User login
Rule identifies women at low risk of VTE recurrence
Results of the REVERSE-II study appear to validate the utility of the HERDOO2 rule to identify women who can safely stop taking anticoagulants after their first unprovoked venous thromboembolism (VTE).
Some women who were classified as low-risk according to HERDOO2 did experience VTE recurrence after they stopped taking anticoagulants.
However, their risk of recurrence was lower than that of women who were classified as high-risk and stopped taking anticoagulants.
These results were published in The BMJ.
“Patients can get very anxious trying to balance the risks of [anticoagulation] with the risks of another blood clot,” said study author Marc Rodger, MD, of Ottawa Hospital and University of Ottawa in Ontario, Canada.
“With this rule, we can confidently tell half of the women we see that they are at low risk of having another blood clot. This means they can stop taking blood thinners once their initial clot is treated, sparing them the cost, inconvenience, and risks of taking life-long medication.”
The HERDOO2 rule suggests a woman has a low risk of VTE if she has 1 or none of the following risk factors:
- HER=Hyperpigmentation, edema, or redness in either leg
- D=High levels of D-dimer in the blood
- O=Obesity (body mass index of 30 kg/m2 or more)
- O=Older age (65+).
To test the rule, Dr Rodgers and his colleagues evaluated 2785 men and women with a first, unprovoked VTE. The patients were recruited between 2008 and 2015 from 44 healthcare centers in 7 countries.
Patients who were found to be at low risk of VTE recurrence were told to stop taking anticoagulants after they completed the initial treatment for their first VTE.
For patients considered at high risk for VTE recurrence, the researchers left the decision of continuing anticoagulation to the patients and their doctors.
Low-risk patients
None of the men in this trial could be identified as low-risk using the HERDOO2 rule.
However, 631 women had a low risk of VTE recurrence according to HERDOO2. Most of these women (n=591) stopped anticoagulant therapy.
Twenty-seven low-risk women decided to continue anticoagulation, 1 patient’s physician decided she required continued anticoagulation, and 3 patients continued for “other” reasons.
Nine patients in this group were lost to follow-up.
High-risk patients
There were 2148 men and women considered at high risk for VTE recurrence. Most of these patients (n=1802) continued anticoagulation.
Of the 323 high-risk patients who stopped anticoagulation, 279 did so because of their own preference, 9 due to physician decision, 15 had a high risk of bleeding, and 20 stopped for “other” reasons.
Twenty-three patients in this group were lost to follow-up.
Results
The researchers followed the patients for a year after they had finished treatment for their first VTE.
The risk of recurrent major VTE per 100 patient-years was:
- 3.0% among low-risk women who discontinued anticoagulants
- 8.1% among men and high-risk women who discontinued anticoagulants
- 7.4% among high-risk women (only) who discontinued anticoagulants
- 1.6% among men and high-risk women who continued anticoagulants.
Among the low-risk women who continued to receive anticoagulants, there were no cases of recurrent, symptomatic VTE.
“We see 2 to 3 patients with unexplained blood clots every day at The Ottawa Hospital,” Dr Rodger said. “If this rule was applied across Canada, we estimate that over 10,000 women a year would be identified as low risk and be able to come off blood thinners.” ![]()
Results of the REVERSE-II study appear to validate the utility of the HERDOO2 rule to identify women who can safely stop taking anticoagulants after their first unprovoked venous thromboembolism (VTE).
Some women who were classified as low-risk according to HERDOO2 did experience VTE recurrence after they stopped taking anticoagulants.
However, their risk of recurrence was lower than that of women who were classified as high-risk and stopped taking anticoagulants.
These results were published in The BMJ.
“Patients can get very anxious trying to balance the risks of [anticoagulation] with the risks of another blood clot,” said study author Marc Rodger, MD, of Ottawa Hospital and University of Ottawa in Ontario, Canada.
“With this rule, we can confidently tell half of the women we see that they are at low risk of having another blood clot. This means they can stop taking blood thinners once their initial clot is treated, sparing them the cost, inconvenience, and risks of taking life-long medication.”
The HERDOO2 rule suggests a woman has a low risk of VTE if she has 1 or none of the following risk factors:
- HER=Hyperpigmentation, edema, or redness in either leg
- D=High levels of D-dimer in the blood
- O=Obesity (body mass index of 30 kg/m2 or more)
- O=Older age (65+).
To test the rule, Dr Rodgers and his colleagues evaluated 2785 men and women with a first, unprovoked VTE. The patients were recruited between 2008 and 2015 from 44 healthcare centers in 7 countries.
Patients who were found to be at low risk of VTE recurrence were told to stop taking anticoagulants after they completed the initial treatment for their first VTE.
For patients considered at high risk for VTE recurrence, the researchers left the decision of continuing anticoagulation to the patients and their doctors.
Low-risk patients
None of the men in this trial could be identified as low-risk using the HERDOO2 rule.
However, 631 women had a low risk of VTE recurrence according to HERDOO2. Most of these women (n=591) stopped anticoagulant therapy.
Twenty-seven low-risk women decided to continue anticoagulation, 1 patient’s physician decided she required continued anticoagulation, and 3 patients continued for “other” reasons.
Nine patients in this group were lost to follow-up.
High-risk patients
There were 2148 men and women considered at high risk for VTE recurrence. Most of these patients (n=1802) continued anticoagulation.
Of the 323 high-risk patients who stopped anticoagulation, 279 did so because of their own preference, 9 due to physician decision, 15 had a high risk of bleeding, and 20 stopped for “other” reasons.
Twenty-three patients in this group were lost to follow-up.
Results
The researchers followed the patients for a year after they had finished treatment for their first VTE.
The risk of recurrent major VTE per 100 patient-years was:
- 3.0% among low-risk women who discontinued anticoagulants
- 8.1% among men and high-risk women who discontinued anticoagulants
- 7.4% among high-risk women (only) who discontinued anticoagulants
- 1.6% among men and high-risk women who continued anticoagulants.
Among the low-risk women who continued to receive anticoagulants, there were no cases of recurrent, symptomatic VTE.
“We see 2 to 3 patients with unexplained blood clots every day at The Ottawa Hospital,” Dr Rodger said. “If this rule was applied across Canada, we estimate that over 10,000 women a year would be identified as low risk and be able to come off blood thinners.” ![]()
Results of the REVERSE-II study appear to validate the utility of the HERDOO2 rule to identify women who can safely stop taking anticoagulants after their first unprovoked venous thromboembolism (VTE).
Some women who were classified as low-risk according to HERDOO2 did experience VTE recurrence after they stopped taking anticoagulants.
However, their risk of recurrence was lower than that of women who were classified as high-risk and stopped taking anticoagulants.
These results were published in The BMJ.
“Patients can get very anxious trying to balance the risks of [anticoagulation] with the risks of another blood clot,” said study author Marc Rodger, MD, of Ottawa Hospital and University of Ottawa in Ontario, Canada.
“With this rule, we can confidently tell half of the women we see that they are at low risk of having another blood clot. This means they can stop taking blood thinners once their initial clot is treated, sparing them the cost, inconvenience, and risks of taking life-long medication.”
The HERDOO2 rule suggests a woman has a low risk of VTE if she has 1 or none of the following risk factors:
- HER=Hyperpigmentation, edema, or redness in either leg
- D=High levels of D-dimer in the blood
- O=Obesity (body mass index of 30 kg/m2 or more)
- O=Older age (65+).
To test the rule, Dr Rodgers and his colleagues evaluated 2785 men and women with a first, unprovoked VTE. The patients were recruited between 2008 and 2015 from 44 healthcare centers in 7 countries.
Patients who were found to be at low risk of VTE recurrence were told to stop taking anticoagulants after they completed the initial treatment for their first VTE.
For patients considered at high risk for VTE recurrence, the researchers left the decision of continuing anticoagulation to the patients and their doctors.
Low-risk patients
None of the men in this trial could be identified as low-risk using the HERDOO2 rule.
However, 631 women had a low risk of VTE recurrence according to HERDOO2. Most of these women (n=591) stopped anticoagulant therapy.
Twenty-seven low-risk women decided to continue anticoagulation, 1 patient’s physician decided she required continued anticoagulation, and 3 patients continued for “other” reasons.
Nine patients in this group were lost to follow-up.
High-risk patients
There were 2148 men and women considered at high risk for VTE recurrence. Most of these patients (n=1802) continued anticoagulation.
Of the 323 high-risk patients who stopped anticoagulation, 279 did so because of their own preference, 9 due to physician decision, 15 had a high risk of bleeding, and 20 stopped for “other” reasons.
Twenty-three patients in this group were lost to follow-up.
Results
The researchers followed the patients for a year after they had finished treatment for their first VTE.
The risk of recurrent major VTE per 100 patient-years was:
- 3.0% among low-risk women who discontinued anticoagulants
- 8.1% among men and high-risk women who discontinued anticoagulants
- 7.4% among high-risk women (only) who discontinued anticoagulants
- 1.6% among men and high-risk women who continued anticoagulants.
Among the low-risk women who continued to receive anticoagulants, there were no cases of recurrent, symptomatic VTE.
“We see 2 to 3 patients with unexplained blood clots every day at The Ottawa Hospital,” Dr Rodger said. “If this rule was applied across Canada, we estimate that over 10,000 women a year would be identified as low risk and be able to come off blood thinners.” ![]()
Pilot Has a Flighty Heart
ANSWER
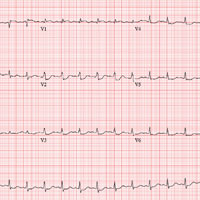
This ECG is consistent with sinus tachycardia, low-voltage QRS complexes, a posterior infarction, and ST- and T-wave abnormalities suggestive of inferior ischemia.
Sinus tachycardia is evidenced by the equal number of P and QRS complexes with a consistent PR interval. QRS complexes of lower amplitude than one would expect for the patient’s body habitus are deemed low voltage.
A posterior infarct is demonstrated by an R wave with no S wave in V1. Additionally, leads V2 and V3 show a pattern of a dominant R wave wit
Finally, inferior ischemia is identified by ST depressions in anterolateral leads I, aVL, V5, and V6, and T-wave inversions in inferior leads III and aVF.
ANSWER
This ECG is consistent with sinus tachycardia, low-voltage QRS complexes, a posterior infarction, and ST- and T-wave abnormalities suggestive of inferior ischemia.
Sinus tachycardia is evidenced by the equal number of P and QRS complexes with a consistent PR interval. QRS complexes of lower amplitude than one would expect for the patient’s body habitus are deemed low voltage.
A posterior infarct is demonstrated by an R wave with no S wave in V1. Additionally, leads V2 and V3 show a pattern of a dominant R wave wit
Finally, inferior ischemia is identified by ST depressions in anterolateral leads I, aVL, V5, and V6, and T-wave inversions in inferior leads III and aVF.
ANSWER
This ECG is consistent with sinus tachycardia, low-voltage QRS complexes, a posterior infarction, and ST- and T-wave abnormalities suggestive of inferior ischemia.
Sinus tachycardia is evidenced by the equal number of P and QRS complexes with a consistent PR interval. QRS complexes of lower amplitude than one would expect for the patient’s body habitus are deemed low voltage.
A posterior infarct is demonstrated by an R wave with no S wave in V1. Additionally, leads V2 and V3 show a pattern of a dominant R wave wit
Finally, inferior ischemia is identified by ST depressions in anterolateral leads I, aVL, V5, and V6, and T-wave inversions in inferior leads III and aVF.
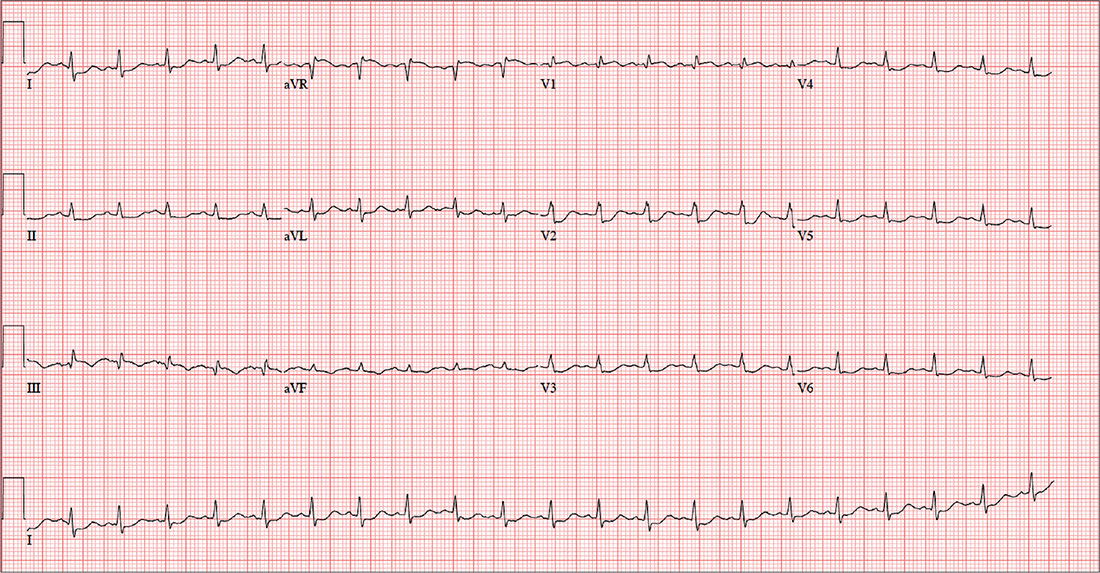
For the past six hours, a 58-year-old man has been experiencing substernal chest discomfort. He is a commercial airline pilot for a regional carrier. Earlier today, he served as co-pilot on a roundtrip flight; about one hour before take-off, he started to feel a dull aching sensation in his chest that he attributed to his recently diagnosed gastroesophageal reflux disease (GERD). After landing, he purchased antacids in the airport terminal and took several, which provided only mild relief for the return trip. The pilot in command suggested he “get checked out” to make certain he didn’t have a bleeding ulcer.
Upon arrival at your facility, the patient appears uncomfortable but denies pain in his chest; there is no radiation to the neck, back, or arm. He denies palpitations, nausea, vomiting, diarrhea, and constipation. He says he feels more short of breath now than he did earlier, adding that “something just doesn’t feel right.”
The patient is a widower; his wife died of breast cancer at age 40. His daughter and son-in-law live in the same neighborhood so he can be close to his grandson. An avid triathlon participant, he has his own gym at home.
His medical history is remarkable for GERD, which was confirmed two months ago by upper endoscopy following several episodes of epigastric pain. He was prescribed a proton pump inhibitor, which he has forgotten to take for the past two days. His last flight physical, performed one year ago, was normal. Surgical history is remarkable for a tonsillectomy and a left inguinal hernia repair, both during childhood.
His current medication list includes lansoprazole and ibuprofen (as needed for musculoskeletal pain). He does not smoke, and he occasionally has a beer on the weekends when he isn’t working. The review of systems is noncontributory apart from his previously detailed symptoms.
Vital signs include a blood pressure of 130/86 mm Hg; pulse, 128 beats/min; respiratory rate, 12 breaths/min-1; and temperature, 97.9°F. His weight is 189 lb and his height, 73 in.
Physical exam reveals an uncomfortable, anxious-appearing male who is otherwise in excellent physical shape. Pertinent findings include a normal thyroid, no evidence of jugular venous distention, and clear lung fields bilaterally. The cardiac exam reveals a regular rate of 130 beats/min with no evidence of murmurs or rubs. The abdomen is flat and nontender, with no organomegaly. Peripheral pulses are equal bilaterally, and there is no peripheral edema. The neurologic exam is grossly intact.
A chest x-ray, ECG, and bloodwork are obtained. The ECG reveals a ventricular rate of 128 beats/min; PR interval, 136 ms; QRS duration, 72 ms; QT/QTc interval, 326/475 ms; P axis, 44°; R axis, 40°; and T axis, –47°. The ECG obtained during his flight physical one year ago showed normal sinus rhythm with nonspecific ST- and T-wave changes but was otherwise normal. With this history in mind, what is your interpretation of today’s ECG?
Defining high reliability
When the Joint Commission on Accreditation of Healthcare Organizations came to our hospital for a survey last fall, our administration was confident that the review would be favorable. The Joint Commission was stressing the reliability of hospitals and so were we. We had chartered a “High-Reliability Organization Enterprise Steering Committee” that was “empowered to make recommendations to the (executive board) on what is needed to achieve the goals of high reliability across the enterprise.” High reliability was a priority for our administration and for the Joint Commission. Unfortunately, nearly no one else knew what high reliability meant.
In 2001, Karl E. Weick and Kathleen M. Sutcliffe published their book, “Managing the Unexpected: Resilient Performance in an Age of Uncertainty,” (Hoboken, N.J.: Jossey-Bass, 2001), which defined high-reliability organizations as those that reliably prevent error. They included examples from the military and from aviation. They proffered five principles to guide those organizations wishing to become highly reliable:
1. Preoccupation with failure.
2. Reluctance to simplify interpretations.
3. Sensitivity to operations.
4. Commitment to resilience.
5. Deference to expertise.
In September 2005, the Agency for Healthcare Research and Quality created a document to adapt the concepts developed by Mr. Weick and Ms. Sutcliffe to the health care industry, where opportunities to avoid error and prevent catastrophe abound. The eventual result has been steady progress in measuring avoidable health care errors, such as avoiding central line–associated blood stream infections and holding health care organizations accountable for their reduction. However, organizational cultures are difficult to change, and there is still a long way to go.
In contrast to large systems, individual providers can change quickly, especially if there is incentive to do so. What principles would increase our own ability to become a high-reliability individuals (HRIs):
• Recognize failure as systemic, not personal. Health care providers are humans, and humans make mistakes. Unfortunately, we come from a tradition that rewards success and penalizes failure. Research shows that is better to recognize failure as something to be prevented next time rather than to be punished now. Admonitions to pay attention, focus more, and remember better rely on fallible humans and reliably fail. Systems solutions, such as checklists, timeouts, and hard stops reliably succeed. HRIs should blame error less often on people, and more often on system failures.
• Simple solutions are preferred to complex requirements. Chemotherapy was once calculated and written by hand. Every cancer center can recall tragic disasters that occurred as a result of errors either by the ordering physician or by interpretations made by pharmacists and nurses. The introduction of electronic chemotherapy ordering has nearly eliminated these mistakes. HRIs can initiate technology solutions to their work to help reduce the risk of errors.
• Sensitivity to patients. Patients often desire to be included as partners in their care. In addition to being present and attentive to patients, why not enlist them as colleagues in care? For example, the patient who has their own calendar of chemotherapy treatments – complete with agents, doses, and schedules – will be more likely to question perceived errors. HRIs are transparent.
• Resilience in character. Learning to accept the potential for error requires acceptance that others also are trying to prevent error and are not judging your competence. The physician who attacks those who are trying to help reduces the psychological safety required for colleagues to speak up when potential errors are identified. Physicians will become HRIs only when they lower their defenses and become more teammates rather than a soloists.
• Deference to evidence. The “way it has always been” must give way to the way things are. Anecdotes and personal conviction do not meet scientific standards and should be abandoned in the face of evidence. Yet, this seemingly obvious principle often is disregarded when clinicians are presented with standardized treatment pathways and limited formularies in the name of autonomy; autonomy is fine until patients are endangered by it. The HRI practices evidence-based medicine.
Marty Makary, MD, explores most of these principles in his book “Unaccountable: What Hospitals Won’t Tell You and How Transparency Can Revolutionize Health Care”(London: Bloomsbury Publishing, 2012). While written from a surgeon’s perspective, Dr. Makary exposes the dangerous state of modern medical care across all specialties. I recommend it as a sobering assessment of the way things are and as a prescription for health care systems and physicians to help them become more reliable.
How are you driving safety in your area? What are some best practices we can share with others? I invite you to reply to [email protected] to initiate a broader discussion of patient safety and reliability. Responses will be posted to hematologynews.com.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When the Joint Commission on Accreditation of Healthcare Organizations came to our hospital for a survey last fall, our administration was confident that the review would be favorable. The Joint Commission was stressing the reliability of hospitals and so were we. We had chartered a “High-Reliability Organization Enterprise Steering Committee” that was “empowered to make recommendations to the (executive board) on what is needed to achieve the goals of high reliability across the enterprise.” High reliability was a priority for our administration and for the Joint Commission. Unfortunately, nearly no one else knew what high reliability meant.
In 2001, Karl E. Weick and Kathleen M. Sutcliffe published their book, “Managing the Unexpected: Resilient Performance in an Age of Uncertainty,” (Hoboken, N.J.: Jossey-Bass, 2001), which defined high-reliability organizations as those that reliably prevent error. They included examples from the military and from aviation. They proffered five principles to guide those organizations wishing to become highly reliable:
1. Preoccupation with failure.
2. Reluctance to simplify interpretations.
3. Sensitivity to operations.
4. Commitment to resilience.
5. Deference to expertise.
In September 2005, the Agency for Healthcare Research and Quality created a document to adapt the concepts developed by Mr. Weick and Ms. Sutcliffe to the health care industry, where opportunities to avoid error and prevent catastrophe abound. The eventual result has been steady progress in measuring avoidable health care errors, such as avoiding central line–associated blood stream infections and holding health care organizations accountable for their reduction. However, organizational cultures are difficult to change, and there is still a long way to go.
In contrast to large systems, individual providers can change quickly, especially if there is incentive to do so. What principles would increase our own ability to become a high-reliability individuals (HRIs):
• Recognize failure as systemic, not personal. Health care providers are humans, and humans make mistakes. Unfortunately, we come from a tradition that rewards success and penalizes failure. Research shows that is better to recognize failure as something to be prevented next time rather than to be punished now. Admonitions to pay attention, focus more, and remember better rely on fallible humans and reliably fail. Systems solutions, such as checklists, timeouts, and hard stops reliably succeed. HRIs should blame error less often on people, and more often on system failures.
• Simple solutions are preferred to complex requirements. Chemotherapy was once calculated and written by hand. Every cancer center can recall tragic disasters that occurred as a result of errors either by the ordering physician or by interpretations made by pharmacists and nurses. The introduction of electronic chemotherapy ordering has nearly eliminated these mistakes. HRIs can initiate technology solutions to their work to help reduce the risk of errors.
• Sensitivity to patients. Patients often desire to be included as partners in their care. In addition to being present and attentive to patients, why not enlist them as colleagues in care? For example, the patient who has their own calendar of chemotherapy treatments – complete with agents, doses, and schedules – will be more likely to question perceived errors. HRIs are transparent.
• Resilience in character. Learning to accept the potential for error requires acceptance that others also are trying to prevent error and are not judging your competence. The physician who attacks those who are trying to help reduces the psychological safety required for colleagues to speak up when potential errors are identified. Physicians will become HRIs only when they lower their defenses and become more teammates rather than a soloists.
• Deference to evidence. The “way it has always been” must give way to the way things are. Anecdotes and personal conviction do not meet scientific standards and should be abandoned in the face of evidence. Yet, this seemingly obvious principle often is disregarded when clinicians are presented with standardized treatment pathways and limited formularies in the name of autonomy; autonomy is fine until patients are endangered by it. The HRI practices evidence-based medicine.
Marty Makary, MD, explores most of these principles in his book “Unaccountable: What Hospitals Won’t Tell You and How Transparency Can Revolutionize Health Care”(London: Bloomsbury Publishing, 2012). While written from a surgeon’s perspective, Dr. Makary exposes the dangerous state of modern medical care across all specialties. I recommend it as a sobering assessment of the way things are and as a prescription for health care systems and physicians to help them become more reliable.
How are you driving safety in your area? What are some best practices we can share with others? I invite you to reply to [email protected] to initiate a broader discussion of patient safety and reliability. Responses will be posted to hematologynews.com.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When the Joint Commission on Accreditation of Healthcare Organizations came to our hospital for a survey last fall, our administration was confident that the review would be favorable. The Joint Commission was stressing the reliability of hospitals and so were we. We had chartered a “High-Reliability Organization Enterprise Steering Committee” that was “empowered to make recommendations to the (executive board) on what is needed to achieve the goals of high reliability across the enterprise.” High reliability was a priority for our administration and for the Joint Commission. Unfortunately, nearly no one else knew what high reliability meant.
In 2001, Karl E. Weick and Kathleen M. Sutcliffe published their book, “Managing the Unexpected: Resilient Performance in an Age of Uncertainty,” (Hoboken, N.J.: Jossey-Bass, 2001), which defined high-reliability organizations as those that reliably prevent error. They included examples from the military and from aviation. They proffered five principles to guide those organizations wishing to become highly reliable:
1. Preoccupation with failure.
2. Reluctance to simplify interpretations.
3. Sensitivity to operations.
4. Commitment to resilience.
5. Deference to expertise.
In September 2005, the Agency for Healthcare Research and Quality created a document to adapt the concepts developed by Mr. Weick and Ms. Sutcliffe to the health care industry, where opportunities to avoid error and prevent catastrophe abound. The eventual result has been steady progress in measuring avoidable health care errors, such as avoiding central line–associated blood stream infections and holding health care organizations accountable for their reduction. However, organizational cultures are difficult to change, and there is still a long way to go.
In contrast to large systems, individual providers can change quickly, especially if there is incentive to do so. What principles would increase our own ability to become a high-reliability individuals (HRIs):
• Recognize failure as systemic, not personal. Health care providers are humans, and humans make mistakes. Unfortunately, we come from a tradition that rewards success and penalizes failure. Research shows that is better to recognize failure as something to be prevented next time rather than to be punished now. Admonitions to pay attention, focus more, and remember better rely on fallible humans and reliably fail. Systems solutions, such as checklists, timeouts, and hard stops reliably succeed. HRIs should blame error less often on people, and more often on system failures.
• Simple solutions are preferred to complex requirements. Chemotherapy was once calculated and written by hand. Every cancer center can recall tragic disasters that occurred as a result of errors either by the ordering physician or by interpretations made by pharmacists and nurses. The introduction of electronic chemotherapy ordering has nearly eliminated these mistakes. HRIs can initiate technology solutions to their work to help reduce the risk of errors.
• Sensitivity to patients. Patients often desire to be included as partners in their care. In addition to being present and attentive to patients, why not enlist them as colleagues in care? For example, the patient who has their own calendar of chemotherapy treatments – complete with agents, doses, and schedules – will be more likely to question perceived errors. HRIs are transparent.
• Resilience in character. Learning to accept the potential for error requires acceptance that others also are trying to prevent error and are not judging your competence. The physician who attacks those who are trying to help reduces the psychological safety required for colleagues to speak up when potential errors are identified. Physicians will become HRIs only when they lower their defenses and become more teammates rather than a soloists.
• Deference to evidence. The “way it has always been” must give way to the way things are. Anecdotes and personal conviction do not meet scientific standards and should be abandoned in the face of evidence. Yet, this seemingly obvious principle often is disregarded when clinicians are presented with standardized treatment pathways and limited formularies in the name of autonomy; autonomy is fine until patients are endangered by it. The HRI practices evidence-based medicine.
Marty Makary, MD, explores most of these principles in his book “Unaccountable: What Hospitals Won’t Tell You and How Transparency Can Revolutionize Health Care”(London: Bloomsbury Publishing, 2012). While written from a surgeon’s perspective, Dr. Makary exposes the dangerous state of modern medical care across all specialties. I recommend it as a sobering assessment of the way things are and as a prescription for health care systems and physicians to help them become more reliable.
How are you driving safety in your area? What are some best practices we can share with others? I invite you to reply to [email protected] to initiate a broader discussion of patient safety and reliability. Responses will be posted to hematologynews.com.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Cosmetic Corner: Dermatologists Weigh in on Products for Hyperhidrosis
To improve patient care and outcomes, leading dermatologists offered their recommendations on hyperhidrosis products. Consideration must be given to:
- Certain Dri Prescription Strength Clinical Roll-On
Clarion Brands Inc
“This over-the-counter antiperspirant has 12% aluminum chloride, making it very effective in treating hyperhidrosis.”—Shari Lipner, MD, PhD, New York, New York
Recommended by Gary Goldenberg, MD, New York, New York
- miraDry
Miramar Labs, Inc
“miraDry offers a noninvasive reduction of sweating of more than 70% after the first treatment in the underarm area.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- SweatBlock Clinical Strength Antiperspirant Towelettes
SweatBlock
“Each of the towelettes contains 14% aluminum chloride, can be applied to any part of the body, and can last up to 7 days.”—Jeannette Graf, MD, New York, New York
Cutis invites readers to send us their recommendations. Products for athlete’s foot, redness reduction, and sensitive skin will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
[polldaddy:9711250]
To improve patient care and outcomes, leading dermatologists offered their recommendations on hyperhidrosis products. Consideration must be given to:
- Certain Dri Prescription Strength Clinical Roll-On
Clarion Brands Inc
“This over-the-counter antiperspirant has 12% aluminum chloride, making it very effective in treating hyperhidrosis.”—Shari Lipner, MD, PhD, New York, New York
Recommended by Gary Goldenberg, MD, New York, New York
- miraDry
Miramar Labs, Inc
“miraDry offers a noninvasive reduction of sweating of more than 70% after the first treatment in the underarm area.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- SweatBlock Clinical Strength Antiperspirant Towelettes
SweatBlock
“Each of the towelettes contains 14% aluminum chloride, can be applied to any part of the body, and can last up to 7 days.”—Jeannette Graf, MD, New York, New York
Cutis invites readers to send us their recommendations. Products for athlete’s foot, redness reduction, and sensitive skin will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
[polldaddy:9711250]
To improve patient care and outcomes, leading dermatologists offered their recommendations on hyperhidrosis products. Consideration must be given to:
- Certain Dri Prescription Strength Clinical Roll-On
Clarion Brands Inc
“This over-the-counter antiperspirant has 12% aluminum chloride, making it very effective in treating hyperhidrosis.”—Shari Lipner, MD, PhD, New York, New York
Recommended by Gary Goldenberg, MD, New York, New York
- miraDry
Miramar Labs, Inc
“miraDry offers a noninvasive reduction of sweating of more than 70% after the first treatment in the underarm area.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- SweatBlock Clinical Strength Antiperspirant Towelettes
SweatBlock
“Each of the towelettes contains 14% aluminum chloride, can be applied to any part of the body, and can last up to 7 days.”—Jeannette Graf, MD, New York, New York
Cutis invites readers to send us their recommendations. Products for athlete’s foot, redness reduction, and sensitive skin will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
[polldaddy:9711250]
New-onset AF boosts bad HFrEF outcomes
WASHINGTON – New onset atrial fibrillation more than doubled the rate of adverse outcomes in patients with heart failure with reduced ejection fraction in a review of more than 15,000 such patients.
In 9,934 patients with heart failure with reduced ejection fraction (HFrEF) and no history of atrial fibrillation (AF), development of new-onset AF was linked with a greater than twofold increased risk of cardiovascular disease death or hospitalization for heart failure during follow-up, compared with HFrEF patients who did not initially have or later develop heart failure, after adjustment for several demographic and clinical variables, John J.V. McMurray, MD, said at the annual meeting of the American College of Cardiology. This difference for the primary endpoint of his analysis was statistically significant.
The 1,645 patients with paroxysmal AF at the start of their follow-up also had a significantly increased rate of cardiovascular death or heart failure hospitalization, but their increased risk when compared with HFrEF patients who didn’t develop AF was a much more modest 20% in his fully adjusted analysis. The patients who began follow-up with paroxysmal AF also had a relatively increased relative stroke rate of 33% when compared with HFrEF patients without AF at baseline who remained AF free, but the all-cause mortality rate among those with paroxysmal AF wasn’t significantly elevated, compared with the comparator group.
The 3,770 patients with persistent or permanent AF at baseline showed no statistically significant spike in their adverse event rates, compared with patients without AF, for any of the examined endpoints. The study group also included 66 patients with an undefined form of AF who weren’t included in these analyses.
“It’s the first episodes and paroxysmal episodes that cause trouble, and the trouble they cause is stroke,” Dr. McMurray said in an interview. Their stroke risk gets exacerbated in clinical practice, because these patients often don’t receive the stroke prevention they need in the form of anticoagulation treatment.
“We find over and over that patients with paroxysmal AF are not anticoagulated as frequently as they should be,” Dr. McMurray said. And HFrEF patients with a first AF episode need anticoagulation, too, as soon as AF is diagnosed, he advised.
He went a step further and speculated that the reason why HFrEF patients with new onset AF did so poorly in his analysis was because they already had several prior, brief AF episodes that had gone undetected. “Many of these patients probably had undiagnosed, clinically unapparent AF episodes” that then resulted in strokes, he suggested.
The upshot is that patients with HFrEF may need more aggressive monitoring for new-onset AF, possibly in the form of small, implanted arrhythmia-detection devices. Dr. McMurray said that he and other researchers are currently testing whether this hypothesis is correct. “We and others are now looking at this because these new data are convincing that new-onset AF is bad news [for HFrEF patients].”
In the analysis, “we looked only at clinically recognized and adjudicated new-onset AF. Goodness knows how many HFrEF patients are having unrecognized paroxysmal AF. Almost certainly there is a lot that is unrecognized” that potentially could be detected using a small implanted arrhythmia monitor, which could then lead to earlier anticoagulant treatment as well as possible treatment with antiarrhythmic drugs or with catheter ablation, Dr. McMurray said. Looking for undetected AF in HFrEF patients “is where the science is moving.”
The findings that Dr. McMurray reported are “something we should act on,” commented Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The comorbidity of AF in HFrEF patients requires “aggressive anticoagulation, and also a review of their heart failure medical treatment to be sure that is optimized, because AF could be a sign of worsening heart failure,” Dr. Hernandez said in an interview. “We may need to more aggressively get HFrEF patients with AF into normal sinus rhythm.”
When Mikhail Kosiborod, MD, treats HFrEF patients with a high risk for AF, such as patients with lower ejection fractions, a dilated left ventricle, or a dilated atrium, “I frequently do 30-day loop recordings in these patients because of their risk for incident AF,” Dr. Kosiborod said in an interview. “We don’t yet have convincing evidence for this, but it makes sense.”
Another finding from his analysis was that the HFrEF patients enrolled in these two trials did not get treatment with a mineralocorticoid receptor antagonist – spironolactone or eplerenone – “as often as they should,” with treatment rates of 44%-48%, compared with use of a beta-blocker in 92%-95% of patients. Treatment with eplerenone (Inspra) “has been shown to reduce the risk for new onset AF, so adding eplerenone or spironolactone is an important step that could be taken to try to prevent AF as well as treat the HFrEF and reduce mortality,” Dr. McMurray said.
PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen. Dr. Hernandez has received honoraria from Amgen, AstraZeneca, Janssen, Merck, and Novartis, and has received research support from Amgen, Bayer, Merck, and Portola. Dr. Kosiborod has been a consultant to several drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Gilead, and Sanofi-Aventis.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – New onset atrial fibrillation more than doubled the rate of adverse outcomes in patients with heart failure with reduced ejection fraction in a review of more than 15,000 such patients.
In 9,934 patients with heart failure with reduced ejection fraction (HFrEF) and no history of atrial fibrillation (AF), development of new-onset AF was linked with a greater than twofold increased risk of cardiovascular disease death or hospitalization for heart failure during follow-up, compared with HFrEF patients who did not initially have or later develop heart failure, after adjustment for several demographic and clinical variables, John J.V. McMurray, MD, said at the annual meeting of the American College of Cardiology. This difference for the primary endpoint of his analysis was statistically significant.
The 1,645 patients with paroxysmal AF at the start of their follow-up also had a significantly increased rate of cardiovascular death or heart failure hospitalization, but their increased risk when compared with HFrEF patients who didn’t develop AF was a much more modest 20% in his fully adjusted analysis. The patients who began follow-up with paroxysmal AF also had a relatively increased relative stroke rate of 33% when compared with HFrEF patients without AF at baseline who remained AF free, but the all-cause mortality rate among those with paroxysmal AF wasn’t significantly elevated, compared with the comparator group.
The 3,770 patients with persistent or permanent AF at baseline showed no statistically significant spike in their adverse event rates, compared with patients without AF, for any of the examined endpoints. The study group also included 66 patients with an undefined form of AF who weren’t included in these analyses.
“It’s the first episodes and paroxysmal episodes that cause trouble, and the trouble they cause is stroke,” Dr. McMurray said in an interview. Their stroke risk gets exacerbated in clinical practice, because these patients often don’t receive the stroke prevention they need in the form of anticoagulation treatment.
“We find over and over that patients with paroxysmal AF are not anticoagulated as frequently as they should be,” Dr. McMurray said. And HFrEF patients with a first AF episode need anticoagulation, too, as soon as AF is diagnosed, he advised.
He went a step further and speculated that the reason why HFrEF patients with new onset AF did so poorly in his analysis was because they already had several prior, brief AF episodes that had gone undetected. “Many of these patients probably had undiagnosed, clinically unapparent AF episodes” that then resulted in strokes, he suggested.
The upshot is that patients with HFrEF may need more aggressive monitoring for new-onset AF, possibly in the form of small, implanted arrhythmia-detection devices. Dr. McMurray said that he and other researchers are currently testing whether this hypothesis is correct. “We and others are now looking at this because these new data are convincing that new-onset AF is bad news [for HFrEF patients].”
In the analysis, “we looked only at clinically recognized and adjudicated new-onset AF. Goodness knows how many HFrEF patients are having unrecognized paroxysmal AF. Almost certainly there is a lot that is unrecognized” that potentially could be detected using a small implanted arrhythmia monitor, which could then lead to earlier anticoagulant treatment as well as possible treatment with antiarrhythmic drugs or with catheter ablation, Dr. McMurray said. Looking for undetected AF in HFrEF patients “is where the science is moving.”
The findings that Dr. McMurray reported are “something we should act on,” commented Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The comorbidity of AF in HFrEF patients requires “aggressive anticoagulation, and also a review of their heart failure medical treatment to be sure that is optimized, because AF could be a sign of worsening heart failure,” Dr. Hernandez said in an interview. “We may need to more aggressively get HFrEF patients with AF into normal sinus rhythm.”
When Mikhail Kosiborod, MD, treats HFrEF patients with a high risk for AF, such as patients with lower ejection fractions, a dilated left ventricle, or a dilated atrium, “I frequently do 30-day loop recordings in these patients because of their risk for incident AF,” Dr. Kosiborod said in an interview. “We don’t yet have convincing evidence for this, but it makes sense.”
Another finding from his analysis was that the HFrEF patients enrolled in these two trials did not get treatment with a mineralocorticoid receptor antagonist – spironolactone or eplerenone – “as often as they should,” with treatment rates of 44%-48%, compared with use of a beta-blocker in 92%-95% of patients. Treatment with eplerenone (Inspra) “has been shown to reduce the risk for new onset AF, so adding eplerenone or spironolactone is an important step that could be taken to try to prevent AF as well as treat the HFrEF and reduce mortality,” Dr. McMurray said.
PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen. Dr. Hernandez has received honoraria from Amgen, AstraZeneca, Janssen, Merck, and Novartis, and has received research support from Amgen, Bayer, Merck, and Portola. Dr. Kosiborod has been a consultant to several drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Gilead, and Sanofi-Aventis.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – New onset atrial fibrillation more than doubled the rate of adverse outcomes in patients with heart failure with reduced ejection fraction in a review of more than 15,000 such patients.
In 9,934 patients with heart failure with reduced ejection fraction (HFrEF) and no history of atrial fibrillation (AF), development of new-onset AF was linked with a greater than twofold increased risk of cardiovascular disease death or hospitalization for heart failure during follow-up, compared with HFrEF patients who did not initially have or later develop heart failure, after adjustment for several demographic and clinical variables, John J.V. McMurray, MD, said at the annual meeting of the American College of Cardiology. This difference for the primary endpoint of his analysis was statistically significant.
The 1,645 patients with paroxysmal AF at the start of their follow-up also had a significantly increased rate of cardiovascular death or heart failure hospitalization, but their increased risk when compared with HFrEF patients who didn’t develop AF was a much more modest 20% in his fully adjusted analysis. The patients who began follow-up with paroxysmal AF also had a relatively increased relative stroke rate of 33% when compared with HFrEF patients without AF at baseline who remained AF free, but the all-cause mortality rate among those with paroxysmal AF wasn’t significantly elevated, compared with the comparator group.
The 3,770 patients with persistent or permanent AF at baseline showed no statistically significant spike in their adverse event rates, compared with patients without AF, for any of the examined endpoints. The study group also included 66 patients with an undefined form of AF who weren’t included in these analyses.
“It’s the first episodes and paroxysmal episodes that cause trouble, and the trouble they cause is stroke,” Dr. McMurray said in an interview. Their stroke risk gets exacerbated in clinical practice, because these patients often don’t receive the stroke prevention they need in the form of anticoagulation treatment.
“We find over and over that patients with paroxysmal AF are not anticoagulated as frequently as they should be,” Dr. McMurray said. And HFrEF patients with a first AF episode need anticoagulation, too, as soon as AF is diagnosed, he advised.
He went a step further and speculated that the reason why HFrEF patients with new onset AF did so poorly in his analysis was because they already had several prior, brief AF episodes that had gone undetected. “Many of these patients probably had undiagnosed, clinically unapparent AF episodes” that then resulted in strokes, he suggested.
The upshot is that patients with HFrEF may need more aggressive monitoring for new-onset AF, possibly in the form of small, implanted arrhythmia-detection devices. Dr. McMurray said that he and other researchers are currently testing whether this hypothesis is correct. “We and others are now looking at this because these new data are convincing that new-onset AF is bad news [for HFrEF patients].”
In the analysis, “we looked only at clinically recognized and adjudicated new-onset AF. Goodness knows how many HFrEF patients are having unrecognized paroxysmal AF. Almost certainly there is a lot that is unrecognized” that potentially could be detected using a small implanted arrhythmia monitor, which could then lead to earlier anticoagulant treatment as well as possible treatment with antiarrhythmic drugs or with catheter ablation, Dr. McMurray said. Looking for undetected AF in HFrEF patients “is where the science is moving.”
The findings that Dr. McMurray reported are “something we should act on,” commented Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The comorbidity of AF in HFrEF patients requires “aggressive anticoagulation, and also a review of their heart failure medical treatment to be sure that is optimized, because AF could be a sign of worsening heart failure,” Dr. Hernandez said in an interview. “We may need to more aggressively get HFrEF patients with AF into normal sinus rhythm.”
When Mikhail Kosiborod, MD, treats HFrEF patients with a high risk for AF, such as patients with lower ejection fractions, a dilated left ventricle, or a dilated atrium, “I frequently do 30-day loop recordings in these patients because of their risk for incident AF,” Dr. Kosiborod said in an interview. “We don’t yet have convincing evidence for this, but it makes sense.”
Another finding from his analysis was that the HFrEF patients enrolled in these two trials did not get treatment with a mineralocorticoid receptor antagonist – spironolactone or eplerenone – “as often as they should,” with treatment rates of 44%-48%, compared with use of a beta-blocker in 92%-95% of patients. Treatment with eplerenone (Inspra) “has been shown to reduce the risk for new onset AF, so adding eplerenone or spironolactone is an important step that could be taken to try to prevent AF as well as treat the HFrEF and reduce mortality,” Dr. McMurray said.
PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen. Dr. Hernandez has received honoraria from Amgen, AstraZeneca, Janssen, Merck, and Novartis, and has received research support from Amgen, Bayer, Merck, and Portola. Dr. Kosiborod has been a consultant to several drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Gilead, and Sanofi-Aventis.
[email protected]
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: Adverse outcomes were more than twice as frequent in HFrEF patients with incident atrial fibrillation, compared with those without AF.
Data source: Post hoc analysis of 15,415 heart failure patients enrolled in the PARADIGM-HF and ATMOSPHERE trials.
Disclosures: PARADIGM-HF and ATMOSPHERE were funded by Novartis. Dr. McMurray has been a consultant to and has received travel and research support from Novartis, and he has received research and travel support from Amgen.
Bioidentical hormone replacement fares well in phase III trial
ORLANDO – An oral combination of naturally-occurring estrogen and progesterone was found safe and effective for treatment of hot flashes in postmenopausal women with an intact uterus.
The phase III trial results represent another step toward approval of a formulation of bioidentical hormone therapy (HT) by the Food and Drug Administration.
“No similar combined HT has been approved in the U.S.; however, compounded bioidentical HT is estimated to have become the most prevalent HT by U.S. prescription volume,” Rogerio Lobo, MD, professor of obstetrics and gynecology at Columbia University, New York, wrote in an abstract accompanying the study. He presented his findings at the annual meeting of the Endocrine Society.
The study enrolled 1,835 patients, of whom 89% completed the efficacy portion of the study. The estrogen-progesterone combination significantly reduced hot flashes, compared with placebo (P less than .05 for all doses at 12 weeks), with the higher two of four different combination doses resulting in significant differences by study week 4. Menopause-related quality of life was also significantly improved by study week 12 for all doses (P less than .05, compared with placebo).
Up to 39 million prescriptions annually may be written for up to 2.5 million women in the United States, Dr. Lobo said. None of the currently available formulations of 17 beta-estradiol and progesterone are FDA approved. The medication studied – dubbed TX-001HR and produced by TherapeuticsMD – combines the two hormones in an oral capsule.
The REPLENISH trial was designed to evaluate the efficacy and safety of four different dose combinations of estradiol (E2) and progesterone (P4), compared with placebo, to treat moderate to severe vasomotor symptoms in postmenopausal women.
The phase III randomized, double-blind, placebo-controlled trial of the E2/P4 combination in postmenopausal women with an intact uterus had an efficacy portion of the study that lasted 12 weeks; endometrial safety was followed for 1 year in a smaller subset of patients.
The dose-ranging study design randomized women 1:1:1:1:1 to one of four combinations of E2 and P4, or to placebo. The four active treatment groups received either 1.0 mg E2/100 mg P4, 0.5 mg E2/100 mg P4, 0.5 mg E2/50 mg P4, or 0.25 mg E2/50 mg P4. There was no active comparator.
The safety portion of the study could include women whose vasomotor severity did not qualify them for the efficacy substudy; there was no placebo in this arm of the study.
Women participating in the vasomotor menopausal symptom (VMS) portion of the study kept a daily symptom diary and completed the Menopause-Specific Quality of Life (MENQOL) questionnaire as an objective measure of menopause-related symptomatology.
The study’s primary efficacy endpoints were VMS frequency and severity, tracked by measuring the mean change from baseline at study weeks 4 and 12. The secondary endpoint was the mean change in VMS frequency and severity week to week, compared with baseline. Patients were included in the modified intention-to-treat population if they took at least one dose of study drug and had at least 5 days of baseline diary data as well as at least 4 days of diary data in one on-treatment week.
The safety cohort included all women who took at least one capsule of the study drug, and tracked the incidence of endometrial hyperplasia out to 12 months for those who participated in the extended safety portion of the trial. The secondary endpoint was the incidence of other adverse events and serious adverse events.
All four dose combinations “provided statistically and clinically significant reduction in the weekly frequency of moderate to severe VMS from baseline at weeks 4 and 12, compared with placebo,” Dr. Lobo said. The lone exception, he said, was the lowest dose combination, which didn’t produce significant VMS reduction until study week 6.
Looking at the week-by-week improvement measure, the 1.0 mg E2/100 mg P4 and the 0.5 mg E2/100 mg P4 formulations improved VMS severity at weeks 4 and 12, compared with placebo.
Quality of life as measured by the MENQOL was significantly improved by all doses by study week 12, compared with placebo. Participants also reported significant improvement on the vasomotor domain of the MENQOL.
There was no endometrial hyperplasia in any study subject, nor were any malignancies detected in any study participant, Dr. Lobo said. The most frequently reported treatment-emergent adverse events were headaches, nasopharyngitis, breast tenderness, upper respiratory tract infection, nausea, back pain, and abdominal pain. Though seven serious treatment-emergent adverse events were considered treatment-related, “no unexpected safety signals were observed,” Dr. Lobo said.
To be included, postmenopausal women aged 40-65 years needed to have an intact uterus and be generally healthy, with a body mass index of less than 35 kg/m2. They also underwent an endometrial biopsy before participating. Their VMS had to occur at least seven times daily, or 50 times in a week, and be moderate to severe in intensity.
Patients with endometrial hyperplasia or melanoma, as well as women with uterine, endometrial, ovarian, or breast cancer, were excluded from the study, as were women with cardiovascular, hepatic, or renal disorders. Women with diabetes and those with thyroid disorders also were excluded.
Though women could have used sex hormone–containing or –modifying medications, they had to cease those medications for a variable washout period before beginning the study. The mean age of study participants was 55, and their mean BMI was 27. Two-thirds of the women were white.
“TX-001HR, if approved, would be a new oral hormone therapy option for postmenopausal women with moderate to severe vasomotor symptoms with an intact uterus,” Dr. Lobo said.
The drug, he said, could present an option in bioidentical hormones – one that has been evaluated for safety and efficacy – for women who are currently using “less regulated and unapproved compounded bioidentical hormone therapy.”
Dr. Lobo reported receiving research support from TherapeuticsMD, which funded the study.
[email protected]
On Twitter @karioakes
ORLANDO – An oral combination of naturally-occurring estrogen and progesterone was found safe and effective for treatment of hot flashes in postmenopausal women with an intact uterus.
The phase III trial results represent another step toward approval of a formulation of bioidentical hormone therapy (HT) by the Food and Drug Administration.
“No similar combined HT has been approved in the U.S.; however, compounded bioidentical HT is estimated to have become the most prevalent HT by U.S. prescription volume,” Rogerio Lobo, MD, professor of obstetrics and gynecology at Columbia University, New York, wrote in an abstract accompanying the study. He presented his findings at the annual meeting of the Endocrine Society.
The study enrolled 1,835 patients, of whom 89% completed the efficacy portion of the study. The estrogen-progesterone combination significantly reduced hot flashes, compared with placebo (P less than .05 for all doses at 12 weeks), with the higher two of four different combination doses resulting in significant differences by study week 4. Menopause-related quality of life was also significantly improved by study week 12 for all doses (P less than .05, compared with placebo).
Up to 39 million prescriptions annually may be written for up to 2.5 million women in the United States, Dr. Lobo said. None of the currently available formulations of 17 beta-estradiol and progesterone are FDA approved. The medication studied – dubbed TX-001HR and produced by TherapeuticsMD – combines the two hormones in an oral capsule.
The REPLENISH trial was designed to evaluate the efficacy and safety of four different dose combinations of estradiol (E2) and progesterone (P4), compared with placebo, to treat moderate to severe vasomotor symptoms in postmenopausal women.
The phase III randomized, double-blind, placebo-controlled trial of the E2/P4 combination in postmenopausal women with an intact uterus had an efficacy portion of the study that lasted 12 weeks; endometrial safety was followed for 1 year in a smaller subset of patients.
The dose-ranging study design randomized women 1:1:1:1:1 to one of four combinations of E2 and P4, or to placebo. The four active treatment groups received either 1.0 mg E2/100 mg P4, 0.5 mg E2/100 mg P4, 0.5 mg E2/50 mg P4, or 0.25 mg E2/50 mg P4. There was no active comparator.
The safety portion of the study could include women whose vasomotor severity did not qualify them for the efficacy substudy; there was no placebo in this arm of the study.
Women participating in the vasomotor menopausal symptom (VMS) portion of the study kept a daily symptom diary and completed the Menopause-Specific Quality of Life (MENQOL) questionnaire as an objective measure of menopause-related symptomatology.
The study’s primary efficacy endpoints were VMS frequency and severity, tracked by measuring the mean change from baseline at study weeks 4 and 12. The secondary endpoint was the mean change in VMS frequency and severity week to week, compared with baseline. Patients were included in the modified intention-to-treat population if they took at least one dose of study drug and had at least 5 days of baseline diary data as well as at least 4 days of diary data in one on-treatment week.
The safety cohort included all women who took at least one capsule of the study drug, and tracked the incidence of endometrial hyperplasia out to 12 months for those who participated in the extended safety portion of the trial. The secondary endpoint was the incidence of other adverse events and serious adverse events.
All four dose combinations “provided statistically and clinically significant reduction in the weekly frequency of moderate to severe VMS from baseline at weeks 4 and 12, compared with placebo,” Dr. Lobo said. The lone exception, he said, was the lowest dose combination, which didn’t produce significant VMS reduction until study week 6.
Looking at the week-by-week improvement measure, the 1.0 mg E2/100 mg P4 and the 0.5 mg E2/100 mg P4 formulations improved VMS severity at weeks 4 and 12, compared with placebo.
Quality of life as measured by the MENQOL was significantly improved by all doses by study week 12, compared with placebo. Participants also reported significant improvement on the vasomotor domain of the MENQOL.
There was no endometrial hyperplasia in any study subject, nor were any malignancies detected in any study participant, Dr. Lobo said. The most frequently reported treatment-emergent adverse events were headaches, nasopharyngitis, breast tenderness, upper respiratory tract infection, nausea, back pain, and abdominal pain. Though seven serious treatment-emergent adverse events were considered treatment-related, “no unexpected safety signals were observed,” Dr. Lobo said.
To be included, postmenopausal women aged 40-65 years needed to have an intact uterus and be generally healthy, with a body mass index of less than 35 kg/m2. They also underwent an endometrial biopsy before participating. Their VMS had to occur at least seven times daily, or 50 times in a week, and be moderate to severe in intensity.
Patients with endometrial hyperplasia or melanoma, as well as women with uterine, endometrial, ovarian, or breast cancer, were excluded from the study, as were women with cardiovascular, hepatic, or renal disorders. Women with diabetes and those with thyroid disorders also were excluded.
Though women could have used sex hormone–containing or –modifying medications, they had to cease those medications for a variable washout period before beginning the study. The mean age of study participants was 55, and their mean BMI was 27. Two-thirds of the women were white.
“TX-001HR, if approved, would be a new oral hormone therapy option for postmenopausal women with moderate to severe vasomotor symptoms with an intact uterus,” Dr. Lobo said.
The drug, he said, could present an option in bioidentical hormones – one that has been evaluated for safety and efficacy – for women who are currently using “less regulated and unapproved compounded bioidentical hormone therapy.”
Dr. Lobo reported receiving research support from TherapeuticsMD, which funded the study.
[email protected]
On Twitter @karioakes
ORLANDO – An oral combination of naturally-occurring estrogen and progesterone was found safe and effective for treatment of hot flashes in postmenopausal women with an intact uterus.
The phase III trial results represent another step toward approval of a formulation of bioidentical hormone therapy (HT) by the Food and Drug Administration.
“No similar combined HT has been approved in the U.S.; however, compounded bioidentical HT is estimated to have become the most prevalent HT by U.S. prescription volume,” Rogerio Lobo, MD, professor of obstetrics and gynecology at Columbia University, New York, wrote in an abstract accompanying the study. He presented his findings at the annual meeting of the Endocrine Society.
The study enrolled 1,835 patients, of whom 89% completed the efficacy portion of the study. The estrogen-progesterone combination significantly reduced hot flashes, compared with placebo (P less than .05 for all doses at 12 weeks), with the higher two of four different combination doses resulting in significant differences by study week 4. Menopause-related quality of life was also significantly improved by study week 12 for all doses (P less than .05, compared with placebo).
Up to 39 million prescriptions annually may be written for up to 2.5 million women in the United States, Dr. Lobo said. None of the currently available formulations of 17 beta-estradiol and progesterone are FDA approved. The medication studied – dubbed TX-001HR and produced by TherapeuticsMD – combines the two hormones in an oral capsule.
The REPLENISH trial was designed to evaluate the efficacy and safety of four different dose combinations of estradiol (E2) and progesterone (P4), compared with placebo, to treat moderate to severe vasomotor symptoms in postmenopausal women.
The phase III randomized, double-blind, placebo-controlled trial of the E2/P4 combination in postmenopausal women with an intact uterus had an efficacy portion of the study that lasted 12 weeks; endometrial safety was followed for 1 year in a smaller subset of patients.
The dose-ranging study design randomized women 1:1:1:1:1 to one of four combinations of E2 and P4, or to placebo. The four active treatment groups received either 1.0 mg E2/100 mg P4, 0.5 mg E2/100 mg P4, 0.5 mg E2/50 mg P4, or 0.25 mg E2/50 mg P4. There was no active comparator.
The safety portion of the study could include women whose vasomotor severity did not qualify them for the efficacy substudy; there was no placebo in this arm of the study.
Women participating in the vasomotor menopausal symptom (VMS) portion of the study kept a daily symptom diary and completed the Menopause-Specific Quality of Life (MENQOL) questionnaire as an objective measure of menopause-related symptomatology.
The study’s primary efficacy endpoints were VMS frequency and severity, tracked by measuring the mean change from baseline at study weeks 4 and 12. The secondary endpoint was the mean change in VMS frequency and severity week to week, compared with baseline. Patients were included in the modified intention-to-treat population if they took at least one dose of study drug and had at least 5 days of baseline diary data as well as at least 4 days of diary data in one on-treatment week.
The safety cohort included all women who took at least one capsule of the study drug, and tracked the incidence of endometrial hyperplasia out to 12 months for those who participated in the extended safety portion of the trial. The secondary endpoint was the incidence of other adverse events and serious adverse events.
All four dose combinations “provided statistically and clinically significant reduction in the weekly frequency of moderate to severe VMS from baseline at weeks 4 and 12, compared with placebo,” Dr. Lobo said. The lone exception, he said, was the lowest dose combination, which didn’t produce significant VMS reduction until study week 6.
Looking at the week-by-week improvement measure, the 1.0 mg E2/100 mg P4 and the 0.5 mg E2/100 mg P4 formulations improved VMS severity at weeks 4 and 12, compared with placebo.
Quality of life as measured by the MENQOL was significantly improved by all doses by study week 12, compared with placebo. Participants also reported significant improvement on the vasomotor domain of the MENQOL.
There was no endometrial hyperplasia in any study subject, nor were any malignancies detected in any study participant, Dr. Lobo said. The most frequently reported treatment-emergent adverse events were headaches, nasopharyngitis, breast tenderness, upper respiratory tract infection, nausea, back pain, and abdominal pain. Though seven serious treatment-emergent adverse events were considered treatment-related, “no unexpected safety signals were observed,” Dr. Lobo said.
To be included, postmenopausal women aged 40-65 years needed to have an intact uterus and be generally healthy, with a body mass index of less than 35 kg/m2. They also underwent an endometrial biopsy before participating. Their VMS had to occur at least seven times daily, or 50 times in a week, and be moderate to severe in intensity.
Patients with endometrial hyperplasia or melanoma, as well as women with uterine, endometrial, ovarian, or breast cancer, were excluded from the study, as were women with cardiovascular, hepatic, or renal disorders. Women with diabetes and those with thyroid disorders also were excluded.
Though women could have used sex hormone–containing or –modifying medications, they had to cease those medications for a variable washout period before beginning the study. The mean age of study participants was 55, and their mean BMI was 27. Two-thirds of the women were white.
“TX-001HR, if approved, would be a new oral hormone therapy option for postmenopausal women with moderate to severe vasomotor symptoms with an intact uterus,” Dr. Lobo said.
The drug, he said, could present an option in bioidentical hormones – one that has been evaluated for safety and efficacy – for women who are currently using “less regulated and unapproved compounded bioidentical hormone therapy.”
Dr. Lobo reported receiving research support from TherapeuticsMD, which funded the study.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: Four different dose combinations of 17 beta estradiol and progesterone improved hot flashes and menopause-related quality of life, compared with placebo (P less than .05 for all).
Data source: Multicenter randomized, double-blind, placebo-controlled study of 1,835 postmenopausal women with an intact uterus.
Disclosures: Dr. Lobo reported receiving research funding from TherapeuticsMD, which sponsored the study.
Eruptive Melanocytic Nevi During Azathioprine Therapy for Antisynthetase Syndrome
Case Report
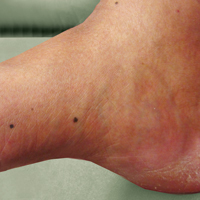
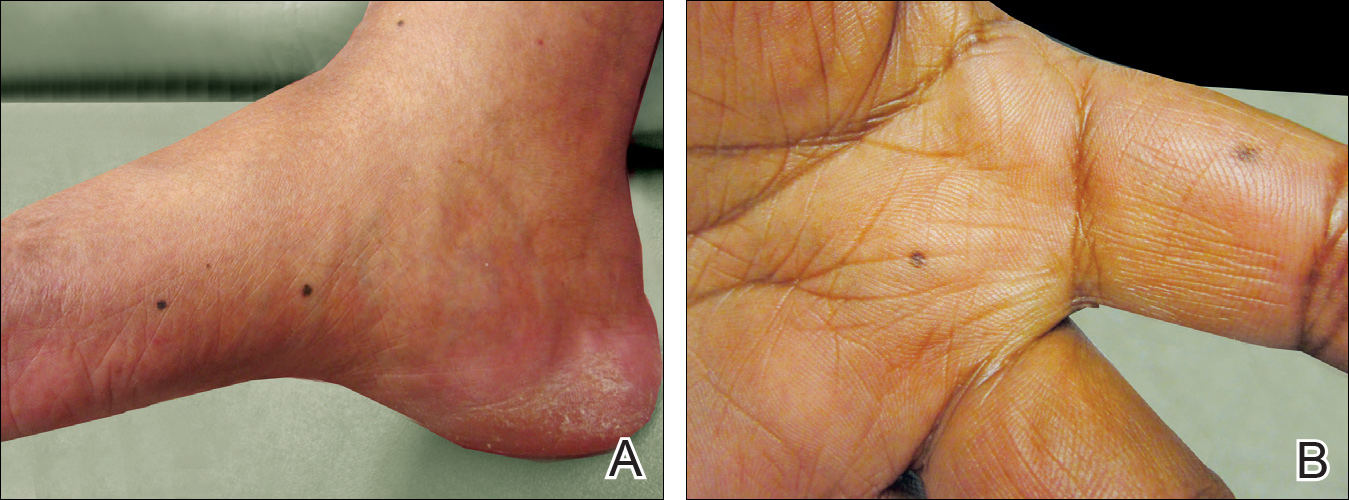
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.
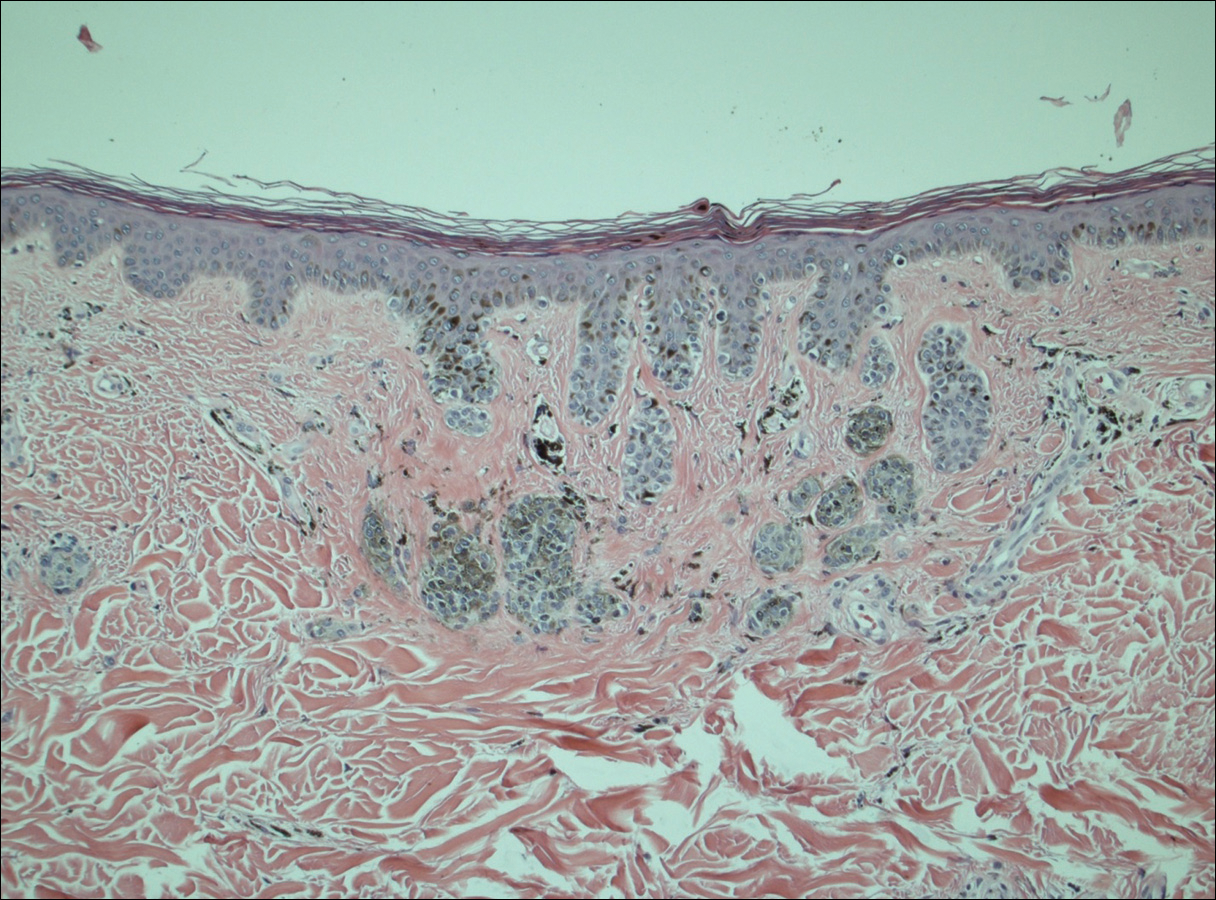
Comment
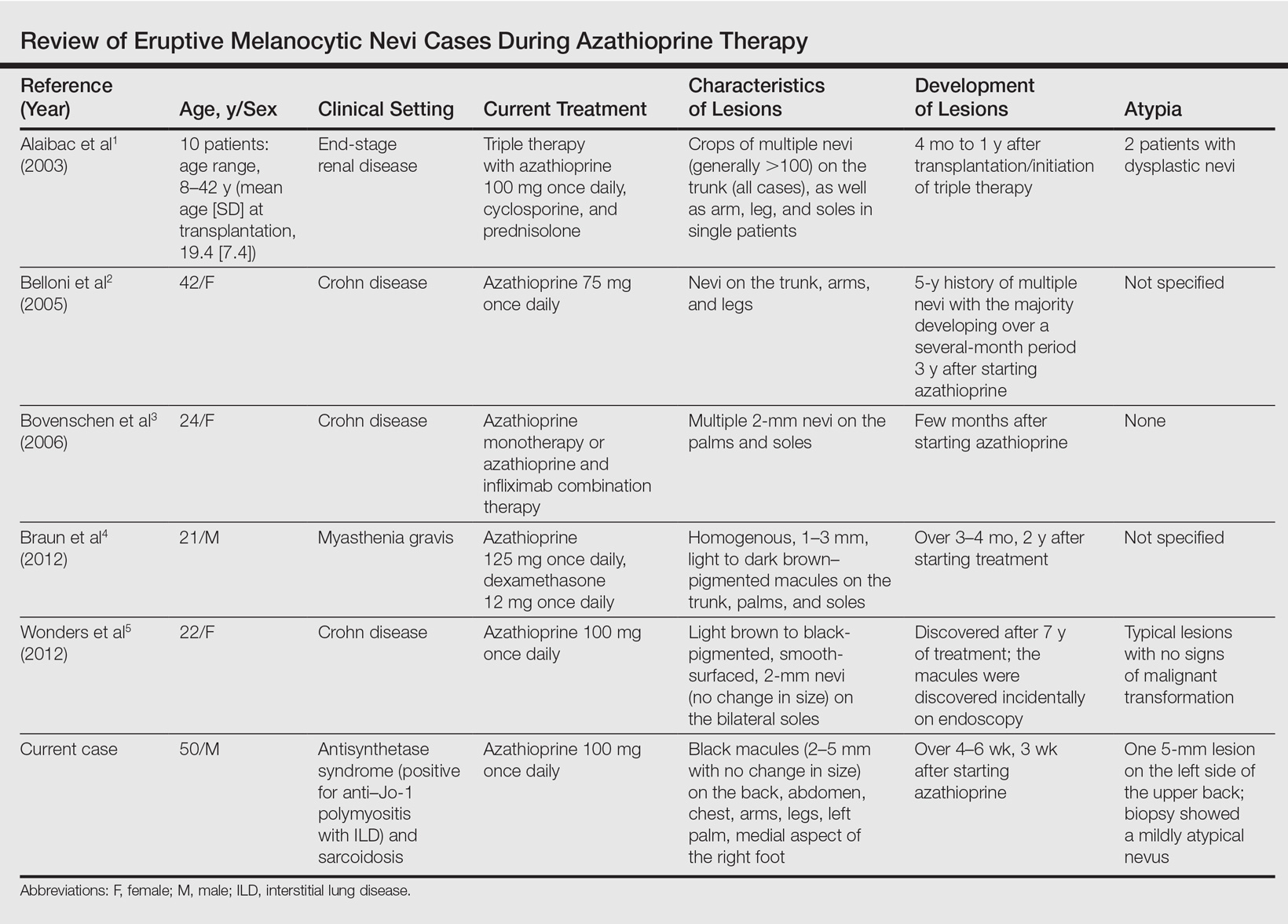
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Practice Points
- A theoretical risk exists in the setting of eruptive melanocytic nevi (EMN) given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.
- Follow patients with EMN with regular skin examinations and biopsies of atypical-appearing lesions given the increased risk for melanoma in this population.
Gliflozins’ heart failure protection in type 2 diabetes confirmed
WASHINGTON – The remarkable and unexpected findings first reported from the EMPA-REG OUTCOME trial in late 2015 – that treatment of type 2 diabetes patients with the SGLT-2 inhibitor empagliflozin led to significantly reduced rates of heart failure hospitalization and all-cause death – received its first major confirmation in an analysis of observational data collected from more than 300,000 patients with type 2 diabetes treated in six countries including the United States.
The new findings also, for the first time, extended the EMPA-REG OUTCOME results (N Engl J Med. 2015 Nov 26;373[22]:2117-28) beyond empagliflozin with evidence that the heart failure and mortality benefit also occurred with other drugs from the sodium glucose cotransporter–2 (SGLT-2) inhibitor class, specifically canagliflozin and dapagliflozin, Mikhail Kosiborod, MD, said at the annual meeting of the American College of Cardiology.
The analysis showed that type 2 diabetes patients who were newly started on treatment with one of these SGLT-2 inhibitors had during follow-up a 39% reduced rate of heart failure hospitalizations, a 51% reduced mortality rate, and a 46% reduced rate of the combined endpoint of heart failure hospitalization or death, compared with patients treated with any other type of oral glucose-lowering drug, reported Dr. Kosiborod. The risk reductions were “remarkably similar to those seen in EMPA-REG OUTCOME,” he noted.
The findings address three “key questions” raised by the EMPA-REG OUTCOME results, Dr. Kosiborod, a cardiologist and professor of medicine at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., said in an interview:
• Is this a class effect? The data “seem to suggest that the benefits seen in the EMPA-REG OUTCOME study are likely a class effect.” The study population of 154,523 patients in the heart failure hospitalization analysis who began treatment with an SGLT-2 inhibitor included 53% who received canagliflozin (Invokana), 42% who received dapagliflozin (Farxiga), and 6% who received empagliflozin (Jardiance) (percentages total 101% because of rounding). Dr. Kosiborod also highlighted that within several of the six countries that contributed data to this analysis – the United States, Denmark, Germany, Norway, Sweden, and the United Kingdom – the percentages of patients on each of these three drugs varied substantially, but despite that the relative reduced risks for heart failure hospitalization and mortality were roughly the same within each country, giving further credence to the notion that a class effect exists.
• Do lower-risk patients benefit? “The benefits of SGLT-2 inhibitor treatment appeared to extend to lower risk patients” than those enrolled in EMPA-REG OUTCOME. In the randomized trial, which enrolled 7,028 patients, more than 99% had established cardiovascular disease. In the new analysis patients had a 13% prevalence of any cardiovascular disease at baseline, and the prevalence of heart failure was 3%.
• Is this relevant to clinical practice? Unlike the highly selected patients entered in the EMPA-REG OUTCOME trial, the patients started on an SGLT-2 inhibitor in the observational study were unselected and came from routine practice situations, “suggesting that the benefits seen in EMPA-REG OUTCOME translate into real-world clinical practice,” Dr. Kosiborod said. “With these data we see for the first time in a large number of patients from multiple countries important evidence suggesting that the SGLT-2 inhibitors may provide in the real world a similar benefit to what was observed in EMPA-REG OUTCOME.”
“The lesson from Dr. Kosiborod’s study is that among patients with type 2 diabetes, treatment with an SGLT-2 inhibitor seems to result in lowered rates of heart failure hospitalizations and mortality, and it’s a safe class of drugs. In the past, we worried about worsening heart failure in patients at risk for developing heart failure” such as patients with type 2 diabetes, said Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The new data make it seem like using an SGLT-2 inhibitor to treat patients with type 2 diabetes “is an appropriate strategy.” But Dr. Hernandez added that in his opinion metformin remains the top drug for type 2 diabetes, while SGLT-2 inhibitors are now “the next drug class to add,” he said in an interview.
Dr. Kosiborod had a somewhat different take. “If a patient with type 2 diabetes did not want to enter a trial or couldn’t get into a trial and fit the profile of a patient who could benefit, I would absolutely treat that patient with an SGLT-2 inhibitor. I’m using these medications clinically as a cardiologist,” he said. “Treatments that have significant benefits for important outcomes should be prioritized over treatments that may reduce hemoglobin A1c but do not have similar benefits.”
The CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors) study used data from adult, previously untreated patients with type 2 diabetes in national registries from the five included European countries. U.S. data were from the Truven Health MarketScan database and from Medicare. This produced a total pool of 160,010 patients who began treatment on an SGLT-2 inhibitor and 1,139,905 patients who began treatment with another oral antidiabetes drug.
Dr. Kosiborod and his associates then performed propensity-score matching to identify 154,523 patients started on an SGLT-2 inhibitor who each closely matched a patient from the other subgroup for baseline demographic and clinical features, producing an analysis dataset of just over 309,000 matched patients. The average age of the included patients was 57 years; 45% were women. During follow-up, 961 patients had a heart failure hospitalization and 1,334 patients died.
Dr. Kosiborod noted that while a potential limitation to his findings is residual confounding not eliminated by the propensity score matching, he was confident about the results because the incidence of other outcomes not expected to be influenced by treatment with SGLT-2 inhibitors were similar in the two study subgroups, suggesting that the linkages between the kind of drug used and differences in heart failure hospitalization rates and in mortality weren’t spurious.
“If there was residual confounding, you’d expect to see similar associations for other endpoints, which we didn’t see,” he said. In addition, the heart failure hospitalization rate differences seen between the SGLT-2 inhibitor recipients and the other patients were consistent in a trio of sensitivity analyses, further buttressing the findings’ plausibility.
CVD-REAL was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Kosiborod has been a consultant to and/or received research funding from AstraZeneca, Boehringer Ingelheim, Sanofi-Aventis and Gilead. Dr. Hernandez has received honoraria and/or research support from AstraZeneca, Amgen, Janssen, Merck, Novartis, and Portola. Several of the coauthors on the CVD-REAL study were AstraZeneca employees.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – The remarkable and unexpected findings first reported from the EMPA-REG OUTCOME trial in late 2015 – that treatment of type 2 diabetes patients with the SGLT-2 inhibitor empagliflozin led to significantly reduced rates of heart failure hospitalization and all-cause death – received its first major confirmation in an analysis of observational data collected from more than 300,000 patients with type 2 diabetes treated in six countries including the United States.
The new findings also, for the first time, extended the EMPA-REG OUTCOME results (N Engl J Med. 2015 Nov 26;373[22]:2117-28) beyond empagliflozin with evidence that the heart failure and mortality benefit also occurred with other drugs from the sodium glucose cotransporter–2 (SGLT-2) inhibitor class, specifically canagliflozin and dapagliflozin, Mikhail Kosiborod, MD, said at the annual meeting of the American College of Cardiology.
The analysis showed that type 2 diabetes patients who were newly started on treatment with one of these SGLT-2 inhibitors had during follow-up a 39% reduced rate of heart failure hospitalizations, a 51% reduced mortality rate, and a 46% reduced rate of the combined endpoint of heart failure hospitalization or death, compared with patients treated with any other type of oral glucose-lowering drug, reported Dr. Kosiborod. The risk reductions were “remarkably similar to those seen in EMPA-REG OUTCOME,” he noted.
The findings address three “key questions” raised by the EMPA-REG OUTCOME results, Dr. Kosiborod, a cardiologist and professor of medicine at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., said in an interview:
• Is this a class effect? The data “seem to suggest that the benefits seen in the EMPA-REG OUTCOME study are likely a class effect.” The study population of 154,523 patients in the heart failure hospitalization analysis who began treatment with an SGLT-2 inhibitor included 53% who received canagliflozin (Invokana), 42% who received dapagliflozin (Farxiga), and 6% who received empagliflozin (Jardiance) (percentages total 101% because of rounding). Dr. Kosiborod also highlighted that within several of the six countries that contributed data to this analysis – the United States, Denmark, Germany, Norway, Sweden, and the United Kingdom – the percentages of patients on each of these three drugs varied substantially, but despite that the relative reduced risks for heart failure hospitalization and mortality were roughly the same within each country, giving further credence to the notion that a class effect exists.
• Do lower-risk patients benefit? “The benefits of SGLT-2 inhibitor treatment appeared to extend to lower risk patients” than those enrolled in EMPA-REG OUTCOME. In the randomized trial, which enrolled 7,028 patients, more than 99% had established cardiovascular disease. In the new analysis patients had a 13% prevalence of any cardiovascular disease at baseline, and the prevalence of heart failure was 3%.
• Is this relevant to clinical practice? Unlike the highly selected patients entered in the EMPA-REG OUTCOME trial, the patients started on an SGLT-2 inhibitor in the observational study were unselected and came from routine practice situations, “suggesting that the benefits seen in EMPA-REG OUTCOME translate into real-world clinical practice,” Dr. Kosiborod said. “With these data we see for the first time in a large number of patients from multiple countries important evidence suggesting that the SGLT-2 inhibitors may provide in the real world a similar benefit to what was observed in EMPA-REG OUTCOME.”
“The lesson from Dr. Kosiborod’s study is that among patients with type 2 diabetes, treatment with an SGLT-2 inhibitor seems to result in lowered rates of heart failure hospitalizations and mortality, and it’s a safe class of drugs. In the past, we worried about worsening heart failure in patients at risk for developing heart failure” such as patients with type 2 diabetes, said Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The new data make it seem like using an SGLT-2 inhibitor to treat patients with type 2 diabetes “is an appropriate strategy.” But Dr. Hernandez added that in his opinion metformin remains the top drug for type 2 diabetes, while SGLT-2 inhibitors are now “the next drug class to add,” he said in an interview.
Dr. Kosiborod had a somewhat different take. “If a patient with type 2 diabetes did not want to enter a trial or couldn’t get into a trial and fit the profile of a patient who could benefit, I would absolutely treat that patient with an SGLT-2 inhibitor. I’m using these medications clinically as a cardiologist,” he said. “Treatments that have significant benefits for important outcomes should be prioritized over treatments that may reduce hemoglobin A1c but do not have similar benefits.”
The CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors) study used data from adult, previously untreated patients with type 2 diabetes in national registries from the five included European countries. U.S. data were from the Truven Health MarketScan database and from Medicare. This produced a total pool of 160,010 patients who began treatment on an SGLT-2 inhibitor and 1,139,905 patients who began treatment with another oral antidiabetes drug.
Dr. Kosiborod and his associates then performed propensity-score matching to identify 154,523 patients started on an SGLT-2 inhibitor who each closely matched a patient from the other subgroup for baseline demographic and clinical features, producing an analysis dataset of just over 309,000 matched patients. The average age of the included patients was 57 years; 45% were women. During follow-up, 961 patients had a heart failure hospitalization and 1,334 patients died.
Dr. Kosiborod noted that while a potential limitation to his findings is residual confounding not eliminated by the propensity score matching, he was confident about the results because the incidence of other outcomes not expected to be influenced by treatment with SGLT-2 inhibitors were similar in the two study subgroups, suggesting that the linkages between the kind of drug used and differences in heart failure hospitalization rates and in mortality weren’t spurious.
“If there was residual confounding, you’d expect to see similar associations for other endpoints, which we didn’t see,” he said. In addition, the heart failure hospitalization rate differences seen between the SGLT-2 inhibitor recipients and the other patients were consistent in a trio of sensitivity analyses, further buttressing the findings’ plausibility.
CVD-REAL was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Kosiborod has been a consultant to and/or received research funding from AstraZeneca, Boehringer Ingelheim, Sanofi-Aventis and Gilead. Dr. Hernandez has received honoraria and/or research support from AstraZeneca, Amgen, Janssen, Merck, Novartis, and Portola. Several of the coauthors on the CVD-REAL study were AstraZeneca employees.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – The remarkable and unexpected findings first reported from the EMPA-REG OUTCOME trial in late 2015 – that treatment of type 2 diabetes patients with the SGLT-2 inhibitor empagliflozin led to significantly reduced rates of heart failure hospitalization and all-cause death – received its first major confirmation in an analysis of observational data collected from more than 300,000 patients with type 2 diabetes treated in six countries including the United States.
The new findings also, for the first time, extended the EMPA-REG OUTCOME results (N Engl J Med. 2015 Nov 26;373[22]:2117-28) beyond empagliflozin with evidence that the heart failure and mortality benefit also occurred with other drugs from the sodium glucose cotransporter–2 (SGLT-2) inhibitor class, specifically canagliflozin and dapagliflozin, Mikhail Kosiborod, MD, said at the annual meeting of the American College of Cardiology.
The analysis showed that type 2 diabetes patients who were newly started on treatment with one of these SGLT-2 inhibitors had during follow-up a 39% reduced rate of heart failure hospitalizations, a 51% reduced mortality rate, and a 46% reduced rate of the combined endpoint of heart failure hospitalization or death, compared with patients treated with any other type of oral glucose-lowering drug, reported Dr. Kosiborod. The risk reductions were “remarkably similar to those seen in EMPA-REG OUTCOME,” he noted.
The findings address three “key questions” raised by the EMPA-REG OUTCOME results, Dr. Kosiborod, a cardiologist and professor of medicine at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., said in an interview:
• Is this a class effect? The data “seem to suggest that the benefits seen in the EMPA-REG OUTCOME study are likely a class effect.” The study population of 154,523 patients in the heart failure hospitalization analysis who began treatment with an SGLT-2 inhibitor included 53% who received canagliflozin (Invokana), 42% who received dapagliflozin (Farxiga), and 6% who received empagliflozin (Jardiance) (percentages total 101% because of rounding). Dr. Kosiborod also highlighted that within several of the six countries that contributed data to this analysis – the United States, Denmark, Germany, Norway, Sweden, and the United Kingdom – the percentages of patients on each of these three drugs varied substantially, but despite that the relative reduced risks for heart failure hospitalization and mortality were roughly the same within each country, giving further credence to the notion that a class effect exists.
• Do lower-risk patients benefit? “The benefits of SGLT-2 inhibitor treatment appeared to extend to lower risk patients” than those enrolled in EMPA-REG OUTCOME. In the randomized trial, which enrolled 7,028 patients, more than 99% had established cardiovascular disease. In the new analysis patients had a 13% prevalence of any cardiovascular disease at baseline, and the prevalence of heart failure was 3%.
• Is this relevant to clinical practice? Unlike the highly selected patients entered in the EMPA-REG OUTCOME trial, the patients started on an SGLT-2 inhibitor in the observational study were unselected and came from routine practice situations, “suggesting that the benefits seen in EMPA-REG OUTCOME translate into real-world clinical practice,” Dr. Kosiborod said. “With these data we see for the first time in a large number of patients from multiple countries important evidence suggesting that the SGLT-2 inhibitors may provide in the real world a similar benefit to what was observed in EMPA-REG OUTCOME.”
“The lesson from Dr. Kosiborod’s study is that among patients with type 2 diabetes, treatment with an SGLT-2 inhibitor seems to result in lowered rates of heart failure hospitalizations and mortality, and it’s a safe class of drugs. In the past, we worried about worsening heart failure in patients at risk for developing heart failure” such as patients with type 2 diabetes, said Adrian F. Hernandez, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C. The new data make it seem like using an SGLT-2 inhibitor to treat patients with type 2 diabetes “is an appropriate strategy.” But Dr. Hernandez added that in his opinion metformin remains the top drug for type 2 diabetes, while SGLT-2 inhibitors are now “the next drug class to add,” he said in an interview.
Dr. Kosiborod had a somewhat different take. “If a patient with type 2 diabetes did not want to enter a trial or couldn’t get into a trial and fit the profile of a patient who could benefit, I would absolutely treat that patient with an SGLT-2 inhibitor. I’m using these medications clinically as a cardiologist,” he said. “Treatments that have significant benefits for important outcomes should be prioritized over treatments that may reduce hemoglobin A1c but do not have similar benefits.”
The CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors) study used data from adult, previously untreated patients with type 2 diabetes in national registries from the five included European countries. U.S. data were from the Truven Health MarketScan database and from Medicare. This produced a total pool of 160,010 patients who began treatment on an SGLT-2 inhibitor and 1,139,905 patients who began treatment with another oral antidiabetes drug.
Dr. Kosiborod and his associates then performed propensity-score matching to identify 154,523 patients started on an SGLT-2 inhibitor who each closely matched a patient from the other subgroup for baseline demographic and clinical features, producing an analysis dataset of just over 309,000 matched patients. The average age of the included patients was 57 years; 45% were women. During follow-up, 961 patients had a heart failure hospitalization and 1,334 patients died.
Dr. Kosiborod noted that while a potential limitation to his findings is residual confounding not eliminated by the propensity score matching, he was confident about the results because the incidence of other outcomes not expected to be influenced by treatment with SGLT-2 inhibitors were similar in the two study subgroups, suggesting that the linkages between the kind of drug used and differences in heart failure hospitalization rates and in mortality weren’t spurious.
“If there was residual confounding, you’d expect to see similar associations for other endpoints, which we didn’t see,” he said. In addition, the heart failure hospitalization rate differences seen between the SGLT-2 inhibitor recipients and the other patients were consistent in a trio of sensitivity analyses, further buttressing the findings’ plausibility.
CVD-REAL was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Kosiborod has been a consultant to and/or received research funding from AstraZeneca, Boehringer Ingelheim, Sanofi-Aventis and Gilead. Dr. Hernandez has received honoraria and/or research support from AstraZeneca, Amgen, Janssen, Merck, Novartis, and Portola. Several of the coauthors on the CVD-REAL study were AstraZeneca employees.
[email protected]
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: Treatment with an SGLT-2 inhibitor was associated with a significant 39% reduction in heart failure hospitalizations compared with other antidiabetes drugs.
Data source: CVD-REAL, which used observational data collected from 309,046 patients with type 2 diabetes in six countries.
Disclosures: CVD-REAL was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Kosiborod has been a consultant to AstraZeneca and to several other drug companies, and he has received research funding from AstraZeneca, Boehringer Ingelheim, Sanofi-Aventis and Gilead. Dr. Hernandez has received honoraria and research support from AstraZeneca and has also received honoraria from Amgen, Janssen, Merck, and Novartis, and has also received research support from Amgen, Bayer, Merck, and Portola. Several of the coauthors on the CVD-REAL study were AstraZeneca employees.
Electronic Collaboration in Dermatology Resident Training Through Social Networking
More than 1.8 billion individuals utilize social media, a number that continues to grow as the social media market expands.1 Social media enables individuals, groups, and organizations to efficiently disperse and access information2-4 and also provides a structure that encourages collaboration between patients, staff, and physicians that cannot be achieved by other communication modalities.4-6 Expert opinions and related educational materials can be shared globally, improving collaboration between dermatologists.6 A structured social networking site for sharing training materials, research, and ideas can help bring the national dermatology community together in a new way.
Other professions have employed social networking tools to accomplish similar goals of organizing training resources; radiology has an electronic database that allows sharing of training materials and incorporates social networking capabilities.7 Their Web software provides functionality for individual file uploading and supports collaboration and sharing, all while maintaining the security of uploaded information. General surgery has already addressed similar concerns via a task force that incorporates all the essential organizations in surgical education.8 Increased satisfaction and academic abilities have been demonstrated with their collaborative curriculum.9 Gastroenterologists also utilize electronic resources; one study showed that using videos to educate patients prior to colonoscopies was superior to face-to-face education.10 In addition, video education may free up time for office staff to accomplish other tasks.
As a specialty, dermatology has not been a leader in the implementation of social networking for collaboration and training purposes. Every dermatologist is an educator. To maintain a successful practice, dermatologists must keep up-to-date on their own clinical knowledge, provide training to their staff, and educate their patients. Although there are numerous educational resources available to dermatologists, an informal survey of 30 dermatology faculty members revealed a practice gap in awareness and utilization of these expanding electronic resources.11
To better understand the needs of the specialty as a whole, we chose to focus on one aspect of dermatology education: resident training. The goal of our study was to survey dermatology residents and faculty to gain a better understanding of how they currently provide education and what online resources and social networking sites they currently use or would be willing to use. The study included 3 central hypotheses: First, residents would be less satisfied with their current curriculum and residents would report greater contributions to the curriculum relative to faculty. Second, both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Lastly, residents would be more willing than faculty to participate in social networking for educational purposes.
Methods
This study was granted institutional review board exemption. Two surveys were developed by the authors to assess the current structure and satisfaction of dermatology residency curriculum and the willingness to participate in social networking to use and share educational materials. The surveys were evaluated for relevance by the survey evaluation team of the Association of Professors of Dermatology (APD). The instrument was not pilot tested.
The surveys were electronically distributed using an online service to dermatology faculty via the APD listserve, which comprised the entirety of the APD membership in 2014. The resident survey was distributed to the dermatology residents via the American Society for Dermatologic Surgery listserve, which included all residents in training (2013-2014 academic year). Second and third invitations to complete the surveys were distributed 3 and 5 weeks later, respectively.
Resident and faculty responses were compared. Additionally, responses were stratified for large (>9 residents) and small programs (≤9 residents) for comparison. Descriptive statistics including means and medians for continuous variables and frequency tables for categorical variables were generated using research and spreadsheet software.
Results
There were 137 survey respondents; 52 of 426 (12.2%) dermatology faculty and 85 of 1539 (5.5%) dermatology residents responded to the survey. Small programs accounted for 24% of total survey responses and 76% were from large programs.
Current Curriculum
The majority of dermatology faculty (44%) and residents (35%) identified 1 to 2 faculty members as contributing to the creation and organization of their respective curricula; however, a notable percentage of residents (9%) reported that no faculty contributed to the organization of the curriculum. Residents noted that senior residents carry twice the responsibility for structuring the curriculum compared to faculty (61% vs 32% of the workload), but faculty described an even split between senior residents and faculty (47% vs 49% of the workload). Faculty believed their residents spend a similar amount of time in resident- and faculty-led instruction (38% vs 35% of their time); however, the majority of residents reported spending too little time in faculty-led instruction (53%). When residents ranked their preference for learning modes, faculty-led and self-study learning were ranked first and second by 48% and 45% of residents, respectively. Resident-led instruction was ranked last by 66% of residents. Likewise, a majority of residents (53%) described their amount of time in faculty-led instruction as too little.
When asked what subjects in dermatology were lacking at their programs, residents reported clinical trials (47%), skin of color (46%), cosmetic dermatology (34%), and aggressive skin cancer/multidisciplinary tumor board (32%). Although 11% of residents reported lacking inpatient dermatology in their curriculum, 0% of faculty reported the same. A notable percentage of faculty reported nothing was lacking compared to residents (25% vs 7%). Despite these different views between residents and faculty on their contributions to and structure of their curriculums, both faculty and residents claimed overall satisfaction (satisfied or very satisfied) with their program’s ability to optimally cover the field of dermatology in 3 years (100% and 91%, respectively).
Large Versus Small Residency Programs
When stratifying the resident responses for small versus large programs, both program sizes reported more time in resident-led instruction than faculty-led instruction. Likewise, residents in both program sizes equally preferred self-study or faculty-led instruction to resident-led instruction. Residents at small programs more often reported lacking instruction in rheumatology, immunobullous diseases, and basic science/skin biology compared to large-program residents. Compared to large-program faculty, small-program faculty reported lacking instruction in cosmetic dermatology.
Faculty at small programs reported spending too little time preparing for their faculty-led instruction compared to faculty at large programs (44% vs 12%). All (100%) of the faculty at small programs were likely to seek out study materials shared by top educators, while 77% of faculty at large programs were likely to do the same. When asked if faculty would translate what their program does well into an electronic format for sharing, 30% of large-program faculty were likely to do so compared to 11% of small-program faculty (Figure 1).
Use of Online Educational Materials and Interest in Collaboration
A majority of faculty and residents stated that they use online educational materials as supplements to traditional classroom lecture and print materials (81% vs 86%); however, almost twice as many residents stated that online educational materials were essential to their current study routines compared to faculty (39% vs 21%).
The majority of faculty (92%) and residents (84%) were either interested or very interested in a collaborative online curriculum. Both residents (85%) and faculty (81%) stated they would be likely to seek out online educational materials shared by top educators. Although both residents and faculty reported many aspects of their curriculums they thought could be beneficial to other dermatology programs (Table 1), only 27% of faculty and 19% of residents were likely to translate those strengths into a shareable electronic format. Several reasons were reported for not contributing to an online curriculum, with lack of time being the most common reason (Table 2).
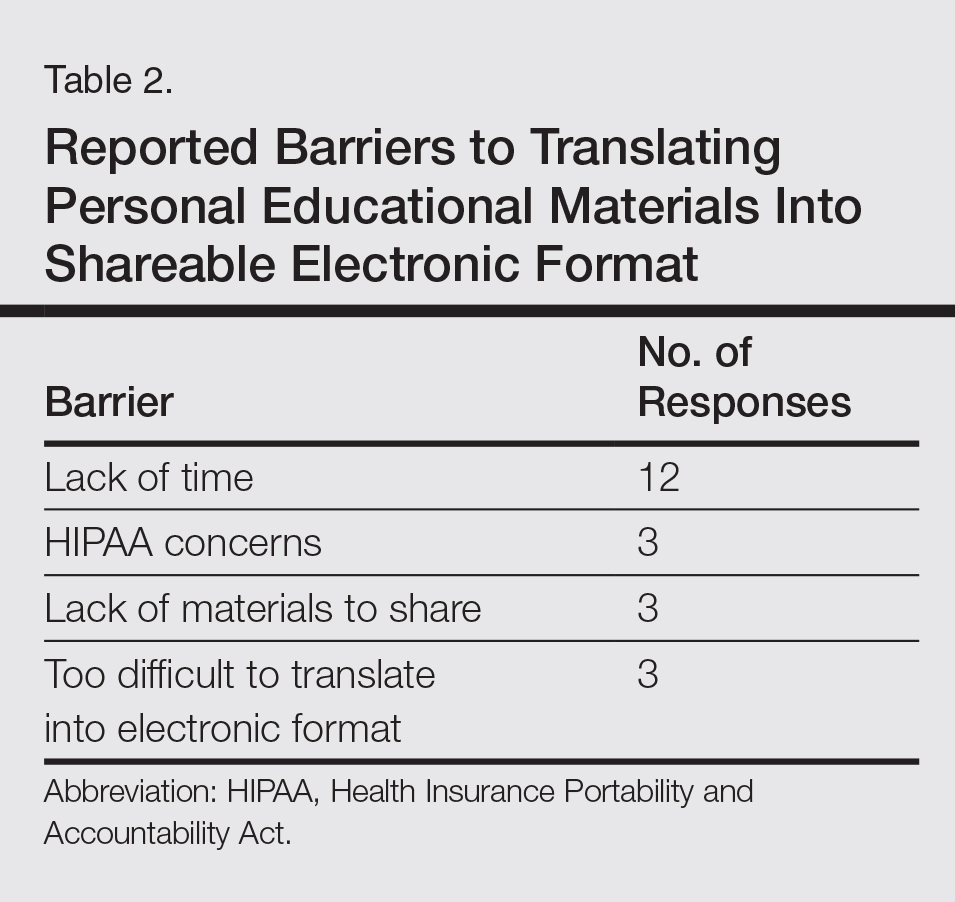
Eighty percent of residents and 88% of faculty reported they were either interested or very interested in being more connected/interactive with their dermatology peers nationally (Figure 2). Likewise, 94% of residents and 87% of faculty agreed that the dermatology community could benefit from a social networking site for educational collaboration. Four times as many residents versus faculty currently use social networking sites (eg, Facebook, LinkedIn, Google Groups) as a primary mode of communication with distant professional peers. The majority of residents (52%) reported they would be likely to participate in a professional social networking site, while the majority of faculty (50%) stated they were neutral on their likelihood of participating. Both residents and faculty reported lack of time as a common reason for being unlikely to utilize a professional social networking site. Other barriers to participation are listed in Table 3.
Comment
This study showed how dermatology faculty and residents currently provide training and what online resources and social networking sites they currently use or would be willing to use. The generalizability of the conclusions is limited by the low response rate for the surveys. The results demonstrated the different views between faculty and residents and between large and small residency programs on various topics. This microcosm of dermatology training can likely be applied to other training scenarios in dermatology, including patient education; training of nurses, physician extenders, and office staff; continuing medical education for physicians; and peer-to-peer collaboration.
Hypothesis 1: Partially Proven
We hypothesized that residents would report less satisfaction with their current curriculum and would report greater resident contributions to the curriculum relative to faculty. Overall, residents and faculty reported satisfaction with their curriculums to provide up-to-date information and breadth in the field of dermatology. Despite their overall satisfaction, more residents reported lacking instruction in several dermatology subtopics compared to faculty. Additionally, residents believed they spend twice as much time structuring their curriculum compared to faculty, with some residents reporting no faculty involvement. Although residents preferred faculty-led instruction, a majority of residents reported they do not have enough faculty-led didactics. The preference for faculty-led training is likely due to the expertise of faculty compared to residents.
Hypothesis 2: Partially Proven
We also hypothesized that both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Although there was no difference in interest between residents at small versus large programs, there was a difference between faculty at small versus large programs. Small-program faculty were more interested in using shared materials than larger programs, while large-program faculty were more likely to share their educational materials. Small-program faculty reported spending too little time preparing their lectures, which is possibly due to a lack of time for preparation. Additionally, residents and faculty at smaller programs report their curriculum was lacking specific dermatology topics compared to large programs. These disparities between program sizes indicate a need for a social networking site for training collaboration in dermatology. Large programs have the ability to share what they do well, which small programs are eager to utilize.
Hypothesis 3: Not Proven
We hypothesized that residents would be more willing than faculty to participate in social networking for educational purposes. The majority of faculty and residents were interested in participating in a collaborative online curriculum and using the shared materials from top educators; however, even though such large majorities favored collaboration and sharing, only 27% of faculty and 19% of residents were likely to translate their own materials into a shareable format. Although lack of time was the most common reason for not sharing materials, electronic methods may have the potential to ultimately save time and remove the burden of content creation. The time it would take to translate selected personal training materials into a shareable form would be made up for by the time saved using another educators’ materials. Updating and customizing shared online educational materials can be much quicker and easier than educators creating materials on their own. Dermatologists would be more efficient facilitators of training via high-quality shared materials while decreasing the time burden associated with resident education.5 Another concern for not sharing or participating in a social networking site was skepticism of information security on such a network. The poor organization and information overload of online resources can compound the already existing time constraints on dermatologists, which may limit their ability to utilize such valuable resources. In addition, quality of online resources is not always guaranteed, and determining the sources that are high quality is sometimes a difficult task.6 For online materials to remain useful, there should be a peer-review process to evaluate quality and assess satisfaction.5
Solution: Create a Dermatology Task Force
A dermatology task force could facilitate the resolution of these challenges of online materials. In addition, a task force could cover the administrative support needed to ensure security and provide maintenance on social networks.
The main limitation to implementing a social network is the presence of the administrative infrastructure to jumpstart its creation. A task force incorporating the essential stakeholders in dermatology training is the first step. With inclusive representation from all of the smaller professional dermatology societies, the American Academy of Dermatology is optimally positioned to create this task force. With existing information technologies, a task force could address the concerns revealed in our survey as well as any future concerns that may arise.
The goal is a single social network for dermatologists that has the capability of improving communication and collaboration between professional peers regardless of their practice setting. Such a network is ideal for the practicing dermatologist for the purposes of staff training, patient education, and obtaining continuing medical education credit. Additionally, peer group collaboration would facilitate the understanding and completion of the evolving requirements for Maintenance of Certification from the American Board of Dermatology. The availability of quality shared materials would save time and increase efficiency of an entire dermatology practice. Materials that aid in patient education would allow office staff to dedicate their time to other tasks, thereby increasing productivity. Shared training materials would decrease the burden of staff education, providing more time for advanced hands-on training. This method of collaborative effort is capable of advancing the field of dermatology as a whole. It can overcome geographical and institutional barriers to connect dermatologists with similar interests worldwide; disseminate advances in diagnosis and treatment; and improve the quality of dermatology training of dermatologists, staff, and patients.
- Statistics and facts about social networks. Statista website. http://www.statista.com/topics/1164/social-networks/. Accessed March 22, 2017.
- Baker RC, Klein M, Samaan Z, et al. Effectiveness of an online pediatric primary care curriculum. Acad Pediatr. 2010;10:131-137.
- Dolev JC, O’Sullivan P, Berger T. The eDerm online curriculum: a randomized study of effective skin cancer teaching to medical students. J Am Acad Dermatol. 2011;65:e165-e171.
- Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150:56-60.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of e-learning in medical education. Acad Med. 2006;81:207-212.
- Hanson AH, Krause LK, Simmons RN, et al. Dermatology education and the internet: traditional and cutting-edge resources. J Am Acad Dermatol. 2011;65:836-842.
- Rowe SP, Siddiqui A, Bonekamp D. The key image and case log application: new radiology software for teaching file creation and case logging that incorporates elements of a social network. Acad Radiol. 2014;21:916-930.
- Bell RH. Surgical council on resident education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007;204:341-346.
- Kirton OC, Reilly P, Staff I, et al. Development and implementation of an interactive, objective, and simulation-based curriculum for general surgery residents. J Surg Educ. 2012;69:718-723.
- Prakash S, Verma S, McGowan J, et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700.
- Carroll BT. eTools for teaching dermatologic surgery. Paper presented at the Association of Professors of Dermatology 2014 Annual Meeting; September 12-13, 2014; Chicago, IL.
More than 1.8 billion individuals utilize social media, a number that continues to grow as the social media market expands.1 Social media enables individuals, groups, and organizations to efficiently disperse and access information2-4 and also provides a structure that encourages collaboration between patients, staff, and physicians that cannot be achieved by other communication modalities.4-6 Expert opinions and related educational materials can be shared globally, improving collaboration between dermatologists.6 A structured social networking site for sharing training materials, research, and ideas can help bring the national dermatology community together in a new way.
Other professions have employed social networking tools to accomplish similar goals of organizing training resources; radiology has an electronic database that allows sharing of training materials and incorporates social networking capabilities.7 Their Web software provides functionality for individual file uploading and supports collaboration and sharing, all while maintaining the security of uploaded information. General surgery has already addressed similar concerns via a task force that incorporates all the essential organizations in surgical education.8 Increased satisfaction and academic abilities have been demonstrated with their collaborative curriculum.9 Gastroenterologists also utilize electronic resources; one study showed that using videos to educate patients prior to colonoscopies was superior to face-to-face education.10 In addition, video education may free up time for office staff to accomplish other tasks.
As a specialty, dermatology has not been a leader in the implementation of social networking for collaboration and training purposes. Every dermatologist is an educator. To maintain a successful practice, dermatologists must keep up-to-date on their own clinical knowledge, provide training to their staff, and educate their patients. Although there are numerous educational resources available to dermatologists, an informal survey of 30 dermatology faculty members revealed a practice gap in awareness and utilization of these expanding electronic resources.11
To better understand the needs of the specialty as a whole, we chose to focus on one aspect of dermatology education: resident training. The goal of our study was to survey dermatology residents and faculty to gain a better understanding of how they currently provide education and what online resources and social networking sites they currently use or would be willing to use. The study included 3 central hypotheses: First, residents would be less satisfied with their current curriculum and residents would report greater contributions to the curriculum relative to faculty. Second, both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Lastly, residents would be more willing than faculty to participate in social networking for educational purposes.
Methods
This study was granted institutional review board exemption. Two surveys were developed by the authors to assess the current structure and satisfaction of dermatology residency curriculum and the willingness to participate in social networking to use and share educational materials. The surveys were evaluated for relevance by the survey evaluation team of the Association of Professors of Dermatology (APD). The instrument was not pilot tested.
The surveys were electronically distributed using an online service to dermatology faculty via the APD listserve, which comprised the entirety of the APD membership in 2014. The resident survey was distributed to the dermatology residents via the American Society for Dermatologic Surgery listserve, which included all residents in training (2013-2014 academic year). Second and third invitations to complete the surveys were distributed 3 and 5 weeks later, respectively.
Resident and faculty responses were compared. Additionally, responses were stratified for large (>9 residents) and small programs (≤9 residents) for comparison. Descriptive statistics including means and medians for continuous variables and frequency tables for categorical variables were generated using research and spreadsheet software.
Results
There were 137 survey respondents; 52 of 426 (12.2%) dermatology faculty and 85 of 1539 (5.5%) dermatology residents responded to the survey. Small programs accounted for 24% of total survey responses and 76% were from large programs.
Current Curriculum
The majority of dermatology faculty (44%) and residents (35%) identified 1 to 2 faculty members as contributing to the creation and organization of their respective curricula; however, a notable percentage of residents (9%) reported that no faculty contributed to the organization of the curriculum. Residents noted that senior residents carry twice the responsibility for structuring the curriculum compared to faculty (61% vs 32% of the workload), but faculty described an even split between senior residents and faculty (47% vs 49% of the workload). Faculty believed their residents spend a similar amount of time in resident- and faculty-led instruction (38% vs 35% of their time); however, the majority of residents reported spending too little time in faculty-led instruction (53%). When residents ranked their preference for learning modes, faculty-led and self-study learning were ranked first and second by 48% and 45% of residents, respectively. Resident-led instruction was ranked last by 66% of residents. Likewise, a majority of residents (53%) described their amount of time in faculty-led instruction as too little.
When asked what subjects in dermatology were lacking at their programs, residents reported clinical trials (47%), skin of color (46%), cosmetic dermatology (34%), and aggressive skin cancer/multidisciplinary tumor board (32%). Although 11% of residents reported lacking inpatient dermatology in their curriculum, 0% of faculty reported the same. A notable percentage of faculty reported nothing was lacking compared to residents (25% vs 7%). Despite these different views between residents and faculty on their contributions to and structure of their curriculums, both faculty and residents claimed overall satisfaction (satisfied or very satisfied) with their program’s ability to optimally cover the field of dermatology in 3 years (100% and 91%, respectively).
Large Versus Small Residency Programs
When stratifying the resident responses for small versus large programs, both program sizes reported more time in resident-led instruction than faculty-led instruction. Likewise, residents in both program sizes equally preferred self-study or faculty-led instruction to resident-led instruction. Residents at small programs more often reported lacking instruction in rheumatology, immunobullous diseases, and basic science/skin biology compared to large-program residents. Compared to large-program faculty, small-program faculty reported lacking instruction in cosmetic dermatology.
Faculty at small programs reported spending too little time preparing for their faculty-led instruction compared to faculty at large programs (44% vs 12%). All (100%) of the faculty at small programs were likely to seek out study materials shared by top educators, while 77% of faculty at large programs were likely to do the same. When asked if faculty would translate what their program does well into an electronic format for sharing, 30% of large-program faculty were likely to do so compared to 11% of small-program faculty (Figure 1).

Use of Online Educational Materials and Interest in Collaboration
A majority of faculty and residents stated that they use online educational materials as supplements to traditional classroom lecture and print materials (81% vs 86%); however, almost twice as many residents stated that online educational materials were essential to their current study routines compared to faculty (39% vs 21%).
The majority of faculty (92%) and residents (84%) were either interested or very interested in a collaborative online curriculum. Both residents (85%) and faculty (81%) stated they would be likely to seek out online educational materials shared by top educators. Although both residents and faculty reported many aspects of their curriculums they thought could be beneficial to other dermatology programs (Table 1), only 27% of faculty and 19% of residents were likely to translate those strengths into a shareable electronic format. Several reasons were reported for not contributing to an online curriculum, with lack of time being the most common reason (Table 2).


Eighty percent of residents and 88% of faculty reported they were either interested or very interested in being more connected/interactive with their dermatology peers nationally (Figure 2). Likewise, 94% of residents and 87% of faculty agreed that the dermatology community could benefit from a social networking site for educational collaboration. Four times as many residents versus faculty currently use social networking sites (eg, Facebook, LinkedIn, Google Groups) as a primary mode of communication with distant professional peers. The majority of residents (52%) reported they would be likely to participate in a professional social networking site, while the majority of faculty (50%) stated they were neutral on their likelihood of participating. Both residents and faculty reported lack of time as a common reason for being unlikely to utilize a professional social networking site. Other barriers to participation are listed in Table 3.


Comment
This study showed how dermatology faculty and residents currently provide training and what online resources and social networking sites they currently use or would be willing to use. The generalizability of the conclusions is limited by the low response rate for the surveys. The results demonstrated the different views between faculty and residents and between large and small residency programs on various topics. This microcosm of dermatology training can likely be applied to other training scenarios in dermatology, including patient education; training of nurses, physician extenders, and office staff; continuing medical education for physicians; and peer-to-peer collaboration.
Hypothesis 1: Partially Proven
We hypothesized that residents would report less satisfaction with their current curriculum and would report greater resident contributions to the curriculum relative to faculty. Overall, residents and faculty reported satisfaction with their curriculums to provide up-to-date information and breadth in the field of dermatology. Despite their overall satisfaction, more residents reported lacking instruction in several dermatology subtopics compared to faculty. Additionally, residents believed they spend twice as much time structuring their curriculum compared to faculty, with some residents reporting no faculty involvement. Although residents preferred faculty-led instruction, a majority of residents reported they do not have enough faculty-led didactics. The preference for faculty-led training is likely due to the expertise of faculty compared to residents.
Hypothesis 2: Partially Proven
We also hypothesized that both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Although there was no difference in interest between residents at small versus large programs, there was a difference between faculty at small versus large programs. Small-program faculty were more interested in using shared materials than larger programs, while large-program faculty were more likely to share their educational materials. Small-program faculty reported spending too little time preparing their lectures, which is possibly due to a lack of time for preparation. Additionally, residents and faculty at smaller programs report their curriculum was lacking specific dermatology topics compared to large programs. These disparities between program sizes indicate a need for a social networking site for training collaboration in dermatology. Large programs have the ability to share what they do well, which small programs are eager to utilize.
Hypothesis 3: Not Proven
We hypothesized that residents would be more willing than faculty to participate in social networking for educational purposes. The majority of faculty and residents were interested in participating in a collaborative online curriculum and using the shared materials from top educators; however, even though such large majorities favored collaboration and sharing, only 27% of faculty and 19% of residents were likely to translate their own materials into a shareable format. Although lack of time was the most common reason for not sharing materials, electronic methods may have the potential to ultimately save time and remove the burden of content creation. The time it would take to translate selected personal training materials into a shareable form would be made up for by the time saved using another educators’ materials. Updating and customizing shared online educational materials can be much quicker and easier than educators creating materials on their own. Dermatologists would be more efficient facilitators of training via high-quality shared materials while decreasing the time burden associated with resident education.5 Another concern for not sharing or participating in a social networking site was skepticism of information security on such a network. The poor organization and information overload of online resources can compound the already existing time constraints on dermatologists, which may limit their ability to utilize such valuable resources. In addition, quality of online resources is not always guaranteed, and determining the sources that are high quality is sometimes a difficult task.6 For online materials to remain useful, there should be a peer-review process to evaluate quality and assess satisfaction.5
Solution: Create a Dermatology Task Force
A dermatology task force could facilitate the resolution of these challenges of online materials. In addition, a task force could cover the administrative support needed to ensure security and provide maintenance on social networks.
The main limitation to implementing a social network is the presence of the administrative infrastructure to jumpstart its creation. A task force incorporating the essential stakeholders in dermatology training is the first step. With inclusive representation from all of the smaller professional dermatology societies, the American Academy of Dermatology is optimally positioned to create this task force. With existing information technologies, a task force could address the concerns revealed in our survey as well as any future concerns that may arise.
The goal is a single social network for dermatologists that has the capability of improving communication and collaboration between professional peers regardless of their practice setting. Such a network is ideal for the practicing dermatologist for the purposes of staff training, patient education, and obtaining continuing medical education credit. Additionally, peer group collaboration would facilitate the understanding and completion of the evolving requirements for Maintenance of Certification from the American Board of Dermatology. The availability of quality shared materials would save time and increase efficiency of an entire dermatology practice. Materials that aid in patient education would allow office staff to dedicate their time to other tasks, thereby increasing productivity. Shared training materials would decrease the burden of staff education, providing more time for advanced hands-on training. This method of collaborative effort is capable of advancing the field of dermatology as a whole. It can overcome geographical and institutional barriers to connect dermatologists with similar interests worldwide; disseminate advances in diagnosis and treatment; and improve the quality of dermatology training of dermatologists, staff, and patients.
More than 1.8 billion individuals utilize social media, a number that continues to grow as the social media market expands.1 Social media enables individuals, groups, and organizations to efficiently disperse and access information2-4 and also provides a structure that encourages collaboration between patients, staff, and physicians that cannot be achieved by other communication modalities.4-6 Expert opinions and related educational materials can be shared globally, improving collaboration between dermatologists.6 A structured social networking site for sharing training materials, research, and ideas can help bring the national dermatology community together in a new way.
Other professions have employed social networking tools to accomplish similar goals of organizing training resources; radiology has an electronic database that allows sharing of training materials and incorporates social networking capabilities.7 Their Web software provides functionality for individual file uploading and supports collaboration and sharing, all while maintaining the security of uploaded information. General surgery has already addressed similar concerns via a task force that incorporates all the essential organizations in surgical education.8 Increased satisfaction and academic abilities have been demonstrated with their collaborative curriculum.9 Gastroenterologists also utilize electronic resources; one study showed that using videos to educate patients prior to colonoscopies was superior to face-to-face education.10 In addition, video education may free up time for office staff to accomplish other tasks.
As a specialty, dermatology has not been a leader in the implementation of social networking for collaboration and training purposes. Every dermatologist is an educator. To maintain a successful practice, dermatologists must keep up-to-date on their own clinical knowledge, provide training to their staff, and educate their patients. Although there are numerous educational resources available to dermatologists, an informal survey of 30 dermatology faculty members revealed a practice gap in awareness and utilization of these expanding electronic resources.11
To better understand the needs of the specialty as a whole, we chose to focus on one aspect of dermatology education: resident training. The goal of our study was to survey dermatology residents and faculty to gain a better understanding of how they currently provide education and what online resources and social networking sites they currently use or would be willing to use. The study included 3 central hypotheses: First, residents would be less satisfied with their current curriculum and residents would report greater contributions to the curriculum relative to faculty. Second, both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Lastly, residents would be more willing than faculty to participate in social networking for educational purposes.
Methods
This study was granted institutional review board exemption. Two surveys were developed by the authors to assess the current structure and satisfaction of dermatology residency curriculum and the willingness to participate in social networking to use and share educational materials. The surveys were evaluated for relevance by the survey evaluation team of the Association of Professors of Dermatology (APD). The instrument was not pilot tested.
The surveys were electronically distributed using an online service to dermatology faculty via the APD listserve, which comprised the entirety of the APD membership in 2014. The resident survey was distributed to the dermatology residents via the American Society for Dermatologic Surgery listserve, which included all residents in training (2013-2014 academic year). Second and third invitations to complete the surveys were distributed 3 and 5 weeks later, respectively.
Resident and faculty responses were compared. Additionally, responses were stratified for large (>9 residents) and small programs (≤9 residents) for comparison. Descriptive statistics including means and medians for continuous variables and frequency tables for categorical variables were generated using research and spreadsheet software.
Results
There were 137 survey respondents; 52 of 426 (12.2%) dermatology faculty and 85 of 1539 (5.5%) dermatology residents responded to the survey. Small programs accounted for 24% of total survey responses and 76% were from large programs.
Current Curriculum
The majority of dermatology faculty (44%) and residents (35%) identified 1 to 2 faculty members as contributing to the creation and organization of their respective curricula; however, a notable percentage of residents (9%) reported that no faculty contributed to the organization of the curriculum. Residents noted that senior residents carry twice the responsibility for structuring the curriculum compared to faculty (61% vs 32% of the workload), but faculty described an even split between senior residents and faculty (47% vs 49% of the workload). Faculty believed their residents spend a similar amount of time in resident- and faculty-led instruction (38% vs 35% of their time); however, the majority of residents reported spending too little time in faculty-led instruction (53%). When residents ranked their preference for learning modes, faculty-led and self-study learning were ranked first and second by 48% and 45% of residents, respectively. Resident-led instruction was ranked last by 66% of residents. Likewise, a majority of residents (53%) described their amount of time in faculty-led instruction as too little.
When asked what subjects in dermatology were lacking at their programs, residents reported clinical trials (47%), skin of color (46%), cosmetic dermatology (34%), and aggressive skin cancer/multidisciplinary tumor board (32%). Although 11% of residents reported lacking inpatient dermatology in their curriculum, 0% of faculty reported the same. A notable percentage of faculty reported nothing was lacking compared to residents (25% vs 7%). Despite these different views between residents and faculty on their contributions to and structure of their curriculums, both faculty and residents claimed overall satisfaction (satisfied or very satisfied) with their program’s ability to optimally cover the field of dermatology in 3 years (100% and 91%, respectively).
Large Versus Small Residency Programs
When stratifying the resident responses for small versus large programs, both program sizes reported more time in resident-led instruction than faculty-led instruction. Likewise, residents in both program sizes equally preferred self-study or faculty-led instruction to resident-led instruction. Residents at small programs more often reported lacking instruction in rheumatology, immunobullous diseases, and basic science/skin biology compared to large-program residents. Compared to large-program faculty, small-program faculty reported lacking instruction in cosmetic dermatology.
Faculty at small programs reported spending too little time preparing for their faculty-led instruction compared to faculty at large programs (44% vs 12%). All (100%) of the faculty at small programs were likely to seek out study materials shared by top educators, while 77% of faculty at large programs were likely to do the same. When asked if faculty would translate what their program does well into an electronic format for sharing, 30% of large-program faculty were likely to do so compared to 11% of small-program faculty (Figure 1).

Use of Online Educational Materials and Interest in Collaboration
A majority of faculty and residents stated that they use online educational materials as supplements to traditional classroom lecture and print materials (81% vs 86%); however, almost twice as many residents stated that online educational materials were essential to their current study routines compared to faculty (39% vs 21%).
The majority of faculty (92%) and residents (84%) were either interested or very interested in a collaborative online curriculum. Both residents (85%) and faculty (81%) stated they would be likely to seek out online educational materials shared by top educators. Although both residents and faculty reported many aspects of their curriculums they thought could be beneficial to other dermatology programs (Table 1), only 27% of faculty and 19% of residents were likely to translate those strengths into a shareable electronic format. Several reasons were reported for not contributing to an online curriculum, with lack of time being the most common reason (Table 2).


Eighty percent of residents and 88% of faculty reported they were either interested or very interested in being more connected/interactive with their dermatology peers nationally (Figure 2). Likewise, 94% of residents and 87% of faculty agreed that the dermatology community could benefit from a social networking site for educational collaboration. Four times as many residents versus faculty currently use social networking sites (eg, Facebook, LinkedIn, Google Groups) as a primary mode of communication with distant professional peers. The majority of residents (52%) reported they would be likely to participate in a professional social networking site, while the majority of faculty (50%) stated they were neutral on their likelihood of participating. Both residents and faculty reported lack of time as a common reason for being unlikely to utilize a professional social networking site. Other barriers to participation are listed in Table 3.


Comment
This study showed how dermatology faculty and residents currently provide training and what online resources and social networking sites they currently use or would be willing to use. The generalizability of the conclusions is limited by the low response rate for the surveys. The results demonstrated the different views between faculty and residents and between large and small residency programs on various topics. This microcosm of dermatology training can likely be applied to other training scenarios in dermatology, including patient education; training of nurses, physician extenders, and office staff; continuing medical education for physicians; and peer-to-peer collaboration.
Hypothesis 1: Partially Proven
We hypothesized that residents would report less satisfaction with their current curriculum and would report greater resident contributions to the curriculum relative to faculty. Overall, residents and faculty reported satisfaction with their curriculums to provide up-to-date information and breadth in the field of dermatology. Despite their overall satisfaction, more residents reported lacking instruction in several dermatology subtopics compared to faculty. Additionally, residents believed they spend twice as much time structuring their curriculum compared to faculty, with some residents reporting no faculty involvement. Although residents preferred faculty-led instruction, a majority of residents reported they do not have enough faculty-led didactics. The preference for faculty-led training is likely due to the expertise of faculty compared to residents.
Hypothesis 2: Partially Proven
We also hypothesized that both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Although there was no difference in interest between residents at small versus large programs, there was a difference between faculty at small versus large programs. Small-program faculty were more interested in using shared materials than larger programs, while large-program faculty were more likely to share their educational materials. Small-program faculty reported spending too little time preparing their lectures, which is possibly due to a lack of time for preparation. Additionally, residents and faculty at smaller programs report their curriculum was lacking specific dermatology topics compared to large programs. These disparities between program sizes indicate a need for a social networking site for training collaboration in dermatology. Large programs have the ability to share what they do well, which small programs are eager to utilize.
Hypothesis 3: Not Proven
We hypothesized that residents would be more willing than faculty to participate in social networking for educational purposes. The majority of faculty and residents were interested in participating in a collaborative online curriculum and using the shared materials from top educators; however, even though such large majorities favored collaboration and sharing, only 27% of faculty and 19% of residents were likely to translate their own materials into a shareable format. Although lack of time was the most common reason for not sharing materials, electronic methods may have the potential to ultimately save time and remove the burden of content creation. The time it would take to translate selected personal training materials into a shareable form would be made up for by the time saved using another educators’ materials. Updating and customizing shared online educational materials can be much quicker and easier than educators creating materials on their own. Dermatologists would be more efficient facilitators of training via high-quality shared materials while decreasing the time burden associated with resident education.5 Another concern for not sharing or participating in a social networking site was skepticism of information security on such a network. The poor organization and information overload of online resources can compound the already existing time constraints on dermatologists, which may limit their ability to utilize such valuable resources. In addition, quality of online resources is not always guaranteed, and determining the sources that are high quality is sometimes a difficult task.6 For online materials to remain useful, there should be a peer-review process to evaluate quality and assess satisfaction.5
Solution: Create a Dermatology Task Force
A dermatology task force could facilitate the resolution of these challenges of online materials. In addition, a task force could cover the administrative support needed to ensure security and provide maintenance on social networks.
The main limitation to implementing a social network is the presence of the administrative infrastructure to jumpstart its creation. A task force incorporating the essential stakeholders in dermatology training is the first step. With inclusive representation from all of the smaller professional dermatology societies, the American Academy of Dermatology is optimally positioned to create this task force. With existing information technologies, a task force could address the concerns revealed in our survey as well as any future concerns that may arise.
The goal is a single social network for dermatologists that has the capability of improving communication and collaboration between professional peers regardless of their practice setting. Such a network is ideal for the practicing dermatologist for the purposes of staff training, patient education, and obtaining continuing medical education credit. Additionally, peer group collaboration would facilitate the understanding and completion of the evolving requirements for Maintenance of Certification from the American Board of Dermatology. The availability of quality shared materials would save time and increase efficiency of an entire dermatology practice. Materials that aid in patient education would allow office staff to dedicate their time to other tasks, thereby increasing productivity. Shared training materials would decrease the burden of staff education, providing more time for advanced hands-on training. This method of collaborative effort is capable of advancing the field of dermatology as a whole. It can overcome geographical and institutional barriers to connect dermatologists with similar interests worldwide; disseminate advances in diagnosis and treatment; and improve the quality of dermatology training of dermatologists, staff, and patients.
- Statistics and facts about social networks. Statista website. http://www.statista.com/topics/1164/social-networks/. Accessed March 22, 2017.
- Baker RC, Klein M, Samaan Z, et al. Effectiveness of an online pediatric primary care curriculum. Acad Pediatr. 2010;10:131-137.
- Dolev JC, O’Sullivan P, Berger T. The eDerm online curriculum: a randomized study of effective skin cancer teaching to medical students. J Am Acad Dermatol. 2011;65:e165-e171.
- Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150:56-60.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of e-learning in medical education. Acad Med. 2006;81:207-212.
- Hanson AH, Krause LK, Simmons RN, et al. Dermatology education and the internet: traditional and cutting-edge resources. J Am Acad Dermatol. 2011;65:836-842.
- Rowe SP, Siddiqui A, Bonekamp D. The key image and case log application: new radiology software for teaching file creation and case logging that incorporates elements of a social network. Acad Radiol. 2014;21:916-930.
- Bell RH. Surgical council on resident education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007;204:341-346.
- Kirton OC, Reilly P, Staff I, et al. Development and implementation of an interactive, objective, and simulation-based curriculum for general surgery residents. J Surg Educ. 2012;69:718-723.
- Prakash S, Verma S, McGowan J, et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700.
- Carroll BT. eTools for teaching dermatologic surgery. Paper presented at the Association of Professors of Dermatology 2014 Annual Meeting; September 12-13, 2014; Chicago, IL.
- Statistics and facts about social networks. Statista website. http://www.statista.com/topics/1164/social-networks/. Accessed March 22, 2017.
- Baker RC, Klein M, Samaan Z, et al. Effectiveness of an online pediatric primary care curriculum. Acad Pediatr. 2010;10:131-137.
- Dolev JC, O’Sullivan P, Berger T. The eDerm online curriculum: a randomized study of effective skin cancer teaching to medical students. J Am Acad Dermatol. 2011;65:e165-e171.
- Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150:56-60.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of e-learning in medical education. Acad Med. 2006;81:207-212.
- Hanson AH, Krause LK, Simmons RN, et al. Dermatology education and the internet: traditional and cutting-edge resources. J Am Acad Dermatol. 2011;65:836-842.
- Rowe SP, Siddiqui A, Bonekamp D. The key image and case log application: new radiology software for teaching file creation and case logging that incorporates elements of a social network. Acad Radiol. 2014;21:916-930.
- Bell RH. Surgical council on resident education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007;204:341-346.
- Kirton OC, Reilly P, Staff I, et al. Development and implementation of an interactive, objective, and simulation-based curriculum for general surgery residents. J Surg Educ. 2012;69:718-723.
- Prakash S, Verma S, McGowan J, et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700.
- Carroll BT. eTools for teaching dermatologic surgery. Paper presented at the Association of Professors of Dermatology 2014 Annual Meeting; September 12-13, 2014; Chicago, IL.
Practice Points
- Educational collaboration between residency programs via social media can result in more well-rounded dermatologists, which will enhance patient care.
- Social media can connect dermatologists nationwide to improve patient care via collaboration.
Osilodrostat maintained cortisol control in Cushing’s syndrome
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
[email protected]
On Twitter @alz_gal
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
[email protected]
On Twitter @alz_gal
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
[email protected]
On Twitter @alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Every patient in the study responded either fully (87.5%) or partially, with a decrease of at least 50% in cortisol from baseline (12.5%), with no loss of control.
Data source: The 31-month extension study comprised 16 patients who had responded well to the drug in a phase II trial.
Disclosures: Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.