User login
Expert to psychiatrists: Collaborative care is here to stay
SCOTTSDALE, ARIZ. – Whether or not the Affordable Care Act (ACA) is repealed, replaced, reviled, or revered should not deter psychiatrists and primary care physicians from seeking to work together, according to a leading expert on integrating mental health care in medical practice.
“Community-based psychiatrists should be focused on finding ways to help create integrated models of care, both in health systems and in other settings, to provide mental health specialty support for primary care providers, regardless of whatever else is going on [in Washington],” Paul Summergrad, MD, said in an interview at the annual meeting of the American College of Psychiatrists.
“If Medicaid expansion is replaced with block grants, that would lead to less care in general for Medicaid recipients,” Dr. Summergrad said in the interview. “It depends on how the states would view their costs of care, and how they would view the medical psychiatric issues.”
Since Medicaid is the largest payer of mental and behavioral health services in the country with reimbursable models of collaborative care, cutting funding for those services would sting. “There’s evidence that addressing mental and behavioral health issues keeps medical costs low,” said Dr. Summergrad, a past president of the American Psychiatric Association.
In his talk, Dr. Summergrad pointed to the now decade-old IMPACT study (Improving Mood: Promoting Access to Collaborative Treatment) which showed that patients screened for depression when presenting with chronic medical issues had lower overall medical costs over time.
He also referred to a more recent study in a large Utah health system that showed overall cost savings, far-fewer emergency department admissions, and better patient outcomes across a wide range of medical issues, which were achieved when mental health services were integrated into routine care: in all, a $12 million investment resulted in $52 million in savings after 4 years (JAMA. 2016;316[8]:826-34). “Utah is not exactly a blue state, but it worked for them,” Dr. Summergrad said. The key was that physicians “embraced normalizing mental health care,” he said.
As for how any changes to the Medicare Access and CHIP Reauthorization Act, which is predicated largely on team-based care for higher reimbursements, Dr. Summergrad said cash-only psychiatrists should be thinking about how to collaborate with primary care providers. “I think the important message at this point is that, however this works, we’ll have to think about integrated care.”
In an interview, Lee H. Beecher, MD, agreed. In fact, Dr. Beecher said that, in his state of Minnesota, engaging with patients on a cash-only basis is the only way a psychiatrist can have a private practice and provide valuable, essential communication with primary care physicians. “Direct-pay physicians are uniquely able to actively facilitate communication,” said Dr. Beecher, president of the nonprofit Minnesota Physician-Patient Alliance. “Most of this is done by phone, directly, with the primary care clinician – rather than inputting and sending an electronic health record.”
Dr. Summergrad had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Whether or not the Affordable Care Act (ACA) is repealed, replaced, reviled, or revered should not deter psychiatrists and primary care physicians from seeking to work together, according to a leading expert on integrating mental health care in medical practice.
“Community-based psychiatrists should be focused on finding ways to help create integrated models of care, both in health systems and in other settings, to provide mental health specialty support for primary care providers, regardless of whatever else is going on [in Washington],” Paul Summergrad, MD, said in an interview at the annual meeting of the American College of Psychiatrists.
“If Medicaid expansion is replaced with block grants, that would lead to less care in general for Medicaid recipients,” Dr. Summergrad said in the interview. “It depends on how the states would view their costs of care, and how they would view the medical psychiatric issues.”
Since Medicaid is the largest payer of mental and behavioral health services in the country with reimbursable models of collaborative care, cutting funding for those services would sting. “There’s evidence that addressing mental and behavioral health issues keeps medical costs low,” said Dr. Summergrad, a past president of the American Psychiatric Association.
In his talk, Dr. Summergrad pointed to the now decade-old IMPACT study (Improving Mood: Promoting Access to Collaborative Treatment) which showed that patients screened for depression when presenting with chronic medical issues had lower overall medical costs over time.
He also referred to a more recent study in a large Utah health system that showed overall cost savings, far-fewer emergency department admissions, and better patient outcomes across a wide range of medical issues, which were achieved when mental health services were integrated into routine care: in all, a $12 million investment resulted in $52 million in savings after 4 years (JAMA. 2016;316[8]:826-34). “Utah is not exactly a blue state, but it worked for them,” Dr. Summergrad said. The key was that physicians “embraced normalizing mental health care,” he said.
As for how any changes to the Medicare Access and CHIP Reauthorization Act, which is predicated largely on team-based care for higher reimbursements, Dr. Summergrad said cash-only psychiatrists should be thinking about how to collaborate with primary care providers. “I think the important message at this point is that, however this works, we’ll have to think about integrated care.”
In an interview, Lee H. Beecher, MD, agreed. In fact, Dr. Beecher said that, in his state of Minnesota, engaging with patients on a cash-only basis is the only way a psychiatrist can have a private practice and provide valuable, essential communication with primary care physicians. “Direct-pay physicians are uniquely able to actively facilitate communication,” said Dr. Beecher, president of the nonprofit Minnesota Physician-Patient Alliance. “Most of this is done by phone, directly, with the primary care clinician – rather than inputting and sending an electronic health record.”
Dr. Summergrad had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Whether or not the Affordable Care Act (ACA) is repealed, replaced, reviled, or revered should not deter psychiatrists and primary care physicians from seeking to work together, according to a leading expert on integrating mental health care in medical practice.
“Community-based psychiatrists should be focused on finding ways to help create integrated models of care, both in health systems and in other settings, to provide mental health specialty support for primary care providers, regardless of whatever else is going on [in Washington],” Paul Summergrad, MD, said in an interview at the annual meeting of the American College of Psychiatrists.
“If Medicaid expansion is replaced with block grants, that would lead to less care in general for Medicaid recipients,” Dr. Summergrad said in the interview. “It depends on how the states would view their costs of care, and how they would view the medical psychiatric issues.”
Since Medicaid is the largest payer of mental and behavioral health services in the country with reimbursable models of collaborative care, cutting funding for those services would sting. “There’s evidence that addressing mental and behavioral health issues keeps medical costs low,” said Dr. Summergrad, a past president of the American Psychiatric Association.
In his talk, Dr. Summergrad pointed to the now decade-old IMPACT study (Improving Mood: Promoting Access to Collaborative Treatment) which showed that patients screened for depression when presenting with chronic medical issues had lower overall medical costs over time.
He also referred to a more recent study in a large Utah health system that showed overall cost savings, far-fewer emergency department admissions, and better patient outcomes across a wide range of medical issues, which were achieved when mental health services were integrated into routine care: in all, a $12 million investment resulted in $52 million in savings after 4 years (JAMA. 2016;316[8]:826-34). “Utah is not exactly a blue state, but it worked for them,” Dr. Summergrad said. The key was that physicians “embraced normalizing mental health care,” he said.
As for how any changes to the Medicare Access and CHIP Reauthorization Act, which is predicated largely on team-based care for higher reimbursements, Dr. Summergrad said cash-only psychiatrists should be thinking about how to collaborate with primary care providers. “I think the important message at this point is that, however this works, we’ll have to think about integrated care.”
In an interview, Lee H. Beecher, MD, agreed. In fact, Dr. Beecher said that, in his state of Minnesota, engaging with patients on a cash-only basis is the only way a psychiatrist can have a private practice and provide valuable, essential communication with primary care physicians. “Direct-pay physicians are uniquely able to actively facilitate communication,” said Dr. Beecher, president of the nonprofit Minnesota Physician-Patient Alliance. “Most of this is done by phone, directly, with the primary care clinician – rather than inputting and sending an electronic health record.”
Dr. Summergrad had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS AT THE AMERICAN COLLEGE OF PSYCHIATRISTS ANNUAL MEETING
Onlay mesh with adhesive just as safe as sublay route
While using sublay mesh continues to be standard practice when performing ventral hernia repair (VHR), a recent study shows that using onlay mesh placement with adhesive can be just as safe, at least in the short term.
“While the use of mesh during VHR is well accepted, the ideal location of mesh placement remains heavily debated,” wrote the study’s authors, adding that the “paucity of high-level data has led the choice of mesh location to reside primarily on the preference of the surgeon rather than grounded in clinical outcomes.”
The investigators identified patients in the Americas Hernia Society Quality Collaborative national registry who were undergoing open, elective VHR and had clean wounds and a wound class I designation based on Centers for Disease Control and Prevention guidelines at any point between January 2013 and January 2016. A total of 1,854 individuals were ultimately selected for inclusion in the study and were divided into two groups: one that received traditional VHR with sublay mesh and one that received onlay mesh with adhesive.
All subjects’ data were analyzed within 30 days for any adverse events related to the wounds from the surgery. These events were surgical site infections, surgical site occurrences – which could include an infection and any skin or soft tissue ischemia, necrosis, or other events – and surgical site occurrences that required procedural intervention, which were defined as any occurrences that required “opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.”
The sublay cohort numbered 1,761 (95.0%), compared with 93 (5.0%) who received the onlay technique. There was no significant difference found in the rate of 30-day adverse incidents between the two cohorts. For surgical site infections, the sublay cohort rate was 2.9%, while the onlay cohort had a 5.5% rate (P = .30). Surgical site occurrences happened in 15.2% of sublay patients versus 7.7% of those in the other group (P = .08), while surgical site occurrences that required procedural intervention were 8.2% in sublay patients but 5.5% in onlay patients (P = .42).
While both approaches fared similarly in terms of comorbidities and average Ventral Hernia Working Group grade, the investigators recommend that “the Chevrel onlay technique be used in nonobese patients without significant comorbidities, with moderate hernia defects, and whose abdominal wall vasculatures are without risk of compromise.” The data were generalizable because of the number of surgeons performing VHR and because the data sample allowed the investigators to control for the hernia width, defect size, and patient comorbidities this case, leading to this conclusion.
“Additional studies are needed to determine the long-term benefits of both approaches with respect to mesh infection rates and hernia recurrence rates, as well as the ideal mesh location for ventral hernia repairs in higher-risk patients,” the authors concluded.
No funding source was disclosed for this study. The investigators reported no relevant financial disclosures.
While using sublay mesh continues to be standard practice when performing ventral hernia repair (VHR), a recent study shows that using onlay mesh placement with adhesive can be just as safe, at least in the short term.
“While the use of mesh during VHR is well accepted, the ideal location of mesh placement remains heavily debated,” wrote the study’s authors, adding that the “paucity of high-level data has led the choice of mesh location to reside primarily on the preference of the surgeon rather than grounded in clinical outcomes.”
The investigators identified patients in the Americas Hernia Society Quality Collaborative national registry who were undergoing open, elective VHR and had clean wounds and a wound class I designation based on Centers for Disease Control and Prevention guidelines at any point between January 2013 and January 2016. A total of 1,854 individuals were ultimately selected for inclusion in the study and were divided into two groups: one that received traditional VHR with sublay mesh and one that received onlay mesh with adhesive.
All subjects’ data were analyzed within 30 days for any adverse events related to the wounds from the surgery. These events were surgical site infections, surgical site occurrences – which could include an infection and any skin or soft tissue ischemia, necrosis, or other events – and surgical site occurrences that required procedural intervention, which were defined as any occurrences that required “opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.”
The sublay cohort numbered 1,761 (95.0%), compared with 93 (5.0%) who received the onlay technique. There was no significant difference found in the rate of 30-day adverse incidents between the two cohorts. For surgical site infections, the sublay cohort rate was 2.9%, while the onlay cohort had a 5.5% rate (P = .30). Surgical site occurrences happened in 15.2% of sublay patients versus 7.7% of those in the other group (P = .08), while surgical site occurrences that required procedural intervention were 8.2% in sublay patients but 5.5% in onlay patients (P = .42).
While both approaches fared similarly in terms of comorbidities and average Ventral Hernia Working Group grade, the investigators recommend that “the Chevrel onlay technique be used in nonobese patients without significant comorbidities, with moderate hernia defects, and whose abdominal wall vasculatures are without risk of compromise.” The data were generalizable because of the number of surgeons performing VHR and because the data sample allowed the investigators to control for the hernia width, defect size, and patient comorbidities this case, leading to this conclusion.
“Additional studies are needed to determine the long-term benefits of both approaches with respect to mesh infection rates and hernia recurrence rates, as well as the ideal mesh location for ventral hernia repairs in higher-risk patients,” the authors concluded.
No funding source was disclosed for this study. The investigators reported no relevant financial disclosures.
While using sublay mesh continues to be standard practice when performing ventral hernia repair (VHR), a recent study shows that using onlay mesh placement with adhesive can be just as safe, at least in the short term.
“While the use of mesh during VHR is well accepted, the ideal location of mesh placement remains heavily debated,” wrote the study’s authors, adding that the “paucity of high-level data has led the choice of mesh location to reside primarily on the preference of the surgeon rather than grounded in clinical outcomes.”
The investigators identified patients in the Americas Hernia Society Quality Collaborative national registry who were undergoing open, elective VHR and had clean wounds and a wound class I designation based on Centers for Disease Control and Prevention guidelines at any point between January 2013 and January 2016. A total of 1,854 individuals were ultimately selected for inclusion in the study and were divided into two groups: one that received traditional VHR with sublay mesh and one that received onlay mesh with adhesive.
All subjects’ data were analyzed within 30 days for any adverse events related to the wounds from the surgery. These events were surgical site infections, surgical site occurrences – which could include an infection and any skin or soft tissue ischemia, necrosis, or other events – and surgical site occurrences that required procedural intervention, which were defined as any occurrences that required “opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.”
The sublay cohort numbered 1,761 (95.0%), compared with 93 (5.0%) who received the onlay technique. There was no significant difference found in the rate of 30-day adverse incidents between the two cohorts. For surgical site infections, the sublay cohort rate was 2.9%, while the onlay cohort had a 5.5% rate (P = .30). Surgical site occurrences happened in 15.2% of sublay patients versus 7.7% of those in the other group (P = .08), while surgical site occurrences that required procedural intervention were 8.2% in sublay patients but 5.5% in onlay patients (P = .42).
While both approaches fared similarly in terms of comorbidities and average Ventral Hernia Working Group grade, the investigators recommend that “the Chevrel onlay technique be used in nonobese patients without significant comorbidities, with moderate hernia defects, and whose abdominal wall vasculatures are without risk of compromise.” The data were generalizable because of the number of surgeons performing VHR and because the data sample allowed the investigators to control for the hernia width, defect size, and patient comorbidities this case, leading to this conclusion.
“Additional studies are needed to determine the long-term benefits of both approaches with respect to mesh infection rates and hernia recurrence rates, as well as the ideal mesh location for ventral hernia repairs in higher-risk patients,” the authors concluded.
No funding source was disclosed for this study. The investigators reported no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point:
Major finding: No significant differences were found between onlay and sublay mesh VHR patients in terms of surgical site infection (P = .30), surgical site occurrences (P = .08), and surgical site occurrences that required intervention (P = .42).
Data source: Retrospective cohort study of 1,854 VHR patients between January 2013 and January 2016.
Disclosures: No funding source disclosed. Authors reported no relevant financial disclosures.
Make the Diagnosis - February 2017
Primary Cutaneous Cryptococcosis
The differential diagnosis upon initial examination may be wide. Patients generally present with a new skin lesion that proves to be refractory to conservative or traditional therapy. The upper extremities are the most common site of PCC. In almost all cases, a previous injury at the site of inoculation can be identified. Risk factors for exposure to Cryptococcus include residence in a rural area and contact with soil contaminated by avian droppings. The average time between skin injury and onset of symptoms has been reported as 2.5 days. Although rare, it is possible for a patient to present with multiple sites of infection in the absence of dissemination. This is almost exclusively seen in immunocompromised hosts.
Because of its widely variable clinical manifestations, PCC is a diagnosis made by culture and histology. Histopathology reveals numerous yeasts and a giant cell inflammatory process. In PCC, serology should not demonstrate Cryptococcus antigen, as the disease is localized to the skin. Patients diagnosed with PCC should undergo proper work-up to rule out the possibility of an underlying immunosuppressive condition, such as infection or malignancy.
Therapy for PCC can be solely medical or a combination of medical and surgical treatments. Surgical de-bulking in combination with 6-12 months of fluconazole is often used. Early treatment is essential, as cases of Cryptococcus dissemination secondary to PCC have been reported.
This patient's workup did not reveal any underlying immunodeficiencies. He worked with heating, ventilation and air condition (HVAC) in Spain. He was referred to infectious disease and admitted to the hospital for intravenous antifungal therapy.
Case and photo courtesy of: Natasha Cowan, BS, UCSD School of Medicine, and Brooke Resh Sateesh, MD, San Deigo Family Dermatology
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Primary Cutaneous Cryptococcosis
The differential diagnosis upon initial examination may be wide. Patients generally present with a new skin lesion that proves to be refractory to conservative or traditional therapy. The upper extremities are the most common site of PCC. In almost all cases, a previous injury at the site of inoculation can be identified. Risk factors for exposure to Cryptococcus include residence in a rural area and contact with soil contaminated by avian droppings. The average time between skin injury and onset of symptoms has been reported as 2.5 days. Although rare, it is possible for a patient to present with multiple sites of infection in the absence of dissemination. This is almost exclusively seen in immunocompromised hosts.
Because of its widely variable clinical manifestations, PCC is a diagnosis made by culture and histology. Histopathology reveals numerous yeasts and a giant cell inflammatory process. In PCC, serology should not demonstrate Cryptococcus antigen, as the disease is localized to the skin. Patients diagnosed with PCC should undergo proper work-up to rule out the possibility of an underlying immunosuppressive condition, such as infection or malignancy.
Therapy for PCC can be solely medical or a combination of medical and surgical treatments. Surgical de-bulking in combination with 6-12 months of fluconazole is often used. Early treatment is essential, as cases of Cryptococcus dissemination secondary to PCC have been reported.
This patient's workup did not reveal any underlying immunodeficiencies. He worked with heating, ventilation and air condition (HVAC) in Spain. He was referred to infectious disease and admitted to the hospital for intravenous antifungal therapy.
Case and photo courtesy of: Natasha Cowan, BS, UCSD School of Medicine, and Brooke Resh Sateesh, MD, San Deigo Family Dermatology
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Primary Cutaneous Cryptococcosis
The differential diagnosis upon initial examination may be wide. Patients generally present with a new skin lesion that proves to be refractory to conservative or traditional therapy. The upper extremities are the most common site of PCC. In almost all cases, a previous injury at the site of inoculation can be identified. Risk factors for exposure to Cryptococcus include residence in a rural area and contact with soil contaminated by avian droppings. The average time between skin injury and onset of symptoms has been reported as 2.5 days. Although rare, it is possible for a patient to present with multiple sites of infection in the absence of dissemination. This is almost exclusively seen in immunocompromised hosts.
Because of its widely variable clinical manifestations, PCC is a diagnosis made by culture and histology. Histopathology reveals numerous yeasts and a giant cell inflammatory process. In PCC, serology should not demonstrate Cryptococcus antigen, as the disease is localized to the skin. Patients diagnosed with PCC should undergo proper work-up to rule out the possibility of an underlying immunosuppressive condition, such as infection or malignancy.
Therapy for PCC can be solely medical or a combination of medical and surgical treatments. Surgical de-bulking in combination with 6-12 months of fluconazole is often used. Early treatment is essential, as cases of Cryptococcus dissemination secondary to PCC have been reported.
This patient's workup did not reveal any underlying immunodeficiencies. He worked with heating, ventilation and air condition (HVAC) in Spain. He was referred to infectious disease and admitted to the hospital for intravenous antifungal therapy.
Case and photo courtesy of: Natasha Cowan, BS, UCSD School of Medicine, and Brooke Resh Sateesh, MD, San Deigo Family Dermatology
Donna Bilu Martin, MD, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
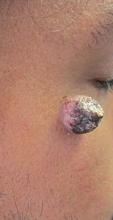
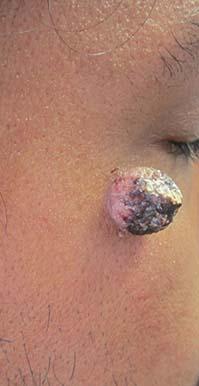
A 34-year-old healthy male with no significant past medical history presented with a rapidly enlarging lesion on the face for 2-3 weeks. He reported bleeding with mild contact/trauma. On physical examination, a 2.3cm pedunculated red-brown plaque with surrounding scattered hyperpigmented papules were present on the right cheek.
Pediatric lupus patients face large burden of serious infection
The burden of serious infection is quite high among children who have systemic lupus erythematosus, with a “striking” preponderance of bacterial pneumonia, according to a report published in Arthritis Care & Research.
Infections are known to be commonplace among systemic lupus erythematosus (SLE) patients in general, and the increased risk is attributed both to the disease and to immunosuppressant therapies. However, most information on this topic comes from studies of adult patients seen at individual academic medical centers, said Linda T. Hiraki, MD, ScD, of the division of rheumatology at The Hospital for Sick Children, Toronto, and her associates.
To examine the nationwide prevalence of serious infections among children with SLE, they analyzed administrative data from a Medicaid database. They focused on 3,500 patients aged 5-18 years, including 1,297 who also had lupus nephritis, who were enrolled in Medicaid during 2000-2006 and followed for a mean of 2.6 years. This yielded a cumulative follow-up of more than 10,100 person-years (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23219).
The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis. By comparison, this overall rate is nearly four times higher than that reported for children with juvenile idiopathic arthritis, and the incidence among children with concomitant lupus nephritis is more than six times higher.
Infection rates were markedly higher among African American (incidence rate ratio [IRR], 1.83) and Native American (IRR, 1.81) children, compared with white children. They also were higher in early adolescence (ages 9-12 years) than earlier in childhood (ages 5-8 years), the investigators said.
Most of the infections (87%) were bacterial, whereas 11% were viral and 1.3% were fungal. (The remaining amount was unknown in the data because of too few numbers for federal reporting.) The most frequent bacterial infections were pneumonia (438 cases), followed by bacteremia (274 cases) and cellulitis (272 cases). Herpes zoster was the most frequent viral infection, accounting for 81 cases. The investigators noted that the low rate of fungal infections may be an artifact of the study protocol, which excluded, for technical reasons, cases of systemic candidiasis.
Not surprisingly, the rate of serious infection was higher among children with a high comorbidity burden than among healthier children.
Overall, the risk of serious infection was 59% higher for SLE patients who took corticosteroids during the study’s 6-month baseline period, in which 67% of patients took them (minimum of 20 mg/day of prednisone equivalent). However, the risk of serious infection was no different between those who used immunosuppressants (31%) or didn’t use them during that period.
A total of 26 children died within 30 days of hospital admission for a serious infection, for an overall mortality of 4.4% among children who developed serious infections. In comparison, 1.6% in the total cohort of 3,500 died. More than half of the children who died had concomitant lupus nephritis. In addition, 77% of those who died were taking corticosteroids when they developed the infections.
It is difficult to distinguish whether the high infection rate could be attributed to SLE itself or to its treatments. More studies are needed to further investigate this, as well as to address the disproportionate incidence among nonwhite children and any potential benefits from prophylactic use of antibiotics and vaccinations, Dr. Hiraki and her associates said.
The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
The study by Hiraki et al. is important because very little is known about the risks of infection in childhood SLE, and there are few sources of data involving large numbers of affected children.
The overall rate of 10.4 serious infections necessitating hospitalization per 100 person-years reported in the study is approximately 10 times higher than the rate in the general Medicaid population. The findings should prompt further study of infection in childhood SLE so we can work toward decreasing this excessive risk.
The investigators unfortunately did not assess medication use throughout the study or try to find factors besides a high SLE risk adjustment index that were associated with infection, and these missed opportunities are the most significant weaknesses of an otherwise well-conducted study because added information about the role of disease activity and medication use would have a greater impact on clinical care than the nonetheless useful knowledge that childhood SLE is associated with a markedly increased infection rate.
Timothy Beukelman, MD, and his associates are with the University of Alabama at Birmingham. They made these remarks in an editorial accompanying Dr. Hiraki and colleagues’ report (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23221). No disclosure information was available with their editorial manuscript.
The study by Hiraki et al. is important because very little is known about the risks of infection in childhood SLE, and there are few sources of data involving large numbers of affected children.
The overall rate of 10.4 serious infections necessitating hospitalization per 100 person-years reported in the study is approximately 10 times higher than the rate in the general Medicaid population. The findings should prompt further study of infection in childhood SLE so we can work toward decreasing this excessive risk.
The investigators unfortunately did not assess medication use throughout the study or try to find factors besides a high SLE risk adjustment index that were associated with infection, and these missed opportunities are the most significant weaknesses of an otherwise well-conducted study because added information about the role of disease activity and medication use would have a greater impact on clinical care than the nonetheless useful knowledge that childhood SLE is associated with a markedly increased infection rate.
Timothy Beukelman, MD, and his associates are with the University of Alabama at Birmingham. They made these remarks in an editorial accompanying Dr. Hiraki and colleagues’ report (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23221). No disclosure information was available with their editorial manuscript.
The study by Hiraki et al. is important because very little is known about the risks of infection in childhood SLE, and there are few sources of data involving large numbers of affected children.
The overall rate of 10.4 serious infections necessitating hospitalization per 100 person-years reported in the study is approximately 10 times higher than the rate in the general Medicaid population. The findings should prompt further study of infection in childhood SLE so we can work toward decreasing this excessive risk.
The investigators unfortunately did not assess medication use throughout the study or try to find factors besides a high SLE risk adjustment index that were associated with infection, and these missed opportunities are the most significant weaknesses of an otherwise well-conducted study because added information about the role of disease activity and medication use would have a greater impact on clinical care than the nonetheless useful knowledge that childhood SLE is associated with a markedly increased infection rate.
Timothy Beukelman, MD, and his associates are with the University of Alabama at Birmingham. They made these remarks in an editorial accompanying Dr. Hiraki and colleagues’ report (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23221). No disclosure information was available with their editorial manuscript.
The burden of serious infection is quite high among children who have systemic lupus erythematosus, with a “striking” preponderance of bacterial pneumonia, according to a report published in Arthritis Care & Research.
Infections are known to be commonplace among systemic lupus erythematosus (SLE) patients in general, and the increased risk is attributed both to the disease and to immunosuppressant therapies. However, most information on this topic comes from studies of adult patients seen at individual academic medical centers, said Linda T. Hiraki, MD, ScD, of the division of rheumatology at The Hospital for Sick Children, Toronto, and her associates.
To examine the nationwide prevalence of serious infections among children with SLE, they analyzed administrative data from a Medicaid database. They focused on 3,500 patients aged 5-18 years, including 1,297 who also had lupus nephritis, who were enrolled in Medicaid during 2000-2006 and followed for a mean of 2.6 years. This yielded a cumulative follow-up of more than 10,100 person-years (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23219).
The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis. By comparison, this overall rate is nearly four times higher than that reported for children with juvenile idiopathic arthritis, and the incidence among children with concomitant lupus nephritis is more than six times higher.
Infection rates were markedly higher among African American (incidence rate ratio [IRR], 1.83) and Native American (IRR, 1.81) children, compared with white children. They also were higher in early adolescence (ages 9-12 years) than earlier in childhood (ages 5-8 years), the investigators said.
Most of the infections (87%) were bacterial, whereas 11% were viral and 1.3% were fungal. (The remaining amount was unknown in the data because of too few numbers for federal reporting.) The most frequent bacterial infections were pneumonia (438 cases), followed by bacteremia (274 cases) and cellulitis (272 cases). Herpes zoster was the most frequent viral infection, accounting for 81 cases. The investigators noted that the low rate of fungal infections may be an artifact of the study protocol, which excluded, for technical reasons, cases of systemic candidiasis.
Not surprisingly, the rate of serious infection was higher among children with a high comorbidity burden than among healthier children.
Overall, the risk of serious infection was 59% higher for SLE patients who took corticosteroids during the study’s 6-month baseline period, in which 67% of patients took them (minimum of 20 mg/day of prednisone equivalent). However, the risk of serious infection was no different between those who used immunosuppressants (31%) or didn’t use them during that period.
A total of 26 children died within 30 days of hospital admission for a serious infection, for an overall mortality of 4.4% among children who developed serious infections. In comparison, 1.6% in the total cohort of 3,500 died. More than half of the children who died had concomitant lupus nephritis. In addition, 77% of those who died were taking corticosteroids when they developed the infections.
It is difficult to distinguish whether the high infection rate could be attributed to SLE itself or to its treatments. More studies are needed to further investigate this, as well as to address the disproportionate incidence among nonwhite children and any potential benefits from prophylactic use of antibiotics and vaccinations, Dr. Hiraki and her associates said.
The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
The burden of serious infection is quite high among children who have systemic lupus erythematosus, with a “striking” preponderance of bacterial pneumonia, according to a report published in Arthritis Care & Research.
Infections are known to be commonplace among systemic lupus erythematosus (SLE) patients in general, and the increased risk is attributed both to the disease and to immunosuppressant therapies. However, most information on this topic comes from studies of adult patients seen at individual academic medical centers, said Linda T. Hiraki, MD, ScD, of the division of rheumatology at The Hospital for Sick Children, Toronto, and her associates.
To examine the nationwide prevalence of serious infections among children with SLE, they analyzed administrative data from a Medicaid database. They focused on 3,500 patients aged 5-18 years, including 1,297 who also had lupus nephritis, who were enrolled in Medicaid during 2000-2006 and followed for a mean of 2.6 years. This yielded a cumulative follow-up of more than 10,100 person-years (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23219).
The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis. By comparison, this overall rate is nearly four times higher than that reported for children with juvenile idiopathic arthritis, and the incidence among children with concomitant lupus nephritis is more than six times higher.
Infection rates were markedly higher among African American (incidence rate ratio [IRR], 1.83) and Native American (IRR, 1.81) children, compared with white children. They also were higher in early adolescence (ages 9-12 years) than earlier in childhood (ages 5-8 years), the investigators said.
Most of the infections (87%) were bacterial, whereas 11% were viral and 1.3% were fungal. (The remaining amount was unknown in the data because of too few numbers for federal reporting.) The most frequent bacterial infections were pneumonia (438 cases), followed by bacteremia (274 cases) and cellulitis (272 cases). Herpes zoster was the most frequent viral infection, accounting for 81 cases. The investigators noted that the low rate of fungal infections may be an artifact of the study protocol, which excluded, for technical reasons, cases of systemic candidiasis.
Not surprisingly, the rate of serious infection was higher among children with a high comorbidity burden than among healthier children.
Overall, the risk of serious infection was 59% higher for SLE patients who took corticosteroids during the study’s 6-month baseline period, in which 67% of patients took them (minimum of 20 mg/day of prednisone equivalent). However, the risk of serious infection was no different between those who used immunosuppressants (31%) or didn’t use them during that period.
A total of 26 children died within 30 days of hospital admission for a serious infection, for an overall mortality of 4.4% among children who developed serious infections. In comparison, 1.6% in the total cohort of 3,500 died. More than half of the children who died had concomitant lupus nephritis. In addition, 77% of those who died were taking corticosteroids when they developed the infections.
It is difficult to distinguish whether the high infection rate could be attributed to SLE itself or to its treatments. More studies are needed to further investigate this, as well as to address the disproportionate incidence among nonwhite children and any potential benefits from prophylactic use of antibiotics and vaccinations, Dr. Hiraki and her associates said.
The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The burden of serious infection is quite high among children who have SLE, with a “striking” preponderance of bacterial pneumonia.
Major finding: The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis.
Data source: A retrospective cohort study using administrative Medicaid data for 3,500 affected U.S. children aged 5-18 years.
Disclosures: The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
Outpatient flu visits down slightly
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.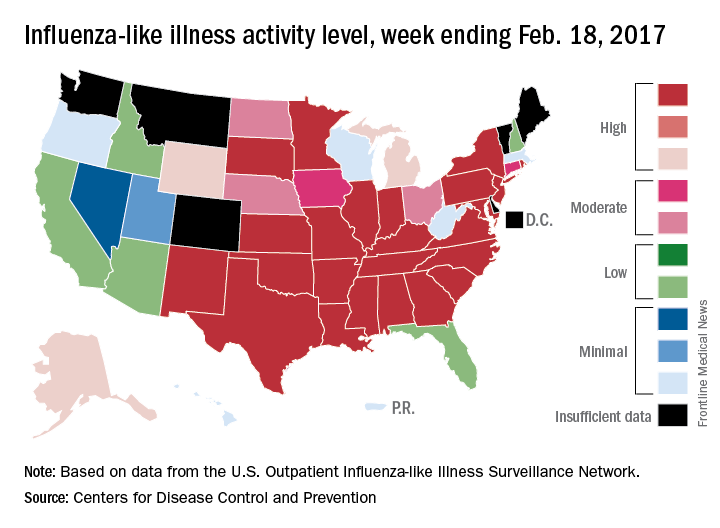
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
Allergic Reaction to Phenylephrine
Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
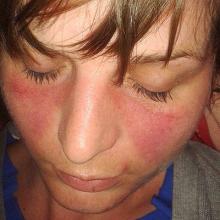
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.
This careful review of past exam notes revealed that phenylephrine and Fluress were the only drops that had not been instilled at the October 2013 visit when no AE was reported. However, Fluress was an unlikely culprit since it was not instilled in October 2014, and the patient still experienced an AE. Therefore, the agent most likely responsible for the allergic reaction in the patient, as confirmed by a review of the past notes and by the aforementioned pharmacologic test, was deemed to be phenylephrine (Table).
Adverse reactions to topical ocular medications and specifically to diagnostic eye drops have long been recognized. Mathias, Camarasa, Barber, Ducombs, and Monsálvezhave reported on variations of conjunctivitis and periorbital erythema with positive patch testing to phenylephrine.1-5 Geyer and colleagues reported on a study of 21 patients who had blepharoconjunctivitis after instillation of phenylephrine.6 In this case study patient, severe keratoconjunctivitis was the clinical manifestation observed.
Villarreal and colleagues studied 31 patients who had a previous reaction to mydriatic drops. The study found that phenylephrine was the drug that most frequently caused an AE (93.5%).7 One patient reacted to the preservative thimerosal, and 1 patient reacted to benoxiprocaine. Tropicamide was demonstrated to be very well tolerated as none of the patients tested positive on either the patch test or the pharmacologic test.
Tropicamide is a nonselective muscarinic antagonist commonly used for mydriasis due to its fast onset and short duration.8 Adverse reactions to tropicamide are rare. Three studies reported on patients who had a positive patch test to tropicamide.9-11 However, the reaction was not provoked by direct instillation of tropicamide into the eye.
Common in-office topical anesthetics, proparacaine, tetracaine, benoxinate, and lidocaine also can cause AEs. Corneal toxicity is a well-known complication with topical anesthetic abuse, whereas allergic reactions are considered rare. The most common symptoms include stingingand discomfort upon instillation. Common signs include punctate corneal epithelial erosionsresulting indirectly from a decrease in reflex tearing, infrequent blinking, and increased tear evaporation.12 Topical anesthetics also inhibit the migration of corneal epithelial cells and cause direct damage to the cells that are present, leading to impaired healing and epithelial defects.13
Manifestations of allergic reaction to topical anesthetics can include conjunctival hyperemia and edema, edematous eyelids, and lacrimation. One published case described a 60-year-old woman who developed eczematous dermatitis of the eyelids after ophthalmic anesthetic drops were instilled prior to laser surgery. Patch testing showed a positive response to benzocaine 5%, proparacaine, and tetracaine 0.5%.14
Preservatives, in general, can cause an allergic reaction. Benzalkonium chloride’s (BAK) cytotoxic sequelae include possible trabecular cell death in glaucoma patients, disruption of tear film stability (even at low concentrations), and immune-allergenic properties. One article reported BAK as one of the 30 most frequent allergens causing allergic periorbital dermatitis.15 Benzalkonium chloride is used in most brands of phenylephrine. However, preservatives in this patient’s case were ruled out as instigating agents since both phenylephrine and tropicamide contain the same preservative, BAK 0.01%, yet this patient did not develop a reaction to tropicamide when used without phenylephrine. Expired medications also were not considered to be a factor as none of the medications used on the patient were indeed expired (the Malcom Randall VAMC clinic maintains a strict policy of discarding medications 28 days after being opened).
Although uncommon, phenylephrine sometimes has been found to cause a type 4 hypersensitivity reaction, also known as cell-mediated or delayed-type hypersensitivity.16 First, helper T cells secrete cytokines. Activation of cytokines recruits and activates cytotoxic T cells, monocytes, and macrophages, leading to inflammation of the surrounding tissue. Examples of cell-mediated hypersensitivity include reactions to the tuberculin skin test and to poison ivy.
Type 1 hypersensitivity reactions, also known as immediate or anaphylactic hypersensitivity reactions, are not triggered by phenylephrine. In this type of reaction, IgE binds to the mast cell on initial exposure to an allergen. On second exposure, the allergen binds to the IgE, causing the mast cell to release mediators of inflammation, triggering physiologic responses. Examples of this type of hypersensitivity include those seen with penicillin, bee stings, hay fever, bronchial asthma, and food allergies, for example, to shellfish.
A toxic reaction’s mechanism differs from that of a type 4 hypersensitivity reaction. Toxic reactions occur due to direct cytotoxicity of a drug caused by a low or high pH and either hyper- or hypo-osmolarity. Toxicity can lead to corneal and conjunctival cell necrosis or induce apoptosis, stimulating inflammatory reactions. Clinically, toxic reactions will present with follicles, whereas allergic reactions will present with papillae.
The definitive diagnostic methods used to determine the allergic agent causing ocular or periocular AEs are patch testing and conjunctival challenge.7 Mathias, Camarasa, Barber, Ducombs,and Monsálvezused patch testing to confirm phenylephrine as the allergic agent in their series of cases. Patch testing entails the application of a small amount of an allergic agent that is taped onto the skin. The allergic agent is confirmed if the patient has a dermal reaction, wherein the area patched will become erythematous. When patch testing is negative or inconclusive, a conjunctival challenge is performed by instillation of the suspected allergic agent into the eye with subsequent observation to determine whether a reaction occurs. The sequelae found in Villarreal’s study included itching, lacrimation, edema, erythema, and sometimes blepharitis.7
A direct conjunctival challenge with the suspected culprit was not pursued in this patient’s case due to the known severity of the potential resulting reaction. The authors instead chose an indirect method of determining the implicating agent and used the process of elimination to whittle down the most likely suspect. A challenge with the medications suspected not to be likely offenders was undertaken. This spared the patient a likely repeat of the AE he had just recovered from.
Management
Allergic reactions can resolve without medical intervention. The first step is to remove the allergen. For delayed hypersensitivity reactions, treatments may include topical decongestants, cool compresses, and corticosteroids.8 The treatment for immediate hypersensitivity reaction differs from that of delayed hypersensitivity reaction in that antihistamines are used.17,18
This patient reported receiving no treatment for his ocular symptoms following eye examinations in the past, yet he experienced complete resolution after each AE. In this case, both a steroid and a prophylactic antibiotic to facilitate a more rapid improvement were used.
Conclusion
Although uncommon, cases of allergic reaction to phenylephrine can occur. The incidence of phenylephrine allergy is 0.6%.6 The case study patient presented with a severe keratoconjunctivitis following routine eye examination with an accompanying history of adverse ocular signs and symptoms following multiple past exams.
It is important for all eye care clinicians to realize that AEs to diagnostic eye drops are possible and can occur following the most routine of visits. Such reactions can be caused by dilating agents, anesthetics, or preservatives, and these may be allergic or toxic. Clinicians should take special care to identify the instigating agent, and if possible, to avoid using such agents on patients during future exams. Clinicians also should understand how best to manage iatrogenic AEs when they encounter them in order to restore a patient’s visual function as quickly as possible.
1. Mathias CG, Maibach HI, Irvine A, Adler W. Allergic contact dermatitis to echothiophate iodide and phenylephrine. Arch Ophthalmol. 1979;97(2):286-287.
2. Camarasa JG. Contact dermatitis to phenylephrine. Contact Dermatitis. 1984;10(3):182.
3. Barber K. Allergic contact eczema to phenylephrine. Contact Dermatitis. 1983;9(4):274-277.
4. Ducombs G, de Casamayor J, Verin P, Maleville J. Allergic contact dermatitis to phenylephrine. Contact Dermatitis. 1986;15(2):107-108.
5. Monsálvez V, Fuertes L, García-Cano I, Vanaclocha F, Ortez de Frutos J. Blepharoconjunctivitis due to phenylephrine [in Spanish]. Actas Dermosifiliogr. 2010;101(5):466-467.
6. Geyer O, Yust I, Lazar M. Allergic blepharoconjunctivitis due to phenylephrine. J Ocul Pharmacol. 1988;4(2):123-126.
7. Villarreal O. Reliability of diagnostic tests for contact allergy to mydriatic eyedrops. Contact Dermatitis. 1998;38(3):150-154.
8. Frazier M, Jaanus SD. Cycloplegics. In: Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th ed. St. Louis, MO: Butterworth-Heinemann; 2009:125-138.
9. Decraene T, Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact Dermatitis. 2001;45(5):309-310.
10. Boukhman MP, Maibach HI. Allergic contact dermatitis from tropicamide ophthalmic solution. Contact Dermatitis. 1999;41(1):47-48.
11. Yoshikawa K, Kawahara S. Contact allergy to atropine and other mydriatic agents. Contact Dermatitis. 1985;12(1):56-57.
12. Mcgee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637-640.
13. Dass BA, Soong HK, Lee B. Effects of proparacaine of actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187-194.
14. Dannaker CJ, Maibach HI, Austin E. Allergic contact dermatitis to proparacaine with subsequent cross-sensitization to tetracaine from ophthalmic preparations. Am J Contact Dermat. 2001;12(3):177-179.
15. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453.
16. Gonzalo-Garijo MA, Pérez-Calderón R, de Argila D, Rodríguez-Nevado I. Erythrodermia to pseudoephedrine in a patient with contact allergy to phenylephrine. Allergol Immunopathol (Madr). 2002;30(4):239-242.
17. Platts-Mills TAE. Immediate hypersensitivity (Type I). In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:423-446.
18. Britton W. Type IV hypersensitivity. In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:477-491.
Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.
This careful review of past exam notes revealed that phenylephrine and Fluress were the only drops that had not been instilled at the October 2013 visit when no AE was reported. However, Fluress was an unlikely culprit since it was not instilled in October 2014, and the patient still experienced an AE. Therefore, the agent most likely responsible for the allergic reaction in the patient, as confirmed by a review of the past notes and by the aforementioned pharmacologic test, was deemed to be phenylephrine (Table).
Adverse reactions to topical ocular medications and specifically to diagnostic eye drops have long been recognized. Mathias, Camarasa, Barber, Ducombs, and Monsálvezhave reported on variations of conjunctivitis and periorbital erythema with positive patch testing to phenylephrine.1-5 Geyer and colleagues reported on a study of 21 patients who had blepharoconjunctivitis after instillation of phenylephrine.6 In this case study patient, severe keratoconjunctivitis was the clinical manifestation observed.
Villarreal and colleagues studied 31 patients who had a previous reaction to mydriatic drops. The study found that phenylephrine was the drug that most frequently caused an AE (93.5%).7 One patient reacted to the preservative thimerosal, and 1 patient reacted to benoxiprocaine. Tropicamide was demonstrated to be very well tolerated as none of the patients tested positive on either the patch test or the pharmacologic test.
Tropicamide is a nonselective muscarinic antagonist commonly used for mydriasis due to its fast onset and short duration.8 Adverse reactions to tropicamide are rare. Three studies reported on patients who had a positive patch test to tropicamide.9-11 However, the reaction was not provoked by direct instillation of tropicamide into the eye.
Common in-office topical anesthetics, proparacaine, tetracaine, benoxinate, and lidocaine also can cause AEs. Corneal toxicity is a well-known complication with topical anesthetic abuse, whereas allergic reactions are considered rare. The most common symptoms include stingingand discomfort upon instillation. Common signs include punctate corneal epithelial erosionsresulting indirectly from a decrease in reflex tearing, infrequent blinking, and increased tear evaporation.12 Topical anesthetics also inhibit the migration of corneal epithelial cells and cause direct damage to the cells that are present, leading to impaired healing and epithelial defects.13
Manifestations of allergic reaction to topical anesthetics can include conjunctival hyperemia and edema, edematous eyelids, and lacrimation. One published case described a 60-year-old woman who developed eczematous dermatitis of the eyelids after ophthalmic anesthetic drops were instilled prior to laser surgery. Patch testing showed a positive response to benzocaine 5%, proparacaine, and tetracaine 0.5%.14
Preservatives, in general, can cause an allergic reaction. Benzalkonium chloride’s (BAK) cytotoxic sequelae include possible trabecular cell death in glaucoma patients, disruption of tear film stability (even at low concentrations), and immune-allergenic properties. One article reported BAK as one of the 30 most frequent allergens causing allergic periorbital dermatitis.15 Benzalkonium chloride is used in most brands of phenylephrine. However, preservatives in this patient’s case were ruled out as instigating agents since both phenylephrine and tropicamide contain the same preservative, BAK 0.01%, yet this patient did not develop a reaction to tropicamide when used without phenylephrine. Expired medications also were not considered to be a factor as none of the medications used on the patient were indeed expired (the Malcom Randall VAMC clinic maintains a strict policy of discarding medications 28 days after being opened).
Although uncommon, phenylephrine sometimes has been found to cause a type 4 hypersensitivity reaction, also known as cell-mediated or delayed-type hypersensitivity.16 First, helper T cells secrete cytokines. Activation of cytokines recruits and activates cytotoxic T cells, monocytes, and macrophages, leading to inflammation of the surrounding tissue. Examples of cell-mediated hypersensitivity include reactions to the tuberculin skin test and to poison ivy.
Type 1 hypersensitivity reactions, also known as immediate or anaphylactic hypersensitivity reactions, are not triggered by phenylephrine. In this type of reaction, IgE binds to the mast cell on initial exposure to an allergen. On second exposure, the allergen binds to the IgE, causing the mast cell to release mediators of inflammation, triggering physiologic responses. Examples of this type of hypersensitivity include those seen with penicillin, bee stings, hay fever, bronchial asthma, and food allergies, for example, to shellfish.
A toxic reaction’s mechanism differs from that of a type 4 hypersensitivity reaction. Toxic reactions occur due to direct cytotoxicity of a drug caused by a low or high pH and either hyper- or hypo-osmolarity. Toxicity can lead to corneal and conjunctival cell necrosis or induce apoptosis, stimulating inflammatory reactions. Clinically, toxic reactions will present with follicles, whereas allergic reactions will present with papillae.
The definitive diagnostic methods used to determine the allergic agent causing ocular or periocular AEs are patch testing and conjunctival challenge.7 Mathias, Camarasa, Barber, Ducombs,and Monsálvezused patch testing to confirm phenylephrine as the allergic agent in their series of cases. Patch testing entails the application of a small amount of an allergic agent that is taped onto the skin. The allergic agent is confirmed if the patient has a dermal reaction, wherein the area patched will become erythematous. When patch testing is negative or inconclusive, a conjunctival challenge is performed by instillation of the suspected allergic agent into the eye with subsequent observation to determine whether a reaction occurs. The sequelae found in Villarreal’s study included itching, lacrimation, edema, erythema, and sometimes blepharitis.7
A direct conjunctival challenge with the suspected culprit was not pursued in this patient’s case due to the known severity of the potential resulting reaction. The authors instead chose an indirect method of determining the implicating agent and used the process of elimination to whittle down the most likely suspect. A challenge with the medications suspected not to be likely offenders was undertaken. This spared the patient a likely repeat of the AE he had just recovered from.
Management
Allergic reactions can resolve without medical intervention. The first step is to remove the allergen. For delayed hypersensitivity reactions, treatments may include topical decongestants, cool compresses, and corticosteroids.8 The treatment for immediate hypersensitivity reaction differs from that of delayed hypersensitivity reaction in that antihistamines are used.17,18
This patient reported receiving no treatment for his ocular symptoms following eye examinations in the past, yet he experienced complete resolution after each AE. In this case, both a steroid and a prophylactic antibiotic to facilitate a more rapid improvement were used.
Conclusion
Although uncommon, cases of allergic reaction to phenylephrine can occur. The incidence of phenylephrine allergy is 0.6%.6 The case study patient presented with a severe keratoconjunctivitis following routine eye examination with an accompanying history of adverse ocular signs and symptoms following multiple past exams.
It is important for all eye care clinicians to realize that AEs to diagnostic eye drops are possible and can occur following the most routine of visits. Such reactions can be caused by dilating agents, anesthetics, or preservatives, and these may be allergic or toxic. Clinicians should take special care to identify the instigating agent, and if possible, to avoid using such agents on patients during future exams. Clinicians also should understand how best to manage iatrogenic AEs when they encounter them in order to restore a patient’s visual function as quickly as possible.
Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.
This careful review of past exam notes revealed that phenylephrine and Fluress were the only drops that had not been instilled at the October 2013 visit when no AE was reported. However, Fluress was an unlikely culprit since it was not instilled in October 2014, and the patient still experienced an AE. Therefore, the agent most likely responsible for the allergic reaction in the patient, as confirmed by a review of the past notes and by the aforementioned pharmacologic test, was deemed to be phenylephrine (Table).
Adverse reactions to topical ocular medications and specifically to diagnostic eye drops have long been recognized. Mathias, Camarasa, Barber, Ducombs, and Monsálvezhave reported on variations of conjunctivitis and periorbital erythema with positive patch testing to phenylephrine.1-5 Geyer and colleagues reported on a study of 21 patients who had blepharoconjunctivitis after instillation of phenylephrine.6 In this case study patient, severe keratoconjunctivitis was the clinical manifestation observed.
Villarreal and colleagues studied 31 patients who had a previous reaction to mydriatic drops. The study found that phenylephrine was the drug that most frequently caused an AE (93.5%).7 One patient reacted to the preservative thimerosal, and 1 patient reacted to benoxiprocaine. Tropicamide was demonstrated to be very well tolerated as none of the patients tested positive on either the patch test or the pharmacologic test.
Tropicamide is a nonselective muscarinic antagonist commonly used for mydriasis due to its fast onset and short duration.8 Adverse reactions to tropicamide are rare. Three studies reported on patients who had a positive patch test to tropicamide.9-11 However, the reaction was not provoked by direct instillation of tropicamide into the eye.
Common in-office topical anesthetics, proparacaine, tetracaine, benoxinate, and lidocaine also can cause AEs. Corneal toxicity is a well-known complication with topical anesthetic abuse, whereas allergic reactions are considered rare. The most common symptoms include stingingand discomfort upon instillation. Common signs include punctate corneal epithelial erosionsresulting indirectly from a decrease in reflex tearing, infrequent blinking, and increased tear evaporation.12 Topical anesthetics also inhibit the migration of corneal epithelial cells and cause direct damage to the cells that are present, leading to impaired healing and epithelial defects.13
Manifestations of allergic reaction to topical anesthetics can include conjunctival hyperemia and edema, edematous eyelids, and lacrimation. One published case described a 60-year-old woman who developed eczematous dermatitis of the eyelids after ophthalmic anesthetic drops were instilled prior to laser surgery. Patch testing showed a positive response to benzocaine 5%, proparacaine, and tetracaine 0.5%.14
Preservatives, in general, can cause an allergic reaction. Benzalkonium chloride’s (BAK) cytotoxic sequelae include possible trabecular cell death in glaucoma patients, disruption of tear film stability (even at low concentrations), and immune-allergenic properties. One article reported BAK as one of the 30 most frequent allergens causing allergic periorbital dermatitis.15 Benzalkonium chloride is used in most brands of phenylephrine. However, preservatives in this patient’s case were ruled out as instigating agents since both phenylephrine and tropicamide contain the same preservative, BAK 0.01%, yet this patient did not develop a reaction to tropicamide when used without phenylephrine. Expired medications also were not considered to be a factor as none of the medications used on the patient were indeed expired (the Malcom Randall VAMC clinic maintains a strict policy of discarding medications 28 days after being opened).
Although uncommon, phenylephrine sometimes has been found to cause a type 4 hypersensitivity reaction, also known as cell-mediated or delayed-type hypersensitivity.16 First, helper T cells secrete cytokines. Activation of cytokines recruits and activates cytotoxic T cells, monocytes, and macrophages, leading to inflammation of the surrounding tissue. Examples of cell-mediated hypersensitivity include reactions to the tuberculin skin test and to poison ivy.
Type 1 hypersensitivity reactions, also known as immediate or anaphylactic hypersensitivity reactions, are not triggered by phenylephrine. In this type of reaction, IgE binds to the mast cell on initial exposure to an allergen. On second exposure, the allergen binds to the IgE, causing the mast cell to release mediators of inflammation, triggering physiologic responses. Examples of this type of hypersensitivity include those seen with penicillin, bee stings, hay fever, bronchial asthma, and food allergies, for example, to shellfish.
A toxic reaction’s mechanism differs from that of a type 4 hypersensitivity reaction. Toxic reactions occur due to direct cytotoxicity of a drug caused by a low or high pH and either hyper- or hypo-osmolarity. Toxicity can lead to corneal and conjunctival cell necrosis or induce apoptosis, stimulating inflammatory reactions. Clinically, toxic reactions will present with follicles, whereas allergic reactions will present with papillae.
The definitive diagnostic methods used to determine the allergic agent causing ocular or periocular AEs are patch testing and conjunctival challenge.7 Mathias, Camarasa, Barber, Ducombs,and Monsálvezused patch testing to confirm phenylephrine as the allergic agent in their series of cases. Patch testing entails the application of a small amount of an allergic agent that is taped onto the skin. The allergic agent is confirmed if the patient has a dermal reaction, wherein the area patched will become erythematous. When patch testing is negative or inconclusive, a conjunctival challenge is performed by instillation of the suspected allergic agent into the eye with subsequent observation to determine whether a reaction occurs. The sequelae found in Villarreal’s study included itching, lacrimation, edema, erythema, and sometimes blepharitis.7
A direct conjunctival challenge with the suspected culprit was not pursued in this patient’s case due to the known severity of the potential resulting reaction. The authors instead chose an indirect method of determining the implicating agent and used the process of elimination to whittle down the most likely suspect. A challenge with the medications suspected not to be likely offenders was undertaken. This spared the patient a likely repeat of the AE he had just recovered from.
Management
Allergic reactions can resolve without medical intervention. The first step is to remove the allergen. For delayed hypersensitivity reactions, treatments may include topical decongestants, cool compresses, and corticosteroids.8 The treatment for immediate hypersensitivity reaction differs from that of delayed hypersensitivity reaction in that antihistamines are used.17,18
This patient reported receiving no treatment for his ocular symptoms following eye examinations in the past, yet he experienced complete resolution after each AE. In this case, both a steroid and a prophylactic antibiotic to facilitate a more rapid improvement were used.
Conclusion
Although uncommon, cases of allergic reaction to phenylephrine can occur. The incidence of phenylephrine allergy is 0.6%.6 The case study patient presented with a severe keratoconjunctivitis following routine eye examination with an accompanying history of adverse ocular signs and symptoms following multiple past exams.
It is important for all eye care clinicians to realize that AEs to diagnostic eye drops are possible and can occur following the most routine of visits. Such reactions can be caused by dilating agents, anesthetics, or preservatives, and these may be allergic or toxic. Clinicians should take special care to identify the instigating agent, and if possible, to avoid using such agents on patients during future exams. Clinicians also should understand how best to manage iatrogenic AEs when they encounter them in order to restore a patient’s visual function as quickly as possible.
1. Mathias CG, Maibach HI, Irvine A, Adler W. Allergic contact dermatitis to echothiophate iodide and phenylephrine. Arch Ophthalmol. 1979;97(2):286-287.
2. Camarasa JG. Contact dermatitis to phenylephrine. Contact Dermatitis. 1984;10(3):182.
3. Barber K. Allergic contact eczema to phenylephrine. Contact Dermatitis. 1983;9(4):274-277.
4. Ducombs G, de Casamayor J, Verin P, Maleville J. Allergic contact dermatitis to phenylephrine. Contact Dermatitis. 1986;15(2):107-108.
5. Monsálvez V, Fuertes L, García-Cano I, Vanaclocha F, Ortez de Frutos J. Blepharoconjunctivitis due to phenylephrine [in Spanish]. Actas Dermosifiliogr. 2010;101(5):466-467.
6. Geyer O, Yust I, Lazar M. Allergic blepharoconjunctivitis due to phenylephrine. J Ocul Pharmacol. 1988;4(2):123-126.
7. Villarreal O. Reliability of diagnostic tests for contact allergy to mydriatic eyedrops. Contact Dermatitis. 1998;38(3):150-154.
8. Frazier M, Jaanus SD. Cycloplegics. In: Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th ed. St. Louis, MO: Butterworth-Heinemann; 2009:125-138.
9. Decraene T, Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact Dermatitis. 2001;45(5):309-310.
10. Boukhman MP, Maibach HI. Allergic contact dermatitis from tropicamide ophthalmic solution. Contact Dermatitis. 1999;41(1):47-48.
11. Yoshikawa K, Kawahara S. Contact allergy to atropine and other mydriatic agents. Contact Dermatitis. 1985;12(1):56-57.
12. Mcgee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637-640.
13. Dass BA, Soong HK, Lee B. Effects of proparacaine of actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187-194.
14. Dannaker CJ, Maibach HI, Austin E. Allergic contact dermatitis to proparacaine with subsequent cross-sensitization to tetracaine from ophthalmic preparations. Am J Contact Dermat. 2001;12(3):177-179.
15. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453.
16. Gonzalo-Garijo MA, Pérez-Calderón R, de Argila D, Rodríguez-Nevado I. Erythrodermia to pseudoephedrine in a patient with contact allergy to phenylephrine. Allergol Immunopathol (Madr). 2002;30(4):239-242.
17. Platts-Mills TAE. Immediate hypersensitivity (Type I). In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:423-446.
18. Britton W. Type IV hypersensitivity. In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:477-491.
1. Mathias CG, Maibach HI, Irvine A, Adler W. Allergic contact dermatitis to echothiophate iodide and phenylephrine. Arch Ophthalmol. 1979;97(2):286-287.
2. Camarasa JG. Contact dermatitis to phenylephrine. Contact Dermatitis. 1984;10(3):182.
3. Barber K. Allergic contact eczema to phenylephrine. Contact Dermatitis. 1983;9(4):274-277.
4. Ducombs G, de Casamayor J, Verin P, Maleville J. Allergic contact dermatitis to phenylephrine. Contact Dermatitis. 1986;15(2):107-108.
5. Monsálvez V, Fuertes L, García-Cano I, Vanaclocha F, Ortez de Frutos J. Blepharoconjunctivitis due to phenylephrine [in Spanish]. Actas Dermosifiliogr. 2010;101(5):466-467.
6. Geyer O, Yust I, Lazar M. Allergic blepharoconjunctivitis due to phenylephrine. J Ocul Pharmacol. 1988;4(2):123-126.
7. Villarreal O. Reliability of diagnostic tests for contact allergy to mydriatic eyedrops. Contact Dermatitis. 1998;38(3):150-154.
8. Frazier M, Jaanus SD. Cycloplegics. In: Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th ed. St. Louis, MO: Butterworth-Heinemann; 2009:125-138.
9. Decraene T, Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact Dermatitis. 2001;45(5):309-310.
10. Boukhman MP, Maibach HI. Allergic contact dermatitis from tropicamide ophthalmic solution. Contact Dermatitis. 1999;41(1):47-48.
11. Yoshikawa K, Kawahara S. Contact allergy to atropine and other mydriatic agents. Contact Dermatitis. 1985;12(1):56-57.
12. Mcgee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637-640.
13. Dass BA, Soong HK, Lee B. Effects of proparacaine of actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187-194.
14. Dannaker CJ, Maibach HI, Austin E. Allergic contact dermatitis to proparacaine with subsequent cross-sensitization to tetracaine from ophthalmic preparations. Am J Contact Dermat. 2001;12(3):177-179.
15. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453.
16. Gonzalo-Garijo MA, Pérez-Calderón R, de Argila D, Rodríguez-Nevado I. Erythrodermia to pseudoephedrine in a patient with contact allergy to phenylephrine. Allergol Immunopathol (Madr). 2002;30(4):239-242.
17. Platts-Mills TAE. Immediate hypersensitivity (Type I). In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:423-446.
18. Britton W. Type IV hypersensitivity. In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:477-491.
Transitions of Care for Emerging Adults with Type 1 Diabetes
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHMP advocates approval of edoxaban product
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization for the same indications as Lixiana.
Both Roteas and Lixiana are oral factor Xa inhibitors that contain the active ingredient edoxaban.
The CHMP has recommended approving Roteas for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
In addition, the CHMP has recommended approving Roteas for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF) who have 1 or more risk factors, such as congestive heart failure, hypertension, age of 75 or older, diabetes mellitus, prior stroke, or transient ischemic attack.
The European Commission approved Lixiana for these indications in June 2015.
If the European Commission decides to approve Roteas as well, the product will be available as film-coated tablets (15 mg, 30 mg, and 60 mg).
The European Commission is expected to make a decision about Roteas within 67 days from the CHMP’s adoption of its opinion (which occurred on February 23).
The application for Roteas was an informed consent application. In this type of application, reference is made to an authorized medicine if the marketing authorization holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The applicant for Roteas is Daiichi Sankyo Europe GmbH, the company that also developed Lixiana.
Lixiana was approved based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic DVT/PE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization for the same indications as Lixiana.
Both Roteas and Lixiana are oral factor Xa inhibitors that contain the active ingredient edoxaban.
The CHMP has recommended approving Roteas for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
In addition, the CHMP has recommended approving Roteas for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF) who have 1 or more risk factors, such as congestive heart failure, hypertension, age of 75 or older, diabetes mellitus, prior stroke, or transient ischemic attack.
The European Commission approved Lixiana for these indications in June 2015.
If the European Commission decides to approve Roteas as well, the product will be available as film-coated tablets (15 mg, 30 mg, and 60 mg).
The European Commission is expected to make a decision about Roteas within 67 days from the CHMP’s adoption of its opinion (which occurred on February 23).
The application for Roteas was an informed consent application. In this type of application, reference is made to an authorized medicine if the marketing authorization holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The applicant for Roteas is Daiichi Sankyo Europe GmbH, the company that also developed Lixiana.
Lixiana was approved based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic DVT/PE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization for the same indications as Lixiana.
Both Roteas and Lixiana are oral factor Xa inhibitors that contain the active ingredient edoxaban.
The CHMP has recommended approving Roteas for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults.
In addition, the CHMP has recommended approving Roteas for the prevention of stroke and systemic embolism in adults with nonvalvular atrial fibrillation (NVAF) who have 1 or more risk factors, such as congestive heart failure, hypertension, age of 75 or older, diabetes mellitus, prior stroke, or transient ischemic attack.
The European Commission approved Lixiana for these indications in June 2015.
If the European Commission decides to approve Roteas as well, the product will be available as film-coated tablets (15 mg, 30 mg, and 60 mg).
The European Commission is expected to make a decision about Roteas within 67 days from the CHMP’s adoption of its opinion (which occurred on February 23).
The application for Roteas was an informed consent application. In this type of application, reference is made to an authorized medicine if the marketing authorization holder of the reference medicine has given consent to the use of their dossier in the application procedure.
The applicant for Roteas is Daiichi Sankyo Europe GmbH, the company that also developed Lixiana.
Lixiana was approved based on data from a pair of phase 3 trials, ENGAGE AF-TIMI 48 and Hokusai-VTE.
Hokusai-VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with DVT and 3319 with PE. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic DVT/PE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
In addition, the incidence of clinically relevant bleeding was significantly lower in the edoxaban arm than the warfarin arm—8.5% and 10.3%, respectively (P=0.004 for superiority).
ENGAGE-AF TIMI 48
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin as prophylaxis for stroke or systemic embolism in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or systemic embolism was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001). ![]()
Oral PrEP works despite bacterial vaginosis
SEATTLE – Bacterial vaginosis does not decrease the effectiveness of oral pre-exposure prophylaxis (PrEP) for HIV prevention, according to an investigation of 1,470 women in Africa.
Daily oral PrEP with tenofovir pills was 77% effective in protecting women with bacterial vaginosis from HIV, and 73% effective in women who did not have BV over the 3-year study. The difference was not statistically significant.
Bacterial vaginosis in PrEP has been a concern ever since it was reported in the summer of 2016 that vaginal tenofovir gel didn’t prevent HIV in women with the condition. “Our results provide reassurance ... We [saw] no evidence that oral PrEP effectiveness was reduced in East African women with Gram stain evidence of BV or vaginal dysbiosis. Oral PrEP delivery to women does not need to be accompanied by testing for BV,” Dr. Heffron said.
It’s likely that Gardnerella vaginalis degrades topical tenofovir when applied vaginally; oral administration bypasses the effect.
The findings come from a subanalysis of the Partners PrEP Study, a phase III trial of daily oral PrEP in Kenya and Uganda. The investigators broke out PrEP results according to BV status, with BV defined as a Nugent score of 7-10, assessed annually. Adherence was high in the 985 women randomized to PrEP, at about 80%.
There were 0.9 cases of newly acquired HIV per 100 person-years among women in the BV PrEP group, versus 3.5 per 100 person-years among BV women not on prep. PrEP was about 63% effective in women with intermediate Nugent scores of 4-6. The differences in HIV protection according to Nugent score were not statistically significant (P = .9).
It didn’t matter if women had Gardnerella, Bacteroides, or Lactobacillus morphotypes. All three are markers of abnormal vaginal microbiota, but PrEP still worked.
The median age in the study was 33 years, and 24% of the women had BV at baseline.
Dr. Heffron did not report any disclosures. The Gates Foundation and other sources funded the work.
SEATTLE – Bacterial vaginosis does not decrease the effectiveness of oral pre-exposure prophylaxis (PrEP) for HIV prevention, according to an investigation of 1,470 women in Africa.
Daily oral PrEP with tenofovir pills was 77% effective in protecting women with bacterial vaginosis from HIV, and 73% effective in women who did not have BV over the 3-year study. The difference was not statistically significant.
Bacterial vaginosis in PrEP has been a concern ever since it was reported in the summer of 2016 that vaginal tenofovir gel didn’t prevent HIV in women with the condition. “Our results provide reassurance ... We [saw] no evidence that oral PrEP effectiveness was reduced in East African women with Gram stain evidence of BV or vaginal dysbiosis. Oral PrEP delivery to women does not need to be accompanied by testing for BV,” Dr. Heffron said.
It’s likely that Gardnerella vaginalis degrades topical tenofovir when applied vaginally; oral administration bypasses the effect.
The findings come from a subanalysis of the Partners PrEP Study, a phase III trial of daily oral PrEP in Kenya and Uganda. The investigators broke out PrEP results according to BV status, with BV defined as a Nugent score of 7-10, assessed annually. Adherence was high in the 985 women randomized to PrEP, at about 80%.
There were 0.9 cases of newly acquired HIV per 100 person-years among women in the BV PrEP group, versus 3.5 per 100 person-years among BV women not on prep. PrEP was about 63% effective in women with intermediate Nugent scores of 4-6. The differences in HIV protection according to Nugent score were not statistically significant (P = .9).
It didn’t matter if women had Gardnerella, Bacteroides, or Lactobacillus morphotypes. All three are markers of abnormal vaginal microbiota, but PrEP still worked.
The median age in the study was 33 years, and 24% of the women had BV at baseline.
Dr. Heffron did not report any disclosures. The Gates Foundation and other sources funded the work.
SEATTLE – Bacterial vaginosis does not decrease the effectiveness of oral pre-exposure prophylaxis (PrEP) for HIV prevention, according to an investigation of 1,470 women in Africa.
Daily oral PrEP with tenofovir pills was 77% effective in protecting women with bacterial vaginosis from HIV, and 73% effective in women who did not have BV over the 3-year study. The difference was not statistically significant.
Bacterial vaginosis in PrEP has been a concern ever since it was reported in the summer of 2016 that vaginal tenofovir gel didn’t prevent HIV in women with the condition. “Our results provide reassurance ... We [saw] no evidence that oral PrEP effectiveness was reduced in East African women with Gram stain evidence of BV or vaginal dysbiosis. Oral PrEP delivery to women does not need to be accompanied by testing for BV,” Dr. Heffron said.
It’s likely that Gardnerella vaginalis degrades topical tenofovir when applied vaginally; oral administration bypasses the effect.
The findings come from a subanalysis of the Partners PrEP Study, a phase III trial of daily oral PrEP in Kenya and Uganda. The investigators broke out PrEP results according to BV status, with BV defined as a Nugent score of 7-10, assessed annually. Adherence was high in the 985 women randomized to PrEP, at about 80%.
There were 0.9 cases of newly acquired HIV per 100 person-years among women in the BV PrEP group, versus 3.5 per 100 person-years among BV women not on prep. PrEP was about 63% effective in women with intermediate Nugent scores of 4-6. The differences in HIV protection according to Nugent score were not statistically significant (P = .9).
It didn’t matter if women had Gardnerella, Bacteroides, or Lactobacillus morphotypes. All three are markers of abnormal vaginal microbiota, but PrEP still worked.
The median age in the study was 33 years, and 24% of the women had BV at baseline.
Dr. Heffron did not report any disclosures. The Gates Foundation and other sources funded the work.
AT CROI
Key clinical point:
Major finding: Daily oral PrEP with tenofovir pills was 77% effective in protecting women with bacterial vaginosis from HIV, and 73% effective in women who did not have BV over the 3-year study.
Data source: Randomized trial of 1,470 women in Kenya and Uganda
Disclosures: The lead investigator did not report any disclosures. The Gates Foundation and other sources funded the work.
Tap patients with suspected HIV neurosyphilis regardless of symptoms
SEATTLE – Neurologic symptoms are an unreliable indicator of neurosyphilis in patients with HIV, according to University of Washington, Seattle, investigators.
After tapping 385 HIV patients with untreated syphilis and possible central nervous system involvement, “the bottom line was that [symptom] sensitivity was so low that not having [symptoms] really shouldn’t reassure you. If you are concerned that your patient could have neurosyphilis because of other factors” – such as an especially high rapid plasma reagin titer or low CD4 count – “you still need to tap them,” said lead investigator Arielle P. Davis, MD, who is in the department of neurology at the university.
In the prospective study, symptoms were simply not sensitive enough to rule anything out. Moderate or greater photophobia had a sensitivity of 6.3% for a reactive cerebrospinal fluid Venereal Disease Research Laboratory (CSF-VDRL) syphilis test; moderate or greater vision loss was 38.1% sensitive for reactive CSF-VDRL, moderate or greater hearing loss was 13.2% sensitive, and moderate or greater gait incoordination was 1.5% sensitive.
Specificity was markedly better for each of those symptoms, and more than 95% for moderate or greater photophobia, hearing loss, and gait incoordination. “In general, as the severity of photophobia, vision loss, hearing loss, and gait incoordination increased, the specificity increased, but at the expense of sensitivity,” the investigators wrote in their poster, which Dr. Davis presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
CSF-VDRL was reactive in 68 subjects (18%). The odds of a reactive CSF-VDRL were significantly higher in patients with vision loss (odds ratio, 2.2; 95% confidence interval, 1.2-4.0; P = .01) and trended toward significance in those with moderate or greater hearing loss (OR, 2.5; 95% CI, 1.0-6.6; P = .06).
Overall, “patients with mild or greater photophobia, vision loss, gait incoordination, and moderate or greater hearing loss were significantly more likely to have a reactive CSF-VDRL, and the presence of any of these four symptoms should [remain] a criterion for pursuing a lumbar puncture.” However, “lack of neurologic symptoms in HIV-infected patients with syphilis should not reassure clinicians that their patients do not have neurosyphilis,” Dr. Davis and her team concluded.
The participants were almost entirely men and mostly white. The average age was about 40 years, and the median rapid plasma reagin titer was 1:64. About 48% of the subjects reported at least mild vision loss, 40% reported mild or worse headache, and 17% reported some degree of hearing loss. Overall, 15.5%-17.2% reported at least mild photophobia, stiff neck, or gait incoordination.
The work was funded by the National Institutes of Health. Dr. Davis did not have any relevant disclosures.
SEATTLE – Neurologic symptoms are an unreliable indicator of neurosyphilis in patients with HIV, according to University of Washington, Seattle, investigators.
After tapping 385 HIV patients with untreated syphilis and possible central nervous system involvement, “the bottom line was that [symptom] sensitivity was so low that not having [symptoms] really shouldn’t reassure you. If you are concerned that your patient could have neurosyphilis because of other factors” – such as an especially high rapid plasma reagin titer or low CD4 count – “you still need to tap them,” said lead investigator Arielle P. Davis, MD, who is in the department of neurology at the university.
In the prospective study, symptoms were simply not sensitive enough to rule anything out. Moderate or greater photophobia had a sensitivity of 6.3% for a reactive cerebrospinal fluid Venereal Disease Research Laboratory (CSF-VDRL) syphilis test; moderate or greater vision loss was 38.1% sensitive for reactive CSF-VDRL, moderate or greater hearing loss was 13.2% sensitive, and moderate or greater gait incoordination was 1.5% sensitive.
Specificity was markedly better for each of those symptoms, and more than 95% for moderate or greater photophobia, hearing loss, and gait incoordination. “In general, as the severity of photophobia, vision loss, hearing loss, and gait incoordination increased, the specificity increased, but at the expense of sensitivity,” the investigators wrote in their poster, which Dr. Davis presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
CSF-VDRL was reactive in 68 subjects (18%). The odds of a reactive CSF-VDRL were significantly higher in patients with vision loss (odds ratio, 2.2; 95% confidence interval, 1.2-4.0; P = .01) and trended toward significance in those with moderate or greater hearing loss (OR, 2.5; 95% CI, 1.0-6.6; P = .06).
Overall, “patients with mild or greater photophobia, vision loss, gait incoordination, and moderate or greater hearing loss were significantly more likely to have a reactive CSF-VDRL, and the presence of any of these four symptoms should [remain] a criterion for pursuing a lumbar puncture.” However, “lack of neurologic symptoms in HIV-infected patients with syphilis should not reassure clinicians that their patients do not have neurosyphilis,” Dr. Davis and her team concluded.
The participants were almost entirely men and mostly white. The average age was about 40 years, and the median rapid plasma reagin titer was 1:64. About 48% of the subjects reported at least mild vision loss, 40% reported mild or worse headache, and 17% reported some degree of hearing loss. Overall, 15.5%-17.2% reported at least mild photophobia, stiff neck, or gait incoordination.
The work was funded by the National Institutes of Health. Dr. Davis did not have any relevant disclosures.
SEATTLE – Neurologic symptoms are an unreliable indicator of neurosyphilis in patients with HIV, according to University of Washington, Seattle, investigators.
After tapping 385 HIV patients with untreated syphilis and possible central nervous system involvement, “the bottom line was that [symptom] sensitivity was so low that not having [symptoms] really shouldn’t reassure you. If you are concerned that your patient could have neurosyphilis because of other factors” – such as an especially high rapid plasma reagin titer or low CD4 count – “you still need to tap them,” said lead investigator Arielle P. Davis, MD, who is in the department of neurology at the university.
In the prospective study, symptoms were simply not sensitive enough to rule anything out. Moderate or greater photophobia had a sensitivity of 6.3% for a reactive cerebrospinal fluid Venereal Disease Research Laboratory (CSF-VDRL) syphilis test; moderate or greater vision loss was 38.1% sensitive for reactive CSF-VDRL, moderate or greater hearing loss was 13.2% sensitive, and moderate or greater gait incoordination was 1.5% sensitive.
Specificity was markedly better for each of those symptoms, and more than 95% for moderate or greater photophobia, hearing loss, and gait incoordination. “In general, as the severity of photophobia, vision loss, hearing loss, and gait incoordination increased, the specificity increased, but at the expense of sensitivity,” the investigators wrote in their poster, which Dr. Davis presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
CSF-VDRL was reactive in 68 subjects (18%). The odds of a reactive CSF-VDRL were significantly higher in patients with vision loss (odds ratio, 2.2; 95% confidence interval, 1.2-4.0; P = .01) and trended toward significance in those with moderate or greater hearing loss (OR, 2.5; 95% CI, 1.0-6.6; P = .06).
Overall, “patients with mild or greater photophobia, vision loss, gait incoordination, and moderate or greater hearing loss were significantly more likely to have a reactive CSF-VDRL, and the presence of any of these four symptoms should [remain] a criterion for pursuing a lumbar puncture.” However, “lack of neurologic symptoms in HIV-infected patients with syphilis should not reassure clinicians that their patients do not have neurosyphilis,” Dr. Davis and her team concluded.
The participants were almost entirely men and mostly white. The average age was about 40 years, and the median rapid plasma reagin titer was 1:64. About 48% of the subjects reported at least mild vision loss, 40% reported mild or worse headache, and 17% reported some degree of hearing loss. Overall, 15.5%-17.2% reported at least mild photophobia, stiff neck, or gait incoordination.
The work was funded by the National Institutes of Health. Dr. Davis did not have any relevant disclosures.
AT CROI
Key clinical point:
Major finding: Moderate or greater photophobia had a sensitivity of 6.3% for a reactive CSF-VDRL syphilis test; moderate or greater vision loss was 38.1% sensitive for reactive CSF-VDRL, moderate or greater hearing loss 13.2% sensitive, and moderate or greater gait incoordination 1.5% sensitive.
Data source: Lumbar punctures of 385 HIV patients with syphilis
Disclosures: The work was funded by the National Institutes of Health. The lead investigator didn’t have any relevant disclosures.