User login
Electronic fetal monitoring: Is it information overload?
There is no question that we are living in the information age where “big data” isn’t just reserved for scientists but is accessible to everyone. Wearable devices have revolutionized when and how often we exercise. Smartphones have changed the way in which we consume news, watch television, take photographs, and record home movies. Online video chatting has allowed people who live miles – or even countries – away to connect on a whole new level. Today’s 7-year-olds have never lived in a world without iPhones and don’t know what life is like without iPads. Technology has improved our daily lives in countless ways. However, is “too much of a good thing” ever just too much?
Last year, we looked back over the preceding 5 decades of ob.gyn. practice. This retrospective analysis demonstrated that today’s practitioners have infinitely more tools at their disposal than many of their mentors did to ensure the best pregnancy outcomes. From prenatal diagnostic approaches, such as ultrasonography and genetic screening, to in utero surgical interventions, our discipline has advanced in leaps and bounds, all over the course of one person’s lifetime.
As technology continues to change and, in many ways, enhance the patient experience, the question we should continually ask is, “just because we can do something, should we do it?” Just because we can perform a chorionic villus sampling, should we perform one? Perhaps not. Just because we can schedule a planned cesarean section, should we? Probably not. The same line of questioning applies to the tools we employ to assist us in labor and delivery, including one of the most ubiquitous ones – the electronic fetal monitor.
The electronic fetal heart rate monitor was developed in the late 1950s to continuously record the fetal heart rate during delivery and to help ob.gyns. identify patterns that might indicate fetal distress. Although the monitors have improved over time, the interpretation of the data obtained, and what measures to employ based on these data, can be unclear. Just because the electronic fetal monitor can detect an abnormal heart rate pattern, should we intervene, and what approaches should we employ?
To help answer these questions, I have invited Dr. Alison G. Cahill, associate professor in the department of obstetrics and gynecology at Washington University, St. Louis, and chief of the division of maternal-fetal medicine, to explore the use, utility, and interpretation of data obtained by electronic fetal monitors.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
There is no question that we are living in the information age where “big data” isn’t just reserved for scientists but is accessible to everyone. Wearable devices have revolutionized when and how often we exercise. Smartphones have changed the way in which we consume news, watch television, take photographs, and record home movies. Online video chatting has allowed people who live miles – or even countries – away to connect on a whole new level. Today’s 7-year-olds have never lived in a world without iPhones and don’t know what life is like without iPads. Technology has improved our daily lives in countless ways. However, is “too much of a good thing” ever just too much?
Last year, we looked back over the preceding 5 decades of ob.gyn. practice. This retrospective analysis demonstrated that today’s practitioners have infinitely more tools at their disposal than many of their mentors did to ensure the best pregnancy outcomes. From prenatal diagnostic approaches, such as ultrasonography and genetic screening, to in utero surgical interventions, our discipline has advanced in leaps and bounds, all over the course of one person’s lifetime.
As technology continues to change and, in many ways, enhance the patient experience, the question we should continually ask is, “just because we can do something, should we do it?” Just because we can perform a chorionic villus sampling, should we perform one? Perhaps not. Just because we can schedule a planned cesarean section, should we? Probably not. The same line of questioning applies to the tools we employ to assist us in labor and delivery, including one of the most ubiquitous ones – the electronic fetal monitor.
The electronic fetal heart rate monitor was developed in the late 1950s to continuously record the fetal heart rate during delivery and to help ob.gyns. identify patterns that might indicate fetal distress. Although the monitors have improved over time, the interpretation of the data obtained, and what measures to employ based on these data, can be unclear. Just because the electronic fetal monitor can detect an abnormal heart rate pattern, should we intervene, and what approaches should we employ?
To help answer these questions, I have invited Dr. Alison G. Cahill, associate professor in the department of obstetrics and gynecology at Washington University, St. Louis, and chief of the division of maternal-fetal medicine, to explore the use, utility, and interpretation of data obtained by electronic fetal monitors.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
There is no question that we are living in the information age where “big data” isn’t just reserved for scientists but is accessible to everyone. Wearable devices have revolutionized when and how often we exercise. Smartphones have changed the way in which we consume news, watch television, take photographs, and record home movies. Online video chatting has allowed people who live miles – or even countries – away to connect on a whole new level. Today’s 7-year-olds have never lived in a world without iPhones and don’t know what life is like without iPads. Technology has improved our daily lives in countless ways. However, is “too much of a good thing” ever just too much?
Last year, we looked back over the preceding 5 decades of ob.gyn. practice. This retrospective analysis demonstrated that today’s practitioners have infinitely more tools at their disposal than many of their mentors did to ensure the best pregnancy outcomes. From prenatal diagnostic approaches, such as ultrasonography and genetic screening, to in utero surgical interventions, our discipline has advanced in leaps and bounds, all over the course of one person’s lifetime.
As technology continues to change and, in many ways, enhance the patient experience, the question we should continually ask is, “just because we can do something, should we do it?” Just because we can perform a chorionic villus sampling, should we perform one? Perhaps not. Just because we can schedule a planned cesarean section, should we? Probably not. The same line of questioning applies to the tools we employ to assist us in labor and delivery, including one of the most ubiquitous ones – the electronic fetal monitor.
The electronic fetal heart rate monitor was developed in the late 1950s to continuously record the fetal heart rate during delivery and to help ob.gyns. identify patterns that might indicate fetal distress. Although the monitors have improved over time, the interpretation of the data obtained, and what measures to employ based on these data, can be unclear. Just because the electronic fetal monitor can detect an abnormal heart rate pattern, should we intervene, and what approaches should we employ?
To help answer these questions, I have invited Dr. Alison G. Cahill, associate professor in the department of obstetrics and gynecology at Washington University, St. Louis, and chief of the division of maternal-fetal medicine, to explore the use, utility, and interpretation of data obtained by electronic fetal monitors.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Novel drugs approved in 2016
The Food and Drug Administration approved 22 new drug products in 12 pharmacologic classes in 2016. Additionally, daclizumab (Zenapax), which was approved several years ago for prophylaxis of acute organ rejection in patients receiving renal transplants, was approved for multiple sclerosis treatment (Zinbryta) last year, and sofosbuvir (Sovaldi), which was approved in 2013 for the treatment of hepatitis C virus, is now combined with velpatasvir (Epclusa) to treat all six major forms of hepatitis C.
There are 22 drugs that can be considered novel drugs. As defined by the FDA, novel drugs have never been approved for human use. There are no human pregnancy data for any of the newly approved drugs or drug combinations. As such, it is important to consider that high molecular weight drugs that probably do not cross the placenta in the first half of pregnancy may do so in late pregnancy.
Antineoplastics
Atezolizumab (Tecentriq) is a programmed death ligand–blocking antibody that is indicated for locally advanced or metastatic urothelial carcinoma and metastatic nonsmall cell lung cancer following platinum-containing chemotherapy. Animal reproduction studies have not been conducted, but, based on its mechanism of action, fetal exposure may increase the risk of developing immune-mediated disorders or altering the normal immune response. The molecular weight is high (145,000) and the terminal half-life is long (27 days).
Olaratumab (Lartruvo) is a platelet-derived growth factor receptor– alpha-blocking antibody. It is indicated, in combination with doxorubicin, for the treatment of soft tissue sarcoma. Although the estimated elimination half-life is long (about 11 days with a range of 6-24 days), the high molecular weight (about 154,000) should limit fetal exposure, at least in the first half of pregnancy. The drug should be avoided in pregnancy, however, based on the animal data, the mechanism of action, and its combination with doxorubicin.
Rucaparib (Rubraca) is a poly (adenosine diphosphate–ribose) polymerase inhibitor indicated for the treatment of ovarian cancer. The drug could cause human fetal harm based on the animal data, mechanism of action, relatively low molecular weight (about 556), and terminal half-life (17 hours).
Venetoclax (Venclexta) is a B-cell lymphoma 2 inhibitor indicated for the treatment of chronic lymphocytic leukemia. Although the animal data, molecular weight (about 868), and elimination half-life (about 26 hours) suggest embryo-fetal risk, the high plasma protein binding (99.9%) should limit the amount crossing the placenta.
Anti-infectives
There are two new monoclonal antibodies in this class. Bezlotoxumab (Zinplava) is used to reduce recurrence of Clostridium difficile. Animal reproduction studies have not been conducted. As the molecular weight is about 148,000, the drug will not cross the placenta, at least not in the first half of pregnancy. However, the drug has a long elimination half-life (about 19 days), so, depending on when it was given, it could cross in late pregnancy. Obiltoxaximab (Anthim), administered as a single IV dose, is indicated for the treatment of inhaled anthrax due to Bacillus anthracis. No fetal harm was observed in animal reproduction studies. The high molecular weight (about 148,000) suggests that the drug will not cross to the embryo and/or fetus, at least not in the first part of pregnancy.
Elbasvir/Grazoprevir (Zepatier) is indicated for the treatment of chronic hepatitis C virus genotype 1 or 4. Animal reproduction studies found no evidence of adverse developmental outcomes. The molecular weights of the two components are about 882 and 767, respectively. Both are extensively bound to plasma proteins, 99.9% and 98.8%, respectively, and the terminal half-lives are 24 and 31 hours. Thus, the product appears to be low risk if used in human pregnancy. However, it is contraindicated if given with ribavirin.
Central nervous system agents
Brivaracetam (Briviact) is an anticonvulsant used to treat partial-onset seizures. Animal reproduction studies suggest moderate risk. The molecular weight (about 212), low plasma protein binding (less than or equal to 20%), and terminal plasma half-life of about 9 hours suggest that the drug will cross the placenta. The manufacturer recommends that the drug should be used in pregnancy only if the potential benefit justifies the potential risk to the embryo/fetus.
Pimavanserin (Nuplazid) is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. Reproduction studies in animals suggest low risk. The molecular weight of the free base (about 428) and the long mean plasma half-lives of the parent drug and active metabolite (57 and 200 hours) suggest that the drug will cross the placenta. However, the high plasma protein binding (about 95%) may limit the exposure. Nevertheless, avoiding the period of organogenesis appears to be best.
Dermatologic agents
Crisaborole (Eucrisa) is indicated for topical treatment of mild to moderate atopic dermatitis. In 33 pediatric subjects (aged 2-17 years) who applied the ointment twice daily for 8 days, low amounts were absorbed systemically with plasma concentrations in the nanogram/milliliter range. Plasma protein binding was 97%. With oral formulations of the drug, animal reproduction studies suggest low risk. Taken in sum, the human pregnancy risk appears to be low.
Ixekizumab (Taltz) is a humanized interleukin-17A antagonist, administered subcutaneously, that is indicated for the treatment of adults with moderate to severe plaque psoriasis. The drug did not cause developmental toxicity in monkeys. The molecular weight for the drug’s protein backbone is 146,158, and the mean elimination half-life was 13 days. The human embryo-fetal risk in the first half of pregnancy appears to be low.
Diagnostic agents
Fluciclovine F 18 (Axumin) is a radioactive diagnostic agent indicated for positron emission tomography imaging in men with suspected prostate cancer. Since the agent is only used in men, there are no human or animal pregnancy data.
Gallium GA 68 dotatate injection, a diagnostic imaging agent to detect rare neuroendocrine tumors, is not yet on the market.
Endocrine/metabolic agents
Lixisenatide (Adlyxin) is a glucagon-like–peptide-1 receptor agonist that is administered subcutaneously. It is indicated as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes. The drug was teratogenic in two animal species. The molecular weight is about 4,859, and the mean terminal half-life was about 3 hours. Since tight control of glucose levels in type 2 diabetes is required during pregnancy, insulin is the treatment of choice. Consequently, lixisenatide should not be used during pregnancy.
Gastrointestinal agents
Obeticholic acid (Ocaliva) is a farnesoid X receptor agonist that is given orally for the treatment of primary biliary cholangitis, in combination with ursodiol, or ursodeoxycholic acid. I have classified ursodiol as compatible in pregnancy in the 10th edition of “Drugs in Pregnancy and Lactation” (2011: Wolters Kluwer Health). The animal reproduction data for both drugs suggest low risk. Based on the molecular weight of obeticholic (about 421), the drug will probably cross to the embryo/fetus, but the high plasma protein binding (greater than 99%) may limit exposure. The elimination half-life is apparently unknown.
Hematologic agents
Defibrotide sodium (Defitelio), given as an intravenous infusion, is an oligonucleotide mixture. It is indicated for the treatment of hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome, with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation. Animal reproduction studies in two species suggest risk. The mean molecular weight is 13,000-20,000. Plasma protein binding is an average 93%, and the elimination half-life is less than 2 hours. It is doubtful if the drug crosses the placenta, especially in the first half of pregnancy. If possible, avoid the drug in the second half of pregnancy.
Immunologics
Two indications have been approved for daclizumab. The first was in 2005 for the prophylaxis of acute organ rejection of renal transplants (Zenapax), and the second was in 2016 for the treatment of relapsing forms of multiple sclerosis (Zinbryta). Reproduction studies in monkeys with Zinbryta can be classified as low risk. The molecular weight (about 144,000) suggests that the drug will not cross the placenta, at least in the first half of pregnancy. However, depending on when the drug is given, the long elimination half-life of 21 days might allow the drug to cross in late pregnancy. Regardless, if the perceived maternal benefit exceeds the potential embryo-fetal risk, the drug should not be withheld because of pregnancy.
Muscular disorder agents
Eteplirsen (Exondys 51) is an antisense oligonucleotide that is given intravenously. It is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the related gene, which is amenable to exon 51 skipping. There are no animal reproduction data. The molecular weight is about 10,306. This suggests that the drug will not cross the placenta, at least in the first half of pregnancy. The elimination half-life is 3-4 hours, and the plasma concentration 24 hours after a dose was 0.07% of the peak plasma concentration. The drug is given once weekly, and waiting for 24 hours or slightly longer after a dose should reduce the exposure, if any, of the embryo-fetus during the first half of pregnancy.
Nusinersen (Spinraza), a survival motor neuron 2 directed–antisense oligonucleotide, is given as an intrathecal dose. It is indicated for the treatment of spinal muscular atrophy. Subcutaneous doses in two animal species caused no developmental toxicity. The molecular weight of 7,501 suggests that the drug will not cross the human placenta, at least not in the first half of pregnancy. The mean terminal elimination half-life in cerebrospinal fluid was 135-177 days and 63-87 days in plasma.
Ophthalmic agents
Lifitegrast (Xiidra) is an ophthalmic solution of a lymphocyte function-associated–antigen-1 antagonist. It is indicated for the treatment of the signs and symptoms of dry eye disease. The animal reproduction data suggest low risk. The molecular weight is about 616, suggesting that the drug would cross the placenta. However, in a Phase III trial conducted before FDA approval, 47 patients with dry eye disease were given 1 drop twice daily for periods up to 360 days. Nine patients (19%) had plasma predose (trough) concentrations above 0.5 ng/mL, the lower limit of quantitation. Trough plasma concentrations in these patients ranged from 0.55 ng/mL to 3.74 ng/mL. These amounts do not appear to represent an embryo-fetal risk.
Respiratory agents
Reslizumab (Cinqair), an interleukin-5 antagonist monoclonal antibody, is given intravenously. It is indicated for add-on maintenance treatment of severe asthma in patients with an eosinophilic phenotype. Animal data in two species suggest low risk. The molecular weight is about 147,000, and the elimination half-life is about 24 days. This suggests that exposure of the embryo and fetus will be minimal, at least in the first half of pregnancy. The maternal benefit appears to outweigh the unknown embryo-fetal risk.
Lactation
None of the above drugs have been studied during breastfeeding. Many drugs, regardless of their molecular weight, will cross into milk in small amounts during the first postpartum week. The effects of this exposure on a nursing infant are unknown. Based on the potential for nursing infant harm, the drugs that probably should not be given during breastfeeding include the four antineoplastics, the atypical antipsychotic pimavanserin, and the diabetes injection lixisenatide.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation,” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The Food and Drug Administration approved 22 new drug products in 12 pharmacologic classes in 2016. Additionally, daclizumab (Zenapax), which was approved several years ago for prophylaxis of acute organ rejection in patients receiving renal transplants, was approved for multiple sclerosis treatment (Zinbryta) last year, and sofosbuvir (Sovaldi), which was approved in 2013 for the treatment of hepatitis C virus, is now combined with velpatasvir (Epclusa) to treat all six major forms of hepatitis C.
There are 22 drugs that can be considered novel drugs. As defined by the FDA, novel drugs have never been approved for human use. There are no human pregnancy data for any of the newly approved drugs or drug combinations. As such, it is important to consider that high molecular weight drugs that probably do not cross the placenta in the first half of pregnancy may do so in late pregnancy.
Antineoplastics
Atezolizumab (Tecentriq) is a programmed death ligand–blocking antibody that is indicated for locally advanced or metastatic urothelial carcinoma and metastatic nonsmall cell lung cancer following platinum-containing chemotherapy. Animal reproduction studies have not been conducted, but, based on its mechanism of action, fetal exposure may increase the risk of developing immune-mediated disorders or altering the normal immune response. The molecular weight is high (145,000) and the terminal half-life is long (27 days).
Olaratumab (Lartruvo) is a platelet-derived growth factor receptor– alpha-blocking antibody. It is indicated, in combination with doxorubicin, for the treatment of soft tissue sarcoma. Although the estimated elimination half-life is long (about 11 days with a range of 6-24 days), the high molecular weight (about 154,000) should limit fetal exposure, at least in the first half of pregnancy. The drug should be avoided in pregnancy, however, based on the animal data, the mechanism of action, and its combination with doxorubicin.
Rucaparib (Rubraca) is a poly (adenosine diphosphate–ribose) polymerase inhibitor indicated for the treatment of ovarian cancer. The drug could cause human fetal harm based on the animal data, mechanism of action, relatively low molecular weight (about 556), and terminal half-life (17 hours).
Venetoclax (Venclexta) is a B-cell lymphoma 2 inhibitor indicated for the treatment of chronic lymphocytic leukemia. Although the animal data, molecular weight (about 868), and elimination half-life (about 26 hours) suggest embryo-fetal risk, the high plasma protein binding (99.9%) should limit the amount crossing the placenta.
Anti-infectives
There are two new monoclonal antibodies in this class. Bezlotoxumab (Zinplava) is used to reduce recurrence of Clostridium difficile. Animal reproduction studies have not been conducted. As the molecular weight is about 148,000, the drug will not cross the placenta, at least not in the first half of pregnancy. However, the drug has a long elimination half-life (about 19 days), so, depending on when it was given, it could cross in late pregnancy. Obiltoxaximab (Anthim), administered as a single IV dose, is indicated for the treatment of inhaled anthrax due to Bacillus anthracis. No fetal harm was observed in animal reproduction studies. The high molecular weight (about 148,000) suggests that the drug will not cross to the embryo and/or fetus, at least not in the first part of pregnancy.
Elbasvir/Grazoprevir (Zepatier) is indicated for the treatment of chronic hepatitis C virus genotype 1 or 4. Animal reproduction studies found no evidence of adverse developmental outcomes. The molecular weights of the two components are about 882 and 767, respectively. Both are extensively bound to plasma proteins, 99.9% and 98.8%, respectively, and the terminal half-lives are 24 and 31 hours. Thus, the product appears to be low risk if used in human pregnancy. However, it is contraindicated if given with ribavirin.
Central nervous system agents
Brivaracetam (Briviact) is an anticonvulsant used to treat partial-onset seizures. Animal reproduction studies suggest moderate risk. The molecular weight (about 212), low plasma protein binding (less than or equal to 20%), and terminal plasma half-life of about 9 hours suggest that the drug will cross the placenta. The manufacturer recommends that the drug should be used in pregnancy only if the potential benefit justifies the potential risk to the embryo/fetus.
Pimavanserin (Nuplazid) is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. Reproduction studies in animals suggest low risk. The molecular weight of the free base (about 428) and the long mean plasma half-lives of the parent drug and active metabolite (57 and 200 hours) suggest that the drug will cross the placenta. However, the high plasma protein binding (about 95%) may limit the exposure. Nevertheless, avoiding the period of organogenesis appears to be best.
Dermatologic agents
Crisaborole (Eucrisa) is indicated for topical treatment of mild to moderate atopic dermatitis. In 33 pediatric subjects (aged 2-17 years) who applied the ointment twice daily for 8 days, low amounts were absorbed systemically with plasma concentrations in the nanogram/milliliter range. Plasma protein binding was 97%. With oral formulations of the drug, animal reproduction studies suggest low risk. Taken in sum, the human pregnancy risk appears to be low.
Ixekizumab (Taltz) is a humanized interleukin-17A antagonist, administered subcutaneously, that is indicated for the treatment of adults with moderate to severe plaque psoriasis. The drug did not cause developmental toxicity in monkeys. The molecular weight for the drug’s protein backbone is 146,158, and the mean elimination half-life was 13 days. The human embryo-fetal risk in the first half of pregnancy appears to be low.
Diagnostic agents
Fluciclovine F 18 (Axumin) is a radioactive diagnostic agent indicated for positron emission tomography imaging in men with suspected prostate cancer. Since the agent is only used in men, there are no human or animal pregnancy data.
Gallium GA 68 dotatate injection, a diagnostic imaging agent to detect rare neuroendocrine tumors, is not yet on the market.
Endocrine/metabolic agents
Lixisenatide (Adlyxin) is a glucagon-like–peptide-1 receptor agonist that is administered subcutaneously. It is indicated as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes. The drug was teratogenic in two animal species. The molecular weight is about 4,859, and the mean terminal half-life was about 3 hours. Since tight control of glucose levels in type 2 diabetes is required during pregnancy, insulin is the treatment of choice. Consequently, lixisenatide should not be used during pregnancy.
Gastrointestinal agents
Obeticholic acid (Ocaliva) is a farnesoid X receptor agonist that is given orally for the treatment of primary biliary cholangitis, in combination with ursodiol, or ursodeoxycholic acid. I have classified ursodiol as compatible in pregnancy in the 10th edition of “Drugs in Pregnancy and Lactation” (2011: Wolters Kluwer Health). The animal reproduction data for both drugs suggest low risk. Based on the molecular weight of obeticholic (about 421), the drug will probably cross to the embryo/fetus, but the high plasma protein binding (greater than 99%) may limit exposure. The elimination half-life is apparently unknown.
Hematologic agents
Defibrotide sodium (Defitelio), given as an intravenous infusion, is an oligonucleotide mixture. It is indicated for the treatment of hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome, with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation. Animal reproduction studies in two species suggest risk. The mean molecular weight is 13,000-20,000. Plasma protein binding is an average 93%, and the elimination half-life is less than 2 hours. It is doubtful if the drug crosses the placenta, especially in the first half of pregnancy. If possible, avoid the drug in the second half of pregnancy.
Immunologics
Two indications have been approved for daclizumab. The first was in 2005 for the prophylaxis of acute organ rejection of renal transplants (Zenapax), and the second was in 2016 for the treatment of relapsing forms of multiple sclerosis (Zinbryta). Reproduction studies in monkeys with Zinbryta can be classified as low risk. The molecular weight (about 144,000) suggests that the drug will not cross the placenta, at least in the first half of pregnancy. However, depending on when the drug is given, the long elimination half-life of 21 days might allow the drug to cross in late pregnancy. Regardless, if the perceived maternal benefit exceeds the potential embryo-fetal risk, the drug should not be withheld because of pregnancy.
Muscular disorder agents
Eteplirsen (Exondys 51) is an antisense oligonucleotide that is given intravenously. It is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the related gene, which is amenable to exon 51 skipping. There are no animal reproduction data. The molecular weight is about 10,306. This suggests that the drug will not cross the placenta, at least in the first half of pregnancy. The elimination half-life is 3-4 hours, and the plasma concentration 24 hours after a dose was 0.07% of the peak plasma concentration. The drug is given once weekly, and waiting for 24 hours or slightly longer after a dose should reduce the exposure, if any, of the embryo-fetus during the first half of pregnancy.
Nusinersen (Spinraza), a survival motor neuron 2 directed–antisense oligonucleotide, is given as an intrathecal dose. It is indicated for the treatment of spinal muscular atrophy. Subcutaneous doses in two animal species caused no developmental toxicity. The molecular weight of 7,501 suggests that the drug will not cross the human placenta, at least not in the first half of pregnancy. The mean terminal elimination half-life in cerebrospinal fluid was 135-177 days and 63-87 days in plasma.
Ophthalmic agents
Lifitegrast (Xiidra) is an ophthalmic solution of a lymphocyte function-associated–antigen-1 antagonist. It is indicated for the treatment of the signs and symptoms of dry eye disease. The animal reproduction data suggest low risk. The molecular weight is about 616, suggesting that the drug would cross the placenta. However, in a Phase III trial conducted before FDA approval, 47 patients with dry eye disease were given 1 drop twice daily for periods up to 360 days. Nine patients (19%) had plasma predose (trough) concentrations above 0.5 ng/mL, the lower limit of quantitation. Trough plasma concentrations in these patients ranged from 0.55 ng/mL to 3.74 ng/mL. These amounts do not appear to represent an embryo-fetal risk.
Respiratory agents
Reslizumab (Cinqair), an interleukin-5 antagonist monoclonal antibody, is given intravenously. It is indicated for add-on maintenance treatment of severe asthma in patients with an eosinophilic phenotype. Animal data in two species suggest low risk. The molecular weight is about 147,000, and the elimination half-life is about 24 days. This suggests that exposure of the embryo and fetus will be minimal, at least in the first half of pregnancy. The maternal benefit appears to outweigh the unknown embryo-fetal risk.
Lactation
None of the above drugs have been studied during breastfeeding. Many drugs, regardless of their molecular weight, will cross into milk in small amounts during the first postpartum week. The effects of this exposure on a nursing infant are unknown. Based on the potential for nursing infant harm, the drugs that probably should not be given during breastfeeding include the four antineoplastics, the atypical antipsychotic pimavanserin, and the diabetes injection lixisenatide.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation,” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
The Food and Drug Administration approved 22 new drug products in 12 pharmacologic classes in 2016. Additionally, daclizumab (Zenapax), which was approved several years ago for prophylaxis of acute organ rejection in patients receiving renal transplants, was approved for multiple sclerosis treatment (Zinbryta) last year, and sofosbuvir (Sovaldi), which was approved in 2013 for the treatment of hepatitis C virus, is now combined with velpatasvir (Epclusa) to treat all six major forms of hepatitis C.
There are 22 drugs that can be considered novel drugs. As defined by the FDA, novel drugs have never been approved for human use. There are no human pregnancy data for any of the newly approved drugs or drug combinations. As such, it is important to consider that high molecular weight drugs that probably do not cross the placenta in the first half of pregnancy may do so in late pregnancy.
Antineoplastics
Atezolizumab (Tecentriq) is a programmed death ligand–blocking antibody that is indicated for locally advanced or metastatic urothelial carcinoma and metastatic nonsmall cell lung cancer following platinum-containing chemotherapy. Animal reproduction studies have not been conducted, but, based on its mechanism of action, fetal exposure may increase the risk of developing immune-mediated disorders or altering the normal immune response. The molecular weight is high (145,000) and the terminal half-life is long (27 days).
Olaratumab (Lartruvo) is a platelet-derived growth factor receptor– alpha-blocking antibody. It is indicated, in combination with doxorubicin, for the treatment of soft tissue sarcoma. Although the estimated elimination half-life is long (about 11 days with a range of 6-24 days), the high molecular weight (about 154,000) should limit fetal exposure, at least in the first half of pregnancy. The drug should be avoided in pregnancy, however, based on the animal data, the mechanism of action, and its combination with doxorubicin.
Rucaparib (Rubraca) is a poly (adenosine diphosphate–ribose) polymerase inhibitor indicated for the treatment of ovarian cancer. The drug could cause human fetal harm based on the animal data, mechanism of action, relatively low molecular weight (about 556), and terminal half-life (17 hours).
Venetoclax (Venclexta) is a B-cell lymphoma 2 inhibitor indicated for the treatment of chronic lymphocytic leukemia. Although the animal data, molecular weight (about 868), and elimination half-life (about 26 hours) suggest embryo-fetal risk, the high plasma protein binding (99.9%) should limit the amount crossing the placenta.
Anti-infectives
There are two new monoclonal antibodies in this class. Bezlotoxumab (Zinplava) is used to reduce recurrence of Clostridium difficile. Animal reproduction studies have not been conducted. As the molecular weight is about 148,000, the drug will not cross the placenta, at least not in the first half of pregnancy. However, the drug has a long elimination half-life (about 19 days), so, depending on when it was given, it could cross in late pregnancy. Obiltoxaximab (Anthim), administered as a single IV dose, is indicated for the treatment of inhaled anthrax due to Bacillus anthracis. No fetal harm was observed in animal reproduction studies. The high molecular weight (about 148,000) suggests that the drug will not cross to the embryo and/or fetus, at least not in the first part of pregnancy.
Elbasvir/Grazoprevir (Zepatier) is indicated for the treatment of chronic hepatitis C virus genotype 1 or 4. Animal reproduction studies found no evidence of adverse developmental outcomes. The molecular weights of the two components are about 882 and 767, respectively. Both are extensively bound to plasma proteins, 99.9% and 98.8%, respectively, and the terminal half-lives are 24 and 31 hours. Thus, the product appears to be low risk if used in human pregnancy. However, it is contraindicated if given with ribavirin.
Central nervous system agents
Brivaracetam (Briviact) is an anticonvulsant used to treat partial-onset seizures. Animal reproduction studies suggest moderate risk. The molecular weight (about 212), low plasma protein binding (less than or equal to 20%), and terminal plasma half-life of about 9 hours suggest that the drug will cross the placenta. The manufacturer recommends that the drug should be used in pregnancy only if the potential benefit justifies the potential risk to the embryo/fetus.
Pimavanserin (Nuplazid) is an atypical antipsychotic indicated for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. Reproduction studies in animals suggest low risk. The molecular weight of the free base (about 428) and the long mean plasma half-lives of the parent drug and active metabolite (57 and 200 hours) suggest that the drug will cross the placenta. However, the high plasma protein binding (about 95%) may limit the exposure. Nevertheless, avoiding the period of organogenesis appears to be best.
Dermatologic agents
Crisaborole (Eucrisa) is indicated for topical treatment of mild to moderate atopic dermatitis. In 33 pediatric subjects (aged 2-17 years) who applied the ointment twice daily for 8 days, low amounts were absorbed systemically with plasma concentrations in the nanogram/milliliter range. Plasma protein binding was 97%. With oral formulations of the drug, animal reproduction studies suggest low risk. Taken in sum, the human pregnancy risk appears to be low.
Ixekizumab (Taltz) is a humanized interleukin-17A antagonist, administered subcutaneously, that is indicated for the treatment of adults with moderate to severe plaque psoriasis. The drug did not cause developmental toxicity in monkeys. The molecular weight for the drug’s protein backbone is 146,158, and the mean elimination half-life was 13 days. The human embryo-fetal risk in the first half of pregnancy appears to be low.
Diagnostic agents
Fluciclovine F 18 (Axumin) is a radioactive diagnostic agent indicated for positron emission tomography imaging in men with suspected prostate cancer. Since the agent is only used in men, there are no human or animal pregnancy data.
Gallium GA 68 dotatate injection, a diagnostic imaging agent to detect rare neuroendocrine tumors, is not yet on the market.
Endocrine/metabolic agents
Lixisenatide (Adlyxin) is a glucagon-like–peptide-1 receptor agonist that is administered subcutaneously. It is indicated as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes. The drug was teratogenic in two animal species. The molecular weight is about 4,859, and the mean terminal half-life was about 3 hours. Since tight control of glucose levels in type 2 diabetes is required during pregnancy, insulin is the treatment of choice. Consequently, lixisenatide should not be used during pregnancy.
Gastrointestinal agents
Obeticholic acid (Ocaliva) is a farnesoid X receptor agonist that is given orally for the treatment of primary biliary cholangitis, in combination with ursodiol, or ursodeoxycholic acid. I have classified ursodiol as compatible in pregnancy in the 10th edition of “Drugs in Pregnancy and Lactation” (2011: Wolters Kluwer Health). The animal reproduction data for both drugs suggest low risk. Based on the molecular weight of obeticholic (about 421), the drug will probably cross to the embryo/fetus, but the high plasma protein binding (greater than 99%) may limit exposure. The elimination half-life is apparently unknown.
Hematologic agents
Defibrotide sodium (Defitelio), given as an intravenous infusion, is an oligonucleotide mixture. It is indicated for the treatment of hepatic veno-occlusive disease, also known as sinusoidal obstruction syndrome, with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation. Animal reproduction studies in two species suggest risk. The mean molecular weight is 13,000-20,000. Plasma protein binding is an average 93%, and the elimination half-life is less than 2 hours. It is doubtful if the drug crosses the placenta, especially in the first half of pregnancy. If possible, avoid the drug in the second half of pregnancy.
Immunologics
Two indications have been approved for daclizumab. The first was in 2005 for the prophylaxis of acute organ rejection of renal transplants (Zenapax), and the second was in 2016 for the treatment of relapsing forms of multiple sclerosis (Zinbryta). Reproduction studies in monkeys with Zinbryta can be classified as low risk. The molecular weight (about 144,000) suggests that the drug will not cross the placenta, at least in the first half of pregnancy. However, depending on when the drug is given, the long elimination half-life of 21 days might allow the drug to cross in late pregnancy. Regardless, if the perceived maternal benefit exceeds the potential embryo-fetal risk, the drug should not be withheld because of pregnancy.
Muscular disorder agents
Eteplirsen (Exondys 51) is an antisense oligonucleotide that is given intravenously. It is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the related gene, which is amenable to exon 51 skipping. There are no animal reproduction data. The molecular weight is about 10,306. This suggests that the drug will not cross the placenta, at least in the first half of pregnancy. The elimination half-life is 3-4 hours, and the plasma concentration 24 hours after a dose was 0.07% of the peak plasma concentration. The drug is given once weekly, and waiting for 24 hours or slightly longer after a dose should reduce the exposure, if any, of the embryo-fetus during the first half of pregnancy.
Nusinersen (Spinraza), a survival motor neuron 2 directed–antisense oligonucleotide, is given as an intrathecal dose. It is indicated for the treatment of spinal muscular atrophy. Subcutaneous doses in two animal species caused no developmental toxicity. The molecular weight of 7,501 suggests that the drug will not cross the human placenta, at least not in the first half of pregnancy. The mean terminal elimination half-life in cerebrospinal fluid was 135-177 days and 63-87 days in plasma.
Ophthalmic agents
Lifitegrast (Xiidra) is an ophthalmic solution of a lymphocyte function-associated–antigen-1 antagonist. It is indicated for the treatment of the signs and symptoms of dry eye disease. The animal reproduction data suggest low risk. The molecular weight is about 616, suggesting that the drug would cross the placenta. However, in a Phase III trial conducted before FDA approval, 47 patients with dry eye disease were given 1 drop twice daily for periods up to 360 days. Nine patients (19%) had plasma predose (trough) concentrations above 0.5 ng/mL, the lower limit of quantitation. Trough plasma concentrations in these patients ranged from 0.55 ng/mL to 3.74 ng/mL. These amounts do not appear to represent an embryo-fetal risk.
Respiratory agents
Reslizumab (Cinqair), an interleukin-5 antagonist monoclonal antibody, is given intravenously. It is indicated for add-on maintenance treatment of severe asthma in patients with an eosinophilic phenotype. Animal data in two species suggest low risk. The molecular weight is about 147,000, and the elimination half-life is about 24 days. This suggests that exposure of the embryo and fetus will be minimal, at least in the first half of pregnancy. The maternal benefit appears to outweigh the unknown embryo-fetal risk.
Lactation
None of the above drugs have been studied during breastfeeding. Many drugs, regardless of their molecular weight, will cross into milk in small amounts during the first postpartum week. The effects of this exposure on a nursing infant are unknown. Based on the potential for nursing infant harm, the drugs that probably should not be given during breastfeeding include the four antineoplastics, the atypical antipsychotic pimavanserin, and the diabetes injection lixisenatide.
Mr. Briggs is a clinical professor of pharmacy at the University of California, San Francisco, and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation,” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Insomnia: Getting to the cause, facilitating relief
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.
Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.
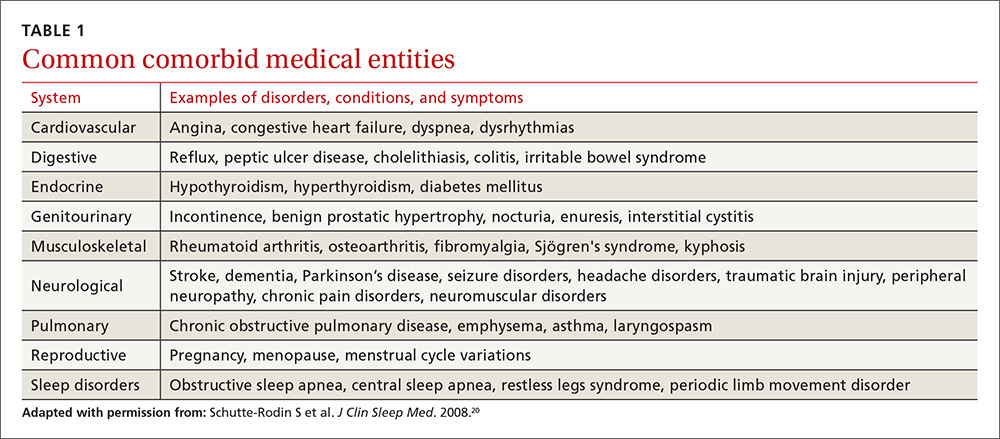
Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.
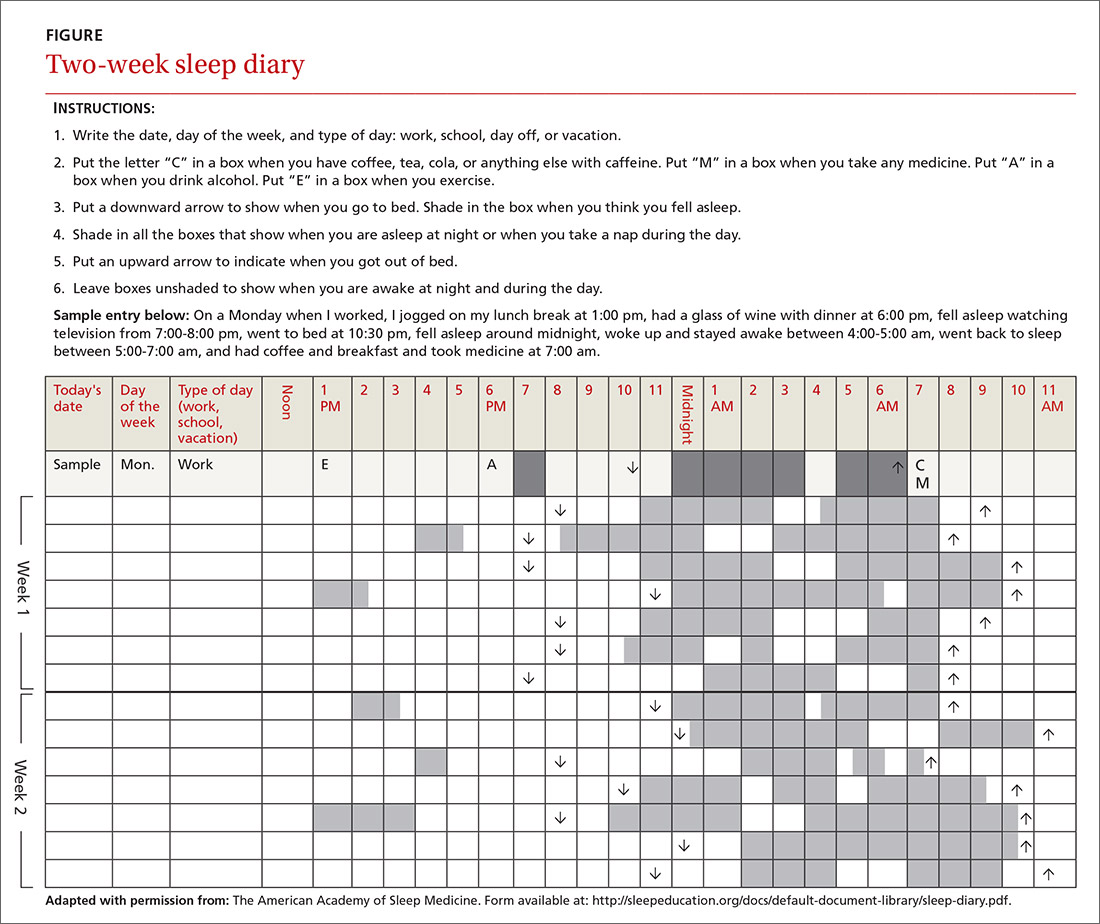
Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
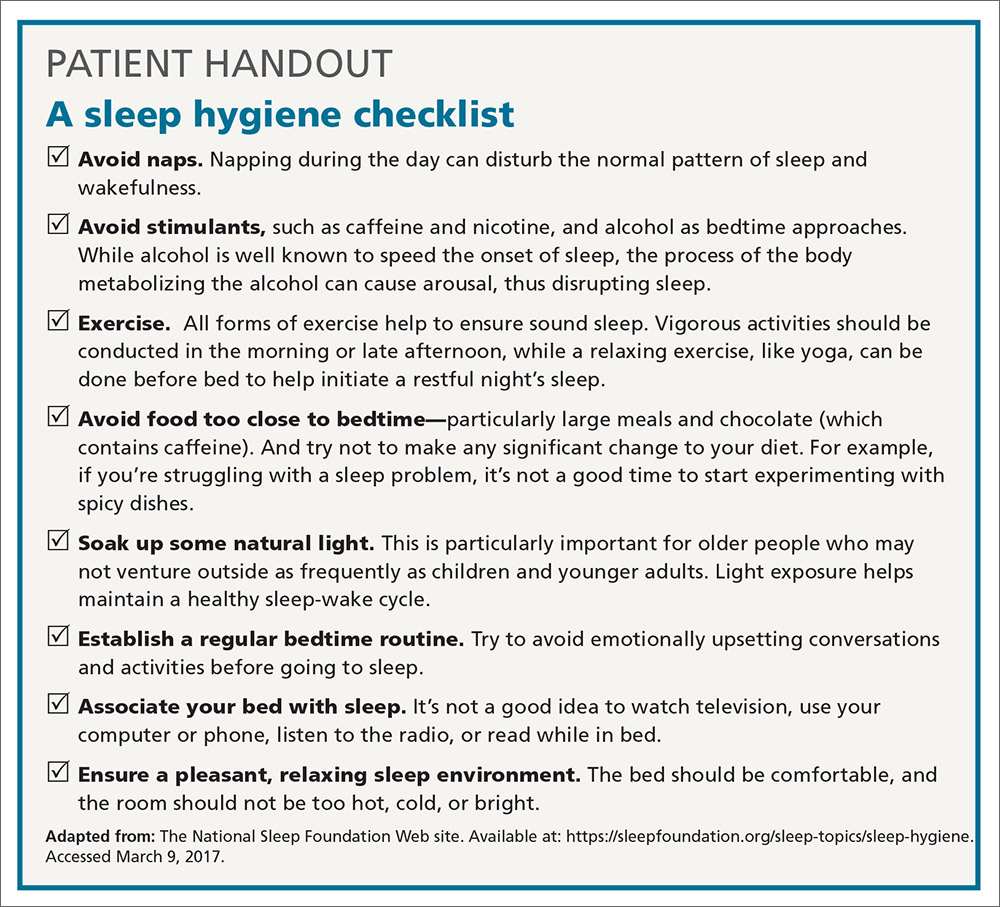
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20
CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.
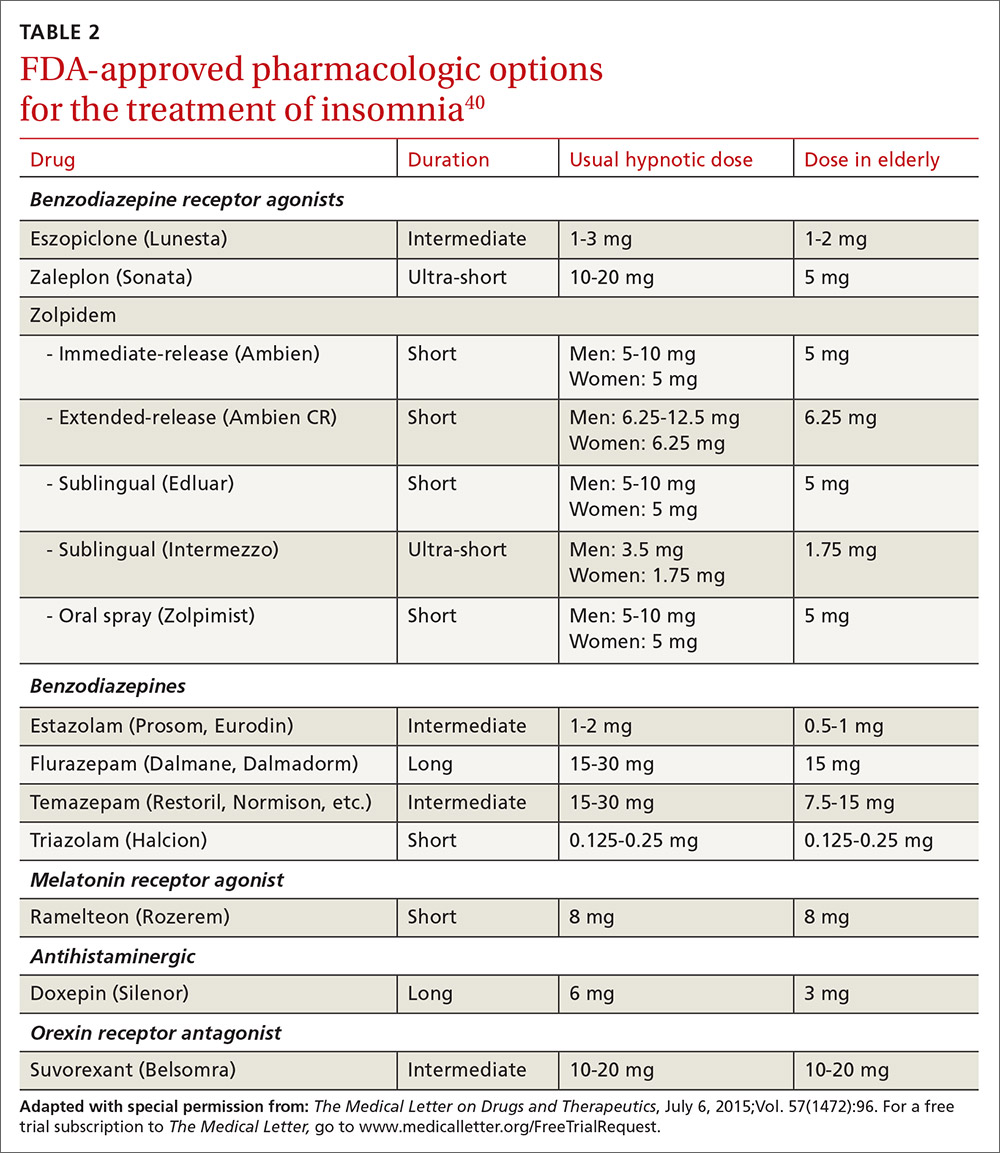
Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.

Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.

Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.

Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20

CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.

Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.

Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.

Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.

Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20

CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.

Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
PRACTICE RECOMMENDATIONS
› Recommend that patients try cognitive behavioral therapy for insomnia (CBT-I), as it is highly effective and some of its techniques can be employed in a busy family medicine clinic with little time commitment. B
› Consider pharmacotherapy for patients with chronic insomnia that persists despite CBT-I, as long as they are properly screened and followed regularly. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Report shows increase in blood cancer incidence and survival
A report on cancer in the US suggests the incidence of leukemia and myeloma has been on the rise in recent years, but the incidence of non-Hodgkin lymphoma (NHL) has been on the decline.
Meanwhile, annual death rates for leukemia and NHL have decreased, and annual death rates for myeloma have decreased in men but not in women.
Furthermore, patients with leukemia, NHL, and myeloma have seen a substantial improvement in 5-year survival rates in recent years relative to patients in the late 1970s.
These findings are part of the Annual Report to the Nation on the Status of Cancer, 1975-2014, which has been published in the Journal of the National Cancer Institute.
This report is released each year, but the current edition includes a special section focused on survival.
“While trends in death rates are the most commonly used measure to assess progress against cancer, survival trends are also an important measure to evaluate progress in improvement of cancer outcomes,” said Ahmedin Jemal, DVM, PhD, of the American Cancer Society.
“We last included a special section on cancer survival in 2004, and, as we found then, survival improved over time for almost all cancers at every stage of diagnosis.”
For the current report, researchers calculated the 5-year average annual percent changes (AAPCs) for 2009 to 2013 for cancer incidence and for 2010 to 2014 for cancer mortality.
Cancer incidence (2009-2013)
In women, the AAPC increased 1.5% for leukemia (P<0.05), decreased 0.5% for NHL (P<0.05), and increased 2.2% for myeloma (P<0.05).
In men, the AAPC increased 1.7% for leukemia (P<0.05), decreased 0.2% for NHL, and increased 2.8% for myeloma (P<0.05).
Cancer mortality (2010-2014)
In women, the AAPC decreased 1.2% for leukemia (P<0.05), decreased 2.2% for NHL (P<0.05), and increased 0.5% for myeloma.
In men, the AAPC decreased 1.0% for leukemia (P<0.05), decreased 2.0% for NHL (P<0.05), and decreased 0.9% for myeloma (P<0.05).
5-year survival
The researchers compared 5-year relative survival for cancers diagnosed from 1975 to 1977 and those diagnosed from 2006 to 2012.
The absolute percentage change over time (for both sexes combined) was 26.1% for NHL, 25.7% for myeloma, and 28.5% for leukemia.
Five-year survival for patients diagnosed in 1975-1977 was 46.5% for NHL, 24.6% for myeloma, and 34.2% for leukemia.
Five-year survival for patients diagnosed in 2006-2012 was 72.6% for NHL, 50.2% for myeloma, and 62.7% for leukemia. ![]()
A report on cancer in the US suggests the incidence of leukemia and myeloma has been on the rise in recent years, but the incidence of non-Hodgkin lymphoma (NHL) has been on the decline.
Meanwhile, annual death rates for leukemia and NHL have decreased, and annual death rates for myeloma have decreased in men but not in women.
Furthermore, patients with leukemia, NHL, and myeloma have seen a substantial improvement in 5-year survival rates in recent years relative to patients in the late 1970s.
These findings are part of the Annual Report to the Nation on the Status of Cancer, 1975-2014, which has been published in the Journal of the National Cancer Institute.
This report is released each year, but the current edition includes a special section focused on survival.
“While trends in death rates are the most commonly used measure to assess progress against cancer, survival trends are also an important measure to evaluate progress in improvement of cancer outcomes,” said Ahmedin Jemal, DVM, PhD, of the American Cancer Society.
“We last included a special section on cancer survival in 2004, and, as we found then, survival improved over time for almost all cancers at every stage of diagnosis.”
For the current report, researchers calculated the 5-year average annual percent changes (AAPCs) for 2009 to 2013 for cancer incidence and for 2010 to 2014 for cancer mortality.
Cancer incidence (2009-2013)
In women, the AAPC increased 1.5% for leukemia (P<0.05), decreased 0.5% for NHL (P<0.05), and increased 2.2% for myeloma (P<0.05).
In men, the AAPC increased 1.7% for leukemia (P<0.05), decreased 0.2% for NHL, and increased 2.8% for myeloma (P<0.05).
Cancer mortality (2010-2014)
In women, the AAPC decreased 1.2% for leukemia (P<0.05), decreased 2.2% for NHL (P<0.05), and increased 0.5% for myeloma.
In men, the AAPC decreased 1.0% for leukemia (P<0.05), decreased 2.0% for NHL (P<0.05), and decreased 0.9% for myeloma (P<0.05).
5-year survival
The researchers compared 5-year relative survival for cancers diagnosed from 1975 to 1977 and those diagnosed from 2006 to 2012.
The absolute percentage change over time (for both sexes combined) was 26.1% for NHL, 25.7% for myeloma, and 28.5% for leukemia.
Five-year survival for patients diagnosed in 1975-1977 was 46.5% for NHL, 24.6% for myeloma, and 34.2% for leukemia.
Five-year survival for patients diagnosed in 2006-2012 was 72.6% for NHL, 50.2% for myeloma, and 62.7% for leukemia. ![]()
A report on cancer in the US suggests the incidence of leukemia and myeloma has been on the rise in recent years, but the incidence of non-Hodgkin lymphoma (NHL) has been on the decline.
Meanwhile, annual death rates for leukemia and NHL have decreased, and annual death rates for myeloma have decreased in men but not in women.
Furthermore, patients with leukemia, NHL, and myeloma have seen a substantial improvement in 5-year survival rates in recent years relative to patients in the late 1970s.
These findings are part of the Annual Report to the Nation on the Status of Cancer, 1975-2014, which has been published in the Journal of the National Cancer Institute.
This report is released each year, but the current edition includes a special section focused on survival.
“While trends in death rates are the most commonly used measure to assess progress against cancer, survival trends are also an important measure to evaluate progress in improvement of cancer outcomes,” said Ahmedin Jemal, DVM, PhD, of the American Cancer Society.
“We last included a special section on cancer survival in 2004, and, as we found then, survival improved over time for almost all cancers at every stage of diagnosis.”
For the current report, researchers calculated the 5-year average annual percent changes (AAPCs) for 2009 to 2013 for cancer incidence and for 2010 to 2014 for cancer mortality.
Cancer incidence (2009-2013)
In women, the AAPC increased 1.5% for leukemia (P<0.05), decreased 0.5% for NHL (P<0.05), and increased 2.2% for myeloma (P<0.05).
In men, the AAPC increased 1.7% for leukemia (P<0.05), decreased 0.2% for NHL, and increased 2.8% for myeloma (P<0.05).
Cancer mortality (2010-2014)
In women, the AAPC decreased 1.2% for leukemia (P<0.05), decreased 2.2% for NHL (P<0.05), and increased 0.5% for myeloma.
In men, the AAPC decreased 1.0% for leukemia (P<0.05), decreased 2.0% for NHL (P<0.05), and decreased 0.9% for myeloma (P<0.05).
5-year survival
The researchers compared 5-year relative survival for cancers diagnosed from 1975 to 1977 and those diagnosed from 2006 to 2012.
The absolute percentage change over time (for both sexes combined) was 26.1% for NHL, 25.7% for myeloma, and 28.5% for leukemia.
Five-year survival for patients diagnosed in 1975-1977 was 46.5% for NHL, 24.6% for myeloma, and 34.2% for leukemia.
Five-year survival for patients diagnosed in 2006-2012 was 72.6% for NHL, 50.2% for myeloma, and 62.7% for leukemia. ![]()
Vaccination reduces risk of flu-associated pediatric deaths
.
“These results support current recommendations for annual influenza vaccination for all children 6 months of age” and older, wrote Brendan Flannery, PhD, and his coauthors at the Centers for Disease Control and Prevention, Atlanta. “To our knowledge, this is the first study to use laboratory-confirmed outcomes to investigate influenza vaccine effectiveness against influenza-associated deaths.”
“Best estimates based on [National Health Interview Survey] data suggested that vaccination reduced the risk of influenza-associated death by half among children with high-risk conditions and by nearly two-thirds among children without high-risk conditions,” Dr. Flannery and his coauthors reported.
Of 358 cases of pediatric death (aged 6 months to 17 years) confirmed to be associated with influenza, 75 (26%) had been vaccinated prior to their disease onset. The case-cohort analysis compared the 358 cases against three cohorts of U.S. children and adolescents: a telephone survey, a household survey, and a health insurance claims database.
The researchers had examined cases that were reported to the U.S. Influenza-Associated Pediatric Mortality Surveillance System from July 2010 to June 2014. They excluded cases of children not yet eligible to be vaccinated or whose disease onset may have occurred before their vaccine had 14 days to take full effect (Pediatrics. 2017 Apr. doi: 10.1542/peds.2016-4244).
.
“These results support current recommendations for annual influenza vaccination for all children 6 months of age” and older, wrote Brendan Flannery, PhD, and his coauthors at the Centers for Disease Control and Prevention, Atlanta. “To our knowledge, this is the first study to use laboratory-confirmed outcomes to investigate influenza vaccine effectiveness against influenza-associated deaths.”
“Best estimates based on [National Health Interview Survey] data suggested that vaccination reduced the risk of influenza-associated death by half among children with high-risk conditions and by nearly two-thirds among children without high-risk conditions,” Dr. Flannery and his coauthors reported.
Of 358 cases of pediatric death (aged 6 months to 17 years) confirmed to be associated with influenza, 75 (26%) had been vaccinated prior to their disease onset. The case-cohort analysis compared the 358 cases against three cohorts of U.S. children and adolescents: a telephone survey, a household survey, and a health insurance claims database.
The researchers had examined cases that were reported to the U.S. Influenza-Associated Pediatric Mortality Surveillance System from July 2010 to June 2014. They excluded cases of children not yet eligible to be vaccinated or whose disease onset may have occurred before their vaccine had 14 days to take full effect (Pediatrics. 2017 Apr. doi: 10.1542/peds.2016-4244).
.
“These results support current recommendations for annual influenza vaccination for all children 6 months of age” and older, wrote Brendan Flannery, PhD, and his coauthors at the Centers for Disease Control and Prevention, Atlanta. “To our knowledge, this is the first study to use laboratory-confirmed outcomes to investigate influenza vaccine effectiveness against influenza-associated deaths.”
“Best estimates based on [National Health Interview Survey] data suggested that vaccination reduced the risk of influenza-associated death by half among children with high-risk conditions and by nearly two-thirds among children without high-risk conditions,” Dr. Flannery and his coauthors reported.
Of 358 cases of pediatric death (aged 6 months to 17 years) confirmed to be associated with influenza, 75 (26%) had been vaccinated prior to their disease onset. The case-cohort analysis compared the 358 cases against three cohorts of U.S. children and adolescents: a telephone survey, a household survey, and a health insurance claims database.
The researchers had examined cases that were reported to the U.S. Influenza-Associated Pediatric Mortality Surveillance System from July 2010 to June 2014. They excluded cases of children not yet eligible to be vaccinated or whose disease onset may have occurred before their vaccine had 14 days to take full effect (Pediatrics. 2017 Apr. doi: 10.1542/peds.2016-4244).
FROM PEDIATRICS
Dynamic Duos: Professional Mentorship
Mentorship, whether through a formal or informal system, plays a significant role in a professional’s life; it fosters the development of professional expertise and is associated with increased job satisfaction. An effective mentor guides a less-experienced colleague by modeling positive behaviors and building trust, while being cognizant that his or her role is to be dependable, engaged, authentic, and attuned to the needs of the mentee. You can probably name one of your mentors off the top of your head right now!
The original “mentor” was a character of that name in Homer’s epic poem The Odyssey, but the word is now used to refer to a trusted advisor, friend, teacher, or wise person. In the story, Mentor served as a friend and advocate to Telemachus, the son of the king of Ithaca, while his father, Odysseus, was away fighting in the Trojan War. In 1699, the novel The Adventures of Telemachus portrayed Mentor as Telemachus’ tutor, and he became the hero of the story.1,2
History holds many examples of mentoring relationships: Socrates and Plato, Haydn and Beethoven, and Freud and Jung. Modern-day duos include Kobe Bryant and Shaquille O’Neal, Kirk and Spock, and—dare I say it?—Brady and Belichick. During the Middle Ages, mentorship—particularly in medicine and nursing—was practiced via apprenticeship, which incorporated support, guidance, socialization, well-being, empowerment, education, and career progression.3
Throughout my career as a PA, I have been fortunate to be guided by competent and willing mentors. What have they had in common? For starters, an internal desire (sometimes called generosity of spirit) to mentor and a commitment to my growth and development as their mentee. Successful professional mentors must also possess the necessary knowledge to help effectively develop their mentee’s skills. Discussions with my colleagues and previous mentors inspired the following compilation of the essential responsibilities and traits of a mentor.
Initiating new ideas. A main aspect of a mentor’s role involves assisting in acquiring the confidence and tools to function and excel in our competitive professional world.4-6 In the early 1970s, when I was a young PA, a wonderful physician and friend, Dr. Burton Brasher, took me under his wing and exemplified what it means to be a clinician. I learned from him that it was also my obligation to mentor others, and I have tried to do this frequently in my four decades as a PA. Through his example, I was shown the importance of cultivating emotional intelligence and sensitivity while still providing an honest assessment of strengths and weaknesses. Here was a physician who was unencumbered by ego. We met often to discuss the care of both of our patients.
Staying the course. In 1995, I mentored James Cannon—a young financial comptroller who desperately wanted to be a PA. I’ve (hopefully) helped him navigate PA school, our mutual time in the military, his time in academia, and his introduction to professional volunteer work. In each stage of his career, we had lengthy conversations about the pros and cons of his decisions. Now, 22 years later, he has become my mentor; he has matured in the profession and is at the forefront of taking it to the next level. It is now very common for me to call on him for his advice as I move into the home stretch of my career. A few years ago, he became a trustee of our university and his skills have advanced the success of our programs. Indeed, the student becomes the teacher.
Networking and articulating cultural norms. Dave Mittman, the co-founder and original publisher of Clinician Reviews, had the experience of hiring his very close friend and PA school classmate, Tom Yackeren. In 1985, Dave was publisher of Physician Assistant Journal (at that time, the official journal of the AAPA). Dave and Tom were business partners and relied on each other’s skills to grow their business. They shared trust, friendship, and a mutual knowledge of professional “culture.” They understood each other and how they could each contribute to their success. Their partnership maintained a complementary balance, each of them able to play to his
Demonstrating honesty, integrity, and enthusiasm. Marie-Eileen Onieal, our NP editor-in-chief, grew up in a household where her father was a firefighter and union organizer. He taught her the value of always paying it forward. While she has mentored many people in her career, she fondly remembers mentoring Lori Fritz through her transition into academia—what Marie-Eileen calls “the precarious journey of an educator.” When she met Lori, she says, they just “clicked,” and that bond has survived to this day. Lori is now an established academician, mentoring new students and professionals, and modeling her experience with Marie-Eileen’s involvement in the profession.
These are examples of when it works. But what happens when the relationship doesn’t “click”? Unfortunately, not all mentorships are fruitful. When mentor and mentee clash, it is paramount to acknowledge that the relationship is not working and to back away appropriately, without regard to ego. No one benefits when the parties are at odds—and this may explain why some of the greatest partnerships form organically.
Above all, in order for a mentorship to be prosperous, mentors must express compassion and remain genuine throughout all interactions with their mentee. It is a long-term commitment. Without this generosity of spirit, the influence and benefit of a professional mentor would be lost. If you have other ideas about what makes a great mentor, or how to foster a more satisfying mentor/mentee relationship, please share them with me at [email protected].
1. Anderson E. 5 Qualities to look for in a mentor. Forbes. www.forbes.com/sites/erikaandersen/2014/09/29/5-qualities-to-look-for-in-a-mentor/#389c58743021. Accessed March 8, 2017.
2. The National Academies Press. Adviser, Teacher, Role Model, Friend: On Being a Mentor to Students in Science and Engineering.
3. Kim YJ. The Odyssey and mentorship today. The Stanford Daily. www.stanforddaily.com/2016/10/27/the-iliad-and-mentorship-today. Accessed March 16, 2017.
4. Wagner AL, Seymour ME. A model of caring mentorship for nursing. J Nurses Staff Dev. 2007;23(5):201-211.
5. University of Wolverhampton Business School. A Managers’ & Mentors Handbook on Mentoring. www2.wlv.ac.uk/registry/qasd/RandV/R&V%2009-10/UWBS/Collab%20Mentoring%20Handbook.pdf. Accessed March 8, 2017.
6. Vivier J, Dana K. The value of mentorship: personal journeys inspired by the teaching philosophy of Chuck Jones. Voice and Speech Review. 2014;8(3):224-249.
Mentorship, whether through a formal or informal system, plays a significant role in a professional’s life; it fosters the development of professional expertise and is associated with increased job satisfaction. An effective mentor guides a less-experienced colleague by modeling positive behaviors and building trust, while being cognizant that his or her role is to be dependable, engaged, authentic, and attuned to the needs of the mentee. You can probably name one of your mentors off the top of your head right now!
The original “mentor” was a character of that name in Homer’s epic poem The Odyssey, but the word is now used to refer to a trusted advisor, friend, teacher, or wise person. In the story, Mentor served as a friend and advocate to Telemachus, the son of the king of Ithaca, while his father, Odysseus, was away fighting in the Trojan War. In 1699, the novel The Adventures of Telemachus portrayed Mentor as Telemachus’ tutor, and he became the hero of the story.1,2
History holds many examples of mentoring relationships: Socrates and Plato, Haydn and Beethoven, and Freud and Jung. Modern-day duos include Kobe Bryant and Shaquille O’Neal, Kirk and Spock, and—dare I say it?—Brady and Belichick. During the Middle Ages, mentorship—particularly in medicine and nursing—was practiced via apprenticeship, which incorporated support, guidance, socialization, well-being, empowerment, education, and career progression.3
Throughout my career as a PA, I have been fortunate to be guided by competent and willing mentors. What have they had in common? For starters, an internal desire (sometimes called generosity of spirit) to mentor and a commitment to my growth and development as their mentee. Successful professional mentors must also possess the necessary knowledge to help effectively develop their mentee’s skills. Discussions with my colleagues and previous mentors inspired the following compilation of the essential responsibilities and traits of a mentor.
Initiating new ideas. A main aspect of a mentor’s role involves assisting in acquiring the confidence and tools to function and excel in our competitive professional world.4-6 In the early 1970s, when I was a young PA, a wonderful physician and friend, Dr. Burton Brasher, took me under his wing and exemplified what it means to be a clinician. I learned from him that it was also my obligation to mentor others, and I have tried to do this frequently in my four decades as a PA. Through his example, I was shown the importance of cultivating emotional intelligence and sensitivity while still providing an honest assessment of strengths and weaknesses. Here was a physician who was unencumbered by ego. We met often to discuss the care of both of our patients.
Staying the course. In 1995, I mentored James Cannon—a young financial comptroller who desperately wanted to be a PA. I’ve (hopefully) helped him navigate PA school, our mutual time in the military, his time in academia, and his introduction to professional volunteer work. In each stage of his career, we had lengthy conversations about the pros and cons of his decisions. Now, 22 years later, he has become my mentor; he has matured in the profession and is at the forefront of taking it to the next level. It is now very common for me to call on him for his advice as I move into the home stretch of my career. A few years ago, he became a trustee of our university and his skills have advanced the success of our programs. Indeed, the student becomes the teacher.
Networking and articulating cultural norms. Dave Mittman, the co-founder and original publisher of Clinician Reviews, had the experience of hiring his very close friend and PA school classmate, Tom Yackeren. In 1985, Dave was publisher of Physician Assistant Journal (at that time, the official journal of the AAPA). Dave and Tom were business partners and relied on each other’s skills to grow their business. They shared trust, friendship, and a mutual knowledge of professional “culture.” They understood each other and how they could each contribute to their success. Their partnership maintained a complementary balance, each of them able to play to his
Demonstrating honesty, integrity, and enthusiasm. Marie-Eileen Onieal, our NP editor-in-chief, grew up in a household where her father was a firefighter and union organizer. He taught her the value of always paying it forward. While she has mentored many people in her career, she fondly remembers mentoring Lori Fritz through her transition into academia—what Marie-Eileen calls “the precarious journey of an educator.” When she met Lori, she says, they just “clicked,” and that bond has survived to this day. Lori is now an established academician, mentoring new students and professionals, and modeling her experience with Marie-Eileen’s involvement in the profession.
These are examples of when it works. But what happens when the relationship doesn’t “click”? Unfortunately, not all mentorships are fruitful. When mentor and mentee clash, it is paramount to acknowledge that the relationship is not working and to back away appropriately, without regard to ego. No one benefits when the parties are at odds—and this may explain why some of the greatest partnerships form organically.
Above all, in order for a mentorship to be prosperous, mentors must express compassion and remain genuine throughout all interactions with their mentee. It is a long-term commitment. Without this generosity of spirit, the influence and benefit of a professional mentor would be lost. If you have other ideas about what makes a great mentor, or how to foster a more satisfying mentor/mentee relationship, please share them with me at [email protected].
Mentorship, whether through a formal or informal system, plays a significant role in a professional’s life; it fosters the development of professional expertise and is associated with increased job satisfaction. An effective mentor guides a less-experienced colleague by modeling positive behaviors and building trust, while being cognizant that his or her role is to be dependable, engaged, authentic, and attuned to the needs of the mentee. You can probably name one of your mentors off the top of your head right now!
The original “mentor” was a character of that name in Homer’s epic poem The Odyssey, but the word is now used to refer to a trusted advisor, friend, teacher, or wise person. In the story, Mentor served as a friend and advocate to Telemachus, the son of the king of Ithaca, while his father, Odysseus, was away fighting in the Trojan War. In 1699, the novel The Adventures of Telemachus portrayed Mentor as Telemachus’ tutor, and he became the hero of the story.1,2
History holds many examples of mentoring relationships: Socrates and Plato, Haydn and Beethoven, and Freud and Jung. Modern-day duos include Kobe Bryant and Shaquille O’Neal, Kirk and Spock, and—dare I say it?—Brady and Belichick. During the Middle Ages, mentorship—particularly in medicine and nursing—was practiced via apprenticeship, which incorporated support, guidance, socialization, well-being, empowerment, education, and career progression.3
Throughout my career as a PA, I have been fortunate to be guided by competent and willing mentors. What have they had in common? For starters, an internal desire (sometimes called generosity of spirit) to mentor and a commitment to my growth and development as their mentee. Successful professional mentors must also possess the necessary knowledge to help effectively develop their mentee’s skills. Discussions with my colleagues and previous mentors inspired the following compilation of the essential responsibilities and traits of a mentor.
Initiating new ideas. A main aspect of a mentor’s role involves assisting in acquiring the confidence and tools to function and excel in our competitive professional world.4-6 In the early 1970s, when I was a young PA, a wonderful physician and friend, Dr. Burton Brasher, took me under his wing and exemplified what it means to be a clinician. I learned from him that it was also my obligation to mentor others, and I have tried to do this frequently in my four decades as a PA. Through his example, I was shown the importance of cultivating emotional intelligence and sensitivity while still providing an honest assessment of strengths and weaknesses. Here was a physician who was unencumbered by ego. We met often to discuss the care of both of our patients.
Staying the course. In 1995, I mentored James Cannon—a young financial comptroller who desperately wanted to be a PA. I’ve (hopefully) helped him navigate PA school, our mutual time in the military, his time in academia, and his introduction to professional volunteer work. In each stage of his career, we had lengthy conversations about the pros and cons of his decisions. Now, 22 years later, he has become my mentor; he has matured in the profession and is at the forefront of taking it to the next level. It is now very common for me to call on him for his advice as I move into the home stretch of my career. A few years ago, he became a trustee of our university and his skills have advanced the success of our programs. Indeed, the student becomes the teacher.
Networking and articulating cultural norms. Dave Mittman, the co-founder and original publisher of Clinician Reviews, had the experience of hiring his very close friend and PA school classmate, Tom Yackeren. In 1985, Dave was publisher of Physician Assistant Journal (at that time, the official journal of the AAPA). Dave and Tom were business partners and relied on each other’s skills to grow their business. They shared trust, friendship, and a mutual knowledge of professional “culture.” They understood each other and how they could each contribute to their success. Their partnership maintained a complementary balance, each of them able to play to his
Demonstrating honesty, integrity, and enthusiasm. Marie-Eileen Onieal, our NP editor-in-chief, grew up in a household where her father was a firefighter and union organizer. He taught her the value of always paying it forward. While she has mentored many people in her career, she fondly remembers mentoring Lori Fritz through her transition into academia—what Marie-Eileen calls “the precarious journey of an educator.” When she met Lori, she says, they just “clicked,” and that bond has survived to this day. Lori is now an established academician, mentoring new students and professionals, and modeling her experience with Marie-Eileen’s involvement in the profession.
These are examples of when it works. But what happens when the relationship doesn’t “click”? Unfortunately, not all mentorships are fruitful. When mentor and mentee clash, it is paramount to acknowledge that the relationship is not working and to back away appropriately, without regard to ego. No one benefits when the parties are at odds—and this may explain why some of the greatest partnerships form organically.
Above all, in order for a mentorship to be prosperous, mentors must express compassion and remain genuine throughout all interactions with their mentee. It is a long-term commitment. Without this generosity of spirit, the influence and benefit of a professional mentor would be lost. If you have other ideas about what makes a great mentor, or how to foster a more satisfying mentor/mentee relationship, please share them with me at [email protected].
1. Anderson E. 5 Qualities to look for in a mentor. Forbes. www.forbes.com/sites/erikaandersen/2014/09/29/5-qualities-to-look-for-in-a-mentor/#389c58743021. Accessed March 8, 2017.
2. The National Academies Press. Adviser, Teacher, Role Model, Friend: On Being a Mentor to Students in Science and Engineering.
3. Kim YJ. The Odyssey and mentorship today. The Stanford Daily. www.stanforddaily.com/2016/10/27/the-iliad-and-mentorship-today. Accessed March 16, 2017.
4. Wagner AL, Seymour ME. A model of caring mentorship for nursing. J Nurses Staff Dev. 2007;23(5):201-211.
5. University of Wolverhampton Business School. A Managers’ & Mentors Handbook on Mentoring. www2.wlv.ac.uk/registry/qasd/RandV/R&V%2009-10/UWBS/Collab%20Mentoring%20Handbook.pdf. Accessed March 8, 2017.
6. Vivier J, Dana K. The value of mentorship: personal journeys inspired by the teaching philosophy of Chuck Jones. Voice and Speech Review. 2014;8(3):224-249.
1. Anderson E. 5 Qualities to look for in a mentor. Forbes. www.forbes.com/sites/erikaandersen/2014/09/29/5-qualities-to-look-for-in-a-mentor/#389c58743021. Accessed March 8, 2017.
2. The National Academies Press. Adviser, Teacher, Role Model, Friend: On Being a Mentor to Students in Science and Engineering.
3. Kim YJ. The Odyssey and mentorship today. The Stanford Daily. www.stanforddaily.com/2016/10/27/the-iliad-and-mentorship-today. Accessed March 16, 2017.
4. Wagner AL, Seymour ME. A model of caring mentorship for nursing. J Nurses Staff Dev. 2007;23(5):201-211.
5. University of Wolverhampton Business School. A Managers’ & Mentors Handbook on Mentoring. www2.wlv.ac.uk/registry/qasd/RandV/R&V%2009-10/UWBS/Collab%20Mentoring%20Handbook.pdf. Accessed March 8, 2017.
6. Vivier J, Dana K. The value of mentorship: personal journeys inspired by the teaching philosophy of Chuck Jones. Voice and Speech Review. 2014;8(3):224-249.
A new addition to JFP: “Behavioral Health Consult”
In this month’s issue of The Journal of Family Practice, we are pleased to launch a new department called “Behavioral Health Consult.” This bimonthly column will feature behavioral and mental health topics such as depression, anxiety, obesity, and substance abuse.
Drawn from real patient encounters. As you read the inaugural item on depression, written by Michael Maksimowski, MD, and Michael Raddock, MD, you'll notice that the article starts with a brief case report. Cases will play an important role in this column and will either describe a single patient whom the author(s) cared for or be an amalgam of several (as was the case this month).
Practical and to the point. We have asked the authors, who are family physicians (FPs) and psychiatrists or psychologists who work closely with FPs, to provide a concentrated and practical summary of the elements of diagnosis and treatment that are most important and pertinent to primary care clinicians.
Addressing an overwhelming need. The need for FPs and other primary care clinicians to stay current on the management of mental and behavioral health issues is obvious. Mood and anxiety disorders (eg, depression, anxiety, panic disorder, agoraphobia) affect almost 30% of the US adult population1 and many of these patients are seen at least initially by their primary care physicians. According to the Centers for Disease Control and Prevention, 4 health risk behaviors—tobacco use, poor nutrition, excess alcohol consumption, and insufficient exercise—cause much of the illness, suffering, and early death related to chronic diseases and conditions.2 My personal experience in our urban Chicago clinic definitely supports these statistics.
No lack of research. I teach several evidence-based medicine courses each year that focus on the review of recent randomized trials and meta-analyses that are important for FPs to know about. Every year, one of my talks is about either mental health or behavioral health research. Every year I wonder whether there will be enough new research to report on, and every year, I find that there is an abundance of research that helps us to better manage these common problems. “Behavioral Health Consult” is this journal’s way of helping to keep you current and informed.
In an effort to make this addition as useful to you as possible, please feel free to email me at [email protected] with suggestions for topics you would like to see in “Behavioral Health Consult.” We look forward to your reactions—and your comments.
1. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
2. Centers for Disease Control and Prevention. Chronic disease prevention and health promotion. Available at: https://www.cdc.gov/chronicdisease/overview/index.htm. Accessed March 19, 2017.
In this month’s issue of The Journal of Family Practice, we are pleased to launch a new department called “Behavioral Health Consult.” This bimonthly column will feature behavioral and mental health topics such as depression, anxiety, obesity, and substance abuse.
Drawn from real patient encounters. As you read the inaugural item on depression, written by Michael Maksimowski, MD, and Michael Raddock, MD, you'll notice that the article starts with a brief case report. Cases will play an important role in this column and will either describe a single patient whom the author(s) cared for or be an amalgam of several (as was the case this month).
Practical and to the point. We have asked the authors, who are family physicians (FPs) and psychiatrists or psychologists who work closely with FPs, to provide a concentrated and practical summary of the elements of diagnosis and treatment that are most important and pertinent to primary care clinicians.
Addressing an overwhelming need. The need for FPs and other primary care clinicians to stay current on the management of mental and behavioral health issues is obvious. Mood and anxiety disorders (eg, depression, anxiety, panic disorder, agoraphobia) affect almost 30% of the US adult population1 and many of these patients are seen at least initially by their primary care physicians. According to the Centers for Disease Control and Prevention, 4 health risk behaviors—tobacco use, poor nutrition, excess alcohol consumption, and insufficient exercise—cause much of the illness, suffering, and early death related to chronic diseases and conditions.2 My personal experience in our urban Chicago clinic definitely supports these statistics.
No lack of research. I teach several evidence-based medicine courses each year that focus on the review of recent randomized trials and meta-analyses that are important for FPs to know about. Every year, one of my talks is about either mental health or behavioral health research. Every year I wonder whether there will be enough new research to report on, and every year, I find that there is an abundance of research that helps us to better manage these common problems. “Behavioral Health Consult” is this journal’s way of helping to keep you current and informed.
In an effort to make this addition as useful to you as possible, please feel free to email me at [email protected] with suggestions for topics you would like to see in “Behavioral Health Consult.” We look forward to your reactions—and your comments.
In this month’s issue of The Journal of Family Practice, we are pleased to launch a new department called “Behavioral Health Consult.” This bimonthly column will feature behavioral and mental health topics such as depression, anxiety, obesity, and substance abuse.
Drawn from real patient encounters. As you read the inaugural item on depression, written by Michael Maksimowski, MD, and Michael Raddock, MD, you'll notice that the article starts with a brief case report. Cases will play an important role in this column and will either describe a single patient whom the author(s) cared for or be an amalgam of several (as was the case this month).
Practical and to the point. We have asked the authors, who are family physicians (FPs) and psychiatrists or psychologists who work closely with FPs, to provide a concentrated and practical summary of the elements of diagnosis and treatment that are most important and pertinent to primary care clinicians.
Addressing an overwhelming need. The need for FPs and other primary care clinicians to stay current on the management of mental and behavioral health issues is obvious. Mood and anxiety disorders (eg, depression, anxiety, panic disorder, agoraphobia) affect almost 30% of the US adult population1 and many of these patients are seen at least initially by their primary care physicians. According to the Centers for Disease Control and Prevention, 4 health risk behaviors—tobacco use, poor nutrition, excess alcohol consumption, and insufficient exercise—cause much of the illness, suffering, and early death related to chronic diseases and conditions.2 My personal experience in our urban Chicago clinic definitely supports these statistics.
No lack of research. I teach several evidence-based medicine courses each year that focus on the review of recent randomized trials and meta-analyses that are important for FPs to know about. Every year, one of my talks is about either mental health or behavioral health research. Every year I wonder whether there will be enough new research to report on, and every year, I find that there is an abundance of research that helps us to better manage these common problems. “Behavioral Health Consult” is this journal’s way of helping to keep you current and informed.
In an effort to make this addition as useful to you as possible, please feel free to email me at [email protected] with suggestions for topics you would like to see in “Behavioral Health Consult.” We look forward to your reactions—and your comments.
1. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
2. Centers for Disease Control and Prevention. Chronic disease prevention and health promotion. Available at: https://www.cdc.gov/chronicdisease/overview/index.htm. Accessed March 19, 2017.
1. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
2. Centers for Disease Control and Prevention. Chronic disease prevention and health promotion. Available at: https://www.cdc.gov/chronicdisease/overview/index.htm. Accessed March 19, 2017.
Screen for bullying—but know what to do next
I read the article, “What family physicians can do to combat bullying” (J Fam Pract. 2017;66:82-89) and Dr. Hickner’s editorial, “It’s time to screen for bullying” (J Fam Pract. 2017;66:66) with great interest. I’m a bullying prevention researcher and the creator of a new bullying prevention program, CirclePoint, which is being piloted in Boston Public Schools. I’m also a featured speaker on bullying in the Massachusetts General Hospital’s life skills after-school program that runs in a dozen area schools.
My work in schools has taught me that as important as it is to identify bullying problems, it is equally important for doctors to know how to counsel patients and caregivers on how to resolve these problems.
Identifying bullying without providing further guidance can actually do more harm than good, both to the child’s health and to the child-physician relationship.
Children often don’t tell adults they are being bullied because the actions that adults take—while well-intended—can sometimes make the situation worse. Further, some caregivers may actually blame the child for being bullied. And a doctor who simply identifies the problem and leaves the next steps to an ill-informed caregiver may lose the patient’s trust.
Also worth noting: Some children who are bullied may not have a clear understanding of what the term “bullying” means. I strongly suggest asking patients about how others are treating them and if anyone is making them upset. Questions about behaviors and feelings are more effective at identifying a bullying problem than questions that use the term “bullying.”
Our program has a free resource that was developed for educators, but can easily be used by physicians to counsel patients and caregivers. It’s designed to convey recommended actions for both the student and caregiver in a matter of minutes.
Doctors who identify a bullying problem bear a responsibility to counsel both the patient and caregiver(s) on what bullying is, why it happens, and, most critically, recommended actions to take to effectively resolve the problem.
Ari Magnusson
Charlestown, Mass
I read the article, “What family physicians can do to combat bullying” (J Fam Pract. 2017;66:82-89) and Dr. Hickner’s editorial, “It’s time to screen for bullying” (J Fam Pract. 2017;66:66) with great interest. I’m a bullying prevention researcher and the creator of a new bullying prevention program, CirclePoint, which is being piloted in Boston Public Schools. I’m also a featured speaker on bullying in the Massachusetts General Hospital’s life skills after-school program that runs in a dozen area schools.
My work in schools has taught me that as important as it is to identify bullying problems, it is equally important for doctors to know how to counsel patients and caregivers on how to resolve these problems.
Identifying bullying without providing further guidance can actually do more harm than good, both to the child’s health and to the child-physician relationship.
Children often don’t tell adults they are being bullied because the actions that adults take—while well-intended—can sometimes make the situation worse. Further, some caregivers may actually blame the child for being bullied. And a doctor who simply identifies the problem and leaves the next steps to an ill-informed caregiver may lose the patient’s trust.
Also worth noting: Some children who are bullied may not have a clear understanding of what the term “bullying” means. I strongly suggest asking patients about how others are treating them and if anyone is making them upset. Questions about behaviors and feelings are more effective at identifying a bullying problem than questions that use the term “bullying.”
Our program has a free resource that was developed for educators, but can easily be used by physicians to counsel patients and caregivers. It’s designed to convey recommended actions for both the student and caregiver in a matter of minutes.
Doctors who identify a bullying problem bear a responsibility to counsel both the patient and caregiver(s) on what bullying is, why it happens, and, most critically, recommended actions to take to effectively resolve the problem.
Ari Magnusson
Charlestown, Mass
I read the article, “What family physicians can do to combat bullying” (J Fam Pract. 2017;66:82-89) and Dr. Hickner’s editorial, “It’s time to screen for bullying” (J Fam Pract. 2017;66:66) with great interest. I’m a bullying prevention researcher and the creator of a new bullying prevention program, CirclePoint, which is being piloted in Boston Public Schools. I’m also a featured speaker on bullying in the Massachusetts General Hospital’s life skills after-school program that runs in a dozen area schools.
My work in schools has taught me that as important as it is to identify bullying problems, it is equally important for doctors to know how to counsel patients and caregivers on how to resolve these problems.
Identifying bullying without providing further guidance can actually do more harm than good, both to the child’s health and to the child-physician relationship.
Children often don’t tell adults they are being bullied because the actions that adults take—while well-intended—can sometimes make the situation worse. Further, some caregivers may actually blame the child for being bullied. And a doctor who simply identifies the problem and leaves the next steps to an ill-informed caregiver may lose the patient’s trust.
Also worth noting: Some children who are bullied may not have a clear understanding of what the term “bullying” means. I strongly suggest asking patients about how others are treating them and if anyone is making them upset. Questions about behaviors and feelings are more effective at identifying a bullying problem than questions that use the term “bullying.”
Our program has a free resource that was developed for educators, but can easily be used by physicians to counsel patients and caregivers. It’s designed to convey recommended actions for both the student and caregiver in a matter of minutes.
Doctors who identify a bullying problem bear a responsibility to counsel both the patient and caregiver(s) on what bullying is, why it happens, and, most critically, recommended actions to take to effectively resolve the problem.
Ari Magnusson
Charlestown, Mass
Is auscultation really better than echocardiography?
In a recent letter to the editor on the role of auscultation and echocardiography, “Point-of-care ultrasound: It’s no replacement for the stethoscope” (J Fam Pract. 2016;65:734), Dr. Fredricks claimed that “doppler ultrasound is not as precise as the stethoscope when used by a practiced listener for identifying the source and subtle characteristics of murmurs.” His citation for this claim was a review article from more than 20 years ago that offered no evidence in support of the superiority of auscultation over echocardiography to characterize murmurs.1 The review did acknowledge the limitations and variability between examiners.
The notion that physical examination is superior to echocardiography is appealing, but likely incorrect. A study of medical students with basic training in echocardiography showed that they were able to characterize murmurs more accurately with point-of-care ultrasound than experienced cardiologists auscultating the murmur.2
The existence of a better test does not obviate the role of the physical examination, but it does highlight the need to understand its limits. Like an ultrasound study, physical examination maneuvers are tests, with sensitivities and specificities. We should approach them as such, and not romanticize their performance.
David Mackenzie, MD
Portland, Me
1. Tavel ME. Cardiac auscultation. A glorious past—but does it have a future? Circulation. 1996;93:1250-1253.
2. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96:1002-1006.
In a recent letter to the editor on the role of auscultation and echocardiography, “Point-of-care ultrasound: It’s no replacement for the stethoscope” (J Fam Pract. 2016;65:734), Dr. Fredricks claimed that “doppler ultrasound is not as precise as the stethoscope when used by a practiced listener for identifying the source and subtle characteristics of murmurs.” His citation for this claim was a review article from more than 20 years ago that offered no evidence in support of the superiority of auscultation over echocardiography to characterize murmurs.1 The review did acknowledge the limitations and variability between examiners.
The notion that physical examination is superior to echocardiography is appealing, but likely incorrect. A study of medical students with basic training in echocardiography showed that they were able to characterize murmurs more accurately with point-of-care ultrasound than experienced cardiologists auscultating the murmur.2
The existence of a better test does not obviate the role of the physical examination, but it does highlight the need to understand its limits. Like an ultrasound study, physical examination maneuvers are tests, with sensitivities and specificities. We should approach them as such, and not romanticize their performance.
David Mackenzie, MD
Portland, Me
In a recent letter to the editor on the role of auscultation and echocardiography, “Point-of-care ultrasound: It’s no replacement for the stethoscope” (J Fam Pract. 2016;65:734), Dr. Fredricks claimed that “doppler ultrasound is not as precise as the stethoscope when used by a practiced listener for identifying the source and subtle characteristics of murmurs.” His citation for this claim was a review article from more than 20 years ago that offered no evidence in support of the superiority of auscultation over echocardiography to characterize murmurs.1 The review did acknowledge the limitations and variability between examiners.
The notion that physical examination is superior to echocardiography is appealing, but likely incorrect. A study of medical students with basic training in echocardiography showed that they were able to characterize murmurs more accurately with point-of-care ultrasound than experienced cardiologists auscultating the murmur.2
The existence of a better test does not obviate the role of the physical examination, but it does highlight the need to understand its limits. Like an ultrasound study, physical examination maneuvers are tests, with sensitivities and specificities. We should approach them as such, and not romanticize their performance.
David Mackenzie, MD
Portland, Me
1. Tavel ME. Cardiac auscultation. A glorious past—but does it have a future? Circulation. 1996;93:1250-1253.
2. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96:1002-1006.
1. Tavel ME. Cardiac auscultation. A glorious past—but does it have a future? Circulation. 1996;93:1250-1253.
2. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96:1002-1006.
When can exercise supplant surgery for degenerative meniscal tears?
ILLUSTRATIVE CASE
A 48-year-old man presents to your office for follow-up of right knee pain that has been bothering him for the last 12 months. He denies any trauma or inciting incident for the pain. On physical exam, he does not have crepitus, but has medial joint line tenderness of his right knee. A magnetic resonance image (MRI) shows a partial, medial meniscal tear. Do you refer him to Physical Therapy (PT) or Orthopedics for arthroscopy and repair?
The meniscus—cartilage in the knee joint that provides support, stability, and lubrication to the joint during activity—can tear during a traumatic event or because of degeneration over time. Traumatic meniscal tears typically happen to younger adults and teens (<30 years of age) during sports, such as basketball and soccer,whereas degenerative meniscal tears generally present in patients ages 40 to 60 years.2,3 The annual incidence of all meniscal tears is 79 per 100,000.4 While some physicians can diagnose traumatic meniscal tears based on history and physical examination, degenerative meniscal tears are generally more challenging, and typically warrant an MRI for confirmation.3
Meniscal tears can be treated either conservatively, with supportive care and exercise, or with surgery. Unfortunately, there are no national orthopedic guidelines available to help direct care. In one observational study of surgery as treatment for both traumatic and degenerative meniscal tears, 95 out of 117 patients (81.2%) were generally satisfied with this treatment at the 4-year follow-up, with higher satisfaction in the traumatic meniscal tear group than in the degenerative tear group.5
Two systematic reviews of surgery vs nonoperative management or sham therapies found no additional benefit of surgery for meniscal tears in a variety of patients with and without osteoarthritis.6,7 However, both studies were of only moderate quality because of the number of patients in the nonoperative groups who ultimately obtained surgery. And neither of the studies directly compared surgery to nonoperative management.6,7
Yet another investigation, a multicenter, randomized, double-blind, sham-controlled study conducted in Finland involving 146 patients, compared sham surgery to arthroscopic partial meniscectomy. Both groups received instruction on performing post-procedure exercises, and both groups had similar and marked improvement in pain and function.8
Clinical practice recommendations devised from a systematic and vast review of the literature recommend that the decision for surgery be based on patient-specific factors such as symptoms, age, mechanism of tear, extent of damage, and occupational/social/activity needs.9
STUDY SUMMARY
Exercise is as good as—and in one way, better than—surgery
The current randomized controlled superiority trial compared exercise therapy to arthroscopic partial meniscectomy in patients ages 35 to 60 years presenting to the orthopedic departments of 2 hospitals in Norway with unilateral knee pain for more than 2 months and an MRI-delineated medial meniscal tear. Patients were included only if they had radiographic evidence of minimal osteoarthritis (Kellgren-Lawrence classification grade ≤2). Exclusion criteria were acute trauma, locked knee, ligament injury, and knee surgery in the same knee within the previous 2 years.
The primary outcomes were change in patient-reported knee function as determined by overall knee injury and osteoarthritis outcome score (KOOS4) after 2 years and thigh muscle strength at 3 months as measured by physiotherapists. The KOOS4 consists of 4 out of the 5 KOOS subscales: pain, other symptoms (swelling, grinding/noise from the joint, ability to straighten and bend), function in sports/recreation, and knee-related quality of life (QOL). This study utilized the average score of each subscale.
Secondary outcomes were the 5 individual KOOS subscales (the 4 previously mentioned plus activities of daily living [ADLs]), as well as thigh muscle strength and lower extremity performance test results.
Methods. Testing personnel were blinded to group allocation; participants wore pants or neoprene sleeves to cover surgical scars. A total of 140 patients were randomized to either 12 weeks (24-36 sessions) of exercise therapy alone or a standardized arthroscopic partial meniscectomy with written and oral encouragement upon discharge to perform simple exercises at home 2 to4 times daily (to regain range of motion and reduce swelling).
Results. The overall mean improvement in KOOS4 score from baseline at 2 years was similar between the exercise group and the meniscectomy group (25.3 points vs 24.4 points, respectively; mean difference [MD], 0.9; 95% confidence interval [CI], -4.3 to 6.1; P=.72). Additionally, muscle strength (measured as peak torque flexion and extension and total work flexion and extension) at both 3 and 12 months showed significant objective improvements favoring exercise therapy.
Secondary outcomes comparing the change from baseline of KOOS subscale scores showed 4 of the 5 having non-significant differences (pain, ADL, sports/recreation, and QOL). Only the symptoms subscale had a significant difference favoring exercise therapy (MD, 5.3 points; 95% CI, 0.5 to 10.2; P=.03), which was likely clinically insignificant when using a grading scale of 0 to 100.
Of those patients allocated to exercise therapy alone, 19% crossed over and underwent surgery during the 2 years of the study.
WHAT'S NEW
Head-to-head comparison adds evidence to previous findings
This is the first trial to directly compare exercise therapy to surgery in patients with meniscal tears. Interestingly, exercise therapy was as effective after a 2-year follow-up period and was superior in the short term for thigh muscle strength.1 The results of this study build on those from the smaller study conducted in Finland mentioned earlier.8 In that study, both groups received instruction for the same graduated exercise plan. The researchers found that exercise was comparable to surgery for meniscal tears in patients with no osteoarthritis.
CAVEATS
Results may not translate to those with more severe osteoarthritis
This trial included patients with only mild to no osteoarthritis in addition to their meniscal tear.1 It is unclear if the results would be maintained in patients with more advanced disease. Additionally, 19% of patients crossed over from the exercise group to the surgery group, even though muscle strength improved. Therefore, education about the risks of surgery and the potential lack of benefit is important.
CHALLENGES TO IMPLEMENTATION
The cost and effort of physical therapy may be a deterrent
The cost of PT can be a barrier for some patients who have adequate insurance coverage for surgery, but inadequate coverage for PT. Additionally, exercise therapy requires significant and ongoing amounts of time and effort, which may be a deterrent for patients with busy lifestyles. Patients and physicians may view surgery as an “easier” fix.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.
2. Beals CT, Magnussen RA, Graham WC, et al. The prevalence of meniscal pathology in asymptomatic athletes. Sports Med. 2016;46:1517-1524.
3. Maffulli N, Longo UG, Campi S, et al. Meniscal tears. Open Access J Sports Med. 2010;1:45-54.
4. Peat G, Bergknut C, Frobell R, et al. Population-wide incidence estimates for soft tissue knee injuries presenting to healthcare in southern Sweden: data from the Skåne Healthcare Register. Arthritis Res Ther. 2014;16:R162.
5. Ghislain NA, Wei JN, Li YG. Study of the clinical outcome between traumatic and degenerative (non-traumatic) meniscal tears after arthroscopic surgery: a 4-years follow-up study. J Clin Diagn Res. 2016;10:RC01-RC04.
6. Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014;186:1057-1064.
7. Monk P, Garfjeld Roberts P, Palmer AJR, et al. The urgent need for evidence in arthroscopic meniscal surgery: a systematic review of the evidence for operative management of meniscal tears. Am J Sports Med. 2016;pii: 0363546516650180. [Epub ahead of print]
8. Sihvonen R, Paavola M, Malmivaara A, et al; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524.
9. Beaufils P, Hulet C, Dhénain M, et al. Clinical practice guidelines for the management of meniscal lesions and isolated lesions of the anterior cruciate ligament of the knee in adults. Orthop Traumatol Surg Res. 2009;95:437-442.
ILLUSTRATIVE CASE
A 48-year-old man presents to your office for follow-up of right knee pain that has been bothering him for the last 12 months. He denies any trauma or inciting incident for the pain. On physical exam, he does not have crepitus, but has medial joint line tenderness of his right knee. A magnetic resonance image (MRI) shows a partial, medial meniscal tear. Do you refer him to Physical Therapy (PT) or Orthopedics for arthroscopy and repair?
The meniscus—cartilage in the knee joint that provides support, stability, and lubrication to the joint during activity—can tear during a traumatic event or because of degeneration over time. Traumatic meniscal tears typically happen to younger adults and teens (<30 years of age) during sports, such as basketball and soccer,whereas degenerative meniscal tears generally present in patients ages 40 to 60 years.2,3 The annual incidence of all meniscal tears is 79 per 100,000.4 While some physicians can diagnose traumatic meniscal tears based on history and physical examination, degenerative meniscal tears are generally more challenging, and typically warrant an MRI for confirmation.3
Meniscal tears can be treated either conservatively, with supportive care and exercise, or with surgery. Unfortunately, there are no national orthopedic guidelines available to help direct care. In one observational study of surgery as treatment for both traumatic and degenerative meniscal tears, 95 out of 117 patients (81.2%) were generally satisfied with this treatment at the 4-year follow-up, with higher satisfaction in the traumatic meniscal tear group than in the degenerative tear group.5
Two systematic reviews of surgery vs nonoperative management or sham therapies found no additional benefit of surgery for meniscal tears in a variety of patients with and without osteoarthritis.6,7 However, both studies were of only moderate quality because of the number of patients in the nonoperative groups who ultimately obtained surgery. And neither of the studies directly compared surgery to nonoperative management.6,7
Yet another investigation, a multicenter, randomized, double-blind, sham-controlled study conducted in Finland involving 146 patients, compared sham surgery to arthroscopic partial meniscectomy. Both groups received instruction on performing post-procedure exercises, and both groups had similar and marked improvement in pain and function.8
Clinical practice recommendations devised from a systematic and vast review of the literature recommend that the decision for surgery be based on patient-specific factors such as symptoms, age, mechanism of tear, extent of damage, and occupational/social/activity needs.9
STUDY SUMMARY
Exercise is as good as—and in one way, better than—surgery
The current randomized controlled superiority trial compared exercise therapy to arthroscopic partial meniscectomy in patients ages 35 to 60 years presenting to the orthopedic departments of 2 hospitals in Norway with unilateral knee pain for more than 2 months and an MRI-delineated medial meniscal tear. Patients were included only if they had radiographic evidence of minimal osteoarthritis (Kellgren-Lawrence classification grade ≤2). Exclusion criteria were acute trauma, locked knee, ligament injury, and knee surgery in the same knee within the previous 2 years.
The primary outcomes were change in patient-reported knee function as determined by overall knee injury and osteoarthritis outcome score (KOOS4) after 2 years and thigh muscle strength at 3 months as measured by physiotherapists. The KOOS4 consists of 4 out of the 5 KOOS subscales: pain, other symptoms (swelling, grinding/noise from the joint, ability to straighten and bend), function in sports/recreation, and knee-related quality of life (QOL). This study utilized the average score of each subscale.
Secondary outcomes were the 5 individual KOOS subscales (the 4 previously mentioned plus activities of daily living [ADLs]), as well as thigh muscle strength and lower extremity performance test results.
Methods. Testing personnel were blinded to group allocation; participants wore pants or neoprene sleeves to cover surgical scars. A total of 140 patients were randomized to either 12 weeks (24-36 sessions) of exercise therapy alone or a standardized arthroscopic partial meniscectomy with written and oral encouragement upon discharge to perform simple exercises at home 2 to4 times daily (to regain range of motion and reduce swelling).
Results. The overall mean improvement in KOOS4 score from baseline at 2 years was similar between the exercise group and the meniscectomy group (25.3 points vs 24.4 points, respectively; mean difference [MD], 0.9; 95% confidence interval [CI], -4.3 to 6.1; P=.72). Additionally, muscle strength (measured as peak torque flexion and extension and total work flexion and extension) at both 3 and 12 months showed significant objective improvements favoring exercise therapy.
Secondary outcomes comparing the change from baseline of KOOS subscale scores showed 4 of the 5 having non-significant differences (pain, ADL, sports/recreation, and QOL). Only the symptoms subscale had a significant difference favoring exercise therapy (MD, 5.3 points; 95% CI, 0.5 to 10.2; P=.03), which was likely clinically insignificant when using a grading scale of 0 to 100.
Of those patients allocated to exercise therapy alone, 19% crossed over and underwent surgery during the 2 years of the study.
WHAT'S NEW
Head-to-head comparison adds evidence to previous findings
This is the first trial to directly compare exercise therapy to surgery in patients with meniscal tears. Interestingly, exercise therapy was as effective after a 2-year follow-up period and was superior in the short term for thigh muscle strength.1 The results of this study build on those from the smaller study conducted in Finland mentioned earlier.8 In that study, both groups received instruction for the same graduated exercise plan. The researchers found that exercise was comparable to surgery for meniscal tears in patients with no osteoarthritis.
CAVEATS
Results may not translate to those with more severe osteoarthritis
This trial included patients with only mild to no osteoarthritis in addition to their meniscal tear.1 It is unclear if the results would be maintained in patients with more advanced disease. Additionally, 19% of patients crossed over from the exercise group to the surgery group, even though muscle strength improved. Therefore, education about the risks of surgery and the potential lack of benefit is important.
CHALLENGES TO IMPLEMENTATION
The cost and effort of physical therapy may be a deterrent
The cost of PT can be a barrier for some patients who have adequate insurance coverage for surgery, but inadequate coverage for PT. Additionally, exercise therapy requires significant and ongoing amounts of time and effort, which may be a deterrent for patients with busy lifestyles. Patients and physicians may view surgery as an “easier” fix.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 48-year-old man presents to your office for follow-up of right knee pain that has been bothering him for the last 12 months. He denies any trauma or inciting incident for the pain. On physical exam, he does not have crepitus, but has medial joint line tenderness of his right knee. A magnetic resonance image (MRI) shows a partial, medial meniscal tear. Do you refer him to Physical Therapy (PT) or Orthopedics for arthroscopy and repair?
The meniscus—cartilage in the knee joint that provides support, stability, and lubrication to the joint during activity—can tear during a traumatic event or because of degeneration over time. Traumatic meniscal tears typically happen to younger adults and teens (<30 years of age) during sports, such as basketball and soccer,whereas degenerative meniscal tears generally present in patients ages 40 to 60 years.2,3 The annual incidence of all meniscal tears is 79 per 100,000.4 While some physicians can diagnose traumatic meniscal tears based on history and physical examination, degenerative meniscal tears are generally more challenging, and typically warrant an MRI for confirmation.3
Meniscal tears can be treated either conservatively, with supportive care and exercise, or with surgery. Unfortunately, there are no national orthopedic guidelines available to help direct care. In one observational study of surgery as treatment for both traumatic and degenerative meniscal tears, 95 out of 117 patients (81.2%) were generally satisfied with this treatment at the 4-year follow-up, with higher satisfaction in the traumatic meniscal tear group than in the degenerative tear group.5
Two systematic reviews of surgery vs nonoperative management or sham therapies found no additional benefit of surgery for meniscal tears in a variety of patients with and without osteoarthritis.6,7 However, both studies were of only moderate quality because of the number of patients in the nonoperative groups who ultimately obtained surgery. And neither of the studies directly compared surgery to nonoperative management.6,7
Yet another investigation, a multicenter, randomized, double-blind, sham-controlled study conducted in Finland involving 146 patients, compared sham surgery to arthroscopic partial meniscectomy. Both groups received instruction on performing post-procedure exercises, and both groups had similar and marked improvement in pain and function.8
Clinical practice recommendations devised from a systematic and vast review of the literature recommend that the decision for surgery be based on patient-specific factors such as symptoms, age, mechanism of tear, extent of damage, and occupational/social/activity needs.9
STUDY SUMMARY
Exercise is as good as—and in one way, better than—surgery
The current randomized controlled superiority trial compared exercise therapy to arthroscopic partial meniscectomy in patients ages 35 to 60 years presenting to the orthopedic departments of 2 hospitals in Norway with unilateral knee pain for more than 2 months and an MRI-delineated medial meniscal tear. Patients were included only if they had radiographic evidence of minimal osteoarthritis (Kellgren-Lawrence classification grade ≤2). Exclusion criteria were acute trauma, locked knee, ligament injury, and knee surgery in the same knee within the previous 2 years.
The primary outcomes were change in patient-reported knee function as determined by overall knee injury and osteoarthritis outcome score (KOOS4) after 2 years and thigh muscle strength at 3 months as measured by physiotherapists. The KOOS4 consists of 4 out of the 5 KOOS subscales: pain, other symptoms (swelling, grinding/noise from the joint, ability to straighten and bend), function in sports/recreation, and knee-related quality of life (QOL). This study utilized the average score of each subscale.
Secondary outcomes were the 5 individual KOOS subscales (the 4 previously mentioned plus activities of daily living [ADLs]), as well as thigh muscle strength and lower extremity performance test results.
Methods. Testing personnel were blinded to group allocation; participants wore pants or neoprene sleeves to cover surgical scars. A total of 140 patients were randomized to either 12 weeks (24-36 sessions) of exercise therapy alone or a standardized arthroscopic partial meniscectomy with written and oral encouragement upon discharge to perform simple exercises at home 2 to4 times daily (to regain range of motion and reduce swelling).
Results. The overall mean improvement in KOOS4 score from baseline at 2 years was similar between the exercise group and the meniscectomy group (25.3 points vs 24.4 points, respectively; mean difference [MD], 0.9; 95% confidence interval [CI], -4.3 to 6.1; P=.72). Additionally, muscle strength (measured as peak torque flexion and extension and total work flexion and extension) at both 3 and 12 months showed significant objective improvements favoring exercise therapy.
Secondary outcomes comparing the change from baseline of KOOS subscale scores showed 4 of the 5 having non-significant differences (pain, ADL, sports/recreation, and QOL). Only the symptoms subscale had a significant difference favoring exercise therapy (MD, 5.3 points; 95% CI, 0.5 to 10.2; P=.03), which was likely clinically insignificant when using a grading scale of 0 to 100.
Of those patients allocated to exercise therapy alone, 19% crossed over and underwent surgery during the 2 years of the study.
WHAT'S NEW
Head-to-head comparison adds evidence to previous findings
This is the first trial to directly compare exercise therapy to surgery in patients with meniscal tears. Interestingly, exercise therapy was as effective after a 2-year follow-up period and was superior in the short term for thigh muscle strength.1 The results of this study build on those from the smaller study conducted in Finland mentioned earlier.8 In that study, both groups received instruction for the same graduated exercise plan. The researchers found that exercise was comparable to surgery for meniscal tears in patients with no osteoarthritis.
CAVEATS
Results may not translate to those with more severe osteoarthritis
This trial included patients with only mild to no osteoarthritis in addition to their meniscal tear.1 It is unclear if the results would be maintained in patients with more advanced disease. Additionally, 19% of patients crossed over from the exercise group to the surgery group, even though muscle strength improved. Therefore, education about the risks of surgery and the potential lack of benefit is important.
CHALLENGES TO IMPLEMENTATION
The cost and effort of physical therapy may be a deterrent
The cost of PT can be a barrier for some patients who have adequate insurance coverage for surgery, but inadequate coverage for PT. Additionally, exercise therapy requires significant and ongoing amounts of time and effort, which may be a deterrent for patients with busy lifestyles. Patients and physicians may view surgery as an “easier” fix.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.
2. Beals CT, Magnussen RA, Graham WC, et al. The prevalence of meniscal pathology in asymptomatic athletes. Sports Med. 2016;46:1517-1524.
3. Maffulli N, Longo UG, Campi S, et al. Meniscal tears. Open Access J Sports Med. 2010;1:45-54.
4. Peat G, Bergknut C, Frobell R, et al. Population-wide incidence estimates for soft tissue knee injuries presenting to healthcare in southern Sweden: data from the Skåne Healthcare Register. Arthritis Res Ther. 2014;16:R162.
5. Ghislain NA, Wei JN, Li YG. Study of the clinical outcome between traumatic and degenerative (non-traumatic) meniscal tears after arthroscopic surgery: a 4-years follow-up study. J Clin Diagn Res. 2016;10:RC01-RC04.
6. Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014;186:1057-1064.
7. Monk P, Garfjeld Roberts P, Palmer AJR, et al. The urgent need for evidence in arthroscopic meniscal surgery: a systematic review of the evidence for operative management of meniscal tears. Am J Sports Med. 2016;pii: 0363546516650180. [Epub ahead of print]
8. Sihvonen R, Paavola M, Malmivaara A, et al; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524.
9. Beaufils P, Hulet C, Dhénain M, et al. Clinical practice guidelines for the management of meniscal lesions and isolated lesions of the anterior cruciate ligament of the knee in adults. Orthop Traumatol Surg Res. 2009;95:437-442.
1. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.
2. Beals CT, Magnussen RA, Graham WC, et al. The prevalence of meniscal pathology in asymptomatic athletes. Sports Med. 2016;46:1517-1524.
3. Maffulli N, Longo UG, Campi S, et al. Meniscal tears. Open Access J Sports Med. 2010;1:45-54.
4. Peat G, Bergknut C, Frobell R, et al. Population-wide incidence estimates for soft tissue knee injuries presenting to healthcare in southern Sweden: data from the Skåne Healthcare Register. Arthritis Res Ther. 2014;16:R162.
5. Ghislain NA, Wei JN, Li YG. Study of the clinical outcome between traumatic and degenerative (non-traumatic) meniscal tears after arthroscopic surgery: a 4-years follow-up study. J Clin Diagn Res. 2016;10:RC01-RC04.
6. Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. CMAJ. 2014;186:1057-1064.
7. Monk P, Garfjeld Roberts P, Palmer AJR, et al. The urgent need for evidence in arthroscopic meniscal surgery: a systematic review of the evidence for operative management of meniscal tears. Am J Sports Med. 2016;pii: 0363546516650180. [Epub ahead of print]
8. Sihvonen R, Paavola M, Malmivaara A, et al; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515-2524.
9. Beaufils P, Hulet C, Dhénain M, et al. Clinical practice guidelines for the management of meniscal lesions and isolated lesions of the anterior cruciate ligament of the knee in adults. Orthop Traumatol Surg Res. 2009;95:437-442.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Recommend supervised exercise therapy to your patients with a medial, degenerative meniscal tear and a minimal history of osteoarthritis because it is as effective as partial meniscectomy, entails little risk, and has the added benefit of increasing muscle strength.1
STRENGTH OF RECOMMENDATION
B: Based on a single, good quality, randomized controlled trial.
Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740.