User login
Nondirected testing for inpatients with severe liver injury
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
© 2017 Society of Hospital Medicine
Judge blocks Texas attempt to defund Planned Parenthood
A federal judge has blocked Texas from withholding Medicaid funds from Planned Parenthood centers in the state, ruling that state officials had no cause to terminate funding for the providers.
In his Feb. 21 decision, Judge Sam Sparks of the U.S. District Court for the Western District of Texas temporarily barred Texas from taking funds from Planned Parenthood while the case proceeds. Restricting the money would deprive Medicaid patients of their right to obtain health care from their chosen providers and would potentially disrupt care for 12,500 Medicaid patients, Judge Sparks wrote.
Texas Attorney General Ken Paxton (R) pledged to appeal the ruling.
“[The] decision is disappointing and flies in the face of basic human decency,” Mr. Paxton said in a statement. “The raw, unedited footage from undercover videos exposed a brazen willingness by Planned Parenthood officials to traffic in fetal body parts, as well as manipulate the timing and method of an abortion ... No taxpayer in Texas should have to subsidize this repugnant and illegal conduct. We should never lose sight of the fact that, as long as abortion is legal in the United States, the potential for these types of horrors will continue.”
Planned Parenthood representatives did not return messages seeking comment on the ruling.
The Texas Health and Human Services Commission (HHSC) terminated funding for its Planned Parenthood providers in the state following a 2015 video by two anti-abortion advocates that purported to show representatives of Planned Parenthood Gulf Coast (Houston) contracting to sell aborted human fetal tissue and body parts. State authorities investigated the facility and found no wrongdoing, but a grand jury indicted the two anti-abortion activists for using fake names and identities.
Separate investigations by the Texas Attorney General’s Office, the Texas Department of State Health Services, and HHSC also found no wrongdoing, according to court documents. In December 2016 however, HHSC sent termination letters to five Texas Planned Parenthood centers, stating that the facilities were not qualified to provide medical services in a “competent, safe, legal and ethical manner” under state and federal law, according to court records. The providers and several patients sued.
In his ruling, Judge Sparks said he found no evidence indicating that an actual program violation occurred warranting termination of funding for the providers.
“After reviewing the evidence currently in the record, the court finds the Inspector General, and thus HHSC, likely acted to disenroll qualified health care providers from Medicaid without cause,” Judge Sparks wrote. “The individual plaintiffs have met their burden to establish a likelihood of success on the merits. The Inspector General did not have prima facie of evidence, or even a scintilla of evidence, to conclude the basis of termination set forth in the final notice merited finding [the plaintiffs] were not qualified.”
Similar efforts were overturned in Virgina after Governor Terry McAuliffe (D) vetoed a bill that sought to restrict state and federal funding from Planned Parenthood providers in the state. In a statement, Gov. McAuliffe said the bill would have harmed Virginians who rely on health care services and programs provided by Planned Parenthood health centers by denying them access to affordable care.
Similar legislation to defund Planned Parenthood providers was introduced by Michigan lawmakers in February.
In addition, efforts are underway at the federal level. On Feb. 16, the House passed H.J.Res.43, which would allow states to withhold Title X family planning funds from providers that offer abortion services, overturning a rule put in place at the end of the Obama administration. The resolution passed 230-188, largely along party lines. It would strike down the Obama-era rule via the 1996 Congressional Review Act, which allows Congress to overturn new regulations within 60 days of their passage. H.J.Res.43 is currently before the Senate.
The American Congress of Obstetricians and Gynecologists expressed disappointment with the House resolution.
“The resolution allows states to discriminate against women’s health care providers for reasons unrelated to qualifications or best practices,” according to an ACOG statement. “Under this resolution, states could disqualify health centers, including Planned Parenthood, from providing Title X contraceptive and preventive care to over 4 million individuals. The Title X program is the only federal grant program exclusively dedicated to providing low-income patients with access to effective family planning and related preventive health services, including contraceptive care. Contraceptive access is essential to helping women achieve greater educational, financial, and professional success and stability. It’s critical to the economic success of this population.”
House Speaker Paul Ryan (R-Wis.) has promised that the bill to repeal the Affordable Care Act will include a measure stripping funds from Planned Parenthood.
[email protected]
On Twitter @legal_med
A federal judge has blocked Texas from withholding Medicaid funds from Planned Parenthood centers in the state, ruling that state officials had no cause to terminate funding for the providers.
In his Feb. 21 decision, Judge Sam Sparks of the U.S. District Court for the Western District of Texas temporarily barred Texas from taking funds from Planned Parenthood while the case proceeds. Restricting the money would deprive Medicaid patients of their right to obtain health care from their chosen providers and would potentially disrupt care for 12,500 Medicaid patients, Judge Sparks wrote.
Texas Attorney General Ken Paxton (R) pledged to appeal the ruling.
“[The] decision is disappointing and flies in the face of basic human decency,” Mr. Paxton said in a statement. “The raw, unedited footage from undercover videos exposed a brazen willingness by Planned Parenthood officials to traffic in fetal body parts, as well as manipulate the timing and method of an abortion ... No taxpayer in Texas should have to subsidize this repugnant and illegal conduct. We should never lose sight of the fact that, as long as abortion is legal in the United States, the potential for these types of horrors will continue.”
Planned Parenthood representatives did not return messages seeking comment on the ruling.
The Texas Health and Human Services Commission (HHSC) terminated funding for its Planned Parenthood providers in the state following a 2015 video by two anti-abortion advocates that purported to show representatives of Planned Parenthood Gulf Coast (Houston) contracting to sell aborted human fetal tissue and body parts. State authorities investigated the facility and found no wrongdoing, but a grand jury indicted the two anti-abortion activists for using fake names and identities.
Separate investigations by the Texas Attorney General’s Office, the Texas Department of State Health Services, and HHSC also found no wrongdoing, according to court documents. In December 2016 however, HHSC sent termination letters to five Texas Planned Parenthood centers, stating that the facilities were not qualified to provide medical services in a “competent, safe, legal and ethical manner” under state and federal law, according to court records. The providers and several patients sued.
In his ruling, Judge Sparks said he found no evidence indicating that an actual program violation occurred warranting termination of funding for the providers.
“After reviewing the evidence currently in the record, the court finds the Inspector General, and thus HHSC, likely acted to disenroll qualified health care providers from Medicaid without cause,” Judge Sparks wrote. “The individual plaintiffs have met their burden to establish a likelihood of success on the merits. The Inspector General did not have prima facie of evidence, or even a scintilla of evidence, to conclude the basis of termination set forth in the final notice merited finding [the plaintiffs] were not qualified.”
Similar efforts were overturned in Virgina after Governor Terry McAuliffe (D) vetoed a bill that sought to restrict state and federal funding from Planned Parenthood providers in the state. In a statement, Gov. McAuliffe said the bill would have harmed Virginians who rely on health care services and programs provided by Planned Parenthood health centers by denying them access to affordable care.
Similar legislation to defund Planned Parenthood providers was introduced by Michigan lawmakers in February.
In addition, efforts are underway at the federal level. On Feb. 16, the House passed H.J.Res.43, which would allow states to withhold Title X family planning funds from providers that offer abortion services, overturning a rule put in place at the end of the Obama administration. The resolution passed 230-188, largely along party lines. It would strike down the Obama-era rule via the 1996 Congressional Review Act, which allows Congress to overturn new regulations within 60 days of their passage. H.J.Res.43 is currently before the Senate.
The American Congress of Obstetricians and Gynecologists expressed disappointment with the House resolution.
“The resolution allows states to discriminate against women’s health care providers for reasons unrelated to qualifications or best practices,” according to an ACOG statement. “Under this resolution, states could disqualify health centers, including Planned Parenthood, from providing Title X contraceptive and preventive care to over 4 million individuals. The Title X program is the only federal grant program exclusively dedicated to providing low-income patients with access to effective family planning and related preventive health services, including contraceptive care. Contraceptive access is essential to helping women achieve greater educational, financial, and professional success and stability. It’s critical to the economic success of this population.”
House Speaker Paul Ryan (R-Wis.) has promised that the bill to repeal the Affordable Care Act will include a measure stripping funds from Planned Parenthood.
[email protected]
On Twitter @legal_med
A federal judge has blocked Texas from withholding Medicaid funds from Planned Parenthood centers in the state, ruling that state officials had no cause to terminate funding for the providers.
In his Feb. 21 decision, Judge Sam Sparks of the U.S. District Court for the Western District of Texas temporarily barred Texas from taking funds from Planned Parenthood while the case proceeds. Restricting the money would deprive Medicaid patients of their right to obtain health care from their chosen providers and would potentially disrupt care for 12,500 Medicaid patients, Judge Sparks wrote.
Texas Attorney General Ken Paxton (R) pledged to appeal the ruling.
“[The] decision is disappointing and flies in the face of basic human decency,” Mr. Paxton said in a statement. “The raw, unedited footage from undercover videos exposed a brazen willingness by Planned Parenthood officials to traffic in fetal body parts, as well as manipulate the timing and method of an abortion ... No taxpayer in Texas should have to subsidize this repugnant and illegal conduct. We should never lose sight of the fact that, as long as abortion is legal in the United States, the potential for these types of horrors will continue.”
Planned Parenthood representatives did not return messages seeking comment on the ruling.
The Texas Health and Human Services Commission (HHSC) terminated funding for its Planned Parenthood providers in the state following a 2015 video by two anti-abortion advocates that purported to show representatives of Planned Parenthood Gulf Coast (Houston) contracting to sell aborted human fetal tissue and body parts. State authorities investigated the facility and found no wrongdoing, but a grand jury indicted the two anti-abortion activists for using fake names and identities.
Separate investigations by the Texas Attorney General’s Office, the Texas Department of State Health Services, and HHSC also found no wrongdoing, according to court documents. In December 2016 however, HHSC sent termination letters to five Texas Planned Parenthood centers, stating that the facilities were not qualified to provide medical services in a “competent, safe, legal and ethical manner” under state and federal law, according to court records. The providers and several patients sued.
In his ruling, Judge Sparks said he found no evidence indicating that an actual program violation occurred warranting termination of funding for the providers.
“After reviewing the evidence currently in the record, the court finds the Inspector General, and thus HHSC, likely acted to disenroll qualified health care providers from Medicaid without cause,” Judge Sparks wrote. “The individual plaintiffs have met their burden to establish a likelihood of success on the merits. The Inspector General did not have prima facie of evidence, or even a scintilla of evidence, to conclude the basis of termination set forth in the final notice merited finding [the plaintiffs] were not qualified.”
Similar efforts were overturned in Virgina after Governor Terry McAuliffe (D) vetoed a bill that sought to restrict state and federal funding from Planned Parenthood providers in the state. In a statement, Gov. McAuliffe said the bill would have harmed Virginians who rely on health care services and programs provided by Planned Parenthood health centers by denying them access to affordable care.
Similar legislation to defund Planned Parenthood providers was introduced by Michigan lawmakers in February.
In addition, efforts are underway at the federal level. On Feb. 16, the House passed H.J.Res.43, which would allow states to withhold Title X family planning funds from providers that offer abortion services, overturning a rule put in place at the end of the Obama administration. The resolution passed 230-188, largely along party lines. It would strike down the Obama-era rule via the 1996 Congressional Review Act, which allows Congress to overturn new regulations within 60 days of their passage. H.J.Res.43 is currently before the Senate.
The American Congress of Obstetricians and Gynecologists expressed disappointment with the House resolution.
“The resolution allows states to discriminate against women’s health care providers for reasons unrelated to qualifications or best practices,” according to an ACOG statement. “Under this resolution, states could disqualify health centers, including Planned Parenthood, from providing Title X contraceptive and preventive care to over 4 million individuals. The Title X program is the only federal grant program exclusively dedicated to providing low-income patients with access to effective family planning and related preventive health services, including contraceptive care. Contraceptive access is essential to helping women achieve greater educational, financial, and professional success and stability. It’s critical to the economic success of this population.”
House Speaker Paul Ryan (R-Wis.) has promised that the bill to repeal the Affordable Care Act will include a measure stripping funds from Planned Parenthood.
[email protected]
On Twitter @legal_med
Forging ahead
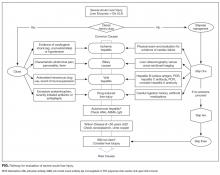
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
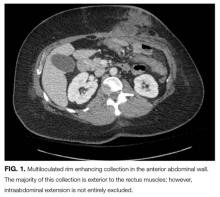
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
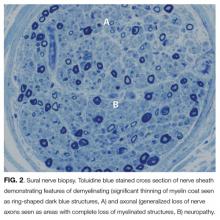
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
© 2017 Society of Hospital Medicine
Health information exchange in US hospitals: The current landscape and a path to improved information sharing
The US healthcare system is highly fragmented, with patients typically receiving treatment from multiple providers during an episode of care and from many more providers over their lifetime.1,2 As patients move between care delivery settings, whether and how their information follows them is determined by a haphazard and error-prone patchwork of telephone, fax, and electronic communication channels.3 The existence of more robust electronic communication channels is often dictated by factors such as which providers share the same electronic health record (EHR) vendor rather than which providers share the highest volume of patients. As a result, providers often make clinical decisions with incomplete information, increasing the chances of misdiagnosis, unsafe or suboptimal treatment, and duplicative utilization.
Providers across the continuum of care encounter challenges to optimal clinical decision-making as a result of incomplete information. These are particularly problematic among clinicians in hospitals and emergency departments (EDs). Clinical decision-making in EDs often involves urgent and critical conditions in which decisions are made under pressure. Time constraints limit provider ability to find key clinical information to accurately diagnose and safely treat patients.4-6 Even for planned inpatient care, providers are often unfamiliar with patients, and they make safer decisions when they have full access to information from outside providers.7,8
Transitions of care between hospitals and primary care settings are also fraught with gaps in information sharing. Clinical decisions made in primary care can set patients on treatment trajectories that are greatly affected by the quality of information available to the care team at the time of initial diagnosis as well as in their subsequent treatment. Primary care physicians are not universally notified when their patients are hospitalized and may not have access to detailed information about the hospitalization, which can impair their ability to provide high quality care.9-11
Widespread and effective electronic health information exchange (HIE) holds the potential to address these challenges.3 With robust, interconnected electronic systems, key pieces of a patient’s health record can be electronically accessed and reconciled during planned and unplanned care transitions. The concept of HIE is simple—make all relevant patient data available to the clinical care team at the point of care, regardless of where that information was generated. The estimated value of nationwide interoperable EHR adoption suggests large savings from the more efficient, less duplicative, and higher quality care that likely results.12,13
There has been substantial funding and activity at federal, state, and local levels to promote the development of HIE in the US. The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act has the specific goal of accelerating adoption and use of certified EHR technology coupled with the ability to exchange clinical information to support patient care.14 The HITECH programs supported specific types of HIE that were believed to be particularly critical to improving patient care and included them in the federally-defined criteria for Meaningful Use (MU) of EHRs (ie, providers receive financial incentives for achieving specific objectives). The MU criteria evolve, moving from data capture in stage 1 to improved patient outcomes in stage 3.15 The HIE criteria focus on sending and receiving summary-of-care records during care transitions.
Despite the clear benefits of HIE and substantial support stated in policy initiatives, the spread of national HIE has been slow. Today, HIE in the US is highly heterogeneous: as a result of multiple federal-, state-, community-, enterprise- and EHR vendor-level efforts, only some provider organizations are able to engage in HIE with the other provider organizations with which they routinely share patients. In this review, we offer a framework and a corresponding set of definitions to understand the current state of HIE in the US. We describe key challenges to HIE progress and offer insights into the likely path to ensure that clinicians have routine, electronic access to patient information.
FOUR KEY DIMENSIONS OF HEALTH INFORMATION EXCHANGE
While the concept of HIE is simple—electronic access to clinical information across healthcare settings—the operationalization of HIE occurs in many different ways.16 While the terms “health information exchange” and “interoperability” are often used interchangeably, they can have different meanings. In this section, we describe 4 important dimensions that serve as a framework for understanding any given effort to enable HIE (Table).
(1) What Is Exchanged? Types of Information
The term “health information exchange” is ambiguous with respect to the type(s) of information that are accessible. Health information exchange may refer to the process of 2 providers electronically sharing a wide range of data, from a single type of information (eg, lab test results), summary of care records, to complete patient records.17 Part of this ambiguity may stem from uncertainty about the scope of information that should be shared, and how this varies based on the type of clinical encounter. For example, critical types of information in the ED setting may differ from those relevant to a primary care team after a referral. While the ability to access only particular types of information will not address all information gaps, providing access to complete patient records may result in information overload that inhibits the ability to find the subset of information relevant in a given clinical encounter.
(2) Who is Exchanging? Relationship Between Provider Organizations
The types of information accessed electronically are effectively agnostic to the relationship between the provider organizations that are sharing information. Traditionally, HIE has been considered as information that is electronically shared among 2 or more unaffiliated organizations. However, there is increasing recognition that some providers may not have electronic access to all information about their patients that exists within their organization, often after a merger or acquisition between 2 providers with different EHR systems.18,19 In these cases, a primary care team in a large integrated delivery system may have as many information gaps as a primary care team in a small, independent practice. Fulfilling clinical information needs may require both intra- and interorganizational HIE, which complicates the design of HIE processes and how the care team approaches incorporating information from both types of organizations into their decision-making. It is also important to recognize that some provider organizations, particularly small, rural practices, may not have the information technology and connectivity infrastructure required to engage in HIE.
(3) How Is Information Exchanged? Types of Electronic Access: Push vs Pull Exchange
To minimize information gaps, electronic access to information from external settings needs to offer both “push” and “pull” options. Push exchange, which can direct information electronically to a targeted recipient, works in scenarios in which there is a known information gap and known information source. The classic use for push exchange is care coordination, such as primary care physician-specialist referrals or hospital-primary care physician transitions postdischarge. Pull exchange accommodates scenarios in which there is a known information gap but the source(s) of information are unknown; it requires that clinical care teams search for and locate the clinical information that exists about the patient in external settings. Here, the classic use is emergency care in which the care team may encounter a new patient and want to retrieve records.
Widespread use of provider portals that offer view-only access into EHRs and other clinical data repositories maintained by external organizations complicate the picture. Portals are commonly used by hospitals to enable community providers to view information from a hospitalization.21 While this does not fall under the commonly held notion of HIE because no exchange occurs, portals support a pull approach to accessing information electronically among care settings that treat the same patients but use different EHRs.
Regardless of whether information is pushed or pulled, this may happen with varying degrees of human effort. This distinction gives rise to the difference between HIE and interoperability. Health information exchange reflects the ability of EHRs to exchange information, while interoperability additionally requires that EHRs be able to use exchanged information. From an operational perspective, the key distinction between HIE and interoperability is the extent of human involvement. Health information exchange requires that a human read and decide how to enter information from external settings (eg, a chart in PDF format sent between 2 EHRs), while interoperability enables the EHR that receives the information to understand the content and automatically triage or reconcile information, such as a medication list, without any human action.21 Health information exchange, therefore, relies on the diligence of the receiving clinician, while interoperability does not.
(4) What Governance Entity Defines the “Rules” of Exchange?
When more than 1 provider organization shares patient-identified data, a governance entity must specify the framework that governs the exchange. While the specifics of HIE governance vary, there are 3 predominant types of HIE networks, based on the type of organization that governs exchange: enterprise HIE networks, EHR vendor HIE networks or community HIE networks.
Enterprise HIE networks exist when 1 or more provider organizations electronically share clinical information to support patient care with some restriction, beyond geography, that dictates which organizations are involved. Typically, restrictions are driven by strategic, proprietary interests.22,23 Although broad-based information access across settings would be in the best interest of the patient, provider organizations are sensitive to the competitive implications of sharing data and may pursue such sharing in a strategic way.24 A common scenario is when hospitals choose to strategically affiliate with select ambulatory providers and exclusively exchange information with them. This should facilitate better care coordination for patients shared by the hospital and those providers but can also benefit the hospital by increasing the referrals from those providers. While there is little direct evidence quantifying the extent to which this type of strategic sharing takes place, there have been anecdotal reports as well as indirect findings that for-profit hospitals in competitive markets are less likely to share patient data.19,25
EHR vendor HIE networks exist when exchange occurs within a community of provider organizations that use an EHR from the same vendor. A subset of EHR vendors have made this capability available; EPIC’s CareEverywhere solution27 is the best-known example. Providers with an EPIC EHR are able to query for and retrieve summary of care records and other documents from any provider organization with EPIC that has activated this functionality. There are also multivendor efforts, such as CommonWell27 and the Sequoia Project’s Carequality collaborative,28 which are initiatives that seek to provide a common interoperability framework across a diverse set of stakeholders, including provider organizations with different EHR systems, in a similar fashion to HIE modules like CareEverywhere. To date, growth in these cross-vendor collaborations has been slow, and they have limited participation. While HIE networks that involve EHR vendors are likely to grow, it is difficult to predict how quickly because they are still in an early phase of development, and face nontechnical barriers such as patient consent policies that vary between providers and across states.
Community HIE networks—also referred to as health information organizations (HIOs) or regional health information organizations (RHIOs)—exist when provider organizations in a community, frequently state-level organizations that were funded through HITECH grants,14 set up the technical infrastructure and governance approach to engage in HIE to improve patient care. In contrast to enterprise or vendor HIE networks that have pursued HIE in ways that appear strategically beneficial, the only restriction on participation in community and state HIE networks is usually geography because they view information exchange as a public good. Seventyone percent of hospital service areas (HSAs) are covered by at least 1 of the 106 operational HIOs, with 309,793 clinicians (licensed prescribers) participating in those exchange networks. Even with early infusions of public and other grant-funding, community HIE networks have experienced significant challenges to sustained operation, and many have ceased operating.29
Thus, for any given provider organization, available HIE networks are primarily shaped by 3 factors:
1. Geographic location, which determines the available community and state HIE networks (as well as other basic information technology and connectivity infrastructure); providers located outside the service areas covered by an operational HIE have little incentive to participate because they do not connect them to providers with whom they share patients. Providers in rural areas may simply not have the needed infrastructure to pursue HIE.
2. Type of organization to which they belong, which determines the available enterprise HIE networks; providers who are not members of large health systems may be excluded from participation in these types of networks.
3. EHR vendor, which determines whether they have access to an EHR vendor HIE network.
ONGOING CHALLENGES
Despite agreement about the substantial potential of HIE to reduce costs and increase the quality of care delivered across a broad range of providers, HIE progress has been slow. While HITECH has successfully increased EHR adoption in hospitals and ambulatory practices,30 HIE has lagged. This is largely because many complex, intertwined barriers must be addressed for HIE to be widespread.
Lack of a Defined Goal
The cost and complexity associated with the exchange of a single type of data (eg, medications) is substantially less than the cost and complexity of sharing complete patient records. There has been little industry consensus on the target goal—do we need to enable sharing of complete patient records across all providers, or will summary of care records suffice? If the latter, as is the focus of the current MU criteria, what types of information should be included in a summary of care record, and should content and/or structure vary depending on the type of care transition? While the MU criteria require the exchange of a summary of care record with defined data fields, it remains unclear whether this is the end state or whether we should continue to push towards broad-based sharing of all patient data as structured elements. Without a clear picture of the ideal end state, there has been significant heterogeneity in the development of HIE capabilities across providers and vendors, and difficulty coordinating efforts to continue to advance towards a nationwide approach. Addressing this issue also requires progress to define HIE usability, that is, how information from external organizations should be presented and integrated into clinical workflow and clinical decisions. Currently, where HIE is occurring and clinicians are receiving summary of care records, they find them long, cluttered, and difficult to locate key information.
Numerous, Complex Barriers Spanning Multiple Stakeholders
In the context of any individual HIE effort, even after the goal is defined, there are a myriad of challenges. In a recent survey of HIO efforts, many identified the following barriers as substantially impeding their development: establishing a sustainable business model, lack of funding, integration of HIE into provider workflow, limitations of current data standards, and working with governmental policy and mandates.30 What is notable about this list is that the barriers span an array of areas, including financial incentives and identifying a sustainable business model, technical barriers such as working within the limitations of data standards, and regulatory issues such as state laws that govern the requirements for patient consent to exchange personal health information. Overcoming any of these issues is challenging, but trying to tackle all of them simultaneously clearly reveals why progress has been slow. Further, resolving many of the issues involve different groups of stakeholders. For example, implementing appropriate patient consent procedures can require engaging with and harmonizing the regulations of multiple states, as well as the Health Insurance Portability and Accountability Act (HIPAA) and regulations specific to substance abuse data.
Weak or Misaligned Incentives
Among the top barriers to HIE efforts are those related to funding and lack of a sustainable business model. This reflects the fact that economic incentives in the current market have not promoted provider engagement in HIE. Traditional fee-for-service payment structures do not reward providers for avoiding duplicative care.31 Further, hospitals perceive patient data as a “key strategic asset, tying physicians and patients to their organization,”24 and are reluctant to share data with competitors. Compounding the problem is that EHR vendors have a business interest in using HIE as a lever to increase revenue. In the short-term, they can charge high fees for interfaces and other HIE-related functionality. In the long-run, vendors may try to influence provider choice of system by making it difficult to engage in cross-vendor exchange.32 Information blocking—when providers or vendors knowingly interfere with HIE33—reflects not only weak incentives, but perverse incentives. While not all providers and vendors experience perverse incentives, the combination of weak and perverse incentives suggests the need to strengthen incentives, so that both types of stakeholders are motivated to tackle the barriers to HIE development. Key to strengthening incentives are payers, who are thought to be the largest beneficiaries of HIE. Payers have been reluctant to make significant investments in HIE without a more active voice in its implementation,34 but a shift to value-based payment may increase their engagement.
THE PATH FORWARD
Despite the continued challenges to nationwide HIE, several policy and technology developments show promise. Stage 3 meaningful use criteria continue to build on previous stages in increasing HIE requirements, raising the threshold for electronic exchange and EHR integration of summary of care documentation in patient transitions. The recently released Medicare Access and CHIP Reauthorization Act (MACRA) Merit-based Incentive Payment System (MIPS) proposed rule replaces stage 3 meaningful use for Medicare-eligible providers with advancing care information (ACI), which accounts for 25% of a provider’s overall incentive reimbursement and includes multiple HIE criteria for providers to report as part of the base and performance score, and follows a very similar framework to stage 3 MU with its criteria regarding HIE.35 While the Centers for Medicare and Medicaid Services (CMS) has not publicly declared that stage 3 MU will be replaced by ACI for hospitals and Medicaid providers, it is likely it will align those programs with the newly announced Medicare incentives.
MACRA also included changes to the Office of the National Coordinator (ONC) EHR certification program in an attempt to further encourage HIE. Vendors and providers must attest that they do not engage in information blocking and will cooperate with the Office’s surveillance programs to that effect. They also must attest that, to the greatest degree possible, their EHR systems allow for bi-directional interoperability with other providers, including those with different EHR vendors, and timely access for patients to view, download, and transmit their health data. In addition, there are emerging federal efforts to pursue a more standardized approach to patient matching and harmonize consent policies across states. These types of new policy initiatives indicate a continued interest in prioritizing HIE and interoperability.21
New technologies may also help spur HIE progress. The newest policy initiatives from CMS, including stage 3 MU and MACRA, have looked to incentivize the creation of application program interfaces (APIs), a set of publicly available tools from EHR vendors to allow developers to build applications that can directly interface with, and retrieve data from, their EHRs. While most patient access to electronic health data to date has been accomplished via patient portals, open APIs would enable developers to build an array of programs for consumers to view, download, and transmit their health data.
Even more promising is the development of the newest Health Level 7 data transmission standard, Fast Healthcare Interoperability Resources (FHIR), which promises to dramatically simplify the technical aspects of interoperability. FHIR utilizes a human-readable, easy to implement modular “resources” standard that may alleviate many technical challenges that come with implementation of an HIE system, enabling cheaper and simpler interoperability.36 A consortium of EHR vendors are working together to test these standards.28 The new FHIR standards also work in conjunction with APIs to allow easier development of consumer-facing applications37 that may empower patients to take ownership of their health data.
CONCLUSION
While HIE holds great promise to reduce the cost and improve the quality of care, progress towards a nationally interoperable health system has been slow. Simply defining HIE and what types of HIE are needed in different clinical scenarios has proven challenging. The additional challenges to implementing HIE in complex technology, legal/regulatory, governance, and incentive environment are not without solutions. Continued policy interventions, private sector collaborations, and new technologies may hold the keys to realizing the vast potential of electronic HIE.
Disclosure
Nothing to report.
1. Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130-1139. PubMed
2. Finnell JT, Overhage JM, Dexter PR, Perkins SM, Lane KA, McDonald CJ. Community clinical data exchange for emergency medicine patients. Paper presented at: AMIA Annual Symposium Proceedings 2003. PubMed
3. Bodenheimer T. Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. PubMed
4. Franczak MJ, Klein M, Raslau F, Bergholte J, Mark LP, Ulmer JL. In emergency departments, radiologists’ access to EHRs may influence interpretations and medical management. Health Aff (Millwood). 2014;33(5):800-806. PubMed
5. Shapiro JS, Kannry J, Kushniruk AW, Kuperman G; New York Clinical Information Exchange (NYCLIX) Clinical Advisory Subcommittee. Emergency physicians’ perceptions of health information exchange. J Am Med Inform Assoc. 2007;14(6):700-705. PubMed
6. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426-432. PubMed
7. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med.. 2004;79(2):186-194. PubMed
8. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(suppl 6):S40-S45. PubMed
9. Smith PC, Araya-Guerra R, Bublitz C, et al. MIssing clinical information during primary care visits. JAMA. 2005;293(5):565-571. PubMed
10. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. PubMed
11. van Walraven C, Taljaard M, Bell CM, et al. A prospective cohort study found that provider and information continuity was low after patient discharge from hospital. J Clin Epidemiol. 2010;63(9):1000-1010. PubMed
12. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood). 2005:(suppl)W5-10-W5-18. PubMed
13. Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep). 2006;132:1-71. PubMed
14. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382-385. PubMed
15. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501-504. PubMed
16. Kuperman G, McGowan J. Potential unintended consequences of health information exchange. J Gen Intern Med. 2013;28(12):1663-1666. PubMed
17. Mathematica Policy Research and Harvard School of Public Health. DesRoches CM, Painter MW, Jha AK, eds. Health Information Technology in the United States, 2015: Transition to a Post-HITECH World (Executive Summary). September 18, 2015. Princeton, NJ: Robert Wood Johnson Foundation; 2015.
18. O’Malley AS, Anglin G, Bond AM, Cunningham PJ, Stark LB, Yee T. Greenville & Spartanburg: Surging Hospital Employment of Physicians Poses Opportunities and Challenges. Washington, DC: Center for Studying Health System Change (HSC); February 2011. 6.
19. Katz A, Bond AM, Carrier E, Docteur E, Quach CW, Yee T. Cleveland Hospital Systems Expand Despite Weak Economy. Washington, DC: Center for Studying Health System Change (HSC); September 2010. 2.
20. Grossman JM, Bodenheimer TS, McKenzie K. Hospital-physician portals: the role of competition in driving clinical data exchange. Health Aff (Millwood). 2006;25(6):1629-1636. PubMed
21. De Salvo KB, Galvez E. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap - Version 1.0. In: Office of the National Coordinator for Health Information Technology. ed 2015. https://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/interoperability-electronic-health-and-medical-records/connecting-health-care-nation-shared-nationwide-interoperability-roadmap-version-10/. Accessed September 3, 2016.
22. Adler-Milstein J, DesRoches C, Jha AK. Health information exchange among US hospitals. Am J Manag Care. 2011;17(11):761-768. PubMed
23. Vest JR. More than just a question of technology: factors related to hospitals’ adoption and implementation of health information exchange. Int J Med Inform. 2010;79(12):797-806. PubMed
24. Grossman JM, Kushner KL, November EA. Creating sustainable local health information exchanges: can barriers to stakeholder participation be overcome? Res Brief. 2008;2:1-12. PubMed
25. Grossman JM, Cohen G. Despite regulatory changes, hospitals cautious in helping physicians purchase electronic medical records. Issue Brief Cent Stud Health Syst Change 2008;123:1-4. PubMed
26. Kaelber DC, Waheed R, Einstadter D, Love TE, Cebul RD. Use and perceived value of health information exchange: one public healthcare system’s experience. Am J Manag Care. 2013;19(10 spec no):SP337-SP343. PubMed
27. Commonwell Health Alliance. http://www.commonwellalliance.org/, 2016. Accessed September 3, 2016.
28. Carequality. http://sequoiaproject.org/carequality/, 2016. Accessed September 3, 2016.
29. Adler-Milstein J, Lin SC, Jha AK. The number of health information exchange efforts is declining, leaving the viability of broad clinical data exchange uncertain. Health Aff (Millwood). 2016;35(7):1278-1285. PubMed
30. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015:34(12):2174-2180. PubMed
31. Health IT Policy Committee Report to Congress: Challenges and Barriers to Interoperability. 2015. https://www.healthit.gov/facas/health-it-policy-committee/health-it-policy-committee-recommendations-national-coordinator-health-it. Accessed September 3, 2016.
32. Everson J, Adler-Milstein J. Engagement in hospital health information exchange is associated with vendor marketplace dominance. Health Aff (MIllwood). 2016;35(7):1286-1293. PubMed
33. Downing K, Mason J. ONC targets information blocking. J AHIMA. 2015;86(7):36-38. PubMed
34. Cross DA, Lin SC, Adler-Milstein J. Assessing payer perspectives on health information exchange. J Am Med Inform Assoc. 2016;23(2):297-303. PubMed
35. Centers for Medicare & Medicaid Services. MACRA: MIPS and APMs. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Accessed September 3, 2016.
36. Raths D. Trend: standards development. Catching FHIR. A new HL7 draft standard may boost web services development in healthcare. Healthc Inform. 2014;31(2):13,16. PubMed
37. Alterovitz G, Warner J, Zhang P, et al. SMART on FHIR genomics: facilitating
The US healthcare system is highly fragmented, with patients typically receiving treatment from multiple providers during an episode of care and from many more providers over their lifetime.1,2 As patients move between care delivery settings, whether and how their information follows them is determined by a haphazard and error-prone patchwork of telephone, fax, and electronic communication channels.3 The existence of more robust electronic communication channels is often dictated by factors such as which providers share the same electronic health record (EHR) vendor rather than which providers share the highest volume of patients. As a result, providers often make clinical decisions with incomplete information, increasing the chances of misdiagnosis, unsafe or suboptimal treatment, and duplicative utilization.
Providers across the continuum of care encounter challenges to optimal clinical decision-making as a result of incomplete information. These are particularly problematic among clinicians in hospitals and emergency departments (EDs). Clinical decision-making in EDs often involves urgent and critical conditions in which decisions are made under pressure. Time constraints limit provider ability to find key clinical information to accurately diagnose and safely treat patients.4-6 Even for planned inpatient care, providers are often unfamiliar with patients, and they make safer decisions when they have full access to information from outside providers.7,8
Transitions of care between hospitals and primary care settings are also fraught with gaps in information sharing. Clinical decisions made in primary care can set patients on treatment trajectories that are greatly affected by the quality of information available to the care team at the time of initial diagnosis as well as in their subsequent treatment. Primary care physicians are not universally notified when their patients are hospitalized and may not have access to detailed information about the hospitalization, which can impair their ability to provide high quality care.9-11
Widespread and effective electronic health information exchange (HIE) holds the potential to address these challenges.3 With robust, interconnected electronic systems, key pieces of a patient’s health record can be electronically accessed and reconciled during planned and unplanned care transitions. The concept of HIE is simple—make all relevant patient data available to the clinical care team at the point of care, regardless of where that information was generated. The estimated value of nationwide interoperable EHR adoption suggests large savings from the more efficient, less duplicative, and higher quality care that likely results.12,13
There has been substantial funding and activity at federal, state, and local levels to promote the development of HIE in the US. The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act has the specific goal of accelerating adoption and use of certified EHR technology coupled with the ability to exchange clinical information to support patient care.14 The HITECH programs supported specific types of HIE that were believed to be particularly critical to improving patient care and included them in the federally-defined criteria for Meaningful Use (MU) of EHRs (ie, providers receive financial incentives for achieving specific objectives). The MU criteria evolve, moving from data capture in stage 1 to improved patient outcomes in stage 3.15 The HIE criteria focus on sending and receiving summary-of-care records during care transitions.
Despite the clear benefits of HIE and substantial support stated in policy initiatives, the spread of national HIE has been slow. Today, HIE in the US is highly heterogeneous: as a result of multiple federal-, state-, community-, enterprise- and EHR vendor-level efforts, only some provider organizations are able to engage in HIE with the other provider organizations with which they routinely share patients. In this review, we offer a framework and a corresponding set of definitions to understand the current state of HIE in the US. We describe key challenges to HIE progress and offer insights into the likely path to ensure that clinicians have routine, electronic access to patient information.
FOUR KEY DIMENSIONS OF HEALTH INFORMATION EXCHANGE
While the concept of HIE is simple—electronic access to clinical information across healthcare settings—the operationalization of HIE occurs in many different ways.16 While the terms “health information exchange” and “interoperability” are often used interchangeably, they can have different meanings. In this section, we describe 4 important dimensions that serve as a framework for understanding any given effort to enable HIE (Table).
(1) What Is Exchanged? Types of Information
The term “health information exchange” is ambiguous with respect to the type(s) of information that are accessible. Health information exchange may refer to the process of 2 providers electronically sharing a wide range of data, from a single type of information (eg, lab test results), summary of care records, to complete patient records.17 Part of this ambiguity may stem from uncertainty about the scope of information that should be shared, and how this varies based on the type of clinical encounter. For example, critical types of information in the ED setting may differ from those relevant to a primary care team after a referral. While the ability to access only particular types of information will not address all information gaps, providing access to complete patient records may result in information overload that inhibits the ability to find the subset of information relevant in a given clinical encounter.
(2) Who is Exchanging? Relationship Between Provider Organizations
The types of information accessed electronically are effectively agnostic to the relationship between the provider organizations that are sharing information. Traditionally, HIE has been considered as information that is electronically shared among 2 or more unaffiliated organizations. However, there is increasing recognition that some providers may not have electronic access to all information about their patients that exists within their organization, often after a merger or acquisition between 2 providers with different EHR systems.18,19 In these cases, a primary care team in a large integrated delivery system may have as many information gaps as a primary care team in a small, independent practice. Fulfilling clinical information needs may require both intra- and interorganizational HIE, which complicates the design of HIE processes and how the care team approaches incorporating information from both types of organizations into their decision-making. It is also important to recognize that some provider organizations, particularly small, rural practices, may not have the information technology and connectivity infrastructure required to engage in HIE.
(3) How Is Information Exchanged? Types of Electronic Access: Push vs Pull Exchange
To minimize information gaps, electronic access to information from external settings needs to offer both “push” and “pull” options. Push exchange, which can direct information electronically to a targeted recipient, works in scenarios in which there is a known information gap and known information source. The classic use for push exchange is care coordination, such as primary care physician-specialist referrals or hospital-primary care physician transitions postdischarge. Pull exchange accommodates scenarios in which there is a known information gap but the source(s) of information are unknown; it requires that clinical care teams search for and locate the clinical information that exists about the patient in external settings. Here, the classic use is emergency care in which the care team may encounter a new patient and want to retrieve records.
Widespread use of provider portals that offer view-only access into EHRs and other clinical data repositories maintained by external organizations complicate the picture. Portals are commonly used by hospitals to enable community providers to view information from a hospitalization.21 While this does not fall under the commonly held notion of HIE because no exchange occurs, portals support a pull approach to accessing information electronically among care settings that treat the same patients but use different EHRs.
Regardless of whether information is pushed or pulled, this may happen with varying degrees of human effort. This distinction gives rise to the difference between HIE and interoperability. Health information exchange reflects the ability of EHRs to exchange information, while interoperability additionally requires that EHRs be able to use exchanged information. From an operational perspective, the key distinction between HIE and interoperability is the extent of human involvement. Health information exchange requires that a human read and decide how to enter information from external settings (eg, a chart in PDF format sent between 2 EHRs), while interoperability enables the EHR that receives the information to understand the content and automatically triage or reconcile information, such as a medication list, without any human action.21 Health information exchange, therefore, relies on the diligence of the receiving clinician, while interoperability does not.
(4) What Governance Entity Defines the “Rules” of Exchange?
When more than 1 provider organization shares patient-identified data, a governance entity must specify the framework that governs the exchange. While the specifics of HIE governance vary, there are 3 predominant types of HIE networks, based on the type of organization that governs exchange: enterprise HIE networks, EHR vendor HIE networks or community HIE networks.
Enterprise HIE networks exist when 1 or more provider organizations electronically share clinical information to support patient care with some restriction, beyond geography, that dictates which organizations are involved. Typically, restrictions are driven by strategic, proprietary interests.22,23 Although broad-based information access across settings would be in the best interest of the patient, provider organizations are sensitive to the competitive implications of sharing data and may pursue such sharing in a strategic way.24 A common scenario is when hospitals choose to strategically affiliate with select ambulatory providers and exclusively exchange information with them. This should facilitate better care coordination for patients shared by the hospital and those providers but can also benefit the hospital by increasing the referrals from those providers. While there is little direct evidence quantifying the extent to which this type of strategic sharing takes place, there have been anecdotal reports as well as indirect findings that for-profit hospitals in competitive markets are less likely to share patient data.19,25
EHR vendor HIE networks exist when exchange occurs within a community of provider organizations that use an EHR from the same vendor. A subset of EHR vendors have made this capability available; EPIC’s CareEverywhere solution27 is the best-known example. Providers with an EPIC EHR are able to query for and retrieve summary of care records and other documents from any provider organization with EPIC that has activated this functionality. There are also multivendor efforts, such as CommonWell27 and the Sequoia Project’s Carequality collaborative,28 which are initiatives that seek to provide a common interoperability framework across a diverse set of stakeholders, including provider organizations with different EHR systems, in a similar fashion to HIE modules like CareEverywhere. To date, growth in these cross-vendor collaborations has been slow, and they have limited participation. While HIE networks that involve EHR vendors are likely to grow, it is difficult to predict how quickly because they are still in an early phase of development, and face nontechnical barriers such as patient consent policies that vary between providers and across states.
Community HIE networks—also referred to as health information organizations (HIOs) or regional health information organizations (RHIOs)—exist when provider organizations in a community, frequently state-level organizations that were funded through HITECH grants,14 set up the technical infrastructure and governance approach to engage in HIE to improve patient care. In contrast to enterprise or vendor HIE networks that have pursued HIE in ways that appear strategically beneficial, the only restriction on participation in community and state HIE networks is usually geography because they view information exchange as a public good. Seventyone percent of hospital service areas (HSAs) are covered by at least 1 of the 106 operational HIOs, with 309,793 clinicians (licensed prescribers) participating in those exchange networks. Even with early infusions of public and other grant-funding, community HIE networks have experienced significant challenges to sustained operation, and many have ceased operating.29
Thus, for any given provider organization, available HIE networks are primarily shaped by 3 factors:
1. Geographic location, which determines the available community and state HIE networks (as well as other basic information technology and connectivity infrastructure); providers located outside the service areas covered by an operational HIE have little incentive to participate because they do not connect them to providers with whom they share patients. Providers in rural areas may simply not have the needed infrastructure to pursue HIE.
2. Type of organization to which they belong, which determines the available enterprise HIE networks; providers who are not members of large health systems may be excluded from participation in these types of networks.
3. EHR vendor, which determines whether they have access to an EHR vendor HIE network.
ONGOING CHALLENGES
Despite agreement about the substantial potential of HIE to reduce costs and increase the quality of care delivered across a broad range of providers, HIE progress has been slow. While HITECH has successfully increased EHR adoption in hospitals and ambulatory practices,30 HIE has lagged. This is largely because many complex, intertwined barriers must be addressed for HIE to be widespread.
Lack of a Defined Goal
The cost and complexity associated with the exchange of a single type of data (eg, medications) is substantially less than the cost and complexity of sharing complete patient records. There has been little industry consensus on the target goal—do we need to enable sharing of complete patient records across all providers, or will summary of care records suffice? If the latter, as is the focus of the current MU criteria, what types of information should be included in a summary of care record, and should content and/or structure vary depending on the type of care transition? While the MU criteria require the exchange of a summary of care record with defined data fields, it remains unclear whether this is the end state or whether we should continue to push towards broad-based sharing of all patient data as structured elements. Without a clear picture of the ideal end state, there has been significant heterogeneity in the development of HIE capabilities across providers and vendors, and difficulty coordinating efforts to continue to advance towards a nationwide approach. Addressing this issue also requires progress to define HIE usability, that is, how information from external organizations should be presented and integrated into clinical workflow and clinical decisions. Currently, where HIE is occurring and clinicians are receiving summary of care records, they find them long, cluttered, and difficult to locate key information.
Numerous, Complex Barriers Spanning Multiple Stakeholders
In the context of any individual HIE effort, even after the goal is defined, there are a myriad of challenges. In a recent survey of HIO efforts, many identified the following barriers as substantially impeding their development: establishing a sustainable business model, lack of funding, integration of HIE into provider workflow, limitations of current data standards, and working with governmental policy and mandates.30 What is notable about this list is that the barriers span an array of areas, including financial incentives and identifying a sustainable business model, technical barriers such as working within the limitations of data standards, and regulatory issues such as state laws that govern the requirements for patient consent to exchange personal health information. Overcoming any of these issues is challenging, but trying to tackle all of them simultaneously clearly reveals why progress has been slow. Further, resolving many of the issues involve different groups of stakeholders. For example, implementing appropriate patient consent procedures can require engaging with and harmonizing the regulations of multiple states, as well as the Health Insurance Portability and Accountability Act (HIPAA) and regulations specific to substance abuse data.
Weak or Misaligned Incentives
Among the top barriers to HIE efforts are those related to funding and lack of a sustainable business model. This reflects the fact that economic incentives in the current market have not promoted provider engagement in HIE. Traditional fee-for-service payment structures do not reward providers for avoiding duplicative care.31 Further, hospitals perceive patient data as a “key strategic asset, tying physicians and patients to their organization,”24 and are reluctant to share data with competitors. Compounding the problem is that EHR vendors have a business interest in using HIE as a lever to increase revenue. In the short-term, they can charge high fees for interfaces and other HIE-related functionality. In the long-run, vendors may try to influence provider choice of system by making it difficult to engage in cross-vendor exchange.32 Information blocking—when providers or vendors knowingly interfere with HIE33—reflects not only weak incentives, but perverse incentives. While not all providers and vendors experience perverse incentives, the combination of weak and perverse incentives suggests the need to strengthen incentives, so that both types of stakeholders are motivated to tackle the barriers to HIE development. Key to strengthening incentives are payers, who are thought to be the largest beneficiaries of HIE. Payers have been reluctant to make significant investments in HIE without a more active voice in its implementation,34 but a shift to value-based payment may increase their engagement.
THE PATH FORWARD
Despite the continued challenges to nationwide HIE, several policy and technology developments show promise. Stage 3 meaningful use criteria continue to build on previous stages in increasing HIE requirements, raising the threshold for electronic exchange and EHR integration of summary of care documentation in patient transitions. The recently released Medicare Access and CHIP Reauthorization Act (MACRA) Merit-based Incentive Payment System (MIPS) proposed rule replaces stage 3 meaningful use for Medicare-eligible providers with advancing care information (ACI), which accounts for 25% of a provider’s overall incentive reimbursement and includes multiple HIE criteria for providers to report as part of the base and performance score, and follows a very similar framework to stage 3 MU with its criteria regarding HIE.35 While the Centers for Medicare and Medicaid Services (CMS) has not publicly declared that stage 3 MU will be replaced by ACI for hospitals and Medicaid providers, it is likely it will align those programs with the newly announced Medicare incentives.
MACRA also included changes to the Office of the National Coordinator (ONC) EHR certification program in an attempt to further encourage HIE. Vendors and providers must attest that they do not engage in information blocking and will cooperate with the Office’s surveillance programs to that effect. They also must attest that, to the greatest degree possible, their EHR systems allow for bi-directional interoperability with other providers, including those with different EHR vendors, and timely access for patients to view, download, and transmit their health data. In addition, there are emerging federal efforts to pursue a more standardized approach to patient matching and harmonize consent policies across states. These types of new policy initiatives indicate a continued interest in prioritizing HIE and interoperability.21
New technologies may also help spur HIE progress. The newest policy initiatives from CMS, including stage 3 MU and MACRA, have looked to incentivize the creation of application program interfaces (APIs), a set of publicly available tools from EHR vendors to allow developers to build applications that can directly interface with, and retrieve data from, their EHRs. While most patient access to electronic health data to date has been accomplished via patient portals, open APIs would enable developers to build an array of programs for consumers to view, download, and transmit their health data.
Even more promising is the development of the newest Health Level 7 data transmission standard, Fast Healthcare Interoperability Resources (FHIR), which promises to dramatically simplify the technical aspects of interoperability. FHIR utilizes a human-readable, easy to implement modular “resources” standard that may alleviate many technical challenges that come with implementation of an HIE system, enabling cheaper and simpler interoperability.36 A consortium of EHR vendors are working together to test these standards.28 The new FHIR standards also work in conjunction with APIs to allow easier development of consumer-facing applications37 that may empower patients to take ownership of their health data.
CONCLUSION
While HIE holds great promise to reduce the cost and improve the quality of care, progress towards a nationally interoperable health system has been slow. Simply defining HIE and what types of HIE are needed in different clinical scenarios has proven challenging. The additional challenges to implementing HIE in complex technology, legal/regulatory, governance, and incentive environment are not without solutions. Continued policy interventions, private sector collaborations, and new technologies may hold the keys to realizing the vast potential of electronic HIE.
Disclosure
Nothing to report.
The US healthcare system is highly fragmented, with patients typically receiving treatment from multiple providers during an episode of care and from many more providers over their lifetime.1,2 As patients move between care delivery settings, whether and how their information follows them is determined by a haphazard and error-prone patchwork of telephone, fax, and electronic communication channels.3 The existence of more robust electronic communication channels is often dictated by factors such as which providers share the same electronic health record (EHR) vendor rather than which providers share the highest volume of patients. As a result, providers often make clinical decisions with incomplete information, increasing the chances of misdiagnosis, unsafe or suboptimal treatment, and duplicative utilization.
Providers across the continuum of care encounter challenges to optimal clinical decision-making as a result of incomplete information. These are particularly problematic among clinicians in hospitals and emergency departments (EDs). Clinical decision-making in EDs often involves urgent and critical conditions in which decisions are made under pressure. Time constraints limit provider ability to find key clinical information to accurately diagnose and safely treat patients.4-6 Even for planned inpatient care, providers are often unfamiliar with patients, and they make safer decisions when they have full access to information from outside providers.7,8
Transitions of care between hospitals and primary care settings are also fraught with gaps in information sharing. Clinical decisions made in primary care can set patients on treatment trajectories that are greatly affected by the quality of information available to the care team at the time of initial diagnosis as well as in their subsequent treatment. Primary care physicians are not universally notified when their patients are hospitalized and may not have access to detailed information about the hospitalization, which can impair their ability to provide high quality care.9-11
Widespread and effective electronic health information exchange (HIE) holds the potential to address these challenges.3 With robust, interconnected electronic systems, key pieces of a patient’s health record can be electronically accessed and reconciled during planned and unplanned care transitions. The concept of HIE is simple—make all relevant patient data available to the clinical care team at the point of care, regardless of where that information was generated. The estimated value of nationwide interoperable EHR adoption suggests large savings from the more efficient, less duplicative, and higher quality care that likely results.12,13
There has been substantial funding and activity at federal, state, and local levels to promote the development of HIE in the US. The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act has the specific goal of accelerating adoption and use of certified EHR technology coupled with the ability to exchange clinical information to support patient care.14 The HITECH programs supported specific types of HIE that were believed to be particularly critical to improving patient care and included them in the federally-defined criteria for Meaningful Use (MU) of EHRs (ie, providers receive financial incentives for achieving specific objectives). The MU criteria evolve, moving from data capture in stage 1 to improved patient outcomes in stage 3.15 The HIE criteria focus on sending and receiving summary-of-care records during care transitions.
Despite the clear benefits of HIE and substantial support stated in policy initiatives, the spread of national HIE has been slow. Today, HIE in the US is highly heterogeneous: as a result of multiple federal-, state-, community-, enterprise- and EHR vendor-level efforts, only some provider organizations are able to engage in HIE with the other provider organizations with which they routinely share patients. In this review, we offer a framework and a corresponding set of definitions to understand the current state of HIE in the US. We describe key challenges to HIE progress and offer insights into the likely path to ensure that clinicians have routine, electronic access to patient information.
FOUR KEY DIMENSIONS OF HEALTH INFORMATION EXCHANGE
While the concept of HIE is simple—electronic access to clinical information across healthcare settings—the operationalization of HIE occurs in many different ways.16 While the terms “health information exchange” and “interoperability” are often used interchangeably, they can have different meanings. In this section, we describe 4 important dimensions that serve as a framework for understanding any given effort to enable HIE (Table).
(1) What Is Exchanged? Types of Information
The term “health information exchange” is ambiguous with respect to the type(s) of information that are accessible. Health information exchange may refer to the process of 2 providers electronically sharing a wide range of data, from a single type of information (eg, lab test results), summary of care records, to complete patient records.17 Part of this ambiguity may stem from uncertainty about the scope of information that should be shared, and how this varies based on the type of clinical encounter. For example, critical types of information in the ED setting may differ from those relevant to a primary care team after a referral. While the ability to access only particular types of information will not address all information gaps, providing access to complete patient records may result in information overload that inhibits the ability to find the subset of information relevant in a given clinical encounter.
(2) Who is Exchanging? Relationship Between Provider Organizations
The types of information accessed electronically are effectively agnostic to the relationship between the provider organizations that are sharing information. Traditionally, HIE has been considered as information that is electronically shared among 2 or more unaffiliated organizations. However, there is increasing recognition that some providers may not have electronic access to all information about their patients that exists within their organization, often after a merger or acquisition between 2 providers with different EHR systems.18,19 In these cases, a primary care team in a large integrated delivery system may have as many information gaps as a primary care team in a small, independent practice. Fulfilling clinical information needs may require both intra- and interorganizational HIE, which complicates the design of HIE processes and how the care team approaches incorporating information from both types of organizations into their decision-making. It is also important to recognize that some provider organizations, particularly small, rural practices, may not have the information technology and connectivity infrastructure required to engage in HIE.
(3) How Is Information Exchanged? Types of Electronic Access: Push vs Pull Exchange
To minimize information gaps, electronic access to information from external settings needs to offer both “push” and “pull” options. Push exchange, which can direct information electronically to a targeted recipient, works in scenarios in which there is a known information gap and known information source. The classic use for push exchange is care coordination, such as primary care physician-specialist referrals or hospital-primary care physician transitions postdischarge. Pull exchange accommodates scenarios in which there is a known information gap but the source(s) of information are unknown; it requires that clinical care teams search for and locate the clinical information that exists about the patient in external settings. Here, the classic use is emergency care in which the care team may encounter a new patient and want to retrieve records.
Widespread use of provider portals that offer view-only access into EHRs and other clinical data repositories maintained by external organizations complicate the picture. Portals are commonly used by hospitals to enable community providers to view information from a hospitalization.21 While this does not fall under the commonly held notion of HIE because no exchange occurs, portals support a pull approach to accessing information electronically among care settings that treat the same patients but use different EHRs.
Regardless of whether information is pushed or pulled, this may happen with varying degrees of human effort. This distinction gives rise to the difference between HIE and interoperability. Health information exchange reflects the ability of EHRs to exchange information, while interoperability additionally requires that EHRs be able to use exchanged information. From an operational perspective, the key distinction between HIE and interoperability is the extent of human involvement. Health information exchange requires that a human read and decide how to enter information from external settings (eg, a chart in PDF format sent between 2 EHRs), while interoperability enables the EHR that receives the information to understand the content and automatically triage or reconcile information, such as a medication list, without any human action.21 Health information exchange, therefore, relies on the diligence of the receiving clinician, while interoperability does not.
(4) What Governance Entity Defines the “Rules” of Exchange?
When more than 1 provider organization shares patient-identified data, a governance entity must specify the framework that governs the exchange. While the specifics of HIE governance vary, there are 3 predominant types of HIE networks, based on the type of organization that governs exchange: enterprise HIE networks, EHR vendor HIE networks or community HIE networks.
Enterprise HIE networks exist when 1 or more provider organizations electronically share clinical information to support patient care with some restriction, beyond geography, that dictates which organizations are involved. Typically, restrictions are driven by strategic, proprietary interests.22,23 Although broad-based information access across settings would be in the best interest of the patient, provider organizations are sensitive to the competitive implications of sharing data and may pursue such sharing in a strategic way.24 A common scenario is when hospitals choose to strategically affiliate with select ambulatory providers and exclusively exchange information with them. This should facilitate better care coordination for patients shared by the hospital and those providers but can also benefit the hospital by increasing the referrals from those providers. While there is little direct evidence quantifying the extent to which this type of strategic sharing takes place, there have been anecdotal reports as well as indirect findings that for-profit hospitals in competitive markets are less likely to share patient data.19,25
EHR vendor HIE networks exist when exchange occurs within a community of provider organizations that use an EHR from the same vendor. A subset of EHR vendors have made this capability available; EPIC’s CareEverywhere solution27 is the best-known example. Providers with an EPIC EHR are able to query for and retrieve summary of care records and other documents from any provider organization with EPIC that has activated this functionality. There are also multivendor efforts, such as CommonWell27 and the Sequoia Project’s Carequality collaborative,28 which are initiatives that seek to provide a common interoperability framework across a diverse set of stakeholders, including provider organizations with different EHR systems, in a similar fashion to HIE modules like CareEverywhere. To date, growth in these cross-vendor collaborations has been slow, and they have limited participation. While HIE networks that involve EHR vendors are likely to grow, it is difficult to predict how quickly because they are still in an early phase of development, and face nontechnical barriers such as patient consent policies that vary between providers and across states.
Community HIE networks—also referred to as health information organizations (HIOs) or regional health information organizations (RHIOs)—exist when provider organizations in a community, frequently state-level organizations that were funded through HITECH grants,14 set up the technical infrastructure and governance approach to engage in HIE to improve patient care. In contrast to enterprise or vendor HIE networks that have pursued HIE in ways that appear strategically beneficial, the only restriction on participation in community and state HIE networks is usually geography because they view information exchange as a public good. Seventyone percent of hospital service areas (HSAs) are covered by at least 1 of the 106 operational HIOs, with 309,793 clinicians (licensed prescribers) participating in those exchange networks. Even with early infusions of public and other grant-funding, community HIE networks have experienced significant challenges to sustained operation, and many have ceased operating.29
Thus, for any given provider organization, available HIE networks are primarily shaped by 3 factors:
1. Geographic location, which determines the available community and state HIE networks (as well as other basic information technology and connectivity infrastructure); providers located outside the service areas covered by an operational HIE have little incentive to participate because they do not connect them to providers with whom they share patients. Providers in rural areas may simply not have the needed infrastructure to pursue HIE.
2. Type of organization to which they belong, which determines the available enterprise HIE networks; providers who are not members of large health systems may be excluded from participation in these types of networks.
3. EHR vendor, which determines whether they have access to an EHR vendor HIE network.
ONGOING CHALLENGES
Despite agreement about the substantial potential of HIE to reduce costs and increase the quality of care delivered across a broad range of providers, HIE progress has been slow. While HITECH has successfully increased EHR adoption in hospitals and ambulatory practices,30 HIE has lagged. This is largely because many complex, intertwined barriers must be addressed for HIE to be widespread.
Lack of a Defined Goal
The cost and complexity associated with the exchange of a single type of data (eg, medications) is substantially less than the cost and complexity of sharing complete patient records. There has been little industry consensus on the target goal—do we need to enable sharing of complete patient records across all providers, or will summary of care records suffice? If the latter, as is the focus of the current MU criteria, what types of information should be included in a summary of care record, and should content and/or structure vary depending on the type of care transition? While the MU criteria require the exchange of a summary of care record with defined data fields, it remains unclear whether this is the end state or whether we should continue to push towards broad-based sharing of all patient data as structured elements. Without a clear picture of the ideal end state, there has been significant heterogeneity in the development of HIE capabilities across providers and vendors, and difficulty coordinating efforts to continue to advance towards a nationwide approach. Addressing this issue also requires progress to define HIE usability, that is, how information from external organizations should be presented and integrated into clinical workflow and clinical decisions. Currently, where HIE is occurring and clinicians are receiving summary of care records, they find them long, cluttered, and difficult to locate key information.
Numerous, Complex Barriers Spanning Multiple Stakeholders
In the context of any individual HIE effort, even after the goal is defined, there are a myriad of challenges. In a recent survey of HIO efforts, many identified the following barriers as substantially impeding their development: establishing a sustainable business model, lack of funding, integration of HIE into provider workflow, limitations of current data standards, and working with governmental policy and mandates.30 What is notable about this list is that the barriers span an array of areas, including financial incentives and identifying a sustainable business model, technical barriers such as working within the limitations of data standards, and regulatory issues such as state laws that govern the requirements for patient consent to exchange personal health information. Overcoming any of these issues is challenging, but trying to tackle all of them simultaneously clearly reveals why progress has been slow. Further, resolving many of the issues involve different groups of stakeholders. For example, implementing appropriate patient consent procedures can require engaging with and harmonizing the regulations of multiple states, as well as the Health Insurance Portability and Accountability Act (HIPAA) and regulations specific to substance abuse data.
Weak or Misaligned Incentives
Among the top barriers to HIE efforts are those related to funding and lack of a sustainable business model. This reflects the fact that economic incentives in the current market have not promoted provider engagement in HIE. Traditional fee-for-service payment structures do not reward providers for avoiding duplicative care.31 Further, hospitals perceive patient data as a “key strategic asset, tying physicians and patients to their organization,”24 and are reluctant to share data with competitors. Compounding the problem is that EHR vendors have a business interest in using HIE as a lever to increase revenue. In the short-term, they can charge high fees for interfaces and other HIE-related functionality. In the long-run, vendors may try to influence provider choice of system by making it difficult to engage in cross-vendor exchange.32 Information blocking—when providers or vendors knowingly interfere with HIE33—reflects not only weak incentives, but perverse incentives. While not all providers and vendors experience perverse incentives, the combination of weak and perverse incentives suggests the need to strengthen incentives, so that both types of stakeholders are motivated to tackle the barriers to HIE development. Key to strengthening incentives are payers, who are thought to be the largest beneficiaries of HIE. Payers have been reluctant to make significant investments in HIE without a more active voice in its implementation,34 but a shift to value-based payment may increase their engagement.
THE PATH FORWARD
Despite the continued challenges to nationwide HIE, several policy and technology developments show promise. Stage 3 meaningful use criteria continue to build on previous stages in increasing HIE requirements, raising the threshold for electronic exchange and EHR integration of summary of care documentation in patient transitions. The recently released Medicare Access and CHIP Reauthorization Act (MACRA) Merit-based Incentive Payment System (MIPS) proposed rule replaces stage 3 meaningful use for Medicare-eligible providers with advancing care information (ACI), which accounts for 25% of a provider’s overall incentive reimbursement and includes multiple HIE criteria for providers to report as part of the base and performance score, and follows a very similar framework to stage 3 MU with its criteria regarding HIE.35 While the Centers for Medicare and Medicaid Services (CMS) has not publicly declared that stage 3 MU will be replaced by ACI for hospitals and Medicaid providers, it is likely it will align those programs with the newly announced Medicare incentives.
MACRA also included changes to the Office of the National Coordinator (ONC) EHR certification program in an attempt to further encourage HIE. Vendors and providers must attest that they do not engage in information blocking and will cooperate with the Office’s surveillance programs to that effect. They also must attest that, to the greatest degree possible, their EHR systems allow for bi-directional interoperability with other providers, including those with different EHR vendors, and timely access for patients to view, download, and transmit their health data. In addition, there are emerging federal efforts to pursue a more standardized approach to patient matching and harmonize consent policies across states. These types of new policy initiatives indicate a continued interest in prioritizing HIE and interoperability.21
New technologies may also help spur HIE progress. The newest policy initiatives from CMS, including stage 3 MU and MACRA, have looked to incentivize the creation of application program interfaces (APIs), a set of publicly available tools from EHR vendors to allow developers to build applications that can directly interface with, and retrieve data from, their EHRs. While most patient access to electronic health data to date has been accomplished via patient portals, open APIs would enable developers to build an array of programs for consumers to view, download, and transmit their health data.
Even more promising is the development of the newest Health Level 7 data transmission standard, Fast Healthcare Interoperability Resources (FHIR), which promises to dramatically simplify the technical aspects of interoperability. FHIR utilizes a human-readable, easy to implement modular “resources” standard that may alleviate many technical challenges that come with implementation of an HIE system, enabling cheaper and simpler interoperability.36 A consortium of EHR vendors are working together to test these standards.28 The new FHIR standards also work in conjunction with APIs to allow easier development of consumer-facing applications37 that may empower patients to take ownership of their health data.
CONCLUSION
While HIE holds great promise to reduce the cost and improve the quality of care, progress towards a nationally interoperable health system has been slow. Simply defining HIE and what types of HIE are needed in different clinical scenarios has proven challenging. The additional challenges to implementing HIE in complex technology, legal/regulatory, governance, and incentive environment are not without solutions. Continued policy interventions, private sector collaborations, and new technologies may hold the keys to realizing the vast potential of electronic HIE.
Disclosure
Nothing to report.
1. Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130-1139. PubMed
2. Finnell JT, Overhage JM, Dexter PR, Perkins SM, Lane KA, McDonald CJ. Community clinical data exchange for emergency medicine patients. Paper presented at: AMIA Annual Symposium Proceedings 2003. PubMed
3. Bodenheimer T. Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. PubMed
4. Franczak MJ, Klein M, Raslau F, Bergholte J, Mark LP, Ulmer JL. In emergency departments, radiologists’ access to EHRs may influence interpretations and medical management. Health Aff (Millwood). 2014;33(5):800-806. PubMed
5. Shapiro JS, Kannry J, Kushniruk AW, Kuperman G; New York Clinical Information Exchange (NYCLIX) Clinical Advisory Subcommittee. Emergency physicians’ perceptions of health information exchange. J Am Med Inform Assoc. 2007;14(6):700-705. PubMed
6. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426-432. PubMed
7. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med.. 2004;79(2):186-194. PubMed
8. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(suppl 6):S40-S45. PubMed
9. Smith PC, Araya-Guerra R, Bublitz C, et al. MIssing clinical information during primary care visits. JAMA. 2005;293(5):565-571. PubMed
10. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. PubMed
11. van Walraven C, Taljaard M, Bell CM, et al. A prospective cohort study found that provider and information continuity was low after patient discharge from hospital. J Clin Epidemiol. 2010;63(9):1000-1010. PubMed
12. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood). 2005:(suppl)W5-10-W5-18. PubMed
13. Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep). 2006;132:1-71. PubMed
14. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382-385. PubMed
15. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501-504. PubMed
16. Kuperman G, McGowan J. Potential unintended consequences of health information exchange. J Gen Intern Med. 2013;28(12):1663-1666. PubMed
17. Mathematica Policy Research and Harvard School of Public Health. DesRoches CM, Painter MW, Jha AK, eds. Health Information Technology in the United States, 2015: Transition to a Post-HITECH World (Executive Summary). September 18, 2015. Princeton, NJ: Robert Wood Johnson Foundation; 2015.
18. O’Malley AS, Anglin G, Bond AM, Cunningham PJ, Stark LB, Yee T. Greenville & Spartanburg: Surging Hospital Employment of Physicians Poses Opportunities and Challenges. Washington, DC: Center for Studying Health System Change (HSC); February 2011. 6.
19. Katz A, Bond AM, Carrier E, Docteur E, Quach CW, Yee T. Cleveland Hospital Systems Expand Despite Weak Economy. Washington, DC: Center for Studying Health System Change (HSC); September 2010. 2.
20. Grossman JM, Bodenheimer TS, McKenzie K. Hospital-physician portals: the role of competition in driving clinical data exchange. Health Aff (Millwood). 2006;25(6):1629-1636. PubMed
21. De Salvo KB, Galvez E. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap - Version 1.0. In: Office of the National Coordinator for Health Information Technology. ed 2015. https://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/interoperability-electronic-health-and-medical-records/connecting-health-care-nation-shared-nationwide-interoperability-roadmap-version-10/. Accessed September 3, 2016.
22. Adler-Milstein J, DesRoches C, Jha AK. Health information exchange among US hospitals. Am J Manag Care. 2011;17(11):761-768. PubMed
23. Vest JR. More than just a question of technology: factors related to hospitals’ adoption and implementation of health information exchange. Int J Med Inform. 2010;79(12):797-806. PubMed
24. Grossman JM, Kushner KL, November EA. Creating sustainable local health information exchanges: can barriers to stakeholder participation be overcome? Res Brief. 2008;2:1-12. PubMed
25. Grossman JM, Cohen G. Despite regulatory changes, hospitals cautious in helping physicians purchase electronic medical records. Issue Brief Cent Stud Health Syst Change 2008;123:1-4. PubMed
26. Kaelber DC, Waheed R, Einstadter D, Love TE, Cebul RD. Use and perceived value of health information exchange: one public healthcare system’s experience. Am J Manag Care. 2013;19(10 spec no):SP337-SP343. PubMed
27. Commonwell Health Alliance. http://www.commonwellalliance.org/, 2016. Accessed September 3, 2016.
28. Carequality. http://sequoiaproject.org/carequality/, 2016. Accessed September 3, 2016.
29. Adler-Milstein J, Lin SC, Jha AK. The number of health information exchange efforts is declining, leaving the viability of broad clinical data exchange uncertain. Health Aff (Millwood). 2016;35(7):1278-1285. PubMed
30. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015:34(12):2174-2180. PubMed
31. Health IT Policy Committee Report to Congress: Challenges and Barriers to Interoperability. 2015. https://www.healthit.gov/facas/health-it-policy-committee/health-it-policy-committee-recommendations-national-coordinator-health-it. Accessed September 3, 2016.
32. Everson J, Adler-Milstein J. Engagement in hospital health information exchange is associated with vendor marketplace dominance. Health Aff (MIllwood). 2016;35(7):1286-1293. PubMed
33. Downing K, Mason J. ONC targets information blocking. J AHIMA. 2015;86(7):36-38. PubMed
34. Cross DA, Lin SC, Adler-Milstein J. Assessing payer perspectives on health information exchange. J Am Med Inform Assoc. 2016;23(2):297-303. PubMed
35. Centers for Medicare & Medicaid Services. MACRA: MIPS and APMs. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Accessed September 3, 2016.
36. Raths D. Trend: standards development. Catching FHIR. A new HL7 draft standard may boost web services development in healthcare. Healthc Inform. 2014;31(2):13,16. PubMed
37. Alterovitz G, Warner J, Zhang P, et al. SMART on FHIR genomics: facilitating
1. Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130-1139. PubMed
2. Finnell JT, Overhage JM, Dexter PR, Perkins SM, Lane KA, McDonald CJ. Community clinical data exchange for emergency medicine patients. Paper presented at: AMIA Annual Symposium Proceedings 2003. PubMed
3. Bodenheimer T. Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. PubMed
4. Franczak MJ, Klein M, Raslau F, Bergholte J, Mark LP, Ulmer JL. In emergency departments, radiologists’ access to EHRs may influence interpretations and medical management. Health Aff (Millwood). 2014;33(5):800-806. PubMed
5. Shapiro JS, Kannry J, Kushniruk AW, Kuperman G; New York Clinical Information Exchange (NYCLIX) Clinical Advisory Subcommittee. Emergency physicians’ perceptions of health information exchange. J Am Med Inform Assoc. 2007;14(6):700-705. PubMed
6. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426-432. PubMed
7. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med.. 2004;79(2):186-194. PubMed
8. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(suppl 6):S40-S45. PubMed
9. Smith PC, Araya-Guerra R, Bublitz C, et al. MIssing clinical information during primary care visits. JAMA. 2005;293(5):565-571. PubMed
10. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. PubMed
11. van Walraven C, Taljaard M, Bell CM, et al. A prospective cohort study found that provider and information continuity was low after patient discharge from hospital. J Clin Epidemiol. 2010;63(9):1000-1010. PubMed
12. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood). 2005:(suppl)W5-10-W5-18. PubMed
13. Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep). 2006;132:1-71. PubMed
14. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382-385. PubMed
15. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501-504. PubMed
16. Kuperman G, McGowan J. Potential unintended consequences of health information exchange. J Gen Intern Med. 2013;28(12):1663-1666. PubMed
17. Mathematica Policy Research and Harvard School of Public Health. DesRoches CM, Painter MW, Jha AK, eds. Health Information Technology in the United States, 2015: Transition to a Post-HITECH World (Executive Summary). September 18, 2015. Princeton, NJ: Robert Wood Johnson Foundation; 2015.
18. O’Malley AS, Anglin G, Bond AM, Cunningham PJ, Stark LB, Yee T. Greenville & Spartanburg: Surging Hospital Employment of Physicians Poses Opportunities and Challenges. Washington, DC: Center for Studying Health System Change (HSC); February 2011. 6.
19. Katz A, Bond AM, Carrier E, Docteur E, Quach CW, Yee T. Cleveland Hospital Systems Expand Despite Weak Economy. Washington, DC: Center for Studying Health System Change (HSC); September 2010. 2.
20. Grossman JM, Bodenheimer TS, McKenzie K. Hospital-physician portals: the role of competition in driving clinical data exchange. Health Aff (Millwood). 2006;25(6):1629-1636. PubMed
21. De Salvo KB, Galvez E. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap - Version 1.0. In: Office of the National Coordinator for Health Information Technology. ed 2015. https://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/interoperability-electronic-health-and-medical-records/connecting-health-care-nation-shared-nationwide-interoperability-roadmap-version-10/. Accessed September 3, 2016.
22. Adler-Milstein J, DesRoches C, Jha AK. Health information exchange among US hospitals. Am J Manag Care. 2011;17(11):761-768. PubMed
23. Vest JR. More than just a question of technology: factors related to hospitals’ adoption and implementation of health information exchange. Int J Med Inform. 2010;79(12):797-806. PubMed
24. Grossman JM, Kushner KL, November EA. Creating sustainable local health information exchanges: can barriers to stakeholder participation be overcome? Res Brief. 2008;2:1-12. PubMed
25. Grossman JM, Cohen G. Despite regulatory changes, hospitals cautious in helping physicians purchase electronic medical records. Issue Brief Cent Stud Health Syst Change 2008;123:1-4. PubMed
26. Kaelber DC, Waheed R, Einstadter D, Love TE, Cebul RD. Use and perceived value of health information exchange: one public healthcare system’s experience. Am J Manag Care. 2013;19(10 spec no):SP337-SP343. PubMed
27. Commonwell Health Alliance. http://www.commonwellalliance.org/, 2016. Accessed September 3, 2016.
28. Carequality. http://sequoiaproject.org/carequality/, 2016. Accessed September 3, 2016.
29. Adler-Milstein J, Lin SC, Jha AK. The number of health information exchange efforts is declining, leaving the viability of broad clinical data exchange uncertain. Health Aff (Millwood). 2016;35(7):1278-1285. PubMed
30. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015:34(12):2174-2180. PubMed
31. Health IT Policy Committee Report to Congress: Challenges and Barriers to Interoperability. 2015. https://www.healthit.gov/facas/health-it-policy-committee/health-it-policy-committee-recommendations-national-coordinator-health-it. Accessed September 3, 2016.
32. Everson J, Adler-Milstein J. Engagement in hospital health information exchange is associated with vendor marketplace dominance. Health Aff (MIllwood). 2016;35(7):1286-1293. PubMed
33. Downing K, Mason J. ONC targets information blocking. J AHIMA. 2015;86(7):36-38. PubMed
34. Cross DA, Lin SC, Adler-Milstein J. Assessing payer perspectives on health information exchange. J Am Med Inform Assoc. 2016;23(2):297-303. PubMed
35. Centers for Medicare & Medicaid Services. MACRA: MIPS and APMs. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Accessed September 3, 2016.
36. Raths D. Trend: standards development. Catching FHIR. A new HL7 draft standard may boost web services development in healthcare. Healthc Inform. 2014;31(2):13,16. PubMed
37. Alterovitz G, Warner J, Zhang P, et al. SMART on FHIR genomics: facilitating
© 2017 Society of Hospital Medicine
Medicare and the 3-inpatient midnight requirement: A statute in need of modernization
On July 30, 1965, Lyndon B. Johnson signed H.R. 6675 into law, establishing Medicare and Medicaid as Title XVIII and Title XIX of the Social Security Act.1 Shortly after, Medicare’s “extended care benefit” began, offering Medicare beneficiaries skilled nursing facility (SNF) care after a qualifying stay of 3 or more consecutive inpatient midnights.2 Fifty years later, the word “inpatient” remains embedded in statute, limiting SNF coverage for Medicare beneficiaries hospitalized as outpatients under observation for part or all of a 3-midnight stay.3
At the individual Medicare beneficiary level, the financial impact of this policy is clear. The Office of Inspector General (OIG) reported a $10,503 beneficiary out-of-pocket cost per uncovered SNF stay following an observation hospitalization in 2012.4 But the actual number of Medicare beneficiaries impacted by this coverage gap is unknown. Using 2009 claims data, Feng et al.5 estimated that 0.75% of previously community dwelling Medicare beneficiaries are discharged to a SNF following an observation hospitalization, and the OIG reported 617,702 beneficiary hospital stays of 3 or more midnights not meeting the 3-midnight inpatient requirement in 2012, with 4% of these beneficiaries discharging to SNFs.4 Yet these studies based on Medicare claims data only capture actual SNF utilization, failing to answer the critical question: How many Medicare beneficiaries need, but forgo, SNF care following a non-qualifying observation hospital stay? In this issue of the Journal of Hospital Medicine, Goldstein et al.6 provide insight to that question. Using chart review of physical therapy and case management recommendations for post-acute SNF care, Goldstein et al.6 compare actual discharge rate to SNF or acute inpatient rehabilitation following an observation stay when such disposition is recommended. In their two-hospital system, fewer than 20% of previously community-dwelling hospitalist patients followed recommendation for post-acute facility stay after observation hospitalization, and more than 40% cited financial concerns as the reason for declining. Patients recommended for SNF also were more likely to be rehospitalized in the subsequent 30 days after discharge, confirming this as a vulnerable patient population. Given Medicare’s original intent to improve health care access for seniors, the case for change seems clear, and the repercussions of not addressing the plight of patients hospitalized under observation is having negative financial and overall detrimental health impacts.
But there are other compelling reasons why this 50-year-old law needs to be improved. Hospital care today is vastly different than when Medicare became law. Average hospital length of stay for patients 65 years and older was 14.2 days in 19657 compared to 5.2 days today,8 clearly a shift in what 3 days of hospital care means. Most importantly, observation stays have become a major part of hospital care. Between 2006 and 2014, per-beneficiary outpatient visits (which include all observation stays) increased 44.2% nationally, while inpatient discharges decreased 19.9%.9 In 2012, the Centers for Medicare & Medicaid Services (CMS) received 1.7 million outpatient observation claims and an additional 700,000 inpatient claims that started with observation days.10 CMS also expected the 2-midnight rule to reduce outpatient observation stays,4 but a recent OIG report11 found that outpatient stays increased 8.1% in the first year (FY 2014) under the new rule, and there were still 748,337 long observation stays (those lasting 2 midnights or longer) in 2014, only a small (2.8%) decrease from the prior year. These factors limit Medicare beneficiary post–acute SNF eligibility in ways that could not have been anticipated when the extended care benefit was created to help seniors access needed health care.
Policymakers must consider cost when considering statutory change. Waiver programs in the 1980s suspending the 3-midnight requirement raised concerns over potential increase in both SNF utilization and associated costs.12 However, more recent data suggest that altering the 3-midnight requirement may not increase post-acute SNF utilization. From 2006 to 2010, Medicare Advantage programs that waived the 3-midnight requirement saw a decrease in hospital length of stay without increased SNF utilization or SNF length of stay, indicating that access to the right level of care at the right time could be cost-saving.13 Recent data from the Bundled Payments for Care Improvement (BPCI) program found savings were largely related to decreased SNF utilization when payments were episode-based,14 a trend that may continue as Medicare moves away from fee-for-service towards bundled payments for more conditions. And although neither example directly tests changing the 3-midnight requirement to include observation midnights, both studies suggest that innovative health care delivery and modification of SNF access did not result in increased SNF utilization or greater post-acute costs. In fact, as Goldstein et al.6 showed, patients recommended for post-acute SNF following observation stay were more likely to be rehospitalized within 30 days, an additional cost that could potentially be avoided if these patients had SNF access. We believe that these correlations strongly support rescinding the 3-
That being said, what can be done? In 2015, the Medicare Payment Advisory Commission (MedPAC) recommended changing the 3-night requirement to require just one of 3 midnights to be inpatient to make a qualifying stay.10 Although an improvement over current law, this proposal would not help the majority of beneficiaries who are exclusively hospitalized under observation status. The “Improving Access to Medicare Coverage Act of 2015”, to be reintroduced in Congress in the coming weeks, would count any midnight spent in the hospital towards the 3-midnight stay requirement, and has bipartisan, bicameral support and cosponsorship.15 In 2015, through unanimous bipartisan, bicameral support, Congress passed the NOTICE Act (PL 114-42), which requires hospitals to inform Medicare beneficiaries hospitalized under observation.16 We believe that the data are clear to both sides of the aisle that Congress should now work together using scientifically-supported research to improve the exact observation policies they felt patients should be informed of. Passing the Improving Access to Medicare Coverage Act is the logical next step in this arena.
Medicare was intended to give seniors access to the healthcare they need. Growth in hospital-based observation care begs for modernization of the statutory 3-inpatient midnight rule. Counting all midnights towards the 3-midnight requirement, whether those midnights are outpatient observation or inpatient, is the right first step.
Disclosures
Representative Courtney is the bill sponsor of the Improving Access to Medicare Coverage Act. The authors report no other conflicts.
1. Medicare & Medicaid Milestones 1937-2015. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf . Accessed September 25, 2016.
2. Loewenstein R. Early effects of Medicare on the health care of the aged. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf. Accessed September 25, 2016.
3. US Social Security Act, Sec. 1861 (i). [42 U.S.C. 1395x]. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. Accessed September 25, 2016.
4. Department of Health and Human Services Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. Available at: https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed September 25, 2016.
5. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care 2014;52:796-800. PubMed
6. Goldstein JN, Schwartz JS, McGraw P, Banks TL, Hicks LS. The unmet need for postacute rehabilitation among medicare observation patients: a single-center study. J Hosp Med. 2017;12(3):168-172.
7. Vital and Health Statistics. Trends in hospital utilization: United States, 1965-1986. https://www.cdc.gov/nchs/data/series/sr_13/sr13_101.pdf. Accessed September 25, 2016.
8. Healthcare Cost and Utilization Project (HCUP). Statistical brief #180. Overview of hospital stays in the United States, 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed September 25, 2016.
9. MedPAC March 2016 Report to the Congress. Chapter 3. Hospital inpatient and outpatient services. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed September 25, 2016.
10. MedPAC. June 2015 Report to the Congress. Chapter 7: Hospital short-stay policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-7-hospital-short-stay-policy-issues-june-2015-report-.pdf?sfvrsn=0 Accessed September 25, 2016.
11. Department of Health and Human Services Office of Inspector General. Vulnerabilities remain under Medicare’s 2-midnight hospital policy, OEI-02-15-00020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed February 19, 2017.
12. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310: 1441-1442. PubMed
13. Grebela R, Keohane L Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs. 2015;34:1324-1330. PubMed
14. Dummit L, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. PubMed
15. HR. 1571 Improving Access to Medicare Coverage Act of 2015. https://www.govtrack.us/congress/bills/114/hr1571/text. Accessed September 25, 2016.
16. PL 114-42. The NOTICE Act. https://www.govtrack.us/congress/bills/114/hr876. Accessed September 25, 2016.
On July 30, 1965, Lyndon B. Johnson signed H.R. 6675 into law, establishing Medicare and Medicaid as Title XVIII and Title XIX of the Social Security Act.1 Shortly after, Medicare’s “extended care benefit” began, offering Medicare beneficiaries skilled nursing facility (SNF) care after a qualifying stay of 3 or more consecutive inpatient midnights.2 Fifty years later, the word “inpatient” remains embedded in statute, limiting SNF coverage for Medicare beneficiaries hospitalized as outpatients under observation for part or all of a 3-midnight stay.3
At the individual Medicare beneficiary level, the financial impact of this policy is clear. The Office of Inspector General (OIG) reported a $10,503 beneficiary out-of-pocket cost per uncovered SNF stay following an observation hospitalization in 2012.4 But the actual number of Medicare beneficiaries impacted by this coverage gap is unknown. Using 2009 claims data, Feng et al.5 estimated that 0.75% of previously community dwelling Medicare beneficiaries are discharged to a SNF following an observation hospitalization, and the OIG reported 617,702 beneficiary hospital stays of 3 or more midnights not meeting the 3-midnight inpatient requirement in 2012, with 4% of these beneficiaries discharging to SNFs.4 Yet these studies based on Medicare claims data only capture actual SNF utilization, failing to answer the critical question: How many Medicare beneficiaries need, but forgo, SNF care following a non-qualifying observation hospital stay? In this issue of the Journal of Hospital Medicine, Goldstein et al.6 provide insight to that question. Using chart review of physical therapy and case management recommendations for post-acute SNF care, Goldstein et al.6 compare actual discharge rate to SNF or acute inpatient rehabilitation following an observation stay when such disposition is recommended. In their two-hospital system, fewer than 20% of previously community-dwelling hospitalist patients followed recommendation for post-acute facility stay after observation hospitalization, and more than 40% cited financial concerns as the reason for declining. Patients recommended for SNF also were more likely to be rehospitalized in the subsequent 30 days after discharge, confirming this as a vulnerable patient population. Given Medicare’s original intent to improve health care access for seniors, the case for change seems clear, and the repercussions of not addressing the plight of patients hospitalized under observation is having negative financial and overall detrimental health impacts.
But there are other compelling reasons why this 50-year-old law needs to be improved. Hospital care today is vastly different than when Medicare became law. Average hospital length of stay for patients 65 years and older was 14.2 days in 19657 compared to 5.2 days today,8 clearly a shift in what 3 days of hospital care means. Most importantly, observation stays have become a major part of hospital care. Between 2006 and 2014, per-beneficiary outpatient visits (which include all observation stays) increased 44.2% nationally, while inpatient discharges decreased 19.9%.9 In 2012, the Centers for Medicare & Medicaid Services (CMS) received 1.7 million outpatient observation claims and an additional 700,000 inpatient claims that started with observation days.10 CMS also expected the 2-midnight rule to reduce outpatient observation stays,4 but a recent OIG report11 found that outpatient stays increased 8.1% in the first year (FY 2014) under the new rule, and there were still 748,337 long observation stays (those lasting 2 midnights or longer) in 2014, only a small (2.8%) decrease from the prior year. These factors limit Medicare beneficiary post–acute SNF eligibility in ways that could not have been anticipated when the extended care benefit was created to help seniors access needed health care.
Policymakers must consider cost when considering statutory change. Waiver programs in the 1980s suspending the 3-midnight requirement raised concerns over potential increase in both SNF utilization and associated costs.12 However, more recent data suggest that altering the 3-midnight requirement may not increase post-acute SNF utilization. From 2006 to 2010, Medicare Advantage programs that waived the 3-midnight requirement saw a decrease in hospital length of stay without increased SNF utilization or SNF length of stay, indicating that access to the right level of care at the right time could be cost-saving.13 Recent data from the Bundled Payments for Care Improvement (BPCI) program found savings were largely related to decreased SNF utilization when payments were episode-based,14 a trend that may continue as Medicare moves away from fee-for-service towards bundled payments for more conditions. And although neither example directly tests changing the 3-midnight requirement to include observation midnights, both studies suggest that innovative health care delivery and modification of SNF access did not result in increased SNF utilization or greater post-acute costs. In fact, as Goldstein et al.6 showed, patients recommended for post-acute SNF following observation stay were more likely to be rehospitalized within 30 days, an additional cost that could potentially be avoided if these patients had SNF access. We believe that these correlations strongly support rescinding the 3-
That being said, what can be done? In 2015, the Medicare Payment Advisory Commission (MedPAC) recommended changing the 3-night requirement to require just one of 3 midnights to be inpatient to make a qualifying stay.10 Although an improvement over current law, this proposal would not help the majority of beneficiaries who are exclusively hospitalized under observation status. The “Improving Access to Medicare Coverage Act of 2015”, to be reintroduced in Congress in the coming weeks, would count any midnight spent in the hospital towards the 3-midnight stay requirement, and has bipartisan, bicameral support and cosponsorship.15 In 2015, through unanimous bipartisan, bicameral support, Congress passed the NOTICE Act (PL 114-42), which requires hospitals to inform Medicare beneficiaries hospitalized under observation.16 We believe that the data are clear to both sides of the aisle that Congress should now work together using scientifically-supported research to improve the exact observation policies they felt patients should be informed of. Passing the Improving Access to Medicare Coverage Act is the logical next step in this arena.
Medicare was intended to give seniors access to the healthcare they need. Growth in hospital-based observation care begs for modernization of the statutory 3-inpatient midnight rule. Counting all midnights towards the 3-midnight requirement, whether those midnights are outpatient observation or inpatient, is the right first step.
Disclosures
Representative Courtney is the bill sponsor of the Improving Access to Medicare Coverage Act. The authors report no other conflicts.
On July 30, 1965, Lyndon B. Johnson signed H.R. 6675 into law, establishing Medicare and Medicaid as Title XVIII and Title XIX of the Social Security Act.1 Shortly after, Medicare’s “extended care benefit” began, offering Medicare beneficiaries skilled nursing facility (SNF) care after a qualifying stay of 3 or more consecutive inpatient midnights.2 Fifty years later, the word “inpatient” remains embedded in statute, limiting SNF coverage for Medicare beneficiaries hospitalized as outpatients under observation for part or all of a 3-midnight stay.3
At the individual Medicare beneficiary level, the financial impact of this policy is clear. The Office of Inspector General (OIG) reported a $10,503 beneficiary out-of-pocket cost per uncovered SNF stay following an observation hospitalization in 2012.4 But the actual number of Medicare beneficiaries impacted by this coverage gap is unknown. Using 2009 claims data, Feng et al.5 estimated that 0.75% of previously community dwelling Medicare beneficiaries are discharged to a SNF following an observation hospitalization, and the OIG reported 617,702 beneficiary hospital stays of 3 or more midnights not meeting the 3-midnight inpatient requirement in 2012, with 4% of these beneficiaries discharging to SNFs.4 Yet these studies based on Medicare claims data only capture actual SNF utilization, failing to answer the critical question: How many Medicare beneficiaries need, but forgo, SNF care following a non-qualifying observation hospital stay? In this issue of the Journal of Hospital Medicine, Goldstein et al.6 provide insight to that question. Using chart review of physical therapy and case management recommendations for post-acute SNF care, Goldstein et al.6 compare actual discharge rate to SNF or acute inpatient rehabilitation following an observation stay when such disposition is recommended. In their two-hospital system, fewer than 20% of previously community-dwelling hospitalist patients followed recommendation for post-acute facility stay after observation hospitalization, and more than 40% cited financial concerns as the reason for declining. Patients recommended for SNF also were more likely to be rehospitalized in the subsequent 30 days after discharge, confirming this as a vulnerable patient population. Given Medicare’s original intent to improve health care access for seniors, the case for change seems clear, and the repercussions of not addressing the plight of patients hospitalized under observation is having negative financial and overall detrimental health impacts.
But there are other compelling reasons why this 50-year-old law needs to be improved. Hospital care today is vastly different than when Medicare became law. Average hospital length of stay for patients 65 years and older was 14.2 days in 19657 compared to 5.2 days today,8 clearly a shift in what 3 days of hospital care means. Most importantly, observation stays have become a major part of hospital care. Between 2006 and 2014, per-beneficiary outpatient visits (which include all observation stays) increased 44.2% nationally, while inpatient discharges decreased 19.9%.9 In 2012, the Centers for Medicare & Medicaid Services (CMS) received 1.7 million outpatient observation claims and an additional 700,000 inpatient claims that started with observation days.10 CMS also expected the 2-midnight rule to reduce outpatient observation stays,4 but a recent OIG report11 found that outpatient stays increased 8.1% in the first year (FY 2014) under the new rule, and there were still 748,337 long observation stays (those lasting 2 midnights or longer) in 2014, only a small (2.8%) decrease from the prior year. These factors limit Medicare beneficiary post–acute SNF eligibility in ways that could not have been anticipated when the extended care benefit was created to help seniors access needed health care.
Policymakers must consider cost when considering statutory change. Waiver programs in the 1980s suspending the 3-midnight requirement raised concerns over potential increase in both SNF utilization and associated costs.12 However, more recent data suggest that altering the 3-midnight requirement may not increase post-acute SNF utilization. From 2006 to 2010, Medicare Advantage programs that waived the 3-midnight requirement saw a decrease in hospital length of stay without increased SNF utilization or SNF length of stay, indicating that access to the right level of care at the right time could be cost-saving.13 Recent data from the Bundled Payments for Care Improvement (BPCI) program found savings were largely related to decreased SNF utilization when payments were episode-based,14 a trend that may continue as Medicare moves away from fee-for-service towards bundled payments for more conditions. And although neither example directly tests changing the 3-midnight requirement to include observation midnights, both studies suggest that innovative health care delivery and modification of SNF access did not result in increased SNF utilization or greater post-acute costs. In fact, as Goldstein et al.6 showed, patients recommended for post-acute SNF following observation stay were more likely to be rehospitalized within 30 days, an additional cost that could potentially be avoided if these patients had SNF access. We believe that these correlations strongly support rescinding the 3-
That being said, what can be done? In 2015, the Medicare Payment Advisory Commission (MedPAC) recommended changing the 3-night requirement to require just one of 3 midnights to be inpatient to make a qualifying stay.10 Although an improvement over current law, this proposal would not help the majority of beneficiaries who are exclusively hospitalized under observation status. The “Improving Access to Medicare Coverage Act of 2015”, to be reintroduced in Congress in the coming weeks, would count any midnight spent in the hospital towards the 3-midnight stay requirement, and has bipartisan, bicameral support and cosponsorship.15 In 2015, through unanimous bipartisan, bicameral support, Congress passed the NOTICE Act (PL 114-42), which requires hospitals to inform Medicare beneficiaries hospitalized under observation.16 We believe that the data are clear to both sides of the aisle that Congress should now work together using scientifically-supported research to improve the exact observation policies they felt patients should be informed of. Passing the Improving Access to Medicare Coverage Act is the logical next step in this arena.
Medicare was intended to give seniors access to the healthcare they need. Growth in hospital-based observation care begs for modernization of the statutory 3-inpatient midnight rule. Counting all midnights towards the 3-midnight requirement, whether those midnights are outpatient observation or inpatient, is the right first step.
Disclosures
Representative Courtney is the bill sponsor of the Improving Access to Medicare Coverage Act. The authors report no other conflicts.
1. Medicare & Medicaid Milestones 1937-2015. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf . Accessed September 25, 2016.
2. Loewenstein R. Early effects of Medicare on the health care of the aged. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf. Accessed September 25, 2016.
3. US Social Security Act, Sec. 1861 (i). [42 U.S.C. 1395x]. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. Accessed September 25, 2016.
4. Department of Health and Human Services Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. Available at: https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed September 25, 2016.
5. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care 2014;52:796-800. PubMed
6. Goldstein JN, Schwartz JS, McGraw P, Banks TL, Hicks LS. The unmet need for postacute rehabilitation among medicare observation patients: a single-center study. J Hosp Med. 2017;12(3):168-172.
7. Vital and Health Statistics. Trends in hospital utilization: United States, 1965-1986. https://www.cdc.gov/nchs/data/series/sr_13/sr13_101.pdf. Accessed September 25, 2016.
8. Healthcare Cost and Utilization Project (HCUP). Statistical brief #180. Overview of hospital stays in the United States, 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed September 25, 2016.
9. MedPAC March 2016 Report to the Congress. Chapter 3. Hospital inpatient and outpatient services. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed September 25, 2016.
10. MedPAC. June 2015 Report to the Congress. Chapter 7: Hospital short-stay policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-7-hospital-short-stay-policy-issues-june-2015-report-.pdf?sfvrsn=0 Accessed September 25, 2016.
11. Department of Health and Human Services Office of Inspector General. Vulnerabilities remain under Medicare’s 2-midnight hospital policy, OEI-02-15-00020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed February 19, 2017.
12. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310: 1441-1442. PubMed
13. Grebela R, Keohane L Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs. 2015;34:1324-1330. PubMed
14. Dummit L, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. PubMed
15. HR. 1571 Improving Access to Medicare Coverage Act of 2015. https://www.govtrack.us/congress/bills/114/hr1571/text. Accessed September 25, 2016.
16. PL 114-42. The NOTICE Act. https://www.govtrack.us/congress/bills/114/hr876. Accessed September 25, 2016.
1. Medicare & Medicaid Milestones 1937-2015. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf . Accessed September 25, 2016.
2. Loewenstein R. Early effects of Medicare on the health care of the aged. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf. Accessed September 25, 2016.
3. US Social Security Act, Sec. 1861 (i). [42 U.S.C. 1395x]. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. Accessed September 25, 2016.
4. Department of Health and Human Services Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. Available at: https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed September 25, 2016.
5. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care 2014;52:796-800. PubMed
6. Goldstein JN, Schwartz JS, McGraw P, Banks TL, Hicks LS. The unmet need for postacute rehabilitation among medicare observation patients: a single-center study. J Hosp Med. 2017;12(3):168-172.
7. Vital and Health Statistics. Trends in hospital utilization: United States, 1965-1986. https://www.cdc.gov/nchs/data/series/sr_13/sr13_101.pdf. Accessed September 25, 2016.
8. Healthcare Cost and Utilization Project (HCUP). Statistical brief #180. Overview of hospital stays in the United States, 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed September 25, 2016.
9. MedPAC March 2016 Report to the Congress. Chapter 3. Hospital inpatient and outpatient services. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed September 25, 2016.
10. MedPAC. June 2015 Report to the Congress. Chapter 7: Hospital short-stay policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-7-hospital-short-stay-policy-issues-june-2015-report-.pdf?sfvrsn=0 Accessed September 25, 2016.
11. Department of Health and Human Services Office of Inspector General. Vulnerabilities remain under Medicare’s 2-midnight hospital policy, OEI-02-15-00020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed February 19, 2017.
12. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310: 1441-1442. PubMed
13. Grebela R, Keohane L Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs. 2015;34:1324-1330. PubMed
14. Dummit L, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. PubMed
15. HR. 1571 Improving Access to Medicare Coverage Act of 2015. https://www.govtrack.us/congress/bills/114/hr1571/text. Accessed September 25, 2016.
16. PL 114-42. The NOTICE Act. https://www.govtrack.us/congress/bills/114/hr876. Accessed September 25, 2016.
© 2017 Society of Hospital Medicine
In reference to “When personality is the problem: Managing patients with difficult personalities on the acute care unit"
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
© 2017 Society of Hospital Medicine
The authors reply, “When personality is the problem: Managing patients with difficult personalities on the acute care unit”
Thank you for the opportunity to reply to Dr. Hunasikatti’s comments regarding our article.1 He brings up some excellent points and we appreciate the opportunity to clarify.
With regards to our example of Cluster A personality, the elderly individual with paranoia, we agree that the differential must include delirium and dementia and an appropriate work-up completed. The intent of the vignette was to illustrate a functional but eccentric individual with paranoid beliefs. The paranoia associated with paranoid personality disorder is classically not responsive to medications—nor are patients typically amenable to such treatment—and behavioral interventions remain paramount, minimizing the negative impact of paranoia on the individual’s care.2,3
Regarding Ms. B, the vignette stated that the pain service was consulted, as Dr. Hunasikatti suggested it should be, but despite aggressive pain control, requests for opiates continued. We agree that appropriate pain management is critical in management of all patients, and pain can exacerbate behavioral issues when insufficiently treated. However, individuals who look to external sources of comfort may continue to request pain medications beyond what is clinically prudent and can benefit from learning additional skills to self-soothe and manage the psychological aspects of pain.4,5
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Hayward BA. Cluster A personality disorders: considering the 'odd-eccentric' in psychiatric nursing. Int J Ment Health Nurs. 2007;16(1):15-21. PubMed
3. Ward RK. Assessment and management of personality disorders. Am Family Physician. 2004;70(8):1505-1512. PubMed
4. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). 2007;4(4):40-46. PubMed
5. Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-152. PubMed
Thank you for the opportunity to reply to Dr. Hunasikatti’s comments regarding our article.1 He brings up some excellent points and we appreciate the opportunity to clarify.
With regards to our example of Cluster A personality, the elderly individual with paranoia, we agree that the differential must include delirium and dementia and an appropriate work-up completed. The intent of the vignette was to illustrate a functional but eccentric individual with paranoid beliefs. The paranoia associated with paranoid personality disorder is classically not responsive to medications—nor are patients typically amenable to such treatment—and behavioral interventions remain paramount, minimizing the negative impact of paranoia on the individual’s care.2,3
Regarding Ms. B, the vignette stated that the pain service was consulted, as Dr. Hunasikatti suggested it should be, but despite aggressive pain control, requests for opiates continued. We agree that appropriate pain management is critical in management of all patients, and pain can exacerbate behavioral issues when insufficiently treated. However, individuals who look to external sources of comfort may continue to request pain medications beyond what is clinically prudent and can benefit from learning additional skills to self-soothe and manage the psychological aspects of pain.4,5
Thank you for the opportunity to reply to Dr. Hunasikatti’s comments regarding our article.1 He brings up some excellent points and we appreciate the opportunity to clarify.
With regards to our example of Cluster A personality, the elderly individual with paranoia, we agree that the differential must include delirium and dementia and an appropriate work-up completed. The intent of the vignette was to illustrate a functional but eccentric individual with paranoid beliefs. The paranoia associated with paranoid personality disorder is classically not responsive to medications—nor are patients typically amenable to such treatment—and behavioral interventions remain paramount, minimizing the negative impact of paranoia on the individual’s care.2,3
Regarding Ms. B, the vignette stated that the pain service was consulted, as Dr. Hunasikatti suggested it should be, but despite aggressive pain control, requests for opiates continued. We agree that appropriate pain management is critical in management of all patients, and pain can exacerbate behavioral issues when insufficiently treated. However, individuals who look to external sources of comfort may continue to request pain medications beyond what is clinically prudent and can benefit from learning additional skills to self-soothe and manage the psychological aspects of pain.4,5
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Hayward BA. Cluster A personality disorders: considering the 'odd-eccentric' in psychiatric nursing. Int J Ment Health Nurs. 2007;16(1):15-21. PubMed
3. Ward RK. Assessment and management of personality disorders. Am Family Physician. 2004;70(8):1505-1512. PubMed
4. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). 2007;4(4):40-46. PubMed
5. Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-152. PubMed
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Hayward BA. Cluster A personality disorders: considering the 'odd-eccentric' in psychiatric nursing. Int J Ment Health Nurs. 2007;16(1):15-21. PubMed
3. Ward RK. Assessment and management of personality disorders. Am Family Physician. 2004;70(8):1505-1512. PubMed
4. Sansone RA, Sansone LA. Borderline personality and the pain paradox. Psychiatry (Edgmont). 2007;4(4):40-46. PubMed
5. Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87(1):144-152. PubMed
© 2017 Society of Hospital Medicine
FDA expands approved indication for lenalidomide
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid).
The drug is now approved for use as maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with multiple myeloma (MM).
The expanded indication makes lenalidomide the first treatment to receive FDA approval for maintenance following auto-HSCT.
The drug was previously FDA-approved for use in combination with dexamethasone to treat patients with MM.
Lenalidomide is also FDA-approved to treat patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes associated with deletion 5q, with or without additional cytogenetic abnormalities.
And lenalidomide is FDA-approved to treat patients with mantle cell lymphoma who have relapsed or progressed after 2 prior therapies, one of which included bortezomib.
Lenalidomide is a product of Celgene.
Studies: Lenalidomide maintenance
The latest approval for lenalidomide was based on results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211.
Results from both studies were published in NEJM in May 2012 (CALGB 100104, IFM 2005-02). The updated data reported here are included in the prescribing information for lenalidomide.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing auto-HSCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen after auto-HSCT, which consisted of lenalidomide monotherapy followed by continuous daily treatment with lenalidomide or placebo until relapse.
Survival
In both studies, the primary efficacy endpoint was progression-free survival (PFS). The PFS data for both studies were updated to reflect results as of March 2015.
In the CALGB study, the median PFS was 5.7 years in the lenalidomide arm and 1.9 years in the placebo arm (hazard ratio [HR]=0.38 [95% CI: 0.28-0.50]).
In the IFM study, the median PFS was 3.9 years in the lenalidomide arm and 2 years in the placebo arm (HR=0.53 [95% CI: 0.44-0.64]).
These studies were not powered for an overall survival (OS) endpoint. However, OS was recorded, and the OS data for both studies were updated to reflect results as of February 2016.
The median OS in the CALGB study was 9.3 years in the lenalidomide arm and 7 years in the placebo arm (HR=0.59 [95% CI: 0.44-0.78]).
In the IFM study, the median OS was 8.8 years in the lenalidomide arm and 7.3 years in the placebo arm (HR=0.90 [95% CI: 0.72-1.13]).
Adverse events
The most frequently reported adverse events in ≥20% of patients in the lenalidomide arm across both studies (CALGB and IFM, respectively) were neutropenia (79%, 61%), thrombocytopenia (72%, 24%), leukopenia (23%, 32%), anemia (21%, 9%), upper respiratory tract infection (27%, 11%), bronchitis (5%, 47%), nasopharyngitis (2%, 35%), cough (10%, 27%), gastroenteritis (0%, 23%), diarrhea (55%, 39%), rash (32%, 8%), fatigue (23%, 11%), asthenia (0%, 30%), muscle spasm (0%, 33%), and pyrexia (8%, 21%).
The most frequently reported grade 3/4 events (more than 20% in the lenalidomide arm) were neutropenia, thrombocytopenia, and leukopenia.
Hematologic second primary malignancies (SPM) occurred in 7.5% of patients receiving lenalidomide maintenance and 3.3% of controls.
The incidence of hematologic plus solid tumor SPM (excluding squamous cell carcinoma and basal cell carcinoma) was 14.9% in the lenalidomide group and 8.8% in the control group, with a median follow-up of 91.5 months.
Non-melanoma skin cancer SPM, including squamous cell carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving lenalidomide maintenance and 2.6% of controls. ![]()
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid).
The drug is now approved for use as maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with multiple myeloma (MM).
The expanded indication makes lenalidomide the first treatment to receive FDA approval for maintenance following auto-HSCT.
The drug was previously FDA-approved for use in combination with dexamethasone to treat patients with MM.
Lenalidomide is also FDA-approved to treat patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes associated with deletion 5q, with or without additional cytogenetic abnormalities.
And lenalidomide is FDA-approved to treat patients with mantle cell lymphoma who have relapsed or progressed after 2 prior therapies, one of which included bortezomib.
Lenalidomide is a product of Celgene.
Studies: Lenalidomide maintenance
The latest approval for lenalidomide was based on results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211.
Results from both studies were published in NEJM in May 2012 (CALGB 100104, IFM 2005-02). The updated data reported here are included in the prescribing information for lenalidomide.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing auto-HSCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen after auto-HSCT, which consisted of lenalidomide monotherapy followed by continuous daily treatment with lenalidomide or placebo until relapse.
Survival
In both studies, the primary efficacy endpoint was progression-free survival (PFS). The PFS data for both studies were updated to reflect results as of March 2015.
In the CALGB study, the median PFS was 5.7 years in the lenalidomide arm and 1.9 years in the placebo arm (hazard ratio [HR]=0.38 [95% CI: 0.28-0.50]).
In the IFM study, the median PFS was 3.9 years in the lenalidomide arm and 2 years in the placebo arm (HR=0.53 [95% CI: 0.44-0.64]).
These studies were not powered for an overall survival (OS) endpoint. However, OS was recorded, and the OS data for both studies were updated to reflect results as of February 2016.
The median OS in the CALGB study was 9.3 years in the lenalidomide arm and 7 years in the placebo arm (HR=0.59 [95% CI: 0.44-0.78]).
In the IFM study, the median OS was 8.8 years in the lenalidomide arm and 7.3 years in the placebo arm (HR=0.90 [95% CI: 0.72-1.13]).
Adverse events
The most frequently reported adverse events in ≥20% of patients in the lenalidomide arm across both studies (CALGB and IFM, respectively) were neutropenia (79%, 61%), thrombocytopenia (72%, 24%), leukopenia (23%, 32%), anemia (21%, 9%), upper respiratory tract infection (27%, 11%), bronchitis (5%, 47%), nasopharyngitis (2%, 35%), cough (10%, 27%), gastroenteritis (0%, 23%), diarrhea (55%, 39%), rash (32%, 8%), fatigue (23%, 11%), asthenia (0%, 30%), muscle spasm (0%, 33%), and pyrexia (8%, 21%).
The most frequently reported grade 3/4 events (more than 20% in the lenalidomide arm) were neutropenia, thrombocytopenia, and leukopenia.
Hematologic second primary malignancies (SPM) occurred in 7.5% of patients receiving lenalidomide maintenance and 3.3% of controls.
The incidence of hematologic plus solid tumor SPM (excluding squamous cell carcinoma and basal cell carcinoma) was 14.9% in the lenalidomide group and 8.8% in the control group, with a median follow-up of 91.5 months.
Non-melanoma skin cancer SPM, including squamous cell carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving lenalidomide maintenance and 2.6% of controls. ![]()
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid).
The drug is now approved for use as maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with multiple myeloma (MM).
The expanded indication makes lenalidomide the first treatment to receive FDA approval for maintenance following auto-HSCT.
The drug was previously FDA-approved for use in combination with dexamethasone to treat patients with MM.
Lenalidomide is also FDA-approved to treat patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes associated with deletion 5q, with or without additional cytogenetic abnormalities.
And lenalidomide is FDA-approved to treat patients with mantle cell lymphoma who have relapsed or progressed after 2 prior therapies, one of which included bortezomib.
Lenalidomide is a product of Celgene.
Studies: Lenalidomide maintenance
The latest approval for lenalidomide was based on results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211.
Results from both studies were published in NEJM in May 2012 (CALGB 100104, IFM 2005-02). The updated data reported here are included in the prescribing information for lenalidomide.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing auto-HSCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen after auto-HSCT, which consisted of lenalidomide monotherapy followed by continuous daily treatment with lenalidomide or placebo until relapse.
Survival
In both studies, the primary efficacy endpoint was progression-free survival (PFS). The PFS data for both studies were updated to reflect results as of March 2015.
In the CALGB study, the median PFS was 5.7 years in the lenalidomide arm and 1.9 years in the placebo arm (hazard ratio [HR]=0.38 [95% CI: 0.28-0.50]).
In the IFM study, the median PFS was 3.9 years in the lenalidomide arm and 2 years in the placebo arm (HR=0.53 [95% CI: 0.44-0.64]).
These studies were not powered for an overall survival (OS) endpoint. However, OS was recorded, and the OS data for both studies were updated to reflect results as of February 2016.
The median OS in the CALGB study was 9.3 years in the lenalidomide arm and 7 years in the placebo arm (HR=0.59 [95% CI: 0.44-0.78]).
In the IFM study, the median OS was 8.8 years in the lenalidomide arm and 7.3 years in the placebo arm (HR=0.90 [95% CI: 0.72-1.13]).
Adverse events
The most frequently reported adverse events in ≥20% of patients in the lenalidomide arm across both studies (CALGB and IFM, respectively) were neutropenia (79%, 61%), thrombocytopenia (72%, 24%), leukopenia (23%, 32%), anemia (21%, 9%), upper respiratory tract infection (27%, 11%), bronchitis (5%, 47%), nasopharyngitis (2%, 35%), cough (10%, 27%), gastroenteritis (0%, 23%), diarrhea (55%, 39%), rash (32%, 8%), fatigue (23%, 11%), asthenia (0%, 30%), muscle spasm (0%, 33%), and pyrexia (8%, 21%).
The most frequently reported grade 3/4 events (more than 20% in the lenalidomide arm) were neutropenia, thrombocytopenia, and leukopenia.
Hematologic second primary malignancies (SPM) occurred in 7.5% of patients receiving lenalidomide maintenance and 3.3% of controls.
The incidence of hematologic plus solid tumor SPM (excluding squamous cell carcinoma and basal cell carcinoma) was 14.9% in the lenalidomide group and 8.8% in the control group, with a median follow-up of 91.5 months.
Non-melanoma skin cancer SPM, including squamous cell carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving lenalidomide maintenance and 2.6% of controls. ![]()
VZV vaccine reduces HZ incidence after HSCT
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes zoster (HZ) in patients who have undergone autologous hematopoietic stem cell transplant (HSCT).
V212 reduced the hazard rate of HZ by an estimated 64% compared to placebo.
The vaccine also reduced the incidence of moderate-to-severe HZ pain and other HZ-related complications, such as hospitalization.
The overall incidence of adverse events (AEs) and the incidence of serious AEs were similar among vaccinated patients and those who received placebo.
Drew J. Winston, MD, of the University of California Los Angeles Medical Center, presented these results as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 6). The trial was sponsored by Merck, the company developing V212.
Treatment
This randomized, double-blind trial enrolled 1230 patients age 18 and older who were undergoing HSCT for any indication and had a history of varicella infection and/or were seropositive for VZV antibody.
The patients were randomized to receive:
- A 4-dose regimen of V212 (n=560) from a consistency lot (a lot having a targeted potency as required by regulators in order to demonstrate that the vaccine can be manufactured consistently)
- A 4-dose regimen of V212 (n=106) from a high-antigen lot (a lot having a higher antigen potency added to assess the safety profile of V212)
- Placebo (n=564).
Randomization was stratified by age (< 50 years vs ≥ 50 years) and by intended duration of post-transplant antiviral prophylaxis (≤3 months vs >3 to 6 months).
Dose 1 of V212 or placebo was given within approximately 30 days before HSCT, and doses 2, 3, and 4 were given approximately 30, 60, and 90 days after HSCT.
Patient characteristics
The median patient age was 57 (range, 19-76) for the consistency lot group, 56 (range, 21-75) for the high-antigen lot group, and 56 (range, 19-79) for the placebo group.
Underlying diseases were non-Hodgkin lymphoma (42%, 40%, and 44%, respectively), Hodgkin lymphoma (10%, 9%, and 9%, respectively), multiple myeloma (44%, 47% and 41%, respectively), acute leukemia (2%, 1%, and 2%, respectively), and “other” diseases (2%, 3% and 4%, respectively).
Roughly 30% of patients in each group received anti-viral agents for 3 months or less after HSCT. Twenty percent to 25% received antiviral therapy for more than 3 months to 6 months.
Thirty-seven percent to 40% received antiviral agents for more than 6 months. And 7% to 12% of patients did not receive any antiviral therapy.
HZ incidence
The average follow-up time for HZ surveillance was approximately 2.3 years (median: 2.6 years) post-vaccination.
Confirmed HZ occurred in 42 of the 538 patients who received V212 from a consistency lot and 113 of the 535 patients who received placebo. (Patients receiving V212 from a high-antigen lot were only included in the safety analysis.)
The estimated efficacy of V212 was 63.8% after adjustment for patient age and the duration of antiviral prophylaxis. Vaccine efficacy against HZ was defined as the relative reduction of hazard rate of HZ in vaccine recipients compared with placebo recipients.
The vaccine met the pre-specified criterion for success, as the lower bound of the 95% confidence interval (CI) was greater than 25%. The 95% CI was 48.4% to 74.6% (P<0.0001).
“The study demonstrates that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr Winston said.
Pain, PHN, and other complications
V212 also reduced the incidence of moderate-to-severe HZ pain—according to the Zoster Brief Pain Inventory (ZBPI) score—by an estimated 69.5% (95% CI, 0.490-0.818).
Nineteen patients in the V212 consistency lot group had moderate-to-severe pain, as did 61 placebo-treated patients.
V212 conferred an estimated 83.7% (95% CI, 0.446-0.952) reduction in the incidence of post-herpetic neuralgia (PHN). Three patients in the V212 consistency lot group and 18 patients in the placebo group had PHN.
PHN was defined as pain in the area of the HZ rash with a “worst pain in the last 24 hours” score of 3 or greater (on a 0-10 scale) on the ZBPI that persists or appears 90 days or beyond after HZ rash onset following HSCT.
Patients who received V212 also saw an estimated 73.5% (95% CI, 0.498-0.860) reduction in “other” HZ complications. Twelve patients in the V212 consistency group and 44 in the placebo group had such complications.
“Other” complications included hospitalization or prolongation of hospitalization due to HZ, disseminated HZ (including disseminated HZ rash or VZV viremia), visceral HZ, ophthalmic HZ, neurological impairment due to HZ, and the administration of intravenous acyclovir therapy for the treatment of HZ post-HSCT.
Safety
All patients who received at least 1 dose of the vaccine or placebo and had follow-up data were included in the safety analysis. Patients were followed for AEs up to 28 days after the fourth vaccination dose.
AEs occurred in 97% of patients who received the vaccine (consistency and high-antigen groups assessed together) and 96.9% of placebo-treated patients. Vaccine-related AEs occurred in 32.6% and 12.6%, respectively.
“Of course, in this population of autologous stem cell transplant patients, adverse events of any type were very common in almost all patients,” Dr Winston said. “However, vaccine-related adverse events were greater in the vaccine recipients compared to the placebo patients, but this was primarily due to an increased incidence of injection-site adverse events in the vaccine recipients.”
Injection-site reactions occurred in 191 vaccinated patients and 36 placebo-treated patients.
The most common systemic AEs—in vaccinated and placebo-treated patients, respectively—were diarrhea (60.1% and 61.9%), nausea (56.5% and 57.8%), pyrexia (49.8% and 46.9%), mucosal inflammation (39.7% and 41.7%), thrombocytopenia (36.4% and 38.4%), febrile neutropenia (33.9% and 28.3%), vomiting (32.6% and 36.6%), anemia (26.6% and 24.4%), neutropenia (25.1% and 23.5%), decreased appetite (23.1% and 23.8%), fatigue (21.8% and 20.7%), hypokalemia (21.3% and 19.9%), and constipation (16.1% and 18.4%).
The incidence of serious AEs was 32.9% in vaccinated patients and 32.7% in the placebo group. The incidence of serious vaccine-related AEs was 0.8% and 0.9%, respectively.
The most common serious AEs—in vaccinated and placebo-treated patients, respectively—were infection (12.3% and 11.9%), relapsed malignancy (7.8% for both), febrile neutropenia (5.3% and 4.9%), pyrexia (3.2% and 4.0%), gastrointestinal disorders (3.2% and 3.6%), respiratory failure (2.7% and 2.2%), cardiac disorders (1.7% and 1.6%), and mucositis (1.2% and 0.9%).
Death occurred in 6.2% of vaccinated patients and 6.3% of placebo-treated patients. Three percent and 3.1%, respectively, discontinued the study due to AEs. ![]()
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes zoster (HZ) in patients who have undergone autologous hematopoietic stem cell transplant (HSCT).
V212 reduced the hazard rate of HZ by an estimated 64% compared to placebo.
The vaccine also reduced the incidence of moderate-to-severe HZ pain and other HZ-related complications, such as hospitalization.
The overall incidence of adverse events (AEs) and the incidence of serious AEs were similar among vaccinated patients and those who received placebo.
Drew J. Winston, MD, of the University of California Los Angeles Medical Center, presented these results as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 6). The trial was sponsored by Merck, the company developing V212.
Treatment
This randomized, double-blind trial enrolled 1230 patients age 18 and older who were undergoing HSCT for any indication and had a history of varicella infection and/or were seropositive for VZV antibody.
The patients were randomized to receive:
- A 4-dose regimen of V212 (n=560) from a consistency lot (a lot having a targeted potency as required by regulators in order to demonstrate that the vaccine can be manufactured consistently)
- A 4-dose regimen of V212 (n=106) from a high-antigen lot (a lot having a higher antigen potency added to assess the safety profile of V212)
- Placebo (n=564).
Randomization was stratified by age (< 50 years vs ≥ 50 years) and by intended duration of post-transplant antiviral prophylaxis (≤3 months vs >3 to 6 months).
Dose 1 of V212 or placebo was given within approximately 30 days before HSCT, and doses 2, 3, and 4 were given approximately 30, 60, and 90 days after HSCT.
Patient characteristics
The median patient age was 57 (range, 19-76) for the consistency lot group, 56 (range, 21-75) for the high-antigen lot group, and 56 (range, 19-79) for the placebo group.
Underlying diseases were non-Hodgkin lymphoma (42%, 40%, and 44%, respectively), Hodgkin lymphoma (10%, 9%, and 9%, respectively), multiple myeloma (44%, 47% and 41%, respectively), acute leukemia (2%, 1%, and 2%, respectively), and “other” diseases (2%, 3% and 4%, respectively).
Roughly 30% of patients in each group received anti-viral agents for 3 months or less after HSCT. Twenty percent to 25% received antiviral therapy for more than 3 months to 6 months.
Thirty-seven percent to 40% received antiviral agents for more than 6 months. And 7% to 12% of patients did not receive any antiviral therapy.
HZ incidence
The average follow-up time for HZ surveillance was approximately 2.3 years (median: 2.6 years) post-vaccination.
Confirmed HZ occurred in 42 of the 538 patients who received V212 from a consistency lot and 113 of the 535 patients who received placebo. (Patients receiving V212 from a high-antigen lot were only included in the safety analysis.)
The estimated efficacy of V212 was 63.8% after adjustment for patient age and the duration of antiviral prophylaxis. Vaccine efficacy against HZ was defined as the relative reduction of hazard rate of HZ in vaccine recipients compared with placebo recipients.
The vaccine met the pre-specified criterion for success, as the lower bound of the 95% confidence interval (CI) was greater than 25%. The 95% CI was 48.4% to 74.6% (P<0.0001).
“The study demonstrates that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr Winston said.
Pain, PHN, and other complications
V212 also reduced the incidence of moderate-to-severe HZ pain—according to the Zoster Brief Pain Inventory (ZBPI) score—by an estimated 69.5% (95% CI, 0.490-0.818).
Nineteen patients in the V212 consistency lot group had moderate-to-severe pain, as did 61 placebo-treated patients.
V212 conferred an estimated 83.7% (95% CI, 0.446-0.952) reduction in the incidence of post-herpetic neuralgia (PHN). Three patients in the V212 consistency lot group and 18 patients in the placebo group had PHN.
PHN was defined as pain in the area of the HZ rash with a “worst pain in the last 24 hours” score of 3 or greater (on a 0-10 scale) on the ZBPI that persists or appears 90 days or beyond after HZ rash onset following HSCT.
Patients who received V212 also saw an estimated 73.5% (95% CI, 0.498-0.860) reduction in “other” HZ complications. Twelve patients in the V212 consistency group and 44 in the placebo group had such complications.
“Other” complications included hospitalization or prolongation of hospitalization due to HZ, disseminated HZ (including disseminated HZ rash or VZV viremia), visceral HZ, ophthalmic HZ, neurological impairment due to HZ, and the administration of intravenous acyclovir therapy for the treatment of HZ post-HSCT.
Safety
All patients who received at least 1 dose of the vaccine or placebo and had follow-up data were included in the safety analysis. Patients were followed for AEs up to 28 days after the fourth vaccination dose.
AEs occurred in 97% of patients who received the vaccine (consistency and high-antigen groups assessed together) and 96.9% of placebo-treated patients. Vaccine-related AEs occurred in 32.6% and 12.6%, respectively.
“Of course, in this population of autologous stem cell transplant patients, adverse events of any type were very common in almost all patients,” Dr Winston said. “However, vaccine-related adverse events were greater in the vaccine recipients compared to the placebo patients, but this was primarily due to an increased incidence of injection-site adverse events in the vaccine recipients.”
Injection-site reactions occurred in 191 vaccinated patients and 36 placebo-treated patients.
The most common systemic AEs—in vaccinated and placebo-treated patients, respectively—were diarrhea (60.1% and 61.9%), nausea (56.5% and 57.8%), pyrexia (49.8% and 46.9%), mucosal inflammation (39.7% and 41.7%), thrombocytopenia (36.4% and 38.4%), febrile neutropenia (33.9% and 28.3%), vomiting (32.6% and 36.6%), anemia (26.6% and 24.4%), neutropenia (25.1% and 23.5%), decreased appetite (23.1% and 23.8%), fatigue (21.8% and 20.7%), hypokalemia (21.3% and 19.9%), and constipation (16.1% and 18.4%).
The incidence of serious AEs was 32.9% in vaccinated patients and 32.7% in the placebo group. The incidence of serious vaccine-related AEs was 0.8% and 0.9%, respectively.
The most common serious AEs—in vaccinated and placebo-treated patients, respectively—were infection (12.3% and 11.9%), relapsed malignancy (7.8% for both), febrile neutropenia (5.3% and 4.9%), pyrexia (3.2% and 4.0%), gastrointestinal disorders (3.2% and 3.6%), respiratory failure (2.7% and 2.2%), cardiac disorders (1.7% and 1.6%), and mucositis (1.2% and 0.9%).
Death occurred in 6.2% of vaccinated patients and 6.3% of placebo-treated patients. Three percent and 3.1%, respectively, discontinued the study due to AEs. ![]()
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes zoster (HZ) in patients who have undergone autologous hematopoietic stem cell transplant (HSCT).
V212 reduced the hazard rate of HZ by an estimated 64% compared to placebo.
The vaccine also reduced the incidence of moderate-to-severe HZ pain and other HZ-related complications, such as hospitalization.
The overall incidence of adverse events (AEs) and the incidence of serious AEs were similar among vaccinated patients and those who received placebo.
Drew J. Winston, MD, of the University of California Los Angeles Medical Center, presented these results as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 6). The trial was sponsored by Merck, the company developing V212.
Treatment
This randomized, double-blind trial enrolled 1230 patients age 18 and older who were undergoing HSCT for any indication and had a history of varicella infection and/or were seropositive for VZV antibody.
The patients were randomized to receive:
- A 4-dose regimen of V212 (n=560) from a consistency lot (a lot having a targeted potency as required by regulators in order to demonstrate that the vaccine can be manufactured consistently)
- A 4-dose regimen of V212 (n=106) from a high-antigen lot (a lot having a higher antigen potency added to assess the safety profile of V212)
- Placebo (n=564).
Randomization was stratified by age (< 50 years vs ≥ 50 years) and by intended duration of post-transplant antiviral prophylaxis (≤3 months vs >3 to 6 months).
Dose 1 of V212 or placebo was given within approximately 30 days before HSCT, and doses 2, 3, and 4 were given approximately 30, 60, and 90 days after HSCT.
Patient characteristics
The median patient age was 57 (range, 19-76) for the consistency lot group, 56 (range, 21-75) for the high-antigen lot group, and 56 (range, 19-79) for the placebo group.
Underlying diseases were non-Hodgkin lymphoma (42%, 40%, and 44%, respectively), Hodgkin lymphoma (10%, 9%, and 9%, respectively), multiple myeloma (44%, 47% and 41%, respectively), acute leukemia (2%, 1%, and 2%, respectively), and “other” diseases (2%, 3% and 4%, respectively).
Roughly 30% of patients in each group received anti-viral agents for 3 months or less after HSCT. Twenty percent to 25% received antiviral therapy for more than 3 months to 6 months.
Thirty-seven percent to 40% received antiviral agents for more than 6 months. And 7% to 12% of patients did not receive any antiviral therapy.
HZ incidence
The average follow-up time for HZ surveillance was approximately 2.3 years (median: 2.6 years) post-vaccination.
Confirmed HZ occurred in 42 of the 538 patients who received V212 from a consistency lot and 113 of the 535 patients who received placebo. (Patients receiving V212 from a high-antigen lot were only included in the safety analysis.)
The estimated efficacy of V212 was 63.8% after adjustment for patient age and the duration of antiviral prophylaxis. Vaccine efficacy against HZ was defined as the relative reduction of hazard rate of HZ in vaccine recipients compared with placebo recipients.
The vaccine met the pre-specified criterion for success, as the lower bound of the 95% confidence interval (CI) was greater than 25%. The 95% CI was 48.4% to 74.6% (P<0.0001).
“The study demonstrates that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr Winston said.
Pain, PHN, and other complications
V212 also reduced the incidence of moderate-to-severe HZ pain—according to the Zoster Brief Pain Inventory (ZBPI) score—by an estimated 69.5% (95% CI, 0.490-0.818).
Nineteen patients in the V212 consistency lot group had moderate-to-severe pain, as did 61 placebo-treated patients.
V212 conferred an estimated 83.7% (95% CI, 0.446-0.952) reduction in the incidence of post-herpetic neuralgia (PHN). Three patients in the V212 consistency lot group and 18 patients in the placebo group had PHN.
PHN was defined as pain in the area of the HZ rash with a “worst pain in the last 24 hours” score of 3 or greater (on a 0-10 scale) on the ZBPI that persists or appears 90 days or beyond after HZ rash onset following HSCT.
Patients who received V212 also saw an estimated 73.5% (95% CI, 0.498-0.860) reduction in “other” HZ complications. Twelve patients in the V212 consistency group and 44 in the placebo group had such complications.
“Other” complications included hospitalization or prolongation of hospitalization due to HZ, disseminated HZ (including disseminated HZ rash or VZV viremia), visceral HZ, ophthalmic HZ, neurological impairment due to HZ, and the administration of intravenous acyclovir therapy for the treatment of HZ post-HSCT.
Safety
All patients who received at least 1 dose of the vaccine or placebo and had follow-up data were included in the safety analysis. Patients were followed for AEs up to 28 days after the fourth vaccination dose.
AEs occurred in 97% of patients who received the vaccine (consistency and high-antigen groups assessed together) and 96.9% of placebo-treated patients. Vaccine-related AEs occurred in 32.6% and 12.6%, respectively.
“Of course, in this population of autologous stem cell transplant patients, adverse events of any type were very common in almost all patients,” Dr Winston said. “However, vaccine-related adverse events were greater in the vaccine recipients compared to the placebo patients, but this was primarily due to an increased incidence of injection-site adverse events in the vaccine recipients.”
Injection-site reactions occurred in 191 vaccinated patients and 36 placebo-treated patients.
The most common systemic AEs—in vaccinated and placebo-treated patients, respectively—were diarrhea (60.1% and 61.9%), nausea (56.5% and 57.8%), pyrexia (49.8% and 46.9%), mucosal inflammation (39.7% and 41.7%), thrombocytopenia (36.4% and 38.4%), febrile neutropenia (33.9% and 28.3%), vomiting (32.6% and 36.6%), anemia (26.6% and 24.4%), neutropenia (25.1% and 23.5%), decreased appetite (23.1% and 23.8%), fatigue (21.8% and 20.7%), hypokalemia (21.3% and 19.9%), and constipation (16.1% and 18.4%).
The incidence of serious AEs was 32.9% in vaccinated patients and 32.7% in the placebo group. The incidence of serious vaccine-related AEs was 0.8% and 0.9%, respectively.
The most common serious AEs—in vaccinated and placebo-treated patients, respectively—were infection (12.3% and 11.9%), relapsed malignancy (7.8% for both), febrile neutropenia (5.3% and 4.9%), pyrexia (3.2% and 4.0%), gastrointestinal disorders (3.2% and 3.6%), respiratory failure (2.7% and 2.2%), cardiac disorders (1.7% and 1.6%), and mucositis (1.2% and 0.9%).
Death occurred in 6.2% of vaccinated patients and 6.3% of placebo-treated patients. Three percent and 3.1%, respectively, discontinued the study due to AEs. ![]()
Acquired mutations may compromise assay
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug sensitivities of leukemic oncogenes,” researchers reported in Oncotarget.
The researchers said the assay “remains an invaluable tool” for validating activating mutations in primary leukemias.
However, the assay is prone to a previously unreported flaw, where Ba/F3 cells can acquire additional mutations.
This issue was discovered by Kevin Watanabe-Smith, PhD, of Oregon Health & Science University in Portland.
He identified the problem while studying a growth-activating mutation in a patient with T-cell leukemia. (The results of that research were published in Leukemia in April 2016).
“When I was sequencing the patient’s DNA to make sure the original, known mutation is there, I was finding additional, unexpected mutations in the gene that I didn’t put there,” Dr Watanabe-Smith said. “And I was getting different mutations every time.”
“After we saw this in several cases, we knew it was worth further study,” added Cristina Tognon, PhD, of Oregon Health & Science University.
So the researchers decided to study Ba/F3 cell lines with and without 4 mutations (found in 3 cytokine receptors) that have “known transformative capacity,” including:
- CSF2RB R461C, a germline mutation found in a patient with T-cell acute lymphoblastic leukemia (ALL)
- CSF3R T618I, a mutation found in chronic neutrophilic leukemia (CNL) and atypical chronic myelogenous leukemia (aCML)
- CSF3R W791X, also found in CNL and aCML
- IL7R 243InsPPCL, which was found in a patient with B-cell ALL.
The researchers said they observed acquired mutations in 1 of 3 CSF2RB wild-type cell lines that transformed and all 4 CSF3R wild-type cell lines, which all transformed.
Furthermore, most CSF2RB R461C lines (4/5) and CSF3R W791X lines (3/4) had additional acquired mutations. However, the CSF3R T618I lines and the IL7R 243InsPPCL lines did not contain acquired mutations.
The researchers said acquired mutations were observed only in weakly transforming oncogenes, which were defined as mutations with a weaker ability to transform cells (less than 1 in every 200 cells) or a slower time to outgrowth (5 days or longer to reach a 5-fold increase over the initial cell number).
The team also said their findings indicate that the majority of the acquired mutations likely exist before IL-3 withdrawal but only expand to levels detectable by Sanger sequencing in the absence of IL-3.
“The potential impact [of these acquired mutations] is that a non-functional mutation could appear functional, and a researcher could publish results that would not be reproducible,” Dr Watanabe-Smith said.
To overcome this problem, Dr Watanabe-Smith and his colleagues recommended taking an additional step when using the Ba/F3 assay—sequencing outgrown cell lines. They also said additional research is needed to devise methods that reduce the incidence of acquired mutations in such assays. ![]()
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug sensitivities of leukemic oncogenes,” researchers reported in Oncotarget.
The researchers said the assay “remains an invaluable tool” for validating activating mutations in primary leukemias.
However, the assay is prone to a previously unreported flaw, where Ba/F3 cells can acquire additional mutations.
This issue was discovered by Kevin Watanabe-Smith, PhD, of Oregon Health & Science University in Portland.
He identified the problem while studying a growth-activating mutation in a patient with T-cell leukemia. (The results of that research were published in Leukemia in April 2016).
“When I was sequencing the patient’s DNA to make sure the original, known mutation is there, I was finding additional, unexpected mutations in the gene that I didn’t put there,” Dr Watanabe-Smith said. “And I was getting different mutations every time.”
“After we saw this in several cases, we knew it was worth further study,” added Cristina Tognon, PhD, of Oregon Health & Science University.
So the researchers decided to study Ba/F3 cell lines with and without 4 mutations (found in 3 cytokine receptors) that have “known transformative capacity,” including:
- CSF2RB R461C, a germline mutation found in a patient with T-cell acute lymphoblastic leukemia (ALL)
- CSF3R T618I, a mutation found in chronic neutrophilic leukemia (CNL) and atypical chronic myelogenous leukemia (aCML)
- CSF3R W791X, also found in CNL and aCML
- IL7R 243InsPPCL, which was found in a patient with B-cell ALL.
The researchers said they observed acquired mutations in 1 of 3 CSF2RB wild-type cell lines that transformed and all 4 CSF3R wild-type cell lines, which all transformed.
Furthermore, most CSF2RB R461C lines (4/5) and CSF3R W791X lines (3/4) had additional acquired mutations. However, the CSF3R T618I lines and the IL7R 243InsPPCL lines did not contain acquired mutations.
The researchers said acquired mutations were observed only in weakly transforming oncogenes, which were defined as mutations with a weaker ability to transform cells (less than 1 in every 200 cells) or a slower time to outgrowth (5 days or longer to reach a 5-fold increase over the initial cell number).
The team also said their findings indicate that the majority of the acquired mutations likely exist before IL-3 withdrawal but only expand to levels detectable by Sanger sequencing in the absence of IL-3.
“The potential impact [of these acquired mutations] is that a non-functional mutation could appear functional, and a researcher could publish results that would not be reproducible,” Dr Watanabe-Smith said.
To overcome this problem, Dr Watanabe-Smith and his colleagues recommended taking an additional step when using the Ba/F3 assay—sequencing outgrown cell lines. They also said additional research is needed to devise methods that reduce the incidence of acquired mutations in such assays. ![]()
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug sensitivities of leukemic oncogenes,” researchers reported in Oncotarget.
The researchers said the assay “remains an invaluable tool” for validating activating mutations in primary leukemias.
However, the assay is prone to a previously unreported flaw, where Ba/F3 cells can acquire additional mutations.
This issue was discovered by Kevin Watanabe-Smith, PhD, of Oregon Health & Science University in Portland.
He identified the problem while studying a growth-activating mutation in a patient with T-cell leukemia. (The results of that research were published in Leukemia in April 2016).
“When I was sequencing the patient’s DNA to make sure the original, known mutation is there, I was finding additional, unexpected mutations in the gene that I didn’t put there,” Dr Watanabe-Smith said. “And I was getting different mutations every time.”
“After we saw this in several cases, we knew it was worth further study,” added Cristina Tognon, PhD, of Oregon Health & Science University.
So the researchers decided to study Ba/F3 cell lines with and without 4 mutations (found in 3 cytokine receptors) that have “known transformative capacity,” including:
- CSF2RB R461C, a germline mutation found in a patient with T-cell acute lymphoblastic leukemia (ALL)
- CSF3R T618I, a mutation found in chronic neutrophilic leukemia (CNL) and atypical chronic myelogenous leukemia (aCML)
- CSF3R W791X, also found in CNL and aCML
- IL7R 243InsPPCL, which was found in a patient with B-cell ALL.
The researchers said they observed acquired mutations in 1 of 3 CSF2RB wild-type cell lines that transformed and all 4 CSF3R wild-type cell lines, which all transformed.
Furthermore, most CSF2RB R461C lines (4/5) and CSF3R W791X lines (3/4) had additional acquired mutations. However, the CSF3R T618I lines and the IL7R 243InsPPCL lines did not contain acquired mutations.
The researchers said acquired mutations were observed only in weakly transforming oncogenes, which were defined as mutations with a weaker ability to transform cells (less than 1 in every 200 cells) or a slower time to outgrowth (5 days or longer to reach a 5-fold increase over the initial cell number).
The team also said their findings indicate that the majority of the acquired mutations likely exist before IL-3 withdrawal but only expand to levels detectable by Sanger sequencing in the absence of IL-3.
“The potential impact [of these acquired mutations] is that a non-functional mutation could appear functional, and a researcher could publish results that would not be reproducible,” Dr Watanabe-Smith said.
To overcome this problem, Dr Watanabe-Smith and his colleagues recommended taking an additional step when using the Ba/F3 assay—sequencing outgrown cell lines. They also said additional research is needed to devise methods that reduce the incidence of acquired mutations in such assays. ![]()