User login
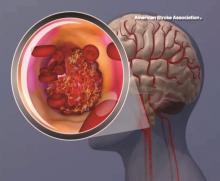
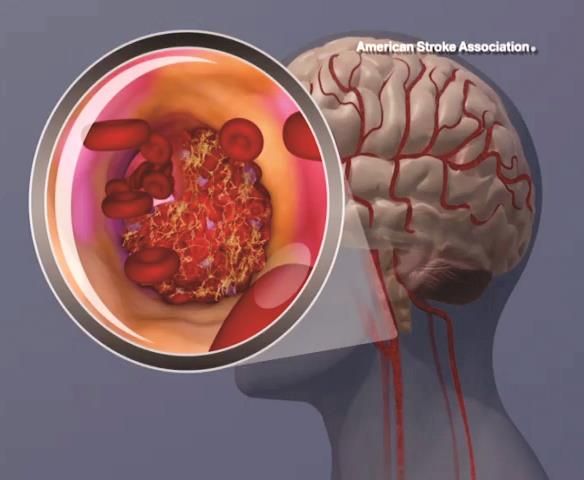
Ticagrelor beats aspirin for recurrent stroke in patients with atherosclerosis
HOUSTON – Ticagrelor outperformed aspirin in preventing a combination of recurrent stroke, heart attack, and death – but only in patients whose index stroke was probably related to atherosclerosis.
The antiplatelet drug reduced the risk of the composite endpoint by 32%, compared with aspirin, in stroke patients with proven ipsilateral atherosclerotic stenosis (hazard ratio, 0.68). But ticagrelor (Brilinta) had no effect at all in those without stenosis (HR, 0.97), Pierre Amarenco, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
The study was simultaneously published in Lancet Neurology (Lancet Neurol. 2017 Feb 23. doi: 10.1016/S1474-4422[17]30038-8). “The interaction that we found suggests what we already know in clinical practice: An understanding of stroke mechanisms and their causes is important to being able to deliver safe and effective treatment of early stroke prevention,” said Dr. Amarenco of Paris-Diderot Sorbonne University, Paris.
The findings come from a preplanned subgroup analysis of the large SOCRATES trial, published in 2016. The study determined that ticagrelor was no better than aspirin in preventing recurrent stroke, heart attack or death in patients who had a transient ischemic attack.
SOCRATES randomized 13,199 patients with a nonsevere ischemic stroke or high-risk transient ischemic attack to ticagrelor (180 mg loading dose on day 1 followed by 90 mg twice daily for days 2-90) or aspirin (300 mg on day 1 followed by 100 mg daily for days 2-90). The primary endpoint was the time to the occurrence of stroke, myocardial infarction, or death within 90 days.
The search for a potentially responsive group made sense, Dr. Amarenco said, because ticagrelor “is an effective antiplatelet therapy in patients with coronary atherosclerotic disease.” Therefore, investigators reasoned, it might be most effective in patients whose strokes were of atherosclerotic origin.
The substudy focused on 3,081 of the patients with proven ipsilateral atherosclerotic stenosis and/or a mobile thrombus or plaque in the aortic arch that was judged to potentially have caused their index stroke. Generally, the atherosclerotic patients were older and more likely to have dyslipidemia, hypertension, diabetes, coronary artery disease, and heart failure than were the patients with strokes of nonatherosclerotic origin. Atherosclerotic patients also were significantly more likely to have had a prior stroke or heart attack.
In the group with atherosclerosis, ticagrelor was significantly more effective at preventing the composite primary endpoint than was aspirin. There were 103 events in 1,542 patients in the ticagrelor group and 147 in 1,539 patients in the aspirin group (6.7% vs. 9.6%) – a “very impressive” risk reduction of 32% (HR, 0.68), Dr. Amarenco said.
In the group of patients without ipsilateral atherosclerotic stenosis, ticagrelor exerted no benefit over aspirin, with an event rate of 6.7% vs. 6.9% (HR, 0.97).
The rate of recurrent ischemic stroke was the driving force behind the significant between-group difference. Ischemic stroke occurred in 6.4% of those taking ticagrelor and 8.5% of those taking aspirin – a significant risk reduction of 27% (HR, 0.73). The drug exerted no benefit for recurrent ischemic stroke over aspirin in the group without atherosclerosis (5.8% vs. 6.1%; HR, 0.93).
There were no differences in the rate of heart attack or death, or in the secondary endpoints of all stroke, disabling stroke, or fatal stroke.
Ticagrelor was not associated with any major bleeding, compared with aspirin in either group, Dr. Amarenco noted.
The higher event rate in the patients with atherosclerosis is not surprising, he said.
“We had the exact same finding in our recent study with TIAregistry.org, which we found that patients with large artery atherosclerosis were at much higher risk than patients with other stroke subtypes.”
Dr. Amarenco disclosed financial relationships with numerous pharmaceutical companies, including AstraZeneca, which sponsored the study.
[email protected]
On Twitter @alz_gal
HOUSTON – Ticagrelor outperformed aspirin in preventing a combination of recurrent stroke, heart attack, and death – but only in patients whose index stroke was probably related to atherosclerosis.
The antiplatelet drug reduced the risk of the composite endpoint by 32%, compared with aspirin, in stroke patients with proven ipsilateral atherosclerotic stenosis (hazard ratio, 0.68). But ticagrelor (Brilinta) had no effect at all in those without stenosis (HR, 0.97), Pierre Amarenco, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
The study was simultaneously published in Lancet Neurology (Lancet Neurol. 2017 Feb 23. doi: 10.1016/S1474-4422[17]30038-8). “The interaction that we found suggests what we already know in clinical practice: An understanding of stroke mechanisms and their causes is important to being able to deliver safe and effective treatment of early stroke prevention,” said Dr. Amarenco of Paris-Diderot Sorbonne University, Paris.
The findings come from a preplanned subgroup analysis of the large SOCRATES trial, published in 2016. The study determined that ticagrelor was no better than aspirin in preventing recurrent stroke, heart attack or death in patients who had a transient ischemic attack.
SOCRATES randomized 13,199 patients with a nonsevere ischemic stroke or high-risk transient ischemic attack to ticagrelor (180 mg loading dose on day 1 followed by 90 mg twice daily for days 2-90) or aspirin (300 mg on day 1 followed by 100 mg daily for days 2-90). The primary endpoint was the time to the occurrence of stroke, myocardial infarction, or death within 90 days.
The search for a potentially responsive group made sense, Dr. Amarenco said, because ticagrelor “is an effective antiplatelet therapy in patients with coronary atherosclerotic disease.” Therefore, investigators reasoned, it might be most effective in patients whose strokes were of atherosclerotic origin.
The substudy focused on 3,081 of the patients with proven ipsilateral atherosclerotic stenosis and/or a mobile thrombus or plaque in the aortic arch that was judged to potentially have caused their index stroke. Generally, the atherosclerotic patients were older and more likely to have dyslipidemia, hypertension, diabetes, coronary artery disease, and heart failure than were the patients with strokes of nonatherosclerotic origin. Atherosclerotic patients also were significantly more likely to have had a prior stroke or heart attack.
In the group with atherosclerosis, ticagrelor was significantly more effective at preventing the composite primary endpoint than was aspirin. There were 103 events in 1,542 patients in the ticagrelor group and 147 in 1,539 patients in the aspirin group (6.7% vs. 9.6%) – a “very impressive” risk reduction of 32% (HR, 0.68), Dr. Amarenco said.
In the group of patients without ipsilateral atherosclerotic stenosis, ticagrelor exerted no benefit over aspirin, with an event rate of 6.7% vs. 6.9% (HR, 0.97).
The rate of recurrent ischemic stroke was the driving force behind the significant between-group difference. Ischemic stroke occurred in 6.4% of those taking ticagrelor and 8.5% of those taking aspirin – a significant risk reduction of 27% (HR, 0.73). The drug exerted no benefit for recurrent ischemic stroke over aspirin in the group without atherosclerosis (5.8% vs. 6.1%; HR, 0.93).
There were no differences in the rate of heart attack or death, or in the secondary endpoints of all stroke, disabling stroke, or fatal stroke.
Ticagrelor was not associated with any major bleeding, compared with aspirin in either group, Dr. Amarenco noted.
The higher event rate in the patients with atherosclerosis is not surprising, he said.
“We had the exact same finding in our recent study with TIAregistry.org, which we found that patients with large artery atherosclerosis were at much higher risk than patients with other stroke subtypes.”
Dr. Amarenco disclosed financial relationships with numerous pharmaceutical companies, including AstraZeneca, which sponsored the study.
[email protected]
On Twitter @alz_gal
HOUSTON – Ticagrelor outperformed aspirin in preventing a combination of recurrent stroke, heart attack, and death – but only in patients whose index stroke was probably related to atherosclerosis.
The antiplatelet drug reduced the risk of the composite endpoint by 32%, compared with aspirin, in stroke patients with proven ipsilateral atherosclerotic stenosis (hazard ratio, 0.68). But ticagrelor (Brilinta) had no effect at all in those without stenosis (HR, 0.97), Pierre Amarenco, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
The study was simultaneously published in Lancet Neurology (Lancet Neurol. 2017 Feb 23. doi: 10.1016/S1474-4422[17]30038-8). “The interaction that we found suggests what we already know in clinical practice: An understanding of stroke mechanisms and their causes is important to being able to deliver safe and effective treatment of early stroke prevention,” said Dr. Amarenco of Paris-Diderot Sorbonne University, Paris.
The findings come from a preplanned subgroup analysis of the large SOCRATES trial, published in 2016. The study determined that ticagrelor was no better than aspirin in preventing recurrent stroke, heart attack or death in patients who had a transient ischemic attack.
SOCRATES randomized 13,199 patients with a nonsevere ischemic stroke or high-risk transient ischemic attack to ticagrelor (180 mg loading dose on day 1 followed by 90 mg twice daily for days 2-90) or aspirin (300 mg on day 1 followed by 100 mg daily for days 2-90). The primary endpoint was the time to the occurrence of stroke, myocardial infarction, or death within 90 days.
The search for a potentially responsive group made sense, Dr. Amarenco said, because ticagrelor “is an effective antiplatelet therapy in patients with coronary atherosclerotic disease.” Therefore, investigators reasoned, it might be most effective in patients whose strokes were of atherosclerotic origin.
The substudy focused on 3,081 of the patients with proven ipsilateral atherosclerotic stenosis and/or a mobile thrombus or plaque in the aortic arch that was judged to potentially have caused their index stroke. Generally, the atherosclerotic patients were older and more likely to have dyslipidemia, hypertension, diabetes, coronary artery disease, and heart failure than were the patients with strokes of nonatherosclerotic origin. Atherosclerotic patients also were significantly more likely to have had a prior stroke or heart attack.
In the group with atherosclerosis, ticagrelor was significantly more effective at preventing the composite primary endpoint than was aspirin. There were 103 events in 1,542 patients in the ticagrelor group and 147 in 1,539 patients in the aspirin group (6.7% vs. 9.6%) – a “very impressive” risk reduction of 32% (HR, 0.68), Dr. Amarenco said.
In the group of patients without ipsilateral atherosclerotic stenosis, ticagrelor exerted no benefit over aspirin, with an event rate of 6.7% vs. 6.9% (HR, 0.97).
The rate of recurrent ischemic stroke was the driving force behind the significant between-group difference. Ischemic stroke occurred in 6.4% of those taking ticagrelor and 8.5% of those taking aspirin – a significant risk reduction of 27% (HR, 0.73). The drug exerted no benefit for recurrent ischemic stroke over aspirin in the group without atherosclerosis (5.8% vs. 6.1%; HR, 0.93).
There were no differences in the rate of heart attack or death, or in the secondary endpoints of all stroke, disabling stroke, or fatal stroke.
Ticagrelor was not associated with any major bleeding, compared with aspirin in either group, Dr. Amarenco noted.
The higher event rate in the patients with atherosclerosis is not surprising, he said.
“We had the exact same finding in our recent study with TIAregistry.org, which we found that patients with large artery atherosclerosis were at much higher risk than patients with other stroke subtypes.”
Dr. Amarenco disclosed financial relationships with numerous pharmaceutical companies, including AstraZeneca, which sponsored the study.
[email protected]
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: The drug cut risk of a combination of recurrent stroke, heart attack, and death by 32% among these patients, but was not better than aspirin for patients without atherosclerosis.
Data source: The subanalysis of the SOCRATES trial, comprising 3,081 patients with atherosclerosis and 10,118 without.
Disclosures: Dr. Amarenco disclosed financial relationships with numerous pharmaceutical companies, including AstraZeneca, which sponsored the study.
Rebecca Gottesman, MD, PhD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Recent increase in subdural hematoma may be linked to antithrombotics
according to data published online Feb. 28 in JAMA.
A retrospective case-control study of 10,010 patients aged 20-89 years with a first-time subdural hematoma, matched by age, sex, and year to 400,380 general controls, showed treatment with a vitamin K antagonist was associated with a 3.69 greater risk of subdural hematoma, compared with controls, and a fourfold increase in risk when used concurrently with an antiplatelet drug (JAMA. 2017;317:836-46. doi: 10.1001/jama.2017.0639).
Low-dose aspirin alone was associated with a 24% increase in the risk of subdural hematoma; clopidogrel was associated with an 87% increase; and a direct oral anticoagulant such as dabigatran etexilate, rivaroxaban, or apixaban was associated with a 73% increase in risk.
Antithrombotic drugs were also associated with an increased risk of death from a subdural hematoma within 30 days after discharge for the hematoma’s diagnosis, an effect most evident with a direct oral anticoagulant or vitamin K antagonist.
Over the course of the Danish population-based study, which covered 2000-2015, the prevalence of antithrombotic drug use more than doubled, from 31 individuals per 1,000 to 76.9 per 1,000.
At the same time, the incidence of subdural hematoma nearly doubled (10.9 per 100,000 person-years to 19 per 100,000 person-years). The increase in subdural hematoma was greatest among older patients, from 55.1 per 100,000 person-years to 99.7 per 100,000 person-years.
“Although use of antithrombotic drugs has long been recognized as a risk factor for subdural hematoma, previous studies were either based exclusively on patients with subdural hematoma (i.e.,with no comparison group) or focused exclusively on patients treated with an anticoagulant,” wrote David Gaist, MD, PhD, of Odense University Hospital in Denmark and coauthors.
While the risk of subdural hematoma was greatest for the shortest duration of treatment with low-dose aspirin, the risk remained steady across all durations of treatment with clopidogrel and did not vary significantly for direct oral anticoagulants or vitamin K antagonists.
Women were more likely to show an increased risk of subdural hematoma with low-dose aspirin or vitamin K antagonist than men.
The analysis also showed that the association between low-dose aspirin and subdural hematoma was significantly higher for individuals aged 75-89 years than for those aged 20-64 years.
“Furthermore, the present results emphasize that the major shifts in patterns of antithrombotic drug treatment for older individuals, and the increasing use of more aggressive antithrombotic regimens, have already had a major effect on subdural hematoma incidence,” the authors wrote.
Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
according to data published online Feb. 28 in JAMA.
A retrospective case-control study of 10,010 patients aged 20-89 years with a first-time subdural hematoma, matched by age, sex, and year to 400,380 general controls, showed treatment with a vitamin K antagonist was associated with a 3.69 greater risk of subdural hematoma, compared with controls, and a fourfold increase in risk when used concurrently with an antiplatelet drug (JAMA. 2017;317:836-46. doi: 10.1001/jama.2017.0639).
Low-dose aspirin alone was associated with a 24% increase in the risk of subdural hematoma; clopidogrel was associated with an 87% increase; and a direct oral anticoagulant such as dabigatran etexilate, rivaroxaban, or apixaban was associated with a 73% increase in risk.
Antithrombotic drugs were also associated with an increased risk of death from a subdural hematoma within 30 days after discharge for the hematoma’s diagnosis, an effect most evident with a direct oral anticoagulant or vitamin K antagonist.
Over the course of the Danish population-based study, which covered 2000-2015, the prevalence of antithrombotic drug use more than doubled, from 31 individuals per 1,000 to 76.9 per 1,000.
At the same time, the incidence of subdural hematoma nearly doubled (10.9 per 100,000 person-years to 19 per 100,000 person-years). The increase in subdural hematoma was greatest among older patients, from 55.1 per 100,000 person-years to 99.7 per 100,000 person-years.
“Although use of antithrombotic drugs has long been recognized as a risk factor for subdural hematoma, previous studies were either based exclusively on patients with subdural hematoma (i.e.,with no comparison group) or focused exclusively on patients treated with an anticoagulant,” wrote David Gaist, MD, PhD, of Odense University Hospital in Denmark and coauthors.
While the risk of subdural hematoma was greatest for the shortest duration of treatment with low-dose aspirin, the risk remained steady across all durations of treatment with clopidogrel and did not vary significantly for direct oral anticoagulants or vitamin K antagonists.
Women were more likely to show an increased risk of subdural hematoma with low-dose aspirin or vitamin K antagonist than men.
The analysis also showed that the association between low-dose aspirin and subdural hematoma was significantly higher for individuals aged 75-89 years than for those aged 20-64 years.
“Furthermore, the present results emphasize that the major shifts in patterns of antithrombotic drug treatment for older individuals, and the increasing use of more aggressive antithrombotic regimens, have already had a major effect on subdural hematoma incidence,” the authors wrote.
Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
according to data published online Feb. 28 in JAMA.
A retrospective case-control study of 10,010 patients aged 20-89 years with a first-time subdural hematoma, matched by age, sex, and year to 400,380 general controls, showed treatment with a vitamin K antagonist was associated with a 3.69 greater risk of subdural hematoma, compared with controls, and a fourfold increase in risk when used concurrently with an antiplatelet drug (JAMA. 2017;317:836-46. doi: 10.1001/jama.2017.0639).
Low-dose aspirin alone was associated with a 24% increase in the risk of subdural hematoma; clopidogrel was associated with an 87% increase; and a direct oral anticoagulant such as dabigatran etexilate, rivaroxaban, or apixaban was associated with a 73% increase in risk.
Antithrombotic drugs were also associated with an increased risk of death from a subdural hematoma within 30 days after discharge for the hematoma’s diagnosis, an effect most evident with a direct oral anticoagulant or vitamin K antagonist.
Over the course of the Danish population-based study, which covered 2000-2015, the prevalence of antithrombotic drug use more than doubled, from 31 individuals per 1,000 to 76.9 per 1,000.
At the same time, the incidence of subdural hematoma nearly doubled (10.9 per 100,000 person-years to 19 per 100,000 person-years). The increase in subdural hematoma was greatest among older patients, from 55.1 per 100,000 person-years to 99.7 per 100,000 person-years.
“Although use of antithrombotic drugs has long been recognized as a risk factor for subdural hematoma, previous studies were either based exclusively on patients with subdural hematoma (i.e.,with no comparison group) or focused exclusively on patients treated with an anticoagulant,” wrote David Gaist, MD, PhD, of Odense University Hospital in Denmark and coauthors.
While the risk of subdural hematoma was greatest for the shortest duration of treatment with low-dose aspirin, the risk remained steady across all durations of treatment with clopidogrel and did not vary significantly for direct oral anticoagulants or vitamin K antagonists.
Women were more likely to show an increased risk of subdural hematoma with low-dose aspirin or vitamin K antagonist than men.
The analysis also showed that the association between low-dose aspirin and subdural hematoma was significantly higher for individuals aged 75-89 years than for those aged 20-64 years.
“Furthermore, the present results emphasize that the major shifts in patterns of antithrombotic drug treatment for older individuals, and the increasing use of more aggressive antithrombotic regimens, have already had a major effect on subdural hematoma incidence,” the authors wrote.
Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
FROM JAMA
Key clinical point: The increasing incidence of subdural hematoma may be linked more common use of antithrombotics.
Major finding: Antithrombotic medication is associated with as much as a fourfold increase in the risk of subdural hematoma.
Data source: A retrospective case-control study of 10,010 patients with a first-ever subdural hematoma.
Disclosures: Four authors declared funds from the pharmaceutical industry, including one advisory board position. No other conflicts of interest were declared.
More than one-third of tumors found on breast cancer screening represent overdiagnosis
The purpose of screening mammography is to detect tumors when they are small and nonpalpable in order to prevent more advanced breast tumors in women. Overdiagnosis, which leads to unnecessary treatment, refers to screen-detected tumors that will not lead to symptoms. Overdiagnosis cannot be measured directly and, therefore, understanding this concept is problematic for both women and clinicians.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Observations from other types of cancer screening put overdiagnosis in perspective
To help us grasp the overall issue of overdiagnosis, we can consider screening mammography alongside cervical cancer screening and colon cancer screening. For instance, screening with cervical cytology has reduced the incidence of and mortality from invasive cervical cancer.1 Likewise, colonoscopy repeatedly has been found to reduce colon cancer mortality.2,3 Decades of media messaging have emphasized the benefits of screening mammograms.4 However, and in contrast with cervical cytology and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic (advanced) disease.5 Likewise, as the Danish authors of a recent study published in Annals of Internal Medicine point out, screening mammography has not achieved the promised reduction in breast cancer mortality.
New data from Denmark highlight overdiagnosis concerns
Jørgensen and colleagues conducted a cohort study to estimate the incidence of screen-detected tumors that would not become clinically relevant (overdiagnosis) among women aged 35 to 84 years between 1980 and 2010 in Denmark.6 This country offers a particularly well-suited backdrop for a study of overdiagnosis because biennial screening mammography was introduced by region beginning in the early 1990s. By 2007, one-fifth of the country’s female population aged 50 to 69 years were invited to participate. In the following years, screening became universal for Danish women in this age group.
For the study, researchers identified the size of all invasive breast cancer tumors diagnosed over the study period and then compared the incidence rates of advanced tumors (more than 20-mm in size at detection) with nonadvanced tumors in screened and unscreened Danish regions. The investigators took into account regional differences not related to screening by assessing the trends in diagnosis of advanced and nonadvanced tumors in screened and unscreened regions among women older and younger than those screened. This gave them a better estimate of the incidence of overdiagnosis.6
Jørgensen and colleagues found that breast cancer screening resulted in an increase in the incidence of nonadvanced tumors, but that it did not reduce the incidence of advanced tumors. They estimated that 39% of the invasive tumors found among women aged 50 to 69 were overdiagnosed.6
These Danish study results, that more than one-third of screen-detected tumors represent overdiagnosis, are similar to those found for studies conducted in the United States and other countries.7,8 The lengthy follow-up after initiation of screening and the assessment of trends in unscreened women represent strengths of the study by Jørgensen and colleagues, and speak to concerns voiced by those skeptical of reported overdiagnosis incidence rates.9
Although breast cancer mortality is declining, the lion’s share of this decline has resulted from improvements in systemic therapy rather than from screening mammography. Widespread screening mammography has resulted in a scenario in which women are more likely to have a breast cancer that was overdiagnosed than in having earlier detection of a tumor destined to grow larger.5 In the future, by targeting higher-risk women, screening may result in a better benefit:risk ratio. However, and as pointed out by Otis Brawley, MD, Chief Medical and Scientific Officer of the American Cancer Society, we must acknowledge that overdiagnosis is common, the benefits of screening have been overstated, and some patients considered as “cured” from breast cancer have in fact been harmed by unneeded treatment.10
Related article:
No surprises from the USPSTF with new guidance on screening mammography
My breast cancer screening approach
As Brawley indicates, we should not abandon screening.10 I continue to recommend screening based on US Preventive Services Taskforce guidance, beginning biennial screens at age 50.11 I also recognize that some women prefer earlier and more frequent screens, while others may prefer less frequent or even no screening.
- Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017-1021.
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-1137.
- Orenstein P. Our feel-good war on breast cancer. New York Times website. http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all& _r=0. Published April 25, 2013. Accessed February 21, 2017.
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer. N Engl J Med. 2016;374(8):596.
- Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017 Jan 10. doi:10.7326/M16-0270.
- Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
- Autier P, Boniol M, Middleton R, et al. Advanced breast cancer incidence following population-based mammographic screening. Ann Oncol. 2011;22(8):1726-1735.
- Kopans DB. Breast-cancer tumor size and screening effectiveness. N Engl J Med. 2017;376(1):93-94.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis [published online ahead of print January 10, 2017]. Ann Intern Med. doi:10.7326/M16-2850.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
The purpose of screening mammography is to detect tumors when they are small and nonpalpable in order to prevent more advanced breast tumors in women. Overdiagnosis, which leads to unnecessary treatment, refers to screen-detected tumors that will not lead to symptoms. Overdiagnosis cannot be measured directly and, therefore, understanding this concept is problematic for both women and clinicians.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Observations from other types of cancer screening put overdiagnosis in perspective
To help us grasp the overall issue of overdiagnosis, we can consider screening mammography alongside cervical cancer screening and colon cancer screening. For instance, screening with cervical cytology has reduced the incidence of and mortality from invasive cervical cancer.1 Likewise, colonoscopy repeatedly has been found to reduce colon cancer mortality.2,3 Decades of media messaging have emphasized the benefits of screening mammograms.4 However, and in contrast with cervical cytology and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic (advanced) disease.5 Likewise, as the Danish authors of a recent study published in Annals of Internal Medicine point out, screening mammography has not achieved the promised reduction in breast cancer mortality.
New data from Denmark highlight overdiagnosis concerns
Jørgensen and colleagues conducted a cohort study to estimate the incidence of screen-detected tumors that would not become clinically relevant (overdiagnosis) among women aged 35 to 84 years between 1980 and 2010 in Denmark.6 This country offers a particularly well-suited backdrop for a study of overdiagnosis because biennial screening mammography was introduced by region beginning in the early 1990s. By 2007, one-fifth of the country’s female population aged 50 to 69 years were invited to participate. In the following years, screening became universal for Danish women in this age group.
For the study, researchers identified the size of all invasive breast cancer tumors diagnosed over the study period and then compared the incidence rates of advanced tumors (more than 20-mm in size at detection) with nonadvanced tumors in screened and unscreened Danish regions. The investigators took into account regional differences not related to screening by assessing the trends in diagnosis of advanced and nonadvanced tumors in screened and unscreened regions among women older and younger than those screened. This gave them a better estimate of the incidence of overdiagnosis.6
Jørgensen and colleagues found that breast cancer screening resulted in an increase in the incidence of nonadvanced tumors, but that it did not reduce the incidence of advanced tumors. They estimated that 39% of the invasive tumors found among women aged 50 to 69 were overdiagnosed.6
These Danish study results, that more than one-third of screen-detected tumors represent overdiagnosis, are similar to those found for studies conducted in the United States and other countries.7,8 The lengthy follow-up after initiation of screening and the assessment of trends in unscreened women represent strengths of the study by Jørgensen and colleagues, and speak to concerns voiced by those skeptical of reported overdiagnosis incidence rates.9
Although breast cancer mortality is declining, the lion’s share of this decline has resulted from improvements in systemic therapy rather than from screening mammography. Widespread screening mammography has resulted in a scenario in which women are more likely to have a breast cancer that was overdiagnosed than in having earlier detection of a tumor destined to grow larger.5 In the future, by targeting higher-risk women, screening may result in a better benefit:risk ratio. However, and as pointed out by Otis Brawley, MD, Chief Medical and Scientific Officer of the American Cancer Society, we must acknowledge that overdiagnosis is common, the benefits of screening have been overstated, and some patients considered as “cured” from breast cancer have in fact been harmed by unneeded treatment.10
Related article:
No surprises from the USPSTF with new guidance on screening mammography
My breast cancer screening approach
As Brawley indicates, we should not abandon screening.10 I continue to recommend screening based on US Preventive Services Taskforce guidance, beginning biennial screens at age 50.11 I also recognize that some women prefer earlier and more frequent screens, while others may prefer less frequent or even no screening.
The purpose of screening mammography is to detect tumors when they are small and nonpalpable in order to prevent more advanced breast tumors in women. Overdiagnosis, which leads to unnecessary treatment, refers to screen-detected tumors that will not lead to symptoms. Overdiagnosis cannot be measured directly and, therefore, understanding this concept is problematic for both women and clinicians.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Observations from other types of cancer screening put overdiagnosis in perspective
To help us grasp the overall issue of overdiagnosis, we can consider screening mammography alongside cervical cancer screening and colon cancer screening. For instance, screening with cervical cytology has reduced the incidence of and mortality from invasive cervical cancer.1 Likewise, colonoscopy repeatedly has been found to reduce colon cancer mortality.2,3 Decades of media messaging have emphasized the benefits of screening mammograms.4 However, and in contrast with cervical cytology and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic (advanced) disease.5 Likewise, as the Danish authors of a recent study published in Annals of Internal Medicine point out, screening mammography has not achieved the promised reduction in breast cancer mortality.
New data from Denmark highlight overdiagnosis concerns
Jørgensen and colleagues conducted a cohort study to estimate the incidence of screen-detected tumors that would not become clinically relevant (overdiagnosis) among women aged 35 to 84 years between 1980 and 2010 in Denmark.6 This country offers a particularly well-suited backdrop for a study of overdiagnosis because biennial screening mammography was introduced by region beginning in the early 1990s. By 2007, one-fifth of the country’s female population aged 50 to 69 years were invited to participate. In the following years, screening became universal for Danish women in this age group.
For the study, researchers identified the size of all invasive breast cancer tumors diagnosed over the study period and then compared the incidence rates of advanced tumors (more than 20-mm in size at detection) with nonadvanced tumors in screened and unscreened Danish regions. The investigators took into account regional differences not related to screening by assessing the trends in diagnosis of advanced and nonadvanced tumors in screened and unscreened regions among women older and younger than those screened. This gave them a better estimate of the incidence of overdiagnosis.6
Jørgensen and colleagues found that breast cancer screening resulted in an increase in the incidence of nonadvanced tumors, but that it did not reduce the incidence of advanced tumors. They estimated that 39% of the invasive tumors found among women aged 50 to 69 were overdiagnosed.6
These Danish study results, that more than one-third of screen-detected tumors represent overdiagnosis, are similar to those found for studies conducted in the United States and other countries.7,8 The lengthy follow-up after initiation of screening and the assessment of trends in unscreened women represent strengths of the study by Jørgensen and colleagues, and speak to concerns voiced by those skeptical of reported overdiagnosis incidence rates.9
Although breast cancer mortality is declining, the lion’s share of this decline has resulted from improvements in systemic therapy rather than from screening mammography. Widespread screening mammography has resulted in a scenario in which women are more likely to have a breast cancer that was overdiagnosed than in having earlier detection of a tumor destined to grow larger.5 In the future, by targeting higher-risk women, screening may result in a better benefit:risk ratio. However, and as pointed out by Otis Brawley, MD, Chief Medical and Scientific Officer of the American Cancer Society, we must acknowledge that overdiagnosis is common, the benefits of screening have been overstated, and some patients considered as “cured” from breast cancer have in fact been harmed by unneeded treatment.10
Related article:
No surprises from the USPSTF with new guidance on screening mammography
My breast cancer screening approach
As Brawley indicates, we should not abandon screening.10 I continue to recommend screening based on US Preventive Services Taskforce guidance, beginning biennial screens at age 50.11 I also recognize that some women prefer earlier and more frequent screens, while others may prefer less frequent or even no screening.
- Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017-1021.
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-1137.
- Orenstein P. Our feel-good war on breast cancer. New York Times website. http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all& _r=0. Published April 25, 2013. Accessed February 21, 2017.
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer. N Engl J Med. 2016;374(8):596.
- Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017 Jan 10. doi:10.7326/M16-0270.
- Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
- Autier P, Boniol M, Middleton R, et al. Advanced breast cancer incidence following population-based mammographic screening. Ann Oncol. 2011;22(8):1726-1735.
- Kopans DB. Breast-cancer tumor size and screening effectiveness. N Engl J Med. 2017;376(1):93-94.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis [published online ahead of print January 10, 2017]. Ann Intern Med. doi:10.7326/M16-2850.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
- Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995;85(6):1017-1021.
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128-1137.
- Orenstein P. Our feel-good war on breast cancer. New York Times website. http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all& _r=0. Published April 25, 2013. Accessed February 21, 2017.
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer. N Engl J Med. 2016;374(8):596.
- Jørgensen KJ, Gøtzsche PC, Kalager M, Zahl PH. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017 Jan 10. doi:10.7326/M16-0270.
- Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
- Autier P, Boniol M, Middleton R, et al. Advanced breast cancer incidence following population-based mammographic screening. Ann Oncol. 2011;22(8):1726-1735.
- Kopans DB. Breast-cancer tumor size and screening effectiveness. N Engl J Med. 2017;376(1):93-94.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis [published online ahead of print January 10, 2017]. Ann Intern Med. doi:10.7326/M16-2850.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
Should the length of treatment for trichomoniasis in women be reconsidered?
EXPERT COMMENTARY
Both the Centers for Disease Control and Prevention and the World Health Organization currently recommend that patients with trichomoniasis be treated with a single 2-g oral dose of metronidazole.1 Following treatment, the reported rates of repeat infection or persistent infection range from 5% to 31%. Repeat infection rates may be even higher in HIV-infected patients.
Repeat infections presumably result from a failure to treat the patient’s sexual partner(s) or from the patient’s exposure to a new partner. Persistent infections, however, may be the result of inadequate primary therapy, even though inherent resistance of the organism to metronidazole is quite rare. To date, no single study has shown that single-dose therapy is inferior to multidose therapy, but most of these studies lack sufficient power to completely exclude the possibility of a type-2 statistical error.2 To compare single-dose with multidose therapy for trichomoniasis in a more systematic manner, Howe and Kissinger conducted a meta-analysis, which was recently published in Sexually Transmitted Diseases.
Related article:
2016 Update on infectious disease
Details of the study
The investigators conducted a comprehensive literature search using Embase, Medline, and ClinicalTrials.gov; 6 articles were included in the final results, 4 of which were randomized controlled trials. Approximately 1,300 participants were included in the 6 trials. All of the patients in the single-dose treatment arms received a 2-g oral dose of metronidazole. In the multidose treatment arms for 2 studies the participants received metronidazole 250 mg orally 3 times daily for 7 days, and for 2 studies the dose was 200 mg 3 times daily for 7 days. The fifth study employed a 500-mg oral dose of metronidazole twice daily for 7 days. The final study used a 400-mg oral dose twice daily for 5 days. The key study end point was treatment failure.
Howe and Kissinger demonstrated that women who received the single 2-g dose were 1.87 times (95% CI, 1.23−2.82; P<.01) more likely to experience a treatment failure compared with women who received a multidose regimen. When the one study that focused only on HIV-infected women was excluded from analysis, the results were similar. The relative risk of treatment failure was 1.80 (95% CI, 1.07−3.02; P<.03).
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
Study limitations
The results of this meta-analysis are interesting and provocative. However, the analysis has several important limitations. Five of the 6 studies were published many years ago (1971, 1972, 1979, 1980, and 1982). The most recent study was published in 2010. The investigators used 4 different multidose regimens, with metronidazole doses ranging from 200 mg to 500 mg and duration of therapy ranging from 5 to 7 days. Four of the six investigations used saline microscopy as the definitive diagnostic test of treatment failure. Compared with culture or DNA testing, microscopy is not as accurate. Moreover, the timing of retesting varied in the studies, and some apparent treatment failures actually may have been due to reinfection. In addition, the studies did not consistently track the adequacy of treatment of the sexual partner.
To be sure, we would benefit from a new comparative study that included a large sample size, a consistent multidose regimen, rigorous treatment of the sexual partner(s), and more sophisticated diagnostic testing to define treatment failure. Pending the publication of such a study, however, I plan to alter my practice pattern and treat infected patients with a multidose regimen of metronidazole. I favor the regimen of 500 mg orally twice daily for 7 days because it is effective against both trichomoniasis and bacterial vaginosis, which is a common co-infection.
The twice-daily regimen is more convenient than the thrice-daily regimen and is not much more expensive than the single-dose regimen ($13 vs $4, http://www.goodrx.com). I will reserve the single 2-g dose of metronidazole for patients in whom treatment adherence is likely to be a problem or for patients in whom an immediate response to treatment is imperative (eg, a patient with preterm premature rupture of membranes or preterm labor).
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29−34.
EXPERT COMMENTARY
Both the Centers for Disease Control and Prevention and the World Health Organization currently recommend that patients with trichomoniasis be treated with a single 2-g oral dose of metronidazole.1 Following treatment, the reported rates of repeat infection or persistent infection range from 5% to 31%. Repeat infection rates may be even higher in HIV-infected patients.
Repeat infections presumably result from a failure to treat the patient’s sexual partner(s) or from the patient’s exposure to a new partner. Persistent infections, however, may be the result of inadequate primary therapy, even though inherent resistance of the organism to metronidazole is quite rare. To date, no single study has shown that single-dose therapy is inferior to multidose therapy, but most of these studies lack sufficient power to completely exclude the possibility of a type-2 statistical error.2 To compare single-dose with multidose therapy for trichomoniasis in a more systematic manner, Howe and Kissinger conducted a meta-analysis, which was recently published in Sexually Transmitted Diseases.
Related article:
2016 Update on infectious disease
Details of the study
The investigators conducted a comprehensive literature search using Embase, Medline, and ClinicalTrials.gov; 6 articles were included in the final results, 4 of which were randomized controlled trials. Approximately 1,300 participants were included in the 6 trials. All of the patients in the single-dose treatment arms received a 2-g oral dose of metronidazole. In the multidose treatment arms for 2 studies the participants received metronidazole 250 mg orally 3 times daily for 7 days, and for 2 studies the dose was 200 mg 3 times daily for 7 days. The fifth study employed a 500-mg oral dose of metronidazole twice daily for 7 days. The final study used a 400-mg oral dose twice daily for 5 days. The key study end point was treatment failure.
Howe and Kissinger demonstrated that women who received the single 2-g dose were 1.87 times (95% CI, 1.23−2.82; P<.01) more likely to experience a treatment failure compared with women who received a multidose regimen. When the one study that focused only on HIV-infected women was excluded from analysis, the results were similar. The relative risk of treatment failure was 1.80 (95% CI, 1.07−3.02; P<.03).
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
Study limitations
The results of this meta-analysis are interesting and provocative. However, the analysis has several important limitations. Five of the 6 studies were published many years ago (1971, 1972, 1979, 1980, and 1982). The most recent study was published in 2010. The investigators used 4 different multidose regimens, with metronidazole doses ranging from 200 mg to 500 mg and duration of therapy ranging from 5 to 7 days. Four of the six investigations used saline microscopy as the definitive diagnostic test of treatment failure. Compared with culture or DNA testing, microscopy is not as accurate. Moreover, the timing of retesting varied in the studies, and some apparent treatment failures actually may have been due to reinfection. In addition, the studies did not consistently track the adequacy of treatment of the sexual partner.
To be sure, we would benefit from a new comparative study that included a large sample size, a consistent multidose regimen, rigorous treatment of the sexual partner(s), and more sophisticated diagnostic testing to define treatment failure. Pending the publication of such a study, however, I plan to alter my practice pattern and treat infected patients with a multidose regimen of metronidazole. I favor the regimen of 500 mg orally twice daily for 7 days because it is effective against both trichomoniasis and bacterial vaginosis, which is a common co-infection.
The twice-daily regimen is more convenient than the thrice-daily regimen and is not much more expensive than the single-dose regimen ($13 vs $4, http://www.goodrx.com). I will reserve the single 2-g dose of metronidazole for patients in whom treatment adherence is likely to be a problem or for patients in whom an immediate response to treatment is imperative (eg, a patient with preterm premature rupture of membranes or preterm labor).
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Both the Centers for Disease Control and Prevention and the World Health Organization currently recommend that patients with trichomoniasis be treated with a single 2-g oral dose of metronidazole.1 Following treatment, the reported rates of repeat infection or persistent infection range from 5% to 31%. Repeat infection rates may be even higher in HIV-infected patients.
Repeat infections presumably result from a failure to treat the patient’s sexual partner(s) or from the patient’s exposure to a new partner. Persistent infections, however, may be the result of inadequate primary therapy, even though inherent resistance of the organism to metronidazole is quite rare. To date, no single study has shown that single-dose therapy is inferior to multidose therapy, but most of these studies lack sufficient power to completely exclude the possibility of a type-2 statistical error.2 To compare single-dose with multidose therapy for trichomoniasis in a more systematic manner, Howe and Kissinger conducted a meta-analysis, which was recently published in Sexually Transmitted Diseases.
Related article:
2016 Update on infectious disease
Details of the study
The investigators conducted a comprehensive literature search using Embase, Medline, and ClinicalTrials.gov; 6 articles were included in the final results, 4 of which were randomized controlled trials. Approximately 1,300 participants were included in the 6 trials. All of the patients in the single-dose treatment arms received a 2-g oral dose of metronidazole. In the multidose treatment arms for 2 studies the participants received metronidazole 250 mg orally 3 times daily for 7 days, and for 2 studies the dose was 200 mg 3 times daily for 7 days. The fifth study employed a 500-mg oral dose of metronidazole twice daily for 7 days. The final study used a 400-mg oral dose twice daily for 5 days. The key study end point was treatment failure.
Howe and Kissinger demonstrated that women who received the single 2-g dose were 1.87 times (95% CI, 1.23−2.82; P<.01) more likely to experience a treatment failure compared with women who received a multidose regimen. When the one study that focused only on HIV-infected women was excluded from analysis, the results were similar. The relative risk of treatment failure was 1.80 (95% CI, 1.07−3.02; P<.03).
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
Study limitations
The results of this meta-analysis are interesting and provocative. However, the analysis has several important limitations. Five of the 6 studies were published many years ago (1971, 1972, 1979, 1980, and 1982). The most recent study was published in 2010. The investigators used 4 different multidose regimens, with metronidazole doses ranging from 200 mg to 500 mg and duration of therapy ranging from 5 to 7 days. Four of the six investigations used saline microscopy as the definitive diagnostic test of treatment failure. Compared with culture or DNA testing, microscopy is not as accurate. Moreover, the timing of retesting varied in the studies, and some apparent treatment failures actually may have been due to reinfection. In addition, the studies did not consistently track the adequacy of treatment of the sexual partner.
To be sure, we would benefit from a new comparative study that included a large sample size, a consistent multidose regimen, rigorous treatment of the sexual partner(s), and more sophisticated diagnostic testing to define treatment failure. Pending the publication of such a study, however, I plan to alter my practice pattern and treat infected patients with a multidose regimen of metronidazole. I favor the regimen of 500 mg orally twice daily for 7 days because it is effective against both trichomoniasis and bacterial vaginosis, which is a common co-infection.
The twice-daily regimen is more convenient than the thrice-daily regimen and is not much more expensive than the single-dose regimen ($13 vs $4, http://www.goodrx.com). I will reserve the single 2-g dose of metronidazole for patients in whom treatment adherence is likely to be a problem or for patients in whom an immediate response to treatment is imperative (eg, a patient with preterm premature rupture of membranes or preterm labor).
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29−34.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29−34.
Rectal cancer proportion in young doubled
The proportion of rectal cancer cases diagnosed in people younger than 55 years doubled over the past 2 decades, according to a report published online in the Journal of the National Cancer Institute.
In contrast, the proportion diagnosed in people older than 55 years has decreased over the last 4 decades, said Rebecca L. Siegel, MPH, strategic director of surveillance information services of surveillance and health services research at the American Cancer Society and her associates.
The incidence of rectal cancer increased by 3.2% per year during the study period among patients aged 20-29 years and in those aged 30-39 years. It didn’t begin rising until the 1990s in adults aged 40-49 years and 50-54 years, and then it rose by a smaller amount – 2.3% per year. In contrast, the incidence of rectal cancer generally declined throughout the 40-year study period among adults aged 55 and older.
Because of these opposing trends, there was a net increase in rectal cancer of 4% per year for people in their twenties together with a net decrease of 2% per year for those aged 75 years and older.
The decreasing rate of rectal cancer in older adults “may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal [CRC] screening. Inherent differences within the colorectum in the way environmental factors initiate and or promote carcinogenesis, as well as the influence of unknown risk factors, may also have contributed,” Ms. Siegel and her associates said.
The temporal pattern was somewhat different for colon cancer. The risk of colon cancer declined “for successive generations during the first half of the twentieth century but has escalated back to the level of those born circa 1890 for current birth cohorts.”
“The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk,” including excess body weight, high intake of processed meat, low intake of dietary fiber, and low levels of physical activity. “New strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles” are needed, the researchers said.
In addition, both clinicians and the public must be educated about the rising probability of the disease in people younger than 55 years. Timely follow-up of symptoms, regardless of patient age, must be emphasized. Younger adults are nearly 60% more likely than are older adults to be diagnosed with advanced CRC, largely because they delay seeking medical care. The disease simply isn’t “on the radar” of young adults or their providers, the investigators added.
This study was supported by the American Cancer Society and the National Institutes of Health. Ms. Siegel and her associates did not provide their conflicts of interest.
AGA Resource
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients: http://www.gastro.org/patient-care/conditions-diseases/colorectal-cancer
Colorectal cancer has been a “good news” story over the past 10-15 years. In the United States we have seen 30% reduction in both incidence and mortality over 10 years. This may be due to many factors, including increased rates of screening. The increased use of aspirin for cardiovascular protection, NSAIDs for joint and muscle pain, use of hormone replacement therapy, and reductions in smoking all likely contribute to the trend in CRC reduction.
New or changing environmental exposures may place younger people at risk. The introduction of industrialized food in our diet over the past 4 decades could have both direct and indirect effects. It is possible that some food chemicals could be carcinogenic, but it is also quite possible that alteration of the microbiome by diet and environmental factors could lead to development of neoplasia in predisposed individuals. The use of antibiotics in our food chain may alter the microbiome.
There is considerable state-to-state variation in rates of CRC incidence and mortality. This is not new, but remains largely unexplained. The highest risk appears to be in the so-called “Rust Belt” and deep South, raising questions about environmental exposures that might predispose to CRC. Lower rates in states like Texas, Colorado, and California may be influenced by the population mix. There is evidence that Hispanics may have lower age-adjusted risk of CRC than blacks and Caucasians, so higher proportions of low-risk groups could impact the statewide risk of CRC. The differences between high-risk (West Virginia’s death rate of 23.4/100,000) and low-risk (Utah’s death rate 8.7/100,000) are too large to be explained by demographic differences alone, and strongly suggests an environmental culprit.
David Lieberman, MD, is professor of medicine; chief of the division of gastroenterology and hepatology, Oregon Health and Science University, Portland; and Vice President of the AGA Institute.
Colorectal cancer has been a “good news” story over the past 10-15 years. In the United States we have seen 30% reduction in both incidence and mortality over 10 years. This may be due to many factors, including increased rates of screening. The increased use of aspirin for cardiovascular protection, NSAIDs for joint and muscle pain, use of hormone replacement therapy, and reductions in smoking all likely contribute to the trend in CRC reduction.
New or changing environmental exposures may place younger people at risk. The introduction of industrialized food in our diet over the past 4 decades could have both direct and indirect effects. It is possible that some food chemicals could be carcinogenic, but it is also quite possible that alteration of the microbiome by diet and environmental factors could lead to development of neoplasia in predisposed individuals. The use of antibiotics in our food chain may alter the microbiome.
There is considerable state-to-state variation in rates of CRC incidence and mortality. This is not new, but remains largely unexplained. The highest risk appears to be in the so-called “Rust Belt” and deep South, raising questions about environmental exposures that might predispose to CRC. Lower rates in states like Texas, Colorado, and California may be influenced by the population mix. There is evidence that Hispanics may have lower age-adjusted risk of CRC than blacks and Caucasians, so higher proportions of low-risk groups could impact the statewide risk of CRC. The differences between high-risk (West Virginia’s death rate of 23.4/100,000) and low-risk (Utah’s death rate 8.7/100,000) are too large to be explained by demographic differences alone, and strongly suggests an environmental culprit.
David Lieberman, MD, is professor of medicine; chief of the division of gastroenterology and hepatology, Oregon Health and Science University, Portland; and Vice President of the AGA Institute.
Colorectal cancer has been a “good news” story over the past 10-15 years. In the United States we have seen 30% reduction in both incidence and mortality over 10 years. This may be due to many factors, including increased rates of screening. The increased use of aspirin for cardiovascular protection, NSAIDs for joint and muscle pain, use of hormone replacement therapy, and reductions in smoking all likely contribute to the trend in CRC reduction.
New or changing environmental exposures may place younger people at risk. The introduction of industrialized food in our diet over the past 4 decades could have both direct and indirect effects. It is possible that some food chemicals could be carcinogenic, but it is also quite possible that alteration of the microbiome by diet and environmental factors could lead to development of neoplasia in predisposed individuals. The use of antibiotics in our food chain may alter the microbiome.
There is considerable state-to-state variation in rates of CRC incidence and mortality. This is not new, but remains largely unexplained. The highest risk appears to be in the so-called “Rust Belt” and deep South, raising questions about environmental exposures that might predispose to CRC. Lower rates in states like Texas, Colorado, and California may be influenced by the population mix. There is evidence that Hispanics may have lower age-adjusted risk of CRC than blacks and Caucasians, so higher proportions of low-risk groups could impact the statewide risk of CRC. The differences between high-risk (West Virginia’s death rate of 23.4/100,000) and low-risk (Utah’s death rate 8.7/100,000) are too large to be explained by demographic differences alone, and strongly suggests an environmental culprit.
David Lieberman, MD, is professor of medicine; chief of the division of gastroenterology and hepatology, Oregon Health and Science University, Portland; and Vice President of the AGA Institute.
The proportion of rectal cancer cases diagnosed in people younger than 55 years doubled over the past 2 decades, according to a report published online in the Journal of the National Cancer Institute.
In contrast, the proportion diagnosed in people older than 55 years has decreased over the last 4 decades, said Rebecca L. Siegel, MPH, strategic director of surveillance information services of surveillance and health services research at the American Cancer Society and her associates.
The incidence of rectal cancer increased by 3.2% per year during the study period among patients aged 20-29 years and in those aged 30-39 years. It didn’t begin rising until the 1990s in adults aged 40-49 years and 50-54 years, and then it rose by a smaller amount – 2.3% per year. In contrast, the incidence of rectal cancer generally declined throughout the 40-year study period among adults aged 55 and older.
Because of these opposing trends, there was a net increase in rectal cancer of 4% per year for people in their twenties together with a net decrease of 2% per year for those aged 75 years and older.
The decreasing rate of rectal cancer in older adults “may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal [CRC] screening. Inherent differences within the colorectum in the way environmental factors initiate and or promote carcinogenesis, as well as the influence of unknown risk factors, may also have contributed,” Ms. Siegel and her associates said.
The temporal pattern was somewhat different for colon cancer. The risk of colon cancer declined “for successive generations during the first half of the twentieth century but has escalated back to the level of those born circa 1890 for current birth cohorts.”
“The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk,” including excess body weight, high intake of processed meat, low intake of dietary fiber, and low levels of physical activity. “New strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles” are needed, the researchers said.
In addition, both clinicians and the public must be educated about the rising probability of the disease in people younger than 55 years. Timely follow-up of symptoms, regardless of patient age, must be emphasized. Younger adults are nearly 60% more likely than are older adults to be diagnosed with advanced CRC, largely because they delay seeking medical care. The disease simply isn’t “on the radar” of young adults or their providers, the investigators added.
This study was supported by the American Cancer Society and the National Institutes of Health. Ms. Siegel and her associates did not provide their conflicts of interest.
AGA Resource
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients: http://www.gastro.org/patient-care/conditions-diseases/colorectal-cancer
The proportion of rectal cancer cases diagnosed in people younger than 55 years doubled over the past 2 decades, according to a report published online in the Journal of the National Cancer Institute.
In contrast, the proportion diagnosed in people older than 55 years has decreased over the last 4 decades, said Rebecca L. Siegel, MPH, strategic director of surveillance information services of surveillance and health services research at the American Cancer Society and her associates.
The incidence of rectal cancer increased by 3.2% per year during the study period among patients aged 20-29 years and in those aged 30-39 years. It didn’t begin rising until the 1990s in adults aged 40-49 years and 50-54 years, and then it rose by a smaller amount – 2.3% per year. In contrast, the incidence of rectal cancer generally declined throughout the 40-year study period among adults aged 55 and older.
Because of these opposing trends, there was a net increase in rectal cancer of 4% per year for people in their twenties together with a net decrease of 2% per year for those aged 75 years and older.
The decreasing rate of rectal cancer in older adults “may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal [CRC] screening. Inherent differences within the colorectum in the way environmental factors initiate and or promote carcinogenesis, as well as the influence of unknown risk factors, may also have contributed,” Ms. Siegel and her associates said.
The temporal pattern was somewhat different for colon cancer. The risk of colon cancer declined “for successive generations during the first half of the twentieth century but has escalated back to the level of those born circa 1890 for current birth cohorts.”
“The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk,” including excess body weight, high intake of processed meat, low intake of dietary fiber, and low levels of physical activity. “New strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles” are needed, the researchers said.
In addition, both clinicians and the public must be educated about the rising probability of the disease in people younger than 55 years. Timely follow-up of symptoms, regardless of patient age, must be emphasized. Younger adults are nearly 60% more likely than are older adults to be diagnosed with advanced CRC, largely because they delay seeking medical care. The disease simply isn’t “on the radar” of young adults or their providers, the investigators added.
This study was supported by the American Cancer Society and the National Institutes of Health. Ms. Siegel and her associates did not provide their conflicts of interest.
AGA Resource
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients: http://www.gastro.org/patient-care/conditions-diseases/colorectal-cancer
Proportion of rectal cancer in young adults doubles
The proportion of rectal cancer cases diagnosed in people younger than 55 years doubled over the past 2 decades, according to a report published online Feb. 28 in the Journal of the National Cancer Institute.
In contrast, the proportion diagnosed in people older than 55 years has decreased over the last 4 decades, said Rebecca L. Siegel, MPH, strategic director of surveillance information services of surveillance and health services research at the American Cancer Society and her associates.
The study population comprised 490,305 patients.
The incidence of rectal cancer increased by 3.2% per year during the study period among patients aged 20-29 years and in those aged 30-39 years. It didn’t begin rising until the 1990s in adults aged 40-49 years and 50-54 years, and then it rose by a smaller amount – 2.3% per year. In contrast, the incidence of rectal cancer generally declined throughout the 40-year study period among adults aged 55 and older.
Because of these opposing trends, there was a net increase in rectal cancer of 4% per year for people in their twenties together with a net decrease of 2% per year for those aged 75 years and older.
The decreasing rate of rectal cancer in older adults “may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal colorectal cancer screening. Inherent differences within the colorectum in the way environmental factors initiate and or promote carcinogenesis, as well as the influence of unknown risk factors, may also have contributed,” Ms. Siegel and her associates said.
The temporal pattern was somewhat different for colon cancer. The risk of colon cancer declined “for successive generations during the first half of the twentieth century but has escalated back to the level of those born circa 1890 for current birth cohorts.”
The rising incidence of both colon and rectal cancers among younger adults is “sobering,” given that such trends “often provide a bellwether of the future disease burden,” they noted.
“The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk,” including excess body weight, high intake of processed meat, low intake of dietary fiber, and low levels of physical activity. “New strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles” are needed, the researchers said.
In addition, both clinicians and the public must be educated about the rising probability of the disease in people younger than 55 years. Timely follow-up of symptoms, regardless of patient age, must be emphasized. Younger adults are nearly 60% more likely than are older adults to be diagnosed with advanced colorectal cancer, largely because they delay seeking medical care. The disease simply isn’t “on the radar” of young adults or their providers, the investigators added.
Colorectal cancer has been a “good news” story over the past 10-15 years. In the United States we have seen 30% reduction in both incidence and mortality over 10 years. This may be due to many factors, including increased rates of screening. The increased use of aspirin for cardiovascular protection, NSAIDs for joint and muscle pain, use of hormone replacement therapy, and reductions in smoking all likely contribute to the trend in CRC reduction.
New or changing environmental exposures may place younger people at risk. The introduction of industrialized food in our diet over the past 4 decades could have both direct and indirect effects. It is possible that some food chemicals could be carcinogenic, but it is also quite possible that alteration of the microbiome by diet and environmental factors could lead to development of neoplasia in predisposed individuals. The use of antibiotics in our food chain may alter the microbiome.
There is considerable state-to-state variation in rates of CRC incidence and mortality. This is not new, but remains largely unexplained. The highest risk appears to be in the so-called “Rust Belt” and deep South, raising questions about environmental exposures that might predispose to CRC. Lower rates in states like Texas, Colorado, and California may be influenced by the population mix. There is evidence that Hispanics may have lower age-adjusted risk of CRC than blacks and Caucasians, so higher proportions of low-risk groups could impact the statewide risk of CRC. The differences between high-risk (West Virginia’s death rate of 23.4/100,000) and low-risk (Utah’s death rate 8.7/100,000) are too large to be explained by demographic differences alone, and strongly suggests an environmental culprit.
David Lieberman, MD, is professor of medicine; chief of the division of gastroenterology and hepatology, Oregon Health and Science University, Portland; and Vice President-elect of AGA.
Colorectal cancer has been a “good news” story over the past 10-15 years. In the United States we have seen 30% reduction in both incidence and mortality over 10 years. This may be due to many factors, including increased rates of screening. The increased use of aspirin for cardiovascular protection, NSAIDs for joint and muscle pain, use of hormone replacement therapy, and reductions in smoking all likely contribute to the trend in CRC reduction.
New or changing environmental exposures may place younger people at risk. The introduction of industrialized food in our diet over the past 4 decades could have both direct and indirect effects. It is possible that some food chemicals could be carcinogenic, but it is also quite possible that alteration of the microbiome by diet and environmental factors could lead to development of neoplasia in predisposed individuals. The use of antibiotics in our food chain may alter the microbiome.
There is considerable state-to-state variation in rates of CRC incidence and mortality. This is not new, but remains largely unexplained. The highest risk appears to be in the so-called “Rust Belt” and deep South, raising questions about environmental exposures that might predispose to CRC. Lower rates in states like Texas, Colorado, and California may be influenced by the population mix. There is evidence that Hispanics may have lower age-adjusted risk of CRC than blacks and Caucasians, so higher proportions of low-risk groups could impact the statewide risk of CRC. The differences between high-risk (West Virginia’s death rate of 23.4/100,000) and low-risk (Utah’s death rate 8.7/100,000) are too large to be explained by demographic differences alone, and strongly suggests an environmental culprit.
David Lieberman, MD, is professor of medicine; chief of the division of gastroenterology and hepatology, Oregon Health and Science University, Portland; and Vice President-elect of AGA.
Colorectal cancer has been a “good news” story over the past 10-15 years. In the United States we have seen 30% reduction in both incidence and mortality over 10 years. This may be due to many factors, including increased rates of screening. The increased use of aspirin for cardiovascular protection, NSAIDs for joint and muscle pain, use of hormone replacement therapy, and reductions in smoking all likely contribute to the trend in CRC reduction.
New or changing environmental exposures may place younger people at risk. The introduction of industrialized food in our diet over the past 4 decades could have both direct and indirect effects. It is possible that some food chemicals could be carcinogenic, but it is also quite possible that alteration of the microbiome by diet and environmental factors could lead to development of neoplasia in predisposed individuals. The use of antibiotics in our food chain may alter the microbiome.
There is considerable state-to-state variation in rates of CRC incidence and mortality. This is not new, but remains largely unexplained. The highest risk appears to be in the so-called “Rust Belt” and deep South, raising questions about environmental exposures that might predispose to CRC. Lower rates in states like Texas, Colorado, and California may be influenced by the population mix. There is evidence that Hispanics may have lower age-adjusted risk of CRC than blacks and Caucasians, so higher proportions of low-risk groups could impact the statewide risk of CRC. The differences between high-risk (West Virginia’s death rate of 23.4/100,000) and low-risk (Utah’s death rate 8.7/100,000) are too large to be explained by demographic differences alone, and strongly suggests an environmental culprit.
David Lieberman, MD, is professor of medicine; chief of the division of gastroenterology and hepatology, Oregon Health and Science University, Portland; and Vice President-elect of AGA.
The proportion of rectal cancer cases diagnosed in people younger than 55 years doubled over the past 2 decades, according to a report published online Feb. 28 in the Journal of the National Cancer Institute.
In contrast, the proportion diagnosed in people older than 55 years has decreased over the last 4 decades, said Rebecca L. Siegel, MPH, strategic director of surveillance information services of surveillance and health services research at the American Cancer Society and her associates.
The study population comprised 490,305 patients.
The incidence of rectal cancer increased by 3.2% per year during the study period among patients aged 20-29 years and in those aged 30-39 years. It didn’t begin rising until the 1990s in adults aged 40-49 years and 50-54 years, and then it rose by a smaller amount – 2.3% per year. In contrast, the incidence of rectal cancer generally declined throughout the 40-year study period among adults aged 55 and older.
Because of these opposing trends, there was a net increase in rectal cancer of 4% per year for people in their twenties together with a net decrease of 2% per year for those aged 75 years and older.
The decreasing rate of rectal cancer in older adults “may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal colorectal cancer screening. Inherent differences within the colorectum in the way environmental factors initiate and or promote carcinogenesis, as well as the influence of unknown risk factors, may also have contributed,” Ms. Siegel and her associates said.
The temporal pattern was somewhat different for colon cancer. The risk of colon cancer declined “for successive generations during the first half of the twentieth century but has escalated back to the level of those born circa 1890 for current birth cohorts.”
The rising incidence of both colon and rectal cancers among younger adults is “sobering,” given that such trends “often provide a bellwether of the future disease burden,” they noted.
“The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk,” including excess body weight, high intake of processed meat, low intake of dietary fiber, and low levels of physical activity. “New strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles” are needed, the researchers said.
In addition, both clinicians and the public must be educated about the rising probability of the disease in people younger than 55 years. Timely follow-up of symptoms, regardless of patient age, must be emphasized. Younger adults are nearly 60% more likely than are older adults to be diagnosed with advanced colorectal cancer, largely because they delay seeking medical care. The disease simply isn’t “on the radar” of young adults or their providers, the investigators added.
The proportion of rectal cancer cases diagnosed in people younger than 55 years doubled over the past 2 decades, according to a report published online Feb. 28 in the Journal of the National Cancer Institute.
In contrast, the proportion diagnosed in people older than 55 years has decreased over the last 4 decades, said Rebecca L. Siegel, MPH, strategic director of surveillance information services of surveillance and health services research at the American Cancer Society and her associates.
The study population comprised 490,305 patients.
The incidence of rectal cancer increased by 3.2% per year during the study period among patients aged 20-29 years and in those aged 30-39 years. It didn’t begin rising until the 1990s in adults aged 40-49 years and 50-54 years, and then it rose by a smaller amount – 2.3% per year. In contrast, the incidence of rectal cancer generally declined throughout the 40-year study period among adults aged 55 and older.
Because of these opposing trends, there was a net increase in rectal cancer of 4% per year for people in their twenties together with a net decrease of 2% per year for those aged 75 years and older.
The decreasing rate of rectal cancer in older adults “may partly reflect detection and removal of precancerous lesions during clinical inspection of the rectum, which was common practice well before formal colorectal cancer screening. Inherent differences within the colorectum in the way environmental factors initiate and or promote carcinogenesis, as well as the influence of unknown risk factors, may also have contributed,” Ms. Siegel and her associates said.
The temporal pattern was somewhat different for colon cancer. The risk of colon cancer declined “for successive generations during the first half of the twentieth century but has escalated back to the level of those born circa 1890 for current birth cohorts.”
The rising incidence of both colon and rectal cancers among younger adults is “sobering,” given that such trends “often provide a bellwether of the future disease burden,” they noted.
“The strong birth cohort effects we observed signal relatively recent changes in exposures that influence risk,” including excess body weight, high intake of processed meat, low intake of dietary fiber, and low levels of physical activity. “New strategies to curb the obesity epidemic and shift Americans toward healthier eating and more active lifestyles” are needed, the researchers said.
In addition, both clinicians and the public must be educated about the rising probability of the disease in people younger than 55 years. Timely follow-up of symptoms, regardless of patient age, must be emphasized. Younger adults are nearly 60% more likely than are older adults to be diagnosed with advanced colorectal cancer, largely because they delay seeking medical care. The disease simply isn’t “on the radar” of young adults or their providers, the investigators added.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: The proportion of rectal cancer cases diagnosed in people younger than age 55 doubled over the past 2 decades.
Key numerical finding: The incidence of rectal cancer increased by 3.2% per year among patients aged 20-29 and 30-39 years, and increased by 2.3% per year in those aged 40-49 years and 50-54 years, but declined among adults aged 55 and older.
Data source: A retrospective cohort study involving 490,305 patients aged 20 years and older diagnosed between 1974 and 2013.
Disclosures: This study was supported by the American Cancer Society and the National Institutes of Health. Ms. Siegel and her associates did not provide their conflicts of interest.
Why are there delays in the diagnosis of endometriosis?
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.
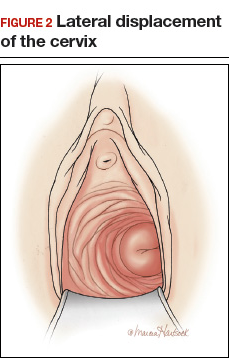
In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.

In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.

In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
She wanted to labor on hands and knees
She wanted to labor on hands and knees
During prenatal visits, a woman, pregnant with her fourth child, discussed undergoing labor and delivery in any position other than on her back; the ObGyn agreed. When she arrived at the hospital in labor, the patient told the nurse that she preferred to labor on her hands and knees. The nurse disagreed because of the fetal heart-rate monitor.
When the patient began hard labor, she turned herself over onto h
Two months later, the patient reported chronic severe pelvic pain and was found to have pudendal neuralgia. She underwent nerve blocks and takes medication for chronic pain.
PATIENT’S CLAIM:
The ObGyn did not assume responsibility when he arrived for the delivery. The nurses did not follow the standard of care. The patient’s injury was the result of tension and compression due to malpositioning of the patient’s legs during delivery.
DEFENDANTS’ DEFENSE:
There was no breach in the standard of care. The patient’s injury, if any, had not been caused by the delivery.
VERDICT:
A $16 million Alabama verdict was returned.
Related article:
10 tips for overcoming common challenges of intrapartum fetal monitoring
Late-term abortion: $1.4M award
Although genetic testing was scheduled for a 37-year-old woman’s 15-week prenatal visit, the ObGyn’s staff failed to draw blood. At 19 weeks’ gestation (April 24), blood was drawn. The ObGyn signed off on test results that showed a high risk for fetal anomaly on May 2, but the patient was not informed until May 22. The ObGyn scheduled amniocentesis for June 3. On May 30, the hospital, based in Illinois, cancelled the test, telling the ObGyn that it was because the patient was over 24 weeks’ pregnant and there was no labor and delivery unit to respond if complications arose. Instead of notifying the patient, the ObGyn arranged for amniocentesis to be performed elsewhere on June 3. The ObGyn saw the amniocentesis results on June 13, but did not tell the patient until July 3, when he advised her to terminate the pregnancy because the baby had severe cardiac defects and Down syndrome; he felt the child would not survive or have very poor quality of life. The ObGyn arranged for the patient to undergo a third-trimester abortion in Kansas and paid all expenses. On July 14, the patient began the 5-day abortion process at 30+ weeks’ gestation.
PATIENT’S CLAIM:
She was never offered additional genetic testing or expedited amniocentesis. She was not told that abortion is illegal in Illinois after 23 6/7 weeks’ gestation. The ObGyn had a motive for paying for her abortion. He never counseled her about options to keep the child. She endured extreme pain and emotional trauma during the abortion and was later found to have posttraumatic stress disorder, multidepressive disorder, and anxiety as a result of the experience. She countered the ObGyn’s contact information claim by saying that her phone number had not changed.
PHYSICIAN’S DEFENSE:
The ObGyn admitted negligence in failing to timely communicate test results but contended that the patient was more than 50% responsible for any delay by failing to update her contact information when she moved. The ObGyn denied causation of any injuries or damage.
VERDICT:
A $1,439,250 Illinois verdict was returned.
Related article:
4 Supreme Court decisions important to ObGyns from the 2015−2016 term
Did delay in delivery cause infant's death?
A woman presented to the hospital in labor. During delivery, the patient’s ObGyn encountered shoulder dystocia. The infant died shortly after birth.
PARENTS’ CLAIM:
The ObGyn and hospital nurses were negligent. The nurses failed to monitor labor and properly communicate with the ObGyn. The ObGyn failed to appreciate the baby’s large size and order a cesarean delivery. The infant’s death was due to a hypoxic event during delivery.
DEFENDANTS’ DEFENSE:
The baby gained an unexpected amount of weight between the last prenatal visit and labor. There was no reason to expect a complication to vaginal delivery. The nurses denied negligence. The child’s sudden death was caused by a genetic cardiac condition.
VERDICT:
A Tennessee defense verdict was returned.
Related article:
Shoulder dystocia: Taking the fear out of management
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
She wanted to labor on hands and knees
During prenatal visits, a woman, pregnant with her fourth child, discussed undergoing labor and delivery in any position other than on her back; the ObGyn agreed. When she arrived at the hospital in labor, the patient told the nurse that she preferred to labor on her hands and knees. The nurse disagreed because of the fetal heart-rate monitor.
When the patient began hard labor, she turned herself over onto h
Two months later, the patient reported chronic severe pelvic pain and was found to have pudendal neuralgia. She underwent nerve blocks and takes medication for chronic pain.
PATIENT’S CLAIM:
The ObGyn did not assume responsibility when he arrived for the delivery. The nurses did not follow the standard of care. The patient’s injury was the result of tension and compression due to malpositioning of the patient’s legs during delivery.
DEFENDANTS’ DEFENSE:
There was no breach in the standard of care. The patient’s injury, if any, had not been caused by the delivery.
VERDICT:
A $16 million Alabama verdict was returned.
Related article:
10 tips for overcoming common challenges of intrapartum fetal monitoring
Late-term abortion: $1.4M award
Although genetic testing was scheduled for a 37-year-old woman’s 15-week prenatal visit, the ObGyn’s staff failed to draw blood. At 19 weeks’ gestation (April 24), blood was drawn. The ObGyn signed off on test results that showed a high risk for fetal anomaly on May 2, but the patient was not informed until May 22. The ObGyn scheduled amniocentesis for June 3. On May 30, the hospital, based in Illinois, cancelled the test, telling the ObGyn that it was because the patient was over 24 weeks’ pregnant and there was no labor and delivery unit to respond if complications arose. Instead of notifying the patient, the ObGyn arranged for amniocentesis to be performed elsewhere on June 3. The ObGyn saw the amniocentesis results on June 13, but did not tell the patient until July 3, when he advised her to terminate the pregnancy because the baby had severe cardiac defects and Down syndrome; he felt the child would not survive or have very poor quality of life. The ObGyn arranged for the patient to undergo a third-trimester abortion in Kansas and paid all expenses. On July 14, the patient began the 5-day abortion process at 30+ weeks’ gestation.
PATIENT’S CLAIM:
She was never offered additional genetic testing or expedited amniocentesis. She was not told that abortion is illegal in Illinois after 23 6/7 weeks’ gestation. The ObGyn had a motive for paying for her abortion. He never counseled her about options to keep the child. She endured extreme pain and emotional trauma during the abortion and was later found to have posttraumatic stress disorder, multidepressive disorder, and anxiety as a result of the experience. She countered the ObGyn’s contact information claim by saying that her phone number had not changed.
PHYSICIAN’S DEFENSE:
The ObGyn admitted negligence in failing to timely communicate test results but contended that the patient was more than 50% responsible for any delay by failing to update her contact information when she moved. The ObGyn denied causation of any injuries or damage.
VERDICT:
A $1,439,250 Illinois verdict was returned.
Related article:
4 Supreme Court decisions important to ObGyns from the 2015−2016 term
Did delay in delivery cause infant's death?
A woman presented to the hospital in labor. During delivery, the patient’s ObGyn encountered shoulder dystocia. The infant died shortly after birth.
PARENTS’ CLAIM:
The ObGyn and hospital nurses were negligent. The nurses failed to monitor labor and properly communicate with the ObGyn. The ObGyn failed to appreciate the baby’s large size and order a cesarean delivery. The infant’s death was due to a hypoxic event during delivery.
DEFENDANTS’ DEFENSE:
The baby gained an unexpected amount of weight between the last prenatal visit and labor. There was no reason to expect a complication to vaginal delivery. The nurses denied negligence. The child’s sudden death was caused by a genetic cardiac condition.
VERDICT:
A Tennessee defense verdict was returned.
Related article:
Shoulder dystocia: Taking the fear out of management
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
She wanted to labor on hands and knees
During prenatal visits, a woman, pregnant with her fourth child, discussed undergoing labor and delivery in any position other than on her back; the ObGyn agreed. When she arrived at the hospital in labor, the patient told the nurse that she preferred to labor on her hands and knees. The nurse disagreed because of the fetal heart-rate monitor.
When the patient began hard labor, she turned herself over onto h
Two months later, the patient reported chronic severe pelvic pain and was found to have pudendal neuralgia. She underwent nerve blocks and takes medication for chronic pain.
PATIENT’S CLAIM:
The ObGyn did not assume responsibility when he arrived for the delivery. The nurses did not follow the standard of care. The patient’s injury was the result of tension and compression due to malpositioning of the patient’s legs during delivery.
DEFENDANTS’ DEFENSE:
There was no breach in the standard of care. The patient’s injury, if any, had not been caused by the delivery.
VERDICT:
A $16 million Alabama verdict was returned.
Related article:
10 tips for overcoming common challenges of intrapartum fetal monitoring
Late-term abortion: $1.4M award
Although genetic testing was scheduled for a 37-year-old woman’s 15-week prenatal visit, the ObGyn’s staff failed to draw blood. At 19 weeks’ gestation (April 24), blood was drawn. The ObGyn signed off on test results that showed a high risk for fetal anomaly on May 2, but the patient was not informed until May 22. The ObGyn scheduled amniocentesis for June 3. On May 30, the hospital, based in Illinois, cancelled the test, telling the ObGyn that it was because the patient was over 24 weeks’ pregnant and there was no labor and delivery unit to respond if complications arose. Instead of notifying the patient, the ObGyn arranged for amniocentesis to be performed elsewhere on June 3. The ObGyn saw the amniocentesis results on June 13, but did not tell the patient until July 3, when he advised her to terminate the pregnancy because the baby had severe cardiac defects and Down syndrome; he felt the child would not survive or have very poor quality of life. The ObGyn arranged for the patient to undergo a third-trimester abortion in Kansas and paid all expenses. On July 14, the patient began the 5-day abortion process at 30+ weeks’ gestation.
PATIENT’S CLAIM:
She was never offered additional genetic testing or expedited amniocentesis. She was not told that abortion is illegal in Illinois after 23 6/7 weeks’ gestation. The ObGyn had a motive for paying for her abortion. He never counseled her about options to keep the child. She endured extreme pain and emotional trauma during the abortion and was later found to have posttraumatic stress disorder, multidepressive disorder, and anxiety as a result of the experience. She countered the ObGyn’s contact information claim by saying that her phone number had not changed.
PHYSICIAN’S DEFENSE:
The ObGyn admitted negligence in failing to timely communicate test results but contended that the patient was more than 50% responsible for any delay by failing to update her contact information when she moved. The ObGyn denied causation of any injuries or damage.
VERDICT:
A $1,439,250 Illinois verdict was returned.
Related article:
4 Supreme Court decisions important to ObGyns from the 2015−2016 term
Did delay in delivery cause infant's death?
A woman presented to the hospital in labor. During delivery, the patient’s ObGyn encountered shoulder dystocia. The infant died shortly after birth.
PARENTS’ CLAIM:
The ObGyn and hospital nurses were negligent. The nurses failed to monitor labor and properly communicate with the ObGyn. The ObGyn failed to appreciate the baby’s large size and order a cesarean delivery. The infant’s death was due to a hypoxic event during delivery.
DEFENDANTS’ DEFENSE:
The baby gained an unexpected amount of weight between the last prenatal visit and labor. There was no reason to expect a complication to vaginal delivery. The nurses denied negligence. The child’s sudden death was caused by a genetic cardiac condition.
VERDICT:
A Tennessee defense verdict was returned.
Related article:
Shoulder dystocia: Taking the fear out of management
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Palliative care ‘in my hands’
A randomized controlled multicenter study published by Carson et al. in JAMA concluded that, for patients with “chronic critical illness” (defined as requiring 7 days of mechanical ventilation), palliative care team-led informational and emotional support meetings did not reduce anxiety or depression for families and may have increased posttraumatic stress disorder symptoms (2016:316[1]:51-62. doi: 10.1001/jama.2016.8474).
This report may surprise surgeons, as well as practitioners in other specialties, as the disconnect between palliative care and critical care services has been previously perceived as an education and access issue, not an outcome problem.
Carson and colleagues point out that fidelity to some components of the meeting “templates” was low, suggesting that there was some flexibility baked into the study design. However, as Russ and Kaufman aptly described, patients and families vary greatly in their appetite for explicit information about prognosis (Cult Med Psychiatry 2005;29[1]:103-23). Conversely, the hypothesis that direct communication about prognosis will be welcomed by families is a core element of the Carson study. The manuscript supplement reports that discussion of the patient’s condition and prognosis took place in 100% of initial meetings. If the same variability in family receptiveness to this information exists in this population as was described by Russ and Kaufman, it is not hard to see why some families experienced negative consequences because of these discussions.
Furthermore, the authors of the Carson study point out that it was not intended to replicate the components of specialist palliative care (JAMA. 2016;316[15]:1598-9).
Essential elements of specialist palliative care include symptom management, a multidisciplinary approach, and fairly close contact in the acute care setting. These features were lacking in the study protocol. Experienced providers of palliative care will often use symptom assessment and symptom management optimization as a conduit for building rapport and to avoid focusing on prognosis until trust has been established. A period of delay before broaching challenging subjects also allows the palliative care team to develop an understanding of the patient’s or surrogates’ preferences regarding the amount and type of information communicated. Palliative care providers benefit from the deepening of relationships with patients and families over time, as much as or possibly more so than providers of other specialties.
The necessity of the multidisciplinary approach to successful palliative care outcomes cannot be overstated. In many programs, patients seen for specialist palliative care consultation are seen by a physician or advanced practitioner, a chaplain, and a social worker within 24-48 hours of initial referral, and these providers have key roles in addressing the sequelae of anxiety, depression, and stress that were the key outcomes in the JAMA study. In the study, the “support and information team” included a palliative care physician and an advanced practice nurse but not a chaplain or social worker, despite the significance of existential/spiritual and social consequences of ventilator withdrawal or progression to tracheostomy for long-term vent support.
Palliative care providers consider the family meeting to be the “procedure” of their field, a belief that may seem incongruous with a surgical understanding of the nature of procedures but is informative as a framework for understanding the results of the Carson study. Just as surgical procedures carry risk of complications or adverse outcomes, family meetings have risk for worsening instead of improving the coping of families and surrogates. And, as surgical technique can be connected to complications, the family meeting technique applied by Carson et al. may be related to its results. Although there was formalized communication between the ICU team and the palliative care team regarding the patient’s condition, prognosis, and treatment plan, there was not a representative from the critical care team present during the majority of the support and information team led family meetings. This represents a marked deviation from common practice at our institution and many others. Our usual practice is to have a member of the ICU team present for discussions focused on patient prognosis, in order to make sure that there is alignment between the messages of the ICU and palliative care teams and also to prevent the crippling of palliative support that occurs when it becomes the sole repository of unwelcome news.
Because the relief of suffering is a core value of surgery and palliative care, there are countless ways these disciplines can inform one another. The outcome of the Carson study is a cautionary tale about the fallibility of the integration of surgical and palliative care teams, both of which would acknowledge the importance of the multidisciplinary approach, relationships developed over time, and symptom management. As surgeons intuitively understand from their operative experience, the “procedure” (the family meeting) has the potential for both risk and benefit, the outcome of which may be determined “in my hands.”
Dr. Rivet is a colon and rectal surgeon with training and board certification in hospice and palliative medicine. She is an assistant professor, departments of surgery and internal medicine, Virginia Commonwealth University, Richmond. She has no disclosures.
A randomized controlled multicenter study published by Carson et al. in JAMA concluded that, for patients with “chronic critical illness” (defined as requiring 7 days of mechanical ventilation), palliative care team-led informational and emotional support meetings did not reduce anxiety or depression for families and may have increased posttraumatic stress disorder symptoms (2016:316[1]:51-62. doi: 10.1001/jama.2016.8474).
This report may surprise surgeons, as well as practitioners in other specialties, as the disconnect between palliative care and critical care services has been previously perceived as an education and access issue, not an outcome problem.
Carson and colleagues point out that fidelity to some components of the meeting “templates” was low, suggesting that there was some flexibility baked into the study design. However, as Russ and Kaufman aptly described, patients and families vary greatly in their appetite for explicit information about prognosis (Cult Med Psychiatry 2005;29[1]:103-23). Conversely, the hypothesis that direct communication about prognosis will be welcomed by families is a core element of the Carson study. The manuscript supplement reports that discussion of the patient’s condition and prognosis took place in 100% of initial meetings. If the same variability in family receptiveness to this information exists in this population as was described by Russ and Kaufman, it is not hard to see why some families experienced negative consequences because of these discussions.
Furthermore, the authors of the Carson study point out that it was not intended to replicate the components of specialist palliative care (JAMA. 2016;316[15]:1598-9).
Essential elements of specialist palliative care include symptom management, a multidisciplinary approach, and fairly close contact in the acute care setting. These features were lacking in the study protocol. Experienced providers of palliative care will often use symptom assessment and symptom management optimization as a conduit for building rapport and to avoid focusing on prognosis until trust has been established. A period of delay before broaching challenging subjects also allows the palliative care team to develop an understanding of the patient’s or surrogates’ preferences regarding the amount and type of information communicated. Palliative care providers benefit from the deepening of relationships with patients and families over time, as much as or possibly more so than providers of other specialties.
The necessity of the multidisciplinary approach to successful palliative care outcomes cannot be overstated. In many programs, patients seen for specialist palliative care consultation are seen by a physician or advanced practitioner, a chaplain, and a social worker within 24-48 hours of initial referral, and these providers have key roles in addressing the sequelae of anxiety, depression, and stress that were the key outcomes in the JAMA study. In the study, the “support and information team” included a palliative care physician and an advanced practice nurse but not a chaplain or social worker, despite the significance of existential/spiritual and social consequences of ventilator withdrawal or progression to tracheostomy for long-term vent support.
Palliative care providers consider the family meeting to be the “procedure” of their field, a belief that may seem incongruous with a surgical understanding of the nature of procedures but is informative as a framework for understanding the results of the Carson study. Just as surgical procedures carry risk of complications or adverse outcomes, family meetings have risk for worsening instead of improving the coping of families and surrogates. And, as surgical technique can be connected to complications, the family meeting technique applied by Carson et al. may be related to its results. Although there was formalized communication between the ICU team and the palliative care team regarding the patient’s condition, prognosis, and treatment plan, there was not a representative from the critical care team present during the majority of the support and information team led family meetings. This represents a marked deviation from common practice at our institution and many others. Our usual practice is to have a member of the ICU team present for discussions focused on patient prognosis, in order to make sure that there is alignment between the messages of the ICU and palliative care teams and also to prevent the crippling of palliative support that occurs when it becomes the sole repository of unwelcome news.
Because the relief of suffering is a core value of surgery and palliative care, there are countless ways these disciplines can inform one another. The outcome of the Carson study is a cautionary tale about the fallibility of the integration of surgical and palliative care teams, both of which would acknowledge the importance of the multidisciplinary approach, relationships developed over time, and symptom management. As surgeons intuitively understand from their operative experience, the “procedure” (the family meeting) has the potential for both risk and benefit, the outcome of which may be determined “in my hands.”
Dr. Rivet is a colon and rectal surgeon with training and board certification in hospice and palliative medicine. She is an assistant professor, departments of surgery and internal medicine, Virginia Commonwealth University, Richmond. She has no disclosures.
A randomized controlled multicenter study published by Carson et al. in JAMA concluded that, for patients with “chronic critical illness” (defined as requiring 7 days of mechanical ventilation), palliative care team-led informational and emotional support meetings did not reduce anxiety or depression for families and may have increased posttraumatic stress disorder symptoms (2016:316[1]:51-62. doi: 10.1001/jama.2016.8474).
This report may surprise surgeons, as well as practitioners in other specialties, as the disconnect between palliative care and critical care services has been previously perceived as an education and access issue, not an outcome problem.
Carson and colleagues point out that fidelity to some components of the meeting “templates” was low, suggesting that there was some flexibility baked into the study design. However, as Russ and Kaufman aptly described, patients and families vary greatly in their appetite for explicit information about prognosis (Cult Med Psychiatry 2005;29[1]:103-23). Conversely, the hypothesis that direct communication about prognosis will be welcomed by families is a core element of the Carson study. The manuscript supplement reports that discussion of the patient’s condition and prognosis took place in 100% of initial meetings. If the same variability in family receptiveness to this information exists in this population as was described by Russ and Kaufman, it is not hard to see why some families experienced negative consequences because of these discussions.
Furthermore, the authors of the Carson study point out that it was not intended to replicate the components of specialist palliative care (JAMA. 2016;316[15]:1598-9).
Essential elements of specialist palliative care include symptom management, a multidisciplinary approach, and fairly close contact in the acute care setting. These features were lacking in the study protocol. Experienced providers of palliative care will often use symptom assessment and symptom management optimization as a conduit for building rapport and to avoid focusing on prognosis until trust has been established. A period of delay before broaching challenging subjects also allows the palliative care team to develop an understanding of the patient’s or surrogates’ preferences regarding the amount and type of information communicated. Palliative care providers benefit from the deepening of relationships with patients and families over time, as much as or possibly more so than providers of other specialties.
The necessity of the multidisciplinary approach to successful palliative care outcomes cannot be overstated. In many programs, patients seen for specialist palliative care consultation are seen by a physician or advanced practitioner, a chaplain, and a social worker within 24-48 hours of initial referral, and these providers have key roles in addressing the sequelae of anxiety, depression, and stress that were the key outcomes in the JAMA study. In the study, the “support and information team” included a palliative care physician and an advanced practice nurse but not a chaplain or social worker, despite the significance of existential/spiritual and social consequences of ventilator withdrawal or progression to tracheostomy for long-term vent support.
Palliative care providers consider the family meeting to be the “procedure” of their field, a belief that may seem incongruous with a surgical understanding of the nature of procedures but is informative as a framework for understanding the results of the Carson study. Just as surgical procedures carry risk of complications or adverse outcomes, family meetings have risk for worsening instead of improving the coping of families and surrogates. And, as surgical technique can be connected to complications, the family meeting technique applied by Carson et al. may be related to its results. Although there was formalized communication between the ICU team and the palliative care team regarding the patient’s condition, prognosis, and treatment plan, there was not a representative from the critical care team present during the majority of the support and information team led family meetings. This represents a marked deviation from common practice at our institution and many others. Our usual practice is to have a member of the ICU team present for discussions focused on patient prognosis, in order to make sure that there is alignment between the messages of the ICU and palliative care teams and also to prevent the crippling of palliative support that occurs when it becomes the sole repository of unwelcome news.
Because the relief of suffering is a core value of surgery and palliative care, there are countless ways these disciplines can inform one another. The outcome of the Carson study is a cautionary tale about the fallibility of the integration of surgical and palliative care teams, both of which would acknowledge the importance of the multidisciplinary approach, relationships developed over time, and symptom management. As surgeons intuitively understand from their operative experience, the “procedure” (the family meeting) has the potential for both risk and benefit, the outcome of which may be determined “in my hands.”
Dr. Rivet is a colon and rectal surgeon with training and board certification in hospice and palliative medicine. She is an assistant professor, departments of surgery and internal medicine, Virginia Commonwealth University, Richmond. She has no disclosures.