User login
Multiple myeloma: Lenalidomide approved as maintenance therapy after auto-HSCT
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
The Food and Drug Administration has approved the use of lenalidomide (Revlimid) for maintenance therapy following autologous hematopoietic stem cell transplant in patients with multiple myeloma.
The expanded indication, announced Feb. 22, makes the immunomodulatory agent the first and only approved treatment for post autologous hematopoietic stem cell transplant (auto-HSCT) maintenance. It was initially approved in 2006 for use in combination with dexamethasone in patients with multiple myeloma who have received at least one prior therapy, and that indication was expanded in 2015 to include those with newly diagnosed multiple myeloma.
According to Celgene, the maker of Revlimid, the latest approval was based on data showing that lenalidomide maintenance therapy delays disease progression following auto-HSCT. Updated phase III randomized controlled trial data from two studies including more than 1,000 patients demonstrated median progression-free survival (PFS) advantages with lenalidomide maintenance vs. no maintenance. In one study – the U.S.-based CALGB 1001014 – median PFS was 5.7 vs. 1.9 years for a difference of 3.8 years (hazard ratio, 0.38). In the second study – the European IFM 2005-02 – median PFS was 3.9 vs. 2 years, for a difference of 1.9 years (HR, 0.53).
In both studies lenalidomide was given as a 10-mg daily oral dose (increased to 15 mg daily after 3 months if tolerated) until disease progression or unacceptable toxicity after auto-HSCT.
Lenalidomide, a derivative of thalidomide, can cause fetal harm and is contraindicated in women who are pregnant. It is available only through a restricted distribution program.
The most frequently reported adverse reactions in the two studies were neutropenia, thrombocytopenia, leukopenia, anemia, upper respiratory tract infection, bronchitis, nasopharyngitis, cough, gastroenteritis, diarrhea, rash, fatigue, muscle spasm, and pyrexia. The most frequently reported grade 3 or 4 reactions occurring in more than 20% of patients in the lenalidomide arms included neutropenia, thrombocytopenia, and leukopenia.
“Autologous stem cell transplant after induction therapy is part of the continuum of care for transplant-eligible multiple myeloma patients. However, most patients will still see their disease recur or progress after this treatment,” Philip McCarthy, MD, of the Roswell Park Cancer Institute in Buffalo, N.Y., said in a Celgene press statement. “Lenalidomide maintenance therapy ... can be considered a standard of care for these patients.”
Annual nailfold videocapillaroscopy found prognostic in systemic sclerosis
Annual nailfold videocapillaroscopy can be used to predict disease progression in systemic sclerosis, according to a report in Seminars in Arthritis & Rheumatism.
Early and diffuse alterations in the microvasculature are a key feature of systemic sclerosis, and nailfold videocapillaroscopy can detect morphologic changes that reflect such alterations, including capillary loss, neoangiogenesis, giant capillaries, microhemorrhages, and the presence of avascular areas. The technique already has an established role in diagnosing systemic sclerosis, said Jérôme Avouac, MD, of the rheumatology department at Cochin Hospital, Paris, and his associates.
Two contiguous fields extending over 1 mm in the middle of each nailfold, corresponding to the distal row of capillaries, were assessed for four features: the number of capillaries, the presence of giant capillaries, microhemorrhages, and neoangiogenesis (defined as meandering, ramified, branching, bushy, bizarre capillaries and those with more than two crossings). A total of 72 patients (51%) showed significant progression of at least one of these features during follow-up.
A progressive loss of capillaries over time strongly predicted overall disease progression (hazard ratio, 4.35). Other significant predictors of overall disease progression included the development of new ischemic digital ulcers (HR, 5.33), progression of lung involvement (HR, 18.53), progression of skin fibrosis (HR, 4.22), and worsening of the Medsger severity score (HR, 5.26). In addition, the presence of neoangiogenesis at baseline, but not the progression of neoangiogenesis over time, also predicted overall disease progression (HR, 2.53), the development of new ischemic digital ulcers (HR, 2.60), progression of lung involvement (HR, 7.38), and worsening of the Medsger severity score (HR, 2.72), Dr. Avouac and his associates said (Semin Arthritis Rheum. 2017 Feb 10. doi: 10.1016/j.semarthrit.2017.02.006).
These findings demonstrate that serial videocapillaroscopy, which they described as a simple, safe, noninvasive, and inexpensive imaging technique, can be used in routine follow-up of systemic sclerosis to improve risk assessment, the investigators said.
The authors reported no financial support for this study and reported having no relevant financial disclosures.
Annual nailfold videocapillaroscopy can be used to predict disease progression in systemic sclerosis, according to a report in Seminars in Arthritis & Rheumatism.
Early and diffuse alterations in the microvasculature are a key feature of systemic sclerosis, and nailfold videocapillaroscopy can detect morphologic changes that reflect such alterations, including capillary loss, neoangiogenesis, giant capillaries, microhemorrhages, and the presence of avascular areas. The technique already has an established role in diagnosing systemic sclerosis, said Jérôme Avouac, MD, of the rheumatology department at Cochin Hospital, Paris, and his associates.
Two contiguous fields extending over 1 mm in the middle of each nailfold, corresponding to the distal row of capillaries, were assessed for four features: the number of capillaries, the presence of giant capillaries, microhemorrhages, and neoangiogenesis (defined as meandering, ramified, branching, bushy, bizarre capillaries and those with more than two crossings). A total of 72 patients (51%) showed significant progression of at least one of these features during follow-up.
A progressive loss of capillaries over time strongly predicted overall disease progression (hazard ratio, 4.35). Other significant predictors of overall disease progression included the development of new ischemic digital ulcers (HR, 5.33), progression of lung involvement (HR, 18.53), progression of skin fibrosis (HR, 4.22), and worsening of the Medsger severity score (HR, 5.26). In addition, the presence of neoangiogenesis at baseline, but not the progression of neoangiogenesis over time, also predicted overall disease progression (HR, 2.53), the development of new ischemic digital ulcers (HR, 2.60), progression of lung involvement (HR, 7.38), and worsening of the Medsger severity score (HR, 2.72), Dr. Avouac and his associates said (Semin Arthritis Rheum. 2017 Feb 10. doi: 10.1016/j.semarthrit.2017.02.006).
These findings demonstrate that serial videocapillaroscopy, which they described as a simple, safe, noninvasive, and inexpensive imaging technique, can be used in routine follow-up of systemic sclerosis to improve risk assessment, the investigators said.
The authors reported no financial support for this study and reported having no relevant financial disclosures.
Annual nailfold videocapillaroscopy can be used to predict disease progression in systemic sclerosis, according to a report in Seminars in Arthritis & Rheumatism.
Early and diffuse alterations in the microvasculature are a key feature of systemic sclerosis, and nailfold videocapillaroscopy can detect morphologic changes that reflect such alterations, including capillary loss, neoangiogenesis, giant capillaries, microhemorrhages, and the presence of avascular areas. The technique already has an established role in diagnosing systemic sclerosis, said Jérôme Avouac, MD, of the rheumatology department at Cochin Hospital, Paris, and his associates.
Two contiguous fields extending over 1 mm in the middle of each nailfold, corresponding to the distal row of capillaries, were assessed for four features: the number of capillaries, the presence of giant capillaries, microhemorrhages, and neoangiogenesis (defined as meandering, ramified, branching, bushy, bizarre capillaries and those with more than two crossings). A total of 72 patients (51%) showed significant progression of at least one of these features during follow-up.
A progressive loss of capillaries over time strongly predicted overall disease progression (hazard ratio, 4.35). Other significant predictors of overall disease progression included the development of new ischemic digital ulcers (HR, 5.33), progression of lung involvement (HR, 18.53), progression of skin fibrosis (HR, 4.22), and worsening of the Medsger severity score (HR, 5.26). In addition, the presence of neoangiogenesis at baseline, but not the progression of neoangiogenesis over time, also predicted overall disease progression (HR, 2.53), the development of new ischemic digital ulcers (HR, 2.60), progression of lung involvement (HR, 7.38), and worsening of the Medsger severity score (HR, 2.72), Dr. Avouac and his associates said (Semin Arthritis Rheum. 2017 Feb 10. doi: 10.1016/j.semarthrit.2017.02.006).
These findings demonstrate that serial videocapillaroscopy, which they described as a simple, safe, noninvasive, and inexpensive imaging technique, can be used in routine follow-up of systemic sclerosis to improve risk assessment, the investigators said.
The authors reported no financial support for this study and reported having no relevant financial disclosures.
Key clinical point: Annual nailfold videocapillaroscopy can be used to predict disease progression in systemic sclerosis.
Major finding: A progressive loss of capillaries over time strongly predicted overall disease progression (HR, 4.35).
Data source: A prospective single-center observational cohort study involving 140 patients followed for up to 5 years.
Disclosures: The authors reported no financial support for this study and reported having no relevant financial disclosures.
ACIP approves minor changes to pediatric hepatitis B vaccine recommendations
Approval to changes of current recommendations for hepatitis B vaccinations for children were voted on by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The 14-member panel voted to approve changes to existing language, which states that infants who are HBsAg negative with anti-HBs levels of less than 10 mIU/mL should be revaccinated with a second three-dose series and retested within 2 months of the series’ final dose. The approved proposal will change that language to state that these infants should receive only one dose, not the entire series of three. However, if anti-HBs levels remain lower than 10 mIU/mL after the one dose, the remaining two vaccinations should be administered, along with testing within 2 months of the final dose.
The other change, a relatively minor one, affects the wording of the recommendations regarding the Vaccines for Children program. In addition to incorporating a language change similar to the aforementioned one – the only difference being that now, the recommendations will explicitly mention postvaccination serologic testing within 2 months of series completion – under the minimum dosing intervals for interrupted vaccination schedules, the second bullet has been modified to say “final dose” instead of “third dose,” as it currently does.
“[This is] to address potential confusion related to different schedules when single-antigen or combination vaccines are used,” explained Jeanne Santoli, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, adding that “the eligible groups are unchanged, schedule and intervals are unchanged, [so] the purpose is to clarify related to dosing intervals and revaccination.”
Both votes were approved of nearly unanimously, with 13 committee members voting to approve while 1 – José R. Romero, MD, who holds the Horace C. Cabe Endowed Chair in Pediatric Infectious Diseases at the University of Arkansas in Little Rock – abstained because of potential conflicts of interest.
Approval by ACIP does not automatically mean that these changes will go into effect; they must first be approved by CDC director Tom Frieden, MD. However, the CDC generally follows ACIP guidance.
Approval to changes of current recommendations for hepatitis B vaccinations for children were voted on by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The 14-member panel voted to approve changes to existing language, which states that infants who are HBsAg negative with anti-HBs levels of less than 10 mIU/mL should be revaccinated with a second three-dose series and retested within 2 months of the series’ final dose. The approved proposal will change that language to state that these infants should receive only one dose, not the entire series of three. However, if anti-HBs levels remain lower than 10 mIU/mL after the one dose, the remaining two vaccinations should be administered, along with testing within 2 months of the final dose.
The other change, a relatively minor one, affects the wording of the recommendations regarding the Vaccines for Children program. In addition to incorporating a language change similar to the aforementioned one – the only difference being that now, the recommendations will explicitly mention postvaccination serologic testing within 2 months of series completion – under the minimum dosing intervals for interrupted vaccination schedules, the second bullet has been modified to say “final dose” instead of “third dose,” as it currently does.
“[This is] to address potential confusion related to different schedules when single-antigen or combination vaccines are used,” explained Jeanne Santoli, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, adding that “the eligible groups are unchanged, schedule and intervals are unchanged, [so] the purpose is to clarify related to dosing intervals and revaccination.”
Both votes were approved of nearly unanimously, with 13 committee members voting to approve while 1 – José R. Romero, MD, who holds the Horace C. Cabe Endowed Chair in Pediatric Infectious Diseases at the University of Arkansas in Little Rock – abstained because of potential conflicts of interest.
Approval by ACIP does not automatically mean that these changes will go into effect; they must first be approved by CDC director Tom Frieden, MD. However, the CDC generally follows ACIP guidance.
Approval to changes of current recommendations for hepatitis B vaccinations for children were voted on by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The 14-member panel voted to approve changes to existing language, which states that infants who are HBsAg negative with anti-HBs levels of less than 10 mIU/mL should be revaccinated with a second three-dose series and retested within 2 months of the series’ final dose. The approved proposal will change that language to state that these infants should receive only one dose, not the entire series of three. However, if anti-HBs levels remain lower than 10 mIU/mL after the one dose, the remaining two vaccinations should be administered, along with testing within 2 months of the final dose.
The other change, a relatively minor one, affects the wording of the recommendations regarding the Vaccines for Children program. In addition to incorporating a language change similar to the aforementioned one – the only difference being that now, the recommendations will explicitly mention postvaccination serologic testing within 2 months of series completion – under the minimum dosing intervals for interrupted vaccination schedules, the second bullet has been modified to say “final dose” instead of “third dose,” as it currently does.
“[This is] to address potential confusion related to different schedules when single-antigen or combination vaccines are used,” explained Jeanne Santoli, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, adding that “the eligible groups are unchanged, schedule and intervals are unchanged, [so] the purpose is to clarify related to dosing intervals and revaccination.”
Both votes were approved of nearly unanimously, with 13 committee members voting to approve while 1 – José R. Romero, MD, who holds the Horace C. Cabe Endowed Chair in Pediatric Infectious Diseases at the University of Arkansas in Little Rock – abstained because of potential conflicts of interest.
Approval by ACIP does not automatically mean that these changes will go into effect; they must first be approved by CDC director Tom Frieden, MD. However, the CDC generally follows ACIP guidance.
Lanadelumab reduced hereditary angioedema attacks by 88%-100%
Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100% in a small, phase I trial.
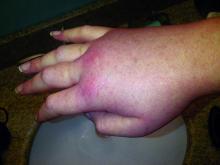
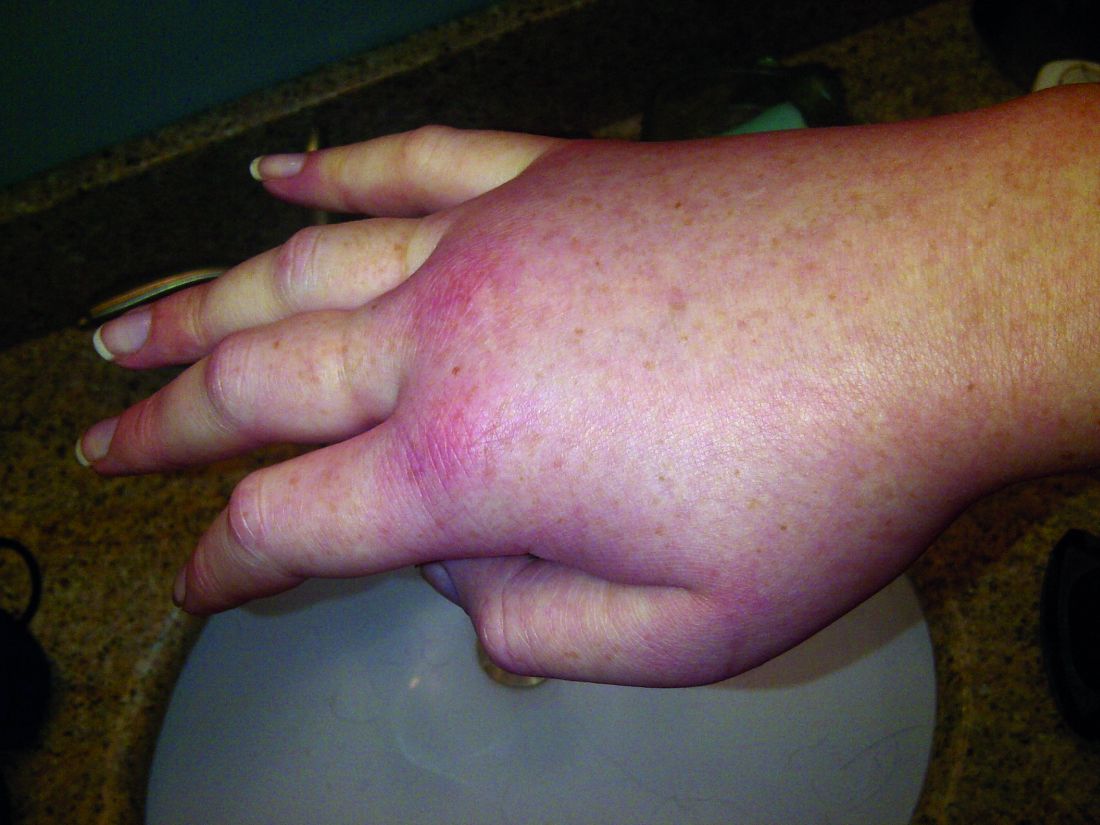
Hereditary angioedema with C1 inhibitor deficiency is a rare disorder characterized by unpredictable, recurrent, and potentially life-threatening episodes of subcutaneous or submucosal swelling, typically affecting the hands and feet, abdomen, face, larynx, or genitourinary tract. It is caused by a deficiency or dysfunction of the C1 inhibitor, which regulates the complement, coagulation, and kallikrein-kinin cascades.
They performed a multicenter, double-blind, randomized study to assess the safety and adverse-effect profile of four doses of this new agent or placebo in 37 adults (aged 18-71 years, mean age, 39.9 years) who received two injections, 2 weeks apart, and were followed for 6 weeks. Given their histories, all the study participants had “a reasonable probability of having one or more attacks” during the study period, the researchers noted.
Four participants received a 30-mg dose, 4 received a 100-mg dose, 5 received a 300-mg dose, 11 received a 400-mg dose, and 13 received placebo.
There were no serious adverse events, no deaths, and no discontinuations of the study medication because of an adverse effect. One patient each developed severe adverse events: pain at the injection site that lasted for 1 minute and headache plus night sweats.
Pharmacodynamic assessments showed that lanadelumab inhibited kallikrein in a linear, dose-dependent manner, and the two higher doses reduced levels of cleaved high-molecular-weight kininogen to those reported in healthy control subjects. At the same time, the two higher doses decreased the number of attacks by 88% and 100%, respectively, compared with placebo.
All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no attacks during the study period, the investigators said.
These findings “provide proof of concept that lanadelumab has the potential to correct the pathophysiological abnormality underlying attacks of angioedema and may be a new therapeutic option for hereditary angioedema with C1 inhibitor deficiency,” Dr. Banerji and her associates said.
The HELP Study, a phase III trial assessing the safety and efficacy of 6 months of lanadelumab treatment, is now underway, they added.
The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing of the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
This preliminary study suggests that a new agent, in injections that would be convenient and widely accessible, could provide an unprecedented level of protection against angioedema.
If these findings are confirmed, and if lanadelumab is affordable, it could transform the way hereditary angioedema is managed and the life prospects for affected families.
Moreover, kallikrein is implicated in other forms of bradykinin-mediated angioedema, such as that associated with ACE inhibitors, and plays a key role in the generation of inflammation and pain. So, the sustained inhibition of kallikrein potentially could be beneficial for a much wider range of disorders.
Hilary J. Longhurst, MD, is at Barts Health National Health Service Trust, London. She reported having ties to BioCryst, CSL Behring, and Shire. Dr. Longhurst made these remarks in an editorial accompanying Dr. Banerji’s report (N Engl J Med. 2017 Feb 23;376[8]:788-9).
This preliminary study suggests that a new agent, in injections that would be convenient and widely accessible, could provide an unprecedented level of protection against angioedema.
If these findings are confirmed, and if lanadelumab is affordable, it could transform the way hereditary angioedema is managed and the life prospects for affected families.
Moreover, kallikrein is implicated in other forms of bradykinin-mediated angioedema, such as that associated with ACE inhibitors, and plays a key role in the generation of inflammation and pain. So, the sustained inhibition of kallikrein potentially could be beneficial for a much wider range of disorders.
Hilary J. Longhurst, MD, is at Barts Health National Health Service Trust, London. She reported having ties to BioCryst, CSL Behring, and Shire. Dr. Longhurst made these remarks in an editorial accompanying Dr. Banerji’s report (N Engl J Med. 2017 Feb 23;376[8]:788-9).
This preliminary study suggests that a new agent, in injections that would be convenient and widely accessible, could provide an unprecedented level of protection against angioedema.
If these findings are confirmed, and if lanadelumab is affordable, it could transform the way hereditary angioedema is managed and the life prospects for affected families.
Moreover, kallikrein is implicated in other forms of bradykinin-mediated angioedema, such as that associated with ACE inhibitors, and plays a key role in the generation of inflammation and pain. So, the sustained inhibition of kallikrein potentially could be beneficial for a much wider range of disorders.
Hilary J. Longhurst, MD, is at Barts Health National Health Service Trust, London. She reported having ties to BioCryst, CSL Behring, and Shire. Dr. Longhurst made these remarks in an editorial accompanying Dr. Banerji’s report (N Engl J Med. 2017 Feb 23;376[8]:788-9).
Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100% in a small, phase I trial.
Hereditary angioedema with C1 inhibitor deficiency is a rare disorder characterized by unpredictable, recurrent, and potentially life-threatening episodes of subcutaneous or submucosal swelling, typically affecting the hands and feet, abdomen, face, larynx, or genitourinary tract. It is caused by a deficiency or dysfunction of the C1 inhibitor, which regulates the complement, coagulation, and kallikrein-kinin cascades.
They performed a multicenter, double-blind, randomized study to assess the safety and adverse-effect profile of four doses of this new agent or placebo in 37 adults (aged 18-71 years, mean age, 39.9 years) who received two injections, 2 weeks apart, and were followed for 6 weeks. Given their histories, all the study participants had “a reasonable probability of having one or more attacks” during the study period, the researchers noted.
Four participants received a 30-mg dose, 4 received a 100-mg dose, 5 received a 300-mg dose, 11 received a 400-mg dose, and 13 received placebo.
There were no serious adverse events, no deaths, and no discontinuations of the study medication because of an adverse effect. One patient each developed severe adverse events: pain at the injection site that lasted for 1 minute and headache plus night sweats.
Pharmacodynamic assessments showed that lanadelumab inhibited kallikrein in a linear, dose-dependent manner, and the two higher doses reduced levels of cleaved high-molecular-weight kininogen to those reported in healthy control subjects. At the same time, the two higher doses decreased the number of attacks by 88% and 100%, respectively, compared with placebo.
All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no attacks during the study period, the investigators said.
These findings “provide proof of concept that lanadelumab has the potential to correct the pathophysiological abnormality underlying attacks of angioedema and may be a new therapeutic option for hereditary angioedema with C1 inhibitor deficiency,” Dr. Banerji and her associates said.
The HELP Study, a phase III trial assessing the safety and efficacy of 6 months of lanadelumab treatment, is now underway, they added.
The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing of the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100% in a small, phase I trial.
Hereditary angioedema with C1 inhibitor deficiency is a rare disorder characterized by unpredictable, recurrent, and potentially life-threatening episodes of subcutaneous or submucosal swelling, typically affecting the hands and feet, abdomen, face, larynx, or genitourinary tract. It is caused by a deficiency or dysfunction of the C1 inhibitor, which regulates the complement, coagulation, and kallikrein-kinin cascades.
They performed a multicenter, double-blind, randomized study to assess the safety and adverse-effect profile of four doses of this new agent or placebo in 37 adults (aged 18-71 years, mean age, 39.9 years) who received two injections, 2 weeks apart, and were followed for 6 weeks. Given their histories, all the study participants had “a reasonable probability of having one or more attacks” during the study period, the researchers noted.
Four participants received a 30-mg dose, 4 received a 100-mg dose, 5 received a 300-mg dose, 11 received a 400-mg dose, and 13 received placebo.
There were no serious adverse events, no deaths, and no discontinuations of the study medication because of an adverse effect. One patient each developed severe adverse events: pain at the injection site that lasted for 1 minute and headache plus night sweats.
Pharmacodynamic assessments showed that lanadelumab inhibited kallikrein in a linear, dose-dependent manner, and the two higher doses reduced levels of cleaved high-molecular-weight kininogen to those reported in healthy control subjects. At the same time, the two higher doses decreased the number of attacks by 88% and 100%, respectively, compared with placebo.
All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no attacks during the study period, the investigators said.
These findings “provide proof of concept that lanadelumab has the potential to correct the pathophysiological abnormality underlying attacks of angioedema and may be a new therapeutic option for hereditary angioedema with C1 inhibitor deficiency,” Dr. Banerji and her associates said.
The HELP Study, a phase III trial assessing the safety and efficacy of 6 months of lanadelumab treatment, is now underway, they added.
The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing of the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Lanadelumab, a monoclonal antibody that inhibits kallikrein, reduced attacks of hereditary angioedema with C1 inhibitor deficiency by 88%-100%.
Major finding: All the patients in the 300-mg group and 9 of the 11 in the 400-mg group had no angioedema attacks during the study period.
Data source: A multicenter, randomized, double-blind, placebo-controlled phase Ib trial involving 37 adults who had hereditary angioedema with C1 inhibitor deficiency.
Disclosures: The trial was sponsored by Dyax, which also participated in the study design, data collection and interpretation, and writing the results. Dr. Banerji reported ties to Alnylam Pharmaceuticals, CSL Behring, Dyax, and Shire; her associates reported ties to numerous industry sources.
Nonthoracic MRI safe in patients with cardiac devices
Nonthoracic MRI was safe in patients who had implanted non–MRI-conditional pacemakers or implantable cardioverter defibrillators, as long as they followed a specific safety protocol before and after the imaging procedure, according to a report published online Feb. 23 in the New England Journal of Medicine.
Patients with implanted cardiac devices have long been advised to avoid MRI because of the potential for the magnetic field to induce heating of the cardiac leads, which could in turn produce thermal injury to the myocardium and adversely affect the device’s function. Certain cardiac devices that have been proved to pose no such hazards have been designated by the Food and Drug Administration as “MRI conditional.” However, an estimated 2 million patients in the United States and another 6 million worldwide have devices that are not MRI conditional, and at least half of these patients are predicted to require an MRI during their lifetimes, said Robert J. Russo, MD, PhD, of Scripps Research Institute and the La Jolla (Calif.) Cardiovascular Research Institute, and his associates.
The MagnaSafe Registry was established to monitor device-related clinical events and device alterations among adults undergoing nonthoracic MRIs at 1.5 T. Dr. Russo and his associates analyzed data in this registry from 19 medical centers during a 5-year period. They assessed 1,000 MRIs in 818 patients with pacemakers and 500 MRIs in 428 patients with implantable cardioverter defibrillators who were followed for 6 months after the imaging procedures. Most of these scans involved the brain or spine, and the median duration of exposure to the magnetic field was 44 minutes.
According to the safety protocol, all devices were interrogated immediately before the MRI and, depending on those results, were programmed to no pacing or asynchronous pacing during the scan with all tachycardia and bradycardia therapies inactivated. Immediately after the scan, all devices were reprogrammed to baseline settings, a full device interrogation was repeated, and, if necessary, further reprogramming was performed to maintain adequate pacing and sensing. A physician, nurse practitioner, or physician’s assistant with cardiac expertise attended each scan.
There were no deaths, lead failures requiring immediate replacement, losses of capture, or full electrical resets associated with any of the 1,500 MRI scans.
Four patients developed atrial fibrillation, and two developed atrial flutter, during or after the MRI; three returned to sinus rhythm while still in the scanning room, and the other three did so within 49 hours. There were six cases requiring partial generator electrical resets. “Changes in device settings were common, but relatively few exceeded our prespecified threshold criteria for a clinically important change,” Dr. Russo and his associates wrote (N Engl J Med. 2017;376[8]:755-64).
Four patients reported feeling discomfort at the implant site during MRI, including one who felt a heating sensation and was removed from the scanner before completing the procedure. None of them had any further problems.
Some experts have suggested that to allow patients with cardiac devices to undergo MRI, the generators and leads could be removed before the procedure and replaced afterward. The findings of this study show that undergoing a nonthoracic MRI using this protocol would likely be a safer alternative, the investigators added.
This work was supported by St. Jude Medical, Biotronik, Boston Scientific, the Hewitt Foundation for Medical Research, and several philanthropic gifts. Dr. Russo reported ties to St. Jude Medical, Biotronik, Boston Scientific, and the Hewitt Foundation, and his associates reported ties to numerous industry sources.
Nonthoracic MRI was safe in patients who had implanted non–MRI-conditional pacemakers or implantable cardioverter defibrillators, as long as they followed a specific safety protocol before and after the imaging procedure, according to a report published online Feb. 23 in the New England Journal of Medicine.
Patients with implanted cardiac devices have long been advised to avoid MRI because of the potential for the magnetic field to induce heating of the cardiac leads, which could in turn produce thermal injury to the myocardium and adversely affect the device’s function. Certain cardiac devices that have been proved to pose no such hazards have been designated by the Food and Drug Administration as “MRI conditional.” However, an estimated 2 million patients in the United States and another 6 million worldwide have devices that are not MRI conditional, and at least half of these patients are predicted to require an MRI during their lifetimes, said Robert J. Russo, MD, PhD, of Scripps Research Institute and the La Jolla (Calif.) Cardiovascular Research Institute, and his associates.
The MagnaSafe Registry was established to monitor device-related clinical events and device alterations among adults undergoing nonthoracic MRIs at 1.5 T. Dr. Russo and his associates analyzed data in this registry from 19 medical centers during a 5-year period. They assessed 1,000 MRIs in 818 patients with pacemakers and 500 MRIs in 428 patients with implantable cardioverter defibrillators who were followed for 6 months after the imaging procedures. Most of these scans involved the brain or spine, and the median duration of exposure to the magnetic field was 44 minutes.
According to the safety protocol, all devices were interrogated immediately before the MRI and, depending on those results, were programmed to no pacing or asynchronous pacing during the scan with all tachycardia and bradycardia therapies inactivated. Immediately after the scan, all devices were reprogrammed to baseline settings, a full device interrogation was repeated, and, if necessary, further reprogramming was performed to maintain adequate pacing and sensing. A physician, nurse practitioner, or physician’s assistant with cardiac expertise attended each scan.
There were no deaths, lead failures requiring immediate replacement, losses of capture, or full electrical resets associated with any of the 1,500 MRI scans.
Four patients developed atrial fibrillation, and two developed atrial flutter, during or after the MRI; three returned to sinus rhythm while still in the scanning room, and the other three did so within 49 hours. There were six cases requiring partial generator electrical resets. “Changes in device settings were common, but relatively few exceeded our prespecified threshold criteria for a clinically important change,” Dr. Russo and his associates wrote (N Engl J Med. 2017;376[8]:755-64).
Four patients reported feeling discomfort at the implant site during MRI, including one who felt a heating sensation and was removed from the scanner before completing the procedure. None of them had any further problems.
Some experts have suggested that to allow patients with cardiac devices to undergo MRI, the generators and leads could be removed before the procedure and replaced afterward. The findings of this study show that undergoing a nonthoracic MRI using this protocol would likely be a safer alternative, the investigators added.
This work was supported by St. Jude Medical, Biotronik, Boston Scientific, the Hewitt Foundation for Medical Research, and several philanthropic gifts. Dr. Russo reported ties to St. Jude Medical, Biotronik, Boston Scientific, and the Hewitt Foundation, and his associates reported ties to numerous industry sources.
Nonthoracic MRI was safe in patients who had implanted non–MRI-conditional pacemakers or implantable cardioverter defibrillators, as long as they followed a specific safety protocol before and after the imaging procedure, according to a report published online Feb. 23 in the New England Journal of Medicine.
Patients with implanted cardiac devices have long been advised to avoid MRI because of the potential for the magnetic field to induce heating of the cardiac leads, which could in turn produce thermal injury to the myocardium and adversely affect the device’s function. Certain cardiac devices that have been proved to pose no such hazards have been designated by the Food and Drug Administration as “MRI conditional.” However, an estimated 2 million patients in the United States and another 6 million worldwide have devices that are not MRI conditional, and at least half of these patients are predicted to require an MRI during their lifetimes, said Robert J. Russo, MD, PhD, of Scripps Research Institute and the La Jolla (Calif.) Cardiovascular Research Institute, and his associates.
The MagnaSafe Registry was established to monitor device-related clinical events and device alterations among adults undergoing nonthoracic MRIs at 1.5 T. Dr. Russo and his associates analyzed data in this registry from 19 medical centers during a 5-year period. They assessed 1,000 MRIs in 818 patients with pacemakers and 500 MRIs in 428 patients with implantable cardioverter defibrillators who were followed for 6 months after the imaging procedures. Most of these scans involved the brain or spine, and the median duration of exposure to the magnetic field was 44 minutes.
According to the safety protocol, all devices were interrogated immediately before the MRI and, depending on those results, were programmed to no pacing or asynchronous pacing during the scan with all tachycardia and bradycardia therapies inactivated. Immediately after the scan, all devices were reprogrammed to baseline settings, a full device interrogation was repeated, and, if necessary, further reprogramming was performed to maintain adequate pacing and sensing. A physician, nurse practitioner, or physician’s assistant with cardiac expertise attended each scan.
There were no deaths, lead failures requiring immediate replacement, losses of capture, or full electrical resets associated with any of the 1,500 MRI scans.
Four patients developed atrial fibrillation, and two developed atrial flutter, during or after the MRI; three returned to sinus rhythm while still in the scanning room, and the other three did so within 49 hours. There were six cases requiring partial generator electrical resets. “Changes in device settings were common, but relatively few exceeded our prespecified threshold criteria for a clinically important change,” Dr. Russo and his associates wrote (N Engl J Med. 2017;376[8]:755-64).
Four patients reported feeling discomfort at the implant site during MRI, including one who felt a heating sensation and was removed from the scanner before completing the procedure. None of them had any further problems.
Some experts have suggested that to allow patients with cardiac devices to undergo MRI, the generators and leads could be removed before the procedure and replaced afterward. The findings of this study show that undergoing a nonthoracic MRI using this protocol would likely be a safer alternative, the investigators added.
This work was supported by St. Jude Medical, Biotronik, Boston Scientific, the Hewitt Foundation for Medical Research, and several philanthropic gifts. Dr. Russo reported ties to St. Jude Medical, Biotronik, Boston Scientific, and the Hewitt Foundation, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Nonthoracic MRI was safe in patients who had implanted non–MRI-conditional pacemakers or ICDs, as long as they followed a specific safety protocol before and after the imaging procedure.
Key numerical finding: No deaths, lead failures requiring immediate replacement, losses of capture, or full electrical resets were tied to any of the 1,500 MRI scans.
Data source: A U.S. registry–based cohort study of 1,000 MRIs involving patients with pacemakers and 500 MRIs involving patients with ICDs, performed during a 5-year period.
Disclosures: This work was supported by St. Jude Medical, Biotronik, Boston Scientific, the Hewitt Foundation for Medical Research, and several philanthropic gifts. Dr. Russo reported ties to St. Jude Medical, Biotronik, Boston Scientific, and the Hewitt Foundation, and his associates reported ties to numerous industry sources.
Mesh cylinder effective for small to medium wide-necked aneurysms
HOUSTON – An expandable mesh cylinder that is approved to treat large, wide-necked carotid aneurysms has now proved successful in treating small lesions of the internal carotid or vertebral artery up to 12 mm in diameter.
The Pipeline Embolization Device (PED, Medtronic) completely occluded 84% of such lesions without significant stenosis or retreatment within 1 year in the PREMIER trial, Ricardo Hanel, MD, PhD, said at the International Stroke Conference sponsored by the American Heart Association.
Overall morbidity and mortality in the year-long trial was very low (2.2%). However, within the first year, three patients had a major stroke in brain regions supplied by the treated artery; one of these was related to device deployment and was fatal, said Dr. Hanel, director of the Baptist Neurological Institute, Jacksonville, Fla.
Counseling patients with these lesions is not easy, Dr. Hanel said. Treatment decisions must take into account not only the patient’s current clinical status and comorbidities, but family history and personal preference. In fact, patient preference was the largest driver of treatment (63%) in the PREMIER study.
In an interview, Dr. Hanel illustrated the importance of individualized decision making. A middle-aged female had been monitored for a small aneurysm for 7 years. When the patient was 6 years old, her mother died during an open operation to treat an aneurysm.
“We had tried to treat this patient with coiling [when the lesion was first detected], but it was unsuccessful,” Dr. Hanel said. “And since her mother had died during surgery, she did not want to go for an open approach. Now, 6 years later, we have the technology to cure her with a single device, and the odds of [recurrence over 10 years] are virtually zero. It is a very personal decision, and we take a lot of factors under consideration before we decide to expose the patient to the risks of this treatment.”
The PED is a flexible 75% cobalt-chromium/25% platinum-tungsten mesh with a braided configuration. It is advanced slightly beyond the aneurysm neck and then deployed. As it opens, it partially occludes the lesion, immediately decreasing the amount of blood entering the sac. Within a month, vascular remodeling is well underway; as endothelium grows throughout the mesh, blood flow into the aneurysm is gradually cut off. Eventually the aneurysmal sac recedes, and the normal vascular architecture is restored.
“Within 4 weeks you can’t see the metal at all,” Dr. Hanel said. “It’s covered by a thin layer of endothelial cells. This device allows the patient’s body to heal and close the aneurysm, and we don’t have to deal with the reoccurrence problem we have with stent coils. The pipeline treats the entire circumference of the vessel.”
The PED is used in combination with dual-antiplatelet therapy (DAP, aspirin/clopidogrel). Dr. Hanel initiates DAP 7 days before the procedure and continues it for 3 months. At that time, clopidogrel may be discontinued. “I advise my patients to then take a baby aspirin every day for the rest of their lives,” and they are regularly monitored, he said. “Aspirin seems to protect against the formation and rupture of aneurysms.”
PREMIER followed 141 patients with unruptured, wide-necked small aneurysms of the internal carotid (up to the terminus) or the vertebral artery segment up to and including the posterior inferior cerebellar artery. The primary efficacy endpoint was complete aneurysm occlusion and absence of significant parent artery stenosis at 1 year. The secondary endpoint was successful device deployment.
The primary safety endpoint was major stroke in the territory supplied by the treated artery or neurologic death at 1 year. There were two secondary safety endpoints: major stroke or neurologic death within 30 days, attributable to procedural complications, and intracerebral hemorrhage more than 30 days later.
The patients were largely female (88%) with a mean age of 55 years. They were asymptomatic with a mean modified Rankin Scale score of 0.2 and National Institutes of Health Stroke Scale score of 0.1. Nearly half of the patients had hypertension, and 38% had hyperlipidemia. About 28% were current smokers, and another 16% had a history of smoking.
The patients’ mean maximal aneurysm diameter was 4.6 mm, with a mean neck width of 3.7 mm. The majority of lesions (84%) were less than 7 mm in diameter, but they ranged up to 12 mm.
Internal carotid artery aneurysms comprised 95% of all in the study; 5% were in the vertebral artery. Most involved the side wall (84%), while the remainder involved a side branch (12%) or were fusiform (4%).
There was only one unsuccessful initial deployment, resulting in a 99.3% deployment success rate. The mean procedure time was 78 minutes. While most patients received just one PED, 10 received multiple devices. The PED completely covered the entire neck of the lesion in 97%. There were no intraoperative aneurysm ruptures and no intraoperative deaths.
At 1 year after implantation, 84% of the aneurysms were completely occluded, with the aneurysmal sac eliminated in 92%. A residual aneurysm remained in 11 patients (8%), and a residual neck in 8 patients (6%). Two patients (1.4%) had arterial stenosis of more than 50%. Three patients (2.2%) required retreatment.
There were three major strokes in the region supplied by the target artery in three patients.
The fatal stroke occurred in a patient who had an aneurysm on the right ophthalmic carotid segment. The first device failed to deploy correctly and was removed. A second device was implanted. The next day the patient developed a facial droop, slurred speech, and a headache. She experienced a distal intraparenchymal hemorrhage and underwent hemicraniotomy, but did not survive.
The second stroke occurred in a patient who needed two devices to occlude a lesion in the left ophthalmic segment of the carotid. The patient developed an intraparenchymal hemorrhage on postoperative day 15. The stroke resolved with sequelae and the clopidogrel dose was increased.
The third stroke was associated with treatment of a right communicating segment aneurysm. The patient stopped taking the recommended DAP and experienced an acute ischemic stroke 169 days after the procedure. This stroke also resolved with sequelae.
Based on the results of PREMIER, Medtronic will pursue Food and Drug Administration approval of the PED for small to medium wide-necked aneurysms, Dr. Hanel noted.
Medtronic sponsored the study. Dr. Hanel is an adviser to the company and has received research funds from it.
[email protected]
On Twitter @alz_gal
HOUSTON – An expandable mesh cylinder that is approved to treat large, wide-necked carotid aneurysms has now proved successful in treating small lesions of the internal carotid or vertebral artery up to 12 mm in diameter.
The Pipeline Embolization Device (PED, Medtronic) completely occluded 84% of such lesions without significant stenosis or retreatment within 1 year in the PREMIER trial, Ricardo Hanel, MD, PhD, said at the International Stroke Conference sponsored by the American Heart Association.
Overall morbidity and mortality in the year-long trial was very low (2.2%). However, within the first year, three patients had a major stroke in brain regions supplied by the treated artery; one of these was related to device deployment and was fatal, said Dr. Hanel, director of the Baptist Neurological Institute, Jacksonville, Fla.
Counseling patients with these lesions is not easy, Dr. Hanel said. Treatment decisions must take into account not only the patient’s current clinical status and comorbidities, but family history and personal preference. In fact, patient preference was the largest driver of treatment (63%) in the PREMIER study.
In an interview, Dr. Hanel illustrated the importance of individualized decision making. A middle-aged female had been monitored for a small aneurysm for 7 years. When the patient was 6 years old, her mother died during an open operation to treat an aneurysm.
“We had tried to treat this patient with coiling [when the lesion was first detected], but it was unsuccessful,” Dr. Hanel said. “And since her mother had died during surgery, she did not want to go for an open approach. Now, 6 years later, we have the technology to cure her with a single device, and the odds of [recurrence over 10 years] are virtually zero. It is a very personal decision, and we take a lot of factors under consideration before we decide to expose the patient to the risks of this treatment.”
The PED is a flexible 75% cobalt-chromium/25% platinum-tungsten mesh with a braided configuration. It is advanced slightly beyond the aneurysm neck and then deployed. As it opens, it partially occludes the lesion, immediately decreasing the amount of blood entering the sac. Within a month, vascular remodeling is well underway; as endothelium grows throughout the mesh, blood flow into the aneurysm is gradually cut off. Eventually the aneurysmal sac recedes, and the normal vascular architecture is restored.
“Within 4 weeks you can’t see the metal at all,” Dr. Hanel said. “It’s covered by a thin layer of endothelial cells. This device allows the patient’s body to heal and close the aneurysm, and we don’t have to deal with the reoccurrence problem we have with stent coils. The pipeline treats the entire circumference of the vessel.”
The PED is used in combination with dual-antiplatelet therapy (DAP, aspirin/clopidogrel). Dr. Hanel initiates DAP 7 days before the procedure and continues it for 3 months. At that time, clopidogrel may be discontinued. “I advise my patients to then take a baby aspirin every day for the rest of their lives,” and they are regularly monitored, he said. “Aspirin seems to protect against the formation and rupture of aneurysms.”
PREMIER followed 141 patients with unruptured, wide-necked small aneurysms of the internal carotid (up to the terminus) or the vertebral artery segment up to and including the posterior inferior cerebellar artery. The primary efficacy endpoint was complete aneurysm occlusion and absence of significant parent artery stenosis at 1 year. The secondary endpoint was successful device deployment.
The primary safety endpoint was major stroke in the territory supplied by the treated artery or neurologic death at 1 year. There were two secondary safety endpoints: major stroke or neurologic death within 30 days, attributable to procedural complications, and intracerebral hemorrhage more than 30 days later.
The patients were largely female (88%) with a mean age of 55 years. They were asymptomatic with a mean modified Rankin Scale score of 0.2 and National Institutes of Health Stroke Scale score of 0.1. Nearly half of the patients had hypertension, and 38% had hyperlipidemia. About 28% were current smokers, and another 16% had a history of smoking.
The patients’ mean maximal aneurysm diameter was 4.6 mm, with a mean neck width of 3.7 mm. The majority of lesions (84%) were less than 7 mm in diameter, but they ranged up to 12 mm.
Internal carotid artery aneurysms comprised 95% of all in the study; 5% were in the vertebral artery. Most involved the side wall (84%), while the remainder involved a side branch (12%) or were fusiform (4%).
There was only one unsuccessful initial deployment, resulting in a 99.3% deployment success rate. The mean procedure time was 78 minutes. While most patients received just one PED, 10 received multiple devices. The PED completely covered the entire neck of the lesion in 97%. There were no intraoperative aneurysm ruptures and no intraoperative deaths.
At 1 year after implantation, 84% of the aneurysms were completely occluded, with the aneurysmal sac eliminated in 92%. A residual aneurysm remained in 11 patients (8%), and a residual neck in 8 patients (6%). Two patients (1.4%) had arterial stenosis of more than 50%. Three patients (2.2%) required retreatment.
There were three major strokes in the region supplied by the target artery in three patients.
The fatal stroke occurred in a patient who had an aneurysm on the right ophthalmic carotid segment. The first device failed to deploy correctly and was removed. A second device was implanted. The next day the patient developed a facial droop, slurred speech, and a headache. She experienced a distal intraparenchymal hemorrhage and underwent hemicraniotomy, but did not survive.
The second stroke occurred in a patient who needed two devices to occlude a lesion in the left ophthalmic segment of the carotid. The patient developed an intraparenchymal hemorrhage on postoperative day 15. The stroke resolved with sequelae and the clopidogrel dose was increased.
The third stroke was associated with treatment of a right communicating segment aneurysm. The patient stopped taking the recommended DAP and experienced an acute ischemic stroke 169 days after the procedure. This stroke also resolved with sequelae.
Based on the results of PREMIER, Medtronic will pursue Food and Drug Administration approval of the PED for small to medium wide-necked aneurysms, Dr. Hanel noted.
Medtronic sponsored the study. Dr. Hanel is an adviser to the company and has received research funds from it.
[email protected]
On Twitter @alz_gal
HOUSTON – An expandable mesh cylinder that is approved to treat large, wide-necked carotid aneurysms has now proved successful in treating small lesions of the internal carotid or vertebral artery up to 12 mm in diameter.
The Pipeline Embolization Device (PED, Medtronic) completely occluded 84% of such lesions without significant stenosis or retreatment within 1 year in the PREMIER trial, Ricardo Hanel, MD, PhD, said at the International Stroke Conference sponsored by the American Heart Association.
Overall morbidity and mortality in the year-long trial was very low (2.2%). However, within the first year, three patients had a major stroke in brain regions supplied by the treated artery; one of these was related to device deployment and was fatal, said Dr. Hanel, director of the Baptist Neurological Institute, Jacksonville, Fla.
Counseling patients with these lesions is not easy, Dr. Hanel said. Treatment decisions must take into account not only the patient’s current clinical status and comorbidities, but family history and personal preference. In fact, patient preference was the largest driver of treatment (63%) in the PREMIER study.
In an interview, Dr. Hanel illustrated the importance of individualized decision making. A middle-aged female had been monitored for a small aneurysm for 7 years. When the patient was 6 years old, her mother died during an open operation to treat an aneurysm.
“We had tried to treat this patient with coiling [when the lesion was first detected], but it was unsuccessful,” Dr. Hanel said. “And since her mother had died during surgery, she did not want to go for an open approach. Now, 6 years later, we have the technology to cure her with a single device, and the odds of [recurrence over 10 years] are virtually zero. It is a very personal decision, and we take a lot of factors under consideration before we decide to expose the patient to the risks of this treatment.”
The PED is a flexible 75% cobalt-chromium/25% platinum-tungsten mesh with a braided configuration. It is advanced slightly beyond the aneurysm neck and then deployed. As it opens, it partially occludes the lesion, immediately decreasing the amount of blood entering the sac. Within a month, vascular remodeling is well underway; as endothelium grows throughout the mesh, blood flow into the aneurysm is gradually cut off. Eventually the aneurysmal sac recedes, and the normal vascular architecture is restored.
“Within 4 weeks you can’t see the metal at all,” Dr. Hanel said. “It’s covered by a thin layer of endothelial cells. This device allows the patient’s body to heal and close the aneurysm, and we don’t have to deal with the reoccurrence problem we have with stent coils. The pipeline treats the entire circumference of the vessel.”
The PED is used in combination with dual-antiplatelet therapy (DAP, aspirin/clopidogrel). Dr. Hanel initiates DAP 7 days before the procedure and continues it for 3 months. At that time, clopidogrel may be discontinued. “I advise my patients to then take a baby aspirin every day for the rest of their lives,” and they are regularly monitored, he said. “Aspirin seems to protect against the formation and rupture of aneurysms.”
PREMIER followed 141 patients with unruptured, wide-necked small aneurysms of the internal carotid (up to the terminus) or the vertebral artery segment up to and including the posterior inferior cerebellar artery. The primary efficacy endpoint was complete aneurysm occlusion and absence of significant parent artery stenosis at 1 year. The secondary endpoint was successful device deployment.
The primary safety endpoint was major stroke in the territory supplied by the treated artery or neurologic death at 1 year. There were two secondary safety endpoints: major stroke or neurologic death within 30 days, attributable to procedural complications, and intracerebral hemorrhage more than 30 days later.
The patients were largely female (88%) with a mean age of 55 years. They were asymptomatic with a mean modified Rankin Scale score of 0.2 and National Institutes of Health Stroke Scale score of 0.1. Nearly half of the patients had hypertension, and 38% had hyperlipidemia. About 28% were current smokers, and another 16% had a history of smoking.
The patients’ mean maximal aneurysm diameter was 4.6 mm, with a mean neck width of 3.7 mm. The majority of lesions (84%) were less than 7 mm in diameter, but they ranged up to 12 mm.
Internal carotid artery aneurysms comprised 95% of all in the study; 5% were in the vertebral artery. Most involved the side wall (84%), while the remainder involved a side branch (12%) or were fusiform (4%).
There was only one unsuccessful initial deployment, resulting in a 99.3% deployment success rate. The mean procedure time was 78 minutes. While most patients received just one PED, 10 received multiple devices. The PED completely covered the entire neck of the lesion in 97%. There were no intraoperative aneurysm ruptures and no intraoperative deaths.
At 1 year after implantation, 84% of the aneurysms were completely occluded, with the aneurysmal sac eliminated in 92%. A residual aneurysm remained in 11 patients (8%), and a residual neck in 8 patients (6%). Two patients (1.4%) had arterial stenosis of more than 50%. Three patients (2.2%) required retreatment.
There were three major strokes in the region supplied by the target artery in three patients.
The fatal stroke occurred in a patient who had an aneurysm on the right ophthalmic carotid segment. The first device failed to deploy correctly and was removed. A second device was implanted. The next day the patient developed a facial droop, slurred speech, and a headache. She experienced a distal intraparenchymal hemorrhage and underwent hemicraniotomy, but did not survive.
The second stroke occurred in a patient who needed two devices to occlude a lesion in the left ophthalmic segment of the carotid. The patient developed an intraparenchymal hemorrhage on postoperative day 15. The stroke resolved with sequelae and the clopidogrel dose was increased.
The third stroke was associated with treatment of a right communicating segment aneurysm. The patient stopped taking the recommended DAP and experienced an acute ischemic stroke 169 days after the procedure. This stroke also resolved with sequelae.
Based on the results of PREMIER, Medtronic will pursue Food and Drug Administration approval of the PED for small to medium wide-necked aneurysms, Dr. Hanel noted.
Medtronic sponsored the study. Dr. Hanel is an adviser to the company and has received research funds from it.
[email protected]
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: The PED completely occluded 84% of aneurysms of the internal carotid or vertebral artery up to 12 mm in diameter at 1 year.
Data source: PREMIER investigators prospectively evaluated the PED in 141 patients.
Disclosures: Medtronic sponsored the study. Dr. Hanel is an adviser to the company and has received research funds from it.
Well-child care: Steady growth in breadth and content
Fifty years ago in 1967, the American Academy of Pediatrics published a “Suggested Schedule for Preventive Child Health Care.” It was, in essence, the first periodicity schedule for well-child visits.
Described by AAP officials at the time as an “amalgamation of schedules used in various clinics and private offices,” it charted out the frequency and basic content of visits from 1 month through 6 years of age, and offered a simple list of items to be considered for guidance and discussion in all visits from 6 years on.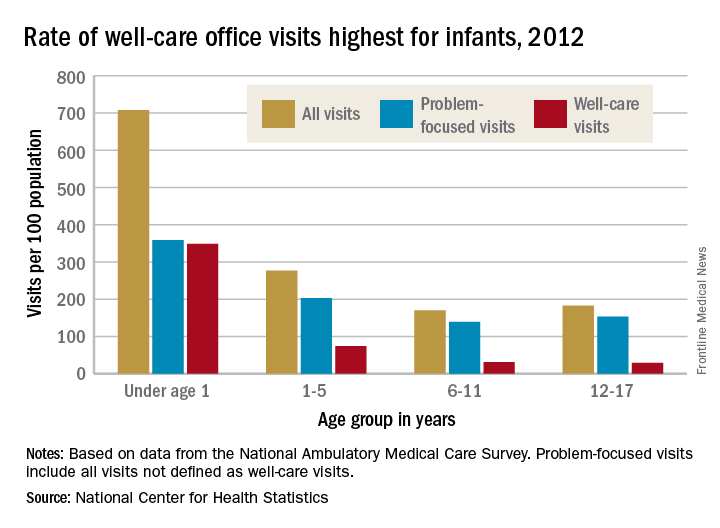
It is updated in real time, and is accompanied by an expansive package of Bright Futures recommendations, guidelines, and tools (including forms, handouts, and questionnaires) for health promotion and guidance. Together, the Periodicity Schedule and Bright Futures guidance reflect decades of steady change in the breadth and content of well-child care – and more recently, in some of its processes.
“When I started practicing [in 1979], developmental surveillance meant asking a few questions about developmental milestones, observing, and maybe lifting a few questions from the Denver Scale [the Denver Developmental Screening Test] to support our surveillance,” said Joseph F. Hagan, Jr., MD, a pediatrician in Burlington, Vt., and coeditor of Bright Futures.
Jack Swanson, MD, a pediatrician in Ames, Iowa, and a member of the Bright Futures Steering Committee, has similar recollections of well-child care in the early 1970s. “The developmental milestones were just questions and nothing more formal. Nutrition was a big [anticipatory guidance] issue, and some safety,” he recalled.
In early pediatric visits, “parents were interested in Dr. [Benjamin] Spock’s recommendations about feeding and raising their baby… and we used to make our own [anticipatory guidance] handouts,” he said. And in the later years, “an adolescent visit used to be every 2 or 3 years.”
“During the Vietnam War, there weren’t enough people who were healthy enough, physically fit enough, to be mustered into the Army,” said Peter Rappo, MD, a pediatrician in Brockton, Mass., who chaired the AAP’s Committee on Practice and Ambulatory Medicine in the late 1990s.
Dr. Rappo became interested in the history of preventive pediatric care after discovering a Children’s Year Campaign (1918-1919) poster in an antiques market. The poster’s message – “The Health of the Child is the Power of the Nation” – remained relevant through the Vietnam War. “I’d like to think that [childhood preventive services] were all about the kids,” he said, “but at the end of the day, it was about military issues too.”
Still, interest in the 1960s in the long-term implications of early-life development fed research that eventually led to an explosion of new science in the 1990s on the importance of early brain development and early life experiences. This scientific literature combined with greater societal interest in school readiness helped drive development of research-based instruments for developmental screening, said pediatrician Edward L. Schor, MD, formerly a vice president at the Commonwealth Fund and now a senior vice president for programs and partnerships at Lucile Packard Foundation for Children’s Health.
“Development was the first topic … of screening instruments,” he said. The tools have “not only increased the quality of care, they also have increased the efficiency of care, because the time to ask and answer these questions was shifted to the waiting room.”
Their use is far from universal, but increasing. Results of the Periodic Surveys administered to a national random sample of AAP members show that pediatricians’ use of at least one formal screening instrument to identify children through 36 months of age at risk for developmental delay increased from 23% in 2002 to 45% in 2009 and 63% in 2016. (And in 2016, 81% reported “always/almost always” using at least one formal screening tool for autism.) The data was presented at the annual meeting of the Society of Developmental and Behavioral Pediatrics September 2016.
For Dr. Rappo’s practice in Massachusetts, the adoption of developmental and behavioral health screening questionnaires for all ages was spurred by a 2007 mandate requiring formal screening for children and adolescents in MassHealth, the state’s combined Medicaid–Children’s Health Insurance Program.
“We all knew intuitively this is what we should be doing, so we also sat down with insurers to talk about why this is important for kids,” he said. Reimbursement improved, and most importantly, he said, use of the tools “has tremendously improved our opportunities for opening up discussions with parents about developmental-behavioral issues.”
The well-child visit of 50 years ago was much more of “a physician-generated, physician-led visit,” said Dr. Swanson. “The pediatrician knew what was needed, and at the end, we’d ask if there were any questions. Today, the first question recommended by Bright Futures is ‘Do you have any questions for the visit?’”
According to a 2009 focus group study involving 282 pediatricians and 41 nurse-practitioners, clinicians agree that eliciting and prioritizing parent concerns is a top priority in well-child care. Yet there’s also some unease. Some said in the focus group discussions that they feel constrained by the Periodicity Schedule, for instance, or feel tension between inviting parents’ concerns while simultaneously addressing the content recommended by professional guidelines (Pediatrics. 2009 Sep;124[3]:849-57).
Indeed, policies and recommendations for health promotion and anticipatory guidance (some consensus-based, some evidence-based or evidence-informed) mushroomed throughout the 1980s and 1990s, Dr. Swanson said. Combined with the increase in recommended screenings through the 1990s and 2000s – and in recent years, the increasing need for discussions to address vaccine concerns, mental and behavioral health issues, and obesity and overweight problems – there are real pulls and tugs.
The time allotted to well-child visits may have increased slightly for some pediatricians – to just over 20 minutes – but overall, visit length hasn’t changed much over the past few decades. “It has pretty much stayed the same, averaging between 15 and 20 minutes,” said Dr. Schor.
Offering guidance to clinicians in prioritizing questions and issues has been a goal in the last two editions (2008 and 2016) of the Bright Futures recommendations – formally called the Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. “The joke was that if you did a Bright Futures well-child visit according to the old Bright Futures, you’d do one in the morning and one in the afternoon,” said Dr. Hagan.
The first edition came out in 1994, after a multidisciplinary group convened by the Maternal and Child Health Bureau at the Health Resources and Services Administration, and the Medicaid Bureau (then part of the Health Care Financing Administration) established the Bright Futures Children’s Health Charter to improve children’s health. The second edition was released in 2000 and updated in 2002, at which point the AAP established the Bright Futures National Center.
Previsit screening tools included in the new edition are aimed at assessing and prioritizing anticipatory guidance issues, Dr. Hagan said, noting too that the Periodicity Schedule and Bright Futures recommendations make distinctions between universal and selective screening. “By design,” he emphasized, “there’s more anticipatory guidance than you might ever accomplish in one visit, because we want to be thorough enough to provide a context – a schema – to deal with the issues.”
Oftentimes, he said, “what parents want to talk about is what you want to talk about.” And pediatricians “develop a skill set to temporize, to figure out what needs to be covered today, and what can be dealt with better at a later time,” Dr. Hagan said. “If you tell kids, for instance, ‘I can help you with this, I just have to get more information,’ they hear that there’s help on the way. Then you follow through.”
Overall, his well-child visits “have gotten much more involved with the emotional well-being of children.” Given that emotional issues and behavioral issues “tend to take a longer time to discuss and unravel,” he sets aside consultation times near the end of the day for families who need to discuss these issues.
And he routinely devotes time – starting at the 2-month visit – to discuss screen time and media use. “I believe that technology is making our children sick,” he said, noting that in his nearly 17 years of practice he has seen increasing numbers of children and adolescents with depression, anxiety, anger, and attention deficit/hyperactivity disorder. “The AAP has done a pretty good job of raising the point, but I don’t think it has hit home with parents yet.”
For Dr. Chung, electronic medical records and systems have enabled him to better flag issues for follow-up over the course of well-child visits, leading to “better longitudinal care.”
Surveys and questionnaires filled out by parents in his practice’s waiting room are scanned into charts, he noted, and adolescents can mark answers on a proprietary confidential risk questionnaire that subsequently gets scrambled so that no one but the provider can understand the responses.
Other potential impacts of electronic systems have yet to be realized, he and others said. Some pediatric practices, Dr. Schor said, have begun engaging with families ahead of well-child visits through the use of a computerized questionnaire that elicits areas and issues of interest. Such outreach may help families feel more invested and committed to attending the visits, particularly those that don’t involve immunizations or school/sport forms, he said.
Families are “not [always] buying what we’re selling [for well-child care],” said Dr. Schor, who has served on AAP committees and has written several well-cited articles on preventive pediatrics care.
Insurance coverage for well-child care got a boost in 2010 when Bright Futures was cited in the Affordable Care Act as the standard of what well-child care should accomplish, and its recommended screenings and services were required to be covered by insurers without cost-sharing.
In the long-run, he said, rethinking the roles of nonphysicians in anticipatory guidance and developmental and psychosocial screening – in interpreting results of questionnaires, for instance – may be essential for well-child care. Outside of large health care systems, “the use of personnel [has been] pretty much been unchanged over the years,” he said. “We need to ask, how can we use each individual’s skills and training most efficiently? How can we retrain and reorganize our patient flow?”
This may be especially important as well-child care increasingly considers family psychosocial issues such as housing, food insecurity, family violence, and other family social stressors. Maternal depression screening made its way into the Periodicity Schedule in February 2016, and Dr. Schor predicts that the schedule will include “family psychosocial risk screening” in another several years.
For now, the newly revised Bright Futures guidelines – and much of well-child care – places an increased emphasis on the social determinants of health, which Dr. Hagan said reflects the “long-standing, logical conclusion that we reached back in the 1990s – that if families are healthy, kids will be healthy … and that family health is also linked to community health.”
Fifty years ago in 1967, the American Academy of Pediatrics published a “Suggested Schedule for Preventive Child Health Care.” It was, in essence, the first periodicity schedule for well-child visits.
Described by AAP officials at the time as an “amalgamation of schedules used in various clinics and private offices,” it charted out the frequency and basic content of visits from 1 month through 6 years of age, and offered a simple list of items to be considered for guidance and discussion in all visits from 6 years on.
It is updated in real time, and is accompanied by an expansive package of Bright Futures recommendations, guidelines, and tools (including forms, handouts, and questionnaires) for health promotion and guidance. Together, the Periodicity Schedule and Bright Futures guidance reflect decades of steady change in the breadth and content of well-child care – and more recently, in some of its processes.
“When I started practicing [in 1979], developmental surveillance meant asking a few questions about developmental milestones, observing, and maybe lifting a few questions from the Denver Scale [the Denver Developmental Screening Test] to support our surveillance,” said Joseph F. Hagan, Jr., MD, a pediatrician in Burlington, Vt., and coeditor of Bright Futures.
Jack Swanson, MD, a pediatrician in Ames, Iowa, and a member of the Bright Futures Steering Committee, has similar recollections of well-child care in the early 1970s. “The developmental milestones were just questions and nothing more formal. Nutrition was a big [anticipatory guidance] issue, and some safety,” he recalled.
In early pediatric visits, “parents were interested in Dr. [Benjamin] Spock’s recommendations about feeding and raising their baby… and we used to make our own [anticipatory guidance] handouts,” he said. And in the later years, “an adolescent visit used to be every 2 or 3 years.”
“During the Vietnam War, there weren’t enough people who were healthy enough, physically fit enough, to be mustered into the Army,” said Peter Rappo, MD, a pediatrician in Brockton, Mass., who chaired the AAP’s Committee on Practice and Ambulatory Medicine in the late 1990s.
Dr. Rappo became interested in the history of preventive pediatric care after discovering a Children’s Year Campaign (1918-1919) poster in an antiques market. The poster’s message – “The Health of the Child is the Power of the Nation” – remained relevant through the Vietnam War. “I’d like to think that [childhood preventive services] were all about the kids,” he said, “but at the end of the day, it was about military issues too.”
Still, interest in the 1960s in the long-term implications of early-life development fed research that eventually led to an explosion of new science in the 1990s on the importance of early brain development and early life experiences. This scientific literature combined with greater societal interest in school readiness helped drive development of research-based instruments for developmental screening, said pediatrician Edward L. Schor, MD, formerly a vice president at the Commonwealth Fund and now a senior vice president for programs and partnerships at Lucile Packard Foundation for Children’s Health.
“Development was the first topic … of screening instruments,” he said. The tools have “not only increased the quality of care, they also have increased the efficiency of care, because the time to ask and answer these questions was shifted to the waiting room.”
Their use is far from universal, but increasing. Results of the Periodic Surveys administered to a national random sample of AAP members show that pediatricians’ use of at least one formal screening instrument to identify children through 36 months of age at risk for developmental delay increased from 23% in 2002 to 45% in 2009 and 63% in 2016. (And in 2016, 81% reported “always/almost always” using at least one formal screening tool for autism.) The data was presented at the annual meeting of the Society of Developmental and Behavioral Pediatrics September 2016.
For Dr. Rappo’s practice in Massachusetts, the adoption of developmental and behavioral health screening questionnaires for all ages was spurred by a 2007 mandate requiring formal screening for children and adolescents in MassHealth, the state’s combined Medicaid–Children’s Health Insurance Program.
“We all knew intuitively this is what we should be doing, so we also sat down with insurers to talk about why this is important for kids,” he said. Reimbursement improved, and most importantly, he said, use of the tools “has tremendously improved our opportunities for opening up discussions with parents about developmental-behavioral issues.”
The well-child visit of 50 years ago was much more of “a physician-generated, physician-led visit,” said Dr. Swanson. “The pediatrician knew what was needed, and at the end, we’d ask if there were any questions. Today, the first question recommended by Bright Futures is ‘Do you have any questions for the visit?’”
According to a 2009 focus group study involving 282 pediatricians and 41 nurse-practitioners, clinicians agree that eliciting and prioritizing parent concerns is a top priority in well-child care. Yet there’s also some unease. Some said in the focus group discussions that they feel constrained by the Periodicity Schedule, for instance, or feel tension between inviting parents’ concerns while simultaneously addressing the content recommended by professional guidelines (Pediatrics. 2009 Sep;124[3]:849-57).
Indeed, policies and recommendations for health promotion and anticipatory guidance (some consensus-based, some evidence-based or evidence-informed) mushroomed throughout the 1980s and 1990s, Dr. Swanson said. Combined with the increase in recommended screenings through the 1990s and 2000s – and in recent years, the increasing need for discussions to address vaccine concerns, mental and behavioral health issues, and obesity and overweight problems – there are real pulls and tugs.
The time allotted to well-child visits may have increased slightly for some pediatricians – to just over 20 minutes – but overall, visit length hasn’t changed much over the past few decades. “It has pretty much stayed the same, averaging between 15 and 20 minutes,” said Dr. Schor.
Offering guidance to clinicians in prioritizing questions and issues has been a goal in the last two editions (2008 and 2016) of the Bright Futures recommendations – formally called the Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. “The joke was that if you did a Bright Futures well-child visit according to the old Bright Futures, you’d do one in the morning and one in the afternoon,” said Dr. Hagan.
The first edition came out in 1994, after a multidisciplinary group convened by the Maternal and Child Health Bureau at the Health Resources and Services Administration, and the Medicaid Bureau (then part of the Health Care Financing Administration) established the Bright Futures Children’s Health Charter to improve children’s health. The second edition was released in 2000 and updated in 2002, at which point the AAP established the Bright Futures National Center.
Previsit screening tools included in the new edition are aimed at assessing and prioritizing anticipatory guidance issues, Dr. Hagan said, noting too that the Periodicity Schedule and Bright Futures recommendations make distinctions between universal and selective screening. “By design,” he emphasized, “there’s more anticipatory guidance than you might ever accomplish in one visit, because we want to be thorough enough to provide a context – a schema – to deal with the issues.”
Oftentimes, he said, “what parents want to talk about is what you want to talk about.” And pediatricians “develop a skill set to temporize, to figure out what needs to be covered today, and what can be dealt with better at a later time,” Dr. Hagan said. “If you tell kids, for instance, ‘I can help you with this, I just have to get more information,’ they hear that there’s help on the way. Then you follow through.”
Overall, his well-child visits “have gotten much more involved with the emotional well-being of children.” Given that emotional issues and behavioral issues “tend to take a longer time to discuss and unravel,” he sets aside consultation times near the end of the day for families who need to discuss these issues.
And he routinely devotes time – starting at the 2-month visit – to discuss screen time and media use. “I believe that technology is making our children sick,” he said, noting that in his nearly 17 years of practice he has seen increasing numbers of children and adolescents with depression, anxiety, anger, and attention deficit/hyperactivity disorder. “The AAP has done a pretty good job of raising the point, but I don’t think it has hit home with parents yet.”
For Dr. Chung, electronic medical records and systems have enabled him to better flag issues for follow-up over the course of well-child visits, leading to “better longitudinal care.”
Surveys and questionnaires filled out by parents in his practice’s waiting room are scanned into charts, he noted, and adolescents can mark answers on a proprietary confidential risk questionnaire that subsequently gets scrambled so that no one but the provider can understand the responses.
Other potential impacts of electronic systems have yet to be realized, he and others said. Some pediatric practices, Dr. Schor said, have begun engaging with families ahead of well-child visits through the use of a computerized questionnaire that elicits areas and issues of interest. Such outreach may help families feel more invested and committed to attending the visits, particularly those that don’t involve immunizations or school/sport forms, he said.
Families are “not [always] buying what we’re selling [for well-child care],” said Dr. Schor, who has served on AAP committees and has written several well-cited articles on preventive pediatrics care.
Insurance coverage for well-child care got a boost in 2010 when Bright Futures was cited in the Affordable Care Act as the standard of what well-child care should accomplish, and its recommended screenings and services were required to be covered by insurers without cost-sharing.
In the long-run, he said, rethinking the roles of nonphysicians in anticipatory guidance and developmental and psychosocial screening – in interpreting results of questionnaires, for instance – may be essential for well-child care. Outside of large health care systems, “the use of personnel [has been] pretty much been unchanged over the years,” he said. “We need to ask, how can we use each individual’s skills and training most efficiently? How can we retrain and reorganize our patient flow?”
This may be especially important as well-child care increasingly considers family psychosocial issues such as housing, food insecurity, family violence, and other family social stressors. Maternal depression screening made its way into the Periodicity Schedule in February 2016, and Dr. Schor predicts that the schedule will include “family psychosocial risk screening” in another several years.
For now, the newly revised Bright Futures guidelines – and much of well-child care – places an increased emphasis on the social determinants of health, which Dr. Hagan said reflects the “long-standing, logical conclusion that we reached back in the 1990s – that if families are healthy, kids will be healthy … and that family health is also linked to community health.”
Fifty years ago in 1967, the American Academy of Pediatrics published a “Suggested Schedule for Preventive Child Health Care.” It was, in essence, the first periodicity schedule for well-child visits.
Described by AAP officials at the time as an “amalgamation of schedules used in various clinics and private offices,” it charted out the frequency and basic content of visits from 1 month through 6 years of age, and offered a simple list of items to be considered for guidance and discussion in all visits from 6 years on.
It is updated in real time, and is accompanied by an expansive package of Bright Futures recommendations, guidelines, and tools (including forms, handouts, and questionnaires) for health promotion and guidance. Together, the Periodicity Schedule and Bright Futures guidance reflect decades of steady change in the breadth and content of well-child care – and more recently, in some of its processes.
“When I started practicing [in 1979], developmental surveillance meant asking a few questions about developmental milestones, observing, and maybe lifting a few questions from the Denver Scale [the Denver Developmental Screening Test] to support our surveillance,” said Joseph F. Hagan, Jr., MD, a pediatrician in Burlington, Vt., and coeditor of Bright Futures.
Jack Swanson, MD, a pediatrician in Ames, Iowa, and a member of the Bright Futures Steering Committee, has similar recollections of well-child care in the early 1970s. “The developmental milestones were just questions and nothing more formal. Nutrition was a big [anticipatory guidance] issue, and some safety,” he recalled.
In early pediatric visits, “parents were interested in Dr. [Benjamin] Spock’s recommendations about feeding and raising their baby… and we used to make our own [anticipatory guidance] handouts,” he said. And in the later years, “an adolescent visit used to be every 2 or 3 years.”
“During the Vietnam War, there weren’t enough people who were healthy enough, physically fit enough, to be mustered into the Army,” said Peter Rappo, MD, a pediatrician in Brockton, Mass., who chaired the AAP’s Committee on Practice and Ambulatory Medicine in the late 1990s.
Dr. Rappo became interested in the history of preventive pediatric care after discovering a Children’s Year Campaign (1918-1919) poster in an antiques market. The poster’s message – “The Health of the Child is the Power of the Nation” – remained relevant through the Vietnam War. “I’d like to think that [childhood preventive services] were all about the kids,” he said, “but at the end of the day, it was about military issues too.”
Still, interest in the 1960s in the long-term implications of early-life development fed research that eventually led to an explosion of new science in the 1990s on the importance of early brain development and early life experiences. This scientific literature combined with greater societal interest in school readiness helped drive development of research-based instruments for developmental screening, said pediatrician Edward L. Schor, MD, formerly a vice president at the Commonwealth Fund and now a senior vice president for programs and partnerships at Lucile Packard Foundation for Children’s Health.
“Development was the first topic … of screening instruments,” he said. The tools have “not only increased the quality of care, they also have increased the efficiency of care, because the time to ask and answer these questions was shifted to the waiting room.”
Their use is far from universal, but increasing. Results of the Periodic Surveys administered to a national random sample of AAP members show that pediatricians’ use of at least one formal screening instrument to identify children through 36 months of age at risk for developmental delay increased from 23% in 2002 to 45% in 2009 and 63% in 2016. (And in 2016, 81% reported “always/almost always” using at least one formal screening tool for autism.) The data was presented at the annual meeting of the Society of Developmental and Behavioral Pediatrics September 2016.
For Dr. Rappo’s practice in Massachusetts, the adoption of developmental and behavioral health screening questionnaires for all ages was spurred by a 2007 mandate requiring formal screening for children and adolescents in MassHealth, the state’s combined Medicaid–Children’s Health Insurance Program.
“We all knew intuitively this is what we should be doing, so we also sat down with insurers to talk about why this is important for kids,” he said. Reimbursement improved, and most importantly, he said, use of the tools “has tremendously improved our opportunities for opening up discussions with parents about developmental-behavioral issues.”
The well-child visit of 50 years ago was much more of “a physician-generated, physician-led visit,” said Dr. Swanson. “The pediatrician knew what was needed, and at the end, we’d ask if there were any questions. Today, the first question recommended by Bright Futures is ‘Do you have any questions for the visit?’”
According to a 2009 focus group study involving 282 pediatricians and 41 nurse-practitioners, clinicians agree that eliciting and prioritizing parent concerns is a top priority in well-child care. Yet there’s also some unease. Some said in the focus group discussions that they feel constrained by the Periodicity Schedule, for instance, or feel tension between inviting parents’ concerns while simultaneously addressing the content recommended by professional guidelines (Pediatrics. 2009 Sep;124[3]:849-57).
Indeed, policies and recommendations for health promotion and anticipatory guidance (some consensus-based, some evidence-based or evidence-informed) mushroomed throughout the 1980s and 1990s, Dr. Swanson said. Combined with the increase in recommended screenings through the 1990s and 2000s – and in recent years, the increasing need for discussions to address vaccine concerns, mental and behavioral health issues, and obesity and overweight problems – there are real pulls and tugs.
The time allotted to well-child visits may have increased slightly for some pediatricians – to just over 20 minutes – but overall, visit length hasn’t changed much over the past few decades. “It has pretty much stayed the same, averaging between 15 and 20 minutes,” said Dr. Schor.
Offering guidance to clinicians in prioritizing questions and issues has been a goal in the last two editions (2008 and 2016) of the Bright Futures recommendations – formally called the Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. “The joke was that if you did a Bright Futures well-child visit according to the old Bright Futures, you’d do one in the morning and one in the afternoon,” said Dr. Hagan.
The first edition came out in 1994, after a multidisciplinary group convened by the Maternal and Child Health Bureau at the Health Resources and Services Administration, and the Medicaid Bureau (then part of the Health Care Financing Administration) established the Bright Futures Children’s Health Charter to improve children’s health. The second edition was released in 2000 and updated in 2002, at which point the AAP established the Bright Futures National Center.
Previsit screening tools included in the new edition are aimed at assessing and prioritizing anticipatory guidance issues, Dr. Hagan said, noting too that the Periodicity Schedule and Bright Futures recommendations make distinctions between universal and selective screening. “By design,” he emphasized, “there’s more anticipatory guidance than you might ever accomplish in one visit, because we want to be thorough enough to provide a context – a schema – to deal with the issues.”
Oftentimes, he said, “what parents want to talk about is what you want to talk about.” And pediatricians “develop a skill set to temporize, to figure out what needs to be covered today, and what can be dealt with better at a later time,” Dr. Hagan said. “If you tell kids, for instance, ‘I can help you with this, I just have to get more information,’ they hear that there’s help on the way. Then you follow through.”
Overall, his well-child visits “have gotten much more involved with the emotional well-being of children.” Given that emotional issues and behavioral issues “tend to take a longer time to discuss and unravel,” he sets aside consultation times near the end of the day for families who need to discuss these issues.
And he routinely devotes time – starting at the 2-month visit – to discuss screen time and media use. “I believe that technology is making our children sick,” he said, noting that in his nearly 17 years of practice he has seen increasing numbers of children and adolescents with depression, anxiety, anger, and attention deficit/hyperactivity disorder. “The AAP has done a pretty good job of raising the point, but I don’t think it has hit home with parents yet.”
For Dr. Chung, electronic medical records and systems have enabled him to better flag issues for follow-up over the course of well-child visits, leading to “better longitudinal care.”
Surveys and questionnaires filled out by parents in his practice’s waiting room are scanned into charts, he noted, and adolescents can mark answers on a proprietary confidential risk questionnaire that subsequently gets scrambled so that no one but the provider can understand the responses.
Other potential impacts of electronic systems have yet to be realized, he and others said. Some pediatric practices, Dr. Schor said, have begun engaging with families ahead of well-child visits through the use of a computerized questionnaire that elicits areas and issues of interest. Such outreach may help families feel more invested and committed to attending the visits, particularly those that don’t involve immunizations or school/sport forms, he said.
Families are “not [always] buying what we’re selling [for well-child care],” said Dr. Schor, who has served on AAP committees and has written several well-cited articles on preventive pediatrics care.
Insurance coverage for well-child care got a boost in 2010 when Bright Futures was cited in the Affordable Care Act as the standard of what well-child care should accomplish, and its recommended screenings and services were required to be covered by insurers without cost-sharing.
In the long-run, he said, rethinking the roles of nonphysicians in anticipatory guidance and developmental and psychosocial screening – in interpreting results of questionnaires, for instance – may be essential for well-child care. Outside of large health care systems, “the use of personnel [has been] pretty much been unchanged over the years,” he said. “We need to ask, how can we use each individual’s skills and training most efficiently? How can we retrain and reorganize our patient flow?”
This may be especially important as well-child care increasingly considers family psychosocial issues such as housing, food insecurity, family violence, and other family social stressors. Maternal depression screening made its way into the Periodicity Schedule in February 2016, and Dr. Schor predicts that the schedule will include “family psychosocial risk screening” in another several years.
For now, the newly revised Bright Futures guidelines – and much of well-child care – places an increased emphasis on the social determinants of health, which Dr. Hagan said reflects the “long-standing, logical conclusion that we reached back in the 1990s – that if families are healthy, kids will be healthy … and that family health is also linked to community health.”
CMS to alert docs of their MIPS status soon
ORLANDO – Want to know if you must participate in the new MIPS program? CMS is about to let you know.
Physicians who are right around the eligibility threshold for participation in the Quality Payment Program “want to know if they are eligible” for the Merit-based Incentive Payment System (MIPS), one of the QPP’s two tracks, Kate Goodrich, MD, said at the annual meeting of the Healthcare Information Management Systems Society. Within the next 6 weeks – about the first week of April – the Centers for Medicare & Medicaid Services will notify practices with less than $30,000 in Medicare payments or that serve less than 100 Medicare patients if they are exempt.
- Do the bare minimum and face no penalties.
- Submit 90 days worth of data and be eligible for a small bonus payment.
- Submit for the full year and be eligible for the full bonus that is to be determined.
Doing absolutely nothing will result in a 4% reduction in Medicare fee schedule payments in 2019.
“We have to expect that we will have some folks who do the minimum” in 2017, Dr. Goodrich, director of the Center for Clinical Standards and Quality and the chief medical officer for CMS, said. “They are just not ready to go beyond that. But even for folks who haven’t participated previously [in reporting programs], we are hearing they want to at least try to do more than just the bare minimum because they want to get ready for future years of the program.”
She said that CMS officials “are definitely hearing from some larger health systems, but even some medium and smaller practices that were really familiar with what we now call legacy programs, so meaningful use and PQRS [Physician Quality Reporting System] and so forth, that they’re ready.”
ORLANDO – Want to know if you must participate in the new MIPS program? CMS is about to let you know.
Physicians who are right around the eligibility threshold for participation in the Quality Payment Program “want to know if they are eligible” for the Merit-based Incentive Payment System (MIPS), one of the QPP’s two tracks, Kate Goodrich, MD, said at the annual meeting of the Healthcare Information Management Systems Society. Within the next 6 weeks – about the first week of April – the Centers for Medicare & Medicaid Services will notify practices with less than $30,000 in Medicare payments or that serve less than 100 Medicare patients if they are exempt.
- Do the bare minimum and face no penalties.
- Submit 90 days worth of data and be eligible for a small bonus payment.
- Submit for the full year and be eligible for the full bonus that is to be determined.
Doing absolutely nothing will result in a 4% reduction in Medicare fee schedule payments in 2019.
“We have to expect that we will have some folks who do the minimum” in 2017, Dr. Goodrich, director of the Center for Clinical Standards and Quality and the chief medical officer for CMS, said. “They are just not ready to go beyond that. But even for folks who haven’t participated previously [in reporting programs], we are hearing they want to at least try to do more than just the bare minimum because they want to get ready for future years of the program.”
She said that CMS officials “are definitely hearing from some larger health systems, but even some medium and smaller practices that were really familiar with what we now call legacy programs, so meaningful use and PQRS [Physician Quality Reporting System] and so forth, that they’re ready.”
ORLANDO – Want to know if you must participate in the new MIPS program? CMS is about to let you know.
Physicians who are right around the eligibility threshold for participation in the Quality Payment Program “want to know if they are eligible” for the Merit-based Incentive Payment System (MIPS), one of the QPP’s two tracks, Kate Goodrich, MD, said at the annual meeting of the Healthcare Information Management Systems Society. Within the next 6 weeks – about the first week of April – the Centers for Medicare & Medicaid Services will notify practices with less than $30,000 in Medicare payments or that serve less than 100 Medicare patients if they are exempt.
- Do the bare minimum and face no penalties.
- Submit 90 days worth of data and be eligible for a small bonus payment.
- Submit for the full year and be eligible for the full bonus that is to be determined.
Doing absolutely nothing will result in a 4% reduction in Medicare fee schedule payments in 2019.
“We have to expect that we will have some folks who do the minimum” in 2017, Dr. Goodrich, director of the Center for Clinical Standards and Quality and the chief medical officer for CMS, said. “They are just not ready to go beyond that. But even for folks who haven’t participated previously [in reporting programs], we are hearing they want to at least try to do more than just the bare minimum because they want to get ready for future years of the program.”
She said that CMS officials “are definitely hearing from some larger health systems, but even some medium and smaller practices that were really familiar with what we now call legacy programs, so meaningful use and PQRS [Physician Quality Reporting System] and so forth, that they’re ready.”
AT HIMSS 2017
PrEP appears to be safe in pregnancy
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
Key clinical point: The study is the first to confirm safety of PrEP in pregnancy.
Major finding: In this study, 16.7% of PrEP-exposed women experienced pregnancy loss versus 23.5% of unexposed.
Data source: Open-label, case-controlled study of 30 PrEP-exposed women and 79 controls.
Disclosures: The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
Online Patient-Reported Reviews of Mohs Micrographic Surgery: Qualitative Analysis of Positive and Negative Experiences
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).
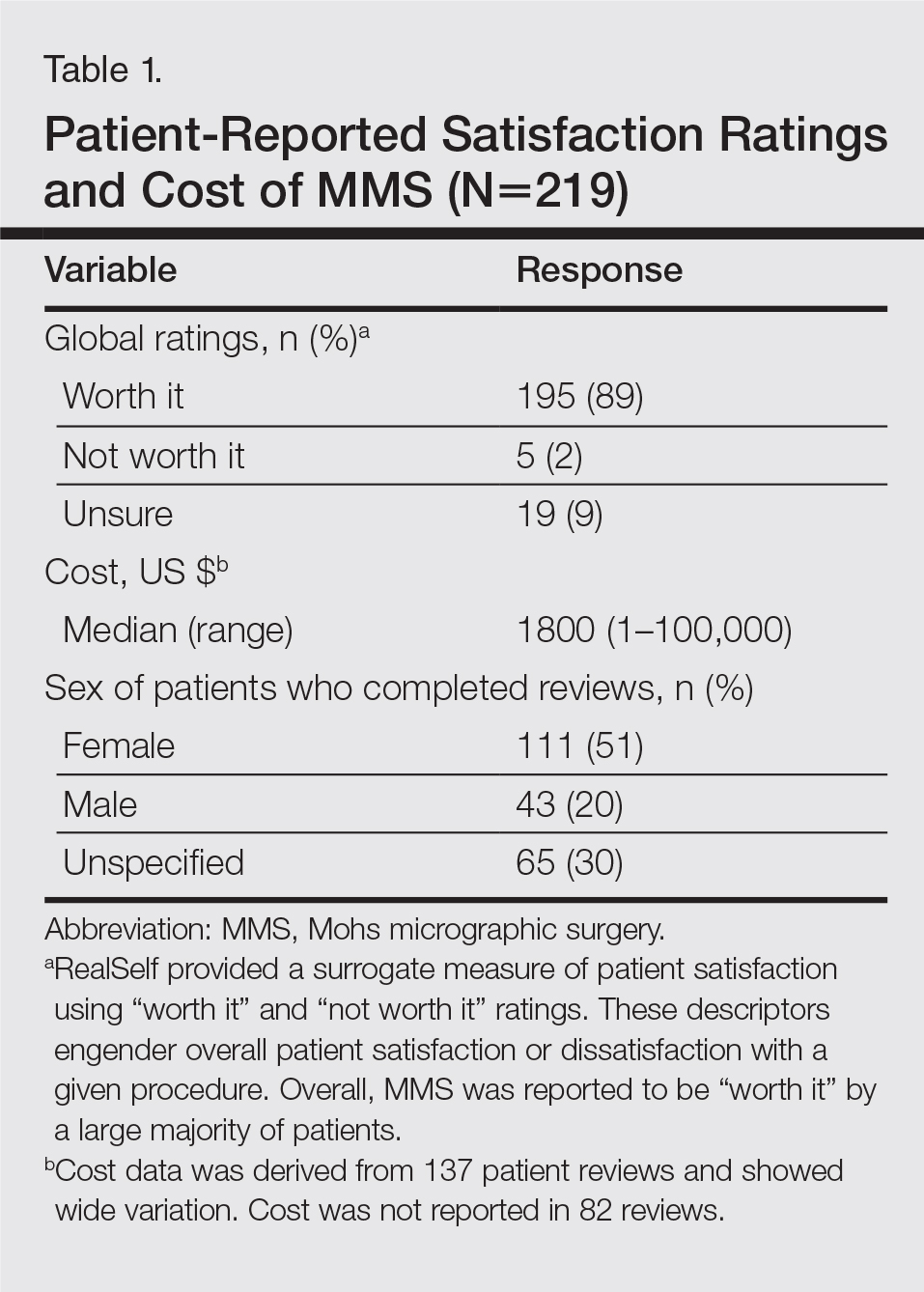

Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).
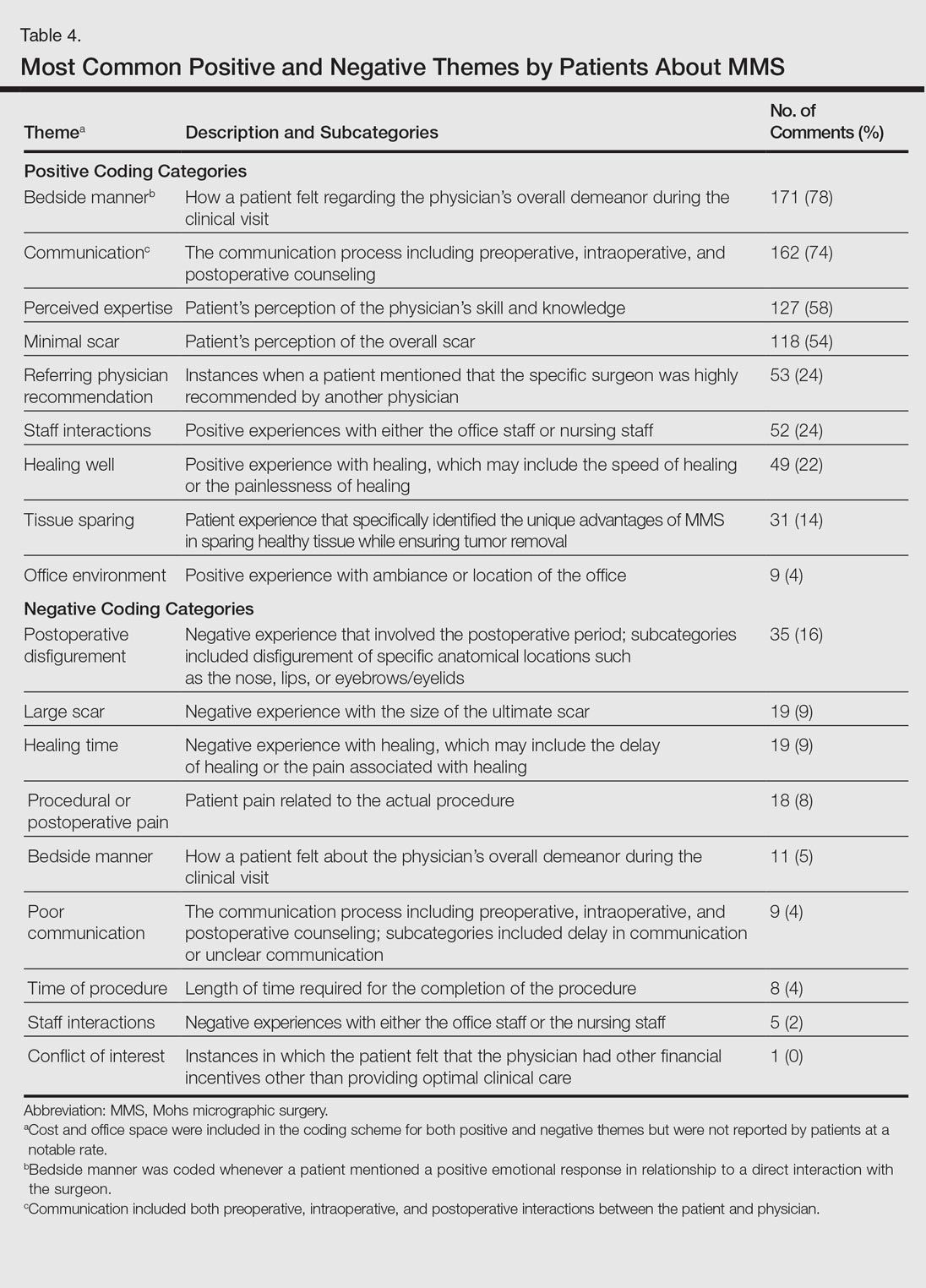
Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).


Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).

Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).


Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).

Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
Resident Pearl
Patients are posting reviews online now more than ever regarding their experiences with dermatologic surgical procedures. Mohs micrographic surgery is rated highly by patients but suspect to missing information and a higher than expected attribution of the procedure to plastic surgeons.