User login
STEMI team repurposed for rapid treatment of pulmonary emboli
WASHINGTON – The in-hospital team responsible for rapid management of ST-elevation myocardial infarction (STEMI) may also be the right team to manage pulmonary embolism (PE), according to a pilot study associating this approach with rapid treatment times and low overall mortality rates.
The data from the pilot study suggest that arming the STEMI team with a protocol for managing PE “is an effective means to care for patients with massive and submassive pulmonary embolism,” said Michael R. Kendall, MD, of the University of Southern California, Los Angeles. He presented the findings at the 2017 Cardiovascular Research Technologies meeting.
There are obvious parallels between STEMI and PE. Like STEMI, PE requires rapid diagnosis, triage, and when appropriate, an endovascular procedure. This led the USC investigators to consider a formal pilot study to test the premise that the STEMI team is in a position to deliver urgent care and good outcomes to PE patients.
The objective of the pilot study was “to evaluate treatment times and clinical outcome for patients with massive and submassive PE using a dedicated PE protocol,” Dr. Kendall explained. Massive PE was defined as hemodynamic instability with systolic blood pressure below 90 mm Hg or requiring inotropic support. Submassive PE was defined as systolic BP greater than 90 mm Hg with right heart dysfunction, such as a dilated right ventricle and elevated troponin levels.
Over an 18-month period beginning in June 2014, 40 PE patients were treated. The average age was 55 years, 50% were obese, 32% had renal insufficiency, 30% had recent surgery or had recently been immobilized, 30% had a history of deep venous thrombosis, and 28% had an active malignancy. At 43%, the largest single source of cases was the emergency department (ED), while 38% were transferred in from other centers, and 19% were already hospitalized at the time of the PE.
All patients underwent computed tomographic pulmonary angiography (CTPA) as part of the diagnostic procedure prior to an invasive angiogram. Patients received one or more different treatments upon confirmation of the PE, including catheter-directed thrombolytics, rheolytic thrombectomy, mechanical fragmentation, mechanical aspiration, and surgical pulmonary embolectomy. Inferior vena cava filters were used as appropriate.
At presentation, 10% were in cardiac arrest, 22% required intubation, and 12% required extracorporeal membrane oxygenation. On the basis of the diagnostic studies, 57% had a massive PE, and the remainder had submassive disease.
The average time from door to CTPA among those presenting to the ED was roughly 5 hours. It took, on average, an additional 2 hours from CTPA to an invasive angiogram, and another 3 hours to treatment, producing a total average door to treatment time of 10 hours.
Most patients received rheolytic thrombectomy, often with another form of treatment, such as catheter-directed thrombolytics, but 15% were treated with anticoagulation alone. Although a few patients improved sufficiently to obviate the need for an invasive procedure, the remainder of the patients received anticoagulation alone because of contraindications for invasive strategies or treatment refusal.
The average length of a hospital stay was 15 days. Bleeding events occurred in 10% of patients and 18% required a blood transfusion. Survival to hospital discharge was 82%. Although there was no control group, this rate of survival was considered favorable in the context of the severity of the PE.
Overall, delivery of urgent care for PE by a STEMI team was found feasible. Even though treatment approaches were not standardized, the protocol for diagnosing and managing PE on an urgent basis produced encouraging times for delivery of care and outcomes, according to the data presented by Dr. Kendall. Because of the differences in the composition and function of the STEMI team, Dr. Kendall indicated that it is difficult to predict similar success at other centers, but the findings overall were considered positive.
The senior author of the study, David M. Shavelle, MD, also at USC, suggested that the program there might be a template. He believes that the STEMI team has the skills to deliver prompt PE care, and he believes that this approach would be appropriate at other centers. He also suggested that such formal programs may be useful in establishing better treatment protocols.
“For PE, there are no clear guidelines for treatment time intervals, such as time from door to endovascular treatment,” Dr. Shavelle observed. He said that the wide variation of devices currently being used for treatment complicates efforts to develop clear clinical protocols and measure outcomes and “would like to see more standardization of treatment and registries to address these areas.”
Dr. Kendall reported no financial relationships.
WASHINGTON – The in-hospital team responsible for rapid management of ST-elevation myocardial infarction (STEMI) may also be the right team to manage pulmonary embolism (PE), according to a pilot study associating this approach with rapid treatment times and low overall mortality rates.
The data from the pilot study suggest that arming the STEMI team with a protocol for managing PE “is an effective means to care for patients with massive and submassive pulmonary embolism,” said Michael R. Kendall, MD, of the University of Southern California, Los Angeles. He presented the findings at the 2017 Cardiovascular Research Technologies meeting.
There are obvious parallels between STEMI and PE. Like STEMI, PE requires rapid diagnosis, triage, and when appropriate, an endovascular procedure. This led the USC investigators to consider a formal pilot study to test the premise that the STEMI team is in a position to deliver urgent care and good outcomes to PE patients.
The objective of the pilot study was “to evaluate treatment times and clinical outcome for patients with massive and submassive PE using a dedicated PE protocol,” Dr. Kendall explained. Massive PE was defined as hemodynamic instability with systolic blood pressure below 90 mm Hg or requiring inotropic support. Submassive PE was defined as systolic BP greater than 90 mm Hg with right heart dysfunction, such as a dilated right ventricle and elevated troponin levels.
Over an 18-month period beginning in June 2014, 40 PE patients were treated. The average age was 55 years, 50% were obese, 32% had renal insufficiency, 30% had recent surgery or had recently been immobilized, 30% had a history of deep venous thrombosis, and 28% had an active malignancy. At 43%, the largest single source of cases was the emergency department (ED), while 38% were transferred in from other centers, and 19% were already hospitalized at the time of the PE.
All patients underwent computed tomographic pulmonary angiography (CTPA) as part of the diagnostic procedure prior to an invasive angiogram. Patients received one or more different treatments upon confirmation of the PE, including catheter-directed thrombolytics, rheolytic thrombectomy, mechanical fragmentation, mechanical aspiration, and surgical pulmonary embolectomy. Inferior vena cava filters were used as appropriate.
At presentation, 10% were in cardiac arrest, 22% required intubation, and 12% required extracorporeal membrane oxygenation. On the basis of the diagnostic studies, 57% had a massive PE, and the remainder had submassive disease.
The average time from door to CTPA among those presenting to the ED was roughly 5 hours. It took, on average, an additional 2 hours from CTPA to an invasive angiogram, and another 3 hours to treatment, producing a total average door to treatment time of 10 hours.
Most patients received rheolytic thrombectomy, often with another form of treatment, such as catheter-directed thrombolytics, but 15% were treated with anticoagulation alone. Although a few patients improved sufficiently to obviate the need for an invasive procedure, the remainder of the patients received anticoagulation alone because of contraindications for invasive strategies or treatment refusal.
The average length of a hospital stay was 15 days. Bleeding events occurred in 10% of patients and 18% required a blood transfusion. Survival to hospital discharge was 82%. Although there was no control group, this rate of survival was considered favorable in the context of the severity of the PE.
Overall, delivery of urgent care for PE by a STEMI team was found feasible. Even though treatment approaches were not standardized, the protocol for diagnosing and managing PE on an urgent basis produced encouraging times for delivery of care and outcomes, according to the data presented by Dr. Kendall. Because of the differences in the composition and function of the STEMI team, Dr. Kendall indicated that it is difficult to predict similar success at other centers, but the findings overall were considered positive.
The senior author of the study, David M. Shavelle, MD, also at USC, suggested that the program there might be a template. He believes that the STEMI team has the skills to deliver prompt PE care, and he believes that this approach would be appropriate at other centers. He also suggested that such formal programs may be useful in establishing better treatment protocols.
“For PE, there are no clear guidelines for treatment time intervals, such as time from door to endovascular treatment,” Dr. Shavelle observed. He said that the wide variation of devices currently being used for treatment complicates efforts to develop clear clinical protocols and measure outcomes and “would like to see more standardization of treatment and registries to address these areas.”
Dr. Kendall reported no financial relationships.
WASHINGTON – The in-hospital team responsible for rapid management of ST-elevation myocardial infarction (STEMI) may also be the right team to manage pulmonary embolism (PE), according to a pilot study associating this approach with rapid treatment times and low overall mortality rates.
The data from the pilot study suggest that arming the STEMI team with a protocol for managing PE “is an effective means to care for patients with massive and submassive pulmonary embolism,” said Michael R. Kendall, MD, of the University of Southern California, Los Angeles. He presented the findings at the 2017 Cardiovascular Research Technologies meeting.
There are obvious parallels between STEMI and PE. Like STEMI, PE requires rapid diagnosis, triage, and when appropriate, an endovascular procedure. This led the USC investigators to consider a formal pilot study to test the premise that the STEMI team is in a position to deliver urgent care and good outcomes to PE patients.
The objective of the pilot study was “to evaluate treatment times and clinical outcome for patients with massive and submassive PE using a dedicated PE protocol,” Dr. Kendall explained. Massive PE was defined as hemodynamic instability with systolic blood pressure below 90 mm Hg or requiring inotropic support. Submassive PE was defined as systolic BP greater than 90 mm Hg with right heart dysfunction, such as a dilated right ventricle and elevated troponin levels.
Over an 18-month period beginning in June 2014, 40 PE patients were treated. The average age was 55 years, 50% were obese, 32% had renal insufficiency, 30% had recent surgery or had recently been immobilized, 30% had a history of deep venous thrombosis, and 28% had an active malignancy. At 43%, the largest single source of cases was the emergency department (ED), while 38% were transferred in from other centers, and 19% were already hospitalized at the time of the PE.
All patients underwent computed tomographic pulmonary angiography (CTPA) as part of the diagnostic procedure prior to an invasive angiogram. Patients received one or more different treatments upon confirmation of the PE, including catheter-directed thrombolytics, rheolytic thrombectomy, mechanical fragmentation, mechanical aspiration, and surgical pulmonary embolectomy. Inferior vena cava filters were used as appropriate.
At presentation, 10% were in cardiac arrest, 22% required intubation, and 12% required extracorporeal membrane oxygenation. On the basis of the diagnostic studies, 57% had a massive PE, and the remainder had submassive disease.
The average time from door to CTPA among those presenting to the ED was roughly 5 hours. It took, on average, an additional 2 hours from CTPA to an invasive angiogram, and another 3 hours to treatment, producing a total average door to treatment time of 10 hours.
Most patients received rheolytic thrombectomy, often with another form of treatment, such as catheter-directed thrombolytics, but 15% were treated with anticoagulation alone. Although a few patients improved sufficiently to obviate the need for an invasive procedure, the remainder of the patients received anticoagulation alone because of contraindications for invasive strategies or treatment refusal.
The average length of a hospital stay was 15 days. Bleeding events occurred in 10% of patients and 18% required a blood transfusion. Survival to hospital discharge was 82%. Although there was no control group, this rate of survival was considered favorable in the context of the severity of the PE.
Overall, delivery of urgent care for PE by a STEMI team was found feasible. Even though treatment approaches were not standardized, the protocol for diagnosing and managing PE on an urgent basis produced encouraging times for delivery of care and outcomes, according to the data presented by Dr. Kendall. Because of the differences in the composition and function of the STEMI team, Dr. Kendall indicated that it is difficult to predict similar success at other centers, but the findings overall were considered positive.
The senior author of the study, David M. Shavelle, MD, also at USC, suggested that the program there might be a template. He believes that the STEMI team has the skills to deliver prompt PE care, and he believes that this approach would be appropriate at other centers. He also suggested that such formal programs may be useful in establishing better treatment protocols.
“For PE, there are no clear guidelines for treatment time intervals, such as time from door to endovascular treatment,” Dr. Shavelle observed. He said that the wide variation of devices currently being used for treatment complicates efforts to develop clear clinical protocols and measure outcomes and “would like to see more standardization of treatment and registries to address these areas.”
Dr. Kendall reported no financial relationships.
AT THE 2017 CRT MEETING
Key clinical point: The same team assembled for rapid response to STEMI can also treat PE, according to a pilot study.
Major finding: In the initial series of patients, of whom 57% had massive PE, in-hospital mortality was 11%.
Data source: A nonrandomized prospective analysis.
Disclosures: Dr. Kendall reported no financial relationships.
Increased schizophrenia, affective disorder risks associated with infections
Increased risks of schizophrenia and affective disorders were found to be associated with infections treated with anti-infective agents and with infections requiring hospitalization, a large-scale study shows.
Researchers examined health records of all 1,015,447 individuals born in Denmark from 1985 to 2002, their history of treatment of infection, and the risk of schizophrenia and affective disorders.
Infections previously have been shown to be associated with increased risks of mental disorders. The goal of the current study was to investigate whether the use of anti-infective agents in primary care settings had a similar association.
And the researchers did find such an association: an increased risk of schizophrenia (hazard ratio, 1.37; 95% confidence interval, 1.2-1.57) and an increased risk of affective disorders (HR, 1.64; 95% CI, 1.48-1.82) associated with the use of anti-infective agents.
“The excess risk was primarily driven by infections treated with antibiotics, whereas infections treated with antivirals, antimycotics, and antiparasitic agents were not significant after mutual adjustment,” wrote Ole Köhler, MD, of Aarhus University Hospital, Risskov, Denmark, and his associates (Acta Psychiatr Scand. 2017 Feb;135[2]:97-105).
An even higher risk of schizophrenia and affective disorders was found to be associated with individuals with infections requiring hospitalization (HR, 2.05; 95% CI, 1.77-2.38; and HR, 2.59; 95% CI, 2.31-2.89; respectively). Find more details about the study here.
Increased risks of schizophrenia and affective disorders were found to be associated with infections treated with anti-infective agents and with infections requiring hospitalization, a large-scale study shows.
Researchers examined health records of all 1,015,447 individuals born in Denmark from 1985 to 2002, their history of treatment of infection, and the risk of schizophrenia and affective disorders.
Infections previously have been shown to be associated with increased risks of mental disorders. The goal of the current study was to investigate whether the use of anti-infective agents in primary care settings had a similar association.
And the researchers did find such an association: an increased risk of schizophrenia (hazard ratio, 1.37; 95% confidence interval, 1.2-1.57) and an increased risk of affective disorders (HR, 1.64; 95% CI, 1.48-1.82) associated with the use of anti-infective agents.
“The excess risk was primarily driven by infections treated with antibiotics, whereas infections treated with antivirals, antimycotics, and antiparasitic agents were not significant after mutual adjustment,” wrote Ole Köhler, MD, of Aarhus University Hospital, Risskov, Denmark, and his associates (Acta Psychiatr Scand. 2017 Feb;135[2]:97-105).
An even higher risk of schizophrenia and affective disorders was found to be associated with individuals with infections requiring hospitalization (HR, 2.05; 95% CI, 1.77-2.38; and HR, 2.59; 95% CI, 2.31-2.89; respectively). Find more details about the study here.
Increased risks of schizophrenia and affective disorders were found to be associated with infections treated with anti-infective agents and with infections requiring hospitalization, a large-scale study shows.
Researchers examined health records of all 1,015,447 individuals born in Denmark from 1985 to 2002, their history of treatment of infection, and the risk of schizophrenia and affective disorders.
Infections previously have been shown to be associated with increased risks of mental disorders. The goal of the current study was to investigate whether the use of anti-infective agents in primary care settings had a similar association.
And the researchers did find such an association: an increased risk of schizophrenia (hazard ratio, 1.37; 95% confidence interval, 1.2-1.57) and an increased risk of affective disorders (HR, 1.64; 95% CI, 1.48-1.82) associated with the use of anti-infective agents.
“The excess risk was primarily driven by infections treated with antibiotics, whereas infections treated with antivirals, antimycotics, and antiparasitic agents were not significant after mutual adjustment,” wrote Ole Köhler, MD, of Aarhus University Hospital, Risskov, Denmark, and his associates (Acta Psychiatr Scand. 2017 Feb;135[2]:97-105).
An even higher risk of schizophrenia and affective disorders was found to be associated with individuals with infections requiring hospitalization (HR, 2.05; 95% CI, 1.77-2.38; and HR, 2.59; 95% CI, 2.31-2.89; respectively). Find more details about the study here.
Robotic PCI success rates higher with radial access
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
AT CRT 2017
Key clinical point: Registry data shows higher success rate for radial versus femoral access in robotic percutaneous coronary interventions.
Major finding: In robotic PCI, the clinical success rate was 99.4% with radial access and 94.7% (P = .002) with femoral access.
Data source: A nonrandomized, retrospective analysis.
Disclosures: Dr. Pourdjabbar reported no financial relationships to disclose.
Preterm infants at higher risk of pertussis
Preterm infants are at greater risk for pertussis than full-term infants, according to a population-based study from the Norwegian Institute of Public Health.
Øystein Rolandsen Riise, MD, PhD, of the institute, and his colleagues identified all live births in Norway from 1998 to 2010 from the Medical Birth Registry of Norway and studied the gestational age of 713,166 infants as an indicator of increased risk of pertussis.
Infants born at 23-27 weeks of gestational age had an incidence rate ratio of pertussis more than fourfold higher than term infants (IRR = 4.49), while those born at 32-34 weeks and 35-36 weeks each had an IRR about 1.5 time higher than term infants (Pediatr Infect Dis J. doi: 10.1097/INF.0000000000001545).
The IRR of preterm infants hospitalized with pertussis was double that of full-term infants (IRR = 1.99).
The increased risk in preterm infants could be attributed to an incomplete transfer of maternal antibodies, specifically the “abundance of IgG [that] is acquired during the last month of full-term pregnancy,” Dr. Riise and colleagues wrote.
While previous studies have found links between preterm birth and pertussis infection, those studies used birth weight instead of gestational age, which leads to possibly inaccurate results, they added.
“Although other studies have used low birth weight to identify infants with increased risk of pertussis, we would have underestimated the number of infants with increased risk by using low birth weight instead of gestational age, since many of the late preterm infants have normal birth weight,” the researchers noted.
Early vaccination is key to protecting preterm infants from pertussis, Dr. Riise and colleagues said, adding that vaccine effectiveness was similar between preterm (93%) and full-term (88.8%) infants.
Preterm infants are at greater risk for pertussis than full-term infants, according to a population-based study from the Norwegian Institute of Public Health.
Øystein Rolandsen Riise, MD, PhD, of the institute, and his colleagues identified all live births in Norway from 1998 to 2010 from the Medical Birth Registry of Norway and studied the gestational age of 713,166 infants as an indicator of increased risk of pertussis.
Infants born at 23-27 weeks of gestational age had an incidence rate ratio of pertussis more than fourfold higher than term infants (IRR = 4.49), while those born at 32-34 weeks and 35-36 weeks each had an IRR about 1.5 time higher than term infants (Pediatr Infect Dis J. doi: 10.1097/INF.0000000000001545).
The IRR of preterm infants hospitalized with pertussis was double that of full-term infants (IRR = 1.99).
The increased risk in preterm infants could be attributed to an incomplete transfer of maternal antibodies, specifically the “abundance of IgG [that] is acquired during the last month of full-term pregnancy,” Dr. Riise and colleagues wrote.
While previous studies have found links between preterm birth and pertussis infection, those studies used birth weight instead of gestational age, which leads to possibly inaccurate results, they added.
“Although other studies have used low birth weight to identify infants with increased risk of pertussis, we would have underestimated the number of infants with increased risk by using low birth weight instead of gestational age, since many of the late preterm infants have normal birth weight,” the researchers noted.
Early vaccination is key to protecting preterm infants from pertussis, Dr. Riise and colleagues said, adding that vaccine effectiveness was similar between preterm (93%) and full-term (88.8%) infants.
Preterm infants are at greater risk for pertussis than full-term infants, according to a population-based study from the Norwegian Institute of Public Health.
Øystein Rolandsen Riise, MD, PhD, of the institute, and his colleagues identified all live births in Norway from 1998 to 2010 from the Medical Birth Registry of Norway and studied the gestational age of 713,166 infants as an indicator of increased risk of pertussis.
Infants born at 23-27 weeks of gestational age had an incidence rate ratio of pertussis more than fourfold higher than term infants (IRR = 4.49), while those born at 32-34 weeks and 35-36 weeks each had an IRR about 1.5 time higher than term infants (Pediatr Infect Dis J. doi: 10.1097/INF.0000000000001545).
The IRR of preterm infants hospitalized with pertussis was double that of full-term infants (IRR = 1.99).
The increased risk in preterm infants could be attributed to an incomplete transfer of maternal antibodies, specifically the “abundance of IgG [that] is acquired during the last month of full-term pregnancy,” Dr. Riise and colleagues wrote.
While previous studies have found links between preterm birth and pertussis infection, those studies used birth weight instead of gestational age, which leads to possibly inaccurate results, they added.
“Although other studies have used low birth weight to identify infants with increased risk of pertussis, we would have underestimated the number of infants with increased risk by using low birth weight instead of gestational age, since many of the late preterm infants have normal birth weight,” the researchers noted.
Early vaccination is key to protecting preterm infants from pertussis, Dr. Riise and colleagues said, adding that vaccine effectiveness was similar between preterm (93%) and full-term (88.8%) infants.
FROM PEDIATRIC INFECTIOUS DISEASE JOURNAL
Key clinical point:
Major finding: The risk of pertussis infection was 4.49-fold higher in infants with a gestational age of 23-27 weeks.
Data source: A cohort study of 713,166 children from the Medical Birth Registry of Norway with a gestation period of 23-37 weeks during 1998-2010.
Disclosures: No funding was secured for this study. The authors had no relevant financial disclosures.
32-year-old woman with pelvic pain and irregular menstrual periods
(A) Paratubal cyst CORRECT
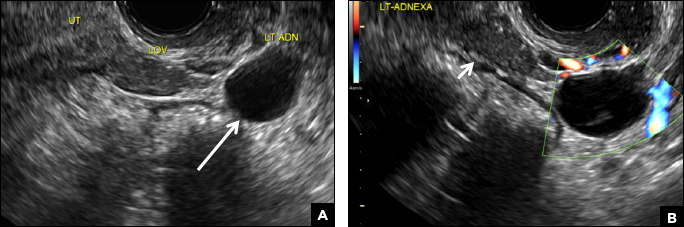
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2
B) Hydrosalpinx INCORRECT
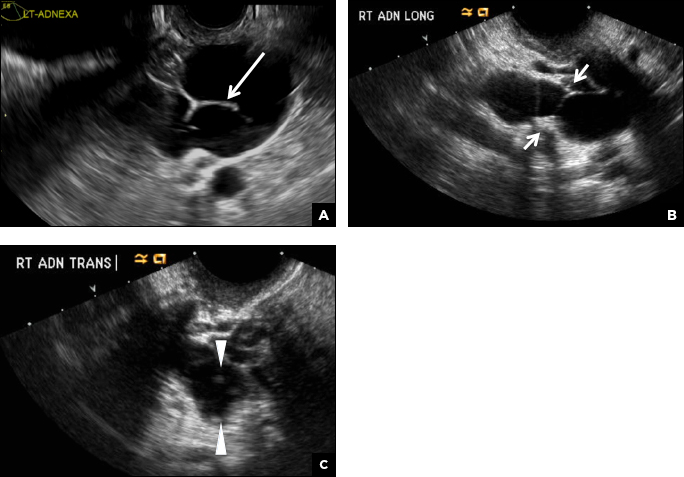
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.
C) Peritoneal inclusion cyst INCORRECT
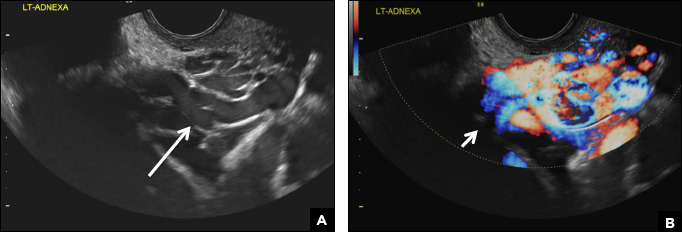
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.
- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2

B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.

C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.

(A) Paratubal cyst CORRECT
Paratubal, or paraovarian, cysts typically are round or oval avascular hypoechoic cysts (long arrow) separate from the adjacent ovary (short arrow). Since they are congenital remnants of the Wolffian duct, they arise from the mesosalpinx, specifically the broad ligament or fallopian tube.1,2 They usually are seen in close proximity to but separate from the ovary without distorting the ovary’s architecture.1,2

B) Hydrosalpinx INCORRECT
A hydrosalpinx appears as an elongated C- or S-shaped, thin-walled tubular serpiginous cystic lesion separate from the ovary. It often has incomplete septations that are infolding of the tube on itself (long arrow).3 Other findings include diametrically opposed indentations (short arrows) of the wall (Waist sign) and short linear mucosal or submucosal folds (arrowhead) that when viewed in cross section appear similar to the spokes of a cogwheel (Cogwheel sign).1–3 Prior tubal infection or gynecologic surgery represent risk factors for hydrosalpinx.

C) Peritoneal inclusion cyst INCORRECT
A peritoneal inclusion cyst appears as an anechoic cystic mass that conforms passively to the shape of the peritoneal cavity/pelvic sidewall (long arrow) and may contain entrapped ovaries (short arrow) within or along the periphery of the fluid collection.1,2 Septations within it are likely from peritoneal adhesions (arrowhead) and may show vascularity.2 Prior (often multiple) gynecologic surgeries represent a risk factor for peritoneal inclusion cysts.

D) Dilated pelvic veins INCORRECT
Dilated pelvic veins appear on sonography as a cluster of elongated and tubular cystic lesions in the adnexa along the broad ligament and demonstrate low level echoes due to sluggish flow (long arrow) and visible red blood cell rouleaux formation. This can be confirmed on color Doppler images (short arrow) and help differentiate it from hydrosalpinx.

- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
- Laing FC, Allison SF. US of the ovary and adnexa: to worry or not to worry? Radiographics. 2012:32(6):1621−1639.
- Moyle PL, Kataoka MY, Nakai A, Takahata A, Reinhold C, Sala E. Nonovarian cystic lesions of the pelvis. Radiographics. 2010;30(4):921−938.
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527−548.
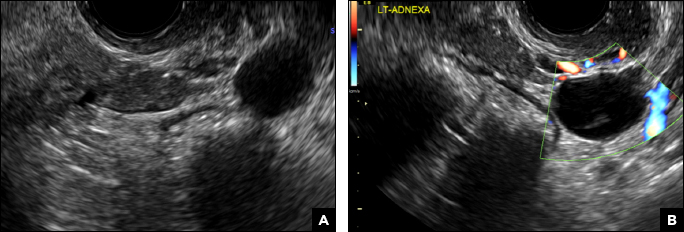
A 32-year-old women presents to her gynecologist’s office reporting pelvic pain and irregular menstrual periods. Results of a urine pregnancy test are negative. Pelvic ultrasonography is performed, with gray scale ( A ) and color Doppler ( B ) images of the left adnexa obtained. Figures shown above.
VIDEO: Clot aspiration equals retrieval for ischemic stroke
HOUSTON – Intracerebral clot aspiration was as safe and effective as stent retriever thrombectomy for restoring cerebral blood flow in a French multicenter, randomized trial with 381 acute ischemic stroke patients.
This study is the “first direct comparison of aspiration versus stent retrieval” as the initial strategy for clot removal in acute ischemic stroke, and it “opens the door to add a new tool” for clot removal, Bertrand Lapergue, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The new results “are the first to show that aspiration first is as good as a stent retriever, but we need to also see the results from COMPASS,” a U.S. multicenter trial that is in the process of making the same comparison, commented Ricardo A. Hanel, MD, a vascular neurosurgeon at Baptist Health in Jacksonville, Fla. The COMPASS Trial: a Direct Aspiration First Pass Technique has now enrolled about two-thirds of its target patient number, and until the study is complete the role of direct aspiration for clot removal in stroke remains investigational for U.S. practice, said Dr. Hanel, a COMPASS investigator.
The aspiration catheter tested in ASTER is marketed by Penumbra and has already received Food and Drug Administration approval for revascularization of ischemic stroke patients. U.S. use of aspiration for treating acute ischemic stroke, however, has remained limited because there is no clear evidence of the method’s efficacy. Dr. Hanel said that he occasionally uses aspiration as an adjunct to clot removal with a stent retriever.
ASTER’s primary endpoint was the percentage of patients who achieved thrombolysis in cerebral infarction (TICI) 2b or 3 flow at the end of treatment, which occurred in 85% of patients treated with aspiration first and in 83% of those treated by clot removal first, a difference that was not statistically significant, Dr. Lapergue reported. The rate of patients who achieved either TICI 2b or 3 flow after the initial strategy only was 63% with aspiration and 68% with clot removal, also a nonsignificant difference. The two strategies also showed no significant difference for any measured safety parameter. The results showed a trend toward more vasospasm with clot removal – a 6% rate, versus 3% with clot aspiration – but this did not reach statistical significance.
Results from additional analyses of the clinical outcomes of patients in the trial and of cost efficacy will be reported later in 2017, Dr. Lapergue said.
ASTER received an unrestricted research grant from Penumbra, a company that markets clot removal aspiration catheters. Dr. Lapergue had no personal disclosures. Dr. Hanel has been a consultant to and received grant support from Medtronic. He has received research grants from MicroVention and has an ownership interest in InNeuroCo.
[email protected]
On Twitter @mitchelzoler
ASTER is an important trial. It shows for the first time that an aspiration device is probably as safe and reasonable for opening an acute occlusion in a large cerebral artery as is a stent retriever.
ASTER, however, was done entirely in a French population, making it uncertain whether the results are applicable to other populations. For example, U.S. acute ischemic stroke patients, especially African Americans and Hispanics, generally have more intracerebral atherostenotic disease than do patients from European countries, while French patients tend to have more embolic disease. Will aspiration be as effective in U.S. patients with atherostenotic blockages? I would love to see this study repeated in a U.S. population of ischemic stroke patients, and that is now happening in the COMPASS trial. It would be helpful to know if there are selected U.S. patients who might be better treated using either aspiration or a stent retriever first.
Although aspiration catheters have already received Food and Drug Administration approval for clot removal in acute ischemic stroke patients, many U.S. interventionalists have moved to deploying stent retrievers based on the very positive results reported with these devices about 2 years ago. For the moment, stent retrievers remain the most prominent devices to open large vessel occlusions.
Ralph L. Sacco, MD, is professor and chairman of neurology at the University of Miami. He had no relevant disclosures. He made these comments in a video interview and during a press conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ASTER is an important trial. It shows for the first time that an aspiration device is probably as safe and reasonable for opening an acute occlusion in a large cerebral artery as is a stent retriever.
ASTER, however, was done entirely in a French population, making it uncertain whether the results are applicable to other populations. For example, U.S. acute ischemic stroke patients, especially African Americans and Hispanics, generally have more intracerebral atherostenotic disease than do patients from European countries, while French patients tend to have more embolic disease. Will aspiration be as effective in U.S. patients with atherostenotic blockages? I would love to see this study repeated in a U.S. population of ischemic stroke patients, and that is now happening in the COMPASS trial. It would be helpful to know if there are selected U.S. patients who might be better treated using either aspiration or a stent retriever first.
Although aspiration catheters have already received Food and Drug Administration approval for clot removal in acute ischemic stroke patients, many U.S. interventionalists have moved to deploying stent retrievers based on the very positive results reported with these devices about 2 years ago. For the moment, stent retrievers remain the most prominent devices to open large vessel occlusions.
Ralph L. Sacco, MD, is professor and chairman of neurology at the University of Miami. He had no relevant disclosures. He made these comments in a video interview and during a press conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ASTER is an important trial. It shows for the first time that an aspiration device is probably as safe and reasonable for opening an acute occlusion in a large cerebral artery as is a stent retriever.
ASTER, however, was done entirely in a French population, making it uncertain whether the results are applicable to other populations. For example, U.S. acute ischemic stroke patients, especially African Americans and Hispanics, generally have more intracerebral atherostenotic disease than do patients from European countries, while French patients tend to have more embolic disease. Will aspiration be as effective in U.S. patients with atherostenotic blockages? I would love to see this study repeated in a U.S. population of ischemic stroke patients, and that is now happening in the COMPASS trial. It would be helpful to know if there are selected U.S. patients who might be better treated using either aspiration or a stent retriever first.
Although aspiration catheters have already received Food and Drug Administration approval for clot removal in acute ischemic stroke patients, many U.S. interventionalists have moved to deploying stent retrievers based on the very positive results reported with these devices about 2 years ago. For the moment, stent retrievers remain the most prominent devices to open large vessel occlusions.
Ralph L. Sacco, MD, is professor and chairman of neurology at the University of Miami. He had no relevant disclosures. He made these comments in a video interview and during a press conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON – Intracerebral clot aspiration was as safe and effective as stent retriever thrombectomy for restoring cerebral blood flow in a French multicenter, randomized trial with 381 acute ischemic stroke patients.
This study is the “first direct comparison of aspiration versus stent retrieval” as the initial strategy for clot removal in acute ischemic stroke, and it “opens the door to add a new tool” for clot removal, Bertrand Lapergue, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The new results “are the first to show that aspiration first is as good as a stent retriever, but we need to also see the results from COMPASS,” a U.S. multicenter trial that is in the process of making the same comparison, commented Ricardo A. Hanel, MD, a vascular neurosurgeon at Baptist Health in Jacksonville, Fla. The COMPASS Trial: a Direct Aspiration First Pass Technique has now enrolled about two-thirds of its target patient number, and until the study is complete the role of direct aspiration for clot removal in stroke remains investigational for U.S. practice, said Dr. Hanel, a COMPASS investigator.
The aspiration catheter tested in ASTER is marketed by Penumbra and has already received Food and Drug Administration approval for revascularization of ischemic stroke patients. U.S. use of aspiration for treating acute ischemic stroke, however, has remained limited because there is no clear evidence of the method’s efficacy. Dr. Hanel said that he occasionally uses aspiration as an adjunct to clot removal with a stent retriever.
ASTER’s primary endpoint was the percentage of patients who achieved thrombolysis in cerebral infarction (TICI) 2b or 3 flow at the end of treatment, which occurred in 85% of patients treated with aspiration first and in 83% of those treated by clot removal first, a difference that was not statistically significant, Dr. Lapergue reported. The rate of patients who achieved either TICI 2b or 3 flow after the initial strategy only was 63% with aspiration and 68% with clot removal, also a nonsignificant difference. The two strategies also showed no significant difference for any measured safety parameter. The results showed a trend toward more vasospasm with clot removal – a 6% rate, versus 3% with clot aspiration – but this did not reach statistical significance.
Results from additional analyses of the clinical outcomes of patients in the trial and of cost efficacy will be reported later in 2017, Dr. Lapergue said.
ASTER received an unrestricted research grant from Penumbra, a company that markets clot removal aspiration catheters. Dr. Lapergue had no personal disclosures. Dr. Hanel has been a consultant to and received grant support from Medtronic. He has received research grants from MicroVention and has an ownership interest in InNeuroCo.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Intracerebral clot aspiration was as safe and effective as stent retriever thrombectomy for restoring cerebral blood flow in a French multicenter, randomized trial with 381 acute ischemic stroke patients.
This study is the “first direct comparison of aspiration versus stent retrieval” as the initial strategy for clot removal in acute ischemic stroke, and it “opens the door to add a new tool” for clot removal, Bertrand Lapergue, MD, said at the International Stroke Conference sponsored by the American Heart Association.
The new results “are the first to show that aspiration first is as good as a stent retriever, but we need to also see the results from COMPASS,” a U.S. multicenter trial that is in the process of making the same comparison, commented Ricardo A. Hanel, MD, a vascular neurosurgeon at Baptist Health in Jacksonville, Fla. The COMPASS Trial: a Direct Aspiration First Pass Technique has now enrolled about two-thirds of its target patient number, and until the study is complete the role of direct aspiration for clot removal in stroke remains investigational for U.S. practice, said Dr. Hanel, a COMPASS investigator.
The aspiration catheter tested in ASTER is marketed by Penumbra and has already received Food and Drug Administration approval for revascularization of ischemic stroke patients. U.S. use of aspiration for treating acute ischemic stroke, however, has remained limited because there is no clear evidence of the method’s efficacy. Dr. Hanel said that he occasionally uses aspiration as an adjunct to clot removal with a stent retriever.
ASTER’s primary endpoint was the percentage of patients who achieved thrombolysis in cerebral infarction (TICI) 2b or 3 flow at the end of treatment, which occurred in 85% of patients treated with aspiration first and in 83% of those treated by clot removal first, a difference that was not statistically significant, Dr. Lapergue reported. The rate of patients who achieved either TICI 2b or 3 flow after the initial strategy only was 63% with aspiration and 68% with clot removal, also a nonsignificant difference. The two strategies also showed no significant difference for any measured safety parameter. The results showed a trend toward more vasospasm with clot removal – a 6% rate, versus 3% with clot aspiration – but this did not reach statistical significance.
Results from additional analyses of the clinical outcomes of patients in the trial and of cost efficacy will be reported later in 2017, Dr. Lapergue said.
ASTER received an unrestricted research grant from Penumbra, a company that markets clot removal aspiration catheters. Dr. Lapergue had no personal disclosures. Dr. Hanel has been a consultant to and received grant support from Medtronic. He has received research grants from MicroVention and has an ownership interest in InNeuroCo.
[email protected]
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Recanalization occurred in 85% of patients treated with aspiration first and 83% treated with clot removal first.
Data source: ASTER, a multicenter, randomized French trial with 381 patients.
Disclosures: ASTER received an unrestricted research grant from Penumbra, a company that markets clot removal aspiration catheters. Dr. Lapergue had no personal disclosures. Dr. Hanel has been a consultant to and received grant support from Medtronic. He has received research grants from MicroVention and has an ownership interest in InNeuroCo.
Low-volume PEG linked to hypokalemia in at-risk patients
FROM GASTROINTESTINAL ENDOSCOPY
Bowel preparation with low-volume polyethylene glycol led to hypokalemia in nearly 25% of high-risk patients who were normokalemic at baseline, according to a first-in-kind large single-center prospective study.
“Hypokalemia is frequently encountered after low-volume PEG bowel cleansing in high-risk patients,” wrote Ankie Reumkens, MD, and her associates at Maastricht University Medical Center, Maastricht, the Netherlands. The report was published online in Gastrointestinal Endoscopy. “Additional large-scale studies are needed on the prevalence of hypokalemia in nonselected populations undergoing bowel cleansing and on the occurrence of potentially very serious side effects in order to decide on screening of high-risk groups in daily clinical practice.”
Good bowel preparation is crucial to colonoscopy. Bowel preparation with both sodium phosphate and high-volume polyethylene glycol (PEG) has caused hypokalemia, but whether this is true of low-volume PEG is unclear, the investigators said. Recently, at their institution, two colonoscopy patients developed severe hypokalemia and died of ventricular arrhythmias after receiving low-volume PEG. These deaths spurred the researchers to prospectively study 1,822 colonoscopy patients who underwent bowel preparation with low-volume PEG in 2014 and who were considered at high risk of hypokalemia by their gastroenterologists or because of hospitalization or diuretic use.
The researchers measured serum potassium levels of all patients before bowel cleansing. After bowel testing, they retested a subgroup of 301 patients who were normokalemic (3.5-5 mmol/L) at baseline (Gastrointest Endosc. 2017 Feb 7. doi: 10.1016/j.gie.2017.01.040).
In all, 77 patients (4%) were hypokalemic before bowel cleansing, the researchers said. Fully one-third were hospitalized, and hospitalization remained a significant risk factor for baseline hypokalemia even after the researchers controlled for diuretic use, age, sex, and reason for colonoscopy (odds ratio, 2.5; 95% confidence interval, 1.5 to 4.2; P less than .001).
Follow-up testing showed that 71 patients (24%) who were normokalemic at baseline became hypokalemic (serum potassium less than 3.5 mmol/L) after bowel preparation with low-volume PEG. Only diuretic use remained significantly associated with this outcome after researchers accounted for age, sex, reason for colonoscopy, and hospitalization status (odds ratio, 2.3; 95% confidence interval, 1.3 to 4.0; P = .004).
This study included preselected groups of diuretic users and hospitalized patients, making it difficult to assess specific and detailed risk factors for hypokalemia, the researchers said. “Despite this limitation, our study clearly shows that hypokalemia may develop in a substantial percentage of patients after the ingestion of low-volume PEG,” they emphasized. But they recommended population-based studies to determine the true prevalence of hypokalemia after colonoscopy, examine risk factors for this outcome, and consider whether it makes sense to screen subgroups at risk.
The protocol at their hospital is to measure serum potassium before bowel cleansing in hospitalized patients and those on diuretics, they noted. Hypokalemic patients then receive oral potassium if their potassium level was 2.5-3.0 mmol/L, and intravenous potassium if their level was below 2.5 mmol/L.
The investigators reported having no funding sources and no competing interests.
FROM GASTROINTESTINAL ENDOSCOPY
Bowel preparation with low-volume polyethylene glycol led to hypokalemia in nearly 25% of high-risk patients who were normokalemic at baseline, according to a first-in-kind large single-center prospective study.
“Hypokalemia is frequently encountered after low-volume PEG bowel cleansing in high-risk patients,” wrote Ankie Reumkens, MD, and her associates at Maastricht University Medical Center, Maastricht, the Netherlands. The report was published online in Gastrointestinal Endoscopy. “Additional large-scale studies are needed on the prevalence of hypokalemia in nonselected populations undergoing bowel cleansing and on the occurrence of potentially very serious side effects in order to decide on screening of high-risk groups in daily clinical practice.”
Good bowel preparation is crucial to colonoscopy. Bowel preparation with both sodium phosphate and high-volume polyethylene glycol (PEG) has caused hypokalemia, but whether this is true of low-volume PEG is unclear, the investigators said. Recently, at their institution, two colonoscopy patients developed severe hypokalemia and died of ventricular arrhythmias after receiving low-volume PEG. These deaths spurred the researchers to prospectively study 1,822 colonoscopy patients who underwent bowel preparation with low-volume PEG in 2014 and who were considered at high risk of hypokalemia by their gastroenterologists or because of hospitalization or diuretic use.
The researchers measured serum potassium levels of all patients before bowel cleansing. After bowel testing, they retested a subgroup of 301 patients who were normokalemic (3.5-5 mmol/L) at baseline (Gastrointest Endosc. 2017 Feb 7. doi: 10.1016/j.gie.2017.01.040).
In all, 77 patients (4%) were hypokalemic before bowel cleansing, the researchers said. Fully one-third were hospitalized, and hospitalization remained a significant risk factor for baseline hypokalemia even after the researchers controlled for diuretic use, age, sex, and reason for colonoscopy (odds ratio, 2.5; 95% confidence interval, 1.5 to 4.2; P less than .001).
Follow-up testing showed that 71 patients (24%) who were normokalemic at baseline became hypokalemic (serum potassium less than 3.5 mmol/L) after bowel preparation with low-volume PEG. Only diuretic use remained significantly associated with this outcome after researchers accounted for age, sex, reason for colonoscopy, and hospitalization status (odds ratio, 2.3; 95% confidence interval, 1.3 to 4.0; P = .004).
This study included preselected groups of diuretic users and hospitalized patients, making it difficult to assess specific and detailed risk factors for hypokalemia, the researchers said. “Despite this limitation, our study clearly shows that hypokalemia may develop in a substantial percentage of patients after the ingestion of low-volume PEG,” they emphasized. But they recommended population-based studies to determine the true prevalence of hypokalemia after colonoscopy, examine risk factors for this outcome, and consider whether it makes sense to screen subgroups at risk.
The protocol at their hospital is to measure serum potassium before bowel cleansing in hospitalized patients and those on diuretics, they noted. Hypokalemic patients then receive oral potassium if their potassium level was 2.5-3.0 mmol/L, and intravenous potassium if their level was below 2.5 mmol/L.
The investigators reported having no funding sources and no competing interests.
FROM GASTROINTESTINAL ENDOSCOPY
Bowel preparation with low-volume polyethylene glycol led to hypokalemia in nearly 25% of high-risk patients who were normokalemic at baseline, according to a first-in-kind large single-center prospective study.
“Hypokalemia is frequently encountered after low-volume PEG bowel cleansing in high-risk patients,” wrote Ankie Reumkens, MD, and her associates at Maastricht University Medical Center, Maastricht, the Netherlands. The report was published online in Gastrointestinal Endoscopy. “Additional large-scale studies are needed on the prevalence of hypokalemia in nonselected populations undergoing bowel cleansing and on the occurrence of potentially very serious side effects in order to decide on screening of high-risk groups in daily clinical practice.”
Good bowel preparation is crucial to colonoscopy. Bowel preparation with both sodium phosphate and high-volume polyethylene glycol (PEG) has caused hypokalemia, but whether this is true of low-volume PEG is unclear, the investigators said. Recently, at their institution, two colonoscopy patients developed severe hypokalemia and died of ventricular arrhythmias after receiving low-volume PEG. These deaths spurred the researchers to prospectively study 1,822 colonoscopy patients who underwent bowel preparation with low-volume PEG in 2014 and who were considered at high risk of hypokalemia by their gastroenterologists or because of hospitalization or diuretic use.
The researchers measured serum potassium levels of all patients before bowel cleansing. After bowel testing, they retested a subgroup of 301 patients who were normokalemic (3.5-5 mmol/L) at baseline (Gastrointest Endosc. 2017 Feb 7. doi: 10.1016/j.gie.2017.01.040).
In all, 77 patients (4%) were hypokalemic before bowel cleansing, the researchers said. Fully one-third were hospitalized, and hospitalization remained a significant risk factor for baseline hypokalemia even after the researchers controlled for diuretic use, age, sex, and reason for colonoscopy (odds ratio, 2.5; 95% confidence interval, 1.5 to 4.2; P less than .001).
Follow-up testing showed that 71 patients (24%) who were normokalemic at baseline became hypokalemic (serum potassium less than 3.5 mmol/L) after bowel preparation with low-volume PEG. Only diuretic use remained significantly associated with this outcome after researchers accounted for age, sex, reason for colonoscopy, and hospitalization status (odds ratio, 2.3; 95% confidence interval, 1.3 to 4.0; P = .004).
This study included preselected groups of diuretic users and hospitalized patients, making it difficult to assess specific and detailed risk factors for hypokalemia, the researchers said. “Despite this limitation, our study clearly shows that hypokalemia may develop in a substantial percentage of patients after the ingestion of low-volume PEG,” they emphasized. But they recommended population-based studies to determine the true prevalence of hypokalemia after colonoscopy, examine risk factors for this outcome, and consider whether it makes sense to screen subgroups at risk.
The protocol at their hospital is to measure serum potassium before bowel cleansing in hospitalized patients and those on diuretics, they noted. Hypokalemic patients then receive oral potassium if their potassium level was 2.5-3.0 mmol/L, and intravenous potassium if their level was below 2.5 mmol/L.
The investigators reported having no funding sources and no competing interests.
Key clinical point. Bowel preparation with low-volume polyethylene glycol (PEG) led to hypokalemia in at-risk patients.
Major finding: In all, 24% of patients who were normokalemic before bowel cleansing developed hypokalemia afterward. Diuretic use was a significant risk factor for hypokalemia (odds ratio, 2.3; P = .004).
Data source: A prospective study of 1,822 colonoscopy patients considered at high risk of hypokalemia.
Disclosures: The investigators reported having no funding sources and no competing interests.
Unrestricted DAA access halved Dutch HCV incidence in HIV
SEATTLE – New hepatitis C infections among HIV-positive men who have sex with men (MSM) were halved in the Netherlands by unrestricted access to direct-acting antivirals (DAAs), primarily ledipasvir/sofosbuvir tablets (Harvoni), according to Dutch investigators.
Since 2015, DAAs have been available to all newly acquired hepatitis C virus (HCV) patients without restriction. Due to the high cost of the drugs, payers in the United States and some other Western countries limit access to only patients with severe liver disease.*
The Dutch government, however, requires insurers to cover them, and has negotiated price discounts with makers. “The price that is paid is secret,” but it’s less than the standard cost of, for instance, €45,000 for a 3-month course of [ledipasvir/sofosbuvir] in the Netherlands, said senior investigator Bart Rijnders, MD, an infectious diseases assistant professor at Erasmus University Medical Center, Rotterdam.
The study compared the incidence of acute HCV (aHCV) in HIV-positive MSM in 2014, before unrestricted access to DAAs, to the incidence of aHCV in 2016, after limits were lifted. The investigators used data from 18 HIV treatment centers spread across the Netherlands, capturing about 80% of Dutch MSM being treated for HIV.
In 2014, there were 93 aHCV infections diagnosed among the men, translating to an incidence of 11.2 cases per 1,000 person-years of follow-up (95% CI, 9.1-13.7 cases). In 2016, there were 49 aHCV cases, an incidence of 5.5 cases per 1,000 person-years (95% CI, 4.1–7.2, P less than .001). At the same time, there was a substantial increase in new syphilis cases, indicating that the 51% reduction in aHCV over 2 years was not due to changes in behavior.
Meanwhile, “within 14 months after these drugs became available to all, 75% of the HIV-positive MSM in the Netherlands were cured of their infection,” Dr. Rijnders said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Ledipasvir/sofosbuvir was the DAA used by about 90% of the men.
In short, unrestricted access to DAAs wiped out the infection so that men were no longer passing it to other men. The results are “an example of what is possible if you search for HCV and treat it as soon as you find it. You cure patients and prevent new infections. In the long run, you may save money,” he said, especially as more DAA options come on the market and prices fall.
Almost all the subjects were seen in their HIV clinic at least twice a year. An uptick in liver enzymes triggered HCV testing. The investigators checked positive results against patients’ own stored blood samples to distinguish new from chronic infections.
The study wasn’t funded, but Dr. Rijnders is a paid researcher for Merck’s DAA option, elbasvir/grazoprevir (Zepatier).
*This story was updated on February 24, 2017.
This is the first proof that early treatment of acute HCV could be a form of prevention. By removing the fibrosis requirement and restrictions forbidding treatment of people who are actively engaged in high-risk behaviors, they are reducing new infections.
We are far behind in the United States; 90% of states have restrictions that don’t allow uniform uptake of hepatitis C treatment and many forbid treatment for people who are actively using drugs. Having restrictions on people that don’t have enough liver disease is like telling a person with HIV they can’t be treated because their CD4 count isn’t below 200.
David Thomas, MD, is professor of medicine and director of the division of infectious diseases at Johns Hopkins University, Baltimore. He wasn’t involved with the work.
This is the first proof that early treatment of acute HCV could be a form of prevention. By removing the fibrosis requirement and restrictions forbidding treatment of people who are actively engaged in high-risk behaviors, they are reducing new infections.
We are far behind in the United States; 90% of states have restrictions that don’t allow uniform uptake of hepatitis C treatment and many forbid treatment for people who are actively using drugs. Having restrictions on people that don’t have enough liver disease is like telling a person with HIV they can’t be treated because their CD4 count isn’t below 200.
David Thomas, MD, is professor of medicine and director of the division of infectious diseases at Johns Hopkins University, Baltimore. He wasn’t involved with the work.
This is the first proof that early treatment of acute HCV could be a form of prevention. By removing the fibrosis requirement and restrictions forbidding treatment of people who are actively engaged in high-risk behaviors, they are reducing new infections.
We are far behind in the United States; 90% of states have restrictions that don’t allow uniform uptake of hepatitis C treatment and many forbid treatment for people who are actively using drugs. Having restrictions on people that don’t have enough liver disease is like telling a person with HIV they can’t be treated because their CD4 count isn’t below 200.
David Thomas, MD, is professor of medicine and director of the division of infectious diseases at Johns Hopkins University, Baltimore. He wasn’t involved with the work.
SEATTLE – New hepatitis C infections among HIV-positive men who have sex with men (MSM) were halved in the Netherlands by unrestricted access to direct-acting antivirals (DAAs), primarily ledipasvir/sofosbuvir tablets (Harvoni), according to Dutch investigators.
Since 2015, DAAs have been available to all newly acquired hepatitis C virus (HCV) patients without restriction. Due to the high cost of the drugs, payers in the United States and some other Western countries limit access to only patients with severe liver disease.*
The Dutch government, however, requires insurers to cover them, and has negotiated price discounts with makers. “The price that is paid is secret,” but it’s less than the standard cost of, for instance, €45,000 for a 3-month course of [ledipasvir/sofosbuvir] in the Netherlands, said senior investigator Bart Rijnders, MD, an infectious diseases assistant professor at Erasmus University Medical Center, Rotterdam.
The study compared the incidence of acute HCV (aHCV) in HIV-positive MSM in 2014, before unrestricted access to DAAs, to the incidence of aHCV in 2016, after limits were lifted. The investigators used data from 18 HIV treatment centers spread across the Netherlands, capturing about 80% of Dutch MSM being treated for HIV.
In 2014, there were 93 aHCV infections diagnosed among the men, translating to an incidence of 11.2 cases per 1,000 person-years of follow-up (95% CI, 9.1-13.7 cases). In 2016, there were 49 aHCV cases, an incidence of 5.5 cases per 1,000 person-years (95% CI, 4.1–7.2, P less than .001). At the same time, there was a substantial increase in new syphilis cases, indicating that the 51% reduction in aHCV over 2 years was not due to changes in behavior.
Meanwhile, “within 14 months after these drugs became available to all, 75% of the HIV-positive MSM in the Netherlands were cured of their infection,” Dr. Rijnders said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Ledipasvir/sofosbuvir was the DAA used by about 90% of the men.
In short, unrestricted access to DAAs wiped out the infection so that men were no longer passing it to other men. The results are “an example of what is possible if you search for HCV and treat it as soon as you find it. You cure patients and prevent new infections. In the long run, you may save money,” he said, especially as more DAA options come on the market and prices fall.
Almost all the subjects were seen in their HIV clinic at least twice a year. An uptick in liver enzymes triggered HCV testing. The investigators checked positive results against patients’ own stored blood samples to distinguish new from chronic infections.
The study wasn’t funded, but Dr. Rijnders is a paid researcher for Merck’s DAA option, elbasvir/grazoprevir (Zepatier).
*This story was updated on February 24, 2017.
SEATTLE – New hepatitis C infections among HIV-positive men who have sex with men (MSM) were halved in the Netherlands by unrestricted access to direct-acting antivirals (DAAs), primarily ledipasvir/sofosbuvir tablets (Harvoni), according to Dutch investigators.
Since 2015, DAAs have been available to all newly acquired hepatitis C virus (HCV) patients without restriction. Due to the high cost of the drugs, payers in the United States and some other Western countries limit access to only patients with severe liver disease.*
The Dutch government, however, requires insurers to cover them, and has negotiated price discounts with makers. “The price that is paid is secret,” but it’s less than the standard cost of, for instance, €45,000 for a 3-month course of [ledipasvir/sofosbuvir] in the Netherlands, said senior investigator Bart Rijnders, MD, an infectious diseases assistant professor at Erasmus University Medical Center, Rotterdam.
The study compared the incidence of acute HCV (aHCV) in HIV-positive MSM in 2014, before unrestricted access to DAAs, to the incidence of aHCV in 2016, after limits were lifted. The investigators used data from 18 HIV treatment centers spread across the Netherlands, capturing about 80% of Dutch MSM being treated for HIV.
In 2014, there were 93 aHCV infections diagnosed among the men, translating to an incidence of 11.2 cases per 1,000 person-years of follow-up (95% CI, 9.1-13.7 cases). In 2016, there were 49 aHCV cases, an incidence of 5.5 cases per 1,000 person-years (95% CI, 4.1–7.2, P less than .001). At the same time, there was a substantial increase in new syphilis cases, indicating that the 51% reduction in aHCV over 2 years was not due to changes in behavior.
Meanwhile, “within 14 months after these drugs became available to all, 75% of the HIV-positive MSM in the Netherlands were cured of their infection,” Dr. Rijnders said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Ledipasvir/sofosbuvir was the DAA used by about 90% of the men.
In short, unrestricted access to DAAs wiped out the infection so that men were no longer passing it to other men. The results are “an example of what is possible if you search for HCV and treat it as soon as you find it. You cure patients and prevent new infections. In the long run, you may save money,” he said, especially as more DAA options come on the market and prices fall.
Almost all the subjects were seen in their HIV clinic at least twice a year. An uptick in liver enzymes triggered HCV testing. The investigators checked positive results against patients’ own stored blood samples to distinguish new from chronic infections.
The study wasn’t funded, but Dr. Rijnders is a paid researcher for Merck’s DAA option, elbasvir/grazoprevir (Zepatier).
*This story was updated on February 24, 2017.
AT CROI
Key clinical point:
Major finding: In 2014, there were 93 acute HCV infections diagnosed among HIV-positive men who have sex with men, translating to an incidence of 11.2 cases per 1,000 person-years of follow-up (95% CI, 9.1-13.7 cases). In 2016, there were 49 cases, an incidence of 5.5 cases per 1,000 person years (95% CI, 4.1–7.2, P less than .001).
Data source: HIV treatment centers in the Netherlands
Disclosures: The study wasn’t funded, but the senior investigator is a paid researcher for Merck’s DAA option, elbasvir/grazoprevir (Zepatier).
Sarcoidosis doubles hospitalized infection risk
Persons with sarcoidosis were found to have double the risk of hospitalization, compared with age-matched controls in a population-based cohort study that also linked glucocorticoid use with an increased risk of hospitalization in this group.
Using data from the Rochester Epidemiology Project record-linkage system, Patompong Ungprasert, MD, an assistant professor of medicine at the Mayo Clinic in Rochester, Minn., and his colleagues identified 345 incident cases of sarcoidosis recorded between 1976 and 2013, confirmed by individual medical records (Ann Am Thorac Soc. 2017 Feb 8. doi: 10.1513/AnnalsATS.201610-750OC). Using random selection, each patient was age- and sex-matched with sarcoidosis-free controls taken from the same database. Medical records across the study were examined for community-acquired infections requiring hospitalization that occurred after the index date or the date of diagnosis.
Dr. Ungprasert and his coinvestigators found that those with sarcoidosis had double the risk of all forms of specific hospitalized infection when compared with controls – a 2.00 hazard ratio (95% confidence interval, 1.41-2.84). The results were similar when adjusted for infection risk factors: 2.13 HR (95% CI, 1.35-3.34).
The risk of hospitalized infection in the sarcoidosis arm was higher than in controls regardless of disease stage: an HR of 1.70 (95% CI, 1.12-2.58, P = .013) in those with Stage I; an HR of 2.00 (95% CI, 1.22-3.29, P = .006) among those with stage II; and an HR of 2.63 (95% CI, 1.58-4.39, P less than .001) in those with Stage III and Stage IV disease.
Biopsies taken in 251 cases resulted in 229 positive results for noncaseating granuloma, and just over half of patients had stage I disease. Stage II disease was found in 29%, Stage III in 15%, and Stage IV in 2%.
Patients in the sarcoidosis group who had not been exposed to immunosuppressive treatment had significantly higher risk of hospitalization with an HR of 1.73 (95% CI, 1.16-2.60; P = .008) when compared with controls. The risk was even higher in study patients who had received immunosuppressive therapy: an HR of 2.41 (95% CI, 1.60-3.64; P less than .001), when compared with controls. Less than half of all sarcoidosis patients required immunosuppressive therapy at any point during follow-up: about 37% by year 30 after original diagnosis. Oral glucocorticoids were the most commonly prescribed medication, used in 113 cases.
A baseline diffusing capacity of the lung for carbon monoxide was associated with an overall increased risk of hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in diffusing capacity of the lung for carbon monoxide (95% CI, 1.01-1.32). A baseline forced vital capacity was associated with an increased hospitalized pneumonia risk with an HR of 1.15 per decrease of 10% predicted in forced vital capacity (95% CI, 1.01-1.32).
Although the use of immunosuppressive agents was not significantly associated with the risk of hospitalized infection (HR, 1.43; 95% CI, 0.94-2.19), current use of oral glucocorticoids, whether alone or as adjunct to immunosuppressive therapy, significantly predicted risk of infection in patients with sarcoidosis, with an HR of 3.03 (95% CI, 1.33-6.90) for oral glucocorticoids up to 10 mg per day, and an HR of 4.48 (95% CI, 1.33-6.90) in patients taking oral glucocorticoids at more than 10 mg per day, when compared with controls.
In an interview, Dr. Ungprasert said the results were not surprising, but provided the following takeaways from this study for physicians caring for patients with sarcoidosis.
“These patients are at an increased risk of serious infection and should seek medical attention as soon as possible when they develop symptoms of infection, such as fever or chills,” he said in an interview. “Keeping current with vaccinations is also important for them.”
Dr. Ungprasert also said the study serves as a reminder to use oral glucocorticoids judiciously. “When considering their use, the physician should keep in mind that a large number of patients with sarcoidosis will have a spontaneous resolution of the disease.”
There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
[email protected]
On Twitter @whitneymcknight
Persons with sarcoidosis were found to have double the risk of hospitalization, compared with age-matched controls in a population-based cohort study that also linked glucocorticoid use with an increased risk of hospitalization in this group.
Using data from the Rochester Epidemiology Project record-linkage system, Patompong Ungprasert, MD, an assistant professor of medicine at the Mayo Clinic in Rochester, Minn., and his colleagues identified 345 incident cases of sarcoidosis recorded between 1976 and 2013, confirmed by individual medical records (Ann Am Thorac Soc. 2017 Feb 8. doi: 10.1513/AnnalsATS.201610-750OC). Using random selection, each patient was age- and sex-matched with sarcoidosis-free controls taken from the same database. Medical records across the study were examined for community-acquired infections requiring hospitalization that occurred after the index date or the date of diagnosis.
Dr. Ungprasert and his coinvestigators found that those with sarcoidosis had double the risk of all forms of specific hospitalized infection when compared with controls – a 2.00 hazard ratio (95% confidence interval, 1.41-2.84). The results were similar when adjusted for infection risk factors: 2.13 HR (95% CI, 1.35-3.34).
The risk of hospitalized infection in the sarcoidosis arm was higher than in controls regardless of disease stage: an HR of 1.70 (95% CI, 1.12-2.58, P = .013) in those with Stage I; an HR of 2.00 (95% CI, 1.22-3.29, P = .006) among those with stage II; and an HR of 2.63 (95% CI, 1.58-4.39, P less than .001) in those with Stage III and Stage IV disease.
Biopsies taken in 251 cases resulted in 229 positive results for noncaseating granuloma, and just over half of patients had stage I disease. Stage II disease was found in 29%, Stage III in 15%, and Stage IV in 2%.
Patients in the sarcoidosis group who had not been exposed to immunosuppressive treatment had significantly higher risk of hospitalization with an HR of 1.73 (95% CI, 1.16-2.60; P = .008) when compared with controls. The risk was even higher in study patients who had received immunosuppressive therapy: an HR of 2.41 (95% CI, 1.60-3.64; P less than .001), when compared with controls. Less than half of all sarcoidosis patients required immunosuppressive therapy at any point during follow-up: about 37% by year 30 after original diagnosis. Oral glucocorticoids were the most commonly prescribed medication, used in 113 cases.
A baseline diffusing capacity of the lung for carbon monoxide was associated with an overall increased risk of hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in diffusing capacity of the lung for carbon monoxide (95% CI, 1.01-1.32). A baseline forced vital capacity was associated with an increased hospitalized pneumonia risk with an HR of 1.15 per decrease of 10% predicted in forced vital capacity (95% CI, 1.01-1.32).
Although the use of immunosuppressive agents was not significantly associated with the risk of hospitalized infection (HR, 1.43; 95% CI, 0.94-2.19), current use of oral glucocorticoids, whether alone or as adjunct to immunosuppressive therapy, significantly predicted risk of infection in patients with sarcoidosis, with an HR of 3.03 (95% CI, 1.33-6.90) for oral glucocorticoids up to 10 mg per day, and an HR of 4.48 (95% CI, 1.33-6.90) in patients taking oral glucocorticoids at more than 10 mg per day, when compared with controls.
In an interview, Dr. Ungprasert said the results were not surprising, but provided the following takeaways from this study for physicians caring for patients with sarcoidosis.
“These patients are at an increased risk of serious infection and should seek medical attention as soon as possible when they develop symptoms of infection, such as fever or chills,” he said in an interview. “Keeping current with vaccinations is also important for them.”
Dr. Ungprasert also said the study serves as a reminder to use oral glucocorticoids judiciously. “When considering their use, the physician should keep in mind that a large number of patients with sarcoidosis will have a spontaneous resolution of the disease.”
There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
[email protected]
On Twitter @whitneymcknight
Persons with sarcoidosis were found to have double the risk of hospitalization, compared with age-matched controls in a population-based cohort study that also linked glucocorticoid use with an increased risk of hospitalization in this group.
Using data from the Rochester Epidemiology Project record-linkage system, Patompong Ungprasert, MD, an assistant professor of medicine at the Mayo Clinic in Rochester, Minn., and his colleagues identified 345 incident cases of sarcoidosis recorded between 1976 and 2013, confirmed by individual medical records (Ann Am Thorac Soc. 2017 Feb 8. doi: 10.1513/AnnalsATS.201610-750OC). Using random selection, each patient was age- and sex-matched with sarcoidosis-free controls taken from the same database. Medical records across the study were examined for community-acquired infections requiring hospitalization that occurred after the index date or the date of diagnosis.
Dr. Ungprasert and his coinvestigators found that those with sarcoidosis had double the risk of all forms of specific hospitalized infection when compared with controls – a 2.00 hazard ratio (95% confidence interval, 1.41-2.84). The results were similar when adjusted for infection risk factors: 2.13 HR (95% CI, 1.35-3.34).
The risk of hospitalized infection in the sarcoidosis arm was higher than in controls regardless of disease stage: an HR of 1.70 (95% CI, 1.12-2.58, P = .013) in those with Stage I; an HR of 2.00 (95% CI, 1.22-3.29, P = .006) among those with stage II; and an HR of 2.63 (95% CI, 1.58-4.39, P less than .001) in those with Stage III and Stage IV disease.
Biopsies taken in 251 cases resulted in 229 positive results for noncaseating granuloma, and just over half of patients had stage I disease. Stage II disease was found in 29%, Stage III in 15%, and Stage IV in 2%.
Patients in the sarcoidosis group who had not been exposed to immunosuppressive treatment had significantly higher risk of hospitalization with an HR of 1.73 (95% CI, 1.16-2.60; P = .008) when compared with controls. The risk was even higher in study patients who had received immunosuppressive therapy: an HR of 2.41 (95% CI, 1.60-3.64; P less than .001), when compared with controls. Less than half of all sarcoidosis patients required immunosuppressive therapy at any point during follow-up: about 37% by year 30 after original diagnosis. Oral glucocorticoids were the most commonly prescribed medication, used in 113 cases.
A baseline diffusing capacity of the lung for carbon monoxide was associated with an overall increased risk of hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in diffusing capacity of the lung for carbon monoxide (95% CI, 1.01-1.32). A baseline forced vital capacity was associated with an increased hospitalized pneumonia risk with an HR of 1.15 per decrease of 10% predicted in forced vital capacity (95% CI, 1.01-1.32).
Although the use of immunosuppressive agents was not significantly associated with the risk of hospitalized infection (HR, 1.43; 95% CI, 0.94-2.19), current use of oral glucocorticoids, whether alone or as adjunct to immunosuppressive therapy, significantly predicted risk of infection in patients with sarcoidosis, with an HR of 3.03 (95% CI, 1.33-6.90) for oral glucocorticoids up to 10 mg per day, and an HR of 4.48 (95% CI, 1.33-6.90) in patients taking oral glucocorticoids at more than 10 mg per day, when compared with controls.
In an interview, Dr. Ungprasert said the results were not surprising, but provided the following takeaways from this study for physicians caring for patients with sarcoidosis.
“These patients are at an increased risk of serious infection and should seek medical attention as soon as possible when they develop symptoms of infection, such as fever or chills,” he said in an interview. “Keeping current with vaccinations is also important for them.”
Dr. Ungprasert also said the study serves as a reminder to use oral glucocorticoids judiciously. “When considering their use, the physician should keep in mind that a large number of patients with sarcoidosis will have a spontaneous resolution of the disease.”
There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
[email protected]
On Twitter @whitneymcknight
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point:
Major finding: 345 patients with sarcoidosis had a hazard ratio of 2.00 (95% CI, 1.41-2.84) for hospitalized infection, compared with controls.
Data source: Olmsted County, Minn. epidemiology records from 1976 to 2013.
Disclosures: There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
New significantly mutated genes detected in prostate cancer
A new large study has identified 78 significantly mutated genes (SMGs) and has enlarged the genetic landscape of prostate cancer. In addition, 37 genes that were not previously reported as SMGs in prostate cancer and 23 that were not previously identified as recurrently altered in cancer, were identified.
“Through aggregation and uniform genomic analysis, we refined the map of somatic mutations in prostate cancer and identified cancer genes and pathways not previously associated with this disease,” said lead author Dr. Joshua Armenia, of the Memorial Sloan Kettering Cancer Center, New York.
“Our findings may inform patient stratification and translational investigation,” Dr. Armenia said in a press briefing held in 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The mutational landscapes of primary and metastatic prostate cancer have been robustly analyzed in multiple whole exome sequencing (WES) studies.
But prostate cancer is a very heterogenous disease, explained Dr. Armenia. Aggregation and uniform genomic analysis of larger cohorts can reveal significantly mutated genes and pathways in the “long tail” (1%-5% of cases).
The power to detect genes is significantly proportional to the number of samples used, and the size of the cohort. “Our knowledge of prostate cancer genes is incomplete,” said Dr. Armenia.
He and his colleagues hypothesized that an aggregate, uniform analysis of all data generated to date would enable discovery of new significantly mutated genes and pathways not previously associated with prostate cancer and shed more light onto the genetic differences between primary and metastatic prostate cancer.
They analyzed 918 tumors, with a total of 583 primary tumors and 335 metastasis, with a mutational significance analysis that uses statistical and biological approaches to determine which genes and pathways are recurrently altered.
This approach led to the identification of 78 SMGs, and 37 of the genes are novel prostate cancer genes implicated in other tumor types; SPEN, SETD2, ARID1A, CUL3 ARID2, SMARCAD1, U2AF1.
“These genes have never been implicated in prostate cancer but are found in other tumor types,” said Dr. Armenia. “We stratified them by pathway, and we found 21% of the tumors show mutations in epigenetic regulators and chromatin remodelers.”
The investigators found that 14% of these tumors show alterations in the ubiquitin pathway in prostate cancer. The novel mutations in CUL3 (M299R hotspot) are mutually exclusive with SPOP mutations.
“Unexpectedly, we also found alterations in splicing pathway alterations in prostate cancer,” Dr. Armenia noted.
There were 38 altered tumors (4% of the 918 total) that had alterations in genes such as U2AF1 (0.5%) and SF3B1 (1%), GEMINS (0.8%), TCERG1 (1.3%), and PRPFB (1.3%).
The researchers found another “interesting” gene, SPEN, which is a novel prostate cancer gene. It is a hormone regulator gene that has been described in breast cancer, where it is associated with tamoxifen resistance. Truncating mutations were found in SPEN, which is a hormone inducible transcriptional repressor, in 2.8% of samples which is a rate similar to the frequency observed in breast tumors. SPEN in prostate cancer may have a similar role to the one it has in breast cancer.
“We performed enrichment analysis of genomic alterations in metastatic tumors to be able to identify markers of advanced disease,” he explained. “And what we found that TP53, AR, PTEN, FOXA1, APC, and BRCA2 alterations are enriched in metastatic samples.”
Alterations in epigenetic regulators (KMT2C, KMT2D) are significantly enriched in metastatic tumors while SPOP mutations and FOXP1/RYBP deletions are enriched in primary tumors.
Dr. Armenia and colleagues also identified a subclass of epigenetically mutated prostate cancer, representing 21% of prostate cancers and insignificantly enriched tumors lacking an ETS fusion.
“We discovered novel pathways in prostate cancer including SW1/SNF and splicing, as well as novel prostate cancer genes that include CUL3 and SPEN, and a set of genomic markers enriched in advanced disease,” he concluded.
A new large study has identified 78 significantly mutated genes (SMGs) and has enlarged the genetic landscape of prostate cancer. In addition, 37 genes that were not previously reported as SMGs in prostate cancer and 23 that were not previously identified as recurrently altered in cancer, were identified.
“Through aggregation and uniform genomic analysis, we refined the map of somatic mutations in prostate cancer and identified cancer genes and pathways not previously associated with this disease,” said lead author Dr. Joshua Armenia, of the Memorial Sloan Kettering Cancer Center, New York.
“Our findings may inform patient stratification and translational investigation,” Dr. Armenia said in a press briefing held in 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The mutational landscapes of primary and metastatic prostate cancer have been robustly analyzed in multiple whole exome sequencing (WES) studies.
But prostate cancer is a very heterogenous disease, explained Dr. Armenia. Aggregation and uniform genomic analysis of larger cohorts can reveal significantly mutated genes and pathways in the “long tail” (1%-5% of cases).
The power to detect genes is significantly proportional to the number of samples used, and the size of the cohort. “Our knowledge of prostate cancer genes is incomplete,” said Dr. Armenia.
He and his colleagues hypothesized that an aggregate, uniform analysis of all data generated to date would enable discovery of new significantly mutated genes and pathways not previously associated with prostate cancer and shed more light onto the genetic differences between primary and metastatic prostate cancer.
They analyzed 918 tumors, with a total of 583 primary tumors and 335 metastasis, with a mutational significance analysis that uses statistical and biological approaches to determine which genes and pathways are recurrently altered.
This approach led to the identification of 78 SMGs, and 37 of the genes are novel prostate cancer genes implicated in other tumor types; SPEN, SETD2, ARID1A, CUL3 ARID2, SMARCAD1, U2AF1.
“These genes have never been implicated in prostate cancer but are found in other tumor types,” said Dr. Armenia. “We stratified them by pathway, and we found 21% of the tumors show mutations in epigenetic regulators and chromatin remodelers.”
The investigators found that 14% of these tumors show alterations in the ubiquitin pathway in prostate cancer. The novel mutations in CUL3 (M299R hotspot) are mutually exclusive with SPOP mutations.
“Unexpectedly, we also found alterations in splicing pathway alterations in prostate cancer,” Dr. Armenia noted.
There were 38 altered tumors (4% of the 918 total) that had alterations in genes such as U2AF1 (0.5%) and SF3B1 (1%), GEMINS (0.8%), TCERG1 (1.3%), and PRPFB (1.3%).
The researchers found another “interesting” gene, SPEN, which is a novel prostate cancer gene. It is a hormone regulator gene that has been described in breast cancer, where it is associated with tamoxifen resistance. Truncating mutations were found in SPEN, which is a hormone inducible transcriptional repressor, in 2.8% of samples which is a rate similar to the frequency observed in breast tumors. SPEN in prostate cancer may have a similar role to the one it has in breast cancer.
“We performed enrichment analysis of genomic alterations in metastatic tumors to be able to identify markers of advanced disease,” he explained. “And what we found that TP53, AR, PTEN, FOXA1, APC, and BRCA2 alterations are enriched in metastatic samples.”
Alterations in epigenetic regulators (KMT2C, KMT2D) are significantly enriched in metastatic tumors while SPOP mutations and FOXP1/RYBP deletions are enriched in primary tumors.
Dr. Armenia and colleagues also identified a subclass of epigenetically mutated prostate cancer, representing 21% of prostate cancers and insignificantly enriched tumors lacking an ETS fusion.
“We discovered novel pathways in prostate cancer including SW1/SNF and splicing, as well as novel prostate cancer genes that include CUL3 and SPEN, and a set of genomic markers enriched in advanced disease,” he concluded.
A new large study has identified 78 significantly mutated genes (SMGs) and has enlarged the genetic landscape of prostate cancer. In addition, 37 genes that were not previously reported as SMGs in prostate cancer and 23 that were not previously identified as recurrently altered in cancer, were identified.
“Through aggregation and uniform genomic analysis, we refined the map of somatic mutations in prostate cancer and identified cancer genes and pathways not previously associated with this disease,” said lead author Dr. Joshua Armenia, of the Memorial Sloan Kettering Cancer Center, New York.
“Our findings may inform patient stratification and translational investigation,” Dr. Armenia said in a press briefing held in 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The mutational landscapes of primary and metastatic prostate cancer have been robustly analyzed in multiple whole exome sequencing (WES) studies.
But prostate cancer is a very heterogenous disease, explained Dr. Armenia. Aggregation and uniform genomic analysis of larger cohorts can reveal significantly mutated genes and pathways in the “long tail” (1%-5% of cases).
The power to detect genes is significantly proportional to the number of samples used, and the size of the cohort. “Our knowledge of prostate cancer genes is incomplete,” said Dr. Armenia.
He and his colleagues hypothesized that an aggregate, uniform analysis of all data generated to date would enable discovery of new significantly mutated genes and pathways not previously associated with prostate cancer and shed more light onto the genetic differences between primary and metastatic prostate cancer.
They analyzed 918 tumors, with a total of 583 primary tumors and 335 metastasis, with a mutational significance analysis that uses statistical and biological approaches to determine which genes and pathways are recurrently altered.
This approach led to the identification of 78 SMGs, and 37 of the genes are novel prostate cancer genes implicated in other tumor types; SPEN, SETD2, ARID1A, CUL3 ARID2, SMARCAD1, U2AF1.
“These genes have never been implicated in prostate cancer but are found in other tumor types,” said Dr. Armenia. “We stratified them by pathway, and we found 21% of the tumors show mutations in epigenetic regulators and chromatin remodelers.”
The investigators found that 14% of these tumors show alterations in the ubiquitin pathway in prostate cancer. The novel mutations in CUL3 (M299R hotspot) are mutually exclusive with SPOP mutations.
“Unexpectedly, we also found alterations in splicing pathway alterations in prostate cancer,” Dr. Armenia noted.
There were 38 altered tumors (4% of the 918 total) that had alterations in genes such as U2AF1 (0.5%) and SF3B1 (1%), GEMINS (0.8%), TCERG1 (1.3%), and PRPFB (1.3%).
The researchers found another “interesting” gene, SPEN, which is a novel prostate cancer gene. It is a hormone regulator gene that has been described in breast cancer, where it is associated with tamoxifen resistance. Truncating mutations were found in SPEN, which is a hormone inducible transcriptional repressor, in 2.8% of samples which is a rate similar to the frequency observed in breast tumors. SPEN in prostate cancer may have a similar role to the one it has in breast cancer.
“We performed enrichment analysis of genomic alterations in metastatic tumors to be able to identify markers of advanced disease,” he explained. “And what we found that TP53, AR, PTEN, FOXA1, APC, and BRCA2 alterations are enriched in metastatic samples.”
Alterations in epigenetic regulators (KMT2C, KMT2D) are significantly enriched in metastatic tumors while SPOP mutations and FOXP1/RYBP deletions are enriched in primary tumors.
Dr. Armenia and colleagues also identified a subclass of epigenetically mutated prostate cancer, representing 21% of prostate cancers and insignificantly enriched tumors lacking an ETS fusion.
“We discovered novel pathways in prostate cancer including SW1/SNF and splicing, as well as novel prostate cancer genes that include CUL3 and SPEN, and a set of genomic markers enriched in advanced disease,” he concluded.
Key clinical point: New findings on prostate cancer genetics may help inform patient stratification and translational research.
Major finding: A total of 78 significantly mutated genes were identified in prostate cancer along with 37 genes that were not previously reported as significantly mutated.
Data source: An experimental study that analyzed 918 tumors obtained from prostate cancer patients.
Disclosures: The study does not list a funding source. Dr. Armenia has no disclosures but several coauthors report relationships with industry.