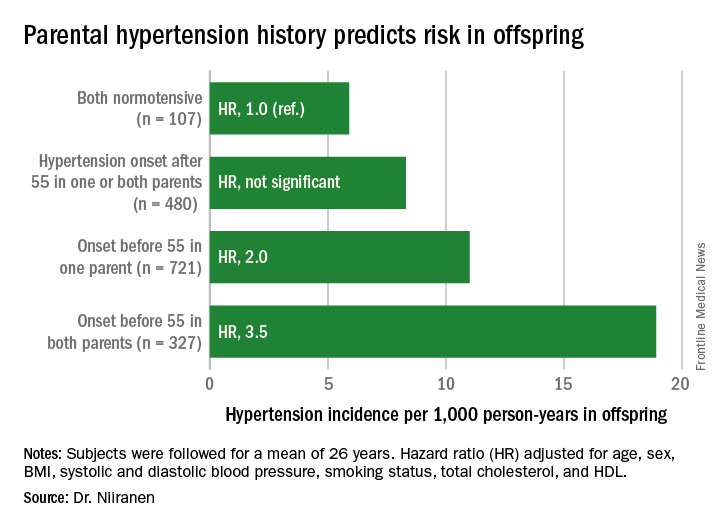
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
Key clinical point:
Major finding: Young adults with two parents who developed hypertension before age 55 years are at 3.5-fold greater risk of subsequently developing hypertension than if both parents were normotensive.
Data source: This was an analysis of the incidence of hypertension during a mean prospective follow-up of 26 years in 1,635 members of the Offspring cohort of the Framingham Heart Study who enrolled as young adults.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study sponsored by the National Heart, Lung, and Blood Institute.
Ocrelizumab Reduces Disease Progression in MS
Ocrelizumab is associated with lower rates of disease activity and progression in patients with relapsing-remitting multiple sclerosis (MS), compared with interferon beta-1a, according to research published in the New England Journal of Medicine. A separate study published in the same journal indicates that ocrelizumab is associated with lower rates of clinical and MRI progression in patients with primary progressive MS, compared with placebo. Ocrelizumab selectively depletes CD20+ B cells, and both studies provide evidence that B cells contribute to the pathogenesis of MS.
“This is the first drug to show a significant effect in slowing disability progression in a phase III trial in primary progressive MS, and therefore the trial represents a landmark study in the field,” said Peter A. Calabresi, MD, Professor of Neurology at Johns Hopkins University School of Medicine in Baltimore, in an accompanying editorial. The articles and editorial were published online ahead of print December 21, 2016.
Ocrelizumab in Relapsing-Remitting MS
Stephen L. Hauser, MD, Chair of Neurology at the University of California, San Francisco School of Medicine, and colleagues conducted OPERA I and OPERA II, two phase III studies to investigate the safety and efficacy of ocrelizumab, compared with interferon beta-1a, in patients with relapsing-remitting MS. The trials had identical protocols, but were conducted at separate trial sites. Eligible participants were between ages 18 and 55, had an Expanded Disability Status Scale (EDSS) score no greater than 5.5 at screening, a history of relapses, and MRI abnormalities consistent with MS. Patients who previously had received a B-cell-targeted therapy or other immunosuppressive medication were excluded.
A total of 821 patients in OPERA I and 835 patients in OPERA II were randomized to 600 mg of IV ocrelizumab every 24 weeks or to 44 µg of subcutaneous interferon beta-1a three times weekly. The treatment period was 96 weeks. Each trial site had separate treating and examining investigators, all of whom were unaware of participants’ treatment assignments. The primary end point was the annualized relapse rate by 96 weeks. Secondary end points included the proportion of patients with disability progression confirmed at 12 weeks, the mean number of gadolinium-enhancing lesions identified on T1-weighted MRI, and the proportion of patients with disability improvement confirmed at 12 weeks.
The annualized relapse rate at 96 weeks in OPERA I was 0.16 in the ocrelizumab group, compared with 0.29 in the interferon beta-1a group. In OPERA II, the annualized relapse rate was 0.16 in the ocrelizumab group, compared with 0.29 in the interferon beta-1a group. These data suggest a 46% lower annualized relapse rate with ocrelizumab in OPERA I and a 47% lower rate with ocrelizumab in OPERA II.
The mean numbers of gadolinium-enhancing lesions in OPERA I were 0.02 with ocrelizumab versus 0.29 with interferon beta-1a. The numbers in OPERA II were 0.02 with ocrelizumab versus 0.42 with interferon beta-1a. The mean numbers of new or newly enlarged T2 hyperintense lesions in OPERA I were 0.32 with ocrelizumab and 1.41 with interferon beta-1a (ie, 77% fewer lesions with ocrelizumab). The values in OPERA II were 0.33 with ocrelizumab versus 1.90 with interferon beta-1a.
In a pooled analysis, 9.1% of patients in the ocrelizumab group had disability progression confirmed at 12 weeks, compared with 13.6% of patients in the interferon beta-1a group. The percentage of patients with disability improvement confirmed at 12 weeks was 20.7% in the ocrelizumab group, compared with 15.6% in the interferon beta-1a group (ie, a 33% higher rate of improvement with ocrelizumab). The effect of ocrelizumab on the rate of confirmed disability improvement was significant in OPERA I, but nonsignificant in OPERA II.
“Additional and extended studies will be required to determine whether the outcomes observed in these 96-week trials, including a near-complete cessation of new plaque formation, as assessed by MRI of the brain, translate into enhanced protection against accrual of disability over the long term,” said Dr. Hauser.
Ocrelizumab in Progressive MS
To examine the safety and efficacy of ocrelizumab in primary progressive MS, Xavier Montalban, MD, PhD, Chair of the Department of Neurology-Neuroimmunology and Director of the MS Centre of Catalonia at the Vall d’Hebron University Hospital in Barcelona, and colleagues conducted the phase III ORATORIO trial. Eligible patients were between ages 18 and 55 and had an EDSS score between 3.0 and 6.5 at screening. Patients with a history of relapsing-remitting MS, secondary progressive MS, or progressive relapsing MS were excluded, as were patients who had had previous treatment with B-cell-targeted therapies and other immunosuppressive medications.
Patients were randomized in a 2:1 ratio to receive 600 mg of IV ocrelizumab or matching placebo every 24 weeks. Double-blinded treatment was administered for at least 120 weeks. As in the OPERA trials, each trial site had separate treating and examining investigators. Patients who completed the blinded treatment phase were eligible to enter an open-label extension phase.
The primary end point was the percentage of patients with disability progression confirmed at 12 weeks in a time-to-event analysis. Secondary end points included the percentage of patients with disability progression confirmed at 24 weeks, change in performance on the timed 25-foot walk from baseline to week 120, change in the total volume of brain lesions on T2-weighted MRI from baseline to week 120, change in brain volume from week 24 to week 120, and change in the Physical Component Summary score of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), version 2, from baseline to week 120.
In all, 488 patients received ocrelizumab, and 244 received placebo. A total of 402 patients in the ocrelizumab arm and 174 controls completed the 120-week treatment period. The percentage of patients with 12-week confirmed disability progression was 32.9% with ocrelizumab versus 39.3% with placebo. The data suggest a 24% reduction in the risk of this outcome with ocrelizumab.
The percentage of patients with 24-week confirmed disability progression was 29.6% with ocrelizumab and 35.7% with placebo (ie, a relative risk reduction of 25% with ocrelizumab). The mean change from baseline to week 120 in performance on the timed 25-foot walk was 38.9% with ocrelizumab versus 55.1% with placebo (ie, a relative reduction of 29.3% with ocrelizumab). The researchers found no significant between-group difference in the change in the SF-36 Physical Component Summary score from baseline to week 120.
In addition, the total volume of hyperintense lesions on T2-weighted images from baseline to week 120 decreased with ocrelizumab and increased with placebo (mean percent change, −3.4% vs 7.4%). The adjusted mean percent change in brain volume from week 24 to week 120 was lower with ocrelizumab than with placebo (–0.90% vs –1.09%).
Infusion-related reactions were more common in the ocrelizumab group than among controls. Neoplasms were reported in 11 of 486 patients (2.3%) in the ocrelizumab group and in two of 239 patients (0.8%) in the placebo group. “Safety will continue to be assessed throughout the open-label extension phase,” said Dr. Montalban.
Unknown Mechanism of Action
The mechanism by which B-cell depletion slows disability progression is not fully understood. It “may be multifunctional, because B cells have important roles in antibody secretion, antigen presentation, and the release of effector cytokines,” said Dr. Calabresi. By virtue of their number, B cells may be more important than other antigen-presenting cells in MS.
One potential reason that ocrelizumab showed positive effects in ORATORIO is that the patient population was relatively young (mean age, 45) and had active MRI scans (more than 25% of the population had gadolinium-enhancing lesions). These factors enabled the demonstration of a measurable anti-inflammatory effect of ocrelizumab in patients with inflammation at an early, reversible stage of the disease. “Another possible explanation is that B cells may mediate pathologic processes by secretion of cytokines or by deposition of immunoglobulins after they enter the CNS,” said Dr. Calabresi. “B cells and plasma cells secrete antibodies that may target CNS antigens such as myelin, neurons, and glia, which could accelerate neurodegeneration or inhibit myelin repair. The continued separation of disability progression curves in the ORATORIO trial beyond 52 weeks, when anti-inflammatory effects have been maximized, and success in the relatively noninflammatory disorder of primary progressive MS suggest that additional mechanisms of action may be operational, and further study is warranted.”
Because ocrelizumab appears to entail a higher risk of herpes reactivation and of neoplasms, “clinicians are urged to carefully consider which patients might benefit the most from ocrelizumab and to stay vigilant with regard to monitoring for side effects that could be managed effectively if detected early,” Dr. Calabresi concluded.
—Erik Greb
Suggested Reading
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Calabresi PA. B-cell depletion - a frontier in monoclonal antibodies for multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Ocrelizumab is associated with lower rates of disease activity and progression in patients with relapsing-remitting multiple sclerosis (MS), compared with interferon beta-1a, according to research published in the New England Journal of Medicine. A separate study published in the same journal indicates that ocrelizumab is associated with lower rates of clinical and MRI progression in patients with primary progressive MS, compared with placebo. Ocrelizumab selectively depletes CD20+ B cells, and both studies provide evidence that B cells contribute to the pathogenesis of MS.
“This is the first drug to show a significant effect in slowing disability progression in a phase III trial in primary progressive MS, and therefore the trial represents a landmark study in the field,” said Peter A. Calabresi, MD, Professor of Neurology at Johns Hopkins University School of Medicine in Baltimore, in an accompanying editorial. The articles and editorial were published online ahead of print December 21, 2016.
Ocrelizumab in Relapsing-Remitting MS
Stephen L. Hauser, MD, Chair of Neurology at the University of California, San Francisco School of Medicine, and colleagues conducted OPERA I and OPERA II, two phase III studies to investigate the safety and efficacy of ocrelizumab, compared with interferon beta-1a, in patients with relapsing-remitting MS. The trials had identical protocols, but were conducted at separate trial sites. Eligible participants were between ages 18 and 55, had an Expanded Disability Status Scale (EDSS) score no greater than 5.5 at screening, a history of relapses, and MRI abnormalities consistent with MS. Patients who previously had received a B-cell-targeted therapy or other immunosuppressive medication were excluded.
A total of 821 patients in OPERA I and 835 patients in OPERA II were randomized to 600 mg of IV ocrelizumab every 24 weeks or to 44 µg of subcutaneous interferon beta-1a three times weekly. The treatment period was 96 weeks. Each trial site had separate treating and examining investigators, all of whom were unaware of participants’ treatment assignments. The primary end point was the annualized relapse rate by 96 weeks. Secondary end points included the proportion of patients with disability progression confirmed at 12 weeks, the mean number of gadolinium-enhancing lesions identified on T1-weighted MRI, and the proportion of patients with disability improvement confirmed at 12 weeks.
The annualized relapse rate at 96 weeks in OPERA I was 0.16 in the ocrelizumab group, compared with 0.29 in the interferon beta-1a group. In OPERA II, the annualized relapse rate was 0.16 in the ocrelizumab group, compared with 0.29 in the interferon beta-1a group. These data suggest a 46% lower annualized relapse rate with ocrelizumab in OPERA I and a 47% lower rate with ocrelizumab in OPERA II.
The mean numbers of gadolinium-enhancing lesions in OPERA I were 0.02 with ocrelizumab versus 0.29 with interferon beta-1a. The numbers in OPERA II were 0.02 with ocrelizumab versus 0.42 with interferon beta-1a. The mean numbers of new or newly enlarged T2 hyperintense lesions in OPERA I were 0.32 with ocrelizumab and 1.41 with interferon beta-1a (ie, 77% fewer lesions with ocrelizumab). The values in OPERA II were 0.33 with ocrelizumab versus 1.90 with interferon beta-1a.
In a pooled analysis, 9.1% of patients in the ocrelizumab group had disability progression confirmed at 12 weeks, compared with 13.6% of patients in the interferon beta-1a group. The percentage of patients with disability improvement confirmed at 12 weeks was 20.7% in the ocrelizumab group, compared with 15.6% in the interferon beta-1a group (ie, a 33% higher rate of improvement with ocrelizumab). The effect of ocrelizumab on the rate of confirmed disability improvement was significant in OPERA I, but nonsignificant in OPERA II.
“Additional and extended studies will be required to determine whether the outcomes observed in these 96-week trials, including a near-complete cessation of new plaque formation, as assessed by MRI of the brain, translate into enhanced protection against accrual of disability over the long term,” said Dr. Hauser.
Ocrelizumab in Progressive MS
To examine the safety and efficacy of ocrelizumab in primary progressive MS, Xavier Montalban, MD, PhD, Chair of the Department of Neurology-Neuroimmunology and Director of the MS Centre of Catalonia at the Vall d’Hebron University Hospital in Barcelona, and colleagues conducted the phase III ORATORIO trial. Eligible patients were between ages 18 and 55 and had an EDSS score between 3.0 and 6.5 at screening. Patients with a history of relapsing-remitting MS, secondary progressive MS, or progressive relapsing MS were excluded, as were patients who had had previous treatment with B-cell-targeted therapies and other immunosuppressive medications.
Patients were randomized in a 2:1 ratio to receive 600 mg of IV ocrelizumab or matching placebo every 24 weeks. Double-blinded treatment was administered for at least 120 weeks. As in the OPERA trials, each trial site had separate treating and examining investigators. Patients who completed the blinded treatment phase were eligible to enter an open-label extension phase.
The primary end point was the percentage of patients with disability progression confirmed at 12 weeks in a time-to-event analysis. Secondary end points included the percentage of patients with disability progression confirmed at 24 weeks, change in performance on the timed 25-foot walk from baseline to week 120, change in the total volume of brain lesions on T2-weighted MRI from baseline to week 120, change in brain volume from week 24 to week 120, and change in the Physical Component Summary score of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), version 2, from baseline to week 120.
In all, 488 patients received ocrelizumab, and 244 received placebo. A total of 402 patients in the ocrelizumab arm and 174 controls completed the 120-week treatment period. The percentage of patients with 12-week confirmed disability progression was 32.9% with ocrelizumab versus 39.3% with placebo. The data suggest a 24% reduction in the risk of this outcome with ocrelizumab.
The percentage of patients with 24-week confirmed disability progression was 29.6% with ocrelizumab and 35.7% with placebo (ie, a relative risk reduction of 25% with ocrelizumab). The mean change from baseline to week 120 in performance on the timed 25-foot walk was 38.9% with ocrelizumab versus 55.1% with placebo (ie, a relative reduction of 29.3% with ocrelizumab). The researchers found no significant between-group difference in the change in the SF-36 Physical Component Summary score from baseline to week 120.
In addition, the total volume of hyperintense lesions on T2-weighted images from baseline to week 120 decreased with ocrelizumab and increased with placebo (mean percent change, −3.4% vs 7.4%). The adjusted mean percent change in brain volume from week 24 to week 120 was lower with ocrelizumab than with placebo (–0.90% vs –1.09%).
Infusion-related reactions were more common in the ocrelizumab group than among controls. Neoplasms were reported in 11 of 486 patients (2.3%) in the ocrelizumab group and in two of 239 patients (0.8%) in the placebo group. “Safety will continue to be assessed throughout the open-label extension phase,” said Dr. Montalban.
Unknown Mechanism of Action
The mechanism by which B-cell depletion slows disability progression is not fully understood. It “may be multifunctional, because B cells have important roles in antibody secretion, antigen presentation, and the release of effector cytokines,” said Dr. Calabresi. By virtue of their number, B cells may be more important than other antigen-presenting cells in MS.
One potential reason that ocrelizumab showed positive effects in ORATORIO is that the patient population was relatively young (mean age, 45) and had active MRI scans (more than 25% of the population had gadolinium-enhancing lesions). These factors enabled the demonstration of a measurable anti-inflammatory effect of ocrelizumab in patients with inflammation at an early, reversible stage of the disease. “Another possible explanation is that B cells may mediate pathologic processes by secretion of cytokines or by deposition of immunoglobulins after they enter the CNS,” said Dr. Calabresi. “B cells and plasma cells secrete antibodies that may target CNS antigens such as myelin, neurons, and glia, which could accelerate neurodegeneration or inhibit myelin repair. The continued separation of disability progression curves in the ORATORIO trial beyond 52 weeks, when anti-inflammatory effects have been maximized, and success in the relatively noninflammatory disorder of primary progressive MS suggest that additional mechanisms of action may be operational, and further study is warranted.”
Because ocrelizumab appears to entail a higher risk of herpes reactivation and of neoplasms, “clinicians are urged to carefully consider which patients might benefit the most from ocrelizumab and to stay vigilant with regard to monitoring for side effects that could be managed effectively if detected early,” Dr. Calabresi concluded.
—Erik Greb
Suggested Reading
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Calabresi PA. B-cell depletion - a frontier in monoclonal antibodies for multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Ocrelizumab is associated with lower rates of disease activity and progression in patients with relapsing-remitting multiple sclerosis (MS), compared with interferon beta-1a, according to research published in the New England Journal of Medicine. A separate study published in the same journal indicates that ocrelizumab is associated with lower rates of clinical and MRI progression in patients with primary progressive MS, compared with placebo. Ocrelizumab selectively depletes CD20+ B cells, and both studies provide evidence that B cells contribute to the pathogenesis of MS.
“This is the first drug to show a significant effect in slowing disability progression in a phase III trial in primary progressive MS, and therefore the trial represents a landmark study in the field,” said Peter A. Calabresi, MD, Professor of Neurology at Johns Hopkins University School of Medicine in Baltimore, in an accompanying editorial. The articles and editorial were published online ahead of print December 21, 2016.
Ocrelizumab in Relapsing-Remitting MS
Stephen L. Hauser, MD, Chair of Neurology at the University of California, San Francisco School of Medicine, and colleagues conducted OPERA I and OPERA II, two phase III studies to investigate the safety and efficacy of ocrelizumab, compared with interferon beta-1a, in patients with relapsing-remitting MS. The trials had identical protocols, but were conducted at separate trial sites. Eligible participants were between ages 18 and 55, had an Expanded Disability Status Scale (EDSS) score no greater than 5.5 at screening, a history of relapses, and MRI abnormalities consistent with MS. Patients who previously had received a B-cell-targeted therapy or other immunosuppressive medication were excluded.
A total of 821 patients in OPERA I and 835 patients in OPERA II were randomized to 600 mg of IV ocrelizumab every 24 weeks or to 44 µg of subcutaneous interferon beta-1a three times weekly. The treatment period was 96 weeks. Each trial site had separate treating and examining investigators, all of whom were unaware of participants’ treatment assignments. The primary end point was the annualized relapse rate by 96 weeks. Secondary end points included the proportion of patients with disability progression confirmed at 12 weeks, the mean number of gadolinium-enhancing lesions identified on T1-weighted MRI, and the proportion of patients with disability improvement confirmed at 12 weeks.
The annualized relapse rate at 96 weeks in OPERA I was 0.16 in the ocrelizumab group, compared with 0.29 in the interferon beta-1a group. In OPERA II, the annualized relapse rate was 0.16 in the ocrelizumab group, compared with 0.29 in the interferon beta-1a group. These data suggest a 46% lower annualized relapse rate with ocrelizumab in OPERA I and a 47% lower rate with ocrelizumab in OPERA II.
The mean numbers of gadolinium-enhancing lesions in OPERA I were 0.02 with ocrelizumab versus 0.29 with interferon beta-1a. The numbers in OPERA II were 0.02 with ocrelizumab versus 0.42 with interferon beta-1a. The mean numbers of new or newly enlarged T2 hyperintense lesions in OPERA I were 0.32 with ocrelizumab and 1.41 with interferon beta-1a (ie, 77% fewer lesions with ocrelizumab). The values in OPERA II were 0.33 with ocrelizumab versus 1.90 with interferon beta-1a.
In a pooled analysis, 9.1% of patients in the ocrelizumab group had disability progression confirmed at 12 weeks, compared with 13.6% of patients in the interferon beta-1a group. The percentage of patients with disability improvement confirmed at 12 weeks was 20.7% in the ocrelizumab group, compared with 15.6% in the interferon beta-1a group (ie, a 33% higher rate of improvement with ocrelizumab). The effect of ocrelizumab on the rate of confirmed disability improvement was significant in OPERA I, but nonsignificant in OPERA II.
“Additional and extended studies will be required to determine whether the outcomes observed in these 96-week trials, including a near-complete cessation of new plaque formation, as assessed by MRI of the brain, translate into enhanced protection against accrual of disability over the long term,” said Dr. Hauser.
Ocrelizumab in Progressive MS
To examine the safety and efficacy of ocrelizumab in primary progressive MS, Xavier Montalban, MD, PhD, Chair of the Department of Neurology-Neuroimmunology and Director of the MS Centre of Catalonia at the Vall d’Hebron University Hospital in Barcelona, and colleagues conducted the phase III ORATORIO trial. Eligible patients were between ages 18 and 55 and had an EDSS score between 3.0 and 6.5 at screening. Patients with a history of relapsing-remitting MS, secondary progressive MS, or progressive relapsing MS were excluded, as were patients who had had previous treatment with B-cell-targeted therapies and other immunosuppressive medications.
Patients were randomized in a 2:1 ratio to receive 600 mg of IV ocrelizumab or matching placebo every 24 weeks. Double-blinded treatment was administered for at least 120 weeks. As in the OPERA trials, each trial site had separate treating and examining investigators. Patients who completed the blinded treatment phase were eligible to enter an open-label extension phase.
The primary end point was the percentage of patients with disability progression confirmed at 12 weeks in a time-to-event analysis. Secondary end points included the percentage of patients with disability progression confirmed at 24 weeks, change in performance on the timed 25-foot walk from baseline to week 120, change in the total volume of brain lesions on T2-weighted MRI from baseline to week 120, change in brain volume from week 24 to week 120, and change in the Physical Component Summary score of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), version 2, from baseline to week 120.
In all, 488 patients received ocrelizumab, and 244 received placebo. A total of 402 patients in the ocrelizumab arm and 174 controls completed the 120-week treatment period. The percentage of patients with 12-week confirmed disability progression was 32.9% with ocrelizumab versus 39.3% with placebo. The data suggest a 24% reduction in the risk of this outcome with ocrelizumab.
The percentage of patients with 24-week confirmed disability progression was 29.6% with ocrelizumab and 35.7% with placebo (ie, a relative risk reduction of 25% with ocrelizumab). The mean change from baseline to week 120 in performance on the timed 25-foot walk was 38.9% with ocrelizumab versus 55.1% with placebo (ie, a relative reduction of 29.3% with ocrelizumab). The researchers found no significant between-group difference in the change in the SF-36 Physical Component Summary score from baseline to week 120.
In addition, the total volume of hyperintense lesions on T2-weighted images from baseline to week 120 decreased with ocrelizumab and increased with placebo (mean percent change, −3.4% vs 7.4%). The adjusted mean percent change in brain volume from week 24 to week 120 was lower with ocrelizumab than with placebo (–0.90% vs –1.09%).
Infusion-related reactions were more common in the ocrelizumab group than among controls. Neoplasms were reported in 11 of 486 patients (2.3%) in the ocrelizumab group and in two of 239 patients (0.8%) in the placebo group. “Safety will continue to be assessed throughout the open-label extension phase,” said Dr. Montalban.
Unknown Mechanism of Action
The mechanism by which B-cell depletion slows disability progression is not fully understood. It “may be multifunctional, because B cells have important roles in antibody secretion, antigen presentation, and the release of effector cytokines,” said Dr. Calabresi. By virtue of their number, B cells may be more important than other antigen-presenting cells in MS.
One potential reason that ocrelizumab showed positive effects in ORATORIO is that the patient population was relatively young (mean age, 45) and had active MRI scans (more than 25% of the population had gadolinium-enhancing lesions). These factors enabled the demonstration of a measurable anti-inflammatory effect of ocrelizumab in patients with inflammation at an early, reversible stage of the disease. “Another possible explanation is that B cells may mediate pathologic processes by secretion of cytokines or by deposition of immunoglobulins after they enter the CNS,” said Dr. Calabresi. “B cells and plasma cells secrete antibodies that may target CNS antigens such as myelin, neurons, and glia, which could accelerate neurodegeneration or inhibit myelin repair. The continued separation of disability progression curves in the ORATORIO trial beyond 52 weeks, when anti-inflammatory effects have been maximized, and success in the relatively noninflammatory disorder of primary progressive MS suggest that additional mechanisms of action may be operational, and further study is warranted.”
Because ocrelizumab appears to entail a higher risk of herpes reactivation and of neoplasms, “clinicians are urged to carefully consider which patients might benefit the most from ocrelizumab and to stay vigilant with regard to monitoring for side effects that could be managed effectively if detected early,” Dr. Calabresi concluded.
—Erik Greb
Suggested Reading
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
Calabresi PA. B-cell depletion - a frontier in monoclonal antibodies for multiple sclerosis. N Engl J Med. 2016 Dec 21 [Epub ahead of print].
STICHES boosts CABG role in severe LV dysfunction
SNOWMASS, COLO. – Coronary artery bypass graft surgery in patients with severe ischemic left ventricular dysfunction is overdue for an upgrade in status in the American College of Cardiology/American Heart Association guidelines on the strength of the landmark STICH trial and its extended follow-up stage known as STICHES, according to Vinod H. Thourani, MD.
Currently, the guidelines give CABG in this large and growing population a class IIb recommendation, meaning it “might be considered.” This undervalues the study’s core lesson: “STICHES showed a clear survival benefit with CABG, so this most likely should become a class IIa recommendation,” Dr. Thourani said at the Annual Cardiovascular Conference at Snowmass.
He went on to describe how he applies the key study findings to individual patients.
At 5 and 10 years of follow-up, the probability of all-cause mortality was reduced by 14% and 16%, respectively, in the CABG group. The surgery provided on average an 18-month extension of life. The price paid for the CABG benefit was a 3.6% mortality rate at 30 days; however, this was overcome by the 2-year mark, at which point survival in the CABG group surpassed that in controls. Thereafter, the all-cause mortality gap between the two groups continued to widen for the duration of follow-up.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization, the CABG group enjoyed a 26% relative risk reduction, compared with optimal medical management alone at 5 years, and a 28% reduction in risk at 10 years. The two study groups diverged in terms of risk of cardiovascular hospitalization after only 3 months.
CABG provided a reduction in the risk of cardiovascular death that was consistent across all ages. In contrast, the reduction in all-cause mortality was not, since a higher proportion of deaths in older patients came from cancer and other noncardiovascular causes (Circulation. 2016 Nov 1;134[18]:1314-24).
There have been no randomized, controlled trials of percutaneous coronary intervention in patients with heart failure.
“An interesting finding in STICHES was that medical therapy had a much higher 10-year all-cause mortality the younger the patient was. So CABG particularly benefits those who are at a younger age – in this study, age 60 or less. As you get older, say, at 80 years of age, I’m not sure there’s a huge benefit in all-cause mortality at that point,” said Dr. Thourani, professor of surgery and medicine, and codirector of the structural heart and valve center at Emory University in Atlanta.
In a STICH substudy, roughly half of participants underwent presurgical myocardial viability testing via single-photon emission CT and/or dobutamine echocardiography. The investigators found that the results didn’t predict mortality benefit for CABG (N Engl J Med. 2011 Apr 28;364[17]:1617-25).
More recently, however, other investigators have reported MRI to have prognostic value. For example, Belgian investigators showed that medical therapy in patients with ischemic heart failure and dysfunctional but viable myocardium on delayed-enhanced MRI was associated with a 4.56-fold increased likelihood of mortality during 3 years of follow-up, compared with complete revascularization via CABG (J Am Coll Cardiol. 2012 Feb 28;59[9]:825-35).
“This observation has been useful for me,” Dr. Thourani said. “My own personal practice is if I have good targets, I don’t do viability testing, but if I have really bad targets where I know I’m going to have a tough time sewing grafts, I try to get an MRI for viability testing.”
One important lesson of STICH is that all patients with heart failure and a low left ventricular ejection fraction should have a coronary angiogram, even if they are free of ischemia on noninvasive testing and have no angina. That’s because the patients enrolled in STICH had little or no angina, the surgeon continued.
These STICH-type patients will benefit greatly from a heart team assessment factoring in an individual’s Society of Thoracic Surgeons’ predicted risk score, based on age, comorbidities, and other factors. For example, if a patient’s STS risk score with CABG is 0.7%, that’s a strong argument for opting for the surgery, since the 30-day operative mortality in STICH was 3.6%. If, on the other hand, the STS score is greater than 7%, that’s a tougher call.
“I think it’s really important that a heart team assessment includes a noninvasive cardiologist as well as an interventional cardiologist and cardiac surgeon because I think interventionalists and cardiac surgeons sometimes get a little goofy in their assessment of these patients,” Dr. Thourani said.
Patients with a low ejection fraction and coronary artery disease who are deemed poor candidates for CABG should be evaluated for a mechanical circulatory support device or a heart transplant.
“I think that’s something we don’t think about enough, quite honestly,” he said.
Dr. Thourani reported serving as a consultant to Abbott Vascular, Edwards Lifesciences, and Gore, and receiving research grants from numerous companies.
SNOWMASS, COLO. – Coronary artery bypass graft surgery in patients with severe ischemic left ventricular dysfunction is overdue for an upgrade in status in the American College of Cardiology/American Heart Association guidelines on the strength of the landmark STICH trial and its extended follow-up stage known as STICHES, according to Vinod H. Thourani, MD.
Currently, the guidelines give CABG in this large and growing population a class IIb recommendation, meaning it “might be considered.” This undervalues the study’s core lesson: “STICHES showed a clear survival benefit with CABG, so this most likely should become a class IIa recommendation,” Dr. Thourani said at the Annual Cardiovascular Conference at Snowmass.
He went on to describe how he applies the key study findings to individual patients.
At 5 and 10 years of follow-up, the probability of all-cause mortality was reduced by 14% and 16%, respectively, in the CABG group. The surgery provided on average an 18-month extension of life. The price paid for the CABG benefit was a 3.6% mortality rate at 30 days; however, this was overcome by the 2-year mark, at which point survival in the CABG group surpassed that in controls. Thereafter, the all-cause mortality gap between the two groups continued to widen for the duration of follow-up.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization, the CABG group enjoyed a 26% relative risk reduction, compared with optimal medical management alone at 5 years, and a 28% reduction in risk at 10 years. The two study groups diverged in terms of risk of cardiovascular hospitalization after only 3 months.
CABG provided a reduction in the risk of cardiovascular death that was consistent across all ages. In contrast, the reduction in all-cause mortality was not, since a higher proportion of deaths in older patients came from cancer and other noncardiovascular causes (Circulation. 2016 Nov 1;134[18]:1314-24).
There have been no randomized, controlled trials of percutaneous coronary intervention in patients with heart failure.
“An interesting finding in STICHES was that medical therapy had a much higher 10-year all-cause mortality the younger the patient was. So CABG particularly benefits those who are at a younger age – in this study, age 60 or less. As you get older, say, at 80 years of age, I’m not sure there’s a huge benefit in all-cause mortality at that point,” said Dr. Thourani, professor of surgery and medicine, and codirector of the structural heart and valve center at Emory University in Atlanta.
In a STICH substudy, roughly half of participants underwent presurgical myocardial viability testing via single-photon emission CT and/or dobutamine echocardiography. The investigators found that the results didn’t predict mortality benefit for CABG (N Engl J Med. 2011 Apr 28;364[17]:1617-25).
More recently, however, other investigators have reported MRI to have prognostic value. For example, Belgian investigators showed that medical therapy in patients with ischemic heart failure and dysfunctional but viable myocardium on delayed-enhanced MRI was associated with a 4.56-fold increased likelihood of mortality during 3 years of follow-up, compared with complete revascularization via CABG (J Am Coll Cardiol. 2012 Feb 28;59[9]:825-35).
“This observation has been useful for me,” Dr. Thourani said. “My own personal practice is if I have good targets, I don’t do viability testing, but if I have really bad targets where I know I’m going to have a tough time sewing grafts, I try to get an MRI for viability testing.”
One important lesson of STICH is that all patients with heart failure and a low left ventricular ejection fraction should have a coronary angiogram, even if they are free of ischemia on noninvasive testing and have no angina. That’s because the patients enrolled in STICH had little or no angina, the surgeon continued.
These STICH-type patients will benefit greatly from a heart team assessment factoring in an individual’s Society of Thoracic Surgeons’ predicted risk score, based on age, comorbidities, and other factors. For example, if a patient’s STS risk score with CABG is 0.7%, that’s a strong argument for opting for the surgery, since the 30-day operative mortality in STICH was 3.6%. If, on the other hand, the STS score is greater than 7%, that’s a tougher call.
“I think it’s really important that a heart team assessment includes a noninvasive cardiologist as well as an interventional cardiologist and cardiac surgeon because I think interventionalists and cardiac surgeons sometimes get a little goofy in their assessment of these patients,” Dr. Thourani said.
Patients with a low ejection fraction and coronary artery disease who are deemed poor candidates for CABG should be evaluated for a mechanical circulatory support device or a heart transplant.
“I think that’s something we don’t think about enough, quite honestly,” he said.
Dr. Thourani reported serving as a consultant to Abbott Vascular, Edwards Lifesciences, and Gore, and receiving research grants from numerous companies.
SNOWMASS, COLO. – Coronary artery bypass graft surgery in patients with severe ischemic left ventricular dysfunction is overdue for an upgrade in status in the American College of Cardiology/American Heart Association guidelines on the strength of the landmark STICH trial and its extended follow-up stage known as STICHES, according to Vinod H. Thourani, MD.
Currently, the guidelines give CABG in this large and growing population a class IIb recommendation, meaning it “might be considered.” This undervalues the study’s core lesson: “STICHES showed a clear survival benefit with CABG, so this most likely should become a class IIa recommendation,” Dr. Thourani said at the Annual Cardiovascular Conference at Snowmass.
He went on to describe how he applies the key study findings to individual patients.
At 5 and 10 years of follow-up, the probability of all-cause mortality was reduced by 14% and 16%, respectively, in the CABG group. The surgery provided on average an 18-month extension of life. The price paid for the CABG benefit was a 3.6% mortality rate at 30 days; however, this was overcome by the 2-year mark, at which point survival in the CABG group surpassed that in controls. Thereafter, the all-cause mortality gap between the two groups continued to widen for the duration of follow-up.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization, the CABG group enjoyed a 26% relative risk reduction, compared with optimal medical management alone at 5 years, and a 28% reduction in risk at 10 years. The two study groups diverged in terms of risk of cardiovascular hospitalization after only 3 months.
CABG provided a reduction in the risk of cardiovascular death that was consistent across all ages. In contrast, the reduction in all-cause mortality was not, since a higher proportion of deaths in older patients came from cancer and other noncardiovascular causes (Circulation. 2016 Nov 1;134[18]:1314-24).
There have been no randomized, controlled trials of percutaneous coronary intervention in patients with heart failure.
“An interesting finding in STICHES was that medical therapy had a much higher 10-year all-cause mortality the younger the patient was. So CABG particularly benefits those who are at a younger age – in this study, age 60 or less. As you get older, say, at 80 years of age, I’m not sure there’s a huge benefit in all-cause mortality at that point,” said Dr. Thourani, professor of surgery and medicine, and codirector of the structural heart and valve center at Emory University in Atlanta.
In a STICH substudy, roughly half of participants underwent presurgical myocardial viability testing via single-photon emission CT and/or dobutamine echocardiography. The investigators found that the results didn’t predict mortality benefit for CABG (N Engl J Med. 2011 Apr 28;364[17]:1617-25).
More recently, however, other investigators have reported MRI to have prognostic value. For example, Belgian investigators showed that medical therapy in patients with ischemic heart failure and dysfunctional but viable myocardium on delayed-enhanced MRI was associated with a 4.56-fold increased likelihood of mortality during 3 years of follow-up, compared with complete revascularization via CABG (J Am Coll Cardiol. 2012 Feb 28;59[9]:825-35).
“This observation has been useful for me,” Dr. Thourani said. “My own personal practice is if I have good targets, I don’t do viability testing, but if I have really bad targets where I know I’m going to have a tough time sewing grafts, I try to get an MRI for viability testing.”
One important lesson of STICH is that all patients with heart failure and a low left ventricular ejection fraction should have a coronary angiogram, even if they are free of ischemia on noninvasive testing and have no angina. That’s because the patients enrolled in STICH had little or no angina, the surgeon continued.
These STICH-type patients will benefit greatly from a heart team assessment factoring in an individual’s Society of Thoracic Surgeons’ predicted risk score, based on age, comorbidities, and other factors. For example, if a patient’s STS risk score with CABG is 0.7%, that’s a strong argument for opting for the surgery, since the 30-day operative mortality in STICH was 3.6%. If, on the other hand, the STS score is greater than 7%, that’s a tougher call.
“I think it’s really important that a heart team assessment includes a noninvasive cardiologist as well as an interventional cardiologist and cardiac surgeon because I think interventionalists and cardiac surgeons sometimes get a little goofy in their assessment of these patients,” Dr. Thourani said.
Patients with a low ejection fraction and coronary artery disease who are deemed poor candidates for CABG should be evaluated for a mechanical circulatory support device or a heart transplant.
“I think that’s something we don’t think about enough, quite honestly,” he said.
Dr. Thourani reported serving as a consultant to Abbott Vascular, Edwards Lifesciences, and Gore, and receiving research grants from numerous companies.
EXPERT ANALYSIS AT THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Patient Expectations May Be Potent Factor in DBS for Parkinson’s Disease
PORTLAND, OR—The discovery that a small amount of electricity delivered to the brain could help treat patients with Parkinson’s disease surprised and delighted neuroscientists. Deep brain stimulation (DBS) can “change people’s lives,” which “creates a lot of expectation,” said Michael S. Okun, MD, Adelaide Lackner Professor and Chairman of Neurology at the University of Florida in Gainesville and National Medical Director of the Parkinson’s Foundation. “But let us not be fooled.… There are many shortcomings.”
Although DBS in its current form may be a powerful treatment for tremor, hyperkinesias, dyskinesias, and motor fluctuations, it is not an effective treatment for gait, freezing, balance, speech, and cognition, he said. As Dr. Okun tells patients, DBS likely will not address problems with “walking, talking, and thinking.”
Various studies indicate that understanding and appropriately managing patients’ expectations regarding DBS therapy is important for achieving optimal outcomes, Dr. Okun said in an overview at the Fourth World Parkinson Congress.
It is important for neurologists to align their expectations with patients’ up front. “What do they have an issue with that is impacting their ability to interface with their world?” he said. Then, the treatment team should tailor the operation to the patient.
Setting Realistic Expectations
Several tools, including the Florida Surgical Questionnaire for Parkinson Disease (FLASQ-PD) and another tool developed by Elena Moro, MD, PhD, Professor of Neurology, Grenoble Alpes University, France, can help identify appropriate candidates for DBS based on patient characteristics. Beyond such instruments, an interdisciplinary team, possibly including a neurologist, psychiatrist, neurosurgeon, and neuropsychologist, should identify a rationale for surgery and choose the best treatment approach. Patients who have contraindications (eg, those who have cognitive impairment or a tremor that does not respond to medication), are over age 70, or need palliation nevertheless may be candidates for DBS, Dr. Okun noted.
Research suggests that interdisciplinary screening may effectively identify patients at risk of poor outcomes. Higuchi et al found that the number and severity of concerns that were raised during interdisciplinary DBS evaluations correlated with unintended hospitalizations and worsened quality of life scores after surgery.
In addition, unrealistic expectations are associated with patients’ subjective outcomes. Maier et al interviewed 30 patients before and three months after surgery and classified patients according to whether their perceived outcome was negative, mixed, or positive. Eight patients with a subjective negative outcome had had unrealistic preoperative expectations, had no postsurgical improvement in quality of life, and had significantly higher presurgical and postsurgical apathy and depression scores. Preoperative apathy and depression predicted the subjective outcomes. Although preoperative apathy and depression are not often measured in clinical practice, “maybe it is something we should be thinking about,” Dr. Okun said.Nisenzon et al surveyed patients to evaluate which treatment outcomes were important to them. Pain, fatigue, emotional distress, walking, posture, and balance were among the domains that had high value to patients. These domains should be addressed with patients when discussing what they can anticipate after DBS surgery, Dr. Okun said.
Medication reduction is another issue that physicians should address with patients when discussing DBS. “A lot of people are expecting DBS to eliminate the need for medications,” which does not happen for 99% of patients, Dr. Okun said.
The National Parkinson Foundation has a free guide to DBS that includes a section detailing what patients can anticipate, he said. Dr. Okun and Pamela R. Zeilman, MSN, ANP-BC, DBS Clinical and Study Coordinator at the Center for Movement Disorders and Neurorestoration, updated the guide in 2014.
Perceptions Shift After Surgery
A study by Hariz et al found that patient perceptions of life shift after stimulation surgery. The investigators interviewed 13 patients who received pallidal DBS. Although certain physical symptoms improved, such as posture and spasms, patients reported that they encountered new challenges after surgery and expressed a need for counseling and support.
Furthermore, expectation may modulate the effect of DBS surgery. In one study, Keitel et al used different scripts to give patients the idea that they were to improve or not improve with various stimulation settings. In a subgroup of patients, tremor decreased or increased by at least 10%, compared with a control condition, when they received the verbal suggestions. Among those who responded to the negative suggestions, semantic verbal fluency also was significantly impaired. “There is an actual potency to the expectations of patients, and it matters how we interact with patients when we are evaluating them both in clinical practice and in the setting of a clinical trial,” Dr. Okun said.
Stimulation Targets and Technology
Multicenter randomized controlled studies of DBS targets have suggested reasons to choose particular stimulation targets over others. For example, stimulation of the subthalamic nucleus (STN) may be better for medication reduction, require fewer battery changes, and have a better economic profile. Meanwhile, stimulation of the globus pallidus internus (GPI) “is an outstanding dyskinesia suppressor … while STN tends to agitate dyskinesia.” GPI devices also may be easier to program.
Pedunculopontine nucleus (PPN) stimulation is a newer technique under investigation that may allow for additional leads to be placed in a patient’s brain. Leads can be implanted on one or both sides of the brain and can be used with GPI or STN leads.
Future DBS devices may enable the remote adjustment of stimulation settings. Nurses and patients also may be able to adjust the devices. DBS increasingly will be tailored to individuals’ symptoms and profiles, and devices may deliver scheduled, responsive, and smart stimulation, Dr. Okun said.
When any new device is introduced to the market, there is “a peak of inflated expectation” about the technology, Dr. Okun said. Then, expectations drop as people discover the device’s limitations. Finally, expectations increase and level as people come to better understand and use the technology. In Parkinson’s disease, neurologists and neurosurgeons are beginning to reach a “plateau of productivity where thousands of people can be helped” by current DBS therapies, he said.
—Jake Remaly
Suggested Reading
Hariz GM, Limousin P, Tisch S, et al. Patients’ perceptions of life shift after deep brain stimulation for primary dystonia--a qualitative study. Mov Disord. 2011;26(11):2101-2106.
Higuchi MA, Martinez-Ramirez D, Morita H, et al. Interdisciplinary Parkinson’s disease deep brain stimulation screening and the relationship to unintended hospitalizations and quality of life. PLoS One. 2016;11(5):e0153785.
Keitel A, Ferrea S, Südmeyer M, et al. Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson’s disease. PLoS One. 2013;8(12):e81878.
Maier F, Lewis CJ, Horstkoetter N, et al. Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: a mixed-method approach. J Neurol Neurosurg Psychiatry. 2013;84(11):1273-1281.
Nisenzon AN, Robinson ME, Bowers D, et al. Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism Relat Disord. 2011;17(2):89-94.
PORTLAND, OR—The discovery that a small amount of electricity delivered to the brain could help treat patients with Parkinson’s disease surprised and delighted neuroscientists. Deep brain stimulation (DBS) can “change people’s lives,” which “creates a lot of expectation,” said Michael S. Okun, MD, Adelaide Lackner Professor and Chairman of Neurology at the University of Florida in Gainesville and National Medical Director of the Parkinson’s Foundation. “But let us not be fooled.… There are many shortcomings.”
Although DBS in its current form may be a powerful treatment for tremor, hyperkinesias, dyskinesias, and motor fluctuations, it is not an effective treatment for gait, freezing, balance, speech, and cognition, he said. As Dr. Okun tells patients, DBS likely will not address problems with “walking, talking, and thinking.”
Various studies indicate that understanding and appropriately managing patients’ expectations regarding DBS therapy is important for achieving optimal outcomes, Dr. Okun said in an overview at the Fourth World Parkinson Congress.
It is important for neurologists to align their expectations with patients’ up front. “What do they have an issue with that is impacting their ability to interface with their world?” he said. Then, the treatment team should tailor the operation to the patient.
Setting Realistic Expectations
Several tools, including the Florida Surgical Questionnaire for Parkinson Disease (FLASQ-PD) and another tool developed by Elena Moro, MD, PhD, Professor of Neurology, Grenoble Alpes University, France, can help identify appropriate candidates for DBS based on patient characteristics. Beyond such instruments, an interdisciplinary team, possibly including a neurologist, psychiatrist, neurosurgeon, and neuropsychologist, should identify a rationale for surgery and choose the best treatment approach. Patients who have contraindications (eg, those who have cognitive impairment or a tremor that does not respond to medication), are over age 70, or need palliation nevertheless may be candidates for DBS, Dr. Okun noted.
Research suggests that interdisciplinary screening may effectively identify patients at risk of poor outcomes. Higuchi et al found that the number and severity of concerns that were raised during interdisciplinary DBS evaluations correlated with unintended hospitalizations and worsened quality of life scores after surgery.
In addition, unrealistic expectations are associated with patients’ subjective outcomes. Maier et al interviewed 30 patients before and three months after surgery and classified patients according to whether their perceived outcome was negative, mixed, or positive. Eight patients with a subjective negative outcome had had unrealistic preoperative expectations, had no postsurgical improvement in quality of life, and had significantly higher presurgical and postsurgical apathy and depression scores. Preoperative apathy and depression predicted the subjective outcomes. Although preoperative apathy and depression are not often measured in clinical practice, “maybe it is something we should be thinking about,” Dr. Okun said.Nisenzon et al surveyed patients to evaluate which treatment outcomes were important to them. Pain, fatigue, emotional distress, walking, posture, and balance were among the domains that had high value to patients. These domains should be addressed with patients when discussing what they can anticipate after DBS surgery, Dr. Okun said.
Medication reduction is another issue that physicians should address with patients when discussing DBS. “A lot of people are expecting DBS to eliminate the need for medications,” which does not happen for 99% of patients, Dr. Okun said.
The National Parkinson Foundation has a free guide to DBS that includes a section detailing what patients can anticipate, he said. Dr. Okun and Pamela R. Zeilman, MSN, ANP-BC, DBS Clinical and Study Coordinator at the Center for Movement Disorders and Neurorestoration, updated the guide in 2014.
Perceptions Shift After Surgery
A study by Hariz et al found that patient perceptions of life shift after stimulation surgery. The investigators interviewed 13 patients who received pallidal DBS. Although certain physical symptoms improved, such as posture and spasms, patients reported that they encountered new challenges after surgery and expressed a need for counseling and support.
Furthermore, expectation may modulate the effect of DBS surgery. In one study, Keitel et al used different scripts to give patients the idea that they were to improve or not improve with various stimulation settings. In a subgroup of patients, tremor decreased or increased by at least 10%, compared with a control condition, when they received the verbal suggestions. Among those who responded to the negative suggestions, semantic verbal fluency also was significantly impaired. “There is an actual potency to the expectations of patients, and it matters how we interact with patients when we are evaluating them both in clinical practice and in the setting of a clinical trial,” Dr. Okun said.
Stimulation Targets and Technology
Multicenter randomized controlled studies of DBS targets have suggested reasons to choose particular stimulation targets over others. For example, stimulation of the subthalamic nucleus (STN) may be better for medication reduction, require fewer battery changes, and have a better economic profile. Meanwhile, stimulation of the globus pallidus internus (GPI) “is an outstanding dyskinesia suppressor … while STN tends to agitate dyskinesia.” GPI devices also may be easier to program.
Pedunculopontine nucleus (PPN) stimulation is a newer technique under investigation that may allow for additional leads to be placed in a patient’s brain. Leads can be implanted on one or both sides of the brain and can be used with GPI or STN leads.
Future DBS devices may enable the remote adjustment of stimulation settings. Nurses and patients also may be able to adjust the devices. DBS increasingly will be tailored to individuals’ symptoms and profiles, and devices may deliver scheduled, responsive, and smart stimulation, Dr. Okun said.
When any new device is introduced to the market, there is “a peak of inflated expectation” about the technology, Dr. Okun said. Then, expectations drop as people discover the device’s limitations. Finally, expectations increase and level as people come to better understand and use the technology. In Parkinson’s disease, neurologists and neurosurgeons are beginning to reach a “plateau of productivity where thousands of people can be helped” by current DBS therapies, he said.
—Jake Remaly
Suggested Reading
Hariz GM, Limousin P, Tisch S, et al. Patients’ perceptions of life shift after deep brain stimulation for primary dystonia--a qualitative study. Mov Disord. 2011;26(11):2101-2106.
Higuchi MA, Martinez-Ramirez D, Morita H, et al. Interdisciplinary Parkinson’s disease deep brain stimulation screening and the relationship to unintended hospitalizations and quality of life. PLoS One. 2016;11(5):e0153785.
Keitel A, Ferrea S, Südmeyer M, et al. Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson’s disease. PLoS One. 2013;8(12):e81878.
Maier F, Lewis CJ, Horstkoetter N, et al. Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: a mixed-method approach. J Neurol Neurosurg Psychiatry. 2013;84(11):1273-1281.
Nisenzon AN, Robinson ME, Bowers D, et al. Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism Relat Disord. 2011;17(2):89-94.
PORTLAND, OR—The discovery that a small amount of electricity delivered to the brain could help treat patients with Parkinson’s disease surprised and delighted neuroscientists. Deep brain stimulation (DBS) can “change people’s lives,” which “creates a lot of expectation,” said Michael S. Okun, MD, Adelaide Lackner Professor and Chairman of Neurology at the University of Florida in Gainesville and National Medical Director of the Parkinson’s Foundation. “But let us not be fooled.… There are many shortcomings.”
Although DBS in its current form may be a powerful treatment for tremor, hyperkinesias, dyskinesias, and motor fluctuations, it is not an effective treatment for gait, freezing, balance, speech, and cognition, he said. As Dr. Okun tells patients, DBS likely will not address problems with “walking, talking, and thinking.”
Various studies indicate that understanding and appropriately managing patients’ expectations regarding DBS therapy is important for achieving optimal outcomes, Dr. Okun said in an overview at the Fourth World Parkinson Congress.
It is important for neurologists to align their expectations with patients’ up front. “What do they have an issue with that is impacting their ability to interface with their world?” he said. Then, the treatment team should tailor the operation to the patient.
Setting Realistic Expectations
Several tools, including the Florida Surgical Questionnaire for Parkinson Disease (FLASQ-PD) and another tool developed by Elena Moro, MD, PhD, Professor of Neurology, Grenoble Alpes University, France, can help identify appropriate candidates for DBS based on patient characteristics. Beyond such instruments, an interdisciplinary team, possibly including a neurologist, psychiatrist, neurosurgeon, and neuropsychologist, should identify a rationale for surgery and choose the best treatment approach. Patients who have contraindications (eg, those who have cognitive impairment or a tremor that does not respond to medication), are over age 70, or need palliation nevertheless may be candidates for DBS, Dr. Okun noted.
Research suggests that interdisciplinary screening may effectively identify patients at risk of poor outcomes. Higuchi et al found that the number and severity of concerns that were raised during interdisciplinary DBS evaluations correlated with unintended hospitalizations and worsened quality of life scores after surgery.
In addition, unrealistic expectations are associated with patients’ subjective outcomes. Maier et al interviewed 30 patients before and three months after surgery and classified patients according to whether their perceived outcome was negative, mixed, or positive. Eight patients with a subjective negative outcome had had unrealistic preoperative expectations, had no postsurgical improvement in quality of life, and had significantly higher presurgical and postsurgical apathy and depression scores. Preoperative apathy and depression predicted the subjective outcomes. Although preoperative apathy and depression are not often measured in clinical practice, “maybe it is something we should be thinking about,” Dr. Okun said.Nisenzon et al surveyed patients to evaluate which treatment outcomes were important to them. Pain, fatigue, emotional distress, walking, posture, and balance were among the domains that had high value to patients. These domains should be addressed with patients when discussing what they can anticipate after DBS surgery, Dr. Okun said.
Medication reduction is another issue that physicians should address with patients when discussing DBS. “A lot of people are expecting DBS to eliminate the need for medications,” which does not happen for 99% of patients, Dr. Okun said.
The National Parkinson Foundation has a free guide to DBS that includes a section detailing what patients can anticipate, he said. Dr. Okun and Pamela R. Zeilman, MSN, ANP-BC, DBS Clinical and Study Coordinator at the Center for Movement Disorders and Neurorestoration, updated the guide in 2014.
Perceptions Shift After Surgery
A study by Hariz et al found that patient perceptions of life shift after stimulation surgery. The investigators interviewed 13 patients who received pallidal DBS. Although certain physical symptoms improved, such as posture and spasms, patients reported that they encountered new challenges after surgery and expressed a need for counseling and support.
Furthermore, expectation may modulate the effect of DBS surgery. In one study, Keitel et al used different scripts to give patients the idea that they were to improve or not improve with various stimulation settings. In a subgroup of patients, tremor decreased or increased by at least 10%, compared with a control condition, when they received the verbal suggestions. Among those who responded to the negative suggestions, semantic verbal fluency also was significantly impaired. “There is an actual potency to the expectations of patients, and it matters how we interact with patients when we are evaluating them both in clinical practice and in the setting of a clinical trial,” Dr. Okun said.
Stimulation Targets and Technology
Multicenter randomized controlled studies of DBS targets have suggested reasons to choose particular stimulation targets over others. For example, stimulation of the subthalamic nucleus (STN) may be better for medication reduction, require fewer battery changes, and have a better economic profile. Meanwhile, stimulation of the globus pallidus internus (GPI) “is an outstanding dyskinesia suppressor … while STN tends to agitate dyskinesia.” GPI devices also may be easier to program.
Pedunculopontine nucleus (PPN) stimulation is a newer technique under investigation that may allow for additional leads to be placed in a patient’s brain. Leads can be implanted on one or both sides of the brain and can be used with GPI or STN leads.
Future DBS devices may enable the remote adjustment of stimulation settings. Nurses and patients also may be able to adjust the devices. DBS increasingly will be tailored to individuals’ symptoms and profiles, and devices may deliver scheduled, responsive, and smart stimulation, Dr. Okun said.
When any new device is introduced to the market, there is “a peak of inflated expectation” about the technology, Dr. Okun said. Then, expectations drop as people discover the device’s limitations. Finally, expectations increase and level as people come to better understand and use the technology. In Parkinson’s disease, neurologists and neurosurgeons are beginning to reach a “plateau of productivity where thousands of people can be helped” by current DBS therapies, he said.
—Jake Remaly
Suggested Reading
Hariz GM, Limousin P, Tisch S, et al. Patients’ perceptions of life shift after deep brain stimulation for primary dystonia--a qualitative study. Mov Disord. 2011;26(11):2101-2106.
Higuchi MA, Martinez-Ramirez D, Morita H, et al. Interdisciplinary Parkinson’s disease deep brain stimulation screening and the relationship to unintended hospitalizations and quality of life. PLoS One. 2016;11(5):e0153785.
Keitel A, Ferrea S, Südmeyer M, et al. Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson’s disease. PLoS One. 2013;8(12):e81878.
Maier F, Lewis CJ, Horstkoetter N, et al. Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: a mixed-method approach. J Neurol Neurosurg Psychiatry. 2013;84(11):1273-1281.
Nisenzon AN, Robinson ME, Bowers D, et al. Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism Relat Disord. 2011;17(2):89-94.










