User login
How’s your postacute network doing?
By now, nearly all hospitals are developing networks of postacute facilities for some or all of their patients, such as those in ACOs, bundled payments, or other value-based programs. Commonly referred to as preferred providers, performance networks, narrow networks, or similar, these networks of skilled nursing facilities (SNFs) and other entities that provide postacute care (like home health agencies) are usually chosen because they have demonstrated that they provide high quality, cost-effective care for patients after they leave the hospital.
While case managers are often the ones who counsel patients and caregivers on the details of the network, hospitalists should have at least a high-level grasp of which facilities are on the list and what the network selection criteria are. I would argue that hospitalists should lead the discussion with patients on postacute facility selection as it relates to which facilities are in the network and why going to a network facility is advantageous. Why? Because as hospitalist practices begin to share clinical and financial risk for patients, or at least become eligible to share in savings as MACRA encourages, they will have a vested interest in network facilities’ performance.
Postacute care network selection criteria
There is a range of criteria – usually incorporating measures of quality and efficiency – for including providers like SNFs in networks. In terms of quality, criteria can include physician/provider availability, star ratings on Nursing Home Compare, care transitions measures, Department of Public Health inspection survey scores, Joint Commission accreditation, etc.
A few caveats regarding specific selection criteria:
Star ratings on Nursing Home Compare
These are derived from nursing staffing ratios, health inspections, and 16 quality measures. More than half of the quality measures pertain to long-stay residents who typically are not in the ACO or bundled payment program for which the network was created (these are usually short-stay patients).
SNF length of stay
High readmission rates from a SNF can actually lower its length of stay, so including “balancing” measures such as readmissions should be considered.
What about patient choice?
Narrow postacute networks are not only becoming the norm, but there is also broad recognition from CMS, MedPAC, and industry leaders that value-based payment programs require such networks to succeed. That said, case managers and other discharge planners may still resist networks on the grounds that they might be perceived as restricting patient choice. One approach to balancing differing views on patient choice is to give patients the traditional longer list of available postacute providers, and also furnish the shorter network list accompanied by an explanation of why certain SNFs are in the network. Thankfully, as ACOs and bundles become widespread, resistance to narrow networks is dying down.
What role should hospitalists play in network referrals?
High functioning hospitalist practices should lead the discussion with patients and the health care team on referrals to network SNFs. Why? Patients are looking for their doctors to guide them on such decisions. Only if the physician opts not to have the discussion will patients look to the case manager for direction on which postacute facility to choose. A better option still would be for the hospitalists to partner with case managers to have the conversation with patients. In such a scenario, the hospitalist can begin the discussion and cover the major points, and the case manager can follow with more detailed information. For less mature hospitalist practices, the case manager can play a larger role in the discussion. In any case, as value-based models become ubiquitous, and shared savings become a driver of hospitalist revenue, hospitalists’ knowledge of and active participation in conversations around narrow networks and referrals will be necessary.
Dr. Whitcomb is chief medical officer at Remedy Partners in Darien, Conn. He is a cofounder and past president of SHM.
By now, nearly all hospitals are developing networks of postacute facilities for some or all of their patients, such as those in ACOs, bundled payments, or other value-based programs. Commonly referred to as preferred providers, performance networks, narrow networks, or similar, these networks of skilled nursing facilities (SNFs) and other entities that provide postacute care (like home health agencies) are usually chosen because they have demonstrated that they provide high quality, cost-effective care for patients after they leave the hospital.
While case managers are often the ones who counsel patients and caregivers on the details of the network, hospitalists should have at least a high-level grasp of which facilities are on the list and what the network selection criteria are. I would argue that hospitalists should lead the discussion with patients on postacute facility selection as it relates to which facilities are in the network and why going to a network facility is advantageous. Why? Because as hospitalist practices begin to share clinical and financial risk for patients, or at least become eligible to share in savings as MACRA encourages, they will have a vested interest in network facilities’ performance.
Postacute care network selection criteria
There is a range of criteria – usually incorporating measures of quality and efficiency – for including providers like SNFs in networks. In terms of quality, criteria can include physician/provider availability, star ratings on Nursing Home Compare, care transitions measures, Department of Public Health inspection survey scores, Joint Commission accreditation, etc.
A few caveats regarding specific selection criteria:
Star ratings on Nursing Home Compare
These are derived from nursing staffing ratios, health inspections, and 16 quality measures. More than half of the quality measures pertain to long-stay residents who typically are not in the ACO or bundled payment program for which the network was created (these are usually short-stay patients).
SNF length of stay
High readmission rates from a SNF can actually lower its length of stay, so including “balancing” measures such as readmissions should be considered.
What about patient choice?
Narrow postacute networks are not only becoming the norm, but there is also broad recognition from CMS, MedPAC, and industry leaders that value-based payment programs require such networks to succeed. That said, case managers and other discharge planners may still resist networks on the grounds that they might be perceived as restricting patient choice. One approach to balancing differing views on patient choice is to give patients the traditional longer list of available postacute providers, and also furnish the shorter network list accompanied by an explanation of why certain SNFs are in the network. Thankfully, as ACOs and bundles become widespread, resistance to narrow networks is dying down.
What role should hospitalists play in network referrals?
High functioning hospitalist practices should lead the discussion with patients and the health care team on referrals to network SNFs. Why? Patients are looking for their doctors to guide them on such decisions. Only if the physician opts not to have the discussion will patients look to the case manager for direction on which postacute facility to choose. A better option still would be for the hospitalists to partner with case managers to have the conversation with patients. In such a scenario, the hospitalist can begin the discussion and cover the major points, and the case manager can follow with more detailed information. For less mature hospitalist practices, the case manager can play a larger role in the discussion. In any case, as value-based models become ubiquitous, and shared savings become a driver of hospitalist revenue, hospitalists’ knowledge of and active participation in conversations around narrow networks and referrals will be necessary.
Dr. Whitcomb is chief medical officer at Remedy Partners in Darien, Conn. He is a cofounder and past president of SHM.
By now, nearly all hospitals are developing networks of postacute facilities for some or all of their patients, such as those in ACOs, bundled payments, or other value-based programs. Commonly referred to as preferred providers, performance networks, narrow networks, or similar, these networks of skilled nursing facilities (SNFs) and other entities that provide postacute care (like home health agencies) are usually chosen because they have demonstrated that they provide high quality, cost-effective care for patients after they leave the hospital.
While case managers are often the ones who counsel patients and caregivers on the details of the network, hospitalists should have at least a high-level grasp of which facilities are on the list and what the network selection criteria are. I would argue that hospitalists should lead the discussion with patients on postacute facility selection as it relates to which facilities are in the network and why going to a network facility is advantageous. Why? Because as hospitalist practices begin to share clinical and financial risk for patients, or at least become eligible to share in savings as MACRA encourages, they will have a vested interest in network facilities’ performance.
Postacute care network selection criteria
There is a range of criteria – usually incorporating measures of quality and efficiency – for including providers like SNFs in networks. In terms of quality, criteria can include physician/provider availability, star ratings on Nursing Home Compare, care transitions measures, Department of Public Health inspection survey scores, Joint Commission accreditation, etc.
A few caveats regarding specific selection criteria:
Star ratings on Nursing Home Compare
These are derived from nursing staffing ratios, health inspections, and 16 quality measures. More than half of the quality measures pertain to long-stay residents who typically are not in the ACO or bundled payment program for which the network was created (these are usually short-stay patients).
SNF length of stay
High readmission rates from a SNF can actually lower its length of stay, so including “balancing” measures such as readmissions should be considered.
What about patient choice?
Narrow postacute networks are not only becoming the norm, but there is also broad recognition from CMS, MedPAC, and industry leaders that value-based payment programs require such networks to succeed. That said, case managers and other discharge planners may still resist networks on the grounds that they might be perceived as restricting patient choice. One approach to balancing differing views on patient choice is to give patients the traditional longer list of available postacute providers, and also furnish the shorter network list accompanied by an explanation of why certain SNFs are in the network. Thankfully, as ACOs and bundles become widespread, resistance to narrow networks is dying down.
What role should hospitalists play in network referrals?
High functioning hospitalist practices should lead the discussion with patients and the health care team on referrals to network SNFs. Why? Patients are looking for their doctors to guide them on such decisions. Only if the physician opts not to have the discussion will patients look to the case manager for direction on which postacute facility to choose. A better option still would be for the hospitalists to partner with case managers to have the conversation with patients. In such a scenario, the hospitalist can begin the discussion and cover the major points, and the case manager can follow with more detailed information. For less mature hospitalist practices, the case manager can play a larger role in the discussion. In any case, as value-based models become ubiquitous, and shared savings become a driver of hospitalist revenue, hospitalists’ knowledge of and active participation in conversations around narrow networks and referrals will be necessary.
Dr. Whitcomb is chief medical officer at Remedy Partners in Darien, Conn. He is a cofounder and past president of SHM.
Hypoperfusion Retinopathy
Cardiovascular diseases are some of the most common conditions found in the geriatric population. Ocular manifestations of systemic cardiovascular conditions often are the initial presentation of the systemic disease. Identifying these findings help reveal the underlying disease and prevent more serious visual and systemic complications or even death.
Hypoperfusion retinopathy can occur as an early manifestation of carotid occlusive disease. It results from poor arterial perfusion pressure secondary to significant or complete carotid artery blockage resulting in retinal cha
Case Report
A 71-year-old white male was referred by his primary care physician (PCP) to the eye clinic for a routine comprehensive eye exam. The patient reported that his current progressive lenses, prescribed 2 years prior, were not strong enough at both distance and near, and that his eyes often felt dry. The symptoms were gradual in onset since his prior exam with no reported flashes, floaters, loss of vision, headaches, or ocular irritations.
The patient’s medical history was significant for morbid obesity, hypertension, borderline diabetes mellitus, and obstructive sleep apnea. His ocular history included recurrent conjunctivitis. At the time of the visit, the patient’s medications included 81 mg aspirin, 10 mg benazepril, 1,000 mg fish oil, 80 mg simvastatin, and use of a continuous positive airway pressure machine.
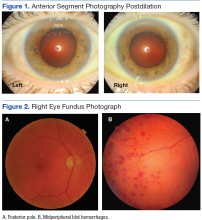
Best-corrected Snellen visual acuity was stable to his last eye exam at 20/25+2 right eye and 20/25-1 left eye with a manifest refraction of +2.25-0.75 × 077, and +2.75-1.25 × 096 in the right and left eye, respectively. Pupils were equally round and reactive to light with no afferent pupillary defect. Extraocular motility and finger counting fields were unremarkable. Anterior segment evaluation revealed lax bilateral upper lid apposition and mild cataracts in both eyes but were otherwise unremarkable (Figure 1). Dilated fundus examination revealed extensive hemorrhaging in the midperipheral retina of the right eye only (Figure 2). The left eye retina showed no abnormalities.
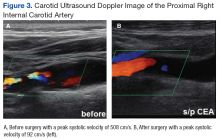
At this point the patient declined any additional symptoms, including eye pain, headache, transient vision loss, jaw claudication, and stroke signs. A complete blood count and hemoglobin A1c (HbA1c) was ordered, and all findings were unremarkable with no evidence of blood dyscrasia and with a HbA1c of 6.0. A carotid ultrasound (CUS) was also performed and revealed severe narrowing of the proximal section of the right internal carotid artery (ICA) with a trickle flow (Figure 3). The peak systolic velocity (PSV) at this level was 508 cm/s. There also was severe narrowing and turbulent flow in both the mid and distal portions of the right ICA. The patient was sent for a vascular evaluation 2 days following the CUS.
Based on the ocular findings and CUS results, the diagnosis of hypoperfusion retinopathy secondary to carotid occlusive disease was made. Because the patient was asymptomatic with no additional ocular sequelae, he was scheduled for an eye clinic follow-up in 2 months. The electrocardiogram, chest X-ray, and exercise stress test results were negative for acute cardiopulmonary disease, ischemia, or arrhythmias. A computed tomography angiography was performed and confirmed a high-grade lesion of the right ICA of > 95%. The vascular surgeon reported an 11% risk of stroke within 5 years and a 1% risk of stroke with surgery. Based on these results the patient underwent a right carotid endarterectomy (CEA) 2 weeks later. A follow-up CUS was performed 1 month post-CEA and revealed no abnormal fluid or significant plaque with a PSV of 92 cm/s (prior to surgery PSV was 508 cm/s) (Figure 3).
The patient returned to the eye clinic 1 month after the CEA. Gonioscopy revealed no neovascularization of the iris or angle and the dilated eye exam showed resolution of the midperipheral blot hemorrhages in his right eye with no evidence of retinal neovascularization.
Discussion
Hypoperfusion retinopathy is characterized by posterior retinal changes secondary to chronic ocular ischemia from decreased arterial perfusion related to significant or complete carotid artery stenosis.1-5 Early literature referred to this condition as venous stasis retinopathy; however, this term is misleading as the condition results from a reduction in arterial perfusion pressure and the term describes venous outflow obstruction.6 The terms carotid ischemic retinopathy, ischemic oculopathy, and hypotensive retinopathy also have been used interchangeably when describing hypoperfusion retinopathy.6
Incidence of hypoperfusion retinopathy is twice as high in males as it is in females due to a higher prevalence of cardiovascular disease.7 Hypoperfusion retinopathy rarely presents before the age of 50 years, with the average age of onset around 65 years.7 The exact rate of occurrence is unknown as this condition often is underdiagnosed because it mimics other vascular conditions, such as venous occlusive disease and diabetic retinopathy.1,7 Patients can present asymptomatically where findings are incidental on a dilated eye exam, or they may present with vision loss that can be gradual, sudden, or transient in nature.5,6,8
Gradual vision loss can follow a period of weeks to months and can occur secondary to posterior ischemia, macular edema, or choroidal hypoperfusion.1,3,8,9 Sudden vision loss can occur from severe hypoperfusion, creating an acute inner layer retinal ischemia. This type of vision loss often is accompanied by a cherry red spot in the macula and can be caused by an embolic plaque.1,8 Transient vision loss (TVL) also can be secondary to a plaque emboli or light induced. Patients with light-induced TVL report poor to blurry vision or prolonged after image when exposed to bright lights. In theory when the retina is exposed to light, there is an increase in metabolic demand that is unmet in those with choroidal vascular insufficiency from significant carotid stenosis.3,8,10
The clinical presentation most often is unilateral. Early stages of the disease generally affect the midperipheral retina but can be found in the posterior pole with chronicity. Early findings include microaneurysms, nerve fiber layer and inner retinal layer hemorrhages, and dilated, but generally not tortuous, veins.5 Chronic stage findings include arteriolar narrowing, extreme venous dilation, occasionally macular edema, and neovascularization of the disc and or retina.5 Disc edema or collaterals usually are not present.5
The mechanism behind hypoperfusion retinopathy results from an overall ischemic cascade and starts with comorbid cardiovascular conditions, such as hypertension, hypercholesterolemia, diabetes, heart disease, and history of smoking.1,2,5 These conditions play a role in creating atherosclerotic buildup in the arterial lumen leading to chronic narrowing and a decrease in arterial perfusion pressure. Over time, a low-grade hypoxic situation is formed, generating vascular endothelial cell damage and pericytes cell loss, thus causing leakage of fluid.1,2,5 With these chronic hypoxic states, angiogenic factor release eventually leads to posterior neovascularization.1,2,5 Further chronicity of carotid occlusive disease can create a panocular ischemia that also involves anterior structures, including iris, conjunctiva, episclera, or cornea. At this point, hypoperfusion retinopathy progresses to a more severe condition called ocular ischemic syndrome (OIS).2,5
Ocular ischemic syndrome can be associated with a 40% mortality rate within 5 years of onset as it is generally found in those with overall poor health.5 Along with posterior neovascularization, anterior structures also are involved. Sixty-seven percent of cases have iris or angle neovascularization of which 35% go on to develop neovascular glaucoma and its complications.1,8 With OIS, 90% of cases have some type of vision loss, and 40% report ipsilateral ocular pain.1,8 Visual loss can be gradual, sudden, or transient. The pain can occur from ocular ischemia, ruptured corneal epithelial microcysts secondary to acute glaucoma, elevated intraocular pressure (IOP) with neovascular glaucoma, or from ipsilateral dural ischemia.1,5,6,8 Fluorescein angiography is commonly used to diagnose and manage OIS, because it allows for the visualization of retinal and choroidal circulation and the detection of neovascular proliferation and ischemic areas.
Diagnostic Imaging
Several diagnostic testing strategies are available to evaluate for carotid occlusive disease. Carotid ultrasonography is a noninvasive, safe, and inexpensive screening tool to evaluate for high-grade stenosis. However, it can sometimes overestimate the degree of stenosis and is not reliable with severe calcifications.8 Computed tomography angiography and magnetic resonance angiography are minimally invasive tools that can be used to screen or confirm the degree of stenosis.8 These can be used in addition or instead of ultrasonography, especially in instances where patients have a short neck or high carotid bifurcation that may affect reliability. Both are contraindicated in those with renal failure as both modalities require the use of a contrast dye. Magnetic resonance angiography is far more expensive, time consuming, and not readily available.8 Carotid angiography is considered the gold standard for imaging the entire carotid artery system because it allows for the evaluation of plaque morphology, atherosclerotic disease, and collateral circulations.8 The disadvantages to this invasive and high-cost procedure include a risk of mortality that can occur secondary to an embolic stroke, myocardial infarction (MI), carotid artery dissection, or arterial thrombosis.8
Treatment
Treatment and management for carotid artery stenosis is focused on combined effort with the patient’s PCP and other specialists, including cardiologist, neurologist, and vascular surgeons.11 Treatment of comorbid conditions, education on healthy lifestyle, and smoking cessation are all imperative to the patient’s well-being. Managing ocular sequelae is based on specific findings and can include intravitreal antivascular edothelial growth factor or steroidal injections, pan retinal photocoagulation, or hypotensive drops.6,7
Restoration of arterial perfusion pressure is the main goal of treatment, and this can be done through CEA or carotid artery stents. Surgical intervention by CEA is determined based on each patient and his or her overall health. A full cardiac workup is required due to surgical risks. The North American Symptomatic Carotid Endarterectomy Trial evaluated symptomatic stenosis and the effectiveness of surgical intervention on stroke prevention. The trial reported that CEA was beneficial in symptomatic patients with 55% to 99% stenosis and especially in those with higher grade stenosis (> 70% up to 95%).5,7,8,12 With regard to asymptomatic patients with high-grade stenosis, CEA has been found to reduce the risk of stroke if there is at least 60% stenosis.5,7,8
Carotid artery stents can be used as an alternative when CEA is not effective or contraindicated due to a history of previous CEA, neck radiation, unstable angina, congestive heart failure, or recent MI.5,7,8 Neither CEA nor stenting is considered effective in complete occlusions due to the high risk of thromboembolism formation.5,7,8
Conclusion
Hypoperfusion retinopathy describes posterior retinal findings that occur secondary to poor arterial perfusion caused by carotid occlusive disease. Early intervention and restoration of this pressure can prevent the risk of developing a more serious condition characterized by a panocular ischemia called OIS. Unlike hypoperfusion retinopathy, OIS also includes anterior segment findings such as iris neovascularization, which may lead to neovascular glaucoma, whereas hypoperfusion retinopathy is localized to the posterior pole. Patients that develop OIS are at a 40% risk of mortality within 5 years due to poor overall health. Understanding the patient’s signs and symptoms can aid in the diagnosis of both conditions. Collaborative management with the patient’s PCP and specialists in treating comorbid conditions is vital to the patients’ well-being.
1. Brown GC, Magargal LE. The ocular ischemic syndrome. Int Ophthalmol. 1988;11(4):239-251.
2. Dahlman AH, McCormack D, Harrison RJ. Bilateral hypoperfuion retinopathy. J R Soc Med. 2001; 94(6):298-299.
3. Dugan JD Jr, Green WR. Ophthalmologic manifestations of carotid occlusive disease. Eye (Lond). 1991;5(pt 2):226-238.
4. Klijn CJ, Kappelle LJ, Tulleken CAF, van Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke. 1997;28(10):2084-2093.
5. McCrary JA III. Venous stasis retinopathy of stenotic or occlusive caroid origin. J Clin Neuroophthalmol. 1989;9(3):195-199.
6. Sanborn GE, Magargal LE. Arterial obstructive disease of the eye. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. 12th ed. Vol 3. Riverwoods, IL: Lippincott Williams & Wilkins; 2013:chap 14.
7. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome–a systematic review. Med Sci Monit. 2012;18(8):RA138-RA144.
8. Atebara NH, Brown GC. The ocular ischemic syndrome. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. 12th ed. Vol 3. Riverwoods, IL: Lippincott Williams & Wilkins; 2013:chap 12.
9. Ho AC, Lieb WE, Flaharty PM, et al. Color Doppler imaging of the ocular ischaemic syndrome. Ophthalmology. 1992;99(9):1453-1462.
10. Kahn M, Green WR, Knox DL, Miller NR. Ocular features of carotid occlusive disease. Retina. 1986;6(4):239-252.
11. Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104(5):859-864.
12. Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30(9):1751-1758.
Cardiovascular diseases are some of the most common conditions found in the geriatric population. Ocular manifestations of systemic cardiovascular conditions often are the initial presentation of the systemic disease. Identifying these findings help reveal the underlying disease and prevent more serious visual and systemic complications or even death.
Hypoperfusion retinopathy can occur as an early manifestation of carotid occlusive disease. It results from poor arterial perfusion pressure secondary to significant or complete carotid artery blockage resulting in retinal cha
Case Report
A 71-year-old white male was referred by his primary care physician (PCP) to the eye clinic for a routine comprehensive eye exam. The patient reported that his current progressive lenses, prescribed 2 years prior, were not strong enough at both distance and near, and that his eyes often felt dry. The symptoms were gradual in onset since his prior exam with no reported flashes, floaters, loss of vision, headaches, or ocular irritations.
The patient’s medical history was significant for morbid obesity, hypertension, borderline diabetes mellitus, and obstructive sleep apnea. His ocular history included recurrent conjunctivitis. At the time of the visit, the patient’s medications included 81 mg aspirin, 10 mg benazepril, 1,000 mg fish oil, 80 mg simvastatin, and use of a continuous positive airway pressure machine.
Best-corrected Snellen visual acuity was stable to his last eye exam at 20/25+2 right eye and 20/25-1 left eye with a manifest refraction of +2.25-0.75 × 077, and +2.75-1.25 × 096 in the right and left eye, respectively. Pupils were equally round and reactive to light with no afferent pupillary defect. Extraocular motility and finger counting fields were unremarkable. Anterior segment evaluation revealed lax bilateral upper lid apposition and mild cataracts in both eyes but were otherwise unremarkable (Figure 1). Dilated fundus examination revealed extensive hemorrhaging in the midperipheral retina of the right eye only (Figure 2). The left eye retina showed no abnormalities.
At this point the patient declined any additional symptoms, including eye pain, headache, transient vision loss, jaw claudication, and stroke signs. A complete blood count and hemoglobin A1c (HbA1c) was ordered, and all findings were unremarkable with no evidence of blood dyscrasia and with a HbA1c of 6.0. A carotid ultrasound (CUS) was also performed and revealed severe narrowing of the proximal section of the right internal carotid artery (ICA) with a trickle flow (Figure 3). The peak systolic velocity (PSV) at this level was 508 cm/s. There also was severe narrowing and turbulent flow in both the mid and distal portions of the right ICA. The patient was sent for a vascular evaluation 2 days following the CUS.
Based on the ocular findings and CUS results, the diagnosis of hypoperfusion retinopathy secondary to carotid occlusive disease was made. Because the patient was asymptomatic with no additional ocular sequelae, he was scheduled for an eye clinic follow-up in 2 months. The electrocardiogram, chest X-ray, and exercise stress test results were negative for acute cardiopulmonary disease, ischemia, or arrhythmias. A computed tomography angiography was performed and confirmed a high-grade lesion of the right ICA of > 95%. The vascular surgeon reported an 11% risk of stroke within 5 years and a 1% risk of stroke with surgery. Based on these results the patient underwent a right carotid endarterectomy (CEA) 2 weeks later. A follow-up CUS was performed 1 month post-CEA and revealed no abnormal fluid or significant plaque with a PSV of 92 cm/s (prior to surgery PSV was 508 cm/s) (Figure 3).
The patient returned to the eye clinic 1 month after the CEA. Gonioscopy revealed no neovascularization of the iris or angle and the dilated eye exam showed resolution of the midperipheral blot hemorrhages in his right eye with no evidence of retinal neovascularization.
Discussion
Hypoperfusion retinopathy is characterized by posterior retinal changes secondary to chronic ocular ischemia from decreased arterial perfusion related to significant or complete carotid artery stenosis.1-5 Early literature referred to this condition as venous stasis retinopathy; however, this term is misleading as the condition results from a reduction in arterial perfusion pressure and the term describes venous outflow obstruction.6 The terms carotid ischemic retinopathy, ischemic oculopathy, and hypotensive retinopathy also have been used interchangeably when describing hypoperfusion retinopathy.6
Incidence of hypoperfusion retinopathy is twice as high in males as it is in females due to a higher prevalence of cardiovascular disease.7 Hypoperfusion retinopathy rarely presents before the age of 50 years, with the average age of onset around 65 years.7 The exact rate of occurrence is unknown as this condition often is underdiagnosed because it mimics other vascular conditions, such as venous occlusive disease and diabetic retinopathy.1,7 Patients can present asymptomatically where findings are incidental on a dilated eye exam, or they may present with vision loss that can be gradual, sudden, or transient in nature.5,6,8
Gradual vision loss can follow a period of weeks to months and can occur secondary to posterior ischemia, macular edema, or choroidal hypoperfusion.1,3,8,9 Sudden vision loss can occur from severe hypoperfusion, creating an acute inner layer retinal ischemia. This type of vision loss often is accompanied by a cherry red spot in the macula and can be caused by an embolic plaque.1,8 Transient vision loss (TVL) also can be secondary to a plaque emboli or light induced. Patients with light-induced TVL report poor to blurry vision or prolonged after image when exposed to bright lights. In theory when the retina is exposed to light, there is an increase in metabolic demand that is unmet in those with choroidal vascular insufficiency from significant carotid stenosis.3,8,10
The clinical presentation most often is unilateral. Early stages of the disease generally affect the midperipheral retina but can be found in the posterior pole with chronicity. Early findings include microaneurysms, nerve fiber layer and inner retinal layer hemorrhages, and dilated, but generally not tortuous, veins.5 Chronic stage findings include arteriolar narrowing, extreme venous dilation, occasionally macular edema, and neovascularization of the disc and or retina.5 Disc edema or collaterals usually are not present.5
The mechanism behind hypoperfusion retinopathy results from an overall ischemic cascade and starts with comorbid cardiovascular conditions, such as hypertension, hypercholesterolemia, diabetes, heart disease, and history of smoking.1,2,5 These conditions play a role in creating atherosclerotic buildup in the arterial lumen leading to chronic narrowing and a decrease in arterial perfusion pressure. Over time, a low-grade hypoxic situation is formed, generating vascular endothelial cell damage and pericytes cell loss, thus causing leakage of fluid.1,2,5 With these chronic hypoxic states, angiogenic factor release eventually leads to posterior neovascularization.1,2,5 Further chronicity of carotid occlusive disease can create a panocular ischemia that also involves anterior structures, including iris, conjunctiva, episclera, or cornea. At this point, hypoperfusion retinopathy progresses to a more severe condition called ocular ischemic syndrome (OIS).2,5
Ocular ischemic syndrome can be associated with a 40% mortality rate within 5 years of onset as it is generally found in those with overall poor health.5 Along with posterior neovascularization, anterior structures also are involved. Sixty-seven percent of cases have iris or angle neovascularization of which 35% go on to develop neovascular glaucoma and its complications.1,8 With OIS, 90% of cases have some type of vision loss, and 40% report ipsilateral ocular pain.1,8 Visual loss can be gradual, sudden, or transient. The pain can occur from ocular ischemia, ruptured corneal epithelial microcysts secondary to acute glaucoma, elevated intraocular pressure (IOP) with neovascular glaucoma, or from ipsilateral dural ischemia.1,5,6,8 Fluorescein angiography is commonly used to diagnose and manage OIS, because it allows for the visualization of retinal and choroidal circulation and the detection of neovascular proliferation and ischemic areas.
Diagnostic Imaging
Several diagnostic testing strategies are available to evaluate for carotid occlusive disease. Carotid ultrasonography is a noninvasive, safe, and inexpensive screening tool to evaluate for high-grade stenosis. However, it can sometimes overestimate the degree of stenosis and is not reliable with severe calcifications.8 Computed tomography angiography and magnetic resonance angiography are minimally invasive tools that can be used to screen or confirm the degree of stenosis.8 These can be used in addition or instead of ultrasonography, especially in instances where patients have a short neck or high carotid bifurcation that may affect reliability. Both are contraindicated in those with renal failure as both modalities require the use of a contrast dye. Magnetic resonance angiography is far more expensive, time consuming, and not readily available.8 Carotid angiography is considered the gold standard for imaging the entire carotid artery system because it allows for the evaluation of plaque morphology, atherosclerotic disease, and collateral circulations.8 The disadvantages to this invasive and high-cost procedure include a risk of mortality that can occur secondary to an embolic stroke, myocardial infarction (MI), carotid artery dissection, or arterial thrombosis.8
Treatment
Treatment and management for carotid artery stenosis is focused on combined effort with the patient’s PCP and other specialists, including cardiologist, neurologist, and vascular surgeons.11 Treatment of comorbid conditions, education on healthy lifestyle, and smoking cessation are all imperative to the patient’s well-being. Managing ocular sequelae is based on specific findings and can include intravitreal antivascular edothelial growth factor or steroidal injections, pan retinal photocoagulation, or hypotensive drops.6,7
Restoration of arterial perfusion pressure is the main goal of treatment, and this can be done through CEA or carotid artery stents. Surgical intervention by CEA is determined based on each patient and his or her overall health. A full cardiac workup is required due to surgical risks. The North American Symptomatic Carotid Endarterectomy Trial evaluated symptomatic stenosis and the effectiveness of surgical intervention on stroke prevention. The trial reported that CEA was beneficial in symptomatic patients with 55% to 99% stenosis and especially in those with higher grade stenosis (> 70% up to 95%).5,7,8,12 With regard to asymptomatic patients with high-grade stenosis, CEA has been found to reduce the risk of stroke if there is at least 60% stenosis.5,7,8
Carotid artery stents can be used as an alternative when CEA is not effective or contraindicated due to a history of previous CEA, neck radiation, unstable angina, congestive heart failure, or recent MI.5,7,8 Neither CEA nor stenting is considered effective in complete occlusions due to the high risk of thromboembolism formation.5,7,8
Conclusion
Hypoperfusion retinopathy describes posterior retinal findings that occur secondary to poor arterial perfusion caused by carotid occlusive disease. Early intervention and restoration of this pressure can prevent the risk of developing a more serious condition characterized by a panocular ischemia called OIS. Unlike hypoperfusion retinopathy, OIS also includes anterior segment findings such as iris neovascularization, which may lead to neovascular glaucoma, whereas hypoperfusion retinopathy is localized to the posterior pole. Patients that develop OIS are at a 40% risk of mortality within 5 years due to poor overall health. Understanding the patient’s signs and symptoms can aid in the diagnosis of both conditions. Collaborative management with the patient’s PCP and specialists in treating comorbid conditions is vital to the patients’ well-being.
Cardiovascular diseases are some of the most common conditions found in the geriatric population. Ocular manifestations of systemic cardiovascular conditions often are the initial presentation of the systemic disease. Identifying these findings help reveal the underlying disease and prevent more serious visual and systemic complications or even death.
Hypoperfusion retinopathy can occur as an early manifestation of carotid occlusive disease. It results from poor arterial perfusion pressure secondary to significant or complete carotid artery blockage resulting in retinal cha
Case Report
A 71-year-old white male was referred by his primary care physician (PCP) to the eye clinic for a routine comprehensive eye exam. The patient reported that his current progressive lenses, prescribed 2 years prior, were not strong enough at both distance and near, and that his eyes often felt dry. The symptoms were gradual in onset since his prior exam with no reported flashes, floaters, loss of vision, headaches, or ocular irritations.
The patient’s medical history was significant for morbid obesity, hypertension, borderline diabetes mellitus, and obstructive sleep apnea. His ocular history included recurrent conjunctivitis. At the time of the visit, the patient’s medications included 81 mg aspirin, 10 mg benazepril, 1,000 mg fish oil, 80 mg simvastatin, and use of a continuous positive airway pressure machine.
Best-corrected Snellen visual acuity was stable to his last eye exam at 20/25+2 right eye and 20/25-1 left eye with a manifest refraction of +2.25-0.75 × 077, and +2.75-1.25 × 096 in the right and left eye, respectively. Pupils were equally round and reactive to light with no afferent pupillary defect. Extraocular motility and finger counting fields were unremarkable. Anterior segment evaluation revealed lax bilateral upper lid apposition and mild cataracts in both eyes but were otherwise unremarkable (Figure 1). Dilated fundus examination revealed extensive hemorrhaging in the midperipheral retina of the right eye only (Figure 2). The left eye retina showed no abnormalities.
At this point the patient declined any additional symptoms, including eye pain, headache, transient vision loss, jaw claudication, and stroke signs. A complete blood count and hemoglobin A1c (HbA1c) was ordered, and all findings were unremarkable with no evidence of blood dyscrasia and with a HbA1c of 6.0. A carotid ultrasound (CUS) was also performed and revealed severe narrowing of the proximal section of the right internal carotid artery (ICA) with a trickle flow (Figure 3). The peak systolic velocity (PSV) at this level was 508 cm/s. There also was severe narrowing and turbulent flow in both the mid and distal portions of the right ICA. The patient was sent for a vascular evaluation 2 days following the CUS.
Based on the ocular findings and CUS results, the diagnosis of hypoperfusion retinopathy secondary to carotid occlusive disease was made. Because the patient was asymptomatic with no additional ocular sequelae, he was scheduled for an eye clinic follow-up in 2 months. The electrocardiogram, chest X-ray, and exercise stress test results were negative for acute cardiopulmonary disease, ischemia, or arrhythmias. A computed tomography angiography was performed and confirmed a high-grade lesion of the right ICA of > 95%. The vascular surgeon reported an 11% risk of stroke within 5 years and a 1% risk of stroke with surgery. Based on these results the patient underwent a right carotid endarterectomy (CEA) 2 weeks later. A follow-up CUS was performed 1 month post-CEA and revealed no abnormal fluid or significant plaque with a PSV of 92 cm/s (prior to surgery PSV was 508 cm/s) (Figure 3).
The patient returned to the eye clinic 1 month after the CEA. Gonioscopy revealed no neovascularization of the iris or angle and the dilated eye exam showed resolution of the midperipheral blot hemorrhages in his right eye with no evidence of retinal neovascularization.
Discussion
Hypoperfusion retinopathy is characterized by posterior retinal changes secondary to chronic ocular ischemia from decreased arterial perfusion related to significant or complete carotid artery stenosis.1-5 Early literature referred to this condition as venous stasis retinopathy; however, this term is misleading as the condition results from a reduction in arterial perfusion pressure and the term describes venous outflow obstruction.6 The terms carotid ischemic retinopathy, ischemic oculopathy, and hypotensive retinopathy also have been used interchangeably when describing hypoperfusion retinopathy.6
Incidence of hypoperfusion retinopathy is twice as high in males as it is in females due to a higher prevalence of cardiovascular disease.7 Hypoperfusion retinopathy rarely presents before the age of 50 years, with the average age of onset around 65 years.7 The exact rate of occurrence is unknown as this condition often is underdiagnosed because it mimics other vascular conditions, such as venous occlusive disease and diabetic retinopathy.1,7 Patients can present asymptomatically where findings are incidental on a dilated eye exam, or they may present with vision loss that can be gradual, sudden, or transient in nature.5,6,8
Gradual vision loss can follow a period of weeks to months and can occur secondary to posterior ischemia, macular edema, or choroidal hypoperfusion.1,3,8,9 Sudden vision loss can occur from severe hypoperfusion, creating an acute inner layer retinal ischemia. This type of vision loss often is accompanied by a cherry red spot in the macula and can be caused by an embolic plaque.1,8 Transient vision loss (TVL) also can be secondary to a plaque emboli or light induced. Patients with light-induced TVL report poor to blurry vision or prolonged after image when exposed to bright lights. In theory when the retina is exposed to light, there is an increase in metabolic demand that is unmet in those with choroidal vascular insufficiency from significant carotid stenosis.3,8,10
The clinical presentation most often is unilateral. Early stages of the disease generally affect the midperipheral retina but can be found in the posterior pole with chronicity. Early findings include microaneurysms, nerve fiber layer and inner retinal layer hemorrhages, and dilated, but generally not tortuous, veins.5 Chronic stage findings include arteriolar narrowing, extreme venous dilation, occasionally macular edema, and neovascularization of the disc and or retina.5 Disc edema or collaterals usually are not present.5
The mechanism behind hypoperfusion retinopathy results from an overall ischemic cascade and starts with comorbid cardiovascular conditions, such as hypertension, hypercholesterolemia, diabetes, heart disease, and history of smoking.1,2,5 These conditions play a role in creating atherosclerotic buildup in the arterial lumen leading to chronic narrowing and a decrease in arterial perfusion pressure. Over time, a low-grade hypoxic situation is formed, generating vascular endothelial cell damage and pericytes cell loss, thus causing leakage of fluid.1,2,5 With these chronic hypoxic states, angiogenic factor release eventually leads to posterior neovascularization.1,2,5 Further chronicity of carotid occlusive disease can create a panocular ischemia that also involves anterior structures, including iris, conjunctiva, episclera, or cornea. At this point, hypoperfusion retinopathy progresses to a more severe condition called ocular ischemic syndrome (OIS).2,5
Ocular ischemic syndrome can be associated with a 40% mortality rate within 5 years of onset as it is generally found in those with overall poor health.5 Along with posterior neovascularization, anterior structures also are involved. Sixty-seven percent of cases have iris or angle neovascularization of which 35% go on to develop neovascular glaucoma and its complications.1,8 With OIS, 90% of cases have some type of vision loss, and 40% report ipsilateral ocular pain.1,8 Visual loss can be gradual, sudden, or transient. The pain can occur from ocular ischemia, ruptured corneal epithelial microcysts secondary to acute glaucoma, elevated intraocular pressure (IOP) with neovascular glaucoma, or from ipsilateral dural ischemia.1,5,6,8 Fluorescein angiography is commonly used to diagnose and manage OIS, because it allows for the visualization of retinal and choroidal circulation and the detection of neovascular proliferation and ischemic areas.
Diagnostic Imaging
Several diagnostic testing strategies are available to evaluate for carotid occlusive disease. Carotid ultrasonography is a noninvasive, safe, and inexpensive screening tool to evaluate for high-grade stenosis. However, it can sometimes overestimate the degree of stenosis and is not reliable with severe calcifications.8 Computed tomography angiography and magnetic resonance angiography are minimally invasive tools that can be used to screen or confirm the degree of stenosis.8 These can be used in addition or instead of ultrasonography, especially in instances where patients have a short neck or high carotid bifurcation that may affect reliability. Both are contraindicated in those with renal failure as both modalities require the use of a contrast dye. Magnetic resonance angiography is far more expensive, time consuming, and not readily available.8 Carotid angiography is considered the gold standard for imaging the entire carotid artery system because it allows for the evaluation of plaque morphology, atherosclerotic disease, and collateral circulations.8 The disadvantages to this invasive and high-cost procedure include a risk of mortality that can occur secondary to an embolic stroke, myocardial infarction (MI), carotid artery dissection, or arterial thrombosis.8
Treatment
Treatment and management for carotid artery stenosis is focused on combined effort with the patient’s PCP and other specialists, including cardiologist, neurologist, and vascular surgeons.11 Treatment of comorbid conditions, education on healthy lifestyle, and smoking cessation are all imperative to the patient’s well-being. Managing ocular sequelae is based on specific findings and can include intravitreal antivascular edothelial growth factor or steroidal injections, pan retinal photocoagulation, or hypotensive drops.6,7
Restoration of arterial perfusion pressure is the main goal of treatment, and this can be done through CEA or carotid artery stents. Surgical intervention by CEA is determined based on each patient and his or her overall health. A full cardiac workup is required due to surgical risks. The North American Symptomatic Carotid Endarterectomy Trial evaluated symptomatic stenosis and the effectiveness of surgical intervention on stroke prevention. The trial reported that CEA was beneficial in symptomatic patients with 55% to 99% stenosis and especially in those with higher grade stenosis (> 70% up to 95%).5,7,8,12 With regard to asymptomatic patients with high-grade stenosis, CEA has been found to reduce the risk of stroke if there is at least 60% stenosis.5,7,8
Carotid artery stents can be used as an alternative when CEA is not effective or contraindicated due to a history of previous CEA, neck radiation, unstable angina, congestive heart failure, or recent MI.5,7,8 Neither CEA nor stenting is considered effective in complete occlusions due to the high risk of thromboembolism formation.5,7,8
Conclusion
Hypoperfusion retinopathy describes posterior retinal findings that occur secondary to poor arterial perfusion caused by carotid occlusive disease. Early intervention and restoration of this pressure can prevent the risk of developing a more serious condition characterized by a panocular ischemia called OIS. Unlike hypoperfusion retinopathy, OIS also includes anterior segment findings such as iris neovascularization, which may lead to neovascular glaucoma, whereas hypoperfusion retinopathy is localized to the posterior pole. Patients that develop OIS are at a 40% risk of mortality within 5 years due to poor overall health. Understanding the patient’s signs and symptoms can aid in the diagnosis of both conditions. Collaborative management with the patient’s PCP and specialists in treating comorbid conditions is vital to the patients’ well-being.
1. Brown GC, Magargal LE. The ocular ischemic syndrome. Int Ophthalmol. 1988;11(4):239-251.
2. Dahlman AH, McCormack D, Harrison RJ. Bilateral hypoperfuion retinopathy. J R Soc Med. 2001; 94(6):298-299.
3. Dugan JD Jr, Green WR. Ophthalmologic manifestations of carotid occlusive disease. Eye (Lond). 1991;5(pt 2):226-238.
4. Klijn CJ, Kappelle LJ, Tulleken CAF, van Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke. 1997;28(10):2084-2093.
5. McCrary JA III. Venous stasis retinopathy of stenotic or occlusive caroid origin. J Clin Neuroophthalmol. 1989;9(3):195-199.
6. Sanborn GE, Magargal LE. Arterial obstructive disease of the eye. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. 12th ed. Vol 3. Riverwoods, IL: Lippincott Williams & Wilkins; 2013:chap 14.
7. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome–a systematic review. Med Sci Monit. 2012;18(8):RA138-RA144.
8. Atebara NH, Brown GC. The ocular ischemic syndrome. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. 12th ed. Vol 3. Riverwoods, IL: Lippincott Williams & Wilkins; 2013:chap 12.
9. Ho AC, Lieb WE, Flaharty PM, et al. Color Doppler imaging of the ocular ischaemic syndrome. Ophthalmology. 1992;99(9):1453-1462.
10. Kahn M, Green WR, Knox DL, Miller NR. Ocular features of carotid occlusive disease. Retina. 1986;6(4):239-252.
11. Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104(5):859-864.
12. Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30(9):1751-1758.
1. Brown GC, Magargal LE. The ocular ischemic syndrome. Int Ophthalmol. 1988;11(4):239-251.
2. Dahlman AH, McCormack D, Harrison RJ. Bilateral hypoperfuion retinopathy. J R Soc Med. 2001; 94(6):298-299.
3. Dugan JD Jr, Green WR. Ophthalmologic manifestations of carotid occlusive disease. Eye (Lond). 1991;5(pt 2):226-238.
4. Klijn CJ, Kappelle LJ, Tulleken CAF, van Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke. 1997;28(10):2084-2093.
5. McCrary JA III. Venous stasis retinopathy of stenotic or occlusive caroid origin. J Clin Neuroophthalmol. 1989;9(3):195-199.
6. Sanborn GE, Magargal LE. Arterial obstructive disease of the eye. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. 12th ed. Vol 3. Riverwoods, IL: Lippincott Williams & Wilkins; 2013:chap 14.
7. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome–a systematic review. Med Sci Monit. 2012;18(8):RA138-RA144.
8. Atebara NH, Brown GC. The ocular ischemic syndrome. In: Tasman W, Jaeger EA, eds. Duane’s Ophthalmology. 12th ed. Vol 3. Riverwoods, IL: Lippincott Williams & Wilkins; 2013:chap 12.
9. Ho AC, Lieb WE, Flaharty PM, et al. Color Doppler imaging of the ocular ischaemic syndrome. Ophthalmology. 1992;99(9):1453-1462.
10. Kahn M, Green WR, Knox DL, Miller NR. Ocular features of carotid occlusive disease. Retina. 1986;6(4):239-252.
11. Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104(5):859-864.
12. Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30(9):1751-1758.
Disease burden impacts outcome of CAR T-cell therapy in B-ALL
WASHINGTON, DC—Results of a retrospective study suggest pretreatment disease burden impacts the outcome of chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Patients who had minimal residual disease (MRD) prior to treatment had superior event-free and overall survival compared to patients who had morphologic disease before treatment.
Patients with MRD were also less likely to experience cytokine release syndrome (CRS) and neurologic toxicity.
Jae Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, presented these results at the AACR Annual Meeting 2017 (abstract CT078).
This study was funded by Juno Therapeutics, the National Cancer Institute, the Terry Fox Foundation, and MSKCC Experimental Therapeutics Center.
“[W]e and other groups have developed and tested CD19-specific [19-28z] CAR T-cell therapy and have reported encouraging results, with high initial complete response rates in patients with B-ALL,” Dr Park said.
“However, relapses are common, even after achieving seemingly deep remission, and severe toxicities have been observed in some patients.”
To gain more insight into these results, Dr Park and his colleagues retrospectively analyzed data from a prospective clinical trial that tested 19-28z CAR T-cell therapy in patients with B-ALL.
All 51 adults in this trial had relapsed after or were refractory to 1 or more conventional multiagent chemotherapy regimens.
The researchers measured disease burden prior to CAR T-cell infusion in all patients and divided them into 2 cohorts:
- 20 patients who had MRD—less than 5% blasts in the bone marrow
- 31 patients who had morphologic disease—5% or more blasts in the bone marrow.
Response and survival
The complete response rate was 95% in the MRD cohort and 77% in the morphologic disease cohort, a difference that was not statistically significant.
At a median follow-up of 18 months, the median event-free survival and overall survival had not been reached for patients in the MRD cohort (because most were still alive and disease-free).
However, for patients in the morphologic disease cohort, the median event-free survival was 6.3 months (P=0.0005), and the median overall survival was 17 months (P=0.0189).
Role of transplant
The researchers found that long-term survival did not improve for patients who proceeded to hematopoietic stem cell transplant (HSCT), regardless of their disease burden at baseline.
“While more patients and longer follow-up will be needed to adequately address the significance of HSCT, the result of this analysis raises a question as to whether 19-28z CAR therapy can be considered as a definitive, curative therapy rather than a bridge to stem cell transplant, at least in a subset of patients,” Dr Park noted.
“Our data suggest that incorporation of 19-28z CAR T cells at the time of MRD following first-line chemotherapy will maximize the durability of CAR T-cell-mediated remissions and survival and can potentially spare these high-risk patients from HSCT, rather than waiting until they relapse morphologically and then trying CAR T-cell therapy when it is less likely to achieve a durable long-term outcome.”
Adverse events and limitations
Patients from the MRD cohort fared better than the morphologic disease cohort in terms of CRS and neurologic toxicity.
Forty-two percent of patients in the morphologic disease cohort developed CRS, compared to 5% of patients in the MRD cohort (P=0.0326).
Neurologic toxicity occurred in 58% of patients in the morphologic disease cohort and 15% of those in the MRD cohort (P=0.0001).
Dr Park noted that a limitation of this study is its retrospective nature, and the findings will need to be validated prospectively.
Furthermore, the analysis on the impact of allogeneic HSCT was limited by a relatively small sample size in each cohort. ![]()
WASHINGTON, DC—Results of a retrospective study suggest pretreatment disease burden impacts the outcome of chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Patients who had minimal residual disease (MRD) prior to treatment had superior event-free and overall survival compared to patients who had morphologic disease before treatment.
Patients with MRD were also less likely to experience cytokine release syndrome (CRS) and neurologic toxicity.
Jae Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, presented these results at the AACR Annual Meeting 2017 (abstract CT078).
This study was funded by Juno Therapeutics, the National Cancer Institute, the Terry Fox Foundation, and MSKCC Experimental Therapeutics Center.
“[W]e and other groups have developed and tested CD19-specific [19-28z] CAR T-cell therapy and have reported encouraging results, with high initial complete response rates in patients with B-ALL,” Dr Park said.
“However, relapses are common, even after achieving seemingly deep remission, and severe toxicities have been observed in some patients.”
To gain more insight into these results, Dr Park and his colleagues retrospectively analyzed data from a prospective clinical trial that tested 19-28z CAR T-cell therapy in patients with B-ALL.
All 51 adults in this trial had relapsed after or were refractory to 1 or more conventional multiagent chemotherapy regimens.
The researchers measured disease burden prior to CAR T-cell infusion in all patients and divided them into 2 cohorts:
- 20 patients who had MRD—less than 5% blasts in the bone marrow
- 31 patients who had morphologic disease—5% or more blasts in the bone marrow.
Response and survival
The complete response rate was 95% in the MRD cohort and 77% in the morphologic disease cohort, a difference that was not statistically significant.
At a median follow-up of 18 months, the median event-free survival and overall survival had not been reached for patients in the MRD cohort (because most were still alive and disease-free).
However, for patients in the morphologic disease cohort, the median event-free survival was 6.3 months (P=0.0005), and the median overall survival was 17 months (P=0.0189).
Role of transplant
The researchers found that long-term survival did not improve for patients who proceeded to hematopoietic stem cell transplant (HSCT), regardless of their disease burden at baseline.
“While more patients and longer follow-up will be needed to adequately address the significance of HSCT, the result of this analysis raises a question as to whether 19-28z CAR therapy can be considered as a definitive, curative therapy rather than a bridge to stem cell transplant, at least in a subset of patients,” Dr Park noted.
“Our data suggest that incorporation of 19-28z CAR T cells at the time of MRD following first-line chemotherapy will maximize the durability of CAR T-cell-mediated remissions and survival and can potentially spare these high-risk patients from HSCT, rather than waiting until they relapse morphologically and then trying CAR T-cell therapy when it is less likely to achieve a durable long-term outcome.”
Adverse events and limitations
Patients from the MRD cohort fared better than the morphologic disease cohort in terms of CRS and neurologic toxicity.
Forty-two percent of patients in the morphologic disease cohort developed CRS, compared to 5% of patients in the MRD cohort (P=0.0326).
Neurologic toxicity occurred in 58% of patients in the morphologic disease cohort and 15% of those in the MRD cohort (P=0.0001).
Dr Park noted that a limitation of this study is its retrospective nature, and the findings will need to be validated prospectively.
Furthermore, the analysis on the impact of allogeneic HSCT was limited by a relatively small sample size in each cohort. ![]()
WASHINGTON, DC—Results of a retrospective study suggest pretreatment disease burden impacts the outcome of chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Patients who had minimal residual disease (MRD) prior to treatment had superior event-free and overall survival compared to patients who had morphologic disease before treatment.
Patients with MRD were also less likely to experience cytokine release syndrome (CRS) and neurologic toxicity.
Jae Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, presented these results at the AACR Annual Meeting 2017 (abstract CT078).
This study was funded by Juno Therapeutics, the National Cancer Institute, the Terry Fox Foundation, and MSKCC Experimental Therapeutics Center.
“[W]e and other groups have developed and tested CD19-specific [19-28z] CAR T-cell therapy and have reported encouraging results, with high initial complete response rates in patients with B-ALL,” Dr Park said.
“However, relapses are common, even after achieving seemingly deep remission, and severe toxicities have been observed in some patients.”
To gain more insight into these results, Dr Park and his colleagues retrospectively analyzed data from a prospective clinical trial that tested 19-28z CAR T-cell therapy in patients with B-ALL.
All 51 adults in this trial had relapsed after or were refractory to 1 or more conventional multiagent chemotherapy regimens.
The researchers measured disease burden prior to CAR T-cell infusion in all patients and divided them into 2 cohorts:
- 20 patients who had MRD—less than 5% blasts in the bone marrow
- 31 patients who had morphologic disease—5% or more blasts in the bone marrow.
Response and survival
The complete response rate was 95% in the MRD cohort and 77% in the morphologic disease cohort, a difference that was not statistically significant.
At a median follow-up of 18 months, the median event-free survival and overall survival had not been reached for patients in the MRD cohort (because most were still alive and disease-free).
However, for patients in the morphologic disease cohort, the median event-free survival was 6.3 months (P=0.0005), and the median overall survival was 17 months (P=0.0189).
Role of transplant
The researchers found that long-term survival did not improve for patients who proceeded to hematopoietic stem cell transplant (HSCT), regardless of their disease burden at baseline.
“While more patients and longer follow-up will be needed to adequately address the significance of HSCT, the result of this analysis raises a question as to whether 19-28z CAR therapy can be considered as a definitive, curative therapy rather than a bridge to stem cell transplant, at least in a subset of patients,” Dr Park noted.
“Our data suggest that incorporation of 19-28z CAR T cells at the time of MRD following first-line chemotherapy will maximize the durability of CAR T-cell-mediated remissions and survival and can potentially spare these high-risk patients from HSCT, rather than waiting until they relapse morphologically and then trying CAR T-cell therapy when it is less likely to achieve a durable long-term outcome.”
Adverse events and limitations
Patients from the MRD cohort fared better than the morphologic disease cohort in terms of CRS and neurologic toxicity.
Forty-two percent of patients in the morphologic disease cohort developed CRS, compared to 5% of patients in the MRD cohort (P=0.0326).
Neurologic toxicity occurred in 58% of patients in the morphologic disease cohort and 15% of those in the MRD cohort (P=0.0001).
Dr Park noted that a limitation of this study is its retrospective nature, and the findings will need to be validated prospectively.
Furthermore, the analysis on the impact of allogeneic HSCT was limited by a relatively small sample size in each cohort. ![]()
FDA clears direct-to-consumer marketing of genetic risk tests
The US Food and Drug Administration (FDA) has authorized marketing of 23andMe Personal Genome Service (PGS) Genetic Health Risk (GHR) tests for 10 medical conditions.
These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual’s genetic predisposition to certain conditions, including factor XI deficiency, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hereditary hemochromatosis, hereditary thrombophilia, and other conditions.
The GHR tests work by isolating DNA from a saliva sample, which is then tested for more than 500,000 genetic variants.
Consumers receive reports of the results, which tell them if they have an increased risk of developing any of the following 10 conditions.
Factor XI deficiency
The 23andMe PGS Genetic Health Risk Report for Factor XI Deficiency is indicated for reporting of the F283L, E117X, and IVS14+1G>A variants in the F11 gene.
This report describes if a person has a variant associated with factor XI deficiency and the potential for a higher risk of excessive bleeding following trauma or surgery, but it does not describe a person’s overall risk for excessive bleeding. This report is most relevant for people of Ashkenazi Jewish descent.
G6PD deficiency
The 23andMe PGS Genetic Health Risk Report for Glucose-6-Phosphate-Dehydrogenase Deficiency is indicated for reporting of the Val68Met variant in the G6PD gene.
This report describes if a person has a variant associated with G6PD deficiency and a higher risk for episodes of anemia, but it does not describe a person’s overall risk of developing anemia. This report is most relevant for people of African descent.
Hereditary hemochromatosis
The 23andMe PGS Genetic Health Risk Report for Hereditary Hemochromatosis is indicated for reporting of the C282Y and H63D variants in the HFE gene.
This report describes if a person has variants associated with hereditary hemochromatosis and a higher risk for iron overload, but it does not describe a person’s overall risk of developing iron overload. This report is most relevant for people of European descent.
Hereditary thrombophilia
The 23andMe PGS Genetic Health Risk Report for Hereditary Thrombophilia is indicated for reporting of the factor V Leiden variant in the F5 gene, as well as the prothrombin G20210A variant in the F2 gene.
This report describes if a person has variants associated with a higher risk of thrombosis, but it does not describe a person’s overall risk of developing thrombosis. This report is most relevant for people of European descent.
Alpha-1 antitrypsin deficiency (AATD)
The 23andMe PGS Genetic Health Risk Report for Alpha-I Antitrypsin Deficiency is indicated for reporting of the PI*Z and PI*S variants in the SERPINA1 gene.
This report describes if a person has variants associated with AATD and a higher risk for lung or liver disease, but it does not describe a person’s overall risk of developing lung or liver disease. This report is most relevant for people of European descent.
Celiac disease
The 23andMe PGS Genetic Health Risk Report for Celiac Disease is indicated for reporting of a variant in the HLA-DQ2.5 haplotype.
The report describes if a person has a haplotype associated with an increased risk of developing celiac disease, but it does not describe a person’s overall risk for developing celiac disease. This report is most relevant for people of European descent.
Early onset primary dystonia (DYT1/TOR1A-related)
The 23andMe PGS Genetic Health Risk Report for Early-Onset Primary Dystonia (DYT1/TOR1A-Related) is indicated for reporting of the deltaE302/303 variant in the DYT1 gene.
This report describes if a person has variants associated with a higher risk for early-onset primary dystonia, but it does not describe a person’s overall risk of developing dystonia. This report is most relevant for people of Ashkenazi Jewish descent.
Gaucher disease
The 23andMe PGS Genetic Health Risk Report for Gaucher Disease Type 1 is indicated for reporting of the N370S, 84GG, and V394L variants in the GBA gene.
This report describes if a person has variants associated with an increased risk for developing carrier status for Gaucher disease type 1 in adults. This report also describes if a result is associated with personal risk for developing symptoms of Gaucher disease type 1, but it does not describe a person’s overall risk of developing Gaucher disease type 1.
This test is most relevant for people of Ashkenazi Jewish descent.
Late-onset Alzheimer’s disease
The 23andMe PGS Genetic Health Risk Report for Late-onset Alzheimer’s Disease is indicated for reporting of the ε4 variant in the APOE gene.
The report describes if a person’s genetic result is associated with an increased risk of developing late-onset Alzheimer’s disease, but it does not describe a person’s overall risk of developing Alzheimer’s disease.
The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Parkinson’s disease
The 23andMe PGS Genetic Health Risk Report for Parkinson’s Disease is indicated for reporting of the G2019S variant in the LRRK2 gene and the N370S variant in the GBA gene.
The report describes if a person’s genetic result is associated with an increased risk of developing Parkinson’s disease, but it does not describe a person’s overall risk of developing Parkinson’s disease. The test is most relevant for people of European, Ashkenazi Jewish, and North African Berber descent.
Access to testing
23andMe, Inc. said it will release its first set of GHR tests—for hereditary thrombophilia, late-onset Alzheimer’s disease, Parkinson’s disease, alpha-1 antitrypsin deficiency, and Gaucher disease—this month. The remaining tests will follow.
New 23andMe Health + Ancestry Service customers in the US will have access to these tests. Current 23andMe customers will be notified directly regarding their eligibility.
About the marketing authorization
The FDA reviewed data for the 23andMe GHR tests through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the tests’ accuracy, reliability, and clinical relevance. These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for these and similar GHR tests.
The FDA intends to exempt additional 23andMe GHR tests from premarket review, and GHR tests from other makers may be exempt after submitting their first premarket notification. A proposed exemption of this kind would allow other, similar tests to enter the market as quickly as possible after a one-time FDA review.
Excluded from the current marketing authorization and any future, related exemption are GHR tests that function as diagnostic tests. ![]()
The US Food and Drug Administration (FDA) has authorized marketing of 23andMe Personal Genome Service (PGS) Genetic Health Risk (GHR) tests for 10 medical conditions.
These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual’s genetic predisposition to certain conditions, including factor XI deficiency, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hereditary hemochromatosis, hereditary thrombophilia, and other conditions.
The GHR tests work by isolating DNA from a saliva sample, which is then tested for more than 500,000 genetic variants.
Consumers receive reports of the results, which tell them if they have an increased risk of developing any of the following 10 conditions.
Factor XI deficiency
The 23andMe PGS Genetic Health Risk Report for Factor XI Deficiency is indicated for reporting of the F283L, E117X, and IVS14+1G>A variants in the F11 gene.
This report describes if a person has a variant associated with factor XI deficiency and the potential for a higher risk of excessive bleeding following trauma or surgery, but it does not describe a person’s overall risk for excessive bleeding. This report is most relevant for people of Ashkenazi Jewish descent.
G6PD deficiency
The 23andMe PGS Genetic Health Risk Report for Glucose-6-Phosphate-Dehydrogenase Deficiency is indicated for reporting of the Val68Met variant in the G6PD gene.
This report describes if a person has a variant associated with G6PD deficiency and a higher risk for episodes of anemia, but it does not describe a person’s overall risk of developing anemia. This report is most relevant for people of African descent.
Hereditary hemochromatosis
The 23andMe PGS Genetic Health Risk Report for Hereditary Hemochromatosis is indicated for reporting of the C282Y and H63D variants in the HFE gene.
This report describes if a person has variants associated with hereditary hemochromatosis and a higher risk for iron overload, but it does not describe a person’s overall risk of developing iron overload. This report is most relevant for people of European descent.
Hereditary thrombophilia
The 23andMe PGS Genetic Health Risk Report for Hereditary Thrombophilia is indicated for reporting of the factor V Leiden variant in the F5 gene, as well as the prothrombin G20210A variant in the F2 gene.
This report describes if a person has variants associated with a higher risk of thrombosis, but it does not describe a person’s overall risk of developing thrombosis. This report is most relevant for people of European descent.
Alpha-1 antitrypsin deficiency (AATD)
The 23andMe PGS Genetic Health Risk Report for Alpha-I Antitrypsin Deficiency is indicated for reporting of the PI*Z and PI*S variants in the SERPINA1 gene.
This report describes if a person has variants associated with AATD and a higher risk for lung or liver disease, but it does not describe a person’s overall risk of developing lung or liver disease. This report is most relevant for people of European descent.
Celiac disease
The 23andMe PGS Genetic Health Risk Report for Celiac Disease is indicated for reporting of a variant in the HLA-DQ2.5 haplotype.
The report describes if a person has a haplotype associated with an increased risk of developing celiac disease, but it does not describe a person’s overall risk for developing celiac disease. This report is most relevant for people of European descent.
Early onset primary dystonia (DYT1/TOR1A-related)
The 23andMe PGS Genetic Health Risk Report for Early-Onset Primary Dystonia (DYT1/TOR1A-Related) is indicated for reporting of the deltaE302/303 variant in the DYT1 gene.
This report describes if a person has variants associated with a higher risk for early-onset primary dystonia, but it does not describe a person’s overall risk of developing dystonia. This report is most relevant for people of Ashkenazi Jewish descent.
Gaucher disease
The 23andMe PGS Genetic Health Risk Report for Gaucher Disease Type 1 is indicated for reporting of the N370S, 84GG, and V394L variants in the GBA gene.
This report describes if a person has variants associated with an increased risk for developing carrier status for Gaucher disease type 1 in adults. This report also describes if a result is associated with personal risk for developing symptoms of Gaucher disease type 1, but it does not describe a person’s overall risk of developing Gaucher disease type 1.
This test is most relevant for people of Ashkenazi Jewish descent.
Late-onset Alzheimer’s disease
The 23andMe PGS Genetic Health Risk Report for Late-onset Alzheimer’s Disease is indicated for reporting of the ε4 variant in the APOE gene.
The report describes if a person’s genetic result is associated with an increased risk of developing late-onset Alzheimer’s disease, but it does not describe a person’s overall risk of developing Alzheimer’s disease.
The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Parkinson’s disease
The 23andMe PGS Genetic Health Risk Report for Parkinson’s Disease is indicated for reporting of the G2019S variant in the LRRK2 gene and the N370S variant in the GBA gene.
The report describes if a person’s genetic result is associated with an increased risk of developing Parkinson’s disease, but it does not describe a person’s overall risk of developing Parkinson’s disease. The test is most relevant for people of European, Ashkenazi Jewish, and North African Berber descent.
Access to testing
23andMe, Inc. said it will release its first set of GHR tests—for hereditary thrombophilia, late-onset Alzheimer’s disease, Parkinson’s disease, alpha-1 antitrypsin deficiency, and Gaucher disease—this month. The remaining tests will follow.
New 23andMe Health + Ancestry Service customers in the US will have access to these tests. Current 23andMe customers will be notified directly regarding their eligibility.
About the marketing authorization
The FDA reviewed data for the 23andMe GHR tests through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the tests’ accuracy, reliability, and clinical relevance. These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for these and similar GHR tests.
The FDA intends to exempt additional 23andMe GHR tests from premarket review, and GHR tests from other makers may be exempt after submitting their first premarket notification. A proposed exemption of this kind would allow other, similar tests to enter the market as quickly as possible after a one-time FDA review.
Excluded from the current marketing authorization and any future, related exemption are GHR tests that function as diagnostic tests. ![]()
The US Food and Drug Administration (FDA) has authorized marketing of 23andMe Personal Genome Service (PGS) Genetic Health Risk (GHR) tests for 10 medical conditions.
These are the first direct-to-consumer tests authorized by the FDA that provide information on an individual’s genetic predisposition to certain conditions, including factor XI deficiency, glucose-6-phosphate dehydrogenase (G6PD) deficiency, hereditary hemochromatosis, hereditary thrombophilia, and other conditions.
The GHR tests work by isolating DNA from a saliva sample, which is then tested for more than 500,000 genetic variants.
Consumers receive reports of the results, which tell them if they have an increased risk of developing any of the following 10 conditions.
Factor XI deficiency
The 23andMe PGS Genetic Health Risk Report for Factor XI Deficiency is indicated for reporting of the F283L, E117X, and IVS14+1G>A variants in the F11 gene.
This report describes if a person has a variant associated with factor XI deficiency and the potential for a higher risk of excessive bleeding following trauma or surgery, but it does not describe a person’s overall risk for excessive bleeding. This report is most relevant for people of Ashkenazi Jewish descent.
G6PD deficiency
The 23andMe PGS Genetic Health Risk Report for Glucose-6-Phosphate-Dehydrogenase Deficiency is indicated for reporting of the Val68Met variant in the G6PD gene.
This report describes if a person has a variant associated with G6PD deficiency and a higher risk for episodes of anemia, but it does not describe a person’s overall risk of developing anemia. This report is most relevant for people of African descent.
Hereditary hemochromatosis
The 23andMe PGS Genetic Health Risk Report for Hereditary Hemochromatosis is indicated for reporting of the C282Y and H63D variants in the HFE gene.
This report describes if a person has variants associated with hereditary hemochromatosis and a higher risk for iron overload, but it does not describe a person’s overall risk of developing iron overload. This report is most relevant for people of European descent.
Hereditary thrombophilia
The 23andMe PGS Genetic Health Risk Report for Hereditary Thrombophilia is indicated for reporting of the factor V Leiden variant in the F5 gene, as well as the prothrombin G20210A variant in the F2 gene.
This report describes if a person has variants associated with a higher risk of thrombosis, but it does not describe a person’s overall risk of developing thrombosis. This report is most relevant for people of European descent.
Alpha-1 antitrypsin deficiency (AATD)
The 23andMe PGS Genetic Health Risk Report for Alpha-I Antitrypsin Deficiency is indicated for reporting of the PI*Z and PI*S variants in the SERPINA1 gene.
This report describes if a person has variants associated with AATD and a higher risk for lung or liver disease, but it does not describe a person’s overall risk of developing lung or liver disease. This report is most relevant for people of European descent.
Celiac disease
The 23andMe PGS Genetic Health Risk Report for Celiac Disease is indicated for reporting of a variant in the HLA-DQ2.5 haplotype.
The report describes if a person has a haplotype associated with an increased risk of developing celiac disease, but it does not describe a person’s overall risk for developing celiac disease. This report is most relevant for people of European descent.
Early onset primary dystonia (DYT1/TOR1A-related)
The 23andMe PGS Genetic Health Risk Report for Early-Onset Primary Dystonia (DYT1/TOR1A-Related) is indicated for reporting of the deltaE302/303 variant in the DYT1 gene.
This report describes if a person has variants associated with a higher risk for early-onset primary dystonia, but it does not describe a person’s overall risk of developing dystonia. This report is most relevant for people of Ashkenazi Jewish descent.
Gaucher disease
The 23andMe PGS Genetic Health Risk Report for Gaucher Disease Type 1 is indicated for reporting of the N370S, 84GG, and V394L variants in the GBA gene.
This report describes if a person has variants associated with an increased risk for developing carrier status for Gaucher disease type 1 in adults. This report also describes if a result is associated with personal risk for developing symptoms of Gaucher disease type 1, but it does not describe a person’s overall risk of developing Gaucher disease type 1.
This test is most relevant for people of Ashkenazi Jewish descent.
Late-onset Alzheimer’s disease
The 23andMe PGS Genetic Health Risk Report for Late-onset Alzheimer’s Disease is indicated for reporting of the ε4 variant in the APOE gene.
The report describes if a person’s genetic result is associated with an increased risk of developing late-onset Alzheimer’s disease, but it does not describe a person’s overall risk of developing Alzheimer’s disease.
The ε4 variant included in this report is found and has been studied in many ethnicities. Detailed risk estimates have been studied the most in people of European descent.
Parkinson’s disease
The 23andMe PGS Genetic Health Risk Report for Parkinson’s Disease is indicated for reporting of the G2019S variant in the LRRK2 gene and the N370S variant in the GBA gene.
The report describes if a person’s genetic result is associated with an increased risk of developing Parkinson’s disease, but it does not describe a person’s overall risk of developing Parkinson’s disease. The test is most relevant for people of European, Ashkenazi Jewish, and North African Berber descent.
Access to testing
23andMe, Inc. said it will release its first set of GHR tests—for hereditary thrombophilia, late-onset Alzheimer’s disease, Parkinson’s disease, alpha-1 antitrypsin deficiency, and Gaucher disease—this month. The remaining tests will follow.
New 23andMe Health + Ancestry Service customers in the US will have access to these tests. Current 23andMe customers will be notified directly regarding their eligibility.
About the marketing authorization
The FDA reviewed data for the 23andMe GHR tests through the de novo premarket review pathway, a regulatory pathway for novel, low-to-moderate-risk devices that are not substantially equivalent to an already legally marketed device.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the tests’ accuracy, reliability, and clinical relevance. These special controls, when met along with general controls, provide reasonable assurance of safety and effectiveness for these and similar GHR tests.
The FDA intends to exempt additional 23andMe GHR tests from premarket review, and GHR tests from other makers may be exempt after submitting their first premarket notification. A proposed exemption of this kind would allow other, similar tests to enter the market as quickly as possible after a one-time FDA review.
Excluded from the current marketing authorization and any future, related exemption are GHR tests that function as diagnostic tests. ![]()
EC publishes guides on patient blood management
The European Commission (EC) has published guides intended to help hospitals and national authorities implement patient blood management (PBM) programs throughout the European Union.
The EC said its guide for hospitals is intended to help them implement PBM in a practical way, building on already recognized best practices.
The guide is the result of the combined expertise of clinicians and PBM professionals and the experience gathered from a 30-month pilot program implementing PBM in 5 European teaching hospitals.
The EC said this guide is relevant for all medical professionals and organizations involved in caring for patients suffering from anemia, blood loss, and medical conditions that might require transfusion.
The EC’s other PBM guide is intended for national authorities. It lists 10 “essential public health operations,” which encompass a range of activities authorities can engage in to aid the implementation of PBM in their health systems.
The essential public health operations (and examples of activities) are:
- Surveillance—eg, continuously collect patient-level data on anemia, transfusion, and outcomes to measure and guide the implementation of PBM as a standard of care
- Monitoring—eg, recommend the monitoring and flagging of too liberal blood component utilization to prevent health hazards
- Health protection—eg, develop information and education materials for clinicians, quality and safety managers, and hospital administrators
- Health promotion—eg, promote awareness of iron deficiency and iron deficiency anemia
- Disease prevention—eg, create a sense of urgency for PBM as a new evidence-based standard of care through professional training and education
- Governance—eg, create and institute a national PBM steering committee under the authority of the Ministry of Health
- Workforce, equipment, and facilities—eg, provide hospital facilities for PBM
- Organization and funding—eg, organize reallocation of funds and resources toward PBM
- Communication—eg, provide PBM webpage sections for patients, health professionals, and health administrators
- Research—eg, conduct PBM-related studies focusing on patient outcomes, cost-effectiveness, etc.
The EC said the publication of their PBM guides is timely, as the journal Transfusion recently published results from a 5-year PBM program in Western Australia, which is the world’s largest PBM program to date. The program included 605,046 patients admitted to 4 major adult tertiary-care hospitals.
The use of blood products was reduced by 41% during the study period. The program also resulted in a 28% reduction in hospital mortality, a 15% reduction in the average hospital length of stay, a 21% decrease in hospital-acquired infections, and a 31% decrease in the incidence of heart attack or stroke. ![]()
The European Commission (EC) has published guides intended to help hospitals and national authorities implement patient blood management (PBM) programs throughout the European Union.
The EC said its guide for hospitals is intended to help them implement PBM in a practical way, building on already recognized best practices.
The guide is the result of the combined expertise of clinicians and PBM professionals and the experience gathered from a 30-month pilot program implementing PBM in 5 European teaching hospitals.
The EC said this guide is relevant for all medical professionals and organizations involved in caring for patients suffering from anemia, blood loss, and medical conditions that might require transfusion.
The EC’s other PBM guide is intended for national authorities. It lists 10 “essential public health operations,” which encompass a range of activities authorities can engage in to aid the implementation of PBM in their health systems.
The essential public health operations (and examples of activities) are:
- Surveillance—eg, continuously collect patient-level data on anemia, transfusion, and outcomes to measure and guide the implementation of PBM as a standard of care
- Monitoring—eg, recommend the monitoring and flagging of too liberal blood component utilization to prevent health hazards
- Health protection—eg, develop information and education materials for clinicians, quality and safety managers, and hospital administrators
- Health promotion—eg, promote awareness of iron deficiency and iron deficiency anemia
- Disease prevention—eg, create a sense of urgency for PBM as a new evidence-based standard of care through professional training and education
- Governance—eg, create and institute a national PBM steering committee under the authority of the Ministry of Health
- Workforce, equipment, and facilities—eg, provide hospital facilities for PBM
- Organization and funding—eg, organize reallocation of funds and resources toward PBM
- Communication—eg, provide PBM webpage sections for patients, health professionals, and health administrators
- Research—eg, conduct PBM-related studies focusing on patient outcomes, cost-effectiveness, etc.
The EC said the publication of their PBM guides is timely, as the journal Transfusion recently published results from a 5-year PBM program in Western Australia, which is the world’s largest PBM program to date. The program included 605,046 patients admitted to 4 major adult tertiary-care hospitals.
The use of blood products was reduced by 41% during the study period. The program also resulted in a 28% reduction in hospital mortality, a 15% reduction in the average hospital length of stay, a 21% decrease in hospital-acquired infections, and a 31% decrease in the incidence of heart attack or stroke. ![]()
The European Commission (EC) has published guides intended to help hospitals and national authorities implement patient blood management (PBM) programs throughout the European Union.
The EC said its guide for hospitals is intended to help them implement PBM in a practical way, building on already recognized best practices.
The guide is the result of the combined expertise of clinicians and PBM professionals and the experience gathered from a 30-month pilot program implementing PBM in 5 European teaching hospitals.
The EC said this guide is relevant for all medical professionals and organizations involved in caring for patients suffering from anemia, blood loss, and medical conditions that might require transfusion.
The EC’s other PBM guide is intended for national authorities. It lists 10 “essential public health operations,” which encompass a range of activities authorities can engage in to aid the implementation of PBM in their health systems.
The essential public health operations (and examples of activities) are:
- Surveillance—eg, continuously collect patient-level data on anemia, transfusion, and outcomes to measure and guide the implementation of PBM as a standard of care
- Monitoring—eg, recommend the monitoring and flagging of too liberal blood component utilization to prevent health hazards
- Health protection—eg, develop information and education materials for clinicians, quality and safety managers, and hospital administrators
- Health promotion—eg, promote awareness of iron deficiency and iron deficiency anemia
- Disease prevention—eg, create a sense of urgency for PBM as a new evidence-based standard of care through professional training and education
- Governance—eg, create and institute a national PBM steering committee under the authority of the Ministry of Health
- Workforce, equipment, and facilities—eg, provide hospital facilities for PBM
- Organization and funding—eg, organize reallocation of funds and resources toward PBM
- Communication—eg, provide PBM webpage sections for patients, health professionals, and health administrators
- Research—eg, conduct PBM-related studies focusing on patient outcomes, cost-effectiveness, etc.
The EC said the publication of their PBM guides is timely, as the journal Transfusion recently published results from a 5-year PBM program in Western Australia, which is the world’s largest PBM program to date. The program included 605,046 patients admitted to 4 major adult tertiary-care hospitals.
The use of blood products was reduced by 41% during the study period. The program also resulted in a 28% reduction in hospital mortality, a 15% reduction in the average hospital length of stay, a 21% decrease in hospital-acquired infections, and a 31% decrease in the incidence of heart attack or stroke. ![]()
Transplantation and ECMO feature prominently at AATS
Saturday at the annual meeting of the American Association for Thoracic Surgery will feature a course dedicated to exploring novel techniques in heart and lung transplant, mechanical circulatory support, and ECMO, among other things.
“The schedule is pretty self-explanatory,” according to course chair Matthew D. Bacchetta, MD, of Columbia University in New York City. “We’ll be getting really good reviews from some of the best centers around the world.”
Following the talks on heart transplants will be a session on lung transplants. This session will include discussions on primary graft dysfunction, techniques for performing transplantations in patients with pulmonary hypertension, and a talk on bioengineered lungs, the latter of which will be given by Harold C. Ott, MD, of Massachusetts General Hospital.
“We’re going to get a very comprehensive update on the use of DCD lung transplantation, meaning donation after cardiac death, which is obviously a hot topic in our field right now,” explained Dr. Bacchetta. DCD will also be discussed in relation to heart transplants.
After lunch, mechanical circulatory support will take center-stage. Presentations will range from dealing with LVAD and BiVAD support, to avoiding and treating pump thrombosis, and techniques for troubleshooting implantable devices. Speakers include Emma Birks, MD, of the University of Louisville, Gert D. Victor Pretorius, MD, of the University of California in San Diego, and Nicholas G. Smedira, MD, of the Cleveland Clinic, among others.
The course will close with a session on extracorporeal membrane oxygenation (ECMO). Dr. Bacchetta will give a presentation on ECMO bridge to transplantation (BTT), while other talks will be about artificial lung development, ECMO transport, management of ambulation during ECMO, and Ex Vivo Lung Perfusion (EVLP). The session, and the course, will end just before 4:00 PM.
Dr. Bacchetta did not report any financial disclosures relevant to this course.
Saturday at the annual meeting of the American Association for Thoracic Surgery will feature a course dedicated to exploring novel techniques in heart and lung transplant, mechanical circulatory support, and ECMO, among other things.
“The schedule is pretty self-explanatory,” according to course chair Matthew D. Bacchetta, MD, of Columbia University in New York City. “We’ll be getting really good reviews from some of the best centers around the world.”
Following the talks on heart transplants will be a session on lung transplants. This session will include discussions on primary graft dysfunction, techniques for performing transplantations in patients with pulmonary hypertension, and a talk on bioengineered lungs, the latter of which will be given by Harold C. Ott, MD, of Massachusetts General Hospital.
“We’re going to get a very comprehensive update on the use of DCD lung transplantation, meaning donation after cardiac death, which is obviously a hot topic in our field right now,” explained Dr. Bacchetta. DCD will also be discussed in relation to heart transplants.
After lunch, mechanical circulatory support will take center-stage. Presentations will range from dealing with LVAD and BiVAD support, to avoiding and treating pump thrombosis, and techniques for troubleshooting implantable devices. Speakers include Emma Birks, MD, of the University of Louisville, Gert D. Victor Pretorius, MD, of the University of California in San Diego, and Nicholas G. Smedira, MD, of the Cleveland Clinic, among others.
The course will close with a session on extracorporeal membrane oxygenation (ECMO). Dr. Bacchetta will give a presentation on ECMO bridge to transplantation (BTT), while other talks will be about artificial lung development, ECMO transport, management of ambulation during ECMO, and Ex Vivo Lung Perfusion (EVLP). The session, and the course, will end just before 4:00 PM.
Dr. Bacchetta did not report any financial disclosures relevant to this course.
Saturday at the annual meeting of the American Association for Thoracic Surgery will feature a course dedicated to exploring novel techniques in heart and lung transplant, mechanical circulatory support, and ECMO, among other things.
“The schedule is pretty self-explanatory,” according to course chair Matthew D. Bacchetta, MD, of Columbia University in New York City. “We’ll be getting really good reviews from some of the best centers around the world.”
Following the talks on heart transplants will be a session on lung transplants. This session will include discussions on primary graft dysfunction, techniques for performing transplantations in patients with pulmonary hypertension, and a talk on bioengineered lungs, the latter of which will be given by Harold C. Ott, MD, of Massachusetts General Hospital.
“We’re going to get a very comprehensive update on the use of DCD lung transplantation, meaning donation after cardiac death, which is obviously a hot topic in our field right now,” explained Dr. Bacchetta. DCD will also be discussed in relation to heart transplants.
After lunch, mechanical circulatory support will take center-stage. Presentations will range from dealing with LVAD and BiVAD support, to avoiding and treating pump thrombosis, and techniques for troubleshooting implantable devices. Speakers include Emma Birks, MD, of the University of Louisville, Gert D. Victor Pretorius, MD, of the University of California in San Diego, and Nicholas G. Smedira, MD, of the Cleveland Clinic, among others.
The course will close with a session on extracorporeal membrane oxygenation (ECMO). Dr. Bacchetta will give a presentation on ECMO bridge to transplantation (BTT), while other talks will be about artificial lung development, ECMO transport, management of ambulation during ECMO, and Ex Vivo Lung Perfusion (EVLP). The session, and the course, will end just before 4:00 PM.
Dr. Bacchetta did not report any financial disclosures relevant to this course.
AATS/AmSECT Welcome Reception
The AATS and AmSECT Welcome Reception will be held on Sunday, April 30, from 5:00 p.m. to 7:00 p.m. in the Exhibit Hall of the Hynes Convention Center. Admission to this event is complimentary to all registered attendees and exhibitors of both the AATS and AmSECT meetings.
Join the AATS and AmSECT, for the official opening to this year’s meeting. Visit with valued exhibitors and supporters in the Exhibition Hall, where you will learn cutting-edge techniques, and discover groundbreaking new products while networking with other attendees.
In celebration of the Centennial for AATS, many exhibits will include historic artifacts in the field of cardiothoracic surgery for all to see. The AATS and AmSECT Exhibition offers a number of exciting learning opportunities:
AATS Mini Theaters: “Deep Dive” presentations of top abstracts from this year’s Annual Meeting as well as product demonstrations by from industry partners.
AATS Learning Center: Innovative surgical procedures as well as webcasts from other AATS events such as the Mitral Conclave meeting.
AmSECT Poster Presentations
AATS Resident Poster CompetitionThe Perioperative/Team-Based Care Poster Competition
The AATS and AmSECT Welcome Reception will be held on Sunday, April 30, from 5:00 p.m. to 7:00 p.m. in the Exhibit Hall of the Hynes Convention Center. Admission to this event is complimentary to all registered attendees and exhibitors of both the AATS and AmSECT meetings.
Join the AATS and AmSECT, for the official opening to this year’s meeting. Visit with valued exhibitors and supporters in the Exhibition Hall, where you will learn cutting-edge techniques, and discover groundbreaking new products while networking with other attendees.
In celebration of the Centennial for AATS, many exhibits will include historic artifacts in the field of cardiothoracic surgery for all to see. The AATS and AmSECT Exhibition offers a number of exciting learning opportunities:
AATS Mini Theaters: “Deep Dive” presentations of top abstracts from this year’s Annual Meeting as well as product demonstrations by from industry partners.
AATS Learning Center: Innovative surgical procedures as well as webcasts from other AATS events such as the Mitral Conclave meeting.
AmSECT Poster Presentations
AATS Resident Poster CompetitionThe Perioperative/Team-Based Care Poster Competition
The AATS and AmSECT Welcome Reception will be held on Sunday, April 30, from 5:00 p.m. to 7:00 p.m. in the Exhibit Hall of the Hynes Convention Center. Admission to this event is complimentary to all registered attendees and exhibitors of both the AATS and AmSECT meetings.
Join the AATS and AmSECT, for the official opening to this year’s meeting. Visit with valued exhibitors and supporters in the Exhibition Hall, where you will learn cutting-edge techniques, and discover groundbreaking new products while networking with other attendees.
In celebration of the Centennial for AATS, many exhibits will include historic artifacts in the field of cardiothoracic surgery for all to see. The AATS and AmSECT Exhibition offers a number of exciting learning opportunities:
AATS Mini Theaters: “Deep Dive” presentations of top abstracts from this year’s Annual Meeting as well as product demonstrations by from industry partners.
AATS Learning Center: Innovative surgical procedures as well as webcasts from other AATS events such as the Mitral Conclave meeting.
AmSECT Poster Presentations
AATS Resident Poster CompetitionThe Perioperative/Team-Based Care Poster Competition
Focus on Thoracic Tumor Management
Managing thoracic tumors will be the focus of a multifaceted course set to take place in Saturday morning’s “General Thoracic Skills: Management of Thoracic Tumors in 2017” session.
“Thoracic surgical approaches and techniques are in constant evolution,” said course chair Virginia R. Litle, MD, of the Boston Medical Center. “Less invasive approaches are available for diagnosing and staging thoracic malignancies, for preoperative planning and avoidance, and for management of complications, [so] we will hear from experts in the field about preoperative planning techniques that are increasingly available.”
Of particular interest will be a brief talk on the use of social media and what boundaries exist, if any, between clinicians and their patients. Brendon M. Stiles, MD, of New York Presbyterian Hospital, will discuss “responsible social media use,” an increasingly relevant concern for their use in health care.
The following session will cover innovative approaches to esophageal replacement, minimally invasive esophagectomy in both prone and lateral orientations, and segmental lung resections, along with talks about helping patients quit smoking and enrolling patients in clinical trials. Shanda H. Blackmon, MD, of the Mayo Clinic, will summarize 3-D printing for operative planning and Yolonda L. Colson, MD, of Brigham & Women’s Hospital, will outline experimental sentinel node mapping for non–small cell lung cancer.
After lunch, there will be an hour of presentations and discussion dedicated to imaging, which will include talks on the optimal surveillance imaging after stereotactic body radiation therapy, image-based therapy for ground glass opacities, and use of the hybrid operating room. The course’s final session will focus on rescue strategies for procedures such as esophagectomies, video and robot-assisted thoracic surgery, and postoperative air leaks in endobronchial valves.
“Management of clinical challenges such as creative esophageal replacement, vascular injuries during robotic surgery, and conduit revision after minimally invasive esophagectomies, will be of interest to thoracic surgeons,” Dr. Litle noted.
Managing thoracic tumors will be the focus of a multifaceted course set to take place in Saturday morning’s “General Thoracic Skills: Management of Thoracic Tumors in 2017” session.
“Thoracic surgical approaches and techniques are in constant evolution,” said course chair Virginia R. Litle, MD, of the Boston Medical Center. “Less invasive approaches are available for diagnosing and staging thoracic malignancies, for preoperative planning and avoidance, and for management of complications, [so] we will hear from experts in the field about preoperative planning techniques that are increasingly available.”
Of particular interest will be a brief talk on the use of social media and what boundaries exist, if any, between clinicians and their patients. Brendon M. Stiles, MD, of New York Presbyterian Hospital, will discuss “responsible social media use,” an increasingly relevant concern for their use in health care.
The following session will cover innovative approaches to esophageal replacement, minimally invasive esophagectomy in both prone and lateral orientations, and segmental lung resections, along with talks about helping patients quit smoking and enrolling patients in clinical trials. Shanda H. Blackmon, MD, of the Mayo Clinic, will summarize 3-D printing for operative planning and Yolonda L. Colson, MD, of Brigham & Women’s Hospital, will outline experimental sentinel node mapping for non–small cell lung cancer.
After lunch, there will be an hour of presentations and discussion dedicated to imaging, which will include talks on the optimal surveillance imaging after stereotactic body radiation therapy, image-based therapy for ground glass opacities, and use of the hybrid operating room. The course’s final session will focus on rescue strategies for procedures such as esophagectomies, video and robot-assisted thoracic surgery, and postoperative air leaks in endobronchial valves.
“Management of clinical challenges such as creative esophageal replacement, vascular injuries during robotic surgery, and conduit revision after minimally invasive esophagectomies, will be of interest to thoracic surgeons,” Dr. Litle noted.
Managing thoracic tumors will be the focus of a multifaceted course set to take place in Saturday morning’s “General Thoracic Skills: Management of Thoracic Tumors in 2017” session.
“Thoracic surgical approaches and techniques are in constant evolution,” said course chair Virginia R. Litle, MD, of the Boston Medical Center. “Less invasive approaches are available for diagnosing and staging thoracic malignancies, for preoperative planning and avoidance, and for management of complications, [so] we will hear from experts in the field about preoperative planning techniques that are increasingly available.”
Of particular interest will be a brief talk on the use of social media and what boundaries exist, if any, between clinicians and their patients. Brendon M. Stiles, MD, of New York Presbyterian Hospital, will discuss “responsible social media use,” an increasingly relevant concern for their use in health care.
The following session will cover innovative approaches to esophageal replacement, minimally invasive esophagectomy in both prone and lateral orientations, and segmental lung resections, along with talks about helping patients quit smoking and enrolling patients in clinical trials. Shanda H. Blackmon, MD, of the Mayo Clinic, will summarize 3-D printing for operative planning and Yolonda L. Colson, MD, of Brigham & Women’s Hospital, will outline experimental sentinel node mapping for non–small cell lung cancer.
After lunch, there will be an hour of presentations and discussion dedicated to imaging, which will include talks on the optimal surveillance imaging after stereotactic body radiation therapy, image-based therapy for ground glass opacities, and use of the hybrid operating room. The course’s final session will focus on rescue strategies for procedures such as esophagectomies, video and robot-assisted thoracic surgery, and postoperative air leaks in endobronchial valves.
“Management of clinical challenges such as creative esophageal replacement, vascular injuries during robotic surgery, and conduit revision after minimally invasive esophagectomies, will be of interest to thoracic surgeons,” Dr. Litle noted.
AmSECT Celebrates Collaboration
The American Society of ExtraCorporeal Technology (AmSECT) is excited to collaborate with AATS for a shared educational program.
Open communication is critical for a high-functioning team, ultimately leading to the best in patient care. During the AmSECT International Conference and AATS Centennial, there will be great opportunities for perfusionists, surgeons, and health care professionals to collaborate, learn together, and make connections. There will be collaborative parallel sessions during the didactic portion of the program on Saturday and Sunday. The combined sessions on Saturday and the Postgraduate Symposia on Sunday include educational content on adult and congenital practice, ethics, transplant and mechanical assist, teamwork, and communication.
[[{"fid":"193783","view_mode":"medstat_image_full_text","attributes":{"height":"73","width":"303","class":"media-element file-medstat-image-full-text","data-delta":"1"},"fields":{"format":"medstat_image_full_text","field_file_image_caption[und][0][value]":"","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_full_text","field_file_image_caption[und][0][value]":"","field_file_image_credit[und][0][value]":""}}}]]AmSECT President Kenny Shann says that AmSECT and the AATS have worked together to eliminate silos of care and bring surgeons and perfusionists together to learn. “We believe that better teams will lead to better outcomes. In my career, learning alongside members of the team, regardless of their specific role, has strengthened my ability to care for patients. Co-learning allows team members to develop a shared mental model and facilitates interdisciplinary communication, which will ultimately result in better patient care.”
Perfusionists: continue the power of collaboration after the Conference by becoming an AmSECT member. In a profession that embraces innovation and ingenuity, our community of connected perfusionists helps you keep up with changing demands and emerging technologies. As a member of AmSECT, you will be supporting efforts to help you do your job better. The AmSECT community focuses on developing practice documents, protocols, best practices, and other value-add guidelines. When the members of AmSECT come together, there is greater potential to meet new opportunities and practice expansions with unbiased results.
Go online to www.amsect.org to learn more and join today.
The American Society of ExtraCorporeal Technology (AmSECT) is excited to collaborate with AATS for a shared educational program.
Open communication is critical for a high-functioning team, ultimately leading to the best in patient care. During the AmSECT International Conference and AATS Centennial, there will be great opportunities for perfusionists, surgeons, and health care professionals to collaborate, learn together, and make connections. There will be collaborative parallel sessions during the didactic portion of the program on Saturday and Sunday. The combined sessions on Saturday and the Postgraduate Symposia on Sunday include educational content on adult and congenital practice, ethics, transplant and mechanical assist, teamwork, and communication.
[[{"fid":"193783","view_mode":"medstat_image_full_text","attributes":{"height":"73","width":"303","class":"media-element file-medstat-image-full-text","data-delta":"1"},"fields":{"format":"medstat_image_full_text","field_file_image_caption[und][0][value]":"","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_full_text","field_file_image_caption[und][0][value]":"","field_file_image_credit[und][0][value]":""}}}]]AmSECT President Kenny Shann says that AmSECT and the AATS have worked together to eliminate silos of care and bring surgeons and perfusionists together to learn. “We believe that better teams will lead to better outcomes. In my career, learning alongside members of the team, regardless of their specific role, has strengthened my ability to care for patients. Co-learning allows team members to develop a shared mental model and facilitates interdisciplinary communication, which will ultimately result in better patient care.”
Perfusionists: continue the power of collaboration after the Conference by becoming an AmSECT member. In a profession that embraces innovation and ingenuity, our community of connected perfusionists helps you keep up with changing demands and emerging technologies. As a member of AmSECT, you will be supporting efforts to help you do your job better. The AmSECT community focuses on developing practice documents, protocols, best practices, and other value-add guidelines. When the members of AmSECT come together, there is greater potential to meet new opportunities and practice expansions with unbiased results.
Go online to www.amsect.org to learn more and join today.
The American Society of ExtraCorporeal Technology (AmSECT) is excited to collaborate with AATS for a shared educational program.
Open communication is critical for a high-functioning team, ultimately leading to the best in patient care. During the AmSECT International Conference and AATS Centennial, there will be great opportunities for perfusionists, surgeons, and health care professionals to collaborate, learn together, and make connections. There will be collaborative parallel sessions during the didactic portion of the program on Saturday and Sunday. The combined sessions on Saturday and the Postgraduate Symposia on Sunday include educational content on adult and congenital practice, ethics, transplant and mechanical assist, teamwork, and communication.
[[{"fid":"193783","view_mode":"medstat_image_full_text","attributes":{"height":"73","width":"303","class":"media-element file-medstat-image-full-text","data-delta":"1"},"fields":{"format":"medstat_image_full_text","field_file_image_caption[und][0][value]":"","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_full_text","field_file_image_caption[und][0][value]":"","field_file_image_credit[und][0][value]":""}}}]]AmSECT President Kenny Shann says that AmSECT and the AATS have worked together to eliminate silos of care and bring surgeons and perfusionists together to learn. “We believe that better teams will lead to better outcomes. In my career, learning alongside members of the team, regardless of their specific role, has strengthened my ability to care for patients. Co-learning allows team members to develop a shared mental model and facilitates interdisciplinary communication, which will ultimately result in better patient care.”
Perfusionists: continue the power of collaboration after the Conference by becoming an AmSECT member. In a profession that embraces innovation and ingenuity, our community of connected perfusionists helps you keep up with changing demands and emerging technologies. As a member of AmSECT, you will be supporting efforts to help you do your job better. The AmSECT community focuses on developing practice documents, protocols, best practices, and other value-add guidelines. When the members of AmSECT come together, there is greater potential to meet new opportunities and practice expansions with unbiased results.
Go online to www.amsect.org to learn more and join today.
Get the 2017 Mobile App
You can get the full AATS meeting experience right in the palm of your hand with the AATS Week Mobile App. Available through the iTunes store, Android Market, and AATS website, the app gives you access to every detail of the AATS Mitral Conclave and the AATS Centennial.
The app features:
- My Schedule and My Notes, which allow you to add your own personalization.
- Complete up-to-date schedule of what is taking place.
- Interactive Exhibit Floor.
- Exhibitors list, with company descriptions, contact information and booth location.
- Floor plans for the New York Hilton Midtown and Boston Hynes Convention Center.
- General meeting information.
You can get the full AATS meeting experience right in the palm of your hand with the AATS Week Mobile App. Available through the iTunes store, Android Market, and AATS website, the app gives you access to every detail of the AATS Mitral Conclave and the AATS Centennial.
The app features:
- My Schedule and My Notes, which allow you to add your own personalization.
- Complete up-to-date schedule of what is taking place.
- Interactive Exhibit Floor.
- Exhibitors list, with company descriptions, contact information and booth location.
- Floor plans for the New York Hilton Midtown and Boston Hynes Convention Center.
- General meeting information.
You can get the full AATS meeting experience right in the palm of your hand with the AATS Week Mobile App. Available through the iTunes store, Android Market, and AATS website, the app gives you access to every detail of the AATS Mitral Conclave and the AATS Centennial.
The app features:
- My Schedule and My Notes, which allow you to add your own personalization.
- Complete up-to-date schedule of what is taking place.
- Interactive Exhibit Floor.
- Exhibitors list, with company descriptions, contact information and booth location.
- Floor plans for the New York Hilton Midtown and Boston Hynes Convention Center.
- General meeting information.