User login
Management of Proximal Biceps Pathology in Overhead Athletes: What Is the Role of Biceps Tenodesis?
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
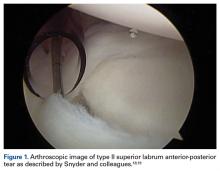
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
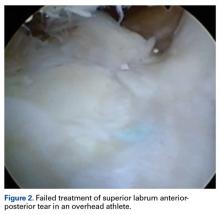
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
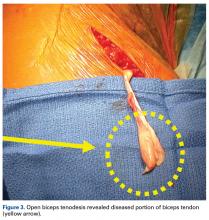
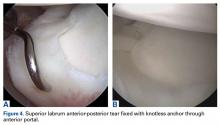
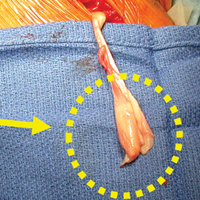
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Take Home Points
- Outcomes after SLAP repair remain guarded.
- Physical examination is key in determining proper management of biceps pathology.
- When performing SLAP repair, knotless technology may prevent future cartilage or rotator cuff injury.
- Revision of SLAP repair is best handled with biceps tenodesis.
- Subpectoral biceps tenodesis avoids residual groove pain.
In recent decades, the long head of the biceps (LHB) tendon has been recognized as a pain generator in the shoulder of throwing athletes. The LHB muscle and its role in glenohumeral kinematics remains largely in question. The LHB tendon varies in size but most commonly is 5 mm to 6mm in diameter and about 9 cm in length, inserting on the superior labrum and supraglenoid tubercle after traveling through the bicipital groove.1 The many conditions that can develop along the course of the biceps tendon include overall biceps tendonitis, biceps tendon subluxation or instability, and injuries to the superior anterior to posterior area of the labrum.
These injuries can occur in young overhead athletes as well as manual laborers and older overhead recreational athletes. Pitching is the most common activity that leads to proximal biceps tendon disorders. The 6 phases of the pitch are linked in a kinetic chain that generates energy that is then translated to high velocity. The amount of force that is exerted on the shoulder during pitching and especially after ball release is impressive, and the athlete’s shoulder changes in many ways as it adapts to the motion.2-5 The late-cocking and deceleration phases are most commonly associated with proximal biceps pathology and the “peel-back” phenomenon. Other common activities that lead to biceps tendon issues in a young population are volleyball, baseball, tennis, softball, swimming, and cricket. Shoulder arthroscopies performed in older patients show degenerative biceps and labrum tears, which should be treated appropriately but perhaps different from how they are treated in overhead athletes.6-8 Further, many professional athletes have asymptomatic superior labrum anterior-posterior (SLAP) tears.9
Mechanism of Injury
Overhead throwing is commonly thought to be the mechanism by which lesions are created in the biceps–labrum complex (BLC). Pitching in particular generates incredible force and torque within the shoulder. In professional pitchers, the resulting throwing speed creates forces regularly in excess of 1000 N.3 These forces effect internal compensatory changes and internal derangement of the BLC. These changes often involve internal rotation deficits and alterations in the rotator cuff, which may contribute to glenohumeral instability and altered joint kinematics.10
Repetitive overhead activity is largely considered the mechanism of injury in this population, though more specific mechanisms have been described, including the peel-back mechanism11 and the posterior superior glenoid impingement. There is little evidence that preventive programs have any effect on decreasing the incidence of SLAP tears in overhead athletes.
Preoperative Evaluation
Preoperative evaluation is arguably the most important step in treating a patient with persistent or recurrent symptoms consistent with a SLAP tear. Evaluation includes thorough history, physical examination, and review of any prior injuries or surgical procedures. The physical examination should focus on maneuvers that define where the problem is occurring. Although SLAP tears are most common in this population, disorders of the biceps tendon within the groove, including inflammation and instability, should be ruled out with physical examination and advanced imaging. Palpation for groove tenderness, impingement-type complaints, internal rotation loss, and SLAP provocative testing are crucial in the diagnosis.12,13 The cause of symptoms may be multifactorial and include the often encountered concomitant pathology of rotator cuff tears, internal impingement, and instability.
Standard radiographs (Grashey anteroposterior, scapular/lateral, axillary lateral) and magnetic resonance imaging (MRI) with or without arthrography can be helpful in identifying and characterizing most SLAP tears as well as failed SLAP tear repairs. However, MRI is often positive for SLAP tears in asymptomatic patients, and diagnosing SLAP tears with MRI is often a challenge.14 MRI can help in determining concomitant pathology, including rotator cuff injury and cysts causing nerve compression. Correlation with clinical examination and patient history is most crucial. Conservative treatment (rest, activity modification, use of oral anti-inflammatory medications) typically is attempted and coordinated with respect to the athlete’s season of play.15,16
Classification
In overhead throwing athletes, SLAP tears typically are associated with anterior shoulder pain. Associated shoulder instability and significant glenohumeral dysfunction are not uncommon in athletes with lesions of the BLC. In 1985, Andrews and colleagues17 were the first to describe SLAP tears in overhead athletes (73 patients). Later, Snyder and colleagues18,19 further classified these lesions into 4 types based on tear stability and location, and they coined the acronym SLAP (Figure 1).
Type I lesions typically are described as fraying at the inner margin of the labrum and are common in throwers, even asymptomatic throwers. Type II lesions, separations of the biceps and labrum from the superior glenoid (≥5 mm of excursion), are the most commonly occurring and treated variant in throwing athletes.20-22 Intraoperative evaluation for a peel-back lesion (placing the arm in abduction with external rotation), rather than for a sulcus of 1 mm to 2 mm, may confirm a type II SLAP tear.20,23,24 It is often important to consider the direction of tear propagation as well. Type III lesions include those with an intact BLC (but with a bucket-handle tear of the superior labral complex and an intact biceps tendon), whereas type IV lesions involve additional extension of the tear into the biceps tendon.18,19The classification systems are well defined. Nevertheless, management of SLAP lesions remains controversial.
Options for Surgical Treatment
SLAP Tear Repair—Outcomes
The incidence of SLAP tear repairs has increased dramatically in recent years.6,25 There are various SLAP tear repair methods, but the most common consists of repairing the labrum and biceps anchor. Management of type II SLAP lesions remains controversial. Several prospective studies have found overall improvement after SLAP tear repair.26-31 Other series have reported less encouraging outcomes, including dissatisfaction with persistent pain and inability to return to throwing.28,32 A 2010 systematic review found that the percentage of patients who returned to their preinjury level of play was only 64%, and outcomes for overhead throwing athletes were even worse—only 22% to 60% of these patients returned to their previous level.33 The right surgery for SLAP tears in this population continues to be an area of uncertainty for many surgeons.
Failed SLAP tear repairs (poor outcomes) have become common in overhead throwing athletes. The reasons for these failed repairs are unclear, but several possible explanations have been offered. One is that labral repair may result in permanent alterations in pitching biomechanics, which may lead to an inability to regain velocity and command.3 Another is that the athlete’s shoulder may remain unstable even after repair.10Hardware complications are a significant concern in this high-level population. Suture anchor pullout or iatrogenic cartilage damage may occur during instrumentation or as a result of suture anchor reactive changes. In addition, there are several reports of glenoid osteochondrolysis (Figure 2) caused by prominent hardware or prominent knots.34-39
Stiffness after SLAP tear repair is a significant problem, with most patients taking up to 6 months to regain full motion.26,48 Overtensioning of the labrum and the glenohumeral ligaments may be the cause, and the solution may be to place anchors posterior (vs anterior) to the biceps insertion. In a large prospective military study, mean forward flexion and external rotation were reduced at final follow-up.31 These outcomes are less acceptable to overhead throwing athletes, who rely on motion for high-end throwing activities.
Primary Biceps Tenodesis—Outcomes
A 2015 database study found a 1.7-fold increase in biceps tenodesis over the preceding 5 years.49 However, relatively few procedures included in the study were performed in patients age younger than 30 years. For many older non-overhead throwers with type II tears, SLAP tear repair has become less popular as a treatment option.32 There is a dearth of knowledge about the outcomes of subpectoral biceps tenodesis as a primary treatment for biceps tendonitis and an associated SLAP tear. Although type I tears historically have been treated with débridement, débridement is seldom used for concomitant biceps tendonitis. It should be coupled with careful clinical examination.
In recent years, biceps tenodesis has been proposed as an alternative to repair for SLAP tears, particularly in older patients.24,44 For obvious reasons, however, there has been some trepidation about performing biceps tenodesis in throwing athletes. Some authors have proposed biceps tenodesis as primary treatment for isolated SLAP tears. Boileau and colleagues44 compared the outcomes of treatment of isolated type II SLAP lesions in 25 consecutive patients. For 10 patients, repair involved suture anchors; for the other 15, arthroscopic biceps tenodesis was performed with an absorbable interference screw. Six of the 10 suture anchor patients were disappointed with their outcome (persistent pain or inability to return to sport), whereas 14 of the 15 biceps tenodesis patients were satisfied. The authors concluded that arthroscopic biceps tenodesis is an effective alternative to repair for type II SLAP lesions, though their study was not isolated to overhead athletes (tenodesis group mean age, 52 years).
In a 2014 series of cases, Ek and colleagues50 reported good outcomes of SLAP tear repair and biceps tenodesis. Again, though, tenodesis was used in older patients, and repair in younger, more active patients, with no high-level athletes in either group. There was no difference in return to sport between groups. In a study of patients who underwent primary biceps tenodesis, Gupta and colleagues51 found 80% excellent outcomes (improved shoulder outcome scores) in select SLAP tear patients, including 8 athletes, 88% of whom were overhead athletes. Gottschalk and colleagues52 reported on differences in prospectively collected outcome data (age, sex, SLAP lesion type II or IV) for primary biceps tenodesis in a series of 33 patients. Twenty-six of the 29 patients who completed follow-up returned to their previous level of activity. These studies suggest that primary biceps tenodesis may be an alternative with lower failure rates in the treatment of SLAP tears in middle-aged patients, and in overhead athletes, though additional specific studies are needed to focus on overhead athletes on a larger scale.
Revision SLAP Tear Repair Versus Biceps Tenodesis
Failed arthroscopic SLAP tear repairs, which are increasingly common, present a unique treatment challenge. In a 2013 prospective cohort series, Gupta and colleagues46 found excellent clinical outcomes of subpectoral biceps tenodesis for failed type II SLAP tears. The authors reported a postoperative SANE (Single Assessment Numeric Evaluation) score of 70.4%, an SST (Simple Shoulder Test) score of 9.33, and an ASES (American Shoulder and Elbow Surgeons) score of 77.96, along with reasonable health-related quality-of-life scores. Werner and colleagues53 evaluated 2-year outcomes of biceps tenodesis performed after SLAP tear repair in 24 patients and found a return to almost normal range of motion as well as good clinical outcome scores. Significantly worse outcomes were found for patients with open worker’s compensation claims.
McCormick and colleagues26 prospectively evaluated the efficacy of biceps tenodesis for failed type II SLAP tear repair in 46 patients. Improvement was noted across all outcome assessments during follow-up (mean, 3.6 years). From these findings, we might conclude that biceps tenodesis is a more predictable option for failed SLAP tear repair, and that it has a relatively low complication rate. However, most investigators have used a heterogeneous patient population, as opposed to overhead athletes specifically. To our knowledge, no one has evaluated the specific population of overhead throwers with failed SLAP tear repairs. In addition, no one has conducted randomized controlled trials comparing débridement, biceps tenodesis, and repair for failed SLAP tear repairs.
Postoperative Considerations
When overhead athletes and their surgeons are considering surgical options, they must take rehabilitation and return to play into account. Many surgeons think the possible marginal clinical benefit of SLAP tear repair may not be worth the protracted rehabilitation. In most practices, rehabilitation after biceps tenodesis is less involved. Discussing the advantages and disadvantages of these 2 procedures can be helpful in decision making.
Dein and colleagues54 reported the case of a middle-aged pitcher who sustained a fracture after biceps tenodesis with an interference screw. Cases like this are concerning. Surgeons should consider altering the rehabilitation regimen when planning postoperative care in cases of biceps tenodesis in throwers. Other reported complications of open tenodesis are deep infection, thrombosis, postoperative stiffness, and nerve injury.55-58
Consequences for Overhead Throwers
The unknown role of the BLC leaves surgeons wary when considering biceps tenodesis for elite athletes. Some have postulated that removing the intra-articular portion of the LHB may cause microinstability and alter joint kinematics.10,59-61 Others have suggested the biceps is desynchronized from the other musculature and is not functionally important.62 Disruption of one portion of the superior labrum may result in instability on the opposite side of the glenoid.10,61 Biomechanical studies, both cadaveric and in vivo, have tried to create proper loads to the LHB and evaluate the kinematics of the shoulder before and after biceps tenodesis and SLAP tear repair.59,60 Using a cadaveric model, Strauss and colleagues63 found that type II SLAP lesions resulted in increased glenohumeral translation compared with baseline. Biceps tenodesis did not restore normal translation, but this did not negatively affect stability in the presence of a SLAP lesion. The consensus is that the role of the biceps is controversial at best.
Several studies have used electromyography (EMG) to evaluate LHB functioning. In 2014, Chalmers and colleagues59 used surface EMG and motion analysis to evaluate 18 pitchers: 6 underwent SLAP tear repair, 5 underwent biceps tenodesis, and 7 were uninjured controls. There were no significant differences in the activity of the LHB muscle, the short head of the biceps muscle, the deltoid, the infraspinatus, or the latissimus among the 3 groups. Motion analysis showed that the normal pattern of muscular activation within the LHB muscle was more closely restored by biceps tenodesis than by SLAP tear repair. In addition, thoracic rotation patterns were significantly more altered in the SLAP tear repair patients than in the uninjured controls. As the authors noted, given the low frequency with which biceps tenodesis is performed in overhead athletes, it is unlikely that larger scale studies will be conducted without a multicenter effort.
Recommendations and Our Preferred Technique
Which surgical option is best for treating symptomatic SLAP lesions in overhead athletes remains unclear. Many athletes struggle to return to high-level play after SLAP tear repair. Whether the same is true after biceps tenodesis is yet to be determined because of the low frequency with which biceps tenodesis is performed in high-level overhead athletes. The options for fixation, technique, and fixation location are equally broad. In this section, we outline our general line of thinking for cases of proximal biceps pathology.
In each case, we perform glenohumeral arthroscopy to evaluate the BLC and identify any other pathology. For overhead athletes who are younger than 30 years and lack bicipital groove pain or signs of gross tendinopathy, we favor arthroscopic SLAP tear repair. Repair is usually performed through an anterior working portal for suture passage and a Wilmington portal for anchor placement. We use knotless technology to achieve stable fixation and stay posterior to the biceps anchor insertion.
For the prevention of any potential pain from the bicipital groove in carefully selected patients—recreational overhead athletes and patients who want a less involved surgical recovery—we favor open subpectoral biceps tenodesis rather than arthroscopic tenodesis. The outcomes of biceps tenodesis are consistent, according to the literature.47,57,64 Moreover, the open approach is favored for the incidence of postoperative stiffness in the arthroscopic population.65 Tendons can be fixed with multiple procedures, including soft-tissue tenodesis, interference screw fixation, and surface anchors. We favor using a tenodesis screw in the subpectoral location, as outlined by Mazzocca and colleagues.64Our algorithm for SLAP lesions is evolving with our understanding of this complex disease process. For young overhead throwers with type II SLAP lesions, we favor arthroscopic SLAP tear repair with knotless technology. For older recreational overhead athletes, we favor biceps tenodesis in the subpectoral region after diagnostic arthroscopy plus biceps tenotomy with or without additional SLAP tear fixation, depending on the stability of the biceps anchor (Figures 4A, 4B).
Conclusion
Overhead athletes who present with symptomatic SLAP lesions often provide a treatment dilemma. Although SLAP tear repair historically has been standard treatment, biceps tenodesis represents a consistent surgical option with low complication rates and low revision rates. It is likely that, as additional data on glenohumeral kinematics and outcomes in young athletes become available, improved decision-making algorithms will follow.
Am J Orthop. 2017;46(1):E71-E78. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
1. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
2. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
3. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
4. Aydin N, Sirin E, Arya A. Superior labrum anterior to posterior lesions of the shoulder: diagnosis and arthroscopic management. World J Orthop. 2014;5(3):344-350.
5. Barber A, Field LD, Ryu R. Biceps tendon and superior labrum injuries: decision-marking. J Bone Joint Surg Am. 2007;89(8):1844-1855.
6. Onyekwelu I, Khatib O, Zuckerman JD, Rokito AS, Kwon YW. The rising incidence of arthroscopic superior labrum anterior and posterior (SLAP) repairs. J Shoulder Elbow Surg. 2012;21(6):728-731.
7. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery Certification Examination Database. Am J Sports Med. 2014;42(8):1904-1910.
8. Walton DM, Sadi J. Identifying SLAP lesions: a meta-analysis of clinical tests and exercise in clinical reasoning. Phys Ther Sport. 2008;9(4):167-176.
9. Lesniak BP, Baraga MG, Jose J, Smith MK, Cunningham S, Kaplan LD. Glenohumeral findings on magnetic resonance imaging correlate with innings pitched in asymptomatic pitchers. Am J Sports Med. 2013;41(9):2022-2027.
10. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
12. Meserve BB, Cleland JA, Boucher TR. A meta-analysis examining clinical test utility for assessing superior labral anterior posterior lesions. Am J Sports Med. 2009;37(11):2252-2258.
13. Pandya NK, Colton A, Webner D, Sennett B, Huffman GR. Physical examination and magnetic resonance imaging in the diagnosis of superior labrum anterior-posterior lesions of the shoulder: a sensitivity analysis. Arthroscopy. 2008;24(3):311-317.
14. Amin MF, Youssef AO. The diagnostic value of magnetic resonance arthrography of the shoulder in detection and grading of SLAP lesions: comparison with arthroscopic findings. Eur J Radiol. 2012;81(9):2343-2347.
15. Cook C, Beaty S, Kissenberth MJ, Siffri P, Pill SG, Hawkins RJ. Diagnostic accuracy of five orthopedic clinical tests for diagnosis of superior labrum anterior posterior (SLAP) lesions. J Shoulder Elbow Surg. 2012;21(1):13-22.
16. Edwards SL, Lee JA, Bell JE, et al. Nonoperative treatment of superior labrum anterior posterior tears: improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
19. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995;4(4):243-248.
20. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
21. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
22. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
23. Chen CH, Hsu KY, Chen WJ, Shih CH. Incidence and severity of biceps long head tendon lesion in patients with complete rotator cuff tears. J Trauma. 2005;58(6):1189-1193.
24. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
25. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
26. McCormick F, Bhatia S, Chalmers P, Gupta A, Verma N, Romeo AA. The management of type II superior labral anterior to posterior injuries. Orthop Clin North Am. 2014;45(1):121-128.
27. Brockmeier SF, Voos JE, Williams RJ 3rd, Altchek DW, Cordasco FA, Allen AA; Hospital for Special Surgery Sports Medicine and Shoulder Service. Outcomes after arthroscopic repair of type-II SLAP lesions. J Bone Joint Surg Am. 2009;91(7):1595-1603.
28. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
29. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
30. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867.
31. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
32. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
33. Gorantla K, Gill C, Wright RW. The outcome of type II SLAP repair: a systematic review. Arthroscopy. 2010;26(4):537-545.
34. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
35. Park MJ, Hsu JE, Harper C, Sennett BJ, Huffman GR. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs. Arthroscopy. 2011;27(10):1335-1340.
36. Sassmannshausen G, Sukay M, Mair SD. Broken or dislodged poly-L-lactic acid bioabsorbable tacks in patients after SLAP lesion surgery. Arthroscopy. 2006;22(6):615-619.
37. Uggen C, Wei A, Glousman RE, et al. Biomechanical comparison of knotless anchor repair versus simple suture repair for type II SLAP lesions. Arthroscopy. 2009;25(10):1085-1092.
38. Weber SC. Surgical management of the failed SLAP repair. Sports Med Arthrosc. 2010;18(3):162-166.
39. Wilkerson JP, Zvijac JE, Uribe JW, Schürhoff MR, Green JB. Failure of polymerized lactic acid tacks in shoulder surgery. J Shoulder Elbow Surg. 2003;12(2):117-121.
40. Weber S. Surgical management of the failed SLAP lesion. Arthroscopy. 2008;24(suppl):e8-e9.
41. Schrøder CP, Skare O, Gjengedal E, Uppheim G, Reikerås O, Brox JI. Long-term results after SLAP repair: a 5-year follow-up study of 107 patients with comparison of patients aged over and under 40 years. Arthroscopy. 2012;28(11):1601-1607.
42. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
43. Mazzocca AD, McCarthy MB, Ledgard FA, et al. Histomorphologic changes of the long head of the biceps tendon in common shoulder pathologies. Arthroscopy. 2013;29(6):972-981.
44. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
45. Boileau P, Krishnan SG, Coste JS, Walch G. Arthroscopic biceps tenodesis: a new technique using bioabsorbable interference screw fixation. Arthroscopy. 2002;18(9):1002-1012.
46. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
47. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
48. McCarty LP 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am. 2013;95(6):507-511.
49. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
50. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
51. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
52. Gottschalk MB, Karas SG, Ghattas TN, Burdette R. Subpectoral biceps tenodesis for the treatment of type II and IV superior labral anterior and posterior lesions. Am J Sports Med. 2014;42(9):2128-2135.
53. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
54. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis [published correction appears in Am J Sports Med. 2014;42(6):NP39]. Am J Sports Med. 2014;42(4):877-879.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Osbahr DC, Diamond AB, Speer KP. The cosmetic appearance of the biceps muscle after long-head tenotomy versus tenodesis. Arthroscopy. 2002;18(5):483-487.
57. Romeo AA, Mazzocca AD, Tauro JC. Arthroscopic biceps tenodesis. Arthroscopy. 2004;20(2):206-213.
58. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
59. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
60. Giphart JE, Elser F, Dewing CB, Torry MR, Millett PJ. The long head of the biceps tendon has minimal effect on in vivo glenohumeral kinematics: a biplane fluoroscopy study. Am J Sports Med. 2012;40(1):202-212.
61. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
62. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2012;30(1):53-60.
63. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
64. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
65. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.
66. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
67. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
Make the Diagnosis - January 2017
Pemphigus vulgaris
Pemphigus vulgaris is the most common type of pemphigus. The average age of onset of 40-60 years. Clinically, patients may present with mucosal blisters and/or erosions. The most common site of mucosal lesions is the oral cavity, where the disease often manifests. Autoantibodies are produced against desmoglein 3 or both desmoglein 1 and desmoglein 3 in pemphigus vulgaris. Blistering is commonly induced with mechanical pressure at th edge of a blister or on normal skin, which is known as the Nikolsky sign. Pemphigus vulgaris has two uncommon clinical variants, pemphigus vegetans and pemphigus herpetiformis. Lack of prompt treatment of pemphigus vulgaris leads to epitope spreading and increased difficulty in management. Treatment with systemic glucocorticoids is the current standard of care to achieve control of the disease, and nonsteroidal immunomodulatory agents such as azathioprine, mycophenolate mofetil, and dapsone can be used in conjunction to help reduce adverse effects associated with long-term glucocorticoid therapy.
Pemphigus foliaceus results from autoantibodies against desmoglein 1. Patients usually present with small, scattered superficial cutaneous blisters that transition into scaly, crusted erosions. The scalp, neck, and trunk are the most common sites of presentation, with sparing of the mucous membranes. Like pemphigus vulgaris, the Nikolsky sign is frequently present in patients with pemphigus foliaceus. However, pemphigus foliaceus is readily distinguished from pemphigus vulgaris by its lack of mucous membrane involvement. The mainstays of treatment of pemphigus foliaceus are similar to those of pemphigus vulgaris, with systemic glucocorticoids and nonsteroidal adjuvant therapies playing a major role in controlling disease.
IgA pemphigus may occur at any age and is characterized by vesicles that progress into pustules commonly present on the trunk and proximal extremities. Erythematous plaques are frequently present alongside the vesicles and pustules. Like pemphigus foliaceus, IgA pemphigus usually spares the mucous membranes. The lesions may be pruritic but can also be asymptomatic. All other types of pemphigus are caused by IgG autoantibodies; however, IgA pemphigus results from IgA autoantibodies against target keratinocyte antigens. Several reports have shown dapsone to be a successful first-line adjuvant therapy for the treatment of IgA pemphigus.
Paraneoplastic pemphigus affects both genders and can occur at any age. It is most commonly the result of a malignancy. It is not clear which autoantibodies are actually responsible for the pathogenicity of most cases of paraneoplastic pemphigus. Clinically, it presents as a combination of severe erosive stomatitis, polymorphous cutaneous lesions, and possible pulmonary involvement. Severe, painful, and erosive mucositis is ubiquitous to the disease, and oral erosions are the most common initial presentation of paraneoplastic pemphigus, with a characteristic involvement of the tongue. Skin lesions commonly manifest after the onset of mucosal lesions. Cutaneous involvement is highly varied from patient to patient, with lesions resembling bullae, inflammatory violaceous papules, targetoid lesions, and desquamation. Management consists of treatment of the underlying neoplasm and control of the disease itself using a variety of agents including immunosuppressants and rituximab.
The patient’s biopsy came back consistent with pemphigus vulgaris. He was started on topical steroid gel for the mucosal lesions, topical steroid cream for the cutaneous lesions, and oral prednisone.
This case and photo were submitted by Natasha Cowan, University of California, San Diego, and Brooke Resh Sateesh, MD, San Diego Family Dermatology.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Pemphigus vulgaris
Pemphigus vulgaris is the most common type of pemphigus. The average age of onset of 40-60 years. Clinically, patients may present with mucosal blisters and/or erosions. The most common site of mucosal lesions is the oral cavity, where the disease often manifests. Autoantibodies are produced against desmoglein 3 or both desmoglein 1 and desmoglein 3 in pemphigus vulgaris. Blistering is commonly induced with mechanical pressure at th edge of a blister or on normal skin, which is known as the Nikolsky sign. Pemphigus vulgaris has two uncommon clinical variants, pemphigus vegetans and pemphigus herpetiformis. Lack of prompt treatment of pemphigus vulgaris leads to epitope spreading and increased difficulty in management. Treatment with systemic glucocorticoids is the current standard of care to achieve control of the disease, and nonsteroidal immunomodulatory agents such as azathioprine, mycophenolate mofetil, and dapsone can be used in conjunction to help reduce adverse effects associated with long-term glucocorticoid therapy.
Pemphigus foliaceus results from autoantibodies against desmoglein 1. Patients usually present with small, scattered superficial cutaneous blisters that transition into scaly, crusted erosions. The scalp, neck, and trunk are the most common sites of presentation, with sparing of the mucous membranes. Like pemphigus vulgaris, the Nikolsky sign is frequently present in patients with pemphigus foliaceus. However, pemphigus foliaceus is readily distinguished from pemphigus vulgaris by its lack of mucous membrane involvement. The mainstays of treatment of pemphigus foliaceus are similar to those of pemphigus vulgaris, with systemic glucocorticoids and nonsteroidal adjuvant therapies playing a major role in controlling disease.
IgA pemphigus may occur at any age and is characterized by vesicles that progress into pustules commonly present on the trunk and proximal extremities. Erythematous plaques are frequently present alongside the vesicles and pustules. Like pemphigus foliaceus, IgA pemphigus usually spares the mucous membranes. The lesions may be pruritic but can also be asymptomatic. All other types of pemphigus are caused by IgG autoantibodies; however, IgA pemphigus results from IgA autoantibodies against target keratinocyte antigens. Several reports have shown dapsone to be a successful first-line adjuvant therapy for the treatment of IgA pemphigus.
Paraneoplastic pemphigus affects both genders and can occur at any age. It is most commonly the result of a malignancy. It is not clear which autoantibodies are actually responsible for the pathogenicity of most cases of paraneoplastic pemphigus. Clinically, it presents as a combination of severe erosive stomatitis, polymorphous cutaneous lesions, and possible pulmonary involvement. Severe, painful, and erosive mucositis is ubiquitous to the disease, and oral erosions are the most common initial presentation of paraneoplastic pemphigus, with a characteristic involvement of the tongue. Skin lesions commonly manifest after the onset of mucosal lesions. Cutaneous involvement is highly varied from patient to patient, with lesions resembling bullae, inflammatory violaceous papules, targetoid lesions, and desquamation. Management consists of treatment of the underlying neoplasm and control of the disease itself using a variety of agents including immunosuppressants and rituximab.
The patient’s biopsy came back consistent with pemphigus vulgaris. He was started on topical steroid gel for the mucosal lesions, topical steroid cream for the cutaneous lesions, and oral prednisone.
This case and photo were submitted by Natasha Cowan, University of California, San Diego, and Brooke Resh Sateesh, MD, San Diego Family Dermatology.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Pemphigus vulgaris
Pemphigus vulgaris is the most common type of pemphigus. The average age of onset of 40-60 years. Clinically, patients may present with mucosal blisters and/or erosions. The most common site of mucosal lesions is the oral cavity, where the disease often manifests. Autoantibodies are produced against desmoglein 3 or both desmoglein 1 and desmoglein 3 in pemphigus vulgaris. Blistering is commonly induced with mechanical pressure at th edge of a blister or on normal skin, which is known as the Nikolsky sign. Pemphigus vulgaris has two uncommon clinical variants, pemphigus vegetans and pemphigus herpetiformis. Lack of prompt treatment of pemphigus vulgaris leads to epitope spreading and increased difficulty in management. Treatment with systemic glucocorticoids is the current standard of care to achieve control of the disease, and nonsteroidal immunomodulatory agents such as azathioprine, mycophenolate mofetil, and dapsone can be used in conjunction to help reduce adverse effects associated with long-term glucocorticoid therapy.
Pemphigus foliaceus results from autoantibodies against desmoglein 1. Patients usually present with small, scattered superficial cutaneous blisters that transition into scaly, crusted erosions. The scalp, neck, and trunk are the most common sites of presentation, with sparing of the mucous membranes. Like pemphigus vulgaris, the Nikolsky sign is frequently present in patients with pemphigus foliaceus. However, pemphigus foliaceus is readily distinguished from pemphigus vulgaris by its lack of mucous membrane involvement. The mainstays of treatment of pemphigus foliaceus are similar to those of pemphigus vulgaris, with systemic glucocorticoids and nonsteroidal adjuvant therapies playing a major role in controlling disease.
IgA pemphigus may occur at any age and is characterized by vesicles that progress into pustules commonly present on the trunk and proximal extremities. Erythematous plaques are frequently present alongside the vesicles and pustules. Like pemphigus foliaceus, IgA pemphigus usually spares the mucous membranes. The lesions may be pruritic but can also be asymptomatic. All other types of pemphigus are caused by IgG autoantibodies; however, IgA pemphigus results from IgA autoantibodies against target keratinocyte antigens. Several reports have shown dapsone to be a successful first-line adjuvant therapy for the treatment of IgA pemphigus.
Paraneoplastic pemphigus affects both genders and can occur at any age. It is most commonly the result of a malignancy. It is not clear which autoantibodies are actually responsible for the pathogenicity of most cases of paraneoplastic pemphigus. Clinically, it presents as a combination of severe erosive stomatitis, polymorphous cutaneous lesions, and possible pulmonary involvement. Severe, painful, and erosive mucositis is ubiquitous to the disease, and oral erosions are the most common initial presentation of paraneoplastic pemphigus, with a characteristic involvement of the tongue. Skin lesions commonly manifest after the onset of mucosal lesions. Cutaneous involvement is highly varied from patient to patient, with lesions resembling bullae, inflammatory violaceous papules, targetoid lesions, and desquamation. Management consists of treatment of the underlying neoplasm and control of the disease itself using a variety of agents including immunosuppressants and rituximab.
The patient’s biopsy came back consistent with pemphigus vulgaris. He was started on topical steroid gel for the mucosal lesions, topical steroid cream for the cutaneous lesions, and oral prednisone.
This case and photo were submitted by Natasha Cowan, University of California, San Diego, and Brooke Resh Sateesh, MD, San Diego Family Dermatology.
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 50 year old Hispanic male presented with a two day history of blisters on his lips, extremities, and upper body. He complained of soreness on his lips. Bullae were flaccid and some had crusting.
Term ultrasound shown unreliable for diagnosing macrosomia
LAS VEGAS – Fetal macrosomia can be challenging to detect by ultrasound performed just before delivery, which had 41% sensitivity and 58% positive predictive value in a prospective study of more than 2,300 pregnancies.
The results also showed that fetal macrosomia (defined as birth weight of more than 4,000 grams) is significantly linked with increased rates of prolonged labor, delivery by either operative vaginal or cesarean approaches, and postpartum hemorrhage, Daniel M. Galvin, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Because all clinicians involved with these deliveries were blinded to the prenatal ultrasound results, the findings suggest that prolonged labor, postpartum hemorrhage, and need for either operative vaginal delivery or cesarean delivery are all outcomes driven by macrosomia itself rather than by clinical actions taken because of an expectation of macrosomia, said Dr. Galvin, an ob.gyn. with Perinatal Ireland, a Dublin-based consortium of eight Irish fetal medicine centers that is examining ways to improve delivery outcomes.
The study used “a pure population of pregnancies with unsuspected fetal macrosomia,” he explained.
Dr. Galvin and his colleagues used data collected in GENESIS, a prospective study run by the Perinatal Ireland multicenter consortium with the primary goal of determining whether late-pregnancy fetal head circumference can predict labor dystocia and intrapartum cesarean delivery. They examined two secondary outcomes: the reliability of ultrasound to estimate fetal size, and the consequences of fetal macrosomia when it is not recognized until delivery is already underway.
The study enrolled 2,336 nulliparous women with singleton pregnancies that ranged from the start of 39 weeks’ gestational age through the end of 40 weeks. The women underwent a standard ultrasound examination to assess fetal biometrics. The study excluded pregnancies with an estimated fetal size greater than 5,000 g. Mothers carrying a fetus estimated to be less than 4,000 g constituted 88% of the study group, with 12% carrying pregnancies with an estimated fetal weight greater than 4,000 g.
The ultrasound examination worked reasonably well for ruling out macrosomia, with an 89% rate of correctly identifying fetuses with a birth weight of less than 4,000 g. Near-term ultrasound was less useful for a positive identification of macrosomia; it flagged 58% of the fetuses born heavier than 4,000 g.
Analysis of delivery mode showed that infants born weighing more than 4,000 g had a statistically significant 56% reduced rate of spontaneous vaginal deliveries compared with smaller neonates, a 63% greater rate of cesarean deliveries, and a 49% greater rate of operative vaginal deliveries, compared with small babies, Dr. Galvin reported. All three between-group differences were statistically significant.
The analysis also showed that compared with the smaller babies, the larger neonates were twice as likely to be born during prolonged labor of more than 12 hours. Delivery of larger neonates was also twice as likely to trigger postpartum hemorrhage. But deliveries of larger babies had no significant link with increased rates of neonatal intensive care admissions, anal sphincter injuries, shoulder dystocias or birth injuries, compared with deliveries of smaller babies.
Dr. Galvin reported having no financial disclosures.
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Fetal macrosomia can be challenging to detect by ultrasound performed just before delivery, which had 41% sensitivity and 58% positive predictive value in a prospective study of more than 2,300 pregnancies.
The results also showed that fetal macrosomia (defined as birth weight of more than 4,000 grams) is significantly linked with increased rates of prolonged labor, delivery by either operative vaginal or cesarean approaches, and postpartum hemorrhage, Daniel M. Galvin, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Because all clinicians involved with these deliveries were blinded to the prenatal ultrasound results, the findings suggest that prolonged labor, postpartum hemorrhage, and need for either operative vaginal delivery or cesarean delivery are all outcomes driven by macrosomia itself rather than by clinical actions taken because of an expectation of macrosomia, said Dr. Galvin, an ob.gyn. with Perinatal Ireland, a Dublin-based consortium of eight Irish fetal medicine centers that is examining ways to improve delivery outcomes.
The study used “a pure population of pregnancies with unsuspected fetal macrosomia,” he explained.
Dr. Galvin and his colleagues used data collected in GENESIS, a prospective study run by the Perinatal Ireland multicenter consortium with the primary goal of determining whether late-pregnancy fetal head circumference can predict labor dystocia and intrapartum cesarean delivery. They examined two secondary outcomes: the reliability of ultrasound to estimate fetal size, and the consequences of fetal macrosomia when it is not recognized until delivery is already underway.
The study enrolled 2,336 nulliparous women with singleton pregnancies that ranged from the start of 39 weeks’ gestational age through the end of 40 weeks. The women underwent a standard ultrasound examination to assess fetal biometrics. The study excluded pregnancies with an estimated fetal size greater than 5,000 g. Mothers carrying a fetus estimated to be less than 4,000 g constituted 88% of the study group, with 12% carrying pregnancies with an estimated fetal weight greater than 4,000 g.
The ultrasound examination worked reasonably well for ruling out macrosomia, with an 89% rate of correctly identifying fetuses with a birth weight of less than 4,000 g. Near-term ultrasound was less useful for a positive identification of macrosomia; it flagged 58% of the fetuses born heavier than 4,000 g.
Analysis of delivery mode showed that infants born weighing more than 4,000 g had a statistically significant 56% reduced rate of spontaneous vaginal deliveries compared with smaller neonates, a 63% greater rate of cesarean deliveries, and a 49% greater rate of operative vaginal deliveries, compared with small babies, Dr. Galvin reported. All three between-group differences were statistically significant.
The analysis also showed that compared with the smaller babies, the larger neonates were twice as likely to be born during prolonged labor of more than 12 hours. Delivery of larger neonates was also twice as likely to trigger postpartum hemorrhage. But deliveries of larger babies had no significant link with increased rates of neonatal intensive care admissions, anal sphincter injuries, shoulder dystocias or birth injuries, compared with deliveries of smaller babies.
Dr. Galvin reported having no financial disclosures.
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Fetal macrosomia can be challenging to detect by ultrasound performed just before delivery, which had 41% sensitivity and 58% positive predictive value in a prospective study of more than 2,300 pregnancies.
The results also showed that fetal macrosomia (defined as birth weight of more than 4,000 grams) is significantly linked with increased rates of prolonged labor, delivery by either operative vaginal or cesarean approaches, and postpartum hemorrhage, Daniel M. Galvin, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Because all clinicians involved with these deliveries were blinded to the prenatal ultrasound results, the findings suggest that prolonged labor, postpartum hemorrhage, and need for either operative vaginal delivery or cesarean delivery are all outcomes driven by macrosomia itself rather than by clinical actions taken because of an expectation of macrosomia, said Dr. Galvin, an ob.gyn. with Perinatal Ireland, a Dublin-based consortium of eight Irish fetal medicine centers that is examining ways to improve delivery outcomes.
The study used “a pure population of pregnancies with unsuspected fetal macrosomia,” he explained.
Dr. Galvin and his colleagues used data collected in GENESIS, a prospective study run by the Perinatal Ireland multicenter consortium with the primary goal of determining whether late-pregnancy fetal head circumference can predict labor dystocia and intrapartum cesarean delivery. They examined two secondary outcomes: the reliability of ultrasound to estimate fetal size, and the consequences of fetal macrosomia when it is not recognized until delivery is already underway.
The study enrolled 2,336 nulliparous women with singleton pregnancies that ranged from the start of 39 weeks’ gestational age through the end of 40 weeks. The women underwent a standard ultrasound examination to assess fetal biometrics. The study excluded pregnancies with an estimated fetal size greater than 5,000 g. Mothers carrying a fetus estimated to be less than 4,000 g constituted 88% of the study group, with 12% carrying pregnancies with an estimated fetal weight greater than 4,000 g.
The ultrasound examination worked reasonably well for ruling out macrosomia, with an 89% rate of correctly identifying fetuses with a birth weight of less than 4,000 g. Near-term ultrasound was less useful for a positive identification of macrosomia; it flagged 58% of the fetuses born heavier than 4,000 g.
Analysis of delivery mode showed that infants born weighing more than 4,000 g had a statistically significant 56% reduced rate of spontaneous vaginal deliveries compared with smaller neonates, a 63% greater rate of cesarean deliveries, and a 49% greater rate of operative vaginal deliveries, compared with small babies, Dr. Galvin reported. All three between-group differences were statistically significant.
The analysis also showed that compared with the smaller babies, the larger neonates were twice as likely to be born during prolonged labor of more than 12 hours. Delivery of larger neonates was also twice as likely to trigger postpartum hemorrhage. But deliveries of larger babies had no significant link with increased rates of neonatal intensive care admissions, anal sphincter injuries, shoulder dystocias or birth injuries, compared with deliveries of smaller babies.
Dr. Galvin reported having no financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Near-term ultrasound identified 58% of fetuses born weighing more than 4,000 g.
Data source: Prospective, multicenter study of 2,336 singleton pregnancies.
Disclosures: Dr. Galvin reported having no financial disclosures.
When the iPad is on the other foot
Sometimes patients take a few notes when I talk, but Niles was different. As I started to spout words of wisdom about his granuloma annulare, he whipped out a tablet and started to type.
“How do you spell that again?” he wanted to know.
I spelled it out, and Niles tapped away. I launched into my usual explanation – how the cause is unknown, how it is roundish but not a fungus, how it usually has no systemic significance, and so on. At each point, looking down at the keyboard, he stopped me.
“Wait, you say it isn’t fungal?”
“No ...”
Typing. “And you don’t know the cause?”
“No, the medical term for that is ‘idiopathic’ ...”
“Wait, how do you spell that?”
I regretted using the word. “I-D-I-O-P-A-T-H-I-C.”
More typing. “Wait, hold on. OK, got it. And what did you say you want to treat it with?”
“A cream. Betamethasone dipropionate.”
“Hold on! How do you spell that?”
I spelled it out, along with “augmented” and “0.05%.”
The interview continued a bit longer. As we concluded, Niles thanked me for seeing him. At no time did he raise his eyes from the tablet, even as he was putting it back into its case. He acted the same way my staff does when I walk into the lunchroom. There I see three or four people sitting around a table with a sandwich or salad in front of them, staring at their smartphones. The same way groups of people do nowadays, everywhere. (A couple of years ago, I took some of my grandchildren out on a rowboat on the Charles River on a sunny summer afternoon. There we saw two young women, oars across their laps, examining their phones.)
When my student and I left the room, I took him aside.
“Did you see anything unusual about how that visit went?” I asked.
When he looked blank, I explained: “The patient didn’t look me in the eye once.”
Yes, come to think of it, the student had noticed that.
“Not very satisfying, was it?” I asked. “It’s hard to talk to somebody who isn’t looking at you. It’s even a little insulting, don’t you think?” He agreed.
“When you’re out in practice in a few years,” I said, “the person in the exam room looking at the computer and not making eye contact is likely to be you. Think about how it felt to watch me talking at the top of the patient’s head, and then imagine how your patients are likely to feel when they’re talking to the top of your head. Unless of course your laptop has a screen that blocks your head altogether.
“I just bring a clipboard with sheets of paper on it into the exam room,” I said. “The way things are working out, I think I’ll be able to make it to the end of my career without being forced to use an electronic device.
“You have your whole career ahead of you, though,” I told him. “I guess you’ll figure out how to make communication work.”
He will too, no doubt. He’ll have to. As the Romans used to say, times change, and we change with them.
No need to spell this out for the younger generation, literally or otherwise.
Just a short addendum from the world of artificial intelligence, as applied to voice recognition software:
Last week I saw Chad, who had seen my colleague a year earlier and come back for a skin check. She had described Chad’s occupation:
“The patient is a flight attendant for Diflucan Airlines.”
Check them out. Their restrooms are so clean you can go barefoot.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Sometimes patients take a few notes when I talk, but Niles was different. As I started to spout words of wisdom about his granuloma annulare, he whipped out a tablet and started to type.
“How do you spell that again?” he wanted to know.
I spelled it out, and Niles tapped away. I launched into my usual explanation – how the cause is unknown, how it is roundish but not a fungus, how it usually has no systemic significance, and so on. At each point, looking down at the keyboard, he stopped me.
“Wait, you say it isn’t fungal?”
“No ...”
Typing. “And you don’t know the cause?”
“No, the medical term for that is ‘idiopathic’ ...”
“Wait, how do you spell that?”
I regretted using the word. “I-D-I-O-P-A-T-H-I-C.”
More typing. “Wait, hold on. OK, got it. And what did you say you want to treat it with?”
“A cream. Betamethasone dipropionate.”
“Hold on! How do you spell that?”
I spelled it out, along with “augmented” and “0.05%.”
The interview continued a bit longer. As we concluded, Niles thanked me for seeing him. At no time did he raise his eyes from the tablet, even as he was putting it back into its case. He acted the same way my staff does when I walk into the lunchroom. There I see three or four people sitting around a table with a sandwich or salad in front of them, staring at their smartphones. The same way groups of people do nowadays, everywhere. (A couple of years ago, I took some of my grandchildren out on a rowboat on the Charles River on a sunny summer afternoon. There we saw two young women, oars across their laps, examining their phones.)
When my student and I left the room, I took him aside.
“Did you see anything unusual about how that visit went?” I asked.
When he looked blank, I explained: “The patient didn’t look me in the eye once.”
Yes, come to think of it, the student had noticed that.
“Not very satisfying, was it?” I asked. “It’s hard to talk to somebody who isn’t looking at you. It’s even a little insulting, don’t you think?” He agreed.
“When you’re out in practice in a few years,” I said, “the person in the exam room looking at the computer and not making eye contact is likely to be you. Think about how it felt to watch me talking at the top of the patient’s head, and then imagine how your patients are likely to feel when they’re talking to the top of your head. Unless of course your laptop has a screen that blocks your head altogether.
“I just bring a clipboard with sheets of paper on it into the exam room,” I said. “The way things are working out, I think I’ll be able to make it to the end of my career without being forced to use an electronic device.
“You have your whole career ahead of you, though,” I told him. “I guess you’ll figure out how to make communication work.”
He will too, no doubt. He’ll have to. As the Romans used to say, times change, and we change with them.
No need to spell this out for the younger generation, literally or otherwise.
Just a short addendum from the world of artificial intelligence, as applied to voice recognition software:
Last week I saw Chad, who had seen my colleague a year earlier and come back for a skin check. She had described Chad’s occupation:
“The patient is a flight attendant for Diflucan Airlines.”
Check them out. Their restrooms are so clean you can go barefoot.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Sometimes patients take a few notes when I talk, but Niles was different. As I started to spout words of wisdom about his granuloma annulare, he whipped out a tablet and started to type.
“How do you spell that again?” he wanted to know.
I spelled it out, and Niles tapped away. I launched into my usual explanation – how the cause is unknown, how it is roundish but not a fungus, how it usually has no systemic significance, and so on. At each point, looking down at the keyboard, he stopped me.
“Wait, you say it isn’t fungal?”
“No ...”
Typing. “And you don’t know the cause?”
“No, the medical term for that is ‘idiopathic’ ...”
“Wait, how do you spell that?”
I regretted using the word. “I-D-I-O-P-A-T-H-I-C.”
More typing. “Wait, hold on. OK, got it. And what did you say you want to treat it with?”
“A cream. Betamethasone dipropionate.”
“Hold on! How do you spell that?”
I spelled it out, along with “augmented” and “0.05%.”
The interview continued a bit longer. As we concluded, Niles thanked me for seeing him. At no time did he raise his eyes from the tablet, even as he was putting it back into its case. He acted the same way my staff does when I walk into the lunchroom. There I see three or four people sitting around a table with a sandwich or salad in front of them, staring at their smartphones. The same way groups of people do nowadays, everywhere. (A couple of years ago, I took some of my grandchildren out on a rowboat on the Charles River on a sunny summer afternoon. There we saw two young women, oars across their laps, examining their phones.)
When my student and I left the room, I took him aside.
“Did you see anything unusual about how that visit went?” I asked.
When he looked blank, I explained: “The patient didn’t look me in the eye once.”
Yes, come to think of it, the student had noticed that.
“Not very satisfying, was it?” I asked. “It’s hard to talk to somebody who isn’t looking at you. It’s even a little insulting, don’t you think?” He agreed.
“When you’re out in practice in a few years,” I said, “the person in the exam room looking at the computer and not making eye contact is likely to be you. Think about how it felt to watch me talking at the top of the patient’s head, and then imagine how your patients are likely to feel when they’re talking to the top of your head. Unless of course your laptop has a screen that blocks your head altogether.
“I just bring a clipboard with sheets of paper on it into the exam room,” I said. “The way things are working out, I think I’ll be able to make it to the end of my career without being forced to use an electronic device.
“You have your whole career ahead of you, though,” I told him. “I guess you’ll figure out how to make communication work.”
He will too, no doubt. He’ll have to. As the Romans used to say, times change, and we change with them.
No need to spell this out for the younger generation, literally or otherwise.
Just a short addendum from the world of artificial intelligence, as applied to voice recognition software:
Last week I saw Chad, who had seen my colleague a year earlier and come back for a skin check. She had described Chad’s occupation:
“The patient is a flight attendant for Diflucan Airlines.”
Check them out. Their restrooms are so clean you can go barefoot.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Exercise can boost cognition after stroke
HOUSTON – Exercise seems to improve some of the cognitive difficulties that can manifest in stroke survivors, even if patients don’t hit the gym for a couple of years after the incident.
A meta-analysis of 14 randomized, controlled studies has determined that the combination of aerobic and weight-bearing exercises is most effective at boosting brain function. The studies showed a moderate, but consistent, effect of exercise in both the acute and chronic phase, Lauren Oberlin said at the International Stroke Conference sponsored by the American Heart Association.
“This is an important message for stroke survivors who have experienced cognitive deficits for a long time: These may not be permanent, and they can be modified with physical activity,” she said.
Long-term cognitive impairment is a very common problem after stroke, she said, occurring to some degree in up to 85% of survivors. The most commonly affected domains are executive function, attention, processing speed, and memory.
“These deficits can also be highly persistent, and remain in the years after a stroke,” Ms. Oberlin said. “And they represent a major health and economic burden. Stroke survivors with cognitive deficits are at an increased risk for long-term disability, functional decline, dependent living, hospitalization, and even mortality. Despite all this, there is an absence of effective treatments. There are no effective pharmaceutical treatments and cognitive rehabilitation training has not been widely successful.”
Exercise has not been as well-studied in stroke as it has in other neurological disorders, including Parkinson’s disease, multiple sclerosis, and more recently, Alzheimer’s disease. Physical activity has also been shown to help maintain and boost cognitive function and memory in healthy aging populations.
Ms. Oberlin and her colleagues conducted a meta-analysis of 14 randomized, controlled studies conducted during 2001-2016. The studies enrolled a total of 736 subjects. All of them randomized subjects to 3-6 months of an active exercise arm or a control arm that did not involve physical training.
The researchers assessed effect sizes by Hedges’ g, calculated separately for intervention and control conditions within each trial. The results were interpreted as follows:
• Small effect = 0.2
• Moderate effect = 0.5
• Large effect = 0.8
Only four of the studies demonstrated a statistically significant benefit between the active and control arms. Seven trended strongly toward the positive, but had wide confidence intervals that crossed the null. Three studies showed no significant benefit. The overall effects size was 0.56 (moderate).
Ms. Oberlin and her colleagues then parsed the data to examine the effect of when the program was initiated after the stroke, the program’s duration, and the type of exercise it studied.
Length of the intervention (less than 3 months and more than 3 months) was not significantly related to cognitive outcome. However, the combination of aerobic and strength training exerted a significantly greater effect size (0.45) than did aerobic training or weight training alone (0.2 and 0.34, respectively). Programs that started more than 3 months after the stroke were also more effective than were those started earlier in recovery (0.45 vs. 0.16).
Finally, in a subset of five studies, she evaluated whether particular cognitive domains benefited most from physical activity. Attention and processing speed improved the most (0.4). Changes in memory and executive function were not significant.
“I will say this was only five studies, so we may have been underpowered to see any effects on these other domains,” Ms. Oberlin said.
The mechanistic link between physical activity and improved cognition has not been fully elucidated in humans, she said. However, animal studies have identified a number of associations. “In rodent studies, we see that exercise improves blood flow to the brain, and promotes both neurogenesis and synaptogenesis. This has been confirmed in imaging studies of healthy older adults. Exercise was associated with increased structural connectivity, changes in functional connectivity, and increases in brain volume in some regions, including the hippocampus and prefrontal cortex.”
She also acknowledged that preaching exercise in clinic is much easier than actually getting patients into the gym. “Stroke survivors often have mobility limitations, and these studies were primarily conducted in subjects who did not have those issues and who could work out on a treadmill or exercise bike. How we can adapt these programs to patients with limited mobility is certainly a challenge.”
Ms. Oberlin had no financial disclosures.
[email protected]
On Twitter @alz_gal
HOUSTON – Exercise seems to improve some of the cognitive difficulties that can manifest in stroke survivors, even if patients don’t hit the gym for a couple of years after the incident.
A meta-analysis of 14 randomized, controlled studies has determined that the combination of aerobic and weight-bearing exercises is most effective at boosting brain function. The studies showed a moderate, but consistent, effect of exercise in both the acute and chronic phase, Lauren Oberlin said at the International Stroke Conference sponsored by the American Heart Association.
“This is an important message for stroke survivors who have experienced cognitive deficits for a long time: These may not be permanent, and they can be modified with physical activity,” she said.
Long-term cognitive impairment is a very common problem after stroke, she said, occurring to some degree in up to 85% of survivors. The most commonly affected domains are executive function, attention, processing speed, and memory.
“These deficits can also be highly persistent, and remain in the years after a stroke,” Ms. Oberlin said. “And they represent a major health and economic burden. Stroke survivors with cognitive deficits are at an increased risk for long-term disability, functional decline, dependent living, hospitalization, and even mortality. Despite all this, there is an absence of effective treatments. There are no effective pharmaceutical treatments and cognitive rehabilitation training has not been widely successful.”
Exercise has not been as well-studied in stroke as it has in other neurological disorders, including Parkinson’s disease, multiple sclerosis, and more recently, Alzheimer’s disease. Physical activity has also been shown to help maintain and boost cognitive function and memory in healthy aging populations.
Ms. Oberlin and her colleagues conducted a meta-analysis of 14 randomized, controlled studies conducted during 2001-2016. The studies enrolled a total of 736 subjects. All of them randomized subjects to 3-6 months of an active exercise arm or a control arm that did not involve physical training.
The researchers assessed effect sizes by Hedges’ g, calculated separately for intervention and control conditions within each trial. The results were interpreted as follows:
• Small effect = 0.2
• Moderate effect = 0.5
• Large effect = 0.8
Only four of the studies demonstrated a statistically significant benefit between the active and control arms. Seven trended strongly toward the positive, but had wide confidence intervals that crossed the null. Three studies showed no significant benefit. The overall effects size was 0.56 (moderate).
Ms. Oberlin and her colleagues then parsed the data to examine the effect of when the program was initiated after the stroke, the program’s duration, and the type of exercise it studied.
Length of the intervention (less than 3 months and more than 3 months) was not significantly related to cognitive outcome. However, the combination of aerobic and strength training exerted a significantly greater effect size (0.45) than did aerobic training or weight training alone (0.2 and 0.34, respectively). Programs that started more than 3 months after the stroke were also more effective than were those started earlier in recovery (0.45 vs. 0.16).
Finally, in a subset of five studies, she evaluated whether particular cognitive domains benefited most from physical activity. Attention and processing speed improved the most (0.4). Changes in memory and executive function were not significant.
“I will say this was only five studies, so we may have been underpowered to see any effects on these other domains,” Ms. Oberlin said.
The mechanistic link between physical activity and improved cognition has not been fully elucidated in humans, she said. However, animal studies have identified a number of associations. “In rodent studies, we see that exercise improves blood flow to the brain, and promotes both neurogenesis and synaptogenesis. This has been confirmed in imaging studies of healthy older adults. Exercise was associated with increased structural connectivity, changes in functional connectivity, and increases in brain volume in some regions, including the hippocampus and prefrontal cortex.”
She also acknowledged that preaching exercise in clinic is much easier than actually getting patients into the gym. “Stroke survivors often have mobility limitations, and these studies were primarily conducted in subjects who did not have those issues and who could work out on a treadmill or exercise bike. How we can adapt these programs to patients with limited mobility is certainly a challenge.”
Ms. Oberlin had no financial disclosures.
[email protected]
On Twitter @alz_gal
HOUSTON – Exercise seems to improve some of the cognitive difficulties that can manifest in stroke survivors, even if patients don’t hit the gym for a couple of years after the incident.
A meta-analysis of 14 randomized, controlled studies has determined that the combination of aerobic and weight-bearing exercises is most effective at boosting brain function. The studies showed a moderate, but consistent, effect of exercise in both the acute and chronic phase, Lauren Oberlin said at the International Stroke Conference sponsored by the American Heart Association.
“This is an important message for stroke survivors who have experienced cognitive deficits for a long time: These may not be permanent, and they can be modified with physical activity,” she said.
Long-term cognitive impairment is a very common problem after stroke, she said, occurring to some degree in up to 85% of survivors. The most commonly affected domains are executive function, attention, processing speed, and memory.
“These deficits can also be highly persistent, and remain in the years after a stroke,” Ms. Oberlin said. “And they represent a major health and economic burden. Stroke survivors with cognitive deficits are at an increased risk for long-term disability, functional decline, dependent living, hospitalization, and even mortality. Despite all this, there is an absence of effective treatments. There are no effective pharmaceutical treatments and cognitive rehabilitation training has not been widely successful.”
Exercise has not been as well-studied in stroke as it has in other neurological disorders, including Parkinson’s disease, multiple sclerosis, and more recently, Alzheimer’s disease. Physical activity has also been shown to help maintain and boost cognitive function and memory in healthy aging populations.
Ms. Oberlin and her colleagues conducted a meta-analysis of 14 randomized, controlled studies conducted during 2001-2016. The studies enrolled a total of 736 subjects. All of them randomized subjects to 3-6 months of an active exercise arm or a control arm that did not involve physical training.
The researchers assessed effect sizes by Hedges’ g, calculated separately for intervention and control conditions within each trial. The results were interpreted as follows:
• Small effect = 0.2
• Moderate effect = 0.5
• Large effect = 0.8
Only four of the studies demonstrated a statistically significant benefit between the active and control arms. Seven trended strongly toward the positive, but had wide confidence intervals that crossed the null. Three studies showed no significant benefit. The overall effects size was 0.56 (moderate).
Ms. Oberlin and her colleagues then parsed the data to examine the effect of when the program was initiated after the stroke, the program’s duration, and the type of exercise it studied.
Length of the intervention (less than 3 months and more than 3 months) was not significantly related to cognitive outcome. However, the combination of aerobic and strength training exerted a significantly greater effect size (0.45) than did aerobic training or weight training alone (0.2 and 0.34, respectively). Programs that started more than 3 months after the stroke were also more effective than were those started earlier in recovery (0.45 vs. 0.16).
Finally, in a subset of five studies, she evaluated whether particular cognitive domains benefited most from physical activity. Attention and processing speed improved the most (0.4). Changes in memory and executive function were not significant.
“I will say this was only five studies, so we may have been underpowered to see any effects on these other domains,” Ms. Oberlin said.
The mechanistic link between physical activity and improved cognition has not been fully elucidated in humans, she said. However, animal studies have identified a number of associations. “In rodent studies, we see that exercise improves blood flow to the brain, and promotes both neurogenesis and synaptogenesis. This has been confirmed in imaging studies of healthy older adults. Exercise was associated with increased structural connectivity, changes in functional connectivity, and increases in brain volume in some regions, including the hippocampus and prefrontal cortex.”
She also acknowledged that preaching exercise in clinic is much easier than actually getting patients into the gym. “Stroke survivors often have mobility limitations, and these studies were primarily conducted in subjects who did not have those issues and who could work out on a treadmill or exercise bike. How we can adapt these programs to patients with limited mobility is certainly a challenge.”
Ms. Oberlin had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Overall, exercise after stroke exerted a moderate effect size of 0.56 on cognition.
Data source: The meta-analysis comprised 14 studies and 736 subjects.
Disclosures: Ms. Oberlin had no financial disclosures.
Inactivated hepatitis A vaccine shows promise in 5-year study
, a study has shown.
In October 2008, a team of investigators in China led by Zhilun Zhang of the Tianjin Center for Disease Control and Prevention, randomly assigned 332 children aged 18-60 months with prevaccination anti-HAV antibody titers of less than 20 mIU/mL to receive either one dose of inactivated hepatitis A vaccine or one dose of live, attenuated hepatitis A vaccine. Both groups were followed through December 2013, with assessments of anti-HAV antibody concentrations at years 1, 2, and 5 post vaccination. In all, 182 successfully completed the study, meeting all requirements, including providing serum samples at each time point.
Titer levels were 76.3% mIU/mL and 66.8mIU/mL for the inactivated and live vaccines at 5 years, respectively. No clinical hepatitis A case was reported.
The study appears online in Human Vaccines & Immunotherapeutics (doi: 10.1080/21645515.2016.1278329).
[email protected]
On Twitter @whitneymcknight
, a study has shown.
In October 2008, a team of investigators in China led by Zhilun Zhang of the Tianjin Center for Disease Control and Prevention, randomly assigned 332 children aged 18-60 months with prevaccination anti-HAV antibody titers of less than 20 mIU/mL to receive either one dose of inactivated hepatitis A vaccine or one dose of live, attenuated hepatitis A vaccine. Both groups were followed through December 2013, with assessments of anti-HAV antibody concentrations at years 1, 2, and 5 post vaccination. In all, 182 successfully completed the study, meeting all requirements, including providing serum samples at each time point.
Titer levels were 76.3% mIU/mL and 66.8mIU/mL for the inactivated and live vaccines at 5 years, respectively. No clinical hepatitis A case was reported.
The study appears online in Human Vaccines & Immunotherapeutics (doi: 10.1080/21645515.2016.1278329).
[email protected]
On Twitter @whitneymcknight
, a study has shown.
In October 2008, a team of investigators in China led by Zhilun Zhang of the Tianjin Center for Disease Control and Prevention, randomly assigned 332 children aged 18-60 months with prevaccination anti-HAV antibody titers of less than 20 mIU/mL to receive either one dose of inactivated hepatitis A vaccine or one dose of live, attenuated hepatitis A vaccine. Both groups were followed through December 2013, with assessments of anti-HAV antibody concentrations at years 1, 2, and 5 post vaccination. In all, 182 successfully completed the study, meeting all requirements, including providing serum samples at each time point.
Titer levels were 76.3% mIU/mL and 66.8mIU/mL for the inactivated and live vaccines at 5 years, respectively. No clinical hepatitis A case was reported.
The study appears online in Human Vaccines & Immunotherapeutics (doi: 10.1080/21645515.2016.1278329).
[email protected]
On Twitter @whitneymcknight
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
Using Gel to Study Effects of Blasts on the Brain
A gel that mimics the texture and mass of the brain, developed by U.S. Army Research Laboratory scientists, may help reveal what happens to the brain during an explosion.
The researchers used pressure-sensitive nanomaterials. The fluorescence intensity of the gel increases or decreases with the amount of pressure applied. Based on how the nanoclusters fluoresce under each pressure, the researchers will be able to gauge what would happen in a “brain situation,” 1 of the researchers says in a Health.mil article. The researchers are planning to create a pressure scale to graph information about the effects of blast pressure from the changes in color.
The laboratory has a working relationship with Japanese medical researchers who are also studying the effects of blast waves. The Japanese team will test the U.S. Army’s samples with a laser-induced shockwave and share the results of that experiment with the U.S. Army.
A gel that mimics the texture and mass of the brain, developed by U.S. Army Research Laboratory scientists, may help reveal what happens to the brain during an explosion.
The researchers used pressure-sensitive nanomaterials. The fluorescence intensity of the gel increases or decreases with the amount of pressure applied. Based on how the nanoclusters fluoresce under each pressure, the researchers will be able to gauge what would happen in a “brain situation,” 1 of the researchers says in a Health.mil article. The researchers are planning to create a pressure scale to graph information about the effects of blast pressure from the changes in color.
The laboratory has a working relationship with Japanese medical researchers who are also studying the effects of blast waves. The Japanese team will test the U.S. Army’s samples with a laser-induced shockwave and share the results of that experiment with the U.S. Army.
A gel that mimics the texture and mass of the brain, developed by U.S. Army Research Laboratory scientists, may help reveal what happens to the brain during an explosion.
The researchers used pressure-sensitive nanomaterials. The fluorescence intensity of the gel increases or decreases with the amount of pressure applied. Based on how the nanoclusters fluoresce under each pressure, the researchers will be able to gauge what would happen in a “brain situation,” 1 of the researchers says in a Health.mil article. The researchers are planning to create a pressure scale to graph information about the effects of blast pressure from the changes in color.
The laboratory has a working relationship with Japanese medical researchers who are also studying the effects of blast waves. The Japanese team will test the U.S. Army’s samples with a laser-induced shockwave and share the results of that experiment with the U.S. Army.
Blood in Urine, Rash on Trunk
Several days ago, a 14-year-old boy suddenly became ill with abdominal pain, fever, and arthralgia. Within 12 hours, a rash developed that covered most of his trunk, arms, and legs but spared his face, palms, and soles. It quickly flared bright red; some lesions were tender to touch. The patient’s legs and scrotum became edematous, and he lost his appetite. The patient developed diarrhea, and bright red blood was seen in his stools.
He was taken to the local emergency department, where examination revealed a fever of 101.5°F, an elevated white blood cell count, and a small amount of blood in his urine. Stool cultures were ordered, and the patient was placed on an unknown antibiotic.
The next day, he consulted his pediatrician, who referred him to dermatology.
EXAMINATION
Today, the patient is afebrile and in no acute distress. He still has a florid rash on his trunk, arms, and legs consisting of very evenly distributed, purpuric lesions that average 3 mm in diameter. A few are palpable, and none are blanchable. A punch biopsy is performed, and an entire lesion is obtained and submitted for pathologic examination.
What is the diagnosis?
DISCUSSION
The report showed leukocytoclastic vasculitis, in which activated lymphocytes attack the inner lining of blood vessels, causing them to leak blood into the surrounding interstitial spaces. Besides the extravasated red blood cells, nuclear dust (remnants of the attacking lymphocytes) is also seen.
These biopsy findings, in context with the patient’s history, help to confirm the diagnosis of Henoch-Schönlein purpura (HSP), an IgA-mediated disease that causes widespread vasculitis of small vessels throughout the body. Besides affecting the skin, this process can injure the gastrointestinal tract, joints, kidneys, and even lungs. As this case illustrates, it almost always presents with a palpable, purpuric, widespread rash, abdominal pain, fever, joint pain, and bloody stools.
HSP is seen primarily in children; in the US, 75% of cases occur in those ages 2 to 5. The most consistent presenting symptoms in this population include rash, abdominal pain, and joint pain. When fever is present, it is typically mild.
A variety factors can trigger HSP, including medications (eg, penicillin, NSAIDs, sulfa) and infection (with organisms such as mycoplasma, mononucleosis, strep, Legionella)—but many cases are simply idiopathic. History of upper respiratory infection, pharyngitis, or intestinal infection is found in 75% of young HSP patients. Antecedent vaccinations have also been reported as a potential trigger.
The diagnosis of HSP is primarily clinical, based on a combination of signs and symptoms and the exclusion of other items in the differential. Besides bloodwork to rule out end-organ (eg, renal) damage, a skin biopsy of the purpuric rash is necessary to establish the type of vasculitis.
Fortunately, most HSP patients recover uneventfully; the exception is the occasional patient with renal complications. The case patient successfully recovered following treatment with oral antibiotics (for presumed strep) and a three-week course of prednisone.
TAKE-HOME LEARNING POINTS
- A purpuric rash should prompt a punch biopsy to search for vasculitis.
- A widespread, palpable, purpuric rash accompanied by systemic symptoms of abdominal pain, arthralgia, fever, and malaise is suggestive of serious disease. In younger patients, Henoch-Schönlein purpura (HSP) should be a major suspect.
- Drugs, bugs, and vaccinations are all possible triggers for HSP.
- Once the diagnosis of HSP is made, monitoring for end-organ damage is essential.
Several days ago, a 14-year-old boy suddenly became ill with abdominal pain, fever, and arthralgia. Within 12 hours, a rash developed that covered most of his trunk, arms, and legs but spared his face, palms, and soles. It quickly flared bright red; some lesions were tender to touch. The patient’s legs and scrotum became edematous, and he lost his appetite. The patient developed diarrhea, and bright red blood was seen in his stools.
He was taken to the local emergency department, where examination revealed a fever of 101.5°F, an elevated white blood cell count, and a small amount of blood in his urine. Stool cultures were ordered, and the patient was placed on an unknown antibiotic.
The next day, he consulted his pediatrician, who referred him to dermatology.
EXAMINATION
Today, the patient is afebrile and in no acute distress. He still has a florid rash on his trunk, arms, and legs consisting of very evenly distributed, purpuric lesions that average 3 mm in diameter. A few are palpable, and none are blanchable. A punch biopsy is performed, and an entire lesion is obtained and submitted for pathologic examination.

What is the diagnosis?
DISCUSSION
The report showed leukocytoclastic vasculitis, in which activated lymphocytes attack the inner lining of blood vessels, causing them to leak blood into the surrounding interstitial spaces. Besides the extravasated red blood cells, nuclear dust (remnants of the attacking lymphocytes) is also seen.
These biopsy findings, in context with the patient’s history, help to confirm the diagnosis of Henoch-Schönlein purpura (HSP), an IgA-mediated disease that causes widespread vasculitis of small vessels throughout the body. Besides affecting the skin, this process can injure the gastrointestinal tract, joints, kidneys, and even lungs. As this case illustrates, it almost always presents with a palpable, purpuric, widespread rash, abdominal pain, fever, joint pain, and bloody stools.
HSP is seen primarily in children; in the US, 75% of cases occur in those ages 2 to 5. The most consistent presenting symptoms in this population include rash, abdominal pain, and joint pain. When fever is present, it is typically mild.
A variety factors can trigger HSP, including medications (eg, penicillin, NSAIDs, sulfa) and infection (with organisms such as mycoplasma, mononucleosis, strep, Legionella)—but many cases are simply idiopathic. History of upper respiratory infection, pharyngitis, or intestinal infection is found in 75% of young HSP patients. Antecedent vaccinations have also been reported as a potential trigger.
The diagnosis of HSP is primarily clinical, based on a combination of signs and symptoms and the exclusion of other items in the differential. Besides bloodwork to rule out end-organ (eg, renal) damage, a skin biopsy of the purpuric rash is necessary to establish the type of vasculitis.
Fortunately, most HSP patients recover uneventfully; the exception is the occasional patient with renal complications. The case patient successfully recovered following treatment with oral antibiotics (for presumed strep) and a three-week course of prednisone.
TAKE-HOME LEARNING POINTS
- A purpuric rash should prompt a punch biopsy to search for vasculitis.
- A widespread, palpable, purpuric rash accompanied by systemic symptoms of abdominal pain, arthralgia, fever, and malaise is suggestive of serious disease. In younger patients, Henoch-Schönlein purpura (HSP) should be a major suspect.
- Drugs, bugs, and vaccinations are all possible triggers for HSP.
- Once the diagnosis of HSP is made, monitoring for end-organ damage is essential.
Several days ago, a 14-year-old boy suddenly became ill with abdominal pain, fever, and arthralgia. Within 12 hours, a rash developed that covered most of his trunk, arms, and legs but spared his face, palms, and soles. It quickly flared bright red; some lesions were tender to touch. The patient’s legs and scrotum became edematous, and he lost his appetite. The patient developed diarrhea, and bright red blood was seen in his stools.
He was taken to the local emergency department, where examination revealed a fever of 101.5°F, an elevated white blood cell count, and a small amount of blood in his urine. Stool cultures were ordered, and the patient was placed on an unknown antibiotic.
The next day, he consulted his pediatrician, who referred him to dermatology.
EXAMINATION
Today, the patient is afebrile and in no acute distress. He still has a florid rash on his trunk, arms, and legs consisting of very evenly distributed, purpuric lesions that average 3 mm in diameter. A few are palpable, and none are blanchable. A punch biopsy is performed, and an entire lesion is obtained and submitted for pathologic examination.

What is the diagnosis?
DISCUSSION
The report showed leukocytoclastic vasculitis, in which activated lymphocytes attack the inner lining of blood vessels, causing them to leak blood into the surrounding interstitial spaces. Besides the extravasated red blood cells, nuclear dust (remnants of the attacking lymphocytes) is also seen.
These biopsy findings, in context with the patient’s history, help to confirm the diagnosis of Henoch-Schönlein purpura (HSP), an IgA-mediated disease that causes widespread vasculitis of small vessels throughout the body. Besides affecting the skin, this process can injure the gastrointestinal tract, joints, kidneys, and even lungs. As this case illustrates, it almost always presents with a palpable, purpuric, widespread rash, abdominal pain, fever, joint pain, and bloody stools.
HSP is seen primarily in children; in the US, 75% of cases occur in those ages 2 to 5. The most consistent presenting symptoms in this population include rash, abdominal pain, and joint pain. When fever is present, it is typically mild.
A variety factors can trigger HSP, including medications (eg, penicillin, NSAIDs, sulfa) and infection (with organisms such as mycoplasma, mononucleosis, strep, Legionella)—but many cases are simply idiopathic. History of upper respiratory infection, pharyngitis, or intestinal infection is found in 75% of young HSP patients. Antecedent vaccinations have also been reported as a potential trigger.
The diagnosis of HSP is primarily clinical, based on a combination of signs and symptoms and the exclusion of other items in the differential. Besides bloodwork to rule out end-organ (eg, renal) damage, a skin biopsy of the purpuric rash is necessary to establish the type of vasculitis.
Fortunately, most HSP patients recover uneventfully; the exception is the occasional patient with renal complications. The case patient successfully recovered following treatment with oral antibiotics (for presumed strep) and a three-week course of prednisone.
TAKE-HOME LEARNING POINTS
- A purpuric rash should prompt a punch biopsy to search for vasculitis.
- A widespread, palpable, purpuric rash accompanied by systemic symptoms of abdominal pain, arthralgia, fever, and malaise is suggestive of serious disease. In younger patients, Henoch-Schönlein purpura (HSP) should be a major suspect.
- Drugs, bugs, and vaccinations are all possible triggers for HSP.
- Once the diagnosis of HSP is made, monitoring for end-organ damage is essential.
Dark line across nose
The FP recognized the dark line on the patient’s face as a hyperpigmented horizontal nasal crease based on the fact that she had the atopic triad and repeatedly wiped her nose in an upward motion (an “allergic salute”) whenever her nose felt itchy. There is no specific treatment for a hyperpigmented horizontal nose crease, except to help control the allergic rhinitis. It also helps to control any atopic dermatitis, which can lead to pruritus.
The patient was happy to know the cause of the condition and did not request treatment for the cosmetic aspect of it. For patients who want treatment, a good place to start is with an over-the-counter 3% hydroquinone bleaching agent, along with 1% hydrocortisone cream. (These can both be applied twice daily.)
The FP in this case also recommended sun protection and sun avoidance to avoid further darkening of the hyperpigmented crease.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized the dark line on the patient’s face as a hyperpigmented horizontal nasal crease based on the fact that she had the atopic triad and repeatedly wiped her nose in an upward motion (an “allergic salute”) whenever her nose felt itchy. There is no specific treatment for a hyperpigmented horizontal nose crease, except to help control the allergic rhinitis. It also helps to control any atopic dermatitis, which can lead to pruritus.
The patient was happy to know the cause of the condition and did not request treatment for the cosmetic aspect of it. For patients who want treatment, a good place to start is with an over-the-counter 3% hydroquinone bleaching agent, along with 1% hydrocortisone cream. (These can both be applied twice daily.)
The FP in this case also recommended sun protection and sun avoidance to avoid further darkening of the hyperpigmented crease.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP recognized the dark line on the patient’s face as a hyperpigmented horizontal nasal crease based on the fact that she had the atopic triad and repeatedly wiped her nose in an upward motion (an “allergic salute”) whenever her nose felt itchy. There is no specific treatment for a hyperpigmented horizontal nose crease, except to help control the allergic rhinitis. It also helps to control any atopic dermatitis, which can lead to pruritus.
The patient was happy to know the cause of the condition and did not request treatment for the cosmetic aspect of it. For patients who want treatment, a good place to start is with an over-the-counter 3% hydroquinone bleaching agent, along with 1% hydrocortisone cream. (These can both be applied twice daily.)
The FP in this case also recommended sun protection and sun avoidance to avoid further darkening of the hyperpigmented crease.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Neuromyelitis Optica Spectrum Disorders: Critical Role of Complement-Dependent Cytotoxicity
Critical Role of Complement-Dependent Cytotoxicity
The complement system was once thought to have a fairly limited role in the immune system, recognizing and eliminating pathogens. Now, however, complement proteins are known to have a much broader role in immunity, and dysregulation of the complement system has been shown to affect the pathogenesis and clinical picture of several autoimmune diseases. This supplement discusses the role of complement-dependent cytotoxicity in the pathophysiology of neuromyelitis optica spectrum disorders.
Click here to read the supplement
US/UNB-NMO/17/0002c
The complement system was once thought to have a fairly limited role in the immune system, recognizing and eliminating pathogens. Now, however, complement proteins are known to have a much broader role in immunity, and dysregulation of the complement system has been shown to affect the pathogenesis and clinical picture of several autoimmune diseases. This supplement discusses the role of complement-dependent cytotoxicity in the pathophysiology of neuromyelitis optica spectrum disorders.
Click here to read the supplement
US/UNB-NMO/17/0002c
The complement system was once thought to have a fairly limited role in the immune system, recognizing and eliminating pathogens. Now, however, complement proteins are known to have a much broader role in immunity, and dysregulation of the complement system has been shown to affect the pathogenesis and clinical picture of several autoimmune diseases. This supplement discusses the role of complement-dependent cytotoxicity in the pathophysiology of neuromyelitis optica spectrum disorders.
Click here to read the supplement
US/UNB-NMO/17/0002c
Critical Role of Complement-Dependent Cytotoxicity
Critical Role of Complement-Dependent Cytotoxicity