User login
Breastfeeding among factors that modify neonatal abstinence syndrome
SAN FRANCISCO – The good news of a prospective, multicenter study presented at the Pediatric Academic Societies meeting was that neonates who were breastfed were less likely to require neonatal abstinence syndrome (NAS) treatment and displayed milder symptoms.
The study also identified other risk factors associated with the need for NAS treatment and the severity of NAS.
“These findings are important because many of these risk factors are modifiable. Prenatal care providers strive to provide the best treatments for both the mother and the fetus. Our findings regarding the association of NAS with some maternal drug exposures can be shared with opiate-dependent mothers during general counseling about tobacco and illicit use in pregnancy and in counseling about NAS,” explained Megan Stover, MD, a fellow in maternal-fetal medicine and genetics at Tufts Medical Center in Boston.
The current standard of care for opioid-dependent pregnant women is medication-assisted treatment with either methadone or buprenorphine. The intervention can be effective in curbing continued opioid abuse and preventing relapse. However, for many of their unborn children, the damage has already been done.
The scope of the problem in the United States is staggering. Between 2004 and 2013, there was a fourfold to fivefold increase in the rate of admissions to neonatal intensive care units (NICUs) for NAS. “It has been estimated that, every minute in the United States, one neonate will require treatment for NAS,” said Dr. Stover. The glum reality for the Tufts researchers is that 50%-80% of opiate-exposed infants will require treatment for NAS. The aim is to reduce this rate.
Dr. Stover was part of a study conducted at hospitals on the U.S. East Coast that aimed to clarify factors before and after birth that were associated with NAS. The enrolled mothers had been treated during their third trimester or following admission for birth for opioid dependence or had received an opioid for relief of chronic pain. They had given birth to their child at term.
Neonates who were born prematurely or who had comorbidities judged to be significant were not part of the analyses. Of the 306 neonates included, 52% required treatment for NAS and 48% did not. The two groups were similar in age of the mother and for neonatal characteristics of gestational age, sex, ethnicity, and body measurements at birth. The severity of NAS was gauged in two ways. One was the number of days of treatment required to free the neonates from the opioid-induced symptoms, with less than 10 days indicating mild NAS, greater than 30 days indicating severe NAS, and the intervening days indicating moderate NAS. Severe NAS also was indicated by the use of two or more medications.
There was good news. Neonates were significantly less likely to require NAS treatment if they were breastfed exclusively, compared with formula fed babies (15% vs 67%; P less than .0001). NAS was usually mild in breastfed babies and often severe in formula-fed babies (P less than .002).
“Our findings regarding the favorable outcomes seen with breastfeeding support recent research regarding the influence of nonpharmacologic approaches to the prevention and management of NAS, namely that more soothing environments, like those outside the NICU, may be more optimal settings for infants undergoing surveillance for NAS,” said Dr. Stover.
Neonatal treatment was more prevalent for women whose opioid substitution therapy involved methadone (54% vs 28% of untreated neonates; P less than .0001). When therapy used buprenorphine, 62% of the neonates did not display NAS. The drug used for substitution therapy had no effect on the length of treatment of the neonates.
“Our data regarding methadone exposure [versus buprenorphine] adds to a growing literature surrounding more favorable neonatal effects seen with this opiate maintenance agent over methadone,” commented Dr. Stover.
NAS treatment was more prevalent for mothers who smoked during pregnancy, compared with those who did not (76% vs 42%; P equal to .02), and for maternal use of illicit drugs (50% vs 34%; P equal to .002), with no effect on length of neonatal treatment.
Maternal psychiatric diagnosis was associated with neonatal NAS (P equal to .03), as was prescription benzodiazepine use in the third trimester of pregnancy (P equal to .02). Benzodiazepine use did not influence the length of treatment. However, maternal alprazolam use did, as it was associated with more severe NAS (P less than .001). Use of selective serotonin reuptake inhibitor during pregnancy was also associated with more severe NAS (P equal to 0.01).
The researchers are currently sifting through the genetic data gathered in the study. The goal is to combine the clinical and genetic data to create a risk score that will be used to tailor care before birth and in the early weeks following birth.
The study was sponsored by Tufts Medical Center and was funded by National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
SAN FRANCISCO – The good news of a prospective, multicenter study presented at the Pediatric Academic Societies meeting was that neonates who were breastfed were less likely to require neonatal abstinence syndrome (NAS) treatment and displayed milder symptoms.
The study also identified other risk factors associated with the need for NAS treatment and the severity of NAS.
“These findings are important because many of these risk factors are modifiable. Prenatal care providers strive to provide the best treatments for both the mother and the fetus. Our findings regarding the association of NAS with some maternal drug exposures can be shared with opiate-dependent mothers during general counseling about tobacco and illicit use in pregnancy and in counseling about NAS,” explained Megan Stover, MD, a fellow in maternal-fetal medicine and genetics at Tufts Medical Center in Boston.
The current standard of care for opioid-dependent pregnant women is medication-assisted treatment with either methadone or buprenorphine. The intervention can be effective in curbing continued opioid abuse and preventing relapse. However, for many of their unborn children, the damage has already been done.
The scope of the problem in the United States is staggering. Between 2004 and 2013, there was a fourfold to fivefold increase in the rate of admissions to neonatal intensive care units (NICUs) for NAS. “It has been estimated that, every minute in the United States, one neonate will require treatment for NAS,” said Dr. Stover. The glum reality for the Tufts researchers is that 50%-80% of opiate-exposed infants will require treatment for NAS. The aim is to reduce this rate.
Dr. Stover was part of a study conducted at hospitals on the U.S. East Coast that aimed to clarify factors before and after birth that were associated with NAS. The enrolled mothers had been treated during their third trimester or following admission for birth for opioid dependence or had received an opioid for relief of chronic pain. They had given birth to their child at term.
Neonates who were born prematurely or who had comorbidities judged to be significant were not part of the analyses. Of the 306 neonates included, 52% required treatment for NAS and 48% did not. The two groups were similar in age of the mother and for neonatal characteristics of gestational age, sex, ethnicity, and body measurements at birth. The severity of NAS was gauged in two ways. One was the number of days of treatment required to free the neonates from the opioid-induced symptoms, with less than 10 days indicating mild NAS, greater than 30 days indicating severe NAS, and the intervening days indicating moderate NAS. Severe NAS also was indicated by the use of two or more medications.
There was good news. Neonates were significantly less likely to require NAS treatment if they were breastfed exclusively, compared with formula fed babies (15% vs 67%; P less than .0001). NAS was usually mild in breastfed babies and often severe in formula-fed babies (P less than .002).
“Our findings regarding the favorable outcomes seen with breastfeeding support recent research regarding the influence of nonpharmacologic approaches to the prevention and management of NAS, namely that more soothing environments, like those outside the NICU, may be more optimal settings for infants undergoing surveillance for NAS,” said Dr. Stover.
Neonatal treatment was more prevalent for women whose opioid substitution therapy involved methadone (54% vs 28% of untreated neonates; P less than .0001). When therapy used buprenorphine, 62% of the neonates did not display NAS. The drug used for substitution therapy had no effect on the length of treatment of the neonates.
“Our data regarding methadone exposure [versus buprenorphine] adds to a growing literature surrounding more favorable neonatal effects seen with this opiate maintenance agent over methadone,” commented Dr. Stover.
NAS treatment was more prevalent for mothers who smoked during pregnancy, compared with those who did not (76% vs 42%; P equal to .02), and for maternal use of illicit drugs (50% vs 34%; P equal to .002), with no effect on length of neonatal treatment.
Maternal psychiatric diagnosis was associated with neonatal NAS (P equal to .03), as was prescription benzodiazepine use in the third trimester of pregnancy (P equal to .02). Benzodiazepine use did not influence the length of treatment. However, maternal alprazolam use did, as it was associated with more severe NAS (P less than .001). Use of selective serotonin reuptake inhibitor during pregnancy was also associated with more severe NAS (P equal to 0.01).
The researchers are currently sifting through the genetic data gathered in the study. The goal is to combine the clinical and genetic data to create a risk score that will be used to tailor care before birth and in the early weeks following birth.
The study was sponsored by Tufts Medical Center and was funded by National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
SAN FRANCISCO – The good news of a prospective, multicenter study presented at the Pediatric Academic Societies meeting was that neonates who were breastfed were less likely to require neonatal abstinence syndrome (NAS) treatment and displayed milder symptoms.
The study also identified other risk factors associated with the need for NAS treatment and the severity of NAS.
“These findings are important because many of these risk factors are modifiable. Prenatal care providers strive to provide the best treatments for both the mother and the fetus. Our findings regarding the association of NAS with some maternal drug exposures can be shared with opiate-dependent mothers during general counseling about tobacco and illicit use in pregnancy and in counseling about NAS,” explained Megan Stover, MD, a fellow in maternal-fetal medicine and genetics at Tufts Medical Center in Boston.
The current standard of care for opioid-dependent pregnant women is medication-assisted treatment with either methadone or buprenorphine. The intervention can be effective in curbing continued opioid abuse and preventing relapse. However, for many of their unborn children, the damage has already been done.
The scope of the problem in the United States is staggering. Between 2004 and 2013, there was a fourfold to fivefold increase in the rate of admissions to neonatal intensive care units (NICUs) for NAS. “It has been estimated that, every minute in the United States, one neonate will require treatment for NAS,” said Dr. Stover. The glum reality for the Tufts researchers is that 50%-80% of opiate-exposed infants will require treatment for NAS. The aim is to reduce this rate.
Dr. Stover was part of a study conducted at hospitals on the U.S. East Coast that aimed to clarify factors before and after birth that were associated with NAS. The enrolled mothers had been treated during their third trimester or following admission for birth for opioid dependence or had received an opioid for relief of chronic pain. They had given birth to their child at term.
Neonates who were born prematurely or who had comorbidities judged to be significant were not part of the analyses. Of the 306 neonates included, 52% required treatment for NAS and 48% did not. The two groups were similar in age of the mother and for neonatal characteristics of gestational age, sex, ethnicity, and body measurements at birth. The severity of NAS was gauged in two ways. One was the number of days of treatment required to free the neonates from the opioid-induced symptoms, with less than 10 days indicating mild NAS, greater than 30 days indicating severe NAS, and the intervening days indicating moderate NAS. Severe NAS also was indicated by the use of two or more medications.
There was good news. Neonates were significantly less likely to require NAS treatment if they were breastfed exclusively, compared with formula fed babies (15% vs 67%; P less than .0001). NAS was usually mild in breastfed babies and often severe in formula-fed babies (P less than .002).
“Our findings regarding the favorable outcomes seen with breastfeeding support recent research regarding the influence of nonpharmacologic approaches to the prevention and management of NAS, namely that more soothing environments, like those outside the NICU, may be more optimal settings for infants undergoing surveillance for NAS,” said Dr. Stover.
Neonatal treatment was more prevalent for women whose opioid substitution therapy involved methadone (54% vs 28% of untreated neonates; P less than .0001). When therapy used buprenorphine, 62% of the neonates did not display NAS. The drug used for substitution therapy had no effect on the length of treatment of the neonates.
“Our data regarding methadone exposure [versus buprenorphine] adds to a growing literature surrounding more favorable neonatal effects seen with this opiate maintenance agent over methadone,” commented Dr. Stover.
NAS treatment was more prevalent for mothers who smoked during pregnancy, compared with those who did not (76% vs 42%; P equal to .02), and for maternal use of illicit drugs (50% vs 34%; P equal to .002), with no effect on length of neonatal treatment.
Maternal psychiatric diagnosis was associated with neonatal NAS (P equal to .03), as was prescription benzodiazepine use in the third trimester of pregnancy (P equal to .02). Benzodiazepine use did not influence the length of treatment. However, maternal alprazolam use did, as it was associated with more severe NAS (P less than .001). Use of selective serotonin reuptake inhibitor during pregnancy was also associated with more severe NAS (P equal to 0.01).
The researchers are currently sifting through the genetic data gathered in the study. The goal is to combine the clinical and genetic data to create a risk score that will be used to tailor care before birth and in the early weeks following birth.
The study was sponsored by Tufts Medical Center and was funded by National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
AT PAS 2017
Key clinical point: Clinical factors associated with the need for treatment of neonatal abstinence syndrome and for its severity were identified.
Major finding: Breastfeeding was also associated with less severe NAS (P equal to .0003).
Data source: Prospective cohort analysis as part of a multisite trial.
Disclosures: The study was sponsored by Tufts Medical Center and was funded by the National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
USPSTF: No thyroid cancer screening for asymptomatic adults
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The rationale for recommending against screening asymptomatic people for thyroid cancer is compelling, and the evidence clearly points to the harms outweighing the benefits.
But that doesn’t mean that the conversation about screening should stop. What the field needs is not a better means to detect thyroid nodules, but a noninvasive measure to distinguish nodules whose cells will leave the thyroid capsule and cause morbidity from nodules whose cells will not. That will spare patients with indolent cancers from unnecessary treatment, while steering the minority of patients with more aggressive cancers to early treatment.
Anne R. Cappola, MD, is in the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and is an associate editor of JAMA. She reported having no relevant financial disclosures. Dr. Cappola made these remarks in an editorial accompanying the recommendation statement and the evidence report (JAMA. 2017 May 9;317[18]:1840-1).
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
The U.S. Preventive Services Task Force recommends against screening asymptomatic adults for thyroid cancer, because the harms of such screening outweigh the benefits, according to a recommendation statement published May 9 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the recommendation statement addresses screening of adults who have no signs or symptoms of thyroid cancer by using neck palpation or ultrasonography, said Kirsten Bibbins-Domingo, MD, PhD, chair of the organization and lead author of the recommendation statement, and her associates (JAMA. 2017 May 9;317[18]:1882-7).
This document is an update of the previous USPSTF recommendation statement issued in 1996 and was undertaken because there have been several major advances related to the disease since that time.
However, despite a comprehensive review of the current literature, the group found no direct evidence supporting a change to their original advice against such screening.
They emphasized that this applies only to asymptomatic adults, not to those who have hoarseness, throat pain, difficulty swallowing, lumps in the neck, swelling, or asymmetry of the neck; nor to those who have a history of exposure to ionizing radiation, a family history of thyroid cancer, or a genetic susceptibility to the disease.
The results of the literature review were summarized in an evidence report by Jennifer S. Lin, MD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates. They examined 67 studies involving nearly 584,000 patients, including 10 studies that addressed screening test performance, 3 that addressed the possible harms of screening, 2 that addressed treatment benefits, and 52 that addressed treatment harms.
No good-quality studies assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes. However, the preponderance of evidence suggested that most thyroid cancers detected by screening are indolent.
So, treatment is likely unnecessary but exposes patients to “nontrivial” harms, including an increased risk of second primary malignancy, permanent adverse effects on the salivary glands, laryngeal nerve injury, hypoparathyroidism, and the need for lifelong thyroid replacement therapy and monitoring for cancer recurrence, Dr. Lin and her associates said in their evidence report (JAMA. 2017 May 9;317[18]:1888-1903).
The task force noted that no professional medical society currently recommends population-based screening for thyroid cancer. The American Cancer Society, American Thyroid Association, American Association of Clinical Endocrinologists, and American College of Endocrinology all have no specific recommendations for screening asymptomatic patients, while the American Academy of Family Physicians recommends against such screening, said Dr. Bibbins-Domingo, who is also a professor of medicine at the University of California, San Francisco, and her associates.
Further information regarding the recommendation statement and the evidence report is available at www.uspreventiveservicestaskforce.org.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
Key clinical point: The USPSTF recommends against screening asymptomatic adults for thyroid cancer because the harms outweigh the benefits.
Major finding: None of the 67 studies reviewed in the evidence report directly assessed the net benefit of thyroid cancer screening, nor whether “early” treatment of screen-detected cancers improved patient outcomes.
Data source: A recommendation statement based on a review of 67 studies published during 1996-2016 involving 583,914 patients.
Disclosures: The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
In pyoderma gangrenosum, cyclosporine is cost effective for large lesions
, based on data from a multicenter, randomized trial published online in the British Journal of Dermatology.
In the STOP-GAP trial, researchers recruited 112 adults with pyoderma gangrenosum and similar baseline demographics. The patients were randomized to receive cyclosporine or prednisolone and were assessed at 6 weeks and 6 months. The researchers also collected quality of life data and cost and resource information.
Healing rates within 6 months were approximately 47% for the cyclosporine and prednisolone groups. Adverse reactions were similar between the two groups at 68% and 66%, respectively.
“Having found no difference for a range of objective and patient-reported outcomes, the trialists concluded that treatment decisions for individual patients should be guided by the different side effect profiles of the two drugs and patient preference,” wrote James M. Mason, MD, of the University of Warwick (England), and his colleagues. Compared with prednisolone, cyclosporine reduced the costs of treatment by 1,100 British pounds, the researchers noted. However, most of that cost savings was seen in patients with lesions of 20 cm2 or greater; the decreased cost for these patients averaged 5,310 British pounds. The cost savings were 1,007 British pounds for patients with lesions less than 20 cm2 (Br J Dermatol. 2017. doi: 10.1111/bjd.15561). “These differences were driven by the pattern of hospitalization, which predominantly occurred in patients receiving prednisolone and may be linked to the occurrence of serious adverse events,” the researchers wrote.
Quality of life was assessed using the EuroQol questionnaire and the Dermatology Life Quality Index.
No significant differences were noted between the two treatments in terms of quality of life scores, although there was a slight increase in quality of life scores among cyclosporine-treated patients over the 24-week follow-up period.
The findings were limited by incomplete data and the use of a base case analysis to complete the gaps, the researchers said. However, the results suggest that cyclosporine may be a cost-effective strategy for patients with larger lesions; no clear advantage was seen for cyclosporine vs. prednisolone treatment in patients with smaller lesions.
The study was supported in part by a grant from the National Institute for Health Research. The researchers had no financial conflicts to disclose.
, based on data from a multicenter, randomized trial published online in the British Journal of Dermatology.
In the STOP-GAP trial, researchers recruited 112 adults with pyoderma gangrenosum and similar baseline demographics. The patients were randomized to receive cyclosporine or prednisolone and were assessed at 6 weeks and 6 months. The researchers also collected quality of life data and cost and resource information.
Healing rates within 6 months were approximately 47% for the cyclosporine and prednisolone groups. Adverse reactions were similar between the two groups at 68% and 66%, respectively.
“Having found no difference for a range of objective and patient-reported outcomes, the trialists concluded that treatment decisions for individual patients should be guided by the different side effect profiles of the two drugs and patient preference,” wrote James M. Mason, MD, of the University of Warwick (England), and his colleagues. Compared with prednisolone, cyclosporine reduced the costs of treatment by 1,100 British pounds, the researchers noted. However, most of that cost savings was seen in patients with lesions of 20 cm2 or greater; the decreased cost for these patients averaged 5,310 British pounds. The cost savings were 1,007 British pounds for patients with lesions less than 20 cm2 (Br J Dermatol. 2017. doi: 10.1111/bjd.15561). “These differences were driven by the pattern of hospitalization, which predominantly occurred in patients receiving prednisolone and may be linked to the occurrence of serious adverse events,” the researchers wrote.
Quality of life was assessed using the EuroQol questionnaire and the Dermatology Life Quality Index.
No significant differences were noted between the two treatments in terms of quality of life scores, although there was a slight increase in quality of life scores among cyclosporine-treated patients over the 24-week follow-up period.
The findings were limited by incomplete data and the use of a base case analysis to complete the gaps, the researchers said. However, the results suggest that cyclosporine may be a cost-effective strategy for patients with larger lesions; no clear advantage was seen for cyclosporine vs. prednisolone treatment in patients with smaller lesions.
The study was supported in part by a grant from the National Institute for Health Research. The researchers had no financial conflicts to disclose.
, based on data from a multicenter, randomized trial published online in the British Journal of Dermatology.
In the STOP-GAP trial, researchers recruited 112 adults with pyoderma gangrenosum and similar baseline demographics. The patients were randomized to receive cyclosporine or prednisolone and were assessed at 6 weeks and 6 months. The researchers also collected quality of life data and cost and resource information.
Healing rates within 6 months were approximately 47% for the cyclosporine and prednisolone groups. Adverse reactions were similar between the two groups at 68% and 66%, respectively.
“Having found no difference for a range of objective and patient-reported outcomes, the trialists concluded that treatment decisions for individual patients should be guided by the different side effect profiles of the two drugs and patient preference,” wrote James M. Mason, MD, of the University of Warwick (England), and his colleagues. Compared with prednisolone, cyclosporine reduced the costs of treatment by 1,100 British pounds, the researchers noted. However, most of that cost savings was seen in patients with lesions of 20 cm2 or greater; the decreased cost for these patients averaged 5,310 British pounds. The cost savings were 1,007 British pounds for patients with lesions less than 20 cm2 (Br J Dermatol. 2017. doi: 10.1111/bjd.15561). “These differences were driven by the pattern of hospitalization, which predominantly occurred in patients receiving prednisolone and may be linked to the occurrence of serious adverse events,” the researchers wrote.
Quality of life was assessed using the EuroQol questionnaire and the Dermatology Life Quality Index.
No significant differences were noted between the two treatments in terms of quality of life scores, although there was a slight increase in quality of life scores among cyclosporine-treated patients over the 24-week follow-up period.
The findings were limited by incomplete data and the use of a base case analysis to complete the gaps, the researchers said. However, the results suggest that cyclosporine may be a cost-effective strategy for patients with larger lesions; no clear advantage was seen for cyclosporine vs. prednisolone treatment in patients with smaller lesions.
The study was supported in part by a grant from the National Institute for Health Research. The researchers had no financial conflicts to disclose.
Key clinical point: Approximately 50% of ulcers in pyoderma gangrenosum patients healed by 6 months on either cyclosporine or prednisolone, but cyclosporine cost less for patients with larger lesions.
Major finding: Cyclosporine was associated with an average cost reduction of 5,310 British pounds for pyoderma gangrenosum patients with lesions 20 cm2 or larger.
Data source: A cost-effectiveness analysis of the STOP-GAP randomized trial of adults with pyoderma gangrenosum.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by a grant from the National Institute for Health Research.
Think twice before recommending partial meniscectomy
LAS VEGAS – Patients with knee osteoarthritis (OA) and a meniscal tear who underwent arthroscopic partial meniscectomy (APM) subsequently experienced accelerated structural progression of their OA, compared with those randomized to physical therapy alone in the randomized METEOR trial.
“In discussing treatment options for symptomatic meniscal tear, patients and providers must weigh the potential benefits and risks of arthroscopic partial meniscectomy, including this increased risk of structural progression,” Jamie E. Collins, PhD, said at the World Congress on Osteoarthritis.
A thorough physician-patient discussion also needs to mention that several large randomized trials have suggested that patients with meniscal tear and osteoarthritic changes experience similar pain relief with APM plus physical therapy (PT) as compared with PT alone, she added at the congress sponsored by the Osteoarthritis Research Society International.
The METEOR trial was a seven-center U.S. randomized trial of APM plus PT or PT alone in patients with MRI or radiographic evidence of OA changes, a meniscal tear on MRI, and mechanical knee symptoms. Dr. Collins reported on 176 randomized patients with baseline and 18-month follow-up MRIs read by a specialist musculoskeletal radiologist. This analysis excluded the 37 patients in the PT group who crossed over to APM within 6 months after randomization.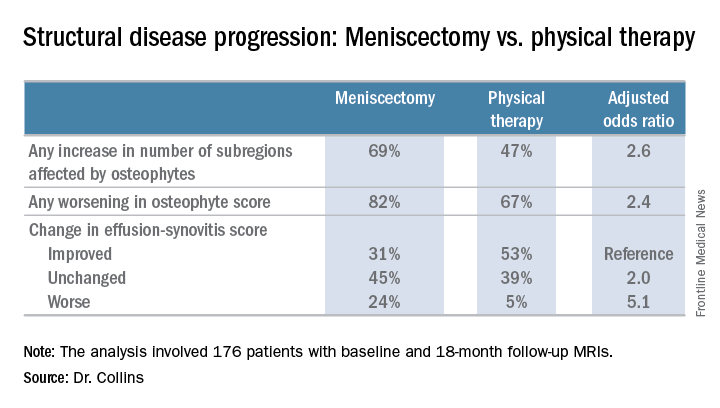
There was no significant difference between the two treatment groups in changes in bone marrow lesions or Hoffa-synovitis.
A secondary analysis that incorporated the 37 crossovers from PT to APM within 6 months showed similarly accelerated OA progression after arthroscopic surgery.
“Future work should focus on determining whether this accelerated structural progression is associated with changes in symptoms and ultimately with a higher risk of total knee replacement. In other words, is 2+ structural worsening something that’s noticeable or important for patients?” Dr. Collins concluded.
She reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
LAS VEGAS – Patients with knee osteoarthritis (OA) and a meniscal tear who underwent arthroscopic partial meniscectomy (APM) subsequently experienced accelerated structural progression of their OA, compared with those randomized to physical therapy alone in the randomized METEOR trial.
“In discussing treatment options for symptomatic meniscal tear, patients and providers must weigh the potential benefits and risks of arthroscopic partial meniscectomy, including this increased risk of structural progression,” Jamie E. Collins, PhD, said at the World Congress on Osteoarthritis.
A thorough physician-patient discussion also needs to mention that several large randomized trials have suggested that patients with meniscal tear and osteoarthritic changes experience similar pain relief with APM plus physical therapy (PT) as compared with PT alone, she added at the congress sponsored by the Osteoarthritis Research Society International.
The METEOR trial was a seven-center U.S. randomized trial of APM plus PT or PT alone in patients with MRI or radiographic evidence of OA changes, a meniscal tear on MRI, and mechanical knee symptoms. Dr. Collins reported on 176 randomized patients with baseline and 18-month follow-up MRIs read by a specialist musculoskeletal radiologist. This analysis excluded the 37 patients in the PT group who crossed over to APM within 6 months after randomization.
There was no significant difference between the two treatment groups in changes in bone marrow lesions or Hoffa-synovitis.
A secondary analysis that incorporated the 37 crossovers from PT to APM within 6 months showed similarly accelerated OA progression after arthroscopic surgery.
“Future work should focus on determining whether this accelerated structural progression is associated with changes in symptoms and ultimately with a higher risk of total knee replacement. In other words, is 2+ structural worsening something that’s noticeable or important for patients?” Dr. Collins concluded.
She reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
LAS VEGAS – Patients with knee osteoarthritis (OA) and a meniscal tear who underwent arthroscopic partial meniscectomy (APM) subsequently experienced accelerated structural progression of their OA, compared with those randomized to physical therapy alone in the randomized METEOR trial.
“In discussing treatment options for symptomatic meniscal tear, patients and providers must weigh the potential benefits and risks of arthroscopic partial meniscectomy, including this increased risk of structural progression,” Jamie E. Collins, PhD, said at the World Congress on Osteoarthritis.
A thorough physician-patient discussion also needs to mention that several large randomized trials have suggested that patients with meniscal tear and osteoarthritic changes experience similar pain relief with APM plus physical therapy (PT) as compared with PT alone, she added at the congress sponsored by the Osteoarthritis Research Society International.
The METEOR trial was a seven-center U.S. randomized trial of APM plus PT or PT alone in patients with MRI or radiographic evidence of OA changes, a meniscal tear on MRI, and mechanical knee symptoms. Dr. Collins reported on 176 randomized patients with baseline and 18-month follow-up MRIs read by a specialist musculoskeletal radiologist. This analysis excluded the 37 patients in the PT group who crossed over to APM within 6 months after randomization.
There was no significant difference between the two treatment groups in changes in bone marrow lesions or Hoffa-synovitis.
A secondary analysis that incorporated the 37 crossovers from PT to APM within 6 months showed similarly accelerated OA progression after arthroscopic surgery.
“Future work should focus on determining whether this accelerated structural progression is associated with changes in symptoms and ultimately with a higher risk of total knee replacement. In other words, is 2+ structural worsening something that’s noticeable or important for patients?” Dr. Collins concluded.
She reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
AT OARSI 2017
Key clinical point:
Major finding: Surgically treated patients were 2.6 times more likely to demonstrate MRI evidence of structural disease progression at 18 months than those who received physical therapy alone.
Data source: This analysis from the multicenter METEOR trial included 176 patients with a symptomatic meniscal tear.
Disclosures: The presenter reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
Subcutaneous rituximab safe, effective for follicular lymphoma
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
Efficacy and safety profiles were similar for subcutaneous and standard IV rituximab when given as first-line therapy to adults with follicular lymphoma, based on results of a phase III clinical trial published online in Lancet Haematology.
Administering rituximab by IV infusion can take up to 6 hours to complete and requires continuous monitoring. Subcutaneous delivery takes approximately 6 minutes using a new rituximab formulation that is 12 times more concentrated to reduce the administered volume. The new formulation is expected to reduce the burden of treatment for patients, as well as for the health care system, said Andrew Davies, PhD, of the Cancer Research UK Centre, Southampton, and his associates.
They compared the two agents in an international open-label trial funded by Hoffmann-La Roche, maker of the subcutaneous formulation. Adult patients at 113 medical centers in 30 countries were randomly assigned to receive either IV (205 patients) or subcutaneous (205 patients) rituximab during induction therapy with six to eight cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CVP (cyclophosphamide, vincristine, and prednisone). They continued with rituximab as maintenance therapy every 2 months for 2 years. The median duration of treatment was 27 months, and median follow-up was 37 months.
The primary efficacy end point – overall (complete or partial) response rate at the end of induction, based on investigator assessment confirmed by an independent review panel of radiologists – was 84.9% with IV and 84.4% with subcutaneous rituximab, a nonsignificant difference. Similarly, the overall response rate at the end of maintenance therapy was not significantly different between the two groups, at 78.1% and 77.9%, respectively.
Progression-free survival (hazard ratio, 0.84) and event-free survival (HR, 0.91) also did not differ significantly between the two study groups, the investigators said (Lancet Haematol. 2017 doi: 10.1016/S2352.3026(17)30078-9).
The rates of adverse events, grade 3 or higher adverse events, and serious adverse events also were similar for IV and subcutaneous formulations of rituximab. “Administration-related reactions were more common in the subcutaneous group but were predominantly mild-to-moderate local injection-site reactions, such as mild pain, swelling and erythema, reflecting the expected change in safety profile when switching to the subcutaneous route of administration,” Dr. Davies and his associates said.
These results indicate that subcutaneous administration of rituximab along with chemotherapy doesn’t compromise the agent’s antilymphoma activity, they added.
FROM LANCET HAEMATOLOGY
Key clinical point: Subcutaneous rituximab had efficacy and safety profiles similar to those of standard IV rituximab when given as first-line therapy to adults with follicular lymphoma.
Major finding: The primary efficacy end point – overall response rate at the end of induction – was 84.9% with IV and 84.4% with subcutaneous rituximab.
Data source: An international randomized controlled phase III trial involving 410 adults followed for 3 years.
Disclosures: This trial was funded by Hoffmann-La Roche, maker of the subcutaneous formulation of rituximab. The pharmaceutical company also was involved in the design and conduct of the trial, collection and interpretation of the data, and writing of the results. Dr. Davies reported ties to Hoffmann-La Roche and numerous other drug companies.
Roux-en-Y bests sleeve gastrectomy for weight loss
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
[email protected]
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: At 5 years post surgery, Roux-en-Y recipients had kept off 25% of their body weight, compared with 20% for sleeve gastrectomy patients (P less than .05).
Data source: Longitudinal follow-up of 716 patients who had one of two surgical procedures for weight loss.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
New ACR-EULAR diagnostic criteria proposed for ANCA-associated vasculitides
BIRMINGHAM, ENGLAND – New criteria for classifying granulomatosis with polyangiitis (GPA) have been proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) .
GPA, a type of antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, was formerly known as Wegener’s granulomatosis. When a small or medium vessel vasculitis has been diagnosed, the new criteria – which are provisional at present – are invoked to confirm the GPA diagnosis. Patients are assessed for five clinical and four laboratory variables.
The new criteria were developed as part of the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study. An international project set up to update the classification criteria for all types of systemic vasculitis, DCVAS involves more than 6,000 patients from 133 sites in 32 countries.
To develop the criteria for GPA, a team of 49 vasculitis experts was asked to review over 1, 400 clinical vignettes of newly diagnosed vasculitis based on cases submitted to the DCVAS. The expert panel was not told the suspected diagnosis. When the panel did not reach consensus on a case, it was further reviewed by seven members of the DCVAS study steering committee.
Using this approach, 85% of 578 cases submitted as GPA were confirmed. Compared with other vasculitides, GPA occurred in younger patients (53 vs. 58 years), was more likely to be proteinase 3–ANCA (81% vs. 3.4%) and c-ANCA (72% vs. 5.5%) positive, and was less likely to be p-ANCA (10.3% vs. 47.3%) and myeloperoxidase-antibody (8% vs. 59.3%) positive.
The next stage was to identify data-driven items that might be used to distinguish GPA from other vasculitides and obtain a clinical consensus on those that were the most important for a diagnosis. Of 1000 possible items that included clinical, laboratory, imaging, and biopsy findings, 91 were retained after regression analysis and 22 appeared independently predictive for GPA.
The top five data-driven items had both c-ANCA and PR3-ANCA antibodies, bloody nasal discharge, nasal ulcers, crusting, or sinonasal congestion or blockage; a high (greater than 1 x 109/L) eosinophil count; and the presence of nasal polyps. There were also some clinical and data-driven items that were considered and used in a final nine-item model, Dr. Robson explained.
A point-based risk score was subsequently developed, with a total score of 5 or more suggesting GPA.
The presence of c-ANCA and PR3-ANCA antibodies, an almost certain indicator of GPA with an odds ratio of 134.8 (95% confidence interval, 62.4–291.1; P less than .001), was given the highest score of 5.
Having bloody or nasal discharge or other nasal symptoms or seeing a granuloma on biopsy were both given a score of 3. A score of 2 was awarded if there were nodules, a mass or cavity on chest imaging, or if there was cartilaginous involvement. A score of 1 was given if there was a loss or reduction in hearing or if the patient had red or painful eyes. Two items – the presence of a high eosinophil count and nasal polyps – were given negative scores (-3 and -4, respectively).
Dr. Robson reported that the nine-item model had an area under the curve of 0.98 and high sensitivity (90.7%) and specificity (93.5%).
Dr. Robson reported having no conflicts of interest.
BIRMINGHAM, ENGLAND – New criteria for classifying granulomatosis with polyangiitis (GPA) have been proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) .
GPA, a type of antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, was formerly known as Wegener’s granulomatosis. When a small or medium vessel vasculitis has been diagnosed, the new criteria – which are provisional at present – are invoked to confirm the GPA diagnosis. Patients are assessed for five clinical and four laboratory variables.
The new criteria were developed as part of the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study. An international project set up to update the classification criteria for all types of systemic vasculitis, DCVAS involves more than 6,000 patients from 133 sites in 32 countries.
To develop the criteria for GPA, a team of 49 vasculitis experts was asked to review over 1, 400 clinical vignettes of newly diagnosed vasculitis based on cases submitted to the DCVAS. The expert panel was not told the suspected diagnosis. When the panel did not reach consensus on a case, it was further reviewed by seven members of the DCVAS study steering committee.
Using this approach, 85% of 578 cases submitted as GPA were confirmed. Compared with other vasculitides, GPA occurred in younger patients (53 vs. 58 years), was more likely to be proteinase 3–ANCA (81% vs. 3.4%) and c-ANCA (72% vs. 5.5%) positive, and was less likely to be p-ANCA (10.3% vs. 47.3%) and myeloperoxidase-antibody (8% vs. 59.3%) positive.
The next stage was to identify data-driven items that might be used to distinguish GPA from other vasculitides and obtain a clinical consensus on those that were the most important for a diagnosis. Of 1000 possible items that included clinical, laboratory, imaging, and biopsy findings, 91 were retained after regression analysis and 22 appeared independently predictive for GPA.
The top five data-driven items had both c-ANCA and PR3-ANCA antibodies, bloody nasal discharge, nasal ulcers, crusting, or sinonasal congestion or blockage; a high (greater than 1 x 109/L) eosinophil count; and the presence of nasal polyps. There were also some clinical and data-driven items that were considered and used in a final nine-item model, Dr. Robson explained.
A point-based risk score was subsequently developed, with a total score of 5 or more suggesting GPA.
The presence of c-ANCA and PR3-ANCA antibodies, an almost certain indicator of GPA with an odds ratio of 134.8 (95% confidence interval, 62.4–291.1; P less than .001), was given the highest score of 5.
Having bloody or nasal discharge or other nasal symptoms or seeing a granuloma on biopsy were both given a score of 3. A score of 2 was awarded if there were nodules, a mass or cavity on chest imaging, or if there was cartilaginous involvement. A score of 1 was given if there was a loss or reduction in hearing or if the patient had red or painful eyes. Two items – the presence of a high eosinophil count and nasal polyps – were given negative scores (-3 and -4, respectively).
Dr. Robson reported that the nine-item model had an area under the curve of 0.98 and high sensitivity (90.7%) and specificity (93.5%).
Dr. Robson reported having no conflicts of interest.
BIRMINGHAM, ENGLAND – New criteria for classifying granulomatosis with polyangiitis (GPA) have been proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) .
GPA, a type of antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, was formerly known as Wegener’s granulomatosis. When a small or medium vessel vasculitis has been diagnosed, the new criteria – which are provisional at present – are invoked to confirm the GPA diagnosis. Patients are assessed for five clinical and four laboratory variables.
The new criteria were developed as part of the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study. An international project set up to update the classification criteria for all types of systemic vasculitis, DCVAS involves more than 6,000 patients from 133 sites in 32 countries.
To develop the criteria for GPA, a team of 49 vasculitis experts was asked to review over 1, 400 clinical vignettes of newly diagnosed vasculitis based on cases submitted to the DCVAS. The expert panel was not told the suspected diagnosis. When the panel did not reach consensus on a case, it was further reviewed by seven members of the DCVAS study steering committee.
Using this approach, 85% of 578 cases submitted as GPA were confirmed. Compared with other vasculitides, GPA occurred in younger patients (53 vs. 58 years), was more likely to be proteinase 3–ANCA (81% vs. 3.4%) and c-ANCA (72% vs. 5.5%) positive, and was less likely to be p-ANCA (10.3% vs. 47.3%) and myeloperoxidase-antibody (8% vs. 59.3%) positive.
The next stage was to identify data-driven items that might be used to distinguish GPA from other vasculitides and obtain a clinical consensus on those that were the most important for a diagnosis. Of 1000 possible items that included clinical, laboratory, imaging, and biopsy findings, 91 were retained after regression analysis and 22 appeared independently predictive for GPA.
The top five data-driven items had both c-ANCA and PR3-ANCA antibodies, bloody nasal discharge, nasal ulcers, crusting, or sinonasal congestion or blockage; a high (greater than 1 x 109/L) eosinophil count; and the presence of nasal polyps. There were also some clinical and data-driven items that were considered and used in a final nine-item model, Dr. Robson explained.
A point-based risk score was subsequently developed, with a total score of 5 or more suggesting GPA.
The presence of c-ANCA and PR3-ANCA antibodies, an almost certain indicator of GPA with an odds ratio of 134.8 (95% confidence interval, 62.4–291.1; P less than .001), was given the highest score of 5.
Having bloody or nasal discharge or other nasal symptoms or seeing a granuloma on biopsy were both given a score of 3. A score of 2 was awarded if there were nodules, a mass or cavity on chest imaging, or if there was cartilaginous involvement. A score of 1 was given if there was a loss or reduction in hearing or if the patient had red or painful eyes. Two items – the presence of a high eosinophil count and nasal polyps – were given negative scores (-3 and -4, respectively).
Dr. Robson reported that the nine-item model had an area under the curve of 0.98 and high sensitivity (90.7%) and specificity (93.5%).
Dr. Robson reported having no conflicts of interest.
AT RHEUMATOLOGY 2017
Key clinical point: Provisional classification criteria have been developed to identify patients with granulomatosis with polyangiitis.
Major finding: The nine-item classification criteria model has an area under the curve of 0.98, with high sensitivity (90.7%) and specificity (93.5%).
Data source: More than 1,400 vasculitis cases submitted to the Diagnostic and Classification of the Systemic Vasculitides (DCVAS) study.
Disclosures: Dr. Robson reported having no conflicts of interest.
Central centrifugal cicatricial alopecia can affect adolescents
Central centrifugal cicatricial alopecia (CCCA) can affect adolescents, and a study of six biopsy-proven cases indicates CCCA has a genetic component, Ariana N. Eginli and her colleagues report in Pediatric Dermatology.
CCCA, a scarring alopecia that disproportionately affects middle-aged women of African descent, has been attributed to hair care and styling practices. In this series, however, five of the six patients had a maternal history of CCCA, and only one had used chemical products or styling tools. “Specifically, the early onset of CCCA in these patients with natural virgin hair raises the possibility of genetic anticipation,” wrote Ms. Eginli of Wake Forest Baptist Health, Winston-Salem, N.C., and her coauthors. “Therefore, recognizing that CCCA can present in children, particularly in those with a positive family history, is of utmost importance in controlling further disease progression and improving their quality of life.”
Two patients had previously undergone scalp surgery, specifically ventriculoperitoneal shunt placement, years before their hair loss began. The authors speculated that the scalp surgery may have contributed to the early development of CCCA.
“We recommend that clinicians check for early signs of CCCA when there are complaints of hair loss on the scalp of offspring of affected women of African descent,” they wrote. “If there is any clinical suspicion of CCCA or any scarring alopecia, a scalp biopsy should be performed.”
Ms. Eginli had no disclosures. One of her colleagues is a consultant for and has received grant support from various drug companies.
[email protected]
On Twitter @eaztweets
Central centrifugal cicatricial alopecia (CCCA) can affect adolescents, and a study of six biopsy-proven cases indicates CCCA has a genetic component, Ariana N. Eginli and her colleagues report in Pediatric Dermatology.
CCCA, a scarring alopecia that disproportionately affects middle-aged women of African descent, has been attributed to hair care and styling practices. In this series, however, five of the six patients had a maternal history of CCCA, and only one had used chemical products or styling tools. “Specifically, the early onset of CCCA in these patients with natural virgin hair raises the possibility of genetic anticipation,” wrote Ms. Eginli of Wake Forest Baptist Health, Winston-Salem, N.C., and her coauthors. “Therefore, recognizing that CCCA can present in children, particularly in those with a positive family history, is of utmost importance in controlling further disease progression and improving their quality of life.”
Two patients had previously undergone scalp surgery, specifically ventriculoperitoneal shunt placement, years before their hair loss began. The authors speculated that the scalp surgery may have contributed to the early development of CCCA.
“We recommend that clinicians check for early signs of CCCA when there are complaints of hair loss on the scalp of offspring of affected women of African descent,” they wrote. “If there is any clinical suspicion of CCCA or any scarring alopecia, a scalp biopsy should be performed.”
Ms. Eginli had no disclosures. One of her colleagues is a consultant for and has received grant support from various drug companies.
[email protected]
On Twitter @eaztweets
Central centrifugal cicatricial alopecia (CCCA) can affect adolescents, and a study of six biopsy-proven cases indicates CCCA has a genetic component, Ariana N. Eginli and her colleagues report in Pediatric Dermatology.
CCCA, a scarring alopecia that disproportionately affects middle-aged women of African descent, has been attributed to hair care and styling practices. In this series, however, five of the six patients had a maternal history of CCCA, and only one had used chemical products or styling tools. “Specifically, the early onset of CCCA in these patients with natural virgin hair raises the possibility of genetic anticipation,” wrote Ms. Eginli of Wake Forest Baptist Health, Winston-Salem, N.C., and her coauthors. “Therefore, recognizing that CCCA can present in children, particularly in those with a positive family history, is of utmost importance in controlling further disease progression and improving their quality of life.”
Two patients had previously undergone scalp surgery, specifically ventriculoperitoneal shunt placement, years before their hair loss began. The authors speculated that the scalp surgery may have contributed to the early development of CCCA.
“We recommend that clinicians check for early signs of CCCA when there are complaints of hair loss on the scalp of offspring of affected women of African descent,” they wrote. “If there is any clinical suspicion of CCCA or any scarring alopecia, a scalp biopsy should be performed.”
Ms. Eginli had no disclosures. One of her colleagues is a consultant for and has received grant support from various drug companies.
[email protected]
On Twitter @eaztweets
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: Of six pediatric patients with biopsy-proven CCCA, five had a family history of CCCA and only one had used chemical products or styling tools.
Data source: A case series of six pediatric patients with biopsy-confirmed CCCA.
Disclosures: Ms. Eginli had no disclosures. One of her colleagues is a consultant for and has received grant support from various drug companies.
Merkel cell carcinoma most likely to recur within 2 years of diagnosis
PORTLAND, ORE. – The first 2 years after diagnosis are crucial when conducting surveillance for recurrence of Merkel cell carcinoma (MCC), Aubriana McEvoy said at the annual meeting of the Society for Investigative Dermatology.
Regardless of stage at diagnosis, the risk of recurrence peaked at about 1 year and leveled off by about year 2 in a retrospective cohort study, according to Ms. McEvoy, a medical student at the University of Washington, Seattle, who conducted the study with colleagues under the mentorship of Paul Nghiem, MD, PhD, professor and head of the division of dermatology. The study also inversely linked primary MCC stage with subsequent recurrence-free survival, highlighted the role of imaging for surveillance of patients who have advanced primary disease, and linked distant metastatic recurrence with significantly worse survival, compared with local or nodal recurrence.
“Patients with Merkel cell carcinoma always ask about recurrence,” Ms. McEvoy said. “Now, for the first time, we have the data to answer their questions.”
Surveillance of MCC is increasingly important, she said: The “treatment landscape is evolving quickly, and immunotherapies such as pembrolizumab can have a good response rate, especially in the setting of lower burden of disease.” But follow-up is costly on several fronts, making it crucial to aim for “enough” and not “too much” surveillance, she added.
“Imaging often costs thousands of dollars, and that’s only one piece of the pie. There’s also the cost of office visits, time spent by the patient and their family, and the emotional investment and uncertainty a patient goes through every time they have to come for a follow-up visit and scan,” Ms. McEvoy said.
Comprehensive, stage-specific guidelines can help clinicians and patients balance the benefits and costs of surveillance, but are lacking in MCC because no published study has characterized recurrence by stage, she said. To fill this gap, she and her associates analyzed 10 years of longitudinal MCC surveillance data on 468 patients who underwent pathologic staging and were followed at the Nghiem laboratory.
The risk of recurrence was highest within the first 2 years after diagnosis, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) MCC. However, the probability of recurrence-free survival correlated inversely with pathologic stage of primary MCC (P = .003). Median recurrence-free survival time was not reached by the 186 patients with local disease and small (2-cm maximum dimension) primary lesions, or by 135 patients with clinically occult nodal disease.
In contrast, median recurrence-free survival was about 6 years among 84 patients with local disease and lesions measuring more than 2 cm; was less than 2 years among 35 patients with clinically apparent, pathologically confirmed nodal disease or in-transit metastases; and was less than 1 year among patients with distant metastatic disease.
The researchers also investigated the risk of distant metastatic recurrence to confirm which patients need most intensive follow-up. Among 138 individuals with available data, 40% of stage I primary MCC patients developed a distant metastatic recurrence, as did 60% of patients with stage IIA or stage IIB primary MCC. And 80% of recurrences among patients with stage IIIA or stage IIIB primary disease were distant metastases. “I think it’s safe to say that stage III patients should receive appropriate, if not vigilant, surveillance,” Ms. McEvoy said. The site of recurrence also was significantly (P less than .001) tied to the risk of subsequent death from recurrent MCC; median survival time was not reached when recurrence was local or nodal, but was less than 2 years when it was distant or metastatic.
Early in 2018, the American Joint Committee on Cancer will update its MCC staging system to distinguish clinical versus pathologic staging. “This is important, because pathologic staging remains the gold standard, providing a much more in-depth view of the patient’s disease,” Ms. McEvoy commented. Ideally, clinicians would use more information to help predict the prognosis of MCC, including sex and immune and viral status, she noted. “But we hope these data provide information for more consistency across the country, so we can catch recurrences earlier, and avoid unnecessary visits and imaging scans for lower-risk patients.”
The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
PORTLAND, ORE. – The first 2 years after diagnosis are crucial when conducting surveillance for recurrence of Merkel cell carcinoma (MCC), Aubriana McEvoy said at the annual meeting of the Society for Investigative Dermatology.
Regardless of stage at diagnosis, the risk of recurrence peaked at about 1 year and leveled off by about year 2 in a retrospective cohort study, according to Ms. McEvoy, a medical student at the University of Washington, Seattle, who conducted the study with colleagues under the mentorship of Paul Nghiem, MD, PhD, professor and head of the division of dermatology. The study also inversely linked primary MCC stage with subsequent recurrence-free survival, highlighted the role of imaging for surveillance of patients who have advanced primary disease, and linked distant metastatic recurrence with significantly worse survival, compared with local or nodal recurrence.
“Patients with Merkel cell carcinoma always ask about recurrence,” Ms. McEvoy said. “Now, for the first time, we have the data to answer their questions.”
Surveillance of MCC is increasingly important, she said: The “treatment landscape is evolving quickly, and immunotherapies such as pembrolizumab can have a good response rate, especially in the setting of lower burden of disease.” But follow-up is costly on several fronts, making it crucial to aim for “enough” and not “too much” surveillance, she added.
“Imaging often costs thousands of dollars, and that’s only one piece of the pie. There’s also the cost of office visits, time spent by the patient and their family, and the emotional investment and uncertainty a patient goes through every time they have to come for a follow-up visit and scan,” Ms. McEvoy said.
Comprehensive, stage-specific guidelines can help clinicians and patients balance the benefits and costs of surveillance, but are lacking in MCC because no published study has characterized recurrence by stage, she said. To fill this gap, she and her associates analyzed 10 years of longitudinal MCC surveillance data on 468 patients who underwent pathologic staging and were followed at the Nghiem laboratory.
The risk of recurrence was highest within the first 2 years after diagnosis, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) MCC. However, the probability of recurrence-free survival correlated inversely with pathologic stage of primary MCC (P = .003). Median recurrence-free survival time was not reached by the 186 patients with local disease and small (2-cm maximum dimension) primary lesions, or by 135 patients with clinically occult nodal disease.
In contrast, median recurrence-free survival was about 6 years among 84 patients with local disease and lesions measuring more than 2 cm; was less than 2 years among 35 patients with clinically apparent, pathologically confirmed nodal disease or in-transit metastases; and was less than 1 year among patients with distant metastatic disease.
The researchers also investigated the risk of distant metastatic recurrence to confirm which patients need most intensive follow-up. Among 138 individuals with available data, 40% of stage I primary MCC patients developed a distant metastatic recurrence, as did 60% of patients with stage IIA or stage IIB primary MCC. And 80% of recurrences among patients with stage IIIA or stage IIIB primary disease were distant metastases. “I think it’s safe to say that stage III patients should receive appropriate, if not vigilant, surveillance,” Ms. McEvoy said. The site of recurrence also was significantly (P less than .001) tied to the risk of subsequent death from recurrent MCC; median survival time was not reached when recurrence was local or nodal, but was less than 2 years when it was distant or metastatic.
Early in 2018, the American Joint Committee on Cancer will update its MCC staging system to distinguish clinical versus pathologic staging. “This is important, because pathologic staging remains the gold standard, providing a much more in-depth view of the patient’s disease,” Ms. McEvoy commented. Ideally, clinicians would use more information to help predict the prognosis of MCC, including sex and immune and viral status, she noted. “But we hope these data provide information for more consistency across the country, so we can catch recurrences earlier, and avoid unnecessary visits and imaging scans for lower-risk patients.”
The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
PORTLAND, ORE. – The first 2 years after diagnosis are crucial when conducting surveillance for recurrence of Merkel cell carcinoma (MCC), Aubriana McEvoy said at the annual meeting of the Society for Investigative Dermatology.
Regardless of stage at diagnosis, the risk of recurrence peaked at about 1 year and leveled off by about year 2 in a retrospective cohort study, according to Ms. McEvoy, a medical student at the University of Washington, Seattle, who conducted the study with colleagues under the mentorship of Paul Nghiem, MD, PhD, professor and head of the division of dermatology. The study also inversely linked primary MCC stage with subsequent recurrence-free survival, highlighted the role of imaging for surveillance of patients who have advanced primary disease, and linked distant metastatic recurrence with significantly worse survival, compared with local or nodal recurrence.
“Patients with Merkel cell carcinoma always ask about recurrence,” Ms. McEvoy said. “Now, for the first time, we have the data to answer their questions.”
Surveillance of MCC is increasingly important, she said: The “treatment landscape is evolving quickly, and immunotherapies such as pembrolizumab can have a good response rate, especially in the setting of lower burden of disease.” But follow-up is costly on several fronts, making it crucial to aim for “enough” and not “too much” surveillance, she added.
“Imaging often costs thousands of dollars, and that’s only one piece of the pie. There’s also the cost of office visits, time spent by the patient and their family, and the emotional investment and uncertainty a patient goes through every time they have to come for a follow-up visit and scan,” Ms. McEvoy said.
Comprehensive, stage-specific guidelines can help clinicians and patients balance the benefits and costs of surveillance, but are lacking in MCC because no published study has characterized recurrence by stage, she said. To fill this gap, she and her associates analyzed 10 years of longitudinal MCC surveillance data on 468 patients who underwent pathologic staging and were followed at the Nghiem laboratory.
The risk of recurrence was highest within the first 2 years after diagnosis, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) MCC. However, the probability of recurrence-free survival correlated inversely with pathologic stage of primary MCC (P = .003). Median recurrence-free survival time was not reached by the 186 patients with local disease and small (2-cm maximum dimension) primary lesions, or by 135 patients with clinically occult nodal disease.
In contrast, median recurrence-free survival was about 6 years among 84 patients with local disease and lesions measuring more than 2 cm; was less than 2 years among 35 patients with clinically apparent, pathologically confirmed nodal disease or in-transit metastases; and was less than 1 year among patients with distant metastatic disease.
The researchers also investigated the risk of distant metastatic recurrence to confirm which patients need most intensive follow-up. Among 138 individuals with available data, 40% of stage I primary MCC patients developed a distant metastatic recurrence, as did 60% of patients with stage IIA or stage IIB primary MCC. And 80% of recurrences among patients with stage IIIA or stage IIIB primary disease were distant metastases. “I think it’s safe to say that stage III patients should receive appropriate, if not vigilant, surveillance,” Ms. McEvoy said. The site of recurrence also was significantly (P less than .001) tied to the risk of subsequent death from recurrent MCC; median survival time was not reached when recurrence was local or nodal, but was less than 2 years when it was distant or metastatic.
Early in 2018, the American Joint Committee on Cancer will update its MCC staging system to distinguish clinical versus pathologic staging. “This is important, because pathologic staging remains the gold standard, providing a much more in-depth view of the patient’s disease,” Ms. McEvoy commented. Ideally, clinicians would use more information to help predict the prognosis of MCC, including sex and immune and viral status, she noted. “But we hope these data provide information for more consistency across the country, so we can catch recurrences earlier, and avoid unnecessary visits and imaging scans for lower-risk patients.”
The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
AT SID 2017
Key clinical point:
Major finding: The risk of recurrence peaked about 1 year after diagnosis and leveled off at about year 2, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) disease.
Data source: A retrospective cohort study of 544 patients with Merkel cell carcinoma (468 with pathologic stage disease).
Disclosures: The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
CRC in Lynch syndrome is lower than previously reported
CHICAGO – Lynch syndrome can predispose individuals to a number of different cancer types, including colorectal tumors, but the results of a preliminary study found that the incidence may be lower than what has been previously reported.
New findings presented at Digestive Disease Week® showed that the incidence of colorectal cancer after screening with colonoscopy ranged from 6.3% to 25.9%, depending on the specific mutated gene. This is in contrast to other reports which have found a 50% increase in the incidence of colorectal cancer in individuals with Lynch syndrome.
“We looked at the incidence of colorectal cancer and other associated cancers in individuals with Lynch syndrome and how screening can have an impact on that,” said study author Ariadna Sanchez, MD, from the Hospital Clínic de Barcelona, Spain.
She emphasized that the results being presented at the meeting are preliminary and, thus, will need further confirmation, but it is a multicenter study and is being conducted in more than 1,100 patients.
“The diagnosis of colorectal cancer with screening colonoscopy is lower than results that have been previously published,” she said. “This finding reinforces the importance of screening colonoscopies in patients with Lynch syndrome.”
As to why their rates are lower, Dr. Sanchez speculated that it may be because precancerous polyps are being removed with screening.
“Our thinking is that, as we perform screening and remove the polyps, then in theory, cancer will be prevented,” she explained, “since they didn’t have the opportunity to continue to progress into cancer.”
In other words, screening this high-risk population may not only identify those who have cancer but may prevent it from developing in others.
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is an inherited disorder that increases the risk of many types of cancer, including colorectal and cancers of the stomach, small intestine, liver, gallbladder ducts, upper urinary tract, brain, skin, ovaries, and endometrium.
Caused by germline mutations in the mismatch DNA repair system (MLH1, MSH2, MSH6, PMS2), it has been difficult to make precise estimates of cancer risk in individuals with the syndrome because of retrospective studies and small cohorts.
Dr. Sanchez and her colleagues conducted a multicenter nation-wide study in Spain with the goal of establishing the cumulative incidence of colorectal cancer and other tumor types in Lynch syndrome and to evaluate the effect of screening surveillance on cancer incidence. Cancer-specific survival will also be assessed.
The cohort included 1,108 patients with Lynch syndrome from 25 centers, who were followed-up for a mean of 67.5 (± 57.8 months).
The first colonoscopy screening detected cancer in 49 patients (MLH1, n = 23/268; MSH2, n = 18/249; MSH6, n = 4/154; PMS2, n = 2/47; EPCAM, n = 2/13), extrapolating to a cumulative incidence of 25.6% for MLH1, 22.1% for MSH2, 6.3% for MSH6, and 25.9% for PMS2 mutation carriers.
Most patients were diagnosed with stage 1 disease (45.7%) and, to a lesser degree, with stage II (28.6%), stage III (22.9%), and stage IV (2.9%).
The 10-year cumulative incidences for subsequent colorectal cancers for patients who had a previous diagnosis of the disease were 9.4% (95% CI, 5-17) for MLH1, 12.6% (95% CI, 5.6-27.6) for MSH2, and 17.2% (95% CI, 6.6-40) for MSH6.
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – Lynch syndrome can predispose individuals to a number of different cancer types, including colorectal tumors, but the results of a preliminary study found that the incidence may be lower than what has been previously reported.
New findings presented at Digestive Disease Week® showed that the incidence of colorectal cancer after screening with colonoscopy ranged from 6.3% to 25.9%, depending on the specific mutated gene. This is in contrast to other reports which have found a 50% increase in the incidence of colorectal cancer in individuals with Lynch syndrome.
“We looked at the incidence of colorectal cancer and other associated cancers in individuals with Lynch syndrome and how screening can have an impact on that,” said study author Ariadna Sanchez, MD, from the Hospital Clínic de Barcelona, Spain.
She emphasized that the results being presented at the meeting are preliminary and, thus, will need further confirmation, but it is a multicenter study and is being conducted in more than 1,100 patients.
“The diagnosis of colorectal cancer with screening colonoscopy is lower than results that have been previously published,” she said. “This finding reinforces the importance of screening colonoscopies in patients with Lynch syndrome.”
As to why their rates are lower, Dr. Sanchez speculated that it may be because precancerous polyps are being removed with screening.
“Our thinking is that, as we perform screening and remove the polyps, then in theory, cancer will be prevented,” she explained, “since they didn’t have the opportunity to continue to progress into cancer.”
In other words, screening this high-risk population may not only identify those who have cancer but may prevent it from developing in others.
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is an inherited disorder that increases the risk of many types of cancer, including colorectal and cancers of the stomach, small intestine, liver, gallbladder ducts, upper urinary tract, brain, skin, ovaries, and endometrium.
Caused by germline mutations in the mismatch DNA repair system (MLH1, MSH2, MSH6, PMS2), it has been difficult to make precise estimates of cancer risk in individuals with the syndrome because of retrospective studies and small cohorts.
Dr. Sanchez and her colleagues conducted a multicenter nation-wide study in Spain with the goal of establishing the cumulative incidence of colorectal cancer and other tumor types in Lynch syndrome and to evaluate the effect of screening surveillance on cancer incidence. Cancer-specific survival will also be assessed.
The cohort included 1,108 patients with Lynch syndrome from 25 centers, who were followed-up for a mean of 67.5 (± 57.8 months).
The first colonoscopy screening detected cancer in 49 patients (MLH1, n = 23/268; MSH2, n = 18/249; MSH6, n = 4/154; PMS2, n = 2/47; EPCAM, n = 2/13), extrapolating to a cumulative incidence of 25.6% for MLH1, 22.1% for MSH2, 6.3% for MSH6, and 25.9% for PMS2 mutation carriers.
Most patients were diagnosed with stage 1 disease (45.7%) and, to a lesser degree, with stage II (28.6%), stage III (22.9%), and stage IV (2.9%).
The 10-year cumulative incidences for subsequent colorectal cancers for patients who had a previous diagnosis of the disease were 9.4% (95% CI, 5-17) for MLH1, 12.6% (95% CI, 5.6-27.6) for MSH2, and 17.2% (95% CI, 6.6-40) for MSH6.
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – Lynch syndrome can predispose individuals to a number of different cancer types, including colorectal tumors, but the results of a preliminary study found that the incidence may be lower than what has been previously reported.
New findings presented at Digestive Disease Week® showed that the incidence of colorectal cancer after screening with colonoscopy ranged from 6.3% to 25.9%, depending on the specific mutated gene. This is in contrast to other reports which have found a 50% increase in the incidence of colorectal cancer in individuals with Lynch syndrome.
“We looked at the incidence of colorectal cancer and other associated cancers in individuals with Lynch syndrome and how screening can have an impact on that,” said study author Ariadna Sanchez, MD, from the Hospital Clínic de Barcelona, Spain.
She emphasized that the results being presented at the meeting are preliminary and, thus, will need further confirmation, but it is a multicenter study and is being conducted in more than 1,100 patients.
“The diagnosis of colorectal cancer with screening colonoscopy is lower than results that have been previously published,” she said. “This finding reinforces the importance of screening colonoscopies in patients with Lynch syndrome.”
As to why their rates are lower, Dr. Sanchez speculated that it may be because precancerous polyps are being removed with screening.
“Our thinking is that, as we perform screening and remove the polyps, then in theory, cancer will be prevented,” she explained, “since they didn’t have the opportunity to continue to progress into cancer.”
In other words, screening this high-risk population may not only identify those who have cancer but may prevent it from developing in others.
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is an inherited disorder that increases the risk of many types of cancer, including colorectal and cancers of the stomach, small intestine, liver, gallbladder ducts, upper urinary tract, brain, skin, ovaries, and endometrium.
Caused by germline mutations in the mismatch DNA repair system (MLH1, MSH2, MSH6, PMS2), it has been difficult to make precise estimates of cancer risk in individuals with the syndrome because of retrospective studies and small cohorts.
Dr. Sanchez and her colleagues conducted a multicenter nation-wide study in Spain with the goal of establishing the cumulative incidence of colorectal cancer and other tumor types in Lynch syndrome and to evaluate the effect of screening surveillance on cancer incidence. Cancer-specific survival will also be assessed.
The cohort included 1,108 patients with Lynch syndrome from 25 centers, who were followed-up for a mean of 67.5 (± 57.8 months).
The first colonoscopy screening detected cancer in 49 patients (MLH1, n = 23/268; MSH2, n = 18/249; MSH6, n = 4/154; PMS2, n = 2/47; EPCAM, n = 2/13), extrapolating to a cumulative incidence of 25.6% for MLH1, 22.1% for MSH2, 6.3% for MSH6, and 25.9% for PMS2 mutation carriers.
Most patients were diagnosed with stage 1 disease (45.7%) and, to a lesser degree, with stage II (28.6%), stage III (22.9%), and stage IV (2.9%).
The 10-year cumulative incidences for subsequent colorectal cancers for patients who had a previous diagnosis of the disease were 9.4% (95% CI, 5-17) for MLH1, 12.6% (95% CI, 5.6-27.6) for MSH2, and 17.2% (95% CI, 6.6-40) for MSH6.
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
Key clinical point: Colorectal cancer screening may lower the incidence of disease in a high-risk population of individuals with Lynch syndrome.
Major finding: The incidence of colorectal cancer was lower than has been previously reported in Lynch syndrome, possibly because of the removal of polyps during screening.
Data source: Prospective multicenter study that included 1,108 patients with Lynch syndrome who underwent colonoscopy screening.
Disclosures: Dr. Sanchez has no disclosures.