User login
“Cold Turkey” Works Best for Smoking Cessation

A 43-year-old man has a 35–pack-year smoking history and currently smokes one pack of cigarettes a day. He is eager to quit smoking since a close friend of his was recently diagnosed with lung cancer. He asks whether he should quit “cold turkey” or gradually. What do you recommend?
Between 2013 and 2014, one in five American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions (both alone and in combination) increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual versus abrupt smoking cessation methods.5
A previous Cochrane review of 10 RCTs demonstrated no significant difference in quit rates between gradual cigarette reduction and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of more than 60,000 smokers who presented to nationwide cessation services. The researchers found that older participants (those 45 and older) and heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
“Cold turkey” is better than gradual cessation at six months
A noninferiority RCT was conducted in England to assess whether gradual smoking cessation is as successful as abrupt cessation.1 The primary outcome was abstinence from smoking at four weeks, assessed using the Russell Standard. This set of six criteria (including validation by exhaled CO concentrations of < 10 ppm) is used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Participants were recruited via letters from their primary care practice inviting them to participate in a smoking cessation study. The 697 subjects were randomized to either the abrupt-cessation group or the gradual-cessation group. Baseline characteristics were similar between groups.
All participants were asked to schedule a quit date for two weeks after their enrollment. Patients assigned to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT; gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the two weeks leading up to the quit date. They were given instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomly assigned to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those two weeks they were given nicotine patches (because the other group received them, and some evidence suggests that precessation NRT increases quit rates) but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, nicotine patches (21 mg/d), and the patient’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice at the following intervals: weekly for two weeks before the quit date; the day before the quit date; weekly for four weeks after the quit date; and eight weeks after the quit date.
The chosen noninferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually, compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for noninferiority; in fact, four-week abstinence was significantly more likely in the abrupt-cessation group than in the gradual-cessation group (49% vs 39.2%; RR, 0.80; number needed to treat [NNT], 10). Similarly, secondary outcomes of eight-week and six-month abstinence rates showed superiority of abrupt over gradual cessation. Six months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR, 0.71; NNT, 15).
Patient preference plays a role
The investigators also found a difference in successful cessation based on the participants’ preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at four weeks than participants who preferred gradual cessation (52.2% vs 38.3%).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation. Four-week abstinence was seen in 34.6% of patients who preferred and were allocated to gradual cessation and in 42% of patients who preferred gradual but were allocated to abrupt cessation.
WHAT’S NEW
Higher quality study; added element of preference
This large, well-designed, noninferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were assigned.

CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at four weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of study participants, along with the relatively small number lost to follow-up, minimizes this weakness.
The majority of participants were white, which may limit generalizability to nonwhite populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly. Therefore, results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Considerable investment in behavioral support
One significant challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse seven times in the first six weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[3]:174-176).
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Prev Med. 2014;63: 96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.

A 43-year-old man has a 35–pack-year smoking history and currently smokes one pack of cigarettes a day. He is eager to quit smoking since a close friend of his was recently diagnosed with lung cancer. He asks whether he should quit “cold turkey” or gradually. What do you recommend?
Between 2013 and 2014, one in five American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions (both alone and in combination) increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual versus abrupt smoking cessation methods.5
A previous Cochrane review of 10 RCTs demonstrated no significant difference in quit rates between gradual cigarette reduction and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of more than 60,000 smokers who presented to nationwide cessation services. The researchers found that older participants (those 45 and older) and heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
“Cold turkey” is better than gradual cessation at six months
A noninferiority RCT was conducted in England to assess whether gradual smoking cessation is as successful as abrupt cessation.1 The primary outcome was abstinence from smoking at four weeks, assessed using the Russell Standard. This set of six criteria (including validation by exhaled CO concentrations of < 10 ppm) is used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Participants were recruited via letters from their primary care practice inviting them to participate in a smoking cessation study. The 697 subjects were randomized to either the abrupt-cessation group or the gradual-cessation group. Baseline characteristics were similar between groups.
All participants were asked to schedule a quit date for two weeks after their enrollment. Patients assigned to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT; gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the two weeks leading up to the quit date. They were given instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomly assigned to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those two weeks they were given nicotine patches (because the other group received them, and some evidence suggests that precessation NRT increases quit rates) but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, nicotine patches (21 mg/d), and the patient’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice at the following intervals: weekly for two weeks before the quit date; the day before the quit date; weekly for four weeks after the quit date; and eight weeks after the quit date.
The chosen noninferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually, compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for noninferiority; in fact, four-week abstinence was significantly more likely in the abrupt-cessation group than in the gradual-cessation group (49% vs 39.2%; RR, 0.80; number needed to treat [NNT], 10). Similarly, secondary outcomes of eight-week and six-month abstinence rates showed superiority of abrupt over gradual cessation. Six months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR, 0.71; NNT, 15).
Patient preference plays a role
The investigators also found a difference in successful cessation based on the participants’ preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at four weeks than participants who preferred gradual cessation (52.2% vs 38.3%).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation. Four-week abstinence was seen in 34.6% of patients who preferred and were allocated to gradual cessation and in 42% of patients who preferred gradual but were allocated to abrupt cessation.
WHAT’S NEW
Higher quality study; added element of preference
This large, well-designed, noninferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were assigned.

CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at four weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of study participants, along with the relatively small number lost to follow-up, minimizes this weakness.
The majority of participants were white, which may limit generalizability to nonwhite populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly. Therefore, results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Considerable investment in behavioral support
One significant challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse seven times in the first six weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[3]:174-176).

A 43-year-old man has a 35–pack-year smoking history and currently smokes one pack of cigarettes a day. He is eager to quit smoking since a close friend of his was recently diagnosed with lung cancer. He asks whether he should quit “cold turkey” or gradually. What do you recommend?
Between 2013 and 2014, one in five American adults reported using tobacco products some days or every day, and 66% of smokers in 2013 made at least one attempt to quit.2,3 The risks of tobacco use and the benefits of cessation are well established, and behavioral and pharmacologic interventions (both alone and in combination) increase smoking cessation rates.4 The US Preventive Services Task Force recommends that health care providers address tobacco use and cessation with patients at regular office visits and offer behavioral and pharmacologic interventions.5 Current guidelines, however, make no specific recommendations regarding gradual versus abrupt smoking cessation methods.5
A previous Cochrane review of 10 RCTs demonstrated no significant difference in quit rates between gradual cigarette reduction and abrupt cessation. The meta-analysis was limited, however, by differences in patient populations, outcome definitions, and types of interventions (both pharmacologic and behavioral).6
In a retrospective cohort study, French investigators reviewed an online database of more than 60,000 smokers who presented to nationwide cessation services. The researchers found that older participants (those 45 and older) and heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly.7
STUDY SUMMARY
“Cold turkey” is better than gradual cessation at six months
A noninferiority RCT was conducted in England to assess whether gradual smoking cessation is as successful as abrupt cessation.1 The primary outcome was abstinence from smoking at four weeks, assessed using the Russell Standard. This set of six criteria (including validation by exhaled CO concentrations of < 10 ppm) is used by the National Centre for Smoking Cessation and Training to decrease variability of reported smoking cessation rates in English studies.8
Participants were recruited via letters from their primary care practice inviting them to participate in a smoking cessation study. The 697 subjects were randomized to either the abrupt-cessation group or the gradual-cessation group. Baseline characteristics were similar between groups.
All participants were asked to schedule a quit date for two weeks after their enrollment. Patients assigned to the gradual-cessation group were provided nicotine replacement patches (21 mg/d) and their choice of short-acting nicotine replacement therapy (NRT; gum, lozenges, nasal spray, sublingual tablets, inhalator, or mouth spray) to use in the two weeks leading up to the quit date. They were given instructions to reduce smoking by half of the baseline amount by the end of the first week, and to a quarter of baseline by the end of the second week.
Patients randomly assigned to the abrupt-cessation group were instructed to continue their current smoking habits until the cessation date; during those two weeks they were given nicotine patches (because the other group received them, and some evidence suggests that precessation NRT increases quit rates) but no short-acting NRT.
Following the cessation date, treatment in both groups was identical, including behavioral support, nicotine patches (21 mg/d), and the patient’s choice of short-acting NRT. Behavioral support consisted of visits with a research nurse at the patient’s primary care practice at the following intervals: weekly for two weeks before the quit date; the day before the quit date; weekly for four weeks after the quit date; and eight weeks after the quit date.
The chosen noninferiority margin was equal to a relative risk (RR) of 0.81 (19% reduction in effectiveness) of quitting gradually, compared with abrupt cessation of smoking. Quit rates in the gradual-reduction group did not reach the threshold for noninferiority; in fact, four-week abstinence was significantly more likely in the abrupt-cessation group than in the gradual-cessation group (49% vs 39.2%; RR, 0.80; number needed to treat [NNT], 10). Similarly, secondary outcomes of eight-week and six-month abstinence rates showed superiority of abrupt over gradual cessation. Six months after the quit date, 15.5% of the gradual-cessation group and 22% of the abrupt-cessation group remained abstinent (RR, 0.71; NNT, 15).
Patient preference plays a role
The investigators also found a difference in successful cessation based on the participants’ preferred method of cessation. Participants who preferred abrupt cessation were more likely to be abstinent at four weeks than participants who preferred gradual cessation (52.2% vs 38.3%).
Patients with a baseline preference for gradual cessation were equally as likely to successfully quit when allocated to abrupt cessation against their preference as when they were allocated to gradual cessation. Four-week abstinence was seen in 34.6% of patients who preferred and were allocated to gradual cessation and in 42% of patients who preferred gradual but were allocated to abrupt cessation.
WHAT’S NEW
Higher quality study; added element of preference
This large, well-designed, noninferiority study showed that abrupt cessation is superior to gradual cessation. The size and design of the study, including a standardized method of assessing cessation and a standardized intervention, make this a higher quality study than those in the Cochrane meta-analysis.6 This study also showed that participants who preferred gradual cessation were less likely to be successful—regardless of the method to which they were assigned.

CAVEATS
Generalizability limited by race and number of cigarettes smoked
Patients lost to follow-up at four weeks (35 in the abrupt-cessation group and 48 in the gradual-cessation group) were assumed to have continued smoking, which may have biased the results toward abrupt cessation. That said, the large number of study participants, along with the relatively small number lost to follow-up, minimizes this weakness.
The majority of participants were white, which may limit generalizability to nonwhite populations. In addition, participants smoked an average of 20 cigarettes per day and, as noted previously, an observational study of tobacco users in France found that heavy smokers (≥ 21 cigarettes/d) were more likely to quit gradually than abruptly. Therefore, results may not be generalizable to heavy smokers.7
CHALLENGES TO IMPLEMENTATION
Considerable investment in behavioral support
One significant challenge is the implementation of such a structured tobacco cessation program in primary care. Both abrupt- and gradual-cessation groups were given considerable behavioral support from research nurses. Participants in this study were seen by a nurse seven times in the first six weeks of the study, and the intervention included nurse-created reduction schedules.
Even if patients in the study preferred one method of cessation to another, they were receptive to quitting either gradually or abruptly. In clinical practice, patients are often set in their desired method of cessation. In that setting, our role is then to inform them of the data and support them in whatever method they choose.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[3]:174-176).
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Prev Med. 2014;63: 96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
1. Lindson-Hawley N, Banting M, West R, et al. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med. 2016;164:585-592.
2. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65:685-691.
3. Lavinghouze SR, Malarcher A, Jama A, et al. Trends in quit attempts among adult cigarette smokers–United States, 2001-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1129-1135.
4. Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608-621.
5. Siu AL; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622-634.
6. Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;11:CD008033.
7. Baha M, Le Faou AL. Gradual versus abrupt quitting among French treatment-seeking smokers. Prev Med. 2014;63: 96-102.
8. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299-303.
Dual targeting effective in HER2+ metastatic colorectal cancer
The combination of trastuzumab (Herceptin) and lapatinib (Tykerb) resulted in an overall objective response rate of 30% in heavily pretreated patients with HER2-positive metastatic colorectal cancer, according to findings presented at the annual meeting of the American Association for Cancer Research.
Of 33 patients, 2 patients achieved complete responses and 8 had partial responses, while 13 patients achieved stable disease. This brought the total clinical benefit rate to 70% at the time of data cutoff on Feb. 28.
“There is a large unmet need in colorectal cancer,” said study author, Silvia Marsoni, MD, director of the precision medicine unit at the Istituto di Candiolo, Fondazione del Piemonte per l’Oncologia–IRCCS in Turin, Italy.
“The combination of drugs really had a great impact on the tumor,” said Dr. Marsoni, who discussed the findings at a presscast. “One of these complete responders is still alive without evidence of disease at almost 36 months from the beginning of his treatment. This was a very good duration of response.”
HER2 amplification is found in 5% of RAS wild-type metastatic colorectal cancer cases. Dual HER2 blockade with both trastuzumab and lapatinib had inhibited tumor growth in preliminary studies, but that has not been the case for either drug used alone.
The HERACLES trial was conducted at four centers in Italy and enrolled 33 patients with RAS wild-type, HER2-positive tumors that were refractory to standard of care treatments, including the epidermal growth factor receptor (EGFR) inhibitors cetuximab and panitumumab.
“These patients were heavily pretreated and 75% had four or more prior treatments,” explained Dr. Marsoni.
All participants received trastuzumab intravenously with a 4 mg/kg loading dose followed by 2 mg/kg weekly, and lapatinib was given orally at 1,000 mg daily. Treatment continued until disease progression.
The primary endpoint was the objective response rate, and secondary endpoints included progression-free survival and safety.
The two patients who achieved a complete response continue to be disease free, and both had tumors that were refractory to cetuximab and resistant to all standard treatment.
Historically, the response rate in metastatic colorectal cancer after second-line treatment is very low, at less than 5% with chemotherapy and about 10%-20% with anti-EGFR therapy.
“There was really no toxicity,” explained Dr. Marsoni. “We used a lapatinib dose that was about 20% less than what is used in breast cancer to avoid GI toxicity.”
Only six patients (18%) experienced grade 3 side effects, which included fatigue, skin rash, and elevated bilirubin.
“In conclusion, we can say that, even if HER2-positive patients in colon cancer are only 3% of the population, they are still a little and important piece of the cake that can be actively targeted with a new chemotherapy-free regimen,” said Dr. Marsoni. “We have a new potential treatment for this population.”
The combination of trastuzumab (Herceptin) and lapatinib (Tykerb) resulted in an overall objective response rate of 30% in heavily pretreated patients with HER2-positive metastatic colorectal cancer, according to findings presented at the annual meeting of the American Association for Cancer Research.
Of 33 patients, 2 patients achieved complete responses and 8 had partial responses, while 13 patients achieved stable disease. This brought the total clinical benefit rate to 70% at the time of data cutoff on Feb. 28.
“There is a large unmet need in colorectal cancer,” said study author, Silvia Marsoni, MD, director of the precision medicine unit at the Istituto di Candiolo, Fondazione del Piemonte per l’Oncologia–IRCCS in Turin, Italy.
“The combination of drugs really had a great impact on the tumor,” said Dr. Marsoni, who discussed the findings at a presscast. “One of these complete responders is still alive without evidence of disease at almost 36 months from the beginning of his treatment. This was a very good duration of response.”
HER2 amplification is found in 5% of RAS wild-type metastatic colorectal cancer cases. Dual HER2 blockade with both trastuzumab and lapatinib had inhibited tumor growth in preliminary studies, but that has not been the case for either drug used alone.
The HERACLES trial was conducted at four centers in Italy and enrolled 33 patients with RAS wild-type, HER2-positive tumors that were refractory to standard of care treatments, including the epidermal growth factor receptor (EGFR) inhibitors cetuximab and panitumumab.
“These patients were heavily pretreated and 75% had four or more prior treatments,” explained Dr. Marsoni.
All participants received trastuzumab intravenously with a 4 mg/kg loading dose followed by 2 mg/kg weekly, and lapatinib was given orally at 1,000 mg daily. Treatment continued until disease progression.
The primary endpoint was the objective response rate, and secondary endpoints included progression-free survival and safety.
The two patients who achieved a complete response continue to be disease free, and both had tumors that were refractory to cetuximab and resistant to all standard treatment.
Historically, the response rate in metastatic colorectal cancer after second-line treatment is very low, at less than 5% with chemotherapy and about 10%-20% with anti-EGFR therapy.
“There was really no toxicity,” explained Dr. Marsoni. “We used a lapatinib dose that was about 20% less than what is used in breast cancer to avoid GI toxicity.”
Only six patients (18%) experienced grade 3 side effects, which included fatigue, skin rash, and elevated bilirubin.
“In conclusion, we can say that, even if HER2-positive patients in colon cancer are only 3% of the population, they are still a little and important piece of the cake that can be actively targeted with a new chemotherapy-free regimen,” said Dr. Marsoni. “We have a new potential treatment for this population.”
The combination of trastuzumab (Herceptin) and lapatinib (Tykerb) resulted in an overall objective response rate of 30% in heavily pretreated patients with HER2-positive metastatic colorectal cancer, according to findings presented at the annual meeting of the American Association for Cancer Research.
Of 33 patients, 2 patients achieved complete responses and 8 had partial responses, while 13 patients achieved stable disease. This brought the total clinical benefit rate to 70% at the time of data cutoff on Feb. 28.
“There is a large unmet need in colorectal cancer,” said study author, Silvia Marsoni, MD, director of the precision medicine unit at the Istituto di Candiolo, Fondazione del Piemonte per l’Oncologia–IRCCS in Turin, Italy.
“The combination of drugs really had a great impact on the tumor,” said Dr. Marsoni, who discussed the findings at a presscast. “One of these complete responders is still alive without evidence of disease at almost 36 months from the beginning of his treatment. This was a very good duration of response.”
HER2 amplification is found in 5% of RAS wild-type metastatic colorectal cancer cases. Dual HER2 blockade with both trastuzumab and lapatinib had inhibited tumor growth in preliminary studies, but that has not been the case for either drug used alone.
The HERACLES trial was conducted at four centers in Italy and enrolled 33 patients with RAS wild-type, HER2-positive tumors that were refractory to standard of care treatments, including the epidermal growth factor receptor (EGFR) inhibitors cetuximab and panitumumab.
“These patients were heavily pretreated and 75% had four or more prior treatments,” explained Dr. Marsoni.
All participants received trastuzumab intravenously with a 4 mg/kg loading dose followed by 2 mg/kg weekly, and lapatinib was given orally at 1,000 mg daily. Treatment continued until disease progression.
The primary endpoint was the objective response rate, and secondary endpoints included progression-free survival and safety.
The two patients who achieved a complete response continue to be disease free, and both had tumors that were refractory to cetuximab and resistant to all standard treatment.
Historically, the response rate in metastatic colorectal cancer after second-line treatment is very low, at less than 5% with chemotherapy and about 10%-20% with anti-EGFR therapy.
“There was really no toxicity,” explained Dr. Marsoni. “We used a lapatinib dose that was about 20% less than what is used in breast cancer to avoid GI toxicity.”
Only six patients (18%) experienced grade 3 side effects, which included fatigue, skin rash, and elevated bilirubin.
“In conclusion, we can say that, even if HER2-positive patients in colon cancer are only 3% of the population, they are still a little and important piece of the cake that can be actively targeted with a new chemotherapy-free regimen,” said Dr. Marsoni. “We have a new potential treatment for this population.”
FROM THE AACR ANNUAL MEETING
Key clinical point: Dual targeting resulted in a 30% objective response rate in heavily pretreated patients with HER2-positive colorectal cancer.
Major finding: Combination trastuzumab and lapatinib resulted in a 70% clinical benefit in heavily pretreated colorectal cancer patients.
Data source: Multicenter prospective study that included 33 patients with HER2-positive metastatic colorectal cancer who had been heavily pretreated.
Disclosures: The study was funded by the Associazione Italiana per la Ricerca sul Cancro, Fondazione Oncologia Niguarda Onlus, and Roche. The study’s lead investigator, Salvatore Siena, MD, has relationships with Amgen, Bayer, Celgene, Eli Lilly, Merck, Merrimack, Novartis, Roche, and Sanofi.
Weight fluctuations linked to higher mortality, CV events
Weight fluctuations – like the typical pattern of weight loss followed by partial or total regain that affects most people attempting to lose weight – are strongly associated with higher mortality, more cardiovascular events, and new-onset diabetes, according to an analysis of the Treating to New Targets trial published online April 6 in the New England Journal of Medicine.
Weight loss is commonly prescribed as a lifestyle intervention to improve health, especially cardiovascular health. However, “the usual pattern for most patients ... is weight loss followed by weight gain” (also termed weight cycling), which has been linked to poor cardiovascular outcomes, especially when the pattern is repeated over time, said Sripal Bangalore, MD, of New York University, and his coinvestigators.
The primary outcome measure – the composite rate of death from coronary heart disease, nonfatal MI, resuscitated cardiac arrest, revascularization, or angina – was significantly associated with weight fluctuations in a dose-dependent fashion so that greater degrees of variability in body weight were linked to higher event rates (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1606148).
“When compared with the lowest quintile, patients with the highest quintile of variability had an increase in the risk of any coronary event of 64%, an increase in the risk of any cardiovascular event of 85%, an increase in the risk of death of 124%, an increase in the risk of MI of 117%, an increase in the risk of stroke of 136%, and an increase in the risk of new-onset diabetes of 78%, independent of traditional risk factors,” Dr. Bangalore and his associates reported.
This association remained strong regardless of the patients’ weight at baseline, consistent among those of normal body weight and those who were overweight or obese. And the association also persisted across multiple sensitivity analyses.
This study was supported by Pfizer. Dr. Bangalore reported serving as a consultant to Pfizer, Daiichi-Sankyo, Boehringer Ingelheim, Merck, Menarini, Gilead, and Abbott Vascular; his associates reported ties to numerous industry sources.
Weight fluctuations – like the typical pattern of weight loss followed by partial or total regain that affects most people attempting to lose weight – are strongly associated with higher mortality, more cardiovascular events, and new-onset diabetes, according to an analysis of the Treating to New Targets trial published online April 6 in the New England Journal of Medicine.
Weight loss is commonly prescribed as a lifestyle intervention to improve health, especially cardiovascular health. However, “the usual pattern for most patients ... is weight loss followed by weight gain” (also termed weight cycling), which has been linked to poor cardiovascular outcomes, especially when the pattern is repeated over time, said Sripal Bangalore, MD, of New York University, and his coinvestigators.
The primary outcome measure – the composite rate of death from coronary heart disease, nonfatal MI, resuscitated cardiac arrest, revascularization, or angina – was significantly associated with weight fluctuations in a dose-dependent fashion so that greater degrees of variability in body weight were linked to higher event rates (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1606148).
“When compared with the lowest quintile, patients with the highest quintile of variability had an increase in the risk of any coronary event of 64%, an increase in the risk of any cardiovascular event of 85%, an increase in the risk of death of 124%, an increase in the risk of MI of 117%, an increase in the risk of stroke of 136%, and an increase in the risk of new-onset diabetes of 78%, independent of traditional risk factors,” Dr. Bangalore and his associates reported.
This association remained strong regardless of the patients’ weight at baseline, consistent among those of normal body weight and those who were overweight or obese. And the association also persisted across multiple sensitivity analyses.
This study was supported by Pfizer. Dr. Bangalore reported serving as a consultant to Pfizer, Daiichi-Sankyo, Boehringer Ingelheim, Merck, Menarini, Gilead, and Abbott Vascular; his associates reported ties to numerous industry sources.
Weight fluctuations – like the typical pattern of weight loss followed by partial or total regain that affects most people attempting to lose weight – are strongly associated with higher mortality, more cardiovascular events, and new-onset diabetes, according to an analysis of the Treating to New Targets trial published online April 6 in the New England Journal of Medicine.
Weight loss is commonly prescribed as a lifestyle intervention to improve health, especially cardiovascular health. However, “the usual pattern for most patients ... is weight loss followed by weight gain” (also termed weight cycling), which has been linked to poor cardiovascular outcomes, especially when the pattern is repeated over time, said Sripal Bangalore, MD, of New York University, and his coinvestigators.
The primary outcome measure – the composite rate of death from coronary heart disease, nonfatal MI, resuscitated cardiac arrest, revascularization, or angina – was significantly associated with weight fluctuations in a dose-dependent fashion so that greater degrees of variability in body weight were linked to higher event rates (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1606148).
“When compared with the lowest quintile, patients with the highest quintile of variability had an increase in the risk of any coronary event of 64%, an increase in the risk of any cardiovascular event of 85%, an increase in the risk of death of 124%, an increase in the risk of MI of 117%, an increase in the risk of stroke of 136%, and an increase in the risk of new-onset diabetes of 78%, independent of traditional risk factors,” Dr. Bangalore and his associates reported.
This association remained strong regardless of the patients’ weight at baseline, consistent among those of normal body weight and those who were overweight or obese. And the association also persisted across multiple sensitivity analyses.
This study was supported by Pfizer. Dr. Bangalore reported serving as a consultant to Pfizer, Daiichi-Sankyo, Boehringer Ingelheim, Merck, Menarini, Gilead, and Abbott Vascular; his associates reported ties to numerous industry sources.
Key clinical point: Weight fluctuations are strongly associated with higher mortality, more cardiovascular events, and new-onset diabetes.
Major finding: Patients with the highest quintile of weight variability had increased risks of coronary events (64%), cardiovascular events (85%), death (124%), MI (117%), stroke (136%), and new-onset diabetes (78%).
Data source: A secondary analysis of data for 9,509 adults with coronary artery disease participating in the Treating to New Targets trial of cholesterol therapy.
Disclosures: This study was supported by Pfizer. Dr. Bangalore reported serving as a consultant to Pfizer, Daiichi-Sankyo, Boehringer Ingelheim, Merck, Menarini, Gilead, and Abbott Vascular; his associates reported ties to numerous industry sources.
Transplantation plus VRD tops VRD alone in myeloma
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: High-dose chemotherapy and autologous stem cell transplantation plus bortezomib, lenalidomide, and dexamethasone (VRD) outperformed consolidation with VRD only in adults with newly diagnosed multiple myeloma.
Major finding: Median progression-free survival was 50 months with transplantation plus VRD and 36 months with VRD only (adjusted hazard ratio for disease progression or death, 0.65; P less than .001).
Data source: A randomized, multicenter, open-label, phase III trial of 700 patients with multiple myeloma.
Disclosures: Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
Fatigue in rheumatoid arthritis declines with pedometer use
Rheumatoid arthritis patients given pedometers reported a decrease in fatigue over a 21-week period, coupled with a small but significant increase in steps walked, according to a study from investigators at the University of California, San Francisco.
“Our results provide evidence that pedometers are effective at increasing physical activity among people with RA and provide support for the hypothesis that increasing physical activity by walking has important effects on fatigue and other RA symptoms,” wrote the researchers, led by Patricia Katz, PhD.
Patients who were given only pedometers increased their walking from baseline to week 21 by an average of 1,441 steps, while those given pedometers plus a step goal walked a mean of 1,656 steps additionally. The control group had a mean decrease of 747 steps.
Scores on the Patient-Reported Outcome Measurement Information System fatigue short form at 21 weeks declined by –3.2 for those who received pedometers only (P = .02) and by –4.8 for those who received pedometers plus a step goal (P = .0002), compared with the control group, which reported a decrease of –1.6 (P = .26).
While both intervention groups saw increased activity when compared with controls, patients given a step goal saw constant improvement, averaging an increase of 22 steps per week, while those without a step goal reported a decrease of 37 steps per week, which the researchers suspected could be from a larger initial increase in activity that could have discouraged a continued increase over time.
Dr. Katz and her associates said that the positive change that a rise in physical activity had on all other aspects of patients’ self-reported assessment shows promising results for the efficacy of exercise in patients, which some physicians had questioned.
“In the past, there was concern that physical activity or exercise might exacerbate rheumatoid arthritis symptoms, [however], our findings are similar to previous reports that exercise did not lead to worsened rheumatoid arthritis,” they wrote.
This study was limited by the small number of participants in the study, as well as the self-reported method of data collection. Researchers were also unable to gather activity intensity, as well as the amount of time patients wore the pedometer, causing the measure of adherence to “only be considered an estimate.”
The researchers reported technical difficulties with the devices, creating missing data.
The study was supported by the Rheumatology Research Foundation. The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
Rheumatoid arthritis patients given pedometers reported a decrease in fatigue over a 21-week period, coupled with a small but significant increase in steps walked, according to a study from investigators at the University of California, San Francisco.
“Our results provide evidence that pedometers are effective at increasing physical activity among people with RA and provide support for the hypothesis that increasing physical activity by walking has important effects on fatigue and other RA symptoms,” wrote the researchers, led by Patricia Katz, PhD.
Patients who were given only pedometers increased their walking from baseline to week 21 by an average of 1,441 steps, while those given pedometers plus a step goal walked a mean of 1,656 steps additionally. The control group had a mean decrease of 747 steps.
Scores on the Patient-Reported Outcome Measurement Information System fatigue short form at 21 weeks declined by –3.2 for those who received pedometers only (P = .02) and by –4.8 for those who received pedometers plus a step goal (P = .0002), compared with the control group, which reported a decrease of –1.6 (P = .26).
While both intervention groups saw increased activity when compared with controls, patients given a step goal saw constant improvement, averaging an increase of 22 steps per week, while those without a step goal reported a decrease of 37 steps per week, which the researchers suspected could be from a larger initial increase in activity that could have discouraged a continued increase over time.
Dr. Katz and her associates said that the positive change that a rise in physical activity had on all other aspects of patients’ self-reported assessment shows promising results for the efficacy of exercise in patients, which some physicians had questioned.
“In the past, there was concern that physical activity or exercise might exacerbate rheumatoid arthritis symptoms, [however], our findings are similar to previous reports that exercise did not lead to worsened rheumatoid arthritis,” they wrote.
This study was limited by the small number of participants in the study, as well as the self-reported method of data collection. Researchers were also unable to gather activity intensity, as well as the amount of time patients wore the pedometer, causing the measure of adherence to “only be considered an estimate.”
The researchers reported technical difficulties with the devices, creating missing data.
The study was supported by the Rheumatology Research Foundation. The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
Rheumatoid arthritis patients given pedometers reported a decrease in fatigue over a 21-week period, coupled with a small but significant increase in steps walked, according to a study from investigators at the University of California, San Francisco.
“Our results provide evidence that pedometers are effective at increasing physical activity among people with RA and provide support for the hypothesis that increasing physical activity by walking has important effects on fatigue and other RA symptoms,” wrote the researchers, led by Patricia Katz, PhD.
Patients who were given only pedometers increased their walking from baseline to week 21 by an average of 1,441 steps, while those given pedometers plus a step goal walked a mean of 1,656 steps additionally. The control group had a mean decrease of 747 steps.
Scores on the Patient-Reported Outcome Measurement Information System fatigue short form at 21 weeks declined by –3.2 for those who received pedometers only (P = .02) and by –4.8 for those who received pedometers plus a step goal (P = .0002), compared with the control group, which reported a decrease of –1.6 (P = .26).
While both intervention groups saw increased activity when compared with controls, patients given a step goal saw constant improvement, averaging an increase of 22 steps per week, while those without a step goal reported a decrease of 37 steps per week, which the researchers suspected could be from a larger initial increase in activity that could have discouraged a continued increase over time.
Dr. Katz and her associates said that the positive change that a rise in physical activity had on all other aspects of patients’ self-reported assessment shows promising results for the efficacy of exercise in patients, which some physicians had questioned.
“In the past, there was concern that physical activity or exercise might exacerbate rheumatoid arthritis symptoms, [however], our findings are similar to previous reports that exercise did not lead to worsened rheumatoid arthritis,” they wrote.
This study was limited by the small number of participants in the study, as well as the self-reported method of data collection. Researchers were also unable to gather activity intensity, as well as the amount of time patients wore the pedometer, causing the measure of adherence to “only be considered an estimate.”
The researchers reported technical difficulties with the devices, creating missing data.
The study was supported by the Rheumatology Research Foundation. The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Mean fatigue scores dropped –3.2 (P = .02) and –4.8 (P = .0002) for patients given only pedometers and pedometers with step goals, respectively.
Data source: Controlled study of 96 rheumatoid arthritis patients observed over a 21-week period.
Disclosures: The study was supported by the Rheumatology Research Foundation. The researchers reported no relevant financial disclosures.
Inpatient cost of atopic dermatitis called ‘substantial’
Inpatient care for atopic dermatitis in the U.S. totaled almost $128 million between 2002 and 2012, indicating a rising financial burden, according to a study funded by the Agency for Healthcare Research and Quality (AHRQ).
Cost of care averaged $8.3 million per year for adults and $3.3 million per year for children, with per-day costs for adult care increasing from $3,200 in 2002 to $3,783 in 2012 and per-day costs for pediatric care increasing from $2,430 to $2,914 in the same period, according to Shanthi Narla, a doctoral research fellow at Northwestern University, Chicago, and her colleagues (J Invest Dermatol. 2017 Mar 1. doi: 10.1016/j.jid.2017.02.975)
Growth in hospitalizations was seen especially in adult AD patients, which rose from 58 million to 76 million over the decade.
Despite such high comparative rates of hospitalization, most adult (80%) and child (97%) AD patients had a shorter average length of stay than those without AD (adults: 2.7 vs. 3.5 days; children: 2.4 vs. 2.7 days; P = .0004),
Growing prevalence of AD hospitalization contributed to the larger financial burden for AD patients, compared with psoriasis and pemphigus, the researchers noted, considering length of stay per AD hospitalization averaged 62.5% and 50% shorter than those for pemphigus or psoriasis.
“The acute signs and symptoms of [AD or eczema]... erythema, oozing/weeping, scaling and pruritus, may resolve faster with optimized treatment than vesicobullae and erosions in pemphigus or ‘lakes of pus’ and skin sloughing in generalized pustular psoriasis,” they wrote.
Additionally, hospitalization costs for AD were 60% and 33% of the cost per psoriasis and pemphigus hospitalizations, respectively, according to Ms. Narla and her colleagues.
The researchers said they were concerned that the prevalence of AD, and subsequently the inpatient financial burden, will continue to increase if left unchecked.
“Prevalences and costs of hospitalization for AD significantly increased during the study period without plateauing, indicating that the total cost of inpatient care for AD may continue to increase,” the researchers asserted.
This study was limited by a lack of information on the severity of patients’ symptoms, as well as whether a dermatologist or other physician diagnosed the patients and by which criteria.
[email protected]
On Twitter @EAZTweets
Inpatient care for atopic dermatitis in the U.S. totaled almost $128 million between 2002 and 2012, indicating a rising financial burden, according to a study funded by the Agency for Healthcare Research and Quality (AHRQ).
Cost of care averaged $8.3 million per year for adults and $3.3 million per year for children, with per-day costs for adult care increasing from $3,200 in 2002 to $3,783 in 2012 and per-day costs for pediatric care increasing from $2,430 to $2,914 in the same period, according to Shanthi Narla, a doctoral research fellow at Northwestern University, Chicago, and her colleagues (J Invest Dermatol. 2017 Mar 1. doi: 10.1016/j.jid.2017.02.975)
Growth in hospitalizations was seen especially in adult AD patients, which rose from 58 million to 76 million over the decade.
Despite such high comparative rates of hospitalization, most adult (80%) and child (97%) AD patients had a shorter average length of stay than those without AD (adults: 2.7 vs. 3.5 days; children: 2.4 vs. 2.7 days; P = .0004),
Growing prevalence of AD hospitalization contributed to the larger financial burden for AD patients, compared with psoriasis and pemphigus, the researchers noted, considering length of stay per AD hospitalization averaged 62.5% and 50% shorter than those for pemphigus or psoriasis.
“The acute signs and symptoms of [AD or eczema]... erythema, oozing/weeping, scaling and pruritus, may resolve faster with optimized treatment than vesicobullae and erosions in pemphigus or ‘lakes of pus’ and skin sloughing in generalized pustular psoriasis,” they wrote.
Additionally, hospitalization costs for AD were 60% and 33% of the cost per psoriasis and pemphigus hospitalizations, respectively, according to Ms. Narla and her colleagues.
The researchers said they were concerned that the prevalence of AD, and subsequently the inpatient financial burden, will continue to increase if left unchecked.
“Prevalences and costs of hospitalization for AD significantly increased during the study period without plateauing, indicating that the total cost of inpatient care for AD may continue to increase,” the researchers asserted.
This study was limited by a lack of information on the severity of patients’ symptoms, as well as whether a dermatologist or other physician diagnosed the patients and by which criteria.
[email protected]
On Twitter @EAZTweets
Inpatient care for atopic dermatitis in the U.S. totaled almost $128 million between 2002 and 2012, indicating a rising financial burden, according to a study funded by the Agency for Healthcare Research and Quality (AHRQ).
Cost of care averaged $8.3 million per year for adults and $3.3 million per year for children, with per-day costs for adult care increasing from $3,200 in 2002 to $3,783 in 2012 and per-day costs for pediatric care increasing from $2,430 to $2,914 in the same period, according to Shanthi Narla, a doctoral research fellow at Northwestern University, Chicago, and her colleagues (J Invest Dermatol. 2017 Mar 1. doi: 10.1016/j.jid.2017.02.975)
Growth in hospitalizations was seen especially in adult AD patients, which rose from 58 million to 76 million over the decade.
Despite such high comparative rates of hospitalization, most adult (80%) and child (97%) AD patients had a shorter average length of stay than those without AD (adults: 2.7 vs. 3.5 days; children: 2.4 vs. 2.7 days; P = .0004),
Growing prevalence of AD hospitalization contributed to the larger financial burden for AD patients, compared with psoriasis and pemphigus, the researchers noted, considering length of stay per AD hospitalization averaged 62.5% and 50% shorter than those for pemphigus or psoriasis.
“The acute signs and symptoms of [AD or eczema]... erythema, oozing/weeping, scaling and pruritus, may resolve faster with optimized treatment than vesicobullae and erosions in pemphigus or ‘lakes of pus’ and skin sloughing in generalized pustular psoriasis,” they wrote.
Additionally, hospitalization costs for AD were 60% and 33% of the cost per psoriasis and pemphigus hospitalizations, respectively, according to Ms. Narla and her colleagues.
The researchers said they were concerned that the prevalence of AD, and subsequently the inpatient financial burden, will continue to increase if left unchecked.
“Prevalences and costs of hospitalization for AD significantly increased during the study period without plateauing, indicating that the total cost of inpatient care for AD may continue to increase,” the researchers asserted.
This study was limited by a lack of information on the severity of patients’ symptoms, as well as whether a dermatologist or other physician diagnosed the patients and by which criteria.
[email protected]
On Twitter @EAZTweets
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point:
Major finding: Hospitalization costs for atopic dermatitis or eczema in adults and children in the U.S. totaled $127.8 million between 2002 and 2012.
Data source: Retrospective analysis of 87 million patient records from the 2002-2012 National Inpatient Sample, with cost of care adjusted for inflation.
Disclosures: Study was sponsored by the Agency for Healthcare Research and Quality (AHRQ), the Dermatology Foundation, and American Medical Association Foundation. The investigators report no relevant financial disclosures.
Cell sheet transplantation improves ischemic cardiomyopathy at 1 year
For patients with ischemic cardiomyopathy (ICM), transplanting sheets of autologous myoblasts onto the epicardial surface was safe and led to “marked” improvements in functional and laboratory measures, investigators reported.
However, cell sheet transplantation was much less effective for patients with dilated cardiomyopathy in this phase I trial, wrote Shigeru Miyagawa, MD, PhD, of Osaka (Japan) University, and his associates in the Journal of the American Heart Association. The findings merit additional follow-up in larger clinical studies, the investigators asserted.
Heart failure remains life threatening even with the best approved treatments. Previously, the researchers transplanted scaffold-free sheets of autologous myoblasts derived from skeletal muscle into small and large animals with heart failure and found that this approach healed severely damaged myocardium. Transplantation caused secreted cytokines to induce native regeneration through paracrine effects, Dr. Miyagawa noted in a prior review article (BioMed Research International. 2013. doi: 10.1155/2013/583912).
To evaluate this approach in humans, the researchers transplanted 15 patients with ischemic cardiomyopathy and 12 patients with dilated cardiomyopathy (DCM). Patients were 20-75 years old, had ejection fractions under 35% and New York Heart Association (NYHA) classifications of II or more, and were responding inadequately to standard treatment with a beta-blocker or ACE inhibitor and an angiotensin receptor blocker. The sole intervention was transplantation of the cell sheet over the left ventricular-free wall during left thoracotomy (J Am Heart Assoc. 2017 Apr 5. doi: 10.1161/JAHA.116.003918). There were no major procedure-related complications during follow-up, the researchers said. For patients with ICM, NYHA classifications dropped significantly at 6 months, from 2.9 at baseline to 2.1 at 6 months and 1.9 at 1 year. Six-Minute Walk Test scores improved significantly, from 416 m at baseline to 475 m at 6 months to 484 m at 1 year. Transplantation also led to significant drops in serum brain natriuretic peptide levels (baseline average, 308 pg/mL; 6 months, 191 pg/mL; 1 year, 182 pg/mL). Additionally, ICM patients improved significantly on measures of pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress.
In contrast, DCM patients did not significantly improve based on the 6-Minute Walk Test, NYHA classification, or brain natriuretic peptide serum levels. Of 12, 3 progressed to NYHA functional class IV by 1-year follow-up and there were five cardiac deaths. There were no cardiac deaths in the ICM group. “It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so cell-sheet implantation may be more suitable for ICM patients, compared with DCM patients, considering pathophysiological aspects,” the researchers wrote.
The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
For patients with ischemic cardiomyopathy (ICM), transplanting sheets of autologous myoblasts onto the epicardial surface was safe and led to “marked” improvements in functional and laboratory measures, investigators reported.
However, cell sheet transplantation was much less effective for patients with dilated cardiomyopathy in this phase I trial, wrote Shigeru Miyagawa, MD, PhD, of Osaka (Japan) University, and his associates in the Journal of the American Heart Association. The findings merit additional follow-up in larger clinical studies, the investigators asserted.
Heart failure remains life threatening even with the best approved treatments. Previously, the researchers transplanted scaffold-free sheets of autologous myoblasts derived from skeletal muscle into small and large animals with heart failure and found that this approach healed severely damaged myocardium. Transplantation caused secreted cytokines to induce native regeneration through paracrine effects, Dr. Miyagawa noted in a prior review article (BioMed Research International. 2013. doi: 10.1155/2013/583912).
To evaluate this approach in humans, the researchers transplanted 15 patients with ischemic cardiomyopathy and 12 patients with dilated cardiomyopathy (DCM). Patients were 20-75 years old, had ejection fractions under 35% and New York Heart Association (NYHA) classifications of II or more, and were responding inadequately to standard treatment with a beta-blocker or ACE inhibitor and an angiotensin receptor blocker. The sole intervention was transplantation of the cell sheet over the left ventricular-free wall during left thoracotomy (J Am Heart Assoc. 2017 Apr 5. doi: 10.1161/JAHA.116.003918). There were no major procedure-related complications during follow-up, the researchers said. For patients with ICM, NYHA classifications dropped significantly at 6 months, from 2.9 at baseline to 2.1 at 6 months and 1.9 at 1 year. Six-Minute Walk Test scores improved significantly, from 416 m at baseline to 475 m at 6 months to 484 m at 1 year. Transplantation also led to significant drops in serum brain natriuretic peptide levels (baseline average, 308 pg/mL; 6 months, 191 pg/mL; 1 year, 182 pg/mL). Additionally, ICM patients improved significantly on measures of pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress.
In contrast, DCM patients did not significantly improve based on the 6-Minute Walk Test, NYHA classification, or brain natriuretic peptide serum levels. Of 12, 3 progressed to NYHA functional class IV by 1-year follow-up and there were five cardiac deaths. There were no cardiac deaths in the ICM group. “It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so cell-sheet implantation may be more suitable for ICM patients, compared with DCM patients, considering pathophysiological aspects,” the researchers wrote.
The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
For patients with ischemic cardiomyopathy (ICM), transplanting sheets of autologous myoblasts onto the epicardial surface was safe and led to “marked” improvements in functional and laboratory measures, investigators reported.
However, cell sheet transplantation was much less effective for patients with dilated cardiomyopathy in this phase I trial, wrote Shigeru Miyagawa, MD, PhD, of Osaka (Japan) University, and his associates in the Journal of the American Heart Association. The findings merit additional follow-up in larger clinical studies, the investigators asserted.
Heart failure remains life threatening even with the best approved treatments. Previously, the researchers transplanted scaffold-free sheets of autologous myoblasts derived from skeletal muscle into small and large animals with heart failure and found that this approach healed severely damaged myocardium. Transplantation caused secreted cytokines to induce native regeneration through paracrine effects, Dr. Miyagawa noted in a prior review article (BioMed Research International. 2013. doi: 10.1155/2013/583912).
To evaluate this approach in humans, the researchers transplanted 15 patients with ischemic cardiomyopathy and 12 patients with dilated cardiomyopathy (DCM). Patients were 20-75 years old, had ejection fractions under 35% and New York Heart Association (NYHA) classifications of II or more, and were responding inadequately to standard treatment with a beta-blocker or ACE inhibitor and an angiotensin receptor blocker. The sole intervention was transplantation of the cell sheet over the left ventricular-free wall during left thoracotomy (J Am Heart Assoc. 2017 Apr 5. doi: 10.1161/JAHA.116.003918). There were no major procedure-related complications during follow-up, the researchers said. For patients with ICM, NYHA classifications dropped significantly at 6 months, from 2.9 at baseline to 2.1 at 6 months and 1.9 at 1 year. Six-Minute Walk Test scores improved significantly, from 416 m at baseline to 475 m at 6 months to 484 m at 1 year. Transplantation also led to significant drops in serum brain natriuretic peptide levels (baseline average, 308 pg/mL; 6 months, 191 pg/mL; 1 year, 182 pg/mL). Additionally, ICM patients improved significantly on measures of pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress.
In contrast, DCM patients did not significantly improve based on the 6-Minute Walk Test, NYHA classification, or brain natriuretic peptide serum levels. Of 12, 3 progressed to NYHA functional class IV by 1-year follow-up and there were five cardiac deaths. There were no cardiac deaths in the ICM group. “It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so cell-sheet implantation may be more suitable for ICM patients, compared with DCM patients, considering pathophysiological aspects,” the researchers wrote.
The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
Key clinical point: Transplanting a sheet of autologous myoblasts onto the epicardial surface was safe and was associated with functional and symptomatic improvements in patients with ischemic cardiomyopathy.
Major finding: Patients improved significantly on the 6-Minute Walk Test, New York Heart Classification, and on measures of brain natriuretic peptide, pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress. These effects did not extend to patients with dilated cardiomyopathy.
Data source: A phase I trial of 27 patients with ischemic or dilated cardiomyopathy.
Disclosures: The study was funded by grants from Regenerative Medicine for Clinical Application, Health and Labor Sciences Research, and the Japan Agency for Medical Research and Development. The investigators disclosed laboratory funding from Terumo for cooperative research in cell sheet.
Pushing the Boundaries of Conventional Surgery
With the myriad procedures and complications that thoracic surgeons are required to be familiar with, the Adult Cardiac Skills course, “100 Years of Training – More Skills Still Needed!” aims to shed some light on the more unconventional procedures that thoracic surgeons see less frequently.
The course will consist of sessions that will each highlight a specific topic: mitral valve repairs. imaging, coronary surgery, and “complex case presentations,” which will feature demonstrations of groundbreaking techniques for challenging presentations a surgeon could encounter.
The mitral valve repair session will focus on bileaflet and endoscopic mitral valve repair, along with talks on right perfusion management, transapical artificial chordae implantation, and using aortic valve repair on a bicuspid aortic valve. These talks – given by Patrick Perier, MD, of the Herz und Gefaess Klinik, Andrea Colli, MD, of the University of Padova, and David Fitzgerald, MD, of the Medical Center of South Carolina, among others – will each last about 15 minutes and will culminate in a panel discussion.
The following hour will be spent discussing imaging in cardiac surgery, where speakers such as Eric E. Roselli, MD, of the Cleveland Clinic, will discuss using imaging in TEVAR to facilitate aortic stenting. Wilson Y. Szeto, MD, of the University of Pennsylvania, will give a talk on transfemoral TAVR, and Mary Beth Brady, MD, of Johns Hopkins University, will discuss the use of transesophageal echocardiogram in thoracic surgery.
After lunch, the coronary surgery session will feature presentations on internal thoracic artery grafts – presented by course chair Dr. Zehr – along with discussions on hybrid revascularization and minimal access off-pump coronary artery bypass. And finally, the last session will highlight the most unusual and rare cases surgeons are likely to come across, such as redoing a mitral valve repair and operating on an infected aortic root. Dr. Zehr hopes the session will address some needs and questions for attendees to improve their practice. “What extra skill sets are necessary to do a few more mitral repairs than you’re already doing and to be a little bit more successful in aortic dissections? Or to know some of the subtleties between doing an open aortic valve versus a minimally invasive transapical or transcathetic aortic valve.”
With the myriad procedures and complications that thoracic surgeons are required to be familiar with, the Adult Cardiac Skills course, “100 Years of Training – More Skills Still Needed!” aims to shed some light on the more unconventional procedures that thoracic surgeons see less frequently.
The course will consist of sessions that will each highlight a specific topic: mitral valve repairs. imaging, coronary surgery, and “complex case presentations,” which will feature demonstrations of groundbreaking techniques for challenging presentations a surgeon could encounter.
The mitral valve repair session will focus on bileaflet and endoscopic mitral valve repair, along with talks on right perfusion management, transapical artificial chordae implantation, and using aortic valve repair on a bicuspid aortic valve. These talks – given by Patrick Perier, MD, of the Herz und Gefaess Klinik, Andrea Colli, MD, of the University of Padova, and David Fitzgerald, MD, of the Medical Center of South Carolina, among others – will each last about 15 minutes and will culminate in a panel discussion.
The following hour will be spent discussing imaging in cardiac surgery, where speakers such as Eric E. Roselli, MD, of the Cleveland Clinic, will discuss using imaging in TEVAR to facilitate aortic stenting. Wilson Y. Szeto, MD, of the University of Pennsylvania, will give a talk on transfemoral TAVR, and Mary Beth Brady, MD, of Johns Hopkins University, will discuss the use of transesophageal echocardiogram in thoracic surgery.
After lunch, the coronary surgery session will feature presentations on internal thoracic artery grafts – presented by course chair Dr. Zehr – along with discussions on hybrid revascularization and minimal access off-pump coronary artery bypass. And finally, the last session will highlight the most unusual and rare cases surgeons are likely to come across, such as redoing a mitral valve repair and operating on an infected aortic root. Dr. Zehr hopes the session will address some needs and questions for attendees to improve their practice. “What extra skill sets are necessary to do a few more mitral repairs than you’re already doing and to be a little bit more successful in aortic dissections? Or to know some of the subtleties between doing an open aortic valve versus a minimally invasive transapical or transcathetic aortic valve.”
With the myriad procedures and complications that thoracic surgeons are required to be familiar with, the Adult Cardiac Skills course, “100 Years of Training – More Skills Still Needed!” aims to shed some light on the more unconventional procedures that thoracic surgeons see less frequently.
The course will consist of sessions that will each highlight a specific topic: mitral valve repairs. imaging, coronary surgery, and “complex case presentations,” which will feature demonstrations of groundbreaking techniques for challenging presentations a surgeon could encounter.
The mitral valve repair session will focus on bileaflet and endoscopic mitral valve repair, along with talks on right perfusion management, transapical artificial chordae implantation, and using aortic valve repair on a bicuspid aortic valve. These talks – given by Patrick Perier, MD, of the Herz und Gefaess Klinik, Andrea Colli, MD, of the University of Padova, and David Fitzgerald, MD, of the Medical Center of South Carolina, among others – will each last about 15 minutes and will culminate in a panel discussion.
The following hour will be spent discussing imaging in cardiac surgery, where speakers such as Eric E. Roselli, MD, of the Cleveland Clinic, will discuss using imaging in TEVAR to facilitate aortic stenting. Wilson Y. Szeto, MD, of the University of Pennsylvania, will give a talk on transfemoral TAVR, and Mary Beth Brady, MD, of Johns Hopkins University, will discuss the use of transesophageal echocardiogram in thoracic surgery.
After lunch, the coronary surgery session will feature presentations on internal thoracic artery grafts – presented by course chair Dr. Zehr – along with discussions on hybrid revascularization and minimal access off-pump coronary artery bypass. And finally, the last session will highlight the most unusual and rare cases surgeons are likely to come across, such as redoing a mitral valve repair and operating on an infected aortic root. Dr. Zehr hopes the session will address some needs and questions for attendees to improve their practice. “What extra skill sets are necessary to do a few more mitral repairs than you’re already doing and to be a little bit more successful in aortic dissections? Or to know some of the subtleties between doing an open aortic valve versus a minimally invasive transapical or transcathetic aortic valve.”
Does laparoscopy have an advantage for emergency VHR?
HOUSTON – The benefits of elective laparoscopic ventral hernia repair over the open approach have been well documented, but, when presented with emergency cases, surgeons still are about 10 times more likely to employ open surgery, possibly exposing patients to greater risk of complications, as well as longer hospital stays, according to an analysis of a national database.
“Despite the benefits of laparoscopic surgery, its utilization in ventral hernia repair (VHR) remains low,” said David Pechman, MD, MBA, of Mount Sinai Beth Israel Medical Center, New York, at the annual meeting of the American Society of Gastrointestinal and Endoscopic Surgeons. “This study suggests that utilization is further decreased in emergency cases. Relative to elective cases, emergency VHR is associated with markedly increased rates of morbidity, giving us more room to improve patient outcomes with the use of laparoscopy in these cases.”
The study analyzed 330 emergency VHR operations in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database for 2012 and 2013. Thirty-two (9.3%) of those operations were performed laparoscopically, and the remainder were open. Because the sample size of laparoscopic operations was so small, drawing statistically significant conclusions from the findings is difficult without a larger, higher-powered study, Dr. Pechman said. “We do think that further research is warranted and believe that analysis of a larger sample would display that increased utilization of laparoscopy in emergency VHR could significantly improve outcomes.”
The analysis found significant differences in outcomes between the laparoscopic and the open operations for emergency VHR. Average hospital stay after laparoscopic emergency VHR was 2.8 days vs. 5.9 days for open VHR (P = .02). Surgical site infection rates were 0% vs. 7.7% (P = .15). Demographics between both laparoscopic and open groups were similar, Dr. Pechman said.
Session moderator E. Matthew Ritter, MD, of Walter Reed National Military Medical Center, Bethesda, Md., noted that the study conclusion is in line with the goals of SAGES’s minimally invasive surgery initiative to increase utilization of laparoscopy. When referring to the findings Dr. Pechman reported, Dr. Ritter said, “This is a remarkably low complication rate for a procedure that could seemingly have some benefit.”
Dr. Pechman’s response acknowledged the concerns of the surgeons doing the procedures: “A lot of this has to do with surgeon comfort and the preoperative decision-making, especially in an emergency setting.”
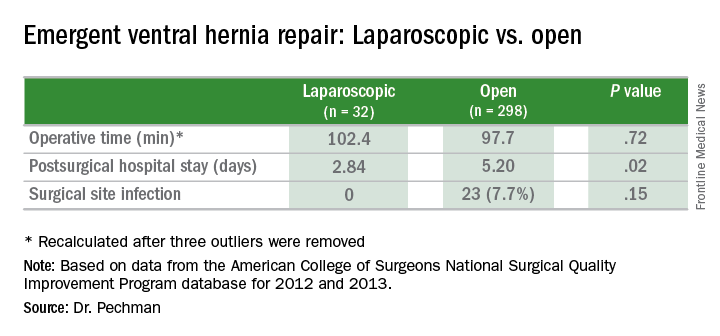
HOUSTON – The benefits of elective laparoscopic ventral hernia repair over the open approach have been well documented, but, when presented with emergency cases, surgeons still are about 10 times more likely to employ open surgery, possibly exposing patients to greater risk of complications, as well as longer hospital stays, according to an analysis of a national database.
“Despite the benefits of laparoscopic surgery, its utilization in ventral hernia repair (VHR) remains low,” said David Pechman, MD, MBA, of Mount Sinai Beth Israel Medical Center, New York, at the annual meeting of the American Society of Gastrointestinal and Endoscopic Surgeons. “This study suggests that utilization is further decreased in emergency cases. Relative to elective cases, emergency VHR is associated with markedly increased rates of morbidity, giving us more room to improve patient outcomes with the use of laparoscopy in these cases.”
The study analyzed 330 emergency VHR operations in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database for 2012 and 2013. Thirty-two (9.3%) of those operations were performed laparoscopically, and the remainder were open. Because the sample size of laparoscopic operations was so small, drawing statistically significant conclusions from the findings is difficult without a larger, higher-powered study, Dr. Pechman said. “We do think that further research is warranted and believe that analysis of a larger sample would display that increased utilization of laparoscopy in emergency VHR could significantly improve outcomes.”
The analysis found significant differences in outcomes between the laparoscopic and the open operations for emergency VHR. Average hospital stay after laparoscopic emergency VHR was 2.8 days vs. 5.9 days for open VHR (P = .02). Surgical site infection rates were 0% vs. 7.7% (P = .15). Demographics between both laparoscopic and open groups were similar, Dr. Pechman said.
Session moderator E. Matthew Ritter, MD, of Walter Reed National Military Medical Center, Bethesda, Md., noted that the study conclusion is in line with the goals of SAGES’s minimally invasive surgery initiative to increase utilization of laparoscopy. When referring to the findings Dr. Pechman reported, Dr. Ritter said, “This is a remarkably low complication rate for a procedure that could seemingly have some benefit.”
Dr. Pechman’s response acknowledged the concerns of the surgeons doing the procedures: “A lot of this has to do with surgeon comfort and the preoperative decision-making, especially in an emergency setting.”

HOUSTON – The benefits of elective laparoscopic ventral hernia repair over the open approach have been well documented, but, when presented with emergency cases, surgeons still are about 10 times more likely to employ open surgery, possibly exposing patients to greater risk of complications, as well as longer hospital stays, according to an analysis of a national database.
“Despite the benefits of laparoscopic surgery, its utilization in ventral hernia repair (VHR) remains low,” said David Pechman, MD, MBA, of Mount Sinai Beth Israel Medical Center, New York, at the annual meeting of the American Society of Gastrointestinal and Endoscopic Surgeons. “This study suggests that utilization is further decreased in emergency cases. Relative to elective cases, emergency VHR is associated with markedly increased rates of morbidity, giving us more room to improve patient outcomes with the use of laparoscopy in these cases.”
The study analyzed 330 emergency VHR operations in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database for 2012 and 2013. Thirty-two (9.3%) of those operations were performed laparoscopically, and the remainder were open. Because the sample size of laparoscopic operations was so small, drawing statistically significant conclusions from the findings is difficult without a larger, higher-powered study, Dr. Pechman said. “We do think that further research is warranted and believe that analysis of a larger sample would display that increased utilization of laparoscopy in emergency VHR could significantly improve outcomes.”
The analysis found significant differences in outcomes between the laparoscopic and the open operations for emergency VHR. Average hospital stay after laparoscopic emergency VHR was 2.8 days vs. 5.9 days for open VHR (P = .02). Surgical site infection rates were 0% vs. 7.7% (P = .15). Demographics between both laparoscopic and open groups were similar, Dr. Pechman said.
Session moderator E. Matthew Ritter, MD, of Walter Reed National Military Medical Center, Bethesda, Md., noted that the study conclusion is in line with the goals of SAGES’s minimally invasive surgery initiative to increase utilization of laparoscopy. When referring to the findings Dr. Pechman reported, Dr. Ritter said, “This is a remarkably low complication rate for a procedure that could seemingly have some benefit.”
Dr. Pechman’s response acknowledged the concerns of the surgeons doing the procedures: “A lot of this has to do with surgeon comfort and the preoperative decision-making, especially in an emergency setting.”

Key clinical point: Outcomes of laparoscopic ventral hernia repair (VHR) may be superior to those for open surgery in the emergency setting.
Major finding: Average hospital stay after laparoscopic emergency VHR was 2.8 days vs. 5.9 days for open surgery, and surgical site infection rates were 0% vs. 7.7%.
Data source: Analysis of 329 cases of emergency VHR enrolled in the American College of Surgeons National Surgical Quality Improvement Program in 2012 and 2013.
Disclosures: Dr. Pechman reported no financial disclosures.
Advanced techniques in cystectomy for mature cystic teratomas

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Novel classification of labial anatomy and evaluation in the treatment of labial agglutination
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Novel classification of labial anatomy and evaluation in the treatment of labial agglutination
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Novel classification of labial anatomy and evaluation in the treatment of labial agglutination
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by