User login
VIDEO: Bacterial DNA predicted infections associated with prednisolone in severe alcoholic hepatitis
High baseline levels of circulating bacterial DNA increased the odds of serious infections by nearly fivefold in patients receiving prednisolone for severe alcoholic hepatitis, even after controlling for MELD score and white blood cell count, investigators reported in the April issue of Gastroenterology (2016 Dec 31. doi: 10.1053/j.gastro.2016.08.029).
“Patients with severe alcoholic hepatitis given prednisolone are at greater risk for developing serious infections and infections after treatment than patients not given prednisolone, which may offset its therapeutic benefit,” Nikhil Vergis, MD, and his associates wrote in Gastroenterology. “Level of circulating bacterial DNA before treatment could identify patients at high risk of infection if given prednisolone, which could be used to select therapies for patients with severe alcoholic hepatitis.”
To further explore rates and predictors of infections in STOPAH, the researchers analyzed longitudinal data on incident infections for 1,092 trial participants who received either prednisolone (40 mg daily) or pentoxifylline (400 mg three times daily). For 731 patients, they also examined whether baseline circulating levels of 16s ribosomal bacterial DNA were associated with infections.
A total of 135 patients (12%) had an infection at baseline, 251 (23%) developed infections during treatment, and 89 (8%) developed infections after treatment, the investigators reported. Prednisolone therapy was not associated with infections during treatment, but was associated with a nearly 30% rise in the odds of serious posttreatment infections compared with pentoxifylline (odds ratio, 1.27; 95% confidence interval, 1.27-2.92; P = .002). Prednisolone recipients who developed infections were significantly more likely to die within 90 days than those who did not, even after controlling for end-stage liver disease or Lille score (OR, 2.5; 95% CI, 1.4-4.3; P = .002). Antibiotic therapy appeared to significantly reduce the risk of mortality among infected prednisolone recipients (13% vs. 52%; OR, 0.13; 95% CI 0.04-0.47; P = .002).
There was “a striking association between bacterial DNA and the development of infection within 7 days in patients treated with prednisolone,” the researchers reported. These patients had a median baseline circulating DNA level of 20.9 pg/mL, while prednisolone recipients who did not develop infections had a median baseline bacterial DNA level of 8.3 pg/mL (P = .004). Bacterial DNA predicted infections with an area under receiver operating characteristic curve of 0.70 (95% CI, 0.58-0.83; P = .003), which substantially exceeded the curve for white blood cell count (0.58).
A cut-off value of 18.5 pg/mL was 80% specific for predicting infection within 7 days of prednisolone therapy, the investigators also reported. Bacterial DNA level did not, however, predict infections within 7 days of pentoxifylline therapy, and pentoxifylline was not linked with infections that were serious, infections during treatment, or infections after treatment. (P =.08).
Using bacterial DNA levels to guide prednisolone prescription also appeared to reduce 90-day mortality in this patient population, although the effect achieved borderline statistical significance, the researchers said. “Larger prospective randomized studies are needed to definitely report whether bacterial DNA-guided therapy can [have an] impact on mortality in severe alcoholic hepatitis, and perhaps in other acute inflammatory conditions” in which immunosuppression is required, they added.
The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators disclosed no conflicts of interest. Senior author Dr. Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
Source: American Gastroenterological Association
High baseline levels of circulating bacterial DNA increased the odds of serious infections by nearly fivefold in patients receiving prednisolone for severe alcoholic hepatitis, even after controlling for MELD score and white blood cell count, investigators reported in the April issue of Gastroenterology (2016 Dec 31. doi: 10.1053/j.gastro.2016.08.029).
“Patients with severe alcoholic hepatitis given prednisolone are at greater risk for developing serious infections and infections after treatment than patients not given prednisolone, which may offset its therapeutic benefit,” Nikhil Vergis, MD, and his associates wrote in Gastroenterology. “Level of circulating bacterial DNA before treatment could identify patients at high risk of infection if given prednisolone, which could be used to select therapies for patients with severe alcoholic hepatitis.”
To further explore rates and predictors of infections in STOPAH, the researchers analyzed longitudinal data on incident infections for 1,092 trial participants who received either prednisolone (40 mg daily) or pentoxifylline (400 mg three times daily). For 731 patients, they also examined whether baseline circulating levels of 16s ribosomal bacterial DNA were associated with infections.
A total of 135 patients (12%) had an infection at baseline, 251 (23%) developed infections during treatment, and 89 (8%) developed infections after treatment, the investigators reported. Prednisolone therapy was not associated with infections during treatment, but was associated with a nearly 30% rise in the odds of serious posttreatment infections compared with pentoxifylline (odds ratio, 1.27; 95% confidence interval, 1.27-2.92; P = .002). Prednisolone recipients who developed infections were significantly more likely to die within 90 days than those who did not, even after controlling for end-stage liver disease or Lille score (OR, 2.5; 95% CI, 1.4-4.3; P = .002). Antibiotic therapy appeared to significantly reduce the risk of mortality among infected prednisolone recipients (13% vs. 52%; OR, 0.13; 95% CI 0.04-0.47; P = .002).
There was “a striking association between bacterial DNA and the development of infection within 7 days in patients treated with prednisolone,” the researchers reported. These patients had a median baseline circulating DNA level of 20.9 pg/mL, while prednisolone recipients who did not develop infections had a median baseline bacterial DNA level of 8.3 pg/mL (P = .004). Bacterial DNA predicted infections with an area under receiver operating characteristic curve of 0.70 (95% CI, 0.58-0.83; P = .003), which substantially exceeded the curve for white blood cell count (0.58).
A cut-off value of 18.5 pg/mL was 80% specific for predicting infection within 7 days of prednisolone therapy, the investigators also reported. Bacterial DNA level did not, however, predict infections within 7 days of pentoxifylline therapy, and pentoxifylline was not linked with infections that were serious, infections during treatment, or infections after treatment. (P =.08).
Using bacterial DNA levels to guide prednisolone prescription also appeared to reduce 90-day mortality in this patient population, although the effect achieved borderline statistical significance, the researchers said. “Larger prospective randomized studies are needed to definitely report whether bacterial DNA-guided therapy can [have an] impact on mortality in severe alcoholic hepatitis, and perhaps in other acute inflammatory conditions” in which immunosuppression is required, they added.
The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators disclosed no conflicts of interest. Senior author Dr. Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
Source: American Gastroenterological Association
High baseline levels of circulating bacterial DNA increased the odds of serious infections by nearly fivefold in patients receiving prednisolone for severe alcoholic hepatitis, even after controlling for MELD score and white blood cell count, investigators reported in the April issue of Gastroenterology (2016 Dec 31. doi: 10.1053/j.gastro.2016.08.029).
“Patients with severe alcoholic hepatitis given prednisolone are at greater risk for developing serious infections and infections after treatment than patients not given prednisolone, which may offset its therapeutic benefit,” Nikhil Vergis, MD, and his associates wrote in Gastroenterology. “Level of circulating bacterial DNA before treatment could identify patients at high risk of infection if given prednisolone, which could be used to select therapies for patients with severe alcoholic hepatitis.”
To further explore rates and predictors of infections in STOPAH, the researchers analyzed longitudinal data on incident infections for 1,092 trial participants who received either prednisolone (40 mg daily) or pentoxifylline (400 mg three times daily). For 731 patients, they also examined whether baseline circulating levels of 16s ribosomal bacterial DNA were associated with infections.
A total of 135 patients (12%) had an infection at baseline, 251 (23%) developed infections during treatment, and 89 (8%) developed infections after treatment, the investigators reported. Prednisolone therapy was not associated with infections during treatment, but was associated with a nearly 30% rise in the odds of serious posttreatment infections compared with pentoxifylline (odds ratio, 1.27; 95% confidence interval, 1.27-2.92; P = .002). Prednisolone recipients who developed infections were significantly more likely to die within 90 days than those who did not, even after controlling for end-stage liver disease or Lille score (OR, 2.5; 95% CI, 1.4-4.3; P = .002). Antibiotic therapy appeared to significantly reduce the risk of mortality among infected prednisolone recipients (13% vs. 52%; OR, 0.13; 95% CI 0.04-0.47; P = .002).
There was “a striking association between bacterial DNA and the development of infection within 7 days in patients treated with prednisolone,” the researchers reported. These patients had a median baseline circulating DNA level of 20.9 pg/mL, while prednisolone recipients who did not develop infections had a median baseline bacterial DNA level of 8.3 pg/mL (P = .004). Bacterial DNA predicted infections with an area under receiver operating characteristic curve of 0.70 (95% CI, 0.58-0.83; P = .003), which substantially exceeded the curve for white blood cell count (0.58).
A cut-off value of 18.5 pg/mL was 80% specific for predicting infection within 7 days of prednisolone therapy, the investigators also reported. Bacterial DNA level did not, however, predict infections within 7 days of pentoxifylline therapy, and pentoxifylline was not linked with infections that were serious, infections during treatment, or infections after treatment. (P =.08).
Using bacterial DNA levels to guide prednisolone prescription also appeared to reduce 90-day mortality in this patient population, although the effect achieved borderline statistical significance, the researchers said. “Larger prospective randomized studies are needed to definitely report whether bacterial DNA-guided therapy can [have an] impact on mortality in severe alcoholic hepatitis, and perhaps in other acute inflammatory conditions” in which immunosuppression is required, they added.
The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators disclosed no conflicts of interest. Senior author Dr. Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: High baseline levels of circulating bacterial DNA predicted serious infections in patients receiving prednisolone for severe alcoholic hepatitis.
Major finding: The odds of serious posttreatment infections were significantly higher for prednisolone compared with pentoxifylline (OR, 1.27; P = .002). High baseline levels of circulating bacterial DNA predicted infection within 7 days of prednisolone therapy (adjusted OR, 4.68; P = .001).
Data source: An analysis of 1,092 patients with severe alcoholic hepatitis from the randomized, double-blind STOPAH trial.
Disclosures: The National Institute for Health Research and Wellcome Trust and Medical Research Council provided funding. Dr. Vergis and 10 coinvestigators reported having no competing interests. Senior author Mark Thursz and one coinvestigator disclosed ties to Gilead, Bristol-Myers Squibb, AbbVie, Abbott, and Norgine.
CHMP recommends new indication for daratumumab
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation has been forwarded to the European Commission, which is expected to make its decision on daratumumab within 2 months.
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
Phase 3 trials
The CHMP’s recommendation regarding daratumumab was based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation has been forwarded to the European Commission, which is expected to make its decision on daratumumab within 2 months.
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
Phase 3 trials
The CHMP’s recommendation regarding daratumumab was based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation has been forwarded to the European Commission, which is expected to make its decision on daratumumab within 2 months.
Daratumumab is a human IgG1k monoclonal antibody that binds to CD38, which is highly expressed on the surface of MM cells.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab.
Phase 3 trials
The CHMP’s recommendation regarding daratumumab was based on data from the phase 3 POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
FDA clears procalcitonin test to hone antibiotic use in LRTI, sepsis
The Food and Drug Administration has cleared the expanded use of a procalcitonin test to help determine antibiotic use in patients with lower respiratory tract infections (LRTI) and sepsis.
The Vidas Brahms PCT Assay (bioMérieux) uses procalcitonin levels to determine whether a patient with a lower respiratory tract infection (LRTI) should begin or remain on antibiotics and when antibiotics should be withdrawn in a patient with sepsis.
The test will be used primarily in hospital settings and emergency departments, according to the FDA. Test levels that are high levels suggest bacterial infection and the need for antibiotics while low levels indicate viral or noninfectious processes. However, concerns exist regarding false-positive or false-negative test results, which can prompt clinicians to prematurely stop or unnecessarily continue an antibiotic regimen in certain patients.
“Health care providers should not rely solely on PCT test results when making treatment decisions but should interpret test results in the context of a patient’s clinical status and other laboratory results,” according to the FDA statement.
The expanded use of the test was approved based on promising data from clinical trials that was presented at an FDA advisory committee meeting in November 2016. The Vidas Brahms test was already approved by the FDA for use in determining a patient’s risk of dying from sepsis. The test was cleared via the FDA 510(k) regulatory pathway, which is meant for tests or devices for which there is already something similar on the market.
Support for the test’s expanded usage comes from published prospective, randomized clinical trials that compared PCT-guided therapy with standard therapy. In those studies, patients who had received PCT-guided therapy experienced significant decreases in antibiotic use without significant affects to their safety.
The Food and Drug Administration has cleared the expanded use of a procalcitonin test to help determine antibiotic use in patients with lower respiratory tract infections (LRTI) and sepsis.
The Vidas Brahms PCT Assay (bioMérieux) uses procalcitonin levels to determine whether a patient with a lower respiratory tract infection (LRTI) should begin or remain on antibiotics and when antibiotics should be withdrawn in a patient with sepsis.
The test will be used primarily in hospital settings and emergency departments, according to the FDA. Test levels that are high levels suggest bacterial infection and the need for antibiotics while low levels indicate viral or noninfectious processes. However, concerns exist regarding false-positive or false-negative test results, which can prompt clinicians to prematurely stop or unnecessarily continue an antibiotic regimen in certain patients.
“Health care providers should not rely solely on PCT test results when making treatment decisions but should interpret test results in the context of a patient’s clinical status and other laboratory results,” according to the FDA statement.
The expanded use of the test was approved based on promising data from clinical trials that was presented at an FDA advisory committee meeting in November 2016. The Vidas Brahms test was already approved by the FDA for use in determining a patient’s risk of dying from sepsis. The test was cleared via the FDA 510(k) regulatory pathway, which is meant for tests or devices for which there is already something similar on the market.
Support for the test’s expanded usage comes from published prospective, randomized clinical trials that compared PCT-guided therapy with standard therapy. In those studies, patients who had received PCT-guided therapy experienced significant decreases in antibiotic use without significant affects to their safety.
The Food and Drug Administration has cleared the expanded use of a procalcitonin test to help determine antibiotic use in patients with lower respiratory tract infections (LRTI) and sepsis.
The Vidas Brahms PCT Assay (bioMérieux) uses procalcitonin levels to determine whether a patient with a lower respiratory tract infection (LRTI) should begin or remain on antibiotics and when antibiotics should be withdrawn in a patient with sepsis.
The test will be used primarily in hospital settings and emergency departments, according to the FDA. Test levels that are high levels suggest bacterial infection and the need for antibiotics while low levels indicate viral or noninfectious processes. However, concerns exist regarding false-positive or false-negative test results, which can prompt clinicians to prematurely stop or unnecessarily continue an antibiotic regimen in certain patients.
“Health care providers should not rely solely on PCT test results when making treatment decisions but should interpret test results in the context of a patient’s clinical status and other laboratory results,” according to the FDA statement.
The expanded use of the test was approved based on promising data from clinical trials that was presented at an FDA advisory committee meeting in November 2016. The Vidas Brahms test was already approved by the FDA for use in determining a patient’s risk of dying from sepsis. The test was cleared via the FDA 510(k) regulatory pathway, which is meant for tests or devices for which there is already something similar on the market.
Support for the test’s expanded usage comes from published prospective, randomized clinical trials that compared PCT-guided therapy with standard therapy. In those studies, patients who had received PCT-guided therapy experienced significant decreases in antibiotic use without significant affects to their safety.
Late-Onset Bexarotene-Induced CD4 Lymphopenia in a Cutaneous T-cell Lymphoma Patient
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
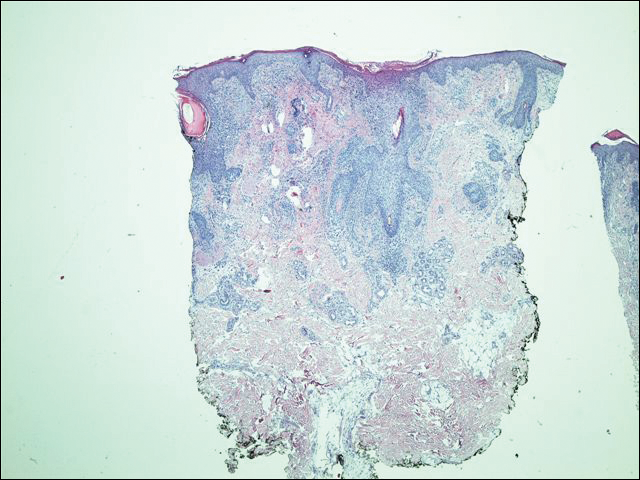
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.
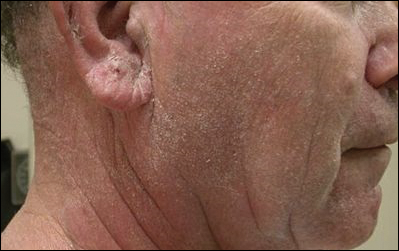

The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
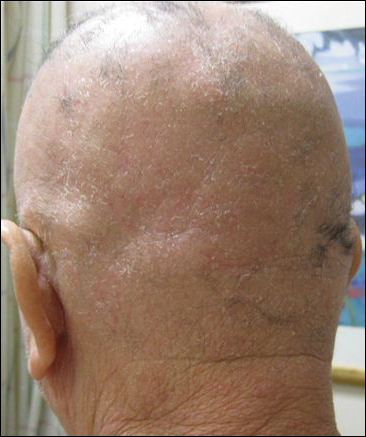
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.
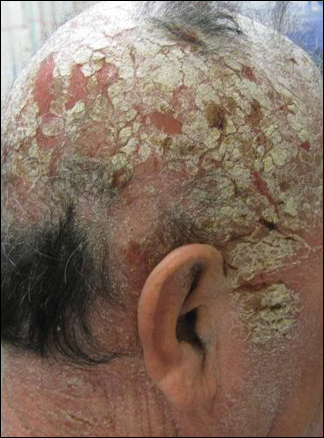
Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.


The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.



Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.


The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.



Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
Practice Points
- Most adverse effects of bexarotene (eg, hypothyroidism, hyperlipidemia, leukopenia) occur within the first several months of therapy.
- Delayed-onset leukopenia, including CD4 lymphopenia, may occur several years after initiating bexarotene therapy, resulting in opportunistic infections.
- Long-term periodic monitoring of T lymphocyte counts at least twice yearly in addition to standard quarterly complete blood cell count with differential are recommended.
Fewer post-transplant CMV infections with novel antiviral prophylaxis
ORLANDO – A first-in-class antiviral drug was safe and effective when used to prevent clinically significant cytomegalovirus infections in adults undergoing hematopoietic cell transplantation.
At 24 weeks post-transplant, 38% (122/325) of those receiving the novel antiviral letermovir were considered treatment failures, compared with 61% (103/170) of those receiving placebo (P less than .0001). All-cause mortality was 10% (n=32/325) for patients receiving letermovir and 16%, (n=27/170) for the placebo group (log rank two-sided P = 0.0317).
The study findings show that “we [can] prevent patients from getting CMV infections from the beginning of the transplant and that [result can] confer a mortality benefit,” lead author Francisco Marty, MD, said in an interview.
Dr. Marty said that letermovir has received fast-track status both from the Food and Drug Administration and from the European Medicines Agency.
HCT recipients who are CMV-positive but who do not have clinically significant disease are not preemptively treated in current practice. “Previously, there wasn’t a primary prevention strategy in bone marrow transplantation. When myelosuppressive drugs were tried such as ganciclovir, any benefit was offset by increased myelosuppression, with resulting increases in bacterial and fungal infections,” said Dr. Marty, professor of medicine at Harvard Medical School, Boston.
Letermovir was generally well-tolerated in the clinical trial; myelotoxicity and nephrotoxicity levels were comparable in patients receiving letermovir and placebo. Letermovir targets the terminase complex, which is a viral replication process specific to CMV and not otherwise present in humans. That fact may explain, in part, letermovir’s limited toxicity, Dr. Marty said. The primary outcome measure of the phase III randomized, double-blind, placebo-controlled trial was the stratum-adjusted proportion of patients who had clinically significant CMV at post-transplant week 24, examining only the patients in the trials who had no detectable CMV DNA at the time of randomization. If patients did not complete the study, or had missing data at week 24, they were considered to have failed the trial.
Overall, 31% of patients were considered at high risk for CMV disease. Half of the patients received myeloablative conditioning, and about a third (35%) received antithymocyte globulin. Donor sources, whose characteristics were balanced between study arms, included 14% mismatched unrelated donors, 13% haploidentical donors, and 4% cord blood.
The multinational study’s 24-week results were presented at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
“This was an international study, conducted at multiple sites in multiple countries. We used two-to-one randomization, and stratified participants by their risk of CMV disease and by study sites. We wanted to make sure the groups were balanced by disease risk and by study sites, to account for regional variations in bone marrow transplant procedures and CMV treatment patterns,” said Dr. Marty.
For the study, clinically significant CMV infection was defined as either CMV disease, such as pneumonia, colitis, or hepatitis, or CMV viremia that would trigger preemptive treatment.
Letermovir, which can be administered orally or intravenously, was dosed at 480 mg per day. Because of the risk for a drug-drug interaction, patients on cyclosporine received 240 mg of letermovir per day. Having intravenous dosing as an option helped patients who were not tolerating oral intake to stay on the study drug during the post-transplant period, he said.
The study drug was begun a median of 9 days post-transplant. Some patients received letermovir or placebo as early as the day of transplant; all patients began the study drug by 28 days post-transplant. The study drug was continued through week 14, or until at least 100 days post-transplant. Overall, 37% of patients had engrafted at the time they began the study drug.
Patients had weekly serum CMV assays performed until week 14, with biweekly sampling done through week 24. If patients developed clinically significant CMV, or if their serum samples yielded CMV DNA warranting preemptive treatment, they discontinued the study drug and began treatment for CMV.
The safety analysis, which was carried through week 48, tracked adverse events from the first dose of study drug until 14 days after discontinuation. Adverse events that were more common with letermovir than placebo included vomiting (19% versus 14%), edema (15% versus 9%), atrial arrhythmias (10% versus 5%), and having alanine aspartate levels more than five times the upper limit of normal (4% versus 2%). Graft versus host disease occurred in 39% of patients in each group; diarrhea and nausea occurred in approximately one fourth of patients in each group.
In response to a question after the presentation, Dr. Marty said, “The higher the risk of CMV disease, the higher the benefit in terms of survival.” Answering another question, about who should receive letermovir. Dr. Marty replied, “Like acyclovir, we should give it during times of risk. And CMV risk is different for different populations. It’s a matter of managing risks and benefits.”
Though letermovir was safe and well-tolerated in this trial, it’s different from acyclovir in that “it’s not a one dollar a day drug,” Dr. Marty acknowledged.
Merck, which plans to market letermovir, was the sponsor of the study and plans to submit applications for approval in both the United States and in the European Union in 2017. Dr. Marty reported receiving research grants from Merck as well as Astellas, Chimerix, and Shire. Additionally, he has received honoraria from Alexion, Chimerix, LFB, Merck, Roche Molecular Diagnostics, and Shire.
This article was updated 2/27/17.
[email protected]
On Twitter @karioakes
ORLANDO – A first-in-class antiviral drug was safe and effective when used to prevent clinically significant cytomegalovirus infections in adults undergoing hematopoietic cell transplantation.
At 24 weeks post-transplant, 38% (122/325) of those receiving the novel antiviral letermovir were considered treatment failures, compared with 61% (103/170) of those receiving placebo (P less than .0001). All-cause mortality was 10% (n=32/325) for patients receiving letermovir and 16%, (n=27/170) for the placebo group (log rank two-sided P = 0.0317).
The study findings show that “we [can] prevent patients from getting CMV infections from the beginning of the transplant and that [result can] confer a mortality benefit,” lead author Francisco Marty, MD, said in an interview.
Dr. Marty said that letermovir has received fast-track status both from the Food and Drug Administration and from the European Medicines Agency.
HCT recipients who are CMV-positive but who do not have clinically significant disease are not preemptively treated in current practice. “Previously, there wasn’t a primary prevention strategy in bone marrow transplantation. When myelosuppressive drugs were tried such as ganciclovir, any benefit was offset by increased myelosuppression, with resulting increases in bacterial and fungal infections,” said Dr. Marty, professor of medicine at Harvard Medical School, Boston.
Letermovir was generally well-tolerated in the clinical trial; myelotoxicity and nephrotoxicity levels were comparable in patients receiving letermovir and placebo. Letermovir targets the terminase complex, which is a viral replication process specific to CMV and not otherwise present in humans. That fact may explain, in part, letermovir’s limited toxicity, Dr. Marty said. The primary outcome measure of the phase III randomized, double-blind, placebo-controlled trial was the stratum-adjusted proportion of patients who had clinically significant CMV at post-transplant week 24, examining only the patients in the trials who had no detectable CMV DNA at the time of randomization. If patients did not complete the study, or had missing data at week 24, they were considered to have failed the trial.
Overall, 31% of patients were considered at high risk for CMV disease. Half of the patients received myeloablative conditioning, and about a third (35%) received antithymocyte globulin. Donor sources, whose characteristics were balanced between study arms, included 14% mismatched unrelated donors, 13% haploidentical donors, and 4% cord blood.
The multinational study’s 24-week results were presented at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
“This was an international study, conducted at multiple sites in multiple countries. We used two-to-one randomization, and stratified participants by their risk of CMV disease and by study sites. We wanted to make sure the groups were balanced by disease risk and by study sites, to account for regional variations in bone marrow transplant procedures and CMV treatment patterns,” said Dr. Marty.
For the study, clinically significant CMV infection was defined as either CMV disease, such as pneumonia, colitis, or hepatitis, or CMV viremia that would trigger preemptive treatment.
Letermovir, which can be administered orally or intravenously, was dosed at 480 mg per day. Because of the risk for a drug-drug interaction, patients on cyclosporine received 240 mg of letermovir per day. Having intravenous dosing as an option helped patients who were not tolerating oral intake to stay on the study drug during the post-transplant period, he said.
The study drug was begun a median of 9 days post-transplant. Some patients received letermovir or placebo as early as the day of transplant; all patients began the study drug by 28 days post-transplant. The study drug was continued through week 14, or until at least 100 days post-transplant. Overall, 37% of patients had engrafted at the time they began the study drug.
Patients had weekly serum CMV assays performed until week 14, with biweekly sampling done through week 24. If patients developed clinically significant CMV, or if their serum samples yielded CMV DNA warranting preemptive treatment, they discontinued the study drug and began treatment for CMV.
The safety analysis, which was carried through week 48, tracked adverse events from the first dose of study drug until 14 days after discontinuation. Adverse events that were more common with letermovir than placebo included vomiting (19% versus 14%), edema (15% versus 9%), atrial arrhythmias (10% versus 5%), and having alanine aspartate levels more than five times the upper limit of normal (4% versus 2%). Graft versus host disease occurred in 39% of patients in each group; diarrhea and nausea occurred in approximately one fourth of patients in each group.
In response to a question after the presentation, Dr. Marty said, “The higher the risk of CMV disease, the higher the benefit in terms of survival.” Answering another question, about who should receive letermovir. Dr. Marty replied, “Like acyclovir, we should give it during times of risk. And CMV risk is different for different populations. It’s a matter of managing risks and benefits.”
Though letermovir was safe and well-tolerated in this trial, it’s different from acyclovir in that “it’s not a one dollar a day drug,” Dr. Marty acknowledged.
Merck, which plans to market letermovir, was the sponsor of the study and plans to submit applications for approval in both the United States and in the European Union in 2017. Dr. Marty reported receiving research grants from Merck as well as Astellas, Chimerix, and Shire. Additionally, he has received honoraria from Alexion, Chimerix, LFB, Merck, Roche Molecular Diagnostics, and Shire.
This article was updated 2/27/17.
[email protected]
On Twitter @karioakes
ORLANDO – A first-in-class antiviral drug was safe and effective when used to prevent clinically significant cytomegalovirus infections in adults undergoing hematopoietic cell transplantation.
At 24 weeks post-transplant, 38% (122/325) of those receiving the novel antiviral letermovir were considered treatment failures, compared with 61% (103/170) of those receiving placebo (P less than .0001). All-cause mortality was 10% (n=32/325) for patients receiving letermovir and 16%, (n=27/170) for the placebo group (log rank two-sided P = 0.0317).
The study findings show that “we [can] prevent patients from getting CMV infections from the beginning of the transplant and that [result can] confer a mortality benefit,” lead author Francisco Marty, MD, said in an interview.
Dr. Marty said that letermovir has received fast-track status both from the Food and Drug Administration and from the European Medicines Agency.
HCT recipients who are CMV-positive but who do not have clinically significant disease are not preemptively treated in current practice. “Previously, there wasn’t a primary prevention strategy in bone marrow transplantation. When myelosuppressive drugs were tried such as ganciclovir, any benefit was offset by increased myelosuppression, with resulting increases in bacterial and fungal infections,” said Dr. Marty, professor of medicine at Harvard Medical School, Boston.
Letermovir was generally well-tolerated in the clinical trial; myelotoxicity and nephrotoxicity levels were comparable in patients receiving letermovir and placebo. Letermovir targets the terminase complex, which is a viral replication process specific to CMV and not otherwise present in humans. That fact may explain, in part, letermovir’s limited toxicity, Dr. Marty said. The primary outcome measure of the phase III randomized, double-blind, placebo-controlled trial was the stratum-adjusted proportion of patients who had clinically significant CMV at post-transplant week 24, examining only the patients in the trials who had no detectable CMV DNA at the time of randomization. If patients did not complete the study, or had missing data at week 24, they were considered to have failed the trial.
Overall, 31% of patients were considered at high risk for CMV disease. Half of the patients received myeloablative conditioning, and about a third (35%) received antithymocyte globulin. Donor sources, whose characteristics were balanced between study arms, included 14% mismatched unrelated donors, 13% haploidentical donors, and 4% cord blood.
The multinational study’s 24-week results were presented at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
“This was an international study, conducted at multiple sites in multiple countries. We used two-to-one randomization, and stratified participants by their risk of CMV disease and by study sites. We wanted to make sure the groups were balanced by disease risk and by study sites, to account for regional variations in bone marrow transplant procedures and CMV treatment patterns,” said Dr. Marty.
For the study, clinically significant CMV infection was defined as either CMV disease, such as pneumonia, colitis, or hepatitis, or CMV viremia that would trigger preemptive treatment.
Letermovir, which can be administered orally or intravenously, was dosed at 480 mg per day. Because of the risk for a drug-drug interaction, patients on cyclosporine received 240 mg of letermovir per day. Having intravenous dosing as an option helped patients who were not tolerating oral intake to stay on the study drug during the post-transplant period, he said.
The study drug was begun a median of 9 days post-transplant. Some patients received letermovir or placebo as early as the day of transplant; all patients began the study drug by 28 days post-transplant. The study drug was continued through week 14, or until at least 100 days post-transplant. Overall, 37% of patients had engrafted at the time they began the study drug.
Patients had weekly serum CMV assays performed until week 14, with biweekly sampling done through week 24. If patients developed clinically significant CMV, or if their serum samples yielded CMV DNA warranting preemptive treatment, they discontinued the study drug and began treatment for CMV.
The safety analysis, which was carried through week 48, tracked adverse events from the first dose of study drug until 14 days after discontinuation. Adverse events that were more common with letermovir than placebo included vomiting (19% versus 14%), edema (15% versus 9%), atrial arrhythmias (10% versus 5%), and having alanine aspartate levels more than five times the upper limit of normal (4% versus 2%). Graft versus host disease occurred in 39% of patients in each group; diarrhea and nausea occurred in approximately one fourth of patients in each group.
In response to a question after the presentation, Dr. Marty said, “The higher the risk of CMV disease, the higher the benefit in terms of survival.” Answering another question, about who should receive letermovir. Dr. Marty replied, “Like acyclovir, we should give it during times of risk. And CMV risk is different for different populations. It’s a matter of managing risks and benefits.”
Though letermovir was safe and well-tolerated in this trial, it’s different from acyclovir in that “it’s not a one dollar a day drug,” Dr. Marty acknowledged.
Merck, which plans to market letermovir, was the sponsor of the study and plans to submit applications for approval in both the United States and in the European Union in 2017. Dr. Marty reported receiving research grants from Merck as well as Astellas, Chimerix, and Shire. Additionally, he has received honoraria from Alexion, Chimerix, LFB, Merck, Roche Molecular Diagnostics, and Shire.
This article was updated 2/27/17.
[email protected]
On Twitter @karioakes
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Cytomegalovirus (CMV) infection or viremia occurred in 38% of patients receiving post-HCT letermovir, compared with 61% of controls.
Data source: Randomized, double-blind, placebo-controlled study of 495 patients seropositive for CMV with no detectable CMV DNA at the time of HCT.
Disclosures: Merck, which plans to market letermovir, was the sponsor of the study. Dr. Marty reported receiving research grants from Merck as well as Astellas, Chimerix, and Shire. Additionally, he has received honoraria from Alexion, Chimerix, LFB, Merck, Roche Molecular Diagnostics, and Shire.
Redefine dysplastic nevi to stratify cancer risk
WAILEA, HAWAII – It is time for a new system of classifying nevi, according to Ashfaq Marghoob, MD, of Memorial Sloan Kettering Cancer Center, New York.
“It was known for the last 30 or 40 years that we do need to subclassify nevi into groups, so as to better stratify for melanoma risk,” identifying groups of individuals who would benefit most from targeted screening, Dr. Marghoob said in a video interview at the meeting, provided by Global Academy for Medical Education/Skin Disease Education Foundation. But it has been clear that there are many flaws in the current classification system, he added.
This is beginning to change as new data emerge about gene mutations and other science that can better stratify “or segregate” the nevi into subsets, and “the hope is we will be better able to predict which subsets are associated with melanoma risk either within the lesion itself or poses an increased risk to the patient,” he explained.
“As our understanding grows, we will start to come out with subsets of nevi that have a certain clinical and dermoscopic morphology,” to help predict which patients would benefit most from being monitored very closely, with the aim of detecting – and curing – melanomas early, said Dr. Marghoob, director of Memorial Sloan Kettering’s regional skin cancer clinic in Hauppauge, N.Y.
He had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – It is time for a new system of classifying nevi, according to Ashfaq Marghoob, MD, of Memorial Sloan Kettering Cancer Center, New York.
“It was known for the last 30 or 40 years that we do need to subclassify nevi into groups, so as to better stratify for melanoma risk,” identifying groups of individuals who would benefit most from targeted screening, Dr. Marghoob said in a video interview at the meeting, provided by Global Academy for Medical Education/Skin Disease Education Foundation. But it has been clear that there are many flaws in the current classification system, he added.
This is beginning to change as new data emerge about gene mutations and other science that can better stratify “or segregate” the nevi into subsets, and “the hope is we will be better able to predict which subsets are associated with melanoma risk either within the lesion itself or poses an increased risk to the patient,” he explained.
“As our understanding grows, we will start to come out with subsets of nevi that have a certain clinical and dermoscopic morphology,” to help predict which patients would benefit most from being monitored very closely, with the aim of detecting – and curing – melanomas early, said Dr. Marghoob, director of Memorial Sloan Kettering’s regional skin cancer clinic in Hauppauge, N.Y.
He had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – It is time for a new system of classifying nevi, according to Ashfaq Marghoob, MD, of Memorial Sloan Kettering Cancer Center, New York.
“It was known for the last 30 or 40 years that we do need to subclassify nevi into groups, so as to better stratify for melanoma risk,” identifying groups of individuals who would benefit most from targeted screening, Dr. Marghoob said in a video interview at the meeting, provided by Global Academy for Medical Education/Skin Disease Education Foundation. But it has been clear that there are many flaws in the current classification system, he added.
This is beginning to change as new data emerge about gene mutations and other science that can better stratify “or segregate” the nevi into subsets, and “the hope is we will be better able to predict which subsets are associated with melanoma risk either within the lesion itself or poses an increased risk to the patient,” he explained.
“As our understanding grows, we will start to come out with subsets of nevi that have a certain clinical and dermoscopic morphology,” to help predict which patients would benefit most from being monitored very closely, with the aim of detecting – and curing – melanomas early, said Dr. Marghoob, director of Memorial Sloan Kettering’s regional skin cancer clinic in Hauppauge, N.Y.
He had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Zika vaccine development expected to last through 2020
Progress continues to be made on creating a Zika vaccine, but taking any of the current candidates all the way through clinical trials and into production could take another few years, according to the latest information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“As we all know, there is no vaccine for Zika, but there are a number of vaccines that have been developed over the last century or so for other flaviviruses, [such as] dengue, yellow fever, Japanese encephalitis, [West Nile], and we know a great deal about flaviviruses in general and the pathology that they have,” explained Gerald R. Kovacs, PhD, of the Biomedical Advanced Research and Development Authority (BARDA). “What we’re doing is using our lessons learned and working with the epidemiologists, with the clinicians, with the nonclinical development people, and using those lessons to develop new vaccines for Zika.”
By next year, the second aim should begin to take shape, which will be the deployment of available vaccines under an appropriate regulatory mechanism to U.S. populations at high risk of exposure.
Finally, by 2020, Dr. Kovacs explained that the government hopes to be partnering with industry to commercialize a Zika vaccine and make it available for broad distribution.
The vaccines being looked at include an inactivated whole-virus vaccine, a live attenuated vaccine that utilizes flavichimeras, a recombinant vaccine, and nucleic acid vaccines, including DNA and mRNA varieties. While each have their pros and cons, only the inactivated whole-virus and live attenuated virus vaccines have licensed human flavivirus vaccines already available for protection against Japanese encephalitis, tick-borne encephalitis, yellow fever, and dengue.
The Zika Purified Inactivated Vaccine (ZPIV) has two candidates in “advanced development,” one by Sanofi Pasteur and the other by Takeda. Currently, the Walter Reed Army Institute of Research and the National Institute of Allergy and Infectious Diseases are conducting phase I clinical trials on both ZPIV candidates to determine their safety and immunogenicity profiles and gathering information on regimen, dosing, and prior flavi immunity. ZPIV has already proven to be fully protective in mice and nonhuman primates. Both the Sanofi and Takeda ZPIVs are expected to enter phase II testing by the middle of next year, and phase III testing at some point in 2019 or 2020.
“Human challenge was discussed at a consultation that the [National Institutes of Health] held a couple of months ago [and] in a nutshell, what the committee found was that there isn’t sufficient information right now on Zika relative to its pathology and how it’s transmitted from humans to humans to support a human clinical study at this time,” said Dr. Kovacs. “But they will, as we accrue more information about this disease, revisit the potential of doing this type of study.”
Dr. Kovacs also highlighted the need for manufacturers to stay in the game as long as possible, urging them not to be discouraged by dwindling interest and funding regarding the Zika vaccine initiative.
“We can develop as many vaccines as possible, but what’s necessary is for these manufacturers to stay in for the long haul,” he explained. “With cuts in funding and less and less enthusiasm for Zika, it becomes challenging for the U.S. government to continue to engage with manufacturers on these types of products [but] we hope that all of our partners will continue on their endeavors with us, but we can’t guarantee that.”
Dr. Kovacs disclosed that he is a consultant for BARDA within the Office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health & Human Services, and that he was speaking at the meeting on behalf of the organization.
Progress continues to be made on creating a Zika vaccine, but taking any of the current candidates all the way through clinical trials and into production could take another few years, according to the latest information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“As we all know, there is no vaccine for Zika, but there are a number of vaccines that have been developed over the last century or so for other flaviviruses, [such as] dengue, yellow fever, Japanese encephalitis, [West Nile], and we know a great deal about flaviviruses in general and the pathology that they have,” explained Gerald R. Kovacs, PhD, of the Biomedical Advanced Research and Development Authority (BARDA). “What we’re doing is using our lessons learned and working with the epidemiologists, with the clinicians, with the nonclinical development people, and using those lessons to develop new vaccines for Zika.”
By next year, the second aim should begin to take shape, which will be the deployment of available vaccines under an appropriate regulatory mechanism to U.S. populations at high risk of exposure.
Finally, by 2020, Dr. Kovacs explained that the government hopes to be partnering with industry to commercialize a Zika vaccine and make it available for broad distribution.
The vaccines being looked at include an inactivated whole-virus vaccine, a live attenuated vaccine that utilizes flavichimeras, a recombinant vaccine, and nucleic acid vaccines, including DNA and mRNA varieties. While each have their pros and cons, only the inactivated whole-virus and live attenuated virus vaccines have licensed human flavivirus vaccines already available for protection against Japanese encephalitis, tick-borne encephalitis, yellow fever, and dengue.
The Zika Purified Inactivated Vaccine (ZPIV) has two candidates in “advanced development,” one by Sanofi Pasteur and the other by Takeda. Currently, the Walter Reed Army Institute of Research and the National Institute of Allergy and Infectious Diseases are conducting phase I clinical trials on both ZPIV candidates to determine their safety and immunogenicity profiles and gathering information on regimen, dosing, and prior flavi immunity. ZPIV has already proven to be fully protective in mice and nonhuman primates. Both the Sanofi and Takeda ZPIVs are expected to enter phase II testing by the middle of next year, and phase III testing at some point in 2019 or 2020.
“Human challenge was discussed at a consultation that the [National Institutes of Health] held a couple of months ago [and] in a nutshell, what the committee found was that there isn’t sufficient information right now on Zika relative to its pathology and how it’s transmitted from humans to humans to support a human clinical study at this time,” said Dr. Kovacs. “But they will, as we accrue more information about this disease, revisit the potential of doing this type of study.”
Dr. Kovacs also highlighted the need for manufacturers to stay in the game as long as possible, urging them not to be discouraged by dwindling interest and funding regarding the Zika vaccine initiative.
“We can develop as many vaccines as possible, but what’s necessary is for these manufacturers to stay in for the long haul,” he explained. “With cuts in funding and less and less enthusiasm for Zika, it becomes challenging for the U.S. government to continue to engage with manufacturers on these types of products [but] we hope that all of our partners will continue on their endeavors with us, but we can’t guarantee that.”
Dr. Kovacs disclosed that he is a consultant for BARDA within the Office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health & Human Services, and that he was speaking at the meeting on behalf of the organization.
Progress continues to be made on creating a Zika vaccine, but taking any of the current candidates all the way through clinical trials and into production could take another few years, according to the latest information presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“As we all know, there is no vaccine for Zika, but there are a number of vaccines that have been developed over the last century or so for other flaviviruses, [such as] dengue, yellow fever, Japanese encephalitis, [West Nile], and we know a great deal about flaviviruses in general and the pathology that they have,” explained Gerald R. Kovacs, PhD, of the Biomedical Advanced Research and Development Authority (BARDA). “What we’re doing is using our lessons learned and working with the epidemiologists, with the clinicians, with the nonclinical development people, and using those lessons to develop new vaccines for Zika.”
By next year, the second aim should begin to take shape, which will be the deployment of available vaccines under an appropriate regulatory mechanism to U.S. populations at high risk of exposure.
Finally, by 2020, Dr. Kovacs explained that the government hopes to be partnering with industry to commercialize a Zika vaccine and make it available for broad distribution.
The vaccines being looked at include an inactivated whole-virus vaccine, a live attenuated vaccine that utilizes flavichimeras, a recombinant vaccine, and nucleic acid vaccines, including DNA and mRNA varieties. While each have their pros and cons, only the inactivated whole-virus and live attenuated virus vaccines have licensed human flavivirus vaccines already available for protection against Japanese encephalitis, tick-borne encephalitis, yellow fever, and dengue.
The Zika Purified Inactivated Vaccine (ZPIV) has two candidates in “advanced development,” one by Sanofi Pasteur and the other by Takeda. Currently, the Walter Reed Army Institute of Research and the National Institute of Allergy and Infectious Diseases are conducting phase I clinical trials on both ZPIV candidates to determine their safety and immunogenicity profiles and gathering information on regimen, dosing, and prior flavi immunity. ZPIV has already proven to be fully protective in mice and nonhuman primates. Both the Sanofi and Takeda ZPIVs are expected to enter phase II testing by the middle of next year, and phase III testing at some point in 2019 or 2020.
“Human challenge was discussed at a consultation that the [National Institutes of Health] held a couple of months ago [and] in a nutshell, what the committee found was that there isn’t sufficient information right now on Zika relative to its pathology and how it’s transmitted from humans to humans to support a human clinical study at this time,” said Dr. Kovacs. “But they will, as we accrue more information about this disease, revisit the potential of doing this type of study.”
Dr. Kovacs also highlighted the need for manufacturers to stay in the game as long as possible, urging them not to be discouraged by dwindling interest and funding regarding the Zika vaccine initiative.
“We can develop as many vaccines as possible, but what’s necessary is for these manufacturers to stay in for the long haul,” he explained. “With cuts in funding and less and less enthusiasm for Zika, it becomes challenging for the U.S. government to continue to engage with manufacturers on these types of products [but] we hope that all of our partners will continue on their endeavors with us, but we can’t guarantee that.”
Dr. Kovacs disclosed that he is a consultant for BARDA within the Office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health & Human Services, and that he was speaking at the meeting on behalf of the organization.
VIDEO: Advances in noninvasive fat reduction include permanent effects
WAILEA, HAWAII – In the past few years, there have been “exciting” advances in noninvasive techniques to permanently remove fat, according to Suzanne Kilmer, MD, of the department of dermatology, University of California, Davis.
There are devices now available to reduce fat “in a permanent way that’s not injurious, it’s not uncomfortable – patients love it,” Dr. Kilmer said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Currently, the three noninvasive modalities she considers most effective for reducing fat are cryolipolysis, the 1,060-nm laser, and focused pulsed ultrasound. In the interview, she discussed the ways these treatments work and their safety.
With all three, “the fat that goes away stays away,” said Dr. Kilmer, who is director of the Laser and Skin Surgery Center of Northern California, Sacramento. Treatment results in release of fat, which is “cleared like it is as if you ate a cheeseburger,” she added. Interestingly, she said, trials that have looked at whether the fat reduction is accompanied by weight loss have found that, in most cases, the patient’s weight remains stable.
Dr. Kilmer’s disclosures included serving as a consultant and/or researcher for Allergan, Cutera, Cynosure, Cytrellis, Kythera, Lumenis, Merz, Miramar, Sebacia, Sienna Labs, Solta, Zeltiq, and Zift.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – In the past few years, there have been “exciting” advances in noninvasive techniques to permanently remove fat, according to Suzanne Kilmer, MD, of the department of dermatology, University of California, Davis.
There are devices now available to reduce fat “in a permanent way that’s not injurious, it’s not uncomfortable – patients love it,” Dr. Kilmer said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Currently, the three noninvasive modalities she considers most effective for reducing fat are cryolipolysis, the 1,060-nm laser, and focused pulsed ultrasound. In the interview, she discussed the ways these treatments work and their safety.
With all three, “the fat that goes away stays away,” said Dr. Kilmer, who is director of the Laser and Skin Surgery Center of Northern California, Sacramento. Treatment results in release of fat, which is “cleared like it is as if you ate a cheeseburger,” she added. Interestingly, she said, trials that have looked at whether the fat reduction is accompanied by weight loss have found that, in most cases, the patient’s weight remains stable.
Dr. Kilmer’s disclosures included serving as a consultant and/or researcher for Allergan, Cutera, Cynosure, Cytrellis, Kythera, Lumenis, Merz, Miramar, Sebacia, Sienna Labs, Solta, Zeltiq, and Zift.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – In the past few years, there have been “exciting” advances in noninvasive techniques to permanently remove fat, according to Suzanne Kilmer, MD, of the department of dermatology, University of California, Davis.
There are devices now available to reduce fat “in a permanent way that’s not injurious, it’s not uncomfortable – patients love it,” Dr. Kilmer said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Currently, the three noninvasive modalities she considers most effective for reducing fat are cryolipolysis, the 1,060-nm laser, and focused pulsed ultrasound. In the interview, she discussed the ways these treatments work and their safety.
With all three, “the fat that goes away stays away,” said Dr. Kilmer, who is director of the Laser and Skin Surgery Center of Northern California, Sacramento. Treatment results in release of fat, which is “cleared like it is as if you ate a cheeseburger,” she added. Interestingly, she said, trials that have looked at whether the fat reduction is accompanied by weight loss have found that, in most cases, the patient’s weight remains stable.
Dr. Kilmer’s disclosures included serving as a consultant and/or researcher for Allergan, Cutera, Cynosure, Cytrellis, Kythera, Lumenis, Merz, Miramar, Sebacia, Sienna Labs, Solta, Zeltiq, and Zift.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Second-generation antipsychotics effective for stuttering
LAS VEGAS – There currently is no Food and Drug Administration–approved medication for the symptomatic treatment of stuttering, but current evidence suggests that second-generation antipsychotics risperidone, olanzapine, and asenapine are beneficial, according to Gerald A. Maguire, MD.
At an annual psychopharmacology update held by the Nevada Psychiatric Association, Dr. Maguire, who is a stutterer himself, said current clinical trials in adults with childhood onset fluency disorder support the dopamine hypothesis of stuttering, where abnormal elevated cerebral dopaminergic activity is implicated.
Older studies found haloperidol to be effective for the treatment of stuttering, but long-term patient outlook is poor because of side effects such as sedation and sexual dysfunction. In a study led by Dr. Maguire, risperidone at a dose of 0.5-2 mg daily for 6 weeks was shown to decrease the percentage of syllables stuttered, time stuttered as a percentage of total time speaking, and overall stuttering severity, compared with placebo (J Clin Psychopharmacol. 2000;20[4]:479-82). A more recent study he also led found that olanzapine at a dose of 2.5-5 mg daily for 90 days showed a significant reduction in symptoms as measured by multiple tools, including the Stuttering Severity Instrument–Third Edition, the Clinical Global Impression scale, and the subject-rated self-assessment of stuttering scale (Ann Clin Psychiatry. 2004;16[2]:63-7).
Dr. Maguire, also associate dean of graduate medical education at the University of California, Riverside medical school, added that findings from several case reports suggest that asenapine and aripiprazole also are effective for managing stuttering symptoms. “Further studies of these drugs are warranted given better side-effect profiles as compared to other second-generation antipsychotics,” he said. There are ongoing investigations of other medications for the treatment of stuttering, including ecopipam, a D-1 dopamine receptor antagonist. An open-label study of ecopipam in adults who stutter is currently under way.
Dr. Maguire disclosed that he has received grant or research support from Intracellular, the Stanley Foundation, and Sunovion. He also is a consultant for Lundbeck, Otsuka, Sunovion, Takeda, and is a member of the speakers’ bureau or has received honoraria from Acadia, Lundbeck, Otsuka, Sunovion, Takeda, and Vanda.
LAS VEGAS – There currently is no Food and Drug Administration–approved medication for the symptomatic treatment of stuttering, but current evidence suggests that second-generation antipsychotics risperidone, olanzapine, and asenapine are beneficial, according to Gerald A. Maguire, MD.
At an annual psychopharmacology update held by the Nevada Psychiatric Association, Dr. Maguire, who is a stutterer himself, said current clinical trials in adults with childhood onset fluency disorder support the dopamine hypothesis of stuttering, where abnormal elevated cerebral dopaminergic activity is implicated.
Older studies found haloperidol to be effective for the treatment of stuttering, but long-term patient outlook is poor because of side effects such as sedation and sexual dysfunction. In a study led by Dr. Maguire, risperidone at a dose of 0.5-2 mg daily for 6 weeks was shown to decrease the percentage of syllables stuttered, time stuttered as a percentage of total time speaking, and overall stuttering severity, compared with placebo (J Clin Psychopharmacol. 2000;20[4]:479-82). A more recent study he also led found that olanzapine at a dose of 2.5-5 mg daily for 90 days showed a significant reduction in symptoms as measured by multiple tools, including the Stuttering Severity Instrument–Third Edition, the Clinical Global Impression scale, and the subject-rated self-assessment of stuttering scale (Ann Clin Psychiatry. 2004;16[2]:63-7).
Dr. Maguire, also associate dean of graduate medical education at the University of California, Riverside medical school, added that findings from several case reports suggest that asenapine and aripiprazole also are effective for managing stuttering symptoms. “Further studies of these drugs are warranted given better side-effect profiles as compared to other second-generation antipsychotics,” he said. There are ongoing investigations of other medications for the treatment of stuttering, including ecopipam, a D-1 dopamine receptor antagonist. An open-label study of ecopipam in adults who stutter is currently under way.
Dr. Maguire disclosed that he has received grant or research support from Intracellular, the Stanley Foundation, and Sunovion. He also is a consultant for Lundbeck, Otsuka, Sunovion, Takeda, and is a member of the speakers’ bureau or has received honoraria from Acadia, Lundbeck, Otsuka, Sunovion, Takeda, and Vanda.
LAS VEGAS – There currently is no Food and Drug Administration–approved medication for the symptomatic treatment of stuttering, but current evidence suggests that second-generation antipsychotics risperidone, olanzapine, and asenapine are beneficial, according to Gerald A. Maguire, MD.
At an annual psychopharmacology update held by the Nevada Psychiatric Association, Dr. Maguire, who is a stutterer himself, said current clinical trials in adults with childhood onset fluency disorder support the dopamine hypothesis of stuttering, where abnormal elevated cerebral dopaminergic activity is implicated.
Older studies found haloperidol to be effective for the treatment of stuttering, but long-term patient outlook is poor because of side effects such as sedation and sexual dysfunction. In a study led by Dr. Maguire, risperidone at a dose of 0.5-2 mg daily for 6 weeks was shown to decrease the percentage of syllables stuttered, time stuttered as a percentage of total time speaking, and overall stuttering severity, compared with placebo (J Clin Psychopharmacol. 2000;20[4]:479-82). A more recent study he also led found that olanzapine at a dose of 2.5-5 mg daily for 90 days showed a significant reduction in symptoms as measured by multiple tools, including the Stuttering Severity Instrument–Third Edition, the Clinical Global Impression scale, and the subject-rated self-assessment of stuttering scale (Ann Clin Psychiatry. 2004;16[2]:63-7).
Dr. Maguire, also associate dean of graduate medical education at the University of California, Riverside medical school, added that findings from several case reports suggest that asenapine and aripiprazole also are effective for managing stuttering symptoms. “Further studies of these drugs are warranted given better side-effect profiles as compared to other second-generation antipsychotics,” he said. There are ongoing investigations of other medications for the treatment of stuttering, including ecopipam, a D-1 dopamine receptor antagonist. An open-label study of ecopipam in adults who stutter is currently under way.
Dr. Maguire disclosed that he has received grant or research support from Intracellular, the Stanley Foundation, and Sunovion. He also is a consultant for Lundbeck, Otsuka, Sunovion, Takeda, and is a member of the speakers’ bureau or has received honoraria from Acadia, Lundbeck, Otsuka, Sunovion, Takeda, and Vanda.
AT THE NPA PSYCHOPHARMACOLOGY UPDATE
VIDEO: Infectious enteritis quadrupled short-term risk of IBS
More than 10% of patients developed irritable bowel syndrome (IBS) within a year after infectious enteritis, which gave them a more than fourfold greater risk than that of controls, according to a systematic review and meta-analysis of 45 studies.
“Protozoal and bacterial enteritis confer the greatest overall risk, although the magnitude of increased risk diminishes with time since exposure,” Fabiane B. Klem, MD, and Akhilesh Wadhwa, MD, of Mayo Clinic in Rochester, Minn., and their associates wrote in the April issue of Gastroenterology. Other significant risk factors for postinfectious IBS (PI-IBS) included female sex, clinically severe infections, antibiotic therapy, and comorbid psychological distress, they said.
Postinfectious IBS can last at least a decade after resolution of campylobacteriosis, shigellosis, salmonellosis, giardiasis, and norovirus infections, even when patients have no other risk factors for IBS, the researchers noted. To update and expand the most recent meta-analysis of this topic (Aliment Pharmacol Ther. 2007;26:535-44), the investigators searched Ovid Medline, EMBASE, Web of Science, and Cochrane Database of Systematic Reviews for studies published from 2006 through Aug. 31, 2015. This search yielded 45 studies, including 30 studies comparing infected patients with controls, who were usually matched by age, sex, and geographic location (Gastroenterology. 2017 Jan 6. doi: 10.1053/j.gastro.2016.12.039).
In all, 10.1% of patients with infectious enteritis developed IBS in the next 12 months (95% confidence interval, 7.2-14.1) – a 4.2-fold increase in risk, compared with that of controls (risk ratio, 4.2; 95% CI, 3.2-5.7). This risk subsequently dropped, but remained significantly elevated (RR, 2.3; 95% CI, 1.8-3.0), compared with controls. “Of patients with enteritis caused by protozoa or parasites, 41.9% developed IBS; of patients with enteritis caused by bacterial infection, 13.8% developed IBS,” the researchers emphasized. Patients with these infections remained at elevated risk of PI-IBS even after 1 year. Viral enteritis also significantly increased the risk of PI-IBS, but risk dropped to baseline levels after a year.
Among 10 pooled studies of IBS subtypes, 46% of patients had mixed IBS, 39% had diarrhea-predominant IBS, and 15% had constipation-predominant IBS. Female sex doubled the odds of PI-IBS (odds ratio, 2.2; 95% CI, 1.6-3.1) in 11 pooled studies. Significant clinical risk factors for PI-IBS included diarrhea lasting more than 7 days (eight studies; OR, 2.6; 95% CI, 1.5-4.6), bloody stool (four studies; OR, 1.9; 95% CI, 1.1-3.0), and antibiotic therapy during infectious enteritis (seven studies; OR, 1.7; 95% CI, 1.2-2.4).
Multiple reports linked PI-IBS to clinical psychological distress at the time of infectious enteritis. Specific risk factors included depression based on the Hospitalization Anxiety and Depression Scale (five studies; OR, 1.5; 95% CI, 1.2-1.9), anxiety based on the Hospital Anxiety and Depression Scale (four studies; OR, 2.0; 95% CI, 1.3-2.9), somatization (four studies; OR, 4.1; 95% CI, 2.7-6.0), and neuroticism (two studies; OR, 3.3; 95% CI, 1.6-6.6). Isolated studies also implicated hypochondriasis, extroversion, negative illness beliefs, stress, sleep disturbance, and adverse life events in the preceding year, the researchers said.
They found no evidence of publication bias, but noted a substantial amount of heterogeneity among studies. Also, some studies did not report multivariate analyses, so individual odds ratios might reflect “a conglomeration of factors,” they said.
The National Institutes of Health and the American Gastroenterological Association funded the work. The investigators reported having no conflicts of interest.
Source: American Gastroenterological Association
The phenomenon of IBS developing after a bout of gastroenteritis (postinfectious [PI]–IBS) was first reported in 1950 and subsequently elaborated by studies from Oxford (Q J Med. 1962;123:307-22), Sheffield (Gut. 1999;44:400-6), and Nottingham (BMJ 1997;314:779-82; Gut. 2000;47:804-11). It has proven to be a fertile area for research, which is the basis for this excellent meta-analysis.
The authors identified 45 studies, 29 in the last decade including a total of 21,421 participants with exposure to gastroenteritis. The pooled prevalence for PI-IBS was 11.5% (95% CI, 8.2%-15.8%) but with considerable heterogeneity, which the authors attempted to explain by a number of subgroup analyses. The authors report that protozoal infection seems to have a higher rate of PI-IBS than bacterial or viral infection, though some caution is warranted, since these figures rely on reports from just one outbreak of giardiasis in Bergen, Norway (Scand J Gastroenterol. 2012;47:956-61). However, if true, this might suggest that a different immune response could be responsible, a feature which others have suggested might predispose particular individuals to PI-IBS (Gut. 2016;65[8]1279-88).
The meta-analysis confirms the consistent increased risk in female patients (odds ratio, 1.69), anxiety (OR, 1.97), and somatization (greatest RR, 4.05), all common risks for the development of IBS but not specific to PI-IBS. Initial disease severity indicators, including bloody stool and more than 7 days of initial illness, which might indicate the severity of underlying damage to the gut, were shown to be significant risk factors. Animal studies of acute infection, particularly parasitic infestation, indicate that significant changes can be seen in both nerve and muscle, but routine histology in PI-IBS patients is normal. Infection produces a striking increase in gut permeability (Gut 2000;47:804-11), a feature of IBS whose molecular basis has been demonstrated by a series of elegant studies (Gut. 2017 Jan 12 [Epub ahead of print]; Gut. 2015;64:1379-88) demonstrating altered tight junctions and immune activation in IBS with diarrhea. The authors found treatment with antibiotics increased the risk of PI-IBS but whether this is attributable to confounding by indication is unclear.
This meta-analysis indicates that PI-IBS also potentially is the most common cause of IBS, given that both the Centers for Disease Control and Prevention in the United States and community surveys in the United Kingdom (BMJ. 1999;318:1046-50) indicate that gastroenteritis affects around 1 in 5 of the population each year. If the incidence of PI-IBS is around 10%, modeling suggests PI-IBS could account for the majority of new cases (J Neurogastroenterol Motil. 2012;18:200-4).
Dr. Robin Spiller is professor of gastroenterology, NIHR Nottingham Digestive Diseases Biomedical Research Unit, Nottingham Digestive Diseases Centre, University of Nottingham, England. He has no relevant conflicts of interest.
The phenomenon of IBS developing after a bout of gastroenteritis (postinfectious [PI]–IBS) was first reported in 1950 and subsequently elaborated by studies from Oxford (Q J Med. 1962;123:307-22), Sheffield (Gut. 1999;44:400-6), and Nottingham (BMJ 1997;314:779-82; Gut. 2000;47:804-11). It has proven to be a fertile area for research, which is the basis for this excellent meta-analysis.
The authors identified 45 studies, 29 in the last decade including a total of 21,421 participants with exposure to gastroenteritis. The pooled prevalence for PI-IBS was 11.5% (95% CI, 8.2%-15.8%) but with considerable heterogeneity, which the authors attempted to explain by a number of subgroup analyses. The authors report that protozoal infection seems to have a higher rate of PI-IBS than bacterial or viral infection, though some caution is warranted, since these figures rely on reports from just one outbreak of giardiasis in Bergen, Norway (Scand J Gastroenterol. 2012;47:956-61). However, if true, this might suggest that a different immune response could be responsible, a feature which others have suggested might predispose particular individuals to PI-IBS (Gut. 2016;65[8]1279-88).
The meta-analysis confirms the consistent increased risk in female patients (odds ratio, 1.69), anxiety (OR, 1.97), and somatization (greatest RR, 4.05), all common risks for the development of IBS but not specific to PI-IBS. Initial disease severity indicators, including bloody stool and more than 7 days of initial illness, which might indicate the severity of underlying damage to the gut, were shown to be significant risk factors. Animal studies of acute infection, particularly parasitic infestation, indicate that significant changes can be seen in both nerve and muscle, but routine histology in PI-IBS patients is normal. Infection produces a striking increase in gut permeability (Gut 2000;47:804-11), a feature of IBS whose molecular basis has been demonstrated by a series of elegant studies (Gut. 2017 Jan 12 [Epub ahead of print]; Gut. 2015;64:1379-88) demonstrating altered tight junctions and immune activation in IBS with diarrhea. The authors found treatment with antibiotics increased the risk of PI-IBS but whether this is attributable to confounding by indication is unclear.
This meta-analysis indicates that PI-IBS also potentially is the most common cause of IBS, given that both the Centers for Disease Control and Prevention in the United States and community surveys in the United Kingdom (BMJ. 1999;318:1046-50) indicate that gastroenteritis affects around 1 in 5 of the population each year. If the incidence of PI-IBS is around 10%, modeling suggests PI-IBS could account for the majority of new cases (J Neurogastroenterol Motil. 2012;18:200-4).
Dr. Robin Spiller is professor of gastroenterology, NIHR Nottingham Digestive Diseases Biomedical Research Unit, Nottingham Digestive Diseases Centre, University of Nottingham, England. He has no relevant conflicts of interest.
The phenomenon of IBS developing after a bout of gastroenteritis (postinfectious [PI]–IBS) was first reported in 1950 and subsequently elaborated by studies from Oxford (Q J Med. 1962;123:307-22), Sheffield (Gut. 1999;44:400-6), and Nottingham (BMJ 1997;314:779-82; Gut. 2000;47:804-11). It has proven to be a fertile area for research, which is the basis for this excellent meta-analysis.
The authors identified 45 studies, 29 in the last decade including a total of 21,421 participants with exposure to gastroenteritis. The pooled prevalence for PI-IBS was 11.5% (95% CI, 8.2%-15.8%) but with considerable heterogeneity, which the authors attempted to explain by a number of subgroup analyses. The authors report that protozoal infection seems to have a higher rate of PI-IBS than bacterial or viral infection, though some caution is warranted, since these figures rely on reports from just one outbreak of giardiasis in Bergen, Norway (Scand J Gastroenterol. 2012;47:956-61). However, if true, this might suggest that a different immune response could be responsible, a feature which others have suggested might predispose particular individuals to PI-IBS (Gut. 2016;65[8]1279-88).
The meta-analysis confirms the consistent increased risk in female patients (odds ratio, 1.69), anxiety (OR, 1.97), and somatization (greatest RR, 4.05), all common risks for the development of IBS but not specific to PI-IBS. Initial disease severity indicators, including bloody stool and more than 7 days of initial illness, which might indicate the severity of underlying damage to the gut, were shown to be significant risk factors. Animal studies of acute infection, particularly parasitic infestation, indicate that significant changes can be seen in both nerve and muscle, but routine histology in PI-IBS patients is normal. Infection produces a striking increase in gut permeability (Gut 2000;47:804-11), a feature of IBS whose molecular basis has been demonstrated by a series of elegant studies (Gut. 2017 Jan 12 [Epub ahead of print]; Gut. 2015;64:1379-88) demonstrating altered tight junctions and immune activation in IBS with diarrhea. The authors found treatment with antibiotics increased the risk of PI-IBS but whether this is attributable to confounding by indication is unclear.
This meta-analysis indicates that PI-IBS also potentially is the most common cause of IBS, given that both the Centers for Disease Control and Prevention in the United States and community surveys in the United Kingdom (BMJ. 1999;318:1046-50) indicate that gastroenteritis affects around 1 in 5 of the population each year. If the incidence of PI-IBS is around 10%, modeling suggests PI-IBS could account for the majority of new cases (J Neurogastroenterol Motil. 2012;18:200-4).
Dr. Robin Spiller is professor of gastroenterology, NIHR Nottingham Digestive Diseases Biomedical Research Unit, Nottingham Digestive Diseases Centre, University of Nottingham, England. He has no relevant conflicts of interest.
More than 10% of patients developed irritable bowel syndrome (IBS) within a year after infectious enteritis, which gave them a more than fourfold greater risk than that of controls, according to a systematic review and meta-analysis of 45 studies.
“Protozoal and bacterial enteritis confer the greatest overall risk, although the magnitude of increased risk diminishes with time since exposure,” Fabiane B. Klem, MD, and Akhilesh Wadhwa, MD, of Mayo Clinic in Rochester, Minn., and their associates wrote in the April issue of Gastroenterology. Other significant risk factors for postinfectious IBS (PI-IBS) included female sex, clinically severe infections, antibiotic therapy, and comorbid psychological distress, they said.
Postinfectious IBS can last at least a decade after resolution of campylobacteriosis, shigellosis, salmonellosis, giardiasis, and norovirus infections, even when patients have no other risk factors for IBS, the researchers noted. To update and expand the most recent meta-analysis of this topic (Aliment Pharmacol Ther. 2007;26:535-44), the investigators searched Ovid Medline, EMBASE, Web of Science, and Cochrane Database of Systematic Reviews for studies published from 2006 through Aug. 31, 2015. This search yielded 45 studies, including 30 studies comparing infected patients with controls, who were usually matched by age, sex, and geographic location (Gastroenterology. 2017 Jan 6. doi: 10.1053/j.gastro.2016.12.039).
In all, 10.1% of patients with infectious enteritis developed IBS in the next 12 months (95% confidence interval, 7.2-14.1) – a 4.2-fold increase in risk, compared with that of controls (risk ratio, 4.2; 95% CI, 3.2-5.7). This risk subsequently dropped, but remained significantly elevated (RR, 2.3; 95% CI, 1.8-3.0), compared with controls. “Of patients with enteritis caused by protozoa or parasites, 41.9% developed IBS; of patients with enteritis caused by bacterial infection, 13.8% developed IBS,” the researchers emphasized. Patients with these infections remained at elevated risk of PI-IBS even after 1 year. Viral enteritis also significantly increased the risk of PI-IBS, but risk dropped to baseline levels after a year.
Among 10 pooled studies of IBS subtypes, 46% of patients had mixed IBS, 39% had diarrhea-predominant IBS, and 15% had constipation-predominant IBS. Female sex doubled the odds of PI-IBS (odds ratio, 2.2; 95% CI, 1.6-3.1) in 11 pooled studies. Significant clinical risk factors for PI-IBS included diarrhea lasting more than 7 days (eight studies; OR, 2.6; 95% CI, 1.5-4.6), bloody stool (four studies; OR, 1.9; 95% CI, 1.1-3.0), and antibiotic therapy during infectious enteritis (seven studies; OR, 1.7; 95% CI, 1.2-2.4).
Multiple reports linked PI-IBS to clinical psychological distress at the time of infectious enteritis. Specific risk factors included depression based on the Hospitalization Anxiety and Depression Scale (five studies; OR, 1.5; 95% CI, 1.2-1.9), anxiety based on the Hospital Anxiety and Depression Scale (four studies; OR, 2.0; 95% CI, 1.3-2.9), somatization (four studies; OR, 4.1; 95% CI, 2.7-6.0), and neuroticism (two studies; OR, 3.3; 95% CI, 1.6-6.6). Isolated studies also implicated hypochondriasis, extroversion, negative illness beliefs, stress, sleep disturbance, and adverse life events in the preceding year, the researchers said.
They found no evidence of publication bias, but noted a substantial amount of heterogeneity among studies. Also, some studies did not report multivariate analyses, so individual odds ratios might reflect “a conglomeration of factors,” they said.
The National Institutes of Health and the American Gastroenterological Association funded the work. The investigators reported having no conflicts of interest.
Source: American Gastroenterological Association
More than 10% of patients developed irritable bowel syndrome (IBS) within a year after infectious enteritis, which gave them a more than fourfold greater risk than that of controls, according to a systematic review and meta-analysis of 45 studies.
“Protozoal and bacterial enteritis confer the greatest overall risk, although the magnitude of increased risk diminishes with time since exposure,” Fabiane B. Klem, MD, and Akhilesh Wadhwa, MD, of Mayo Clinic in Rochester, Minn., and their associates wrote in the April issue of Gastroenterology. Other significant risk factors for postinfectious IBS (PI-IBS) included female sex, clinically severe infections, antibiotic therapy, and comorbid psychological distress, they said.
Postinfectious IBS can last at least a decade after resolution of campylobacteriosis, shigellosis, salmonellosis, giardiasis, and norovirus infections, even when patients have no other risk factors for IBS, the researchers noted. To update and expand the most recent meta-analysis of this topic (Aliment Pharmacol Ther. 2007;26:535-44), the investigators searched Ovid Medline, EMBASE, Web of Science, and Cochrane Database of Systematic Reviews for studies published from 2006 through Aug. 31, 2015. This search yielded 45 studies, including 30 studies comparing infected patients with controls, who were usually matched by age, sex, and geographic location (Gastroenterology. 2017 Jan 6. doi: 10.1053/j.gastro.2016.12.039).
In all, 10.1% of patients with infectious enteritis developed IBS in the next 12 months (95% confidence interval, 7.2-14.1) – a 4.2-fold increase in risk, compared with that of controls (risk ratio, 4.2; 95% CI, 3.2-5.7). This risk subsequently dropped, but remained significantly elevated (RR, 2.3; 95% CI, 1.8-3.0), compared with controls. “Of patients with enteritis caused by protozoa or parasites, 41.9% developed IBS; of patients with enteritis caused by bacterial infection, 13.8% developed IBS,” the researchers emphasized. Patients with these infections remained at elevated risk of PI-IBS even after 1 year. Viral enteritis also significantly increased the risk of PI-IBS, but risk dropped to baseline levels after a year.
Among 10 pooled studies of IBS subtypes, 46% of patients had mixed IBS, 39% had diarrhea-predominant IBS, and 15% had constipation-predominant IBS. Female sex doubled the odds of PI-IBS (odds ratio, 2.2; 95% CI, 1.6-3.1) in 11 pooled studies. Significant clinical risk factors for PI-IBS included diarrhea lasting more than 7 days (eight studies; OR, 2.6; 95% CI, 1.5-4.6), bloody stool (four studies; OR, 1.9; 95% CI, 1.1-3.0), and antibiotic therapy during infectious enteritis (seven studies; OR, 1.7; 95% CI, 1.2-2.4).
Multiple reports linked PI-IBS to clinical psychological distress at the time of infectious enteritis. Specific risk factors included depression based on the Hospitalization Anxiety and Depression Scale (five studies; OR, 1.5; 95% CI, 1.2-1.9), anxiety based on the Hospital Anxiety and Depression Scale (four studies; OR, 2.0; 95% CI, 1.3-2.9), somatization (four studies; OR, 4.1; 95% CI, 2.7-6.0), and neuroticism (two studies; OR, 3.3; 95% CI, 1.6-6.6). Isolated studies also implicated hypochondriasis, extroversion, negative illness beliefs, stress, sleep disturbance, and adverse life events in the preceding year, the researchers said.
They found no evidence of publication bias, but noted a substantial amount of heterogeneity among studies. Also, some studies did not report multivariate analyses, so individual odds ratios might reflect “a conglomeration of factors,” they said.
The National Institutes of Health and the American Gastroenterological Association funded the work. The investigators reported having no conflicts of interest.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point.
Major finding: A total of 10.1% of patients with infectious enteritis developed IBS in the next 12 months, a 4.2-fold increase in risk, compared with that of controls.
Data source: A systematic review and meta-analysis of 45 studies.
Disclosures: The National Institutes of Health and the American Gastroenterological Association funded the work. The investigators reported having no conflicts of interest.