User login
ABIM turns MOC page with open-book 2-year exams
SAN DIEGO – The way the president of the American Board of Internal Medicine, Richard J. Baron, MD, sees it, maintenance of certification is more important than ever, because trust in the medical profession “is under assault right now in all kinds of ways.”
So, to help “bring clarity to uncertainty,” ABIM is continuing its makeover of the maintenance of certification (MOC) process. Beginning in 2018, an open-book option to test every 2 years will be available for physicians who are certified in internal medicine and for those in the subspecialty of nephrology.
Similar maintenance of certification changes are scheduled to be rolled out to other medical specialties by 2020.
Known as the “Knowledge Check-In,” the 2-year assessment is a shorter, “lower stakes” option that can be taken at home, in an office, or at a testing facility. The check-ins will be scheduled 4-6 times per year, with 10-year exams remaining available twice per year. The open-book 2-year assessments will be about 3 hours in length.
“It’s a more continuous way of learning and assessing, because the way we’ll do feedback is going to change,” explained Dr. Ejnes, who practices in Cranston, R.I. “Specifically, you’ll know right away whether you were successful or not with the assessment, as opposed to having to wait a couple of months, which happens with the 10-year assessment. Then you’ll get more feedback later helping to identify areas where you may be a little weaker and need to work out things.”
In general, physicians will need to either take the 2-year assessments or pass the 10-year assessment within 10 years of their last pass of the 10-year exam. Those who fail two successive 2-year assessments will have to take the 10-year exam. However, unsuccessful performance on the 2-year assessment in 2018 will not have a negative impact on certification or MOC participation status.
“It won’t count as one of the two opportunities you have before you have to go to the 10-year exam,” Dr. Ejnes said. “It allows people to try it out and lets us learn from what happens and do whatever we need to do to make things better.”
Why a 2-year period instead of a 5-year option, for example? A shorter time frame will allow the ABIM to move to a more modular approach to test material, Dr. Ejnes explained. For now, the 2-year assessments will be breadth-of-discipline exams.
Physicians whose certification expires in 2017 will need to take the 10-year exam – as Dr. Ejnes noted he himself was forced to do. “You cannot wait until 2018,” he cautioned. “That’s important, because if you let your certification lapse, you can’t enter the certification pathway. The prerequisite is that you need to be in good standing with your certification.”
The open-book Knowledge Check-Ins and 10-year assessments are slated to expand to eight subspecialties in 2019 and nine more in 2020.
Linking MOC and trust
Speaking at the annual meeting of the American College of Physicians, Dr. Baron said that false and misleading information circulated widely on Facebook and other social media channels runs the gamut of health issues, from falsified studies about purported links between vaccines and autism and public health scares on impostor websites, to stories of miracle cures for any number of ailments.
“It’s not just vaccines people are questioning,” said Dr. Baron, ABIM’s president and CEO. “There are erosions of trust in government, and there’s the tenacity and power of wildly inaccurate information. You will be dealing with patients who tenaciously believe things that you know not to be true. You will need to find ways to build trust, credibility, and relationships based on their trusting that what you’re saying is really in their interest.”
U.S. physicians aren’t secure in the shaky trust landscape. In fact, globally, the United States ranks 24th in public trust level of physicians by country (N. Eng. J. Med. 2014 Oct 23;371[17]:1570-2).
“The confidence in the medical system today is lower than the confidence in police or in small business,” Dr. Baron said. “That’s [the view] people are bringing into your offices every day. I don’t think we can assume that deference and trust are given to doctors, that the privileged role that society affords us is something that we’re going to have forever. We all have to think how trust is built in the new world.”
Will patients value MOC?
During a question and answer session at the ACP session, Anne Cummings, MD, an internist who practices in Greenbrae, Calif., asked the ABIM for support in educating the general public about what it means to be treated by a board-certified physician.
“I had a naturopath tell me the other day that she had the same training as I had,” Dr. Cummings said. “I was floored, but I think that patients don’t know the difference [between board-certified and not board-certified].”
Dr. Baron agreed ABIM needs to do more to promote the value of certification among patients. But he also called on board-certified physicians to deliver the value message directly to their own patients.
Other attendees recommended that ABIM expand the number of ways physicians can earn MOC points, and they expressed concern about the time MOC takes away from their daily practice.
For regular updates on the MOC process, physicians can subscribe to the ABIM’s blog at transforming.abim.org.
SAN DIEGO – The way the president of the American Board of Internal Medicine, Richard J. Baron, MD, sees it, maintenance of certification is more important than ever, because trust in the medical profession “is under assault right now in all kinds of ways.”
So, to help “bring clarity to uncertainty,” ABIM is continuing its makeover of the maintenance of certification (MOC) process. Beginning in 2018, an open-book option to test every 2 years will be available for physicians who are certified in internal medicine and for those in the subspecialty of nephrology.
Similar maintenance of certification changes are scheduled to be rolled out to other medical specialties by 2020.
Known as the “Knowledge Check-In,” the 2-year assessment is a shorter, “lower stakes” option that can be taken at home, in an office, or at a testing facility. The check-ins will be scheduled 4-6 times per year, with 10-year exams remaining available twice per year. The open-book 2-year assessments will be about 3 hours in length.
“It’s a more continuous way of learning and assessing, because the way we’ll do feedback is going to change,” explained Dr. Ejnes, who practices in Cranston, R.I. “Specifically, you’ll know right away whether you were successful or not with the assessment, as opposed to having to wait a couple of months, which happens with the 10-year assessment. Then you’ll get more feedback later helping to identify areas where you may be a little weaker and need to work out things.”
In general, physicians will need to either take the 2-year assessments or pass the 10-year assessment within 10 years of their last pass of the 10-year exam. Those who fail two successive 2-year assessments will have to take the 10-year exam. However, unsuccessful performance on the 2-year assessment in 2018 will not have a negative impact on certification or MOC participation status.
“It won’t count as one of the two opportunities you have before you have to go to the 10-year exam,” Dr. Ejnes said. “It allows people to try it out and lets us learn from what happens and do whatever we need to do to make things better.”
Why a 2-year period instead of a 5-year option, for example? A shorter time frame will allow the ABIM to move to a more modular approach to test material, Dr. Ejnes explained. For now, the 2-year assessments will be breadth-of-discipline exams.
Physicians whose certification expires in 2017 will need to take the 10-year exam – as Dr. Ejnes noted he himself was forced to do. “You cannot wait until 2018,” he cautioned. “That’s important, because if you let your certification lapse, you can’t enter the certification pathway. The prerequisite is that you need to be in good standing with your certification.”
The open-book Knowledge Check-Ins and 10-year assessments are slated to expand to eight subspecialties in 2019 and nine more in 2020.
Linking MOC and trust
Speaking at the annual meeting of the American College of Physicians, Dr. Baron said that false and misleading information circulated widely on Facebook and other social media channels runs the gamut of health issues, from falsified studies about purported links between vaccines and autism and public health scares on impostor websites, to stories of miracle cures for any number of ailments.
“It’s not just vaccines people are questioning,” said Dr. Baron, ABIM’s president and CEO. “There are erosions of trust in government, and there’s the tenacity and power of wildly inaccurate information. You will be dealing with patients who tenaciously believe things that you know not to be true. You will need to find ways to build trust, credibility, and relationships based on their trusting that what you’re saying is really in their interest.”
U.S. physicians aren’t secure in the shaky trust landscape. In fact, globally, the United States ranks 24th in public trust level of physicians by country (N. Eng. J. Med. 2014 Oct 23;371[17]:1570-2).
“The confidence in the medical system today is lower than the confidence in police or in small business,” Dr. Baron said. “That’s [the view] people are bringing into your offices every day. I don’t think we can assume that deference and trust are given to doctors, that the privileged role that society affords us is something that we’re going to have forever. We all have to think how trust is built in the new world.”
Will patients value MOC?
During a question and answer session at the ACP session, Anne Cummings, MD, an internist who practices in Greenbrae, Calif., asked the ABIM for support in educating the general public about what it means to be treated by a board-certified physician.
“I had a naturopath tell me the other day that she had the same training as I had,” Dr. Cummings said. “I was floored, but I think that patients don’t know the difference [between board-certified and not board-certified].”
Dr. Baron agreed ABIM needs to do more to promote the value of certification among patients. But he also called on board-certified physicians to deliver the value message directly to their own patients.
Other attendees recommended that ABIM expand the number of ways physicians can earn MOC points, and they expressed concern about the time MOC takes away from their daily practice.
For regular updates on the MOC process, physicians can subscribe to the ABIM’s blog at transforming.abim.org.
SAN DIEGO – The way the president of the American Board of Internal Medicine, Richard J. Baron, MD, sees it, maintenance of certification is more important than ever, because trust in the medical profession “is under assault right now in all kinds of ways.”
So, to help “bring clarity to uncertainty,” ABIM is continuing its makeover of the maintenance of certification (MOC) process. Beginning in 2018, an open-book option to test every 2 years will be available for physicians who are certified in internal medicine and for those in the subspecialty of nephrology.
Similar maintenance of certification changes are scheduled to be rolled out to other medical specialties by 2020.
Known as the “Knowledge Check-In,” the 2-year assessment is a shorter, “lower stakes” option that can be taken at home, in an office, or at a testing facility. The check-ins will be scheduled 4-6 times per year, with 10-year exams remaining available twice per year. The open-book 2-year assessments will be about 3 hours in length.
“It’s a more continuous way of learning and assessing, because the way we’ll do feedback is going to change,” explained Dr. Ejnes, who practices in Cranston, R.I. “Specifically, you’ll know right away whether you were successful or not with the assessment, as opposed to having to wait a couple of months, which happens with the 10-year assessment. Then you’ll get more feedback later helping to identify areas where you may be a little weaker and need to work out things.”
In general, physicians will need to either take the 2-year assessments or pass the 10-year assessment within 10 years of their last pass of the 10-year exam. Those who fail two successive 2-year assessments will have to take the 10-year exam. However, unsuccessful performance on the 2-year assessment in 2018 will not have a negative impact on certification or MOC participation status.
“It won’t count as one of the two opportunities you have before you have to go to the 10-year exam,” Dr. Ejnes said. “It allows people to try it out and lets us learn from what happens and do whatever we need to do to make things better.”
Why a 2-year period instead of a 5-year option, for example? A shorter time frame will allow the ABIM to move to a more modular approach to test material, Dr. Ejnes explained. For now, the 2-year assessments will be breadth-of-discipline exams.
Physicians whose certification expires in 2017 will need to take the 10-year exam – as Dr. Ejnes noted he himself was forced to do. “You cannot wait until 2018,” he cautioned. “That’s important, because if you let your certification lapse, you can’t enter the certification pathway. The prerequisite is that you need to be in good standing with your certification.”
The open-book Knowledge Check-Ins and 10-year assessments are slated to expand to eight subspecialties in 2019 and nine more in 2020.
Linking MOC and trust
Speaking at the annual meeting of the American College of Physicians, Dr. Baron said that false and misleading information circulated widely on Facebook and other social media channels runs the gamut of health issues, from falsified studies about purported links between vaccines and autism and public health scares on impostor websites, to stories of miracle cures for any number of ailments.
“It’s not just vaccines people are questioning,” said Dr. Baron, ABIM’s president and CEO. “There are erosions of trust in government, and there’s the tenacity and power of wildly inaccurate information. You will be dealing with patients who tenaciously believe things that you know not to be true. You will need to find ways to build trust, credibility, and relationships based on their trusting that what you’re saying is really in their interest.”
U.S. physicians aren’t secure in the shaky trust landscape. In fact, globally, the United States ranks 24th in public trust level of physicians by country (N. Eng. J. Med. 2014 Oct 23;371[17]:1570-2).
“The confidence in the medical system today is lower than the confidence in police or in small business,” Dr. Baron said. “That’s [the view] people are bringing into your offices every day. I don’t think we can assume that deference and trust are given to doctors, that the privileged role that society affords us is something that we’re going to have forever. We all have to think how trust is built in the new world.”
Will patients value MOC?
During a question and answer session at the ACP session, Anne Cummings, MD, an internist who practices in Greenbrae, Calif., asked the ABIM for support in educating the general public about what it means to be treated by a board-certified physician.
“I had a naturopath tell me the other day that she had the same training as I had,” Dr. Cummings said. “I was floored, but I think that patients don’t know the difference [between board-certified and not board-certified].”
Dr. Baron agreed ABIM needs to do more to promote the value of certification among patients. But he also called on board-certified physicians to deliver the value message directly to their own patients.
Other attendees recommended that ABIM expand the number of ways physicians can earn MOC points, and they expressed concern about the time MOC takes away from their daily practice.
For regular updates on the MOC process, physicians can subscribe to the ABIM’s blog at transforming.abim.org.
AT ACP INTERNAL MEDICINE
April 2017 Digital Edition
Click here to access the April 2017 Digital Edition.
Table of Contents
- Write for Us, Right for You
- A Heart Failure Management Program Using Shared Medical Appointments
- Suspected Clozapine-Induced Cardiomyopathy and Heart Failure With Reduced Ejection Fraction
- Military Sexual Trauma and Sexual Health: Practice and Future Research for Mental Health Professionals
- Veterans as Caregivers: Those Who Continue to Serve
- Conjoint Sessions With Clinical Pharmacy and Health Psychology for Chronic Pain
- Hypoperfusion Retinopathy
- William Beaumont: A Pioneer of Physiology

Click here to access the April 2017 Digital Edition.
Table of Contents
- Write for Us, Right for You
- A Heart Failure Management Program Using Shared Medical Appointments
- Suspected Clozapine-Induced Cardiomyopathy and Heart Failure With Reduced Ejection Fraction
- Military Sexual Trauma and Sexual Health: Practice and Future Research for Mental Health Professionals
- Veterans as Caregivers: Those Who Continue to Serve
- Conjoint Sessions With Clinical Pharmacy and Health Psychology for Chronic Pain
- Hypoperfusion Retinopathy
- William Beaumont: A Pioneer of Physiology

Click here to access the April 2017 Digital Edition.
Table of Contents
- Write for Us, Right for You
- A Heart Failure Management Program Using Shared Medical Appointments
- Suspected Clozapine-Induced Cardiomyopathy and Heart Failure With Reduced Ejection Fraction
- Military Sexual Trauma and Sexual Health: Practice and Future Research for Mental Health Professionals
- Veterans as Caregivers: Those Who Continue to Serve
- Conjoint Sessions With Clinical Pharmacy and Health Psychology for Chronic Pain
- Hypoperfusion Retinopathy
- William Beaumont: A Pioneer of Physiology

Drug granted fast track designation for PNH
The US Food and Drug Administration (FDA) has granted fast track designation to Coversin™ for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients who have polymorphisms conferring eculizumab resistance.
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9, and also independently inhibits LTB4 activity.
Coversin is being developed by Akari Therapeutics.
Akari is evaluating Coversin in a pair of phase 2 trials.
In the first trial, researchers are evaluating Coversin in patients with PNH who have never received a complement-blocking therapy. Interim results from this ongoing trial are scheduled to be presented at Akari’s Research and Development Day on April 24 in New York, New York.
In the second phase 2 trial, researchers are evaluating Coversin in patients with PNH who have C5 polymorphisms that confer resistance to eculizumab.
One patient has been enrolled in this trial and has received Coversin for over a year. The treatment has resulted in significant lactate dehydrogenase reduction and complete complement blockade.
About fast track designation
The FDA created the fast track program to facilitate the development and expedite the review of drugs that show promise for treating serious or life-threatening diseases and address unmet medical needs.
Companies developing drugs that receive fast track designation benefit from more frequent communications and meetings with the FDA to review their drug’s development plan, including the design of proposed clinical trials, use of biomarkers, and the extent of data needed for approval.
Drugs with fast track designation may qualify for priority review as well, if relevant criteria are met. Priority review shortens the FDA review process from 10 months to 6 months.
Fast track designation also allows for a rolling review process, whereby completed sections of the investigational new drug application can be submitted for FDA review as they become available, instead of waiting for all sections to be completed. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to Coversin™ for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients who have polymorphisms conferring eculizumab resistance.
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9, and also independently inhibits LTB4 activity.
Coversin is being developed by Akari Therapeutics.
Akari is evaluating Coversin in a pair of phase 2 trials.
In the first trial, researchers are evaluating Coversin in patients with PNH who have never received a complement-blocking therapy. Interim results from this ongoing trial are scheduled to be presented at Akari’s Research and Development Day on April 24 in New York, New York.
In the second phase 2 trial, researchers are evaluating Coversin in patients with PNH who have C5 polymorphisms that confer resistance to eculizumab.
One patient has been enrolled in this trial and has received Coversin for over a year. The treatment has resulted in significant lactate dehydrogenase reduction and complete complement blockade.
About fast track designation
The FDA created the fast track program to facilitate the development and expedite the review of drugs that show promise for treating serious or life-threatening diseases and address unmet medical needs.
Companies developing drugs that receive fast track designation benefit from more frequent communications and meetings with the FDA to review their drug’s development plan, including the design of proposed clinical trials, use of biomarkers, and the extent of data needed for approval.
Drugs with fast track designation may qualify for priority review as well, if relevant criteria are met. Priority review shortens the FDA review process from 10 months to 6 months.
Fast track designation also allows for a rolling review process, whereby completed sections of the investigational new drug application can be submitted for FDA review as they become available, instead of waiting for all sections to be completed. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to Coversin™ for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients who have polymorphisms conferring eculizumab resistance.
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9, and also independently inhibits LTB4 activity.
Coversin is being developed by Akari Therapeutics.
Akari is evaluating Coversin in a pair of phase 2 trials.
In the first trial, researchers are evaluating Coversin in patients with PNH who have never received a complement-blocking therapy. Interim results from this ongoing trial are scheduled to be presented at Akari’s Research and Development Day on April 24 in New York, New York.
In the second phase 2 trial, researchers are evaluating Coversin in patients with PNH who have C5 polymorphisms that confer resistance to eculizumab.
One patient has been enrolled in this trial and has received Coversin for over a year. The treatment has resulted in significant lactate dehydrogenase reduction and complete complement blockade.
About fast track designation
The FDA created the fast track program to facilitate the development and expedite the review of drugs that show promise for treating serious or life-threatening diseases and address unmet medical needs.
Companies developing drugs that receive fast track designation benefit from more frequent communications and meetings with the FDA to review their drug’s development plan, including the design of proposed clinical trials, use of biomarkers, and the extent of data needed for approval.
Drugs with fast track designation may qualify for priority review as well, if relevant criteria are met. Priority review shortens the FDA review process from 10 months to 6 months.
Fast track designation also allows for a rolling review process, whereby completed sections of the investigational new drug application can be submitted for FDA review as they become available, instead of waiting for all sections to be completed. ![]()
Tetanus: Debilitating Infection
CE/CME No: CR-1704
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize patients who are at risk for tetanus.
• Describe the clinical presentation of tetanus.
• Discuss proper treatment for a patient with tetanus.
• Promote widespread vaccination against tetanus.
FACULTY
Timothy W. Ferrarotti is the Director of Didactic Education and Assistant Professor in the PA Studies Program at the University of Saint Joseph, West Hartford, Connecticut.
The author has no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of April 2017.
Article begins on next page >>
Tetanus is a devastating disease that can be prevented by proper immunization and wound care. Although the incidence is low in the United States due to widespread routine vaccination, immunization coverage remains below target, especially in older adults. Since outcome is influenced by the clinician's ability to make a timely diagnosis and initiate appropriate care, continued appreciation of tetanus is warranted.
Tetanus is a neurologic disorder resulting from infection by the gram-positive, spore-forming anaerobic bacillus Clostridium tetani. The bacterium, in spore form, typically enters the body through a contaminated soft-tissue wound. Ubiquitous in the environment, C tetani spores are found throughout the world—in soil as well as in animal feces and saliva—and are resistant to temperature extremes and antiseptics. Tetanus is infectious but not contagious (not transmitted person-to-person).1 Wounds with devitalized tissue or those supporting anaerobic conditions, such as bites, puncture wounds, burns, and gangrene, are conducive to the development of tetanus. Infection can also occur following dental extractions, abortions, and illicit drug injection.1 Although vaccination programs have decreased the incidence of tetanus in the United States, C tetani infection remains an ongoing clinical concern because the spores are omnipresent, universal vaccination coverage has not been achieved, and vaccine-immunity wanes over time, placing individuals at risk.
PATHOPHYSIOLOGY
C tetani produces two toxins: tetanolysin and tetanospasmin (tetanus toxin). Tetanolysin may have a role in promoting the diffusion of tetanospasmin in soft tissues.2 Tetanospasmin is a highly potent toxin, with a lethal dose in humans of less than 2.5 ng/kg of body weight.3 The toxin enters the peripheral nerve at the site of injury and migrates to the central nervous system (CNS). There, it causes unopposed α-motor neuron firing by preventing the release of inhibitory neurotransmitters such as γ-aminobutyric acid (GABA), resulting in muscle spasms and excess reflexive response to sensory stimuli.4 It also leads to excessive catecholamine release from the adrenal medulla.1
Tetanospasmin binds to neurons in the spinal cord and brainstem. Because toxin binding is irreversible, resolution of tetanus requires the neurons to grow new axon terminals. The effects of tetanus can persist for six to eight weeks until new terminals develop.3,5 Patients often require several weeks of ventilator support during this time.3

EPIDEMIOLOGY
Tetanus continues to be a serious cause of morbidity and mortality worldwide. The majority of cases (80%) occur in Africa and Southeast Asia.6 Incidence is much lower in the United States (0.1 cases per million persons annually) because of widespread vaccination, with only 233 cases of tetanus reported between 2001 and 2008.7 However, in the absence of confirmatory tests, the diagnosis is a clinical one; furthermore, there is no laboratory reporting program for tetanus. As a result, more cases may occur in the US than are detected or reported.
In developed countries, tetanus is primarily a disease of the elderly or the unvaccinated. Older persons, especially non-veterans, are less likely to have received the primary series. Because immunity decreases with age, even those who completed the primary series but have not received booster doses are at increased risk.8 Home-schooled children, who are not subject to school-entry vaccination requirements, are also at risk if unvaccinated.9
Neonatal tetanus is an ongoing problem in undeveloped countries that lack maternal vaccination programs. (Maternal immunization successfully reduces neonatal tetanus via passive immunity, and maternal tetanus via active immunity.) Unvaccinated women who undergo nonmedical abortions or unhygienic childbirth are at increased risk for tetanus.3,10
Other risk factors for tetanus include wound contamination with soil, saliva, or devitalized tissue; injection drug use; and exposure to anthropogenic or natural disasters.1 C tetani spores can contaminate heroin and may grow in abscesses of heroin users.4 Small outbreaks of tetanus among injection drug users have been reported, even among younger adults who had some immunity from childhood vaccination.7,11 In addition, patients with diabetes are at increased risk for tetanus. These patients may have chronic wounds due to slowed healing and poor vascularity, which can lead to lower oxygen tension in their wounds and create an environment conducive to anaerobic infection. These chronic wounds are often ignored as a potential nidus for tetanus; instead, focus is placed on plantar puncture wounds or lacerations.7
Though tetanus risk is greatest for those who were never fully immunized, cases have been reported in persons who were immunized in the remote past but had not received a recent booster. Such cases show that tetanus immunity is not absolute and does wane over time.12-14 Among the 233 tetanus cases reported in the US during 2001-2008, vaccination status was reported for 92. Of these, 24 patients had a complete series and 31 patients had at least one prior dose of tetanus vaccine.7 Furthermore, six cases occurred in patients known to have had the four-dose series and a booster within 10 years of diagnosis. Similarly, a 14-year-old boy who was fully vaccinated developed cephalic tetanus from a stingray wound.14 Given these data, clinicians should not assume that a patient who reports having had “a tetanus shot” is completely protected; a full series and regular boosters are required, and, in rare cases, tetanus can occur despite full vaccination.
PATIENT PRESENTATION AND TETANUS TYPES
The CDC describes tetanus as “the acute onset of hypertonia and/or painful muscle contractions (usually of the muscles of the jaw and neck) and generalized muscle spasms without other apparent medical cause.”15 Clinicians should always consider tetanus in patients with dysphagia and trismus, especially if the patient has a wound, had not received primary vaccination, or has not had a booster in several decades. Tetanus cannot be ruled out based on the lack of a wound, however, since up to 25% of patients who develop tetanus have no obvious site of inoculation.16 The incubation period ranges from 3 to 21 days, with more severe cases having shorter incubation periods (< 8 days).10 The closer the site of inoculation is to the CNS, the more serious the disease usually is—and the shorter the incubation period will be.1
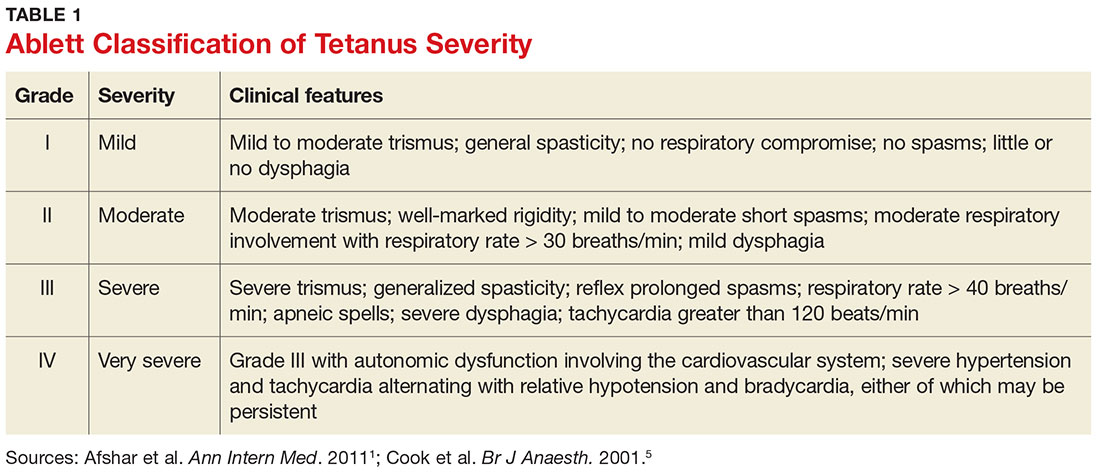
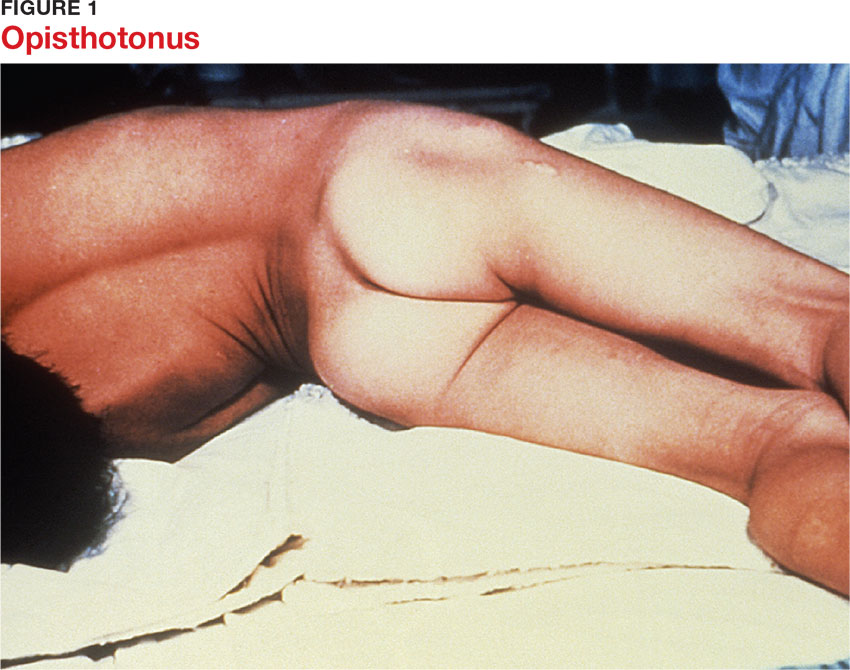
Presentation depends on the time elapsed since inoculation, the severity of illness (determined by the Ablett classification; see Table 1), and the form of tetanus involved. The patient may present early when the infection and toxin are localized to the wound and have not progressed to the CNS (localized tetanus). There may be a wound with signs of infection, including erythema, induration, edema, warmth, tenderness, and drainage. If the injury is on the head or neck, cephalic tetanus may occur, causing the patient to present with painful spasms of the extra-ocular, facial, and/or neck muscles; trismus; dysphagia; or even a Horner-like syndrome. The patient with more advanced, generalized tetanus may have decorticate posturing, abdominal wall rigidity, or opisthotonus.1,2,5,17
Four types of tetanus have been described: generalized, localized, cephalic, and neonatal.
Generalized tetanus is the most common form, accounting for approximately 80% of cases.10 It may involve contractions of the masseter muscles, producing trismus; facial muscles, producing risus sardonicus (sardonic smile); neck and shoulder muscles; abdominal wall muscles, mimicking guarding; and back muscles, producing opisthotonus (arching of the back, neck, and head; see Figure 1) and decorticate posturing (flexion and adduction of the arms, clenched fists, and extension of the lower extremities).1,5,6 Patients with generalized tetanus often exhibit hyperresponsiveness to the environment. As a result, noises and sudden light changes may result in acute spasms. In addition, patients may experience painful spasms when affected muscles are palpated. Affected reflex arcs are usually hyperresponsive to stimuli.1 Intermittent spasms of the thoracic, pharyngeal, and/or laryngeal muscles may cause periods of apnea. Autonomic effects of tetanus mimic those associated with the catecholamine excess of pheochromocytoma. Patients exhibit restlessness, irritability, diaphoresis, fever, excessive salivation, gastric stasis, hypertension, tachycardia, and arrhythmia. There may be interposed hypotension and bradycardia.1,5,17
Localized tetanus involves painful spastic contraction of muscles at or near the site of inoculation. It often evolves into generalized tetanus as the toxin spreads further into the CNS.
Cephalic tetanus involves facial and laryngeal muscles. It is rare, accounting for 1% to 3% of tetanus cases.6 Patients may initially have flaccid paralysis, mimicking stroke, rather than spasm, because the toxin has not completely migrated up the peripheral nerve into the CNS. As the toxin enters the CNS and induces the typical spasm (trismus), the diagnosis will be more obvious. The presence of trismus or a subacute wound on the head may be used to discriminate tetanus from stroke. Cephalic tetanus often evolves into generalized tetanus, affecting more of the body in a caudal direction.5
Neonatal tetanus develops within one week after birth. The neonate with tetanus is usually born to a mother lacking immunization. Typically, the infant sucks and feeds for the first couple of days, then develops inability/refusal to suck/feed, has difficulty opening his/her mouth, becomes weak, and develops muscle spasms.3 The affected child may develop risus sardonicus, clenched hands, dorsiflexion of the feet, and opisthotonus.3
DIFFERENTIAL DIAGNOSIS
The clinician should consider other CNS conditions in the differential diagnosis (see Table 2). Although similar to generalized seizures, tetanus causes painful spasms and does not produce a loss of consciousness.1,17 Tetanus, intracranial bleed, and meningitis all can cause meningismus; meningitis, however, is more likely to manifest with other symptoms of infection, such as headache and fever. Although the autonomic dysfunction of tetanus can cause pyrexia, fever would usually coincide with other sympathetic symptoms, such as hypertension, tachycardia, and diaphoresis. Intracranial bleeding tends to have a more rapid onset than tetanus and produces headache and mental status changes. Seventh nerve palsy produces muscle flaccidity, not spasm, and is usually painless unless associated with herpetic inflammation.1,5,6,14,17
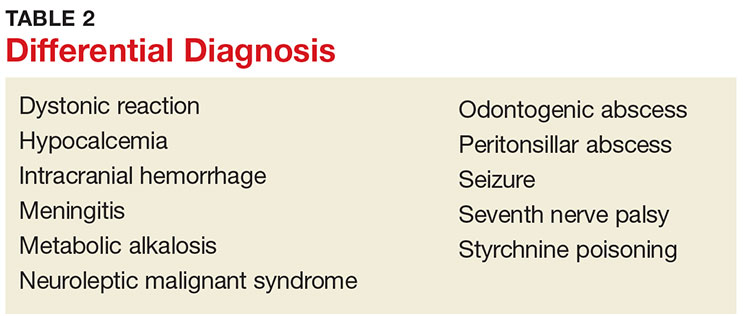
Poisoning and medication effects should also be considered. Strychnine poisoning manifests similar to tetanus but occurs without a wound.5 Blood and urine assays for strychnine can be diagnostic. Dystonic reactions resulting from neuroleptic medications—such as phenothiazines—include torticollis, oropharyngeal muscle spasms, and deviation of the eyes. Unlike tetanus, drug-induced dystonia does not cause reflex spasms and often resolves with benztropine or diphenhydramine administration.1 Neuroleptic malignant syndrome can also cause muscular rigidity and autonomic instability, but unlike tetanus, it often causes altered mental status; it should be considered in patients who recently received a causative medication.5,17
Tetanus often manifests with reflexive muscle spasms similar to those seen in electrolyte and acid-base abnormalities. Hypocalcemia may produce a reflexive spasm of the facial muscles when the facial nerve is percussed (Chvostek sign), while alkalemia may produce reflexive spasm of the hand and wrist muscles (Trousseau sign).1 Lab tests can rule out these diagnoses.1,5
A patient with an odontogenic abscess may have pain and muscle spasm/trismus, but the infection is usually easily detected on exam. The clinician should be cautious in attributing the trismus solely to the swelling, however, as C tetani has been found in odontogenic abscesses and the patient may have both.1,17 Peritonsillar abscess will often produce trismus. When abscess is the cause, careful examination of the oropharynx will usually demonstrate tonsillar exudate, hypertrophy, soft tissue erythema, and tenderness, as well as a misplaced uvula.1
DIAGNOSIS
Tetanus is a clinical diagnosis, usually made based on the findings described. Confirmatory lab tests are not readily available. The organism is infrequently recovered in cultures of specimens from suspected wounds (30% of cases).10,11 Serologic testing on specimens drawn before administration of tetanus immunoglobulin (TIG) may indicate very low or undetectable antitetanus antibody levels, but tetanus can still occur when “protective” levels of antibodies are present.11 Detection of tetanus toxin in plasma or a wound with bioassays and polymerase chain reaction might be possible, but these tests are only available in a few settings.3
THE MULTIFACETED CARE PLAN
The primary care provider should refer a patient with suspected tetanus to an emergency department, preferably a tertiary care center with the necessary specialists. Patients are likely to require prolonged hospitalization. In a recent series of tetanus cases in California, the median length of hospitalization was 18 days.12 Treatment is multifaceted; interventions include immunization, wound care, administration of antibiotics and other pharmacologic agents, and supportive therapy (see Table 3).
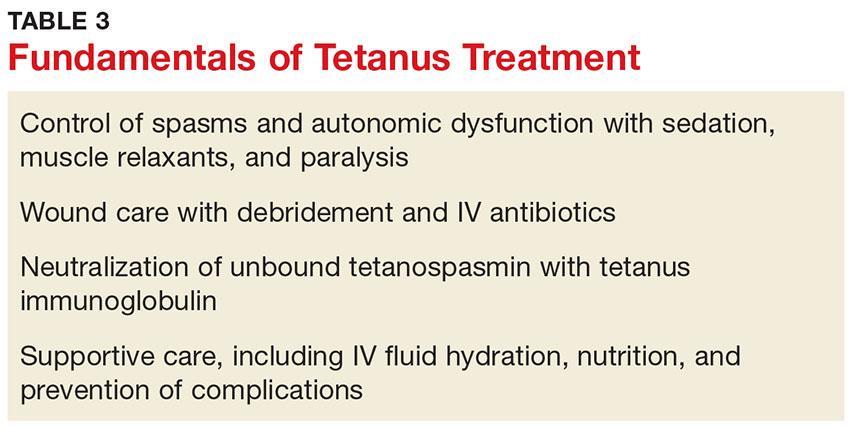
Immunization
All patients with suspected tetanus should immediately receive both passive (with TIG) and active (tetanus toxoid–containing vaccines) immunization. Because of the extremely high potency of tetanus toxin, the very small amount of toxin that is required to cause tetanus is insufficient to prompt an immune response that would confer immunity. Therefore, treatment is the same regardless of whether the patient had prior disease.10
TIG binds to and neutralizes unbound tetanospasmin, preventing progression of the disease. As noted, TIG will not reverse the binding of the toxin to nerve structures.5 Due to a lack of prospective studies, there is disagreement regarding TIG dosage: Doses as high as 3,000-6,000 U have been recommended, but case studies indicate that the dosage recommended by the CDC (500 U) is likely effective.13 The full CDC recommendation is 500 U of human-derived TIG intramuscularly administered at locations near and away from the wound (but always away from the tetanus toxoid injection site).10,17 Outside the US, equine-based TIG may be the only option. Animal-derived TIG is less desirable because of increased allergy risk; when used, a small amount (0.1 mL) should be first administered as an intradermal test.17
Tetanus toxoid immunization produces active immunity. It is currently available in combination antigen forms (tetanus and diphtheria vaccine [Td], tetanus-diphtheria-acellular pertussis [Tdap] vaccine). The dose of either is 0.5 mL. Patients with tetanus should receive three doses given intramuscularly: immediately, at 4 weeks, and at 6 to 12 months.10
Wound care
Wound care should include incision and drainage, removal of foreign bodies, debridement, and irrigation. These steps are taken in order to ensure an aerobic environment in the wound, ultimately decreasing C tetani survival.1
Antibiotics
The preferred antimicrobial agent for treating tetanus infection is metronidazole 500 mg intravenously (IV) every 6 hours.1,3,17 Penicillin (2 to 4 million U IV every 4 to 6 hours) is effective against C tetani, but it is a GABA-receptor antagonist and may worsen tetanus by further inhibiting the release of GABA.1,14,18 GABA-receptor antagonism may also occur with cephalosporins; however, these broader-spectrum agents may be necessary to treat mixed infections.17 Alternatives include doxycycline, macrolides, and clindamycin.1
Other pharmacologic treatment
Benzodiazepines (eg, diazepam 10 to 30 mg IV) can help control rigidity and muscle spasms and are a mainstay of tetanus treatment.18 Benzodiazepines and propofol both act on GABA receptors, producing sedation in addition to controlling muscle spasms.19 Traditionally, more severe spasms, such as opisthotonus, have required induction of complete paralysis with nondepolarizing paralytics, such as pancuronium or vecuronium. However, paralysis is not optimal therapy since it necessitates sedation, intubation, and mechanical ventilation. Because tetanus does not resolve for 6 to 8 weeks, patients who require mechanical ventilation will also require tracheostomy to prevent laryngotracheal stenosis. Paralysis and mechanical ventilation can also lead to deep venous thrombosis, decubitus ulcers, and pneumonia.5 The ideal treatment would reduce the spasms and autonomic instability without the risks associated with deep sedation and paralysis.5
Other agents used in the treatment of tetanus include magnesium sulfate, which can decrease muscle spasm and ameliorate the effects of autonomic dysfunction, and intrathecal baclofen, which can decrease muscle spasm.19,20 Patients with persistent autonomic dysfunction may require combined α- and ß-adrenergic receptor blockade.1,17-20
Supportive care
It is important to implement supportive care, including limiting auditory and tactile stimulation, as well as providing adequate hydration and nutritional support. IV fluids, parenteral feeding, and enteral feeding are required. Measures should be taken to prevent complications of prolonged immobility, paralysis, and mechanical ventilation, including deep venous thrombosis, pulmonary embolism, and pressure ulcers. The quality of supportive care and the swiftness with which the diagnosis is made and appropriate treatment is initiated are key factors that determine an individual patient’s outcome.21
COMPLICATIONS AND MORTALITY
Tetanus can lead to many complications, including long bone and spine fractures from severe muscle spasms, as well as renal failure and aspiration. Most spinal fractures involve the thoracic spine, but lumbar spine fractures have been reported.22 Burst-type fractures of the vertebrae may cause cauda equina syndrome or directly injure the spinal cord if fragments are retropulsed.22 Persistent muscle spasm can also cause rhabdomyolysis and renal failure. Lab test results, including elevated levels of creatine phosphokinase and myoglobin (rhabdomyolysis) as well as blood urea nitrogen and creatinine (renal failure), can indicate presence of complications. Muscle relaxation and hydration are key to prevention.
Patients with trismus are often unable to swallow and maintain oral hygiene, leading to caries and dental abscess. The trismus itself can also cause dental or jaw fractures.2,13 Aspiration can occur when laryngeal muscles are affected, resulting in pneumonia in 50% to 70% of autopsied cases of tetanus.10 Additionally, the paralyzed patient receiving ventilatory support can develop pneumonia, deep vein thrombosis, and pulmonary embolism.5,13 Neonatal tetanus often results in complications such as cerebral palsy or cognitive delay.1
A number of factors influence the severity and outcome of tetanus. Untreated tetanus is typically fatal, with respiratory failure the most common cause of death in settings where mechanical ventilation is unavailable.1 Where mechanical ventilation is accessible, autonomic dysfunction accounts for most deaths.20 Ventilation aside, the case-fatality rate varies according to the medical system. The rate is often less than 20% where modern ICUs are available but can exceed 50% in undeveloped countries with limited facilities.1,5 A review of outcomes data for 197 of the 233 tetanus cases reported in the US during 2001-2008 (modern medical care was provided in all) showed an overall case-fatality rate of 13.2%.7
Age and vaccination status also affect outcomes, with higher case-fatality rates seen in older (18% in those ≥ 60, 31% in those ≥ 65) and unvaccinated (22%) patients. 7,10 In the study of tetanus cases from 2001-2008, the fatality rate was five times higher in patients ages 65 or older compared with patients younger than 65.7 This study also showed that severity of tetanus may be inversely related to the number of vaccine doses the individual has received, and that having previous vaccination was associated with improved survival, as only four of the 26 deaths occurred in patients with prior vaccination.7
Patients who survive the first two weeks of tetanus have a better chance of recovery. Those with multiple chronic comorbidities, such as chronic obstructive pulmonary disease (COPD), diabetes, or cardiovascular disease, are more likely to die because of the physiologic stress of the illness and its treatment.1,7,12 The provision of ventilator support is more complicated in those with COPD; similarly, the autonomic effects of tetanus can be more problematic for patients with chronic cardiac disease or neurologic complications of chronic diabetes.13
PATIENT EDUCATION
Widespread vaccination against tetanus, which began in the US in the mid-20th century, has greatly reduced disease incidence.7 However, vaccination coverage rates remain below target.
In 2012, only 82.5% of children ages 19 to 35 months received the recommended four doses of diphtheria-tetanus-pertussis (DTaP) vaccine, and 94.3% received at least three doses.23 Only 84.6% of teens ages 13 to 17 years received the primary four doses as well as the recommended booster dose.24 The same year, only 55% of patients ages 65 and older and 64% of adults ages 19 to 64 had received a tetanus booster within the previous 10 years.25
Vaccination rates are lower for black, Hispanic, and Asian adults in the US.25 Clinicians should proactively recommend tetanus booster immunization to all adults.
CONCLUSION
Although few clinicians in developed countries will see a case of tetanus, all should be alert for it. Elderly patients and those not fully vaccinated are at risk. Routine immunization decreases but does not eliminate the risk. Tetanus differs from other illnesses controlled by national immunization efforts in that unvaccinated persons do not benefit from herd immunity, because the disease is not contagious. The diagnosis is clinical and should always be considered in patients with trismus, dysphagia, and/or adrenergic excess. Wounds that place a patient at risk for tetanus involve devitalized tissues and anaerobic conditions. Prompt diagnosis is essential, because it allows for early neutralization of unbound tetanospasmin. Wound care including debridement, antibiotic therapy, control of muscle spasms and the effects of autonomic instability, and airway care are fundamental to the treatment of tetanus.
1. Afshar M, Raju M, Ansell D, Bleck TP. Narrative review: tetanus—a health threat after natural disasters in developing countries. Ann Intern Med. 2011;154(5):329-335.
2. Demir NA, Sumer S, Ural O, et al. An alternative treatment approach in tetanus: botulinum toxin. Trop Doct. 2015;45(1): 46-48.
3. Thwaites CL, Beeching NJ, Newton CR. Maternal and neonatal tetanus. Lancet. 2015;385(9965):362-370.
4. Aronoff DM. Clostridium novyi, sordellii, and tetani: mechanisms of disease. Anaerobe. 2013;24:98-101.
5. Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87(3):477-487.
6. Doshi A, Warrell C, Dahdaleh D, Kullmann D. Just a graze? Cephalic tetanus presenting as a stroke mimic. Pract Neurol. 2014;14(1):39-41.
7. CDC. Tetanus surveillance—United States, 2001-2008. MMWR Morb Mortal Wkly Rep. 2011;60(12):365-369.
8. McCabe J, La Varis T, Mason D. Cephalic tetanus complicating geriatric fall. N Z Med J. 2014;127(1400):98-100.
9. Johnson MG, Bradley KK, Mendus S, et al. Vaccine-preventable disease among homeschooled children: two cases of tetanus in Oklahoma. Pediatrics. 2013;132(6):e1686-e1689.
10. CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington, DC: Public Health Foundation; 2015.
11. Tiwari TS. Chapter 16: Tetanus. In: CDC. Manual for Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: CDC; 2012.
12. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis—California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015; 64(9):243-246.
13. Aksoy M, Celik EC, Ahiskalioglu A, Karakaya MA. Tetanus is still a deadly disease: a report of six tetanus cases and reminder of our knowledge. Trop Doct. 2014;44(1):38-42.
14. Felter RA, Zinns LE. Cephalic tetanus in an immunized teenager. Pediatr Emerg Care. 2015;31(7):511-513.
15. CDC. Tetanus (Clostridium tetani) 1996 case definition. www.cdc.gov/nndss/conditions/tetanus/case-definition/1996/. Accessed February 17, 2017.
16. Thwaites CL, Farrar JJ. Preventing and treating tetanus [commentary]. BMJ. 2003;326(7381):117-118.
17. Sexton DJ. Tetanus. UpToDate. www.uptodate.com/contents/tetanus?topicKey=ID%2F5525. Accessed February 17, 2017.
18. Rodrigo C, Fernando D, Rajapakse S. Pharmacological management of tetanus: an evidence-based review. Crit Care. 2014;18(2):217.
19. Santos ML, Mota-Miranda A, Alves-Pereira A, et al. Intrathecal baclofen for the treatment of tetanus. Clin Infect Dis. 2004;38(3):321-328.
20. Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomized controlled trial. Lancet. 2006;368:1436-1443.
21. Govindaraj GM, Riyaz A. Current practice in the management of tetanus. Crit Care. 2014;18(3):145.
22. Wilson TJ, Orringer DA, Sullivan SE, Patil PG. An L-2 burst fracture and cauda equina syndrome due to tetanus. J Neurosurg Spine. 2012;16(1):82-85.
23. CDC. National, state and local area vaccination coverage among children aged 19-35 months—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(36):733-740.
24. CDC. National and state vaccination coverage among adolescents aged 13-17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685-693.
25. Williams WW, Lu PJ, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(5):95-102.
CE/CME No: CR-1704
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize patients who are at risk for tetanus.
• Describe the clinical presentation of tetanus.
• Discuss proper treatment for a patient with tetanus.
• Promote widespread vaccination against tetanus.
FACULTY
Timothy W. Ferrarotti is the Director of Didactic Education and Assistant Professor in the PA Studies Program at the University of Saint Joseph, West Hartford, Connecticut.
The author has no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of April 2017.
Article begins on next page >>
Tetanus is a devastating disease that can be prevented by proper immunization and wound care. Although the incidence is low in the United States due to widespread routine vaccination, immunization coverage remains below target, especially in older adults. Since outcome is influenced by the clinician's ability to make a timely diagnosis and initiate appropriate care, continued appreciation of tetanus is warranted.
Tetanus is a neurologic disorder resulting from infection by the gram-positive, spore-forming anaerobic bacillus Clostridium tetani. The bacterium, in spore form, typically enters the body through a contaminated soft-tissue wound. Ubiquitous in the environment, C tetani spores are found throughout the world—in soil as well as in animal feces and saliva—and are resistant to temperature extremes and antiseptics. Tetanus is infectious but not contagious (not transmitted person-to-person).1 Wounds with devitalized tissue or those supporting anaerobic conditions, such as bites, puncture wounds, burns, and gangrene, are conducive to the development of tetanus. Infection can also occur following dental extractions, abortions, and illicit drug injection.1 Although vaccination programs have decreased the incidence of tetanus in the United States, C tetani infection remains an ongoing clinical concern because the spores are omnipresent, universal vaccination coverage has not been achieved, and vaccine-immunity wanes over time, placing individuals at risk.
PATHOPHYSIOLOGY
C tetani produces two toxins: tetanolysin and tetanospasmin (tetanus toxin). Tetanolysin may have a role in promoting the diffusion of tetanospasmin in soft tissues.2 Tetanospasmin is a highly potent toxin, with a lethal dose in humans of less than 2.5 ng/kg of body weight.3 The toxin enters the peripheral nerve at the site of injury and migrates to the central nervous system (CNS). There, it causes unopposed α-motor neuron firing by preventing the release of inhibitory neurotransmitters such as γ-aminobutyric acid (GABA), resulting in muscle spasms and excess reflexive response to sensory stimuli.4 It also leads to excessive catecholamine release from the adrenal medulla.1
Tetanospasmin binds to neurons in the spinal cord and brainstem. Because toxin binding is irreversible, resolution of tetanus requires the neurons to grow new axon terminals. The effects of tetanus can persist for six to eight weeks until new terminals develop.3,5 Patients often require several weeks of ventilator support during this time.3

EPIDEMIOLOGY
Tetanus continues to be a serious cause of morbidity and mortality worldwide. The majority of cases (80%) occur in Africa and Southeast Asia.6 Incidence is much lower in the United States (0.1 cases per million persons annually) because of widespread vaccination, with only 233 cases of tetanus reported between 2001 and 2008.7 However, in the absence of confirmatory tests, the diagnosis is a clinical one; furthermore, there is no laboratory reporting program for tetanus. As a result, more cases may occur in the US than are detected or reported.
In developed countries, tetanus is primarily a disease of the elderly or the unvaccinated. Older persons, especially non-veterans, are less likely to have received the primary series. Because immunity decreases with age, even those who completed the primary series but have not received booster doses are at increased risk.8 Home-schooled children, who are not subject to school-entry vaccination requirements, are also at risk if unvaccinated.9
Neonatal tetanus is an ongoing problem in undeveloped countries that lack maternal vaccination programs. (Maternal immunization successfully reduces neonatal tetanus via passive immunity, and maternal tetanus via active immunity.) Unvaccinated women who undergo nonmedical abortions or unhygienic childbirth are at increased risk for tetanus.3,10
Other risk factors for tetanus include wound contamination with soil, saliva, or devitalized tissue; injection drug use; and exposure to anthropogenic or natural disasters.1 C tetani spores can contaminate heroin and may grow in abscesses of heroin users.4 Small outbreaks of tetanus among injection drug users have been reported, even among younger adults who had some immunity from childhood vaccination.7,11 In addition, patients with diabetes are at increased risk for tetanus. These patients may have chronic wounds due to slowed healing and poor vascularity, which can lead to lower oxygen tension in their wounds and create an environment conducive to anaerobic infection. These chronic wounds are often ignored as a potential nidus for tetanus; instead, focus is placed on plantar puncture wounds or lacerations.7
Though tetanus risk is greatest for those who were never fully immunized, cases have been reported in persons who were immunized in the remote past but had not received a recent booster. Such cases show that tetanus immunity is not absolute and does wane over time.12-14 Among the 233 tetanus cases reported in the US during 2001-2008, vaccination status was reported for 92. Of these, 24 patients had a complete series and 31 patients had at least one prior dose of tetanus vaccine.7 Furthermore, six cases occurred in patients known to have had the four-dose series and a booster within 10 years of diagnosis. Similarly, a 14-year-old boy who was fully vaccinated developed cephalic tetanus from a stingray wound.14 Given these data, clinicians should not assume that a patient who reports having had “a tetanus shot” is completely protected; a full series and regular boosters are required, and, in rare cases, tetanus can occur despite full vaccination.
PATIENT PRESENTATION AND TETANUS TYPES
The CDC describes tetanus as “the acute onset of hypertonia and/or painful muscle contractions (usually of the muscles of the jaw and neck) and generalized muscle spasms without other apparent medical cause.”15 Clinicians should always consider tetanus in patients with dysphagia and trismus, especially if the patient has a wound, had not received primary vaccination, or has not had a booster in several decades. Tetanus cannot be ruled out based on the lack of a wound, however, since up to 25% of patients who develop tetanus have no obvious site of inoculation.16 The incubation period ranges from 3 to 21 days, with more severe cases having shorter incubation periods (< 8 days).10 The closer the site of inoculation is to the CNS, the more serious the disease usually is—and the shorter the incubation period will be.1
Presentation depends on the time elapsed since inoculation, the severity of illness (determined by the Ablett classification; see Table 1), and the form of tetanus involved. The patient may present early when the infection and toxin are localized to the wound and have not progressed to the CNS (localized tetanus). There may be a wound with signs of infection, including erythema, induration, edema, warmth, tenderness, and drainage. If the injury is on the head or neck, cephalic tetanus may occur, causing the patient to present with painful spasms of the extra-ocular, facial, and/or neck muscles; trismus; dysphagia; or even a Horner-like syndrome. The patient with more advanced, generalized tetanus may have decorticate posturing, abdominal wall rigidity, or opisthotonus.1,2,5,17

Four types of tetanus have been described: generalized, localized, cephalic, and neonatal.
Generalized tetanus is the most common form, accounting for approximately 80% of cases.10 It may involve contractions of the masseter muscles, producing trismus; facial muscles, producing risus sardonicus (sardonic smile); neck and shoulder muscles; abdominal wall muscles, mimicking guarding; and back muscles, producing opisthotonus (arching of the back, neck, and head; see Figure 1) and decorticate posturing (flexion and adduction of the arms, clenched fists, and extension of the lower extremities).1,5,6 Patients with generalized tetanus often exhibit hyperresponsiveness to the environment. As a result, noises and sudden light changes may result in acute spasms. In addition, patients may experience painful spasms when affected muscles are palpated. Affected reflex arcs are usually hyperresponsive to stimuli.1 Intermittent spasms of the thoracic, pharyngeal, and/or laryngeal muscles may cause periods of apnea. Autonomic effects of tetanus mimic those associated with the catecholamine excess of pheochromocytoma. Patients exhibit restlessness, irritability, diaphoresis, fever, excessive salivation, gastric stasis, hypertension, tachycardia, and arrhythmia. There may be interposed hypotension and bradycardia.1,5,17

Localized tetanus involves painful spastic contraction of muscles at or near the site of inoculation. It often evolves into generalized tetanus as the toxin spreads further into the CNS.
Cephalic tetanus involves facial and laryngeal muscles. It is rare, accounting for 1% to 3% of tetanus cases.6 Patients may initially have flaccid paralysis, mimicking stroke, rather than spasm, because the toxin has not completely migrated up the peripheral nerve into the CNS. As the toxin enters the CNS and induces the typical spasm (trismus), the diagnosis will be more obvious. The presence of trismus or a subacute wound on the head may be used to discriminate tetanus from stroke. Cephalic tetanus often evolves into generalized tetanus, affecting more of the body in a caudal direction.5
Neonatal tetanus develops within one week after birth. The neonate with tetanus is usually born to a mother lacking immunization. Typically, the infant sucks and feeds for the first couple of days, then develops inability/refusal to suck/feed, has difficulty opening his/her mouth, becomes weak, and develops muscle spasms.3 The affected child may develop risus sardonicus, clenched hands, dorsiflexion of the feet, and opisthotonus.3
DIFFERENTIAL DIAGNOSIS
The clinician should consider other CNS conditions in the differential diagnosis (see Table 2). Although similar to generalized seizures, tetanus causes painful spasms and does not produce a loss of consciousness.1,17 Tetanus, intracranial bleed, and meningitis all can cause meningismus; meningitis, however, is more likely to manifest with other symptoms of infection, such as headache and fever. Although the autonomic dysfunction of tetanus can cause pyrexia, fever would usually coincide with other sympathetic symptoms, such as hypertension, tachycardia, and diaphoresis. Intracranial bleeding tends to have a more rapid onset than tetanus and produces headache and mental status changes. Seventh nerve palsy produces muscle flaccidity, not spasm, and is usually painless unless associated with herpetic inflammation.1,5,6,14,17

Poisoning and medication effects should also be considered. Strychnine poisoning manifests similar to tetanus but occurs without a wound.5 Blood and urine assays for strychnine can be diagnostic. Dystonic reactions resulting from neuroleptic medications—such as phenothiazines—include torticollis, oropharyngeal muscle spasms, and deviation of the eyes. Unlike tetanus, drug-induced dystonia does not cause reflex spasms and often resolves with benztropine or diphenhydramine administration.1 Neuroleptic malignant syndrome can also cause muscular rigidity and autonomic instability, but unlike tetanus, it often causes altered mental status; it should be considered in patients who recently received a causative medication.5,17
Tetanus often manifests with reflexive muscle spasms similar to those seen in electrolyte and acid-base abnormalities. Hypocalcemia may produce a reflexive spasm of the facial muscles when the facial nerve is percussed (Chvostek sign), while alkalemia may produce reflexive spasm of the hand and wrist muscles (Trousseau sign).1 Lab tests can rule out these diagnoses.1,5
A patient with an odontogenic abscess may have pain and muscle spasm/trismus, but the infection is usually easily detected on exam. The clinician should be cautious in attributing the trismus solely to the swelling, however, as C tetani has been found in odontogenic abscesses and the patient may have both.1,17 Peritonsillar abscess will often produce trismus. When abscess is the cause, careful examination of the oropharynx will usually demonstrate tonsillar exudate, hypertrophy, soft tissue erythema, and tenderness, as well as a misplaced uvula.1
DIAGNOSIS
Tetanus is a clinical diagnosis, usually made based on the findings described. Confirmatory lab tests are not readily available. The organism is infrequently recovered in cultures of specimens from suspected wounds (30% of cases).10,11 Serologic testing on specimens drawn before administration of tetanus immunoglobulin (TIG) may indicate very low or undetectable antitetanus antibody levels, but tetanus can still occur when “protective” levels of antibodies are present.11 Detection of tetanus toxin in plasma or a wound with bioassays and polymerase chain reaction might be possible, but these tests are only available in a few settings.3
THE MULTIFACETED CARE PLAN
The primary care provider should refer a patient with suspected tetanus to an emergency department, preferably a tertiary care center with the necessary specialists. Patients are likely to require prolonged hospitalization. In a recent series of tetanus cases in California, the median length of hospitalization was 18 days.12 Treatment is multifaceted; interventions include immunization, wound care, administration of antibiotics and other pharmacologic agents, and supportive therapy (see Table 3).

Immunization
All patients with suspected tetanus should immediately receive both passive (with TIG) and active (tetanus toxoid–containing vaccines) immunization. Because of the extremely high potency of tetanus toxin, the very small amount of toxin that is required to cause tetanus is insufficient to prompt an immune response that would confer immunity. Therefore, treatment is the same regardless of whether the patient had prior disease.10
TIG binds to and neutralizes unbound tetanospasmin, preventing progression of the disease. As noted, TIG will not reverse the binding of the toxin to nerve structures.5 Due to a lack of prospective studies, there is disagreement regarding TIG dosage: Doses as high as 3,000-6,000 U have been recommended, but case studies indicate that the dosage recommended by the CDC (500 U) is likely effective.13 The full CDC recommendation is 500 U of human-derived TIG intramuscularly administered at locations near and away from the wound (but always away from the tetanus toxoid injection site).10,17 Outside the US, equine-based TIG may be the only option. Animal-derived TIG is less desirable because of increased allergy risk; when used, a small amount (0.1 mL) should be first administered as an intradermal test.17
Tetanus toxoid immunization produces active immunity. It is currently available in combination antigen forms (tetanus and diphtheria vaccine [Td], tetanus-diphtheria-acellular pertussis [Tdap] vaccine). The dose of either is 0.5 mL. Patients with tetanus should receive three doses given intramuscularly: immediately, at 4 weeks, and at 6 to 12 months.10
Wound care
Wound care should include incision and drainage, removal of foreign bodies, debridement, and irrigation. These steps are taken in order to ensure an aerobic environment in the wound, ultimately decreasing C tetani survival.1
Antibiotics
The preferred antimicrobial agent for treating tetanus infection is metronidazole 500 mg intravenously (IV) every 6 hours.1,3,17 Penicillin (2 to 4 million U IV every 4 to 6 hours) is effective against C tetani, but it is a GABA-receptor antagonist and may worsen tetanus by further inhibiting the release of GABA.1,14,18 GABA-receptor antagonism may also occur with cephalosporins; however, these broader-spectrum agents may be necessary to treat mixed infections.17 Alternatives include doxycycline, macrolides, and clindamycin.1
Other pharmacologic treatment
Benzodiazepines (eg, diazepam 10 to 30 mg IV) can help control rigidity and muscle spasms and are a mainstay of tetanus treatment.18 Benzodiazepines and propofol both act on GABA receptors, producing sedation in addition to controlling muscle spasms.19 Traditionally, more severe spasms, such as opisthotonus, have required induction of complete paralysis with nondepolarizing paralytics, such as pancuronium or vecuronium. However, paralysis is not optimal therapy since it necessitates sedation, intubation, and mechanical ventilation. Because tetanus does not resolve for 6 to 8 weeks, patients who require mechanical ventilation will also require tracheostomy to prevent laryngotracheal stenosis. Paralysis and mechanical ventilation can also lead to deep venous thrombosis, decubitus ulcers, and pneumonia.5 The ideal treatment would reduce the spasms and autonomic instability without the risks associated with deep sedation and paralysis.5
Other agents used in the treatment of tetanus include magnesium sulfate, which can decrease muscle spasm and ameliorate the effects of autonomic dysfunction, and intrathecal baclofen, which can decrease muscle spasm.19,20 Patients with persistent autonomic dysfunction may require combined α- and ß-adrenergic receptor blockade.1,17-20
Supportive care
It is important to implement supportive care, including limiting auditory and tactile stimulation, as well as providing adequate hydration and nutritional support. IV fluids, parenteral feeding, and enteral feeding are required. Measures should be taken to prevent complications of prolonged immobility, paralysis, and mechanical ventilation, including deep venous thrombosis, pulmonary embolism, and pressure ulcers. The quality of supportive care and the swiftness with which the diagnosis is made and appropriate treatment is initiated are key factors that determine an individual patient’s outcome.21
COMPLICATIONS AND MORTALITY
Tetanus can lead to many complications, including long bone and spine fractures from severe muscle spasms, as well as renal failure and aspiration. Most spinal fractures involve the thoracic spine, but lumbar spine fractures have been reported.22 Burst-type fractures of the vertebrae may cause cauda equina syndrome or directly injure the spinal cord if fragments are retropulsed.22 Persistent muscle spasm can also cause rhabdomyolysis and renal failure. Lab test results, including elevated levels of creatine phosphokinase and myoglobin (rhabdomyolysis) as well as blood urea nitrogen and creatinine (renal failure), can indicate presence of complications. Muscle relaxation and hydration are key to prevention.
Patients with trismus are often unable to swallow and maintain oral hygiene, leading to caries and dental abscess. The trismus itself can also cause dental or jaw fractures.2,13 Aspiration can occur when laryngeal muscles are affected, resulting in pneumonia in 50% to 70% of autopsied cases of tetanus.10 Additionally, the paralyzed patient receiving ventilatory support can develop pneumonia, deep vein thrombosis, and pulmonary embolism.5,13 Neonatal tetanus often results in complications such as cerebral palsy or cognitive delay.1
A number of factors influence the severity and outcome of tetanus. Untreated tetanus is typically fatal, with respiratory failure the most common cause of death in settings where mechanical ventilation is unavailable.1 Where mechanical ventilation is accessible, autonomic dysfunction accounts for most deaths.20 Ventilation aside, the case-fatality rate varies according to the medical system. The rate is often less than 20% where modern ICUs are available but can exceed 50% in undeveloped countries with limited facilities.1,5 A review of outcomes data for 197 of the 233 tetanus cases reported in the US during 2001-2008 (modern medical care was provided in all) showed an overall case-fatality rate of 13.2%.7
Age and vaccination status also affect outcomes, with higher case-fatality rates seen in older (18% in those ≥ 60, 31% in those ≥ 65) and unvaccinated (22%) patients. 7,10 In the study of tetanus cases from 2001-2008, the fatality rate was five times higher in patients ages 65 or older compared with patients younger than 65.7 This study also showed that severity of tetanus may be inversely related to the number of vaccine doses the individual has received, and that having previous vaccination was associated with improved survival, as only four of the 26 deaths occurred in patients with prior vaccination.7
Patients who survive the first two weeks of tetanus have a better chance of recovery. Those with multiple chronic comorbidities, such as chronic obstructive pulmonary disease (COPD), diabetes, or cardiovascular disease, are more likely to die because of the physiologic stress of the illness and its treatment.1,7,12 The provision of ventilator support is more complicated in those with COPD; similarly, the autonomic effects of tetanus can be more problematic for patients with chronic cardiac disease or neurologic complications of chronic diabetes.13
PATIENT EDUCATION
Widespread vaccination against tetanus, which began in the US in the mid-20th century, has greatly reduced disease incidence.7 However, vaccination coverage rates remain below target.
In 2012, only 82.5% of children ages 19 to 35 months received the recommended four doses of diphtheria-tetanus-pertussis (DTaP) vaccine, and 94.3% received at least three doses.23 Only 84.6% of teens ages 13 to 17 years received the primary four doses as well as the recommended booster dose.24 The same year, only 55% of patients ages 65 and older and 64% of adults ages 19 to 64 had received a tetanus booster within the previous 10 years.25
Vaccination rates are lower for black, Hispanic, and Asian adults in the US.25 Clinicians should proactively recommend tetanus booster immunization to all adults.
CONCLUSION
Although few clinicians in developed countries will see a case of tetanus, all should be alert for it. Elderly patients and those not fully vaccinated are at risk. Routine immunization decreases but does not eliminate the risk. Tetanus differs from other illnesses controlled by national immunization efforts in that unvaccinated persons do not benefit from herd immunity, because the disease is not contagious. The diagnosis is clinical and should always be considered in patients with trismus, dysphagia, and/or adrenergic excess. Wounds that place a patient at risk for tetanus involve devitalized tissues and anaerobic conditions. Prompt diagnosis is essential, because it allows for early neutralization of unbound tetanospasmin. Wound care including debridement, antibiotic therapy, control of muscle spasms and the effects of autonomic instability, and airway care are fundamental to the treatment of tetanus.
CE/CME No: CR-1704
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize patients who are at risk for tetanus.
• Describe the clinical presentation of tetanus.
• Discuss proper treatment for a patient with tetanus.
• Promote widespread vaccination against tetanus.
FACULTY
Timothy W. Ferrarotti is the Director of Didactic Education and Assistant Professor in the PA Studies Program at the University of Saint Joseph, West Hartford, Connecticut.
The author has no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of April 2017.
Article begins on next page >>
Tetanus is a devastating disease that can be prevented by proper immunization and wound care. Although the incidence is low in the United States due to widespread routine vaccination, immunization coverage remains below target, especially in older adults. Since outcome is influenced by the clinician's ability to make a timely diagnosis and initiate appropriate care, continued appreciation of tetanus is warranted.
Tetanus is a neurologic disorder resulting from infection by the gram-positive, spore-forming anaerobic bacillus Clostridium tetani. The bacterium, in spore form, typically enters the body through a contaminated soft-tissue wound. Ubiquitous in the environment, C tetani spores are found throughout the world—in soil as well as in animal feces and saliva—and are resistant to temperature extremes and antiseptics. Tetanus is infectious but not contagious (not transmitted person-to-person).1 Wounds with devitalized tissue or those supporting anaerobic conditions, such as bites, puncture wounds, burns, and gangrene, are conducive to the development of tetanus. Infection can also occur following dental extractions, abortions, and illicit drug injection.1 Although vaccination programs have decreased the incidence of tetanus in the United States, C tetani infection remains an ongoing clinical concern because the spores are omnipresent, universal vaccination coverage has not been achieved, and vaccine-immunity wanes over time, placing individuals at risk.
PATHOPHYSIOLOGY
C tetani produces two toxins: tetanolysin and tetanospasmin (tetanus toxin). Tetanolysin may have a role in promoting the diffusion of tetanospasmin in soft tissues.2 Tetanospasmin is a highly potent toxin, with a lethal dose in humans of less than 2.5 ng/kg of body weight.3 The toxin enters the peripheral nerve at the site of injury and migrates to the central nervous system (CNS). There, it causes unopposed α-motor neuron firing by preventing the release of inhibitory neurotransmitters such as γ-aminobutyric acid (GABA), resulting in muscle spasms and excess reflexive response to sensory stimuli.4 It also leads to excessive catecholamine release from the adrenal medulla.1
Tetanospasmin binds to neurons in the spinal cord and brainstem. Because toxin binding is irreversible, resolution of tetanus requires the neurons to grow new axon terminals. The effects of tetanus can persist for six to eight weeks until new terminals develop.3,5 Patients often require several weeks of ventilator support during this time.3

EPIDEMIOLOGY
Tetanus continues to be a serious cause of morbidity and mortality worldwide. The majority of cases (80%) occur in Africa and Southeast Asia.6 Incidence is much lower in the United States (0.1 cases per million persons annually) because of widespread vaccination, with only 233 cases of tetanus reported between 2001 and 2008.7 However, in the absence of confirmatory tests, the diagnosis is a clinical one; furthermore, there is no laboratory reporting program for tetanus. As a result, more cases may occur in the US than are detected or reported.
In developed countries, tetanus is primarily a disease of the elderly or the unvaccinated. Older persons, especially non-veterans, are less likely to have received the primary series. Because immunity decreases with age, even those who completed the primary series but have not received booster doses are at increased risk.8 Home-schooled children, who are not subject to school-entry vaccination requirements, are also at risk if unvaccinated.9
Neonatal tetanus is an ongoing problem in undeveloped countries that lack maternal vaccination programs. (Maternal immunization successfully reduces neonatal tetanus via passive immunity, and maternal tetanus via active immunity.) Unvaccinated women who undergo nonmedical abortions or unhygienic childbirth are at increased risk for tetanus.3,10
Other risk factors for tetanus include wound contamination with soil, saliva, or devitalized tissue; injection drug use; and exposure to anthropogenic or natural disasters.1 C tetani spores can contaminate heroin and may grow in abscesses of heroin users.4 Small outbreaks of tetanus among injection drug users have been reported, even among younger adults who had some immunity from childhood vaccination.7,11 In addition, patients with diabetes are at increased risk for tetanus. These patients may have chronic wounds due to slowed healing and poor vascularity, which can lead to lower oxygen tension in their wounds and create an environment conducive to anaerobic infection. These chronic wounds are often ignored as a potential nidus for tetanus; instead, focus is placed on plantar puncture wounds or lacerations.7
Though tetanus risk is greatest for those who were never fully immunized, cases have been reported in persons who were immunized in the remote past but had not received a recent booster. Such cases show that tetanus immunity is not absolute and does wane over time.12-14 Among the 233 tetanus cases reported in the US during 2001-2008, vaccination status was reported for 92. Of these, 24 patients had a complete series and 31 patients had at least one prior dose of tetanus vaccine.7 Furthermore, six cases occurred in patients known to have had the four-dose series and a booster within 10 years of diagnosis. Similarly, a 14-year-old boy who was fully vaccinated developed cephalic tetanus from a stingray wound.14 Given these data, clinicians should not assume that a patient who reports having had “a tetanus shot” is completely protected; a full series and regular boosters are required, and, in rare cases, tetanus can occur despite full vaccination.
PATIENT PRESENTATION AND TETANUS TYPES
The CDC describes tetanus as “the acute onset of hypertonia and/or painful muscle contractions (usually of the muscles of the jaw and neck) and generalized muscle spasms without other apparent medical cause.”15 Clinicians should always consider tetanus in patients with dysphagia and trismus, especially if the patient has a wound, had not received primary vaccination, or has not had a booster in several decades. Tetanus cannot be ruled out based on the lack of a wound, however, since up to 25% of patients who develop tetanus have no obvious site of inoculation.16 The incubation period ranges from 3 to 21 days, with more severe cases having shorter incubation periods (< 8 days).10 The closer the site of inoculation is to the CNS, the more serious the disease usually is—and the shorter the incubation period will be.1
Presentation depends on the time elapsed since inoculation, the severity of illness (determined by the Ablett classification; see Table 1), and the form of tetanus involved. The patient may present early when the infection and toxin are localized to the wound and have not progressed to the CNS (localized tetanus). There may be a wound with signs of infection, including erythema, induration, edema, warmth, tenderness, and drainage. If the injury is on the head or neck, cephalic tetanus may occur, causing the patient to present with painful spasms of the extra-ocular, facial, and/or neck muscles; trismus; dysphagia; or even a Horner-like syndrome. The patient with more advanced, generalized tetanus may have decorticate posturing, abdominal wall rigidity, or opisthotonus.1,2,5,17

Four types of tetanus have been described: generalized, localized, cephalic, and neonatal.
Generalized tetanus is the most common form, accounting for approximately 80% of cases.10 It may involve contractions of the masseter muscles, producing trismus; facial muscles, producing risus sardonicus (sardonic smile); neck and shoulder muscles; abdominal wall muscles, mimicking guarding; and back muscles, producing opisthotonus (arching of the back, neck, and head; see Figure 1) and decorticate posturing (flexion and adduction of the arms, clenched fists, and extension of the lower extremities).1,5,6 Patients with generalized tetanus often exhibit hyperresponsiveness to the environment. As a result, noises and sudden light changes may result in acute spasms. In addition, patients may experience painful spasms when affected muscles are palpated. Affected reflex arcs are usually hyperresponsive to stimuli.1 Intermittent spasms of the thoracic, pharyngeal, and/or laryngeal muscles may cause periods of apnea. Autonomic effects of tetanus mimic those associated with the catecholamine excess of pheochromocytoma. Patients exhibit restlessness, irritability, diaphoresis, fever, excessive salivation, gastric stasis, hypertension, tachycardia, and arrhythmia. There may be interposed hypotension and bradycardia.1,5,17

Localized tetanus involves painful spastic contraction of muscles at or near the site of inoculation. It often evolves into generalized tetanus as the toxin spreads further into the CNS.
Cephalic tetanus involves facial and laryngeal muscles. It is rare, accounting for 1% to 3% of tetanus cases.6 Patients may initially have flaccid paralysis, mimicking stroke, rather than spasm, because the toxin has not completely migrated up the peripheral nerve into the CNS. As the toxin enters the CNS and induces the typical spasm (trismus), the diagnosis will be more obvious. The presence of trismus or a subacute wound on the head may be used to discriminate tetanus from stroke. Cephalic tetanus often evolves into generalized tetanus, affecting more of the body in a caudal direction.5
Neonatal tetanus develops within one week after birth. The neonate with tetanus is usually born to a mother lacking immunization. Typically, the infant sucks and feeds for the first couple of days, then develops inability/refusal to suck/feed, has difficulty opening his/her mouth, becomes weak, and develops muscle spasms.3 The affected child may develop risus sardonicus, clenched hands, dorsiflexion of the feet, and opisthotonus.3
DIFFERENTIAL DIAGNOSIS
The clinician should consider other CNS conditions in the differential diagnosis (see Table 2). Although similar to generalized seizures, tetanus causes painful spasms and does not produce a loss of consciousness.1,17 Tetanus, intracranial bleed, and meningitis all can cause meningismus; meningitis, however, is more likely to manifest with other symptoms of infection, such as headache and fever. Although the autonomic dysfunction of tetanus can cause pyrexia, fever would usually coincide with other sympathetic symptoms, such as hypertension, tachycardia, and diaphoresis. Intracranial bleeding tends to have a more rapid onset than tetanus and produces headache and mental status changes. Seventh nerve palsy produces muscle flaccidity, not spasm, and is usually painless unless associated with herpetic inflammation.1,5,6,14,17

Poisoning and medication effects should also be considered. Strychnine poisoning manifests similar to tetanus but occurs without a wound.5 Blood and urine assays for strychnine can be diagnostic. Dystonic reactions resulting from neuroleptic medications—such as phenothiazines—include torticollis, oropharyngeal muscle spasms, and deviation of the eyes. Unlike tetanus, drug-induced dystonia does not cause reflex spasms and often resolves with benztropine or diphenhydramine administration.1 Neuroleptic malignant syndrome can also cause muscular rigidity and autonomic instability, but unlike tetanus, it often causes altered mental status; it should be considered in patients who recently received a causative medication.5,17
Tetanus often manifests with reflexive muscle spasms similar to those seen in electrolyte and acid-base abnormalities. Hypocalcemia may produce a reflexive spasm of the facial muscles when the facial nerve is percussed (Chvostek sign), while alkalemia may produce reflexive spasm of the hand and wrist muscles (Trousseau sign).1 Lab tests can rule out these diagnoses.1,5
A patient with an odontogenic abscess may have pain and muscle spasm/trismus, but the infection is usually easily detected on exam. The clinician should be cautious in attributing the trismus solely to the swelling, however, as C tetani has been found in odontogenic abscesses and the patient may have both.1,17 Peritonsillar abscess will often produce trismus. When abscess is the cause, careful examination of the oropharynx will usually demonstrate tonsillar exudate, hypertrophy, soft tissue erythema, and tenderness, as well as a misplaced uvula.1
DIAGNOSIS
Tetanus is a clinical diagnosis, usually made based on the findings described. Confirmatory lab tests are not readily available. The organism is infrequently recovered in cultures of specimens from suspected wounds (30% of cases).10,11 Serologic testing on specimens drawn before administration of tetanus immunoglobulin (TIG) may indicate very low or undetectable antitetanus antibody levels, but tetanus can still occur when “protective” levels of antibodies are present.11 Detection of tetanus toxin in plasma or a wound with bioassays and polymerase chain reaction might be possible, but these tests are only available in a few settings.3
THE MULTIFACETED CARE PLAN
The primary care provider should refer a patient with suspected tetanus to an emergency department, preferably a tertiary care center with the necessary specialists. Patients are likely to require prolonged hospitalization. In a recent series of tetanus cases in California, the median length of hospitalization was 18 days.12 Treatment is multifaceted; interventions include immunization, wound care, administration of antibiotics and other pharmacologic agents, and supportive therapy (see Table 3).

Immunization
All patients with suspected tetanus should immediately receive both passive (with TIG) and active (tetanus toxoid–containing vaccines) immunization. Because of the extremely high potency of tetanus toxin, the very small amount of toxin that is required to cause tetanus is insufficient to prompt an immune response that would confer immunity. Therefore, treatment is the same regardless of whether the patient had prior disease.10
TIG binds to and neutralizes unbound tetanospasmin, preventing progression of the disease. As noted, TIG will not reverse the binding of the toxin to nerve structures.5 Due to a lack of prospective studies, there is disagreement regarding TIG dosage: Doses as high as 3,000-6,000 U have been recommended, but case studies indicate that the dosage recommended by the CDC (500 U) is likely effective.13 The full CDC recommendation is 500 U of human-derived TIG intramuscularly administered at locations near and away from the wound (but always away from the tetanus toxoid injection site).10,17 Outside the US, equine-based TIG may be the only option. Animal-derived TIG is less desirable because of increased allergy risk; when used, a small amount (0.1 mL) should be first administered as an intradermal test.17
Tetanus toxoid immunization produces active immunity. It is currently available in combination antigen forms (tetanus and diphtheria vaccine [Td], tetanus-diphtheria-acellular pertussis [Tdap] vaccine). The dose of either is 0.5 mL. Patients with tetanus should receive three doses given intramuscularly: immediately, at 4 weeks, and at 6 to 12 months.10
Wound care
Wound care should include incision and drainage, removal of foreign bodies, debridement, and irrigation. These steps are taken in order to ensure an aerobic environment in the wound, ultimately decreasing C tetani survival.1
Antibiotics
The preferred antimicrobial agent for treating tetanus infection is metronidazole 500 mg intravenously (IV) every 6 hours.1,3,17 Penicillin (2 to 4 million U IV every 4 to 6 hours) is effective against C tetani, but it is a GABA-receptor antagonist and may worsen tetanus by further inhibiting the release of GABA.1,14,18 GABA-receptor antagonism may also occur with cephalosporins; however, these broader-spectrum agents may be necessary to treat mixed infections.17 Alternatives include doxycycline, macrolides, and clindamycin.1
Other pharmacologic treatment
Benzodiazepines (eg, diazepam 10 to 30 mg IV) can help control rigidity and muscle spasms and are a mainstay of tetanus treatment.18 Benzodiazepines and propofol both act on GABA receptors, producing sedation in addition to controlling muscle spasms.19 Traditionally, more severe spasms, such as opisthotonus, have required induction of complete paralysis with nondepolarizing paralytics, such as pancuronium or vecuronium. However, paralysis is not optimal therapy since it necessitates sedation, intubation, and mechanical ventilation. Because tetanus does not resolve for 6 to 8 weeks, patients who require mechanical ventilation will also require tracheostomy to prevent laryngotracheal stenosis. Paralysis and mechanical ventilation can also lead to deep venous thrombosis, decubitus ulcers, and pneumonia.5 The ideal treatment would reduce the spasms and autonomic instability without the risks associated with deep sedation and paralysis.5
Other agents used in the treatment of tetanus include magnesium sulfate, which can decrease muscle spasm and ameliorate the effects of autonomic dysfunction, and intrathecal baclofen, which can decrease muscle spasm.19,20 Patients with persistent autonomic dysfunction may require combined α- and ß-adrenergic receptor blockade.1,17-20
Supportive care
It is important to implement supportive care, including limiting auditory and tactile stimulation, as well as providing adequate hydration and nutritional support. IV fluids, parenteral feeding, and enteral feeding are required. Measures should be taken to prevent complications of prolonged immobility, paralysis, and mechanical ventilation, including deep venous thrombosis, pulmonary embolism, and pressure ulcers. The quality of supportive care and the swiftness with which the diagnosis is made and appropriate treatment is initiated are key factors that determine an individual patient’s outcome.21
COMPLICATIONS AND MORTALITY
Tetanus can lead to many complications, including long bone and spine fractures from severe muscle spasms, as well as renal failure and aspiration. Most spinal fractures involve the thoracic spine, but lumbar spine fractures have been reported.22 Burst-type fractures of the vertebrae may cause cauda equina syndrome or directly injure the spinal cord if fragments are retropulsed.22 Persistent muscle spasm can also cause rhabdomyolysis and renal failure. Lab test results, including elevated levels of creatine phosphokinase and myoglobin (rhabdomyolysis) as well as blood urea nitrogen and creatinine (renal failure), can indicate presence of complications. Muscle relaxation and hydration are key to prevention.
Patients with trismus are often unable to swallow and maintain oral hygiene, leading to caries and dental abscess. The trismus itself can also cause dental or jaw fractures.2,13 Aspiration can occur when laryngeal muscles are affected, resulting in pneumonia in 50% to 70% of autopsied cases of tetanus.10 Additionally, the paralyzed patient receiving ventilatory support can develop pneumonia, deep vein thrombosis, and pulmonary embolism.5,13 Neonatal tetanus often results in complications such as cerebral palsy or cognitive delay.1
A number of factors influence the severity and outcome of tetanus. Untreated tetanus is typically fatal, with respiratory failure the most common cause of death in settings where mechanical ventilation is unavailable.1 Where mechanical ventilation is accessible, autonomic dysfunction accounts for most deaths.20 Ventilation aside, the case-fatality rate varies according to the medical system. The rate is often less than 20% where modern ICUs are available but can exceed 50% in undeveloped countries with limited facilities.1,5 A review of outcomes data for 197 of the 233 tetanus cases reported in the US during 2001-2008 (modern medical care was provided in all) showed an overall case-fatality rate of 13.2%.7
Age and vaccination status also affect outcomes, with higher case-fatality rates seen in older (18% in those ≥ 60, 31% in those ≥ 65) and unvaccinated (22%) patients. 7,10 In the study of tetanus cases from 2001-2008, the fatality rate was five times higher in patients ages 65 or older compared with patients younger than 65.7 This study also showed that severity of tetanus may be inversely related to the number of vaccine doses the individual has received, and that having previous vaccination was associated with improved survival, as only four of the 26 deaths occurred in patients with prior vaccination.7
Patients who survive the first two weeks of tetanus have a better chance of recovery. Those with multiple chronic comorbidities, such as chronic obstructive pulmonary disease (COPD), diabetes, or cardiovascular disease, are more likely to die because of the physiologic stress of the illness and its treatment.1,7,12 The provision of ventilator support is more complicated in those with COPD; similarly, the autonomic effects of tetanus can be more problematic for patients with chronic cardiac disease or neurologic complications of chronic diabetes.13
PATIENT EDUCATION
Widespread vaccination against tetanus, which began in the US in the mid-20th century, has greatly reduced disease incidence.7 However, vaccination coverage rates remain below target.
In 2012, only 82.5% of children ages 19 to 35 months received the recommended four doses of diphtheria-tetanus-pertussis (DTaP) vaccine, and 94.3% received at least three doses.23 Only 84.6% of teens ages 13 to 17 years received the primary four doses as well as the recommended booster dose.24 The same year, only 55% of patients ages 65 and older and 64% of adults ages 19 to 64 had received a tetanus booster within the previous 10 years.25
Vaccination rates are lower for black, Hispanic, and Asian adults in the US.25 Clinicians should proactively recommend tetanus booster immunization to all adults.
CONCLUSION
Although few clinicians in developed countries will see a case of tetanus, all should be alert for it. Elderly patients and those not fully vaccinated are at risk. Routine immunization decreases but does not eliminate the risk. Tetanus differs from other illnesses controlled by national immunization efforts in that unvaccinated persons do not benefit from herd immunity, because the disease is not contagious. The diagnosis is clinical and should always be considered in patients with trismus, dysphagia, and/or adrenergic excess. Wounds that place a patient at risk for tetanus involve devitalized tissues and anaerobic conditions. Prompt diagnosis is essential, because it allows for early neutralization of unbound tetanospasmin. Wound care including debridement, antibiotic therapy, control of muscle spasms and the effects of autonomic instability, and airway care are fundamental to the treatment of tetanus.
1. Afshar M, Raju M, Ansell D, Bleck TP. Narrative review: tetanus—a health threat after natural disasters in developing countries. Ann Intern Med. 2011;154(5):329-335.
2. Demir NA, Sumer S, Ural O, et al. An alternative treatment approach in tetanus: botulinum toxin. Trop Doct. 2015;45(1): 46-48.
3. Thwaites CL, Beeching NJ, Newton CR. Maternal and neonatal tetanus. Lancet. 2015;385(9965):362-370.
4. Aronoff DM. Clostridium novyi, sordellii, and tetani: mechanisms of disease. Anaerobe. 2013;24:98-101.
5. Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87(3):477-487.
6. Doshi A, Warrell C, Dahdaleh D, Kullmann D. Just a graze? Cephalic tetanus presenting as a stroke mimic. Pract Neurol. 2014;14(1):39-41.
7. CDC. Tetanus surveillance—United States, 2001-2008. MMWR Morb Mortal Wkly Rep. 2011;60(12):365-369.
8. McCabe J, La Varis T, Mason D. Cephalic tetanus complicating geriatric fall. N Z Med J. 2014;127(1400):98-100.
9. Johnson MG, Bradley KK, Mendus S, et al. Vaccine-preventable disease among homeschooled children: two cases of tetanus in Oklahoma. Pediatrics. 2013;132(6):e1686-e1689.
10. CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington, DC: Public Health Foundation; 2015.
11. Tiwari TS. Chapter 16: Tetanus. In: CDC. Manual for Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: CDC; 2012.
12. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis—California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015; 64(9):243-246.
13. Aksoy M, Celik EC, Ahiskalioglu A, Karakaya MA. Tetanus is still a deadly disease: a report of six tetanus cases and reminder of our knowledge. Trop Doct. 2014;44(1):38-42.
14. Felter RA, Zinns LE. Cephalic tetanus in an immunized teenager. Pediatr Emerg Care. 2015;31(7):511-513.
15. CDC. Tetanus (Clostridium tetani) 1996 case definition. www.cdc.gov/nndss/conditions/tetanus/case-definition/1996/. Accessed February 17, 2017.
16. Thwaites CL, Farrar JJ. Preventing and treating tetanus [commentary]. BMJ. 2003;326(7381):117-118.
17. Sexton DJ. Tetanus. UpToDate. www.uptodate.com/contents/tetanus?topicKey=ID%2F5525. Accessed February 17, 2017.
18. Rodrigo C, Fernando D, Rajapakse S. Pharmacological management of tetanus: an evidence-based review. Crit Care. 2014;18(2):217.
19. Santos ML, Mota-Miranda A, Alves-Pereira A, et al. Intrathecal baclofen for the treatment of tetanus. Clin Infect Dis. 2004;38(3):321-328.
20. Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomized controlled trial. Lancet. 2006;368:1436-1443.
21. Govindaraj GM, Riyaz A. Current practice in the management of tetanus. Crit Care. 2014;18(3):145.
22. Wilson TJ, Orringer DA, Sullivan SE, Patil PG. An L-2 burst fracture and cauda equina syndrome due to tetanus. J Neurosurg Spine. 2012;16(1):82-85.
23. CDC. National, state and local area vaccination coverage among children aged 19-35 months—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(36):733-740.
24. CDC. National and state vaccination coverage among adolescents aged 13-17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685-693.
25. Williams WW, Lu PJ, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(5):95-102.
1. Afshar M, Raju M, Ansell D, Bleck TP. Narrative review: tetanus—a health threat after natural disasters in developing countries. Ann Intern Med. 2011;154(5):329-335.
2. Demir NA, Sumer S, Ural O, et al. An alternative treatment approach in tetanus: botulinum toxin. Trop Doct. 2015;45(1): 46-48.
3. Thwaites CL, Beeching NJ, Newton CR. Maternal and neonatal tetanus. Lancet. 2015;385(9965):362-370.
4. Aronoff DM. Clostridium novyi, sordellii, and tetani: mechanisms of disease. Anaerobe. 2013;24:98-101.
5. Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87(3):477-487.
6. Doshi A, Warrell C, Dahdaleh D, Kullmann D. Just a graze? Cephalic tetanus presenting as a stroke mimic. Pract Neurol. 2014;14(1):39-41.
7. CDC. Tetanus surveillance—United States, 2001-2008. MMWR Morb Mortal Wkly Rep. 2011;60(12):365-369.
8. McCabe J, La Varis T, Mason D. Cephalic tetanus complicating geriatric fall. N Z Med J. 2014;127(1400):98-100.
9. Johnson MG, Bradley KK, Mendus S, et al. Vaccine-preventable disease among homeschooled children: two cases of tetanus in Oklahoma. Pediatrics. 2013;132(6):e1686-e1689.
10. CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington, DC: Public Health Foundation; 2015.
11. Tiwari TS. Chapter 16: Tetanus. In: CDC. Manual for Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: CDC; 2012.
12. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis—California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015; 64(9):243-246.
13. Aksoy M, Celik EC, Ahiskalioglu A, Karakaya MA. Tetanus is still a deadly disease: a report of six tetanus cases and reminder of our knowledge. Trop Doct. 2014;44(1):38-42.
14. Felter RA, Zinns LE. Cephalic tetanus in an immunized teenager. Pediatr Emerg Care. 2015;31(7):511-513.
15. CDC. Tetanus (Clostridium tetani) 1996 case definition. www.cdc.gov/nndss/conditions/tetanus/case-definition/1996/. Accessed February 17, 2017.
16. Thwaites CL, Farrar JJ. Preventing and treating tetanus [commentary]. BMJ. 2003;326(7381):117-118.
17. Sexton DJ. Tetanus. UpToDate. www.uptodate.com/contents/tetanus?topicKey=ID%2F5525. Accessed February 17, 2017.
18. Rodrigo C, Fernando D, Rajapakse S. Pharmacological management of tetanus: an evidence-based review. Crit Care. 2014;18(2):217.
19. Santos ML, Mota-Miranda A, Alves-Pereira A, et al. Intrathecal baclofen for the treatment of tetanus. Clin Infect Dis. 2004;38(3):321-328.
20. Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomized controlled trial. Lancet. 2006;368:1436-1443.
21. Govindaraj GM, Riyaz A. Current practice in the management of tetanus. Crit Care. 2014;18(3):145.
22. Wilson TJ, Orringer DA, Sullivan SE, Patil PG. An L-2 burst fracture and cauda equina syndrome due to tetanus. J Neurosurg Spine. 2012;16(1):82-85.
23. CDC. National, state and local area vaccination coverage among children aged 19-35 months—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(36):733-740.
24. CDC. National and state vaccination coverage among adolescents aged 13-17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685-693.
25. Williams WW, Lu PJ, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(5):95-102.
AGA releases POWER – an obesity practice guide for gastroenterologists
The obesity epidemic has reached critical proportions. A new practice guide from the American Gastroenterological Association aims to help gastroenterologists engage in a multidisciplinary effort to tackle the problem.
The guide, entitled “POWER: Practice Guide on Obesity and Weight Management, Education and Resources,” includes a comprehensive clinical process for assessing and safely and effectively managing patients with obesity, as well as a framework focused on helping practitioners navigate the business operational issues related to the management of obesity. Both are in press for the May issue of Clinical Gastroenterology and Hepatology (2016. doi: 10.1016/j.cgh.2016.10.023).
The POWER model recognizes obesity as an epidemic and as an economic and societal burden that should be embraced as a chronic, relapsing disease best managed across a flexible care cycle using a team approach.
“Every single gastroenterologist is at the front line of this obesity epidemic. Before patients develop diabetes or joint problems or cardiovascular disease, they are already in our clinics, they already have [gastroesophageal reflux disease], they have nonalcoholic fatty liver disease, they have colon cancer – and those conditions present even earlier than the other complications of obesity,” said Dr. Acosta of the Mayo Clinic, Rochester, Minn.
The guide is a model for addressing obesity – the root cause of many of these conditions – rather than simply treating its symptoms, he added.
The approach to obesity management promoted by POWER involves four phases along a continuum of care: assessment, intensive weight loss intervention, weight stabilization and reintensification when needed, and prevention of weight regain.
Lifestyle changes are the cornerstones of obesity management and maintenance of weight loss, but the POWER model includes much more, as it incorporates guidance on the use of pharmacotherapy, bariatric endoscopy, and surgery.
“We tried to make it extremely simple, bringing it down to the busy clinician level,” Dr. Acosta said. “We want to be able to embrace and tackle obesity... in a very straightforward manner.”
Gastroenterologists shouldn’t be afraid of taking on obesity, he added.
“We feel comfortable managing extremely complicated medications, so we should be able to handle the obesity medications. We are already endoscopists... so we want all gastroenterologists to say, ‘I can do this, too; I can incorporate this into my practice,’ ” he said.
Further, gastroenterologists already have a relationship with bariatric surgeons, so referring those with obesity for surgery if appropriate is also simple, he added.
When it comes to moving through the four phases of care, each should be addressed separately using the best evidence available. Realistic goals should be set, and only when those goals are met should care move to the next phase, according to the guide. Learn how to implement the AGA Obesity Practice Guide at www.gastro.org/obesity.
The assessment phase should include a medical evaluation to identify underlying etiologies, screen for causes of secondary weight gain, and identify related comorbidities. A nutrition evaluation should focus not only on nutritional status and appetite, but also on the patient’s relationship with food, food allergies and intolerances, and food environment. A physical activity/exercise evaluation should explore the patient’s activity level and preferences, as well as limiting factors such as joint disease.
A psychosocial evaluation is particularly important, as behavioral modification is a critical component of successful obesity management, and some patients – such as those with a low score on the weight Efficacy Lifestyle Questionnaire Short-Form – may benefit from referral to a health care professional experienced in obesity counseling and behavioral therapy.
Gastroenterologists already work with other specialists, including nutritionists, psychiatrists, and psychologists within their institutions and communities, so the POWER model is an extension of that.
“That’s what this proposes – a multidisciplinary team effort,” he said.
The approach to treatment should be based on the findings of these assessments.
“Physicians should discuss all the appropriate options and their expected weight loss, potential side effects, and figure in the patient’s wishes and goals. Furthermore, physicians should recognize special comorbidities that may favor one intervention over another,” the authors wrote.
The intensive weight loss intervention phase should be based on modest initial weight loss goals, which increase the likelihood of success, increase patient confidence, and encourage ongoing efforts to lose weight. Further, modest weight loss vs. larger amounts of weight loss is more easily achieved and maintained. In addition to lifestyle changes, an evaluation of whether other interventions are needed is important, particularly in patients with weight regain or plateaus in weight loss.
The weight stabilization and intensification therapy for relapse phase is essential to prevent weight regain and its associated consequences. This phase introduces patients to the attitudes and behaviors that are likely to lead to long-term maintenance of weight loss, the authors note.
The prevention of weight regain phase – a maintenance phase – is unique among obesity care guidelines, and is a critical component of obesity management, Dr. Acosta said.
“Helping patients lose weight and keep it off requires a comprehensive and sustained effort that involves devising an individualized approach to diet, behavior, and exercise,” he and his colleagues wrote.
In addition to detailed steps and tips for moving through this care cycle, the POWER guide also details the various tools to facilitate adherence to a healthier diet and lifestyle. Various medications, including phentermine, extended-release phentermine/topiramate, lorcaserin, and liraglutide are described, as are various types of bariatric endoscopy and bariatric surgery.
A section on addressing the unique needs of obese children and adolescents is also included in the guide for those gastroenterologists who treat children.
“Obesity really begins in childhood, so it is a pediatric disease in its origin, so it was important to us to incorporate issues unique to children for our pediatric GI colleagues,” Dr. Streett said.
Importantly, the practice guide was developed with input from the Society of American Gastrointestinal and Endoscopic Surgeons, The Obesity Society, the Academy of Nutrition and Dietetics, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. and the program has been endorsed with additional input by the American Society for Gastrointestinal Endoscopy, American Society for Metabolic and Bariatric Surgery, American Association for the Study of Liver Diseases, and the Obesity Medicine Association.
This collaborative approach is also unique among existing guidelines, and is important, given the need for practitioners across the care spectrum to work together to address obesity, she said.
“What we’ve been doing [individually] hasn’t worked successfully, so that is something that people recognize in the field of medicine: Obesity is something that has physiological, nutritional, dietetic, socioeconomic, and behavioral aspects and we need to have a multipronged approach for success. We need patients to be hearing similar messages and having their care integrated,” she said, adding that “as we move toward a value-based schema, this is the perfect disorder to address in that way.”
Dr. Acosta is a stockholder of Gila Therapeutics and serves on the scientific advisory board or board of directors of Gila Therapeutics, Inversago, and General Mills. Dr. Streett reported having no disclosures.
The obesity epidemic has reached critical proportions. A new practice guide from the American Gastroenterological Association aims to help gastroenterologists engage in a multidisciplinary effort to tackle the problem.
The guide, entitled “POWER: Practice Guide on Obesity and Weight Management, Education and Resources,” includes a comprehensive clinical process for assessing and safely and effectively managing patients with obesity, as well as a framework focused on helping practitioners navigate the business operational issues related to the management of obesity. Both are in press for the May issue of Clinical Gastroenterology and Hepatology (2016. doi: 10.1016/j.cgh.2016.10.023).
The POWER model recognizes obesity as an epidemic and as an economic and societal burden that should be embraced as a chronic, relapsing disease best managed across a flexible care cycle using a team approach.
“Every single gastroenterologist is at the front line of this obesity epidemic. Before patients develop diabetes or joint problems or cardiovascular disease, they are already in our clinics, they already have [gastroesophageal reflux disease], they have nonalcoholic fatty liver disease, they have colon cancer – and those conditions present even earlier than the other complications of obesity,” said Dr. Acosta of the Mayo Clinic, Rochester, Minn.
The guide is a model for addressing obesity – the root cause of many of these conditions – rather than simply treating its symptoms, he added.
The approach to obesity management promoted by POWER involves four phases along a continuum of care: assessment, intensive weight loss intervention, weight stabilization and reintensification when needed, and prevention of weight regain.
Lifestyle changes are the cornerstones of obesity management and maintenance of weight loss, but the POWER model includes much more, as it incorporates guidance on the use of pharmacotherapy, bariatric endoscopy, and surgery.
“We tried to make it extremely simple, bringing it down to the busy clinician level,” Dr. Acosta said. “We want to be able to embrace and tackle obesity... in a very straightforward manner.”
Gastroenterologists shouldn’t be afraid of taking on obesity, he added.
“We feel comfortable managing extremely complicated medications, so we should be able to handle the obesity medications. We are already endoscopists... so we want all gastroenterologists to say, ‘I can do this, too; I can incorporate this into my practice,’ ” he said.
Further, gastroenterologists already have a relationship with bariatric surgeons, so referring those with obesity for surgery if appropriate is also simple, he added.
When it comes to moving through the four phases of care, each should be addressed separately using the best evidence available. Realistic goals should be set, and only when those goals are met should care move to the next phase, according to the guide. Learn how to implement the AGA Obesity Practice Guide at www.gastro.org/obesity.
The assessment phase should include a medical evaluation to identify underlying etiologies, screen for causes of secondary weight gain, and identify related comorbidities. A nutrition evaluation should focus not only on nutritional status and appetite, but also on the patient’s relationship with food, food allergies and intolerances, and food environment. A physical activity/exercise evaluation should explore the patient’s activity level and preferences, as well as limiting factors such as joint disease.
A psychosocial evaluation is particularly important, as behavioral modification is a critical component of successful obesity management, and some patients – such as those with a low score on the weight Efficacy Lifestyle Questionnaire Short-Form – may benefit from referral to a health care professional experienced in obesity counseling and behavioral therapy.
Gastroenterologists already work with other specialists, including nutritionists, psychiatrists, and psychologists within their institutions and communities, so the POWER model is an extension of that.
“That’s what this proposes – a multidisciplinary team effort,” he said.
The approach to treatment should be based on the findings of these assessments.
“Physicians should discuss all the appropriate options and their expected weight loss, potential side effects, and figure in the patient’s wishes and goals. Furthermore, physicians should recognize special comorbidities that may favor one intervention over another,” the authors wrote.
The intensive weight loss intervention phase should be based on modest initial weight loss goals, which increase the likelihood of success, increase patient confidence, and encourage ongoing efforts to lose weight. Further, modest weight loss vs. larger amounts of weight loss is more easily achieved and maintained. In addition to lifestyle changes, an evaluation of whether other interventions are needed is important, particularly in patients with weight regain or plateaus in weight loss.
The weight stabilization and intensification therapy for relapse phase is essential to prevent weight regain and its associated consequences. This phase introduces patients to the attitudes and behaviors that are likely to lead to long-term maintenance of weight loss, the authors note.
The prevention of weight regain phase – a maintenance phase – is unique among obesity care guidelines, and is a critical component of obesity management, Dr. Acosta said.
“Helping patients lose weight and keep it off requires a comprehensive and sustained effort that involves devising an individualized approach to diet, behavior, and exercise,” he and his colleagues wrote.
In addition to detailed steps and tips for moving through this care cycle, the POWER guide also details the various tools to facilitate adherence to a healthier diet and lifestyle. Various medications, including phentermine, extended-release phentermine/topiramate, lorcaserin, and liraglutide are described, as are various types of bariatric endoscopy and bariatric surgery.
A section on addressing the unique needs of obese children and adolescents is also included in the guide for those gastroenterologists who treat children.
“Obesity really begins in childhood, so it is a pediatric disease in its origin, so it was important to us to incorporate issues unique to children for our pediatric GI colleagues,” Dr. Streett said.
Importantly, the practice guide was developed with input from the Society of American Gastrointestinal and Endoscopic Surgeons, The Obesity Society, the Academy of Nutrition and Dietetics, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. and the program has been endorsed with additional input by the American Society for Gastrointestinal Endoscopy, American Society for Metabolic and Bariatric Surgery, American Association for the Study of Liver Diseases, and the Obesity Medicine Association.
This collaborative approach is also unique among existing guidelines, and is important, given the need for practitioners across the care spectrum to work together to address obesity, she said.
“What we’ve been doing [individually] hasn’t worked successfully, so that is something that people recognize in the field of medicine: Obesity is something that has physiological, nutritional, dietetic, socioeconomic, and behavioral aspects and we need to have a multipronged approach for success. We need patients to be hearing similar messages and having their care integrated,” she said, adding that “as we move toward a value-based schema, this is the perfect disorder to address in that way.”
Dr. Acosta is a stockholder of Gila Therapeutics and serves on the scientific advisory board or board of directors of Gila Therapeutics, Inversago, and General Mills. Dr. Streett reported having no disclosures.
The obesity epidemic has reached critical proportions. A new practice guide from the American Gastroenterological Association aims to help gastroenterologists engage in a multidisciplinary effort to tackle the problem.
The guide, entitled “POWER: Practice Guide on Obesity and Weight Management, Education and Resources,” includes a comprehensive clinical process for assessing and safely and effectively managing patients with obesity, as well as a framework focused on helping practitioners navigate the business operational issues related to the management of obesity. Both are in press for the May issue of Clinical Gastroenterology and Hepatology (2016. doi: 10.1016/j.cgh.2016.10.023).
The POWER model recognizes obesity as an epidemic and as an economic and societal burden that should be embraced as a chronic, relapsing disease best managed across a flexible care cycle using a team approach.
“Every single gastroenterologist is at the front line of this obesity epidemic. Before patients develop diabetes or joint problems or cardiovascular disease, they are already in our clinics, they already have [gastroesophageal reflux disease], they have nonalcoholic fatty liver disease, they have colon cancer – and those conditions present even earlier than the other complications of obesity,” said Dr. Acosta of the Mayo Clinic, Rochester, Minn.
The guide is a model for addressing obesity – the root cause of many of these conditions – rather than simply treating its symptoms, he added.
The approach to obesity management promoted by POWER involves four phases along a continuum of care: assessment, intensive weight loss intervention, weight stabilization and reintensification when needed, and prevention of weight regain.
Lifestyle changes are the cornerstones of obesity management and maintenance of weight loss, but the POWER model includes much more, as it incorporates guidance on the use of pharmacotherapy, bariatric endoscopy, and surgery.
“We tried to make it extremely simple, bringing it down to the busy clinician level,” Dr. Acosta said. “We want to be able to embrace and tackle obesity... in a very straightforward manner.”
Gastroenterologists shouldn’t be afraid of taking on obesity, he added.
“We feel comfortable managing extremely complicated medications, so we should be able to handle the obesity medications. We are already endoscopists... so we want all gastroenterologists to say, ‘I can do this, too; I can incorporate this into my practice,’ ” he said.
Further, gastroenterologists already have a relationship with bariatric surgeons, so referring those with obesity for surgery if appropriate is also simple, he added.
When it comes to moving through the four phases of care, each should be addressed separately using the best evidence available. Realistic goals should be set, and only when those goals are met should care move to the next phase, according to the guide. Learn how to implement the AGA Obesity Practice Guide at www.gastro.org/obesity.
The assessment phase should include a medical evaluation to identify underlying etiologies, screen for causes of secondary weight gain, and identify related comorbidities. A nutrition evaluation should focus not only on nutritional status and appetite, but also on the patient’s relationship with food, food allergies and intolerances, and food environment. A physical activity/exercise evaluation should explore the patient’s activity level and preferences, as well as limiting factors such as joint disease.
A psychosocial evaluation is particularly important, as behavioral modification is a critical component of successful obesity management, and some patients – such as those with a low score on the weight Efficacy Lifestyle Questionnaire Short-Form – may benefit from referral to a health care professional experienced in obesity counseling and behavioral therapy.
Gastroenterologists already work with other specialists, including nutritionists, psychiatrists, and psychologists within their institutions and communities, so the POWER model is an extension of that.
“That’s what this proposes – a multidisciplinary team effort,” he said.
The approach to treatment should be based on the findings of these assessments.
“Physicians should discuss all the appropriate options and their expected weight loss, potential side effects, and figure in the patient’s wishes and goals. Furthermore, physicians should recognize special comorbidities that may favor one intervention over another,” the authors wrote.
The intensive weight loss intervention phase should be based on modest initial weight loss goals, which increase the likelihood of success, increase patient confidence, and encourage ongoing efforts to lose weight. Further, modest weight loss vs. larger amounts of weight loss is more easily achieved and maintained. In addition to lifestyle changes, an evaluation of whether other interventions are needed is important, particularly in patients with weight regain or plateaus in weight loss.
The weight stabilization and intensification therapy for relapse phase is essential to prevent weight regain and its associated consequences. This phase introduces patients to the attitudes and behaviors that are likely to lead to long-term maintenance of weight loss, the authors note.
The prevention of weight regain phase – a maintenance phase – is unique among obesity care guidelines, and is a critical component of obesity management, Dr. Acosta said.
“Helping patients lose weight and keep it off requires a comprehensive and sustained effort that involves devising an individualized approach to diet, behavior, and exercise,” he and his colleagues wrote.
In addition to detailed steps and tips for moving through this care cycle, the POWER guide also details the various tools to facilitate adherence to a healthier diet and lifestyle. Various medications, including phentermine, extended-release phentermine/topiramate, lorcaserin, and liraglutide are described, as are various types of bariatric endoscopy and bariatric surgery.
A section on addressing the unique needs of obese children and adolescents is also included in the guide for those gastroenterologists who treat children.
“Obesity really begins in childhood, so it is a pediatric disease in its origin, so it was important to us to incorporate issues unique to children for our pediatric GI colleagues,” Dr. Streett said.
Importantly, the practice guide was developed with input from the Society of American Gastrointestinal and Endoscopic Surgeons, The Obesity Society, the Academy of Nutrition and Dietetics, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. and the program has been endorsed with additional input by the American Society for Gastrointestinal Endoscopy, American Society for Metabolic and Bariatric Surgery, American Association for the Study of Liver Diseases, and the Obesity Medicine Association.
This collaborative approach is also unique among existing guidelines, and is important, given the need for practitioners across the care spectrum to work together to address obesity, she said.
“What we’ve been doing [individually] hasn’t worked successfully, so that is something that people recognize in the field of medicine: Obesity is something that has physiological, nutritional, dietetic, socioeconomic, and behavioral aspects and we need to have a multipronged approach for success. We need patients to be hearing similar messages and having their care integrated,” she said, adding that “as we move toward a value-based schema, this is the perfect disorder to address in that way.”
Dr. Acosta is a stockholder of Gila Therapeutics and serves on the scientific advisory board or board of directors of Gila Therapeutics, Inversago, and General Mills. Dr. Streett reported having no disclosures.
Latest weekly flu data show no decline in visits
Outpatient visits for influenza-like illness (ILI) held steady for the week ending March 25, but the number of states at the “high” range of activity dropped from 12 from 10 the previous week, according to the Centers for Disease Prevention and Control.
The proportion of outpatient visits for ILI was 3.2% for the second consecutive week, which halted the slowdown in activity that began the week ending Feb. 18. That 3.2% represents just under 25,000 visits for ILI of the almost 747,000 total visits reported to the Outpatient Influenza-like Illness Surveillance Network (ILINet) for the week ending March 25. By age, the largest groups with ILI visits for the week were individuals aged 5-24 years (41%) and those aged 4 years and under (20%), the CDC reported.
There were six flu-related pediatric deaths reported during the week ending March 25, but all occurred in earlier weeks. The total number of such deaths is now 61 for the 2016-2017 season, the CDC said.
Outpatient visits for influenza-like illness (ILI) held steady for the week ending March 25, but the number of states at the “high” range of activity dropped from 12 from 10 the previous week, according to the Centers for Disease Prevention and Control.
The proportion of outpatient visits for ILI was 3.2% for the second consecutive week, which halted the slowdown in activity that began the week ending Feb. 18. That 3.2% represents just under 25,000 visits for ILI of the almost 747,000 total visits reported to the Outpatient Influenza-like Illness Surveillance Network (ILINet) for the week ending March 25. By age, the largest groups with ILI visits for the week were individuals aged 5-24 years (41%) and those aged 4 years and under (20%), the CDC reported.
There were six flu-related pediatric deaths reported during the week ending March 25, but all occurred in earlier weeks. The total number of such deaths is now 61 for the 2016-2017 season, the CDC said.
Outpatient visits for influenza-like illness (ILI) held steady for the week ending March 25, but the number of states at the “high” range of activity dropped from 12 from 10 the previous week, according to the Centers for Disease Prevention and Control.
The proportion of outpatient visits for ILI was 3.2% for the second consecutive week, which halted the slowdown in activity that began the week ending Feb. 18. That 3.2% represents just under 25,000 visits for ILI of the almost 747,000 total visits reported to the Outpatient Influenza-like Illness Surveillance Network (ILINet) for the week ending March 25. By age, the largest groups with ILI visits for the week were individuals aged 5-24 years (41%) and those aged 4 years and under (20%), the CDC reported.
There were six flu-related pediatric deaths reported during the week ending March 25, but all occurred in earlier weeks. The total number of such deaths is now 61 for the 2016-2017 season, the CDC said.
What’s new at HM17
There is only one annual meeting dedicated to hospitalists, designed by hospitalists, and focusing purely on issues important to hospitalists. But even that isn’t enough to make sure more hospitalists show up every year.
That’s because a yearly conference can’t just be a rehash of the last one.
A valuable conference, certainly one worth spending the bulk of a continuing medical budget on, offers something new every year. Or, to look at the schedule for HM17, a lot of new every year.
That’s an unlikely complaint this year. The annual meeting schedule for May 1-4 at Mandalay Bay Resort and Casino includes five new educational tracks: High Value Care, Clinical Updates, Health Policy, Diagnostic Reasoning, and Medical Education.
“We’re really excited to be able to offer more clinical content,” said HM17 course director Lenny Feldman, MD, FAAP, FACP, SFHM.
Dr. Feldman sees each of the new tracks as filling separate and specific needs of HM attendees who vary from nonphysician providers to hospitalists to medical students.
Take, for instance, the High Value Care, Clinical Updates, and Diagnostic Reasoning sessions that are debuting.
“We wanted to make sure that we had as many clinically oriented sessions as possible,” Dr. Feldman said. “Which meant we needed to increase the amount of clinical content we have offered compared to the past few years. The new clinical track allows us to add probably 12 or so different sessions that will fill the needs of our attendees.”
The Diagnostic Reasoning and High Value Care tracks, in particular, highlight the annual meeting’s continued evolution toward a focus on evidence-based care, as that mantra becomes a bedrock of clinical treatment.
“Training our hospitalists to use the best dialogistic reasoning in their approach to their patients is a big push in hospital medicine right now,” Dr. Feldman said, “Hopefully, a track on that topic will excite people who love thinking about medicine, who got into medicine because of the mystery and want a renewed focus on how to be a great diagnostician.”
Dr. Feldman also noted that the High Value Care track should be a hot topic, as hospitalists want to learn how to provide high quality and high value care to patients at the same time. The new tracks should appeal to different groups and make the annual meeting more appealing to a variety of attendees, not just rank-and-file doctors.
The mini Medical Education track, for instance, is a subset of a half-dozen sessions tailored directly to medical educators in academic settings who face different challenges than their counterparts in community settings. The same goes for the Health Policy track, which will offer a handful of sessions suitable for novices looking to learn more in an age of reform, or policy wonks hoping to expand their knowledge.
Meeting evolving needs
New offerings aren’t limited to the main conference schedule. The 2017 roster of pre-courses includes one titled, “Bugs, Drugs and You: Infectious Diseases ‘Boot Camp’ for Hospitalists.” This daylong session hasn’t been held since 2013, and copresenter Jennifer Hanrahan, DO, associate professor of medicine at Case Western Reserve University in Cleveland, says the timing is good.
“I don’t know that the percentage of people hospitalized for infection has increased,” she said. “Because we are doing things more quickly than we did in the past, length of stays are shorter and there is a lot of pressure to get patients out of the hospital. There is a lot of consultation with Infectious Disease.”
Dr. Hanrahan, who also serves as medical director of infection prevention at Cleveland’s MetroHealth Medical Center, says that with so many patients hospitalized for infections, the value of updating one’s knowledge every few years is critical.
“I’ve been an infectious disease physician for 18 years and I’m also a hospitalist,” she said. “The types of questions I get vary a great deal depending on the experience of the hospitalist. My hope would be that we would be able to provide a basic level of understanding so that people would be more confident in approaching these problems.”
Another new feature this year is offer some of the most popular sessions at multiple times. In years past, popular sessions – such as “Update and Pearls in Infectious Diseases” and “Non–Evidence-Based Medicine: Things We Do for No Reason” – are standing room only events with attendees sitting on floors or gathered to eavesdrop from doorways.
“That says something about the content that’s being delivered, but that’s not very comfortable for folks who want to sit through a session,” Dr. Feldman said. “We’ve decided to add repeat sessions of popular presentations. We want everyone to be comfortable while they’re learning the important clinical content that’s being delivered at these sessions.”
The 2017 focus on healthcare policy is also new. Educational sessions on the policy landscape will be formally buttressed by plenary presentations from Patrick Conway, MD, MSc, MHM, deputy administrator for Innovation and Quality at the Centers for Medicare & Medicaid Services and director of the Center for Medicare and Medicaid Innovation, and Karen DeSalvo, MD, MPH, MSc, a former acting assistant secretary for health at the U.S. Department of Health and Human Services and national coordinator for health information technology.
“There’s a thirst for (policy news) among members of the Society of Hospital Medicine,” Dr. Feldman said. “It is easy to get lost in the day-to-day work that we do, but I think most of us really enjoy hearing about the bigger picture, especially when the bigger picture is in flux.”
“Right now, this is critical,” added Dr. Finn. “Health insurance coverage has a huge impact on hospitals. I think all practicing hospitalists will need to engage with the hospital C-suite if insurance and coverage changes. Since we are hospital based, we are directly tied to anything that the federal government does in terms of health care changes. It’s important for hospitalists to be knowledgeable about health policy.”
One major highlight of the meeting calendar – less new and more historically under-appreciated, in Dr. Feldman’s view – should be the 18 workshop presentations, which are essentially 90-minute dissertations, whittled down from roughly 150 submissions.
“These are the best submissions that we received,” Dr. Feldman said. “We worked hard to make sure that the workshops encompass the breadth and depth of hospital medicine. It is not just one area that’s covered in every workshop. We’ll have workshops ranging from clinical reasoning and communication with patients, to quality improvement issues and high value care discussions, as well as a case-based approach to inpatient dermatology.”
While annual meetings’ new offerings are always an important draw, Dr. Feldman says that the annual “standbys,” such as practice management and pediatrics, are necessary to keep attendees up to date on best practices in changing times.
“It’s pretty self-evident that if we’re going to be an important specialty, we need to serve those who are caring for patients day in and day out, as well as folks who are researching how we can do it better,” he said. “Then we must make sure that data is disseminated to all of us who are taking care of patients. That’s one of the really important parts of this meeting: dissemination of the important work.”
There is only one annual meeting dedicated to hospitalists, designed by hospitalists, and focusing purely on issues important to hospitalists. But even that isn’t enough to make sure more hospitalists show up every year.
That’s because a yearly conference can’t just be a rehash of the last one.
A valuable conference, certainly one worth spending the bulk of a continuing medical budget on, offers something new every year. Or, to look at the schedule for HM17, a lot of new every year.
That’s an unlikely complaint this year. The annual meeting schedule for May 1-4 at Mandalay Bay Resort and Casino includes five new educational tracks: High Value Care, Clinical Updates, Health Policy, Diagnostic Reasoning, and Medical Education.
“We’re really excited to be able to offer more clinical content,” said HM17 course director Lenny Feldman, MD, FAAP, FACP, SFHM.
Dr. Feldman sees each of the new tracks as filling separate and specific needs of HM attendees who vary from nonphysician providers to hospitalists to medical students.
Take, for instance, the High Value Care, Clinical Updates, and Diagnostic Reasoning sessions that are debuting.
“We wanted to make sure that we had as many clinically oriented sessions as possible,” Dr. Feldman said. “Which meant we needed to increase the amount of clinical content we have offered compared to the past few years. The new clinical track allows us to add probably 12 or so different sessions that will fill the needs of our attendees.”
The Diagnostic Reasoning and High Value Care tracks, in particular, highlight the annual meeting’s continued evolution toward a focus on evidence-based care, as that mantra becomes a bedrock of clinical treatment.
“Training our hospitalists to use the best dialogistic reasoning in their approach to their patients is a big push in hospital medicine right now,” Dr. Feldman said, “Hopefully, a track on that topic will excite people who love thinking about medicine, who got into medicine because of the mystery and want a renewed focus on how to be a great diagnostician.”
Dr. Feldman also noted that the High Value Care track should be a hot topic, as hospitalists want to learn how to provide high quality and high value care to patients at the same time. The new tracks should appeal to different groups and make the annual meeting more appealing to a variety of attendees, not just rank-and-file doctors.
The mini Medical Education track, for instance, is a subset of a half-dozen sessions tailored directly to medical educators in academic settings who face different challenges than their counterparts in community settings. The same goes for the Health Policy track, which will offer a handful of sessions suitable for novices looking to learn more in an age of reform, or policy wonks hoping to expand their knowledge.
Meeting evolving needs
New offerings aren’t limited to the main conference schedule. The 2017 roster of pre-courses includes one titled, “Bugs, Drugs and You: Infectious Diseases ‘Boot Camp’ for Hospitalists.” This daylong session hasn’t been held since 2013, and copresenter Jennifer Hanrahan, DO, associate professor of medicine at Case Western Reserve University in Cleveland, says the timing is good.
“I don’t know that the percentage of people hospitalized for infection has increased,” she said. “Because we are doing things more quickly than we did in the past, length of stays are shorter and there is a lot of pressure to get patients out of the hospital. There is a lot of consultation with Infectious Disease.”
Dr. Hanrahan, who also serves as medical director of infection prevention at Cleveland’s MetroHealth Medical Center, says that with so many patients hospitalized for infections, the value of updating one’s knowledge every few years is critical.
“I’ve been an infectious disease physician for 18 years and I’m also a hospitalist,” she said. “The types of questions I get vary a great deal depending on the experience of the hospitalist. My hope would be that we would be able to provide a basic level of understanding so that people would be more confident in approaching these problems.”
Another new feature this year is offer some of the most popular sessions at multiple times. In years past, popular sessions – such as “Update and Pearls in Infectious Diseases” and “Non–Evidence-Based Medicine: Things We Do for No Reason” – are standing room only events with attendees sitting on floors or gathered to eavesdrop from doorways.
“That says something about the content that’s being delivered, but that’s not very comfortable for folks who want to sit through a session,” Dr. Feldman said. “We’ve decided to add repeat sessions of popular presentations. We want everyone to be comfortable while they’re learning the important clinical content that’s being delivered at these sessions.”
The 2017 focus on healthcare policy is also new. Educational sessions on the policy landscape will be formally buttressed by plenary presentations from Patrick Conway, MD, MSc, MHM, deputy administrator for Innovation and Quality at the Centers for Medicare & Medicaid Services and director of the Center for Medicare and Medicaid Innovation, and Karen DeSalvo, MD, MPH, MSc, a former acting assistant secretary for health at the U.S. Department of Health and Human Services and national coordinator for health information technology.
“There’s a thirst for (policy news) among members of the Society of Hospital Medicine,” Dr. Feldman said. “It is easy to get lost in the day-to-day work that we do, but I think most of us really enjoy hearing about the bigger picture, especially when the bigger picture is in flux.”
“Right now, this is critical,” added Dr. Finn. “Health insurance coverage has a huge impact on hospitals. I think all practicing hospitalists will need to engage with the hospital C-suite if insurance and coverage changes. Since we are hospital based, we are directly tied to anything that the federal government does in terms of health care changes. It’s important for hospitalists to be knowledgeable about health policy.”
One major highlight of the meeting calendar – less new and more historically under-appreciated, in Dr. Feldman’s view – should be the 18 workshop presentations, which are essentially 90-minute dissertations, whittled down from roughly 150 submissions.
“These are the best submissions that we received,” Dr. Feldman said. “We worked hard to make sure that the workshops encompass the breadth and depth of hospital medicine. It is not just one area that’s covered in every workshop. We’ll have workshops ranging from clinical reasoning and communication with patients, to quality improvement issues and high value care discussions, as well as a case-based approach to inpatient dermatology.”
While annual meetings’ new offerings are always an important draw, Dr. Feldman says that the annual “standbys,” such as practice management and pediatrics, are necessary to keep attendees up to date on best practices in changing times.
“It’s pretty self-evident that if we’re going to be an important specialty, we need to serve those who are caring for patients day in and day out, as well as folks who are researching how we can do it better,” he said. “Then we must make sure that data is disseminated to all of us who are taking care of patients. That’s one of the really important parts of this meeting: dissemination of the important work.”
There is only one annual meeting dedicated to hospitalists, designed by hospitalists, and focusing purely on issues important to hospitalists. But even that isn’t enough to make sure more hospitalists show up every year.
That’s because a yearly conference can’t just be a rehash of the last one.
A valuable conference, certainly one worth spending the bulk of a continuing medical budget on, offers something new every year. Or, to look at the schedule for HM17, a lot of new every year.
That’s an unlikely complaint this year. The annual meeting schedule for May 1-4 at Mandalay Bay Resort and Casino includes five new educational tracks: High Value Care, Clinical Updates, Health Policy, Diagnostic Reasoning, and Medical Education.
“We’re really excited to be able to offer more clinical content,” said HM17 course director Lenny Feldman, MD, FAAP, FACP, SFHM.
Dr. Feldman sees each of the new tracks as filling separate and specific needs of HM attendees who vary from nonphysician providers to hospitalists to medical students.
Take, for instance, the High Value Care, Clinical Updates, and Diagnostic Reasoning sessions that are debuting.
“We wanted to make sure that we had as many clinically oriented sessions as possible,” Dr. Feldman said. “Which meant we needed to increase the amount of clinical content we have offered compared to the past few years. The new clinical track allows us to add probably 12 or so different sessions that will fill the needs of our attendees.”
The Diagnostic Reasoning and High Value Care tracks, in particular, highlight the annual meeting’s continued evolution toward a focus on evidence-based care, as that mantra becomes a bedrock of clinical treatment.
“Training our hospitalists to use the best dialogistic reasoning in their approach to their patients is a big push in hospital medicine right now,” Dr. Feldman said, “Hopefully, a track on that topic will excite people who love thinking about medicine, who got into medicine because of the mystery and want a renewed focus on how to be a great diagnostician.”
Dr. Feldman also noted that the High Value Care track should be a hot topic, as hospitalists want to learn how to provide high quality and high value care to patients at the same time. The new tracks should appeal to different groups and make the annual meeting more appealing to a variety of attendees, not just rank-and-file doctors.
The mini Medical Education track, for instance, is a subset of a half-dozen sessions tailored directly to medical educators in academic settings who face different challenges than their counterparts in community settings. The same goes for the Health Policy track, which will offer a handful of sessions suitable for novices looking to learn more in an age of reform, or policy wonks hoping to expand their knowledge.
Meeting evolving needs
New offerings aren’t limited to the main conference schedule. The 2017 roster of pre-courses includes one titled, “Bugs, Drugs and You: Infectious Diseases ‘Boot Camp’ for Hospitalists.” This daylong session hasn’t been held since 2013, and copresenter Jennifer Hanrahan, DO, associate professor of medicine at Case Western Reserve University in Cleveland, says the timing is good.
“I don’t know that the percentage of people hospitalized for infection has increased,” she said. “Because we are doing things more quickly than we did in the past, length of stays are shorter and there is a lot of pressure to get patients out of the hospital. There is a lot of consultation with Infectious Disease.”
Dr. Hanrahan, who also serves as medical director of infection prevention at Cleveland’s MetroHealth Medical Center, says that with so many patients hospitalized for infections, the value of updating one’s knowledge every few years is critical.
“I’ve been an infectious disease physician for 18 years and I’m also a hospitalist,” she said. “The types of questions I get vary a great deal depending on the experience of the hospitalist. My hope would be that we would be able to provide a basic level of understanding so that people would be more confident in approaching these problems.”
Another new feature this year is offer some of the most popular sessions at multiple times. In years past, popular sessions – such as “Update and Pearls in Infectious Diseases” and “Non–Evidence-Based Medicine: Things We Do for No Reason” – are standing room only events with attendees sitting on floors or gathered to eavesdrop from doorways.
“That says something about the content that’s being delivered, but that’s not very comfortable for folks who want to sit through a session,” Dr. Feldman said. “We’ve decided to add repeat sessions of popular presentations. We want everyone to be comfortable while they’re learning the important clinical content that’s being delivered at these sessions.”
The 2017 focus on healthcare policy is also new. Educational sessions on the policy landscape will be formally buttressed by plenary presentations from Patrick Conway, MD, MSc, MHM, deputy administrator for Innovation and Quality at the Centers for Medicare & Medicaid Services and director of the Center for Medicare and Medicaid Innovation, and Karen DeSalvo, MD, MPH, MSc, a former acting assistant secretary for health at the U.S. Department of Health and Human Services and national coordinator for health information technology.
“There’s a thirst for (policy news) among members of the Society of Hospital Medicine,” Dr. Feldman said. “It is easy to get lost in the day-to-day work that we do, but I think most of us really enjoy hearing about the bigger picture, especially when the bigger picture is in flux.”
“Right now, this is critical,” added Dr. Finn. “Health insurance coverage has a huge impact on hospitals. I think all practicing hospitalists will need to engage with the hospital C-suite if insurance and coverage changes. Since we are hospital based, we are directly tied to anything that the federal government does in terms of health care changes. It’s important for hospitalists to be knowledgeable about health policy.”
One major highlight of the meeting calendar – less new and more historically under-appreciated, in Dr. Feldman’s view – should be the 18 workshop presentations, which are essentially 90-minute dissertations, whittled down from roughly 150 submissions.
“These are the best submissions that we received,” Dr. Feldman said. “We worked hard to make sure that the workshops encompass the breadth and depth of hospital medicine. It is not just one area that’s covered in every workshop. We’ll have workshops ranging from clinical reasoning and communication with patients, to quality improvement issues and high value care discussions, as well as a case-based approach to inpatient dermatology.”
While annual meetings’ new offerings are always an important draw, Dr. Feldman says that the annual “standbys,” such as practice management and pediatrics, are necessary to keep attendees up to date on best practices in changing times.
“It’s pretty self-evident that if we’re going to be an important specialty, we need to serve those who are caring for patients day in and day out, as well as folks who are researching how we can do it better,” he said. “Then we must make sure that data is disseminated to all of us who are taking care of patients. That’s one of the really important parts of this meeting: dissemination of the important work.”
Report: Psychiatry workforce needs better training, delivery models
More work needs to be done to address the shortage of psychiatrists, including improvements in training and models of health care delivery, according to a new report from the National Council for Behavioral Health’s Medical Director Institute.
In framing the problem, Joseph Parks, MD, a psychiatrist who serves as medical director of the National Council, said during a March 28 teleconference to introduce the report that “55% of the counties in the United States have no psychiatrist in them” and “77% of the counties report a severe shortage.” He noted that the number of psychiatrists available declined by 10% between 2003 and 2013 and that the average age of practicing psychiatrists is in the mid-50s. In other medical specialties, the average age is in the mid-40s, he said.
“This has resulted in people having long wait times and being unable to get psychiatric services,” Dr. Parks said. Those factors are leading patients to pursue psychiatric care in alternative places, such as in primary care physician practices and emergency departments.
In emergency departments, the average wait for dispositions for some psychiatric patients can reach 23 hours, the report says. And more people are going to EDs for care.
“There has been a 42% increase in patients going to the emergency rooms for psychiatric services in the past 3 years,” Dr. Parks said. “But most of them aren’t staffed with psychiatrists. So people end up stuck in the emergency rooms for hours – two to three times as long as they spend for general medical conditions.”
The report looks at causes, and makes actionable recommendations for payers and providers. It also makes recommendations about the infrastructure needed to train future psychiatrists.
A key part of the problem is the increased demand, which is partly attributable to the expansion of health care coverage through the Affordable Care Act’s Medicaid expansion provisions as well as the normalization of views on behavioral health.
“People want psychiatric services,” said Dr. Parks, who has practiced medicine and worked as a policy maker in Missouri. “They know treatment works. It’s less stigmatized than it used to be, so people are more willing to accept and seek treatment.”
Among the trends cited by the report is a shortage of new psychiatrists coming out of medical schools.
“There are problems with not enough training capacity,” he said. “We’ve had increases in federal support for increased training capacity for ob.gyns. and primary care, but we’ve not had that same increase and support, and there are [fewer] supports for training of psychiatrists and fewer slots.”
Burnout is another problem facing psychiatrists.
“Psychiatrists who are practicing are in many cases forced to do it at lower than usual reimbursements [and] are having short visits,” Dr. Parks said. “They are rushed ... they don’t get the same supports that other physicians get. They don’t get the same ancillary staff to assist them in caring for the patients.”
Elaborating on the issues surrounding reimbursement, Dr. Parks noted that 40% of psychiatrists work on a cash-only basis, and 75% of behavioral health organizations lose money on fees collected for psychiatric services.
An ongoing workforce concern, especially in light of changes to the H-1B program, is that 50% of new trainees are foreign medical graduates.
“Luckily, there is a broad range of solutions, and there is something for all the major players to do here,” Dr. Parks said, noting that the report highlights many of these solutions.
“We need to change the care delivery system so it’s not the psychiatrist seeing everybody continuously,” he said. “Psychiatrists need to be used more as expert consultants. People need to be identified using data analytics as opposed to waiting for the patient to complain. And they need to be working more in teams, so they are doing the essential things that only a psychiatrist can do.”
Dr. Parks added that psychiatrists need to delegate “other parts of care and follow-up for people who are stable or for services that can be done by other professionals, such as psychiatric nurses or perhaps physician assistants. “We need an increase not only in more training capacity for psychiatrists but [also in] more alternative providers.”
Patrick Runnels, MD, a psychiatrist who cochairs the Medical Director Institute, highlighted several of the training issues.
“[W]e determined that psychiatrists also bear responsibility for improving this workforce crisis,” Dr. Runnels said during the call. “That starts with making our training consistent with the emerging needs and models of care that are attractive to potential trainees.”
And getting more clinicians into areas of high need, like psychiatry, starts at the medical school level.
“We were able to determine [that] in medical school, medical students were more likely to be recruited into psychiatry based on two characteristics – that the medical school had a strong reputation within their psychiatry department, particularly a strong rotation that was well rated by medical students in psychiatry, and that the length of the rotation was longer,” he said. “When those two things are put together, more students choose to go into psychiatry residency.”
In addition, more exposure is needed to aspects of practice that fit with the way in which medical care is being delivered, including better training in team-based collaborative care and medication-assisted treatment for substance use disorders.
“We also think that residents need to be placed in a range of settings, some settings in which they don’t get very much placement right now, including federally qualified health centers, patient-centered medical homes, [and] experience with telepsychiatry,” said Dr. Runnels, who also serves as medical director of the Centers for Families and Children in Cleveland.
“On top of that, we need our psychiatry residents to graduate with skills in health care data analysis, particularly at the population level,” Dr. Runnels continued. “We need our residents to understand the impact of the treatments that we have on entire populations and how to best allocate resources to deal with the whole population. Those things are hugely important.”
The National Council, based in Washington, is made up of 2,900 member organizations across the country that serve 10 million adults, children, and families who are living with mental health and substance use disorders.
More work needs to be done to address the shortage of psychiatrists, including improvements in training and models of health care delivery, according to a new report from the National Council for Behavioral Health’s Medical Director Institute.
In framing the problem, Joseph Parks, MD, a psychiatrist who serves as medical director of the National Council, said during a March 28 teleconference to introduce the report that “55% of the counties in the United States have no psychiatrist in them” and “77% of the counties report a severe shortage.” He noted that the number of psychiatrists available declined by 10% between 2003 and 2013 and that the average age of practicing psychiatrists is in the mid-50s. In other medical specialties, the average age is in the mid-40s, he said.
“This has resulted in people having long wait times and being unable to get psychiatric services,” Dr. Parks said. Those factors are leading patients to pursue psychiatric care in alternative places, such as in primary care physician practices and emergency departments.
In emergency departments, the average wait for dispositions for some psychiatric patients can reach 23 hours, the report says. And more people are going to EDs for care.
“There has been a 42% increase in patients going to the emergency rooms for psychiatric services in the past 3 years,” Dr. Parks said. “But most of them aren’t staffed with psychiatrists. So people end up stuck in the emergency rooms for hours – two to three times as long as they spend for general medical conditions.”
The report looks at causes, and makes actionable recommendations for payers and providers. It also makes recommendations about the infrastructure needed to train future psychiatrists.
A key part of the problem is the increased demand, which is partly attributable to the expansion of health care coverage through the Affordable Care Act’s Medicaid expansion provisions as well as the normalization of views on behavioral health.
“People want psychiatric services,” said Dr. Parks, who has practiced medicine and worked as a policy maker in Missouri. “They know treatment works. It’s less stigmatized than it used to be, so people are more willing to accept and seek treatment.”
Among the trends cited by the report is a shortage of new psychiatrists coming out of medical schools.
“There are problems with not enough training capacity,” he said. “We’ve had increases in federal support for increased training capacity for ob.gyns. and primary care, but we’ve not had that same increase and support, and there are [fewer] supports for training of psychiatrists and fewer slots.”
Burnout is another problem facing psychiatrists.
“Psychiatrists who are practicing are in many cases forced to do it at lower than usual reimbursements [and] are having short visits,” Dr. Parks said. “They are rushed ... they don’t get the same supports that other physicians get. They don’t get the same ancillary staff to assist them in caring for the patients.”
Elaborating on the issues surrounding reimbursement, Dr. Parks noted that 40% of psychiatrists work on a cash-only basis, and 75% of behavioral health organizations lose money on fees collected for psychiatric services.
An ongoing workforce concern, especially in light of changes to the H-1B program, is that 50% of new trainees are foreign medical graduates.
“Luckily, there is a broad range of solutions, and there is something for all the major players to do here,” Dr. Parks said, noting that the report highlights many of these solutions.
“We need to change the care delivery system so it’s not the psychiatrist seeing everybody continuously,” he said. “Psychiatrists need to be used more as expert consultants. People need to be identified using data analytics as opposed to waiting for the patient to complain. And they need to be working more in teams, so they are doing the essential things that only a psychiatrist can do.”
Dr. Parks added that psychiatrists need to delegate “other parts of care and follow-up for people who are stable or for services that can be done by other professionals, such as psychiatric nurses or perhaps physician assistants. “We need an increase not only in more training capacity for psychiatrists but [also in] more alternative providers.”
Patrick Runnels, MD, a psychiatrist who cochairs the Medical Director Institute, highlighted several of the training issues.
“[W]e determined that psychiatrists also bear responsibility for improving this workforce crisis,” Dr. Runnels said during the call. “That starts with making our training consistent with the emerging needs and models of care that are attractive to potential trainees.”
And getting more clinicians into areas of high need, like psychiatry, starts at the medical school level.
“We were able to determine [that] in medical school, medical students were more likely to be recruited into psychiatry based on two characteristics – that the medical school had a strong reputation within their psychiatry department, particularly a strong rotation that was well rated by medical students in psychiatry, and that the length of the rotation was longer,” he said. “When those two things are put together, more students choose to go into psychiatry residency.”
In addition, more exposure is needed to aspects of practice that fit with the way in which medical care is being delivered, including better training in team-based collaborative care and medication-assisted treatment for substance use disorders.
“We also think that residents need to be placed in a range of settings, some settings in which they don’t get very much placement right now, including federally qualified health centers, patient-centered medical homes, [and] experience with telepsychiatry,” said Dr. Runnels, who also serves as medical director of the Centers for Families and Children in Cleveland.
“On top of that, we need our psychiatry residents to graduate with skills in health care data analysis, particularly at the population level,” Dr. Runnels continued. “We need our residents to understand the impact of the treatments that we have on entire populations and how to best allocate resources to deal with the whole population. Those things are hugely important.”
The National Council, based in Washington, is made up of 2,900 member organizations across the country that serve 10 million adults, children, and families who are living with mental health and substance use disorders.
More work needs to be done to address the shortage of psychiatrists, including improvements in training and models of health care delivery, according to a new report from the National Council for Behavioral Health’s Medical Director Institute.
In framing the problem, Joseph Parks, MD, a psychiatrist who serves as medical director of the National Council, said during a March 28 teleconference to introduce the report that “55% of the counties in the United States have no psychiatrist in them” and “77% of the counties report a severe shortage.” He noted that the number of psychiatrists available declined by 10% between 2003 and 2013 and that the average age of practicing psychiatrists is in the mid-50s. In other medical specialties, the average age is in the mid-40s, he said.
“This has resulted in people having long wait times and being unable to get psychiatric services,” Dr. Parks said. Those factors are leading patients to pursue psychiatric care in alternative places, such as in primary care physician practices and emergency departments.
In emergency departments, the average wait for dispositions for some psychiatric patients can reach 23 hours, the report says. And more people are going to EDs for care.
“There has been a 42% increase in patients going to the emergency rooms for psychiatric services in the past 3 years,” Dr. Parks said. “But most of them aren’t staffed with psychiatrists. So people end up stuck in the emergency rooms for hours – two to three times as long as they spend for general medical conditions.”
The report looks at causes, and makes actionable recommendations for payers and providers. It also makes recommendations about the infrastructure needed to train future psychiatrists.
A key part of the problem is the increased demand, which is partly attributable to the expansion of health care coverage through the Affordable Care Act’s Medicaid expansion provisions as well as the normalization of views on behavioral health.
“People want psychiatric services,” said Dr. Parks, who has practiced medicine and worked as a policy maker in Missouri. “They know treatment works. It’s less stigmatized than it used to be, so people are more willing to accept and seek treatment.”
Among the trends cited by the report is a shortage of new psychiatrists coming out of medical schools.
“There are problems with not enough training capacity,” he said. “We’ve had increases in federal support for increased training capacity for ob.gyns. and primary care, but we’ve not had that same increase and support, and there are [fewer] supports for training of psychiatrists and fewer slots.”
Burnout is another problem facing psychiatrists.
“Psychiatrists who are practicing are in many cases forced to do it at lower than usual reimbursements [and] are having short visits,” Dr. Parks said. “They are rushed ... they don’t get the same supports that other physicians get. They don’t get the same ancillary staff to assist them in caring for the patients.”
Elaborating on the issues surrounding reimbursement, Dr. Parks noted that 40% of psychiatrists work on a cash-only basis, and 75% of behavioral health organizations lose money on fees collected for psychiatric services.
An ongoing workforce concern, especially in light of changes to the H-1B program, is that 50% of new trainees are foreign medical graduates.
“Luckily, there is a broad range of solutions, and there is something for all the major players to do here,” Dr. Parks said, noting that the report highlights many of these solutions.
“We need to change the care delivery system so it’s not the psychiatrist seeing everybody continuously,” he said. “Psychiatrists need to be used more as expert consultants. People need to be identified using data analytics as opposed to waiting for the patient to complain. And they need to be working more in teams, so they are doing the essential things that only a psychiatrist can do.”
Dr. Parks added that psychiatrists need to delegate “other parts of care and follow-up for people who are stable or for services that can be done by other professionals, such as psychiatric nurses or perhaps physician assistants. “We need an increase not only in more training capacity for psychiatrists but [also in] more alternative providers.”
Patrick Runnels, MD, a psychiatrist who cochairs the Medical Director Institute, highlighted several of the training issues.
“[W]e determined that psychiatrists also bear responsibility for improving this workforce crisis,” Dr. Runnels said during the call. “That starts with making our training consistent with the emerging needs and models of care that are attractive to potential trainees.”
And getting more clinicians into areas of high need, like psychiatry, starts at the medical school level.
“We were able to determine [that] in medical school, medical students were more likely to be recruited into psychiatry based on two characteristics – that the medical school had a strong reputation within their psychiatry department, particularly a strong rotation that was well rated by medical students in psychiatry, and that the length of the rotation was longer,” he said. “When those two things are put together, more students choose to go into psychiatry residency.”
In addition, more exposure is needed to aspects of practice that fit with the way in which medical care is being delivered, including better training in team-based collaborative care and medication-assisted treatment for substance use disorders.
“We also think that residents need to be placed in a range of settings, some settings in which they don’t get very much placement right now, including federally qualified health centers, patient-centered medical homes, [and] experience with telepsychiatry,” said Dr. Runnels, who also serves as medical director of the Centers for Families and Children in Cleveland.
“On top of that, we need our psychiatry residents to graduate with skills in health care data analysis, particularly at the population level,” Dr. Runnels continued. “We need our residents to understand the impact of the treatments that we have on entire populations and how to best allocate resources to deal with the whole population. Those things are hugely important.”
The National Council, based in Washington, is made up of 2,900 member organizations across the country that serve 10 million adults, children, and families who are living with mental health and substance use disorders.
What do you call a general medicine hospitalist who focuses on comanaging with a single medical subspecialty?
For more than 2 decades, U.S. health systems have drawn on hospitalists’ expertise to lower length of stay and enhance safety for general medical patients. Many hospital medicine groups have extended this successful practice model across a growing list of services, stretching the role of generalists as far as it can go. While a diverse scope of practice excites some hospitalists, others find career satisfaction with a specific patient population. Some even balk at rotating through all of the possible primary and comanagement services staffed by their group. A growing number of job opportunities have emerged for individuals who are drawn to a specialized patient population but either remain generalist at heart or don’t want to complete a fellowship.
The latest State of Hospital Medicine (SoHM) report provides new insight into this trend, which brings our unique talents to subspecialty populations.
To understand the prevalence of this practice style, the following topic was added to the 2016 SoHM survey: “Some hospital medicine groups include hospitalists who focus their practice exclusively or predominantly in a single medical subspecialty area (e.g., a general internist who exclusively cares for patients on an oncology service in collaboration with oncologists).” Groups were asked to report whether one or more members of their group practiced this way and with which specialty. Although less than a quarter of groups responded to this question, we learned that a substantial portion of respondent groups employ such individuals (see table below).
We look forward to tracking this area with subsequent surveys. Already, national meetings are developing for specialty hospitalists (for example, in oncology), and we see opportunities for specialty hospitalists to network through the Society of Hospital Medicine annual meeting and HMX online. My prediction is for growth in the number of groups reporting the employment of specialty hospitalists, but only time will tell. Hospital medicine group leaders should consider both participating in the next SOHM survey and digging into the details of the current report as ways to advance the best practices for developing specialty hospitalist positions.
Dr. White is associate professor of medicine at the University of Washington, Seattle, and a member of SHM’s Practice Analysis Committee.
For more than 2 decades, U.S. health systems have drawn on hospitalists’ expertise to lower length of stay and enhance safety for general medical patients. Many hospital medicine groups have extended this successful practice model across a growing list of services, stretching the role of generalists as far as it can go. While a diverse scope of practice excites some hospitalists, others find career satisfaction with a specific patient population. Some even balk at rotating through all of the possible primary and comanagement services staffed by their group. A growing number of job opportunities have emerged for individuals who are drawn to a specialized patient population but either remain generalist at heart or don’t want to complete a fellowship.
The latest State of Hospital Medicine (SoHM) report provides new insight into this trend, which brings our unique talents to subspecialty populations.
To understand the prevalence of this practice style, the following topic was added to the 2016 SoHM survey: “Some hospital medicine groups include hospitalists who focus their practice exclusively or predominantly in a single medical subspecialty area (e.g., a general internist who exclusively cares for patients on an oncology service in collaboration with oncologists).” Groups were asked to report whether one or more members of their group practiced this way and with which specialty. Although less than a quarter of groups responded to this question, we learned that a substantial portion of respondent groups employ such individuals (see table below).

We look forward to tracking this area with subsequent surveys. Already, national meetings are developing for specialty hospitalists (for example, in oncology), and we see opportunities for specialty hospitalists to network through the Society of Hospital Medicine annual meeting and HMX online. My prediction is for growth in the number of groups reporting the employment of specialty hospitalists, but only time will tell. Hospital medicine group leaders should consider both participating in the next SOHM survey and digging into the details of the current report as ways to advance the best practices for developing specialty hospitalist positions.
Dr. White is associate professor of medicine at the University of Washington, Seattle, and a member of SHM’s Practice Analysis Committee.
For more than 2 decades, U.S. health systems have drawn on hospitalists’ expertise to lower length of stay and enhance safety for general medical patients. Many hospital medicine groups have extended this successful practice model across a growing list of services, stretching the role of generalists as far as it can go. While a diverse scope of practice excites some hospitalists, others find career satisfaction with a specific patient population. Some even balk at rotating through all of the possible primary and comanagement services staffed by their group. A growing number of job opportunities have emerged for individuals who are drawn to a specialized patient population but either remain generalist at heart or don’t want to complete a fellowship.
The latest State of Hospital Medicine (SoHM) report provides new insight into this trend, which brings our unique talents to subspecialty populations.
To understand the prevalence of this practice style, the following topic was added to the 2016 SoHM survey: “Some hospital medicine groups include hospitalists who focus their practice exclusively or predominantly in a single medical subspecialty area (e.g., a general internist who exclusively cares for patients on an oncology service in collaboration with oncologists).” Groups were asked to report whether one or more members of their group practiced this way and with which specialty. Although less than a quarter of groups responded to this question, we learned that a substantial portion of respondent groups employ such individuals (see table below).

We look forward to tracking this area with subsequent surveys. Already, national meetings are developing for specialty hospitalists (for example, in oncology), and we see opportunities for specialty hospitalists to network through the Society of Hospital Medicine annual meeting and HMX online. My prediction is for growth in the number of groups reporting the employment of specialty hospitalists, but only time will tell. Hospital medicine group leaders should consider both participating in the next SOHM survey and digging into the details of the current report as ways to advance the best practices for developing specialty hospitalist positions.
Dr. White is associate professor of medicine at the University of Washington, Seattle, and a member of SHM’s Practice Analysis Committee.
HM17’s ‘must-see sessions’
LAS VEGAS — Not to sound like a Sin City come on, but pick a course, any course.
No, seriously.
Hospitalists and other attendees at the Hospitalist Medicine 2017 meeting next month will do well to figure out what sessions they want to attend before arriving at the Mandalay Bay Resort and Casino. This 4-day Super Bowl of hospital medicine prides itself on offering more than any attendee can find time for. This year is no exception, as the annual meeting has added five new educational tracks: High-Value Care, Clinical Updates, Health Policy, Diagnostic Reasoning, and Medical Education.
The committee does its job to fill the meeting with best-in-class educational sessions. Here are some of the group’s recommendations for this year’s meeting:
1. “The Hospitalist’s Role in the Opioid Epidemic” – Tuesday, May 2; 1:35 p.m.–2:35 p.m.
2. “Opioids for Acute Pain Management in the Seriously Ill – How to Safely Prescribe” – Wednesday, May 3; 2:50 p.m.–3:30 p.m.
3. “Non-opiate Pain Management for the Hospitalist” – Wednesday, May 3; 4:20 p.m.–5:00 p.m.
Elizabeth Cook, MD, medical director of the hospitalist division of Medical Associates of Central Virginia in Lynchburg, said, “The historical emphasis on pain control has helped contributed to the current epidemic of opioid abuse, overdoses, and deaths. Hospitalists have a need to use these medications for care of the hospitalized patient but have an important part to play in leading the way to appropriate use and patient education regarding the dangers of these medications. These sessions will provide hospitalists with some tools to use in beginning to effect a shift in pain management strategies and responsible use of narcotic pain medications.”
Miguel Angel Villagra, MD, FACP, FHM, hospitalist department program medical director at White River Medical Center in Batesville, Ark., said, “As primary front-line providers in the acute care setting, we face the everyday struggles in the management of chronic opioid users. Acquiring some general guidelines can help us tailor our approach within an ethical focus to improve the care of this population.”
Sarah Stella, MD, an academic hospitalist at Denver Health, said, “This is a crucial and timely topic. Hospitalists have had a hand in perpetuating the opioid epidemic and can play an important role in helping to end it. In this regard, there are many opportunities to do good, such as judicious prescribing and tapering medications for acute pain, starting eligible patients on Suboxone [buprenorphine] in-house, and arranging substance abuse treatment follow-up.”
4. “Focus on POCUS - Introduction to Point-of-Care Ultrasound for Pediatric Hospitalists” – Tuesday, May 2; 10:35 a.m.–11:35 a.m.
Weijen Chang, MD, SFHM, FAAP, chief of the division of pediatric hospital medicine, Baystate Medical Center/Baystate Children’s Hospital, Springfield, Mass., said, “This is the first pediatric POCUS session offered at SHM ever. And it does not require an additional cost ... the pediatric track is critically important, as a substantial number of athlete attendees are either Peds or MedPeds. I think SHM aims to create a pediatric track that discusses topics that are less covered in other meetings, such as the value equation and issues facing women leaders in HM.”
6. “Foundations of a Hospital Medicine Telemedicine Program” – Wednesday, May 3; 415 p.m.–5:20 p.m.
Dr. Villagra added, “Telemedicine is a new innovative technology with the promise of overcoming geographical barriers to health care providers. A lot of new companies and software development has made this technology more user/patient friendly.”
7. “Hot Topics in Health Policy for Hospitalists” – Thursday, May 4; 7:40 a.m.–8:35 a.m.
8. “The Impact of the New Administration on Health Care Reform” – Thursday, May 4; 8:45 a.m.–9:40 a.m.
9. “Health Care Payment Reform for Hospitalist 2017: Tips for MIPS and Beyond” – Thursday, May 4; 9:50 a.m.–10:45 a.m.
Dr. Stella said, “As a safety-net hospitalist in Colorado, a state which largely expanded Medicare under the Affordable Care Act (ACA), I am concerned about the impact repealing the ACA would have on my patients as well as on safety-net hospitals such as my own. I hope that these sessions will increase my understanding of the issues and my ability to advocate for my patients.”
Dr. Cook said, “The U.S. government is functioning in historically unprecedented ways with major shifts in health care policy expected to occur over the next 4 years. It is essential that physician leaders play an active role in shaping the discussion around these important topics ... hospitalists have an opportunity to provide leadership in this arena, and these sessions will help participants to build the knowledge about these complex issues that is crucial to being an active part of the dialogue.”
10. “Workshop: Hospitalists as Leaders in Patient Flow and Hospital Throughput” – Thursday, May 4; 10 a.m.–11:30 a.m.
Dr. Stella said, “Recently, I was appointed to a leadership role on a major initiative to improve hospital patient flow at my institution. We are concentrating on several different areas, including avoidable hospitalizations, preventable excess days, delayed discharges, and variable access to services. I was excited to see a workshop this year dedicated to how hospitalists can successfully lead such initiatives. I will definitely be attending this session as I am interested in what others are doing in their institutions to creatively overcome patient flow challenges.”
11. “Hospitalist Careers: So Many Options” – Tuesday, May 2; 10:35 a.m.–11:15 a.m.
Dr. Villagra said, “Hospital medicine has so many pathways for a full career development and is not a pit stop before fellowship. Early- and mid-career hospitalists can benefit from interactions with senior hospitalists for the understanding of what hospital medicine has to offer for their professional growth.”
Richard Quinn is a freelance writer in New Jersey.
LAS VEGAS — Not to sound like a Sin City come on, but pick a course, any course.
No, seriously.
Hospitalists and other attendees at the Hospitalist Medicine 2017 meeting next month will do well to figure out what sessions they want to attend before arriving at the Mandalay Bay Resort and Casino. This 4-day Super Bowl of hospital medicine prides itself on offering more than any attendee can find time for. This year is no exception, as the annual meeting has added five new educational tracks: High-Value Care, Clinical Updates, Health Policy, Diagnostic Reasoning, and Medical Education.
The committee does its job to fill the meeting with best-in-class educational sessions. Here are some of the group’s recommendations for this year’s meeting:
1. “The Hospitalist’s Role in the Opioid Epidemic” – Tuesday, May 2; 1:35 p.m.–2:35 p.m.
2. “Opioids for Acute Pain Management in the Seriously Ill – How to Safely Prescribe” – Wednesday, May 3; 2:50 p.m.–3:30 p.m.
3. “Non-opiate Pain Management for the Hospitalist” – Wednesday, May 3; 4:20 p.m.–5:00 p.m.
Elizabeth Cook, MD, medical director of the hospitalist division of Medical Associates of Central Virginia in Lynchburg, said, “The historical emphasis on pain control has helped contributed to the current epidemic of opioid abuse, overdoses, and deaths. Hospitalists have a need to use these medications for care of the hospitalized patient but have an important part to play in leading the way to appropriate use and patient education regarding the dangers of these medications. These sessions will provide hospitalists with some tools to use in beginning to effect a shift in pain management strategies and responsible use of narcotic pain medications.”
Miguel Angel Villagra, MD, FACP, FHM, hospitalist department program medical director at White River Medical Center in Batesville, Ark., said, “As primary front-line providers in the acute care setting, we face the everyday struggles in the management of chronic opioid users. Acquiring some general guidelines can help us tailor our approach within an ethical focus to improve the care of this population.”
Sarah Stella, MD, an academic hospitalist at Denver Health, said, “This is a crucial and timely topic. Hospitalists have had a hand in perpetuating the opioid epidemic and can play an important role in helping to end it. In this regard, there are many opportunities to do good, such as judicious prescribing and tapering medications for acute pain, starting eligible patients on Suboxone [buprenorphine] in-house, and arranging substance abuse treatment follow-up.”
4. “Focus on POCUS - Introduction to Point-of-Care Ultrasound for Pediatric Hospitalists” – Tuesday, May 2; 10:35 a.m.–11:35 a.m.
Weijen Chang, MD, SFHM, FAAP, chief of the division of pediatric hospital medicine, Baystate Medical Center/Baystate Children’s Hospital, Springfield, Mass., said, “This is the first pediatric POCUS session offered at SHM ever. And it does not require an additional cost ... the pediatric track is critically important, as a substantial number of athlete attendees are either Peds or MedPeds. I think SHM aims to create a pediatric track that discusses topics that are less covered in other meetings, such as the value equation and issues facing women leaders in HM.”
6. “Foundations of a Hospital Medicine Telemedicine Program” – Wednesday, May 3; 415 p.m.–5:20 p.m.
Dr. Villagra added, “Telemedicine is a new innovative technology with the promise of overcoming geographical barriers to health care providers. A lot of new companies and software development has made this technology more user/patient friendly.”
7. “Hot Topics in Health Policy for Hospitalists” – Thursday, May 4; 7:40 a.m.–8:35 a.m.
8. “The Impact of the New Administration on Health Care Reform” – Thursday, May 4; 8:45 a.m.–9:40 a.m.
9. “Health Care Payment Reform for Hospitalist 2017: Tips for MIPS and Beyond” – Thursday, May 4; 9:50 a.m.–10:45 a.m.
Dr. Stella said, “As a safety-net hospitalist in Colorado, a state which largely expanded Medicare under the Affordable Care Act (ACA), I am concerned about the impact repealing the ACA would have on my patients as well as on safety-net hospitals such as my own. I hope that these sessions will increase my understanding of the issues and my ability to advocate for my patients.”
Dr. Cook said, “The U.S. government is functioning in historically unprecedented ways with major shifts in health care policy expected to occur over the next 4 years. It is essential that physician leaders play an active role in shaping the discussion around these important topics ... hospitalists have an opportunity to provide leadership in this arena, and these sessions will help participants to build the knowledge about these complex issues that is crucial to being an active part of the dialogue.”
10. “Workshop: Hospitalists as Leaders in Patient Flow and Hospital Throughput” – Thursday, May 4; 10 a.m.–11:30 a.m.
Dr. Stella said, “Recently, I was appointed to a leadership role on a major initiative to improve hospital patient flow at my institution. We are concentrating on several different areas, including avoidable hospitalizations, preventable excess days, delayed discharges, and variable access to services. I was excited to see a workshop this year dedicated to how hospitalists can successfully lead such initiatives. I will definitely be attending this session as I am interested in what others are doing in their institutions to creatively overcome patient flow challenges.”
11. “Hospitalist Careers: So Many Options” – Tuesday, May 2; 10:35 a.m.–11:15 a.m.
Dr. Villagra said, “Hospital medicine has so many pathways for a full career development and is not a pit stop before fellowship. Early- and mid-career hospitalists can benefit from interactions with senior hospitalists for the understanding of what hospital medicine has to offer for their professional growth.”
Richard Quinn is a freelance writer in New Jersey.
LAS VEGAS — Not to sound like a Sin City come on, but pick a course, any course.
No, seriously.
Hospitalists and other attendees at the Hospitalist Medicine 2017 meeting next month will do well to figure out what sessions they want to attend before arriving at the Mandalay Bay Resort and Casino. This 4-day Super Bowl of hospital medicine prides itself on offering more than any attendee can find time for. This year is no exception, as the annual meeting has added five new educational tracks: High-Value Care, Clinical Updates, Health Policy, Diagnostic Reasoning, and Medical Education.
The committee does its job to fill the meeting with best-in-class educational sessions. Here are some of the group’s recommendations for this year’s meeting:
1. “The Hospitalist’s Role in the Opioid Epidemic” – Tuesday, May 2; 1:35 p.m.–2:35 p.m.
2. “Opioids for Acute Pain Management in the Seriously Ill – How to Safely Prescribe” – Wednesday, May 3; 2:50 p.m.–3:30 p.m.
3. “Non-opiate Pain Management for the Hospitalist” – Wednesday, May 3; 4:20 p.m.–5:00 p.m.
Elizabeth Cook, MD, medical director of the hospitalist division of Medical Associates of Central Virginia in Lynchburg, said, “The historical emphasis on pain control has helped contributed to the current epidemic of opioid abuse, overdoses, and deaths. Hospitalists have a need to use these medications for care of the hospitalized patient but have an important part to play in leading the way to appropriate use and patient education regarding the dangers of these medications. These sessions will provide hospitalists with some tools to use in beginning to effect a shift in pain management strategies and responsible use of narcotic pain medications.”
Miguel Angel Villagra, MD, FACP, FHM, hospitalist department program medical director at White River Medical Center in Batesville, Ark., said, “As primary front-line providers in the acute care setting, we face the everyday struggles in the management of chronic opioid users. Acquiring some general guidelines can help us tailor our approach within an ethical focus to improve the care of this population.”
Sarah Stella, MD, an academic hospitalist at Denver Health, said, “This is a crucial and timely topic. Hospitalists have had a hand in perpetuating the opioid epidemic and can play an important role in helping to end it. In this regard, there are many opportunities to do good, such as judicious prescribing and tapering medications for acute pain, starting eligible patients on Suboxone [buprenorphine] in-house, and arranging substance abuse treatment follow-up.”
4. “Focus on POCUS - Introduction to Point-of-Care Ultrasound for Pediatric Hospitalists” – Tuesday, May 2; 10:35 a.m.–11:35 a.m.
Weijen Chang, MD, SFHM, FAAP, chief of the division of pediatric hospital medicine, Baystate Medical Center/Baystate Children’s Hospital, Springfield, Mass., said, “This is the first pediatric POCUS session offered at SHM ever. And it does not require an additional cost ... the pediatric track is critically important, as a substantial number of athlete attendees are either Peds or MedPeds. I think SHM aims to create a pediatric track that discusses topics that are less covered in other meetings, such as the value equation and issues facing women leaders in HM.”
6. “Foundations of a Hospital Medicine Telemedicine Program” – Wednesday, May 3; 415 p.m.–5:20 p.m.
Dr. Villagra added, “Telemedicine is a new innovative technology with the promise of overcoming geographical barriers to health care providers. A lot of new companies and software development has made this technology more user/patient friendly.”
7. “Hot Topics in Health Policy for Hospitalists” – Thursday, May 4; 7:40 a.m.–8:35 a.m.
8. “The Impact of the New Administration on Health Care Reform” – Thursday, May 4; 8:45 a.m.–9:40 a.m.
9. “Health Care Payment Reform for Hospitalist 2017: Tips for MIPS and Beyond” – Thursday, May 4; 9:50 a.m.–10:45 a.m.
Dr. Stella said, “As a safety-net hospitalist in Colorado, a state which largely expanded Medicare under the Affordable Care Act (ACA), I am concerned about the impact repealing the ACA would have on my patients as well as on safety-net hospitals such as my own. I hope that these sessions will increase my understanding of the issues and my ability to advocate for my patients.”
Dr. Cook said, “The U.S. government is functioning in historically unprecedented ways with major shifts in health care policy expected to occur over the next 4 years. It is essential that physician leaders play an active role in shaping the discussion around these important topics ... hospitalists have an opportunity to provide leadership in this arena, and these sessions will help participants to build the knowledge about these complex issues that is crucial to being an active part of the dialogue.”
10. “Workshop: Hospitalists as Leaders in Patient Flow and Hospital Throughput” – Thursday, May 4; 10 a.m.–11:30 a.m.
Dr. Stella said, “Recently, I was appointed to a leadership role on a major initiative to improve hospital patient flow at my institution. We are concentrating on several different areas, including avoidable hospitalizations, preventable excess days, delayed discharges, and variable access to services. I was excited to see a workshop this year dedicated to how hospitalists can successfully lead such initiatives. I will definitely be attending this session as I am interested in what others are doing in their institutions to creatively overcome patient flow challenges.”
11. “Hospitalist Careers: So Many Options” – Tuesday, May 2; 10:35 a.m.–11:15 a.m.
Dr. Villagra said, “Hospital medicine has so many pathways for a full career development and is not a pit stop before fellowship. Early- and mid-career hospitalists can benefit from interactions with senior hospitalists for the understanding of what hospital medicine has to offer for their professional growth.”
Richard Quinn is a freelance writer in New Jersey.