User login
Vaccine can fight different malaria strains
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine, according to a phase 1 study published in PNAS.
The vaccine, known as the PfSPZ Vaccine, contains weakened Plasmodium falciparum sporozoites that are able to generate a protective immune response against live malaria infection.
Prior research showed that the PfSPZ Vaccine can provide long-term protection against a single malaria strain matched to the vaccine.
The new study has shown that the PfSPZ Vaccine can protect against a different strain of P falciparum as well.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said Anthony S. Fauci, MD, of the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
“These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The PfSPZ Vaccine was developed by Sanaria Inc. The company designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes for this trial. The NIAID supported the development of the vaccine through several grants.
Study details
The study enrolled 31 healthy, malaria-naive adults ages 19 to 45.
Fifteen subjects were scheduled to receive 3 doses of the PfSPZ Vaccine—9.0 × 105 PfSPZ administered intravenously 3 times at 8-week intervals. The remaining subjects served as controls.
Nineteen weeks after receiving the final dose of the test vaccine, vaccinated subjects and controls were exposed to bites from mosquitoes infected with the same strain of P falciparum parasites (NF54) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 subjects (64%) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites. All 6 of the non-vaccinated subjects who were challenged at the same time had malaria parasites in their blood.
Of the 9 subjects who showed no evidence of malaria, 6 subjects were again exposed to mosquito bites, this time from mosquitoes infected with a different strain of P falciparum (Pf7G8), 33 weeks after the final immunization.
In this group, 5 of the 6 subjects (83%) were protected against malaria infection. None of the 6 control subjects who were challenged were protected.
“Achieving durable protection against a malaria strain different from the vaccine strain, over 8 months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, MD, of NIAID.
“If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The researchers found the PfSPZ Vaccine activated T cells and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both malaria challenge strains, providing some insight into how the vaccine was mediating protection.
Ongoing research should determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations.
A phase 2 trial testing 3 different dosages in a 3-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy of the vaccine against natural infection. ![]()
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine, according to a phase 1 study published in PNAS.
The vaccine, known as the PfSPZ Vaccine, contains weakened Plasmodium falciparum sporozoites that are able to generate a protective immune response against live malaria infection.
Prior research showed that the PfSPZ Vaccine can provide long-term protection against a single malaria strain matched to the vaccine.
The new study has shown that the PfSPZ Vaccine can protect against a different strain of P falciparum as well.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said Anthony S. Fauci, MD, of the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
“These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The PfSPZ Vaccine was developed by Sanaria Inc. The company designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes for this trial. The NIAID supported the development of the vaccine through several grants.
Study details
The study enrolled 31 healthy, malaria-naive adults ages 19 to 45.
Fifteen subjects were scheduled to receive 3 doses of the PfSPZ Vaccine—9.0 × 105 PfSPZ administered intravenously 3 times at 8-week intervals. The remaining subjects served as controls.
Nineteen weeks after receiving the final dose of the test vaccine, vaccinated subjects and controls were exposed to bites from mosquitoes infected with the same strain of P falciparum parasites (NF54) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 subjects (64%) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites. All 6 of the non-vaccinated subjects who were challenged at the same time had malaria parasites in their blood.
Of the 9 subjects who showed no evidence of malaria, 6 subjects were again exposed to mosquito bites, this time from mosquitoes infected with a different strain of P falciparum (Pf7G8), 33 weeks after the final immunization.
In this group, 5 of the 6 subjects (83%) were protected against malaria infection. None of the 6 control subjects who were challenged were protected.
“Achieving durable protection against a malaria strain different from the vaccine strain, over 8 months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, MD, of NIAID.
“If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The researchers found the PfSPZ Vaccine activated T cells and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both malaria challenge strains, providing some insight into how the vaccine was mediating protection.
Ongoing research should determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations.
A phase 2 trial testing 3 different dosages in a 3-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy of the vaccine against natural infection. ![]()
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine, according to a phase 1 study published in PNAS.
The vaccine, known as the PfSPZ Vaccine, contains weakened Plasmodium falciparum sporozoites that are able to generate a protective immune response against live malaria infection.
Prior research showed that the PfSPZ Vaccine can provide long-term protection against a single malaria strain matched to the vaccine.
The new study has shown that the PfSPZ Vaccine can protect against a different strain of P falciparum as well.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said Anthony S. Fauci, MD, of the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland.
“These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The PfSPZ Vaccine was developed by Sanaria Inc. The company designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes for this trial. The NIAID supported the development of the vaccine through several grants.
Study details
The study enrolled 31 healthy, malaria-naive adults ages 19 to 45.
Fifteen subjects were scheduled to receive 3 doses of the PfSPZ Vaccine—9.0 × 105 PfSPZ administered intravenously 3 times at 8-week intervals. The remaining subjects served as controls.
Nineteen weeks after receiving the final dose of the test vaccine, vaccinated subjects and controls were exposed to bites from mosquitoes infected with the same strain of P falciparum parasites (NF54) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 subjects (64%) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites. All 6 of the non-vaccinated subjects who were challenged at the same time had malaria parasites in their blood.
Of the 9 subjects who showed no evidence of malaria, 6 subjects were again exposed to mosquito bites, this time from mosquitoes infected with a different strain of P falciparum (Pf7G8), 33 weeks after the final immunization.
In this group, 5 of the 6 subjects (83%) were protected against malaria infection. None of the 6 control subjects who were challenged were protected.
“Achieving durable protection against a malaria strain different from the vaccine strain, over 8 months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, MD, of NIAID.
“If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The researchers found the PfSPZ Vaccine activated T cells and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both malaria challenge strains, providing some insight into how the vaccine was mediating protection.
Ongoing research should determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations.
A phase 2 trial testing 3 different dosages in a 3-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy of the vaccine against natural infection. ![]()
AAD Plenary session topics range from telemedicine to Ebola
Features of the plenary session (P151) at this year’s American Academy of Dermatology annual meeting include the Clarence S. Livingood, MD Memorial Award and Lectureship, on “Telemedicine and the Future of Medicine,” presented by Carrie L. Kovarik, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia.
Dr. Kovarik’s presentation will be followed by the AAD president’s address, by outgoing president Abel Torres, MD, JD, professor and chairman of the department of dermatology at Case Western Reserve University MetroHealth Systems, Cleveland; and professor and chairman of the department of dermatology at Loma Linda University Medical Center, Loma Linda, California.
Christine Léauté-Labrèze, MD, of the Hôpital Pellegrin–Enfants, Centre Hospitalier Universitaire, Bordeaux, France, will then present the Eugene J. Van Scott Award for Innovative Therapy of the Skin and Phillip Frost Leadership Lecture: “Propranolol in Infantile Hemangiomas: A Successful Drug Repurposing.” Dr. Léauté-Labrèze was the lead author of the multicenter, randomized controlled study that evaluated the efficacy and safety of oral propranolol for treating infantile hemangiomas (N Engl J Med. 2015;372:735-46).
Incoming AAD president Henry W. Lim, MD, will follow, with the president-elect’s address. Dr. Lim is the chairman of the department of dermatology and Clarence S. Livingood Chair in Dermatology at Henry Ford Health System in Detroit.
Boris C. Bastian, MD, professor of dermatology and pathology, University of California, San Francisco, will give the Lila and Murray Gruber Memorial Cancer Research Award and Lectureship, on “How Moles Become Cancer.” Dr. Bastian is also the director of the Clinical Cancer Genomics Laboratory at UCSF.
This year, the Marion B. Sulzberger, MD, Memorial Award and Lectureship – titled “Getting to the Heart (and other Comorbidities) of Psoriasis” – will be given by Joel M. Gelfand, MD, professor of dermatology, University of Pennsylvania.
Finally, this year’s guest speaker is the Center for Disease Control and Prevention’s Jordan W. Tappero, MD, whose lecture is titled: “The West African Ebola Epidemic and the Global Health Security Agenda.” Dr. Tappero is director of the Division of Global Health Protection, in the CDC’s Center for Global Health.
The plenary session is scheduled for Sunday March 5, 8 AM to 11:30 AM, in the Chapin Theater at the Orange County Convention Center, Orlando.
Features of the plenary session (P151) at this year’s American Academy of Dermatology annual meeting include the Clarence S. Livingood, MD Memorial Award and Lectureship, on “Telemedicine and the Future of Medicine,” presented by Carrie L. Kovarik, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia.
Dr. Kovarik’s presentation will be followed by the AAD president’s address, by outgoing president Abel Torres, MD, JD, professor and chairman of the department of dermatology at Case Western Reserve University MetroHealth Systems, Cleveland; and professor and chairman of the department of dermatology at Loma Linda University Medical Center, Loma Linda, California.
Christine Léauté-Labrèze, MD, of the Hôpital Pellegrin–Enfants, Centre Hospitalier Universitaire, Bordeaux, France, will then present the Eugene J. Van Scott Award for Innovative Therapy of the Skin and Phillip Frost Leadership Lecture: “Propranolol in Infantile Hemangiomas: A Successful Drug Repurposing.” Dr. Léauté-Labrèze was the lead author of the multicenter, randomized controlled study that evaluated the efficacy and safety of oral propranolol for treating infantile hemangiomas (N Engl J Med. 2015;372:735-46).
Incoming AAD president Henry W. Lim, MD, will follow, with the president-elect’s address. Dr. Lim is the chairman of the department of dermatology and Clarence S. Livingood Chair in Dermatology at Henry Ford Health System in Detroit.
Boris C. Bastian, MD, professor of dermatology and pathology, University of California, San Francisco, will give the Lila and Murray Gruber Memorial Cancer Research Award and Lectureship, on “How Moles Become Cancer.” Dr. Bastian is also the director of the Clinical Cancer Genomics Laboratory at UCSF.
This year, the Marion B. Sulzberger, MD, Memorial Award and Lectureship – titled “Getting to the Heart (and other Comorbidities) of Psoriasis” – will be given by Joel M. Gelfand, MD, professor of dermatology, University of Pennsylvania.
Finally, this year’s guest speaker is the Center for Disease Control and Prevention’s Jordan W. Tappero, MD, whose lecture is titled: “The West African Ebola Epidemic and the Global Health Security Agenda.” Dr. Tappero is director of the Division of Global Health Protection, in the CDC’s Center for Global Health.
The plenary session is scheduled for Sunday March 5, 8 AM to 11:30 AM, in the Chapin Theater at the Orange County Convention Center, Orlando.
Features of the plenary session (P151) at this year’s American Academy of Dermatology annual meeting include the Clarence S. Livingood, MD Memorial Award and Lectureship, on “Telemedicine and the Future of Medicine,” presented by Carrie L. Kovarik, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia.
Dr. Kovarik’s presentation will be followed by the AAD president’s address, by outgoing president Abel Torres, MD, JD, professor and chairman of the department of dermatology at Case Western Reserve University MetroHealth Systems, Cleveland; and professor and chairman of the department of dermatology at Loma Linda University Medical Center, Loma Linda, California.
Christine Léauté-Labrèze, MD, of the Hôpital Pellegrin–Enfants, Centre Hospitalier Universitaire, Bordeaux, France, will then present the Eugene J. Van Scott Award for Innovative Therapy of the Skin and Phillip Frost Leadership Lecture: “Propranolol in Infantile Hemangiomas: A Successful Drug Repurposing.” Dr. Léauté-Labrèze was the lead author of the multicenter, randomized controlled study that evaluated the efficacy and safety of oral propranolol for treating infantile hemangiomas (N Engl J Med. 2015;372:735-46).
Incoming AAD president Henry W. Lim, MD, will follow, with the president-elect’s address. Dr. Lim is the chairman of the department of dermatology and Clarence S. Livingood Chair in Dermatology at Henry Ford Health System in Detroit.
Boris C. Bastian, MD, professor of dermatology and pathology, University of California, San Francisco, will give the Lila and Murray Gruber Memorial Cancer Research Award and Lectureship, on “How Moles Become Cancer.” Dr. Bastian is also the director of the Clinical Cancer Genomics Laboratory at UCSF.
This year, the Marion B. Sulzberger, MD, Memorial Award and Lectureship – titled “Getting to the Heart (and other Comorbidities) of Psoriasis” – will be given by Joel M. Gelfand, MD, professor of dermatology, University of Pennsylvania.
Finally, this year’s guest speaker is the Center for Disease Control and Prevention’s Jordan W. Tappero, MD, whose lecture is titled: “The West African Ebola Epidemic and the Global Health Security Agenda.” Dr. Tappero is director of the Division of Global Health Protection, in the CDC’s Center for Global Health.
The plenary session is scheduled for Sunday March 5, 8 AM to 11:30 AM, in the Chapin Theater at the Orange County Convention Center, Orlando.
COVER to COVER: Connecting Older Veterans (Especially Rural) to Community or Veteran-Eligible Resources
According to the VA, 23% of veterans in the U.S., nearly 5.2 million individuals, live in rural areas.1 The VHA serves more than 3 million rural veterans, and 56% of those enrolled in the VA system are aged ≥ 65 years.1 Thus, aging veterans in rural areas constitute a substantial group who need support and assistance from the VA. Fortunately, the VA offers numerous benefits for veterans that support aging in place and improve quality of life through the VHA, Veterans Benefits Administration (VBA), and National Cemetery Administration (NCA).
Despite the opportunities, many VA benefits go unclaimed. In some cases, veterans simply do not know these benefits exist.2 In a 2010 VA report, only 41% of veterans indicated that they understood their benefits “a lot” or “some.”2 However, their understanding of specific benefits tended to be lower. For example, many veterans stated that they had “heard about” burial options at VA cemeteries (41.5%), but few understood specific benefits, such as cash burial allowances (10.6%).2
Many veterans also hold misperceptions about eligibility, which prevents them from applying. For example, some veterans believe that a high income or lack of combat service disqualifies them from receiving VA benefits.3 Some veterans believe that others are more deserving of VA services, and they don’t want to “take a spot someone else needs.”3 Finally, some veterans hold negative attitudes about the VA, making them less likely to claim VA benefits, such as health care.4
For rural veterans, accessing benefits can be especially difficult, because most VA facilities that offer assistance are in urban centers.5 Though online access to benefit information is improving through programs like My HealtheVet and public facing websites, some older adults do not use computers, and Internet and mobile phone connectivity are often limited in rural communities.6 Nearly 43% of rural veterans do not have broadband Internet in their homes.1 Moreover, the complexity of navigating benefits information via the Internet can be a frustrating and confusing process for older veterans.6
Accessing services and benefits in the community is similarly difficult. For more than 30 years, community organizations have noted the frustration that clients experienced with navigating a complex network of community providers who provide long-term services and supports (LTSS).7,8 In 2003, the Administration on Aging and the Centers for Medicare and Medicaid Services developed the Aging and Disability Resource Center (ADRC) program to promote a “no wrong door” approach for LTSS. Aging and Disability Resource Centers are a single point of entry into a network of community, state, and federal LTSS for older adults and individuals with disabilities.8 Options counselors at ADRCs provide information, counseling, and assistance with connecting to a vast network of programs such as Social Security, Medicaid, local transportation, Meals on Wheels, and housing assistance through a single office.
Backround
In 2012, VHA Office of Rural Health Resource (ORH) and Utah ADRC conducted a national survey of ADRC sites about their experiences working with veterans and found that 95% of ADRCs always or usually asked clients about their veteran status. The survey found that veteran clients present to ADRCs with diverse needs, many of which could be addressed through a VA benefit. However, the majority (58%) of ADRC respondents reported that they had never attempted to help a veteran apply for VA benefits (unpublished data, 2012).
Respondents reported a limited understanding of VA benefits, infrequent contact with VA, and frustrations with the VA system. Although familiar with several sources for information about VA benefits (eg, toll-free number, websites, local VA facilities, etc), respondents generally found these sources unhelpful and insufficient for answering their questions. The only positive anecdotal comments that respondents made regarding VA were from those with personal relationships with employees at the VA who could help with veteran needs. Finally, all respondents reported a need for more information about VA benefits and to assist them with helping veteran clients.
Survey Response
In 2013, the ORH and the VA Salt Lake City Geriatric Research Education and Clinical Center (GRECC), under the sponsorship of the VA Office of Geriatrics and Extended Care (GEC), developed a collaborative demonstration with the Utah ADRC to address the needs identified in this survey. Connecting Older Veterans (Especially Rural) to Community or Veteran Eligible Resources (COVER to COVER) is a demonstration project designed to create a new access point for VA benefits for veterans living in rural areas. The pilot had 2 aims: (1) train ADRC options counselors as Veteran Benefits Specialists (VBSs); and (2) build relationships between the ADRCs and VA to facilitate information and referral.
Between 2013 and 2015, the demonstration was housed at the VA Salt Lake City Health Care System GRECC as part of the clinical demonstration portfolio. The GRECC staff provided administrative support and mentorship for the developing partnerships. Subsequently, the demonstration was selected as a Promising Practice for enterprise-wide implementation. Both ORH and GEC coordinated opportunities for broad dissemination.
Program Elements
In Utah, 5 pilot ADRC agencies cover 19 counties, 14 of which are entirely rural. The remaining counties contain populations that are 20% to 49% urbanized (1 county), 50% to 80% urbanized (1 county), and 80% to 100% urbanized (3 counties). More than 95,000 veterans (12,857 in the 14 rural counties) live in the participating counties. The average income for veterans in all participating counties is $36,699 for men and $30,915 for women.9 Furthermore, about 53% of veterans in all these counties are aged > 65 years.9
For this pilot, each ADRC site assigned an existing options counselor as a dedicated VBS. Each VBS completed 80 to 100 hours of training in VA benefits. To facilitate the amount of training required to become experts, the ORH funded a portion of the salary for each VBS.
An outreach specialist at the VA Salt Lake City Regional Benefit Office, a geriatric social worker at the VA Salt Lake City Health Care System, and an outreach specialist at the Utah Department of Veterans and Military Affairs (UDVMA) were primary trainers for this pilot. Trainers provided 15 training sessions between February 2013 and September 2015, totaling 74 hours. The 5 designated VBSs attended all trainings, but meetings were opened to all ADRC staff and other community organizations; 115 individuals from Utah, Idaho, Nevada, New Mexico, and Wyoming attended at least 1 training. In the first year and a half, trainings ranged from 1.5 to 4.5 hours and provided a general overview of benefits. As the value of these trainings increased among the ADRCs and other community providers, longer seminars were offered, the longest lasting 2 days, which provided in-depth training.
Training topics comprised the following 4 general categories:
- Core—VA structure, military culture
- VHA—health care, enrollment and eligibility, in-home services
- VBA—pension, aid and attendance, disability compensation, nursing home, dependency and indemnity compensation
- NCA burial benefits
In response to participant requests for training on other VA benefits, additional VA staff presented topics such as mental health, homelessness, telehealth, Vet Centers, and My HealtheVet. Information on the Veterans Choice Program was incorporated into later trainings.
In addition to the training provided by COVER to COVER, the 5 ADRC VBSs completed the 25-hour Training Responsibility Involvement and Preparation of Claims (TRIP) online course. This coursework qualified them to take the examination to become certified veterans service officers.
With the information received in training, ADRC VBSs assist veteran clients and their families to learn about and apply for VA benefits. Veterans or family members contact the ADRC with a variety of needs, such as difficulty paying utilities, functional limitations, etc. All ADRC staff screen callers for veteran status and refer willing veterans or family members to the VBS who provides information about LTSS options and screens for eligibility for VA benefits.
Through these training events, VBSs also formed relationships with the VA trainers, resulting in the ability to refer to and coordinate with the VA on cases when needed. The VBSs often work closely with the UDVMA by helping veterans organize needed documents and coordinate with UDVMA staff to complete VA benefit claims. Furthermore, VBSs can help veterans navigate the VA system and advocate for their needs in coordination with the VA trainers.
The VBAs have described numerous positive outcomes from the COVER to COVER program. They universally report improved knowledge and confidence in assisting veteran clients. In many cases, simply identifying clients’ veteran status in the normal ADRC intake protocol has placed them in touch with many veterans without any significant change in their workload. One specialist reported that COVER to COVER has improved the quality of services she can provide to veterans and that connecting veteran clients to VA frees public resources for other clients in need. Finally, they report that the trainings introduced them to key VA contacts and laid the groundwork for developing relationships with new partners. The following case is representative of the types of client experiences VBSs routinely describe.
Case Study
“Larry,” a 94 year-old World War II veteran who had never applied for VA benefits, presented to a rural ADRC for assistance with paying his utility bills. Larry had numerous health issues, including early stage dementia. He relied on his 96-year-old wife, “Sandy,” to assist him with activities of daily living (ADLs) and instrumental activities of living (IADLs). However, Sandy also had health problems that limited her ability to help. The couple wanted to stay in their home but worried they could not do it without help.
An ADRC staff member referred Larry and Sandy to the VBS, who helped the couple enroll in a community LTSS program called Aging Alternatives for in-home services. During this time, Sandy passed away, but the VBS continued to work with Larry and helped him apply for VA disability compensation, enroll in VHA health care, and connect to VA’s Veteran Directed Home and Community Based Services (VDHCBS) program for in-home services.
Larry received a 70% service-connected disability rating and started receiving monthly compensation from the VA. Although Larry wants to stay at home, the rating of 70% service connection allows VA to cover nursing home placement should it be needed. He established a VA primary care physician and uses VDHCBS to purchase in-home services. Since Larry receives in-home services from the VHA, he was discharged from the Aging Alternatives program. This allowed the ADRC to reallocate this resource to another person in need. Larry is still living at home.
Future Directions
This case study highlights the benefits for veterans of COVER to COVER program through its emphasis on productive relationships between VA and community partners. More extensive data collection related to veteran outcomes is ongoing and will be essential for sustaining the program locally and to support broader dissemination to other states. Ideally, expansion to other sites will include temporary pilot funding to offset the time needed to gain the knowledgeand skills to become a VBS and to provide consultation and training to other ADRC staff. Once the pilot funding ends, the ADRC staff would have the necessary knowledge, skills, and relationships to continue providing services to veterans.
Acknowledgments
This project was supported by the VHA Office of Rural Health. The authors thank all ADRC, UDVMA, and VHA staff who participated in the project.
1. VHA Office of Rural Health. Office of rural health annual report: thrive 2015. http://www.rural-health.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed January 9, 2017.
2. National Center for Veterans Analysis and Statistics. 2010 national survey of veterans: understanding and knowledge of VA benefits and services. http://www.va.gov/VETDATA/docs/SpecialReports/2010NSV_Awareness_FINAL.pdf. Published November 2011. Accessed January 9, 2017.
3. Wittrock S, Ono S, Stewart K, Reisinger HS, Charlton M. Unclaimed health care benefits: a mixed-method analysis of rural veterans. J Rural Health. 2015;31(1):35-46.
4. Fox AB, Meyer EC, Vogt D. Attitudes about the VA health-care setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58.
5. Helm MD. System worth saving report on rural healthcare 2012. http://archive.legion.org/handle/123456789/1951. Published 2012. Accessed January 9, 2017.
6. Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the Internet. Comput Human Behav. 2011;27(3):1231-1237.
7. Kane RL, Kane RA. A guide through the maze of long-term care. West J Med. 1981;135(6):503-510.
8. O’Shaughnessy CV. Aging and disability resource centers can help consumers navigate the maze of long-term services and supports. Generations. 2011;35(1):64-68.
9. United States Census Bureau. Veteran status: 2010-2014 American community survey 5-year estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_S2101&prodType=table. Accessed January 9, 2017.
According to the VA, 23% of veterans in the U.S., nearly 5.2 million individuals, live in rural areas.1 The VHA serves more than 3 million rural veterans, and 56% of those enrolled in the VA system are aged ≥ 65 years.1 Thus, aging veterans in rural areas constitute a substantial group who need support and assistance from the VA. Fortunately, the VA offers numerous benefits for veterans that support aging in place and improve quality of life through the VHA, Veterans Benefits Administration (VBA), and National Cemetery Administration (NCA).
Despite the opportunities, many VA benefits go unclaimed. In some cases, veterans simply do not know these benefits exist.2 In a 2010 VA report, only 41% of veterans indicated that they understood their benefits “a lot” or “some.”2 However, their understanding of specific benefits tended to be lower. For example, many veterans stated that they had “heard about” burial options at VA cemeteries (41.5%), but few understood specific benefits, such as cash burial allowances (10.6%).2
Many veterans also hold misperceptions about eligibility, which prevents them from applying. For example, some veterans believe that a high income or lack of combat service disqualifies them from receiving VA benefits.3 Some veterans believe that others are more deserving of VA services, and they don’t want to “take a spot someone else needs.”3 Finally, some veterans hold negative attitudes about the VA, making them less likely to claim VA benefits, such as health care.4
For rural veterans, accessing benefits can be especially difficult, because most VA facilities that offer assistance are in urban centers.5 Though online access to benefit information is improving through programs like My HealtheVet and public facing websites, some older adults do not use computers, and Internet and mobile phone connectivity are often limited in rural communities.6 Nearly 43% of rural veterans do not have broadband Internet in their homes.1 Moreover, the complexity of navigating benefits information via the Internet can be a frustrating and confusing process for older veterans.6
Accessing services and benefits in the community is similarly difficult. For more than 30 years, community organizations have noted the frustration that clients experienced with navigating a complex network of community providers who provide long-term services and supports (LTSS).7,8 In 2003, the Administration on Aging and the Centers for Medicare and Medicaid Services developed the Aging and Disability Resource Center (ADRC) program to promote a “no wrong door” approach for LTSS. Aging and Disability Resource Centers are a single point of entry into a network of community, state, and federal LTSS for older adults and individuals with disabilities.8 Options counselors at ADRCs provide information, counseling, and assistance with connecting to a vast network of programs such as Social Security, Medicaid, local transportation, Meals on Wheels, and housing assistance through a single office.
Backround
In 2012, VHA Office of Rural Health Resource (ORH) and Utah ADRC conducted a national survey of ADRC sites about their experiences working with veterans and found that 95% of ADRCs always or usually asked clients about their veteran status. The survey found that veteran clients present to ADRCs with diverse needs, many of which could be addressed through a VA benefit. However, the majority (58%) of ADRC respondents reported that they had never attempted to help a veteran apply for VA benefits (unpublished data, 2012).
Respondents reported a limited understanding of VA benefits, infrequent contact with VA, and frustrations with the VA system. Although familiar with several sources for information about VA benefits (eg, toll-free number, websites, local VA facilities, etc), respondents generally found these sources unhelpful and insufficient for answering their questions. The only positive anecdotal comments that respondents made regarding VA were from those with personal relationships with employees at the VA who could help with veteran needs. Finally, all respondents reported a need for more information about VA benefits and to assist them with helping veteran clients.
Survey Response
In 2013, the ORH and the VA Salt Lake City Geriatric Research Education and Clinical Center (GRECC), under the sponsorship of the VA Office of Geriatrics and Extended Care (GEC), developed a collaborative demonstration with the Utah ADRC to address the needs identified in this survey. Connecting Older Veterans (Especially Rural) to Community or Veteran Eligible Resources (COVER to COVER) is a demonstration project designed to create a new access point for VA benefits for veterans living in rural areas. The pilot had 2 aims: (1) train ADRC options counselors as Veteran Benefits Specialists (VBSs); and (2) build relationships between the ADRCs and VA to facilitate information and referral.
Between 2013 and 2015, the demonstration was housed at the VA Salt Lake City Health Care System GRECC as part of the clinical demonstration portfolio. The GRECC staff provided administrative support and mentorship for the developing partnerships. Subsequently, the demonstration was selected as a Promising Practice for enterprise-wide implementation. Both ORH and GEC coordinated opportunities for broad dissemination.
Program Elements
In Utah, 5 pilot ADRC agencies cover 19 counties, 14 of which are entirely rural. The remaining counties contain populations that are 20% to 49% urbanized (1 county), 50% to 80% urbanized (1 county), and 80% to 100% urbanized (3 counties). More than 95,000 veterans (12,857 in the 14 rural counties) live in the participating counties. The average income for veterans in all participating counties is $36,699 for men and $30,915 for women.9 Furthermore, about 53% of veterans in all these counties are aged > 65 years.9
For this pilot, each ADRC site assigned an existing options counselor as a dedicated VBS. Each VBS completed 80 to 100 hours of training in VA benefits. To facilitate the amount of training required to become experts, the ORH funded a portion of the salary for each VBS.
An outreach specialist at the VA Salt Lake City Regional Benefit Office, a geriatric social worker at the VA Salt Lake City Health Care System, and an outreach specialist at the Utah Department of Veterans and Military Affairs (UDVMA) were primary trainers for this pilot. Trainers provided 15 training sessions between February 2013 and September 2015, totaling 74 hours. The 5 designated VBSs attended all trainings, but meetings were opened to all ADRC staff and other community organizations; 115 individuals from Utah, Idaho, Nevada, New Mexico, and Wyoming attended at least 1 training. In the first year and a half, trainings ranged from 1.5 to 4.5 hours and provided a general overview of benefits. As the value of these trainings increased among the ADRCs and other community providers, longer seminars were offered, the longest lasting 2 days, which provided in-depth training.
Training topics comprised the following 4 general categories:
- Core—VA structure, military culture
- VHA—health care, enrollment and eligibility, in-home services
- VBA—pension, aid and attendance, disability compensation, nursing home, dependency and indemnity compensation
- NCA burial benefits
In response to participant requests for training on other VA benefits, additional VA staff presented topics such as mental health, homelessness, telehealth, Vet Centers, and My HealtheVet. Information on the Veterans Choice Program was incorporated into later trainings.
In addition to the training provided by COVER to COVER, the 5 ADRC VBSs completed the 25-hour Training Responsibility Involvement and Preparation of Claims (TRIP) online course. This coursework qualified them to take the examination to become certified veterans service officers.
With the information received in training, ADRC VBSs assist veteran clients and their families to learn about and apply for VA benefits. Veterans or family members contact the ADRC with a variety of needs, such as difficulty paying utilities, functional limitations, etc. All ADRC staff screen callers for veteran status and refer willing veterans or family members to the VBS who provides information about LTSS options and screens for eligibility for VA benefits.
Through these training events, VBSs also formed relationships with the VA trainers, resulting in the ability to refer to and coordinate with the VA on cases when needed. The VBSs often work closely with the UDVMA by helping veterans organize needed documents and coordinate with UDVMA staff to complete VA benefit claims. Furthermore, VBSs can help veterans navigate the VA system and advocate for their needs in coordination with the VA trainers.
The VBAs have described numerous positive outcomes from the COVER to COVER program. They universally report improved knowledge and confidence in assisting veteran clients. In many cases, simply identifying clients’ veteran status in the normal ADRC intake protocol has placed them in touch with many veterans without any significant change in their workload. One specialist reported that COVER to COVER has improved the quality of services she can provide to veterans and that connecting veteran clients to VA frees public resources for other clients in need. Finally, they report that the trainings introduced them to key VA contacts and laid the groundwork for developing relationships with new partners. The following case is representative of the types of client experiences VBSs routinely describe.
Case Study
“Larry,” a 94 year-old World War II veteran who had never applied for VA benefits, presented to a rural ADRC for assistance with paying his utility bills. Larry had numerous health issues, including early stage dementia. He relied on his 96-year-old wife, “Sandy,” to assist him with activities of daily living (ADLs) and instrumental activities of living (IADLs). However, Sandy also had health problems that limited her ability to help. The couple wanted to stay in their home but worried they could not do it without help.
An ADRC staff member referred Larry and Sandy to the VBS, who helped the couple enroll in a community LTSS program called Aging Alternatives for in-home services. During this time, Sandy passed away, but the VBS continued to work with Larry and helped him apply for VA disability compensation, enroll in VHA health care, and connect to VA’s Veteran Directed Home and Community Based Services (VDHCBS) program for in-home services.
Larry received a 70% service-connected disability rating and started receiving monthly compensation from the VA. Although Larry wants to stay at home, the rating of 70% service connection allows VA to cover nursing home placement should it be needed. He established a VA primary care physician and uses VDHCBS to purchase in-home services. Since Larry receives in-home services from the VHA, he was discharged from the Aging Alternatives program. This allowed the ADRC to reallocate this resource to another person in need. Larry is still living at home.
Future Directions
This case study highlights the benefits for veterans of COVER to COVER program through its emphasis on productive relationships between VA and community partners. More extensive data collection related to veteran outcomes is ongoing and will be essential for sustaining the program locally and to support broader dissemination to other states. Ideally, expansion to other sites will include temporary pilot funding to offset the time needed to gain the knowledgeand skills to become a VBS and to provide consultation and training to other ADRC staff. Once the pilot funding ends, the ADRC staff would have the necessary knowledge, skills, and relationships to continue providing services to veterans.
Acknowledgments
This project was supported by the VHA Office of Rural Health. The authors thank all ADRC, UDVMA, and VHA staff who participated in the project.
According to the VA, 23% of veterans in the U.S., nearly 5.2 million individuals, live in rural areas.1 The VHA serves more than 3 million rural veterans, and 56% of those enrolled in the VA system are aged ≥ 65 years.1 Thus, aging veterans in rural areas constitute a substantial group who need support and assistance from the VA. Fortunately, the VA offers numerous benefits for veterans that support aging in place and improve quality of life through the VHA, Veterans Benefits Administration (VBA), and National Cemetery Administration (NCA).
Despite the opportunities, many VA benefits go unclaimed. In some cases, veterans simply do not know these benefits exist.2 In a 2010 VA report, only 41% of veterans indicated that they understood their benefits “a lot” or “some.”2 However, their understanding of specific benefits tended to be lower. For example, many veterans stated that they had “heard about” burial options at VA cemeteries (41.5%), but few understood specific benefits, such as cash burial allowances (10.6%).2
Many veterans also hold misperceptions about eligibility, which prevents them from applying. For example, some veterans believe that a high income or lack of combat service disqualifies them from receiving VA benefits.3 Some veterans believe that others are more deserving of VA services, and they don’t want to “take a spot someone else needs.”3 Finally, some veterans hold negative attitudes about the VA, making them less likely to claim VA benefits, such as health care.4
For rural veterans, accessing benefits can be especially difficult, because most VA facilities that offer assistance are in urban centers.5 Though online access to benefit information is improving through programs like My HealtheVet and public facing websites, some older adults do not use computers, and Internet and mobile phone connectivity are often limited in rural communities.6 Nearly 43% of rural veterans do not have broadband Internet in their homes.1 Moreover, the complexity of navigating benefits information via the Internet can be a frustrating and confusing process for older veterans.6
Accessing services and benefits in the community is similarly difficult. For more than 30 years, community organizations have noted the frustration that clients experienced with navigating a complex network of community providers who provide long-term services and supports (LTSS).7,8 In 2003, the Administration on Aging and the Centers for Medicare and Medicaid Services developed the Aging and Disability Resource Center (ADRC) program to promote a “no wrong door” approach for LTSS. Aging and Disability Resource Centers are a single point of entry into a network of community, state, and federal LTSS for older adults and individuals with disabilities.8 Options counselors at ADRCs provide information, counseling, and assistance with connecting to a vast network of programs such as Social Security, Medicaid, local transportation, Meals on Wheels, and housing assistance through a single office.
Backround
In 2012, VHA Office of Rural Health Resource (ORH) and Utah ADRC conducted a national survey of ADRC sites about their experiences working with veterans and found that 95% of ADRCs always or usually asked clients about their veteran status. The survey found that veteran clients present to ADRCs with diverse needs, many of which could be addressed through a VA benefit. However, the majority (58%) of ADRC respondents reported that they had never attempted to help a veteran apply for VA benefits (unpublished data, 2012).
Respondents reported a limited understanding of VA benefits, infrequent contact with VA, and frustrations with the VA system. Although familiar with several sources for information about VA benefits (eg, toll-free number, websites, local VA facilities, etc), respondents generally found these sources unhelpful and insufficient for answering their questions. The only positive anecdotal comments that respondents made regarding VA were from those with personal relationships with employees at the VA who could help with veteran needs. Finally, all respondents reported a need for more information about VA benefits and to assist them with helping veteran clients.
Survey Response
In 2013, the ORH and the VA Salt Lake City Geriatric Research Education and Clinical Center (GRECC), under the sponsorship of the VA Office of Geriatrics and Extended Care (GEC), developed a collaborative demonstration with the Utah ADRC to address the needs identified in this survey. Connecting Older Veterans (Especially Rural) to Community or Veteran Eligible Resources (COVER to COVER) is a demonstration project designed to create a new access point for VA benefits for veterans living in rural areas. The pilot had 2 aims: (1) train ADRC options counselors as Veteran Benefits Specialists (VBSs); and (2) build relationships between the ADRCs and VA to facilitate information and referral.
Between 2013 and 2015, the demonstration was housed at the VA Salt Lake City Health Care System GRECC as part of the clinical demonstration portfolio. The GRECC staff provided administrative support and mentorship for the developing partnerships. Subsequently, the demonstration was selected as a Promising Practice for enterprise-wide implementation. Both ORH and GEC coordinated opportunities for broad dissemination.
Program Elements
In Utah, 5 pilot ADRC agencies cover 19 counties, 14 of which are entirely rural. The remaining counties contain populations that are 20% to 49% urbanized (1 county), 50% to 80% urbanized (1 county), and 80% to 100% urbanized (3 counties). More than 95,000 veterans (12,857 in the 14 rural counties) live in the participating counties. The average income for veterans in all participating counties is $36,699 for men and $30,915 for women.9 Furthermore, about 53% of veterans in all these counties are aged > 65 years.9
For this pilot, each ADRC site assigned an existing options counselor as a dedicated VBS. Each VBS completed 80 to 100 hours of training in VA benefits. To facilitate the amount of training required to become experts, the ORH funded a portion of the salary for each VBS.
An outreach specialist at the VA Salt Lake City Regional Benefit Office, a geriatric social worker at the VA Salt Lake City Health Care System, and an outreach specialist at the Utah Department of Veterans and Military Affairs (UDVMA) were primary trainers for this pilot. Trainers provided 15 training sessions between February 2013 and September 2015, totaling 74 hours. The 5 designated VBSs attended all trainings, but meetings were opened to all ADRC staff and other community organizations; 115 individuals from Utah, Idaho, Nevada, New Mexico, and Wyoming attended at least 1 training. In the first year and a half, trainings ranged from 1.5 to 4.5 hours and provided a general overview of benefits. As the value of these trainings increased among the ADRCs and other community providers, longer seminars were offered, the longest lasting 2 days, which provided in-depth training.
Training topics comprised the following 4 general categories:
- Core—VA structure, military culture
- VHA—health care, enrollment and eligibility, in-home services
- VBA—pension, aid and attendance, disability compensation, nursing home, dependency and indemnity compensation
- NCA burial benefits
In response to participant requests for training on other VA benefits, additional VA staff presented topics such as mental health, homelessness, telehealth, Vet Centers, and My HealtheVet. Information on the Veterans Choice Program was incorporated into later trainings.
In addition to the training provided by COVER to COVER, the 5 ADRC VBSs completed the 25-hour Training Responsibility Involvement and Preparation of Claims (TRIP) online course. This coursework qualified them to take the examination to become certified veterans service officers.
With the information received in training, ADRC VBSs assist veteran clients and their families to learn about and apply for VA benefits. Veterans or family members contact the ADRC with a variety of needs, such as difficulty paying utilities, functional limitations, etc. All ADRC staff screen callers for veteran status and refer willing veterans or family members to the VBS who provides information about LTSS options and screens for eligibility for VA benefits.
Through these training events, VBSs also formed relationships with the VA trainers, resulting in the ability to refer to and coordinate with the VA on cases when needed. The VBSs often work closely with the UDVMA by helping veterans organize needed documents and coordinate with UDVMA staff to complete VA benefit claims. Furthermore, VBSs can help veterans navigate the VA system and advocate for their needs in coordination with the VA trainers.
The VBAs have described numerous positive outcomes from the COVER to COVER program. They universally report improved knowledge and confidence in assisting veteran clients. In many cases, simply identifying clients’ veteran status in the normal ADRC intake protocol has placed them in touch with many veterans without any significant change in their workload. One specialist reported that COVER to COVER has improved the quality of services she can provide to veterans and that connecting veteran clients to VA frees public resources for other clients in need. Finally, they report that the trainings introduced them to key VA contacts and laid the groundwork for developing relationships with new partners. The following case is representative of the types of client experiences VBSs routinely describe.
Case Study
“Larry,” a 94 year-old World War II veteran who had never applied for VA benefits, presented to a rural ADRC for assistance with paying his utility bills. Larry had numerous health issues, including early stage dementia. He relied on his 96-year-old wife, “Sandy,” to assist him with activities of daily living (ADLs) and instrumental activities of living (IADLs). However, Sandy also had health problems that limited her ability to help. The couple wanted to stay in their home but worried they could not do it without help.
An ADRC staff member referred Larry and Sandy to the VBS, who helped the couple enroll in a community LTSS program called Aging Alternatives for in-home services. During this time, Sandy passed away, but the VBS continued to work with Larry and helped him apply for VA disability compensation, enroll in VHA health care, and connect to VA’s Veteran Directed Home and Community Based Services (VDHCBS) program for in-home services.
Larry received a 70% service-connected disability rating and started receiving monthly compensation from the VA. Although Larry wants to stay at home, the rating of 70% service connection allows VA to cover nursing home placement should it be needed. He established a VA primary care physician and uses VDHCBS to purchase in-home services. Since Larry receives in-home services from the VHA, he was discharged from the Aging Alternatives program. This allowed the ADRC to reallocate this resource to another person in need. Larry is still living at home.
Future Directions
This case study highlights the benefits for veterans of COVER to COVER program through its emphasis on productive relationships between VA and community partners. More extensive data collection related to veteran outcomes is ongoing and will be essential for sustaining the program locally and to support broader dissemination to other states. Ideally, expansion to other sites will include temporary pilot funding to offset the time needed to gain the knowledgeand skills to become a VBS and to provide consultation and training to other ADRC staff. Once the pilot funding ends, the ADRC staff would have the necessary knowledge, skills, and relationships to continue providing services to veterans.
Acknowledgments
This project was supported by the VHA Office of Rural Health. The authors thank all ADRC, UDVMA, and VHA staff who participated in the project.
1. VHA Office of Rural Health. Office of rural health annual report: thrive 2015. http://www.rural-health.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed January 9, 2017.
2. National Center for Veterans Analysis and Statistics. 2010 national survey of veterans: understanding and knowledge of VA benefits and services. http://www.va.gov/VETDATA/docs/SpecialReports/2010NSV_Awareness_FINAL.pdf. Published November 2011. Accessed January 9, 2017.
3. Wittrock S, Ono S, Stewart K, Reisinger HS, Charlton M. Unclaimed health care benefits: a mixed-method analysis of rural veterans. J Rural Health. 2015;31(1):35-46.
4. Fox AB, Meyer EC, Vogt D. Attitudes about the VA health-care setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58.
5. Helm MD. System worth saving report on rural healthcare 2012. http://archive.legion.org/handle/123456789/1951. Published 2012. Accessed January 9, 2017.
6. Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the Internet. Comput Human Behav. 2011;27(3):1231-1237.
7. Kane RL, Kane RA. A guide through the maze of long-term care. West J Med. 1981;135(6):503-510.
8. O’Shaughnessy CV. Aging and disability resource centers can help consumers navigate the maze of long-term services and supports. Generations. 2011;35(1):64-68.
9. United States Census Bureau. Veteran status: 2010-2014 American community survey 5-year estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_S2101&prodType=table. Accessed January 9, 2017.
1. VHA Office of Rural Health. Office of rural health annual report: thrive 2015. http://www.rural-health.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed January 9, 2017.
2. National Center for Veterans Analysis and Statistics. 2010 national survey of veterans: understanding and knowledge of VA benefits and services. http://www.va.gov/VETDATA/docs/SpecialReports/2010NSV_Awareness_FINAL.pdf. Published November 2011. Accessed January 9, 2017.
3. Wittrock S, Ono S, Stewart K, Reisinger HS, Charlton M. Unclaimed health care benefits: a mixed-method analysis of rural veterans. J Rural Health. 2015;31(1):35-46.
4. Fox AB, Meyer EC, Vogt D. Attitudes about the VA health-care setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58.
5. Helm MD. System worth saving report on rural healthcare 2012. http://archive.legion.org/handle/123456789/1951. Published 2012. Accessed January 9, 2017.
6. Lee B, Chen Y, Hewitt L. Age differences in constraints encountered by seniors in their use of computers and the Internet. Comput Human Behav. 2011;27(3):1231-1237.
7. Kane RL, Kane RA. A guide through the maze of long-term care. West J Med. 1981;135(6):503-510.
8. O’Shaughnessy CV. Aging and disability resource centers can help consumers navigate the maze of long-term services and supports. Generations. 2011;35(1):64-68.
9. United States Census Bureau. Veteran status: 2010-2014 American community survey 5-year estimates. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_14_5YR_S2101&prodType=table. Accessed January 9, 2017.
FDA clears push-button blood draw device
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP.
It provides “virtually painless, simple, and fast” blood collection, according to Seventh Sense Biosystems, Inc., the company marketing the device.
The FDA clearance allows healthcare workers to use TAP only for the collection of capillary blood for hemoglobin A1c testing.
However, Seventh Sense Biosystems said it is working with the FDA to expand the use of TAP to add additional tests, as well as at-home blood collection.
How TAP works
The TAP device is placed on a patient’s upper arm, and blood collection starts with the press of a button. The process typically takes 2 to 3 minutes.
When TAP is placed on the arm, the base of the device generates a seal with the skin to create a vacuum. When the user presses the button, TAP deploys microneedles that puncture the skin above the dermis.
The vacuum draws capillary blood out of the micropunctures and through microfluidic channels into a collection chamber prefilled with lithium heparin.
TAP collects up to 100 μL of blood. To transfer the blood to an analyzer, a precision pipette is placed in an access port at the bottom of the device.
Seventh Sense Biosystems said it will launch TAP over the coming months. For up-to-date information, visit www.7sbio.com. ![]()
Paid Sick Days Help Reduce Flu Exposure (For Some)
Research has confirmed what many people know from experience: that paid sick days can make the workplace healthier. In 1 study, researchers found universal access to paid sick days (PSD) reduced influenza in the workplace by 6%. To drill down on the influence PSD access has on decisions to stay home from work, or to stay home with a child who has flu or influenza-like-illness, researchers from University of Pittsburgh analyzed data from the 2009 Medical Expenditure Panel Survey for 12,901 households and 12,044 employees. They chose the 2009 survey because the numbers of influenza-like-illness and influenza cases were likely to have been higher due to the 2009 H1N1 pandemic.
Of the workers surveyed, 64% had access to PSD. Access was associated significantly with gender, race/ethnicity, income, education, and number of employees in the workplace. In the group of 4,911 employees who had children, 68% had PSD.
The study highlighted some subgroups that face barriers to following CDC recommendations, such as staying home for up to 24 hours after symptoms subside. Hispanics, for instance, were significantly less likely to stay home when ill, but this was not necessarily an ethnic difference, the researchers say. Rather, they suggest, it may have had more to do with job security and workplace culture. They cite a survey done during the 2009 H1N1 pandemic, in which Hispanics reported fewer resources at work than non-Hispanic whites, including paid sick leave, job security, and ability to work from home.
Women tend to be the main caregivers for children. In this study, women had a higher prevalence of staying home for a child’s illness than men, even after the researchers controlled for PSD access. Yet, in a different survey women also were more likely to report for work when ill, or when a child was ill. The researchers called this “presenteeism.”
The researchers underscore the importance of PSD laws in reducing the economic burden of healthy behavior in families. They note that in 2015, 35% of employees did not have access to PSD, and those employees were usually people with low income. Only 34% of those in the lowest-income group had access to PSD, compared with 89% in the highest income groups.
Source:
Piper K, Youk A, James AE, III, Kumar S. PLoS ONE. 2017;12(2): e0170698.
doi:10.1371/journal.pone.0170698
Research has confirmed what many people know from experience: that paid sick days can make the workplace healthier. In 1 study, researchers found universal access to paid sick days (PSD) reduced influenza in the workplace by 6%. To drill down on the influence PSD access has on decisions to stay home from work, or to stay home with a child who has flu or influenza-like-illness, researchers from University of Pittsburgh analyzed data from the 2009 Medical Expenditure Panel Survey for 12,901 households and 12,044 employees. They chose the 2009 survey because the numbers of influenza-like-illness and influenza cases were likely to have been higher due to the 2009 H1N1 pandemic.
Of the workers surveyed, 64% had access to PSD. Access was associated significantly with gender, race/ethnicity, income, education, and number of employees in the workplace. In the group of 4,911 employees who had children, 68% had PSD.
The study highlighted some subgroups that face barriers to following CDC recommendations, such as staying home for up to 24 hours after symptoms subside. Hispanics, for instance, were significantly less likely to stay home when ill, but this was not necessarily an ethnic difference, the researchers say. Rather, they suggest, it may have had more to do with job security and workplace culture. They cite a survey done during the 2009 H1N1 pandemic, in which Hispanics reported fewer resources at work than non-Hispanic whites, including paid sick leave, job security, and ability to work from home.
Women tend to be the main caregivers for children. In this study, women had a higher prevalence of staying home for a child’s illness than men, even after the researchers controlled for PSD access. Yet, in a different survey women also were more likely to report for work when ill, or when a child was ill. The researchers called this “presenteeism.”
The researchers underscore the importance of PSD laws in reducing the economic burden of healthy behavior in families. They note that in 2015, 35% of employees did not have access to PSD, and those employees were usually people with low income. Only 34% of those in the lowest-income group had access to PSD, compared with 89% in the highest income groups.
Source:
Piper K, Youk A, James AE, III, Kumar S. PLoS ONE. 2017;12(2): e0170698.
doi:10.1371/journal.pone.0170698
Research has confirmed what many people know from experience: that paid sick days can make the workplace healthier. In 1 study, researchers found universal access to paid sick days (PSD) reduced influenza in the workplace by 6%. To drill down on the influence PSD access has on decisions to stay home from work, or to stay home with a child who has flu or influenza-like-illness, researchers from University of Pittsburgh analyzed data from the 2009 Medical Expenditure Panel Survey for 12,901 households and 12,044 employees. They chose the 2009 survey because the numbers of influenza-like-illness and influenza cases were likely to have been higher due to the 2009 H1N1 pandemic.
Of the workers surveyed, 64% had access to PSD. Access was associated significantly with gender, race/ethnicity, income, education, and number of employees in the workplace. In the group of 4,911 employees who had children, 68% had PSD.
The study highlighted some subgroups that face barriers to following CDC recommendations, such as staying home for up to 24 hours after symptoms subside. Hispanics, for instance, were significantly less likely to stay home when ill, but this was not necessarily an ethnic difference, the researchers say. Rather, they suggest, it may have had more to do with job security and workplace culture. They cite a survey done during the 2009 H1N1 pandemic, in which Hispanics reported fewer resources at work than non-Hispanic whites, including paid sick leave, job security, and ability to work from home.
Women tend to be the main caregivers for children. In this study, women had a higher prevalence of staying home for a child’s illness than men, even after the researchers controlled for PSD access. Yet, in a different survey women also were more likely to report for work when ill, or when a child was ill. The researchers called this “presenteeism.”
The researchers underscore the importance of PSD laws in reducing the economic burden of healthy behavior in families. They note that in 2015, 35% of employees did not have access to PSD, and those employees were usually people with low income. Only 34% of those in the lowest-income group had access to PSD, compared with 89% in the highest income groups.
Source:
Piper K, Youk A, James AE, III, Kumar S. PLoS ONE. 2017;12(2): e0170698.
doi:10.1371/journal.pone.0170698
When the Diagnosis Hurts
ANSWER
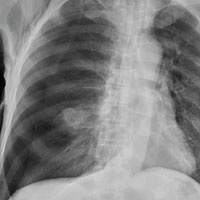
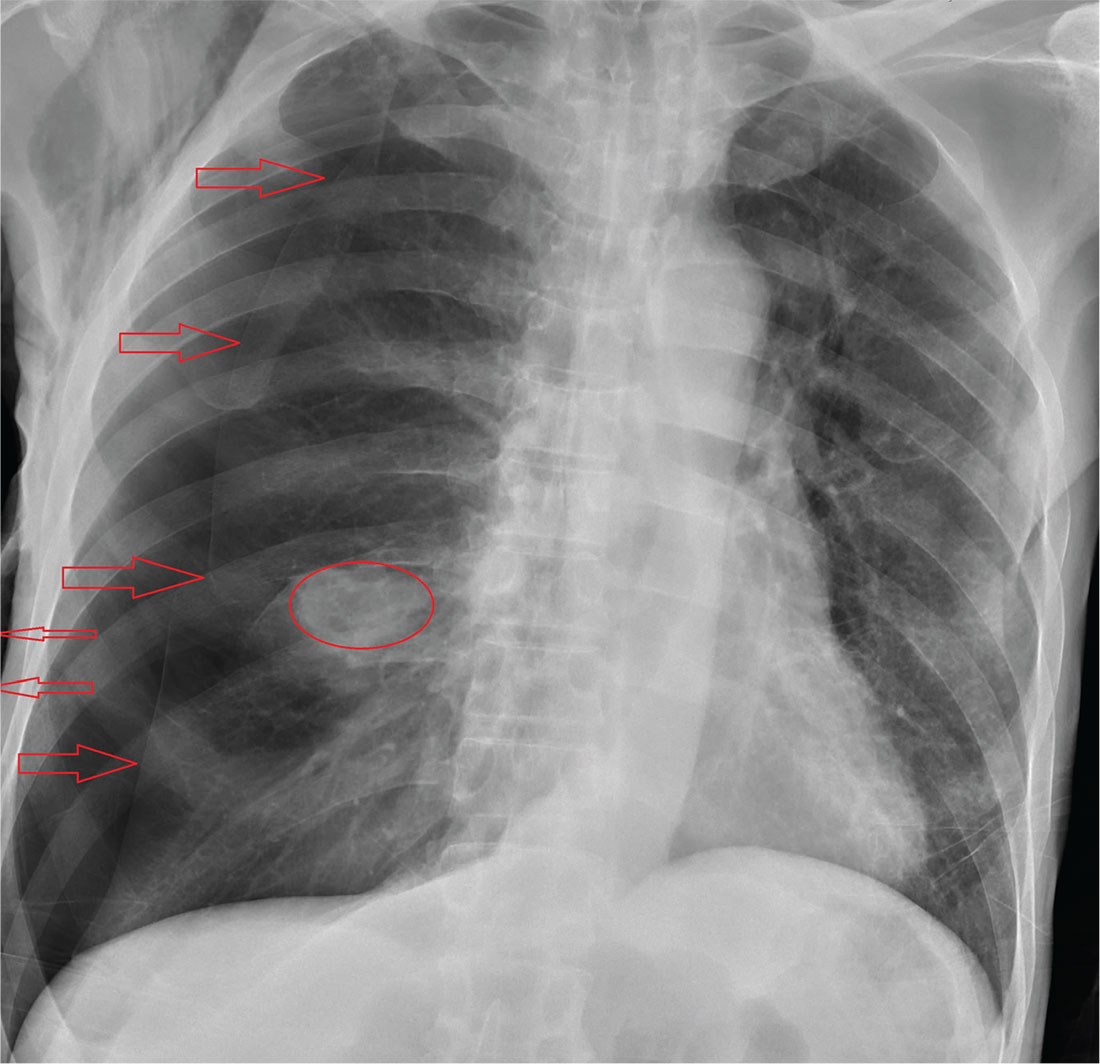
The radiograph shows an oval hyperdensity within the right mid lung, presumably the known lung mass. Of note, however, is an approximate 50% pneumothorax of the right lung. It is creating mild tension, indicated by the slightly displaced trachea. There is also evidence of subcutaneous air in the right lateral chest.
These findings likely result from a complication of the aforementioned biopsy. The patient underwent chest tube placement and was admitted for further treatment.

ANSWER
The radiograph shows an oval hyperdensity within the right mid lung, presumably the known lung mass. Of note, however, is an approximate 50% pneumothorax of the right lung. It is creating mild tension, indicated by the slightly displaced trachea. There is also evidence of subcutaneous air in the right lateral chest.
These findings likely result from a complication of the aforementioned biopsy. The patient underwent chest tube placement and was admitted for further treatment.

ANSWER
The radiograph shows an oval hyperdensity within the right mid lung, presumably the known lung mass. Of note, however, is an approximate 50% pneumothorax of the right lung. It is creating mild tension, indicated by the slightly displaced trachea. There is also evidence of subcutaneous air in the right lateral chest.
These findings likely result from a complication of the aforementioned biopsy. The patient underwent chest tube placement and was admitted for further treatment.
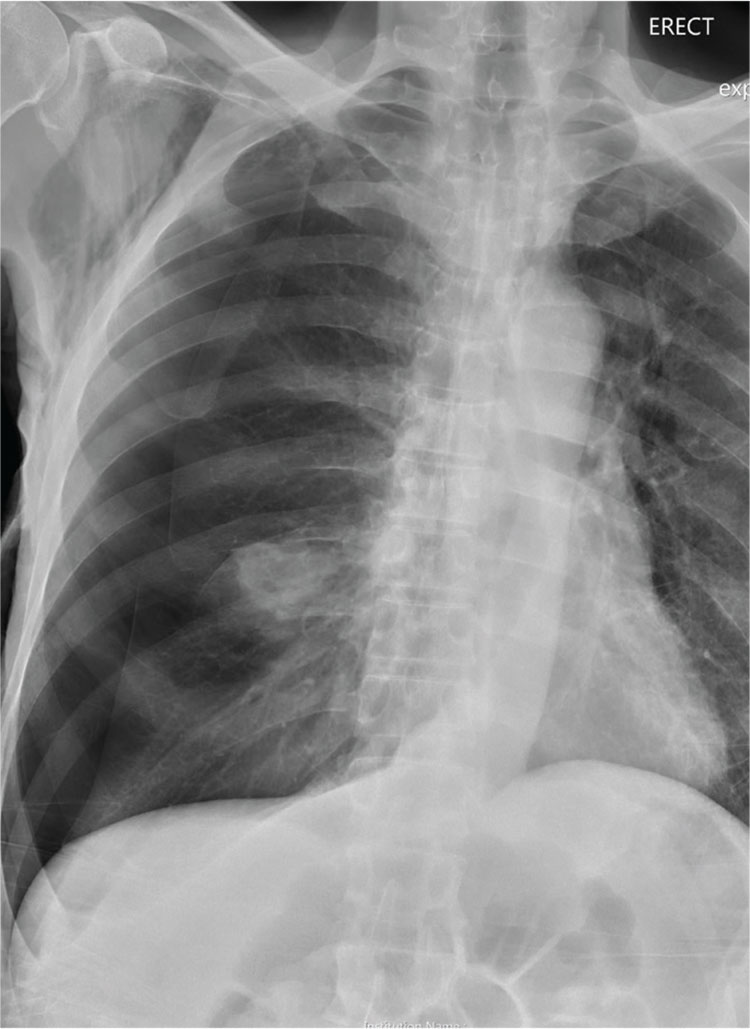
A 60-year-old man presents to the emergency department for evaluation of chest pain that began a few hours ago. He denies injury and has no associated nausea or shortness of breath. Earlier today, he underwent biopsy of a recently discovered mass in his right lung. Otherwise, his medical history is only significant for hypertension. He is a former pack-a-day smoker but quit three months ago.
On physical exam, you note an uncomfortable male in no obvious distress. He is afebrile, with normal vital signs. His O2 saturation is 96% on room air. Breath sounds appear to be clear bilaterally, although the patient expresses some discomfort with inhalation. Heart sounds are normal as well.
While the nurse and tech place an IV, a portable chest radiograph is obtained. What is your impression?
Inpatient palliative care improves QOL for HSCT patients
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
CHMP recommends authorization of antiemetic agent
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
Late-breaking research presented March 4-5 at AAD meeting
Dermatology News will be on site later this week at the annual meeting of the American Academy of Dermatology in Orlando. Look for the latest news in medical, surgical, and aesthetic dermatology starting Friday, March 3. The late-breaker sessions are on Saturday and Sunday March 4 and 5.
The late-breaking research session on clinical trials on March 4 will highlight:
- 16-week results from two phase III studies of certolizumab for chronic plaque psoriasis.
- Phase III results on the long-term management of moderate to severe atopic dermatitis (AD) with dupilumab plus topical corticosteroids.
Additional late breakers focus on pediatric, procedural, and pathology studies:
- Data from the Pediatric Eczema Elective Registry, on racial/ethnic disparities in health care utilization and school attendance among children with AD.
- Results of a prospective study evaluating the efficacy and systemic absorption of topical timolol for infantile hemangioma.
- Results of a randomized, placebo-controlled, double-blind study of oral tranexamic acid for treating moderate to severe melasma.
- The efficacy of adjuvant treatment with the long-pulsed 1064nm Nd:YAG Laser for toe onychomycosis.
- Risk factors associated with a recent change in the size, shape or color of moles among participants in the AAD SPOTme® Program (2009-2010).
- A survey-based study on the management of longitudinal melanonychia among attending and resident dermatologists.
Dermatology News will be on site later this week at the annual meeting of the American Academy of Dermatology in Orlando. Look for the latest news in medical, surgical, and aesthetic dermatology starting Friday, March 3. The late-breaker sessions are on Saturday and Sunday March 4 and 5.
The late-breaking research session on clinical trials on March 4 will highlight:
- 16-week results from two phase III studies of certolizumab for chronic plaque psoriasis.
- Phase III results on the long-term management of moderate to severe atopic dermatitis (AD) with dupilumab plus topical corticosteroids.
Additional late breakers focus on pediatric, procedural, and pathology studies:
- Data from the Pediatric Eczema Elective Registry, on racial/ethnic disparities in health care utilization and school attendance among children with AD.
- Results of a prospective study evaluating the efficacy and systemic absorption of topical timolol for infantile hemangioma.
- Results of a randomized, placebo-controlled, double-blind study of oral tranexamic acid for treating moderate to severe melasma.
- The efficacy of adjuvant treatment with the long-pulsed 1064nm Nd:YAG Laser for toe onychomycosis.
- Risk factors associated with a recent change in the size, shape or color of moles among participants in the AAD SPOTme® Program (2009-2010).
- A survey-based study on the management of longitudinal melanonychia among attending and resident dermatologists.
Dermatology News will be on site later this week at the annual meeting of the American Academy of Dermatology in Orlando. Look for the latest news in medical, surgical, and aesthetic dermatology starting Friday, March 3. The late-breaker sessions are on Saturday and Sunday March 4 and 5.
The late-breaking research session on clinical trials on March 4 will highlight:
- 16-week results from two phase III studies of certolizumab for chronic plaque psoriasis.
- Phase III results on the long-term management of moderate to severe atopic dermatitis (AD) with dupilumab plus topical corticosteroids.
Additional late breakers focus on pediatric, procedural, and pathology studies:
- Data from the Pediatric Eczema Elective Registry, on racial/ethnic disparities in health care utilization and school attendance among children with AD.
- Results of a prospective study evaluating the efficacy and systemic absorption of topical timolol for infantile hemangioma.
- Results of a randomized, placebo-controlled, double-blind study of oral tranexamic acid for treating moderate to severe melasma.
- The efficacy of adjuvant treatment with the long-pulsed 1064nm Nd:YAG Laser for toe onychomycosis.
- Risk factors associated with a recent change in the size, shape or color of moles among participants in the AAD SPOTme® Program (2009-2010).
- A survey-based study on the management of longitudinal melanonychia among attending and resident dermatologists.
AAD 2017: Top tips to managing your time at the annual meeting
Dermatology News editorial advisor Adam Friedman, MD, provided the following recommendations for navigating the upcoming American Academy of Dermatology annual meeting, at the Orange County Convention Center in Orlando, March 3-6:
Don’t spread yourself too thin
Who’s ready to be overwhelmed by the almost 500 session options at the annual AAD meeting? It’s that time again, so here is my advice on how to stay on track during this circus. First, download the AAD mobile app. This makes life a lot easier when trying to find your room or search for an ongoing session. The paper program is so 2008. The AAD has created various tracks to enable dermatologists of varying stages in career or clinical foci to easily go after their interests. Follow those bread crumbs.
My general advice when deciding what to attend at this meeting is pick two or three specific topics or skills you want to expand upon or develop and go after sessions pertaining to them. Don’t spread yourself too thin as learning a little about a lot won’t strengthen your knowledge base.
Residents: Look at practice-based sessions
For residents, there are multiple professional development and board prep courses available. At this stage in your career, it’s important to acquire practical tips on topics from contract negotiation to speaker training as many of these skills are beyond the scope of residency training. Whether you are preparing for the in-training exam or the boards, programs such as “Boards Blitz” (S049) and “Conquer the Boards: An Experiential Review” (C006) will help you along the way. If you want practical career-building advice, hit up “Boards and Beyond” (F116) and “Careers in Academic Dermatology” (D004).
Get scorched in Orlando
Interested in what’s hot right now in dermatology? Look no further. There are multiple “Hot Topics” sessions scheduled throughout the meeting, with the main event on Saturday from 1 p.m. to 4 p.m. (S024) led by none other than AAD presidential candidate Kenneth J. Tomecki, MD. Other Hot Topic sessions include: “Hot Topics in Acute and Inpatient Pediatric Dermatology” (F007), “Hot Topics in Translational Dermatology” (U065), and “FDA Hot Topics: The Evolving Regulatory Landscape” (F005).
What about playing catch up on this year’s hottest publications? Check out “New Medical Dermatology Literature that Changed my Approach to Practice” (U025) with legendary academician Warren Heymann, MD. Not to be missed!
Dr. Friedman is director of the residency program and director of translational research in the department of dermatology, George Washington University, Washington.
Dermatology News editorial advisor Adam Friedman, MD, provided the following recommendations for navigating the upcoming American Academy of Dermatology annual meeting, at the Orange County Convention Center in Orlando, March 3-6:
Don’t spread yourself too thin
Who’s ready to be overwhelmed by the almost 500 session options at the annual AAD meeting? It’s that time again, so here is my advice on how to stay on track during this circus. First, download the AAD mobile app. This makes life a lot easier when trying to find your room or search for an ongoing session. The paper program is so 2008. The AAD has created various tracks to enable dermatologists of varying stages in career or clinical foci to easily go after their interests. Follow those bread crumbs.
My general advice when deciding what to attend at this meeting is pick two or three specific topics or skills you want to expand upon or develop and go after sessions pertaining to them. Don’t spread yourself too thin as learning a little about a lot won’t strengthen your knowledge base.
Residents: Look at practice-based sessions
For residents, there are multiple professional development and board prep courses available. At this stage in your career, it’s important to acquire practical tips on topics from contract negotiation to speaker training as many of these skills are beyond the scope of residency training. Whether you are preparing for the in-training exam or the boards, programs such as “Boards Blitz” (S049) and “Conquer the Boards: An Experiential Review” (C006) will help you along the way. If you want practical career-building advice, hit up “Boards and Beyond” (F116) and “Careers in Academic Dermatology” (D004).
Get scorched in Orlando
Interested in what’s hot right now in dermatology? Look no further. There are multiple “Hot Topics” sessions scheduled throughout the meeting, with the main event on Saturday from 1 p.m. to 4 p.m. (S024) led by none other than AAD presidential candidate Kenneth J. Tomecki, MD. Other Hot Topic sessions include: “Hot Topics in Acute and Inpatient Pediatric Dermatology” (F007), “Hot Topics in Translational Dermatology” (U065), and “FDA Hot Topics: The Evolving Regulatory Landscape” (F005).
What about playing catch up on this year’s hottest publications? Check out “New Medical Dermatology Literature that Changed my Approach to Practice” (U025) with legendary academician Warren Heymann, MD. Not to be missed!
Dr. Friedman is director of the residency program and director of translational research in the department of dermatology, George Washington University, Washington.
Dermatology News editorial advisor Adam Friedman, MD, provided the following recommendations for navigating the upcoming American Academy of Dermatology annual meeting, at the Orange County Convention Center in Orlando, March 3-6:
Don’t spread yourself too thin
Who’s ready to be overwhelmed by the almost 500 session options at the annual AAD meeting? It’s that time again, so here is my advice on how to stay on track during this circus. First, download the AAD mobile app. This makes life a lot easier when trying to find your room or search for an ongoing session. The paper program is so 2008. The AAD has created various tracks to enable dermatologists of varying stages in career or clinical foci to easily go after their interests. Follow those bread crumbs.
My general advice when deciding what to attend at this meeting is pick two or three specific topics or skills you want to expand upon or develop and go after sessions pertaining to them. Don’t spread yourself too thin as learning a little about a lot won’t strengthen your knowledge base.
Residents: Look at practice-based sessions
For residents, there are multiple professional development and board prep courses available. At this stage in your career, it’s important to acquire practical tips on topics from contract negotiation to speaker training as many of these skills are beyond the scope of residency training. Whether you are preparing for the in-training exam or the boards, programs such as “Boards Blitz” (S049) and “Conquer the Boards: An Experiential Review” (C006) will help you along the way. If you want practical career-building advice, hit up “Boards and Beyond” (F116) and “Careers in Academic Dermatology” (D004).
Get scorched in Orlando
Interested in what’s hot right now in dermatology? Look no further. There are multiple “Hot Topics” sessions scheduled throughout the meeting, with the main event on Saturday from 1 p.m. to 4 p.m. (S024) led by none other than AAD presidential candidate Kenneth J. Tomecki, MD. Other Hot Topic sessions include: “Hot Topics in Acute and Inpatient Pediatric Dermatology” (F007), “Hot Topics in Translational Dermatology” (U065), and “FDA Hot Topics: The Evolving Regulatory Landscape” (F005).
What about playing catch up on this year’s hottest publications? Check out “New Medical Dermatology Literature that Changed my Approach to Practice” (U025) with legendary academician Warren Heymann, MD. Not to be missed!
Dr. Friedman is director of the residency program and director of translational research in the department of dermatology, George Washington University, Washington.