User login
Long-term durability low for nonmesh vaginal prolapse repair
SAN ANTONIO – At 5-year follow-up, outcomes were slightly better on most measures for transvaginal uterosacral ligament suspension versus transvaginal sacrospinous ligament fixation for apical prolapse, but the differences were not statistically significant, according to the first randomized trial to compare the two techniques.
Quality of life improvements were durable, but the overall 5-year success rate – defined as the absence of descent of the vaginal apex more than one-third into the vagina; anterior or posterior vaginal wall descent beyond the hymen; bothersome vaginal bulge symptoms; and further treatment for prolapse – was 39% in the 109 women randomized to bilateral uterosacral ligament suspension (ULS) and 30% in the 109 women randomized to unilateral sacrospinous ligament fixation (SSLF).
But there was a notable finding in the study. If women failed to meet all the requirements for success at any one visit, they were classified as surgical failures. However, many who missed the mark at one visit met all the requirements for success on other visits, including their last follow-up.
“We don’t think as surgeons that a bulge comes and goes on a yearly basis, but people actually moved in and out of success and failure over time, and that’s new,” Dr. Jelovsek said. “We just don’t understand the dynamic variables of anatomic prolapse, because no one’s looked at it. The assumption of ‘once a failure, always a failure’ may underestimate success rates.”
Nonetheless, using that approach in the study, the investigators found that the anatomic success was 54% in the ULS and 38% in the SSLF groups at 5 years, and 37% of women in the ULS group reported bothersome vaginal bulge symptoms, versus 42% of women with SSLF. A total of 12% of women with ULS and 8% of women with SSLF had undergone POP retreatment at 5 years, either by pessary or secondary surgery but, again, the differences were not statistically significant.
Of the 145 anatomic failures in the study, 41% were stage 3 or 4.
Quality of life improvements, assessed annually by phone, “were maintained over 5 years despite progressive increases in surgical failure rates over time,” with about a 70-point improvement in the Pelvic Organ Prolapse Distress Inventory and similar gains in other measures in both groups, Dr. Jelovsek reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
There were no between-group differences in suture exposure (about 25% in both groups) or sling erosion (about 3%) at 5 years.
There was a difference in granulation tissue: 28.9% with ULS and 18.8% with SSLF (odds ratio with ULS, 1.9; 95% confidence interval 1-3.7). The majority of adverse events occurred within 2 years of surgery.
Early pelvic floor muscle training made no difference in outcomes for the women randomized to it.
The women in the study had stage 2-4 prolapse at baseline. In addition to vaginal suspension surgery, they had vaginal hysterectomies if there was uterine prolapse, and all the women had concomitant retropubic midurethral sling surgery for stress incontinence.
At 2 years, composite success rates were about 60% in both groups (JAMA. 2014 Mar 12;311[10]:1023-34).
The study didn’t identify risk factors for failure, but they would be helpful to know, Dr. Jelovsek said. High-risk women might benefit from a more durable mesh repair. For now at least, “most women say the risk” of pain and other serious mesh complications “completely outweighs the bulge symptoms,” he said.
The trial, an extension of OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss), was conducted at nine U.S. centers in the Pelvic Floor Disorders Network, which is funded by the National Institutes of Health. Dr. Jelovsek reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – At 5-year follow-up, outcomes were slightly better on most measures for transvaginal uterosacral ligament suspension versus transvaginal sacrospinous ligament fixation for apical prolapse, but the differences were not statistically significant, according to the first randomized trial to compare the two techniques.
Quality of life improvements were durable, but the overall 5-year success rate – defined as the absence of descent of the vaginal apex more than one-third into the vagina; anterior or posterior vaginal wall descent beyond the hymen; bothersome vaginal bulge symptoms; and further treatment for prolapse – was 39% in the 109 women randomized to bilateral uterosacral ligament suspension (ULS) and 30% in the 109 women randomized to unilateral sacrospinous ligament fixation (SSLF).
But there was a notable finding in the study. If women failed to meet all the requirements for success at any one visit, they were classified as surgical failures. However, many who missed the mark at one visit met all the requirements for success on other visits, including their last follow-up.
“We don’t think as surgeons that a bulge comes and goes on a yearly basis, but people actually moved in and out of success and failure over time, and that’s new,” Dr. Jelovsek said. “We just don’t understand the dynamic variables of anatomic prolapse, because no one’s looked at it. The assumption of ‘once a failure, always a failure’ may underestimate success rates.”
Nonetheless, using that approach in the study, the investigators found that the anatomic success was 54% in the ULS and 38% in the SSLF groups at 5 years, and 37% of women in the ULS group reported bothersome vaginal bulge symptoms, versus 42% of women with SSLF. A total of 12% of women with ULS and 8% of women with SSLF had undergone POP retreatment at 5 years, either by pessary or secondary surgery but, again, the differences were not statistically significant.
Of the 145 anatomic failures in the study, 41% were stage 3 or 4.
Quality of life improvements, assessed annually by phone, “were maintained over 5 years despite progressive increases in surgical failure rates over time,” with about a 70-point improvement in the Pelvic Organ Prolapse Distress Inventory and similar gains in other measures in both groups, Dr. Jelovsek reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
There were no between-group differences in suture exposure (about 25% in both groups) or sling erosion (about 3%) at 5 years.
There was a difference in granulation tissue: 28.9% with ULS and 18.8% with SSLF (odds ratio with ULS, 1.9; 95% confidence interval 1-3.7). The majority of adverse events occurred within 2 years of surgery.
Early pelvic floor muscle training made no difference in outcomes for the women randomized to it.
The women in the study had stage 2-4 prolapse at baseline. In addition to vaginal suspension surgery, they had vaginal hysterectomies if there was uterine prolapse, and all the women had concomitant retropubic midurethral sling surgery for stress incontinence.
At 2 years, composite success rates were about 60% in both groups (JAMA. 2014 Mar 12;311[10]:1023-34).
The study didn’t identify risk factors for failure, but they would be helpful to know, Dr. Jelovsek said. High-risk women might benefit from a more durable mesh repair. For now at least, “most women say the risk” of pain and other serious mesh complications “completely outweighs the bulge symptoms,” he said.
The trial, an extension of OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss), was conducted at nine U.S. centers in the Pelvic Floor Disorders Network, which is funded by the National Institutes of Health. Dr. Jelovsek reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – At 5-year follow-up, outcomes were slightly better on most measures for transvaginal uterosacral ligament suspension versus transvaginal sacrospinous ligament fixation for apical prolapse, but the differences were not statistically significant, according to the first randomized trial to compare the two techniques.
Quality of life improvements were durable, but the overall 5-year success rate – defined as the absence of descent of the vaginal apex more than one-third into the vagina; anterior or posterior vaginal wall descent beyond the hymen; bothersome vaginal bulge symptoms; and further treatment for prolapse – was 39% in the 109 women randomized to bilateral uterosacral ligament suspension (ULS) and 30% in the 109 women randomized to unilateral sacrospinous ligament fixation (SSLF).
But there was a notable finding in the study. If women failed to meet all the requirements for success at any one visit, they were classified as surgical failures. However, many who missed the mark at one visit met all the requirements for success on other visits, including their last follow-up.
“We don’t think as surgeons that a bulge comes and goes on a yearly basis, but people actually moved in and out of success and failure over time, and that’s new,” Dr. Jelovsek said. “We just don’t understand the dynamic variables of anatomic prolapse, because no one’s looked at it. The assumption of ‘once a failure, always a failure’ may underestimate success rates.”
Nonetheless, using that approach in the study, the investigators found that the anatomic success was 54% in the ULS and 38% in the SSLF groups at 5 years, and 37% of women in the ULS group reported bothersome vaginal bulge symptoms, versus 42% of women with SSLF. A total of 12% of women with ULS and 8% of women with SSLF had undergone POP retreatment at 5 years, either by pessary or secondary surgery but, again, the differences were not statistically significant.
Of the 145 anatomic failures in the study, 41% were stage 3 or 4.
Quality of life improvements, assessed annually by phone, “were maintained over 5 years despite progressive increases in surgical failure rates over time,” with about a 70-point improvement in the Pelvic Organ Prolapse Distress Inventory and similar gains in other measures in both groups, Dr. Jelovsek reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
There were no between-group differences in suture exposure (about 25% in both groups) or sling erosion (about 3%) at 5 years.
There was a difference in granulation tissue: 28.9% with ULS and 18.8% with SSLF (odds ratio with ULS, 1.9; 95% confidence interval 1-3.7). The majority of adverse events occurred within 2 years of surgery.
Early pelvic floor muscle training made no difference in outcomes for the women randomized to it.
The women in the study had stage 2-4 prolapse at baseline. In addition to vaginal suspension surgery, they had vaginal hysterectomies if there was uterine prolapse, and all the women had concomitant retropubic midurethral sling surgery for stress incontinence.
At 2 years, composite success rates were about 60% in both groups (JAMA. 2014 Mar 12;311[10]:1023-34).
The study didn’t identify risk factors for failure, but they would be helpful to know, Dr. Jelovsek said. High-risk women might benefit from a more durable mesh repair. For now at least, “most women say the risk” of pain and other serious mesh complications “completely outweighs the bulge symptoms,” he said.
The trial, an extension of OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss), was conducted at nine U.S. centers in the Pelvic Floor Disorders Network, which is funded by the National Institutes of Health. Dr. Jelovsek reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
Key clinical point:
Major finding: The overall 5-year success rate was 39% in women randomized to bilateral uterosacral ligament suspension and 30% in women randomized to unilateral sacrospinous ligament fixation.
Data source: The first randomized trial to compare the two commonly used techniques was conducted among 218 women at nine U.S. centers in the Pelvic Floor Disorders Network.
Disclosures: The Pelvic Floor Disorders Network is funded by the National Institutes of Health. The lead investigator reported having no relevant financial disclosures.
Chronic Obstructive Pulmonary Disease: Epidemiology, Clinical Presentation, and Evaluation
From the Department of Preventive Medicine and Environmental Health, University of Kentucky College of Public Health, Lexington, KY.
Abstract
- Objective: To review the classification, epidemiology, clinical presentation, and evaluation of patients with chronic obstructive pulmonary disease (COPD).
- Methods: Review of the literature.
- Results: While smoking remains the most important risk factor for COPD in much of the developed world, other risk factors, including genetic factors and occupational or environmental exposures, remain important. COPD is the third leading cause of death in the United States. In 2011, 13.7 million adults aged ≥ 25 years were diagnosed with COPD in the United States, and as many as 12 million adults may have COPD that is undiagnosed. In 2010, COPD was responsible for an estimated 10.3 million physician office visits and 1.5 million emergency room visits and was estimated to be the second leading cause of disability-adjusted life years lost among the US population. COPD has primary, secondary, and tertiary prevention strategies. The treatment of COPD has improved in recent years, with new therapies improving patient quality of life.
- Conclusion: COPD remains a serious public health problem that is often underdiagnosed, particularly in its early stages.
Key words: Chronic obstructive pulmonary disease; epidemiology; mortality; smoking; evaluation.
Chronic obstructive pulmonary disease (COPD) is characterized by fixed airflow obstruction with breathing-related symptoms, such as chronic cough, exertional dyspnea, expectoration, and wheeze [1]. These symptoms may occur in conjunction with airway hyperresponsiveness and overlap with other chronic diseases such as asthma. Although COPD is a nonspecific term referring to a set of conditions that develop progressively as a result of a number of different disease processes, it most commonly refers to chronic bronchitis and emphysema. These conditions can be present with or without significant physical impairment. Despite being a very common disease and the third leading cause of death in the United States [2], COPD often is a silent and unrecognized disease, particularly in its early phases [3], and may go untreated.
In this article, we review the classification, epidemiology, clinical presentation, and assessment of patients with COPD.
Definition and Classification
Several different definitions have existed for COPD [4–8]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD), an international collaboration of leading experts in COPD launched in the late 90s with a goal to develop evidence-based recommendations for diagnosis and management of COPD [4], currently defines COPD as “a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases” [4].
Severity of COPD has typically been determined using the degree of lung function impairment, although the wisdom of this approach has been questioned,
Previous definitions of COPD differentiated between chronic bronchitis, asthma, and emphysema, acknowledging that there is frequently overlap between these disease entities [12,13]. The GOLD definition of COPD does not differentiate between chronic bronchitis and emphysema but does note that although asthma and COPD can coexist [4], the largely reversible airflow limitation in asthma merits different therapeutic approaches than the largely irreversible airflow limitation of COPD. The overlap of asthma and COPD in a significant proportion of patients has been the focus of recent work [14].
Epidemiology
Prevalence of COPD
The Behavioral Risk Factor Surveillance System (BRFSS) is an ongoing national random-digit-dialed telephone survey of landline and cellphone households designed to measure behavioral risk factors for the noninstitutionalized adult population of the US [15]. An affirmative response to the following question was defined as physician-diagnosed COPD: “Have you ever been told by a doctor or other health professional that you have chronic obstructive pulmonary disease (COPD), emphysema, or bronchitis?”[16]. Based on 2011 BRFSS survey, 13.7 million adults aged ≥ 25 years were estimated to have a self-reported physician diagnosis of COPD in the United States. The greatest age-adjusted prevalence was found to be clustered along the Ohio River Valley and the southern states [16].
The National Health Interview Survey (NHIS) is an annually conducted, nationally representative survey of the civilian noninstitutionalized population aged 18 years and older. A positive response to one or both of the following questions was used to define COPD: “Have you ever been told by a doctor or other health professional that you had emphysema?” and “During the past 12 months, have you been told by a doctor or other health professional that you had chronic bronchitis?” Age-adjusted COPD prevalence estimates showed significant interyear variation during 1999–2011 period, and were higher in women than in men with the highest prevalence noted in 2001 for both genders [16].
The NHIS estimates for COPD have 2 important limitations. First, these estimates depend on the proper recognition and diagnosis of COPD by both the study participants and their health care providers. This would tend to bias the estimates toward counting fewer cases than actually exist. A bias in the opposite direction, however, is that the term chronic bronchitis in this survey is not precisely defined and could be interpreted as recurrent episodes of acute bronchitis. The finding that “chronic bronchitis” has been reported in 3% to 4% of children supports the presence of this potential bias. The second limitation is that this survey is not able to validate, through physiologic evaluation, whether airway obstruction is present or absent.
These limitations were addressed, in part, by separate nationally representative US surveys that include an examination component, such as the National Health and Nutrition Examination Surveys (NHANES) [17]. An analysis of these data from 1988–1994 and 2007–2012 [18] demonstrated that over 70% of people with evidence of obstruction (based on an FEV1/FVC < 70%) did not have a diagnosis of lung disease (COPD or asthma). In addition, people with evidence of obstruction had a higher risk of mortality whether or not they had diagnosed lung disease [18].
Evaluation of “reversibility” of the airway obstruction requires the administration of bronchodilator, which is not a part of most population-based studies. A subset of participants in the NHANES 2007–2012 survey received a bronchodilator, with a decrease in the estimated prevalence of obstruction from 20.9% to 14.0% [19]. However, a closer look at similar data from a study where all people got a bronchodilator reveal that only a small proportion of people with “reversibility” actually had a significant response to the bronchodilator [20]. In a clinic-based study of subjects with COPD who were aged 69 years and older, 31% demonstrated reversibility, defined as a 15% improvement (from baseline) in FVC and FEV1 following administration of an inhaled bronchodilator [21]. In this study, subjects with more severe obstruction were more likely to have reversibility but would also be more likely to continue to have diminished lung function after maximum improvement was obtained, thus being classified as having “partial reversibility.”
The presence of significant reversibility or partial reversibility in patients with COPD [15] and nonreversible airflow obstruction in asthma patients [22] demonstrates that these diseases can coexist or, alternatively, that there is overlap and imprecision in the ways that these diseases are clinically diagnosed.
Morbidity and Mortality
COPD is a leading cause of disease morbidity and mortality in the United States. The National Center for Health Statistics (NCHS) conducts ongoing surveillance of several health indicators nationally. The NCHS collects physician office visit data using the National Ambulatory Medical Care Survey [23], emergency department visit data and hospital outpatient data using the National Hospital Ambulatory Medical Care Survey [24], hospitalization data using the National Hospital Discharge Survey [25], and death data using the mortality component of the National Vital Statistics System [26]. The following data include the number and rate of COPD events in adults in the United States (using International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM], codes 490, 491, 492 and 496) in these data sets for the most recent years available.
In 2010, COPD was responsible for an estimated 10.3 million physician office visits, with a resulting age-adjusted rate of 494.8 per 10,000 US civilian population [16]. COPD was also responsible for an estimated 1.5 million emergency room visits, with a resulting age-adjusted rate of 72 visits per 10,000 population [16].
COPD is a leading cause of hospitalization in US adults, particularly in older populations. In 2010, almost 699,000 hospitalizations, were attributed to COPD. The age-adjusted rate of COPD hospitalizations (as the primary cause of hospitalization) was 32.2 per 10,000 population in 2010 [16].
Deaths due to or associated with COPD have not significantly changed since 1999. While the age-adjusted death rate among men declined during 1999–2010 (P = 0.001), the rate among women has not changed significantly (P = 0.127). In 2010, 63, 778 men and 69, 797 women aged ≥ 25 years died of COPD [26]. One of the limitations of using the mortality component of the National Vital Statistics System is that it is based on the underlying cause of death as reported on the death certificate; however, many decedents with COPD listed on the death certificate have their death attributed to another cause [27]. The significance of COPD as a contributor to death is undefined when it is present with diseases more likely to be attributed as the underlying cause of death, such as myocardial infarction or lung cancer [28].
COPD is a very costly disease, with estimated direct medical costs in 2004 of $20.9 billion. The estimated indirect costs related to morbidity (loss of work time and productivity) and premature mortality is an additional $16.3 billion, for a total of $37.2 billion [29]. Because COPD may be present but not listed as the underlying cause of death or the primary reason for hospitalization, these cited estimates may underestimate the true cost of COPD. For example, in another analysis of COPD costs in the US, the total for 2010 was estimated at $32.1 billion [30], but could be up to $100 billion [31] depending on the assumptions surrounding comorbid disease.
Another manifestation of the importance of COPD is its effect on the burden of disease in a population determined using disability-adjusted life-years (DALYs). DALYs for a disease or condition are calculated as the sum of the years of life lost due to premature mortality in the population and the years of life lost due to disability [32]. In 2010, COPD was estimated to be the second leading cause of DALYs lost among the North American population [33]. Worldwide, COPD is expected to move up from being the twelfth leading cause of DALYs lost in 1990 to the fifth leading cause in 2020 [34].
Gender Differences
Smoking-related diseases such as COPD and lung cancer are continuing to increase among women in the United States [35,36], while they have plateaued or are decreasing among men [27,37]. Some evidence has emerged that compared with men at a similar level of tobacco smoking, women may be more likely to develop COPD [38] or that the severity of COPD in women may be increased [39–41].
In the Lung Health Study, which evaluated patients with mild COPD, more women than men demonstrated increased airway responsiveness, although this difference was thought to be related to airway caliber rather than gender [42]. Adult women are more likely to both develop and die of asthma than are men [43–45]. In NHANES III, whereas women reported more physician-diagnosed COPD and asthma than men, men and women had similar rates of decreased lung function, and a similar proportion of both men and women with low lung function had undiagnosed lung disease [3]. The current evidence is inadequate to determine whether women who smoke are more likely to develop COPD or have more severe COPD than men, although this question is being studied by various groups.
Risk Factors and Etiology
Smoking is the dominant risk factor for the development and progression of COPD; however, not all smokers develop COPD, and COPD does occur in persons who have never smoked [1], suggesting that other factors are important in the etiology of COPD. Alpha1-antitrypsin deficiency is an important cause of COPD in a very small percentage of cases [46]. Other undefined genetic factors certainly play an important role in COPD development [38]. The role of infections in both the development and progression of COPD is receiving increased attention, including the role of adenoviral infections in emphysema [47–49].
Occupational and environmental exposures to various pollutants (eg, particulate matter, agricultural dusts) are also important factors in the development of COPD [50,51]. Exposure to indoor air pollutants such as smoke from solid biomass fuels is a major risk factor for COPD especially among women and children in low- and middle-income countries [52,53]. Occupational exposure to fumes and dusts remains an important cause for COPD globally [53,54]. Exposure to outdoor air pollution is associated with a risk of development of COPD as well as exacerbation of the existing disease [53,55].
Clinical Presentation
COPD is heterogeneous in its presentation. Based on data from NHANES III, 44% of patients with severe airflow limitation (FEV1 < 50% of predicted) may not report symptoms [3]. Among patients with severe airflow limitation who do report symptoms, the symptoms reported most frequently include wheezing (64%) and shortness of breath (65%).
In recent years, COPD has been increasingly recognized as a systemic illness, with effects on nutritional status, muscle wasting, and depression [56–58]. A large proportion of patients probably have components of chronic bronchitis, asthma, and emphysema occurring together. Although some of this overlap may be related to misdiagnosis, some of it may be a measure of the presence of airflow limitation reversibility, as described above. Better defining individuals in these groups may ultimately help tailor better interventions.
Some of the barriers to COPD diagnosis and subsequent treatment often include insufficient knowledge and awareness about COPD especially among primary care physicians, misdiagnosis of COPD as other respiratory diseases such asthma, as well as patient-related barriers involving lack of awareness of early symptoms of COPD and considering them to be related to aging or smoking [59].
Evaluation
The evaluation of a patient with suspected COPD is oriented toward establishing the correct diagnosis and, once this has occurred, determining the extent of the impairment such that therapy can be appropriately targeted.
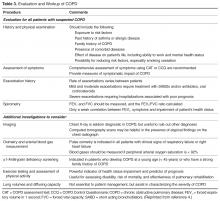
Components in the evaluation of COPD are listed in Table 3. Every patient with suspected COPD should undergo a thorough history and physical examination. The history should pay particular attention to the following: exposure to risk factors; past history of asthma or allergic disease; family history of COPD; presence of comorbid diseases; effect of disease on the patient’s life, including ability to work and mental health status; and possibilities for reducing risk factors, especially smoking cessation [4]. The physical examination is rarely diagnostic in COPD because most physical abnormalities do not occur until the advanced stages of the disease. Physical examination findings in
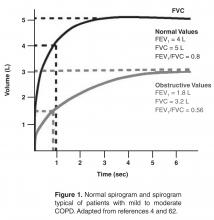
Pulmonary function testing is a critical part of the evaluation of suspected COPD. Whereas most patients with COPD can be managed by a primary care physician, patients with moderate or severe COPD should be evaluated by a specialist [4].
Once the diagnosis of moderate or severe COPD has been established, further testing, including chest radiograph, arterial blood gas determination, screening for α1-antitrypsin deficiency, 6-minute walk testing or exercise oxymetry may be indicated based on the patient’s history and/or clinical findings. Data from computed tomography scans are useful in some advanced cases.
Prognosis of COPD is often influenced by presence of various comorbidities including extrapulmonary, such as osteoporosis, metabolic syndrome, and depression that may be seen as parts of multimorbidity associated with aging [60,61]. Therefore, it is advised to look for comorbidities in COPD patients with any severity of airflow obstruction and treat them accordingly [4].
Therapy for COPD targets reducing risk factors, improving symptoms, and decreasing the risk of exacerbations [10]. Interventions include smoking cessation, vaccinations, decreasing exposures to occupational and environmental pollutants, pulmonary rehabilitation, bronchodilators, and corticosteroids. Select patients with advanced COPD may benefit from other interventions, such as surgical reduction of lung size, lung transplant, the phosphodiesterase inhibitor roflumilast and chronic treatment with antibiotics such as macrolides.
Conclusion
COPD is a common disease that is a leading cause of morbidity and mortality, both in the United States and worldwide. Most cases of COPD are attributable to smoking. Although its incidence among men has plateaued, it continues to increase among women. COPD, particularly in its early stages, is under-diagnosed in the United States. An increased awareness among physicians of the prevalence of mild COPD and the importance of spirometry in diagnosing the disease is important in combating the disease.
Corresponding author: David M. Mannino, MD, Department of Preventive Medicine and Environmental Health, University of Kentucky College of Public Health, 111 Washington Avenue, Lexington, KY 40536, [email protected].
Financial disclosures: Dr. Mannino has received fees from GlaxoSmithKline, Novartis, AstraZeneca, Sunovion, and Boehringer Ingelheim for advisory board services.
1. Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest 1998;113(4 Suppl):235S–41S.
2. National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD; 2016.
3. Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000;160:1683–9.
4. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available at http://goldcopd.org.
5. Vestbo J, Hurd SS, Rodriguez-Roisin R. The 2011 revision of the global strategy for the diagnosis, management and prevention of COPD (GOLD) – why and what? Clin Respir J 2012;6:208–14.
6. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995;152(5 Pt 2):S77–121.
7. Siafakas N, Vermeire P, Pride Na, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995;8:1398–420.
8. Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46.
9. Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008;63:1046–51.
10. Bestall J, Paul E, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6.
11. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
12. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1256–76.
13. Dirksen A, Christensen H, Evald T, et al. Bronchodilator and corticosteroid reversibility in ambulatory patients with airways obstruction. Danish Med Bull 1991;38:486–9.
14. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664–73.
15. Hansen EF, Phanareth K, Laursen LC, et al. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159(4 Pt 1):1267–71.
16. Ford ES, Croft JB, Mannino DM, et al. COPD surveillance—United States, 1999-2011. Chest 2013;144:284–305.
17. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital and health statistics Ser 1, Programs and collection procedures. 1994:1–407.
18. Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed Obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc 2015;12:1788–95.
19. Tilert T, Dillon C, Paulose-Ram R, et al. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007-2010. Respir Res 2013;14:103.
20. Prentice HA, Mannino DM, Caldwell GG, Bush HM. Significant bronchodilator responsiveness and “reversibility” in a population sample. COPD 2010;7:323–30.
21. Chang JT, Moran MB, Cugell DW, Webster JR. COPD in the elderly: a reversible cause of functional impairment. Chest 1995;108:736–40.
22. Ulrik C, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J 1999;14:892–6.
23. Hing E, Hall MJ, Ashman JJ, Xu J. National hospital ambulatory medical care survey: 2007 outpatient department summary. Natl Health Stat Report 2010;28:1–32.
24. Niska R, Bhuiya F, Xu J. National hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Report 2010;26:1–31.
25. Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital and health statistics Series 13, Data from the National Health Survey. 2006(162):1–209.
26. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;61:1–117.
27. Mannino DM, Brown C, Giovino GA. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997;156(3 Pt 1):814–8.
28. Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol 1991;133:795–800.
29. Miller JD, Foster T, Boulanger L, et al. Direct Costs of COPD in the U.S.: An Analysis of Medical Expenditure Panel Survey (MEPS) Data. COPD 2005;2:311–8.
30. Ford ES, Murphy LB, Khavjou O, et al. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest 2015;147:31–45.
31. Mannino DM. Counting costs in COPD: what do the numbers mean? Chest 2015;147:3–5.
32. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223.
33. Murray CJ, Abraham J, Ali MK, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–606.
34. Lopez AD, Murray CC. The global burden of disease 1990–2020. Nat Med 1998;4:1241–3.
35. Cohen SB-Z, Paré PD, Man SFP, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women. Am J Respir Crit Care Med 2007;176:113–20.
36. Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–84.
37. Tanoue LT. Cigarette smoking and women’s respiratory health. Clin Chest Med 2000;21:47–65, viii.
38. Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:2152–8.
39. Carter R, Nicotra B, Huber G. Differing effects of airway obstruction on physical work capacity and ventilation in men and women with COPD. Chest 1994;106:1730–9.
40. Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med 2011;184:414–20.
41. Sørheim I-C, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65:480–5.
42. Kanner RE, Connett JE, Altose MD, Buist AS, Lee WW, Tashkin DP, et al. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med 1994;150:956–61.
43. De Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women: a retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med 2000;162:68–74.
44. Moorman JE, Moorman J, Mannino DM. Increasing US asthma mortality rates: who is really dying? J Asthma 2001;38:65–71.
45. Mannino DM, Homa DM, Pertowski CA, et al. Surveillance for asthma—United States, 1960–1995. MMWR CDC Surveill Summ 1998;47:1–27.
46. Snider GL. Molecular epidemiology: a key to better understanding of chronic obstructive lung disease. Monaldi Arch Chest Dis 1995;50:3–6.
47. Hogg JC. Chronic bronchitis: the role of viruses. Semin Respir Infect 2000;15:32–40.
48. Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 1998;158:998–1001.
49. Hegele RG, Hayashi S, Hogg JC, Paré PD. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory trad infections. Am J Respir Crit Care Med 1995;151:1659–65.
50. Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009;64:6–12.
51. Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respi Dis 1989;140(3 Pt 2):S85–S91.
52. Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011;66:232–9.
53. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–73.
54. Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003;22:462–9.
55. Andersen ZJ, Hvidberg M, Jensen SS, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution. Am J Respir Crit Care Med 2011;183:455–61.
56. Agusti À, Soriano JB. COPD as a systemic disease. COPD 2008;5:133–8.
57. Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med 2008;121:789–96.
58. Cekerevac I, Lazic Z, Petrovic M, Novkovic L. COPD and depression. Eur Respir J 2012;40(Suppl 56).
59. Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med 2011;4:729–39.
60. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013;22:454–75.
61. Barnes PJ. Gold 2017: A new report. Chest 2017;151:245–6.
62. Choo C. Combination therapy options in Stable COPD. US Pharm 2010;35:31–7.
From the Department of Preventive Medicine and Environmental Health, University of Kentucky College of Public Health, Lexington, KY.
Abstract
- Objective: To review the classification, epidemiology, clinical presentation, and evaluation of patients with chronic obstructive pulmonary disease (COPD).
- Methods: Review of the literature.
- Results: While smoking remains the most important risk factor for COPD in much of the developed world, other risk factors, including genetic factors and occupational or environmental exposures, remain important. COPD is the third leading cause of death in the United States. In 2011, 13.7 million adults aged ≥ 25 years were diagnosed with COPD in the United States, and as many as 12 million adults may have COPD that is undiagnosed. In 2010, COPD was responsible for an estimated 10.3 million physician office visits and 1.5 million emergency room visits and was estimated to be the second leading cause of disability-adjusted life years lost among the US population. COPD has primary, secondary, and tertiary prevention strategies. The treatment of COPD has improved in recent years, with new therapies improving patient quality of life.
- Conclusion: COPD remains a serious public health problem that is often underdiagnosed, particularly in its early stages.
Key words: Chronic obstructive pulmonary disease; epidemiology; mortality; smoking; evaluation.
Chronic obstructive pulmonary disease (COPD) is characterized by fixed airflow obstruction with breathing-related symptoms, such as chronic cough, exertional dyspnea, expectoration, and wheeze [1]. These symptoms may occur in conjunction with airway hyperresponsiveness and overlap with other chronic diseases such as asthma. Although COPD is a nonspecific term referring to a set of conditions that develop progressively as a result of a number of different disease processes, it most commonly refers to chronic bronchitis and emphysema. These conditions can be present with or without significant physical impairment. Despite being a very common disease and the third leading cause of death in the United States [2], COPD often is a silent and unrecognized disease, particularly in its early phases [3], and may go untreated.
In this article, we review the classification, epidemiology, clinical presentation, and assessment of patients with COPD.
Definition and Classification
Several different definitions have existed for COPD [4–8]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD), an international collaboration of leading experts in COPD launched in the late 90s with a goal to develop evidence-based recommendations for diagnosis and management of COPD [4], currently defines COPD as “a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases” [4].
Severity of COPD has typically been determined using the degree of lung function impairment, although the wisdom of this approach has been questioned,
Previous definitions of COPD differentiated between chronic bronchitis, asthma, and emphysema, acknowledging that there is frequently overlap between these disease entities [12,13]. The GOLD definition of COPD does not differentiate between chronic bronchitis and emphysema but does note that although asthma and COPD can coexist [4], the largely reversible airflow limitation in asthma merits different therapeutic approaches than the largely irreversible airflow limitation of COPD. The overlap of asthma and COPD in a significant proportion of patients has been the focus of recent work [14].
Epidemiology
Prevalence of COPD
The Behavioral Risk Factor Surveillance System (BRFSS) is an ongoing national random-digit-dialed telephone survey of landline and cellphone households designed to measure behavioral risk factors for the noninstitutionalized adult population of the US [15]. An affirmative response to the following question was defined as physician-diagnosed COPD: “Have you ever been told by a doctor or other health professional that you have chronic obstructive pulmonary disease (COPD), emphysema, or bronchitis?”[16]. Based on 2011 BRFSS survey, 13.7 million adults aged ≥ 25 years were estimated to have a self-reported physician diagnosis of COPD in the United States. The greatest age-adjusted prevalence was found to be clustered along the Ohio River Valley and the southern states [16].
The National Health Interview Survey (NHIS) is an annually conducted, nationally representative survey of the civilian noninstitutionalized population aged 18 years and older. A positive response to one or both of the following questions was used to define COPD: “Have you ever been told by a doctor or other health professional that you had emphysema?” and “During the past 12 months, have you been told by a doctor or other health professional that you had chronic bronchitis?” Age-adjusted COPD prevalence estimates showed significant interyear variation during 1999–2011 period, and were higher in women than in men with the highest prevalence noted in 2001 for both genders [16].
The NHIS estimates for COPD have 2 important limitations. First, these estimates depend on the proper recognition and diagnosis of COPD by both the study participants and their health care providers. This would tend to bias the estimates toward counting fewer cases than actually exist. A bias in the opposite direction, however, is that the term chronic bronchitis in this survey is not precisely defined and could be interpreted as recurrent episodes of acute bronchitis. The finding that “chronic bronchitis” has been reported in 3% to 4% of children supports the presence of this potential bias. The second limitation is that this survey is not able to validate, through physiologic evaluation, whether airway obstruction is present or absent.
These limitations were addressed, in part, by separate nationally representative US surveys that include an examination component, such as the National Health and Nutrition Examination Surveys (NHANES) [17]. An analysis of these data from 1988–1994 and 2007–2012 [18] demonstrated that over 70% of people with evidence of obstruction (based on an FEV1/FVC < 70%) did not have a diagnosis of lung disease (COPD or asthma). In addition, people with evidence of obstruction had a higher risk of mortality whether or not they had diagnosed lung disease [18].
Evaluation of “reversibility” of the airway obstruction requires the administration of bronchodilator, which is not a part of most population-based studies. A subset of participants in the NHANES 2007–2012 survey received a bronchodilator, with a decrease in the estimated prevalence of obstruction from 20.9% to 14.0% [19]. However, a closer look at similar data from a study where all people got a bronchodilator reveal that only a small proportion of people with “reversibility” actually had a significant response to the bronchodilator [20]. In a clinic-based study of subjects with COPD who were aged 69 years and older, 31% demonstrated reversibility, defined as a 15% improvement (from baseline) in FVC and FEV1 following administration of an inhaled bronchodilator [21]. In this study, subjects with more severe obstruction were more likely to have reversibility but would also be more likely to continue to have diminished lung function after maximum improvement was obtained, thus being classified as having “partial reversibility.”
The presence of significant reversibility or partial reversibility in patients with COPD [15] and nonreversible airflow obstruction in asthma patients [22] demonstrates that these diseases can coexist or, alternatively, that there is overlap and imprecision in the ways that these diseases are clinically diagnosed.
Morbidity and Mortality
COPD is a leading cause of disease morbidity and mortality in the United States. The National Center for Health Statistics (NCHS) conducts ongoing surveillance of several health indicators nationally. The NCHS collects physician office visit data using the National Ambulatory Medical Care Survey [23], emergency department visit data and hospital outpatient data using the National Hospital Ambulatory Medical Care Survey [24], hospitalization data using the National Hospital Discharge Survey [25], and death data using the mortality component of the National Vital Statistics System [26]. The following data include the number and rate of COPD events in adults in the United States (using International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM], codes 490, 491, 492 and 496) in these data sets for the most recent years available.
In 2010, COPD was responsible for an estimated 10.3 million physician office visits, with a resulting age-adjusted rate of 494.8 per 10,000 US civilian population [16]. COPD was also responsible for an estimated 1.5 million emergency room visits, with a resulting age-adjusted rate of 72 visits per 10,000 population [16].
COPD is a leading cause of hospitalization in US adults, particularly in older populations. In 2010, almost 699,000 hospitalizations, were attributed to COPD. The age-adjusted rate of COPD hospitalizations (as the primary cause of hospitalization) was 32.2 per 10,000 population in 2010 [16].
Deaths due to or associated with COPD have not significantly changed since 1999. While the age-adjusted death rate among men declined during 1999–2010 (P = 0.001), the rate among women has not changed significantly (P = 0.127). In 2010, 63, 778 men and 69, 797 women aged ≥ 25 years died of COPD [26]. One of the limitations of using the mortality component of the National Vital Statistics System is that it is based on the underlying cause of death as reported on the death certificate; however, many decedents with COPD listed on the death certificate have their death attributed to another cause [27]. The significance of COPD as a contributor to death is undefined when it is present with diseases more likely to be attributed as the underlying cause of death, such as myocardial infarction or lung cancer [28].
COPD is a very costly disease, with estimated direct medical costs in 2004 of $20.9 billion. The estimated indirect costs related to morbidity (loss of work time and productivity) and premature mortality is an additional $16.3 billion, for a total of $37.2 billion [29]. Because COPD may be present but not listed as the underlying cause of death or the primary reason for hospitalization, these cited estimates may underestimate the true cost of COPD. For example, in another analysis of COPD costs in the US, the total for 2010 was estimated at $32.1 billion [30], but could be up to $100 billion [31] depending on the assumptions surrounding comorbid disease.
Another manifestation of the importance of COPD is its effect on the burden of disease in a population determined using disability-adjusted life-years (DALYs). DALYs for a disease or condition are calculated as the sum of the years of life lost due to premature mortality in the population and the years of life lost due to disability [32]. In 2010, COPD was estimated to be the second leading cause of DALYs lost among the North American population [33]. Worldwide, COPD is expected to move up from being the twelfth leading cause of DALYs lost in 1990 to the fifth leading cause in 2020 [34].
Gender Differences
Smoking-related diseases such as COPD and lung cancer are continuing to increase among women in the United States [35,36], while they have plateaued or are decreasing among men [27,37]. Some evidence has emerged that compared with men at a similar level of tobacco smoking, women may be more likely to develop COPD [38] or that the severity of COPD in women may be increased [39–41].
In the Lung Health Study, which evaluated patients with mild COPD, more women than men demonstrated increased airway responsiveness, although this difference was thought to be related to airway caliber rather than gender [42]. Adult women are more likely to both develop and die of asthma than are men [43–45]. In NHANES III, whereas women reported more physician-diagnosed COPD and asthma than men, men and women had similar rates of decreased lung function, and a similar proportion of both men and women with low lung function had undiagnosed lung disease [3]. The current evidence is inadequate to determine whether women who smoke are more likely to develop COPD or have more severe COPD than men, although this question is being studied by various groups.
Risk Factors and Etiology
Smoking is the dominant risk factor for the development and progression of COPD; however, not all smokers develop COPD, and COPD does occur in persons who have never smoked [1], suggesting that other factors are important in the etiology of COPD. Alpha1-antitrypsin deficiency is an important cause of COPD in a very small percentage of cases [46]. Other undefined genetic factors certainly play an important role in COPD development [38]. The role of infections in both the development and progression of COPD is receiving increased attention, including the role of adenoviral infections in emphysema [47–49].
Occupational and environmental exposures to various pollutants (eg, particulate matter, agricultural dusts) are also important factors in the development of COPD [50,51]. Exposure to indoor air pollutants such as smoke from solid biomass fuels is a major risk factor for COPD especially among women and children in low- and middle-income countries [52,53]. Occupational exposure to fumes and dusts remains an important cause for COPD globally [53,54]. Exposure to outdoor air pollution is associated with a risk of development of COPD as well as exacerbation of the existing disease [53,55].
Clinical Presentation
COPD is heterogeneous in its presentation. Based on data from NHANES III, 44% of patients with severe airflow limitation (FEV1 < 50% of predicted) may not report symptoms [3]. Among patients with severe airflow limitation who do report symptoms, the symptoms reported most frequently include wheezing (64%) and shortness of breath (65%).
In recent years, COPD has been increasingly recognized as a systemic illness, with effects on nutritional status, muscle wasting, and depression [56–58]. A large proportion of patients probably have components of chronic bronchitis, asthma, and emphysema occurring together. Although some of this overlap may be related to misdiagnosis, some of it may be a measure of the presence of airflow limitation reversibility, as described above. Better defining individuals in these groups may ultimately help tailor better interventions.
Some of the barriers to COPD diagnosis and subsequent treatment often include insufficient knowledge and awareness about COPD especially among primary care physicians, misdiagnosis of COPD as other respiratory diseases such asthma, as well as patient-related barriers involving lack of awareness of early symptoms of COPD and considering them to be related to aging or smoking [59].
Evaluation
The evaluation of a patient with suspected COPD is oriented toward establishing the correct diagnosis and, once this has occurred, determining the extent of the impairment such that therapy can be appropriately targeted.
Components in the evaluation of COPD are listed in Table 3. Every patient with suspected COPD should undergo a thorough history and physical examination. The history should pay particular attention to the following: exposure to risk factors; past history of asthma or allergic disease; family history of COPD; presence of comorbid diseases; effect of disease on the patient’s life, including ability to work and mental health status; and possibilities for reducing risk factors, especially smoking cessation [4]. The physical examination is rarely diagnostic in COPD because most physical abnormalities do not occur until the advanced stages of the disease. Physical examination findings in
Pulmonary function testing is a critical part of the evaluation of suspected COPD. Whereas most patients with COPD can be managed by a primary care physician, patients with moderate or severe COPD should be evaluated by a specialist [4].
Once the diagnosis of moderate or severe COPD has been established, further testing, including chest radiograph, arterial blood gas determination, screening for α1-antitrypsin deficiency, 6-minute walk testing or exercise oxymetry may be indicated based on the patient’s history and/or clinical findings. Data from computed tomography scans are useful in some advanced cases.
Prognosis of COPD is often influenced by presence of various comorbidities including extrapulmonary, such as osteoporosis, metabolic syndrome, and depression that may be seen as parts of multimorbidity associated with aging [60,61]. Therefore, it is advised to look for comorbidities in COPD patients with any severity of airflow obstruction and treat them accordingly [4].
Therapy for COPD targets reducing risk factors, improving symptoms, and decreasing the risk of exacerbations [10]. Interventions include smoking cessation, vaccinations, decreasing exposures to occupational and environmental pollutants, pulmonary rehabilitation, bronchodilators, and corticosteroids. Select patients with advanced COPD may benefit from other interventions, such as surgical reduction of lung size, lung transplant, the phosphodiesterase inhibitor roflumilast and chronic treatment with antibiotics such as macrolides.
Conclusion
COPD is a common disease that is a leading cause of morbidity and mortality, both in the United States and worldwide. Most cases of COPD are attributable to smoking. Although its incidence among men has plateaued, it continues to increase among women. COPD, particularly in its early stages, is under-diagnosed in the United States. An increased awareness among physicians of the prevalence of mild COPD and the importance of spirometry in diagnosing the disease is important in combating the disease.
Corresponding author: David M. Mannino, MD, Department of Preventive Medicine and Environmental Health, University of Kentucky College of Public Health, 111 Washington Avenue, Lexington, KY 40536, [email protected].
Financial disclosures: Dr. Mannino has received fees from GlaxoSmithKline, Novartis, AstraZeneca, Sunovion, and Boehringer Ingelheim for advisory board services.
From the Department of Preventive Medicine and Environmental Health, University of Kentucky College of Public Health, Lexington, KY.
Abstract
- Objective: To review the classification, epidemiology, clinical presentation, and evaluation of patients with chronic obstructive pulmonary disease (COPD).
- Methods: Review of the literature.
- Results: While smoking remains the most important risk factor for COPD in much of the developed world, other risk factors, including genetic factors and occupational or environmental exposures, remain important. COPD is the third leading cause of death in the United States. In 2011, 13.7 million adults aged ≥ 25 years were diagnosed with COPD in the United States, and as many as 12 million adults may have COPD that is undiagnosed. In 2010, COPD was responsible for an estimated 10.3 million physician office visits and 1.5 million emergency room visits and was estimated to be the second leading cause of disability-adjusted life years lost among the US population. COPD has primary, secondary, and tertiary prevention strategies. The treatment of COPD has improved in recent years, with new therapies improving patient quality of life.
- Conclusion: COPD remains a serious public health problem that is often underdiagnosed, particularly in its early stages.
Key words: Chronic obstructive pulmonary disease; epidemiology; mortality; smoking; evaluation.
Chronic obstructive pulmonary disease (COPD) is characterized by fixed airflow obstruction with breathing-related symptoms, such as chronic cough, exertional dyspnea, expectoration, and wheeze [1]. These symptoms may occur in conjunction with airway hyperresponsiveness and overlap with other chronic diseases such as asthma. Although COPD is a nonspecific term referring to a set of conditions that develop progressively as a result of a number of different disease processes, it most commonly refers to chronic bronchitis and emphysema. These conditions can be present with or without significant physical impairment. Despite being a very common disease and the third leading cause of death in the United States [2], COPD often is a silent and unrecognized disease, particularly in its early phases [3], and may go untreated.
In this article, we review the classification, epidemiology, clinical presentation, and assessment of patients with COPD.
Definition and Classification
Several different definitions have existed for COPD [4–8]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD), an international collaboration of leading experts in COPD launched in the late 90s with a goal to develop evidence-based recommendations for diagnosis and management of COPD [4], currently defines COPD as “a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases” [4].
Severity of COPD has typically been determined using the degree of lung function impairment, although the wisdom of this approach has been questioned,
Previous definitions of COPD differentiated between chronic bronchitis, asthma, and emphysema, acknowledging that there is frequently overlap between these disease entities [12,13]. The GOLD definition of COPD does not differentiate between chronic bronchitis and emphysema but does note that although asthma and COPD can coexist [4], the largely reversible airflow limitation in asthma merits different therapeutic approaches than the largely irreversible airflow limitation of COPD. The overlap of asthma and COPD in a significant proportion of patients has been the focus of recent work [14].
Epidemiology
Prevalence of COPD
The Behavioral Risk Factor Surveillance System (BRFSS) is an ongoing national random-digit-dialed telephone survey of landline and cellphone households designed to measure behavioral risk factors for the noninstitutionalized adult population of the US [15]. An affirmative response to the following question was defined as physician-diagnosed COPD: “Have you ever been told by a doctor or other health professional that you have chronic obstructive pulmonary disease (COPD), emphysema, or bronchitis?”[16]. Based on 2011 BRFSS survey, 13.7 million adults aged ≥ 25 years were estimated to have a self-reported physician diagnosis of COPD in the United States. The greatest age-adjusted prevalence was found to be clustered along the Ohio River Valley and the southern states [16].
The National Health Interview Survey (NHIS) is an annually conducted, nationally representative survey of the civilian noninstitutionalized population aged 18 years and older. A positive response to one or both of the following questions was used to define COPD: “Have you ever been told by a doctor or other health professional that you had emphysema?” and “During the past 12 months, have you been told by a doctor or other health professional that you had chronic bronchitis?” Age-adjusted COPD prevalence estimates showed significant interyear variation during 1999–2011 period, and were higher in women than in men with the highest prevalence noted in 2001 for both genders [16].
The NHIS estimates for COPD have 2 important limitations. First, these estimates depend on the proper recognition and diagnosis of COPD by both the study participants and their health care providers. This would tend to bias the estimates toward counting fewer cases than actually exist. A bias in the opposite direction, however, is that the term chronic bronchitis in this survey is not precisely defined and could be interpreted as recurrent episodes of acute bronchitis. The finding that “chronic bronchitis” has been reported in 3% to 4% of children supports the presence of this potential bias. The second limitation is that this survey is not able to validate, through physiologic evaluation, whether airway obstruction is present or absent.
These limitations were addressed, in part, by separate nationally representative US surveys that include an examination component, such as the National Health and Nutrition Examination Surveys (NHANES) [17]. An analysis of these data from 1988–1994 and 2007–2012 [18] demonstrated that over 70% of people with evidence of obstruction (based on an FEV1/FVC < 70%) did not have a diagnosis of lung disease (COPD or asthma). In addition, people with evidence of obstruction had a higher risk of mortality whether or not they had diagnosed lung disease [18].
Evaluation of “reversibility” of the airway obstruction requires the administration of bronchodilator, which is not a part of most population-based studies. A subset of participants in the NHANES 2007–2012 survey received a bronchodilator, with a decrease in the estimated prevalence of obstruction from 20.9% to 14.0% [19]. However, a closer look at similar data from a study where all people got a bronchodilator reveal that only a small proportion of people with “reversibility” actually had a significant response to the bronchodilator [20]. In a clinic-based study of subjects with COPD who were aged 69 years and older, 31% demonstrated reversibility, defined as a 15% improvement (from baseline) in FVC and FEV1 following administration of an inhaled bronchodilator [21]. In this study, subjects with more severe obstruction were more likely to have reversibility but would also be more likely to continue to have diminished lung function after maximum improvement was obtained, thus being classified as having “partial reversibility.”
The presence of significant reversibility or partial reversibility in patients with COPD [15] and nonreversible airflow obstruction in asthma patients [22] demonstrates that these diseases can coexist or, alternatively, that there is overlap and imprecision in the ways that these diseases are clinically diagnosed.
Morbidity and Mortality
COPD is a leading cause of disease morbidity and mortality in the United States. The National Center for Health Statistics (NCHS) conducts ongoing surveillance of several health indicators nationally. The NCHS collects physician office visit data using the National Ambulatory Medical Care Survey [23], emergency department visit data and hospital outpatient data using the National Hospital Ambulatory Medical Care Survey [24], hospitalization data using the National Hospital Discharge Survey [25], and death data using the mortality component of the National Vital Statistics System [26]. The following data include the number and rate of COPD events in adults in the United States (using International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM], codes 490, 491, 492 and 496) in these data sets for the most recent years available.
In 2010, COPD was responsible for an estimated 10.3 million physician office visits, with a resulting age-adjusted rate of 494.8 per 10,000 US civilian population [16]. COPD was also responsible for an estimated 1.5 million emergency room visits, with a resulting age-adjusted rate of 72 visits per 10,000 population [16].
COPD is a leading cause of hospitalization in US adults, particularly in older populations. In 2010, almost 699,000 hospitalizations, were attributed to COPD. The age-adjusted rate of COPD hospitalizations (as the primary cause of hospitalization) was 32.2 per 10,000 population in 2010 [16].
Deaths due to or associated with COPD have not significantly changed since 1999. While the age-adjusted death rate among men declined during 1999–2010 (P = 0.001), the rate among women has not changed significantly (P = 0.127). In 2010, 63, 778 men and 69, 797 women aged ≥ 25 years died of COPD [26]. One of the limitations of using the mortality component of the National Vital Statistics System is that it is based on the underlying cause of death as reported on the death certificate; however, many decedents with COPD listed on the death certificate have their death attributed to another cause [27]. The significance of COPD as a contributor to death is undefined when it is present with diseases more likely to be attributed as the underlying cause of death, such as myocardial infarction or lung cancer [28].
COPD is a very costly disease, with estimated direct medical costs in 2004 of $20.9 billion. The estimated indirect costs related to morbidity (loss of work time and productivity) and premature mortality is an additional $16.3 billion, for a total of $37.2 billion [29]. Because COPD may be present but not listed as the underlying cause of death or the primary reason for hospitalization, these cited estimates may underestimate the true cost of COPD. For example, in another analysis of COPD costs in the US, the total for 2010 was estimated at $32.1 billion [30], but could be up to $100 billion [31] depending on the assumptions surrounding comorbid disease.
Another manifestation of the importance of COPD is its effect on the burden of disease in a population determined using disability-adjusted life-years (DALYs). DALYs for a disease or condition are calculated as the sum of the years of life lost due to premature mortality in the population and the years of life lost due to disability [32]. In 2010, COPD was estimated to be the second leading cause of DALYs lost among the North American population [33]. Worldwide, COPD is expected to move up from being the twelfth leading cause of DALYs lost in 1990 to the fifth leading cause in 2020 [34].
Gender Differences
Smoking-related diseases such as COPD and lung cancer are continuing to increase among women in the United States [35,36], while they have plateaued or are decreasing among men [27,37]. Some evidence has emerged that compared with men at a similar level of tobacco smoking, women may be more likely to develop COPD [38] or that the severity of COPD in women may be increased [39–41].
In the Lung Health Study, which evaluated patients with mild COPD, more women than men demonstrated increased airway responsiveness, although this difference was thought to be related to airway caliber rather than gender [42]. Adult women are more likely to both develop and die of asthma than are men [43–45]. In NHANES III, whereas women reported more physician-diagnosed COPD and asthma than men, men and women had similar rates of decreased lung function, and a similar proportion of both men and women with low lung function had undiagnosed lung disease [3]. The current evidence is inadequate to determine whether women who smoke are more likely to develop COPD or have more severe COPD than men, although this question is being studied by various groups.
Risk Factors and Etiology
Smoking is the dominant risk factor for the development and progression of COPD; however, not all smokers develop COPD, and COPD does occur in persons who have never smoked [1], suggesting that other factors are important in the etiology of COPD. Alpha1-antitrypsin deficiency is an important cause of COPD in a very small percentage of cases [46]. Other undefined genetic factors certainly play an important role in COPD development [38]. The role of infections in both the development and progression of COPD is receiving increased attention, including the role of adenoviral infections in emphysema [47–49].
Occupational and environmental exposures to various pollutants (eg, particulate matter, agricultural dusts) are also important factors in the development of COPD [50,51]. Exposure to indoor air pollutants such as smoke from solid biomass fuels is a major risk factor for COPD especially among women and children in low- and middle-income countries [52,53]. Occupational exposure to fumes and dusts remains an important cause for COPD globally [53,54]. Exposure to outdoor air pollution is associated with a risk of development of COPD as well as exacerbation of the existing disease [53,55].
Clinical Presentation
COPD is heterogeneous in its presentation. Based on data from NHANES III, 44% of patients with severe airflow limitation (FEV1 < 50% of predicted) may not report symptoms [3]. Among patients with severe airflow limitation who do report symptoms, the symptoms reported most frequently include wheezing (64%) and shortness of breath (65%).
In recent years, COPD has been increasingly recognized as a systemic illness, with effects on nutritional status, muscle wasting, and depression [56–58]. A large proportion of patients probably have components of chronic bronchitis, asthma, and emphysema occurring together. Although some of this overlap may be related to misdiagnosis, some of it may be a measure of the presence of airflow limitation reversibility, as described above. Better defining individuals in these groups may ultimately help tailor better interventions.
Some of the barriers to COPD diagnosis and subsequent treatment often include insufficient knowledge and awareness about COPD especially among primary care physicians, misdiagnosis of COPD as other respiratory diseases such asthma, as well as patient-related barriers involving lack of awareness of early symptoms of COPD and considering them to be related to aging or smoking [59].
Evaluation
The evaluation of a patient with suspected COPD is oriented toward establishing the correct diagnosis and, once this has occurred, determining the extent of the impairment such that therapy can be appropriately targeted.
Components in the evaluation of COPD are listed in Table 3. Every patient with suspected COPD should undergo a thorough history and physical examination. The history should pay particular attention to the following: exposure to risk factors; past history of asthma or allergic disease; family history of COPD; presence of comorbid diseases; effect of disease on the patient’s life, including ability to work and mental health status; and possibilities for reducing risk factors, especially smoking cessation [4]. The physical examination is rarely diagnostic in COPD because most physical abnormalities do not occur until the advanced stages of the disease. Physical examination findings in
Pulmonary function testing is a critical part of the evaluation of suspected COPD. Whereas most patients with COPD can be managed by a primary care physician, patients with moderate or severe COPD should be evaluated by a specialist [4].
Once the diagnosis of moderate or severe COPD has been established, further testing, including chest radiograph, arterial blood gas determination, screening for α1-antitrypsin deficiency, 6-minute walk testing or exercise oxymetry may be indicated based on the patient’s history and/or clinical findings. Data from computed tomography scans are useful in some advanced cases.
Prognosis of COPD is often influenced by presence of various comorbidities including extrapulmonary, such as osteoporosis, metabolic syndrome, and depression that may be seen as parts of multimorbidity associated with aging [60,61]. Therefore, it is advised to look for comorbidities in COPD patients with any severity of airflow obstruction and treat them accordingly [4].
Therapy for COPD targets reducing risk factors, improving symptoms, and decreasing the risk of exacerbations [10]. Interventions include smoking cessation, vaccinations, decreasing exposures to occupational and environmental pollutants, pulmonary rehabilitation, bronchodilators, and corticosteroids. Select patients with advanced COPD may benefit from other interventions, such as surgical reduction of lung size, lung transplant, the phosphodiesterase inhibitor roflumilast and chronic treatment with antibiotics such as macrolides.
Conclusion
COPD is a common disease that is a leading cause of morbidity and mortality, both in the United States and worldwide. Most cases of COPD are attributable to smoking. Although its incidence among men has plateaued, it continues to increase among women. COPD, particularly in its early stages, is under-diagnosed in the United States. An increased awareness among physicians of the prevalence of mild COPD and the importance of spirometry in diagnosing the disease is important in combating the disease.
Corresponding author: David M. Mannino, MD, Department of Preventive Medicine and Environmental Health, University of Kentucky College of Public Health, 111 Washington Avenue, Lexington, KY 40536, [email protected].
Financial disclosures: Dr. Mannino has received fees from GlaxoSmithKline, Novartis, AstraZeneca, Sunovion, and Boehringer Ingelheim for advisory board services.
1. Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest 1998;113(4 Suppl):235S–41S.
2. National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD; 2016.
3. Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000;160:1683–9.
4. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available at http://goldcopd.org.
5. Vestbo J, Hurd SS, Rodriguez-Roisin R. The 2011 revision of the global strategy for the diagnosis, management and prevention of COPD (GOLD) – why and what? Clin Respir J 2012;6:208–14.
6. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995;152(5 Pt 2):S77–121.
7. Siafakas N, Vermeire P, Pride Na, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995;8:1398–420.
8. Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46.
9. Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008;63:1046–51.
10. Bestall J, Paul E, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6.
11. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
12. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1256–76.
13. Dirksen A, Christensen H, Evald T, et al. Bronchodilator and corticosteroid reversibility in ambulatory patients with airways obstruction. Danish Med Bull 1991;38:486–9.
14. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664–73.
15. Hansen EF, Phanareth K, Laursen LC, et al. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159(4 Pt 1):1267–71.
16. Ford ES, Croft JB, Mannino DM, et al. COPD surveillance—United States, 1999-2011. Chest 2013;144:284–305.
17. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital and health statistics Ser 1, Programs and collection procedures. 1994:1–407.
18. Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed Obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc 2015;12:1788–95.
19. Tilert T, Dillon C, Paulose-Ram R, et al. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007-2010. Respir Res 2013;14:103.
20. Prentice HA, Mannino DM, Caldwell GG, Bush HM. Significant bronchodilator responsiveness and “reversibility” in a population sample. COPD 2010;7:323–30.
21. Chang JT, Moran MB, Cugell DW, Webster JR. COPD in the elderly: a reversible cause of functional impairment. Chest 1995;108:736–40.
22. Ulrik C, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J 1999;14:892–6.
23. Hing E, Hall MJ, Ashman JJ, Xu J. National hospital ambulatory medical care survey: 2007 outpatient department summary. Natl Health Stat Report 2010;28:1–32.
24. Niska R, Bhuiya F, Xu J. National hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Report 2010;26:1–31.
25. Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital and health statistics Series 13, Data from the National Health Survey. 2006(162):1–209.
26. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;61:1–117.
27. Mannino DM, Brown C, Giovino GA. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997;156(3 Pt 1):814–8.
28. Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol 1991;133:795–800.
29. Miller JD, Foster T, Boulanger L, et al. Direct Costs of COPD in the U.S.: An Analysis of Medical Expenditure Panel Survey (MEPS) Data. COPD 2005;2:311–8.
30. Ford ES, Murphy LB, Khavjou O, et al. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest 2015;147:31–45.
31. Mannino DM. Counting costs in COPD: what do the numbers mean? Chest 2015;147:3–5.
32. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223.
33. Murray CJ, Abraham J, Ali MK, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–606.
34. Lopez AD, Murray CC. The global burden of disease 1990–2020. Nat Med 1998;4:1241–3.
35. Cohen SB-Z, Paré PD, Man SFP, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women. Am J Respir Crit Care Med 2007;176:113–20.
36. Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–84.
37. Tanoue LT. Cigarette smoking and women’s respiratory health. Clin Chest Med 2000;21:47–65, viii.
38. Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:2152–8.
39. Carter R, Nicotra B, Huber G. Differing effects of airway obstruction on physical work capacity and ventilation in men and women with COPD. Chest 1994;106:1730–9.
40. Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med 2011;184:414–20.
41. Sørheim I-C, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65:480–5.
42. Kanner RE, Connett JE, Altose MD, Buist AS, Lee WW, Tashkin DP, et al. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med 1994;150:956–61.
43. De Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women: a retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med 2000;162:68–74.
44. Moorman JE, Moorman J, Mannino DM. Increasing US asthma mortality rates: who is really dying? J Asthma 2001;38:65–71.
45. Mannino DM, Homa DM, Pertowski CA, et al. Surveillance for asthma—United States, 1960–1995. MMWR CDC Surveill Summ 1998;47:1–27.
46. Snider GL. Molecular epidemiology: a key to better understanding of chronic obstructive lung disease. Monaldi Arch Chest Dis 1995;50:3–6.
47. Hogg JC. Chronic bronchitis: the role of viruses. Semin Respir Infect 2000;15:32–40.
48. Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 1998;158:998–1001.
49. Hegele RG, Hayashi S, Hogg JC, Paré PD. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory trad infections. Am J Respir Crit Care Med 1995;151:1659–65.
50. Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009;64:6–12.
51. Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respi Dis 1989;140(3 Pt 2):S85–S91.
52. Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011;66:232–9.
53. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–73.
54. Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003;22:462–9.
55. Andersen ZJ, Hvidberg M, Jensen SS, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution. Am J Respir Crit Care Med 2011;183:455–61.
56. Agusti À, Soriano JB. COPD as a systemic disease. COPD 2008;5:133–8.
57. Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med 2008;121:789–96.
58. Cekerevac I, Lazic Z, Petrovic M, Novkovic L. COPD and depression. Eur Respir J 2012;40(Suppl 56).
59. Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med 2011;4:729–39.
60. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013;22:454–75.
61. Barnes PJ. Gold 2017: A new report. Chest 2017;151:245–6.
62. Choo C. Combination therapy options in Stable COPD. US Pharm 2010;35:31–7.
1. Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest 1998;113(4 Suppl):235S–41S.
2. National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD; 2016.
3. Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2000;160:1683–9.
4. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available at http://goldcopd.org.
5. Vestbo J, Hurd SS, Rodriguez-Roisin R. The 2011 revision of the global strategy for the diagnosis, management and prevention of COPD (GOLD) – why and what? Clin Respir J 2012;6:208–14.
6. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995;152(5 Pt 2):S77–121.
7. Siafakas N, Vermeire P, Pride Na, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995;8:1398–420.
8. Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46.
9. Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008;63:1046–51.
10. Bestall J, Paul E, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6.
11. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
12. Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1256–76.
13. Dirksen A, Christensen H, Evald T, et al. Bronchodilator and corticosteroid reversibility in ambulatory patients with airways obstruction. Danish Med Bull 1991;38:486–9.
14. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664–73.
15. Hansen EF, Phanareth K, Laursen LC, et al. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159(4 Pt 1):1267–71.
16. Ford ES, Croft JB, Mannino DM, et al. COPD surveillance—United States, 1999-2011. Chest 2013;144:284–305.
17. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital and health statistics Ser 1, Programs and collection procedures. 1994:1–407.
18. Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed Obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc 2015;12:1788–95.
19. Tilert T, Dillon C, Paulose-Ram R, et al. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007-2010. Respir Res 2013;14:103.
20. Prentice HA, Mannino DM, Caldwell GG, Bush HM. Significant bronchodilator responsiveness and “reversibility” in a population sample. COPD 2010;7:323–30.
21. Chang JT, Moran MB, Cugell DW, Webster JR. COPD in the elderly: a reversible cause of functional impairment. Chest 1995;108:736–40.
22. Ulrik C, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J 1999;14:892–6.
23. Hing E, Hall MJ, Ashman JJ, Xu J. National hospital ambulatory medical care survey: 2007 outpatient department summary. Natl Health Stat Report 2010;28:1–32.
24. Niska R, Bhuiya F, Xu J. National hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Report 2010;26:1–31.
25. Kozak LJ, DeFrances CJ, Hall MJ. National hospital discharge survey: 2004 annual summary with detailed diagnosis and procedure data. Vital and health statistics Series 13, Data from the National Health Survey. 2006(162):1–209.
26. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;61:1–117.
27. Mannino DM, Brown C, Giovino GA. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997;156(3 Pt 1):814–8.
28. Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol 1991;133:795–800.
29. Miller JD, Foster T, Boulanger L, et al. Direct Costs of COPD in the U.S.: An Analysis of Medical Expenditure Panel Survey (MEPS) Data. COPD 2005;2:311–8.
30. Ford ES, Murphy LB, Khavjou O, et al. Total and state-specific medical and absenteeism costs of COPD among adults aged >/= 18 years in the United States for 2010 and projections through 2020. Chest 2015;147:31–45.
31. Mannino DM. Counting costs in COPD: what do the numbers mean? Chest 2015;147:3–5.
32. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223.
33. Murray CJ, Abraham J, Ali MK, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–606.
34. Lopez AD, Murray CC. The global burden of disease 1990–2020. Nat Med 1998;4:1241–3.
35. Cohen SB-Z, Paré PD, Man SFP, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women. Am J Respir Crit Care Med 2007;176:113–20.
36. Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–84.
37. Tanoue LT. Cigarette smoking and women’s respiratory health. Clin Chest Med 2000;21:47–65, viii.
38. Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:2152–8.
39. Carter R, Nicotra B, Huber G. Differing effects of airway obstruction on physical work capacity and ventilation in men and women with COPD. Chest 1994;106:1730–9.
40. Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med 2011;184:414–20.
41. Sørheim I-C, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65:480–5.
42. Kanner RE, Connett JE, Altose MD, Buist AS, Lee WW, Tashkin DP, et al. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med 1994;150:956–61.
43. De Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women: a retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med 2000;162:68–74.
44. Moorman JE, Moorman J, Mannino DM. Increasing US asthma mortality rates: who is really dying? J Asthma 2001;38:65–71.
45. Mannino DM, Homa DM, Pertowski CA, et al. Surveillance for asthma—United States, 1960–1995. MMWR CDC Surveill Summ 1998;47:1–27.
46. Snider GL. Molecular epidemiology: a key to better understanding of chronic obstructive lung disease. Monaldi Arch Chest Dis 1995;50:3–6.
47. Hogg JC. Chronic bronchitis: the role of viruses. Semin Respir Infect 2000;15:32–40.
48. Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 1998;158:998–1001.
49. Hegele RG, Hayashi S, Hogg JC, Paré PD. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory trad infections. Am J Respir Crit Care Med 1995;151:1659–65.
50. Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax 2009;64:6–12.
51. Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respi Dis 1989;140(3 Pt 2):S85–S91.
52. Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011;66:232–9.
53. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–73.
54. Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J 2003;22:462–9.
55. Andersen ZJ, Hvidberg M, Jensen SS, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution. Am J Respir Crit Care Med 2011;183:455–61.
56. Agusti À, Soriano JB. COPD as a systemic disease. COPD 2008;5:133–8.
57. Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med 2008;121:789–96.
58. Cekerevac I, Lazic Z, Petrovic M, Novkovic L. COPD and depression. Eur Respir J 2012;40(Suppl 56).
59. Fromer L. Diagnosing and treating COPD: understanding the challenges and finding solutions. Int J Gen Med 2011;4:729–39.
60. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013;22:454–75.
61. Barnes PJ. Gold 2017: A new report. Chest 2017;151:245–6.
62. Choo C. Combination therapy options in Stable COPD. US Pharm 2010;35:31–7.
New antibody shows potential as prognostic marker in early rheumatoid arthritis
Individuals with rheumatoid arthritis who have antifibrillar collagen type II antibodies appear to have a unique disease phenotype characterized by acute but transient inflammation around the time of diagnosis, as well as a favorable prognosis, according to findings from a retrospective cohort study.
The antifibrillar collagen type II (anti-CII) phenotype also differed from the phenotype of individuals with anti–cyclic citrullinated peptide 2 (anti-CCP2) antibodies who typically exhibit inflammatory markers and higher disease activity later in the disease course, reported Vivek Anand Manivel of Uppsala (Sweden) University and his colleagues (Ann Rheum Dis. 2017 Mar 23. doi: 10.1136/annrheumdis-2016-210873).
Overall, the investigators detected anti-CII positivity in 6.6% and anti-CCP2 in 57.9%, including only anti-CII antibodies in 2.6%, only anti-CCP2 in 54%, double positivity in 3.9%, and double negativity in 39.4%. A control group of 960 patients from the Swedish general population who were matched for age, locality, and sex were positive for anti-CII in 1.6% and anti-CCP2 in 1.7%.
Significant differences were discovered between anti-CII positive and negative groups for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC), and Disease Activity Score encompassing 28 joints (DAS28). Anti-CII seropositive patients had higher CRP at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months.
“Anti-CCP2 [seropositivity] on the other hand was associated with higher disease activity later during the 5-year follow-up, and with CRP and ESR during the full period,” the investigators wrote.
The same respective phenotypic patterns occurred when the comparisons were limited to patients with anti-CII positivity alone or anti-CCP2 positivity alone, the investigators noted. However, “patients in the anti-CII and anti-CCP2 double-positive group mainly followed the anti-CII pattern, with early but not late increased values for CRP, ESR, DAS28, and DAS28CRP, but with a mixed pattern for SJC.”
In comparisons where baseline clinical and laboratory measures were associated with anti-CII or anti-CCP2 positivity, anti-CII was more often associated with favorable late changes in CRP, ESR, SJC, TJC, DAS28, DAS28CRP, Health Assessment Questionnaire, and pain and global measurements on a visual analog scale when compared with antibody double-negative patients. There were fewer changes in clinical and laboratory measures associated with anti-CCP2 positivity. These significant changes associated with anti-CII positivity were always larger improvements than were seen for antibody double-negative patients, whereas the significant changes observed with anti-CCP2 patients were always smaller improvements than those for antibody double-negative patients.
Moderate or good European League Against Rheumatism responses were significantly associated with anti-CII positivity at 12, 24, 36, and 60 months, whereas they were negatively associated with anti-CCP2 positivity at 36 and 60 months.
Overall, the findings of the study show that since “anti-CII-positive patients with RA have an acute onset, but favorable prognosis” and anti-CCP2 is associated with poor prognosis, “the combined analysis of anti-CII and ACPA/anti-CCP2 may be a new two-dimensional tool for predicting the prognosis and choosing therapy in newly diagnosed patients with RA,” the authors suggested.
The study was funded by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. Dr. Manivel and his coauthors reported no relevant financial disclosures.
Individuals with rheumatoid arthritis who have antifibrillar collagen type II antibodies appear to have a unique disease phenotype characterized by acute but transient inflammation around the time of diagnosis, as well as a favorable prognosis, according to findings from a retrospective cohort study.
The antifibrillar collagen type II (anti-CII) phenotype also differed from the phenotype of individuals with anti–cyclic citrullinated peptide 2 (anti-CCP2) antibodies who typically exhibit inflammatory markers and higher disease activity later in the disease course, reported Vivek Anand Manivel of Uppsala (Sweden) University and his colleagues (Ann Rheum Dis. 2017 Mar 23. doi: 10.1136/annrheumdis-2016-210873).
Overall, the investigators detected anti-CII positivity in 6.6% and anti-CCP2 in 57.9%, including only anti-CII antibodies in 2.6%, only anti-CCP2 in 54%, double positivity in 3.9%, and double negativity in 39.4%. A control group of 960 patients from the Swedish general population who were matched for age, locality, and sex were positive for anti-CII in 1.6% and anti-CCP2 in 1.7%.
Significant differences were discovered between anti-CII positive and negative groups for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC), and Disease Activity Score encompassing 28 joints (DAS28). Anti-CII seropositive patients had higher CRP at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months.
“Anti-CCP2 [seropositivity] on the other hand was associated with higher disease activity later during the 5-year follow-up, and with CRP and ESR during the full period,” the investigators wrote.
The same respective phenotypic patterns occurred when the comparisons were limited to patients with anti-CII positivity alone or anti-CCP2 positivity alone, the investigators noted. However, “patients in the anti-CII and anti-CCP2 double-positive group mainly followed the anti-CII pattern, with early but not late increased values for CRP, ESR, DAS28, and DAS28CRP, but with a mixed pattern for SJC.”
In comparisons where baseline clinical and laboratory measures were associated with anti-CII or anti-CCP2 positivity, anti-CII was more often associated with favorable late changes in CRP, ESR, SJC, TJC, DAS28, DAS28CRP, Health Assessment Questionnaire, and pain and global measurements on a visual analog scale when compared with antibody double-negative patients. There were fewer changes in clinical and laboratory measures associated with anti-CCP2 positivity. These significant changes associated with anti-CII positivity were always larger improvements than were seen for antibody double-negative patients, whereas the significant changes observed with anti-CCP2 patients were always smaller improvements than those for antibody double-negative patients.
Moderate or good European League Against Rheumatism responses were significantly associated with anti-CII positivity at 12, 24, 36, and 60 months, whereas they were negatively associated with anti-CCP2 positivity at 36 and 60 months.
Overall, the findings of the study show that since “anti-CII-positive patients with RA have an acute onset, but favorable prognosis” and anti-CCP2 is associated with poor prognosis, “the combined analysis of anti-CII and ACPA/anti-CCP2 may be a new two-dimensional tool for predicting the prognosis and choosing therapy in newly diagnosed patients with RA,” the authors suggested.
The study was funded by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. Dr. Manivel and his coauthors reported no relevant financial disclosures.
Individuals with rheumatoid arthritis who have antifibrillar collagen type II antibodies appear to have a unique disease phenotype characterized by acute but transient inflammation around the time of diagnosis, as well as a favorable prognosis, according to findings from a retrospective cohort study.
The antifibrillar collagen type II (anti-CII) phenotype also differed from the phenotype of individuals with anti–cyclic citrullinated peptide 2 (anti-CCP2) antibodies who typically exhibit inflammatory markers and higher disease activity later in the disease course, reported Vivek Anand Manivel of Uppsala (Sweden) University and his colleagues (Ann Rheum Dis. 2017 Mar 23. doi: 10.1136/annrheumdis-2016-210873).
Overall, the investigators detected anti-CII positivity in 6.6% and anti-CCP2 in 57.9%, including only anti-CII antibodies in 2.6%, only anti-CCP2 in 54%, double positivity in 3.9%, and double negativity in 39.4%. A control group of 960 patients from the Swedish general population who were matched for age, locality, and sex were positive for anti-CII in 1.6% and anti-CCP2 in 1.7%.
Significant differences were discovered between anti-CII positive and negative groups for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC), and Disease Activity Score encompassing 28 joints (DAS28). Anti-CII seropositive patients had higher CRP at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months.
“Anti-CCP2 [seropositivity] on the other hand was associated with higher disease activity later during the 5-year follow-up, and with CRP and ESR during the full period,” the investigators wrote.
The same respective phenotypic patterns occurred when the comparisons were limited to patients with anti-CII positivity alone or anti-CCP2 positivity alone, the investigators noted. However, “patients in the anti-CII and anti-CCP2 double-positive group mainly followed the anti-CII pattern, with early but not late increased values for CRP, ESR, DAS28, and DAS28CRP, but with a mixed pattern for SJC.”
In comparisons where baseline clinical and laboratory measures were associated with anti-CII or anti-CCP2 positivity, anti-CII was more often associated with favorable late changes in CRP, ESR, SJC, TJC, DAS28, DAS28CRP, Health Assessment Questionnaire, and pain and global measurements on a visual analog scale when compared with antibody double-negative patients. There were fewer changes in clinical and laboratory measures associated with anti-CCP2 positivity. These significant changes associated with anti-CII positivity were always larger improvements than were seen for antibody double-negative patients, whereas the significant changes observed with anti-CCP2 patients were always smaller improvements than those for antibody double-negative patients.
Moderate or good European League Against Rheumatism responses were significantly associated with anti-CII positivity at 12, 24, 36, and 60 months, whereas they were negatively associated with anti-CCP2 positivity at 36 and 60 months.
Overall, the findings of the study show that since “anti-CII-positive patients with RA have an acute onset, but favorable prognosis” and anti-CCP2 is associated with poor prognosis, “the combined analysis of anti-CII and ACPA/anti-CCP2 may be a new two-dimensional tool for predicting the prognosis and choosing therapy in newly diagnosed patients with RA,” the authors suggested.
The study was funded by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. Dr. Manivel and his coauthors reported no relevant financial disclosures.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: RA patients with anti-CII seropositivity had significantly higher C-reactive protein levels at 0-6 months, higher ESR at 0-3 months, a higher SJC at 0-6 months, and a higher DAS28 at 0-3 months than did anti-CII seronegative patients.
Data source: Retrospective cohort study of EIRA study patients and 960 controls.
Disclosures: Study funded by Swedish Research Council, Swedish Rheumatism Association, King Gustav Vth 80-Year Foundation, and Uppsala County Council. The authors reported no relevant financial disclosures.
Suicide Risk in Older Adults: The Role and Responsibility of Primary Care
From the Primary Care Institute, Gainesville, FL.
Abstract
- Objective: To provide primary care practitioners with the knowledge required to identify and address older adult suicide risk in their practice.
- Methods: Review of the literature and good clinical practices.
- Results: Primary care practitioners play an important role in older adult suicide prevention and must have knowledge about older adult suicide risk, including risk factors and warning signs in this age-group. Practitioners also must appropriately screen for and manage suicide risk. Older adults, particularly older men, are at high risk for suicide, though they may be less likely to report suicide ideation. Additionally, older adults frequently see primary care practitioners within a month prior to death by suicide. A number of older adult–specific risk factors are reviewed, and appropriate screening and intervention for the primary care setting are discussed.
- Conclusion: Primary care practitioners are uniquely qualified to address a broad range of potential risk factors and should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide.
Key words: suicide; older adults; risk factors; screening; safety planning.
Primary care practitioners play an important role in older adult suicide prevention and have a responsibility to identify and address suicide risk among older adults. To do so, practitioners must understand the problem of older adult suicide, recognize risk factors for suicide in older adults, screen for suicide risk, and appropriately assess and manage suicide risk. Primary care practitioners may face challenges in completing these tasks; the goal of this article is to assist practitioners in addressing these challenges.
Suicide in Older Adults
The United States has recently seen increases in suicide rates across the lifespan; from 1999 to 2014, the suicide rate rose by 24% across all ages [3]. Among both men and women aged 65 to 74, the suicide rate increased in this time period [3]. The high suicide rate among older adults is particularly important to address given the increasing numbers of older adults in the United States. By 2050, the older adult population in the United States is expected to reach 88.5 million, more than double the older adult population in 2010 [4]. Additionally, the generation that is currently aging into older adulthood has historically had higher rates of suicide across their lifespan [5]. Given that suicide rates also increase in older adulthood for men, the coming decades may evidence even higher rates of suicide among older adults than previously and it is critical that older adult suicide prevention becomes a public health priority.
It is also essential to discuss other suicide-related outcomes among older adults, including suicide attempts and suicide ideation. This is critical particularly because the ratio of suicide attempts to deaths by suicide in this age-group is 4 to 1 [1]. This is in contrast to the ratio of attempts to deaths across all ages, which is 25 suicide attempts per death by suicide [1]. This means that suicide prevention must occur before a first suicide attempt is made; suicide attempts cannot be used a marker of elevated suicide risk in older adults or an indication that intervention is needed. Intervention is required prior to suicide risk becoming elevated to the point of a suicide attempt.
It is also critical to recognize that despite the fact that suicide rates rise with age, reports of suicide ideation decrease with age [7,8]. Across all ages, 3.9% of Americans report past-year suicide ideation; however, only 2.7% of older adults report thoughts of suicide [9]. The discrepancy with the increasing rates of death by suicide with age suggest that suicide risk, and thereby opportunities for intervention, may be missed in this age-group [10].
However, older adults may be more willing to report death ideation, as research has found that over 15% of older adults endorse death ideation [11–13]. Death ideation is a desire for death without a specific desire to end one’s own life, and is an important suicide-related outcome, as older adults with death ideation appear the same as those with suicide ideation in terms of depression, hopelessness, and history of suicidal behavior [14]. Additionally, older adults with death ideation had more hospitalizations, more outpatient visits, and more medical issues than older adults with suicide ideation [15]. Therefore, death ideation should be taken as seriously as suicide ideation in older adults [14]. In sum, the high rates of death by suicide, the likelihood of death on a first or early suicide attempt, and the discrepancy between decreasing reports of suicide ideation and increasing rates of death by suicide among older adults indicate that older adult suicide is an important public health problem.
Suicide Prevention Strategies
Many suicide prevention strategies to date have focused on indicated prevention, which concentrates on individuals already identified at high risk (eg, those with suicide ideation or who have made a suicide attempt) [16]. However, because older adults may not report suicide ideation or survive a first suicide attempt, indicated prevention is likely not enough to be effective in older adult suicide prevention. A multilevel suicide prevention strategy [17] is required to prevent older adult suicide [18]. Older adult suicide prevention must include indicated prevention but must also include selective and universal prevention [16]. Selective prevention focuses on groups who may be at risk for suicide (eg, individuals with depression, older adults) and universal prevention focuses on the entire population (eg, interventions to reduce mental health stigma) [16]. To prevent older adult suicide, crisis intervention is critical, but suicide prevention efforts upstream of the development of a suicidal crisis are also essential.
The Importance of Primary Care
Research indicates that primary care is one of the best settings in which to engage in older adult suicide prevention [18]. Older adults are significantly less likely to receive specialty mental health care than younger adults, even when they have depressive symptoms [19]. Additionally, among older adults who died by suicide, 58% had contact with a primary care provider within a month of their deaths, compared to only 11% who had contact with a mental health specialist [20]. Among older adults who died by suicide, 67% saw any provider in the 4 weeks prior to their death [21]. Approximately 10% of older adults saw an outpatient mental health provider, 11% saw a primary care physician for a mental health issue, and 40% saw a primary care physician for a non-mental health issue [21]. Therefore, because older adults are less likely to receive specialty mental health treatment and so often seen a primary care practitioner prior to death by suicide, primary care may be the ideal place for older adult suicide risk to be detected and addressed, especially as many older adults visit primary care without a mental health presenting concern prior to their death by suicide.
Additionally, older adults may be more likely to disclose suicide ideation to primary care practitioners, with whom they are more familiar, than physicians in other settings (eg, emergency departments). Research has shown that familiarity with a primary care physician significantly increases the likelihood of patient disclosure of psychosocial issues to the physician [22]. Primary care providers also have a critical role as care coordinators; many older adults also see specialty physicians and use the emergency department. In fact, older adults are more likely to use the emergency department than younger adults, but emergency departments are not equipped to navigate the complex care needs of this population [23]. Primary care practitioners are important in ensuring that health issues of older adults are addressed by coordinating with specialists, hospitals (eg, inpatient stays, emergency department visits, surgery) and other health services (eg, home health care, physical therapy). Approximately 35% of older adults in the United States experience a lack of care coordination [24], which can negatively impact their health and leave issues such as suicide ideation unaddressed. Primary care practitioners may be critical in screening for mental health issues and suicide risk during even routine visits because of their familiarity with patients, and also play an important role in coordinating care for older adults to improve well-being and to ensure that critical issues, such as suicide ideation, are appropriately addressed.
Primary care practitioners can also be key in upstream prevention. Primary care practitioners are in a unique role to address risk factors for suicide prior to the development of a suicidal crisis. Because older adults frequently see primary care practitioners, such practitioners may have more opportunities to identify risk factors (eg, chronic pain, depression). Primary care practitioners are also trained to treat a broad range of conditions, providing the skills to address many different risk factors.
Finally, primary care is a setting in which screening for depression and suicide ideation among older adults is recommended. The US Preventive Services Task Force recommends screening for depression in all adults and older adults and provides recommended screening instruments, some of which include questions about self-harm or suicide risk [25]. However, this same group has concluded that there is insufficient evidence to support a recommendation for suicide risk screening [26]. Despite this, the Joint Commission recently released an alert that recommends screening for suicide risk in all settings, including primary care [27]. The Joint Commission requirement for ambulatory care that is relevant to suicide is PC.04.01.01: The organization has a process that addresses the patient’s need for continuing care, treatment, or services after discharge or transfer; behavioral health settings have additional suicide-specific requirements. The recommendations, though, go far beyond this requirement for primary care. The Joint Commission specifically notes that primary care clinicians play an important role in detecting suicide ideation and recommends that primary care practitioners review each patient’s history for suicide risk factors, screen all patients for suicide risk, review screenings before patients leave appointments, and take appropriate actions to address suicide risk when needed [27]. Further details are available in the Joint Commission’s Sentinel Event Alert titled, “Detecting and treating suicide ideation in all settings” [27]. Given these recommendations, primary care is an important setting in which to identify and address suicide risk.
Risk Factors for Older Adult Suicide
Numerous reviews exist that cover many risk factors for suicide in older adults [18,28]. This article will focus briefly on risk factors that are likely to be recognized and potentially addressed by primary care practitioners. Risk factors that apply across the lifespan can be recalled through a mnemonic: IS PATH WARM [29]. These risk factors include suicide Ideation, Substance abuse, Purposelessness, Anxiety (including agitation and poor sleep), feeling Trapped, Hopelessness, social Withdrawal, Anger or rage, Recklessness (ie, engaging in risky activities), and Mood changes. The National Suicide Prevention Lifeline also includes being in unbearable physical pain, perceiving one’s self as a burden to others, and seeking revenge on others as risk factors [30]. More specific to older adults, Conwell notes 5 categories or domains of risk factors with strong research support: psychiatric symptoms, somatic illness, functional impairment, social integration, and personality traits and coping [18,31].
Affective or mood disorders, particularly depression and depressive symptoms, are some of the most well-studied and strongest risk factors for older adult suicide [31]; 71% to 97% of all older adults who die by suicide have psychiatric illnesses [28]. Mood disorders, including major depressive episodes, are most consistently linked to older adult suicide risk; there is evidence as well for anxiety disorders and substance abuse disorders as risk factors, though it is somewhat mixed [28]. Therefore, screening for depression, anxiety, and substance abuse may be key to recognizing potential suicide risk. However, depression and anxiety do not present similarly in younger and older adults [32,33]. Depressive symptoms in older adults may be more somatic (eg, agitation, gastrointestinal symptoms) [32] and may reflect more anhedonia than mood changes [33]. Anxiety in older adults tends to be reported as stress or tension, whereas younger adults report feeling anxious or worried [33]. Additionally, substance abuse is often underrecognized, underdiagnosed, and undertreated in older adults [34]. Proactive screening for substance abuse is important as it may not interfere with work or other obligations in older adults, and therefore substance abuse may not be identified by older adults or others in their lives.
Physical illness may also be a risk factor for suicide [28,31]. Numerous diagnoses have been linked to suicide risk, including cancers, neurodegenerative diseases (eg, amyotrophic lateral sclerosis, Huntington disease), spinal cord injury, cardiovascular disease, and pulmonary disease [28,35]. However, overall illness burden (ie, number of chronic illnesses) [28] and self-perceived health [36] appear to be stronger risk factors than any specific illness. Additionally, authors have suggested that illness itself may not be a particularly strong risk factor, but the effect of illness on depressive symptoms [35], functioning, pain, or hopelessness due to the potential for decline over time [28] may increase suicide risk in older adults. Pain itself has been identified as a risk factor for suicide, as have perceptions of burden to others, hopelessness, and functional impairment [28].
In terms of functional impairment, research has shown that impairment in completing instrumental activities of daily living is associated with higher risk for death by suicide, and cognitive impairment may also be associated with elevated suicide risk [28]. However, there are some discrepant findings regarding the role of dementia in suicide risk, which may reflect medical and psychiatric comorbidities, as well as different stages of dementia or levels of cognitive impairment (eg, hopelessness about cognitive decline may increase suicide risk shortly after diagnosis, whereas lack of insight may decrease risk later in the course of the illness) [37]. Related to functional or cognitive impairment is perceived burdensomeness (ie, the perception that one is a liability or burden to others, to the point that others would be better off if one was gone) [38], which may also be associated with suicide risk in older adults [39,40]. Researchers have found that the interaction between perceived burdensomeness and thwarted belongingness (ie, a belief that one lacks reciprocal caring relationships and does not belong) identified older adults who were likely experiencing suicide ideation but did not report it [41]. These findings indicate that perceived burdensomeness and thwarted belongingness may be key in identifying older adults at risk for suicide.
Thwarted belongingness has also been linked to suicide ideation in older adults [41]. In fact, studies suggest that social integration is especially important for reducing suicide risk in this population [28,31,42]. A larger social network, living with others, and being active in the community are each protective against suicide [28]. Bereavement, which can reduce social connectedness and acts as a significant life stressor, is also an important risk factor [31]. Retirement may also reduce social connectedness, and employment changes have been identified as a suicide risk factor for older adults [28]. Retirement has been linked to risk for death by suicide in this population [43], and may not only serve to reduce social connectedness, but for some older adults may also be a significant role loss or loss of sense of purpose that can influence suicide risk.
Finally, rigid personality traits or coping styles are a risk factor for suicide among older adults [28,31]. As older adults face potential losses, health changes, and functional decline, effective positive coping strategies and flexibility are key to maintaining well-being. If older adults are unable to flexibly cope with these challenges, their risk for suicide increases [28].
In addition to risk factors, which confer suicide risk but do not necessarily suggest that an older adult is thinking about suicide, warning signs exist that indicate that suicide risk is imminent. These include suicidal communication (ie, talking or writing about suicide), seeking access to means, and making preparations for suicide (eg, ensuring a will is in place, giving away prized possessions). One important note is that discussing and preparing for death may be developmentally appropriate for older adults, particularly those with chronic illnesses; however, such appropriate preparation is critically different from talking about suicide or a desire for death.
Additionally, a lack of planning for the future may be a warning sign. For example, older adults who decline to schedule medical follow-up or do not wish to refill needed prescriptions may be exhibiting warning signs that should be addressed. Similarly, not following needed medical regimens (eg, an older adult with diabetes no longer taking insulin) is also a warning sign. Other, potentially more subtle warning signs may include significant changes in mood, sleep, or social interactions. Older adults may become agitated and sleep less when they are considering suicide, or may feel more at ease after they have made the decision to die by suicide and their sleep or mood may improve. Withdrawing from valued others may also be a warning sign. Finally, recent major changes (eg, loss of a spouse, moving to an assisted living facility) may be triggers for suicide risk and can serve as warning signs themselves.
Specific Screening Strategies
Given the numerous risk factors and warning signs for older adult suicide, as well as the time limitations that primary care practitioners face [44,45], it would be impractical to comprehensively assess each older adult who presents at a primary care practice. Therefore, more specific screening is necessary. Most importantly, every older adult should be screened for suicide ideation and death ideation at every visit. Screening at every visit is critical because suicide ideation may develop at any point. Previous research has included screening of over 29,000 older adults in 11 primary care settings for suicide ideation, risk of alcohol misuse, and mental health disorders [15], suggesting that suicide risk screening is feasible. Other studies have also successfully used widespread screening for depression and suicide ideation among older adults in primary care [46–48]. Additionally, in an emergency department setting, universal suicide risk screening has been associated with significantly improved risk detection [49], indicating that improved screening may be beneficial in identifying suicide risk. Importantly, asking about suicide does not cause thoughts of suicide [50]. Additionally, it is a myth that those who talk about suicide ideation will not act on these thoughts [51].
When primary care practitioners inquire about suicide ideation, they should also ask about death ideation; though some may believe that death ideation is not as significant in terms of suicide risk as suicide ideation, recall that research has not found differences in previous suicide attempts or current hopelessness among older adults with death ideation versus suicide ideation [14]. Therefore, screening for death ideation should be completed as part of every suicide risk screening.
Screening can take many forms. Screening may be oral; asking an older adult if he or she is having thoughts of suicide or is experiencing a desire to die is a brief, 2-question screening that may provide valuable information (eg, “Are you having thoughts about your own death or wanting to die?”, “Are you having thoughts of killing yourself or thinking about suicide?”). This screening may be conducted by medical assistants, nurses, care managers, or physicians, with the patient’s responses documented. Importantly, a standard procedure should be implemented to ensure older adults are consistently asked about suicide risk at each visit, but do not feel inundated by such questions from numerous staff.
If verbal questions are asked, they must be asked appropriately. Euphemisms or indirect language should not be used during a screening; older adults should be directly asked about thoughts of death and suicide, not simply asked questions such as, “Have you ever had thoughts of harming or hurting yourself?” A question like this does not adequately assess current suicide risk, as it does not assess current thoughts, nor does it specifically inquire about suicide ideation (ie, killing one’s self). It is also important to phrase questions in a manner that invites honest responses and conveys an openness to listening. For example, asking, “You’re not thinking about suicide, are you?” suggests that the practitioner wants the older adult to say no and is not comfortable with the older adult endorsing suicide ideation. Open questions that allow endorsement or denial (eg, “Are you having thoughts of killing yourself?”) imply that the practitioner is receptive to either an endorsement or denial of suicide ideation.
Alternatively, a written screening can be used; older adults may complete a questionnaire prior to their appointment or while waiting to see their practitioner. Such an assessment may be a brief screening (eg, using similar yes/no questions to an oral screening), or may be a standardized measure. For example, the Geriatric Suicide Ideation Scale [52] is a 31-item self-report measure that provides scores for suicide ideation, death ideation, loss of personal and social worth, and perceived meaning in life. Though there are not standard cutoffs that suggest high versus low suicide risk, responses can be reviewed to identify whether older adults are reporting suicide ideation or death ideation, and can also be compared to norms (ie, average scores) from other older adults [52]. This measure also has the benefit of 2 subscales that do not specifically require reporting thoughts of suicide or death (ie, loss of personal and social worth, perceived meaning in life), which may give practitioners an indication of an older adult’s suicide risk even if the older adult is not comfortable disclosing suicide ideation, as has been shown in previous research [7,8].
Similarly, the Geriatric Depression Scale, which has a validated 15-item version [53], does not directly ask about suicide ideation but has a 5-item subscale that has been found to be highly correlated with reported suicide ideation [54]. When administered to older adult primary care patients, this subscale was an effective measure of suicide ideation; a score of ≥ 1 was the best cutoff for determining whether an older adult reported suicide ideation [55].
Additionally, as noted previously, the interaction between perceived burdensomeness and thwarted belongingness may identify older adults who are potentially experiencing, but not reporting, suicide ideation [41]. The Interpersonal Needs Questionnaire [56] is the validated assessment for both perceived burdensomeness and thwarted belongingness. Perceived burdensomeness is assessed via 6 self-report items, and thwarted belongingness is assessed via 9 self-report items on this measure [56]. There are not specific cutoffs that determine high versus low perceived burdensomeness or thwarted belongingness, but older adults’ responses can provide information about their experiences of these constructs. Administration of the Interpersonal Needs Questionnaire can provide information about potential risk for suicide among older adults who may otherwise deny thoughts of suicide or death.
If the screening for suicide ideation or death ideation is positive (ie, the older adult endorses thoughts of suicide or death), the treating primary care practitioner must then follow up with additional questions to determine current level of suicide risk. To make this determination, at a minimum, follow-up questions should focus on whether the older adult has any intent to die by suicide (eg, “Do you have any intent to act on your thoughts of suicide?”), as well as whether he or she has a plan to die by suicide (eg, “Have you begun formulating a plan to die by suicide?”). When asking about a plan, it is important to determine how specific the plan is. For example, an older adult with a specific method identified and date selected to implement the plan is at much higher risk than an older adult with a relatively vague idea. It is also critical to assess for the older adult’s access to means for suicide. If an older adult has a specific plan and has the capability to carry out the plan (eg, plans to overdose on prescription medication and has large quantities of medication or high-lethality medication at home), he or she is more likely to die by suicide than an older adult who does not have access to means (eg, only has small quantities of low-lethality medication available). A general assessment of risk factors and previous suicidal behavior (ie, any previous suicide attempts) also informs decisions about level of risk and interventions.
After a screening or assessment is completed, a risk determination must be made and documented. Acute suicide risk can be categorized as low, moderate, or high. It is not appropriate to say that there is “no” suicide risk present. Low risk occurs when there is no current suicide ideation, no plan to die by suicide, and no intent to act on suicidal thoughts, especially when the patient has no history of suicidal behavior and few risk factors [57]. Moderate risk is evident when there is current suicide ideation, but no specific plan to die by suicide or intent to act on suicidal thoughts. There are likely warning signs or risk factors, which may include previous suicidal behaviors, present in moderate suicide risk [57]. High risk is indicated by current suicide ideation with plan to die by suicide and suicidal intent. There are significant warning signs and risk factors present; there may also be a recent suicide attempt, though this is not a requirement for a high risk determination [57]. Undetermined suicide risk occurs when a practitioner cannot accurately assess risk, but concern regarding suicide is present; this is primarily used when a patient refuses to answer questions about suicide. Undetermined risk should be treated as at least moderate risk. Because research shows that death ideation has similar outcomes to suicide ideation in older adults [14], death ideation should also be factored into determinations of suicide risk; reports of death ideation may indicate low or moderate risk in older adults, dependent upon other risk factors, suicidal intent, and plan.
After a risk determination is made, it must be documented in the medical record. The level of risk and rationale for that determination must be included [58]. Stating only the level of risk without a rationale (ie, the older adult’s responses to questions) is not adequate, and documenting only the older adult’s responses without a determination of risk is also not sufficient. Finally, it is critical to document the intervention that occurred or steps taken after the level of risk was determined.
Critically, stating only that there was no indication of suicide risk is inadequate. For example, documenting “No evidence of suicide risk” is not appropriate. This documentation does not indicate that the older adult was specifically asked about suicide ideation, death ideation, suicidal intent, or plan to die by suicide. It also does not indicate a level of suicide risk. Examples of appropriate documentation include:
Patient was asked about suicide risk. She denied current suicide ideation but reported death ideation. She denied any current suicidal intent or plan. She also denied any previous suicide attempts. Therefore, acute suicide risk was deemed to be low. Provided patient with wallet card about the National Suicide Prevention Lifeline. Also called the Friendship Line while in the room with the patient to connect her with services. Finally, provided a brief list of local mental health professionals to patient; the patient reported she would like to see Dr. Smith. Called and left a message for Dr. Smith with referral information with patient during appointment.
Patient was asked about suicide risk. He reported both death ideation and suicide ideation. He also reported a nonspecific plan (ie, causing a single-vehicle motor vehicle accident, with no specific plan for the motor vehicle accident or timeframe) and denied any intent to act on his thoughts of suicide. He reported one previous suicide attempt, at age 22, by overdose on over-the-counter medication. He reported that this attempt did not require medical attention. Therefore, acute suicide risk was determined to be moderate. Patient was introduced to the behavioral health specialist, who met with the patient during the appointment to conduct further assessment and intervention.
Specific Intervention Strategies
Despite the fact that the pace of the primary care setting often does not allow for time-intensive intervention, there are ways to address suicide risk in this setting. Importantly, no-suicide contracts should not be used at any time [59,60]. No-suicide contracts are documents that patients who are experiencing suicide ideation are required to sign that state that they will not die by suicide while under the care of the practitioner. These contracts have no evidence of effectiveness, and some researchers argue that they may in fact damage the relationship with patients and serve the practitioner’s needs more than the patient’s needs [59].
One of the best options for older adults at low acute suicide risk is to provide resources and referrals. The National Suicide Prevention Lifeline can be reached at 1-800-273-TALK (8255); trained counselors are available to speak to patients at all times. Wallet cards with information about the National Suicide Prevention Lifeline are available at no charge from the US Substance Abuse and Mental Health Services Administration online store. The Friendship Line is another service available free to adults ages 60 and older, 24 hours per day, 7 days per week; this line can be reached at 1-800-971-0016. The Friendship Line, which is managed by the Institute on Aging, also provides outreach calls to older adults who may be isolated or lonely, increasing connectedness and potentially reducing suicide risk.
Having a ready list of local mental health professionals with expertise in geriatrics and suicide risk to provide to the patient is also beneficial. Recall, though, that older adults are less likely to seek out and receive mental health services [19]; therefore, connecting the patient with resources or referrals during the appointment is critical. If the practitioner does not have time to do this, having a medical assistant or other staff member that the patient knows engage in this step may be appropriate. For example, the patient can call the Friendship Line or National Suicide Prevention Lifeline while in the room with the practitioner, which may reduce anxiety or stigma about doing so and connect the patient with services. Similarly, calling a local mental health professional to make a referral during the appointment may increase the likelihood that the older adult will follow up on the referral.
The most ideal method of intervention for moderate or high acute suicide risk is a warm handoff to a behavioral or mental health specialist. As primary care and behavioral health become more integrated and financially viable as reimbursement through the Centers for Medicare and Medicaid Services improves [61], it is becoming increasingly likely that such a specialist will be on-site and available. Research has found that collaborative care in primary care reduces suicide risk in older adults [46–48,62]. Mental health specialists can conduct more comprehensive assessments and spend more time intervening to reduce suicide risk among older adults with death or suicide ideation. If an on-site behavioral health specialist is not available, older adults at high suicide risk may need to be referred to an emergency department for further evaluation and follow-up. Each state has its own laws and procedures regarding this process, which should be incorporated into a practice’s procedures for addressing high suicide risk. The procedure often involves ensuring that the older adult is accompanied at all times (ie, not left alone in a room), alerting emergency services (usually via phone call to an emergency line, such as 911), and completion of paperwork by a practitioner asserting that the patient is a danger to self. Police or other emergency personnel are then responsible for transporting the patient for further evaluation and determination of whether hospitalization is required.
If more time is available, either via the treating primary care practitioner or other patient care staff in the office, other brief interventions may be beneficial. First, means safety discussions are critical, particularly for older adults with plans for suicide or access to highly lethal means. In such discussions, patients are encouraged to restrict access to the methods that they may use to die by suicide. Plans for restricting access are developed, and when possible, a support person is enlisted to ensure that the plans are carried out. For example, if an older adult has access to firearms (eg, keeps a loaded weapon in his or her nightstand), he or she is encouraged to restrict his or her access to it. Ideally, this is through removing the weapon from the home, either permanently or until suicide risk reduces (eg, giving it to a friend, turning it over to police), but more safe storage may also be an option if the older adult is not willing to remove the weapon from the home. This may mean using a gun lock or storing the weapon in a gun safe, storing ammunition separately from an unloaded weapon, removing the firing pin, or otherwise disassembling the weapon. Means safety counseling has been shown to be effective in reducing suicide rates [63] and is acceptable to patients [64]. Studies indicate that over 90% of individuals who make a suicide attempt and survive do not go on to die by suicide [65]; therefore, reducing access to highly lethal means during a suicidal crisis may be key in reducing suicide rates. Though an in-depth review of means safety counseling is outside the scope of this article, readers are directed to Bryan, Stone, and Rudd’s article for a practical overview of means safety discussions [66].
Second, safety planning is a brief intervention that may be beneficial in the primary care setting [67,68]. The goal of a safety plan is to create an individualized plan to remain safe during a suicidal crisis. Means safety discussion is the last of 6 steps in the safety plan [68]. The first 5 steps include identifying warning signs, using internal coping strategies, social connectedness as distraction, social support for the crisis, and professionals that can be used as resources. When patients can identify specific, individualized warning signs that occur prior to a crisis, they can then use strategies to cope and prevent the crisis from worsening. Coping strategies that are encouraged are first internal (ie, those that can be done without relying on anyone else), such as exercise or journaling. If those do not improve the patient’s mood, then he or she is encouraged to use people or social settings as a distraction (eg, people watching at the mall, calling an acquaintance to chat), and if he or she is still feeling bad, encouraged to get social support for the crisis (eg, calling a family member to discuss the crisis and get support). Finally, if all of these steps are not effective, the older adult is encouraged to reach out to professional supports, such as a mental health provider, the National Suicide Prevention Lifeline, or 911 (or go to an emergency room). Readers are encouraged to review Stanley and Brown’s articles for comprehensive details about safety planning as an intervention [67,68]. Additionally, an article with specific adaptations for safety planning with older adults is forthcoming [69].
Conclusion
Older adults, particularly older men, are at high risk for suicide [1,2], and primary care practitioners are a critical component of older adult suicide prevention. Older adults frequently see primary care practitioners within a month prior to death by suicide [20,21]; primary care practitioners are uniquely qualified to address a broad range of potential risk factors, and may have more interactions and familiarity with older adults at risk for suicide than other medical professionals [20–22]. Primary care practitioners should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide. Screening can consist of standardized written questionnaires or oral questioning, and interventions may include providing resources and referrals, discussions about means safety, safety planning, and handoff to a mental health specialist. Interventions for suicide risk are likely feasible and acceptable in primary care [46–48]. Primary care practitioners have an important role to play in older adult suicide prevention, and must be prepared to interact with older adults who may be at risk for suicide.
Corresponding author: Danielle R. Jahn, PhD, Primary Care Institute, 605 NE 1st St, Gainesville, FL 32605, [email protected].
Financial disclosures: None reported.
1. American Association of Suicidology. U.S.A. suicide: 2014official final data. 2016. Accessed at www.suicidology.org/Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf.
2. Centers for Disease Control and Prevention. Leading causes of death reports, national and regional, 1999-2015. 2016. Accessed at https://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html.
3. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief, No 241. Hyattsville, MD: National Center for Health Statistics; 2016.
4. US Census Bureau. The next four decades. The older population in the United States: 2010 to 2050. 2010. Accessed at www.census.gov/prod/2010pubs/p25-1138.pdf.
5. Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep 2010;125:680–8.
6. Substance Abuse and Mental Health Services Administration. Issue brief 4: preventing suicide in older adults. 2012. Accessed at https://aoa.acl.gov/AoA_Programs/HPW/Behavioral/docs2/Issue%20Brief%204%20Preventing%20Suicide.pdf.
7. Duberstein PR, Conwell Y, Seidlitz L, et al. Age and suicidal ideation in older depressed inpatients. Am J Geriatr Psychiatry 1999;7:289–96.
8. Lynch TR, Johnson CS, Mendelson T, et al. Correlates of suicidal ideation among an elderly depressed sample. J Affect Disord 1999;56:9–15.
9. Centers for Disease Control and Prevention. Suicide: facts at a glance 2015. 2015. Accessed at www.cdc.gov/violenceprevention/pdf/suicide-datasheet-a.pdf.
10. Cukrowicz KC, Duberstein PR, Vannoy SD, et al. What factors determine disclosure of suicide ideation in adults 60 and older to a treatment provider? Suicide Life Threat Behav 2014;44:331–7.
11. Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int J Psychiatry Med 2006;36:183–98.
12. Scocco P, Fantoni G, Rapattoni M, et al. Death ideas, suicidal thoughts, and plans among nursing home residents. J Geriatr Psychiatry Neurol 2009;22:141–8.
13. Scocco P, Meneghel G, Caon F, et al. Death ideation and its correlates: survey of an over-65-year-old population. J Nerv Ment Dis 2001;189:210–8.
14. Szanto K, Reynolds III CF, Frank E, et al. Suicide in elderly patients: is active vs. passive suicidal ideation a clinically valid distinction? Am J Geriatr Psychiatry, 2002;4:197–207.
15. Bartels SJ, Coakley E, Oxman TE, et al. Suicidal and death ideation in older primary care patients with depression, anxiety, and at-risk alcohol use. Am J Geriatr Psychiatry 2002;10:417–27.
16. Yip PSF. A public health approach to suicide prevention. Hong Kong J Psychiatry 2005;15:29–31.
17. van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 2011;32:319–33.
18. Conwell Y. Suicide and suicide prevention in later life. Focus 2013;11:39–47.
19. Crabb R, Hunsley J. Utilization of mental health services among older adults with depression. J Clin Psychol 2006;62:299–312.
20. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry 2002;159:909–16.
21. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med 2014;29:870–7.
22. Robinson JW, Roter DL. Psychosocial problem disclosure by primary care patients. Soc Sci Med 1999;48:1353–62.
23. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47.
24. Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff 2014;33:2247–55.
25. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380–7.
26. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:719–26.
27. The Joint Commission. Detecting and treating suicide risk in all settings. Sentinel Event Alert 2016;56:1–7.
28. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68.
29. American Association of Suicidology. Know the warning signs of suicide. 2016. Accessed at www.suicidology.org/resources/warning-signs.
30. National Suicide Prevention Lifeline. Suicide warning signs. 2011. Accessed at www.suicidepreventionlifeline.org/App_Files/Media/PDF/NSPL_WalletCard.pdf.
31. Conwell Y. Suicide later in life: challenges and priorities for prevention. Am J Prev Med 2014;47:S244–50.
32. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
33. Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: comparison between older and younger clinical samples. Int Psychogeriatr 2015;27:1523–32.
34. Substance Abuse and Mental Health Services Administration. Substance abuse among older adults. Treatment Improvement Protocol (TIP) Series, No. 26. HHS Publication No. (SMA) 12-3918. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998.
35. Fiske A, O’Riley AA, Widoe RK. Physical health and suicide in late life: an evaluative review. Clin Gerontologist 2008;31:31–50.
36. Duberstein PR, Conwell Y, Conner KR, et al. Suicide at 50 years of age and older: perceived physical illness, family discord, and financial strain. Psychol Med 2004;34:137–46.
37. Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement 2010;6:75–82.
38. Joiner T. Why people die by suicide. Cambridge: Harvard University Press; 2005.
39. Jahn DR, Cukrowicz KC. The impact of the nature of relationships on perceived burdensomeness and suicide ideation in a community sample of older adults. Suicide Life Threat Behav 2011;41:635–49.
40. Jahn DR, Cukrowicz KC, Linton K, Prabhu F. The mediating effect of perceived burdensomeness on the relation between depressive symptoms and suicide ideation in a community sample of older adults. Aging Ment Health 2011;15:214–20.
41. Cukrowicz KC, Jahn DR, Graham RD, et al. Suicide risk in older adults: evaluating models of risk and predicting excess zeros in a primary care sample. J Abnorm Psychol 2013;122:1021–30.
42. Fassberg MM, Van Orden KA, Duberstein, P, et al. A systematic review of social factors and suicidal behavior in older adulthood. Int J Environ Res Public Health 2012;9:722–45.
43. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elderly: a psychological autopsy study in a north Italy area (1994-2004). Am J Geriatr Psychiatry 2008;16:727–35.
44. Konrad TR, Link CL, Shackelton RJ, et al. It’s about time: physicians’ perceptions of time constraints in primary medical practice in three national healthcare systems. Med Care 2010;48:95–100.
45. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2006;42:1871–94.
46. Bruce ML, Ten Have TR, Reynolds III CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91.
47. Alexopoulos GS, Reynolds CF III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
48. Unutzer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc 2006;54:1550–6.
49. Boudreaux ED, Camargo Jr CA, Arias SA, et al. Improving suicide risk screening and detection in the emergency department. Am J Prev Med 2016;50:445–53.
50. Mathias CW, Furr RM, Sheftall AH, et al. What’s the harm in asking about suicidal ideation? Suicide Life Threat Behav 2012;42:341–51.
51. Joiner T. Myths about suicide. Cambridge: Harvard University Press; 2011.
52. Heisel MJ, Flett GL. The development and initial validation of the Geriatric Suicide Ideation Scale. Am J Geriatr Psychiatry 2006;14:742–51.
53. Sheikh JL, Yesavage JA. Geriatric Depression Scale: recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Howarth Press; 1986: 165–73.
54. Heisel MJ, Flett GL, Duberstein PR, Lyness JM. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry 2005;13:876–83.
55. Heisel MJ, Duberstein PR, Lyness JM, Feldman MD. Screening for suicide ideation among older primary care patients. J Am Board Fam Med 2010;23:260–9.
56. Van Orden KA, Cukrowicz KC, Witte TK, Joiner Jr TE. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 2012;24;197–215.
57. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. Accessed at www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf.
58. Freedenthal S. Documentation: do it well, for the client’s sake and yours. 2013. Accessed at www.speakingofsuicide.com/2013/05/25/documentation/.
59. McMyler C, Pryjmachuk S. Do ‘no-suicide’ contracts work? J Psychiatr Ment Hlt 2008;15:512–22.
60. Rudd MD, Mandrusiak M, Joiner Jr TE. The case against no-suicide contracts: the commitment to treatment statement as a practice alternative. J Clin Psychol 2006;62:243–51.
61. National Institute of Mental Health. Adding better mental health care to primary care: a new era of behavioral health integration. 2016. Accessed at www.nimh.nih.gov/news/science-news/2016/adding-better-mental-health-care-to-primary-care.shtml.
62. Lapierre S, Erlangsen A, Waern M, et al. A systematic review of elderly suicide prevention programs. Crisis 2011;32;88–98.
63. Hawton K. Restricting access to methods of suicide: rationale and evaluation of this approach to suicide prevention. Crisis 2007;28:4–9.
64. Walters H, Kulkarni M, Forman J, et al. Feasibility and acceptability of interventions to delay gun access in VA mental health settings. Gen Hosp Psychiatry 2012;34:692–8.
65. Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. Brit J Psychiatry 2002;181:193–9.
66. Bryan CJ, Stone SL, Rudd MD. A practical, evidence-based approach for means-restriction counseling with suicidal patients. Prof Psychol Res Pr 2011;42:339–46.
67. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. 2008. Accessed at www.mentalhealth.va.gov/docs/va_safety_planning_manual.pdf.
68. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–64.
69. Jahn DR, Conti EC, Simons KV, et al. Evidence and considerations for safety planning as a suicide prevention strategy for older adults. 2017. Manuscript in preparation.
70. Knox KL, Stanley B, Currier GW, et al. An emergency department-based brief intervention for veterans at risk for suicide (SAFE VET). Am J Public Health 2012;102:S33–7.
From the Primary Care Institute, Gainesville, FL.
Abstract
- Objective: To provide primary care practitioners with the knowledge required to identify and address older adult suicide risk in their practice.
- Methods: Review of the literature and good clinical practices.
- Results: Primary care practitioners play an important role in older adult suicide prevention and must have knowledge about older adult suicide risk, including risk factors and warning signs in this age-group. Practitioners also must appropriately screen for and manage suicide risk. Older adults, particularly older men, are at high risk for suicide, though they may be less likely to report suicide ideation. Additionally, older adults frequently see primary care practitioners within a month prior to death by suicide. A number of older adult–specific risk factors are reviewed, and appropriate screening and intervention for the primary care setting are discussed.
- Conclusion: Primary care practitioners are uniquely qualified to address a broad range of potential risk factors and should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide.
Key words: suicide; older adults; risk factors; screening; safety planning.
Primary care practitioners play an important role in older adult suicide prevention and have a responsibility to identify and address suicide risk among older adults. To do so, practitioners must understand the problem of older adult suicide, recognize risk factors for suicide in older adults, screen for suicide risk, and appropriately assess and manage suicide risk. Primary care practitioners may face challenges in completing these tasks; the goal of this article is to assist practitioners in addressing these challenges.
Suicide in Older Adults
The United States has recently seen increases in suicide rates across the lifespan; from 1999 to 2014, the suicide rate rose by 24% across all ages [3]. Among both men and women aged 65 to 74, the suicide rate increased in this time period [3]. The high suicide rate among older adults is particularly important to address given the increasing numbers of older adults in the United States. By 2050, the older adult population in the United States is expected to reach 88.5 million, more than double the older adult population in 2010 [4]. Additionally, the generation that is currently aging into older adulthood has historically had higher rates of suicide across their lifespan [5]. Given that suicide rates also increase in older adulthood for men, the coming decades may evidence even higher rates of suicide among older adults than previously and it is critical that older adult suicide prevention becomes a public health priority.
It is also essential to discuss other suicide-related outcomes among older adults, including suicide attempts and suicide ideation. This is critical particularly because the ratio of suicide attempts to deaths by suicide in this age-group is 4 to 1 [1]. This is in contrast to the ratio of attempts to deaths across all ages, which is 25 suicide attempts per death by suicide [1]. This means that suicide prevention must occur before a first suicide attempt is made; suicide attempts cannot be used a marker of elevated suicide risk in older adults or an indication that intervention is needed. Intervention is required prior to suicide risk becoming elevated to the point of a suicide attempt.
It is also critical to recognize that despite the fact that suicide rates rise with age, reports of suicide ideation decrease with age [7,8]. Across all ages, 3.9% of Americans report past-year suicide ideation; however, only 2.7% of older adults report thoughts of suicide [9]. The discrepancy with the increasing rates of death by suicide with age suggest that suicide risk, and thereby opportunities for intervention, may be missed in this age-group [10].
However, older adults may be more willing to report death ideation, as research has found that over 15% of older adults endorse death ideation [11–13]. Death ideation is a desire for death without a specific desire to end one’s own life, and is an important suicide-related outcome, as older adults with death ideation appear the same as those with suicide ideation in terms of depression, hopelessness, and history of suicidal behavior [14]. Additionally, older adults with death ideation had more hospitalizations, more outpatient visits, and more medical issues than older adults with suicide ideation [15]. Therefore, death ideation should be taken as seriously as suicide ideation in older adults [14]. In sum, the high rates of death by suicide, the likelihood of death on a first or early suicide attempt, and the discrepancy between decreasing reports of suicide ideation and increasing rates of death by suicide among older adults indicate that older adult suicide is an important public health problem.
Suicide Prevention Strategies
Many suicide prevention strategies to date have focused on indicated prevention, which concentrates on individuals already identified at high risk (eg, those with suicide ideation or who have made a suicide attempt) [16]. However, because older adults may not report suicide ideation or survive a first suicide attempt, indicated prevention is likely not enough to be effective in older adult suicide prevention. A multilevel suicide prevention strategy [17] is required to prevent older adult suicide [18]. Older adult suicide prevention must include indicated prevention but must also include selective and universal prevention [16]. Selective prevention focuses on groups who may be at risk for suicide (eg, individuals with depression, older adults) and universal prevention focuses on the entire population (eg, interventions to reduce mental health stigma) [16]. To prevent older adult suicide, crisis intervention is critical, but suicide prevention efforts upstream of the development of a suicidal crisis are also essential.
The Importance of Primary Care
Research indicates that primary care is one of the best settings in which to engage in older adult suicide prevention [18]. Older adults are significantly less likely to receive specialty mental health care than younger adults, even when they have depressive symptoms [19]. Additionally, among older adults who died by suicide, 58% had contact with a primary care provider within a month of their deaths, compared to only 11% who had contact with a mental health specialist [20]. Among older adults who died by suicide, 67% saw any provider in the 4 weeks prior to their death [21]. Approximately 10% of older adults saw an outpatient mental health provider, 11% saw a primary care physician for a mental health issue, and 40% saw a primary care physician for a non-mental health issue [21]. Therefore, because older adults are less likely to receive specialty mental health treatment and so often seen a primary care practitioner prior to death by suicide, primary care may be the ideal place for older adult suicide risk to be detected and addressed, especially as many older adults visit primary care without a mental health presenting concern prior to their death by suicide.
Additionally, older adults may be more likely to disclose suicide ideation to primary care practitioners, with whom they are more familiar, than physicians in other settings (eg, emergency departments). Research has shown that familiarity with a primary care physician significantly increases the likelihood of patient disclosure of psychosocial issues to the physician [22]. Primary care providers also have a critical role as care coordinators; many older adults also see specialty physicians and use the emergency department. In fact, older adults are more likely to use the emergency department than younger adults, but emergency departments are not equipped to navigate the complex care needs of this population [23]. Primary care practitioners are important in ensuring that health issues of older adults are addressed by coordinating with specialists, hospitals (eg, inpatient stays, emergency department visits, surgery) and other health services (eg, home health care, physical therapy). Approximately 35% of older adults in the United States experience a lack of care coordination [24], which can negatively impact their health and leave issues such as suicide ideation unaddressed. Primary care practitioners may be critical in screening for mental health issues and suicide risk during even routine visits because of their familiarity with patients, and also play an important role in coordinating care for older adults to improve well-being and to ensure that critical issues, such as suicide ideation, are appropriately addressed.
Primary care practitioners can also be key in upstream prevention. Primary care practitioners are in a unique role to address risk factors for suicide prior to the development of a suicidal crisis. Because older adults frequently see primary care practitioners, such practitioners may have more opportunities to identify risk factors (eg, chronic pain, depression). Primary care practitioners are also trained to treat a broad range of conditions, providing the skills to address many different risk factors.
Finally, primary care is a setting in which screening for depression and suicide ideation among older adults is recommended. The US Preventive Services Task Force recommends screening for depression in all adults and older adults and provides recommended screening instruments, some of which include questions about self-harm or suicide risk [25]. However, this same group has concluded that there is insufficient evidence to support a recommendation for suicide risk screening [26]. Despite this, the Joint Commission recently released an alert that recommends screening for suicide risk in all settings, including primary care [27]. The Joint Commission requirement for ambulatory care that is relevant to suicide is PC.04.01.01: The organization has a process that addresses the patient’s need for continuing care, treatment, or services after discharge or transfer; behavioral health settings have additional suicide-specific requirements. The recommendations, though, go far beyond this requirement for primary care. The Joint Commission specifically notes that primary care clinicians play an important role in detecting suicide ideation and recommends that primary care practitioners review each patient’s history for suicide risk factors, screen all patients for suicide risk, review screenings before patients leave appointments, and take appropriate actions to address suicide risk when needed [27]. Further details are available in the Joint Commission’s Sentinel Event Alert titled, “Detecting and treating suicide ideation in all settings” [27]. Given these recommendations, primary care is an important setting in which to identify and address suicide risk.
Risk Factors for Older Adult Suicide
Numerous reviews exist that cover many risk factors for suicide in older adults [18,28]. This article will focus briefly on risk factors that are likely to be recognized and potentially addressed by primary care practitioners. Risk factors that apply across the lifespan can be recalled through a mnemonic: IS PATH WARM [29]. These risk factors include suicide Ideation, Substance abuse, Purposelessness, Anxiety (including agitation and poor sleep), feeling Trapped, Hopelessness, social Withdrawal, Anger or rage, Recklessness (ie, engaging in risky activities), and Mood changes. The National Suicide Prevention Lifeline also includes being in unbearable physical pain, perceiving one’s self as a burden to others, and seeking revenge on others as risk factors [30]. More specific to older adults, Conwell notes 5 categories or domains of risk factors with strong research support: psychiatric symptoms, somatic illness, functional impairment, social integration, and personality traits and coping [18,31].
Affective or mood disorders, particularly depression and depressive symptoms, are some of the most well-studied and strongest risk factors for older adult suicide [31]; 71% to 97% of all older adults who die by suicide have psychiatric illnesses [28]. Mood disorders, including major depressive episodes, are most consistently linked to older adult suicide risk; there is evidence as well for anxiety disorders and substance abuse disorders as risk factors, though it is somewhat mixed [28]. Therefore, screening for depression, anxiety, and substance abuse may be key to recognizing potential suicide risk. However, depression and anxiety do not present similarly in younger and older adults [32,33]. Depressive symptoms in older adults may be more somatic (eg, agitation, gastrointestinal symptoms) [32] and may reflect more anhedonia than mood changes [33]. Anxiety in older adults tends to be reported as stress or tension, whereas younger adults report feeling anxious or worried [33]. Additionally, substance abuse is often underrecognized, underdiagnosed, and undertreated in older adults [34]. Proactive screening for substance abuse is important as it may not interfere with work or other obligations in older adults, and therefore substance abuse may not be identified by older adults or others in their lives.
Physical illness may also be a risk factor for suicide [28,31]. Numerous diagnoses have been linked to suicide risk, including cancers, neurodegenerative diseases (eg, amyotrophic lateral sclerosis, Huntington disease), spinal cord injury, cardiovascular disease, and pulmonary disease [28,35]. However, overall illness burden (ie, number of chronic illnesses) [28] and self-perceived health [36] appear to be stronger risk factors than any specific illness. Additionally, authors have suggested that illness itself may not be a particularly strong risk factor, but the effect of illness on depressive symptoms [35], functioning, pain, or hopelessness due to the potential for decline over time [28] may increase suicide risk in older adults. Pain itself has been identified as a risk factor for suicide, as have perceptions of burden to others, hopelessness, and functional impairment [28].
In terms of functional impairment, research has shown that impairment in completing instrumental activities of daily living is associated with higher risk for death by suicide, and cognitive impairment may also be associated with elevated suicide risk [28]. However, there are some discrepant findings regarding the role of dementia in suicide risk, which may reflect medical and psychiatric comorbidities, as well as different stages of dementia or levels of cognitive impairment (eg, hopelessness about cognitive decline may increase suicide risk shortly after diagnosis, whereas lack of insight may decrease risk later in the course of the illness) [37]. Related to functional or cognitive impairment is perceived burdensomeness (ie, the perception that one is a liability or burden to others, to the point that others would be better off if one was gone) [38], which may also be associated with suicide risk in older adults [39,40]. Researchers have found that the interaction between perceived burdensomeness and thwarted belongingness (ie, a belief that one lacks reciprocal caring relationships and does not belong) identified older adults who were likely experiencing suicide ideation but did not report it [41]. These findings indicate that perceived burdensomeness and thwarted belongingness may be key in identifying older adults at risk for suicide.
Thwarted belongingness has also been linked to suicide ideation in older adults [41]. In fact, studies suggest that social integration is especially important for reducing suicide risk in this population [28,31,42]. A larger social network, living with others, and being active in the community are each protective against suicide [28]. Bereavement, which can reduce social connectedness and acts as a significant life stressor, is also an important risk factor [31]. Retirement may also reduce social connectedness, and employment changes have been identified as a suicide risk factor for older adults [28]. Retirement has been linked to risk for death by suicide in this population [43], and may not only serve to reduce social connectedness, but for some older adults may also be a significant role loss or loss of sense of purpose that can influence suicide risk.
Finally, rigid personality traits or coping styles are a risk factor for suicide among older adults [28,31]. As older adults face potential losses, health changes, and functional decline, effective positive coping strategies and flexibility are key to maintaining well-being. If older adults are unable to flexibly cope with these challenges, their risk for suicide increases [28].
In addition to risk factors, which confer suicide risk but do not necessarily suggest that an older adult is thinking about suicide, warning signs exist that indicate that suicide risk is imminent. These include suicidal communication (ie, talking or writing about suicide), seeking access to means, and making preparations for suicide (eg, ensuring a will is in place, giving away prized possessions). One important note is that discussing and preparing for death may be developmentally appropriate for older adults, particularly those with chronic illnesses; however, such appropriate preparation is critically different from talking about suicide or a desire for death.
Additionally, a lack of planning for the future may be a warning sign. For example, older adults who decline to schedule medical follow-up or do not wish to refill needed prescriptions may be exhibiting warning signs that should be addressed. Similarly, not following needed medical regimens (eg, an older adult with diabetes no longer taking insulin) is also a warning sign. Other, potentially more subtle warning signs may include significant changes in mood, sleep, or social interactions. Older adults may become agitated and sleep less when they are considering suicide, or may feel more at ease after they have made the decision to die by suicide and their sleep or mood may improve. Withdrawing from valued others may also be a warning sign. Finally, recent major changes (eg, loss of a spouse, moving to an assisted living facility) may be triggers for suicide risk and can serve as warning signs themselves.
Specific Screening Strategies
Given the numerous risk factors and warning signs for older adult suicide, as well as the time limitations that primary care practitioners face [44,45], it would be impractical to comprehensively assess each older adult who presents at a primary care practice. Therefore, more specific screening is necessary. Most importantly, every older adult should be screened for suicide ideation and death ideation at every visit. Screening at every visit is critical because suicide ideation may develop at any point. Previous research has included screening of over 29,000 older adults in 11 primary care settings for suicide ideation, risk of alcohol misuse, and mental health disorders [15], suggesting that suicide risk screening is feasible. Other studies have also successfully used widespread screening for depression and suicide ideation among older adults in primary care [46–48]. Additionally, in an emergency department setting, universal suicide risk screening has been associated with significantly improved risk detection [49], indicating that improved screening may be beneficial in identifying suicide risk. Importantly, asking about suicide does not cause thoughts of suicide [50]. Additionally, it is a myth that those who talk about suicide ideation will not act on these thoughts [51].
When primary care practitioners inquire about suicide ideation, they should also ask about death ideation; though some may believe that death ideation is not as significant in terms of suicide risk as suicide ideation, recall that research has not found differences in previous suicide attempts or current hopelessness among older adults with death ideation versus suicide ideation [14]. Therefore, screening for death ideation should be completed as part of every suicide risk screening.
Screening can take many forms. Screening may be oral; asking an older adult if he or she is having thoughts of suicide or is experiencing a desire to die is a brief, 2-question screening that may provide valuable information (eg, “Are you having thoughts about your own death or wanting to die?”, “Are you having thoughts of killing yourself or thinking about suicide?”). This screening may be conducted by medical assistants, nurses, care managers, or physicians, with the patient’s responses documented. Importantly, a standard procedure should be implemented to ensure older adults are consistently asked about suicide risk at each visit, but do not feel inundated by such questions from numerous staff.
If verbal questions are asked, they must be asked appropriately. Euphemisms or indirect language should not be used during a screening; older adults should be directly asked about thoughts of death and suicide, not simply asked questions such as, “Have you ever had thoughts of harming or hurting yourself?” A question like this does not adequately assess current suicide risk, as it does not assess current thoughts, nor does it specifically inquire about suicide ideation (ie, killing one’s self). It is also important to phrase questions in a manner that invites honest responses and conveys an openness to listening. For example, asking, “You’re not thinking about suicide, are you?” suggests that the practitioner wants the older adult to say no and is not comfortable with the older adult endorsing suicide ideation. Open questions that allow endorsement or denial (eg, “Are you having thoughts of killing yourself?”) imply that the practitioner is receptive to either an endorsement or denial of suicide ideation.
Alternatively, a written screening can be used; older adults may complete a questionnaire prior to their appointment or while waiting to see their practitioner. Such an assessment may be a brief screening (eg, using similar yes/no questions to an oral screening), or may be a standardized measure. For example, the Geriatric Suicide Ideation Scale [52] is a 31-item self-report measure that provides scores for suicide ideation, death ideation, loss of personal and social worth, and perceived meaning in life. Though there are not standard cutoffs that suggest high versus low suicide risk, responses can be reviewed to identify whether older adults are reporting suicide ideation or death ideation, and can also be compared to norms (ie, average scores) from other older adults [52]. This measure also has the benefit of 2 subscales that do not specifically require reporting thoughts of suicide or death (ie, loss of personal and social worth, perceived meaning in life), which may give practitioners an indication of an older adult’s suicide risk even if the older adult is not comfortable disclosing suicide ideation, as has been shown in previous research [7,8].
Similarly, the Geriatric Depression Scale, which has a validated 15-item version [53], does not directly ask about suicide ideation but has a 5-item subscale that has been found to be highly correlated with reported suicide ideation [54]. When administered to older adult primary care patients, this subscale was an effective measure of suicide ideation; a score of ≥ 1 was the best cutoff for determining whether an older adult reported suicide ideation [55].
Additionally, as noted previously, the interaction between perceived burdensomeness and thwarted belongingness may identify older adults who are potentially experiencing, but not reporting, suicide ideation [41]. The Interpersonal Needs Questionnaire [56] is the validated assessment for both perceived burdensomeness and thwarted belongingness. Perceived burdensomeness is assessed via 6 self-report items, and thwarted belongingness is assessed via 9 self-report items on this measure [56]. There are not specific cutoffs that determine high versus low perceived burdensomeness or thwarted belongingness, but older adults’ responses can provide information about their experiences of these constructs. Administration of the Interpersonal Needs Questionnaire can provide information about potential risk for suicide among older adults who may otherwise deny thoughts of suicide or death.
If the screening for suicide ideation or death ideation is positive (ie, the older adult endorses thoughts of suicide or death), the treating primary care practitioner must then follow up with additional questions to determine current level of suicide risk. To make this determination, at a minimum, follow-up questions should focus on whether the older adult has any intent to die by suicide (eg, “Do you have any intent to act on your thoughts of suicide?”), as well as whether he or she has a plan to die by suicide (eg, “Have you begun formulating a plan to die by suicide?”). When asking about a plan, it is important to determine how specific the plan is. For example, an older adult with a specific method identified and date selected to implement the plan is at much higher risk than an older adult with a relatively vague idea. It is also critical to assess for the older adult’s access to means for suicide. If an older adult has a specific plan and has the capability to carry out the plan (eg, plans to overdose on prescription medication and has large quantities of medication or high-lethality medication at home), he or she is more likely to die by suicide than an older adult who does not have access to means (eg, only has small quantities of low-lethality medication available). A general assessment of risk factors and previous suicidal behavior (ie, any previous suicide attempts) also informs decisions about level of risk and interventions.
After a screening or assessment is completed, a risk determination must be made and documented. Acute suicide risk can be categorized as low, moderate, or high. It is not appropriate to say that there is “no” suicide risk present. Low risk occurs when there is no current suicide ideation, no plan to die by suicide, and no intent to act on suicidal thoughts, especially when the patient has no history of suicidal behavior and few risk factors [57]. Moderate risk is evident when there is current suicide ideation, but no specific plan to die by suicide or intent to act on suicidal thoughts. There are likely warning signs or risk factors, which may include previous suicidal behaviors, present in moderate suicide risk [57]. High risk is indicated by current suicide ideation with plan to die by suicide and suicidal intent. There are significant warning signs and risk factors present; there may also be a recent suicide attempt, though this is not a requirement for a high risk determination [57]. Undetermined suicide risk occurs when a practitioner cannot accurately assess risk, but concern regarding suicide is present; this is primarily used when a patient refuses to answer questions about suicide. Undetermined risk should be treated as at least moderate risk. Because research shows that death ideation has similar outcomes to suicide ideation in older adults [14], death ideation should also be factored into determinations of suicide risk; reports of death ideation may indicate low or moderate risk in older adults, dependent upon other risk factors, suicidal intent, and plan.
After a risk determination is made, it must be documented in the medical record. The level of risk and rationale for that determination must be included [58]. Stating only the level of risk without a rationale (ie, the older adult’s responses to questions) is not adequate, and documenting only the older adult’s responses without a determination of risk is also not sufficient. Finally, it is critical to document the intervention that occurred or steps taken after the level of risk was determined.
Critically, stating only that there was no indication of suicide risk is inadequate. For example, documenting “No evidence of suicide risk” is not appropriate. This documentation does not indicate that the older adult was specifically asked about suicide ideation, death ideation, suicidal intent, or plan to die by suicide. It also does not indicate a level of suicide risk. Examples of appropriate documentation include:
Patient was asked about suicide risk. She denied current suicide ideation but reported death ideation. She denied any current suicidal intent or plan. She also denied any previous suicide attempts. Therefore, acute suicide risk was deemed to be low. Provided patient with wallet card about the National Suicide Prevention Lifeline. Also called the Friendship Line while in the room with the patient to connect her with services. Finally, provided a brief list of local mental health professionals to patient; the patient reported she would like to see Dr. Smith. Called and left a message for Dr. Smith with referral information with patient during appointment.
Patient was asked about suicide risk. He reported both death ideation and suicide ideation. He also reported a nonspecific plan (ie, causing a single-vehicle motor vehicle accident, with no specific plan for the motor vehicle accident or timeframe) and denied any intent to act on his thoughts of suicide. He reported one previous suicide attempt, at age 22, by overdose on over-the-counter medication. He reported that this attempt did not require medical attention. Therefore, acute suicide risk was determined to be moderate. Patient was introduced to the behavioral health specialist, who met with the patient during the appointment to conduct further assessment and intervention.
Specific Intervention Strategies
Despite the fact that the pace of the primary care setting often does not allow for time-intensive intervention, there are ways to address suicide risk in this setting. Importantly, no-suicide contracts should not be used at any time [59,60]. No-suicide contracts are documents that patients who are experiencing suicide ideation are required to sign that state that they will not die by suicide while under the care of the practitioner. These contracts have no evidence of effectiveness, and some researchers argue that they may in fact damage the relationship with patients and serve the practitioner’s needs more than the patient’s needs [59].
One of the best options for older adults at low acute suicide risk is to provide resources and referrals. The National Suicide Prevention Lifeline can be reached at 1-800-273-TALK (8255); trained counselors are available to speak to patients at all times. Wallet cards with information about the National Suicide Prevention Lifeline are available at no charge from the US Substance Abuse and Mental Health Services Administration online store. The Friendship Line is another service available free to adults ages 60 and older, 24 hours per day, 7 days per week; this line can be reached at 1-800-971-0016. The Friendship Line, which is managed by the Institute on Aging, also provides outreach calls to older adults who may be isolated or lonely, increasing connectedness and potentially reducing suicide risk.
Having a ready list of local mental health professionals with expertise in geriatrics and suicide risk to provide to the patient is also beneficial. Recall, though, that older adults are less likely to seek out and receive mental health services [19]; therefore, connecting the patient with resources or referrals during the appointment is critical. If the practitioner does not have time to do this, having a medical assistant or other staff member that the patient knows engage in this step may be appropriate. For example, the patient can call the Friendship Line or National Suicide Prevention Lifeline while in the room with the practitioner, which may reduce anxiety or stigma about doing so and connect the patient with services. Similarly, calling a local mental health professional to make a referral during the appointment may increase the likelihood that the older adult will follow up on the referral.
The most ideal method of intervention for moderate or high acute suicide risk is a warm handoff to a behavioral or mental health specialist. As primary care and behavioral health become more integrated and financially viable as reimbursement through the Centers for Medicare and Medicaid Services improves [61], it is becoming increasingly likely that such a specialist will be on-site and available. Research has found that collaborative care in primary care reduces suicide risk in older adults [46–48,62]. Mental health specialists can conduct more comprehensive assessments and spend more time intervening to reduce suicide risk among older adults with death or suicide ideation. If an on-site behavioral health specialist is not available, older adults at high suicide risk may need to be referred to an emergency department for further evaluation and follow-up. Each state has its own laws and procedures regarding this process, which should be incorporated into a practice’s procedures for addressing high suicide risk. The procedure often involves ensuring that the older adult is accompanied at all times (ie, not left alone in a room), alerting emergency services (usually via phone call to an emergency line, such as 911), and completion of paperwork by a practitioner asserting that the patient is a danger to self. Police or other emergency personnel are then responsible for transporting the patient for further evaluation and determination of whether hospitalization is required.
If more time is available, either via the treating primary care practitioner or other patient care staff in the office, other brief interventions may be beneficial. First, means safety discussions are critical, particularly for older adults with plans for suicide or access to highly lethal means. In such discussions, patients are encouraged to restrict access to the methods that they may use to die by suicide. Plans for restricting access are developed, and when possible, a support person is enlisted to ensure that the plans are carried out. For example, if an older adult has access to firearms (eg, keeps a loaded weapon in his or her nightstand), he or she is encouraged to restrict his or her access to it. Ideally, this is through removing the weapon from the home, either permanently or until suicide risk reduces (eg, giving it to a friend, turning it over to police), but more safe storage may also be an option if the older adult is not willing to remove the weapon from the home. This may mean using a gun lock or storing the weapon in a gun safe, storing ammunition separately from an unloaded weapon, removing the firing pin, or otherwise disassembling the weapon. Means safety counseling has been shown to be effective in reducing suicide rates [63] and is acceptable to patients [64]. Studies indicate that over 90% of individuals who make a suicide attempt and survive do not go on to die by suicide [65]; therefore, reducing access to highly lethal means during a suicidal crisis may be key in reducing suicide rates. Though an in-depth review of means safety counseling is outside the scope of this article, readers are directed to Bryan, Stone, and Rudd’s article for a practical overview of means safety discussions [66].
Second, safety planning is a brief intervention that may be beneficial in the primary care setting [67,68]. The goal of a safety plan is to create an individualized plan to remain safe during a suicidal crisis. Means safety discussion is the last of 6 steps in the safety plan [68]. The first 5 steps include identifying warning signs, using internal coping strategies, social connectedness as distraction, social support for the crisis, and professionals that can be used as resources. When patients can identify specific, individualized warning signs that occur prior to a crisis, they can then use strategies to cope and prevent the crisis from worsening. Coping strategies that are encouraged are first internal (ie, those that can be done without relying on anyone else), such as exercise or journaling. If those do not improve the patient’s mood, then he or she is encouraged to use people or social settings as a distraction (eg, people watching at the mall, calling an acquaintance to chat), and if he or she is still feeling bad, encouraged to get social support for the crisis (eg, calling a family member to discuss the crisis and get support). Finally, if all of these steps are not effective, the older adult is encouraged to reach out to professional supports, such as a mental health provider, the National Suicide Prevention Lifeline, or 911 (or go to an emergency room). Readers are encouraged to review Stanley and Brown’s articles for comprehensive details about safety planning as an intervention [67,68]. Additionally, an article with specific adaptations for safety planning with older adults is forthcoming [69].
Conclusion
Older adults, particularly older men, are at high risk for suicide [1,2], and primary care practitioners are a critical component of older adult suicide prevention. Older adults frequently see primary care practitioners within a month prior to death by suicide [20,21]; primary care practitioners are uniquely qualified to address a broad range of potential risk factors, and may have more interactions and familiarity with older adults at risk for suicide than other medical professionals [20–22]. Primary care practitioners should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide. Screening can consist of standardized written questionnaires or oral questioning, and interventions may include providing resources and referrals, discussions about means safety, safety planning, and handoff to a mental health specialist. Interventions for suicide risk are likely feasible and acceptable in primary care [46–48]. Primary care practitioners have an important role to play in older adult suicide prevention, and must be prepared to interact with older adults who may be at risk for suicide.
Corresponding author: Danielle R. Jahn, PhD, Primary Care Institute, 605 NE 1st St, Gainesville, FL 32605, [email protected].
Financial disclosures: None reported.
From the Primary Care Institute, Gainesville, FL.
Abstract
- Objective: To provide primary care practitioners with the knowledge required to identify and address older adult suicide risk in their practice.
- Methods: Review of the literature and good clinical practices.
- Results: Primary care practitioners play an important role in older adult suicide prevention and must have knowledge about older adult suicide risk, including risk factors and warning signs in this age-group. Practitioners also must appropriately screen for and manage suicide risk. Older adults, particularly older men, are at high risk for suicide, though they may be less likely to report suicide ideation. Additionally, older adults frequently see primary care practitioners within a month prior to death by suicide. A number of older adult–specific risk factors are reviewed, and appropriate screening and intervention for the primary care setting are discussed.
- Conclusion: Primary care practitioners are uniquely qualified to address a broad range of potential risk factors and should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide.
Key words: suicide; older adults; risk factors; screening; safety planning.
Primary care practitioners play an important role in older adult suicide prevention and have a responsibility to identify and address suicide risk among older adults. To do so, practitioners must understand the problem of older adult suicide, recognize risk factors for suicide in older adults, screen for suicide risk, and appropriately assess and manage suicide risk. Primary care practitioners may face challenges in completing these tasks; the goal of this article is to assist practitioners in addressing these challenges.
Suicide in Older Adults
The United States has recently seen increases in suicide rates across the lifespan; from 1999 to 2014, the suicide rate rose by 24% across all ages [3]. Among both men and women aged 65 to 74, the suicide rate increased in this time period [3]. The high suicide rate among older adults is particularly important to address given the increasing numbers of older adults in the United States. By 2050, the older adult population in the United States is expected to reach 88.5 million, more than double the older adult population in 2010 [4]. Additionally, the generation that is currently aging into older adulthood has historically had higher rates of suicide across their lifespan [5]. Given that suicide rates also increase in older adulthood for men, the coming decades may evidence even higher rates of suicide among older adults than previously and it is critical that older adult suicide prevention becomes a public health priority.
It is also essential to discuss other suicide-related outcomes among older adults, including suicide attempts and suicide ideation. This is critical particularly because the ratio of suicide attempts to deaths by suicide in this age-group is 4 to 1 [1]. This is in contrast to the ratio of attempts to deaths across all ages, which is 25 suicide attempts per death by suicide [1]. This means that suicide prevention must occur before a first suicide attempt is made; suicide attempts cannot be used a marker of elevated suicide risk in older adults or an indication that intervention is needed. Intervention is required prior to suicide risk becoming elevated to the point of a suicide attempt.
It is also critical to recognize that despite the fact that suicide rates rise with age, reports of suicide ideation decrease with age [7,8]. Across all ages, 3.9% of Americans report past-year suicide ideation; however, only 2.7% of older adults report thoughts of suicide [9]. The discrepancy with the increasing rates of death by suicide with age suggest that suicide risk, and thereby opportunities for intervention, may be missed in this age-group [10].
However, older adults may be more willing to report death ideation, as research has found that over 15% of older adults endorse death ideation [11–13]. Death ideation is a desire for death without a specific desire to end one’s own life, and is an important suicide-related outcome, as older adults with death ideation appear the same as those with suicide ideation in terms of depression, hopelessness, and history of suicidal behavior [14]. Additionally, older adults with death ideation had more hospitalizations, more outpatient visits, and more medical issues than older adults with suicide ideation [15]. Therefore, death ideation should be taken as seriously as suicide ideation in older adults [14]. In sum, the high rates of death by suicide, the likelihood of death on a first or early suicide attempt, and the discrepancy between decreasing reports of suicide ideation and increasing rates of death by suicide among older adults indicate that older adult suicide is an important public health problem.
Suicide Prevention Strategies
Many suicide prevention strategies to date have focused on indicated prevention, which concentrates on individuals already identified at high risk (eg, those with suicide ideation or who have made a suicide attempt) [16]. However, because older adults may not report suicide ideation or survive a first suicide attempt, indicated prevention is likely not enough to be effective in older adult suicide prevention. A multilevel suicide prevention strategy [17] is required to prevent older adult suicide [18]. Older adult suicide prevention must include indicated prevention but must also include selective and universal prevention [16]. Selective prevention focuses on groups who may be at risk for suicide (eg, individuals with depression, older adults) and universal prevention focuses on the entire population (eg, interventions to reduce mental health stigma) [16]. To prevent older adult suicide, crisis intervention is critical, but suicide prevention efforts upstream of the development of a suicidal crisis are also essential.
The Importance of Primary Care
Research indicates that primary care is one of the best settings in which to engage in older adult suicide prevention [18]. Older adults are significantly less likely to receive specialty mental health care than younger adults, even when they have depressive symptoms [19]. Additionally, among older adults who died by suicide, 58% had contact with a primary care provider within a month of their deaths, compared to only 11% who had contact with a mental health specialist [20]. Among older adults who died by suicide, 67% saw any provider in the 4 weeks prior to their death [21]. Approximately 10% of older adults saw an outpatient mental health provider, 11% saw a primary care physician for a mental health issue, and 40% saw a primary care physician for a non-mental health issue [21]. Therefore, because older adults are less likely to receive specialty mental health treatment and so often seen a primary care practitioner prior to death by suicide, primary care may be the ideal place for older adult suicide risk to be detected and addressed, especially as many older adults visit primary care without a mental health presenting concern prior to their death by suicide.
Additionally, older adults may be more likely to disclose suicide ideation to primary care practitioners, with whom they are more familiar, than physicians in other settings (eg, emergency departments). Research has shown that familiarity with a primary care physician significantly increases the likelihood of patient disclosure of psychosocial issues to the physician [22]. Primary care providers also have a critical role as care coordinators; many older adults also see specialty physicians and use the emergency department. In fact, older adults are more likely to use the emergency department than younger adults, but emergency departments are not equipped to navigate the complex care needs of this population [23]. Primary care practitioners are important in ensuring that health issues of older adults are addressed by coordinating with specialists, hospitals (eg, inpatient stays, emergency department visits, surgery) and other health services (eg, home health care, physical therapy). Approximately 35% of older adults in the United States experience a lack of care coordination [24], which can negatively impact their health and leave issues such as suicide ideation unaddressed. Primary care practitioners may be critical in screening for mental health issues and suicide risk during even routine visits because of their familiarity with patients, and also play an important role in coordinating care for older adults to improve well-being and to ensure that critical issues, such as suicide ideation, are appropriately addressed.
Primary care practitioners can also be key in upstream prevention. Primary care practitioners are in a unique role to address risk factors for suicide prior to the development of a suicidal crisis. Because older adults frequently see primary care practitioners, such practitioners may have more opportunities to identify risk factors (eg, chronic pain, depression). Primary care practitioners are also trained to treat a broad range of conditions, providing the skills to address many different risk factors.
Finally, primary care is a setting in which screening for depression and suicide ideation among older adults is recommended. The US Preventive Services Task Force recommends screening for depression in all adults and older adults and provides recommended screening instruments, some of which include questions about self-harm or suicide risk [25]. However, this same group has concluded that there is insufficient evidence to support a recommendation for suicide risk screening [26]. Despite this, the Joint Commission recently released an alert that recommends screening for suicide risk in all settings, including primary care [27]. The Joint Commission requirement for ambulatory care that is relevant to suicide is PC.04.01.01: The organization has a process that addresses the patient’s need for continuing care, treatment, or services after discharge or transfer; behavioral health settings have additional suicide-specific requirements. The recommendations, though, go far beyond this requirement for primary care. The Joint Commission specifically notes that primary care clinicians play an important role in detecting suicide ideation and recommends that primary care practitioners review each patient’s history for suicide risk factors, screen all patients for suicide risk, review screenings before patients leave appointments, and take appropriate actions to address suicide risk when needed [27]. Further details are available in the Joint Commission’s Sentinel Event Alert titled, “Detecting and treating suicide ideation in all settings” [27]. Given these recommendations, primary care is an important setting in which to identify and address suicide risk.
Risk Factors for Older Adult Suicide
Numerous reviews exist that cover many risk factors for suicide in older adults [18,28]. This article will focus briefly on risk factors that are likely to be recognized and potentially addressed by primary care practitioners. Risk factors that apply across the lifespan can be recalled through a mnemonic: IS PATH WARM [29]. These risk factors include suicide Ideation, Substance abuse, Purposelessness, Anxiety (including agitation and poor sleep), feeling Trapped, Hopelessness, social Withdrawal, Anger or rage, Recklessness (ie, engaging in risky activities), and Mood changes. The National Suicide Prevention Lifeline also includes being in unbearable physical pain, perceiving one’s self as a burden to others, and seeking revenge on others as risk factors [30]. More specific to older adults, Conwell notes 5 categories or domains of risk factors with strong research support: psychiatric symptoms, somatic illness, functional impairment, social integration, and personality traits and coping [18,31].
Affective or mood disorders, particularly depression and depressive symptoms, are some of the most well-studied and strongest risk factors for older adult suicide [31]; 71% to 97% of all older adults who die by suicide have psychiatric illnesses [28]. Mood disorders, including major depressive episodes, are most consistently linked to older adult suicide risk; there is evidence as well for anxiety disorders and substance abuse disorders as risk factors, though it is somewhat mixed [28]. Therefore, screening for depression, anxiety, and substance abuse may be key to recognizing potential suicide risk. However, depression and anxiety do not present similarly in younger and older adults [32,33]. Depressive symptoms in older adults may be more somatic (eg, agitation, gastrointestinal symptoms) [32] and may reflect more anhedonia than mood changes [33]. Anxiety in older adults tends to be reported as stress or tension, whereas younger adults report feeling anxious or worried [33]. Additionally, substance abuse is often underrecognized, underdiagnosed, and undertreated in older adults [34]. Proactive screening for substance abuse is important as it may not interfere with work or other obligations in older adults, and therefore substance abuse may not be identified by older adults or others in their lives.
Physical illness may also be a risk factor for suicide [28,31]. Numerous diagnoses have been linked to suicide risk, including cancers, neurodegenerative diseases (eg, amyotrophic lateral sclerosis, Huntington disease), spinal cord injury, cardiovascular disease, and pulmonary disease [28,35]. However, overall illness burden (ie, number of chronic illnesses) [28] and self-perceived health [36] appear to be stronger risk factors than any specific illness. Additionally, authors have suggested that illness itself may not be a particularly strong risk factor, but the effect of illness on depressive symptoms [35], functioning, pain, or hopelessness due to the potential for decline over time [28] may increase suicide risk in older adults. Pain itself has been identified as a risk factor for suicide, as have perceptions of burden to others, hopelessness, and functional impairment [28].
In terms of functional impairment, research has shown that impairment in completing instrumental activities of daily living is associated with higher risk for death by suicide, and cognitive impairment may also be associated with elevated suicide risk [28]. However, there are some discrepant findings regarding the role of dementia in suicide risk, which may reflect medical and psychiatric comorbidities, as well as different stages of dementia or levels of cognitive impairment (eg, hopelessness about cognitive decline may increase suicide risk shortly after diagnosis, whereas lack of insight may decrease risk later in the course of the illness) [37]. Related to functional or cognitive impairment is perceived burdensomeness (ie, the perception that one is a liability or burden to others, to the point that others would be better off if one was gone) [38], which may also be associated with suicide risk in older adults [39,40]. Researchers have found that the interaction between perceived burdensomeness and thwarted belongingness (ie, a belief that one lacks reciprocal caring relationships and does not belong) identified older adults who were likely experiencing suicide ideation but did not report it [41]. These findings indicate that perceived burdensomeness and thwarted belongingness may be key in identifying older adults at risk for suicide.
Thwarted belongingness has also been linked to suicide ideation in older adults [41]. In fact, studies suggest that social integration is especially important for reducing suicide risk in this population [28,31,42]. A larger social network, living with others, and being active in the community are each protective against suicide [28]. Bereavement, which can reduce social connectedness and acts as a significant life stressor, is also an important risk factor [31]. Retirement may also reduce social connectedness, and employment changes have been identified as a suicide risk factor for older adults [28]. Retirement has been linked to risk for death by suicide in this population [43], and may not only serve to reduce social connectedness, but for some older adults may also be a significant role loss or loss of sense of purpose that can influence suicide risk.
Finally, rigid personality traits or coping styles are a risk factor for suicide among older adults [28,31]. As older adults face potential losses, health changes, and functional decline, effective positive coping strategies and flexibility are key to maintaining well-being. If older adults are unable to flexibly cope with these challenges, their risk for suicide increases [28].
In addition to risk factors, which confer suicide risk but do not necessarily suggest that an older adult is thinking about suicide, warning signs exist that indicate that suicide risk is imminent. These include suicidal communication (ie, talking or writing about suicide), seeking access to means, and making preparations for suicide (eg, ensuring a will is in place, giving away prized possessions). One important note is that discussing and preparing for death may be developmentally appropriate for older adults, particularly those with chronic illnesses; however, such appropriate preparation is critically different from talking about suicide or a desire for death.
Additionally, a lack of planning for the future may be a warning sign. For example, older adults who decline to schedule medical follow-up or do not wish to refill needed prescriptions may be exhibiting warning signs that should be addressed. Similarly, not following needed medical regimens (eg, an older adult with diabetes no longer taking insulin) is also a warning sign. Other, potentially more subtle warning signs may include significant changes in mood, sleep, or social interactions. Older adults may become agitated and sleep less when they are considering suicide, or may feel more at ease after they have made the decision to die by suicide and their sleep or mood may improve. Withdrawing from valued others may also be a warning sign. Finally, recent major changes (eg, loss of a spouse, moving to an assisted living facility) may be triggers for suicide risk and can serve as warning signs themselves.
Specific Screening Strategies
Given the numerous risk factors and warning signs for older adult suicide, as well as the time limitations that primary care practitioners face [44,45], it would be impractical to comprehensively assess each older adult who presents at a primary care practice. Therefore, more specific screening is necessary. Most importantly, every older adult should be screened for suicide ideation and death ideation at every visit. Screening at every visit is critical because suicide ideation may develop at any point. Previous research has included screening of over 29,000 older adults in 11 primary care settings for suicide ideation, risk of alcohol misuse, and mental health disorders [15], suggesting that suicide risk screening is feasible. Other studies have also successfully used widespread screening for depression and suicide ideation among older adults in primary care [46–48]. Additionally, in an emergency department setting, universal suicide risk screening has been associated with significantly improved risk detection [49], indicating that improved screening may be beneficial in identifying suicide risk. Importantly, asking about suicide does not cause thoughts of suicide [50]. Additionally, it is a myth that those who talk about suicide ideation will not act on these thoughts [51].
When primary care practitioners inquire about suicide ideation, they should also ask about death ideation; though some may believe that death ideation is not as significant in terms of suicide risk as suicide ideation, recall that research has not found differences in previous suicide attempts or current hopelessness among older adults with death ideation versus suicide ideation [14]. Therefore, screening for death ideation should be completed as part of every suicide risk screening.
Screening can take many forms. Screening may be oral; asking an older adult if he or she is having thoughts of suicide or is experiencing a desire to die is a brief, 2-question screening that may provide valuable information (eg, “Are you having thoughts about your own death or wanting to die?”, “Are you having thoughts of killing yourself or thinking about suicide?”). This screening may be conducted by medical assistants, nurses, care managers, or physicians, with the patient’s responses documented. Importantly, a standard procedure should be implemented to ensure older adults are consistently asked about suicide risk at each visit, but do not feel inundated by such questions from numerous staff.
If verbal questions are asked, they must be asked appropriately. Euphemisms or indirect language should not be used during a screening; older adults should be directly asked about thoughts of death and suicide, not simply asked questions such as, “Have you ever had thoughts of harming or hurting yourself?” A question like this does not adequately assess current suicide risk, as it does not assess current thoughts, nor does it specifically inquire about suicide ideation (ie, killing one’s self). It is also important to phrase questions in a manner that invites honest responses and conveys an openness to listening. For example, asking, “You’re not thinking about suicide, are you?” suggests that the practitioner wants the older adult to say no and is not comfortable with the older adult endorsing suicide ideation. Open questions that allow endorsement or denial (eg, “Are you having thoughts of killing yourself?”) imply that the practitioner is receptive to either an endorsement or denial of suicide ideation.
Alternatively, a written screening can be used; older adults may complete a questionnaire prior to their appointment or while waiting to see their practitioner. Such an assessment may be a brief screening (eg, using similar yes/no questions to an oral screening), or may be a standardized measure. For example, the Geriatric Suicide Ideation Scale [52] is a 31-item self-report measure that provides scores for suicide ideation, death ideation, loss of personal and social worth, and perceived meaning in life. Though there are not standard cutoffs that suggest high versus low suicide risk, responses can be reviewed to identify whether older adults are reporting suicide ideation or death ideation, and can also be compared to norms (ie, average scores) from other older adults [52]. This measure also has the benefit of 2 subscales that do not specifically require reporting thoughts of suicide or death (ie, loss of personal and social worth, perceived meaning in life), which may give practitioners an indication of an older adult’s suicide risk even if the older adult is not comfortable disclosing suicide ideation, as has been shown in previous research [7,8].
Similarly, the Geriatric Depression Scale, which has a validated 15-item version [53], does not directly ask about suicide ideation but has a 5-item subscale that has been found to be highly correlated with reported suicide ideation [54]. When administered to older adult primary care patients, this subscale was an effective measure of suicide ideation; a score of ≥ 1 was the best cutoff for determining whether an older adult reported suicide ideation [55].
Additionally, as noted previously, the interaction between perceived burdensomeness and thwarted belongingness may identify older adults who are potentially experiencing, but not reporting, suicide ideation [41]. The Interpersonal Needs Questionnaire [56] is the validated assessment for both perceived burdensomeness and thwarted belongingness. Perceived burdensomeness is assessed via 6 self-report items, and thwarted belongingness is assessed via 9 self-report items on this measure [56]. There are not specific cutoffs that determine high versus low perceived burdensomeness or thwarted belongingness, but older adults’ responses can provide information about their experiences of these constructs. Administration of the Interpersonal Needs Questionnaire can provide information about potential risk for suicide among older adults who may otherwise deny thoughts of suicide or death.
If the screening for suicide ideation or death ideation is positive (ie, the older adult endorses thoughts of suicide or death), the treating primary care practitioner must then follow up with additional questions to determine current level of suicide risk. To make this determination, at a minimum, follow-up questions should focus on whether the older adult has any intent to die by suicide (eg, “Do you have any intent to act on your thoughts of suicide?”), as well as whether he or she has a plan to die by suicide (eg, “Have you begun formulating a plan to die by suicide?”). When asking about a plan, it is important to determine how specific the plan is. For example, an older adult with a specific method identified and date selected to implement the plan is at much higher risk than an older adult with a relatively vague idea. It is also critical to assess for the older adult’s access to means for suicide. If an older adult has a specific plan and has the capability to carry out the plan (eg, plans to overdose on prescription medication and has large quantities of medication or high-lethality medication at home), he or she is more likely to die by suicide than an older adult who does not have access to means (eg, only has small quantities of low-lethality medication available). A general assessment of risk factors and previous suicidal behavior (ie, any previous suicide attempts) also informs decisions about level of risk and interventions.
After a screening or assessment is completed, a risk determination must be made and documented. Acute suicide risk can be categorized as low, moderate, or high. It is not appropriate to say that there is “no” suicide risk present. Low risk occurs when there is no current suicide ideation, no plan to die by suicide, and no intent to act on suicidal thoughts, especially when the patient has no history of suicidal behavior and few risk factors [57]. Moderate risk is evident when there is current suicide ideation, but no specific plan to die by suicide or intent to act on suicidal thoughts. There are likely warning signs or risk factors, which may include previous suicidal behaviors, present in moderate suicide risk [57]. High risk is indicated by current suicide ideation with plan to die by suicide and suicidal intent. There are significant warning signs and risk factors present; there may also be a recent suicide attempt, though this is not a requirement for a high risk determination [57]. Undetermined suicide risk occurs when a practitioner cannot accurately assess risk, but concern regarding suicide is present; this is primarily used when a patient refuses to answer questions about suicide. Undetermined risk should be treated as at least moderate risk. Because research shows that death ideation has similar outcomes to suicide ideation in older adults [14], death ideation should also be factored into determinations of suicide risk; reports of death ideation may indicate low or moderate risk in older adults, dependent upon other risk factors, suicidal intent, and plan.
After a risk determination is made, it must be documented in the medical record. The level of risk and rationale for that determination must be included [58]. Stating only the level of risk without a rationale (ie, the older adult’s responses to questions) is not adequate, and documenting only the older adult’s responses without a determination of risk is also not sufficient. Finally, it is critical to document the intervention that occurred or steps taken after the level of risk was determined.
Critically, stating only that there was no indication of suicide risk is inadequate. For example, documenting “No evidence of suicide risk” is not appropriate. This documentation does not indicate that the older adult was specifically asked about suicide ideation, death ideation, suicidal intent, or plan to die by suicide. It also does not indicate a level of suicide risk. Examples of appropriate documentation include:
Patient was asked about suicide risk. She denied current suicide ideation but reported death ideation. She denied any current suicidal intent or plan. She also denied any previous suicide attempts. Therefore, acute suicide risk was deemed to be low. Provided patient with wallet card about the National Suicide Prevention Lifeline. Also called the Friendship Line while in the room with the patient to connect her with services. Finally, provided a brief list of local mental health professionals to patient; the patient reported she would like to see Dr. Smith. Called and left a message for Dr. Smith with referral information with patient during appointment.
Patient was asked about suicide risk. He reported both death ideation and suicide ideation. He also reported a nonspecific plan (ie, causing a single-vehicle motor vehicle accident, with no specific plan for the motor vehicle accident or timeframe) and denied any intent to act on his thoughts of suicide. He reported one previous suicide attempt, at age 22, by overdose on over-the-counter medication. He reported that this attempt did not require medical attention. Therefore, acute suicide risk was determined to be moderate. Patient was introduced to the behavioral health specialist, who met with the patient during the appointment to conduct further assessment and intervention.
Specific Intervention Strategies
Despite the fact that the pace of the primary care setting often does not allow for time-intensive intervention, there are ways to address suicide risk in this setting. Importantly, no-suicide contracts should not be used at any time [59,60]. No-suicide contracts are documents that patients who are experiencing suicide ideation are required to sign that state that they will not die by suicide while under the care of the practitioner. These contracts have no evidence of effectiveness, and some researchers argue that they may in fact damage the relationship with patients and serve the practitioner’s needs more than the patient’s needs [59].
One of the best options for older adults at low acute suicide risk is to provide resources and referrals. The National Suicide Prevention Lifeline can be reached at 1-800-273-TALK (8255); trained counselors are available to speak to patients at all times. Wallet cards with information about the National Suicide Prevention Lifeline are available at no charge from the US Substance Abuse and Mental Health Services Administration online store. The Friendship Line is another service available free to adults ages 60 and older, 24 hours per day, 7 days per week; this line can be reached at 1-800-971-0016. The Friendship Line, which is managed by the Institute on Aging, also provides outreach calls to older adults who may be isolated or lonely, increasing connectedness and potentially reducing suicide risk.
Having a ready list of local mental health professionals with expertise in geriatrics and suicide risk to provide to the patient is also beneficial. Recall, though, that older adults are less likely to seek out and receive mental health services [19]; therefore, connecting the patient with resources or referrals during the appointment is critical. If the practitioner does not have time to do this, having a medical assistant or other staff member that the patient knows engage in this step may be appropriate. For example, the patient can call the Friendship Line or National Suicide Prevention Lifeline while in the room with the practitioner, which may reduce anxiety or stigma about doing so and connect the patient with services. Similarly, calling a local mental health professional to make a referral during the appointment may increase the likelihood that the older adult will follow up on the referral.
The most ideal method of intervention for moderate or high acute suicide risk is a warm handoff to a behavioral or mental health specialist. As primary care and behavioral health become more integrated and financially viable as reimbursement through the Centers for Medicare and Medicaid Services improves [61], it is becoming increasingly likely that such a specialist will be on-site and available. Research has found that collaborative care in primary care reduces suicide risk in older adults [46–48,62]. Mental health specialists can conduct more comprehensive assessments and spend more time intervening to reduce suicide risk among older adults with death or suicide ideation. If an on-site behavioral health specialist is not available, older adults at high suicide risk may need to be referred to an emergency department for further evaluation and follow-up. Each state has its own laws and procedures regarding this process, which should be incorporated into a practice’s procedures for addressing high suicide risk. The procedure often involves ensuring that the older adult is accompanied at all times (ie, not left alone in a room), alerting emergency services (usually via phone call to an emergency line, such as 911), and completion of paperwork by a practitioner asserting that the patient is a danger to self. Police or other emergency personnel are then responsible for transporting the patient for further evaluation and determination of whether hospitalization is required.
If more time is available, either via the treating primary care practitioner or other patient care staff in the office, other brief interventions may be beneficial. First, means safety discussions are critical, particularly for older adults with plans for suicide or access to highly lethal means. In such discussions, patients are encouraged to restrict access to the methods that they may use to die by suicide. Plans for restricting access are developed, and when possible, a support person is enlisted to ensure that the plans are carried out. For example, if an older adult has access to firearms (eg, keeps a loaded weapon in his or her nightstand), he or she is encouraged to restrict his or her access to it. Ideally, this is through removing the weapon from the home, either permanently or until suicide risk reduces (eg, giving it to a friend, turning it over to police), but more safe storage may also be an option if the older adult is not willing to remove the weapon from the home. This may mean using a gun lock or storing the weapon in a gun safe, storing ammunition separately from an unloaded weapon, removing the firing pin, or otherwise disassembling the weapon. Means safety counseling has been shown to be effective in reducing suicide rates [63] and is acceptable to patients [64]. Studies indicate that over 90% of individuals who make a suicide attempt and survive do not go on to die by suicide [65]; therefore, reducing access to highly lethal means during a suicidal crisis may be key in reducing suicide rates. Though an in-depth review of means safety counseling is outside the scope of this article, readers are directed to Bryan, Stone, and Rudd’s article for a practical overview of means safety discussions [66].
Second, safety planning is a brief intervention that may be beneficial in the primary care setting [67,68]. The goal of a safety plan is to create an individualized plan to remain safe during a suicidal crisis. Means safety discussion is the last of 6 steps in the safety plan [68]. The first 5 steps include identifying warning signs, using internal coping strategies, social connectedness as distraction, social support for the crisis, and professionals that can be used as resources. When patients can identify specific, individualized warning signs that occur prior to a crisis, they can then use strategies to cope and prevent the crisis from worsening. Coping strategies that are encouraged are first internal (ie, those that can be done without relying on anyone else), such as exercise or journaling. If those do not improve the patient’s mood, then he or she is encouraged to use people or social settings as a distraction (eg, people watching at the mall, calling an acquaintance to chat), and if he or she is still feeling bad, encouraged to get social support for the crisis (eg, calling a family member to discuss the crisis and get support). Finally, if all of these steps are not effective, the older adult is encouraged to reach out to professional supports, such as a mental health provider, the National Suicide Prevention Lifeline, or 911 (or go to an emergency room). Readers are encouraged to review Stanley and Brown’s articles for comprehensive details about safety planning as an intervention [67,68]. Additionally, an article with specific adaptations for safety planning with older adults is forthcoming [69].
Conclusion
Older adults, particularly older men, are at high risk for suicide [1,2], and primary care practitioners are a critical component of older adult suicide prevention. Older adults frequently see primary care practitioners within a month prior to death by suicide [20,21]; primary care practitioners are uniquely qualified to address a broad range of potential risk factors, and may have more interactions and familiarity with older adults at risk for suicide than other medical professionals [20–22]. Primary care practitioners should be prepared to identify risk factors and warning signs for older adult suicide, ask appropriate questions to screen for suicide risk, and intervene to prevent suicide. Screening can consist of standardized written questionnaires or oral questioning, and interventions may include providing resources and referrals, discussions about means safety, safety planning, and handoff to a mental health specialist. Interventions for suicide risk are likely feasible and acceptable in primary care [46–48]. Primary care practitioners have an important role to play in older adult suicide prevention, and must be prepared to interact with older adults who may be at risk for suicide.
Corresponding author: Danielle R. Jahn, PhD, Primary Care Institute, 605 NE 1st St, Gainesville, FL 32605, [email protected].
Financial disclosures: None reported.
1. American Association of Suicidology. U.S.A. suicide: 2014official final data. 2016. Accessed at www.suicidology.org/Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf.
2. Centers for Disease Control and Prevention. Leading causes of death reports, national and regional, 1999-2015. 2016. Accessed at https://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html.
3. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief, No 241. Hyattsville, MD: National Center for Health Statistics; 2016.
4. US Census Bureau. The next four decades. The older population in the United States: 2010 to 2050. 2010. Accessed at www.census.gov/prod/2010pubs/p25-1138.pdf.
5. Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep 2010;125:680–8.
6. Substance Abuse and Mental Health Services Administration. Issue brief 4: preventing suicide in older adults. 2012. Accessed at https://aoa.acl.gov/AoA_Programs/HPW/Behavioral/docs2/Issue%20Brief%204%20Preventing%20Suicide.pdf.
7. Duberstein PR, Conwell Y, Seidlitz L, et al. Age and suicidal ideation in older depressed inpatients. Am J Geriatr Psychiatry 1999;7:289–96.
8. Lynch TR, Johnson CS, Mendelson T, et al. Correlates of suicidal ideation among an elderly depressed sample. J Affect Disord 1999;56:9–15.
9. Centers for Disease Control and Prevention. Suicide: facts at a glance 2015. 2015. Accessed at www.cdc.gov/violenceprevention/pdf/suicide-datasheet-a.pdf.
10. Cukrowicz KC, Duberstein PR, Vannoy SD, et al. What factors determine disclosure of suicide ideation in adults 60 and older to a treatment provider? Suicide Life Threat Behav 2014;44:331–7.
11. Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int J Psychiatry Med 2006;36:183–98.
12. Scocco P, Fantoni G, Rapattoni M, et al. Death ideas, suicidal thoughts, and plans among nursing home residents. J Geriatr Psychiatry Neurol 2009;22:141–8.
13. Scocco P, Meneghel G, Caon F, et al. Death ideation and its correlates: survey of an over-65-year-old population. J Nerv Ment Dis 2001;189:210–8.
14. Szanto K, Reynolds III CF, Frank E, et al. Suicide in elderly patients: is active vs. passive suicidal ideation a clinically valid distinction? Am J Geriatr Psychiatry, 2002;4:197–207.
15. Bartels SJ, Coakley E, Oxman TE, et al. Suicidal and death ideation in older primary care patients with depression, anxiety, and at-risk alcohol use. Am J Geriatr Psychiatry 2002;10:417–27.
16. Yip PSF. A public health approach to suicide prevention. Hong Kong J Psychiatry 2005;15:29–31.
17. van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 2011;32:319–33.
18. Conwell Y. Suicide and suicide prevention in later life. Focus 2013;11:39–47.
19. Crabb R, Hunsley J. Utilization of mental health services among older adults with depression. J Clin Psychol 2006;62:299–312.
20. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry 2002;159:909–16.
21. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med 2014;29:870–7.
22. Robinson JW, Roter DL. Psychosocial problem disclosure by primary care patients. Soc Sci Med 1999;48:1353–62.
23. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47.
24. Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff 2014;33:2247–55.
25. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380–7.
26. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:719–26.
27. The Joint Commission. Detecting and treating suicide risk in all settings. Sentinel Event Alert 2016;56:1–7.
28. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68.
29. American Association of Suicidology. Know the warning signs of suicide. 2016. Accessed at www.suicidology.org/resources/warning-signs.
30. National Suicide Prevention Lifeline. Suicide warning signs. 2011. Accessed at www.suicidepreventionlifeline.org/App_Files/Media/PDF/NSPL_WalletCard.pdf.
31. Conwell Y. Suicide later in life: challenges and priorities for prevention. Am J Prev Med 2014;47:S244–50.
32. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
33. Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: comparison between older and younger clinical samples. Int Psychogeriatr 2015;27:1523–32.
34. Substance Abuse and Mental Health Services Administration. Substance abuse among older adults. Treatment Improvement Protocol (TIP) Series, No. 26. HHS Publication No. (SMA) 12-3918. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998.
35. Fiske A, O’Riley AA, Widoe RK. Physical health and suicide in late life: an evaluative review. Clin Gerontologist 2008;31:31–50.
36. Duberstein PR, Conwell Y, Conner KR, et al. Suicide at 50 years of age and older: perceived physical illness, family discord, and financial strain. Psychol Med 2004;34:137–46.
37. Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement 2010;6:75–82.
38. Joiner T. Why people die by suicide. Cambridge: Harvard University Press; 2005.
39. Jahn DR, Cukrowicz KC. The impact of the nature of relationships on perceived burdensomeness and suicide ideation in a community sample of older adults. Suicide Life Threat Behav 2011;41:635–49.
40. Jahn DR, Cukrowicz KC, Linton K, Prabhu F. The mediating effect of perceived burdensomeness on the relation between depressive symptoms and suicide ideation in a community sample of older adults. Aging Ment Health 2011;15:214–20.
41. Cukrowicz KC, Jahn DR, Graham RD, et al. Suicide risk in older adults: evaluating models of risk and predicting excess zeros in a primary care sample. J Abnorm Psychol 2013;122:1021–30.
42. Fassberg MM, Van Orden KA, Duberstein, P, et al. A systematic review of social factors and suicidal behavior in older adulthood. Int J Environ Res Public Health 2012;9:722–45.
43. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elderly: a psychological autopsy study in a north Italy area (1994-2004). Am J Geriatr Psychiatry 2008;16:727–35.
44. Konrad TR, Link CL, Shackelton RJ, et al. It’s about time: physicians’ perceptions of time constraints in primary medical practice in three national healthcare systems. Med Care 2010;48:95–100.
45. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2006;42:1871–94.
46. Bruce ML, Ten Have TR, Reynolds III CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91.
47. Alexopoulos GS, Reynolds CF III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
48. Unutzer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc 2006;54:1550–6.
49. Boudreaux ED, Camargo Jr CA, Arias SA, et al. Improving suicide risk screening and detection in the emergency department. Am J Prev Med 2016;50:445–53.
50. Mathias CW, Furr RM, Sheftall AH, et al. What’s the harm in asking about suicidal ideation? Suicide Life Threat Behav 2012;42:341–51.
51. Joiner T. Myths about suicide. Cambridge: Harvard University Press; 2011.
52. Heisel MJ, Flett GL. The development and initial validation of the Geriatric Suicide Ideation Scale. Am J Geriatr Psychiatry 2006;14:742–51.
53. Sheikh JL, Yesavage JA. Geriatric Depression Scale: recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Howarth Press; 1986: 165–73.
54. Heisel MJ, Flett GL, Duberstein PR, Lyness JM. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry 2005;13:876–83.
55. Heisel MJ, Duberstein PR, Lyness JM, Feldman MD. Screening for suicide ideation among older primary care patients. J Am Board Fam Med 2010;23:260–9.
56. Van Orden KA, Cukrowicz KC, Witte TK, Joiner Jr TE. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 2012;24;197–215.
57. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. Accessed at www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf.
58. Freedenthal S. Documentation: do it well, for the client’s sake and yours. 2013. Accessed at www.speakingofsuicide.com/2013/05/25/documentation/.
59. McMyler C, Pryjmachuk S. Do ‘no-suicide’ contracts work? J Psychiatr Ment Hlt 2008;15:512–22.
60. Rudd MD, Mandrusiak M, Joiner Jr TE. The case against no-suicide contracts: the commitment to treatment statement as a practice alternative. J Clin Psychol 2006;62:243–51.
61. National Institute of Mental Health. Adding better mental health care to primary care: a new era of behavioral health integration. 2016. Accessed at www.nimh.nih.gov/news/science-news/2016/adding-better-mental-health-care-to-primary-care.shtml.
62. Lapierre S, Erlangsen A, Waern M, et al. A systematic review of elderly suicide prevention programs. Crisis 2011;32;88–98.
63. Hawton K. Restricting access to methods of suicide: rationale and evaluation of this approach to suicide prevention. Crisis 2007;28:4–9.
64. Walters H, Kulkarni M, Forman J, et al. Feasibility and acceptability of interventions to delay gun access in VA mental health settings. Gen Hosp Psychiatry 2012;34:692–8.
65. Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. Brit J Psychiatry 2002;181:193–9.
66. Bryan CJ, Stone SL, Rudd MD. A practical, evidence-based approach for means-restriction counseling with suicidal patients. Prof Psychol Res Pr 2011;42:339–46.
67. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. 2008. Accessed at www.mentalhealth.va.gov/docs/va_safety_planning_manual.pdf.
68. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–64.
69. Jahn DR, Conti EC, Simons KV, et al. Evidence and considerations for safety planning as a suicide prevention strategy for older adults. 2017. Manuscript in preparation.
70. Knox KL, Stanley B, Currier GW, et al. An emergency department-based brief intervention for veterans at risk for suicide (SAFE VET). Am J Public Health 2012;102:S33–7.
1. American Association of Suicidology. U.S.A. suicide: 2014official final data. 2016. Accessed at www.suicidology.org/Portals/14/docs/Resources/FactSheets/2014/2014datapgsv1b.pdf.
2. Centers for Disease Control and Prevention. Leading causes of death reports, national and regional, 1999-2015. 2016. Accessed at https://webappa.cdc.gov/sasweb/ncipc/leadcaus10_us.html.
3. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief, No 241. Hyattsville, MD: National Center for Health Statistics; 2016.
4. US Census Bureau. The next four decades. The older population in the United States: 2010 to 2050. 2010. Accessed at www.census.gov/prod/2010pubs/p25-1138.pdf.
5. Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep 2010;125:680–8.
6. Substance Abuse and Mental Health Services Administration. Issue brief 4: preventing suicide in older adults. 2012. Accessed at https://aoa.acl.gov/AoA_Programs/HPW/Behavioral/docs2/Issue%20Brief%204%20Preventing%20Suicide.pdf.
7. Duberstein PR, Conwell Y, Seidlitz L, et al. Age and suicidal ideation in older depressed inpatients. Am J Geriatr Psychiatry 1999;7:289–96.
8. Lynch TR, Johnson CS, Mendelson T, et al. Correlates of suicidal ideation among an elderly depressed sample. J Affect Disord 1999;56:9–15.
9. Centers for Disease Control and Prevention. Suicide: facts at a glance 2015. 2015. Accessed at www.cdc.gov/violenceprevention/pdf/suicide-datasheet-a.pdf.
10. Cukrowicz KC, Duberstein PR, Vannoy SD, et al. What factors determine disclosure of suicide ideation in adults 60 and older to a treatment provider? Suicide Life Threat Behav 2014;44:331–7.
11. Kim YA, Bogner HR, Brown GK, Gallo JJ. Chronic medical conditions and wishes to die among older primary care patients. Int J Psychiatry Med 2006;36:183–98.
12. Scocco P, Fantoni G, Rapattoni M, et al. Death ideas, suicidal thoughts, and plans among nursing home residents. J Geriatr Psychiatry Neurol 2009;22:141–8.
13. Scocco P, Meneghel G, Caon F, et al. Death ideation and its correlates: survey of an over-65-year-old population. J Nerv Ment Dis 2001;189:210–8.
14. Szanto K, Reynolds III CF, Frank E, et al. Suicide in elderly patients: is active vs. passive suicidal ideation a clinically valid distinction? Am J Geriatr Psychiatry, 2002;4:197–207.
15. Bartels SJ, Coakley E, Oxman TE, et al. Suicidal and death ideation in older primary care patients with depression, anxiety, and at-risk alcohol use. Am J Geriatr Psychiatry 2002;10:417–27.
16. Yip PSF. A public health approach to suicide prevention. Hong Kong J Psychiatry 2005;15:29–31.
17. van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 2011;32:319–33.
18. Conwell Y. Suicide and suicide prevention in later life. Focus 2013;11:39–47.
19. Crabb R, Hunsley J. Utilization of mental health services among older adults with depression. J Clin Psychol 2006;62:299–312.
20. Luoma JB, Martin CE, Pearson JL. Contact with mental health and primary care providers before suicide: a review of the evidence. Am J Psychiatry 2002;159:909–16.
21. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med 2014;29:870–7.
22. Robinson JW, Roter DL. Psychosocial problem disclosure by primary care patients. Soc Sci Med 1999;48:1353–62.
23. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47.
24. Osborn R, Moulds D, Squires D, et al. International survey of older adults finds shortcomings in access, coordination, and patient-centered care. Health Aff 2014;33:2247–55.
25. Siu AL, US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;315:380–7.
26. LeFevre ML, U.S. Preventive Services Task Force. Screening for suicide risk in adolescents, adults, and older adults in primary care: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:719–26.
27. The Joint Commission. Detecting and treating suicide risk in all settings. Sentinel Event Alert 2016;56:1–7.
28. Conwell Y, Van Orden K, Caine ED. Suicide in older adults. Psychiatr Clin North Am 2011;34:451–68.
29. American Association of Suicidology. Know the warning signs of suicide. 2016. Accessed at www.suicidology.org/resources/warning-signs.
30. National Suicide Prevention Lifeline. Suicide warning signs. 2011. Accessed at www.suicidepreventionlifeline.org/App_Files/Media/PDF/NSPL_WalletCard.pdf.
31. Conwell Y. Suicide later in life: challenges and priorities for prevention. Am J Prev Med 2014;47:S244–50.
32. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012;200:275–81.
33. Wuthrich VM, Johnco CJ, Wetherell JL. Differences in anxiety and depression symptoms: comparison between older and younger clinical samples. Int Psychogeriatr 2015;27:1523–32.
34. Substance Abuse and Mental Health Services Administration. Substance abuse among older adults. Treatment Improvement Protocol (TIP) Series, No. 26. HHS Publication No. (SMA) 12-3918. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998.
35. Fiske A, O’Riley AA, Widoe RK. Physical health and suicide in late life: an evaluative review. Clin Gerontologist 2008;31:31–50.
36. Duberstein PR, Conwell Y, Conner KR, et al. Suicide at 50 years of age and older: perceived physical illness, family discord, and financial strain. Psychol Med 2004;34:137–46.
37. Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement 2010;6:75–82.
38. Joiner T. Why people die by suicide. Cambridge: Harvard University Press; 2005.
39. Jahn DR, Cukrowicz KC. The impact of the nature of relationships on perceived burdensomeness and suicide ideation in a community sample of older adults. Suicide Life Threat Behav 2011;41:635–49.
40. Jahn DR, Cukrowicz KC, Linton K, Prabhu F. The mediating effect of perceived burdensomeness on the relation between depressive symptoms and suicide ideation in a community sample of older adults. Aging Ment Health 2011;15:214–20.
41. Cukrowicz KC, Jahn DR, Graham RD, et al. Suicide risk in older adults: evaluating models of risk and predicting excess zeros in a primary care sample. J Abnorm Psychol 2013;122:1021–30.
42. Fassberg MM, Van Orden KA, Duberstein, P, et al. A systematic review of social factors and suicidal behavior in older adulthood. Int J Environ Res Public Health 2012;9:722–45.
43. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elderly: a psychological autopsy study in a north Italy area (1994-2004). Am J Geriatr Psychiatry 2008;16:727–35.
44. Konrad TR, Link CL, Shackelton RJ, et al. It’s about time: physicians’ perceptions of time constraints in primary medical practice in three national healthcare systems. Med Care 2010;48:95–100.
45. Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2006;42:1871–94.
46. Bruce ML, Ten Have TR, Reynolds III CF, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. J Am Med Assoc 2004;291:1081–91.
47. Alexopoulos GS, Reynolds CF III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009;166:882–90.
48. Unutzer J, Tang L, Oishi S, et al. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc 2006;54:1550–6.
49. Boudreaux ED, Camargo Jr CA, Arias SA, et al. Improving suicide risk screening and detection in the emergency department. Am J Prev Med 2016;50:445–53.
50. Mathias CW, Furr RM, Sheftall AH, et al. What’s the harm in asking about suicidal ideation? Suicide Life Threat Behav 2012;42:341–51.
51. Joiner T. Myths about suicide. Cambridge: Harvard University Press; 2011.
52. Heisel MJ, Flett GL. The development and initial validation of the Geriatric Suicide Ideation Scale. Am J Geriatr Psychiatry 2006;14:742–51.
53. Sheikh JL, Yesavage JA. Geriatric Depression Scale: recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Howarth Press; 1986: 165–73.
54. Heisel MJ, Flett GL, Duberstein PR, Lyness JM. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry 2005;13:876–83.
55. Heisel MJ, Duberstein PR, Lyness JM, Feldman MD. Screening for suicide ideation among older primary care patients. J Am Board Fam Med 2010;23:260–9.
56. Van Orden KA, Cukrowicz KC, Witte TK, Joiner Jr TE. Thwarted belongingness and perceived burdensomeness: construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol Assess 2012;24;197–215.
57. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for assessment and management of patients at risk for suicide. 2013. Accessed at www.healthquality.va.gov/guidelines/MH/srb/VADODCP_SuicideRisk_Full.pdf.
58. Freedenthal S. Documentation: do it well, for the client’s sake and yours. 2013. Accessed at www.speakingofsuicide.com/2013/05/25/documentation/.
59. McMyler C, Pryjmachuk S. Do ‘no-suicide’ contracts work? J Psychiatr Ment Hlt 2008;15:512–22.
60. Rudd MD, Mandrusiak M, Joiner Jr TE. The case against no-suicide contracts: the commitment to treatment statement as a practice alternative. J Clin Psychol 2006;62:243–51.
61. National Institute of Mental Health. Adding better mental health care to primary care: a new era of behavioral health integration. 2016. Accessed at www.nimh.nih.gov/news/science-news/2016/adding-better-mental-health-care-to-primary-care.shtml.
62. Lapierre S, Erlangsen A, Waern M, et al. A systematic review of elderly suicide prevention programs. Crisis 2011;32;88–98.
63. Hawton K. Restricting access to methods of suicide: rationale and evaluation of this approach to suicide prevention. Crisis 2007;28:4–9.
64. Walters H, Kulkarni M, Forman J, et al. Feasibility and acceptability of interventions to delay gun access in VA mental health settings. Gen Hosp Psychiatry 2012;34:692–8.
65. Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm: systematic review. Brit J Psychiatry 2002;181:193–9.
66. Bryan CJ, Stone SL, Rudd MD. A practical, evidence-based approach for means-restriction counseling with suicidal patients. Prof Psychol Res Pr 2011;42:339–46.
67. Stanley B, Brown GK. Safety plan treatment manual to reduce suicide risk: veteran version. 2008. Accessed at www.mentalhealth.va.gov/docs/va_safety_planning_manual.pdf.
68. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–64.
69. Jahn DR, Conti EC, Simons KV, et al. Evidence and considerations for safety planning as a suicide prevention strategy for older adults. 2017. Manuscript in preparation.
70. Knox KL, Stanley B, Currier GW, et al. An emergency department-based brief intervention for veterans at risk for suicide (SAFE VET). Am J Public Health 2012;102:S33–7.
CMS recognizes Society of Hospital Medicine’s Center for Quality Improvement
PHILADELPHIA – The Society of Hospital Medicine (SHM)’s Center for Quality Improvement (QI) has been distinguished by the Centers for Medicare & Medicaid Services for maintaining an ongoing collaborative partnership with CMS to enhance patient safety.
The letter of recognition from Paul McGann, MD, Jean Moody-Williams, RN, MPP, and Dennis Wagner, MPA, of the CMS, to Jenna Goldstein, MA, director of SHM’s Center for QI, and Kevin Vuernick, MPA, senior project manager, noted: “Over the last several years, our team has been privileged to partner with you and the Society of Hospital Medicine on the work of quality improvement and patient safety. Without relationships like these, the results in the reduction of patient harm we have seen at a national scale, saving 87,000 lives and nearly $20 billion in cost savings, would never have been possible.”
In August 2016, CMS’ Hospital Improvement Innovation Networks contacted SHM to participate in their weekly Partnership for Patients (PfP) Pacing Event webinar to present strategies for reducing opioid use and preventing adverse drug events, including SHM’s Mentored Implementation pilot program on Reducing Adverse Drug Events Related to Opioids (RADEO). SHM’s contribution to this webinar was twofold: Thomas W. Frederickson, MD, the lead author of the RADEO guide and one of two program mentors, spoke about the development of the RADEO program and its importance in the acute care setting. Matthew Jared, MD, a hospitalist at St. Anthony Hospital in Oklahoma City, one of the five pilot RADEO sites, discussed his experience implementing specific RADEO interventions as well as the mentoring provided by Dr. Frederickson of the department of hospital medicine at CHI Health in Omaha, Neb.
As a result of this successful partnership, SHM was contacted in January to provide its perspective on best practices in managing inpatients receiving opioids and adverse drug event data collection. At that time, Mr. Vuernick discussed the lessons learned between RADEO’s pilot program and the second iteration of RADEO, which launched in November 2016.
For more information about SHM’s Center for QI, please visit www.hospitalmedicine.org/QI. For more information about SHM and hospital medicine, visit www.hospitalmedicine.org and follow SHM on Twitter at @SHMLive.
PHILADELPHIA – The Society of Hospital Medicine (SHM)’s Center for Quality Improvement (QI) has been distinguished by the Centers for Medicare & Medicaid Services for maintaining an ongoing collaborative partnership with CMS to enhance patient safety.
The letter of recognition from Paul McGann, MD, Jean Moody-Williams, RN, MPP, and Dennis Wagner, MPA, of the CMS, to Jenna Goldstein, MA, director of SHM’s Center for QI, and Kevin Vuernick, MPA, senior project manager, noted: “Over the last several years, our team has been privileged to partner with you and the Society of Hospital Medicine on the work of quality improvement and patient safety. Without relationships like these, the results in the reduction of patient harm we have seen at a national scale, saving 87,000 lives and nearly $20 billion in cost savings, would never have been possible.”
In August 2016, CMS’ Hospital Improvement Innovation Networks contacted SHM to participate in their weekly Partnership for Patients (PfP) Pacing Event webinar to present strategies for reducing opioid use and preventing adverse drug events, including SHM’s Mentored Implementation pilot program on Reducing Adverse Drug Events Related to Opioids (RADEO). SHM’s contribution to this webinar was twofold: Thomas W. Frederickson, MD, the lead author of the RADEO guide and one of two program mentors, spoke about the development of the RADEO program and its importance in the acute care setting. Matthew Jared, MD, a hospitalist at St. Anthony Hospital in Oklahoma City, one of the five pilot RADEO sites, discussed his experience implementing specific RADEO interventions as well as the mentoring provided by Dr. Frederickson of the department of hospital medicine at CHI Health in Omaha, Neb.
As a result of this successful partnership, SHM was contacted in January to provide its perspective on best practices in managing inpatients receiving opioids and adverse drug event data collection. At that time, Mr. Vuernick discussed the lessons learned between RADEO’s pilot program and the second iteration of RADEO, which launched in November 2016.
For more information about SHM’s Center for QI, please visit www.hospitalmedicine.org/QI. For more information about SHM and hospital medicine, visit www.hospitalmedicine.org and follow SHM on Twitter at @SHMLive.
PHILADELPHIA – The Society of Hospital Medicine (SHM)’s Center for Quality Improvement (QI) has been distinguished by the Centers for Medicare & Medicaid Services for maintaining an ongoing collaborative partnership with CMS to enhance patient safety.
The letter of recognition from Paul McGann, MD, Jean Moody-Williams, RN, MPP, and Dennis Wagner, MPA, of the CMS, to Jenna Goldstein, MA, director of SHM’s Center for QI, and Kevin Vuernick, MPA, senior project manager, noted: “Over the last several years, our team has been privileged to partner with you and the Society of Hospital Medicine on the work of quality improvement and patient safety. Without relationships like these, the results in the reduction of patient harm we have seen at a national scale, saving 87,000 lives and nearly $20 billion in cost savings, would never have been possible.”
In August 2016, CMS’ Hospital Improvement Innovation Networks contacted SHM to participate in their weekly Partnership for Patients (PfP) Pacing Event webinar to present strategies for reducing opioid use and preventing adverse drug events, including SHM’s Mentored Implementation pilot program on Reducing Adverse Drug Events Related to Opioids (RADEO). SHM’s contribution to this webinar was twofold: Thomas W. Frederickson, MD, the lead author of the RADEO guide and one of two program mentors, spoke about the development of the RADEO program and its importance in the acute care setting. Matthew Jared, MD, a hospitalist at St. Anthony Hospital in Oklahoma City, one of the five pilot RADEO sites, discussed his experience implementing specific RADEO interventions as well as the mentoring provided by Dr. Frederickson of the department of hospital medicine at CHI Health in Omaha, Neb.
As a result of this successful partnership, SHM was contacted in January to provide its perspective on best practices in managing inpatients receiving opioids and adverse drug event data collection. At that time, Mr. Vuernick discussed the lessons learned between RADEO’s pilot program and the second iteration of RADEO, which launched in November 2016.
For more information about SHM’s Center for QI, please visit www.hospitalmedicine.org/QI. For more information about SHM and hospital medicine, visit www.hospitalmedicine.org and follow SHM on Twitter at @SHMLive.
PSYCHIATRY UPDATE 2017
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 500 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, March 30 to April 1, 2017, at the Marriott Chicago Magnificent Mile in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us at Psychiatry Update Encore in Las Vegas, December 10 to 12, 2017 or next year in Chicago, March 22 to 24, 2018.
View summaries from the event on the following pages.
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Greg W. Mattingly, MD, Midwest Research Group and St. Charles Psychiatric Associates, St. Charles, Missouri.
In an industry-sponsored symposium, Dr. Mattingly reported that in the STAR-D study, approximately one-half of patients with major depressive disorder (MDD) did not experience adequate response to an initial selective serotonin reuptake inhibitor and 3 of 4 of those non-responding patients did not achieve full response with a second antidepressant, which prompts consideration of an adjunctive agent. Brexpiprazole (Rexulti) is a partial agonist for serotonin, dopamine, and noradrenergic systems. In pivotal trials as an adjunctive treatment in MDD, brexpiprazole, 2 mg/d, resulted in a statistically significant decrease in Montgomery-Åsburg Depression Rating Scale scores compared with placebo. Most common adverse reactions observed in ≥5% of patients and at least twice the rate of placebo included akathisia and weight increase.
Essentials of Malingering Assessment
Douglas Mossman, MD, University of Cincinnati
Malingering is intentional lying with an external incentive, such as avoiding work or obtaining drugs. Dr. Mossman gave 2 examples of malingered posttraumatic stress disorder and psychosis. Although lying cannot be detected by careful examination of facial expressions or gestures, a detailed evaluation can reveal malingering. An individual who is malingering psychosis may describe symptoms, such as “I talk to voices all the time,” but clinicians never observe such behavior. Signs of malingering include using “textbook” terms for symptoms; inconsistencies in their history or symptoms; sudden onset of delusions; exaggerating; and being unpleasant, dishonest, or demanding.
Beyond Efficacy and Effectiveness: Neurotoxicity vs Neuroprotection are the REAL Differences Between Typical and Atypical Antipsychotics
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Dr. Nasrallah discussed the difference between typical vs atypical antipsychotics—the former is neurotoxic, the latter is neuroprotective. Because patients with schizophrenia experience a loss of brain volume and cerebral grey matter and increased lateral ventricle volume, consider atypical antipsychotics for their neuroprotective properties.
In several studies typical antipsychotics, such as haloperidol, have been found to be neurotoxic, causing apoptosis and decreased cell viability. Atypical antipsychotics may be beneficial for patients with schizophrenia because they:
- stimulate production of new brain cells and increase neurotropic factors
- reverse PCP-induced changes in gene expression and loss of dendritic spines in the frontal cortex
- are neuroprotective against ischemic stroke damage
- prevent oligodendrocyte damage caused by interferon gamma-stimulated microglia.
Medicolegal Hazards in the Information Age: Malpractice and More
Douglas Mossman, MD, University of Cincinnati
Dr. Mossman began by answering the question, “What should I do if a patient ‘friended’ me on Facebook?” Such online relationships can blur boundaries or risk breaching confidentiality, therefore medical organizations recommend ignoring a friend request. Telemedicine via Skype is cost effective and enhances outreach to patients in rural areas or who cannot travel to the office, but online clinical encounters lack multidimensional aspects of the interpersonal encounter and might not be HIPAA compliant. E-mail carries some of the same concerns, such as confidentially of personal information, although the practice—when employed appropriately—is supported by some medical associations, including the American Psychiatric Association.
Treatment-Resistance and Suicidality in Schizophrenia: 2 Major Management Challenges
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Patients can seem treatment-resistant because of inadequate antipsychotic dosing, smoking, substance-induced relapse, nonadherence, or a general medical condition. Dr. Nasrallah discussed how to recognize true treatment-resistant schizophrenia and rule of spurious treatment resistance. If your patient is truly treatment-resistant, what do you do when everything else fails?
Risk factors for suicide include male sex, depressed mood, substance use, and social isolation. Clozapine, the only drug FDA-approved for refractory schizophrenia and suicidality, is underutilized for such patients. Dr. Nasrallah also presented evidence for the use of adjunctive modalities, such as lamotrigine, steroids, omega-3 fatty acids, NSAIDs, antidepressants, glutamatergic agents, and rTMS, as well as psychotherapy.
Luncheon Symposium
Depression, Its impact, and the Importance of Recognition and Treatment
Faculty: Jon Winston Draud, MS, MD, University of Tennessee Health Science Center
Major depressive disorder (MDD) is the most commonly diagnosed condition, second to cardiovascular disease. Dr. Draud recommended using a wellness screen such as the WHO-5 (World Health Organization, 5 item well-being index) in addition to a depression screening tool such as the PHQ-9. He emphasized that the goal of treatment should not be merely remission—but remission without residual symptoms. Cognitive impairment is the most common residual symptom. Patients with residual symptoms relapse earlier (5.5 times faster) and at a greater rate than patients without residual symptoms (76% vs 25%, respectively). Continue treatment and monitoring even after symptoms appear to subside. Recommended treatment is multi-modal and should include cognitive therapy and exercise.
New and Old Treatments for Opioid Abuse and Dependence
Mark S. Gold, MD, Washington University
Each day more than 1,000 people are treated in emergency departments for improper use of prescription opioids. But is naloxone saving lives or is overdose reversal nothing more than CPR? Dr. Gold spoke about the need for psychiatric assessment after a patient has been revived. Historically, treatment has stopped at abstinence or overdose treatment, but patients need ongoing treatment. Family therapy, vocational assistance, and psychotherapy are essential.
Dr. Gold reviewed established and newer treatments, including naloxone and naltrexone. Methadone and buprenorphine-naloxone can be effective for adherent patients who abuse only one drug. Naltrexone gives patients time to get their lives on track. Probuphine has comparable efficacy with buprenorphine-naloxone and methadone.
Impact of a Personality Disorder in Management of Comorbid Disorder
Donald W. Black, MD, University of Iowa
Personality disorder (PD) indicates patterns of long-term functioning and are not limited to episodes of illness. Abnormal personality traits are common among the general population, but are not considered a personality disorder unless they are inflexible, maladaptive, persisting, and cause distress either for the patient or the family. There are few cases of “pure” PDs without a comorbid psychiatric disorder. Personality disorders are not as stable as once understood; they wax and wane in response to stressors or depressed mood or anxiety. When a PD is comorbid with another disorder, patients are less likely to respond to medication and to experience remission from the comorbid psychiatric disorder.
Evaluation and Treatment of Patients Who Abuse Methamphetamine or Cocaine
Mark S. Gold, MD, Washington University
There are no FDA-approved medications or advancement in treatment for cocaine overdose—primary treatment is still ice baths. When assessing cocaine use, consider the route of ingestion and duration of use, which influence severity. Stimulants, whether methamphetamine or cocaine, cause changes in dopamine that are difficult to reverse. Substitute stimulants, such as modafinil, or vaccines have been proposed for cocaine abuse, but the evidence is not robust. Methamphetamine produces a schizophrenia-like illness, but antipsychotics are not effective. Naltrexone and bupropion showed some efficacy but was not statistically significant. There are no effective treatments for overdose or relapse prevention other than traditional group and residential treatment approaches.
Risks in Using Cannabis
Kevin Hill, MD, MHS, McLean Hospital
Although only 9% of Cannabis users become dependent, Dr. Hill recommended talking to all patients who use Cannabis about the risks, such as problems with work, school, and relationships. When treating patients with Cannabis use disorder, explore reasons that the individual would want to stop using Cannabis, take a careful history, and most importantly, build a good therapeutic alliance.
The most robust data for medical Cannabis is for chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis; however, there are more than 70 indications among the 28 states that allow its use. Dr. Hill suggests having a written policy, engage in conversation about why the patient wants medical Cannabis, be open to evaluating such a patient, and consider treating the patient’s symptoms with traditional modalities.

Marlene P. Freeman, MD, Massachusetts General Hospital
Dr. Freeman discussed the important role mental health providers play in helping women during pregnancy decrease medical and obstetrical risks, such as nutrition and maintaining a healthy weight. Because one-half of pregnancies in the United States are unplanned, consider medications that are compatible with pregnancy, and recommend omega-3 fatty acids and lifestyle changes such as diet.
To diagnose premenstrual dysphoric disorder, Dr. Freeman recommends asking your patient to document and rate daily moods using a mobile app or calendar. In perimenopause, the risk of depression increases because estrogen has antidepressant effects. Although, there are no guidelines for treating depression in women in perimenopause, consider serotonergic antidepressants, supplements such as omega-3 fatty acids, isoflavones, and black cohosh, and sleep aids for patients with insomnia—a common feature of menopause.
ADHD, Bipolar Disorder, and Depression in Children
Jeffrey R. Strawn, MD, FAACP, University of Cincinnati
Attention-deficit/hyperactivity disorder (ADHD) and bipolar disorder (BD) may share an underlying biological etiology, Dr. Strawn explained. Shared risk factors include in utero events, dietary factors, and genetics. Differentiating ADHD from BD depends on the developmental stage of the patient. Symptoms overlap, which could lead to overdiagnosis of ADHD in youths with BD.
Dr. Strawn discussed how children with depression might display mood lability and irritability, rather than verbalizing feelings because they do not use language effectively until age 7. Children may have somatic symptoms early and irritability might decrease into adolescence. Anxiety disorder in children emerges early—usually as a phobia—around age 12 to 14, with an increase in onset of depressive disorders. Dr. Strawn reviewed screening tools to diagnose and track anxiety symptoms, as well as the pros and cons of pharmacological treatments.

George T. Grossberg, MD, Saint Louis University
Psychotic symptoms could be common in older adults; therefore it is important to evaluate whether these symptoms cause emotional suffering or impairment in daily function. Dr. Grossberg recommended that when treating psychotic disorders in geriatric patients to first evaluate and treat underlying medical problems and identify offending medications or environmental or psychosocial triggers, then consider psychosocial or environmental interventions. Consider antipsychotics for patients who are experiencing severe emotional distress or those who pose a high safety risk. If antipsychotics are necessary, pick an agent based on side effects, “start low, go slow,” and discuss the risks and benefits with the family.
Role of Psychiatrists in Long-term Care Facilities
In his presentation on the role of psychiatrists in long-term care facilities, Dr. Grossberg described common disorders including the behavioral and psychiatric symptoms of dementia, as well as risk for depression. Overprescribing is common in long-term care facilities; therefore when considering a patient’s medication regimen, often less is more. Dr. Grossberg also discussed common undertreated or undercorrected physical health problems, including hearing or vision deficits, obstructive sleep apnea, and malnutrition.
Treating Somatizing Patients
Alexander W. Thompson, MD, MBA, MPH, University of Iowa Carver College of Medicine
Somatizing patients experience symptoms all of the time, whether a headache or nausea, but most symptoms do not have an organic cause, and they might seek treatment for any or all symptoms. The goal of treating somatizing patients is to not harm them with unneeded workup and treatment. Dr. Thomspon recommends providing a letter to the patient’s primary care physician with your recommendations, which can reduce medical costs and improve physical function. Although there are no clear pharmacotherapies, cognitive-behavioral therapy focused on health and anxiety can help.
Fatigue
Fatigue experienced by patients with chronic fatigue syndrome is unrelenting, is not the result of ongoing exertion, and is unrelieved by rest. When approaching a patient with extreme fatigue, start with a thorough evaluation in collaboration with a primary care physician, Dr. Thompson said. Establish a rapport with the patient, limit iatrogenic harm, and treat chronic fatigue as you would any chronic condition. Rintatolimod and valganciclovir have showed some evidence of benefit, and graded exercise therapy has shown success.
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 500 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, March 30 to April 1, 2017, at the Marriott Chicago Magnificent Mile in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us at Psychiatry Update Encore in Las Vegas, December 10 to 12, 2017 or next year in Chicago, March 22 to 24, 2018.
View summaries from the event on the following pages.
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Greg W. Mattingly, MD, Midwest Research Group and St. Charles Psychiatric Associates, St. Charles, Missouri.
In an industry-sponsored symposium, Dr. Mattingly reported that in the STAR-D study, approximately one-half of patients with major depressive disorder (MDD) did not experience adequate response to an initial selective serotonin reuptake inhibitor and 3 of 4 of those non-responding patients did not achieve full response with a second antidepressant, which prompts consideration of an adjunctive agent. Brexpiprazole (Rexulti) is a partial agonist for serotonin, dopamine, and noradrenergic systems. In pivotal trials as an adjunctive treatment in MDD, brexpiprazole, 2 mg/d, resulted in a statistically significant decrease in Montgomery-Åsburg Depression Rating Scale scores compared with placebo. Most common adverse reactions observed in ≥5% of patients and at least twice the rate of placebo included akathisia and weight increase.
Essentials of Malingering Assessment
Douglas Mossman, MD, University of Cincinnati
Malingering is intentional lying with an external incentive, such as avoiding work or obtaining drugs. Dr. Mossman gave 2 examples of malingered posttraumatic stress disorder and psychosis. Although lying cannot be detected by careful examination of facial expressions or gestures, a detailed evaluation can reveal malingering. An individual who is malingering psychosis may describe symptoms, such as “I talk to voices all the time,” but clinicians never observe such behavior. Signs of malingering include using “textbook” terms for symptoms; inconsistencies in their history or symptoms; sudden onset of delusions; exaggerating; and being unpleasant, dishonest, or demanding.
Beyond Efficacy and Effectiveness: Neurotoxicity vs Neuroprotection are the REAL Differences Between Typical and Atypical Antipsychotics
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Dr. Nasrallah discussed the difference between typical vs atypical antipsychotics—the former is neurotoxic, the latter is neuroprotective. Because patients with schizophrenia experience a loss of brain volume and cerebral grey matter and increased lateral ventricle volume, consider atypical antipsychotics for their neuroprotective properties.
In several studies typical antipsychotics, such as haloperidol, have been found to be neurotoxic, causing apoptosis and decreased cell viability. Atypical antipsychotics may be beneficial for patients with schizophrenia because they:
- stimulate production of new brain cells and increase neurotropic factors
- reverse PCP-induced changes in gene expression and loss of dendritic spines in the frontal cortex
- are neuroprotective against ischemic stroke damage
- prevent oligodendrocyte damage caused by interferon gamma-stimulated microglia.
Medicolegal Hazards in the Information Age: Malpractice and More
Douglas Mossman, MD, University of Cincinnati
Dr. Mossman began by answering the question, “What should I do if a patient ‘friended’ me on Facebook?” Such online relationships can blur boundaries or risk breaching confidentiality, therefore medical organizations recommend ignoring a friend request. Telemedicine via Skype is cost effective and enhances outreach to patients in rural areas or who cannot travel to the office, but online clinical encounters lack multidimensional aspects of the interpersonal encounter and might not be HIPAA compliant. E-mail carries some of the same concerns, such as confidentially of personal information, although the practice—when employed appropriately—is supported by some medical associations, including the American Psychiatric Association.
Treatment-Resistance and Suicidality in Schizophrenia: 2 Major Management Challenges
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Patients can seem treatment-resistant because of inadequate antipsychotic dosing, smoking, substance-induced relapse, nonadherence, or a general medical condition. Dr. Nasrallah discussed how to recognize true treatment-resistant schizophrenia and rule of spurious treatment resistance. If your patient is truly treatment-resistant, what do you do when everything else fails?
Risk factors for suicide include male sex, depressed mood, substance use, and social isolation. Clozapine, the only drug FDA-approved for refractory schizophrenia and suicidality, is underutilized for such patients. Dr. Nasrallah also presented evidence for the use of adjunctive modalities, such as lamotrigine, steroids, omega-3 fatty acids, NSAIDs, antidepressants, glutamatergic agents, and rTMS, as well as psychotherapy.
Luncheon Symposium
Depression, Its impact, and the Importance of Recognition and Treatment
Faculty: Jon Winston Draud, MS, MD, University of Tennessee Health Science Center
Major depressive disorder (MDD) is the most commonly diagnosed condition, second to cardiovascular disease. Dr. Draud recommended using a wellness screen such as the WHO-5 (World Health Organization, 5 item well-being index) in addition to a depression screening tool such as the PHQ-9. He emphasized that the goal of treatment should not be merely remission—but remission without residual symptoms. Cognitive impairment is the most common residual symptom. Patients with residual symptoms relapse earlier (5.5 times faster) and at a greater rate than patients without residual symptoms (76% vs 25%, respectively). Continue treatment and monitoring even after symptoms appear to subside. Recommended treatment is multi-modal and should include cognitive therapy and exercise.
New and Old Treatments for Opioid Abuse and Dependence
Mark S. Gold, MD, Washington University
Each day more than 1,000 people are treated in emergency departments for improper use of prescription opioids. But is naloxone saving lives or is overdose reversal nothing more than CPR? Dr. Gold spoke about the need for psychiatric assessment after a patient has been revived. Historically, treatment has stopped at abstinence or overdose treatment, but patients need ongoing treatment. Family therapy, vocational assistance, and psychotherapy are essential.
Dr. Gold reviewed established and newer treatments, including naloxone and naltrexone. Methadone and buprenorphine-naloxone can be effective for adherent patients who abuse only one drug. Naltrexone gives patients time to get their lives on track. Probuphine has comparable efficacy with buprenorphine-naloxone and methadone.
Impact of a Personality Disorder in Management of Comorbid Disorder
Donald W. Black, MD, University of Iowa
Personality disorder (PD) indicates patterns of long-term functioning and are not limited to episodes of illness. Abnormal personality traits are common among the general population, but are not considered a personality disorder unless they are inflexible, maladaptive, persisting, and cause distress either for the patient or the family. There are few cases of “pure” PDs without a comorbid psychiatric disorder. Personality disorders are not as stable as once understood; they wax and wane in response to stressors or depressed mood or anxiety. When a PD is comorbid with another disorder, patients are less likely to respond to medication and to experience remission from the comorbid psychiatric disorder.
Evaluation and Treatment of Patients Who Abuse Methamphetamine or Cocaine
Mark S. Gold, MD, Washington University
There are no FDA-approved medications or advancement in treatment for cocaine overdose—primary treatment is still ice baths. When assessing cocaine use, consider the route of ingestion and duration of use, which influence severity. Stimulants, whether methamphetamine or cocaine, cause changes in dopamine that are difficult to reverse. Substitute stimulants, such as modafinil, or vaccines have been proposed for cocaine abuse, but the evidence is not robust. Methamphetamine produces a schizophrenia-like illness, but antipsychotics are not effective. Naltrexone and bupropion showed some efficacy but was not statistically significant. There are no effective treatments for overdose or relapse prevention other than traditional group and residential treatment approaches.
Risks in Using Cannabis
Kevin Hill, MD, MHS, McLean Hospital
Although only 9% of Cannabis users become dependent, Dr. Hill recommended talking to all patients who use Cannabis about the risks, such as problems with work, school, and relationships. When treating patients with Cannabis use disorder, explore reasons that the individual would want to stop using Cannabis, take a careful history, and most importantly, build a good therapeutic alliance.
The most robust data for medical Cannabis is for chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis; however, there are more than 70 indications among the 28 states that allow its use. Dr. Hill suggests having a written policy, engage in conversation about why the patient wants medical Cannabis, be open to evaluating such a patient, and consider treating the patient’s symptoms with traditional modalities.

Marlene P. Freeman, MD, Massachusetts General Hospital
Dr. Freeman discussed the important role mental health providers play in helping women during pregnancy decrease medical and obstetrical risks, such as nutrition and maintaining a healthy weight. Because one-half of pregnancies in the United States are unplanned, consider medications that are compatible with pregnancy, and recommend omega-3 fatty acids and lifestyle changes such as diet.
To diagnose premenstrual dysphoric disorder, Dr. Freeman recommends asking your patient to document and rate daily moods using a mobile app or calendar. In perimenopause, the risk of depression increases because estrogen has antidepressant effects. Although, there are no guidelines for treating depression in women in perimenopause, consider serotonergic antidepressants, supplements such as omega-3 fatty acids, isoflavones, and black cohosh, and sleep aids for patients with insomnia—a common feature of menopause.
ADHD, Bipolar Disorder, and Depression in Children
Jeffrey R. Strawn, MD, FAACP, University of Cincinnati
Attention-deficit/hyperactivity disorder (ADHD) and bipolar disorder (BD) may share an underlying biological etiology, Dr. Strawn explained. Shared risk factors include in utero events, dietary factors, and genetics. Differentiating ADHD from BD depends on the developmental stage of the patient. Symptoms overlap, which could lead to overdiagnosis of ADHD in youths with BD.
Dr. Strawn discussed how children with depression might display mood lability and irritability, rather than verbalizing feelings because they do not use language effectively until age 7. Children may have somatic symptoms early and irritability might decrease into adolescence. Anxiety disorder in children emerges early—usually as a phobia—around age 12 to 14, with an increase in onset of depressive disorders. Dr. Strawn reviewed screening tools to diagnose and track anxiety symptoms, as well as the pros and cons of pharmacological treatments.

George T. Grossberg, MD, Saint Louis University
Psychotic symptoms could be common in older adults; therefore it is important to evaluate whether these symptoms cause emotional suffering or impairment in daily function. Dr. Grossberg recommended that when treating psychotic disorders in geriatric patients to first evaluate and treat underlying medical problems and identify offending medications or environmental or psychosocial triggers, then consider psychosocial or environmental interventions. Consider antipsychotics for patients who are experiencing severe emotional distress or those who pose a high safety risk. If antipsychotics are necessary, pick an agent based on side effects, “start low, go slow,” and discuss the risks and benefits with the family.
Role of Psychiatrists in Long-term Care Facilities
In his presentation on the role of psychiatrists in long-term care facilities, Dr. Grossberg described common disorders including the behavioral and psychiatric symptoms of dementia, as well as risk for depression. Overprescribing is common in long-term care facilities; therefore when considering a patient’s medication regimen, often less is more. Dr. Grossberg also discussed common undertreated or undercorrected physical health problems, including hearing or vision deficits, obstructive sleep apnea, and malnutrition.
Treating Somatizing Patients
Alexander W. Thompson, MD, MBA, MPH, University of Iowa Carver College of Medicine
Somatizing patients experience symptoms all of the time, whether a headache or nausea, but most symptoms do not have an organic cause, and they might seek treatment for any or all symptoms. The goal of treating somatizing patients is to not harm them with unneeded workup and treatment. Dr. Thomspon recommends providing a letter to the patient’s primary care physician with your recommendations, which can reduce medical costs and improve physical function. Although there are no clear pharmacotherapies, cognitive-behavioral therapy focused on health and anxiety can help.
Fatigue
Fatigue experienced by patients with chronic fatigue syndrome is unrelenting, is not the result of ongoing exertion, and is unrelieved by rest. When approaching a patient with extreme fatigue, start with a thorough evaluation in collaboration with a primary care physician, Dr. Thompson said. Establish a rapport with the patient, limit iatrogenic harm, and treat chronic fatigue as you would any chronic condition. Rintatolimod and valganciclovir have showed some evidence of benefit, and graded exercise therapy has shown success.
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 500 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, March 30 to April 1, 2017, at the Marriott Chicago Magnificent Mile in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us at Psychiatry Update Encore in Las Vegas, December 10 to 12, 2017 or next year in Chicago, March 22 to 24, 2018.
View summaries from the event on the following pages.
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Greg W. Mattingly, MD, Midwest Research Group and St. Charles Psychiatric Associates, St. Charles, Missouri.
In an industry-sponsored symposium, Dr. Mattingly reported that in the STAR-D study, approximately one-half of patients with major depressive disorder (MDD) did not experience adequate response to an initial selective serotonin reuptake inhibitor and 3 of 4 of those non-responding patients did not achieve full response with a second antidepressant, which prompts consideration of an adjunctive agent. Brexpiprazole (Rexulti) is a partial agonist for serotonin, dopamine, and noradrenergic systems. In pivotal trials as an adjunctive treatment in MDD, brexpiprazole, 2 mg/d, resulted in a statistically significant decrease in Montgomery-Åsburg Depression Rating Scale scores compared with placebo. Most common adverse reactions observed in ≥5% of patients and at least twice the rate of placebo included akathisia and weight increase.
Essentials of Malingering Assessment
Douglas Mossman, MD, University of Cincinnati
Malingering is intentional lying with an external incentive, such as avoiding work or obtaining drugs. Dr. Mossman gave 2 examples of malingered posttraumatic stress disorder and psychosis. Although lying cannot be detected by careful examination of facial expressions or gestures, a detailed evaluation can reveal malingering. An individual who is malingering psychosis may describe symptoms, such as “I talk to voices all the time,” but clinicians never observe such behavior. Signs of malingering include using “textbook” terms for symptoms; inconsistencies in their history or symptoms; sudden onset of delusions; exaggerating; and being unpleasant, dishonest, or demanding.
Beyond Efficacy and Effectiveness: Neurotoxicity vs Neuroprotection are the REAL Differences Between Typical and Atypical Antipsychotics
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Dr. Nasrallah discussed the difference between typical vs atypical antipsychotics—the former is neurotoxic, the latter is neuroprotective. Because patients with schizophrenia experience a loss of brain volume and cerebral grey matter and increased lateral ventricle volume, consider atypical antipsychotics for their neuroprotective properties.
In several studies typical antipsychotics, such as haloperidol, have been found to be neurotoxic, causing apoptosis and decreased cell viability. Atypical antipsychotics may be beneficial for patients with schizophrenia because they:
- stimulate production of new brain cells and increase neurotropic factors
- reverse PCP-induced changes in gene expression and loss of dendritic spines in the frontal cortex
- are neuroprotective against ischemic stroke damage
- prevent oligodendrocyte damage caused by interferon gamma-stimulated microglia.
Medicolegal Hazards in the Information Age: Malpractice and More
Douglas Mossman, MD, University of Cincinnati
Dr. Mossman began by answering the question, “What should I do if a patient ‘friended’ me on Facebook?” Such online relationships can blur boundaries or risk breaching confidentiality, therefore medical organizations recommend ignoring a friend request. Telemedicine via Skype is cost effective and enhances outreach to patients in rural areas or who cannot travel to the office, but online clinical encounters lack multidimensional aspects of the interpersonal encounter and might not be HIPAA compliant. E-mail carries some of the same concerns, such as confidentially of personal information, although the practice—when employed appropriately—is supported by some medical associations, including the American Psychiatric Association.
Treatment-Resistance and Suicidality in Schizophrenia: 2 Major Management Challenges
Henry A. Nasrallah, MD, Saint Louis University School of Medicine
Patients can seem treatment-resistant because of inadequate antipsychotic dosing, smoking, substance-induced relapse, nonadherence, or a general medical condition. Dr. Nasrallah discussed how to recognize true treatment-resistant schizophrenia and rule of spurious treatment resistance. If your patient is truly treatment-resistant, what do you do when everything else fails?
Risk factors for suicide include male sex, depressed mood, substance use, and social isolation. Clozapine, the only drug FDA-approved for refractory schizophrenia and suicidality, is underutilized for such patients. Dr. Nasrallah also presented evidence for the use of adjunctive modalities, such as lamotrigine, steroids, omega-3 fatty acids, NSAIDs, antidepressants, glutamatergic agents, and rTMS, as well as psychotherapy.
Luncheon Symposium
Depression, Its impact, and the Importance of Recognition and Treatment
Faculty: Jon Winston Draud, MS, MD, University of Tennessee Health Science Center
Major depressive disorder (MDD) is the most commonly diagnosed condition, second to cardiovascular disease. Dr. Draud recommended using a wellness screen such as the WHO-5 (World Health Organization, 5 item well-being index) in addition to a depression screening tool such as the PHQ-9. He emphasized that the goal of treatment should not be merely remission—but remission without residual symptoms. Cognitive impairment is the most common residual symptom. Patients with residual symptoms relapse earlier (5.5 times faster) and at a greater rate than patients without residual symptoms (76% vs 25%, respectively). Continue treatment and monitoring even after symptoms appear to subside. Recommended treatment is multi-modal and should include cognitive therapy and exercise.
New and Old Treatments for Opioid Abuse and Dependence
Mark S. Gold, MD, Washington University
Each day more than 1,000 people are treated in emergency departments for improper use of prescription opioids. But is naloxone saving lives or is overdose reversal nothing more than CPR? Dr. Gold spoke about the need for psychiatric assessment after a patient has been revived. Historically, treatment has stopped at abstinence or overdose treatment, but patients need ongoing treatment. Family therapy, vocational assistance, and psychotherapy are essential.
Dr. Gold reviewed established and newer treatments, including naloxone and naltrexone. Methadone and buprenorphine-naloxone can be effective for adherent patients who abuse only one drug. Naltrexone gives patients time to get their lives on track. Probuphine has comparable efficacy with buprenorphine-naloxone and methadone.
Impact of a Personality Disorder in Management of Comorbid Disorder
Donald W. Black, MD, University of Iowa
Personality disorder (PD) indicates patterns of long-term functioning and are not limited to episodes of illness. Abnormal personality traits are common among the general population, but are not considered a personality disorder unless they are inflexible, maladaptive, persisting, and cause distress either for the patient or the family. There are few cases of “pure” PDs without a comorbid psychiatric disorder. Personality disorders are not as stable as once understood; they wax and wane in response to stressors or depressed mood or anxiety. When a PD is comorbid with another disorder, patients are less likely to respond to medication and to experience remission from the comorbid psychiatric disorder.
Evaluation and Treatment of Patients Who Abuse Methamphetamine or Cocaine
Mark S. Gold, MD, Washington University
There are no FDA-approved medications or advancement in treatment for cocaine overdose—primary treatment is still ice baths. When assessing cocaine use, consider the route of ingestion and duration of use, which influence severity. Stimulants, whether methamphetamine or cocaine, cause changes in dopamine that are difficult to reverse. Substitute stimulants, such as modafinil, or vaccines have been proposed for cocaine abuse, but the evidence is not robust. Methamphetamine produces a schizophrenia-like illness, but antipsychotics are not effective. Naltrexone and bupropion showed some efficacy but was not statistically significant. There are no effective treatments for overdose or relapse prevention other than traditional group and residential treatment approaches.
Risks in Using Cannabis
Kevin Hill, MD, MHS, McLean Hospital
Although only 9% of Cannabis users become dependent, Dr. Hill recommended talking to all patients who use Cannabis about the risks, such as problems with work, school, and relationships. When treating patients with Cannabis use disorder, explore reasons that the individual would want to stop using Cannabis, take a careful history, and most importantly, build a good therapeutic alliance.
The most robust data for medical Cannabis is for chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis; however, there are more than 70 indications among the 28 states that allow its use. Dr. Hill suggests having a written policy, engage in conversation about why the patient wants medical Cannabis, be open to evaluating such a patient, and consider treating the patient’s symptoms with traditional modalities.

Marlene P. Freeman, MD, Massachusetts General Hospital
Dr. Freeman discussed the important role mental health providers play in helping women during pregnancy decrease medical and obstetrical risks, such as nutrition and maintaining a healthy weight. Because one-half of pregnancies in the United States are unplanned, consider medications that are compatible with pregnancy, and recommend omega-3 fatty acids and lifestyle changes such as diet.
To diagnose premenstrual dysphoric disorder, Dr. Freeman recommends asking your patient to document and rate daily moods using a mobile app or calendar. In perimenopause, the risk of depression increases because estrogen has antidepressant effects. Although, there are no guidelines for treating depression in women in perimenopause, consider serotonergic antidepressants, supplements such as omega-3 fatty acids, isoflavones, and black cohosh, and sleep aids for patients with insomnia—a common feature of menopause.
ADHD, Bipolar Disorder, and Depression in Children
Jeffrey R. Strawn, MD, FAACP, University of Cincinnati
Attention-deficit/hyperactivity disorder (ADHD) and bipolar disorder (BD) may share an underlying biological etiology, Dr. Strawn explained. Shared risk factors include in utero events, dietary factors, and genetics. Differentiating ADHD from BD depends on the developmental stage of the patient. Symptoms overlap, which could lead to overdiagnosis of ADHD in youths with BD.
Dr. Strawn discussed how children with depression might display mood lability and irritability, rather than verbalizing feelings because they do not use language effectively until age 7. Children may have somatic symptoms early and irritability might decrease into adolescence. Anxiety disorder in children emerges early—usually as a phobia—around age 12 to 14, with an increase in onset of depressive disorders. Dr. Strawn reviewed screening tools to diagnose and track anxiety symptoms, as well as the pros and cons of pharmacological treatments.

George T. Grossberg, MD, Saint Louis University
Psychotic symptoms could be common in older adults; therefore it is important to evaluate whether these symptoms cause emotional suffering or impairment in daily function. Dr. Grossberg recommended that when treating psychotic disorders in geriatric patients to first evaluate and treat underlying medical problems and identify offending medications or environmental or psychosocial triggers, then consider psychosocial or environmental interventions. Consider antipsychotics for patients who are experiencing severe emotional distress or those who pose a high safety risk. If antipsychotics are necessary, pick an agent based on side effects, “start low, go slow,” and discuss the risks and benefits with the family.
Role of Psychiatrists in Long-term Care Facilities
In his presentation on the role of psychiatrists in long-term care facilities, Dr. Grossberg described common disorders including the behavioral and psychiatric symptoms of dementia, as well as risk for depression. Overprescribing is common in long-term care facilities; therefore when considering a patient’s medication regimen, often less is more. Dr. Grossberg also discussed common undertreated or undercorrected physical health problems, including hearing or vision deficits, obstructive sleep apnea, and malnutrition.
Treating Somatizing Patients
Alexander W. Thompson, MD, MBA, MPH, University of Iowa Carver College of Medicine
Somatizing patients experience symptoms all of the time, whether a headache or nausea, but most symptoms do not have an organic cause, and they might seek treatment for any or all symptoms. The goal of treating somatizing patients is to not harm them with unneeded workup and treatment. Dr. Thomspon recommends providing a letter to the patient’s primary care physician with your recommendations, which can reduce medical costs and improve physical function. Although there are no clear pharmacotherapies, cognitive-behavioral therapy focused on health and anxiety can help.
Fatigue
Fatigue experienced by patients with chronic fatigue syndrome is unrelenting, is not the result of ongoing exertion, and is unrelieved by rest. When approaching a patient with extreme fatigue, start with a thorough evaluation in collaboration with a primary care physician, Dr. Thompson said. Establish a rapport with the patient, limit iatrogenic harm, and treat chronic fatigue as you would any chronic condition. Rintatolimod and valganciclovir have showed some evidence of benefit, and graded exercise therapy has shown success.
Hormonal IUDs have higher expulsion rates immediately postpartum
Hormonal intrauterine devices inserted immediately postpartum had a nearly six times greater likelihood of expulsion compared with copper IUDs, but most women who requested any type of long-acting reversible contraception (LARC) postpartum were still using it half a year later, a recent study found.
“With more than eight out of ten women continuing use at 6 months, in-hospital placement of postpartum LARC devices is a worthwhile intervention,” reported Jennifer L. Eggebroten, MD, and her associates at the University of Utah. “More than half of women who experienced IUD expulsion without commencement of another highly effective contraceptive went on to become pregnant within 2 years, highlighting the need for appropriate counseling prior to device placement and backup contraception planning.”
Ninety percent of the patients were Hispanic, 87% had prior children, and 87% had an income below $24,000. Most (77%) had a vaginal delivery. Those who requested the copper IUD tended to be older and have more children compared with those who asked for the hormonal IUD or implant.
Among the 289 patients who completed the 6 months of follow-up, 17% of those with a hormonal IUD had an expulsion, compared with 4% of women with copper IUDs. That translated to a 5.8 times greater risk of expulsion for hormonal IUDs than for copper ones after the researchers accounted for age, mode of delivery, parity, and any breastfeeding. Expulsion rates were statistically similar between those who had vaginal deliveries and those who had cesarean deliveries.
Just 8% of the women requested removal of their device during the 6 months of follow-up. Most (67%) of the 21 women who had expulsions asked for a replacement. Cost of the device delayed or prevented replacement in some cases. Over the next 2 years, 6 of the 11 women who did not get replacement devices became pregnant.
Meanwhile, 81% of women with a hormonal IUD (88% including replacements), 83% with a copper IUD (86% including replacements), and 90% with an implant were still using that device 6 months later. A quarter of the women who completed the study follow-up reported that they did not return to their providers for their postpartum exams.
“For patients at high risk of rapid repeat pregnancy or who may not return for a postpartum visit, the benefit of placement of a highly effective method of contraception in the hospital prior to discharge may outweigh the increased risk of expulsion,” the researchers wrote. “In some states, by 8 weeks, public insurance coverage may expire and women face much more challenging obstacles to affordable, highly effective birth control options.”
The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
Hormonal intrauterine devices inserted immediately postpartum had a nearly six times greater likelihood of expulsion compared with copper IUDs, but most women who requested any type of long-acting reversible contraception (LARC) postpartum were still using it half a year later, a recent study found.
“With more than eight out of ten women continuing use at 6 months, in-hospital placement of postpartum LARC devices is a worthwhile intervention,” reported Jennifer L. Eggebroten, MD, and her associates at the University of Utah. “More than half of women who experienced IUD expulsion without commencement of another highly effective contraceptive went on to become pregnant within 2 years, highlighting the need for appropriate counseling prior to device placement and backup contraception planning.”
Ninety percent of the patients were Hispanic, 87% had prior children, and 87% had an income below $24,000. Most (77%) had a vaginal delivery. Those who requested the copper IUD tended to be older and have more children compared with those who asked for the hormonal IUD or implant.
Among the 289 patients who completed the 6 months of follow-up, 17% of those with a hormonal IUD had an expulsion, compared with 4% of women with copper IUDs. That translated to a 5.8 times greater risk of expulsion for hormonal IUDs than for copper ones after the researchers accounted for age, mode of delivery, parity, and any breastfeeding. Expulsion rates were statistically similar between those who had vaginal deliveries and those who had cesarean deliveries.
Just 8% of the women requested removal of their device during the 6 months of follow-up. Most (67%) of the 21 women who had expulsions asked for a replacement. Cost of the device delayed or prevented replacement in some cases. Over the next 2 years, 6 of the 11 women who did not get replacement devices became pregnant.
Meanwhile, 81% of women with a hormonal IUD (88% including replacements), 83% with a copper IUD (86% including replacements), and 90% with an implant were still using that device 6 months later. A quarter of the women who completed the study follow-up reported that they did not return to their providers for their postpartum exams.
“For patients at high risk of rapid repeat pregnancy or who may not return for a postpartum visit, the benefit of placement of a highly effective method of contraception in the hospital prior to discharge may outweigh the increased risk of expulsion,” the researchers wrote. “In some states, by 8 weeks, public insurance coverage may expire and women face much more challenging obstacles to affordable, highly effective birth control options.”
The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
Hormonal intrauterine devices inserted immediately postpartum had a nearly six times greater likelihood of expulsion compared with copper IUDs, but most women who requested any type of long-acting reversible contraception (LARC) postpartum were still using it half a year later, a recent study found.
“With more than eight out of ten women continuing use at 6 months, in-hospital placement of postpartum LARC devices is a worthwhile intervention,” reported Jennifer L. Eggebroten, MD, and her associates at the University of Utah. “More than half of women who experienced IUD expulsion without commencement of another highly effective contraceptive went on to become pregnant within 2 years, highlighting the need for appropriate counseling prior to device placement and backup contraception planning.”
Ninety percent of the patients were Hispanic, 87% had prior children, and 87% had an income below $24,000. Most (77%) had a vaginal delivery. Those who requested the copper IUD tended to be older and have more children compared with those who asked for the hormonal IUD or implant.
Among the 289 patients who completed the 6 months of follow-up, 17% of those with a hormonal IUD had an expulsion, compared with 4% of women with copper IUDs. That translated to a 5.8 times greater risk of expulsion for hormonal IUDs than for copper ones after the researchers accounted for age, mode of delivery, parity, and any breastfeeding. Expulsion rates were statistically similar between those who had vaginal deliveries and those who had cesarean deliveries.
Just 8% of the women requested removal of their device during the 6 months of follow-up. Most (67%) of the 21 women who had expulsions asked for a replacement. Cost of the device delayed or prevented replacement in some cases. Over the next 2 years, 6 of the 11 women who did not get replacement devices became pregnant.
Meanwhile, 81% of women with a hormonal IUD (88% including replacements), 83% with a copper IUD (86% including replacements), and 90% with an implant were still using that device 6 months later. A quarter of the women who completed the study follow-up reported that they did not return to their providers for their postpartum exams.
“For patients at high risk of rapid repeat pregnancy or who may not return for a postpartum visit, the benefit of placement of a highly effective method of contraception in the hospital prior to discharge may outweigh the increased risk of expulsion,” the researchers wrote. “In some states, by 8 weeks, public insurance coverage may expire and women face much more challenging obstacles to affordable, highly effective birth control options.”
The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: Hormonal IUDs were 5.8 times more likely to be expelled than were copper ones, but at least 80% of women who received a LARC still had it 6 months later.
Data source: A prospective cohort of 325 women who received a hormonal or copper IUD or a contraceptive implant immediately postpartum between October 2013 and February 2016.
Disclosures: The University of Utah and the Eunice Kennedy Shriver National Institute of Child Health and Human Development funded the research. The University of Utah receives research funding from LARC manufacturers and one of the coauthors reported financial relationships with LARC manufacturers.
Liver disease likely to become increasing indication for bariatric surgery
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
AGA Resource
The AGA Obesity Practice Guide provides tools for gastroenterologists to lead a multidisciplinary team of health-care professionals for the management of patients with obesity. Learn more at www.gastro.org/obesity.
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
AGA Resource
The AGA Obesity Practice Guide provides tools for gastroenterologists to lead a multidisciplinary team of health-care professionals for the management of patients with obesity. Learn more at www.gastro.org/obesity.
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
AGA Resource
The AGA Obesity Practice Guide provides tools for gastroenterologists to lead a multidisciplinary team of health-care professionals for the management of patients with obesity. Learn more at www.gastro.org/obesity.
AT DIGESTIVE DISEASES: NEW ADVANCES
Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.
A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.
The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.

After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.
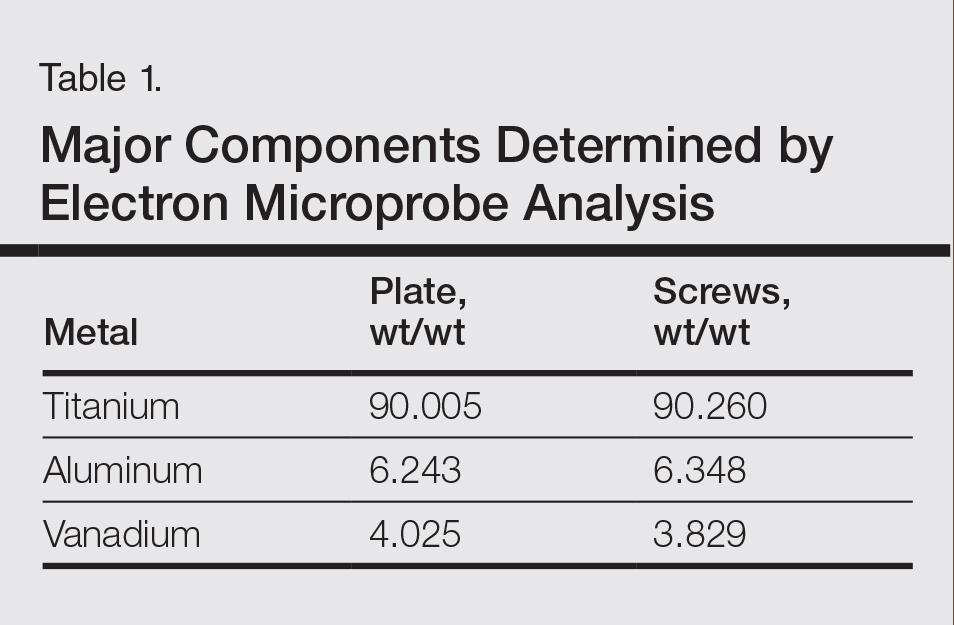
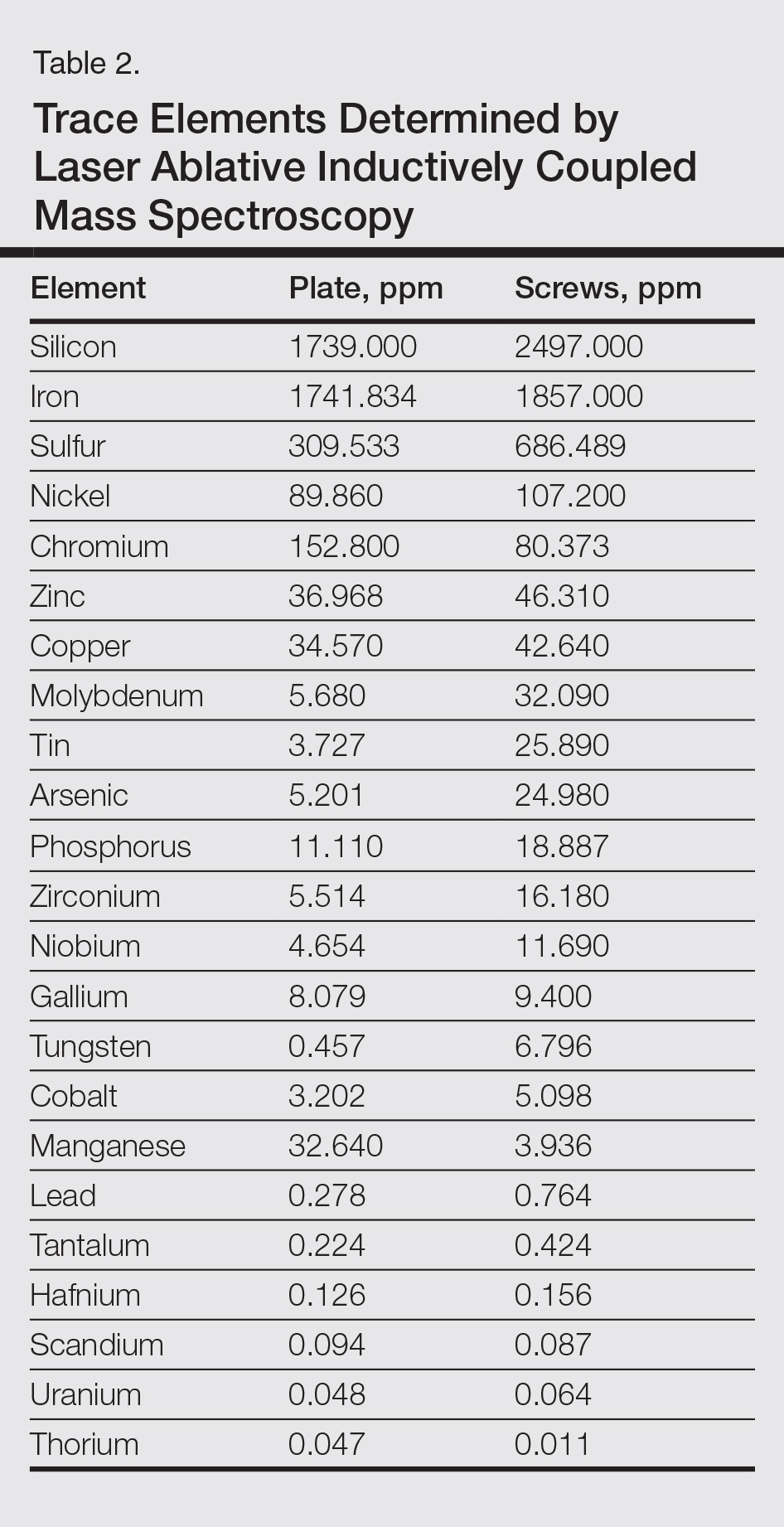
Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.


Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
Metal allergy in patients with orthopedic implants can cause serious problems including dermatitis and implant failure.1 As life expectancy increases, the general population ages, and more metallic orthopedic implants are placed,2 allergy to these implants is expected to be a problem of greater significance. Uncertainty remains regarding best practice for patients with suspected metal implant allergy.1 The major questions are: Who should be tested? When should they be tested? What are the optimal tests to diagnose metal allergy?3-8
We report the case of a patient with vanadium allergy who developed a diffuse eczematous dermatitis and implant failure after receiving a vanadium-containing titanium alloy orthopedic implant in the left foot. This case is remarkable because hypersensitivity reactions to titanium-based hardware are rare, as they traditionally have not been thought to provoke allergic reactions.9
Case Report
A 62-year-old woman who was otherwise healthy presented with an eruption of more than 80 pruritic, nummular, eczematous plaques on the arms, legs, back, and buttocks of 3 weeks’ duration (Figure 1). She had a history of allergy to metal used in costume jewelry. Six weeks prior, the patient underwent implantation of a titanium alloy plate in the left foot for surgical repair of painful deforming osteoarthritis. A radiograph of the foot showed appropriate placement. According to the manufacturer, the plate was composed of the compound Ti6Al4V, which contained 90% titanium, 6% aluminum, and 4% vanadium. The lesions developed on the skin close to but not directly over the surgical site.

A punch biopsy of one of the lesions on the shoulder showed lymphoeosinophilic spongiosis consistent with a delayed hypersensitivity reaction (Figure 2). There was mild clinical improvement of the eruption with topical steroids. A course of prednisone for systemic effect resulted in clearing of the eruption, but it promptly recurred on cessation of the steroids. The patient was then patch tested using the North American 80 Comprehensive Series, with an additional 59 common textile, shampoo, fragrance, and several metal allergens, all of which were negative.

The patient had persistent pain and swelling at the surgical site, and radiographs taken postoperatively at 6 months showed implant failure (Figure 3). The hardware was surgically removed 8 months after implantation (Figure 4) and the plate and screws were submitted to the Institute for Mineral Resources Geosciences LA-ICP-MS Facility and the Lunar and Planetary Laboratory at the University of Arizona (Tucson, Arizona) for analysis. The skin lesions began to improve days after the hardware was removed and the eruption cleared over the following 3 weeks with no additional treatment.


After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.


Comment
Titanium is an attractive metal to use in orthopedic implants. It has a high strength-to-weight ratio, a low modulus of elasticity, and good resistance to corrosion. Titanium can be categorized as either commercially pure titanium (cp-Ti) or a titanium alloy. Colloquially, both cp-Ti and titanium alloys are often referred to simply as titanium, but the distinction is important when it comes to medical implants and devices. Commercially pure titanium is more than 99% pure titanium, but up to 1% of its volume can be comprised of impurities.10 In titanium alloys, the alloy elements are intentionally added to create a material with optimal properties. The 2 most common types of titanium that are used for orthopedic implants are cp-Ti and Ti6Al4V, a titanium alloy containing approximately 90% titanium, 6% aluminum, and 4% vanadium. Similar to cp-Ti, titanium alloys also can contain impurities such as aluminum, beryllium, cobalt, chromium, iron, nickel, and palladium, among many others. Although these impurities often are considered negligible from a metallurgy perspective, as they do not change the properties of the material, these trace elements may be present in large enough quantities to cause hypersensitivity reactions.11
Several weeks after implantation of a titanium alloy metal plate in the left foot, a widespread eczematous eruption developed in our patient who had no prior skin disease. The eruption was steroid responsive but did not clear until the plate was removed. Detailed metallurgy analysis confirmed that vanadium was present and was not homogeneously distributed in the plate. The plate also was different in composition from the screws. Additional studies showed that galvanic corrosion between the plate and the chemically different screws might have contributed to the release of vanadium in the tissue.
Vanadium is known to be allergenic, especially in the presence of implant failure.12,13 In our patient, patch testing with more than 100 allergens was negative, except for vanadium trichloride 1%. Our patient’s presentation strongly suggested that she developed a vanadium allergy manifesting as systemic allergic contact dermatitis. She demonstrated no history of skin disease, a widespread eczematous eruption after exposure, histology consistent with systemic contact allergy, a positive patch test to vanadium, and clearance of the eruption on removal of the antigen, which have been proposed as objective criteria that support a diagnosis of metal implant allergy.14 She refused our suggestion to reimplant a portion of the remaining plate under the skin without screws and monitor for recurrence of the eruption. She did not have a lesion overlying the surgical site, but she did develop lesions near the surgical scar. The literature indicates that cutaneous manifestations of allergy to metallic implants can be both localized and generalized.14
Although reports are rare, other researchers have found vanadium allergy in patients with metal orthopedic implants.5,12,13,15 The scarcity of literature on vanadium allergy seems to suggest that it is a rare entity, but we believe that it may be more common. Vanadium allergy may be underdiagnosed because it is not a standard patch test allergen. Furthermore, many of those who do choose to test for it use what we believe to be ineffective formulas of vanadium when patch testing patients. Our patient demonstrated a positive patch test reaction only to vanadium trichloride and not to pure vanadium, which is consistent with the small number of other studies that investigated vanadium allergy.5,12,13,15 We believe that vanadium trichloride is more water soluble than elemental vanadium,16 and thus more likely to identify true vanadium allergy than other test materials.
Although reports of vanadium allergy in patients with metal implants are rare in the medical literature, the material science literature clearly states that vanadium is toxic and that vanadium-containing implants are problematic.17-20 It has been shown that although Ti6Al4V implants are considered highly resistant to corrosion, they will slowly and continuously corrode in a physiologic environment and release titanium, aluminum, and vanadium ions, both systemically and into the peri-implant space.11 To address these problems with vanadium, vanadium-free titanium alloys such as Ti6Al7Nb have specifically been developed for medical use to address the problems caused by vanadium. Ti6Al7Nb contains 7% niobium rather than vanadium and appears to have some improved qualities in surgical implants.17
There is still a great deal of uncertainty around metal implant allergy. Allergy to metal implants can be difficult to diagnose for several reasons. Some metals are not conducive to patch testing because of their low bioavailability. Additionally, we lack validated and standardized patch test formulas for metals that can be diagnosed by patch testing. Furthermore, there is uncertainty about what to do after allergy to a metal implant is diagnosed; in some cases (eg, with more extensive procedures such as total joint replacements), removal or replacement of the implant may be associated with increased risk of further complications.6,21
Conclusion
We suggest that manufacturers consider vanadium-free alloys such as Ti7Al6Nb, which contains niobium instead of vanadium, in their surgical implants,22 and if surgeons have a choice, they should consider using titanium implants with niobium rather than vanadium.10 We suggest that clinicians consider vanadium allergy in patients with Ti6Al4V surgical implants and signs of a hypersensitivity reaction, and include vanadium trichloride 1% when patch testing.
Acknowledgment
The authors would like to thank Nicholas R. Krasnow, PhD (Tucson, Arizona), for his invaluable help coordinating, performing, and interpreting the metal analyses.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
- Thyssen JP, Johansen JD, Menné T, et al. Hypersensitivity reactions from metallic implants: a future challenge that needs to be addressed. Br J Dermatol. 2010;162:235-236.
- Aquino M, Mucci T. Systemic contact dermatitis and allergy to biomedical devices. Curr Allergy Asthma Rep. 2013;13:518-527.
- Krecisz B, Kiec-Swierczynska M, Chomiczewska-Skora D. Allergy to orthopedic metal implants—a prospective study. Int J Occup Med Environ Health. 2012;25:463-469.
- Atanaskova Mesinkovska N, Tellez A, Molina L, et al. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693.
- Frigerio E, Pigatto PD, Guzzi G, et al. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279.
- Amini M, Mayes WH, Tzeng TH, et al. Evaluation and management of metal hypersensitivity in total joint arthroplasty: a systematic review. J Long Term Eff Med Implants. 2014;24:25-36.
- Thomas P, Bandl WD, Maier S, et al. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis. 2006;55:199-202.
- Wood MM, Warshaw EM. Hypersensitivity reactions to titanium: diagnosis and management. Dermatitis. 2015;26:7-25.
- Cadosch D, Chan E, Gautschi OP, et al. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91:1252-1262.
- Granchi D, Cenni E, Trisolino G, et al. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-264.
- Granchi D, Cenni E, Tigani D, et al. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-1500.
- Thyssen JP, Menné T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Kręcisz B, Kieć-Świerczyńska M, Bąkowicz-Mitura K. Allergy to metals as a cause of orthopedic implant failure. Int J Occup Med Environ Health. 2006;19:178-180.
- Costigan M, Cary R, Dobson S. Vanadium Pentoxide and Other Inorganic Vanadium Compounds. Geneva, Switzerland: World Health Organization; 2001.
- Challa VS, Mali S, Misra RD. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J Biomed Mater Res A. 2013;101:2083-2089.
- Okazaki Y, Rao S, Ito Y, et al. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197-1215.
- Paszenda Z, Walke W, Jadacka S. Electrochemical investigations of Ti6Al4V and Ti6Al7Nb alloys used on implants in bone surgery. J Achievements Materials Manufacturing Eng. 2010;38:24-32.
- Wang K. The use of titanium for medical applications in the USA. Materials Sci Eng A. 1996:134-137.
- Haseeb M, Butt MF, Altaf T, et al. Indications of implant removal: a study of 83 cases. Int J Health Sci (Qassim). 2017;11:1-7.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress Materials Sci. 2009;54:397-425.
Practice Points
- Vanadium may be an underrecognized allergen in patients with metal implants.
- Consider vanadium allergy in those with surgical implants and signs of hypersensitivity reaction.
- Test for allergy with vanadium trichloride.
- Niobium is an alternative for implants in vanadium-allergic patients.
Refractory vasovagal syncope responds to DDD-CLS pacing
Washington – Help is finally at hand for the subgroup of patients who have severe refractory vasovagal syncope that recurs frequently and unpredictably, and is associated with a strong cardioinhibitory response on tilt table testing, Gonzalo Baron-Esquivias, MD, said at the annual meeting of the American College of Cardiology.
Results of the randomized, multicenter, double-blind, crossover, placebo-controlled SPAIN trial showed that implantation of a permanent pacemaker programmed to a closed loop stimulation (DDD-CLS) algorithm resulted in an 89% decrease in the risk of syncopal recurrences compared with sham DDI pacing, reported Dr. Baron-Esquivias, chief of clinical cardiology at Virgen del Rocio University Hospital in Seville, Spain.
Moreover, the number needed to treat with DDD-CLS pacing for 1 year to prevent a syncopal episode was a mere 2.7 patients in the SPAIN trial, he noted.
The study included 46 patients who met strict criteria for participation and received a permanent pacemaker for which they had no conventional indication. In half of the subjects, the device was programmed to DDD-CLS pacing, while the other half received sham DDI pacing, making them a placebo-treated control group. After 12 months, each group was switched over to the other form of pacing.
Dr. Baron-Esquivias emphasized that the tough SPAIN eligibility criteria enabled investigators to accurately define a specific CSL-responsive subgroup. This is not a form of therapy that’s appropriate for most patients who experience vasovagal syncope. In the right population, however, DDD-CLS pacing is life transforming. The SPAIN investigators documented severely impaired quality of life in the study population, with dramatic improvement on CLS pacing.
Vasovagal syncope is by far the most common cause of syncope. But roughly 70 out of every 100 patients who present to the emergency department with vasovagal syncope will never have a repeat episode. And of the 30 who do, perhaps only 10 will have frequent recurrences refractory to conventional measures and that impose a severe effect on quality of life. This is the subgroup for whom consideration of pacemaker therapy is appropriate.
To be eligible for the SPAIN trial, patients had to have at least five previous episodes of neuromediated vasovagal syncope, including at least two within the past year. They also had to be at least 40 years old, since CLS-responsive vasovagal syncope is skewed toward an older age group. In addition, all participants had to have normal results on a complete physical examination that included an orthostatic test, 12-lead echocardiogram, two-dimensional echocardiography, carotid sinus massage, and 24-hour Holter monitoring. Only then were they eligible for a tilt-table test. A positive head-up tilt test demonstrating a cardioinhibitory response required a heart rate drop to less than 40 bpm for at least 10 sec or a greater than 3-sec pause.
The average age of the 46 subjects who fulfilled all of these criteria was 56 years – several decades older than most patients who present with syncope. They averaged 12 prior syncopal episodes, including 4.5 during the prior 12 months. Three-quarters of the patients demonstrated asystole during the tilt test, with an average duration of 15 sec.
Four of the 46 patients experienced a syncopal recurrence during their 12 months on DDD-CLS pacing, while 21 of the same group of 46 had recurrent syncope during their year on DDI sham pacing. This translated into an absolute 37% reduction in syncopal episodes with active pacemaker therapy. Patients had an 8.8-fold greater risk of syncopal recurrence while on DDI sham pacing compared with DDD-CLS.
Dr. Baron-Esquivias explained that the DDD-CLS algorithm is designed to detect impending syncope at an early and actionable stage. The algorithm identifies the combination of increased myocardial contractility and reduced right ventricular intracardiac impedance, which heralds the first stage of vasovagal syncope. It then directs the pacemaker to activate high-rate atrioventricular sequential pacing to prevent arterial hypotension, bradycardia, and overt syncope.
Discussant Kenneth A. Ellenbogen, MD, applauded Dr. Baron-Esquivias and coinvestigators for “having the strength and conviction to attack this problem where so many prior studies have failed.”
For example, the earlier European SYNPACE trial showed no benefit for dual-chamber pacemaker therapy in DDD-RDR (rate-drop response) mode in patients with recurrent severe cardioinhibitory vasovagal syncope (Eur Heart J. 2004 Oct;25[19]:1741-8), probably due to a combination of a less robust early-syncope detection algorithm than that of DDD-CLS and less restrictive patient selection criteria, observed Dr. Ellenbogen, chairman of the division of cardiology and director of clinical cardiology electrophysiology and pacing at the Medical College of Virginia, Richmond.
The SPAIN trial was funded by the Spanish Society of Cardiology. Dr. Baron-Esquivias reported having no financial conflicts.
A considerably larger randomized trial known as BIOSync CLS, sponsored by Biotronic, which developed the CLS program, is ongoing.
Washington – Help is finally at hand for the subgroup of patients who have severe refractory vasovagal syncope that recurs frequently and unpredictably, and is associated with a strong cardioinhibitory response on tilt table testing, Gonzalo Baron-Esquivias, MD, said at the annual meeting of the American College of Cardiology.
Results of the randomized, multicenter, double-blind, crossover, placebo-controlled SPAIN trial showed that implantation of a permanent pacemaker programmed to a closed loop stimulation (DDD-CLS) algorithm resulted in an 89% decrease in the risk of syncopal recurrences compared with sham DDI pacing, reported Dr. Baron-Esquivias, chief of clinical cardiology at Virgen del Rocio University Hospital in Seville, Spain.
Moreover, the number needed to treat with DDD-CLS pacing for 1 year to prevent a syncopal episode was a mere 2.7 patients in the SPAIN trial, he noted.
The study included 46 patients who met strict criteria for participation and received a permanent pacemaker for which they had no conventional indication. In half of the subjects, the device was programmed to DDD-CLS pacing, while the other half received sham DDI pacing, making them a placebo-treated control group. After 12 months, each group was switched over to the other form of pacing.
Dr. Baron-Esquivias emphasized that the tough SPAIN eligibility criteria enabled investigators to accurately define a specific CSL-responsive subgroup. This is not a form of therapy that’s appropriate for most patients who experience vasovagal syncope. In the right population, however, DDD-CLS pacing is life transforming. The SPAIN investigators documented severely impaired quality of life in the study population, with dramatic improvement on CLS pacing.
Vasovagal syncope is by far the most common cause of syncope. But roughly 70 out of every 100 patients who present to the emergency department with vasovagal syncope will never have a repeat episode. And of the 30 who do, perhaps only 10 will have frequent recurrences refractory to conventional measures and that impose a severe effect on quality of life. This is the subgroup for whom consideration of pacemaker therapy is appropriate.
To be eligible for the SPAIN trial, patients had to have at least five previous episodes of neuromediated vasovagal syncope, including at least two within the past year. They also had to be at least 40 years old, since CLS-responsive vasovagal syncope is skewed toward an older age group. In addition, all participants had to have normal results on a complete physical examination that included an orthostatic test, 12-lead echocardiogram, two-dimensional echocardiography, carotid sinus massage, and 24-hour Holter monitoring. Only then were they eligible for a tilt-table test. A positive head-up tilt test demonstrating a cardioinhibitory response required a heart rate drop to less than 40 bpm for at least 10 sec or a greater than 3-sec pause.
The average age of the 46 subjects who fulfilled all of these criteria was 56 years – several decades older than most patients who present with syncope. They averaged 12 prior syncopal episodes, including 4.5 during the prior 12 months. Three-quarters of the patients demonstrated asystole during the tilt test, with an average duration of 15 sec.
Four of the 46 patients experienced a syncopal recurrence during their 12 months on DDD-CLS pacing, while 21 of the same group of 46 had recurrent syncope during their year on DDI sham pacing. This translated into an absolute 37% reduction in syncopal episodes with active pacemaker therapy. Patients had an 8.8-fold greater risk of syncopal recurrence while on DDI sham pacing compared with DDD-CLS.
Dr. Baron-Esquivias explained that the DDD-CLS algorithm is designed to detect impending syncope at an early and actionable stage. The algorithm identifies the combination of increased myocardial contractility and reduced right ventricular intracardiac impedance, which heralds the first stage of vasovagal syncope. It then directs the pacemaker to activate high-rate atrioventricular sequential pacing to prevent arterial hypotension, bradycardia, and overt syncope.
Discussant Kenneth A. Ellenbogen, MD, applauded Dr. Baron-Esquivias and coinvestigators for “having the strength and conviction to attack this problem where so many prior studies have failed.”
For example, the earlier European SYNPACE trial showed no benefit for dual-chamber pacemaker therapy in DDD-RDR (rate-drop response) mode in patients with recurrent severe cardioinhibitory vasovagal syncope (Eur Heart J. 2004 Oct;25[19]:1741-8), probably due to a combination of a less robust early-syncope detection algorithm than that of DDD-CLS and less restrictive patient selection criteria, observed Dr. Ellenbogen, chairman of the division of cardiology and director of clinical cardiology electrophysiology and pacing at the Medical College of Virginia, Richmond.
The SPAIN trial was funded by the Spanish Society of Cardiology. Dr. Baron-Esquivias reported having no financial conflicts.
A considerably larger randomized trial known as BIOSync CLS, sponsored by Biotronic, which developed the CLS program, is ongoing.
Washington – Help is finally at hand for the subgroup of patients who have severe refractory vasovagal syncope that recurs frequently and unpredictably, and is associated with a strong cardioinhibitory response on tilt table testing, Gonzalo Baron-Esquivias, MD, said at the annual meeting of the American College of Cardiology.
Results of the randomized, multicenter, double-blind, crossover, placebo-controlled SPAIN trial showed that implantation of a permanent pacemaker programmed to a closed loop stimulation (DDD-CLS) algorithm resulted in an 89% decrease in the risk of syncopal recurrences compared with sham DDI pacing, reported Dr. Baron-Esquivias, chief of clinical cardiology at Virgen del Rocio University Hospital in Seville, Spain.
Moreover, the number needed to treat with DDD-CLS pacing for 1 year to prevent a syncopal episode was a mere 2.7 patients in the SPAIN trial, he noted.
The study included 46 patients who met strict criteria for participation and received a permanent pacemaker for which they had no conventional indication. In half of the subjects, the device was programmed to DDD-CLS pacing, while the other half received sham DDI pacing, making them a placebo-treated control group. After 12 months, each group was switched over to the other form of pacing.
Dr. Baron-Esquivias emphasized that the tough SPAIN eligibility criteria enabled investigators to accurately define a specific CSL-responsive subgroup. This is not a form of therapy that’s appropriate for most patients who experience vasovagal syncope. In the right population, however, DDD-CLS pacing is life transforming. The SPAIN investigators documented severely impaired quality of life in the study population, with dramatic improvement on CLS pacing.
Vasovagal syncope is by far the most common cause of syncope. But roughly 70 out of every 100 patients who present to the emergency department with vasovagal syncope will never have a repeat episode. And of the 30 who do, perhaps only 10 will have frequent recurrences refractory to conventional measures and that impose a severe effect on quality of life. This is the subgroup for whom consideration of pacemaker therapy is appropriate.
To be eligible for the SPAIN trial, patients had to have at least five previous episodes of neuromediated vasovagal syncope, including at least two within the past year. They also had to be at least 40 years old, since CLS-responsive vasovagal syncope is skewed toward an older age group. In addition, all participants had to have normal results on a complete physical examination that included an orthostatic test, 12-lead echocardiogram, two-dimensional echocardiography, carotid sinus massage, and 24-hour Holter monitoring. Only then were they eligible for a tilt-table test. A positive head-up tilt test demonstrating a cardioinhibitory response required a heart rate drop to less than 40 bpm for at least 10 sec or a greater than 3-sec pause.
The average age of the 46 subjects who fulfilled all of these criteria was 56 years – several decades older than most patients who present with syncope. They averaged 12 prior syncopal episodes, including 4.5 during the prior 12 months. Three-quarters of the patients demonstrated asystole during the tilt test, with an average duration of 15 sec.
Four of the 46 patients experienced a syncopal recurrence during their 12 months on DDD-CLS pacing, while 21 of the same group of 46 had recurrent syncope during their year on DDI sham pacing. This translated into an absolute 37% reduction in syncopal episodes with active pacemaker therapy. Patients had an 8.8-fold greater risk of syncopal recurrence while on DDI sham pacing compared with DDD-CLS.
Dr. Baron-Esquivias explained that the DDD-CLS algorithm is designed to detect impending syncope at an early and actionable stage. The algorithm identifies the combination of increased myocardial contractility and reduced right ventricular intracardiac impedance, which heralds the first stage of vasovagal syncope. It then directs the pacemaker to activate high-rate atrioventricular sequential pacing to prevent arterial hypotension, bradycardia, and overt syncope.
Discussant Kenneth A. Ellenbogen, MD, applauded Dr. Baron-Esquivias and coinvestigators for “having the strength and conviction to attack this problem where so many prior studies have failed.”
For example, the earlier European SYNPACE trial showed no benefit for dual-chamber pacemaker therapy in DDD-RDR (rate-drop response) mode in patients with recurrent severe cardioinhibitory vasovagal syncope (Eur Heart J. 2004 Oct;25[19]:1741-8), probably due to a combination of a less robust early-syncope detection algorithm than that of DDD-CLS and less restrictive patient selection criteria, observed Dr. Ellenbogen, chairman of the division of cardiology and director of clinical cardiology electrophysiology and pacing at the Medical College of Virginia, Richmond.
The SPAIN trial was funded by the Spanish Society of Cardiology. Dr. Baron-Esquivias reported having no financial conflicts.
A considerably larger randomized trial known as BIOSync CLS, sponsored by Biotronic, which developed the CLS program, is ongoing.
At ACC 17
Key clinical point:
Major finding: The number of patients with severe recurrent cardioinhibitory reflex vasovagal syncope needed to be treated with DDD-CLS pacing for 1 year to prevent a recurrent episode is 2.7.
Data source: SPAIN, a 2-year randomized, multicenter, double-blind, prospective, placebo-controlled crossover trial of 46 patients with severe recurrent vasovagal syncope treated via pacemaker therapy.
Disclosures: The Spanish Society of Cardiology funded the study. The presenter reported having no financial conflicts.