User login
What are the chances?
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
Two weeks after undergoing a below-knee amputation (BKA) and 10 days after being discharged to a skilled nursing facility (SNF), an 87-year-old man returned to the emergency department (ED) for evaluation of somnolence and altered mental state. In the ED, he was disoriented and unable to provide a detailed history.
The differential diagnosis for acute confusion and altered consciousness is broad. Initial possibilities include toxic-metabolic abnormalities, medication side effects, and infections. Urinary tract infection, pneumonia, and surgical-site infection should be assessed for first, as they are common causes of postoperative altered mentation. Next to be considered are subclinical seizure, ischemic stroke, and infectious encephalitis or meningitis, along with hemorrhagic stroke and subdural hematoma.
During initial assessment, the clinician should ascertain baseline mental state, the timeline of the change in mental status, recent medication changes, history of substance abuse, and concern about any recent trauma, such as a fall. Performing the physical examination, the clinician should assess vital signs and then focus on identifying localizing neurologic deficits.
First steps in the work-up include a complete metabolic panel, complete blood cell count, urinalysis with culture, and a urine toxicology screen. If the patient has a “toxic” appearance, blood cultures should be obtained. An electrocardiogram should be used to screen for drug toxicity or evidence of cardiac ischemia. If laboratory test results do not reveal an obvious infectious or metabolic cause, a noncontrast computed tomography (CT) of the head should be obtained. In terms of early interventions, a low glucose level should be treated with thiamine and then glucose, and naloxone should be given if there is any suspicion of narcotic overdose.
More history was obtained from the patient’s records. The BKA was performed to address a nonhealing transmetatarsal amputation. Two months earlier, the transmetatarsal amputation had been performed as treatment for a diabetic forefoot ulcer with chronic osteomyelitis. The patient’s post-BKA course was uncomplicated. He was started on intravenous (IV) ertapenem on postoperative day 1, and on postoperative day 4 was discharged to the SNF to complete a 6-week course of antibiotics for osteomyelitis. Past medical history included paroxysmal atrial fibrillation, coronary artery disease, congestive heart failure (ejection fraction 40%), and type 2 diabetes mellitus. Medications given at the SNF were oxycodone, acetaminophen, cholecalciferol, melatonin, digoxin, ondansetron, furosemide, gabapentin, correctional insulin, tamsulosin, senna, docusate, warfarin, and metoprolol. While there, the patient’s family expressed concern about his diminishing “mental ability.” They reported he had been fully alert and oriented on arrival at the SNF, and living independently with his wife before the BKA. Then, a week before the ED presentation, he started becoming more somnolent and forgetful. The gabapentin and oxycodone dosages were reduced to minimize their sedative effects, but he showed no improvement. At the SNF, a somnolence work-up was not performed.
Several of the patient’s medications can contribute to altered mental state. Ertapenem can cause seizures as well as profound mental status changes, though these are more likely in the setting of poor renal function. The mental status changes were noticed about a week into the patient’s course of antibiotics, which suggests a possible temporal correlation with the initiation of ertapenem. An electroencephalogram is required to diagnose nonconvulsive seizure activity. Narcotic overdose should still be considered, despite the recent reduction in oxycodone dosage. Digoxin toxicity, though less likely when the dose is stable and there are no changes in renal function, can cause a confused state. Concurrent use of furosemide could potentiate the toxic effects of digoxin.
Non-medication-related concerns include hypoglycemia, hyperglycemia, and, given his history of atrial fibrillation, cardioembolic stroke. Although generalized confusion is not a common manifestation of stroke, a thalamic stroke can alter mental state but be easily missed if not specifically considered. Additional lab work-up should include a digoxin level and, since he is taking warfarin, a prothrombin time/international normalized ratio (PT/INR). If the initial laboratory studies and head CT do not explain the altered mental state, magnetic resonance imaging (MRI) of the brain should be performed to further assess for stroke.
On physical examination in the ED, the patient was resting comfortably with eyes closed, and arousing to voice. He obeyed commands and participated in the examination. His Glasgow Coma Scale score was 13; temperature, 36.8°C, heart rate, 80 beats per minute; respiratory rate, 16 breaths per minute; blood pressure, 90/57 mm Hg; and 100% peripheral capillary oxygen saturation while breathing ambient air. He appeared well developed. His heart rhythm was irregularly irregular, without murmurs, rubs, or gallops. Respiratory and abdominal examination findings were normal. The left BKA incision was well approximated, with no drainage, dehiscence, fluctuance, or erythema. On neurologic examination, the patient was intermittently oriented only to self. Pupils were equal, round, and reactive to light; extraocular movements were intact; face was symmetric; tongue was midline; sensation on face was equal bilaterally; and shoulder shrug was intact. Strength was 5/5 and symmetric in the elbow and hip and 5/5 in the right knee and ankle (not tested on left because of BKA). Deep tendon reflexes were 3+ and symmetrical at the biceps, brachioradialis, and triceps tendons and 3+ in the right patellar and Achilles tendons. Sensation was intact and symmetrical in the upper and lower extremities. The patient’s speech was slow and slurred, and his answers were unrelated to the questions being asked.
The patient’s mental state is best described as lethargic. As he is only intermittently oriented, he meets the criteria for delirium. He is not obtunded or comatose, and his pupils are at least reactive, not pinpoint, so narcotic overdose is less likely. Thalamic stroke remains in the differential diagnosis; despite the seemingly symmetrical sensation examination, hemisensory deficits cannot be definitively ruled out given the patient’s mental state. A rare entity such as carcinomatosis meningitis or another diffuse, infiltrative neoplastic process could be causing his condition. However, because focal deficits other than abnormal speech and diffuse hyperreflexia are absent, toxic, infectious, or metabolic causes are more likely than structural abnormalities. Still possible is a medication toxicity, such as ertapenem toxicity or, less likely, digoxin toxicity. In terms of infectious possibilities, urinary tract infection could certainly present in this fashion, especially if the patient had a somewhat low neurologic reserve at baseline, and hypotension could be secondary to sepsis. Encephalitis or meningitis remains in the differential diagnosis, though the patient appears nontoxic, and therefore a bacterial etiology is very unlikely.
The patient’s hyperreflexia may be an important clue. Although the strength of his reflexes at baseline is unknown, seizures can cause transiently increased reflexes as well as a confused, lethargic mental state. Reflexes can also be increased by a drug overdose that has caused serotonin syndrome. Of the patient’s medications, only ondansetron can cause this reaction. Hyperthyroidism can cause brisk reflexes and confusion, though more typically it causes agitated confusion. A thyroid-stimulating hormone level should be added to the initial laboratory panel.
A complete blood count revealed white blood cell count 11.86 K/uL with neutrophilic predominance and immature granulocytes, hemoglobin 11.5 g/dL, and platelet count 323 K/uL. Serum sodium was 141 mEq/L, potassium 4.2 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, creatinine 1.14 mg/dL (prior baseline of 0.8-1.0 mg/dL), blood urea nitrogen 26 mg/dL, blood glucose 159 mg/dL, and calcium 9.1 mg/dL. His digoxin level was 1.3 ng/mL (reference range 0.5-1.9 mg/mL) and troponin was undetectable. INR was 2.7 and partial thromboplastin time (PTT) 60 seconds. Vitamin B12 level was 674 pg/mL (reference range >180). A urinalysis had 1+ hyaline casts and was negative for nitrites, leukocyte esterase, blood, and bacteria. An ECG revealed atrial fibrillation with a ventricular rate of 80 beats per minute. A chest radiograph showed clear lung fields. A CT of the head without IV contrast had no evidence of an acute intracranial abnormality. In the ED, 1 liter of IV normal saline was given and blood pressure improved to 127/72 mm Hg.
The head CT does not show intracranial bleeding, and, though it is reassuring that INR is in the therapeutic range, ischemic stroke must remain in the differential diagnosis. Sepsis is less likely given that the criteria for systemic inflammatory response syndrome are not met, and hypotension was rapidly corrected with administration of IV fluids. Urinary tract infection was ruled out with the negative urinalysis. Subclinical seizures remain possible, as does medication-related or other toxicity. A medication overdose, intentional or otherwise, should also be considered.
The patient was admitted to the hospital. On reassessment by the inpatient team, he was oriented only to self, frequently falling asleep, and not recalling earlier conversations when aroused. His speech remained slurred and difficult to understand. Neurologic examination findings were unchanged since the ED examination. On additional cerebellar examination, he had dysmetria with finger-to-nose testing bilaterally and dysdiadochokinesia (impaired rapid alternating movements) of the left hand.
His handedness is not mentioned; the dysdiadochokinesia of the left hand may reflect the patient’s being right-handed, or may signify a focal cerebellar lesion. The cerebellum is also implicated by the bilateral dysmetria. Persistent somnolence in the absence of CT findings suggests a metabolic or infectious process. Metabolic processes that can cause bilateral cerebellar ataxia and somnolence include overdose of a drug or medication. Use of alcohol or a medication such as phenytoin, valproic acid, or a benzodiazepine can cause the symptoms in this case, but was not reported by the family, and there was no documentation of it in the SNF records. Wernicke encephalopathy is rare and is not well supported by the patient’s presentation but should be considered, as it can be easily treated with thiamine. Meningoencephalitis affecting the cerebellum remains possible, but infection is less likely. Both electroencephalogram and brain MRI should be performed, with a specific interest in possible cerebellar lesions. If the MRI is unremarkable, a lumbar puncture should be performed to assess opening pressure and investigate for infectious etiologies.
MRI of the brain showed age-related volume loss and nonspecific white matter disease without acute changes. Lack of a clear explanation for the neurologic findings led to suspicion of a medication side effect. Ertapenem was stopped on admission because it has been reported to rarely cause altered mental status. IV moxifloxacin was started for the osteomyelitis. Over the next 2 days, symptoms began resolving; within 24 hours of ertapenem discontinuation, the patient was awake, alert, and talkative. On examination, he remained dysarthric but was no longer dysmetric. Within 48 hours, the dysarthria was completely resolved, and he was returned to the SNF to complete a course of IV moxifloxacin.
DISCUSSION
Among elderly patients presenting to the ED, altered mental status is a common complaint, accounting for 10% to 30% of visits.1 Medications are a common cause of altered mental status among the elderly and are responsible for 40% of delirium cases.1 The risk of adverse drug events (ADEs) rises with the number of medications prescribed.1-3 Among patients older than 60 years, the incidence of polypharmacy (defined as taking >5 prescription medications) increased from roughly 20% in 1999 to 40% in 2012.4,5 The most common ADEs in the ambulatory setting (25%) are central nervous system (CNS) symptoms, including dizziness, sleep disturbances, and mood changes.6 A medication effect should be suspected in any elderly patient presenting with altered mental state.
The present patient developed a constellation of neurologic symptoms after starting ertapenem, one of the carbapenem antibiotics, which is a class of medications that can cause CNS ADEs. Carbapenems are renally cleared, and adjustments must be made for acute or chronic changes in kidney function. Carbapenems are associated with increased risk of seizure; the incidence of seizure with ertapenem is 0.2%.7,8 Food and Drug Administration postmarketing reports have noted ertapenem can cause somnolence and dyskinesia,9 and several case reports have described ertapenem-associated CNS side effects, including psychosis and encephalopathy.10-13 Symptoms and examination findings can include confusion, disorientation, garbled speech, dysphagia, hallucinations, miosis, myoclonus, tremor, and agitation.10-13 Although reports of dysmetria and dysdiadochokinesia are lacking, suspicion of an ADE in this case was heightened by the timing of the exposure and the absence of alternative infectious, metabolic, and vascular explanations for bilateral cerebellar dysfunction.
The Naranjo Adverse Drug Reaction (ADR) scale may help clinicians differentiate ADEs from other etiologies of symptoms. It uses 10 weighted questions (Table) to estimate the probability that an adverse clinical event is caused by a drug reaction.14 The present case was assigned 1 point for prior reports of neurologic ADEs associated with ertapenem, 2 for the temporal association, 1 for resolution after medication withdrawal, 2 for lack of alternative causes, and 1 for objective evidence of neurologic dysfunction—for a total of 7 points, indicating ertapenem was probably the cause of the patient’s neurologic symptoms. Of 4 prior cases in which carbapenem toxicity was suspected and the Naranjo scale was used, 3 found a probable relationship, and the fourth a highly probable one.10,12 Confusion, disorientation, hallucinations, tangential thoughts, and garbled speech were reported in the 3 probable cases of ADEs. In the highly probable case, tangential thoughts, garbled speech, and miosis were noted on examination, and these findings returned after re-exposure to ertapenem. Of note, these ADEs occurred in patients with normal and abnormal renal function, and in middle-aged and elderly patients.10,11,13
Most medications have a long list of low-frequency and rarely reported adverse effects. The present case reminds clinicians to consider rare adverse effects, or variants of previously reported adverse effects, in a patient with unexplained symptoms. To estimate the probability that a drug is causing harm to a patient, using a validated tool such as the Naranjo scale helps answer the question, What are the chances?
KEY TEACHING POINTS
Clinicians should include rare adverse effects of common medications in the differential diagnosis.
The Naranjo score is a validated tool that can be used to systematically assess the probability of an adverse drug effect at the bedside.
- The presentation of ertapenem-associated neurotoxicity may include features of bilateral cerebellar dysfunction.
Disclosure
Nothing to report.
1. Inouye SK, Fearing MA, Marcantonio ER. Delirium. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009.
2. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46(5):1517-1533. PubMed
3. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55(1):29-34. PubMed
4. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. PubMed
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed
6. Thomsen LA, Winterstein AG, Søndergaard B, Haugbølle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411-1426. PubMed
7. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. PubMed
8. Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043-2055. PubMed
9. US Food and Drug Administration. Invanz (ertapenem) injection [safety information]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm196605.htm. Published July 2013. Accessed July 6, 2015.
10. Oo Y, Packham D, Yau W, Munckhof WJ. Ertapenem-associated psychosis and encephalopathy. Intern Med J. 2014;44(8):817-819. PubMed
11. Wen MJ, Sung CC, Chau T, Lin SH. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol. 2013;80(6):474-478. PubMed
12. Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother. 2011;45(1):e6. PubMed
13. Kong V, Beckert L, Awunor-Renner C. A case of beta lactam-induced visual hallucination. N Z Med J. 2009;122(1298):76-77. PubMed
14. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
Two weeks after undergoing a below-knee amputation (BKA) and 10 days after being discharged to a skilled nursing facility (SNF), an 87-year-old man returned to the emergency department (ED) for evaluation of somnolence and altered mental state. In the ED, he was disoriented and unable to provide a detailed history.
The differential diagnosis for acute confusion and altered consciousness is broad. Initial possibilities include toxic-metabolic abnormalities, medication side effects, and infections. Urinary tract infection, pneumonia, and surgical-site infection should be assessed for first, as they are common causes of postoperative altered mentation. Next to be considered are subclinical seizure, ischemic stroke, and infectious encephalitis or meningitis, along with hemorrhagic stroke and subdural hematoma.
During initial assessment, the clinician should ascertain baseline mental state, the timeline of the change in mental status, recent medication changes, history of substance abuse, and concern about any recent trauma, such as a fall. Performing the physical examination, the clinician should assess vital signs and then focus on identifying localizing neurologic deficits.
First steps in the work-up include a complete metabolic panel, complete blood cell count, urinalysis with culture, and a urine toxicology screen. If the patient has a “toxic” appearance, blood cultures should be obtained. An electrocardiogram should be used to screen for drug toxicity or evidence of cardiac ischemia. If laboratory test results do not reveal an obvious infectious or metabolic cause, a noncontrast computed tomography (CT) of the head should be obtained. In terms of early interventions, a low glucose level should be treated with thiamine and then glucose, and naloxone should be given if there is any suspicion of narcotic overdose.
More history was obtained from the patient’s records. The BKA was performed to address a nonhealing transmetatarsal amputation. Two months earlier, the transmetatarsal amputation had been performed as treatment for a diabetic forefoot ulcer with chronic osteomyelitis. The patient’s post-BKA course was uncomplicated. He was started on intravenous (IV) ertapenem on postoperative day 1, and on postoperative day 4 was discharged to the SNF to complete a 6-week course of antibiotics for osteomyelitis. Past medical history included paroxysmal atrial fibrillation, coronary artery disease, congestive heart failure (ejection fraction 40%), and type 2 diabetes mellitus. Medications given at the SNF were oxycodone, acetaminophen, cholecalciferol, melatonin, digoxin, ondansetron, furosemide, gabapentin, correctional insulin, tamsulosin, senna, docusate, warfarin, and metoprolol. While there, the patient’s family expressed concern about his diminishing “mental ability.” They reported he had been fully alert and oriented on arrival at the SNF, and living independently with his wife before the BKA. Then, a week before the ED presentation, he started becoming more somnolent and forgetful. The gabapentin and oxycodone dosages were reduced to minimize their sedative effects, but he showed no improvement. At the SNF, a somnolence work-up was not performed.
Several of the patient’s medications can contribute to altered mental state. Ertapenem can cause seizures as well as profound mental status changes, though these are more likely in the setting of poor renal function. The mental status changes were noticed about a week into the patient’s course of antibiotics, which suggests a possible temporal correlation with the initiation of ertapenem. An electroencephalogram is required to diagnose nonconvulsive seizure activity. Narcotic overdose should still be considered, despite the recent reduction in oxycodone dosage. Digoxin toxicity, though less likely when the dose is stable and there are no changes in renal function, can cause a confused state. Concurrent use of furosemide could potentiate the toxic effects of digoxin.
Non-medication-related concerns include hypoglycemia, hyperglycemia, and, given his history of atrial fibrillation, cardioembolic stroke. Although generalized confusion is not a common manifestation of stroke, a thalamic stroke can alter mental state but be easily missed if not specifically considered. Additional lab work-up should include a digoxin level and, since he is taking warfarin, a prothrombin time/international normalized ratio (PT/INR). If the initial laboratory studies and head CT do not explain the altered mental state, magnetic resonance imaging (MRI) of the brain should be performed to further assess for stroke.
On physical examination in the ED, the patient was resting comfortably with eyes closed, and arousing to voice. He obeyed commands and participated in the examination. His Glasgow Coma Scale score was 13; temperature, 36.8°C, heart rate, 80 beats per minute; respiratory rate, 16 breaths per minute; blood pressure, 90/57 mm Hg; and 100% peripheral capillary oxygen saturation while breathing ambient air. He appeared well developed. His heart rhythm was irregularly irregular, without murmurs, rubs, or gallops. Respiratory and abdominal examination findings were normal. The left BKA incision was well approximated, with no drainage, dehiscence, fluctuance, or erythema. On neurologic examination, the patient was intermittently oriented only to self. Pupils were equal, round, and reactive to light; extraocular movements were intact; face was symmetric; tongue was midline; sensation on face was equal bilaterally; and shoulder shrug was intact. Strength was 5/5 and symmetric in the elbow and hip and 5/5 in the right knee and ankle (not tested on left because of BKA). Deep tendon reflexes were 3+ and symmetrical at the biceps, brachioradialis, and triceps tendons and 3+ in the right patellar and Achilles tendons. Sensation was intact and symmetrical in the upper and lower extremities. The patient’s speech was slow and slurred, and his answers were unrelated to the questions being asked.
The patient’s mental state is best described as lethargic. As he is only intermittently oriented, he meets the criteria for delirium. He is not obtunded or comatose, and his pupils are at least reactive, not pinpoint, so narcotic overdose is less likely. Thalamic stroke remains in the differential diagnosis; despite the seemingly symmetrical sensation examination, hemisensory deficits cannot be definitively ruled out given the patient’s mental state. A rare entity such as carcinomatosis meningitis or another diffuse, infiltrative neoplastic process could be causing his condition. However, because focal deficits other than abnormal speech and diffuse hyperreflexia are absent, toxic, infectious, or metabolic causes are more likely than structural abnormalities. Still possible is a medication toxicity, such as ertapenem toxicity or, less likely, digoxin toxicity. In terms of infectious possibilities, urinary tract infection could certainly present in this fashion, especially if the patient had a somewhat low neurologic reserve at baseline, and hypotension could be secondary to sepsis. Encephalitis or meningitis remains in the differential diagnosis, though the patient appears nontoxic, and therefore a bacterial etiology is very unlikely.
The patient’s hyperreflexia may be an important clue. Although the strength of his reflexes at baseline is unknown, seizures can cause transiently increased reflexes as well as a confused, lethargic mental state. Reflexes can also be increased by a drug overdose that has caused serotonin syndrome. Of the patient’s medications, only ondansetron can cause this reaction. Hyperthyroidism can cause brisk reflexes and confusion, though more typically it causes agitated confusion. A thyroid-stimulating hormone level should be added to the initial laboratory panel.
A complete blood count revealed white blood cell count 11.86 K/uL with neutrophilic predominance and immature granulocytes, hemoglobin 11.5 g/dL, and platelet count 323 K/uL. Serum sodium was 141 mEq/L, potassium 4.2 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, creatinine 1.14 mg/dL (prior baseline of 0.8-1.0 mg/dL), blood urea nitrogen 26 mg/dL, blood glucose 159 mg/dL, and calcium 9.1 mg/dL. His digoxin level was 1.3 ng/mL (reference range 0.5-1.9 mg/mL) and troponin was undetectable. INR was 2.7 and partial thromboplastin time (PTT) 60 seconds. Vitamin B12 level was 674 pg/mL (reference range >180). A urinalysis had 1+ hyaline casts and was negative for nitrites, leukocyte esterase, blood, and bacteria. An ECG revealed atrial fibrillation with a ventricular rate of 80 beats per minute. A chest radiograph showed clear lung fields. A CT of the head without IV contrast had no evidence of an acute intracranial abnormality. In the ED, 1 liter of IV normal saline was given and blood pressure improved to 127/72 mm Hg.
The head CT does not show intracranial bleeding, and, though it is reassuring that INR is in the therapeutic range, ischemic stroke must remain in the differential diagnosis. Sepsis is less likely given that the criteria for systemic inflammatory response syndrome are not met, and hypotension was rapidly corrected with administration of IV fluids. Urinary tract infection was ruled out with the negative urinalysis. Subclinical seizures remain possible, as does medication-related or other toxicity. A medication overdose, intentional or otherwise, should also be considered.
The patient was admitted to the hospital. On reassessment by the inpatient team, he was oriented only to self, frequently falling asleep, and not recalling earlier conversations when aroused. His speech remained slurred and difficult to understand. Neurologic examination findings were unchanged since the ED examination. On additional cerebellar examination, he had dysmetria with finger-to-nose testing bilaterally and dysdiadochokinesia (impaired rapid alternating movements) of the left hand.
His handedness is not mentioned; the dysdiadochokinesia of the left hand may reflect the patient’s being right-handed, or may signify a focal cerebellar lesion. The cerebellum is also implicated by the bilateral dysmetria. Persistent somnolence in the absence of CT findings suggests a metabolic or infectious process. Metabolic processes that can cause bilateral cerebellar ataxia and somnolence include overdose of a drug or medication. Use of alcohol or a medication such as phenytoin, valproic acid, or a benzodiazepine can cause the symptoms in this case, but was not reported by the family, and there was no documentation of it in the SNF records. Wernicke encephalopathy is rare and is not well supported by the patient’s presentation but should be considered, as it can be easily treated with thiamine. Meningoencephalitis affecting the cerebellum remains possible, but infection is less likely. Both electroencephalogram and brain MRI should be performed, with a specific interest in possible cerebellar lesions. If the MRI is unremarkable, a lumbar puncture should be performed to assess opening pressure and investigate for infectious etiologies.
MRI of the brain showed age-related volume loss and nonspecific white matter disease without acute changes. Lack of a clear explanation for the neurologic findings led to suspicion of a medication side effect. Ertapenem was stopped on admission because it has been reported to rarely cause altered mental status. IV moxifloxacin was started for the osteomyelitis. Over the next 2 days, symptoms began resolving; within 24 hours of ertapenem discontinuation, the patient was awake, alert, and talkative. On examination, he remained dysarthric but was no longer dysmetric. Within 48 hours, the dysarthria was completely resolved, and he was returned to the SNF to complete a course of IV moxifloxacin.
DISCUSSION
Among elderly patients presenting to the ED, altered mental status is a common complaint, accounting for 10% to 30% of visits.1 Medications are a common cause of altered mental status among the elderly and are responsible for 40% of delirium cases.1 The risk of adverse drug events (ADEs) rises with the number of medications prescribed.1-3 Among patients older than 60 years, the incidence of polypharmacy (defined as taking >5 prescription medications) increased from roughly 20% in 1999 to 40% in 2012.4,5 The most common ADEs in the ambulatory setting (25%) are central nervous system (CNS) symptoms, including dizziness, sleep disturbances, and mood changes.6 A medication effect should be suspected in any elderly patient presenting with altered mental state.
The present patient developed a constellation of neurologic symptoms after starting ertapenem, one of the carbapenem antibiotics, which is a class of medications that can cause CNS ADEs. Carbapenems are renally cleared, and adjustments must be made for acute or chronic changes in kidney function. Carbapenems are associated with increased risk of seizure; the incidence of seizure with ertapenem is 0.2%.7,8 Food and Drug Administration postmarketing reports have noted ertapenem can cause somnolence and dyskinesia,9 and several case reports have described ertapenem-associated CNS side effects, including psychosis and encephalopathy.10-13 Symptoms and examination findings can include confusion, disorientation, garbled speech, dysphagia, hallucinations, miosis, myoclonus, tremor, and agitation.10-13 Although reports of dysmetria and dysdiadochokinesia are lacking, suspicion of an ADE in this case was heightened by the timing of the exposure and the absence of alternative infectious, metabolic, and vascular explanations for bilateral cerebellar dysfunction.
The Naranjo Adverse Drug Reaction (ADR) scale may help clinicians differentiate ADEs from other etiologies of symptoms. It uses 10 weighted questions (Table) to estimate the probability that an adverse clinical event is caused by a drug reaction.14 The present case was assigned 1 point for prior reports of neurologic ADEs associated with ertapenem, 2 for the temporal association, 1 for resolution after medication withdrawal, 2 for lack of alternative causes, and 1 for objective evidence of neurologic dysfunction—for a total of 7 points, indicating ertapenem was probably the cause of the patient’s neurologic symptoms. Of 4 prior cases in which carbapenem toxicity was suspected and the Naranjo scale was used, 3 found a probable relationship, and the fourth a highly probable one.10,12 Confusion, disorientation, hallucinations, tangential thoughts, and garbled speech were reported in the 3 probable cases of ADEs. In the highly probable case, tangential thoughts, garbled speech, and miosis were noted on examination, and these findings returned after re-exposure to ertapenem. Of note, these ADEs occurred in patients with normal and abnormal renal function, and in middle-aged and elderly patients.10,11,13
Most medications have a long list of low-frequency and rarely reported adverse effects. The present case reminds clinicians to consider rare adverse effects, or variants of previously reported adverse effects, in a patient with unexplained symptoms. To estimate the probability that a drug is causing harm to a patient, using a validated tool such as the Naranjo scale helps answer the question, What are the chances?
KEY TEACHING POINTS
Clinicians should include rare adverse effects of common medications in the differential diagnosis.
The Naranjo score is a validated tool that can be used to systematically assess the probability of an adverse drug effect at the bedside.
- The presentation of ertapenem-associated neurotoxicity may include features of bilateral cerebellar dysfunction.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
Two weeks after undergoing a below-knee amputation (BKA) and 10 days after being discharged to a skilled nursing facility (SNF), an 87-year-old man returned to the emergency department (ED) for evaluation of somnolence and altered mental state. In the ED, he was disoriented and unable to provide a detailed history.
The differential diagnosis for acute confusion and altered consciousness is broad. Initial possibilities include toxic-metabolic abnormalities, medication side effects, and infections. Urinary tract infection, pneumonia, and surgical-site infection should be assessed for first, as they are common causes of postoperative altered mentation. Next to be considered are subclinical seizure, ischemic stroke, and infectious encephalitis or meningitis, along with hemorrhagic stroke and subdural hematoma.
During initial assessment, the clinician should ascertain baseline mental state, the timeline of the change in mental status, recent medication changes, history of substance abuse, and concern about any recent trauma, such as a fall. Performing the physical examination, the clinician should assess vital signs and then focus on identifying localizing neurologic deficits.
First steps in the work-up include a complete metabolic panel, complete blood cell count, urinalysis with culture, and a urine toxicology screen. If the patient has a “toxic” appearance, blood cultures should be obtained. An electrocardiogram should be used to screen for drug toxicity or evidence of cardiac ischemia. If laboratory test results do not reveal an obvious infectious or metabolic cause, a noncontrast computed tomography (CT) of the head should be obtained. In terms of early interventions, a low glucose level should be treated with thiamine and then glucose, and naloxone should be given if there is any suspicion of narcotic overdose.
More history was obtained from the patient’s records. The BKA was performed to address a nonhealing transmetatarsal amputation. Two months earlier, the transmetatarsal amputation had been performed as treatment for a diabetic forefoot ulcer with chronic osteomyelitis. The patient’s post-BKA course was uncomplicated. He was started on intravenous (IV) ertapenem on postoperative day 1, and on postoperative day 4 was discharged to the SNF to complete a 6-week course of antibiotics for osteomyelitis. Past medical history included paroxysmal atrial fibrillation, coronary artery disease, congestive heart failure (ejection fraction 40%), and type 2 diabetes mellitus. Medications given at the SNF were oxycodone, acetaminophen, cholecalciferol, melatonin, digoxin, ondansetron, furosemide, gabapentin, correctional insulin, tamsulosin, senna, docusate, warfarin, and metoprolol. While there, the patient’s family expressed concern about his diminishing “mental ability.” They reported he had been fully alert and oriented on arrival at the SNF, and living independently with his wife before the BKA. Then, a week before the ED presentation, he started becoming more somnolent and forgetful. The gabapentin and oxycodone dosages were reduced to minimize their sedative effects, but he showed no improvement. At the SNF, a somnolence work-up was not performed.
Several of the patient’s medications can contribute to altered mental state. Ertapenem can cause seizures as well as profound mental status changes, though these are more likely in the setting of poor renal function. The mental status changes were noticed about a week into the patient’s course of antibiotics, which suggests a possible temporal correlation with the initiation of ertapenem. An electroencephalogram is required to diagnose nonconvulsive seizure activity. Narcotic overdose should still be considered, despite the recent reduction in oxycodone dosage. Digoxin toxicity, though less likely when the dose is stable and there are no changes in renal function, can cause a confused state. Concurrent use of furosemide could potentiate the toxic effects of digoxin.
Non-medication-related concerns include hypoglycemia, hyperglycemia, and, given his history of atrial fibrillation, cardioembolic stroke. Although generalized confusion is not a common manifestation of stroke, a thalamic stroke can alter mental state but be easily missed if not specifically considered. Additional lab work-up should include a digoxin level and, since he is taking warfarin, a prothrombin time/international normalized ratio (PT/INR). If the initial laboratory studies and head CT do not explain the altered mental state, magnetic resonance imaging (MRI) of the brain should be performed to further assess for stroke.
On physical examination in the ED, the patient was resting comfortably with eyes closed, and arousing to voice. He obeyed commands and participated in the examination. His Glasgow Coma Scale score was 13; temperature, 36.8°C, heart rate, 80 beats per minute; respiratory rate, 16 breaths per minute; blood pressure, 90/57 mm Hg; and 100% peripheral capillary oxygen saturation while breathing ambient air. He appeared well developed. His heart rhythm was irregularly irregular, without murmurs, rubs, or gallops. Respiratory and abdominal examination findings were normal. The left BKA incision was well approximated, with no drainage, dehiscence, fluctuance, or erythema. On neurologic examination, the patient was intermittently oriented only to self. Pupils were equal, round, and reactive to light; extraocular movements were intact; face was symmetric; tongue was midline; sensation on face was equal bilaterally; and shoulder shrug was intact. Strength was 5/5 and symmetric in the elbow and hip and 5/5 in the right knee and ankle (not tested on left because of BKA). Deep tendon reflexes were 3+ and symmetrical at the biceps, brachioradialis, and triceps tendons and 3+ in the right patellar and Achilles tendons. Sensation was intact and symmetrical in the upper and lower extremities. The patient’s speech was slow and slurred, and his answers were unrelated to the questions being asked.
The patient’s mental state is best described as lethargic. As he is only intermittently oriented, he meets the criteria for delirium. He is not obtunded or comatose, and his pupils are at least reactive, not pinpoint, so narcotic overdose is less likely. Thalamic stroke remains in the differential diagnosis; despite the seemingly symmetrical sensation examination, hemisensory deficits cannot be definitively ruled out given the patient’s mental state. A rare entity such as carcinomatosis meningitis or another diffuse, infiltrative neoplastic process could be causing his condition. However, because focal deficits other than abnormal speech and diffuse hyperreflexia are absent, toxic, infectious, or metabolic causes are more likely than structural abnormalities. Still possible is a medication toxicity, such as ertapenem toxicity or, less likely, digoxin toxicity. In terms of infectious possibilities, urinary tract infection could certainly present in this fashion, especially if the patient had a somewhat low neurologic reserve at baseline, and hypotension could be secondary to sepsis. Encephalitis or meningitis remains in the differential diagnosis, though the patient appears nontoxic, and therefore a bacterial etiology is very unlikely.
The patient’s hyperreflexia may be an important clue. Although the strength of his reflexes at baseline is unknown, seizures can cause transiently increased reflexes as well as a confused, lethargic mental state. Reflexes can also be increased by a drug overdose that has caused serotonin syndrome. Of the patient’s medications, only ondansetron can cause this reaction. Hyperthyroidism can cause brisk reflexes and confusion, though more typically it causes agitated confusion. A thyroid-stimulating hormone level should be added to the initial laboratory panel.
A complete blood count revealed white blood cell count 11.86 K/uL with neutrophilic predominance and immature granulocytes, hemoglobin 11.5 g/dL, and platelet count 323 K/uL. Serum sodium was 141 mEq/L, potassium 4.2 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, creatinine 1.14 mg/dL (prior baseline of 0.8-1.0 mg/dL), blood urea nitrogen 26 mg/dL, blood glucose 159 mg/dL, and calcium 9.1 mg/dL. His digoxin level was 1.3 ng/mL (reference range 0.5-1.9 mg/mL) and troponin was undetectable. INR was 2.7 and partial thromboplastin time (PTT) 60 seconds. Vitamin B12 level was 674 pg/mL (reference range >180). A urinalysis had 1+ hyaline casts and was negative for nitrites, leukocyte esterase, blood, and bacteria. An ECG revealed atrial fibrillation with a ventricular rate of 80 beats per minute. A chest radiograph showed clear lung fields. A CT of the head without IV contrast had no evidence of an acute intracranial abnormality. In the ED, 1 liter of IV normal saline was given and blood pressure improved to 127/72 mm Hg.
The head CT does not show intracranial bleeding, and, though it is reassuring that INR is in the therapeutic range, ischemic stroke must remain in the differential diagnosis. Sepsis is less likely given that the criteria for systemic inflammatory response syndrome are not met, and hypotension was rapidly corrected with administration of IV fluids. Urinary tract infection was ruled out with the negative urinalysis. Subclinical seizures remain possible, as does medication-related or other toxicity. A medication overdose, intentional or otherwise, should also be considered.
The patient was admitted to the hospital. On reassessment by the inpatient team, he was oriented only to self, frequently falling asleep, and not recalling earlier conversations when aroused. His speech remained slurred and difficult to understand. Neurologic examination findings were unchanged since the ED examination. On additional cerebellar examination, he had dysmetria with finger-to-nose testing bilaterally and dysdiadochokinesia (impaired rapid alternating movements) of the left hand.
His handedness is not mentioned; the dysdiadochokinesia of the left hand may reflect the patient’s being right-handed, or may signify a focal cerebellar lesion. The cerebellum is also implicated by the bilateral dysmetria. Persistent somnolence in the absence of CT findings suggests a metabolic or infectious process. Metabolic processes that can cause bilateral cerebellar ataxia and somnolence include overdose of a drug or medication. Use of alcohol or a medication such as phenytoin, valproic acid, or a benzodiazepine can cause the symptoms in this case, but was not reported by the family, and there was no documentation of it in the SNF records. Wernicke encephalopathy is rare and is not well supported by the patient’s presentation but should be considered, as it can be easily treated with thiamine. Meningoencephalitis affecting the cerebellum remains possible, but infection is less likely. Both electroencephalogram and brain MRI should be performed, with a specific interest in possible cerebellar lesions. If the MRI is unremarkable, a lumbar puncture should be performed to assess opening pressure and investigate for infectious etiologies.
MRI of the brain showed age-related volume loss and nonspecific white matter disease without acute changes. Lack of a clear explanation for the neurologic findings led to suspicion of a medication side effect. Ertapenem was stopped on admission because it has been reported to rarely cause altered mental status. IV moxifloxacin was started for the osteomyelitis. Over the next 2 days, symptoms began resolving; within 24 hours of ertapenem discontinuation, the patient was awake, alert, and talkative. On examination, he remained dysarthric but was no longer dysmetric. Within 48 hours, the dysarthria was completely resolved, and he was returned to the SNF to complete a course of IV moxifloxacin.
DISCUSSION
Among elderly patients presenting to the ED, altered mental status is a common complaint, accounting for 10% to 30% of visits.1 Medications are a common cause of altered mental status among the elderly and are responsible for 40% of delirium cases.1 The risk of adverse drug events (ADEs) rises with the number of medications prescribed.1-3 Among patients older than 60 years, the incidence of polypharmacy (defined as taking >5 prescription medications) increased from roughly 20% in 1999 to 40% in 2012.4,5 The most common ADEs in the ambulatory setting (25%) are central nervous system (CNS) symptoms, including dizziness, sleep disturbances, and mood changes.6 A medication effect should be suspected in any elderly patient presenting with altered mental state.
The present patient developed a constellation of neurologic symptoms after starting ertapenem, one of the carbapenem antibiotics, which is a class of medications that can cause CNS ADEs. Carbapenems are renally cleared, and adjustments must be made for acute or chronic changes in kidney function. Carbapenems are associated with increased risk of seizure; the incidence of seizure with ertapenem is 0.2%.7,8 Food and Drug Administration postmarketing reports have noted ertapenem can cause somnolence and dyskinesia,9 and several case reports have described ertapenem-associated CNS side effects, including psychosis and encephalopathy.10-13 Symptoms and examination findings can include confusion, disorientation, garbled speech, dysphagia, hallucinations, miosis, myoclonus, tremor, and agitation.10-13 Although reports of dysmetria and dysdiadochokinesia are lacking, suspicion of an ADE in this case was heightened by the timing of the exposure and the absence of alternative infectious, metabolic, and vascular explanations for bilateral cerebellar dysfunction.
The Naranjo Adverse Drug Reaction (ADR) scale may help clinicians differentiate ADEs from other etiologies of symptoms. It uses 10 weighted questions (Table) to estimate the probability that an adverse clinical event is caused by a drug reaction.14 The present case was assigned 1 point for prior reports of neurologic ADEs associated with ertapenem, 2 for the temporal association, 1 for resolution after medication withdrawal, 2 for lack of alternative causes, and 1 for objective evidence of neurologic dysfunction—for a total of 7 points, indicating ertapenem was probably the cause of the patient’s neurologic symptoms. Of 4 prior cases in which carbapenem toxicity was suspected and the Naranjo scale was used, 3 found a probable relationship, and the fourth a highly probable one.10,12 Confusion, disorientation, hallucinations, tangential thoughts, and garbled speech were reported in the 3 probable cases of ADEs. In the highly probable case, tangential thoughts, garbled speech, and miosis were noted on examination, and these findings returned after re-exposure to ertapenem. Of note, these ADEs occurred in patients with normal and abnormal renal function, and in middle-aged and elderly patients.10,11,13
Most medications have a long list of low-frequency and rarely reported adverse effects. The present case reminds clinicians to consider rare adverse effects, or variants of previously reported adverse effects, in a patient with unexplained symptoms. To estimate the probability that a drug is causing harm to a patient, using a validated tool such as the Naranjo scale helps answer the question, What are the chances?
KEY TEACHING POINTS
Clinicians should include rare adverse effects of common medications in the differential diagnosis.
The Naranjo score is a validated tool that can be used to systematically assess the probability of an adverse drug effect at the bedside.
- The presentation of ertapenem-associated neurotoxicity may include features of bilateral cerebellar dysfunction.
Disclosure
Nothing to report.
1. Inouye SK, Fearing MA, Marcantonio ER. Delirium. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009.
2. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46(5):1517-1533. PubMed
3. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55(1):29-34. PubMed
4. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. PubMed
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed
6. Thomsen LA, Winterstein AG, Søndergaard B, Haugbølle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411-1426. PubMed
7. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. PubMed
8. Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043-2055. PubMed
9. US Food and Drug Administration. Invanz (ertapenem) injection [safety information]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm196605.htm. Published July 2013. Accessed July 6, 2015.
10. Oo Y, Packham D, Yau W, Munckhof WJ. Ertapenem-associated psychosis and encephalopathy. Intern Med J. 2014;44(8):817-819. PubMed
11. Wen MJ, Sung CC, Chau T, Lin SH. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol. 2013;80(6):474-478. PubMed
12. Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother. 2011;45(1):e6. PubMed
13. Kong V, Beckert L, Awunor-Renner C. A case of beta lactam-induced visual hallucination. N Z Med J. 2009;122(1298):76-77. PubMed
14. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. PubMed
1. Inouye SK, Fearing MA, Marcantonio ER. Delirium. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009.
2. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46(5):1517-1533. PubMed
3. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55(1):29-34. PubMed
4. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. PubMed
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed
6. Thomsen LA, Winterstein AG, Søndergaard B, Haugbølle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411-1426. PubMed
7. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. PubMed
8. Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043-2055. PubMed
9. US Food and Drug Administration. Invanz (ertapenem) injection [safety information]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm196605.htm. Published July 2013. Accessed July 6, 2015.
10. Oo Y, Packham D, Yau W, Munckhof WJ. Ertapenem-associated psychosis and encephalopathy. Intern Med J. 2014;44(8):817-819. PubMed
11. Wen MJ, Sung CC, Chau T, Lin SH. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol. 2013;80(6):474-478. PubMed
12. Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother. 2011;45(1):e6. PubMed
13. Kong V, Beckert L, Awunor-Renner C. A case of beta lactam-induced visual hallucination. N Z Med J. 2009;122(1298):76-77. PubMed
14. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. PubMed
© 2017 Society of Hospital Medicine
Safe and effective bedside thoracentesis: A review of the evidence for practicing clinicians
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
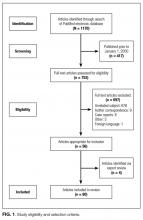
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
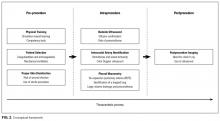
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.
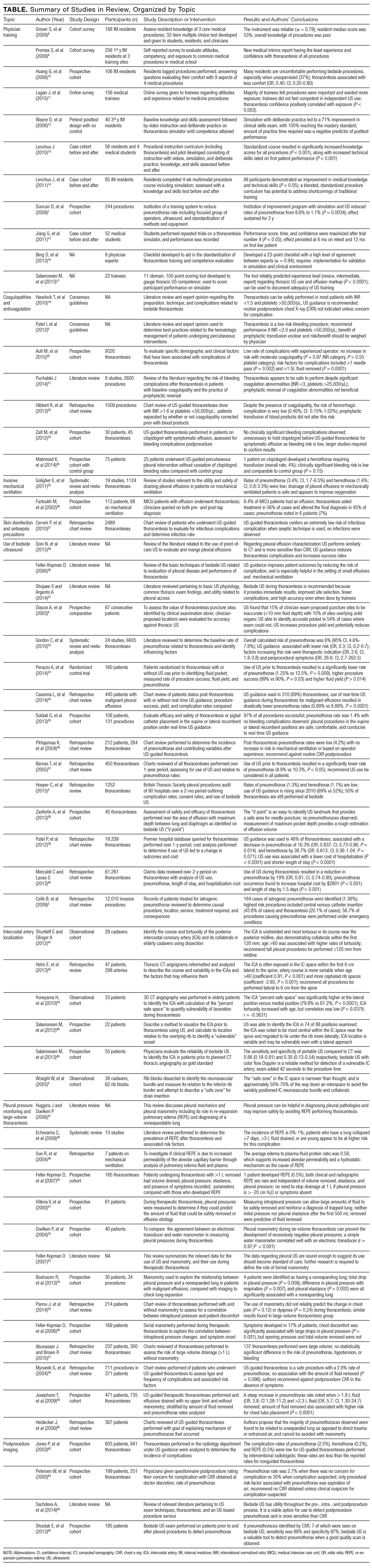
PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
© 2017 Society of Hospital Medicine
Hospital medicine and perioperative care: A framework for high-quality, high-value collaborative care
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
© 2017 Society of Hospital Medicine
Advances in Targeted Therapy for Breast Cancer
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
Psyllium cut frequency of abdominal pain in pediatric IBS trial
Consuming psyllium fiber significantly reduced the frequency, but not the severity, of abdominal pain in children with irritable bowel syndrome in a randomized, double-blind, placebo-controlled trial reported in the May issue of Clinical Gastroenterology and Hepatology (2016 Nov;14[11]:1667).
Psyllium therapy did not reduce the self-reported severity of abdominal pain, Robert J. Shulman, MD, of Baylor College of Medicine in Houston reported with his associates in Clinical Gastroenterology and Hepatology. Psyllium was associated with shifts in intestinal microbiota, compared with baseline, although the changes did not reach statistical significance when compared with placebo, the researchers added. “Further studies are needed to investigate the potential mechanism whereby psyllium decreases abdominal pain frequency in children with irritable bowel syndrome [IBS],” they wrote.
IBS affects up to 20% of school-aged children. Consuming psyllium is thought to improve abdominal pain and stooling symptoms in adults with IBS, but data are inconclusive, and few randomized trials have evaluated fiber in childhood IBS. Therefore, the investigators randomly assigned 103 children (average age, 13 years; standard deviation, 3 years) with IBS who had responded inadequately to an 8-day carbohydrate elimination diet to receive a single daily dose of either psyllium or placebo maltodextrin for 6 weeks. Children aged 7-11 years received 6 g of fiber, while those aged 12-18 years received 12 g of fiber. Patients filled out a daily pain and stool diary during a 2-week baseline assessment period and again during the final 2 weeks of the trial. They also underwent breath hydrogen and methane testing, gut permeability testing, and a stool microbiota assessment during the final weekend of treatment.
At baseline, the trial arms resembled each other in terms of frequency and severity of abdominal pain, psychological characteristics, percentage of normal stools, baseline hydrogen production, and gastrointestinal permeability, the researchers said. During the final 2 weeks of treatment, the psyllium arm reported an average of 8.2 (standard deviation, 1.2) fewer episodes of abdominal pain, compared with baseline, while the control arm reported a mean reduction of 4.1 (SD, 1.3) episodes of abdominal pain (P = .03). At the end of treatment, the arms did not significantly differ in percentage of breath hydrogen or methane production, gastrointestinal permeability, or percentage of normal stools or diarrhea. However, controls had a significantly greater reduction in constipation compared with the psyllium group (P = .048).
Stool microbiome assessments of 33 children revealed a trend toward a greater increase in Bacteroidetes and a greater decrease in Firmicutes bacteria in the fiber group, compared with the control group (P = .068). The fiber group was also “marginally enriched” in bacteria of class Bacteroidia, while the placebo group was enriched in bacteria of class Clostridia (P = .094). However, the groups did not differ at narrower taxonomic levels, the researchers said. A larger sample size might have facilitated better detection of differences between groups, such as in breath hydrogen production or interactions between abdominal pain and psychological symptoms, they added.
The study was supported in part by the National Institutes of Health, the Daffy’s Foundation, and the USDA/ARS. The investigators reported having no conflicts of interest.
Consuming psyllium fiber significantly reduced the frequency, but not the severity, of abdominal pain in children with irritable bowel syndrome in a randomized, double-blind, placebo-controlled trial reported in the May issue of Clinical Gastroenterology and Hepatology (2016 Nov;14[11]:1667).
Psyllium therapy did not reduce the self-reported severity of abdominal pain, Robert J. Shulman, MD, of Baylor College of Medicine in Houston reported with his associates in Clinical Gastroenterology and Hepatology. Psyllium was associated with shifts in intestinal microbiota, compared with baseline, although the changes did not reach statistical significance when compared with placebo, the researchers added. “Further studies are needed to investigate the potential mechanism whereby psyllium decreases abdominal pain frequency in children with irritable bowel syndrome [IBS],” they wrote.
IBS affects up to 20% of school-aged children. Consuming psyllium is thought to improve abdominal pain and stooling symptoms in adults with IBS, but data are inconclusive, and few randomized trials have evaluated fiber in childhood IBS. Therefore, the investigators randomly assigned 103 children (average age, 13 years; standard deviation, 3 years) with IBS who had responded inadequately to an 8-day carbohydrate elimination diet to receive a single daily dose of either psyllium or placebo maltodextrin for 6 weeks. Children aged 7-11 years received 6 g of fiber, while those aged 12-18 years received 12 g of fiber. Patients filled out a daily pain and stool diary during a 2-week baseline assessment period and again during the final 2 weeks of the trial. They also underwent breath hydrogen and methane testing, gut permeability testing, and a stool microbiota assessment during the final weekend of treatment.
At baseline, the trial arms resembled each other in terms of frequency and severity of abdominal pain, psychological characteristics, percentage of normal stools, baseline hydrogen production, and gastrointestinal permeability, the researchers said. During the final 2 weeks of treatment, the psyllium arm reported an average of 8.2 (standard deviation, 1.2) fewer episodes of abdominal pain, compared with baseline, while the control arm reported a mean reduction of 4.1 (SD, 1.3) episodes of abdominal pain (P = .03). At the end of treatment, the arms did not significantly differ in percentage of breath hydrogen or methane production, gastrointestinal permeability, or percentage of normal stools or diarrhea. However, controls had a significantly greater reduction in constipation compared with the psyllium group (P = .048).
Stool microbiome assessments of 33 children revealed a trend toward a greater increase in Bacteroidetes and a greater decrease in Firmicutes bacteria in the fiber group, compared with the control group (P = .068). The fiber group was also “marginally enriched” in bacteria of class Bacteroidia, while the placebo group was enriched in bacteria of class Clostridia (P = .094). However, the groups did not differ at narrower taxonomic levels, the researchers said. A larger sample size might have facilitated better detection of differences between groups, such as in breath hydrogen production or interactions between abdominal pain and psychological symptoms, they added.
The study was supported in part by the National Institutes of Health, the Daffy’s Foundation, and the USDA/ARS. The investigators reported having no conflicts of interest.
Consuming psyllium fiber significantly reduced the frequency, but not the severity, of abdominal pain in children with irritable bowel syndrome in a randomized, double-blind, placebo-controlled trial reported in the May issue of Clinical Gastroenterology and Hepatology (2016 Nov;14[11]:1667).
Psyllium therapy did not reduce the self-reported severity of abdominal pain, Robert J. Shulman, MD, of Baylor College of Medicine in Houston reported with his associates in Clinical Gastroenterology and Hepatology. Psyllium was associated with shifts in intestinal microbiota, compared with baseline, although the changes did not reach statistical significance when compared with placebo, the researchers added. “Further studies are needed to investigate the potential mechanism whereby psyllium decreases abdominal pain frequency in children with irritable bowel syndrome [IBS],” they wrote.
IBS affects up to 20% of school-aged children. Consuming psyllium is thought to improve abdominal pain and stooling symptoms in adults with IBS, but data are inconclusive, and few randomized trials have evaluated fiber in childhood IBS. Therefore, the investigators randomly assigned 103 children (average age, 13 years; standard deviation, 3 years) with IBS who had responded inadequately to an 8-day carbohydrate elimination diet to receive a single daily dose of either psyllium or placebo maltodextrin for 6 weeks. Children aged 7-11 years received 6 g of fiber, while those aged 12-18 years received 12 g of fiber. Patients filled out a daily pain and stool diary during a 2-week baseline assessment period and again during the final 2 weeks of the trial. They also underwent breath hydrogen and methane testing, gut permeability testing, and a stool microbiota assessment during the final weekend of treatment.
At baseline, the trial arms resembled each other in terms of frequency and severity of abdominal pain, psychological characteristics, percentage of normal stools, baseline hydrogen production, and gastrointestinal permeability, the researchers said. During the final 2 weeks of treatment, the psyllium arm reported an average of 8.2 (standard deviation, 1.2) fewer episodes of abdominal pain, compared with baseline, while the control arm reported a mean reduction of 4.1 (SD, 1.3) episodes of abdominal pain (P = .03). At the end of treatment, the arms did not significantly differ in percentage of breath hydrogen or methane production, gastrointestinal permeability, or percentage of normal stools or diarrhea. However, controls had a significantly greater reduction in constipation compared with the psyllium group (P = .048).
Stool microbiome assessments of 33 children revealed a trend toward a greater increase in Bacteroidetes and a greater decrease in Firmicutes bacteria in the fiber group, compared with the control group (P = .068). The fiber group was also “marginally enriched” in bacteria of class Bacteroidia, while the placebo group was enriched in bacteria of class Clostridia (P = .094). However, the groups did not differ at narrower taxonomic levels, the researchers said. A larger sample size might have facilitated better detection of differences between groups, such as in breath hydrogen production or interactions between abdominal pain and psychological symptoms, they added.
The study was supported in part by the National Institutes of Health, the Daffy’s Foundation, and the USDA/ARS. The investigators reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Compared with placebo maltodextrin, consuming psyllium fiber significantly reduced the self-reported frequency of abdominal pain in children with irritable bowel syndrome.
Major finding: Children who received psyllium reported an average of 8.2 fewer pain episodes, compared with baseline, while controls reported a mean reduction of 4.1 pain episodes (P = .03).
Data source: A randomized, double-blind trial of 103 children aged 12-18 years of age with irritable bowel syndrome.
Disclosures: The study was supported in part by the National Institutes of Health, the Daffy’s Foundation, and the USDA/ARS. The investigators reported having no conflicts of interest.
ACS New SSR Offers Webinar Training Sessions
The American College of Surgeons (ACS) has announced the launch of the new Surgeon Specific Registry (SSR), hosted by QuintilesIMS. The new SSR is set to go live in this spring. The latest version of the registry will have several enhanced features, including improved reporting capabilities, delegate-level access to enter data, and the ability to add custom fields for additional relevant variables.
To help you prepare for this transition, the SSR team will host several educational webinars to demonstrate the new system’s capabilities and features. The ACS encourages both current and potential users to participate. To view the available times and register for one of the upcoming webinars, visit the SSR News and Updates web page at facs.org/quality-programs/ssr/news.
Contact [email protected] if you have any questions.
The American College of Surgeons (ACS) has announced the launch of the new Surgeon Specific Registry (SSR), hosted by QuintilesIMS. The new SSR is set to go live in this spring. The latest version of the registry will have several enhanced features, including improved reporting capabilities, delegate-level access to enter data, and the ability to add custom fields for additional relevant variables.
To help you prepare for this transition, the SSR team will host several educational webinars to demonstrate the new system’s capabilities and features. The ACS encourages both current and potential users to participate. To view the available times and register for one of the upcoming webinars, visit the SSR News and Updates web page at facs.org/quality-programs/ssr/news.
Contact [email protected] if you have any questions.
The American College of Surgeons (ACS) has announced the launch of the new Surgeon Specific Registry (SSR), hosted by QuintilesIMS. The new SSR is set to go live in this spring. The latest version of the registry will have several enhanced features, including improved reporting capabilities, delegate-level access to enter data, and the ability to add custom fields for additional relevant variables.
To help you prepare for this transition, the SSR team will host several educational webinars to demonstrate the new system’s capabilities and features. The ACS encourages both current and potential users to participate. To view the available times and register for one of the upcoming webinars, visit the SSR News and Updates web page at facs.org/quality-programs/ssr/news.
Contact [email protected] if you have any questions.
Applications for 2018 Alliance Scholar Awards Accepted through June 30
Applications for 2018 Alliance Scholar Awards Accepted through June 30
The Alliance for Clinical Trials in Oncology Foundation is accepting applications for the 2018 Alliance Scholar Awards. Applications must be submitted by 12:00 midnight (CST) on June 30.
Alliance Scholar Award applicants must be oncology junior faculty at Alliance institutions within five years of training (rank below associate professor) and have completed training in an oncology clinical specialty (medical, surgical, radiation, gynecologic, and so on). Additionally, proposals must include a letter of support from the appropriate Alliance Scientific Committee Chair to ensure the proposal is closely tied to the Alliance’s research agenda of the Alliance.
Alliance Scholar Award recipients will receive a two-year, non-renewable cancer research grant of $40,000 in direct costs per year, plus 10 percent in indirect costs for each of the two years. Successful applicants will be announced at the plenary session at the 2017 Alliance Fall Group Meeting held in Chicago, IL, November 2–4. Funding will begin approximately January 1, 2018. For application requirements and the link to the online submission portal, visit the Alliance Scholar Awards page on the Alliance website at http://bit.ly/1JMXkwS.
The Alliance/American College of Surgeons Clinical Research Program offers opportunities for surgeons to become involved in the research and development of evidence-based practices in surgical oncology. If you would like to participate in oncology clinical research or oncology-related projects, contact [email protected].
Applications for 2018 Alliance Scholar Awards Accepted through June 30
The Alliance for Clinical Trials in Oncology Foundation is accepting applications for the 2018 Alliance Scholar Awards. Applications must be submitted by 12:00 midnight (CST) on June 30.
Alliance Scholar Award applicants must be oncology junior faculty at Alliance institutions within five years of training (rank below associate professor) and have completed training in an oncology clinical specialty (medical, surgical, radiation, gynecologic, and so on). Additionally, proposals must include a letter of support from the appropriate Alliance Scientific Committee Chair to ensure the proposal is closely tied to the Alliance’s research agenda of the Alliance.
Alliance Scholar Award recipients will receive a two-year, non-renewable cancer research grant of $40,000 in direct costs per year, plus 10 percent in indirect costs for each of the two years. Successful applicants will be announced at the plenary session at the 2017 Alliance Fall Group Meeting held in Chicago, IL, November 2–4. Funding will begin approximately January 1, 2018. For application requirements and the link to the online submission portal, visit the Alliance Scholar Awards page on the Alliance website at http://bit.ly/1JMXkwS.
The Alliance/American College of Surgeons Clinical Research Program offers opportunities for surgeons to become involved in the research and development of evidence-based practices in surgical oncology. If you would like to participate in oncology clinical research or oncology-related projects, contact [email protected].
Applications for 2018 Alliance Scholar Awards Accepted through June 30
The Alliance for Clinical Trials in Oncology Foundation is accepting applications for the 2018 Alliance Scholar Awards. Applications must be submitted by 12:00 midnight (CST) on June 30.
Alliance Scholar Award applicants must be oncology junior faculty at Alliance institutions within five years of training (rank below associate professor) and have completed training in an oncology clinical specialty (medical, surgical, radiation, gynecologic, and so on). Additionally, proposals must include a letter of support from the appropriate Alliance Scientific Committee Chair to ensure the proposal is closely tied to the Alliance’s research agenda of the Alliance.
Alliance Scholar Award recipients will receive a two-year, non-renewable cancer research grant of $40,000 in direct costs per year, plus 10 percent in indirect costs for each of the two years. Successful applicants will be announced at the plenary session at the 2017 Alliance Fall Group Meeting held in Chicago, IL, November 2–4. Funding will begin approximately January 1, 2018. For application requirements and the link to the online submission portal, visit the Alliance Scholar Awards page on the Alliance website at http://bit.ly/1JMXkwS.
The Alliance/American College of Surgeons Clinical Research Program offers opportunities for surgeons to become involved in the research and development of evidence-based practices in surgical oncology. If you would like to participate in oncology clinical research or oncology-related projects, contact [email protected].
Nominate an Inspiring Woman for WiSC Award
The American College of Surgeons (ACS) Women in Surgery Committee (WiSC) is accepting nominations for the second annual Dr. Mary Edwards Walker Inspiring Women in Surgery Award, which will be presented at Clinical Congress 2017 in San Diego, CA. The award will be accorded in recognition of an individual’s significant contributions to the advancement of women in the field of surgery. Nominations are due April 30.
The award honors Dr. Mary Edwards Walker for the example she set for future generations as the first woman surgeon to serve as a U.S. Army physician and the only woman to ever receive the U.S. Armed Forces Medal of Honor for bravery.
All nominations must be accompanied by the following documents:
• A letter of nomination outlining how the candidate has contributed to the advancement of women in the field of surgery
• An up-to-date curriculum vitae
Self-nominations are acceptable and should include a letter of reference. Nominations and questions should be submitted to Connie Bura at [email protected].
The American College of Surgeons (ACS) Women in Surgery Committee (WiSC) is accepting nominations for the second annual Dr. Mary Edwards Walker Inspiring Women in Surgery Award, which will be presented at Clinical Congress 2017 in San Diego, CA. The award will be accorded in recognition of an individual’s significant contributions to the advancement of women in the field of surgery. Nominations are due April 30.
The award honors Dr. Mary Edwards Walker for the example she set for future generations as the first woman surgeon to serve as a U.S. Army physician and the only woman to ever receive the U.S. Armed Forces Medal of Honor for bravery.
All nominations must be accompanied by the following documents:
• A letter of nomination outlining how the candidate has contributed to the advancement of women in the field of surgery
• An up-to-date curriculum vitae
Self-nominations are acceptable and should include a letter of reference. Nominations and questions should be submitted to Connie Bura at [email protected].
The American College of Surgeons (ACS) Women in Surgery Committee (WiSC) is accepting nominations for the second annual Dr. Mary Edwards Walker Inspiring Women in Surgery Award, which will be presented at Clinical Congress 2017 in San Diego, CA. The award will be accorded in recognition of an individual’s significant contributions to the advancement of women in the field of surgery. Nominations are due April 30.
The award honors Dr. Mary Edwards Walker for the example she set for future generations as the first woman surgeon to serve as a U.S. Army physician and the only woman to ever receive the U.S. Armed Forces Medal of Honor for bravery.
All nominations must be accompanied by the following documents:
• A letter of nomination outlining how the candidate has contributed to the advancement of women in the field of surgery
• An up-to-date curriculum vitae
Self-nominations are acceptable and should include a letter of reference. Nominations and questions should be submitted to Connie Bura at [email protected].
Everything We Say and Do: Discussing advance care planning
Editor’s note: “Everything We Say and Do” is an informational series developed by the Society of Hospital Medicine’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experiences of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I empower all of my patients by giving them the opportunity to consider advance care planning.
Why I do it
Everyone deserves advance care planning, and every health care encounter, including a hospitalization, is an opportunity to better identify and document patients’ wishes for care should they become unable to express them. If we wait for patients to develop serious advanced illness before having advance care planning conversations, we risk depriving them of the care they would want in these situations. Additionally, we place a huge burden on family members who may struggle with excruciatingly difficult decisions in the absence of guidance about their loved one’s wishes.
How I do it
I start by identifying which components of advance care planning each patient needs, using a simple algorithm (see figure). All of my patients are queried about code status, and I give them the opportunity to better understand the value of having a healthcare proxy and advance directives, if they are not already in place.
For the remainder of this column, I’m going to focus on patients who have an acute and/or chronic treatable illness – those who require simpler advance-care-planning conversations.
To comfortably initiate the conversation about advance care planning, I always start by asking permission. I commonly say, “There are a couple of important items I discuss with all of my patients to make sure they get the care they want. Would it be okay for us to talk about those now?” This respectfully puts the patient in control. I then initiate a discussion of code status by saying, “It’s important that all of us on your care team know what you would like us to do if you got so sick that we couldn’t communicate with you. I’m not expecting this to happen, but I ask all my patients this question so that we have your instructions.” From there, the conversation evolves depending on whether the patient has any familiarity with this question and its implications.
To introduce the concept of a health care proxy and advance directives, I ask, “Have you ever thought about who you might choose to make medical decisions on your behalf if you became too sick to make those decisions yourself?” Then, finally, I share the following information, usually referring to the blank advance directives document they received in their admission packet: “There is a valuable way to put your wishes about specific care options in writing so others will know your wishes if you’re unable to communicate with them. Would you like to talk about that right now?” Again, this gives the patient control of the situation and an opportunity to decline the conversation if they are not interested or comfortable at that time.
It’s important to document the nature and outcome of these conversations. Keep in mind, advance care planning discussions need not occur at the time of admission. In fact, admission may be the worst time for some patients, further underscoring the importance of documentation so that subsequent providers can see whether advance care planning has been addressed during the hospital stay.
Note: For useful educational resources that address goals-of-care conversations in patients toward the end of life, the Center to Advance Palliative Care (www.capc.org) has a number of educational courses that address these important communication skills.
Dr. Rudolph is vice president of physician development and patient experience for Sound Physicians, Tacoma, Wash. and chair of the SHM Patient Experience Committee .
Reference
1. Moss, A.H., Ganjoo, J, Sharma S, et al. Utility of the “Surprise” Question to Identify Dialysis Patients with High Mortality. Clinical Journal of the American Society of Nephrology: CJASN. 2008;3(5):1379-84. doi:10.2215/CJN.00940208.
Editor’s note: “Everything We Say and Do” is an informational series developed by the Society of Hospital Medicine’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experiences of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I empower all of my patients by giving them the opportunity to consider advance care planning.
Why I do it
Everyone deserves advance care planning, and every health care encounter, including a hospitalization, is an opportunity to better identify and document patients’ wishes for care should they become unable to express them. If we wait for patients to develop serious advanced illness before having advance care planning conversations, we risk depriving them of the care they would want in these situations. Additionally, we place a huge burden on family members who may struggle with excruciatingly difficult decisions in the absence of guidance about their loved one’s wishes.
How I do it
I start by identifying which components of advance care planning each patient needs, using a simple algorithm (see figure). All of my patients are queried about code status, and I give them the opportunity to better understand the value of having a healthcare proxy and advance directives, if they are not already in place.

For the remainder of this column, I’m going to focus on patients who have an acute and/or chronic treatable illness – those who require simpler advance-care-planning conversations.
To comfortably initiate the conversation about advance care planning, I always start by asking permission. I commonly say, “There are a couple of important items I discuss with all of my patients to make sure they get the care they want. Would it be okay for us to talk about those now?” This respectfully puts the patient in control. I then initiate a discussion of code status by saying, “It’s important that all of us on your care team know what you would like us to do if you got so sick that we couldn’t communicate with you. I’m not expecting this to happen, but I ask all my patients this question so that we have your instructions.” From there, the conversation evolves depending on whether the patient has any familiarity with this question and its implications.
To introduce the concept of a health care proxy and advance directives, I ask, “Have you ever thought about who you might choose to make medical decisions on your behalf if you became too sick to make those decisions yourself?” Then, finally, I share the following information, usually referring to the blank advance directives document they received in their admission packet: “There is a valuable way to put your wishes about specific care options in writing so others will know your wishes if you’re unable to communicate with them. Would you like to talk about that right now?” Again, this gives the patient control of the situation and an opportunity to decline the conversation if they are not interested or comfortable at that time.
It’s important to document the nature and outcome of these conversations. Keep in mind, advance care planning discussions need not occur at the time of admission. In fact, admission may be the worst time for some patients, further underscoring the importance of documentation so that subsequent providers can see whether advance care planning has been addressed during the hospital stay.
Note: For useful educational resources that address goals-of-care conversations in patients toward the end of life, the Center to Advance Palliative Care (www.capc.org) has a number of educational courses that address these important communication skills.
Dr. Rudolph is vice president of physician development and patient experience for Sound Physicians, Tacoma, Wash. and chair of the SHM Patient Experience Committee .
Reference
1. Moss, A.H., Ganjoo, J, Sharma S, et al. Utility of the “Surprise” Question to Identify Dialysis Patients with High Mortality. Clinical Journal of the American Society of Nephrology: CJASN. 2008;3(5):1379-84. doi:10.2215/CJN.00940208.
Editor’s note: “Everything We Say and Do” is an informational series developed by the Society of Hospital Medicine’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experiences of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I empower all of my patients by giving them the opportunity to consider advance care planning.
Why I do it
Everyone deserves advance care planning, and every health care encounter, including a hospitalization, is an opportunity to better identify and document patients’ wishes for care should they become unable to express them. If we wait for patients to develop serious advanced illness before having advance care planning conversations, we risk depriving them of the care they would want in these situations. Additionally, we place a huge burden on family members who may struggle with excruciatingly difficult decisions in the absence of guidance about their loved one’s wishes.
How I do it
I start by identifying which components of advance care planning each patient needs, using a simple algorithm (see figure). All of my patients are queried about code status, and I give them the opportunity to better understand the value of having a healthcare proxy and advance directives, if they are not already in place.

For the remainder of this column, I’m going to focus on patients who have an acute and/or chronic treatable illness – those who require simpler advance-care-planning conversations.
To comfortably initiate the conversation about advance care planning, I always start by asking permission. I commonly say, “There are a couple of important items I discuss with all of my patients to make sure they get the care they want. Would it be okay for us to talk about those now?” This respectfully puts the patient in control. I then initiate a discussion of code status by saying, “It’s important that all of us on your care team know what you would like us to do if you got so sick that we couldn’t communicate with you. I’m not expecting this to happen, but I ask all my patients this question so that we have your instructions.” From there, the conversation evolves depending on whether the patient has any familiarity with this question and its implications.
To introduce the concept of a health care proxy and advance directives, I ask, “Have you ever thought about who you might choose to make medical decisions on your behalf if you became too sick to make those decisions yourself?” Then, finally, I share the following information, usually referring to the blank advance directives document they received in their admission packet: “There is a valuable way to put your wishes about specific care options in writing so others will know your wishes if you’re unable to communicate with them. Would you like to talk about that right now?” Again, this gives the patient control of the situation and an opportunity to decline the conversation if they are not interested or comfortable at that time.
It’s important to document the nature and outcome of these conversations. Keep in mind, advance care planning discussions need not occur at the time of admission. In fact, admission may be the worst time for some patients, further underscoring the importance of documentation so that subsequent providers can see whether advance care planning has been addressed during the hospital stay.
Note: For useful educational resources that address goals-of-care conversations in patients toward the end of life, the Center to Advance Palliative Care (www.capc.org) has a number of educational courses that address these important communication skills.
Dr. Rudolph is vice president of physician development and patient experience for Sound Physicians, Tacoma, Wash. and chair of the SHM Patient Experience Committee .
Reference
1. Moss, A.H., Ganjoo, J, Sharma S, et al. Utility of the “Surprise” Question to Identify Dialysis Patients with High Mortality. Clinical Journal of the American Society of Nephrology: CJASN. 2008;3(5):1379-84. doi:10.2215/CJN.00940208.
Celebrating our accomplishments
I recently had the good fortune to read a commentary written by Dr. Peter Angelos in ACS Surgery News entitled, The Right Choice? Surgeons, confidence, and humility (2017, February, p. 11). The essay touches on the philosophy, psychology, and attitudes that surgeons adopt and express in their daily interactions with the public.
The article refers to “the balance between lack of confidence and overconfidence, and between thoughtful introspection and paralyzing fear of future complications.” This is a critically important struggle in the mind of the surgeon. I would like to propose an exercise to bolster self-esteem in the psyche of the surgeon, particularly in the formative stages of one’s career, without fostering false or pathological bravado.
I certainly see the benefit in this tradition of analyzing and reviewing surgical misadventures and discussing the proper management of uninvited complications. It is a process rooted in the concepts of honesty, transparency, introspection, reflection, collaboration, and trust.
What I would like to propose is not the cessation of the M & M conference, but the addition of a complementary conference, which I refer to as Success and Survival conference. This meeting would showcase clinical scenarios in which a given patient should have succumbed to his illness but, instead, thrived as a result of the exemplary care provided by the surgical team involved. This would shine a bright spotlight on what it is that we do, and why our profession is so extraordinary. It would serve as a wonderful reminder for surgeons at all stages of their careers as to why we chose such a rigorous, challenging, and difficult vocation as our life’s work.
Such a venue would provide young surgeons an opportunity – not to flaunt – but to share and take well-deserved pride in their victories. I believe this conference would be as effective in terms of its educational value as the M & M, but it would not be associated with negative emotions of guilt, shame, and fear. The S & S would be a setting in which the young surgeon could shine in front of his or her peers as well as the attending staff and faculty.
The academic culture that prides itself on adages such as, “Whatever doesn’t kill you makes you stronger,” “The only problem with being on call every other night is that you miss half the pathology,” and “Eat when you can, sleep when you can, and don’t mess with the pancreas,” is long overdue in celebrating the accomplishments of surgeons publicly and on a regular basis
In the end, we should want to promote future generations of surgeons who are technically sound, demonstrate excellent judgment under the most difficult circumstances, and who are able to achieve, ideally, their full surgical potential by arriving at a true harmony between self-assurance and uncertainty.
Dr. Chuback is a vascular surgeon in private practice in Paramus, N.J.
I recently had the good fortune to read a commentary written by Dr. Peter Angelos in ACS Surgery News entitled, The Right Choice? Surgeons, confidence, and humility (2017, February, p. 11). The essay touches on the philosophy, psychology, and attitudes that surgeons adopt and express in their daily interactions with the public.
The article refers to “the balance between lack of confidence and overconfidence, and between thoughtful introspection and paralyzing fear of future complications.” This is a critically important struggle in the mind of the surgeon. I would like to propose an exercise to bolster self-esteem in the psyche of the surgeon, particularly in the formative stages of one’s career, without fostering false or pathological bravado.
I certainly see the benefit in this tradition of analyzing and reviewing surgical misadventures and discussing the proper management of uninvited complications. It is a process rooted in the concepts of honesty, transparency, introspection, reflection, collaboration, and trust.
What I would like to propose is not the cessation of the M & M conference, but the addition of a complementary conference, which I refer to as Success and Survival conference. This meeting would showcase clinical scenarios in which a given patient should have succumbed to his illness but, instead, thrived as a result of the exemplary care provided by the surgical team involved. This would shine a bright spotlight on what it is that we do, and why our profession is so extraordinary. It would serve as a wonderful reminder for surgeons at all stages of their careers as to why we chose such a rigorous, challenging, and difficult vocation as our life’s work.
Such a venue would provide young surgeons an opportunity – not to flaunt – but to share and take well-deserved pride in their victories. I believe this conference would be as effective in terms of its educational value as the M & M, but it would not be associated with negative emotions of guilt, shame, and fear. The S & S would be a setting in which the young surgeon could shine in front of his or her peers as well as the attending staff and faculty.
The academic culture that prides itself on adages such as, “Whatever doesn’t kill you makes you stronger,” “The only problem with being on call every other night is that you miss half the pathology,” and “Eat when you can, sleep when you can, and don’t mess with the pancreas,” is long overdue in celebrating the accomplishments of surgeons publicly and on a regular basis
In the end, we should want to promote future generations of surgeons who are technically sound, demonstrate excellent judgment under the most difficult circumstances, and who are able to achieve, ideally, their full surgical potential by arriving at a true harmony between self-assurance and uncertainty.
Dr. Chuback is a vascular surgeon in private practice in Paramus, N.J.
I recently had the good fortune to read a commentary written by Dr. Peter Angelos in ACS Surgery News entitled, The Right Choice? Surgeons, confidence, and humility (2017, February, p. 11). The essay touches on the philosophy, psychology, and attitudes that surgeons adopt and express in their daily interactions with the public.
The article refers to “the balance between lack of confidence and overconfidence, and between thoughtful introspection and paralyzing fear of future complications.” This is a critically important struggle in the mind of the surgeon. I would like to propose an exercise to bolster self-esteem in the psyche of the surgeon, particularly in the formative stages of one’s career, without fostering false or pathological bravado.
I certainly see the benefit in this tradition of analyzing and reviewing surgical misadventures and discussing the proper management of uninvited complications. It is a process rooted in the concepts of honesty, transparency, introspection, reflection, collaboration, and trust.
What I would like to propose is not the cessation of the M & M conference, but the addition of a complementary conference, which I refer to as Success and Survival conference. This meeting would showcase clinical scenarios in which a given patient should have succumbed to his illness but, instead, thrived as a result of the exemplary care provided by the surgical team involved. This would shine a bright spotlight on what it is that we do, and why our profession is so extraordinary. It would serve as a wonderful reminder for surgeons at all stages of their careers as to why we chose such a rigorous, challenging, and difficult vocation as our life’s work.
Such a venue would provide young surgeons an opportunity – not to flaunt – but to share and take well-deserved pride in their victories. I believe this conference would be as effective in terms of its educational value as the M & M, but it would not be associated with negative emotions of guilt, shame, and fear. The S & S would be a setting in which the young surgeon could shine in front of his or her peers as well as the attending staff and faculty.
The academic culture that prides itself on adages such as, “Whatever doesn’t kill you makes you stronger,” “The only problem with being on call every other night is that you miss half the pathology,” and “Eat when you can, sleep when you can, and don’t mess with the pancreas,” is long overdue in celebrating the accomplishments of surgeons publicly and on a regular basis
In the end, we should want to promote future generations of surgeons who are technically sound, demonstrate excellent judgment under the most difficult circumstances, and who are able to achieve, ideally, their full surgical potential by arriving at a true harmony between self-assurance and uncertainty.
Dr. Chuback is a vascular surgeon in private practice in Paramus, N.J.